Abstract
Introduction
In general, women more often experience metformin-associated adverse drug reactions (ADRs) than men.
Objectives
We aimed to assess whether sex differences in reported ADRs for metformin are observed at different times after initiation, and to explore their concurrence with sex differences in the dose of metformin over time. This may guide future studies in assessing the involved mechanisms of sex differences in metformin-associated ADRs and may guide sex-specific management of ADRs in clinical practice.
Methods
This study has a longitudinal design using data about patients initiating metformin collected by the Dutch National Pharmacovigilance Center Lareb through their Intensive Monitoring program. Patients were asked to complete a web-based questionnaire six times after initiation (i.e., at 2 weeks, 6 weeks and at 3, 6, 9, and 12 months). The outcome variables were the proportion of patients reporting any ADR (primary) and the dose of metformin (secondary). Sex differences in the proportions of ADRs and in the dose were tested at each assessment using Pearson Chi-Squared tests and Wilcoxon rank-sum tests, respectively. Using Bonferroni adjustment for multiple testing, a p value < 0.01 was considered statistically significant.
Results
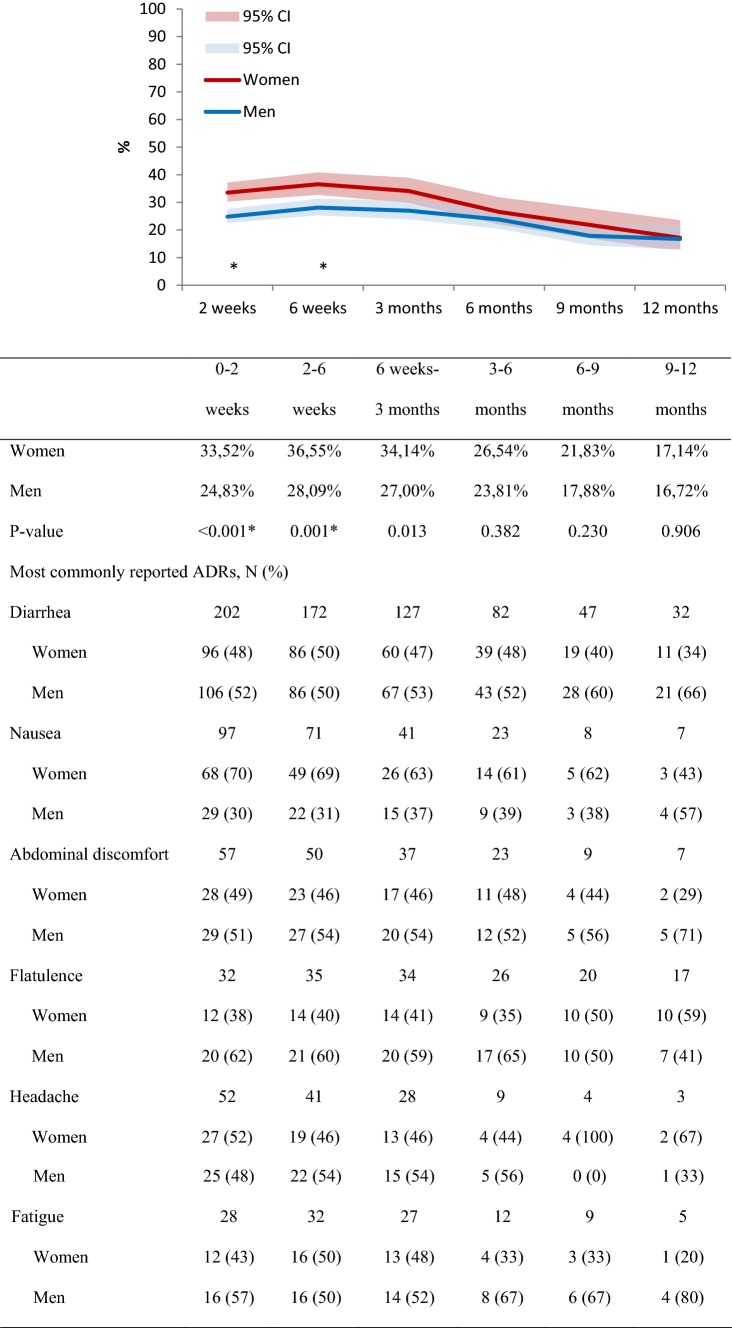
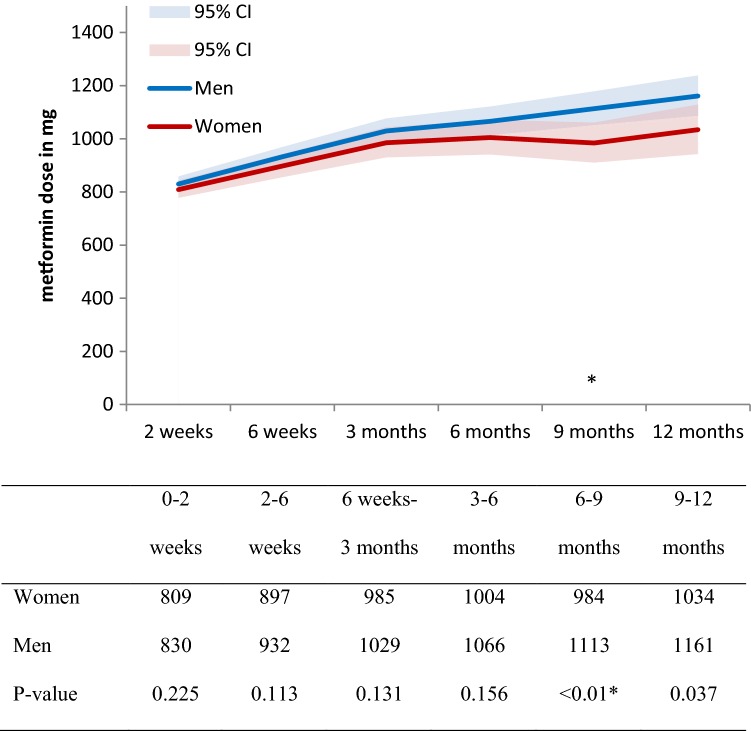
The number of included patients was 1712 (40.9% women). Women reported an ADR more often than men, which was statistically significant at the assessment at 2 weeks (34% vs 25%, p < 0.001), and 6 weeks (37% vs 28%, p = 0.001) after initiation. In general, women were reported to be prescribed a lower dose than men, which became statistically significant at the 9-month assessment (p < 0.01).
Conclusions
Sex differences in reported ADRs were seen in the first weeks after metformin initiation, whereas statistically significant differences in self-reported prescribed dosing were observed after several months. Patients, in particular women, might benefit from being prescribed lower metformin doses at treatment initiation.
Electronic supplementary material
The online version of this article (10.1007/s40264-020-00913-8) contains supplementary material, which is available to authorized users.
Key Points
| A higher proportion of women reporting metformin-associated adverse drug reactions (ADRs) is seen, particularly at early stages after initiation. |
| The reduction of sex differences in ADR reporting over time is accompanied by a lower self-reported dose increase of metformin among women at later stages after initiation. |
| Patients, in particular women, might benefit from being prescribed lower metformin doses at treatment initiation. |
Introduction
Metformin is the most commonly prescribed and guideline-recommended initial glucose-lowering drug for people with type 2 diabetes mellitus due to its well-known safety profile, demonstrated cardiovascular benefits, and low costs [1–4]. Nevertheless, metformin-associated adverse drug reactions (ADRs) are common, particularly gastrointestinal complaints such as diarrhea, nausea, and abdominal discomfort. A previous study showed that 35% of patients initiating metformin reported at least one ADR during a 1-year follow-up period [5]. This proportion was higher among women than among men (40% vs 31%) [5].
In general, metformin-associated ADRs are transient and dose-related and can be minimized by initiating at a low dose, taking the drug with or after a meal, slowly increasing the dose, or if necessary, switching to the extended-release preparation [6–9]. Given the higher proportion of women experiencing ADRs of metformin than men, it seems that ADRs may be less transient in women or that minimization strategies are not sufficiently applied for women. An analysis of prescription data from general practitioners suggests that women are generally prescribed a lower metformin dose at initiation and during a 1-year follow-up period than men [see Electronic Supplementary Material (ESM) 1], but its association with experienced ADRs over time has not been assessed.
The aim of our study was to extend our knowledge on sex differences in metformin-associated ADRs and the association with drug dosing. More specifically, we aimed to assess whether sex differences in reported ADRs for metformin are observed at different times after initiation, and to explore their concurrence with sex differences in the self-reported prescribed dose of metformin over time. Information about the course of sex differences in ADRs and drug dosing over time may guide future studies in assessing the involved mechanisms of sex differences in metformin-associated ADRs and may guide sex-specific management of ADRs in clinical practice.
Methods
Study Design and Data Source
This study has a longitudinal design in which data from the Lareb Intensive Monitoring (LIM) program of the Dutch National Pharmacovigilance Center Lareb were used. In LIM, patients were included when they signed up for the study after having received a leaflet from the pharmacist during the first dispensing of a drug of interest. Several antidiabetic drugs have been assessed using LIM, including metformin. Patients in LIM were followed for a maximum period of 12 months, in which they were asked to complete (six times) a web-based questionnaire about experienced ADRs and additional questions including the dose of the prescribed drug. The questionnaire was sent 2 weeks, 6 weeks, 3 months, 6 months, 9 months, and 12 months after treatment initiation. Patients were asked to report possible ADRs experienced in the period since the previous assessment (i.e., 0–2 weeks, 2–6 weeks, 6 weeks to 3 months, 3–6 months, 6–9 months, and 9–12 months). The answers given by the patients on a previous questionnaire were presented in the next questionnaire so that the patients only needed to adapt the aspects that changed during follow-up. Possible ADRs were events considered to be related to the use of the drug under study by the patient. The LIM procedures have been described in more detail previously [10–12].
Population
Patients participating in LIM and initiating metformin between February 2008 and May 2012 were included in this study. Some patients did not complete the questionnaire at all six assessments. Data for these patients were included for the available follow-up period from inclusion until the assessment with missing data. This implies that questionnaires completed by these patients after the missing assessment were excluded. Furthermore, patients were excluded when they completed a follow-up questionnaire for an antidiabetic drug other than metformin.
Outcomes and Determinant
The primary outcome was the proportion of patients reporting any ADR. ADRs reported in the questionnaires by the patients were classified by trained assessors at the pharmacovigilance center according to the Medical Dictionary for Regulatory Activities (MedDRA), version 20.0 [13].
The secondary outcome was the dose of metformin. The daily dose of metformin was based on the information on the dose and the frequency per day reported by the patients in the questionnaire. A reported value for the dose that is implausible (i.e., lower than the start dose of 500 mg or higher than the maximum recommended dose of 3000 mg in the Netherlands) [14] and values other than numbers for the frequency (i.e., “continuously”, “according to scheme”, and “if necessary”) were coded as missing.
The determinant used in this study was the sex (i.e., women vs men) of the patients.
Analyses
Descriptive statistics were used to present the proportion of patients reporting an ADR, the most commonly reported ADRs, and the self-reported prescribed dose of metformin over time. Sex differences in the proportions of reported ADRs and in the dose of metformin were tested at each assessment using Pearson Chi-Squared tests and Wilcoxon rank-sum tests, respectively. Sensitivity analyses were conducted in which only patients who completed the questionnaire at all six assessments were included. The analyses were conducted using Stata® version 14 (Stata Corp., College Station, TX, USA). Using Bonferroni adjustment for multiple testing, a p value < 0.01 was considered statistically significant. Figures were made using Microsoft Excel® 2010 (Microsoft Corp., Redmond, WA, USA).
Results
In total, 1712 patients participated in this study (average age 58 years (SD 12), 40.9% women) (Table 1). Men were, on average, 60 years of age at the time of metformin initiation, whereas women were, on average, 55 years of age (Table 1). The questionnaire was completed at all six assessments by 474 patients (average age 59 years (SD 11), 36.9% women). Of all patients, 41% reported at least one ADR during follow-up (37% of the males and 46% of the females), with a total of 2673 ADRs at the Preferred Term level of the MedDRA (1395 by males and 1278 by females). The most commonly reported ADRs were diarrhea, nausea, abdominal discomfort, flatulence, headache, and fatigue (Table 1).
Table 1.
Descriptive statistics of the included patients
| Total | Men | Women | |
|---|---|---|---|
| Number of patients (%) | 1712 | 1011 (59.1) | 701 (40.9) |
| Average age in years (SD) | 58 (12) | 60 (10) | 55 (12) |
| Number of patients (%) per time period | |||
| 2 weeks | 1712 | 1011 (59.1) | 701 (40.9) |
| 6 weeks | 1336 | 808 (60.5) | 528 (39.5) |
| 3 months | 1050 | 637 (60.7) | 413 (39.3) |
| 6 months | 813 | 504 (62.0) | 309 (38.0) |
| 9 months | 615 | 386 (62.8) | 229 (37.2) |
| 12 months | 474 | 299 (63.1) | 175 (36.9) |
| Most commonly reported ADRs, N (%) | |||
| Diarrhea | 662 | 351 (53.0) | 311 (47.0) |
| Nausea | 247 | 82 (33.2) | 165 (66.8) |
| Abdominal discomfort | 183 | 98 (53.6) | 85 (46.4) |
| Flatulence | 164 | 95 (57.9) | 69 (42.1) |
| Headache | 137 | 68 (49.6) | 69 (50.4) |
| Fatigue | 113 | 64 (56.6) | 49 (43.4) |
Sex Differences in the Proportion of Reported ADRs Over Time
In the first period of 2 weeks after metformin initiation, 25% of the men reported to have experienced an ADR compared with 34% of the women. These numbers were somewhat higher in the periods between 2 and 6 weeks and between 6 weeks and 3 months after initiation and decreased in the following assessments. The proportion of women and men was relatively similar over time for the most commonly reported ADRs. Women generally reported an ADR more often than men, which was statistically significant for the first two assessments (at 2 weeks: 34% vs 25%, p < 0.001; at 6 weeks: 37% vs 28%, p = 0.001 for women vs men, respectively; Fig. 1). A similar pattern was seen in the sensitivity analysis in which only patients who completed all six assessments were included, although it was only considered statistically significant at 6 weeks (Fig. S1 in ESM 2).
Fig. 1.
Proportion with 95% confidence interval (CI) of women and men reporting an adverse drug reaction (ADR) for metformin and an overview of the most commonly reported ADRs at each assessment. *Statistically significant differences between women and men
Sex Differences in Metformin Dose Over Time
The average dose of metformin increased in the study population from 821 mg at 2 weeks to 1115 mg at 12 months (Table S1 in ESM 2). Average doses increased slightly more for men (from 830 to 1161 mg) than for women (from 809 to 1034 mg) (Fig. 2; Table S1 in ESM 2). Doses appeared to be higher for men at each assessment, but this was considered statistically significant only at the 9-month assessment (p < 0.01; Fig. 2). In the sensitivity analyses that included only patients who completed all six assessments, the doses for women were significantly lower than the doses for men at the 2-week assessment (Fig. S2 and Table S2 in ESM 2). The average initial dose for women completing all six assessments was 717 mg, whereas this was 836 mg for men.
Fig. 2.
Average dose in milligrams and 95% confidence interval (CI) of self-reported dose of metformin at each assessment and per sex.*Statistically significant differences between women and men
Discussion
A higher number of women reporting an ADR was primarily seen in the first weeks after metformin initiation. The reduction of sex differences in ADR reporting over time was accompanied by a lower dose of metformin among women at later stages after initiation.
Previous studies indicate that women more often report an ADR than men for drugs in general [15–18], as well as for metformin specifically [5, 19]. The current study adds to this knowledge that sex differences in metformin-associated ADRs are particularly shown at early stages after initiation. There could be several explanations for this finding. First, the experience of ADRs may lead to discontinuation of metformin treatment and therefore a loss to follow-up of the patients experiencing ADRs in the current study. The reasons for drop-out in our study are unknown but the proportion of men who completed all six assessments in this study was somewhat higher than the proportion of women. However, the number of both men and women reporting an ADR was somewhat higher for those who completed all six assessments (25.1% and 36.0%, respectively, at the first assessment) than those in the total study population (24.8% vs 33.5%, respectively, at the first assessment), refuting that patients experiencing an ADR in the first weeks after treatment initiation dropped out of the study. Also, the same pattern of sex differences in ADRs over time was shown in the sensitivity analyses, in which only patients who completed the six assessments were included.
Another explanation could lie in sex differences in the dose of metformin. The most commonly reported ADRs among both men and women were diarrhea, nausea, abdominal discomfort, flatulence, headache, and fatigue. Most of these are Type A effects, suggesting that they could be avoided by using the appropriate dose for an individual [20]. The average dose of metformin at initiation was somewhat lower for women than for men. Over time, this difference became larger, suggesting that women received fewer up titrations and/or more dose reductions, and was accompanied by fewer ADRs reported by women in these later months. In the sensitivity analysis, women who completed all assessments initiated on lower doses than men who completed all assessments, but also on lower doses than women who did not complete all assessments. This suggests that women starting on low doses are more likely to tolerate metformin treatment up to 1 year of follow up. A post-hoc analysis showed that both men and women who reported an ADR were generally on a higher dose than those who did not report an ADR (Fig. S3 in ESM 2). This indicates that the advice to start with a low dose and up titrate slowly [6, 7, 9] is relevant for both women and men, and that healthcare professionals should pay attention to this advice to reduce the burden of ADRs among their patients. Further studies are needed to assess the underlying factors of the potential sex difference of metformin dose on experiencing ADRs. A factor that may be important to take into account is someone’s weight. Previously it was shown that sex differences in pharmacokinetics can be due to weight differences [21].
Finally, it could also be that ADRs among women are more often of a transient nature or that women adapt to or handle ADRs differently than men. A previous study showed that ADRs experienced by people with diabetes can be transient or fluctuating over time [22] but it is not clear whether this differs between men and women. This could in part be a gender-related factor, since social, behavioral, and cultural differences between men and women may influence the experience and reporting of ADRs [15, 23, 24]. Women and men have different risk perceptions [25] and it has been shown that women more often read patient information leaflets than men [26]. Such differences should be included when investigating explanations for the observed sex differences in ADRs associated with metformin that were shown particularly at early stages after initiation.
The main strength of this study is the longitudinal data collection where patients were followed for a period of 12 months. Therefore, sex differences could be assessed at different times after treatment initiation. A limitation of the study is the low number of patients that completed all six assessments (i.e., 28% of the patients). These numbers are somewhat lower than intensive monitoring studies of duloxetine (39%) and pregabalin (38%) [10, 27]. The follow-up period in these studies was, however, shorter (i.e., 6 months), so a further decrease in the number of participants in our study may have been due to the longer follow-up. In general, patients experiencing an ADR may be more motivated to complete a questionnaire about ADRs [5]. This might have led to more women completing all questionnaires, but that was not the case in our study. Another limitation of this study is the unavailable information about other patient characteristics (e.g., body weight, diabetes duration, and glomerular filtration rate), for which we could not adjust. Further studies should assess the role of such characteristics on the observed differences between men and women in ADRs. We did not conduct a formal causality assessment since the focus is on patient-reported ADRs for metformin. A general limitation of studies using questionnaires is the representativeness of the responders. A previous study showed some differences between the patient population in the LIM diabetes study and an external reference population [12]. More specifically, the LIM population was somewhat younger, healthier, and included more men than the reference population, which may have led to an underestimation of ADRs.
Conclusion
Sex differences in reported ADRs were mainly observed during the first weeks after metformin initiation, whereas differences in self-reported prescribed dosing became significant after several months. Patients, in particular women, might benefit from being prescribed a lower dose of metformin at initiation to reduce the risk of ADR occurrence.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
All authors contributed to the development and formulation of the research question. CE and EPP extracted/collected the data. All authors were involved in the analyses plan. STVconducted the analyses. All authors contributed to the interpretation of the data. The first draft of the manuscript was written by STV and all authors commented on previous versions of the manuscript. All authors have read and approved the final manuscript.
Compliance with Ethical Standards
Funding
This study was funded by ZonMW—The Netherlands Organization for Health Research and Development (Project Number 849100006). In addition, funding was received from the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No 754425.
Conflict of interest
Sieta T. de Vries, Petra Denig, Corine Ekhart, Peter G.M. Mol, and Eugene P. van Puijenbroek declare that they have no conflict of interest.
Ethical approval
No ethical approval was necessary for this study in which observational data from a questionnaire-based prospective cohort study was used.
Data availability statement
This study was part of a wider project assessing gender differences in adverse drug reactions. The data used in the project, including the data that support the findings of this study, are described in DataverseNL available at https://hdl.handle.net/10411/NG8CRG.
References
- 1.American Diabetes Association. 9 Pharmacologic approaches to glycemic treatment: Standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S90–S102. doi: 10.2337/dc19-S009. [DOI] [PubMed] [Google Scholar]
- 2.International Diabetes Federation (IDF). Clinical practice recommendations for managing type 2 diabetes in primary care - 2017. [online]. https://www.idf.org/e-library/guidelines/128-idf-clinical-practice-recommendations-for-managing-type-2-diabetes-in-primary-care.html. Accessed 1 July 2019.
- 3.Nederlands Huisartsen Genootschap (NHG) [Dutch College of General Practitioners]. NHG-Standaard Diabetes mellitus type 2 (Vierde (partiële) herziening) - 2018. [online]. https://www.nhg.org/standaarden/volledig/nhg-standaard-diabetes-mellitus-type-2. Accessed 1 July 2019.
- 4.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2015;58(3):429–442. doi: 10.1007/s00125-014-3460-0. [DOI] [PubMed] [Google Scholar]
- 5.de Jong L, Härmark L, van Puijenbroek E. Time course, outcome and management of adverse drug reactions associated with metformin from patient’s perspective: a prospective, observational cohort study in the Netherlands. Eur J Clin Pharmacol. 2016;72(5):615–622. doi: 10.1007/s00228-016-2019-z. [DOI] [PubMed] [Google Scholar]
- 6.Bonnet F, Scheen A. Understanding and overcoming metformin gastrointestinal intolerance. Diabetes Obes Metab. 2017;19(4):473–481. doi: 10.1111/dom.12854. [DOI] [PubMed] [Google Scholar]
- 7.Bailey CJ. Biguanides and NIDDM. Diabetes Care. 1992;15(6):755–772. doi: 10.2337/diacare.15.6.755. [DOI] [PubMed] [Google Scholar]
- 8.Bailey CJ, Turner RC. Metformin. N Engl J Med. 1996;334(9):574–579. doi: 10.1056/NEJM199602293340906. [DOI] [PubMed] [Google Scholar]
- 9.Marshall SM. 60 years of metformin use: a glance at the past and a look to the future. Diabetologia. 2017;60(9):1561–1565. doi: 10.1007/s00125-017-4343-y. [DOI] [PubMed] [Google Scholar]
- 10.Härmark L, van Puijenbroek E, Straus S, van Grootheest K. Intensive monitoring of pregabalin: results from an observational, web-based, prospective cohort study in the Netherlands using patients as a source of information. Drug Saf. 2011;34(3):221–231. doi: 10.2165/11585030-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 11.Härmark L, Puijenbroek E, Grootheest K. Longitudinal monitoring of the safety of drugs by using a web-based system: the case of pregabalin. Pharmacoepidemiol Drug Saf. 2011;20(6):591–597. doi: 10.1002/pds.2135. [DOI] [PubMed] [Google Scholar]
- 12.Härmark L, Alberts S, van Puijenbroek E, Denig P, van Grootheest K. Representativeness of diabetes patients participating in a web-based adverse drug reaction monitoring system. Pharmacoepidemiol Drug Saf. 2013;22(3):250–255. doi: 10.1002/pds.3341. [DOI] [PubMed] [Google Scholar]
- 13.Brown EG, Wood L, Wood S. The medical dictionary for regulatory activities (MedDRA) Drug Saf. 1999;20(2):109–117. doi: 10.2165/00002018-199920020-00002. [DOI] [PubMed] [Google Scholar]
- 14.Geneesmiddeleninformatiebank [Medicines information repository of the Dutch Medicines Evaluation Board]. Summary of Product Characteristics. Metformine HCl TEVA 500 - 850 - 1000 MG. [online]. https://www.geneesmiddeleninformatiebank.nl/smpc/h10500_smpc.pdf. Accessed 1 July 2019.
- 15.de Vries ST, Denig P, Ekhart C, Burgers JS, Kleefstra N, Mol PGM, van Puijenbroek EP. Sex differences in adverse drug reactions reported to the national pharmacovigilance centre in the Netherlands: an explorative observational study. Br J Clin Pharmacol. 2019;85(7):1507–1515. doi: 10.1111/bcp.13923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holm L, Ekman E, Jorsater Blomgren K. Influence of age, sex and seriousness on reporting of adverse drug reactions in Sweden. Pharmacoepidemiol Drug Saf. 2017;26(3):335–343. doi: 10.1002/pds.4155. [DOI] [PubMed] [Google Scholar]
- 17.Zopf Y, Rabe C, Neubert A, Gassmann KG, Rascher W, Hahn EG, Brune K, Dormann H. Women encounter ADRs more often than do men. Eur J Clin Pharmacol. 2008;64(10):999–1004. doi: 10.1007/s00228-008-0494-6. [DOI] [PubMed] [Google Scholar]
- 18.Tran C, Knowles SR, Liu BA, Shear NH. Gender differences in adverse drug reactions. J Clin Pharmacol. 1998;38(11):1003–1009. doi: 10.1177/009127009803801103. [DOI] [PubMed] [Google Scholar]
- 19.Walker EA, Molitch M, Kramer MK, Kahn S, Ma Y, Edelstein S, Smith K, Johnson MK, Kitabchi A, Crandall J. Adherence to preventive medications: predictors and outcomes in the Diabetes Prevention Program. Diabetes Care. 2006;29(9):1997–2002. doi: 10.2337/dc06-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyboom RHB, Gribnau FWJ, Hekster YA, de Koning GHP, Egberts ACG. Characteristics of topics in pharmacovigilance in The Netherlands. Clin Drug Invest. 1996;12(4):207–219. doi: 10.2165/00044011-199612040-00006. [DOI] [Google Scholar]
- 21.Parekh A, Fadiran EO, Uhl K, Throckmorton DC. Adverse effects in women: implications for drug development and regulatory policies. Expert Rev Clin Pharmacol. 2011;4(4):453–466. doi: 10.1586/ecp.11.29. [DOI] [PubMed] [Google Scholar]
- 22.Denig P, van Puijenbroek EP, Soliman N, Mol PGM, de Vries ST. Adverse drug event patterns experienced by patients with diabetes: a diary study in primary care. Pharmacoepidemiol Drug Saf. 2019;28(9):1175–1179. doi: 10.1002/pds.4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kautzky-Willer A, Harreiter J. Sex and gender differences in therapy of type 2 diabetes. Diabetes Res Clin Pract. 2017;131:230–241. doi: 10.1016/j.diabres.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 24.Kautzky-Willer A, Harreiter J, Pacini G. Sex and gender differences in risk, pathophysiology and complications of Type 2 Diabetes Mellitus. Endocr Rev. 2016;37(3):278–316. doi: 10.1210/er.2015-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gustafson PE. Gender differences in risk perception: theoretical and methodological perspectives. Risk Anal. 1998;18(6):805–811. doi: 10.1023/B:RIAN.0000005926.03250.c0. [DOI] [PubMed] [Google Scholar]
- 26.Hammar T, Nilsson A, Hovstadius B. Patients’ views on electronic patient information leaflets. Pharmacy Practice. 2016;14(2):702. doi: 10.18549/PharmPract.2016.02.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Härmark L, van Puijenbroek E, van Grootheest K. Intensive monitoring of duloxetine: results of a web-based intensive monitoring study. Eur J Clin Pharmacol. 2013;69(2):209–215. doi: 10.1007/s00228-012-1313-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study was part of a wider project assessing gender differences in adverse drug reactions. The data used in the project, including the data that support the findings of this study, are described in DataverseNL available at https://hdl.handle.net/10411/NG8CRG.