Abstract
Background & Aims
We compared clinical, laboratory, radiological, and outcome features of patients with SARS-CoV-2 infection (COVID-19) with pneumonia, with vs without diarrhea.
Methods
We performed a retrospective, single-center analysis of 84 patients with SARS-CoV-2 pneumonia in Wuhan Union Hospital, China, from January 19 through February 7, 2020. Cases were confirmed by real-time reverse-transcriptase PCR of nasal and pharyngeal swab specimens for SARS-CoV-2 RNA. Blood samples were analyzed for white blood cell count, lymphocyte count, alanine aminotransferase, creatine kinase, lactate dehydrogenase, D-dimer, C-reactive protein, and in some cases, immunoglobulins, complement, lymphocyte subsets, and cytokines. Virus RNA was detected in stool samples by real-time PCR.
Results
Of the 84 patients with SARS-CoV-2 pneumonia, 26 (31%) had diarrhea. The duration of fever and dyspnea in patients with diarrhea was significantly longer than those without diarrhea (all P < .05). Stool samples from a higher proportion of patients with diarrhea tested positive for virus RNA (69%) than from patients without diarrhea (17%) (P < .001). As of February 19, a lower proportion of patients with diarrhea had a negative result from the latest throat swab for SARS-CoV-2 (77%) than patients without diarrhea (97%) (P = .010), during these patients’ hospitalization. Of 76 patients with a negative result from their latest throat swab test during hospitalization, a significantly higher proportion of patients with diarrhea had a positive result from the retest for SARS-CoV-2 in stool (45%) than patients without diarrhea (20%) (P = .039).
Conclusions
At a single center in Wuhan, China, 31% of patients with SARS-CoV-2 pneumonia had diarrhea. A significantly higher proportion of patients with diarrhea have virus RNA in stool than patients without diarrhea. Elimination of SARS-CoV-2 from stool takes longer than elimination from the nose and throat.
Keywords: COVID-19, SARS-CoV-2, Diarrhea, Pneumonia
Abbreviations used in this paper: ACE2, angiotensin-converting enzyme 2; COVID-19, corona virus disease 2019; CT, computed tomography; RT-PCR, reverse transcriptase polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; WHO, World Health Organization
What You Need to Know.
Background
Studies are needed to compare the clinical, laboratory, and outcome features of patients with SARS-CoV-2 infection (COVID-19) with pneumonia, with vs without diarrhea.
Findings
At a single center in Wuhan, China, 31% of patients with SARS-CoV-2 pneumonia had diarrhea. A significantly higher proportion of patients with diarrhea have virus RNA in stool than patients without diarrhea.
Implications for patient care
Elimination of SARS-CoV-2 from stool takes longer than elimination from the nose and throat.
Since December 2019, pneumonia caused by novel coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has broken out in Wuhan, Hubei province, China.1 The novel coronavirus has spread to other cities in China and even around the world.1, 2, 3, 4 As of April 10, 2020, 1,521,252 confirmed cases have been reported globally. Among them, 83,305 confirmed cases were from China; furthermore, 3345 cases had died.5 The clinical manifestations of novel coronavirus, infected pneumonia (corona virus disease 2019 [COVID-19]), have been reported in several recent studies.1 , 6, 7, 8, 9 Some COVID-19 patients had gastrointestinal symptoms, especially diarrhea. It has been reported that the stools from confirmed cases were tested positive by reverse transcriptase polymerase chain reaction (RT-PCR) for SARS-CoV-2, suggesting the possibility of fomite transmission.1 , 3 However, the difference in clinical characteristics between diarrhea and non-diarrhea cases has not been reported.
In this study, we analyzed the differences in the clinical characteristics, laboratory examinations, imaging manifestations, and outcomes between COVID-19 patients with diarrhea and those without diarrhea.
Methods
Sources of Data
Consecutive patients with confirmed COVID-19 who were admitted to Wuhan Union Hospital from January 19 to February 7, 2020 were enrolled retrospectively. All patients were diagnosed according to World Health Organization (WHO) interim guidance. A confirmed case was defined as a positive result to real-time RT-PCR assay of nasal and pharyngeal swab specimens for SARS-CoV-2 RNA. Only the confirmed cases were enrolled in this study. Stool samples were also tested for SARS-CoV-2 RNA by RT-PCR. The definition of diarrhea by the WHO is having 3 or more loose or liquid stools per day or having more stools than a person’s health condition.
All medical records of the enrolled patients were collected, including clinical symptoms, laboratory findings, imaging manifestations, and outcomes. The clinical outcomes (ie, discharges, mortality) were monitored up to February 19, 2020, the final date of follow-up.
Data collection and analysis of cases were determined by the National Health Commission of the People’s Republic of China to be part of a continuing public health outbreak investigation and were thus considered exempt from institutional review board approval.
Laboratory Confirmation
The SARS-CoV-2 laboratory test assays were performed for throat swab specimens and stool samples on the basis of the previous WHO recommendation.10 RNA in patients’ specimens was extracted and tested for SARS-CoV-2 by real-time RT-PCR using the same protocol as previously described in the studies from Wuhan Jinyintan Hospital.6 , 7
Statistical Analysis
Continuous variables were expressed as the means and standard deviations. Categorical variables were summarized as the counts and percentages in each category. Comparisons were determined by unpaired t test or χ2 tests as appropriate. All statistical analyses were performed with SPSS 22.0 (SPSS Inc, Chicago, IL).
All authors had access to the study data and reviewed and approved the final manuscript.
Results
Clinical Characteristics
A total of 84 hospitalized health care workers with confirmed COVID-19 were enrolled in this study population, including 17 doctors, 66 nurses, and 1 allied health worker. All the doctors worked in the wards and went to outpatient clinic intermittently for 2 half-days per week. Most of the nurses worked in the wards, except for 3 nurses who worked in the fever clinic. The only allied health worker worked in the Network Center of Union Hospital. All of these patients were admitted to isolation wards. The median age of the patients was 37 years (range, 24–74 years), and 28 of the 84 patients (33%) were male. Among them, 26 patients (31%) had diarrhea; the remaining 58 patients had no diarrhea. The comparison of clinical characteristics between these 2 groups is shown in Table 1 . Several clinical symptoms were more common in diarrhea group as compared with non-diarrhea group, including headache (58% vs 22%, P = .003), myalgia or fatigue (65% vs 34%, P = .010), cough (85% vs 45%, P < .001), sputum production (54% vs 21%, P = .004), nausea (38% vs 10%, P = .005), and vomiting (19% vs 2%, P = .010).
Table 1.
Characteristics of COVID-19 Patients
| Characteristic | All patients (n = 84) | Diarrhea group (n = 26) | Non-diarrhea group (n = 58) | P value |
|---|---|---|---|---|
| Male sex, n (%) | 28 (33) | 8 (31) | 20 (35) | .806 |
| Age, y | ||||
| Median | 37 | 38.5 | 37 | |
| Range | 24–74 | 29–74 | 24–67 | |
| Age ≥50 y, n (%) | 23 (27) | 7 (27) | 16 (28) | >.999 |
| Suspected case contact exposure, n (%) | 57 (68) | 19 (73) | 38 (66) | .616 |
| Current smoking, n (%) | 5 (6) | 2 (8) | 3 (5) | .643 |
| Underlying illness, n (%) | 13 (16) | 3 (12) | 10 (17) | .746 |
| Respiratory disease | 3 (4) | 0 | 3 (5) | .549 |
| Diabetes mellitus | 6 (7) | 2 (8) | 4 (7) | >.999 |
| Hypertension | 4 (5) | 1 (4) | 3 (5) | >.999 |
| Symptoms | ||||
| Fever | 72 (86) | 22 (85) | 50 (86) | >.999 |
| Headache | 28 (33) | 15 (58) | 13 (22) | .003 |
| Myalgia or fatigue | 37 (44) | 17 (65) | 20 (34) | .010 |
| Cough | 48 (57) | 22 (85) | 26 (45) | <.001 |
| Sputum production | 26 (31) | 14 (54) | 12 (21) | .004 |
| Dyspnea | 32 (38) | 9 (35) | 23 (40) | .809 |
| Nausea | 16 (19) | 10 (38) | 6 (10) | .005 |
| Vomiting | 6 (7) | 5 (19) | 1 (2) | .010 |
| Abdominal pain | 2 (2) | 2 (8) | 0 (0) | .093 |
| Abdominal distention | 3 (4) | 2 (8) | 1 (2) | .225 |
| Tenesmus | 1 (1) | 1 (4) | 0 (0) | .310 |
COVID-19, corona virus disease 2019.
Laboratory and Radiologic Findings on Admission to Hospital
Table 2 shows the comparison of laboratory findings between the diarrhea group and non-diarrhea group at the time of hospitalization. Most of the laboratory findings had no difference between these 2 groups, including white blood cell count, lymphocyte count, alanine aminotransferase, creatine kinase, lactate dehydrogenase, D-dimer, and C-reactive protein. Some of the 84 patients were tested for immunoglobulins, complement, lymphocyte subsets, and cytokines. As shown in Supplementary Table 1, several cytokines including interleukin 2, interleukin 4, and interferon-γ decreased significantly in diarrhea group as compared with non-diarrhea group (2.26 ± 0.27 vs 2.59 ± 0.24 pg/mL, P = .001; 1.54 ± 0.37 vs 2.11 ± 0.44 pg/mL, P = .001; 1.90 ± 0.49 vs 2.85 ± 1.27 pg/mL, P = .012, respectively). However, there were no differences in immunoglobulins, complement, and lymphocyte subsets between these 2 groups, as shown in Supplementary Table 2, Supplementary Table 3.
Table 2.
Laboratory Findings of 84 Patients Infected With SARS-CoV-2 on Admission to Hospital
| All patients (n = 84) | Diarrhea group (n = 26) | Non-diarrhea group (n = 58) | P value | |
|---|---|---|---|---|
| White blood cell count, ×109/L | 4.0 ± 1.5 | 3.7 ± 1.6 | 4.2 ± 1.5 | .170 |
| <3.5, n (%) | 36 (43) | 16 (62) | 20 (35) | .055 |
| 3.5–9.5, n (%) | 46 (55) | 10 (38) | 36 (62) | |
| >9.5, n (%) | 2 (2) | 0 (0) | 2 (3) | |
| Neutrophil count, ×109/L | 2.4 ± 1.1 | 2.3 ± 1.3 | 2.5 ± 1.0 | .443 |
| Lymphocyte count, ×109/L | 1.3 ± 0.6 | 1.1 ± 0.4 | 1.3 ± 0.7 | .178 |
| <1.1, n (%) | 33 (39) | 14 (54) | 19 (33) | .143 |
| 1.1–3.2, n (%) | 49 (58) | 12 (46) | 37 (64) | |
| >3.2, n (%) | 2 (2) | 0 | 2 (3) | |
| Platelet count, ×109/L | 180.1 ± 58.2 | 164.6 ± 55.4 | 187.1 ± 58.8 | .103 |
| Hemoglobin, g/L | 128.3 ± 15.6 | 128.8 ± 15.5 | 128.1 ± 15.8 | .851 |
| ALT, U/L | 28.3 ± 19.3 | 20.6 ± 7.5 | 31.6 ± 21.8 | .014 |
| AST, U/L | 29.0 ± 13.1 | 24.9 ± 6.4 | 30.8 ± 14.8 | .055 |
| ALB, g/L | 40.4 ± 4.1 | 40.5 ± 4.7 | 40.3 ± 3.8 | .837 |
| T-BIL, μmol/L | 9.2 ± 3.0 | 8.3 ± 2.4 | 9.7 ± 3.2 | .050 |
| D-BIL, μmol/L | 3.0 ± 2.2 | 2.5 ± 1.5 | 3.2 ± 2.4 | .175 |
| CREA, μmol/L | 69.0 ± 18.9 | 66.4 ± 15.5 | 70.1 ± 20.3 | .411 |
| BUN, mmol/L | 3.7 ± 1.3 | 3.6 ± 1.3 | 3.8 ± 1.3 | .516 |
| CK, U/L | 109.5 ± 149.3 | 72.9 ± 45.3 | 125.9 ± 175.4 | .134 |
| LDH, U/L | 238.1 ± 98.5 | 213.1 ± 49.6 | 249.3 ± 112.6 | .121 |
| PT, sec | 13.3 ± 1.5 | 13.8 ± 2.6 | 13.1 ± 0.5 | .051 |
| APTT, sec | 38.8 ± 3.5 | 38.8 ± 4.2 | 38.7 ± 3.2 | .905 |
| D-dimer, mg/L | 0.47 ± 0.41 | 0.49 ± 0.51 | 0.46 ± 0.36 | .758 |
| CRP, mg/L, n (%) | ||||
| <8.0 | 35 (42) | 9 (35) | 26 (45) | .475 |
| ≥8.0 | 49 (58) | 17 (65) | 32 (55) | |
| PCT, μg/L, n (%) | ||||
| <0.5 | 82 (98) | 25 (96) | 57 (98) | .526 |
| ≥0.5 | 2 (2) | 1 (4) | 1 (2) | |
| ESR, mm/h | 21.9 ± 18.7 | 19.2 ± 14.1 | 23.0 ± 20.3 | .390 |
ALB, albumin; ALT, alanine aminotransferase; APTT, activated partial thromboplastin time; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CK, creatine kinase; CREA, creatinine; CRP, C-reactive protein; D-BIL, direct bilirubin; ESR, erythrocyte sedimentation rate; LDH, lactic dehydrogenase; PCT, procalcitonin; PT, prothrombin time; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; T-BIL, total bilirubin.
The most common finding in computed tomography (CT) images was ground-glass opacifications (96%), as shown in Supplementary Table 4. There were no differences in the location or number of ground-glass opacifications between these 2 groups. On admission to hospital, all confirmed COVID patients were tested for SARS-CoV-2 RNA from stool samples. Stool samples from a higher proportion of patients with diarrhea (69%) tested positive for virus RNA than from patients without diarrhea (17%) (P < .001) (Table 3 ).
Table 3.
Outcome of SARS-CoV-2 RT-PCR for Stool in Patients at Admission
| SARS-CoV-2 (RT-PCR) | All patients (n = 84) | Diarrhea group (n = 26) | Non-diarrhea group (n = 58) | P value |
|---|---|---|---|---|
| Negative, n (%) | 56 (67) | 8 (31) | 48 (83) | <.001 |
| Positive, n (%) | 28 (33) | 18 (69) | 10 (17) |
RT-PCR, reverse transcriptase polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Clinical Outcomes
As of February 19, 2020, none of these 84 patients had died or had been admitted to intensive care unit; 63 patients had been discharged, and 21 patients were still in hospital. All patients received antibiotics and antiviral agents during hospitalization; 39 patients (46%) received 2 kinds of antibiotics. After a period of time for treatment, throat swab specimens from patients were regathered and tested for SARS-CoV-2. If the result of real-time RT-PCR was negative 2 times consecutively, body temperature returned to normal for more than 3 days, and respiratory symptoms and lung lesions on CT scan improved significantly, patients were permitted to be discharged.
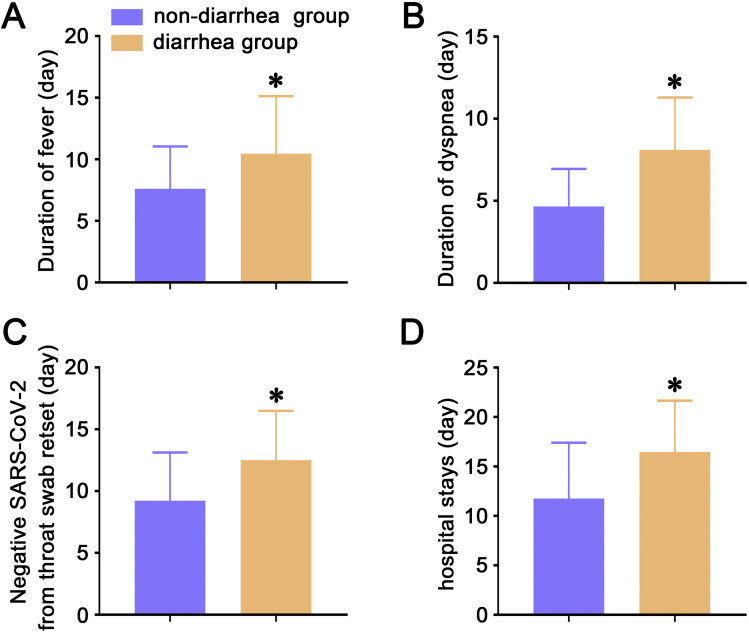
The duration of fever and dyspnea in patients with diarrhea was significantly longer than in those without diarrhea (10.5 ± 4.7 vs 7.6 ± 3.4 days, P = .005; 8.1 ± 3.2 vs 4.7 ± 2.3 days, P = .002, respectively) (Figure 1 A and B). By the end of February 19, 2020, a lower proportion of patients with diarrhea (77%) had a negative result from the latest throat swab for SARS-CoV-2 than patients without diarrhea (97%) (P = .010) during these patients’ hospitalization (Table 4 ). The mean time of SARS-CoV-2 in throat swab turning to be negative was longer in diarrhea group as compared with non-diarrhea group (12.5 ± 4.0 vs 9.2 ± 3.9 days, P = .002) (Figure 1 C). Patients’ stool specimens were also retested for SARS-CoV-2. Of 76 COVID-19 patients who had a negative result from their latest throat swab test during hospitalization, a significantly higher proportion of patients with diarrhea (45%) had a positive result from the retest for SARS-CoV-2 in stool than in patients without diarrhea (20%) (P = .039) (Table 5 ). All patients were reexamined with a CT scan during hospitalization. By the end of February 19, 2020, the improvement rate and deterioration rate showed no differences between diarrhea group and non-diarrhea group, as shown in Supplementary Table 5. Discharged patients were more common in non-diarrhea group as compared with diarrhea group (83% vs 58%, P = .028) (Supplementary Table 6). Meanwhile, the hospital stays were longer in diarrhea group than in non-diarrhea group (16.5 ± 5.2 vs 11.8 ± 5.6 days, P < .001) (Figure 1 D).
Figure 1.
(A) The lasting days of fever in COVID-19 patients. (B) The lasting days of dyspnea in COVID-19 patients. (C) Time from the day of the COVID-19 patients’ admission to patients’ SARS-CoV-2 in throat swab showing negative. (D) Hospital stays in COVID-19 patients. Data are presented as means ± standard deviation. ∗P < .05. Comparisons were determined by unpaired t test. COVID-19, corona virus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Table 4.
Outcome of SARS-CoV-2 RT-PCR Latest Examination for Throat Swab in Patients Infected With SARS-CoV-2 During Hospitalization
| SARS-CoV-2 RT-PCR latest examination | All patients (n = 84) | Diarrhea group (n = 26) | Non-diarrhea group (n=58) | P value |
|---|---|---|---|---|
| Negative, n (%) | 76 (90) | 20 (77) | 56 (97) | .010 |
| Positive, n (%) | 8 (10) | 6 (23) | 2 (3) |
RT-PCR, reverse transcriptase polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Table 5.
Outcome of SARS-CoV-2 RT-PCR Reexamination of Stool in Patients Whose Throat Swab Latest Test for SARS-CoV-2 Has Shown Negative
| SARS-CoV-2 (RT-PCR) | All patients (n = 76) | Diarrhea group (n = 20) | Non-diarrhea group (n = 56) | P value |
|---|---|---|---|---|
| Negative, n (%) | 56 (74) | 11 (55) | 45 (80) | .039 |
| Positive, n (%) | 20 (26) | 9 (45) | 11 (20) |
RT-PCR, reverse transcriptase polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
The mean diarrhea time of the 26 patients was 3.7 days. The smear for stool fungus was positive in 7 patients (27%), and stool occult blood was positive in 3 patients (12%). After treatment of intestinal microecological modulator, the visual analogue scale scores for diarrhea, frequency of defecation, and Bristol scores decreased significantly in patients with diarrhea (6.8 ± 1.1 vs 3.0 ± 1.0, 5.7 ± 2.8 vs 2.1 ± 0.8, 5.9 ± 0.6 vs 3.7 ± 0.7, respectively; all P < .0001), as shown in Supplementary Table 7.
Discussion
This study has shown that diarrhea occurred in 31% of patients with SARS-CoV-2 infectious pneumonia and focuses on the difference between COVID-19 patients with diarrhea and those without. Although most of the laboratory and radiologic findings showed no difference between these 2 groups, we did find some characteristics that differed between them.
The COVID-19 patients with diarrhea experienced headache, myalgia or fatigue, cough, sputum production, nausea, and vomiting more frequently than those patients without diarrhea, but they seldom experienced abdominal pain, abdominal distention, and tenesmus. The characteristics of diarrhea in SARS-CoV-2 pneumonia patients included increased defecation frequency (3–14 times per day) and pasty stool with no mucus or purulent blood. The diarrhea in some patients gradually alleviated and disappeared during hospitalization, but in other patients, the frequency of diarrhea increased, and smear for stool fungus and stool test for occult blood showed positive.
The intestinal epithelial injury caused by the infection of novel coronavirus might be an important cause of the diarrhea in COVID-19 patients. Full-genome sequencing and phylogenic analysis showed that SARS-CoV-2 and SARS-CoV belong to the same genus of coronaviruses (Betacoronaviruses), with about 80% sequence identity.11 , 12 SARS viral particles and genomic sequence were detected in the mucosa of the intestine.13 Recently, researchers from Guangzhou Institute of Respiratory Health successfully isolated SARS-CoV-2 from a COVID-19 patient’s stool (unpublished data, February 2020). Studies indicated that SARS-CoV-2 and SARS-CoV use the same receptor, angiotensin-converting enzyme 2 (ACE2), to get access into host cells.11 , 14 Through single-cell RNA sequencing technology, Zhao et al15 found that the expression of ACE2 was concentrated in a small population of type II alveolar cells (AT2 cells) in the normal human lungs. However, the lung AT2 cells were not the only highly expressing ACE2 cells; they were also in esophagus upper and stratified epithelial cells and absorptive enterocytes from ileum and colon.16 The digestive system is also a potential pathway for SARS-CoV-2 infection. As a common symptom in COVID-19 patients, diarrhea also indicates the involvement of the digestive system. The absorptive enterocytes were the most vulnerable intestinal epithelial cells and can be invaded by coronavirus and norovirus, leading to malabsorption, unbalanced intestinal secretion, and activated enteric nervous system, resulting in diarrhea finally.17 , 18 The diarrhea symptoms may be caused by the invaded ACE2-expressing enterocytes. The underlying molecular pathogenesis needs to be further investigated.
Another reason for the diarrhea in COVID-19 patients might be antibiotic-associated diarrhea. Broad-spectrum antibiotic use can disrupt the gastrointestinal microbiota, resulting in diarrhea.19 All patients in this study (100%) had received oral or intravenous antibiotics, and some (46%) had received 2 antibiotics. Because this is a small size study, we failed to analyze the correlation between antibiotic and diarrhea. The diarrhea in these patients was significantly relieved after taking intestinal probiotics, indicating that the use of antimicrobial drugs might be an important cause of diarrhea in COVID-19 patients.
We also found that the duration of symptoms in patients with diarrhea was significantly longer than in those without diarrhea, including fever and dyspnea. Patients with diarrhea took much more time to eliminate SARS-CoV-2 from respiratory system, leading to longer hospital stay time.
The frequency of positive rate for testing SARS-CoV-2 from stool was higher in patients with diarrhea, as compared with patients without diarrhea at admission. This indicated that SARS-CoV-2 infection in digestive system may be more common and more severe in patients with diarrhea. Moreover, stool specimens retested for SARS-CoV-2 in patients with persistent diarrhea persisted as positive, even after the throat swab testing for SARS-CoV-2 had turned out to be negative. The elimination of SARS-CoV-2 from digestive system may be much later and harder than that from respiratory system. Patients with negative throat swab test for SARS-CoV-2 may still be able to spread infection to other people through fomite transmission.
We suppose the delayed elimination of SARS-CoV-2 in digestive system might be partly related to the use of antibiotic. Studies indicated that antibiotics had profound effect on gut microbiota, leading to altered immune system, including antibody production and T-cell differentiation.20, 21, 22 Additional studies are needed to investigate the relationship between gut microbiota and antibiotics in COVID-19 patients.
The proportion of patients with diarrhea (31%) in this study is much higher than what was reported in other series.1 , 7 , 8 The possible reasons are the precise and timely descriptions of symptoms of the 84 patients, who were all infected medical staff. According to their descriptions, we noted 26 patients complained of diarrhea among the total 84 patients. However, the sample size was relatively small, and it could not represent the overall situation. The collection and analysis of such data are still ongoing in our group, and we expect to give a more comprehensive explanation in the near future.
None of these 84 infected health care workers died or required intensive care unit admission. One explanation might be that almost all the medical staff with COVID-19 pneumonia in our cohort were younger people and had mild disease. Another explanation might be that our patients did not have significant underlying chronic diseases and received timely treatment.
This study has several limitations. First, the sample size of COVID-19 patients with diarrhea was relatively smaller than those without diarrhea (26 vs 58). Second, all of the 84 patients were confirmed with throat swab specimens, and no paired lower respiratory tract specimens were obtained to see the difference in the viral RNA detection rate between them.
Conclusions
In this single-center case series of 84 confirmed COVID-19 patients in Wuhan, China, 26 patients (31%) experienced diarrhea. COVID-19 patients with diarrhea experienced discomfort longer, as compared with COVID-19 patients without diarrhea. In patients with diarrhea, stool specimens testing for SARS-CoV-2 may persist as positive, even after the throat swab testing for SARS-CoV-2 has turned out to be negative.
Acknowledgments
The authors thank all the patients involved in the study. The authors especially thank Dr Hui Zhang for his dedication to data analysis and encouragement.
CRediT Authorship Contributions
Xiao-Shan Wei (Data curation: Lead; Formal analysis: Lead)
Xu Wang (Investigation: Equal)
Yi-Ran Niu (Validation: Equal)
Lin-Lin Ye (Validation: Equal)
Wen-Bei Peng (Visualization: Equal)
Zi-Hao Wang (Validation: Equal)
Wei-Bing Yang (Writing – original draft: Equal)
Bo-Han Yang (Writing – original draft: Equal)
Jian-Chu Zhang (Writing – original draft: Equal)
Wan-Li Ma (Writing – review & editing: Equal)
Xiao-Rong Wang (Writing – review & editing: Equal)
Qiong Zhou (Conceptualization: Lead; Writing – review & editing: Lead)
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding Supported by National Natural Science Foundation of China (No. 81973990; No. 81900096; No. 81770090) and by the Fundamental Research Funds for the Central Universities, HUST COVID-19 Rapid Response Call (No. 2020kfyXGYJ030).
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at https://doi.org/10.1016/j.cgh.2020.04.030.
Supplementary Material
Supplementary Table 1.
Cytokine Detection Assays in Patients Infected With SARS-CoV-2
| Characteristic | Patients (n = 30) | Diarrhea group (n = 15) | Non-diarrhea group (n = 15) | P value |
|---|---|---|---|---|
| IL2 (pg/mL; normal range 0.10–4.10) | 2.41 ± 0.30 | 2.26 ± 0.27 | 2.59 ± 0.24 | .001 |
| IL4 (pg/mL; normal range 0.10–3.20) | 1.80 ± 0.49 | 1.54 ± 0.37 | 2.11 ± 0.44 | .001 |
| IL6 (pg/mL; normal range 0.10–2.90) | 13.27 ± 15.16 | 15.87 ± 18.78 | 10.10 ± 9.16 | .294 |
| Increased, n (%) | 27 (90) | 13 (87) | 14 (93) | >.999 |
| IL10 (pg/mL; normal range 0.10–5.00) | 4.62 ± 2.59 | 3.84 ± 1.64 | 5.57 ± 3.27 | .078 |
| Increased, n (%) | 8 (27) | 3 (20) | 5 (33) | .682 |
| TNF-α (pg/mL; normal range 0.10–23.00) | 2.41 ± 1.90 | 2.68 ± 2.56 | 2.08 ± 0.36 | .376 |
| IFN-γ (pg/mL; normal range 0.10–18.00) | 2.33 ± 1.02 | 1.90 ± 0.49 | 2.85 ± 1.27 | .012 |
IFN, interferon; IL, interleukin; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TNF, tumor necrosis factor.
Supplementary Table 2.
Identification of Immunoglobulins and Complement in Patients Infected With SARS-CoV-2
| Characteristic | Patients (n = 30) | Diarrhea group (n = 15) | Non-diarrhea group (n = 15) | P value |
|---|---|---|---|---|
| IgE (IU/mL; normal range 1–190) | 92.02 ± 110.29 | 79.36 ± 120.60 | 99.25 ± 108.02 | .638 |
| Increased, n (%) | 6 (20) | 2 (13) | 4 (27) | .651 |
| IgG (g/L; normal range 7.51–15.60) | 11.85 ± 2.04 | 11.45 ± 1.87 | 12.09 ± 2.17 | .394 |
| IgA (g/L; normal range 0.82–4.53) | 1.95 ± 0.75 | 2.14 ± 0.93 | 1.83 ± 0.63 | .294 |
| IgM (g/L; normal range 0.460–3.040) | 1.34 ± 0.74 | 1.29 ± 0.34 | 1.43 ± 0.90 | .578 |
| C3 (g/L; normal range 0.790–1.520) | 0.81 ± 0.19 | 0.79 ± 0.20 | 0.82 ± 0.19 | .677 |
| Decreased, n (%) | 14 (47) | 6 (40) | 8 (53) | .715 |
| C4 (g/L; normal range 0.160–0.380) | 0.27 ± 0.07 | 0.25 ± 0.08 | 0.28 ± 0.07 | .284 |
C, complement; IG, immunoglobulin; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Supplementary Table 3.
Analysis of Lymphocyte Subsets in Patients Infected With SARS-CoV-2
| Characteristic | Patients (n = 30) | Diarrhea group (n = 15) | Non-diarrhea group (n = 15) | P value |
|---|---|---|---|---|
| CD3+ T cells (%; normal range 58.17–84.22) | 74.74 ± 8.15 | 74.69 ± 6.41 | 74.80 ± 10.59 | .973 |
| CD4+ T cells (%; normal range 24.34–51.37) | 42.41 ± 10.20 | 42.12 ± 12.61 | 42.82 ± 6.33 | .849 |
| CD8+ T cells (%; normal range 14.23–38.95) | 27.55 ± 10.13 | 27.66 ± 11.45 | 27.39 ± 8.75 | .943 |
| B cells (%; normal range 4.10–18.31) | 12.15 ± 3.26 | 11.64 ± 2.69 | 12.96 ± 4.09 | .305 |
| NK cells (%; normal range 3.33–30.47) | 9.96 ± 7.06 | 10.50 ± 6.78 | 9.10 ± 7.95 | .608 |
| CD4+/CD8+ ratio (normal range 0.41–2.72) | 2.11 ± 2.22 | 2.40 ± 2.91 | 1.72 ± 0.58 | .382 |
NK, natural killer; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Supplementary Table 4.
Pulmonary Computed Tomography of Patients With COVID-19 Pneumonia on Admission to Hospital
| Pulmonary computed tomography | Patients (n = 84) | Diarrhea group (n = 26) | Non-diarrhea group (n = 58) | P value |
|---|---|---|---|---|
| Ground-glass opacification | 81 (96) | 24 (92) | 57 (98) | .225 |
| Side of ground-glass opacification | .556 | |||
| Left | 5 (6) | 2 (8) | 3 (5) | |
| Right | 4 (5) | 2 (8) | 2 (4) | |
| Both | 72 (89) | 20 (83) | 52 (91) | |
| Location of ground-glass opacification | .315 | |||
| Peripheral parenchyma | 54 (67) | 14 (58) | 40 (70) | |
| Central parenchyma | 0 | 0 | 0 | |
| Both | 27 (33) | 10 (42) | 17 (30) | |
| Number of ground-glass opacification | >.999 | |||
| Single | 9 (11) | 3 (13) | 6 (11) | |
| Multifocal | 72 (89) | 21 (88) | 51 (89) | |
| Patchy consolidation | 34 (40) | 14 (54) | 20 (34) | .148 |
| Reticular change | 12 (14) | 3 (12) | 9 (16) | .746 |
NOTE. Data shown as n (%).
COVID-19, corona virus disease 2019.
Supplementary Table 5.
Outcome of Computed Tomography Imaging Reexamination in Patients Infected With SARS-CoV-2
| Computed tomography imaging reexamination | All patients (n = 84) | Diarrhea group (n = 26) | Non-diarrhea group (n = 58) | P value |
|---|---|---|---|---|
| Improvement, n (%) | 64 (76) | 17 (65) | 47 (81) | .115 |
| Deterioration, n (%) | 12 (14) | 4 (15) | 8 (14) | |
| Similarity, n (%) | 8 (10) | 5 (19) | 3 (5) |
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Supplementary Table 6.
Number of Discharged and Hospitalized Patients as of February 19, 2020
| All patients (n = 84) | Diarrhea group (n = 26) | Non-diarrhea group (n = 58) | P value | |
|---|---|---|---|---|
| Discharged, n (%) | 63 (75) | 15 (58) | 48 (83) | .028 |
| Hospitalized, n (%) | 21 (25) | 11 (42) | 10 (17) |
Supplementary Table 7.
Outcome of Diarrhea Patients Before and After Probiotics Treatment
| Before probiotics treatment (n = 26) | After probiotics treatment (n = 26) | P value | |
|---|---|---|---|
| VAS scores for diarrhea | 6.8 ± 1.1 | 3.0 ± 1.0 | <.0001 |
| Defecating frequency | 5.7 ± 2.8 | 2.1 ± 0.8 | <.0001 |
| Bristol score | 5.9 ± 0.6 | 3.7 ± 0.7 | <.0001 |
| Stool occult blood positive, n (%) | 3 (12) | 0 (0) | .235 |
| Smear for stool fungus positive, n (%) | 7 (27) | 0 (0) | .010 |
NOTE. VAS, visual analogue scale/score (0, no diarrhea; ≤3, slight diarrhea; 4–6, moderate diarrhea; 7–10, severe diarrhea); Bristol score: 1, separate hard lumps (like nuts); 2, sausage-shaped but lumpy; 3, like a sausage or snake, but with cracks on its surface; 4, like a sausage or snake and smooth and soft; 5, soft blobs with a clear cut edge; 6, fluffy pieces with ragged edges and mushy; 7, watery with no solid pieces.
References
- 1.Guan W.-j., Ni Z.-y., Hu Y. Clinical characteristics of 2019 novel coronavirus infection in China. medRxiv. 2020 2020.02.06.20020974. [Google Scholar]
- 2.Rothe C., Schunk M., Sothmann P. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382:970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holshue M.L., DeBolt C., Lindquist S. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.nCo VNIRST. 2019-nCoV acute respiratory disease, Australia: epidemiology report 1 (reporting week 26 January - 1 February 2020) Commun Dis Intell (2018) 2020:44. doi: 10.33321/cdi.2020.44.13. [DOI] [PubMed] [Google Scholar]
- 5.WHO main website. https://www.who.int Available at:
- 6.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Y., Lu Q., Liu M. Epidemiological and clinical features of the 2019 novel coronavirus outbreak in China. medRxiv. 2020 2020.02.10.20021675. [Google Scholar]
- 10.Coronavirus disease (COVID-19) technical guidance: laboratory testing for 2019-nCoV in humans. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/laboratory-guidance Available at:
- 11.Zhou P., Yang X.-L., Wang X.-G. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu F., Zhao S., Yu B. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gu J., Gong E., Zhang B. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202:415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann M., Kleine-Weber H., Krüger N. The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. bioRxiv. 2020 2020.01.31.929042. [Google Scholar]
- 15.Zhao Y., Zhao Z., Wang Y. Single-cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019-nCov. bioRxiv. 2020 2020.01.26.919985. [Google Scholar]
- 16.Zhang H., Kang Z., Gong H. The digestive system is a potential route of 2019-nCov infection: a bioinformatics analysis based on single-cell transcriptomes. bioRxiv. 2020 2020.01.30.927806. [Google Scholar]
- 17.Ettayebi K., Crawford S.E., Murakami K. Replication of human noroviruses in stem cell–derived human enteroids. Science. 2016;353:1387–1393. doi: 10.1126/science.aaf5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desmarets L.M.D., Theuns T., Roukaerts I.D. Role of sialic acids in feline enteric coronavirus infections. J Gen Virol. 2014;95:1911–1918. doi: 10.1099/vir.0.064717-0. [DOI] [PubMed] [Google Scholar]
- 19.Evans M., Salewski R.P., Christman M.C. Effectiveness of Lactobacillus helveticus and Lactobacillus rhamnosus for the management of antibiotic-associated diarrhoea in healthy adults: a randomised, double-blind, placebo-controlled trial. Br J Nutr. 2016;116:94–103. doi: 10.1017/S0007114516001665. [DOI] [PubMed] [Google Scholar]
- 20.de Gunzburg J., Ghozlane A., Ducher A. Protection of the human gut microbiome from antibiotics. J Infect Dis. 2018;217:628–636. doi: 10.1093/infdis/jix604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez-Perez G., Hicks A.L., Tekieli T.M. Maternal antibiotic treatment impacts development of the neonatal intestinal microbiome and antiviral immunity. J Immunol. 2016;196:3768–3779. doi: 10.4049/jimmunol.1502322. [DOI] [PubMed] [Google Scholar]
- 22.McAleer J.P., Kolls J.K. Contributions of the intestinal microbiome in lung immunity. Eur J Immunol. 2018;48:39–49. doi: 10.1002/eji.201646721. [DOI] [PMC free article] [PubMed] [Google Scholar]