Significance
Stimulation of lateral hypothalamic (LH) neurons produces eating in sated animals and increases activity of dopamine neurons. The present study shows that the activation of dopamine neurons failed to induce eating. Rather, food approach and eating were observed via activation of LH fibers that project through the VTA, continuing caudally and terminating in a brainstem region medial to the locus coeruleus (LC). We found that activation of GABA neurons in this peri-LC region is both necessary and sufficient for LH stimulation-induced eating, whereas their role in normal homeostatic feeding appears negligible. These findings suggest that this circuit orchestrates just one of the multiple aspects of eating: a compulsive consumption of food in the absence of a physiological stimulus of hunger.
Keywords: eating, reward, LH, VTA, LC
Abstract
Electrical or optogenetic stimulation of lateral hypothalamic (LH) GABA neurons induces rapid vigorous eating in sated animals. The dopamine system has been implicated in the regulation of feeding. Previous work has suggested that a subset of LH GABA neurons projects to the ventral tegmental area (VTA) and targets GABA neurons, inhibiting them and thereby disinhibiting dopaminergic activity and release. Furthermore, stimulation-induced eating is attenuated by dopamine lesions or receptor antagonists. Here we explored the involvement of dopamine in LH stimulation-induced eating. LH stimulation caused sated mice to pick up pellets of standard chow with latencies that varied based on stimulation intensity; once food was picked up, animals ate for the remainder of the 60-s stimulation period. However, lesion of VTA GABA neurons failed to disrupt this effect. Moreover, direct stimulation of VTA or substantia nigra dopamine cell bodies failed to induce food approach or eating. Looking further, we found that some LH GABA fibers pass through the VTA to more caudal sites, where they synapse onto neurons near the locus coeruleus (LC). Similar eating was induced by stimulation of LH GABA terminals or GABA cell bodies in this peri-LC region. Lesion of peri-LC GABA neurons blocked LH stimulation-induced eating, establishing them as a critical downstream circuit element for LH neurons. Surprisingly, lesions did not alter body weight, suggesting that this system is not involved in the hunger or satiety mechanisms that govern normal feeding. Thus, we present a characterization of brain circuitry that may promote overeating and contribute to obesity.
The initiation of eating is a basic behavior crucial for survival. It can be induced in sated animals by prolonged stimulation of agouti-related peptide (AgRP) neurons in the arcuate nucleus of the hypothalamus (1, 2). These neurons act to regulate feeding in a homeostatic manner, increasing their activity during fasting (3) and inhibiting it throughout presentation and consumption of food (4, 5). In contrast to the slow and homeostatic action of AgRP neurons, a distinct group of GABA neurons in the perifornical region of the LH can control eating on a very rapid time scale. During the daytime, sated animals approach food within seconds upon electrical (6, 7) or optogenetic stimulation (8–12) of LH neurons. This eating response is time locked: it initiates and ceases immediately after the stimulation (11, 13).
Dopamine is widely implicated in the control of feeding. Widespread disruption of dopamine function through chemical lesion or genetic knockout causes severe aphagia (14–18). Temporally precise inhibition of VTA dopamine cell firing through optogenetic stimulation of VTA GABA neurons disrupts licking for sucrose solutions in hungry mice (19). Similarly, stimulation-induced eating in sated animals is attenuated by dopamine receptor antagonists at low doses that preserve locomotor function (20, 21). Although dopamine is clearly necessary for the initiation of eating, its ability to promote eating in the absence of physiological need is unclear. Although amphetamine administration can cause weight loss (22), at lower doses it enhances sensitivity of LH stimulation-induced eating (23). Indeed, intra-VTA infusion of ghrelin or morphine at doses that increase dopamine release in terminal sites stimulates eating in sated animals (24–26).
Recent studies have shown that LH GABA neurons preferentially synapse onto VTA GABA neurons (9). Activation of GABAergic LH-VTA projections disinhibits VTA dopamine neurons, increasing dopamine release in terminal regions (10). Thus, it has been proposed that LH stimulation may elicit compulsive eating through the disinhibition of dopamine neurons (10, 27). A requirement of this model is that selective activation of midbrain dopamine neurons should be sufficient to induce eating in sated animals. Surprisingly, this has not been tested yet, using modern optogenetic tools. The initial aim of this study was to confirm this hypothesis using optogenetic stimulation in mice causally. Surprisingly, we found that activation of VTA or SNc dopamine neurons was not responsible for the vigorous eating observed during LH stimulation. Quite the opposite: Our data suggest that eating elicited by stimulation of LH neurons is mediated by a projection that passes through the VTA, continuing caudally to terminate in a brainstem structure adjacent to the locus coeruleus (LC).
Results
Direct Stimulation of Midbrain Dopamine Neurons Is Rewarding but Does Not Induce Food Approach or Eating.
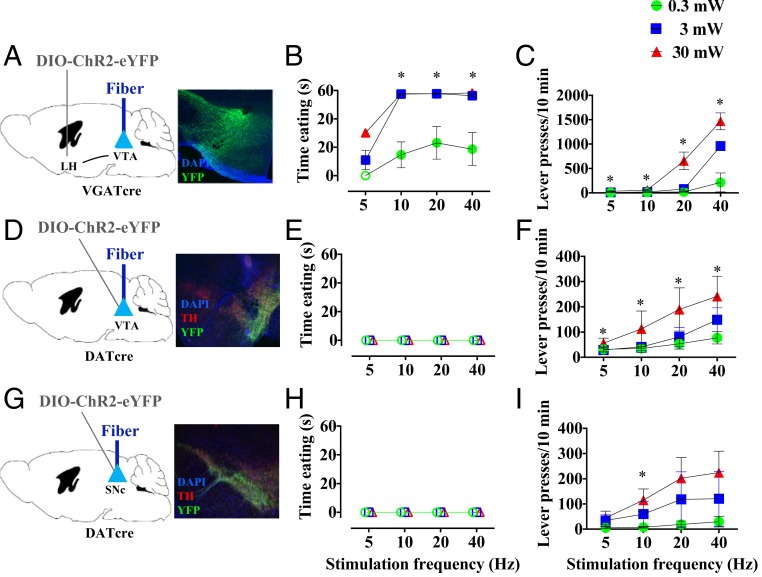
In our first set of experiments (Fig. 1), we unilaterally targeted Channelrhodopsin2 (ChR2) stimulation to LH GABA projections in the VTA (the circuit presumed to drive the behavior) or VTA or SNc dopamine cell bodies (hypothesized target neurons). During stimulation, we measured initiation of “eating,” which we define operationally to include picking up, chewing, and swallowing of food. Animals were sated at the time of testing, such that no animal ate during the 60-s intervals between stimulation periods. Stimulation of LH-VTA GABA projections produced robust and rapid eating (Fig. 1 A and B), whereas mice with stimulation of VTA (Fig. 1 D and E) or SNc (Fig. 1 G and H) neurons failed to eat food within the 60-s stimulation periods under all stimulation conditions tested (main effect of brain region, F2,12 = 14.49; P < 0.0001). Thus, increased activity of dopamine neurons does not seem capable of underlying the rapid eating seen with LH stimulation. In the LH animals, when each animal picked up a food pellet, it immediately began chewing it, swallowing much of what was chewed for the remainder of each 60-s period of stimulation. We thus had two measures of relevance: latency to pick up food and time of eating the food. Because no other activity such as grooming or locomotion intervened once eating was initiated, the sum of these measures always equaled 60. Faster approach to food was associated with increased stimulation frequency (significant brain region × frequency interaction; F2, 156 = 7.83; P = 0.0006); post hoc tests indicated significant effects of LH stimulation at 10, 20, or 40 Hz, using 3 or 30 mW laser power. These frequency effects are consistent with previous reports (9, 11, 13; but see ref. 12). Direct optogenetic manipulations of VTA GABA and glutamate cell bodies also failed to elicit feeding in sated animals (SI Appendix, Fig. S2).
Fig. 1.
Optogenetic stimulation of VTA or SNc cell bodies produces reward but does not replicate eating effects of LH-VTA pathway stimulation. (A) VGAT LH-VTA circuit targeting. eYFP-expressing (green) fibers in VTA tissue (magnification: 10×). (B) Photostimulation of VGAT LH-VTA projection significantly produces robust eating in sated animals. (C) VGAT LH-VTA-ChR2 mice support self-stimulation. (D) DAT VTA-ChR2-eYFP (green) and TH staining (red) (magnification: 10×). (E) Photostimulation of DAT VTA does not produce eating in sated animals. (F) DAT VTA:ChR2 mice support self-stimulation. (G) DAT SNc-ChR2-eYFP (green) and TH staining (red) (magnification: 10×). (H) Photostimulation of DAT SNc does not produce eating in sated animals. (I) DAT VTA:ChR2 mice support self-stimulation. *P < 0.05 post hoc comparison vs. null value (B, E, and H) or inactive lever (C, F, and I) Open shapes in eating (B, E, and H) indicate conditions in which no eating was observed.
To confirm the efficacy of optogenetic dopamine stimulation, we tested animals for self-stimulation, which is well-established as being mediated by midbrain dopamine system activation (28–30); each mouse pressed a lever to obtain a half-second photostimulation. All three brain regions strongly supported self-stimulation (Fig. 1 C, F, and I), with a significant brain region × frequency × power interaction (F2, 156 = 17.27; P < 0.0001). Post hoc comparisons of active versus inactive lever presses showed significant rewarding effects with 0.3 mW LH stimulation at 5 or 10 Hz; 3 mW stimulation at 5, 10, or 40 Hz; and 30 mW stimulation at all frequencies tested. VTA lever presses were significantly affected with 0.3 mW stimulation at all four frequencies, 3 mW stimulation at 10 or 20 Hz, and 30 mW stimulation at 5, 20, or 40 Hz. SNc lever presses were significantly affected only with 30 mW and 10 Hz. Lever pressing for LH-VTA stimulation was substantially higher in part because the rate of presses increased within the timeout (SI Appendix, Fig. S1A). Moreover, the higher number of presses was not a result of an increased nonspecific locomotor activity (SI Appendix, Fig. S1B). On the contrary, the stimulation of LH-VTA caused a reduction in locomotion in a frequency and laser power-dependent manner (SI Appendix, Fig. S1C). Taken together, these experiments suggest that effective stimulation of midbrain dopamine neurons is not sufficient to rapidly induce eating in sated animals.
To exclude the hypothesis that stimulation of the LH GABA system was simply overcoming the inhibitory effects of anxiety caused by lighting, we tested wild-type animals for eating under different conditions: fasted or sated and under white light or in darkness. Fasted mice ate with shorter latency than sated mice, but lighting intensity did not significantly affect eating latency in our testing paradigm (SI Appendix, Fig. S3).
Eating Is Induced by LH GABA Projections that Pass through the VTA and Terminate Near the LC.
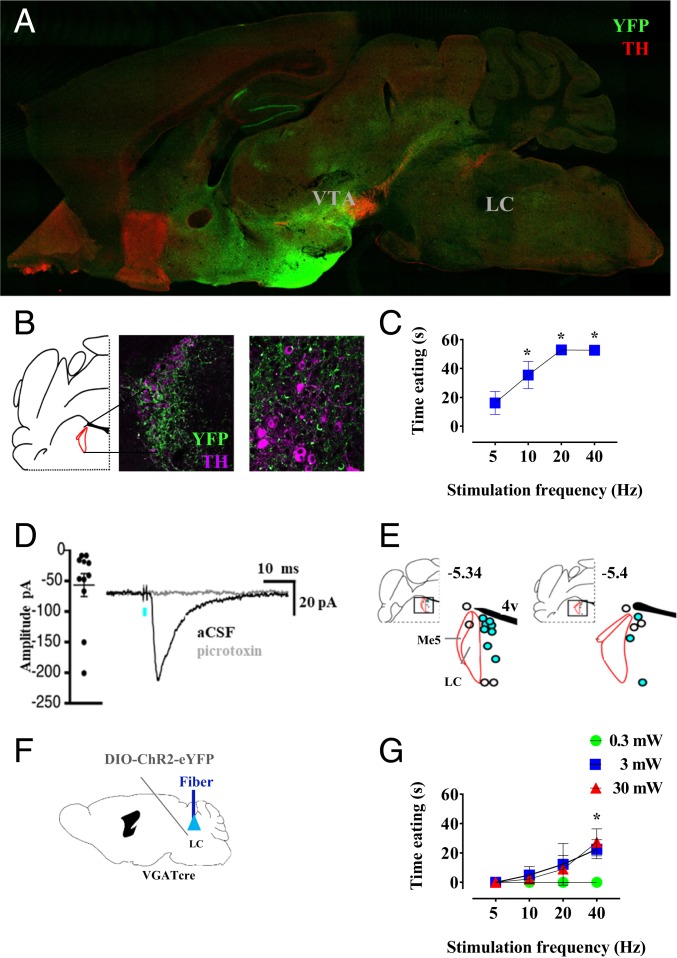
The preceding results led us to hypothesize that stimulation of LH-VTA GABA fibers may cause eating by activating fibers of passage continuing through the VTA to more caudal sites. Thus, we injected mice with DIO-ChR2-YFP, as before, and sagittally cut brains to visualize more thoroughly the distribution of the LH GABA fiber pathways (Fig. 2A). We found strong rostral projections anterior to the bed nucleus of the stria terminalis, dentate gyrus, and lateral habenula. The caudal projections went to and through the VTA and rostromedial tegmental nucleus (RMTg), continuing to the periaqueductal gray, with further posterior projections splitting dorsally toward the LC, and ventrally toward the dorsal inferior olive. Closer inspection of LC tissue, however, indicated that YFP-expressing axons are distributed around the LC (Fig. 2B and SI Appendix, Fig. S4B), a region we refer to here as peri-LC.
Fig. 2.
LH GABA neurons project caudally to the locus coeruleus, where terminal stimulation elicits eating and evokes GABA receptor-mediated currents in nonnoradrenergic neurons. (A) Sagittal representative image of eYFP-expressing (green) fibers projecting from LH and TH staining (red). (B) Representative coronal section showing fluorescence of LH terminals in peri-LC and fiber placement. (C) Photostimulation of VGAT LH-peri-LC-ChR2 produces eating. (D) Photostimulation of LH-peri-LC produces GABA-A synaptic current blocked by picrotoxin. Inward currents were measured from Vhold = −88 mV. (E) Synaptically connected cells (blue) were all located medial to the LC. (F and G) Photostimulation of VGAT LC-ChR2-eYFP produces eating. *P < 0.05 post hoc comparison vs. null value.
To test the ability of these various LH GABA projections to induce eating, we injected mice bilaterally with ChR2 virus and implanted three to four optical fibers per mouse, each targeting different putative terminal regions as identified earlier. Although some animals tested showed additional fluorescence lateral to the peri-LC in the parabrachial nucleus, electrical stimulation medial to the LC, but not in the more lateral parabrachial nucleus, induces eating (31); therefore, we focused efforts at this level of the pons to peri-LC. Because increased laser power causes light to spread throughout more brain tissue (32), our ability to draw anatomical conclusions is dependent on selecting appropriate laser power. Although we tested multiple laser powers as in previous experiments, we limited the analysis to tests carried out at 3 mW, which we found to be the minimum power sufficient to drive behavioral effects reliably (Fig. 1 and SI Appendix, Fig. S2). As expected, stimulation of VTA and RMTg projections produced eating (SI Appendix, Fig. S4A); importantly, stimulation of peri-LC terminals also produced eating (Fig. 2C), whereas none of the forebrain regions did so (significant main effects of brain region; F7,17 = 30.11; P < 0.0001). Post hoc tests indicated significant eating after stimulation of VTA, RMTg, and peri-LC tissue at 10, 20, and 40 Hz. Notably, stimulation of the peri-LC fibers produced eating with latencies similar to those produced by fibers in the VTA. Although periaqueductal gray stimulation produced trends for effects, they did not reach statistical significance (SI Appendix, Fig. S4A). Interestingly, it was recently shown that stimulation of ascending GABAergic projections from the LH to the diagonal band of Broca could elicit eating in sated mice (33). We did not observe significant fluorescence in this region in our animals, which may be a consequence of exact LH injection coordinates. Furthermore, in sated VGAT mice, we stimulated the LH-peri-LC projection for 2 consecutive hours (SI Appendix, Fig. S9). Our results show that the mice approached the food immediately after the laser was turned on, and continued without interruption for the duration of the stimulation. This behavior disappeared right after the end of the light delivery.
To test for synaptic connectivity of LH GABA neurons with cells in the peri-LC region, we performed whole-cell patch clamp electrophysiology in mice (Fig. 2D and SI Appendix, Figs. S5 and S8). Stimulation of the GABA LH-peri LC pathway in brain slices using 1-ms pulses of 473-nm light failed to evoke synaptic currents in all 9 noradrenergic neurons tested within the LC (SI Appendix, Fig. S5A), identified by responding to the alpha 2-adrengerigc receptor agonist UK-14304 (SI Appendix, Fig. S5A). In contrast, recordings from nonnoradrenergic neurons surrounding the LC identified fast synaptic inhibitory currents evoked by LH pathway stimulation in 11 of 18 neurons, which were blocked by the GABAA receptor antagonist picrotoxin (Fig. 2D and SI Appendix, Fig. S8). Recorded neurons were filled with neurobiotin to determine their location post hoc. All synaptically coupled neurons were all found medial to the LC (Fig. 2E). In addition, trains of stimulation (5 pulses at 60 Hz) produced slow inhibitory postsynaptic currents in five of six neurons, which were blocked by the GABAB receptor antagonist CGP55845 (SI Appendix, Fig. S5B). Thus, GABA inputs from the LH innervate a subset of nonnoradrenergic neurons just medial to the LC; activation of this pathway in vivo is sufficient to induce eating in sated mice. Furthermore, as this projection is likely to pass through the medial forebrain bundle, photostimulation of the VTA in mice expressing ChR2 throughout axons of LH neurons would be expected to stimulate this pathway as well.
Given our findings that direct optogenetic control of VTA neuronal populations did not induce eating (Fig. 1), we reasoned that it was important to test our putative brain region, peri-LC, for the ability to directly evoke eating behavior. We tested this by optogenetically stimulating or inhibiting GABAergic, glutamatergic, or noradrenergic cell bodies in and around LC tissue. Eating was significantly induced by stimulation of GABAergic cells (Fig. 2 F and G), main effect of cell type (F2,10 = 32.12; P < 0.001). Post hoc analysis showed significant eating responses at 40 Hz stimulation with 3 and 30 mW laser power. Stimulation or inhibition of glutamatergic or noradrenergic cells in this region failed to induce eating (SI Appendix, Fig. S6).
GABAergic peri-LC Cell Bodies Are Necessary and Sufficient for Stimulation-Induced Eating.
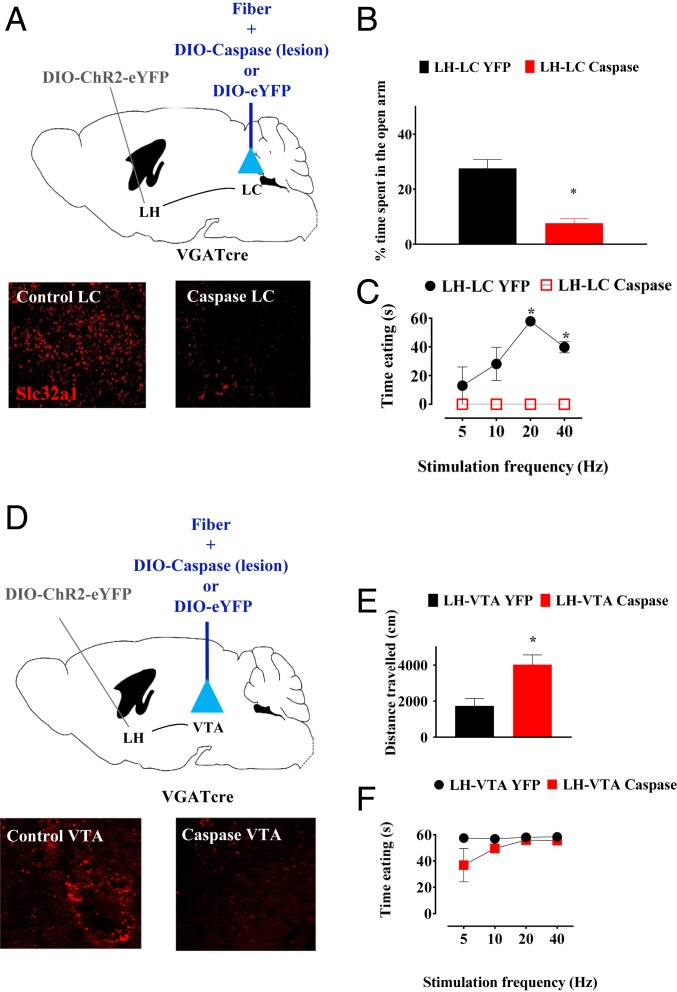
Our data thus far support the hypothesis that GABAergic peri-LC neurons are a critical downstream target of LH GABA neurons in mediating stimulation-induced eating. This is in contrast with previously published suggestions that VTA GABA neurons are the critical downstream circuit element (10, 27). To directly test these hypotheses, we conducted two sequential lesion experiments to test the necessity of peri-LC (Fig. 3A) or VTA (Fig. 3D) GABA neurons in LH stimulation-induced eating. Lesion of GABA neurons in target tissue was accomplished with bilateral injection of a virus encoding Cre-inducible caspase (34), with YFP as a control. Mice were also injected with DIO-ChR2-eYFP unilaterally in the LH and had a permanent fiber optic implant above target tissue. Mice with lesion of peri-LC GABA neurons (n = 4) showed 88.4 ± 2.05% decrease in number of neurons in the peri-LC expressing Slc32a1, the mRNA that encodes VGAT (t = 5.134; df = 6; P = 0.0021). Strikingly, mice with lesion of peri-LC GABA neurons completely failed to eat in response to LH stimulation (F1, 6 = 98.16, P < 0.0001; Fig. 3C). Post hoc comparisons of 3-mW stimulation trials showed significant differences between caspase and YFP control at 20 and 40 Hz. Lesions did not significantly affect body weights of mice with chronic ad libitum access to chow (SI Appendix, Fig. S7), indicating that the loss of stimulation-induced eating was not a result of general impairments in the ability to consume food. In contrast, mice with lesion of VTA GABA neurons responded to LH stimulation by eating with latencies similar to YFP controls (F1,6 =3.58; P = 0.11; Fig. 3F). Mice with lesions of VTA GABA neurons (n = 4) showed 74.64 ± 3.12% decrease in number of neurons expressing Slc32a1 within the VTA when compared with the controls (t = 13.3; df = 6; P < 0.0001). Interestingly, lesioned mice in both experiments showed other gross behavioral differences to controls even in the absence of laser, suggesting effectiveness of lesion. Peri-LC lesioned mice showed greater anxiety-like behavior in the elevated plus maze (Fig. 3B; t = 3.35; df = 21; P < 0.0001). VTA lesioned mice were hyperlocomotive (t = 3.35; df = 6; P = 0.0154; Fig. 3E) These data indicate that peri-LC GABA neurons are a critical downstream step in the LH circuitry underlying stimulation-induced eating, whereas VTA GABA neurons are not.
Fig. 3.
Lesion of peri-LC GABA neurons inhibits eating elicited by LH-LC stimulation, while lesion of VTA GABA neurons does not have an effect on the latency to eat evoked by LH-VTA stimulation. (A) VGAT LH-ChR2/LC-cre–inducible caspase/YFP circuit target. Representative image of Slc32a1 expression (red) (magnification: 10×). (B) Cre-inducible lesion of peri-LC GABA neurons results in a significant decrease of time spent in the open arm of an elevated plus-maze. (C) Photostimulation VGAT LH-ChR2/LC-cre–inducible caspase does not produce eating, with a significant effect of the Caspase virus. Open shapes indicate conditions in which no eating was observed. (D) VGAT LH-ChR2/VTA-cre–inducible caspase/YFP circuit target. Representative image of Slc32a1 expression (red) (magnification: 10×). (E) Cre-inducible lesion of VTA GABA neurons increases basal locomotor activity. (F) Photostimulation VGAT LH-ChR2/VTA-cre–inducible caspase produces eating without any significative difference when compared with the VGAT LH-ChR2/VTA-YFP control. *P < 0.05 t test (B and E) or post hoc comparison of YFP control vs. caspase lesion (C).
Discussion
In this study, we explored the brain circuitry involved in the compulsive eating that can be induced by electrical (13) or optogenetic (8, 9) stimulation of GABAergic cells of the lateral hypothalamus. Such stimulation promotes the rapid approach to food and causes eating in physiologically sated rodents. A variety of literature had implicated dopamine in this phenomenon; however, our study shows that LH projections to the VTA are not involved in the induced eating, and that the critical fibers project through the VTA and synapse on a nonnoradrenergic target medial to locus coeruleus. We found these peri-LC neurons to be both necessary and sufficient for LH stimulation-induced eating; in contrast, cells in the VTA fulfilled neither of these conditions. Interestingly, lesions of the peri-LC region did not alter body weight, suggesting that this system orchestrates just one of the multiple aspects of eating: a compulsive consumption of food in absence of a physiological stimulus of hunger. Indeed, the eating induced by LH or peri-LC stimulation occurs despite the hormonal controls that inhibit eating during periods of satiety. Two findings underscore this inference. First, experienced animals ate during the period of stimulation. Second, the animals ceased eating as soon as stimulation terminated and never ate in the absence of stimulation.
Stimulation of the LH elicits both eating and reward, and several lines of evidence from electrical stimulation studies suggest that these distinct behavioral effects are driven by a single neurobiological substrate. The boundaries of LH tissue (35) and the refractory periods (36) of corresponding fibers supporting the two behaviors are the same. Because LH stimulation increases dopamine release (10, 30), and each behavior is attenuated by dopamine antagonists (20, 21, 37), it seemed reasonable that this was a common mechanistic element driving both responses. Indeed, early in vivo optogenetic studies have demonstrated convincingly that stimulation of midbrain dopamine neurons is sufficient for reward. No published study to date had tested the ability of optogenetic stimulation of dopamine cell bodies to elicit eating, and we were surprised to find that it did not. The critical target of LH GABA neurons in our study appears to be neurons medial to the LC. Future studies of the heterogeneity of the peri-LC GABA population with regard to their inputs, outputs, and microcircuitry are needed to increase our understanding of their functional contribution to eating.
Eating can be induced by stimulation of multiple hypothalamic groups, including LH GABA neurons and AgRP neurons of the arcuate nucleus. Their regulation of eating differs in several regards. The eating elicited by LH stimulation begins within seconds of stimulation onset and terminates immediately on its cessation. In contrast, eating does not begin until after several minutes of AgRP neuron stimulation (38, 39) and can continue for tens of minutes after stimulation has ceased (38, 39). In our experiments, we observed similar behavior when we stimulated a small population of gabaergic neurons in the arcuate nucleus (SI Appendix, Fig. S9), which would be expected to include AgRP neurons, which coexpress VGAT (40).
A second major difference between these cell populations is their role in regulating normal feeding, controlled by physiological signals for hunger and satiety. Destruction of arcuate AgRP neurons in adult mice causes total starvation (41), indicating an essential role in normal feeding. In the LH, nonspecific lesion in rats (7) or genetically targeted lesion of GABA neurons in mice (8) reduce normal food intake and cause weight loss, but not death. Interestingly, we found that one target of LH GABA neurons in the peri-LC are sufficient to induce eating, but not necessary for normal feeding. This suggests that there exist multiple subpopulations of LH neurons that control feeding in different ways, an idea that is reinforced by heterogeneity of LH GABA cell population observed in other recent studies (8, 9).
In conclusion, we provide data showing that the hypothalamus is capable of driving compulsive eating through direct projections to a brainstem structure medial to the LC, and fails to drive eating through interaction with VTA GABA or dopamine neurons. Future studies are necessary to further understand the role of peri-LC neurons in normal eating and their potential role in governing compulsive food and perhaps drug seeking.
Materials and Methods
Subjects.
All procedures were conducted in accordance with the NIH Animal Care and Use Committee guidelines (42). Experiments involved male and female adult mice (10 to 20 wk old at the time of surgery) maintained under a 12-h light/dark cycle with food and water available ad libitum. Animals were group housed before surgery and single-housed after surgery. Complete information are provided in SI Appendix, Experimental Procedures.
Surgical Procedures.
Mice were anesthetized using ketamine and xylazine, surgically injected with viral vectors, and implanted with fiber optic cables. Complete microinjection methods are described in SI Appendix, Experimental Procedures.
Behavioral Testing.
For all behavioral experiments, mice were moved from the vivarium to the behavioral facility and allowed to acclimate in a holding area for 30 min before the start of the test. Complete behavioral test experiments are described in SI Appendix, Experimental Procedures.
Histology.
After behavioral testing, all mice were deeply anesthetized and transcardially perfused with 4% paraformaldehyde in PBS at pH 7.4. For immunohistochemistry, fixed brains were sectioned on a vibratome at 40 μm. Complete histology methods are described in SI Appendix, Experimental Procedures.
Electrophysiology.
Mice were deeply anesthetized with isoflurane and killed by decapitation. Brains were quickly removed and placed in warmed (30 °C) modified Krebs buffer. Complete methods are described in SI Appendix, Experimental Procedures.
Statistical Analysis.
Complete information are described in SI Appendix, Experimental Procedures.
Data Availability Statement.
All data discussed in the paper are available in Mendeley Data (43).
Supplementary Material
Acknowledgments
We thank the National Institute on Drug Abuse (NIDA) breeding staff for breeding the transgenic animals, Hugo Tejeda and Leonardo Bontempi at NIDA Intramural Research Program (IRP), and Dennis R. Sparta and Sonia Aroni at the University of Maryland for their critical reading of the manuscript. This research was supported by the NIDA Intramural Research Program.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: Behavioral and electrophysiology data are available in Mendeley Data (https://doi.org/10.17632/wrk3krr9hv.1).
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1909340117/-/DCSupplemental.
References
- 1.Aponte Y., Atasoy D., Sternson S. M., AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat. Neurosci. 14, 351–355 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atasoy D., Betley J. N., Su H. H., Sternson S. M., Deconstruction of a neural circuit for hunger. Nature 488, 172–177 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kong D., et al. , A postsynaptic AMPK→p21-Activated kinase pathway drives fasting-induced synaptic plasticity in AgRP neurons. Neuron 91, 25–33 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burnett C. J., et al. , Hunger-driven motivational state competition. Neuron 92, 187–201 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Su Z., Alhadeff A. L., Betley J. N., Nutritive, post-ingestive signals are the primary regulators of AgRP neuron activity. Cell Rep. 21, 2724–2736 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morgane P. J., Evidence of a ‘hunger motivational’ system in the lateral hypothalamus of the rat. Nature 191, 672–674 (1961). [DOI] [PubMed] [Google Scholar]
- 7.Hoebel B. G., Teitelbaum P., Hypothalamic control of feeding and self-stimulation. Science 135, 375–377 (1962). [DOI] [PubMed] [Google Scholar]
- 8.Jennings J. H., et al. , Visualizing hypothalamic network dynamics for appetitive and consummatory behaviors. Cell 160, 516–527 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nieh E. H., et al. , Decoding neural circuits that control compulsive sucrose seeking. Cell 160, 528–541 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nieh E. H., et al. , Inhibitory input from the lateral hypothalamus to the ventral tegmental area disinhibits dopamine neurons and promotes behavioral activation. Neuron 90, 1286–1298 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gigante E. D., Benaliouad F., Zamora-Olivencia V., Wise R. A., Optogenetic activation of a lateral hypothalamic-ventral tegmental drive-reward pathway. PLoS One 11, e0158885 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barbano M. F., Wang H. L., Morales M., Wise R. A., Feeding and reward are differentially induced by activating GABAergic lateral hypothalamic projections to VTA. J. Neurosci. 36, 2975–2985 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wise R. A., Lateral hypothalamic electrical stimulation: Does it make animals ‘hungry’? Brain Res. 67, 187–209 (1974). [DOI] [PubMed] [Google Scholar]
- 14.Ungerstedt U., Adipsia and aphagia after 6-hydroxydopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiol. Scand. Suppl. 367, 95–122 (1971). [DOI] [PubMed] [Google Scholar]
- 15.Zigmond M. J., Stricker E. M., Recovery of feeding and drinking by rats after intraventricular 6-hydroxydopamine or lateral hypothalamic lesions. Science 182, 717–720 (1973). [DOI] [PubMed] [Google Scholar]
- 16.Fibiger H. C., Zis A. P., McGeer E. G., Feeding and drinking deficits after 6-hydroxydopamine administration in the rat: Similarities to the lateral hypothalamic syndrome. Brain Res. 55, 135–148 (1973). [DOI] [PubMed] [Google Scholar]
- 17.Zhou Q. Y., Palmiter R. D., Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell 83, 1197–1209 (1995). [DOI] [PubMed] [Google Scholar]
- 18.Cannon C. M., Palmiter R. D., Reward without dopamine. J. Neurosci. 23, 10827–10831 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Zessen R., Phillips J. L., Budygin E. A., Stuber G. D., Activation of VTA GABA neurons disrupts reward consumption. Neuron 73, 1184–1194 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phillips A. G., Nikaido R. S., Disruption of brain stimulation-induced feeding by dopamine receptor blockade. Nature 258, 750–751 (1975). [DOI] [PubMed] [Google Scholar]
- 21.Streather A., Bozarth M. A., Effect of dopamine-receptor blockade on stimulation-induced feeding. Pharmacol. Biochem. Behav. 27, 521–524 (1987). [DOI] [PubMed] [Google Scholar]
- 22.Dobrzanski S., Doggett N. S., On the relation between hypodipsia and anorexia induced by (+)-amphetamine in the mouse. J. Pharm. Pharmacol. 28, 922–924 (1976). [DOI] [PubMed] [Google Scholar]
- 23.Colle L., Wise R. A., Concurrent facilitory and inhibitory effects of amphetamine on stimulation-induced eating. Brain Res. 459, 356–360 (1988). [DOI] [PubMed] [Google Scholar]
- 24.Mucha R. F., Iversen S. D., Increased food intake after opioid microinjections into nucleus accumbens and ventral tegmental area of rat. Brain Res. 397, 214–224 (1986). [DOI] [PubMed] [Google Scholar]
- 25.Naleid A. M., Grace M. K., Cummings D. E., Levine A. S., Ghrelin induces feeding in the mesolimbic reward pathway between the ventral tegmental area and the nucleus accumbens. Peptides 26, 2274–2279 (2005). [DOI] [PubMed] [Google Scholar]
- 26.Abizaid A., et al. , Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J. Clin. Invest. 116, 3229–3239 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stuber G. D., Wise R. A., Lateral hypothalamic circuits for feeding and reward. Nat. Neurosci. 19, 198–205 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsai H. C., et al. , Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science 324, 1080–1084 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wise R. A., McDevitt R. A., Drive and reinforcement circuitry in the brain: Origins, neurotransmitters, and projection fields. Neuropsychopharmacology 43, 680–689 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Witten I. B., et al. , Recombinase-driver rat lines: Tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron 72, 721–733 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Micco D. J., Jr, Complex behaviors elicited by stimulation of the dorsal pontine tegmentum in rats. Brain Res. 75, 172–176 (1974). [DOI] [PubMed] [Google Scholar]
- 32.Yizhar O., Fenno L. E., Davidson T. J., Mogri M., Deisseroth K., Optogenetics in neural systems. Neuron 71, 9–34 (2011). [DOI] [PubMed] [Google Scholar]
- 33.Cassidy R. M., et al. , A lateral hypothalamus to basal forebrain neurocircuit promotes eating by suppressing responses to anxiogenic environmental cues. Sci. Adv. 5, eaav1640 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang C. F., et al. , Sexually dimorphic neurons in the ventromedial hypothalamus govern mating in both sexes and aggression in males. Cell 153, 896–909 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Margules D. L., Olds J., Identical “feeding” and “rewarding” systems in the lateral hypothalamus of rats. Science 135, 374–375 (1962). [DOI] [PubMed] [Google Scholar]
- 36.Gratton A., Wise R. A., Comparisons of refractory periods for medial forebrain bundle fibers subserving stimulation-induced feeding and brain stimulation reward: A psychophysical study. Brain Res. 438, 256–263 (1988). [DOI] [PubMed] [Google Scholar]
- 37.Fouriezos G., Wise R. A., Pimozide-induced extinction of intracranial self-stimulation: Response patterns rule out motor or performance deficits. Brain Res. 103, 377–380 (1976). [DOI] [PubMed] [Google Scholar]
- 38.Campos C. A., Bowen A. J., Schwartz M. W., Palmiter R. D., Parabrachial CGRP neurons control meal termination. Cell Metab. 23, 811–820 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y., Lin Y. C., Zimmerman C. A., Essner R. A., Knight Z. A., Hunger neurons drive feeding through a sustained, positive reinforcement signal. eLife 5, e18640 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tong Q., Ye C. P., Jones J. E., Elmquist J. K., Lowell B. B., Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat. Neurosci. 11, 998–1000 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luquet S., Perez F. A., Hnasko T. S., Palmiter R. D., NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science 310, 683–685 (2005). [DOI] [PubMed] [Google Scholar]
- 42.National Research Council , Guide for the Care and Use of Laboratory Animals (National Academies Press, Washington, DC, ed. 8, 2011). [Google Scholar]
- 43.Marino R. A., Control of food approach and eating by a GABAergic projection from lateral hypothalamus to dorsal pons. Mendeley Data. 10.17632/wrk3krr9hv.1. Deposited 11 March 2020. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data discussed in the paper are available in Mendeley Data (43).