Abstract
Neoplastically transformed astrocytes express functionally active cell surface β adrenergic receptors (βARs). Treatment of glioma models in vitro and in vivo with β adrenergic agonists variably amplifies or attenuates cellular proliferation. In the majority of in vivo models, β adrenergic agonists generally reduce cellular proliferation. However, treatment with β adrenergic agonists consistently reduces tumor cell invasive potential, angiogenesis, and metastasis. β adrenergic agonists induced decreases of invasive potential are chiefly mediated through reductions in the expression of matrix metalloproteinases types 2 and 9. Treatment with β adrenergic agonists also clearly reduce tumoral neoangiogenesis, which may represent a putatively useful mechanism to adjuvantly amplify the effects of bevacizumab. Bevacizumab is a monoclonal antibody targeting the vascular endothelial growth factor receptor. We may accordingly designate βagonists to represent an enhancer of bevacizumab. The antiangiogenic effects of β adrenergic agonists may thus effectively render an otherwise borderline effective therapy to generate significant enhancement in clinical outcomes. β adrenergic agonists upregulate expression of the major histocompatibility class II DR alpha gene, effectively potentiating the immunogenicity of tumor cells to tumor surveillance mechanisms. Authors have also demonstrated crossmodal modulation of signaling events downstream from the β adrenergic cell surface receptor and microtubular polymerization and depolymerization. Complex effects and desensitization mechanisms of the β adrenergic signaling may putatively represent promising therapeutic targets. Constant stimulation of the β adrenergic receptor induces its phosphorylation by β adrenergic receptor kinase (βARK), rendering it a suitable substrate for alternate binding by β arrestins 1 or 2. The binding of a β arrestin to βARK phosphorylated βAR promotes receptor mediated internalization and downregulation of cell surface receptor and contemporaneously generates a cell surface scaffold at the βAR. The scaffold mediated activation of extracellular regulated kinase 1/2, compared with protein kinase A mediated activation, preferentially favors cytosolic retention of ERK1/2 and blunting of nuclear translocation and ensuant pro-transcriptional activity. Thus, βAR desensitization and consequent scaffold assembly effectively retains the cytosolic homeostatic functions of ERK1/2 while inhibiting its pro-proliferative effects. We suggest these mechanisms specifically will prove quite promising in developing primary and adjuvant therapies mitigating glioma growth, angiogenesis, invasive potential, and angiogenesis. We suggest generating compounds and targeted mutations of the β adrenergic receptor favoring β arrestin binding and scaffold facilitated activation of ERK1/2 may hold potential promise and therapeutic benefit in adjuvantly treating most or all cancers. We hope our discussion will generate fruitful research endeavors seeking to exploit these mechanisms.
Keywords: β adrenergic receptor, β adrenergic receptor kinase, β arrestin, Glioma, Glioblastoma, Tumor
Introduction
Untransformed and malignantly transformed astroglial cells widely express neurolemmal cell surface β adrenergic receptors [1–3]. Human (e.g., U-251-MG, LM, and 1321 N1 astrocytoma cell lines) and rat (e.g., C6, C62B) glioma cells widely overexpress pharmacologically-stimulable and functionally active cell surface β adrenergic receptors (βARs) [4, 5]. In mice transfected with U87 cells in order to induce gliomagenesis in vivo, tumors overexpress β2ARs by approximately two-fold compared with cells of nearby healthy parenchyma [6]. Accordingly, β adrenergic receptor modulated signaling regulates intracellular signal transduction pathways implicated in the initiation, promotion, and progression of carcinogenesis. Studies have extensively indicated β adrenergic signaling powerfully modulates tumor cell proliferation, angiogenesis, invasiveness, and metastasis [7]. Authors have collectively elucidated these effects in glioma models in vitro [8, 9] and in vivo [10]. We extensively discuss differential signal transduction pathways conveying β adrenergic signaling to cytosolic and nuclear mechanisms mediating cell surface receptor desensitization in untransformed and neoplastically transformed glioma cells [11–16]. Our molecularly-oriented discourse will shed light on the apparent paradoxical behavior of carcinomas in response to pharmacological agonism or antagonism of β adrenergic receptor modulated signaling in vitro and in vivo. In so doing, we effectively illumine the potential utility of developing compounds modulating β adrenergic receptor modulated signaling in the treatment of cerebral gliomas [10]. The development of a thorough understanding of these mechanisms will pave the way and enhance our capacity to develop novel therapeutic approaches to induce log cell eradication of malignantly transformed astrocytes constituting cerebral gliomas [11–16].
β adrenergic receptor modulated signaling
We present an integrated framework detailing and conceptualizing the effects of β adrenergic receptor modulated signaling upon intracellular signal transduction pathways [11–16], constituted by specific and sequential phosphorylation-dependent conformational protein modifications, mechanisms blunting βAR-G protein coupling and promoting receptor internalization [14, 17], and candidate therapeutic molecular targets modulating downstream signaling effects [18]. β adrenergic receptors constitute a family of heteromultimeric heptahelical transmembrane proteins (Fig. 1) [16], which modulate cellular processes by promoting G protein-mediated signal transduction (Fig. 2) [19] and alternately upregulating [20, 21] or downregulating [22] the catalytic enzymatic activity of adenylate cyclase, which generates cyclic adenosine monophosphate (cyclic AMP or cAMP) from the high-energy substrate adenosine triphosphate (ATP) [23]. Cyclic AMP allosterically activates protein kinase A (PKA) by binding its regulatory subunits and physically releasing its catalytic subunits [24–27]. Ligand binding mediated promotion of β adrenergic receptor modulated signaling concurrently potentiates the catalytic enzymatic activity of phospholipase C, generating diacylglycerol (DAG) and inositol triphosphate (IP3) from the precursor phospholipid phosphatidyl inositol diphosphate (PIP2). DAG allosterically activates protein kinase C, which phosphorylatively modulates a host of intracellular signal transduction pathways. Binding of IP3 to receptors studding the phospholipid bilayer membrane of the sarcoplasmic reticulum enhances the release of divalent cationic calcium from abundant organellar stores to the cytosol. Ligand-activated β adrenergic receptors transactivate intracellular tyrosine, serine-threonine, or SRC kinase-coupled membrane protein growth factor receptors [28–31]. C-terminal phosphorylated β adrenergic receptor β arrestin complexes constituting nidal signaling scaffolds may selectively and specifically potentiate ERK1/2 activity and a set of variably related intracellular signal transduction pathways (Figs. 3, 4) [32–34].
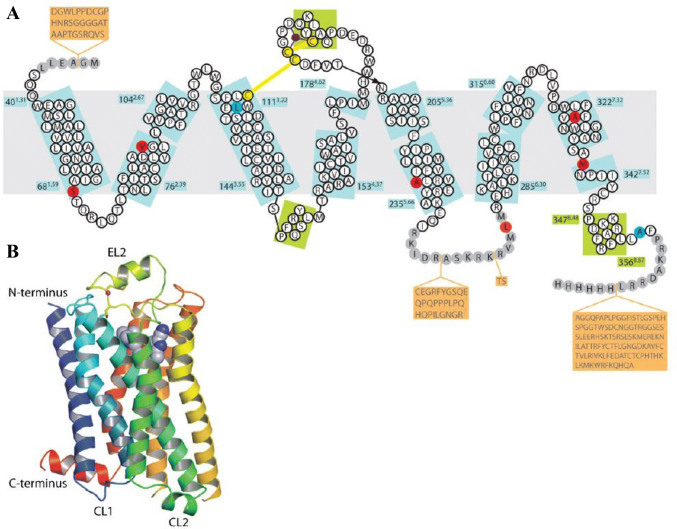
Fig. 1.
β1 adrenergic receptor structure schematic diagram. A Turkey β1 adrenergic receptor sequence illustrated in relation to secondary structural elements (these refer to alpha helices, beta pleated sheets, and beta bends). Amino acid sequence indicated in white circles demonstrate regions which are not well ordered, with sequences not resolved indicated by grey circles. Amino acid sequences on an orange background were deleted in order to generate the β1 adrenergic receptor construct for expression. Thermostabilising (red), C116L (mediating an increase in functional expression), and C358A (eliminating the palmitoylation site) (blue) mutations, and Na+ ion (purple) indicated by color. Numbers refer to the first and last amino acid residues contained within each helix (blue boxes), with the Ballesteros-Weinstein numbering indicated in superscript. Helices were defined utilizing the Kabasch & Sander algorithm, with helix distortions being defined as residues maintaining chain torsion angles differing by more than 40° from the standard α-helix values (− 60°, − 40°). B Ribbon representation of the 1 adrenergic receptor structure demonstrated in rainbow colouration, with N-terminus (blue), C-terminus (red), Na+ ion (pink), and two disulphide bonds (yellow) indicated. Cyanopindolol is indicated as a space-filling model. Extracellular loop 2 (EL2) and cytoplasmic loops 1 and 2 (CL1, CL2) are labelled. Reprinted with permission from Warne et al. [16]. (Color figure online)
Fig. 2.
β adrenergic receptor G protein cycle. A Extracellular agonist binds to the β adrenergic receptor effecting conformational changes within the cytoplasmic ends of the receptor transmembrane domains, allowing the heterotrimeric G protein to bind the β adrenergic receptor. G protein binding to the β adrenergic receptor facilitates conformational changes promoting GTP-GDP exchange by the α subunit, facilitating dissociation of the catalytic α and noncatalytic βgamma subunit. The G protein catalytic α and noncatalytic βgamma subunits mediate various effector activities. The G protein activity stimulates adenylate cyclase activity and the noncatalytic βgamma subunit is demonstrated activating membrane calcium channels mediating entry of extracellular calcium to the cytosol. The α subunit subsequently catalyzes hydrolysis of bound guanosine triphosphate to guanosine diphosphate, mediating G protein α and βgamma subunit reconstitution. The G protein βgamma noncatalytic subunit also ferries the β adrenergic receptor kinase towards the β adrenergic receptor. B The purified β adrenergic receptor Gs protein complex free of nucleotides is maintained in detergent micelles. The Gα subunit consists of the Ras domain (αRas) with GTPase activity and the α-helical domain (αAH). Both subunits are involved in nucleotide binding. In the nucleotide-free condition, the α helical domain has a variable position relative to the Gα Ras domain. Reprinted with permission from Rasmussen et al. [81]
Fig. 3.
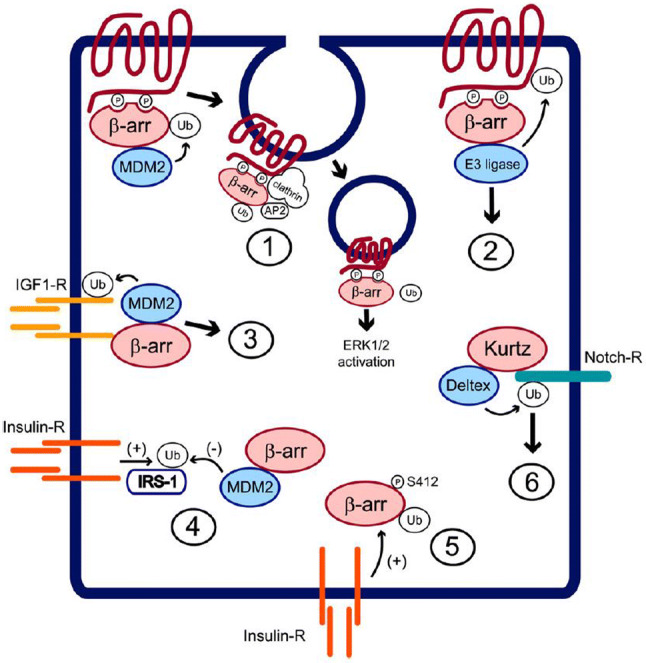
β-Arrestin contributes to ubiquitinylation and receptor mediated signaling. (1) MDM2 binds and mediates ubiquitinylation of receptor-associated β arrestin, promoting recruitment of clathrin and AP2, internalization of membrane bound receptors, and β arrestin-mediated scaffold facilitated signaling. (2) β arrestins facilitate ubiquitinylation of receptors by forming scaffolds comprised of E3 ligase, bringing these enzymes into close proximity with the receptors, thereby promoting receptor ubiquitinylation and trafficking to lysosomes for degradation. (3) β arrestin1 serves as an adaptor protein bringing the E3 ligase MDM2 to the activated insulin like growth factor-1 receptor, thereby promoting ubiquitinylation of receptor and subsequent proteasomal degradation. (4) β arrestins compete with insulin receptor substrate 1 for MDM2, thereby reducing insulin-induced MDM2-mediated ubiquitinylation of insulin receptor substrate 1 and proteasomal degradation. Insulin receptor substrate 1. β arrestins thus enhance sensitivity to insulin signaling. (5) Stimulation by insulin through tyrosine kinase receptors promotes phosphorylation of β-arrestin, ubiquitinylation, and receptor downregulation, thereby augmenting heptahelical transmembrane receptor-mediated G protein signaling and reducing signaling facilitated by the adapter function of β arrestin promoting scaffold assembly. β-arrestin-mediated signaling (e.g., to extracellular regulated kinase 1/2). (6) In Drosophila melanogaster, Kurtz, the nonvisual homologin of arrestin, interacts with the ubiquitin ligase Deltex in order to facilitate Notch ubiquitinylation. Notch ubiquitinylation promotes its proteasomal degradation. Reprinted with permission from Lefkowitz et al. [13]
Fig. 4.

Conventional compared to biased heptahelical transmembrane receptor signaling. A Agonist-stimulated heptahelical transmembrane receptor signaling is mediated via both heterotrimeric G proteins and β arrestins. B Conventional antagonists bind heptahelical transmembrane receptor proteins and prevent agonist stimulated signaling through both heterotrimeric G proteins and β arrestins. C Soi-disant biased agonists/antagonists (e.g., SII-angiotensin II) prevent heterotrimeric G protein signaling mediated by agonist stimulation of G protein coupled receptor while promoting β arrestin facilitated scaffold signaling. Reprinted with permission from Lefkowitz et al. [13]
Desensitization of β adrenergic receptor ligand binding-effector coupling (Fig. 3) heads a pseudo-dichotomous signal transduction pathway switch [13, 35, 36] (Fig. 4). (N.B. C6 glioma cells undergo downregulation of cell surface β adrenergic receptor expression when grown in serum [37]). Agonist binding to the β adrenergic receptor renders it suitable to undergo carboxyl terminal phosphorylation by β adrenergic receptor kinase (βARK) [11, 14], β arrestin 1 and/or 2 binding of phosphorylated β adrenergic receptor C-terminal sterically hinders βAR-G protein coupling [66, 81]. The adapter function of β arrestin proteins promotes binding of clathrin to the internal layer of phospholipid zones surrounding βARs, which effects clathrin-coated pit-mediated receptor endocytosis [13]. The βAR-β arrestin scaffold promotes binding of ERK1/2, c Jun N terminal kinase 3 (JNK3), Raf, cRaf1, and MEK1 (Figs. 3, 4) [11, 12, 15]. Preferential activation of these signaling proteins which classically promote cellular proliferation when activated by protein kinase A by the scaffold mechanism coordinately favors cytosolic retention and effects of these proteins and prevents nuclear translocation and pro-transcriptional activity-mediated promotion of deoxyribonucleic acid and proteins constituting the mitotic machinery [11, 12]. β arrestin 1 exhibits preferentially stable binding kinetics with the βAR compared with β arrestin 2. Binding of the amino terminus of β arrestin 1 to the carboxyl terminus of βAR generates stable receptor internalization and slow βAR GPCR dephosphorylation, slowing return to the cell membrane [15]. Stable β arrestin 1 βAR binding favors scaffold assembly and scaffold mediated activation of the pleiotropically-acting kinase ERK1/2 [12, 15]. Thus, the same set of mechanisms which mediates desensitization and internalization of the β adrenergic receptor [15] coordinately contributes to modulating the effects mediated by ERK1/2 [11, 12, 15].
Thus, instead of conceiving of β arrestin to represent a general inhibitor of β adrenergic signaling, it may be more appropriate and prudent to conceptualize this protein to modulate β adrenergic receptor modulated signaling, coordinately attenuating G protein-mediated effects and preferentially shifting signaling towards the non-proliferative actions of ERK1/2 (Fig. 4) [11, 12, 15]. Kinetics of β arrestin dissociation from GPCRs powerfully determine receptor conformational changes and dictate effects of downstream signaling [15]. Angiotensin 1A, vasopressin 2, neurotensin, and dopamine receptor carboxyl termini bind β arrestin 2 stably with slower dissociation kinetics compared with the carboxyl terminal of βARs, generating stable clathrin coated pit-mediated internalization with slower dephosphorylation and return to the cell membrane [15]. The stable binding preferentially favors the cytosolic retention and activity ERK1/2, while downregulating the nuclear effects of the kinase [11, 12]. β arrestin 2 binds the α1b and β2 adrenergic receptors transiently with more rapid dissociation kinetics. Rapid dissociation kinetics generates equivalently rapid removal of phosphate moieties from the G protein-coupled receptor (GPCR) and return of endocytosed receptor to the plasmalemmal phospholipid bilayer [15] and preferentially enhances G protein mediated effects of G protein coupled receptor activation and comparatively attenuates scaffold mediated effects upon signal transduction pathways, coordinately promoting nuclear translocation of, and transcriptional upregulation mediated by, activated ERK1/2.
Modulation of cellular proliferation by β adrenergic signaling
Malignantly transformed astroglia overexpress pharmacologically stimulabe and functionally active β adrenergic receptors [5]. Studies have provided evidence indicating ligand activation of β adrenergic receptor modulated signaling may either promote or blunt proliferation of malignantly transformed cells in glioma models [4, 38–44] and extra-neuraxial carcinoma [45–49]. Specifically, ligand activation of β adrenergic receptors potently amplifies cellular proliferation in lung [7], gastric [50], hepatocellular [51], pancreatic [52], colorectal [53], breast [54, 55], ovarian [56, 57], and prostatic [49] carcinoma models in vitro. Paradoxically, pharmacological antagonism of β adrenergic receptors also potently attenuates cellular proliferation in hemangioblastoma [58] and hepatic [55], pancreatic [59], gastric [50], colorectal [46], breast [54, 55], ovarian, and prostatic [60] carcinoma models in vitro. β antagonists reduce cellular proliferation and migration in neuroblastoma cell lines [8], enhance therapeutic concentrations of co-administered medications [8], and reduce expression of P-glycoprotein inhibitors [61]. β adrenergic receptor agonists were shown to reduce the proliferation of MDA-MB-231 human breast cancer cells [48, 118]. Succinctly, blunting of tumor cell proliferation in vitro by β adrenergic agonists results from desensitization and by β adrenergic antagonists results directly from receptor antagonism [62]. Studies have alternately demonstrated improved [63] or reduced [64] survival in patients harboring ovarian carcinoma receiving pharmacological antagonists of β adrenergic receptors. The bitopic agonist and GPR55 antagonist (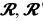 )-4′-methoxy-1-naphthylfenoterol, which may be designated as (
)-4′-methoxy-1-naphthylfenoterol, which may be designated as (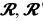 )-MNF, significantly reduces mitogenic potential in melanoma by modulating cyclic AMP protein kinase A-dependent pathways [65]. (
)-MNF, significantly reduces mitogenic potential in melanoma by modulating cyclic AMP protein kinase A-dependent pathways [65]. (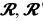 )-4-methoxy-1-naphthylfenoterol reduces HepG2 and PANC-1 tumor cell migratory capacity through actions upon GPR55 [66].
)-4-methoxy-1-naphthylfenoterol reduces HepG2 and PANC-1 tumor cell migratory capacity through actions upon GPR55 [66].
Treatment with the β adrenergic agonist isoproterenol dose-dependently enhances U251MG glioblastoma cellular proliferation by promoting the phosphorylation and enzymatic activity of ERK1/2 in vitro [67]. Norepinephrine reduces cellular proliferation and uptake of l-arginine in rat glial cells [68] and 1,25-dihydroxycholecalciferol-induced apoptosis of glioma cells in vitro [69]. The bitopic compound (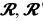 )-fenoterol inhibits proliferation of, and reduces l-arginine uptake in, N1321 astrocytoma and U118 glioblastoma cells [9]. Stimulation of purinergic receptor (P2Y12) modulated signaling inhibits cyclic AMP from tonically inhibiting protein kinase B, which in turn tonically restricts C6 glioma cells from undergoing differentiation [70]. Thus, we may, by extension, consider promoting the enzymatic catalytic activity of adenylate cyclase enhances the synthesis of cyclic adenosine monophosphate and restricts protein kinase B from tonically inhibiting proliferation of C6 glioma cells. Similarly, phosphatidylinositol-3-kinase (PI3K) mediated enhancement of cyclic AMP synthesis would concurrently promote cellular differentiation [70]. Though our best understanding of molecular pathways converging upon, and diverging through, protein kinase A, would lead us to surmise enhanced levels of intracellular cyclic adenosine monophosphate and activity of ERK1/2 (i.e., MAPK) signaling correlates with enhanced cellular proliferation and reduced levels correlate with the converse complementary set of effects, Kurino et al. paradoxically demonstrated C6 glioma cells experience paradoxical inhibition of MAPK by growth factor-mediated upregulation of cyclic AMP several decades ago [71].
)-fenoterol inhibits proliferation of, and reduces l-arginine uptake in, N1321 astrocytoma and U118 glioblastoma cells [9]. Stimulation of purinergic receptor (P2Y12) modulated signaling inhibits cyclic AMP from tonically inhibiting protein kinase B, which in turn tonically restricts C6 glioma cells from undergoing differentiation [70]. Thus, we may, by extension, consider promoting the enzymatic catalytic activity of adenylate cyclase enhances the synthesis of cyclic adenosine monophosphate and restricts protein kinase B from tonically inhibiting proliferation of C6 glioma cells. Similarly, phosphatidylinositol-3-kinase (PI3K) mediated enhancement of cyclic AMP synthesis would concurrently promote cellular differentiation [70]. Though our best understanding of molecular pathways converging upon, and diverging through, protein kinase A, would lead us to surmise enhanced levels of intracellular cyclic adenosine monophosphate and activity of ERK1/2 (i.e., MAPK) signaling correlates with enhanced cellular proliferation and reduced levels correlate with the converse complementary set of effects, Kurino et al. paradoxically demonstrated C6 glioma cells experience paradoxical inhibition of MAPK by growth factor-mediated upregulation of cyclic AMP several decades ago [71].
Carvedilol exerts a pleiotropic set of effects upon C6 glioma cells in vitro, enhancing the proportional fraction of cells in the soi-disant S and G2 phases at 24 h and the proportional fraction of cells in the G0 and G1 phases at 72 h [72]. These differential dynamics are consistent with initial promotion of β adrenergic receptor modulated signaling, enhancement of the catalytic enzymatic activity of adenylate cyclase, and increased cyclic adenosine monophosphate levels, protein kinase A activity, and extracellular regulated kinase 1/2 mediated phosphorylation of target nuclear proteins, enhancing cellular proliferation, followed by β adrenergic receptor desensitization of ligand effector coupling, reducing cellular proliferation [72]. Coadministration of carvedilol enhanced imatinib-induced cellular apoptosis (5% and 2% at 24 h and 72 h in a monolayer of C6 glioma cells), mitochondrial lysis, and retention of P-glycoprotein inhibitor [72]. Treatment with the bitopic βagonist GPR55 antagonist (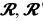 )-MNF reduces cellular proliferation (by inducing G1 cell cycle arrest), cell motility, phosphorylation of molecular substrates of protein kinase A, and activity through ERK1/2 and Akt pathways. High concentrations of (
)-MNF reduces cellular proliferation (by inducing G1 cell cycle arrest), cell motility, phosphorylation of molecular substrates of protein kinase A, and activity through ERK1/2 and Akt pathways. High concentrations of (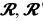 )-MNF reduces glioma cell motility [72].
)-MNF reduces glioma cell motility [72].
In seeking to evaluate the effects of promoting βAR modulated signaling upon the behavior of gliomas in vivo, Yoshida et al. generated extra-neuraxial models of glioma and meningeal gliomatosis by subcutaneously implanting C6 glioma cells [74]. Treatment with the β1 and β2 adrenergic receptor agonist isoproterenol, which may elicit cellular pro-proliferative effects through the activation of adenylate cyclase-cyclic AMP-protein kinase A-ERK1/2 signaling in vitro, paradoxically reduced tumor growth and improved animal survival in vivo [74]. These effects were synergistically enhanced by treatment with the phosphodiesterase inhibitor papaverine, implicating cyclic AMP mediates the effects generated by β agonists [74]. Isoproterenol was shown to attenuate C6 glioma cellular proliferation in vitro, an effect synergistically promoted by inhibition of the enzymatic degradative activity of phosphodiesterase by papaverine [75]. The findings collectively indicate βAR modulation may reduce growth of gliomas in human patients.
Differential effects mediated by β adrenergic agonists, and the congruent effects paradoxically mediated by pharmacological antagonists of β adrenergic receptor modulated signaling, upon non-malignantly transformed and glioma cellular proliferation may result from differential activation of downstream intracellular signal transduction pathways promoted by agonist ligand binding to, and/or desensitization of βAR and phospho-βAR-β arrestin scaffold assembly [6, 72, 76–80]. Stimulation of βAR stimulates the AC-cAMP-PKA-ERK1/2 pathway, effectively promoting cellular proliferation [81]. However, sustained βAR activation generates receptor desensitization, clathrin coated pit mediated receptor endocytosis and internalization, parallel increases of cytosolic calcium concentrations, and upregulation of the synthesis of phosphodiesterase enzyme [13]. The effects collectively attenuate the adenylate cyclase-cAMP-PKA pathways, preferentially promote scaffold facilitated activation of ERK1/2 rather than PKA mediated phosphorylative activation, reducing nuclear translocation and pro-transcriptional effects augmenting cellular proliferation, and amplifying the enzymatic cleavage capacity of phosphodiesterase to reduce cyclic AMP levels [11, 12]. Recent work conducted by O’Hayre et al. indicates β2AR-mediated activation of ERK absolutely requires β arrestins [82].
Intracellular effects of β adrenergic signaling in glioma models
βAR agonists enhance C6 glioma cellular proliferation and motility by promoting PKA and ERK1/2 signaling [83], which we believe to represent the chief and most likely direct effect of appropriately augmenting β adrenergic receptor modulated signaling. β antagonists reduce glioma cellular proliferation by inducing glioma cell cycle arrest and attenuate cyclic AMP mediated activation of ERK1/2 [6, 72, 77, 79, 80]. Differential and divergent effects mediated by β2 adrenergic receptor stimulation in vitro could be attributed to alternate coupling to either or both Gs or Gi proteins [84]. Gαs protein activates, and Gαi protein inhibits, the enzymatic activity of adenylate cyclase. Transfection of with Go1 alpha protein complementary DNA reduced isoproterenol- (βAR agonist) and forskolin (adenylate cyclase activator)-mediated enhancement of cytosolic increases of cyclic adenosine monophosphate and isoproterenol mediated transient increases of cytosolic calcium and calcium mediated enhancement of cytosolic accumulation of cyclic adenosine monophosphate [83]. (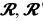 )-MNF activates either or both Gs or Gi coupled β2 ARs, whereas (
)-MNF activates either or both Gs or Gi coupled β2 ARs, whereas (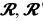 )-Fen selectively enhances the activity of Gαs-coupled β2 ARs [65, 73, 85, 86]. These properties of the bitopic compounds fenoterol stereoisomers (
)-Fen selectively enhances the activity of Gαs-coupled β2 ARs [65, 73, 85, 86]. These properties of the bitopic compounds fenoterol stereoisomers (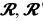 )-MNF and (
)-MNF and (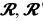 )-Fen cause these agents to mediate more effects upon cellular proliferation and dynamic behavior compared with pure β adrenergic agonists (Fig. 5).
)-Fen cause these agents to mediate more effects upon cellular proliferation and dynamic behavior compared with pure β adrenergic agonists (Fig. 5).
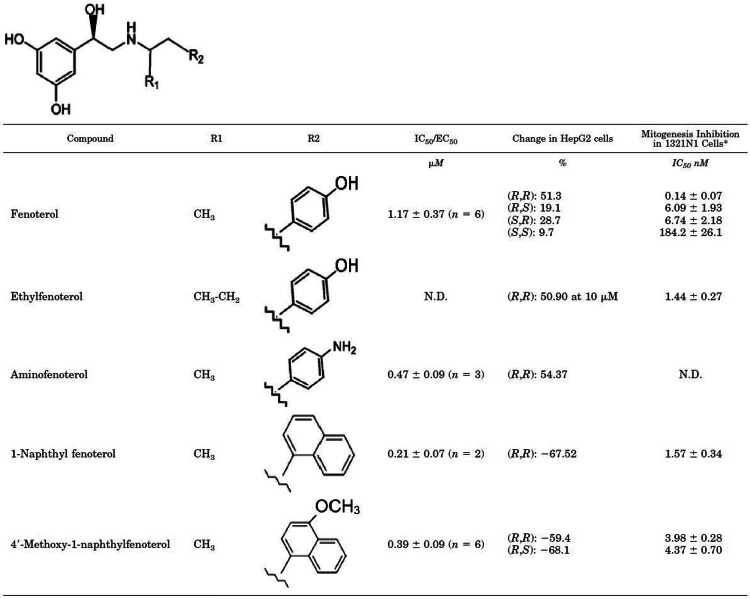
Fig. 5.
Fenoterol structure, chemical activity, and biological actions. Fenoterols represent ideal candidate molecular structures which could be chemically modified in order to optimize agonist potency and generate specific beta adrenergic receptor conformations conducive of tighter binding of β arrestins. The fenoterol core structure consists of a bisubstituted meta dihydroxphenyl moiety and an ethanolamine side chain. The side chain attachments include a methyl or ethyl group in the R1 position and various ring form modifications of substituted (hydroxy, amino, methoxy) benzyl or naphthyl rings. IC50/EC50 ratios are inversely proportional to potency of inhibition of tritiated thymidine incorporation, a measure of DNA synthesis and thus cellular proliferative capacity. Lower ratios between the IC50 and EC50 correlate with lower concentrations of drug necessary to attenuates rates of synthetic thymidine incorporation into DNA. These fenoterol derivates effect potent inhibition of cellular proliferative capacity and effect cellular apoptosis. Different fenoterol stereoisomers generate differential percentage changes of HepG2 cells and inhibition of 1321 N astrocytoma cell mitogenic capacity, as measured by tritiated thymidine incorporation. The α carbon and γ amine groups represent steroisomerically active centers [51, 65, 66, 73, 85, 86]. Reprinted with permission from Paul et al. [51]
Gi protein-coupled receptors (e.g., GABAB, opioid, cannabinoid, α2 adrenergic) commonly converge on attenuating the enzymatic activity of adenylate cyclase, blunting the generation of cyclic AMP and reducing cyclic AMP-mediated enhancement of cellular proliferation, invasion, and metastasis [87, 88]. Cross-talk between βAR with Gi protein-coupled receptors may contribute to differential effects mediated by β adrenergic receptor modulated signaling. For example, ligand activation of GABAB receptors inhibits isoproterenol-mediated enhancement of pancreatic cancer cell proliferation [89], providing evidence indicating a critical importance of crosstalk amongst β adrenergic and the complement constituents of the family of G protein-coupled receptors. Crossmodal modulation of cell surface receptor activation, desensitization, and scaffold-mediated effects may critically contribute to differential effects generated by alternate stable or transient ligand binding of pharmacological agonists or antagonists to βARs in different tumor cell lines [88]. The described effects may also explain β adrenergic agonist and antagonist-mediated attenuation of glioma tumor cell migration [72, 79] and enhance drug sensitivity to imatinib [72].
Mechanisms underlying desensitization of β adrenergic receptor modulated signaling in glioma cell lines
Continuous β adrenergic receptor agonist stimulation desensitizes ligand binding-effector coupling, promotes clathrin-coated pit mediated receptor cytosolic internalization, and downregulates nascent messenger ribonucleic acid (RNA) transcripts in non-malignantly-transformed astrocytes [13] and glioma cell lines [90]. β adrenergic receptor kinase phosphorylates βAR carboxyl terminus amino acid moieties, to which β arrestin binds, coordinately reducing the efficacy of ligand binding-effector coupling [13], reduces βAR-mediated cytosolic calcium rises, and preferentially attenuating cAMP-PKA facilitated activation of ERK1/2 [15] and favoring βAR-β arrestin scaffold facilitated ERK1/2 activation [15]. Scaffold-mediated activation of ERK1/2 favors cytosolic retention and attenuates nuclear translocation and pro-transcriptional activity, preserving the housekeeping homeostatic function of ERK1/2 while preventing its promotion of cellular proliferation (Figs. 3, 4). Elevations of cytosolic calcium effectively attenuate βAR stimulation- and adenylate cyclase stimulation- (forskolin) mediated enhancement of cytosolic cyclic AMP concentration in a C62B glioma model in vitro [91], perhaps by promoting the de novo synthesis of phosphodiesterase [44], prevented by treatment with the RNA polymerase II inhibitor α-amanitin.
Isoproterenol βAR stimulation mediated cyclic AMP rises downregulate βAR messenger RNA transcription (and enhance phosphodiesterase synthesis [42]), inhibited by treatment with colchicine, though unaltered by the microtubule depolymerization inhibitor taxol-mediated enhancement of cytosolic concentrations of cyclic AMP [92]. A cyclic AMP response element (CRE) nested within DNA encoding the βAR subjects the gene to modulation by cyclic AMP concentrations. Treatment with the myelosuppressive non-neuropathic microtubule synthesis inhibitor vinblastine at doses insufficient to modulate protein synthesis prevents isoproterenol mediated enhancement of phosphodiesterase synthesis, though fails to prevent β agonist-mediated upregulation of nerve growth factor [42]. Crossmodal modulation between molecular compounds modulating polymerization and depolymerization of microtubules and βAR modulated signaling may be critically implicated in glioma initiation, promotion, progression, invasion, and metastasis [93]. NG 108-15 rat neuroblastoma cells express βARK isotypes 1 and 2 mRNA and exhibit Gβγ-dependent phosphorylation of rhodopsin and agonist-bound delta opioid receptor, recapitulating effects mediated by βAR activation in non-transformed cells [94, 95]. Glioma cells may exhibit differential kinetics of βAR desensitization compared with non-malignantly-transformed cells. C6 glioma cells undergoing comparatively fewer cycles of replication exhibit enhanced βAR ligand binding-effector coupling, evidenced by comparatively greater rises of cytosolic cAMP and calcium in response to treatment with the nonselective βagonist isoproterenol [96]; C6 glioma neoplastic astrocytes having undergone cellular senescence effectively amplify cyclic AMP levels in response to stimulation of βAR modulated signaling only in the presence of a pharmacological inhibitors of phosphodiesterase [96].
βAR activation conformationally modifies rat-derived C6 glioma cellular phenotype from fibroblastic to astrocytic [97], presumably via cyclic AMP mediated effects upon the state and dynamics of the cytoskeleton, effects potently inhibited in the presence of serum containing lysophosphatidic acid in a GTP-binding protein-dependent manner [97]. Enhancement of cytosolic calcium concentrations by treatment with thrombin reverts cellular morphology from astrocytic- to epithelial-like [98], presumably via calcium-mediated downregulation of βAR-mediated enhancement of cytosolic concentrations of cyclic AMP. Treatment with the direct thrombin inhibitor hirudin, but not with antithrombin III [98], inhibited βAR activation mediated cellular morphological transformation. Thrombin effects upon cellular morphology are likely mediated through activation of cell surface platelet activated receptors (PARs). The experimental findings collectively indicate β adrenergic receptor agonists and thrombin coordinately converge on modulating intracellular signal transduction pathways affecting dynamic microtubular architecture by modulating cyclic AMP levels through ligand binding mediated effector coupling of allosterically activated membrane surface receptors [97, 98]. Pharmacological antagonism of the mGlu3 receptor attenuates glioma cellular proliferation and enhances transformation of glioma cells from a fibroblastic to an astrocytic phenotype [55]. The described behavior may play a critical role in invasion and metastasis of cerebral glioma cells through crossmodal modulation amongst and between Gs and Gi protein coupled receptors [55].
Modulation of matrix metalloproteinase expression by β adrenergic signaling
The apical inter-endothelial tight junction-coupled basement membrane (BM), glycosaminoglycan- and protein-rich extracellular matrix (ECM), and blood brain barrier (BBB) collectively constitute initially formidable obstacles to tumor cell invasion, dissemination, metastasis, and distant implantation [99–101]. Matrix metalloproteinases (MMPs) modulate cellular proliferative capacity, cellular migration, and neoangiogenesis and enhance glioma cell capacity to invade and metastasize by enzymatically degrading the basement membrane and extracellular matrix [6]. MMP-2 and MMP-9 represent the predominantly extracellularly-liberated isoforms implicated in enhancing invasion and metastasis by glioma cells [102]. Human brain microvascular endothelial cells (HBMECs) maintain the microarchitectural integrity of the blood brain barrier [103]. Treatment of HBMECs grown on collagen I, collagen IV, fibronectin, laminin, or hyaluronic acid with cyclic AMP supplements enhances microarchitectural junctional continuity and expression of zona occludin 1, VE-cadherin, and claudin 5 [103]. Inhibition of MMP-9 effectively forestalls HBMEC neoangiogenesis [104], invasiveness [104], and metastasis [105] in vitro. Treatment of rat C6 glioma cells with eugenol encapsulated chitosan nanopolymers reduces tumor cell metastatic potential by reducing the expression of MMP-9 [105]. Tissue hypoxia may promote the expression and proteolytic enzymatic activity of MMP, effects which could conceivably contribute to potentiating BBB disruption in hypoxic regions of glioma tumor masses [106]. Thus, enhancing cerebral blood flow via spinal cord stimulation in patients harboring intracranial gliomas [46] may reduce tumor invasive potential by reducing hypoxia-induced augmentation of MMP secretion [46].
A host of membrane receptor tyrosine kinases (RTKs) and G protein-coupled receptors (GPCRs) [67] and membrane bound ectodomain proteolytic metalloproteinases (e.g., ADAM17; 34,110] regulate the expression and/or degradative enzymatic activity of matrix metalloproteases in non-malignantly-transformed astrocytes, human brain microvascular endothelial cells [6], and neoplastically-transformed astroglia, effects coordinately or alternately facilitated via ERK1/2 [67] and/or epidermal growth factor receptor (EGFR)-PI3K-serine-threonine kinase signaling [107] Specifically, pharmacological antagonism of βAR modulated signaling attenuates the expression of MMP-2 and MMP-9 in HBMECs [6] and reduces MMP-9 expression in tumors treated with the tumor promoting agent phorbol 12-myristate 13-acetate [108]. Norepinephrine enhances the activity and/or expression of MMP-9 and VEGF in HONE-1, HNE-1, and CNE-1 nasopharyngeal carcinoma cells [74] and metastasis in PC3 prostate carcinoma cells [60]. Treatment with propranolol reduces norepinephrine and stress-induced conferring of metastatic potential upon EG, SKOV3, and 222 ovarian carcinoma cells [56]. Concurrent inhibition of βAR modulated signaling and cyclooxygenase 2 significantly reduces the risk of metastasis and generates potent immunomodulatory effects [109]. HuR protein, overexpressed in cancers, stabilizes the MMP-9 mRNA transcript [6]. Propranolol attenuates the expression of MMP-9 (but not MMP-2] and generates cytosolic retention of HuR, reducing stability of the MMP-9 transcript [6]. HuR expression may also be suppressed via the green tea polyphenol epigallocatechin gallate and the isothiocyanate sulforaphane, effects exploitable therapeutically in the adjuvant treatment of carcinomas, by forestalling angiogenesis, invasive potential, and metastasis [6, 110].
Since hypoxia enhances glioma cell invasion through the upregulation of MMP-2 and MMP-9 in human and rat models in vitro and in xenograft models in vivo, there may exist cross-pathway communication between βAR modulated signaling, AC/cAMP/PKA, EGFR/PI3K/Akt, PTEN, mTOR, and VEGF pathways [111]. We detail a subset of the findings relevant to the emergent acquisition of an integrated and cohesive conceptual framework from which to understand the crossmodal interactions of these pathways by, and satisfaction of, the distinguished reader [111]. Hypoxia [1% O2] upregulates the expression of HIF-1α, MMP-2, and MMP-9 downregulated expression of TIMP-1 in U87MG, U251MG, U373MG, and LN18 human glioma cell lines related to normoxic [21% O2] conditions [111]. Treatment with HIF-1α small interfering ribonucleic acid (siRNA) reduced expression of HIF-1α, MMP-2, and MMP-9 and blunted tumor cell invasion in glioma spheroids co-cultured with rat-derived brain slices; the magnitude of these effects was preferentially amplified under normoxic conditions (1%) [111]. The results collectively indicate hypoxia enhances glioma tumor migration and invasive potential by upregulating the expression of MMP-2 and MMP-9 in a HIF-1α-dependent manner [111]. Tumor necrosis factor α-converting enzyme/a disintegrin and metalloproteinase 17, colloquially termed ADAM17 amongst molecular oncologists, proteolytically sheds phospholipid membrane bilayer-bound receptor, growth factor, and cytokine ectodomains [107].
Hypoxia upregulates the expression of ADAM17, activity of which correlates with 9L rat gliosarcoma and human U87 human glioma cell invasive potential, via EGFR-phosphatidylinositol-3-kinase-serine threonine kinase signaling, though independently of MMP-2 and MMP-9 levels [107]. Protease inhibitor-mediated attenuation of ADAM17 proteolytic enzymatic activity or siRNA mediated downregulation of ADAM17 expression reduces hypoxia-mediated enhancement of 9L rat gliosarcoma and U87 human glioma cell invasiveness [107]. Molecular inhibition of the mammalian target of rapamycin induced G1 cell cycle arrest, reduced synthesis of VEGF, and downregulated the expression of MMP-2 and/or MMP-9 in PTEN (phosphatase and tensin homolog deleted from chromosome 10)-null U87MG and D54 human glioma cells, but not PTEN-null HOG oligodendroglioma cells [77]. Treatment of U87 xenografts in vivo induces glioma regression, presumably indicating cellular apoptosis, reduces tumoral VEGF expression, and blunts the expression of MMP-2 [77]. Treatment with fentanyl reduces cellular proliferation, migration, and invasion of gastric cancer MGC-803 cells in vitro, attenuates PI3K/Akt signaling, reduces expression of MMP-9, and enhances expression of the pro-apoptotic proteins caspase-9 and death-associated protein kinase 1 (DAPK1) [105], the latter pair of effects synergistically enhanced by treatment with the PI3K molecular inhibitor LY294002 and MMP-9 molecular inhibitor SB-3CT. Accordingly, pharmacological modulation of β adrenergic receptor modulated signaling may be exploited to blunt tumor cell invasion by reducing MMP expression levels in human intracranial (e.g., glioma, glioblastoma, gliosarcoma) and extra-neuraxial (e.g., melanoma, breast cancer, gastric cancer, pancreatic cancer, colorectal cancer, prostate cancer, ovarian cancer) carcinomas and sarcomas. These effects may be synergistically enhanced by coordinately administering βAR modulators with mTOR inhibitors, HIF-1α pathway modulators, the serine protease inhibitor and tryptase inhibitor nafamostat mesylate, conventional cytotoxic chemotherapy, monoclonal antibodies to tumor-specific growth factor receptors, tumor-specific cytotoxic CD3+ CD8+ T cells.
Modulation of angiogenesis by β adrenergic signaling
Cerebral, brainstem, and cerebellar gliomas exhibit heterogeneous arteriolar density [112]. Tumor neoangiogenesis promotes glioma growth, promotion, progression, invasion, and metastasis of gliomas [6, 76] and extra-neuraxial [113, 114] carcinomas, subject to modulation by β adrenergic receptor modulated signaling. Treatment with norepinephrine [115] or dopamine [116] and stress promote angiogenesis in ovarian carcinomas by potentiating βAR mediated attenuation of PPARγ signaling and thus disinhibiting the synthesis of VEGF and FGF2, molecular behavior putatively extending to cerebral gliomas [116]. Reciprocally, pharmacological antagonist of β adrenergic receptor modulated signaling specifically forestalls incipient endothelial tubulogenesis and emergent angiogenesis, sans altering cell viability or migratory capacity, by reducing the expression of matrix metalloproteases in HBMECs in vitro [6]. Chronic stress attenuates PPARγ-mediated signaling via upregulating activity through β adrenergic receptor modulated pathways, effectively disinhibiting the synthesis of VEGF and FGF2 and precluding angiogenesis in models of ovarian carcinoma, a set of effects attenuated through the use of pioglitazone [113]. To this end, pediatricians now commonly espouse the use of propranolol to effect involution of the vascular endothelium in infants harboring benign hemangiomas [6]. The revealed set of molecular effects may be exploited to therapeutic benefit to generate marked reductions in glioma [6, 76] and extra-neuraxial [113, 114] hypervascular carcinoma growth potential, invasiveness, and angiogenesis. The effects of anti-angiogenic compounds are characteristically amplified in the presence of ionizing radiation [117].
Immunomodulation by β adrenergic receptor modulated signaling
Immune effector responses mediating homeostatic antimicrobial and tumor cell surveillance and those contributing to the pathogenesis of neurodegenerative diseases, may occur within parenchyma contained within both the cranial cavity and vertebral column, alternately or coordinately recruiting innate and/or adaptive (cellular and humoral effector arms) mechanisms [118–120]. Major histocompatibility (MHC) class II (dimer; each monomer constituted by α and βdomains)-complexed non-native glycoprotein antigen fragments (endocytosed and processed by antigen presenting cells [macrophages, dendritic cells, B cells]) are presented to effector CD3+ CD4+ helper T cells and MHC class I (α1, α2, β1, β2-microglobulin domains)-complexed non-native glycoprotein antigen fragments (endogenously synthesized and modified by any cell type except nucleate spermatozoa and anucleate erythrocytes) are presented to CD3+ CD8+ cytotoxic T cells [120], constituting cell-mediated immunity. B cell generated immunoglobulins, antigen-potentiated immunoglobulin class isotype switching, and antigen-dependent maintenance of clonal plasma cell populations generating functional antibody against nonnative antigens constitutes ‘humoral’ immunity [120]. Immune effector mechanisms surveille and eradicate incipiently transformed neoplastic tumor seed cells. CD3+ CD8+ and natural killer (NK) cells eradicate mutationally transformed cells generating MHC I-complexed tumor-specific antigens via cytotoxic CD3+ CD8+ T cells, effectively preventing the progression and promotion of carcinogenically-mutated cells [120]. Abnormalities of these mechanisms could contribute to tumor initiation, promotion, and progression [121–123]. MHC II-bearing immunologically active astroglia and/or microglia abundantly populate malignant cerebral, brainstem, cerebellar, and spinal cord glioblastomas and astrocytomas [124]. Accordingly, brain microglial MHC class II expression antigen-specifically enhances immune responses within neural tissue [124], offering a set of therapeutic targets by which to eradicate glioma cells by enhancing intrinsic antitumor response mechanisms [125]. MHC class II cell surface proteins may be found complexed with endocytosed- and endogenously-modified non-native antigens and are expressed in macrophages, plasma cells, and dendritic cells [119]. These antigen presenting cells interact with Th1 and Th2 subtypes of CD3+ CD4+ T cell effector arms and mediate differential host immune responses [118].
βAR modulated signaling and downstream target pathways play critical immunomodulatory roles by regulating MHC class II expression human glioma cell lines [124]. In differentiated U-373-MG, U-105-MG, and D-54-MG glioblastoma cells, treatment with the βAR agonist isoproterenol (1 × 10–6 to 5 × 10–6 M), adenylate cyclase activator forskolin, or cyclic AMP analogue deoxybromo-cyclic AMP (DBcAMP), enhance membrane cell surface expression of MHC class II DR molecules, effects generally mediated by enhanced synthesis of transcriptionally-nascent messenger ribonucleic acid transcripts [124]. For example, treatment with norepinephrine and isoproterenol upregulate MHC class II cell surface expression in U-373-MG differentiated glioblastoma cells [124]. Treatment with isoproterenol enhances expression of MHC class II in U-373-MG cells to a greater extent compared with norepinephrine, given concurrent selective stimulation of βAR by the former and concurrent stimulation of β and α adrenergic receptors by the latter. IFN-γ enhances MHC class II expression in U-105-MG (1.5-fold increase) and D-54-MG (2.5-fold increase) glioblastoma cell lines to a greater extent compared with the upregulation of MHC class II synthesis elicited by IFN-γ in U-373-MG cells [124]. Treatment with IFN-γ coordinately enhances neuroblastoma membrane cell surface expression of MHC class I β2-microglobulin complexed tetradomain multimers, an effect not generated by treatment with DBcAMP [126]. Treatment with the decarboxylated [3,4-DOPA decarboxylase; cofactor biotin) hydroxylated (dopamine-β-hydroxylase; cofactor tetrahydrobiopterin) 3,4-dihydroxphenylalanine catecholamine derivative norepinephrine prevents IFN-γ mediated enhancement of MHC class II cell surface expression [127]. The finding perhaps collectively indicates norepinephrine- and IFNγ-mediated enhancement of MHC class II expression share a common and overlapping downstream set of mediators, likely converging upon, and diverging through, cyclic AMP and protein kinase A. Thus, β adrenergic agonists and interferon-γ may generate therapeutically exploitable immunomodulatory effects in treating gliomas by upregulating cellular mediated gliomatotoxic immune responses through adenylate cyclase-cAMP-protein kinase A-dependent upregulation of membrane cell surface expression of MHC class II complexed-tumoral antigens and thus putatively represent effective adjuvants which may enhance the effects of tumor therapies enhancing host immune mechanisms (tumor antigen-specific antibodies, CD3+ CD8+ cytotoxic T cells, and NK cells) curtailing proliferation, angiogenesis, invasion, and metastasis of glioma cells. We present the caveat that treatment with neither isoproterenol nor forskolin upregulated DRα gene expression in HL-60 promyelocytic leukemic cells [128], evidencing possible heterogeneity of the effect according to specific tumor cell type or inter-experimental differences.
Treatment with βAR agonists or TNF-α promotes proliferation of C6 glioma cells in vitro, with the latter coordinately upregulating βAR cell surface density via βAR-dependent and PKC-mediated signaling [124], effects indicating crossmodal interaction between βAR signaling and molecular immune mediators. The findings of Lung et al. collectively indicate TNF promotes proliferation of C6 glioma cells through β adrenergic receptor activation [39]. The secreted pro-inflammatory protein cytokine tumor necrosis factor α (TNF-α), synthesized and elaborated by macrophages and microglia, binds membrane cell surface receptors possessing intracellular receptor tyrosine kinase activity and potentiates and mediates a spectrum of effects on cellular genetic transcription and tissue physiology. TNF-α enhances macrophage synthesis of IL-1, hypothalamic synthesis of prostaglandins and pyrogen proteins, hepatically-synthesized acute phase reactants (IL-6, mannose binding protein), vascular endothelial expression of inter-endothelial cellular adhesion- and vascular cellular adhesion molecule-1 and synergistically potentiate adaptive immune effector and memory mechanisms. TNF-α amplifies pyrogenic signaling in hypothalamic nuclei by raising the thermic set point, enhancing equilibria of biochemical metabolism, promoting non-shivering thermogenesis, and augmenting innate and adaptive immune responses, effects we suggest potentiate host immune mediated eradication of malignantly-transformed tumor cells.
β agonists synergistically enhance, diminish, or fail to alter TNF-mediated upregulation of proteins (see Table 1 of [129]). Specifically, isoproterenol was shown to synergistically enhance TNF-mediated upregulation of A20 and IL-6, attenuates TNF-mediated downregulation of LEF1, with a non-statistically significant tendency towards blunting TNF-mediated upregulation of ICAM-1 and VCAM-1 in cultured astrocytes [129]. The biological mechanisms upon which these effects are predicated, investigated in the context of glioma, may be extended to rational therapeutic design of medications designed to treat systemic inflammatory response syndrome, sepsis, severe sepsis, septic shock, and multiorgan dysfunction syndrome [129]. As an aside, βagonists enhance the synthesis of alveolar surfactant and compliance of the pulmonary parenchyma, a therapeutically exploitable corollary effect of βagonists upon pulmonary mechanics [130]. In the author’s anecdotal experience in the critical care unit, maintaining a very low dose of norepinephrine [1–2 μg/kg/min) seems to correlate with improved metrics of tissue oxygenation (oxygenation index; PaO2:FIO2 ratio) in patients experiencing severe acute lung injury occurring in the context of septic shock.
Clinical relevance
Johansen et al. describe a retrospective series of 218 patients unfortunately afflicted with glioblastoma, all of whom received the anti-VEGF monoclonal antibody bevacizumab (most common adverse effects: arterial hypertension, bleeding diathesis, delayed wound healing) and alternately received β antagonists or placebo [61]. Inclusion of β antagonists in therapeutic regimens yielded no enhancement of survival. Retrospectivity and non-randomization of patients receiving βantagonist treatment and comparison groups limits the study [61]. A study evaluating the utility of β antagonists excluding bevacizumab in patients with newly diagnosed low and high grade glioma sans multifocal disease or extra-neuraxial metastases may effectively unveil whether the observed effects are chiefly attributable to reducing angiogenesis [61]. β adrenergic receptor blockade significantly improves clinical outcomes and survival in patients harboring breast, ovarian, and prostate carcinoma and melanoma [131]. These agents reduce the risk of developing prostate carcinoma [132] and hepatocellular carcinoma in patients infected with hepatitis C [133] and prolong survival in patients with breast cancer [134].
Drug development
Malignant potential of glioma cells depends critically on their capacity to transgress through the basement membrane [135, 136], migrate through the extracellular matrix [137], reach and enter proximally located microvasculature, travel to distant sites [138], exit the microvasculature, and implant and grow in distant microenvironments [139]. Neoangiogenesis induced by protein factors released from glioma cells contributes to sustaining tumoral growth [140]. Evasion of immune responses by downregulation of cell surface expression of tumor specific antigens and negative immunomodulators contributes to immune evasion by glioma cells [125]. In this regard, β adrenergic signaling multi-mechanistically modulates immune mechanisms [124], local tumoral angiogenesis [6], and processes contributing to invasion and metastasis by neoplastic tumors [139]. Modulation of β adrenergic receptor modulated signaling by various compounds may thus be exploited to enhance immune responses to tumor, by increasing the cell surface expression of tumor specific antigens complexed with MHC class II homodimers [124] and thus promote antigen-specific tumor responses [124], inhibiting tumoral angiogenesis [6] and thus blunting the capacity for tumoral growth, and downregulate the expression and secretion of extracellular matrix degrading matrix metalloproteinases [6].
Fenoterols represent useful candidate molecular compounds which may be chemically modified in order to optimize agonist potency and generate specific β adrenergic receptor conformations favoring β arrestin binding [65, 73]. Typical agonists or bitopic agonist-antagonists, such as (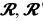 )-MNF, exhibiting contemporaneous effects on GPR55 signaling, may exert cytostatic effects proving therapeutically beneficial in the adjuvant treatment of gliomas and extra-neuraxial malignancies [65]. Reinartz et al. identified the (
)-MNF, exhibiting contemporaneous effects on GPR55 signaling, may exert cytostatic effects proving therapeutically beneficial in the adjuvant treatment of gliomas and extra-neuraxial malignancies [65]. Reinartz et al. identified the ( ), as well as the (
), as well as the ( )-stereoisomers of the bitopic agent 4′-methoxy-1-naphthyl-fenoterol to exhibit preferential binding to βARs coupling to Gs protein [86]. Since these ligands preferentially favored G protein-mediated signaling in response to βAR activation, disfavoring phosphorylation of the carboxyl terminal of the βAR and β arrestin binding, these agents represent a unique set of βagonists to which desensitization develops slowly, and may be exploited therapeutically in the treatment of common medical conditions in lieu of classically utilized βagonists, postulates subjectable to rigorous empirical interrogation. The specific stereoisomeric conformation of fenoterol derivatives and composition of the aminoalkyl moiety dictates binding affinity to β2 adrenoceptor-Gsα fusion proteins [85]. The efforts of medicinal chemists to further modify these agents will arm us with the capacity to develop compounds uniquely and preferentially generating carboxyl terminal βARK-phosphorylated βAR-β arrestin complexes preferentially favoring scaffold-mediated ERK1/2 activation [11, 12].
)-stereoisomers of the bitopic agent 4′-methoxy-1-naphthyl-fenoterol to exhibit preferential binding to βARs coupling to Gs protein [86]. Since these ligands preferentially favored G protein-mediated signaling in response to βAR activation, disfavoring phosphorylation of the carboxyl terminal of the βAR and β arrestin binding, these agents represent a unique set of βagonists to which desensitization develops slowly, and may be exploited therapeutically in the treatment of common medical conditions in lieu of classically utilized βagonists, postulates subjectable to rigorous empirical interrogation. The specific stereoisomeric conformation of fenoterol derivatives and composition of the aminoalkyl moiety dictates binding affinity to β2 adrenoceptor-Gsα fusion proteins [85]. The efforts of medicinal chemists to further modify these agents will arm us with the capacity to develop compounds uniquely and preferentially generating carboxyl terminal βARK-phosphorylated βAR-β arrestin complexes preferentially favoring scaffold-mediated ERK1/2 activation [11, 12].
For whatever reason, our instinctual faculties lead us to believe developing pharmaco-molecular switches favoring βAR-β arrestin scaffold facilitated activation of ERK1/2 may represent a pleiotropically effective panacea in the treatment of gliomas and extra-neuraxial carcinomas: the cytosolic homeostatic functions mediated by ERK1/2 are preserved, eschewing physiological compromise of metabolically active epithelia, with concurrent blunting of its nuclear pro-transriptional activity, representing the most empirically plausible anti-carcinogenic therapeutic mechanism [11, 12, 85, 86]. The prudent modulation of βAR modulated signaling, putatively employing combinatorial therapeutic strategies exploiting bitopic fenoterol derivative compounds and nafamostat mesylate, may effectively blunt the progression of macular degeneration and retino-degenerative diseases [85, 86]. Molecular pharmacological enhancers or inhibitors of protein machinery contributing to desensitization of β adrenergic receptors and modulators of the scaffold promoted effects of distal signal transduction pathways of β adrenergic receptor may generate potent antitumoral effects [141, 142]. Studies have thoroughly demonstrated and elucidated the structural conformations of cyto-transductively active and inactive conformations of the β adrenergic receptor [13, 14, 16, 81]. This information may be exploited in order to genetically engineer chimeric β adrenergic receptor constructs, for example, exhibiting more stable binding dynamics with β arrestin, thus promoting scaffold-promoted effects of the G protein-coupled receptor β arrestin [13, 85, 86], including cytosolic retention of activated ERK1/2 and inhibition of its nuclear translocation, thus preventing cellular proliferation consequent to enhanced transcriptional activity [11, 12]. Precedence for these effects was shown by Tohgo et al., who generated chimeric constructs of the vasopressin receptor by replacing its native carboxyl terminal amino acid sequence with that of the carboxyl terminal end of the β adrenergic receptor [9]. Further studies utilizing targeted genetic mutations of the carboxyl terminal chain of amino acid residues of the β adrenergic receptor and amino terminal chain of amino acid residues of the β arrestin protein may enhance our capacity to generate genetically-modified stable constructs promoting scaffold-mediated activation of ERK1/2, chimeric constructs transfectable utilizing adenoviral vectors [11, 12].
β arrestin binds βARK-phosphorylated β adrenergic receptor carboxyl terminal amino acid moieties [14]. The Gβγ subunit of the Gs protein promotes βARK translocation from the cytosolic pool towards the membrane and promotes βARK-mediated phosphorylation of the βAR [9, 16, 66]. High affinity binding of β adrenergic receptor kinase with a yet to be identified microsomal membrane protein through electrostatic interactions putatively indicates an important contribution of the interaction to mechanistically modulate β adrenergic receptor kinase activity [14]. Subcellular compartmentalization of the β adrenergic receptor kinase may represent a prominent mechanism regulating β adrenergic receptor desensitization [14]. Pharmacological G protein stimulators enhance the kinase activity of microsomal membrane protein-bound β adrenergic receptor kinase, but not binding affinity [14]. Upregulation of G protein expression and enhancement of Gβγ activity through viral transfection of genetic constructs covalently linked to, and continuous with, a high activity promoter or treatment with pharmacological G protein stimulators (mastoparan/GTPγS or aluminum fluoride) could be employed to therapeutic advantage to augment β adrenergic receptor kinase activity, consequently promoting β arrestin binding to β adrenergic receptor carboxyl terminal phosphorylated amino acid moieties and βAR-β arrestin scaffold-mediated facilitation of ERK1/2 activity [14]. Combinatorial therapeutic approaches seeking to contemporaneously upregulate β adrenergic receptor kinase-mediated phosphorylation of the β adrenergic receptor carboxyl terminal chain of amino acid moieties and enhance β adrenergic receptor-β arrestin binding stability could represent a promising therapeutic strategy in the adjuvant treatment of gliomas and other cancers.
Strategies which may enhance the stability of β arrestin-G protein coupled receptor interaction would preferentially force the equilibrium from PKA- to scaffold-mediated activation of ERK1/2 [11, 12]. These effects would coordinately promote cytosolic retention of ERK1/2 and reduce ERK1/2-meidated nuclear pro-transcriptional activity (though possible via ERK1/2 mediated phosphorylation of nuclear translocable enzymes) therapeutically promotable via drug-mediated stabilization and adenoviral transfection with stable proximal peptide chain terminal generating more stable interactions with the β adrenergic receptor carboxyl terminal domain [32, 33, 142]. Adenoviral vector delivery of a high activity promoter linked to β arrestin may enhance the expression of the protein, enhancing scaffold-mediated activation, and cytosolic retention, of ERK1/2 and reduce pro-transcriptional activity mediated by the phosphorylating phosphorylated conformation of the enzyme [86, 143, 144]. We believe this will prove to be a safe and effective strategy in preventing the onset, and ameliorating and attenuating the progression, of carcinogenesis and atherogenesis, by reducing the extracellular regulated kinase 1/2 mediated promotion of vascular smooth muscle cell proliferation. However, there may exist some difficulty in the technical challenge of achieving stable transfection of cells with adenoviral vectors and modulating the extent and distribution of cellular expression of transfected βAR GPCRs or β arrestin constructs [145]. Self-targeted oncolytic adenoviral nanospheres may successfully enhance adenoviral transfection of target cells with chimeric beta adrenergic receptor (vasopressin or angiotensin carboxyl-terminal substituted carboxyl terminals) or (N-terminal modified) β arrestin complexes [146].
Small interfering RNA mediated downregulation of β arrestin 1 and 2 expression reduced isoproterenol-mediated enhancement of ERK1/2 activation in HEK293 cells, though CRISPR/Cas9-mediated deletion of β arrestins and membrane G proteins had variable effects on ERK1/2 responsivitiy to β adrenergic stimulation [147]. We accordingly suggest evaluating the utility of fenoterol derivatives in utilizing CRISPR/Cas9 to mediate targeted deletions of β arrestin 1, β arrestin 2, Gas protein, and/or Gai protein and/or targeted knock-ins of chimeric constructs of βAR or β arrestin in HEK293, PC12, C6 rat-derived glioma, and human U87MG, U251MG, U373MG, and LN18 [147]. We further suggest intracerebrally implanting CRISPR/Cas9-mutated or adenovirally-transfected glioma cells to generate glioma models in vivo [147]. We may accordingly exploit these models to more precisely evaluate the role of variably modified fenoterol derivatives upon tumor cell proliferation, migratory capacity, invasion, angiogenesis, and metastasis [147].
The approach will require extensive preclinical studies in order to elucidate the full complementary spectrum of biological effects of administering adenoviral vectors containing β adrenergic receptor constructs. Multimodal strategies seeking to optimize the development of compounds promoting stable GPCR-β arrestin interactions and contemporaneous treatment with specific ERK inhibitors may maximize the actualized survival benefit in patients harboring gliomas and extra-neuraxial malignancy [9, 14, 111]. These therapies may prove of clinical utility in curtailing initiation, promotion, and progression of gliomas and may prove to represent a useful general adjuvant to multimodal therapy of glioblastoma [6, 76, 111]. Immunomodulatory effects of β adrenergic signaling, prominently regulating cell surface expression of MHC class II, suggests manipulating these pathways may represent an effective adjuvant technique to be utilized in conjunction with various immunotherapeutic approaches, including generation of tumor specific antibodies, cytotoxic T cells, and NK cells in a variety of cancers [124].[N.B.: As a brief aside, our empirically derived instinctual conceptualization leads us to surmise coordinate treatment with modulators of β adrenergic signaling, the bitopic compounds (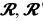 )-MNF and (
)-MNF and (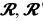 )-fenoterol, and/or the serine protease inhibitor nafamostat mesylate may exert synergistically therapeutic effects in the setting of cerebral glioma and extra-neuraxial carcinoma, neurovascular disease, and septic shock (Patent Pending, Ghali and Ghali, authors of the present work) and coronavirus COVID-19 responsible for the emerging international pandemic [148]. The sequential activity of the proteases furin, transmembrane protease serine 2 (TMPRSS2), and cathepsins cause sequential cleavage of the Middle East respiratory syndrome coronavirus (MERS-CoV) envelope protein, ‘S’, which fuses with host cell CD26, co-expressed with TMPRSS2 in target cells. The serine protease inhibitor nafamostat mesylate interferes with pro-S protein cleavage, preventing effective fusion of the Middle East respiratory coronavirus with host eukaryotic target cells [149]. Nafamostat mesylate was shown to prevent ‘S’-mediated membrane fusion according to a Renilla luciferase assay and prevent MERS-CoV infection in vitro in a preparation of Calu3 cells [149]. Nafamostat mesylate interferes with the proteolytic cleavage of Ebola virus envelope proteins necessary for virus-host cell fusion by reducing the proteolytic release of CatB from rat pancreas [150] and microvascular leakage in patients with Dengue hemorrhage fever and shock through tryptase inhibition, blocking vascular leakage in vivo [151].
)-fenoterol, and/or the serine protease inhibitor nafamostat mesylate may exert synergistically therapeutic effects in the setting of cerebral glioma and extra-neuraxial carcinoma, neurovascular disease, and septic shock (Patent Pending, Ghali and Ghali, authors of the present work) and coronavirus COVID-19 responsible for the emerging international pandemic [148]. The sequential activity of the proteases furin, transmembrane protease serine 2 (TMPRSS2), and cathepsins cause sequential cleavage of the Middle East respiratory syndrome coronavirus (MERS-CoV) envelope protein, ‘S’, which fuses with host cell CD26, co-expressed with TMPRSS2 in target cells. The serine protease inhibitor nafamostat mesylate interferes with pro-S protein cleavage, preventing effective fusion of the Middle East respiratory coronavirus with host eukaryotic target cells [149]. Nafamostat mesylate was shown to prevent ‘S’-mediated membrane fusion according to a Renilla luciferase assay and prevent MERS-CoV infection in vitro in a preparation of Calu3 cells [149]. Nafamostat mesylate interferes with the proteolytic cleavage of Ebola virus envelope proteins necessary for virus-host cell fusion by reducing the proteolytic release of CatB from rat pancreas [150] and microvascular leakage in patients with Dengue hemorrhage fever and shock through tryptase inhibition, blocking vascular leakage in vivo [151].
Conclusions
Authors have extensively detailed and elucidated mechanisms contributing to β adrenergic receptor modulated signaling, dynamics, and regulation [11–16, 47, 154], pharmacological modulation of which may powerfully modify tumor cell proliferation, motility, immunogenicity, elaboration of protein mediators promoting angiogenesis, and invasive and metastatic potential [124, 154]. Studies have alternately demonstrated amplification or attenuation of cellular proliferation of gliomas [6, 39, 40] and extra-neuraxial carcinomas in response to pharmacological enhancement of β adrenergic receptor modulated signaling [8, 45, 46, 49, 51, 52, 54, 57, 63, 152]. The character of βagonist utilized, tumor model and preparation type, receptor regulation dynamics, and differential distal signal transduction mechanisms may explain inter-experimental differences. The wise development of a set of experiments designed to more precisely characterize the full complement of effects mediated by β adrenergic receptor modulated signaling in carcinogenic initiation, promotion, and progression, immunogenic modulation, angiogenesis, and tumor cell tissue invasion and metastasis, specifically [6]. Crystallographic studies will further characterize inactive, transitional, and active tridimensional conformations of the β adrenergic receptor and specific conformational modifications induced by treatment with various agonists and antagonists of the heptahelical transmembrane G protein coupled receptor [14, 16]. Conformational protein modifications may differentially stabilize or destabilize binding between β adrenergic receptor carboxyl termini and β arrestin amino termini, thus generating differential effects upon desensitization, receptor endocytosis, and scaffold formation [11, 12, 14, 16]. Rational drug design and mathematical models of βAR-drug binding will identify drug-specific and tumor cell-specific factors rendering β adrenergic receptor modulated signaling more likely to promote or inhibit cellular proliferation, unveil determinants contributing to preferential Gs versus Gi activation or inhibition, and identify optimal bio-organic compounds modulating the conformational state of β adrenergic receptors in staying the progression of glioblastoma [85, 86, 131, 132]. Adenoviral transfection with chimeric constructs of β adrenergic receptors possessing carboxyl termini with high binding affinity to β arrestin amino termini and/or β arrestins possessing amino termini with high ligand binding affinity to GPCR carboxyl termini targeted specifically to glioma cells and high activity promoters may effectively preferentially promote scaffold-mediated activation of ERK1/2, blunting its nuclear translocation and retaining its cytosolic homeostatic effects, putatively proving to be a useful primary or adjuvant therapeutic approach enhancing the currently employed regimen of maximal safe resection, external beam radiotherapy, as well as concurrent and adjuvant temozolomide [21, 153]. We suggest a panoply of multimodal strategies designed to modulate β adrenergic signaling represent promising therapeutic approaches to be exploited in the treatment of glioblastoma [65, 73, 85, 86, 153]. Preclinical studies will prove necessary in order to develop compounds exhibiting the specific and desired effects upon β adrenergic receptor modulated signaling. Clinical studies will prove necessary in order to evaluate the safety and efficacy of these medications [65, 73, 85, 86]. Preclinical in vitro and in vivo studies and clinical studies will emergently cultivate an appreciation of the influence of pharmacological agonists, inverse agonists, antagonists of β adrenergic receptor modulated signaling, and fenoterol derivative bitopics upon the biomolecular mechanistic underpinnings of β adrenergic receptor modulated signaling upon molecular behavior of glioma cells and dynamic patterns of glioma growth, invasion, angiogenesis, and metastasis, and effects on survival metrics [65, 73, 85, 86, 151] (Table 1).
Table 1.
Effects of βAR signaling upon glioma
| Effect | Mechanisms |
|---|---|
| Promotes tumor cell proliferation and growth | AC-cAMP-PKA-ERK1/2-CREB → promotes cellular proliferation |
| Attenuates tumor cell proliferation and growth |
βAR → phospho-βAR via βARK → binding of β arrestin Promotes receptor internalization Promotes scaffold facilitated ERK1/2 activation ERK1/2 cytosolically retained ERK1/2 nuclear translocation prevented PLC → DAG + IP3 DAG → PKC IP3 → sarcoplasmic [Ca2+]i release [Ca2+]i → blunts cAMP-PKA signaling Upregulation of PDE degrades cAMP |
| Reduces tumor invasive potential | Decreases activity and expression of MMP-2 and MMP-9 |
| Reduces tumor neoangiogenesis | Decreases tubulogenesis |
| Reduces tumor metastatic potential | Decreases invasive potential and angiogenesis |
| Amplifies anti-tumor cellular adaptive immunity | Upregulates cell surface expression of MHC class II nonnative antigens |
The acute effects of βAR modulated signaling chiefly include promotion of tumor cell proliferation, invasion, angiogenesis, and metastasis. Prolonged administration of βAR agonists rapidly promotes phosphorylation of the carboxyl terminal by β adrenergic receptor kinase and binding of β arresting, weakening ligand binding-effector coupling and enhancing scaffold mediated activation of ERK1/2
AC adenylate cyclase, βAR β adrenergic receptor, βARK β adrenergic receptor kinase, cAMP cyclic adenosine monophosphate, CREB cyclic AMP response element binding protein, DAG diacylglycerol, ERK1/2 extracellular regulated kinase ½, IP3 inositol triphosphate, MMP-2 matrix metalloproteinase 2, MMP-9 matrix metalloproteinase-9, PKA protein kinase A, PKC protein kinase C, PDE phosphodiesterase, PLC phospholipase C
Funding
No funding was received for this study.
Compliance with ethical standards
Conflict of interest
No conflict of interest to disclose.
Ethical approval
All procedures performed in the studies were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1007/s11033-022-07301-8"
Change history
3/8/2022
This article has been retracted. Please see the Retraction Notice for more detail: 10.1007/s11033-022-07301-8
References
- 1.Harden TK, McCarthy KD. Identification of the β adrenergic receptor subtype on astroglia purified from rat brain. J Pharmacol Exp Ther. 1982;222(3):600–605. [PubMed] [Google Scholar]
- 2.Terasaki WL, Brooker G. [125I]Iodohydroxybenzylpindolol binding sites on intact rat glioma cells. Evidence for β-adrenergic receptors of high coupling efficiency. J Biol Chem. 1978;253(15):5418–5425. [PubMed] [Google Scholar]
- 3.Vardjan N, Kreft M, Zorec R. Dynamics of β-adrenergic/cAMP signaling and morphological changes in cultured astrocytes. Glia. 2014;62(4):566–579. doi: 10.1002/glia.22626. [DOI] [PubMed] [Google Scholar]
- 4.Conroy WG, Peoples RW, Isom GE. Identification of functional β-adrenergic receptors on AC glioma cells. Biochem Pharmacol. 1989;38(19):3175–3178. doi: 10.1016/0006-2952(89)90610-2. [DOI] [PubMed] [Google Scholar]
- 5.Sardi I, Giunti L, Bresci C, Buccoliero AM, Degl'innocenti D, Cardellicchio S, Baroni G, Castiglione F, Ros MD, Fiorini P, Giglio S, Genitori L, Aricò M, Filippi L. Expression of β-adrenergic receptors in pediatric malignant brain tumors. Oncol Lett. 2013;5(1):221–225. doi: 10.3892/ol.2012.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Annabi B, Lachambre MP, Plouffe K, Moumdjian R, Béliveau R. Propranolol adrenergic blockade inhibits human brain endothelial cells tubulogenesis and matrix metalloproteinase-9 secretion. Pharmacol Res. 2009;60:438–445. doi: 10.1016/j.phrs.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Hu P, He J, Liu S, Wang M, Pan B, Zhang W. ß2-adrenergic receptor activation promotes the proliferation of A549 lung cancer cells via the ERK1/2/CREB pathway. Oncol Rep. 2016;36:1757–1763. doi: 10.3892/or.2016.4966. [DOI] [PubMed] [Google Scholar]
- 8.Pasquier E, Street J, Pouchy C, Carre M, Gifford AJ, Murray J, Norris MD, Trahair T, Andre N, Kavallaris M. β-blockers increase response to chemotherapy via direct antitumour and anti-angiogenic mechanisms in neuroblastoma. Br J Cancer. 2013;108(12):2485–2494. doi: 10.1038/bjc.2013.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toll L, Jimenez L, Waleh N, Jozwiak K, Woo AY, Xiao RP, Bernier M, Wainer IW. {Beta}2-adrenergic receptor agonists inhibit the proliferation of 1321N1 astrocytoma cells. J Pharmacol Exp Ther. 2011;336(2):524–532. doi: 10.1124/jpet.110.173971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshida T, Shimizu K, Ushio Y, Hayakawa T, Mogami H, Sakamoto Y, Egawa T. Treatment of rat glioma with a β-adrenergic agonist and a phosphodiesterase inhibitor in vivo. No To Shinkei. 1987;39(8):719–723. [PubMed] [Google Scholar]
- 11.DeFea KA, Vaughn ZD, O'Bryan EM, Nishijima D, Déry O, Bunnett NW. The proliferative and antiapoptotic effects of substance P are facilitated by formation of a beta-arrestin-dependent scaffolding complex. Proc Natl Acad Sci USA. 2000;97(20):11086–11091. doi: 10.1073/pnas.190276697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeFea KA, Zalevsky J, Thoma MS, Déry O, Mullins RD, Bunnett NW. Beta-arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J Cell Biol. 2000;148(6):1267–1281. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lefkowitz RJ, Rajagopal K, Whalen EJ. New roles for beta-arrestins in cell signaling: not just for seven-transmembrane receptors. Mol Cell. 2006;24(5):643–652. doi: 10.1016/j.molcel.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Murga C, Ruiz-Gómez A, García-Higuera I, Kim CM, Benovic JL, Mayor F., Jr High affinity binding of beta-adrenergic receptor kinase to microsomal membranes. Modulation of the activity of bound kinase by heterotrimeric G protein activation. J Biol Chem. 1996;271(2):985–994. doi: 10.1074/jbc.271.2.985. [DOI] [PubMed] [Google Scholar]
- 15.Tohgo A, Choy EW, Gesty-Palmer D, Pierce KL, Laporte S, Oakley RH, Caron MG, Lefkowitz RJ, Luttrell LM. The stability of the G protein-coupled receptor-beta-arrestin interaction determines the mechanism and functional consequence of ERK activation. J Biol Chem. 2003;278(8):6258–6267. doi: 10.1074/jbc.M212231200. [DOI] [PubMed] [Google Scholar]
- 16.Warne T, Serrano-Vega MJ, Baker JG, Moukhametzianov R, Edwards PC, Henderson R, Leslie AG, Tate CG, Schertler GF. Structure of a beta1-adrenergic G-protein-coupled receptor. Nature. 2008;454(7203):486–491. doi: 10.1038/nature07101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thissen JA, Casey P. Microsomal membranes contain a high affinity binding site for prenylated peptides. J Biol Chem. 1993;268(19):13780–13783. [PubMed] [Google Scholar]
- 18.Brady AE, Wang Q, Allen PB, Rizzo M, Greengard P, Limbird LE. Α2-adrenergic agonist enrichment of spinophilin at the cell surface involves β gamma subunits of Gi proteins and is preferentially induced by the α 2A-subtype. Mol Pharmacol. 2005;67(5):1690–1696. doi: 10.1124/mol.104.005215. [DOI] [PubMed] [Google Scholar]
- 19.Steyaert J, Kobilka BK. Nanobody stabilization of G protein-coupled receptor conformational states. Curr Opin Struct Biol. 2011;21(4):567–572. doi: 10.1016/j.sbi.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alexander RW, Davis JN, Lefkowitz RJ. Direct identification and characterisation of beta-adrenergic receptors in rat brain. Nature. 1975;258(5534):437–440. doi: 10.1038/258437a0. [DOI] [PubMed] [Google Scholar]
- 21.Marchi F, Sahnane N, Cerutti R, Cipriani D, Barizzi J, Stefanini FM, Epistolio S, Cerati M, Balbi S, Mazzucchelli L, Sessa F, Pesce GA, Reinert M, Frattini M. The impact of surgery in IDH 1 wild type glioblastoma in relation with the MGMT deregulation. Front Oncol. 2020;9:1569. doi: 10.3389/fonc.2019.01569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brust TF, Conley JM, Watts VJ. Gα(i/o)-coupled receptor-mediated sensitization of adenylyl cyclase: 40 years later. Eur J Pharmacol. 2015;763(Pt B):223–232. doi: 10.1016/j.ejphar.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Böhm M. Alterations of β-adrenoceptor-G-protein-regulated adenylyl cyclase in heart failure. Mol Cell Biochem. 1995;147(1–2):147–160. [PubMed] [Google Scholar]
- 24.Galello F, Portela P, Moreno S, Rossi S. Characterization of substrates that have a differential effect on Saccharomyces cerevisiae protein kinase A holoenzyme activation. J Biol Chem. 2010;285(39):29770–29779. doi: 10.1074/jbc.M110.120378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerlits O, Weiss KL, Blakeley MP, Veglia G, Taylor SS, Kovalevsky A. Zooming in on protons: neutron structure of protein kinase A trapped in a product complex. Sci Adv. 2019;5(3):eaav0482. doi: 10.1126/sciadv.aav0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gold MG, Fowler DM, Means CK, Pawson CT, Stephany JJ, Langeberg LK, Fields S, Scott JD. Engineering A-kinase anchoring protein (AKAP)-selective regulatory subunits of protein kinase A (PKA) through structure-based phage selection. J Biol Chem. 2013;288(24):17111–17121. doi: 10.1074/jbc.M112.447326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamaguchi T, Nakamuta S, Funahashi Y, Takano T, Nishioka T, Shohag MH, Yura Y, Kaibuchi K, Amano M. In vivo screening for substrates of protein kinase A using a combination of proteomic approaches and pharmacological modulation of kinase activity. Cell Struct Funct. 2015;40(1):1–12. doi: 10.1247/csf.14014. [DOI] [PubMed] [Google Scholar]
- 28.Burch ML, Osman N, Getachew R, Al-Aryahi S, Poronnik P, Zheng W, Hill MA, Little PJ. G protein coupled receptor transactivation: extending the paradigm to include serine/threonine kinase receptors. Int J Biochem Cell Biol. 2012;44(5):722–727. doi: 10.1016/j.biocel.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 29.Słomiany BL, Słomiany A. Gastric mucin secretion in response to β-adrenergic G protein-coupled receptor activation is mediated by SRC kinase-dependent epidermal growth factor receptor transactivation. J Physiol Pharmacol. 2005;56(2):247–258. [PubMed] [Google Scholar]
- 30.Slomiany BL, Slomiany A. Secretion of gastric mucus phospholipids in response to β-adrenergic G protein-coupled receptor activation is mediated by SRC kinase-dependent epidermal growth factor receptor transactivation. J Physiol Pharmacol. 2004;55(3):627–638. [PubMed] [Google Scholar]
- 31.Slomiany BL, Slomiany A. Src-kinase-dependent epidermal growth factor receptor transactivation in salivary mucin secretion in response to β-adrenergic G-protein-coupled receptor activation. Inflammopharmacology. 2004;12(3):233–245. doi: 10.1163/1568560042342329. [DOI] [PubMed] [Google Scholar]
- 32.Bolger GB, Baillie GS, Li X, Lynch MJ, Herzyk P, Mohamed A, Mitchell LH, McCahill A, Hundsrucker C, Klussmann E, Adams DR, Houslay MD. Scanning peptide array analyses identify overlapping binding sites for the signalling scaffold proteins, β-arrestin and RACK1, in cAMP-specific phosphodiesterase PDE4D5. Biochem J. 2006;398(1):23–36. doi: 10.1042/BJ20060423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith KJ, Baillie GS, Hyde EI, Li X, Houslay TM, McCahill A, Dunlop AJ, Bolger GB, Klussmann E, Adams DR, Houslay MD. 1H NMR structural and functional characterisation of a cAMP-specific phosphodiesterase-4D5 (PDE4D5) N-terminal region peptide that disrupts PDE4D5 interaction with the signalling scaffold proteins, β-arrestin and RACK1. Cell Signal. 2007;19(12):2612–2624. doi: 10.1016/j.cellsig.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 34.Xiao LJ, Lin P, Lin F, Liu X, Qin W, Zou HF, Guo L, Liu W, Wang SJ, Yu XG. ADAM17 targets MMP-2 and MMP-9 via EGFR-MEK-ERK pathway activation to promote prostate cancer cell invasion. Int J Oncol. 2012;40:1714–1724. doi: 10.3892/ijo.2011.1320. [DOI] [PubMed] [Google Scholar]
- 35.Dibner MD, Insel PA. Serum catecholamines desensitize β-adrenergic receptors of cultured C6 glioma cells. J Biol Chem. 1981;256(14):7343–7346. [PubMed] [Google Scholar]
- 36.Franklin TJ, Twose PA. Desensitization of β-adrenergic receptors of glioma cells: studies with intact and broken cell preparations. FEBS Lett. 1976;66(2):225–229. doi: 10.1016/0014-5793(76)80509-1. [DOI] [PubMed] [Google Scholar]
- 37.Dibner MD, Insel PA. Growth of C6 glioma cells in serum-containing medium decreases β-adrenergic receptor number. J Cell Physiol. 1981;109(2):309–315. doi: 10.1002/jcp.1041090214. [DOI] [PubMed] [Google Scholar]
- 38.Koschel K, Muenzel P. Persistent paramyxovirus infections and behaviour of β-adrenergic receptors in C-6 rat glioma cells. J Gen Virol. 1980;47(2):513–517. doi: 10.1099/0022-1317-47-2-513. [DOI] [PubMed] [Google Scholar]
- 39.Lung HL, Shan SW, Tsang D, Leung KN. Tumor necrosis factor-alpha mediates the proliferation of rat C6 glioma cells via beta-adrenergic receptors. J Neuroimmunol. 2005;166(1–2):102–112. doi: 10.1016/j.jneuroim.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 40.Pianet I, Canioni P, Labouesse J, Merle M. Β-adrenergic stimulation of C6 glioma cells: effects of cAMP overproduction on cellular metabolites. A multinuclear NMR study. Eur J Biochem. 1992;209(2):707–715. doi: 10.1111/j.1432-1033.1992.tb17339.x. [DOI] [PubMed] [Google Scholar]
- 41.Schwartz JP, Costa E. Protein kinase translocation following β-adrenergic receptor activation in C6 glioma cells. J Biol Chem. 1980;255(7):2943–2948. [PubMed] [Google Scholar]
- 42.Schwartz JP, Costa E. β Adrenergic receptor-mediated regulation of cyclic nucleotide phosphodiesterase in C6 glioma cells: vinblastine blockade of isoproterenol induction. J Pharmacol Exp Ther. 1980;212(3):569–572. [PubMed] [Google Scholar]
- 43.Schwartz JP, Onali P. Β-adrenergic receptor regulation of a cyclic AMP phosphodiesterase in C6 glioma cells. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;16:195–203. [PubMed] [Google Scholar]
- 44.Schwartz JP. RNA polymerase II in C6 glioma cells. Alpha-amanitin blockade of cAMP phosphodiesterase induction by beta-adrenergic stimulation. Exp Cell Res. 1982;137(1):39–45. doi: 10.1016/0014-4827(82)90005-2. [DOI] [PubMed] [Google Scholar]
- 45.Carie AE, Sebti SM. A chemical biology approach identifies a β-2 adrenergic receptor agonist that causes human tumor regression by blocking the Raf-1/Mek-1/Erk1/2 pathway. Oncogene. 2007;26:3777–3788. doi: 10.1038/sj.onc.1210172. [DOI] [PubMed] [Google Scholar]
- 46.Coelho M, Moz M, Correia G, Teixeira A, Medeiros R, Ribeiro L. Antiproliferative effects of ß-blockers on human colorectal cancer cells. Oncol Rep. 2015;33(5):2513–2520. doi: 10.3892/or.2015.3874. [DOI] [PubMed] [Google Scholar]
- 47.Montoya A, Varela-Ramirez A, Dickerson E, Pasquier E, Torabi A, Aguilera R, Nahleh Z, Bryan B. The beta adrenergic receptor antagonist propranolol alters mitogenic and apoptotic signaling in late stage breast cancer. Biomed J. 2019;42(3):155–165. doi: 10.1016/j.bj.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Slotkin TA, Zhang J, Dancel R, Garcia SJ, Willis C, Seidler FJ. β-adrenoceptor signaling and its control of cell replication in MDA-MB-231 human breast cancer cells. Breast Cancer Res Treat. 2000;60:153–166. doi: 10.1023/a:1006338232150. [DOI] [PubMed] [Google Scholar]
- 49.Zhang P, He X, Tan J, Zhou X, Zou L. β-arrestin2 mediates β-2 adrenergic receptor signaling inducing prostate cancer cell progression. Oncol Rep. 2011;26(6):1471–1477. doi: 10.3892/or.2011.1417. [DOI] [PubMed] [Google Scholar]
- 50.Zhang X, Zhang Y, He Z, Yin K, Li B, Zhang L, Xu Z. Chronic stress promotes gastric cancer progression and metastasis: an essential role for ADRB2. Cell Death Dis. 2019;10(11):788. doi: 10.1038/s41419-019-2030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paul RK, Ramamoorthy A, Scheers J, Wersto RP, Toll L, Jimenez L, Bernier M, Wainer IW. Cannabinoid receptor activation correlates with the proapoptotic action of the ß2-adrenergic agonist (R, R')-4-methoxy-1-naphthylfenoterol in HepG2 hepatocarcinoma cells. J Pharmacol Exp Ther. 2012;343(1):157–166. doi: 10.1124/jpet.112.195206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiao MB, Jin DD, Jiao YJ, Ni WK, Liu JX, Qu LS, Lu CH, Ni RZ, Jiang F, Chen WC. β2-AR regulates the expression of AKR1B1 in human pancreatic cancer cells and promotes their proliferation via the ERK1/2 pathway. Mol Biol Rep. 2018;45(6):1863–1871. doi: 10.1007/s11033-018-4332-3. [DOI] [PubMed] [Google Scholar]
- 53.Tatsuta M, Iishi H, Yamamura H, Baba M, Taniguchi H. Inhibition by isoproterenol and neostigmine of experimental carcinogenesis in rat colon by azoxymethane. Br J Cancer. 1988;58(5):619–620. doi: 10.1038/bjc.1988.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ouyang X, Zhu Z, Yang C, Wang L, Ding G, Jiang F. Epinephrine increases malignancy of breast cancer through p38 MAPK signaling pathway in depressive disorders. Int J Clin Exp Pathol. 2019;12(6):1932–1946. [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou J, Liu Z, Zhang L, Hu X, Wang Z, Ni H, Wang Y, Qin J. Activation of β2-adrenergic receptor promotes growth and angiogenesis in breast cancer by down-regulating PPARγ. Cancer Res Treat. 2020 doi: 10.4143/crt.2019.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sood AK, Bhatty R, Kamat AA, Landen CN, Han L, Thaker PH, Li Y, Gershenson DM, Lutgendorf S, Cole SW. Stresshormone-mediated invasion of ovarian cancer cells. Clin Cancer Res. 2006;12(2):369–375. doi: 10.1158/1078-0432.CCR-05-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thaker PH, Sood AK, Ramondetta LM. Importance of adrenergic pathways in women's cancers. Cancer Biomark. 2013;13(3):145–154. doi: 10.3233/CBM-130324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Albiñana V, de Las Heras KVG, Serrano-Heras G, Segura T, Perona-Moratalla AB, Mota-Pérez M, de Campos JM, Botella LM. Propranolol reduces viability and induces apoptosis in hemangioblastoma cells from von Hippel-Lindau patients. Orphanet J Rare Dis. 2015;10:118. doi: 10.1186/s13023-015-0343-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Al-Wadei HA, Al-Wadei MH, Schuller HM. Prevention of pancreatic cancer by the beta-blocker propranolol. Anticancer Drugs. 2009;20(6):477–482. doi: 10.1097/CAD.0b013e32832bd1e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Palm D, Lang K, Niggemann B, Drell TL, 4th, Masur K, Zaenker KS, et al. The norepinephrine-driven metastasis development of PC-3 human prostate cancer cells in BALB/c nude mice is inhibited by β-blockers. Int J Cancer. 2006;118:2744–2749. doi: 10.1002/ijc.21723. [DOI] [PubMed] [Google Scholar]
- 61.Johansen MD, Urup T, Holst CB, Christensen IJ, Grunnet K, Lassen U, Friis S, Poulsen HS. Outcome of bevacizumab therapy in patients with recurrent glioblastoma treated with angiotensin system inhibitors. Cancer Investig. 2018;36:512–519. doi: 10.1080/07357907.2018.1544639. [DOI] [PubMed] [Google Scholar]
- 62.Terasaki WL, Brooker G, de Vellis J, Inglish D, Hsu CY, Moylan RD. Involvement of cyclic amp and protein synthesis in catecholamine refractoriness. Adv Cyclic Nucleotide Res. 1978;9:33–52. [PubMed] [Google Scholar]
- 63.Ramondetta LM, Hu W, Thaker PH, Urbauer DL, Chisholm GB, Westin SN, Sun Y, Ramirez PT, Fleming N, Sahai SK, Nick AM, Arevalo JMG, Dizon T, Coleman RL, Cole SW, Sood AK. Prospective pilot trial with combination of propranolol with chemotherapy in patients with epithelial ovarian cancer and evaluation on circulating immune cell gene expression. Gynecol Oncol. 2019;154(3):524–530. doi: 10.1016/j.ygyno.2019.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Couttenier A, Lacroix O, Silversmit G, Vaes E, De Schutter H, Robert A. Beta-blocker use and mortality following ovarian cancer diagnosis: a population-based study. Cancer Epidemiol. 2019;62:101579. doi: 10.1016/j.canep.2019.101579. [DOI] [PubMed] [Google Scholar]
- 65.Wnorowski A, Sadowska M, Paul RK, Singh NS, Boguszewska-Czubara A, Jimenez L, Abdelmohsen K, Toll L, Jozwiak K, Bernier M, Wainer IW. Activation of β2-adrenergic receptor by (R, R′)-4′-methoxy-1-naphthylfenoterol inhibits proliferation and motility of melanoma cells. Cell Signal. 2015;27:997–1007. doi: 10.1016/j.cellsig.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Paul RK, Wnorowski A, Gonzalez-Mariscal I, Nayak SK, Pajak K, Moaddel R, Indig FE, Bernier M, Wainer IW. (R, R')-4'-methoxy-1-naphthylfenoterol targets GPR55-mediated ligand internalization and impairs cancer cell motility. Biochem Pharmacol. 2014;87(4):547–561. doi: 10.1016/j.bcp.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.He JJ, Zhang WH, Liu SL, Chen YF, Liao CX, Shen QQ, Hu P. Activation of ß-adrenergic receptor promotes cellular proliferation in human glioblastoma. Oncol Lett. 2017;14:3846–3852. doi: 10.3892/ol.2017.6653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Feinstein DL, Rozelman E. Norepinephrine suppresses l-arginine uptake in rat glial cells. Neurosci Lett. 1997;223(1):37–40. doi: 10.1016/s0304-3940(97)13402-4. [DOI] [PubMed] [Google Scholar]
- 69.Canova C, Baudet C, Chevalier G, Brachet P, Wion D. Noradrenaline inhibits the programmed cell death induced by 1,25-dihydroxyvitamin D3 in glioma. Eur J Pharmacol. 1997;319(2–3):365–368. doi: 10.1016/s0014-2999(96)00942-9. [DOI] [PubMed] [Google Scholar]
- 70.Van Kolen K, Slegers H. P2Y12 receptor stimulation inhibits beta-adrenergic receptor-induced differentiation by reversing the cyclic AMP-dependent inhibition of protein kinase B. J Neurochem. 2004;89(2):442–453. doi: 10.1111/j.1471-4159.2004.02339.x. [DOI] [PubMed] [Google Scholar]
- 71.Kurino M, Fukunaga K, Ushio Y, Miyamoto E. Cyclic AMP inhibits activation of mitogen-activated protein kinase and cell proliferation in response to growth factors in cultured rat cortical astrocytes. J Neurochem. 1996;67(6):2246–2255. doi: 10.1046/j.1471-4159.1996.67062246.x. [DOI] [PubMed] [Google Scholar]
- 72.Erguven M, Yazihan N, Aktas E, Sabanci A, Chiang JLI, Oktem G, Bilir A. Carvedilol in glioma treatment alone and with imatinib in vitro. Int J Oncol. 2010;36:857–866. doi: 10.3892/ijo00000563. [DOI] [PubMed] [Google Scholar]
- 73.Wnorowski A, Suchb J, Paula RK, Werstoc RP, Indigd FE, Jozwiakb K, Berniere M, Wainera IW. Concurrent activation of ß2-adrenergic receptor and blockage of GPR55 disrupts pro-oncogenic signaling in glioma cells. Cell Signal. 2017;36:176–188. doi: 10.1016/j.cellsig.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang EV, Sood AK, Chen M, Li Y, Eubank TD, Marsh CB, Jewell S, Flavahan NA, Morrison C, Yeh PE, Lemeshow S, Glaser R. Norepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer Res. 2006;66(21):10357–10364. doi: 10.1158/0008-5472.CAN-06-2496. [DOI] [PubMed] [Google Scholar]
- 75.Chelmicka-Schorr E, Arnason BG, Holshouser SJ. C-6 glioma growth in rats: suppression with a β-adrenergic agonist and a phosphodiesterase inhibitor. Ann Neurol. 1980;8(4):447–449. doi: 10.1002/ana.410080421. [DOI] [PubMed] [Google Scholar]
- 76.Ahir BK, Engelhard HH, Lakka SS. Tumor development and angiogenesis in adult brain tumor: glioblastoma. Mol Neurobiol. 2020 doi: 10.1007/s12035-020-01892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Heimberger AB, Wang E, McGary EC, Hess KR, Henry VK, Shono T, Cohen Z, Gumin J, Sawaya R, Conrad CA, Lang FF. Mechanisms of action of rapamycin in gliomas. Neuro Oncol. 2005;7(1):1–11. doi: 10.1215/S1152851704000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Homburger V, Pantaloni C, Lucas M, Gozlan H, Bockaert J. Β adrenergic receptor repopulation of C6 glioma cells after irreversible blockade and down regulation. J Cell Physiol. 1984;121(3):589–597. doi: 10.1002/jcp.1041210318. [DOI] [PubMed] [Google Scholar]
- 79.Pavlova O, Shirokov A, Fomin A, Navolokin N, Terskov A, Khorovodov A, Namykin A, Pavlov A, Tuchin V, Semyachkina Glushkovskaya O. Optical in vivo and ex vivo imaging of glioma cells migration via the cerebral vessels: prospective clinical application of the beta2-adrenoreceptors blockade for glioma treatment. J Innov Opt Health Sci. 2018;11:1850025. doi: 10.1142/S1793545818500256. [DOI] [Google Scholar]
- 80.Sokolowska P, Nowak JZ. Constitutive activity of β-adrenergic receptors in C6 glioma cells. Pharmacol Rep. 2005;57:659–663. [PubMed] [Google Scholar]
- 81.Rasmussen SG, DeVree BT, Zou Y, Kruse AC, Chung KY, Kobilka TS, Thian FS, Chae PS, Pardon E, Calinski D, Mathiesen JM, Shah ST, Lyons JA, Caffrey M, Gellman SH, Steyaert J, Skiniotis G, Weis WI, Sunahara RK, Kobilka BK. Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature. 2011;477(7366):549–555. doi: 10.1038/nature10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.O'Hayre M, Eichel K, Avino S, Zhao X, Steffen DJ, Feng X, Kawakami K, Aoki J, Messer K, Sunahara R, Inoue A, von Zastrow M, Gutkind JS. Genetic evidence that β-arrestins are dispensable for the initiation of β2-adrenergic receptor signaling to ERK. Sci Signal. 2017;10(484):eaal3395. doi: 10.1126/scisignal.aal3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Charpentier N, Prézeau L, Carrette J, Bertorelli R, Le Cam G, Manzoni O, Bockaert J, Homburger V. Transfected Go1 α inhibits the calcium dependence of β-adrenergic stimulated cAMP accumulation in C6 glioma cells. J Biol Chem. 1993;268(12):8980–8989. [PubMed] [Google Scholar]
- 84.Woo AY, Wang TB, Zeng X, Zhu W, Abernethy DR, Wainer IW, Xiao RP. Mol Pharmacol. 2009;75:158–165. doi: 10.1124/mol.108.051078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Reinartz MT, Kälble S, Wainer IW, Seifert R. Interaction of fenoterol stereoisomers with β2-adrenoceptor-G sα fusion proteins: antagonist and agonist competition binding. Naunyn Schmiedebergs Arch Pharmacol. 2015;388(5):517–524. doi: 10.1007/s00210-015-1086-5. [DOI] [PubMed] [Google Scholar]
- 86.Reinartz MT, Kälble S, Littmann T, Ozawa T, Dove S, Kaever V, Wainer IW, Seifert R. Structure-bias relationships for fenoterol stereoisomers in six molecular and cellular assays at the β2-adrenoceptor. Naunyn Schmiedebergs Arch Pharmacol. 2015;388(1):51–65. doi: 10.1007/s00210-014-1054-5. [DOI] [PubMed] [Google Scholar]
- 87.Cottingham C, Lu R, Jiao K, Wang Q. Cross-talk from ß-adrenergic receptors modulates a2A-adrenergic receptor endocytosis in sympathetic neurons via protein kinase A and spinophilin. J Biol Chem. 2013;288:29193–29205. doi: 10.1074/jbc.M113.469494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schuller HM. Inhibitory role of Gi-coupled receptors on cAMP-driven cancers with focus on opioid receptors in lung adenocarcinoma and its stem cells. Vitam Horm. 2019;111:299–311. doi: 10.1016/bs.vh.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 89.Schuller HM, Al-Wadei HA, Majidi M. GABA B receptor is a novel drug target for pancreatic cancer. Cancer. 2008;112:767–778. doi: 10.1002/cncr.23231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zaremba TG, Fishman PH. Desensitization of catecholamine-stimulated adenylate cyclase and down-regulation of β-adrenergic receptors in rat glioma C6 cells. Role of cyclic AMP and protein synthesis. Mol Pharmacol. 1984;26(2):206–211. [PubMed] [Google Scholar]
- 91.Debernardi MA, Munshi R, Brooker G. Ca2+ inhibition of β-adrenergic receptor- and forskolin-stimulated cAMP accumulation in C6–2B rat glioma cells is independent of protein kinase C. Mol Pharmacol. 1993;43(3):451–458. [PubMed] [Google Scholar]
- 92.Hough C, Fukamauchi F, Chuang DM. Regulation of β-adrenergic receptor mRNA in rat C6 glioma cells is sensitive to the state of microtubule assembly. J Neurochem. 1994;62(2):421–430. doi: 10.1046/j.1471-4159.1994.62020421.x. [DOI] [PubMed] [Google Scholar]
- 93.Yan K, Popova JS, Moss A, Shah B, Rasenick MM. Tubulin stimulates adenylyl cyclase activity in C6 glioma cells by bypassing the β-adrenergic receptor: a potential mechanism of G protein activation. J Neurochem. 2001;76(1):182–190. doi: 10.1046/j.1471-4159.2001.00013.x. [DOI] [PubMed] [Google Scholar]
- 94.Ghahary A, Cheng KW. Identification and characterization of the β-adrenergic receptor on neuroblastoma x glioma hybrid NG108-15 cells. Cell Mol Neurobiol. 1990;10(3):337–350. doi: 10.1007/BF00711179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schulz K, Müller S, Belke-Louis G, Schulz R. Rat β-adrenergic receptor kinases 1 and 2 in mouse neuroblastoma X rat glioma NG 108–15 hybrid cells. Biochem Pharmacol. 1998;55(1):65–70. doi: 10.1016/s0006-2952(97)00380-8. [DOI] [PubMed] [Google Scholar]
- 96.Mallorga P, Tallman JF, Fishman PH. Differences in the β-adrenergic responsiveness between high and low passage rat glioma C6 cells. Biochim Biophys Acta. 1981;678(2):221–229. doi: 10.1016/0304-4165(81)90210-5. [DOI] [PubMed] [Google Scholar]
- 97.Koschel K, Tas PW. Lysophosphatidic acid reverts the β-adrenergic agonist-induced morphological response in C6 rat glioma cells. Exp Cell Res. 1993;206(1):162–166. doi: 10.1006/excr.1993.1133. [DOI] [PubMed] [Google Scholar]
- 98.Tas PW, Koschel K. Thrombin reverts the β-adrenergic agonist-induced morphological response in rat glioma C6 cells. Exp Cell Res. 1990;189(1):22–27. doi: 10.1016/0014-4827(90)90251-5. [DOI] [PubMed] [Google Scholar]
- 99.Warren KE. Beyond the blood: brain barrier: the importance of central nervous system (CNS) pharmacokinetics for the treatment of CNS tumors, including diffuse intrinsic pontine glioma. Front Oncol. 2018;3(8):239. doi: 10.3389/fonc.2018.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang F, Xu CL, Liu CM. Drug delivery strategies to enhance the permeability of the blood-brain barrier for treatment of glioma. Drug Des Dev Ther. 2015;9(9):2089–2100. doi: 10.2147/DDDT.S79592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang L, Zhang S, Yao J, Lowery FJ, Zhang Q, Huang WC, Li P, Li M, Wang X, Zhang C, Wang H, Ellis K, Cheerathodi M, McCarty JH, Palmieri D, Saunus J, Lakhani S, Huang S, Sahin AA, Aldape KD, Steeg PS, Yu D. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015;527(7576):100–104. doi: 10.1038/nature15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Park JY, Kim H, Lim DW, Kim JE, Park WH, Park SD. Ethanol Extract of Lycopodium serratum Thunb. attenuates lipopolysaccharide-induced C6 glioma cells migration via matrix metalloproteinase-9 expression. Chin J Integr Med. 2018;24(11):860–866. doi: 10.1007/s11655-017-2923-9. [DOI] [PubMed] [Google Scholar]
- 103.Gray KM, Jung JW, Inglut CT, Huang HC, Stroka KM. Quantitatively relating brain endothelial cell-cell junction phenotype to global and local barrier properties under varied culture conditions via the junction analyzer program. Fluids Barriers CNS. 2020;17(1):16. doi: 10.1186/s12987-020-0177-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jadhav U, Chigurupati S, Lakka SS, Mohanam S. Inhibition of matrix metalloproteinase-9 reduces in vitro invasion and angiogenesis in human microvascular endothelial cells. Int J Oncol. 2004;25:1407–1414. [PubMed] [Google Scholar]
- 105.Li Z, Veeraraghavan VP, Mohan SK, Bolla SR, Lakshmanan H, Kumaran S, Aruni W, Aladresi AAM, Shair OHM, Alharbi SA, Chinnathambi A. Apoptotic induction and anti-metastatic activity of eugenol encapsulated chitosan nanopolymer on rat glioma C6 cells via alleviating the MMP signaling pathway. J Photochem Photobiol B. 2020;203:111773. doi: 10.1016/j.jphotobiol.2019.111773. [DOI] [PubMed] [Google Scholar]
- 106.Kleinman ME, Greives MR, Churgin SS, Blechman KM, Chang EI, Ceradini DJ, et al. Hypoxia-inducedmediators of stem/progenitor cell trafficking are increased in children with hemangioma. Arterioscler Thromb Vasc Biol. 2007;27:2664–2670. doi: 10.1161/ATVBAHA.107.150284. [DOI] [PubMed] [Google Scholar]
- 107.Zheng HL, Yang J, Hou Y, Sun B, Zhang Q, Mou Y, Wand L, Wu C. Oligomer procyanidins (F2) isolated from grape seeds inhibits tumor angiogenesis and cell invasion by targeting HIF-1a in vitro. Int J Oncol. 2015;46(2):708–720. doi: 10.3892/ijo.2014.2744. [DOI] [PubMed] [Google Scholar]
- 108.Annabi B, Rojas-Sutterlin S, Laroche M, Lachambre MP, Moumdjian R, Béliveau R. The diet-derived sulforaphane inhibitsmatrixmetalloproteinase-9-activated human brain microvascular endothelial cell migration and tubulogenesis. Mol Nutr Food Res. 2008;52:692–700. doi: 10.1002/mnfr.200700434. [DOI] [PubMed] [Google Scholar]
- 109.Benish M, Bartal I, Goldfarb Y, Levi B, Avraham R, Raz A, et al. Perioperative use of β-blockers and COX-2 inhibitors may improve immune competence and reduce the risk of tumor metastasis. Ann Surg Oncol. 2008;15:2042–2052. doi: 10.1245/s10434-008-9890-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McLaughlin N, Annabi B, Lachambre MP, Kim KS, Bahary JP, Moumdjian R, et al. Combined low dose ionizing radiation and green tea-derived epigallocatechin-3-gallate treatment induces human brain endothelial cells death. J Neurooncol. 2006;80:111–121. doi: 10.1007/s11060-006-9171-8. [DOI] [PubMed] [Google Scholar]
- 111.Fujiwara S, Nakagawa K, Harada H, Nagato S, Furukawa K, Teraoka M, Seno T, Oka K, Iwata S, Ohnishi T. Silencing hypoxia-inducible factor-1alpha inhibits cell migration and invasion under hypoxic environment in malignant gliomas. Int J Oncol. 2007;30(4):793–802. [PubMed] [Google Scholar]
- 112.Stadlbauer A, Zimmermann M, Oberndorfer S, Doerfler A, Buchfelder M, Heinz G, Roessler K. Vascular hysteresis loops and vascular architecture mapping in patients with glioblastoma treated with antiangiogenic therapy. Sci Rep. 2017;7(1):8508. doi: 10.1038/s41598-017-09048-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mitra S, Bal A, Kashyap D, Kumar S, Shrivastav S, Das A, Singh G. Tumour angiogenesis and c-Met pathway activation—impications in breast cancer. APMIS. 2020 doi: 10.1111/apm.13031. [DOI] [PubMed] [Google Scholar]
- 114.Sie ZL, Li RY, Sampurna BP, Hsu PJ, Liu SC, Wang HD, Huang CL, Yuh CH. WNK1 kinase stimulates angiogenesis to promote tumor growth and metastasis. Cancers (Basel) 2020;12(3):E575. doi: 10.3390/cancers12030575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Moreno-Smith M, Lutgendorf SK, Sood AK. Impact of stress on cancer metastasis. Future Oncol. 2010;6(12):1863–1881. doi: 10.2217/fon.10.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Moreno-Smith M, Lee SJ, Lu C, Nagaraja AS, He G, Rupaimoole R, Han HD, Jennings NB, Roh JW, Nishimura M, Kang Y, Allen JK, Armaiz GN, Matsuo K, Shahzad MM, Bottsford-Miller J, Langley RR, Cole SW, Lutgendorf SK, Siddik ZH, Sood AK. Biologic effects of dopamine on tumor vasculature in ovarian carcinoma. Neoplasia. 2013;15(5):502–510. doi: 10.1593/neo.121412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McGee MC, Hamner JB, Williams RF, Rosati SF, Sims TL, Ng CY, Gaber MW, Calabrese C, Wu J, Nathwani AC, Duntsch C, Merchant TE, Davidoff AM. Improved intratumoral oxygenation through vascular normalization increases glioma sensitivity to ionizing radiation. Int J Radiat Oncol Biol Phys. 2010;76(5):1537–1545. doi: 10.1016/j.ijrobp.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Catalano M, D'Alessandro G, Trettel F, Limatola C. Role of infiltrating microglia/macrophages in glioma. Adv Exp Med Biol. 2020;1202:281–298. doi: 10.1007/978-3-030-30651-9_14. [DOI] [PubMed] [Google Scholar]
- 119.Domingues P, González-Tablas M, Otero Á, Pascual D, Miranda D, Ruiz L, Sousa P, Ciudad J, Gonçalves JM, Lopes MC, Orfao A, Tabernero MD. Tumor infiltrating immune cells in gliomas and meningiomas. Brain Behav Immun. 2016;53:1–15. doi: 10.1016/j.bbi.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 120.Sharonov GV, Serebrovskaya EO, Yuzhakova DV, Britanova OV, Chudakov DM. B cells, plasma cells and antibody repertoires in the tumour microenvironment. Nat Rev Immunol. 2020 doi: 10.1038/s41577-019-0257-x. [DOI] [PubMed] [Google Scholar]
- 121.Eisemann T, Costa B, Peterziel H, Angel P. Podoplanin positive myeloid cells promote glioma development by immune suppression. Front Oncol. 2019;9:187. doi: 10.3389/fonc.2019.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen Q, Han B, Meng X, Duan C, Yang C, Wu Z, Magafurov D, Zhao S, Safin S, Jiang C, Cai J. Immunogenomic analysis reveals LGALS1 contributes to the immune heterogeneity and immunosuppression in glioma. Int J Cancer. 2019;145(2):517–530. doi: 10.1002/ijc.32102. [DOI] [PubMed] [Google Scholar]
- 123.Qian J, Luo F, Yang J, Liu J, Liu R, Wang L, Wang C, Deng Y, Lu Z, Wang Y, Lu M, Wang JY, Chu Y. TLR2 promotes glioma immune Evasion by downregulating MHC class II molecules in microglia. Cancer Immunol Res. 2018;6(10):1220–1233. doi: 10.1158/2326-6066.CIR-18-0020. [DOI] [PubMed] [Google Scholar]
- 124.Basta PV, Moore TL, Yokota S, Ting JP. A β-adrenergic agonist modulates DR α gene transcription via enhanced cAMP levels in a glioblastoma multiforme line. J Immunol. 1989;142:2895–2901. [PubMed] [Google Scholar]
- 125.Vismara MFM, Donato A, Malara N, Presta I, Donato G. Immunotherapy in gliomas: are we reckoning without the innate immunity? Int J Immunopathol Pharmacol. 2019;33:2058738419843378. doi: 10.1177/2058738419843378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gross N, Beck D, Favre S, Carrel S. In vitro modulation of human neuroblastoma cells induced by IFN-gamma, retinoic acid, and dibutyryl cyclic AMP. Int J Cancer. 1987;39:521. doi: 10.1002/ijc.2910390420. [DOI] [PubMed] [Google Scholar]
- 127.Frohman EM, Vayuvegula B, van den Noort S, Gupta S. Norepinephrine inhibits gamma-interferon-induced MHC class II (Ia) antigen expression on cultured brain astrocytes. J Neuroimmunol. 1988;17:89. doi: 10.1016/0165-5728(88)90017-3. [DOI] [PubMed] [Google Scholar]
- 128.Blomhoff HK, Ruud E, Fundunderud S, Godal T Distinct effect of forskolin and interferon-gamma on cell proliferation and regulation of histocompatibility antigen expression in hematopoietic cells. Biochim. Biophys. Acta. 1986;887:150. doi: 10.1016/0167-4889(86)90049-2. [DOI] [PubMed] [Google Scholar]
- 129.Laureys G, Gerlo S, Spooren A, Demol F, De Keyser J, Aerts JL. ß2-adrenergic agonists modulate TNF-a induced astrocytic inflammatory gene expression and brain inflammatory cell populations. J Neuroinflamm. 2014;30(11):21. doi: 10.1186/1742-2094-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.McAuley DF, Matthay MA. Is there a role for beta-adrenoceptor agonists in the management of acute lung injury and the acute respiratory distress syndrome? Treat Respir Med. 2005;4(5):297–307. doi: 10.2165/00151829-200504050-00001. [DOI] [PubMed] [Google Scholar]
- 131.Vojvodic A, Vojvodic P, Vlaskovic-Jovicevic T, Sijan G, Dimitrijevic S, Peric-Hajzler Z, Matovic D, Wollina U, Tirant M, Thuong NV, Fioranelli M, Lotti T. Beta blockers and melanoma. Open Access Maced J Med Sci. 2019;7(18):3110–3112. doi: 10.3889/oamjms.2019.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Perron L, Bairati I, Harel F, Meyer F. Antihypertensive drug use and the risk of prostate cancer (Canada) Cancer Causes Control. 2004;15(6):535–541. doi: 10.1023/B:CACO.0000036152.58271.5e. [DOI] [PubMed] [Google Scholar]
- 133.Nkontchou G, Aout M, Mahmoudi A, Roulot D, Bourcier V, Grando-Lemaire V, Ganne-Carrie N, Trinchet JC, Vicaut E, Beaugrand M. Effect of long-term propranolol treatment on hepatocellular carcinoma incidence in patients with HCV associated cirrhosis. Cancer Prev Res (Phila) 2012;5:1007–1014. doi: 10.1158/1940-6207.CAPR-11-0450. [DOI] [PubMed] [Google Scholar]
- 134.Childers WK, Hollenbeak CS, Cheriyath P. Beta-blockers reduce breast cancer recurrence and breast cancer death: a metaanalysis. Clin Breast Cancer. 2015;15:426–431. doi: 10.1016/j.clbc.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 135.Amenta PS, Hadad S, Lee MT, Barnard N, Li D, Myers JC. Loss of types XV and XIX collagen precedes basement membrane invasion in ductal carcinoma of the female breast. J Pathol. 2003;199(3):298–308. doi: 10.1002/path.1303. [DOI] [PubMed] [Google Scholar]
- 136.Baba K, Kuwano H, Kitamura K, Sugimachi K. Carcinomatous invasion and lymphocyte infiltration in early esophageal carcinoma with special regard to the basement membrane. Immunohistochem Study Hepatogastroenterol. 1993;40(3):226–231. [PubMed] [Google Scholar]
- 137.Varga I, Hutóczki G, Petrás M, Scholtz B, Mikó E, Kenyeres A, Tóth J, Zahuczky G, Bognár L, Hanzély Z, Klekner A. Expression of invasion-related extracellular matrix molecules in human glioblastoma versus intracerebral lung adenocarcinoma metastasis. Cent Eur Neurosurg. 2010;71(4):173–180. doi: 10.1055/s-0030-1249698. [DOI] [PubMed] [Google Scholar]
- 138.van Wyk HC, Roxburgh CS, Horgan PG, Foulis AF, McMillan DC. The detection and role of lymphatic and blood vessel invasion in predicting survival in patients with node negative operable primary colorectal cancer. Crit Rev Oncol Hematol. 2014;90(1):77–90. doi: 10.1016/j.critrevonc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 139.Zeng Q, Michael IP, Zhang P, Saghafinia S, Knott G, Jiao W, McCabe BD, Galván JA, Robinson HPC, Zlobec I, Ciriello G, Hanahan D. Synaptic proximity enables NMDAR signalling to promote brain metastasis. Nature. 2019;573(7775):526–531. doi: 10.1038/s41586-019-1576-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhu C, Kros JM, Cheng C, Mustafa D. The contribution of tumor-associated macrophages in glioma neo-angiogenesis and implications for anti-angiogenic strategies. Neuro Oncol. 2017;19(11):1435–1446. doi: 10.1093/neuonc/nox081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fuwa M, Kageyama M, Ohashi K, Sasaoka M, Sato R, Tanaka M, Tashiro K. Nafamostat and sepimostat identified as novel neuroprotective agents via NR2B N-methyl-d-aspartate receptor antagonism using a rat retinal excitotoxicity model. Sci Rep. 2019;9(1):20409. doi: 10.1038/s41598-019-56905-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Guo C, Whitmarsh AJ. The β-arrestin-2 scaffold protein promotes c-Jun N-terminal kinase-3 activation by binding to its nonconserved N terminus. J Biol Chem. 2008;283(23):15903–15911. doi: 10.1074/jbc.M710006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bauer R, Enns H, Jungmann A, Leuchs B, Volz C, Schinkel S, Koch WJ, Raake PW, Most P, Katus HA, Müller OJ. Various effects of AAV9-mediated βARKct gene therapy on the heart in dystrophin-deficient (mdx) mice and δ-sarcoglycan-deficient (Sgcd-/-) mice. Neuromuscul Disord. 2019;29(3):231–241. doi: 10.1016/j.nmd.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 144.Chen SH, Sun JM, Chen BM, Lin SC, Chang HF, Collins S, Chang D, Wu SF, Lu YC, Wang W, Chen TC, Kasahara N, Wang HE, Tai CK. Efficient prodrug activator gene therapy by retroviral replicating vectors prolongs survival in an immune-competent intracerebral glioma model. Int J Mol Sci. 2020;21(4):E1433. doi: 10.3390/ijms21041433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Joshi CR, Labhasetwar V, Ghorpade A. Destination brain: the past, present, and future of therapeutic gene delivery. J Neuroimmune Pharmacol. 2017;12(1):51–83. doi: 10.1007/s11481-016-9724-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ran H, Quan G, Huang Y, Zhu C, Lu C, Liu W, Pan X, Wu C. The practical self-targeted oncolytic adenoviral nanosphere based on immuno-obstruction method via polyprotein surface precipitation technique enhances transfection efficiency for virotherapy. Biochem Biophys Res Commun. 2019;508(3):791–796. doi: 10.1016/j.bbrc.2018.10.162. [DOI] [PubMed] [Google Scholar]
- 147.Luttrell LM, Wang J, Plouffe B, Smith JS, Yamani L, Kaur S, Jean-Charles PY, Gauthier C, Lee MH, Pani B, Kim J, Ahn S, Rajagopal S, Reiter E, Bouvier M, Shenoy SK, Laporte SA, Rockman HA, Lefkowitz RJ. Manifold roles of β-arrestins in GPCR signaling elucidated with siRNA and CRISPR/Cas9. Sci Signal. 2018;11(549):eaat7650. doi: 10.1126/scisignal.aat7650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chen X, Xu Z, Zeng S, Wang X, Liu W, Qian L, Wei J, Yang X, Shen Q, Gong Z, Yan Y. The molecular aspect of antitumor effects of protease inhibitor nafamostat mesylate and its role in potential clinical applications. Front Oncol. 2019;3(9):852. doi: 10.3389/fonc.2019.00852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yamamoto M, Matsuyama S, Li X, Takeda M, Kawaguchi Y, Inoue JI, Matsuda Z. Identification of nafamostat as a potent inhibitor of middle east respiratory syndrome coronavirus S protein-mediated membrane fusion using the split-protein-based cell-cell fusion assay. Antimicrob Agents Chemother. 2016;60(11):6532–6539. doi: 10.1128/AAC.01043-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nishimura H, Yamaya M. A synthetic serine protease inhibitor, nafamostat mesilate, is a drug potentially applicable to the treatment of ebola virus disease. Tohoku J Exp Med. 2015;237(1):45–50. doi: 10.1620/tjem.237.45. [DOI] [PubMed] [Google Scholar]
- 151.Rathore AP, Mantri CK, Aman SA, Syenina A, Ooi J, Jagaraj CJ, Goh CC, Tissera H, Wilder-Smith A, Ng LG, Gubler DJ, St John AL. Dengue virus-elicited tryptase induces endothelial permeability and shock. J Clin Invest. 2019;2(130):4180–4193. doi: 10.1172/JCI128426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Coelho M, Soares-Silva C, Brandão D, Marino F, Cosentino M, Ribeiro L. β-Adrenergic modulation of cancer cell proliferation: available evidence and clinical perspectives. J Cancer Res Clin Oncol. 2017;143(2):275–291. doi: 10.1007/s00432-016-2278-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Stupp R, Weber DC. The role of radio- and chemotherapy in glioblastoma. Onkologie. 2005;28:315–317. doi: 10.1159/000085575. [DOI] [PubMed] [Google Scholar]
- 154.Marshall NJ, von Borcke S, Ekins RP. Independence of β-adrenergic and thyrotropin receptors linked to adenylate cyclase in the thyroid. Nature. 1976;261(5561):603–604. doi: 10.1038/261603a0. [DOI] [PubMed] [Google Scholar]