Abstract
Summary: Humoral immunity following vaccination or infection is mainly derived from two types of cells: memory B cells and plasma cells. Memory B cells do not actively secrete antibody but instead maintain their immunoglobulin in the membrane‐bound form that serves as the antigen‐specific B‐cell receptor. In contrast, plasma cells are terminally differentiated cells that no longer express surface‐bound immunoglobulin but continuously secrete antibody without requiring further antigenic stimulation. Pre‐existing serum or mucosal antibody elicited by plasma cells (or other intermediate antibody‐secreting cells) represents the first line of defense against reinfection and is critical for protection against many microbial diseases. However, the mechanisms involved with maintaining long‐term antibody production are not fully understood. Here, we examine several models of long‐term humoral immunity and present a new model, described as the ‘Imprinted Lifespan’ model of plasma cell longevity. The foundation of this model is that plasma cells are imprinted with a predetermined lifespan based on the magnitude of B‐cell signaling that occurs during the induction of an antigen‐specific humoral immune response. This represents a testable hypothesis and may explain why some antigen‐specific antibody responses fade over time whereas others are maintained essentially for life.
Keywords: immunological memory, plasma cell, memory B cell, antibody
Memory B‐cell‐dependent and ‐independent models of long‐term antibody production
Several models of humoral immunity have been developed to explain or predict the duration of humoral immunity following infection or vaccination ( Fig. 1 ). Memory B‐cell‐dependent models are based on the requirement of memory B cells to become activated, proliferate, and differentiate into antibody‐secreting daughter cells that intermittently or continuously repopulate the antibody‐secreting plasma cell pool. These models are based on either antigen‐specific stimulation via chronic infection, repeated infection, booster vaccination, persisting antigen in the form of immune complexes, or non‐antigen‐specific polyclonal B‐cell stimulation via Toll‐like receptor (TLR) engagement or bystander T‐cell activation. There are also two models of memory B‐cell‐independent maintenance of long‐term antibody production, and these are based on the concept that memory B cells and plasma cells represent two independently regulated B‐cell populations and that the duration of antibody production by plasma cells is determined either by competition for space in immunological niches, such as the bone marrow, or is based on the principle that antigen‐specific plasma cells have a predetermined lifespan.
Figure 1.
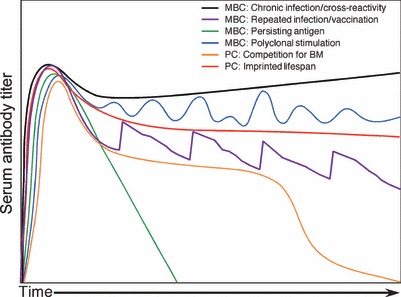
Models of sustained humoral immunity. Several memory B‐cell (MBC)‐dependent and ‐independent models have been developed to explain how long‐term antibody responses are maintained. In this figure, we illustrate how antigen‐specific antibody responses might be maintained under the different proposed models. Chronic infection or cross‐reactivity to either self or environmental antigens is expected to stimulate memory B cells to proliferate and differentiate into antibody‐secreting daughter cells and result in increasing antibody responses over time due to continuous stimulation and accumulation of memory B cells and plasma cells. Repeated infection or booster vaccination will likely lead to periodic increases in antigen‐specific memory B‐cell activation and subsequent increases in antibody responses that would decline during the intervening periods between outbreaks or vaccinations. Persisting antigen in the form of antibody:antigen immune complexes on the surface of follicular dendritic cells (FDCs) will stimulate memory B cells in an antigen‐specific manner, resulting in antibody responses that will decline at the rate of antigen decay or consumption by the memory B‐cell pool. Non‐antigen‐specific polyclonal memory B‐cell stimulation by Toll‐like receptor (TLR) engagement or bystander T‐cell activation will trigger antibody responses to spike during heterologous infections or vaccinations and increase antibody responses to all pre‐existing antibody specificities. Alternatively, long‐term antibody responses may be maintained by long‐lived plasma cells (PCs), and two models are proposed. One model is based on plasma cell competition for space in the bone marrow in which pre‐existing plasma cells are dislodged by incoming plasmablasts, and antibody responses decline as a function of plasma cell displacement. Since there is finite space in the bone marrow, this model would suggest that antibody responses will decline more rapidly during advanced age as a function of increased competition in the bone marrow compartment. Another model of long‐lived plasma cells is based on the theory that plasma cells are imprinted with a specified lifespan, which is determined during the induction phase of the antigen‐specific antibody response.
To decipher which model (or models) best depict the outcome of humoral immune responses over time, we compared these models to biological observations made during the longitudinal analysis of serum antibody responses against eight different virus and vaccine antigens in human subjects ( Fig. 2 ) monitored for up to 26 years (1). In these studies, we examined serological memory in 45 subjects who provided a total of 630 serum samples that had been banked at the Oregon National Primate Research Center (ONPRC), and the immunological profiles of two representative subjects are presented here. By comparing the various models of humoral immunity ( Fig. 1 ) to the observations made during these longitudinal experiments ( Fig. 2 ), we hope to gain a better understanding of the different factors that are involved with maintaining long‐term antibody responses.
Figure 2.
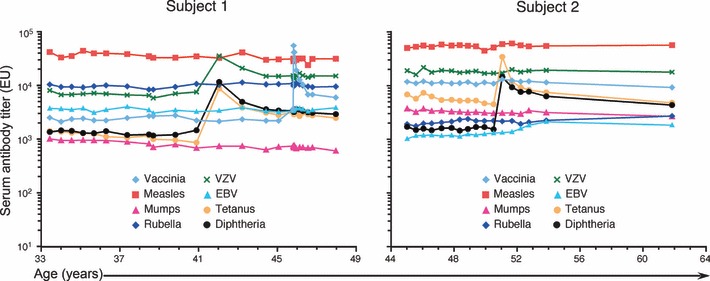
Longitudinal analysis of antigen‐specific antibody responses in two representative subjects. Antigen‐specific serum antibody responses were followed in two subjects from the Oregon National Primate Research Center (ONPRC) cohort (1) and show the durability of humoral immunity during middle age (Subject 1) and more advanced age (Subject 2). Both subjects were vaccinated against tetanus and diphtheria, and Subject 1 also received smallpox vaccination (i.e. vaccinia virus infection). Antibody responses were determined by ELISA as previously described (1). VZV, varicella‐zoster virus; EBV, Epstein–Barr virus.
Chronic infection or cross‐reactive antigen models
One mechanism for maintaining prolonged antibody responses is through continued antigen presentation or internal ‘boosting’ of the immune response by viruses that cause chronic infection ( Fig. 1 ). This model represents only a subset of potential immunological experiences, because many pathogens are unable to sustain a chronic or persistent infection. In these cases, memory B cells exposed to chronic viral or microbial antigens would be triggered to proliferate and differentiate into antibody‐secreting plasma cells and either maintain or even inflate serological memory through a memory B‐cell‐dependent mechanism. Alternatively, idiotypic networks or cross‐reactivity to environmental or self‐antigens may result in chronic stimulation of an antibody response. The potential for these latter models is debatable, since it would be difficult to envision how antigen‐specific affinity maturation would occur if the antibodies are reacting to other antibody molecules [i.e. idiotypic networks (2)] or to other self or environmental antigens, given that affinity maturation ceases shortly after vaccination and is resumed only after booster vaccination with the same antigen (3). Moreover, if continuous cross‐reactive B‐cell activation is the mechanism for maintaining long‐term antibody responses, then it is difficult to explain why some antibody responses decline whereas others are maintained for decades or in some cases for life. These types of discrepancies may indicate why the cross‐reactive models of humoral immunity have largely fallen out of favor in recent years.
We studied the duration of humoral immunity against two common chronic/latent viral infections, Epstein–Barr virus (EBV) and varicella zoster virus (VZV). EBV‐specific antibody responses were maintained at steady‐state levels (T 1/2 = 11 552 years; 95% CI, 63 years to infinity) ( Table 1 ) with no significant decrease in titer over time [P = 0.99, (1)]. Human cytomegalovirus (CMV) causes a persistent infection that not only induces strong antiviral antibody responses but also causes ‘immunological memory inflation’ with virus‐specific antibody titers that continue to increase incrementally for years after infection (IJ Amanna and MK Slifka, unpublished data). In contrast, antibody responses against VZV declined slowly but significantly over time [P = 0.005, (1)] with an estimated 50‐year half‐life (95% CI, 30–153 years) ( Table 1 ). This time was substantially shorter than the calculated antibody half‐life of antibody responses mounted against other viruses that cause acute infection ( Table 1 ) and indicates that a latent infection does not necessarily elicit the most durable antibody response. From a virological perspective, these results also indicate that when VZV is latent, it appears to be truly sequestered from the host immune system. This observation may also explain why pediatric vaccination against VZV, which is based on a live, attenuated herpesvirus (Varivax®, Merck, Whitehouse Station, NJ, USA) that can form latency (4, 5, 6) still requires booster vaccination in order to sustain protective immunity against natural VZV infection (1, 7).
Table 1.
Duration of antibody responses after infection or vaccination*
| Virus/vaccine antigen | Mean antibody half‐life (95% confidence interval) |
|---|---|
| Tetanus | 11 (10–14) |
| Diphtheria | 19 (14–33) |
| Varicella zoster virus | 50 (30–153) |
| Vaccinia | 92 (46–∞) |
| Rubella | 114 (48–∞) |
| Mumps | 542 (90–∞) |
| Measles | 3014 (104–∞) |
| Epstein–Barr virus | 11 552 (63–∞) |
*Duration of antigen‐specific antibody responses (in years) was determined by a mixed‐effect model of longitudinal analysis.
Adapted from Ref. (1).
Repeated infection or booster vaccination models
Prior to vaccine‐mediated control of measles, mumps, and rubella, the prolonged immunity induced by these acute viral infections was thought to be maintained, at least in part, by periodic reinfection during the course of intermittent outbreaks ( Fig. 1 ). In this model, antibody responses would uniformly decline until the next outbreak/exposure, resulting in a sharp spike in antibody titer that would again be followed by fading immunological memory and then the cycle would be repeated. Although reinfection or revaccination will trigger either a brief spike in antibody titers (1, 8) or prolonged antibody responses that are raised to a higher maintained level (1, 8), it is becoming clear that repetitive antigenic exposure is not an absolute requirement for maintaining long‐term antibody responses. Following longitudinal analysis over the course of up to 26 years (1), antibody responses were maintained for decades in the absence of local outbreaks of measles, mumps, and rubella. This observation is consistent with historical evidence of protective long‐term immunity in the absence of endemic virus exposure (9, 10).
Booster vaccination is another approach to maintaining long‐term immunity and booster vaccination against tetanus is recommended every 10 years due to the possibility that immunity is lost relatively quickly in the absence of revaccination. However, in cases of high levels of pre‐existing antibody, booster vaccination only provides a temporary increase in antibody production with antibody titers often declining back to nearly the original prebooster vaccination levels within 6 months (8) or within 2–3 years (1) ( Fig. 2 ). Both of the subjects presented in Fig. 2 received booster tetanus vaccination, which coincidentally results in booster diphtheria vaccination, since >90% of adult tetanus shots in the US also contain diphtheria toxoid. Subject 1 demonstrated a nearly 10‐fold increase in tetanus‐specific antibody titers following vaccination, and these levels declined relatively quickly for about 2 years before reaching a new plateau by 3 years postvaccination. At this point, the antibody response against both tetanus and diphtheria reached homeostasis at levels that were about two‐ to threefold higher than the prebooster vaccination levels. Similar to Subject 1, Subject 2 demonstrated a robust anamnestic antibody response against both tetanus and diphtheria following booster vaccination, and the antibody responses likewise decayed rapidly for the first 3 years after vaccination before reaching a plateau with more durable antibody production. Interestingly, once reaching homeostasis, the diphtheria‐specific antibody titers of Subject 2 stabilized at levels that were nearly fourfold higher than the original diphtheria‐specific titers, whereas the tetanus‐specific antibody levels (which were already relatively high) stabilized at titers that were less than twofold higher than their original level. This is consistent with previous studies on tetanus vaccination demonstrating that booster vaccination increased the frequency of memory B cells without necessarily increasing tetanus‐specific antibody titers (11). Once antibody responses following tetanus and diphtheria vaccination have stabilized, they decline with a half‐life of approximately 11 years (1, 12) and 19 years (1), respectively ( Table 1 ). In addition to booster vaccination against tetanus and diphtheria, Subject 1 experienced reactivation/re‐exposure to VZV as well as receiving smallpox revaccination. In response to either natural infection (VZV) or smallpox vaccination (i.e. vaccinia virus), there was an initial spike in virus‐specific antibody titers that eventually decayed to within a twofold difference of the original antibody levels ( Fig. 2 ). This result is not unique to this individual; two independent studies have demonstrated that booster smallpox vaccination has only minor or no effect on the levels of long‐term antibody production (13, 14). These results indicate that booster vaccination or infection can correct a relatively low pre‐existing antibody response but is unlikely to continue boosting high antibody levels to new plateaus because there appears to be a physiological limit to the proportion of the antibody response that is directed to any given antigen or pathogen. Understanding the mechanism(s) that govern how these immunological plateaus are established or manipulated would represent a breakthrough in B‐cell biology and play an important role in future vaccine development.
Perhaps the most compelling direct evidence for long‐term antibody production in the absence of reinfection or revaccination comes from analysis of immunity following smallpox vaccination (1) ( Fig. 2 ). In this case, reinfection or re‐exposure to vaccinia virus (the virus used in the smallpox vaccine) is unlikely to occur due to cessation of routine smallpox vaccination in 1972 (15, 16). Moreover, the last case of smallpox in the US occurred in 1949 (17), and it represented the only other orthopoxvirus in North America known to infect humans. Moreover, vaccinia is not known to spread systemically (18) or form chronic or latent infection in healthy individuals, so analysis of immunity to vaccinia provides a unique opportunity to measure the duration of antibody responses in the absence of these potential contributing factors. Several studies have identified long‐term antibody responses following smallpox vaccination (13, 14, 19, 20, 21, 22, 23) but quantitation of antibody half‐life was difficult to determine due to the nature of observational cross‐sectional studies and the long duration of humoral immunity that was observed. Longitudinal analysis of the ONPRC cohort has provided insight into this question, and the half‐life of antibody responses following smallpox vaccination (i.e. vaccinia virus infection) was estimated at 92 years (95% CI; 46 years to infinity) ( Table 1 ). Together, these studies show that repeated vaccination or infection by endemic pathogens is not a requirement for maintaining long‐term, even lifelong immunity.
Persisting antigen model
One of the oldest and most commonly described models used to explain long‐term antibody responses is the persisting antigen model ( Fig. 1 ). In this scenario, foreign protein antigens persist on the surface of follicular dendritic cells (FDCs) in the form of immune complexes, and these antigen:antibody complexes continuously stimulate resident memory B cells to proliferate and differentiate into antibody‐secreting plasma cells. Extensive studies on persisting antigen and the role of FDCs were performed in the 1970s and 1980s (24, 25) with some of the most influential research performed by Tew and Mandel (26). Using radiolabeled antigens, they demonstrated that injected protein does indeed persist in vivo and that the antigens appear to follow a biphasic rate of decay. Initially, greater than 99% of antigen is lost or degraded within the first 2–4 days with an antigen half‐life of ≤40 h (27). The next phase of decay is much slower, with an estimated half‐life of approximately 8 weeks (95% CI: 5.1–20 weeks). If antibody levels are maintained by continued or intermittent activation of memory B cells via persisting antigen in the form of immune complexes, and if one assumes that the ability of memory B cells to capture antigen, proliferate, and differentiate into antibody‐secreting cells (ASCs) does not decline over time, then the decay rate of serum antibody will be proportional to the decay rate of persisting antigen ( Fig. 1 ). If we use the high end of the 95% confidence interval for antigen persistence in vivo (i.e. 20 weeks or approximately 0.5 year), then antigen‐driven B‐cell activation should decline steadily over time and antibody responses should decay with approximately a 0.5‐year half‐life. This is in stark contrast to the durable antibody responses observed in human subjects following acute viral infection or immunization with tetanus or diphtheria toxoid vaccines ( Fig. 2 , Table 1 ).
There is no doubt that antigen can persist in the form of immune complexes, and this property is important for the germinal center reaction as well as affinity maturation of the memory B‐cell response (24, 25, 28, 29, 30, 31, 32). Indeed, antigen can be detected in lymphoid organs for weeks or months after injection (25, 31, 33), which is associated with prolonged germinal center reactions (30, 33). Although antigen can clearly persist, it is unlikely to persist forever. For instance, there is a finite limit to how much antigen can be bound to a particular FDC. According to published studies (27), it is estimated that approximately 300 000 antigen molecules can bind to the surface of an FDC. As noted previously (9), if antigen decays with an average of an 8‐week half‐life, then only one molecule of antigen would remain on the surface of an FDC after 3 years. Dendritic cell lifespan may also be a factor to consider, since these cells have an estimated lifespan of only days or weeks (34, 35, 36). Although memory B cells do not necessarily require CD4+ T‐cell help to persist (37, 38), if antigen is stimulating memory B cells, then the B cells are likely to consume the antigen (39, 40) and present it to CD4+ T cells. The maintenance of CD4+ T‐cell memory should result as an added benefit. However, studies have shown in mice (41) and in humans (13, 22, 42) that virus‐specific CD4+ T‐cell memory declines over time, even though antiviral antibody responses remain stable. Comparative analysis of CD4+ T‐cell memory and virus‐specific antibody responses following smallpox vaccination further showed that there is no correlation between the levels of T‐cell memory and antibody titers (13). Taken together, it is thus suggested that immune complexes and persisting antigen can play an important role in eliciting and maintaining the early stages of a humoral immune response, but it is unlikely to be the sole mechanism for maintaining antibody responses that can last >50 years (1, 13, 43).
Polyclonal stimulation model
Unlike other memory B‐cell‐dependent models of humoral immunity, the polyclonal stimulation model is based on non‐antigen‐specific, polyclonal stimulation of memory B cells through bystander T‐cell activation or TLR engagement. In this model, serum antibody production is maintained at levels that are proportional to the frequency of antigen‐specific memory B cells (44, 45). Ongoing or intermittent infection is thought to elicit cytokines and/or TLR activation of the memory B‐cell pool, which, in turn, triggers the B cells to proliferate and differentiate into antibody‐secreting plasma cells with an estimated 40‐day half‐life (44, 45). This model is based, in part, on compelling in vitro data demonstrating that memory B cells can be activated to divide and differentiate into ASCs following exposure to combinations of cytokines and TLR agonists such as CpG oligonucleotides (44). Indeed, TLR‐induced memory B‐cell activation (in conjunction with immunoglobulin crosslinking) is an effective means for measuring memory B‐cell frequencies in bulk culture (22, 46) or in limiting dilution assays (1, 47). In vivo, however, there is conflicting evidence for polyclonal stimulation of B cells playing a role in maintaining pre‐existing antibody responses. In the original study, tetanus vaccination was examined due to its ability to induce memory T‐cell activation and bystander T‐cell help. Within 5–7 days after booster tetanus vaccination, the frequency of ASCs in the peripheral blood that was specific for unrelated antigens such as measles or T. gondii appeared to increase by 5‐ to 10‐fold. Although serum titers to these antigens were not examined, one would predict that there would be a concomitant increase in serum antibody titers if these ASCs were involved with maintaining or increasing serum antibody levels ( Fig. 1 ). When tetanus‐specific antibody titers were examined, there was an expected antigen‐specific response with antibody titers increasing by about 80‐fold at the peak of the response and then declining over the ensuing weeks with an approximate 40‐day half‐life (44). Similar to other memory B‐cell‐dependent models, the polyclonal B‐cell activation theory represents a complex scenario in which continued infection or immune activation will be required in order to sustain antigen‐specific antibody levels for decades ( Fig. 2 ). Moreover, it requires a careful balance in proliferation with an equal distribution of daughter cells that enter the plasma cell pool while also repopulating the memory B‐cell pool to their original numbers – which remain remarkably stable over time (1, 22). It is unlikely that chronic infection is responsible for this form of non‐antigen‐specific memory B‐cell stimulation, since this would be expected to trigger memory B cells of all antigen specificities to proliferate/differentiate into antibody‐secreting daughter cells with a similar antibody half‐life. This model, however, does not fully explain the differences in antigen‐specific antibody maintenance observed in vivo ( Table 1 ). If repeated acute heterologous infection is required to maintain antibody responses, then this may result in substantial variability in antibody responses over time, especially if plasma cells and their coinciding antibody levels have a 40‐day half‐life (44). Such a rapid decay rate, followed by spikes in reactivation/repopulation of peripheral plasma cells, would likely differ from year to year as well as between seasons within a given year if based on the frequency of contagious infections such as rhinoviruses, coronaviruses, influenza, etc. However, longitudinal studies of multiple subjects over the course of one to two decades have indicated that antibody responses are not erratic ( Fig. 1 ) but instead undergo stable trends unless altered by antigen‐specific vaccination or infection‐based events ( Fig. 2 ). For instance, when analysis of antigen‐specific antibody responses to eight different antigens was performed for the first several weeks following smallpox vaccination (Subject 1, Fig. 2 ), we found little or no effect on the levels of antibody responses to unrelated antigens (1). This finding is consistent with other independent studies conducted in mice (38, 48, 49) and in human subjects (8) in which an in vivo effect of heterologous vaccination or infection on pre‐existing serum antibody responses to unrelated antigens has not been observed. Although it appears unlikely that polyclonal memory B‐cell stimulation through heterologous infection‐related TLR engagement or bystander T‐cell activation is required for maintenance of long‐term antibody responses, it is still an interesting phenomenon. Learning why memory B cells do (or do not) respond to these types of inflammatory signals will be important for developing better vaccines, especially since TLR agonists represent an exciting area of future adjuvant development (50).
Plasma cell niche competition model
There are several lines of evidence indicating that plasma cells represent an independent B‐cell population that can be long‐lived in the absence of repopulation by memory B cells. First, studies involving tritium incorporation (51) or bromodeoxyuridine (BrdU) incorporation (52) have shown that plasma cells can survive for long periods of time in the absence of cell division. Second, although memory B cells themselves are also quite long‐lived (1, 22), for each of the memory B‐cell‐dependent models of long‐term antibody production to be feasible, there needs to be a direct correlation between memory B‐cell numbers and antibody levels. In this regard, there are some studies that have identified this correlation (22, 44), whereas there are others who do not find a consistent correlation between memory B‐cell frequencies and antibody levels (1, 11, 53). Third, when memory B cells are depleted in animal models (54, 55, 56), plasma cell populations and antibody levels continue to persist for long periods of time. This is further supported by anecdotal evidence of sustained antibody responses to vaccine antigens in human subjects undergoing B‐cell ablative therapy with anti‐CD20 depletion (57). Although memory B cells may not be required for the long‐term maintenance of plasma cell populations, they still play a vital role in protective immunity by mounting secondary responses following exposure or re‐exposure to their specific antigen. For instance, adoptive transfer studies clearly show that memory B cells will rapidly expand and repopulate plasma cell numbers after booster vaccination with their specific antigen but will remain dormant and not respond to vaccination with an irrelevant antigen (38).
If plasma cells can be long‐lived, as these data suggest, then how is the duration of antibody production determined and regulated? The plasma cell competition model is based on the theory that plasma cells survive for long periods of time when residing in the appropriate niche or microenvironment but that they are eventually displaced through competition with newly generated migratory plasmablasts that compete with the older resident plasma cells for finite space in the survival niche (58). Once displaced, the terminally differentiated plasma cells are believed to be unable to regain localization within the niche and subsequently die. Unlike the polyclonal stimulation model that predicts an increase in antibody responses to unrelated antigens during or shortly after infection/vaccination, this model would predict that each new infection or vaccination would result in the loss of pre‐existing humoral immunity to unrelated antigens through the process of the new plasmablasts displacing pre‐existing plasma cells of other specificities. It is believed that 80–90% of long‐lived plasma cells reside in the bone marrow compartment (58, 59, 60, 61, 62), and one could argue that the rates of cell death by displacement would increase as the competition for limited space increases. Plasma cells accumulate in the bone marrow as a function of age and thus one would predict that plasma cell competition would also increase with age. Continuing this line of thought, one would further predict that antibody responses should decay at a more rapid rate as a function of age, since plasma cells would be destroyed at a more rapid rate proportional to the loss of available space and proportionally higher competition in the bone marrow niche ( Fig. 1 ). In contrast, when longitudinal analysis of antigen‐specific antibody responses were performed in subjects in their 20s to 30s or in their 50s to 60s, there was no observable change in antibody maintenance patterns (1) ( Fig. 2 ). In other words, antibody responses against tetanus and diphtheria maintained more rapid decay rates regardless of an individual’s age, whereas durable antibody responses against viruses such as measles, mumps, or rubella remained long‐lived regardless of age ( Table 1 ). Based on these in vivo observations, it is difficult to conclude that plasma cell competition for space will adequately explain the differences in antibody response patterns ( Table 1 ) and does not predict the durability of humoral immunity during advanced age (1) ( Fig. 2 ).
Plasma cell imprinted lifespan model
All antibody responses are not created equal. T‐cell‐independent antibody responses are known to be short‐lived, whereas T‐cell‐dependent antigens elicit immunity that lasts for decades or in some cases may be maintained for a lifetime ( Table 1 ). In an attempt to explain the differential lifespan of antibody responses observed against different virus and vaccine antigens, we have developed a model of plasma cell longevity that is based on individual plasma cells being imprinted with a specific lifespan at the time of induction ( Fig. 3 ). Epigenetic imprinting of function is not a unique concept; we have previously demonstrated that individual virus‐specific memory B cells will differ with regard to the number of antibody‐secreting daughter cells that are produced following antigenic stimulation, and the antibody‐secreting daughter cells appear to have different antibody secretion rates that are unique to their cellular lineage (63). More recent studies have shown that B cells that receive T‐cell help will produce antibody‐secreting progeny cells that produce significantly more antibody on a per cell basis than B cells that were activated by antigen in the absence of T‐cell help (64). This finding suggests that the microenvironment and availability of T‐cell help can have a profound impact on the function that is imprinted on the plasma cells produced from a particular memory B‐cell.
Figure 3.
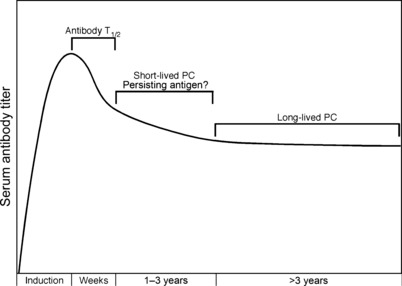
The Imprinted Lifespan Model of long‐term antibody maintenance. As shown in Table 1 , antibody responses to specific virus and vaccine antigens have significantly different lifespans. Since plasma cells secrete antibody and consequently lose expression of the membrane‐bound forms of immunoglobulin, it is unlikely that they can detect or respond to specific antigen in their environment. It is thus suggested that the antigen‐specific imprinting of the antibody response must occur during the interface between B cells and antigen during the induction of the humoral immune response. In this model, the combined signals through the B‐cell receptor and signals obtained through CD4+ T‐cell help will dictate the lifespan of antibody‐secreting plasma cells. For instance, if B cells are exposed to soluble, non‐repetitive self protein or an unconjugated free chemical hapten, then there is no cross‐linking of the B‐cell receptor (BCR) and no T‐cell help, resulting in weak and short‐lived antibody responses. If the antigen is highly repetitive, then the BCR will be cross‐linked and increase signal strength to the responding B cell. However, without concomitant T‐cell help, the T‐cell‐independent antibody response remains short‐lived – in the order of a few weeks or months. In contrast, if the antigen contains foreign protein, then the responding B‐cell will receive critical CD4+ T‐cell help and induce an antibody response that could last for decades (e.g. tetanus toxoid). If the foreign protein antigen is also highly repetitive (e.g. a protein on the surface of a virus particle), then the combination of increased signaling through the BCR together with CD4+ T‐cell help will induce plasma cell progeny with an extended lifespan and potentially provide life‐long immunity against reinfection. Since many microbes have highly repetitive structures (identified through Toll‐like receptor as well as other pattern recognition receptors), the potential risk to the mammalian host is determined by the repetitive nature of the antigen and the availability of T‐cell help.
There are two underlying tenets with the imprinted lifespan model. First, unlike memory B cells or intermediate ASC types such as plasmablasts [that maintain the ability to proliferate as well as secrete antibody (65)], plasma cells are terminally differentiated cells that by definition can no longer divide. Therefore in the absence of reconstitution by memory B cells, the duration of antigen‐specific serum antibody responses during the intervals between vaccinations or infections will be directly proportional to the lifespan of the antigen‐specific plasma cell populations. Second, unlike memory B cells, plasma cells lose the ability to sense and respond to antigenic changes in their environment. Memory B cells are highly adept at antigen capture due to maintaining high affinity antigen‐specific antibody molecules on their surface (66). Likewise, memory B cells are able to elicit and respond to T‐cell help through the expression of major histocompatibility complex (MHC) class II as well as a number of adhesion molecules such as CD80 and CD86 (53). In contrast, plasma cells lose the expression of many mature B‐cell markers including MHC class II molecules (54), thus reducing their ability to elicit and respond to cognate T‐cell help. Although plasma cells will be directed to sites of inflammation (58), long‐term antibody production after resolution of infection or vaccination is often maintained by plasma cells in the bone marrow (60). The bone marrow compartment is also sequestered from the main sites of initial infection (skin, respiratory, and mucosal surfaces), thus further reducing but not eliminating antigenic exposure. Bearing these factors in mind, we believe that plasma cells will be unable to differentially respond to antigenic changes in their microenvironment and modify their specified lifespan.
What will determine the lifespan of antigen‐specific plasma cells? It may be based on the strength of the combined B‐cell signals that together provide an adjustable form of ‘risk assessment’ of the antigenic exposure incurred during initiation of the humoral immune response. If a naive B‐cell is exposed to soluble self protein or an unconjugated chemical molecule such as a free hapten, then little or no antibody response is induced, because there is no B‐cell receptor (BCR) crosslinking and no T‐cell help ( Fig. 3 ). During the germinal center reaction, soluble protein induces apoptosis, further eliminating antibody responses to soluble antigens (67). Even memory B cells are poorly responsive to soluble foreign proteins in the absence of T‐cell help (38), and this property may be important because it reduces the amount of antibody directed towards self‐antigens or induction of antibody responses to environmental or irrelevant antigens. For instance, B cells will respond effectively to a repetitive or particulate antigen such as the CMV gB protein on the surface of intact virus particles but are unable to respond effectively to gB protein in its soluble form (38). This may be one reason why protein aggregates complexed with adjuvants such as alum are much more immunogenic than soluble proteins administered in the absence of adjuvant.
In contrast to free haptens or soluble proteins, if the BCR is crosslinked by a highly repetitive non‐protein antigen such as a hapten conjugated to LPS or ficoll, then a T‐cell‐independent antibody response is induced. T‐cell‐independent antibody responses are typically short‐lived and maintained for only a few weeks or months. By comparison, T‐cell‐dependent antibody responses are typically longer lived than T‐cell‐independent responses. One example of a non‐repetitive T‐cell‐dependent antigen is tetanus toxoid. This antigen represents a foreign antigen in which CD4+ T‐cell responses will be induced and provide help to antigen‐specific B cells. Tetanus is not a repetitive antigen, and so there is low BCR crosslinking. Combined with the B‐cell signals obtained through cognate CD4+ T‐cell help, an antibody response will be induced that lasts for years or potentially for decades ( Table 1 ). To achieve an even longer lifespan, the antigen‐specific B‐cell would require the combination of a foreign protein antigen (thus eliciting antigen‐specific CD4+ T‐cell help) with a highly repetitive antigenic structure to trigger capping of the BCR and presumably stronger signal transduction in the responding B cell. A virus particle provides an example of this form of repetitive antigen that would induce B cells to proliferate and differentiate into plasma cells with an increased lifespan. The underlying theory is that highly repetitive foreign antigens are associated with the presence of a microbial infection and that induction of a long‐term antibody response to microbial antigens provides a selective advantage to the host because it will prevent reinfection, in some cases for life (9). Foreign proteins that are not repetitive may still be from an infectious agent (e.g. tetanus toxin), but as a general rule, the programmed lifespan against these proteins would be more limited. An alternative explanation for the differences observed in the duration of humoral immunity against viruses versus vaccine antigens, such as tetanus and diphtheria ( Table 1 ), is that live infections may trigger an inflammatory microenvironment that provides better stimulation or ‘programming’ than what might occur following vaccination with these particular non‐replicating antigens formulated in alum. In this regard, we have begun to examine the duration of immunity against another bacterial toxin, pertussis toxin, following natural infection with Bordetella pertussis, a Gram‐negative bacterium. Preliminary results indicate that the duration of immunity to the non‐repetitive pertussis toxin is similar to that observed for vaccine‐induced immunity against tetanus and diphtheria antigens (IJ Amanna and MK Slifka, unpublished data) and suggests that microbial infection and TLR activation may not be sufficient for inducing a lifelong antibody response. Although these studies are only preliminary and more work needs to be done, these data lead us to believe that the repetitive nature of the antigen (in combination with T‐cell help) is more critical than an inflammatory microenvironment in eliciting the most long‐lived antibody responses.
There are at least three reasons to limit the duration of an antibody response. First, the initial antibody response is often not class‐switched and is typically of low affinity. It would be unwise to make a long‐term low‐affinity antibody response if the host is capable of mounting a better, more specific antibody response after somatic mutation and affinity maturation. Interestingly, when the anti‐apoptotic gene Bcl‐xL was overexpressed in transgenic mice, the size or number of germinal centers was unchanged, but antigen‐specific antibody responses were mounted by a substantially higher proportion of low‐affinity ASCs (68). This observation indicates an important role for selection and apoptosis in culling suboptimal B cells and antibody‐secreting daughter cells from the long‐lived plasma cell pool. Second, if the antigen is non‐repetitive, then it might not be a microbial antigen or it might not be a protective surface antigen. For instance, mounting an antibody response to a highly repetitive glycoprotein on the surface of a virion is more likely to have neutralizing activity or other antiviral functions (69) and be more protective than mounting a long‐term antibody response to a non‐repetitive viral protein such as a viral DNA polymerase or RNA polymerase. It is clear that antibody responses to different human immunodeficiency virus (HIV) proteins will demonstrate dramatically different half‐lives (12), and it remains to be determined whether this is unique to HIV or whether there is a range of antibody half‐lives mounted against different components of a particular virus (or other microbe) in other model systems as well. Third, since there is a finite amount of space in the bone marrow for sustaining plasma cell numbers, it is important to modify the duration of the immune response and only direct lifelong immunity to the antigens that have the greatest likelihood of representing infectious agents. This way the bone marrow compartment is not overly populated with plasma cells of limited protective value. By allowing a range of plasma cell life spans to be developed, the loss of some relatively short‐lived plasma cells against low‐priority targets would allow more flexible space for the development and maintenance of new long‐lived plasma cells to new infectious agents. One potential outcome of this model is that as we age and the bone marrow compartment accumulates long‐lived plasma cells, it may be more difficult to induce a long‐lived antibody response to new antigens. Although only speculation, it may be one of the explanations for the difficulty in effectively vaccinating elderly individuals and opens an interesting area of future investigation.
Future directions
Time frame of analysis dictates the estimation of antibody lifespan
A common but largely unrecognized complication with measurements of humoral immunity is that the calculated duration of serum antibody responses can be dramatically altered by which time points are used to measure antigen‐specific antibody kinetics ( Fig. 4 ). For instance, if serum antibody responses are measured during the first days or weeks after the peak of the response, then antibody decay rates will often be in the order of 20–40 days – suggesting induction of only a short‐lived antibody response. Since many of the early ASCs induced during vaccination or infection have a half‐life of only a few days (70, 71, 72, 73), the associated antibody response measured at early time points will be dictated largely by the lifespan of the immunoglobulin (Ig) molecules themselves. Studies have shown that although human IgG molecules have a half‐life of approximately 17.5–26 days (74, 75, 76), this differs among individuals, and antibody half‐life estimates of as high as 42 days (75) to as long as 80 days (77) have been observed under some circumstances. This recognition indicates that when antibody responses are measured immediately after vaccination and are determined to be less than 3–4 weeks duration, then the estimated lifespan of the surviving plasma cell populations will likely be skewed by the protein half‐life of IgG molecules and may not be indicative of the plasma cell half‐life per se. Within a few months after vaccination or acute infection, the rates of antibody decay will slow dramatically as the effects of free Ig are removed from the circulation through IgG catabolism (78) and antibody kinetics become more representative of the lifespan of the underlying plasma cell populations producing the antibody of interest ( Fig. 4 ). Interestingly, even if one waits 1–2 years after vaccination or infection before measuring the duration of immunity, it may still indicate an artificially short plasma cell lifespan. Previous studies have shown that antibody responses in humans do not reach steady state levels until approximately 2–3 years after infection or vaccination (79, 80), which is also apparent in the representative subjects shown in Fig. 2 . For Subject 1, the antibody responses to VZV, tetanus, diphtheria, and vaccinia declined rapidly during the first 3 years after the serospike and then were maintained with a longer half‐life with similar decay rates as that observed prior to the spike in antibody titers. Similar results were observed following tetanus and diphtheria vaccination of Subject 2; antibody responses declined most rapidly during the 2–3 years following vaccination before stabilizing into more long‐lived maintenance of serum antibody titers thereafter. There are a number of mechanisms that may explain these observations. First, it is possible that during the first 2–3 years following vaccination or infection, the antibody response is maintained by a combination of memory B‐cell‐dependent mechanisms (e.g. persisting antigen in the form of immune complexes on the surface of FDCs) and memory B‐cell‐independent mechanisms (i.e. long‐lived plasma cells). As antigen levels slowly decline, the memory B‐cell‐dependent pathway would subside, leaving only long‐lived plasma cells to remain and continue the production of antigen‐specific serum antibodies. Plasma cell repopulation by persisting antigen and memory B‐cell stimulation may explain why plasma cells at 6 weeks postimmunization were only temporarily depleted by coblockade of leukocyte function‐associated antigen‐1 (LFA‐1) and very late antigen‐4 (VLA‐4), whereas they remained depleted for up to 10 weeks when blockade of LFA‐1 and VLA‐4 was accompanied by depletion of CD20+ memory B cells (55). In addition, it is likely that there is a combination of short‐lived and long‐lived plasma cells that are generated during the course of a humoral immune response (70, 71, 72, 73, 81). Over time, the short‐lived plasma cells will decline, and this decline will eventually select for the plasma cells with the longest inherent lifespan. Unfortunately, it is not always feasible to monitor antigen‐specific antibody responses for multiple years after vaccination or infection, and it will be very useful if mathematical models can be developed that accurately predict the duration of an antibody response based on the kinetics of the immune response measured at earlier time points.
Figure 4.
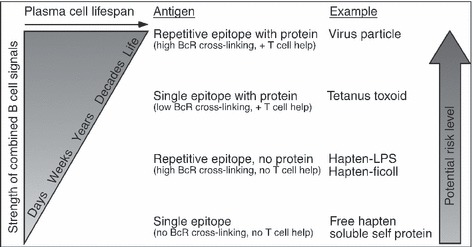
Kinetics of a prototypical serum antibody response. The kinetics of an antigen‐specific antibody response does not typically follow a single rate of decay but instead will often follow a pattern involving at least three phases of antibody half‐life kinetics. Shortly after the peak in antibody production following acute infection or vaccination, antibody responses will decline rapidly, often at the rate of free IgG protein catabolism and removal from the serum [T 1/2 = 17.5–26 days (74, 75, 76)]. For the next 1–3 years, antibody responses will be more long‐lived but will still decline more rapidly than antibody responses analyzed at >3 years after the antigenic insult when antibody production has reached steady‐state levels (1, 80). During the first 1–3 years after vaccination or infection, the antibody response may be maintained by a combination of memory B‐cell‐dependent mechanisms (e.g. persisting antigen on the surface of FDCs) and memory B‐cell‐independent mechanisms (i.e. long‐lived plasma cells), and in this case, the final, steady state antibody response would only become apparent once the antigen depot has been exhausted and is no longer contributing to the plasma cell pool. An alternative model that is not mutually exclusive to the persisting antigen model is that antibody responses elicited during the first few years after vaccination or infection may be produced by a mixed population of long‐lived and short‐lived plasma cells. Plasma cells with the shortest lifespan will decline over time, which will eventually select for the subset of plasma cells with the longest inherent lifespan. Bearing this in mind, the longevity of a given antigen‐specific antibody response might be considered short‐lived, moderately long‐lived, or very long‐lived, depending on the time frame in which the analysis is performed.
Role of host genetic factors
We have found significant differences in the duration of immunity to different virus and vaccine antigens ( Table 1 ) and propose that the duration of immunity is dictated largely by the repetitive nature of the antigen and the availability of T‐cell help ( Fig. 3 ). However, these characteristics may not be the sole factors in determining how long an immune response will be maintained by a specific individual. During our analysis of the ONPRC cohort, we found one subject (Subject 4) in particular who mounted antibody responses of similar duration, regardless of the antigen tested (1). With the exception of antiviral responses to EBV that showed no evidence of decline, the antibody responses to the seven other virus and vaccine antigens declined with similar kinetics (estimated half‐life of 14–31 years). This finding was not due to general immune suppression or an inability to mount high antibody responses, since this subject demonstrated normal levels of humoral immunity to each of the antigens in addition to demonstrating a strong anamnestic antibody response following booster smallpox vaccination. However, this person was largely incapable of mounting the lifelong antibody response that was observed among other subjects following acute viral infections such as measles, mumps, rubella, or vaccinia ( Table 1 ). Targeted genetic analysis of this individual (and perhaps others if identified in future studies) may provide clues into the complex cellular and molecular mechanisms that distinguish long‐term humoral immunity from lifelong humoral immunity.
Molecular mechanisms of long‐lived plasma cell maintenance
Long‐lived plasma cells are able to maintain antibody responses for extensive periods of time, but the underlying molecular mechanisms that allow for such extended survival still remain unresolved. The longevity of these bone marrow‐resident cells is all the more impressive considering the stress of continually producing 10 000 to 20 000 molecules of Ig per second (82, 83, 84, 85). Current models of plasma cell longevity suggest that survival niches exist in the bone marrow that specifically support prolonged survival (86). For instance, plasma cells migrate to specific locations such as the bone marrow through chemokine signaling (e.g. CXCR4‐CXCL12) and are then supported through local molecular interactions (e.g. LFA‐1/VLA‐4 adhesion, or IL‐5, IL‐6 signaling, etc.).
Despite substantial evidence that antigen‐specific plasma cells reside primarily in the bone marrow (60, 61), many of the assumptions concerning molecular factors important for migration and survival are based on in vitro studies of isolated plasma cells. Few studies have focused on the in vivo effects of specific genetic deletions, especially on established antibody responses. For example, IL‐6 was determined to be an important survival factor for bone marrow‐derived plasma cells in vitro, yet IL‐6‐deficient mice show no long‐term humoral defects after immunization with ovalbumin, a prototypical T‐cell‐dependent antigen (87). This conclusion was supported by examining both ovalbumin‐specific antibody levels as well as bone marrow‐derived ASCs at 20–21 weeks postimmunization. Similarly, although newly formed plasma cells appear to be responsive to CXCL12 (the ligand for CXCR4) for a short period of time following emigration from the spleen (88), the importance of CXCR4 in orchestrating and maintaining long‐term antibody responses is less apparent. Initial results in a CXCR4−/− chimeric mouse model suggested a defect in plasma cell accumulation in the bone marrow at days 7 to 14 postimmunization (89). Nevertheless, a conditional B‐lineage‐specific CXCR4‐deficient model also showed early defects in bone marrow accumulation of plasma cells following a T‐cell‐dependent response to NP‐KLH (day 9 postimmunization), but the mice demonstrated no long‐term defects in antibody levels, antibody affinity maturation, or plasma cell accumulation at 90 days postimmunization (90). This study indicates that these specific chemokines and cytokines are not absolutely required for plasma cell survival and illustrates the difficulty in dissecting individual mechanisms in a system wherein biological redundancy may be involved.
Despite the lack of clarity in terms of chemokine and cytokine requirements, several important factors supporting plasma cell maintenance have been established. Blimp‐1 has long been known as a critical factor in terminal B‐cell differentiation, with the ability to act as a master gene regulator by directing sequence‐specific chromatin remodeling (91). Through its downregulation of Pax5 and upregulation of XBP1 (among many other roles), it is thought to simultaneously induce antibody secretion in plasma cells and initiate the unfolded‐protein response to deal with the stress of increased protein production (58). More recently, Blimp‐1 has been shown to have a direct effect upon established humoral immune responses. By employing a conditional Blimp‐1 knockout, investigators demonstrated that a previously established T‐cell‐dependent antibody response to NP‐KLH decreased significantly over time when compared to controls following loss of Blimp‐1 expression (92). The loss of plasma cell function was verified independently by adoptive transfer studies. Nevertheless, while substantial progress has been made in determining the signals that induce Blimp‐1 expression (LPS, CpG oligonucleotides, IL‐2, IL‐5, IL‐6, IL‐10, IL‐21, etc.) (93), the in vivo signals that act directly on plasma cells remain undefined.
Another set of important plasma cell maintenance factors have emerged recently among the tumor necrosis factor (TNF) family members, BAFF (B‐cell‐activating factor of the TNF family) and APRIL (a proliferation‐inducing ligand). Both of these proteins have the ability to bind the TNF receptors, TACI (transmembrane activator and calcium modulator ligand interactor) and BCMA (B‐cell maturation antigen), with the additional interaction of BAFF binding exclusively to the BAFF receptor (BAFF‐R) (94). The BAFF system is a well known survival and maturation network involved in peripheral B‐cell development, but its role in plasma cell development has been less characterized, due primarily to its upstream effects on normal B‐cell development. However, genetic deletion of BCMA in mice demonstrated that defects in this receptor led to decreased maintenance of long‐lived plasma cells in response to a T‐cell‐dependent antigen (95) despite antibody responses that appeared normal during the first 2 weeks of the immune response. This finding indicates that BCMA was not required for induction of a normal antibody response but was essential for its maintenance. Additional data implicating BAFF and/or APRIL in plasma cell maintenance has recently been described utilizing in vivo blockade with soluble versions of the BAFF receptor and TACI (which blocks both BAFF and APRIL) (96). In these experiments, mice were immunized with a T‐cell‐dependent antigen, rested for 3 months, and then subjected to in vivo blockade of BAFF or APRIL signaling. The blockade of either individual protein had no effect, but the simultaneous blockade of both BAFF and APRIL together led to a significant decrease in antigen‐specific plasma cells, strongly indicating a redundant and supportive role for both molecules in the long‐term survival of antigen‐specific plasma cells. Similar results were observed following in vivo blockade of the adhesion molecules LFA‐1 and VLA‐4 (55).
Although the studies of Blimp‐1, BAFF/APRIL, and LFA‐1/VLA‐4 have yielded substantial insight into the molecular mechanisms that directly effect plasma cell maintenance, questions still remain as to why some populations of plasma cells are more long‐lived than others. Two independent studies have shown that long‐lived antibody responses against protein antigens (e.g. tetanus or diphtheria) range with half‐lives of about 10–20 years, whereas responses against acute viral infections show little to no decline (1, 12). One possibility is that the long‐term fate of each plasma cell is imprinted at the time of activation, perhaps related to BCR cross‐linking ( Fig. 3 ). This is an important biological mechanism, because it could be detrimental to the host if all antibody responses (especially low affinity responses or antibody responses cross‐reactive to self antigens) were long‐lived. Perhaps the additional BCR cross‐linking afforded by repetitive T‐cell‐dependent epitopes (such as viral particles) enhances the downstream molecular mechanisms required for long‐term survival. Additional signals delivered to the B‐cell at the time of activation could also play key roles in downstream longevity. For instance, mice deficient in the adapter protein SAP (SLAM‐associated protein) can initially mount normal levels of antibody, but long‐term antibody maintenance is greatly impaired (97). Adoptive transfer experiments demonstrated that the defect was T‐cell intrinsic, because SAP‐deficient B cells mounted normal antibody responses in a normal host, whereas wildtype B cells could not mount long‐term antibody responses if T‐cell help came from SAP‐deficient T cells. More recent studies (98) have shown that SAP‐deficient T cells are suboptimal at forming long‐lived B‐cell:T‐cell conjugates in vivo, and it is presumed that such brief cell‐to‐cell interactions result in poor T‐cell help. This may explain why antibody responses in SAP‐deficient animals appear to mimic the kinetics and duration of T‐cell‐independent responses. T cells, however, are unlikely to be needed for long‐term plasma cell maintenance, since established long‐term antibody responses can be maintained even in the absence of T cells (38) or after T‐cell memory has declined (13). While progress has been made in dissecting the factors associated with development and maintenance of plasma cells, a stepwise roadmap of the signals that are necessary for this process remains to be determined.
References
- 1. Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med 2007;357:1903–1915. [DOI] [PubMed] [Google Scholar]
- 2. Jerne NK. Idiotypic networks and other preconceived ideas. Immunol Rev 1984;79:5–24. [DOI] [PubMed] [Google Scholar]
- 3. MacLennan IC, Casamayor‐Palleja M, Toellner KM, Gulbranson‐Judge A, Gordon J. Memory B‐cell clones and the diversity of their members. Semin Immunol 1997;9:229–234. [DOI] [PubMed] [Google Scholar]
- 4. Plotkin SA, Starr SE, Connor K, Morton D. Zoster in normal children after varicella vaccine. J Infect Dis 1989;159:1000–1001. [DOI] [PubMed] [Google Scholar]
- 5. Hardy I, Gershon AA, Steinberg SP, LaRussa P. The incidence of zoster after immunization with live attenuated varicella vaccine. A study in children with leukemia. Varicella Vaccine Collaborative Study Group. N Engl J Med 1991;325:1545–1550. [DOI] [PubMed] [Google Scholar]
- 6. Krause PR, Klinman DM. Varicella vaccination: evidence for frequent reactivation of the vaccine strain in healthy children. Nat Med 2000;6:451–454. [DOI] [PubMed] [Google Scholar]
- 7. Chaves SS, et al. Loss of vaccine‐induced immunity to varicella over time. N Engl J Med 2007;356:1121–1129. [DOI] [PubMed] [Google Scholar]
- 8. Di Genova G, Roddick J, McNicholl F, Stevenson FK. Vaccination of human subjects expands both specific and bystander memory T cells but antibody production remains vaccine specific. Blood 2006;107:2806–2813. [DOI] [PubMed] [Google Scholar]
- 9. Slifka MK, Ahmed R. Long‐term humoral immunity against viruses: revisiting the issue of plasma cell longevity. Trends Microbiol 1996;4:394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Slifka MK. Immunological memory to viral infection. Curr Opin Immunol 2004;16:443–450. [DOI] [PubMed] [Google Scholar]
- 11. Nanan R, Heinrich D, Frosch M, Kreth HW. Acute and long‐term effects of booster immunisation on frequencies of antigen‐specific memory B‐lymphocytes. Vaccine 2001;20:498–504. [DOI] [PubMed] [Google Scholar]
- 12. Bonsignori M, et al. HIV‐1 envelope induces memory B cell responses that correlate with plasma antibody levels after envelope gp120 protein vaccination or HIV‐1 infection. J Immunol 2009;183:2708–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hammarlund E, et al. Duration of antiviral immunity after smallpox vaccination. Nat Med 2003;9:1131–1137. [DOI] [PubMed] [Google Scholar]
- 14. Taub DD, et al. Immunity from smallpox vaccine persists for decades: a longitudinal study. Am J Med 2008;121:1058–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Neff JM, Lane JM, Fulginiti VA, Henderson DA. Contact vaccinia – transmission of vaccinia from smallpox vaccination. JAMA 2002;288:1901–1905. [DOI] [PubMed] [Google Scholar]
- 16. Sepkowitz KA. How contagious is vaccinia? N Engl J Med 2003;348:439–446. [DOI] [PubMed] [Google Scholar]
- 17. Irons JV, Sullivan TD, Cook EBM, Cox GW, Hale RA. Outbreak of smallpox in the lower Rio Grande valley of Texas in 1949. Am J Public Health Nations Health 1953;43:25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cummings JF, Polhemus ME, Hawkes C, Klote M, Ludwig GV, Wortmann G. Lack of vaccinia viremia after smallpox vaccination. Clin Infect Dis 2004;38:456–458. [DOI] [PubMed] [Google Scholar]
- 19. El‐Ad B, et al. The persistence of neutralizing antibodies after revaccination against smallpox. J Infect Dis 1990;161:446–448. [DOI] [PubMed] [Google Scholar]
- 20. Stienlauf S, et al. Kinetics of formation of neutralizing antibodies against vaccinia virus following re‐vaccination. Vaccine 1999;17:201–204. [DOI] [PubMed] [Google Scholar]
- 21. Frey SE, Newman FK, Yan L, Belshe RB. Response to smallpox vaccine in persons immunized in the distant past. JAMA 2003;289:3295–3299. [DOI] [PubMed] [Google Scholar]
- 22. Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long‐term B cell memory in humans after smallpox vaccination. J Immunol 2003;171:4969–4973. [DOI] [PubMed] [Google Scholar]
- 23. Putz MM, Alberini I, Midgley CM, Manini I, Montomoli E, Smith GL. Prevalence of antibodies to Vaccinia virus after smallpox vaccination in Italy. J Gen Virol 2005;86:2955–2960. [DOI] [PubMed] [Google Scholar]
- 24. Liu YJ, Grouard G, De Bouteiller O, Banchereau J. Follicular dendritic cells and germinal centers. Int Rev Cytol 1996;166:139–179. [DOI] [PubMed] [Google Scholar]
- 25. MacLennan IC. Germinal centers. Annu Rev Immunol 1994;12:117–139. [DOI] [PubMed] [Google Scholar]
- 26. Tew JG, Kosco MH, Burton GF, Szakal AK. Follicular dendritic cells as accessory cells. Immunol Rev 1990;117:185–211. [DOI] [PubMed] [Google Scholar]
- 27. Tew JG, Mandel TE. Prolonged antigen half‐life in the lymphoid follicles of specifically immunized mice. Immunology 1979;37:69–76. [PMC free article] [PubMed] [Google Scholar]
- 28. Kelsoe G. The germinal center: a crucible for lymphocyte selection. Semin Immunol 1996;8:179–184. [DOI] [PubMed] [Google Scholar]
- 29. McHeyzer‐Williams MG, Ahmed R. B cell memory and the long‐lived plasma cell. Curr Opin Immunol 1999;11:172–179. [DOI] [PubMed] [Google Scholar]
- 30. Dogan I, et al. Multiple layers of B cell memory with different effector functions. Nat Immunol 2009;10:1292–1299. [DOI] [PubMed] [Google Scholar]
- 31. Mandel TE, Phipps RP, Abbot A, Tew JG. The follicular dendritic cell: long term antigen retention during immunity. Immunol Rev 1980;53:29–59. [DOI] [PubMed] [Google Scholar]
- 32. Tarlinton D. Germinal centers: form and function. Curr Opin Immunol 1998;10:245–251. [DOI] [PubMed] [Google Scholar]
- 33. Bachmann MF, Odermatt B, Hengartner H, Zinkernagel RM. Induction of long‐lived germinal centers associated with persisting antigen after viral infection. J Exp Med 1996;183:2259–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Salomon B, Cohen JL, Masurier C, Klatzmann D. Three populations of mouse lymph node dendritic cells with different origins and dynamics. J Immunol 1998;160:708–717. [PubMed] [Google Scholar]
- 35. Kamath AT, et al. The development, maturation, and turnover rate of mouse spleen dendritic cell populations. J Immunol 2000;165:6762–6770. [DOI] [PubMed] [Google Scholar]
- 36. Ruedl C, Koebel P, Bachmann M, Hess M, Karjalainen K. Anatomical origin of dendritic cells determines their life span in peripheral lymph nodes. J Immunol 2000;165:4910–4916. [DOI] [PubMed] [Google Scholar]
- 37. Vieira P, Rajewsky K. Persistence of memory B cells in mice deprived of T cell help. Int Immunol 1990;2:487–494. [DOI] [PubMed] [Google Scholar]
- 38. Hebeis BJ, et al. Activation of virus‐specific memory B cells in the absence of T cell help. J Exp Med 2004;199:593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Puaux AL, et al. A very rapid and simple assay based on trogocytosis to detect and measure specific T and B cell reactivity by flow cytometry. Eur J Immunol 2006;36:779–788. [DOI] [PubMed] [Google Scholar]
- 40. Aucher A, Magdeleine E, Joly E, Hudrisier D. Capture of plasma membrane fragments from target cells by trogocytosis requires signaling in T cells but not in B cells. Blood 2008;111:5621–5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Homann D, Teyton L, Oldstone MB. Differential regulation of antiviral T‐cell immunity results in stable CD8+ but declining CD4+ T‐cell memory. Nat Med 2001;7:913–919. [DOI] [PubMed] [Google Scholar]
- 42. Amara RR, Nigam P, Sharma S, Liu J, Bostik V. Long‐lived poxvirus immunity, robust CD4 help, and better persistence of CD4 than CD8 T cells. J Virol 2004;78:3811–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sawyer WA. Persistence of yellow fever immunity. Am J Prev Med 1931;5:413–428. [Google Scholar]
- 44. Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science 2002;298:2199–2202. [DOI] [PubMed] [Google Scholar]
- 45. Traggiai E, Puzone R, Lanzavecchia A. Antigen dependent and independent mechanisms that sustain serum antibody levels. Vaccine 2003;21(Suppl. 2):S35–S37. [DOI] [PubMed] [Google Scholar]
- 46. Crotty S, Aubert RD, Glidewell J, Ahmed R. Tracking human antigen‐specific memory B cells: a sensitive and generalized ELISPOT system. J Immunol Methods 2004;286:111–122. [DOI] [PubMed] [Google Scholar]
- 47. Amanna IJ, Slifka MK. Quantitation of rare memory B cell populations by two independent and complementary approaches. J Immunol Methods 2006;317:175–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Benson MJ, et al. Distinction of the memory B cell response to cognate antigen versus bystander inflammatory signals. J Exp Med 2009;206:2013–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Richard K, Pierce SK, Song W. The agonists of TLR4 and 9 are sufficient to activate memory B cells to differentiate into plasma cells in vitro but not in vivo . J Immunol 2008;181:1746–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kanzler H, Barrat FJ, Hessel EM, Coffman RL. Therapeutic targeting of innate immunity with Toll‐like receptor agonists and antagonists. Nat Med 2007;13:552–559. [DOI] [PubMed] [Google Scholar]
- 51. Miller JJ. An autoradiographic study of plasma cell and lymphocyte survival in rat popliteal lymph nodes. J Immunol 1964;92:673–681. [PubMed] [Google Scholar]
- 52. Manz RA, Thiel A, Radbruch A. Lifetime of plasma cells in the bone marrow. Nature 1997;388:133–134. [DOI] [PubMed] [Google Scholar]
- 53. Leyendeckers H, et al. Correlation analysis between frequencies of circulating antigen‐specific IgG‐bearing memory B cells and serum titers of antigen‐specific IgG. Eur J Immunol 1999;29:1406–1417. [DOI] [PubMed] [Google Scholar]
- 54. Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long‐lived plasma cells. Immunity 1998;8:363–372. [DOI] [PubMed] [Google Scholar]
- 55. DiLillo DJ, et al. Maintenance of long‐lived plasma cells and serological memory despite mature and memory B cell depletion during CD20 immunotherapy in mice. J Immunol 2008;180:361–371. [DOI] [PubMed] [Google Scholar]
- 56. Ahuja A, Anderson SM, Khalil A, Shlomchik MJ. Maintenance of the plasma cell pool is independent of memory B cells. Proc Natl Acad Sci USA 2008;105:4802–4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cambridge G, et al. Serologic changes following B lymphocyte depletion therapy for rheumatoid arthritis. Arthritis Rheum 2003;48:2146–2154. [DOI] [PubMed] [Google Scholar]
- 58. Radbruch A, et al. Competence and competition: the challenge of becoming a long‐lived plasma cell. Nat Rev Immunol 2006;6:741–750. [DOI] [PubMed] [Google Scholar]
- 59. Hill SW. Distribution of plaque‐forming cells in the mouse for a protein antigen. Evidence for highly active parathymic lymph nodes following intraperitoneal injection of hen lysozyme. Immunology 1976;30:895–906. [PMC free article] [PubMed] [Google Scholar]
- 60. Benner R, Hijmans W, Haaijman JJ. The bone marrow: the major source of serum immunoglobulins, but still a neglected site of antibody formation. Clin Exp Immunol 1981;46:1–8. [PMC free article] [PubMed] [Google Scholar]
- 61. Slifka MK, Matloubian M, Ahmed R. Bone marrow is a major site of long‐term antibody production after acute viral infection. J Virol 1995;69:1895–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hyland L, Sangster M, Sealy R, Coleclough C. Respiratory virus infection of mice provokes a permanent humoral immune response. J Virol 1994;68:6083–6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Slifka MK, Ahmed R. Limiting dilution analysis of virus‐specific memory B cells by an ELISPOT assay. J Immunol Methods 1996;199:37–46. [DOI] [PubMed] [Google Scholar]
- 64. Taillardet M, et al. The thymus‐independent immunity conferred by a pneumococcal polysaccharide is mediated by long‐lived plasma cells. Blood 2009;114:4432–4440. [DOI] [PubMed] [Google Scholar]
- 65. Claflin AJ, Smithies O. Antibody‐producing cells in division. Science 1967;157:1561–1562. [PubMed] [Google Scholar]
- 66. Lanzavecchia A. Antigen‐specific interaction between T and B cells. Nature 1985;314:537–539. [DOI] [PubMed] [Google Scholar]
- 67. Nossal GJ. B lymphocyte physiology: the beginning and the end. Ciba Found Symp 1997;204:220–230. [DOI] [PubMed] [Google Scholar]
- 68. Takahashi Y, et al. Relaxed negative selection in germinal centers and impaired affinity maturation in bcl‐xL transgenic mice. J Exp Med 1999;190:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Burton DR. Opinion: antibodies, viruses and vaccines. Nat Rev Immunol 2002;2:706–713. [DOI] [PubMed] [Google Scholar]
- 70. Cooper EH. Production of lymphocytes and plasma cells in the rat following immunization with human serum albumin. Immunology 1961;4:219–231. [PMC free article] [PubMed] [Google Scholar]
- 71. Schooley JC. Autoradiographic observations of plasma cell formation. J Immunol 1961;86:331–337. [PubMed] [Google Scholar]
- 72. Makela O, Nossal GJV. Autoradiographic studies on the immune response. J Exp Med 1962;115:231–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Levy M, Vieira P, Coutinho A, Freitas A. The majority of “natural” immunoglobulin‐secreting cells are short‐lived and the progeny of cycling lymphocytes. Eur J Immunol 1987;17:849–854. [DOI] [PubMed] [Google Scholar]
- 74. Scheiermann N, Kuwert EK. Uptake and elimination of hepatitis B immunoglobulins after intramuscular application in man. Dev Biol Stand 1983;54:347–355. [PubMed] [Google Scholar]
- 75. Hopkins RJ, et al. Safety and pharmacokinetic evaluation of intravenous vaccinia immune globulin in healthy volunteers. Clin Infect Dis 2004;39:759–766. [DOI] [PubMed] [Google Scholar]
- 76. Adner N, Leibl H, Enzersberger O, Kirgios M, Wahlberg T. Pharmacokinetics of human tick‐borne encephalitis virus antibody levels after injection with human tick‐borne encephalitis immunoglobulin, solvent/detergent treated, FSME‐BULIN S/D in healthy volunteers. Scand J Infect Dis 2001;33:843–847. [DOI] [PubMed] [Google Scholar]
- 77. Bruss JB, et al. Treatment of severe pertussis: a study of the safety and pharmacology of intravenous pertussis immunoglobulin. Pediatr Infect Dis J 1999;18:505–511. [DOI] [PubMed] [Google Scholar]
- 78. Ghetie V, Ward ES. Transcytosis and catabolism of antibody. Immunol Res 2002;25:97–113. [DOI] [PubMed] [Google Scholar]
- 79. Lee MS, Chien LJ, Yueh YY, Lu CF. Measles seroepidemiology and decay rate of vaccine‐induced measles IgG titers in Taiwan, 1995–1997. Vaccine 2001;19:4644–4651. [DOI] [PubMed] [Google Scholar]
- 80. Herrmann KL, Halstead SB, Wiebenga NH. Rubella antibody persistence after immunization. JAMA 1982;247:193–196. [PubMed] [Google Scholar]
- 81. Ho F, Lortan JE, MacLennan I, Khan M. Distinct short‐lived and long‐lived antibody‐producing cell populations. Eur J Immunol 1986;16:1297–1301. [DOI] [PubMed] [Google Scholar]
- 82. Conrad RE, Ingraham JS. Rate of hemolytic antibody production by single cells in vivo in rabbits. J Immunol 1974;112:17–25. [PubMed] [Google Scholar]
- 83. Helmreich E, Kern M, Eisen HN. The secretion of antibody by isolated lymph node cells. J Biol Chem 1961;236:464–473. [PubMed] [Google Scholar]
- 84. Helmreich E, Kern M, Eisen HN. Observations on the mechanism of secretion of g‐globulins by isolated lymph node cells. J Biol Chem 1962;237:1925–1931. [PubMed] [Google Scholar]
- 85. Hibi T, Dosch HM. Limiting dilution analysis of the B cell compartment in human bone marrow. Eur J Immunol 1986;16:139–145. [DOI] [PubMed] [Google Scholar]
- 86. Tokoyoda K, Zehentmeier S, Chang HD, Radbruch A. Organization and maintenance of immunological memory by stroma niches. Eur J Immunol 2009;39:2095–2099. [DOI] [PubMed] [Google Scholar]
- 87. Cassese G, et al. Plasma cell survival is mediated by synergistic effects of cytokines and adhesion‐dependent signals. J Immunol 2003;171:1684–1690. [DOI] [PubMed] [Google Scholar]
- 88. Hauser AE, et al. Chemotactic responsiveness toward ligands for CXCR3 and CXCR4 is regulated on plasma blasts during the time course of a memory immune response. J Immunol 2002;169:1277–1282. [DOI] [PubMed] [Google Scholar]
- 89. Hargreaves DC, et al. A coordinated change in chemokine responsiveness guides plasma cell movements. J Exp Med 2001;194:45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Nie Y, Waite J, Brewer F, Sunshine MJ, Littman DR, Zou YR. The role of CXCR4 in maintaining peripheral B cell compartments and humoral immunity. J Exp Med 2004;200:1145–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kallies A, Nutt SL. Terminal differentiation of lymphocytes depends on Blimp‐1. Curr Opin Immunol 2007;19:156–162. [DOI] [PubMed] [Google Scholar]
- 92. Shapiro‐Shelef M, Lin KI, Savitsky D, Liao J, Calame K. Blimp‐1 is required for maintenance of long‐lived plasma cells in the bone marrow. J Exp Med 2005;202:1471–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Martins G, Calame K. Regulation and functions of Blimp‐1 in T and B lymphocytes. Annu Rev Immunol 2008;26:133–169. [DOI] [PubMed] [Google Scholar]
- 94. Mackay F, Schneider P. Cracking the BAFF code. Nat Rev Immunol 2009;9:491–502. [DOI] [PubMed] [Google Scholar]
- 95. O’Connor BP, et al. BCMA is essential for the survival of long‐lived bone marrow plasma cells. J Exp Med 2004;199:91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Benson MJ, et al. Cutting edge: the dependence of plasma cells and independence of memory B cells on BAFF and APRIL. J Immunol 2008;180:3655–3659. [DOI] [PubMed] [Google Scholar]
- 97. Crotty S, Kersh EN, Cannons J, Schwartzberg PL, Ahmed R. SAP is required for generating long‐term humoral immunity. Nature 2003;421:282–287. [DOI] [PubMed] [Google Scholar]
- 98. Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP‐controlled T–B cell interactions underlie germinal centre formation. Nature 2008;455:764–769. [DOI] [PMC free article] [PubMed] [Google Scholar]


