Abstract
Extracorporeal membrane oxygenation (ECMO) has become a mainstay of therapy for patients suffering from severe respiratory failure. Ambulatory ECMO systems aim to provide long-term out-of-hospital respiratory support. As a patient’s activity level changes, the required level of ECMO support varies with oxygen consumption and metabolic fluctuations. To compensate for such changes, an Auto-Regulatory ECMO system (AR-ECMO) has been developed and its performance was evaluated as a proof-of-concept in an acute ovine model.
The AR-ECMO system consists of a regular ECMO circuit and an electromechanical control system. A custom fuzzy-logic control algorithm was implemented to adjust the blood flow and sweep gas flow of the ECMO circuit to meet the varying respiratory demand by utilizing two noninvasive sensors for venous oxyhemoglobin saturation and the oxygenator exhaust gas CO2 concentration. Disturbance responses of the AR-ECMO to induced acute respiratory distress were assessed for six hours in four juvenile sheep cannulated with a veno-pulmonary artery ECMO configuration, including acute ventilator shutoff, ventilator step-change (off-on-off), and forced desaturation.
All sheep survived for the study duration. The AR-ECMO system was able to respond and maintain stable hemodynamics and physiological blood gas contents (SpO2 = 96.3 % ± 4.29, pH = 7.44 ± 0.09, pCO2 = 38.9 ± 9.9 mmHg, and pO2 =237.9 ± 123.6 mmHg) during simulated respiratory distress. Acceptable correlation between oxygenator exhaust gas CO2 and oxygenator outlet pCO2 were observed (R2= 0.84). In summary, the AR-ECMO system successfully maintained physiologic control of peripheral oxygenation and carbon dioxide over the study period, utilizing only measurements taken directly from the ECMO circuit. The range of system response necessitates an adaptable system in the setting of variable metabolic demands. The ability of this system to respond to significant disturbances in ventilator support is encouraging. Future work to evaluate our AR-ECMO system in long-term, awake animal studies is necessary for further refinement.
Keywords: Respiratory distress, extracorporeal membrane oxygenation (ECMO), artificial lung, auto-regulatory control
Introduction
Acute respiratory failure is responsible for nearly 2 million hospitalizations annually1, with chronic obstructive pulmonary disease (COPD) ranking as the 4th leading cause of death in the United States2. For many of these patients, mechanical ventilation is insufficient at providing long-term respiratory support, and extracorporeal membrane oxygenation (ECMO) must be utilized. Over the past decade, the use of ECMO has seen a greater than four-fold increase in use, with nearly 11,408 cases performed worldwide in 20173. This widespread adoption of ECMO as a therapy for severe respiratory failure has led to maturation in ECMO technology and treatment strategies, with patient survival improving in lockstep. One advance in the delivery of ECMO includes the use of ambulation for patients on ECMO therapy, with studies reporting improved outcomes for select patients ambulating on ECMO4–8.
Currently, there is no truly ambulatory ECMO system that is deployable without intensive supervision. Patients ambulating on traditional ECMO require assistance by a team of nurses, perfusionists, and physical therapists during ambulation. Innovative ambulatory ECMO solutions are on the horizon, however, with encouraging results in animal studies9–12. These devices – often referred to as “Artificial Lungs” – are compact and are specifically designed for mobility and long-term use. However, current implementations of the Artificial Lung are unable to automatically adjust the oxygen delivery and carbon dioxide removal to meet the varying need of respiratory support while the patient is active and ambulating. In simulation studies of patients supported on ECMO with severe end-stage interstitial lung disease, it was found that ECMO blood flow was required to change from 2.5 LPM to 8.5 LPM as the simulated patient’s activity level increased from rest to exercise13. An Artificial Lung that requires manual adjustment of blood flow or sweep gas flow will require either complicated patient training, or assistance by a skilled caretaker.
To overcome this problem, an automatic control system could be designed to facilitate adaptation of the level of ECMO support to the patient’s physiologic requirements. Indeed, there has been some effort in the development of a closed-loop system for the automation of such a system over the past decade. Kopp et al describe a cascaded control system, termed SmartECLA, that maintains SpO2 and pCO2 in pigs on venovenous (VV) ECMO, while delivering a hypoxic (FiO2 of 15%) gas mixture to the native lungs14. Similarly, a venoarterial (VA) ECMO control system is described by Mendoza Garcia et al, comparing adaptive to non-adaptive fuzzy control systems in simulation and in acute animal studies15. Despite the promise of these described approaches, they are implemented for use in the stationary critically-ill patient. These adaptive systems are not targeted at the ambulatory patient who may benefit from an automatic lung-assist device. Additionally, their control solutions have a common reliance on an invasive peripheral blood gas monitoring unit, which limits their practical applicability. As such, we sought to develop a closed-loop, auto-regulatory ECMO control system (AR-ECMO) for use on an ambulatory artificial lung that is under development at our institution. Our AR-ECMO implementation was designed to adjust the level of support based on physiologic measurements taken directly from the ECMO circuit, without the need for additional invasive sensors. Our system implements a fuzzy-logic control system, which translates real-world measurements into degrees of participation in multiple “fuzzy” response rules. The system then computes a response to the error as a combination of these rules, and generates a real-world output in return.
Methods
Auto-Regulatory ECMO System
The AR-ECMO system was developed by utilizing an electromechanical control system to adjust the blood flow and sweep gas flow of a VV-ECMO circuit according to desired physiologic targets. The AR-ECMO system utilizes the mixed venous oxyhemoglobin saturation (SvO2) at the oxygenator inlet as the physiologic target for oxygenation, and the oxygenator exhaust gas CO2 concentration (ExCO2) as the physiologic target for CO2 removal. The AR-ECMO system configuration requires specification of the target SvO2, the target oxygenator return pCO2, a regression equation relating oxygenator return pCO2 to ExCO2, and upper and lower limits for blood and gas flows. The AR-ECMO control system utilizes two inter-related fuzzy control algorithms – one for blood flow, and one for sweep gas flow. The blood flow control algorithm measures instant SvO2 at the oxygenator inlet, and determines the error (difference) between this measurement and the target SvO2, as well as the rate of change of this error. The specific error may represent either a deficit in oxygenation, or a surplus of oxygenator support. Using the SvO2 error and its rate of change as inputs, the fuzzy control algorithm determines the blood flow change required to return the measured SvO2 to the target. The blood flow fuzzy control algorithm is self-improving, and implements a fuzzy model reference learning controller (FMRLC). The FMRLC compares the SvO2 response curve to an “ideal” response, pre-specified as a second-order response to a step disturbance that settles over a 10 second period of time. Deviations between the actual SvO2 response and the “ideal” SvO2 response cause the algorithm to adjust the magnitude of the blood flow response calculations, such that over time the system improves its performance automatically. The sweep gas flow control loop implements a fuzzy control algorithm, but does not implement a similar FMRLC. The sweep gas flow is calculated as a function of a fuzzy control algorithm that uses both the oxygenator return pCO2 error and its rate of change as inputs.
The fuzzy control system is implemented as a custom software solution (Microsoft C# .NET, v4.0), calculates the appropriate changes to blood flow or sweep gas flow based on the measured physiologic inputs. The fuzzy Control system communicates with a 32‐bit ARM core microcontroller (Arduino Due, www.arduino.cc, Somerville, MA), which interfaces directly with the hardware components. The communication between the fuzzy control system and the Arduino Due is bi-directional, with target flows set by the fuzzy control system, and measured flow rates and ExCO2 concentration transmitted from the Arduino Due to the fuzzy control system. The interface with the oxyhemoglobin saturation meter is implemented in the fuzzy control system directly. The Arduino Due implements two independent software-based proportional-integral-derivative (PID) controllers to maintain the blood flow and sweep gas flow at the desired settings. The blood flow setting is adjusted via communication with an electronically controllable motor driver for the blood pump. The sweep gas flow is adjusted by varying the position of a stepper motor that is coupled to a needle valve which throttles the sweep gas source. We constructed the VV-ECMO circuit using a custom centrifugal blood pump with a custom polymethylpentene hollow fiber membrane adult oxygenator. Both the pump and oxygenator were developed at our laboratory for ambulatory respiratory support in partnership with Breethe, Inc. (Halethorpe, MD). Their performances meet the requirements for adult respiratory support. For the range of blood flow rates in this study, the oxygen saturation level at the outlet of the custom oxygenator was always higher than 99%. Specifically, we use an optical oxyhemoglobin saturation meter that measures the ECMO circuit inlet and outlet oxyhemoglobin saturation levels (BioTrend oxygen saturation and hematocrit monitor, Medtronic plc, Minneapolis, MN).
The relationship between the ExCO2 and the oxygenator outlet pCO2 is determined via a linear regression which was established based on the data collected from previously experimentally measured ExCO2 and oxygenator outlet pCO2. The high-level software allows for specification of a regression equation for the estimated oxygenator outlet pCO2 as a function of the measured ExCO2. The AR-ECMO system was evaluated in four acute experiments in an ovine model as described below. Of note, the oxygenator exhaust gas pathway was modified after the first experiment by adding a collecting cap and 2-meters of exhaust tubing to the oxygenator outlet to prevent uncontrolled diffusion of CO2 to the atmosphere. As such, ExCO2 results for Sheep 1 are not included in this report.
In-Vivo Evaluation
Surgical Procedure
Four healthy juvenile Dorset hybrid sheep (male, 33~52 kg) bred for laboratory research (Archer Farms, Darlington, MD) were used in the study. Each sheep was examined physically with basic laboratory tests (complete blood counts, white blood cell differential, serum chemistry, C-reactive protein and coagulation panel) to ensure the physical health status. All sheep were hosted in dedicated rooms with 12 hours of daytime light, free to access a supply of fresh water and fed with a balanced diet including roughage (hay), manufactured feed. All sheep received humane care in accordance with the Guide for Care and Use of Laboratory Animals (Eighth Edition, 2011).
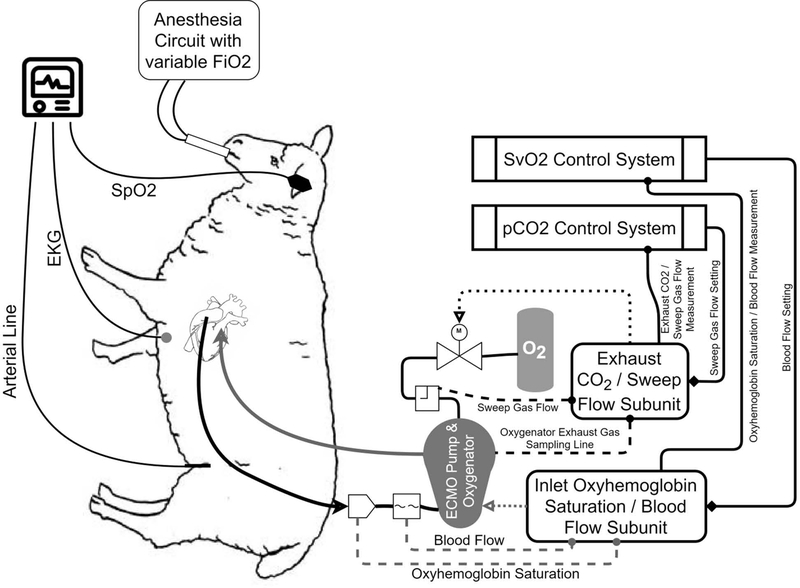
The sheep were induced with Ketamine (3–5 mg/kg)/ Xylazine (0.03–0.05mg/kg) through intramuscular injection. Then, the sheep were intubated and anesthetized using mechanical ventilation with 1–3% isoflurane or propofol as supplemental anesthetic drug at a dose of 100–400 μg/kg/min (IV). Following induction of general anesthesia, the sheep underwent placement of a femoral arterial line for arterial pressure measurement and arterial blood gas samples, followed by left thoracotomy and central cannulation of the right atrium (32Fr, venous drainage, Medtronic, Minneapolis, MN) and pulmonary artery (27Fr, arterial return, Medtronic, Minneapolis, MN). Cannulae were attached to the AR-ECMO circuit which was pre-assembled and primed with sterile heparinized saline (heparin: 2 U/mL). After the implantation of the AR-ECMO circuit, the upper limit of the ECMO blood flow was determined by increasing the pump speed in a stepwise fashion until inlet starvation (suckdown) was encountered. Anesthesia was provided via endotracheal isoflurane prior to initiation of the AR-ECMO support and was transitioned to propofol (100–400 μg/kg/min) and ketamine (3–5 mg/kg) (IV) continuous-rate infusions after ventilator support was stopped. Experimental setup and a logical block diagram of the AR-ECMO system are depicted in Figure 1. The surgical procedure and care were carried out according to the approved protocol by the Institutional Animal Care and Use Committee (IACUC) of the University of Maryland School of Medicine.
Figure 1.
Experimental setup and AR-ECMO logical block diagram.
Transition to AR-ECMO Support
The respiratory needs of each sheep were initially provided with mechanical ventilation delivered via an endotracheal tube, with an FiO2 of 100% and a minute ventilation of 6 L/min. A series of stepwise reductions in FiO2 down to 0% were first initiated over a 10-minute period, followed by stepwise reduction in minute ventilation over a 10-minute period. After the reduction of FiO2 to 0% and minute ventilation to 0 L/min, the ventilator was shut off and the sheep was supported entirely on the AR-ECMO.
Disturbance-Response Assessment
Three separate disturbances in ventilator support provided to the native lungs of the sheep were designed to evaluate the limits of performance of the AR-ECMO system. In all scenarios, step-wise changes in ventilator support were implemented in order to assess the AR-ECMO system’s response to the most extreme disturbances, anticipating that disturbances in real patients are more likely to be gradual. In scenario 1, a rapid respiratory distress was produced by a sudden shutoff of the mechanical ventilation. Initially, the sheep was supported simultaneously by both the ventilator with 100% FiO2 and 6 L/min minute ventilation through the native lungs, and the AR-ECMO system which equilibrated to these ventilator settings. The mechanical ventilation was suddenly stopped, and the response of the AR-ECMO system to this sudden change in respiratory support was tracked.
In Scenario 2, a step-change was employed in ventilator support, where the AR-ECMO system was initially stabilized with no native ventilator support. The ventilator was then abruptly switched on to provide 6 L/min of minute ventilation with 100% FiO2. After 5 minutes of ventilator support, the ventilator was abruptly switched off again. The AR-ECMO system response was tracked during this series of events.
The response of the AR-ECMO system to an acute respiratory failure created by a forced desaturation event was also assessed in Scenario 3. In this assessment, the AR-ECMO system was placed in a fixed mode, and both the ECMO blood flow and sweep gas flow rates were set to 1 L/min while the ventilator was turned off and providing no native lung support. This blood flow rate was intentionally chosen to be inadequate for support of the oxygenation needs of the sheep, and the peripheral oxyhemoglobin saturation (SpO2) declined accordingly. Once the animal’s SpO2 reached a level below 85%, the AR-ECMO system was switched to auto-regulatory mode, and the system was permitted to rescue the sheep.
In all disturbance response assessments we continuously recorded the blood flow rates (Transonic flow meter, model T402, Transonic Systems, Ithaca, NY), sweep gas flow rates (Fathoms gas flow meter, GR Series, Fathoms Technology, Georgetown, TX), SvO2 (BioTrend oxygen saturation and hematocrit monitor, Medtronic plc, Minneapolis, MN), SpO2 (Oximax N-65, Nellcor Medtronic, Minneapolis, MN), Heart Rate, Arterial Pressure (indwelling femoral arterial line, Patient monitor Solar 8000, GE Healthcare, Chicago, IL) and exhaust gas CO2 concentration (SprintIR GC-0017, CO2 Meter, Ormond Beach, FL). Arterial blood gas samples were taken from the femoral arterial line every 15 minutes for the first hour of AR-ECMO support, and at 30 minute intervals for hours 2 through 6. The data were recorded at a sample rate of 250 Hz.
Data Analysis
Data are presented as means with SD. Longitudinal data are graphed with respect to time. Simple linear regression was used to relate ExCO2 to oxygenator outlet pCO2. All data analysis was performed in Microsoft Excel (2010 Version) or SAS 9.4 (SAS Ins., Cary, NC) as necessary.
Results
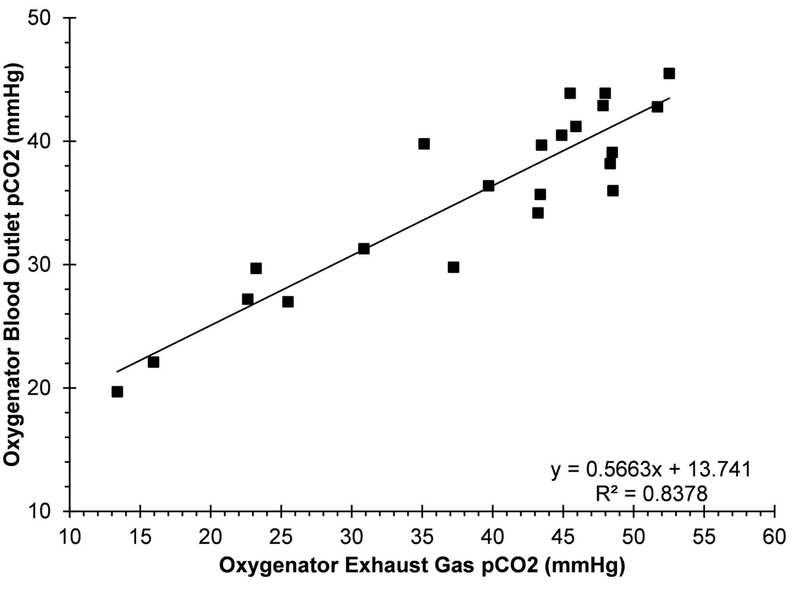
Overall, 4 sheep underwent surgical cannulation without any technical issue and were placed on a VV-ECMO support mode with the AR-ECMO system. All sheep survived for the duration of the 6-hour experiment. The maximum-achievable blood flow-rate, peripheral SpO2, heart rate, mean arterial pressure and blood gas parameters are summarized in Table 1. In combination, 93.7% of measurements showed a SpO2 of at least 90% when the sheep were on the AR-ECMO support. The pCO2 in the peripheral blood was between 35–45 mmHg in 48.9% of measurements overall when the target oxygenator exhaust CO2 concentration ranged from 30–45 mmHg. When the oxygenator exhaust CO2 was set to a specific target of 40 mmHg, the peripheral CO2 concentration showed a mean of 37.7±3.88 mmHg and was within 35–45 mmHg in 66.7% of measurements. Consistent with our earlier findings, the ExCO2 correlated well with oxygenator outlet pCO2 (R2= 0.84) for sweep gas flows above 1.0 L/min (Figure 2). Disturbance-response assessments for individual animals are detailed below.
Table 1 -.
Measured Physiologic and Device Parameters by Experiment
| Sheep No. | Weight [kg] |
Maximum Achievable Blood Flow [L/min] |
SpO2 [%] | HR [BPM] |
MAP [mmHg] |
pH | pCO2
[mmHg] |
pCO2
from 40 mmHg |
pO2 [mmHg] |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 52 | 4.09 | 96.6±2.88 | 103±16 | 102±11 | 7.39±0.06 | 37.1±6.4 | 1.31 | 173.7±66.3 |
| 2 | 40 | 3.58 | 96.8±3.74 | 108±14 | 83±35 | 7.46±0.13 | 40.2±16.1 | 3.38 | 287.9±145.3 |
| 3 | 33 | 4.93 | 94.0±5.60 | 101±8 | 97±19 | 7.46±0.05 | 37.7±5.1 | 1.56 | 173.4±82.2 |
| 4 | 38 | 3.17 | 98.0±1.45 | 111±6 | 106±35 | 7.46±0.05 | 40.1±6.5 | 1.06 | 300.3±115.3 |
| Overall | 40.8 | 3.94±0.66 | 96.3±4.29 | 107±11 | 93±32 | 7.44±0.09 | 38.9±9.9 | 1.10 | 237.9±123.6 |
Data: mean ± SD
Figure 2.
The relationship between measured pCO2 at the oxygenator blood outlet and oxygenator exhaust gas CO2 concentration for animals 2–4 (sweep gas glow rate of >1.0 LPM).
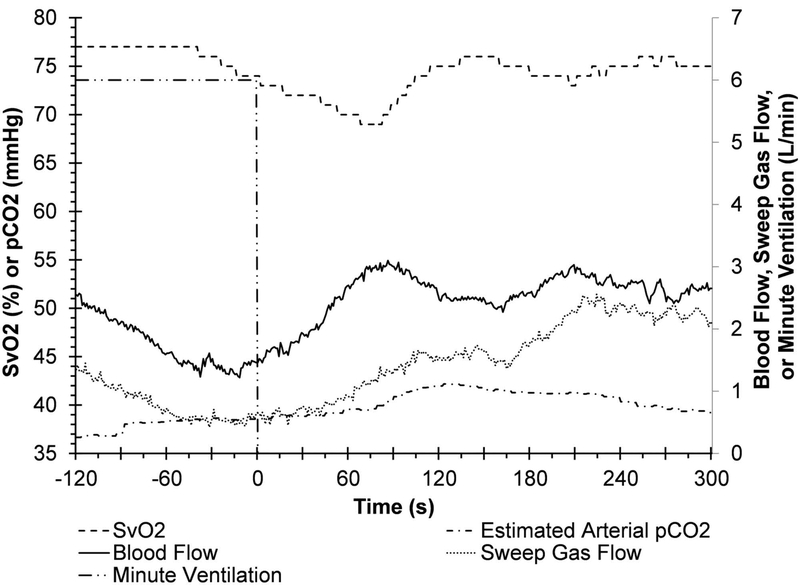
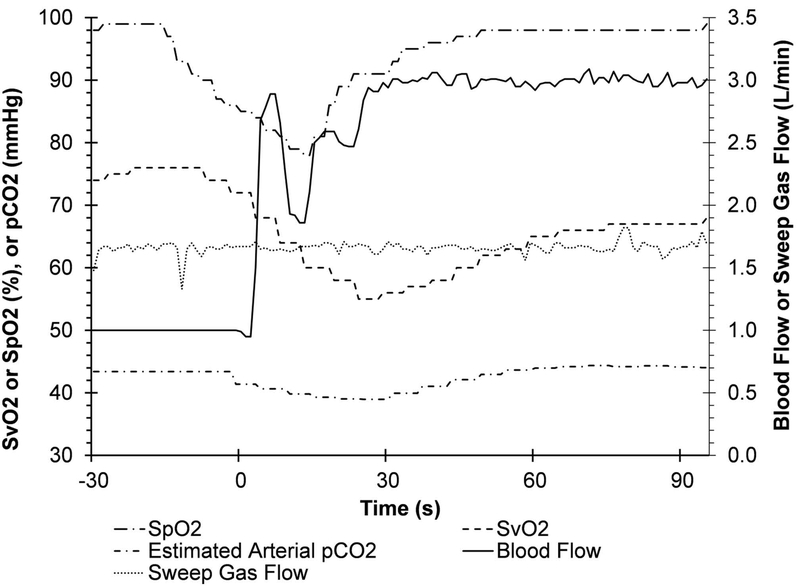
A representative system response to an acute ventilator shutoff (Scenario 1) is shown in Figure 3 for Sheep 4. Initially, the sheep was supported on 6 L/min of ventilation (100% FiO2) via the native lungs (shown by long-dash dot-dot line). At time zero, the ventilator support was stopped, and the AR-ECMO system was free to adapt to the change in native lung support. In this scenario, the system successfully increased blood flow from 1.2 L/min to 2.5 L/min over a period of 60 seconds, and a peak flow of 3.04 L/min at 90 seconds (solid line). The sheep’s SvO2 initially fell to a nadir of 69% within 70 seconds of ventilator shutoff. The AR-ECMO self-regulated increase in blood flow mitigated further peripheral hypoxemia and returned the SvO2 to the target of 75% within 120 seconds (dash line). The sheep’s peripheral SpO2 remained above 98% during this period (not plotted).
Figure 3.
Representative traces of the measured blood flow rate, SvO2, sweep gas flow rate, and the estimated arterial pCO2 during the response of the AR-ECMO to ventilator Shutoff (Scenario 1) (targeted SvO2: 75%, targeted pCO2: 40 mmHg, ventilator FiO2: 100%. SpO2 > 98% throughout this period).
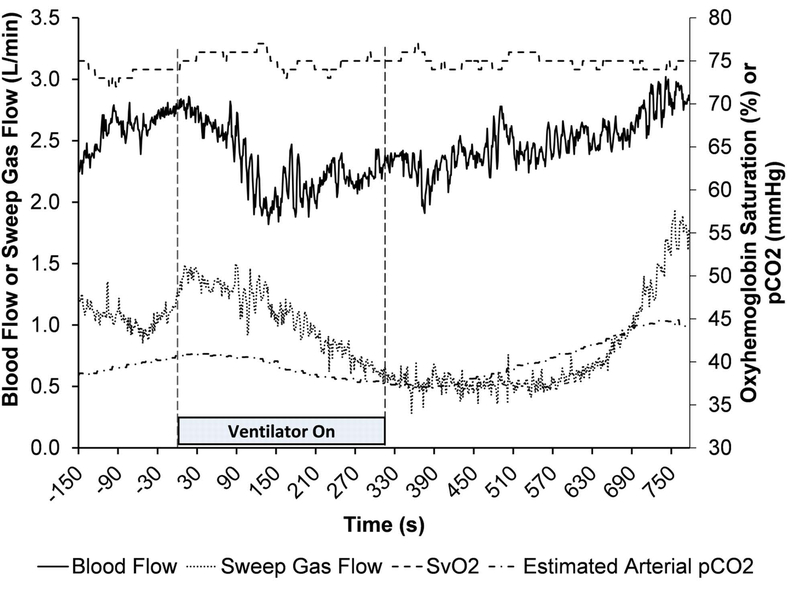
With Sheep 3 supported by the AR-ECMO system without native lung ventilation, the system maintained relative stability around the set-points, with SvO2 73% to 77% and blood flow ranging from 2.25 L/min to 2.81 L/min. With these settings, the estimated oxygenator exhaust CO2 ranged from 38.5 mmHg to 40.7 mmHg, and the sweep gas ranged from 0.88 to 1.35 L/min. This stabilized status was then provoked by activating the ventilator support to 6 L/min of minute ventilation at 100% FiO2 (Scenario 2). To compensate this respiratory support change, the AR-ECMO system decreased the blood flow rate to a nadir of 1.82 L/min, and the sweep gas flow rate to 0.6 L/min. Cessation of mechanical ventilation then prompted an increase in the VV-ECMO blood flow rate to 3.02 L/min to maintain the SvO2 near the set-point, and an increase in sweep gas flow rate to 1.94 L/min during this time. The SpO2 remained above 95% during this experiment. Representative traces of the measured blood flow rate, sweep gas flow rate, SvO2, and estimated pCO2 during the step change in ventilator support are depicted in Figure 4 for Sheep 3.
Figure 4.
Representative traces of the measured blood flow rate, SvO2, sweep gas flow rate, and the estimated arterial pCO2 during the response of the AR-ECMO to step changes in ventilator support (Scenario 2)
In Scenario 3, forced desaturation was induced as a system input disturbance (Figure 5, Sheep 4), with the SpO2 decreasing from 99% to 85% in 16 seconds (dash dot line) while Sheep 4 was purposely receiving insufficient VV-ECMO support (1.0 L/min blood flow) without ventilation of the native lungs. The SvO2 decreased from 76% to 72% during this period (dash line). At time t=0, the AR-ECMO system was switched from the manual mode to the auto-regulatory mode, and was allowed to recover the sheep. An immediate increase in blood flow from 1.0 L/min to 2.89 L/min was observed over 7 seconds (solid line), with an eventual increase to 3.0 L/min within 30 seconds. The SpO2 initially continued to decrease to 78% over the first 14 seconds after the AR-ECMO support started; however, there was recovery to SpO2 greater than 90% within 22 seconds, and greater than 95% within 35 seconds of auto-regulatory support. The SvO2 continued to decrease during the initial 25 seconds of the auto-regulatory support, reaching a nadir of 55%. Recovery of the SvO2 was delayed in comparison to SpO2, and the SvO2 reached only 68% within 90 seconds of recovery. Of note, there was initial oscillation in the blood flow during the early stage of recovery which settled by 27 seconds. The sweep gas and estimated arterial pCO2 remained relatively stable throughout this experiment.
Figure 5.
Representative traces of the measured blood flow rate, SvO2, sweep gas flow rate, and the estimated arterial pCO2 during the response of the AR-ECMO to forced desaturation (Scenario 3).
Discussion
This paper reports the results from using the AR-ECMO system in total artificial lung support of an ovine model in the acute setting. Through these experiments, the AR-ECMO system demonstrated the ability to maintain appropriate oxygenation and carbon dioxide management in the sheep through a series of respiratory disturbances. The AR-ECMO system was challenged with the extremes of native ventilator support, ranging from full 6 L/min minute ventilation of 100% FiO2, followed by immediate cessation of native ventilation. In this setting, the AR-ECMO system appropriately increased the blood flow support in response to progressive decrease in systemic oxygenation, and similarly adjusted the sweep gas flow in response to hyper- or hypocarbia. We predict that in clinical use, an AR-ECMO system would be subject to less-extreme changes to native ventilator support; however, the active and ambulating patient will have alterations in metabolic demands that are not captured with our acute studies. In addition to ambulatory use, we envision an automated ECMO system as a lung-rescue device, with the potential for ECMO initiation by non-experts. Our forced-desaturation experiment evaluates the performance of our system in such a setting, and we demonstrated the ability to recover the hypoxic sheep.
The closed-loop control of ECMO and cardiopulmonary bypass systems has been proposed over the past decade, with some groups reporting progress. Kopp et al. reported a study in a series of six pigs that underwent veno-venous cannulation for ECMO, and were supported with their SmartECLA device14. They report that 98% of SpO2 readings were at least 90%, and pCO2 readings were between 30.9 and 42.7 mmHg on blood gas analysis. Notably, the lowest endotracheal FiO2 delivered was 15%, and they delivered no fewer than 7 respirations per minute with 8mL/kg tidal volumes. In contrast, the sheep in our experiment were completely supported by the ECMO circuit and were entirely weaned from respiratory support via their native lungs. We report similar performance of oxygenation, with 93.7% of our SpO2 readings at 90% or above. Our management of pCO2 requires additional refinement, however, with only 66.7% of our pCO2 measurements between 35 and 45 mmHg (with target pCO2 40 mmHg). To control pCO2, it is critical to have accurate measurement of ExCO2 since the control algorithm is based on the relationship between the ExCO2 and oxygenator outlet pCO2. We found that the ExCO2 was unreliable at low sweep gas flow rates (below 1.0 L/min). The phenomenon of room-air contamination in the measurement of oxygenator exhaust gas is established, and Potger et al determined it to be a significant factor at low sweep gas flows16.
We designed our AR-ECMO system to control parameters that are measured directly from the VV-ECMO circuit, without the need for additional invasive sensors. Although a continuously-sampling online blood gas monitor would allow for direct management of peripheral oxygen and carbon dioxide concentrations, our ultimate goal is for long-term ambulatory ECMO support. The minimization of the use of non-durable blood-contacting sensors supports this development goal. Reducing the reliance on invasive sensors not only has the potential to improve patient comfort and mobility, but also decreases the potential risk for infection or malfunction. We believe that achieving this level of system performance when measuring only two physiologic parameters on the VV-ECMO circuit represents a significant advancement in automation of ECMO support. Additionally, our AR-ECMO system was designed to balance performance and cost, utilizing the Arduino Due for our electromechanical interface. The high-level control system is software-based and facilitates expedient development and refinement; however, the logic of this system could be implemented in the low-level microprocessor system for increased portability.
This proof-of-concept study has several limitations, including the small number of animals and relatively short duration of evaluation. We have significant experience in the long-term evaluation of our manually-controlled ECMO system in awake and ambulating sheep, and we aim to evaluate our AR-ECMO system in long-term sheep studies in the future. In the present experiments we utilized the ventilator as our system disturbance. In reality, however, acute changes to metabolic demand will be the source of system disturbance, and the AR-ECMO system must demonstrate an ability to adapt in a variety of scenarios. Future work to this end, includes utilization of our AR-ECMO system for total respiratory support in awake, exercising sheep with compromised native lung function, and have begun preliminary experimentation. An alternative area of future experimentation involves acute studies of the AR-ECMO system with either pharmacologic or electrical stimulation to increase oxygen demand. Our AR-ECMO system is in a prototype stage, with manually assembled circuit boards and componentry. For long-term studies we intend to refine our design to ensure durability. It is noted that our right atrium/pulmonary artery cannulation strategy provides a unique circumstance in which there is no recirculation between cannula, and our right atrial cannula directly samples mixed-venous blood. Although a tunneled version of this central cannulation strategy has been proposed for long-term ambulatory ECMO, we anticipate that our control system software could be modified to function in a pure veno-venous configuration, and could accommodate for recirculatory blood flow. Naturally, additional studies with alternative cannulation modes would be necessary to confirm satisfactory performance in such configurations. The system is not compared to manual adjustment performed by a skilled technician. Although this comparison would be informative, it is most critical that the AR-ECMO system satisfactorily meets the metabolic needs of the patient at this stage of development. In future studies, demonstration of non-inferiority in comparison to manual adjustment would be necessary. Perhaps the most significant shortcoming of our system is the degree of error in our CO2 management. We designed our system to be free from the blood-contacting online blood-gas monitor in an effort to improve system longevity, reliability, portability, and cost. Unfortunately, these initial studies showed deficiency in managing pCO2, as we achieve only 66.7% of readings within a normal range. We envision a refined device that would maintain a normal pCO2 over 90% of the time, and would implement alarm features if such performance was not achieved. Despite these challenges in CO2 management, we have found satisfactory correlation between oxygenator exhaust CO2 and oxygenator outlet pCO2 which suggests that improvements are needed in the control algorithm, not in the measurement technique.
The ultimate goal of medical innovation is to provide therapeutic benefit to patients. To fulfil this goal, our AR-ECMO system must undergo further refinement and validation prior to pursuing clinical application. We have implemented safeguards for automatically correcting pump-inlet starvation (suckdown) and for preventing conditions of inadequate CO2 removal in the setting of adequate oxygenation. The AR-ECMO system would be considered a Physiologic Closed Loop Controlled Medical Device as defined by the United States Food and Drug Administration17, and approval of such a device requires satisfactory demonstration of system performance and stability. Although seeking approval of such a device poses a substantial challenge, we believe that automatic adaptation is a necessary feature of an out-of-hospital artificial lung, and we aim to provide patients with such a device.
Conclusions
The therapeutic options for patients with severe respiratory failure continues to expand, with ambulatory respiratory support devices on the horizon. For out-of-hospital and active patients on respiratory support, we anticipate a need for an automatically adapting ECMO system, and our AR-ECMO system aims to satisfy this need. In our acute ovine studies we demonstrated satisfactory management of oxygenation, with a promising carbon dioxide management strategy. Our system maintains physiologic targets using the measurements from the VV-ECMO circuit as inputs, reducing the need for invasive sensors. Although this technology requires additional refinement, our acute ovine studies provide an encouraging basis for the future of ambulatory respiratory support devices.
Acknowledgement
This work was supported by the National Institutes of Health (grant numbers: R01HL118372 and R01HL141817).
Footnotes
Conflict of Interest Statement
Z.J. W. and B.P.G. disclose ownership interest in Breethe, Inc. R.G.C, J. Z., Z.J.W. and B.P.G disclose intellectual property for the ECMO control system and blood oxygenators. All other authors declare that they have no conflict of interest in the subject matter or materials discussed in this study.
References
- 1.Stefan MS, Shieh M-S, Pekow PS, et al. Epidemiology and outcomes of acute respiratory failure in the United States, 2001 to 2009: A national survey: Acute Respiratory Failure Epidemiology. J Hosp Med. 2013;8(2):76–82. doi: 10.1002/jhm.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy SL, Xu J, Kochanek KD, Arias E. Mortality in the United States, 2017. 2018. [PubMed] [Google Scholar]
- 3.The Extracorporeal Life Support Organization (ELSO). ECLS Registry Report - International Summary- January, 2019. https://www.elso.org/Registry/Statistics.aspx
- 4.Hartwig MG, Zanotti G, Rehder K, Turner DA, Lin SS, Davis RD. Ambulatory ECMO Provides a Superior Bridge to Lung Transplantation Compared to Conventional ECMO. J Heart Lung Transplant. 2012;31(4):S59. [Google Scholar]
- 5.Abrams D, Javidfar J, Farrand E, et al. Early mobilization of patients receiving extracorporeal membrane oxygenation: a retrospective cohort study. Crit Care. 2014;18(1):R38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia JP, Iacono A, Kon ZN, Griffith BP. Ambulatory extracorporeal membrane oxygenation: A new approach for bridge-to-lung transplantation. J Thorac Cardiovasc Surg. 2010;139(6):e137–e139. doi: 10.1016/j.jtcvs.2009.12.021 [DOI] [PubMed] [Google Scholar]
- 7.Lehr CJ, Zaas DW, Cheifetz IM, Turner DA. Ambulatory Extracorporeal Membrane Oxygenation as a Bridge to Lung Transplantation. Chest. 2015;147(5):1213–1218. doi: 10.1378/chest.14-2188 [DOI] [PubMed] [Google Scholar]
- 8.Garcia JP, Kon ZN, Evans C, et al. Ambulatory veno-venous extracorporeal membrane oxygenation: Innovation and pitfalls. J Thorac Cardiovasc Surg. 2011;142(4):755–761. doi: 10.1016/j.jtcvs.2011.07.029 [DOI] [PubMed] [Google Scholar]
- 9.Madhani SP, Frankowski BJ, Ye S-H, et al. In Vivo 5 Day Animal Studies of a Compact, Wearable Pumping Artificial Lung: ASAIO J. 2019;65(1):94–100. doi: 10.1097/MAT.0000000000000740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu ZJ, Zhang T, Bianchi G, et al. Thirty-Day In-Vivo Performance of a Wearable Artificial Pump-Lung for Ambulatory Respiratory Support. Ann Thorac Surg. 2012;93(1):274–281. doi: 10.1016/j.athoracsur.2011.08.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madhani SP, Frankowski BJ, Burgreen GW, et al. In vitro and in vivo evaluation of a novel integrated wearable artificial lung. J Heart Lung Transplant. 2017;36(7):806–811. doi: 10.1016/j.healun.2017.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou X, Wang D, Sumpter R, Pattison G, Ballard-Croft C, Zwischenberger JB. Long-term support with an ambulatory percutaneous paracorporeal artificial lung. J Heart Lung Transplant. 2012;31(6):648–654. doi: 10.1016/j.healun.2012.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chicotka S, Burkhoff D, Dickstein ML, Bacchetta M. Extracorporeal Membrane Oxygenation for End-Stage Interstitial Lung Disease With Secondary Pulmonary Hypertension at Rest and Exercise: Insights From Simulation Modeling. ASAIO J. 2018;64(2):203–210. doi: 10.1097/MAT.0000000000000646 [DOI] [PubMed] [Google Scholar]
- 14.Kopp R, Bensberg R, Stollenwerk A, et al. Automatic Control of Veno-Venous Extracorporeal Lung Assist: Automatic Control of ECLA. Artif Organs. 2016;40(10):992–998. doi: 10.1111/aor.12664 [DOI] [PubMed] [Google Scholar]
- 15.Mendoza García A, Krane M, Baumgartner B, et al. Automation of a portable extracorporeal circulatory support system with adaptive fuzzy controllers. Med Eng Phys. 2014;36(8):981–990. doi: 10.1016/j.medengphy.2014.04.009 [DOI] [PubMed] [Google Scholar]
- 16.Potger Kieron C., McMillan Darryl, Southwell Joanne, Dando Hayden, O’Shaughnessy Killian. Membrane Oxygenator Exhaust Capnography for Continuously Estimating Arterial Carbon Dioxide Tension During Cardiopulmonary Bypass. J Extra Corpor Technol. 2003;35(3):218-. [PubMed] [Google Scholar]
- 17.Physiological Closed-Loop Controlled (PCLC) Medical Devices Discussion Paper. In: White Oak, Maryland; 2015. [Google Scholar]