Summary
Monoclonal antibodies (mAb) to the transmissible gastroenteritis virus (TGEV) nucleoprotein (N) and membrane protein (M) were prepared and used for the comparative assessment of three blocking ELISA variants to detect TGEV. The competitive blocking ELISA format showed the highest sensitivity, allowing detection of 103 TCID50 TGEV/ml in culture medium. Ninety‐nine porcine field faecal samples obtained from 37 herds affected with diarrhoea were examined, and various TGEV levels were found in nine samples from six herds. However, only in three samples were significant TGEV concentrations demonstrated. The relationship between incidence of TGEV gastroenteritis and the spread of porcine respiratory coronavirus infection in pig farms is discussed.
Introduction
Rotaviruses and coronaviruses rank, along with bacterial infections, among the most significant causal agents of gastroenteritis in pigs. The two coronaviruses causing transmissible gastroenteritis (TGE) and porcine epidemic diarrhoea (PED) are morphologically identical, but they are antigenically different. TGE gastroenteritis, occurring mostly in the enzootic form, is not such a problem in Europe as it was in the past (Štěpánek et al., 1972; Pritchard et al., 1999). Although different results on the prevalence of TGEV infection were given (Kim and Cho, 1998; Chae et al., 2000), this infection still may be topical in some countries. Decreased incidence of TGE gastroenteritis is largely influenced by the spread of porcine respiratory coronavirus (PRCV) infection in pig herds (Bernard et al., 1989; Šesták et al., 1996). High level of nucleotide sequence homology (98%) of the two viruses was proven by genome analysis (Britton et al., 1991) with major deletions in genome coding S protein of PRCV. Therefore, it is presumed that PRCV is a spike (S) gene deletion mutant of TGEV, and minor alterations of the viral genome led to distinct viral tropism (Rasschaert et al., 1990; Šesták et al., 1996; Ballesteros et al., 1997; Constantini et al., 2004).
Three major structural proteins occur in coronaviruses: the glycoprotein S (Mr 150–220 K) found in the viral protrusions (corona), the nucleocapsid phosphoprotein N (Mr 45–57 K) and the membrane protein M (Mr 20–30 K) (Saif, 1993; Utiger et al., 1995). While viral antigens N and M of TGEV and PRCV are identical, differences were demonstrated in glycoprotein S epitopes, which do not trigger production of neutralizing antibodies. Monoclonal antibodies (mAb) to those epitopes are currently used to distinguish between TGEV and PRCV antibodies (Simkins et al., 1993). However the situation is different when detection of the virus in faecal samples is considered.
Although the presence of PRCV in faecal samples was demonstrated by extremely sensitive RT‐PCR technique (Constantini et al., 2004), little if any intestinal multiplication of PRCV was proven (Cox et al., 1990a, 1990b, 1990c). It follows that the method of lower sensitivity based on the use of antibodies to both TGEV and PRCV antigens could be suitable for specific demonstration of TGEV in faeces. Therefore the objective of our study was to check such mAb in modifications of blocking ELISA method, and to implement the optimal one for TGEV demonstration.
Materials and Methods
Viral and control antigens
The strain of TGEV, CAPM V‐344 (Collection of Animal Pathogenic Microorganisms, Brno, Czech Republic) was propagated in the porcine kidney cell line (PK‐15) in Eagle‐MEM medium. After development of cytopathic effect and centrifugation (3000 g for 15 min) of the medium, the pellet was resuspended in 1/100 of the original medium volume in phosphate‐buffered saline (PBS) as crude viral antigen (V‐Ag). Crude control antigen (C‐Ag) was prepared similarly from uninfected cells. Purified V‐Ag was prepared from the supernatant of the infectious medium by ultracentrifugation on cushions of 20 and 45% sucrose according to Hofmann and Wyler (1990). Antigens were kept at −80°C before use. Culture media with known tissue culture infectious dose (TCID50/ml) were used for the determination of blocking ELISA method sensitivity. To exclude possible crossreactions with other agents, crude V‐Ag (TGE, PED and rota A) were tested by all ELISA methods used. The reactivities of mAb with five TGEV strains (V‐344, Shizuoka, Purdue, and two of our field isolates Cz‐1970 and Cz‐1995) were also examined.
Experimental infection
Two hysterectomy derived, colostrum‐deprived 19‐day‐old piglets were kept in sterile conditions, and orally infected with 2 × 104.8 TCID50 TGEV. Diarrhoea appeared 24 hpi in both piglets; samples of faeces were collected during seven consecutive days. Seven weeks post‐infection piglets were challenged with 5 × 104.8 TCID50 TGEV and killed 12 days later by exsanguination under total anaesthesia. The titre of TGEV antibodies in serum obtained (SwSpos.) was determined by indirect ELISA (204 800). TGEV‐negative swine blood serum (SwSneg.) was obtained from 21‐day‐old uninfected piglet. The immunoglobulin fraction (SwATGEV) prepared from positive serum was used as a binding antibody in the blocking ELISA methods.
Electron microscopic examination
Rota‐ and coronaviruses were detected by electron microscopic examination of faecal samples and culture media after negative staining with 2% ammonium molybdate solution in water, pH 7.0 (Šmíd et al., 1993).
Immunoperoxidase test
Monolayers of infected (TGEV, PEDV, rotavirus A) and uninfected cells were fixed with acetone 15 min at 20°C. After inhibition of endogenous peroxidase (Li et al., 1987), the TGEV was detected by mAb by direct and indirect immunoperoxidase (IP) tests. The reactions were read after 3–5 min incubation in a substrate solution containing chromogen AEC (3‐amino‐9‐ethylcarbazole; Sigma, St Louis, MO, USA).
SDS‐PAGE and Western blot analysis
Low molecular weight standard (LMW; Pharmacia, Uppsala, Sweden) and purified TGE V‐Ag were separated by discontinuous electrophoresis (Laemmli, 1970) in 12% polyacrylamide gel (Miniprotean 3 Cell; Bio‐Rad Labs, Hercules, CA, USA). After transfer to a nitrocellulose (NC) membrane (0.2 μm; Bio‐Rad) and separation of the NC lanes, the lane containing LMW was stained with colloid gold (Moeremans et al., 1985), and lanes with V‐Ag were used for Western blot (WB) analysis. After incubation (1 h/20°C) with the tested sera or mAb incubations with peroxidase conjugates and subsequently in a chromogen solution DAB (3,3′‐diaminobenzidine; Sigma) followed.
Monoclonal antibodies
Hybridomas producing mAb to TGEV (mAbTGEV) were prepared according to Galfrè and Milstein (1981) by fusion of splenic lymphoid cells of immunized mice of the line BALB/c with cells of the myeloma line Sp 2/0. Hybridomas producing mAb with optimal results in indirect ELISA were checked by WB and IP tests and selected mAb were used for preparation of ascitic fluids. After purification by ion exchange chromatography, mAbTGEV obtained were stored at −18°C in 50% glycerol or used for the preparation of peroxidase conjugates.
Peroxidase conjugates
Rabbit antibodies to swine and mouse immunoglobulins (RASwIg, RAMoIg) or swine antibodies to mouse immunoglobulins (SwAMoIg) were purified from hyperimmune sera by affinity chromatography. These antibodies and mAb were conjugated with horseradish peroxidase (HRPO, type VI‐A; Sigma) using the periodate method (Boorsma and Streefkerk, 1979). Stock conjugate solutions adjusted to 1 mg of immunoglobulin/ml were further diluted for IP, WB and ELISA tests 100× to 10 000× in PBS containing 0.05% Tween 20 and 0.5% lactalbuminhydrolysate (PBST‐LAH).
Indirect ELISA
TGEV antibodies in tested sera, in culture media of hybridomas and in purified mAbTGEV preparations were assayed using the indirect ELISA method. Pairs of wells of microtitre plates (Nunc‐Immunoplate; PolySorp, Roskilde, Denmark), alternatingly precoated with TGE crude V‐Ag and C‐Ag were incubated with diluted tested samples. After second incubation with conjugates (always 1 h/37°C), the reactions were visualized in a substrate solution with chromogen TMB (3,3′,5,5′‐tetramethyl‐benzidine; Sigma). After 15 min, the reaction was stopped by addition of 1 m H2SO4, and absorbances were measured spectrophotometrically at 450 nm. Control wells filled with the diluent (blank) at the first incubation, or with control negative and positive serum were included in each examination. Samples showing a difference in optical density of at least 0.1 (after subtraction of absorbances of blank wells) were classified as positive.
Blocking ELISA
Three variants of ELISA method with specificity checked by blocking test were compared by box titration using faeces of experimentally infected piglets 24 hpi. Two double antibody sandwich ELISA variants (DAS‐ELISA) and competitive blocking ELISA (CB‐ELISA) method were compared. Microtitre plate wells (Nunc‐Immuno Plate; MaxiSorp, Roskilde, Denmark) precoated with binding antibodies was used. Pairs of wells filled with 50 μl of mixtures of faeces and SwSneg. or SwSpos. fivefold diluted 1 : 2 to 1 : 250 or 1 : 20 to 1 : 500, respectively, were examined. The second incubation was performed using the detection antibodies (conjugate). PBST‐LAH containing 0.5 m NaCl and 1 mg EDTA.Na2/ml for samples dilution at the first incubation and rinsing of wells four times with PBST between incubations at 37°C were used. Pairs of wells filled with the diluent (blank) or the mixtures of crude V‐Ag (TGEV, PEDV, rotavirus A) and SwSneg./pos. during the first incubation were included in each analysis. The following variants of the blocking ELISA method were investigated.
Variant 1. Double antibody sandwich ELISA
Binding antibody mAbTGEV was used. The first incubation with antigen test samples was performed for 2 h, the second incubation with the conjugate HRPO‐mAb‐TGEV for 1 h.
Variant 2. Competitive blocking ELISA
Binding antibody SwATGEV was used. After 1 h of incubation with the antigen test samples, the wells were supplemented with 50 μl diluent containing 0.5 μg mAbTGEV/ml, and the incubation continued for another hour. The second 1 h incubation was done using the conjugate HRPO‐SwAMoIg.
Variant 3. Double antibody sandwich ELISA
Binding antibody SwATGEV was used. The first incubation with the antigen test samples was performed for 2 h and the second 1 h incubation was conducted with the conjugate HRPO‐mAbTGEV.
The samples were regarded as positive if the net absorbance (NA), i.e. the difference of average absorbances in the wells incubated with SwSneg./pos. was >0.1, and the reactions were blocked by >50% in the wells with SwSpos. Blocking percentages were determined using the formula: %B = 100 − [(ASwSpos. × 100):(ASwSneg.)].
TGEV and PRCV antibodies in blood sera
Antibodies to PRCV and TGEV were assessed and differentiated in 81 blood serum samples of sows from randomly selected five herds. Commercial kit (Ingezim Corona Diferencial; INGENASA, Madrid, Spain) was used for examination. Sample preparation and evaluation of the results was carried out according to the manufacturer's instructions.
Field faecal samples
In total, 99 faecal samples from piglets with diarrhoea on 37 farms were examined. Most samples were from piglets younger than 21 days. After delivery, they were diluted in two to three volumes of Earle's medium, centrifuged (15 min, 3000 g), and the supernatants examined by electron microscopy and CB‐ELISA method. Samples were kept at −80°C before ELISA analysis.
Results
Monoclonal antibodies
After verification of the specificity of hybridomas producing mAbTGEV by WB, ELISA and IP tests, four preparations (D7/G7; D3/G6; B7/F7 and B4/F2) were selected for further use. All were of the isotype IgG1 and reacted with the membrane protein M (D7/G7) and nucleoprotein N (B7/F7) in WB analysis. Weak reactivity of mAb D3/G6 with epitopes present on protein M and nucleoprotein N were detected (Fig. 1). Conformational changes of viral antigens resulted in markedly decreased or even absent reactiveness of mAb B4/F2 in WB analysis. All mAb used reacted specifically with native TGEV strains in IP (Fig. 2) and ELISA tests according to results given in Table 1. Indirect ELISA titres of individual mAbTGEV solutions containing 5 mg Ig/ml ranged between 2 × 105 and 4 × 106. Cross reactivity of mAb with other viral antigens (rotavirus A and PEDV) could not be detected by any of methods used. The reactivity of individual mAb with five TGEV strains was checked by CB‐ELISA examination of infectious culture media. According to obtained positive NA and %B values, the mAb D7/G7 and B7/F7 react with all TGEV strains, while mAb D3/G6 and B4/F2 react with strains V‐344 and Cz‐1995 only. By the use of mAb D7/G7 alone or mixture of mAb in CB‐ELISA examination of various TGEV strains (Table 1) or positive field faecal samples (results not shown) the same results were obtained. Only a little lower absorbance values with D7/G7 were detected. Therefore the mixture of equal concentrations (w/v) of all mAb was selected for routine CB‐ELISA examination of field samples.
Figure 1.
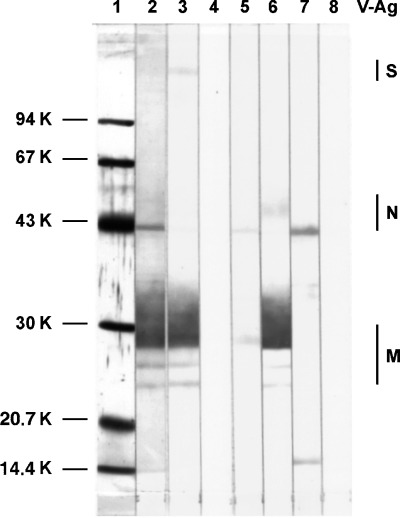
Western blot analysis of TGEV antibodies. After separation in 12% polyacrylamide gel, purified TGEV and a low molecular weight standard (LMW) were transferred to a nitrocellulose membrane. A part of the membrane with the LMW standard was stained with colloidal gold (lane 1). The other lanes with TGEV were incubated with swine antisera to TGEV (lanes 2 and 3), mAbTGEV D3/G6 (lane 5), D7/G7 (lane 6), B7/F7 (lane 7) or with diluting solution alone (lanes 4 and 8). After incubation with peroxidase conjugates to swine (lanes 2–4) and mouse (lanes 5–8) immunoglobulins, the reaction was visualised by incubation in a substrate solution containing chromogen DAB. The localization of TGEV antigens S, N and M is indicated.
Figure 2.
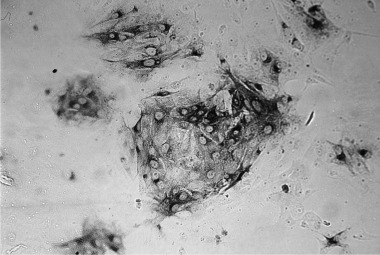
Detection of TGEV in infected cells by immunoperoxidase test. Direct immunoperoxidase detection of TGEV in a cell line PK‐15 12 hpi using the conjugate HRPO‐mAbTGEV D7/G7. Intensive staining of the cytoplasm proves propagation of TGEV in infected cells.
Table 1.
CB‐ELISA detection of mAb reactivity with TGEV strains
| mAb TGEV | TGEV strain | ||||
|---|---|---|---|---|---|
| Shizuoka | Purdue | Cz‐1970 | Cz‐1995 | V‐344 | |
| D7/G7 | |||||
| A | 0.737/0.094 | 0.659/0.035 | 0.606/0.027 | 0.701/0.021 | 0.507/0.026 |
| NA | 0.643 | 0.624 | 0.579 | 0.680 | 0.481 |
| %B | 87.2 | 94.7 | 95.5 | 97.0 | 94.9 |
| B7/F7* | |||||
| A | 1.004/0.397 | 1.168/0.563 | 0.829/0.292 | 1.051/0.210 | 1.281/0.773 |
| NA | 0.607 | 0.605 | 0.537 | 0.841 | 0.508 |
| %B | 60.5 | 51.8 | 64.8 | 80.0 | 39.7 |
| D3/G6 | |||||
| A | 0.023/0.006 | 0.033/0.011 | 0.017/0.002 | 0.483/0.011 | 0.542/0.028 |
| NA | 0.017 | 0.022 | 0.015 | 0.472 | 0.514 |
| %B | 73.9 | 66.7 | 88.2 | 97.7 | 94.8 |
| B4/F2 | |||||
| A | 0.128/0.034 | 0.141/0.045 | 0.106/0.021 | 0.649/0.013 | 0.596/0.047 |
| NA | 0.094 | 0.096 | 0.085 | 0.636 | 0.549 |
| %B | 73.4 | 68.1 | 80.2 | 98.0 | 92.1 |
| mAb mix | |||||
| A | 0.937/0.176 | 0.938/0.224 | 0.774/0.128 | 0.990/0.099 | 1.070/0.318 |
| NA | 0.761 | 0.714 | 0.646 | 0.891 | 0.752 |
| %B | 81.2 | 76.1 | 83.5 | 90.0 | 70.3 |
A, average absorbances obtained in wells containing SwSneg./pos; NA, net absorbance; %B, percentage of the reaction blocking in wells incubated with SwSpos; mAb mix, mixture of equal concentrations (w/v) of individual mAb.
Positive A, NA and %B values are given in bold.
The culture medium of the strain V‐344 was frozen 48 hpi (after development of CPE) and examined at working dilution 1 : 4. The remaining media were frozen 24 hpi (before CPE) and examined diluted 1 : 2.
*The culture medium of the strain V‐344 incubated with mAb B7/F7 was clearly positive when diluted 1 : 8 and 1 : 16 with the A, NA and %B values 0.992/0.379, 0.613 and 61.8%, and 0.415/0.095, 0.314 and 76.8% respectively.
Comparison of blocking ELISA methods
Sensitivity of the three blocking ELISA variants was checked by box titration of mixtures of faces of an experimentally infected piglet 24 hpi and SwSneg./SwSpos. Competitive blocking ELISA variant (CB‐ELISA) was selected as optimal (Table 2). Working dilutions of faecal samples 1 : 2, SwSneg./pos. 1 : 40, mAbTGEV mixture containing 5 mgIg/ml 1 : 10 000 and HRPO‐SwAMoIg 1 : 10 000 were used for routine CB‐ELISA examinations. High TGEV concentration in the tested sample is connected with high absorbance values obtained, and the other way round. It follows also, that evaluation of samples with different TGEV concentrations is influenced significantly both by sample and SwSneg./pos. dilutions. In Table 2 results with SwSneg./pos. diluted 20× and 100× only are given.
Table 2.
Detection of TGEV in a faecal sample of an experimentally infected piglet 24 hpi using three variants of monoclonal blocking ELISA methods
| ELISA variant | Absorbances and % of blocking obtained with different dilutions of faces and swine sera | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Faeces dilution | SwS dilution 20× | SwS dilution 100× | |||||||
| Neg. | Pos. | NA | %B | Neg. | Pos. | NA | %B | ||
| 1 DAS‐ELISA *mAbTGEV †HRPO‐mAbTGEV | 2× | 0.577 | 0.167 | 0.410 | 71.1 | 0.334 | 0.264 | 0.070 | 21.0 |
| 10× | 0.418 | 0.113 | 0.305 | 73.0 | 0.442 | 0.140 | 0.302 | 68.3 | |
| 50× | 0.178 | 0.072 | 0.106 | 59.6 | 0.213 | 0.059 | 0.154 | 72.3 | |
| 250× | 0.142 | 0.058 | 0.084 | 59.2 | 0.054 | 0.049 | 0.005 | 9.3 | |
| 2 CB‐ELISA *SwATGEV †HRPO‐SwAMoIg | 2× | 1.392 | 0.606 | 0.786 | 56.5 | 1.309 | 1.049 | 0.260 | 19.9 |
| 10× | 1.225 | 0.153 | 1.072 | 87.5 | 1.379 | 0.640 | 0.739 | 53.6 | |
| 50× | 0.304 | 0.022 | 0.282 | 92.8 | 1.056 | 0.134 | 0.922 | 87.3 | |
| 250× | 0.032 | 0.018 | 0.014 | 43.7 | 0.202 | 0.025 | 0.177 | 87.6 | |
| 3 DAS‐ELISA *SwATGEV †HRPO‐mAbTGEV | 2× | 1.743 | 0.873 | 0.870 | 49.9 | 1.573 | 1.314 | 0.259 | 16.5 |
| 10× | 1.482 | 0.270 | 1.212 | 81.8 | 1.543 | 0.829 | 0.714 | 46.3 | |
| 50× | 0.441 | 0.099 | 0.342 | 77.6 | 1.197 | 0.204 | 0.993 | 83.0 | |
| 250× | 0.142 | 0.073 | 0.069 | 48.6 | 0.215 | 0.063 | 0.152 | 70.7 | |
Dilutions of samples with positive values NA (>0.1) and %B (>50.0) are given in bold.
NA, net absorbance. Differences of mean absorbances in wells incubated with SwSneg./pos.
%B, % of absorbance blocking in the wells incubated with SwSpos. in comparison with the wells incubated with SwSneg.
*Binding antibodies.
†Detection antibodies (conjugate) used in respective blocking ELISA method variants.
CB‐ELISA sensitivity and specificity
The culture medium from PK‐15 infected cells containing 104.8 TCID50 TGEV/ml was analysed by box titration. Mixtures of medium diluted 1 : 4 to 1 : 64 and porcine sera diluted 1 : 20 to 1 : 500 respectively, were examined. Positive NA and %B values were obtained at all dilutions of both medium and SwS (results not shown). The sensitivity of the CB‐ELISA method was therefore estimated to exceed 103 TCID50 TGEV/ml. CB‐ELISA method specificity was confirmed by examination of crude V‐Ag (TGEV, PEDV, rotavirus A). Positive results were obtained with TGE V‐Ag only with average absorbances in the wells incubated with SwSneg./pos. 1.211/0.278 (NA = 0.933, %B = 77.0). In the wells with crude PEDV and rotavirus A the absorbances were −0.004/0.003 and 0.012/0.013 respectively.
TGEV and PRCV antibodies in blood sera
By examination of 81 blood serum samples of sows from five herds, TGEV antibodies were detected only in a single sample. PRCV antibodies were detected in 47 (58%) samples in all herds. Percentage of PRCV‐positive animals in respective herds ranged between 8 and 93% (results not shown).
Experimental infection
The TGEV was detected by CB‐ELISA in all faecal samples, taken between 1 and 7 days after experimental infection of piglets. The propagation of TGEV in the intestine thus exceeds 7 dpi. According to absorbance values obtained, highest TGEV concentrations were detected between 3 and 5 dpi (Table 3).
Table 3.
Detection of TGEV in faecal samples obtained between 1 and 7 days after experimental infection. The influence of faecal sample dilutions on the CB‐ELISA results
| Faeces (dpi) | Faeces dilution 2× | Faeces dilution 10× | Faeces dilution 50× | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SwS neg. | SwS pos. | NA | %B | SwS neg. | SwS pos. | NA | %B | SwS neg. | SwS pos. | NA | %B | |
| 1 | 1.410 | 0.727 | 0.683 | 48.4 | 1.286 | 0.194 | 1.092 | 84.9 | 0.477 | 0.011 | 0.466 | 97.7 |
| 2 | 1.423 | 0.896 | 0.527 | 37.0 | 1.518 | 0.325 | 1.193 | 78.6 | 0.486 | 0.012 | 0.474 | 97.5 |
| 3 | 1.330 | 1.137 | 0.193 | 14.5 | 1.402 | 0.619 | 0.783 | 55.8 | 1.219 | 0.098 | 1.121 | 92.0 |
| 4 | 1.083 | 0.910 | 0.173 | 16.0 | 1.157 | 0.508 | 0.649 | 56.1 | 1.060 | 0.071 | 0.989 | 93.3 |
| 5 | 1.075 | 0.769 | 0.306 | 28.5 | 1.298 | 0.445 | 0.853 | 65.7 | 1.084 | 0.051 | 1.033 | 95.3 |
| 6 | 1.139 | 0.724 | 0.415 | 36.4 | 1.313 | 0.365 | 0.948 | 72.2 | 0.791 | 0.039 | 0.752 | 95.1 |
| 7 | 0.885 | 0.330 | 0.555 | 62.7 | 0.837 | 0.098 | 0.739 | 88.3 | 0.269 | 0.027 | 0.242 | 90.0 |
dpi, The day post‐infection.
CB‐ELISA examination was performed at various working dilutions of faecal samples (2×, 10×, 50×) and constant dilutions of SwSneg./pos. (40×), mAbTGEV mixture (10 000×) and HRPO‐SwAMoIg (10 000×).
Samples with positive NA (>0.1) and %B (>50.0) values are given in bold.
Field samples of faeces
Altogether 99 field faecal samples from 37 herds were examined by CB‐ELISA. Various TGEV concentrations were found in nine samples from six herds. However, high TGEV concentrations confirmed by absorbances 0.941–1.335 (NA = 0.900–1.286, %B = 95.6–98.2) were detected in three samples only. The maximum absorbances in the remaining faecal samples indicating low TGEV concentration reached 0.371.
Electron microscopic examinations
The results obtained by EM and CB‐ELISA were poorly correlated reaching the values of about 20%. Similarly, as in our previous study concerning PEVD detection (Rodák et al., 2005) corona‐like particles without typical structures (corona) were frequently detected.
Discussion
Gastroenteritis caused by TGEV is less important at present in comparison with the 1960s and 1970s when mortality of piglets reached 80–100% in affected herds (Štěpánek et al., 1972). In addition to possible decreased TGEV virulence, it is explained by the spread of PRCV infection in pig herds and by protection of piglets by crossreacting maternal antibodies (Saif et al., 1994; Lanza et al., 1995).
For diagnosis of gastroenteritis caused by coronaviruses ELISA methods are used in addition to conventional virological procedures and molecular virology methods (Paton et al., 1997; Pritchard et al., 1999; Kim et al., 2001). The ‘DAS‐ELISA’ with specificity checked by blocking test has been used for both TGEV and PEDV demonstration in faeces (van Nieuwstadt et al., 1988; Carvajal et al., 1995).
Therefore, the detection of TGEV in faecal samples by blocking ELISA method was the objective of our study. We supposed that mAb against N and M antigens of various TGEV strains could be useful for this purpose in spite of their crossreactivity with PRCV. Faecal shedding of PRCV after infection was demonstrated by nested‐RT‐PCR (Constantini et al., 2004). However, the demonstration of ingested virus cannot be excluded. Little if any enteric multiplication of PRCV was demonstrated by immunofluorescence after experimental infection of 1‐week‐old piglets only, and it remained limited to a few unidentified cells located in or underneath the epithelial layer of the villi and/or crypts (Cox et al., 1990a, 1990b, 1990c). The RT‐PCR is extremely sensitive allowing RNA detection of only occasional viral particles in the sample. Although the sensitivity of TGEV detection by CB‐ELISA in faecal samples is sufficient (103 TCID50 TGEV/ml), we suppose that the possibility to demonstrate traces of PRCV is relatively low. Nevertheless, this assumption will be further checked by application of RT‐PCR in following experiments.
The sensitivities of TGEV detection by DAS‐ELISA and CB‐ELISA were compared and higher sensitivity and specificity of the last method was proven. While in DAS‐ELISA conjugated mAb are used, CB‐ELISA method is based on the use of unconjugated mAb. The results obtained also indicate that some faecal samples may be wrongly assessed under constant dilutions of the reaction components. The samples with high concentrations of viral antigen, giving high absorbance values, assessed as negative for %B < 50, are clearly positive under changed dilutions of faeces or SwS. The evaluation of samples with low antigen concentrations and low absorbance values are affected by SwS dilutions as well (2, 3). This was confirmed by examination of 99 field faecal samples; nine of them were TGEV positive. However, high TGEV concentrations, suggesting that the virus is an important causative agent of gastroenteritis, were detected in three samples only. Moreover, in all TGEV‐positive samples, the presence of rotavirus A and/or PEDV was demonstrated, indicating the importance of mixed enteric viral infections (results not shown). The demonstration of TGEV antibodies in only one of 81 randomly selected field sow serum samples indicates that the animals produce antibodies if the TGEV antigenic stimulation is high enough to compete with the stimulation by other enteric pathogens. The results also correlate with the assumption that the immunity of sows to PRCV leads to decreased occurrence of TGEV gastroenteritis (Bernard et al., 1989; Lanza et al., 1995; Šesták et al., 1996).
Effectivity of virus detection in faecal samples may be affected by the time of sample collection after the first symptoms of diarrhoea had emerged and the conditions of their shipment. Marked destruction of coronaviruses occurs in the gastric and intestinal contents (Aynaud and Bottreau, 1984). It follows, that characteristic structures of coronaviruses are less frequent by EM examination and cellular substructures may imitate ‘corona‐like’ particles. This can explain low correlation between EM and CB‐ELISA results.
The results described, confirm suitability and sufficient sensitivity of the CB‐ELISA for routine demonstration of TGEV in field faecal samples. CB‐ELISA methods were also successfully applied in detection of group A rotavirus and PEDV in faeces (Rodák et al., 2004, 2005). In spite of higher sensitivity of RT‐PCR technique, the advantages of CB‐ELISA examinations are lower costs, the possibility to demonstrate simultaneously three most important causal agents of viral gastroenteritis by uniform method and to examine sufficient number (20–40) of samples. This can give information both on their incidence in pig herds and on the effectiveness of immunoprophylactic measures.
Acknowledgements
This work was supported by projects QF 4051 and MZE 0002716201 of the Ministry of Agriculture of the Czech Republic. The authors wish to thank Mrs Farníková, Mrs Licková, Miss Hoydenová and Miss Bačinská for their skilful technical assistance.
References
- Aynaud, J. M. , and Bottreau E., 1984: Transmissible gastroenteritis of swine: stability of coronavirus in gastric and intestinal contents. Ann. Rech. Vet. 15, 359–364. [PubMed] [Google Scholar]
- Ballesteros, M. L. , Sanchez C. M., and Enjuanes L., 1997: Two amino acid changes at the N‐terminus of transmissible gastroenteritis coronavirus spike protein result in the loss of enteric tropism. Virology 227, 378–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard, S. , Bottreau E., Aynaud J. M., Have P., and Szymansky J., 1989: Natural infection with the porcine respiratory coronavirus induces protective lactogenic immunity against transmissible gastroenteritis. Vet. Microbiol. 21, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorsma, D. M. , and Streefkerk J. G., 1979: Periodate or glutaraldehyde for preparing peroxidase conjugates? J. Immunol. Methods 30, 245–255. [DOI] [PubMed] [Google Scholar]
- Britton, P. , Mawditt K. L., and Page K. W., 1991: The cloning and sequencing of the virion protein genes from a British isolate of porcine respiratory coronavirus: comparison with transmissible gastroenteritis virus genes. Virus Res. 21, 181–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvajal, A. , Lanza I., Diego R., Rubio P., and Cármenes P., 1995: Evaluation of a blocking ELISA using monoclonal antibodies for the detection of porcine epidemic diarrhea virus and its antibodies. J. Vet. Diagn. Invest. 7, 60–64. [DOI] [PubMed] [Google Scholar]
- Chae, C. , Kim O., Choi C., Min K., Cho W.‐S., Kim J., and Tai J. H., 2000: Prevalence of porcine epidemic diarrhoea virus and transmissible gastroenteritis virus infection in Korean pigs. Vet. Rec. 18, 606–608. [DOI] [PubMed] [Google Scholar]
- Constantini, V. , Lewis P., Alsop J., Templeton C., and Saif L. J., 2004: Respiratory and fecal shedding of porcine respiratory coronavirus (PRCV) in sentinel weaned pigs and sequence of the partial S‐gene of the PRCV isolates. Arch. Virol. 149, 957–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, E. , Hooyberghs J., and Pensaert M. B., 1990a: Sites of replication of a porcine respiratory coronavirus related to transmissible gastroenteritis virus. Res. Vet. Sci. 48, 165–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, E. , Pensaert M. B., Callebaut P., and van Deun K., 1990b: Intestinal replication of a porcine respiratory coronavirus closely related antigenically to the enteric transmissible gastroenteritis virus. Vet. Microbiol. 23, 237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, E. , Pensaert M. B., Hooyberghs J., and van Deun K., 1990c: Sites of replication of a porcine respiratory coronavirus in 5‐week‐old pigs with or without maternal antibodies. Adv. Exp. Med. Biol. 276, 429–433. [DOI] [PubMed] [Google Scholar]
- Galfrè, G. , and Milstein C., 1981: Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 73, 3–46. [DOI] [PubMed] [Google Scholar]
- Hofmann, M. , and Wyler R., 1990: Enzyme‐linked immunosorbent assay for the detection of porcine epidemic diarrhea coronavirus antibodies in swine sera. Vet. Microbiol. 21, 263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, B. H. , and Cho K. H., 1998: Transmissible gastroenteritis of pigs in southeastern Korea in 1994–1996: prevalence and factors associated with the infection In: Done S., Thomson S., and Varley M. (eds) Proceedings of the 15th IPVS Congress, Birmingham, UK, 5–9 July, p. 146. Nottingham University Press, Manor farm, Nottingham. [Google Scholar]
- Kim, S. Y. , Song D. S., and Park B. K., 2001: Differential detection of transmissible gastroenteritis virus and porcine epidemic diarrhea virus by duplex RT‐PCR. J. Vet. Diagn. Invest. 13, 516–520. [DOI] [PubMed] [Google Scholar]
- Laemmli, U. K. , 1970: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Lanza, I. , Shoup D. I., and Saif L. J., 1995: Lactogenic immunity and milk antibody isotypes to transmissible gastroenteritis virus in sows exposed to porcine respiratory coronavirus during pregnancy. Am. J. Vet. Res. 56, 739–748. [PubMed] [Google Scholar]
- Li, C. Y. , Ziesmer S. C., and Lazcano‐Villareal O., 1987: Use of azide and hydrogen peroxide as an inhibitor for endogeneous peroxidase in the immunoperoxidase method. J. Histochem. Cytochem. 35, 1457–1460. [DOI] [PubMed] [Google Scholar]
- Moeremans, M. , Daniels G., and de Mey J., 1985: Sensitive colloidal (gold or silver) staining of protein blots on nitrocellulose membranes. Anal. Biochem. 145, 315–321. [DOI] [PubMed] [Google Scholar]
- van Nieuwstadt, A. P. , Cornelissen J. B., and Zetstra T., 1988: Comparison of two methods for detection of transmissible gastroenteritis virus in feces of pigs with experimentally induced infection. Am. J. Vet. Res. 49, 1836–1843. [PubMed] [Google Scholar]
- Paton, D. , Ibata G., Sands J., and McGoldrick A., 1997: Detection of transmissible gastroenteritis virus by RT‐PCR and differentiation from porcine respiratory coronavirus. J. Virol. Methods 66, 303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard, G. C. , Paton D. J., Wibberley G., and Ibata G., 1999: Transmissible gastroenteritis and porcine epidemic diarrhoea in Britain. Vet. Rec. 144, 616–618. [DOI] [PubMed] [Google Scholar]
- Rasschaert, D. , Duarte M., and Laude H., 1990: Porcine respiratory coronavirus differs from transmissible gastroenteritis virus by a few genomic deletions. J. Gen. Virol. 71, 2599–2607. [DOI] [PubMed] [Google Scholar]
- Rodák, L. , Šmíd B., Nevoránková Z., Smítalová R., and Valíček L., 2004: Verification of sensitivity and specificity of group A rotavirus detection in piglets faeces with monoclonal blocking ELISA methods. J. Vet. Med. B 51, 160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodák, L. , Valíček L. Šmíd B., and Nevoránková Z., 2005: An ELISA optimized for porcine epidemic diarrhoea virus detection in faeces. Vet. Microbiol. 105, 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saif, L. J. , 1993. Coronavirus immunogens. Vet. Microbiol. 37, 285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saif, L. J , van Cott J. L., and Brim T. A., 1994: Immunity to transmissible gastroenteritis virus and porcine respiratory coronavirus infections in swine. Vet. Immunol. Immunopathol. 43, 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šesták, K. , Lanza I., Park S. K., Weilnau P. A., and Saif L. J., 1996: Contribution of passive immunity to porcine respiratory coronavirus to protection against transmissible gastroenteritis virus challenge exposure in suckling pigs. Am. J. Vet. Res. 57, 664–671. [PubMed] [Google Scholar]
- Simkins, R. A. , Weilnau P. A., van Cott J., Brim T. A., and Saif L. J., 1993: Competition ELISA, using monoclonal antibodies to the transmissible gastroenteritis virus (TGEV) S protein, for serologic differentiation of pigs infected with TGEV or porcine respiratory coronavirus. Am. J. Vet. Res. 54, 254–259. [PubMed] [Google Scholar]
- Šmíd, B. , Valíček L., Rodák L., and Kudrna J., 1993: Electron microscopic demonstration of porcine epidemic diarrhea virus in the Czech Republic. Vet. Med. Czech. 38, 333–341 (in Czech). [PubMed] [Google Scholar]
- Štěpánek, J. , Menšík J., Rozkošný V., and Mesároš E., 1972: Transmissible gastroenteritis of swine (TGE). Physicochemical and biological properties of strains isolated in Czechoslovakia. Acta Vet. (Brno), 41, 435–445. [Google Scholar]
- Utiger, A. , Tobler K., Bridgen A., Suter M., Singh M., and Ackermann M., 1995: Identification of proteins specified by porcine epidemic diarrhoea virus In: Talbot P. J., and Levy G. A. (eds), Corona – and Related Viruses, pp. 287–290. Plenum Press, New York. [DOI] [PubMed] [Google Scholar]


