Significance
Spontaneous arousals from sleep are associated with tachycardia and blood pressure responses excessive to physiological need. The prevailing view is that stereotyped autonomic activity is generated by feedforward inputs from cortical and subcortical systems implicated in the arousal, akin to autonomic activation with emotional behavior or cognitive effort. This remains an inadequate explanation, and mechanisms that augment arousal and autonomic functions in sleep remain enigmatic. We identified that swallows trigger rapid, robust, and patterned tachycardia conserved across wake, sleep, and arousal states. Nocturnal swallowing and glottic adduction—essential airway defense mechanisms—were also causally linked to prolonged, intense arousals. These findings identify a fundamental mechanism driving both autonomic activation and heightened arousal via cortical feedback from brainstem swallow networks.
Keywords: central pattern generation, arousal from sleep, arousal tachycardia, swallow
Abstract
Cortical arousal from sleep is associated with autonomic activation and acute increases in heart rate. Arousals vary considerably in their frequency, intensity/duration, and physiological effects. Sleep and arousability impact health acutely (daytime cognitive function) and long-term (cardiovascular outcomes). Yet factors that modify the arousal intensity and autonomic activity remain enigmatic. In this study of healthy human adults, we examined whether reflex airway defense mechanisms, specifically swallowing or glottic adduction, influenced cardiac autonomic activity and cortical arousal from sleep. We found, in all subjects, that swallows trigger rapid, robust, and patterned tachycardia conserved across wake, sleep, and arousal states. Tachycardia onset was temporally matched to glottic adduction—the first phase of swallow motor program. Multiple swallows increase the magnitude of tachycardia via temporal summation, and blood pressure increases as a function of the degree of tachycardia. During sleep, swallows were overwhelmingly associated with arousal. Critically, swallows were causally linked to the intense, prolonged cortical arousals and marked tachycardia. Arousal duration and tachycardia increased in parallel as a function of swallow incidence. Our findings suggest that cortical feedback and tachycardia are integrated responses of the swallow motor program. Our work highlights the functional influence of episodic, involuntary airway defense reflexes on sleep and vigilance and cardiovascular function in healthy individuals.
The ability to arouse from sleep is essential for survival (1–3). Arousals are characterized by abrupt changes in the activity of the central nervous system, as observed by electroencephalogram (EEG). Cortical EEG arousals can occur spontaneously or be elicited by virtually any sensory stimuli, if of adequate magnitude. They are accompanied by rapid marked activation of the autonomic and respiratory systems, resulting in transient increases in heart rate (HR), blood pressure (BP), sympathetic vasomotor activity, and ventilation which then decline in subsequent sleep or wakefulness (4–7). These short-lived bursts of autonomic activity do not have a clear physiological function and are driven by integrated mechanisms that remain incompletely understood.
Several chronic sleep disorders are characterized by excessive arousals and fragmented sleep and lead to daytime cognitive impairments, including obstructive sleep apnea (OSA), Cheyne–Stokes breathing, restless leg syndrome, and periodic limb movement disorder. Frequent arousals and associated autonomic activation may also contribute to cardiovascular and metabolic comorbidities that are a common feature of these sleep disorders (8–11). The number of arousals per hour of sleep and the arousing stimulus are useful clinical measures of sleep quality. Arousals also greatly vary in intensity, duration, and EEG morphology, and in their physiological consequences. More intense arousals are longer in duration (12), impair sleep resumption and sleep stability (13, 14), and are associated with greater surges in cardiorespiratory activity (5, 15) that may contribute to OSA pathogenesis (16–18). Little is known about the factors that modify the arousal intensity or magnitude of the cardiac autonomic response to arousal. Interestingly, respiratory stimuli (hypercapnia, hypoxemia, resistive loading) have minimal effect on arousal intensity (12) or the heart rate response (6, 19–24). We wondered whether the reflex motor acts that protect the airway during sleep, principally swallowing, but also laryngeal adduction, influence arousability, arousal intensity, or the cardiac autonomic response to arousal.
Swallowing is a vital protective response against aspiration, including aspiration of saliva during sleep (25). It is a complex motor act, requiring precisely coordinated sequential activation and inhibition of various respiratory and nonrespiratory muscles regulated by a brain stem neuronal network, the so-called swallow central pattern generator (CPG) (26–28). Swallowing occurs episodically, triggered by sensory inputs or cortical commands, and is coordinated with rhythmic breathing, mastication, and other motor behaviors by the brainstem CPGs (27), and by feedback to higher level centers for sensorimotor planning and control (29, 30). Thus, during quiet wakefulness, involuntary or reflexive swallows produce patterns of cortical and subcortical activation via sensory and central feedback (29–34). Swallows also trigger autonomic activation, including a rapid, transient increase in heart rate (35–37) and increased sweat secretion (sympathetic sudomotor activation) (38). During sleep, swallowing occurs episodically in association with cortical EEG arousal, and rarely in stable sleep (39–41). The effects of sleep on cortical and autonomic activation by swallowing are unknown.
The relationship between swallowing, arousal, and arousal tachycardia is not clear. Our basic hypothesis is that spontaneous swallowing in sleep causes cortical arousal and tachycardia. Our objectives were to 1) determine the effects of swallowing on tachycardia and identify the mechanism, and 2) examine the temporal relationship between swallowing, arousal from sleep, and tachycardia.
Results
Effects of Swallowing on Heart Rate and Blood Pressure during Quiet Wakefulness.
The hemodynamic effects of swallowing have not been described. Here, we determined the blood pressure (BP) and heart rate responses to swallows in 14 participants during wakefulness, resting supine. In all individuals, volitional swallowing evoked a rapid, robust, and highly reproducible tachycardia (Fig. 1 A and B) with a small increase in systolic BP (SBP), diastolic BP (DBP), and mean arterial BP (MBP). A single swallow produced an increase in heart rate of 12 ± 5 beats per minute (bpm) (mean, SD) (Fig. 1A). Two swallows in quick succession had an additive effect (Fig. 1B) and produced a larger, stereotypic increase in heart rate of 17 ± 7 beats per minute from rest, with larger pressor responses (see SI Appendix, Table S2 for all resting and peak values). The change (Δ) in all hemodynamic measures was positively linearly related to the magnitude of the swallow tachycardia, including ∆mean BP (Fig. 1C) (r2 = 0.60, P < 0.001), ∆DBP (r2 = 0.64, P < 0.0001), and ∆SBP (r2 = 0.46, P < 0.001). For every +1 ∆HR (bpm) above a threshold of +6 bpm (∆MBP = 0 [x-intercept]), blood pressure variables increased +1 mmHg (Fig. 1C).
Fig. 1.
Swallows elicit a robust, patterned tachycardia and pressor response. (A) Individual examples of heart rate and blood pressure measured by finger photoplethysmography showing rapid reproducible tachycardia and pressor responses elicited by a single swallow. (B) Two swallows in quick succession produce larger increases in heart rate and blood pressure from the same individual in A. (C) Group data from 14 participants plotting average increase in heart rate (∆HR) and mean blood pressure (∆Mean BP) with one swallow (unfilled circle) or two swallows in quick succession (filled circle). Note mean blood pressure increased as function of the swallow tachycardia (∆HR).
Coordination of Breathing, Swallowing, and Cardiac Activation during Wakefulness: Swallow Tachycardia Mechanism.
Swallowing requires a precise sequence of oropharyngeal–esophageal motor patterning. The timing of the tachycardia and its relationship to specific phases of swallowing and breathing have not been examined and may yield insight to the mechanism(s) that trigger tachycardia.
During wakefulness and sleep, swallows were observed in expiration in all individuals. For some, swallows occurred in early expiration, followed by brief exhalation. In others, swallows occurred in mid-to-late expiration, preceding inspiration. The onset of the tachycardia coincided with earliest phase of swallowing, the glottic closure and the cessation of airflow (Fig. 2). During wakefulness, resting supine, the latency from the onset of the glottic adduction and tachycardia to the peak increase in epiglottic pressure (pharyngeal constriction peristaltic phase) was 0.9 ± 0.4 s and 0.8 ± 0.4 s, respectively.
Fig. 2.
Coordination of breathing, swallow, and tachycardia. Single (Left) and ensemble averages (Right) of heart rate (HR), airflow (Flow), and epiglottic pressure (Pepi) recorded from one supine participant during a single dry swallow. Note the rapid increase in heart rate occurs with glottic adduction (zero flat-lining airflow; gray shading) and precedes changes in epiglottic pressure. Mean (black line) ± SE (red lines). bpm, beats per minute; ECG, electrocardiogram; L/s, liters per second; Vt, tidal volume.
The swallow tachycardia was observed as a rapid, automatic, and patterned response initiated simultaneously with glottic closure, the first phase of the involuntary pharyngeal swallow motor act (27).
Nocturnal Swallowing Effects on Cortical Arousal and the Arousal Tachycardia.
Participant sleep and respiratory parameters.
Baseline sleep and respiratory characteristics are reported in SI Appendix, Table S1. Average total sleep time was 369 min (range, 277 to 491 min), with normal sleep stage distribution and EEG arousals randomly occurring across all sleep stages. Most EEG arousals were spontaneous (86%), with a small proportion preceded by respiratory events, including hypopnea (12%) or apnea (2%) (SI Appendix, Table S1). The total number of EEG arousal events per individual varied considerably (range, 29 to 227).
Nocturnal swallows increase cortical arousal duration and arousal tachycardia.
Arousal intensity and the arousal tachycardia varied considerably within an individual. Yet, factors that influence the arousal intensity and/or arousal tachycardia remain enigmatic (5, 6, 12, 42). Next, we examined if swallows in sleep cause arousal or modify arousal durations or the heart rate response to arousal.
Most swallows in 10 individuals (98%) occurred in association with spontaneous EEG arousal. All swallows were in expiration, and most occurred within the first few breaths of the EEG arousal in a probabilistic pattern: 58% of all swallows occurred within two breaths of EEG arousal; 76%, <3 breaths; 88%, <4 breaths; and 94%, <5 breaths (Fig. 3 A and D–G) (see also SI Appendix, Fig. S1 for the temporal pattern of swallows and arousal for each participant). A minority of swallows coincided with onset of the arousal, and a minority of swallows preceded the onset of arousal (SI Appendix, Figs. S1 and S4). The coincidence of swallowing with EEG arousal varied between individuals from 8 to 67% of all EEG arousals, and this proportion was inversely associated with the arousal index (4 to 38 EEG arousal per hour; r2 = 0.66; P = 0.004) (SI Appendix, Fig. S2).
Fig. 3.
Nocturnal swallows occur with arousal from sleep; swallows cause marked increases in heart rate and prolong EEG arousal duration. (A) This summary displays the overnight sleep stage hypnogram, arousals, swallow incidence, and heart rate (beats per min [bpm]) in a single participant. Swallows occurred with arousal, and not in stable sleep. Single swallows were most frequently observed during an EEG arousal. Two or more swallows observed on occasion. (B) Electroencephalography (EEG) arousal duration stratified by the incidence of involuntary swallowing with arousal from the same participant displayed in A (i.e., no swallow, single swallow, two or more swallows). Arousals associated with one or more swallows were significantly longer in duration (P < 0.0001; one-way ANOVA), mean ± SD. (C) Ensemble averages of the heart rate responses to all EEG arousals from participant A grouped by swallow incidence (no swallow, single swallow, two or more swallows). The arousal tachycardia increases as a function of the swallow incidence. Mean (black line) ± SE (gray). (D–G) Four example traces of EEG arousal duration (gray shading), heart rate, and respiratory responses to spontaneous EEG arousal with no swallow (D), one (E and F), or multiple swallows (G). Note the rapid increase in heart rate is triggered by the swallow (i.e., compare D and E). (F) Note the timing of the marked increase in heart rate is linked to the swallow, not the EEG arousal. (G) Note the effects of multiple swallows. EEG, electroencephalography (EEG electrodes: O2/A1, C3/A2); ECG, electrocardiogram; Pepi, epiglottic pressure (cmH2O); submental EMG (Chin); Pmask, pressure of nasal mask; Flow, airflow measured by pneumotach. Reflex glottic adduction identified by the “flatlining” (zero value) on the airflow and mask pressure waveforms. Swallows identified by the positive spike in epiglottic pressure and increase in submental EMG.
The heart rate response to arousal increased markedly if swallowing also occurred (Fig. 3 C–G). The arousal tachycardia further increased as a function of the frequency of swallows, as depicted in Fig. 3C. The swallows were temporally linked with the large increases in heart rate, more so than the arousal itself. Fig. 3 D–G shows four examples of arousal and the effects of the timing and frequency of swallowing on the arousal tachycardia. (Note the timing of the swallow tachycardia in Fig. 3F and the effects of multiple swallows in Fig. 3G.)
For all individuals, spontaneous arousal without swallows modestly increased heart rate by 6 ± 3 bpm (n = 10) (Fig. 4A) whereas EEG arousal with a single swallow increased heart rate by 18 ± 4 bpm (n = 10) (Fig. 4A), EEG arousal with two swallows increased heart rate by 26 ± 5 bpm (n = 10) (Fig. 4A), and EEG arousal with >2 swallows further increased the magnitude of tachycardia, as observed in a subset of participants (n = 5 of 10) (Fig. 4A). The effect of swallows on the magnitude of the arousal tachycardia was similar across all sleep stages (Fig. 4B). In the absence of a swallow, arousal from rapid eye movement (REM) sleep was associated with a greater magnitude of tachycardia compared to arousal from non-REM sleep (Fig. 4B).
Fig. 4.
Effects of swallow incidence and sleep stage on the arousal tachycardia and arousal duration. (A) The arousal tachycardia increases as a function of the swallow incidence. (B) A single swallow increased the arousal tachycardia in all sleep stages. In the absence of swallows, arousals from REM sleep produced a significantly larger tachycardia than arousals from non-REM sleep (N1 to N3) (C) Arousal duration increases as a function of the swallow incidence. (D) A single swallow increased the arousal duration in all sleep stages. N1, stage 1 non-REM sleep; N2, stage 2 non-REM sleep; N3, stage 3 non-REM sleep; REM, rapid eye movement sleep. Mean ± SD. *P < 0.05; **P < 0.005; ***P < 0.001; ****P < 0.0001.
Arousal duration also increased as a function of swallows, as observed in the individual examples (Fig. 3 B and D–G) and at the group level (Fig. 4C). Swallows were associated with prolonged arousal durations across all sleep stages (Fig. 4D). Arousals longer than 15 s are considered full awakenings by American Academy of Sleep Medicine (AASM) scoring rules (43). Only 11 ± 6% of all EEG arousals without swallows lasted >15 s (SI Appendix, Fig. S3). By contrast, 55 ± 9% of arousals with a single swallow were >15 s, 85 ± 7% of arousal with two swallows >15 s, and 91 ± 8% of arousals with more than two swallows >15 s in duration (P < 0.001 for the effect of swallow incidence on EEG arousals >15 s).
Reflex Swallows in Sleep Trigger Tachycardia and EEG Arousal.
Swallow-related feedback stimuli may be sufficient to produce EEG arousal. We observed in six of the 10 participants examples of swallowing immediately prior to clear cortical arousal from sleep (SI Appendix, Figs. S1 and S4). No other arousing stimuli were detected, including respiratory, auditory/snort, and body/leg movements that might explain the tachycardia in these instances.
Swallows without cortical arousal were rare during sleep (2% of all nocturnal swallows) but were observed in six of the 10 participants (SI Appendix, Fig. S5). The swallow tachycardia in stable sleep exhibited the stereotypic, rapid pattern elicited during wakefulness although the peak increase in heart rate was less in sleep (SI Appendix, Fig. S4). Thus, swallowing triggers a patterned increase in heart rate that is highly reproducible across wakefulness and sleep and in association with arousal from sleep.
Reflex Glottic Closure without Swallowing during EEG Arousal Also Increases the Tachycardia and Arousal Duration.
The laryngeal adductor reflex, or glottic closure reflex, is vital for preventing aspiration of materials into the trachea and lungs. Adduction occurs prior to the spontaneous swallow. We observed that the onset of the swallow tachycardia was temporally matched to glottic adduction, the first involuntary phase of swallow motor program. Next, we examined all cases of reflex glottic adduction not accompanied by swallowing to assess the associated tachycardia and arousal.
Reflex glottic closures were frequently observed during EEG arousal events that also triggered swallowing activity (Fig. 3 F and G). Arousal with reflex glottic closure without swallows occurred in only 7% of all arousals (range, 1 to 11%) in eight of the 10 participants studied. Arousal duration and arousal tachycardia were significantly greater when associated with glottic closure without swallows versus spontaneous arousals (Fig. 5). The magnitude of this effect was comparable to that observed when glottic closure was accompanied by a swallow.
Fig. 5.
Increased arousal tachycardia and arousal duration associated with arousal and reflex glottic adduction (no swallow). Arousal tachycardia and arousal duration were significantly greater when associated with reflex glottic adduction vs. spontaneous arousals without reflex glottic adduction. Mean ± SD. *P < 0.05; ***P < 0.001.
Discussion
The major finding of this study in healthy adults is that swallowing during sleep drives patterned cardiac and cortical activation that produces marked increases in heart rate, blood pressure, and prolonged arousal. We describe a robust and reproducible tachycardia that is evoked by swallowing that persists across wakefulness, stable sleep, and during arousal from sleep. The tachycardia is a rapid, patterned response, with an onset linked to glottic adduction, the earliest phase of involuntary swallowing. We show that repeated swallows increase the magnitude of the tachycardia via temporal summation and that blood pressure increases as a function of the degree of tachycardia. During sleep, swallowing was overwhelmingly associated with arousal. Most swallows follow arousal, some occur simultaneously, and few precede it. Importantly, the swallows were temporally linked to marked tachycardia and EEG arousals of longer duration. We also found occasional examples where swallowing during sleep appeared sufficient to evoke EEG arousal. Reflex laryngeal adduction with EEG arousal was also associated with marked tachycardia and prolonged EEG arousal, similar to the effects of swallowing. Collectively, these observations suggest that a common trigger for the cardiac and cortical activation is the reflex motor action which closes the glottis, stops airflow, and protects the lower airway from tracheal aspiration.
Thus, we propose that the central integration of reflex swallowing triggers patterned cardiac and cortical responses that evoke marked tachycardia and arousal. The physiological significance of these responses remains to be elucidated. We suggest 1) the rapid increase in heart rate and blood pressure synchronized with reflex laryngeal adduction and swallowing boosts brain blood flow during apnea, and 2) arousal from sleep enables other motor and behavioral responses to airway protection, like coughing, if required. In summary, our study makes a fundamental discovery by identifying reflex swallowing, an essential protective mechanism, as the cause of large increases in heart rate, awake and asleep, and to more intense, prolonged arousals from sleep in healthy individuals.
Swallow Tachycardia Mechanism and Significance.
A stereotyped tachycardia with swallowing has been observed in humans consistent with the present study (Fig. 1) (35, 37). The mechanism(s) responsible, and the functional significance, remain poorly understood. Several different concepts have been proposed, and there remains issue as to whether the tachycardia is centrally generated by the swallow motor program, or is reflexively triggered by feedback from pharyngeal and/or esophageal sensory afferents activated with swallowing (27). There is also considerable literature on a rare clinical disorder of cardiac tachyarrhythmia with swallowing food more so than liquids (44), for which several distinct reflexive mechanisms have been identified. These include vagovagal reflexes triggered by normal esophageal peristalsis (45, 46), vasovagal reflex originating from pulmonary veins (47), a cardio-sympathetic effect (ill-defined) (48), or mechanical stimulation of the left atrium by a distended esophagus (49). However, there has been little research investigating mechanisms for the swallow tachycardia.
The current study supports the model of a central, patterned heart rate response generated by the brainstem swallow central pattern generator. This is based on the following findings. 1) Through simultaneous recordings of airflow and epiglottic pressure with ECG, we observed the onset of the tachycardia to be temporally linked to glottic closure, the first sequence of the involuntary swallow motor program. 2) The tachycardia onset precedes the pharyngeal constriction (peristatic) phase by ∼1 s. Peak muscle activity and marked positive airway pressures (+100 cm H2O) are generated during pharyngeal peristalsis, and afferents encoding muscle force, pressure, and touch are robustly activated here. Our findings do not make a temporal link to pharyngeal afferent mechanisms, or esophageal feedback thereafter, in driving reflexive increases in heart rate. 3) The tachycardia associated with volitional or spontaneous swallowing in our awake participants was essentially identical. This shows that volitional motor effort is not a factor (50). 4) The tachycardia was reliably evoked across different behavioral states—wakefulness, sleep, and arousal from sleep—and exhibited a highly stereotypic temporal pattern and peak response. This high degree of fidelity would not be expected if dependent on afferent drive, or corticobulbar loops, or central circuits with several layers of sensory filtering. Therefore, we conclude that this is a fundamental feature of the swallow motor program.
The rapid onset of the swallow tachycardia and its rapid reversal to resting heart rate are in all likelihood due to acute changes in cardiovagal tone which is responsible for the beat-to-beat variability (51). The kinetics of the sympathetic cardiac tone are much more gradual (52) although it is quite possible that parallel activation of cardiovascular sympathetic outflow occurs, like esophageal and skin sudomotor activation (38).
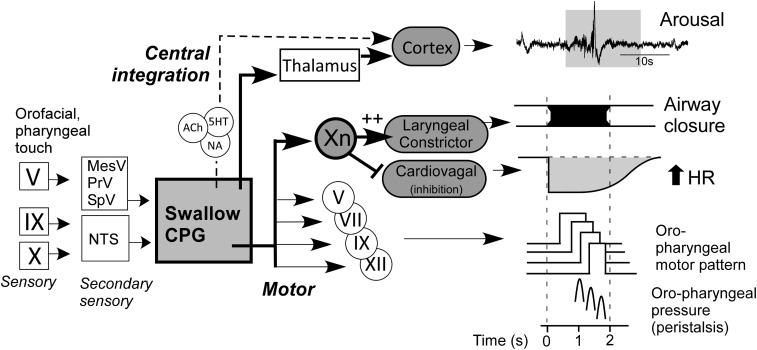
Our model of the swallow motor program (Fig. 6) posits that cardiovagal motor neurons of nucleus ambiguus are acutely inhibited in parallel with the activation of laryngeal constrictor motor neurons. This pattern of activation and inhibition couples the glottic adduction and the tachycardia. It is evidenced by the recordings of nucleus ambiguus vagal motor neurons in sheep that identify several distinct clusters of motor neurons acutely activated or inhibited by swallow (53). The inhibition of cardiovagal tone is likely a direct, patterned command from the swallow central pattern generator, and not indirectly related to a pause in breathing. Normal cardiovagal–respiratory coupling activates cardiovagal motor neurons in expiration to cause bradycardia and inhibits in inspiration (respiratory sinus arrhythmia) (51, 54). In humans, the swallow tachycardia supersedes the bradycardia of diving or breath holding (37) and is further evidence for robust central patterning.
Fig. 6.
Schematic model of swallow sensorimotor program incorporating effects on cardiac vagal tone, heart rate, and cortical EEG arousal. Inhibition of cardiovagal tone and increase in heart rate occur in parallel with glottic closure and precede the pharyngeal constriction. EEG arousal occurs via ascending feedback from the swallow central pattern generator (CPG) via thalamic relay, and possibly via brainstem monoamine arousal promoting networks, including noradrenergic (NA) locus coeruleus, serotoninergic (5HT) dorsal and median raphe, and acetylcholine (Ach) mesopontine tegmentum neurons (71). Roman numerals depict the cranial afferent nerves (□), central sensory nuclei (□), and motorneuronal nuclei (○) involved in swallowing. NTS, nucleus tractus solitarius.
Our observations identify unique features of the swallow motor program, with differential commands to vagal motor neurons with cardiac function (inhibition) versus airway striated muscle or enteric smooth muscle function (activation). In the clinical setting, cardiac vagal tone is notoriously difficult to measure noninvasively and is usually done by measures of heart rate variability (respiratory sinus arrhythmia), which remains a controversial measure of vagal tone, in part due to influences of breathing effort and rate. On the other hand, swallow tachycardia appears to be exclusively vagal, and a simple test could be incorporated into clinical practice. The range of heart rate increases we observed with a single swallow, or a quick series of swallows, represents the full dynamic range of resting vagal tone (i.e., 65 to 100 bpm).
Swallow Feedback Increases Arousal Intensity and Arousability.
Functional brain imaging studies in awake humans consistently show reflex swallowing engages higher level cortical and subcortical sensorimotor integration and cognition (reviewed in ref. 31). Cortical feedback arises via the thalamus from ascending sensory afferents and from the central feedback from the brainstem swallow CPG. The thalamus is an essential locus for integration and filtering of all sensory inputs relayed to the cortex. Interestingly, oro-pharyngeal and tracheal airway mechanoreceptive afferents undergo minimal thalamic filtering (55, 56), unlike auditory and somatosensory tactile afferents (57). This suggests that afferents encoding airway pressures and touch are highly salient stimuli and that maintaining airway patency involves higher order vigilance. The swallow motor act is under constant somatosensory cortical surveillance (29), with neuronal activity increasing immediately prior to an automatic saliva swallow, and two peaks of activation that relate to the pharyngeal (2 s) and esophageal (15 to 20 s) swallow phases (29). Experts suggest that cortical representation is an important modifier of the involuntary swallow motor act and note problems with swallowing (dysphagia) following focal hemispheric stroke or with various neurodegenerative disorders (30). The effects of sleep on swallow-related thalamic and cortical feedback is unknown and needs clarification. Sleep reduces the frequency of spontaneous swallowing (39). This relates, in part, to reduced saliva production with sleep (58), but also to sleep state-dependent increase in the thresholds required to initiate swallowing (59), a common feature of sleep on neural reflexes (60). Nocturnal spontaneous swallows are mostly associated with arousal from sleep in adult humans (39, 41, 59, 61) and mammalians (25, 62). This strong association supports our thesis that the central integration of reflexively triggered swallowing includes the activation of vigilance-regulating neuronal networks sufficient to produce arousal from sleep and/or prolong arousal and delay sleep resumption.
Our findings support a causal link between spontaneous swallowing and arousal-promoting mechanisms. We suggest that the following four observations establish causality. First, virtually all swallows are associated with cortical arousal. Second, a single swallow prolonged the arousal durations by ∼100% compared to arousal with no swallow. This effect was reliably found in arousals across all sleep states. Third, a stimulus-response relationship exists: Cortical arousal duration increased as a function of the number of swallows. Fourth, in a minority of cases, swallows preceded arousals in which no other arousing stimuli are identifiable. Further evidence for reflex swallowing precipitating arousal from sleep is from studies infusing a liquid bolus into the pharyngeal cavity, as described in piglets (25), adult cats (62), and humans (59).
Reflex Glottic Adduction during Arousal Is Also Associated with Increased Arousal Duration and Tachycardia.
Reflex glottic adduction and swallowing provide the primary defense against airway aspiration. Both motor behaviors adduct the vocal cords to abruptly close the airway. They share a common sensorimotor control system and function as an integrated or tiered response to pharyngeal chemo- or mechanosensitive stimuli. Prior studies reveal that stimulation of the pharyngeal mucosa with small volumes of water induces vocal cord adduction at low threshold volumes of ∼0.15 mL, and reflexive swallowing triggered with a threshold ∼0.5 mL of water during wakefulness in healthy young humans (63). This relationship is preserved in healthy elderly adults although it requires larger volumes of water (∼0.35 mL for glottic adduction and 1.7 mL to trigger swallowing) (63). Experimental models in cat and rat also observe threshold-dependent coactivation of reflex glottic adduction and swallowing with pharyngeal provocation (64–66). Both reflexes can be induced in all phases of the respiratory cycle and exert a potent suppression of inspiratory CPG activity (28, 66).
We observed reflex glottic adduction during EEG arousal, most commonly during arousal events that also featured swallowing (Fig. 3 F and G). Reflex adduction was readily identified by the clear, invariable zero flatlining observed in the airflow, mask, and epiglottic pressure waveforms, without the spike in epiglottic pressure that defines swallows. Arousals with reflex glottic closure without swallows were relatively few (present in 7% of all arousals; range, 1 to 11%; n = 8 of 10 participants studied). Arousal duration and arousal tachycardia were significantly greater when associated with glottic adduction without swallows versus spontaneous arousals. The magnitude of this effect was comparable to arousals with swallowing. As valving the airway shut is the common motor outcome for both behaviors, we suggest that the tachycardia and heighted arousal are the consequence of triggering this shared mechanism, perhaps to boost blood flow and vigilance during an airway protective apnea. Our findings notably contrast with observations of passive airway closure in obstructive sleep apnea patients for which the airway collapse itself - nor the various airway structures that can obstruct the airway e.g., tongue, epiglottis, soft palate - does not trigger tachycardia (67–69).
Methodological Limitations: EEG Arousal and Swallow Interactions.
We highlight three methodological considerations for our study and for the field. First, we did not perform fine-grained analyses of the EEG power spectra to quantify arousal intensity because of an associated muscle artifact with swallowing visible in a subset of participants . The artifact is transient, but it does superimpose fast frequency waves in EEG signals in the beta and gamma range. However, it is well-established that arousal intensity covaries with arousal duration (6, 12, 14). Second, temporal accuracy of manual EEG arousal scoring is challenging and vulnerable to error. However, scoring in this study was performed by a single experienced sleep technician blinded to the pressure and flow signals who paid careful attention to score arousal timing as accurately as possible. Nonetheless, it remains possible that EEG arousals occurred with different temporal timing at other regions of the cortex that were not covered by our electrode array, which could influence our temporal relation findings. Third, one of the tenets of AASM criteria scores sleep/wake in 30-s epochs and class arousals lasting longer than 15 s (>50% of 30-s epoch) as wakefulness/awakenings. If we constrain our data to arousals <15 s, we arbitrarily exclude a significant proportion of arousals with accompanying swallows and/or reflex glottic adduction. Note that earlier studies have excluded or stratified arousals lasting longer than 15 s (15). There is growing interest in defining the arousal intensity spectrum (13, 42). Despite this, factors influencing arousal intensity remain largely unknown or are inconsequential (i.e., age, sex, sleep stage, and respiratory feedback) (12, 15). For instance, the arousal intensity and duration do not differ between healthy individuals and people with OSA (15), nor between spontaneous EEG arousals and those preceded by hypopnea or apnea within an individual (12). Our findings identify reflex glottic adduction and swallowing as motor acts causally linked to prolonged arousals. Thus, future studies should consider arousals >15 s as part of the continuous spectrum of EEG arousals and monitor respiratory variables for signs of glottic adduction and swallowing, especially if the study involves spectral analysis and quantifying the beta power of EEG arousal.
Conclusion.
In summary, our data suggest that swallowing-induced tachycardia makes up a large component of the cardiovascular activation during arousal from sleep and may also explain a considerable degree of the variation in arousal duration and intensity. We propose that the central integration of reflex swallowing triggers patterned cardiac and cortical responses that evoke marked tachycardia and prolonged arousal. The physiological significance of these responses and their contribution to the cardiovascular and cognitive sequelae of sleep disorders need further examination.
Methods
Participants.
Swallow tachycardia was examined in 24 healthy adults (13 female; age 18 to 65 y), recruited from the community and local sleep clinics via advertisement. Two subgroups were studied: One group (n = 14; 8 female) was examined for supine blood pressure and heart rate responses to swallows during wakefulness. The second group (n = 10; 5 female) undertook overnight polysomnography to examine the relationships between EEG arousal, swallows, heart rate, and breathing during sleep. Participants were excluded if they were taking any medications that may affect breathing or sleep; were pregnant or nursing mothers; or were currently using sleep apnea therapies, either continuous positive airway pressure (CPAP) or mandibular advancement splints. Group participant characteristics are reported in Table 1.
Table 1.
Participant characteristics
| Experiment | ||
| HR + BP measures (wake) | Polysomnography | |
| N | 14 | 10 |
| Sex (F:M) | 8:6 | 5:5 |
| Age (range) | 31 ± 6 (24 to 45 y) | 35 ± 15 (20 to 63 y) |
| BMI (range) | 24.7 ± 1.7 (22.1 to 28.7) | 24 ± 3 (19.8 to 29.1) |
Group anthropometric data acquired from participants undertaking the overnight sleep study or blood pressure measurements. Values are means ± SD of the mean. BMI, body mass index (kg/m2); F, female; M, male.
The study was approved by the South Eastern Sydney Local Health District Human Research Ethics Committee and satisfied the Declaration of Helsinki. All participants provided written informed consent prior to taking part.
Measurements and Equipment.
Cardiovascular measures.
Noninvasive measurements of beat-to-beat blood pressure (BP) were made via finger photoplethysmography (Finapres Medical Systems, The Netherlands), verified by manual brachial artery sphygmomanometry. Continuous measurements of BP, electrocardiogram (ECG) were acquired via an analog-to-digital converter (Spike2; Cambridge Electronic Design, United Kingdom) at 500 Hz per channel and used for offline analysis to determine beat-to-beat values for heart rate (HR), systolic BP, diastolic BP, and mean arterial pressure (MAP). Heart rate was derived from the ECG R-R interval.
Polysomnography, respiratory measures, and detection of swallows.
Polysomnography was performed using established methods (70). The electroencephalogram (EEG) was recorded continuously from electrodes placed according to the 10–20 System, referenced to electrodes attached to the mastoids. EEG signals were sampled at a rate of 250 Hz. Left and right electrooculograms, submentalis electromyogram (EMG), pulse oximetry, and body position (infrared video monitoring) were also recorded to determine sleep stage and cortical arousals.
Participants were instructed to sleep on their back as much as possible and were monitored for ∼8 h.
Respiratory parameters were monitored with a pneumotachograph (model 3700A; Hans Rudolph Inc., Kansas City, KS) connected to an unvented nasal mask (Respironics, Murrysville, PA) and a differential pressure transducer (Validyne Corp., Northbridge, CA). Nasal mask pressures were also monitored via ports in the mask. The epiglottic pressure (Pepi) catheter (model MCP-500; Millar, Houston, TX) was inserted through a decongested (0.5% phenylephrine) and anesthetized (4% lidocaine) nostril until the pressure-sensing tip was located 1 to 2 cm caudal to the base of the tongue.
Data Analysis.
All signals were acquired using an analog-to-digital convertor with Spike2 software and analyzed off-line. Sleep stages, arousal onset and duration, and respiratory events were scored using standard American Academy of Sleep Medicine guidelines (43) by an experienced sleep technician naive to the study objectives. Arousals were scored by the following: 1) preceded by a minimum of 30 s of continuous sleep, defined by EEG theta activity, and 2) defined by a visually discernible shift to higher frequency EEG (alpha) activity lasting a minimum of 3 s. Arousal onset was defined by the first occurrence of EEG alpha activity. Arousals with ECG artifacts from bodily movements were excluded in one subject (four movement arousals excluded from 163 arousals). Reflex glottic closures were identified by the “flatlining” (zero value) on the airflow and mask pressure waveforms. Swallows were detected as a spike in epiglottic pressure (Pepi) as measured with a catheter with increase in submental surface electromyogram (EMG) recordings of geniohyoid, mylohyoid, and genioglossus muscle activity (26, 27). Temporal relationships between swallowing, breathing, and tachycardia were determined from ensemble averages of airflow, epiglottic pressure, and heart rate triggered to peak epiglottic pressure during the swallow. The latencies between onset of glottic adduction (airflow at “zero”), the onset of the tachycardia (rising threshold two SDs above the mean), and peak epiglottic pressure during the swallow were measured.
The effects of swallows on resting heart rate and blood pressure during quiet wake were determined by averaging 30 s of each variable preceding the swallow (resting) and the peak response immediately after the swallow. Peak response was the average of three fastest heart beats. Similarly, the effects of arousal on heart rate were determined by averaging 30 s prior to the arousal (resting) and then measuring the peak response following arousal. Data are mostly reported as change from baseline (i.e., ∆HR = peak HR − resting HR) and are stratified by incidence of swallow. Data are reported as mean ± standard deviation (SD).
Statistical Comparisons.
Analyses consisted of the following: 1) Analyses of the effects of spontaneous swallows and BP or HR were made using a two-tailed paired Student’s t test; 2) the relationship between the swallow tachycardia and the increase in BP was examined by linear regression analysis; 3) the effects of swallow incidence on arousal tachycardia and arousal duration were determined by one-way repeated measures ANOVA; and 4) the sleep-state dependent effects of swallows on arousal tachycardia and arousal duration were determined by two-way repeated measures ANOVA. All datasets were tested for normality. P < 0.05 was considered statistically significant. All statistical analyses were performed using PRISM software (version 7; GraphPad Software).
Data Availability.
All data are available in the main text.
Supplementary Material
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1907393117/-/DCSupplemental.
References
- 1.Halász P., Terzano M., Parrino L., Bódizs R., The nature of arousal in sleep. J. Sleep Res. 13, 1–23 (2004). [DOI] [PubMed] [Google Scholar]
- 2.Guyenet P. G., Abbott S. B., Chemoreception and asphyxia-induced arousal. Respir. Physiol. Neurobiol. 188, 333–343 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilmartin G. S., Thomas R. J., Mechanisms of arousal from sleep and their consequences. Curr. Opin. Pulm. Med. 10, 468–474 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Catcheside P. G., Chiong S. C., Mercer J., Saunders N. A., McEvoy R. D., Noninvasive cardiovascular markers of acoustically induced arousal from non-rapid-eye-movement sleep. Sleep 25, 797–804 (2002). [DOI] [PubMed] [Google Scholar]
- 5.Sforza E., Jouny C., Ibanez V., Cardiac activation during arousal in humans: Further evidence for hierarchy in the arousal response. Clin. Neurophysiol. 111, 1611–1619 (2000). [DOI] [PubMed] [Google Scholar]
- 6.Trinder J., et al. , On the nature of cardiovascular activation at an arousal from sleep. Sleep 26, 543–551 (2003). [PubMed] [Google Scholar]
- 7.Jordan A. S., Eckert D. J., Catcheside P. G., McEvoy R. D., Ventilatory response to brief arousal from non-rapid eye movement sleep is greater in men than in women. Am. J. Respir. Crit. Care Med. 168, 1512–1519 (2003). [DOI] [PubMed] [Google Scholar]
- 8.Sulit L., Storfer-Isser A., Kirchner H. L., Redline S., Differences in polysomnography predictors for hypertension and impaired glucose tolerance. Sleep 29, 777–783 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Koo B. B., et al. ; Osteoporotic Fractures in Men (MrOS) Study Group , Association of incident cardiovascular disease with periodic limb movements during sleep in older men: Outcomes of sleep disorders in older men (MrOS) study. Circulation 124, 1223–1231 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leung R. S., Diep T. M., Bowman M. E., Lorenzi-Filho G., Bradley T. D., Provocation of ventricular ectopy by cheyne-stokes respiration in patients with heart failure. Sleep 27, 1337–1343 (2004). [DOI] [PubMed] [Google Scholar]
- 11.Ekstedt M., Akerstedt T., Söderström M., Microarousals during sleep are associated with increased levels of lipids, cortisol, and blood pressure. Psychosom. Med. 66, 925–931 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Amatoury J., et al. , Arousal intensity is a distinct pathophysiological trait in obstructive sleep apnea. Sleep 39, 2091–2100 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Younes M., et al. , Odds ratio product of sleep EEG as a continuous measure of sleep state. Sleep 38, 641–654 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Younes M., Hanly P. J., Immediate postarousal sleep dynamics: An important determinant of sleep stability in obstructive sleep apnea. J. Appl. Physiol. (1985) 120, 801–808 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Azarbarzin A., Ostrowski M., Hanly P., Younes M., Relationship between arousal intensity and heart rate response to arousal. Sleep 37, 645–653 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eckert D. J., Younes M. K., Arousal from sleep: Implications for obstructive sleep apnea pathogenesis and treatment. J. Appl. Physiol. (1985) 116, 302–313 (2014). [DOI] [PubMed] [Google Scholar]
- 17.Younes M., Role of arousals in the pathogenesis of obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 169, 623–633 (2004). [DOI] [PubMed] [Google Scholar]
- 18.Loredo J. S., Ziegler M. G., Ancoli-Israel S., Clausen J. L., Dimsdale J. E., Relationship of arousals from sleep to sympathetic nervous system activity and BP in obstructive sleep apnea. Chest 116, 655–659 (1999). [DOI] [PubMed] [Google Scholar]
- 19.Horner R. L., Brooks D., Kozar L. F., Tse S., Phillipson E. A., Immediate effects of arousal from sleep on cardiac autonomic outflow in the absence of breathing in dogs. J. Appl. Physiol. (1985) 79, 151–162 (1995). [DOI] [PubMed] [Google Scholar]
- 20.Trinder J., et al. , Cardiac and respiratory activity at arousal from sleep under controlled ventilation conditions. J. Appl. Physiol. (1985) 90, 1455–1463 (2001). [DOI] [PubMed] [Google Scholar]
- 21.Goff E. A., et al. , The effect of flow limitation on the cardiorespiratory response to arousal from sleep under controlled conditions of chemostimulation in healthy older adults. J. Sleep Res. 21, 718–723 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Trinder J., Ivens C., Kleiman J., Kleverlaan D., White D. P., The cardiorespiratory activation response at an arousal from sleep is independent of the level of CO(2). J. Sleep Res. 15, 174–182 (2006). [DOI] [PubMed] [Google Scholar]
- 23.O’Driscoll D. M., Meadows G. E., Corfield D. R., Simonds A. K., Morrell M. J., Cardiovascular response to arousal from sleep under controlled conditions of central and peripheral chemoreceptor stimulation in humans. J. Appl. Physiol. (1985) 96, 865–870 (2004). [DOI] [PubMed] [Google Scholar]
- 24.Catcheside P. G., et al. , Acute cardiovascular responses to arousal from non-REM sleep during normoxia and hypoxia. Sleep 24, 895–902 (2001). [DOI] [PubMed] [Google Scholar]
- 25.Page M., Jeffery H. E., Marks V., Post E. J., Wood A. K., Mechanisms of airway protection after pharyngeal fluid infusion in healthy sleeping piglets. J. Appl. Physiol. (1985) 78, 1942–1949 (1995). [DOI] [PubMed] [Google Scholar]
- 26.Ertekin C., Aydogdu I., Neurophysiology of swallowing. Clin. Neurophysiol. 114, 2226–2244 (2003). [DOI] [PubMed] [Google Scholar]
- 27.Jean A., Brain stem control of swallowing: Neuronal network and cellular mechanisms. Physiol. Rev. 81, 929–969 (2001). [DOI] [PubMed] [Google Scholar]
- 28.Bautista T. G., Sun Q. J., Pilowsky P. M., The generation of pharyngeal phase of swallow and its coordination with breathing: Interaction between the swallow and respiratory central pattern generators. Prog. Brain Res. 212, 253–275 (2014). [DOI] [PubMed] [Google Scholar]
- 29.Kamarunas E., Mulheren R., Palmore K., Ludlow C., Timing of cortical activation during spontaneous swallowing. Exp. Brain Res. 236, 475–484 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Leopold N. A., Daniels S. K., Supranuclear control of swallowing. Dysphagia 25, 250–257 (2010). [DOI] [PubMed] [Google Scholar]
- 31.Malandraki G. A., Johnson S., Robbins J., Functional MRI of swallowing: From neurophysiology to neuroplasticity. Head Neck 33 (suppl. 1), S14–S20 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin R. E., Goodyear B. G., Gati J. S., Menon R. S., Cerebral cortical representation of automatic and volitional swallowing in humans. J. Neurophysiol. 85, 938–950 (2001). [DOI] [PubMed] [Google Scholar]
- 33.Kern M., et al. , Swallow-related cerebral cortical activity maps are not specific to deglutition. Am. J. Physiol. Gastrointest. Liver Physiol. 280, G531–G538 (2001). [DOI] [PubMed] [Google Scholar]
- 34.Kern M. K., Jaradeh S., Arndorfer R. C., Shaker R., Cerebral cortical representation of reflexive and volitional swallowing in humans. Am. J. Physiol. Gastrointest. Liver Physiol. 280, G354–G360 (2001). [DOI] [PubMed] [Google Scholar]
- 35.Sherozia O. P., Ermishkin V. V., Lukoshkova E. V., Dynamics of swallowing-induced cardiac chronotropic responses in healthy subjects. Bull. Exp. Biol. Med. 135, 322–326 (2003). [DOI] [PubMed] [Google Scholar]
- 36.Nitta E., Iwasa Y., Sugita M., Hirono C., Shiba Y., Role of mastication and swallowing in the control of autonomic nervous activity for heart rate in different postures. J. Oral Rehabil. 30, 1209–1215 (2003). [DOI] [PubMed] [Google Scholar]
- 37.Gandevia S. C., McCloskey D. I., Potter E. K., Reflex bradycardia occurring in response to diving, nasopharyngeal stimulation and ocular pressure, and its modification by respiration and swallowing. J. Physiol. 276, 383–394 (1978). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arıcı Ş., et al. , Sympathetic skin responses in adult humans during sequential swallowing. Neurophysiol. Clin. 43, 11–17 (2013). [DOI] [PubMed] [Google Scholar]
- 39.Lichter I., Muir R. C., The pattern of swallowing during sleep. Electroencephalogr. Clin. Neurophysiol. 38, 427–432 (1975). [DOI] [PubMed] [Google Scholar]
- 40.Lear C. S., Flanagan J. B. Jr, Moorrees C. F., The frequency of deglutition in man. Arch. Oral Biol. 10, 83–100 (1965). [DOI] [PubMed] [Google Scholar]
- 41.Sato K., Nakashima T., Human adult deglutition during sleep. Ann. Otol. Rhinol. Laryngol. 115, 334–339 (2006). [DOI] [PubMed] [Google Scholar]
- 42.Azarbarzin A., et al. , Arousal responses during overnight polysomnography and their reproducibility in healthy young adults. Sleep 38, 1313–1321 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berry R. B., et al. ; American Academy of Sleep Medicine ; Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine , Rules for scoring respiratory events in sleep: Update of the 2007 AASM manual for the scoring of sleep and associated events. J. Clin. Sleep Med. 8, 597–619 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tada H., et al. , Swallowing-induced atrial tachyarrhythmias: Prevalence, characteristics, and the results of the radiofrequency catheter ablation. Pacing Clin. Electrophysiol. 30, 1224–1232 (2007). [DOI] [PubMed] [Google Scholar]
- 45.Tandeter H., Kobal S., Katz A., Swallowing-induced atrial tachyarrhythmia triggered by salbutamol: Case report and review of the literature. Clin. Cardiol. 33, E116–E120 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suarez L. D., Chiozza M. A., Foye R., Mosso H., Perosio A. M., Swallowing-dependent atrial tachyarrhythmias. Their mechanism. J. Electrocardiol. 13, 301–305 (1980). [DOI] [PubMed] [Google Scholar]
- 47.Yokoshiki H., Mitsuyama H., Watanabe M., Tsutsui H., Swallowing-induced multifocal atrial tachycardia originating from right pulmonary veins. J. Electrocardiol 44, 395.e1–395.e5 (2011). [DOI] [PubMed] [Google Scholar]
- 48.Shirayama T., et al. , Swallowing-induced tachycardia; three modalities of autonomic nervous effects. Jpn. J. Med. 28, 647–650 (1989). [DOI] [PubMed] [Google Scholar]
- 49.Tanoue K., Sonoda M., Yamashita E., Tanaka H., Nuruki N., Swallowing-induced atrial tachyarrhythmia triggered by solid foods. Circulation 130, e113–e115 (2014). [DOI] [PubMed] [Google Scholar]
- 50.Jones C. L., et al. , Neuroanatomical substrates for the volitional regulation of heart rate. Front. Psychol. 6, 300 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Farmer D. G., Dutschmann M., Paton J. F., Pickering A. E., McAllen R. M., Brainstem sources of cardiac vagal tone and respiratory sinus arrhythmia. J. Physiol. 594, 7249–7265 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dampney R. A., Functional organization of central pathways regulating the cardiovascular system. Physiol. Rev. 74, 323–364 (1994). [DOI] [PubMed] [Google Scholar]
- 53.Zoungrana O. R., Amri M., Car A., Roman C., Intracellular activity of motoneurons of the rostral nucleus ambiguus during swallowing in sheep. J. Neurophysiol. 77, 909–922 (1997). [DOI] [PubMed] [Google Scholar]
- 54.Bautista T. G., Burke P. G., Sun Q. J., Berkowitz R. G., Pilowsky P. M., The generation of post-inspiratory activity in laryngeal motoneurons: A review. Adv. Exp. Med. Biol. 669, 143–149 (2010). [DOI] [PubMed] [Google Scholar]
- 55.Wheeler-Hegland K., Pitts T., Davenport P. W., Cortical gating of oropharyngeal sensory stimuli. Front. Physiol. 1, 167 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ruehland W. R., Rochford P. D., Trinder J., Spong J., O’Donoghue F. J., Evidence against a subcortical gate preventing conscious detection of respiratory load stimuli. Respir. Physiol. Neurobiol. 259, 93–103 (2019). [DOI] [PubMed] [Google Scholar]
- 57.Horner R. L., Sanford L. D., Pack A. I., Morrison A. R., Activation of a distinct arousal state immediately after spontaneous awakening from sleep. Brain Res. 778, 127–134 (1997). [DOI] [PubMed] [Google Scholar]
- 58.Thie N. M., Kato T., Bader G., Montplaisir J. Y., Lavigne G. J., The significance of saliva during sleep and the relevance of oromotor movements. Sleep Med. Rev. 6, 213–227 (2002). [DOI] [PubMed] [Google Scholar]
- 59.Okuno K., et al. , Sleep stage coordination of respiration and swallowing: A preliminary study. Dysphagia 31, 579–586 (2016). [DOI] [PubMed] [Google Scholar]
- 60.Burke P. G., et al. , State-dependent control of breathing by the retrotrapezoid nucleus. J. Physiol. 593, 2909–2926 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Don G. W., Waters K. A., Influence of sleep state on frequency of swallowing, apnea, and arousal in human infants. J. Appl. Physiol. (1985) 94, 2456–2464 (2003). [DOI] [PubMed] [Google Scholar]
- 62.Anderson C. A., Dick T. E., Orem J., Swallowing in sleep and wakefulness in adult cats. Sleep 18, 325–329 (1995). [DOI] [PubMed] [Google Scholar]
- 63.Shaker R., et al. , Pharyngoglottal closure reflex: Characterization in healthy young, elderly and dysphagic patients with predeglutitive aspiration. Gerontology 49, 12–20 (2003). [DOI] [PubMed] [Google Scholar]
- 64.Bautista T. G., Dutschmann M., Ponto-medullary nuclei involved in the generation of sequential pharyngeal swallowing and concomitant protective laryngeal adduction in situ. J. Physiol. 592, 2605–2623 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shaker R., et al. , Pharyngoglottal closure reflex: Identification and characterization in a feline model. Am. J. Physiol. 275, G521–G525 (1998). [DOI] [PubMed] [Google Scholar]
- 66.Toor R. U. A. S., et al. , Neurons in the intermediate reticular nucleus coordinate postinspiratory activity, swallowing, and respiratory-sympathetic coupling in the rat. J. Neurosci. 39, 9757–9766 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jordan A. S., et al. , Termination of respiratory events with and without cortical arousal in obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 184, 1183–1191 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Azarbarzin A., Ostrowski M., Moussavi Z., Hanly P., Younes M., Contribution of arousal from sleep to postevent tachycardia in patients with obstructive sleep apnea. Sleep 36, 881–889 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Catcheside P. G., Jordan A. S., Reflex tachycardia with airway opening in obstructive sleep apnea. Sleep 36, 819–821 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carter S. G., et al. , Zopiclone increases the arousal threshold without impairing genioglossus activity in obstructive sleep apnea. Sleep 39, 757–766 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saper C. B., Scammell T. E., Lu J., Hypothalamic regulation of sleep and circadian rhythms. Nature 437, 1257–1263 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in the main text.