Abstract
This study determines the relative survival (RS) of Bacillus subtilis spores loaded on an N95 filtering facepiece respirator (FFR) after decontamination by five methods under worst‐case conditions. Relative survival was obtained by testing after decontamination and after storing the FFRs at 37°C and 95% relative humidity for 24 hours. The decontamination methods involved ethanol, bleach, ultraviolet irradiation (UVA 365 nm, UVC 254 nm), an autoclave, and a traditional electric rice cooker (TERC) that was made in Taiwan. Without decontamination, 59 ± 8% of the loaded spores survived for 24 hours. When 70% ethanol was added to the N95 FFR at a packing density of 0.23, the RS was 73 ± 5% initially and decayed to 22 ± 8% in 24 hours. Relative survival remained above 20% after 20 minutes of UVA irradiation. The other four decontamination measures achieved 99%‐100% biocidal efficacy, as measured immediately after the methods were applied to the test FFRs. Relative survival is a useful parameter for measuring sterilization or degree of disinfection. Bleach, UVC, an autoclave, and a TERC provide better biocidal efficacy than ethanol and UVA. Not only a higher filter quality but also a lower value of RS produced the most decontaminated FFR.
Keywords: bioefficacy, decontamination, N95, respirator, spore, survive
Practical Implications.
The survival of bacteria of reclaimed National Institute for Occupational Safety and Health‐certified N95 filtering facepiece respirators (FFRs) after decontamination is important, especially for healthcare workers.
Safe respirator usage after decontamination using various methods improves infection control and protection against biohazards.
The optimal dosages of decontamination methods are important for determining a comprehensive infection control strategy.
Our work addresses the potential for cross‐contamination of reused respirators with a view to overcoming FFR shortages and so to increase capacity for controlling future outbreaks.
1. INTRODUCTION
Filtering facepiece respirators (FFR) should be discarded after use for one work shift to control infection,1 especially if they come into contact with airborne pathogens, such as Mycobacterium tuberculosis,2 or influenza virus.3, 4 During the severe acute respiratory syndrome (SARS) epidemic outbreak, consumers’ demand for N95 respirators increased owing to their high collection efficiency. During the outbreak of Middle East respiratory syndrome (MERS) in Korea, pharmacies in South Korea sold many times more N95 FFRs than usual. However, these FFRs are sometimes reused, especially during a shortage or when their distribution is delayed.5 Economic considerations may also apply.6 The price of certified FFRs, such as National Institute for Occupational Safety and Health (NIOSH)‐approved N95 FFRs, typically exceeds those of non‐certified masks. Affordability considerations favor reuse.
National Institute for Occupational Safety and Health recommends practices for the extended use and limited reuse of NIOSH‐certified N95 FFRs.1 NIOSH defines reuse as the use of the same N95 respirator for multiple encounters with patients but removing it (“doffing”) after each encounter. The respirator is stored between encounters to be put on again (“donned”) before the next encounter with a patient. To prevent tuberculosis, the CDC recommends that a disposable respirator can be reused by the same worker as long as it maintains its physical integrity and its proper use provides protection (exposure reduction) consistent with the assigned protection factor for respirator of its class.1 Furthermore, NIOSH requires that, between uses, used respirators should be hung in a designated storage area or kept in a clean, breathable container such as a paper bag. To minimize potential cross‐contamination, respirators are stored without touching each other and the use of the respirator is clearly identified. Storage containers should be disposed of or cleaned regularly.1 The FDA defines three kinds of reuse: between patients with adequate reprocessing, reuse by the same person with adequate reprocessing/decontamination, and repeated use by the same person over a period with or without reprocessing.7, 8
Before FFRs are reused, they may be decontaminated to control the growth of microorganisms on them. However, whether a decontaminated N95 FFR can be reused is an issue that requires detailed consideration. In some cases, the use of chemical disinfectants may require that an employer train workers on protecting themselves against chemical hazards and on complying with OSHA's Hazard Communication, 29 CFR 1910.1200, and other standards.9 Contaminated objects with porous surfaces that cannot be disinfected may have to be disposed of.9 All personnel, clothing, equipment, and samples that leave a contaminated area (generally referred to as the Exclusion Zone) must be decontaminated to remove any harmful chemicals or infectious organisms that may be attached to them.10 Decontamination methods (i) physically remove contaminants, (ii) inactivate contaminants by chemical detoxification or disinfection/sterilization, or (iii) remove contaminants by a combination of both physical and chemical methods.10 NIOSH has published a series of research articles on mask decontamination.11, 12, 13, 14
In selecting decontamination methods, both decontamination and protective capability are considered.15 For example, ultraviolet germicidal irradiation (UVGI) and bleach reportedly do not significantly reduce the protective capability (penetration by contaminants) of FFRs.13, 14 Bergman et al13 tested many methods, involving UVGI and bleach, and found that FFRs that were treated with these two decontaminants and control samples exhibited expected filter aerosol penetration (<5%) and filter airflow resistance. Physical damage varied with treatment method. Further research is needed before any particular decontamination methods can be recommended. Other chemical and energetic methods also have potential for decontaminating FFRs,11, 12, 16 but few studies have addressed the elimination of viable microorganisms from FFRs. UVGI had been reported effectively to eliminate H5N117 or MS2 coliphages18 from FFRs and was found not to affect drastically the filtration efficiency of FFRs. Related studies have not evaluated the efficiency with which decontamination methods destroy bacteria. Therefore, objective experimentally obtained information concerning the destruction of bacteria using various decontamination methods is required to support the reuse of FFRs.
The FDA has not cleared alcohol as the main active ingredient in liquid chemical sterilants or high‐level disinfectants, because alcohol is rapidly bactericidal rather than bacteriostatic against vegetative forms of bacteria, and it does not destroy bacterial spores.15, 19 However, ethanol or isopropanol can eliminate the electrostatic charges on filters which were used before the test of particle penetration through the electret masks.20, 21, 22
In subtropical areas, such as Taiwan, temperatures and humidity are high all year round, favoring the growth of bacteria. Therefore, this study compares the cultivation of airborne Bacillus subtilis spores that are loaded on N95 FFRs after treatment by a commercially available decontamination method with that after storage at a constant worst‐case temperature and relative humidity (RH) to elucidate the survival and reproduction of bacteria on N95 FFRs. The potential for N95 reuse during a shortage of epidemic‐preventive supplies is evaluated, and recommendations concerning decontamination methods and FFR reuse criteria are made to increase public health.
OSHA has recommended that decontamination methods include chemical disinfection, irradiation, gas/vapor sterilization or steam sterilization, and dry heat sterilization.10 To understand the biological effect of decontamination on FFRs, the following five decontamination methods were compared: low‐temperature chemical decontamination using (i) ethanol and (ii) bleach, and physical decontamination using (iii) UVGI, (iv) an autoclave to provide moist heat, and (v) a traditional electric rice cooker (TERC), which was made in Taiwan, to provide dry heat. The first four methods are preferred methods for the disinfection or sterilization of patient‐care medical devices.15 TERC is frequently used in hospitals in Taiwan.22 The filter quality of FFRs, including particle penetration and pressure drop, was originally published elsewhere.22
2. MATERIALS AND METHODS
2.1. Test system and decontamination methods
The main test variable in this study is the survival of bacteria that were loaded on N95 FFRs that were decontaminated by various methods under worst‐case temperature and humidity, which prevail when an FFR is placed in a zipper bag in a healthcare worker's pocket with the goal of preventing cross‐contamination,21 and touching of the respirator.1, 23 In the experiment, B. subtilis spores were the tested microbial strain; a six‐jet Collison nebulizer (BGI, Waltham, MA) sprayed the spores into a test system, shown in Figure 1, where they were loaded on N95 FFRs by suction to simulate the respiratory flow of workers during intensive activity.21 The experimental FFR was an N95 FFR (8210, 3M, St. Paul, MN), certified by NIOSH. It was divided into six pieces, to which five decontamination methods were applied; they involved ethanol, bleach, UV, an autoclave, and a traditional electric rice cooker (TERC), made in Taiwan, without steam. The treatment proceeded as follows.
Figure 1.

Experimental system setup.21, 22 A filter holder contains one piece of N95FFR
Ethanol: Ethanol with various concentrations and volumes was added to the center of the surface of the N95 FFR using a pipette,21 the FFR was then dried in a petri dish that was placed in a biosafety cabinet (BSC) for 10 minutes.
Bleach: A 0.4 mL volume of bleach with various concentrations (5.4% (w/w) as Cl2: original; 2.7%: one part bleach to one part of deionized water; 0.54%: one part bleach to nine parts of deionized water13) was added to the center of the surface of the N95 FFR using a pipette,21 the FFR was then dried in a petri dish in a BSC for 10 minutes.
UV: An N95 FFR was placed 10 cm below a 6 W handheld UV lamp (model UVGL‐58, VUP LLC, Upland, CA) that emitted a wavelength of 254 nm (UVC, 18.9 mW/cm2) or 365 nm (UVA, 31.2 mW/cm2). Both sides of each N95 FFR were exposed for different times ‐ 1, 2, 5, 10 and 20 minutes ‐ in a BSC. The UV intensity was measured using a handheld laser power and energy meter (OPHIR NOVAII, model Nova II PD300‐UV) and was reported as a mean of five measurements over a 10 × 10 mm aperture with a swivel mount and a removable filter.
Autoclave: The N95 FFR was heated for 15 minutes at 121°C and 103 kPa.
TERC: The N95 FFR was placed in an electric rice cooker for dry heating for 3 minutes (149‐164°C, without added water).22
2.2. Sampling procedure
Each N95 FFR was placed into the system (Figure 1) for 30 minutes of bacterial bioaerosol sampling. The respiratory flow (85 L/min) of workers during high‐intensity activities was used,24 and the face velocity for the whole N95 FFR was calculated as 8.3 cm/s. The N95 FFRs were cut into pieces with a diameter of 45 mm. Each had an effective diameter of 40 mm and a filtration area of 12.6 cm2. The sampling flow rate of the pump was 6.3 L/min, which produced the desired face velocity.17, 21, 25
Bacillus subtilis prototype strains (CCRC 12145, Taiwan Food Industry Research and Development Institute) were used to prepare an endospore suspension liquid for generating bacterial bioaerosols.21 The suspension was centrifuged at 1917 g for 5 minutes. The supernatant was discarded and the pellet was resuspended in sterile distilled water. This washing process was repeated two times,26 and spores were resuspended in approximately 55 mL of sterile distilled water to yield a uniform mixture which was poured into the Collison nebulizer.21 The spores were aerosolized at a pressure of 25 psi when the dilution air flow rate was 80 L/min, as presented in Figure 1. The stability of the bioaerosol concentration in the system was verified using an Andersen single‐stage sampler (Andersen Inc., Atlanta, GA).21
The aqueous packing density (αaq) of the retained liquid decontaminants was modified that in a previous report, in which αaq was the volume fraction of the filter27; it is calculated using the equation,
| (1) |
where V aq is the volume of liquid disinfectant that was spiked onto the test N95 FFR. The volume of the test N95 FFR (V f) was 1.84 mL, which was estimated from the volume of water that was displaced by it. When 0.15, 0.4, 0.8, and 1.6 mL of ethanol were spiked onto the surface of the test N95, αaq values of 0.082, 0.23, 0.44, and 0.87, respectively, were obtained.
After spores were loaded onto the FFRs for 30 minutes, the FFRs were decontaminated using one of the five aforementioned methods, and then placed in an incubator (Model: HONG‐YU, HRM‐80, Taichung, Taiwan) at the worst‐case scenario temperature of 37°C (similar to body temperature) and 95% RH (the maximum feasible RH value), respectively, for 24 hours for another day of usage. Each batch test was conducted in triplicate. Figure 2 displays the sampling procedure.
Figure 2.
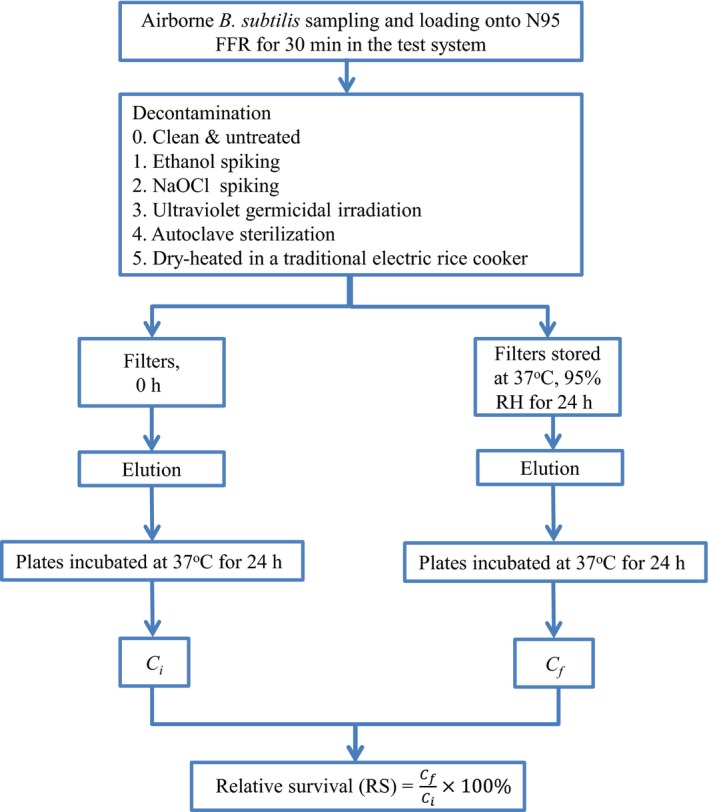
Sample treatment flowchart
After decontamination, elution was performed. It involved placing the test filter in a 50 mL centrifuge tube and then adding 20 mL of sterilized water to soak the filters completely.21 A centrifuge was used to recover the loaded spores by desorption from the FFRs at a centrifugation speed of 3500 rpm for 10 minutes, followed by 1 minutes of vortexing. The centrifuged and vortexed suspension (0.1 mL) was uniformly applied to the TSA and then placed in an incubator for 24 hours. Colony forming units (CFUs) were counted and their relative survival (RS) was calculated as follows.21, 28, 29
| (2) |
where C f is the number of CFUs after decontamination and C i is the number of CFUs before decontamination (Figure 2).
3. RESULTS
Figure 3 presents the decontaminating effect of 0.4 mL (αaq = 0.23) ethanol at various concentrations on the RS of B. subtilis spores that were loaded on the N95 FFR. An RS of 89 ± 6% was obtained after spiking with 50% ethanol, and 73 ± 5% was obtained after spiking with 70% ethanol. The lowest RS of 68 ± 3% was obtained when the concentration of ethanol was 80%. The result that was obtained using 95% ethanol (RS = 73 ± 7%) was close to that obtained using 70% ethanol although the samples that were spiked with 95% ethanol sometimes yielded slightly higher values of RS than were obtained using the 80% ethanol samples. An RS of 59 ± 8% was obtained in 24 hours without decontamination. The 50%, 70%, 80%, and 95% ethanol‐treated samples had RS values of 33 ± 8%, 22 ± 8%, 20 ± 2% and 26 ± 7% after 24 hours, respectively.
Figure 3.

Relative survival as a function of concentration of 0.4 mL ethanol for Bacillus subtilis spores loaded on N95 filtering facepiece respirator. Error bars represent one standard deviation
Figure 4 shows the effect of 70% ethanol on the RS of B. subtilis spores. Just after spiking with ethanol, the RS was found to have declined from 100% to 68%‐75%. When 0.4 mL (αaq = 0.23) of 70% ethanol was applied, the RS fell to 22% in 24 hours. The RS fell to 20% when 80% ethanol was used.
Figure 4.

Relative survival as a function of aqueous packing density (αaq) of 70% ethanol for Bacillus subtilis spores loaded on N95 filtering facepiece respirator. Error bars represent one standard deviation
In the bleach decontamination test, no colony was recovered after 5.4%, 2.7% or 0.54% NaOCl was used, constituting no dilution, twofold, and 10‐fold dilution,9 respectively (Figure 5). This study found that NaOCl, even when diluted 10‐fold from standard bleach, had a strong decontamination effect, with a 100% bactericidal effect.
Figure 5.
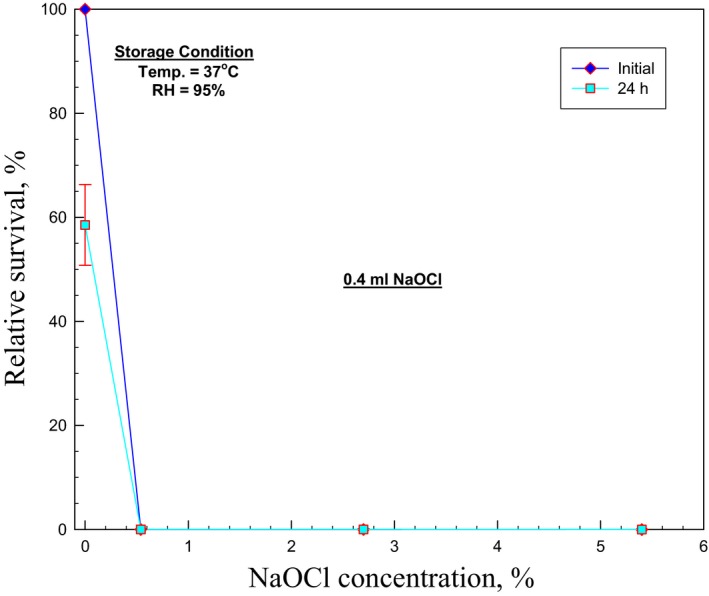
Relative survival as a function of concentration of 0.4 mL NaOCl for Bacillus subtilis spores loaded on N95 filtering facepiece respirator. Error bars represent one standard deviation
Similar results were achieved using UVC. No colony was recovered after exposure to UVC for as little as 5 minutes (Figure 6). However, RS remained above 20% after 20 minutes of irradiation by UVA, exponentially decaying with increased exposure time (Figure 6).
Figure 6.

Relative survival as a function of wavelength and duration of exposure to ultraviolet for Bacillus subtilis spores loaded on N95 filtering facepiece respirator. Error bars represent one standard deviation
Figure 7 presents the RS values that were achieved using the five decontamination methods. Four of the methods ‐ involving 0.54% NaOCl, UVC, an autoclave and a TERC ‐ effectively sterilized almost 100% of the bacteria. Decontamination with 70% ethanol yielded an initial RS of approximately 75% and an RS that remained above 20% after 24 hours of storage.
Figure 7.

Relative survival of Bacillus subtilis spores loaded on N95 filtering facepiece respirator. Error bars represent one standard deviation. (TERC: Taiwan traditional electric rice cooker)
4. DISCUSSION
Decontamination using ethanol yielded higher RS of spores than the other four decontamination methods (Figure 7). The other four methods yielded RS values of close to zero, indicating effective sterilization. The biosafety manual that was published by the World Health Organization (WHO) notes that alcohols are effective against vegetative bacteria but not spores.30 The US CDC also states that alcohols can eliminate many or even all pathogenic microorganisms except for bacterial spores.15 Our findings are consistent with the recommendations of the WHO and the US CDC.
In this study, 59 ± 8% of the loaded spores survived on N95 FFRs for 24 hours without decontamination. This result is consistent with some previous studies of the survival rates of B. subtilis spores at 37°C and an RH of 85%31 or 95%21 Moreover, the results of Sagripanti and Bonifacino32 suggest that the nature of the challenged surface may affect the sporicidal activity of some chemical agents. For example, a study by Li et al revealed that the average values of RS (%) that were obtained by elution from a Nuclepore filter for hardy B. subtilis were 64% and 48% at sampling times of 1 and 30 minutes, respectively. The average RS (%) values of B. subtilis from a gelatin filter were 128% and 108% at sampling times of 1 and 30 minutes, respectively.26 Our results were comparable to those obtained for Nuclepore filter samples.
The WHO biosafety manual mentions that alcohols should be used at concentrations of approximately 70% (v/v) in water to maximize their germicidal effectiveness. The WHO and the US CDC both recommend the use of 70% alcohol.15, 30 The US CDC also notes that the biocidal activity of alcohol diminishes sharply at dilutions of weaker than 50% (v/v), and the optimal bactericidal concentration is 60%‐90% (v/v).15 From the result of quadratic polynomial regression, the lowest RS occurred at the 83% and 76% alcohol for the initial and 24 hours samples, respectively (Figure 3). The RS values of approximately 70%‐95% (v/v) (Figure 3) that were obtained in this study support the US CDC's recommendation.
The optimal bactericidal concentrations for various microorganisms may vary.15 Pseudomonas aeruginosa was destroyed by ethanol at concentrations of 30%‐100%, and Serratia marcescens, E. coli, and Salmonella typhosa were destroyed by ethanol at concentrations of 40%‐100%. Gram‐positive organisms, such as Staphylococcus aureus and Streptococcus pyogenes, were slightly more resistant to ethanol, being destroyed by ethanol concentrations of 60% to 95%. When the effect of ethanol against M. tuberculosis was evaluated, 95% ethanol was found to kill the tubercle bacilli in water or sputum suspension within 15 seconds. As the challenge bioaerosol in the current study is B. subtilis spore, the RS remained at 75 ± 17% (Figure 7) indicating that the B. subtilis spores were more resistant than those mentioned above, including tubercle bacilli. Ethanol does not affect the viability of Bacillus spores in current guidance,1, 10, 15, 30 and a comprehensive examination of disinfection against different target microorganisms should therefore be performed.
The average RS (%) values for hardy B. subtilis that were obtained by elution from FFRs decayed to 23 ± 8% in 24 hours of treatment with 70% ethanol but to 59 ± 8% without ethanol (Figure 7). The decay to 59% was caused mainly by the nature of the FFR surface and effects of storage. The combined effect of FFR surface, storage, and ethanol treatment might have been expected to yield an RS of 44% (59% × 75%), but was only 22% in this study. As ethanol should not have this much of an effect on spores,1, 10, 15, 30 the nature of the FFR surface, treatment with ethanol, and the storage conditions may have an interaction effect. Therefore, the mechanism by which ethanol affects the amounts of spores that survive on FFRs should be further investigated.
Figure 4 presents the effect of the αaq of 70% ethanol on the RS of B. subtilis spores. When αaq exceeded 0.23, the initial RS was around 70% and that after 24 hours was about 20%. Lin et al21 found that when 1.5 mL of artificial saliva was dropped onto an N95, its surface tension caused it to form a sphere‐like droplet that was attached to the hydrophobic first layer, before it slowly penetrated the second and third layers. However, in the present study, which is based on observation, 70% ethanol penetrated the filter rapidly and quickly evaporated to the air. Accordingly, increasing αaq had little effect on the RS of B. subtilis spores and may have been responsible for the rapid evaporation of 70% ethanol.
About 60%‐70% of the B. subtilis spores that were loaded on N95 FFRs survived after 24 hours of storage without decontamination, whereas only approximately 20% of spores retained their cultivability after 20 minutes of irradiation by UVA (Figure 6), whose disinfection effect was comparable to that of ethanol (Figure 4). From the result of exponential decay regression, the half‐life (the value of RS reduces to 50%) was 10 and 0.17 minutes for the UVA and UVC irradiation, respectively (Figure 6). Although UVA could not decontaminate as effectively as UVC, it did have some decontaminating effect. This finding warrants further study.
The results in our study verify the biocidal efficacy of bleach (0.54% NaOCl), UVC, and an autoclave, which are well known means of sterilization.15, 30 Interestingly, the TERC exhibited biocidal efficacy as a sterilizing device. In the WHO biosafety manual,30 heat is regarded as one of the most commonly used physical agents for decontamination against pathogens. “Dry” heat, which is non‐corrosive, is applied to many items of laboratory‐ware, which can withstand temperatures of at least 160°C for 2‐4 hours. In this study, the TERC is used as a dry heating device and was found to exhibit a biocidal efficacy that reaches effective sterilization in 3 minutes. The results achieved using the TERC provide useful information regarding effective means for decontaminating and reusing FFRs.
Notably, when an N95 FFR is reused, the biocidal efficacy of the decontamination treatment, filter quality, fit factor (which is affected by physical damage to the frame or rubber strap), and toxic residual chemicals on FFR14 must all be considered. For example, bleach can harm the wearer if not properly used to decontaminate an N95 FFR before reuse.15 Safe disposal of spent bleach is important, and users may decide to neutralize the microbicidal activity of the bleach before disposal. Solutions can be neutralized by reaction with chemicals such as sodium bisulfite, or glycine.15 Considering the potential health risks, the method of decontamination using bleach must be modified such as by the use of chemical methods for neutralizing residuals.12
The RS is a function of decontamination and the biological characteristics of pathogens. The filter quality combines penetration and pressure drop and is affected by the physical characteristics of the FFR. However, this study focused on RS because it is a useful metric for quantifying sterilization or degree of disinfection. In summary, bleach, UVC, the autoclave, and the TERC provide effective sterilization. However, ethanol and UVA are ineffective and not cleared as high‐level disinfectant by US FDA.19 A better reuse FFR has a higher filter quality and a lower RS.
ACKNOWLEDGEMENTS
This work was supported by grants from the Institute of Labor, Occupational Safety and Health, Ministry of Labor, and from the Ministry of Science and Technology, Republic of China, Taiwan.
Lin T‐H, Tang F‐C, Hung P‐C, Hua Z‐C, Lai C‐Y. Relative survival of Bacillus subtilis spores loaded on filtering facepiece respirators after five decontamination methods. Indoor Air. 2018;28:754–762. 10.1111/ina.12475
REFERENCES
- 1. National Institute for Occupational Safety and Health (NIOSH) . Recommended guidance for extended use and limited reuse of N95 filtering facepiece respirators in healthcare settings. 2014.
- 2. Nazaroff WW, Nicas M, Miller SL. Framework for evaluating measures to control nosocomial tuberculosis transmission. Indoor Air. 1998;8:205‐218. [Google Scholar]
- 3. Milton DK, Fabian MP, Cowling BJ, Grantham ML, McDevitt JJ. Influenza virus aerosols in human exhaled breath: particle size, culturability, and effect of surgical masks. PLoS Pathog. 2013;9:e1003205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carias C, Rainisch G, Shankar M, et al. Potential demand for respirators and surgical masks during a hypothetical influenza pandemic in the United States. Clin Infect Dis. 2015;60:S42‐S51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fisher EM, Shaffer RE. Considerations for recommending extended use and limited reuse of filtering facepiece respirators in health care settings. J Occup Environ Hyg. 2014;11:D115‐D128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rivera P, Louther J, Mohr J, Campbell A, DeHovitz J, Sepkowitz KA. Does a cheaper mask save money? The cost implementing a respiratory personal protective equipment program. Infect Control Hosp Epidemiol. 1997;18:24‐27. [DOI] [PubMed] [Google Scholar]
- 7. IOM . Reusability of Facemasks During an Influenza Pandemic: Facing the Flu. Washington, DC: National Academies Press; 2006. [Google Scholar]
- 8. Lin CS. FDA regulation of surgical masks and respirators. 2006. [Online]. http://www.iom.edu/~/media/Files/ActivityFiles/PublicHealth/ReusableFluMasks/FDApresentation12306.ashx. Accessed December 22, 2017.
- 9. Occupational Safety and Health Administration . Cleaning and decontamination of ebola on surfaces. Guidance for workers and employers in non‐healthcare/non‐laboratory settings. https://www.osha.gov/Publications/OSHA_FS-3756.pdf. Accessed December 22, 2017.
- 10. Occupational Safety and Health Administration . Decontamination. https://www.osha.gov/SLTC/hazardouswaste/training/decon.html. Accessed December 22, 2017.
- 11. Viscusi DJ, King WP, Shaffer RE. Effect of decontamination on the filtration efficiency of two filtering facepiece respirator models. J Int Soc Resp Prot. 2007;24:93‐107. [Google Scholar]
- 12. Viscusi DJ, Bergman MS, Eimer BC, Shaffer RE. Evaluation of five decontamination methods for filtering facepiece respirators. Ann Occup Hyg. 2009;53:815‐827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bergman MS, Viscusi DJ, Heimbuch BK, Wander JD, Sambol AR, Shaffer RE. Evaluation of multiple (3‐cycle) decontamination processing for filtering facepiece respirators. JEFF. 2010;4:33‐41. [Google Scholar]
- 14. Viscusi DJ, Bergman MS, Novak DA, et al. Impact of three biological decontamination methods on filtering facepiece respirator fit, odor, comfort, and donning ease. J Occup Environ Hyg. 2011;8:426‐436. [DOI] [PubMed] [Google Scholar]
- 15. Rutala WA, Weber DJ, The Healthcare Infection Control Practices Advisory Committee (HICPAC) . Guideline for disinfection and sterilization in healthcare facilities. Centers for Disease Control (US), 2008.
- 16. Salter W, Kinney K, Wallace W, Lumley L, Heimbuch BK, Wander JD. Analysis of residual chemical on filtering facepiece respirators after decontamination. J Occup Environ Hyg. 2010;7:437‐445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lore MB, Heimbuch BK, Brown TL, Wander JD, Hinrichs SH. Effectiveness of three decontamination treatments against influenza virus applied to filtering facepiece respirators. Ann Occup Hyg. 2012;56:92‐101. [DOI] [PubMed] [Google Scholar]
- 18. Fisher EM, Shaffer RE. A method to determine the available UV‐C dose for the decontamination of filtering facepiece respirators. J Appl Microbial. 2010;110:287‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Food and Drug Administration . FDA‐cleared sterilants and high level disinfectants with general claims for processing reusable medical and dental devices. 2015.
- 20. Chen CC, Huang SH. The effects of particle charge on the performance of a filtering facepiece. Am Ind Hyg Assoc J. 1998;59:227‐233. [DOI] [PubMed] [Google Scholar]
- 21. Lin TH, Tang FC, Chiang CH, Chang CP, Lai CY. Recovery of bacteria in filtering facepiece respirators and effects of artificial saliva/perspiration on bacterial survival and performance of respirators. Aerosol Air Qual Res. 2017;17:187‐197. [Google Scholar]
- 22. Lin TH, Chen CC, Huang SH, Kuo CW, Lai CY, Lin WY. Filter quality of electret masks in filtering 14.6–594 nm aerosol particles: Effects of five decontamination methods. PLoS ONE. 2017;12:e0186217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rebmann T, Carrico R, Wang J. Physiologic and other effects and compliance with long‐term respirator use among medical intensive care unit nurses. Am J Infect Control. 2013;41:1218‐1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Respiratory protection devices: final rule and notice. (42 CFR Part 84) Federal Register June 8, 1995; 60:30336‐30404. [Google Scholar]
- 25. Harnish DA, Heimbuch BK, Husband M, et al. Challenge of N95 filtering facepiece respirators with viable H1N1 influenza aerosols. Infect Control Hosp Epidemiol. 2013;34:494‐499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li CS, Hao ML, Lin WH, Chang CW, Wang CS. Evaluation of microbial samplers for bacterial microorganisms. Aerosol Sci Technol. 1999;30:100‐108. [Google Scholar]
- 27. Lee KW, Liu BYH. Theoretical study of aerosol filtration by fibrous filters. Aerosol Sci Technol. 1982;1:147‐161. [Google Scholar]
- 28. Lin WH, Li CS. The effect of sampling time and flow rates on the bioefficiency of three fungal spore sampling methods. Aerosol Sci Technol. 1998;28:511‐522. [Google Scholar]
- 29. Lin WH, Li CS. Influence of storage on the fungal concentration determination of impinger and filter samples. Am Ind Hyg Assoc J. 2003;64:102‐107. [DOI] [PubMed] [Google Scholar]
- 30. World Health Organization . Laboratory Biosafety Manual. Geneva: World Health Organization; 2004. [Google Scholar]
- 31. Wang Z, Reponen T, Willeke K, Grinspun S. Survival of bacteria on respirator filters. Aerosol Sci Technol. 1999;30:300‐308. [Google Scholar]
- 32. Sagripanti JL, Bonifacino A. Comparative sporicidal effect of liquid chemical germicides on three medical devices contaminated with spores of Bacillus subtilis . Am J Infect Control. 1996;24:364‐371. [DOI] [PubMed] [Google Scholar]


