Abstract
The objectives of this study were to assess the effectiveness of an ultraviolet (UV‐C, 254 nm) irradiation system and the spray‐drying method as two independent safety steps on inactivation of Escherichia coli K88 and K99 spiked in porcine plasma at 6·46 ± 0·04 log10 ml−1 and 6·78 ± 0·67 log10 ml−1 respectively for UV‐C method, and at 7·31 ± 0·39 log10 ml−1 and 7·66 ± 0·11 log10 ml−1, respectively for the spray‐drying method. The UV‐C method was performed at different UV light doses (from 750 to 9000 J l−1) using a pilot plant UV‐C device working under turbulent flow. Spray‐drying treatment was done at inlet temperature 220 ± 1°C and two different outlet temperatures, 80 ± 1°C or 70 ± 1°C. Results indicated that UV‐C treatment induced a 4 log10 viability reduction for both E. coli at 3000 J l−1. Full inactivation of both E. coli strains was achieved in all spray‐dried samples dehydrated at both outlet temperatures. The special UV‐C system design for turbid liquid porcine plasma is a novel treatment that can provide an additional redundant biosafety feature that can be incorporated into the manufacturing process for spray‐dried animal plasma.
Significance and Impact of the Study
The safety of raw materials from animal origin such as spray‐dried porcine plasma (SDPP) may be a concern for the swine industry. Ultraviolet treatment at 254 nm (UV‐C) of liquid plasma has been proposed as an additional biosafety feature in the manufacturing process of SDPP. We found that UV‐C exposure in the liquid plasma at 3000 J l−1 reduces about 4 log10 ml−1 for E. coli K88 and K99. Full inactivation of both E. coli strains was achieved in all spray‐dried samples. The incorporation of UV‐C treatment to liquid plasma improves the robustness of the SDPP manufacturing process.
Keywords: blood derivatives, Escherichia coli, porcine plasma, spray‐drying, ultraviolet irradiation
Significance and Impact of the Study: The safety of raw materials from animal origin such as spray‐dried porcine plasma (SDPP) may be a concern for the swine industry. Ultraviolet treatment at 254 nm (UV‐C) of liquid plasma has been proposed as an additional biosafety feature in the manufacturing process of SDPP. We found that UV‐C exposure in the liquid plasma at 3000 J l−1 reduces about 4 log10 ml−1 for E. coli K88 and K99. Full inactivation of both E. coli strains was achieved in all spray‐dried samples. The incorporation of UV‐C treatment to liquid plasma improves the robustness of the SDPP manufacturing process.

Introduction
Spray‐dried animal plasma (SDP) is a protein source extensively used in pig feed due to its functional components that contribute to improved post‐weaning performance and survival (Torrallardona 2010). However, the safety of raw materials from animal origins is a concern for the swine industry. Ultraviolet (UV) treatment of liquid plasma has been proposed to introduce an additional redundant inactivation step in the manufacturing process of SDP to further enhance biosafety of the final spray‐dried product (Polo et al. 2015; Blázquez et al. 2017).
Ultraviolet exposure at a wavelength of 254 nm (UV‐C) is a nonthermal process that has a germicidal effect by causing thymine‐thymine and thymine‐cytosine dimers in DNA and thymine‐uracil dimers in RNA, which disrupts microbial reproduction (Jagger 1967). During the spray‐drying process, thermal inactivation, high pressure and rapid dehydration are the phenomena involved in microbial inactivation. Although the most important site of damage caused by dehydration is the cytoplasmic membrane (Crowe et al. 1987; Lievense & van 't Riet 1994; Perdana et al. 2013; Huang et al. 2017), dehydration also produces damage to DNA/RNA and protein (Lievense 1992). Thus, the sequential action of both methods for the plasma production process is promising to inactivate micro‐organisms, since both damage different targets involved in microbial inactivation.
Enterotoxigenic Escherichia coli is one of the main causes of enteric disease and death in newborn and weaned pigs (David 2002) and is the major cause of neonatal diarrhoea in calves (Acres 1985). E. coli requires the expression of adhesion fimbriae (adhesins), which are encoded in plasmids, to be adhered to the intestinal epithelium. E. coli expressing K88 adhesin is mainly found in pigs (Gaastra and De Graaf 1982), while K99 is the main adhesion antigen found in bovine species (Tzipori 1981), although K99 can also be found in ovine and porcine species (Gaastra and De Graaf 1982).
The aim of this study was to assess the effectiveness of a UV‐C treatment system on E. coli inactivation after inoculation in fresh unconcentrated liquid porcine plasma. In addition, a second objective was to test the effectiveness of the spray‐drying process on the inactivation of E. coli at two different outlet temperatures, at the regular outlet temperature normally used by the industry (80°C) and at lower outlet temperature (70°C).
Results and discussion
UV‐C test
Plasma inoculated with E.coli K88 strain had an initial count of 6·46 ± 0·04 log10 ml−1. After UV‐C treatment at 3000 J l−1, bacterial counts showed a significant reduction of 4·34 log, describing a curve adjusted to the log linear plus tail model (Fig. 1) with a regression coefficient of R 2 = 0·95 (Table 1). At doses of 6000 and 9000 J l−1, residual E. coli populations of 1·18 ± 0·30 and 1·12 ± 0·30 log10 ml−1 were counted, respectively. The UV‐C doses required to have 4 log10 reduction (log10R) was predicted to be 3105 J l−1.
Figure 1.
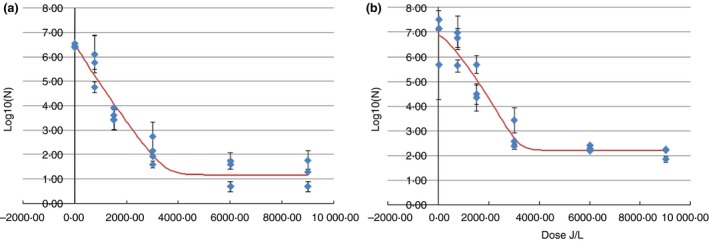
Inactivation kinetics of the strains Escherichia coli K88 (a) and E. coli K99 (b) after UV‐C irradiation. Escherichia coli K88 presented a Log linear plus tail inactivation kinetics while E. coli K99 showed a Weibull plus tail inactivation kinetics. ‘Measured’ indicate the real data obtained during the experiment. ‘Identified’ is the best model fit for prediction kinetics obtained by the GInaFiT program  Measured ;
Measured ;  Identified [Colour figure can be viewed at http://wileyonlinelibrary.com]
Identified [Colour figure can be viewed at http://wileyonlinelibrary.com]
Table 1.
Statistical parameters of the two models for inactivation applied to results obtained with strains Escherichia coli K88 and K99
| E. coli K88 | E. coli K99 | |||
|---|---|---|---|---|
| Log linear plus tail | Weibull plus tail | Log linear plus tail | Weibull plus tail | |
| MSEa | 0·2594 | 0·2686 | 0·3874 | 0·3835 |
| RMSEb | 0·5093 | 0·5182 | 0·6224 | 0·6193 |
| R‐square | 0·9504 | 0·9511 | 0·9167 | 0·9235 |
| R‐square adjusted | 0·9457 | 0·9438 | 0·9048 | 0·9058 |
| 4D reductionc reached at (J l−1) | 3105·9 | 3105·9 | 3427·2 | 3427·2 |
MSE: Mean sum of squared error.
RMSE: Root mean sum of squared error. The lowest RMSE value determines the inactivation model with the best fit. The lowest RMSE value is showed in bold in the table.
4D reduction: UV‐C dose irradiation in J l−1 at which a 4 Log reduction was achieved.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Plasma inoculated with the strain E. coli K99 had an initial count of 6·78 ± 0·67 log10 ml−1. After UV‐C treatment, bacterial counts decreased significantly, showing a curve adjusted with the Weibull plus tail model, with a regression coefficient of R 2 = 0·923 (Table 1). There was a 3·97 log10 ml−1 reduction of the initial count between 0 and 3000 J l−1 (Fig. 1). Residual populations of 2·30 ± 0·08 and 2·11 ± 0·15 log10 ml−1 were counted after irradiation at doses of 6000 and 9000 J l−1, respectively. The 4 log10R was predicted to be achieved at 3427 J l−1.
Spray‐drying test
Full inactivation of strains E. coli K88 and K99 was achieved in all spray‐dried samples dehydrated at an inlet temperature of 220 ± 1°C and both outlet temperatures of 80 ± 1°C or 70 ± 1°C (Table 2).
Table 2.
Effect of the outlet temperature on the inactivation of each Escherichia coli strain tested
| E. coli K88 strain (CFU log10 g−1 solids) ± SD | E. coli K99 strain (CFU log10 g−1 solids) ± SD | |
|---|---|---|
| 80°C outlet air temperature | ||
| Inoculated plasma | 7·31 ± 0·39 | 7·66 ± 0·11 |
| Spray‐dried plasma (SDP) | <1 | <1 |
| 70°C Outlet air temperature | ||
| Inoculated plasma | 6·93 ± 0·5 | 7·44 ± 0·42 |
| SDP | <1 | <1 |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Numerous studies have demonstrated the effectiveness of the spray‐drying process used during the manufacturing of SDP, providing evidence that SDP is a biologically safe product relative to multiple pathogens of concern for the swine industry (Polo et al. 2005; Pujols et al. 2007, 2008, 2014; Gerber et al. 2014). However, it is prudent to evaluate additional biosafety features that may further enhance the robustness of the SDP production process. Exposure to UV‐C is extensively used for the disinfection of liquid media and surfaces due to its germicidal activity (Guerrero‐Beltran 2004; Lin et al. 2012). Previous research has demonstrated that UV‐C treatment of liquid plasma was effective to inactivate Porcine parvovirus (Polo et al. 2015) and Salmonella spp. (Blázquez et al. 2017) inoculated in liquid plasma. During the spray‐drying process, temperature and dehydration are the mechanisms that contribute to microbial mortality (Perdana et al. 2013; Huang et al. 2017), whereas, UV‐C treatment causes damage to nucleic acids (Jagger 1967) and protein‐DNA cross links (Smith 1962).
In this study, UV‐C inactivation kinetics of two strains of E. coli from porcine (K88) and bovine (K99) origins were very similar, although such kinetics fit better to different models, as indicated by the lower RMSE in each case. For both strains of E. coli, a rapid decrease in bacterial count was observed between 0 and 3000 J l−1 of UV‐C, with the appearance of a residual population (Nres) afterwards. These results agree with other UV‐C inactivation studies performed with E. coli (Hijnen 2006). The reduction of the inactivation rate at high UV fluencies (tailing) could be caused by micro‐organism aggregation, appearance of a resistant subpopulation, hydraulic design (Hijnen 2006), matrix effect or particle size effect (Winward 2008). Porcine plasma is a dense, coloured, liquid matrix with 8–10% solids, and contains a complex blend of different proteins with some of the proteins having binding properties (Burnouf 2007). Therefore, the matrix and particle size effects of porcine plasma may have had a special impact on the tailing effects of UV‐C treatment in the present study.
The residual population of E. coli after UV‐C treatment should apparently be eliminated in the subsequent spray‐drying process based on the total inactivation results by the spray‐drying methods at the two outlet temperatures tested (Table 2). The outlet spray‐drying temperature is 80°C for commercial manufacturing of SDP (Pérez‐Bosque et al. 2016) and results of the present study suggest that both E. coli strains are very susceptible to spray‐drying even at a lower outlet temperature (70°C). These results provide confidence that current commercial spray‐drying conditions are highly effective for inactivation of E. coli.
Processing steps should be able to remove or inactivate a wide range of pathogens, according to the World Health Organization (WHO, 2004) guidelines on viral inactivation and removal procedures intended to assure the viral safety of human blood plasma products. These guidelines recommend that two or more robust, effective and reliable processes will be able to remove or inactivate 4 logs or more of viruses. Although the inactivation of viruses has to be considered separately, these guidelines used for virus safety in human plasma transfusion products can be applied to pathogens in general that affect animal blood then UV‐C light treatment at 3000 J l−1 and spray‐drying can be considered two robust safety procedures in the production of SDP since both methods individually inactivated at least 4 log10 E. coli.
In addition, the manufacturing process of SDP has other safety features, such as blood collection from healthy animals declared fit at slaughter for human consumption, pooling of inherent neutralizing antibodies (NA) against potential pathogens, and post‐packaging storage in a dry environment at room temperature for at least 14 days (Pérez‐Bosque et al. 2016). Plasma pooling is also a recognized safety step in the production of certain human plasma products (Solheim et al. 2000, 2006, 2008), since there is successful neutralization of antigens in the presence of NA. Some micro‐organisms in dehydrated form and stored under appropriate constant conditions can remain viable in a unique vitrified state for very long times, even years (Perdana et al. 2013). Spray‐dried plasma has a water activity of <0·6 and is packaged and stored in mild temperatures. The storage conditions for SDP held at room temperature (c. 20°C) for 14 days has been demonstrated effective to inactivate Porcine epidemic diarrhea virus, Porcine reproductive and respiratory syndrome virus and other coronaviruses when these viruses were experimentally inoculated on spray‐dried plasma (Pujols and Segalés 2014; Sampedro et al. 2015). However, it is unknown if these storage conditions affect E. coli or other bacteria survival in spray‐dried plasma. In the present study, the SDP storage temperature effect (20°C for 14 days) on E. coli survival was not tested because both E. coli strains did not survive the spray‐drying process. All the above‐mentioned safety features involved in the manufacturing process of SDP use different inactivation mechanisms, and collectively ensure the biosafety of SDP.
In conclusion, this study provides evidence that affordable levels of UV‐C treatment (3000 J l−1) of liquid porcine plasma can significantly decrease E. coli bacterial counts (4 log10 ml−1 at 3000 J l−1). Furthermore, the study indicated that both UV‐C treatment and spray‐drying as independent safety procedures are very effective for inactivating E. coli K88 and K99. This novel UV‐C technology can be adapted to further enhance the robustness of the manufacturing process for assuring the biosafety of spray‐dried plasma.
Materials and methods
Bacterial strains and test products
Two strains of E. coli were used in the present study: an isolate from swine expressing the K88 adhesin, and a second isolate from bovine expressing the K99 adhesin (both isolates were kindly provided by Dr. Antonio Juárez. University of Barcelona, Spain). A 0·3 ml volume of E. coli isolates was cultured in 100 ml of LB media (Sigma‐Aldrich) at 37°C and 150 rev min−1 for 18 h. The cells were subsequently concentrated by centrifuging (1000g for 20 min at 4°C) using sterilized 40 ml tubes containing 20 ml of culture media. The remaining culture media was removed by resuspending the cell precipitate in 20 ml of sterile 0·01 g mol−1 phosphate buffer saline (PBS). After resuspension, it was centrifuged again as described above and the resulting cell precipitate that was resuspended again in 500 ml of PBS reaching a final titre of 8·98 log10 CFU per ml for K88 and 8·91 log10 CFU per ml for K99 .
Fresh liquid porcine plasma from the production plant of APC Europe S.A., (Granollers, Spain) was used for these trials. This plasma was obtained by centrifugation of blood from pigs processed at a local officially inspected abattoir.
Settings of pilot scale UV‐C system
The UV‐C reactor system (SP1) was designed and manufactured by Sure Pure Operation AG (Zug, Switzerland) that has already been described by Blázquez et al. (2017). The configuration of the pilot scale UV‐C reactor consisted of a closed system with one low pressure mercury UV lamp (30 UV‐C Watts, 254 nm) surrounded by a quartz crystal. The plasma flowed through a steel tube containing a vortex (internal grooved spiral tube that generated a turbulent flow) between the spiral tube and the quartz sleeve. The tangential inlet of the reactor created high velocity and turbulence in the inlet chamber improving liquid contact with the UV‐C light. The liquid was pumped from the inlet chamber into the reactor at a constant flow rate of 4000 l h−1 to achieve a Reynolds value greater than 2800 which is indicative of a turbulent flow (Simmons et al. 2012). Plasma was pumped from the tank to the UV‐C lamp and recirculated many times through this circuit to achieve the required UV‐C dose vs time. The time spent by the liquid to pass through the system once was 7·2 s, delivering 22·95 J l−1 or 23·40 mJ cm−2 per cycle.
UV‐C test
A total of 60 kg of plasma were used for the present study, 30 kg for each of the tested E. coli strains. For each isolate, the 30‐kg batch was divided into three 10‐kg sub‐batches to conduct tests in triplicate. Because liquid fresh plasma from the abattoir may contain different micro‐organisms, the initial 60 kg of plasma product was treated by UV‐C at 10 000 J l−1 for 1 h to inactivate any potential bacteria prior to artificial inoculation with E. coli.
Plasma was spiked with an inoculum of 90 ml of E. coli K88 (ratio 1/330) and 220 ml in the case of E. coli K99 (ratio 1/138). After inoculation, the liquid was recirculated through the UV‐C device for 3 min before activating the UV lamp. At time 0, a non‐processed sample was taken and the UV lamp was activated. During the UV‐C treatment, 150 ml samples were taken when doses reached 750, 1500, 3000, 6000 and 9000 J l−1 (equivalent to 4′47″, 9′51″, 18′54″, 37′34″, 56′00″).
After each UV‐C irradiation dose, 1 mL samples were 10‐fold diluted in peptone water and 0·1 ml inoculated by duplicates onto TBX agar plates (Sigma‐Aldrich) and incubated for 24 h at 37°C. Plates with more than 20 and <300 colonies were counted and results expressed as log10 ml−1.
Spray‐drying test
A total of 7 kg of fresh plasma from a commercial manufacturing plant was previously UV‐C treated at 10 000 J l−1 prior to inoculation with the E. coli strains to eliminate any other bacteria present in the initial raw material. Half amount (3·5 kg) of this UV treated plasma was spiked with the swine E. coli K88 isolate at a ratio of 1/47 reaching a final titer of 7·31 ± 0·39 log10 ml−1 and the other half with the bovine E. coli K99 isolate, at a ratio 1/18 reaching a final titre of 7·66 ± 0·11 log10 ml−1. From each of the 3·5 kg inoculated plasma aliquots, two bottles of 750 ml were obtained and spray‐dried in a lab drier (Büchi Mini Spray Dryer B‐290, Büchi Labortechnik, Switzerland) at two different conditions: inlet temperature at 220 ± 1°C and outlet temperature at 80 ± 1°C or 70 ± 1°C, after stabilization of the spray‐drier with water and non‐inoculated control plasma. All studies were performed in triplicate. Air flow through the column was set at 20–27 m3 h−1 at 20°C. Estimated dwell time was <1 s.
Once SDP was obtained at the two designated outlet temperatures, three tubes containing 0·5 g of dried plasma for each condition were obtained and the dry samples were resuspended in water at a ratio of 1 : 9. From this resuspension, 0·1 ml was seeded in TBX agar for 24 h at 37°C. Colony counting was performed as indicated in the previous section. Results were expressed as a log10 g−1 of solids according to the equation: log10 g−1 = log10(CFU per ml)/[(% solid content of resuspended sample)/100].
Modelling of inactivation
The GInaFiT software was used to calculate and plot nonlinear E. coli survival curves. The log linear plus tail (Geeraerd et al. 2000) and Weibull plus tail (Albert and Mafart 2005) models were tested. The log linear plus tail model (Geeraerd et al. 2005) follows the equation (1):
| (1) |
where k max is the inactivation rate of the log linear part of the curve; N0 is the initial bacterial concentration; t is time and N res is the number of resistant bacteria sub‐population.
The Weibull model plus tail (Albert and Mafart 2005) uses the equation (2):
| (2) |
where N0 is the initial bacterial concentration; t is time; δ parameter represents the time of the first decimal reduction concentration for the part of the population not belonging to N res; p parameter allows to determine concavity or convexity of the curve; and N res is the number of resistant bacteria sub‐population.
Statistical analysis
Data were expressed by means of Log10 values and standard deviations of three independent experimental batches. Mean, standard deviations, anova and F‐test for comparisons were calculated with Excel 2007 (Microsoft Office). The LSD (Least Significant Difference) test was calculated with Statgraphics Centurion XV ver. 15.2.14 (©StatPoint Technologies Inc, Warrenton, Virginia) to determine significant differences between treatments. Differences at P < 0·05 were considered significant.
Mean square error (MSE), goodness of fit in terms of root mean square error (RMSE), correlation coefficient (R 2) and adjusted correlation coefficient (adj‐R 2) values were calculated with the GInaFiT software (Geeraerd et al. 2005). The smallest RMSE determined the inactivation model with the best fit (Geeraerd et al. 2005).
Conflict of Interest
Elena Blázquez, Carmen Rodríguez, Jesús Ródenas and Javier Polo are employed by APC Europe, S.L.U. Joy Campbell and Javier Polo are employed by APC Inc. Both companies manufacture and sell spray‐dried animal plasma. Joan Pujols, Ana Pérez de Rozas and Joaquim Segalés declare no conflict of interest.
Acknowledgements
This study was partly supported by Secretaria de Universitats i Recerca del Departament d'Economia i Coneixement de la Generalitat de Catalunya (2014 DI 066). The funding from CERCA Programme (Generalitat de Catalunya) to IRTA is also acknowledged.
References
- Acres, S. (1985) Enterotoxigenic Escherichia coli infections in newborn calves: a review. J Dairy Sci 68, 229–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert, I. and Mafart, P. (2005) A modified Weibull model for bacterial inactivation. Int J Food Microbiol 100, 197–211. [DOI] [PubMed] [Google Scholar]
- Blázquez, E. , Rodríguez, C. , Ródenas, J. , Pérez de Rozas, A. , Segalés, J. , Pujols, J. and Polo, J. (2017) Ultraviolet (UV‐C) inactivation of Enterococcus faecium, Salmonella choleraesuis and Salmonella typhimurium in porcine plasma. PLoS ONE 12, e0175289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnouf, T. (2007) Modern plasma fractionation. Transfus Med Rev 21, 101–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe, J.H. , Crowe, L.M. , Carpenter, J.F. and Aurell Wistrom, C. (1987) Stabilization of dry phospholipid bilayers and proteins by sugars. Biochem J 242, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David, D. (2002) Enterotoxigenic Escherichia coli infection in pigs and its diagnosis. Swine Heal Prod 10, 171–175. [Google Scholar]
- Gaastra, W. and De Graaf, F.K. (1982) Host‐specific fimbrial adhesins of noninvasive enterotoxigenic Escherichia coli strains. Microbiol Rev 46, 129–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geeraerd, A.H. , Herremans, C.H. and Van Impe, J.F. (2000) Structural model requirements to describe microbial inactivation during a mild heat treatment. Int J Food Microbiol 59, 185–209. [DOI] [PubMed] [Google Scholar]
- Geeraerd, A.H. , Valdramidis, V.P. and Van Impe, J.F. (2005) GInaFiT, a freeware tool to assess non‐log‐linear microbial survivor curves. Int J Food Microbiol 102, 95–105. [DOI] [PubMed] [Google Scholar]
- Gerber, P.F. , Xiao, C.T. , Chen, Q. , Zhang, J. , Halbur, P.G. and Opriessnig, T. (2014) The spray‐drying process is sufficient to inactivate infectious porcine epidemic diarrhea virus in plasma. Vet Microbiol 174, 86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero‐Beltran, J.A. (2004) Advantages and limitations on processing foods by UV light. Food Sci Technol Int 10, 137–147. [Google Scholar]
- Hijnen, W.A.M. (2006) Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo)cysts in water: a review. Water Res 40, 3–22. [DOI] [PubMed] [Google Scholar]
- Huang, S. , Vignolles, M.L. , Chen, X.D. , Le Loir, Y. , Jan, G. , Schuck, P. and Jeantet, R. (2017) Spray drying of probiotics and other food‐grade bacteria: a review. Trends Food Sci Technol 63, 1–17. [Google Scholar]
- Jagger, J. (1967) Introduction to Research in Ultraviolet Photobiology. Englewood, NJ: Prentice Hall Inc. [Google Scholar]
- Lievense, L.C. and van 't Riet, K. (1994) Convective drying of bacteria. II. Factors influencing survival. Adv Biochem Eng Biotechnol 51, 71–89. [PubMed] [Google Scholar]
- Lievense, L.C. (1992) The inactivation of Bacillus plantarum . Chem Eng Sci 47, 87–90. [Google Scholar]
- Lin, C.H. , Yu, R.F. , Cheng, W.P. and Liu, C.R. (2012) Monitoring and control of UV and UV‐TiO2 disinfections for municipal wastewater reclamation using artificial neural networks. J Hazard Mater 209–210, 348–354. [DOI] [PubMed] [Google Scholar]
- Perdana, J. , Bereschenko, L. , Fox, M.B. , Kuperus, J.H. , Kleerebezem, M. , Boom, R.M. and Schutyser, M.A.I. (2013) Dehydration and thermal inactivation of Lactobacillus plantarum WCFS1: comparing single droplet drying to spray and freeze drying. Food Res Int 54, 1351–1359. [Google Scholar]
- Pérez‐Bosque, A. , Polo, J. and Torrallardona, D. (2016) Spray dried plasma as an alternative to antibiotics in piglet feeds, mode of action and biosafety. Porc Heal Manag 2, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo, J. , Quigley, J.D. , Russell, L.E. , Campbell, J.M. , Pujols, J. and Lukert, P.D. (2005) Efficacy of spray‐drying to reduce infectivity of pseudorabies and porcine reproductive and respiratory syndrome (PRRS) viruses and seroconversion in pigs fed diets containing spray‐dried animal plasma. J Anim Sci 83, 1933–1938. [DOI] [PubMed] [Google Scholar]
- Polo, J. , Rodríguez, C. , Ródenas, J. , Russell, L.E. , Campbell, J.M. , Crenshaw, J.D. , Torrallardona, D. and Pujols, J. (2015) Ultraviolet light (UV) inactivation of porcine parvovirus in liquid plasma and effect of UV irradiated spray dried porcine plasma on performance of weaned pigs. PLoS ONE 10(7), e0133008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujols, J. and Segalés, J. (2014) Survivability of porcine epidemic diarrhea virus (PEDV) in bovine plasma submitted to spray drying processing and held at different time by temperature storage conditions. Vet Microbiol 174, 427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujols, J. , Rosell, R. , Russell, L. , Campbell, J. and Crenshaw, J. (2007) Inactivation of swine vesicular disease virus in porcine plasma by spray‐drying. Am Assoc Swine Vet Perry, IA, pp. 281–284. [Google Scholar]
- Pujols, J. , Lopez‐Soria, S. , Segales, J. , Fort, M. , Sibila, M. , Rosell, R. , Solanes, D. , Russell, L. et al (2008) Lack of transmission of porcine circovirus type 2 to weanling pigs by feeding them spray‐dried porcine plasma. Vet Rec 163, 536–538. [DOI] [PubMed] [Google Scholar]
- Pujols, J. , Rodríguez, C. , Navarro, N. , Pina‐Pedrero, S. , Campbell, J.M. , Crenshaw, J. and Polo, J. (2014) No transmission of hepatitis E virus in pigs fed diets containing commercial spray‐dried porcine plasma: a retrospective study of samples from several swine trials. Virol J 11, 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampedro, F. , Snider, T. , Bueno, I. , Bergeron, J. , Urriola, P.E. and Davies, P.R. (2015) Risk assessment of feed ingredients of porcine origin as vehicles for transmission of Porcine Epidemic Diarrhea Virus (PEDv). Natl Pork Board, 1–117. http://research.pork.org/FileLibrary/ResearchDocuments/14-164-DAVIES-UofMN.pdf (Accessed September 4, 2018). [Google Scholar]
- Simmons, M.J.H. , Alberini, F. , Tsoligkas, A.N. , Gargiuli, J. , Parker, D.J. , Fryer, P.J. and Robinson, S. (2012) Development of a hydrodynamic model for the UV‐C treatment of turbid food fluids in a novel “SurePure turbulator™” swirl‐tube reactor. Innov Food Sci Emerg Technol 14, 122–134. [Google Scholar]
- Smith, K. (1962) Dose dependent decrease in extractability of DNA from bacteria following irradiation with ultraviolet light or with visible light plus dye. Biochem Biophys Res Commun 8, 157–163. [DOI] [PubMed] [Google Scholar]
- Solheim, B. , Rollag, H. , Svennevig, J.L. , Arafa, O. , Fosse, E. and Bergerud, U. (2000) Viral safety of solvent/detergent‐treated plasma. Transfusion 40, 84–90. [DOI] [PubMed] [Google Scholar]
- Solheim, B.G. , Cid, J. and Ossealer, J‐C. (2006) Pathogen reduction technologies In: Global Perspectives in Transfusion Medicine. Lozano M, Contreras M, Blajchman M, editors. AABB Press; pp. 103–148. Bethesda, MD, USA. [Google Scholar]
- Solheim, B.G. , Chetty, R. and Flesland, O. (2008) Indications for use and cost‐effectiveness of pathogen‐reduced ABO‐universal plasma. Curr Opin Hematol 15, 612–617. [DOI] [PubMed] [Google Scholar]
- Torrallardona, D. (2010) Spray dried animal plasma as an alternative to antibiotics in weanling pigs. Asian‐Australasian J Anim Sci 23, 131–148. [Google Scholar]
- Tzipori, S. (1981) The aetiology and diagnosis of calf diarrhea. Vet Rec 108, 510–514. [DOI] [PubMed] [Google Scholar]
- WHO (2004) Annex 4 Guidelines on viral inactivation and removal procedures intended to assure the viral safety of human blood plasma products. World Heal Organ 924, 150–224. [Google Scholar]
- Winward, G.P. (2008) Ultraviolet (UV) disinfection of grey water: particle size effects. Environ Technol 29, 235–244. [DOI] [PubMed] [Google Scholar]


