Abstract
Objective
This study aimed to conduct a systematic review of the clinical outcomes reported for pregnant patients with coronavirus disease 2019.
Data Sources
The PubMed, CINAHL, and Scopus databases were searched using a combination of key words such as “Coronavirus and/or pregnancy,” “COVID and/or pregnancy,” “COVID disease and/or pregnancy,” and “COVID pneumonia and/or pregnancy.” There was no restriction of language to allow collection of as many cases as possible.
Study Eligibility Criteria
All studies of pregnant women who received a coronavirus disease 2019 diagnosis using acid nucleic test, with reported data about pregnancy, and, in case of delivery, reported outcomes, were included.
Study Appraisal and Synthesis Methods
All the studies included have been evaluated according to the tool for evaluating the methodological quality of case reports and case series described by Murad et al.
Results
Six studies that involved 51 pregnant women were eligible for the systematic review. At the time of the report, 3 pregnancies were ongoing; of the remaining 48 pregnant women, 46 gave birth by cesarean delivery, and 2 gave birth vaginally; in this study, 1 stillbirth and 1 neonatal death were reported.
Conclusion
Although vertical transmission of severe acute respiratory syndrome coronavirus 2 infection has been excluded thus far and the outcome for mothers and neonates has been generally good, the high rate of preterm delivery by cesarean delivery is a reason for concern. Cesarean delivery was typically an elective surgical intervention, and it is reasonable to question whether cesarean delivery for pregnant patients with coronavirus disease 2019 was warranted. Coronavirus disease 2019 associated with respiratory insufficiency in late pregnancies certainly creates a complex clinical scenario.
Key words: cesarean delivery, coronavirus pneumonia, COVID-19, fetal death, neonatal outcomes, preterm birth, SARS-CoV-2, stillbirth, vertical transmission novel coronavirus, viral pneumonia
Introduction
On March 12, 2020, on the basis of more than 20,000 confirmed cases and almost 1000 deaths in Europe, the World Health Organization announced the new coronavirus outbreak pandemic. At the end of December 2019, a cluster of cases of pneumonia of unknown cause was reported in Wuhan, Hubei Province, China.1 At the beginning of January 2020, a novel coronavirus, called 2019-nCoV, was identified as the etiologic agent by Chinese authorities. Other coronavirus infections included the common cold (HCoV-229E, NL63, OC43, and HKU1), Middle East respiratory syndrome (MERS-CoV), and severe acute respiratory syndrome (SARS-CoV). A study reviewed the epidemiologic, clinical, laboratory, and radiologic features, as well as treatment and clinical outcomes, of patients with pneumonia caused by laboratory-confirmed coronavirus disease 2019 (COVID-19).2
AJOG at a Glance.
Why was this study conducted?
Coronavirus disease 2019 (COVID-19) is a pandemic and will affect a large number of pregnant patients in the near future. Preliminary experience with pregnant patients with COVID-19 suggests that the clinical outcome of the mother and neonate is often favorable, but it is unclear how and when these patients gave birth. The aim of this study was to conduct a systematic review of the clinical outcomes reported for pregnant patients with COVID-19.
Key findings
The median gestational age was 36.5 weeks (interquartile range, 35–38), with 15 cases of preterm birth (39%); cesarean delivery was reported in 96% of the cases, but the indications were not clearly described.
What does this add to what is known?
Most pregnant patients with COVID-19 thus far have given birth preterm by cesarean delivery; in some cases, cesarean deliveries were elective. Whether cesarean delivery is warranted or not remains to be established.
The presence of COVID-19 in a pregnant patient raises concerns, as other types of coronaviruses were frequently associated with adverse outcomes.3 Professional organizations have rapidly published preliminary documents providing advice on diagnosis and management of pregnant patients with COVID-19.4 , 5 However, the scientific literature on the subject is scanty.
Objective
The aim of this study was to collect and review the available information about the impact of COVID-19 on mothers and neonates and to focus on time and mode of delivery. In addition, we conducted a systematic review of the available literature in English and Chinese languages.2 , 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16
Materials and methods
Study design
To determine eligible articles, the following electronic databases were screened from March 14, 2020, to March 16, 2020: PubMed, Scopus, and CINAHL. To retrieve articles related with the theme of interest, the following terms were used to search the electronic databases: “coronavirus and/or pregnancy,” “COVID and/or pregnancy,” “COVID disease and/or pregnancy,” and “COVID pneumonia and/or pregnancy.”
Criteria for study selection
Reports included in this review consisted of case series, case reports, and retrospective studies. No randomized controlled trials were found. Only reports describing management of pregnancies complicated by COVID-19 were included in this systematic review. We registered this review on the International Prospective Register of Systematic Reviews. We did not provide contacts with the corresponding authors because of the time constraints and the importance to have immediate results.
Assessment of risk of bias
Two study investigators (A.N.D.G. and R.R.) independently conducted the primary literature research using the main search terms. When the studies not conforming to the preestablished eligibility and inclusion criteria were excluded, the same study investigators (A.N.D.G. and R.R.) independently reviewed the data collection forms in a second screening time. At the third review time, the remaining reports were further analyzed for compatibility. In case of any disagreement between the examiners after independent evaluation, consensus was reached by reevaluation and discussion with a more experienced author (G.S.). The remaining studies were introduced into the final review step of qualitative synthesis.
Data quality assessment
Two independent examiners (A.N.D.G. and R.R.) applied the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) for the data extraction and quality assessment.11 The examiners (A.N.D.G. and R.R.) independently assessed the methodologies of the studies according to the tool for evaluating the methodological quality of case reports and case series described by Murad et al.13 The tool considers 4 domains (selection, ascertainment, causality, and reporting) and provides 8 questions to aid quality score (Table 1 ). If all of the domains were satisfied, the study would be classified as “good quality”; if 3 of the domains were satisfied, the study would be classified as “fair quality”; and if only 2 or 1 of the domains were satisfied, the study would be classified as “poor quality.”
Table 1.
Table tool used for the evaluation of the methodological quality of case reports and case series13
| Domains | Leading explanatory questions |
|---|---|
| Selection |
|
| Ascertainment |
|
|
|
| Causality |
|
|
|
|
|
|
|
| Reporting |
|
Della Gatta. COVID-19 during pregnancy: a systematic review. Am J Obstet Gynecol 2020.
Data extraction and synthesis
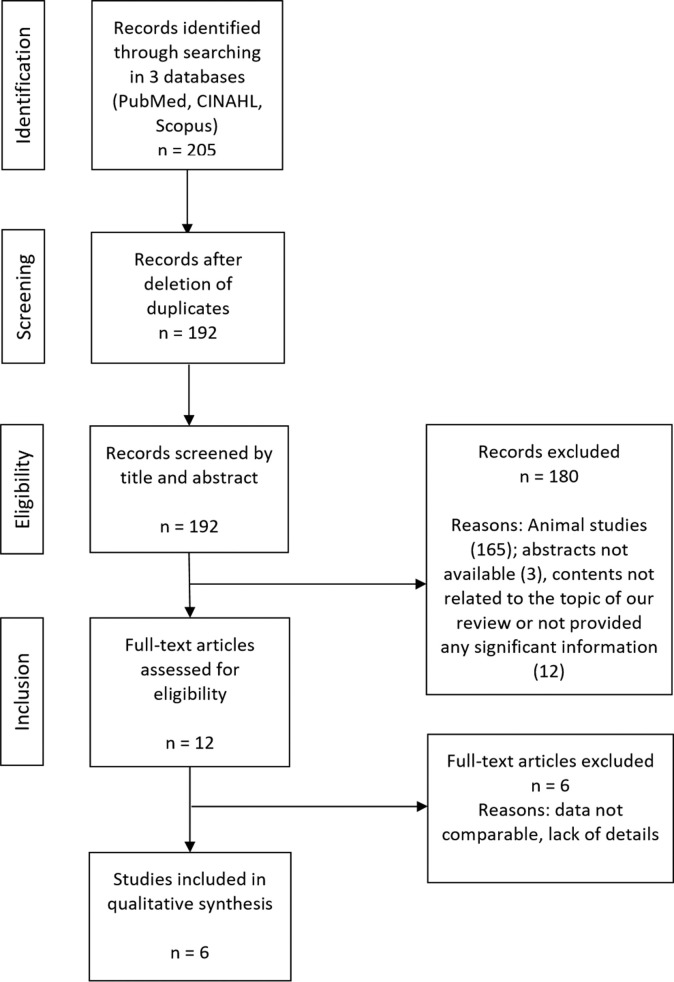
A total of 205 articles with publication dates from 1969 to 2020 were found through the PubMed, CINAHL, and Scopus databases. The selection process followed the PRISMA workflow. We excluded 180 articles for the following reasons: animal studies (165), abstract not available (3), contents not related to the topic of our review, or significant information not provided (12). To collect as many cases as possible, no restriction on language (Chinese and English) was applied in the selection of articles. We included only studies in which the diagnosis was based on the criteria provided by the New Coronavirus Pneumonia Prevention and Control Program (4th edition and subsequent editions) published by the National Health Commission of China.5 After deletion of duplicates, a total number of 12 references were selected; 6 full-text articles were excluded because data were not comparable. We subsequently reviewed the 6 articles describing clinical presentation, pathogenesis, macroscopic and histopathologic aspects, natural history, diagnosis, and treatment. Furthermore, we selected all reported cases with pregnant patients aged ≥20 years and analyzed the following aspects: clinical features, symptoms, associated diseases, fetal characteristics, time of delivery, type of delivery, and follow-up. Two study investigators (A.N.D.G. and R.R.) independently reviewed the data collection forms to verify data accuracy.
Results
A total of 6 studies were eventually selected for analysis (Figure ; Table 2 ). From a methodological point of view, only 1 study6 fulfilled all of the domains, 3 studies10 , 15 , 16 were classified of fair quality, and 2 studies7 , 8 were judged to be of poor quality because the selection criteria of the cases were unclear. Most articles did not report follow-up of the pregnant women after delivery, and 1 study included 3 ongoing pregnancies.10 We found a total of 51 cases of pregnancies with COVID-19. In 50 patients, the diagnosis of COVID-19 was confirmed by quantitative reverse transcriptase polymerase chain reaction on samples from the respiratory tract. In 1 patient,16 diagnosis was based on clinical symptoms and a chest computed tomography scan typical for viral interstitial pneumonia. Because of the exclusion of other diseases that could cause fever and lung infection, the local Center for Disease Control registered her as a confirmed 2019-nCoV case.
Figure.
Search strategy flowchart
Della Gatta. COVID-19 during pregnancy: a systematic review. Am J Obstet Gynecol 2020.
Table 2.
List of studies included in the analysis
| First author | Judgmenta | Cases | Type of study | Setting |
|---|---|---|---|---|
| Chen et al6 | Good | 9 | Retrospective | Department of Gynaecology and Obstetrics, Zhongnan Hospital of Wuhan University, Wuhan, Hubei, China |
| Liu et al10 | Fair | 13 | Retrospective | Hospital of Sun Yat-sen University, Guangzhou, Guangdong 510080, China |
| Chen et al7 | Poor | 3 | Retrospective | Department of Obstetrics and Gynecology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, China |
| Zhang et al15 | Fair | 16 | Retrospective with control group | Department of Obstetrics, the Central Hospital of Qianjiang City, Qianjiang 433199, China |
| Zhu et al16 | Fair | 9 | Retrospective | Department of Neonatology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, China |
| Li et al8 | Poor | 1 | Retrospective | The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, China |
Della Gatta. COVID-19 during pregnancy: a systematic review. Am J Obstet Gynecol 2020.
Using the tool described by Murad at al.13
Median maternal age was 30 years (interquartile range [IQR], 27.5–33). Median gestational age at diagnosis was 36 weeks (IQR, 35–37.5). Of 48 pregnant patients who delivered, the median gestational age was 36.5 weeks (IQR, 35–38), and 15 patients (31%) delivered before 37 weeks’ gestation. In 22 cases, the interval between symptoms onset and delivery6, 7, 8 , 16 ranged between 1 and 7 days (median 2; IQR, 1–4). In 3 cases, COVID-19 symptoms appeared after delivery.16
Symptoms at onset of COVID-19 infection were reported for 35 pregnant women (69%), and the symptoms were similar to those described in nonpregnant patients (Table 3 )2:
-
•
Seventeen pregnant women (48%) presented with fever at hospital admission.
-
•
Sixteen women (46%) indicated dry cough (considered alone or in association with other symptoms).
-
•
Eight patients (23%) had fever only in the postpartum period.
Table 3.
Reported symptoms at diagnosis
| Onset symptoms | Reported only for 35 of 51 patients, often in combination (%) |
|---|---|
| Dry cough | 16/35 (45.7) |
| Fever admission | 17/35 (48.6) |
| Postpartum fever | 8/35 (22.9) |
| Myalgias | 3/35 (8.6) |
| Malaise | 2/35 (5.7) |
| Dyspnea | 4/35 (11.4) |
| Sore throat | 5/35 (14.3) |
| Diarrhea | 2/35 (5.7) |
| Fatigue | 3/35 (8.6) |
| Cholecystitis | 2/35 (5.7) |
Della Gatta. COVID-19 during pregnancy: a systematic review. Am J Obstet Gynecol 2020.
Less frequent symptoms included sore throat (5 cases), dyspnea (4 cases), fatigue (3 cases), myalgia (3 cases), malaise (2 cases), diarrhea (2 cases), and cholecystitis (2 cases).
No pregestational comorbidities such as hypertension, diabetes, or cardiovascular disease were reported. There was 1 case of gestational hypertension at 27 weeks’ gestation and 1 case of preeclampsia at 31 weeks’ gestation; in both cases, symptoms appeared after the diagnosis of COVID-19. In addition to COVID-19 during pregnancy, there was 1 case of influenza infection at admission in the hospital for respiratory difficulties.6 Four patients had a previous cesarean delivery, 1 patient a previous stillbirth, and 1 patient placenta previa.7
We found no cases of coronavirus infection during the first trimester of pregnancy, 2 cases of infection in the second trimester, and 49 cases of infection in the third trimester. The 2 cases in the second trimester and the 1 case in the third trimester (33 weeks’ gestation) were reported as ongoing.10 Of the remaining 48 women, 2 had a spontaneous vaginal delivery: 1 at gestational age of 34 weeks and 2 days (in this case, diagnosis of COVID-19 was done only after delivery) and 1 at gestational age of 31 weeks (this was a case of twin pregnancy).16 The remaining 46 patients underwent cesarean delivery. The indications to the cesarean delivery were not clearly reported in all cases. Available data regarding indications to cesarean delivery are shown in Table 4 . It is noteworthy that premature rupture of the membranes occurred in at least 9 of 34 patients (26%).
Table 4.
Indications to cesarean delivery in 34 cases
| Indication | Cases, n (%) |
|---|---|
| COVID-19 pneumonia | 19 (55.9) |
| PROM | 9 (26.5) |
| Fetal distress | 6 (17.6) |
| Preterm labor | 4 (11.8) |
| Previous cesarean delivery | 3 (8.8) |
| Previous stillbirth | 2 (5.9) |
| Pregnancy at term | 2 (5.9) |
| Elevated liver enzymes | 1 (2.9) |
| Preeclampsia | 1 (2.9) |
| Placenta previa | 1 (2.9) |
| Abruptio placentae | 1 (2.9) |
| Multiple organ dysfunction syndrome | 1 (2.9) |
| Oligohydramnios | 1 (2.9) |
| Psychosocial factors | 1 (2.9) |
COVID-19, coronavirus disease 2019; PROM, premature rupture of membrane.
Della Gatta. COVID-19 during pregnancy: a systematic review. Am J Obstet Gynecol 2020.
Furthermore, a 30-year-old patient with no comorbidities received a diagnosis of COVID-19 at 34 weeks’ gestation and developed severe pneumonia. Her condition worsened during hospitalization, requiring intensive care unit (ICU) admission and multiple organ dysfunction syndrome associated with acute respiratory distress syndrome requiring intubation and mechanical ventilation. Further complications occurred including acute hepatic failure, acute renal failure, and septic shock. A intrauterine fetal demise occurred in this case. At the time of publication of the original work, this patient was still in the support of extracorporeal membrane oxygenation.10 Another patient was admitted to the ICU after delivery for developing severe pneumonia and for worsening of general condition; she was discharged at the time of publication of the original paper.15 Not all studies reported data for the radiologic tests performed in pregnant women: among 51 cases of COVID-19 infection during pregnancy, only 22 patients were reported with a chest computed tomography confirmatory test for typical signs of viral infection; furthermore, 1 patient had a negative chest x-ray result for pneumonia, and 1 patient had a positive chest x-ray result for pneumonia. Data from laboratory tests were not complete for all cases. Authors reported increased concentrations of alanine aminotransferase and aspartate aminotransferase for 2 patients.6 , 15
One fetal death occurred in a critically ill patient.10 The remaining 48 neonates (with 1 set of twins16) were in good condition at the time of birth. However, not all reviewed studies reported a 5-minute Apgar score. In all cases, neonatal throat swab samples were collected within 72 hours after birth and tested negative, with the exception of 1 positive infant who was tested 36 hours after birth. One neonate was delivered by cesarean delivery at gestational age of 34 weeks and 5 days; was adequate for gestational age; was admitted to the neonatal intensive care unit (NICU) 30 minutes after delivery because of shortness of breath and moaning; developed thrombocytopenia, liver dysfunction, and multiple organ failure; and died 9 days after delivery. The authors stated that although a throat swab in this neonate was negative for COVID-19, a perinatal infection cannot be excluded.16 Admission to the NICU in the remaining infants was not clearly described.
Comments
Principal finding
Thus far, pregnant patients with COVID-19 have almost invariably delivered by cesarean delivery and frequently before term gestation. This is a reason for concern because the COVID-19 pandemic is spreading around the world, and most likely, many pregnant women will be affected.17 However, one may question whether the choices made by the obstetricians thus far were modified. In most cases, the indication for cesarean delivery was not clearly stated, and it is certainly possible that the decision was influenced by the understandable anxiety toward the potential consequences of a new viral infection.18, 19, 20 As a matter of fact, in our analysis of the available literature, the clinical outcome has been generally favorable for both mothers and neonates, although a word of caution is necessary. Of the 51 cases we analyzed, at least 1 mother was severely compromised, and in general follow-up, data were scanty. We confirm that there was no evidence of vertical transmission, but previous experience with infections, caused by similar pathogens, such as SARS and MERS,21 , 22 indicates that vertical transmission is not the exclusive cause of fetal morbidity and mortality. Out of 48 neonates that were delivered, there was 1 stillbirth in a severely compromised mother, and there was also 1 neonatal death that may not be independent from the infection. At present, the available evidence does not provide insight as to whether these patients require or do not require a different approach from a standard one. It would be important that in the near future, studies around the implications of COVID-19 in pregnancy contain thorough information about both the maternal and fetal conditions at the time of delivery and the rationale behind obstetrical interventions.
Strengths and limitations
The main strength of our analysis is that we have provided thus far the largest series on pregnancies with COVID-19. The main weakness is that the available literature around the obstetrical implications of COVID-19 is limited, both in numbers and in quality. The justification behind our study is that the spread of the disease dictates the need to rapidly evaluate and discuss the evidence that has been generated.
Clinical implications
Authorities and professional societies, such as the Italian Health Council,5 the English Royal College of Obstetricians and Gynaecologists,3 and the Society for Maternal-Fetal Medicine,4 have taken a stance that COVID-19 is not a contraindication to vaginal delivery. In light of the absence of vertical transmission and of the outcome that preliminary experience has been generally good, the stance of the authors appears reasonable. However, thus far, virtually all patients had undergone elective cesarean delivery soon after the diagnosis. Whether this contributed to the favorable results that were observed seems unlikely, but it cannot be excluded with certainty. In nonpregnant patients, COVID-19 spans along a wide spectrum of severity. Most patients, particularly those of young age, are asymptomatic or not respiratory compromised. In these cases, standard obstetrical care seems sufficient, with the only caveat that fetal distress was described in almost 20% of cases. Whether this was related to maternal compromise or not is not known, but it seems reasonable to provide continuous fetal monitoring in labor. In a minority of patients (1 case in our series of 51), severe respiratory compromise will be present. In such cases, after fetal viability, cesarean delivery may be life-saving for both the mother and the neonate. The most difficult scenario is certainly the intermediate case, a patient with compensated respiratory insufficiency, that may deteriorate in the following days. In preterm pregnancies, balancing the pros and cons of a conservative management vs expediting the delivery is difficult, and determining the optimal mode of delivery of a mother with hypoxia is also a difficult decision. The issue of obstetrical complications is also relevant. The high rate of premature delivery in our review appears to be mostly the consequence of elective interventions. However, COVID-19 seems to be associated with spontaneous preterm birth; in our review, preterm labor was reported in at least 6 of 48 cases, and premature rupture of the membranes was reported in 9 of 34 cases.
Conclusion
The available data on pregnant patients with COVID-19 do not provide a clear conclusion into the clinical implications for the mother and neonate. The outcome thus far described is favorable, but fetal and maternal risks should not be underestimated. Although preterm delivery was mostly the consequence of elective interventions, a trend toward spontaneous prematurity is present. It is essential that future studies provide more detailed information on maternal and fetal conditions, as well as the rationale for obstetrical interventions. Experience, thus far, is limited to patients who developed the disease in late gestation and patients who delivered shortly after the diagnosis. The fetal consequences of long-standing infections occurring in early gestation are unknown.
Acknowledgments
The authors acknowledge the contribution of Ms Erika Bonaccorso for translating Chinese reports to English.
Footnotes
The authors report no conflict of interest.
References
- 1.World Health Organization Coronavirus disease (COVID-19) pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 Available at:
- 2.Wu Y.C., Chen C.S., Chan Y.J. The outbreak of COVID-19: an overview. J Chin Med Assoc. 2020;83:217–220. doi: 10.1097/JCMA.0000000000000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Mascio D., Khalil A., Saccone G., et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2020 doi: 10.1016/j.ajogmf.2020.100107. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Royal College of Obstetricians & Gynaecologists Coronavirus (COVID-19) infection in pregnancy. Information for healthcare professionals. Version 8. 2020. https://www.rcog.org.uk/globalassets/documents/guidelines/2020-04-17-coronavirus-covid-19-infection-in-pregnancy.pdf Available at:
- 5.National Health Commission of China New coronavirus pneumonia prevention and control program [in Chinese]. 4th ed. 2020. http://www.gov.cn/zhengce/zhengceku/2020-01/28/5472673/files/0f96c10cc09d4d36a6f9a9f0b42d972b.pdf Available at:
- 6.Chen H., Guo J., Wang C., et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen S., Huang B., Luo D.J., et al. Pregnant women with new coronavirus infection: a clinical characteristics and placental pathological analysis of three cases. Zhonghua Bing Li Xue Za Zhi. 2020;49:E005. doi: 10.3760/cma.j.cn112151-20200225-00138. [DOI] [PubMed] [Google Scholar]
- 8.Li Y., Zhao R., Zheng S., et al. Lack of vertical transmission of severe acute respiratory syndrome coronavirus 2, China. Emerg Infect Dis J. 2020 doi: 10.3201/eid2606.200287. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang H., Acharya G. Novel corona virus disease (COVID-19) in pregnancy: what clinical recommendations to follow? Acta Obstet Gynecol Scand. 2020;99:439–442. doi: 10.1111/aogs.13836. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y., Chen H., Tang K., Guo Y. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy. J Infect. 2020 doi: 10.1016/j.jinf.2020.02.028. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D., Liberati A., Tetzlaff J., et al. and the PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 12.Mullins E, Evans D, Viner RM, O’Brien P, Morris E. Coronavirus in pregnancy and delivery: rapid review. Ultrasound Obstet Gynecol [In press]. [DOI] [PubMed]
- 13.Murad M.H., Sultan S., Haffar S., Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23:60–63. doi: 10.1136/bmjebm-2017-110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang H., Wang C., Poon L.C. Novel coronavirus infection and pregnancy. Ultrasound Obstet Gynecol. 2020;55:435–437. doi: 10.1002/uog.22006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang L., Jiang Y., Wei M., et al. Analysis of the pregnancy outcomes in pregnant women with COVID-19 in Hubei Province. Zhonghua Fu Chan Ke Za Zhi. 2020;55:E009. doi: 10.3760/cma.j.cn112141-20200218-00111. [DOI] [PubMed] [Google Scholar]
- 16.Zhu H., Wang L., Fang C., et al. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020;9:51–60. doi: 10.21037/tp.2020.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schuchat A. Reflections on pandemics, past and present. Am J Obstet Gynecol. 2011;204(6 Suppl 1):S4–S6. doi: 10.1016/j.ajog.2011.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adhikari E.H., Nelson D.B., Johnson K.A., et al. Infant outcomes among women with Zika virus infection during pregnancy: results of a large prenatal Zika screening program. Am J Obstet Gynecol. 2017;216:292.e1–292.e8. doi: 10.1016/j.ajog.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 19.Avram C.M., Greiner K.S., Tilden E., Caughey A.B. Point-of-care HIV viral load in pregnant women without prenatal care: a cost-effectiveness analysis. Am J Obstet Gynecol. 2019;221:265.e1–265.e9. doi: 10.1016/j.ajog.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 20.Avram C.M., Greiner K.S., Tilden E., Caughey A.B. Additional considerations regarding point-of-care HIV viral load in pregnant women without prenatal care. Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2019.06.021. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Schwartz D.A., Graham A.L. Potential Maternal and Infant Outcomes from (Wuhan) Coronavirus 2019-nCoV Infecting Pregnant Women: Lessons from SARS, MERS, and Other Human Coronavirus Infections. Viruses. 2020;12 doi: 10.3390/v12020194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rasmussen S.A., Smulian J.C., Lednicky J.A., Wen T.S., Jamieson D.J. Coronavirus disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.02.017. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]