Significance
Cellulosic biofuels have not yet reached cost parity with conventional petroleum fuels. One strategy to address this challenge is to generate valuable coproducts alongside biofuels. Engineering bioenergy crops to generate value-added bioproducts in planta can reduce input requirements relative to microbial chassis and skip costly deconstruction and conversion steps. Although rapid progress has been made in plant metabolic engineering, there has been no systematic analysis devoted to quantifying the impact of such engineered bioenergy crops on biorefinery economics. Here, we provide new insights into how bioproduct accumulation in planta affects biofuel selling prices. We present the range of bioproduct selling prices and accumulation rates needed to compensate for additional extraction steps and reach a target $2.50/gal minimum biofuel selling price.
Keywords: in planta accumulation, bioproduct, technoeconomic analysis, bioenergy crop, biofuel
Abstract
Coproduction of high-value bioproducts at biorefineries is a key factor in making biofuels more cost-competitive. One strategy for generating coproducts is to directly engineer bioenergy crops to accumulate bioproducts in planta that can be fractionated and recovered at biorefineries. Here, we develop quantitative insights into the relationship between bioproduct market value and target accumulation rates by investigating a set of industrially relevant compounds already extracted from plant sources with a wide range of market prices and applications, including <$10/kg (limonene, latex, and polyhydroxybutyrate [PHB]), $10 to $100/kg (cannabidiol), and >$100/kg (artemisinin). These compounds are used to identify a range of mass fraction thresholds required to achieve net economic benefits for biorefineries and the additional amounts needed to reach a target $2.50/gal biofuel selling price, using cellulosic ethanol production as a test case. Bioproduct market prices and recovery costs determine the accumulation threshold; we find that moderate- to high-value compounds (i.e., cannabidiol and artemisinin) offer net economic benefits at accumulation rates of just 0.01% dry weight (dwt) to 0.02 dwt%. Lower-value compounds, including limonene, latex, and PHB, require at least an order-of-magnitude greater accumulation to overcome additional extraction and recovery costs (0.3 to 1.2 dwt%). We also find that a diversified approach is critical. For example, global artemisinin demand could be met with fewer than 10 biorefineries, while global demand for latex is equivalent to nearly 180 facilities. Our results provide a roadmap for future plant metabolic engineering efforts aimed at increasing the value derived from bioenergy crops.
Cellulosic biofuel production in the United States has fallen behind federally mandated levels and, at the current trajectory, is not on track to meet the 16 billion gallons per year target by 2022 (1). This lag is due to several factors, including the recent global economic recession in the late 2000s and the dramatic drop in oil prices in the mid-2010s, but one of the most important contributing factors is that cellulosic biofuels remain more expensive to produce than conventional fossil fuels when negative externalities are not fully accounted for. Improving biofuel yields and optimizing the deconstruction and conversion processes that produce them are important for reducing biofuel production costs and increasing market uptake, but some previous studies have shown that these approaches to process optimization alone are not necessarily sufficient to reach minimum selling prices competitive with current conventional fuel prices, even at near-theoretical yields from sugars (2). One strategy to overcome these challenges is the generation of valuable coproducts alongside biofuels by a cellulosic biorefinery (3). Several approaches have been proposed in directly engineering bioenergy crops as a promising means to improve the economics of biorefineries (e.g., increasing the hexose/pentose ratio of the cell wall polysaccharides, modifying lignin properties, decreasing cell wall recalcitrance) (4). Although all of these approaches target various biomass traits useful for increasing fuel yields, there has been recent interest in the use of plants for the photosynthetic production of high-value products (5). Significant work has gone into plant metabolic engineering efforts, yet there has been no systematic analysis devoted to quantifying the impact on biofuel production costs and the minimum in planta accumulation needed to make such engineered approaches worthwhile at commercial scale.
Plant systems have many potential advantages over microbial chassis in the accumulation and production of engineered bioproducts: 1) plants require fewer inputs to grow than microbial chassis, 2) direct extraction of compounds from plants may skip costly deconstruction and conversion steps, and 3) plants may already have the endogenous machinery needed for the biosynthesis of specific classes of compounds (e.g., lignans, cytochrome P450-derived compounds, and so forth). Even if production of the biofuel or recovery of the bioproduct alone would not be economically attractive, this strategy would allow a biorefinery to spread its single largest operating cost—the biomass feedstock—across multiple products, thus improving the economics of the entire system relative to a single-product strategy. Humans have a long history of using traditional breeding methods to increase in planta production of target small molecules for a variety of applications (e.g., artemisinin) (6). For a more limited set of molecules, studies have also demonstrated comparable or higher accumulation of value-added products through plant metabolic engineering, including artemisinin (7), polyhydroxybutyrate (PHB) (8), and limonene (9). Although there are many potential advantages to utilizing bioenergy crops as a chassis for producing valuable bioproducts, a critical evaluation of the costs, benefits, and limitations can provide valuable insight to help prioritize molecular targets, set minimum accumulation goals, and understand the potential scale of production relative to market sizes.
To quantify the impacts of coproducts on biofuel economics, we use technoeconomic analysis, which is a combination of engineering design, process simulation, and scenario analysis. Total capital investment (TCI) and annual operating cost (AOC) are estimated and used to calculate a standard metric referred to as the minimum biofuel selling price (10). We use an ionic liquid-based forage sorghum-to-ethanol conversion process as a baseline model, with variations to incorporate extraction and purification of different coproducts prior to pretreatment. Mass accumulation rates are calculated as a fraction of total above-ground harvested biomass. We identified five representative compounds with a wide range of market prices and applications, including <$10/kg (limonene, latex, and PHB), $10 to $100/kg (cannabidiol), and >$100/kg (artemisinin). Accumulation and recovery of each compound was modeled separately to investigate the impact on the minimum selling price of bioethanol (SI Appendix, Table S1). The primary goal of this study is to investigate to what extent in planta bioproducts will improve the economics of biorefineries, what levels of accumulation are necessary to compensate for the additional extraction costs required to recover them, and the levels required to achieve a target cellulosic ethanol selling price of $2.50/gal (11).
Results
Simulated Cost of Cellulosic Fuel Production and Coproduct Recovery.
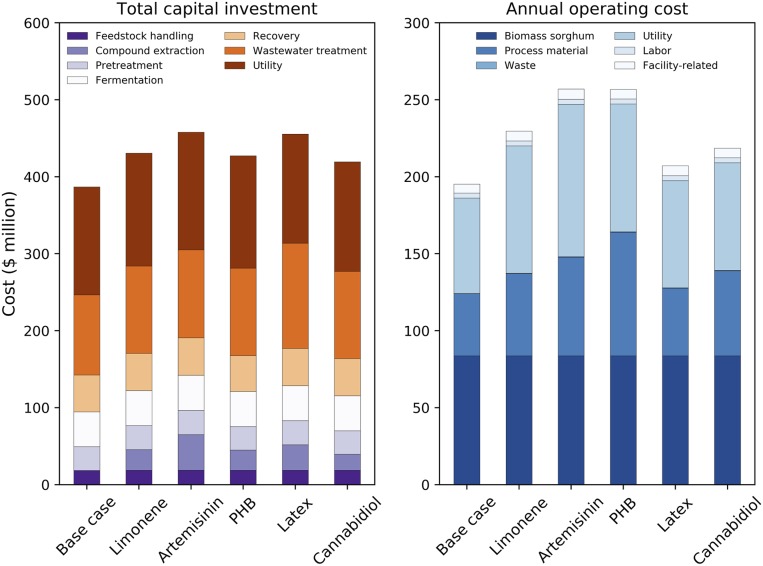
To estimate the cost of recovering each of the five representative products (limonene, latex, PHB, cannabidiol, and artemisinin), we developed separate process simulation models in SuperPro Designer. In each case, chopped, dried sorghum biomass is delivered to the biorefinery at a cost of $95 per dry metric ton, which is based on the costs of cultivating, harvesting, and transporting sorghum (2). In the base case, this biomass is pretreated, undergoes simultaneous saccharification and fermentation, and the ethanol is recovered and sold as fuel. Wastewater is treated onsite and the resulting biogas is combusted alongside lignin to generate process heat and electricity for the facility. TCI and AOC for the base case biorefinery are about $390 million and $190 million, respectively. Each coproduct is extracted upstream of pretreatment and the specific recovery processes are documented in Materials and Methods and SI Appendix, Figs. S1–S5 and Tables S1 and S2.
We find that artemisinin requires the largest capital investment for recovery (approximately $26 million) due to its complex purification processes; these processes are also energy-intensive and thus substantially increase utilities costs (12). The latex accumulation scenario results in higher TCI than any coproduction scenario in this study, totaling approximately $500 million, but the lowest AOC (Fig. 1). Wastewater treatment is capital-intensive and, because the latex extraction process requires large quantities of water, the latex-producing biorefinery requires roughly $100 million for onsite wastewater treatment as compared to about $50 million in the base case. The multistage centrifugation steps required to recover latex after the extraction process are also a key contributor to the TCI. In contrast, the biorefinery with coproduction of cannabidiol proves to be the least capital-intensive at $431 million. The biorefinery modeled with coproduction of limonene, similar to cannabidiol, requires mild extraction conditions and a relatively cheap extraction solvent (hexane). The biorefinery modeled with coproduction of PHB requires a more expensive solvent, butyl acetate, at a higher solvent-to-biomass ratio, which results in higher operating costs.
Fig. 1.
TCI and AOC of the modeled cellulosic ethanol production facility with and without high-value bioproducts. In the base case scenario, the nonengineered biomass sorghum feedstock is utilized and no value-added bioproduct is produced. Detailed modeled costs can be found in SI Appendix, Table S5.
Regardless of the selected high-value compound, the delivered biomass feedstock cost is the largest contributor to the AOC (∼40%), followed by utilities and other process chemicals. The utilities include electricity, heating, and cooling agents, which could further be reduced by minimizing the extraction time and temperature. The process chemical costs are largely influenced by their loading rates (solvent-to-biomass ratio) and purchase prices. Sensitivity analyses (SI Appendix, Fig. S6) on minimum ethanol selling price (MESP) indicate that biomass sorghum feedstock cost is one of the most sensitive parameters in all of the scenarios considered in this study. For instance, by reducing the biomass sorghum feedstock cost by 25%, the MESP could be reduced by around $0.2/gal. If one focuses exclusively on the bioproduct extraction process, the purchase price and loading ratio of solvents are the two most influential parameters to the MESP. The fraction of bioproduct recovered from the biomass is also a critical parameter, and the economic impact is dependent on the market price, as shown in Fig. 2.
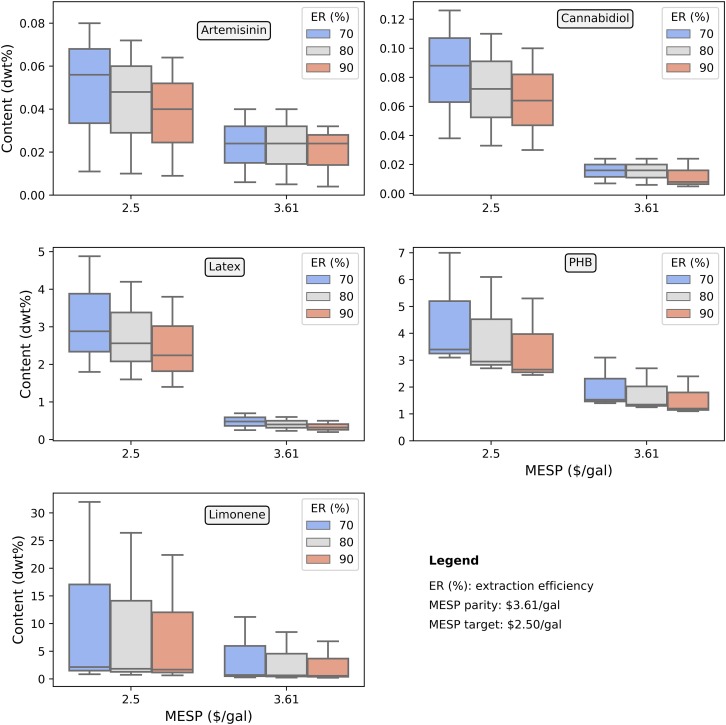
Fig. 2.
MESP with the selected bioproducts under different extraction efficiencies. Content labeled in the y axis refers to the amount (dry basis) of value-added bioproducts required in biomass sorghum. The sensitivity bars denote the range of content for reaching cost-parity and targeted MESP based on the expected distribution of market prices for each bioproduct (SI Appendix, Table S1).
Minimum In Planta Accumulation for Biorefinery Cost Parity and a $2.50/gal Fuel Target.
Based on the technoeconomic models developed in this study, we quantified the minimum in planta accumulation of each value-added compound required to reach cost parity with the base case scenario (MESP of $3.61/gal) and the targeted MESP of $2.50/gal. The results are presented in Fig. 2, considering three different extraction efficiencies (70%, 80%, and 90%). These extraction efficiencies are reported in previous studies (13, 14) and are selected in this study to demonstrate their potential impacts on the process economics. For example, the reported recovery of limonene is above 90% (13); however, the overall extraction efficiency of artemisinin for large-scale production is in the range of 62 to 70% (14).
Because of their higher market values (∼$100/kg), artemisinin and cannabidiol require relatively lower in planta accumulation rates to achieve cost parity and the targeted MESP. For the cost-parity scenario, cannabidiol, artemisinin, latex, limonene, and PHB require accumulation rates of around 0.01% dry weight (dwt%), 0.02 dwt%, 0.3 dwt%, 0.6 dwt%, and 1.2 dwt%, respectively, to reach the breakeven price at 90% extraction efficiency. At the same 90% extraction efficiency, artemisinin, cannabidiol, PHB, limonene, and latex require in planta accumulation of 0.04 dwt%, 0.06 dwt%, 2.7 dwt%, 1.7 dwt%, and 2.2 dwt%, respectively, to reach the $2.50/gal MESP target. These results were consistent with their current market prices except for PHB because of its higher AOC, as indicated in Fig. 1.
The threshold values of bioproducts accumulated in planta are also dependent on their extraction efficiencies, which are uncertain and dependent on where in the plant the bioproduct is accumulated. In this study, we assume the product is accumulated in the entirety of the above-ground sorghum biomass and can be extracted without depolymerizing the cell wall and prematurely liberating sugars or other compounds that may complicate product recovery. We also assume that the bioproduct is stable and does not break down during crop senescence or long-term storage of dried biomass. Any of these factors could impact the fraction of accumulated bioproduct that is ultimately recovered and thus the minimum amount required to offer net value. As an example, at a recovery efficiency of 70%, the amount of limonene required to achieve cost parity with the base case increases from 0.6 dwt% (in the 90% recovery scenario) to 0.7 dwt%. The required amount of limonene, latex, and PHB to achieve cost parity and the $2.50/gal target were all an order-of-magnitude higher than what is required for artemisinin and cannabidiol due to their much lower market prices. At a 70% recovery efficiency, the minimum required in planta accumulation of latex, PHB, and limonene require 2.9 dwt%, 3.4 dwt%, and 2.2 dwt%, respectively, to reach the targeted MESP.
In addition to uncertainty associated with recovery efficiencies for each bioproduct, the market prices will also fluctuate over time, so we conducted a sensitivity analysis based on historical maximum and minimum market prices for each (results shown as the sensitivity bars in Fig. 2). The huge difference in the historical price of limonene ($0.4/kg around 2005 to $11/kg in 2011) (15) resulted in the largest variation in the accumulation amount (dry basis) for reaching both cost parity and the target MESP when compared to other bioproducts. In order to reach the target MESP, the accumulation level of limonene ranges from 0.7 dwt% to 22.4 dwt% at 90% extraction efficiency due to the price fluctuations. The relatively consistent historical price of natural latex, in the range of $1.57/kg to $4.82/kg (from 2010 to 2018) (https://www.statista.com/statistics/727582/price-of-rubber-per-pound/), led to the smallest variation in the MESP for all scenarios.
Accumulation of Target Bioproducts in Native Sources and Engineered Plants.
Although engineering efforts specific to the accumulation of bioproducts in high-yield bioenergy crops are limited, it is worthwhile to compare the target mass contents calculated as part of our analysis with the mass fractions achieved in planta for each as reported across the literature. Based on the mass fractions shown in Fig. 3, it is reasonable to expect that each bioproduct explored in this study could be accumulated at rates that provide substantial net economic benefits to cellulosic biorefineries.
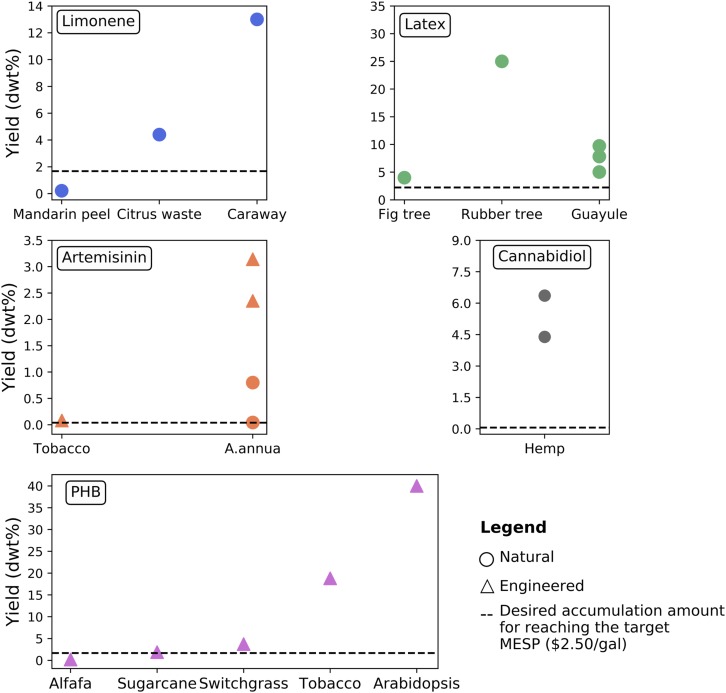
Fig. 3.
Reported in planta accumulation amount (dry basis) of the selected bioproducts in various crops. Black dashed line is the desired in planta accumulation amount obtained in this study for reaching the target MESP ($2.50/gal). Limonene yields from citrus waste (16) and mandarin peel (63) are based on the total raw materials amount. Yield of limonene from caraway is based on the caraway fruit dry weight (9). Artemisinin yields from A. annua are based on the plant dry weight (6, 28). Artemisinin yield in tobacco is based on the dry weight of leaves (30). Yields of artemisinin from the engineered A. annua are the percentage of the leaves dry weight (7, 29). Latex yields from guayule are based on the stem dry weight (26). Yield of natural latex from rubber tree is the percentage of natural latex content on the whole plant (25). Latex yield from fig tree is based on the whole plant (27). PHB yield from alfalfa (21), sugarcane (22), switchgrass (23), tobacco (24), and Arabidopsis (8) are based on the leaves dry weight. Yields of cannabidiol from hemp (32) are based on the leaves dry weight.
Limonene is naturally available in citrus peel and other plant tissues, but the accumulation amount depends on the citrus varieties. Bouwmeester et al. (9) reported that the young caraway fruit accumulated high levels of limonene (13 dwt% fruit dry weight) prior to its conversion to carvone as the fruit matures. In citrus waste, as much as 4.4 dwt% of limonene can be extracted in citrus waste-to-ethanol facility (16). Lohrasbi et al. (17) found that the elevated concentration of limonene in citrus waste does decrease the minimum ethanol selling price if sold as a high-value coproduct. One unintended negative consequence of engineering plants to accumulate more limonene could be a decrease in air quality; limonene and other terpenes are emitted naturally by some trees and bushes, and these volatile organic compounds undergo photochemical reactions with other air pollutants to form fine particulate matter (aerosols) and photochemical smog (17). In citrus and other specialized limonene-producing plants, the cytotoxic compound is stored in secretory cavities lined by specialized epithelial cell (18). Current efforts to engineer limonene in planta have resulted in relatively low accumulation (e.g., 143 ng/g fresh weight) (19), although there was a side-advantage in that the plants are more resistant to pathogens (20). Strategies to mitigate these problems include targeting in planta production to specific cell-types, glycosylating it, or producing a related but less cytotoxic or volatile compound that could be converted in the biorefinery, such as cineole.
PHB production has been engineered in a wide variety of plants (Fig. 3). With the expression of three genes: phaA or bktB (3-ketothiolase), phaB (acetoacetyle-CoA reductase), and phaC (PHA synthase), Arabidopsis has the ability to accumulate PHB up to 4% of their fresh weight (∼40 dwt%) in the leaf chloroplasts (8). PHB has also been produced in alfalfa (1.80 dwt% in the leaves) (21), sugarcane (1.88 dwt% in the leaves) (22), and switchgrass (3.72 dwt% in the leaves) (23). In the chloroplast of tobacco, PHB can be accumulated up to 18.8 dwt% in the leaf tissue (24). The current progress of engineered PHB production in plants and the minimum required amount of PHB obtained in this study of 2.2 dwt% demonstrate that the minimum required PHB accumulation in bioenergy crops is already biologically achievable.
Natural latex content in a rubber tree (Hevea) accounts for about 2 dwt% of the total rubber tree (25). In the flowering shrub guayule (Parthenium argentatum), the latex content varies from 1 to 10 dwt% in the branches depending on the harvesting seasons (26). Ficus carica (fig tree) has also been developed as an alternative to the rubber crop because it can generate a large volume of latex (4.1 dwt%) (27). Bioenergy crops would need to accumulate about 2.9 dwt% of latex to achieve the targeted MESP, which is within the range already reported in other plants. However, there are significant challenges in targeting latex as a bioproduct, specifically considering the unique tissues and physiology required for latex-producing plants. This highlights some of the considerations necessary in pursuing bioproduct engineering efforts in feedstock crops.
Compared to limonene, latex, and PHB, artemisinin content in Artemisia annua is relatively low, ranging from 0.04 dwt% to 0.8 dwt% of the plant dry weight (6, 28). With overexpression of several genes involved in artemisinin biosynthesis in A. annua, artemisinin yield could be increased significantly (7, 29). Lv et al. (7) observed that overexpression of the AaNAC1 gene not only enhanced the yield of artemisinin, but also increased the drought tolerance of A. annua. Apart from A. annua, tobacco is the only plant that has been used as the platform to produce artemisinin. Malhotra et al. (30) engineered two metabolic pathways into three different cellular compartments, resulting in threefold enhancement in artemisinin yield in tobacco. However, due to the complex glycosylation response in Nicotiana and the regulation in artemisinin biosynthesis pathway, the production level in tobacco still remains low (31). Although only a small amount of artemisinin (0.06 dwt%) must be accumulated in bioenergy crops to offset the recovery costs, the complex metabolic biosynthesis pathway of artemisinin may limit its application in biorefineries. Nonetheless, the levels of artemisinin reported from engineered A. annua lines suggest that plant metabolic engineering efforts have already achieved concentrations of artemisinin that would hit the target $2.50/gal MESP if translated into a higher-yielding bioenergy crop.
Cannabidiol is naturally accumulated in Cannabis sativa; researchers reported up to 7.50 dwt% cannabidiol accumulated in hemp (a strain of C. sativa grown for industrial uses) (32). No efforts to engineer cannabidiol production in other plants have been reported in the literature to-date, although interest in increasing cannabidiol production in C. sativa is likely to increase given hemp’s removal from the Controlled Substances Act in 2018 and projected increases in demand. However, even the amounts naturally accumulated in hemp are greater than the minimum required to provide net value to biorefineries, if the same mass fractions can be achieved in sorghum or other bioenergy crops (0.09 dwt%).
Relationship between Bioproduct Price and Required In Planta Accumulation.
Although the cost and expected yields for product extraction and recovery vary from product-to-product, a crucial question is whether our analysis of five representative compounds can be translated into a more generalizable relationship between market value and minimum targeted in planta accumulation. Fig. 4 provides this price vs. mass fraction relationship, incorporating ranges in each value based on varying separations costs (all assuming 90% extraction efficiency). Detailed selling prices of each bioproduct under different accumulation rates and ethanol selling price scenarios are documented in SI Appendix, Figs. S7 and S8.
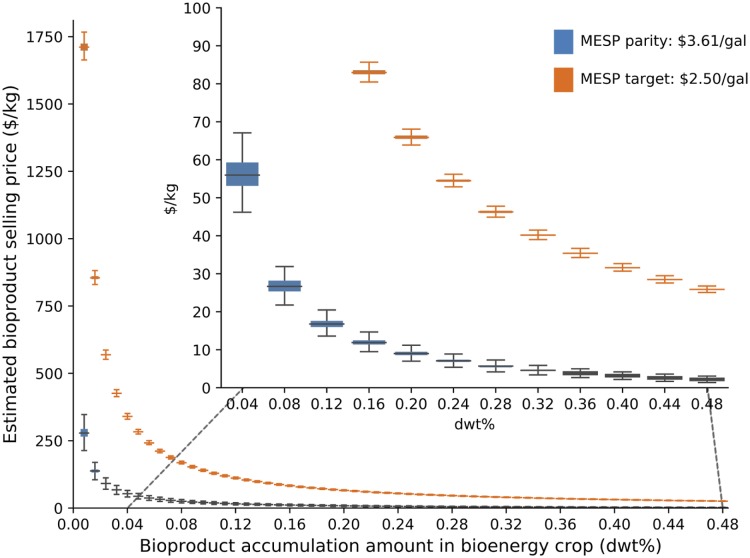
Fig. 4.
Minimum required selling price ranges for bioproducts ($/kg) under different in planta accumulation amount (dry basis) in order to reach the MESP parity ($3.61/gal) and targeted selling price of ethanol ($2.50/gal). The Inset shows the estimated bioproduct selling price of less than $100/kg.
There is a notable gap between the bioproduct selling price required to achieve cost parity with the base case biorefinery (MESP of $3.61/gal) versus the selling price needed to subsidize biofuel production such that it reaches the targeted MESP ($2.50/gal). For example, a bioproduct accumulated at 0.2 dwt% needs to sell for only around $10/kg if the goal is to reach cost-parity with a typical cellulosic biorefinery, whereas that same product must sell for $65/kg to enable an MESP of $2.50/gal. As expected, order-of-magnitude increases in the accumulation rate of bioproducts have a dramatic effect on the minimum selling price necessary to make the extraction economically viable; if less than 0.01 dwt% of a bioproduct is produced in bioenergy crops, the required selling price of bioproduct must be ∼$300/kg to achieve MESP parity; in the same scenario, the selling price of the bioproduct could be reduced to $10/kg if the in planta accumulation level reaches 0.2 dwt%. The required selling price of a bioproduct is reduced from ∼$1,700/kg to $10/kg if the in planta accumulation rate of the bioproduct is increased from 0.01 dwt% to 1.0 dwt%.
An additional question not yet fully addressed is whether a facility would profit more from simply extracting the bioproduct and discarding the remaining biomass, as opposed to converting the biomass to fuel. The answer hinges on numerous uncertain factors, including whether the remaining biomass postextraction can be sold in a fiber, feed, or more conventional energy (e.g., anaerobic digestion) market and at what price, as opposed to requiring disposal at a net cost. In turn, that question of how residual biomass can be handled relates to what solvents and other contaminants may remain after extraction. Even if the biomass is sent to an anaerobic digester, there will be substantial residual solids to be managed. If the biomass disposal cost/revenue is (hypothetically) zero, a roughly equivalent question is: Does the selling price of a liquid biofuel justify the marginal cost of converting biomass to fuel? Depending on the fuel molecule of interest, a feedstock cost of zero could translate into a minimum biofuel price as low as $1.2 to 1.5/gal (10, 33). A key assumption embedded in this analysis is that making renewable liquid fuels to replace petroleum is valuable, as opposed to envisioning alternatives that do not produce liquid biofuels but may be more profitable in the short-term absent any policy intervention.
Discussion
Coproducts are likely to play a pivotal role in making a future biorefinery economically feasible. Petroleum refineries maximize profits by maintaining the flexibility to crack heavier products into lighter, high-value coproducts in response to shifting market prices. Future cellulosic biorefineries must diversify in a similar manner; the traditional configuration, in which electricity is the sole coproduct, is not likely to be competitive given the low value of electricity exports to the grid (34). The best strategy for achieving this diversification remains a hotly debated topic, with recommendations ranging from fractionation and highly specialized conversion to “biological funneling” of heterogeneous mixture (4, 35). Previous studies on coproduction of commodity chemicals, such as limonene recovery in citrus waste-to-ethanol plant (16) and the integrated production of PHB and ethanol production in a sugarcane mill (36), demonstrate the potential value of using higher-value bioproducts to improve the economics of biofuel production, but none of these previous studies provided generalizable guidance on levels of accumulation in planta. Our results provide insight into the role of bioproduct accumulation for increasing the value derived from engineered bioenergy crops. An important caveat is that ongoing development or improvement of microbial routes can impact future bioproduct market prices. The competitiveness of microbial routes will vary on a product-specific basis. For example, semisynthetic production of artemisinin is already commercialized and can achieve production costs at the very low end of the market price range (around $350/kg) (37, 38). Microbial production of limonene in the United States based on Amyris technology achieves costs on par with the 2016 market price of around $4.5/kg while other recent studies report far greater costs of nearly $20/kg (39, 40). Microbial PHB production costs vary considerably ($2 to 15/kg) because of the diverse range of carbon sources used (41–43), while microbial cannabidiol production appears to be in its infancy and the only reported cost is a future target of $1,000/kg (44). Comparing on the basis of production costs alone can miss additional nuances and tradeoffs. For example, reaching very high purities required for food and pharmaceutical products may present engineering challenges that favor microbial or in planta production, depending on the specific target and extraction method. Additionally, many crops of interest are currently either not genetically tractable or have very limited tools for complex metabolic engineering in comparison to microbial systems, so future development of the plant engineering field will be essential to achieving bioproduct accumulation in commercially relevant bioenergy crops.
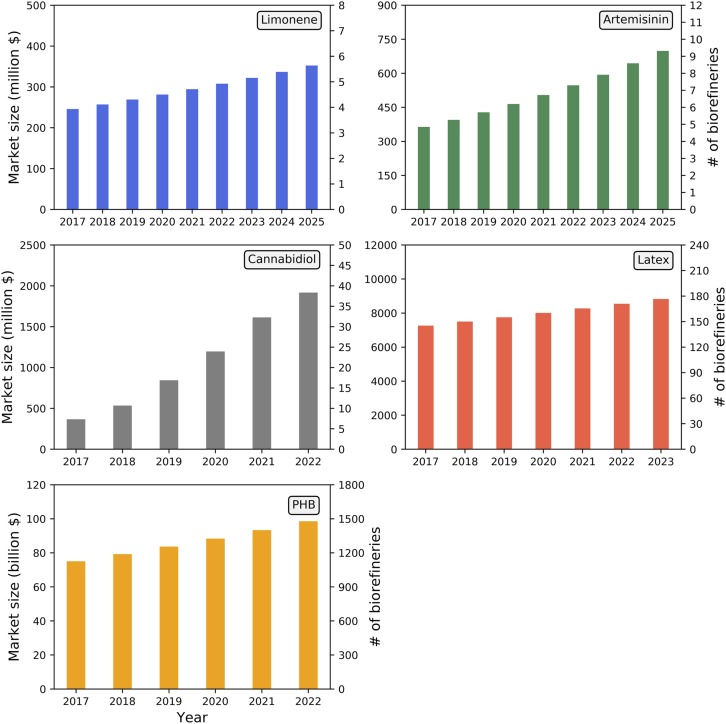
Another potential criticism of integrating high-value and lower-volume chemical production into biorefineries is the mismatch in scale; for very small-volume pharmaceutical markets, a single biorefinery could generate enough of a bioproduct to easily overwhelm the market. This study is not meant to suggest that all bioenergy crops and all biorefineries should be dedicated to coproducing a single high-value product. Rather, plant engineering efforts should be diversified across a variety of biorefining processes and bioproducts that allow for the industry to generate a wider range of commercially viable targets. To gauge the potential scale of production relative to the global market size for each bioproduct explored in this study, we summed the number of commercial-scale cellulosic biorefineries required to meet the entirety of both current and projected future demand, assuming sufficient accumulation to reach the $2.50/gal MESP target (Fig. 5). To meet limonene market demand, excluding its yet unrealized potential as a jet fuel and diesel blendstock after upgrading (2), fewer than six commercial-scale biorefineries would meet the projected 2025 market demand. Global artemisinin demand would be fully met with approximately nine biorefineries. This result indicates that, while very high-value compounds such as pharmaceuticals are attractive, even a single biorefinery would likely flood the market and push the price down. Unlike the linear growth expected for other products, the market demand for cannabidiol is expected to grow by an order-of-magnitude by 2025, at which point nearly 40 biorefineries would be required to meet this demand. Latex and PHB are the two highest-volume markets, which would require the output from 180 and 1,500 biorefineries, respectively. For comparison, there are ∼200 corn ethanol facilities operating in the United States currently and these facilities may ultimately be retrofitted or expanded to produce cellulosic biofuels (45). It is worth noting that this study does not consider the cost of entering consumer markets, which will vary depending on the product.
Fig. 5.
Projected global market size for each bioproduct and maximum number of biorefineries needed to meet total global demand, based on per facility coproduction required to bring MESP to the $2.50/gal target. Market projections: Global limonene market (64), artemisinin combination therapy market (65), cannabidiol sales in the United States (61), global styrene butadiene latex market (66), and global PP market (67).
Our analysis suggests that, although no single bioproduct will be sufficiently high-volume and high-value to improve the economics of all biofuel production at the enterprise level, bioenergy crops can be engineered to produce a variety of high-value products in planta at concentrations well beyond the minimum needed to be cost-effective and have a significant impact on the commercial viability of cellulosic biorefineries. The targeted bioproduct titers mapped to market prices provide a blueprint for the next generation of feedstocks, biorefineries, and a more robust bioeconomy in the United States and across the globe. Our results demonstrate the critical role that technoeconomic models will play in guiding the metabolic goals of future plant engineering efforts toward targets with the potential to make the greatest impact on society.
Materials and Methods
Bioethanol Production Process Simulation.
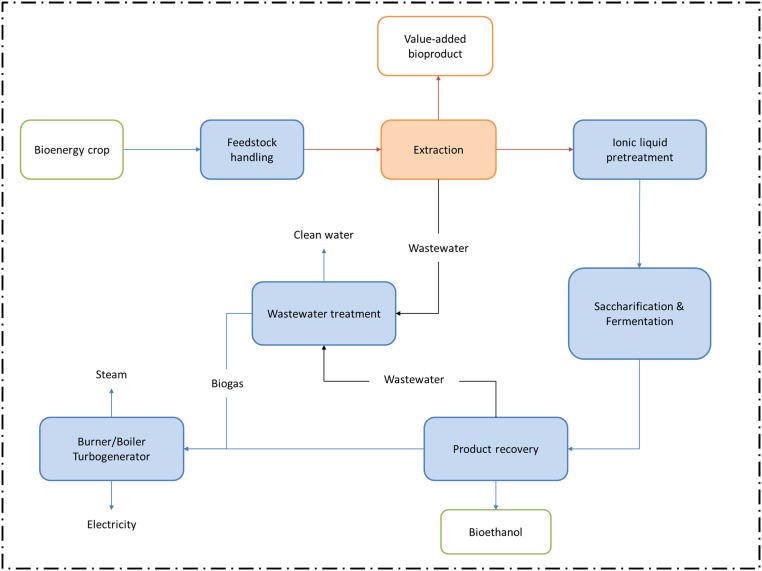
The cellulosic bioethanol production process, which is common across all scenarios, involves feedstock handling, ionic liquid (IL) pretreatment, enzymatic hydrolysis and fermentation, product recovery, wastewater treatment, and lignin combustion sections. In every case, the value-added bioproduct extraction process is added before IL pretreatment as shown in Fig. 6. In this study, biomass sorghum is used as a representative biomass feedstock, although results will not be substantially different for any other biomass feedstocks. The selected value-added compounds—including limonene, artemisinin, PHB, latex, and cannabidiol—are coproducts (with ethanol being the primary, high-volume product) in their respective scenarios. We assume that the coproduct is accumulated in the entirety of the above-ground sorghum biomass, it is stable in its final active form, and it does not break down during crop senescence or long-term storage of dried biomass. We also assume that the coproduct can be extracted without depolymerizing the cell wall and prematurely liberating sugars or other compounds that may complicate product recovery. The feedstock handling section of the biorefinery includes conveyors and short-term storage units. Each value-added compound considered in this study is extracted by chemical solvents, using unit processes tailored for each product type (Table 1) and stored onsite. The remaining biomass sorghum after coproduct extraction is mixed with ILs and water at an IL-to-biomass ratio of 0.29 wt% and pretreated at 140 °C for 3 h (46). Cholinium lysinate ([Ch][Lys]) is used as a representative IL due to its effectiveness in depolymerizing biomass and compatibility with enzyme and microbes (2). Following the pretreatment, H2SO4 is added to adjust the pH to around 5 and the slurry is routed to the enzymatic hydrolysis unit. The enzymatic hydrolysis is carried out at the enzyme loading rate of 10 mg/g of glucan and at a temperature of 50 °C for 48 h in order to convert the majority of the cellulose and hemicellulose into fermentable sugars. The glucan-to-glucose and xylan-to-xylose conversion rates are assumed to be 90% (46). The assumptions and modeling conditions associated with the seed production, fermentation, and other downstream processes, including ethanol recovery, wastewater treatment and lignin combustion, are consistent with the National Renewable Energy Laboratory (NREL) report (10). The glucose-to-ethanol conversion rate is assumed to be 95% of stoichiometric theoretical yield, and the xylose-to-ethanol conversion rate is set to 85% of theoretical yield (10). The IL is separated after the fermentation process and recycled back to the pretreatment process (46). Detailed input parameters for the base case scenario and sensitivity analyses are summarized in SI Appendix, Table S2.
Fig. 6.
A schematic of bioethanol production process with the value-added bioproduct and the integrated one-pot high gravity ionic liquid-based pretreatment process. Biomass sorghum is used as a representative bioenergy crop. In this study, the selected value-added bioproducts are limonene, artemisinin, PHB, latex, and cannabidiol.
Table 1.
Operating conditions for the selected extraction processes
| Compound | Extraction solvent | Extraction conditions | Source |
| Limonene | Hexane | a. Solvent: biomass = 2:1 (g/g) | (55) |
| b. 30 min at 20 °C | |||
| c. Solvent: biomass = 2:1 (g/g) | |||
| d. 30 min at 20 °C | |||
| Artemisinin | Hexane | a. Solvent: biomass = 4:1 (g/g) | (14) |
| b. 8 h at 40 °C | |||
| c. Solvent: biomass = 4:1 (g/g) | |||
| d. 8 h at 40 °C | |||
| Latex | Buffer (0.1% Na2SO3, 0.2% NH3 and 0.1% casein) | a. Solvent: biomass = 2 mL: 1 g | (53) |
| b. 30 min at room temperaure | |||
| c. Solvent: biomass = 2 mL: 1 g | |||
| d. 30 min at room temperature | |||
| PHB | Butyl acetate | a. Solvent: biomass = 100 mL: 1 g | (54) |
| b. 30 min at 103 °C | |||
| c. Solvent: biomass = 100 mL: 1 g | |||
| d. 30 min at 103 °C | |||
| Cannabidiol | Methanol: hexane (9:1) | a. Solvent: biomass = 1 mL: 15 mg | (59) |
| b. 30 min at room temperature | |||
| c. Solvent: biomass = 1 mL: 15 mg | |||
| d. 30 min at room temperature |
Selection of Value-Added Bioproducts.
Among the selected compounds, limonene is one of the largest secondary metabolites produced in plants and a potential jet fuel precursor (47) because of its high energy density, low freezing point, and good chemical stability (48). Another compound of interest is a biopolymer, PHB, which has been commercially produced in bacterial systems and is considered as an ideal replacement for conventional plastics due to its biodegradability (49). In bacterial hosts, PHB can be accumulated up to 80% of their cell dry mass (50) but the fermentation process still costs at least 5 to 10 times more than the production of conventional plastics like polyethylene (8). Given that PHB production has been successfully demonstrated in crops including alfalfa (21), sugarcane (22), and switchgrass (23), it is reasonable to speculate that additional next-generation bioenergy crops could be engineered for PHB production. Latex is another commonly used material analyzed in this study, which may require a unique extraction process due to its viscous characteristics. Cannabidiol, the primary active compound of hemp oil, is the fourth compound selected in this study. In 2018, Agricultural Improvement Act of 2018 removed hemp from the Controlled Substances Act and considered hemp as an agricultural product; it also allowed states to establish the regulatory of hemp production in their state (51).
Compounds intended for pharmaceutical applications are investigated in this study because they represent an approximate upper bound on bioproduct market value. Artemisinin, an effective antimalarial drug, is recommended by the World Health Organization to treat uncomplicated malaria since 2012 (31). However, due to the unstable availability of its natural source, A. annua, the supply and price of artemisinin fluctuated dramatically (37). Although engineering plants to produce pharmaceutical compounds remains challenging, products like Ebola vaccines have been successfully produced in transgenic tobacco (52).
Value-Added Bioproduct Extraction Process.
Careful selection of the bioproduct extraction and separation process is crucial to achieve a high bioproduct recovery rate without any negative impact on the downstream biomass conversion process. Although biomass will ultimately need to be depolymerized for saccharification and ethanol production, the bioproduct should be extracted prior to depolymerization to minimize the need for costly separation and purification processes. Therefore, we do not consider any bioproduct extraction methods that will depolymerize the biomass feedstock, such as steam distillation, acid hydrolysis, and supercritical carbon dioxide. Detailed process flow diagrams for the selected value-added compounds and corresponding extraction process are presented in SI Appendix, Figs. S1–S5. In general, the bioproduct extraction process starts with biomass sorghum grinding and a successive solvent-based extraction process. The desired compound is extracted based on reported process conditions, which are summarized in Table 1. After extraction, the solvent is evaporated, condensed, and recycled. The extracted material (value-added bioproduct) is collected, purified, and stored onsite. Regardless of the selected value-added coproducts, 5% of the extracted bioproduct is assumed to be lost during the purification process (36) and 1% dry matter loss of biomass is assumed during the extraction process. Additionally, the purity of all compounds is assumed to be ∼99% (12, 36, 53, 54).
Hexane is used to extract limonene from biomass sorghum because it is cheap and requires relatively little energy for recovery (14, 55). Pure n-hexane is not suitable for commercial applications due to its flammability and explosive characteristics (https://pubchem.ncbi.nlm.nih.gov/compound/Hexane). The commercial hexane is a mixture of isomers with similar chemical properties as n-hexane but has lower boiling and melting points, which makes it widely used in vegetable oil production (56). Hexane is also used for extracting artemisinin in this study since it is an established technology in the countries where A. annua is processed (55). However, hexane is toxic to microorganisms [concentration of 13 g/L was used in toxicity test (56)], which may inhibit ethanol production (55). Thus, hexane residue must be evaporated as much as possible prior to fermentation to avoid negatively impacting fuel yield. In the PHB extraction process, butyl acetate is employed as the extraction solvent. Compared with a standard halogenated solvent, such as chloroform, this nonhalogenated solvent is less hazardous to human and ecological health (57) and results in a higher PHB recovery rate (∼96% from Cupriavidus necator) (54). Similar to hexane, butyl acetate is moderately toxic to microbes. Laboratory results indicate that butyl acetate concentrations >4 g/L are toxic to Escherichia coli and should be maintained below 2.5 g/L (58). For latex, it is not feasible to tap small plants to extract latex, which is the standard extraction method for rubber trees (53). Thus, researchers developed a flow method, which uses an aqueous extraction buffer (0.2% NH3 and 0.1% Na2SO4), to recover latex (26, 53). The recovery process for latex requires multiple stages of centrifugation after extraction due to its high viscosity (53). Cannabidiol is extracted from hemp by using supercritical carbon dioxide, hydrocarbons, and ethanol. This study considers cannabidiol extraction using a mixture of methanol and hexane (9:1) as solvent (59).
Technoeconomic Analysis.
For each scenario, a commercial-scale sorghum-to-ethanol (with bioproduct extraction) model is constructed and simulated using the process modeling software SuperPro Designer v10 (Intelligen, Inc.). The capacity of the modeled biorefinery is 2,000 dry metric tons of biomass per day and the annual operating time is 8,410 h per year. Biomass sorghum with a moisture content of 20% is considered as the biomass feedstock and its structural composition is summarized in SI Appendix, Table S2. Ethanol is the main product along with each of the selected value-added compounds produced as a coproduct in their respective scenarios. After performing a mass and energy balance analysis in SuperPro Designer, TCI, AOC, and MESP are determined. The TCI includes the fixed capital investment (FCI), land cost, working capital, and start-up cost. FCI is the sum of the installed equipment cost, warehouse, field expenses, construction fee, project contingency fee, and other costs including piping cost (10). Equipment purchase prices and installation multipliers (SI Appendix, Table S3) are based on an “nth plant” assumption, and the price index and scaling factors are harmonized with the widely cited 2011 NREL corn stover-to-ethanol report (10). Working capital and start-up costs are assumed to be 5% of the FCI. The cost factors used to determine the direct and indirect costs, such as installation, piping, site development, land, warehouse, field expenses, project contingency, construction fees, and other costs, are consistent with the 2011 NREL report (10) and can be found in SI Appendix, Table S4.
The AOC includes raw materials cost, utility cost, labor cost, and facility-dependent cost. The delivered biomass sorghum feedstock cost is estimated to be $95.0/dry ton (33). Although the supply cost of biomass sorghum is not specific for engineered biomass sorghum, the feedstock cost is higher than the US Department of Energy-targeted lignocellulosic biomass supply cost of $80.0/ton (60). While biomass harvesting, transportation, and storage costs remain the same regardless of engineered or nonengineered biomass sorghum, the engineered biomass sorghum may have different nutrient requirements, or could be more or less susceptible to various biotic or abiotic stresses. These factors can only be further explored once engineered plants are produced and tested in the greenhouse and field. To capture these sources of uncertainty, we conducted sensitivity analysis on feedstock supply cost. Other raw materials prices are listed in SI Appendix, Table S2. The required process steam is generated onsite from the combustion of lignin and remaining solids as well as biogas generated from the wastewater treatment section. Labor requirements are consistent with the 2011 NREL report (10); the corresponding salaries are obtained from the 2018 labor market. The facility-dependent cost includes maintenance and insurance costs, which are assumed to be 3% and 0.7% of the installed equipment cost, respectively (10).
The MESP is determined by using discounted cash flow analysis. Biorefinery plant life is assumed to be 30 years, with the internal rate of return of 10%. Depreciation is accounted for using the modified accelerated cost recovery system. The construction time for the biorefinery is set to be 36 mo and the start-up time is assumed to be 6 mo (10). The MESP is calculated to be the ethanol selling price required to reach a net present value of zero for each facility. Current market prices of the selected value-added compounds, with the exception of cannabidiol and PHB, are based on literature and industry reports (SI Appendix, Table S1). The cannabidiol price is obtained from hemp-derived cannabidiol oil products (61). The price of polypropylene (PP) is used as a proxy for the PHB price because PHB produced in the biorefinery eventually will ultimately compete with conventional plastics and its properties are most similar to PP (62). The historical prices of each compound as well as its distribution (SI Appendix, Table S1) were used to estimate the ranges of accumulation levels required to reach ethanol cost parity with the baseline and to reach the lower target MESP of $2.50/gal. After estimating the range of costs for each bioproducts’ extraction and recovery process, we used these values to create a triangular distribution for the purpose of estimating a generalized minimum bioproduct selling price as a function of mass fraction in the feedstock. The single-point sensitivity analysis was carried out using the minimum and maximum values of each input parameter as listed in SI Appendix, Table S2. Several input factors, including but not limited to IL pretreatment conditions, raw materials costs, coproduct extraction conditions, and feedstock price were investigated in order to identify the most influential input parameter on the final MESP in each scenario. All costs in this study are reported in 2018 US dollars.
Data Availability Statement.
All data discussed in the paper are published in SI Appendix.
Supplementary Material
Acknowledgments
This work was part of the Department of Energy, Joint BioEnergy Institute (http://www.jbei.org) supported by the US Department of Energy, Office of Science, Office of Biological and Environmental Research, through Contract DE-AC02-05CH11231 between Lawrence Berkeley National Laboratory and the US Department of Energy. The United States Government retains and the publisher, by accepting the article for publication, acknowledges that the United States Government retains a nonexclusive, paid-up, irrevocable, world-wide license to publish or reproduce the published form of this manuscript, or allow others to do so, for United States Government purposes. P.M.S. was supported by the Department of Energy (DE-AC02-05CH11231) and start-up funding provided by the University of California, Davis.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2000053117/-/DCSupplemental.
References
- 1.U.S. Environmental Protection Agency , Regulation of Fuels and Fuel Additives: Changes to Renewable Fuel Standard Program (U.S. Environmental Protection Agency, 2010). [Google Scholar]
- 2.Baral N. R., et al. , Techno-economic analysis and life-cycle greenhouse gas mitigation cost of five routes to bio-jet fuel blendstocks. Energy Environ. Sci. 12, 807–824 (2019). [Google Scholar]
- 3.Budzianowski W. M., High-value low-volume bioproducts coupled to bioenergies with potential to enhance business development of sustainable biorefineries. Renew. Sustain. Energy Rev. 70, 793–804 (2017). [Google Scholar]
- 4.Baral N. R., et al. , Approaches for more efficient biological conversion of lignocellulosic feedstocks to biofuels and bioproducts. ACS Sustain. Chem.& Eng. 7, 9062–9079 (2019). [Google Scholar]
- 5.Bioenergy Technology Office , Strategic Plan. For a Thriving and Sustainable Bioeconomy (US Department of Energy, 2016). [Google Scholar]
- 6.Duke M. V., Paul R. N., Elsohly H. N., Sturtz G., Duke S. O., Localization of artemisinin and artemisitene in foliar tissues of glanded and glandless biotypes of Artemisia annua L. Int. J. Plant Sci. 155, 365–372 (1994). [Google Scholar]
- 7.Lv Z., et al. , Overexpression of a novel NAC domain-containing transcription factor gene (AaNAC1) enhances the content of artemisinin and increases tolerance to drought and botrytis cinerea in Artemisia annua. Plant Cell Physiol. 57, 1961–1971 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Bohmert K., et al. , Transgenic Arabidopsis plants can accumulate polyhydroxybutyrate to up to 4% of their fresh weight. Planta 211, 841–845 (2000). [DOI] [PubMed] [Google Scholar]
- 9.Bouwmeester H. J., Gershenzon J., Konings M. C. J. M., Croteau R., Biosynthesis of the monoterpenes limonene and carvone in the fruit of caraway. I. Demonstration of enzyme activities and their changes with development. Plant Physiol. 117, 901–912 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Humbird D., et al. , Process Design and Economics for Biochemical Conversion of Lignocellulosic Biomass to Ethanol (National Renewable Energy Laboratory, 2011). [Google Scholar]
- 11.Bioenergy Technologies Office , Multi-Year Program Plan (US Department of Energy, 2016). [Google Scholar]
- 12.Piletska E. V., Karim K., Cutler M., Piletsky S. A., Development of the protocol for purification of artemisinin based on combination of commercial and computationally designed adsorbents. J. Sep. Sci. 36, 400–406 (2013). [DOI] [PubMed] [Google Scholar]
- 13.John I., Muthukumar K., Arunagiri A., A review on the potential of citrus waste for D-limonene, pectin, and bioethanol production. Int. J. Green Energy 14, 599–612 (2017). [Google Scholar]
- 14.Lapkin A. A., Plucinski P. K., Cutler M., Comparative assessment of technologies for extraction of artemisinin. J. Nat. Prod. 69, 1653–1664 (2006). [DOI] [PubMed] [Google Scholar]
- 15.Ciriminna R., Lomeli-Rodriguez M., Demma Carà P., Lopez-Sanchez J. A., Pagliaro M., Limonene: A versatile chemical of the bioeconomy. Chem. Commun. (Camb.) 50, 15288–15296 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Lohrasbi M., Pourbafrani M., Niklasson C., Taherzadeh M. J., Process design and economic analysis of a citrus waste biorefinery with biofuels and limonene as products. Bioresour. Technol. 101, 7382–7388 (2010). [DOI] [PubMed] [Google Scholar]
- 17.Filipsson A. F., Bard J., Karlsson S., Concise International Chemical Assessment Document 5: Limonene (World Health Organization, Geneva, Switzerland, 1998). [Google Scholar]
- 18.Voo S. S., Grimes H. D., Lange B. M., Assessing the biosynthetic capabilities of secretory glands in citrus peel. Plant Physiol. 159, 81–94 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohara K., Ujihara T., Endo T., Sato F., Yazaki K., Limonene production in tobacco with Perilla limonene synthase cDNA. J. Exp. Bot. 54, 2635–2642 (2003). [DOI] [PubMed] [Google Scholar]
- 20.Chen X., et al. , The rice terpene synthase gene OsTPS19 functions as an (S)-limonene synthase in planta, and its overexpression leads to enhanced resistance to the blast fungus Magnaporthe oryzae. Plant Biotechnol. J. 16, 1778–1787 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saruul P., Srienc F., Somers D. A., Samac D. A., Production of a biodegradable plastic polymer, poly-β-hydroxybutyrate, in transgenic alfalfa. Crop Sci. 42, 919 (2002). [Google Scholar]
- 22.Petrasovits L. A., Purnell M. P., Nielsen L. K., Brumbley S. M., Production of polyhydroxybutyrate in sugarcane. Plant Biotechnol. J. 5, 162–172 (2007). [DOI] [PubMed] [Google Scholar]
- 23.Somleva M. N., et al. , Production of polyhydroxybutyrate in switchgrass, a value-added co-product in an important lignocellulosic biomass crop. Plant Biotechnol. J. 6, 663–678 (2008). [DOI] [PubMed] [Google Scholar]
- 24.Bohmert-Tatarev K., McAvoy S., Daughtry S., Peoples O. P., Snell K. D., High levels of bioplastic are produced in fertile transplastomic tobacco plants engineered with a synthetic operon for the production of polyhydroxybutyrate. Plant Physiol. 155, 1690–1708 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Backhaus R. A., Rubber formation in plants—A mini review. Isr. J. Bot. 34, 283–293 (1985). [Google Scholar]
- 26.Cornish K., Chapman M. H., Nakayama F. S., Vinyard S. H., Whitehand L. C., Latex quantification in guayule shrub and homogenate. Ind. Crops Prod. 10, 121–136 (1999). [Google Scholar]
- 27.Kang H., Kang M. Y., Han K. H., Identification of natural rubber and characterization of rubber biosynthetic activity in fig tree. Plant Physiol. 123, 1133–1142 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu B., Wang H., Du Z., Li G., Ye H., Metabolic engineering of artemisinin biosynthesis in Artemisia annua L. Plant Cell Rep. 30, 689–694 (2011). [DOI] [PubMed] [Google Scholar]
- 29.Zhang L., et al. , Development of transgenic Artemisia annua (Chinese wormwood) plants with an enhanced content of artemisinin, an effective anti-malarial drug, by hairpin-RNA-mediated gene silencing. Biotechnol. Appl. Biochem. 52, 199–207 (2009). [DOI] [PubMed] [Google Scholar]
- 30.Malhotra K., et al. , Compartmentalized metabolic engineering for artemisinin biosynthesis and effective malaria treatment by oral delivery of plant cells. Mol. Plant 9, 1464–1477 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ikram N. K. B. K., Simonsen H. T., A review of biotechnological artemisinin production in plants. Front. Plant Sci. 8, 1966 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pacifico D., Miselli F., Carboni A., Moschella A., Mandolino G., Time course of cannabinoid accumulation and chemotype development during the growth of Cannabis sativa L. Euphytica 160, 231–240 (2008). [Google Scholar]
- 33.Baral N. R., et al. , Greenhouse gas footprint, water-intensity, and, production cost of bio-based isopentenol as a renewable transportation fuel. ACS Sustain. Chem.& Eng. 7, 15434–15444 (2019). [Google Scholar]
- 34.Shen R., Tao L., Yang B., Techno‐economic analysis of jet‐fuel production from biorefinery waste lignin. Biofuels Bioprod. Biorefin. 13, 486–501 (2019). [Google Scholar]
- 35.Linger J. G., et al. , Lignin valorization through integrated biological funneling and chemical catalysis. Proc. Natl. Acad. Sci. U.S.A. 111, 12013–12018 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nonato R. V., Mantelatto P. E., Rossell C. E., Integrated production of biodegradable plastic, sugar and ethanol. Appl. Microbiol. Biotechnol. 57, 1–5 (2001). [DOI] [PubMed] [Google Scholar]
- 37.Paddon C. J., Keasling J. D., Semi-synthetic artemisinin: A model for the use of synthetic biology in pharmaceutical development. Nat. Rev. Microbiol. 12, 355–367 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Peplow M., Synthetic biology’s first malaria drug meets market resistance. Nature 530, 389–390 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Porter M., Haynes M., Biorenewable Insights Isoprene and Isoprenoids (Nexant Inc., 2016). [Google Scholar]
- 40.Sun C., Theodoropoulos C., Scrutton N. S., Techno-economic assessment of microbial limonene production. Bioresour. Technol. 300, 122666 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi J., Lee S. Y., Process analysis and economic evaluation for Poly(3-hydroxybutyrate) production by fermentation. Bioprocess Eng. 17, 335–342 (1997). [Google Scholar]
- 42.Listewnik H. F., Wendlandt K. D., Jechorek M., Mirschel G., Process design for the microbial synthesis of poly-β-hydroxybutyrate (PHB) from natural gas. Eng. Life Sci. 7, 278–282 (2007). [Google Scholar]
- 43.Mudliar S. N., Vaidya A. N., Suresh Kumar M., Dahikar S., Chakrabarti T., Techno-economic evaluation of PHB production from activated sludge. Clean Technol. Environ. Policy 10, 255–262 (2008). [Google Scholar]
- 44.Dolgin E., The bioengineering of cannabis. Nature 572, S5–S7 (2019). [Google Scholar]
- 45.Cui X., Kavvada O., Huntington T., Scown C. D., Strategies for near-term scale-up of cellulosic biofuel production using sorghum and crop residues in the U.S. Environ. Res. Lett. 13, 124002 (2018). [Google Scholar]
- 46.Sundstrom E., et al. , Demonstrating a separation-free process coupling ionic liquid pretreatment, saccharification, and fermentation with Rhodosporidium toruloides to produce advanced biofuels. Green Chem. 20, 2870–2879 (2018). [Google Scholar]
- 47.Mewalal R., et al. , Plant-derived terpenes: A feedstock for specialty biofuels. Trends Biotechnol. 35, 227–240 (2017). [DOI] [PubMed] [Google Scholar]
- 48.Chuck C., Donnelly J., The compatibility of potential bioderived fuels with Jet A-1 aviation kerosene. Appl. Energy 118, 83–91 (2014). [Google Scholar]
- 49.Wang Y., et al. , Synthesis of medium-chain-length-polyhydroxyalkanoates in tobacco via chloroplast ge-netic engineering. Chin. Sci. Bull. 50, 1113–1120 (2005). [Google Scholar]
- 50.Anderson A. J., Dawes E. A., Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol. Rev. 54, 450–472 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.National Conference of State Legislatures , State industrial hemp statutes (2019). https://www.ncsl.org/research/agriculture-and-rural-development/state-industrial-hemp-statutes.aspx. Accessed 31 July 2019.
- 52.Laere E., et al. , Plant-based vaccines: Production and challenges. J. Bot. 2016, 1–11 (2016). [Google Scholar]
- 53.Buranov A. U., Elmuradov B. J., Extraction and characterization of latex and natural rubber from rubber-bearing plants. J. Agric. Food Chem. 58, 734–743 (2010). [DOI] [PubMed] [Google Scholar]
- 54.Aramvash A., Gholami-Banadkuki N., Moazzeni-Zavareh F., Hajizadeh-Turchi S., An environmentally friendly and efficient method for extraction of PHB biopolymer with non-halogenated solvents. J. Microbiol. Biotechnol. 25, 1936–1943 (2015). [DOI] [PubMed] [Google Scholar]
- 55.Wikandari R., Nguyen H., Millati R., Niklasson C., Taherzadeh M. J., Improvement of biogas production from orange peel waste by leaching of limonene. BioMed Res. Int. 2015, 494182 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheng M.-H., Rosentrater K. A., Economic feasibility analysis of soybean oil production by hexane extraction. Ind. Crops Prod. 108, 775–785 (2017). [Google Scholar]
- 57.U.S. EPA , Chloroform. https://www.epa.gov/sites/production/files/2016-09/documents/chloroform.pdf. Accessed 29 July 2019.
- 58.Wilbanks B., Trinh C. T., Comprehensive characterization of toxicity of fermentative metabolites on microbial growth. Biotechnol. Biofuels 10, 262 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ambach L., et al. , Simultaneous quantification of delta-9-THC, THC-acid A, CBN and CBD in seized drugs using HPLC-DAD. Forensic Sci. Int. 243, 107–111 (2014). [DOI] [PubMed] [Google Scholar]
- 60.Brandt C. C., et al. , 2016 Billion-Ton Report: Advancing Domestic Resources for a Thriving Bioeconomy, Volume 1: Economic Availability of Feedstocks (Oak Ridge National Laboratory, Oak Ridge, TN, 2016). [Google Scholar]
- 61.Carcer G. A. D., Murphy S., Kagia J., Ooyen C., McCoy J. J., “The CBD report 2018 industry outlook” (New Frontier Data, 2018). [Google Scholar]
- 62.Markl E., Grünbichler H., Lackner M., PHB - Bio Based and Biodegradable Replacement for PP: A Review. Nov. Tech. Nutr. Food Sci. 2, 206–209 (2018). [Google Scholar]
- 63.Choi I. S., Kim J.-H., Wi S. G., Kim K. H., Bae H.-J., Bioethanol production from Mandarin (Citrus unshiu) peel waste using popping pretreatment. Appl. Energy 102, 204–210 (2013). [Google Scholar]
- 64.Carcer G. A. D., Murphy S., Kagia J., Ooyen C., McCoy J. J., “The CBD report 2018 industry outlook” (New Frontier Data, 2018) Accessed 25 June 2019.
- 65.Grand View Research , Artemisinin combination therapy market size, share & trends analysis report by type (Artemether+Lumefantrine, Artesunate+Amodiaquine), by region, and segment forecasts, 2018–2025. https://www.grandviewresearch.com/industry-analysis/artemisinin-combination-therapy-act-market. Accessed 25 June 2019.
- 66.Transparency Market Research , Global styrene butadiene latex market to reach US$9,120.3 mn rising at CAGR of 3.3% by 2023 owing to extensive research and development—TMR. https://www.globenewswire.com/news-release/2018/09/06/1566257/0/en/Global-Styrene-Butadiene-Latex-Market-to-reach-US-9-120-3-mn-Rising-at-CAGR-of-3-3-by-2023-Owing-to-Extensive-Research-and-Development-TMR.html. Accessed 25 June 2019.
- 67.Markets and Markets , Polypropylene Market Analysis, Recent Market Developments, Industry Forecast to 2017-2022. https://www.marketsandmarkets.com/Market-Reports/polypropylene-market-64103589.html?gclid=CjwKCAiAlO7uBRANEiwA_vXQ-82x4wMmadcIXs2S3rGU-FHb1LMadasMuIO-_ZudKLAUelWtuHpNOBoCecYQAvD_BwE. Accessed 25 November 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data discussed in the paper are published in SI Appendix.