Abstract
Irisin is a myokine secreted mainly from skeletal muscle that is known for having beneficial metabolic effects via enhancement of energy expenditure and insulin sensitivity. Studies show that irisin also acts as an autocrine/paracrine to promote myogenesis and muscle growth. However, the protective role of irisin against muscular wasting remains unclear. We confirmed that irisin secretion was upregulated by electrical pulse stimulation an in vitro exercise mimetic model. Next, we tested if irisin exerted an anti-atrophic effect on cultured C2C12 myotubes treated with dexamethasone (DEX), a representative inducer of muscular atrophy. Treatment of cultured myotubes with DEX reduced myotube size and increased proteasome activity, which were attenuated by irisin. Also, irisin effectively prevented dephosphorylation of forkhead box O (FoxO) 3α and upregulation of muscle-specific ubiquitin ligases in DEX-treated myotubes. The protective effect of irisin on DEX-mediated myotube atrophy was partially regulated by insulin-like growth factor-1-dependent signaling. These results suggested that irisin may prevent glucocorticoid-induced muscle atrophy by inhibiting FoxO-mediated ubiquitin-proteasome overactivity.
Electronic supplementary material
The online version of this article (10.1007/s00424-020-02367-4) contains supplementary material, which is available to authorized users.
Keywords: Muscle atrophy, Irisin, Glucocorticoid, FoxO3α, Atrogin-1/MAFbx, MuRF-1
Introduction
Skeletal muscle atrophy is a debilitating consequence of physiological processes and conditions such as disuse, malnutrition, and aging. It is also a prominent pathological feature of chronic illnesses including cardiac or renal failure, chronic obstructive pulmonary disease, liver cirrhosis, and cancer [6, 11]. Muscle atrophy causes exercise intolerance and an inability to perform daily activity because of muscle weakness and fatigue, which leads to poor quality of life [15, 25]. Excessive loss of muscle mass can exacerbate disease complications due to impaired efficacy of different therapeutic treatments, thus increasing morbidity and mortality [1].
Irisin, a 112-amino acid, hormone-like molecule cleaved from fibronectin type III domain containing 5 (FNDC5) is predominantly secreted from skeletal muscle [20]. Irisin is strongly implicated in muscle growth. Circulating irisin is upregulated by both endurance and strength exercise, and its levels positively correlate with indicators of skeletal muscle mass and levels of insulin-like growth factor-1 (IGF-1), an anabolic hormone of skeletal muscle [10, 13, 18]. Our previous study showed a negative correlation between circulating irisin levels and incidence of pre-sarcopenia and sarcopenia [5]. Correspondingly, mice with null mutations in myostatin, a main negative regulator of muscle growth, display elevated irisin levels and increased muscle growth-related gene expression and skeletal musculature [30]. An increase in irisin secretion has also been observed in vitro, using conditional knockdown of myostatin in C2C12 muscle cells [8]. Recent evidence suggests that irisin exerts its myogenic effects by enhancing the myoblast fusion and protein synthesis pathway [14, 26]. Given the relationship between irisin and skeletal musculature, we questioned if irisin protects against muscular wasting along with being a promyogenic factor.
Dexamethasone (DEX), a synthetic glucocorticoid, is widely used to induce proteolytic muscle atrophy in both in vivo and in vitro models [28]. DEX is commonly used to treat medical conditions such as inflammatory and autoimmune disorders [32]. It also causes a reduction in protein synthesis and promotes the breakdown of proteins related to skeletal muscle mass via the ubiquitin-proteasome system [22, 28]. Because of aggravating catabolic effects concomitant with glucocorticoid treatments, therapeutic approaches that prevent DEX-induced muscle wasting have important clinical implications.
The effect of irisin on glucocorticoid-induced muscle wasting has not yet been established, with studies mostly focused on its myogenic differentiation and regulation of muscle growth-related factors. Therefore, we investigated the hypothesis that irisin, an exercise-responsive myokine, exerts a protective effect against DEX-mediated muscular atrophy in cultured C2C12 myotubes.
Materials and methods
Reagents
Dulbecco’s modified Eagles medium, fetal bovine serum, and penicillin/streptomycin were purchased from Hyclone (Logan, UT, USA), and horse serum was from Gibco (Grand Island, NY, USA). Trypsin and TRIzol reagents were purchased from Invitrogen (Carlsbad, CA, USA). DEX and IGF-1 were purchased from Sigma (St Louis, MO, USA), and recombinant irisin (r-irisin) was from Adipogen (Seoul, Korea).
Cell culture and differentiation induction
C2C12 myoblast cells from the American Type Culture Collection (ATCC, Manassas, VA, USA) were cultivated in Dulbecco’s modified Eagles medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. For each experimental condition, myoblasts were plated in 100-mm culture dishes (for the measurement of cell diameter), 6-well plates (for western blots), or 96-well plates (for proteasome activity assays). At approximately 90% confluence, 10% fetal bovine serum was replaced with 2% horse serum for 6 days to induce C2C12 myoblast cells to differentiate into myotubes. Cell cultures were maintained in a humidified chamber with 5% CO2 at 37 °C, and culture media were changed every other day.
Chemical and electrical pulse stimulation
As an in vitro exercise model, myotubes were subjected to electrical pulse stimulation (EPS) using a C-dish with carbon electrodes combined with a pulse generator (C-Pace 100; IonOptix, Milton, MA, USA). Either low (1 Hz with 2 ms duration) or high (99 Hz with 1 ms duration, 20 pulses every 20 s) frequency was applied with both EPS modes set to 11.5 V intensity for a cumulative number of pulses for 24 h. Following washing twice with phosphate-buffered saline, EPS treatment was performed under serum-free conditions and conditioned media, and cell lysates were harvested immediately after EPS. For irisin treatment, differentiated C2C12 myotubes were serum starved for 24 h and treated with multiple doses of r-irisin or with an effective dose at different timepoints. To identify anti-atrophic effects of irisin, C2C12 myotubes were subdivided into four groups: (i) control, with cells incubated in serum-free medium containing 100 U/ml penicillin and 100 mg/ml streptomycin; (ii) DEX, with cells treated with 100 μM DEX; (iii) irisin, with cells treated with 100 ng/ml r-irisin; and (iv) DEX + irisin, with cells co-treated with 100 μM DEX and 100 ng/ml r-irisin. All incubations were for 24 or 48 h prior to harvesting cells and performing experiments.
Western blots
C2C12 myotubes were lysed in RIPA buffer containing protease inhibitor and protein phosphatase inhibitor cocktail. Protein concentrations were determined using a BCA Protein Assay kit (Thermo Fisher Scientific, Waltham, MA, USA). Proteins were separated on 4–12% polyacrylamide gel using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride membranes. Membranes were blocked in 3 or 5% (w/v) skim milk in Tris-buffered saline with Tween 20 (TBST), followed by incubation overnight at 4 °C with primary antibody against irisin (Adipogen), FNDC5, IGF-1, muscle atrophy F-box (atrogin-1), muscle RING finger-1 (MuRF-1) (Abcam, Cambridge, UK), Ser473-phosphorylated and total Akt, Thr202/Tyr204-phosphorylated and total ERK1/2, Tyr1135-phosphorylated and total IGF-1 receptor (IGF-1R) β, Ser318/321-phosphorylated and total FoxO3α, Thr172-phosphorylated and total AMPKα (Cell Signaling Technology, Danvers, MA, USA), or GAPDH (Santa Cruz Biotechnology, Dallas, Texas, USA). After washing three times for 10 min in TBST, membranes were incubated with peroxidase-conjugated secondary antibody for 1 h followed by washing. Detection of proteins used ECL Western Blotting Substrate (Thermo Fisher Scientific) on ChemiDoc XRS + Systems (Bio-Rad, Hercules, CA, USA). To detect irisin secretion levels, myotubes were incubated for 24 h with serum-free media containing only antibiotics after indicated treatments and washing. Conditioned media were collected and centrifuged at 1000 rpm for 5 min, and supernatants were concentrated using 10-kDa molecular weight cutoff spin filters (Amicon, Millipore, MA, USA). Concentrated culture media samples were loaded onto 15% SDS-PAGE.
Proteasome activity analysis
A Proteasome-GloTM Chymotrypsin-like Cell-Based Assays kit (Promega, Madison, WI, USA) was used on intact myotubes attached to culture plates according to the manufacturer’s instructions with slight modifications. Myoblasts were seeded at 10,000 cells per well in 100 μl and differentiated in 96-well plates with clear optical bottoms. After 48-h DEX and/or r-irisin treatment of differentiated myotubes, an equal volume of luminogenic substrate specified for chymotrypsin-like protease activity was added to samples. After shaking at 700 rpm using a plate shaker for 2 min and incubation at room temperature for 10 min, luminescence was detected by a luminometer (BioTek Instruments, VT, USA). To confirm assay specificity, the same number of samples was pretreated for 1 h with 10 μM epoxomicin, a proteasome inhibitor. For each sample, proteasome activity was normalized with epoxomicin-pretreated luminescence as the background signal.
Cell size determination after DEX and/or irisin treatment
After 48-h DEX and/or r-irisin treatment, myotubes were fixed with 4% paraformaldehyde in phosphate-buffered saline. Images were from a Nikon Eclipse TE2000U microscope (Nikon, Avon, MA), captured using Photometrics Cool SNAP CCD camera (Roper Scientific, Tucson, AZ, USA) under phase-contrast microscopy at × 100 magnification. Diameters of individual myotubes were analyzed using MetaMorph 6.1 software (Molecular Devices, Sunnyvale, CA, USA). Average diameters of at least 200 myotubes were determined for each condition at three points separated by 50 μm along the myotube.
Statistical analysis
All values are presented as mean ± standard error of the mean (SEM) or standard deviation (SD) from at least three separate experiments, and analyses were performed using IBM SPSS Statistics version 23 (IBM Corp., Armonk, NY, USA). P values below 0.05 were considered statistically significant. One-way analysis of variance (ANOVA) was used to compare means among multiple groups, followed by Tukey’s post hoc test.
Results
Increased irisin expression and secretion in C2C12 myotubes in response to exercise-like conditions
To evaluate expression and secretion levels of FNDC5/irisin in response to excitation-contraction coupling, we applied EPS for 24 h to C2C12 myotubes as an in vitro exercise mimetic model. Continuous repetitive contraction and relaxation of myotubes by EPS was observed, and no morphological changes were detected after EPS completion. The exercise-like condition of EPS-induced contraction was confirmed by the ratio of phosphorylated/total AMPK (Fig. 1A and B), which indicated exercise- and contraction-mediated increase in ATP consumption in skeletal muscle cells and tissues. Both low- and high-frequency EPS modalities resulted in increased FNDC5 and irisin protein levels in myotubes (Fig. 1C) and increased irisin secreted into the culture medium (Fig. 1D).
Fig. 1.
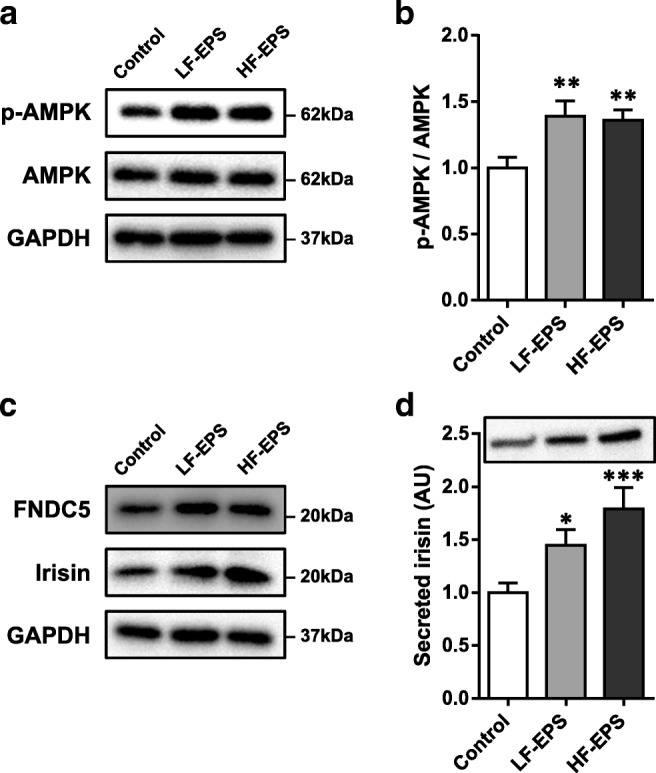
Irisin expression and secretion were upregulated in response to an exercise-like condition in C2C12 myotubes. A Representative immunoblot of phosphorylated AMPK (p-AMPK) and total AMPK in C2C12 myotubes treated for 24 h without and with low- or high-frequency mode electrical pulse stimulation (LF-EPS or HF-EPS). B Densitometric quantification of relative protein expression of p-AMPK normalized by total AMPK. GAPDH was the loading control. C Protein expression and D secretion levels of FNDC5/irisin in EPS-treated myotubes. Relative secretion levels in culture medium were normalized to total protein concentration of cell lysates. Bars represent the mean ± SEM of (B) or SD of four (D) separate experiments. AU, arbitrary unit. *p < 0.05, **p < 0.01, ***p < 0.001 vs. control
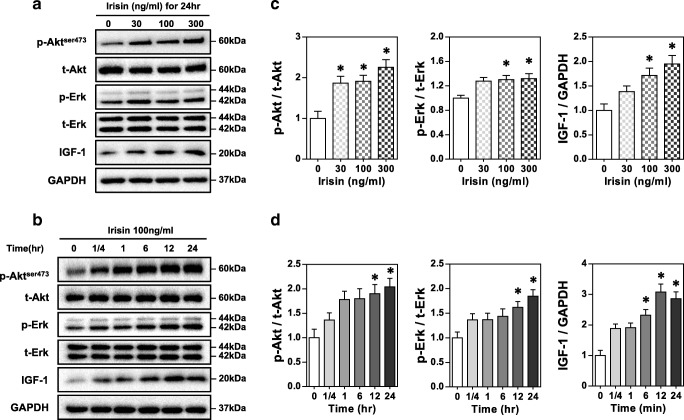
Irisin activates Akt and ERK1/2 signaling and upregulates IGF-1 expression
To determine the signaling mediators underlying the myotrophic effects of irisin, we used an in vitro model of r-irisin on C2C12 myotubes starved of serum for 24 h. Western blots showed that 24 h of r-irisin treatment increased IGF-1 protein and phosphorylation of downstream effectors Akt and ERK1/2 in a dose-dependent manner (Fig. 2A and C). Time-dependent increases were also seen at 100 ng/ml r-irisin (Fig. 2B and D). These results indicated that the myotrophic potential of irisin occurred via regulating muscle anabolic factors and downstream signaling.
Fig. 2.
Irisin activates Akt and ERK1/2 signaling and upregulates IGF-1 expression. Representative immunoblot of dose-dependent (A) and time-dependent (B) effect of irisin treatment on phosphorylation of Akt and ERK1/2 and IGF-1 expression in differentiated C2C12 myotubes. C and D Densitometric quantification of results in panels A and B, respectively. GAPDH was the loading control. Bars represent the mean ± SEM of three separate experiments (n = 3). *p < 0.05 vs. without irisin treatment
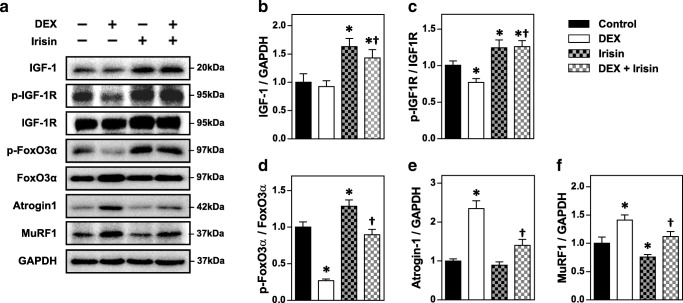
Irisin ameliorates atrophic signaling in DEX-induced myotube atrophy
To determine if irisin exerted myogenic effects during atrophic conditions caused by a glucocorticoid, we evaluated expression of IGF-1 and activity of its receptor in DEX-treated myotubes with and without r-irisin. DEX treatment resulted in decreased phosphorylation of IGF-1R, indicating reduced receptor activity. In r-irisin-treated myotubes, DEX had no significant effect on IGF-1 expression or IGF-1R activity (Fig. 3A–C). To investigate the suppressive effect of irisin on DEX-mediated atrophic signaling, we assessed expression of the muscle-specific ubiquitin ligases (atrogenes), atrogin-1 and MuRF-1, and the transcriptional activity of FoxO3α, a critical mediator of atrogenes. DEX treatment increased activation of FoxO3α, as evidenced by decreased phosphorylation (p-FoxO3α) and increased atrogin-1 and MuRF-1 expression. In contrast, r-irisin treatment of myotubes resulted in decreased atrogene signaling and slightly higher FoxO3α phosphorylation and decreased MuRF1 expression. When r-irisin was added to DEX-treated myotubes, the decreased in p-FoxO3α with DEX treatment was abolished, and p-FoxO3α was close to control levels. The increased atrogene expression due to DEX was also significantly lower, suggesting attenuation of ubiquitin-mediated atrophic signaling (Fig. 3A and D–F).
Fig. 3.
Irisin ameliorates IGF-1 signaling transduction and attenuates FoxO-mediated expression of muscle-specific ubiquitin ligases in dexamethasone-induced myotube atrophy. A Effect of irisin treatment on expression of IGF-1, atrogin-1, and MuRF-1 and phosphorylation of IGF-1 receptor (p-IGF-1R) and FoxO3α (p-FoxO3α) in DEX-treated C2C12 myotubes. B–F Densitometric quantification of protein levels of IGF-1 (B), p-IGF-1R (C), p-FoxO3α (D), atrogin-1 (E), and MuRF-1 (F). Bars represent the mean ± SEM of three to four separate experiments (n = 3–4). DEX, dexamethasone. * p < 0.05 vs. control; † p < 0.05 vs. DEX
Irisin attenuates DEX-induced proteolytic activity and myotube atrophy
Next, we investigated if attenuation of muscle atrophy- and hypertrophy-related signaling by r-irisin prevented muscular wasting. We measured the chymotrypsin-like activity of 26S proteasome, considered to be representative of the proteasome proteolytic capacity. Consistent with the molecular alterations, r-irisin treatment reduced baseline proteasome activity. It also attenuated the elevated proteasome activity with DEX in C2C12 myotubes (Fig. 4A). Finally, to confirm the effectiveness of irisin on preserving myotube size, morphological differences were observed among experimental groups of control or treated with DEX and/or r-irisin. Representative photos of myotubes with corresponding treatments are in Fig. 4B. Compared to the control group, exposure to 100 uM DEX for 24 h reduced myotube diameter by 37.4%, whereas treatment with 100 ng/ml r-irisin increased myotube diameters by 19.9%. Myotube diameter was largely restored in the DEX + r-irisin group to 87.8% of control values (Fig. 4C). These results showed that irisin treatment prevented myotube wasting and suggested that this effect correlated with an ubiquitin-proteasome proteolytic pathway.
Fig. 4.
Irisin attenuates dexamethasone-induced proteolytic activity and myotube atrophy. A Comparison of the chymotrypsin-like activity of the 26S proteasome determined via cultured cell-based luminescent assay among the four groups. B Representative photographs of C2C12 myotubes for control, DEX, irisin, and DEX + irisin treatments. C Comparison of myotube diameters among the four groups. Bars represent the mean ± SD of three (A, 4 wells per group in each experiment) or four (C, 50 myotubes in each experiment) separate cultures. RLU, relative light units. DEX, dexamethasone. * p < 0.05 vs. control; † p < 0.05 vs. DEX
Discussion
Controversy about the existence of human irisin was resolved after circulating human irisin was quantitatively confirmed in sedentary and trained individuals by mass spectrometry assays [16]. Reports indicate that irisin positively affects myogenesis by enhancing intramuscular anabolic signaling and protects against unloading- or denervation-induced muscle wasting [7, 26]. However, the endocrine/paracrine effect of this myokine on glucocorticoid-induced myopathy is still unknown. We reasoned that irisin alleviated glucocorticoid-mediated muscular atrophy, since it is a myogenic and exercise-induced factor. To our knowledge, this is the first evidence that irisin has an anti-atrophic potential on a myopathy model caused by a glucocorticoid.
EPS-treated myotubes are confirmed to be closely comparable to muscles of trained mice in an exercise-activated signaling pathway, mitochondrial biogenesis, and substrate metabolism [3, 23]. Emerging evidence indicates that EPS augments the expression and secretion of several myokines in cultured myotubes [23], but whether that was true for irisin was unclear. Only two studies used in vitro models to study irisin, using pharmacological compounds to mimic acute exercise responses [10]. In those studies, despite the increase in PGC-1α mRNA, irisin production remained unaltered or even decreased [17, 27]. By contrast, EPS-evoked myotube contraction in this study resulted in increased irisin expression and secretion. These discrepancies among study results may be attributed to variation in the ability of different approaches to trigger exercise-activated signaling and adaptive responses. Repetitive mechanical stress and intracellular calcium transients do not occur in pharmacological simulation of exercise, which may be crucial factors for irisin production.
The results of this study were consistent with studies that showed irisin stimulates phosphorylation of Akt and ERK1/2 in murine muscle cell and tissue [26, 33]. These molecules are considered important intracellular signaling molecules of IGF-1-mediated muscle growth and hypertrophy [12]. These results, along with increased IGF-1 expression, suggest that irisin leads to stimulation of muscle protein anabolism in an IGF-1-dependent manner [31].
High levels of glucocorticoid in pathological and pharmacological conditions can cause muscle breakdown due to the increased rate of protein degradation, which results from reduced PI3K-Akt signaling and consequent increase in FoxO-mediated proteolysis [6, 9]. Among the three FoxO isoforms in skeletal muscle, the activation of FoxO3 through dephosphorylation is sufficient to promote two crucial ubiquitin ligases, atrogin-1 and MuRF-1. This event leads to the hyperactivity of the proteolytic system [4, 28]. Our results showed that irisin prevented the phosphorylation of FoxO3α and suppressed the expression of atrogenes during normal conditions as well as during DEX treatment. Irisin also attenuated chymotrypsin-like enzyme activity, the major proteolytic activity of 26S proteasome, that serves as the principal machine for regulated protein degradation in eukaryotic cells [19]. Interestingly, reduced functional activity of the proteasome by irisin was observed even in basal condition without DEX treatment. Overall, these results implied that the reduced activities of FoxO subsequently down-regulated ubiquitin ligases. This muscle-specific signaling pathway was at least partly attributable to enhanced IGF-1 signaling through irisin treatment [21]. Finally, we confirmed that the decrease in myotube diameter induced by DEX was prevented by co-treating with irisin.
Although this study was conducted in an in vitro system, previous studies show that DEX-mediated changes in protein turnover rates are quite similar in cultured myotubes and in vivo muscle tissues [29]. We therefore assume that our results predict a preventive effect of irisin on glucocorticoid-induced muscle atrophy in vivo. Further research is needed, however, to understand the exact mechanism of irisin-mediated effects. Identifying the irisin receptors will help in understanding the signaling mechanism which results in the elevation of IGF-1 and its related intracellular signaling. Consequently, this study also suggests that the enhancement of muscle anabolic signaling may also be associated with the mechanisms reported in the recent works where irisin was identified as a novel myokine that improves the glucose dysregulation as well as abnormal lipid metabolism [2, 24].
Taken together, these findings suggest that irisin exerts a protective effect against muscle wasting by counteracting the effect of DEX on FoxO-mediated ubiquitin-proteasome overactivity and restoration of muscular atrophy through IGF-1-mediated signaling. We believe this study provides the basis for understanding the beneficial effects of exercise or pharmacological approaches using myokines to manage glucocorticoid-induced muscle wasting.
Electronic supplementary material
(PDF 4767 kb)
Acknowledgments
The authors gratefully acknowledge Dr. Kyu-Sang Park and Dr. Jun Namkung for their thoughtful advice and suggestions and thank to Dr. Kyoung-hye Yoon and Sohyun Kim for proofreading.
Abbreviations
- FNDC5
Fibronectin type III domain containing 5
- AKT
Protein kinase B
- ERK
Extracellular signal-regulated kinase
- IGF-1
Insulin-like growth factor-1
- DEX
Dexamethasone
- FoxO
Forkhead box O
- Atrogin-1
Muscle atrophy F-box protein
- MuRF-1
Muscle RING-finger protein-1
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Jae Seung Chang. The first draft of the manuscript was written by Jae Seung Chang, and In Deok Kong commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding information
This study was supported by the National Research Foundation of Korea grant funded by the Korea government (NRF-2018R1C1B6005036 and NRF-2017R1A5A2015369) and in part by the Yonsei University Research Fund of 2018.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bonaldo P, Sandri M. Cellular and molecular mechanisms of muscle atrophy. Dis Model Mech. 2013;6:25–39. doi: 10.1242/dmm.010389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Hojlund K, Gygi SP, Spiegelman BM. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burch N, Arnold AS, Item F, Summermatter S, Brochmann Santana Santos G, Christe M, Boutellier U, Toigo M, Handschin C. Electric pulse stimulation of cultured murine muscle cells reproduces gene expression changes of trained mouse muscle. PLoS One. 2010;5:e10970. doi: 10.1371/journal.pone.0010970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calnan DR, Brunet A. The FoxO code. Oncogene. 2008;27:2276–2288. doi: 10.1038/onc.2008.21. [DOI] [PubMed] [Google Scholar]
- 5.Chang JS, Kim TH, Nguyen TT, Park KS, Kim N, Kong ID. Circulating irisin levels as a predictive biomarker for sarcopenia: a cross-sectional community-based study. Geriatr Gerontol Int. 2017;17:2266–2273. doi: 10.1111/ggi.13030. [DOI] [PubMed] [Google Scholar]
- 6.Cohen S, Nathan JA, Goldberg AL. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat Rev Drug Discov. 2015;14:58–74. doi: 10.1038/nrd4467. [DOI] [PubMed] [Google Scholar]
- 7.Colaianni G, Mongelli T, Cuscito C, Pignataro P, Lippo L, Spiro G, Notarnicola A, Severi I, Passeri G, Mori G, Brunetti G, Moretti B, Tarantino U, Colucci SC, Reseland JE, Vettor R, Cinti S, Grano M. Irisin prevents and restores bone loss and muscle atrophy in hind-limb suspended mice. Sci Rep. 2017;7:2811. doi: 10.1038/s41598-017-02557-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong J, Dong Y, Dong Y, Chen F, Mitch WE, Zhang L. Inhibition of myostatin in mice improves insulin sensitivity via irisin-mediated cross talk between muscle and adipose tissues. Int J Obes. 2016;40:434–442. doi: 10.1038/ijo.2015.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eley HL, Tisdale MJ. Skeletal muscle atrophy, a link between depression of protein synthesis and increase in degradation. J Biol Chem. 2007;282:7087–7097. doi: 10.1074/jbc.M610378200. [DOI] [PubMed] [Google Scholar]
- 10.Fatouros IG. Is irisin the new player in exercise-induced adaptations or not? A 2017 update. Clin Chem Lab Med. 2018;56:525–548. doi: 10.1515/cclm-2017-0674. [DOI] [PubMed] [Google Scholar]
- 11.Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, Cederholm T, Chandler J, De Meynard C, Donini L, Harris T, Kannt A, Keime Guibert F, Onder G, Papanicolaou D, Rolland Y, Rooks D, Sieber C, Souhami E, Verlaan S, Zamboni M. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glass DJ. Molecular mechanisms modulating muscle mass. Trends Mol Med. 2003;9:344–350. doi: 10.1016/S1471-4914(03)00138-2. [DOI] [PubMed] [Google Scholar]
- 13.Huh JY, Panagiotou G, Mougios V, Brinkoetter M, Vamvini MT, Schneider BE, Mantzoros CS. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism. 2012;61:1725–1738. doi: 10.1016/j.metabol.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huh JY, Dincer F, Mesfum E, Mantzoros CS. Irisin stimulates muscle growth-related genes and regulates adipocyte differentiation and metabolism in humans. Int J Obes. 2014;38:1538–1544. doi: 10.1038/ijo.2014.42. [DOI] [PubMed] [Google Scholar]
- 15.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 16.Jedrychowski MP, Wrann CD, Paulo JA, Gerber KK, Szpyt J, Robinson MM, Nair KS, Gygi SP, Spiegelman BM. Detection and quantitation of circulating human irisin by tandem mass spectrometry. Cell Metab. 2015;22:734–740. doi: 10.1016/j.cmet.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurdiova T, Balaz M, Mayer A, Maderova D, Belan V, Wolfrum C, Ukropec J, Ukropcova B. Exercise-mimicking treatment fails to increase Fndc5 mRNA & irisin secretion in primary human myotubes. Peptides. 2014;56:1–7. doi: 10.1016/j.peptides.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Kurdiova T, Balaz M, Vician M, Maderova D, Vlcek M, Valkovic L, Srbecky M, Imrich R, Kyselovicova O, Belan V, Jelok I, Wolfrum C, Klimes I, Krssak M, Zemkova E, Gasperikova D, Ukropec J, Ukropcova B. Effects of obesity, diabetes and exercise on Fndc5 gene expression and irisin release in human skeletal muscle and adipose tissue: in vivo and in vitro studies. J Physiol. 2014;592:1091–1107. doi: 10.1113/jphysiol.2013.264655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livneh I, Cohen-Kaplan V, Cohen-Rosenzweig C, Avni N, Ciechanover A. The life cycle of the 26S proteasome: from birth, through regulation and function, and onto its death. Cell Res. 2016;26:869–885. doi: 10.1038/cr.2016.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez Munoz IY, Camarillo Romero EDS, Garduno Garcia JJ. Irisin a novel metabolic biomarker: present knowledge and future directions. Int J Endocrinol. 2018;2018:7816806. doi: 10.1155/2018/7816806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mavalli MD, DiGirolamo DJ, Fan Y, Riddle RC, Campbell KS, van Groen T, Frank SJ, Sperling MA, Esser KA, Bamman MM, Clemens TL. Distinct growth hormone receptor signaling modes regulate skeletal muscle development and insulin sensitivity in mice. J Clin Invest. 2010;120:4007–4020. doi: 10.1172/JCI42447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menconi M, Gonnella P, Petkova V, Lecker S, Hasselgren PO. Dexamethasone and corticosterone induce similar, but not identical, muscle wasting responses in cultured L6 and C2C12 myotubes. J Cell Biochem. 2008;105:353–364. doi: 10.1002/jcb.21833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nikolic N, Gorgens SW, Thoresen GH, Aas V, Eckel J, Eckardt K. Electrical pulse stimulation of cultured skeletal muscle cells as a model for in vitro exercise - possibilities and limitations. Acta Physiol (Oxf) 2017;220:310–331. doi: 10.1111/apha.12830. [DOI] [PubMed] [Google Scholar]
- 24.Perakakis N, Triantafyllou GA, Fernandez-Real JM, Huh JY, Park KH, Seufert J, Mantzoros CS. Physiology and role of irisin in glucose homeostasis. Nat Rev Endocrinol. 2017;13:324–337. doi: 10.1038/nrendo.2016.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Powers SK, Lynch GS, Murphy KT, Reid MB, Zijdewind I. Disease-induced skeletal muscle atrophy and fatigue. Med Sci Sports Exerc. 2016;48:2307–2319. doi: 10.1249/MSS.0000000000000975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reza MM, Subramaniyam N, Sim CM, Ge X, Sathiakumar D, McFarlane C, Sharma M, Kambadur R. Irisin is a pro-myogenic factor that induces skeletal muscle hypertrophy and rescues denervation-induced atrophy. Nat Commun. 2017;8:1104. doi: 10.1038/s41467-017-01131-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanchez J, Nozhenko Y, Palou A, Rodriguez AM. Free fatty acid effects on myokine production in combination with exercise mimetics. Mol Nutr Food Res. 2013;57:1456–1467. doi: 10.1002/mnfr.201300126. [DOI] [PubMed] [Google Scholar]
- 28.Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. doi: 10.1016/S0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schakman O, Gilson H, Thissen JP. Mechanisms of glucocorticoid-induced myopathy. J Endocrinol. 2008;197:1–10. doi: 10.1677/JOE-07-0606. [DOI] [PubMed] [Google Scholar]
- 30.Shan T, Liang X, Bi P, Kuang S. Myostatin knockout drives browning of white adipose tissue through activating the AMPK-PGC1alpha-Fndc5 pathway in muscle. FASEB J. 2013;27:1981–1989. doi: 10.1096/fj.12-225755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell. 2004;14:395–403. doi: 10.1016/S1097-2765(04)00211-4. [DOI] [PubMed] [Google Scholar]
- 32.WHO Expert Committee on the Selection and Use of Essential Medicines (19th: 2013: Geneva Switzerland), World Health Organization. (2014) The selection and use of essential medicines: report of the WHO Expert Committee, 2013 (including the 18th WHO Model List of Essential Medicines and the 4th WHO Model List of Essential Medicines for Children). WHO technical report series,, vol 985. World Health Organization, Geneva, Switzerland
- 33.Yang Z, Chen X, Chen Y, Zhao Q. Decreased irisin secretion contributes to muscle insulin resistance in high-fat diet mice. Int J Clin Exp Pathol. 2015;8:6490–6497. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 4767 kb)