Abstract
In this overview, the authors have discussed the potential advantages of the association between mycorrhizae and plants, their mutual accelerated growth under favorable conditions and their role in nutrient supply. In addition, methods for isolating mycorrhizae are described and spore morphologies and their adaptation to various conditions are outlined. Further, the significant participation of controlled greenhouses and other supported physiological environments in propagating mycorrhizae is detailed. The reviewed information supports the lack of host- and niche-specificity by arbuscular mycorrhizae, indicating that these fungi are suitable for use in a wide range of ecological conditions and with propagules for direct reintroduction. Regarding their prospective uses, the extensive growth of endomycorrhizal fungi suggests it is suited for poor-quality and low-fertility soils.
Keywords: Mycorrhiza, Arbuscule, Greenhouse, Plant-fungal association, Propagule
Introduction
Recently, the looming food security problem has highlighted plant science as an emerging discipline and led to a commitment to devising new strategies to enhance crop productivity. Biotic and abiotic stresses, such as drought, salinity, flooding, plant pathogens, nutrient deficiency and toxicity, which limit global crop productivity, are reasons for food scarcity. Given the potential for food shortages, strategies should be adopted to achieve maximum productivity and economic crop returns (Ahmad et al. 2012). The application of fertilizer is one of the methods used to obtain optimum yields, although this may cause environmental issues. If chemical fertilizers are applied continuously, they may lead to the deterioration of soil characteristics and fertility, and heavy metals can accumulate in plant tissues; eventually, the nutrition value and edibility of fruits will be affected (Mosa et al. 2014). Instead of using chemical fertilizers, biological fertilizers, such as animal manure, the decaying remains of organic matter, domestic sewage, excess crops and microorganisms (e.g., bacteria and fungi), can be used as a more sustainable alternative. Furthermore, plant growth can be improved through microbial inoculation, including inoculation with plant growth promoting rhizobacteria (PGPR) and mycorrhizal fungi. These microbes play significant roles in the promotion of plant growth through the regulation of nutrition and hormonal balances, production of plant growth regulators, solubilization of nutrients and induction of resistance against plant pathogens. In addition, plant interactions with these microbes have shown synergistic as well as antagonistic interactions with other microbes in the rhizosphere. These interactions are important for sustainable agriculture because they maintain plant growth and development through biological processes rather than through agrochemicals.
Moreover, mycorrhizae can be found in all the soils where plants can grow, and these fungi facilitate the absorption of water and nutrients by plants. Plants send sugars from their leaves to fungi as food. Further, root surface area can be increased by mycorrhizae, allowing plants to uptake water and nutrients more efficiently from a large soil volume (Nadeem et al. 2014). In addition, it has been shown that different mycorrhizal species exhibit a variety of responses depending on the plant species with which they are associated (Ortas and Ustuner 2014). This is because there is a wide range of mycorrhizal fungal species that could change the strength of plant-plant interactions, and plant growth will vary. It is widely accepted that there are higher growth rates in plants inoculated with mycorrhizae than in control plants because of the increase in photosynthetic activities. Mycorrhizae also play an important role in the supply of essential nutrients to their associated plant; interestingly, fungicides or herbicides will not affect the growth of mycorrhizae.
Glomeromycota, referred to as arbuscular mycorrhizal fungi, are one of the most significant fungi because they form mutualistic relationships with the roots of almost 90% of plant species (Stajich et al. 2009). Arbuscular mycorrhizae can be seen in the belowground parts of the earliest plant fossils and facilitate nutrient acquisition by plants in exchange for photosynthate. They are also vital to plant fitness and may determine the compositions of plant communities. Arbuscular mycorrhizae are hyphal and produce highly branched haustoria, which promotes nutrient exchange with their host root cells.
Mycorrhizal fungi
According to the definition by Brundrett in 2002, mutualistic relationships are formed between the modified absorptive organs of mycorrhizae, which mainly consist of plant roots (photobiont) and fungal hyphae (mycobiont). The main purpose of this relationship is to transfer nutrients between the organisms (Brundrett 2002). Mycorrhizal symbiosis plays an important role in ecosystems because mycorrhizae affect plant productivity and plant diversity. Usually, plant productivity is improved by mycorrhizal interactions, but this is not always the case; under different environmental conditions, symbiosis can span various species interactions, from mutualism to parasitism (Maherali 2014). Mycorrhizal fungi can even have parasitic interactions with plants when the net benefits of the symbiosis are lower than the net cost. Mycorrhizal associations are complex; thus, an understanding of the several parameters affecting the function of mycorrhizae, such as the morphology and physiology of both symbionts and biotic and abiotic factors at the rhizosphere, community and ecosystem levels, is required. In the commercial production of infected plants, several species of fungi are used, and seedling inoculation with either spores or mycelial cultures is usually the starting point (Grimm et al. 2005).
Information on fungi
Fungi are macroscopic and microscopic organisms, such as mushrooms, truffles, puffballs, and glomeromycetes (soil fungi that connect to roots); they are totally separate from the cell walls of plants and are capable of secreting a range of enzymes (Anbu et al. 2004, 2005, 2007; Gopinath et al. 2002, 2003, 2005; Kumarevel et al. 2005; Lee et al. 2015; Zaragoza 2017). Fungi can reproduce by both sexual and asexual reproduction and typically produce spores. Fungi are categorized into taxonomic ranks based mainly on morphologies that are described by their structure and phylogeny. They are further categorized by their genetic differences. However, the procedures to classify fungi according to genetic differences are complicated (Ellison et al. 2014; Grimm et al. 2005).
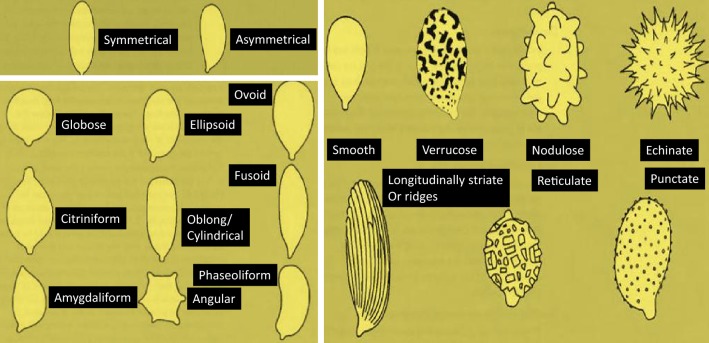
To understand fungi and simplify their identification, methods have been developed to isolate fungi, such as the pour plate and dilution-based spread plate techniques and spore isolation (Magnet et al. 2013). All isolated fungi must be obtained in pure cultures by using a standard technique (Rohilla and Salar 2011). Additionally, caution must be taken when performing isolation to avoid cross-contamination, which would significantly affect the final isolation results. Among mycorrhizal fungi, various types of spores have been found to occur (Fig. 1).
Fig. 1.
Various shapes and textures of mycorrhizal spores. Different types, which are widely distributed across a range of plants, are displayed
Wet sieving and decanting method
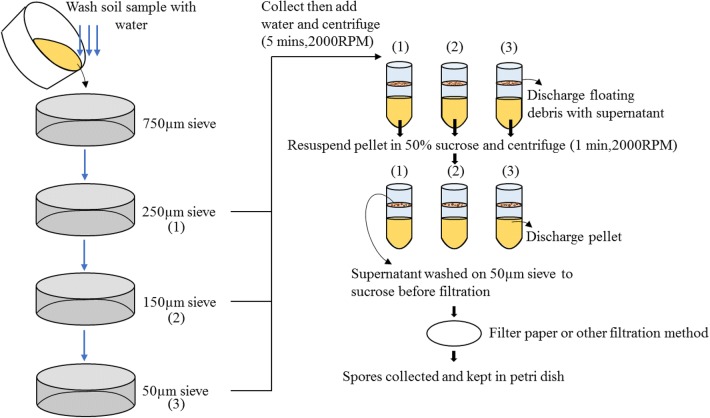
Wet sieving and decanting methods are simple spore isolation techniques introduced by Gerdemann and Nicolson to extract fungal spores from soil samples (Gerdemann and Nicolson 1963) (Fig. 2). This technique was used for sieving the coarse particles of the soil and retaining the fungal spores and organic particles on sieves of different sizes. Then, the spores are removed and collected for observation under a microscope. There are different types of spore isolation methods, such as the sucrose centrifugation method, adhesion flotation method, and capillary rise method. However, in the study of Shamini and Amutha, wet sieving and decanting methods were shown to obtain a large number of spores (Shamini and Amutha 2015).
Fig. 2.
Representation of wet sieving and decanting methods. The steps involved are shown. Both methods have been used in the past and have advantages for the separation of mycorrhizal spores. Wet sieving captures smaller spores
Analysis of fungal morphology
The analysis of fungal morphology is mainly based on fungal structures and sizes. Most fungi are composed of structures called mycelia (thread-like hyphae) and grow from the tips of fungi, similar to the binary branching of trees. Fungi can be differentiated because of their lack of a hyphal septum. Spores are the main characteristic used to identify the types of fungi. Particular representative fungi must be isolated, and then their morphologies must be observed (Tsuneo watanabe 2002). Generally, fungi are observed by using microscopes, such as stereomicroscopes, compound microscopes and scanning electron microscopes. Moreover, advances in image and particle analysis and micromechanical devices have improved morphological data (Krull et al. 2013), which means that the structures of microorganisms can be seen more clearly and in greater detail. In addition, the identification of fungi depends not only on morphology but also on fungal cultures, which can be obtained from single spore isolations (Choi et al. 1999).
Spore morphology
According to Levetin, the following characteristics can be used to identify fungi: the spore size and shape, the number of cells in the spore, the spore wall thickness, the spore color and surface ornamentation, and the attachment of scars. Compared with the sizes and shapes of spores, the spore wall characteristics are very useful for the identification of fungi. Additionally, spore walls can be smooth or consist of various ornamentations, which usually occur on the outermost surface and appear spore-like spiny, punctate, warted, striated, ridged, or reticulate (Fig. 1). Therefore, ornamentation can also be used for identification.
Types of mycorrhizae
Mycorrhizae are divided into two types based on the structure of their hyphae. The fungal hyphae that do not penetrate the individual cells within the roots are known as ectomycorrhizal fungi, while the hyphae of fungi that penetrate the cell wall and invaginate the cell membrane are called endomycorrhizal fungi (Szabo et al. 2014). In addition, according to Heijden and Martin, four main types of mycorrhizal fungi have been classified: arbuscular mycorrhizae, ectomycorrhizae, orchid mycorrhizae and ericoid mycorrhizae (van der Heijden et al. 2015). Furthermore, endomycorrhizae include the arbuscular, ericoid, and orchid mycorrhizae, while arbutoid mycorrhizae can be classified as ectomycorrhizae and monotropoid mycorrhizae form a special category.
Arbuscular mycorrhizae
Vesicular–arbuscular mycorrhizal fungi (VAM) and soil fungi are alternative terms for arbuscular mycorrhizal fungi (Vogelsang et al. 2004). These fungi belong to the Glomeromycota and are believed to have an asexual reproductive strategy. Plants depend heavily on these fungi to reach their optimal growth potential. Arbuscular mycorrhizal symbiosis is the most common non-pathogenic symbiosis in the soil and is found in 80% of vascular plant roots (Brundrett 2002). Additionally, arbuscular mycorrhizae only grow in association with appropriate host plants and plant species and vary by host. Arbuscules are fungal structures growing into individual plant cells (Fig. 3). Arbuscular mycorrhizal (AM) fungi can be found within almost all phyla in the Angiosperms (Duhoux et al. 2001). According to Miranda and Jennifer, AM fungi not only improve phosphorus nutrition to plants but also enhance the uptake of zinc, copper, nitrogen and iron (Hart and Forsythe 2012). They are also resistant to some root diseases and drought tolerant.
Fig. 3.
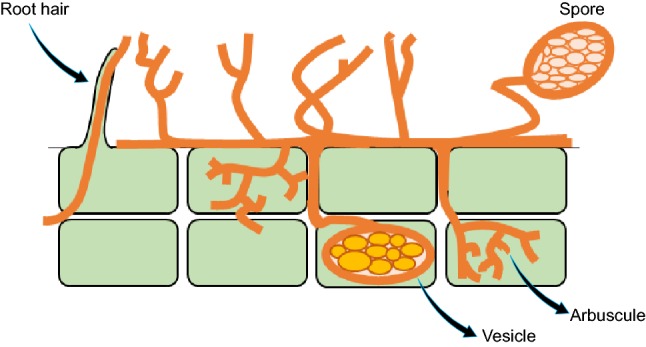
Growth pattern of arbuscular mycorrhizae. The formation of vesicles is shown. Arbuscular mycorrhizae are widely reported and commonly used. This is mainly due to their attraction to phosphorous sources. The arbuscules form a vesicle
Ectomycorrhizae (EM)
Ectomycorrhizae are a large group (Szabo et al. 2014) with a widespread distribution but only 3–4% of the vascular plant families are associated with these fungi (Brundrett 2004). Chiefly, EM are members of the phyla Ascomycota and Basidiomycota, and EM mutualism is thought to be independently derived multiple times from saprophytic lineages (Merckx 2012). Plant species that form EM mutualisms have been shown to have antimicrobial components that protect the plants from root pathogens. These fungi are characterized by their growth on the exterior surfaces of roots (Schnepf et al. 2008). Thus, roots are covered by fungal tissue, and the covering is known as a hyphal mantle (Fig. 4). Strands of mycelium, the fungal filaments, extend from the hyphal mantle into the soil and act as the roots of the plant, absorbing minerals and nutrients.
Fig. 4.
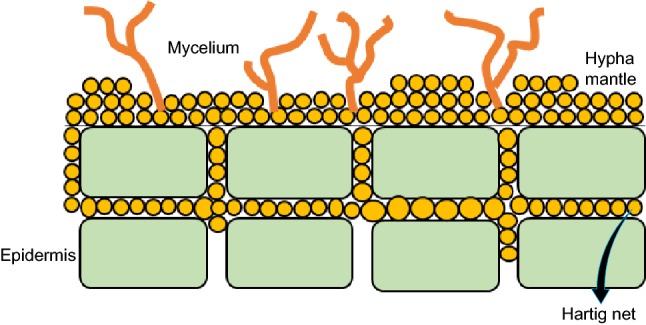
The growth pattern of endomycorrhizae. The structure of endomycorrhizae is different from that of ectomycorrhizae
Formation of mycorrhizae
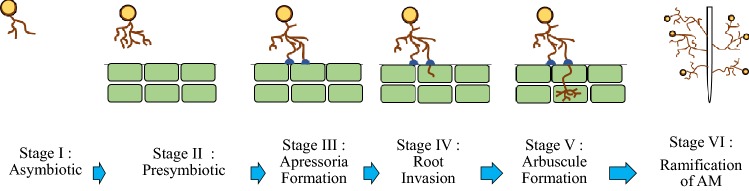
Arbuscular mycorrhizal fungi go through several developmental stages in the formation of mycorrhizae (Fig. 5). During the symbiotic stage, spores are germinated and limited hyphal development by arbuscular mycorrhizal fungi has been found due to the absence of host plants. However, after germination, the spores enter the presymbiotic stage, which is characterized by extensive hyphal branching when root exudates are present (Kuo et al. 2014). In addition, appressoria are formed once the fungus contacts a root surface and before the hyphae penetrate the root epidermis. This is followed by the symbiotic colonization of the root cortex tissue, which involves the formation of intracellular arbuscules (tree-like, heavily branched structures) or hyphal coils, and concomitantly, the production of a unique extraradical mycelium occurs. Host plants play a primary role in orchestrating the arbuscular mycorrhizal infection process, and it is tempting to speculate that similar changes occur during the colonization of the cortical cells. Overall, these developmental processes require molecular communication between the arbuscular mycorrhizae and the plant, including the exchange and perception of signals by the symbiotic partners.
Fig. 5.
Developmental stages of arbuscular mycorrhizae. The different stages are indicated. Generally, six stages are involved in the formation of a complete association
Mycorrhizal plant interactions
There are two nutrient uptake pathways for roots colonized by mycorrhizae: the plant uptake pathway (PP) and the mycorrhizal uptake pathway (MP) (Fig. 6). The PP involves the uptake of nutrients directly through the transporter and occurs in the root epidermis and root hairs. In the MP, nutrients are indirectly transferred via fungal transporters in the extraradical mycelium (ERM) of the fungus and transported to the hartig net in EM interactions or to the intraradical mycelium (IRM) in arbuscular mycorrhizal interactions (shown in the mycorrhizal interface). The uptake by mycorrhiza-inducible plant transporters occurs in the periarbuscular membrane from the interfacial apoplast. The displayed fungal structures indicate the colonization of one host root by multiple fungal species, which can differ in their efficiency. Through these processes, the fungi are able to obtain nutrients from the soil and transfer these nutrients to their hosts (Bucking et al. 2012).
Fig. 6.
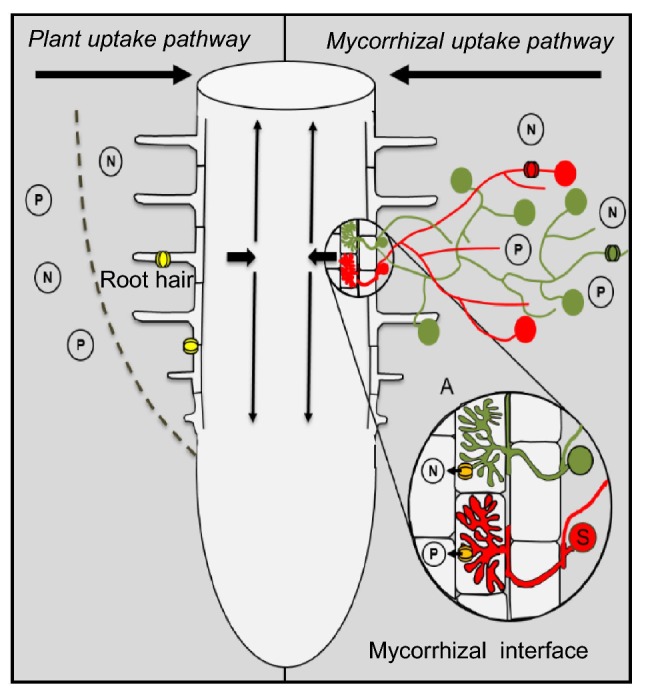
Nutrient uptake pathways. The involvement of the plant uptake pathway and mycorrhizal uptake pathway is shown. The major nutrients are indicated, and the directions of the movements are displayed
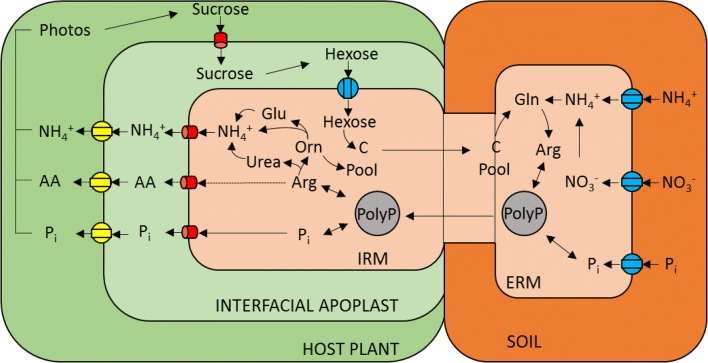
The rapid uptake of nutrients, for example, ‘P’, by roots leads to the formation of a depletion zone, and fungal hyphae extend beyond the depletion zones to penetrate and exploit a larger volume of soil to uptake nutrients. From the uptake by the hyphae, nutrients are translocated within the hyphal network to the fungal sheath, intercellularly to the hartig net and with the exception of ectomycorrhizae and monotropoid mycorrhizae, intracellularly into plant cells. The fungal sheath is able to store nutrients, supporting the fungi by continuing to provide nutrients to the plant host when the soil nutrient levels decrease. When receiving extra nutrients, mycorrhizal plants lose between 10 and 20% of the photosynthates they produce; these photosynthates are used by the mycorrhizal fungi and their associated structures for development, maintenance and operation.
Nutrients are moved by the fungal ERM via Pi, NO3− or NH4+ transporters (blue); ‘N’ is assimilated into Arg through the anabolic arm of the urea cycle (this is shown only for arbuscular mycorrhizae); and Pi is converted into polyP in the ERM. The polyP is then transported from the ERM to the IRM. The polyP is hydrolyzed and Arg and Pi are released in the IRM. Arg breaks down to NH4+ in the catabolic arm of the urea cycle (this is shown only for arbuscular mycorrhizae). This process is facilitated by Pi, NH4+, and potential amino acid (AA, possibly only in the EM) efflux through the fungal plasma membrane (red) into the interfacial apoplast. The nutrients are taken up by the plant from the mycorrhizal interface through mycorrhiza-inducible Pi or NH4+ transporters. Photosynthesis is stimulated by the improved nutrient supply and is facilitated by the efflux of sucrose through the plant plasma membrane into the interfacial apoplast, sucrose hydrolysis in the interfacial apoplast via an apoplastic plant invertase, and the uptake of hexoses by the mycorrhizal fungi through the fungal monosaccharide transporters.
Phosphate uptake
Mycorrhizal symbioses are recognized for their importance in plant nutrition and ionic transport, particularly in phosphorus uptake (Fig. 7). To maintain crop yields, modern agricultural systems are highly dependent on the continual inputs of phosphate-based fertilizers. These fertilizers are processed from phosphate rock, which is a non-renewable natural resource. Therefore, the world could soon face a resource scarcity crisis that might affect global food security. Arbuscular mycorrhizae form a symbiosis with the roots of nearly all vascular plants and could play a key role in solving the phosphate shortage problem. Additionally, by improving the efficiency of nutrient uptake and by increasing plant resistance to pathogens and abiotic stresses, mycorrhizal symbiosis can enhance plant growth and therefore reduce the need for phosphate-based fertilizers. Herein, we provide an update of recent findings and reports on mycorrhizae as the cornerstone of a "second green revolution" and the types of mycorrhizae that enhance plant growth. Different types of mycorrhizae may be obtained from different plant growth materials.
Fig. 7.
Transport mechanisms in arbuscular and ectomycorrhizal interactions. The transports in the plants and soils are shown. The molecules and ions generally involved in transport are displayed
Plant growth under physiological conditions
Numerous parameters can be used to measure plant growth. The most common and simple measurements are the number of leaves, plant height, and plant color. These parameters may be seen by the naked eye or using tools for daily measurement. Most farmers evaluate the changes in these parameters to assess the growth of their plants because they are easy to observe. In addition, some complicated parameters, such as leaf dry matter content, stem specific density, and the pH of green leaves, can be used to evaluate plant growth (Pérez-Harguindeguy et al. 2013). The use of these parameters requires specific formulas and calculations.
Plant growth under greenhouse conditions
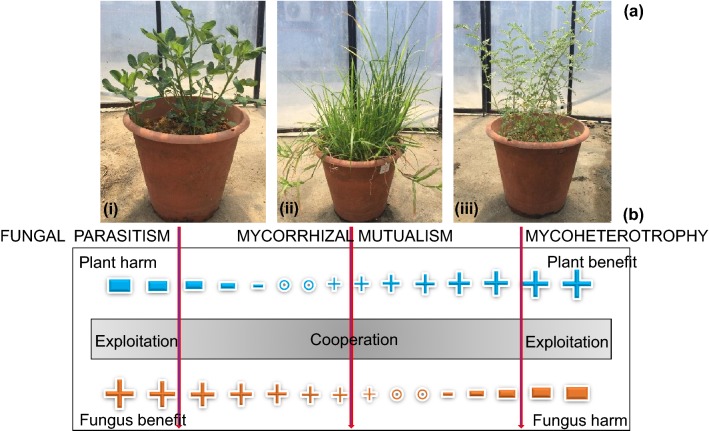
Plants are protected from adverse conditions and growers are able to control growth conditions when plants are grown in greenhouses (Fig. 8a). A high yield, good quality, uniformity, and precise timing of delivery can be achieved with good control. A variety of crops are grown in greenhouses, the products of which include leaves, roots, bulbs, tubers, flowers, fruits, seeds, young plants, and mature plants (herbs, salad plants, bedding plants, potted plants, and garden plants). Technology is required to control the growing conditions in greenhouses, primarily for heating and venting and optionally for root-zone heating, cooling, fogging, misting, CO2 enrichment, shading, assimilation light, day-length extension and black-out timing. Light, temperature, and humidity are three growth factors that have been identified (Nederhoff 2007). These factors influence plant growth and can be controlled in greenhouses.
Fig. 8.
a Mycorrhizal-associated plants grown in a greenhouse. (i) groundnut; (ii) onion; and (iii) chickpea. b Classifying symbiotic interactions. The classes include mutualism, parasitism, and commensalism. The mutualistic plant-fungus interaction is the midpoint of the continuum of interactions and the exploitation of a plant by a mycorrhizal fungus is the endpoint
Study lines with greenhouses
Greenhouse technology is used for sustainable crop production in regions with adverse climatic conditions. High summer temperatures are a major issue for successful greenhouse crop production throughout the year (Kumar et al. 2009). Greenhouse technology refers to the production of plants for economic use in a covered structure, which allows the rapid harvesting of solar radiation and the modification of agroclimatic conditions conducive to plant growth and development. This technology embraces infrastructure modeling, selection of plants for adaptation, production economics, agronomic management and commercial potential. Therefore, greenhouse crop productivity is largely independent of outdoor environmental conditions (Reddy 2016).
Enhanced mycorrhizal associations under controlled greenhouse conditions
The definition of symbiosis (two or more organisms living together) can be applied to all mycorrhizal associations (Brundrett 2004; Crops 2015). The term mutualism indicates mutual benefits in associations involving two or more living organisms. In a review by Brundrett, the terms ‘balanced’ and ‘exploitative’ are proposed for mutualistic and non-mutualistic mycorrhizal associations, respectively (Brundrett 2004). Most mycorrhizal associations, except mycoheterotrophic associations, are ‘balanced’ mutualistic associations, in which the fungus and plant benefit from the association. Mycoheterotrophic plants have an ‘exploitative’ mycorrhizal association that benefits only the plants. Thus, exploitative associations are symbiotic but are not mutualistic. In the research of Bronstein, it was proposed that rather than classifying symbiotic interactions into distinct categories (e.g., mutualism, parasitism, and commensalism), they should be viewed as dynamic points along a continuum (Merckx 2012). The mutualistic plant–fungus interaction is the midpoint, and the exploitation of a plant by a mycorrhizal fungus is the endpoint (Fig. 8b). In mycoheterotrophic interactions, the mycorrhizal fungi are exploited by the plants to obtain carbon and other nutrients (Merckx 2012).
Creating a beneficial environment for endomycorrhizal (VAM) colonies requires a plant to have a symbiotic relationship with a VAM. First, the amount of inorganic phosphorus should be low in the soil solution; mycorrhizae will not grow or colonize roots when the phosphorus level is high. This is because the relationship between plants and fungi evolved to help the plants access low levels of phosphorus in the soil. Mycorrhizae cannot grow or establish when phosphorus levels are above 10 ppm in the soil. The mycorrhizae are not killed; they create an environment in which they do not germinate or grow and are rendered ineffective. When plant roots release sugars and hormones and form an association with mycorrhizal spores, the spores start to germinate. This trigger allows the spores to stay dormant, suspended in the soil, until plants actively grow. Thus, the shelf life of mycorrhizae is typically longer (up to two years) than that of other biological additives. As far as it is known, the typical lime addition rates and moderate pH levels of professional growing medium products do not have a significant positive or negative effect on the growth and colonization of mycorrhizae. Mycorrhizae can be used with other bioproducts. There are other helper bacteria or fungi that are often added to mycorrhizal blends, which stimulate and support the growth of the mycorrhizal colonies. In addition, at the beginning of production, chemical fungicides should be avoided until time has passed to allow root colonization (Miller 2012). Although there is a long history of mycorrhizal research, it is still continuing due to the potential of mycorrhizae to benefit humans and society and boost economies (Bauer et al. 2020; Quiroga et al. 2020; Wulantuya et al. 2020).
Prospective uses
In the agricultural field, there are several variables when growing plants outdoors, including weather, watering, fertilization and soil quality. These variables can introduce the potential for greater plant stress and therefore highly benefit from endomycorrhizal fungi. Endomycorrhizae can be incorporated directly into the soil, but if plants are grown in a growth medium, their roots will continue to be colonized even after transplanting into the soil. The benefits are the same as those potentially seen by the grower and include resistance to transplant shock and increased numbers of fruits and flowers. Unlike roots, endomycorrhizal fungi establish quickly in new soil environments. Therefore, they can ease transplant shock by providing water and nutrients for the plant and serve as a buffer to help the plant adjust to its new soil environment. Plants reach their optimum growth rate with endomycorrhizae as a result of reduced stress; therefore, edible plants have the ability and resources to produce more vegetables/fruits and larger vegetables/fruits per plant and flowering plants often produce more flowers. Plants such as beech, willow, birch, pine, fir, oak, and spruce receive many benefits from mycorrhizal associations, and the association of legume and cereal plants with mycorrhizae increases the benefits of these plants to humans. Overall, plants are often larger when grown with endomycorrhizal fungi, especially if plants are grown in poor-quality and low-fertility soils.
Acknowledgements
The author would like to acknowledge the support from Malaysia Fundamental Research Grant Scheme (FRGS) to H.I.Z. (Grant number 9003-00750) and Short Term Grant by Universiti Malaysia Perlis to S.C.B.G. (Grant number 9001-00558).
Author contributions
All the authors contributed to the preparation of the manuscript and discussion. Both authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
On behalf of all the authors, the corresponding author states that there is no conflict of interest.
References
- Ahmad P, Kumar A, Gupta A, Hu X, Hakeem R, Azooz MM, Sharma S. Crop production for agricultural improvement. Crop Prod Agric Improv. 2012 doi: 10.1007/978-94-007-4116-4. [DOI] [Google Scholar]
- Anbu P, Hilda A, Gopinath SCB. Keratinophilic fungi of poultry farm and feather dumping soil in Tamil Nadu, India. Mycopathologia. 2004;158:303–309. doi: 10.1007/s11046-004-3465-1. [DOI] [PubMed] [Google Scholar]
- Anbu P, Gopinath SCB, Hilda A, Lakshmi T, Annadurai G. Purification of keratinase from poultry farm isolate-Scopulariopsis brevicaulis and statistical optimization of enzyme activity. Enzyme Microb Tech. 2005;36:639–647. doi: 10.1016/j.enzmictec.2004.07.019. [DOI] [Google Scholar]
- Anbu P, Gopinath SCB, Hilda A, Lakshmipriya T, Annadurai G. Optimization of extracellular keratinase production by poultry farm isolate Scopulariopsis brevicaulis. Bioresour Technol. 2007;98:1298–1303. doi: 10.1016/j.biortech.2006.05.047. [DOI] [PubMed] [Google Scholar]
- Bauer JT, Koziol L, Bever JD. Local adaptation of mycorrhizae communities changes plant community composition and increases aboveground productivity. Oecologia. 2020 doi: 10.1007/s00442-020-04598-9. [DOI] [PubMed] [Google Scholar]
- Brundrett MC. Coevolution of roots and mycorrhizas of land plants. New Phytol. 2002;154:275–304. doi: 10.1046/j.1469-8137.2002.00397.x. [DOI] [PubMed] [Google Scholar]
- Brundrett M. Diversity and classification of mycorrhizal associations. Biol Rev Camb Philos Soc. 2004;79:473–495. doi: 10.1017/S1464793103006316. [DOI] [PubMed] [Google Scholar]
- Bucking H, Liepold E, Ambilwade P (2012) The role of the mycorrhizal symbiosis in nutrient uptake of plants and the regulatory mechanisms underlying these transport processes. World â€TM s largest Science, Technology and Medicine Open Access book publisher. Intech Open
- Choi Y, Hyde KD, Ho WWH. Single spore isolation of fungi. Fungal Divers. 1999;3:29–38. [Google Scholar]
- Crops V. Effect of four mycorrhizal products on Fusarium root rot on different vegetable crops. Plant Pathol Microbiol. 2015;6:2–6. doi: 10.4172/2157-7471.1000255. [DOI] [Google Scholar]
- Duhoux E, Rinaudo G, Diem HG, Auguy F, Fernandez D, Bogusz D, Franche C, Dommergues Y, Huguenin B. Angiosperm Gymnostoma trees produce root nodules colonized by arbuscular mycorrhizal fungi related to Glomus. New Phytologist. 2001;149:115–125. doi: 10.1046/j.1469-8137.2001.00005.x. [DOI] [PubMed] [Google Scholar]
- Ellison CE, Kowbel D, Glass NL, Taylor JW, Brem RB. Discovering functions of unannotated genes from a transcriptome survey of wild fungal isolates. MBio. 2014;5:e01046–e1113. doi: 10.1128/mBio.01046-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdemann JW, Nicolson TH. Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans Br Mycol Soc. 1963;46:235–244. doi: 10.1016/S0007-1536(63)80079-0. [DOI] [Google Scholar]
- Gopinath SCB, Hilda A, Priya TL, Annadurai G. Purification of lipase from Cunninghamella verticillata and optimization of enzyme activity using response surface methodology. World J Microbiol Biotechnol. 2002;18:449–458. doi: 10.1023/A:1015579121800. [DOI] [Google Scholar]
- Gopinath SCB, Hilda A, Lakshmi Priya T, Annadurai G, Anbu P. Purification of lipase from Geotrichum candidum: conditions optimized for enzyme production using Box-Behnken design. World J Microbiol Biotechnol. 2003;19:681–689. doi: 10.1023/A:1025119222925. [DOI] [Google Scholar]
- Gopinath SCB, Anbu P, Hilda A. Extracellular enzymatic activity profiles in fungi isolated from oil-rich environments. Mycoscience. 2005;46:119–126. doi: 10.1007/s10267-004-0221-9. [DOI] [Google Scholar]
- Grimm LH, Kelly S, Krull R, Hempel DC. Morphology and productivity of filamentous fungi. Appl Microbiol Biotechnol. 2005;69:375–384. doi: 10.1007/s00253-005-0213-5. [DOI] [PubMed] [Google Scholar]
- Hart MM, Forsythe JA. Scientia Horticulturae Using arbuscular mycorrhizal fungi to improve the nutrient quality of crops; nutritional benefits in addition to phosphorus. Sci Hortic (Amsterdam) 2012;148:206–214. doi: 10.1016/j.scienta.2012.09.018. [DOI] [Google Scholar]
- Krull R, Wucherpfennig T, Esfandabadi ME, Walisko R, Melzer G, Hempel DC, Kampen I, Kwade A, Wittmann C. Characterization and control of fungal morphology for improved production performance in biotechnology. J Biotechnol. 2013;163:112–123. doi: 10.1016/j.jbiotec.2012.06.024. [DOI] [PubMed] [Google Scholar]
- Kumarevel TS, Gopinath SCB, Hilda A, Gautham N, Ponnusamy MN. Purification of lipase from Cunninghamella verticillata by stepwise precipitation and optimized conditions for crystallization. World J Microbiol Biotechnol. 2005;21:23–26. doi: 10.1007/s11274-004-1005-2. [DOI] [Google Scholar]
- Kumar KS, Tiwari KN, Jha MK. Design and technology for greenhouse cooling in tropical and subtropical regions: a review. Energy Build. 2009;41:1269–1275. doi: 10.1016/j.enbuild.2009.08.003. [DOI] [Google Scholar]
- Kuo A, Kohler A, Martin FM, Grigoriev IV. Expanding genomics of mycorrhizal symbiosis. Front Microbiol. 2014;5:1–7. doi: 10.3389/fmicb.2014.00582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LP, Karbul HM, Citartan M, Gopinath SCB, Lakshmipriya T, Tang TH. Lipase-secreting Bacillus species in an oil-contaminated habitat : promising strains to alleviate oil pollution. BioMed Res Int. 2015;2015:820575. doi: 10.1155/2015/820575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnet MH, Sarkar D, Ahmed Z. Samples Of different industry side in Dhaka city, Bangladesh. Int J Innov Res Dev. 2013;2:338–339. [Google Scholar]
- Maherali H. Is there an association between root architecture and mycorrhizal growth response? New Phytol. 2014;204:192–200. doi: 10.1111/nph.12927. [DOI] [PubMed] [Google Scholar]
- Merckx VSFT. Mycoheterotrophy: the biology of plants living on fungi. Mycoheterotrophy Biol Plants Living Fungi. 2012 doi: 10.1007/978-1-4614-5209-6. [DOI] [Google Scholar]
- Miller BM (2012) Using mycorrhizae in a professional mix.
- Mosa W, Paszt L, EL-Megeed N. The role of bio-fertilization in improving fruits productivity-a review. Adv Microbiol. 2014;4:1057–1064. doi: 10.4236/aim.2015.51003. [DOI] [Google Scholar]
- Nadeem SM, Ahmad M, Zahir ZA, Javaid A, Ashraf M. The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol Adv. 2014;32:429–448. doi: 10.1016/j.biotechadv.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Nederhoff EM (2007) Using a greenhouse for controlling plant growth© 57: 126–133.
- Ortas I, Ustuner O. Determination of different growth media and various mycorrhizae species on citrus growth and nutrient uptake. Sci Hortic (Amsterdam) 2014;166:84–90. doi: 10.1016/j.scienta.2013.12.014. [DOI] [Google Scholar]
- Pérez-Harguindeguy N, Díaz S, Garnier E, et al. New handbook for standardised measurement of plant functional traits worldwide. Aust J Bot. 2013;61:167–234. doi: 10.1071/BT12225. [DOI] [Google Scholar]
- Quiroga G, Erice G, Aroca R, Delgado-Huertas A, Ruiz-Lozano JM. Elucidating the possible involvement of maize aquaporins and arbuscular mycorrhizal symbiosis in the plant ammonium and urea transport under drought stress conditions. Plants (Basel) 2020;9:E148. doi: 10.3390/plants9020148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PP. Sustainable crop protection under protected cultivation. Sustain Crop Prot under Prot Cultiv. 2016 doi: 10.1007/978-981-287-952-3. [DOI] [Google Scholar]
- Rohilla SK, Salar RK. Isolation and characterization of various fungal strains from agricultural soil contaminated with pesticides. Res J Recent Sci. 2011;1:297–303. doi: 10.1016/j.scienta.2009.07.019. [DOI] [Google Scholar]
- Schnepf A, Roose T, Schweiger P. Growth model for arbuscular mycorrhizal fungi. J R Soc Interface. 2008;5:773–784. doi: 10.1098/rsif.2007.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamini S, Amutha K (2015) Techniques for extraction. Int J Front Sci Technol pp 0–6
- Stajich J, Berbee ML, Blackwell M, et al. The fungi. Curr Biol. 2009;19:R840–R845. doi: 10.1016/j.cub.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo K, Böll S, Erős-Honti ZS. Applying artificial mycorrhizae in planting urban trees. Appl Ecol. 2014;12:835–853. doi: 10.15666/aeer/1204. [DOI] [Google Scholar]
- Tsuneo W. Pictorial atlas of soil and seed fungi: morphologies of cultured fungi and key to species. 2. Boca Raton: CRC Press; 2002. [Google Scholar]
- van der Heijden MGA, Martin FM, Selosse MA, Sanders IR. Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytol. 2015;205:1406–1423. doi: 10.1111/nph.13288. [DOI] [PubMed] [Google Scholar]
- Vogelsang KM, Bever JD, Griswold M, Schultz PA (2004) The use of mycorrhizal fungi in erosion control applications. Contract 1–150.
- Wulantuya MK, Bayandala FY, Matsukura K, Seiwa K. Gap creation alters the mode of conspecific distance-dependent seedling establishment via changes in the relative influence of pathogens and mycorrhizae. Oecologia. 2020;192:449–462. doi: 10.1007/s00442-020-04596-x. [DOI] [PubMed] [Google Scholar]
- Zaragoza O. Mycology. Ref Modul Life Sci. 2017 doi: 10.1016/B978-0-12-809633-8.12378-7. [DOI] [Google Scholar]