Abstract
The endothelial surface is a highly flexible signaling hub which is able to sense the hemodynamic forces of the streaming blood. The subsequent mechanosignaling is basically mediated by specific structures, like the endothelial glycocalyx building the top surface layer of endothelial cells as well as mechanosensitive ion channels within the endothelial plasma membrane. The mechanical properties of the endothelial cell surface are characterized by the dynamics of cytoskeletal proteins and play a key role in the process of signal transmission from the outside (lumen of the blood vessel) to the interior of the cell. Thus, the cell mechanics directly interact with the function of mechanosensitive structures and ion channels. To precisely maintain the vascular tone, a coordinated functional interdependency between endothelial cells and vascular smooth muscle cells is necessary. This is given by the fact that mechanosensitive ion channels are expressed in both cell types and that signals are transmitted via autocrine/paracrine mechanisms from layer to layer. Thus, the outer layer of the endothelial cells can be seen as important functional mechanosensitive and reactive cellular compartment. This review aims to describe the known mechanosensitive structures of the vessel building a bridge between the important role of physiological mechanosignaling and the proper vascular function. Since mutations and dysfunction of mechanosensitive proteins are linked to vascular pathologies such as hypertension, they play a potent role in the field of channelopathies and mechanomedicine.
Keywords: Mechanosensitive ion channels, Glycocalyx, Mechanotransduction, Shear stress sensor, Nanomechanics
Introduction
Maintaining vascular homeostasis and keeping blood pressure variations in an optimal physiological range are a lifelong challenge which among others ensure a sufficient blood flow and supply of oxygen and nutrients to peripheral organs. Therefore, pump function of the heart, vascular resistance and renal water, and salt homeostasis are closely monitored by various physiological mechanisms, which reconcile metabolic demand and supply on an acute and long-term scale. Endothelial cells (EC) are located at the innermost layer of all blood and lymphatic vessels. They are constantly exposed to mechanical forces mediated by the blood flow, thereby maintaining a selective permeable barrier between the tissue and intravascular lumen. In addition to this transport barrier, EC contribute to the regulation of blood pressure and represent a multifunctional signal-transducing surface. EC function can thereby be modified by a bench of biochemical signals (catecholamines, neurotransmitter, cytokines, growth factors) [100, 173, 186, 196] as well as mechanical stimuli coming from the blood stream itself [38, 72, 134]. Blood flow induced hemodynamic forces such as shear stress, hydrostatic pressure, and circumferential stretch can be sensed by EC through mechanosensors and transferred into signaling pathways, modifying gene and protein expression and endothelial function [113].
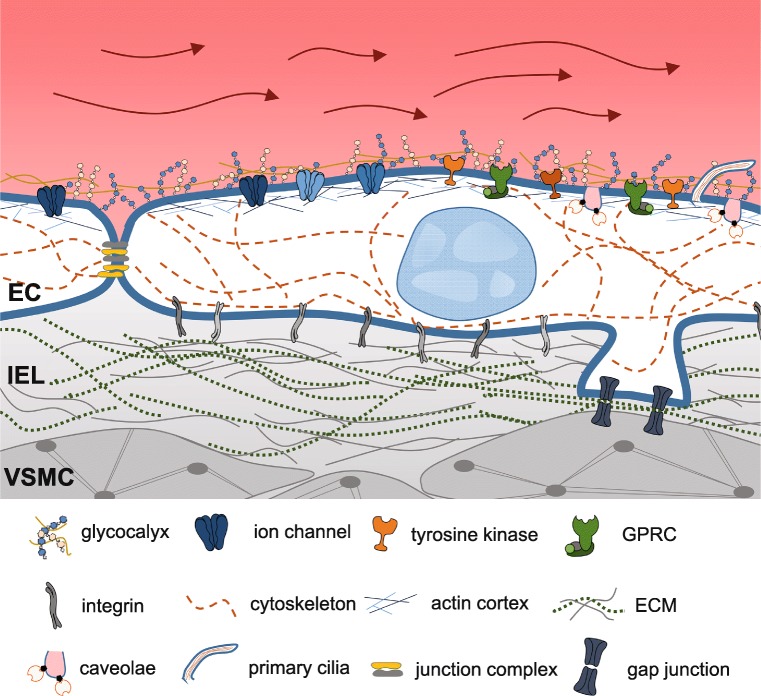
The different hemodynamic forces vary depending on, e.g., physical activity, different vessels types, vessel location (bifurcation sites), and—temporally—on the pulsatile cardiac action. Even at the level of EC, there is a distinct spatial distribution of the external forces acting on different cellular mechanosensors. These mechanical forces are sensed and translated into biochemical signals by specific structures and proteins located in the membranes of endothelial cells. During the last years, a number of potential cellular mechanosensitive and responsive structures have been identified so far, including cell adhesion proteins (like VE-Cadherin, PECAM-1), ion channels, tyrosine kinase receptors (VEGF receptor 2), G-protein coupled receptors (GPCR), caveolae, primary cilia, cytoskeletal actin, nesprins, integrins, and the endothelial glycocalyx (eGC) [43, 81, 193].
After being sensed by the EC, the mechanical forces are encoded and transmitted to the vascular smooth muscle cells (VSMC), which either respond with relaxation or contraction. In fact, a close functional interaction between EC and the neighboring VSMC is responsible for the regulation of the vascular tone and the ability of cells to react on different biochemical and mechanical stimuli from the streaming blood. During the last years, it became clear that in particular the mechanical properties of EC (i) depend on flow-mediated forces and (ii) determine the contraction status of VSMC. This well-described mechanism is mainly based on the ability of the EC to release nitric oxide (NO) in a shear stress-dependent manner, which diffuses to adjacent VSMCs where it triggers vasodilation via cGMP-dependent pathways [154]. A reduction in NO is strongly associated with increased levels of reactive oxygen species (ROS) generated by NAD(P)H oxidase, xanthine oxidase, or uncoupled endothelial nitric oxide synthase (eNOS) within the vascular wall, leading not only to scavenging of NO but also to disruption of some signaling pathways that mediate its production [16]. Hence, the tight interplay between EC and VSMC controls vascular function and vessel tone. Primarily the ability of the EC to change their mechanical properties, i.e., to alternate between “stiff” and “soft” conditions, is an important physiological feature of the endothelium. Endothelial cells which have lost this ability and are arrested in chronic stiffening can be seen as dysfunctional [95].
This review mainly focuses on the impact of the endothelial glycocalyx and mechanosensitive ion channels in endothelial mechanosensing. The endothelial cell surface, including glycocalyx, plasma membrane, cortex, and ion channels, can be seen in total as important functional mechanosensitive and reactive cellular compartment. Since mutations and dysfunction of mechanosensitive structures are linked to vascular pathologies such as hypertension [83, 127, 169], they play a potent role in the field of channelopathies and mechanomedicine.
Shear stress-mediated mechanosignaling
Due to their position, EC sense and react to changes in shear stress caused by the blood stream, which is substantial for a proper physiological vascular function [5, 31]. It is generally accepted that shear forces lead to an EC-mediated vasodilation due to secretion of vasoactive substances like NO [154]. Other known shear stress-induced mediators involved in the control of vascular tone are prostacyclin, a potent vasodilator [18, 111, 139], and endothelin, a strong vasoconstrictor and different mitogenic molecules [198]. This vasomodulatory secretion can mediate increase as well as decrease in vessel diameter. This mechanism is also known as flow-mediated dilation (FMD) and its impairment, caused on a decreased NO production, which can be seen as a hallmark of endothelial dysfunction [46, 55, 95]. In line with this, FMD was found to be markedly reduced in hypertensive patients and diseases like hyperaldosteronism [129, 138].
Shear stress is a tangential force arising due to the friction of the blood volume and the vessel wall (in fact the EC). It varies over the vascular tree from 1 dyne/cm2 at venous ECs up to 40 dyne/cm2 in arterial vessels [87, 193]. Another type of force is the blood pressure itself, exerting a variable magnitude, ranging from 120 to almost 0 mmHg (MAP; mean arterial pressure) depending on different types and location of the blood vessels. Both forces mediate the third type of force, the so-called circumferential stretch, acting through transmural pressure differences distending the vessel wall [144]. EC response to hemodynamical variations of the blood flow ranges from acute adaptations in ion channel function to long-lasting gene regulatory events [33, 67]. EC can also respond to shear stress with cytoskeletal remodeling by increasing actin stress fibers [14]. Here, it is important to differentiate between laminar and non-laminar (turbulent) forms of shear stress [98], since these different forms of shear stress modulate many different effects in the vascular system. Laminar shear stress (LSS) physiologically occurs mainly at straight parts of the blood vessels and is known to mediate protective properties such as down-regulation of inflammatory cytokines, adhesion molecules, and oxidative stress [69, 76, 102]. These positive effects are mainly caused by the physical properties of LSS as an ideal-typical parabola shape flow, where the shear rate is decreased at the center of the lumen of the blood vessel and gradually increased toward the wall [18]. Disturbances of the hemodynamic homeostasis are associated with cardiovascular diseases [22]. Especially, pathologic changes in the rheology of the blood lead to and maintain atherogenic processes – especially in the branching regions of blood vessels where non-laminar shear stress (NLSS) occurs.
In contrast to LSS, NLSS is defined as the flow in which the blood velocity varies continuously over the course of time, even though the overall flow may remain steady [18]. This can explain the pathophysiology of atherosclerotic lesion, which non-random distribution can be attributed to the alterations of local function of vascular ECs by a disturbed flow pattern like flow separation, recirculation, reattachment, low and reciprocal shear stress, and high spatial and temporal gradients of shear stress [22].
To understand these flow-mediated alterations in EC function and dysfunction, a detailed knowledge of EC mechanosignaling is crucial. The following chapters will focus on different mechanosensitive structures within the endothelium (see Fig. 1 for overview).
Fig. 1.
Mechanosensitive structures of the endothelium. Blood flow-induced hemodynamic forces such as shear stress, hydrostatic pressure, and circumferential stretch can be sensed by EC through mechanosensors. These structures sense the mechanical forces and translate them to biochemical signals by specific proteins located on/in the membranes of endothelial cells. Potential cellular mechanosensitive and responsive structures are depicted in this figure. EC, endothelial cell; IEL, internal elastic lamina; VSMC, vascular smooth muscle cell
Mechanosensitive structures in the endothelium
The cellular tensegrity model has been proposed to explain transduction of mechanical forces to biochemical signals [79]. It is based on the concept that complementary mechanical forces arising from the cytoskeleton and extracellular tethering sites to the ECM or neighboring cells are balanced. A shift in this equilibrium mediates mechanosensing and signal transduction [80]. Based on a cellular level, a hierarchical and multi-modular tensegrity structure is postulated. In line with this model, traction force microscopy analyzes cell tension (= cell adhesion) exerted from cytoskeletal parts to its anchoring points of the ECM on a flexible polyacrylamide substrate [137]. Sims and colleagues were able to show that EC exert force to the substrate which can be revoked by trypsin treatment [160]. Individual stress fibers are tensed by actomyosin motors and confer the forces to the ECM, thereby modulating a cellular pre-stress which is transmitted to and balanced by traction forces that act at the cell-anchoring points to the substrate [80, 93].
Tyrosine kinase receptors (e.g., VEGFR2 or Tie-2) are activated in ECs after shear stress exposure in a ligand-independent manner [85, 97, 176]. The mechanism which leads to phosphorylation of VEGFR2 in response to shear is still not well understood. VEGFR2 seems to work in a network along with PECAM-1 and VE-cadherin, mediating the intramembrane binding to the whole mechanosensory complex [27]. The eGC could be identified as another interaction partner of VEGFR2, thereby regulating receptor endocytosis and activation in response to eGC composition [96]. Of note, the endothelium-stabilizing receptor Tie-2 was found to be deactivated during sepsis, leading to an eGC breakdown, and could be prevented by Tie-2 activation and blockage of Tie-2 antagonist angiopoietin [40].
GPCR and G-proteins have been identified in shear stress signal transduction in various studies. For example, GPR68 could be identified in a shear stress RNAi library screen as a necessary component for flow-mediated dilation in small resistance arteries [192]. Additionally, G-proteins can be activated by shear stress independently from GPRC activation. The G-protein Gαq/11, for example, could be activated by shear in the presence of GPCR antagonists in the human coronary artery endothelial cells [36].
Caveolae, small cholesterol and glycosphingolipid-rich flask-shaped membrane invagination, form membrane microdomains containing various signaling molecules, including the aforementioned kinases, GPCR, and ion channels [15, 54, 157]. eNOS is associated with caveolae and its positioning is coupled to proper NO production [61, 156]. Redistribution of eNOS away from the plasma membrane depends on cholesterol composition of the caveolae. Oxidized low-density lipid and cholesterol depletion lead to reduce NO production [177]. Proteins within the caveolae like caveolin-1 thereby inhibit eNOS function and participate in EC-mediated vasodilation [19]. In addition, caveolin-1 stabilizes eNOS expression level and is proposed to be an important determinant of endothelial vasodilatory functions [20].
The endothelial barrier is formed by tight junctions, VE-cadherin and PECAM-1 [176]. The vascular permeability is thereby mainly controlled by VE-cadherin in a Ca2+-dependent manner [35]. Cadherin complexes are connected to the cytoskeleton via catenin and vinculin and can remodel in response to mechanical stimuli [75]. Activation of vinculin can lead to F-actin polymerization, and VE-cadherin and PECAM-1 protein complexes can be altered in response to shear stress [26, 172]. Cell-matrix interactions via integrins are also discussed to be part of the mechanosensitive complex in EC [21]. However, evidences for a direct activation of integrins by shear stress are limited. Integrins more likely are activated by biochemical and not force-based signals arising from other primary mechanosensors [105, 174].
Primary, non-motile cilia are protrusions of the apical cell membrane with an extend up to 5 μm and consist of microtubule bundles, which are connected to the intracellular cytoskeleton [45]. Cilia are sensitive to shear stress and can be disassembled by LSS (15 dyne/cm2), accompanied by major rearrangement of the cytoskeleton [82]. Cilia mediated shear stress sensing coupled to Ca2+ signaling and nitric oxide production. Knockdown of cilia proteins lead to disturbed mechanosignaling [125, 126].
The cytoskeleton is composed of three major filament types, namely (i) the microfilaments, (ii) intermediate filaments, and (iii) microtubules. This cytoskeletal scaffold can be deformed and transmits force/tension via focal adhesion sites, integrins, cellular junctions, and extracellular matrix to the interior of the cell [32]. Microfilaments consist of actin polymers, which can be rearranged highly dynamically by change from filamentous actin (F-actin) to globular actin (G-actin) and can be connected between cellular structures. De/stabilization is mainly mediated by members of the Rho family of small GTP-binding proteins like Rho and Rac GTPases. To counteract external tensile forces, actin can polymerize in response to tensile forces, leading to stress fiber formation, which are composed of actin and myosin II filaments [71, 132, 178].
Intermediate filament proteins like laminin form the nuclear scaffold adjacent to the inner nuclear membrane. It thereby contributes to chromatin regulation and signaling pathways affecting gene expression [84]. It is discussed, that laminins act as a “mechanostat” that is able to sense extracellular forces and respond by reinforcing the cytoskeleton and the extracellular matrix, e.g., by directly transducing external forces to the nucleus which alters gene expression [123, 135]. Microtubules are involved in shear stress-derived cell polarity and are interconnected as well as linked to membrane proteins throughout the cell [175, 176]. Recently, it could be shown that microtubules also interact with integrin-based focal adhesions and myosin IIA filaments [141]. This connection of external contact, adhesion receptors, and cytoskeletal structures serves as a potent mechanotransducer for inside-out as well as outside-in signaling pathways.
The following chapters will mainly focus on the impact of the endothelial glycocalyx and connected mechanosensitive ion channels in the vascular mechanosensing. Being mechanosensitive switches, ion channels convert mechanical stimuli attaining the cell membrane (pressure, stretch, shear) into electrical and biochemical signals, which affect the cellular and physiological reactions.
Endothelial glycocalyx
The glycocalyx is the top surface layer of all living cells, including endothelial cells, and is built by a negatively charged, brush-like structure, with a functional height up to 500 nm. This membrane-bound carbohydrate-rich layer covers the luminal membrane of endothelial cells (endothelial glycocalyx, eGC) and is associated with different plasma proteins [187]. Together with the cortical actin, a thin actin mesh directly underneath the plasma membrane, and membrane proteins, like mechanosensitive ion channels, the eGC build a highly dynamic hub for intra- and extracellular signals [52, 88]. eGC functions range from modulation of leukocyte adhesion, regulation of blood coagulation, maintaining vascular permeability barrier, and mediating flow-induced NO release. So, it has been recognized as an important vasculoprotective nanobarrier [28, 64]. For a detailed overview of the eGC nanomechanics and functions, we refer to a recent review from our group [28].
The eGC is formed by glycoproteins and proteoglycans like heparan and chondroitin sulfate as well as hydrophilic hyaluronic acid [146, 151, 170]. The components are covalently anchored, and transmembrane proteins like syndecan link the eGC with the intracellular actin cortex [143]. This enables the eGC to transduce extracellular signals into intracellular biochemical signaling pathways. In the same time, because of its intrinsic charge, other negatively charged molecules (or cells) from the plasma are hindered from passing this first barrier [25]. From this point of view, the eGC acts as an effective cation buffering and barrier system [41, 153]. Under healthy physiological conditions, the eGC structure is in a steady state of permanent turnover caused by flow-mediated degradation and reorganization by biosynthesis of new eGC components. The exact turnover of eGC can hardly be analyzed, known values range from 6 h in enterocytes to 5 days in rat uterine epithelial cells [58, 86]. However, eGC must be seen as a highly flexible and inhomogeneous structure in dependence of EC (and eGC) positioning along the vessel tree as well as due to various electrostatic and biochemical interactions between its constituents [121, 189].
eGC as mechanosensor
Due to its unique localization as an interface between the blood stream and tissue, the eGC has been identified to function as a mechanosensor as well as mechanotransducer [6, 166, 187]. For example, Yen and colleagues showed that flow-induced NO production in post-capillary venules and arterioles of rat mesenteric arteries can be abolished by enzymatic removal of heparan sulfate by heparanase III treatment. The authors postulate that the eGC acts as a mechanotransducer and participates in the regulation of NO production [194]. Dragovich and colleagues showed in brain microvascular endothelial cells that enzymatic removal of eGC components lead to perpetuated Ca2+ signals and eNOS activity [39] accompanied by the remodeling of cytoskeletal structures [3]. In fact, the eGC itself can be modulated in structure and function in response to changes in blood flow [68, 187]. Shear stress induces remodeling of the eGC, by increasing heparan sulfate, chondroitin sulfate, glypican-1, and syndecan-1 at the cell surface, thereby influencing the integrity of the glycocalyx and its ability of sensing shear stress [195]. In addition, shear stress acting on the EC stabilizes the eGC, which is important for proper endothelial function and NO production [168, 194]. For example, laminar shear stress induces a recruitment of hyaluronan synthase 2 to the endothelial plasma membrane and increases hyaluronan expression, a major structural eGC component [184]. In line with this, the presence of heparan sulfate, and thus an intact eGC, is necessary for flow-induced NO production in aortic EC [57]. eGC breakdown by antagonism of endothelium-stabilizing receptor Tie-2 leads to plasma leakage and increased leukocyte recruitment in vivo [109].
These findings strengthen the idea of a vasculoprotective function of the eGC [64, 189]. However, it is postulated that stabilization and turnover of the eGC by shear stress might rather be a physiological response to mechanotransductory changes under flow conditions. We were able to show that moderate laminar shear stress (LSS, 8 dyne/cm2) increased the amount of heparan sulfate at the surface of endothelial cells, while treatment with heparanase I leads to a significant reduction of the eGC under shear stress conditions. Delgadillo and colleagues also showed shear-mediated effect on the physical nanobarrier function of eGC. Comparisons of different shear rates on HUVECs lead to higher eGC thickness and decreased neutrophil adhesion under high (10 dyne/cm2) vs. low (0.5 dyne/cm2) shear stress [37]. In addition, moderate LSS leads to increase F-actin polymerization within the actin cortex (unpublished data of our group). This illustrates that physiological shear stress is obligatory for a proper eGC structure and plasticity to fulfill mechanosensory function within the vascular system.
As described above, from the pathophysiological point of view, a damaged eGC exerts a disturbed mechanotransduction to intracellular components like the endothelial actin cortex and will change membrane characteristics including the presence of mechanosensitive ion channel, adhesion molecules, and cytoskeletal anchor proteins [197]. Different authors postulate feedback reinforcement between damaged eGC and progression of endothelial dysfunction [48, 159, 197].
First observation of a pathophysiological damage of eGC was done by Van den Berg. He screened atheroprone regions of mouse internal carotid arteries and observed a reduced eGC thickness in disease predilection compared with common carotid arteries [179]. Others found higher eGC component synthesis (because of higher eGC turnover) in arteries exposed to higher shear stress compared with low shear stress [64]. Different non-cardiovascular as well as cardiovascular diseases are accompanied by disturbed eGC mechanosensing. In an in vitro model of hyperglycemia, a disturbed flow-mediated alignment of EC was accompanied by loss of heparan sulfate (major eGC component), as well as reduced NO production in response to shear stress application [17, 108]. Dialysis patients show impaired eGC structure and shedded hyaluronic acid as well as syndecan-1 in the blood [180]. Also the impact of eGC damage on glomerulus filtration and development of albuminuria are widely discussed [149]. High ox-LDL levels induce degradation of the eGC and subsequently increased leukocyte adhesion in cremaster venules [25]. Knockdown of syndecan-1, an important eGC component, lead to impaired mechanosignaling in injured carotid arteries, larger neointimal hyperplasia, and increased VSMC proliferation [59]. Lack of syndecan-1 is associated with impaired migration and enhanced adhesion of macrophages, as well as increased inflammation and atherosclerotic plaque formation [4].
In conclusion, disturbance of the eGC structure, e.g., by changes in blood flow parameters lead to altered mechanosignaling in EC. These results strengthen the concept of a mutually interacting signaling hub of eGC, cortical actin, and ion channel within the endothelial cell.
Mechanosensitive channels in the endothelium
In response to shear stress or flow-mediated membrane stretch, opening of mechanosensitive ion channels is the very first step in cellular mechanosignaling [24, 115, 122]. These ion channels show partially opposed characteristics, ranging from hyperpolarization by K+ selective TREK channels to depolarization by Ca2+ and Na+ permeable Piezo1 channels. Here, we mainly focus on mechanosensitive cation permeable ion channels, leading to Ca2+ influx into the cell. There is substantial evidence that the increase in intracellular Ca2+ is one of the earliest events in response to shear stress. In endothelium, increased Ca2+ subsequently activate eNOS and intermediate conductance Ca2+-activated K+ channels (IKCa), resulting in vasodilation through eNOS-mediated NO release and/or membrane hyperpolarization. Ion channels seem particularly well suited to perceive physical forces and are strongly suggested as key players in the sensing of shear stress [77].
Piezo1 and Piezo2 are mechanically activated cation channels which mediate as large homomultimeric complexes cation currents in various tissues [29, 181]. Both isoforms are mechanically gated and confer nonselective (Na+, K+, and Ca2+) currents with fast activation kinetics. Whereas Piezo2 is mainly expressed in tactile epithelial cells (Merkel) [190] and mechanosensory neurons [122], Piezo1 has been reported to mediates mechanically induces currents in various cell types, including endothelial cells and smooth muscle cells [142, 145]. Piezo1 was shown to be an important sensor of shear force in EC and involved in cell alignment in flow direction [103]. Laminar flow-mediated activation of Piezo1 mediates flow-induced release of ATP from endothelial cells, resulting in the activation of the Gq/G11-coupled purinergic P2Y2 receptor [182, 183]. P2Y2/receptor and Gq/G11 cascades lead to activation of AKT and eNOS and mediate flow-induced vasodilation. Both laminar and disturbed flows activate the same initial mechanosignaling pathway involving Piezo1- and Gq/G11-mediated signaling [2]. Accordingly, Piezo1 channel activator Yoda1 induces NO-mediated relaxation of murine intrapulmonary arteries [101].
Transient receptor potential (TRP) channels are non-voltage gated cation channels, regulated by polymodal stimuli and implicated in a variety of cellular functions [128]. At least ten TRP channels (TRPC1, 5, 6; TRPV1, 2, 4; TRPM3, 7; TRPA1; TRPP2) have been proposed to be mechanosensitive [24, 81, 112, 158, 171]. TRP can mediate Ca2+ signaling but also can be Ca2+ regulated by directly Ca2+ binding to the channel or Ca2+-calmodulin complex mediated activation. In VSMC, Ca2+ influx through TRP channels leads to membrane depolarization and forced influx through voltage-gated Ca2+ channels (L-type or T-type Ca2+ channels, CaV1.2 / CaV3.1). Ca2+-calmodulin complex activates myosin light chain kinase and initiates the contractile process [73].
In many cases, it is still not finally clarified how this mechanosensivity is mediated, although a number of studies support the mechanosensitive characteristics of TRP channels [73]. The following principles are discussed: (i) direct activation by extracellular forces like membrane stretch and shear-induced changes in lipid bilayer conformation and subsequent deformation of channel domains [60, 114, 162], (ii) tethering of ion channel structures with cellular component like ECM, proteins, or intracellular cytoskeleton [9, 117], and (iii) indirect activation by other primary mechanosensors and subsequent biochemical transduction to effector TRP channels [91, 107, 119]. In the following sections, some mechanosensitive candidates of the TRP family will be discussed.
TRPV4 has been proposed to be a candidate for the molecular blood flow sensor inducing the flow-induced vasodilation, a response to increased blood flow velocity or viscosity [31]. Consistently, TRPV4 was identified to be activated under hypertonic conditions and cell swelling-mediated membrane stretch [104]. On the other hand, cell-attached patch clamp approaches were not able to directly activate TRPV4 by pipette suction, suggesting an indirect activation of TRPV4 through force-sensitive signaling cascades [43]. Kohler and colleagues showed that in rat carotid artery, endothelial cells agonist- or shear stress-induced activation of TRPV4 leads to dilation of rat gracilis arteries. eNOS blockade attenuates this TRPV4-mediated effect [91]. The same group was able to show that TRPV4 knockout showed significantly reduced flow-induced vasodilation [70]. In line with this, Mendoza and colleagues found a TRPV4-dependent relaxation involving NO and EDHFs and Ca2+ influx through endothelial TRPV4 channels in response to flow [120]. Shear stress also leads to exocytosis-mediated recruitment of TRPV4 channels and endothelial sensitization to mechanical stress [8].
TRPV4 was found to be co-localized with TRPC1 proteins in EC from rabbit mesenteric arteries. Analysis of (high external) Ca2+-induced EC-dependent vasodilation showed TRPV4- and TRPC1-dependent Ca2+-influx and induction of NO production. Activation of TRPV4 (agonist) induced NO production, and subsequent vasodilation could be prevented by L-NAME (N(ω)-nitro-L-arginine methyl ester, eNOS inhibitor), TRPV4 antagonist (RN1734), or TRPC1 antagonism (T1E3, blocking peptide). Heteromeric TRPV4 and TRPC1 channels mediate calcium-sensing receptor induced vasorelaxation through NO production [65].
The TRPC1 channel is the first cloned member of the mammalian TRP superfamily [188]. TRPC1 function is generally associated with regulation of store-operated Ca2+ channels (SOC) and receptor-operated Ca2+ channels (ROCC) via interaction with STIM1, ORAI1, and IP3 receptors [10, 34]. Mechanical (tonic) stretch application for 14 h to human myometrial smooth muscle cells leads to increased expression (qPCR and WB) of TRPC3 and C4, but not of TRPC1 or C6 [30]. On the other hand, up-regulation of TRPC1, C3, and C6 could be found in pressurized hearts after aortic constriction, suggesting mechano-responsive expression pattern of TRPC1 channels [94, 133]. Nevertheless, TRPC1 as mechano-sensitive channel has been a subject of controversial debates [11, 63]. Overexpression of TRPC1 in frog oocytes increased the number of stretch-activated ion channels in patch clamp experiments, which can be diminished by siRNA approach [114]. In cancer-associated fibroblasts, TRPC1 is involved in responding to an increase of the ambient pressure [53], whereas in MDCK-F cells, TRPC1 also contributes to mechano-signaling during cell migration [47]. TRPC1 has also been identified as a component of biomechanical signaling in the development of pressure-induced heart failure and hypertrophy [44, 155].
It is controversially discussed if TRPC1 acts as homomeric or at least as a heteromeric channel together with TRPC3/4/5 or TRPV4 [63, 110, 163, 165]. In HUVECs (primary human umbilical vein endothelial cells), agonist-mediated stimulation of calcium-sensing receptor (CaSR) leads to a TRPC1-dependent increase in intracellular Ca2+ and enhances NO production. The authors postulate a coupling of TRPC1 to CaSR and TRPC1-mediated store-operated Ca2+ entry (SOCE) mechanisms for Ca2+ influx [140]. TRPC1 is also co-localized with TRPV4 in EC from mesenteric arteries. This heteromeric channel is activated by CaSR and induces an increase in NO production and vasorelaxation [65, 66].
TRPC6 is potentially a mechanosensitive TRP channel, which can be activated directly by diacylglycerol (DAG) [74, 92]. TRPC6 is important for regulating endothelial permeability in response to pro-inflammatory cytokines and inflammatory markers [99, 161]. In EC of the pulmonary arteries, TRPC6 knockout diminished the TRPC6 agonist-mediated increase in intracellular Ca2+, vascular filtration, and edema formation [150]. Fleming and colleagues showed that cytochrome P450 (CYP)-derived epoxyeicosatrienoic acids (EETs), one amongst other mechanically produced metabolites, supports translocation of TRPC6 to caveolin-1-rich cell membrane areas [56]. The direct mechanical activation of TRPC6 is discussed controversially. Inoue et al. proposed a synergistic activation by a combined mechanical and muscarinic receptor agonist carbachol-mediated stimulation [148].
TRPM7 expression could be shown in HUVECs by Baldoli and colleagues [7], where it has been linked to magnesium transport. TRPM7 is somehow unique in comparison with other TRP because it possesses a regulatory kinase domain at the C-terminus [147]. The mechanosensitive potential of TRPM7 could be shown in pressure-loading patch clamp approaches [191] as well as in fluid shear stress experiments in mesenchymal stromal cells [106].
TRPP2 (also known as polycystin-2 and polycystic kidney disease 2, PKD2) has been linked to mechanosensitive functions of primary cilia. Reduced expression of TRPP2 leads to decreased NO production in murine EC [1]. Knockdown of TRPP2 leads to an inability of EC to transduce extracellular shear stress into intracellular Ca2+ signaling and biochemical nitric oxide synthesis [125]. Also an interaction between TRPP2 and TRPC1 and a potential role in stretch-induced injury of blood-brain barrier endothelial cells is postulated [12, 136]. Additionally, it was observed that only a heteromeric channel composition of TRPP2, TRPC1, and TRPV4 is able to mediate flow-induced cation currents [42].
The epithelial sodium channel ENaC has primarily been described in principal cells of the distal nephron in the kidney, where it is mainly involved in salt and water homeostasis [13, 62]. Now it is obvious that ENaC is expressed in a variety of different tissues where it fulfills diverse functions. In particular, ENaC was identified in the vascular endothelium, where it controls endothelial nanomechanics [50, 167]. ENaC, like many other ion channels, is linked to cytoskeletal components and these interactions are used for mechanotransduction [51, 78, 118, 185]. It is proposed that an increase in ENaC activity in EC and thus an enhanced sodium influx stabilizes cortical actin in its filamentous form (F-actin), leading to a more rigid cell cortex [131, 185]. Unpublished data from our group show that functional inhibition of ENaC provokes a shift from F- to G-actin which in turn leads to a softening of the cell cortex. In contrast, chemical stabilization of the actin cytoskeleton abrogates this effect. Hence, ENaC function and actin dynamics are strongly correlated in EC.
In the case of the epithelial ENaC, a flow-modulated stimulation of ENaC activity and sodium absorption is mediated by an increase in hydrostatic pressure, suggesting a flow-sensitive way of channel gating [152]. In addition, Guo and colleagues showed that ENaC can be activated by flow and increased hydrostatic pressure, and increased intracellular sodium levels lead to reduce NO production in EC [67]. In line with these findings, we were able to show that ENaC is inserted into the membrane in response to acute shear stress modulations (unpublished data from our group). This leads to increase Na+ influx into the EC and polymerization of the cortical actin. A recent publication shows that ENaC shear force sensing is dependent on sugar residue interaction with the eGC. Extracellular N-glycosylated asparagine residues of ENaC interconnect the channel with the ECM as well as eGC, and removal of these N-glycans lead to decreased shear force-induced ENaC currents [90]. These data support the idea of a tight interaction and interdependence of eGC, ion channel function, and cytoskeleton as coupled mechanosensors of the endothelium.
Interaction between mechanosensitive ion channels in the VSMC and EC
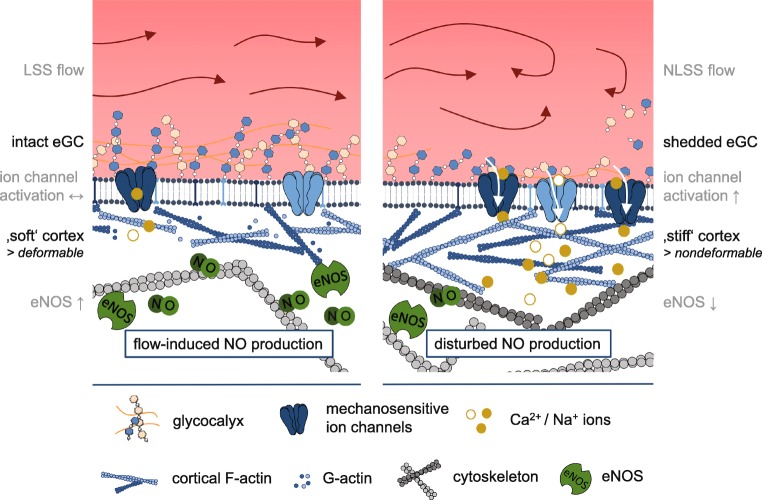
The regulation of the vascular tone is basically mediated by processes within the vessel wall. As mentioned before, EC and VSMC are in close physical vicinity and their functions are tightly coupled. Hence, biochemical as well as mechanical signals from the streaming blood are recognized by the endothelial surface structures and conducted to the VSMC. One of the best described paracrine mechanism of EC-VSMC interplay is the EC-derived NO release which directly affects the contraction status of the VSMC: A high production of NO in EC leads to relaxation of the VSMC and decreased vessel tone, while a reduction of NO release causes contraction of the VSMC and increased vessel tone. This in turn is directly linked to the mechanical properties of endothelial cells: A soft endothelial cell cortex is easily deformable by the streaming blood and thus the endothelial cell releases higher amounts of NO in contrast to a stiff cell cortex [51, 130]. The mechanical properties of the endothelial surface and the regulation of the vascular tone are mediated by ion channels (see Fig. 2). Of note, many typical EC mechanosensitive ion channels are also identified in the VSMC, but the functional interaction of them is only sparsely described.
Fig. 2.
Model of eGC- and ion channel-mediated mechanosignaling. Physiological LSS is accompanied by an intact eGC structure and a “soft” and deformable actin cortex. EC can react to changes in blood flow with increased eNOS activity and NO-mediated vasodilation (left figure). Pathophysiological increase of shear stress (e.g., by NLSS) leads to a disturbed eGC structure, increased Ca2+, and Na+ influx and stiffening of the cell cortex. This is accompanied by reduced eNOS activity and impaired flow-mediated vasodilation (right figure). The ability of the EC to change their mechanical properties, i.e., to alternate between “stiff” and “soft” conditions, is an important physiological feature. Loss of this plasticity leads to a dysfunctional endothelium
Here, some examples of mechanosensitive ion channels are described which are expressed in different cell layers of the vessel and seem to interact to maintain signal transduction and function within the vessel.
In EC, the mechanosensitive ENaC plays a crucial role in the orchestrated mechanism of vascular tone control. The plasma membrane insertion of the channel leads to stiffening of the endothelial surface which is mechanistically linked to the polymerization of the cortical actin leading to a subsequent reduction of NO release upon stimulation with shear stress [49]. In VSMC, ENaC is part of the transduction pathway of constriction response to pressure and acts as potential mechanosensor as it mediates pressure-induced vasoconstriction [41, 89]. Constitutive absence of the endothelial αENaC subunit leads to drastically decreased flow-dependent dilation of mouse mesenteric arteries, indicating that ENaC acts as mechanosensor [167]. Mutations in endothelial β- and γENaC contribute to severe forms of arterial hypertension [83]. Whether VSMC ENaC plays a role in this situation is not known yet. However, the presence of the channel in both cell types and similar regulatory mechanisms [185] let us assume a concerted action in the control of blood vessel tone.
Another example of a mechanosensitive ion channel which is expressed in both EC and VSMC is Piezo1. This non-selective cation channel is activated by mechanical stimuli, such as membrane stretch or shear stress. In EC, Piezo1 is activated by shear stress and leads to Ca2+ influx and phosphorylation of AKT and eNOS which results in an increased NO production and subsequent VSMC-mediated vasodilation [183]. In contrast, in VSMC, Piezo1 is activated by stretch and involved in processes of vascular remodeling under pathological conditions leading to a decrease in vessel diameter [122, 145]. Together, both Piezo1-dependent mechanisms effectively maintain basal blood pressure regulation.
As mentioned before, many members of the TRP channel family are also expressed in both EC and VSMC. In the vascular endothelium, TRP channels are known to act as stretch mechanosensors and to be involved in Ca2+ signaling. In VSMC, Ca2+ influx through TRP channels in general leads to membrane depolarization. Hence, they play a role in myogenic tone response and vasoconstriction. If and how TRP channels in EC and VSMC do interact is not really resolved at the moment.
In general, there is increasing evidence that the communication between EC and VSMC is not “one-way” from the endothelium to the muscle cells but rather a mutual interaction between both layers. However, shear- or stretch-induced responses in VSMC-free capillaries depend on the mechanosensing by the endothelial cell layer, while the pressure-dependent myogenic response can be attributed to the VSMC.
Recently, myoendothelial junctions have been identified as morphologically distinct structures which are formed by the membranes of both EC and VSMC and appropriate gap junctions between them. These gap junctions are composed of two connexons, composed of at least six connexin proteins. They basically serve as signaling microdomains to enable cross talk between EC and VSMC. Dilating substances, such as NO, are delivered from the EC to the VSMC, whereas IP3 diffusion from VSMC to EC provokes a Ca2+-response and leads to constriction. The latter pathway most likely activates intracellular Ca2+ stores through TRPV4 (for review see [164]). Thus, via myoendothelial junctions, the cross talk between the endothelium and smooth muscle is facilitated.
In an elegant study by Chiu et al., it was demonstrated that vascular EC function is influenced by the neighboring VSMC. In a co-culture shear stress model, the alignment of EC under flow occurs more rapidly than under static conditions. Furthermore, they conclude that shear stress may lead to a down-regulation of pathophysiological relevant genes and thus may exert vasculoprotective effects [23]. This again is a strong indicator of the functional and physiological relevant interaction between the vascular layers, maintaining vascular tone and reactivity.
Conclusion and perspectives
Proper regulation of the vessel tone is the basis of cardiovascular health. One of the major mechanisms which contribute to the fine tuning of vasodilation, or contraction is the sensing of mechanical stresses exerted on the vessel wall. In particular, endothelial cells immediately react with a change of their nanomechanical properties and conduct biochemical and/or mechanical signals to the vascular smooth muscle cells. Only the close interaction between all layers of the vascular wall (i) glycocalyx on top of endothelial cells, (ii) endothelial cells, and (iii) smooth muscle cells can maintain vessel tonus, regulate the expression of genes and proteins, and can cause morphological changes. Important mediators of the mechanosignaling are mechanosensitive ion channels expressed in both cell types. Disruption of these ion channel-mediated mechanisms may cause various diseases, such as hypertension and atherosclerosis, commonly described as channelopathies. Gain-of-function mutations in ENaC, for example, lead to a sustained stiffening of the endothelial cell cortex which might contribute to the severe hypertension in patients and mice [83].
There is evidence that TRP channels also contribute to the pathogenesis of hypertension, and it is reported that mutations in TRPC channel genes can be linked to cardiovascular diseases [127]. Expression of TRPC3 for example is elevated in patients with malignant hypertension in the vascular endothelium [169]. TRPM4 may be also involved in the control of blood pressure as TRPM4-deficient mice showed a hypertensive phenotype [116]. In this context, Keiji Naruse introduced the term “mechanomedicine.” This includes the investigation and characterization, but also the therapeutical benefit of this knowledge [124]. Especially, in the cardiovascular system, the mechanosensitive structures could serve as both predictors and pharmaceutical targets in cardiovascular pathologies.
Acknowledgment
Open Access funding provided by Projekt DEAL. The authors wish to thank the past and present members of their laboratories whose worked and contributed to developing the concepts described in this review. We would like to thank Carl Vahldieck for the revision and proofreading of the manuscript.
Funding information
K.K.V. acknowledges the support from the Deutsche Forschungsgemeinschaft (DFG; KU 1496/7-1, KU 1496/7-3).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.AbouAlaiwi WA, Takahashi M, Mell BR, Jones TJ, Ratnam S, Kolb RJ, Nauli SM. Ciliary polycystin-2 is a mechanosensitive calcium channel involved in nitric oxide signaling cascades. Circ Res. 2009;104:860–869. doi: 10.1161/CIRCRESAHA.108.192765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albarrán-Juárez J, Iring A, Wang S, Joseph S, Grimm M, Strilic B, Wettschureck N, Althoff TF, Offermanns S. Piezo1 and Gq/G11 promote endothelial inflammation depending on flow pattern and integrin activation. J Exp Med. 2018;215:2655–2672. doi: 10.1084/jem.20180483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ando J, Yamamoto K. Flow detection and calcium signalling in vascular endothelial cells. Cardiovasc Res. 2013;99:260–268. doi: 10.1093/cvr/cvt084. [DOI] [PubMed] [Google Scholar]
- 4.Angsana J, Chen J, Smith S, Xiao J, Wen J, Liu L, Haller CA, Chaikof EL. Syndecan-1 modulates the motility and resolution responses of macrophages. Arterioscler Thromb Vasc Biol. 2015;35:332–340. doi: 10.1161/ATVBAHA.114.304720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashley Z, Mugloo S, McDonald FJ, Fronius M. Epithelial Na+ channel differentially contributes to shear stress-mediated vascular responsiveness in carotid and mesenteric arteries from mice. Am J Physiol Heart Circ Physiol. 2018;314:H1022–H1032. doi: 10.1152/ajpheart.00506.2017. [DOI] [PubMed] [Google Scholar]
- 6.Bai K, Wang W. Spatio-temporal development of the endothelial glycocalyx layer and its mechanical property in vitro. J R Soc Interface. 2012;9:2290–2298. doi: 10.1098/rsif.2011.0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baldoli E, Castiglioni S, Maier JAM. Regulation and function of TRPM7 in human endothelial cells: TRPM7 as a potential novel regulator of endothelial function. PloS One. 2013;8:e59891. doi: 10.1371/journal.pone.0059891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baratchi S, Almazi JG, Darby W, Tovar-Lopez FJ, Mitchell A, McIntyre P. Shear stress mediates exocytosis of functional TRPV4 channels in endothelial cells. Cell Mol Life Sci CMLS. 2016;73:649–666. doi: 10.1007/s00018-015-2018-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Becker D, Bereiter-Hahn J, Jendrach M. Functional interaction of the cation channel transient receptor potential vanilloid 4 (TRPV4) and actin in volume regulation. Eur J Cell Biol. 2009;88:141–152. doi: 10.1016/j.ejcb.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Beech DJ. TRPC1: store-operated channel and more. Pflüg Arch Eur J Physiol. 2005;451:53–60. doi: 10.1007/s00424-005-1441-3. [DOI] [PubMed] [Google Scholar]
- 11.Beech DJ, Xu SZ, McHugh D, Flemming R. TRPC1 store-operated cationic channel subunit. Cell Calcium. 2003;33:433–440. doi: 10.1016/S0143-4160(03)00054-X. [DOI] [PubMed] [Google Scholar]
- 12.Berrout J, Jin M, O’Neil RG. Critical role of TRPP2 and TRPC1 channels in stretch-induced injury of blood-brain barrier endothelial cells. Brain Res. 2012;1436:1–12. doi: 10.1016/j.brainres.2011.11.044. [DOI] [PubMed] [Google Scholar]
- 13.Bhalla V, Hallows KR. Mechanisms of ENaC regulation and clinical implications. J Am Soc Nephrol JASN. 2008;19:1845–1854. doi: 10.1681/ASN.2008020225. [DOI] [PubMed] [Google Scholar]
- 14.Birukov KG, Birukova AA, Dudek SM, Verin AD, Crow MT, Zhan X, DePaola N, Garcia JGN. Shear stress-mediated cytoskeletal remodeling and cortactin translocation in pulmonary endothelial cells. Am J Respir Cell Mol Biol. 2002;26:453–464. doi: 10.1165/ajrcmb.26.4.4725. [DOI] [PubMed] [Google Scholar]
- 15.Boyd NL, Park H, Yi H, Boo YC, Sorescu GP, Sykes M, Jo H. Chronic shear induces caveolae formation and alters ERK and Akt responses in endothelial cells. Am J Physiol-Heart Circ Physiol. 2003;285:H1113–H1122. doi: 10.1152/ajpheart.00302.2003. [DOI] [PubMed] [Google Scholar]
- 16.Brandes RP, Weissmann N, Schröder K. Nox family NADPH oxidases in mechano-transduction: mechanisms and consequences. Antioxid Redox Signal. 2014;20:887–898. doi: 10.1089/ars.2013.5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brower JB, Targovnik JH, Caplan MR, Massia SP. High glucose-mediated loss of cell surface heparan sulfate proteoglycan impairs the endothelial shear stress response. Cytoskelet Hoboken NJ. 2010;67:135–141. doi: 10.1002/cm.20430. [DOI] [PubMed] [Google Scholar]
- 18.Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J Am Coll Cardiol. 2007;49:2379–2393. doi: 10.1016/j.jacc.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 19.Chen Z, Bakhshi FR, Shajahan AN, Sharma T, Mao M, Trane A, Bernatchez P, van Nieuw Amerongen GP, Bonini MG, Skidgel RA, Malik AB, Minshall RD. Nitric oxide-dependent Src activation and resultant caveolin-1 phosphorylation promote eNOS/caveolin-1 binding and eNOS inhibition. Mol Biol Cell. 2012;23:1388–1398. doi: 10.1091/mbc.E11-09-0811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Z, SDS O, Zimnicka AM, Jiang Y, Sharma T, Chen S, Lazarov O, Bonini MG, Haus JM, Minshall RD. Reciprocal regulation of eNOS and caveolin-1 functions in endothelial cells. Mol Biol Cell. 2018;29:1190–1202. doi: 10.1091/mbc.E17-01-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chistiakov DA, Orekhov AN, Bobryshev YV. Effects of shear stress on endothelial cells: go with the flow. Acta Physiol Oxf Engl. 2017;219:382–408. doi: 10.1111/apha.12725. [DOI] [PubMed] [Google Scholar]
- 22.Chiu J-J, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91:327–387. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiu J-J, Chen L-J, Chen C-N, Lee P-L, Lee C-I. A model for studying the effect of shear stress on interactions between vascular endothelial cells and smooth muscle cells. J Biomech. 2004;37:531–539. doi: 10.1016/j.jbiomech.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Christensen AP, Corey DP. TRP channels in mechanosensation: direct or indirect activation? Nat Rev Neurosci. 2007;8:510–521. doi: 10.1038/nrn2149. [DOI] [PubMed] [Google Scholar]
- 25.Constantinescu AA, Vink H, Spaan JAE. Endothelial cell glycocalyx modulates immobilization of leukocytes at the endothelial surface. Arterioscler Thromb Vasc Biol. 2003;23:1541–1547. doi: 10.1161/01.ATV.0000085630.24353.3D. [DOI] [PubMed] [Google Scholar]
- 26.Conway DE, Coon BG, Budatha M, Arsenovic PT, Orsenigo F, Wessel F, Zhang J, Zhuang Z, Dejana E, Vestweber D, Schwartz MA. VE-cadherin phosphorylation regulates endothelial fluid shear stress responses through the polarity protein LGN. Curr Biol CB. 2017;27:2219–2225.e5. doi: 10.1016/j.cub.2017.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coon BG, Baeyens N, Han J, Budatha M, Ross TD, Fang JS, Yun S, Thomas J-L, Schwartz MA. Intramembrane binding of VE-cadherin to VEGFR2 and VEGFR3 assembles the endothelial mechanosensory complex. J Cell Biol. 2015;208:975–986. doi: 10.1083/jcb.201408103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cosgun ZC, Fels B, Kusche-Vihrog K (2020) Nanomechanics of the endothelial glycocalyx: from structure to function. Am J Pathol. 10.1016/j.ajpath.2019.07.021 [DOI] [PubMed]
- 29.Coste B, Xiao B, Santos JS, Syeda R, Grandl J, Spencer KS, Kim SE, Schmidt M, Mathur J, Dubin AE, Montal M, Patapoutian A. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature. 2012;483:176–181. doi: 10.1038/nature10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dalrymple A, Mahn K, Poston L, Songu-Mize E, Tribe RM. Mechanical stretch regulates TRPC expression and calcium entry in human myometrial smooth muscle cells. Mol Hum Reprod. 2007;13:171–179. doi: 10.1093/molehr/gal110. [DOI] [PubMed] [Google Scholar]
- 31.Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995;75:519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med. 2009;6:16–26. doi: 10.1038/ncpcardio1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davies PF, Robotewskyj A, Griem ML, Dull RO, Polacek DC. Hemodynamic forces and vascular cell communication in arteries. Arch Pathol Lab Med. 1992;116:1301–1306. [PubMed] [Google Scholar]
- 34.de Souza LB, Ambudkar IS. Trafficking mechanisms and regulation of TRPC channels. Cell Calcium. 2014;56:43–50. doi: 10.1016/j.ceca.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 35.Dejana E. Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol. 2004;5:261–270. doi: 10.1038/nrm1357. [DOI] [PubMed] [Google Scholar]
- 36.Dela Paz NG, Melchior B, Frangos JA. Shear stress induces Gαq/11 activation independently of G protein-coupled receptor activation in endothelial cells. Am J Physiol Cell Physiol. 2017;312:C428–C437. doi: 10.1152/ajpcell.00148.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Delgadillo LF, Marsh GA, Waugh RE (2020) Endothelial glycocalyx layer properties and its ability to limit leukocyte adhesion. Biophys J. 10.1016/j.bpj.2020.02.010 [DOI] [PMC free article] [PubMed]
- 38.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 39.Dragovich MA, Chester D, Fu BM, Wu C, Xu Y, Goligorsky MS, Zhang XF. Mechanotransduction of the endothelial glycocalyx mediates nitric oxide production through activation of TRP channels. Am J Physiol Cell Physiol. 2016;311:C846–C853. doi: 10.1152/ajpcell.00288.2015. [DOI] [PubMed] [Google Scholar]
- 40.Drost CC, Rovas A, Kusche-Vihrog K, Van Slyke P, Kim H, Hoang VC, Maynes JT, Wennmann DO, Pavenstädt H, Linke W, Lukasz A, Hesse B, Kümpers P. Tie2 Activation promotes protection and reconstitution of the endothelial glycocalyx in human sepsis. Thromb Haemost. 2019;119:1827–1838. doi: 10.1055/s-0039-1695768. [DOI] [PubMed] [Google Scholar]
- 41.Drummond HA, Jernigan NL, Grifoni SC. Sensing tension: epithelial sodium channel/acid-sensing ion channel proteins in cardiovascular homeostasis. Hypertens Dallas Tex 1979. 2008;51:1265–1271. doi: 10.1161/HYPERTENSIONAHA.107.093401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Du J, Ma X, Shen B, Huang Y, Birnbaumer L, Yao X. TRPV4, TRPC1, and TRPP2 assemble to form a flow-sensitive heteromeric channel. FASEB J Off Publ Fed Am Soc Exp Biol. 2014;28:4677–4685. doi: 10.1096/fj.14-251652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Earley S, Brayden JE. Transient receptor potential channels in the vasculature. Physiol Rev. 2015;95:645–690. doi: 10.1152/physrev.00026.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eder P, Molkentin JD. TRPC channels as effectors of cardiac hypertrophy. Circ Res. 2011;108:265–272. doi: 10.1161/CIRCRESAHA.110.225888. [DOI] [PubMed] [Google Scholar]
- 45.Egorova AD, van der Heiden K, Poelmann RE, Hierck BP. Primary cilia as biomechanical sensors in regulating endothelial function. Differ Res Biol Divers. 2012;83:S56–S61. doi: 10.1016/j.diff.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 46.Endemann DH, Schiffrin EL. Endothelial dysfunction. J Am Soc Nephrol JASN. 2004;15:1983–1992. doi: 10.1097/01.ASN.0000132474.50966.DA. [DOI] [PubMed] [Google Scholar]
- 47.Fabian A, Bertrand J, Lindemann O, Pap T, Schwab A. Transient receptor potential canonical channel 1 impacts on mechanosignaling during cell migration. Pflüg Arch Eur J Physiol. 2012;464:623–630. doi: 10.1007/s00424-012-1169-9. [DOI] [PubMed] [Google Scholar]
- 48.Fels J, Kusche-Vihrog K (2018) Endothelial nanomechanics in the context of endothelial (Dys)function and inflammation. Antioxid Redox Signal. 10.1089/ars.2017.7327 [DOI] [PMC free article] [PubMed]
- 49.Fels J, Callies C, Kusche-Vihrog K, Oberleithner H. Nitric oxide release follows endothelial nanomechanics and not vice versa. Pflugers Arch. 2010;460:915–923. doi: 10.1007/s00424-010-0871-8. [DOI] [PubMed] [Google Scholar]
- 50.Fels J, Oberleithner H, Kusche-Vihrog K. Ménage à trois: aldosterone, sodium and nitric oxide in vascular endothelium. Biochim Biophys Acta (BBA) - Mol Basis Dis. 2010;1802:1193–1202. doi: 10.1016/j.bbadis.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 51.Fels J, Jeggle P, Kusche-Vihrog K, Oberleithner H. Cortical actin nanodynamics determines nitric oxide release in vascular endothelium. PloS One. 2012;7:e41520. doi: 10.1371/journal.pone.0041520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fels J, Jeggle P, Liashkovich I, Peters W, Oberleithner H. Nanomechanics of vascular endothelium. Cell Tissue Res. 2014;355:727–737. doi: 10.1007/s00441-014-1853-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fels B, Nielsen N, Schwab A (2016) Role of TRPC1 channels in pressure-mediated activation of murine pancreatic stellate cells. Eur Biophys J:1–14. 10.1007/s00249-016-1176-4 [DOI] [PubMed]
- 54.Filippini A, Sica G, D’Alessio A. The caveolar membrane system in endothelium: from cell signaling to vascular pathology. J Cell Biochem. 2018;119:5060–5071. doi: 10.1002/jcb.26793. [DOI] [PubMed] [Google Scholar]
- 55.Fleming I, Busse R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1–R12. doi: 10.1152/ajpregu.00323.2002. [DOI] [PubMed] [Google Scholar]
- 56.Fleming I, Rueben A, Popp R, Fisslthaler B, Schrodt S, Sander A, Haendeler J, Falck JR, Morisseau C, Hammock BD, Busse R. Epoxyeicosatrienoic acids regulate Trp channel dependent Ca2+ signaling and hyperpolarization in endothelial cells. Arterioscler Thromb Vasc Biol. 2007;27:2612–2618. doi: 10.1161/ATVBAHA.107.152074. [DOI] [PubMed] [Google Scholar]
- 57.Florian JA, Kosky JR, Ainslie K, Pang Z, Dull RO, Tarbell JM. Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ Res. 2003;93:e136–e142. doi: 10.1161/01.RES.0000101744.47866.D5. [DOI] [PubMed] [Google Scholar]
- 58.Fraser JR, Laurent TC, Laurent UB. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med. 1997;242:27–33. doi: 10.1046/j.1365-2796.1997.00170.x. [DOI] [PubMed] [Google Scholar]
- 59.Fukai N, Kenagy RD, Chen L, Gao L, Daum G, Clowes AW. Syndecan-1: an inhibitor of arterial smooth muscle cell growth and intimal hyperplasia. Arterioscler Thromb Vasc Biol. 2009;29:1356–1362. doi: 10.1161/ATVBAHA.109.190132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gao X, Wu L, O’Neil RG. Temperature-modulated Diversity of TRPV4 Channel Gating activation by physical stresses and phorbol ester derivatives through protein kinase C-dependent and -independent pathways. J Biol Chem. 2003;278:27129–27137. doi: 10.1074/jbc.M302517200. [DOI] [PubMed] [Google Scholar]
- 61.García-Cardeña G, Oh P, Liu J, Schnitzer JE, Sessa WC. Targeting of nitric oxide synthase to endothelial cell caveolae via palmitoylation: implications for nitric oxide signaling. Proc Natl Acad Sci U S A. 1996;93:6448–6453. doi: 10.1073/pnas.93.13.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Garty H, Palmer LG. Epithelial sodium channels: function, structure, and regulation. Physiol Rev. 1997;77:359–396. doi: 10.1152/physrev.1997.77.2.359. [DOI] [PubMed] [Google Scholar]
- 63.Gottlieb P, Folgering J, Maroto R, Raso A, Wood TG, Kurosky A, Bowman C, Bichet D, Patel A, Sachs F, Martinac B, Hamill OP, Honoré E. Revisiting TRPC1 and TRPC6 mechanosensitivity. Pflüg Arch Eur J Physiol. 2008;455:1097–1103. doi: 10.1007/s00424-007-0359-3. [DOI] [PubMed] [Google Scholar]
- 64.Gouverneur M, Berg B, Nieuwdorp M, Stroes E, Vink H. Vasculoprotective properties of the endothelial glycocalyx: effects of fluid shear stress. J Intern Med. 2006;259:393–400. doi: 10.1111/j.1365-2796.2006.01625.x. [DOI] [PubMed] [Google Scholar]
- 65.Greenberg HZE, Carlton-Carew SRE, Khan DM, Zargaran AK, Jahan KS, Vanessa Ho W-S, Albert AP. Heteromeric TRPV4/TRPC1 channels mediate calcium-sensing receptor-induced nitric oxide production and vasorelaxation in rabbit mesenteric arteries. Vasc Pharmacol. 2017;96–98:53–62. doi: 10.1016/j.vph.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Greenberg HZE, Carlton-Carew SRE, Zargaran AK, Jahan KS, Birnbaumer L, Albert AP (2019) Heteromeric TRPV4/TRPC1 channels mediate calcium-sensing receptor-induced relaxations and nitric oxide production in mesenteric arteries: comparative study using wild-type and TRPC1-/- mice. Channels (Austin):1–14. 10.1080/19336950.2019.1673131 [DOI] [PMC free article] [PubMed]
- 67.Guo D, Liang S, Wang S, Tang C, Yao B, Wan W, Zhang H, Jiang H, Ahmed A, Zhang Z, Gu Y. Role of epithelial Na+ channels in endothelial function. J Cell Sci. 2016;129:290–297. doi: 10.1242/jcs.168831. [DOI] [PubMed] [Google Scholar]
- 68.Haase K, Pelling AE (2015) Investigating cell mechanics with atomic force microscopy. J R Soc Interface 12. 10.1098/rsif.2014.0970 [DOI] [PMC free article] [PubMed]
- 69.Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H. Role of oxidative stress in atherosclerosis. Am J Cardiol. 2003;91:7A–11A. doi: 10.1016/s0002-9149(02)03144-2. [DOI] [PubMed] [Google Scholar]
- 70.Hartmannsgruber V, Heyken W-T, Kacik M, Kaistha A, Grgic I, Harteneck C, Liedtke W, Hoyer J, Köhler R. Arterial response to shear stress critically depends on endothelial TRPV4 expression. PloS One. 2007;2:e827. doi: 10.1371/journal.pone.0000827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hayakawa K, Tatsumi H, Sokabe M. Actin filaments function as a tension sensor by tension-dependent binding of cofilin to the filament. J Cell Biol. 2011;195:721–727. doi: 10.1083/jcb.201102039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hecker M, Mülsch A, Bassenge E, Förstermann U, Busse R. Subcellular localization and characterization of nitric oxide synthase(s) in endothelial cells: physiological implications. Biochem J. 1994;299:247–252. doi: 10.1042/bj2990247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hill-Eubanks DC, Gonzales AL, Sonkusare SK, Nelson MT. Vascular TRP channels: performing under pressure and going with the flow. Physiol Bethesda Md. 2014;29:343–360. doi: 10.1152/physiol.00009.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- 75.Huveneers S, Oldenburg J, Spanjaard E, van der Krogt G, Grigoriev I, Akhmanova A, Rehmann H, de Rooij J. Vinculin associates with endothelial VE-cadherin junctions to control force-dependent remodeling. J Cell Biol. 2012;196:641–652. doi: 10.1083/jcb.201108120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hwang J, Ing MH, Salazar A, Lassègue B, Griendling K, Navab M, Sevanian A, Hsiai TK. Pulsatile versus oscillatory shear stress regulates NADPH oxidase subunit expression: implication for native LDL oxidation. Circ Res. 2003;93:1225–1232. doi: 10.1161/01.RES.0000104087.29395.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hyman AJ, Tumova S, Beech DJ. Piezo1 channels in vascular development and the sensing of shear stress. Curr Top Membr. 2017;79:37–57. doi: 10.1016/bs.ctm.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 78.Ilatovskaya DV, Pavlov TS, Levchenko V, Negulyaev YA, Staruschenko A. Cortical actin binding protein cortactin mediates ENaC activity via Arp2/3 complex. FASEB J Off Publ Fed Am Soc Exp Biol. 2011;25:2688–2699. doi: 10.1096/fj.10-167262. [DOI] [PubMed] [Google Scholar]
- 79.Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J Off Publ Fed Am Soc Exp Biol. 2006;20:811–827. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- 80.Ingber DE, Wang N, Stamenovic D. Tensegrity, cellular biophysics, and the mechanics of living systems. Rep Prog Phys Phys Soc G B. 2014;77:046603. doi: 10.1088/0034-4885/77/4/046603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Inoue R, Jian Z, Kawarabayashi Y. Mechanosensitive TRP channels in cardiovascular pathophysiology. Pharmacol Ther. 2009;123:371–385. doi: 10.1016/j.pharmthera.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 82.Iomini C, Tejada K, Mo W, Vaananen H, Piperno G. Primary cilia of human endothelial cells disassemble under laminar shear stress. J Cell Biol. 2004;164:811–817. doi: 10.1083/jcb.200312133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jeggle P, Callies C, Tarjus A, Fassot C, Fels J, Oberleithner H, Jaisser F, Kusche-Vihrog K. Epithelial sodium channel stiffens the vascular endothelium in vitro and in Liddle mice. Hypertens Dallas Tex 1979. 2013;61:1053–1059. doi: 10.1161/HYPERTENSIONAHA.111.199455. [DOI] [PubMed] [Google Scholar]
- 84.Ji JY. Endothelial nuclear lamina in mechanotransduction under shear stress. Adv Exp Med Biol. 2018;1097:83–104. doi: 10.1007/978-3-319-96445-4_5. [DOI] [PubMed] [Google Scholar]
- 85.Jin Z-G, Ueba H, Tanimoto T, Lungu AO, Frame MD, Berk BC. Ligand-independent activation of vascular endothelial growth factor receptor 2 by fluid shear stress regulates activation of endothelial nitric oxide synthase. Circ Res. 2003;93:354–363. doi: 10.1161/01.RES.0000089257.94002.96. [DOI] [PubMed] [Google Scholar]
- 86.Jones BJ, Murphy CR. A high resolution study of the glycocalyx of rat uterine epithelial cells during early pregnancy with the field emission gun scanning electron microscope. J Anat. 1994;185(Pt 2):443–446. [PMC free article] [PubMed] [Google Scholar]
- 87.Kamiya A, Bukhari R, Togawa T. Adaptive regulation of wall shear stress optimizing vascular tree function. Bull Math Biol. 1984;46:127–137. doi: 10.1016/S0092-8240(84)80038-5. [DOI] [PubMed] [Google Scholar]
- 88.Kasas S, Wang X, Hirling H, Marsault R, Huni B, Yersin A, Regazzi R, Grenningloh G, Riederer B, Forrò L, Dietler G, Catsicas S. Superficial and deep changes of cellular mechanical properties following cytoskeleton disassembly. Cell Motil Cytoskeleton. 2005;62:124–132. doi: 10.1002/cm.20086. [DOI] [PubMed] [Google Scholar]
- 89.Kim E-C, Choi S-K, Lim M, Yeon S-I, Lee Y-H. Role of endogenous ENaC and TRP channels in the myogenic response of rat posterior cerebral arteries. PloS One. 2013;8:e84194. doi: 10.1371/journal.pone.0084194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Knoepp F, Ashley Z, Barth D, Baldin J-P, Jennings M, Kazantseva M, Saw EL, Katare R, Alvarez de la Rosa D, Weissmann N, Fronius M. Shear force sensing of epithelial Na+ channel (ENaC) relies on N-glycosylated asparagines in the palm and knuckle domains of αENaC. Proc Natl Acad Sci U S A. 2020;117:717–726. doi: 10.1073/pnas.1911243117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Köhler R, Heyken W-T, Heinau P, Schubert R, Si H, Kacik M, Busch C, Grgic I, Maier T, Hoyer J. Evidence for a functional role of endothelial transient receptor potential V4 in shear stress-induced vasodilatation. Arterioscler Thromb Vasc Biol. 2006;26:1495–1502. doi: 10.1161/01.ATV.0000225698.36212.6a. [DOI] [PubMed] [Google Scholar]
- 92.Kress M, Karasek J, Ferrer-Montiel AV, Scherbakov N, Haberberger RV. TRPC channels and diacylglycerol dependent calcium signaling in rat sensory neurons. Histochem Cell Biol. 2008;130:655–667. doi: 10.1007/s00418-008-0477-9. [DOI] [PubMed] [Google Scholar]
- 93.Kumar S, Maxwell IZ, Heisterkamp A, Polte TR, Lele TP, Salanga M, Mazur E, Ingber DE. Viscoelastic retraction of single living stress fibers and its impact on cell shape, cytoskeletal organization, and extracellular matrix mechanics. Biophys J. 2006;90:3762–3773. doi: 10.1529/biophysj.105.071506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kuwahara K, Wang Y, McAnally J, Richardson JA, Bassel-Duby R, Hill JA, Olson EN. TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J Clin Invest. 2006;116:3114–3126. doi: 10.1172/JCI27702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lang F (2011) Stiff endothelial cell syndrome in vascular inflammation and mineralocorticoid excess. Hypertension [DOI] [PubMed]
- 96.LeBlanc ME, Saez-Torres KL, Cano I, Hu Z, Saint-Geniez M, Ng Y-S, D’Amore PA. Glycocalyx regulation of vascular endothelial growth factor receptor 2 activity. FASEB J Off Publ Fed Am Soc Exp Biol. 2019;33:9362–9373. doi: 10.1096/fj.201900011R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee HJ, Koh GY. Shear stress activates Tie2 receptor tyrosine kinase in human endothelial cells. Biochem Biophys Res Commun. 2003;304:399–404. doi: 10.1016/s0006-291x(03)00592-8. [DOI] [PubMed] [Google Scholar]
- 98.Lee J, Packard RRS, Hsiai TK. Blood flow modulation of vascular dynamics. Curr Opin Lipidol. 2015;26:376–383. doi: 10.1097/MOL.0000000000000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Leung P-C, Cheng K-T, Liu C, Cheung W-T, Kwan H-Y, Lau K-L, Huang Y, Yao X. Mechanism of non-capacitative Ca2+ influx in response to bradykinin in vascular endothelial cells. J Vasc Res. 2006;43:367–376. doi: 10.1159/000094096. [DOI] [PubMed] [Google Scholar]
- 100.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 101.Lhomme A, Gilbert G, Pele T, Deweirdt J, Henrion D, Baudrimont I, Campagnac M, Marthan R, Guibert C, Ducret T, Savineau J-P, Quignard J-F. Stretch-activated Piezo1 channel in endothelial cells relaxes mouse intrapulmonary arteries. Am J Respir Cell Mol Biol. 2019;60:650–658. doi: 10.1165/rcmb.2018-0197OC. [DOI] [PubMed] [Google Scholar]
- 102.Li R, Mittelstein D, Lee J, Fang K, Majumdar R, Tintut Y, Demer LL, Hsiai TK. A dynamic model of calcific nodule destabilization in response to monocyte- and oxidized lipid-induced matrix metalloproteinases. Am J Physiol Cell Physiol. 2012;302:C658–C665. doi: 10.1152/ajpcell.00313.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li J, Hou B, Tumova S, Muraki K, Bruns A, Ludlow MJ, Sedo A, Hyman AJ, McKeown L, Young RS, Yuldasheva NY, Majeed Y, Wilson LA, Rode B, Bailey MA, Kim HR, Fu Z, Carter DA, Bilton J, Imrie H, Ajuh P, Dear TN, Cubbon RM, Kearney MT, Prasad RK, Evans PC, Ainscough JF, Beech DJ. Piezo1 integration of vascular architecture with physiological force. Nature. 2014;515:279–282. doi: 10.1038/nature13701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liedtke W, Choe Y, Martí-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103:525–535. doi: 10.1016/S0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu Y, Sweet DT, Irani-Tehrani M, Maeda N, Tzima E. Shc coordinates signals from intercellular junctions and integrins to regulate flow-induced inflammation. J Cell Biol. 2008;182:185–196. doi: 10.1083/jcb.200709176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu Y-S, Liu Y-A, Huang C-J, Yen M-H, Tseng C-T, Chien S, Lee OK. Mechanosensitive TRPM7 mediates shear stress and modulates osteogenic differentiation of mesenchymal stromal cells through Osterix pathway. Sci Rep. 2015;5:16522. doi: 10.1038/srep16522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Loot AE, Popp R, Fisslthaler B, Vriens J, Nilius B, Fleming I. Role of cytochrome P450-dependent transient receptor potential V4 activation in flow-induced vasodilatation. Cardiovasc Res. 2008;80:445–452. doi: 10.1093/cvr/cvn207. [DOI] [PubMed] [Google Scholar]
- 108.Lopez-Quintero SV, Cancel LM, Pierides A, Antonetti D, Spray DC, Tarbell JM. High glucose attenuates shear-induced changes in endothelial hydraulic conductivity by degrading the glycocalyx. PloS One. 2013;8:e78954. doi: 10.1371/journal.pone.0078954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lukasz A, Hillgruber C, Oberleithner H, Kusche-Vihrog K, Pavenstädt H, Rovas A, Hesse B, Goerge T, Kümpers P. Endothelial glycocalyx breakdown is mediated by angiopoietin-2. Cardiovasc Res. 2017;113:671–680. doi: 10.1093/cvr/cvx023. [DOI] [PubMed] [Google Scholar]
- 110.Ma X, Cheng K-T, Wong C-O, O’Neil RG, Birnbaumer L, Ambudkar IS, Yao X. Heteromeric TRPV4-C1 channels contribute to store-operated Ca(2+) entry in vascular endothelial cells. Cell Calcium. 2011;50:502–509. doi: 10.1016/j.ceca.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA. 1999;282:2035–2042. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- 112.Malsch P, Andratsch M, Vogl C, Link AS, Alzheimer C, Brierley SM, Hughes PA, Kress M. Deletion of interleukin-6 signal transducer gp130 in small sensory neurons attenuates mechanonociception and down-regulates TRPA1 expression. J Neurosci. 2014;34:9845–9856. doi: 10.1523/JNEUROSCI.5161-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mammoto A, Mammoto T, Ingber DE. Mechanosensitive mechanisms in transcriptional regulation. J Cell Sci. 2012;125:3061–3073. doi: 10.1242/jcs.093005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Maroto R, Raso A, Wood TG, Kurosky A, Martinac B, Hamill OP. TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nat Cell Biol. 2005;7:179–185. doi: 10.1038/ncb1218. [DOI] [PubMed] [Google Scholar]
- 115.Martino F, Perestrelo AR, Vinarský V, Pagliari S, Forte G (2018) Cellular mechanotransduction: from tension to function. Front Physiol 9. 10.3389/fphys.2018.00824 [DOI] [PMC free article] [PubMed]
- 116.Mathar I, Vennekens R, Meissner M, Kees F, Van der Mieren G, Camacho Londoño JE, Uhl S, Voets T, Hummel B, van den Bergh A, Herijgers P, Nilius B, Flockerzi V, Schweda F, Freichel M. Increased catecholamine secretion contributes to hypertension in TRPM4-deficient mice. J Clin Invest. 2010;120:3267–3279. doi: 10.1172/JCI41348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Matthews BD, Thodeti CK, Tytell JD, Mammoto A, Overby DR, Ingber DE. Ultra-rapid activation of TRPV4 ion channels by mechanical forces applied to cell surface beta1 integrins. Integr Biol Quant Biosci Nano Macro. 2010;2:435–442. doi: 10.1039/c0ib00034e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mazzochi C, Bubien JK, Smith PR, Benos DJ. The carboxyl terminus of the alpha-subunit of the amiloride-sensitive epithelial sodium channel binds to F-actin. J Biol Chem. 2006;281:6528–6538. doi: 10.1074/jbc.M509386200. [DOI] [PubMed] [Google Scholar]
- 119.Mederos y Schnitzler M, Storch U, Meibers S, Nurwakagari P, Breit A, Essin K, Gollasch M, Gudermann T. Gq-coupled receptors as mechanosensors mediating myogenic vasoconstriction. EMBO J. 2008;27:3092–3103. doi: 10.1038/emboj.2008.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mendoza SA, Fang J, Gutterman DD, Wilcox DA, Bubolz AH, Li R, Suzuki M, Zhang DX. TRPV4-mediated endothelial Ca2+ influx and vasodilation in response to shear stress. Am J Physiol Heart Circ Physiol. 2010;298:H466–H476. doi: 10.1152/ajpheart.00854.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mulivor AW, Lipowsky HH. Inflammation- and ischemia-induced shedding of venular glycocalyx. Am J Physiol Heart Circ Physiol. 2004;286:H1672–H1680. doi: 10.1152/ajpheart.00832.2003. [DOI] [PubMed] [Google Scholar]
- 122.Murthy SE, Dubin AE, Patapoutian A. Piezos thrive under pressure: mechanically activated ion channels in health and disease. Nat Rev Mol Cell Biol. 2017;18:771–783. doi: 10.1038/nrm.2017.92. [DOI] [PubMed] [Google Scholar]
- 123.Naetar N, Ferraioli S, Foisner R. Lamins in the nuclear interior - life outside the lamina. J Cell Sci. 2017;130:2087–2096. doi: 10.1242/jcs.203430. [DOI] [PubMed] [Google Scholar]
- 124.Naruse K. Mechanomedicine. Biophys Rev. 2018;10:1257–1262. doi: 10.1007/s12551-018-0459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nauli SM, Kawanabe Y, Kaminski JJ, Pearce WJ, Ingber DE, Zhou J. Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation. 2008;117:1161–1171. doi: 10.1161/CIRCULATIONAHA.107.710111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nauli SM, Jin X, AbouAlaiwi WA, El-Jouni W, Su X, Zhou J. Non-motile primary cilia as fluid shear stress mechanosensors. Methods Enzymol. 2013;525:1–20. doi: 10.1016/B978-0-12-397944-5.00001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nilius B, Owsianik G. Transient receptor potential channelopathies. Pflugers Arch. 2010;460:437–450. doi: 10.1007/s00424-010-0788-2. [DOI] [PubMed] [Google Scholar]
- 128.Nilius B, Owsianik G. The transient receptor potential family of ion channels. Genome Biol. 2011;12:218. doi: 10.1186/gb-2011-12-3-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nishizaka MK, Amin ZM, Green SA, Renfroe KY, Calhoun DA. Impaired endothelium-dependent flow-mediated vasodilation in hypertensive subjects with hyperaldosteronism. Circulation. 2004;109:2857–2861. doi: 10.1161/01.CIR.0000129307.26791.8E. [DOI] [PubMed] [Google Scholar]
- 130.Oberleithner H, Callies C, Kusche-Vihrog K, Schillers H, Shahin V, Riethmüller C, Macgregor GA, de Wardener HE. Potassium softens vascular endothelium and increases nitric oxide release. Proc Natl Acad Sci U S A. 2009;106:2829–2834. doi: 10.1073/pnas.0813069106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Oda T, Makino K, Yamashita I, Namba K, Maéda Y. Distinct structural changes detected by X-ray fiber diffraction in stabilization of F-actin by lowering pH and increasing ionic strength. Biophys J. 2001;80:841–851. doi: 10.1016/S0006-3495(01)76063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ohashi K, Fujiwara S, Mizuno K. Roles of the cytoskeleton, cell adhesion and rho signalling in mechanosensing and mechanotransduction. J Biochem (Tokyo) 2017;161:245–254. doi: 10.1093/jb/mvw082. [DOI] [PubMed] [Google Scholar]
- 133.Ohba T, Watanabe H, Murakami M, Takahashi Y, Iino K, Kuromitsu S, Mori Y, Ono K, Iijima T, Ito H. Upregulation of TRPC1 in the development of cardiac hypertrophy. J Mol Cell Cardiol. 2007;42:498–507. doi: 10.1016/j.yjmcc.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 134.Orr AW, Murphy-Ullrich JE. Regulation of endothelial cell function BY FAK and PYK2. Front Biosci J Virtual Libr. 2004;9:1254–1266. doi: 10.2741/1239. [DOI] [PubMed] [Google Scholar]
- 135.Osmanagic-Myers S, Dechat T, Foisner R. Lamins at the crossroads of mechanosignaling. Genes Dev. 2015;29:225–237. doi: 10.1101/gad.255968.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Patel A, Sharif-Naeini R, Folgering JRH, Bichet D, Duprat F, Honoré E. Canonical TRP channels and mechanotransduction: from physiology to disease states. Pflüg Arch Eur J Physiol. 2010;460:571–581. doi: 10.1007/s00424-010-0847-8. [DOI] [PubMed] [Google Scholar]
- 137.Pelham RJ, Wang Y l. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci U S A. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pemp B, Weigert G, Karl K, Petzl U, Wolzt M, Schmetterer L, Garhofer G. Correlation of flicker-induced and flow-mediated vasodilatation in patients with endothelial dysfunction and healthy volunteers. Diabetes Care. 2009;32:1536–1541. doi: 10.2337/dc08-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Qiu Y, Tarbell JM. Interaction between wall shear stress and circumferential strain affects endothelial cell biochemical production. J Vasc Res. 2000;37:147–157. doi: 10.1159/000025726. [DOI] [PubMed] [Google Scholar]
- 140.Qu Y-Y, Wang L-M, Zhong H, Liu Y-M, Tang N, Zhu L-P, He F, Hu Q-H. TRPC1 stimulates calcium-sensing receptor-induced store-operated Ca2+ entry and nitric oxide production in endothelial cells. Mol Med Rep. 2017;16:4613–4619. doi: 10.3892/mmr.2017.7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rafiq NBM, Nishimura Y, Plotnikov SV, Thiagarajan V, Zhang Z, Shi S, Natarajan M, Viasnoff V, Kanchanawong P, Jones GE, Bershadsky AD. A mechano-signalling network linking microtubules, myosin IIA filaments and integrin-based adhesions. Nat Mater. 2019;18:638–649. doi: 10.1038/s41563-019-0371-y. [DOI] [PubMed] [Google Scholar]
- 142.Ranade SS, Qiu Z, Woo S-H, Hur SS, Murthy SE, Cahalan SM, Xu J, Mathur J, Bandell M, Coste B, Li Y-SJ, Chien S, Patapoutian A. Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc Natl Acad Sci U S A. 2014;111:10347–10352. doi: 10.1073/pnas.1409233111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Reitsma S, Slaaf DW, Vink H, van Zandvoort MAMJ, oude Egbrink MGA. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch. 2007;454:345–359. doi: 10.1007/s00424-007-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Reneman RS, Arts T, Hoeks APG. Wall shear stress--an important determinant of endothelial cell function and structure--in the arterial system in vivo. Discrepancies with theory. J Vasc Res. 2006;43:251–269. doi: 10.1159/000091648. [DOI] [PubMed] [Google Scholar]
- 145.Retailleau K, Duprat F, Arhatte M, Ranade SS, Peyronnet R, Martins JR, Jodar M, Moro C, Offermanns S, Feng Y, Demolombe S, Patel A, Honoré E. Piezo1 in smooth muscle cells is involved in hypertension-dependent arterial remodeling. Cell Rep. 2015;13:1161–1171. doi: 10.1016/j.celrep.2015.09.072. [DOI] [PubMed] [Google Scholar]
- 146.Rosenberg RD, Shworak NW, Liu J, Schwartz JJ, Zhang L. Heparan sulfate proteoglycans of the cardiovascular system. Specific structures emerge but how is synthesis regulated? J Clin Invest. 1997;99:2062–2070. doi: 10.1172/JCI119377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ryazanova LV, Hu Z, Suzuki S, Chubanov V, Fleig A, Ryazanov AG. Elucidating the role of the TRPM7 alpha-kinase: TRPM7 kinase inactivation leads to magnesium deprivation resistance phenotype in mice. Sci Rep. 2014;4:1–11. doi: 10.1038/srep07599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ryuji I, Jensen LJ, Zhong J, Juan S, Lin H, Lurie AI, Henriksen FH, Max S, Hiromitsu M, Yasuhiro K, Masayuki M, Yasuo M, Yushi I. Synergistic activation of vascular TRPC6 channel by receptor and mechanical stimulation via phospholipase C/diacylglycerol and phospholipase A2/ω-hydroxylase/20-HETE pathways. Circ Res. 2009;104:1399–1409. doi: 10.1161/CIRCRESAHA.108.193227. [DOI] [PubMed] [Google Scholar]
- 149.Salmon AHJ, Satchell SC. Endothelial glycocalyx dysfunction in disease: albuminuria and increased microvascular permeability. J Pathol. 2012;226:562–574. doi: 10.1002/path.3964. [DOI] [PubMed] [Google Scholar]
- 150.Samapati R, Yang Y, Yin J, Stoerger C, Arenz C, Dietrich A, Gudermann T, Adam D, Wu S, Freichel M, Flockerzi V, Uhlig S, Kuebler WM. Lung endothelial Ca2+ and permeability response to platelet-activating factor is mediated by acid sphingomyelinase and transient receptor potential classical 6. Am J Respir Crit Care Med. 2012;185:160–170. doi: 10.1164/rccm.201104-0717OC. [DOI] [PubMed] [Google Scholar]
- 151.Sarrazin S, Lamanna WC, Esko JD (2011) Heparan sulfate proteoglycans. Cold Spring Harb Perspect Biol 3. 10.1101/cshperspect.a004952 [DOI] [PMC free article] [PubMed]
- 152.Satlin LM, Sheng S, Woda CB, Kleyman TR. Epithelial Na(+) channels are regulated by flow. Am J Physiol Renal Physiol. 2001;280:F1010–F1018. doi: 10.1152/ajprenal.2001.280.6.F1010. [DOI] [PubMed] [Google Scholar]
- 153.Schierke F, Wyrwoll MJ, Wisdorf M, Niedzielski L, Maase M, Ruck T, Meuth SG, Kusche-Vihrog K. Nanomechanics of the endothelial glycocalyx contribute to Na+-induced vascular inflammation. Sci Rep. 2017;7:46476. doi: 10.1038/srep46476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sessa WC. eNOS at a glance. J Cell Sci. 2004;117:2427–2429. doi: 10.1242/jcs.01165. [DOI] [PubMed] [Google Scholar]
- 155.Seth M, Zhang Z-S, Mao L, Graham V, Burch J, Stiber J, Tsiokas L, Winn M, Abramowitz J, Rockman HA, Birnbaumer L, Rosenberg P. TRPC1 channels are critical for hypertrophic signaling in the heart. Circ Res. 2009;105:1023–1030. doi: 10.1161/CIRCRESAHA.109.206581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Shaul PW, Smart EJ, Robinson LJ, German Z, Yuhanna IS, Ying Y, Anderson RG, Michel T. Acylation targets emdothelial nitric-oxide synthase to plasmalemmal caveolae. J Biol Chem. 1996;271:6518–6522. doi: 10.1074/jbc.271.11.6518. [DOI] [PubMed] [Google Scholar]
- 157.Shin H, Haga JH, Kosawada T, Kimura K, Li YS, Chien S, Schmid-Schönbein GW. Fine control of endothelial VEGFR-2 activation: caveolae as fluid shear stress shelters for membrane receptors. Biomech Model Mechanobiol. 2019;18:5–16. doi: 10.1007/s10237-018-1063-2. [DOI] [PubMed] [Google Scholar]
- 158.Sidi S, Friedrich RW, Nicolson T. NompC TRP channel required for vertebrate sensory hair cell mechanotransduction. Science. 2003;301:96–99. doi: 10.1126/science.1084370. [DOI] [PubMed] [Google Scholar]
- 159.Sieve I, Münster-Kühnel AK, Hilfiker-Kleiner D. Regulation and function of endothelial glycocalyx layer in vascular diseases. Vasc Pharmacol. 2018;100:26–33. doi: 10.1016/j.vph.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 160.Sims JR, Karp S, Ingber DE. Altering the cellular mechanical force balance results in integrated changes in cell, cytoskeletal and nuclear shape. J Cell Sci. 1992;103(Pt 4):1215–1222. doi: 10.1242/jcs.103.4.1215. [DOI] [PubMed] [Google Scholar]
- 161.Singh I, Knezevic N, Ahmmed GU, Kini V, Malik AB, Mehta D. Galphaq-TRPC6-mediated Ca2+ entry induces RhoA activation and resultant endothelial cell shape change in response to thrombin. J Biol Chem. 2007;282:7833–7843. doi: 10.1074/jbc.M608288200. [DOI] [PubMed] [Google Scholar]
- 162.Spassova MA, Hewavitharana T, Xu W, Soboloff J, Gill DL. A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc Natl Acad Sci U S A. 2006;103:16586–16591. doi: 10.1073/pnas.0606894103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Storch U, Forst A-L, Philipp M, Gudermann T, Mederos y Schnitzler M. Transient receptor potential channel 1 (TRPC1) reduces calcium permeability in heteromeric channel complexes. J Biol Chem. 2012;287:3530–3540. doi: 10.1074/jbc.M111.283218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Straub AC, Zeigler AC, Isakson BE. The myoendothelial junction: connections that deliver the message. Physiol Bethesda Md. 2014;29:242–249. doi: 10.1152/physiol.00042.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Strübing C, Krapivinsky G, Krapivinsky L, Clapham DE. TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron. 2001;29:645–655. doi: 10.1016/s0896-6273(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 166.Tarbell JM, Simon SI, Curry F-RE. Mechanosensing at the vascular interface. Annu Rev Biomed Eng. 2014;16:505–532. doi: 10.1146/annurev-bioeng-071813-104908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tarjus A, Maase M, Jeggle P, Martinez-Martinez E, Fassot C, Loufrani L, Henrion D, Hansen PBL, Kusche-Vihrog K, Jaisser F. The endothelial αENaC contributes to vascular endothelial function in vivo. PloS One. 2017;12:e0185319. doi: 10.1371/journal.pone.0185319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Thi MM, Tarbell JM, Weinbaum S, Spray DC. The role of the glycocalyx in reorganization of the actin cytoskeleton under fluid shear stress: a “bumper-car” model. Proc Natl Acad Sci U S A. 2004;101:16483–16488. doi: 10.1073/pnas.0407474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Thilo F, Loddenkemper C, Berg E, Zidek W, Tepel M. Increased TRPC3 expression in vascular endothelium of patients with malignant hypertension. Mod Pathol. 2009;22:426–430. doi: 10.1038/modpathol.2008.200. [DOI] [PubMed] [Google Scholar]
- 170.Tkachenko E, Rhodes JM, Simons M. Syndecans: new kids on the signaling block. Circ Res. 2005;96:488–500. doi: 10.1161/01.RES.0000159708.71142.c8. [DOI] [PubMed] [Google Scholar]
- 171.Tobin DM, Madsen DM, Kahn-Kirby A, Peckol EL, Moulder G, Barstead R, Maricq AV, Bargmann CI. Combinatorial expression of TRPV channel proteins defines their sensory functions and subcellular localization in C. elegans neurons. Neuron. 2002;35:307–318. doi: 10.1016/S0896-6273(02)00757-2. [DOI] [PubMed] [Google Scholar]
- 172.Tolbert CE, Thompson PM, Superfine R, Burridge K, Campbell SL. Phosphorylation at Y1065 in vinculin mediates actin bundling, cell spreading, and mechanical responses to force. Biochemistry. 2014;53:5526–5536. doi: 10.1021/bi500678x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.True AL, Rahman A, Malik AB. Activation of NF-kappaB induced by H(2)O(2) and TNF-alpha and its effects on ICAM-1 expression in endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2000;279:L302–L311. doi: 10.1152/ajplung.2000.279.2.L302. [DOI] [PubMed] [Google Scholar]
- 174.Tzima E, del Pozo MA, Shattil SJ, Chien S, Schwartz MA. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. EMBO J. 2001;20:4639–4647. doi: 10.1093/emboj/20.17.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tzima E, Kiosses WB, del Pozo MA, Schwartz MA. Localized cdc42 activation, detected using a novel assay, mediates microtubule organizing center positioning in endothelial cells in response to fluid shear stress. J Biol Chem. 2003;278:31020–31023. doi: 10.1074/jbc.M301179200. [DOI] [PubMed] [Google Scholar]
- 176.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 177.Uittenbogaard A, Shaul PW, Yuhanna IS, Blair A, Smart EJ. High density lipoprotein prevents oxidized low density lipoprotein-induced inhibition of endothelial nitric-oxide synthase localization and activation in caveolae. J Biol Chem. 2000;275:11278–11283. doi: 10.1074/jbc.275.15.11278. [DOI] [PubMed] [Google Scholar]
- 178.Uyeda TQP, Iwadate Y, Umeki N, Nagasaki A, Yumura S (2011) Stretching actin filaments within cells enhances their affinity for the myosin II motor domain. PLoS One 6. 10.1371/journal.pone.0026200 [DOI] [PMC free article] [PubMed]
- 179.van den Berg BM, Spaan JAE, Rolf TM, Vink H. Atherogenic region and diet diminish glycocalyx dimension and increase intima-to-media ratios at murine carotid artery bifurcation. Am J Physiol Heart Circ Physiol. 2006;290:H915–H920. doi: 10.1152/ajpheart.00051.2005. [DOI] [PubMed] [Google Scholar]
- 180.Vlahu CA, Lemkes BA, Struijk DG, Koopman MG, Krediet RT, Vink H. Damage of the endothelial glycocalyx in dialysis patients. J Am Soc Nephrol JASN. 2012;23:1900–1908. doi: 10.1681/ASN.2011121181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Volkers L, Mechioukhi Y, Coste B. Piezo channels: from structure to function. Pflüg Arch Eur J Physiol. 2015;467:95–99. doi: 10.1007/s00424-014-1578-z. [DOI] [PubMed] [Google Scholar]
- 182.Wang S, Iring A, Strilic B, Juárez JA, Kaur H, Troidl K, Tonack S, Burbiel JC, Müller CE, Fleming I, Lundberg JO, Wettschureck N, Offermanns S. P2Y2 and Gq/G11 control blood pressure by mediating endothelial mechanotransduction. J Clin Invest. 2015;125:3077–3086. doi: 10.1172/JCI81067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wang S, Chennupati R, Kaur H, Iring A, Wettschureck N, Offermanns S. Endothelial cation channel PIEZO1 controls blood pressure by mediating flow-induced ATP release. J Clin Invest. 2016;126:4527–4536. doi: 10.1172/JCI87343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wang G, Kostidis S, Tiemeier GL, Sol WMPJ, de Vries MR, Giera M, Carmeliet P, van den Berg BM, Rabelink TJ. Shear stress regulation of endothelial glycocalyx structure is determined by glucobiosynthesis. Arterioscler Thromb Vasc Biol. 2020;40:350–364. doi: 10.1161/ATVBAHA.119.313399. [DOI] [PubMed] [Google Scholar]
- 185.Warnock DG, Kusche-Vihrog K, Tarjus A, Sheng S, Oberleithner H, Kleyman TR, Jaisser F. Blood pressure and amiloride-sensitive sodium channels in vascular and renal cells. Nat Rev Nephrol. 2014;10:146–157. doi: 10.1038/nrneph.2013.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wassmann S, Stumpf M, Strehlow K, Schmid A, Schieffer B, Böhm M, Nickenig G. Interleukin-6 induces oxidative stress and endothelial dysfunction by overexpression of the angiotensin II type 1 receptor. Circ Res. 2004;94:534–541. doi: 10.1161/01.RES.0000115557.25127.8D. [DOI] [PubMed] [Google Scholar]
- 187.Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng. 2007;9:121–167. doi: 10.1146/annurev.bioeng.9.060906.151959. [DOI] [PubMed] [Google Scholar]
- 188.Wes PD, Chevesich J, Jeromin A, Rosenberg C, Stetten G, Montell C. TRPC1, a human homolog of a Drosophila store-operated channel. Proc Natl Acad Sci U S A. 1995;92:9652–9656. doi: 10.1073/pnas.92.21.9652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wiesinger A, Peters W, Chappell D, Kentrup D, Reuter S, Pavenstädt H, Oberleithner H, Kümpers P. Nanomechanics of the endothelial glycocalyx in experimental sepsis. PloS One. 2013;8:e80905. doi: 10.1371/journal.pone.0080905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Woo S-H, Ranade S, Weyer AD, Dubin AE, Baba Y, Qiu Z, Petrus M, Miyamoto T, Reddy K, Lumpkin EA, Stucky CL, Patapoutian A. Piezo2 is required for Merkel-cell mechanotransduction. Nature. 2014;509:622–626. doi: 10.1038/nature13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Xiao E, Yang HQ, Gan Y-H, Duan D-H, He L-H, Guo Y, Wang SQ, Zhang Y. Brief reports: TRPM7 Senses mechanical stimulation inducing osteogenesis in human bone marrow mesenchymal stem cells. Stem Cells Dayt Ohio. 2015;33:615–621. doi: 10.1002/stem.1858. [DOI] [PubMed] [Google Scholar]
- 192.Xu J, Mathur J, Vessières E, Hammack S, Nonomura K, Favre J, Grimaud L, Petrus M, Francisco A, Li J, Lee V, Xiang F-L, Mainquist JK, Cahalan SM, Orth AP, Walker JR, Ma S, Lukacs V, Bordone L, Bandell M, Laffitte B, Xu Y, Chien S, Henrion D, Patapoutian A. GPR68 senses flow and is essential for vascular physiology. Cell. 2018;173:762–775.e16. doi: 10.1016/j.cell.2018.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yamamoto K, Ando J. New molecular mechanisms for cardiovascular disease: blood flow sensing mechanism in vascular endothelial cells. J Pharmacol Sci. 2011;116:323–331. doi: 10.1254/jphs.10R29FM. [DOI] [PubMed] [Google Scholar]
- 194.Yen W, Cai B, Yang J, Zhang L, Zeng M, Tarbell JM, Fu BM. Endothelial surface glycocalyx can regulate flow-induced nitric oxide production in microvessels in vivo. PLoS One. 2015;10:e0117133. doi: 10.1371/journal.pone.0117133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zeng Y, Tarbell JM. The adaptive remodeling of endothelial glycocalyx in response to fluid shear stress. PLoS One. 2014;9:e86249. doi: 10.1371/journal.pone.0086249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhang C, Hein TW, Wang W, Ren Y, Shipley RD, Kuo L. Activation of JNK and xanthine oxidase by TNF-alpha impairs nitric oxide-mediated dilation of coronary arterioles. J Mol Cell Cardiol. 2006;40:247–257. doi: 10.1016/j.yjmcc.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 197.Zhang X, Sun D, Song JW, Zullo J, Lipphardt M, Coneh-Gould L, Goligorsky MS. Endothelial cell dysfunction and glycocalyx - a vicious circle. Matrix Biol J Int Soc Matrix Biol. 2018;71–72:421–431. doi: 10.1016/j.matbio.2018.01.026. [DOI] [PubMed] [Google Scholar]
- 198.Ziegler T, Bouzourène K, Harrison VJ, Brunner HR, Hayoz D. Influence of oscillatory and unidirectional flow environments on the expression of endothelin and nitric oxide synthase in cultured endothelial cells. Arterioscler Thromb Vasc Biol. 1998;18:686–692. doi: 10.1161/01.atv.18.5.686. [DOI] [PubMed] [Google Scholar]