Abstract
Interoception (the sensing of inner-body signals) is a multi-faceted construct with major relevance for basic and clinical neuroscience research. However, the neurocognitive signatures of this domain (cutting across behavioral, electrophysiological, and fMRI connectivity levels) are rarely reported in convergent or systematic fashion. Additionally, various controversies in the field might reflect the caveats of standard interoceptive accuracy (IA) indexes, mainly based on heartbeat detection (HBD) tasks. Here we profit from a novel IA index (md) to provide a convergent multidimensional and multi-feature approach to cardiac interoception. We found that outcomes from our IA-md index are associated with –and predicted by– canonical markers of interoception, including the hd-EEG-derived heart-evoked potential (HEP), fMRI functional connectivity within interoceptive hubs (insular, somatosensory, and frontal networks), and socio-emotional skills. Importantly, these associations proved more robust than those involving current IA indexes. Furthermore, this pattern of results persisted when taking into consideration confounding variables (gender, age, years of education, and executive functioning). This work has relevant theoretical and clinical implications concerning the characterization of cardiac interoception and its assessment in heterogeneous samples, such as those composed of neuropsychiatric patients.
Keywords: Interoception, heartbeat detection task, cardiac frequency, heart-evoked potential, functional connectivity, emotion
1. Introduction
Interoception (the sensing of inner body signals) is a multi-faceted construct, encompassing diverse markers at neurophysiological, neuroanatomical, hemodynamic, cognitive, and behavioral levels (1). Accruing investigation on this domain has influenced accounts of varied psychobiological phenomena, such as socio-emotional processes (2–8), memory (9, 10), and decision making (11–13). Furthermore, interoception has become a hotspot for research on neuropsychiatric disorders due to its therapeutic potential (14–22). Notwithstanding, evidence on its neurocognitive signatures proves controversial. For instance, reported associations between interoception and social cognition domains, such as empathy (23) or theory of mind (24), are not always replicated (25). The same is true for interoceptive alterations in pathological conditions, including anxiety (26, 27) and depersonalization-derealization disorder (28, 29). These inconsistences might reflect the limitations of unidimensional approaches and the methodological pitfalls of mainstream procedures, which mainly rely on heartbeat detection (HBD) tasks to provide interoceptive accuracy (IA) scores (30–33). Therefore, a need arises for new, robust frameworks in the field. Against this background, we introduce a multidimensional and multi-feature approach, supported by a promising interoceptive index based on a motor-tracking HBD task (34), to provide a convergent characterization of cardiac interoception cutting across behavioral, electrophysiological, and hemodynamic levels.
Mainstream interoceptive tasks require subjects to track their cardiac bumps through silent counting (e.g., 35) or motor tapping (e.g., 36, 37). In this approach, IA is typically calculated as the difference between perceived and actual heartbeats (i.e., Schandry’s index). Despite its simplicity, this index has been severely criticized (33, 38–40) mainly because responses may be guided by an estimation of the average heart rate rather than the actual tracking of relevant signals (41–43). Furthermore, this index is biased by the total number of responses, such that a higher number of tracked heartbeats leads to a higher IA even if body signals are not actually perceived. Indeed, people with high IA do not show a corresponding high correlation between responses and actual heartbeats, which suggests that they over-report heartbeat perception (38).
Motor-tracking HBD tasks can yield a more robust IA index based on Signal Detection Theory (SDT) (44–46) –i.e., d’ index. This framework allows estimating the subject’s sensitivity and specificity in discriminating signal (heartbeats) from noise, penalizing correct responses made by chance. Nevertheless, this method also faces major limitations. In particular, it requires a definition of a window time-locked to the heartbeat to consider a response as correct (‘hit’) or incorrect (‘false alarm’), but heartbeat perception hardly occurs in the same timespan for all individuals (33).
More importantly, the approaches above share an additional and critical shortcoming: they are blind to the effect of heart rate changes on behavioral responses during the task. Indeed, heart rate modulates heartbeat counting (38) and detection (47). As explained above, Schandry’s index is based on a single number comparing the subject’s total perceived and actual heartbeats. For its part, the d’ index weighs correct and incorrect motor responses to heartbeats depending on their occurrence in a fixed time-window that remains constant throughout the task. Thus, they both fail to account for on-the-fly behavioral adjustments to heart rate fluctuations, potentially produced by changes in respiration (48), temperature (49), or arousal or stress levels (50). Those indexes, then, are suboptimal to determine whether subjects are following their hearts’ rhythm or other sensations (51).
Furthermore, heartbeat perception may also be affected by potential confounding variables, such as demographic (i.e., gender, age, years of education) or domain-general cognitive factors (e.g., executive functioning), which typically modulate results in any task. In fact, some studies have reported higher IA in men than women (52, 53), but others have found no evidence for gender-based differences (54, 55). Additionally, although aging seems to have a detrimental effect on IA (55), the lack of longitudinal data precludes excluding sample- or task-specific confounds (56). In any case, most available research has not accounted for these potentially relevant factors.
In this context, we recently developed a new IA index, called ‘mean distance’ (md) (34), that captures the oscillatory coupling between subjects’ responses and cardiac frequency during motor-tracking HBD tasks (15, 57, 58). This metric presents important advantages. First, md is mostly uncontaminated by the subjects’ beliefs about their average heart rate since it compares motor responses and heartbeat frequencies in multiple overlapping time-windows rather than a single time-span. Second, md is unaffected by the total number of responses because subjects who tap repeatedly do not obtain higher IA unless their response frequency is close to their cardiac frequency. Third, md does not rely on arbitrary time windows to consider a response as correct or incorrect, as it assesses heartbeat frequency rather than individual heartbeats. Finally, unlike all previous IA procedures, md captures dynamic behavioral adjustments driven by cardiac frequency changes.
Using this new index, we developed a multidimensional and multi-feature approach to robustly characterize cardiac interoception (Figure 1.A and B). We assessed a large sample of 114 healthy subjects with a validated HBD task (15, 57, 58), and tested the association of our md index with canonical neurocognitive markers of interoception, including the heart-evoked potential (HEP) –here derived from high-density electroencephalography (hd-EEG) (15, 36, 51, 52, 54, 57, 59–62)– and functional connectivity signatures from resting-state functional magnetic resonance imaging (fMRI) (15, 57). Also, given the intimate links between interoception and socio-emotional skills (2–8), we tested the association of our md index and emotion recognition tasks. Then, for comparison, we repeated all analyses with the two mainstream indexes described above: a modified version of Schandry’s index (mSI) (35) (Supplementary Material 1.1), and a d’ score based on SDT (44–46) (Supplementary Material 1.2). Finally, to explore whether the combination of ongoing brain measures (HEP), resting-state interoceptive brain network correlates, and behavioral data (emotion recognition scores) predicts each IA index, we applied a data-driven multivariate computational analysis. Thereupon, we explored whether ensuing predictions were affected when adding potential confounding variables (i.e., gender, age, years of education, and executive functioning) (Figure 1.C), which is critical to evaluate interoception in heterogeneous populations. Based on previous findings, we expected to find significant associations between IA-md and canonical neurocognitive markers of interoception (i.e., HEP, fMRI networks, emotion recognition). Furthermore, we hypothesized that these associations would be stronger for md than standard IA indexes (mSI and d’). Finally, we expected to find null associations between interoceptive markers and exteroceptive accuracy (EA) –the control condition of the motor-tracking HBD task–, which would support the construct validity of IA.
Figure 1. Experimental procedure and data analysis.
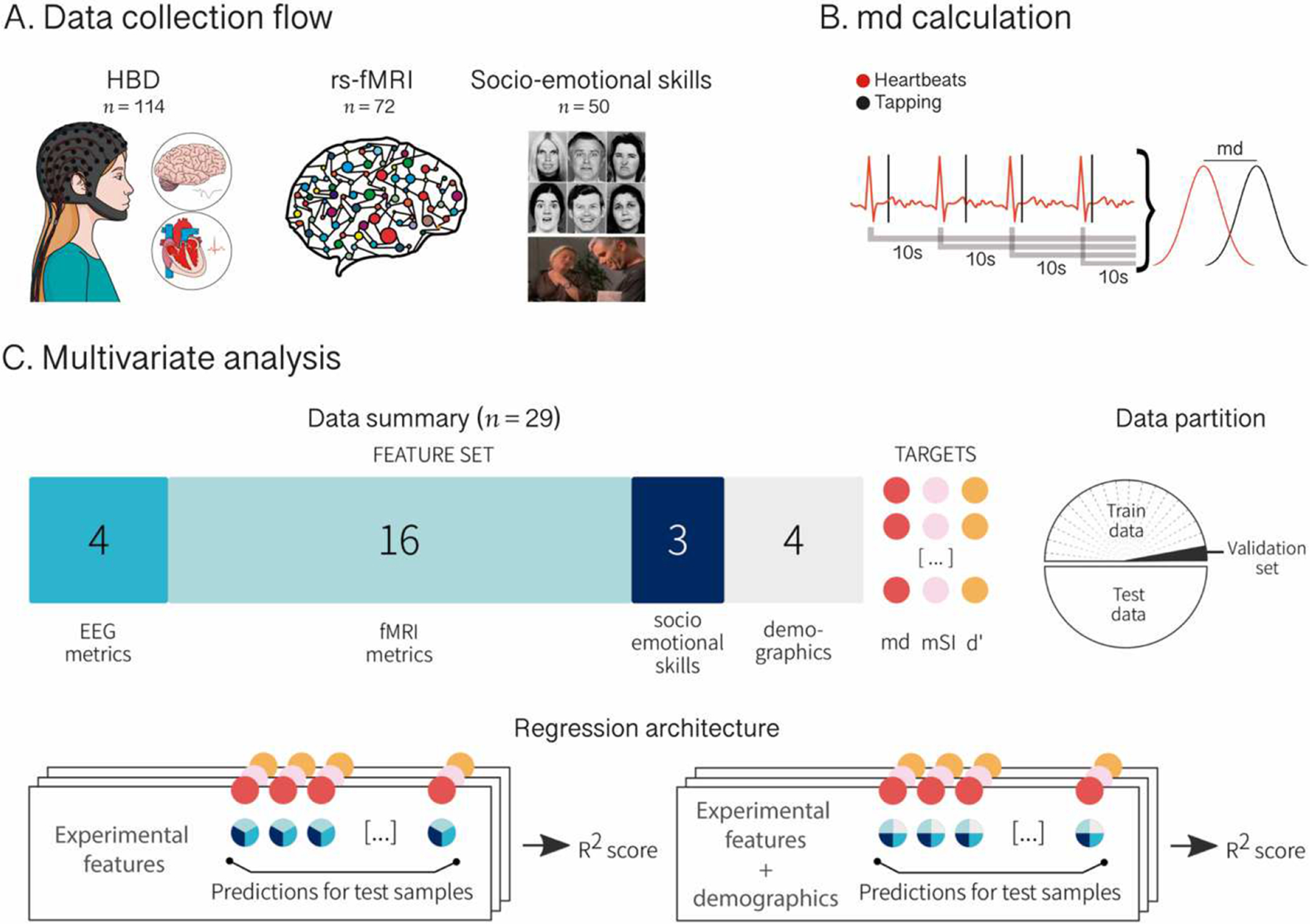
A. Data collection flow. Participants performed a heartbeat detection (HBD) task in which they were instructed to tap a key following their own heartbeats while electrocardiographic (ECG) and high-density electroencephalographic (hd-EEG) signals were recorded. This was done twice (two 2-min blocks). Then, a subsample of participants underwent a resting-state functional magnetic resonance imaging (fMRI) session and a socio-emotional skills assessment involving emotion recognition tasks (Ekman-35 and TASIT-EET). B. md calculation. During the HBD task, tapping responses and ECG signals were recorded and logged as marks in time. To calculate IA-md, blocks were subdivided in overlapping 10-second windows starting at each individual heartbeat. The absolute difference between cardiac frequency and response frequency (md) was computed for each individual window and averaged over all windows comprising both blocks. C. Multivariate analysis. Four heart-evoked potential (HEP) modulation metrics from EEG recordings, 16 functional connectivity metrics from fMRI registers, and three emotion recognition scores from the socio-emotional skills assessment were introduced as selected features in a linear regression model to test their power in predicting IA-md score as well as two other indexes for comparison: a modified version of Schandry’s index (mSI) and a d’ score. The regression was then repeated including four demographic and executive functioning features (‘demographics’). For both analyses, data were split in 50–50 train and test partition and optimized over a validation set bootstrapped from train partition. We assessed the coefficient of determination (R2) between the target and the predicted value for data in test partition.
2. Materials and methods1
2.1. Participants
The study comprised 114 volunteers (59 female; 5.5 % left-handed) between 17 and 84 years old (M = 40.81, SD = 20.54). They had a mean of 14.64 years of education (SD = 3.95) and declared no history of psychiatric or neurological conditions, substance abuse disorder or heart diseases. Furthermore, they underwent a standard clinical examination comprising neurological, neuropsychiatric, and neuropsychological assessments by expert professionals –Supplementary Material 2.1. The INECO Frontal Screening (IFS) battery (63), a brief tool to evaluate executive functioning, revealed preserved scores across the sample (N = 108, M = 25.05, SD = 2.82). The IFS assesses three executive functions: response inhibition and set shifting, abstraction capacity, and working memory. Total IFS scores range from 0 to 30 (with higher scores representing better executive functioning) (63) –more details about this test are provided in Supplementary Material 2.2. The discrepancy between the entire sample size (N = 114) and the subsample with IFS scores (N = 108) reflects missing data. All participants signed an informed consent in accordance with the Declaration of Helsinki. The study was approved by the Ethics Committee of the host institution.
2.2. Interoceptive performance: Heartbeat detection task
We assessed cardiac interoception through a validated HBD task (14, 15, 26, 29, 34, 46, 51, 57–59, 64) –available online at http://bit.ly/2EpfGrq. The task comprises two conditions (15, 57, 58). The exteroceptive condition provides a control measure assessing the subjects’ capacity to attend to external stimuli –i.e., EA. Participants were binaurally presented with an audio of a recorded heartbeat (digitally constructed from an actual electrocardiogram record of a researcher), which they had to follow by pressing a key with their dominant hand. They were given the following instructions: “In this part of the test, you will hear the beating of a heart recorded from another person. You must follow every heartbeat by tapping the “z” key on the laptop keyboard. Do not try to anticipate your responses by guessing the recorded heart rhythm; instead, tap as fast as you can after each beat you hear”. This condition comprised two blocks lasting 2 minutes each. In the first block, recorded heartbeats were presented at a constant and regular frequency (60 bpm), while in the second block, recorded heartbeats were manipulated to have the same overall frequency (60 bpm) but at irregular intervals. Both blocks of the exteroceptive condition were always presented in the same order, before moving on to the interoceptive condition.
The interoceptive condition provides an objective measure of the subjects’ ability to track their own heartbeats (i.e., IA) (30). Participants were asked to tap a key with their dominant hand following their own heartbeats. They were instructed not to use any external cues, as stated in the instructions: “Now, you must follow the beating of your own heart by tapping the “z” key for every beat you feel. You should not guide your responses by checking your arterial pulse in your wrists or neck. If you are unable to feel these sensations, you should appeal to your intuition trying to respond whenever you think your heart is beating”. The interoceptive condition also included two blocks of 2 minutes each, with identical instructions.
While subjects performed the HBD task, we recorded the electrocardiographic signals to register the heartbeats alongside motor responses over time. We also obtained hd-EEG recordings to analyze HEP modulations during the task, as detailed in Section 2.3.2.
To estimate the subjects’ accuracy across each condition, we calculated the md index (34), which is based on the comparison between the frequencies of heartbeats and motor responses (Figure 1.B). First, for each condition, we subdivided each block in overlapping windows starting at each individual heartbeat and extending for 10 seconds. Then, for each window, we computed the absolute difference (md) between cardiac frequency (measured as 1/mean R-R) and response frequency (1/mean inter response intervals). This process is represented in the following equation:
where fc is the average cardiac frequency in a window of w duration centered at time i, fr is the average response frequency in the same window and time, and N is the number of heartbeats in the block.
In addition, to control for possible periods during which subjects may have lost concentration, a coefficient of variation (CV) was estimated to assess the regularity of the motor responses inside each individual 10-second window (34). To compute the CV, we calculated the ratio of the standard deviation to the mean of the participant’s time-intervals between motor responses. The CV estimate was used for thresholding. Windows with CV > 0.5 were not used in the estimation of md because they would fall above the expected values to reflect delivered signal detection (34, 65).
Finally, the absolute difference between cardiac and response frequencies was averaged across all windows comprising each block of each condition. More specifically, the averaged md of the windows that make up blocks one and two resulted in the EA index, while the averaged md of the windows that make up blocks three and four resulted in the IA index. Since md is a distance index, its minimum score is 0, indicating a perfect match between motor responses and cardiac frequencies, with higher scores indicating higher distances, and thus, worse performance.
We also followed canonical procedures to compute other IA indexes for comparison: a modified version of Schandry’s index (mSI) (35), and a d’ score calculated by means of the SDT (44–46). These are described in Supplementary Material 1.1 and 1.2, respectively.
2.3. EEG data
2.3.1. Signal acquisition and preprocessing
For all participants (N = 114), we recorded hd-EEG signals during the HBD task using a Biosemi Active-two 128-channel system at 1024 Hz. To acquire electrocardiographic data, two external Ag/Ag-Cl adhesive electrodes placed in lead-II were included as references. Data were band-pass filtered during recording (0.1–100 Hz) and offline (0.5–30 Hz) in order to remove undesired frequency components. The signal was re-referenced offline to averaged mastoids. Ocular movement artifacts were removed through independent components analysis and visual inspection, as done in previous works (14, 15, 59).
2.3.2. HEP analysis
The HEP is a negative deflection that emerges from 200 to 500 ms post R-wave in frontal-central topographies (15, 36, 51, 52, 54, 57, 59–62). Since the HEP constitutes a canonical marker of interoceptive attention to heartbeats (52, 59), its analysis was circumscribed to the interoceptive condition, as done in other works (14, 62).
To analyze the HEP, we implemented a PeakFinder function on Matlab (66) to detect the R-wave-electrocardiographic values, allowing to segment continuous EEG data (14, 15, 34, 51, 57–59, 67). Epochs were segmented from 300 ms prior to the onset of the R-wave onset to 500 ms after, and baseline-corrected relative to a −300 to −200 ms time window. Noisy epochs were rejected using an automated procedure, which excludes data points as artifacts if the probability of the epoch exceeds a threshold of 2.5 SDs from the mean probability distribution calculated from all trials or by measuring the kurtosis of probability distribution (34, 68) and visual inspection.
Following previous research (57), HEP modulations were calculated in an extended frontal region of interest (ROI) comprising 30 electrodes (see Figure 2.A), and analyses were repeated in three subdivisions of that ROI: a left-frontal ROI (Biosemi C26, C27, C28, C31, C32, D3, D4, D5, D6, D7), a central-frontal ROI (Biosemi C11, C12, C18, C19, C20, C21, C22, C23, C24, C25), and a right-frontal ROI (Biosemi C26, C27, C28, C31, C32, D3, D4, D5, D6, D7). We calculated the average HEP amplitude per subject in the mentioned ROIs circumscribed to two temporal windows: 200–300 ms and 300–400 ms after the R-wave, as peak HEP amplitudes have been reported in those latencies (54, 59–61). Time-segments post 200 ms after the R-wave are the less vulnerable to the potential influence of the cardiac field artifact (69–71).
Figure 2. Results.
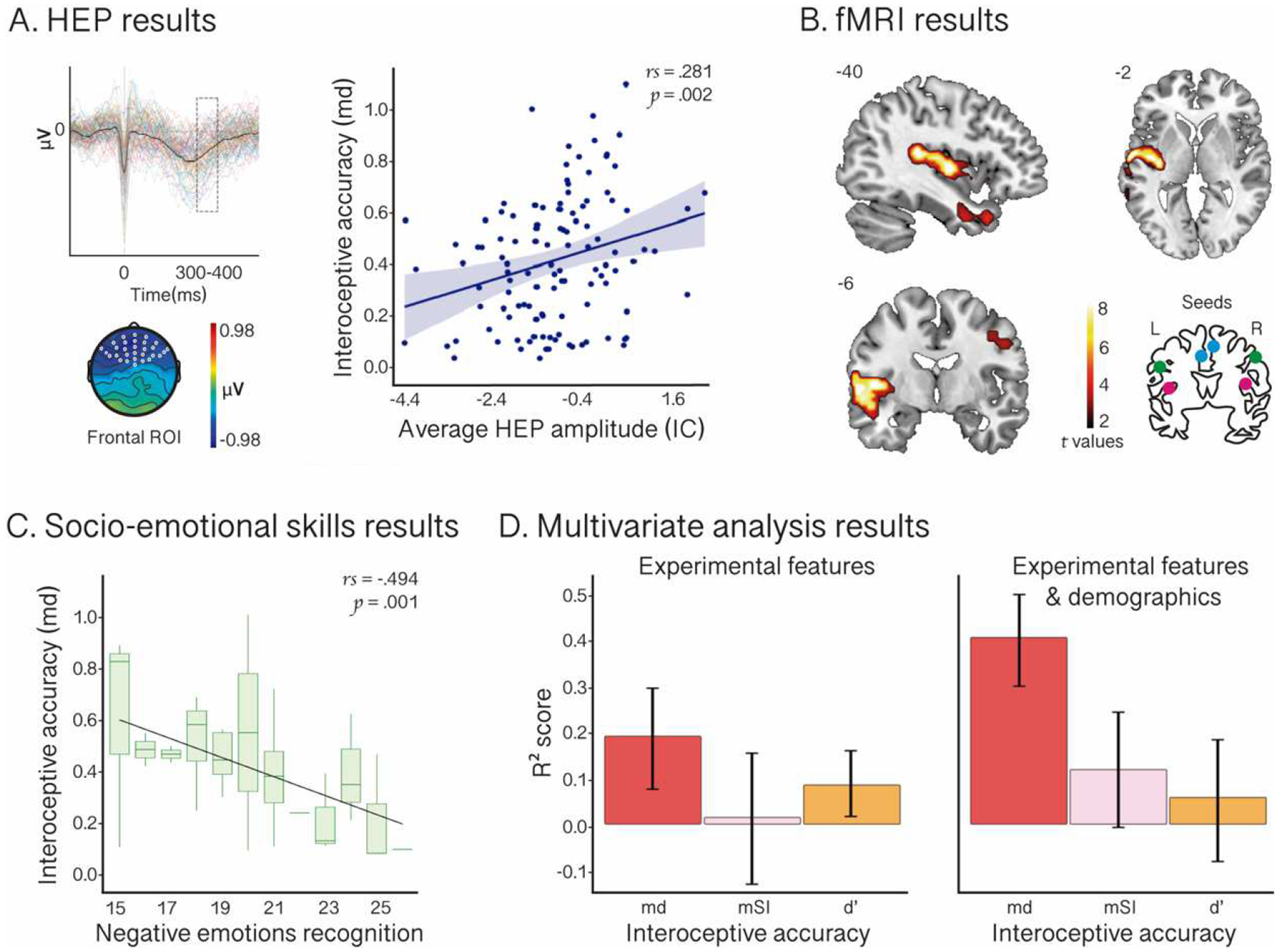
A. HEP results. The HEP diagram illustrates the modulation of this component for each subject. Outliers were excluded for visualization purposes. The scalp topography shows the sample’s average amplitude (microvolts) in the epoch (−300 to 500 ms). The graph on the right displays the correlation between IA-md and the average HEP amplitude during the interoceptive condition of the HBD in a window time-locked to 300–400 ms after the R-wave (shadowed box in the HEP diagram) in an extended frontal-central region of interest (ROI) –white dots in the scalp topography. B. fMRI results. Functional connectivity between insular, frontal, superior-temporal, postcentral, precentral, and inferior parietal cortical regions and interoceptive seeds significantly associated with IA-md. Results for all seeds are plotted together. The brain diagram on the bottom right illustrates the seeds: left and right insula (pink), left and right anterior cingulate cortex (blue), and left and right postcentral cortex (green). L: left; R: right. C. Socio-emotional skills results. Correlation between IA-md and negative emotion recognition, as measured through the sum of the performance in the Ekman-35 and the TASIT-EET global scores. Boxplots indicate the median and range of subjects’ IA-md performance. D. Multivariate analysis results. Combined HEP, fMRI, and socio-emotional skills metrics (i.e., experimental features) yielded a greater coefficient of determination for IA-md than for IA-mSI and IA-d’ (left panel), and these results persisted when adding demographic features, even improving for IA-md (right panel). Regressor performance is shown on test data.
To explore the association of IA indexes (md, mSI and d’) and HEP modulations in selected ROIs, we performed non-parametric correlation tests (Spearman’s rho). Results were considered significant using a statistical threshold of p < 0.05. In order to show the specificity of the IA construct, analyses were repeated to test the expected null association between EA indexes (md, mSI and d’) and HEP modulation.
2.4. fMRI data
As in previous works (15, 57), we explored the association between the IA indexes (md, mSI and d’) and the patterns of fMRI co-activation of key interoceptive regions, namely the insula, the postcentral cortex, and the anterior cingulate cortex (ACC), which are proposed to subserve interoceptive processing (5, 7, 72). We also tested the expected null associations among functional connectivity and EA indexes (md, mSI and d’).
2.4.1. Image acquisition and preprocessing
The fMRI acquisition protocol and the description of preprocessing steps are reported in accordance with the practical guide from the Organization for Human Brain Mapping (73, 74). We obtained 10-min resting-state fMRI recordings from a subsample of 72 participants (see Supplementary Table 1 for demographics and executive functioning information about this subsample, and Supplementary Table 2 for overlap between subsamples). Images were acquired in a 1.5 T Phillips Intera scanner with a standard head coil (8 channels). We acquired functional spin echo volumes in a sequentially ascending order, parallel to the anterior-posterior commissures, covering the whole brain. The following parameters were used: TR = 2777 ms; TE = 50 ms; flip angle = 908; 33 slices, matrix dimension = 64 × 64; voxel size in plane = 3.6 mm × 3.6 mm; slice thickness = 4 mm; number of volumes = 209. Participants were instructed to lying still, keep their eyes closed, avoid falling asleep, and not to think about anything in particular.
Before preprocessing, we discarded the first five volumes of each subject’s resting-state recording to ensure that magnetization achieved a steady state. Images were then preprocessed using the Data Processing Assistant for Resting-State fMRI (DPARSF V2.3) (75), an open-access toolbox that generates automatic pipeline for fMRI analysis. DPARFS works by calling the Statistical Parametric Mapping (SPM 12) and the Resting-State fMRI Data Analysis Toolkit (REST V.1.7). As in previous studies (15, 57), preprocessing steps included slice-timing correction (using middle slice of each volume as the reference scan) and realignment to the first scan of the session to correct head movement (SPM functions). We regressed out six motion parameters, CFS, and WM signals to reduce the effect of motion and physiological artifacts such as cardiac and respiration effects (REST V1.7 toolbox). Motion parameters were estimated during realignment, and CFS and WM masks were derived from the tissue segmentation of each subject’s T1 scan in native space with SPM12 (after co-registration of each subject’s structural image with the functional image). Then, images were normalized to the MNI space using the echo-planar imaging (EPI) template from SPM (76), smoothed using a 8-mm full-width-at-half-maximum isotropic Gaussian kernel (SPM functions), and bandpass filtered between 0.01–0.08 Hz. None of the participants showed movements greater than 3 mm (M = 0.1, SD = 0.06) and/or rotations higher than 3° (M = 0.08, SD = 0.07).
2.4.2. Seed analysis
To explore the association between IA indexes (md, mSI and d’) and the functional connectivity of interoceptive hubs, we selected a-priori six spherical 5-mm seeds based on MNI space: left insula (x = −40, y = 10, z = 0) (72), right insula (x = 42, y = 8, z = 2) (72), left ACC (x = −2, y = 6, z = 32) (5), right ACC (x = 6, y = −2, z = 48) (7), left postcentral cortex (x = −58, y = −14, z = 24) (5), and right postcentral cortex (x = 56, y = −24, z = 36) (5) –see Figure 2.B. For each participant, we extracted the temporal course of the BOLD signal of the voxels comprising each seed region and correlated these data with the temporal course of the BOLD signal of every voxel of the rest of the brain (Pearson’s correlation coefficient; DPARSF toolbox). Then, we performed a Fisher z-transformation. The resulting connectivity maps for each seed were used to perform multiple regression analyses in SPM 12, including IA score as the regressor of interest and age as a nuisance covariate. To further account for aging effects in fMRI results (e.g., 77), the main analysis (i.e., the association between IA-md and the functional connectivity of the seeds) was also performed in the subsample of subjects < 55 years old (N = 46), with a mean age of 29.26 (SD = 13.43, range = 17–54).
To consider results as statistically significant, the alpha level was set at p < 0.001, uncorrected (78–81), with an extent threshold of 30 voxels (78, 81). These parameters, reported in previous works (78, 81), aim to prevent spurious findings, such as those that could be obtained with thresholds of 10 voxels (74).
In order to show the specificity of the IA construct, analyses were repeated to test the expected null associations between EA indexes (md, mSI and d’) and the functional connectivity within interoceptive hubs.
2.5. Socio-emotional tasks
2.5.1. Facial emotion recognition task (Ekman-35)
A subsample of 50 participants completed this task (Supplementary Tables 1 and 2), which consists in identifying basic facial emotional expressions in static pictures from the Ekman series (82). Stimuli were displayed on a computer screen, and participants were given the following instructions: “I will present you with various faces, one by one, expressing one of the following emotions: happiness, surprise, sadness, fear, disgust, or anger. You have to tell me which emotion is expressed by each face. You may respond “neutral” when no emotion can be identified. This is not a speed test, but try not to dwell on your answer for too long”. The seven possible response options were written at the bottom of the screen in each trial. Stimuli remained static until the participant gave a verbal response, which the examiner had to write down. Answers given at latencies longer than 12 seconds were omitted from the analyses. In total, 35 different face stimuli were presented, five corresponding to each of the six basic emotion categories (sadness, fear, anger, disgust, surprise, happiness), and an additional five corresponding to neutral expressions. One point was given for each correct response.
To perform correlational analyses with IA indexes (md, mSI and d’), we computed three global scores: a negative emotion recognition score (corresponding to the sum of sadness, fear, anger, and disgust scores), a positive emotion recognition score (the sum of surprise and happiness), and a total score (the sum of all correct responses). The association between IA indexes and the described global scores were performed using non-parametric correlation tests (Spearman rho), considering an alpha threshold of p < 0.05. Correlations between EA indexes (md, mSI and d’) and the global scores were also performed to test the specificity of these markers.
2.5.2. The Awareness of Social Inference Test (TASIT) – Emotion Evaluation Test (EET)
Forty-seven participants performed this task (Supplementary Tables 1 and 2), which assesses the ability to infer basic emotions in videotaped vignettes representing actors interacting in naturalistic situations (83). Given that the verbal scripts are neutral in content, the emotions must be inferred from a combination of various clues, including prosody, facial expressions, body language, and the social situation surrounding the emotional expression. This particularity makes the TASIT-EET a more ecological task than picture-based ones (such as Ekman’s), since it resembles more precisely the types of interactions people encounter in real life situations. Some scenes depict only one actor talking (on the telephone or directly to the camera), while others show two actors and instructions are given to focus on one of them. Before visualizing each tape, the following instructions were given: “I will show you some short scenes. Please observe each one carefully. After each scene, I will write down the emotion that you tell me that best describes the feeling of the person in the scene. You have to select 1 of 5 emotions from the list that will appear on the screen after each scene. The first will be a practice trial”. Thus, the participant was asked to verbally identify the emotion displayed by the target actor within five options that appear written in the computer screen at the offset of the video: sadness, fear, anger, disgust, surprise, obtaining one point for each correct response. In total, ten short (15–60 seconds) videos were presented, two per each emotion category.
For correlational analysis, we computed a negative emotion recognition score (corresponding to the sum of sadness, fear, anger, and disgust scores) and a total score (the sum of all correct responses). We tested the association between IA indexes (md, mSI and d’) and the global scores through non-parametric correlation tests (Spearman rho), considering an alpha level of p < 0.05. Correlations between EA indexes (md, mSI and d’) and the global scores were also performed to test the specificity of these markers.
2.6. Multivariate analysis
After univariate analysis, we explored how robustly the different IA indexes (md, mSI, d’) were predicted by the combination of measures tapping ongoing brain markers (hd-EEG-HEP), resting-state functional connectivity, and socio-emotional skills. To this end, we used a data-driven multidimensional and multi-feature computational analysis using the subsample that included the cases that completed all sessions of the experimental design (i.e., EEG, fMRI, and socio-emotional skills assessments) (n = 29) (Figure 1.C). For each target variable (IA-md, IA-mSI, IA-d’), we performed a linear regression with an L2 regularization (84) using as input all experimental features that yielded significant associations with any IA index in the previous analyses (i.e., HEP modulation in the extended frontal ROI and its subdivisions, the average functional connectivity of each seed associated with each IA index, and Ekman-35 and TASIT-EET scores) –Section 3.5.1 for details. We used the statistical criteria as filter method of feature selection because this is a standard practice in machine learning studies (46, 85–87).
Then, to explore how confounding variables influenced the predictions, we implemented another linear regression with an L2 regularization (84) for each target (IA-md, IA-mSI, IA-d’), adding demographic (gender, age, and years of education) and executive functioning (total IFS score) measures to the previously mentioned features (Section 3.5.1).
For both analyses, we split the data in 50–50 train and test partition. Regardless of the regularization parameter, the process was optimized over a validation set (20%) bootstrapped from train partition. We assessed the coefficient of determination (R2) between the target and the predicted value for data in test partition. To get a more realistic estimation, we performed the regression 30 times and informed the mean and standard deviation.
Although our sample size is small (N = 29), as recommended (88, 89), we explicitly avoided using the leave-one-out cross-validation (LOOCV) method, since the coefficient of determination (R2) –the models’ performance score– needs a large set of test samples to be computed. While it would be possible to accumulate the dependent variable’s prediction over the LOOCV procedure and then compute the R2, this would not allow us to assess the variance of the score (the standard deviation) due to changes in the training data. Thus, to know how precise the model’s performance score is when we change the data used to train it, we opted for a random sampling procedure, training with one partition and testing in other, various times, always sampling from different random partitions (46, 90).
3. Results
3.1. Heartbeat detection task results and associations with sample demographics and executive functioning profiles
The md index was estimated including only ‘good windows’ (those that met the requirement of CV < 0.5 in the regularity of motor responses) –see Section 2.2 for details about this procedure. Analyses revealed that the mean percentage of good windows was 96% (SD = 0.06) for the interoceptive condition, and 97% (SD = 0.07) for the exteroceptive condition, with no significant difference between them (t = −1.666; p = 0.097). This result indicates that subjects maintained a comparable level of concentration in both conditions of the HBD task.
Regarding performance, as expected for interoceptive measures, IA-md scores (M = 0.43; SD = 0.25) were higher (and thus, worse) than EA-md scores (M = 0.06; SD = 0.09) across the sample (t = 15.196; p = 0.000). This result was also found for the comparison indexes (mSI and d’) –see Supplementary Table 3 for details. In addition, subjects’ IA-md scores were more variable (IQR = 1.61) than EA-md scores (IQR = 0.50). This variability pattern was also captured by mSI, but not d’ (Supplementary Table 3).
Regarding demographic information, there were no gender differences in either IA-md (t = 1.075; p = 0.285) or EA-md (t = −0.242; p = 0.810). Null results were also found for mSI and d’ (Supplementary Table 4). Lastly, the IA-md index was not associated with age (rs = −0.036; p = 0.702), years of education (rs = −0.135; p = 0.153) or executive functions as tapped by the IFS (rs = −0.040; p = 0.683), indicating that interoceptive performance could not be explained by these confounding factors when taken separately. Similar results were obtained for IA-mSI and IA-d’ (Supplementary Table 5). On the other hand, the number of years of education and the total IFS score were significantly correlated with EA-md (rs = −0.288; p = 0.003 and rs = −0.255; p = 0.013, respectively), possibly reflecting the demands of attending to external stimuli. These results were replicated for EA-d’, but not for the EA-mSI index (Supplementary Table 5).
3.2. HEP results
As expected, we found a significant positive correlation between IA-md scores and HEP amplitude in a window of 300–400 ms after the R-wave peak in the defined extended ROI comprising 30 fronto-central electrodes (rs = 0.281; p = 0.002) (Figure 2.A). Since the md index is an error score, this result indicates that lower (thus, better) IA-dm scores are associated with more negative HEP modulations. Similar results were obtained when tested in the subdivisions of that ROI (Supplementary Table 6). However, IA-md was not associated with HEP amplitude in the earlier 200–300-time window (rs = 0.148; p = 0.117). In addition, no significant association was found between EA-md and HEP modulation. Finally, IA and EA scores derived from mSI and d’ did not correlate with HEP modulation in any window or ROI (Supplementary Table 6; Supplementary Figures 1.A and 2.A).
3.3. Functional connectivity results
Seed analysis revealed significant associations between IA-md and the functional connectivity of key interoceptive hubs, mainly in the left hemisphere (Figure 2.B). More specifically, md was negatively associated with the strength of the correlation between the temporal course of the BOLD signal of the selected seeds (bilateral insula, ACC, and postcentral cortex) and the temporal course of the BOLD signal in insular, frontal, temporal, postcentral, precentral, and inferior parietal cortical regions (Supplementary Table 7). Repeating this analysis in the subsample of subjects < 55 years old yielded a consistent though more widespread pattern of results (Supplementary Table 8 and Supplementary Figure 3). Results were also replicated for IA-mSI (Supplementary Table 9 and Supplementary Figure 1.B), although the strength of association was significantly lower than that for IA-md (t = −9.14; p = 0.000) – Supplementary Figure 4. For its part, the IA-d’ index correlated with the functional connectivity between the seeds and ACC, precentral, postcentral, frontal and temporal regions (Supplementary Table 10 and Supplementary Figure 2.B). In contrast, no significant associations were found for EA measured as md and mSI (Supplementary Figures 5 and 6). Lastly, while the functional connectivity of some seeds appeared significantly correlated with EA-d’, these do not belong to interoceptive networks, but comprise occipital, precuneus, and cerebellar regions (Supplementary Table 11 and Supplementary Figure 7). All fMRI results were considered significant with a statistical threshold of p < 0.001, uncorrected, extent threshold = 30 voxels (78, 81).
3.4. Socio-emotional skills results
The subjects’ performance in emotion recognition tasks is displayed in Supplementary Table 12. We found significant associations between IA-md scores and measures of negative emotion recognition. More specifically, better performance (lower IA-md scores) correlated with higher scores in the recognition of negative emotions in the two tasks administered: Ekman-35 (rs = −0.323; p = 0.022) and TASIT-EET (rs = −0.328; p = 0.034). For visualization purposes, Figure 2.C displays the correlation between IA-md and a composite negative emotion recognition score, comprised by the sum of the subjects’ performance in both tasks. In addition, we found a significant negative correlation between IA-md and TASIT-EET total score (rs = −0.403; p = 0.005), and a trend toward significance in the association between IA-md and Ekman-35 total score (rs = −0.263; p = 0.065). In contrast, IA-md was not correlated with positive emotion recognition –as measured with Ekman-35 (rs = 0.088; p = 0.543). Results concerning TASIT (negative emotion recognition and total scores) were replicated for IA-d’, but not for mSI. Additionally, IA-mSI and IA-d’ were not associated with positive emotion recognition. Furthermore, no significant associations were found between EA –as measured by md, mSI and d’– and emotion recognition measures (All these results are provided in Supplementary Table 13).
3.5. Multivariate analysis results
3.5.1. Feature selection
For our first multivariate regression architecture (Section 2.6 and Figure 1.C, bottom left diagram), we included as predictor features the experimental variables that yielded significant associations with any IA index in the previous analyses. In total, we included:
Four EEG metrics: HEP amplitude values in the 300–400 ms-window after the R-wave peak in the extended ROI comprising 30 fronto-central electrodes, and in the left-frontal, central-frontal, and right-frontal subdivisions of that ROI (since all these variables were significantly associated with IA-md);
Sixteen fMRI metrics: the average functional connectivity of each seed that showed a significant association with each IA index (i.e., 6 features corresponding to the functional connectivity of the 6 seeds that showed significant associations with IA-md –Supplementary Table 7, 5 features corresponding to the functional connectivity of the 5 seeds that showed significant associations with IA-mSI –Supplementary Table 9, and 5 features corresponding to the functional connectivity of the 5 seeds that showed significant associations with IA-d’ –Supplementary Table 10); and
Three socio-emotional skills metrics: Ekman-35 negative emotion recognition score (since this variable was significantly correlated with IA-md), and TASIT-EET negative emotion recognition and total scores (since these last two variables were significantly correlated with IA-md and IA-d’) –Supplementary Table 13.
For our second multivariate regression architecture (Section 2.6 and Figure 1.C, bottom right diagram), we added to the previously mentioned features three demographic variables (gender, age, and years of education) and one executive functioning variable (total IFS score) – collectively called ‘demographics’.
3.5.2. Multiple linear regressions results
The combined experimental features (HEP, fMRI, and socio-emotional skills metrics) resulted in a higher coefficient of determination for IA-md than for the comparison indexes, IA-mSI and IA-d’ (Table 1 and Figure 2.D, left panel). When adding demographics to the experimental features, the coefficient of determination for IA-md improved, and it remained higher than for IA-mSI –which also improved– and IA-d’ (Table 1 and Figure 2.D, right panel).
Table 1.
Multiple linear regressions results
| Predicted IA index | ||||
|---|---|---|---|---|
| md | mSI | d’ | ||
| Features included in the model | Experimental variables (HEP, fMRI, and socio-emotional skills metrics) |
R2 = 0.196 SD = 0.306 |
R2 = 0.018 SD = 0.389 |
R2 = 0.090 SD = 0.201 |
| Experimental variables + demographics (gender, age, years of education, and executive functioning) |
R2 = 0.410 SD = 0.286 |
R2 = 0.125 SD = 0.359 |
R2 = 0.063 SD = 0.388 |
|
fMRI: functional magnetic resonance imaging; HEP: heart-evoked potential; IA: interoceptive accuracy.
4. Discussion
This work provides, for the first time, a systematic multidimensional approach to cardiac interoception in combination with a dynamic and sensitive IA index (i.e., md) during a validated motor-tracking HBD task (34). We showed that this metric is associated with canonical neurocognitive markers of interoception, including the HEP, functional connectivity within interoceptive hubs, and socio-emotional skills. Furthermore, using a multivariate regression model, we showed that IA-md can be predicted by those markers better than by mainstream IA indexes (mSI and d’). Lastly, while IA-md was not directly associated with the sample’s demographic variables (age, gender, and years of education) and overall executive functioning, adding these features to the multivariate regression model increased predictive precision, suggesting that IA-md is more sensitive to non-interoceptive variables that may partially account for subjects’ performance in the HBD task. Therefore, our approach represents a robust framework for the field, since the IA-md index overcomes several methodological limitations of mainstream alternatives, including Schandry’s index and the d’ index.
First, we assessed whether our md index yielded predictable behavioral results by discriminating between interoceptive and exteroceptive abilities. We found poorer performance in the former condition when measured with md, but also with mSI and d’. Note, in this sense, that the interoceptive condition of the HBD task (where participants are asked to follow their own heartbeats without taking their pulse) involves high uncertainty, usually resulting in floor-level scores regardless of the method used to quantify IA (40). In addition, IA-md scores were more variable than EA-md scores, again reflecting the high degree of uncertainty of the interoceptive condition and the dispersion found in interoceptive ability in the general population (22, 30, 91).
Regarding the relationship between md and neurophysiological markers of interoception, we found a significant correlation between IA scores and HEP modulation (the better IA-md score, the more negative the amplitude of the HEP). The negative-going modulation of the HEP is considered a canonical marker of interoception since it (i) captures allocation of attention to body signals (52, 59, 92, 93), (ii) distinguishes between good and bad heartbeat perceivers (54, 61), and (iii) has sources in interoceptive hubs (61). However, the association between HEP amplitude and behavioral performance in HBD tasks have proven elusive (15, 94, 95). Similarly, in our study, HEP modulation was not significantly associated with either IA-mSI or IA-d’ outcomes. Importantly, EA was not related to HEP amplitude regardless of the method used, highlighting the specificity of the result for the IA-md index.
Results concerning hemodynamic markers of interoception also support the sensitivity of our md index. Indeed, IA-md was related to functional connectivity among interoceptive networks. Specifically, we found that, the better IA-md score, the stronger the resting-state functional connectivity among insular, somatosensory (i.e., postcentral), frontal, temporal, and ACC regions. These results are in line with previous studies from active (7, 8, 72) and resting-state (14, 15) fMRI experiments consistently implicating those cortical structures in interoception. Particularly, the insular and somatosensory cortices play a key role in mapping the physiological condition of the body and in using that information to generate subjective feeling states (6, 7). Connections within interoceptive seeds and frontal regions (i.e., middle and superior frontal gyrus) may reflect the allocation of attention to endogenous stimuli needed for decision making (i.e., tapping responses) during the task (96). In contrast to previous evidence (5, 7), the involvement of the ACC was minor in the present study. However, this is not surprising since this region might be more relevant for top-down executive monitoring (97), while a primary tracking of bodily changes would occur in insular and somatosensory cortices (98).
It is worth noting that our functional connectivity results showed a bilateral but more left-lateralized insular involvement. This finding would seem to clash with previous reports of predominantly right-sided insular activity in the processing of interoceptive signals (7, 72, 99). However, meta-analytic evidence of interoception (5, 72, 100) has revealed a significant engagement of the left insula, slightly below that of the right insula. Moreover, in Adolfi’s study (5) while the greatest likelihood of activation was found within the right insular cortex (BA13), additional significant clusters in the left insula (BA13) comprised a greater number of voxels, suggesting a greater spatial extent in that region. Bilateral modulations of the insula (7, 99, 101–106) and the neighboring Rolandic operculum (107) have also been consistently reported during active cardiac interoceptive tasks. In fact, motor-tracking HBD tasks similar to ours have yielded activations not only in the right anterior insula/frontal operculum (8), but also (and exclusively) in the left insula (108). Finally, and more pertinent to our results, previous associations between resting-state fMRI connectivity and IA in HBD tasks have yielded mixed results. Chong et al. (109) reported a significant correlation between heartbeat counting scores and salience network connectivity in the right posterior insula, but also a trend towards a positive association in the left posterior insula, suggesting the involvement of a bilateral insular pattern in cardiac monitoring. More specifically, using the same motor-tracking HBD task as ours, positive associations have been found between IA scores and the functional connectivity of the left or bilateral insula (14, 34, 57). Taken together, all this evidence supports the bilateral involvement of the insula in cardiac interoception, even in experimental settings very similar to the present one.
In particular, the specific left (and bilateral) insula involvement during motor-tracking HBD performance could be interpreted in light of the embodied predictive interoception coding (EPIC) model (110), which proposes an active inference account of interoception. According to the EPIC model, the interoceptive system in the brain is composed by agranular visceromotor regions (e.g., anterior insula, posterior ventromedial prefrontal cortex, cingulate cortex) that generate interoceptive predictions and prediction errors from actual sensory signals (related from the body to the granular layer IV of the primary insular interoceptive cortex). The prediction errors can in turn act as a forward model to prime motor responses. Thus, the mid-posterior insula would compute the interoceptive prediction error and propagate it back to the deep layers of the visceromotor regions where the predictions originated. In this context, we propose a forward model based on intra-hemispheric insular-motor system connections: Insular hubs may convey information from interoceptive predictions errors to adjust motor actions (here, tapping responses to heartbeats). Since the majority of our subjects were right-handed, the lateralization of results to the left insula could be explained by the intra-hemispheric connections with the left motor system corresponding to the dominant hand-movements. However, further research is required to directly test the hypothesis of this forward model.
The pattern of functional connectivity results described above was replicated when excluding older adults (> 55 years old) from the analysis (Supplementary Table 8 and Supplementary Figure 3), suggesting common mechanisms across a very large age-range. Results were also replicated for IA-mSI, although less robustly. Regarding the functional connectivity associated with IA-d’, it did not involve the insular cortex, a key interoceptive hub (6). Thus, fMRI results favor our IA-md index. Importantly, all reported associations were specific for IA (as opposed to EA) scores, supporting the construct validity of IA-md index as a measure of interoceptive ability.
The link between interoception and socio-emotional processing is grounded in strong theoretical frameworks (4, 6, 111–113), with embodied simulation accounts suggesting that individuals might be able to recognize others’ emotions by means of body resonance and by interpreting the corresponding interoceptive signals (114). However, these ideas have received sparse empirical support from HBD tasks, with some studies reporting associations between IA and the sensitivity to facial emotions (115), empathy (23), or affective theory of mind (24), and others providing incongruent findings regarding emotion perception (116) and various socio-emotional skills (25). We suggest this might be due to the index used to quantify interoception. In fact, here we found significant associations between interoceptive ability and socio-emotional skills when IA was measured with md, but not with mSI or d’. More specifically, IA-md correlated with the recognition of negative emotions in others in two tasks: one consisting on identifying facial emotions in static pictures (i.e., Ekman-35) (82), and another with greater contextual load, consisting in recognizing emotions in naturalistic social scenarios (i.e., TASIT-EET) (83), which implicates social cognition skills in general, and theory of mind in particular. Additionally, associations with interoception were specific for negative (as opposed to positive) emotion recognition, in accordance to previous research (7). This specificity may reflect common neural substrates between interoception and the processing of negative affective states, such as disgust (117), pain processing (118), empathy for pain (118), envy (119), and social exclusion (120), among others, all of which converge in the insular cortex and the ACC. Thus, the md index may be more sensitive to capture the theoretical role of interoception in the vicarious experience of emotional states. Note that we found no relationship between EA and emotion recognition, underscoring the specificity of results for our IA-md index.
After univariate analysis, we aimed to test how the combination of multiple dimensions (i.e., electrophysiological, hemodynamic, behavioral) explained the variance in the sample’s IA scores when measured by each index (md, mSI and d’). Thus, we performed a data-driven multivariate regression including as selected features all the variables yielding significant associations with IA in the previous analyses. Results revealed that prediction was more accurate for md, indicating that our measure better captures interoceptive features across dimensions. Furthermore, these results persisted (and even increased for md and mSI) when adding confounding variables to the model, including demographics and executive functioning information. Thus, domain-general factors may interact with specific interoceptive dimensions in explaining the variance in IA scores. Indeed, interoceptive performance might prove better in male (52, 53) and young (55) subjects, in relation to mediating factors such as body composition (percentage of body fat) (121). In addition, cognitive abilities (indexed here as executive functioning) may also impact on HBD performance. Educational level can also influence interoceptive outcomes through its relationship with cognitive functioning (122). Importantly, we did not find associations between IA and any of those factors when assessed with univariate methods (i.e., Spearman correlations). In contrast, EA was related to executive performance and years of education, reflecting the capacity to attend to external stimuli, as expected. However, when these variables were included in the multivariate model alongside interoceptive markers, they increased predictions for IA-md, suggesting our measure outperforms other measures in capturing interoceptive variability induced by confounding factors. This finding has relevant implications concerning the assessment of interoception in heterogeneous samples, such as those composed of neuropsychiatric patients.
In sum, this work represents a robust approach combining different dimensions (i.e., electrophysiological, hemodynamic, behavioral) to evaluate HBD-derived IA with different measures. Results also support the validity of our newly developed index (i.e., md), which overcomes major limitations of other widely used alternatives. As this measure is based on capturing synchrony, it is less contaminated by confounding factors such as heart rate estimations (which affects Schandry’s index), and it avoids arbitrary definitions of time-lapses to determine correct responses (which affects the d’ index). More importantly, in contrast to other metrics, IA-md accounts for heart changes effects in subjects’ online performance during motor-tracking HBD tasks. This aspect might be crucial in making IA-md a more sensible index of interoceptive ability. Indeed, interoceptive stimuli (i.e., heartbeats) are variable and temporally inconsistent by nature. As the literature on action-perception coupling shows, expert individuals are indeed more efficient at tracking unexpected changes in task-relevant exteroceptive stimuli (e.g., a ball moving in a sport context) (123). Analogously, individuals with good interoceptive abilities could prove better at detecting the changing rhythm of inner stimuli (i.e., their heart rate), and IA-md is designed to capture such ability.
Also, our results are relevant for the assessment of interoception with clinical aims. In fact, the literature concerning interoceptive alterations in neuropsychiatry are partially inconsistent (e.g., 26, 27), contrasting its theoretical relevance and therapeutic potential (17). The md index, whose validity and sensitivity are supported by its associations with multiple dimensional canonical markers of interoception, could be helpful in this regard.
Future works should also assess whether our results reflect the neurocognitive correlates of interoception beyond the cardiac domain, and whether our measure (md) is sensitive to tap interoceptive abilities related to other systems. Indeed, interoception has been mainly studied through HBD tasks because heartbeats are discrete and frequent internal events that can be easily, non-invasively, and objectively measured (30) and/or manipulated (40). However, interoception is not limited to cardiac sensations, but also includes the monitoring of other internal signals, such as thermoceptive, nociceptive, respiratory, and gastrointestinal (GI) stimuli (6, 17, 121, 124–126). Based on evidence showing an overlap between cardiac and non-cardiac –particularly GI– interoceptive abilities (127, 128), we hope our results could be extrapolated to other interoceptive modalities. Notwithstanding, more research is needed to effectively test the assumption that interoceptive signal detection and awareness work in a coherent and coordinated fashion across different systems (see, for example, 129, 130–132). Here we have provided a systematic framework that, although based on heartbeat detection, has the potential to be used in other contexts. In principle, our index can be implemented in any setting involving self-detectable organs’ signals. To illustrate, the GI system, as the heart, also generates its own rhythm (125, 133), which can be measured through non-invasive electrogastrography (e.g., 127).
Moving forward from the cardiovascular system to study other interoceptive modalities –and how they influence and are influenced by cognition and emotion– is necessary to create “interoceptive profiles” (17) and expand our knowledge about the mechanisms by which individuals sense their physiological condition in health and disease (17). Moreover, since heartbeat detection is itself difficult (with approximately 40% of subjects reporting not being able to consciously register their heartbeats at all) (40), the development of experimental paradigms aimed at assessing other interoceptive modalities would be promising.
Some limitations must be acknowledged. First, its correlational approach prevents us from making causal claims. Future studies should include experimental manipulations to directly assess the impact of cardiac frequency changes in HBD performance. Second, our fMRI analysis was based on resting-state spontaneous fluctuations of the BOLD signal, which constitute only indirect evidence of the neural correlates of interoception. The use of active fMRI tasks would be useful to more precisely detect the cortical regions involved in online interoceptive processing. Finally, note that we used a permissive alpha value for our functional connectivity analyses (p < 0.001 uncorrected, extent threshold = 30 voxels) (78, 81). However, our analyses were hypothesis-driven and results actually align with previous literature, suggesting that we found a true effect that could have been missed with a more conservative approach (134).
In conclusion, here we provided evidence for a multidimensional and multi-feature framework to interoception combined with a new IA index (md) capturing oscillatory couplings between heartbeats and responses during a validated HBD task. Comparisons of this index with other commonly used ones, alongside multivariate analysis, suggest the IA-md index would constitute a better proxy of interoceptive dynamics, even in highly heterogeneous samples. These results pave the way for new theoretical and clinical breakthroughs in the study of interoception.
Supplementary Material
Funding:
This work was supported by grants from CONICET, FONCYT-PICT (2017-1818, 2017-1820), CONICYT/FONDECYT Regular (1170010), CONICYT/FONDECYT (1171200), FONDAP (15150012), the Global Brain Health Initiative, the INECO Foundation, the Inter-American Development Bank (IDB), and the National Institute On Aging of the National Institutes of Health under Award Number R01AG057234 (to IA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: None to declare.
All data, metadata, and code are available from the corresponding author on reasonable request.
References
- 1.Tsakiris M, Critchley H. Interoception beyond homeostasis: affect, cognition and mental health. Philos Trans R Soc Lond B Biol Sci. 2016;371(1708). Epub 2017/01/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Critchley HD, Garfinkel SN. Interoception and emotion. Curr Opin Psychol. 2017;17:7–14. Epub 2017/09/28. [DOI] [PubMed] [Google Scholar]
- 3.Ondobaka S, Kilner J, Friston K. The role of interoceptive inference in theory of mind. Brain Cogn. 2017;112:64–8. Epub 2015/08/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiens S. Interoception in emotional experience. Curr Opin Neurol. 2005;18(4):442–7. Epub 2005/07/09. [DOI] [PubMed] [Google Scholar]
- 5.Adolfi F, Couto B, Richter F, Decety J, Lopez J, Sigman M, et al. Convergence of interoception, emotion, and social cognition: A twofold fMRI meta-analysis and lesion approach. Cortex. 2017;88:124–42. Epub 2017/01/16. [DOI] [PubMed] [Google Scholar]
- 6.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3(8):655–66. Epub 2002/08/03. [DOI] [PubMed] [Google Scholar]
- 7.Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7(2):189–95. Epub 2004/01/20. [DOI] [PubMed] [Google Scholar]
- 8.Zaki J, Davis JI, Ochsner KN. Overlapping activity in anterior insula during interoception and emotional experience. Neuroimage. 2012;62(1):493–9. Epub 2012/05/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Werner NS, Peres I, Duschek S, Schandry R. Implicit memory for emotional words is modulated by cardiac perception. Biol Psychol. 2010;85(3):370–6. Epub 2010/09/04. [DOI] [PubMed] [Google Scholar]
- 10.Umeda S, Tochizawa S, Shibata M, Terasawa Y. Prospective memory mediated by interoceptive accuracy: a psychophysiological approach. Philos Trans R Soc Lond B Biol Sci. 2016;371(1708). Epub 2017/01/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunn BD, Galton HC, Morgan R, Evans D, Oliver C, Meyer M, et al. Listening to your heart. How interoception shapes emotion experience and intuitive decision making. Psychol Sci. 2010;21(12):1835–44. Epub 2010/11/26. [DOI] [PubMed] [Google Scholar]
- 12.Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci. 2009;13(8):334–40. Epub 2009/08/01. [DOI] [PubMed] [Google Scholar]
- 13.Gu X, FitzGerald TH. Interoceptive inference: homeostasis and decision-making. Trends Cogn Sci. 2014;18(6):269–70. Epub 2014/03/04. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Cordero I, Sedeno L, de la Fuente L, Slachevsky A, Forno G, Klein F, et al. Feeling, learning from and being aware of inner states: interoceptive dimensions in neurodegeneration and stroke. Philos Trans R Soc Lond B Biol Sci. 2016;371(1708). Epub 2017/01/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salamone PC, Esteves S, Sinay VJ, Garcia-Cordero I, Abrevaya S, Couto B, et al. Altered neural signatures of interoception in multiple sclerosis. Hum Brain Mapp. 2018;39(12):4743–54. Epub 2018/08/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paulus MP, Stein MB. Interoception in anxiety and depression. Brain Struct Funct. 2010;214(5–6):451–63. Epub 2010/05/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khalsa SS, Lapidus RC. Can Interoception Improve the Pragmatic Search for Biomarkers in Psychiatry? Front Psychiatry. 2016;7:121 Epub 2016/08/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Lernia D, Serino S, Riva G. Pain in the body. Altered interoception in chronic pain conditions: A systematic review. Neurosci Biobehav Rev. 2016;71:328–41. Epub 2016/10/30. [DOI] [PubMed] [Google Scholar]
- 19.Quattrocki E, Friston K. Autism, oxytocin and interoception. Neurosci Biobehav Rev. 2014;47:410–30. Epub 2014/10/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naqvi NH, Bechara A. The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Struct Funct. 2010;214(5–6):435–50. Epub 2010/06/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van den Stock J, Kumfor F. Behavioural variant frontotemporal dementia: At the interface of interoception, emotion and social cognition? Cortex. 2017;115:335–40. Epub 2017/09/09. [DOI] [PubMed] [Google Scholar]
- 22.Marshall CR, Hardy CJD, Russell LL, Clark CN, Dick KM, Brotherhood EV, et al. Impaired Interoceptive Accuracy in Semantic Variant Primary Progressive Aphasia. Front Neurol. 2017;8:610 Epub 2017/12/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grynberg D, Pollatos O. Perceiving one’s body shapes empathy. Physiol Behav. 2015;140:54–60. Epub 2014/12/17. [DOI] [PubMed] [Google Scholar]
- 24.Shah P, Catmur C, Bird G. From heart to mind: Linking interoception, emotion, and theory of mind. Cortex. 2017;93:220–3. Epub 2017/05/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ainley V, Maister L, Tsakiris M. Heartfelt empathy? No association between interoceptive awareness, questionnaire measures of empathy, reading the mind in the eyes task or the director task. Front Psychol. 2015;6:554 Epub 2015/05/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoris A, Esteves S, Couto B, Melloni M, Kichic R, Cetkovich M, et al. The roles of interoceptive sensitivity and metacognitive interoception in panic. Behav Brain Funct. 2015;11:14 Epub 2015/04/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ehlers A, Breuer P. How good are patients with panic disorder at perceiving their heartbeats? Biol Psychol. 1996;42(1–2):165–82. Epub 1996/01/05. [DOI] [PubMed] [Google Scholar]
- 28.Michal M, Reuchlein B, Adler J, Reiner I, Beutel ME, Vogele C, et al. Striking discrepancy of anomalous body experiences with normal interoceptive accuracy in depersonalization-derealization disorder. PLoS One. 2014;9(2):e89823 Epub 2014/03/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sedeno L, Couto B, Melloni M, Canales-Johnson A, Yoris A, Baez S, et al. How do you feel when you can’t feel your body? Interoception, functional connectivity and emotional processing in depersonalization-derealization disorder. PLoS One. 2014;9(6):e98769 Epub 2014/06/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garfinkel SN, Seth AK, Barrett AB, Suzuki K, Critchley HD. Knowing your own heart: distinguishing interoceptive accuracy from interoceptive awareness. Biol Psychol. 2015;104:65–74. Epub 2014/12/03. [DOI] [PubMed] [Google Scholar]
- 31.Ring C, Brener J. Heartbeat counting is unrelated to heartbeat detection: A comparison of methods to quantify interoception. Psychophysiology. 2018;55(9):e13084 Epub 2018/04/11. [DOI] [PubMed] [Google Scholar]
- 32.Schulz A, Lass-Hennemann J, Sutterlin S, Schachinger H, Vogele C. Cold pressor stress induces opposite effects on cardioceptive accuracy dependent on assessment paradigm. Biol Psychol. 2013;93(1):167–74. Epub 2013/01/29. [DOI] [PubMed] [Google Scholar]
- 33.Brener J, Ring C. Towards a psychophysics of interoceptive processes: the measurement of heartbeat detection. Philos Trans R Soc Lond B Biol Sci. 2016;371(1708). Epub 2017/01/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de la Fuente A, Sedeno L, Vignaga SS, Ellmann C, Sonzogni S, Belluscio L, et al. Multimodal neurocognitive markers of interoceptive tuning in smoked cocaine. Neuropsychopharmacology. 2019. Epub 2019/03/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schandry R. Heart beat perception and emotional experience. Psychophysiology. 1981;18(4):483–8. Epub 1981/07/01. [DOI] [PubMed] [Google Scholar]
- 36.Canales-Johnson A, Silva C, Huepe D, Rivera-Rei A, Noreika V, Garcia Mdel C, et al. Auditory Feedback Differentially Modulates Behavioral and Neural Markers of Objective and Subjective Performance When Tapping to Your Heartbeat. Cereb Cortex. 2015;25(11):4490–503. Epub 2015/04/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McFarland RA. Heart rate perception and heart rate control. Psychophysiology. 1975;12(4):402–5. Epub 1975/07/01. [DOI] [PubMed] [Google Scholar]
- 38.Zamariola G, Maurage P, Luminet O, Corneille O. Interoceptive accuracy scores from the heartbeat counting task are problematic: Evidence from simple bivariate correlations. Biol Psychol. 2018;137:12–7. Epub 2018/06/27. [DOI] [PubMed] [Google Scholar]
- 39.Murphy J, Brewer R, Hobson H, Catmur C, Bird G. Is alexithymia characterised by impaired interoception? Further evidence, the importance of control variables, and the problems with the Heartbeat Counting Task. Biol Psychol. 2018;136:189–97. Epub 2018/05/29. [DOI] [PubMed] [Google Scholar]
- 40.Khalsa SS, Rudrauf D, Sandesara C, Olshansky B, Tranel D. Bolus isoproterenol infusions provide a reliable method for assessing interoceptive awareness. Int J Psychophysiol. 2009;72(1):34–45. Epub 2008/10/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Windmann S, Schonecke OW, Frohlig G, Maldener G. Dissociating beliefs about heart rates and actual heart rates in patients with cardiac pacemakers. Psychophysiology. 1999;36(3):339–42. Epub 1999/06/03. [DOI] [PubMed] [Google Scholar]
- 42.Murphy J, Millgate E, Geary H, Ichijo E, Coll MP, Brewer R, et al. Knowledge of resting heart rate mediates the relationship between intelligence and the heartbeat counting task. Biol Psychol. 2018;133:1–3. Epub 2018/01/30. [DOI] [PubMed] [Google Scholar]
- 43.Ring C, Brener J, Knapp K, Mailloux J. Effects of heartbeat feedback on beliefs about heart rate and heartbeat counting: a cautionary tale about interoceptive awareness. Biol Psychol. 2015;104:193–8. Epub 2015/01/03. [DOI] [PubMed] [Google Scholar]
- 44.Macmillan NA, Creelman CD. Detection theory: A user’s guide: Psychology press; 2004. [Google Scholar]
- 45.Killeen PR. Signal detection theory. Encyclopedia of theory in psychology. 2015;2:855–9. [Google Scholar]
- 46.Gonzalez Campo C, Salamone PC, Rodríguez-Arriagada N, Richter F, Herrera E, Bruno D, et al. Fatigue in multiple sclerosis is associated with multimodal interoceptive abnormalities. Multiple Sclerosis Journal. 2019:1352458519888881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Knapp-Kline K, Kline JP. Heart rate, heart rate variability, and heartbeat detection with the method of constant stimuli: slow and steady wins the race. Biol Psychol. 2005;69(3):387–96. Epub 2005/06/01. [DOI] [PubMed] [Google Scholar]
- 48.Novak V, Novak P, de Champlain J, Le Blanc A, Martin R, Nadeau R. Influence of respiration on heart rate and blood pressure fluctuations. Journal of Applied Physiology. 1993;74(2):617–26. [DOI] [PubMed] [Google Scholar]
- 49.Shin H. Ambient temperature effect on pulse rate variability as an alternative to heart rate variability in young adult. Journal of clinical monitoring and computing. 2016;30(6):939–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taelman J, Vandeput S, Spaepen A, Van Huffel S, editors. Influence of mental stress on heart rate and heart rate variability. 4th European conference of the international federation for medical and biological engineering; 2009: Springer. [Google Scholar]
- 51.Couto B, Salles A, Sedeno L, Peradejordi M, Barttfeld P, Canales-Johnson A, et al. The man who feels two hearts: the different pathways of interoception. Soc Cogn Affect Neurosci. 2014;9(9):1253–60. Epub 2013/07/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Montoya P, Schandry R, Muller A. Heartbeat evoked potentials (HEP): topography and influence of cardiac awareness and focus of attention. Electroencephalogr Clin Neurophysiol. 1993;88(3):163–72. Epub 1993/05/01. [DOI] [PubMed] [Google Scholar]
- 53.Grabauskaite A, Baranauskas M, Griskova-Bulanova I. Interoception and gender: What aspects should we pay attention to? Conscious Cogn. 2017;48:129–37. Epub 2016/11/21. [DOI] [PubMed] [Google Scholar]
- 54.Pollatos O, Schandry R. Accuracy of heartbeat perception is reflected in the amplitude of the heartbeat-evoked brain potential. Psychophysiology. 2004;41(3):476–82. Epub 2004/04/23. [DOI] [PubMed] [Google Scholar]
- 55.Khalsa SS, Rudrauf D, Tranel D. Interoceptive awareness declines with age. Psychophysiology. 2009;46(6):1130–6. Epub 2009/07/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murphy J, Brewer R, Catmur C, Bird G. Interoception and psychopathology: A developmental neuroscience perspective. Dev Cogn Neurosci. 2017;23:45–56. Epub 2017/01/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yoris A, Abrevaya S, Esteves S, Salamone P, Lori N, Martorell M, et al. Multilevel convergence of interoceptive impairments in hypertension: New evidence of disrupted body-brain interactions. Hum Brain Mapp. 2017;39(4):1563–81. Epub 2017/12/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yoris A, Garcia AM, Traiber L, Santamaria-Garcia H, Martorell M, Alifano F, et al. The inner world of overactive monitoring: neural markers of interoception in obsessive-compulsive disorder. Psychol Med. 2017;47(11):1957–70. Epub 2017/04/05. [DOI] [PubMed] [Google Scholar]
- 59.Garcia-Cordero I, Esteves S, Mikulan EP, Hesse E, Baglivo FH, Silva W, et al. Attention, in and Out: Scalp-Level and Intracranial EEG Correlates of Interoception and Exteroception. Front Neurosci. 2017;11:411 Epub 2017/08/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fukushima H, Terasawa Y, Umeda S. Association between interoception and empathy: evidence from heartbeat-evoked brain potential. Int J Psychophysiol. 2011;79(2):259–65. Epub 2010/11/09. [DOI] [PubMed] [Google Scholar]
- 61.Pollatos O, Kirsch W, Schandry R. Brain structures involved in interoceptive awareness and cardioafferent signal processing: a dipole source localization study. Hum Brain Mapp. 2005;26(1):54–64. Epub 2005/04/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pollatos O, Herbert BM, Mai S, Kammer T. Changes in interoceptive processes following brain stimulation. Philos Trans R Soc Lond B Biol Sci. 2016;371(1708). Epub 2017/01/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Torralva T, Roca M, Gleichgerrcht E, Lopez P, Manes F. INECO Frontal Screening (IFS): a brief, sensitive, and specific tool to assess executive functions in dementia. J Int Neuropsychol Soc. 2009;15(5):777–86. Epub 2009/07/29. [DOI] [PubMed] [Google Scholar]
- 64.Melloni M, Sedeno L, Couto B, Reynoso M, Gelormini C, Favaloro R, et al. Preliminary evidence about the effects of meditation on interoceptive sensitivity and social cognition. Behav Brain Funct. 2013;9:47 Epub 2013/12/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Werner G, Mountcastle VB. The variability of central neural activity in a sensory system, and its implications for the central reflection of sensory events. Journal of Neurophysiology. 1963;26(6):958–77. [DOI] [PubMed] [Google Scholar]
- 66.Kruczyk M, Umer HM, Enroth S, Komorowski J. Peak Finder Metaserver - a novel application for finding peaks in ChIP-seq data. BMC Bioinformatics. 2013;14:280 Epub 2013/09/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Couto B, Adolfi F, Velasquez M, Mesow M, Feinstein J, Canales-Johnson A, et al. Heart evoked potential triggers brain responses to natural affective scenes: A preliminary study. Auton Neurosci. 2015;193:132–7. Epub 2015/07/21. [DOI] [PubMed] [Google Scholar]
- 68.Zich C, Debener S, Kranczioch C, Bleichner MG, Gutberlet I, De Vos M. Real-time EEG feedback during simultaneous EEG-fMRI identifies the cortical signature of motor imagery. Neuroimage. 2015;114:438–47. Epub 2015/04/19. [DOI] [PubMed] [Google Scholar]
- 69.Kern M, Aertsen A, Schulze-Bonhage A, Ball T. Heart cycle-related effects on event-related potentials, spectral power changes, and connectivity patterns in the human ECoG. Neuroimage. 2013;81:178–90. Epub 2013/05/21. [DOI] [PubMed] [Google Scholar]
- 70.Dirlich G, Dietl T, Vogl L, Strian F. Topography and morphology of heart action-related EEG potentials. Electroencephalogr Clin Neurophysiol. 1998;108(3):299–305. Epub 1998/06/02. [DOI] [PubMed] [Google Scholar]
- 71.Park HD, Correia S, Ducorps A, Tallon-Baudry C. Spontaneous fluctuations in neural responses to heartbeats predict visual detection. Nat Neurosci. 2014;17(4):612–8. Epub 2014/03/13. [DOI] [PubMed] [Google Scholar]
- 72.Schulz SM. Neural correlates of heart-focused interoception: a functional magnetic resonance imaging meta-analysis. Philos Trans R Soc Lond B Biol Sci. 2016;371(1708). Epub 2017/01/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nichols TE, Das S, Eickhoff SB, Evans AC, Glatard T, Hanke M, et al. Best practices in data analysis and sharing in neuroimaging using MRI. Nat Neurosci. 2017;20(3):299–303. Epub 2017/02/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Poldrack RA, Baker CI, Durnez J, Gorgolewski KJ, Matthews PM, Munafo MR, et al. Scanning the horizon: towards transparent and reproducible neuroimaging research. Nature reviews Neuroscience. 2017;18(2):115–26. Epub 2017/01/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chao-Gan Y, Yu-Feng Z. DPARSF: A MATLAB Toolbox for “Pipeline” Data Analysis of Resting-State fMRI. Front Syst Neurosci. 2010;4:13 Epub 2010/06/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Hum Brain Mapp. 1999;7(4):254–66. Epub 1999/07/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Varangis E, Habeck CG, Razlighi QR, Stern Y. The Effect of Aging on Resting State Connectivity of Predefined Networks in the Brain. Frontiers in aging neuroscience. 2019;11:234 Epub 2019/09/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moguilner S, Garcia AM, Mikulan E, Hesse E, Garcia-Cordero I, Melloni M, et al. Weighted Symbolic Dependence Metric (wSDM) for fMRI resting-state connectivity: A multicentric validation for frontotemporal dementia. Scientific reports. 2018;8(1):11181 Epub 2018/07/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sedeno L, Piguet O, Abrevaya S, Desmaras H, Garcia-Cordero I, Baez S, et al. Tackling variability: A multicenter study to provide a gold-standard network approach for frontotemporal dementia. Human brain mapping. 2017;38(8):3804–22. Epub 2017/05/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.d’Ambrosio A, Hidalgo de la Cruz M, Valsasina P, Pagani E, Colombo B, Rodegher M, et al. Structural connectivity-defined thalamic subregions have different functional connectivity abnormalities in multiple sclerosis patients: Implications for clinical correlations. Human brain mapping. 2017;38(12):6005–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Loitfelder M, Filippi M, Rocca M, Valsasina P, Ropele S, Jehna M, et al. Abnormalities of resting state functional connectivity are related to sustained attention deficits in MS. PloS one. 2012;7(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bertoux M, Volle E, de Souza LC, Funkiewiez A, Dubois B, Habert MO. Neural correlates of the mini-SEA (Social cognition and Emotional Assessment) in behavioral variant frontotemporal dementia. Brain Imaging Behav. 2014;8(1):1–6. Epub 2013/10/01. [DOI] [PubMed] [Google Scholar]
- 83.McDonald S, Flanagan S, Rollins J, Kinch J. TASIT: A new clinical tool for assessing social perception after traumatic brain injury. The Journal of head trauma rehabilitation. 2003;18(3):219–38. Epub 2003/06/13. [DOI] [PubMed] [Google Scholar]
- 84.Alpaydin E. Introduction to machine learning: MIT press; 2009. [Google Scholar]
- 85.Kassraian-Fard P, Matthis C, Balsters JH, Maathuis MH, Wenderoth N. Promises, pitfalls, and basic guidelines for applying machine learning classifiers to psychiatric imaging data, with autism as an example. Front Psychiatry. 2016;7:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Anderson JS, Nielsen JA, Froehlich AL, DuBray MB, Druzgal TJ, Cariello AN, et al. Functional connectivity magnetic resonance imaging classification of autism. Brain. 2011;134(12):3742–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dottori M, Sedeño L, Caro MM, Alifano F, Hesse E, Mikulan E, et al. Towards affordable biomarkers of frontotemporal dementia: A classification study via network’s information sharing. Sci Rep. 2017;7(1):3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Friedman J, Hastie T, Tibshirani R. The elements of statistical learning: Springer series in statistics New York; 2001. [Google Scholar]
- 89.Kvålseth TO. Cautionary note about R 2. The American Statistician. 1985;39(4):279–85. [Google Scholar]
- 90.Donnelly-Kehoe PA, Pascariello GO, García AM, Hodges JR, Miller B, Rosen H, et al. Robust automated computational approach for classifying frontotemporal neurodegeneration: Multimodal/multicenter neuroimaging. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring. 2019;11:588–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tsakiris M, Tajadura-Jimenez A, Costantini M. Just a heartbeat away from one’s body: interoceptive sensitivity predicts malleability of body-representations. Proc Biol Sci. 2011;278(1717):2470–6. Epub 2011/01/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yuan H, Yan HM, Xu XG, Han F, Yan Q. Effect of heartbeat perception on heartbeat evoked potential waves. Neurosci Bull. 2007;23(6):357–62. Epub 2007/12/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Petzschner FH, Weber LA, Wellstein KV, Paolini G, Do CT, Stephan KE. Focus of attention modulates the heartbeat evoked potential. Neuroimage. 2019;186:595–606. Epub 2018/11/26. [DOI] [PubMed] [Google Scholar]
- 94.Terhaar J, Viola FC, Bar KJ, Debener S. Heartbeat evoked potentials mirror altered body perception in depressed patients. Clin Neurophysiol. 2012;123(10):1950–7. Epub 2012/05/01. [DOI] [PubMed] [Google Scholar]
- 95.Schulz A, Koster S, Beutel ME, Schachinger H, Vogele C, Rost S, et al. Altered patterns of heartbeat-evoked potentials in depersonalization/derealization disorder: neurophysiological evidence for impaired cortical representation of bodily signals. Psychosom Med. 2015;77(5):506–16. Epub 2015/05/20. [DOI] [PubMed] [Google Scholar]
- 96.Farb NA, Segal ZV, Anderson AK. Attentional modulation of primary interoceptive and exteroceptive cortices. Cereb Cortex. 2013;23(1):114–26. Epub 2012/01/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4(6):215–22. Epub 2000/05/29. [DOI] [PubMed] [Google Scholar]
- 98.Critchley HD, Mathias CJ, Dolan RJ. Neuroanatomical basis for first- and second-order representations of bodily states. Nat Neurosci. 2001;4(2):207–12. Epub 2001/02/15. [DOI] [PubMed] [Google Scholar]
- 99.Pollatos O, Schandry R, Auer DP, Kaufmann C. Brain structures mediating cardiovascular arousal and interoceptive awareness. Brain research. 2007;1141:178–87. [DOI] [PubMed] [Google Scholar]
- 100.Salvato G, Richter F, Sedeño L, Bottini G, Paulesu E. Building the bodily self-awareness: Evidence for the convergence between interoceptive and exteroceptive information in a multilevel kernel density analysis study. Hum Brain Mapp. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kleint NI, Wittchen H-U, Lueken U. Probing the interoceptive network by listening to heartbeats: an fMRI study. PLoS One. 2015;10(7):e0133164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Caseras X, Murphy K, Mataix-Cols D, López-Solà M, Soriano-Mas C, Ortriz H, et al. Anatomical and functional overlap within the insula and anterior cingulate cortex during interoception and phobic symptom provocation. Hum Brain Mapp. 2013;34(5):1220–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ernst J, Northoff G, Böker H, Seifritz E, Grimm S. Interoceptive awareness enhances neural activity during empathy. Hum Brain Mapp. 2013;34(7):1615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Simmons WK, Avery JA, Barcalow JC, Bodurka J, Drevets WC, Bellgowan P. Keeping the body in mind: insula functional organization and functional connectivity integrate interoceptive, exteroceptive, and emotional awareness. Hum Brain Mapp. 2013;34(11):2944–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tan Y, Wei D, Zhang M, Yang J, Jelinčić V, Qiu J. The role of mid-insula in the relationship between cardiac interoceptive attention and anxiety: evidence from an fMRI study. Scientific reports. 2018;8(1):17280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yao S, Becker B, Zhao W, Zhao Z, Kou J, Ma X, et al. Oxytocin modulates attention switching between interoceptive signals and external social cues. Neuropsychopharmacology. 2018;43(2):294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Blefari ML, Martuzzi R, Salomon R, Bello-Ruiz J, Herbelin B, Serino A, et al. Bilateral Rolandic operculum processing underlying heartbeat awareness reflects changes in bodily self-consciousness. European Journal of Neuroscience. 2017;45(10):1300–12. [DOI] [PubMed] [Google Scholar]
- 108.Klabunde M, Juszczak H, Jordan T, Baker J, Bruno J, Carrion V, et al. Functional neuroanatomy of interoceptive processing in children and adolescents: a pilot study. Scientific reports. 2019;9(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chong JSX, Ng GJP, Lee SC, Zhou J. Salience network connectivity in the insula is associated with individual differences in interoceptive accuracy. Brain Structure and Function. 2017;222(4):1635–44. [DOI] [PubMed] [Google Scholar]
- 110.Barrett LF, Simmons WK. Interoceptive predictions in the brain. Nat Rev Neurosci. 2015;16(7):419–29. Epub 2015/05/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.James W. What is an Emotion?: Simon and Schuster; 2013. [Google Scholar]
- 112.Damasio AR. Descartes’ error: Random House; 2006. [Google Scholar]
- 113.Barrett LF, Russell JA. The psychological construction of emotion: Guilford Publications; 2014. [Google Scholar]
- 114.Gallese V, Sinigaglia C. What is so special about embodied simulation? Trends Cogn Sci. 2011;15(11):512–9. Epub 2011/10/11. [DOI] [PubMed] [Google Scholar]
- 115.Terasawa Y, Moriguchi Y, Tochizawa S, Umeda S. Interoceptive sensitivity predicts sensitivity to the emotions of others. Cognition and Emotion. 2014;28(8):1435–48. [DOI] [PubMed] [Google Scholar]
- 116.Ferguson ML, Katkin ES. Visceral perception, anhedonia, and emotion. Biol Psychol. 1996;42(1–2):131–45. [DOI] [PubMed] [Google Scholar]
- 117.Wicker B, Keysers C, Plailly J, Royet J-P, Gallese V, Rizzolatti G. Both of us disgusted in My insula: the common neural basis of seeing and feeling disgust. Neuron. 2003;40(3):655–64. [DOI] [PubMed] [Google Scholar]
- 118.Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 2011;54(3):2492–502. [DOI] [PubMed] [Google Scholar]
- 119.Takahashi H, Kato M, Matsuura M, Mobbs D, Suhara T, Okubo Y. When your gain is my pain and your pain is my gain: neural correlates of envy and schadenfreude. Science. 2009;323(5916):937–9. [DOI] [PubMed] [Google Scholar]
- 120.Eisenberger NI, Lieberman MD. Why rejection hurts: a common neural alarm system for physical and social pain. Trends Cogn Sci. 2004;8(7):294–300. [DOI] [PubMed] [Google Scholar]
- 121.Cameron OG. Interoception: the inside story—a model for psychosomatic processes. Psychosom Med. 2001;63(5):697–710. [DOI] [PubMed] [Google Scholar]
- 122.Hackman DA, Farah MJ, Meaney MJ. Socioeconomic status and the brain: mechanistic insights from human and animal research. Nature reviews neuroscience. 2010;11(9):651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mallek M, Benguigui N, Dicks M, Thouvarecq R. Sport expertise in perception-action coupling revealed in a visuomotor tracking task. Eur J Sport Sci. 2017;17(10):1270–8. Epub 2017/09/30. [DOI] [PubMed] [Google Scholar]
- 124.Hölzl R, Erasmus L-P, Möltner A. Detection, discrimination and sensation of visceral stimuli. Biological Psychology. 1996;42(1–2):199–214. [DOI] [PubMed] [Google Scholar]
- 125.Azzalini D, Rebollo I, Tallon-Baudry C. Visceral signals shape brain dynamics and cognition. Trends in cognitive sciences. 2019. [DOI] [PubMed] [Google Scholar]
- 126.Alfonsi P, Adam F, Bouhassira D. Thermoregulation and pain perception: evidence for a homoeostatic (interoceptive) dimension of pain. European Journal of Pain. 2016;20(1):138–48. [DOI] [PubMed] [Google Scholar]
- 127.Herbert BM, Muth ER, Pollatos O, Herbert C. Interoception across modalities: on the relationship between cardiac awareness and the sensitivity for gastric functions. PloS one. 2012;7(5):e36646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Whitehead WE, Drescher VM. Perception of gastric contractions and self-control of gastric motility. Psychophysiology. 1980;17(6):552–8. [DOI] [PubMed] [Google Scholar]
- 129.Steptoe A, Noll A. The perception of bodily sensations, with special reference to hypochondriasis. Behaviour research and therapy. 1997;35(10):901–10. [DOI] [PubMed] [Google Scholar]
- 130.Kollenbaum V-E, Dahme B, Kirchner G. ‘Interoception’of heart rate, blood pressure, and myocardial metabolism during ergometric work load in healthy young subjects. Biological Psychology. 1996;42(1–2):183–97. [DOI] [PubMed] [Google Scholar]
- 131.Mauss IB, Levenson RW, McCarter L, Wilhelm FH, Gross JJ. The tie that binds? Coherence among emotion experience, behavior, and physiology. Emotion. 2005;5(2):175. [DOI] [PubMed] [Google Scholar]
- 132.Ferentzi E, Bogdány T, Szabolcs Z, Csala B, Horváth Á, Köteles F. Multichannel investigation of interoception: Sensitivity is not a generalizable feature. Frontiers in human neuroscience. 2018;12:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sanders KM, Koh SD, Ward SM. Interstitial cells of Cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2006;68:307–43. [DOI] [PubMed] [Google Scholar]
- 134.Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Social cognitive and affective neuroscience. 2009;4(4):423–8. Epub 2009/12/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


