Abstract
The elite athlete has a potentially increased sensitivity to respiratory infections, rendering protective measures particularly important. Some other infections that may appear in clusters in the sports setting, such as gastroenteritis, leptospirosis, herpes simplex and viral hepatitis, also require special precautionary attention. Strenuous exercise during ongoing infection and fever may be hazardous and should always be avoided. In addition, early symptoms of infection warrant caution until the nature and severity of the infection become apparent. Because myocarditis may or may not be accompanied by fever, malaise or catarrhal symptoms, athletes should be informed about the symptoms suggestive of this disease. Although sudden unexpected death resulting from myocarditis is rare, exercise should be avoided whenever myocarditis is suspected. Guidelines are suggested for the management and counselling of athletes suffering from infections, including recommendations on when to resume training. Acute febrile infections are associated with decreased performance resulting from muscle wasting, circulatory deregulation and impaired motor coordination, which require variable amounts of time to become normalized once the infection is over.
Keywords: bacterial infection, exercise, myocarditis, performance, viral infection
Introduction
As compared with a sedentary lifestyle, the practice of moderate, regular physical training is generally considered to be associated with improved health, including, for example, lower blood pressure and bodyweight, improved glucose tolerance and possibly a decreased sensitivity to upper respiratory tract (URT) infections. 1 However, a single bout of strenuous endurance exercise is followed by temporary functional immunodepression, the extent and duration of which are related to that of the effort exerted (for review, see Mackinnon 2 ). During that ‘open window’, 3 the sensitivity to URT 4 , 5 , 6 and possibly also to other infections is potentially increased. If resting periods between such exercise sessions/ competitions are not long enough to allow the immune function to recover, an increased sensitivity to infectious diseases may be present for a prolonged period of time. 7 , 8 The potential for such a situation truly exists among the high‐ performing elite within several areas of sports and notably so in seasonal endurance sports. For URT infections, it has been suggested that a J‐shaped curve best describes the relationship between the intensity of physical activity, ranging from a sedentary lifestyle to the activity of the high‐performance endurance athlete (along the x axis), and the sensitivity to infections (along the y axis). 9 , 10 Interestingly, a recent study in elite swimmers has indicated that measurement of salivary IgA levels over a training season may be predictive for athletes at risk of infection. 11
In the sports setting, air‐ and droplet‐borne pathogens, such as common cold viruses, are easily transmitted by aerosol or contact, especially in team sports, posing a threat not least to the elite. Infections by pathogens that are transmitted by inoculation, such as the herpes and hepatitis B viruses, tend to accumulate within certain sports.
In the present article, infectious diseases in high‐ performance athletes will be discussed, including commonly encountered infections such as URT infections, as well as some other infections that tend to occur in clusters or epidemics, and also myocarditis, which may become aggravated by exercise. Furthermore, some guidelines will be proposed for the management and counselling of the individual athlete experiencing symptoms suggestive of an infection. First, some aspects of the host's metabolic response to infections and the interaction between infection and exercise will be considered.
Metabolic responses to infections
Acute infections in general evoke a multitude of host responses, some of which are specific for the causative microorganism, including specific cellular and humoral immunity, whereas others are stereotypic and similar in various infections and occur as a systemic acute phase reaction to the invading microorganisms. 12 , 13 The non‐specific, cytokine‐mediated host responses to infections have the purpose of increasing the host's potential for survival by causing a shift in the metabolism in order to mobilize nutrients from body tissues, predominantly amino acids from muscle tissue (Figure 1). This is to satisfy the increased nutritional needs of the activated immune system and to provide substrates for the accelerated energy production during fever, which is generally associated with anorexia and decreased food intake. The elevated insulin levels during infection and fever generally hamper the mobilization of fatty acids from fat depots. 12 , 13
Figure 1.
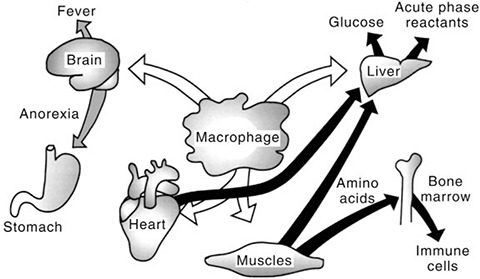
Schematic description of cytokine effects in acute infectious diseases. Cytokines stimulate muscle protein degradation by mediating release of amino acids from muscles and increased uptake of amino acids in the liver and other organs. Similarly, cytokines are involved in the fever response and in the development of anorexia during infection and fever.
An important feature of the acute phase reaction is the induction of a generalized catabolism of muscle protein, which progresses throughout the acute phase of the infection. In humans, muscle protein degradation and tissue wasting was originally demonstrated in nitrogen balance studies in experimental viral, bacterial and protozoan infections (Figure 2). 14 Thus, from a qualitative aspect, these responses are essentially similar regardless of the causative organism, whereas their magnitude is related to the virulence of the organism and the incubated dose, as well as to the susceptibility/immune status of the host. Several mild infections, however, such as an uncomplicated common cold, may not give rise to any significant systemic acute phase reaction. This array of acute phase responses, one of which is the occurrence of fever, is triggered by the release of cytokines from activated macrophages and monocytes. 12 , 13 Similarly, exercise is accompanied by increased body temperature and increased immune activity. 15 However, although exercise has been shown to be associated with the release to plasma of endogenous pyrogen, 16 a majority of studies do not support the idea that exercise hyperthermia is the result of a change in the body temperature set point, 17 as is the case in infection.
Figure 2.
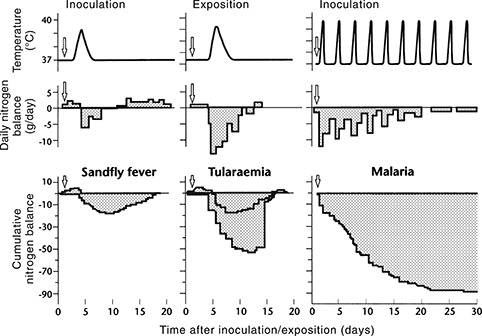
Body temperature, daily nitrogen balance and cumulative nitrogen balance during experimental sandfly fever, tularaemia and malaria in human volunteers (adapted from Beisel et al. 14 ).
The neuromuscular system in infections
The skeletal muscles, which constitute approximately 45% of bodyweight, are the major source of amino acids, 14 but the heart muscle also contributes (Figure 1). 18 , 19 , 20 Thus, a decrease in the protein content of the skeletal muscles, as well as in that of the heart muscle, is a general feature in acute infectious diseases, the magnitude of which is related to the magnitude and duration of the systemic acute phase reaction of the host. In acute myocarditis, this wasting of myocardial muscle protein occurs in parallel with the microorganism‐ and inflammation‐related damage to the heart. This cytokine‐ triggered muscle protein loss is gradually replenished when the infection has resolved.
Several microorganisms may cause meningo‐encephalitis, whereas infections located in peripheral nerves are rare. However, the neuromuscular physiology and function may be compromised as a result of the host response to infections located outside the neuromuscular system and in the absence of disease‐specific symptoms or pathology within this system. In one study, for example, a reduced safety margin of the neuromuscular transmission in the motor end‐plate was noted in the early phase of uncomplicated influenza, echovirus infection and mumps. 21 In addition, in the early course of a prototype experimental viral infection (sandfly fever), a decrease in speed and coordination in the performance of motor skills at submaximal force was observed, which could be quickly reversed by cyclo‐oxygenase inhibitors. 22 Although the pathogenesis of these changes has not been clarified, these results may have a bearing on sports in which coordination and precision are crucial. In addition, the impaired coordination and ‘motor precision’ may increase the risk of ankle sprains, dislocations and so on in many sports.
Performance during and after infections
During ongoing infection and fever the aerobic exercise capacity, as determined from the heart rate response during submaximal exercise, is decreased. This has been shown in healthy human volunteers injected with pyrogens, 23 as well as in volunteers receiving live organisms and tested on the first day of fever. 24 , 25 By contrast, the observed maximal oxygen uptake (maximal aerobic power) has been found to be uninfluenced during the flush phase of short‐lasting experimental pyrogen‐induced fever. 23 Similarly, in another study, the maximal aerobic power did not decrease as a result of thermal dehydration. 26 Thus, during fever, the maximal aerobic power may be underestimated if calculated from the heart rate response during submaximal exercise tests. To the best of our knowledge, there are no published studies in which the maximal oxygen uptake has been measured during ongoing infection and fever, obviously for ethical reasons. Thus, the rate and magnitude of the possible decrease in the maximal aerobic power during ongoing febrile infections in humans are not known.
In one study, it has been found that on the first day of fever in experimental sandfly fever both the isometric and the isotonic muscle strength decreased by up to 30%, depending on the tested muscle group, as compared with pre‐infection baselines. 25 The muscle biopsies carried out showed that no significant muscle protein degradation had yet occurred at this early stage of the infection to account for the impairment. However, the magnitude of the impairment was significantly correlated with the individual's own ratings of subjective symptoms, including myalgia. Thus, whether causally related or coexisting, a person's perception or experience of a febrile illness seems to negatively influence their ability to perform muscle work.
When an acute infection is over, the decline in muscle strength and endurance from the individual's baseline conditions when healthy is correlated to the muscle protein loss caused by the infection. In otherwise healthy young male adults, a week‐long uncomplicated febrile infection of a severity that required ‘clinical bed rest’ resulted in a decrease in the isometric strength by 5–15%, 27 and in the isometric endurance by 13–18%, 28 in various major muscle groups tested shortly after the disappearance of clinical symptoms of disease as compared with subsequent repeated individual baseline results when the patients were healthy. Furthermore, both in young male and young female adults, such infections cause a decrease in the aerobic exercise capacity, as determined from the heart rate response during submaximal exercise, of approximately 25%. 29 The greater decrease in the aerobic capacity compared with that in the muscle capacity is due to circulatory deregulation associated both with the infection and with bed rest, both of which contribute to deterioration of the aerobic capacity; the circulatory deregulation, in turn, is partly an effect of a decreased blood volume resulting from the bed rest. 30
The aerobic exercise capacity is dependent on central factors, such as the blood volume, total haemoglobin and myocardial function, as well as on peripheral factors, that is, the state of the skeletal muscles. 31 All of these factors may be negatively influenced by the infection or by the clinical bed rest that is part of the treatment, or both. 29 , 30 The deterioration of isometric strength and endurance may, however, be almost entirely ascribable to the infection‐induced catabolic effects on muscle. 27 , 28
Interaction between infection and exercise
Only few studies have focused on the question of whether, prior to the time of exposure to microorganisms, there is a critical period during which exercise stress may influence the proneness to develop subsequent disease. These few experimental studies on bacterial infections in rodents have shown that one bout of vigorous exercise shortly prior to infection drastically increases the resistance to the infection. Conversely, when the infection is contracted before the time of exercise, strenuous exercise is detrimental to the host. All animal experiments of which we are aware concerning non‐myocarditic bacterial and viral infections have shown that strenuous exercise during the febrile phase of an infection may be hazardous. For obvious ethical reasons, analogous experiments in humans are not feasible. For a more extensive review, see Friman and Ilbäck. 13 Interestingly, however, the muscle trainability seems to be preserved during some but not other infections. In experimental tularaemia in the rat, for example, strenuous exercise during the acute febrile course counteracted, but did not eliminate, the infection‐ associated muscle catabolism, yielding a net result of more limited protein degradation than in resting infected animals, 18 whereas in experimental influenza no anabolic effect of the exercise was seen. 32 In both experiments, exercise during ongoing febrile infection caused complications or increased lethality.
Among clinical conditions where microorganisms cause disease in the central nervous system, poliomyelitis has been studied in relation to exercise. This disease may become aggravated by exercise when performed in the early, preparalytic stage, resulting in more severe paralysis in muscles that have been involved in the exercise (for review, see Pedersen 33 ). In acute viral hepatitis, controlled studies have shown no adverse short‐ or long‐term effects of exercise of various, though not maximal, intensities during the early course. 34 , 35 , 36 , 37 Likewise, in patients suffering from febrile respiratory infections, a mild activity programme of letting the patient get out of bed every half‐hour during waking hours throughout the entire febrile course resulted in a smaller blood volume reduction and a smaller decrease in aerobic capacity compared to conventional bed rest. 38
In conclusion, in febrile infections in general there is no scientific basis for recommending other than rest or only moderate activity programmes, such as frequently getting out of bed, during the acute phase of the infection. In acute viral hepatitis, submaximal exercise seems to be safe.
Infection, performance and exercise interaction in the trained (conditioned) host
The effects of the trained state on the clinical course and metabolic responses to bacterial and viral infections have been studied experimentally. Cannon and Kluger have shown that the lethality in Salmonella typhimurium infection is lower in trained than in untrained rats. 39 Ilbäck et al., in a study of tularaemia and influenza in the mouse, have found a similar effect with influenza, whereas with tularaemia no difference in lethality is seen between trained and untrained animals. 32 Furthermore, in both tularaemia and influenza, the catabolic effects on myocardial protein and performance‐related oxidative muscle enzyme activities are significantly less pronounced in trained animals. In another study, it has been shown that the improved metabolic status achieved by a pre‐infection training programme was partly preserved during subsequent Streptococcus pneumoniae infection in the trained host, whereas in the infected untrained host the situation was more unfavourable. 40 The trained animals burned more fat, showed better ketonaemic adaptation and saved more glycogen than did the untrained animals during this infection. Furthermore, in studies in humans, trained individuals have been found to have a higher level of resting natural immunity than untrained ones (for review, see Pedersen 8 ). Exercise during ongoing URT infection may cause bronchial hyperreactivity. 41
To the best of our knowledge, there are no reports on adequately controlled studies on the effects of acute infectious diseases on performance, nor on the interaction between infection and exercise, in elite athletes.
Exercise in the convalescent phase: Reconditioning
During infections that evoke a systemic acute phase reaction, the magnitude of the resulting muscle protein catabolism is the major determinant of the length of the convalescent period, provided there is no complicating myocarditis. Following a brief, flu‐like illness with about 36 h of fever, as in sandfly fever, as long as 2 weeks is required for the accumulated muscle protein losses to be replenished in individuals not engaging in systematic training programmes. This period is much longer after more long‐lasting or severe infections, such as tularaemia or malaria (Figure 2). 14
Muscle trainability is preserved in early malnutrition, 42 as well as during fasting plus bacterial infection 18 and, as shown in healthy individuals, a weaker stimulus is required to evoke a training response in sedentary or deconditioned individuals than in well‐trained ones. 31 , 43 The situation after an acute febrile infection represents a deconditioned state as compared with that prior to the infection. Thus, although not confirmed in studies on patients who have suffered from various infectious diseases, available evidence suggests that the time to full replenishment of the muscle protein and resumed performance can be shortened by physical training once the acute phase response to the infection is over.
Myocarditis
In sports medicine, infections that are located in such tissues that are metabolically or mechanically activated during exercise, such as poliomyelitis (as mentioned earlier) and myopericarditis, pose a particular problem. In myocarditis, multiple experimental studies have shown exercise to be detrimental. 44 , 45 , 46 , 47 , 48
Presenting clinical features and diagnosis
The presenting features of acute infectious myocarditis are summarized in Table 1. It may be estimated that the majority of myocarditis episodes are subclinical, that is, the patient experiences no cardiac symptoms (Figure 3). 49 , 50 Chest symptoms may range from vague oppression to sharp pain, which becomes worse when taking a deep breath and sometimes radiates to the jaws, left shoulder and arm. Pain is correlated to coexisting pericarditis. Palpitation is common, the most frequent dysrhythmia being ventricular extrasystoles. Acute congestive heart failure is an infrequent presenting feature, as is sudden unexpected death. 51 , 52 , 53 The diagnosis of acute infectious myopericarditis can usually be made with reasonable certainty by means of clinical examination and readily available clinical tests, such as serial electrocardiographic recordings and serum markers of a myocyte lesion, although other, more sophisticated methods are sometimes needed (Table 2). 54 The clinical management of acute infectious myopericarditis in athletes is described in Table 3. This usually benign condition, which has a favourable long‐term prognosis in the great majority of cases, 55 , 56 , 57 should be differentiated from subacute and chronic myocarditis, the epidemiology and pathogenesis of which are less well known and where a myocardial biopsy may be required to establish the diagnosis (‘histopathological myocarditis’; Figure 3). The definitions and classification of the cardiomyopathies have recently been revised; inflammatory cardiomyopathy is now defined as myocarditis in association with cardiac dysfunction. 58
Table 1.
Presenting features of acute infectious myocarditis (adapted from Karjalainen 53 )
| Mimics myocardial infarction (chest pain/discomfort/oppression) |
| Dysrhythmia (risk of sudden death) |
| Congestive heart failure |
| No cardiac symptoms |
Figure 3.
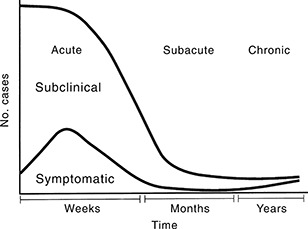
Spectrum of infectious myocarditis/cardiomyopathy. Reproduced with permission from Friman and Ilbäck. 13
Table 2.
Diagnosis of acute infectious myopericarditis (adapted from Karjalainen and Heikkila 104 and Friman et al. 54 )
| Method | Features |
|---|---|
| ECG, definite myopericarditis | Typically evolving ST‐T changes on serial ECG recordings 104 |
| ECG, possible myopericarditis | Conduction defects and dysrhythmia triggered by infection, gradually evolving T wave changes, unresponsive to beta‐blockade |
| Serum markers of myocardial injury (transiently elevated in most cases of definite myopericarditis) | Troponin T or I (high myocardium specificity) Creatine kinase, isozyme MB* (acceptable myocardium specificity) Aspartate aminotransferase (AST)* (low myocardium specificity) |
| Echocardiography | Transient global or regional hypokinesis of left ventricle (LV), transient thickening of LV wall, LV dilatation (usually mild), pericardial effusion if concomitant pericarditis, may be normal despite elevated serum levels of markers of myocardial injury |
| Endomyocardial biopsy | May be considered in severe, complicated or subchronic cases |
Note that in highly trained healthy athletes, aspartate aminotransferase (AST) and even creatine kinase isozyme MB (CK‐MB) may be
moderately elevated! ECG, electrocardiogram.
Table 3.
Management of acute infectious myopericarditis in athletes (adapted from Friman et al. 54 )
| Patients with definite myopericarditis | |
| Observe in hospital during period of elevated serum levels of myocardial injury markers | |
| Treat complex dysrhythmias and heart failure | |
| Test for specific infections, treat bacterial infections | |
| After first week, avoid strenuous exercise until ECG at rest has normalized or stabilized (which occurs within 2 months in most mild to moderate cases) | |
| In convalescent period, beta‐blocking drugs may be considered if hyperkinetic heart symptoms are present | |
| Maximal exercise ECG test before resuming sport activities or other major heart‐taxing efforts | |
| Patients with possible myopericarditis | |
| Avoid strenuous exercise until myopericarditis has been excluded | |
| Common conditions often wrongly diagnosed as myocarditis | |
| Misinterpreted ECG, e.g. hyperadrenergic state with T‐wave changes (beta‐blockade normalizes ECG) or early repolarization pattern in ECG, both common in athletes | |
| Overtraining syndrome, especially when combined with findings significant to the athlete's heart | |
| ‘Heart neurosis’ to which various symptoms and signs often contribute | |
ECG, electrocardiogram.
Aetiology
Although several non‐infectious causes are known, the majority of cases of myocarditis are considered to have an infectious origin. 59 , 60 The infection may solely affect the heart, either the myocardium or pericardium or both, or the myopericarditis may be part, or a complication, of an infection located elsewhere, most commonly in the upper respiratory/ digestive tract. With few exceptions, 60 clinical studies have incriminated the coxsackieviruses as the most common aetiological factors in acute infectious myopericarditis, being involved in approximately 50% of the cases. 51 , 52 , 61 The coxsackieviruses belong to the enteroviruses, encompassing some 70 different serotypes. These viruses are ubiquitous and usually give rise to only mild throat symptoms or gastrointestinal upsets, or no symptoms at all, and virtually everybody suffers several such infections during their lifetime. Occasionally the enteroviruses affect the heart. Furthermore, a host of other viruses, especially respiratory viruses, such as influenza and cytomegalovirus, may give rise to myocarditis, whereas the most common aetiologies of the ‘common cold’, the rhinoviruses, have not been unequivocally documented as causes of myocarditis. 62
Cardiac involvement in HIV infection is described in the section ‘The athlete and HIV infection’.
Several bacteria, such as beta‐haemolytic streptococci, a common cause of tonsillopharyngitis, may give rise to toxic myocarditis. 63 Thus, patients with a diagnosis of streptococcal tonsillopharyngitis should refrain from strenuous exercise during the first week of antibiotic treatment even if no chest symptoms are present. Not uncommonly, Mycoplasma pneumoniae infection is complicated by myocarditis, usually occurring as a mild complication in pneumonia. In one study, M. pneumoniae was incriminated in 6% of consecutive military conscripts suffering from unequivocal myocarditis and 1–2% of conscripts hospitalized with mycoplasmal infection had concomitant myocardial involvement. 64 In Chlamydia pneumoniae infection, which strikes a majority of the adult population worldwide, myocarditis has been reported. 65 , 66 However, the incidence of this complication in that common infection has not been systematically studied.
In some sports, such as orienteering or adventure races, the athletes may be heavily exposed to mosquitoes, ticks and other insects. Borrelia burgdorferi, which is transmitted by ticks, causes Lyme disease, occasionally including myocarditis. In South America, Trypanosoma cruzi, the protozoa causing Chagas' disease, is a common cause of cardiomyopathy and when affecting young sportsmen carries an increased risk of sudden cardiac death. 67
Sometimes elite athletes try to enhance their performance by taking drugs, such as the central stimulants amphetamine and cocaine, although cocaine is mostly taken for social reasons, also by athletes. Myocarditis is one of the various cardiovascular effects of cocaine abuse and in one study was found at autopsy in 20% of subjects in whom cocaine was present in the blood. 68 The possible use of central stimulants should thus be considered in elite athletes with cardiac involvement. 69 , 70
Pathogenesis and interaction with exercise
Much of the current knowledge on the pathogenesis of myocarditis has been obtained from experimental studies in coxsackievirus mouse models. The myocardial tissue lesion in acute viral myocarditis in the immunologically mature individual is partly affected by the replicating virus causing cytolysis and subsequent scavenging of infected cells by virus‐specific cytotoxic T cells, and partly by the action of cross‐reactive (autoimmune) cytotoxic T cells, as well as cross‐reactive autoantibodies, targeted at non‐infected myocardial cells and thus increasing the tissue damage. 71 , 72 In addition, infected myocardial cells may cause a shutdown of the metabolism in adjacent non‐infected cells by means of a toxic metabolite. 72 Thus, in the immunologically mature individual, the tissue lesion in viral myocarditis is the result of several mechanisms acting in concert, where cardiac damage induced by the virus is the primary event. 73
Acute infectious myocarditis is accompanied by a systemic acute phase reaction varying in severity, including both myocardial and skeletal muscle protein degradation.
Exercise stress during the early phase of coxsackie myocarditis in suckling mice has been found to be associated with an increased viral replication rate within myocardial cells, resulting in increased cytolysis and tissue damage. 44 , 45 Exercise stress during the early phase of this disease in adult mice also enhances the immune mechanisms, resulting in an increased inflammatory lesion and necrosis. 47
Myocarditis and sudden unexpected death
Several epidemiological studies have shown that sudden unexpected death (SUD) in the young (below 35 years of age) may be caused by several underlying conditions and is often precipitated by exercise. 74 , 75 In a recent study on athletes, hypertrophic cardiomyopathy was the most frequent single cause of SUD and was found at autopsy in 26% of white athletes and in 48% of black athletes, whereas myocarditis was the suggestive cause in only 5% of the cases. 75 Several previous studies have shown that an average of 10% of cases of SUD in young athletes have been caused by myocarditis 74 and in only few studies has myocarditis been a more frequent cause of SUD. 76 , 77 Clustering of SUD is rare, but has been reported to have occurred among males having roots in South‐East Asia. The SUD occurred during sleep and at post‐mortem investigations there were few abnormal findings. 78
During 1979–92, an increased rate of SUD was reported among young Swedish orienteers; there were 15 men and one woman (Figure 4) and myocarditis was the most frequent histopathological feature. 77 , 79 The inflammatory process was subacute to chronic. All but two of the SUD were associated with exercise. It has previously been found that loss of performance may be the only symptom of ongoing myocarditis, 80 but all of these deceased people had performed at, or close to, their individual maximum shortly before they died. Five of them, however, had experienced heart‐related symptoms prior to death, such as fainting during exercise, tachycardia or chest pain, but the majority had not reported any prior warning symptoms. Since late 1992 there have been no new SUD among young Swedish orienteers. It seems plausible that the various measures taken at that time, including the introduction of a 6 month intermission in training and competition in early 1993, may have had a favourable effect in changing behavioural patterns; previously, the training habits among young elite orienteers had been extreme. 77
Figure 4.
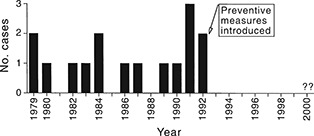
Sudden unexpected cardiac deaths in young Swedish orienteers 1979–99 (n = 16); adapted from Wesslén et al., Eur. Heart J. 1996; 17: 902–10. 77 ©1996 WB Saunders, A Harcourt Health Sciences Company.
Trace elements and cardiomyopathy
Some decades ago it was suspected that Keshan disease, a cardiomyopathy occurring in selenium‐deficient areas of China, was also linked to infection, because of its seasonal appearance. Subsequently, Chinese scientists have been able to isolate a number of viruses from Keshan disease victims, including a coxsackievirus type B4. This virus causes more heart damage when inoculated into selenium‐deficient mice than when given to mice with adequate selenium levels. For references, see Levander and Beck. 81 In addition, selenium supplementation in adequately fed mice has been found to increase NK cell activity and reduce the severity of coxsackie B3 myocarditis. 82 , 83 Since that time, Beck et al. have confirmed the early Chinese reports of an aggravated cardiac lesion in coxsackie myocarditis in the selenium‐deficient host, which they found to be caused by mutation of the virus into a variant previously known to be cardiovirulent. 84 Likewise, a vitamin E‐deficient diet selects for a stable cardiovirulent coxsackie variant. 85 Low‐dose exposure to several environmental pollutants, such as nickel, methyl mercury and dioxin, has been found to be correlated to aggravated experimental coxsackievirus infection. 86 , 87 It would seem that some environmental pollutants may compete with trace elements necessary for an optimal immune function, such as zinc 88 and selenium, in the target organ of the infection, resulting in increased disease severity. 83 , 86
In athletes residing in selenium‐deficient areas, adequate selenium supplementation would seem advisable, although selenium deficiency has not been linked to increased morbidity from infectious diseases in human studies.
The athlete and HIV infection
The main characteristics of HIV infection and AIDS are progressively deteriorating immune function, opportunistic infections, muscle wasting, anxiety and depression. The literature on exercise and immune function in HIV infection has recently been extensively reviewed by Shephard. 89 He has concluded that although the studies performed hitherto have been limited in scale and have often lacked appropriate controls, the available information strongly suggests that exercise is a beneficial activity for the HIV‐infected person. Exercise has been found to reduce anxiety and depression in such individuals 90 and muscle trainability seems to persist, at least into early AIDS. In one recent study, for example, short‐term, high‐intensity, progressive resistance training was found to significantly increase the lean body mass and muscle strength in HIV infection. 91 Other recent findings in HIV‐positive homosexual men suggest that exercising 3–4 times per week is associated with a temporary slowing down of the progression toward AIDS, compared with the situation in a non‐exercising control group. 92 Taken together, however, the available data do not allow any firm conclusions to be drawn regarding possible beneficial or detrimental effects of exercise training on the immune system in HIV‐positive individuals. 93
Thus, although the case of ‘Magic’ Johnson highlights the fact that HIV infection is an issue also within elite sports and athletics, the current knowledge does not allow for any scientifically based guidelines regarding the advisability for the individual asymptomatic athlete to continue practising their sport at the elite level.
Increasingly, HIV infection is recognized as an important cause of myocarditis and dilated cardiomyopathy. Early studies have revealed the presence of myocarditis in 45–52% of AIDS patients. 94 , 95 In a recent study of 952 asymptomatic HIV‐positive patients, Barbaro et al. found that 76 patients (8%) fulfilled echocardiographic criteria of dilated cardiomyopathy. 96 By means of endomyocardial biopsy, a histological diagnosis of myocarditis was made in 63 (83%) of the 76 patients, and in 58 (76%) of the patients nucleotide sequences of HIV were found. Furthermore, nucleic acids of coxsackieviruses coexisted in the myocytes in several of the patients and cytomegalovirus and Epstein–Barr virus nucleic acids were found in a few.
Clusters of infectious diseases in the sports setting
The lion's share of infections in elite athletes, as in others, consists of URT infections, especially in team sports where close contact between teammates promotes transmission. Historically, some infectious diseases have shown a clear accumulation among athletes within certain sports, such as hepatitis B among Swedish orienteers 97 and numerous outbreaks of herpes gladiatorum among wrestlers and other contact sportsmen, 98 , 99 , 100 due to efficient modes of transmission of the causative microorganisms. Large international sport events put a strain on infrastructure functioning, such as food handling or shared hygienic facilities both for athletes and spectators. Table 4 presents some infections of which accumulations or an increased risk among athletes has been documented.
Table 4.
Documented outbreaks or infections to which athletes are especially prone
| Disease | Transmission | Sport at risk | Reference | |
|---|---|---|---|---|
| Leptospirosis: 74 cases in 961 participants of triathlon race, Illinois, USA | Swimming in contaminated water | Triathlon | 105 , 106 | |
| Canoe/kayak Outdoor aquatic sports | 107 | |||
| Gastroenteritis: increased risk within 1 week after swimming in race compared with cyclists, The Netherlands | Swimming in contaminated water | Triathlon | 108 | |
| Schistosomiasis: increased risk noted for canoeists, South Africa. 80% of triathletes in Zimbabwe tested positive | Contaminated water | Canoe/kayak Triathlon | 109 110 | |
| Hepatitis B: | ||||
| outbreak with 1500 cases among 10 000 participants, Sweden. | Multiple bloody scratches and primitive washing facilities | Orienteering | 97 | |
| Outbreak among sumo wrestlers, Japan | Wrestling | 111 | ||
| Herpes gladiatorum: numerous outbreaks of herpes simplex virus infection of the skin | Close body contact | Wrestling Contact sports | 98 , 112 , 99 100 | |
| Tinea gladiatorum: cutaneous manifestations, numerous outbreaks | Close body contact | Wrestling Contact sports | 113 , 114 115 | |
| Measles: | Airborne, highly contagious | All sports | ||
| Three participants from New Zealand fell ill at an international gymnastics competition in IN, USA | 116 | |||
| A track and field athlete from Argentina caused 25 secondary cases among US residents from seven states | 117 | |||
| Purulent meningitis: Outbreak tracked to rugby sports club | Person‐to‐person | All sports | 118 | |
| Aseptic meningitis: Outbreaks among football teams in different states, USA | Often person‐to‐person | All sports | 119 , 120 , 121 | |
| Spotted fever rickettsiosis: Rickettsia africae infection, 13 cases among returning French competitors of adventure race in South Africa | Tick‐borne | Adventure race, orienteering | 122 | |
| Lyme disease | Tick‐borne | Outdoor sports taking place in vegetation | 123 , 124 | |
| Gastroenteritis (food poisoning): numerous individual cases. Unacceptable source of inferior results for athletes. | Food‐ or waterborne | All sports | ||
| Over 500 participants were affected by Campylobacter‐contaminated raw cow's milk in a drink during a jogging rally, Switzerland | 125 | |||
| During sports day for handicapped, 485 of 1094 fell ill, Thailand | 126 | |||
| A salmonella outbreak at the Junior World Rowing Championships, impaired performance of several teams, Poland | 127 | |||
| Foodborne outbreaks during the Barcelona Olympic Games, Spain | 128 | |||
| Athlete's foot: interdigital fungal infection of the horny layer predisposing to bacterial superinfection. Predisposition also from sweating in tight shoes or staying in an aquatic milieu. | Some transmission via wet sufaces such as at public baths or swimming pools | Universal but more predominant in water sports | 129 , 130 | |
| Swimmer's ear: otitis externa | Aquatic activities | |||
| HIV: three published cases Hepatitis C: one case Hepatitis B: one case Dermal abscesses | Contaminated drugs or injection devices | Athletes injecting legitimate drugs such as vitamins or various prohibited performance enhancing drugs | 131 , 132 , 133 | |
The 2000 Sydney Olympic games will take place in September and will thus coincide with the annual influenza A epidemic that usually occurs during May–September in southern Australia. During the games, the organisers will distribute 200 000 condoms among the competitors to lower the risk of sexually transmitted diseases, including HIV.
Conclusions and guidelines
In general, it may be said that physical activity in connection with infections is associated with certain medical risks, both for the infected individual and for fellow sportsmen, who may in turn become infected. The latter risk has relevance mainly in team sports, but is also possible in other sports where the participants are in close physical contact before, during or after the sporting event. Both of these aspects will be discussed. Furthermore, some guidelines for the management and counselling of athletes, primarily intended for the general practitioner, will be proposed, mainly with reference to adults. Several previous publications include pertinent information on the handling of infections in athletes and on what to tell the patient. 33 , 89 , 90 , 101 , 102 , 103
The risk to the individual
The risks of deleterious effects of physical activity in an infected person vary considerably, depending both on the location, degree and cause of the infection and on the intensity and type of physical activity. Vigorous or prolonged physical activity as well as intensive mental stress can lower defences against infection. Furthermore, a subclinical complication of infection, such as myocarditis, can be aggravated by physical exertion. The risk level is generally higher in a training and competing sportsperson than in the ordinary jogger, for example. The physician's advice to different patients therefore needs to be individualized.
The muscular and cardiac capacity is reduced in connection with most infections, especially if the infection is accompanied by fever. Continuing to train during the infection cannot generally prevent this temporary impairment of physical capacity. On the contrary, training during the course of an infection can lead to further reduction of the capacity, infection complications and other damage.
The nervous system is affected in general by infection and fever, with impairment of coordination ability (the ‘motor precision’). This can influence physical performance, especially in sports that demand high precision. In addition, the risk of damage to joints, ligaments and tendons is increased.
Physical exertion in the presence of fever implies an increased haemodynamic load on the heart compared with such exertion in the healthy individual. This may lead to the manifestation, sometimes in the form of fatal dysrhythmia, of perhaps previously undiagnosed heart disease, for example coronary sclerosis or hypertrophic cardiomyopathy. Furthermore, for example in respiratory tract infections, a heavy physical load may aggravate the infection, with more pronounced or prolonged symptoms, or development of complications, such as sinusitis and pneumonia. This applies even in the absence of fever. Myocarditis is the complication that has been discussed most in this regard. It should be pointed out, however, that in the majority of cases infectious myocarditis resolves, when properly attended to, without residual symptoms and that in myocarditis sudden death is rare.
A large number of different viruses and bacteria can infect the heart and give rise to myocarditis. Some microorganisms are more prone than others to attack the heart, while others do this very rarely. The former group includes enteroviruses (mainly coxsackievirus) and the latter group includes common cold viruses (rhinovirus and coronavirus). There is no quick test for demonstrating these viruses in an infected person. Moreover, many other microorganisms associated with a varying degree of risk of myocarditis often give rise to similar symptoms. There are certain rules of thumb (see later), but their precision varies. In clinical practice, therefore, conclusions drawn from the symptomatology and clinical picture regarding the microbial cause and hence the risk of myocarditis can be uncertain. As a general rule, the physician should adopt a more cautious attitude towards physical activity in infected sportsmen who are under ‘pressure’ to achieve their maximal performance than in infected joggers, for example, because mental stress can also lower the defence against infection. Special attention must be paid to elite athletes, where the environmental demands and expectations regarding participation and success, as well as their own demands, are particularly high. In certain sports the impairment of performance associated with the infection can be compensated by the sportsman's routine and skill, which may lead to increased risk taking.
Mononucleosis can often be diagnosed by a rapid test. This disease can be associated with myocarditis. Furthermore, mononucleosis is often accompanied by splenic enlargement, which has an impact on counselling, especially of persons practising contact sports and weightlifting.
Tonsillopharyngitis with beta‐streptococci can also be diagnosed by a quick test and penicillin therapy should be given. There is some risk of myocarditis, despite treatment, during the first week.
Gastroenteritis is associated with fluid losses, which reduce performance as a result of a decreased plasma volume. Fluid losses through sweating in connection with physical exercise accentuate this effect and increase the risk of collapse and of manifestation of undiagnosed heart diseases. Myocarditis can sometimes occur as a complication of gastroenteritis.
Skin infections of different kinds are common in sports, usually in the form of infected chafing sores, athlete's foot, infected eczema and plantar warts. Dermal borreliosis (erythema migrans in Lyme disease) is common among sportspeople who are exposed to ticks. Myocarditis is a well‐known complication of borreliosis. Sometimes even minor skin infections, on account of their location, can form a hindrance to sports activities and in occasional cases can constitute a port of entrance of bacteria that give rise to septicaemia. Small superficial skin infections are seldom contraindications to training and competitions. One exception is herpes infection in the skin, particularly in wrestlers. During wrestling, viruses can easily be transmitted to other wrestlers via skin lesions.
Genital infections give rise to local and general symptoms of varying degrees of severity or may be asymptomatic. It is uncommon for the microorganisms involved in such infections to lead to myocarditis.
There is currently no evidence that the course of the disease in asymptomatic HIV infection is influenced unfavourably by physical activity and sports. On the contrary, it has been documented that training and competitive activities have an important effect in improving the quality of life in many HIV patients.
Suggestions for guidelines for management and counselling
In people with fever (38°C or more), rest should always be recommended. People who know their normal temperature and pulse curves should rest if their resting temperature has increased by 0.5–1°C or more and at the same time their resting pulse has risen by 10 b.p.m. or more, in combination with general symptoms (malaise, muscle pains, muscle tenderness, diffuse joint pains, headache).
General malaise of acute onset, especially in combination with muscle pains, muscle tenderness, diffuse joint pains and headache, should give reason to recommend rest, even when the body temperature is normal.
In all infections, caution should be observed during the first 1–3 days of symptoms, even with a normal body temperature, until the body defence against infection has had time to become mobilized and until the further development of the infection becomes clear. Serious infections often have prodromal symptoms and in such cases it may take 1–3 days before the serious nature of the infection becomes evident.
In people with nasal catarrh without a sore throat, cough or general symptoms, caution is recommended during the first 1–3 days, after which training can gradually be resumed if the symptoms do not become intensified. If a cold is accompanied by further symptoms, for example, sore throat, hoarseness or cough, the recommendation should be more restrictive, depending on the degree and development of the symptoms.
In people with a sore throat without any other manifestations, caution is advised until this has begun to improve. In cases of beta‐streptococcal tonsillopharyngitis, which should be treated with penicillin for 10 days, rest is recommended until the symptoms have disappeared and caution is recommended during the first week of treatment even in the absence of symptoms.
In cases of mononucleosis, the general recommendation is rest until the symptoms have disappeared. Sometimes, however, fatigue can persist for several months after this disease and in such cases a suitable time for resuming training has to be judged individually. Furthermore, mononucleosis is often accompanied by splenic enlargement and an enlarged spleen in mononucleosis is fragile and can rupture if it is subjected to a blow or increased pressure. Thus, people engaged in contact sports, such as football, wrestling and so on, and weightlifting should wait 1 month after they have become free from fever before taking up these sports again.
In gastroenteritis, heavy physical exercise should be avoided.
In skin infections, the recommendations need to be individualized. All athletes should observe caution in episodes of herpes accompanied by regional lymphadenitis or general symptoms. Individuals practising contact sports should be screened for dermal herpes lesions and infected individuals should wait until the vesicles have dried before resuming these activities. Erythema migrans should be treated with penicillin or doxycycline for 10 days and caution should be recommended during the first week.
In cystitis, which mainly affects women, strenuous physical exertion should be avoided until the symptoms have subsided.
People with ongoing genital infections should avoid strenuous physical exertion. In asymptomatic genital chlamydial infection, it seems reasonable to restrict the physical activity during the period of antibiotic therapy.
Asymptomatic HIV infection constitutes no hindrance to exercise and sports (as far as the infected person is concerned; see later).
In most cases of febrile infectious diseases, training can be resumed as soon as the fever has abated. It is important, however, that the training should be resumed gradually, and with attention paid to the ‘body signals’. If unexpected symptoms referable to the heart should appear, for example pain, a sense of pressure or discomfort in the chest, irregular heart beats, abnormal breathlessness or fatigue or exertional syncope, the training should be discontinued and a physician consulted, because myocarditis can occur in connection with a number of different infections. It is important to point out that myocarditis can develop even without prior symptoms of infection. In people who have reached middle age, the possibility of coronary disease or myocardial infarction should also be considered with symptoms of this type.
In general, it may be said that in infections, as in other situations, it is important to ‘listen to the body signals’.
Risks to the environment: Epidemiological aspects
Plantar warts are readily spread via shower floors and changing rooms. These warts should therefore be treated quickly in athletes.
Respiratory tract infections can readily be transmitted both by droplet infection (sneezing/cough) and by contact (direct skin contact such as hand‐shaking or indirect contact via objects) among sportspeople who are in close proximity before, during or after a training or competitive event. Examples of this are countless. In addition, the fact that strenuous or prolonged physical exertion can reduce the defence against infection increases the susceptibility to respiratory tract infection.
Because prevention of exposure is the only prophylactic measure available in the vast majority of respiratory tract infections, the above aspects should be carefully considered by the individual athlete, as well as by trainers and sports leaders, before an infected individual allows himself or is allowed to meet his fellow participants prior to important training and competitive events. Annual immunization against influenza should be recommended for elite athletes.
Generally, athletes with HIV infection should be allowed to participate in sports just like any others. Physicians of HIV patients who are engaged in sports associated with a risk of exposure of blood, such as wrestling, boxing, football and so on, should inform the patients concerned of the theoretical risk that the infection can be transmitted further and strongly advise against their continuing to take part in sports of that kind. It is important to consider the anonymity aspects and to see that the infection status of the person concerned does not come to the knowledge of the leaders or teammates unless the individual has given their consent.
Acknowledgements
Our thanks are due to Professors Lars‐Magnus Engström, Bente Klarlund‐Pedersen and Christer Rolf for reviewing the suggestions for guidelines included in this article.
References
- 1. Nieman DC, Nehlsen‐Cannarella SL, Markoff PA. The effects of moderate exercise training on natural killer cells and acute upper respiratory tract infections. Int. J. Sports Med. 1990; 11: 467–73. [DOI] [PubMed] [Google Scholar]
- 2. Mackinnon LT. Immunity in athletes. Int. J. Sports Med. 1997; 18 (Suppl. 1): S62–8. [DOI] [PubMed] [Google Scholar]
- 3. Pedersen BK, Ullum H. NK‐cell response to physical activity: Possible mechanisms of action. Med. Sci. Sports Exerc. 1994; 26: 140–6. [DOI] [PubMed] [Google Scholar]
- 4. Peters E, Bateman E. Ultramarathon running and upper respiratory tract infections. An epidemiological study. S. Afr. Med. J. 1983; 64: 582–4. [PubMed] [Google Scholar]
- 5. Nieman DC, Johanssen LM, Lee JW, Arabatzis K. Infectious episodes in runners before and after the Los Angeles Marathon. J. Sports Med. Phys. Fitness 1990; 30: 316–28. [PubMed] [Google Scholar]
- 6. Heath GW, Ford ES, Craven TE, Macera CA, Jackson KL, Pate RR. Exercise and the incidence of upper respiratory tract infections. Med. Sci. Sports Exerc. 1991; 23: 152–7. [PubMed] [Google Scholar]
- 7. Fitzgerald L. Exercise and the immune system. Immunol. Today 1991; 9: 337–9. [DOI] [PubMed] [Google Scholar]
- 8. Pedersen BK. Chronic exercise and the immune system. In: Pedersen BK.). Exercise Immunology. New York: Springer, 1997; 113–22. [Google Scholar]
- 9. Nieman DC, Nehlsen‐Cannarella SL. Exercise and infection In: Watson RR, Eisinger M.). Exercise and Disease. Boca Raton (FL): CRC Press, 1992; 122–48. [Google Scholar]
- 10. Nieman DC. Exercise, upper respiratory tract infection and the immune system. Med. Sci. Sports Exerc. 1994; 26: 128–39. [DOI] [PubMed] [Google Scholar]
- 11. Gleeson M, McDonald WA, Pyne DB et al Salivary IgA levels and infection risk in elite swimmers. Med. Sci. Sports Exerc. 1999; 31: 67–73. [DOI] [PubMed] [Google Scholar]
- 12. Beisel WR. Metabolic response of host to infections In: Feigin RD, Cherry JD. (eds). Textbook of Pediatric Infectious Diseases, 4th edn. Philadelphia: WB Saunders Co., 1998; 54–69. [Google Scholar]
- 13. Friman G, Ilbäck N‐G. Acute infection: Metabolic responses, effects on performance, interaction with exercise, and myocarditis. Int. J. Sports Med. 1998; 19: 172–82. [DOI] [PubMed] [Google Scholar]
- 14. Beisel WR, Sawyer WD, Ryll ED, Crozier D. Metabolic effects of intracellular infection in man. Ann. Intern. Med. 1967; 67: 744–79. [DOI] [PubMed] [Google Scholar]
- 15. Pedersen BK. Acute exercise and the immune system In: Pedersen BK.). Exercise Immunology. New York: Springer, 1997; 5–38. [Google Scholar]
- 16. Cannon JG, Kluger MJ. Endogenous pyrogen activity in human plasma after exercise. Science 1983; 220: 617–19. [DOI] [PubMed] [Google Scholar]
- 17. Pedersen BK, Klokker M, Kappel M. Possible role of hyperthermia and hypoxia in exercise‐induced immunomodulation In: Pedersen BK.). Exercise Immunology. New York: Springer, 1997; 61–73. [Google Scholar]
- 18. Friman G, Ilbäck N‐G, Beisel WR, Crawford DJ. The effects of strenuous exercise on infection with Francisella tularensis in rats. J. Infect. Dis. 1982; 14: 706–14. [DOI] [PubMed] [Google Scholar]
- 19. Ilbäck N‐G, Friman G, Beisel WR. Biochemical responses of the myocardium and red skeletal muscle to Salmonella typhimurium infection in the rat. Clin. Physiol. 1983; 3: 551–63. [DOI] [PubMed] [Google Scholar]
- 20. Ilbäck N‐G, Friman G, Beisel WR, Johnson AJ. Sequential metabolic alterations in the myocardium during influenza and tularemia in mice. Infect. Immun. 1984; 45: 491–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Friman G, Schiller H, Schwartz M. Disturbed neuromuscular transmission in viral infections. Scand. J. Infect. Dis. 1977; 9: 99–103. [DOI] [PubMed] [Google Scholar]
- 22. Alluisi EA, Beisel WR, Morgan BB, Caldwell LS. Effects of sandfly fever on isometric muscular strength, endurance and recovery. J. Motor Behav. 1980; 12: 1–11. [DOI] [PubMed] [Google Scholar]
- 23. Grimby G, Exercise in man during pyrogen‐induced fever. Scand. J. Clin. Lab. Invest. 1962; 14: (Suppl. 67). [PubMed] [Google Scholar]
- 24. Daniels WL, Sharp DS, Wright JE et al Effects of virus infection on physical performance in man. Mil. Med. 1985; 150: 8–14. [PubMed] [Google Scholar]
- 25. Friman G, Wright J, Ilbäck N‐G et al Does fever or myalgia indicate reduced physical performance capacity in viral infections ? Acta Med. Scand. 1985; 217: 353–61. [DOI] [PubMed] [Google Scholar]
- 26. Saltin B. Circulatory response to submaximal and maximal exercise after thermal dehydration. J. Appl. Physiol. 1964; 19: 1125, [DOI] [PubMed] [Google Scholar]
- 27. Friman G. Effect of acute infectious disease on isometric muscle strength. Scand. J. Clin. Lab. Invest. 1977; 37: 303–8. [DOI] [PubMed] [Google Scholar]
- 28. Friman G. Effect of acute infectious disease on human isometric muscle endurance. Uppsala J. Med. Sci. 1978; 83: 105–8. [DOI] [PubMed] [Google Scholar]
- 29. Friman G. Effects of acute infectious disease on circulatory function. Acta Med. Scand. 1976; 592: 1–62. [PubMed] [Google Scholar]
- 30. Friman G. Effect of clinical bed rest for seven days on physical performance. Acta Med. Scand. 1979; 205: 389–93. [DOI] [PubMed] [Google Scholar]
- 31. Åstrand P‐O, Rodahl K. Textbook of work physiology. Physiological Bases of Exercise. 3rd edn. New York: McGraw‐Hill Book Company, 1986. [Google Scholar]
- 32. Ilbäck N‐G, Friman G, Beisel WR, Johnson AJ, Berendt RF. Modifying effects of exercise on clinical course and biochemical response of the myocardium in influenza and tularemia in mice. Infect. Immun. 1984; 45: 498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pedersen BK. Exercise and infection In: Pedersen BK.). Exercise Immunology. New York: Springer, 1997; 133–47. [Google Scholar]
- 34. Chalmers TC, Eckhardt RD, Reynolds WE et al The treatment of acute infectious hepatitis. Controlled studies of the effects of diet, rest, and physical reconditioning on the acute course of the disease and on the incidence of relapses and residual abnormalities. J. Clin. Invest. 1955; 34: 1163, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nefzger MD, Chalmers TC. The treatment of acute infectious hepatitis. Am. J. Med. 1963; 35: 299–309. [DOI] [PubMed] [Google Scholar]
- 36. Repsher LH, Freebern RK. Effects of early and vigorous exercise on recovery from infectious hepatitis. N. Engl. J. Med. 1969; 281: 1393–6. [DOI] [PubMed] [Google Scholar]
- 37. Edlund Å. The effect of defined physical exercise in the early convalescence of viral hepatitis. Scand. J. Infect. Dis. 1971; 3: 189–96. [DOI] [PubMed] [Google Scholar]
- 38. Hedin G, Friman G. Orthostatic reactions and blood volumes after moderate physical activation during acute febrile infections. Int. Rehab. Med. 1982; 4: 107–9. [DOI] [PubMed] [Google Scholar]
- 39. Cannon JG, Kluger MJ. Exercise enhances survival rate in mice infected with Salmonella typhimurium (41830). Proc. Soc. Exp. Biol. Med. 1984; 175: 518–21. [DOI] [PubMed] [Google Scholar]
- 40. Ilbäck N‐G, Friman G, Crawford DJ, Neufeld HA. Effects of training on metabolic responses and performance capacity in Streptococcus pneumoniae infected rats. Med. Sci. Sports Exerc. 1991; 23: 422–7. [PubMed] [Google Scholar]
- 41. Heir T, Aanestad G, Carlsen KH, Larsen S. Respiratory tract infection and bronchial responsiveness in elite athletes and sedentary control subjects. Scand. J. Med. Sci. Sports 1995; 5: 94–9. [DOI] [PubMed] [Google Scholar]
- 42. Raju N. Effect of exercise during rehabilitation on swimming performance, metabolism and function of muscle in rats. Br. J. Nutr. 1977; 38: 157–65. [DOI] [PubMed] [Google Scholar]
- 43. Adolfsson G, Circulatory and respiratory function in relation to physical activity in female patients before and after cholecystectomy. Acta Chir. Scand. 1969; Suppl. 401. [PubMed] [Google Scholar]
- 44. Gatmaitan BG, Chason JL, Lerner AM. Augmentation of the virulence of murine coxsackievirus B‐3 myocardiopathy by exercise. J. Exp. Med. 1970; 131: 1121–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lerner AM, Wilson FM. Virus myocardiopathy. Progr. Med. Virol. 1973; 15: 63–91. [PubMed] [Google Scholar]
- 46. Ilbäck N‐G, Friman G, Squibb RL, Johnson AJ, Ballentine DA, Beisel WR. The effect of exercise and fasting on the myocardial protein and lipid metabolism in experimental bacterial myocarditis. Acta Pathol. Microbiol. Immunol. Scand. [A] 1984; 92: 195–204. [DOI] [PubMed] [Google Scholar]
- 47. Ilbäck N‐G, Fohlman J, Friman G. Exercise in Coxsackie B3 myocarditis affects heart lymphocyte subpopulations and the inflammatory reaction. Am. Heart J. 1989; 117: 1298–302. [DOI] [PubMed] [Google Scholar]
- 48. Kiel RJ, Smith FE, Chason J, Khatib R, Reyes MP. Coxsackievirus B3 myocarditis in C3H/HeJ mice: Description of an inbred virulence. Eur. J. Epidemiol. 1989; 3: 348–50. [DOI] [PubMed] [Google Scholar]
- 49. Karjalainen J, Heikkilä J, Nieminen M et al Etiology of mild acute infectious myocarditis. Relation to clinical features. Acta Med. Scand. 1983; 213: 65–73. [DOI] [PubMed] [Google Scholar]
- 50. Gravanis MG, Sternby NH. Incidence of myocarditis. Arch. Pathol. Lab. Med. 1991; 15: 390–2. [PubMed] [Google Scholar]
- 51. Woodruff JF. Viral myocarditis. Am. J. Pathol. 1980; 101: 424–79. [PMC free article] [PubMed] [Google Scholar]
- 52. Vikerfors T, Stjerna A, Olcén P, Malmcrona R, Magnius L. Acute myocarditis. Serologic diagnosis, clinical findings and follow‐up. Acta Med. Scand. 1988; 223: 45–52. [PubMed] [Google Scholar]
- 53. Karjalainen J. Clinical diagnosis of myocarditis and dilated cardiomyopathy. Scand. J. Infect. Dis. 1993; 25 (Suppl. 88): 33–43. [PubMed] [Google Scholar]
- 54. Friman G, Wesslén L, Karjalainen J, Rolf C. Infectious and lymphocytic myocarditis: Epidemiology and factors relevant to sports medicine. Scand. J. Med. Sci. Sports 1995; 5: 269–78. [DOI] [PubMed] [Google Scholar]
- 55. Bergström K, Erikson U, Nordbring F, Nordgren B, Parrow A. Acute non‐rheumatic myopericarditis: A follow‐up study. Scand. J. Infect. Dis. 1970; 2: 7–16. [DOI] [PubMed] [Google Scholar]
- 56. Giesecke J. The long‐term prognosis in acute myocarditis. Eur. Heart J. 1987; Suppl. J: 251–3. [Google Scholar]
- 57. Remes J, Helin M, Vaino P, Rautio P. Clinical outcome and left ventricular function 23 years after acute Coxsackie virus myopericarditis. Eur. Heart J. 1990; 11: 182–8. [DOI] [PubMed] [Google Scholar]
- 58. Richardson P, McKenna W, Bristow M. Report of the 1995 World Health Organization/International Society and Federation of Cardiology task force on the definition and classification of cardiomyopathies. Circulation 1996; 93: 841–2. [DOI] [PubMed] [Google Scholar]
- 59. Brodison A, Swann JW. Myocarditis: A review. J. Infect. 1998; 37: 99–103. [DOI] [PubMed] [Google Scholar]
- 60. Karjalainen J, Heikkilä J. Incidence of three presentations of acute myocarditis in young men in military service: A 20‐year experience. Eur. Heart J. 1999; 20: 1120–5. [DOI] [PubMed] [Google Scholar]
- 61. Frisk G, Torfason EG, Diderholm H. Reverse radioimmunoassays of IgM and IgG antibodies to Coxsackie B viruses in patients with acute myopericarditis. J. Med. Virol. 1984; 14: 191–200. [DOI] [PubMed] [Google Scholar]
- 62. Friman G, Wesslén L, Fohlman J, Karjalainen J, Rolf C. The epidemiology of infectious myocarditis, lymphocytic myocarditis and dilated cardiomyopathy. Eur. Heart J. 1995; 16 (Suppl. O): 36–41. [DOI] [PubMed] [Google Scholar]
- 63. Karjalainen J. Streptococcal tonsillitis and acute nonrheumatic myopericarditis. Chest 1989; 95: 359–63. [DOI] [PubMed] [Google Scholar]
- 64. Karjalainen J. A loud third heart sound and asymptomatic myocarditis during Mycoplasma pneumoniae infection. Eur. Heart J. 1990; 11: 960–3. [DOI] [PubMed] [Google Scholar]
- 65. Frydén A, Kihlström E, Maller R, Persson K, Romanus V, Ånséhn S. A clinical and epidemiological study of ‘Ornithosis’ caused by Chlamydia psittaci and Chlamydia pneumoniae (strain TWAR). Scand. J. Infect. Dis. 1989; 21: 681–91. [DOI] [PubMed] [Google Scholar]
- 66. Odeh M, Oliven A. Chlamydial infections of the heart. Eur. J. Clin. Microbiol. Infect. Dis. 1992; 11: 885–93. [DOI] [PubMed] [Google Scholar]
- 67. Rossi L. Structural and non‐structural disease underlying high‐risk cardiac arrhythmias relevant to sports medicine. J. Sports Med. Phys. Fitness 1995; 35: 79–86. [PubMed] [Google Scholar]
- 68. Virmani R, Rabinowitz M, Smialek JE, Smyth DF. Cardiovascular effects of cocaine: An autopsy study of 40 patients. Am. Heart J. 1988; 155: 1068–76. [DOI] [PubMed] [Google Scholar]
- 69. Nademanee K. Cardiovascular effects and toxicities of cocaine. J. Addict. Dis. 1992; 11: 71–82. [DOI] [PubMed] [Google Scholar]
- 70. Das G. Review: Cardiovascular effects of cocaine abuse. Int. J. Clin. Pharmacol. Ther. Toxicol. 1993; 31: 521–8. [PubMed] [Google Scholar]
- 71. Beisel KW, Srinivasappa J, Prabhakar BS. Molecular cloning of a heart antigen that cross‐reacts with a neutralizing antibody to coxsackievirus B4. Eur. Heart J. 1991; 12 (Suppl. D): 60–4. [DOI] [PubMed] [Google Scholar]
- 72. Huber SA, Gauntt CJ, Sakkinen P. Enteroviruses and myocarditis: Viral pathogenesis through replication, cytokine induction, and immunopathogenicity. Adv. Virus Res. 1998; 51: 35–80. [DOI] [PubMed] [Google Scholar]
- 73. Rose N. Viral damage or ‘molecular mimicry’ – Placing the blame in myocarditis. Nat. Med. 2000; 6: 631–2. [DOI] [PubMed] [Google Scholar]
- 74. McCaffrey FM, Braconier DS, Strong WB. Sudden cardiac death in young athletes. Am. J. Dis. Child. 1991; 145: 177–83. [DOI] [PubMed] [Google Scholar]
- 75. Maron B, Shirani J, Poliac L, Mathenge R, Roberts W, Mueller F. Sudden death in young competitive athletes. JAMA 1996; 276: 199–204. [PubMed] [Google Scholar]
- 76. Phillips M, Robinowitz M, Higgins JR, Boran KJ, Reed T, Virmani R. Sudden cardiac death in air force recruits. JAMA 1986; 256: 2696–9. [PubMed] [Google Scholar]
- 77. Wesslén L, Påhlson C, Lindquist O et al An increase in sudden unexpected cardiac deaths among young Swedish orienteers during 1979–92. Eur. Heart J. 1996; 17: 902–10. [DOI] [PubMed] [Google Scholar]
- 78. Parrish R, Tucker M, Ing R, Encarnacion C, Eberhardt M. Sudden unexplained death syndrome in Southeast Asian refugees: A review of CDC surveillance. Mor. Mortal. Wkly Rep. CDC Surveill. Summ. 1987; 36: 43SS–53SS. [PubMed] [Google Scholar]
- 79. Larsson E, Wesslén L, Lindquist O et al Sudden unexpected cardiac deaths among young Swedish orienteers – Morphological changes in hearts and other organs. APMIS 1999; 107: 325–36. [DOI] [PubMed] [Google Scholar]
- 80. Roberts JA. Viral illnesses and sports performance. Sports Med. 1986; 3: 296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Levander OA, Beck MA. Interacting nutritional and infectious etiologies of Keshan Disease. Insights from coxsackie virus B‐induced myocarditis in mice deficient in selenium or vitamin E. Biol. Trace Elem. Res. 1997; 56: 5–21. [DOI] [PubMed] [Google Scholar]
- 82. Ilbäck N‐G, Fohlman J, Friman G. Protective effect of selenium on the development of Coxsackie virus B3 induced inflammatory lesions in the murine myocardium. J. Trace Elem. Exp. Med. 1989; 2: 257–66. [Google Scholar]
- 83. Ilbäck N‐G, Fohlman J, Friman G. Effects of selenium supplementation on virus‐induced inflammatory heart lesions. Biol. Trace Elem. Res. 1998; 63: 51–66. [DOI] [PubMed] [Google Scholar]
- 84. Beck MA, Shi Q, Morris CV, Levander OA. Rapid genomic evolution of a non‐virulent Coxsackievirus B3 in selenium‐ deficient mice results in selection of identical virulent isolates. Nat. Med. 1995; 5: 433–6. [DOI] [PubMed] [Google Scholar]
- 85. Levander OA, Ager AL, Beck MA. Vitamin E and selenium: Contrasting and interacting nutritional determinants of host resistance to parasitic and viral infections. Proc. Nutr. Soc. 1995; 54: 475–87. [DOI] [PubMed] [Google Scholar]
- 86. Ilbäck N‐G, Lindh U, Fohlman J, Friman G. New aspects of murine coxsackie B3 myocarditis – Focus on heavy metals. Eur. Heart J. 1995; 16: (Suppl. O): 20–4. [DOI] [PubMed] [Google Scholar]
- 87. Funseth E, Wicklund‐Glynn A, Friman G, Ilbäck N‐G. Redistribution of accumulated 2,3,7,8‐tetrachloro‐dibenzo‐p‐dioxin during coxsackievirus B3 infection in the mouse. Toxicol. Lett. 2000; (in press). [DOI] [PubMed] [Google Scholar]
- 88. Gainer JH. Effects of heavy metals and of deficiency of zinc on mortality rates in mice infected with encephalomyocarditis virus. Am. J. Vet. Res. 1977; 38: 869–72. [PubMed] [Google Scholar]
- 89. Shephard RJ. Exercise, immune function and HIV infection. J. Sports Med. Phys. Fitness 1998; 38: 101–10. [PubMed] [Google Scholar]
- 90. Eichner ER. Infection, immunity, and exercise. Phys. Sportsmed. 1993; 21: 125–35. [DOI] [PubMed] [Google Scholar]
- 91. Roubenoff R, McDermott A, Weiss L et al Short‐term progressive resistance training increases strength and lean body mass in adults infected with human immunodeficiency virus. AIDS 1999; 13: 231–9. [DOI] [PubMed] [Google Scholar]
- 92. Mustafa T, Sy FS, Macera CA et al Association between exercise and HIV disease progression in a cohort of homosexual men. Ann. Epidemiol. 1999; 9: 127–31. [DOI] [PubMed] [Google Scholar]
- 93. Pedersen BK, Exercise in Patients with HIV Infection. In: Pedersen BK.). Exercise Immunology. New York: Springer, 1997; 123–32. [Google Scholar]
- 94. Reilly JM, Cunnion RE, Anderson DW et al Frequency of myocarditis, left ventricular dysfunction and ventricular tachycardia in the acquired immune deficiency syndrome. Am. J. Cardiol. 1988; 62: 789–93. [DOI] [PubMed] [Google Scholar]
- 95. Anderson DW, Virmani R, Reilly JM et al Prevalent myocarditis at necropsy in the acquired immunodeficiency syndrome. J. Am. Coll. Cardiol. 1988; 11: 792–9. [DOI] [PubMed] [Google Scholar]
- 96. Barbaro G, Di Lorenzo G, Grisorio G, Barbarini G. Incidence of dilated cardiomyopathy and detection of HIV in myocardial cells of HIV‐positive patients. N. Engl. J. Med. 1998; 339: 1093–9. [DOI] [PubMed] [Google Scholar]
- 97. Ringertz O. Serum hepatitis in Swedish track‐finders. Scand. J. Infect. Dis. 1971; 2 (Suppl. 2): 3–25. [DOI] [PubMed] [Google Scholar]
- 98. Dyke LM, Merikangas UR, Bruton OC, Trask SG, Hetrick FM. Skin infection in wrestlers due to herpes simplex virus. JAMA 1965; 194: 1001–2. [PubMed] [Google Scholar]
- 99. Skinner GR, Davies J, Ahmad A, McLeish P, Buchan A. An outbreak of herpes rugbiorum managed by vaccination of players and sociosexual contacts. J. Infect. 1996; 33: 163–7. [DOI] [PubMed] [Google Scholar]
- 100. Anderson BJ. The effectiveness of valacyclovir in preventing reactivation of herpes gladiatorum in wrestlers. Clin. J. Sport Med. 1999; 9: 86–90. [DOI] [PubMed] [Google Scholar]
- 101. Simon HB. Exercise and infection. Phys. Sportsmed. 1987; 15: 135–41. [Google Scholar]
- 102. Brenner IKM, Shek PN, Shephard RJ. Infection in athletes. Sports Med. 1994; 17: 86–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Nieman DC. Exercise immunology: Practical applications. Int. J. Sports Med. 1997; 18 (Suppl. 1): 91–100. [DOI] [PubMed] [Google Scholar]
- 104. Karjalainen J, Heikkila J. ‘Acute pericarditis’: Myocardial enzyme release as evidence for myocarditis. Am. Heart J. 1986; 111: 546–52. [DOI] [PubMed] [Google Scholar]
- 105. Outbreak of acute febrile illness among athletes participating in triathlons – Wisconsin and Illinois, 1998 [published erratum appears in MMWR Morb. Mortal. Wkly Rep. 1998; 47: 619]. MMWR Morb. Mortal. Wkly Rep. 1998; 47: 585–8. [PubMed] [Google Scholar]
- 106. Update: Leptospirosis and unexplained acute febrile illness among athletes participating in triathlons – Illinois and Wisconsin, 1998. MMWR Morb. Mortal. Wkly Rep. 1998; 47: 673–6. [PubMed] [Google Scholar]
- 107. Shaw RD. Kayaking as a risk factor for leptospirosis. Mo. Med. 1992; 89: 354–7. [PubMed] [Google Scholar]
- 108. van Asperen IA, Medema G, Borgdorff MW, Sprenger MJ, Havelaar AH. Risk of gastroenteritis among triathletes in relation to faecal pollution of fresh waters. Int. J. Epidemiol. 1998; 27: 309–15. [DOI] [PubMed] [Google Scholar]
- 109. Taylor MB, Becker PJ, Van Rensburg EJ, Harris BN, Bailey IW, Grabow WO. A serosurvey of water‐borne pathogens amongst canoeists in South Africa. Epidemiol. Infect. 1995; 115: 299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Jeans AK, Schwellnus MP. The risk of schistosomiasis in Zimbabwean triathletes. S. Afr. Med. J. 1994; 84: 756–8. [PubMed] [Google Scholar]
- 111. Kashiwagi S, Hayashi J, Ikematsu H, Nishigori S, Ishihara K, Kaji M. An outbreak of hepatitis B in members of a high school sumo wrestling club. JAMA 1982; 248: 213–14. [PubMed] [Google Scholar]
- 112. Holland EJ, Mahanti RL, Belongia EA et al Ocular involvement in an outbreak of herpes gladiatorum. Am. J. Ophthalmol. 1992; 114: 680–4. [DOI] [PubMed] [Google Scholar]
- 113. Beller M, Gessner BD. An outbreak of tinea corporis gladiatorum on a high school wrestling team. J. Am. Acad. Dermatol. 1994; 31: (2 Part 1): 197–201. [DOI] [PubMed] [Google Scholar]
- 114. Piqu E, Copado R, Cabrera A et al An outbreak of tinea gladiatorum in Lanzarote. Clin. Exp. Dermatol. 1999; 24: 7–9. [PubMed] [Google Scholar]
- 115. Kohl TD, Martin DC, Berger MS. Comparison of topical and oral treatments for tinea gladiatorum. Clin. J. Sport Med. 1999; 9: 161–6. [DOI] [PubMed] [Google Scholar]
- 116. Measles at an international gymnastics competition – Indiana, 1991. MMWR Morb. Mortal. Wkly Rep. 1992; 41: 109–11. [PubMed] [Google Scholar]
- 117. Ehresmann KR, Hedberg CW, Grimm MB, Norton CA, MacDonald KL, Osterholm MT. An outbreak of measles at an international sporting event with airborne transmission in a domed stadium. J. Infect. Dis. 1995; 171: 679–83. [DOI] [PubMed] [Google Scholar]
- 118. Koh YM, Barnes GH, Kaczmarski E, Stuart JM. Outbreak of meningococcal disease linked to a sports club. Lancet 1998; 352: 706–7. [DOI] [PubMed] [Google Scholar]
- 119. Aseptic meningitis in a high school football team – Ohio. MMWR Morb. Mortal. Wkly Rep. 1981; 29: 631–7. [PubMed] [Google Scholar]
- 120. Baron RC, Hatch MH, Kleeman K, MacCormack JN. Aseptic meningitis among members of a high school football team. An outbreak associated with echovirus 16 infection. JAMA 1982; 248: 1724–7. [PubMed] [Google Scholar]
- 121. Moore M, Baron RC, Filstein MR et al Aseptic meningitis and high school football players. 1978 and 1980. JAMA 1983; 249: 2039–42. [PubMed] [Google Scholar]
- 122. Fournier PE, Roux V, Caumes E, Donzel M, Raoult D. Outbreak of Rickettsia africae infections in participants of an adventure race in South Africa. Clin. Infect. Dis. 1998; 27: 316–23. [DOI] [PubMed] [Google Scholar]
- 123. Fahrer H, Sauvain MJ, v.d. Linden S, Zhioua E, Gern L, Aeschlimann A. [Prevalence of Lyme borreliosis in a Swiss population at risk]. Schweiz. Med. Wochenschr. 1988; 118: 65–9. [PubMed] [Google Scholar]
- 124. Gustafson R, Forsgren M, Gardulf A, Granstrom M, Svenungsson B. Antibody prevalence and clinical manifestations of Lyme borreliosis and tick‐borne encephalitis in Swedish orienteers. Scand. J. Infect. Dis. 1993; 25: 605–11. [DOI] [PubMed] [Google Scholar]
- 125. Stalder H, Isler R, Stutz W, Salfinger M, Lauwers S, Vischer W. [Contribution to the epidemiology of Campylobacter jejuni. From asymptomatic excretion by a cow in the cowshed to overt disease in over 500 persons]. Schweiz. Med. Wochenschr. 1983; 113: 245–9. [PubMed] [Google Scholar]
- 126. Thaikruea L, Pataraarechachai J, Savanpunyalert P, Naluponjiragul U. An unusual outbreak of food poisoning. Southeast Asian J. Trop. Med. Public Health 1995; 26: 78–85. [PubMed] [Google Scholar]
- 127. Anderson AC. Outbreak of Salmonella food poisoning at Junior World Rowing Championships. Br. J. Sports Med. 1996; 30: 347–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Panella H, Plasencia A, Sanz M, Cayla JA. [An evaluation of the epidemiological surveillance system for infectious diseases in the Barcelona Olympic Games of 1992]. Gac. Sanit. 1995; 9: 84–90. [DOI] [PubMed] [Google Scholar]
- 129. Byrd OE. Athlete's foot. West Med. Med. J. West 1966; 7: 142–4. [PubMed] [Google Scholar]
- 130. Kamihama T, Kimura T, Hosokawa JI, Ueji M, Takase T, Tagami K. Tinea pedis outbreak in swimming pools in Japan. Public Health 1997; 111: 249–53. [DOI] [PubMed] [Google Scholar]
- 131. Rich JD, Dickinson BP, Feller A, Pugatch D, Mylonakis E. The infectious complications of anabolic‐androgenic steroid injection. Int. J. Sports Med. 1999; 20: 563–6. [DOI] [PubMed] [Google Scholar]
- 132. Parana R, Lyra L, Trepo C. Intravenous vitamin complexes used in sporting activities and transmission of HCV in Brazil. Am. J. Gastroenterol. 1999; 94: 857–8. [DOI] [PubMed] [Google Scholar]
- 133. Rich JD, Dickinson BP, Flanigan TP, Valone SE. Abscess related to anabolic‐androgenic steroid injection. Med. Sci. Sports Exerc. 1999; 31: 207–9. [DOI] [PubMed] [Google Scholar]


