Abstract
The ε4 allele of the apolipoprotein E gene (APOE), a risk factor for cognitive decline, is associated with alterations in medial temporal lobe (MTL) structure and function, yet little research has been dedicated to understanding how these alterations might interact to negatively impact cognition. To bridge this gap, the present study employed linear regression models to determine the extent to which APOE genotype (ε4+, ε4−) modifies interactive effects of baseline arterial spin labeling MRI-measured cerebral blood flow (CBF) and FreeSurfer-derived cortical thickness/volume (CT/Vo) in two MTL regions of interest (entorhinal cortex, hippocampus) on memory change in 98 older adults who were cognitively normal at baseline. Baseline entorhinal CBF was positively associated with memory change, but only among ε4 carriers with lower entorhinal CT. Similarly, baseline entorhinal CT was positively associated with memory change, but only among ε4 carriers with lower entorhinal CBF. Findings suggest that APOE ε4 carriers may experience concomitant alterations in neurovascular function and morphology in the MTL that interact to negatively affect cognition prior to the onset of overt clinical symptoms. Results also suggest the presence of distinct multimodal neural signatures in the entorhinal cortex that may signal relative risk for cognitive decline among this group, perhaps reflecting different stages of cerebrovascular compensation (early effective vs later ineffective).
Keywords: aging, APOE ε4, cerebral blood flow, cognitive decline, cortical thickness
INTRODUCTION
The ε4 allele of the apolipoprotein E (APOE) gene increases risk for pathologic age-related cognitive decline (Bretsky et al., 2003; Richard J. Caselli et al., 2009; Schiepers et al., 2012; Tsuang et al., 2013), but the mechanistic link between APOE ε4 and cognitive decline is still poorly understood. APOE-related alterations in cognition have been consistently reported, with older adult ε4 carriers demonstrating worse cognitive performance (Adamson et al., 2010; De Blasi et al., 2009; Honea, Vidoni, Harsha, & Burns, 2009; Kukolja, Thiel, Eggermann, Zerres, & Fink, 2010; Tuminello & Han, 2011) and accelerated cognitive decline, most notably in episodic memory ability (Bretsky et al., 2003; R. J. Caselli et al., 2004; Richard J. Caselli et al., 2009; Hayden et al., 2009; C.-C. Liu, Kanekiyo, Xu, & Bu, 2013; Schiepers et al., 2012; Whitehair et al., 2010). There is also converging evidence of APOE-related alterations in brain function and structure in medial temporal lobe (MTL) regions (Burggren et al., 2008; Cohen, Small, Lalonde, Friz, & Sunderland, 2001; den Heijer et al., 2002; Hays, Zlatar, & Wierenga, 2016; Jak, Houston, Nagel, Corey-Bloom, & Bondi, 2007). However, very little research has focused on understanding how alterations in MTL structure and function might interact to negatively impact cognition among APOE ε4 carriers. Successful attempts to characterize these complex interactions could lead to identification of earlier and more reliable markers of incipient cognitive decline or treatments with the potential to delay, or even prevent, age-related changes in cognition.
Older adult APOE ε4 carriers demonstrate reduced cortical thickness and accelerated gray matter atrophy in MTL regions when compared to non-carriers, most notably in the entorhinal cortex and hippocampus (Burggren et al., 2008; Cohen et al., 2001; den Heijer et al., 2002; Donix et al., 2013, 2010; Jak et al., 2007; Tohgi et al., 1997). Moreover, reduced structural integrity in these brain regions is linked to detriments in cognition and conversion to MCI and AD (Honea et al., 2009; Lind et al., 2006; Pacheco, Goh, Kraut, Ferrucci, & Resnick, 2015; Soldan et al., 2015), suggesting that the ε4 allele may exert negative impacts on cognition, at least in part, through altered MTL structure. APOE ε4 carriers also demonstrate alterations in cerebral blood flow (CBF), or the rate of delivery of arterial blood to the capillary bed in a volume of tissue, across widespread medial temporal, frontal, and parietal regions (Tai et al., 2016; Wierenga, Hays, & Zlatar, 2014). More specifically, ε4 carriers tend to exhibit higher resting CBF than non-carriers in early adulthood and middle-age, but lower resting CBF in old age (Thambisetty, Beason-Held, An, Kraut, & Resnick, 2010; Wierenga et al., 2013). The biphasic nature of CBF among ε4 carriers has been attributed to cerebrovascular compensation, with early increases reflecting attempts to compensate for the deleterious effects of APOE ε4 (e.g., impaired repair mechanisms, neurovascular disruption) and subsequent decreases reflecting a relative breakdown of this compensation (Dai et al., 2009; Hays et al., 2016; Koizumi et al., 2018; Luckhaus et al., 2008; Ostergaard et al., 2013; Wierenga et al., 2012). Notably, cross-sectional investigations of cerebral perfusion in cognitively normal older adult ε4 carriers have produced mixed results, with reports of both increased and decreased CBF, relative to non-carriers (Bangen et al., 2009; Filippini et al., 2011, 2011; Thambisetty et al., 2010; Wierenga et al., 2013; Zlatar et al., 2016), and both positive and negative associations between CBF and cognition (Bangen et al., 2012; Wierenga et al., 2012; Zlatar et al., 2016).
Although mixed findings with regard to links between CBF, APOE, and cognition may be partially accounted for by differences in sample characteristics or methodology, recent evidence by our group (Hays et al., 2019) suggests another possibility; namely, that these inconsistencies reflect a failure to account for underlying structural integrity within the same anatomical region. We found evidence of an interactive effect of MTL CBF, structural integrity, and APOE on cognition among a sample of cognitively normal older adults, whereby the relationship between entorhinal CBF and memory performance varied as a function of APOE genotype and entorhinal cortical thickness. More specifically, observed relationships between entorhinal CBF and memory performance were negative in ε4 carriers with lower entorhinal cortical thickness, positive in non-carriers with lower entorhinal cortical thickness, and negative in non-carriers with higher entorhinal cortical thickness. We propose that differential relationships among these variables can be understood in the context of cerebrovascular compensation, with compensatory increases in CBF being invoked among individuals with lower cortical reserve in the same region, and differences in the direction of the relationship between CBF and cognition representing different points along a trajectory of compensation. For example, the observed positive relationship between entorhinal CBF and cognition (higher CBF associated with better memory) among non-carriers with lower entorhinal cortical reserve may reflect successful cerebrovascular compensation, whereas the negative relationship between entorhinal CBF and cognition (higher CBF associated with worse memory) among ε4 carriers with lower entorhinal cortical reserve may reflect a relative breakdown of cerebrovascular compensation, such that compensatory increases in CBF are maximally invoked and not supportive of concurrent memory function. Taken together, these data suggest that ε4 carriers with a combined pattern of higher CBF and lower CT in MTL regions might be at elevated risk for future cognitive decline. However, it remains unclear whether this pattern also predicts future cognitive decline. For example, although relative hyperperfusion among this group does not appear supportive of concurrent memory function, it might be supportive of memory function over time (e.g., greater memory stability). Exploring links between APOE-related alterations in MTL structure and function and their impacts on longitudinal changes in cognition represents a critical next step toward improving our understanding of the mechanistic link between APOE and future cognitive decline.
Together, previous evidence suggests that relationships between MTL CBF and concurrent memory performance vary by APOE genotype and underlying structural integrity. However, to our knowledge, no study has explored the interactive effects of these variables as they relate to longitudinal changes in memory performance. In order to bridge this gap in the literature, the current study used arterial spin labeling (ASL) magnetic resonance imaging (MRI) and a high-resolution structural scan among a well-characterized sample of cognitively normal older adults with serial cognitive data to determine the extent to which APOE genotype (ε4+ vs. ε4−) modifies interactive effects of baseline MTL resting CBF and brain structure (cortical thickness [CT], volume [Vo]) on longitudinal changes in verbal episodic memory performance. Based on our cross-sectional findings, we predicted that APOE genotype would modify the interactive effects of baseline CBF and brain structure on memory change, such that higher CBF (reflecting maximally invoked compensatory response) and lower CT/Vo in MTL regions (entorhinal cortex [EC], hippocampus [Hc]) at baseline would be associated with greater memory decline among e4 carriers, but not among non-carriers.
MATERIALS AND METHODS
Participants
See Table 1 for participant demographic and cognitive characteristics. Participants were community-dwelling older adult volunteers enrolled in a longitudinal study of aging at the VA San Diego Healthcare System (VASDHS). A total of 98 cognitively normal participants between the ages of 64 and 89 (mean age = 72.8, SD = 6.0) with available data were included in the current analyses. Thirty-one participants were carriers of the APOE e4 allele (ε3/ε4 = 28, ε4/ε4 = 3) and 67 were non-carriers (ε3/ε3 = 58, ε3/ε2 = 9). All participants were administered a full neuropsychological battery and an MRI scan at baseline (mean time interval between neuropsychological testing and MRI scan = 16 days) and received follow-up neuropsychological testing at one-year intervals (time interval between baseline neuropsychological testing and most recent follow-up: mean = 2.5 years, min = 0.9, max = 5.5 years). To ensure all participants had normal cognitive function at baseline, participants were excluded if baseline performance on more than one measure within a cognitive domain was more than one standard deviation below age-appropriate norms, consistent with the empirically-derived criteria for diagnosis of MCI developed by Jak and colleagues (Jak et al., 2009), or if overall performance on the Dementia Rating Scale (DRS) was more than 1 standard deviation below age-appropriate norms (see Table 3 in the Appendix for specific cognitive tests, domains, and normative data). Potential participants were excluded if they had a dementia or MCI diagnosis at baseline, a history of severe head injury, major vascular event, uncontrolled hypertension, or a DSM-IV Axis I diagnosis of learning disability, attention deficit disorder, mood disorder, or substance abuse. In addition, participants were excluded if they had contraindications to MRI scanning such as ferrous implants or a pacemaker. All participants provided written informed consent prior to enrollment and data were collected in accordance with all ethical standards as stipulated by the UC San Diego and VA San Diego Healthcare System institutional review board-approved procedures.
Table 1.
Participant demographic, cognitive, and brain characteristics.
| APOE ε4− (N=67) | APOE ε4+ (N=31) | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Mean | SD | Mean | SD | t or χ2 | df | p |
| Age (years) | 72.94 | 6.36 | 72.58 | 5.96 | 0.27 | 96 | 0.786 |
| Sex (male/female) | 23/44 | -- | 13/18 | -- | 0.53 | 1 | 0.467 |
| Education (years) | 16.62 | 2.27 | 16.00 | 2.22 | 1.28 | 96 | 0.203 |
| FSRP Stroke Risk Percent | 9.65 | 6.71 | 9.83 | 7.74 | 0.11 | 95 | 0.909 |
| NP and MRI time interval (days) | 22.94 | 84.5 | 1.58 | 94.3 | 1.12 | 96 | 0.264 |
| NP Baseline and follow-up interval (years) | 2.43 | 1.12 | 2.67 | 1.06 | 1.00 | 96 | 0.319 |
| Baseline DRS Total Score | 141 | 2.76 | 141 | 2.85 | 0.00 | 96 | 0.999 |
| Baseline Memory Composite | 0.150 | 0.80 | −0.325 | 0.85 | 2.66 | 96 | 0.009** |
| Baseline WMS-R LM Immediate Recall | 31.55 | 6.38 | 28.00 | 5.62 | 2.66 | 96 | 0.009** |
| Baseline WMS-R LM Delayed Recall | 28.64 | 7.23 | 25.00 | 7.46 | 2.29 | 96 | 0.023* |
| Baseline CVLT-II List 1–5 total | 51.22 | 9.65 | 45.38 | 10.4 | 2.71 | 96 | 0.007** |
| Baseline CVLT-II SD Free Recall | 10.34 | 3.06 | 9.35 | 2.90 | 1.51 | 96 | 0.134 |
| Baseline CVLT-II LD Free Recall | 11.40 | 2.86 | 10.12 | 3.10 | 1.99 | 96 | 0.048* |
| Memory-CS-Composite | −0.07 | 0.59 | 0.185 | 0.97 | 1.36 | 96 | 0.179 |
| CS WMS-R LM Immediate Recall | 0.238 | 4.99 | 0.967 | 7.34 | 0.50 | 96 | 0.618 |
| CS WMS-R LM Delayed Recall | 0.985 | 5.42 | 1.10 | 7.84 | 0.07 | 96 | 0.943 |
| CS CVLT-II List 1–5 total | −0.076 | 8.78 | 5.548 | 10.5 | 2.59 | 95 | 0.013* |
| CS CVLT-II SD Free Recall | −0.348 | 2.65 | 1.096 | 3.25 | 2.16 | 95 | 0.035* |
| CS CVLT-II LD Free Recall | −0.045 | 2.32 | 0.323 | 3.37 | 0.55 | 95 | 0.585 |
| Entorhinal CBF | 43.32 | 15.3 | 43.4 | 14.7 | 0.01 | 85 | 0.987 |
| Entorhinal CT | 3.31 | 0.32 | 3.27 | 0.31 | 0.60 | 96 | 0.549 |
| Hippocampal CBF | 47.79 | 11.9 | 50.78 | 9.90 | 1.16 | 90 | 0.248 |
| Hippocampal Vo | 3698 | 451 | 3653 | 482 | 0.44 | 96 | 0.656 |
APOE= Apolipoprotein E; NP= Neuropsychological testing; DRS= Mattis Dementia Rating Scale; WMS= Wechsler Memory Scale; LM= Logical Memory; SS= Scaled score; CVLT= California Verbal Learning Test; SD= Short delay; LD= Long delay; CS= change score
Significance at p<0.05
Significance at p< 0.01.
Raw scores are presented unless denoted otherwise.#
Cognition
To estimate neuropsychological change in individuals, we calculated simple change scores (follow-up raw score minus baseline raw score) for the following cognitive tests: trials 1–5, short delay free-recall, and long delay free-recall raw scores from the California Verbal Learning Test – Second Edition (CVLT-II), measuring word list recall, and the Logical Memory immediate and delayed recall subtests of the Wechsler Memory Scale-Revised (WMS-R), measuring story recall. These tests were selected based on results from a principal component analysis previously reported by our group on a similar sample of older adults (Wierenga et al., 2012). A verbal memory change score composite (Memory-CS-Composite) was calculated by averaging the z-scores for simple change for each of the tests for the entire sample. A baseline verbal memory composite score was derived by averaging the z-scores at baseline for each of the tests for the entire sample. In order to account for potential influence of practice effects and regression to the mean (Cysique et al., 2009; Heaton et al., 2001), we also ran all analyses using standardized regression-based change scores and results were similar (please see Appendix for methods and results [Table 4, Table 5]).
Apolipoprotein E genotyping
Genotyping was performed by the ADCS Biomarker Core at UCSD using real time PCR Restriction Fragment Length Polymorphism analysis. Genomic DNA was collected from participants using buccal swab and extracted using Qiamp DNA blood mini kit (Qiagen) followed by PCR amplification (Wierenga, et al., 2012). Those with at least one ε4 allele (i.e., ε2/ε4, ε3/ε4, ε4/ε4) were classified as APOE e4 carriers (ε4+) and those without an ε4 allele (i.e., ε2/ε2, ε2/ε3, ε3/ε3) were classified as non-carriers (ε4−). Given that the APOE ε2 allele is thought to have protective effects (Suri, Heise, Trachtenberg, & Mackay, 2013), all analyses were run including and excluding ε2 carriers from the ε4+ group and the pattern of results was similar. Therefore, results from the entire sample are presented. An exact test of Hardy-Weinberg Equilibrium was not significant (p= 0.62), suggesting that the distribution of APOE genotype in the sample included in this manuscript does not differ significantly from the expected distribution in the general population.
MRI acquisition
Imaging data were acquired on a GE Discovery MR750 3T whole body system with a body transmit coil and an 8-channel receive-only head coil at the University of California San Diego Center for Functional MRI. The structural brain sequence consisted of a high-resolution T1-weighted Fast Spoiled Gradient Recall (3D FSPGR) scan: 172 1 mm contiguous sagittal slices, FOV = 25 cm, TR = 8 ms, TE = 3.1 ms, flip angle = 12, TI = 600 ms, 256 × 192 matrix, Bandwidth = 31.25 kHz, frequency direction = S-I, NEX = 1, scan time = 8 min and 13 sec. Resting CBF was acquired with the Multiphase Pseudocontinuous Arterial Spin Labeling (MPPCASL) sequence, which is optimized for robust CBF quantification (Jung, Wong, & Liu, 2010): tagging duration = 2 sec, post-labeling delay = 1.6 sec, TR = 4.2 sec, TE = 3 ms, reps = 64, FOV = 24 × 24 cm, 64×64 matrix, 3.75 mm x 3.75 mm in-plane resolution, 20 5 mm axial slices with a single shot 2D spiral acquisition, collecting 8 cycles where each cycle consists of 8 images acquired with unique phase offsets, acquisition time = 4:46 minutes. A CSF calibration scan was also obtained using a spiral readout with TR = 4 sec and TE = 3.4 ms and comprised nine 90° excitation pulses which were turned off for the first eight repetitions to generate PD-weighted contrast (scan time: 36 sec) to obtain an estimate of the equilibrium magnetization of cerebral spinal fluid, which is used to convert the perfusion signal into calibrated CBF units (mL blood/100g tissue/min). Finally, a minimum contrast image (TE = 11 ms, TR = 2000 ms, 8-shot interleaved spiral acquisition) was acquired to adjust for transmit and receive coil inhomogeneities. Two field map scans were also acquired and used for off-line field map correction to help correct for signal bunching and dropouts in the frontal/medial temporal lobes.
MRI pre-processing
CBF
ASL image processing was performed with Analysis of Functional NeuroImages (AFNI, afni.nimh.nih.gov)(Cox, 1996), FMRIB Software Library (FSL, Oxford, United Kingdom), and locally created Matlab scripts. Field map correction was applied to the ASL time series prior to co-registration to the middle time point to minimize the effects of participant motion. Coil inhomogeneities were corrected using data from the minimum contrast image and the approach described in (Wang, Qiu, & Constable, 2005). For each participant, a mean ASL difference image was formed from the average difference of the control and tag images and slice timing delays were accounted for in order to make the post-labeling delay time slice specific (T. T. Liu & Wong, 2005). In other words, with the slices acquired in an ascending sequential fashion and an inter-slice timing delay of 28 ms, the slice-specific post-labeling delays ranged from 1600 ms for the most inferior slice to 2132 ms for the most superior slice. The mean ASL image was then converted to absolute units of CBF (mL/100g tissue/min) using an estimate of the equilibrium magnetization of CSF as a reference signal (Chalela et al., 2000). This procedure resulted in a calibrated perfusion value for each voxel. Additional details on the CBF quantification process, including the equation used, are provided in (Jung et al., 2010). Skull stripping of the high-resolution T1-weighted image was performed using AFNI’s 3dSkullStrip. Tissue segmentation was performed using FSL’s Automated Segmentation Tool (FAST) algorithm (http://fsl.fmrib.ox.ac.uk/fsl/) to define cerebrospinal fluid (CSF), gray matter (GM) and white matter (WM) regions. The high-resolution T1-weighted image and partial volume segmentations were registered to ASL space, and the CBF data were resampled to the same resolution as the T1-weighted image. The partial volume estimates were used to perform partial volume correction of the high-resolution CBF data using a linear regression approach with kernel size of 3 (Asllani et al., 2008; Zhao et al., 2017) as implemented by the ASL file function in the BASIL toolset of FSL (Chappell, Groves, Whitcher, & Woolrich, 2009). The choice of the 3×3 kernel size was motivated the findings of (Zhao et al., 2017) indicating that a 3×3 kernel provides higher accuracy as compared to a larger 5×5 kernel. The partial volume corrected gray matter CBF data were then resampled back to their native resolution and registered to FreeSurfer space. Quality assurance of ASL data was performed prior to analysis using outlier detection, inspection of CBF histograms, and visual checks of the CBF maps, with removal of values for regions with poor CBF map coverage. This resulted in removal of 5% of the data. CBF values were extracted from each anatomically defined FreeSurfer ROI (see below for FreeSurfer methods). Because we did not aim to explore laterality and there were significant and robust correlations between hemispheres (all r > 0.57, p < 0.0001), we averaged CBF values across left and right hemispheres. Average EC and Hc CBF were used in subsequent analyses.
CT and Vo
Cortical thickness and volume analysis were performed using FreeSurfer version 5.3 (http://surfer.nmr.mgh.harvard.edu), with the technical details of these procedures described in prior publications (Dale, Fischl, & Sereno, 1999; Dale & Sereno, 1993; B. Fischl & Dale, 2000; B. Fischl, Liu, & Dale, 2001; B. Fischl, Sereno, Tootell, & Dale, 1999; Bruce Fischl et al., 2002; Bruce Fischl, Salat, et al., 2004; Bruce Fischl, van der Kouwe, et al., 2004; Reuter, Rosas, & Fischl, 2010; Reuter, Schmansky, Rosas, & Fischl, 2012). Cortical thickness was extracted from the left and right EC and volume was extracted from the left and right Hc (measures of thickness are not available for subcortical brain structures). A quality assurance protocol was performed before analysis using the ENIGMA guidelines (http://enigma.usc.edu/protocols/imaging-protocols) and included visual checks of the cortical segmentations and region-by-region removal of values for segmentations found to be incorrect. Histograms of all regions’ values were also computed for visual inspection. Because we did not aim to explore laterality and there were significant and robust correlations between hemispheres (all r > 0.62, p < 0.0001), we averaged CT values across left and right hemispheres. Average EC CT and average Hc Vo were used in subsequent analyses.
Statistical analyses
t-tests were used to compare groups on age, years of education, brain variables (CBF, CT/Vo), and cognitive variables. A χ2-test was used to compare groups on sex. CBF and CT/Vo were extracted from FreeSurfer-derived regions of interest (i.e., CT of the EC, Vo of the Hc) and multiple linear regression models were employed in R with the Memory-CS-Composite as the dependent variable and APOE genotype and brain variables (CBF, CT/Vo) as independent variables. The following 3-way interactions were explored: 1) APOE genotype x EC CBF x EC CT, and 2) APOE genotype x Hc CBF x Hc Vo. A Bonferroni correction was applied to correct for the two comparisons, and coefficients were only considered significant at p<0.025. All analyses statistically adjusted for the effects of age, sex, education, and the cognitive follow-up interval (time between baseline cognitive testing and the most recent follow-up cognitive testing). Analyses including measures of Hc Vo also adjusted for the effects of total intracranial volume. Non-multicollinearity between independent variables was confirmed by application of the multicollinearity index VIF (all VIF<2), linearity was confirmed by residuals versus fits plots, normality was confirmed by Q-Q plots, non-heteroskedasticity was confirmed by scale location plots, and no influential cases were identified through examinations of residuals versus leverage plots.
RESULTS
Group differences in demographic and assessment variables
Groups (ε4+, ε4−) did not differ significantly on age, sex, or years of education, nor did they differ significantly in the time interval between baseline neuropsychological testing and neuroimaging or the cognitive follow-up interval (all ps > 0.05; see Table 1). APOE ε4 carriers demonstrated worse performance on the baseline memory composite than did non-carriers. They also performed worse on four of the five subtests that were used to create the baseline memory composite (i.e., CVLT-II trials 1–5, long delay free recall; WMS Logical Memory Immediate and Delayed Recall; see Table 1). Groups (ε4+, ε4−) did not differ significantly on the Memory-CS-Composite, but ε4 carriers demonstrated less decline on two of the five subtests (CVLT-II trials 1–5 and short-delay free recall, see Table 1). No other significant group differences in cognitive performance were found (all ps > 0.05). No group differences in resting CBF or CT/Vo were found in brain regions of interest (i.e., Hc, EC; all ps > .05; see Table 1).
APOE, CBF, and CT/Vo on memory change
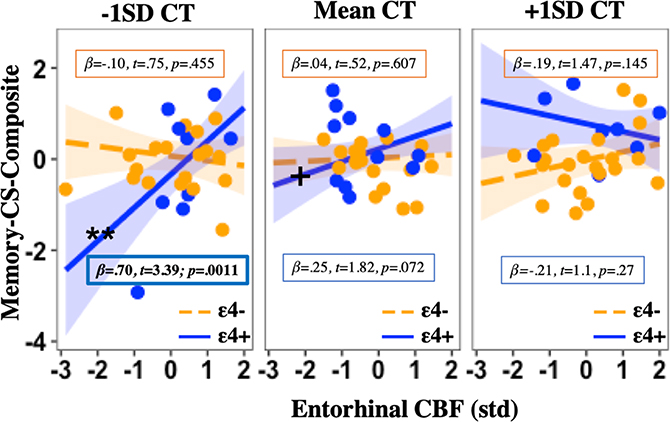
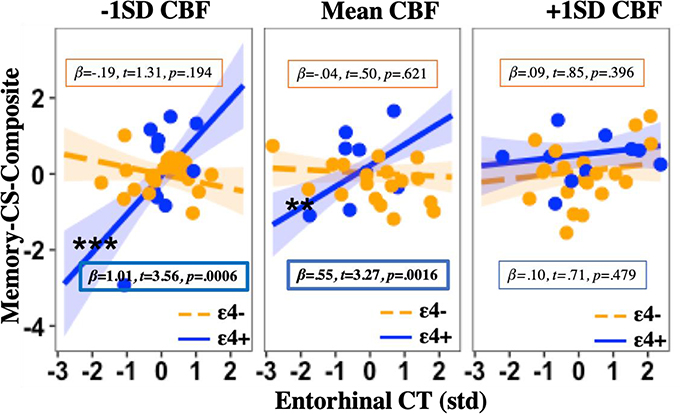
A significant three-way interaction was found in the EC (β= −.59, t= 3.47, p= 0.0008; see Table 2), whereby there was a positive relationship between EC CBF and memory change, but only among ε4 carriers with lower baseline EC CT. Similarly, there was a positive relationship between EC CT and memory change, but only among ε4 carriers with lower baseline EC CBF (see Fig. 1). There was not a statistically significant three-way interaction observed in the Hc (see Table 2).
Table 2.
Effects of CBF, CT/Vo, and APOE, on memory change.
| Independent Variable | β | s.e. | t | p-value |
|---|---|---|---|---|
| APOE | 0.1972 | 0.1594 | 1.237 | 0.2198 |
| Entorhinal CT | −0.0448 | 0.0902 | 0.497 | 0.6207 |
| Entorhinal CBF | 0.0362 | 0.0897 | 0.405 | 0.6869 |
| APOE*Entorhinal CT | 0.5996 | 0.1883 | 3.184 | 0.0021** |
| APOE*Entorhinal CBF | 0.2413 | 0.1613 | 1.496 | 0.1388 |
| Entorhinal CT*Entorhinal CBF | 0.1426 | 0.0931 | 1.531 | 0.1300 |
| APOE*Entorhinal CT*Entorhinal CBF | −0.5962 | 0.1717 | 3.471 | 0.0008*** |
| APOE | 0.1247 | 0.174604 | 0.715 | 0.4769 |
| Hippocampal Vo | −0.0069 | 0.104691 | −0.067 | 0.9468 |
| Hippocampal CBF | 0.0066 | 0.100248 | 0.067 | 0.9470 |
| APOE*Hippocampal Vo | 0.3895 | 0.180638 | 2.156 | 0.0340 |
| APOE*Hippocampal CBF | 0.1401 | 0.19019 | 0.737 | 0.4633 |
| Hc Vo*Hippocampal CBF | 0.0016 | 0.100275 | 0.016 | 0.9871 |
| APOE*Hippocampal Vo*Hippocampal CBF | −0.1779 | 0.17253 | −1.032 | 0.3053 |
Only variables of interest are included; Continuous independent variables were standardized; APOE=Apolipoprotein E gene; CBF= Cerebral blood flow; CT= Cortical thickness; Vo= Volume; β= Standardized regression coefficient; t= t-statistic; p= p=value
Significance at p<0.025
Significance at p<0.01
Significance at p<0.001
Fig. 1a. Effects of entorhinal CT, CBF, and APOE on memory change.
Three-way interaction plot with simple slopes (mean ±1SD) showing that the relationship between baseline entorhinal CBF and memory change differs significantly by APOE genotype and CT, such that lower baseline entorhinal CBF is associated with greater memory decline, but only among ε4 carriers with lower baseline entorhinal CT, with higher CT appearing relatively protective against future cognitive decline. Note: EC= Entorhinal cortex; APOE= Apolipoprotein E gene; CBF= Cerebral blood flow; CT= Cortical thickness; std= Z-score standardization; SD= standard deviation; t= t-statistic; p= p-value; **Simple slope significance at p<0.01; simple slope significance at p<0.001
Post hoc analyses
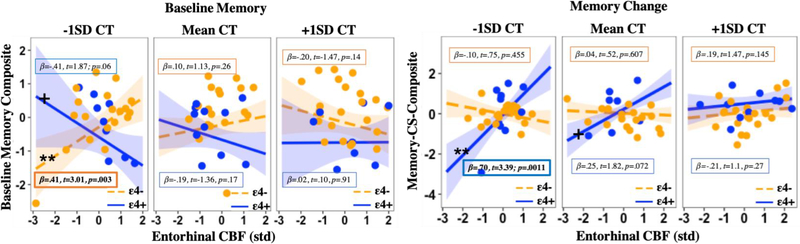
Aspects of the current longitudinal results differ from our previously reported cross-sectional study from which the current sample was selected (Hays et al., 2019). Cross-sectionally, we found that higher (rather than lower) CBF in the context of lower CT among ε4 carriers was associated with worse baseline memory performance. As such, we thought it important to confirm that the prior cross-sectional findings were replicable in this smaller subset of participants and that differential findings indeed reflect differences in the dependent variable (e.g., baseline memory performance versus longitudinal change in memory performance), rather than sampling error or minor methodological differences (i.e., reduced sample size, averaging across left and right hemispheres). Post hoc analyses of the current sample with baseline memory as the dependent variable largely replicated the prior three-way interaction in the EC (including directionality), such that independent associations between EC CBF and CT on baseline memory performance were dependent on APOE genotype and baseline brain measures (β=.51, t= 2.86, p= 0.005; see Table 6 in the Appendix). With regard to ε4 carriers, higher CBF and lower CT was associated with worse baseline memory performance (see Fig. 2 for a side by side comparison of results with regard to baseline memory versus longitudinal memory change).
Fig. 2. Differential effects of CBF, CT, and APOE on baseline memory and memory change.
Three-way interaction plots with simple slopes (mean ±1SD) showing that the combination of higher CBF and lower CT among ε4 carriers is associated with worse memory performance at baseline, but with greater memory stability over time. In contrast, the combination of lower CBF and lower CT at baseline among ε4 carriers is associated with greater memory decline over time. These differential findings suggest that compensatory increases in CBF at baseline among ε4 carriers with lower cortical reserve (although associated with worse baseline memory) are supportive of memory function over time, whereas relative reductions in CBF among this same group are associated with memory decline, likely reflecting an absence or breakdown of cerebrovascular compensatory mechanisms. Note: EC= Entorhinal cortex; APOE= Apolipoprotein E gene; CBF= Cerebral blood flow; CT= Cortical thickness; std= Z-score standardization; SD= standard deviation; t= t-statistic; p= p-value; n.s.= not significant; +Denotes marginal simple slope significance at p<0.10; **Denotes simple slope significance at p<0.01
DISCUSSION
Results showed that lower baseline CBF is associated with greater memory decline, while higher baseline CBF is associated with greater memory stability, but only for ε4 carriers who show lower baseline CT in this same anatomical region. Similarly, baseline levels of EC CT are positively associated with future memory change, but only for carriers who show lower baseline EC CBF. These findings suggest that ε4 carriers who demonstrate lower CBF and lower CT in the EC at baseline are at elevated risk for future cognitive decline, whereas carriers who show higher levels of at least one of these brain indicators may be relatively protected against future cognitive decline. The localization of this interaction within the EC is highly suggestive of AD-related neuropathological processes, rather than exacerbation or acceleration of normal aging processes, as the EC is one of the first regions to be affected by AD pathology (Braak & Braak, 1991), but is relatively spared in normal aging (Fjell et al., 2009; Good et al., 2001; Raz et al., 2005; Raz, Rodrigue, & Haacke, 2007).
The current results extend our prior cross-sectional cognitive findings to show that interactions among MTL CBF, CT, and APOE also predict longitudinal changes in memory performance. Notably, the longitudinal cognitive results differ from our recent cross-sectional findings (Hays et al., 2019) in an important way; the longitudinal results showed that the combination of lower CBF and lower CT among APOE ε4 carriers was associated with worse memory function over time (memory decline), whereas the cross-sectional results showed that the combination of higher CBF and lower CT was associated with worse memory function at baseline. Of note, post hoc analyses within the current sample replicated these prior cross-sectional findings (see Fig. 2 for a comparison of results [baseline memory versus longitudinal change in memory as dependent variable]). Together, these data suggest that higher baseline EC CBF among this group, although not supportive of memory function at baseline, has beneficial effects on memory function over time (e.g., greater memory stability), whereas lower baseline EC CBF has detrimental effects on memory function over time (e.g., greater memory decline). Although speculative, these findings may be appreciated in the context of cerebrovascular compensation, whereby ε4 carriers with lower entorhinal CT are engaged in a process of cerebrovascular compensation, with relative increases in CBF reflecting effective attempts to maintain adequate brain oxygenation in the face of vascular aging and/or neuropathological damage (e.g., higher CBF and lower CT being associated with worse baseline memory but greater memory stability over time) and relative reductions in CBF reflecting ineffective cerebrovascular compensation (e.g., lower CBF and lower CT associated with greater memory decline). Within this framework, the current results could suggest that different stages of cerebrovascular compensation are indeed detectable in cognitively normal ε4 carriers with lower CT and may hold predictive utility with regard to cognitive trajectories. This notion is largely consistent with the Capillary Dysfunction Hypothesis of Alzheimer’s disease (Jespersen & Østergaard, 2012; Ostergaard et al., 2013), which posits that ε4 carriers experience increased heterogeneity of capillary blood flow, which reduces the amount of oxygen that can diffuse into tissue. This reduction in oxygen diffusion necessitates a compensatory increase in CBF to maintain adequate brain oxygenation. However, progressive increases in this heterogeneity of flow is thought to result in low tissue oxygen tension, a state which paradoxically benefits (due to increased blood-tissue oxygen concentration gradients) from suppression of CBF. Although essential for the maintenance of oxygen availability, this compensatory reduction in cerebral perfusion may ultimately lead to oxidative stress, activation of inflammatory pathways, and increased amyloid levels in the brain. In this context, the observed pattern of lower cross-sectional EC CBF and CT among ε4 carriers being associated with future cognitive decline could reflect the negative downstream effects of vascular dysfunction, with lower CBF reflecting suppression of perfusion and lower CT reflecting subsequent neurodegeneration due to the damaging effects of this suppression (e.g., oxidative stress, neuroinflammation, amyloid aggregation). Alternatively, lower CT among this group could represent long-standing APOE-related alterations in brain structure that are independent of capillary dysfunction and/or alterations in CBF but may confer additive risk for cognitive decline by way of lower cortical reserve. However, it is important to note that the current study did not explore CBF or CT/Vo longitudinally, therefore, serial neuroimaging studies are needed to directly test hypotheses relating to cerebrovascular compensation.
Taken together, the current results suggest that cognitively normal ε4 carriers who demonstrate a combined pattern of lower CBF and lower CT in the EC may be at elevated risk for future memory decline due to ineffective cerebrovascular compensation, whereas those who demonstrate higher levels of at least one of these measures may be relatively protected against decline. It should be noted, however, that the average follow-up interval in the current study was only 2.5 years. As such, ε4 carriers showing lower levels of either CBF or CT in the EC may still be at increased risk for cognitive decline beyond several years, relative to those with higher levels of both brain measures. This may be particularly true for ε4 carriers showing a combination of higher CBF and lower CT in the entorhinal cortex, as this pattern suggests the presence of cerebrovascular compensatory mechanisms that, although appearing supportive of memory function over several years, are likely to breakdown over time (e.g., increased CBF followed later by suppressed CBF). Interestingly, this suggests that there are distinct multimodal neural signatures in the entorhinal cortex that may be sensitive to relative risk for future cognitive decline among cognitively normal ε4 carriers (e.g., high risk= lower CBF and lower CT; moderate risk= higher CBF and lower CT; low risk= higher CBF and higher CT), reflecting different stages of cerebrovascular compensation. Another question that remains unanswered is whether the interaction of longitudinal changes in MTL CBF and CT might provide even earlier and more reliable detection of risk for cognitive decline among ε4 carriers, as longitudinal changes in perfusion may capture more accurate information about where ε4 carries lie on this trajectory of compensation (e.g., longitudinal increases reflecting early compensation, longitudinal decreases reflecting breakdown of compensation) when compared to cross-sectional measures.
Strengths and limitations
The strongest limitation of the current study is our use of cross-sectional brain measures (CBF, CT/Vo) which restricted our ability to draw causal conclusions or infer directionality. It is also important to note that our average cognitive follow-up interval of 2.5 years is relatively short, and we did not observe a significant decline in the Memory-CS-Composite in either group, nor did groups differ significantly on this measure. Although somewhat unexpected, these findings suggest that a longer follow-up interval is necessary to detect widespread decline in the current sample, which was highly educated and relatively healthy (no major vascular events, no brain injuries, no uncontrolled hypertension, no major mental health diagnoses, cognitively normal at baseline). This relatively short interval also limited our ability to determine whether the effects of entorhinal CBF and CT on cognition among ε4 carriers represents normal aging or pathologic processes. Future studies should attempt to replicate these findings using longer cognitive follow-up intervals. Our sample was also characterized by relatively high levels of education, and although this demographic factor was not associated with APOE genotype, its limited range may reduce the generalizability of these findings. Although data collection for this study was initiated prior to 2015, future ASL studies of older adults should consider using longer post-labeling delay as recommended by the ISMRM perfusion study group in 2015 (Alsop et al., 2015). Although groups did not differ significantly in the average time interval between cognitive testing and MRI scanning, decreasing the time interval between cognitive testing and MRI may improve accuracy of brain-behavior associations. Moreover, our sample of 98 included only 31 APOE ε4 carriers, which may have limited our ability to detect a statistically significant three-way interaction in the Hc. Future investigations should include longitudinal designs with larger, more diverse samples to replicate and extend the current finding and establish a range of effect size to inform power for study design. Future studies should also investigate whether interactive effects of CBF and CT/Vo on cognition extend to regions outside the MTL. Lastly, investigation of interactions between longitudinal changes in CBF and CT on cognitive decline, and the inclusion of additional markers of AD, such as CSF biomarkers may help better characterize APOE ε4 carriers.
Strengths of the current study include the use of non-invasive ASL MRI to measure partial volume corrected CBF, and the use of a high-resolution structural MRI scan to examine regional CT and Vo. Furthermore, use of FreeSurfer offers advantages over traditional voxel-based morphometry methods, as it also allows for examination of the components of volume separately (thickness and surface area), providing a direct index of cortical morphology that is less susceptible to variations in individual positioning (Kim et al., 2005) and allowing for sub-voxel precision with thickness values being assigned to individual vertices rather than voxels (B. Fischl & Dale, 2000). Moreover, the extraction of CBF from FreeSurfer-derived brain regions allowed us to directly investigate CBF (and brain structure) in regions that are defined by each individual’s anatomy, rather than atlas-defined regions which are less sensitive to individual anatomical differences because they require that data are first aligned and warped to a generic anatomic template. Another strength includes the use of longitudinal cognitive data and standardized regression-based change scores to account for practice effect and regression toward the mean. Lastly, the current study benefited from the inclusion of a well-controlled and well-characterized sample of cognitive normal older adults, which included the use of several cognitive test performances to characterize cognitive status.
Conclusions
The current findings indicate that older adult APOE ε4 carriers may experience concomitant alterations in neurovascular function and morphology in the MTL that interact with one another to negatively affect cognition prior to the onset of overt clinical symptoms, providing insight into the mechanistic link between APOE ε4 and cognitive decline. Results also suggest the presence of distinct multimodal neural signatures in the entorhinal cortex that may signal relative risk for cognitive decline among this group (e.g., high risk= lower CBF and lower CT; moderate risk= higher CBF and lower CT; low risk= higher CBF and higher CT), likely reflecting different stages of cerebrovascular compensation. Such early detection could inform candidate selection and study design for future clinical trials. On a broader scale, the current results add to accumulating evidence supporting the early role of vascular dysregulation in AD (de la Torre, 2010; Iadecola, 2004; Iturria-Medina et al., 2016; Zlokovic, 2011) and could lead to the identification of vasoprotective treatments with the potential to delay or prevent the onset of age-related cognitive decline and/or AD.
Fig. 1b. Effects of entorhinal CT, CBF, and APOE on memory change.
Three-way interaction plot with simple slopes (mean ±1SD) showing that the relationship between baseline entorhinal CT and memory change differs significantly by APOE genotype and CBF, such that lower baseline entorhinal CT is associated with greater memory decline, but only among ε4 carriers with average or lower baseline entorhinal CBF, with higher CBF appearing protective against future cognitive decline. Note: EC= Entorhinal cortex; APOE= Apolipoprotein E gene; CBF= Cerebral blood flow; CT= Cortical thickness; std= Z-score standardization; SD= standard deviation; t= t-statistic; p= p-value; **Simple slope significance at p<0.01
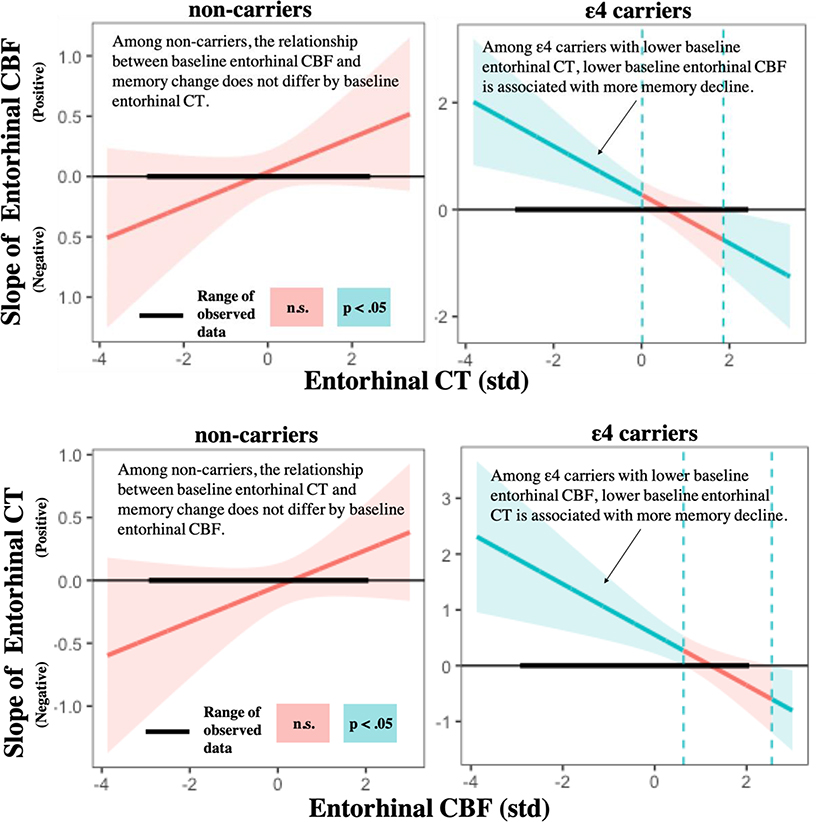
Fig. 1c. Effects of entorhinal CT, CBF, and APOE on memory change.
Johnson-Neyman plots showing the intervals (depicted in blue) for which the interaction of EC CBF and CT on memory change is statically significant for ε4 carriers and non-carriers. Lower baseline entorhinal CBF is associated with greater memory decline, but only among ε4 carriers with lower (≤ 0.01SD) baseline entorhinal CT. Similarly, lower baseline CT is associated with greater cognitive decline, but only among ε4 carriers with lower (≤ 0.63SD) baseline entorhinal CBF. These findings suggest that, among ε4 carriers, the combination of lower CT and lower CBF in the entorhinal cortex is associated with future cognitive decline, whereas having higher levels of at least one of these brain measures (CBF or CT) at baseline is relatively protective against future cognitive decline. Note: EC= Entorhinal cortex; APOE= Apolipoprotein E gene; CBF= Cerebral blood flow; CT= Cortical thickness; std= Z-score standardization; n.s.= not significant
Acknowledgements
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by VA CSR&D Merit Award [5I01CX000565 C.E.W.], the National Science Foundation Graduate Research Fellowship Program [2015207525 C.C.H.], and the National Institute on Aging of the National Institutes of Health [K23AG049906 Z.Z.Z.], [K24AG026431 M.W.B], [R01AG054049 M.W.B]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the VA, National Science Foundation, or National Institutes of Health. We would also like to acknowledge Dr. Robert Rissman, Associate Professor of Neurosciences at the University of California San Diego for his contributions to this project.
APPENDIX
Table 3.
Cognitive tests, domains, and normative data.
| Test | Cognitive Domain | Normative Data |
|---|---|---|
| DRS | Global Cognition | (Mattis, 1988) |
| WMS-R Logical Memory | Memory | (Ivnik et al., 1992) |
| WMS-R Visual Reproduction Recall | Memory | (Ivnik et al., 1992) |
| CVLT-II | Memory | (Delis, Kramer, & Ober, 2000) |
| WCST-48 card version | Executive Functioning | (Lineweaver, Bond, Thomas, & Salmon, 1999) |
| DKEFS Trail Making | Executive Functioning | (Delis, Kaplan, & Kramer, 2001) |
| DKEFS CW Interference | Executive Functioning | (Delis et al., 2001) |
| DKEFS Letter Fluency | Language | (Delis et al., 2001) |
| DKEFS Category Fluency | Language | (Delis et al., 2001) |
| Boston Naming Test | Language | (Heaton & Psychological Assessment Resources, 2004) |
| MiNT | Language | (Ivanova, Salmon, & Gollan, 2013) |
| DKEFS Trails Visual Scanning | Visuospatial Functioning | (Delis et al., 2001) |
| WMS-R Visual Reproduction Copy | Visuospatial Functioning | (Ivnik et al., 1992) |
| Clock Drawing Test | Visuospatial Functioning | N/A |
| DKEFS CW Interference Word Reading | Attention/Processing Speed | (Delis et al., 2001) |
| WAIS-R Digit Symbol | Attention/Processing Speed | (Ivnik et al., 1992) |
| WAIS-R Digit Span | Attention/Processing Speed | (Ivnik et al., 1992) |
DRS= Mattis Dementia Rating Scale; WMS-R= Wechsler Memory Scale Revised; CVLT= California Verbal Learning Test; WCST= Wisconsin Card Sorting Test; DKEFS= Delis-Kaplan Executive Function System; CW= Color-Word; WAIS-R= Wechsler Adult Intelligence Scale Revised; MINT= Multilingual Naming Test
Regression-based cognitive change score
As an alternative approach to account for practice effect and regression toward the mean, standardized change scores were also calculate to estimate neuropsychological change in individuals(Cysique et al., 2009; Heaton et al., 2001). To develop the mean scaled score regression change score (MSR-CS), the first step consisted of defining a reference group for which no neuropsychological change is expected beyond practice effect: we used a sample of 77 cognitively normal older adults who were also assessed serially at one-year intervals. The demographic characteristics of the reference group were as follows: mean age of 73.3 years (SD = 8.0); with a mean level of education of 16.3 years (SD = 2.1); 36% were men. These demographic characteristics were not statistically different from the target sample. All subjects in the reference group were cognitively normal (using the same criteria described above for the target sample) at baseline and remained cognitively normal at all follow-up timepoints. The second step of the MSR-CS development was as follows: the reference group yielded a set of regression equations (see Table 4 in the Appendix) from baseline to first follow-up (mean time between baseline to first follow-up in reference sample = 1.3 years, SD = 0.77), statistically adjusting for age, sex, education, and the follow-up interval, which was then used to derive predicted follow-up scores in the target sample. Subtracting the predicted follow-up score from individual’s observed follow-up score and dividing that result by the standard deviation of the residuals from the reference group regression models provided a standard score of cognitive change. This score was then used as a continuous variable; the MSR-CS (i.e., significant neuropsychological decline as: MSR-CS ≤−1.04 based on a two-tailed 70% confidence interval, MSR-CS ≤−1.28 based on a two-tailed 80% confidence interval, MSR-CS ≤−1.645 based on a two-tailed 90% confidence interval). The methods above were applied to the following cognitive test scores: trials 1–5, short delay free-recall, and long delay free-recall raw scores from the California Verbal Learning Test – Second Edition (CVLT-II), measuring word list recall, and the Logical Memory immediate and delayed recall subtests of the Wechsler Memory Scale-Revised (WMS-R), measuring story recall. These tests were selected based on results from a principal component analysis previously reported by our group on a similar sample of older adults (Wierenga et al., 2012). A verbal memory change score composite (Memory-CS-Composite) was calculated by averaging the MSR-CS’s for the five verbal memory tests.
Table 4.
Regression equations for predicting follow-up scores on CVLT-II and WMS-R Logical Memory variables.
| CVLT-II variable | R2 | SEE | a | b | c | d | e | f |
| Total 1–5 | .39 | 8.55 | 10.04 | .73 | −.02 | .27 | .88 | 1.14 |
| Short delay free recall | .42 | 2.45 | 8.18 | .63 | −.07 | −.14 | −.38 | −.17 |
| Long delay free recall | .42 | 2.19 | 4.40 | .54 | −.03 | .25 | .35 | −.38 |
| WMS-R Logical Memory variable | R2 | SEE | a | b | c | d | e | f |
| Logical Memory I | .40 | 4.53 | 10.68 | .48 | −.03 | .49 | −.91 | 1.11 |
| Logical Memory II | .41 | 4.56 | 11.59 | .49 | −.09 | .46 | −.33 | 1.04 |
CVLT-II = California Verbal Learning Test- second edition; WMS-R= Wechsler Memory Scale- Revised; SEE = standard error of the estimate; a= Constant; b= Unstandardized b weight for time 1 index score; c=Unstandardized b weight for age; d= Unstandardized b weight for education; e= Unstandardized b weight for gender; f= Unstandardized b weight for retest interval; Age is in years; Education is in years; Gender is coded as 0 for female and 1 for male; Retest interval is in years
Table 5.
Effects of CBF, CT/Vo, and APOE, on regression-based memory change.
| Independent Variable | β | s.e. | t | p-value |
|---|---|---|---|---|
| APOE | 0.132 | 0.206 | 0.643 | 0.522 |
| Entorhinal CT | −0.052 | 0.112 | −0.468 | 0.640 |
| Entorhinal CBF | 0.082 | 0.112 | 0.734 | 0.465 |
| APOE*Entorhinal CT | 0.720 | 0.233 | 3.09 | 0.002** |
| APOE*Entorhinal CBF | 0.197 | 0.204 | 0.967 | 0.336 |
| Entorhinal CT*Entorhinal CBF | 0.090 | 0.122 | 0.738 | 0.462 |
| APOE*Entorhinal CT*Entorhinal CBF | −0.590 | 0.223 | −2.647 | 0.009** |
| APOE | 0.024 | 0.213 | 0.114 | 0.909 |
| Hippocampal Vo | −0.027 | 0.012 | −0.218 | 0.828 |
| Hippocampal CBF | 0.033 | 0.119 | 0.279 | 0.781 |
| APOE*Hippocampal Vo | 0.519 | 0.215 | 2.417 | 0.017* |
| APOE*Hippocampal CBF | 0.055 | 0.228 | 0.243 | 0.808 |
| Hc Vo*Hippocampal CBF | −0.001 | 0.119 | −0.014 | 0.988 |
| APOE*Hippocampal Vo*Hippocampal CBF | −0.222 | 0.205 | −1.082 | 0.282 |
Only variables of interest are included; Continuous independent variables were standardized; APOE=Apolipoprotein E gene; CBF= Cerebral blood flow; CT= Cortical thickness; Vo= Volume; β= Standardized regression coefficient; t= t-statistic; p= p=value
Significance at p<0.025
Significance at p<0.01
Table 6.
Post hoc analysis of EC CBF, CT/Vo, and APOE, on baseline memory.
| Independent Variable | β | s.e. | t | p-value |
|---|---|---|---|---|
| APOE | −0.448 | 0.168 | −2.666 | 0.009** |
| Entorhinal CT | 0.067 | 0.095 | 0.708 | 0.481 |
| Entorhinal CBF | 0.128 | 0.094 | 1.352 | 0.180 |
| APOE*Entorhinal CT | −0.147 | 0.198 | −0.743 | 0.460 |
| APOE*Entorhinal CBF | −0.337 | 0.170 | −1.983 | 0.050+ |
| Entorhinal CT*Entorhinal CBF | −0.304 | 0.098 | −3.101 | 0.002** |
| APOE*Entorhinal CT*Entorhinal CBF | 0.518 | 0.181 | 2.864 | 0.005** |
Note: Only variables of interest are included in table; All continuous independent variables were standardized; APOE= Apolipoprotein E gene; CBF= Cerebral blood flow; CT= Cortical thickness; Vo= Volume; β= Standardized regression coefficient; t= t-statistic; p= p=value
Denotes marginal significance at p<0.10
Denotes significance at p<0.05
Denotes significance at p<0.01
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Compliance with Ethical Standards: The authors declare that they have no conflict of interest. Informed consent was obtained from all individual participants included in the study and all procedures were in accordance with the ethical standards of the UC San Diego and VA San Diego Healthcare System institutional review board and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Data availability: The data that support the findings of this study may be available on request from the corresponding author [CEW]. The data are not publicly available due to them containing information that could compromise research participant privacy/consent.
REFERENCES
- Adamson MM, Landy KM, Duong S, Fox-Bosetti S, Ashford JW, Murphy GM, … Taylor JL (2010). Apolipoprotein E epsilon4 influences on episodic recall and brain structures in aging pilots. Neurobiology of Aging, 31(6), 1059–1063. 10.1016/j.neurobiolaging.2008.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsop DC, Detre JA, Golay X, Günther M, Hendrikse J, Hernandez- Garcia L, … Zaharchuk G (2015). Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magnetic Resonance in Medicine, 73(1), 102–116. 10.1002/mrm.25197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asllani I, Habeck C, Scarmeas N, Borogovac A, Brown TR, & Stern Y (2008). Multivariate and univariate analysis of continuous arterial spin labeling perfusion MRI in Alzheimer’s disease. Journal of Cerebral Blood Flow and Metabolism: Official Journal of the International Society of Cerebral Blood Flow and Metabolism, 28(4), 725–736. 10.1038/sj.jcbfm.9600570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangen KJ, Restom K, Liu TT, Jak AJ, Wierenga CE, Salmon DP, & Bondi MW (2009). Differential age effects on cerebral blood flow and BOLD response to encoding: Associations with cognition and stroke risk. Neurobiology of Aging, 30(8), 1276–1287. 10.1016/j.neurobiolaging.2007.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangen KJ, Restom K, Liu TT, Wierenga CE, Jak AJ, Salmon DP, & Bondi MW (2012). Assessment of Alzheimer’s disease risk with functional magnetic resonance imaging: an arterial spin labeling study. Journal of Alzheimer’s Disease: JAD, 31 Suppl 3, S59–74. 10.3233/JAD-2012-120292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, & Braak E (1991). Demonstration of amyloid deposits and neurofibrillary changes in whole brain sections. Brain Pathology (Zurich, Switzerland), 1(3), 213–216. [DOI] [PubMed] [Google Scholar]
- Bretsky P, Guralnik JM, Launer L, Albert M, Seeman TE, & MacArthur Studies of Successful Aging. (2003). The role of APOE-epsilon4 in longitudinal cognitive decline: MacArthur Studies of Successful Aging. Neurology, 60(7), 1077–1081. [DOI] [PubMed] [Google Scholar]
- Burggren AC, Zeineh MM, Ekstrom AD, Braskie MN, Thompson PM, Small GW, & Bookheimer SY (2008). Reduced cortical thickness in hippocampal subregions among cognitively normal apolipoprotein E e4 carriers. NeuroImage, 41(4), 1177–1183. 10.1016/j.neuroimage.2008.03.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caselli RJ, Dueck AC, Osborne D, Sabbagh MN, Connor DJ, Ahern GL, … Reiman EM (2009). Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. The New England Journal of Medicine, 361(3), 255–263. 10.1056/NEJMoa0809437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caselli RJ, Reiman EM, Osborne D, Hentz JG, Baxter LC, Hernandez JL, & Alexander GG (2004). Longitudinal changes in cognition and behavior in asymptomatic carriers of the APOE e4 allele. Neurology, 62(11), 1990–1995. [DOI] [PubMed] [Google Scholar]
- Chalela JA, Alsop DC, Gonzalez-Atavales JB, Maldjian JA, Kasner SE, & Detre JA (2000). Magnetic Resonance Perfusion Imaging in Acute Ischemic Stroke Using Continuous Arterial Spin Labeling. Stroke, 31(3), 680–687. 10.1161/01.STR.31.3.680 [DOI] [PubMed] [Google Scholar]
- Chappell MA, Groves AR, Whitcher B, & Woolrich MW (2009). Variational Bayesian Inference for a Nonlinear Forward Model. IEEE Transactions on Signal Processing, 57(1), 223–236. 10.1109/TSP.2008.2005752 [DOI] [Google Scholar]
- Cohen RM, Small C, Lalonde F, Friz J, & Sunderland T (2001). Effect of apolipoprotein E genotype on hippocampal volume loss in aging healthy women. Neurology, 57(12), 2223–2228. [DOI] [PubMed] [Google Scholar]
- Cox RW (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, an International Journal, 29(3), 162–173. [DOI] [PubMed] [Google Scholar]
- Cysique LA, Vaida F, Letendre S, Gibson S, Cherner M, Woods SP, … Ellis RJ (2009). Dynamics of cognitive change in impaired HIV-positive patients initiating antiretroviral therapy. Neurology, 73(5), 342–348. 10.1212/WNL.0b013e3181ab2b3b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai W, Lopez OL, Carmichael OT, Becker JT, Kuller LH, & Gach HM (2009). Mild Cognitive Impairment and Alzheimer Disease: Patterns of Altered Cerebral Blood Flow at MR Imaging. Radiology, 250(3), 856–866. 10.1148/radiol.2503080751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, & Sereno MI (1999). Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage, 9(2), 179–194. 10.1006/nimg.1998.0395 [DOI] [PubMed] [Google Scholar]
- Dale AM, & Sereno MI (1993). Improved Localizadon of Cortical Activity by Combining EEG and MEG with MRI Cortical Surface Reconstruction: A Linear Approach. Journal of Cognitive Neuroscience, 5(2), 162–176. 10.1162/jocn.1993.5.2.162 [DOI] [PubMed] [Google Scholar]
- De Blasi S, Montesanto A, Martino C, Dato S, De Rango F, Bruni AC, … Passarino G (2009). APOE polymorphism affects episodic memory among non demented elderly subjects. Experimental Gerontology, 44(3), 224–227. 10.1016/j.exger.2008.11.005 [DOI] [PubMed] [Google Scholar]
- de la Torre JC (2010). The Vascular Hypothesis of Alzheimer’s Disease: Bench to Bedside and Beyond. Neurodegenerative Diseases, 7(1–3), 116–121. 10.1159/000285520 [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, & Kramer JH (2001). Delis-Kaplan Executive Function System (D-KEFS). San Antonia, TX: The Psychological Corporation. [Google Scholar]
- Delis DC, Kramer JH, & Ober BA (2000). The California Verbal Learning Test - Second Edition. San Antonia, TX: The Psychological Corporation. [Google Scholar]
- den Heijer T, Oudkerk M, Launer LJ, van Duijn CM, Hofman A, & Breteler MMB (2002). Hippocampal, amygdalar, and global brain atrophy in different apolipoprotein E genotypes. Neurology, 59(5), 746–748. [DOI] [PubMed] [Google Scholar]
- Donix M, Burggren AC, Scharf M, Marschner K, Suthana NA, Siddarth P, … Bookheimer SY (2013). APOE associated hemispheric asymmetry of entorhinal cortical thickness in aging and Alzheimer’s disease. Psychiatry Research, 214(3). 10.1016/j.pscychresns.2013.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donix M, Burggren AC, Suthana NA, Siddarth P, Ekstrom AD, Krupa AK, … Bookheimer SY (2010). Longitudinal changes in medial temporal cortical thickness in normal subjects with the APOE-4 polymorphism. NeuroImage, 53(1), 37–43. 10.1016/j.neuroimage.2010.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini N, Ebmeier KP, MacIntosh BJ, Trachtenberg AJ, Frisoni GB, Wilcock GK, … Mackay CE (2011). Differential effects of the APOE genotype on brain function across the lifespan. NeuroImage, 54(1), 602–610. 10.1016/j.neuroimage.2010.08.009 [DOI] [PubMed] [Google Scholar]
- Fischl B, & Dale AM (2000). Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America, 97(20), 11050–11055. 10.1073/pnas.200033797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Liu A, & Dale AM (2001). Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Transactions on Medical Imaging, 20(1), 70–80. 10.1109/42.906426 [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, … Dale AM (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron, 33(3), 341–355. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJW, Makris N, Ségonne F, Quinn BT, & Dale AM (2004). Sequence-independent segmentation of magnetic resonance images. NeuroImage, 23 Suppl 1, S69–84. 10.1016/j.neuroimage.2004.07.016 [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, & Dale AM (1999). High-resolution intersubject averaging and a coordinate system for the cortical surface. Human Brain Mapping, 8(4), 272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, … Dale AM (2004). Automatically parcellating the human cerebral cortex. Cerebral Cortex (New York, N.Y.: 1991), 14(1), 11–22. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Fennema-Notestine C, McEvoy LK, Hagler DJ, Holland D, … Dale AM (2009). One year brain atrophy evident in healthy aging. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 29(48), 15223–15231. 10.1523/JNEUROSCI.3252-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, & Frackowiak RS (2001). A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage, 14(1 Pt 1), 21–36. 10.1006/nimg.2001.0786 [DOI] [PubMed] [Google Scholar]
- Hayden KM, Zandi PP, West NA, Tschanz JT, Norton MC, Corcoran C, … Welsh-Bohmer KA (2009). Effects of Family History and Apolipoprotein E ε4 Status on Cognitive Decline in the Absence of Alzheimer Dementia: The Cache County Study. Archives of Neurology, 66(11), 1378–1383. 10.1001/archneurol.2009.237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays CC, Zlatar ZZ, Meloy MJ, Bondi MW, Gilbert PE, Liu TT, … Wierenga CE (2019). APOE modifies the interaction of entorhinal cerebral blood flow and cortical thickness on memory function in cognitively normal older adults. NeuroImage, 202, 116162 10.1016/j.neuroimage.2019.116162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays CC, Zlatar ZZ, & Wierenga CE (2016). The Utility of Cerebral Blood Flow as a Biomarker of Preclinical Alzheimer’s Disease. Cellular and Molecular Neurobiology, 36(2), 167–179. 10.1007/s10571-015-0261-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, & Psychological Assessment Resources, I. (2004). Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults, Professional Manual. Retrieved from https://books.google.com/books?id=u5rfnAEACAAJ
- Heaton RK, Temkin N, Dikmen S, Avitable N, Taylor MJ, Marcotte TD, & Grant I (2001). Detecting change: A comparison of three neuropsychological methods, using normal and clinical samples. Archives of Clinical Neuropsychology: The Official Journal of the National Academy of Neuropsychologists, 16(1), 75–91. [PubMed] [Google Scholar]
- Honea RA, Vidoni E, Harsha A, & Burns JM (2009). Impact of APOE on the Healthy Aging Brain: A Voxel-Based MRI and DTI Study. Journal of Alzheimer’s Disease : JAD, 18(3), 553–564. 10.3233/JAD-2009-1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C (2004). Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci, 5(5), 347–360. [DOI] [PubMed] [Google Scholar]
- Iturria-Medina Y, Sotero RC, Toussaint PJ, Mateos-Pérez JM, Evans AC, & Alzheimer’s Disease Neuroimaging Initiative. (2016). Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nature Communications, 7, 11934 10.1038/ncomms11934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova I, Salmon DP, & Gollan TH (2013). The multilingual naming test in Alzheimer’s disease: clues to the origin of naming impairments. Journal of the International Neuropsychological Society: JINS, 19(3), 272–283. 10.1017/S1355617712001282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivnik RJ, Malec JF, Smith GE, Tangalos EG, Petersen RC, Kokmen E, & Kurland LT (1992). Mayo’s older americans normative studies: WMS-R norms for ages 56 to 94. Clinical Neuropsychologist, 6(sup001), 49–82. 10.1080/13854049208401879 [DOI] [Google Scholar]
- Jak AJ, Bondi MW, Delano-Wood L, Wierenga C, Corey-Bloom J, Salmon DP, & Delis DC (2009). Quantification of five neuropsychological approaches to defining mild cognitive impairment. The American Journal of Geriatric Psychiatry : Official Journal of the American Association for Geriatric Psychiatry, 17(5), 368–375. 10.1097/JGP.0b013e31819431d5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jak AJ, Houston WS, Nagel BJ, Corey-Bloom J, & Bondi MW (2007). Differential Cross-Sectional and Longitudinal Impact of APOE Genotype on Hippocampal Volumes in Nondemented Older Adults. Dementia and Geriatric Cognitive Disorders, 23(6), 382–389. 10.1159/000101340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jespersen SN, & Østergaard L (2012). The roles of cerebral blood flow, capillary transit time heterogeneity, and oxygen tension in brain oxygenation and metabolism. Journal of Cerebral Blood Flow & Metabolism, 32(2), 264–277. 10.1038/jcbfm.2011.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y, Wong EC, & Liu TT (2010). Multiphase pseudocontinuous arterial spin labeling (MP-PCASL) for robust quantification of cerebral blood flow. Magnetic Resonance in Medicine, 64(3), 799–810. 10.1002/mrm.22465 [DOI] [PubMed] [Google Scholar]
- Kim JS, Singh V, Lee JK, Lerch J, Ad-Dab’bagh Y, MacDonald D, … Evans AC (2005). Automated 3-D extraction and evaluation of the inner and outer cortical surfaces using a Laplacian map and partial volume effect classification. NeuroImage, 27(1), 210–221. 10.1016/j.neuroimage.2005.03.036 [DOI] [PubMed] [Google Scholar]
- Koizumi K, Hattori Y, Ahn SJ, Buendia I, Ciacciarelli A, Uekawa K, … Iadecola C (2018). Apoε4 disrupts neurovascular regulation and undermines white matter integrity and cognitive function. Nature Communications, 9(1), 3816 10.1038/s41467-018-06301-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukolja J, Thiel CM, Eggermann T, Zerres K, & Fink GR (2010). Medial temporal lobe dysfunction during encoding and retrieval of episodic memory in non-demented APOE ε4 carriers. Neuroscience, 168(2), 487–497. 10.1016/j.neuroscience.2010.03.044 [DOI] [PubMed] [Google Scholar]
- Lind J, Larsson A, Persson J, Ingvar M, Nilsson L-G, Bäckman L, … Nyberg L (2006). Reduced hippocampal volume in non-demented carriers of the apolipoprotein E ɛ4: Relation to chronological age and recognition memory. Neuroscience Letters, 396(1), 23–27. 10.1016/j.neulet.2005.11.070 [DOI] [PubMed] [Google Scholar]
- Lineweaver TT, Bond MW, Thomas RG, & Salmon DP (1999). A normative study of Nelson’s (1976) modified version of the Wisconsin Card Sorting Test in healthy older adults. The Clinical Neuropsychologist, 13(3), 328–347. 10.1076/clin.13.3.328.1745 [DOI] [PubMed] [Google Scholar]
- Liu C-C, Kanekiyo T, Xu H, & Bu G (2013). Apolipoprotein E and Alzheimer disease: risk, mechanisms, and therapy. Nature Reviews. Neurology, 9(2), 106–118. 10.1038/nrneurol.2012.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TT, & Wong EC (2005). A signal processing model for arterial spin labeling functional MRI. NeuroImage, 24(1), 207–215. 10.1016/j.neuroimage.2004.09.047 [DOI] [PubMed] [Google Scholar]
- Luckhaus C, Flüβ MO, Wittsack H-J, Grass-Kapanke B, Jänner M, Khalili-Amiri R, … Cohnen M (2008). Detection of changed regional cerebral blood flow in mild cognitive impairment and early Alzheimer’s dementia by perfusion-weighted magnetic resonance imaging. NeuroImage, 40(2), 495–503. 10.1016/j.neuroimage.2007.11.053 [DOI] [PubMed] [Google Scholar]
- Mattis S (1988). Dementia Rating Scale: DRS: Professional Manual. Odessa, FL: PAR. [Google Scholar]
- Ostergaard L, Aamand R, Gutierrez-Jimenez E, Ho YC, Blicher JU, Madsen SM, … West MJ (2013). The capillary dysfunction hypothesis of Alzheimer’s disease. Neurobiol Aging, 34(4), 1018–1031. [DOI] [PubMed] [Google Scholar]
- Pacheco J, Goh JO, Kraut MA, Ferrucci L, & Resnick SM (2015). Greater cortical thinning in normal older adults predicts later cognitive impairment. Neurobiology of Aging, 36(2), 903–908. 10.1016/j.neurobiolaging.2014.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, … Acker JD (2005). Regional Brain Changes in Aging Healthy Adults: General Trends, Individual Differences and Modifiers. Cerebral Cortex, 15(11), 1676–1689. 10.1093/cercor/bhi044 [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, & Haacke EM (2007). Brain aging and its modifiers: insights from in vivo neuromorphometry and susceptibility weighted imaging. Annals of the New York Academy of Sciences, 1097, 84–93. 10.1196/annals.1379.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Rosas HD, & Fischl B (2010). Highly Accurate Inverse Consistent Registration: A Robust Approach. NeuroImage, 53(4), 1181–1196. 10.1016/j.neuroimage.2010.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Schmansky NJ, Rosas HD, & Fischl B (2012). Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage, 61(4), 1402–1418. 10.1016/j.neuroimage.2012.02.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiepers OJG, Harris SE, Gow AJ, Pattie A, Brett CE, Starr JM, & Deary IJ (2012). APOE E4 status predicts age-related cognitive decline in the ninth decade: longitudinal follow-up of the Lothian Birth Cohort 1921. Molecular Psychiatry, 17(3), 315–324. 10.1038/mp.2010.137 [DOI] [PubMed] [Google Scholar]
- Soldan A, Pettigrew C, Lu Y, Wang M-C, Selnes O, Albert M, … Miller MI (2015). Relationship of medial temporal lobe atrophy, APOE genotype, and cognitive reserve in preclinical Alzheimer’s disease. Human Brain Mapping, 36(7), 2826–2841. 10.1002/hbm.22810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri S, Heise V, Trachtenberg AJ, & Mackay CE (2013). The forgotten APOE allele: A review of the evidence and suggested mechanisms for the protective effect of APOE ɛ2. Neuroscience & Biobehavioral Reviews, 37(10, Part 2), 2878–2886. 10.1016/j.neubiorev.2013.10.010 [DOI] [PubMed] [Google Scholar]
- Tai LM, Thomas R, Marottoli FM, Koster KP, Kanekiyo T, Morris AW, & Bu G (2016). The Role of APOE in Cerebrovascular Dysfunction. Acta Neuropathologica, 131(5), 709–723. 10.1007/s00401-016-1547-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thambisetty M, Beason-Held L, An Y, Kraut MA, & Resnick SM (2010). APOE epsilon4 genotype and longitudinal changes in cerebral blood flow in normal aging. Archives of Neurology, 67(1), 93–98. 10.1001/archneurol.2009.913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohgi H, Takahashi S, Kato E, Homma A, Niina R, Sasaki K, … Sasaki M (1997). Reduced size of right hippocampus in 39- to 80-year-old normal subjects carrying the apolipoprotein E epsilon4 allele. Neuroscience Letters, 236(1), 21–24. [DOI] [PubMed] [Google Scholar]
- Tsuang D, Leverenz JB, Lopez OL, Hamilton RL, Bennett DA, Schneider JA, … Zabetian CP (2013). APOE ϵ4 Increases Risk for Dementia in Pure Synucleinopathies. JAMA Neurology, 70(2), 223–228. 10.1001/jamaneurol.2013.600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuminello ER, & Han SD (2011). The Apolipoprotein E Antagonistic Pleiotropy Hypothesis: Review and Recommendations [Research article]. 10.4061/2011/726197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Qiu M, & Constable RT (2005). In vivo method for correcting transmit/receive nonuniformities with phased array coils. Magnetic Resonance in Medicine, 53(3), 666–674. 10.1002/mrm.20377 [DOI] [PubMed] [Google Scholar]
- Whitehair DC, Sherzai A, Emond J, Raman R, Aisen PS, Petersen RC, … Alzheimer’s Disease Cooperative Study. (2010). Influence of apolipoprotein E varepsilon4 on rates of cognitive and functional decline in mild cognitive impairment. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 6(5), 412–419. 10.1016/j.jalz.2009.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga CE, Clark LR, Dev SI, Shin DD, Jurick SM, Rissman RA, … Bondi MW (2013). Interaction of age and APOE genotype on cerebral blood flow at rest. Journal of Alzheimer’s Disease: JAD, 34(4), 921–935. 10.3233/JAD-121897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga CE, Dev SI, Shin DD, Clark LR, Bangen KJ, Jak AJ, … Bondi MW (2012). Effect of mild cognitive impairment and APOE genotype on resting cerebral blood flow and its association with cognition. Journal of Cerebral Blood Flow & Metabolism, 32(8), 1589–1599. 10.1038/jcbfm.2012.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga CE, Hays CC, & Zlatar ZZ (2014). Cerebral blood flow measured by arterial spin labeling MRI as a preclinical marker of Alzheimer’s disease. Journal of Alzheimer’s Disease: JAD, 42 Suppl 4, S411–419. 10.3233/JAD-141467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao MY, Mezue M, Segerdahl AR, Okell TW, Tracey I, Xiao Y, & Chappell MA (2017). A systematic study of the sensitivity of partial volume correction methods for the quantification of perfusion from pseudo-continuous arterial spin labeling MRI. NeuroImage, 162, 384–397. 10.1016/j.neuroimage.2017.08.072 [DOI] [PubMed] [Google Scholar]
- Zlatar ZZ, Bischoff-Grethe A, Hays CC, Liu TT, Meloy MJ, Rissman RA, … Wierenga CE (2016). Higher Brain Perfusion May Not Support Memory Functions in Cognitively Normal Carriers of the ApoE ε4 Allele Compared to Non-Carriers. Frontiers in Aging Neuroscience, 151 10.3389/fnagi.2016.00151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV (2011). Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nature Reviews. Neuroscience, 12(12), 723–738. 10.1038/nrn3114 [DOI] [PMC free article] [PubMed] [Google Scholar]