Abstract
In order to evaluate the prevalence of Isospora suis in conventional piglet production in Germany, pooled faecal samples from 327 pig litters from 18 pig production units (20–320 sows each) were examined. At least 10 litters from each farm were investigated. I. suis was present on 83% of the farms and 42.5% of the litters, the infection rate being highest in the third week of age (48.2%). I. suis was found more frequently in samples of diarrhoea than in firm faeces (49.2% compared to 22.2%). Twenty naturally infected piglets from six of these farms underwent examination post mortem, including histology, virology and bacteriology. Histological examination revealed atrophy of the villi in various degrees, mild crypt hyperplasia, fusion of the villi, metaplastic epithelium, erosions and necrosis, especially in the medium and the posterior jejunum and in the ileum. Asexual and sexual developmental stages of the parasite were found in varying numbers in the epithelium of the whole of the small intestine. Bacteria and viruses were mostly excluded as the cause of diarrhoea, and it was concluded that I. suis was the primary pathogen inducing distinct changes and clinical symptoms of diarrhoea.
Introduction
Isospora suis has been described as an important cause of diarrhoea in young suckling piglets in the USA, Canada and Denmark (Bergeland, 1977; Sanford and Josephson, 1981; Henriksen et al., 1989). Various studies have shown that the parasite is also common in German piglet production and that parasitological findings correlate with diarrhoea (Mathea, 1993; Otten et al., 1996; Ilieff, 1997; Meyer et al., 1999). However, these coccidia are often neglected as a primary pathogen since clinical findings are not specific and detection of oocysts can be difficult and is rarely attempted. Piglets with isosporosis develop diarrhoea of varying consistency, mostly at 7 to 15 days of age; overall depression and dehydration can occur. In particular, reduced weight gain and overall poor performance of infected animals are of economic importance, while death occurs only rarely (Sangster et al., 1978; Stuart et al., 1980; Sanford and Josephson, 1981; Lindsay, 1989; Driesen et al., 1993). The aim of the present study was to investigate whether field infections with I. suis are associated with pathomorphological changes in the gut that are related to the clinical symptoms of diarrhoea.
Materials and Methods
From February 1998 to June 1999 pooled faecal samples from 327 pig litters were examined coproscopically for I. suis, and 20 piglets from litters naturally infected with I. suis were examined post mortem. Faecal samples were collected from 18 piglet production units in Eastern Westphalia with herd sizes between 20 and 320 sows and variable management practices. On some farms piglet diarrhoea was a health problem, while on others it occurred only sporadically. On each farm at least 10 litters, preferably those with diarrhoea, were examined between 5 and 28 days of age. Individual faecal samples were taken mostly rectally, from three to five piglets of each litter and pooled. For coproscopy a combined sedimentation‐flotation method was used. Two to three grams of faeces were suspended in tap water and sieved (mesh size: 250 μm) into a 100‐ml glass beaker. After 3 minutes of sedimentation the supernatant was discarded, and the sediment was transferred to a 10‐ml tube and centrifuged (400 × g, 5 min). The supernatant was discarded again and the sediment was resuspended in sodium chloride/zinc chloride solution (specific gravity: 1.53 g/ml; Takla, 1985) with subsequent centrifugation as above. The tube was then filled with salt solution up to a convex meniscus. After 10 min of flotation the meniscus was lifted with a microscopical slide and examined microscopically (100 × magnification). In samples of the necropsied piglets the numbers of oocysts per gram faeces (opg) were counted using a conventional McMaster technique.
Twenty I. suis infected piglets from six farms with a history of isosporosis were examined post mortem, 19 suckling piglets (8–20 days old) and one weaner of unknown age. Animals were chosen according to their clinical symptoms and, in some cases, on the basis of positive results of coproscopy of the litter sample. Immediately before necropsy the piglets were killed by carbon dioxide fumigation or intracardial injection of sodium pentobarbital (EUTHA 77®, Essex Animal Health, Friesoythe, Germany) followed by necropsy, histological, parasitological and, for differential diagnosis, bacteriological and virological examinations. For histology samples were taken from the following locations: duodenum (pars descendens duodeni), cranial, medial and caudal jejunum, ileum, caecum (caudal third) and colon (flexura centralis). Samples were fixed in 10% buffered formaline, embedded in paraffin, cut (3 μm thickness) and stained with haematoxilin‐eosin (HE). Coccidia stages were counted in 10 fields (400 × magnification) and assigned to the developmental (sexual or asexual) stages. Microbiological examination was performed by direct cultivation on blood agar with subsequent subcultivation on MacConkey agar and finally further differentiation on endo‐agar. Salmonella were enriched in Preuß liquid medium, and material was also transferred to liver bouillon to test for clostridia. In positive cases clostridia were differentiated further by toxin typing with a Bio‐X‐Enterotoxaemia ELISA kit (BioX, Brussels, Belgium). Using Bacillus subtilis as the test organism a test for antibiotic residues (three‐plate‐test) was carried out. Using a Slidex Rota‐Kit 2 (Bio Mérieux SA, Lyon, France) and, in parallel, cell culture (cell line MA‐104), rectal or colonic contents were tested for rotavirus. Freeze sections of the caudal jejunum and duodenum were investigated with direct immunofluorescence for the presence of transmissible gastroenteritis (TGE) virus or epizootic virus diarrhoe (EVD) virus.
The χ2 test was applied to compare percentages; for low numbers of cases, Fisher's exact test was used. Differences were considered significant at P ≤ 0.05.
Results
Coproscopical examinations in piglet production units
Isospora suis was found in 15 (83%) of the 18 units investigated. In all, 42.5% of the 327 litters were positive (Table 1). Prevalence was highest in pigs at 15–21 days of age (48.2%). At the age of 5–14 days 40.1% of the litters excreted oocysts; 35.7% of the litters at 22–28 days old were positive. With regard to the farm size I. suis was found more often in small and medium‐sized herds than in large herds (Table 1). Litters kept in farrowing units with straw bedding (n=152) were infected significantly (P ≤ 0.05) more often (49.3%) than those on slatted floors (36.6%; n=175). Occurrence of I. suis was inversely related to the proportion of the perforated floor area. While in units where 50% or less of the floor area was perforated 51.3% of the litters shed I. suis, only 8.3% of the litters on mainly perforated floors did so (P ≤ 0.001).
Table 1.
. Numbers of piglet production farms and percentages of I. suis positive litters

Litters with diarrhoea (i.e. pasty or liquid faeces) were significantly more often infected with I. suis (P ≤ 0.001) than those without. While 49.2% of the litters with diarrhoea (n=264) were Isospora‐positive only 22% of the litters without diarrhoea (n=81) shed oocysts. Similarly, litters from 10 farms where diarrhoea was considered to be a herd problem had higher prevalence rates (53.3% of 246 litters) than those from other farms (9.9% of 81 litters) (P ≤ 0.001).
Strongyloides ransomi eggs were found in five samples (1.5%), Eimeria oocysts in one (0.3%) of 327 litters; all six samples were from one farm.
Examination of Isospora‐positive piglets
Clinical and parasitological findings
Of the 20 piglets, 14 were 8–15 days old, five 17–20 days, one was older than 21 days. Ten animals were slightly depressed, one was severely depressed and nine behaved normally. The nutritional status was poor in 17 cases. Eight piglets were dehydrated. Eighteen animals had diarrhoea (Fig. 1). Isospora was found in 11 faecal samples, nine of which were diarrhoeic. Diarrhoeic samples which were negative for I. suis were mostly liquid or watery, while the positive samples were of pasty or semi‐liquid consistency and yellow‐grey or light yellow in colour. Oocyst counts showed values of 102–105 opg, being highest in samples with pasty consistence and yellow‐grey colour.
Figure 1.

. Diarrhoea, dehydration and emaciation during acute isosporosis in a suckling piglet.
Macroscopical findings
At gross post‐mortem examination 18 piglets showed diffuse hyperaemia of the small intestinal wall with liquid, yellow contents which in some cases contained mucus or small milk curds. In one case the posterior part of the small intestine was covered with a thick yellow‐grey pseudo‐membranous fibrin layer; another animal showed small flakes of fibrin in the liquid intestinal contents. The mesenteric lymph nodes were enlarged in 13 cases; 19 animals had a well‐filled stomach.
Microscopical findings (Fig. 2A–D)
Figure 2.
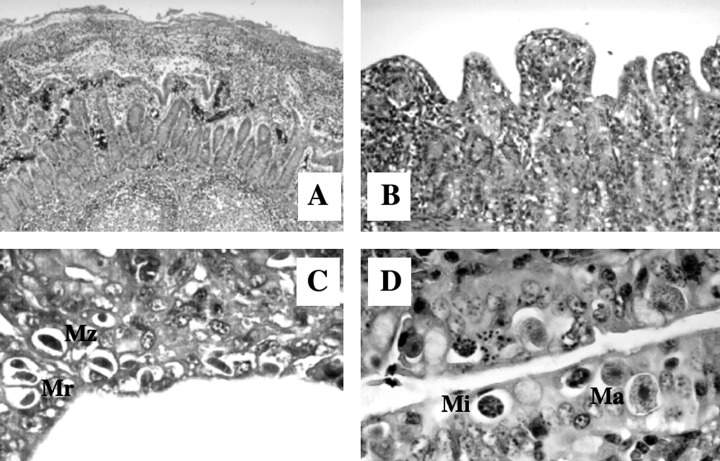
. Porcine isosporosis in naturally infected piglets at 8–14 days of age. HE staining. (A) Fibrinous, necrotic enteritis of the caudal jejunum (10 × magnification). (B) Villous atrophy and fusion with epithelial flattening and crypt hyperplasia of the medial jejunum (63 × magnification). (C) Merozoites (Mz) and type I meronts (Mr) in the epithelium of the medial jejunum (250 × magnification). (D) Macrogamonts (Ma) and microgamonts (Mi) in the ileal epithelium (250 × magnification).
Histological examination revealed atrophy of the villi in different grades (often severe), especially in the medial and caudal jejunum and in the ileum, accompanied by mild crypt hyperplasia. The villi were reduced and frequently fused, covered with metaplastic, flat to cubic epithelium or with focal to multifocal loss of epithelium or necrosis. Occasionally, the superficial epithelium was covered with thin layers of fibrinous threads, detritus, inflammatory cells and bacteria. One piglet suffered from diphtheroid‐necrotic enteritis. The lamina propria showed mild to moderate accumulation of mononuclear cells as well as a moderate to high focal to multifocal infiltration with neutrophilic and eosinophilic granulocytes. Mild oedema was found in some cases, and in one it was severe.
Endogenous stages of I. suis could be found in the ileum and jejunum, and in one case also in the caecum and colon. The medial (93%) and caudal (85%) jejunum and the ileum (75%) were affected significantly (P ≤ 0.05) more often than the cranial jejunum (33%). The parasitic stages, which were surrounded by a parasitophorous vacuole, were mainly found in the epithelium of the distal ends of the villi; at high levels of infection parasites were also found at the base of the villi and occasionally in the epithelium of the crypts. In one case few goblet cells were also infected. In areas with extensive epithelial damage, few or no parasites were found. In all, more asexual (84.8%) than sexual (15.2%) stages were found in the epithelium. Of the nine piglets which had been negative at coproscopy, three harboured exclusively asexual stages, three mainly asexual stages, and three were only weakly infected. The piglets that had been coproscopically positive for Isospora all contained sexual stages in their epithelium.
Differential diagnostic findings
Sixteen out of 20 I. suis‐positive piglets had infections with one or more bacterial or viral agents. E. coli could be isolated from 12 animals, with three isolates growing with haemolysis on blood agar (E. coli vs. h). Five out of 10 samples tested for C. perfringens type A were positive. Two bacteriologically negative animals were positive for antibiotic residues. Rotavirus was found in one case, while neither TGE nor EDV viruses were found in the 19 animals examined. Neither S. ransomi nor Cryptosporidium parvum were found.
Discussion
In concordance with Otten et al. (1996) and Meyer et al. (1999) the results of the present study show that in Germany I. suis is widely distributed amongst young suckling piglets and that infection is associated with diarrhoea. In other studies, coccidia were also found in 60–80% of the farms investigated (Driesen et al., 1993; Eysker et al., 1994; Ilieff, 1997). The average litter infection rate lies in the range of those (36.3%) found in the Netherlands by Eysker et al. (1994). Higher (cumulative) infection rates of 53.8–62.2% were found when litters were examined several times in weekly intervals (Eysker et al., 1994; Otten et al., 1996; Meyer et al., 1999). We found that the highest rates of infection of the litters was in the third week of age, which is in concordance with Otten et al. (1996). In contrast, Meyer et al. (1999) found that in large piglet production units with good management the prevalence increased until the fourth week, which Daugschies et al. (1999) put down to slower spread of the infection on farms with good hygiene management. According to Lindsay et al. (1992) and Otten et al. (1996) isosporosis can occur on any farm, independently of the size or management. However, we found that I. suis was regularly found in small to medium‐sized units and on farms with straw bedding. Additionally, the prevalence was negatively correlated with the percentage of perforated floor areas, perhaps due to less contact between the piglets and their faeces, which is in concordance with the results of other authors (Sayd and Kawazone, 1996; Meyer et al., 1999). Sangster et al. (1978) speculated that isosporosis is more common in units with straw bedding due to a poor hygienic status. Meyer et al. (1999), however, frequently found the parasite also in large, well‐managed piglet production units, while above‐average hygiene measures in a nucleus herd were associated with low infection rates. Contaminated farrowing units are probably the most important source of infection of the piglets (Lindsay, 1989), and consequently hygiene is of utmost importance to lower the infection pressure; however, even after thorough cleaning and disinfection few oocysts can remain which are sufficient for the spread of infection (Christensen and Henriksen, 1994).
The necropsied piglets mostly displayed signs of disease described in the literature. Only two animals had no diarrhoea despite oocyst excretion; however, the course of disease can also be subclinical (Matuschka and Heydorn, 1980; Stuart et al., 1982; Otten et al., 1996; Meyer et al., 1999). As previously described (Otten et al., 1996; Meyer et al., 1999) oocysts were found most frequently in faecal samples of pasty consistence and yellow‐grey colour. This should be taken into consideration for diagnostic sampling; however, it has to be kept in mind that the ingestion of pre‐starter feed or turf can influence the colour of the faeces.
At necropsy we found that, in concordance with other authors (Sangster et al., 1978; Eustis and Nelson, 1981), in cases of isosporosis macroscopic alteration is often only discrete and obvious presudomembranous fibrinous layers are rare. Histological lesions were found mainly in the medial and caudal jejunum and in the ileum, and in these sections parasite stages in the epithelium were most common. We therefore recommend that several tissue samples be removed from these areas of the small intestines. The microscopic alterations of the intestinal mucosa were also described by other authors for piglets with natural (Eustis and Nelson, 1981; Vitovec and Koudela, 1987) or experimental (Matuschka and Heydorn, 1980) isosporosis. In cases of field infections more asexual than sexual stages were found in the epithelium (Sanford and Josephson, 1981; Chae et al., 1998), which is in agreement with our findings. Piglets infected primarily with asexual stages at necropsy displayed the most prominent pathohistological changes of the intestinal mucosa, as described by Stuart et al. (1980) and Robinson et al. (1983), and the stages can be considered to be the more pathogenic. The position of the endogenous stages in the epithelium was identical to previous observations (Lindsay et al., 1980; Matuschka and Heydorn, 1980; Harleman and Meyer, 1984); occasionally the large intestines are affected without significant histological alterations (Sangster et al., 1978; Sanford and Josephson, 1981; Harleman and Meyer, 1984). Several animals harboured only few parasites in the different intestinal sections despite distinct mucosal alterations, as previously described by Current (1987) and Lindsay et al. (1999). According to Stuart et al. (1982) the degree and distribution of the Isospora‐induced alterations depend on the age of the piglet and the infection dose. Since in cases of field infections time and dose of infection are unknown, we could not correlate the pathohistological changes to the infection rate of the epithelium. However, it could be demonstrated that infection of the intestinal epithelium with coccidia is unambiguously correlated with pathohistological changes of varying degrees. Isosporosis was diagnosed more often by histology than by coproscopy only, so that the histological examination of piglets sacrificed for diagnostic necropsy should be part of Isospora diagnosis.
In the cases we investigated other enteropathogens were probably of only minor importance as causes of diarrhoea. Faecal examination of the litters showed that S. ransomi and Eimeria spp. occured only rarely in suckling piglets and, as is the case for Cryptosporidium, were negligible as causative agents for diarrhoea. Similarly, viruses were found only rarely in our investigations. Coronaviruses, which cause TGE and EVD, could not be isolated. In general, these agents seem to have declined in importance since PRCV (porcine respiratory coronavirus) was found in Europe (Paul et al., 1994) and are more common in weaners (Heinritzi et al., 1990). Rotavirus was found in a 20‐day‐old piglet; however, this animal did not display more distinct lesions of the small intestine than those with pure Isospora infections as described by some authors (Eustis and Nelson, 1981; Vitovec and Koudela, 1987), which is probably due to the age of the animal. Pure rotavirus infections often do not cause any symptoms due to the ubiquity of the virus and the immune protection of the piglets (Waldmann and Plonait, 1997).
Bacteriological testing excluded Salmonella infections. C. perfringens type A can lead to mild villous atrophy, but it can also be found in the faeces of healthy animals (Waldmann and Plonait, 1997). C. perfringens type A was found in five out of 10 animals examined. A synergistic effect of these bacteria with I. suis, as described by Nabuurs et al. (1983) for rotaviruses, cannot be excluded and requires further investigations. E. coli was found in 12 animals. Enterotoxic E. coli (ETEC) are amongst the most important causes for diarrhoea in suckling piglets and weaners; however, the cause for diarrhoea is hypersecretion with little or no mucosal lesions of the mucosa (Waldmann and Plonait, 1997). Only three of the 12 E. coli isolates were haemolytic and have to be considered as pathogenic for piglets. In previous works ETEC were also only rarely found in Isospora‐infected piglets (Ilieff, 1997; Chae et al., 1998). The rare occurrence of pathogenic E. coli in our study is probably due to vaccination of the sows.
While Driesen et al. (1993) and Ilieff (1997) diagnosed I. suis infections mainly as monoinfections, Meyer et al. (1999) showed that isosporosis is frequently associated with E. coli infections. Bergeland (1977) hypothesized that necrotic enteritis in the course of isosporosis is due to multicause infections with viruses and bacteria. We only found necrotic enteritis in one case of isosporosis with concurrent infection with C. perfringens type A. It can be assumed that secondary pathogens can exacerbate an Isospora infection, and that the infection rate of the epithelium with parasite stages causing primary damage plays a crucial role for the course of the disease. Competing infectious agents make diagnosis more difficult and may be misinterpreted as primary causes of diarrhoea (Greve, 1985; Lindsay et al., 1992), so that a complete diagnosis even after isolation of one enteropathogen is important (Chae et al., 1998).
The importance of I. suis as a primary pathogen has been demonstrated by several experimental studies (Stuart et al., 1980; Robinson et al., 1983; Harleman and Meyer, 1984; Vitovec and Koudela, 1990) and is substantiated by the present work. The results of coproscopy and necropsy make it clear that under managment practices common in Germany I. suis infections are correlated with diarrhoea. Higgins (1999) hypothesized that in the United Kingdom isosporosis is commonly overlooked, and in cases of diarrhoea and poor performance of suckling piglets Isospora‐directed diagnosis should be strongly considered. The increasing awareness of I. suis as an important enteropathogen in suckling piglets led to the recommendation of Martineau and Del Castillo (1999) to use toltrazuril (Baycox®., Bayer) for `diagnostic therapy' in cases where clinical and epidemiological findings are characteristic. The present investigation substantiates the impression that I. suis is also a primary cause of diarrhoea under the conditions of piglet production in Germany.
References
- 1. Bergeland, E. M. , 1977: Necrotic enteritis in nursing piglets. Proc. Annu. Meet. Am. Assoc. Vet. Laboratory Diagn. 20 , 151–158. [Google Scholar]
- 2. Chae, C. , Kwon D., Kim O., Min K., Dheon D. S., Chois C., Kim B., and Suh J., 1998: Diarrhoea in nursing piglets associated with coccidiosis: prevalence, microscopic lesions and coexisting microorganisms. Vet. Rec. 143 , 417–420. [DOI] [PubMed] [Google Scholar]
- 3. Christensen, J. B. P. , and Henriksen S. A., 1994: Shedding of oocysts in piglets experimentaly infected with Isospora suis . Acta Vet. Scand. 35 , 165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Current, W. L. , 1987: Neonatal swine coccidiosis. Anim. Health Nutr. 42 , 8–10. [Google Scholar]
- 5. Daugschies, A. , Meyer C., and Joachim A., 1999: Vorkommen von Isospora suis . Ferkelerzeuger- Ferkelaufzuchtbetrieben. Prakt. Tierarzt 80 , 530–537. [Google Scholar]
- 6. Driesen, S. J. , Carland P. G., and Fahy V. A., 1993: Studies on preweaning piglet diarrhoea. Aust. Vet. J. 70 , 259–263. [DOI] [PubMed] [Google Scholar]
- 7. Eustis, S. L. , and Nelson D. T., 1981: Lesions associated with coccidiosis in nursing piglets. Vet. Pathol. 18 , 21–28. [DOI] [PubMed] [Google Scholar]
- 8. Eysker, M. Boerdam G. A., Hollanders W., and Verheijden J. H. M., 1994: The prevalence of Isospora suis and Strongyloides ransomi in suckling piglets in the Netherlands. Vet. Q. 16 , 203–205. [DOI] [PubMed] [Google Scholar]
- 9. Greve, E. , 1985: Isospora suis species in a Danish SPF herd. Nord. Veterinaermed. 37 , 140–144. [PubMed] [Google Scholar]
- 10. Harleman, J. H ., and R. C. Meyer , 1984: Life cycle of Isospora suis in gnotobiotic and conventionalized piglets. Vet. Parasitol. 17 , 27–39. [DOI] [PubMed] [Google Scholar]
- 11. Harleman, J. H. , and Meyer R. C., 1985: Pathogenicity of Isospora suis in gnotobiotic and conventionalised piglets. Vet. Rec. 116 , 561–565. [DOI] [PubMed] [Google Scholar]
- 12. Heinritzi, K. , Plank G., and Eichhorn W., 1990: Neue Aspekte im klinischen Verlauf der Coronavirusinfektion der Schweine. Tierärztl. Umsch. 45 , 39–44. [Google Scholar]
- 13. Henriksen, S. A. , Christensen J. P. B., and Yvore P., 1989: Coccidiosis in piglets in Denmark. Shedding of oocysts of Isospora suis in relation to the age of the host. In: Yvore, P., (ed.), Coccidia and Intestinal Coccidimorphs, pp. 489–492. INRA, Paris.
- 14. Higgins, R. J. , 1999: Diagnosis of porcine coccidiosis. Pig J. 43 , 80–87. [Google Scholar]
- 15. Ilieff, A. , 1997: Untersuchungen zur Verbreitung und zur Epidemiologie von enteropathogenen Infektionserregern bei durchfallkranken Saug‐ und Absatzferkeln. Gießen, Justus‐Liebig‐University, Fachber. Veterinärmed., Diss.
- 16. Lindsay, D. S. , 1989: Diagnosing and controlling Isospora suis in nursing pigs. Vet. Med. 84 , 443–446. [Google Scholar]
- 17. Lindsay, D. S. , Blagburn B. L., and Dubey J. P., 1999: Coccidia and other protozoa. In: B. E. Straw, S. D'Allaire, W. L. Mengeling, and D. J. Taylor (eds.), Disease of Swine, 8th edn., pp. 655–660. Iowa State University Press, Ames, Iowa.
- 18. Lindsay, D. S. , Blagburn B. L., and Powe T. A., 1992: Enteric coccidial infections and coccidiosis in swine. Comp. Contin. Educ. Pract. Vet. 14 , 698–702. [Google Scholar]
- 19. Lindsay, D. S. , Stuart P., Wheat B. E., and Ernst J. V., 1980: Endogenous development of the swine coccidium Isospora suis Biester 1934. J. Parasitol. 66 , 771–779. [PubMed] [Google Scholar]
- 20. Martineau, G. P. , and Del Castillo J., 1999: Epidemiological, clinical and control investigations on field porcine coccidiosis: Clinical, epidemiological and parasitological paradigms?. Diseases related to Protozoa and Possibilities for Treatment. 17th International Conference on WAAVP. Copenhagen, Bayer Workshop, 9–12. [DOI] [PubMed]
- 21. Mathea, J. , 1993: Vorkommen von Isospora suis und Cryptosporidium parvum beim Ferkel in Schweinebeständen mit unterschiedlichen Haltungsbedingungen. Berlin: Freie University, Fachber. Veterinärmed., Diss.
- 22. Matuschka, F. R. , and Heydorn O. A., 1980: Die Entwicklung von Isospora suis Biester und Murray 1934, (Sporozoa: Coccidia: Eimeriidae) im Schwein. Zool. Beiträge 26 , 405–476. [Google Scholar]
- 23. Meyer, C. , Joachim A., and Daugschies A., 1999: Occurrenc of Isospora suis in larger piglet production units and on specialized piglet rearing farms. Vet. Parasitol. 82 , 277–284. [DOI] [PubMed] [Google Scholar]
- 24. Nabuurs, M. J. A. , Haagsma J., Van der Molen E. J., and Van der Heijden J., 1983: Diarrhea in one to three week‐old piglets associated with Clostridium perfringens Typ A. Ann. Rech. Vet. 14 , 408–411. [PubMed] [Google Scholar]
- 25. Otten, A. , Takla M., Daugschies A., and Rommel M., 1996: Untersuchungen zur Epizootiologie und pathogenen Bedeutung von Infektionen mit Isospora suis in zehn Ferkelerzeugerbetrieben in Nordrhein‐Westfalen. Berl. Münch. Tierärztl. Wschr. 109 , 220–223. [PubMed] [Google Scholar]
- 26. Paul, P. S. , Halbur P. G., and Vaughn E. M., 1994: Significance of porcine respiratory coronavirus infection. Com. Cont. Educ. Pract. Vet. 16 , 1223–1234. [Google Scholar]
- 27. Robinson, Y. , Morin M., Girard C., and Higgins R., 1983: Experimental transmission of intestinal coccidiosis to piglets. Can. J. Comp. Med. 47 , 401–407. [PMC free article] [PubMed] [Google Scholar]
- 28. Sanford, S. E. , and Josephson G. K. A., 1981: Porcine neonatal coccidiosis. Can. Vet. J. 22 , 282–285. [PMC free article] [PubMed] [Google Scholar]
- 29. Sangster, L. T. , Stuart B. P., Williams D. J., and Bedell D. M., 1978: Coccidiosis associated with scours in baby pigs. Vet. Med. Small Anim. Clin. 73 , 1317–1319. [PubMed] [Google Scholar]
- 30. Sayd, S. M. O. , and Kawazone U., 1996: Prevalence of porcine neonatal isosporosis in Brazil. Vet. Parasitol. 67 , 196–174. [DOI] [PubMed] [Google Scholar]
- 31. Stuart, B. P. , Gosser H. S., Allen C. B., and Bedell D. M., 1982: Coccidiosis in swine: dose and age response to Isospora suis . Can. J. Comp. Med. 46 , 317–320. [PMC free article] [PubMed] [Google Scholar]
- 32. Stuart, B. P. , Lindsay D. S., Ernst J. V., and Gosser H. S., 1980: Isospora suis enteritis in piglets. Vet. Pathol. 17 , 84–93. [DOI] [PubMed] [Google Scholar]
- 33. Takla, M. , 1985: Coproscopical diagnosis of fluke eggs in cattle – an experimental report. Zbl. Bakt. Hyg. A. 260 , 419 419. [Google Scholar]
- 34. Vitovec, J. , and Koudela B., 1987: Pathology of natural isosporosis in nursing piglets. Folia Parasitol. 34 , 199–204. [PubMed] [Google Scholar]
- 35. Vitovec, J ., and B. Koudela , 1990: Double alteration of the small intestine in conventional and gnotobiotic piglets experimentally infected with coccidium Isospora suis . Folia Parasitol. 37 , 211–215. [PubMed] [Google Scholar]
- 36. Waldmann, K. H. , and H., Plonait , 1997: Erkrankungen der Verdauungsorgane und des Abdomens. In: Plonait, H., and. K. Bickhardt (eds.), Lehrbuch der Schweinekrankheiten. pp. 307–386. Paul Parey, Berlin.


