Abstract
The flushing of toilets generates contaminated aerosols, the transmission of which may cause the spread of disease, particularly in the immunocompromised or the elderly. This study investigated the emission strength of three types of airborne bacteria, namely Staphylococcus epidermidis, Escherichia coli, and Pseudomonas alcaligenes, during toilet flushing in a custom‐built toilet under a controlled environment. Flushing was activated by a flushometer operated at two pressure levels, 400 kPa (high pressure [HP]) and 200 kPa (low pressure [LP]), and by a water cistern tank placed 95 cm (high tank [HT]) and 46 cm (low tank [LT]) above the toilet seat. The pathogens emitted by the first flush were calculated, with the correlations between airborne pathogen emissions and droplet concentration (HP, r=0.944, P<.001; LP, r=0.803, P<.001, HT, r=0.885, P<.05) and bacterial size (HP, r=−0.919, P<.001; LP, r=−0.936, P<.001; HT, r=−0.967, P<.05) in the different conditions then tested. The emission strength in the HP condition was statistically greater than that in the LP condition, whereas the cistern tank system produced less emissions than the flushometer system, and tank height was not found to be a sensitive parameter.
Keywords: airborne transmitted pathogens, emission strength, infectious diseases, size‐resolved emissions, toilet flushing‐generated water droplets, toilet hygiene
Practical Implications.
Emission strength is probably the single most important parameter for estimating the bacterial exposure risk from toilet flushing. This study found emission strength and bacterial size to be negatively correlated, which is valuable information for estimating the risks associated with small viruses or large bacteria. It also documented a correlation between droplet concentration and emission strength. Finally, it found a cistern tank design to be the preferred toilet flushing option because of its minimal generation of potentially infectious small aerosols.
1. INTRODUCTION
The human‐to‐human transmission of airborne pathogens, such as tuberculosis, smallpox, severe acute respiratory syndrome coronavirus (SARS‐CoV), Middle East respiratory syndrome coronavirus, avian influenza, and Ebola,1, 2 presents a challenge to the prevention, management, and containment of emerging infectious disease outbreaks and infection control worldwide. A number of studies have investigated human‐to‐human transmission via expiratory droplets inside built environments with a view to minimizing it, but there are also other airborne pathways that can transmit pathogens. When external energy is used to disintegrate liquid into airborne droplets, the process is referred to as atomization. The splashing of water droplets during the flushing process is commonly observed when toilets are poorly designed or the flushing pressure is too high. In addition to the relatively large droplets that are visible (~ millimeters in size), the toilet flushing process is also known to atomize numerous smaller airborne droplets. It was demonstrated as long ago as the 1960s that droplets with airborne pathogens can be generated during the toilet flushing process, thereby creating infectious hazards.3 More recent studies have revealed a very high viral load, up to 1011 viral particles per gram of fecal matter.4 If feces containing pathogenic organisms are shed by an infected person and contaminate toilet fluid, the atomized droplets produced may well be infectious.5, 6, 7 The results of previous studies have raised concern over the aerosolization of excreta containing such intestinal or urinary pathogenic microorganisms as Aeromonas sp., Bacillus sp., Campylobacter sp., Clostridium sp., Escherichia sp., Klebsiella sp., Pseudomonas sp., Salmonella sp., Serratia sp., Shigella sp., and Staphylococcus sp. presenting either as colonization or infection.8 Among all pathogens concerned, noroviruses have received the greatest attention, particularly in elderly and healthcare settings.9 In addition, pathogens other than the typical intestinal pathogens, including the deadly SARS‐CoV and Ebola virus, have also been detected in excreta, thus further raising concerns about the potentially infectious pathogen‐laden aerosols generated by toilet flushing.
When the fecal shedding of pathogenic organisms takes place in the toilet bowl of a public washroom, flushing the toilet may expose subsequent washroom users through primary exposure to contaminated aerosols via direct inhalation, and/or secondary exposure may occur via contact with contaminated surfaces.10 Large droplets settle within 1‐2 m,11 and are highly likely to contaminate such environmental surfaces as door handles, banisters, and flush handles.12 Small droplets generally refer to droplet nuclei with a diameter in the range of less than 5 μm. As they settle slowly in the air, they can travel farther and for a much longer duration.13 It has been reported that contaminated droplet nuclei can be dispersed to other areas of a washroom,14 and they have even been found outside a mock‐up toilet cubicle.3
Barker and Jones6 conducted a toilet seeding experiment in which they measured the level of bioaerosols generated by the flushing of a residential toilet. They reported the amount of Serratia marcescens in the air to increase sharply from zero colony‐forming units per cubic meter (CFU/m3) to 1370 CFU/m3 after the first flush. However, a later study15 recorded a much smaller number of size‐resolved flushing‐generated airborne droplets than that observed by Barker and Jones.5 A similar study was recently conducted in a hospital to determine the concentration of Clostridium difficile following flushing. It found that even with the toilet lid down, aerosolized droplets could be recovered 10 cm above the seat within 60 minutes of flushing.16
Quantification of the potential transmission risk of pathogen‐laden aerosols is important. Emission strength is the most important of all known parameters for such quantification,17 but has rarely been investigated. Quantifying the risk of potential transmission based on indirect evidence without carrying out controlled experiments is unreliable. It is believed that flushing systems (and related parameters) exert the strongest effect on airborne pathogen emission. A significant association between bacterial emission strength at different degrees of flushing energy and flushing systems was reported in a recent study focusing on the initial droplet size distribution generated by flushing an experimental toilet system with various flushing mechanisms.14 However, submicrometer monodisperse spheres were used as surrogates for microorganisms.
The flushing of a toilet bowl can be achieved either by supplying water pressure through a flushometer or by gravity through a cistern system. Flushometer‐valve toilets are typically used in commercial and office buildings because the recharge/refilling time is very short, meaning the toilet is available for the next user almost immediately. Cistern systems (or simple tank systems), in contrast, take a few minutes to recharge after each flush, causing inconvenience in high‐frequency settings such as shopping arcades or other public buildings. However, cistern systems remain popular in residential and educational buildings, partly because their design and maintenance are simpler and less costly than those for flushometer‐based systems.
The aim of this study was to investigate the emission strength of the first flush for the two flushing systems. Parameters such as water pressure, tank height, and bacterial size were varied, and the correlations between airborne droplet concentration and bacterial emission strength were investigated.
2. MATERIALS AND METHODS
2.1. Experimental setup
The test‐rig comprised a toilet bowl incorporating a flushometer, a cistern tank, a storage tank, a booster pump, a pneumatic pressure vessel, and tailor‐made pipework (Figure S1). A ceramic wash‐down‐type toilet bowl was installed (C19P, Econax) because it is the most common toilet bowl type used in Hong Kong households. All public housing in Hong Kong, which accommodates ~30% of the approximately 7.3 million population, is equipped with such toilets. The flushometer was designed to supply 14 L per flush (Naval Exposed Water Closet, Sloan, USA). Because no direct pressurized city water was available in the laboratory, the water supply was specially arranged and designed with a closed‐loop system. The outlet of the bowl was connected to a stainless steel water tank (with a capacity of 50 L), and a booster pump with a pneumatic pressure vessel was installed to deliver water from the storage tank to the flushometer, thereby forming a closed‐loop circuit in which water discharged from the water closet was recirculated.
The cistern tank system was simpler in design. A standard cistern tank was purchased from a local plumbing store and filled with 14 L of tap water for each flush. A tailor‐made tracking system was fabricated to allow the cistern height to be adjusted with a handle. The two flushing systems could be rendered interchangeable in less than a minute via a few simple valve‐changing steps.
The temporal profiles of the different‐sized droplets generated during toilet flushing were recorded using an optical particle counter (3330, MN, TSI) with the sampling rate set to 1 seconds. The counter allowed six user‐defined bins to be selected from sizes ranging from 0.3 to 10 μm: (i) 0.3‐0.6 μm, (ii) 0.6‐1 μm, (iii) 1‐1.5 μm, (iv) 1.5‐2 μm, (v) 2‐6 μm, and (vi) 6‐10 μm. The rationale for these sizes is discussed in the Results section 3.3. Airborne bacteria were collected by a single‐stage viable Andersen cascade sampler (Thermo Fisher Scientific Inc.) operating at 28.3 L/min.
To enhance the accuracy of the bacterial counts, instead of random sampling inside the entire environmental chamber, a small transparent acrylic box of 45×45×45 cm (L×W×H) in size was placed upright on the toilet bowl seat surface, with a 33‐cm‐diameter circular opening in one side facing the toilet bowl (Figure 1). To ensure that the air was well‐mixed inside the box, and to avoid unnecessarily vigorous mixing to prevent high‐turbulence deposition loss, preliminary trial tests were run to determine whether any mixing fans were required. Four small computer muffin fans were hung in each upper corner to provoke mixing. As the results indicated no significant differences in the air samples, no mixing fans were used subsequently. Also, the electrostatic and diffusion losses were found to be negligible, with the smallest droplet size concerned being just 0.3 μm.
Figure 1.
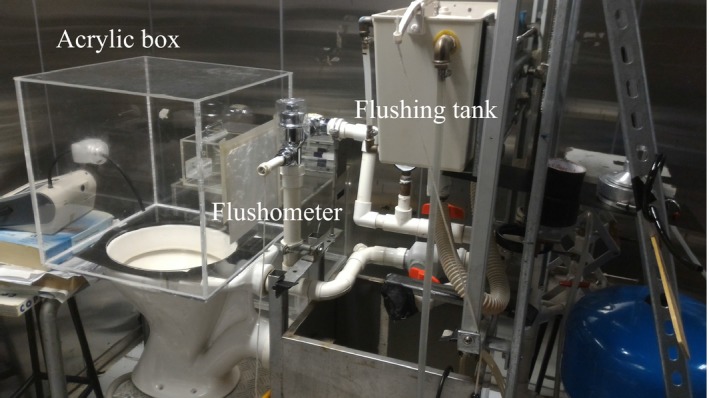
Photograph showing the setup of the toilet and transparent acrylic box measuring 45×45×45 cm (L×H×W)
To balance the pressure differential created by the sampling pump, another circular opening of 15 cm in diameter was made in one of the vertical walls. Electrostatic filter paper was fixed at the opening to allow filtered air to flow through without affecting the bacterial count. Another small opening of 3.5 cm in diameter was made to allow particle counter measurements through the conductive sampling tube. Finally, a standalone split‐type air‐conditioning system operated in the chamber throughout the experiments, and thus, the temperature and relative humidity were maintained at 22±0.5°C and 55±5%, respectively.
2.2. Parameters
Such parameters as the static pressure of the water supply and cistern height were selected on the basis of practical application. By adjusting the water pressure switch, two levels of water supply pressure, high pressure (HP, 400 kPa) and low pressure (LP, 200 kPa), were achieved, and the pressurized water was fed to the flushometer. This range is the conventional water pressure range for flushometer systems.14 Two cistern heights, as measured from the surface of the toilet seat to the bottom of the tank, were selected: high tank (HT, 95 cm) and low tank (LT, 46 cm). The HT system represented the systems used in old residential buildings, whereas the LT system represented that used in newer residential and commercial buildings.
The quantity of water used during flushing was also measured. For the cistern system, such measurement was a straightforward matter of measuring the drop in water level before and after flushing, which was estimated to be 10 L. For the flushometer system, the water used per flush was estimated to be 14 L.
2.3. Selection of microorganisms
Similar to a previous study,18 the selection criteria for the microorganisms were based on biosafety issues and physical characteristics rather than pathogenic properties. Three biosafety levels for bacteria of different sizes and shapes were selected as surrogates for the pathogenic species commonly found in bioaerosols. Further details can be found in the Supporting Information (Table S1).
Staphylococcus epidermidis (ATCC 12228) are gram‐positive cocci with a spherical shape and diameter around 0.96 μm that are arranged in clusters. E. coli (ATCC 10536) and P. alcaligenes (ATCC 14909) are rod‐shaped, gram‐negative bacilli. Of the three, S. epidermidis is the smallest in size, and P. alcaligenes (ATCC 14909) is the largest, with approximately 2 μm per axis.
2.4. Procedure for measuring bacterial emission strength
Bacterial emission strength was measured in the order of S. epidermidis, E. coli, and P. alcaligenes. The first two types of bacteria were tested in all four scenarios (HP, LP, HT, and LT), whereas P. alcaligenes was tested in the HP and LP conditions alone. Each type of bacteria was inoculated onto a nutrient agar (NA) plate from frozen stock. After incubation at 30 or 37°C for 24 to 72 hour, a colony from the NA plate was inoculated into a 200 mL nutrient broth (BD), and incubated in an orbital shaker at 30 or 37°C for 24 to 48 hour to reach a stationary phase. The suspensions were used immediately for the emission strength experiments. The input of each bacterial suspension was 109 to 1010 cells, and the suspension volumes used are shown in Table S2 in the Supporting Information.
For the background experiment, the acrylic box was placed upright on the toilet seat, and flushing was activated. The Andersen sampler was loaded with a 90‐mm Petri dish containing NA and operated for 5 minutes. The dish was incubated at 30 or 37°C for 24 to 72 hour. The CFUs obtained were counted manually after cultivation.
For the bacterial emission experiments, each bacterial suspension was splashed onto the porcelain surface of the toilet bowl using a syringe. The sampling procedure was the same as that for the background experiment. At least three independent experiments were conducted for each flushing scenario.
To overcome the main drawback of the closed‐loop system, that is, that after flushing a certain amount of bacteria can flow back into the water tank, the entire system was thoroughly disinfected between experiments by pouring 100 mL of household bleach (5.25% sodium hypochlorite by mass) into the toilet bowl, followed by 12 mL of 5% sodium thiosulfate to neutralize the bleach residue, and then flushing with plenty of fresh water. Water samples collected after disinfection were found to have no bacterial cross‐contamination.
2.5. Measurement of airborne water droplet concentrations
An approximately 10‐cm conductive sampling tube was connected from the sampling inlet of the counter to the center of the acrylic box. Preliminary observation indicated that 15 minutes was sufficient for the particles to decay back to their background levels (Figures 2 and S2). For the first measurement, samples were collected for 5 mins to determine the background level before flushing, and the second flushing was activated 15 minutes after the first. As noted, measurements were repeated at least four times for each scenario. Although the particle counter was not calibrated for each specific aerosol examined, and hence, the readings are not the actual gravimetric values, it is believed that such a limitation did not affect the study's results because they were normalized by the results collected by the same counter.
Figure 2.
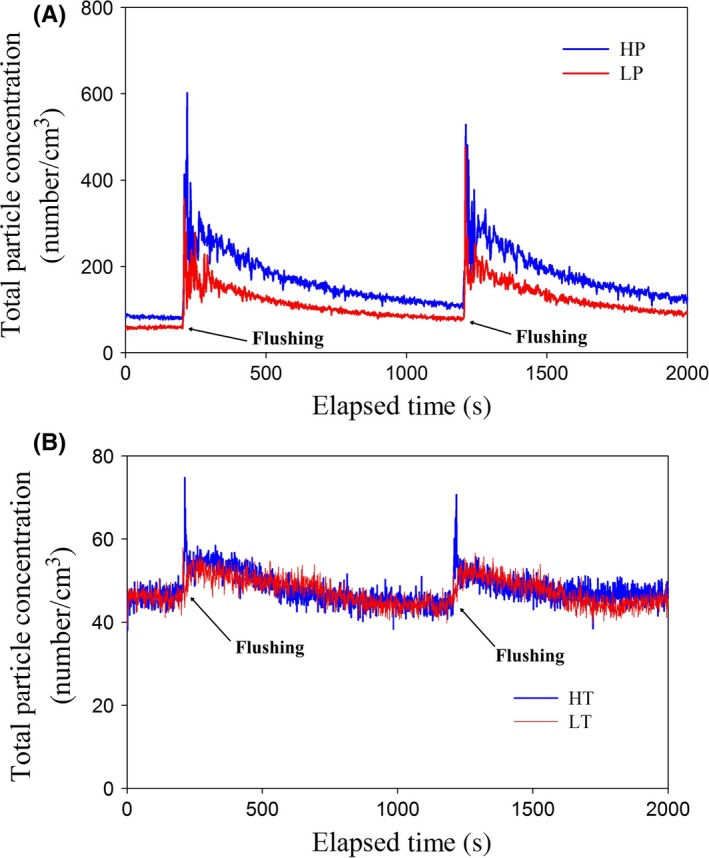
Temporal profile of total particle (0.3‐10 μm) emission strength upon toilet flushing in the (A) high‐pressure (HP, in blue) and low‐pressure (LP, in red) and (B) high‐tank (HT, in blue) and low‐tank (LT, in red) conditions
2.6. Statistical analysis
Comparisons of the emission strengths of the different types of bacteria and the different scenarios were computed by the Mann‐Whitney U test and Kruskal‐Wallis test, respectively. Pearson correlation coefficients were used to test the strength of the association between two parameters. The IBM statistical package SPSS (version 16.0) was used for all of the statistical computations.
3. RESULTS
3.1. Aerosol droplet concentrations and emission strength
Before detailed analysis of the size‐resolved concentration profiles, preliminary results on the total emissions generated by the different flushing systems in the four scenarios were obtained. Figure 2 presents the temporal profile of the total particle emissions of the four scenarios. Two salient points can be observed. First, the flushometer system produced a significantly higher droplet concentration than the tank system regardless of the water pressure or tank height. Second, emission was detected immediately after flushing was activated, and it took less than 15 seconds for the droplet concentration to increase to peak level.
The water pressure for the cistern tank was 4.9 and 9.8 kPa for the LT and HT conditions, respectively, whereas that for the flushometer was 200 and 400 kPa. Hence, the pressure produced by the cistern tank was, at most, 5% (9.8/200) of that produced by the flushometer, which suggests that cistern systems create much less energy for the atomization of droplets, and thus generate significantly fewer droplets. This observation is supported by the results of this study and previous studies.14
Total and size‐resolved droplet emissions per flush were determined by the summation of all temporal particle counts minus the background counts for 15 seconds post‐flush.14 Because the emission time (~15 seconds) was much shorter than the decay time, droplet emissions could be approximated by Equation (1):
| (1) |
where E is droplet emissions per flush; Q is the air sampling flow of the optical particle counter, that is, 1.67×10−5 m3/s; β is the particle loss rate (s−1); and V is the volume of the acrylic box, that is, 0.091 m3. β depends on such factors as particle size, turbulence intensity, and deposition surface,19 whereas Q+βV is estimated from the decay of the droplet profiles, and falls within the range of 0.9×10−5 m3/s to 6×10−5 m3/s. The simple approach of using the average of the range, that is, 3.0×10−5 m3/s, was adopted in calculating Equation (1). The droplet emission strengths per flush are shown in Table 1. It can be seen that the total emission strength in the HP condition was approximately 3.6, 21.0, and 19.8 times greater than that in the LP, HT, and LT conditions, respectively. These results are comparable to those published by Johnston et al.,14 who found the highest number of droplets, that is, 145 000, to be yielded by a flushometer operating at >350 kPa among the four flushing mechanisms they tested. However, that droplet number is only approximately half that found in the present study. The discrepancy may be attributable to their neglect of droplet loss inside the bowl. Their working pressure was also less than 400 kPa, and the different particle counters used in the two studies may also have affected the concentration results. Nonetheless, the two measurements are in fairly good agreement.
Table 1.
Results of total and size‐resolved droplet emissions per flush in different of size ranges and pressure conditions (rounded to the nearest 100 except for the last column in which the numbers are rounded to the nearest 10). The errors are one standard deviation
| Flushing Scenario | Total | Range of droplet sizes | |||
|---|---|---|---|---|---|
| 0.3‐0.6 μm | >0.6‐10 μm | >1.5‐10 μm | >2‐10 μm | ||
| High Pressure | 287 400±32 700 | 245 700±29 400 | 41 700±3300 | 8100±510 | 1980±120 |
| Low Pressure | 80 200±6900 | 61 800±5600 | 18 300±1204 | 6100±257 | 1270±100 |
| High Tank | 13 700±3000 | 8400±2100 | 5300±852 | 800±135 | 290±30 |
| Low Tank | 14 500±2100 | 8600±1300 | 5900±756 | 700±53 | 380±30 |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
3.2. Size‐resolved aerosol concentrations
To obtain more details on the emission characteristics, the temporal profile of the size‐resolved droplet concentrations was investigated, with the results presented in Figure S2 (Supporting Information). In proposing a simple correlation between droplet size and emission strength, it was hypothesized that a droplet of diameter d can embed a pathogen whose size is at most diameter d. In light of this hypothesis, the concentration count from 0.6 to 10 μm was used to correlate the emission strength per flush for S. epidermidis, and those from 1.5 μm‐10 μm and 2 μm‐10 μm for E. coli and P. alcaligenes, respectively, as elaborated upon in the next section. Droplets with the smallest diameter range (0.3‐0.6 μm) were included to highlight the concentrations of different sizes.
The size‐resolved droplet concentrations are also shown in Table 1. The ratio of the average droplet emission strength was calculated. The HP to LP ratios for 0.3‐0.6 μm, 0.6‐10 μm, 1.5‐10 μm, and 2‐10 μm were 3.9, 2.3, 1.3, and 1.6, respectively, which are in line with the ratio of 3.6 for the total concentration. The magnitude of the ratios for the cistern tanks was very different. For droplet diameters in the range of 0.3‐0.6 μm, the HP/HT droplet emission ratio was 29.2 times, whereas that for those ranging from 0.6 to 10 μm, 1.5 to 10 μm, and 2 to 10 μm were 7.8, 10.2, and 6.8, respectively. Hence, compared with the flushometer, the droplet emission concentration produced by the cistern tank was only 3.4% for droplets sized ≤0.6 μm, whereas it ranged from 10% to 14% for droplets ≥1.5 μm. This finding has important implications, showing that an exposure risk still exists even for tank flushing (with a low degree of atomization energy) with a low level of total droplet emissions.
3.3. Pathogen emission strength of first flush
The pathogen emission strength of the first flush in the four scenarios was calculated by Equation (2), with the results shown in Figure 3.
Figure 3.
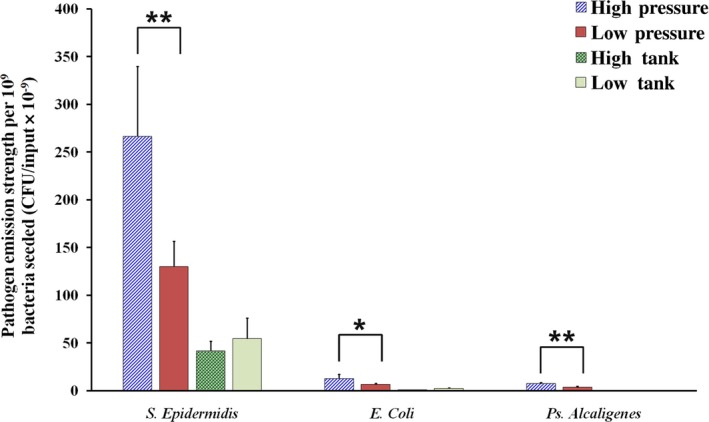
Emission strength of different types of bacteria at the first flush in the high (HP, 400 kPa)‐ and low (LP, 200 kPa)‐pressure conditions and at the two tank heights (high tank=95 cm and low tank=46 cm above the toilet seat). The emission strengths of each bacterial type, as reflected by the number of bacteria (CFU/input×10−9) differed significantly in the two pressure conditions, as indicated by the asterisks (*P<.05, **P<.001; P‐value<.05 denotes statistical significance). The emission strength of S. epidermidis was statistically greater than that of E. coli (HP, P<.005; LP, P<.005) and P. alcaligenes (HP, P<.005; LP, P<.01) in both the HP and LP conditions, whereas no statistical difference in emission strength was found between E. coli and P. alcaligenes in either pressure condition
| (2) |
where CFU is the count in the Petri dish with bacteria added to the toilet bowl, and CFU(BG) is the count without bacteria added. Positive hole correction was applied for all samples.20 Because the order of magnitude of the CFU counts and bacteria was significantly dispersed, the results were normalized by 109.
The emission strength of S. epidermidis was statistically greater than that of E. coli and P. alcaligenes in the HP (P<.05) and LP (P<.05) conditions, respectively. For all three bacteria, the emission strength under HP was statistically greater than that under LP: S. epidermidis (P<.001), E. coli (P<.05), and P. alcaligenes (P<.001). The strengths recorded under HP were almost double those recorded under LP. For E. coli tested in the HT and LT scenarios, the respective CFU results were very low. As similarly low or even lower CFU results were anticipated for P. alcaligenes, only the HP and LP scenarios were tested.
The correlations between the airborne pathogen concentrations and quantity of airborne droplets are reported in Table 2. It can be seen that there was a positive correlation between the droplet and pathogen concentrations in the HP, LP, and HT conditions, whereas a non‐intuitive result was obtained for LT, that is, the emission strength was higher than in the HT condition. The exact reason for this result is unclear, but may be attributable to the very low concentration of aerosols in the HT and LT conditions. The explanation is further clouded by the highly complex water flow (atomization process).
Table 2.
Correlations between emission and droplet concentrations and bacterial size in the high (400 kPa) and low (200 kPa) pressure and high (95 cm) and low tank (46 cm) scenarios
| Net emission concentration: flushometer operated at different pressure levels or in different cistern tanks | Correlation coefficient (r) | P‐valuea | |
|---|---|---|---|
| Droplet concentration | |||
| High pressure | 0.944 | <.001 | |
| Low pressure | 0.803 | <.001 | |
| High tank | 0.885 | <.05 | |
| Low tank | 0.707 | >.05 | |
| Types of bacteria of different bacterial sizes | |||
| High pressure | −0.919 | <.001 | |
| Low pressure | −0.936 | <.001 | |
| High tank | −0.967 | <.05 | |
| Low tank | −0.924 | <.05 |
Denotes statistical significance at P‐value<.05.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
The correlations between emission strength and bacterial size are also shown in Table 2, with a negative such correlation found in both the HP and LP scenarios. S. epidermidis exhibited the greater emission strength, 21 times that of E. coli. Very limited information on emission strength can be found in the literature, and no study to date has investigated its correlation with bacterial size. Gerba et al.7 commented that owing to the small size of viruses, the emission strength of MS2 is greater than that of E. coli.
Our work confirms the correlation between a threshold droplet size and emission strength, thereby also supporting our hypothesis, that is, that a droplet of diameter d can embed a pathogen whose size is at most diameter d. As shown in Table 1, the emission of smaller droplet overwhelms larger droplets. Combining these two observations, it can explain the airborne emission strength of pathogens decreases with the their sizes.
It can be inferred from these results that low flushing pressure is better in practice than high pressure. However, additional factors such as refilling time and cleaning performance also need to be considered, and hence, the best level of in‐use flushing pressure for minimizing the generation of emissions cannot be determined solely on the basis of these results. In practice, an overly low pressure may be insufficient to remove the waste adhering to the bowl surface, which raises issues of hygiene. Nevertheless, drainage system designers would be advised to take our results into consideration in conjunction with the need to provide satisfactory drainage performance, particularly in facilities for the elderly and other special groups and in hospitals and clinical settings, to reduce the risk of transmitting potentially infectious contaminated aerosols to high‐risk persons.
4. DISCUSSION
The World Health Organization21 reported that during the SARS outbreak inadequate plumbing systems allowed infectious SARS droplets to enter buildings via sewage and drainage systems, blaming in particular strong upwards airflows, inadequate traps, and non‐functional water seals. It has thus been recommended that drainage systems be designed to prevent the evaporation of fluids within U‐traps.22 However, this preventive measure can minimize the risk of exposure only through the pathways of building drainage systems. Even when water seals function properly, the atomization of airborne pathogens occurs on the toilet bowl surface during flushing.
There are many commercially available products designed to improve toilet hygiene, including paper toilet seat covers, gel/foam disinfectants, and automatic toilet bowl cleaners or tablets, but none is capable of completely preventing infection through the aerosolization of fecal matter during toilet flushing. Toilet seat covers and surface disinfectants are effective in preventing contact with and the transmission of surface contaminants to other users, but they cannot prevent airborne transmission. A high degree of disinfection efficacy has been observed for automatic toilet bowl tablets,23 which work through the continuous release of disinfectants to kill microorganisms. However, their short lifespan and efficacy only for cistern systems are two concerns with their use. Before any mitigation measures can be proposed, the exposure risk must be quantified, and the most important factor in such risk is source emission strength.
Although comprehensive measurements were performed in this research, there were a number of limitations. First, feces vary in consistency from watery to formed, and also vary from individual to individual and within individuals at different times. Moreover, there are also many more toilet bowl designs than those investigated in this research, such as wash‐down and siphonic designs. Both of these factors may affect emission dynamics, but did not fall into our study objectives and hence are not addressed herein. Nonetheless, our results on total droplet generation are in good agreement with the results of other studies, which strengthens the conclusions drawn herein. Second, the study could have been improved by considering a wider droplet size range in testing our hypothesis. Finally, an attempt was made to test the yeast species Saccharomyces cerevisiae in the size range of 3.0‐8.0×5.0‐10.0 μm. However, very few surface or airborne CFUs were found, and the test was therefore discontinued and is not reported in detail.
5. CONCLUSION
The pilot study reported herein was the first to measure the emission strength of airborne pathogens and to demonstrate a correlation between airborne CFUs and flush droplet counts. It investigated the two most popular flushing systems, flushometer and cistern systems, at two different water pressures (HP=400 kPa and LP=200 kPa) and tank heights (HT=95 cm and LT=46 cm) and tested three types of bacteria representing small‐, medium‐ and large‐sized pathogens. Several salient observations were made. First, atomization energy notably influenced droplet and bacterial emission. Second, the total droplet concentration under HP was approximately 21 times that at HT. Third, in the HP, LP and HT conditions, (1) droplet concentration and pathogen emission were positively correlated, and (2) pathogen emission and bacterial size were negatively correlated. Finally, in all flushing conditions the emission strength of S. epidermidis was greatest, followed by E. coli and then P. alcaligenes.
This paper also highlights the need for greater concern over the transmission via toilet flushing of aerosols containing pathogenic organisms, which poses particular for the immunocompromised, children and the elderly. Finally, our findings also imply that a cistern tank design is preferable to a flushometer design with respect to aerosol generation.
Supporting information
ACKNOWLEDGEMENTS
The work described in this paper was fully supported by a General Research Fund from the Research Grants Council of the Hong Kong Special Administrative Region of China [CityU 115213].
Lai ACK, Tan TF, Li WS, Ip DKM. Emission strength of airborne pathogens during toilet flushing. Indoor Air. 2018;28:73–79. 10.1111/ina.12406
REFERENCES
- 1. Osterholm MT, Moore KA, Kelley NS, et al. Transmission of Ebola viruses: what we know and what we do not know. MBio. 2015;6:e01154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yu ITS, Li Y, Wong TS, et al. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N Engl J Med. 2004;350:1731‐1739. [DOI] [PubMed] [Google Scholar]
- 3. Darlow HM, Bale WR. Infective hazards of water‐closets. Lancet. 1959;6:1196‐1200. [DOI] [PubMed] [Google Scholar]
- 4. Lee N, Chan MC, Wong B, et al. Fecal viral concentration and diarrhea in norovirus gastroenteritis. Emerg Infect Dis. 2007;13:1399‐1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barker J, Bloomfield SF. Survival of Salmonella in bathrooms and toilets in domestic homes following salmonellosis. J Appl Microbiol. 2000;89:137‐144. [DOI] [PubMed] [Google Scholar]
- 6. Barker J, Jones MV. The potential spread of infection caused by aerosol contamination of surfaces after flushing a domestic toilet. J Appl Microbiol. 2005;99:339‐347. [DOI] [PubMed] [Google Scholar]
- 7. Gerba CP, Wallis C, Melnick JL. Microbiological hazards of household toilets: droplet production and the fate of residual organisms. Appl Environ Microbiol. 1975;30:229‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Getto L, Zeserson E, Breyer M. Vomiting, diarrhea, constipation, and gastroenteritis. Emerg Med Clin North Am. 2011;29:211‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bonifait L, Charlebois R, Vimont A, et al. Detection and quantification of airborne norovirus during outbreaks in healthcare facilities. Clin Infect Dis. 2015;61:299‐304. [DOI] [PubMed] [Google Scholar]
- 10. Floree GE, Bates ST, Knights D, et al. Microbial biogeography of public restroom surfaces. PLoS ONE. 2012;6:e28132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cowling BJ, Ip DKM, Fang VJ, et al. Aerosol transmission is an important mode of influenza A virus spread. Nat Commun. 2013;4:1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Best EL, Fawley WN, Parnell P, et al. The potential for airborne dispersal of Clostridium difficile from symptomatic patients. Clin Infect Dis. 2010;50:1450‐1457. [DOI] [PubMed] [Google Scholar]
- 13. Nicas M, Nazaroff WW, Hubbard A. Toward understanding the risk of secondary airborne infection: emission of respirable pathogens. J Occup Environ Hyg. 2005;2:143‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Johnson D, Lynch R, Marshall C, et al. Aerosol generation by modern flush toilets. Aerosol Sci Technol. 2013;47:1047‐1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. O'Toole J, Keywood M, Sinclair M, et al. Risk in the mist? Deriving data to quantify microbial health risks associated with aerosol generation by water‐efficient devices during typical domestic water‐using activities. Water Sci Technol. 2009;60:2913‐2920. [DOI] [PubMed] [Google Scholar]
- 16. Best EL, Sandoe JA, Wilcox MH. Potential for aerosolization of Clostridium difficile after flushing toilets: the role of toilet lids in reducing environmental contamination risk. J Hosp Infect. 2012;80:1‐5. [DOI] [PubMed] [Google Scholar]
- 17. Qian J, Hospodsky D, Yamamoto N, et al. Size‐resolved emission rates of airborne bacteria and fungi in an occupied classroom. Indoor Air. 2012;22:339‐351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lai ACK, Cheung ACT, Wong MML, et al. Evaluation of cold plasma disinfection efficacy against different airborne bacteria in ventilation duct flow. Build Environ. 2016;98:39‐46. [Google Scholar]
- 19. Lai ACK. Particle deposition indoors: a review. Indoor Air. 2002;12:211‐214. [DOI] [PubMed] [Google Scholar]
- 20. Macher JM. Positive‐hole correction of multiple‐jet impactors for collecting viable microorganisms. Am Ind Hyg Assoc J. 1989;50:561‐568. [DOI] [PubMed] [Google Scholar]
- 21. World Health Organization (WHO) . Inadequate plumbing systems likely contributed to SARS transmission. WHO Media Center news release, September 26, 2003; http://www.who.int/mediacentre/news/releases/2003/pr70/en/.
- 22. Gormley M, Templeton KE, Kelly DA, et al. Environmental conditions and the prevalence of norovirus in hospital building drainage system wastewater and airflows. Build Serv Eng Res T. 2014;35:244‐253. [Google Scholar]
- 23. Yahya MT, Cassells JM, Straub TM, et al. Reduction of microbial aerosols by automatic toilet bowl cleaners. J Environ Health. 1992;55:32‐34. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


