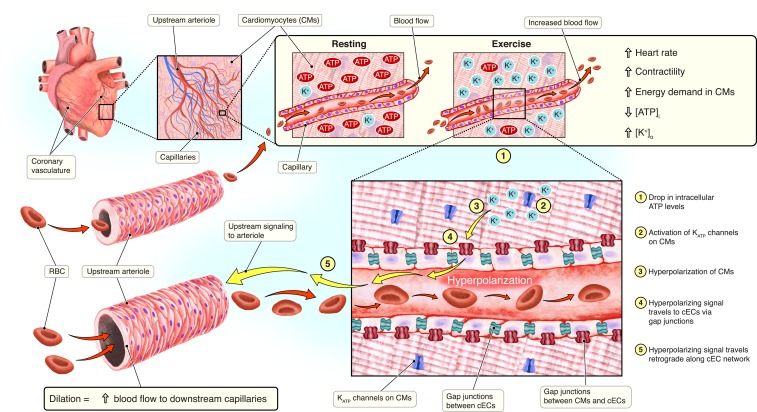
The heart is a metabolically demanding organ with limited energetic reserves to sustain its monumental workload. An efficient blood supply via the coronary vascular network is therefore critical for maintaining efficient cardiac muscle function. The coronary vascular network originates just above the aortic valve, where the right and left main coronary arteries connect to the ascending aorta. These arteries branch out into smaller-diameter arterioles that connect to capillaries, where oxygen exchange takes place (Fig. 1). In PNAS, Zhao et al. (1) describe a signaling pathway in the heart that couples metabolic changes in ventricular myocytes to hyperpolarization of capillary endothelial cells (cECs). This hyperpolarizing signal rapidly propagates throughout the arteriolar tree, causing vasodilation. The resulting upstream vasodilatory response is necessary to increase blood flow during periods of cardiac muscle metabolic stress to match oxygen supply with metabolic demands. The physiological implications of these findings are described below.
Fig. 1.
Metabolic–electrical signaling in the coronary vasculature. During resting conditions, cardiomyocytes (CMs) have high levels of intracellular ATP ([ATP]i) and low levels of extracellular K+ ([K+]o). Upon increased myocardial contraction, levels of [ATP]i decrease and [K+]o increases. Decreased [ATP]I levels trigger the activation of KATP channels expressed in the CM plasma membrane. Efflux of K+ ions induces hyperpolarization of CMs. Hyperpolarizing current passes to electrically coupled capillary endothelial cells (cECs) via gap junctions. The hyperpolarizing signal then travels in a retrograde direction along the cEC network to an upstream arteriole. This hyperpolarizing signal induces relaxation of the smooth muscle cells that encapsulate the coronary vasculature.
A central tenet in cardiac physiology is that oxygen extraction efficiency is almost at saturation in the coronary circulation (2). Increasing oxygen demand is rapidly matched by increased blood flow via changes in perfusion pressure or vascular resistance. The diameter of coronary arteries, which is controlled by the contractile state of the smooth muscle cells lining walls of these vessels, determines vascular resistance. Coronary smooth muscle cells intrinsically respond to increases in intravascular pressure by contracting. This myogenic response is critical for autoregulation of blood flow (3).
In addition to intrinsic control of vessel diameter, coronary artery tone, and hence vascular resistance, is influenced by multiple extrinsic factors. These include compressive forces from the contracting myocardium, perfusion pressure, neurohormonal factors (including sympathetic nerve activation), endothelial, and metabolic factors such as adenosine, reactive oxygen species, H+, and high extracellular K+ (4) acting directly on vascular smooth muscle cells.
The mechanisms underlying local metabolic regulation of coronary vascular resistance have been extensively investigated (for a review, see ref. 3). This work indicates that in the coronary vasculature, both arterial myocytes and endothelial cells that line vessels act as sensors of metabolism. These sensing pathways are particularly critical during bouts of stress and exercise when cardiomyocyte oxygen demand increases. In a number of proposed mechanisms linking metabolism to changes in luminal diameter and coronary blood flow, the partial pressure of oxygen (pO2) in capillaries is the initiating signal. During increased oxygen consumption, pO2 in capillaries falls, essentially generating a local, hypoxic environment.
Previous studies have shed light on the role of adenine nucleotides in regulating coronary blood flow. The “adenine-nucleotide hypothesis” postulates that ATP is liberated from red blood cells when in a low pO2 environment (5). ATP is hydrolyzed within the capillary lumen by nucleotidases and converted to ADP, AMP, and adenosine. These adenine nucleotides activate purinergic (P2Y1) receptors on cECs to induce a retrograde vasodilatory response (5). An alternate, but complementary hypothesis, termed the “adenosine hypothesis of coronary blood flow control,” proposes that adenosine is released from cardiomyocytes during hypoxia (6, 7). In this model, adenosine is released from cardiomyocytes and directly stimulates receptors on coronary smooth muscle cells to initiate a retrograde vasodilatory cascade (6, 7). However, recent studies have suggested that adenosine concentrations are not augmented during increased myocardial O2 consumption (8).
In their study, Zhao et al. (1) provide evidence for an additional adenine nucleotide-mediated pathway to regulate coronary blood flow (Fig. 1). The authors suggest that KATP channels, expressed on cardiomyocytes, represent the metabolic sensor. KATP channels are activated by a decrease in intracellular ATP ([ATP]i) concentrations (9). Therefore, any reduction in [ATP]i will result in an efflux of K+ from cardiomyocytes and subsequent hyperpolarization. Since [ATP]i levels drop during increased metabolic activity in cardiomyocytes, KATP channels provide the necessary link between metabolism and the production of an electrical signal. To arrive at their conclusions, the authors used a multidisciplinary approach to dissect the role of cardiomyocytes and cECs in this sensory-signaling cascade.
First, Zhao et al. (1) utilized an elegant ex vivo ventricular tissue preparation to determine the consequences of KATP channel activation on arterial diameter. Coronary arteries were cannulated and pressurized within the tissue and the vasculature labeled for diameter analysis. During KATP channel activation, arterial and capillary diameters increased, suggesting that KATP activation leads to a vasodilatory response. Evidence from the wider literature has demonstrated expression of KATP channels in arterial myocytes and cECs (9). To address confounding influence from these cell types, Zhao et al. (1) confirmed that responses to KATP channel activation were still present in arterial myocyte-specific dominant-negative KATP tissue preparations. These data suggest that during heightened metabolic demand, decreasing cardiomyocyte [ATP]i triggers activation of KATP channels.
Next, Zhao et al. (1) conducted experiments to determine whether the KATP-induced hyperpolarizing signal could travel from cardiomyocytes to neighboring capillaries. The authors cocultured isolated ventricular myocytes and cECs. Whole-cell patch-clamp recordings and fluorescent dye tracing studies established that ventricular myocyte and cEC pairs were electrically connected to each other. This electrical coupling permitted the flow of hyperpolarizing current from ventricular myocytes to cECs. The authors then determined the consequences of activating KATP channels in ventricular myocytes and the effect on coupled cECs. Cell pairs that were electrically connected generated a hyperpolarizing current in cECs. Contrastingly, when cells were physically disconnected, this current was abolished. Furthermore, disconnected, isolated cECs displayed no hyperpolarizing current in the presence of KATP channel openers.
Together, these studies suggest that cardiomyocytes are capable of signaling their metabolic state to electrically coupled cECs. The electrical signaling unit formed by cardiomyocytes and coupled cECs permits a rapid, extremely local response to changes in cardiomyocyte [ATP]i concentrations. This signaling unit could trigger a long-range vasodilatory response to increase blood flow to metabolically demanding cardiomyocytes. In the context of the wider field, this retrograde vasodilatory response would be faster compared to hydrolysis of ATP and activation of purinergic G-protein–coupled receptors. Indeed, it is likely that multiple, parallel mechanisms exist in the heart to ensure metabolic requirements are met with an upstream dilatory response. A fast and a slow hyperpolarizing signal could be required to sustain dilation in upstream arterioles. The importance of having many mechanisms in place to sense and transmit metabolic information, particularly during cardiovascular stress, may be critical to prevent myocardial ischemia.
In addition, Zhao et al. (1) discovered that an inwardly rectifying K+ channel, Kir2.1, is also involved in metabolic sensing. Kir2.1 channel conductance is augmented by elevated extracellular K+ concentrations ([K+]o). Increased [K+]o is already an established mediator of metabolic signaling in the myocardium (4, 10) and in many other organs and tissues. In the brain, Kir2.1 channels have been implicated in facilitating neurovascular coupling (11, 12). During the neuronal repolarization phase, K+ ions are released into the interstitium and act on Kir2.1 channels. Therefore, changes in [K+]o reflect neuronal activity. In respect to the heart, during increased cardiomyocyte contractility, the myocardium effluxes high concentrations of [K+]o (10). Zhao et al. (1) studied the effects of high [K+]o on cECs. They found that upon elevation of [K+]o, cECs elicited a transient membrane hyperpolarization mediated by Kir2.1. The authors speculate that elevations of [K+]o are a direct result of K+ efflux from KATP channels and other K+ channels expressed on the cardiomyocyte plasma membrane. This would provide the necessary spatial elevations in [K+]o concentrations to activate Kir2.1. Determining how sustained or transient the Kir2.1-mediated response is, is important to elucidate. Evidence from other coronary vascular studies suggests the vasodilator response to high [K+]o is transient and higher concentrations elicit vasoconstriction (4). Therefore, it is unlikely that Kir2.1 activation is generating a sustained, steady-state dilatory responses. Instead, Kir2.1-mediated hyperpolarization could be important when other metabolic sensing mechanisms fail, therefore providing a compensatory pathway to prevent local ischemia during cardiomyocyte stress.
The experiments carried out by Zhao et al. (1) demonstrate two distinct, but complementary metabolic sensing pathways that initiate upstream arteriole vasodilation. Activation of KATP channels, as a result of diminished cardiomyocyte [ATP]i levels, provides a fast electrical signal to changes in metabolism (Fig. 1). This signaling cascade has single-cardiomyocyte–level precision. In light of their important findings, many questions need to be addressed. Future investigation should focus its attention on whether this metabolic sensing pathway is a global phenomenon across all coronary vessels in the heart. For example, do these mechanisms also exist in the pacemaker and atrial regions of the heart? Further study should also seek out imaging approaches to visualize the spreading hyperpolarization across the vascular tree. This may provide details on the kinetics and strength of the vasodilatory response and the threshold required to sustain it. It will also be fruitful to design and conduct studies to measure [ATP]i concentrations in cardiomyocytes, while simultaneously observing electrical responses in neighboring cECs. To further dissect the role of KATP channels in coronary blood flow regulation, transgenic approaches must be used to specifically manipulate KATP channel activity. Similarly, specific genetic ablation of gap junctions between cardiomyocytes and cECs will illuminate the necessity of these electrical conduits for transmitting the hyperpolarizing signal.
Acknowledgments
We thank Mr. Joshua Tulman for help with the illustration. This work was supported by NIH Grants 1R01HL144071 and 1OT2OD026580.
Footnotes
The authors declare no competing interest.
See companion article, “ATP- and voltage-dependent electro-metabolic signaling regulates blood flow in heart,” 10.1073/pnas.1922095117.
References
- 1.Zhao G., Joca H. C., Nelson M. T., Lederer W. J., ATP- and voltage-dependent electro-metabolic signaling regulates blood flow in heart. Proc. Natl. Acad. Sci. U.S.A. 117, 7461–7470 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolff C. B., Normal cardiac output, oxygen delivery and oxygen extraction. Adv. Exp. Med. Biol. 599, 169–182 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Bayliss W. M., On the local reactions of the arterial wall to changes of internal pressure. J. Physiol. 28, 220–231 (1902). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodwill A. G., Dick G. M., Kiel A. M., Tune J. D., Regulation of coronary blood flow. Compr. Physiol. 7, 321–382 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorman M. W., et al. , Adenine nucleotide control of coronary blood flow during exercise. Am. J. Physiol. Heart Circ. Physiol. 299, H1981–H1989 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feigl E. O., Berne’s adenosine hypothesis of coronary blood flow control. Am. J. Physiol. Heart Circ. Physiol. 287, H1891–H1894 (2004). [DOI] [PubMed] [Google Scholar]
- 7.Berne R. M., Regulation of coronary blood flow. Physiol. Rev. 44, 1–29 (1964). [DOI] [PubMed] [Google Scholar]
- 8.Tune J. D., Richmond K. N., Gorman M. W., Olsson R. A., Feigl E. O., Adenosine is not responsible for local metabolic control of coronary blood flow in dogs during exercise. Am. J. Physiol. Heart Circ. Physiol. 278, H74–H84 (2000). [DOI] [PubMed] [Google Scholar]
- 9.Foster M. N., Coetzee W. A., KATP channels in the cardiovascular system. Physiol. Rev. 96, 177–252 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katz L. N., Lindner E., The action of excess Na, Ca and K on the coronary vessels. Am. J. Physiol. Legacy Content 124, 155–160 (1938). [Google Scholar]
- 11.Longden T. A., et al. , Capillary K+-sensing initiates retrograde hyperpolarization to increase local cerebral blood flow. Nat. Neurosci. 20, 717–726 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Longden T. A., Nelson M. T., Vascular inward rectifier K+ channels as external K+ sensors in the control of cerebral blood flow. Microcirculation 22, 183–196 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]