Highlights
-
•
Pulmonary CT features of the 2019 novel coronavirus disease were diverse.
-
•
This disease changed rapidly at the early stage, and lasted for a long time.
-
•
CT could provide semi-quantitative analysis of pulmonary damage severity.
Keywords: Computed tomographic, COVID-19, Ground-glass opacities, Crazy-paving pattern
Abstract
Purpose
To evaluate lung abnormalities on thin-section computed tomographic (CT) scans in patients with COVID-19 and correlate findings to duration of symptoms.
Methods
In total, 348 CT scans in 112 patients were classified according to the time after the onset of the initial symptoms, namely stage-1 (0–4 days); stage-2 (5–9 days); stage-3 (10–14 days); stage-4 (15–21 days); stage-5 (22–28 days); and stage-6 (>28 days). Each lung lobe was evaluated for extent affected by ground-glass opacities (GGO), crazy-paving pattern and consolidation, in five categories of percentual severity. Summation of scores from all five lung lobes provided the total CT score (maximal CT score, 25).
Results
The predominant patterns of lung abnormalities were GGOs, crazy-paving pattern, consolidation and linear opacities. The frequency of crazy-paving pattern, consolidation and linear opacities peaked at stage-3 (62.7 %), stage-4 (75.0 %) and stage-5 (83.1 %), respectively, and decreased thereafter. Total CT scores increased from stage-1 to stage-2 (2.8 ± 3.1, vs. 6.5 ± 4.6, respectively, P < 0.01), and thereafter remained high. The lower lobes were more inclined to be involved with higher CT scores except for stage-1. At stage-6 98.1 % of CT scans still showed abnormalities (CT score 7.5 ± 4.1).
Conclusion
Thin-section CT could provide semi-quantitative analysis of pulmonary damage severity. This disease changed rapidly at the early stage, then tended to be stable and lasted for a long time.
1. Introduction
Since December 2019, many unexplained pneumonia’s had been reported, initially related to exposure at the Huanan Seafood Market in Wuhan city, Hubei province, China [1]. The pathogen was soon identified as a coronavirus, similar to those involved in severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV). The novel coronavirus was named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by international committee on taxonomy of viruses (ICTV), capable of infecting humans, on 11 February 2020 and the disease was termed COVID-19 by world health organization (WHO) [1]. Based on the current epidemiological surveys, the incubation period of this disease is 1–14 days, mostly 3–7 days. Fever, dry cough and fatigue are the main manifestations. A few patients have symptoms such as nasal obstruction, runny nose, sore throat, myalgia and diarrhea. Seriously ill patients may rapidly progress to ARDS. The outbreak of COVID-19 has been declared a public health emergency of international concern by WHO. As of March 18, 2020, a total of 186,777 confirmed cases with COVID-19 pneumonia have been reported globally, including 7480 deaths (4.0 %). A specific viral nucleic acid assay using real time reverse transcription - polymerase chain reaction (RT-PCR) was quickly developed to confirm the diagnosis of COVID-19 [2,3]. However, from the recently published literature, some patients with likely 2019-nCoV infection might have initial negative RT-PCR results [4]. Reasons for false negative RT-PCR tests may include insufficient cellular material for detection and improper extraction of nucleic acid from clinical materials [5,6]. According to current experience, lung CT imaging may manifest abnormalities earlier than RT-PCR testing. Currently, high-resolution CT has been included as one of the main tools for screening, primary diagnosis, and evaluation of disease severity [7].
The aim of this study, therefore, was to evaluate lung abnormalities on thin-section computed tomographic (CT) scans in patients with COVID-19 and correlate findings to duration of symptoms.
2. Materials and methods
2.1. Study population
Institutional review board approval was obtained, and informed consent for this retrospective study was waived. The anonymous data were collected and analyzed to facilitate better clinical decisions and treatment. In total, 112 patients (mean age, 55.8 years; range, 12–89 years), including 51 males (mean age, 58.3 years; range, 25–89 years) and 61 females (mean age, 53.7 years; range, 12–86 years) with confirmed COVID-19 pneumonia, were included in our study between February 2020 and March 2020 (Table 1 ). All patients underwent a series of chest CT scans to evaluate the severity of the disease and observe the change of the disease until the acute exudative lesions were obviously absorbed or disappeared.
Table 1.
Demographic and clinical data of the patient cohort.
| Clinical characteristics and laboratory data | All patients (n = 112) |
|---|---|
| Gender | |
| Male | 51 (45.54 %) |
| Female | 61 (54.46 %) |
| Age (y) | 55.8 ± 16.1 (12–89) |
| Male | 58.3 ± 16.2 (25–89) |
| Female | 53.7 ± 15.9 (12–86) |
| Inital symptoms | |
| Fever | 84 (75.0 %) |
| Low grade fever (37.3−38.0) | 46 (41.1 %) |
| Moderate grade fever (38.1−39.0) | 31 (27.6 %) |
| High grade fever (>39.0) | 7 (6.2 %) |
| Dry Cough | 48 (42.8 %) |
| Expectoration | 15 (13.3 %) |
| Throat pain | 4 (3.5 %) |
| Chest distress | 24 (21.4 %) |
| Dyspnea | 18 (16.1 %) |
| Fatigue | 38 (33.9 %) |
| Nausea and vomiting | 4 (3.5 %) |
| Abdominal pain and diarrhea | 14 (12.5 %) |
| Myalgia | 18 (16.1 %) |
| Headache | 8 (7.1 %) |
| Dizziness | 2 (1.7 %) |
| Palpitation | 2 (1.7 %) |
| Laboratory investigations | |
| White blood cell count, WBC (109/L) | 5.6 ± 2.9 (0.9–16.4) |
| Neutrophil count, N (109/L) | 4.2 ± 2.9 (0.4–15.7) |
| Neutrophil percentage (%) | 68.7 ± 14.6 (39.6–97.1) |
| Lymphocyte count, L (109/L) | 1.0 ± 0.5 (0.1–2.3) |
| Lymphocyte percentage (%) | 21.6 ± 12.0 (1.5–47.1) |
| Monocytes count, M (109/L) | 0.4 ± 0.2 (0.1–1.1) |
| Monocytes percentage (%) | 8.6 ± 4.2 (1.0–23.5) |
| Red blood cell count, RBC (1012/L) | 4.1 ± 0.5 (2.9–6.1) |
| Blood platelet, PLT (109/L) | 210.4 ± 91.1 (62.0–469.0) |
| Hemoglobin, HGB (g/L) | 130.0 ± 15.5 (78.8–184.0) |
| C-reactive protein, CRP (mg/L) | 51.5 ± 81.8 (0.2–646.0) |
| Erythrocyte sedimentation rate, ESR (mm/h) | 29.1 ± 25.6 (2.0–119.0) |
| Alanine aminotransferase, ALT (U/L) | 34.8 ± 31.6 (8.0–175.0) |
| Aspartate aminotransferase, AST (U/L) | 32.3 ± 18.5 (9.0–90.0) |
| Total bilirubin, TBIL (μmol/L) | 13.2 ± 6.3 (4.6–38.2) |
| Albumin, ALB (g/L) | 37.5 ± 6.5 (23.3–74.3) |
| Globulin, GLB (g/L) | 30.0 ± 4.3 (22.1–43.5) |
| Urea (mmol/L) | 4.8 ± 2.0 (1.4–14.3) |
| Creatinine, Crea (μmol/L) | 68.4 ± 19.2 (34.4–158.7) |
| Fasting blood glucose, FBG (mmol/L) | 6.8 ± 2.6 (3.7–16.8) |
| Lactate dehydrogenase, LDH (U/L) | 260.5 ± 175.7 (46.0–1305.0) |
| Creatine kinase, CK (U/L) | 119.4 ± 149.5 (1.7–882.0) |
| Creatine kinase isoenzyme MB, CK-MB (U/L) | 13.6 ± 6.6 (2.0–44.0) |
| lnterleukin-6, IL-6 (pg/mL) | 30.6 ± 50.4 (0.1–309.0) |
| Prothrombin time, PT (s) | 12.9 ± 1.9 (10.4–27.4) |
| D-dimer (ng/mL) | 2405.8 ± 7079.0 (33.0–43142.0) |
| The period between the onset of initial symptoms and the hospitalized (d) | 8.0 ± 5.2 (1–22) |
| The frequency of CT scans | |
| 1 scan | 13 (11.6 %) |
| 2 scans | 30 (26.8 %) |
| 3 scans | 31 (27.7 %) |
| 4 scans | 19 (16.9 %) |
| 5 scans | 12 (10.7 %) |
| 6 scans | 5 (4.5 %) |
| 7 scans | 0 (0%) |
| 8 scans | 2 (1.8 %) |
| Total number of CT scans | 348 |
| Average number of CT scans per person | 3.1 ± 1.4 (1–8) |
2.2. Diagnostic and cure criteria of COVID-19 pneumonia
Based on the preliminary diagnosis and treatment protocols from the National Health Commission of the People’s Republic of China, the diagnostic criteria of COVID-19 pneumonia were as follows : 1. Epidemiological history: travel or residential history of Wuhan city and surrounding areas, or other communities with case reports within 14 days before the onset of the disease; exposure history to infected persons with 2019-nCoV (positive for nucleic acid detection) within 14 days before onset; 2. Clinical manifestations and laboratory indicators: fever and / or respiratory symptoms, imaging characteristics of pneumonia, and / or normal or decreased white blood cells count or decreased lymphocyte count; and 3. Confirmed diagnosis: real-time RT-PCR detection of COVID-19 in throat swabs or lower respiratory tract; highly homologous with other novel coronavirus through virus gene sequencing [7,8]. The patients with confirmed COVID-19 pneumonia were all hospitalized and isolated for treatment. The discharge criteria were: 1. Afebrile for more than 3 days; 2. Respiratory symptoms significantly improved; 3. Obvious improvement of acute exudative lesions on chest CT; and 4. Two consecutive negative COVID-19 nucleic acid tests at least 24 h apart [7].
2.3. CT protocol
CT scans were performed at the end-inspiration level with patients in supine position and arms raised. Two CT systems were used with the following parameters: GE Discovery CT750 HD, 1.25 mm section thickness for reconstruction, 1.25 mm gap, tube voltage 120 kV with automatic tube current modulation, DFOV 40.0 × 46.6 cm; Siemens Somatom Definition, Siemens Healthineers, Germany, 1.0 mm section thickness for reconstruction, 1.0 mm gap, tube voltage 120 kV with automatic tube current modulation, DFOV 36.8 × 42.9 cm.
2.4. Chest CT image analysis
Two experienced radiologists with 13 and 11 years of clinical experience in chest CT radiology respectively reviewed the thin-section CT images respectively and reached a decision in consensus. The observers categorized the predominant patterns on CT scans as ground-glass opacification (GGO, hazy areas of increased attenuation without obscuration of the underlying vessels), crazy-paving pattern (GGO with interlobular and intralobular septal thickening), consolidation (homogeneous opacification of the parenchyma with obscuration of the underlying vessels), and linear opacities (disordered arrangement of coarse linear or curvilinear opacities or fine subpleural reticulation). On the scans, some other minor signs such as air bronchogram, cavitation, bronchiectasia, pleural effusion, pericardial effusion, pneumothorax and mediastinal lymphadenopathy (defined as a lymph node greater than 1 cm in short-axis diameter) were also noted. The distribution of pulmonary lesions was noted as peripheral (predominantly subpleural, involving mainly the peripheral one-third of the lung), central (predominantly lung hilum, involving mainly the central two-third of the lung), and diffuse (both subpleural and central regions). The involvement of pulmonary lesions was also noted as single lobe, unilateral multilobe and bilateral multilobe.
After evaluation, the scans were categorized according to the period between the onset of initial symptoms and the CT scans: stage-1 (0–4 days, n = 47); stage-2 (5–9 days, n = 54); stage-3 (10–14 days, n = 67); stage-4 (15–21 days, n = 68); stage-5 (22–28 days, n = 59); and stage-6 (>28 days, n = 53) [8]. The severity of disease according to the scope of GGO, crazy-paving pattern and consolidation at thin-section CT was also evaluated. Bilateral lungs were divided into five lung zones according to the anatomical structure of lung: left upper lobe, left lower lobe, right upper lobe, right middle lobe and right lower lobe. Each lung lobe was assigned a score which was based on the following criteria: score 0, 0% involvement; score 1, less than 5% involvement; score 2, 5% to less than 25 % involvement; score 3, 25 % to less than 50 % involvement; score 4, 50 % to less than 75 % involvement; and score 5, 75 % or greater involvement. Summation of scores provided a semi-quantitative evaluation for overall lung involvement (maximal CT score for both lungs was 25) [8,9].
2.5. Statistical analysis
Statistical analyses were performed using SPSS version 23.0 (SPSS, Inc., Chicago, IL) and Graphpad prism version 7.0 (GraphPad software Inc., USA). Quantitative data were presented as mean ± standard deviation (SD) (minimum - maximum). The counting data were presented as count (percentage of total). Quantitative data were tested first with the Kolmogorov - Smirnov test for normality and Levene test for homogeneity of variance. The comparisons of paired and non-paired quantitative data were evaluated using Wilcoxon test and Mann-Whitney U test between two groups, and Friedman test and Kruskal-Wallis test among multiple groups. Differences were considered significant at P < .05.
3. Results
3.1. Clinical characteristics and laboratory data
The most prevalent presenting symptoms at onset of illness were fever (75.0 %), dry cough (42.8 %), fatigue (33.9 %), chest distress (21.4 %), dyspnea (16.1 %) and myalgia (16.1 %). Less common symptoms were expectoration, abdominal pain and diarrhea, headache, nausea and vomiting, throat pain, dizziness and palpitation (Table 1).
The first laboratory tests of all hospitalized patients were recorded. Most patients had normal white blood cell count (WBC, 5.6 ± 2.9 × 109/L) and decreased lymphocyte count (1.0 ± 0.5 × 109/L), with a mildly elevated C-reactive protein (CRP, 51.5 ± 81.8 mg/L), erythrocyte sedimentation rate (ESR, 29.1 ± 25.6 mm/h) and lnterleukin-6 (IL-6, 30.6 ± 50.4 pg/mL). Some patients had elevated alanine aminotransferase (ALT, 34.8 ± 31.6 U/L), aspartate aminotransferase (AST, 32.3 ± 18.5 U/L), total bilirubin (TBIL 13.2 ± 6.3 μmol/L), urea (4.8 ± 2.0 mmol/L), creatinine(Crea, 68.4 ± 19.2 μmol/L), lactate dehydrogenase (LDH, 260.5 ± 175.7 U/L), creatine kinase (CK, 119.4 ± 149.5 U/L) and creatine kinase isoenzyme MB (CK-MB, 13.6 ± 6.6 U/L) (Table 1). A total of 348 pulmonary CT scans were performed and each patient underwent an average of 3.1 ± 1.4 CT scans (range: 1–8). The rates of patients who had 1–8 CT scans were 11.6 % (13), 26.8 % (30), 27.7 % (31), 16.9 % (19), 10.7 % (12), 4.5 % (5), 0% (0) and 1.8 % (2), respectively (Table 1).
3.2. Chest CT image findings
The distribution of pulmonary lesions on CT in COVID-19 pneumonia patients was mostly peripheral at all stages with the highest rate (66.6 %) at stage-2 (Table 2 , Fig. 1 ). With the development of the disease, the lesions gradually spread from the periphery to the center. Only a very small number of patients had originally a central lesion (2.1 %) (Table 2, Fig. 2 ).
Table 2.
Distribution and frequency of the pulmonary lesions on CT at different stages.
| Stage-1 (n = 47) |
Stage-2 (n = 54) |
Stage-3 (n = 67) |
Stage-4 (n = 68) |
Stage-5 (n = 59) |
Stage-6 (n = 53) |
|
|---|---|---|---|---|---|---|
| Distribution of pulmonary lesions | ||||||
| No lesion | 10 (21.2 %) | 1 (1.8 %) | 2 (2.9 %) | 2 (2.9 %) | 1 (1.7 %) | 1 (1.8 %) |
| Peripheral | 30 (63.8) | 36 (66.6 %) | 41 (61.2 %) | 37 (54.4 %) | 31 (52.4 %) | 30 (56.6 %) |
| Central | 1 (2.1 %) | 1 (1.8 %) | 1 (1.5 %) | 0 (0%) | 1 (1.7 %) | 0 (0 %) |
| Diffuse | 6 (12.7 %) | 16 (29.6 %) | 23 (34.3 %) | 29 (42.6 %) | 26 (44.1 %) | 22 (41.5 %) |
| Involvement of the lung | ||||||
| No involvement | 10 (21.2 %) | 1 (1.8 %) | 2 (2.9 %) | 2 (2.9 %) | 1 (1.7 %) | 1 (1.8 %) |
| Single lobe | 16 (34.0 %) | 11 (20.3 %) | 8 (11.9 %) | 1 (1.5 %) | 4 (6.8 %) | 2 (3.8 %) |
| Unilateral multilobe | 1 (2.1 %) | 1 (1.8 %) | 2 (2.9 %) | 0 (0 %) | 1 (1.7 %) | 0 (0 %) |
| Bilateral multilobe | 20 (42.5 %) | 41 (75.7 %) | 55 (82.1 %) | 65 (95.6 %) | 53 (89.8 %) | 50 (94.3 %) |
| GGO | 36 (76.5 %) | 48 (88.8 %) | 61 (91.0 %) | 59 (86.7 %) | 57 (96.6 %) | 52 (98.1 %) |
| Crazy-paving pattern | 17 (36.1 %) | 31 (57.4 %) | 42 (62.7 %) | 38 (55.9 %) | 33 (55.9 %) | 28 (52.8 %) |
| Consolidation | 12 (25.5 %) | 37 (68.5 %) | 42 (62.7 %) | 51 (75.0 %) | 30 (50.8 %) | 16 (30.2 %) |
| Linear opacities | 3 (6.3 %) | 14 (25.9 %) | 32 (47.7 %) | 51 (75.0 %) | 49 (83.1 %) | 39 (73.6 %) |
| Air bronchogram | 8 (17.0 %) | 27 (50.0 %) | 27 (40.3 %) | 34 (50.0 %) | 18 (30.5 %) | 13 (24.5 %) |
| Cavitation | 0 (0 %) | 0 (0 %) | 0 (0 %) | 0 (0 %) | 0 (0 %) | 0 (0 %) |
| Bronchiectasia | 3 (6.3 %) | 7 (12.9 %) | 13 (19.4 %) | 23 (33.8 %) | 19 (32.2 %) | 24 (45.2 %) |
| Pleural effusion | 2 (4.2 %) | 5 (9.2 %) | 14 (20.9 %) | 19 (27.9 %) | 11(18.6 %) | 8 (15.1 %) |
| Pericardial effusion | 0 (0 %) | 0 (0 %) | 3 (4.4 %) | 1 (1.5 %) | 1 (1.7 %) | 0 (0 %) |
| Lymphadenopathy | 0 (0 %) | 0 (0 %) | 0 (0 %) | 0 (0 %) | 0 (0 %) | 0 (0 %) |
| Pneumothorax | 0 (0 %) | 0 (0 %) | 1 (1.5 %) | 0 (0 %) | 1 (1.7 %) | 2 (3.8 %) |
Note: The counting data were presented as count (percentage of total).
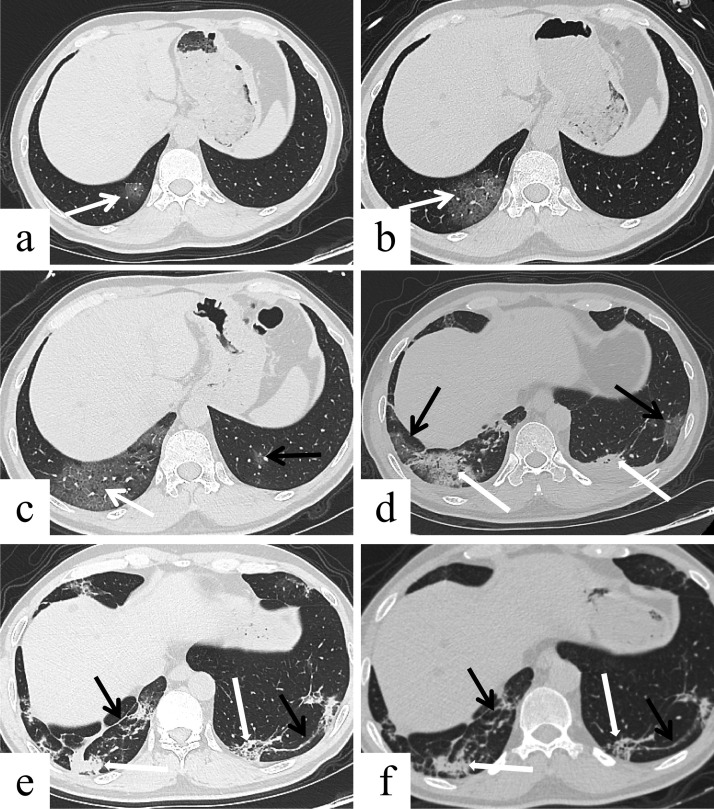
Fig. 1.
Male, 48 years, transverse thin-section CT scan. (a) 4 days after the onset of initial symptoms (stage-1): subpleural GGO (arrow) in the right lower lobe. (b) 8 days after the onset of initial symptoms (stage-2): more severe lesions with GGO and crazy-paving pattern (arrow) in the right lower lobe. (c) 12 days after the onset of initial symptoms (stage-3): again increased severity of GGO and crazy-paving pattern (white arrow) in the right lower lobe, and GGO (black arrow) also appeared in the left lower lobe. (d) 18 days after the onset of initial symptoms (stage-4): The predominant pattern of abnormality was consolidation (thick white arrow), accompanied by a small GGO (thin black arrow) in both lower lobes. (e) 26 days after the onset of initial symptoms (stage-5): the extent of the lesions was reduced with consolidation (thick white arrow) and linear opacities (thin black arrow) in the right lower lobe, and crazy-paving pattern (thick white arrow) and linear opacities (thin black arrow) in the left lower lobe. (f) 38 days after the onset of initial symptoms (stage-6): There was no significant change in the extent and composition of lesions compared with the directly prior CT result, with consolidation (thick white arrow) and linear opacities (thin black arrow) in the right lower lobe, and crazy-paving pattern (thick white arrow) and linear opacities (thin black arrow) in the left lower lobe.
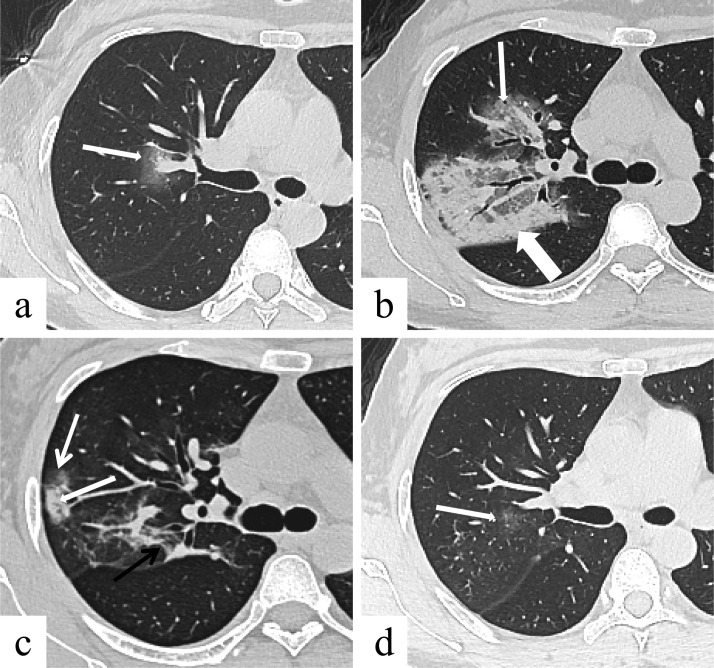
Fig. 2.
Female, 41 years, transverse thin-section CT scan. (a) 0 day after the onset of initial symptoms (stage-1): pure GGO in the right upper lobe near the hilum (arrow). (b) 7 days after the onset of initial symptoms (stage-2): increased severity of the lesions with GGO (thin arrow), crazy-paving pattern (thin arrow) and consolidation (thick arrow). (c) 14 days after the onset of initial symptoms (stage-3): reduced extent of GGO (thin white arrow) and consolidation (thick white arrow), and linear opacities appeared (thin black arrow). (d) 25 days after the onset of initial symptoms (stage-5): only a small residual pure GGO (arrow).
In most patients, the lesions were present in bilaterally, in multiple lobes, with the lowest rate (42.5 %) at stage-1 and the highest rate (95.6 %) at stage-4. In the early stage of the disease (Stage-1), CT scans showed in 34 % lesions that involved a single lung lobe and in 21.2 % no lung involvement (Table 2).
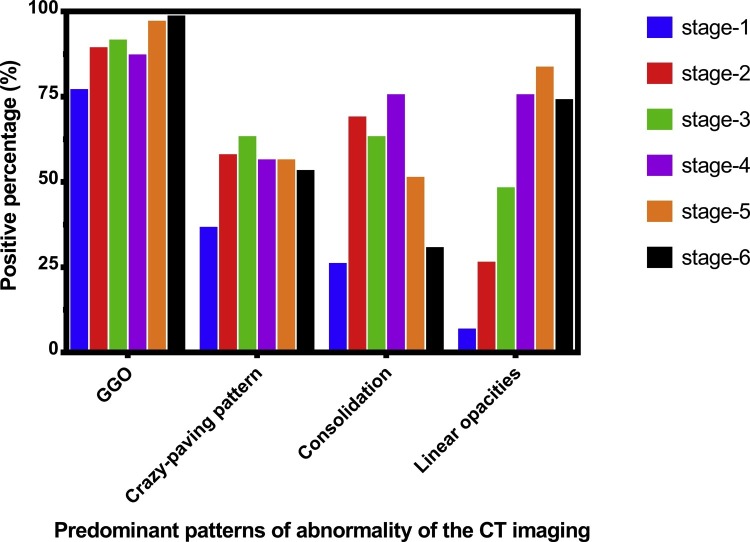
The most frequent CT findings of COVID-19 pneumonia were GGO, crazy-paving pattern, consolidation and linear opacities (Figs. 1, 2). The predominant patterns of abnormality changed over time. In the early stage (Stage-1), GGO was the most important imaging manifestation (76.5 %), and in some patients, crazy-paving pattern were present (36.1 %), as well as consolidation (25.5 %), or linear opacities (6.3 %). The frequency of GGO, crazy-paving pattern, consolidation and linear opacities were highest at stage-6 (98.1 %), stage-3 (62.7 %), stage-4 (75.0 %) and stage-5 (83.1 %) respectively, and tended to decrease in later stages, and at stage-6 98.1 % of CT scans still showed abnormalities (Fig. 3 ). Other CT findings of COVID-19 pneumonia included air bronchogram, bronchiectasis and pleural effusion with their maximum frequencies occurring in stage-2/4 (50.0 %), stage-6 (45.2 %) and stage-4 (27.9 %) respectively. Only a few patients had pericardial effusion (4.4 %) and pneumothorax (3.8 %). No mediastinal lymphadenopathy was observed on CT scanning (Table 2).
Fig. 3.
The frequency of GGO, crazy-paving pattern, consolidation and linear opacities at six stages.
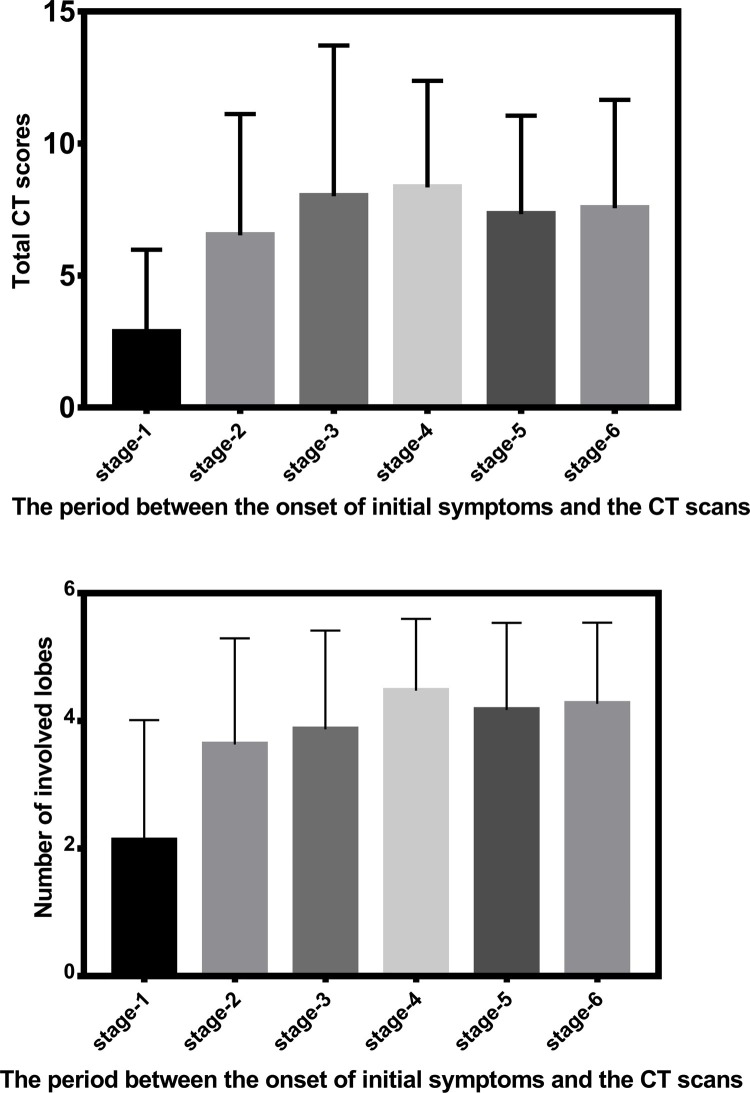
The lower lobes were more inclined to be involved, with higher CT scores at any stages (P < 0.01) except for stage-1 (P > 0.05) (Table 3 ). The CT scores of the bilateral lungs, right / left lung and each lobe were lowest at stage-1 than at other stages (Table 3, P < 0.05), except for CT scores of right middle lobe between stage-1 and stage-2 (0.4 ± 0.5 vs. 0.9 ± 0.8, P = 0.063). There were no significant difference in CT scores among other stages except for CT scores of left lung and left lower lobe between stage-2 and stage-4 (2.4 ± 2.0 vs. 3.4 ± 1.7, P = 0.028 ; 1.4 ± 1.3 vs. 1.9 ± 1.1, P = 0.036) (Table 3, Fig. 4 ).
Table 3.
The CT scores of the pulmonary involvement at different stages.
| Stage-1 (n = 47) |
Stage-2 (n = 54) |
Stage-3 (n = 67) |
Stage-4 (n = 68) |
Stage-5 (n = 59) |
Stage-6 (n = 53) |
|
|---|---|---|---|---|---|---|
| CT score of the right lung | 1.7 ± 1.8 (0–8) |
4.1 ± 2.7 (0–14) |
4.7 ± 3.3 (0–15) |
4.9 ± 2.5 (0–14) |
4.2 ± 2.1 (0–10) |
4.5 ± 2.4 (0–9) |
| CT score of the left lung | 1.1 ± 1.4 (0–6) |
2.4 ± 2.0 (0–8) |
3.3 ± 2.5 (0–10) |
3.4 ± 1.7 (0–9) |
3.1 ± 1.8 (0–7) |
3.0 ± 1.8 (0–7) |
| CT score in each lobe | ||||||
| Right upper lobe | 0.5 ± 0.8 (0–3) |
1.3 ± 1.1 (0–5) |
1.5 ± 1.3 (0–5) |
1.6 ± 1.0 (0–5) |
1.4 ± 1.0 (0–4) |
1.4 ± 0.9 (0–4) |
| Right middle lobe | 0.4 ± 0.5 (0–2) |
0.9 ± 0.8 (0–4) |
1.2 ± 1.1 (0–5) |
1.2 ± 0.8 (0–5) |
0.9 ± 0.8 (0–4) |
1.1 ± 0.8 (0–3) |
| Right lower lobe | 0.8 ± 0.7 (0–3) |
1.9 ± 1.2 (0–5) |
2.0 ± 1.3 (0–5) |
2.1 ± 1.1 (0–5) |
1.8 ± 0.7 (0–3) |
2.0 ± 1.0 (0–4) |
| Left upper lobe | 0.4 ± 0.6 (0–3) |
1.0 ± 0.9 (0–3) |
1.3 ± 1.2 (0–5) |
1.4 ± 0.8 (0–5) |
1.3 ± 0.9 (0–4) |
1.2 ± 0.8 (0–3) |
| Left lower lobe | 0.6 ± 0.8 (0–3) |
1.4 ± 1.3 (0–5) |
1.9 ± 1.4 (0–5) |
1.9 ± 1.1 (0–5) |
1.7 ± 1.0 (0–4) |
1.7 ± 1.1 (0–4) |
Note: Quantitative data were presented as mean ± SD (min – max). Friedman test showed significant difference of the CT score among right upper lobe, right middle lobe and right lower lobe (P < 0.01); Wilcoxon test showed significant difference of the CT score between left upper lobe and left lower lobe (P < 0.01); Kruskal-Wallis test showed significant difference of the CT score among different stages (P < 0.01). Differences were considered significant at P < 0.05.
Fig. 4.
(a) The total CT scores of all stages based the period between the onset of initial symptoms and the CT scans. (b) The number of involved lobes of all stages based the period between the onset of initial symptoms and the CT scans.
4. Discussion
In this study, we analyzed the chest CT features and scores of patients with COVID-19 in different stages of disease.
In the early stage of symptomatic COVID-19 (0–4 days), 21.2 % of the CT scans showed no abnormalities. In case of CT abnormalities, peripheral GGO was the most important imaging manifestation (76.5 %), indicating the disease may mainly invade the terminal respiratory bronchi or alveoli at first. Some patients also showed crazy-paving pattern (36.1 %), consolidation (25.5 %), or linear opacities (6.3 %) on early stage CT, which indicated either the rapid progress of the disease with poor prognosis or shorter course with good prognosis. Consistent with one study [10], some patients in our study (21.2 %) presented with negative chest CT at initial presentation (0–4 days symptoms). In the progressive stage (5–9 days), the CT features of the lesions were variable. Crazy-paving pattern, consolidation and linear opacities increased significantly, indicating interstitial edema and alveolar exudation. The frequency of crazy-paving pattern, consolidation and linear opacities peaked at stage-3 (10–14 days), stage-4 (15–21 days) and stage-5 (22–28 days), respectively, and decreased thereafter, reflecting variable patterns of pulmonary abnormality by stage and a long process of disease development. In addition, the changes of chest CT in patients with COVID-19 at different stages may reflect the pathological changes to some extent [8].
Semi-quantitative CT scoring could reflect the severity of different stages of this disease. The total CT scores of the bilateral lungs were lowest at stage-1 compared to other stages, but there was no significant difference among other stages, indicating that the disease changed rapidly within 10 days after the onset of the initial symptom, then tended to be stable and lasted for a long time. In addition, there was a higher CT score in the lower lobes, consistent with other studies [8].
This study had several limitations. First, because of the relatively few cases, the results of our imaging findings might be biased. Second, follow-up observations of lung changes at Thin-Section CT of the disease are needed after discharge, but were not performed in this study. Third, there was a small number of pediatric population. Finally, no lung tissue biopsies were available to assess the correlation between radiological and histopathologic findings.
In summary, in our study with serial CT scanning, the CT features of the COVID-19 pneumonia were diverse and changed with duration of symptoms. The study provided thin-section CT evidence of semi-quantitative analysis of pulmonary damage severity for this potentially fatal disease. Our data suggested that thin-section CT could serve as a useful tool for evaluating the change of pulmonary abnormalities in patients during the acute and convalescent periods of the illness.
Funding sources
None.
CRediT authorship contribution statement
Xun Ding: Conceptualization, Methodology, Software, Data curation, Writing - original draft. Jia Xu: Resources, Software, Data curation. Jun Zhou: Formal analysis, Methodology. Qingyun Long: Supervision, Validation.
Declaration of Competing Interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled “Chest CT findings of COVID-19 pneumonia by duration of symptoms”
Acknowledgements
The authors gratefully thank the following doctors for their support with providing language help and clinical case analysis: Bing Liu and Wei Wang, the development of respiratory, Zhongnan Hospital of Wuhan University, Wuhan University, Wuhan, China.
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K.W., Bleicker T., Brunink S., Schneider J., Schmidt M.L., Mulders D., Haagmans B.L., van der Veer B., van den Brink S., Wijsman L., Goderski G., Romette J.L., Ellis J., Zambon M., Peiris M., Goossens H., Reusken C., Koopmans M.P.G., Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3) doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fang Y., Zhang H., Xie J., Lin M., Ying L., Pang P., Ji W. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology. 2020 doi: 10.1148/radiol.2020200432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ye G., Li Y., Lu M., Chen S., Luo Y., Wang S., Wang Y., Wang X. Experience of different upper respiratory tract sampling strategies for detection of COVID-19. J. Hosp. Infect. 2020 doi: 10.1016/j.jhin.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie X., Zhong Z., Zhao W., Zheng C., Wang F., Liu J. Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing. Radiology. 2020 doi: 10.1148/radiol.2020200343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shigemura J., Ursano R.J., Morganstein J.C., Kurosawa M., Benedek D.M. Public responses to the novel 2019 coronavirus (2019-nCoV) in Japan: mental health consequences and target populations. Psychiatry Clin. Neurosci. 2020 doi: 10.1111/pcn.12988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan Y., Guan H., Zhou S., Wang Y., Li Q., Zhu T., Hu Q., Xia L. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur. Radiol. 2020 doi: 10.1007/s00330-020-06731-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan F., Ye T., Sun P., Gui S., Liang B., Li L., Zheng D., Wang J., Hesketh R.L., Yang L., Zheng C. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. 2020 doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang Y.C., Yu C.J., Chang S.C., Galvin J.R., Liu H.M., Hsiao C.H., Kuo P.H., Chen K.Y., Franks T.J., Huang K.M., Yang P.C. Pulmonary sequelae in convalescent patients after severe acute respiratory syndrome: evaluation with thin-section CT. Radiology. 2005;236(3):1067–1075. doi: 10.1148/radiol.2363040958. [DOI] [PubMed] [Google Scholar]
- 10.Ling Z., Xu X., Gan Q., Zhang L., Luo L., Tang X., Liu J. Asymptomatic SARS-CoV-2 infected patients with persistent negative CT findings. Eur. J. Radiol. 2020;126 doi: 10.1016/j.ejrad.2020.108956. [DOI] [PMC free article] [PubMed] [Google Scholar]