Abstract
Adaptation capacity is critical for maintaining cognition, yet it is understudied in groups at risk for dementia. Autonomic nervous system (ANS) is critical for neurovisceral integration and is a key contributor to adaptation capacity. To determine the central nervous system’s top-down regulation on ANS, we conducted a mechanistic randomized controlled trial study, using a 6-week processing speed and attention (PS/A) targeted intervention. Eighty-four older adults with amnestic mild cognitive impairment (aMCI) were randomized to a 6-week PS/A-targeted intervention or an active control without PS/A. Utilizing repeated measures (i.e., PS/A test different from the intervention, resting and cognitive task-based ECG, and resting fMRI) at baseline, immediately post-intervention (post-test), and 6-month follow-up, we aimed to test whether there is a causal influence of PS/A on vagal control of ANS via their shared central neural pathways in aMCI. We indexed vagal control of ANS using high-frequency heart rate variability (HF-HRV) extracted from ECG data. Functional brain connectivity patterns were extracted from fMRI using advanced statistical tools. Compared to the control group, the intervention group showed significant improvement in PS/A, HF-HRV, salience network (SN), central executive network (CEN), and frontal parietal network (FPN) connectivity at post-test; the effect on SN, CEN, and FPN remained at 6-month follow-up. Changes in PS/A and SN connectivity significantly predicted change in HF-HRV from baseline to post-test and/or 6-month-follow-up. Age, neurodegeneration, nor sex did not affect these relationships. This work provides novel support for top-down regulation of PS/A and associated SN on vagal control of ANS. Intervening PS/A may be a viable approach for promoting adaptation capacity in groups at risk for dementia.
Keywords: Vagal control, autonomic flexibility, processing speed and attention, aging, salience network
INTRODUCTION
Adaptation capacity is critical for health and survival (Epel and Lithgow, 2014). In response to environmental and biological challenges or demanding stimuli, a dynamic neurophysiological regulatory process, dominated by autonomic nervous system (ANS), promotes ongoing regulation and adaptation to stimuli (Mulcahy et al., 2019). Vagal influences on cardiac control, indexed by high-frequency heart rate variability (HF-HRV), has emerged as a key marker for such regulation and adaptation in younger and healthy adults (Zahn et al., 2016). Notably, autonomic dysfunction can compromise adaptation to demands or stressors, a phenomenon common in older adults, particularly those with cognitive impairment (Femminella et al., 2014; Jandackova et al., 2016). Additionally, maladaptation to stressors has been shown to further deteriorate brain and cognitive function in old age, thereby accelerating neurodegeneration (McEwen and Morrison, 2013). This evidence points to the need for interventions that can simultaneously target brain and cognitive function, and strengthen the capacity for adaptation to promote healthy cognitive aging.
Compared to other cognitive domains, processing speed and attention (PS/A) are particularly relevant to vagal control in older age (Mahinrad et al., 2016; Williams et al., 2016). In addition to primary regulation from the hypothalamic-pituitary-adrenal axis, ANS is regulated by various cortical regions, from insula and anterior cingulate cortex (ACC) to medial prefrontal cortex (PFC), via a complex hierarchy in nervous system regions (Lin et al., 2017a; Lin et al., 2016b; Mak et al., 2017; Park et al., 2013; Seeley et al., 2007; Smith et al., 2017). According to the neurovisceral integration model (Mulcahy et al., 2019; Smith et al., 2017; Thayer et al., 2009; Thayer and Lane, 2000), intrinsic brain networks [e.g., somatosensory network (SA), salience network (SN), frontal-parietal network (FPN), central executive network (CEN), and default mode network (DMN)], seeded in insular, anterior cingulate, and prefrontal cortices, may contribute to this vagal control hierarchy of ANS flexibility. Of note, despite the structural similarity in regulating ANS, there are functional differences in these networks when regulating moment-to-moment changes in ANS: SN, FPN, CEN, and DMN are involved to varying extents in the regulation of cognitive effort, such as PS/A (Amso and Scerif, 2015); whereas, SA merely engages in the internal regulation of bodily perception (e.g.,(Kern et al., 2013). Meanwhile, there are regions merely engaging in regulating sensory input during PS/A task but not ANS flexibility, such as those involved in the visual network (VN) (Amso and Scerif, 2015). We suspect that intervening to strengthen PS/A via cognitive training, which can reinforce the connectivity of select brain networks (Lin et al., 2016a; O’Brien et al., 2013), may promote capacity for adaptation to environmental challenges, including ANS flexibility.
In our mechanistic randomized controlled trial (RCT) study, we aimed to examine the causal influence of top-down PS/A regulation on ANS. Here, we proposed to intervene on PS/A in older adults with amnestic mild cognitive impairment (aMCI), using a neuroplasticity-based cognitive training [vision-based speed of processing (VSOP)], to examine the relationship between changes in PS/A and vagal control of ANS flexibility, as well as the underlying neural linkage. We examined the following intrinsic brain networks based on their relationships to ANS and PS/A: ANS-specific (SA); PS/A-specific (VN)]; and shared networks (FPN, CEN, DMN, and SN). We hypothesized that VSOP training would induce changes in PS/A, which, in turn, would catalyze changes in HF-HRV by modifying their shared brain networks.
METHODS
Participants
Eighty-four subjects diagnosed with aMCI were recruited from University-affiliated memory, internal, and geriatric clinics. All clinics used 2011 diagnostic criteria for aMCI: a) must have a memory deficit (1–1.5 SD below age- and education-corrected population norms); b) may have deficits in other cognitive domains, such as executive function; c) preserved basic activities of daily living, defined as requiring occasional assistance on less than two items on the Minimum Data Set-Home Care interview; d) absence of dementia using NINCDS-ADRDA criteria. Due to the heterogeneity in battery tests that clinics used to evaluate non-amnestic domains, we differentiated single- (amnestic deficit only) vs. multi-domain (amnestic and non-amnestic deficits) phenotypes using the EXAMINER executive function composite score, a uniform measure of critical non-amnestic domains (i.e., disinhibition, verbal fluency, and working memory), above or below 0 during enrollment (Kramer et al., 2014).
Other inclusion criteria included (1) if prescribed AD medication (i.e., memantine or cholinesterase inhibitors), no changes in dose(s) in the 3 months prior to recruitment; (2) capacity to give consent based on clinician assessment; and (3) other: age ≥60 years, English-speaking, adequate visual acuity for testing, and community-dwelling. Exclusion criteria included (1) current enrollment in another cognitive improvement study; (2) major depression: change in antipsychotic, antiseizure, antidepressant or anxiolytic medications in the past 3 months prior to recruitment; (3) MRI contraindications (including pacemakers); (4) major vascular disease that can interfere with the interpretation of ECG data(e.g., stroke, myocardial infarction, congestive heart failure). The study was approved by the University of Rochester Research Subject Review Board. Written consent was obtained from each participant.
Interventions and randomization
A double-blinded RCT was conducted. Participants were randomly assigned to VSOP or an active control mental leisure activities (MLA) group at a ratio of 2:1 following the baseline assessment. Outcome assessors and participants were blinded to group randomization. We have described our intervention in an earlier pilot study (Lin et al., 2016a). The description of the single-blinded randomized control trial is publicly available (clinicaltrial.gov: NCT02559063) and the CONSORT form is included in Supplemental Material.
Briefly, a suite of five BrainHQ (Posit Science, CA) exercises that targeted PS/A was used for VSOP training. In Eye for Detail, a series of 3 to 5 images (e.g., butterflies) were briefly presented one at a time in different locations on the screen. Participants were required to identify the locations of the stimuli identical in appearance. Difficulty level changed as a function of the number of stimuli, as well as the contrast and distance between the stimuli. In Hawk Eye, a cluster of birds flashed briefly on the screen in peripheral vision. Participants were required to identify the location of the target bird that differed from the other distractor birds. Difficulty level varied in contrast between the birds and the background, stimulus duration, and diameter of the bird clusters. In Visual Sweeps, two sweep patterns (movement of bars) were presented simultaneously. Participants were required to determine whether each pattern moved inward or outward. Difficulty level changed as a function of color and luminance, orientation, and spatial frequency. In Double Decision, participants were required to identify which of two vehicle stimuli appeared in the center of the screen, as well as the location of a peripheral road sign. Difficulty level changed as a function of contrast between the center stimuli, number of peripheral stimuli, diameter of the field of view, and complexity of the background. In Target Tracker, a few target objects (e.g. bubbles) are displayed on the screen, followed by additional “distractors” identical in appearance. Participants were required to track the target objects as all of the objects moved in Brownian motion, then select the targets after the stimuli stopped moving. Difficulty level varied in number of target objects, speed and duration of object motion, and contrast between the objects and the background. All tasks shared visual components, and the tasks became increasingly more difficult as subjects’ training progressed, thus requiring faster reaction times. Responses were based on object type or location and orientation on the screen. The training automatically adjusted the difficulty of each task based on each participant’s performance, ensuring that participants always trained near their maximum capacity on each task.
MLA group performed online leisure activities, including word search, Sudoku, and solitaire. These games were selected to control for prior computer and internet exposure, visual stimuli, as well as provide daily mental stimulation to minimize dropout risk. Participants were permitted to play any combination of the games during each training session.
The online platforms for VSOP and MLA were identical. We provided orientation and two check-in sessions in-person. All other sessions were administered by subjects at home. The training period lasted 6 weeks with participants being encouraged to complete four 1-hour sessions per week. Within each training session, VSOP group was asked to play five 10-minute tasks with brief breaks between them: the type and order of task for a particular session was generated by BrainHQ in a random order, but the difficulty level of a task was based on a participant’s record on the same task. MLA group was asked to play the three games in any combination, difficulty level, or length of time for a total of one hour per session. Of note, the training session used in the orientation was identical in the order and difficulty level (i.e., entry level) for participants within a group. Also, during orientation and each check-in session, a participant’s training sessions during that period was planned with the interventionist according to a his/her schedule. Individual adherence for both groups was tracked through the online platforms. During the self-administration period, a 24/7 hotline was available for technical support. No adverse effects were associated with the interventions.
Assessment
Relevant to the current paper, data on PS/A, ANS measures, and brain networks were collected at baseline, post-test (i.e., the week following completion of the intervention), and 6-month follow-up.
Computerized Useful Field of View (UFOV)
Computerized Useful Field of View (UFOV) measures PS/A via three subtests targeting visual processing speed, selective attention, and divided attention, respectively (Ball et al., 1988). Visual and attentional demands of UFOV are similar (although not identical) to the task demands of VSOP training (Lin et al., 2013; Melnick et al., 2013). In subtest 1 (processing speed), participants must identify the target stimulus from two center stimuli (i.e., car AND truck), then recall the location of the target on a subsequent screen. In subtest 2 (divided attention), participants perform a variation of subtest 1, where they must identify the location of a peripheral target (i.e., truck only), in addition to the center target (i.e., car OR truck), then recall the correct locations of both targets on a subsequent screen. In subtest 3 (selective attention), participants perform a variation of subtest 2, with additional distractor stimuli surrounding the center and peripheral stimuli. UFOV utilizes a two-step adaptive staircase algorithm to estimate performance thresholds and vary subtest difficulty (i.e., stimulus duration). Threshold performance for each subtest is displayed in milliseconds after test completion. Performance scores range from 17 to 500 ms, with corresponding descriptive cutoffs ranging from “normal” to “severe.” Lower scores indicate better performance. Consistent with other clinical trials (Ball et al., 2002; Wolinsky et al., 2009), a composite score was developed by averaging the reaction times of the three subtests, then natural log-transforming to address skewness. Low composite scores indicate shorter reaction time and better performance.
Auditory Consonant Trigrams (ACT)
Auditory Consonant Trigrams (ACT) (Brown, 1958; Shura et al., 2016), a task with high working memory load, served as the cognitive challenge. Participants received a three-consonant trigram (e.g. HJI) verbally, at a rate of one letter per second, followed immediately by a random three-digit number. Participants were asked to recall the trigram after a time delay (9, 18, or 36 seconds), during which they performed simple arithmetic aloud and backwards from the given number by 3’s to minimize rehearsal effects. The task consisted of three randomized trials for each time delay. Participants’ response times during ACT varied. Before ACT, a 5-minute acclimation (rest) period was administered. We selected ACT as the cognitive challenge task due to its high cognitive load, which may simulate a stressful situation (Shura et al., 2016); however, accuracy while performing ACT (auditory-based working memory) was not directly relevant here.
Electrocardiography (ECG) data
Electrocardiography (ECG) data were acquired with Mindware 2-Slot BioNex model and BioLab software. HRV was monitored continuously using a standard lead-II electrode configuration during the rest and ACT task periods. HF-HRV data were preprocessed with Mindware HRV analysis software (v3.1), using methods described previously (Berntson et al., 1997). Briefly, consecutive R-R intervals were preprocessed using a filter at 0.15–0.40 Hz to generate HF-HRV, then natural log-transformed. We extracted 15-second segments and removed ectopic beats and artifacts using consistent visual inspection between two raters. The first and last four segments, as well as incomplete segments (i.e., <15 seconds), of each recording during rest and task were excluded from analysis. Null values from motion and arrhythmic artifacts in the remaining data were excluded by dividing the number of null-absent segments by the total number of segments to obtain the percentage of usable data for each participant. A threshold of 70% was applied to identify subjects with valid rest and task data. Average HF-HRV was calculated from the surviving HF-HRV_rest data. Surviving task data were partitioned into three blocks for each participant: average HF-HRV during the refined first and last minutes (blocks 1 and 3, respectively); and average HF-HRV across the remaining refined segments (block 2). The three blocks represented the initial, middle, and end of HF-HRV reactivity. Average HF-HRV across the three blocks was calculated as the HF-HRV_task score.
Resting-state fMRI data acquisition:
Imaging data were collected using a research dedicated 3T Siemens TrioTIM scanner (Erlangen, Germany) with a 32-channel head coil. Each magnetic resonance session began with a scout image, followed by an MPRAGE scan (TI = 1100ms, TE/TR = 3.44ms/2530 ms, 1-mm isotropic resolution, 256×256 matrix, FA = 7, 1-mm slice thickness, 192 slices), which provides high-resolution structural-weighted anatomical images for image-registration purposes. A 2D axial fast Gradient-Recalled Echo pulse sequence was used to generate field maps to correct for field inhomogeneity distortions in echo-planar imaging sequences. Blood-oxygen-level-dependent (BOLD) functional data were collected using a gradient echo-planar imaging sequence (TE/TR = 30ms/2500 ms, 4-mm isotropic resolution, 64×64 matrix, FA = 90, 4-mm slice thickness, 37 contiguous axial slices). Participants were instructed to relax with their eyes open, without falling asleep. An in-scanner camera was used to ensure compliance. Of note, fMRI data were collected on a separate day, but within a one-week window, from the ACT task to avoid potential stress-related effects.
Imaging data processing:
The first 10 functional volumes were excluded to allow for signal callibration effects. Resting-state functional MRI data were then preprocessed using FMRIB Software Library (FSL version 5.0.6). Preprocessing of the function data included: slice timing, head-motion corrected, co-registered to structural image in native space, normalized to Montreal Neurological Institute (MNI) standard space, and resampling (3 × 3 × 3 mm). After that, the data were spatially smoothed with a 5-mm FWHM isotropic Gaussian kernel, and temporally filtered with a band-pass filter (0.01–0.08 Hz). Nuisance covariates were regressed out, including 6 head motion parameters, white matter signal, and cerebrospinal fluid signal. Of note, related to the head motion correction, 6 head motion parameters (3 translation and 3 rotation) were regressed out from data. All subjects were included since their head movement was within 3 mm on any axis and head rotation was less than 4 degree.
Background information
Background information was collected at baseline, including demographics, health history, depressive symptoms in the past 7 days per Geriatric Depression Scale (GDS), and cortical thickness signature for Alzheimer’s disease-associated neurodegeneration (ADSCT) from structure-weighted anatomical images (Jack et al., 2015). Details can be found in Table 1.
Table 1.
Baseline characteristics
| Total (N = 84) | VSOP group (n = 56) | MLA group (n = 28) | t or χ2, df (p) | |
|---|---|---|---|---|
| Age, mean (SD) | 74.71 (7.30) | 75.23 (7.49) | 73.68 (6.92) | 0.92, 82 (.36) |
| Years of education, mean (SD) | 16.34 (2.55) | 16.17 (2.39) | 16.68 (2.87) | −0.86, 82 (.39) |
| Male, n (%) | 45 (53.6) | 32 (57.1) | 13 (46.4) | 0.86, 1 (.35) |
| Non-Hispanic White, n (%) | 74 (88.1) | 52 (92.9) | 22 (78.6) | 3.63, 1 (.06) |
| Married, n (%) | 62 (73.8) | 42 (75.0) | 20 (71.4) | 0.12, 1 (.73) |
| MOCA, mean (SD) | 24.05 (2.62) | 23.89 (2.75) | 24.36 (2.33) | −0.77, 82 (.45) |
| GDS, mean (SD) | 2.04 (2.23) | 2.18 (2.15) | 1.75 (2.40) | 0.83, 82 (.41) |
| Single-domain aMCI, n (%) | 37 (44) | 22 (39.3) | 15 (53.6) | 1.55, 1 (.21) |
| First-degree family history of Alzheimer’s dementia, n (%) | 43 (51.2) | 28 (50.0) | 15 (53.6) | 0.10, 1 (.76) |
| ADSCT, mean (SD) | 2.77 (0.16) | 2.75 (0.17) | 2.81 (0.14) | −1.84, 82 (.07) |
| • ADSCT ≤ 2.77mm3/neurodegeneration, n (%) | 41 (48.8) | 31 (55.4) | 10 (35.7) | 2.88, 1 (.09) |
| Taking AD medication, n (%) | 11 (13.1) | 8 (14.3) | 3 (10.7) | 0.21, 1 (.65) |
| Taking beta-blockers, n (%) | 16 (19) | 10 (17.9) | 6 (21.4) | 0.15, 1 (.69) |
| BMI, mean (SD) | 26.67 (4.64) | 26.57 (4.75) | 26.86 (4.50) | −0.27, 82 (.79) |
| Chronic condition index, mean (SD) | 4.46 (2.21) | 4.38 (2.15) | 4.64 (2.34) | −0.52, 82 (.60) |
| • Hypertension, n (%) | 45 (54.2) | 27 (49.1) | 18 (64.3) | 1.73, 1 (.19) |
| • Diabetes, n (%) | 10 (11.9) | 9 (16.1) | 1 (3.6) | 2.78, 1 (.10) |
| Baseline UFOV, mean (SD) | 5.89 (0.51) | 5.89 (0.51) | 5.89 (0.50) | −0.08, 80 (.94) |
| Baseline HF-HRV_rest, mean (SD) | 4.26 (1.70) | 4.28 (1.90) | 4.24 (1.26) | 0.10, 75 (.92) |
| Baseline HF-HRV_task, mean (SD) | 4.33 (1.66) | 4.16 (1.81) | 4.68 (1.25) | 0.82, 1 (.33)* |
Note.
controlled for baseline HF-HRV_rest
Data analysis
Overview:
We focused on intention-to-treat (ITT) analysis (VSOP = 49, MLA = 28). Of note, we purposely sampled a larger intervention group to determine characteristics explaining VSOP training effect, which was not a purpose for this particular paper. To addressed potential bias from the unbalanced sample, however, we provided corrected effect size (Hedge’s g) for the main variables. The effect size of training for each outcome was calculated using standardized mean difference with 95% CI [(Mtraining – Mcontrol at later time) – (Mtraining – Mcontrol at baseline)] / intra-subject standard deviation. Sample size calculations were performed using G*power. Based on parameter assumptions outlined in the protocol, the sample size (VSOP = 49, MLA = 28) at α = 0.05, provided 80% power to detect an improvement at Cohen’s d = 0.67.
Our primary analyses were focused on the comparison or change between baseline and post-test because the intervention was implemented during this period. The change from baseline to 6-month follow-up was considered additional validation for the relationships between PS/A, brain networks, and HF-HRV, albeit we did not expect significant group-by-time intervention effects to last for 6 months.
Imaging data analysis:
Independent component analysis.
To identify intrinsic brain networks, we used resting-state fMRI data across all subjects and all available time points using group independent component analysis (ICA). Group-ICA was conducted using the MELODIC algorithm of FSL with probabilistic ICA approach. Specifically, individual data were concatenated across the time course to identify independent components (n = 14, auto-estimated by MELODIC). An average z-score of 2.5 < z < 8 was defined as the threshold for the group-ICA maps. Six components (VN, CEN, SA, SN, FPN, and DMN; see Figure 1) relevant to our research questions were identified by two raters who visually compared our components to ICA results from other relevant studies (Beckmann et al., 2005; Sorg et al., 2007; van Oort et al., 2017; Wang et al., 2008).
Figure 1.
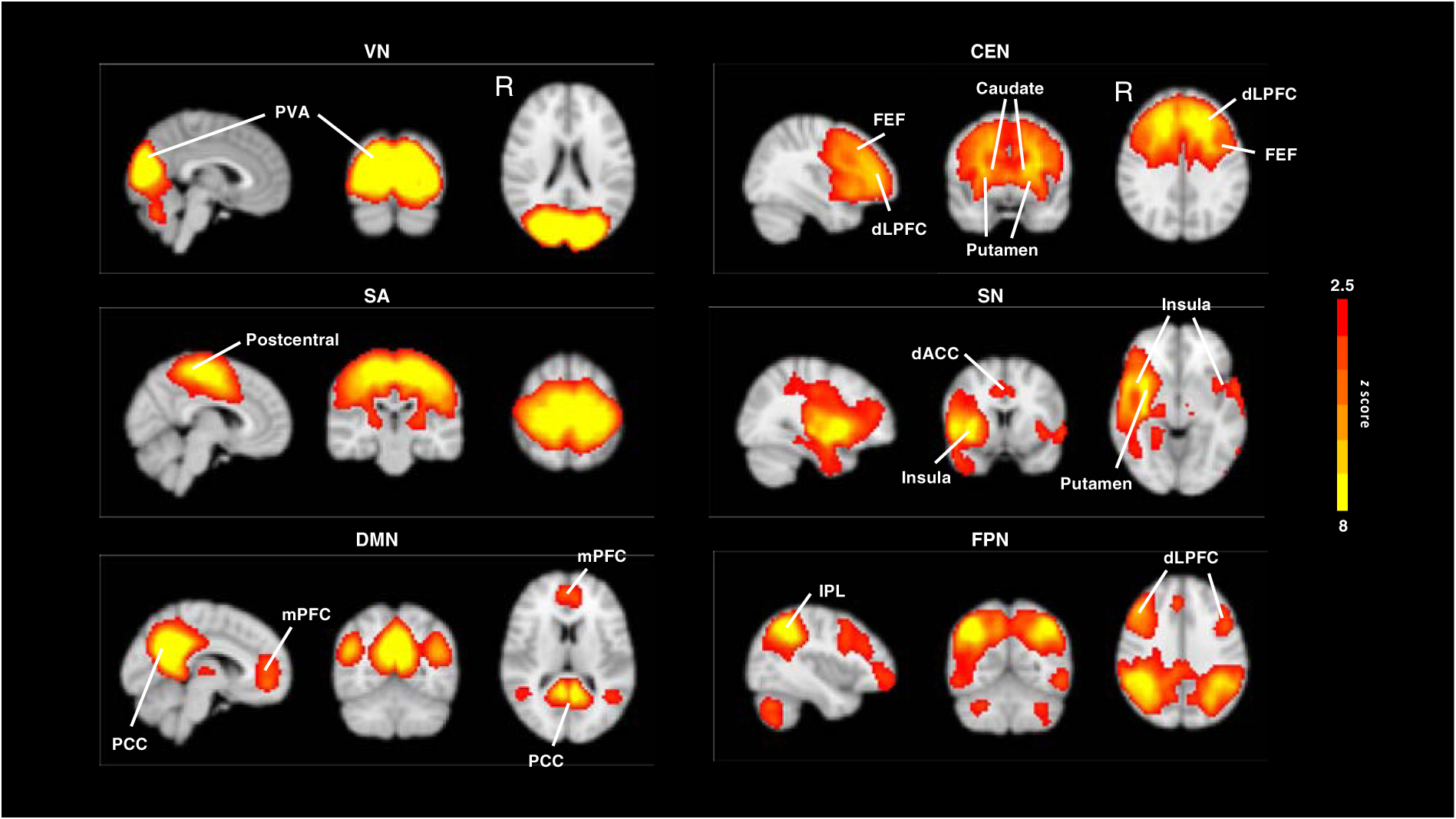
ICA results of intrinsic brain networks. NOTE: CEN = central executive network; DLPFC = dorsolateral prefrontal cortex; dACC = dorsal anterior cingulate cortex; DMN = default mode network; FEF = frontal eye fields; FPN = frontoparietal network. IPL = inferior parietal lobule; mPFC = medial prefrontal cortex; PCC = posterior cingulate cortex; PVA = primary visual areas; SA = somatosensory network; SN = salience network; VN = visual network;
Group-level analyses.
The intervention effect on brain networks was primarily analyzed within the change from baseline to post-test; the significant intervention effect was defined as the significant group-by-time difference within a particular network. An FSL dual regression technique was applied to compare the group-by-time differences within each of the six networks (Nickerson et al., 2017). We first used group-ICA spatial maps as spatial regressors against each subject’s 4D resting-state fMRI data to calculate subject-specific time courses, then used subject-specific time courses as temporal regressors to determine their respective parameter estimate (PE) spatial maps. We subsequently fit a General Linear Model to test the PE-spatial maps for group-by-time interaction. We applied the masks from the Group-ICA result, avoiding voxels outside the functional networks. To adjust for multiple comparisons at the cluster level, the statistical map threshold was set at z > 2.3, with voxel correction at p < 0.01 and cluster correction at p < 0.05, assuming a Gaussian random field for the z-statistics. For voxels showing significant group-by-time differences with cluster correction, we created masks that pinpointed the locations of these voxels. These masks were overlaid on subject-specific spatial maps to extract mean ICA PE-values for subsequent analyses. Of note, for those networks without significant intervention effects (in our case, DMN and VN), no mask could be created. Additionally, the mask identified from the baseline and post-test comparison was used as the reference for extracting PE-values from subjects’ respective 6-month follow-up brain networks.
Other data analyses
Other data analyses were conducted using SPSS 24.0. We compared baseline characteristics to determine equivalency, using independent t-tests for continuous variables and χ2 tests for categorical variables. For the within-group intervention effect, paired t-tests was used to compare baseline and post-test or follow-up.
Generalized Estimating Equation (GEE) modeling was used to analyze the between-group intervention effect on main variables (Equation 1) and relationships between changes in main variables across assessments (Equation 2).
| Equation 1: |
where “Visit” was considered a categorical variable, with baseline as the reference. Any significant main effect of “Visit” suggests significant differences in post-test or follow-up outcome from baseline. MLA was considered a reference for “Group.” Any significant main effect of “Group” suggests significantly different levels in outcome of the VSOP group relative to the MLA group. Any significant interaction effect between “Visit” and “Group” suggests significant changes in outcome of the VSOP group relative to the MLA group at post-test or follow-up from baseline. “ԑ” represents the variability across participants and within the same participant over time, using the AR(1) error structure to minimize practice effects across assessments.
| Equation 2: |
where x here refers to time-dependent UFOV or intrinsic network; y refers to time-dependent HF-HRV (at rest or during task while controlling for corresponding at-rest data); and the remaining parameters refer to the same ones defined in Equation 1. When examining models with additional variables (i.e., age, sex, and ADSCT), the variables were added to Equation 2. When examining models within group, “Group” variable was removed from Equation 2.
Of note, α for correlational analyses involving intrinsic networks was set at false discovery rate (FDR) corrected p = 0.05; in the present study, four networks survived group-level analyses. For other analyses, was set at 0.05. A two-tailed test was used.
Data availability
A complete database has been created to host all data. Data will be stripped of any identifiers and stored pursuant to University Research Subject Review Board protocols for purposes of analysis and manuscript preparation. We will make the data (demographics, health history, behavioral, ECG, and imaging data) and associated documentation available to users under a data-sharing user agreement approved by the University Research Subject Review Board 24 months after the completion of the R01 grant (6/1/2022).
RESULTS
Baseline characteristics
VSOP and MLA groups showed no significant differences in any of the baseline measures or demographics (Table 1).
The immediate effect of the intervention and relationships between main variables
Figure 2 displays group-by-time comparison of UFOV and ANS measures. There was a significantly larger improvement of UFOV in the VSOP group compared to MLA (B = −0.21, SE = 0.07, χ2 = 7.94, p = .005; Hedge’s g = 0.51). VSOP group showed a significantly greater training-related improvement in HF-HRV_task compared to MLA, controlled for HF-HRV_rest (B = 0.59, SE = 0.21, χ2 = 7.64, p = .006; Hedge’s g = 0.42), albeit there was no group-by-time difference in HF-HRV_rest (B = 0.53, SE = 0.36, χ2 = 2.14, p = .14). Within HF-HRV_task, block 2 (middle stage of reactivity) showed the greatest significant group-by-time difference (B = 0.77, SE = 0.22, χ2 = 11.96, p = .001 vs. χ2 = 0.08 and χ2 = 1.72 for other two blocks).
Figure 2.
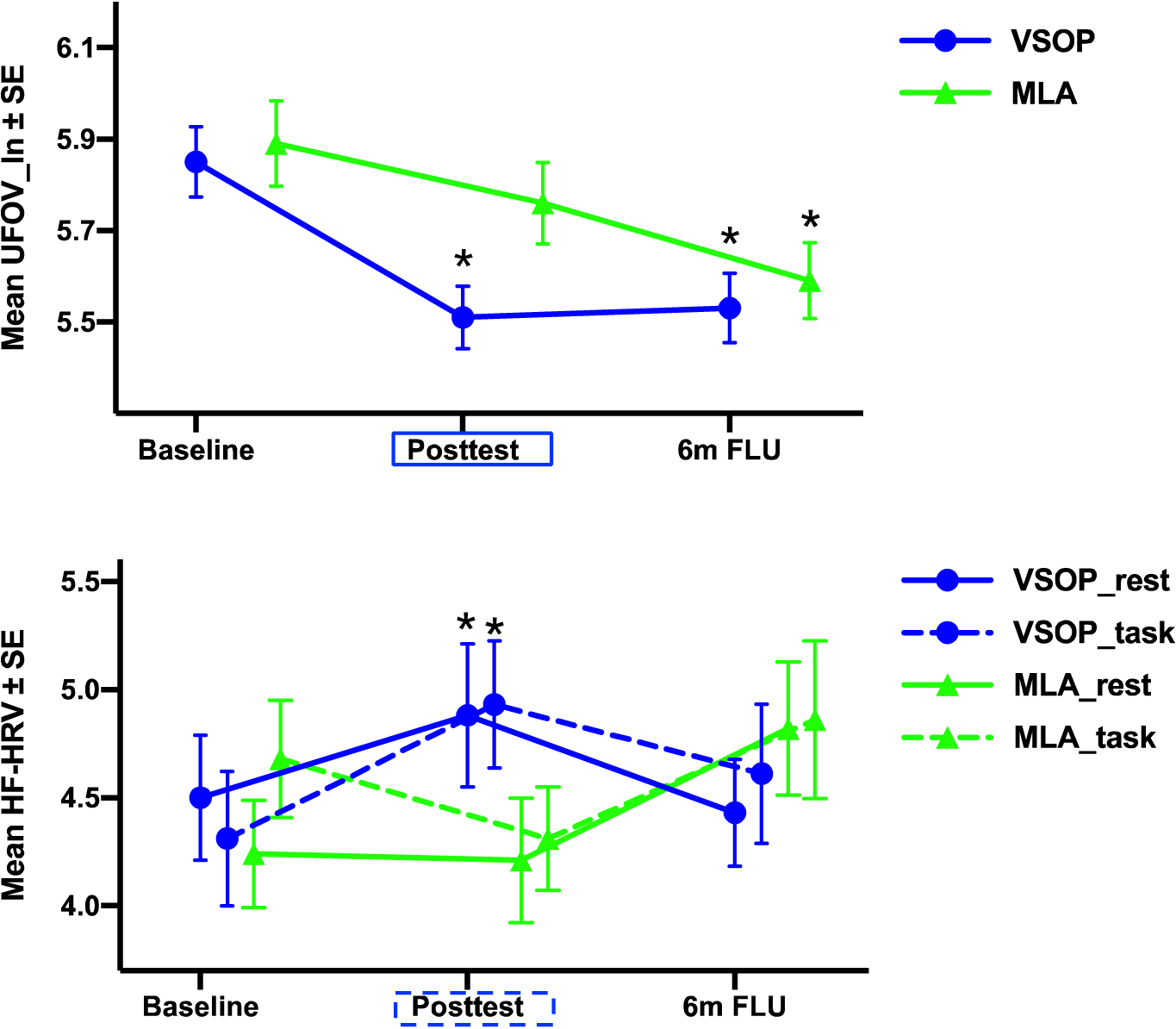
Group (VSOP vs. MLA)-by-time (Baseline vs. Post-test) comparison of UFOV and HF-HRV. NOTE: Rectangle represents significant between-group difference that was higher in VSOP (blue) or MLA (green) group; Star represents significant within-group change from baseline. Both between- and within-group analyses were based on a two-tailed test. UFOV_ln = log-transformed Useful Field of View test; HF-HRV = high frequency heart rate variability; _rest = HF-HRV at rest; _task = HF-HRV in response to the cognitive challenge task.
Figure 3 displays group-by-time comparison on imaging measures. The significant group-by-time effect was observed for functional connectivity (FC) in CEN (seeded in cingulate gyrus, MNI: −12, 6, 33, number of voxels = 668), SN (seed in insula, MNI: 54, −3, 36, number of voxels = 221), FPN (seed in inferior parietal lobule, MNI: 6, 21, 48, number of voxels = 565), and SA (seed in postcentral gyrus, MNI: 57, −12, 36, number of voxels = 155).
Figure 3.
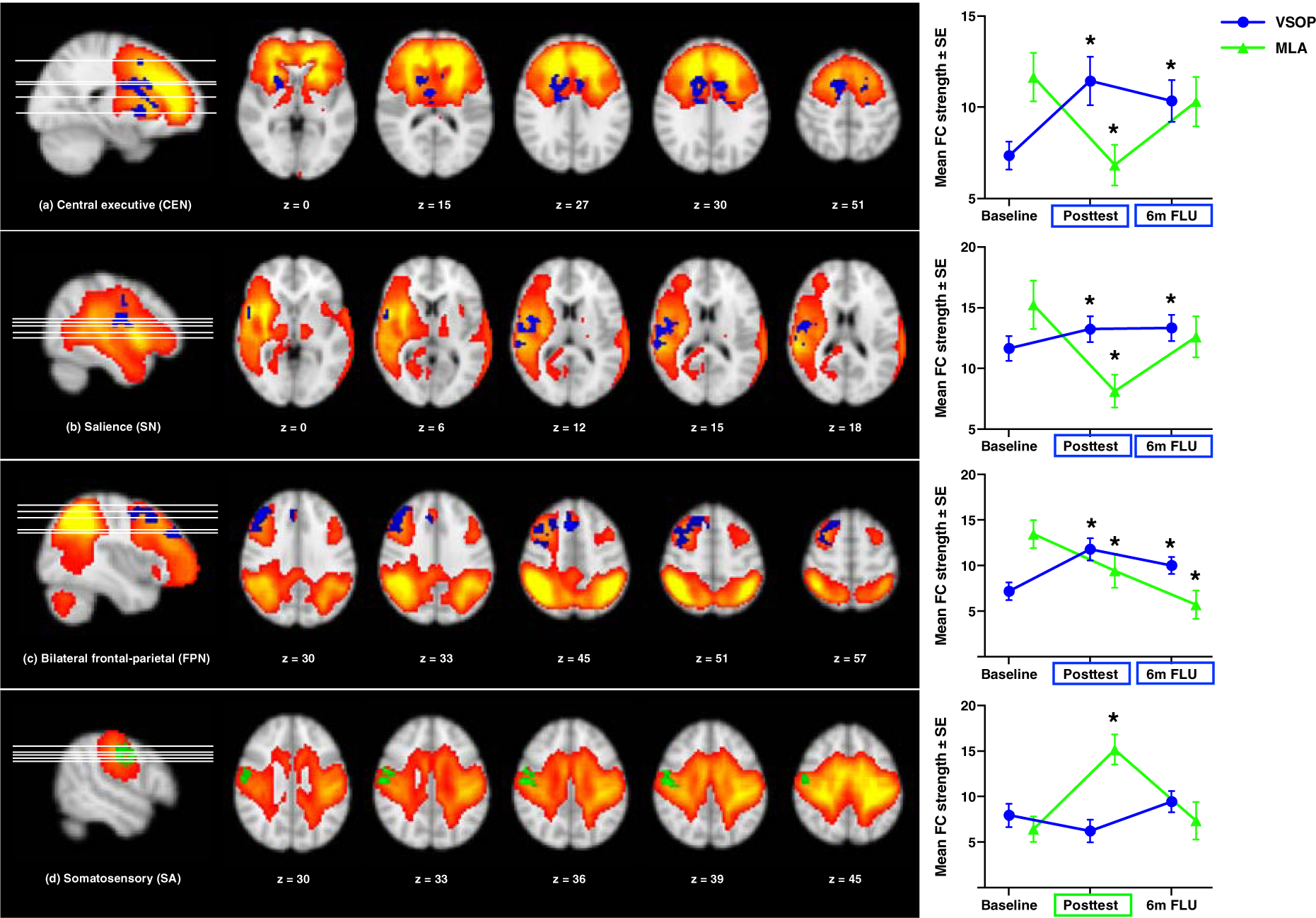
Significant group (VSOP vs. MLA)-by-time (Baseline vs. Post-test) interaction effects on intrinsic networks. Note: the statistical maps threshold was z > 2.3, with voxel correction at p < 0.01 and cluster correction at p < 0.05, assuming a Gaussian random field for the z-statistics. There was a significant group-by-time effect in CEN (seed in cingulate gyrus, MNI (xyz): −12, 6, 33, number of voxels = 668), SN (seed in insula, MNI: 54, −3, 36, number of voxels = 221), FPN (seed in inferior parietal lobule, MNI: 6, 21, 48, number of voxels = 565), and SA (seed in postcentral gyrus, MNI: 57, −12, 36, number of voxels = 155). Rectangle represents significant between-group difference that was higher in VSOP (blue) or MLA (green) group; star represents significant within-group change from baseline. Both between- and within-group analyses were based on a two-tailed test.
A total of 40 subjects in the VSOP group (71.4%) vs. 26 in MLA (92.9%) complied with the intervention (i.e., completed >12 hours of intervention). According to cumulative literature (Ball et al., 2002; Wolinsky et al., 2013), ≥ 10 hours VSOP training is sufficient to stimulate significant PS/A improvement in old age. This difference in compliance rate was significant (χ2 = 6.00, df = 2, p = 0.050). There was no relationship between training time and UFOV, ANS, or imaging measures across groups at post-test.
Relationships between main variables from baseline to post-test
UFOV and SN had significant relationships with HF-HRV_task, as derived from GEE Equation 2. That is, greater improvement (i.e., decrease in reaction time) in UFOV (B = −0.33, SE = 0.17, χ2 = 3.61, p = .057), as well as greater enhancement in FC strength in SN (B = 0.03, SE = 0.01, χ2 = 14.79, corrected p < .001), but not other networks over time was related to greater improvement (i.e., increase) in HF-HRV_task when controlling for HRV_rest. These key results did not change when controlling for age, sex, and ADSCT (between UFOV and HF-HRV_task: B = −0.31, SE = 0.17, χ2 = 3.33, p = .068; between SN and HF-HRV_task: B = 0.04, SE = 0.01, χ2 = 17.22, corrected p < .001). When analyzing the relationships within each group, the relationship between UFOV and HF-HRV_task was not significant, while the relationship between SN and HF-HRV_task remained significant for VSOP group (B = 0.04, SE = 0.01, χ2 = 7.32, corrected p = .007).
In the sample that complied with the intervention (VSOP: n = 40, MLA: n = 26), the significant correlations between HF-HRV_task and UFOV (B = −0.33, SE = 0.17, χ2 = 3.96, p = .047) and SN (B = 0.03, SE = 0.01, χ2 = 11.87, corrected p < .001) remained.
The 6-month follow-up effect of the intervention and relationships between main variables from baseline to 6-month follow-up
As validation, we examined the changes, as well as the relationships, between ANS measures, brain networks (based on brain maps derived from the comparison of baseline and post-test), and UFOV from baseline to 6-month follow-up. There was no significant intervention effect on ANS measures or UFOV (Figure 2), but the significance on the brain networks remained similar (Figures 3), with slightly weaker correlations (between UFOV and HF-HRV_task: B = −0.22, SE = 0.17, χ2 = 1.72, p = .18; between SN and HF-HRV_task: B = 0.03, SE = 0.01, χ2 = 8.89, corrected p = .003).
DISCUSSION
In the present study of older adults with aMCI, we examined the causal relationship between PS/A and vagal control underlying ANS flexibility by experimentally manipulating PS/A, using a double-blinded RCT design to compare VSOP training and MLA. The two interventions differed mainly by practicing PS/A or not. We found that VSOP training resulted in significant, or a trend in, improvement in PS/A, functional connectivity of several brain networks (i.e., CEN, SN, and FPN), and HF-HRV in response to a cognitive challenge task from baseline to post-test, compared to MLA training. The significant intervention effect for SN, CEN, and FPN remained evident at 6-month follow-up. More importantly, improvements in PS/A and SN, but not other networks, were significantly related to improvement in HF-HRV_task from baseline to post-test, as well as 6-month follow-up.
There are two major implications for the results. First, we have provided evidence for a top-down pathway linking PS/A and vagal control in ANS flexibility. VSOP training includes five tasks that target PS/A, whereas MLA contains neither timed visual stimuli nor reaction time constraints. Cumulative correlational literature suggests PS/A, in particular, are related to vagal control (Mahinrad et al., 2016; Williams et al., 2016); our finding that PS/A-targeted training enhances vagal cardiac control corroborates this literature. Furthermore, our neuroimaging analyses provide additional support for this top-down regulation of ANS by revealing a potential neural linkage via SN. That is, VSOP training improved the FC strength of SN, with such improvement related to increased HF-HRV reactivity in response to a stressful event. SN, seeded in insula, ACC and surrounding subcortical regions, is considered a “switch” between resting and task engagement, particularly during tasks with interoceptive or environmental stimuli that are considered salient (Menon and Uddin, 2010; Seeley et al., 2007). An emerging study also revealed the role of SN in supporting excellent cognitive aging (Zhang et al., 2019). Compared to other networks that participate in regulating PS/A (e.g., DMN, CEN and FPN), perception (e.g., VN), or somatosensory (SA), SN seems to coordinate multiple systems for goal-oriented behaviors (Menon and Uddin, 2010), such as adaptation to environmental stimuli in the current context. Furthermore, VSOP had no effect on SA nor was there a linkage between changes in SA and vagal control, suggesting that SA may not be relevant to PS/A. Notably, HF-HRV during a demanding cognitive task (especially block 2, the stage requiring greatest cognitive engagement), but not resting HF-HRV, was affected by the PS/A intervention. Changes in task-related HF-HRV, but not resting HF-HRV, were also related to the cognitive and neural changes in response to intervention. Collectively, these findings may suggest that integration of cognitive- and ANS-supporting networks is most evident under conditions of high cognitive load in cognitively vulnerable older adults at risk for dementia. Synthesizing these findings, our data contribute to existing literature on integrated cognitive and neural pathways by further demonstrating a causal pathway between PS/A, SN, and vagal cardiac control during demanding cognitive challenges or stressful events.
Second, we found evidence that VSOP training strengthens cognitive and neural efficiency, with concomitant vagal regulation of cardiac control, suggesting overall better capacity for adaptation to stress. There are several categories of interventions that can explicitly improve the central autonomic networks (e.g., SN), resulting in improved vagal-mediated ANS flexibility and cognitive function. Such interventions include physical exercise, non-invasive brain stimulation (e.g., transcranial magnetic and direct current stimulations) (Dedoncker et al., 2016; Makovac et al., 2017; Martin et al., 2017). We and others have previously demonstrated that both ANS function and PS/A-related neural signals predict the cognitive effects of VSOP training (Lin et al., 2017a; O’Brien et al., 2013). Here, we are among the first to reveal that a cognitive training paradigm may be a viable behavioral intervention for strengthening ANS flexibility by targeting the linkage underlying the shared neural mechanism between ANS and PS/A.
On contrast, MLA group had significant enhancement in the SA network strength immediately after intervention relatively to VSOP group. The relaxation nature or enjoyment from these leisure activities may help explain MLA group’s neural change (Wijaya et al., 2020). The relatively weaker network strength in CEN, SN, and FPN in MLA group after intervention may be explained by the lower cognitive load associated with the MLA tasks compared to VSOP. Noticeably, we are cautious toward interpreting any within-group network strength changes immediately after intervention since the imaging analysis focused on identifying between-group difference.
Age, sex, and neurodegeneration did not affect causal relationships between PS/A or SN and ANS flexibility, which aligns with our previous finding that ACC completely mediates the relationship between neurodegeneration and HF-HRV response to cognitive challenges (Lin et al., 2017b). However, the impact of Alzheimer’s disease pathology on the relationships remains unclear. Although it is known that an amyloid deposition-associated increase in acetylcholinesterase activity can affect multiple networks supporting PS/A or vagal control (Grothe and Teipel, 2016; Lin et al., 2017b; Rub et al., 2016; Santos et al., 2017; Szili-Torok et al., 2001), limited literature addresses whether VSOP training modulates HF-HRV merely through the shared neural networks of PS/A and vagal control, or through additional mechanisms. For example, VSOP training has been reported to mitigate depressive symptoms in older adults (Wolinsky et al., 2015); and lower depressive symptomatology and depressive treatment response are associated with higher HF-HRV (Chambers and Allen, 2002; Cyranowski et al., 2011). To further understand effects of VSOP training on ANS flexibility, as well as ANS contributions to stress adaptation, additional pathways should be investigated, such as emotional regulation, depressive and anxiety symptomatology, and/or perceived stress. Importantly, the long-term effect of VSOP training on ANS flexibility and other relevant outcomes requires further research. Finally, issues of adherence to VSOP may be addressed by adding high-order executive complexity to the training, enriching stimulus variability, and incorporating motivational techniques during assessments (Fiszdon et al., 2016).
Before reaching a conclusion on the causal relationship between PS/A, brain networks, and vagal control of ANS flexibility, several domains related to the cognitive training or neurovisceral integration literature should be further explored. First, there are cognitive training paradigms built upon PS/A that monitor brain regions or networks involved in regulating ANS, with additional cognitive components [e.g. video gaming can modify insula (Gong et al., 2015), ACC (Gong et al., 2019b), or CEN (Gong et al., 2019a)]. Future studies should examine the intervention effects of these paradigms on ANS monitoring, which may help understand other cognitive domain contributions to ANS flexibility. Second, the updated neurovisceral integration theory proposed that the involvement of several cortical networks (i.e., SA, CEN, FPN, and DMN), in addition to SN, supports ANS. In the present study, VSOP training improved CEN and FPN, whereas MLA improved SA; nonetheless, we did not find any relationship between these networks and HF-HRV with a working memory task, suggesting that the brain’s involvement in vagal control of ANS may be scenario specific. However, we caution defaulting hastily back to the vagal control hierarchy, as HF-HRV should first be assessed in response to other stress task protocols (e.g., somatosensory, emotional regulation, and decision making).
Regardless, our study adds to the growing evidence for the integration of cognitive and ANS regulation by shared neural mechanisms. Our findings support a novel, causal pathway from improvements in PS/A to neurophysiological underpinnings of ANS regulation, all of which can support efficacious adaptation to environmental changes and stressors. Further consideration is needed regarding the promise of VSOP training to promote stress adaptation, cognitive health, and well-being in the context of Alzheimer’s disease and associated risk of dementia.
Supplementary Material
Acknowledgments
Study funded by NIH/NINR R01 NR015452 to Feng Lin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosure: None
Statistical analysis conducted by Dr. Lin, Dr. Chen, and Ms. Ye, University of Rochester
References
- Amso D, Scerif G, 2015. The attentive brain: insights from developmental cognitive neuroscience. Nat Rev Neurosci 16, 606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball K, Berch DB, Helmers KF, Jobe JB, Leveck MD, Marsiske M, Morris JN, Rebok GW, Smith DM, Tennstedt SL, Unverzagt FW, Willis SL, Advanced Cognitive Training for, I., Vital Elderly Study, G., 2002. Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA 288, 2271–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball KK, Beard BL, Roenker DL, Miller RL, Griggs DS, 1988. Age and visual search: Expanding the useful field of view. JOSA A 5, 2210–2219. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM, 2005. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci 360, 1001–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT Jr., Eckberg DL, Grossman P, Kaufmann PG, Malik M, Nagaraja HN, Porges SW, Saul JP, Stone PH, van der Molen MW, 1997. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology 34, 623–648. [DOI] [PubMed] [Google Scholar]
- Brown J, 1958. Some tests of the decay theory of immediate memory. Quarterly Journal of Experimental Psychology 10, 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers AS, Allen JJ, 2002. Vagal tone as an indicator of treatment response in major depression. Psychophysiology 39, 861–864. [DOI] [PubMed] [Google Scholar]
- Cyranowski JM, Hofkens TL, Swartz HA, Salomon K, Gianaros PJ, 2011. Cardiac vagal control in nonmedicated depressed women and nondepressed controls: impact of depression status, lifetime trauma history, and respiratory factors. Psychosom Med 73, 336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedoncker J, Brunoni AR, Baeken C, Vanderhasselt MA, 2016. A Systematic Review and Meta-Analysis of the Effects of Transcranial Direct Current Stimulation (tDCS) Over the Dorsolateral Prefrontal Cortex in Healthy and Neuropsychiatric Samples: Influence of Stimulation Parameters. Brain Stimul 9, 501–517. [DOI] [PubMed] [Google Scholar]
- Epel ES, Lithgow GJ, 2014. Stress biology and aging mechanisms: toward understanding the deep connection between adaptation to stress and longevity. J Gerontol A Biol Sci Med Sci 69 Suppl 1, S10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Femminella GD, Rengo G, Komici K, Iacotucci P, Petraglia L, Pagano G, de Lucia C, Canonico V, Bonaduce D, Leosco D, Ferrara N, 2014. Autonomic dysfunction in Alzheimer’s disease: tools for assessment and review of the literature. J Alzheimers Dis 42, 369–377. [DOI] [PubMed] [Google Scholar]
- Fiszdon JM, Kurtz MM, Choi J, Bell MD, Martino S, 2016. Motivational Interviewing to Increase Cognitive Rehabilitation Adherence in Schizophrenia. Schizophr Bull 42, 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong D, He H, Liu D, Ma W, Dong L, Luo C, Yao D, 2015. Enhanced functional connectivity and increased gray matter volume of insula related to action video game playing. Sci Rep 5, 9763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong D, Ma W, Liu T, Yan Y, Yao D, 2019a. Electronic-Sports Experience Related to Functional Enhancement in Central Executive and Default Mode Areas. Neural Plast 2019, 1940123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong D, Yao Y, Gan X, Peng Y, Ma W, Yao D, 2019b. A Reduction in Video Gaming Time Produced a Decrease in Brain Activity. Front Hum Neurosci 13, 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grothe MJ, Teipel SJ, 2016. Spatial patterns of atrophy, hypometabolism, and amyloid deposition in Alzheimer’s disease correspond to dissociable functional brain networks. Hum Brain Mapp 37, 35–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr., Wiste HJ, Weigand SD, Knopman DS, Mielke MM, Vemuri P, Lowe V, Senjem ML, Gunter JL, Reyes D, Machulda MM, Roberts R, Petersen RC, 2015. Different definitions of neurodegeneration produce similar amyloid/neurodegeneration biomarker group findings. Brain 138, 3747–3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jandackova VK, Scholes S, Britton A, Steptoe A, 2016. Are Changes in Heart Rate Variability in Middle-Aged and Older People Normative or Caused by Pathological Conditions? Findings From a Large Population-Based Longitudinal Cohort Study. J Am Heart Assoc 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern M, Aertsen A, Schulze-Bonhage A, Ball T, 2013. Heart cycle-related effects on event-related potentials, spectral power changes, and connectivity patterns in the human ECoG. NeuroImage 81, 178–190. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Mungas D, Possin KL, Rankin KP, Boxer AL, Rosen HJ, Bostrom A, Sinha L, Berhel A, Widmeyer M, 2014. NIH EXAMINER: conceptualization and development of an executive function battery. J Int Neuropsychol Soc 20, 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Chen DG, Vance D, Mapstone M, 2013. Trajectories of combined laboratory- and real world-based speed of processing in community-dwelling older adults. J Gerontol B Psychol Sci Soc Sci 68, 364–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Heffner KL, Ren P, Tadin D, 2017a. A Role of the Parasympathetic Nervous System in Cognitive Training. Curr Alzheimer Res 14, 784–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Heffner KL, Ren P, Tivarus ME, Brasch J, Chen DG, Mapstone M, Porsteinsson AP, Tadin D, 2016a. Cognitive and Neural Effects of Vision-Based Speed-of-Processing Training in Older Adults with Amnestic Mild Cognitive Impairment: A Pilot Study. J Am Geriatr Soc 64, 1293–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Ren P, Cotton K, Porsteinsson A, Mapstone M, Heffner KL, 2016b. Mental Fatigability and Heart Rate Variability in Mild Cognitive Impairment. Am J Geriatr Psychiatry 24, 374–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Ren P, Wang X, Anthony M, Tadin D, Heffner KL, 2017b. Cortical thickness is associated with altered autonomic function in cognitively impaired and non-impaired older adults. J Physiol 595, 6969–6978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahinrad S, Jukema JW, van Heemst D, Macfarlane PW, Clark EN, de Craen AJ, Sabayan B, 2016. 10-Second heart rate variability and cognitive function in old age. Neurology 86, 1120–1127. [DOI] [PubMed] [Google Scholar]
- Mak LE, Minuzzi L, MacQueen G, Hall G, Kennedy SH, Milev R, 2017. The Default Mode Network in Healthy Individuals: A Systematic Review and Meta-Analysis. Brain Connect 7, 25–33. [DOI] [PubMed] [Google Scholar]
- Makovac E, Thayer JF, Ottaviani C, 2017. A meta-analysis of non-invasive brain stimulation and autonomic functioning: Implications for brain-heart pathways to cardiovascular disease. Neurosci Biobehav Rev 74, 330–341. [DOI] [PubMed] [Google Scholar]
- Martin AK, Meinzer M, Lindenberg R, Sieg MM, Nachtigall L, Floel A, 2017. Effects of Transcranial Direct Current Stimulation on Neural Networks Structure in Young and Older Adults. J Cogn Neurosci 29, 1817–1828. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Morrison JH, 2013. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron 79, 16–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick MD, Harrison BR, Park S, Bennetto L, Tadin D, 2013. A strong interactive link between sensory discriminations and intelligence. Curr Biol 23, 1013–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ, 2010. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 214, 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulcahy JS, Larsson DEO, Garfinkel SN, Critchley HD, 2019. Heart rate variability as a biomarker in health and affective disorders: A perspective on neuroimaging studies. NeuroImage 202, 116072. [DOI] [PubMed] [Google Scholar]
- Nickerson LD, Smith SM, Ongur D, Beckmann CF, 2017. Using Dual Regression to Investigate Network Shape and Amplitude in Functional Connectivity Analyses. Front Neurosci 11, 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien JL, Edwards JD, Maxfield ND, Peronto CL, Williams VA, Lister JJ, 2013. Cognitive training and selective attention in the aging brain: an electrophysiological study. Clin Neurophysiol 124, 2198–2208. [DOI] [PubMed] [Google Scholar]
- Park G, Vasey MW, Van Bavel JJ, Thayer JF, 2013. Cardiac vagal tone is correlated with selective attention to neutral distractors under load. Psychophysiology 50, 398–406. [DOI] [PubMed] [Google Scholar]
- Rub U, Stratmann K, Heinsen H, Turco DD, Seidel K, Dunnen W, Korf HW, 2016. The Brainstem Tau Cytoskeletal Pathology of Alzheimer’s Disease: A Brief Historical Overview and Description of its Anatomical Distribution Pattern, Evolutional Features, Pathogenetic and Clinical Relevance. Curr Alzheimer Res 13, 1178–1197. [DOI] [PubMed] [Google Scholar]
- Santos CY, Machan JT, Wu WC, Snyder PJ, 2017. Autonomic Cardiac Function in Preclinical Alzheimer’s Disease. J Alzheimers Dis 59, 1057–1065. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD, 2007. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27, 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shura RD, Rowland JA, Miskey HM, 2016. Auditory Consonant Trigrams: A Psychometric Updatedagger. Arch Clin Neuropsychol 31, 47–57. [DOI] [PubMed] [Google Scholar]
- Smith R, Thayer JF, Khalsa SS, Lane RD, 2017. The hierarchical basis of neurovisceral integration. Neurosci Biobehav Rev 75, 274–296. [DOI] [PubMed] [Google Scholar]
- Sorg C, Riedl V, Muhlau M, Calhoun VD, Eichele T, Laer L, Drzezga A, Forstl H, Kurz A, Zimmer C, Wohlschlager AM, 2007. Selective changes of resting-state networks in individuals at risk for Alzheimer’s disease. Proc Natl Acad Sci U S A 104, 18760–18765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szili-Torok T, Kalman J, Paprika D, Dibo G, Rozsa Z, Rudas L, 2001. Depressed baroreflex sensitivity in patients with Alzheimer’s and Parkinson’s disease. Neurobiol Aging 22, 435–438. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Hansen AL, Saus-Rose E, Johnsen BH, 2009. Heart rate variability, prefrontal neural function, and cognitive performance: the neurovisceral integration perspective on self-regulation, adaptation, and health. Ann Behav Med 37, 141–153. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD, 2000. A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disord 61, 201–216. [DOI] [PubMed] [Google Scholar]
- van Oort J, Tendolkar I, Hermans EJ, Mulders PC, Beckmann CF, Schene AH, Fernandez G, van Eijndhoven PF, 2017. How the brain connects in response to acute stress: A review at the human brain systems level. Neurosci Biobehav Rev 83, 281–297. [DOI] [PubMed] [Google Scholar]
- Wang K, Jiang T, Yu C, Tian L, Li J, Liu Y, Zhou Y, Xu L, Song M, Li K, 2008. Spontaneous activity associated with primary visual cortex: a resting-state FMRI study. Cereb Cortex 18, 697–704. [DOI] [PubMed] [Google Scholar]
- Wijaya M, Lau D, Horrocks S, McGlone F, Ling H, Schirmer A, 2020. The human “feel” of touch contributes to its perceived pleasantness. J Exp Psychol Hum Percept Perform 46, 155–171. [DOI] [PubMed] [Google Scholar]
- Williams DP, Thayer JF, Koenig J, 2016. Resting cardiac vagal tone predicts intraindividual reaction time variability during an attention task in a sample of young and healthy adults. Psychophysiology 53, 1843–1851. [DOI] [PubMed] [Google Scholar]
- Wolinsky FD, Vander Weg MW, Howren MB, Jones MP, Dotson MM, 2009. A randomized controlled trial of cognitive training using a visual speed of processing intervention in middle aged and older adults. PLoS One 8, e61624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky FD, Vander Weg MW, Howren MB, Jones MP, Dotson MM, 2013. A randomized controlled trial of cognitive training using a visual speed of processing intervention in middle aged and older adults. PLoS One 8, e61624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky FD, Vander Weg MW, Howren MB, Jones MP, Dotson MM, 2015. The effect of cognitive speed of processing training on the development of additional IADL difficulties and the reduction of depressive symptoms: results from the IHAMS randomized controlled trial. J Aging Health 27, 334–354. [DOI] [PubMed] [Google Scholar]
- Zahn D, Adams J, Krohn J, Wenzel M, Mann CG, Gomille LK, Jacobi-Scherbening V, Kubiak T, 2016. Heart rate variability and self-control--A meta-analysis. Biol Psychol 115, 9–26. [DOI] [PubMed] [Google Scholar]
- Zhang J, Andreano JM, Dickerson BC, Touroutoglou A, Barrett LF, 2019. Stronger Functional Connectivity in the Default Mode and Salience Networks Is Associated With Youthful Memory in Superaging. Cereb Cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
A complete database has been created to host all data. Data will be stripped of any identifiers and stored pursuant to University Research Subject Review Board protocols for purposes of analysis and manuscript preparation. We will make the data (demographics, health history, behavioral, ECG, and imaging data) and associated documentation available to users under a data-sharing user agreement approved by the University Research Subject Review Board 24 months after the completion of the R01 grant (6/1/2022).


