Abstract
Background
Community-acquired pneumonia (CAP) is a leading cause of sepsis and common presentation to emergency department (ED) with a high mortality rate. The prognostic prediction value of sequential organ failure assessment (SOFA) and quick SOFA (qSOFA) scores in CAP in ED has not been validated in detail. The aim of this research is to investigate the prognostic prediction value of SOFA, qSOFA, and admission lactate compared with that of other commonly used severity scores (CURB65, CRB65, and PSI) in septic patients with CAP in ED.
Methods
Adult septic patients with CAP admitted between Jan. 2017 and Jan. 2019 with increased admission SOFA ≥ 2 from baseline were enrolled. The primary outcome was 28-day mortality. The secondary outcome included intensive care unit (ICU) admission, mechanical ventilation, and vasopressor use. Prognostic prediction performance of the parameters above was compared using receiver operating characteristic (ROC) curves. Kaplan–Meier survival curves were compared using optimal cutoff values of qSOFA and admission lactate.
Results
Among the 336 enrolled septic patients with CAP, 89 patients died and 247 patients survived after 28-day follow-up. The CURB65, CRB65, PSI, SOFA, qSOFA, and admission lactate levels were statistically significantly higher in the death group (P < 0.001). qSOFA and SOFA were superior and the combination of qSOFA + lactate and SOFA + lactate outperformed other combinations of severity score and admission lactate in predicting both primary and secondary outcomes. Patients with admission qSOFA < 2 or lactate ≤ 2 mmol/L showed significantly prolonged survival than those patients with qSOFA ≥ 2 or lactate > 2 mmol/L (log-rank χ2 = 59.825, P < 0.001). The prognostic prediction performance of the combination of qSOFA and admission lactate was comparable to the full version of SOFA (AUROC 0.833 vs. 0.795, Z = 1.378, P=0.168 in predicting 28-day mortality; AUROC 0.868 vs. 0.895, Z = 1.022, P=0.307 in predicting ICU admission; AUROC 0.868 vs. 0.845, Z = 0.921, P=0.357 in predicting mechanical ventilation; AUROC 0.875 vs. 0.821, Z = 2.12, P=0.034 in predicting vasopressor use).
Conclusion
qSOFA and SOFA were superior to CURB65, CRB65, and PSI in predicting 28-day mortality, ICU admission, mechanical ventilation, and vasopressor use for septic patients with CAP in ED. Admission qSOFA with lactate is a convenient and useful predictor. Admission qSOFA ≥ 2 or lactate > 2 mmol/L would be very helpful in discriminating high-risk patients with a higher mortality rate.
1. Introduction
Community-acquired pneumonia (CAP), a major cause of sepsis and the 8th leading cause of death, is a common respiratory tract infection encountered in the emergency department (ED) [1]. CAP is caused by virus, bacteria, or fungi, and its symptoms include cough, chest pain, fever, and dyspnea. Diagnosis of CAP is determined by symptoms, physical examinations, laboratory findings, and chest radiographs, as well as etiologic agent culture. Severity evaluation is of vital importance for treatment location selection, empirical antimicrobial initiation, and adjunctive and supportive treatment adoption [2]. For CAP severity assessment, the CURB65 (confusion, urea > 7 mmol/L, respiratory rate ≥ 30/min, blood pressure < 90 mmHg systolic and/or ≤60 mmHg diastolic, and age ≥ 65 years) and pneumonia severity index (PSI) score are most widely used worldwide [3, 4]. Preliminary research studies demonstrated that there were no significant differences in overall test performance between CURB65 and PSI [5].
CAP accounts for substantial mortality worldwide, with a high risk of developing respiratory failure and septic shock. The third international consensus definitions for sepsis and septic shock generated new definitions and updated the clinical criteria [6]. Sepsis was defined as life-threatening organ dysfunction caused by a dysregulated host response to infection, and organ dysfunction was defined as an increase in the sequential organ failure assessment (SOFA) score of 2 or higher [6], highlighting the clinical implication of (SOFA) score and qSOFA (respiratory rate ≥ 22/min, altered mentation, and systolic blood pressure ≥ 100 mmHg) score [6]. Lactate has been utilized as a marker to evaluate the acid-base homeostasis and perfusion status of patients. Higher lactate levels are reported to be associated with higher hospital mortalities and longer length of ED and hospital stay in unselected patients [7, 8]. However, the prognostic value of SOFA score, qSOFA score, and lactate in patients with CAP in the emergency department (ED) has not been fully elucidated.
In the present study, we explored the prognostic prediction value of SOFA, qSOFA, and admission lactate in patients with CAP in ED, and the results were compared with that of other commonly used CAP severity scores (CURB65, CRB65, PSI).
2. Patients and Methods
This was a single-center, retrospective cohort study carried out in Beijing Chao-yang Hospital, Capital Medical University, which is a tertiary referral hospital located in the northern region of China with approximately 250,000 annual ED visits per year. The Institutional Review Board and Medical Ethics Committee have approved this study, and written informed consent was waived because of the retrospective design of this study.
Adult patients with the discharge diagnosis of CAP admitted between Jan. 2017 and Jan. 2019 were screened. The inclusion criteria were age ≥ 18 years, new infiltrates on chest radiograph, and two or more symptoms including cough, fever, dyspnea, sputum production, breathlessness, and/or chest pain [9]. In accordance with sepsis 3.0 criteria, we only enrolled CAP patients with increased SOFA score ≥ 2 from baseline. The following patients were excluded from this study: (1) age < 18 years; (2) patients with metastatic tumor, acquired immunodeficiency syndrome (AIDS), active tuberculosis, previous transplantation, immunosuppressive therapy, and pregnancy; (3) patients transferred from other hospitals or diagnosed with hospital acquired pneumonia; (4) patients with incomplete clinical, laboratory, or radiographic records; (5) patients with increased admission SOFA score < 2 from baseline.
Demographic characteristics of all enrolled patients on ED arrival were collected and recorded by trained triage nurses on admission. Past history, comorbidities, and vital signs (blood pressure, heart rate, breath rate, and state of consciousness) were also recorded. Laboratory parameters on admission including full blood count, hemoglobin level (HGB), hemocrit (HCT), platelet level (PLT), albumin (ALB), hepatic function (aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin (TBIL), direct bilirubin (DBIL)), renal function (creatinine (CREA), blood urea nitrogen (BUN)), electrolytes, and arterial blood gas including lactate level were assessed and collected. CURB65, CRB65, PSI, SOFA, and qSOFA scores on admission for each patient were calculated according to international criteria and analyzed, utilizing data collected on ED arrival.
All patients were followed up for 28 days through medical records, and 28-day mortality was the primary study end point. According to their prognosis after 28-day admission, patients were divided into death group and survival group. The secondary outcome included ICU admission, mechanical ventilation use, and vasopressor use.
All analyses were performed using SPSS 22.0 statistical software package (SPSS Inc, Chicago, IL, USA). Data with normal distribution were described as mean ± standard deviation and compared using Student's t-test. Data with skewed distribution were expressed as median (interquartile range) and compared using the Mann–Whitney U nonparametric test. The categorical variables were described as percentages and compared using the chi-squared test or Fisher's exact test. Receiver operating characteristics (ROC) curves for each predictor were constructed, and the area under the curve (AUC) was determined to assess their predictive values. Combination models of severity score and lactate were established using several logistic regressions to save the predicted probabilities. ROC curve analysis was performed using the saved probabilities as a new indicator. Comparisons of each predictor were conducted using MedCalc 15.0 Software (Acacialaan, Ostend, Belgium). A Z-test was used for comparing the AUCs between different curves. For comparison of the AUCs, Z = (A1−A2)/ was used, the test values being Z0.05 = 1.96 and Z0.01 = 2.58. Based on the cutoff values, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were also calculated. Kaplan–Meier survival curves were drawn using cutoff values of qSOFA and lactate. A two-tailed value of P < 0.05 was considered statistically significant.
3. Results
A total of 561 patients were screened at recruitment, and 225 patients were excluded (Figure 1). A total of 336 patients were finally included in our study group. Of the 225 excluded patients, 16 patients' age < 18 years old, 74 patients were transferred from other hospitals, 10 patients were with a history of previous transplantation, 5 patients were finally diagnosed with pulmonary tuberculosis, 3 patients were with pulmonary thromboembolism, 6 patients were with lung cancer, 4 patients were with HIV, 57 patients were with incomplete medical records, 30 patients were with admission SOFA score < 2 from baseline, and 20 patients were lost to follow-up with unknown prognosis.
Figure 1.
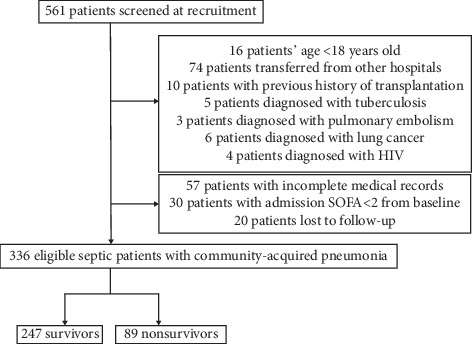
Flow chart of patients enrolled in our study.
Of the 336 patients, 89 patients were dead and 247 patients survived after 28-day follow-up, and the total mortality rate was 26.5% (Table 1). The total mean age was 76 (61, 84) years, and the male-to-female ratio was 1.73 : 1. Comorbidities of enrolled patients include chronic obstructive pulmonary disease (COPD) (11.9%), cardiovascular disease (CDVD) (14.3%), cerebrovascular disease (CBVD) (26.2%), diabetes (22.9%), chronic renal disease (CRD) (8.9%), and hepatobiliary disease (HBD) (7.1%). There were no significant differences between the death and survival groups in age (P=0.136) and male-female ratio (P=0.914). CBVD was the most common comorbidity, and a previous history of diabetes mellitus was more common among nonsurvivors (Table 1). 61 patients were admitted in ICU, and their age was older than those who were not admitted in ICU (Table 1). As to laboratory parameters, the ALB level was significantly lower, while the creatine and blood urea nitrogen (BUN) levels were significantly higher in the death group, the patients admitted in ICU, and the patients who use mechanical ventilation or vasopressors, respectively (P < 0.05) (Tables 1 and 2). The vital signs of the nonsurvivors, the patients admitted in ICU, and the patients used mechanical ventilation or vasopressors were more unstable (P < 0.05) (Tables 1 and 2). The CURB65, CRB65, PSI, SOFA, qSOFA, and admission lactate levels were significantly higher in the death group, the ICU admission group, the mechanical ventilation group, and the vasopressors use group (P < 0.05) (Tables 1 and 2).
Table 1.
Baseline characteristics of enrolled patients with CAP in ED.
| All cohort | Death | Survival | P | ICU admission | Non-ICU admission | P | |
|---|---|---|---|---|---|---|---|
| N (%) | 336 | 89 (26.5) | 247 (73.5) | 61 (18.2) | 275 (81.8) | ||
| Age (yrs) | 76 (61, 84) | 78 (67, 85) | 75 (61, 83) | 0.136 | 81 (70, 88) | 74 (61, 83) | 0.003 |
| Male, n (%) | 213 (63.4) | 56 (62.9) | 157 (63.6) | 0.914 | 40 (65.6) | 173 (62.9) | 0.696 |
| Comorbidities, n (%) | |||||||
| COPD | 40 (11.9) | 12 (13.5) | 28 (11.3) | 0.592 | 16 (26.2) | 24 (8.7) | <0.001 |
| CDVD | 48 (14.3) | 18 (20.2) | 30 (12.1) | 0.062 | 14 (23.0) | 34 (12.4) | 0.033 |
| CBVD | 88 (26.2) | 28 (31.5) | 60 (24.3) | 0.187 | 18 (29.5) | 70 (25.5) | 0.515 |
| Diabetes | 77 (22.9) | 31 (34.8) | 46 (18.6) | 0.002 | 24 (39.3) | 53 (19.3) | 0.001 |
| CRD | 30 (8.9) | 9 (10.1) | 21 (8.5) | 0.648 | 8 (13.1) | 22 (8.0) | 0.205 |
| HBD | 24 (7.1) | 5 (5.6) | 19 (7.7) | 0.515 | 4 (6.6) | 20 (7.3) | 0.844 |
| Healthy | 28 (8.3) | 6 (6.7) | 22 (8.9) | 0.526 | 8 (13.1) | 20 (7.3) | 0.135 |
| Laboratory results | |||||||
| WBC (×109/L) | 10.0 (6.7, 14.1) | 11.0 (6.7, 15.9) | 9.8 (6.8, 13.9) | 0.406 | 11.7 (7.5, 15.9) | 9.7 (6.6, 13.9) | 0.046 |
| HGB (g/L) | 126 (115, 137) | 123 (111, 134) | 127 (117, 138) | 0.125 | 123 ± 22 | 127 (117, 138) | 0.367 |
| HCT (%) | 36.9 (31.8, 40.8) | 34.7 ± 9.1 | 37.3 (32.9, 40.9) | 0.037 | 35.5 ± 9.3 | 37.3 (32.5) | 0.368 |
| PLT (×109/L) | 184 (131, 251) | 163 (123, 249) | 191 (136, 251) | 0.311 | 155 (123, 230) | 191 (137, 254) | 0.116 |
| ALB (g/L) | 36.2 (32.1, 39.1) | 34.0 ± 5.9 | 36.7 (33, 39.3) | 0.001 | 34.3 ± 6.0 | 36.5 (32.5, 39.1) | 0.044 |
| CREA (μmol/L) | 80.5 (60.8, 114.3) | 92 (63.6, 140) | 75.9 (60.4, 105.8) | 0.011 | 95.5 (74.6, 150) | 75.9 (60.2, 105) | <0.001 |
| BUN (mmol/L) | 7.4 (5.3, 11.0) | 8.7 (6.0, 13.4) | 6.8 (5.1, 10.4) | 0.010 | 10.4 (6.7, 16.1) | 6.8 (5.1, 10.4) | <0.001 |
| AST (U/L) | 30 (20, 56) | 33 (23, 68) | 30 (20, 53) | 0.174 | 36 (23, 64) | 30 (20, 54) | 0.240 |
| ALT (U/L) | 20 (14, 37) | 17 (12, 35) | 21 (14, 37) | 0.225 | 16 (11, 32) | 21 (14, 38) | 0.167 |
| TBIL (μmol/L) | 14.8 (9.6, 23.0) | 15.3 (10.3, 23.5) | 14.3 (9.5, 22.7) | 0.318 | 15.1 (9.6, 23.6) | 14.7 (9.8, 22.9) | 0.778 |
| DBIL (μmol/L) | 6.4 (4.0, 10.6) | 6.8 (4.4, 11.3) | 6.1 (3.8, 10.2) | 0.089 | 6.1 (4.3, 11.5) | 6.4 (4.0, 10.4) | 0.503 |
| K+ (mmol/L) | 3.8 (3.5, 4.2) | 3.9 (3.5, 4.4) | 3.8 (3.5, 4.2) | 0.252 | 4.0 (3.6, 4.4) | 3.8 (3.5, 4.2) | 0.050 |
| PaO2/FiO2 | 303 (262, 352) | 285 (246, 328) | 313 (273, 363) | <0.001 | 286 (241, 328) | 310 (268, 361) | 0.007 |
| Vital signs | |||||||
| SBP (mmHg) | 132 (119, 139) | 122 (97, 135) | 134 (125, 143) | <0.001 | 122 (96, 135) | 132 (122, 142) | <0.001 |
| DBP (mmHg) | 68 (65, 76) | 63 (55, 70) | 70 (66, 78) | <0.001 | 65 ± 11 | 69 (65, 76) | <0.001 |
| HR (times/min) | 86 (78, 94) | 87 (79, 106) | 86 (78, 94) | 0.009 | 86 (79, 106) | 86 (78, 94) | 0.024 |
| Severity scores | |||||||
| CURB65 | 2 (2, 3) | 3 (3, 4) | 2 (1, 3) | <0.001 | 3 (3, 4) | 2 (2, 3) | <0.001 |
| CRB65 | 2 (1, 3) | 3 (2, 3) | 2 (1, 2) | <0.001 | 3 (2, 3) | 2 (1, 2) | <0.001 |
| PSI | 128 ± 40 | 157 ± 35 | 120 ± 38 | <0.001 | 174 (150, 191) | 121 ± 36 | <0.001 |
| SOFA | 3 (2, 5) | 6 (4, 8) | 3 (2, 4) | <0.001 | 7 (6, 9) | 3 (2, 4) | <0.001 |
| qSOFA | 2 (1, 2) | 3 (2, 3) | 1 (1, 2) | <0.001 | 3 (2, 3) | 1 (1, 2) | <0.001 |
| Lactate (mmol/L) | 1.4 (1.1, 2.1) | 1.8 (1.3, 2.9) | 1.3 (1.1, 1.8) | <0.001 | 2.5 (1.6, 4.2) | 1.3 (1.1, 1.8) | <0.001 |
Data are presented as n, n (%), or median (QL, QU). CAP: community-acquired pneumonia; ED: emergency department; SCAP: severe community-acquired pneumonia; NSCAP: nonsevere community-acquired pneumonia; COPD: chronic obstructive pulmonary disease; CDVD: cardiovascular disease; CBVD: cerebrovascular disease; CRD: chronic renal disease; HBD: hepatobiliary disease; WBC: white blood cell; HGB: hemoglobin; HCT: hematocrit; PLT: platelet; ALB: albumin; CREA: creatinine; BUN: blood urea nitrogen; AST: aspartate aminotransferase; ALT: alanine aminotransferase; TBIL: total bilirubin; DBIL: direct bilirubin; SBP: systolic blood pressure; DBP: diastolic blood pressure; HR: heart rate; CURB65: confusion, urea > 7 mmol/L, respiratory rate ≥ 30/min, blood pressure < 90 mmHg systolic and/or ≤60 mmHg diastolic, and age ≥ 65 years; CRB65: confusion, respiratory rate ≥ 30/min, blood pressure < 90 mmHg systolic and/or ≤60 mmHg diastolic, and age ≥ 65 years; PSI: pneumonia severity index; SOFA: sequential organ failure assessment; qSOFA: quick sequential organ failure assessment.
Table 2.
Baseline characteristics of enrolled patients with CAP in ED.
| All cohorts | MV | NMV | P | Use of vasopressors | Nonuse of vasopressors | P | |
|---|---|---|---|---|---|---|---|
| N (%) | 336 | 83 (24.7) | 253 (75.3) | 108 (32.1) | 228 (67.9) | ||
| Age (yrs) | 76 (61, 84) | 78 (67, 84) | 76 (61, 84) | 0.584 | 78 (66, 87) | 75 (61, 83) | 0.065 |
| Male, n (%) | 213 (63.4) | 54 (60.7) | 159 (64.4) | 0.535 | 71 (65.7) | 142 (62.3) | 0.539 |
| Comorbidities, n (%) | |||||||
| COPD | 40 (11.9) | 14 (15.7) | 26 (10.5) | 0.194 | 15 (13.9) | 25 (11.0) | 0.440 |
| CDVD | 48 (14.3) | 14 (15.7) | 34 (13.8) | 0.650 | 18 (16.7) | 30 (13.2) | 0.391 |
| CBVD | 88 (26.2) | 20 (22.5) | 68 (27.5) | 0.352 | 30 (27.8) | 58 (25.4) | 0.649 |
| Diabetes | 77 (22.9) | 15 (16.9) | 62 (25.1) | 0.112 | 35 (32.4) | 42 (18.4) | 0.004 |
| CRD | 30 (8.9) | 9 (10.1) | 21 (8.5) | 0.648 | 12 (11.1) | 18 (7.9) | 0.334 |
| HBD | 24 (7.1) | 6 (6.7) | 18 (7.3) | 0.864 | 4 (3.7) | 20 (8.8) | 0.092 |
| Healthy | 28 (8.3) | 7 (7.9) | 21 (8.5) | 0.852 | 5 (4.6) | 23 (10.1) | 0.091 |
| Laboratory results | |||||||
| WBC (×109/L) | 10.0 (6.7, 14.1) | 10.4 (6.7, 15.8) | 9.7 (6.8, 13.9) | 0.244 | 10.8 (6.9, 16.0) | 9.8 (6.6, 13.9) | 0.180 |
| HGB (g/L) | 126 (115, 137) | 126 (115, 137) | 126 (116, 137) | 0.781 | 126 (113, 136) | 127 (117, 138) | 0.314 |
| HCT (%) | 36.9 (31.8, 40.8) | 35.4 ± 9.2 | 37.2 (32.7, 40.7) | 0.356 | 34.9 ± 9.1 | 37.3 (32.9, 40.9) | 0.092 |
| PLT (×109/L) | 184 (131, 251) | 155 (117, 241) | 192 (141, 251) | 0.109 | 162 (123, 245) | 193 (141, 252) | 0.082 |
| ALB (g/L) | 36.2 (32.1, 39.1) | 34 ± 6 | 36.7 (32.8, 39.3) | 0.002 | 34.2 ± 5.7 | 36.8 (33.0, 39.4) | <0.001 |
| CREA (μmol/L) | 80.5 (60.8, 114.3) | 92.5 (71.2, 149.2) | 75.5 (59.9, 104) | <0.001 | 92 (69, 145.4) | 75.4 (60, 101.4) | <0.001 |
| BUN (mmol/L) | 7.4 (5.3, 11.0) | 9.5 (6.5, 15.0) | 6.8 (5.0, 10.3) | <0.001 | 9.0 (6.0, 13.6) | 6.8 (5.1, 10.0) | <0.001 |
| AST (U/L) | 30 (20, 56) | 36 (23, 81) | 29 (20, 53) | 0.097 | 33 (23, 75) | 29 (20, 53) | 0.122 |
| ALT (U/L) | 20 (14, 37) | 17 (12, 45) | 21 (14, 36) | 0.694 | 18 (12, 40) | 21 (14, 37) | 0.220 |
| TBIL (μmol/L) | 14.8 (9.6, 23.0) | 16.1 (10.6, 25) | 14 (9.5, 22.3) | 0.164 | 16.0 (10.2, 25.9) | 13.9 (9.5, 21.9) | 0.089 |
| DBIL (μmol/L) | 6.4 (4.0, 10.6) | 7 (4.4, 12.3) | 6.1 (3.9, 9.7) | 0.037 | 7.0 (4.4, 12.0) | 6.0 (3.8, 9.6) | 0.020 |
| K+ (mmol/L) | 3.8 (3.5, 4.2) | 4 (3.6, 4.5) | 3.8 (3.5, 4.1) | 0.006 | 4.0 (3.5, 4.3) | 3.8 (3.5, 4.2) | 0.220 |
| PaO2/FiO2 | 303 (262, 352) | 285 (244, 326) | 313 (272, 363) | <0.001 | 287 (246, 333) | 315 (274, 363) | 0.002 |
| Vital signs | |||||||
| SBP (mmHg) | 132 (119, 139) | 122 (100, 135) | 134 (125, 143) | <0.001 | 113 (97, 133) | 135 (126, 145) | <0.001 |
| DBP (mmHg) | 68 (65, 76) | 65 (55, 71) | 70 (66, 78) | <0.001 | 62 (55, 69) | 72 (68, 78) | <0.001 |
| HR (times/min) | 86 (78, 94) | 86 (79, 104) | 86 (78, 94) | 0.033 | 89 (79, 106) | 86 (78, 92) | <0.001 |
| Severity scores | |||||||
| CURB65 | 2 (2, 3) | 3 (3, 4) | 2 (1, 3) | <0.001 | 3 (2.5, 4) | 2 (1, 3) | <0.001 |
| CRB65 | 2 (1, 3) | 3 (2, 3) | 2 (1, 2) | <0.001 | 3 (2, 3) | 2 (1, 2) | <0.001 |
| PSI | 128 ± 40 | 161 ± 37 | 120 ± 36 | <0.001 | 156 ± 38 | 118 ± 36 | <0.001 |
| SOFA | 3 (2, 5) | 6 (5, 9) | 3 (2, 4) | <0.001 | 6 (4, 8) | 3 (2, 4) | <0.001 |
| qSOFA | 2 (1, 2) | 3 (2, 3) | 1 (1, 2) | <0.001 | 2 (2, 3) | 1 (1, 2) | <0.001 |
| Lactate (mmol/L) | 1.4 (1.1, 2.1) | 1.9 (1.3, 4.2) | 1.4 (1.1, 1.8) | <0.001 | 2.3 (1.4, 4.1) | 1.3 (1.0, 1.6) | <0.001 |
Data are presented as n, n (%), or median (QL, QU). CAP: community-acquired pneumonia; MV: mechanical ventilation; NMV: nonmechanical ventilation; ED: emergency department; SCAP: severe community-acquired pneumonia; NSCAP: nonsevere community-acquired pneumonia; COPD: chronic obstructive pulmonary disease; CDVD: cardiovascular disease; CBVD: cerebrovascular disease; CRD: chronic renal disease; HBD: hepatobiliary disease; WBC: white blood cell; HGB: hemoglobin; HCT: hematocrit; PLT: platelet; ALB: albumin; CREA: creatinine; BUN: blood urea nitrogen; AST: aspartate aminotransferase; ALT: alanine aminotransferase; TBIL: total bilirubin; DBIL: direct bilirubin; SBP: systolic blood pressure; DBP: diastolic blood pressure; HR: heart rate; CURB65: confusion, urea > 7 mmol/L, respiratory rate ≥ 30/min, blood pressure < 90 mmHg systolic and/or ≤60 mmHg diastolic, and age ≥ 65 years; CRB65: confusion, respiratory rate ≥ 30/min, blood pressure < 90 mmHg systolic and/or ≤60 mmHg diastolic, and age ≥ 65 years; PSI: pneumonia severity index; SOFA: sequential organ failure assessment; qSOFA: quick sequential organ failure assessment.
In predicting 28-day mortality, ROC curve comparisons showed that the AUROC of qSOFA (0.807) was the highest among single predictors (Table 3, Figure 2), followed by SOFA (0.795), PSI (0.768), CURB65 (0.744), CRB65 (0.737), and admission lactate (0.679). Moreover, the qSOFA score was with the highest sensitivity (91%) and negative predictive value (NPV) (94.3%), which highlighted the prediction performance of qSOFA. However, among the combinations of severity score and admission lactate, the AUROC of qSOFA + lactate (0.833) was the highest, followed by the combination of SOFA + lactate (0.803), CRB65 + lactate (0.776), PSI + lactate (0.774), and CURB65 + lactate (0.772). Multiple pairwise comparisons among single predictors showed that there were no significant differences between qSOFA and SOFA (Z = 0.373, P=0.709), qSOFA and PSI (Z = 1.296, P=0.195), CURB65 and PSI (Z = 0.940, P=0.347), CURB65 and SOFA (Z = 1.499, P=0.134), and PSI and SOFA (Z = 0.835, P=0.404). There were significant differences between qSOFA and CURB65 (Z = 2.333, P=0.020), qSOFA and CRB65 (Z = 2.504, P=0.012), qSOFA and lactate (Z = 3.246, P=0.001), and SOFA and lactate (Z = 3.143, P=0.002), whereas pairwise comparisons among combinations of severity scores and lactate demonstrated that the combination of qSOFA + lactate demonstrated superiority over other combinations except the combination of SOFA + lactate. There were no significant differences between CURB65 + lactate and CRB65 + lactate (Z = 0.244, P=0.807), CURB65 + lactate and PSI + lactate (Z = 0.062, P=0.951), CURB65 + lactate and SOFA + lactate (Z = 1.054, P=0.292), PSI + lactate and SOFA + lactate (Z = 0.993, P=0.321), and SOFA + lactate and qSOFA + lactate (Z = 1.187, P=0.235).
Table 3.
ROC curve comparisons between CAP severity scores and admission lactate in predicting 28-day mortality.
| AUC (95% CI) | P value | Cutoff value | Sensi (%) | Speci (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|---|---|---|
| CURB65 | 0.744 (0.685–0.802) | <0.001 | 3.0 | 75.3 | 61.9 | 41.6 | 87.4 |
| CRB65 | 0.737 (0.675–0.799) | <0.001 | 3.0 | 56.2 | 83.0 | 54.4 | 84.0 |
| PSI | 0.768 (0.711–0.824) | <0.001 | 131 | 77.5 | 64.0 | 43.7 | 88.8 |
| SOFA | 0.795 (0.740–0.850) | <0.001 | 4.0 | 84.3 | 64.4 | 46.0 | 91.9 |
| qSOFA | 0.807 (0.754–0.859) | <0.001 | 2.0 | 91.0 | 53.8 | 41.5 | 94.3 |
| Lactate | 0.679 (0.612–0.745) | <0.001 | 2.0 | 48.3 | 78.1 | 44.3 | 80.7 |
| CURB65 + lactate | 0.772 (0.718–0.827) | <0.001 | 0.18 | 83.1 | 59.5 | 42.5 | 90.7 |
| CRB65 + lactate | 0.776 (0.719–0.833) | <0.001 | 0.25 | 68.5 | 77.7 | 52.5 | 87.3 |
| PSI + lactate | 0.774 (0.719–0.828) | <0.001 | 0.21 | 80.9 | 61.9 | 43.3 | 90.0 |
| SOFA + lactate | 0.803 (0.749–0.857) | <0.001 | 0.23 | 74.2 | 76.5 | 53.2 | 89.2 |
| qSOFA + lactate | 0.833 (0.786–0.881) | <0.001 | 0.29 | 64.0 | 87.9 | 65.6 | 87.1 |
ROC: area under the curve; CAP: community-acquired pneumonia; Sensi: sensitivity; Speci: specificity; PPV: positive predictive value; NPV: negative predictive value; CURB65: confusion, urea > 7 mmol/L, respiratory rate ≥ 30/min, blood pressure < 90 mmHg systolic and/or ≤60 mmHg diastolic, and age ≥ 65 years; CRB65: confusion, respiratory rate ≥30/min, blood pressure < 90 mmHg systolic and/or ≤60 mmHg diastolic, and age ≥ 65 years; PSI: pneumonia severity index; SOFA: sequential organ failure assessment; qSOFA: quick sequential organ failure assessment.
Figure 2.
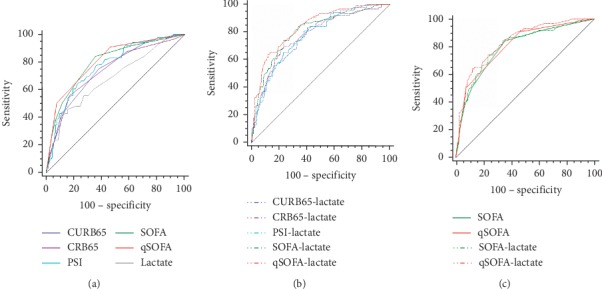
ROC curve comparisons of (a) CURB65, CRB65, PSI, SOFA, qSOFA, and admission lactate in predicting 28-day mortality; (b) different combinations of CAP severity scores and admission lactate in predicting 28-day mortality; and (c) SOFA, qSOFA, SOFA + lactate, and qSOFA + lactate in predicting 28-day mortality.
The ability to predict ICU admission was higher when the SOFA was used rather than either CURB65, CRB65, qSOFA, or PSI (Table 4, Figure 3). These differences were statistically significant. SOFA achieved the highest AUROC (0.895) in predicting ICU admission, followed by PSI (0.837), qSOFA (0.822), CURB65 (0.774), lactate (0.742), and CRB65 (0.738). As to the comparisons of combinations of severity scores and lactate, SOFA + lactate achieved the highest AUROC (0.902), followed by qSOFA + lactate (0.868), PSI + lactate (0.840), CURB65 + lactate (0.818), and CRB65 + lactate (0.806). There were no significant differences between SOFA + lactate and qSOFA + lactate (Z = 1.476, P=0.140) and SOFA + lactate and PSI + lactate (Z = 1.949, P=0.051).
Table 4.
ROC curve comparisons among severity scores and admission lactate in predicting ICU admission.
| AUC (95% CI) | P value | Cutoff value | Sensi (%) | Speci (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|---|---|---|
| CURB65 | 0.774 (0.709–0.840) | <0.001 | 3.0 | 83.6 | 60.0 | 31.7 | 94.3 |
| CRB65 | 0.738 (0.667–0.810) | <0.001 | 3.0 | 59.0 | 79.6 | 39.1 | 89.7 |
| PSI | 0.837 (0.777–0.896) | <0.001 | 155 | 73.8 | 83.6 | 49.9 | 93.5 |
| SOFA | 0.895 (0.846–0.943) | <0.001 | 6.0 | 77.0 | 90.2 | 63.5 | 94.6 |
| qSOFA | 0.822 (0.769–0.874) | <0.001 | 2.0 | 96.7 | 50.5 | 30.2 | 98.6 |
| Lactate | 0.742 (0.669–0.816) | <0.001 | 2.0 | 55.7 | 77.1 | 35.0 | 88.7 |
| CURB65 + lactate | 0.818 (0.759–0.877) | <0.001 | 0.21 | 67.2 | 82.2 | 45.6 | 91.9 |
| CRB65 + lactate | 0.806 (0.741–0.870) | <0.001 | 0.16 | 77.0 | 74.9 | 40.5 | 93.6 |
| PSI + lactate | 0.840 (0.782–0.898) | <0.001 | 0.25 | 72.1 | 86.2 | 53.7 | 93.3 |
| SOFA + lactate | 0.902 (0.857–0.947) | <0.001 | 0.18 | 82.0 | 87.6 | 59.5 | 95.6 |
| qSOFA + lactate | 0.868 (0.823–0.912) | <0.001 | 0.14 | 83.6 | 78.2 | 46.0 | 95.6 |
ROC: area under the curve; CAP: community-acquired pneumonia; SCAP: severe community-acquired pneumonia; Sensi: sensitivity; Speci: specificity; PPV: positive predictive value; NPV: negative predictive value; CURB65: confusion, urea > 7 mmol/L, respiratory rate ≥ 30/min, blood pressure < 90 mmHg systolic and/or ≤60 mmHg diastolic, and age ≥ 65 years; CRB65: confusion, respiratory rate ≥ 30/min, blood pressure < 90 mmHg systolic and/or ≤60 mmHg diastolic, and age ≥ 65 years; PSI: pneumonia severity index; SOFA: sequential organ failure assessment; qSOFA: quick sequential organ failure assessment.
Figure 3.
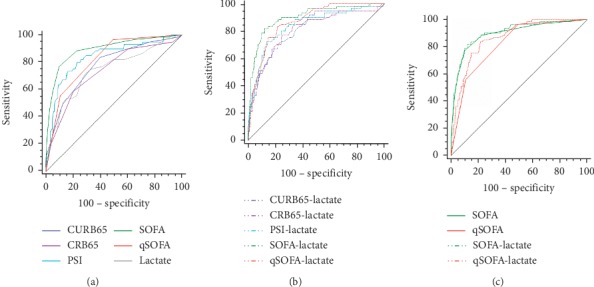
ROC curve comparisons of (a) CURB65, CRB65, PSI, SOFA, qSOFA, and admission lactate in predicting ICU admission; (b) different combinations of CAP severity scores and admission lactate in predicting ICU admission; and (c) SOFA, qSOFA, SOFA + lactate, and qSOFA + lactate in predicting ICU admission.
In predicting mechanical ventilation (Table 5, Figure 4), SOFA achieved the highest AUROC (0.845), followed by qSOFA (0.838), PSI (0.780), CURB65 (0.771), and CRB65 (0.758). SOFA and qSOFA had similar prediction performance (Z = 0.215, P=0.830). As to the combinations of severity scores and lactate, SOFA + lactate (AUROC = 0.851) and qSOFA + lactate (AUROC = 0.868) also had similar prediction value (Z = 0.746, P=0.456) and demonstrated superiority over other combinations. Among the single predictors in predicting vasopressor use (Table 6, Figure 5), the AUROC of SOFA (0.821) and qSOFA (0.820) was higher than that of PSI (0.767), CURB65 (0.759), CRB65 (0.746), and lactate (0.758), while the difference between SOFA and qSOFA was not statistically significant (Z = 0.046, P=0.964). In terms of the combinations of severity scores and lactate, SOFA + lactate (AUROC = 0.848) and qSOFA + lactate (AUROC = 0.875) also had similar prediction value (Z = 1.426, P=0.154) and demonstrated superiority over other combinations.
Table 5.
ROC curve comparisons between CAP severity scores and admission lactate in predicting mechanical ventilation.
| AUC (95% CI) | P value | Cutoff value | Sensi (%) | Speci (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|---|---|---|
| CURB65 | 0.771 (0.713–0.829) | <0.001 | 3.0 | 78.3 | 62.1 | 40.4 | 89.7 |
| CRB65 | 0.758 (0.697–0.820) | <0.001 | 3.0 | 59.0 | 83.0 | 53.2 | 86.1 |
| PSI | 0.780 (0.722–0.837) | <0.001 | 150 | 63.9 | 80.6 | 51.9 | 87.2 |
| SOFA | 0.845 (0.794–0.895) | <0.001 | 5.0 | 75.9 | 78.7 | 53.9 | 90.9 |
| qSOFA | 0.838 (0.789–0.887) | <0.001 | 2.0 | 94.0 | 53.8 | 40.0 | 96.5 |
| Lactate | 0.698 (0.628–0.768) | <0.001 | 2.0 | 49.4 | 77.9 | 42.3 | 82.4 |
| CURB65 + lactate | 0.804 (0.752–0.857) | <0.001 | 0.26 | 63.9 | 80.6 | 51.9 | 87.2 |
| CRB65 + lactate | 0.809 (0.755–0.864) | <0.001 | 0.21 | 75.9 | 76.7 | 51.7 | 90.7 |
| PSI + lactate | 0.796 (0.741–0.850) | <0.001 | 0.23 | 71.1 | 75.1 | 48.4 | 88.8 |
| SOFA + lactate | 0.851 (0.801–0.902) | <0.001 | 0.21 | 79.5 | 77.9 | 54.1 | 92.1 |
| qSOFA + lactate | 0.868 (0.826–0.911) | <0.001 | 0.26 | 71.1 | 88.9 | 67.8 | 90.4 |
ROC: area under the curve; CAP: community-acquired pneumonia; Sensi: sensitivity; Speci: specificity; PPV: positive predictive value; NPV: negative predictive value; CURB65: confusion, urea > 7 mmol/L, respiratory rate ≥ 30/min, blood pressure < 90 mmHg systolic and/or ≤60 mmHg diastolic, and age ≥ 65 years; CRB65: confusion, respiratory rate≥30/min, blood pressure < 90 mmHg systolic and/or ≤60 mmHg diastolic, and age ≥ 65 years; PSI: pneumonia severity index; SOFA: sequential organ failure assessment; qSOFA: quick sequential organ failure assessment.
Figure 4.

ROC curve comparisons of(a) CURB65, CRB65, PSI, SOFA, qSOFA, and admission lactate in predicting mechanical ventilation; (b) different combinations of CAP severity scores and admission lactate in predicting mechanical ventilation; and (c) SOFA, qSOFA, SOFA + lactate and qSOFA + lactate in predicting mechanical ventilation.
Table 6.
ROC curve comparisons between CAP severity scores and admission lactate in predicting vasopressor use.
| AUC (95% CI) | P value | Cutoff value | Sensi (%) | Speci (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|---|---|---|
| CURB65 | 0.759 (0.705–0.814) | <0.001 | 3.0 | 75.0 | 64.9 | 50.3 | 84.6 |
| CRB65 | 0.746 (0.688–0.803) | <0.001 | 3.0 | 53.7 | 85.1 | 63.1 | 79.5 |
| PSI | 0.767 (0.712–0.823) | <0.001 | 150.0 | 60.2 | 83.8 | 63.8 | 81.6 |
| SOFA | 0.821 (0.772–0.871) | <0.001 | 4.0 | 84.3 | 68.4 | 55.8 | 90.2 |
| qSOFA | 0.820 (0.772–0.868) | <0.001 | 2.0 | 90.7 | 57.5 | 50.3 | 92.9 |
| Lactate | 0.758 (0.700–0.817) | <0.001 | 2.0 | 57.4 | 84.6 | 68.7 | 81.3 |
| CURB65 + lactate | 0.824 (0.778–0.871) | <0.001 | 0.24 | 84.3 | 67.1 | 54.8 | 90.0 |
| CRB65 + lactate | 0.830 (0.782–0.877) | <0.001 | 0.31 | 75.0 | 80.3 | 64.3 | 87.1 |
| PSI + lactate | 0.820 (0.773–0.867) | <0.001 | 0.28 | 76.9 | 76.3 | 60.6 | 87.5 |
| SOFA + lactate | 0.848 (0.801–0.894) | <0.001 | 0.29 | 75.0 | 82.5 | 67.0 | 87.4 |
| qSOFA + lactate | 0.875 (0.835–0.915) | <0.001 | 0.38 | 69.4 | 89.9 | 76.5 | 86.1 |
ROC: area under the curve; CAP: community-acquired pneumonia; Sensi: sensitivity; Speci: specificity; PPV: positive predictive value; NPV: negative predictive value; CURB65: confusion, urea > 7 mmol/L, respiratory rate ≥ 30/min, blood pressure < 90 mmHg systolic and/or ≤60 mmHg diastolic, and age ≥ 65 years; CRB65: confusion, respiratory rate ≥ 30/min, blood pressure < 90 mmHg systolic and/or ≤60 mmHg diastolic, and age ≥ 65 years; PSI: pneumonia severity index; SOFA: sequential organ failure assessment; qSOFA: quick sequential organ failure assessment.
Figure 5.
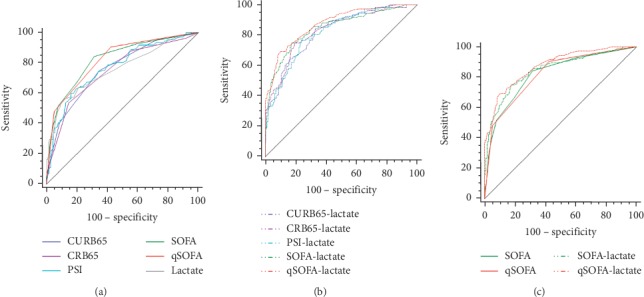
ROC curve comparisons of(a) CURB65, CRB65, PSI, SOFA, qSOFA, and admission lactate in predicting vasopressor use; (b) different combinations of CAP severity scores and admission lactate in predicting vasopressor use; and (c) SOFA, qSOFA, SOFA + lactate, and qSOFA + lactate in predicting vasopressor use.
As to the comparison of the prediction ability of SOFA and the combination of qSOFA and admission lactate, no significant differences were found between them in predicting 28-day mortality (0.795 vs. 0.833, Z = 1.378, P=0.168), ICU admission (0.895 vs. 0.868, Z = 1.022, P=0.307), and mechanical ventilation (0.845 vs. 0.868, Z = 0.921, P=0.357), while qSOFA + lactate was significantly superior to SOFA in predicting vasopressor use (0.821 vs. 0.875, Z = 2.12, P=0.034).
The mortality of CAP patients in ED who had a qSOFA ≥ 2 or lactate ≥ 2 mmol/L was 37.7%, which was significant higher than those patients who had qSOFA < 2 or lactate < 2 mmol/L (5.2%) (Table 7). For the secondary outcomes, significant difference was also found between the two groups in terms of ICU admission (27.3% vs. 0.9%), mechanical ventilation (36.4% vs. 2.6%), and vasopressor use (46.4% vs. 5.2%). Moreover, Kaplan–Meier survival curve analyses revealed that the CAP patients in ED with admission qSOFA < 2 or lactate ≤ 2 mmol/L showed significantly prolonged survival than those patients with qSOFA ≥ 2 or lactate > 2 mmol/L (Log-rank χ2 = 59.825, P < 0.001) (Figure 6).
Table 7.
Comparisons of severity scores and different outcomes in patients with CAP using qSOFA and lactate.
| qSOFA ≥ 2 or lactate > 2 | P value | ||
|---|---|---|---|
| Yes (n = 216) | No (n = 120) | ||
| Severity scores | |||
| CURB65 | 3 (2, 4) | 2 (1, 2) | <0.001 |
| CRB65 | 2 (2, 3) | 1 (1, 2) | <0.001 |
| PSI | 144 ± 37 | 104 ± 32 | <0.001 |
| SOFA | 4 (3, 6) | 2 (2, 3) | <0.001 |
| qSOFA | 2 (2, 3) | 1 (1, 1) | <0.001 |
| Lactate | 1.8 (1.2, 2.8) | 1.2 (1.0, 1.4) | <0.001 |
|
| |||
| Primary outcome, n (%) | |||
| 28-day mortality | 83 (38.4) | 6 (5.0) | <0.001 |
|
| |||
| Secondary outcome, n (%) | |||
| ICU admission | 60 (27.8) | 1 (0.8) | <0.001 |
| Mechanical ventilation | 80 (37.0) | 3 (2.5) | <0.001 |
| Use of vasopressors | 102 (47.2) | 6 (5.0) | <0.001 |
Data are presented as n, n (%), or median (QL, QU). CAP: community-acquired pneumonia; CURB65: confusion, urea > 7 mmol/L, respiratory rate ≥ 30/min, blood pressure < 90 mmHg systolic and/or ≤60 mmHg diastolic, and age ≥ 65 years; CRB65: confusion, respiratory rate ≥ 30/min, blood pressure < 90 mmHg systolic and/or ≤60 mmHg diastolic, and age ≥ 65 years; PSI: pneumonia severity index; SOFA: sequential organ failure assessment; qSOFA: quick sequential organ failure assessment; ICU: intensive care unit.
Figure 6.
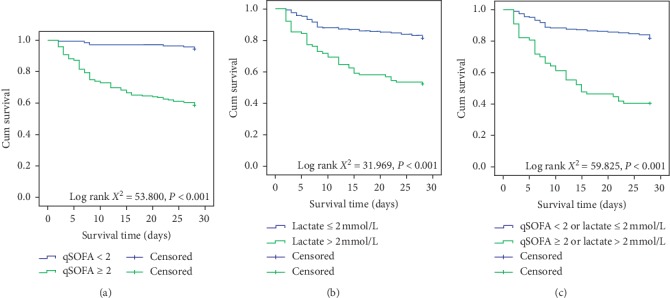
Kaplan–Meier survival curve comparison: (a) between septic CAP patients with admission qSOFA < 2 and septic CAP patients with admission qSOFA ≥ 2; (b) between septic CAP patients with admission lactate ≤ 2 mmol/L and septic CAP patients with admission lactate > 2 mmol/L; (c) between septic CAP patients with admission qSOFA < 2 or lactate ≤ 2 mmol/L and septic CAP patients with admission qSOFA ≥ 2 or lactate > 2 mmol/L.
4. Discussion
CAP is one of the most common infections seen in ED with a wide spectrum of severity and pathogens. Delayed treatment may result in serious consequences and even death. Therefore, timely diagnosis, assessment of severity, and treatment can improve prognosis. Severity evaluation is of vital significance in the initial management of CAP. Various scoring systems of CAP exist. They differ from each other, and they are used as tools to aid clinical diagnosis and treatment; however, doctors should take clinical experience into consideration [2].
As far as the components are concerned, the CURB65 score is very similar to qSOFA score. It comprises of five variables and divided patients into three broad risk groups as follows: Scores 0-1: low risk for 30-day mortality; Score 2: intermediate risk of 30-day mortality; Scores 3–5: high risk of 30-day mortality [3]. It was primarily designed to predict mortality and identify low-risk patients potentially suitable for ambulatory management and has been widely utilized in patients with CAP [10]. The CURB65 score has been extensively validated and performed similarly to the PSI score in predicting 30-day mortality of CAP patients [11], although previous study revealed that CURB65 may be more suitable for identifying high-risk patients, while PSI had advantage in the identification of low-risk patients [5]. The simplicity of calculation of CURB65 demonstrated superiority over other complex severity scores utilized in crowded emergency rooms. Furthermore, the CRB65 score, which does not require a blood urea level, is more suitable for use in gross-roots hospitals. Previous research studies demonstrated that CURB65/CRB65 does not incorporate an assessment of oxygenation and thus underestimated the risk of death and severity of influenza pneumonia [2, 12, 13].
Comparatively, the PSI score consisted of 20 different parameters with different weights including demographics, comorbidities, and clinical and laboratory findings [4]. The PSI score is heavily weighted by age and comorbidities, which shows that it underestimates severity of young CAP patients and it is not advised to guide intensive care unit admission [13, 14]. Moreover, the underlying health conditions may strongly influence mortality based on pneumonia severity in aged populations [15]. A study by Zhang et al. demonstrated that PSI performed better than CURB65 for mortality prediction, while its discriminative power decreased with advancing age [16]. Regrettably, the complexity of PSI limited its clinical application in ED.
The stratified and prognostic performance of SOFA and qSOFA in CAP has not yet been evaluated in detail, and only few studies have investigated its application in CAP in ED. Our previous research proved that SOFA is superior to CURB65, PSI, qSOFA, and procalcitonin in predicting 28-day mortality, with an AUROC of 0.913 [17], while Kim reported AUROC of SOFA and qSOFA to be 0.83 and 0.81, respectively [18]. Comparatively, qSOFA only requires a few items and vital signs. Previous research findings proved that it presented better clinical usefulness as prompt tools for ED or nonrespiratory specialists [19, 20].
Serum lactate is a well-known prognostic marker for patients with sepsis, and initial ED lactate is reported to be a useful marker to risk-stratify critically ill patients presenting to ED [7]. Sepsis 3.0 guidelines recommend lactate > 2.0 mmol/L with the requirement of vasopressor use to maintain a mean arterial blood pressure of 65 mmHg as the new definition of septic shock [6]. Obtaining blood for measuring lactate is recommended by surviving sepsis campaign bundle update, and if lactate > 2 mmol/L, it should be remeasured within 2–4 hours to guide resuscitation [21]. Nonetheless, the prognostic role of lactate on CAP patients has not been well studied. Gwak et al. enrolled 397 CAP patients and proved that the initial lactate level is independently associated with mortality in hospitalized patients with CAP [22]. Chen and Li enrolled 1641 patients and investigated the predictive performance of lactate, CURB65, and the combination of lactate and CURB65 for predicting mortality and ICU admission in pneumonia patients in ED, while results indicated that lactate is superior to CURB65 in predicting mortality, hospitalization, and intensive care unit (ICU) admission and lactate-CURB65 combination improves the predictive value of single CURB65 [10]. A similar study by Frenzen and colleagues concluded that admission lactate levels significantly improved the prognostic value (need for mechanical ventilation, vasopressors, ICU admission, or hospital mortality) of CRB/CURB65 scores in CAP patients with an optimal cutoff value of 1.8 mmol/L [23]. Song et al. enrolled 443 patients with CAP in ED, and results showed that the AUROC of qSOFA and SOFA for prediction of mortality was 0.720 and 0.845, while the combination of qSOFA and lactate was not significantly different from SOFA [24].
To the best of our knowledge, not many studies have explored the prognostic prediction performance of qSOFA score and admission lactate in septic CAP patients in ED before. Our results revealed that qSOFA has the highest sensitivity and negative predictive value in predicting both primary (28-day mortality) and secondary outcomes (ICU admission, mechanical ventilation, and vasopressor use). qSOFA outperformed other single predictors (CURB65, CRB65, PSI, SOFA, and lactate) in predicting 28-day mortality, although the AUROC of qSOFA was not significantly different from SOFA. Similar to previous studies, the combination of qSOFA + lactate was proved comparable to full version of SOFA in predicting primary and secondary outcomes in our research [25, 26]. Moreover, qSOFA + lactate was not statistically significant from SOFA + lactate (Z = 1.187, P=0.235). Regrettably, unlike the results of previous study [24], our research indicated that qSOFA + lactate and SOFA + lactate did not improve the prognostic prediction performance of single qSOFA (Z = 1.886, P=0.059) or single SOFA (Z = 1.066, P=0.286), respectively. The relatively small sample size may explain the result heterogeneity between our research and previous studies. Nonetheless, in view of the simplicity and convenience of qSOFA, it can be a better choice as tools for prognostic evaluation of septic CAP patients in ED. Furthermore, in predicting both primary and secondary outcomes (28-day mortality, ICU admission, mechanical ventilation, and vasopressor use), the optimal cutoff value for qSOFA was 2 and optimal cutoff value for admission lactate was 2 mmol/L. Our study revealed that septic CAP patients in ED with qSOFA < 2 or lactate ≤ 2 mmol/L demonstrated significantly prolonged survival than those patients with qSOFA ≥ 2 or admission lactate > 2 mmol/L, which could help physicians in ED enhance their awareness when treating these kind of septic CAP patients. A qSOFA of 2 or more would be very helpful and useful in discriminating high-risk patients with a high mortality rate.
Our research is among the very few studies that have explored the prognostic performance of CURB65, CRB65, PSI, SOFA, qSOFA, and admission lactate at the same time. Our results highlighted the superiority of qSOFA and SOFA in predicting 28-day mortality, ICU admission, mechanical ventilation, and vasopressor use. However, there are some limitations in our study. First, the relatively small sample size and the retrospective and single-center design may result in selection bias and do not allow for analysis of all clinical data. Our results should be verified by more multicenter, prospective studies with larger sample size. Second, our research only enrolled septic patients with CAP in ED. These patients are of older age and with more complications, which could have a higher mortality rate and may influence the final results.
5. Conclusions
In conclusion, we analyzed the prognostic prediction value of CURB65, CRB65, PSI, SOFA, qSOFA, and admission lactate at the same time in patients with ED in our solitary center. We found that qSOFA and SOFA were superior to CURB65, CRB65, and PSI in predicting 28-day mortality, ICU admission, mechanical ventilation, and vasopressor use for septic CAP patients in ED. The prediction performance of the combination of qSOFA and admission lactate was comparable to full version of SOFA. qSOFA with admission lactate could be a convenient, valuable, and practical tool for prognostic prediction. Further multicenter studies with larger sample size are needed to validate our results.
Abbreviations
- CAP:
Community-acquired pneumonia
- ED:
Emergency department
- PSI:
Pneumonia severity index
- SOFA:
Sequential organ failure assessment
- CURB65:
Confusion, urea > 7 mmol/L, respiratory rate ≥ 30/min, blood pressure <90 mmHg systolic and or ≤60 mmHg diastolic, and age ≥ 65 years
- CRB65:
Confusion, respiratory rate ≥ 30/min, blood pressure <90 mmHg systolic and/or ≤60 mmHg diastolic, and age ≥ 65 years
- HGB:
Hemoglobin
- HCT:
Hemocrit
- PLT:
Platelet
- ALB:
Albumin
- AST:
Aspartate aminotransferase
- ALT:
Alanine aminotransferase
- TBIL:
Total bilirubin
- DBIL:
Direct bilirubin
- CREA:
Creatinine
- BUN:
Blood urea nitrogen
- WBC:
White blood cell
- ROC:
Receiver operating characteristics
- AUC:
Area under the curve
- AUROC:
Area under the receiver operating characteristics curve
- PPV:
Positive predictive value
- NPV:
Negative predictive value
- COPD:
Chronic obstructive pulmonary disease
- CDVD:
Cardiovascular disease
- CBVD:
Cerebral-vascular disease
- CRD:
Chronic renal disease
- HBD:
Hepatobiliary disease
- ICU:
Intensive care unit.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Heron M. Death: leading causes for 2014. National Vital Statistics Reports. 2016;65(5):1–96. [PubMed] [Google Scholar]
- 2.Cao B., Huang Y., She D.-Y., et al. Diagnosis and treatment of community-acquired pneumonia in adults: 2016 clinical practice guidelines by the Chinese Thoracic Society, Chinese Medical Association. The Clinical Respiratory Journal. 2018;12(4):1320–1360. doi: 10.1111/crj.12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim W. S., van der Eerden M. M., Laing R., et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377–382. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fine M. J., Auble T. E., Yealy D. M., et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. New England Journal of Medicine. 1997;336(4):243–250. doi: 10.1056/nejm199701233360402. [DOI] [PubMed] [Google Scholar]
- 5.Chalmers J. D., Singanayagam A., Akram A. R., et al. Severity assessment tools for predicting mortality in hospitalised patients with community-acquired pneumonia. Systematic review and meta-analysis. Thorax. 2010;65(10):878–883. doi: 10.1136/thx.2009.133280. [DOI] [PubMed] [Google Scholar]
- 6.Singer M., Deutschman C. S., Seymour C. W., et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bou Chebl R., El Khuri C., Shami A., et al. Serum lactate is an independent predictor of hospital mortaltiy in critically ill patients in the emergency department: a retrospective study. Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine. 2017;25(1):p. 69. doi: 10.1186/s13049-017-0415-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park Y. J., Kim D. H., Kim S. C., et al. Serum lactate upon emergency department arrival as a predictor of 30-day in-hospital mortality in an unselected population. PLoS One. 2018;13(1) doi: 10.1371/journal.pone.0190519.e0190519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mandell L. A., Wunderink R. G., Anzueto A., et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clinical Infectious Diseases. 2007;44(Supplement_2):S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y.-X., Li C.-S. Lactate on emergency department arrival as a predictor of mortality and site-of-care in pneumonia patients: a cohort study. Thorax. 2015;70(5):404–410. doi: 10.1136/thoraxjnl-2014-206461. [DOI] [PubMed] [Google Scholar]
- 11.Lim W. S., Baudouin S. V., George R. C., et al. Pneumonia guidelines committee of the BTS standards of care committee. BTS guidelines for the management of community acquired pneumonia in adults: updates 2009. Thorax. 2009;64(Suppl 3):iii1–iii55. doi: 10.1136/thx.2009.121434. [DOI] [PubMed] [Google Scholar]
- 12.Mulrennan S., Tempone S. S., Ling I. T., et al. Pandemic influenza (H1N1) 2009 pneumonia: CURB-65 score for predicting severity and nasopharyngeal sampling for diagnosis are unreliable. PLoS One. 2010;5(9) doi: 10.1371/journal.pone.0012849.e12849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chalmers J. D., Singanayagam A., Hill A. T. Predicting the need for mechanical ventilation and/or inotropic support for young adults admitted to the hospital with community-acquired pneumonia. Clinical Infectious Diseases. 2008;47(12):1571–1574. doi: 10.1086/593195. [DOI] [PubMed] [Google Scholar]
- 14.Chalmers J. D., Mandal P., Singanayagam A., et al. Severity assessment tools to guide ICU admission in community-acquired pneumonia: systematic review and meta-analysis. Intensive Care Medicine. 2011;37(9):1409–1420. doi: 10.1007/s00134-011-2261-x. [DOI] [PubMed] [Google Scholar]
- 15.Hamaguchi S., Suzuki M., Sasaki K., et al. Adult pneumonia study group–Japan. Six underlying health conditions strongly influence mortality based on pneumonia severity in an ageing population of Japan: a prospective cohort study. BMC Pulmonary Medicine. 2018;18(1):p. 88. doi: 10.1186/s12890-018-0648-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Z., Yong Y., Tan W., Shen L., Ng H., Fong K. Prognostic factors for mortality due to pneumonia among adults from different age groups in Singapore and mortality predictions based on PSI and CURB-65. Singapore Medical Journal. 2018;59(4):190–198. doi: 10.11622/smedj.2017079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou H., Guo S., Lan T., Ma S., Zhang F., Zhao Z. Risk stratification and prediction value of procalcitonin and clinical severity scores for community-acquired pneumonia in ED. The American Journal of Emergency Medicine. 2018;36(12):2155–2160. doi: 10.1016/j.ajem.2018.03.050. [DOI] [PubMed] [Google Scholar]
- 18.Kim M. W., Lim J. Y., Oh S. H. Mortality prediction using serum biomarkers and various clinical risk scales in community-acquired pneumonia. Scandinavian Journal of Clinical and Laboratory Investigation. 2017;77(7):486–492. doi: 10.1080/00365513.2017.1344298. [DOI] [PubMed] [Google Scholar]
- 19.Ranzani O. T., Prina E., Menéndez R., et al. New sepsis definition (Sepsis-3) and community-acquired pneumonia mortality. A validation and clinical decision-making study. American Journal of Respiratory and Critical Care Medicine. 2017;196(10):1287–1297. doi: 10.1164/rccm.201611-2262oc. [DOI] [PubMed] [Google Scholar]
- 20.Tokioka F., Okamoto H., Yamazaki A., Itou A., Ishida T. The prognostic performance of qSOFA for community-acquired pneumonia. Journal of Intensive Care. 2018;6(1):p. 46. doi: 10.1186/s40560-018-0307-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levy M. M., Evans L. E., Rhodes A. The surviving sepsis campaign bundle. Critical Care Medicine. 2018;46(6):997–1000. doi: 10.1097/ccm.0000000000003119. [DOI] [PubMed] [Google Scholar]
- 22.Gwak M. H., Jo S., Jeong T., et al. Initial serum lactate level is associated with inpatient mortality in patients with community-acquired pneumonia. The American Journal of Emergency Medicine. 2015;33(5):685–690. doi: 10.1016/j.ajem.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Frenzen F. S., Kutschan U., Meiswinkel N., Schulte-Hubbert B., Ewig S., Kolditz M. Admission lactate predicts poor prognosis independently of the CRB/CURB-65 scores in community-acquired pneumonia. Clinical Microbiology and Infection. 2018;24(3):306.e1–306.e6. doi: 10.1016/j.cmi.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Song H., Moon H. G., Kim S. H. Efficacy of quick sequential organ failure assessment with lactate concentration for predicting mortality in patients with community-acquired pneumonia in the emergency department. Clinical and Experimental Emergency Medicine. 2019;6(1):1–8. doi: 10.15441/ceem.17.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ho K. M., Lan N. S. H. Combining quick sequential organ failure assessment with plasma lactate concentration is comparable to standard sequential organ failure assessment score in predicting mortality of patients with and without suspected infection. Journal of Critical Care. 2017;38:1–5. doi: 10.1016/j.jcrc.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Zhou H., Lan T., Guo S. Stratified and prognostic value of admission lactate and severity scores in patients with community-acquired pneumonia in emergency department: a single-center retrospective cohort study. Medicine (Baltimore) October 2019;98(41) doi: 10.1097/md.0000000000017479.e17479 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request


