Significance
Humans are unusually long-lived; thus, there must have been important changes to the aging process during our evolutionary history. Recent lifestyle shifts in the developed world have also affected aging. Chimpanzees are valuable comparative models for aging given that they share a close evolutionary relationship with humans and are among the longest-lived nonhuman mammals. In this study, comprising over 20 y of longitudinal data from a population of wild chimpanzees, we report that chimpanzees and humans exhibit common signatures of aging in a key system that regulates physiological responses to stressors. This suggests that aging of the stress response is neither a side effect of extended life span in the human species nor a by-product of recent shifts in lifestyle.
Keywords: glucocorticoids, primates, senescence, circadian rhythms, stress
Abstract
Cortisol, a key product of the stress response, has critical influences on degenerative aging in humans. In turn, cortisol production is affected by senescence of the hypothalamic–pituitary–adrenal (HPA) axis, leading to progressive dysregulation and increased cortisol exposure. These processes have been studied extensively in industrialized settings, but few comparative data are available from humans and closely related species living in natural environments, where stressors are very different. Here, we examine age-related changes in urinary cortisol in a 20-y longitudinal study of wild chimpanzees (n = 59 adults) in the Kanyawara community of Kibale National Park, Uganda. We tested for three key features of HPA aging identified in many human studies: increased average levels, a blunted diurnal rhythm, and enhanced response to stressors. Using linear mixed models, we found that aging was associated with a blunting of the diurnal rhythm and a significant linear increase in cortisol, even after controlling for changes in dominance rank. These effects did not differ by sex. Aging did not increase sensitivity to energetic stress or social status. Female chimpanzees experienced their highest levels of cortisol during cycling (versus lactation), and this effect increased with age. Male chimpanzees experienced their highest levels when exposed to sexually attractive females, but this effect was diminished by age. Our results indicate that chimpanzees share some key features of HPA aging with humans. These findings suggest that impairments of HPA regulation are intrinsic to the aging process in hominids and are side effects neither of extended human life span nor of atypical environments.
Glucocorticoids are produced by the adrenal cortex in response to a variety of internal and external stressors and have far-reaching physiological consequences. While these actions are adaptive in coordinating short-term responses to threats, chronic activation of the stress response, specifically the action of glucocorticoids, can have deleterious long-term effects on multiple systems (1). Thus, glucocorticoids are intimately involved in the degenerative processes of aging (2).
Glucocorticoids have been implicated in multiple pathways that lead to adverse health outcomes with age, including neurodegeneration, muscle atrophy, immunosuppression, cardiovascular disease, and bone loss (2–6). The damaging systemic effects of glucocorticoids during aging are exacerbated if glucocorticoid production itself increases with age. Aging in humans and animal models is frequently associated with dysfunction in regulation of the hypothalamic–pituitary–adrenal (HPA) axis, resulting in delayed termination of the stress response and overproduction of glucocorticoids (2, 7, 8). Aging is also commonly associated with blunting of the diurnal rhythm of glucocorticoid production, which typically peaks shortly before waking and declines throughout the day (9–11). Glucocorticoids may also increase with age because somatic senescence creates more stressors for the HPA axis to respond to. For example, the cumulative burden of repeated responses to pathogens contributes to senescence of the immune system, a phenomenon that not only increases vulnerability to infectious disease but leads to increasing levels of background inflammation (12). Glucocorticoids become chronically elevated as an anti-inflammatory response (12, 13). Thus, glucocorticoids play a central role in the vicious cycle between stressors, senescence, and disease.
Human studies have yielded inconsistent findings about the association of age and glucocorticoid production. For example, some studies report increases in baseline or average levels of cortisol production but no differences in response to challenge (14, 15). Other studies report that aging is associated with increased responsiveness and/or impaired feedback regulation but not necessarily with increases in baseline cortisol production (16–19). Still others find no evidence of age-related changes in HPA function (20). One reasonable conclusion is that altered HPA function is not an inevitable consequence of aging but may be contingent on a panoply of genetic and lifestyle risk factors (13). Recent studies indicate that maintaining high physical fitness can attenuate the effects of aging on inflammation and HPA dysregulation (21, 22), as can maintenance of strong social networks and positive affect (23).
As with most aspects of human senescence, aging of the HPA axis has been studied primarily within clinical samples in the developed world, in environments that are epidemiologically, nutritionally, and socially different from those in which human physiology and our aging process evolved. Thus, broader comparative data are necessary to better understand whether adrenal pathologies are intrinsically linked to the aging of the human organism or are influenced by novel environments. For example, studies of small-scale subsistence populations have recently called into question the conventional understanding of the links between aging, inflammation, and disease (24–27). While individuals in these settings maintain high levels of physical activity, which reduces the harmful effects of inflammation, they also experience greater ecological stress, including high rates of infectious disease and unstable nutrition. Several studies of small-scale societies fail to find increases in basal cortisol with age (28–31), though age-related blunting of the cortisol diurnal rhythm is consistent across populations (9, 29, 30).
Evidence on aging of the HPA axis in primates is scant and inconsistent (32–41). The glaring problem in interpreting findings across human and nonhuman studies is that most studies are small and cross-sectional, often representing each subject with few hormone measures. Consequently, most studies fail to distinguish between the effects of biological aging and those that may stem from variation in stress experience across the life course. In wild primate populations and small-scale human societies, old individuals are often underrepresented, and there is a strong potential for heterogeneity in mortality risk to yield a biased sample of healthy elders (42). Robust longitudinal studies involving repetitive sampling are rare (39) but necessary to detect how aging influences physiology within individuals.
In the current study, we used 20 y of longitudinal data to examine age-related changes in glucocorticoids (urinary cortisol) in wild chimpanzees in the Kanyawara community in Kibale National Park, Uganda. The Kanyawara chimpanzees are not provisioned, and medical interventions are rare (e.g., removal of poachers’ snares); thus, these chimpanzees experience the full spectrum of ecologically relevant stressors, including seasonal climate fluctuations, infectious disease, food scarcity, and interactions with conspecifics. Given their close evolutionary relationship to humans, we tested the hypothesis that adult chimpanzees exhibit senescence of the HPA axis, with age-associated characteristics similar to what has been observed in human clinical studies. Specifically, we predicted that older chimpanzees would exhibit 1) an altered circadian rhythm for cortisol excretion, characterized by a shallower rate of decline across the day; 2) higher cortisol levels, even after controlling for changes in age-varying sociodemographic characteristics; and 3) higher sensitivity to stressors.
To address predictions 2 and 3, we determined whether age changes in cortisol production were independent of and/or interacted with measures of dietary quality, dominance rank, and reproductive effort, which are commonly reported stressors in wild primates (43). Because chimpanzee distribution and behavior are constrained by the availability of ripe fruit (44, 45), we predicted that chimpanzees would have increased cortisol during periods of low dietary quality, defined by low ripe fruit consumption (46). We predicted that older chimpanzees would be particularly affected because these individuals are likely to experience physical constraints on competitive success and mobility. Dominance rank is often associated with variation in glucocorticoid levels because it determines access to resources and exposure to stressors, such as aggression (47). While low social status is typically associated with increased stress and poor health outcomes in humans, studies of other social mammals often find the reverse (47, 48). Based on prior short-term studies of Kanyawara chimpanzees, we predicted that high rank would be associated with elevated cortisol in males (46) and lower cortisol in females (49) and that these effects would be magnified with age. Finally, we predicted that chimpanzees of both sexes would exhibit higher cortisol during periods of higher reproductive effort and that this effect would increase as chimpanzees aged. We predicted that as male chimpanzees aged, they would experience increasing stress in response to mating competition, while aging females would experience increasing stress in response to the high energetic demands of lactation.
Methods
Observations and urine sampling of wild chimpanzees were conducted from late 1997 to early 2017 using noninvasive methods. Over this time, the Kanyawara study community comprised 49 to 54 chimpanzees, including 9 to 11 adult males and 13 to 18 adult females. All field research was conducted with the approval of the Institutional Animal Care and Use Committees of Harvard University and the University of New Mexico.
Field staff of the Kibale Chimpanzee Project conducted daily, full-day chimpanzee follows as often as possible, often targeting two chimpanzee subgroups in different locations. The current study comprised 6,538 unique study days and 75,212 h of observation. Briefly, protocols of relevance to the current study comprised 1) daily records of demographic events, health, and female reproductive status; 2) 15 min scan sampling for group-level data, including individuals present and feeding behavior; 3) detailed all-occurrence sampling of social behavior, including all instances of aggression and formal dominance signals used to calculate dominance ranks; and 4) opportunistic collection of urine samples. See SI Appendix for expanded methods for observational measures (dietary quality, dominance rank, and reproductive effort) and for further details on sampling and assay procedures.
We examined immunoreactive cortisol using previously validated enzyme–immunoassay procedures (SI Appendix and refs. 49 and 50–52). This analysis considers only adult individuals, defined as those physically, socially, and sexually mature. This comprises males of at least 15 y of age and females from the time that they first exhibited maximally tumescent sexual swellings, an indication that ovarian cycling has begun (53, 54). Samples from pregnant females were excluded because glucocorticoid levels increase markedly over the course of pregnancy and may be influenced by interactions between maternal and fetal HPA axes (55). Our dataset comprised 7,888 samples from 39 adult females (mean = 217 samples/female, range = 1 to 782) and 8,029 samples from 20 adult males (mean = 401 samples/male, range: 17 to 1,395).
Our analysis comprised four linear mixed models (LMMs); see SI Appendix for complete details. The first model examined effects of age and sex on the diurnal rhythm of cortisol excretion. In subsequent analyses, we modeled cortisol values that were corrected for time of excretion. The second model used the combined sample from males and females as an initial test of the prediction that time-corrected cortisol production increases with age, and we tested for an interaction between age and sex. A third pair of models was conducted for males and females separately and tested the prediction that effects of age persist after controlling for social and energetic variables (dietary quality, dominance rank, and reproductive effort) that had not been included in the combined sex model. Next, in order to test the prediction that aging increases responsiveness to stressors, we used log-likelihood ratio tests (LLRTs) to evaluate, one at a time, whether interactions between age and either dietary quality, dominance rank, or reproductive effort improved model fits. While most samples (60%) were analyzed within 3 y of collection, all models included a control for the interval between collection and analysis. While inclusion of this term improves model fits, it does not alter outcomes related to other predictors. All models also incorporate random effects to control for variable sampling across individuals and over time (SI Appendix).
Data Availability.
Datasets are available in Dryad (56).
Results
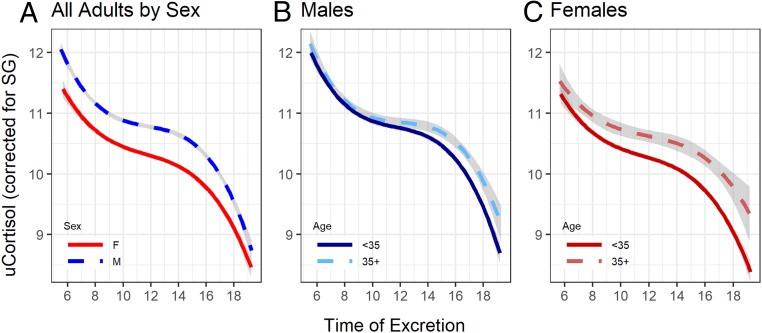
Urinary cortisol excretion declined across the day, closely approximating a third-order polynomial regression (SI Appendix, Fig. S1). Thus, to test the prediction that age influenced the diurnal rhythm, we modeled time as a cubic effect and examined the interaction between age and time of excretion (SI Appendix, Table S1). As predicted, older chimpanzees exhibited a significantly shallower rate of decline driven by higher levels at the end of the day (Fig. 1). Males exhibited higher cortisol concentrations than females across the day. None of the interactions involving chimpanzee sex were significant (P > 0.1).
Fig. 1.
Diurnal pattern of glucocorticoid excretion in wild adult chimpanzees (n = 15,917 samples, 20 individuals). (A) Males excrete higher levels of glucocorticoids (blue dashed line), but the diurnal pattern is not significantly different from that of females (red solid line); in both males (B, blue) and females (C, red), older individuals (35+ years, dashed lines) exhibit a significant blunting of diurnal rhythm compared to younger adults (solid lines). Urinary cortisol (uCortisol) levels are corrected for specific gravity (SG) and log transformed. While discrete age categories are shown, all statistical models used a continuous specification of age. Shaded bands indicate 95% CIs.
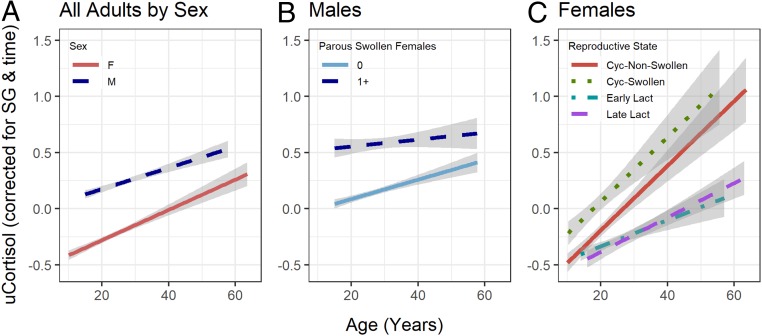
Before constructing multivariate models for each sex separately, we undertook a preliminary LMM on the combined sample to determine whether there was a net effect of age on time-corrected cortisol. Indeed, cortisol increased significantly with age (estimate = 0.025, SE = 0.005, X2 = 26.6, P < 0.00001). While males had higher average cortisol than females (estimate = 0.294, SE = 0.084, X2 = 12.3, P = 0.0005), the slope of the age relationship did not differ by sex (female = 0.029 vs. male = 0.021, LLRT: X2 = 0.5, P = 0.472, Fig. 2A).
Fig. 2.
Urinary cortisol (uCortisol) variation by age, sex, and reproductive effort. uCortisol concentrations are the residual from time of day, after correction for urine specific gravity and log transformation. (A) While each sex produces more cortisol as they age and males (blue dashed line, n = 8,029 samples, 20 individuals) produce significantly higher levels than females (red solid line, n = 7,888, 39 individuals), the age effect was statistically similar between males and females. (B) Males produce more cortisol on days when they are in association with at least one parous, swollen female (dashed line) than on days when they are not (solid line), but the effect of age is reduced when parous, swollen females are present. (C) Females exhibit higher overall cortisol production and a steeper increase with age when they are cycling (green dotted line: cycling with maximal swelling; red solid line: cycling without maximal swelling) than when in early lactation (aqua dot-dashed line: first 2 y after parturition) or late lactational amenorrhea (purple dashed line). Shaded bands indicate 95% CIs.
We predicted that if aging was an intrinsic influence on cortisol production, age would remain a positive and significant predictor of cortisol after controlling for sociodemographic and ecological predictors: dietary quality, dominance rank, and reproductive effort. This prediction was supported in multivariate models for both males and females, where the effect of age remained positive and significant (Tables 1 and 2). Dietary quality, defined as the proportion of ripe fruit in the chimpanzees’ diet in the previous 14 d, did not influence cortisol concentrations of either sex. Dominance rank influenced the cortisol concentrations of males but not females. Males who were higher ranking for their age produced higher cortisol. Reproductive effort affected cortisol concentrations in both sexes. For males, cortisol concentrations were significantly increased on days of high reproductive effort, defined as those in which a maximally swollen, parous female was present in a party with the sampled male. For females, cortisol varied significantly by reproductive state. Pairwise tests indicated that females had higher cortisol concentrations on days when they were cycling with a maximal swelling, compared to when they were cycling and nonswollen (Tukey’s pairwise test, z = 5.977, P < 0.001), in early lactation (z = 7.619, P < 0.001), or in late lactational amenorrhea (z = 6.786, P < 0.001).
Table 1.
Linear mixed model for time-corrected cortisol for adult male chimpanzees
| Model predictors | Estimate | Variance | SE | SD | X2 | P |
| Fixed predictors | ||||||
| (Intercept) | 0.288 | 0.088 | 10.7 | 0.001 | ||
| Age | 0.010 | 0.004 | 8.1 | 0.004 | ||
| Dominance rank | 0.004 | 0.001 | 8.1 | 0.004 | ||
| Diet quality | <0.001 | 0.001 | 0.0 | 0.951 | ||
| Reproductive effort* | 0.174 | 0.023 | 34.7 | <0.0001 | ||
| Years to assay | 73.7 | <0.0001 | ||||
| 4–6 y | −0.424 | 0.063 | ||||
| 7–9 y | −0.540 | 0.081 | ||||
| Random effects | ||||||
| ChimpID | 0.018 | 0.134 | 14.1 | 0.0002 | ||
| Age*ChimpID | <0.001 | 0.007 | 2.6 | 0.108 | ||
| Rank*ChimpID | <0.001 | 0.004 | 4.3 | 0.038 | ||
| Year of Assay | 0.026 | 0.160 | 10.3 | 0.001 | ||
| Month-Year | 0.222 | 0.472 | 1597.9 | <0.00001 | ||
| Interaction effects | ||||||
| Age*Dietary Quality | <0.001 | <0.001 | 2.6 | 0.109 | ||
| Age*Dominance Rank | <0.001 | <0.001 | 2.1 | 0.151 | ||
| Age*Reproductive Effort | −0.006 | 0.002 | 6.1 | 0.014 |
n = 8,029 urine samples, 20 individuals. Significance of fixed effects was determined via type III Wald chi-square tests. Significance of random effects and interactions was determined via LLRTs. Interactions were tested one at a time against the base model.
For males, reproductive effort was defined as the presence or absence of parous swollen females.
Table 2.
Linear mixed model for time-corrected cortisol for adult female chimpanzees
| Model predictors | Estimate | Variance | SE | SD | X2 | P |
| Fixed predictors | ||||||
| (Intercept) | 5.768 | 0.087 | 4433.6 | <0.00001 | ||
| Age | 0.027 | 0.007 | 15.7 | 0.00007 | ||
| Dominance rank | 0.007 | 0.002 | 0.9 | 0.332 | ||
| Diet quality | 0.001 | 0.001 | 1.3 | 0.260 | ||
| Reproductive effort* | 62.2 | <0.00001 | ||||
| Cycling, swollen | 0.251 | 0.042 | ||||
| Early lactation | −0.074 | 0.034 | ||||
| Late lactation | −0.084 | 0.042 | ||||
| Years to assay | 76.6 | <0.00001 | ||||
| −4–6 y | −0.353 | 0.050 | ||||
| −7–9 y | −0.577 | 0.079 | ||||
| Random effects | ||||||
| ChimpID | 0.047 | 0.217 | 42.2 | <0.00001 | ||
| Age*ChimpID | 0.001 | 0.025 | 14.9 | 0.0001 | ||
| Rank*ChimpID | <0.001 | 0.006 | 20.6 | <0.00001 | ||
| Year of Assay | 0.016 | 0.127 | 77.6 | <0.00001 | ||
| Month-Year | 0.271 | 0.521 | 1361.8 | <0.00001 | ||
| Interaction effects | ||||||
| Age*Dietary Quality | <0.001 | <0.001 | 0.2 | 0.624 | ||
| Age*Dominance Rank | <0.001 | <0.001 | 1.1 | 0.304 | ||
| Age*Reproductive Effort | 17.1 | 0.0007 | ||||
| Cycling, swollen | −0.003 | 0.005 | ||||
| Early lactation | −0.017 | 0.004 | ||||
| Late lactation | −0.011 | 0.004 |
n = 7,888 urine samples, 39 individuals. Significance of fixed effects was determined via type III Wald chi-square tests. Significance of random effects and interactions was determined via LLRTs. Interactions were tested one at a time against the base model.
For females, reproductive effort was defined as reproductive state, with cycling-nonswollen females as the reference category.
To test the prediction that older chimpanzees exhibit a greater glucocorticoid response to stressors, we tested for interactions between age and the other model predictors. We found no significant interactions between age and either dietary quality or dominance rank (Tables 1 and 2). Interactions between age and reproductive effort were observed in both sexes. Contrary to our prediction, the effects of reproductive effort in males decreased rather than increased with age (Fig. 2B). Across all ages, males exhibited elevated cortisol on days of high reproductive effort, but this represented a smaller absolute and relative change for older males whose cortisol levels were already high. Among females, the effects of age were steeper among cycling females and shallower among lactating females (Fig. 2C). Significant pairwise differences in slope were detected early lactation and either cycling condition (vs. nonswollen: z = 4.0, P = 0.0004; vs. swollen: z = 2.7, P = 0.033).
Discussion
We examined a large, longitudinal dataset on urinary cortisol in wild chimpanzees, finding that glucocorticoid production increased reliably with age. While aging may be associated with shifts in the experience of stressors, several lines of evidence suggest that our findings were due principally to intrinsic aging of the HPA axis. First, age-related increases in glucocorticoids were accompanied by a blunting of the diurnal rhythm, a feature that characterizes impaired regulation of the HPA axis due to aging or other factors in humans (9–11). Second, the increase in glucocorticoids with age could not be explained by age-related changes in social status or reproductive effort, nor could any longitudinal changes in diet quality explain the increase. Third, while males and females experience different rank trajectories across the life course and differ markedly in the intensity of status competition (57, 58), the sexes exhibited statistically indistinguishable age changes in glucocorticoid production and in diurnal rhythms. As has been observed in humans, changes in HPA function occurred linearly across adulthood, rather than just at extreme ages. This suggests that changes in HPA function are a normative feature of senescence in wild chimpanzees and not a specific indicator of age-related pathology.
Though studies are inconsistent, human subjects often exhibit increases in the glucocorticoid response to stressors, characterized by an increased magnitude or duration of response or both (18, 59). Our study found limited support for this aspect of aging in chimpanzees. Neither dominance rank nor dietary quality interacted significantly with age to affect cortisol levels. While exposure to sexually receptive females was a potent stressor for males of all ages, the effect on cortisol was diminished among older males whose cortisol levels were already high. In females, the effect of reproductive state on cortisol production was moderated by age. While we had expected that older females would produce an enhanced cortisol response to the energetic challenges of lactation, it was cycling females who exhibited both higher cortisol levels overall and a greater change with age. Steroid hormones interact with the HPA axis (60); thus, it is plausible that these effects could have been influenced by higher estrogen levels among cycling versus lactating females. Results from other primates suggest this explanation is insufficient, as cycling females are typically found to have similar or lower glucocorticoid levels than lactating females (61–66). It should be noted that across all interactions in both males and females, the effects of age on cortisol were consistently positive. While aging led to a reduced response to mating effort in males and an increased response to sexual cycling in females, peak cortisol production for both sexes was predicted by the combination of old age and the mating context (Fig. 2). The mating environment poses a particular physiological challenge for aging chimpanzees.
Given the wild setting, we could control neither the nature of the stressor nor the timing of sampling relative to the stressor. The interaction effects we observed could, in theory, speak to changes in the nature of the behavioral stressors associated with reproductive effort rather than a change in sensitivity of the HPA axis. Older males may exhibit a reduced cortisol response to mating days if they invest less in costly behaviors when exposed to receptive females. Doing so may be an important strategy to avoid stress and other risks as males age. By contrast, older females appear to be less able to control their exposure to stressors in the mating environment, particularly as they become more sexually attractive to males as they age (67). Sexually attractive females elicit larger aggregations of males (68, 69), which compromises their foraging success (70). They also experience high rates of sexually coercive aggression throughout cycling, and this elicits stress responses (49, 52). Thus, the interaction effect for females may not indicate age changes in HPA regulation, as we did not find broader support for increases in stress reactivity with age.
Male Kanyawara chimpanzees produced higher cortisol levels than females at any age (∼33% higher), but aging had parallel effects in males and females. This similarity is notable because the life histories of males and females differ in important ways. Male chimpanzees generally experience a peak in dominance rank during prime adulthood (∼25 to 35 y), after which they decline in rank, engage in less frequent aggressive competition, and sire fewer offspring (71–73). The current study corroborated prior findings from this community that high dominance rank is associated with relatively high cortisol levels for males, perhaps due to the high costs of physical competition and associated testosterone production (46). Thus, the age pattern of cortisol increase for males occurs despite, rather than because of, age changes in rank. Female chimpanzees, on the other hand, reliably rise in rank as they age (57, 74), but rank did not contribute significantly to cortisol variation. Thus, in neither sex could the robust age increase in cortisol levels be attributed merely to changes in social status.
In our study, male chimpanzees had higher urinary cortisol levels than females across all ages. Because the gonadal and adrenal axes interact at many levels, sex hormones have the potential to contribute to variation in HPA function. Estrogen generally stimulates increased HPA response, while testosterone has the opposite effect (60, 75, 76). Accordingly, studies of humans and rodent models have often reported increased HPA sensitivity in females versus males, yielding more pronounced and/or prolonged glucocorticoid response to direct (pharmacological) stimulation (60, 76–78). In the face of behavioral stressors, sex differences are far less consistent, suggesting that perception of stress has an important influence (15, 79–82). In that vein, the fact that the main effect of sex in our study reverses the expected pattern could be explained by differences in the nature and frequency of stressors experienced by males and females. In humans, the effects of aging on HPA responses are consistently more pronounced in women than in men across all kinds of stressors (17, 18, 83, 84). Despite estrogen’s role in enhancing HPA activity, the withdrawal of estrogen during human menopause is implicated in accelerated HPA dysregulation and an accompanying increase in affective disorders (75, 85). In our study, the effect sizes of age on cortisol were consistently higher for females than for males, but our combined models did not yield significant age by sex interactions. In contrast to humans, chimpanzees rarely experience menopause (86), and even the eldest females in our sample remained reproductively active. It is plausible that this difference in life history may attenuate the sex difference in age-related HPA dysregulation in chimpanzees relative to what has been observed in humans.
A key cause for higher glucocorticoids in our sample of aging chimpanzees was a blunting of the diurnal rhythm, leading to higher levels late in the day. We could not directly address the time course of cortisol production, only its excretion in urine; thus, our data will be influenced by a time lag and integration of cortisol production over several hours (87), depending on hydration. Nevertheless, chimpanzees and humans exhibit strong similarities not only in the overall diurnal rhythm but also in the specific effects of aging. Similar effects, characterized by an elevation of afternoon glucocorticoid production, have been reported in captive rhesus macaques (88, 89) and marmosets (90), suggesting that this may be a common signature of aging across primates. Comparative data are scant but indicate that this pattern is not consistent across mammals (91–94). Shifts in circadian regulation of the HPA axis have been linked to negative health effects of aging, including compromised antioxidant defense (89), inflammation (95), metabolic disorders (96), and neurodegenerative diseases (97). Moreover, altered circadian production of glucocorticoids is unlikely to occur in isolation. Our findings are highly suggestive of broader changes in circadian regulation, symptomatic of wider health effects (98, 99).
Our data show that there are some key similarities in aging of the HPA axis between humans and chimpanzees, most likely shared through common descent. These signal two important processes: a progressive increase in glucocorticoid exposure with age and aging of the HPA axis itself. Our findings lend support to the hypothesis that age-related changes in glucocorticoid regulation are, in fact, an ancient feature of aging in hominids and are not simply an emergent by-product of extended human life span or of the unusual environments in which most humans have been studied. This is in contrast to other aspects of aging, such as cardiovascular health, which are dramatically impacted by industrialized environments and differ fundamentally between apes and humans (26, 100, 101). Additionally, our findings clarify that senescent dysregulation of the HPA axis does not require atypical exposure to stressors but can occur in the species’ natural environment, informing a debate that has arisen from laboratory models (102–104). Finally, given that glucocorticoids play a central role in the biology of human aging, our findings suggest broader similarities in the mechanisms underlying senescence in chimpanzees, about which we know very little. It remains critically important to address how these processes unfold in long-lived species and under conditions that resemble those in which the species evolved, facing selection pressures like energy limitation, immune challenges, and social competition.
Supplementary Material
Acknowledgments
This study was facilitated through support and permissions from the Makerere University Biological Field Station, the Uganda National Council for Science and Technology, and the Uganda Wildlife Authority. We sincerely thank the Kibale Chimpanzee Project field staff and project managers for daily data collection. For laboratory and database assistance, we thank Lindsey Hagberg, Megan Cole, Heather Moles, Jayda Patterson, Sarah Schmidt, Nicole Thompson, Lara Saipe Durgavitch, and Jillian Rutherford. Long-term research has been supported by an active grant from NIH (National Institute on Aging and Office for Research on Women’s Health Grant R01-AG049395) and past grants from the US National Science Foundation (Grants BCS-0849380, BCS-1355014, and IOS-LTREB-0416125), NIH (Grant AI058715), the Leakey Foundation, and the Wenner-Gren Foundation.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Dryad database, https://datadryad.org/stash (DOI:10.5061/dryad.h18931zgr).
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1920593117/-/DCSupplemental.
References
- 1.Sapolsky R. M., “Neuroendocrinology of the stress response” in Behavioral Endocrinology, Becker J. B., Breedlove S. M., Crews D., Eds. (MIT Press, Cambridge, MA, 1992), pp. 284–324. [Google Scholar]
- 2.Sapolsky R., Armanini M., Packan D., Tombaugh G., Stress and glucocorticoids in aging. Endocrinol. Metab. Clin. North Am. 16, 965–980 (1987). [PubMed] [Google Scholar]
- 3.Hasan K. M. M., Rahman M. S., Arif K. M. T., Sobhani M. E., Psychological stress and aging: Role of glucocorticoids (GCs). Age (Dordr.) 34, 1421–1433 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manolagas S. C., From estrogen-centric to aging and oxidative stress: A revised perspective of the pathogenesis of osteoporosis. Endocr. Rev. 31, 266–300 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walker B. R., Glucocorticoids and cardiovascular disease. Eur. J. Endocrinol. 157, 545–559 (2007). [DOI] [PubMed] [Google Scholar]
- 6.Brotman D. J., Golden S. H., Wittstein I. S., The cardiovascular toll of stress. Lancet 370, 1089–1100 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Aguilera G., HPA axis responsiveness to stress: Implications for healthy aging. Exp. Gerontol. 46, 90–95 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilkinson C. W., Peskind E. R., Raskind M. A., Decreased hypothalamic-pituitary-adrenal axis sensitivity to cortisol feedback inhibition in human aging. Neuroendocrinology 65, 79–90 (1997). [DOI] [PubMed] [Google Scholar]
- 9.Nyberg C. H., Diurnal cortisol rhythms in Tsimane’ Amazonian foragers: New insights into ecological HPA axis research. Psychoneuroendocrinology 37, 178–190 (2012). [DOI] [PubMed] [Google Scholar]
- 10.Sharma M., et al. , Circadian rhythms of melatonin and cortisol in aging. Biol. Psychiatry 25, 305–319 (1989). [DOI] [PubMed] [Google Scholar]
- 11.Van Cauter E., Leproult R., Kupfer D. J., Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. J. Clin. Endocrinol. Metab. 81, 2468–2473 (1996). [DOI] [PubMed] [Google Scholar]
- 12.Franceschi C., et al. , Inflammaging and anti-inflammaging: A systemic perspective on aging and longevity emerged from studies in humans. Mech. Ageing Dev. 128, 92–105 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Baylis D., Bartlett D. B., Patel H. P., Roberts H. C., Understanding how we age: Insights into inflammaging. Longev. Healthspan 2, 8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pavlov E. P., Harman S. M., Chrousos G. P., Loriaux D. L., Blackman M. R., Responses of plasma adrenocorticotropin, cortisol, and dehydroepiandrosterone to ovine corticotropin-releasing hormone in healthy aging men. J. Clin. Endocrinol. Metab. 62, 767–772 (1986). [DOI] [PubMed] [Google Scholar]
- 15.Nicolson N., Storms C., Ponds R., Sulon J., Salivary cortisol levels and stress reactivity in human aging. J. Gerontol. A Biol. Sci. Med. Sci. 52, M68–M75 (1997). [DOI] [PubMed] [Google Scholar]
- 16.Born J., Ditschuneit I., Schreiber M., Dodt C., Fehm H. L., Effects of age and gender on pituitary-adrenocortical responsiveness in humans. Eur. J. Endocrinol. 132, 705–711 (1995). [DOI] [PubMed] [Google Scholar]
- 17.Greenspan S. L., Rowe J. W., Maitland L. A., McAloon-Dyke M., Elahi D., The pituitary-adrenal glucocorticoid response is altered by gender and disease. J. Gerontol. 48, M72–M77 (1993). [DOI] [PubMed] [Google Scholar]
- 18.Otte C., et al. , A meta-analysis of cortisol response to challenge in human aging: Importance of gender. Psychoneuroendocrinology 30, 80–91 (2005). [DOI] [PubMed] [Google Scholar]
- 19.Rohleder N., Wolf J. M., Kirschbaum C., Glucocorticoid sensitivity in humans-interindividual differences and acute stress effects. Stress 6, 207–222 (2003). [DOI] [PubMed] [Google Scholar]
- 20.Waltman C., Blackman M. R., Chrousos G. P., Riemann C., Harman S. M., Spontaneous and glucocorticoid-inhibited adrenocorticotropic hormone and cortisol secretion are similar in healthy young and old men. J. Clin. Endocrinol. Metab. 73, 495–502 (1991). [DOI] [PubMed] [Google Scholar]
- 21.Traustadóttir T., Bosch P. R., Matt K. S., The HPA axis response to stress in women: Effects of aging and fitness. Psychoneuroendocrinology 30, 392–402 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Lucertini F., et al. , High cardiorespiratory fitness is negatively associated with daily cortisol output in healthy aging men. PLoS One 10, e0141970 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaffey A. E., Bergeman C. S., Clark L. A., Wirth M. M., Aging and the HPA axis: Stress and resilience in older adults. Neurosci. Biobehav. Rev. 68, 928–945 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gurven M., Kaplan H., Winking J., Finch C., Crimmins E. M., Aging and inflammation in two epidemiological worlds. J. Gerontol. A Biol. Sci. Med. Sci. 63, 196–199 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gurven M., et al. , Inflammation and infection do not promote arterial aging and cardiovascular disease risk factors among lean horticulturalists. PLoS One 4, e6590 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaplan H., et al. , Coronary atherosclerosis in indigenous South American Tsimane: A cross-sectional cohort study. Lancet 389, 1730–1739 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raichlen D. A., et al. , Physical activity patterns and biomarkers of cardiovascular disease risk in hunter-gatherers. Am. J. Hum. Biol. 29, e22919 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Pontzer H., et al. , Energy expenditure and activity among Hadza hunter-gatherers. Am. J. Hum. Biol. 27, 628–637 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Amir D., Ellison P. T., Hill K. R., Bribiescas R. G., Diurnal variation in salivary cortisol across age classes in Ache Amerindian males of Paraguay. Am. J. Hum. Biol. 27, 344–348 (2015). [DOI] [PubMed] [Google Scholar]
- 30.Urlacher S. S., Liebert M. A., Konečná M., Global variation in diurnal cortisol rhythms: Evidence from Garisakang forager-horticulturalists of lowland Papua New Guinea. Stress 21, 101–109 (2018). [DOI] [PubMed] [Google Scholar]
- 31.von Rueden C. R., et al. , Political influence associates with cortisol and health among egalitarian forager-farmers. Evol. Med. Public Health 2014, 122–133 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chambers K. C., Resko J. A., Phoenix C. H., Correlation of diurnal changes in hormones with sexual behavior and age in male rhesus macaques. Neurobiol. Aging 3, 37–42 (1982). [DOI] [PubMed] [Google Scholar]
- 33.Strier K. B., Ziegler T. E., Wittwer D. J., Seasonal and social correlates of fecal testosterone and cortisol levels in wild male muriquis (Brachyteles arachnoides). Horm. Behav. 35, 125–134 (1999). [DOI] [PubMed] [Google Scholar]
- 34.Bales K. L., French J. A., Hostetler C. M., Dietz J. M., Social and reproductive factors affecting cortisol levels in wild female golden lion tamarins (Leontopithecus rosalia). Am. J. Primatol. 67, 25–35 (2005). [DOI] [PubMed] [Google Scholar]
- 35.Goncharova N. D., Lapin B. A., Age-related endocrine dysfunction in nonhuman primates. Ann. N. Y. Acad. Sci. 1019, 321–325 (2004). [DOI] [PubMed] [Google Scholar]
- 36.Goncharova N. D., Lapin B. A., Effects of aging on hypothalamic-pituitary-adrenal system function in non-human primates. Mech. Ageing Dev. 123, 1191–1201 (2002). [DOI] [PubMed] [Google Scholar]
- 37.Downs J. L., Mattison J. A., Ingram D. K., Urbanski H. F., Effect of age and caloric restriction on circadian adrenal steroid rhythms in rhesus macaques. Neurobiol. Aging 29, 1412–1422 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fourie N. H., Jolly C. J., Phillips-Conroy J. E., Brown J. L., Bernstein R. M., Variation of hair cortisol concentrations among wild populations of two baboon species (Papio anubis, P. hamadryas) and a population of their natural hybrids. Primates 56, 259–272 (2015). [DOI] [PubMed] [Google Scholar]
- 39.Alberts S. C., et al. , “The male-female health-survival paradox: A comparative perspective on sex differences in aging and mortality” in Sociality, Hierarchy, Health: Comparative Biodemography: A Collection of Papers, Weinstein M., MA Lane, Eds. (National Academies Press, Washington, DC, 2014), pp. 339–364. [PubMed] [Google Scholar]
- 40.Sapolsky R. M., Altmann J., Incidence of hypercortisolism and dexamethasone resistance increases with age among wild baboons. Biol. Psychiatry 30, 1008–1016 (1991). [DOI] [PubMed] [Google Scholar]
- 41.Hämäläinen A., Heistermann M., Kraus C., The stress of growing old: Sex- and season-specific effects of age on allostatic load in wild grey mouse lemurs. Oecologia 178, 1063–1075 (2015). [DOI] [PubMed] [Google Scholar]
- 42.Vaupel J. W., Manton K. G., Stallard E., The impact of heterogeneity in individual frailty on the dynamics of mortality. Demography 16, 439–454 (1979). [PubMed] [Google Scholar]
- 43.Beehner J. C., Bergman T. J., The next step for stress research in primates: To identify relationships between glucocorticoid secretion and fitness. Horm. Behav. 91, 68–83 (2017). [DOI] [PubMed] [Google Scholar]
- 44.Wrangham R. W., Chapman C. A., Clark A. P., Isabirye-Basuta G., “Social ecology of Kanyawara chimpanzees: Implications for understanding the costs of great ape groups” in Great Ape Societies, McGrew W. C., Marchant L. F., Nishida T., Eds. (Cambridge University Press, Cambridge, UK, 1996), pp. 45–57. [Google Scholar]
- 45.Balcomb S. R., Chapman C. A., Wrangham R. W., Relationship between chimpanzee (Pan troglodytes) density and large, fleshy-fruit tree density: Conservation implications. Am. J. Primatol. 51, 197–203 (2000). [DOI] [PubMed] [Google Scholar]
- 46.Muller M. N., Wrangham R. W., Dominance, cortisol and stress in wild chimpanzees (Pan troglodytes schweinfurthii). Behav. Ecol. Sociobiol. 55, 332–340 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sapolsky R. M., Social status and health in humans and other animals. Annu. Rev. Anthropol. 33, 393–418 (2004). [Google Scholar]
- 48.Creel S. F., Dominance, aggression, and glucocorticoid levels in social carnivores. J. Mammal. 86, 255–264 (2005). [Google Scholar]
- 49.Emery Thompson M., Muller M. N., Kahlenberg S. M., Wrangham R. W., Dynamics of social and energetic stress in wild female chimpanzees. Horm. Behav. 58, 440–449 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sabbi K. H., et al. , Human-like adrenal development in wild chimpanzees: A longitudinal study of cortisol and DHEAS. Am. J. Primatol., 10.1002/ajp.23064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kahlenberg S. M., Emery Thompson M., Muller M. N., Immigration costs for female chimpanzees and male protection as an immigrant counterstrategy to intrasexual aggression. Anim. Behav. 76, 1497–1509 (2008). [Google Scholar]
- 52.Muller M. N., Kahlenberg S. M., Emery Thompson M., Wrangham R. W., Male coercion and the costs of promiscuous mating for female chimpanzees. Proc. Biol. Sci. 274, 1009–1014 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Emery Thompson M., Sabbi K. H., Evolutionary demography of the great apes. https://osf.io/d2thj/. Accessed 5 September 2019.
- 54.Muller M. N., Testosterone and reproductive effort in male primates. Horm. Behav. 91, 36–51 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith R., Thomson M., Neuroendocrinology of the hypothalamo-pituitary-adrenal axis in pregnancy and the puerperium. Baillieres Clin. Endocrinol. Metab. 5, 167–186 (1991). [DOI] [PubMed] [Google Scholar]
- 56.Emery Thompson M., et al. , Data from “Wild chimpanzees exhibit humanlike aging of glucocorticoid regulation.” Dryad, 10.5061/dryad.h18931zgr. Deposited 4 March 2020. [DOI] [PMC free article] [PubMed]
- 57.Foerster S., et al. , Chimpanzee females queue but males compete for social status. Sci. Rep. 6, 35404 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Muller M. N., Mitani J. C., Conflict and cooperation in wild chimpanzees. Adv. Stud. Behav. 35, 275–331 (2005). [Google Scholar]
- 59.Seeman T. E., Robbins R. J., Aging and hypothalamic-pituitary-adrenal response to challenge in humans. Endocr. Rev. 15, 233–260 (1994). [DOI] [PubMed] [Google Scholar]
- 60.Heck A. L., Handa R. J., Sex differences in the hypothalamic-pituitary-adrenal axis’ response to stress: An important role for gonadal hormones. Neuropsychopharmacology 44, 45–58 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Foerster S., Cords M., Monfort S. L., Seasonal energetic stress in a tropical forest primate: Proximate causes and evolutionary implications. PLoS One 7, e50108 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ziegler T. E., Scheffler G., Snowdon C. T., The relationship of cortisol levels to reproductive functioning in female cotton-top tamarins, Saguinus oedipus. Horm. Behav. 29, 407–424 (1995). [DOI] [PubMed] [Google Scholar]
- 63.Weingrill T., Gray D. A., Barrett L., Henzi S. P., Fecal cortisol levels in free-ranging female chacma baboons: Relationship to dominance, reproductive state and environmental factors. Horm. Behav. 45, 259–269 (2004). [DOI] [PubMed] [Google Scholar]
- 64.Carnegie S. D., Fedigan L. M., Ziegler T. E., Social and environmental factors affecting fecal glucocorticoids in wild, female white-faced capuchins (Cebus capucinus). Am. J. Primatol. 73, 861–869 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gesquiere L. R., et al. , Coping with a challenging environment: Effects of seasonal variability and reproductive status on glucocorticoid concentrations of female baboons (Papio cynocephalus). Horm. Behav. 54, 410–416 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Setchell J. M., Smith T., Wickings E. J., Knapp L. A., Factors affecting fecal glucocorticoid levels in semi-free-ranging female mandrills (Mandrillus sphinx). Am. J. Primatol. 70, 1023–1032 (2008). [DOI] [PubMed] [Google Scholar]
- 67.Muller M. N., Thompson M. E., Wrangham R. W., Male chimpanzees prefer mating with old females. Curr. Biol. 16, 2234–2238 (2006). [DOI] [PubMed] [Google Scholar]
- 68.Emery Thompson M., Wrangham R. W., “Comparison of sex differences in gregariousness in fission-fusion species: Reducing bias by standardizing for party size” in Primates of Western Uganda, Newton-Fisher N. E., Notman H., Reynolds V., Paterson J., Eds. (Developments in Primatology: Progress and Prospects, Springer, New York, NY, 2006), pp. 209–226. [Google Scholar]
- 69.Wrangham R. W., “Why are male chimpanzees more gregarious than mothers? A scramble competition hypothesis” in Primate Males: Causes and Consequences of Variation in Group Composition, Kappeler P. M., Ed. (Cambridge University Press, Cambridge, UK, 2000), pp. 248–258. [Google Scholar]
- 70.Emery Thompson M., Muller M. N., Wrangham R. W., Male chimpanzees compromise the foraging success of their mates in Kibale National Park, Uganda. Behav. Ecol. Sociobiol. 68, 1973–1983 (2014). [Google Scholar]
- 71.Watts D. P., Male dominance relationships in an extremely large chimpanzee community at Ngogo, Kibale National Park, Uganda. Behaviour 155, 969–1009 (2018). [Google Scholar]
- 72.Wroblewski E. E., et al. , Male dominance rank and reproductive success in chimpanzees, Pan troglodytes schweinfurthii. Anim. Behav. 77, 873–885 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Foerster S., et al. , Chimpanzee females queue but males compete for social status. Sci. Rep. 6, 35404 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kahlenberg S. M., Emery Thompson M., Wrangham R. W., Female competition over core areas in Pan troglodytes schweinfurthii, Kibale National Park, Uganda. Int. J. Primatol. 29, 931–947 (2008). [Google Scholar]
- 75.Toufexis D., Rivarola M. A., Lara H., Viau V., Stress and the reproductive axis. J. Neuroendocrinol. 26, 573–586 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bale T. L., Epperson C. N., Sex differences and stress across the lifespan. Nat. Neurosci. 18, 1413–1420 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gallucci W. T., et al. , Sex differences in sensitivity of the hypothalamic-pituitary-adrenal axis. Health Psychol. 12, 420–425 (1993). [DOI] [PubMed] [Google Scholar]
- 78.Goel N., Workman J. L., Lee T. T., Innala L., Viau V., Sex differences in the HPA axis. Compr. Physiol. 4, 1121–1155 (2014). [DOI] [PubMed] [Google Scholar]
- 79.Kudielka B. M., Kirschbaum C., Sex differences in HPA axis responses to stress: A review. Biol. Psychol. 69, 113–132 (2005). [DOI] [PubMed] [Google Scholar]
- 80.Seeman T. E., Singer B., Charpentier P., Gender differences in patterns of HPA axis response to challenge: Macarthur studies of successful aging. Psychoneuroendocrinology 20, 711–725 (1995). [DOI] [PubMed] [Google Scholar]
- 81.Kelly M. M., Tyrka A. R., Anderson G. M., Price L. H., Carpenter L. L., Sex differences in emotional and physiological responses to the trier social stress test. J. Behav. Ther. Exp. Psychiatry 39, 87–98 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stroud L. R., Salovey P., Epel E. S., Sex differences in stress responses: Social rejection versus achievement stress. Biol. Psychiatry 52, 318–327 (2002). [DOI] [PubMed] [Google Scholar]
- 83.Heuser I. J., et al. , Age-associated changes of pituitary-adrenocortical hormone regulation in humans: Importance of gender. Neurobiol. Aging 15, 227–231 (1994). [DOI] [PubMed] [Google Scholar]
- 84.Seeman T. E., Singer B., Wilkinson C. W., McEwen B., Gender differences in age-related changes in HPA axis reactivity. Psychoneuroendocrinology 26, 225–240 (2001). [DOI] [PubMed] [Google Scholar]
- 85.Young E., Korszun A., Sex, trauma, stress hormones and depression. Mol. Psychiatry 15, 23–28 (2010). [DOI] [PubMed] [Google Scholar]
- 86.Emery Thompson M., et al. , Aging and fertility patterns in wild chimpanzees provide insights into the evolution of menopause. Curr. Biol. 17, 2150–2156 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bahr N. I., Palme R., Möhle U., Hodges J. K., Heistermann M., Comparative aspects of the metabolism and excretion of cortisol in three individual nonhuman primates. Gen. Comp. Endocrinol. 117, 427–438 (2000). [DOI] [PubMed] [Google Scholar]
- 88.Gust D. A., et al. , Activity of the hypothalamic-pituitary-adrenal axis is altered by aging and exposure to social stress in female rhesus monkeys. J. Clin. Endocrinol. Metab. 85, 2556–2563 (2000). [DOI] [PubMed] [Google Scholar]
- 89.Goncharova N. D., Shmaliy A. V., Bogatyrenko T. N., Koltover V. K., Correlation between activity of antioxidant enzymes and circadian rhythms of corticosteroids in Macaca mulatta monkeys of different age. Exp. Gerontol. 41, 778–783 (2006). [DOI] [PubMed] [Google Scholar]
- 90.Cross N., Rogers L. J., Diurnal cycle in salivary cortisol levels in common marmosets. Dev. Psychobiol. 45, 134–139 (2004). [DOI] [PubMed] [Google Scholar]
- 91.Hauger R. L., Thrivikraman K. V., Plotsky P. M., Age-related alterations of hypothalamic-pituitary-adrenal axis function in male Fischer 344 rats. Endocrinology 134, 1528–1536 (1994). [DOI] [PubMed] [Google Scholar]
- 92.Honma S., Katsuno Y., Abe H., Honma K., Aging affects development and persistence of feeding-associated circadian rhythm in rat plasma corticosterone. Am. J. Physiol. 271, R1514–R1520 (1996). [DOI] [PubMed] [Google Scholar]
- 93.Palazzolo D. L., Quadri S. K., The effects of aging on the circadian rhythm of serum cortisol in the dog. Exp. Gerontol. 22, 379–387 (1987). [DOI] [PubMed] [Google Scholar]
- 94.Cordero M., Brorsen B. W., McFarlane D., Circadian and circannual rhythms of cortisol, ACTH, and α-melanocyte-stimulating hormone in healthy horses. Domest. Anim. Endocrinol. 43, 317–324 (2012). [DOI] [PubMed] [Google Scholar]
- 95.Cutolo M., et al. , Circadian rhythms: Glucocorticoids and arthritis. Ann. N. Y. Acad. Sci. 1069, 289–299 (2006). [DOI] [PubMed] [Google Scholar]
- 96.Pasquali R., Vicennati V., Cacciari M., Pagotto U., The hypothalamic-pituitary-adrenal axis activity in obesity and the metabolic syndrome. Ann. N. Y. Acad. Sci. 1083, 111–128 (2006). [DOI] [PubMed] [Google Scholar]
- 97.Ferrari E., et al. , Age-related changes of the adrenal secretory pattern: Possible role in pathological brain aging. Brain Res. Brain Res. Rev. 37, 294–300 (2001). [DOI] [PubMed] [Google Scholar]
- 98.Paschos G. K., FitzGerald G. A., Circadian clocks and metabolism: Implications for microbiome and aging. Trends Genet. 33, 760–769 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chung S., Son G. H., Kim K., Circadian rhythm of adrenal glucocorticoid: Its regulation and clinical implications. Biochim. Biophys. Acta 1812, 581–591 (2011). [DOI] [PubMed] [Google Scholar]
- 100.Finch C. E., Evolution in health and medicine Sackler colloquium: Evolution of the human lifespan and diseases of aging: Roles of infection, inflammation, and nutrition. Proc. Natl. Acad. Sci. U.S.A. 107, 1718–1724 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Varki N., et al. , Heart disease is common in humans and chimpanzees, but is caused by different pathological processes. Evol. Appl. 2, 101–112 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Meaney M. J., Aitken D. H., Sharma S., Viau V., Basal ACTH, corticosterone and corticosterone-binding globulin levels over the diurnal cycle, and age-related changes in hippocampal type I and type II corticosteroid receptor binding capacity in young and aged, handled and nonhandled rats. Neuroendocrinology 55, 204–213 (1992). [DOI] [PubMed] [Google Scholar]
- 103.Morano M. I., Vázquez D. M., Akil H., The role of the hippocampal mineralocorticoid and glucocorticoid receptors in the hypothalamo-pituitary-adrenal axis of the aged Fisher rat. Mol. Cell. Neurosci. 5, 400–412 (1994). [DOI] [PubMed] [Google Scholar]
- 104.Sapolsky R. M., Do glucocorticoid concentrations rise with age in the rat? Neurobiol. Aging 13, 171–174 (1992). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Datasets are available in Dryad (56).