Abstract
Germline BRCA1 and BRCA2 mutations confer an increased lifetime risk for breast cancer and ovarian cancer. Several studies have investigated prognosis among BRCA1/2 mutation carriers and noncarriers, but the prognostic impact on outcomes of breast cancer patients has not been determined. The aim of this study was to determine the prognosis of TNBC patients with and without BRCA1/2 germline mutation. Among 502 patients diagnosed with TNBC between 2005 and 2008, 124 patients with a strong family history of breast cancer or ovarian cancer as well as TNBC patients diagnosed under 45 years were referred to the Genetic Counseling Unit for genetic counselling and genetic tests. In 30 (24%) of them, the BRCA1/2 mutation was detected (the most common 5382insC in 18 (60%) patients). The median follow-up of the entire group was 60 months. BRCA1/2 mutation carriers were statistically significantly younger at TNBC diagnosis compared with nonmutation patients (41 vs 47 years, respectively). Patients with the BRCA1/2 mutation had smaller tumors (stage I: 47% vs 24.5% in noncarriers), but there was no significant difference in the regional nodal status (58.5–63% with cN0). Contralateral breast cancer developed in 26.5% of BRCA1/2 mutation carriers and in 14% of noncarriers. Other primary cancers were also slightly more common in BRCA1/2 mutation carriers (16.5% vs 9.5%). The performed analysis did not show any significant differences between the groups in recurrence-free survival (p=0.312). There was no significant difference between patients with or without BRCA1/2 mutation as regards overall survival (p=0.649) and the risk of TNBC death (p=0.333). The survival from detection of metastases was similar in two groups (p=0.865). Our study demonstrated that the BRCA1 mutation does not affect TNBC patients' outcomes.
1. Introduction
BRCA1 and BRCA2 are tumor suppressor genes involved in DNA damage repair, cell cycle control, gene transcription regulation, and apoptosis. The common germline mutations of the BRCA1 gene are 5382insC, 185delAG, 3819del5, and 4153delA and of BRCA2 are 4075delGT and 580del4 [1]. In the western population, about 5% of the breast cancer patients may carry heritable cancer susceptibility gene mutations, with BRCA1 being the most common mutation [2]. The mutation rate can be higher in Ashkenazi Jews [3, 4]. Interestingly, BRCA1/2 mutation rates in Asians are lower than those in whites [5].
1.1. Prevalence of Breast/Ovarian Cancer
Germline BRCA1 and BRCA2 mutations confer an increased lifetime risk for breast cancer and ovarian cancer. Women with BRCA1/2 germline mutations have a higher incidence of breast cancer than those without these genetic abnormalities. The cumulative incidence of breast cancer by age 70–80 years in female mutation carriers is 71.4–87% for the BRCA1 mutation and 77–88% for the BRCA2 mutation [6–8]. The ovarian cancer risk is 59–65% for the BRCA1 mutation and 34.5–37% for the BRCA2 mutation [6, 8]. The high lifetime risk of breast and ovarian cancers in BRCA1/2 carriers is crucial for counselling, intensive breast and ovarian screening (annual MRI commenced from the age of 25 with the additional annual mammography from the age of 30, 6-monthly ovarian cancer screening with transvaginal ultrasound, and Ca125 serum measure started at the age of 30), and risk-reducing surgery (bilateral salpingo-oophorectomy and bilateral risk-reducing mastectomy including skin-sparing and nipple-sparing mastectomy) [9, 10].
Compared to BRCA2 carriers and noncarriers, BRCA1-associated breast cancers are often high-grade and poorly differentiated infiltrating ductal carcinoma and are more often triple-negative with higher expressions of cytokeratin 5/6, cyclin E, and p53. Patients with BRCA1-associated breast cancers are younger than those with the BRCA2 mutation and those without mutation [11, 12].
1.2. Prognosis
Several studies have investigated prognosis among BRCA1/2 mutation carriers and noncarriers, but the prognostic impact on outcomes of breast cancer patients has not been definitely determined. It is controversial whether BRCA1/2 mutations in breast cancer are associated with poor prognosis. Some studies revealed that BRCA1/2 mutation carriers with breast cancer had worse overall survival (OS) than noncarriers [13–15], others showed no difference [16–20], and some studies indicated that BRCA1/2 mutation carriers had better survival than noncarriers [21–23]. Differences could be partly the result of the analysis of different ethnic populations (Ashkenazi Jewish population [24], central-eastern population [15], western population [19], or Asian population [20, 25]), small study group with mutations, variations in mutation assay techniques, mutation types, cancer treatment modalities, or length of follow-up.
Among all biological subtypes of breast cancer, triple-negative breast cancer (TNBC) is more likely to harbor a germline BRCA1/2 mutation, with reported prevalence rates varying from about 10% to 20% [20, 22, 26, 27]. The effect of the BRCA1/2 mutation on the prognosis in TNBC patients has not been well examined, with divergent findings reported in the previous studies [18, 20, 22, 28–30].
2. Aim
The aim of this study was to determine the prognosis of TNBC patients with and without BRCA1/2 germline mutation.
3. Materials and Methods
Five hundred two consecutive TNBC patients treated at the Department of Breast Cancer and Reconstructive Surgery, Maria Skłodowska-Curie Institute–Cancer Center (MSCI), Warsaw, Poland, between 2005 and 2008, were selected and analyzed to assess risk factors of recurrence, recurrence-free survival (RFS), and OS. Among them, 124 patients with a strong family history of breast cancer or ovarian cancer as well as TNBC patients diagnosed under 45 years were referred to the Genetic Counseling Unit of Cancer Prevention Department in MSCI, Warsaw, for genetic counselling and genetic tests. The patients were tested for the following BRCA1/2 mutations: BRCA1 gene: c.5266dupC (5382insC), c.181T>G (C61G, 300T>G), c.3700_3704delGTAAA (3819del5), c.68_69delAG (185delAG), c.676delT (p.Cys226Valfs), c.1687C>T (p.Gln563Ter), c.3756_3759delGTCT (3875del4), c.4035delA (4153delA), c.5251C>T (5370C>T), and c.5345G>A (p.Trp1782X) and BRCA2 gene: c.658_659del GT (p.Val220fs), c.5946delT (6174delT), c.9371A>T (p.Asn3124Ile), and c.5744C>T (C5972T). Characteristics of the whole group of 502 TNBC patients and 124 patients in whom genetic tests were performed are presented in Tables 1 and 2. The Ki-67 expression and vimentin expression were conducted additionally due to the fact that, in the analyzed period of time, these markers were not assessed as standard practice (vimentin still remains as an experimental biomarker, expressed more often in mesenchymal tumors). The decisions on therapy were made regardless of the BRCA1/2 mutation status.
Table 1.
Characteristics of 502 TNBC patients.
| Factor | Rate (%) | |
|---|---|---|
| Number of patients | 502 | 100 |
|
| ||
| Age at diagnosis (years) | ||
| Median | 55 | |
| Mean | 56 | |
| Range | 24–98 | |
|
| ||
| Clinical staging (cTNM) | ||
| I | 97 | 19.5 |
| II | 246 | 49 |
| III | 132 | 26 |
| IV | 27 | 5.5 |
|
| ||
| Initial clinical tumor staging | ||
| cT0 | 7 | 1 |
| cT1 | 111 | 22 |
| cT2 | 248 | 49.5 |
| cT3 | 58 | 12 |
| cT4 | 76 | 15 |
| No available data | 2 | 0.5 |
|
| ||
| Initial clinical node staging | ||
| cN0 | 243 | 48 |
| cN1 | 180 | 36 |
| cN2 | 58 | 11.5 |
| cN3 | 19 | 4 |
| No available data | 2 | 0.5 |
|
| ||
| HER2 expression | ||
| 0 or 1+ | 431 | 86 |
| 2+, FISH negative | 71 | 14 |
|
| ||
| Histological type | ||
| NST | 416 | 83 |
| Lobular | 25 | 5 |
| Medullar | 11 | 2 |
| Apocrine | 11 | 2 |
| Metaplastic | 20 | 4 |
| Others | 20 | 4 |
|
| ||
| G | ||
| 1 | 21 | 4 |
| 2 | 165 | 33 |
| 3 | 310 | 62 |
| No available data | 6 | 1 |
|
| ||
| Ki-67 expression | ||
| <14% | 140 | 28 |
| 14–30% | 183 | 36.5 |
| >30% | 133 | 26.5 |
| No available data | 46 | 9 |
|
| ||
| Vimentin expression assessed | ||
| Yes | 443 | 88 |
| No | 59 | 12 |
|
| ||
| Vimentin | ||
| Positive | 71/443 | 16 |
| Negative | 372/443 | 84 |
|
| ||
| Contralateral breast cancer | 41 | 8 |
| Other primary cancer (other than contralateral breast cancer) | 45 | 9 |
FISH: fluorescence in situ hybridization.
Table 2.
Characteristics of 124 TNBC patients assessed for BRCA1/2 mutations.
| Factor | Patients tested for BRCA mutations | p value (BRCA-positive vs BRCA-negative) | |||
|---|---|---|---|---|---|
| BRCA noncarriers | Rate (%) | BRCA carriers | Rate (%) | ||
| Number of patients | 94 | 100 | 30 | 100 | |
|
| |||||
| Age at diagnosis (years) | |||||
| Median | 49 | 40 | 0.0115 | ||
| Mean | 47.5 | 41.4 | |||
| Range | 25–67 | 24–76 | |||
|
| |||||
| Clinical staging (cTNM) | |||||
| I | 23 | 24.5 | 14 | 47 | 0.0006 |
| II | 51 | 54 | 13 | 43 | |
| III | 19 | 20 | 2 | 7 | |
| IV | 1 | <0.5 | 1 | 3 | |
|
| |||||
| Initial clinical tumor staging | |||||
| cT0 | 0 | 0 | 0 | 0 | 0.0004 |
| cT1 | 28 | 30 | 16 | 53 | |
| cT2 | 56 | 59.5 | 9 | 30 | |
| cT3 | 4 | 4 | 2 | 7 | |
| cT4 | 6 | 6.5 | 3 | 10 | |
| No available data | 0 | 0 | 0 | 0 | |
|
| |||||
| Initial clinical node staging | |||||
| cN0 | 55 | 58.5 | 19 | 63 | 0.1063 |
| cN1 | 27 | 28.5 | 10 | 33 | |
| cN2 | 9 | 9.5 | 1 | 4 | |
| cN3 | 3 | 3.5 | 0 | 0 | |
| No available data | 0 | 0 | 0 | 0 | |
|
| |||||
| HER2 expression | |||||
| 0 or 1+ | 79 | 84 | 29 | 97 | 0.0091 |
| 2+, FISH negative | 15 | 16 | 1 | 3 | |
|
| |||||
| Histological type | |||||
| NST | 80 | 21 | 70 | 0.0023 | |
| Lobular | 5 | 85 | 1 | 3.5 | |
| Medullar | 5 | 5.5 | 1 | 3.5 | |
| Apocrine | 2 | 5.5 | 1 | 3.5 | |
| Metaplastic | 2 | 2 | 2 | 6 | |
| Others | 0 | 2 | 4 | 13.5 | |
|
| |||||
| G | |||||
| 1 | 0 | 0 | 2 | 6.5 | 0.0065 |
| 2 | 29 | 30 | 12 | 40 | |
| 3 | 64 | 68 | 16 | 53.5 | |
| No available data | 1 | 2 | 0 | 0 | |
|
| |||||
| Ki-67 expression | |||||
| <14% | 26 | 27.5 | 5 | 16.5 | 0.0761 |
| 14–30% | 29 | 31 | 10 | 33.5 | |
| >30% | 28 | 30 | 13 | 43.5 | |
| No available data | 11 | 11.5 | 2 | 6.5 | |
|
| |||||
| Vimentin expression assessed | |||||
| Yes | 82 | 87 | 26 | 86.5 | 0.8361 |
| No | 12 | 13 | 4 | 13.5 | |
|
| |||||
| Vimentin | |||||
| Positive | 14 | 15 | 8 | 26.5 | 0.0372 |
| Negative | 68 | 85 | 18 | 73.5 | |
|
| |||||
| Contralateral breast cancer | 13 | 14 | 8 | 26.5 | 0.0228 |
| Other primary cancer (other than contralateral breast cancer) | 9 | 9.5 | 5 | 16.5 | 0.1475 |
FISH: fluorescence in situ hybridization.
3.1. Statistical Analysis
Univariate analysis was performed in order to compare patient and tumor characteristics (age at diagnosis, clinical stage, HER2 expression, histological grade G, Ki-67 expression, and vimentin expression) as well as therapy (type of surgery, radiotherapy, and (neo)adjuvant chemotherapy) depending on the BRCA1/2 mutation status. R Development Core Team (R 3.1.3., 2009) software was used for these analyses.
The following definitions of events were used:
RFS—time from TNBC diagnosis to recurrence
OS—time from TNBC diagnosis to death from any cause
Breast cancer-specific survival (BCSS)—time from TNBC diagnosis to death from breast cancer
Survival from dissemination—time from recurrence to death from any cause
Then, RFS, OS, and survival from dissemination of the disease in both groups were assessed. Additionally, risk of breast cancer death using the competing risk method was evaluated. Finally, the BRCA1/2 mutation was assessed as one of the seven prognostic factors for recurrence and survival in multivariate analysis using the multistep Cox model. The other prognostic factors in the Cox model were age at diagnosis, TNM stage (I, II, or III), Ki-67 expression, vimentin expression, histological grade G (G1, G2, or G3), and histological type (no special type—NST or others).
4. Results
Finally, 124 (25%) out of 502 TNBC patients had undergone genetic counselling with BRCA1/2 mutation tests and were included for further analysis. In 30 (24%) of them, the BRCA1/2 mutation was detected. Only in one case, the mutation of the BRCA2 gene was found, and for the BRCA1 gene, 29 mutated cases were detected. The following BRCA1 mutations were found: c.5266dupC (5382insC) in 18 patients, c.181T>G (C61G, 300T>G) in 5 patients, c.3700_3704delGTAAA (3819del5) in 2 patients, and c.5251C>T (5370C>T), c.5345G>A (p.Trp1782X), c.3756_3759delGTCT (3875del4), and c.68_69delAG (185delAG) in 1 patient each, respectively. One patient harbored BRCA2 gene mutation c.5744C>T (C5972T). The comparison between BRCA1/2 mutation carriers and noncarriers is presented in Table 2. The median follow-up of the entire group was 60 months. BRCA1/2 mutation carriers were statistically significantly younger at TNBC diagnosis compared with nonmutation patients (41 vs 47 years, respectively). Patients with the BRCA1/2 mutation had smaller tumors (stage I: 47% vs 24.5% in noncarriers), but there was no significant difference in the regional nodal status (58.5–63% with cN0). The most common histological type was NST in both groups with a similar rate of medullar cancer (3.5–5.5%). Noncarriers had more often G3 tumors. Contralateral breast cancer developed in 26.5% of BRCA1/2 mutation carriers and in 14% of noncarriers. In both groups, almost half contralateral breast cancers developed before TNBC diagnosis. Other primary cancers were also slightly more common in BRCA1/2 mutation carriers (16.5% vs 9.5%). Almost all cases occurred after TNBC diagnosis in both groups (only 2 cases of lymphoma and one ovarian cancer developed before TNBC). The summary of these results is presented in Table 2.
In 72 patients (58% of all TNBC), the primary operation was performed. In other 47 (38%) patients, surgery was carried out after neoadjuvant chemotherapy. Breast-conserving surgery was more common in BRCA1/2 mutation carriers (41.5% vs 33.5%). Adjuvant chemotherapy was performed in 87 patients (90% after primary surgery). Overall, (neo)adjuvant chemotherapy was performed in a similar percentage of patients with or without BRCA1/2 mutation. The summary of patient therapy is presented in Table 3.
Table 3.
Therapy of 124 TNBC patients assessed for BRCA1/2 mutations.
| Type of therapy | Patients tested for BRCA mutations | p value (BRCA-positive vs BRCA-negative) | |||
|---|---|---|---|---|---|
| BRCA noncarriers | Rate (%) | BRCA carriers | Rate (%) | ||
| Number of patients | 94 | 100 | 30 | 100 | |
|
| |||||
| Surgery | |||||
| Yes | 90 | 96 | 29 | 97 | 0.7004 |
| No | 4 | 4 | 1 | 3 | |
|
| |||||
| Type of surgery | |||||
| Mastectomy | 60/90 | 66.5 | 17/29 | 58.5 | 0.2438 |
| Breast-conserving surgery | 30/90 | 33.5 | 12/29 | 41.5 | |
|
| |||||
| Radiotherapy | |||||
| Yes | 55 | 58.5 | 17 | 56.5 | 0.7751 |
| No | 39 | 41.5 | 13 | 43.5 | |
|
| |||||
| Radiotherapy | |||||
| After mastectomy | 27/55 | 49 | 5/17 | 29.5 | 0.0044 |
| After breast-conserving surgery | 28/55 | 51 | 12/17 | 70.5 | |
|
| |||||
| Neoadjuvant chemotherapy | |||||
| Yes | 20 | 21.5 | 4 | 13.5 | 0.0940 |
| No | 74 | 78.5 | 26 | 86.5 | |
|
| |||||
| Regimens in neoadjuvant chemotherapy | |||||
| AT⟶CMF | 5/20 | 25 | 1/4 | 25 | <0.0001 |
| Anthracycline + taxane | 9/20 | 45 | 2/4 | 50 | |
| Anthracycline | 5/20 | 5 | 1/4 | 25 | |
| Others | 1/20 | 25 | 0 | 0 | |
|
| |||||
| Adjuvant chemotherapy | |||||
| Yes | 64 | 68 | 23 | 76.5 | 0.1541 |
| No | 30 | 32 | 7 | 23.5 | |
|
| |||||
| Regimens in adjuvant chemotherapy | |||||
| Anthracycline (AC) | 41/64 | 64 | 12/23 | 52 | 0.0574 |
| FEC/FAC | 11/64 | 17.5 | 4/23 | 17.5 | |
| Anthracycline + taxane | 8/64 | 12.5 | 5/23 | 21.5 | |
| CMF | 2/64 | 3 | 0 | 0 | |
| Taxane | 2/64 | 3 | 2/23 | 9 | |
We compared RFS, OS, risk of breast cancer death, and survival from distant metastases in BRCA1/2 carriers and noncarriers. The performed analysis did not show any significant differences between the groups in RFS (p=0.312), also after taking into account the clinical stage of TNBC (in patients in the following stages: I: p=1.0, II: p=0.454, and III: p=0.197) or (neo)adjuvant chemotherapy (p > 0.05). The risk of the recurrence depending on the BRCA1/2 mutation status is shown in Figure 1. There was no significant difference between patients with or without BRCA1/2 mutation regarding overall survival (p=0.649). The BRCA1/2 mutation was not a prognostic factor of patient survival. The results are presented in Figure 2. The risk of TNBC death did not differ significantly in both groups (Figure 3).
Figure 1.
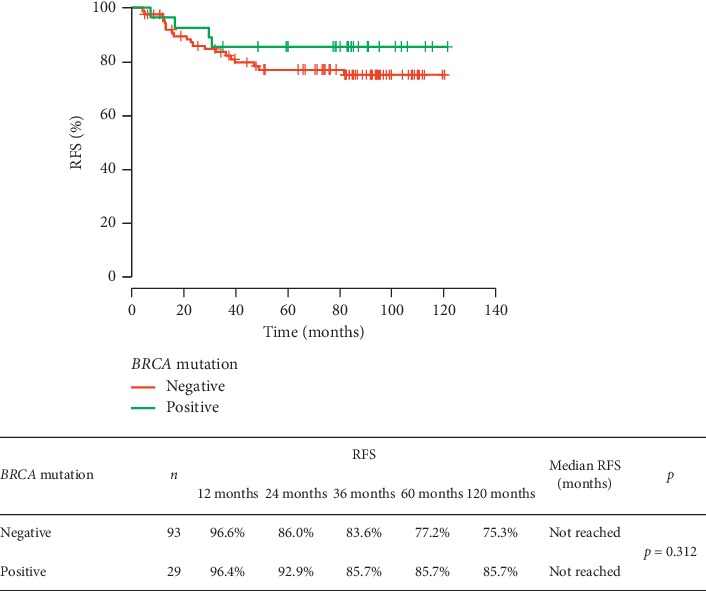
Risk of recurrence in TNBC patients depending on the BRCA mutation status.
Figure 2.
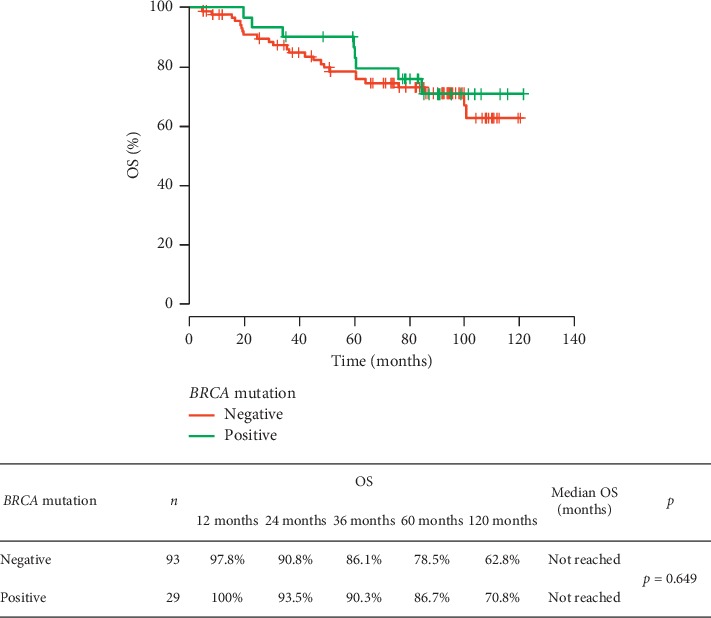
Risk of death in TNBC patients depending on the BRCA mutation status.
Figure 3.
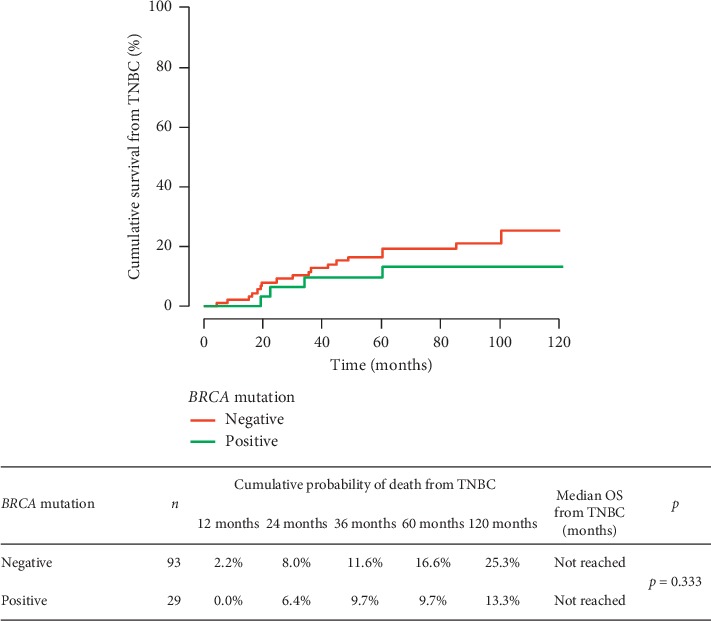
Relationship between the presence of the BRCA1/2 mutation and the risk of death due to TNBC.
In 13% (4/30) of BRCA1/2 mutation patients and in 21% (20/94) of noncarriers, the recurrence of the disease was detected. In both groups, there was one patient with primary metastatic TNBC. There was no significant difference in survival from detection of metastases between these two groups (p=0.865). The results are presented in Figure 4.
Figure 4.
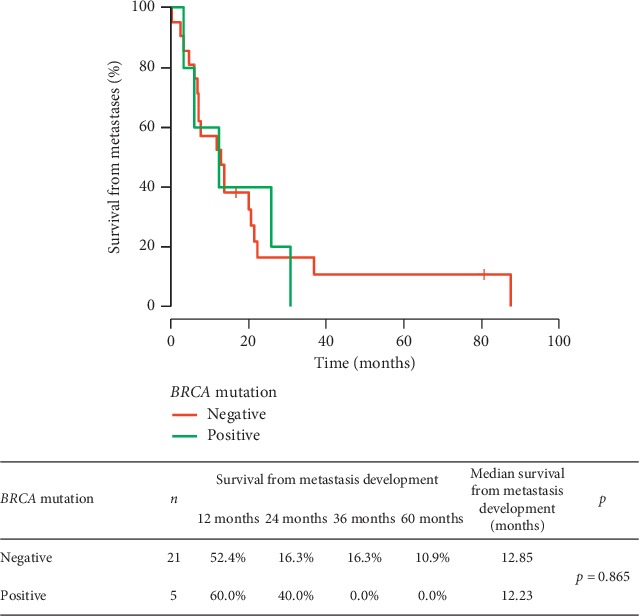
Survival time counted from relapse depending on the BRCA1/2 mutation status.
Among seven variables taken in multivariate analysis, TNM stage was the only factor significantly influencing recurrence and death. There was no correlation between RFS or OS and other analyzed risk factors, including the BRCA1/2 germline mutation. The results are shown in Tables 4 and 5.
Table 4.
Multivariate analysis: final model for RFS.
| Factor | HR | 95% CI | p | |
|---|---|---|---|---|
| Clinical stage: I or II | Reference | |||
|
| ||||
| Clinical stage: III | 43.26 | 2.13 | 880.64 | 0.014 |
Table 5.
Multivariate analysis: final model for OS.
| Factor | HR | 95% CI | p | |
|---|---|---|---|---|
| Clinical stage: I | Reference | |||
|
| ||||
| Clinical stage: II | 2.359 | 1.385 | 4.016 | 0.002 |
|
| ||||
| Clinical stage: III | 8.353 | 4.918 | 14.188 | <0.001 |
5. Discussion
Our study showed that the outcome of TNBC patients did not differ depending on the BRCA mutation status. We aimed to clarify the prognostic value of BRCA1/2 mutations on breast cancer-specific outcomes after conventional treatment. In our study, RFS, OS, and risk of death from TNBC were similar between patients with breast cancer and BRCA1 germline mutation and noncarriers. Because of the fact that among our patients with BRCA1/2 mutations only one had BRCA2 mutation, the results and discussion concern about patients with breast cancer and BRCA1 mutation.
5.1. All Biological Types of Breast Cancer
The meta-analysis of 11 studies performed by Lee et al. revealed that patients with breast cancer and BRCA1 mutation had worse OS compared to noncarriers (HR = 1.92). The BRCA2 mutation did not affect survival in patients with breast cancer (HR = 1.30) [31].
In meta-analysis by Zhong et al. [32], based on 13 studies with 10 016 women with breast cancer, concerning breast cancer survival, the BRCA1 mutation carriers had worse OS than noncarriers (HR = 1.5, p=0.009) but were not significantly different from noncarriers in terms of progression-free survival (HR = 1.35, p=0.09).
In other meta-analysis performed by Zhu et al. [3], based on 34 studies, event-free survival (EFS), OS, and BCSS were compared in three groups of breast cancer patients: BRCA1 carriers, BRCA2 carriers, and BRCA1/2 noncarriers. In patients with BRCA1 and BRCA2 mutations, OS was worse than that in patients without mutation (p < 0.001 and p=0.034, respectively) but did not translate into poor BCSS (p=0.448 and p=0.401, respectively) or EFS (p=0.438 and p=0.558, respectively) [3]. The BRCA1 mutation was significantly associated with worse OS in studies conducted in Europe (p < 0.001) and studies assessing patients diagnosed before 1995 (p < 0.007).
The POSH prospective cohort study analyzed patients with young-onset breast cancer (≤40 years) regarding the BRCA1/2 mutation status [33]. Recently published results indicated no significant difference in OS or distant disease-free survival between patients carrying BRCA1/2 mutations and patients without those mutations after a diagnosis of breast cancer.
A study by Wang et al. performed on the Chinese cohort revealed that patients with BRCA1/2 mutations had worse survival outcomes than noncarriers [25]. BRCA1/2 mutation carriers were more likely to have lymph node involvement at initial diagnosis than noncarriers [25]. In our study, we did not observe these kinds of relations.
5.2. Triple-Negative Breast Cancer
Studies that have evaluated the prognostic role of the BRCA1/2 mutation in patients with TNBC have shown inconclusive results, but the newest and larger ones are in line with our study.
In the study performed by Yadav et al. [34], 266 TNBC patients had undergone BRCA1/2 mutation tests. In 27% of them, BRCA1/2 mutations were detected. No statistically significant difference was found in locoregional recurrence, distant recurrence, RFS, and OS between the breast cancer patients with and without BRCA1/2 mutations. 5-year OS for BRCA1/2-positive and BRCA1/2-negative breast cancer patients was 83% and 90% and 5-year RFS was 83% and 80%, respectively. The differences were not statistically significant [34].
In the study by Gonzales-Angulo et al. [22], based on 77 TNBC patients, RFS was better for patients with the BRCA1/2 mutation and OS was similar between carriers and noncarriers.
In another study, Maksimenko et al. [30] compared the outcomes of 78 TNBC patients without BRCA1 mutation with those of 38 TNBC patients with the BRCA1 mutation. The BCSS and distant recurrence were significantly lower in the BRCA1-positive patients. In 4 other larger studies, there was no difference found in recurrence and survival between TNBC carriers and noncarriers of BRCA1/2 mutations [18, 20, 28, 29]. A meta-analysis of 11 papers performed by Xie et al. also revealed that RFS and OS in TNBC patients with and without BRCA1/2 mutations did not differ [20].
Baretta et al. [24] performed a meta-analysis concerning the relation between BRCA1/2 mutation and prognosis of breast cancer based on 105 220 breast cancer patients including 3588 (3.4%) BRCA1/2 mutation carriers. OS, BCSS, RFS, and distant metastasis-free survival (DMFS) were estimated. The authors found that BRCA1 mutation carriers had a 30% higher risk of dying than BRCA1-negative/sporadic cases (OS), but they did not find association between BRCA1 and the risk of death from breast cancer (BCSS). Contrary to patients with all subtypes of breast cancer, 1748 patients with TNBC and BRCA1/2 mutations had better OS than BRCA1/2-negative ones (HR = 0.49) [24]. The risk of recurrence in TNBC was not statistically different between BRCA1/2 carriers and BRCA1/2 noncarriers (p=0.82). BCSS and DMFS of BRCA1 mutation carriers did not differ from those of BRCA1-negative TNBC patients (p=0.76 and p=0.65, respectively) [24].
In the present study, all investigated TNBC cases were diagnosed and treated in one breast cancer department. The used methods did not differ depending on the BRCA1/2 mutation status, and patients had a long time of follow-up (up to 10 years). Nowadays, new drugs such as poly(adenosine diphosphate-ribose) polymerase (PARP) inhibitors (olaparib and talazoparib) are dedicated to metastatic BRCA1/2-positive TNBC as well as immunotherapy for PDL-1-positive metastatic TNBC [35–37]. These drugs can influence the survival of BRCA1/2 carriers with TNBC in the future. In the analyzed cohort with metastatic disease, the survival did not depend on the BRCA1/2 mutation status. In contrast, Larson et al. showed that BRCA carriers with metastatic TNBC had clinically significant improved OS at 3 years compared to patients without BRCA mutations (3-year OS of 63% vs 28%). In that study also, no patients received treatment with the PARP inhibitor [38].
6. Limitations of the Study
The retrospective nature of the study and a small number of recurrences or deaths in patients who had undergone genetic tests are two main limitations of this study.
Out of 502 consecutive TNBC patients referred to MSCI between the years 2005 and 2008, only 124 (25%) patients underwent genetic tests for the BRCA1/2 mutation. From them, the BRCA1/2 mutation was found only in 30 cases, which gives 6% (30/502) BRCA1/2 carriers among 502 TNBC patients. According to the current NCCN guideline and ESMO recommendations, 65% of all TNBC patients from our analysis met the genetic test criteria solely by their age at diagnosis of TNBC (up to 60 years); therefore, the tests should be performed [10, 39]. This number might be even higher considering other criteria such as a strong family history of breast/ovarian cancer. In the years 2005–2008, genetic tests were offered at our institution only for patients with a strong family history of breast/ovarian cancer and for those under 45 years at the initial diagnosis of breast cancer.
7. Conclusion
Our study demonstrated that the BRCA1 mutation does not affect RFS and OS in patients diagnosed with TNBC. The outcome of breast cancer in BRCA1 carriers and noncarriers was comparable. The BRCA1 germline mutation did not influence the prognosis of the TNBC patients.
Acknowledgments
This research was supported by the grant from National Science Centre (NCN), Poland (UMO-2014/15/B/NZ5/03532).
Data Availability
The data used to support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this article.
References
- 1.Wang F., Fang Q., Ge Z., Yu N., Xu S., Fan X. Common BRCA1 and BRCA2 mutations in breast cancer families: a meta-analysis from systematic review. Molecular Biology Reports. 2012;39(3):2109–2118. doi: 10.1007/s11033-011-0958-0. [DOI] [PubMed] [Google Scholar]
- 2.Tung N., Battelli C., Allen B., et al. Frequency of mutations in individuals with breast cancer referred for BRCA1 and BRCA2 testing using next-generation sequencing with a 25-gene panel. Cancer. 2015;121(1):25–33. doi: 10.1002/cncr.29010. [DOI] [PubMed] [Google Scholar]
- 3.Zhu Y., Wu J., Zhang C., et al. BRCA mutations and survival in breast cancer: an updated systematic review and meta-analysis. Oncotarget. 2016;7:70113–70127. doi: 10.18632/oncotarget.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warner E., Foulkes W., Goodwin P., et al. Prevalence and penetrance of BRCA1 and BRCA2 gene mutations in unselected Ashkenazi Jewish women with breast cancer. JNCI Journal of the National Cancer Institute. 1999;91(14):1241–1247. doi: 10.1093/jnci/91.14.1241. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J., Pei R., Pang Z., et al. Prevalence and characterization of BRCA1 and BRCA2 germline mutations in Chinese women with familial breast cancer. Breast Cancer Research and Treatment. 2012;132(2):421–428. doi: 10.1007/s10549-011-1596-x. [DOI] [PubMed] [Google Scholar]
- 6.Evans D. G., Shenton A., Woodward E., Lalloo F., Howell A., Maher E. R. Penetrance estimates for BRCA1 and BRCA2 based on genetic testing in a clinical cancer genetics service setting: risks of breast/ovarian cancer quoted should reflect the cancer burden in the family. BMC Cancer. 2008;8(1) doi: 10.1186/1471-2407-8-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ford D., Easton D. F., Stratton M., et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The breast cancer linkage consortium. American Journal of Human Genetics. 1998;62(62):676–689. doi: 10.1086/301749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van der Kolk D. M., de Bock G. H., Leegte B. K., et al. Penetrance of breast cancer, ovarian cancer and contralateral breast cancer in BRCA1 and BRCA2 families: high cancer incidence at older age. Breast Cancer Research and Treatment. 2010;124(3):643–651. doi: 10.1007/s10549-010-0805-3. [DOI] [PubMed] [Google Scholar]
- 9.Paluch-Shimon S., Sessa C., Cardoso M. J., Gilbert F., Senkus E. Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO Clinical Practice Guidelines for cancer prevention and screening. Annals of Oncology. 2016;27(suppl 5):v103–v110. doi: 10.1093/annonc/mdw327. [DOI] [PubMed] [Google Scholar]
- 10.NCCN Clinical Practice Guidelines in Oncology. Genetic/Familial High Risk Assessment: Breast and Ovarian. Version 3. Fort Washington, PA, USA: NCCN; 2019. [Google Scholar]
- 11.Krammer J., Pinker-Domenig K., Robson M. E., et al. Breast cancer detection and tumor characteristics in BRCA1 and BRCA2 mutation carriers. Breast Cancer Research and Treatment. 2017;163(3):565–571. doi: 10.1007/s10549-017-4198-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bordeleau L., Panchal S., Goodwin P. Prognosis of BRCA-associated breast cancer: a summary of evidence. Breast Cancer Research and Treatment. 2010;119(1):13–24. doi: 10.1007/s10549-009-0566-z. [DOI] [PubMed] [Google Scholar]
- 13.Stoppa-Lyonnet D., Ansquer Y., Dreyfus H., et al. Familial invasive breast cancers: worse outcome related to BRCA1 mutations. Journal of Clinical Oncology. 2000;18(24):4053–4059. doi: 10.1200/jco.2000.18.24.4053. [DOI] [PubMed] [Google Scholar]
- 14.Brekelmans C. T. M., Seynaeve C., Menke-Pluymers M., et al. Survival and prognostic factors in BRCA1-associated breast cancer. Annals of Oncology. 2006;17(3):391–400. doi: 10.1093/annonc/mdj095. [DOI] [PubMed] [Google Scholar]
- 15.Huzarski T., Byrski T., Gronwald J., et al. Ten-year survival in patients with BRCA1-negative and BRCA1-positive breast cancer. Journal of Clinical Oncology. 2013;31(26):3191–3196. doi: 10.1200/jco.2012.45.3571. [DOI] [PubMed] [Google Scholar]
- 16.Jóhannsson O. T., Ranstam J., Borg A., Olsson H. Survival of BRCA1 breast and ovarian cancer patients: a population-based study from southern Sweden. Journal of Clinical Oncology. 1998;16(2):397–404. doi: 10.1200/jco.1998.16.2.397. [DOI] [PubMed] [Google Scholar]
- 17.Rennert G., Bisland-Naggan S., Barnett-Griness O., et al. Clinical outcomes of breast cancer in carriers of BRCA1 and BRCA2 mutations. New England Journal of Medicine. 2007;357(2):115–123. doi: 10.1056/nejmoa070608. [DOI] [PubMed] [Google Scholar]
- 18.Lee L. J., Alexander B., Schnitt S. J., et al. Clinical outcome of triple negative breast cancer in BRCA1 mutation carriers and noncarriers. Cancer. 2011;117(14):3093–3100. doi: 10.1002/cncr.25911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodwin P. J., Phillips K.-A., West D. W., et al. Breast cancer prognosis in BRCA1 and BRCA2 mutation carriers: an international prospective breast cancer family registry population-based cohort study. Journal of Clinical Oncology. 2012;30(1):19–26. doi: 10.1200/jco.2010.33.0068. [DOI] [PubMed] [Google Scholar]
- 20.Xie Y., Gou Q., Wang Q., et al. The role of BRCA status on prognosis in patients with triple-negative breast cancer. Oncotarget. 2017;8(50):87151–87162. doi: 10.18632/oncotarget.19895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veronesi A., de Giacomi C., Magri M. D., et al. Familial breast cancer: characteristics and outcome of BRCA1-2 positive and negative cases. BMC Cancer. 2005;5(1) doi: 10.1186/1471-2407-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalez-Angulo A. M., Timms K. M., Liu S., et al. Incidence and outcome of BRCA mutations in unselected patients with triple receptor-negative breast cancer. Clinical Cancer Research. 2011;17(5):1082–1089. doi: 10.1158/1078-0432.ccr-10-2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cortesi L., Masini C., Cirilli C., et al. Favorable ten-year overall survival in a Caucasian population with high probability of hereditary breast cancer. BMC Cancer. 2010;10(1) doi: 10.1186/1471-2407-10-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baretta Z., Mocellin S., Goldin E., et al. Effect of BRCA germline mutations on breast cancer prognosis. Medicine. 2016;95(40) doi: 10.1097/md.0000000000004975.e4975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y. A., Jian J.-W., Hung Ch-F., et al. Germline breast cancer susceptibility gene mutations and breast cancer outcomes. BMC Cancer. 2018;18(1):315–327. doi: 10.1186/s12885-018-4229-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hartman A.-R., Kaldate R. R., Sailer L. M., et al. Prevalence of BRCA mutations in an unselected population of triple-negative breast cancer. Cancer. 2012;118(11):2787–2795. doi: 10.1002/cncr.26576. [DOI] [PubMed] [Google Scholar]
- 27.Wong-Brown M. W., Meldrum C. J., Carpenter J. E., et al. Prevalence of BRCA1 and BRCA2 germline mutations in patients with triple-negative breast cancer. Breast Cancer Research and Treatment. 2015;150(1):71–80. doi: 10.1007/s10549-015-3293-7. [DOI] [PubMed] [Google Scholar]
- 28.Bayraktar S., Gutierrez-Barrera A. M., Liu D., et al. Outcome of triple-negative breast cancer in patients with or without deleterious BRCA mutations. Breast Cancer Research and Treatment. 2011;130(1):145–153. doi: 10.1007/s10549-011-1711-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tung N., Gaughan E., Hacker M. R., et al. Outcome of triple negative breast cancer: comparison of sporadic and BRCA1-associated cancers. Breast Cancer Research and Treatment. 2014;146(1):175–182. doi: 10.1007/s10549-014-2995-6. [DOI] [PubMed] [Google Scholar]
- 30.Maksimenko J., Irmejs A., Nakazawa-Miklasevica M., et al. Prognostic role of BRCA1 mutation in patients with triple-negative breast cancer. Oncology Letters. 2014;7(1):278–284. doi: 10.3892/ol.2013.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee E.-H., KOHBRA Research Group, Park S. K., et al. Effect of BRCA1/2 mutation on short-term and long-term breast cancer survival: a systematic review and meta-analysis. Breast Cancer Research and Treatment. 2010;122(1):11–25. doi: 10.1007/s10549-010-0859-2. [DOI] [PubMed] [Google Scholar]
- 32.Zhong Q., Peng H.-L., Zhao X., Zhang L., Hwang W.-T. Effects of BRCA1- and BRCA2-related mutations on ovarian and breast cancer survival: a meta-analysis. Clinical Cancer Research. 2015;21(1):211–220. doi: 10.1158/1078-0432.ccr-14-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Copson E. R., Maishman T. C., Tapper W. J., et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): a prospective cohort study. The Lancet Oncology. 2018;19(2):169–180. doi: 10.1016/s1470-2045(17)30891-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yadav S., Ladkany R., Yadav D., et al. Impact of BRCA mutation status on survival of women with triple-negative breast cancer. Clinical Breast Cancer. 2017;18(5):e1229–e1235. doi: 10.1016/j.clbc.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 35.Robson M., Im S.-A., Senkus E., et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. New England Journal of Medicine. 2017;377(6):523–533. doi: 10.1056/nejmoa1706450. [DOI] [PubMed] [Google Scholar]
- 36.Litton J. K., Rugo H. S., Ettl J., et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. New England Journal of Medicine. 2018;379(8):753–763. doi: 10.1056/nejmoa1802905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmid P., Adams S., Rugo H. S., et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. New England Journal of Medicine. 2018;379(22):2108–2121. doi: 10.1056/nejmoa1809615. [DOI] [PubMed] [Google Scholar]
- 38.Larson K., Wang Y. Y., Finke K., et al. Impact of germline BRCA mutation status on survival in women with metastatic triple negative breast cancer. Proceedings of the 2018 San Antonio Breast Cancer Symposium; December 2018; San Antonio, TX, USA. AACR Cancer Research; [Google Scholar]
- 39.Cardoso F., Kyriakides S., Ohno S., et al. Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Annals of Oncology. 2019;30(8):1194–1220. doi: 10.1093/annonc/mdz173. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon reasonable request.


