Summary
Aims
To synthesise the evidence relating influenza and influenza‐like symptoms to the risks of myocardial infarction (MI), heart failure (HF) and stroke.
Methods
We conducted a systematic review and meta‐analysis of the evidence relating influenza and influenza‐like symptoms to the risks of MI, HF and stroke. We systematically searched all MEDLINE and EMBASE entries up to August 2014 for studies of influenza vs. the cardiovascular outcomes above. We conducted random effects meta‐analysis using inverse variance method for pooled odds ratios (OR) and evaluated statistical heterogeneity using the I 2 statistic.
Results
We identified 12 studies with a total of 84,003 participants. The pooled OR for risk of MI vs. influenza (serologically confirmed) was 1.27 (95% CI, confidence interval 0.54–2.95), I 2 = 47%, which was significant for the only study that adjusted for confounders (OR 5.50, 95% CI 1.31–23.13). The pooled OR for risk of MI vs. influenza‐like symptoms was 2.17 (95% CI 1.68–2.80), I 2 = 0%, which was significant for both unadjusted (OR 2.23, 95% CI 1.65–3.01, five studies) and adjusted studies (OR 2.01, 95% CI 1.24–3.27, two studies). We found one study that evaluated stroke risk, one study in patients with HF, and one that evaluated mortality from MI – all of these studies suggested increased risks of events with influenza‐like symptoms.
Conclusions
There is an association between influenza‐like illness and cardiovascular events, but the relationship is less clear with serologically diagnosed influenza. We recommend renewed efforts to apply current clinical guidelines and maximise the uptake of annual influenza immunisation among patients with cardiovascular diseases, to decrease their risks of MI and stroke.
Review criteria
We conducted a systematic review and meta‐analysis of the evidence relating influenza and influenza‐like symptoms to the risks of myocardial infarction (MI), heart failure (HF) and stroke.
We systematically searched all MEDLINE and EMBASE entries up to August 2014 for studies of influenza and influenza‐like symptoms vs. the cardiovascular outcomes above.
We conducted random effects meta‐analysis using inverse variance method for pooled odds ratios (OR) and evaluated statistical heterogeneity using the I 2 statistic.
Message for the clinic
There is an apparent association between influenza‐like illness and adverse cardiovascular events.
We recommend renewed efforts to apply current clinical guidelines and maximise the uptake of annual influenza immunisation among patients with cardiovascular diseases, to decrease their risks of MI and stroke.
Introduction
During a typical flu season in England and Wales, there are around 1.1 million extra consultations for acute respiratory infections, over 3000 excess hospital admissions, and around 12,500 deaths 1. In the H1N1 influenza pandemic, around 540,000 people in England had symptomatic H1N1 infection, with a case fatality rate of 26 deaths per 100,000 cases 2.
There is some observational evidence that major cardiovascular events are a prominent mechanism in deaths linked to influenza 3, 4. Excess mortality during influenza epidemics in Europe and the USA in the early 1900s was indeed attributed to causes other than influenza, such as heart disease 5, a finding replicated in more contemporary studies 6, 7, 8. Stroke also appears to be more common after a respiratory infection 9, 10, 11.
While some studies suggests that influenza may be a precursor to incident cardiovascular events, there remain inconsistencies in the literature. Several reviews have evaluated the potential for influenza to trigger cardiovascular events 3, 8, 12, 13, 14. These articles have summarised cardiovascular manifestations of influenza and described the direct effects of the virus on the myocardium 3, 14, as well as the potential mechanisms of acute coronary syndrome with infection 13.
While existing studies have shown some evidence that influenza may be associated with cardiovascular disease (CVD) there has been no published meta‐analysis of the association. In addition, these studies focused on coronary heart disease, while less is known about stroke and heart failure (HF).
In view of the uncertainty regarding the association between influenza and influenza‐like symptoms and risk of CVD, we conducted a systematic review and meta‐analysis to quantify the risk of myocardial infarction (MI), HF and stroke among patients recorded as having influenza and influenza‐like symptoms.
Methods
Eligibility criteria
We selected studies that evaluated the association between influenza and adverse cardiovascular outcomes. We included all study designs and placed no restriction on the definition of influenza that could be based on recording flu‐like symptoms or serologically confirmed cases. The end‐points were considered were MI, HF, stroke and cardiovascular death.
Search strategy
We searched (via OVID) MEDLINE and EMBASE from inception up to the end of August 2014 with no language limitations and using the broad free‐text and indexing search terms [(influenza or flu) AND ((myocardial infarction OR acute coronary syndrome OR ischemic heart disease OR ischaemic heart disease) OR (heart failure or cardiac failure or left ventricular impairment) OR (stroke OR cerebrovascular disease or cerebrovascular accident) OR (cardiac death OR cardiovascular death OR cardiovascular mortality)]. Additional relevant studies were identified by checking the bibliographies of included articles.
Study selection and data extraction
Two reviewers (CSK and SU) evaluated all titles and abstracts for studies that met the inclusion criteria and studies that did not clearly meet the selection criteria were excluded. One senior author (YKL) checked the potential inclusions and full reports (where available) of potentially relevant studies were retrieved and independently checked for eligibility. Two reviewers independently extracted data from included studies into a proforma spreadsheet covering study design; study location; characteristics of participants; definition of influenza; outcome ascertainment and results. The spreadsheet data were then checked for completeness and accuracy by a senior reviewer (YKL). Two reviewers (CSK and SU) then independently extracted the number of events and denominators from the sources papers, thereby enabling calculations of crude (unadjusted) estimates of the risk of adverse events among patients with influenza. Where raw results were not available, we collected results that were the most adjusted risk estimates [adjusted relative risk, odds ratio (OR), or hazard ratios] for cardiovascular events with influenza. Any differences were reconciled through the arbitration and further investigation of a senior clinical researcher (YKL). Authors of manuscripts were contacted to seek further information where necessary.
Assessment or risk of bias
The quality of included studies was evaluated using a risk of bias assessment including: ascertainment of exposure to influenza, ascertainment of selected cardiovascular outcomes and adjustments for potential confounders. Overall risk of bias of studies was deemed to be low if all three categories were satisfied and deemed to be moderate risk of bias if two categories were satisfied and high risk of bias if zero or one categories were satisfied. Where there was no evidence of substantial heterogeneity and more than 10 studies available for meta‐analysis, we used funnel plots to assess publication bias 15.
Statistical analysis
We used RevMan 5.2. (Nordic Cochrane Centre, Copenhagen, Denmark) to conduct a DerSimonian–Laird random effects meta‐analysis using an inverse variance weighting approach, to calculate a pooled OR. We assumed asymptotic convergence of risk ratio and OR as the proportion of adverse outcomes was low 16. We evaluated both adjusted and unadjusted data from primary studies, although we preferentially used adjusted data where available. We stratified the main analysis based on the measures of ascertaining influenza (e.g. laboratory serology tests or based on clinical presentation suggesting influenza‐like illness) and use of adjustments to account for potential confounders. If different types of influenza were reported, we used the category with the largest number of patients.
Statistical heterogeneity was assessed using I 2 statistic 17, with I 2 values of 30–60% representing a moderate level of heterogeneity.
Statistical results are presented as the main effect with 95% confidence intervals (CIs) in braces unless otherwise specified.
Results
Study design and participant characteristics
We identified 12 studies that met the inclusion criteria 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29. Details of the study selection are shown in Figure S1. There were seven case–control studies, two case‐cross‐over studies, two cohort studies and one self‐controlled case‐series study. There were a total of 84,003 participants. The mean age from four studies ranged from 42 to 65 years and median age ranged from 64 to 75 years from four other studies. The percentage of male patients ranged from 42% to 100%. Details are shown in Table 1.
Table 1.
Study design and participant characteristics
| Study ID | Study design | Country | Year | Participants | Mean age | % Male | Participant inclusion criteria |
|---|---|---|---|---|---|---|---|
| (a) Studies with influenza confirmed by laboratory testing | |||||||
| Nicholls 1977 | Cohort study | UK | May 1975 to July 1975 | 59 | NS | NS | Participants were admitted to the special coronary‐care unit of the King Edward VIII Hospital |
| Ponka 1981 | Case–control study | Finland | Jan 1980 to Mar 1980 | 49 cases of MI, 37 controls | 65 years (63 years in cases, 68 years in controls) | 55 (57% in cases and 51% in controls) | Participant cases had diagnosis of MI based on clinical history, electrocardiographical changes and elevated serum levels of MB fraction of creatine phosphokinase. Control patients had been admitted with acute non‐cardiac reason |
| Porter 1999 | Case–control study | USA | June 1978 to June 1980 | 118 cases, 20 controls | NS | NS | Participant cases had died with a malignant tumour and received chemotherapy. Participant controls died from acute myocardial infarction and were autopsied within 1 week |
| Guan 2012 | Case–control study | China | Oct 2005 to Mar 2006 and Oct 2006 to Mar 2007 | 102 cases, 150 controls | 56 years | 62 | Participants were inpatients and outpatients at a large teaching hospital of Harbin Medical University in China during influenza season of Oct 2005 to Mar 2007. Patients were newly diagnosed AMI inpatients at the Cardiac Care Unit from Nov 2005 to Jan 2006. Controls were current employees or retirees and received physical examinations every 2–3 years as required by their employers |
| Macintyre 2013 | Case–control study | Australia | 2008–2010 | 275 cases, 284 controls | 56% ≥ 65 years | 62 | Participant cases were age ≥ 40 years admitted with AMI to the cardiology unit during the influenza season, while controls were age ≥ 40 years attending orthopaedic or ophthalmic outpatient clinics |
| (b) Studies with influenza based on clinical features of influenza like illness | |||||||
| Mattila 1989 | Case–control study | Finland | NS | 40 cases of AMI, 41 controls, 30 chronic coronary heart disease | 42 years | 100 | Participant cases were admitted to the Helsinki University Central Hospital because of AMI and controls were patients with chronic coronary disease, who had been admitted to the hospital for coronary angiography or a random group of inhabitants of Helsinki |
| Zheng 1998 | Case cross‐over study | USA | NS | 2264 | NS | NS | Participants were in a database of an NHLBI‐sponsored study that has characterised triggering of MI by anger, physical exertion and sexual activity |
| Madjid 2007 | Cohort study | Russia | 1993–2000 | 34,892 | Median 75 years for women and 65 years for men | 43 | Participants were in residents of 10 districts of St Petersburg, Russia. Data from Public Health Service was used to determine weekly mortality based on autopsy results. Groups split into AMI and chronic ischaemic heart disease |
| Pesonen 2008 | Case–control study | Sweden | March 1999 to April 2002 | 110 cases, 323 controls | 63 years | 78 | Participant cases were admitted to the coronary‐care unit of Lund University Hospital for AMI or unstable angina pectoris and controls were selected from the population register and matched with respect to age, sex, living area, parish, season and the epidemiological status in the community |
| Warren‐Gash 2012 | Self‐controlled Case Series Study | UK | Jan 2003 to July 2009 | 3927 | Median age 73 years | 60 | Participants had records of both AMI and consultation for acute respiratory infection and were recorded in both the General Practice Register Database and MI National Audit Programme |
| Warren‐Gash 2013 | Case–control study | UK | Sept 2009 to Feb 2010 | 70 cases, 64 controls | Median 64 years | 79 | Participants were inpatients at the Royal Free London NHS Foundation Trust and cases had experienced AMI and controls were aged ≥ 40 years admitted with an acute surgical condition with no history of myocardial infarction within the past month |
| Luna 2014 | Case cross‐over study | USA | 2007–2009 | 41,148 | Median 74 years | 48 | Participants had HCUP/AHRQ administrative claims from all non‐federal hospitals in California |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Quality assessment of included studies
The study quality assessment is shown in Table S1. The methods for ascertaining exposure to influenza included the use of questionnaires, clinical assessments, review of patient records and laboratory testing. Seven studies used well‐characterised methods of ascertaining influenza exposure blood tests 18, 20, 22, 23, 24, lung tissue testing 26 and a validated algorithm 19. Apart from one study where ascertainment of outcome was unclear 29, all studies reported methods that could reliably identify cardiovascular events. Three studies reported use of adjustments for more than two potential confounders 18, 27, 28. In terms of risk of bias, one study was classified as low risk of bias 18 and three studies were classified as high risk of bias 21, 25, 29.
Influenza exposure and cardiovascular outcomes
Influenza exposure and cardiovascular outcome evaluation results are shown in Table 2. Influenza exposure was defined by serology or laboratory tests in four studies while in the other five studies it was based on symptoms and questionnaires. In Madjid et al., influenza epidemics were defined as weekly acute respiratory disease morbidity exceeding the predefined epidemic thresholds 21. The reporting of the follow‐up or timing of influenza exposure was variable. Three studies did not report any information on this 18, 20, 26, but among the other studies, influenza exposure ranged from the preceding week to preceding 3 months. Finally, nine studies evaluated MI as the cardiovascular outcome while one study evaluated death from MI 21, one study evaluated ischaemic stroke 19 and one study evaluated HF 24.
Table 2.
Exposures, outcomes and results of included studies
| Study ID | Exposure (influenza/infection) definition | Outcome definition | Timing of assessment | Result |
|---|---|---|---|---|
| (a) Studies with influenza confirmed by laboratory testing | ||||
| Nicholls 1977 | Influenza‐like illness within 7 days of admission with serological evidence of influenza infection | MI | Influenza‐like illness within 7 days before admission |
Influenza‐like illness: MI 13/13 vs. 25/46 control, OR 1.7 (0.5–5.6) Serological test for influenza A: MI 8/8 vs. 30/51 control Serological test for influenza A: heart failure (LVF or CCF) 3/8 vs. 4/51 control Influenza from antibodies: OR 0.9 (0.2–3.1) |
| Ponka 1981 | Participants were tested for influenza A using complement‐fixing antibodies by a standard microtest. Participants were asked if they had symptoms of infectious disease on admission or during the preceding 3 weeks | MI | Samples of blood taken on admission and 2 weeks later |
The frequency of flu‐like in MI group is 6/49 and in control group is 4/37, OR 1.2 (0.3–4.4) Frequency of influenza A on serological testing in MI group is 3/49 and in control group is 4/37, OR 0.5 (0.1–2.6) |
| Porter 1999 | Lung tissue samples from participants were grown on MDCK cells with added trypsin to test for influenza viruses | MI | Unclear |
Influenza A: MI 1/6 vs. 19/132 Influenza B: MI 0/1 vs. 20/137 Influenza (viral antigens in lung tissue): 1.0 (0.1–1.86) |
| Guan 2012 | Influenza virus A and B infection based on blood testing for serum IgG antibodies | AMI | Not reported | AMI and influenza A adjusted OR 5.5 (1.3–23.0), influenza B adjusted OR 20.3 (5.6–40.8) |
| Macintyre 2013 | Laboratory evidence of influenza | AMI | Not reported | Evidence of recent influenza infection and risk of AMI‐adjusted OR 1.07 (0.53–2.19) |
| (b) Studies with influenza based on clinical features of influenza‐like illness | ||||
| Mattila 1989 | Influenza‐like illness defined as the occurrence of a sore throat, nasal congestion or cough together with fever | AMI | Infectious symptoms within the preceding 3‐month period | Influenza‐like illness: AMI 11/40 vs. population control 4/41 vs. chronic coronary disease 4/30, OR 3.0 (1.1–8.2) |
| Zheng 1998 | Trained nurse used a standardised questionnaire to determine if participant had any flu‐like illness with a fever and sore throat or any other infection | MI | Infection during previous week | OR of MI for infections 1 day prior to MI onset OR 2.4 (1.7–3.4) compared with the seventh day prior to the onset |
| Madjid 2007 | Influenza epidemics defined as the weekly acute respiratory disease morbidity exceeded the predefined epidemic thresholds | AMI death | Deaths during influenza epidemics over a 7‐year period | Influenza epidemic and odds of death from AMI OR 1.30 (1.08–1.56) |
| Pesonen 2008 | Participants were asked about the presence of infection symptoms during the preceding 4 weeks using a questionnaire | MI | Symptoms within the previous 4 weeks | Symptoms of influenza‐like illness 2–3 vs. 1 or none and risk of MI OR 3.8 (1.4–10.8) |
| Warren‐Gash 2012 | Acute respiratory infection was extracted from data on primary care consultation | AMI | Acute respiratory infection up to 91 days previously | Risk of acute respiratory infection and AMI‐adjusted incidence ratio 1–3 days 4.19 (3.18–5.53), 4–7 days 2.69 (1.99–3.63), 8–14 days 1.66 (1.24–2.23), 15–28 days 1.41 (1.12–1.77), 29–91 days 1.05 (0.92–1.21) |
| Warren‐Gash 2013 | Influenza‐like illness defined by history of feeling feverish with either cough or sore throat within the last month from questionnaire and additional exposures were nasopharyngeal and throat swabs testing positive for influenza and IgA antibodies in serum | AMI | Influenza‐like illness within the last month | Influenza‐like illness and AMI‐adjusted OR 3.17 (0.61–16.47) |
| Luna 2014 | Influenza‐like illness with unclear definition | Ischaemic stroke | Influenza‐like illness up to 90 days previously | Influenza‐like illness and ischaemic stroke 15‐days adjusted OR 6.5 (2.2–19.7), 30‐days adjusted OR 3.7 (1.7–8.3), 90‐days adjusted OR 3.3 (1.9–5.8) |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Risk of myocardial infarction with influenza
Of the nine studies with a MI outcome, we excluded the study by Pesonen et al. since it compared the risk of MI at different influenza symptom levels, rather than symptoms vs. no symptoms 25. The remaining eight studies were then stratified into four studies, which used serology or laboratory tests to confirm influenza and seven studies that diagnosed influenza based on symptoms or clinical presentation. The pooled OR for the risk of MI with serologically diagnosed influenza was OR 1.27 (95% CI, 0.54–2.95), I 2 = 47% (956 participants), which was significant for the only study that adjusted for confounders (OR 5.50, 95% CI 1.31–23.13; Figure 1). The pooled OR for risk of MI with influenza‐like symptoms was 2.17 (95% CI 1.68–2.80), I 2 = 0%, 6658 participants, which was significant for both unadjusted OR 2.23 (95% CI 1.65–3.01, five studies, 2597 participants) and adjusted (OR 2.01, 95% CI 1.24–3.27, two studies, 4061 participants) studies – see Figure 2.
Figure 1.
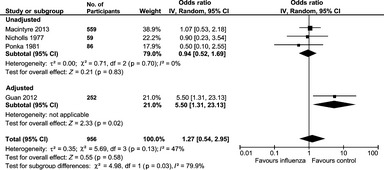
Risk of myocardial infarction with influenza based on serology or laboratory tests
Figure 2.
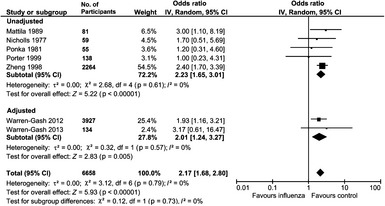
Risk of myocardial infarction with influenza‐like illness
Pesonen et al. found that two to three vs. one or no symptoms of influenza‐like illness was associated with increased risk of MI (OR 3.8, 95% CI 1.4–10.8) 25.
Risk of HF with influenza
The study by Nicholls et al. was the only study that evaluated the risk of HF with influenza 24. This study found that three of eight participants who tested positive for influenza A had HF (left ventricular failure or congestive cardiac failure) while four of the other 51 participants had HF. The crude OR for risk of HF from this study was 7.05 (95% CI 1.22–40.90).
Risk of stroke with influenza
The study by Luna et al. evaluated the timing of flu‐like illness and risk of ischaemic stroke 19. They found that the greatest risk was present within the first 15 days (adjusted OR 6.5, 95% CI 2.2–19.7) and decreased in magnitude with prolonged duration to adjusted OR 3.3 (95% CI 1.9–5.8) at 90 days.
Risk of cardiovascular mortality with influenza
Madjid et al. conducted a study that was not included in the meta‐analysis because it evaluated outcomes indirectly, namely autopsy rates of CVD vs. influenza epidemics 21. This was the only study that evaluated risk of cardiovascular mortality and found that that influenza epidemics were associated with increased odds of death from acute MI (OR 1.30, 95% CI 1.08–1.56).
Discussion
Our meta‐analysis suggests that influenza‐like illness is associated with a twofold increase in MI. The risk of MI with serologically defined influenza is less evident, however, the only study that adjusted for potential confounders showed an increased risk of MI with positive influenza tests. In addition, there is limited evidence that flu‐like illness precipitates both stroke and HF events, although these observations are based on just single publications, thus warranting further study.
A major challenge of studies which associate flu‐like symptoms and CVD is the spectrum of viral infections that can initiate flu‐like symptoms. A review of influenza‐like illness suggests that respiratory syncytial virus, rhinovirus, adenovirus, parainfluenza viruses and human coronaviruses can present similar to influenza 30. A study of influenza‐like illnesses over successive winters in the UK found that 480 of 2226 swabs (21.6%) were positive for respiratory syncytial virus 31. A smaller study in Scotland found that picornavirus was also an important cause of influenza‐like illness 32. Other studies have also identified rhinovirus 33, adenovirus 33, human corona virus 33 and parainfluenza virus 34 as important causes of influenza‐like illness.
Our findings suggest that exposure to influenza‐like illnesses may be associated with increased risk of cardiovascular events, hence measures such as influenza vaccination should be supported in line with current recommendations, particularly among patients who are at risk of CVDs.
The definition of influenza varied among studies in the current analysis since both serological tests and clinical assessments were used. We have observed that influenza‐like illness defined by clinical features, which may be caused by both influenza and other viral infections such as those highlighted above are associated with an increased risk of MI. This association appears to be absent in influenza cases defined by serology. There are a number of possible explanations that might account for this. The clinical features of influenza may be secondary to other viral infections so using clinical features may include other non‐influenza infections. Participants in whom influenza was diagnosed by serology may represent a different biological cohort than those patients in whom influenza diagnosis is based on clinical features. This may be because participants identified from serology may be asymptomatic, have atypical or milder symptoms because of infection with less virulent genotypes of influenza virus while participants who are diagnosed with influenza on clinical grounds might have more severe influenza infections which could manifest cardiovascular complications. Inclusion of asymptomatic cases may lead to a cohort with fewer cardiovascular events and reduced likelihood of detecting differences when compared with control group. Second, the timing of the serology test is important to determine if it is associated with cardiovascular event. Serology tests are able to measure the antibodies that develop in response to influenza virus but this response develops within 2–3 weeks of infection 35, 36, 37. Once developed, antibodies remain at detectable levels for months 38, thus making the test reliable in confirming influenza infection exposure. Consequently, if serological testing took place in the acute phase infection, the test result may represent a false negative. The quality of the serology test for influenza may also present a problem as high quality and standardised methods should be used to minimise variability in results. It is notable that two of the studies of serology took place in 1977 and 1981 and it is unclear, if the laboratory tests are as reliable and robust as those having been conducted more recently. Serological tests may detect cross‐reactive antibodies generated by previous exposure to antigenically similar viruses leading to false positive results. The current gold standard for laboratory confirmation of influenza virus infection is reverse transcription‐polymerase chain reaction or viral culture although this methodology was not used in studies included in the current analysis.
It is notable that there are differences between influenza and influenza‐like illness. True influenza, which is confirmed by serology, will not include other flu‐like mimics unless there is dual infection. However, serologically defined influenza infection will also include asymptomatic cases that may not be clinically relevant and may be missed unless a study defines a cohort and tests all participants regardless of whether they are symptomatic. Influenza‐like illness on the other hand may include other viral illnesses as previously described. This population will represent the clinically relevant cohort and may be larger than the true influenza cohort. However, it may be more heterogeneous in clinical features than the influenza cohort because it may include infections with other viruses. The ideal study should actually consider both in parallel. This can be conducted by taking a defined population and testing all participants for influenza and then prospectively collect data on whether participants develop symptoms of influenza. This study will allow understanding of the specificity of the influenza‐like illness that is unclear.
While we identified 12 studies of CVDs and patients with influenza and influenza‐like illness, and built on existing reviews. This study was the first to quantify the risk, to consider serological diagnosis separately from symptomatic diagnoses and to review the limited evidence for risk of stroke and HF with influenza‐like illness. However, many of the studies were of low quality and more than 10‐years old, so their findings may not generalise well to current clinical practice and settings.
While we found some evidence to support the role of influenza‐like illnesses as a trigger for cardiovascular events the precise mechanism is unclear. It has been suggested that influenza and influenza‐like illnesses may increase the risk of acute MI by mechanisms such as: antigenic cross‐reactivity; increased pro‐inflammatory and pro‐thrombotic cytokines; loss of anti‐inflammatory properties of HDL particles; increased trafficking of macrophages into the arterial wall; pronounced expression of inflammatory cytokines by infected monocytes; reduced clotting times and induction of pro‐coagulant activity by infected endothelial cells and increased expression of tissue factor 18. Coagulopathy and inflammation are thought to be key factors 39. Inflammation is also an important part of the atherosclerotic process and inflammatory cytokines such as TNF‐α and IL‐6 are increased in the context of studies of influenza in mice 40. Repeated influenza infection may injure the vascular endothelial cells and initiate the inflammatory response that is required to accelerate and enhance atherosclerosis development 18. Furthermore, viral infections can trigger the production of inflammatory cytokines, which could destabilise existing vulnerable plaques leading to MI 41.
It is worth noting that included studies were of high risk of bias because of their retrospective nature and study designs, which were case–control or case cross‐over. In addition, bias may arise from the variation in the way influenza was diagnosed which ranged from symptoms associated with respiratory infection to diagnoses based on serology tests. The most reliable diagnostic methodology is laboratory serological tests, as other chest infections or atypical respiratory infections may have clinical features consistent with influenza infection. However, even when serological tests were used to ascertain influenza infection, it is possible that patients who did not have serology positive influenza did not have flu and this may account for the dissimilarity between the two large cohorts evaluating public health records and the remaining studies. Furthermore, adjustments for potential confounders are important to reduce risk of bias. Only a limited number of studies in the current review adjusted for baseline variables and there is always the risk of unmeasured confounders.
Reporting bias represents a limitation in some of the studies included in the current review. Data from large databases may fail to capture asymptomatic cases of influenza, which do not present the health services or patients who self medicate and do not seek medical attention. Another limitation relates to the inclusion of older studies that used different diagnostic criteria for MI. The diagnostic criteria for MI in older studies were based mainly on clinical criteria, echocardiography, electrocardiographical changes and possible enzyme rises of creatinine kinase that are not as sensitive as high sensitivity troponin assays used in contemporary practice to detect myonecrosis, which may have resulted in under‐reporting of incident MI in older studies
Further studies should be conducted to evaluate the risk of CVD with influenza. These observational studies should be prospective in design with outcomes linked to a CVD register. Once potential cases of influenza are identified, further laboratory tests should be performed to confirm diagnosis of influenza. In addition, important potential confounders such as smoking, socioeconomic status, underlying chronic respiratory disease and other cardiovascular risk factors need to be accounted for in risk‐adjusted models.
Conclusions
In conclusion, there is an apparent association between influenza‐like illness and adverse cardiovascular events. This association is less clear for serologically defined influenza, which may be a limitation of published data and study designs – there are many biologically plausible mechanisms to explain the relationship. We recommend renewed efforts to apply current clinical guidelines and maximise the uptake of annual influenza immunisation among patients with CVDs, to decrease their risks of MI and stroke.
Author contributions
MAM conceptualised the review. CSK and YKL performed the literature search. CSK and SU screened the search results for relevant studies and extracted the data. CSK, EK and YKL were involved in the data‐analysis. CSK drafted the manuscript and all authors contributed in writing of the paper.
Supporting information
Table S1. Risk of bias assessment of included studies.
Figure S1. Flow diagram of study selection.
Disclosures
The authors have no conflicts of interest to declare.
References
- 1. Fleming DM. The contribution of influenza to combined acute respiratory infections, hospital admissions, and deaths in winter. Commun Dis Public Health 2000; 3: 32–8. [PubMed] [Google Scholar]
- 2. Donaldson LJ, Rutter PD, Ellis BM et al. Mortality from pandemic A/H1N1 2009 influenza in England: public health surveillance study. Br Med J 2009; 339: b5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mamas MA, Fraser D, Neyses L. Cardiovascular manifestations associated with influenza virus infection. Int J Cardiol 2008; 130: 304–9. [DOI] [PubMed] [Google Scholar]
- 4. Warren‐Gash C. Influenza and ischaemic heart disease: research challenges and future directions. Heart 2013; 99: 1795–6. [DOI] [PubMed] [Google Scholar]
- 5. Collins SC. Excess mortality form cause other than influenza and pneumonia during influenza epidemics. Public Health Rep 1932; 47: 2159–80.19315373 [Google Scholar]
- 6. Tillett HE, Smith JW, Gooch CD. Excess deaths attributed to influenza in England and Wales: age at death and certified cause. Int J Epidemiol 1983; 12: 344–52. [DOI] [PubMed] [Google Scholar]
- 7. Warren‐Gash C, Bhaskaran K, Hayward A et al. Circulating influenza virus, climatic factors, and acute myocardial infarction: a time series study in England and Wales and Hong Kong. J Infect Dis 2011; 203: 1710–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Madjid M, Naghavi M, Litovsky S et al. Influenza and cardiovascular disease: a new opportunity for prevention and the need for further studies. Circulation 2003; 108: 255–62. [DOI] [PubMed] [Google Scholar]
- 9. Smeeth L, Thomas SL, Hall AJ et al. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med 2004; 351: 2611–8. [DOI] [PubMed] [Google Scholar]
- 10. Grau AJ, Preusch MR, Palm F et al. Association of symptoms of chronic bronchitis and frequent flu‐like illnesses with stroke. Stroke 2009; 40: 3206–10. [DOI] [PubMed] [Google Scholar]
- 11. Pinol Ripoll G, de la Puerta I, Santos S et al. Chronic bronchitis and acute infections as new risk factor for ischemic stroke and the lack of protection offered by the influenza vaccination. Cerebrovasc Dis 2008; 26: 339–47. [DOI] [PubMed] [Google Scholar]
- 12. Warren‐Gash C, Smeeth L, Hayward AC. Influenza as a trigger for acute myocardial infarction or death from cardiovascular disease: a systematic review. Lancet Infect Dis 2009; 9: 601–10. [DOI] [PubMed] [Google Scholar]
- 13. Corrales‐Medina VF, Madjid M, Musher DM. Role of acute infection in triggering acute coronary syndrome. Lancet Infect Dis 2010; 10: 83–92. [DOI] [PubMed] [Google Scholar]
- 14. Estrabragh ZR, Mamas MA. The cardiovascular manifestations of influenza: a systematic review. Int J Cardiol 2013; 167: 2397–403. [DOI] [PubMed] [Google Scholar]
- 15. Ioannidis JP, Trikalinos TA. The appropriateness of asymmetry tests for publication bias in meta‐analysis: a large survey. CMAJ 2007; 176: 1091–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Davies HT, Crombie IK, Tavakoli M. When can odds ratios mislead? Br Med J 1998; 316: 989–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Higgins JP, Thompson SG, Deeks JJ et al. Measuring inconsistency in meta‐analysis. Br Med J 2003; 327: 557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guan X, Yang W, Sun X et al. Association of influenza virus infection and inflammatory cytokines with acute myocardial infarction. Inflamm Res 2012; 61: 591–8. [DOI] [PubMed] [Google Scholar]
- 19. Luna JM, Kamel H, Willey J et al. Influenza‐like illness is associated with risk of ischemic stroke: a case‐crossover analysis. Stroke 2014; 45: ATP163. [Google Scholar]
- 20. Macintyre CR, Heywood AE, Kovoor P et al. Ischaemic heart disease, influenza and influenza vaccination: a prospective case control study. Heart 2013; 99: 1843–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Madjid M, Millder CC, Zarubaev VV et al. Influenza epidemics and acute respiratory disease activity are associated with a surge in autopsy‐confirmed coronary heart disease death: results from 8 years of autopsies in 34892 subjects. Eur Heart J 2007; 28: 1205–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mattila KJ. Viral and bacterial infections in patients with acute myocardial infarction. J Intern Med 1989; 225: 293–6. [DOI] [PubMed] [Google Scholar]
- 23. Ponka A, Jalanko H, Ponka T et al. Viral and mycoplasmal antibodies in patients with myocardial infarction. Ann Clin Res 1981; 13: 429–32. [PubMed] [Google Scholar]
- 24. Nicholls AC, Thomas M. Coxsackie virus infection in acute myocardial infarction. Lancet 1977; 1: 883–4. [DOI] [PubMed] [Google Scholar]
- 25. Pesonen E, Andsberg E, Grubb A et al. Elevated infection parameters and infection symptoms predict an acute coronary events. Ther Adv Cardiovasc Dis 2008; 2: 419–24. [DOI] [PubMed] [Google Scholar]
- 26. Porter DD, Porter HG. Respiratory viral antigens in autopsy lung tissue specimens from patients with cancer of myocardial infarction. Clin Infect Dis 1999; 29: 437–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Warren‐Gash C, Hayward AC, Hemingway H et al. Influenza infection and risk of acute myocardial infarction in England and Wales: a CALIBER self‐controlled case series study. J Infect Dis 2012; 206: 1652–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Warren‐Gash C, Geretti AM, Hamilton G et al. Influenza‐like illness in acute myocardial infarction patients during the winter wave of the influenza A H1N1 pandemic in London: a case‐control study. BMJ Open 2013; 3: piie002604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zheng ZJ, Mittelman M, Tofler G et al. Triggers of myocardial infarction and sudden death. J Am Coll Cardiol 1998; 31: 132A. [Google Scholar]
- 30. Kelly H, Birch C. The causes and diagnosis of influenza‐like illness. Aust Fam Physician 2004; 33: 305–9. [PubMed] [Google Scholar]
- 31. Zambon MC, Stockton JD, Clewley JP et al. Contribution of influenza and respiratory syncytial virus to community cases of influenza‐like illness: an observational study. Lancet 2001; 358: 1410–6. [DOI] [PubMed] [Google Scholar]
- 32. Wallace LA, Collins TC, Douglas JD et al. Virological surveillance of influenza‐like illness in the community using PCR and serology. J Clin Virol 2004; 31: 40–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Huo X, Qin Y, Qi X et al. Surveillance of 16 respiratory viruses in patients with influenza‐like illness in Nanjing China. J Med Virol 2012; 84: 1980–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Iwane MK, Edwards KM, Szilagyi PG et al. Population‐based surveillance of hospitalizations associated with respiratory syncytial virus, influenza virual and parainfluenza viruses among young children. Pediatrics 2004; 113: 1758–64. [DOI] [PubMed] [Google Scholar]
- 35. Miller E, Hoschler K, Hardelid P et al. Incidence of 2009 pandemic influenza A H1N1 infection in England: a cross‐sectional serological study. Lancet 2010; 375: 1100–8. [DOI] [PubMed] [Google Scholar]
- 36. Vequilla V, Hancock K, Schiffer J et al. Sensitivity and specificity of serologic assays for detection of human infection with 2009 pandemic H1N1 virus in U.S. populations. J Clin Microbiol 2011; 49: 2210–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen MI, Barr IG, Koh GCH et al. Serological response in RT‐PCR confirmed H1N1‐2009 influenza A by hemagglutination inhibition and virus neutralization assays: an observational study. PLoS ONE 2010; 5: e12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Laurie KL, Huston P, Riley S et al. Influenza serological studies to inform public health action: best practices to optimise timing, quality and reporting. Influenza Other Respir Viruses 2013; 7: 211–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Marsden PA. Inflammation and coagulation in the cardiovascular system: the contribution of influenza. Circ Res 2006; 99: 1152–3. [DOI] [PubMed] [Google Scholar]
- 40. Szretter KJ, Gangappa S, Lu X et al. Role of host cytokine responses in the pathogenesis of avian H5N1 influenza viruses in mice. J Virol 2007; 81: 2736–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Naghavi M, Barlas Z, Siadaty S et al. Association of influenza vaccination and reduced risk of recurrent myocardial infarction. Circulation 2000; 102: 3039–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Risk of bias assessment of included studies.
Figure S1. Flow diagram of study selection.


