Abstract
Background
Acute lymphocytic leukemia (ALL) is a common blood cancer which induces high mortality in children. Bromodomains and extra-terminal (BET) protein inhibitors, such as JQ1 and ARV-825, are promising cancer therapeutic agents that can be used by targeting c-Myc. A recent work reported that JQ1 effectively attenuates ALL in vitro by suppressing cell proliferation and accelerating apoptosis. The purpose of this research was to probe into the potential mechanism of how JQ1 inhibits ALL cell proliferation in vitro.
Material/Methods
Cell viability of ALL cells were measured by CTG after treatment by JQ1. Cell cycle analysis was done by EdU and PI staining. Cell apoptosis was assessed by Annexin V/PI staining. Glycolysis was detected using Seahorse and LC-MS kits. The expression of glycolytic rate-limiting enzymes was assessed by RNA-seq, qRT-PCR, and Western blot.
Results
JQ1 suppressed cell proliferation by arresting the cell cycle and inducing the apoptosis of acute lymphocytic leukemia cells. JQ1 inhibited cell proliferation of B-ALL cells by restraining glycolysis. Conversely, the cell cycle block of B-ALL cells induced by JQ1 was partially abolished after pretreatment with 2-Deoxy-D-glucose (2-DG), an inhibitor of glycolysis. Furthermore, JQ1 restrained the glycolysis of B-ALL cell lines by remarkably downregulating the rate-limiting enzymes of glycolysis, such as hexokinase 2, phosphofructokinase, and lactate dehydrogenase A. Moreover, the cell cycle arrest was reversed in B-ALL cells with overexpressed c-Myc treated by JQ1, which is involved in the enhancement of glycolysis.
Conclusions
The BET inhibitor JQ1 suppresses the proliferation of ALL by inhibiting c-Myc-mediated glycolysis, thus providing a new strategy for the treatment of ALL.
MeSH Keywords: Cell Cycle; Genes, myc; Glycolysis; Precursor Cell Lymphoblastic Leukemia-Lymphoma
Background
Acute lymphocytic leukemia (ALL) is a common blood cancer which causes high mortality in children [1]. Although the survival period of most patients can be extended through chemotherapy treatment, some individuals still show no response to chemotherapy or cannot tolerate the adverse effects of long-term chemotherapy [2]. Therefore, studies should explore new treatment strategies for ALL from an innovative perspective [3]. Tumor cells reprogram their metabolism from catabolism to anabolism to fuel cell rapid proliferation [4]. Similar to other solid tumors, previous studies have shown that ALL cells prefer glycolysis as the main energy source under sufficient oxygen conditions, a phenomenon called the Warburg effect [5]. This metabolic process can create substantial intracellular biomass, providing intermediates for the synthesis of proteins, lipids, nucleic acids, and other macromolecules required for cell proliferation [6–8].
Bromodomain-containing protein 4 (BRD4) belongs to the bromodomains and extra-terminal (BET) protein family. BET proteins regulate tumor development by regulating the expression of multiple transcription factors, including c-Myc and Bcl-2 [9–11]. c-Myc is encoded by proto-oncogene c-MYC, a metabolic sensor, and functions as a central regulator of proliferation, differentiation, and apoptosis [12,13]. Overexpressed c-Myc promotes cell rapid proliferation and accelerates the metastasis process in a variety of malignant tumors, whereas BRD4-specific inhibitor JQ1 can induce cell apoptosis and significantly restrain progression of malignancy, suggesting that BRD4 can mediate the fate of tumor cells by c-Myc [14–16]. Also, a recent work reported that JQ1 effectively attenuates ALL in vitro by suppressing cell proliferation and accelerating apoptosis [17], but how JQ1 affects the metabolism of ALL and its underlying mechanisms has never been reported.
In our research, we found that c-Myc promoted rapid cell proliferation and improved the glycolysis ability of B-ALL cell lines, while JQ1 restrained the proliferation of ALL by suppressing c-Myc-mediated glycolysis. Our results may provide new therapeutic strategies for ALL.
Material and Methods
Cell lines
Human B-ALL cell lines (NALM6, REH, SEM, and RS411) and 293T cell lines were obtained from the ATCC (American Type Culture Collection). B-ALL and 293T cell lines were cultured in PRMI1640 and DMEM (HyClone) containing 10% fetal bovine serum (Thermo Fisher) and 1% penicillin-streptomycin (Gibco).
RNA extraction and quantitative reverse transcription-polymerase chain reaction (qRT-PCR) analysis
Total cellular RNA was extracted by TRIzol reagent (Life Technologies). Reverse transcription and real-time RT-PCR was conducted according to the protocol of the PrimeScript RT reagent Kit (Takara). After the cDNA was synthesized, qRT-PCR was performed following the protocol of the QIAGEN SYBR Green PCR kit. The primers used were:
-
β-actin: forward (F):
5′-TCTGGCACCACACCTTCTACAAT-3′ and
reverse (R): 5′-TGGGGTGTTGAAGGTCTCAAA-3′;
-
hexokinase 2 (HK2):
F: 5′-CTCTC TGCAACCAGTTCTCTG-3′ and
R: 5′-CCAGGCATTCGGCAATGTG-3′;
-
lactate dehydrogenase A (LDHA):
F: 5′-ATGGCAACTCTAAAGGATCAGC-3′ and
R: 5′-CCAACCCCAACAACTGTAATCT-3;
-
pyruvate kinase M2 (PKM2):
F: 5′-ATGTCGAAGCCCCATAGTGAA-3′ and
R: 5′-TGGGTGGTG AATCAATGTCCA-3′.
Western blot analysis
Western blot analysis was conducted by the conventional method [15]. The primary antibodies mainly used were HK2 (CST #2867), LDHA (CST #3582), Phosphofructokinase, Platelet (PFKP) (CST #8164), PKM2 (CST #4053), C-MYC (CST # 9402S), BCL-2 (Abcam ab32124), cleaved caspase 9 (Abcam ab3253), β-actin (HuaAn M1201-2), GAPDH (CST #51740), and β-tubulin (HuaAn EM0103). The antibodies were subsequently detected by fluorescently labeled or horseradish peroxidase-linked secondary antibodies.
Cell viability assay
Cell viability was assessed with CellTiter-Glo Luminescent Cell Viability Assay (Promega). The cells were plated and treated with gradient concentrations of JQ1 (Selleck, USA). After 72 h, the concentration of JQ1 was analyzed based on the fluorescence value when the drug killed 50% of the cells.
Cell proliferation assay
The cells were plated and treated with JQ1, and the cells were counted after trypan blue staining from days 1 to 5.
Cell cycle analysis
This assay was conducted following instructions of the Click-iT EdU Flow Cytometry Assay Kit (Life Technologies). The cells were plated and treated with JQ1. After 48 h, EdU was added to the cells and incubated for 1 h. After fixation, permeabilization, and staining, the cells were labeled with propidium iodide (PI). The analysis was made using a flow cytometer (Becton Dickinson).
Cell apoptosis analysis
The assay was measured following the protocol of the Annexin V Apoptosis Detection Kit (BD). The samples were assessed through flow cytometry.
Construction of overexpression stable cell lines
The c-Myc and vector plasmids were acquired from Dr. Y Li (Wuhan University, China). The package and concentration of virus were determined as previously reported [17]. NALM6 and REH were cultured with the enriched virus medium and selected with puromycin to construct stable cell lines. Finally, the overexpression efficiency was verified.
Quantification of lactate and ATP analysis
The intracellular lactate and ATP of JQ1 or dimethyl sulfoxide (DMSO)-treated cells were determined following the instructions of the Glycolysis Cell-Based Assay Kit (Cayman) and Enhanced ATP Assay Kit (Beyotime Biotechnology), respectively.
Glucose uptake assay
2-NBDG (Invitrogen) was used to test the glucose uptake ability of the cells. Briefly, the treated cells were labeled with fluorescent 2-NBDG for 30 min in a 5% CO2 incubator. After removing all media and washing with PBS, the samples were measured by flow cytometric analysis.
Seahorse analysis
First, the cells were plated and treated with JQ1. Subsequently, the cells were resuspended in running buffer for 30 min and analyzed by an XF96 extracellular flux analyzer (Agilent Technologies). Based on the time points of glucose, oligomycin, and 2-DG injection, the basic conditions of glycolysis, maximal glycolytic capacity, and non-glycolytic activity were detected.
Metabolite analysis
First, a total of 1×107 cells were harvested. Next, the cells were cracked in ice-cold 80% methanol and centrifuged to obtain the supernatant. Finally, the supernatant was analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS).
RNA-seq analysis
RNA extraction was done using TRIzol. The experimental process included the RNA-seq protocols suggested by BGI (China). First, total RNA was reversed to cDNA for constructing the library, and the sequencing was conducted on the cDNA library. The raw reads were filtered and clean reads were mapped according to the Bowtie2 and HISAT. Then, the gene expression level (FPKM) was calculated. The data were analyzed using the BGI online system.
Statistical analysis
The data are presented as the mean±standard deviation from at least 3 independent experiments. The t test was used for comparisons between 2 groups. P<0.05 was viewed as statistically significant.
Results
JQ1 restrained the proliferation of B-ALL cell lines
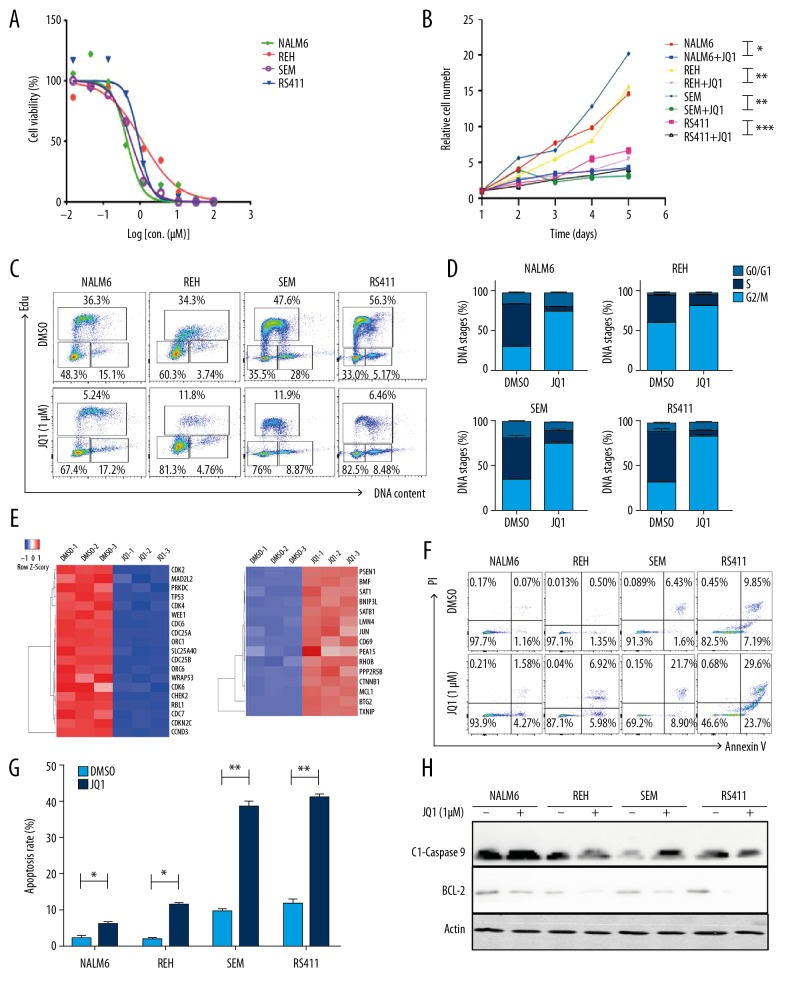
To explore the cytotoxic effects of JQ1 on B-ALL cells, we tested the cell viability after NALM6, REH, SEM, and RS411 cell lines were treated with a gradient concentration of JQ1 for 72 h. The half-maximal inhibitory concentration of REH, NALM6, SEM, and RS411 reached 1.16, 0.93, 0.45, and 0.57 μM, respectively (Figure 1A). The cell viability of all cell lines significantly declined in a time-dependent manner after 1-μM JQ1 treatment for 5 days (Figure 1B). Next, we measured the cell cycle by staining with EdU and PI. The vast majority of B-ALL cells were distributed in the S/G2/M phase (Figure 1C), whereas the cell populations of G0/1 phase dramatically increased after treatment with JQ1 for 48 h (Figure 1D), suggesting that JQ1 restrained the proliferation of B-ALL cells through blocking the cell cycle. RNA-seq data showed that the mRNA level of the cell cycle-related genes cyclin-dependent kinase 2 (CDK2) and CDK4 decreased after JQ1 treatment. The expression of apoptotic genes such as MCL1 was increased by JQ1 treatment (Figure 1E). Moreover, assessment of cell apoptosis of B-ALL cells treated by JQ1 for 48 h showed that the apoptotic rates of B-ALL cell lines increased, especially those of SEM and RS411, indicating that JQ1 induced apoptosis in B-ALL cells (Figure 1F, 1G). Meanwhile, the expression of apoptotic protein cleaved caspase 9 and anti-apoptotic protein Bcl-2 were assessed by Western blot. The results displayed that cleaved caspase 9 was markedly upregulated and Bcl-2 was observably downregulated in JQ1-treated B-ALL cells (Figure 1H).
Figure 1.
JQ1 restrained the proliferation of B-ALL cell lines. (A) Drug sensitivity assay of NALM6, REH, SEM, and RS411 cell lines after treatment with gradient concentrations of JQ1 for 72 h. (B) The cell growth curve of NALM6, REH, SEM, and RS411 cells after treatment with DMSO or JQ1 (1 μM) for 5 days. (C) EdU- and PI-labeled cell cycle of NALM6, REH, SEM, and RS411 cells were analyzed after treatment with DMSO or JQ1 (1 μM) for 48 h. (D) Statistical analysis of cell cycle distributions in C. (E) Heatmap of cell cycle and apoptosis-related genes detected by RNA-seq in NALM6 cells after treatment with DMSO or JQ1 (1 μM) for 48 h. (F) Annexin V- and PI-labeled cell apoptosis of NALM6, REH, SEM, and RS411 cells analyzed by flow cytometry after DMSO or JQ1 (1 μM) treatment for 48 h. (G) Statistical analysis of cell apoptosis rates in F. (H) Expressions of cleaved caspase 9 and BCL-2 detected by Western blot in NALM6, REH, SEM, and RS411 cells after treatment with DMSO or JQ1 (1 μM) for 48 h.* P<0.05; ** P<0.01.
JQ1 suppressed the growth of B-ALL cell lines by inhibiting glycolysis
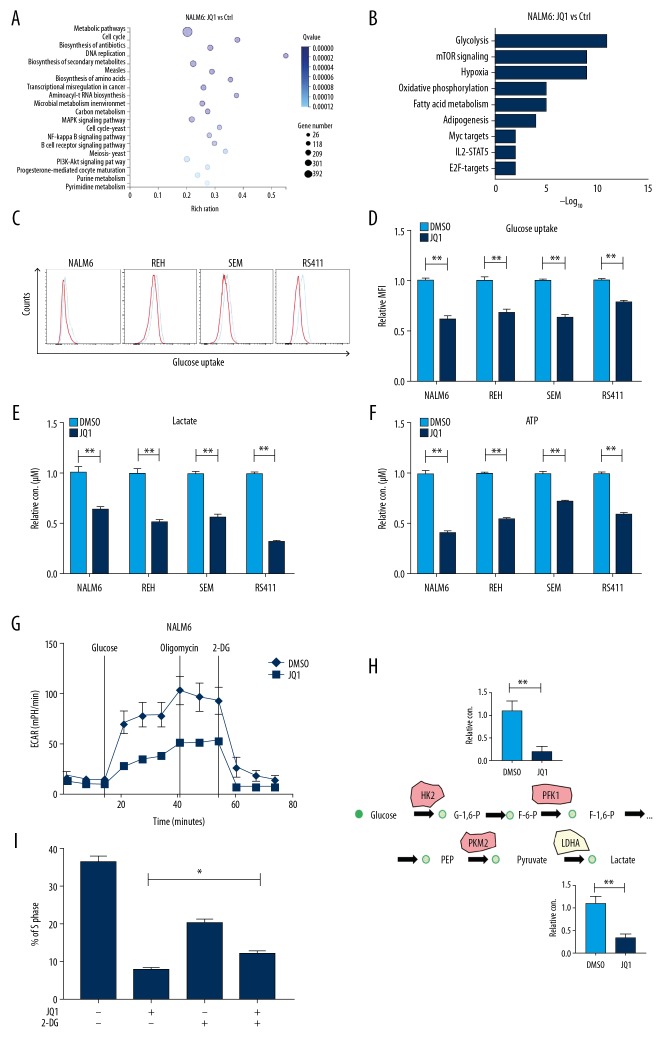
The proliferation of tumor cells needs sufficient nutrients, including glucose, lipids, and amino acids. Therefore, tumor cells reprogram their metabolism from catabolism to anabolism to fuel cell proliferation [18]. However, the influence of JQ1 on the energy metabolism of B-ALL cells has not been previously reported. We analyzed the RNA-seq data and discovered that JQ1 significantly downregulated the mRNA level of metabolic pathways, cell cycle, and DNA replication (Figure 2A). In addition, the downregulated genes were enriched in glycolysis, mammalian target of rapamycin signaling, and oxidative phosphorylation (Figure 2B), indicating that JQ1 significantly inhibited the glycolysis of B-ALL cells. To confirm that JQ1 impedes anaerobic glycolysis of B-ALL cells, we measured the glucose uptake of B-ALL cells after treatment with JQ1 for 48 h. The results demonstrated that JQ1 clearly reduced the glucose uptake capacity of B-ALL cell lines in varying degrees, suggesting that glucose metabolism was affected (Figure 2C, 2D). Lactate production and ATP content of B-ALL cells were determined by relevant reagent kits. The levels of lactate and ATP notably declined after JQ1 treatment for 48 h, indicating that JQ1 inhibits glycolysis of leukemia cells (Figure 2E, 2F, respectively). Furthermore, we used the extracellular acidification rate (ECAR) to directly determine the glycolysis capacity of NALM6 cells, and found that the maximal glycolytic capacity of cells dramatically decreased after JQ1 treatment (Figure 2G). Moreover, to further confirm that JQ1 reduced the anaerobic glycolysis of B-ALL cells, we measured the intermediate products of glycolysis in JQ1-treated B-ALL cells by LC-MS/MS, and found that the levels of metabolic intermediates of glycolysis, such as glucose-6-phosphate (G-6-P) and lactate, were significantly lower in JQ1-treated B-ALL cells (Figure 2H). To verify that JQ1 suppressed the proliferation of B-ALL cell lines by inhibiting glycolysis, the cell cycle of B-ALL cells was measured after treatment with 2-Deoxy-D-glucose (2-DG), a glycolysis inhibitor. We observed the cell cycle block after JQ1 treatment was partially abolished in 2-DG-pretreated cells (Figure 2I).
Figure 2.
JQ1 suppressed the growth of B-ALL cell lines by inhibiting glycolysis. (A) Bubble diagram of enrichment analysis in NALM6 cells measured by RNA-seq after treatment with DMSO or JQ1 (1 μM) for 48 h. (B) Pathway analysis of downregulated metabolic genes (False Discovery Rate <0.05) in JQ1-treated NALM6 cells. (C) Glucose uptake of NALM6, REH, SEM, and RS411 cells detected by flow cytometry after treatment with DMSO or JQ1 (1 μM) for 48 h. (D) Statistical analysis of NALM6, REH, SEM, and RS411 cells estimated using the related kits after DMSO or JQ1 (1 μM) treatment for 48 h. (E) Quantification of lactate production in NALM6, REH, SEM, and RS411 cells assessed using the related kit after treatment with DMSO or JQ1 (1 μM) for 48 h. (F) Quantification of intracellular ATP in NALM6, REH, SEM, and RS411 cells assessed using the related kits after treatment with DMSO or JQ1 (1 μM) for 48 h. (G) Detection of ECAR in NALM6 cell after treatment with DMSO or JQ1 (1 μM) for 48 h by seahorse. (H) Schematic map of glycolytic metabolism and the metabolic intermediates of glycolysis measured by LC-MS/MS. (I) The cell cycle of JQ1 (2 uM)-treated NALM6 cells analyzed by flow cytometry after pretreatment with 2DG (5mM) for 12 h. * P<0.05; ** P<0.01.
JQ1 restrained the glycolysis of B-ALL cell lines by downregulating the expression of metabolic enzymes
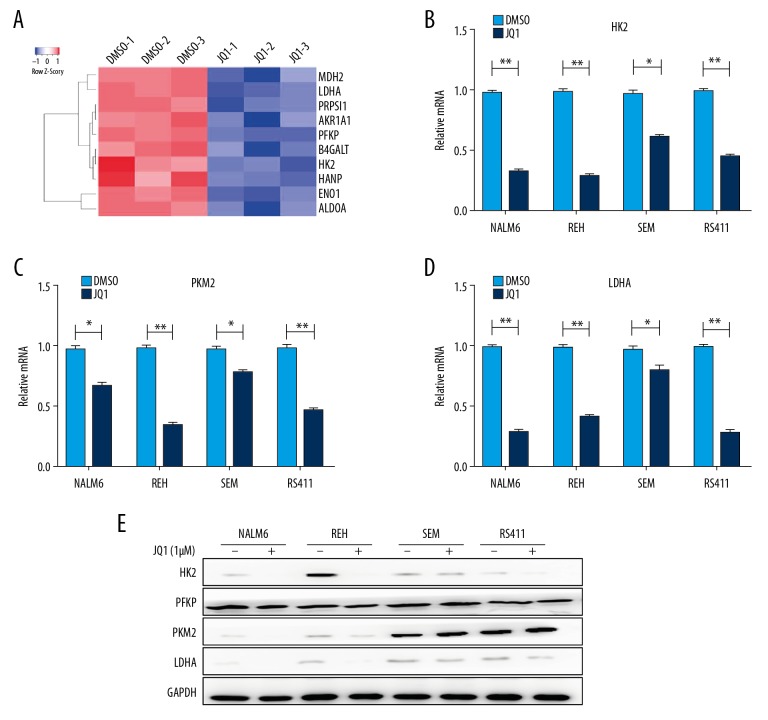
Glycolysis is the initial step in glucose metabolism and involves 9 reactions, each of which is catalyzed by distinct rate-limiting enzymes, such as HK, phosphofructokinase, and pyruvate kinase [19]. To determine whether JQ1 represses the glycolytic metabolism of B-ALL cells through downregulating the expression of metabolic enzymes, we reanalyzed the RNA-seq data and found that JQ1 remarkably downregulated the rate-limiting enzymes of glycolytic metabolism, such as HK2, PFKP, and LDHA (Figure 3A). We validated the expression of glycolytic rate-limiting enzymes by qRT-PCR and revealed that JQ1 dramatically inhibited the expression of HK2, PKM2, and LDHA (Figure 3B–3D). The protein expressions of the rate-limiting enzymes of glycolytic metabolism were detected by Western blot, showing that JQ1 significantly downregulated the expressions of HK2, PFKP, PKM2, and LDHA (Figure 3E).
Figure 3.
JQ1 restrained the glycolysis of B-ALL cell lines by downregulating the expression of metabolic enzymes. (A) Heatmap of glycolysis-related genes analyzed by RNA-seq in NALM6 cells after treatment with DMSO or JQ1 (1 μM) for 48 h. (B–D) Relative mRNA expression levels of HK2, PKM2, and LDHA measured by qRT-PCR in NALM6, REH, SEM, and RS411 cells after treatment with DMSO or JQ1 (1 μM) for 48 h. (E) Protein expressions of HK2, PFKP, PKM2, and LDHA detected by Western blot in NALM6, REH, SEM, and RS411 cells after DMSO or JQ1 (1 μM) treatment for 48 h.* P<0.05; ** P<0.01.
JQ1 induced cell cycle block by inhibiting c-Myc-mediated glycolysis
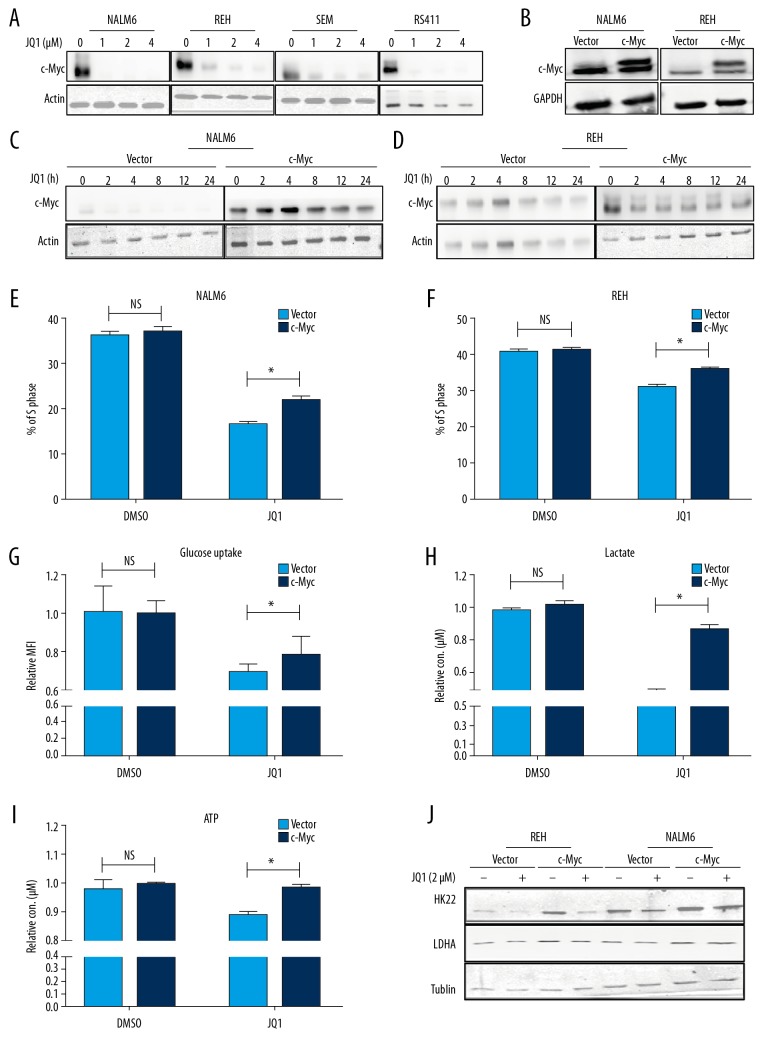
C-Myc is the most commonly reported target of BET inhibitors [20]. c-Myc regulates the expression of many glycolysis-related genes such as PKM2 and LDHA, and it concomitantly activates cell division cycle protein 25A (CDC25A) gene expression [21–23]. We hypothesized that JQ1 leads to cell cycle block by repressing the expression of c-Myc-mediated glycolytic genes. To test this hypothesis, we first confirmed that JQ1 suppressed the proliferation of B-ALL cells by downregulating c-Myc (Figure 4A). To test whether the reduction of glycolysis of leukemia cells induced by JQ1 is mediated through c-Myc, we overexpressed c-Myc on NALM6 and REH cell lines by lentivirus infection and confirmed that the protein levels of c-Myc were upregulated in NALM6 and REH cells (Figure 4B). The overexpression of c-Myc could not be inhibited by treatment with JQ1 (Figure 4C, 4D). We discovered that the cell populations in the S phase were reversed in c-Myc-overexpressing B-ALL cells after treatment with JQ1 (Figure 4E, 4F). We also discovered that the levels of glucose uptake, intracellular lactate, and ATP were dramatically rescued in c-Myc-overexpressing B-ALL cells after treatment with JQ1 (Figure44G–4I), indicating that JQ1 restrained the glycolysis of B-ALL cells by inhibiting c-Myc expression. Finally, we assessed the protein levels of glycolysis key enzymes in c-Myc-overexpressing cells after JQ1 treatment, and observed that the protein expressions of these enzymes were rescued in c-Myc-overexpressing B-ALL cells (Figure 4J).
Figure 4.
JQ1 induced cell cycle arrest by inhibiting c-Myc-mediated glycolysis. (A) Protein expression of c-Myc determined by Western blot in NALM6, REH, SEM, and RS411 cells after treatment with DMSO or JQ1 (1 μM) (1, 2, and 4 μM) for 48 h. (B) Overexpression level of c-Myc in NALM6 and REH cells was checked by Western blot. (C, D) The expression level of c-Myc in NALM6 and REH cells tested by Western blot after treatment with DMSO or JQ1 (1 μM) in a time course. (E, F) EdU- and PI-labeled cell cycle of c-Myc-overexpressing NALM6 and REH cells analyzed by flow cytometry after DMSO or JQ1 (2 μM) treatment for 24 h. (G) Quantification of intracellular lactate in vector and c-Myc-overexpressing NALM6 cells after DMSO or JQ1 (2 μM) treatment for 24 h. (H) Quantification of intracellular ATP in vector and c-Myc-overexpressing NALM6 cells after DMSO or JQ1 (2 μM) treatment for 24 h. (I) Glucose uptake of vector and c-Myc-overexpressing NALM6 cells analyzed by flow cytometry after treatment with DMSO or JQ1 (2 μM) for 24 h. (J) Levels of HK2 and LDHA in c-Myc-overexpressing NALM6 and REH cells tested by Western blot after treatment with DMSO or JQ1 (2 μM) for 24 h.* P<0.05.
Discussion
The 5-year survival rate of patients with ALL has reached over 80% through use of risk-stratified combination chemotherapy or bone marrow transplantation and improved supportive care [24]. However, almost 20% of ALL patients in remission still have relapse, which leads to treatment failure [25]. Therefore, therapeutic strategies must be developed to overcome drug resistance and ALL relapse. Our study shows that JQ1 can effectively attenuate B-ALL in vitro by suppressing cell proliferation and accelerating apoptosis. These results are consistent with previous observations on solid tumors [11,14,26,27]. Moreover, our data indicate that triggering cell cycle arrest, which results in more cells in the resting state, is the key to treatment with JQ1 [28]. The rapid proliferation of tumor cells is accompanied by enhanced glycolysis in most cases [4,7,29–31]. However, the effect of JQ1 on the metabolism of acute lymphocytic leukemia cells is unknown. In the present study, we first noted that JQ1 can significantly affect the glycolytic metabolism of B-ALL cells by inhibiting glucose absorption and metabolic process and eventually causing the reduction of metabolic intermediates, such as lactate and ATP, which are the main materials and energy sources for cell synthesis. According to the results of RNA-seq, JQ1 suppressed the glycolytic process by inhibiting the expression of glycolysis key enzymes, including hexokinase 2, phosphofructokinase, and lactate dehydrogenase A. We also found that the glycolysis inhibitor 2-DG blocked the cell cycle arrest of B-ALL cells induced by JQ1, suggesting JQ1 suppressed the proliferation of B-ALL by partially inhibiting glycolysis. JQ1 not only affected glycolysis, but also altered the mitochondrial oxidative phosphorylation metabolism of B-ALL cells, but the mechanism is unclear.
As a BRD4-specific inhibitor, JQ1 can mediate the proliferation and apoptosis of a variety of tumor cells by c-Myc [26,32]. In our study, the level of c-Myc decreased in B-ALL cells after JQ1 treatment. Because c-Myc plays an important part in cell metabolism [12,13,33,34], we hypothesized that glycolysis repression mediated by JQ1 is due to the reduction of the c-Myc level. Then, we carried out a rescue experiment by overexpressing c-Myc, and observed that the effect of JQ1 decreased, accompanied by the relief of cell cycle arrest and glycolysis, suggesting that the inhibited glycolysis and cell cycle arrest of JQ1 was mediated by c-Myc.
Through detecting the therapeutic effect of JQ1 on B-ALL cell lines, we confirmed that glycolysis is involved in these leukemic cells, and further extended understanding of the mechanism of JQ1 in the treatment of B-ALL by inhibiting the c-Myc-mediated glycolysis. These findings offer a new treatment strategy for clinical practice in ALL.
Conclusions
Our study verified that c-Myc promoted rapid cell proliferation and improved the glycolysis ability of B-ALL cell lines, whereas JQ1 restrained the proliferation of ALL by inhibiting c-Myc-mediated glycolysis. Our results are clinically significant and may provide a new treatment strategy for ALL.
Footnotes
Conflicts of interest
None.
Source of support: This work was supported by the National Key R&D Program of China, Stem Cell and Translation Research (No. 2016YFA0102000), the National Natural Science Foundation of China (No. 81570121; 81870082), the Hospital-Public Cross-Link [2] Project of Shanghai Jiao Tong University (No. YG2017MS31), and the “Dawn” Program of Shanghai Education Commission, China (No. 19SG14)
References
- 1.Wu C, Li W. Genomics and pharmacogenomics of pediatric acute lymphoblastic leukemia. Crit Rev Oncol Hematol. 2018;126:100–11. doi: 10.1016/j.critrevonc.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Uderzo C, Dini G, Locatelli F, et al. Treatment of childhood acute lymphoblastic leukemia after the first relapse: Curative strategies. Haematologica. 2000;85:47–53. [PubMed] [Google Scholar]
- 3.Kamel-Reid S, Letarte M, Doedens M, et al. Bone marrow from children in relapse with pre-B acute lymphoblastic leukemia proliferates and disseminates rapidly in scid mice. Blood. 1991;78:2973–81. [PubMed] [Google Scholar]
- 4.Bose S, Le A. Glucose metabolism in cancer. Adv Exp Med Biol. 2018;1063:3–12. doi: 10.1007/978-3-319-77736-8_1. [DOI] [PubMed] [Google Scholar]
- 5.Vaupel P, Schmidberger H, Mayer A. The Warburg effect: Essential part of metabolic reprogramming and central contributor to cancer progression. Int J Radiat Biol. 2019;95:912–19. doi: 10.1080/09553002.2019.1589653. [DOI] [PubMed] [Google Scholar]
- 6.Ishibashi K, Egami R, Nakai K, et al. An Anti-tumorigenic role of the Warburg effect at emergence of transformed cells. Cell Struct Funct. 2018;43:171–76. doi: 10.1247/csf.18018. [DOI] [PubMed] [Google Scholar]
- 7.Ferreira LM. Cancer metabolism: The Warburg effect today. Exp Mol Pathol. 2010;89:372–80. doi: 10.1016/j.yexmp.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Spencer NY, Stanton RC. The Warburg effect, lactate, and nearly a century of trying to cure cancer. Semin Nephrol. 2019;39:380–93. doi: 10.1016/j.semnephrol.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Ghoshal A, Yugandhar D, Srivastava AK. BET inhibitors in cancer therapeutics: A patent review. Expert Opin Ther Pat. 2016;26:505–22. doi: 10.1517/13543776.2016.1159299. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Wang B, Zhao N, et al. Pharmacological targeting of BET bromodomains inhibits lens fibrosis via downregulation of MYC expression. Invest Ophthalmol Vis Sci. 2019;60:4748–58. doi: 10.1167/iovs.19-27596. [DOI] [PubMed] [Google Scholar]
- 11.Fiskus W, Cai T, DiNardo CD, et al. Superior efficacy of cotreatment with BET protein inhibitor and BCL2 or MCL1 inhibitor against AML blast progenitor cells. Blood Cancer J. 2019;9:4. doi: 10.1038/s41408-018-0165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stine ZE, Walton ZE, Altman BJ, et al. MYC, metabolism, and cancer. Cancer Discov. 2015;5:1024–39. doi: 10.1158/2159-8290.CD-15-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dang CV, Le A, Gao P. MYC-induced cancer cell energy metabolism and therapeutic opportunities. Clin Cancer Res. 2009;15:6479–83. doi: 10.1158/1078-0432.CCR-09-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang G, Deng W, Liu Y, et al. General mechanism of JQ1 in inhibiting various types of cancer. Mol Med Rep. 2020;21:1021–34. doi: 10.3892/mmr.2020.10927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Otto C, Schmidt S, Kastner C, et al. Targeting bromodomain-containing protein 4 (BRD4) inhibits MYC expression in colorectal cancer cells. Neoplasia. 2019;21:1110–20. doi: 10.1016/j.neo.2019.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park I, Yang HN, Jeon SY, et al. Anti-tumor activity of BET inhibitors in androgen-receptor-expressing triple-negative breast cancer. Sci Rep. 2019;9:13305. doi: 10.1038/s41598-019-49366-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Costa DD, Agathanggelou A, Perry T, et al. BET inhibition as a single or combined therapeutic approach in primary paediatric B-precursor acute lymphoblastic leukaemia. Blood Cancer J. 2013;3:e126. doi: 10.1038/bcj.2013.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lebelo MT, Joubert AM, Visagie MH. Warburg effect and its role in tumourigenesis. Arch Pharm Res. 2019;42:833–47. doi: 10.1007/s12272-019-01185-2. [DOI] [PubMed] [Google Scholar]
- 19.Counihan JL, Grossman EA, Nomura DK. Cancer metabolism: Current understanding and therapies. Chem Rev. 2018;118:6893–923. doi: 10.1021/acs.chemrev.7b00775. [DOI] [PubMed] [Google Scholar]
- 20.Andrieu G, Belkina AC, Denis GV. Clinical trials for BET inhibitors run ahead of the science. Drug Discov Today Technol. 2016;19:45–50. doi: 10.1016/j.ddtec.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang J, Cao R, Zhang Y, et al. PKM2 dephosphorylation by Cdc25A promotes the Warburg effect and tumorigenesis. Nat Commun. 2016;7:12431. doi: 10.1038/ncomms12431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Icard P, Fournel L, Wu Z, et al. Interconnection between metabolism and cell cycle in cancer. Trends Biochem Sci. 2019;44:490–501. doi: 10.1016/j.tibs.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Roy D, Gao Y, Herve S, et al. Interplay between cancer cell cycle and metabolism: Challenges, targets and therapeutic opportunities. Biomed Pharmacother. 2017;89:288–96. doi: 10.1016/j.biopha.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 24.Dinner S, Lee D, Liedtk M. Current therapy and novel agents for relapsed or refractory acute lymphoblastic leukemia. Leuk Lymphoma. 2014;55:1715–72. doi: 10.3109/10428194.2013.856428. [DOI] [PubMed] [Google Scholar]
- 25.Kato M, Manabe A. Treatment and biology of pediatric acute lymphoblastic leukemia. Pediatr Int. 2018;60:4–12. doi: 10.1111/ped.13457. [DOI] [PubMed] [Google Scholar]
- 26.Zhou S, Zhang S, Wang L, et al. BET protein inhibitor JQ1 downregulates chromatin accessibility and suppresses metastasis of gastric cancer via inactivating RUNX2/NID1 signaling. Oncogenesis. 2020;9:33. doi: 10.1038/s41389-020-0218-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maggisano V, Celano M, Malivindi R, et al. Nanoparticles loaded with the BET inhibitor JQ1 block the growth of triple negative breast cancer cells in vitro and in vivo. Cancers (Basel) 2019;12 doi: 10.3390/cancers12010091. pii: E91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bandopadhayay P, Piccioni F, O’Rourke R, et al. Neuronal differentiation and cell-cycle programs mediate response to BET-bromodomain inhibition in MYC-driven medulloblastoma. Nat Commun. 2019;10:2400. doi: 10.1038/s41467-019-10307-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fortunato S, Bononi G, Granchi C, et al. An update on patents covering agents that interfere with the cancer glycolytic cascade. Chem Med Chem. 2018;13:2251–65. doi: 10.1002/cmdc.201800447. [DOI] [PubMed] [Google Scholar]
- 30.Yang J, Ren B, Yang G, et al. The enhancement of glycolysis regulates pancreatic cancer metastasis. Cell Mol Life Sci. 2020;77:305–21. doi: 10.1007/s00018-019-03278-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim J, DeBerardinis R. Mechanisms and implications of metabolic heterogeneity in cancer. Cell Metab. 2019;30:434–46. doi: 10.1016/j.cmet.2019.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li W, Gupta SK, Han W, et al. Targeting MYC activity in double-hit lymphoma with MYC and BCL2 and/or BCL6 rearrangements with epigenetic bromodomain inhibitors. J Hematol Oncol. 2019;12:73. doi: 10.1186/s13045-019-0761-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsieh AL, Walton ZE, Altman BJ, et al. MYC and metabolism on the path to cancer. Semin Cell Dev Biol. 2015;43:11–21. doi: 10.1016/j.semcdb.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Secombe J, Pierce SB, Eisenman RN. Myc: A weapon of mass destruction. Cell. 2004;117:153–56. doi: 10.1016/s0092-8674(04)00336-8. [DOI] [PubMed] [Google Scholar]