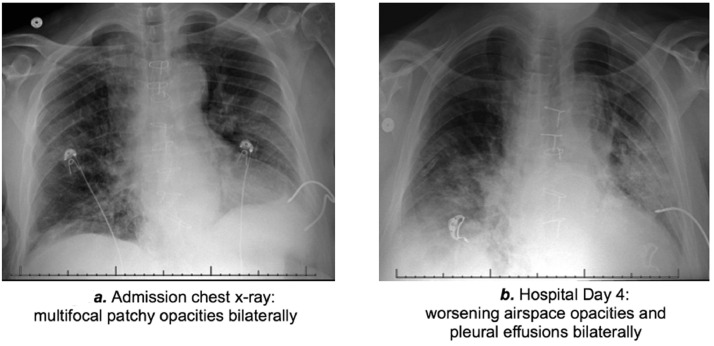
A 61-year-old African American man with a history of hypertension, coronary artery disease, end-stage renal disease (on hemodialysis since 2014), and end-stage heart failure secondary to arrhythmogenic right ventricular cardiomyopathy underwent dual organ heart–kidney transplantation in May 2019. Approximately 2 months after transplant, mycophenolate was discontinued owing to episodes of pancreatitis, leukopenia, and detectable BK polyomavirus. Since then, the patient was maintained on tacrolimus (goal level of 8 ng/ml) and low-dose prednisone (5 mg/day). Eight months after transplant, the patient developed a mild influenza A infection that was treated with oseltamivir for 10 days. After 6 weeks, approximately 10 months after heart–kidney transplantation, the patient re-presented with cough productive of yellow sputum for 3 days, associated with pleuritic chest pain, dyspnea, nasal congestion, and subjective fevers. He denied travel or exposure to known individuals infected with coronavirus disease 2019 (COVID-19). Initial vital signs were within normal limits and physical examination was unremarkable. The blood oxygen saturation on room air was 96%. Respiratory viral panel was negative, and white blood cell count and blood lactate were normal. Absolute lymphocyte count was reduced (700 /µl; reference range: 1,300–3,600 /µl). C-reactive protein was elevated (15.8 mg/liter; reference range: 0–5 mg/liter). The initial chest X-ray revealed multifocal pneumonia (Figure 1 a). The patient was started empirically on vancomycin, piperacillin–tazobactam, and azithromycin. The baseline immunosuppression was reduced: prednisone was held, and tacrolimus dose was decreased to a lower goal level of 6–8 ng/ml. Prophylaxis for opportunistic infection was continued with ganciclovir and atovaquone. Oropharyngeal and nasopharyngeal swabs were sent for severe acute respiratory syndrome coronavirus 2 reverse transcriptase–polymerase chain reaction testing, and the patient was admitted to an airborne isolation bed. On hospital Day 4, the patient remained clinically stable with blood oxygen saturation ∼95% on room air, but radiographic worsening was noted (Figure 1b). His severe acute respiratory syndrome coronavirus 2 reverse transcriptase–polymerase chain reaction test returned positive, and he was started on lopinavir/ritonavir 400/100 mg every 12 hours and nitazoxanide 500 mg every 12 hours for 7 days. He was also given 1 dose of 40 g intravenous immunoglobulin. Tacrolimus levels were followed daily, and given the known drug–drug interaction with ritonavir, a decreased tacrolimus clearance was observed, and no tacrolimus dose was administered or required for a week. Anti-bacterial therapy was discontinued. The patient improved and by hospital Day 14, experienced only intermittent cough with scant sputum production. His C-reactive protein decreased to 8.1 mg/liter. He was discharged home to self-care.
Figure 1.
Radiographic assessment of the patient. (a) Admission chest X-ray showing bilateral multifocal patchy opacities. (b) Chest X-ray on hospital Day 4 showing bilateral worsening airspace opacities and pleural effusions.
This patient with COVID-19 exhibited a relatively mild form of the disease, remained afebrile, and maintained good oxygen saturation throughout his hospital course. His presentation was similar to that reported in non-immunosuppressed patients, and similar presentations were reported in 2 and 3 COVID-19–positive heart transplant recipients from China1 and Italy, respectively. Because respiratory viral illness represents a significant cause of morbidity and mortality in the aging and immunocompromised transplant populations,2 these patients would likely benefit from early screening and aggressive treatment wherever possible. Currently, there is no proven targeted therapy available for COVID-19. The regimen of lopinavir/ritonavir, nitazoxanide, and intravenous immunoglobulin was chosen for our patient with a history of dual organ transplantation and COVID-19 on the basis of in vitro data,3 limited clinical data,4 and drug availability at our institution at the time of this patient's diagnosis. A recent randomized, controlled assessment of lopinavir/ritonavir in adults hospitalized with severe COVID-19 reported no significant benefit.5 However, it should be emphasized that the patients in this study had relatively few comorbidities and may have received treatment relatively late in the disease process. It is unclear whether these findings can be extrapolated to the transplant population. An important challenge and consideration for use of lopinavir/ritonavir in transplant recipients is significant drug–drug interactions with tacrolimus. There have been limited data supporting the use of the anti-protozoal agent nitazoxanide as an anti-viral drug and immunomodulator, with several case series reporting positive outcomes in transplant patients with a viral illness.6 In addition, small studies suggest a benefit for using intravenous immunoglobulin replacement in transplant recipients with low-level immunoglobulin and severe infections (our patient's IgG levels were at the lower limit of normal).7 Prospective evaluation of potential therapies will be important for tailoring treatment for the COVID-19–positive transplant population as the pandemic continues to grow.
References
- 1.Li F, Cai J, Dong N. First cases of COVID-19 in heart transplantation from China [e-pub ahead of print]. J Heart Lung Transplant. doi: 10.1016/j.healun.2020.03.006, accessed 29 March 2020. [DOI] [PMC free article] [PubMed]
- 2.Law N, Kumar D. Post-transplant viral respiratory infections in the older patient: epidemiology, diagnosis and management. Drugs Aging. 2017;34:743–754. doi: 10.1007/s40266-017-0491-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang M, Cao R, Zhang L. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F, Yu T, Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19 [e-pub ahead of print]. N Engl J Med. doi: 10.1056/NEJMoa2001282, accessed 29 March 2020. [DOI] [PMC free article] [PubMed]
- 6.Avery RK, Lonze BE, Kraus ES, Marr KA, Montgomery RA. Severe chronic norovirus diarrheal disease in transplant recipients: clinical features of an under-recognized syndrome [e-pub ahead of print]. Transpl Infect Dis. doi: 10.1111/tid.12674, accessed 29 March 2020. [DOI] [PubMed]
- 7.Carbone J, Sarmiento E, Del Pozo N. Restoration of humoral immunity after intravenous immunoglobulin replacement therapy in heart transplant recipients with post-transplant antibody deficiency and severe infections. Clin Transpl. 2012;26:E277–E283. doi: 10.1111/j.1399-0012.2012.01653.x. [DOI] [PubMed] [Google Scholar]