Significance
Defective intestinal barrier function and enhanced lipopolysaccharide (LPS)/bacterial translocation is a key pathogenic factor in many human diseases, including obesity. To date, the molecular mechanisms leading to intestinal barrier defects are not well understood, and there are no available therapeutic approaches to target intestinal barrier function. Here we show that soluble epoxide hydrolase (sEH) could be a novel therapeutic target of obesity-induced intestinal barrier dysfunction and LPS/bacterial translocation, and that sEH inhibitors, which have been evaluated in human clinical trials targeting other disorders, could be promising agents for prevention or treatment.
Keywords: obesity, intestinal barrier dysfunction, soluble epoxide hydrolase
Abstract
Intestinal barrier dysfunction, which leads to translocation of bacteria or toxic bacterial products from the gut into bloodstream and results in systemic inflammation, is a key pathogenic factor in many human diseases. However, the molecular mechanisms leading to intestinal barrier defects are not well understood, and there are currently no available therapeutic approaches to target intestinal barrier function. Here we show that soluble epoxide hydrolase (sEH) is an endogenous regulator of obesity-induced intestinal barrier dysfunction. We find that sEH is overexpressed in the colons of obese mice. In addition, pharmacologic inhibition or genetic ablation of sEH abolishes obesity-induced gut leakage, translocation of endotoxin lipopolysaccharide or bacteria, and bacterial invasion-induced adipose inflammation. Furthermore, systematic treatment with sEH-produced lipid metabolites, dihydroxyeicosatrienoic acids, induces bacterial translocation and colonic inflammation in mice. The actions of sEH are mediated by gut bacteria-dependent mechanisms, since inhibition or genetic ablation of sEH fails to attenuate obesity-induced gut leakage and adipose inflammation in mice lacking gut bacteria. Overall, these results support that sEH is a potential therapeutic target for obesity-induced intestinal barrier dysfunction, and that sEH inhibitors, which have been evaluated in human clinical trials targeting other human disorders, could be promising agents for prevention and/or treatment.
Obesity is a serious health problem in the United States and other Western countries; currently, more than 35% of adults and nearly 17% of children in the United States are obese (1, 2). It is well established that obese individuals are at increased risk of developing many diseases, including but not limited to metabolic diseases, diabetes, cardiovascular diseases, and several types of cancers (3). The mechanisms for obesity-associated diseases are not fully understood, and emerging research supports that obesity-induced intestinal barrier dysfunction plays a central role in the pathogenesis of various obesity-induced diseases (4–7). Indeed, animal and human studies have shown that obese subjects have impaired intestinal barrier function, leading to enhanced translocation of bacteria or toxic bacterial products from the gut into the bloodstream and distant organs, resulting in systemic inflammation, insulin resistance, and tissue dysfunction (4–7). Besides obesity, barrier dysfunction is also a key pathogenic factor in many other human diseases, including inflammatory bowel disease, gastric ulcers, cancer, and celiac disease (8). However, the molecular mechanisms leading to intestinal barrier dysfunction are not well understood, and currently there are no available therapeutic approaches for prevention and/or treatment (8, 9).
Using a liquid chromatography-tandem mass spectrometry (LC-MS/MS)-based lipidomics, our recent research supports that soluble epoxide hydrolase (sEH), an enzyme involved in eicosanoid metabolism (10, 11), is a potential therapeutic target for obesity-induced colonic inflammation (12). We found that sEH is up-regulated and that sEH-derived metabolites, such as dihydroxyeicosatrienoic acids (DHETs), are increased in the colon tissues of diet-induced obese mice. Furthermore, inhibition or genetic ablation of sEH attenuates obesity-induced immune cell infiltration and expression of inflammatory cytokines in the colon tissues (12). It is well established that colonic inflammation, particularly that due to proinflammatory cytokines, can disrupt barrier function and result in intestinal permeability (8, 13, 14). Therefore, we hypothesize that sEH could be an endogenous regulator of intestinal barrier function, and that sEH inhibitors, which are currently being evaluated in human clinical trials targeting other disorders (10, 15, 16), could be promising agents for preventing or treating intestinal barrier dysfunction and its resulting pathologies. To this end, here we studied the roles of sEH and sEH inhibitors in obesity-induced barrier dysfunction in mouse models.
Results
Genetic Ablation of sEH Abolishes Obesity-Induced Gut Leakage and Bacterial Translocation.
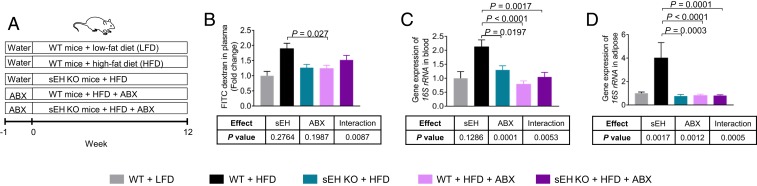
Consistent with our previous study (12), we found that compared with wild-type (WT) C57BL/6 mice treated with a low-fat diet (LFD), WT mice treated with a high-fat diet (HFD) had elevated expression of Ephx2 (encoding sEH) in the colon (SI Appendix, Fig. S1). We then used sEH knockout (KO) mice to determine the roles of sEH in obesity-induced gut leakage. We treated WT mice and sEH KO mice with an LFD or HFD for 3 months (experimental scheme in Fig. 1A) and found that genetic ablation of sEH did not dramatically change HFD-induced body weight increase (SI Appendix, Fig. S2) but did abolish obesity-induced gut leakage and bacterial translocation (Fig. 1 B–D). Indeed, using a fluorescein isothiocyanate (FITC)-dextran–based permeability assay, we found that in WT mice, the HFD treatment increased leakage of orally administered FITC-dextran from the gut into the bloodstream, while this effect was abolished in the sEH KO mice (Fig. 1B). Consistent with the FITC-dextran assay, HFD treatment increased the bacterial load in both the blood and the adipose tissue of the WT mice, while such effects were abolished in the sEH KO mice (Fig. 1 C and D). Together, these results demonstrate that genetic ablation of sEH attenuates obesity-induced gut leakage and bacterial translocation.
Fig. 1.
Genetic ablation of sEH abolishes obesity-induced gut leakage and bacterial translocation. (A) Scheme of animal experiment. (B) FITC-dextran–based permeability assay (n = 3 to 4 mice per group). (C) Quantification of bacterial load in blood (assessed by gene expression of 16S rRNA; n = 6 to 8 mice per group). (D) Quantification of bacterial load in gonadal adipose tissue (n = 5 to 8 mice per group). The data are mean ± SEM.
To explore the roles of gut microbiota in the actions of sEH, we determined the extent to which antibiotic mixture (ABX)-mediated suppression of gut microbiota modulates the actions of sEH (Fig. 1A). We used an ABX used in previous studies (6, 17) and found that treatment with the ABX caused a dramatic reduction of fecal bacteria (SI Appendix, Fig. S3), validating its suppressing effects on the microbiota. In the presence of the microbiota (without the ABX treatment), genetic ablation of sEH abolished obesity-induced gut leakage and bacterial translocation; however, with depleted microbiota (with the ABX treatment), genetic ablation of sEH failed to attenuate these obesity-induced disorders (Fig. 1 B–D). A nested two-way ANOVA demonstrated a significant interaction between sEH (sEH KO mice vs. WT mice) and gut microbiota (water vs. ABX) on gut leakage (P < 0.05 for interaction; Fig. 1 B–D). These results support that the presence of the microbiota is critical for these biological actions of sEH.
Genetic Ablation of sEH Abolishes Obesity-Induced Adipose Inflammation via Gut Bacteria-Dependent Mechanisms.
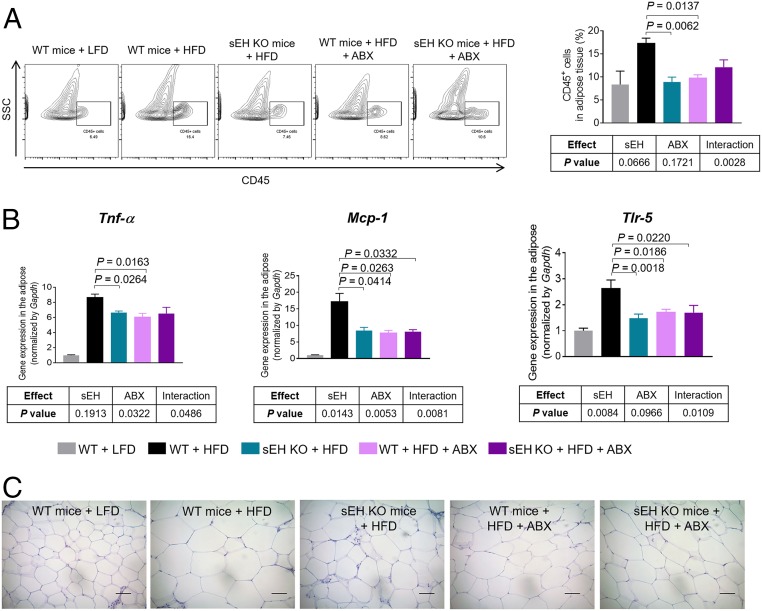
After demonstrating that genetic ablation of sEH abolishes obesity-induced bacterial translocation into the adipose tissue (Fig. 1D), we tested the effects of sEH on adipose inflammation. In the WT mice, treatment with an HFD increased the infiltration of CD45+ immune cells, enhanced the expression of proinflammatory genes (Tnf-α, Mcp-1, and Tlr-5), and enlarged adipocytes in the adipose tissue (Fig. 2 A–C and SI Appendix, Fig. S6), demonstrating enhanced adipose inflammation. Such effects were attenuated in the sEH KO mice (Fig. 2 A–C), demonstrating that genetic ablation of sEH suppresses obesity-induced adipose inflammation.
Fig. 2.
Genetic ablation of sEH abolishes obesity-induced adipose inflammation via gut bacteria-dependent mechanisms. (A) FACS quantification of CD45+ immune cells in gonadal adipose tissue (n = 4 to 5 mice per group). (Left) Representative FACS images. (Right) Quantification of immune cells in gonadal adipose tissue. (B) Expression of proinflammatory genes Tnf-α, Mcp-1, and Tlr-5 in gonadal adipose tissue (n = 5 to 8 mice per group). (C) Representative hematoxylin and eosin (H&E)-stained histological sections of gonadal adipose tissue. The data are mean ± SEM. (Scale bars: 50 μm.)
To validate whether genetic ablation of sEH suppresses obesity-induced adipose inflammation via gut bacteria-dependent mechanisms, we determined the extent to which ABX-mediated suppression of gut bacteria modulates the actions of sEH (Fig. 1A). We found that in the presence of the microbiota (without the ABX treatment), genetic ablation of sEH suppressed obesity-induced adipose inflammation, but with depleted microbiota (via the ABX treatment), genetic ablation of sEH failed to attenuate these obesity-induced disorders (Fig. 2 A–C). Nested two-way ANOVA revealed a significant interaction between sEH (sEH KO mice vs. WT mice) and gut bacteria (water vs. ABX) on adipose inflammation (P < 0.05 for interaction; Fig. 2 A–C). Together, these results demonstrate that the presence of gut bacteria is critical for the actions of sEH on intestinal barrier functions.
Pharmacologic Inhibition of sEH Abolishes Obesity-Induced LPS/Bacterial Translocation.
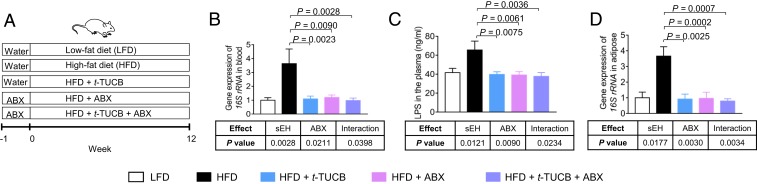
To date, there are no available therapeutic approaches to target intestinal barrier function (8). Given our findings that genetic ablation of sEH abolishes obesity-induced intestinal barrier dysfunction, we studied the effects of trans-4-{4-[3-(4-trifluoromethoxyphenyl) ureido] cyclohexyloxy} benzoic acid (t-TUCB), a potent and selective sEH inhibitor with an IC50 of 0.9 nM for human sEH and an IC50 of 1.3 nM for mouse sEH (18), on intestinal barrier functions. We treated C57BL/6 WT mice with an LFD or HFD, as well as with vehicle or 10 mg/L t-TUCB (scheme of animal experiment in Fig. 3A). Consistent with the results obtained in sEH KO mice, we found that t-TUCB had little effect on HFD-induced body weight change (SI Appendix, Fig. S4), while it abolished HFD-induced LPS/bacterial translocation (Fig. 3 B–D). HFD treatment increased the concentration of LPS in plasma and enhanced the bacterial load in both blood and adipose tissue, while such effects were abolished by treatment with t-TUCB (Fig. 3 B–D). Furthermore, as expected, when combined with ABX treatment to deplete the gut bacteria (Fig. 3A), treatment with t-TUCB had little effect on LPS/bacterial translocation (Fig. 3 B–D). Together, these results suggest that pharmacologic inhibition of sEH suppresses obesity-induced LPS/bacterial translocation.
Fig. 3.
Pharmacologic inhibition of sEH abolishes obesity-induced LPS/bacterial translocation. (A) Scheme of the animal experiment. (B) Quantification of bacterial load in blood. (C) Concentration of LPS in plasma. (D) Quantification of bacterial load in gonadal adipose tissue. The data are mean ± SEM, n = 6 to 10 mice per group.
To explore the cellular mechanisms by which t-TUCB modulates intestinal barrier function, we studied colonic expression of tight junction proteins, which are critical regulators of intestinal permeability (8). qRT-PCR and immunohistochemical staining showed that HFD treatment reduced colonic expression of Claudin-1 (SI Appendix, Fig. S5), an important tight junction protein (8). Such effects were abolished by treatment with t-TUCB in a gut bacteria-dependent manner (SI Appendix, Fig. S5). Together, these results support that t-TUCB modulates obesity-induced intestinal barrier dysfunction, in part through regulating the colonic expression of tight junction proteins.
Pharmacologic Inhibition of sEH Abolishes Obesity-Induced Adipose Inflammation via Gut Bacteria-Dependent Mechanisms.
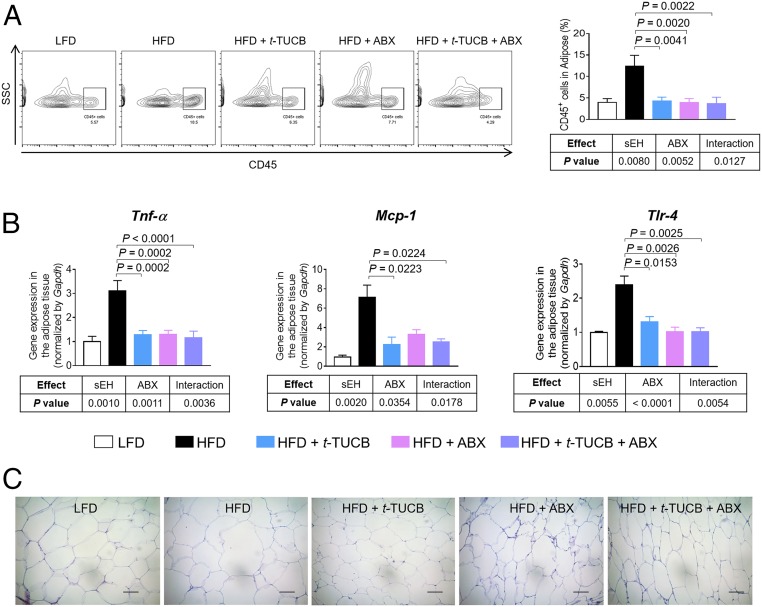
We further studied the effects of t-TUCB on obesity-induced adipose inflammation. Treatment with t-TUCB abolished HFD-induced adipose inflammation, with reduced infiltration of immune cells, attenuated expression of proinflammatory genes, and smaller adipocytes, in the adipose tissue (Fig. 4 A–C). In addition, we found that the effects of t-TUCB on adipose inflammation require the presence of the microbiota; in the presence of the microbiota (without ABX treatment), inhibition of sEH suppressed obesity-induced adipose inflammation, but with depleted microbiota (via ABX treatment), inhibition of sEH failed to attenuate these obesity-induced disorders (Fig. 4 A–C). These results agree with our findings obtained in sEH KO mice (Fig. 2), supporting that pharmacologic inhibition of sEH attenuates obesity-induced adipose inflammation by suppressing translocation of bacterial products from the gut into adipose tissue.
Fig. 4.
Pharmacologic inhibition of sEH abolishes obesity-induced adipose inflammation via gut bacteria-dependent mechanisms. (A) FACS quantification of CD45+ immune cells in gonadal adipose tissue (n = 5 mice per group). (Left) Representative FACS images. (Right) Quantification of immune cells in adipose tissue. (B) Gene expression of Tnf-α, Mcp-1, and Tlr-4 in gonadal adipose tissue (n = 8 to 10 mice per group). (C) Representative H&E histology of gonadal adipose tissue. The data are mean ± SEM. (Scale bars: 50 μm.)
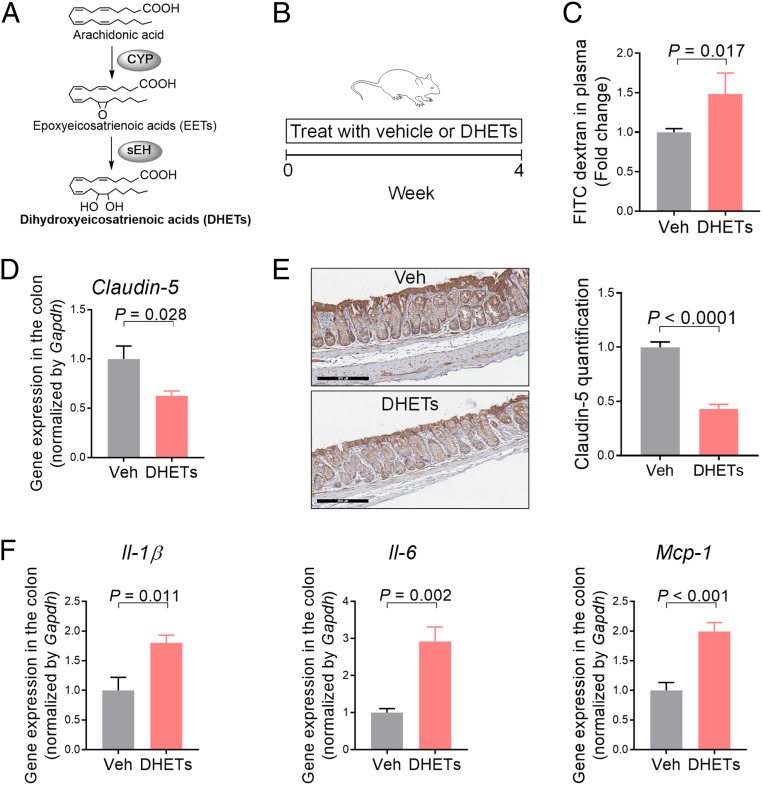
Treatment with sEH-Produced Lipid Metabolites, DHETs, Induces Colonic Inflammation and Gut Leakage in Mice.
To explore the molecular mechanism by which sEH promotes gut leakage, we studied the effects of DHETs, metabolites produced by sEH from arachidonic acid (Fig. 5A), on gut leakage in mice. To this end, we treated mice with 2 mg/kg/d DHETs via a minipump for 4 wk (Fig. 5B). Using an FITC-dextran–based permeability assay, we found that treatment with DHETs increased leakage of the orally administered FITC-dextran from the gut into the bloodstream (Fig. 5C), illustrating enhanced gut leakage. Consistent with the permeability assay, qRT-PCR and immunohistochemistry showed that DHETs reduced the colonic expression of Claudin-5, a tight-junction protein involved in regulating intestinal barrier function (8) (Fig. 5 D and E). Furthermore, we found that DHETs increased the colonic expression of proinflammatory cytokines Il-1β, and Il-6, Mcp-1, important regulators of inflammation and intestinal permeability (8, 13, 14) (Fig. 5F). Together, these results support that sEH-produced DHETs could contribute to the actions of sEH.
Fig. 5.
Treatment with sEH-produced lipid metabolites DHETs induces colonic inflammation and gut leakage in mice. (A) Biochemistry of the CYP-sEH pathway. (B) Scheme of the animal experiment. (C) FITC-dextran–based permeability assay. (D) Gene expression of Claudin-5 in colon. (E) Immunohistochemistry of Claudin-5 in colon. (Scale bars: 200 μm.) (F) Gene expression of proinflammatory cytokines in colon. The data are mean ± SEM, n = 4 to 6 mice per group.
Discussion
Substantial studies have shown that intestinal barrier dysfunction plays a central role in the pathogenesis of various obesity-induced diseases, making this cellular system a promising target for preventing or treating obesity-associated disorders (4–7). However, the mechanism by which obesity leads to intestinal barrier dysfunction is not well understood, and currently there are no therapeutic approaches for prevention or treatment. Besides obesity, barrier dysfunction is also a key pathogenic factor in many other human diseases, including inflammatory bowel disease, gastric ulcers, cancer, and celiac disease (8). Therefore, there is an urgent need to better understand the underlying mechanisms of barrier dysfunction to develop mechanism-based strategies for its prevention and/or treatment. Here our central finding is that sEH, a proinflammatory enzyme involved in fatty acid metabolism (10), is an endogenous regulator of obesity-induced barrier dysfunction (SI Appendix, Fig. S7). We found that inhibition or genetic ablation of sEH abolished obesity-induced gut leakage, LPS/bacterial translocation, and bacterial invasion-induced adipose inflammation, supporting that sEH could be a promising therapeutic target for obesity-induced leaky gut syndrome. This finding could be clinically important, because the sEH inhibitors have been tested in human clinical trials targeting other disorders (16).
The sEH enzyme catalyzes the hydrolysis of fatty acid epoxides, lipid metabolites produced by the actions of cytochrome P450 (CYP) monooxygenases, to generate the corresponding fatty acid diols, such as DHETs (10). Here we have shown that continuous infusion with DHETs via a minipump induced colonic inflammation and intestinal barrier dysfunction in mice, suggesting an important role of DHETs in mediating the actions of sEH on barrier function. This finding is consistent with recent studies showing that sEH-derived fatty acid diols can cause barrier defects and/or inflammation. Hu et al. (19) showed that 19,20-dihydroxydocosapentaenoic acid (19,20-DHDP), an sEH metabolite derived from docosahexaenoic acid (DHA; 22:6ω-3), induces endothelial cell permeability. Levan et al. (20) showed that 12,13-dihydroxyoctadecenoic acid (12,13-DiHOME), an sEH metabolite derived from linoleic acid (18:2ω-6), is increased in the feces of asthma patients and exaggerates lung inflammation in mice. These studies support that the sEH-produced fatty acid diols, once thought to be biologically inactive (10), have potent actions with biological/clinical importance. Many lipid metabolites act via binding to specific G protein coupled receptors (21). The receptors of the sEH-derived fatty acid diols are unknown at present, hindering further studies to elucidate the cellular targets of these lipid metabolites in promoting barrier dysfunction. A better understanding of the mechanism by which the sEH-produced fatty acid diols regulate barrier function could aid the development of novel strategies for preventing or treating barrier dysfunction.
Our results support a model in which the inhibition or genetic ablation of sEH suppresses obesity-induced intestinal barrier dysfunction and attenuates the translocation of LPS/bacteria from the gut into the adipose tissue, resulting in attenuation of adipose inflammation (SI Appendix, Fig. S7). Indeed, the presence of gut bacteria is critical for the actions of sEH, since inhibition or genetic ablation of sEH fails to attenuate obesity-induced adipose inflammation in ABX-treated mice.
There are several possible mechanisms by which sEH regulates intestinal barrier function. We have shown that the inhibition or genetic ablation of sEH abolishes obesity-induced production of proinflammatory cytokines in the colon (12). Many of these cytokines are important regulators of barrier function and inducers of intestinal permeability (8, 13, 14). Therefore, it is feasible that genetic ablation or inhibition of sEH attenuates obesity-induced intestinal barrier dysfunction, in part through suppressing colonic inflammation. In addition, our data support that genetic ablation of sEH could alter obesity-associated gut microbiota; compared with WT mice on an LFD, the WT mice on an HFD had a lower total fecal microbial biomass, and such an effect was attenuated in sEH KO mice (SI Appendix, Fig. S3C), although more detailed studies are needed to characterize the impact of sEH on the microbiota. Previous studies have shown that the microbiota is critical for regulating gut permeability (6); therefore, it is feasible that genetic ablation or inhibition of sEH attenuates obesity-induced intestinal barrier dysfunction, in part through modulating the microbiota.
Most importantly, our results show that oral administration of low dose of an sEH inhibitor via drinking water abolishes obesity-induced gut leakage, LPS/bacterial translocation, and bacterial invasion-induced adipose inflammation. These results support that the sEH inhibitors, which have been evaluated in human clinical trials targeting other disorders (10, 15, 16), could be promising agents for prevention and/or treatment. This is important because there currently are no available therapeutic approaches that target intestinal barrier function (8). GlaxoSmithKline has developed a drug candidate, GSK2256294, a potent, selective, and slowly reversible sEH inhibitor with IC50 values of 27 pM for human sEH and 189 pM for mouse sEH (22). Two Phase I human clinical trials have shown that this compound is well tolerated and causes sustained inhibition of sEH in humans, without observable adverse effects (15). Other sEH inhibitors are also being considered for human trials (16). These resources could greatly help human translations to use sEH inhibitors for preventing or treating obesity-induced barrier dysfunction. Notably, previous studies have shown that obese individuals have higher concentrations of LPS, a well-established biomarker of gut leakage, in the circulation (23, 24). It would be feasible to run a small-scale human clinical trial to test whether treatment with sEH inhibitors reduce the circulating concentration of LPS in obese individuals.
In summary, our results support that sEH is an endogenous regulator of obesity-induced intestinal barrier dysfunction and associated disorders, and that sEH inhibitors could be promising agents for preventing or treating obesity-induced barrier defects. Besides obesity, intestinal barrier dysfunction is also a key pathogenic factor in other human diseases (8), and it would be important to test the roles of sEH in barrier function in other disorders.
Materials and Methods
Details of the experimental protocols are given in SI Appendix, Materials and Methods.
Animal Experiments.
All animal experiments were conducted in accordance with the protocols approved by the Institutional Animal Care and Use Committee of the University of Massachusetts Amherst and the University of California Davis. C57BL/6 male mice were purchased from Charles River Laboratories and maintained in a standard animal facility. The experimental diets, LFD (10 kcal% fat, containing 1.9% wt/wt lard and 2.4 wt/wt % soybean oil; catalog no. D12450J) and HFD (60% kcal% fat, containing 31.7 wt/wt % lard and 3.2 wt/wt % soybean oil; catalog no. D12492), were purchased from Research Diet.
Animal protocol 1: Effect of pharmacologic inhibition of sEH on HFD-induced LPS/bacteria translocation and adipose inflammation.
C57BL/6 male mice were assigned at random to five groups (n = 11 to 12 mice per group) and treated with the LFD or HFD diet, with drinking water containing 2% (vol/vol) polyethylene glycol 400 (PEG 400) as vehicle, and/or 10 mg/L t-TUCB and/or an ABX containing 1 g/L ampicillin, 0.5 g/L neomycin, and 4 g/L Sweet’N Low Zero Calorie Sweetener (Cumberland Packing Corporation). After 12 wk, the mice were euthanized for analysis.
Animal protocol 2: Effect of genetic ablation of sEH on HFD-induced LPS/bacteria translocation and adipose inflammation.
WT and sEH KO mice established on the C57BL/6 background were treated with the LFD or HFD, with drinking water with or without an ABX containing 1 g/L ampicillin, 0.5 g/L neomycin, and 4 g/L Sweet’N Low Zero Calorie Sweetener. After 12 wk, the mice were euthanized for analysis.
Animal protocol 3: Effect of DHETs on induced gut leakage and colon inflammation.
C57BL/6 male mice were s.c. implanted with an osmotic minipump (Durect; model 1004) that delivered vehicle (a 1:1 vol/vol mixed solution of DMSO and PEG 400) or DHETs (2 mg/kg/d). During the experiment, the mice were maintained on standard mouse chow. After 4 wk, the mice were euthanized for analysis.
Data Analysis.
Data are expressed as mean ± SEM. The comparison of vehicle and DHETs (Fig. 5) was performed using either Student’s t test or the Wilcoxon–Mann–Whitney test. Analysis of sEH (WT mice vs. sEH KO mice or vehicle vs. t-TUCB) and antibiotics (water vs. ABX) under the HFD along with a control group (WT mice + LFD) was performed by nested two-way ANOVA (with homogeneous/heterogeneous variance) designed for the factorial plus control experiment, followed by a test between the WT + LFD group and the WT + HFD group and then Tukey’s multiple comparison test for the comparisons among four groups: WT + HFD, sEH KO + HFD, WT + HFD + ABX, and sEH KO + HFD + ABX or HFD, HFD + t-TUCB, HFD + ABX, and HFD + t-TUCB + ABX. The validity of the nested two-way ANOVA model assumptions was assessed by the analysis of residuals and a Q-Q plot. For the analysis of 16S rRNA in both blood and adipose tissue (Figs. 1D and 3 B and D) and gene expression of Clauind-1 in the colon (SI Appendix, Fig. S5), log transformation was applied before analyzing the data to satisfy the assumptions of the nested two-way ANOVA.
Data Availability Statement.
All data discussed in the paper are included in the main text and SI Appendix.
Supplementary Material
Acknowledgments
We thank Dr. Patricia I. Oteiza (University of California Davis) for help with the FITC-dextran permeability assay, Jane (Qian) Chen (University of California Davis Center for Immunology and Infectious Diseases) for assistance with the immunohistochemistry staining, and the staff at the Flow Cytometry Core Facility of the University of Massachusetts Amherst for FACS analysis. This work was supported by US Department of Agriculture (USDA) National Institute of Food and Agriculture Grants 2016-67017-24423 and 2019-67017-29248, National Cancer Institute Grants R03CA218520 and R03CA237795, USDA Hatch Grant MAS00492 (to G.Z.), National Institute of Environmental Health Sciences (NIEHS) Grant R35 ES030443 and NIEHS Superfund Research Program P42 ES004699 (to B.D.H.), and NSF Grant DMS-1761320 and NIEHS Grant R00 ES024806 (to K.S.S.L.).
Footnotes
Competing interest statement: B.D.H. is a coauthor on patents from the University of California on the use of sEH inhibitors to treat inflammatory diseases but not intestinal barrier dysfunction and associated diseases.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1916189117/-/DCSupplemental.
References
- 1.Flegal K. M., Carroll M. D., Kit B. K., Ogden C. L., Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA 307, 491–497 (2012). [DOI] [PubMed] [Google Scholar]
- 2.Ogden C. L., Carroll M. D., Kit B. K., Flegal K. M., Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA 307, 483–490 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bray G. A., Medical consequences of obesity. J. Clin. Endocrinol. Metab. 89, 2583–2589 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Cani P. D., et al. , Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56, 1761–1772 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Neves A. L., Coelho J., Couto L., Leite-Moreira A., Roncon-Albuquerque R. Jr, Metabolic endotoxemia: A molecular link between obesity and cardiovascular risk. J. Mol. Endocrinol. 51, R51–R64 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Cani P. D., et al. , Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57, 1470–1481 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Boutagy N. E., McMillan R. P., Frisard M. I., Hulver M. W., Metabolic endotoxemia with obesity: Is it real and is it relevant? Biochimie 124, 11–20 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bischoff S. C., et al. , Intestinal permeability—a new target for disease prevention and therapy. BMC Gastroenterol. 14, 189 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thaiss C. A., et al. , Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science 359, 1376–1383 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Zhang G., Kodani S., Hammock B. D., Stabilized epoxygenated fatty acids regulate inflammation, pain, angiogenesis and cancer. Prog. Lipid Res. 53, 108–123 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J., Sanidad K. Z., Zhang G., Cytochrome P450 monooxygenase/soluble epoxide hydrolase-mediated eicosanoid pathway in colorectal cancer and obesity-associated colorectal cancer. Oncoscience 6, 371–375 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang W., et al. , Lipidomic profiling reveals soluble epoxide hydrolase as a therapeutic target of obesity-induced colonic inflammation. Proc. Natl. Acad. Sci. U.S.A. 115, 5283–5288 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruewer M., et al. , Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J. Immunol. 171, 6164–6172 (2003). [DOI] [PubMed] [Google Scholar]
- 14.Turner J. R., Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 9, 799–809 (2009). [DOI] [PubMed] [Google Scholar]
- 15.Lazaar A. L., et al. , Pharmacokinetics, pharmacodynamics and adverse event profile of GSK2256294, a novel soluble epoxide hydrolase inhibitor. Br. J. Clin. Pharmacol. 81, 971–979 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McReynolds C., Schmidt W. K., Wagner K., Hammock B. D., Advancing soluble epoxide hydrolase inhibitors through the valley of death into phase 1 clinical trials for treating painful diabetic neuropathy by utilizing university partnerships, collaborations, and NIH support. FASEB J. 30 (suppl. 1), 1272–1276 (2016). [Google Scholar]
- 17.Vijay-Kumar M., et al. , Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science 328, 228–231 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang S. H., Tsai H. J., Liu J. Y., Morisseau C., Hammock B. D., Orally bioavailable potent soluble epoxide hydrolase inhibitors. J. Med. Chem. 50, 3825–3840 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu J., et al. , Inhibition of soluble epoxide hydrolase prevents diabetic retinopathy. Nature 552, 248–252 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levan S. R., et al. , Elevated faecal 12,13-diHOME concentration in neonates at high risk for asthma is produced by gut bacteria and impedes immune tolerance. Nat. Microbiol. 4, 1851–1861 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Funk C. D., Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science 294, 1871–1875 (2001). [DOI] [PubMed] [Google Scholar]
- 22.Podolin P. L., et al. , In vitro and in vivo characterization of a novel soluble epoxide hydrolase inhibitor. Prostaglandins other lipid mediat. 104-105, 25–31 (2013). [DOI] [PubMed] [Google Scholar]
- 23.Trøseid M., et al. , Plasma lipopolysaccharide is closely associated with glycemic control and abdominal obesity: Evidence from bariatric surgery. Diabetes Care 36, 3627–3632 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clemente-Postigo M., et al. , Endotoxin increase after fat overload is related to postprandial hypertriglyceridemia in morbidly obese patients. J. Lipid Res. 53, 973–978 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data discussed in the paper are included in the main text and SI Appendix.