Abstract
Acute lymphoblastic leukemia (ALL) is the most common pediatric cancer. Vincristine is a core chemotherapeutic agent for patients with ALL; unfortunately, approximately 78% will develop vincristine-induced peripheral neuropathy (VIPN). VIPN can result in vincristine dose reductions that decrease therapeutic efficacy: making it important to understand which children are at highest risk for VIPN. We hypothesized that pediatric ALL patients who were obese at diagnosis would develop worse VIPN than healthy weight children with ALL within the first year. Our results confirmed that obese pediatric patients have significantly (p=0.03) worse VIPN than patients of healthy weight.
Keywords: Cancer, Neuropathy, Pediatrics, Obesity, Vincristine
INTRODUCTION
The most common pediatric cancer is leukemia (26%); acute lymphoblastic leukemia (ALL) is the most prevalent subtype (75%)1. Vincristine is one of several chemotherapies included in standard ALL treatment1. Although improved treatments have led to a 90% 5-year survival rate1, approximately 78% of children develop significant peripheral neuropathy due to vincristine-induced peripheral neuropathy (VIPN)2. VIPN is observed early in treatment3 and is characterized by progressive motor, sensory and autonomic impairment. VIPN may lead to vincristine dose reduction with decreased therapeutic exposure and efficacy4–6.
VIPN not only jeopardizes therapeutic efficacy, but may negatively affect quality of life for children long after treatment completion7–9. A study of 531 adult survivors of pediatric cancers revealed evidence that exposure to cumulative doses of vinca-alkaloids significantly increases the risk of motor, but not sensory, impairment in adulthood8. Additionally, by age 45, the majority of survivors (95.5%) will experience at least one chronic condition such as obesity, type-2 diabetes, metabolic syndrome, and cardiovascular disease, and most (80.5%) will also develop at least one severe or life-threatening condition in adulthood10.
There are many reasons some children experience worse VIPN. One explanation may be genetics. Results from our previous four-center genetic association study demonstrated that vincristine is metabolized more efficiently by CYP3A5 enzyme as compared to CYP3A411,12. Furthermore, the severity of VIPN is significantly worse in Caucasian children due to the fact they are more likely not to have the CYP3A5*1 allele13, which is responsible for producing fully functioning CYP3A5 enzyme13,14.
Other reasons may be non-genetic. For example, in a study examining risk factors for neuropathy in diabetic patients, it was determined that obesity and hypertriglyceridemia significantly increased the risk for peripheral neuropathy independent of glucose control. Moreover, these factors had more deleterious effects on the small unmyelinated C fibers involved with sensation as compared to the large myelinated fibers associated with motor conduction velocity15. Unmyelinated C fibers are responsible for mechanical, thermal, and chemical pain, identical to the type of pain observed in our patient population with VIPN.
Given the findings of this study and the fact we observed differences in the severity of VIPN in children being treated for ALL based on their body weight, our research team conducted an analysis of a subset of data from our genetic association study specifically examining the association between body mass index (BMI) at time of diagnosis and severity of neuropathy over the first 18 months of treatment.
METHODS
We screened data from our original genetic study for patients classified as either healthy or obese at time of their diagnosis of ALL. A cohort of 49 patients between the ages of 2 – 18 was used for secondary analysis (21 male and 28 female Caucasians). All patients had a diagnosis of precursor B cell ALL and were receiving vincristine as part of his/her primary therapy and the only neurotoxic drug in their schedule. All patients had standard vincristine which was capped at 2mg. Enrollment occurred prior to or within 7 days of initiation of chemotherapy.
Patients were assigned to a weight category based on baseline BMI percentile for age prior to any treatment. The categories were: Healthy (5th – 85th %); Obese (> 95th %). Utilizing these categories, 40 patients were in the healthy weight category (20 male; 20 female) and 9 patients were in the obese category (1 male; 8 female). Due to the small patient numbers for the obese as compared to the healthy group, we could not look at gender or age as a covariate. Additionally, since all patients were Caucasian, we could not examine race as a risk factor either. For a summary of patient demographic data see Table 1. Neuropathy was measured at baseline, weekly during induction, approximately monthly during consolidation, quarterly during the first two years of maintenance chemotherapy, and annually thereafter. Neuropathy was quantified using the Total Neuropathy Score – Pediatric Vincristine (TNS-PV), a pediatric-focused variation of the TNS validated by our research team2. The neuropathy assessments were performed by pediatric neurologist-trained evaluators. Inter-rater reliability was also measured and determined to be strong2. The TNS-PV assesses the proximal extension of numbness, tingling, neuropathic pain, vibration and temperature, muscle strength, and tendon reflexes, as well as constipation and vocal cord function. Assessment methods have been previously described3.
Table 1:
Demographic data for all subjects included in the study.
| Females (28) | Males (21) | |
|---|---|---|
| Healthy | 20 | 20 |
| Obese | 8 | 1 |
| Caucasian | 28 | 21 |
| Healthy BMI Percentile (average) | 0.46 | 0.44 |
| Obese BMI Percentile (average) | 0.98 | 0.96 |
RESULTS
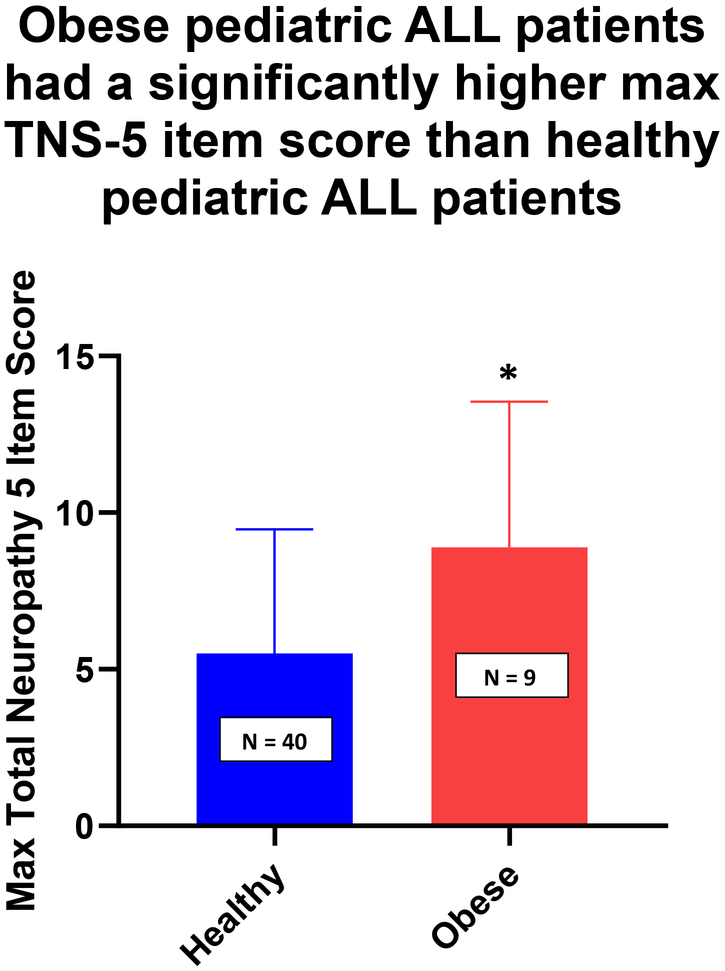
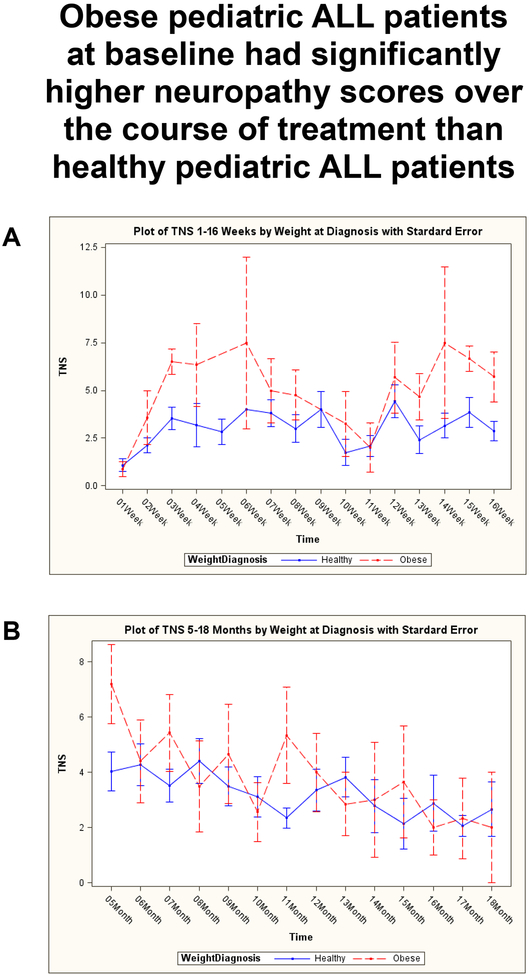
Analysis with an unpaired t-test revealed obese patients had significantly worse maximum neuropathy severity scores as compared to healthy weight patients during the first year of treatment for ALL (Figure 1, Mean: Healthy = 5.5, Obese = 8.9; SD: Healthy = 0.6, Obese = 1.5; p = 0.03). Further analysis of the data using a mixed effect model, indicated obese patients also experienced worse neuropathy over an 18 month treatment timeframe (Figure 2, p <0.00001). However, there is a significant negative interaction effect of obesity on neuropathy scores over time as noted in Table 2 (Month*Weight, p = 0.03). The findings indicate that the neuropathy scores of the obese patients decrease to be more in-line with the healthy weight group by 18 months. A second major finding of the mixed effect model was that if at any time point throughout the treatment a patient was categorized as obese, then they showed an increase in their neuropathy score (Table 2, Weight). Although not significant, there was a strong trend (p = 0.08). A correlation analysis for CYP3A5 and weight showed no significant difference in genotypes between the two groups (data not shown). Additional analysis compared the average doses between healthy and obese patients at each measurement time (1w-3w, 1m-12m) as well as comparing the average dose over all measurements (Dose Average_month) and no significant difference was found between the doses of vincristine administered to either group (data not shown).
Fig 1:
Average Total Neuropathy Score-5 for healthy and obese ALL patients during the first 12 months of treatment with vincristine. Data are presented as average score ± SD. *p= 0.03.
Fig 2:
Average Total Neuropathy Score – 5 for healthy and obese ALL patients during the first 18 months of treatment with vincristine. A) Shown for individual weeks 1–16 and B) shown for individual months 5–18. Analysis with a mixed effect model indicated that subjects who were classified as obese at time of diagnosis had significantly greater neuropathy scores over treatment time p<0.00001
Table 2:
ALL patients categorized as obese at any point during treatment trended to have worse neuropathy. A mixed effect model was used to look at the effect of weight over time on TNS over time. At any time point during treatment if a patient was categorized as obese, they showed a trend in having worse neuropathy (p=0.0772 marginal significance). The effect of obesity on TNS decreases over time (significant interaction effect with a negative sign p=0.0218).
| Effect | Estimate | Standard Error | DF | T Value | P-value |
|---|---|---|---|---|---|
| Month | 0.03633 | 0.02845 | 397 | 1.28 | 0.2024 |
| Weight (Obese vs. Healthy) | 1.1195 | 0.6317 | 397 | 1.77 | 0.0772 |
| Month*Weight (Obese vs. Healthy) | −0.1293 | 0.05614 | 397 | −2.30 | 0.0218 |
DISCUSSION
Approximately eight in ten children with ALL will experience significant VIPN2. Given the high survival rate, most of these children will become adult cancer survivors and subsequently develop health issues at a greater rate and at earlier ages than individuals without a history of cancer7–9. We hypothesize that chronic neuropathy may be associated with long-term health complications such as obesity. Thus, it is important to understand what traits, genetic or non-genetic, can lead to worse neuropathy in children receiving chemotherapy.
The findings of our study support the hypothesis that obesity (based on BMI percentile) is an underlying factor associated with worse VIPN in children diagnosed with ALL. Conversely, a retrospective study conducted by Hijiya and colleagues using the St. Jude Total Therapy studies and examining the effect of body weight on pharmacokinetics, toxicity, and outcome of treatment in children with ALL did not find any difference in neuropathy based on BMI16. The St. Jude study focused on children receiving high dose methotrexate and, while we know that methotrexate can also have toxic effects, severe neuropathy induced by this drug is very rare (<1%) and manifests as reversible optic neuropathy, not peripheral neuropathy17. In addition, the common terminology criteria v2.0 was used to assess toxicity in that study while we used TNS-PV, a selective neuropathy assessment with greater sensitivity. Therefore, even though both studies assessed neuropathy and obesity in children with ALL, the chemotherapeutic treatments and assessment tools were quite different and most likely the reason for dissimilar findings.
Since vincristine dosing is based on body surface area, which accounts for weight and height, we also hypothesized that young obese children develop worse neuropathy because they received higher doses than healthy weight children. However, since vincristine doses are capped at 2 mg regardless of BMI, we concluded that dose was not likely a contributing factor to the differences in VIPN. Furthermore, our analysis showed that there was no difference in vincristine dosage at any time point or cumulatively between the groups.
It is well-established that adipose tissue is not just an energy store, but also an endocrine organ18. Humans and animals studies show that obesity is a pro-inflammatory state, producing and releasing many pro-inflammatory factors19. It is possible in obese subjects that there is greater release of pro-inflammatory cytokines and other factors which, in turn, enhance vincristine’s neuropathic effects. Subsequently, once the body has time to compensate for the inflammatory response and subsequently the effects on the peripheral nerves, obese subjects would begin to appear more like healthy subjects over time. This hypothesis could explain why when a subject moved into the obese category, their neuropathy scores went up.
As mentioned previously, a study in diabetic patients demonstrated an effect of obesity and hypertriglyceridemia as factors leading to worse peripheral neuropathy independent of glucose control15. We know hypertriglyceridemia can be induced with exposure to asparaginase and steroids, both of which are normal treatment for ALL, so it is possible that they are contributing factors. However, given the standardization of these treatments both groups would have been exposed to the same amount thus, it is probably not a major contributing factor.
Another possible reason for our findings is that vincristine is stored in the adipose tissue and therefore remains in the body longer resulting in greater exposure to the peripheral nerves over time in obese patients. A recent mouse study using obese and control mice demonstrated that by the third hour after vincristine injection, drug levels were significantly higher in adipose tissue of obese mice20.
Our study resulted in significant findings; however, due to the limitations of the study, such as size of each group and the inability to stratify by age or sex, we cannot conclude that other variables may be important as well. We will repeat this analysis in a larger cohort to understand other specific factors which may contribute to increased neuropathy in an obese population.
Overall, the data from the current study provide evidence of interplay between obesity and maximal VIPN in children with ALL receiving vincristine. The next key step is to understand the safest, most effective approach to dosing in order to minimize these risks. Future studies are warranted that test interventions which can decrease obesity in this population and be implemented early in cancer treatment. It should be noted here that there is data suggesting that when patients lose weight during therapy (even if obese), it can be associated with worse outcomes. So future studies should be conducted to better evaluate pharmacokinetics and drug distribution of vincristine in obese pediatric patients. This will allow the administration of vincristine in obese patients with minimized toxicity.
Acknowledgements
The authors wish to thank Celia Bridges, BSN, MA, RN, Robert Knoerl, PhD, RN, and James P. Kelly, BS from the University of Michigan for their assistance in conducting neuropathy assessments and collecting biospecimens. J.R. also gratefully acknowledges funding from the NIH grant R01 HD062484.
Conflicts of Interest and Source of Funding:
The authors whose names are listed on the title page certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript. This study was supported by the National Institutes of Health National Institute of Childhood Health and Disease R01 HD062484.
REFERENCES
- 1.Society AC. Cancer Facts and Figures. 2014; http://www.cancer.org/acs/groups/content/@research/documents/webcontent/acspc-041787.pdf. Accessed August 4, 2016.
- 2.Lavoie Smith EM, Li L, Hutchinson RJ, et al. Measuring vincristine-induced peripheral neuropathy in children with acute lymphoblastic leukemia. Cancer Nurs. 2013;36(5):E49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lavoie Smith EM, Li L, Chiang C, et al. Patterns and severity of vincristine-induced peripheral neuropathy in children with acute lymphoblastic leukemia. J Peripher Nerv Syst. 2015;20(1):37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gidding CE, Meeuwsen-de Boer GJ, Koopmans P, Uges DR, Kamps WA, de Graaf SS. Vincristine pharmacokinetics after repetitive dosing in children. Cancer Chemother Pharmacol. 1999;44(3):203–209. [DOI] [PubMed] [Google Scholar]
- 5.Gomber S, Dewan P, Chhonker D. Vincristine induced neurotoxicity in cancer patients. Indian J Pediatr. 2010;77(1):97–100. [DOI] [PubMed] [Google Scholar]
- 6.Toopchizadeh V, Barzegar M, Rezamand A, Feiz A. Electrophysiological consequences of vincristine contained chemotherapy in children: A cohort study. J Pediatric Neurology. 2009;7(4):351–356. [Google Scholar]
- 7.Lehtinen SS, Huuskonen UE, Harila-Saari AH, Tolonen U, Vainionpaa LK, Lanning BM. Motor nervous system impairment persists in long-term survivors of childhood acute lymphoblastic leukemia. Cancer. 2002;94(9):2466–2473. [DOI] [PubMed] [Google Scholar]
- 8.Ness KK, Jones KE, Smith WA, et al. Chemotherapy-related neuropathic symptoms and functional impairment in adult survivors of extracranial solid tumors of childhood: results from the St. Jude Lifetime Cohort Study. Arch Phys Med Rehabil. 2013;94(8):1451–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Postma TJ, Benard BA, Huijgens PC, Ossenkoppele GJ, Heimans JJ. Long-term effects of vincristine on the peripheral nervous system. J Neurooncol. 1993;15(1):23–27. [DOI] [PubMed] [Google Scholar]
- 10.Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309(22):2371–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dennison JB, Jones DR, Renbarger JL, Hall SD. Effect of CYP3A5 expression on vincristine metabolism with human liver microsomes. J Pharmacol Exp Ther. 2007;321(2):553–563. [DOI] [PubMed] [Google Scholar]
- 12.Dennison JB, Kulanthaivel P, Barbuch RJ, Renbarger JL, Ehlhardt WJ, Hall SD. Selective metabolism of vincristine in vitro by CYP3A5. Drug Metab Dispos. 2006;34(8):1317–1327. [DOI] [PubMed] [Google Scholar]
- 13.Renbarger JL, McCammack KC, Rouse CE, Hall SD. Effect of race on vincristine-associated neurotoxicity in pediatric acute lymphoblastic leukemia patients. Pediatr Blood Cancer. 2008;50(4):769–771. [DOI] [PubMed] [Google Scholar]
- 14.Egbelakin A, Ferguson MJ, MacGill EA, et al. Increased risk of vincristine neurotoxicity associated with low CYP3A5 expression genotype in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2011;56(3):361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith AG, Singleton JR. Obesity and hyperlipidemia are risk factors for early diabetic neuropathy. J Diabetes Complications. 2013;27(5):436–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hijiya N, Panetta JC, Zhou Y, et al. Body mass index does not influence pharmacokinetics or outcome of treatment in children with acute lymphoblastic leukemia. Blood. 2006;108(13):3997–4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clare G, Colley S, Kennett R, Elston JS. Reversible optic neuropathy associated with low-dose methotrexate therapy. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2005;25(2):109–112. [DOI] [PubMed] [Google Scholar]
- 18.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89(6):2548–2556. [DOI] [PubMed] [Google Scholar]
- 19.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115(5):911–919; quiz 920. [DOI] [PubMed] [Google Scholar]
- 20.Behan JW, Avramis VI, Yun JP, Louie SG, Mittelman SD. Diet-induced obesity alters vincristine pharmacokinetics in blood and tissues of mice. Pharmacol Res. 2010;61(5):385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]