Abstract
Antimicrobial resistance (AMR) continues to threaten global health. Although global and national AMR action plans are in place, infection prevention and control is primarily discussed in the context of health care facilities with home and everyday life settings barely addressed. As seen with the recent global SARS-CoV-2 pandemic, everyday hygiene measures can play an important role in containing the threat from infectious microorganisms. This position paper has been developed following a meeting of global experts in London, 2019. It presents evidence that home and community settings are important for infection transmission and also the acquisition and spread of AMR. It also demonstrates that the targeted hygiene approach offers a framework for maximizing protection against colonization and infections, thereby reducing antibiotic prescribing and minimizing selection pressure for the development of antibiotic resistance. If combined with the provision of clean water and sanitation, targeted hygiene can reduce the circulation of resistant bacteria in homes and communities, regardless of a country's Human Development Index (overall social and economic development). Achieving a reduction of AMR strains in health care settings requires a mirrored reduction in the community. The authors call upon national and international policy makers, health agencies, and health care professionals to further recognize the importance of targeted hygiene in the home and everyday life settings for preventing and controlling infection, in a unified quest to tackle AMR.
Key Words: Targeted hygiene, COVID-19, Antimicrobial resistance, Global health
Antimicrobial resistance (AMR) is one of the greatest threats to global health today.1 Although global and national AMR action plans are in place, infection prevention and control is primarily discussed in the context of health care facilities with home and everyday life settings barely addressed.
As seen with the recent global pandemic of coronavirus SARS-CoV-2, everyday hygiene measures can play an important role in containing and delaying the threat from infectious microorganisms and when employed at other times, are likely to reduce disease for which antibiotics would be prescribed or even mis-prescribed.
At the time of writing this review and according to Dr Maarten van Dongen, founder of AMR Insights, the global deaths from SARS-CoV-2 between November 2019 and March 2020 reached 16,500 with many affected succumbing to secondary bacterial infections. In comparison, 2,000 people worldwide die as a result of resistant bacteria every day,8 amounting to 258,000 people dying from the effects of AMR within a similar time-frame.
The latest analysis by the World Health Organization's (WHO) Global Antimicrobial Resistance Surveillance program, which included data from 49 countries, found high levels of AMR in Escherichia coli, Klebsiella pneumoniae, Salmonella spp., Acinetobacter spp., Staphylococcus aureus, and Streptococcus pneumoniae in every WHO region.2
The global impact is already profound and expected to intensify, particularly among the poorest nations.3 , 4 The main driver is overuse and misuse of antibiotics in medicine and agriculture including unregulated over-the-counter sales, while global spread of resistant bacteria or resistance genes is attributed to poor infection prevention and control in health care facilities, and suboptimal hygiene and sanitation in communities, confounded by poor infrastructure and weak governance.5 In the United States, between 80% and 90% of the volume of human antibiotic use occurs in the outpatient setting, with nearly 50% considered to be inappropriate or unnecessary.6 Without prompt action, it is estimated that rates of AMR to commonly used antibiotics could exceed 40%-60% in some countries by 2030,7 and by 2050, around 10 million people could die each year as a result of resistance to antibiotics and other antimicrobial agents.8 Almost 9 million of these will be in Africa and Asia.8
In 2015, an alliance of the WHO, the Food and Agriculture Organization of the United Nations and the World Organization for Animal Health developed a Global Action Plan (GAP) to: “improve awareness and understanding of AMR through effective communication, education and training, strengthen the knowledge and evidence base through surveillance and research, reduce the incidence of infection through effective sanitation, hygiene and infection prevention measures, optimize the use of antimicrobial medicines in human and animal health, and, develop the economic case for sustainable investment that takes account of the needs of all countries and to increase investment in new medicines, diagnostic tools, vaccines and other interventions.”9 The GAP emphasizes the need for society-wide engagement, with a clear focus on “prevention first.”9 One of the 5 strategic objectives is a reduction in the incidence of infection through improved sanitation, hygiene, and infection prevention.9 At least 120 countries have finalized national action plans, with the plans of more than 60 other countries under development.10
What is striking is that the GAP and national plans discuss infection prevention and control primarily in the context of health care facilities. (See https://www.who.int/antimicrobialresistance/national-action-plans/library/en/). By contrast, the latest 2019 UK national action plan, which sets out a 20-year vision11 and a 5-year plan12 for how the United Kingdom will contribute to controlling AMR by 2040, offers guidelines on infection prevention in health care settings, but also highlights the role of the community, noting that, when it comes to infections in the community, the public have a huge part to play.12 The plan emphasizes the importance of e-bug (www.e-bu.eu), an educational program developed by Public Health England which aims to ensure that all children across Europe leave school with an understanding of AMR and the role of hand, food, and respiratory hygiene in prevention of infections.
This paper is the output of a scientific meeting commissioned by The Global Hygiene Council involving an invited group of experts from the fields of microbiology, AMR, hygiene, and public health. The paper is not a systematic review, which has already been undertaken by others, including the International Scientific Forum on Home Hygiene (http://www.ifhhomehygiene.org), but presents key evidence that the home and other everyday life settings are important for the transmission of infections and the acquisition and spread of AMR, and that home and everyday life hygiene should be given greater consideration in global and national action plans. It is also important to acknowledge that while hygiene measures are a vital part of infection prevention, addressing the rising threat of AMR requires a holistic approach which includes reducing the use of antibiotics in agriculture, introducing tighter regulations of over the counter sales and improving access to clean water and sanitation.13
Adopting a targeted hygiene approach in our homes and everyday lives (including workplaces, schools, nurseries, on public transport, during leisure activities etc.) offers a way to maximize protection against colonization and infection, at the times and places where there is the greatest risk of transmission. This in turn reduces the need for antibiotics, thereby minimizing the selection pressure for development of AMR.
Importance of the home and everyday life settings in the spread of infection
In recent years, demographic changes and changes in health service structure mean that the number of people living in the community needing special care, because they are at greater risk of infection, has significantly increased. The largest proportion of these are the elderly, who generally have reduced immunity to infection which is often exacerbated by other illnesses like diabetes and malignant illnesses. A decrease in immunity usually starts from 50 years old.
Other infection-susceptible groups include the very young, patients recently discharged from hospital, and family members with invasive devices such as catheters, as well as those whose immune competence is impaired as a result of chronic and degenerative illnesses (including HIV/AIDS) or because they are receiving immunosuppressant drugs or other therapies. Immunosuppressed individuals are often also on other medications such as antibiotics, to help protect them from infection but can further increase susceptibility to infections such as Clostridium difficile.
Home and everyday life settings provide multiple opportunities for spread of infection. Everyday life settings include locations where normally there is no mandated hygiene policy as is typically found in clinical and educational settings; for example, work places, public transport, gyms, child day-care facilities, and shopping centers.
Poor hygiene is considered a major factor in the transmission of community-based infections, including gastrointestinal (GI) and respiratory tract (RT) infections such as colds and influenza, and skin infections caused by S. aureus.14 For the elderly, communal living environments, combined with problems of fecal incontinence, create an environment in which enteric and foodborne pathogens are easily spread. As a result, the incidence of salmonellosis and Campylobacter diarrhea appears to be higher among the elderly in these situations.
More vulnerable “at risk” members of society are now being looked after outside hospital settings. For example, in Germany, it is estimated that approximately three-quarters of all people in need of care are currently being cared for at home.15 In the community, the immunocompromised are also at risk from opportunistic pathogens such as E. coli, Klebsiella spp., and Pseudomonas aeruginosa, which are considered as hospital related.15
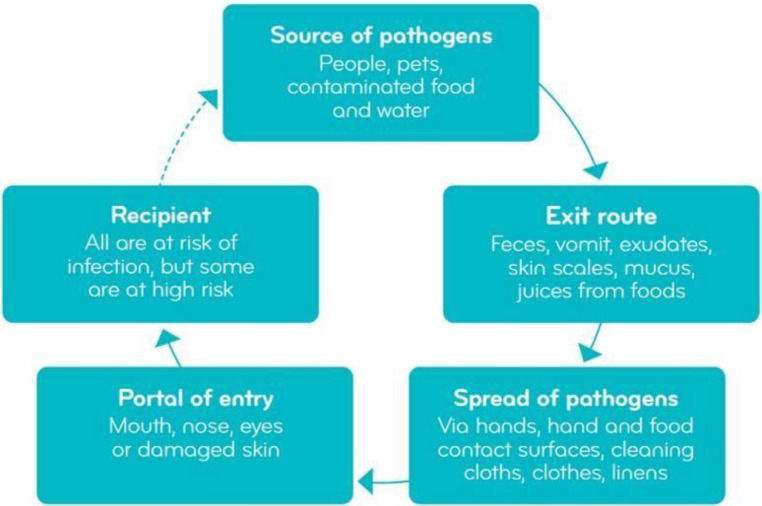
The key steps in preventing the spread of infection, known as breaking the chain of infection, are the same regardless of setting. In the home, pathogens may have been brought home from hospital settings or enter the home via colonized or infected people, pets/domestic animals, or through contaminated food and water.15 , 16 Pathogens and other microbes are shed constantly from these sources, with rapid transmission around the home mainly via hands, hand and food contact surfaces, cleaning utensils and in the air (Fig 1 ).15
Fig 1.
The chain of infection in the home and everyday environments (adapted with permission from the IFH, see reference 15).
Multiple studies conducted in home and everyday life situations demonstrate that pathogens on hands and surfaces can be transferred from an infected person spread via hands and surfaces, from feces, via respiratory droplets and from handling and preparing contaminated food, and can survive and disseminate in numbers sufficient to cause infection.15 , 16 These studies involve species including C. difficile, 17 Salmonella spp., Campylobacter spp., S. aureus, E. coli,18, 19, 20, 21, 22 Listeria monocytogenes, 23 , 24 norovirus,25, 26, 27 rhinovirus,28 influenza virus,27 , 29 and SARS-CoV-2.30
A study18 involving preparation of Salmonella and Campylobacter-contaminated chickens (N = 20) in domestic kitchens showed transfer of the pathogen to 10.1% and 7.2%, respectively, of contact surfaces (hands, cleaning cloths, chopping boards, utensils, tap handles, and cupboard door handles). In a follow-up study,31 hands, chopping board, and cloth samples yielded Most Probable Number counts of >100 and >1,000 Salmonella per sample from 13.3% and 8%, respectively. For Campylobacter, counts of >100 and >1,000 were isolated from 5% and 1.7%, respectively. This is a concern, since it is estimated that 80% of Salmonella infections originate in the home,32 and a UK study detected Campylobacter spp. in 56% of chilled retail chickens, with 7% of samples containing >1,000 colony forming units (CFU)/g of skin.33 The infectious dose of Campylobacter is estimated at <500 CFU.34 Chaidez et al35 demonstrated that the risk of Salmonella transmission from cleaning cloths via hands to mouth was far higher than the guideline levels for acceptable risk.
Since most pathogenic organisms die relatively rapidly, particularly on dry surfaces, the greatest risk of human exposure presents immediately after shedding from an infected or contaminated source. However some species, including S. aureus, E. coli, and other organisms such as fungal species, rhinovirus, and norovirus can survive for long periods even on dry surfaces.36 Audit studies suggest that some gram-negative organisms can form permanent reservoirs or secondary sources of contamination, particularly where moisture is present such as in sinks and drains, kitchen cleaning cloths, and sponges.37, 38, 39, 40, 41 To what extent these reservoirs of contamination might include potentially harmful species is not known. However, a study conducted in Culiacan, Mexico, detected Salmonella in 173/180 (96%) kitchen cleaning cloths taken from domestic homes, with an average Most Probable Number Salmonella count of 661.35 Although further investigation is required, this suggests that Salmonella may be multiplying in the cloths to form a permanent reservoir.
Infection risk depends on the level of exposure to pathogenic organisms. The “infectious dose” of bacteria and viruses (ie, the number of bacterial cells or virus particles required to cause a significant risk of infection) varies for different organisms and in different situations.42 , 43 The infectious dose for commonly encountered bacteria such as Campylobacter, Shigella, enterohemorrhagic E. coli and C. difficile, and viruses such as norovirus and rhinovirus, may be small (1-500 particles or cells).44, 45, 46, 47, 48, 49 For others (eg, Salmonella), it can be far greater (∼106 organisms).44 The dose also depends on host susceptibility and mode of entry, and may be lower for at-risk groups in the community such as children, the elderly, and people with compromised immunity.44
Although care of increasing numbers of patients in the community, including at home can help alleviate over-burdened health systems, it can be undermined by inadequate infection control in the home, and urgent focus is now needed on infection transmission in homes and community settings in addition to health care settings.
AMR bacteria in the home and community environments
Although multidrug-resistant (MDR) bacteria (ie, bacteria that have acquired resistance to at least 1 agent in 3 or more antimicrobial classes) are typically hospital-acquired,50 studies conducted primarily in high-income countries show that some have become quite prevalent in the community.51 Infected or colonized patients discharged from health care settings can remain persistent skin carriers of methicillin-resistant S. aureus (MRSA), or fecal carriers of enterobacteria strains which carry MDR factors (eg, New Delhi metallo-beta-lactamase 1 [NDM-1] or extended spectrum beta-lactamase [ESBL] enzymes). Many community-onset infections are associated with recent discharge from a health care setting.52, 53, 54, 55, 56 MDR strains can be passed to other family members who become infected or colonized56 although carrier status is often not apparent as colonization does not necessary results in clinical disease.
Factors affecting the spread of MDR bacteria into home and everyday life settings are complex. MRSA is probably the most important MDR bacterium to transition from health care settings to the community. The ease of transfer of MRSA from hospitals to the home via health care workers and others in contact with hospitals has been demonstrated in multiple studies.52, 53, 54, 55, 56 This is illustrated by a study57 where significant levels of community and hospital strains of MRSA were recovered from high-frequency touch surfaces (door handles, toilet seats, reception areas, public washrooms, corridors, and lifts) in public areas in the community and in London hospitals, suggesting cross-contamination between the 2 settings. Once in the home, MRSA can colonize and/or cause infection among family members.58, 59, 60
Other studies show the spread of MRSA from a colonized index case (eg, a nurse) at work to the home environment and other family members.56 Antibiotic treatment of family members failed to eradicate colonization because they became recolonized from contaminated environmental surfaces. Eradication was only successful when antibiotic treatment was combined with rigorous cleaning of the home environment.56 Further studies on spread of MRSA in the home environment are reviewed by Bloomfield et al, 2012.43
Since 2000, we have seen the emergence of new “community acquired” strains of MRSA (CA-MRSA). While health care-associated strains are mainly a risk to vulnerable people, for CA-MRSA, any family member is at risk and it is more prevalent among children and young adults where they cause infections of cuts, wounds, and abrasions. US experience suggests the risk is greatest among those engaging skin-to-skin contact activities and contact with contaminated objects such as towels, sheets, and sports equipment. Transmission is common in settings such as prisons, schools, and sports teams.43 A study assessing the transmission of CA-MRSA in a university in the United States, found multidrug-resistant USA300 responsible for diseases including necrotizing pneumonia, severe sepsis, and necrotizing fasciitis, on common touch surfaces at the university, student homes, and local community settings. This suggests transfer between different locations within the community.61
Enterobacterales are a common cause of community-associated infections, including urinary tract infections and bacteremia as well as GI infections.52 Resistance in community-associated Enterobacterales mediated by extended spectrum β-lactamases is now common in Asia, the Middle East, South America, and some parts of Europe. A 2012 study in Birmingham, UK, indicated that the proportion of E. coli carrying CTX-M ESBL genotypes in a community population was 11.3%,62 while a 2006-2011 study63 reported a 10-fold increase (from 0.6% to 6%) in ESBL-producing E. coli fecal carriage in healthy subjects in a Parisian community. Lower-prevalence regions include North America, parts of Northern Europe, Australia, and New Zealand.52
According to the WHO, foodborne pathogens, including most commonly Salmonella, Campylobacter, and E.coli affect millions of people globally every year, causing diarrhea or debilitating infections.64 Data from 18 European countries suggest that about 31% of foodborne outbreaks occur in private homes.16 Foodborne infections associated with these pathogens can be effectively treated by a course of antibiotics, but, for infections caused by consumption of foods contaminated with antibiotic resistant strains, this is not possible.
Recent reports indicate the importance of food as a potential source of ESBL-producing organisms, explaining in part the spread of such organisms to community settings.65 Poor hygiene with contaminated chopping boards and sponges have been found to be the potential cause of the spread of ESBLs in community settings. For example, in a study conducted in Switzerland, France, and Germany, where 144 cutting boards used in domestic homes were examined after preparation of chicken, meat, or game, ESBL-producing E. coli was recovered from 5 samples (3.5%).66 An Italian study, of 100 “in-use” kitchen sponges, found high levels of Enterobacteriaceae (5.89 log CFU/g). Identification of enterobacteria revealed several opportunistic and pathogenic agents, including Enterobacter cloacae (28%), Citrobacter freundii (23%), and Cronobacter sakazakii (15.1%). In total, 69/309 (22%) of enterobacteria strains tested were ESBL-positive.67 Kitchen sponges act not only as reservoirs of microorganisms but also as disseminators over domestic surfaces, which can lead to cross-contamination of hands and food, which is considered a main cause of foodborne disease outbreaks.
Carbapenem-resistant Enterobacteriaceae are also on the rise globally, but, to date, most carbapenem-resistant Enterobacteriaceae infections in the United States and Europe have been health care-associated.68 , 70 Although data from Asia are sparse, carbapenemases have been found in bacteria recovered from drinking water in India and in food-producing animals in China.69 , 71 , 72 In European studies during the 1990s, vancomycin-resistant enterococci were detected in the stools of healthy volunteers.73, 74, 75, 76, 77 However, rates of vancomycin-resistant enterococci, carbapenem resistance in Acinetobacter infections, and MDR P. aeruginosa are thought to be low in individuals living in the community.52
Overall, the evidence suggests that MDR strains of bacteria, like any other strains of bacteria, can enter the home or other settings via people who are infected or colonized or via contaminated food and can be spread to other members of the family via hands and contaminated surfaces.
The role of targeted home and everyday life hygiene in tackling antibiotic resistance
If implemented effectively, home and everyday life hygiene has the potential to reduce rates of infection and the need for antibiotic prescriptions, thereby reducing the selective pressure for the development and subsequent dissemination of resistance.16 , 78 As witnessed in the recent global efforts to contain SARS-CoV-2 virus and delay the spread of Covid-19, hygiene practices including handwashing are the first line of defense to reduce the transmission of infection. Targeted hygiene also helps to reduce spread of bacterial species with a low degree of pathogenicity (opportunistic pathogens) such as, enterococci that are known to contain MDR determinants. These can form reservoirs of resistance determinants, which can be disseminated by horizontal transfer to other pathogenic species.79
What is targeted hygiene?
Targeted hygiene is a risk management system developed for home and everyday life settings during the 1980s.80 It is based on scientifically validated systems developed by the food and other manufacturing sectors as the most effective means to protect products from contamination. Studies on consumer understanding of hygiene show that the public are very confused about hygiene and what hygiene really means, tending to equate it with eradication of dirt, assumed to be the major source of harmful microbes.81 , 82 Targeted hygiene means focusing hygiene practices in places and at times (referred to as “risk moments”) when harmful microbes are most likely to be spread, in order to break the chain of infection transmission. The key risk moments within home and everyday settings include food handling, using the toilet and changing a baby's diaper/nappy, touching surfaces frequently touched by others, coughing, sneezing and nose blowing, handling and laundering clothing and household linen, caring for domestic animals, disposing of refuse, and caring for an infected family member who is shedding infectious microbes into the environment.15 , 16 , 83 , 84
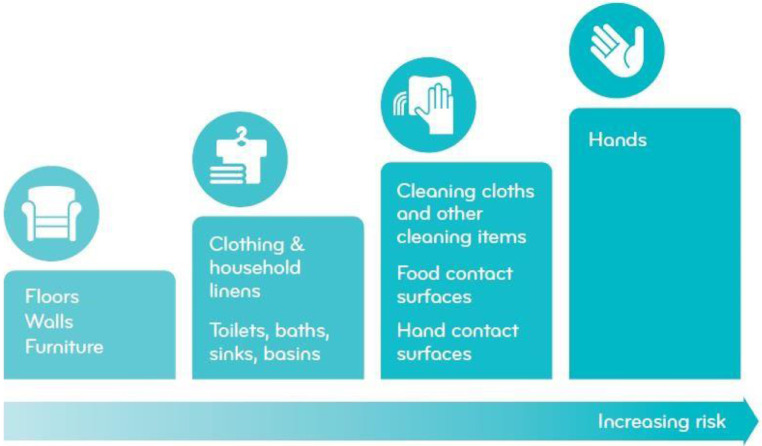
Microbiological data15 , 16 suggest that the surfaces that are most often responsible for spread of harmful microbes, at key moments include the hands themselves, hand contact surfaces, food contact surfaces, and cleaning cloths and other cleaning items (Fig 2 ). These surfaces are referred to as critical surfaces or critical control points. Clothing, household linen, toilets, sinks, and bath surfaces may also contribute to establishing a chain of infection; however, the risks associated with these surfaces are typically lower as they rely on the hands and other “chain links” to disseminate infectious microbes to cause human exposure.
Fig 2.
Critical surfaces in the home, ranked by risk of infection transmission (adapted with permission from the IFH, see ref. 15)
Breaking the chain of infection
An important aspect of targeted hygiene is hygienic cleaning—as opposed to visible cleaning—to break the chain of infection. This is achieved using hygiene procedures (products plus process) to reduce pathogenic microorganisms on critical surfaces to a level where they are no longer harmful to health, thereby preventing ongoing spread.16 , 84 Several methods exist to achieve such reduction in potential pathogens: mechanical/physical removal using dry wiping, soap or detergent-based cleaning together with adequate rinsing, inactivation or eradication using a disinfectant on hard surfaces or an alcohol-based sanitizer on the hands, or a physical process such as heating (to ≥60°C/140°F) or ultraviolet treatment. Most frequently, a combination of these approaches is likely to be used.16 , 84
When developing hygiene procedures aimed at breaking the chain of infection, the goal should be to ensure that each procedure is appropriate to its intended use. In recent years, risk modeling has been developed in order to achieve this.80 Quantitative Microbial Risk Assessment (QMRA) was originally developed for ensuring water quality and is increasingly being used to develop infection prevention control strategies in other settings, including health care.85 , 86
QMRA is a scientifically validated approach that uses published data to model the chain of infection and estimate safe residual level of contamination at critical points in the chain.84 , 87 This information is then used to estimate the log reduction required to reduce contamination to a safe level. Based on these estimates, tests modeling use conditions can be used to develop effective hygiene procedures to achieve the required reduction. The approach is set out in more detail by Bloomfield et al.84
In the past, recommendations on selection of hygiene procedures for home and everyday life were based on the health status of family members, and it is still argued by some that disinfectants should only be used in situations where people are infected or at increased risk of infection.87, 88, 89 However, if home and everyday life hygiene is to be effective, one has to take into account the established evidence from models simulating use conditions showing that, in some risk situations, hygiene procedures that involve just wiping or detergent-based cleaning are insufficient.19 , 36 , 90 , 91
Chaidez et al demonstrated, using QMRA, that twice-daily soaking of kitchen cleaning cloths in sodium hypochlorite disinfectant solution (2.1%) reduced the average 6-week probability of acquiring a Salmonella infection from handling cloths by almost 100-fold, compared with usual cleaning practices.36
Although there are data to show that hygiene is important in preventing transmission of MRSA colonization and infection in the domestic environment, further investigation is required to demonstrate the full extent to which poor home hygiene may contribute to the burden of foodborne infection associated with antibiotic-resistant strains.
Assessing the impact of hygiene on infection rates and antibiotic prescribing
Quantifying the impact of hygiene on the burden of infection in home and everyday life is challenging because of the large population sizes required to generate significant results, and difficulties in conducting studies involving multiple interventions. Most data have been generated from single intervention studies—primarily hand hygiene—where meta-analyses show a positive impact on GI and RT infections.91, 92, 93
Children who attend day-care centers have significantly more infections than those who do not. The most common are RT and GI infections, and the risk of otitis media is almost twice that of children remaining at home.94 Studies in day-care centers and schools in which hand hygiene was combined with cleaning and/or disinfection of environmental surfaces indicate a positive impact on illness rates and reduction in the use of antibiotics.94, 95, 96, 97
In an intervention study96 in a preschool setting involving enhanced environmental cleaning and disinfection with an emphasis on toys, the number of courses of antibiotics administered to children aged 6 weeks to 5 years was reduced from a median of 0.33 (25%-75%; interquartile range 0.25-0.67) per child per month to 0.28 (25%-75%; interquartile range 0.17-0.42; P < .05). Another 15-month study in 10 day-care centers involving intensified handwashing, use of alcohol-based disinfectant and disposable towels, cleaning of the day-care center, and regular washing of toys, resulted in 24% (95% confidence interval [CI] 22%, 27%) fewer prescriptions of antimicrobials among the children and staff (P < .001).94 A 2018 hand hygiene study at child care centers, reported a 30% reduction of antibiotic prescriptions for RT infections in a group who used hand sanitizers compared with a control group.98 Another 2018 intervention study99 found that children were prescribed antibiotics for significantly fewer weeks in day-care centers using specific disinfecting products and cleaning protocols than centers that continued to use their standard procedures and products (RR = 0.68; 95% CI 0.54, 0.86; P = .001)—a relative risk reduction of almost one-third.
To the best of our knowledge, only 1 study on the impact of targeted hygiene in the home has been conducted.100 This study, conducted among low-income communities in Cape Town, South Africa, evaluated the impact of hygiene education alone and education in combination with handwashing with soap at critical times, bathing at least 3 times a week, cleaning/disinfecting household surfaces at critical times, and proper waste disposal.100 The study reported that children under 5 years of age from the education only households were 2.5 times more likely to experience GI illness (hazard ratio [HR] 2.5; 95% CI 1.17, 4.91) and 4.6 times more likely to experience RT illness (HR 4.6, 95% CI 1.97, 10.54) than those where households implemented the additional hygiene measures. This study suggests the important role a multicomponent intervention, including hygiene education and the use of hygiene product, plays in reducing the risk of infections in a home setting. However, according to a recent literature review of 29 studies assessing behavior change interventions designed to increase hand-hygiene and environmental disinfecting in settings likely to include children, in order to widely implement such approaches and see better infection outcomes, significant hygiene behavior change is still needed for communities.101
QMRA is also now being used to estimate the impact of hygiene interventions on infection in community settings.102 Haas et al103 used QMRA to compare the impact of hand hygiene on transmission from hand to mouth of E. coli O157:H7 following hand contact with ground/minced beef. It was determined that, if no handwashing was performed, this would result in 0.7 infections per year. By contrast, if all individuals washed their hands with soap following contact with the ground/minced beef, producing a 0.3 log reduction on hands, this would result in an estimated 0.014 infections per year (98% median risk reduction compared with no handwashing). If all individuals used an alcohol-based hand sanitizer following contact with the beef, producing a 4.3 log reduction on the hands, this would result in an estimated 0.00005 infections/year—a 99.9996% median risk reduction for the hand sanitizer compared with handwashing.
Duff et al104 developed a computer-based model to estimate the cost-effectiveness of a disinfection program that targeted high-risk food preparation activities in household kitchens. The model estimated that approximately 80,000 Salmonella, Campylobacter, and E. coli O157 infections could be prevented annually in US homes, resulting in $138 million in direct medical cost savings and a gain of 15,845 quality-adjusted life-years. Set against a program cost of $788 million, the program was associated with a favorable cost-effectiveness ratio of $41,021/quality-adjusted life-year gained. Results were similar for households in Canada and the United Kingdom.
Could use of microbicides in the home and everyday life select for AMR and its dissemination?
Concern has been expressed as to whether expanding use of microbicidal products, in the home and everyday life may contribute to the rise in AMR.105 Sublethal levels of microbicides can induce stress on bacterial cells, causing expression of mechanisms that reduce the biocide concentration at the bacterial target site further and allow the bacterial cell to repair.106 , 107 These include overexpression of an efflux system, membrane regulatory changes, and changes in membrane permeability and composition.107 These same mechanisms can produce changes in the susceptibility profile to unrelated antimicrobials.107 In other words, the use of microbicides may cross-select for antibiotic resistance and be associated with reduced antibiotic susceptibility to clinically significant levels (recently reviewed by Maillard107).
Factors inherent to the microbicide (ie, concentration, formulation, mechanism of action), the microorganisms (ie, type/strain, metabolism, resistance mechanisms), and product usage (eg, concentration, exposure time), all impact on product efficacy.107 Decreases in efficacy, for example, following shorter contact time or product dilution, will lead to bacterial survival—antimicrobial damage caused by a sublethal concentration of a microbicide is likely to be repairable.107
A number of expert reports commissioned in the last 10 years have highlighted laboratory studies linking microbicide use with reduced antibiotic susceptibility. However, these reports conclude that there is little evidence for this effect occurring in real-life clinical practice, and have called for further research into whether microbicide use influences antibiotic resistance in the community.108, 109, 110, 111
Rutala et al112 found that the frequency of occurrence of antibiotic resistance in environmental isolates from homes was much lower than for clinical isolates from a hospital intensive care unit and an outpatient setting where there was routine extensive use of antibiotics.
Two studies were carried out to investigate whether antibiotic-resistant strains were more likely to be found in homes where antibacterial products were used, compared with homes where they were not.113 , 114 Samples were collected from houses in the United States and United Kingdom of 30 users and nonusers of antibacterials. Susceptibility tests against antibiotics and antibacterial agents (triclosan, pine oil, BAC, and para-chloro-meta-xylenol) were carried out on the bacteria isolated. The authors concluded that there was no evidence that antibiotic-resistant strains occurred more frequently in user homes compared with nonuser homes. A 1-year study by Aiello et al (2005) also showed that household use of antibacterial cleaning products was not a significant risk factor for occurrence of antibiotic-resistant isolates from hands.115
Despite more than 20 years of research, there is still no conclusive resolution to the question of whether and to what extent microbicides might contribute to AMR in clinical practice. In light of laboratory data, which indicate that microbicide-induced AMR is biologically plausible for some types of microbicides, it is concluded that use of microbicides needs to be prudent and appropriate and that the products containing them must be used at recommended concentrations and with the appropriate contact time.
Targeted hygiene works to ensure that use of disinfectants and hand sanitizers (ie, microbicides used at the correct concentration and contact time) are confined to situations where there is identifiable risk of spread of harmful microorganisms, ensuring that they play an essential role in tackling AMR. The need for antibiotic prescribing may in fact increase if disinfectants and hand sanitizers are not used as indicated, due to the increased risk of infection and survival of bacteria bearing AMR determinants. These could potentially spread to other areas in the home and on into the community. It is important to note also that preventing viral infections as well as bacterial infections, such as those that cause respiratory and GI infections, can also have a role in reducing AMR as this will eliminate the potential for mis-prescribing or misuse of antibiotics.
The need for targeted hygiene in low- and middle-income countries
In low- and middle-income countries (LMICs) and all countries, regardless of their Health Development Index (measurement of a country's overall social and economic development), the principles of targeted hygiene are the same as those for high-income countries: pathogens from infected or contaminated sources are introduced into the home and other settings and spread via critical surfaces such as hands, frequently touched and food contact surfaces. The major differences are that there are more risk factors for infection, including drug-resistant infections in the community in LMICs as a result of suboptimal environmental sanitation, lack of environmental regulations, high-density housing, and inadequate access to clean water due to contamination during collection, transportation, storage, and use.116 These issues can lead to situations such as those found in India, where unregulated over-the-counter sales of antibiotics, poor sanitation, and environmental antibiotic pollution, alongside other behavioral, cultural, and social factors, have created ideal conditions for a rapid rise in MDR infections.117 , 118
In 2015, an estimated 663 million people around the world were drinking from unimproved water sources, and 2.4 billion had no access to improved sanitation—the vast majority of these were in sub-Saharan Africa and South Asia.119 It is estimated that 2.3 billion people lack the use of sanitation facilities which are not shared with other households,120 and 892 million people still defecate in the open, leading to contamination of drinking water sources and spread of disease.121 Higher AMR rates in LMICs in which per-capita antibiotic consumption is far lower than in high-income countries, suggests that environmental and other factors are contributing to the increased prevalence of AMR.5
A number of audit studies have been conducted evaluating the occurrence of microbes in homes in LMICs, mostly reporting on the presence of coliforms or total counts (see review by Bloomfield et al).43 As with studies conducted in high-income countries, the highest levels of contamination in LMICs are typically found in moist locations such as kitchen sponges and dishcloths.122, 123, 124 The key question, however, is whether, and to what extent, the incidence and levels of potentially harmful pathogens (and thus infection risks) are higher in homes without access to adequate water and sanitation.
Sinclair and Gerba124 monitored fecal coliforms, total coliforms, E. coli and heterotrophic plate count bacteria on household surfaces in 8 homes that had improved latrines (ie, a pour-flush latrine) in a rural village of Cambodia, and compared the results with similar data from homes in the United States39 and Japan.40 Fecal coliform levels in Cambodia were found to be highest in moist locations such as the plastic ladle used for sink water, the toilet seat surface, and the cutting board surface.124 For E. coli, the mean log CFU per 4 cm2 ranged from 0.5 to 4.0, with highest counts found on the top of the squat toilet, the wash basin, and the floor around the toilet. Fecal coliform levels were 100-fold higher on these surfaces in Cambodia than on equivalent surfaces in the US and Japanese studies.
In LMICs, due to a lack of basic sanitation, good hand hygiene is of vital importance.116
Globally, it has been estimated that only 19% of the population washes its hands with soap after contact with excreta.93 Observations show that handwashing with soap is undertaken in an ad hoc manner,116 with many households having no access to handwashing facilities.125 Unsurprisingly, studies in LMICs have reported high levels of fecal indicator bacteria on the hands of household members,126, 127, 128 with 1 study correlating presence of fecal contamination on the hands with the prevalence of GI and respiratory symptoms within the household.126 A Cochrane review showed that improving handwashing practices probably reduces diarrhea episodes in child day-care centers in both high-income countries and among communities living in low- to middle-income countries by as much as 30%.92
Conclusions
The evidence set out in this paper suggests that, if combined with measures ensuring clean water and adequate sanitation, targeted hygiene practices in home and everyday life settings could make a significant contribution to tackling AMR through infection prevention and a consequential reduction in antibiotic prescribing. This is true in all areas of the world including low-income countries.
Additionally, the evidence suggests that hygiene promotion would contribute to preventing the transmission of resistant bacteria from the home and everyday life settings, into health care settings, and back into the community. Further research is still needed to evaluate the extent to which this might occur, especially in communities in low-income countries.
To be effective, hygiene interventions need to consider all aspects that are likely to affect the outcome. This includes a reduction of antibiotics from the food chain and the environment, improved hygiene education and availability of appropriate products as well as the provision of clean water and improved sanitation.
Based on these findings, the authors of this paper issue a call to action to national and international health policy makers, health agencies, and health care professionals to give greater recognition to the importance of hygiene in the home and everyday life and development and promotion or more effective codes of practice for hygiene in the home and everyday life as part of national action plans to tackle AMR. Although the precise impact of hygiene on transmission of infection between community and health care settings needs further investigation, it is important to recognize that reducing the need for antibiotic prescribing and the circulation of AMR strains in health care settings cannot be achieved without also reducing circulation of infections and AMR strains in the community. We cannot allow hygiene in home and everyday life settings to become the weak link in the chain.
Footnotes
Conflicts of interest: Professor Jean-Yves Maillard is the Director for Biocide Consult Ltd.
Professor Essack is Chairperson of the Global Respiratory Infection Partnership, sponsored by an unrestricted educational grant from Reckitt and Benckiser, UK.
Professor Charles P. has received payments from the following companies for consultancy, advisory boards and grants: Allied Biosciences, Clorox, Ecolab, Georgia Pacific, GOJO Industries, Guest Services, Kimberly Clark, Kohler, Proctor and Gamble, Purethread, Reckitt Benckiser, and Unilever India.
Joseph R. Rubino holds the position of R&D Shared Services Director, RB Inc., Germ Protection, and Personal Care.
Disclosures: The development of this position paper was supported by an educational grant from Reckitt Benckiser who funded the activity of the Global Hygiene Council. Medical writing support was provided by the medical communications agency, Spink, which runs the secretariat for the GHC.
References
- 1.World Health Organization. Antibiotic resistance: key facts. 2018. Available at:https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance. Accessed June 17, 2019.
- 2.World Health Organization . 2019. Global antimicrobial resistance surveillance system (GLASS) Report 20172018. Available at: https://www.who.int/glass/resources/publications/earlyimplementation-report-2017-2018/en/. Accessed June 18, 2019. [Google Scholar]
- 3.The World Bank/International Bank for Reconstruction and Development. Drug-resistant infections: a threat to our economic future. 2017. Available at:https://www.worldbank.org/en/topic/health/publication/drug-resistant-infections-a-threat-to-our-economicfuture. Accessed July 1, 2019.
- 4.Gandra S, Tseng KK, Arora A, et al. The mortality burden of multidrug-resistant pathogens in India: a retrospective observational study. Clin Infect Dis. 2018;69:563–570. doi: 10.1093/cid/ciy955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collignon P, Beggs JJ, Walsh TR, Gandra S, Laxminarayan R. Anthropological and socioeconomic factors contributing to global antimicrobial resistance: a univariate and multivariable analysis. Lancet Planet Health. 2018;2:e398–e405. doi: 10.1016/S2542-5196(18)30186-4. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Antibiotic prescribing and use in doctor's offices: measuring outpatient antibiotic prescribing. Available at: https://www.cdc.gov/antibioticuse/community/programs-measurement/measuring-antibiotic-prescribing.html#f10. Accessed November 7, 2019.
- 7.Organisation for Economic Co-operation and Development (OECD). Stemming the superbug tide: just a few dollars more. Policy Brief. Available at:https://www.oecd.org/health/stemming-the-superbug-tide9789264307599-en.htm. Accessed July 2, 2019.
- 8.O'Neil J. Review on antimicrobial resistance: tackling drug-resistant infections globally. 2014. Available at:https://amr-review.org/Publications.html. Accessed May 11, 2020.
- 9.World Health Organization. Global action plan on antimicrobial resistance. 2015. Geneva, Switzerland. Available at:https://www.who.int/antimicrobial-resistance/global-action-plan/en/. Accessed June 18, 2019.
- 10.World Health Organization. Seventy-second World Health Assembly. Provisional agenda item 11.8. 2019. Available at:http://apps.who.int/gb/ebwha/pdf_files/WHA72/A72_18-en.pdf. Accessed June 19, 2019.
- 11.Department of Health and Social Care. UK 20-year vision for antimicrobial resistance. 2019. Available at:https://www.gov.uk/government/publications/uk-20-year-vision-for-antimicrobial-resistance. Accessed September 20, 2019.
- 12.Department of Health and Social Care. UK 5-year action plan for antimicrobial resistance 2019 to 2024. (Online). 2019. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/784894/UK_AMR_5_year_national_action_plan.pdf. Accessed September 20, 2019.
- 13.Food and Agriculture Organization of the United Nations . 2018. Antimicrobial Resistance Policy Review and Development Framework; p. iv. [Google Scholar]
- 14.Scott E. Community-based infections and the potential role of common touch surfaces as vectors for the transmission of infectious agents in home and community settings. Am J Infect Control. 2013;41:1087–1092. doi: 10.1016/j.ajic.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 15.International Scientific Forum on Home Hygiene. Containing the burden of infectious diseases is everyone's responsibility. 2018. Available at:https://www.ifh-homehygiene.org/sites/default/files/publications/IFH%20White%20Paper-10-18.pdf. Accessed April 6, 2020.
- 16.Liu CM, Stegger M, Aziz M, et al. Escherichia coli ST131H22 as a foodborne uropathogen. mBio. 2018;9 doi: 10.1128/mBio.00470-18. e00470-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim KH, Fekety R, Batts DH, et al. Isolation of Clostridium difficile from the environment and contacts of patients with antibiotic associated colitis. J Infect Dis. 1981;143:42–50. doi: 10.1093/infdis/143.1.42. [DOI] [PubMed] [Google Scholar]
- 18.Cogan TA, Bloomfield SF, Humphrey TJ. The effectiveness of hygiene procedures for prevention of cross-contamination from chicken carcasses in the domestic kitchen. Lett Appl Microbiol. 1999;29:3548. doi: 10.1046/j.1472-765x.1999.00656.x. [DOI] [PubMed] [Google Scholar]
- 19.Gorman R, Bloomfield S, Adley CC. A study of cross-contamination of food-borne pathogens in the domestic kitchen in the Republic of Ireland. Int J Food Microbiol. 2002;76:143–150. doi: 10.1016/s0168-1605(02)00028-4. [DOI] [PubMed] [Google Scholar]
- 20.van Asselt ED, de Jong AEI, de Jonge R, Nauta MJ. Cross-contamination in the kitchen: estimation of transfer rates for cutting boards, hands and knives. J Appl Microbiol. 2008;105:1392–1401. doi: 10.1111/j.1365-2672.2008.03875.x. [DOI] [PubMed] [Google Scholar]
- 21.de Jong AE, Verhoeff-Bakkenes L, Nauta MJ, de Jonge R. Cross-contamination in the kitchen: effect of hygiene measures. J Appl Microbiol. 2008;105:615–624. doi: 10.1111/j.1365-2672.2008.03778.x. [DOI] [PubMed] [Google Scholar]
- 22.Tang JYH, Nishibuchi M, Nakaguchi Y, Ghazali FM, Saleha AA, Son R. Transfer of Campylobacter jejuni from raw to cooked chicken via wood and plastic cutting boards. Lett Appl Microbiol. 2011;52:581–588. doi: 10.1111/j.1472-765X.2011.03039.x. [DOI] [PubMed] [Google Scholar]
- 23.Evans EW, Redmond EC. Domestic kitchen microbiological contamination and self-reported food hygiene practices of older adult consumers. J Food Protection. 2019;82:1326–1335. doi: 10.4315/0362-028X.JFP-18-533. [DOI] [PubMed] [Google Scholar]
- 24.Borrusso PA, Quinlan JJ. Prevalence of pathogens and indicator organisms in home kitchens and correlation with unsafe food handling practices and conditions. J Food Protection. 2017;80:590–597. doi: 10.4315/0362-028X.JFP-16-354. [DOI] [PubMed] [Google Scholar]
- 25.Barker J, Vipond IB, Bloomfield SF. The effects of cleaning and disinfection in reducing the spread of Norwalk-like virus contamination via environmental surfaces. J Hosp Infect. 2004;58:42–49. doi: 10.1016/j.jhin.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 26.Jones EL, Kramer A, Gaither M, Gerba CP. Role of fomites contamination during an outbreak of norovirus on houseboats. Int J Environ Health Res. 2007;17:123–131. doi: 10.1080/09603120701219394. [DOI] [PubMed] [Google Scholar]
- 27.Bright KR, Boone SA, Gerba CP. Occurrence of bacteria and viruses on elementary classroom surfaces and the potential role of classroom hygiene in the spread of infectious diseases. J School Nurs. 2010;26:33–41. doi: 10.1177/1059840509354383. [DOI] [PubMed] [Google Scholar]
- 28.Winther B, Mccue K, Ashe K, Rubino J, Hendley JO. Abstract of the Ann Conf Antimicrob Agents Chemotherapy. 2006. Contamination of environmental surfaces during normal daily activities of hotel guest with rhinovirus colds. V-1693. [Google Scholar]
- 29.Boone SA, Gerba CP. The occurrence of influenza A virus on household and day care center fomites. J Infect. 2005;51:103–109. doi: 10.1016/j.jinf.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 30.Kampf G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 2020;104:246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cogan TA, Slader J, Bloomfield SF, Humphrey TJ. Achieving hygiene in the domestic kitchen: the effectiveness of commonly used cleaning procedures. J Appl Microbiol. 2002;92:885–892. doi: 10.1046/j.1365-2672.2002.01598.x. [DOI] [PubMed] [Google Scholar]
- 32.Scott E. Hygiene issues in the home. Am J Infect Control. 1999;27:S22–S25. doi: 10.1016/s0196-6553(99)70038-6. [DOI] [PubMed] [Google Scholar]
- 33.Public Health England. Year 4 report. A microbiological survey of Campylobacter contamination in fresh while UK-produced chilled chickens at retail sale. 2019. Available at:https://www.food.gov.uk/research/foodborne-diseases/a-microbiological-survey-of-campylobactercontamination-in-fresh-whole-uk-produced-chilled-chickens-at-retail-sale-y234. Accessed September 24, 2019.
- 34.Centers for Disease Control and Prevention . CDC Yellow Book. 2020. Campylobacteriosis.https://wwwnc.cdc.gov/travel/yellowbook/2020/travel-related-infectiousdiseases/campylobacteriosis Available at. Accessed August 8, 2019. [Google Scholar]
- 35.Chaidez C, Soto-Beltran M, Gerba CP, Tamimi AH. Reduction of risk of Salmonella infection from kitchen cleaning clothes by use of sodium hypochlorite disinfectant cleaner. Lett Appl Microbiol. 2014;59:487–492. doi: 10.1111/lam.12321. [DOI] [PubMed] [Google Scholar]
- 36.Kramer A, Schwebke I, Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis. 2006;6:130. doi: 10.1186/1471-2334-6-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Josephson KL, Rubino JR, Pepper IL. Characterization and quantification of bacterial pathogens and indicator organisms in household kitchens with and without the use of a disinfectant cleaner. J Appl Microbiol. 1997;83:737–750. doi: 10.1046/j.1365-2672.1997.00308.x. [DOI] [PubMed] [Google Scholar]
- 38.Rusin P, Orosz-Coughlin P, Gerba C. Reduction of fecal coliform, coliform and heterotrophic plate count bacteria in the household kitchen and bathroom by disinfection with hypochlorite cleaners. J Appl Microbiol. 1998;85:819–828. doi: 10.1046/j.1365-2672.1998.00598.x. [DOI] [PubMed] [Google Scholar]
- 39.Ojima M, Toshima Y, Koya E, Ara K, Kawai S, Ueda N. Bacterial contamination of Japanese households and related concern about sanitation. Int J Environ Health Res. 2002;12:41–52. doi: 10.1080/09603120120110040. [DOI] [PubMed] [Google Scholar]
- 40.Enriquez CE, Enriquez-Gordillo R, Kennedy DI, Gerba C. Bacteriological survey of used cellulose sponges and cotton dishcloths from domestic kitchens. Dairy Food Environ Sanit. 1997;17:20–24. [Google Scholar]
- 41.Gerba CP, Tamimi AH, Maxwell S, Sifuentes LY, Hoffman DR, Koenig DW. Bacterial occurrence in kitchen hand towels. Food Prot Trends. 2014;34:312–317. [Google Scholar]
- 42.Bloomfield SF, Exner M, Signorelli C, Nath KJ, Scott EA. International Scientific Forum on Home Hygiene; 2012. The Chain of Infection Transmission in the Home and Everyday Life Settings, and the Role of Hygiene in Reducing the Risk of Infection.http://www.ifhhomehygiene.org/sites/default/files/publications/IFHinfectiontransmissionreviewFINAL.pdf Available at: Accessed July 1, 2019. [Google Scholar]
- 43.Yezli S, Otter JA. Minimum infective dose of the major human respiratory and enteric viruses transmitted through food and the environment. Food Environ Virol. 2011;3:1–30. doi: 10.1007/s12560-011-9056-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kothary MH, Babu US. Infective dose of foodborne pathogens in volunteers: a review. J Food Saf. 2001;21:49–73. [Google Scholar]
- 45.Larson HE, Price AB, Honour P, Borriello SP. Clostridium difficile and the aetiology of pseudomembranous colitis. Lancet. 1978;311:1063–1066. doi: 10.1016/s0140-6736(78)90912-1. [DOI] [PubMed] [Google Scholar]
- 46.Robilotti E, Deresinski S, Pinsky BA. Norovirus. Clin Microbiol Rev. 2015;28:134–164. doi: 10.1128/CMR.00075-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Winther B. Rhinovirus infections in the upper airway. Proc Am Thorac Soc. 2011;8:79–89. doi: 10.1513/pats.201006-039RN. [DOI] [PubMed] [Google Scholar]
- 48.Atmar RL, Opekun AR, Gilger MA, et al. Determination of the 50% human infectious dose for Norwalk virus. J Infect Dis. 2014;209:1016–1022. doi: 10.1093/infdis/jit620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haas CN, Rose JB, Gerba CP. 2nd ed. Wiley Blackwell; New York: 2014. Quantitative Microbial Risk Assessment. [Google Scholar]
- 50.Mody L, Washer LL, Kaye KS, et al. Multidrug-resistant organisms in hospitals: what is on patient hands and in their rooms? Clin Infect Dis. 2019;69:1837–1844. doi: 10.1093/cid/ciz092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Duin D, Paterson DL. Multidrug-resistant bacteria in the community: trends and lessons learned. Infect Dis Clin North Am. 2016;30:377–390. doi: 10.1016/j.idc.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Masterton RG, Coia JE, Notman AW, Kempton-Smith L, Cookson BD. Refractory methicillin-resistant Staphylococcus aureus carriage associated with contamination of the home environment. J Hosp Infect. 1995;29:318–319. doi: 10.1016/0195-6701(95)90284-8. [DOI] [PubMed] [Google Scholar]
- 53.Allen KD, Anson JJ, Parsons LA, Frost NG. Staff carriage of methicillin-resistant Staphylococcus aureus (EMRSA 15) and the home environment: a case report. J Hosp Infect. 1997;35:307–311. doi: 10.1016/s0195-6701(97)90225-5. [DOI] [PubMed] [Google Scholar]
- 54.Calfee DP, Durbin LJ, Germanson TP, Toney DM, Smith EB, Farr BM. Spread of methicillin-resistant Staphylococcus aureus (MRSA) among household contacts of individuals with nosocomially acquired MRSA. Infect Control Hosp Epidemiol. 2003;24:422–426. doi: 10.1086/502225. [DOI] [PubMed] [Google Scholar]
- 55.Kniehl E, Becker A, Forster DH, et al. The influence of methicillin-resistant Staphylococcus aureus (MRSA) carriers in a nursery and transmission of MRSA to their households. J Hosp Infect. 1999;42:45–51. doi: 10.1053/jhin.1998.0551. [DOI] [PubMed] [Google Scholar]
- 56.Bloomfield SF. Spread of antibiotic resistant strains in the home and community. Int Sci Forum Home Hyg. 2013 Available at: http://www.ifh-homehygiene.org/review/spread-antibiotic-resistant-strains-home-and-community. Accessed May 12, 2020. [Google Scholar]
- 57.Cave R, Misra R, Chen J, Wang S, Mkrtchyan H. Whole genome sequencing revealed new molecular characteristics in multidrug resistant staphylococci recovered from high frequency touched surfaces in London. Nat Sci Rep. 2019;9:9637. doi: 10.1038/s41598-019-45886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hollis RJ, Barr JL, Doebbeling BN, Pfaller MA, Wenzel RP. Familial carriage of methicillin-resistant Staphylococcus aureus and subsequent infection in a premature neonate. Clin Infect Dis. 1995;21:328–332. doi: 10.1093/clinids/21.2.328. [DOI] [PubMed] [Google Scholar]
- 59.Hollyoak V, Gunn A. Methicillin-resistant Staphylococcus aureus (MRSA) in the community. Lancet. 1995;346:513. [PubMed] [Google Scholar]
- 60.Shahin R, Johnson IL, Jamieson F, McGeer A, Tolkin J, Ford-Jones EL. Methicillin-resistant Staphylococcus aureus carriage in a child care center following a case of disease. Toronto Child Care Center Study Group. Arch Pediatr Adolesc Med. 1999;153:864–868. doi: 10.1001/archpedi.153.8.864. [DOI] [PubMed] [Google Scholar]
- 61.Roberts MC, Soge OO, No D, Helgeson SE, Meschke JS. Student Homes and Local Community; 2011. Aureus isolated form public surfaces on a University Campus. [DOI] [PubMed] [Google Scholar]
- 62.Wickramasinghe NH, Xu L, Eustace A, Shabir S, Saluja T, Hawkey PM. High community faecal carriage rates of CTX-M ESBL-producing Escherichia coli in a specific population group in Birmingham, UK. J Antimicrob Chemother. 2012;67:1108–1113. doi: 10.1093/jac/dks018. [DOI] [PubMed] [Google Scholar]
- 63.Nicolas-Chanoine MH, Gruson C, Bialek-Davenet S, et al. 10-fold increase (2006-11) in the rate of healthy subjects with extended-spectrum β-lactamase-producing Escherichia coli faecal carriage in a Parisian check-up centre. J Antimicrob Chemother. 2013;68:562–568. doi: 10.1093/jac/dks429. [DOI] [PubMed] [Google Scholar]
- 64.Rocourt J, Boy G, Vierk K, Schlundt J. The present state of food borne disease in OECD countries. Available at: www.who.int/foodsafety/publications/foodborne-disease/oecdfbd.pdf. Accessed May 11, 2020.
- 65.Founou LL, Founou RC, Essack SY. Antibiotic resistance in the food chain: a developing country perspective. Front Microbiol. 2016;7:1881. doi: 10.3389/fmicb.2016.01881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tschudin-Sutter S, Frei R, Stephan R, Hächler H, Nogarth D, Widmer AF. Extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae: a threat from the kitchen. Infect Control Hosp Epidemiol. 2014;35:581–584. doi: 10.1086/675831. [DOI] [PubMed] [Google Scholar]
- 67.Marotta SM, Giarratana F, Calvagna A, Ziino G, Giuffrida A, Panebianco A. Study on microbial communities in domestic kitchen sponges: Evidence of Cronobacter sakazakii and Extended Spectrum Beta Lactamase (ESBL) producing bacteria. Ital J Food Saf. 2019;7:7672. doi: 10.4081/ijfs.2018.7672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Epstein L, Hunter JC, Arwady MA, et al. New Delhi metallo-β-lactamaseproducing carbapenem-resistant Escherichia coli associated with exposure to duodenoscopes. JAMA. 2014;312:1447–1455. doi: 10.1001/jama.2014.12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Khajuria A, Praharaj AK, Kumar M, Grover N. Emergence of Escherichia coli, co-producing NDM-1 and OXA-48 carbapenemases, in urinary isolates, at a Tertiary Care Centre at Central India. J Clin Diagn Res. 2014;8 doi: 10.7860/JCDR/2014/7952.4413. DC01-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bhargava A, Hayakawa K, Silverman E, et al. Risk factors for colonization due to carbapenemresistant Enterobacteriaceae among patients exposed to long-term acute care and acute care facilities. Infect Control Hosp Epidemiol. 2014;35:398–405. doi: 10.1086/675614. [DOI] [PubMed] [Google Scholar]
- 71.Walsh TR, Weeks J, Livermore DM, Toleman MA. Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: an environmental point prevalence study. Lancet Infect Dis. 2011;11:355–362. doi: 10.1016/S1473-3099(11)70059-7. [DOI] [PubMed] [Google Scholar]
- 72.Wang Y, Wu C, Zhang Q, et al. Identification of New Delhi metallo-β-lactamase 1 in Acinetobacter lwoffii of food animal origin. PLoS One. 2012;7:e37152. doi: 10.1371/journal.pone.0037152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jordens JZ, Bates J, Griffiths DT. Faecal carriage and nosocomial spread of vancomycin-resistant Enterococcus faecium. J Antimicrob Chemother. 1994;34:515–528. doi: 10.1093/jac/34.4.515. [DOI] [PubMed] [Google Scholar]
- 74.Donnelly JP, Voss A, Witte W, Murray BE. Does the use in animals of antimicrobial agents, including glycopeptide antibiotics, influence the efficacy of antimicrobial therapy in humans? J Antimicrob Chemother. 1996;37:389–392. doi: 10.1093/jac/37.2.389. [DOI] [PubMed] [Google Scholar]
- 75.Gordts B, Claeys K, Jannes H, Van Landuyt HW. Program and Abstracts of the 34th Interscience Conference on Antimicrobial Agents and Chemotherapy, Orlando. American Society for Microbiology; WashingtonDC: 1994. Are vancomycin resistant enterococci (VRE) normal inhabitants of the GI tract of hospitalized patients? p. 145. [Abstract] [Google Scholar]
- 76.Endtz HP, van den Braak N, van Belkum A, et al. Program and Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy, New Orleans. American Society for Microbiology; Washington (DC): 1996. Prevalence of vancomycin-resistant enterococci in hospital and community-based patients in the Netherlands; p. 37. [Abstract] [Google Scholar]
- 77.Bogaard A, London N, Driessen C, Stobberingh E. Program and Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy, New Orleans. American Society for Microbiology; Washington (DC): 1996. Prevalence of resistant fecal bacteria in turkeys, turkey farmers and turkey slaughterers; p. 86. [Abstract] [Google Scholar]
- 78.Scott E, Bloomfield SF. Vol. 37. Alliance for the Prudent Use of Antibiotics Newsletter; 2017. The hygiene hypothesis misnomer and its potential impact on strategies to tackle the global problem of antibiotic resistance; pp. 10–12. (The hygiene hypothesis misnomer and its potential impact on strategies to tackle the global problem of antibiotic resistance). [Google Scholar]
- 79.Bengtsson-Palme J, Kristiansoon E, Larsson DGJ. Environmental factors influencing the development and spread of antibiotic resistance. FEMS Microbiol Rev. 2018;42:68–80. doi: 10.1093/femsre/fux053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bloomfield SF, Scott E. In: Emmerson AM, editor. Vol. 19. 1997. A risk assessment approach to the use of disinfectant procedures in the community; pp. 37–47. (Development of Consensus in Global Infection Control. Research and Clinical Forums). [Google Scholar]
- 81.Royal Society for Public Health. Too clean or not too clean? The case for targeted hygiene in the home and everyday life. 2019. Available at: https://www.rsph.org.uk/our-work/policy/infectioncontrol/too-clean-or-not-too-clean.html. Accessed July 2, 2019.
- 82.Royal Society for Public Health. RSPH calls for clean up of public attitudes to hygiene. 2019. Available at: https://www.rsph.org.uk/about-us/news/rsph-calls-for-clean-up-of-public-attitudes-to-hygiene.html. Accessed November 8, 2019.
- 83.Bloomfield SF, Scott EA. A risk assessment approach to use of antimicrobials in the home to prevent spread of infection. Am J Infect Control. 2013;41(5 suppl):S87–S93. doi: 10.1016/j.ajic.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 84.Bloomfield SF, Carling PC, Exner M. A unified framework for developing effective hygiene procedures for hands, environmental surfaces and laundry in healthcare, domestic, food handling and other settings. GMS Hyg Infect Control. 2017;12 doi: 10.3205/dgkh000293. Doc 08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Perez V, Mena KD, Watson HN, Prater RB, McIntyre JL. Evaluation and quantitative microbial risk assessment of a unique antimicrobial agent for hospital surface treatment. Am J Infect Control. 2015;43:1201–1207. doi: 10.1016/j.ajic.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 86.World Health Organization . 2017. Quantitative Microbial Risk Assessment: Application for Water Safety Management.https://www.who.int/water_sanitation_health/publications/qmra/en/ Geneva, Switzerland. Available at. [Google Scholar]
- 87.Gebel J, Exner M, French G, et al. The role of surface disinfection in infection prevention. GMS Hyg Infect Control. 2013;8 doi: 10.3205/dgkh000210. Doc10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dancer SJ. Controlling hospital-acquired infection: focus on the role of the environment and new technologies for decontamination. Clin Microbiol Rev. 2014;27:665–690. doi: 10.1128/CMR.00020-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Exner M, Vacata V, Hornei B, Dietlein E, Gebel J. Household cleaning and surface disinfection: new insights and strategies. J Hosp Infect. 2004;56(suppl 2):S70–S75. doi: 10.1016/j.jhin.2003.12.037. [DOI] [PubMed] [Google Scholar]
- 90.Sifuentes LY, Koenig DW, Phillips RL, Reynolds KA, Gerba CP. Use of hygiene protocols to control the spread of viruses in a hotel. Food Environ Virol. 2014;6:175–181. doi: 10.1007/s12560-014-9158-0. [DOI] [PubMed] [Google Scholar]
- 91.Aiello AE, Coulborn RM, Perez V, Larson EL. Effect of hand hygiene on infectious disease risk in the community setting: a meta-analysis. Am J Public Health. 2008;98:1372–1381. doi: 10.2105/AJPH.2007.124610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ejemot-Nwadiaro RI, Ehiri JE, Arikpo D, Meremikwu MM, Critchley JA. Hand washing promotion for preventing diarrhoea. Cochrane Database Syst Rev. 2015;19:906–916. doi: 10.1002/14651858.CD004265.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Freeman MC, Stocks ME, Cumming O, et al. Hygiene and health: systematic review of handwashing practices worldwide and update of health effects. Trop Med Int Health. 2014;19:906–916. doi: 10.1111/tmi.12339. [DOI] [PubMed] [Google Scholar]
- 94.Uhari M, Möttönen M. An open randomized controlled trial of infection prevention in child day-care centers. Pediatr Infect Dis J. 1999;18:672–677. doi: 10.1097/00006454-199908000-00004. [DOI] [PubMed] [Google Scholar]
- 95.Ban HQ, Li T, Shen J, et al. Effects of multiple cleaning and disinfection interventions on infectious diseases in children: a group randomized trial in China. Biomed Environ Sci. 2015;28:779–787. doi: 10.3967/bes2015.109. [DOI] [PubMed] [Google Scholar]
- 96.Krilov LR, Barone SR, Mandel FS, Cusack TM, Gaber DJ, Rubino JR. Impact of an infection control program in a specialized preschool. Am J Infect Control. 1996;24:167–173. doi: 10.1016/s0196-6553(96)90008-5. [DOI] [PubMed] [Google Scholar]
- 97.Sandora TJ, Shih MC, Goldmann DA. Reducing absenteeism from gastrointestinal and respiratory illness in elementary school students: a randomized, controlled trial of an infection-control intervention. Pediatrics. 2008;121:e1555–e1562. doi: 10.1542/peds.2007-2597. [DOI] [PubMed] [Google Scholar]
- 98.Azor-Martinez E, Yui-Hifume R. Effectiveness of a hand hygiene program at child care centers: a cluster randomized trial. Pediatrics. 2018;142 doi: 10.1542/peds.2018-1245. [DOI] [PubMed] [Google Scholar]
- 99.Bronson-Lowe DL, Strazdas LA, Rawiel U, Orosz-Coghlan P, Gerba CP, Lebowitz MD. Antibiotic use in child care centers: the impact of an enhanced environmental hygiene intervention. Available at:https://cals.arizona.edu/sites/cals.arizona.edu.main/files/reports/Antibiotics%20use%20in%20daycare%20centers.pdf. Accessed June 19, 2019.
- 100.Cole EC, Hawkley M, Rubino JR, et al. Comprehensive family hygiene promotion in peri-urban Cape Town: gastrointestinal and respiratory illness and skin infection reduction in children aged under 5. South Afr J Child Health. 2017;6:109–117. http://www.sajch.org.za/index.php/SAJCH/article/view/459/359 Available at: Accessed July 1, 2019. [Google Scholar]
- 101.Staniford LJ, Schmidtke KA. A systematic review of hand-hygiene and environmental-disinfection interventions in settings with children. BMC Public Health. 2020;20:195. doi: 10.1186/s12889-020-8301-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Canales RA, Reynolds KA, Wilson AM, et al. Modeling the role of fomites in a norovirus outbreak. J Occup Environ Hyg. 2019;16:16–26. doi: 10.1080/15459624.2018.1531131. [DOI] [PubMed] [Google Scholar]
- 103.Haas CN, Marie JR, Rose JB, Gerba CP. Assessment of benefits from use of antimicrobial hand products: reduction in risk from handling ground beef. Int J Hyg Environ Health. 2005;208:461–466. doi: 10.1016/j.ijheh.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 104.Duff SB, Scott EA, Mafilios MS, et al. Cost-effectiveness of a targeted disinfection program in household kitchens to prevent foodborne illnesses in the United States, Canada, and the United Kingdom. J Food Prot. 2003;66:2103–2115. doi: 10.4315/0362-028x-66.11.2103. [DOI] [PubMed] [Google Scholar]
- 105.U.S. Food and Drug Administration. FDA issues final rule on safety and effectiveness of antibacterial soaps. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm517478.htm. 2016. Accessed August 9, 2019.
- 106.Maillard JY. Resistance of bacteria to biocides [e-pub ahead of print]. Microbiol Spectr. 10.1128/microbiolspec.ARBA-0006-2017. Accessed May 11, 2020 [DOI] [PMC free article] [PubMed]
- 107.Maillard J-Y. In: Block's Disinfection, Sterilization and Preservation, 6e. McDonnell G, Hansen J, editors. Wolters Kluwer; Philadelphia: 2019. Bacterial resistance to biocides. in press. [Google Scholar]
- 108.Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) European Commission; 2009. Assessment of the Antibiotic Resistance Effects of Biocides. Available at: https://ec.europa.eu/health/ph_risk/committees/04_scenihr/docs/scenihr_o_021.pdf. Accessed May 11, 2020. [Google Scholar]
- 109.Oggioni MR, Furi L, Coelho JR, Maillard JY, Martínez JL. Recent advances in the potential interconnection between antimicrobial resistance to biocides and antibiotics. Expert Rev Anti Infect Ther. 2013;11:363–366. doi: 10.1586/eri.13.16. [DOI] [PubMed] [Google Scholar]
- 110.Donaghy JA, Jagadeesan B, Goodburn K, et al. Relationship of sanitizers, disinfectants, and cleaning agents with antimicrobial resistance. J Food Prot. 2019;82:889–902. doi: 10.4315/0362-028X.JFP-18-373. [DOI] [PubMed] [Google Scholar]
- 111.Food and Agriculture Organization of the United Nations. Biocides and Antimicrobial Resistance . FAO Antimicrobial Resistance Working Group; 2018. Summary Report of an FAO Meeting of Experts. Available at: http://www.fao.org/3/BU655en/bu655en.pdf. Accessed May 11, 2020. [Google Scholar]
- 112.Rutala WA, Weber DJ, Barbee SI, Gergen MF, Sobsey MD. Evaluation of antibiotic resistant bacteria in home kitchens and bathrooms. Inf Cont Epidemiol. 2000;21:132. doi: 10.1016/j.ajic.2023.04.005. [DOI] [PubMed] [Google Scholar]
- 113.Marshall BM, Roblet E, Dumont T, et al. Abstracts Ann Meet Am Soc Microbiol. 2003. The frequency of bacteria and antibiotic resistance in homes that use and do not use surface antibacterial agents; pp. A–147. [Google Scholar]
- 114.Cole EC, Addison RA, Rubino JR., et al. Investigation of antibiotic and antibacterial agent cross resistance in target bacteria from homes of antibacterial product users and non users. J Appl Microbiol. 2003;95:664–676. doi: 10.1046/j.1365-2672.2003.02022.x. [DOI] [PubMed] [Google Scholar]
- 115.Aiello A.E., Marshall B., Levy S.B., Della-Latta P, Lin SX, Larson E. Antibacterial cleaning products and drug resistance. Emerg Infect Dis. 2005;11:1565–1570. doi: 10.3201/eid1110.041276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kumwenda S. In: The Relevance of Hygiene to Health in Developing Countries. Potgieter N, Traore AN, editors. InTechOpen; 2019. Challenges to hygiene improvement in developing countries.https://www.intechopen.com/books/the-relevance-of-hygiene-to-health-in-developingcountries/challenges-to-hygiene-improvement-in-developing-countries Available at. Accessed August 9, 2019. [Google Scholar]
- 117.Gandra S, Joshi J, Trett A, Lamkang AS, Laxminarayan R. Center for Disease Dynamics, Economics & Policy; Washington, DC: 2017. Scoping Report on Antimicrobial Resistance in India. [Google Scholar]
- 118.Laxminarayan R, Chaudhury RR. Antibiotic resistance in India: drivers and opportunities for action. PLoS Med. 2016;13 doi: 10.1371/journal.pmed.1001974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.The World Bank . Clean water and sanitation; 2017. Atlas of Sustainable Development Goals 2017.http://datatopics.worldbank.org/sdgatlas/archive/2017/SDG-06-clean-water-andsanitation.html Available at. [Google Scholar]
- 120.United Nations Educational, Scientific and Cultural Organization (UNESCO) World Water Assessment Programme. The United Nations World Water Development Report 2019: Leaving No One Behind. Paris, UNESCO. Available at: https://www.unwater.org/publications/world-water-development-report-2019//. Accessed 27 September 2019.
- 121.Carrasco L, Mena KD, Mota LC, et al. Occurrence of faecal contamination in households along the US-Mexico border. Lett Appl Microbiol. 2008;46:682–687. doi: 10.1111/j.1472-765X.2008.02378.x. [DOI] [PubMed] [Google Scholar]
- 122.Medrano-Félix A, Martínez C, Castro-del Campo N, et al. Impact of prescribed cleaning and disinfectant use on microbial contamination in the home. J Appl Microbiol. 2011;110:463–471. doi: 10.1111/j.1365-2672.2010.04901.x. [DOI] [PubMed] [Google Scholar]
- 123.Keshav V, Krüger CA, Mathee A, Naicker N, Swart A, Barnard TG. E. coli from dishcloths as an indicator of hygienic status in households. J Water Sanitat Hyg Dev. 2015;5:351–358. [Google Scholar]
- 124.Sinclair RG, Gerba CP. Microbial contamination in kitchens and bathrooms of rural Cambodian village households. Lett Appl Microbiol. 2011;52:144–149. doi: 10.1111/j.1472-765X.2010.02978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.World Health Organization/United Nations Children's Fund . 2017. Progress on Drinking Water, Sanitation and Hygiene: 2017 Update and SDG Baselines.https://www.unicef.org/publications/index_96611.html Available at. [Google Scholar]
- 126.Pickering AJ, Davis J, Walters SP, et al. Hands, water, and health: fecal contamination in Tanzanian communities with improved, non-networked water supplies. Environ Sci Technol. 2010;44:3267–3272. doi: 10.1021/es903524m. [DOI] [PubMed] [Google Scholar]
- 127.Luby SP, Kadir MA, Yushuf Sharker MA, Yeasmin F, Unicomb L, Sirajul Islam M. A community randomised controlled trial promoting waterless hand sanitizer and handwashing with soap, Dhaka, Bangladesh. Trop Med Int Health. 2010;15:1508–1516. doi: 10.1111/j.1365-3156.2010.02648.x. [DOI] [PubMed] [Google Scholar]
- 128.Pickering AJ, Julian TR, Mamuya S, Boehm AB, Davis J. Bacterial hand contamination among Tanzanian mothers varies temporally and following household activities. Trop Med Int Health. 2011;16:233–239. doi: 10.1111/j.1365-3156.2010.02677.x. [DOI] [PubMed] [Google Scholar]