Abstract
Background and aim
A novel coronavirus severe acute respiratory syndrome corona virus 2 (SARS-CoV-2) caused pneumonia, Coronavirus Disease 2019 (COVID-19), broke out in Wuhan, China in December 2019, and spread all over the world. Patients with COVID-19 showed huge differences in the hospital stay, progression, and prognosis. As reported, the comorbidities may play an important role in COVID-19. Here, we aim to address the role of cardiovascular disease (CVD) in the progression and prognosis of COVID-19.
Methods and results
Eighty-three confirmed COVID-19 patients were divided into CVD (n = 42) and non-CVD (n = 41) group according to their medical history. Medical records including demographic data, medical history, clinical characteristics, laboratory examinations, chest computed tomography (CT), and treatment measures were collected, analyzed, and compared between the two groups. COVID-19 patients with CVD showed (1) more severe pathological changes in the lungs, (2) elevated injury-related enzymes including α-hydroxybutyrate dehydrogenase (HDBH), lactic dehydrogenase (LDH), γ-glutamyltransferase (GGT), creatine kinase (CK), and alanine aminotransferase (ALT), (3) significantly increased uncontrolled inflammation related markers, such as c-reactive protein (CRP), interleukin (IL)-6, serum ferritin, erythrocyte sedimentation rate (ESR), and serum amyloid A (SAA), (4) serious hypercoagulable status reflected by increased D-dimer and serum fibrinogen (FIB), and (5) higher mortality, compared to COVID-19 patients without CVD.
Conclusions
Our data indicated that CVD is a strong risk factor for rapid progression and bad prognosis of COVID-19. More intensive medical care should be applied to patients with CVD to prevent rapid deterioration of the disease.
Keywords: COVID-19, Cardiovascular disease, Prognosis
Highlights
-
•
The COVID-19 patients with CVD experienced more severe pneumonia.
-
•
The COVID-19 patients with CVD showed more tissue injury-related enzymes release.
-
•
The COVID-19 patients with CVD were at higher risk of cytokine storm.
-
•
The COVID-19 patients with CVD were at higher risk of hypercoagulable state.
-
•
CVD is a risk factor for the progression and prognosis of COVID-19.
Introduction
Corona Virus Disease 2019 (COVID-19) was first reported in December, 2019 in Wuhan, China [1]. This novel beta-coronavirus was initially designated as 2019-nCoV and renamed as SARS-CoV-2 after global consensus [2]. By Mar 31, 2020, the disease has spread to more than 200 countries, including the USA, Italy, Iran, etc., with 662,037 confirmed patients and 37,819 deaths. Most patients are mildly ill and have flu-like symptoms, such as fever, cough, etc., however, there were also many patients developing acute respiratory distress syndrome (ARDS) or multiple organ failure (MOF) in a short time, and the mortality of critical patients has been reported to be as high as 50% [[3], [4], [5]].
Previous studies have found that patients with comorbidities infected with SARS-CoV or MERS-CoV were more likely to progress to ARDS or even death [[6], [7], [8], [9]]. As dramatically increased numbers of patients are infected by SARS-CoV-2, clinicians are paying more and more attention to the impact of underlying diseases on the prognosis of COVID-19. A descriptive study of 1099 cases with COVID-19 showed that 23.7% patients had coexisting disorders, among which hypertension (15%), diabetes (7.4%), and coronary heart disease (2.5%) were the top three underlying diseases [3]. Yang X. et al. reported that older patients (>65 years) with comorbidities and ARDS are at increasing risk of death. Therefore, the comorbidities had been confirmed to be correlated with the severity of COVID-19 and could lead to poor prognosis or death [5].
CVD is the leading global cause of death, accounting for more than 17.3 million deaths in 2013, and the toll might reach 23.6 million by 2030 [10,11]. Several investigations have demonstrated a higher susceptibility to MERS-CoV and human papillomavirus infection in CVD patients, probably owing to endothelial dysfunction, metabolic abnormalities, and increased pro-inflammatory cytokine, which eventually lead to impaired immune function [12]. In addition, CVD is a risk factor for poor prognosis and significantly increases the mortality of MERS [9,13]. Several clinical studies have shown that CVD is the most common comorbidity in patients with COVID-19 and that the proportion of COVID-19 patients with CVD is higher in critically ill and dead cases [14].
To determine whether CVD is a potential risk factor and to what extent it influences the progression and prognosis of COVID-19, 83 COVID-19 patients including 42 with CVD and 41 without CVD who were admitted to Wuhan Union Hospital from February 1, 2020 to February 20, 2020 were included in this study according to the inclusion criteria. Their basic information, laboratory examinations, chest CT scans, and treatments were collected and analyzed. We found that CVD is associated with the progression and poor prognosis of COVID-19.
Methods
Ethical statement
As a retrospective study, data analysis was performed anonymously. The study was approved by the Institutional Ethics Board of Wuhan Union Hospital of Tongji Medical College, Huazhong University of Science and Technology. The requirement for informed consent was waived by the Ethics Commission of the designated hospital for emerging infectious diseases.
Study design and participants
The admitted COVID-19 patients in Wuhan Union hospital from February 1, 2020 to February 20, 2020 were enrolled into this retrospective single-center study. The treatments of all patients were according to the Diagnosis and Treatment Scheme of COVID-19 by National Health Commission and National Administration of Traditional Chinese Medicine of China. Severe COVID-19 was designated when the patients met one of the following criteria: 1) Respiratory distress with respiratory frequency ≥30/min; 2) Pulse Oximeter Oxygen Saturation ≤93% at rest; 3) Oxygenation index (artery partial pressure of oxygen/inspired oxygen fraction, PaO2/FiO2) ≤ 300 mmHg. And the clinical outcomes were monitored up. Medical records of all enrolled patients including demographic data, medical history, clinical characteristics, laboratory examinations, chest CT scans, and treatment measures were obtained.
We screened 178 confirmed COVID-19 patients. In order to avoid the interference of other diseases on the prognosis of COVID-19, patients with other underlying diseases were excluded in this study. At the end, 42 patients with CVD and 41 without CVD were enrolled in this study. In addition, CT imaging scores were used to quantify the pathological changes of lungs in COVID-19 patients. Each lobe (5 lobes in total) was assigned a score as described previously [15]. The total CT score of every patient was the consensus of two physicians who were blinded to clinical information of the patients.
Statistical analysis
The data analysis was performed of patients with CVD and patients without CVD. The categorical variables were summarized as counts or percentages, and continuous variables were expressed as medians and interquartile ranges (IQR) or simple ranges, as appropriate. No imputation was made for missing data. The categorical variables were compared using χ2 test, and the Fisher exact test was used when the data were limited. Continuous variables were compared using independent group t test when the data were normally distributed; otherwise, the Mann–Whitney test was used. The risk factor analysis of COVID-19 is based on logistic regression analysis. Data statistics was applied using SPSS 20.0 software, and P < 0.05 was regarded as a significant difference.
Results
Patient characteristics
The demographic and clinical characteristics of the patients are shown in Table 1 . The median age of the patients was 43 years (IQR, 32–62) and 41% of the patients were male. For all patients, the two most common CVD were hypertension and coronary heart disease, and the top five symptoms were fever, shortness of breath, poor appetite, cough, and sputum production, whereas myalgia, chill, pharyngalgia, and headache were relatively rare. Compared with patients without CVD, patients with CVD were older and had poorer appetite, more nausea and vomiting, more severe cases, and higher mortality. However, there were no significant differences in gender, other baseline symptoms, and the time from onset of symptom to hospital admission between the two groups (Table 1). As the CVD group was older, in order to assess the impact of age on the progression of COVID-19, we performed a logistic regression analysis. The results showed that CVD was the risk factor for a severe event, but not age was not a risk factor in our study (Table S1).
Table 1.
Demographics and baseline characteristics of patients infected with SARS-CoV-2.
| No.(%) |
||||
|---|---|---|---|---|
| Total (n = 83) | Non-CVD (n = 41) | CVD (n = 42) | P-valuea | |
| Age, median (IQR), y | 43 (32–62) | 32 (30–37) | 62 (50–68) | <0.01 |
| Gender | ||||
| Male | 34 (41) | 16 (39) | 18 (42.9) | 0.72 |
| Female | 49 (59) | 25 (61) | 24 (57.1) | |
| BMI | 23.89 (21.97–25.3) | 22.82 (20.42–24.2) | 25.3 (23.96–26.65) | <0.01 |
| Hypertension | 33 (39.8) | 0 | 33 (78.6) | <0.01 |
| Coronary heart disease | 5 (6) | 0 | 5 (11.9) | 0.07 |
| Signs and symptoms | ||||
| Fever | 70 (84.3) | 35 (85.4) | 35 (83.3) | 0.80 |
| Highest temperature, °C | ||||
| <37.3 | 13 (15.7) | 6 (14.6) | 7 (16.7) | 0.84 |
| 37.3–38.0 | 22 (26.5) | 13 (31.7) | 9 (21.4) | 0.29 |
| 38.1–39.0 | 38 (45.7) | 21 (51.2) | 17 (40.5) | 0.33 |
| >39.0 | 10 (12.1) | 1 (2.5) | 9 (21.4) | 0.02 |
| Shortness of breath | 31 (37.3) | 14 (34.1) | 17 (40.5) | 0.55 |
| Poor appetite | 30 (36.1) | 5 (12.2) | 25 (59.5) | <0.01 |
| Cough | 29 (34.9) | 17 (41.5) | 12 (28.6) | 0.22 |
| Sputum production | 17 (20.5) | 8 (19.5) | 9 (21.4) | 0.83 |
| Myalgia | 8 (9.6) | 7 (17.1) | 1 (2.4) | 0.06 |
| Hypoxemia | 9 (10.8) | 2 (4.9) | 7 (16.7) | 0.17 |
| Chill | 6 (7.2) | 4 (9.8) | 2 (4.8) | 0.65 |
| Pharyngalgia | 6 (7.2) | 5 (12.2) | 1 (2.4) | 0.19 |
| Headache | 6 (7.2) | 4 (9.8) | 2 (4.8) | 0.65 |
| Nausea and Vomiting | 6 (7.2) | 0 | 6 (14.3) | 0.04 |
| Chest pain | 3 (3.6) | 3 (7.3) | 0 | 0.23 |
| Dizziness | 2 (2.4) | 2 (4.9) | 0 | 0.15 |
| Onset of symptom to, median (IQR), d | ||||
| Hospital admission | 7 (5–12) | 7 (5–13.8) | 8 (5–10) | 0.31 |
| Severe case | 18 (21.7) | 5 (12.2) | 13 (31.0) | 0.04 |
| Mortality | 6 (7.2) | 0 | 6 (14.3) | 0.04 |
P values indicate differences between CVD and non-CVD patients. P < 0.05 was considered statistically significant.
CVD patients showed more serious lung injury, multiple enzyme release, inflammation storm, and hypercoagulability
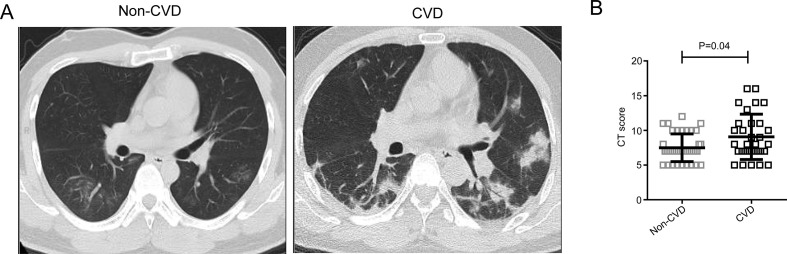
CT scans were performed at the time of admission, and the most common characteristics of chest CT were ground-glass opacity and bilateral patchy shadowing (Fig. 1 A). Moreover, patients with CVD had more prominent radiologic abnormalities than those without CVD. Furthermore, the severity of pathological changes was evaluated by the quantifiable score system described before. We found that the patients with CVD presented higher CT imaging score compared with the patients without CVD (Fig. 1B).
Figure 1.
CT results of the patients with CVD and patients without CVD. A. The representative CT images of the patients with CVD and patients without CVD. B. The CT score of the patients with CVD and patients without CVD. P < 0.05 was considered statistically significant.
For all patients, there were some abnormal laboratory test results (Table 2 ), including serum amyloid A (SAA), c-reactive protein (CRP), serum ferritin, erythrocyte sedimentation rate (ESR), interleukin (IL)-6, and fibrinogen (FIB).
Table 2.
Comparison of laboratory parameters between CVD and non-CVD COVID-19 patients.
| Normal Range | Median (IQR) |
||||
|---|---|---|---|---|---|
| Total (n = 83) | Non-CVD (n = 41) | CVD (n = 42) | P Valuea | ||
| HBDH (U/L) | 72–182 | 154 (134.5–205.0) | 142 (125.5–167.5) | 176 (146.5–226.0) | <0.01 |
| ALT (U/L) | 5–35 | 21.5 (16–33.25) | 19 (14.5–31.5) | 26 (17–42) | <0.01 |
| LDH (U/L) | 109–245 | 207 (178.5–282) | 187 (173–229) | 258 (205.75–321.75) | 0.01 |
| GGT (U/L) | 11–50 | 16 (12.5–26.5) | 13 (11–16) | 24.5 (16–47.5) | <0.01 |
| AST (U/L) | 8–40 | 23 (19–28.5) | 21.5 (17–26) | 26 (21.5–45.5) | 0.03 |
| ALP (U/L) | 40–150 | 56 (47–73) | 50 (46–63) | 64 (55–81) | <0.01 |
| CK (U/L) | 38–174 | 63 (47–90.5) | 57 (41.5–74.5) | 76.5 (54.75–109) | <0.01 |
| SAA (mg/dL) | <10 | 47.35 (5.23–430.83) | 15.7 (3.8–73.1) | 339.4 (34.55–692.95) | <0.01 |
| TP (mg/L) | 64–83 | 64.85 (59.53–70.68) | 68.75 (63.85–72.775) | 60.75 (57.675–65.3) | <0.01 |
| Prealbumin (mg/L) | 0.17–0.42 | 0.208 (0.14–0.25) | 0.212 (0.2–0.25) | 0.166 (0.12–0.23) | 0.02 |
| ALB (mg/L) | 35–55 | 39.25 (35.98–42.53) | 41.4 (39.25–43.7) | 36.9 (34.4–38.9) | <0.01 |
| ALB/GLB | 1.5–2.5 | 1.6 (1.3–1.8) | 1.6 (1.4–1.8) | 1.5 (1.2–1.8) | 0.53 |
| Lymphocytes (×109/L) | 1.1–3.2 | 1.15 (0.695–1.545) | 1.4 (1.08–1.81) | 0.805 (0.47–1.173) | <0.01 |
| Neutrophils (×109/L) | 1.8–6.3 | 2.78 (2.015–4.115) | 2.55 (2.01–3.22) | 3.23 (2.023–5.7) | 0.03 |
| RBC (×1012/L) | 3.8–5.1 | 4.16 (3.87–4.44) | 4.3 (4.09–4.66) | 3.94 (3.763–4.218) | <0.01 |
| Hemoglobin (g/dL) | 115–150 | 124 (117–135) | 132 (119–141) | 120 (115–129) | <0.01 |
| CRP (mg/L) | <8 | 9.625 (3.14–34.95) | 5.575 (3.14–13) | 21.35 (6.143–85.825) | <0.01 |
| Serum ferritin (ng/ml) | 21.8–275 | 294.35 (88.28–713.85) | 99.9 (55.9–185.8) | 539.6 (313.1–914.5) | <0.01 |
| ESR (mm/h) | <15 | 18.5 (7–40) | 10 (7–24) | 48 (25–76) | <0.01 |
| IL-6 (pg/ml) | 0.1–2.9 | 6.69 (3.33–15.535) | 3.69 (2.963–9.72) | 13.73 (6.56–24.13) | <0.01 |
| IL-10 (pg/ml) | 0.1–5 | 4.03 (3.425–4.75) | 3.58 (3.173–4.268) | 4.54 (3.75–4.83) | <0.01 |
| D-dimer (μg/L) | <0.5 | 0.41 (0.22–1.125) | 0.28 (0.22–0.348) | 1.105 (0.435–1.548) | <0.01 |
| FIB (g/L) | 2.0–4.0 | 4.01 (3.235–5.27) | 3.505 (2.82–4.07) | 5.01 (3.965–6.135) | <0.01 |
Abbreviation: IQR, interquartile range; COVID-19, coronavirus disease 2019; HBDH, α-Hydroxybutyrate Dehydrogenase; ALT, alanine aminotransferase; LDH, lactic dehydrogenase; GGT, γ-glutamyltransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; CK, creatine kinase; SAA, serum amyloid A; TP, total protein; ALB, albumin; GLB, globulin; RBC, red blood cells; CRP, c-reactive protein; ESR, erythrocyte sedimentation rate; FIB, fibrinogen.
P values indicate differences between CVD and non-CVD patients. P < 0.05 was considered statistically significant.
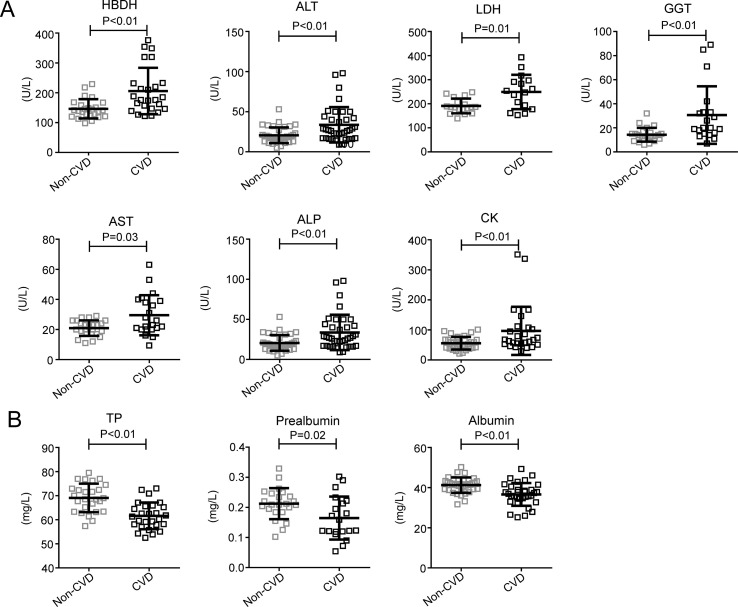
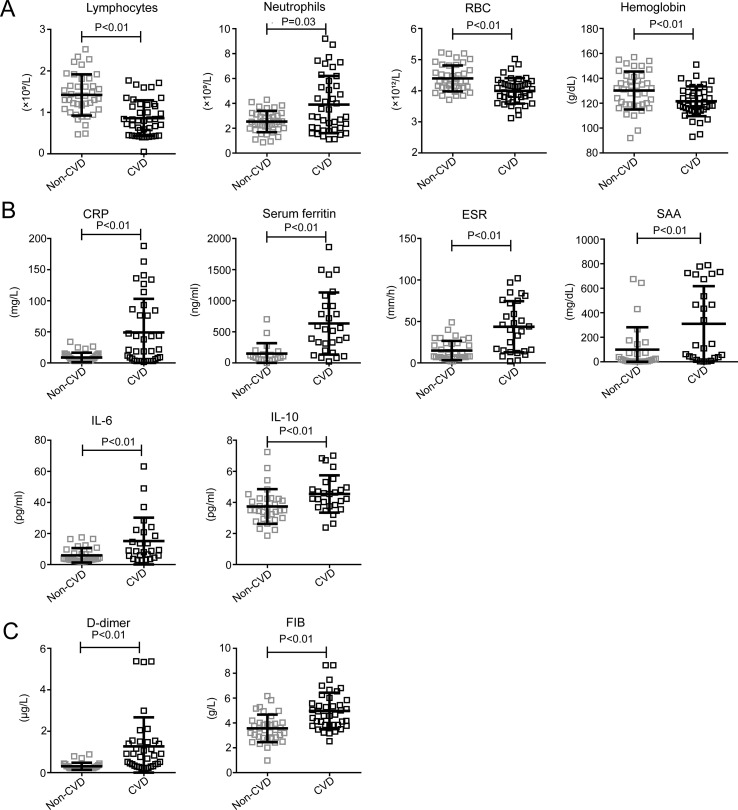
It is worth noting that the levels of enzymes such as α-hydroxybutyrate dehydrogenase (HBDH), alanine aminotransferase (ALT), lactic dehydrogenase (LDH), γ-glutamyltransferase (GGT), alkaline phosphatase (ALP), and creatine kinase (CK) were higher in patients with CVD (Table 2, Fig. 2 A). Furthermore, the levels of neutrophils, SAA, CRP, serum ferritin, ESR, IL-6, IL-10, and D-dimer, FIB were also significantly higher in the patients with CVD (Table 2, Fig. 3 B and C). Further, the levels of total protein (TP), prealbumin, and albumin (ALB) (Table 2, Fig. 2B), the absolute counts of lymphocytes and red blood cells (RBC), and the level of hemoglobin (Table 2, Fig. 3A) were significantly lower in the CVD group compared to the non-CVD group. These data showed that the COVID-19 patients with CVD were at higher risk of tissue injury-related enzymes release, excessive uncontrolled inflammation responses, and hypercoagulable state, which may contribute to the poorer prognosis of COVID-19.
Figure 2.
Biochemical examination results of the patients with CVD and patients without CVD.P < 0.05 was considered statistically significant.
Figure 3.
Other laboratory tests of the patients with CVD and patients without CVD. A. Blood test results of the patients with CVD and patients without CVD. B. Inflammation-related laboratory results of the patients with CVD and patients without CVD. C. Coagulation-related laboratory results of the patients with CVD and patient without CVD. P < 0.05 was considered statistically significant.
Discussion
The prognosis of COVID-19 patients is regulated by many factors, and patients with underlying diseases such as tumors and CVD are more likely to have adverse outcomes [16,17]. However, the pathological features of the COVID-19 patients with CVD are not well understood. In order to address the role of CVD in the progression and prognosis of COVID-19, the clinical characteristics of these COVID-19 patients should be identified, which can provide a foundation for risk factor management. Therefore, in this study, we retrospectively analyzed the differences in basic characteristics and laboratory tests between the COVID-19 patients with and without CVD and we tried to explain the differences.
Previous studies suggested that age, gender, and obesity are risk factors for CVD, as well as the poor prognosis of COVID-19 [5]. In this study, we did found that the patients with CVD showed an older median age and a higher BMI score compared with those without CVD, while age was not the risk factor for the severe condition, and there was no difference in their gender composition. In addition to common symptoms, we found that gastrointestinal symptoms including poor appetite, nausea, and vomiting were more likely to occur in the patients with CVD, which may be related to the drugs that patients used. Some patients with CVD control blood pressure using ACEI/ARB drugs which inhibit the activity of ACE. However, despite the similarity in structure between ACE and ACE2, ACEI drugs cannot inhibit the activity of ACE2 [18]. Further, Ferrario et al. found that the expressions of ACE2 in a rat model are increased by 4.7-fold and 2.8-fold after administration with ACEI and ARB drugs, respectively [19]. SARS-CoV-2 enters cells by binding to ACE2 receptors on their surface, and ACE2 is highly expressed in mucosal epithelial cells of the respiratory and digestive tracts [[20], [21], [22]]. Therefore, those patients with CVD using ACEI/ARB drugs to control blood pressure may be more vulnerable to the virus invade and have digestive symptoms after infection. Furthermore, the use of ACEI drugs in patients with viral pneumonia does not reduce the incidence of adverse outcomes, and it is not suggested that they be used in patients with COVID-19 [19].
Chest CT images can be used as a reference of disease severity assessment. Next, we analyzed the changes in chest CT images in the CVD and the non-CVD group. Predictably, the CT image scores of patients with CVD were higher than those without CVD, which means that CVD patients have more severe infection. Indeed, the mortality of patients in the CVD group was significantly higher than that in the non-CVD group.
The adverse outcome of COVID-19 patient is associated with the damage of multiple organs, which was reflected by the elevated enzymes. Although our results showed that some enzyme levels are at normal range, the levels of enzymes in the CVD group are significantly higher than those in the non-CVD group, which means that the patients with CVD are more likely to suffer from multiple organ dysfunction syndrome (MODS). In addition, other researchers found that SRAS-CoV-2 could bind to ACE2 on myocardial cells, leading to a significant increase in myocardial injury markers. However, due to pathological changes, myocardial cells in patients with CVD are more susceptible to the damage of SARS-CoV-2, which may be the reason why there are differences in multiple enzyme release between the two groups of patients.
Furthermore, the levels of total protein, albumin, prealbumin, red blood cells, and hemoglobin in patients with CVD are significantly lower compared with those without CVD, which indicates potential immune dysfunction. Our data did display a lower lymphocyte count and a higher pro-inflammatory cytokine level in the CVD group, which signifies an increased occurrence of cytokine storm in these patients. According to previous research, cytokine storm plays an important role in the pathogenesis of SARS and MERS, and is also an important cause of death [23,24]. In addition, cytokine storm is linked to the damage of myocardial cells and may be related to cardiovascular complications in patients with SARS-CoV infection [25]. After infection, the levels of inflammation indicators like CRP, serum ferritin, ESR, and SSA were increased in COVID-19 patients and associated with the severity of the disease, and levels of these indicators in the patients with CVD were significantly higher than those without CVD. All these results above indicate that COVID-19 patients with CVD are more likely to form an inflammatory storm, which eventually leads to rapid deterioration of these patients' conditions.
During the process of infection, pulmonary inflammation prevents the oxygen exchange in alveoli, leading to hypoxia in the body, which activates the fibrinolytic system. Compared with the non-CVD group, the levels of D-dimer and FIB were higher in patients of the CVD group, which means that they were more prone to hypercoagulability. It is worth noting that a hypercoagulable state is more likely to cause cardiovascular events such as acute myocardial infarction (AMI), and two patients suffered from AMI during their hospitalization in the CVD group (data not shown). Additionally, a hypercoagulable state also increases the risk of pulmonary embolism, which can explain the sudden occurrence of complications such as hypoxia and heart failure. In this study, all six death cases occurred in the CVD group and were caused by respiratory failure and circulatory failure, which is consistent with the prediction of the laboratory tests.
To sum up, our data indicate that the COVID-19 patients with CVD showed more severe clinical symptoms, lung injury, tissue injury-related enzymes release, excessive uncontrolled cytokine storm, and hypercoagulable state compared with the non-CVD patients. Therefore, CVD can be regarded as a risk factor for the progression and prognosis of COVDI-19. CVD makes it difficult for the treatment of COVID-19, and we should pay more attention to CVD patients to prevent the rapid deterioration caused by the disease.
Funding
This study was funded by the grants from the project of Thousand Youth Talents for D.H. and from the National Natural Science Foundation of China (No. 31770983 and 81974249 to D.H.).
Author contributions
D.H. and S.L. designed the study. W.G., R.Z., Y.D., H.Z., Z.Z., and C.T. researched data. W.G. and M.L. contributed to the data analysis. L.W., K.D., H.Z., and Z.Z. contributed to the discussion. W.G., Y.D., and M.L. wrote the manuscript. H.W., L.Z., H.F., S.L., and D.H. reviewed/edited the manuscript.
Declaration of Competing Interest
The authors have no conflicts of interest to note.
Handling Editor: A. Siani
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.numecd.2020.04.013.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early transmission dynamics in wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet (London, England) 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Resp Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badawi A., Ryoo S.G. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int J Infect Dis. 2016;49:129–133. doi: 10.1016/j.ijid.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Booth C.M., Matukas L.M., Tomlinson G.A., Rachlis A.R., Rose D.B., Dwosh H.A. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. Jama. 2003;289:2801–2809. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- 8.Yang Y.M., Hsu C.Y., Lai C.C., Yen M.F., Wikramaratna P.S., Chen H.H. Impact of comorbidity on fatality rate of patients with Middle East respiratory syndrome. Sci Rep. 2017;7:11307. doi: 10.1038/s41598-017-10402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Assiri A., Al-Tawfiq J.A., Al-Rabeeah A.A., Al-Rabiah F.A., Al-Hajjar S., Al-Barrak A. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13:752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benjamin E.J., Blaha M.J., Chiuve S.E., Cushman M., Das S.R., Deo R. Heart disease and stroke statistics-2017 update: a report from the American heart association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roth G.A., Forouzanfar M.H., Moran A.E., Barber R., Nguyen G., Feigin V.L. Demographic and epidemiologic drivers of global cardiovascular mortality. N Engl J Med. 2015;372:1333–1341. doi: 10.1056/NEJMoa1406656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joo E.J., Chang Y., Kwon M.J., Cho A., Cheong H.S., Ryu S. High-risk human papillomavirus infection and the risk of cardiovascular disease in Korean women. Circ Res. 2019;124:747–756. doi: 10.1161/CIRCRESAHA.118.313779. [DOI] [PubMed] [Google Scholar]
- 13.Rahman A., Sarkar A. Risk factors for fatal Middle East respiratory syndrome coronavirus infections in Saudi Arabia: analysis of the WHO line list, 2013-2018. Am J Publ Health. 2019;109:1288–1293. doi: 10.2105/AJPH.2019.305186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. Jama. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ooi G.C., Khong P.L., Muller N.L., Yiu W.C., Zhou L.J., Ho J.C. Severe acute respiratory syndrome: temporal lung changes at thin-section CT in 30 patients. Radiology. 2004;230:836–844. doi: 10.1148/radiol.2303030853. [DOI] [PubMed] [Google Scholar]
- 16.W L., W G., R C., W W., J L., K X. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu R., Ming X., Xu O., Zhou J., Peng H., Xiang N. Association of cardiovascular manifestations with in-hospital outcomes in patients with COVID-19: a hospital staff data. medRxiv. 2020;(2020.02) 29.20029348. [Google Scholar]
- 18.Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000;87:E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 19.Ferrario C.M., Jessup J., Chappell M.C., Averill D.B., Brosnihan K.B., Tallant E.A. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 20.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel V.B., Zhong J.C., Grant M.B., Oudit G.Y. Role of the ACE2/angiotensin 1-7 Axis of the renin-angiotensin system in heart failure. Circ Res. 2016;118:1313–1326. doi: 10.1161/CIRCRESAHA.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tipnis S.R., Hooper N.M., Hyde R., Karran E., Christie G., Turner A.J. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000;275:33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 23.Mahallawi W.H., Khabour O.F., Zhang Q., Makhdoum H.M., Suliman B.A. MERS-CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine. 2018;104:8–13. doi: 10.1016/j.cyto.2018.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong C.K., Lam C.W., Wu A.K., Ip W.K., Lee N.L., Chan I.H. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136:95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu C.M., Wong R.S., Wu E.B., Kong S.L., Wong J., Yip G.W. Cardiovascular complications of severe acute respiratory syndrome. Postgrad Med J. 2006;82:140–144. doi: 10.1136/pgmj.2005.037515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.