Abstract
The indoor environment of a mechanically ventilated hospital building controls infection rates as well as influences patients’ healing processes and overall medical outcomes. This review covers the scientific research that has assessed patients’ medical outcomes concerning at least one indoor environmental parameter related to building heating, ventilation, and air conditioning (HVAC) systems, such as indoor air temperature, relative humidity, and indoor air ventilation parameters. Research related to the naturally ventilated hospital buildings was outside the scope of this review article. After 1998, a total of 899 papers were identified that fit the inclusion criteria of this study. Of these, 176 papers have been included in this review to understand the relationship between the health outcomes of a patient and the indoor environment of a mechanically ventilated hospital building. The purpose of this literature review was to summarize how indoor environmental parameters related to mechanical ventilation systems of a hospital building are impacting patients. This review suggests that there is a need for future interdisciplinary collaborative research to quantify the optimum range for HVAC parameters considering airborne exposures and patients’ positive medical outcomes.
Keywords: ambient temperature, indoor air quality, indoor environment of hospital buildings, mechanical ventilation, patient outcomes, relative humidity
Practical Implications.
The indoor environment of a mechanically ventilated hospital building controls infectious disease transmission and influences patients’ outcomes.
A summary of the recommended optimum ranges of temperature, relative humidity, ventilation rates, air filtration, differential pressure control, and ventilation strategies will be beneficial for the patients’ wellbeing.
These findings from the published scientific literature will be helpful for researchers, policy‐makers, healthcare and building design professionals in enhancing standards related to the HVAC systems considering patients’ outcomes.
Future interdisciplinary collaborative research has been identified and can lead to specific optimum ranges for indoor environmental parameters in different spaces for hospital buildings.
1. INTRODUCTION
Surgical site infections (SSIs) are infections that occur after surgery,1 and they are the most common nosocomial infections.2, 3 According to a Centers for Disease Control (CDC) and Prevention report, 31% of hospitalized patients get infected by SSIs among all other health care‐associated infections (HAI).3 This nosocomial infection can cause morbidity and even contribute to mortality.4, 5 SSI incidence is dependent on surgical sterilization procedures, mechanical ventilation systems, high‐efficiency particulate absorbing (HEPA) filters, ultraviolet radiation, air renewal, humidity control, temperature, differential air pressure, particle count, surface colony count, and antibiotic prophylaxis.6, 7, 8, 9
The importance of the indoor environment on patients’ healing processes was initially mentioned by Florence Nightingale10 and is one of the major issues to which healthcare professionals, environmental psychologists, consultants, and architects are giving priority.11, 12, 13, 14, 15, 16, 17, 18, 19 The indoor environment controls infection rates and influences the overall patient outcome.19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34 Appropriately designed building heating, ventilation, and air conditioning (HVAC) systems can enhance patients’ recovery process,35 can reduce the length of hospital stay,15, 18, 25, 36, 37 can lessen medical errors and infection rates,20, 23, 38 and can improve the indoor air quality (IAQ) and minimize HAI.39 An improved indoor environment of a hospital building can reduce costs associated with airborne illnesses by 9%‐20%.40
To establish the relationship between health outcomes and the physical environment, Rubin et al30 identified 85 relevant studies where parameters including room size, room privacy, interior design of a room, patient control of his/her environment, music, lighting, exposure to sunlight, window view of nature, ventilation system contaminants, humidity, and temperature have been reviewed. Among these studies on the effect of healthcare environment on patient outcomes, seven were on humidity; four on air filtration system; four on ventilation system; two on temperature; one on (increase outside air changes, improve filter efficiency, maintain constant temperature and humidity, increase positive pressure of operating room air). They30 concluded that there is convincing evidence linking patients’ clinical outcomes and built environment parameters. Zimring et al32 identified a connection between the hospital indoor environment and patient and staff outcomes with respect to four sectors: staff stress and fatigue reduction and increased effectiveness in delivering care; patient safety improvement; stress reduction and improvement in patient outcomes; and overall healthcare quality improvement. Dijkstra et al23 summarized literature on environmental stimuli (eg, furniture, art, color, nature, plants, gardens, carpeting, room size, spatial layout, private rooms, noise, music, odor, television/video, light, windows, and view from a window) and their impact on patients’ psychological outcomes rather than on direct physiological outcomes. Huisman et al25 reviewed hospital interior layouts and their relationship with medical staff error, patient falls, infection rates, indoor quality, comfort, building materials, visual comfort, acoustics, view, and privacy. They covered the indoor environment (eg, ambient temperature, humidity, ventilation strategies, and air quality) under the subtopic of safety and security, not patients’ medical outcomes.25
Previous studies related to the hospital building's indoor environment discretely focused on patients’ thermal comfort,35, 41, 42, 43, 44, 45 acoustic comfort,46 visual comfort, and IAQ.47, 48, 49, 50 The impacts of single or multiple indoor environmental parameters related to the mechanical ventilation system on overall patient outcomes have not been summarized yet. Hence, this review covers the findings from the published scientific literature on the associations of temperature, relative humidity, ventilation rate, air filter, differential pressure, and ventilation strategy, with patient outcomes. This literature review considered articles published after 1998 and was restricted to codes, guidelines, and standards published by professional societies, licensing agencies, and regulatory organizations. Studies related to natural ventilation for infection control are outside of the scope of this review. Additionally, parameters related to building design, architecture, interior design, noise, aroma, and lighting are being excluded from the scope of this literature review, since this review exclusively focuses on parameters related to the HVAC systems.
This review will help researchers, policymakers, healthcare, and building design professionals to understand the importance of indoor environmental parameters and provide information for enhancing standards related to the HVAC systems in attaining positive medical outcomes for patients. The review also identified avenues for future interdisciplinary collaborative research to quantify the optimum range for indoor environmental parameters considering patients’ positive medical outcomes.
2. METHODOLOGY OF THE REVIEW PROCESS
A multidisciplinary reviewing process was adopted to find out both quantitative and qualitative academic research evidence on indoor environmental parameters and their impact on patients’ medical outcomes. PubMed [Medline], JSTOR, ScienceDirect, Scopus bibliographic databases, Google Scholar, and Texas A&M University Library databases were searched for 58 keywords. Combination keywords, such as “ambient temperature AND patient outcomes,” “mechanical ventilation system AND patient outcomes,” “indoor air quality AND patient outcomes,” “airflow AND patient,” “mechanical ventilation AND infection,” “physical environment AND patient outcomes,” and others were used as well to confine the search area. Scientific publications in the fields of both hospital buildings and parameters related to the HVAC system of a mechanically ventilated building were reviewed. Also, the citations in each study found during the main search were reviewed for potential relevance. This paper includes relevant articles that were published after the review done by Rubin et al.30
Through a systematic review process, a total of 1871 abstracts were screened, with a total of 899 papers being identified as relevant to the scope of this study. These articles went through a full‐text review process and were excluded if the patient outcomes were not biological and physiological, or if the built environment parameters are not related to the building HVAC system, variables such as noise, aroma, light, and other building layouts and interior design. Additionally, research on naturally ventilated hospital buildings was excluded as well since this review article solely focuses on the parameters related to the mechanical ventilation system of a hospital building. As a result, 176 articles have been included in this review paper. However, articles that partially fulfilled the objective of this review were included. For example, papers concentrating on both patient and staff outcomes were evaluated, though only patients’ outcomes related study were included in this review paper. Among 176 papers in this review, 133 investigated patients’ outcomes as a function of at least one indoor environmental parameter related to building HVAC systems.
The article selection process is shown in Figure 1. All the references of these articles were verified and crosschecked.
Figure 1.
Diagram showing the methodology of the review process
3. INDOOR ENVIRONMENTAL PARAMETERS IMPACTING PATIENT OUTCOMES
Patient outcomes are dependent on the indoor environment of a hospital building.19, 33, 51, 52, 53 Previous research on evidence‐based design for healthcare facilities has established that hospital‐acquired infection rates are directly related to IAQ.17, 25, 45, 54, 55, 56, 57 Patients’ psychological health is affected by poor indoor environment.58, 59 Studies have demonstrated an association between environmental variables and “Sick building syndrome” (SBS).60, 61, 62 Patients and elderly in hospitals and nursing homes are sensitive to these specific building‐related illness caused by SBS; they are hypersensitivity pneumonitis; building‐related asthma; and legionellosis.61 Environmental parameters can be modified to improve the physical environment and promote patients’ positive health outcomes.15, 16, 32, 33
Kameel and Khalil63 have mentioned that HVAC supply air temperature and RH can inhibit the growth of bacteria and activate or deactivate viruses. Lutz et al64 identified a positive correlation between infection rates and parameters related to the indoor environment (eg, type of air filter, the direction of airflow and air pressure, air changes per hour (ACH) in a room, humidity, and ventilation system cleaning and maintenance). Codinhoto et al22 summarized the built environment parameters relating to health outcomes of both patient and healthcare professionals by proposing a framework and grouped these environmental parameters to “ergonomics,” “fabric and ambient,” “art and esthetics,” and “services.” To propose a framework based on a cause‐effect relationship, they categorized patient health outcomes under three sections: psychological, physical, and physiological.22 They concluded that the indoor environment of a healthcare facility has a considerable impact on patients’ health outcomes.22 Rashid and Zimring65 have developed a framework relating indoor environment with patient stress as an outcome. Ulrich et al66 analyzed nine built environment parameters (eg, audio environment, visual environment, safety enhancement, way‐finding system, sustainability, patient room, family support spaces, staff support spaces, and physician support spaces) with respect to patient recovery.
This review focuses on summarizing published literature that investigates the impact of each or multiple indoor environmental parameters on patient outcomes. The following section will categorize studies related to patient medical outcomes under indoor air temperature, relative humidity, indoor air ventilation rate, air filtration system, differential pressure control, and mechanical ventilation strategies.
3.1. Indoor air temperature
3.1.1. Indoor air temperature and nosocomial pathogens
Potential airborne pathogens such as bacteria, viruses, and fungi can pose severe health effects.67 The susceptibility of patients to nosocomial pathogens depends on the pathogen's survivability on various surfaces.68, 69 Temperature is one of the major factors that influence the transmission and survivability of these microorganisms.70, 71, 72
Low ambient temperature increases influenza virus transmission since the survivability of infectious agents rises.73, 74 The optimum temperature to control the survival of airborne influenza viruses is as high as 30°C (86°F) at 50% relative humidity,75 which will create an uncomfortable indoor environment as per ASHRAE1 Standard 55.76 Through an experimental study, Lowen et al73, 77 concluded that at 20°C (68°F) influenza virus transmission is dependent on humidity, but at the higher temperature (30°C; 86°F), the transmission was eliminated regardless of relative humidity. Low temperature is associated with longer persistence of most viruses, such as the astrovirus,78 adenovirus, poliovirus, herpes simplex virus, and hepatitis A virus.68, 69 Most bacteria, fungi, and viruses are more stable and persist longer at low temperatures, such as 4°C (39.2°F) or 6°C (42.8°F).69 Tang75 focusing on the disease‐oriented evidence reviewed the survival of airborne infectious bacteria in relation to indoor air temperature. He concluded that temperatures above about 24°C decrease the survival of gram‐negative, gram‐positive, and intracellular bacteria.75 Clinically relevant airborne fungi, potentially life‐threatening for immunocompromised patients are Aspergillus species (Aspergillus flavus and Aspergillus fumigatus)75, 79; Blastomyces; Coccidioides; Cryptococcus; and Histoplasma species.80 Unlike the laboratory‐based testing for viruses and bacteria, air sampling testing to identify the presence or absence of fungi and their spores in natural settings revealed higher spore counts at a higher temperature,75, 81, 82 although, based on the literature review, Kramer et al69, 71 concluded opposite findings.
3.1.2. Indoor air temperature and thermal comfort perception
Indoor air temperature is important for patients’ thermal comfort perception.83 Thermal comfort has an impact on patients’ healing processes,35, 84 satisfaction with surgical care,85 well‐being, and safety.35, 86, 87, 88 Due to medication and drug use, a patient's thermoregulatory system affects the overall perception of thermal comfort.89 Uncomfortable environments have negative effects on patients, such as sleeplessness and restlessness,86 and can cause shivering, inattentiveness, and muscular and joint tension.63, 85, 90, 91, 92, 93, 94 Maintaining thermal comfort in an operating room (OR) is a challenge since the situation varies with the surgery types, various patient requirements, various activity levels of hospital staff, different interior settings of lights and equipment, and the total number of occupied people at a certain time.43, 95 It is recommended to modulate the OR temperature according to the need of each surgery type for optimum comfort level.19, 35, 43
3.1.3. Optimum temperature and patient's thermal risks
In hospitals, researchers recommended separate thermal zones to address the different needs of patients, and their separate thermal preferences43, 45, 96, 97, 98 summarize the recommended optimum temperatures to prevent the thermal risk of patients (Table 1). Patients’ thermal status99, 100 and a low ambient OR temperature101, 102, 103, 104, 105 are the main reasons that cause patients’ intraoperative hypothermia. Studies have identified the correlation between low ambient room temperature and hypothermia among patients during the perianesthesia or perioperative period.101, 106, 107, 108 Since high ambient temperature (>23°C or 73.4°F) is required to avoid perioperative hypothermia, it may be found uncomfortable for the OR personnel.109, 110
Table 1.
Recommended optimum temperature for different spaces in a hospital building
| Spaces in a hospital building | Optimum temperature set point | References |
|---|---|---|
| Operating room | >26°C (78.8°F) | De Witte and Sessler235 |
| 24‐26°C (74.2‐78.8°F) | Balaras et al,128 Sadrizadeh, and Loomans137 | |
| ≥21°C (69.8°F) | Melhado et al,43 Khodakarami, and Nasrollahi35 | |
| Postoperative care area/room | ≥24°C (75°F) | Hooper et al236 |
| Intraoperative area/room | 20‐25°C (68‐77°F) | Hooper et al,236 Association of Perioperative Registered Nurses,237 Morris,104 Morris and Wilkey,105 Wang et al238 |
| Delivery room | ≥26°C (78.8°F) | Knobel et al,239 Knobel and Holditch240 |
| Nursery (for infants) | Around 28°C (82.4°F) | Lyon and Freer125 |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Surgical site infection is one of the leading effects of even mild hypothermia, where a 1.9°C (3.42°F) reduction in core body temperature increases the chance of SSI three times in a patient after colorectal surgery.90 Mild perioperative core hypothermia may increase the risk of wound infection,90, 111 increase the length of hospital stay,90, 112, 113 increase blood loss,114 cause cardiac complications,112 and cause a prolonged post‐anesthetic recovery.113 Perioperative hypothermia poses a relative risk of severe complications, such as cardiac events,115, 116, 117, 118 blood loss,114 impaired wound healing,115, 116, 117 wound infections,119 an increased rate of morbidity and mortality,114, 115, 120, 121 length of hospital stay, and the cost of treatment.115, 116, 117
Higher ambient temperature is recommended during anesthetic induction and surgical skin preparation; conversely, a lower ambient temperature is recommended before surgical incision.19 However, this method has limited effectiveness among adult patients because of the time interval for warming the room is relatively brief and requires wide swings in temperature to have a significant clinical effect.122 A study on critically ill trauma patients confirmed that there is no correlation between the decrease of ambient OR temperature and patient core body temperature with effective use of active warming strategies on patients.123, 124 Controlling indoor air temperature is crucial for other severely ill patients (eg, burn victims) where the application of active warming strategy is difficult.19, 86 Thermal stability is important for preterm infants to reduce harmful side effects, such as delayed adaptation to extrauterine life, hypoglycemia, respiratory distress, hypoxia, metabolic acidosis, coagulation defects, acute renal failure, necrotizing enterocolitis, and failure to gain weight or weight loss and morbidity.125, 126, 127
3.2. Relative humidity (RH)
3.2.1. Influence of relative humidity on infectious disease transmission
In hospital buildings, evidence has confirmed that RH affects infection control because it is related to the growth and transfer of airborne bacteria,128 some strains of viruses, and fungi.35, 83, 129 A strong correlation has been found between the transmission of viruses and absolute humidity,74 and viruses outbreak when the vapor content of the air decrease.130 Several controlled studies concluded that both RH and humidity ratio values influence the survival of viruses and bacteria.69, 74, 131, 132, 133, 134
An RH level higher than 45%‐50% promotes fungal growth indoors75, 83, 135, 136 and affects the concentrations of allergens, bacteria,98, 137 and increase the settling rate of aerosols.136 Most fungal species cannot grow when RH is below 60%.69 High humidity levels support microbial growth due to moisture absorption by building materials.136 Pathogenic microorganisms can adhere quickly to moist and slick or damaged walls and ceilings138 which can affect patient and staff well‐being.139, 140, 141, 142
Relative humidity has an impact on the viability of both airborne and droplet transmission of viruses.75, 143 However, this relationship is quite complex. Both lipid‐enveloped and non‐lipid‐enveloped viruses are less stable at relative humidities between 40% and 70%,143 while an ideal range for the airborne influenza survival is 23%‐81%.73 A study131 found that at 23% RH, the infectivity of influenza virus is as high as 71% to 77%, whereas at higher RH level (43% RH) the infectivity found 16% to 22%. Based on an experimental study, Lowen et al73 concluded that airborne transmission of influenza virus was maximum at 20%‐35% RH, and poor at 50% RH. Tang et al134 summarized that higher RH (>50%) is favorable to viruses without a lipid envelope, for example, poliovirus.144 However, lipid‐enveloped viruses, for example, influenza,73, 74 Lassa fever virus, and human coronavirus survive longer in low RH (<50%) and their persistence eliminate at RH >80%.134 Findings on the effects of RH on the survival of airborne bacteria appear to be more complicated than with viruses.75, 143, 144 A literature review69 on the persistence of nosocomial pathogens on any intimate surfaces concluded that at higher humidity most types of bacteria persist longer and spore concentrations of most fungi were higher. However, conflicting results were found on the on the persistence of clinically relevant pathogens. Table 2 summarizes research findings on favorable RH ranges for the growth and survivability of various nosocomial pathogens and microorganisms.
Table 2.
Favorable relative humidity (RH) ranges for the survivability of nosocomial pathogens and microorganisms
| Example | Relative humidity (RH) | References | |
|---|---|---|---|
| Virus | |||
| Viruses (with lipid envelops) | Respiratory viruses (Influenza virus, Para‐Influenza virus, Corona virus, Respiratory syncytial virus, Herpes simplex virus, Measles virus, Rubella virus, and Varicella zoster virus) | Lower RH (20%‐30% RH) | Tang,75 Kramer et al69 |
| Influenza, Lassa fever virus, and Human coronavirus (hCV) | Lower RH (<50% RH) | Tang et al,134 Noti et al131 | |
| Influenza | 20%‐35% RH | Lowen et al73 | |
| Influenza | 23%‐43% RH | Noti et al131 | |
| Viruses (non‐lipid enveloped) | Adenovirus, Enterovirus, and Rhinoviruses | Higher RH (70%‐90% RH) | Tang,75 Kramer et al69 |
| Poliovirus | Higher RH (>50% RH) | Tang et al134 | |
| Bacteria | |||
| Gram‐negative bacteria | Pseudomonas spp., Enterobacter spp., Klebsiella spp., Salmonella seftenberg, Pseudomonas aeruginosa, Chlamydia trachomatis | Higher RH | Tang,75 Tang et al134 |
| Serratia marcescens, Escherichia coli, Klebsiella pneumoniae, and Proteus vulgaris | Lower RH (<50% RH) | Tang,75 Kramer et al,69 Tang et al,134 Cox144 | |
| Airborne gram‐positive bacteria | Staphylococcus epidermidis, Streptococcus haemolyticus, Bacillus subtilis, and Streptococcus pneumoniae | Lower RH (<50% RH) | Tang,75 Tang et al134 |
| Enterococcus faecalis | Lower RH | Robine et al241 | |
| Listeria monocytogenes | Higher RH | Kramer et al,69 Tang et al134 | |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
3.2.2. Impact on indoor comfort
In a hospital building, RH levels are related to patients’ indoor thermal comfort and hygiene of spaces.35, 128 Low humidity levels can affect patients' indoor comfort perception145, 146 and can cause irritations,63, 146 dry skin and nose,35, 63 and throat irritation.35, 63, 145, 146 Dryness can promote blood coagulation, which is undesirable for patients during surgery.35, 128, 137 Research confirmed that for preterm infants, higher humidity levels along with a warm temperature are recommended to control evaporative heat loss.147, 148 A study confirmed that if the humidity of the incubator decreased below 60%, then the infant body temperature decreased by as much as 1°C (33.8°F) within 5 minutes.148 To reduce evaporative heat loss among newborns, increasing the humidity level is the most effective option.125 Balaras et al128 suggested the recommended RH range for a hospital building should be 30% to 60%.
3.3. Indoor air ventilation
Pathogens and other respiratory viruses, such as influenza,134, 149, 150, 151 SARS‐associated coronavirus (SARS‐CoV),134, 152, 153 tuberculosis,134, 152, 154 Q‐fever, and measles152 can be transmitted through an airborne route. There is sufficient evidence to support that indoor air ventilation can contribute to the spread of airborne infectious disease in hospitals.152, 155, 156, 157, 158, 159, 160, 161, 162 Several literature reviews covering both naturally and mechanically ventilated buildings have examined the impact of ventilation on health outcomes.152, 159, 163, 164, 165, 166 Some of these covered other building types along with hospitals.152, 163, 164, 165
The following subsections summarize suggested ventilation requirements considering airborne pathogen transmission. Primarily, based on previous research findings, this will review the optimum range of ventilation rates or flow rates for positive patient outcomes. Additionally, ventilation strategies, air filtration systems, and desirable room pressurization with respect to the adjacent areas in a hospital building will be reviewed. This review excluded all published standards and guidelines (eg, ANSI/ASHRAE/ASHE Standard 170,167 FGI,168 AIA,169 ASHRAE Handbook,170 2013 ASHRAE HVAC Design Manual for Hospitals and Clinics171). that specify mandatory or recommended requirements including ventilation rates, filtration, and pressure relationships. Additionally, studies on naturally ventilated hospital buildings are being excluded since this review exclusively focuses on parameters related to the mechanical ventilation systems.
3.3.1. Ventilation rate
Ventilation rates are measured as ACH, that is, how many times the air in a defined space is replaced per hour. Several comprehensive literature reviews on ACH and infectious disease transmission concluded that there is insufficient evidence to specify the minimum and maximum ventilation requirements in hospitals based on infection control risk to patients.152, 164, 172, 173 English166 reviewed the ventilation guidelines of US hospitals and concluded that the effect of ventilation requirements on general infection rates is still unidentified except in ORs174 and airborne isolation rooms.175 English166 showed a chronological summary of air change rates in hospitals, where establishing the relationship with patient outcomes was outside of his scope.
Although many studies observed that lower ventilation rates could increase the risk of airborne cross‐infection,154, 160, 174, 175, 176 increasing the ACH only may not always be advantageous for the patients’ well‐being from the infection risk perspective.164, 173, 177, 178, 179, 180, 181, 182 Using numerical modeling through computational fluid dynamics (CFD) analysis, Memarzadeh and Xu177 suggested that in an enclosed mechanically ventilated room (eg, an isolation room) increasing the airflow rate (ie, ACH) may not be the major contributing factor to control infections transmission. Instead, the ventilation system design and the distance from the contaminant source are important factors than flow rate.177 They177 suggested uninterrupted path between the contaminant source and the exhaust to control contaminants. Grosskopf and Mousavi183 had a similar conclusion for general patient and isolation patient test room studies. Results from another study conducted in the field environmental chamber concluded that increasing supply ACH might escalate the airborne infection risk transmission under certain circumstances (eg, position of the source and the susceptible person in relation to the supply and return air grills).179 A study in a simulated two‐bed hospital isolation room with mixing air distribution system showed that the elevated ventilation rates might increase the risk of airborne cross‐infection.173 The exposure level depended on the positioning and distance from the source, and posture of the infected patients.173 The recommended 12 ACH in the present standards and guidelines resulted in draft discomfort within the occupied zone due to higher air velocities.173
Conversely, across‐sectional observational study by Menzies et al154 showed a higher tuberculosis infection risk for healthcare workers in non‐isolation rooms (eg, general patient rooms) with ventilation rates of less than 2 ACH. Another study87 of ventilation performance in patient rooms showed that a ventilation rate of 4 ACH with supplemental heating and cooling would be favorable in terms of thermal comfort, uniformity, and ventilation effectiveness. Results also suggested that six ACH is optimum, and similar conclusions have been found for hospital isolation rooms.87 Through a CFD study, Memarzadeh and Jiang175 suggested that total ventilation rates over 10 ACH will not be effective for Airborne Infection Isolation Rooms (AIIR) since this flow rate did not decrease the exposure to infectious disease transmission. Based on the review on aerosol‐transmitted infections in the isolation room, Tang et al134 recommended minimum 12 ACH, so that the air moves from healthcare workers to a patient. They134 also suggested that placing patients close to the exhaust vent will reduce the cross‐contamination risks. A study following airflow modeling and particle tracking methodologies concluded that an OR with ceiling heights between 2.74 m (9 ft) and 3.66 m (12 ft) should maintain 20‐25 ACH for contamination control.184 A study on 4‐bed patient rooms showed a minor reduction in infectious disease transmission through hand colonization when ventilation rates change from four ACH to six ACH.185 The results of a CFD simulation in the general wards of Hong Kong hospitals showed that a flow rate of nine ACH effectively minimized infection risk of three respiratory viruses.186 Table 3 below summarizes the recommended minimum total ACH of the supply air and outside air comparison between Ninomura and Bartley187 and English.166
Table 3.
Comparison of total ACH of the supply air and outside air recommendation in different hospital building spaces
| Different spaces in a hospital building | Minimum total ACH/outside ACH | |
|---|---|---|
| Ninomura and Bartley187 | English166 | |
| Patient room | 6‐4 ACH/2 ACH | 4 ACH/2 ACH |
| Labor/Delivery/Recovery/Postpartum | 6‐4 ACH/2 ACH | 6 ACH/2 ACH |
| AIIR | 12 ACH/2 ACH | – |
| Emergency rooms and radiology—waiting and triage rooms | 12 ACH/2 ACH | – |
| Procedure rooms/operating rooms | 15 ACH/3 ACH | 6 ACH/2 ACH |
| Nursery | – | 6 ACH/2 ACH |
| Anesthetic storage | – | 8 ACH/– |
| Patient corridor | – | 2 ACH/– |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Based on results discussed above, it is evident that the current air distribution methods practiced today are insufficient in order to control the spread of infectious disease within a hospital environment. Along with ACH, the contamination risk depends on the (a) positioning and distance of the susceptible person (eg, caregiver; patient) from the infected source; (b) position of both susceptible person and infected source in relation to the supply and return air grills; (c) posture of the infected source; (d) air velocities; and (e) air distribution pattern.
3.3.2. Ventilation strategies
The ventilation strategy and air distribution pattern in a hospital building are correlated with the airborne transmission of infectious agents.152, 158, 188, 189, 190 This section will evaluate the role of various ventilation strategies in removing airborne pathogens from different spaces in hospitals.
CFD analysis found that in patient rooms, displacement ventilation made larger bioaerosols (>10 μm) suspend in the air for longer periods, whereas smaller particles were able to escape the space.191 Another experimental study concluded that in multiple bed patient rooms, the spacing between beds should be farther apart with the displacement ventilation strategy compared with mixing the air.192 Qian et al192 also concluded that the exhaled nuclei droplet from infected patients penetrates long distances during displacement ventilation, and takes longer to dissipate than mixing ventilation strategies.
A comparative experimental study for hospital wards showed that the displacement ventilation system would have higher contaminant concentration than a mixing ventilation system if the auxiliary exhaust is located to the lower part of the wall.193 However, when the exhaust was relocated at the upper part of the wall, the displacement ventilation at 4 ACH showed lower contaminant concentration than traditional mixing ventilation at six ACH.193 Another study with a similar conclusion added that, for better performance, supply air diffusers should be unobstructed and located at a lower level and toilet transfer grilles at a high level.194 Compared with pure mixing ventilation, the displacement ventilation increases the cross‐infection risk 12 times when two persons (source and target) are at face‐to‐face and face‐to‐side position at 0.35 m distance.181 The contaminant concentration profile showed that the displacement ventilation showed higher risk of transmission close to the contaminant source and exhaust, compared with a mixing ventilation system.193, 194, 195 Guity et al194 also concluded that the displacement ventilation delivers much lower contaminant concentration in areas further away from the patient.
Conversely, a study on two floor‐supply‐type ventilation flow patterns showed that unidirectional–upward system was more efficient in removing the smallest droplet nuclei (<1.5 μm), but the single‐side‐floor system was effective at removing large droplets and droplet nuclei.188 Another study on supply air inlet locations showed that the underfloor air distribution system performs better in reducing bioaerosol concentration than the ceiling type and side wall supply systems.196
Experimental test chamber results and Eulerian‐Lagrangian computations revealed that the mixing ventilation system has a positive influence on bioaerosol dispersion.176 A simulation of the hospital ward with a ceiling‐mixing type ventilation system showed that the dispersions of airborne contaminants were significantly affected by the location of the exhaust air vents.197 They also concluded that the decay rate of contaminant concentration is exponential with a complete mixing ventilation system.197 Using an engineering computational technique for the isolation room, researchers have found that the parallel‐directional airflow pattern and staggered air‐supply and exhaust vents positioning can efficiently control infectious disease contamination.198 This study also concluded that the ceiling to floor level ventilation airflow resulted in poor infection control.198 An analytical CFD study in hospital ward showed that when the air was supplied and extracted through the ceiling, it was more effective in removing airborne pathogens compared with other strategies.189
After reviewing 20 ORs, Balaras et al128 summarized that for quicker dissipation of contaminated air, laminar downward airflow was found to be effective with air changes ranging from 3.2 to 58 ACH. Another simulated study showed that positioning the ventilation grills at the ceiling removes the aerosol more quickly than the wall ventilation system.189 For downward air movement, Khalil199 recommended the supply air outlets need to be located at the ceiling and the exhaust inlets on the opposite walls. The optimum location of supply outlets is crucial to reduce the residence time of pollutants efficiently.95 In ORs, laminar airflow can limit surgical‐site infections by lessening the bacterial air contamination.65, 200, 201, 202 Based on routine surveillance data on hospital ORs, Brandt et al8 concluded that ventilation with vertical laminar airflow was associated with a higher risk for severe SSIs. To control the contamination through microbiological organisms, a laminar airflow system promotes high ACH at low supply air speed.200
Based on above discussion, while designing ventilation strategy in any spaces within a hospital building, the posture and distance between two persons (source and target), the location of diffusers (inlet and outlet vents), and air change rates are important factors in reducing contaminant concentration. It is worth to mention that these test results are either simulation‐based or an outcome from the experimental test chamber, which are usually done in a static environment without considering real‐world scenarios (eg, provider traffic).
3.3.3. Air filtration system
A high‐efficiency particulate air (HEPA) filtration system can reduce the load of bacteria, which is the most common cause of hospital‐associated infections203 and other infectious particles.204, 205, 206, 207, 208, 209, 210, 211 HEPA filters were found to be effective for patients having environmental fungal contamination, such as invasive fungal infections (IFIs) caused by construction near hospitals.207, 211, 212, 213, 214, 215, 216 This filtration system can decrease the airborne concentrations of aerosolized pathogens and viruses.217, 218 Full outside air ventilation along with return air through HEPA filtration is capable of reducing the concentration of droplet nuclei with 30%‐90% effectiveness.219 However, HEPA filter within ducts can become a breeding ground of microorganisms which may significantly contaminate the filtered air.64, 220, 221, 222 Lutz et al64 found that the insulation and filter materials are vulnerable to fungal degradation under high humidity. Due to the ineffectiveness of HEPA filters in reducing environmental fungal spore counts,223 researcher emphasized the importance of terminal filtrations system,64 photocatalytic oxidation application,221 and portable HEPA filtration units223 in areas with vulnerable immunocompromised patients.
HEPA filters can add pressure losses, which results in higher fan power, greater water quality control (for legionella), and moisture control due to the impact of moisture content on the survival of pathogens.83, 167 Memarzadeh70 suggested that for effective patient outcomes, these filtration systems should be paired with higher ACH.
3.3.4. Differential pressure control
Differential pressure is important to control the contamination of airborne infectious agents through airflows between the protective and less protective spaces of a hospital building.157, 224, 225, 226 In a hospital building, pressurization or depressurization relative to its surroundings needs to be maintained in the microbiology laboratories, the anteroom to AIIRs, AIIRs, autopsy suites, bronchoscopy rooms, emergency department and radiology waiting rooms, and ORs or surgical rooms.226 AIIRs need to be maintained at negative differential air pressure (“negative” means that the air pressure of the area is lower than the adjacent spaces) to avoid contamination from patients with highly infectious diseases (ASHRAE 170‐2008 as cited in Aliabadi et al161). Sterilizing spaces and service zones, such as laundry and bathrooms, should be negatively pressurized.161 In contrast, surgery rooms should continuously maintain positive differential air pressure to avoid particle infiltration.161, 227 Protective environment rooms for immunocompromised patients (eg, AIDS) need to be kept at positive differential air pressure.161, 168, 224 To control the IAQ, Ninomura and Bartley187 summarized that the critical care; AIIRs; bronchoscopy rooms; and endoscopy rooms should be negatively pressurized with respect to its adjacent areas, whereas, diagnostic and treatment area; ORs; pharmacy; sterile storage; and clean linen storage should be positively pressurized.187
After reviewing international standards, Kao and Yang198 mentioned that the minimum pressure differential requirements between the isolation and non‐isolation zone varied from 2.5 to 30 Pa. US guidelines recommend the minimum pressure difference for the AIIR should be 2.5 Pa (0.01 in. of H2O).157, 169, 224, 225, 228 The Curry International Tuberculosis Center229 mentioned that a small negative pressure might not be adequately maintained due to external factors, such as fluctuating air currents caused by elevators, doors, or windows to the outside. They suggested a minimum of 7.5 Pa (0.03 in. of H2O) differential pressure between the isolation room and the anteroom following the recommendation from the California Division of Occupational Safety and Health (Cal/OSHA).229 Depending on the need and restrictions for surgery rooms and adjacent spaces, 5‐15 Pa (0.02‐0.06 in. of H2O) differential pressure was recommended,227 while others suggested up to 20‐25 Pa (0.08‐0.1 in. of H2O).230
Experimental tracer gas studies performed in a test chamber simulating an AIIR showed that the pressure differential −15.0 Pa (0.06 in. of H2O) could effectively reduce the risk of infectious disease contamination.231 According to Streifel,228 the minimum differential pressure for ORs and protective environment rooms should be 0.25 Pa (0.001 in. of H2O). A multi‐zone airflow simulation study concluded that the leakage in room pressure control could significantly affect contaminant transfer.232 This emphasizes the importance of considering leakage in achieving design pressure differentials.232 Results from the tracer containment testing of AIIR showed that due to people's movement, differential pressure 15 Pa (0.06 in. of H2O) might not even effectively prevent the migration of air volume.233 Adams et al234 compared the containment efficiency in an anteroom‐equipped hospital AIIR at varied differential pressure (2.5, 11, and 20 Pa) in the presence or absence of care provider movement. They concluded that the higher pressure differential (>2.5 Pa or 0.01 in. of H2O) would control the contamination effectively with and without provider traffic.234
Based on the above discussion, it is important to note that in a hospital building critically important spaces need to be pressurized or depressurized with respect to its surroundings. To prevent contamination by infected patients, negative air pressure inside the room (eg, AIIR) relative to the surrounding areas should be maintained by mechanically removing (exhausting) more air than is supplied. Conversely, to protect patients (eg, immunocompromised patients in OR) from airborne infectious agents, the room needs to be positively pressurized. While designing and controlling this inlet and outlet airflows disequilibrium in a mechanically ventilated hospital building, the movement of people and leakages through doors, windows, or cracks need to be considered.
4. CONCLUSION
4.1. Summary of literature findings
In a hospital building, the built environment can have a beneficial impact on patients’ healing processes.12, 22, 23, 25, 29, 30, 32, 34, 35, 38, 43 This review covered the published research that has assessed patients’ medical outcomes with respect to at least one indoor environmental parameter related to the mechanical ventilation system of a building including temperature, relative humidity, and overall IAQ. Scientific publications in the fields of both healthcare and building HVAC systems published after the review paper of Rubin et al30 have been included in this review process. Studies related to the natural ventilation system, building design, architecture, interior design, noise, aroma, and lighting were outside of the scope of this review. This review summarized peer‐reviewed papers on how indoor environmental parameters related to the mechanical ventilation systems of a hospital building impact patient outcomes.
Higher indoor air temperature is recommended to control the survivability and transmission of most bacteria,75 fungi,69, 71 and viruses.68, 69, 73, 74, 75, 78 Low ambient OR temperatures are critical for patients, which lead researchers to recommend the optimum temperature to prevent thermal risk, with a minimum suggested temperature for ORs of 21°C (69.8°F),35, 43 and a maximum of 26°C (78.8°F)128, 137, 235 or higher.235 Based on the existing literature, this recommended range varies depending on the types of surgery, patient demographics (eg, age, gender), and OR personnel.
Most bacteria, fungi, and viruses persist longer at higher humidity (eg, >70%) and infectivity lessens exposure in the RH range between 40% and 70%.69 Higher RH (50% RH or higher) is recommended to control the transmission of lipid‐enveloped viruses, for example, influenza.73, 74, 131 However, lower RH (50% RH or lower) is suggested for viruses without a lipid envelope.75 For most gram‐negative bacteria (eg, Escherichia coli; Klebsiella pneumonia), higher RH (50% RH or higher) is detrimental to their survival,69, 75, 134, 144 except a few (eg, Pseudomonas spp., Enterobacter spp., Klebsiella spp., and Salmonella Seftenberg).75, 134 Similarly, higher RH is recommended to control airborne gram‐positive bacteria.75, 134 Figure 2 summarizes the favorable RH ranges for the survivability of nosocomial pathogens and microorganisms. Higher RH is also suggested for hospitals to avoid thermal discomfort145, 146 and other negative consequences due to dryness.35, 63, 128, 145, 146 It is evident from these findings that maintaining certain RH ranges may be detrimental for some microorganisms but favorable for others. Guidelines for humidity ranges in any hospital areas should consider both survival and infectivity of airborne‐transmitted infectious disease control and thermal comfort.
Figure 2.
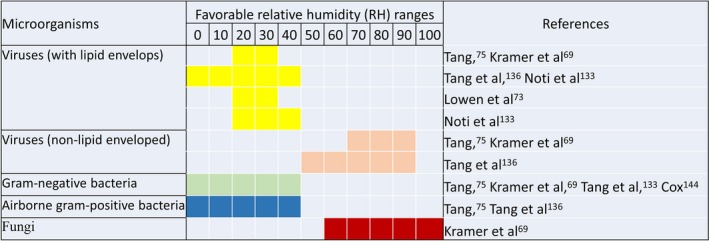
Diagram of favorable RH ranges for different microorganisms
This review found that ventilation rates, ventilation strategies, air filtration, and differential pressure control can contribute to the spread of airborne infectious disease in hospitals, although specific recommendations are institution specific. The evidence indicates that higher ventilation rates may reduce the infection rate in several situations. However, the maximum required ventilation rates (above which there is no further reduction of infection risk) at different spaces in hospitals are yet unknown,164 and it depends on other parameters, such as air distribution pattern; position and distance of the susceptible person from both source and air diffusers; and position and posture of infected source.134, 173, 177, 179, 183
Results from both experimental and computational studies confirmed that for patient rooms, the mixing ventilation strategies showed better contamination control and lowered the infectious disease transmission risks.176, 181, 189, 192, 193, 194, 195 For multiple bed patient rooms, displacement ventilation strategies may not be suitable unless beds are placed apart; the exhaust is located at the upper part of the wall; and unobstructed supply air diffusers are located at a lower level of the wall. Additionally, the posture and distance between two persons (source and target) have impacts on the contaminant concentration profile. These results are highly dependent on a subject's position and distance from the source of the contaminant181, 194; location of the vents189, 193, 194, 196, 197, 198, 199; and ventilation rates.128, 176 Along with appropriate ventilation strategies, the HEPA filtration system can effectively reduce the contamination load.204, 205, 206, 207, 208, 209, 210, 211, 217, 218, 219 However, maintaining the differential air pressure with respect to the adjacent spaces is very critical since the HEPA filters within ducts may have limited control over airborne nosocomial infections due to contaminated air from adjacent spaces.220 In order to control airborne contamination through pressurization‐depressurization in critical areas of a hospital building, important considerations are leakage in room pressure control232 and provider traffic.233, 234 The comprehensive ventilation guidelines for different zones within a hospital require patient‐oriented evidence incorporating ACH along with the (a) positioning and distance of the susceptible person (eg, caregiver; patient) from the infected source; (b) position of both susceptible person and contaminated source in relation to the supply and return air grills; (c) posture of the infected source; (d) air velocities; (e) air distribution pattern; and (f) location of the air filtration system.
4.2. Recommendations
Based on this literature review, temperature, humidity, and the indoor air ventilation system in hospital buildings affect various infectious organisms, which then have an effect on patient outcomes. Published results contain contradictory findings, which made the comparative assessment difficult due to inconsistency in experimental design, choice of variables, location and settings, demographics, diseases, patients, and the types of outcome measurements. Hence, it is impossible to make evidence‐based decisions regarding the optimum ranges to improve patient‐oriented outcomes such as symptoms, morbidity, quality of life, or mortality. These contradictory results of the current research suggest that all indoor environmental parameters related to the HVAC system need to be measured or included in the comparative analysis of each study. Additionally, a common set of variables need to be defined for comparative analysis.
A few epidemiological studies have been undertaken specifically to investigate the suitable ranges of multiple indoor environmental parameters (eg, temperature, RH, ACH); there is little patient‐oriented evidence to formulate guidelines for hospitals. While extensive simulation‐based research has been performed, very little patient‐oriented evidence has been produced. For validation, simulations and experiments need to correlate by physical measurements. Additional multidisciplinary studies including researchers, patients, building owners, facility managers, and maintenance staffs studies are needed, which would address evidence‐based decisions regarding the optimum ranges to improve patient‐oriented outcomes.
Studies that look at nosocomial infection rates, the spread of infection within hospitals, and associated costs are potential avenues of research. A multidisciplinary study combining available molecular biology testing, advanced computer modeling, experimental testing, and on‐site experimental designs could provide evidence to identify optimum ranges for temperature, humidity, and ACH along with appropriate ventilation design strategies. It is also necessary to address these variables as a function of spaces within a hospital since each zone has unique occupants and different functionality. Additionally, the structural variation of infectious agents (ie, viruses, bacteria, and fungi) may need to be considered separately when investigating airborne survival since each will have differing conditions under which they may be optimally suppressed. Finally, the relationship between IEQ variables, thermal comfort perception of patients, and airborne contamination need to be investigated. The health effects of ventilation in locations with highly polluted outdoor air and other diverse outdoor conditions present an important area of future research.
ACKNOWLEDGEMENTS
We specially acknowledge the Energy Systems Laboratory, Texas A&M University for support.
Shajahan A, Culp CH, Williamson B. Effects of indoor environmental parameters related to building heating, ventilation, and air conditioning systems on patients' medical outcomes: A review of scientific research on hospital buildings. Indoor Air. 2019;29:161–176. 10.1111/ina.12531
ENDNOTE
ASHRAE was formed as the American Society of Heating, Refrigerating, and Air‐Conditioning Engineers and from 2012, ASHRAE began doing business as “ASHRAE.” https://www.ashrae.org/about://www.ashrae.org/about.
REFERENCES
- 1. Centers for Disease Control and Prevention (CDC) . [CDC website]. May 17, 2012. https://www.cdc.gov/hai/ssi/ssi.html. Accessed January 31, 2017.
- 2. Ananda BB, Raj S, Ramesh BS. Clinical study of causative factors, precautionary measures and the treatment of surgical site infections (SSIs) in elective general surgery cases at Dr BR AMCH. Organ. 2016;5:8‐92. [Google Scholar]
- 3. Magill SS, Hellinger W, Cohen J, et al. Prevalence of healthcare‐associated infections in acute care hospitals in Jacksonville, Florida. Infect Control Hosp Epidemiol. 2012;33:283‐291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Horan TC, Culver DH, Gaynes RP, Jarvis WR, Edwards JR, Reid CR, National Nosocomial Infections Surveillance (NNIS) System . Nosocomial infections in surgical patients in the United States, January 1986‐June 1992. Infect Control Hosp Epidemiol. 1993;14:73–80. [DOI] [PubMed] [Google Scholar]
- 5. Poulsen KB, Wachmann CH, Bremmelgaard A, Sörensen AI, Raahave D, Petersen JV. Survival of patients with surgical wound infection: a case‐control study of common surgical interventions. Br J Surg. 1995;82:208–209. [DOI] [PubMed] [Google Scholar]
- 6. Kampf G. The six golden rules to improve compliance in hand hygiene. J Hosp Infect. 2004;56:3–5. [DOI] [PubMed] [Google Scholar]
- 7. Tumia N, Ashcroft GP. Convection warmers‐a possible source of contamination in laminar airflow operating theatres? J Hosp Infect. 2002;52(3):171–174. [DOI] [PubMed] [Google Scholar]
- 8. Brandt C, Hott U, Sohr D, Daschner F, Gastmeier P, Rüden H. Operating room ventilation with laminar airflow shows no protective effect on the surgical site infection rate in orthopedic and abdominal surgery. Ann Surg. 2008;248:695–700. [DOI] [PubMed] [Google Scholar]
- 9. Alfonso‐Sanchez JL, Martinez IM, Martín‐Moreno JM, González RS, Botía F. Analyzing the risk factors influencing surgical site infections: the site of environmental factors. Can J Surg. 2017;60:155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nightingale F. Notes on Nursing. London: Harrison; 1859:59. [Google Scholar]
- 11. Ulrich RS. How design impacts wellness. Healthc Forum J. 1992;35:20–25. [PubMed] [Google Scholar]
- 12. Ulrich RS. Effects of interior design on wellness: theory and recent scientific research. J Health Care Interior Des. 1991;3:97–109. [PubMed] [Google Scholar]
- 13. Ulrich RS. Environmental research and critical care In: Hamilton DK, ed. ICU 2010: Design for the Future. Houston: Center for Innovation in Health Facilities; 2000:195–207. [Google Scholar]
- 14. Ulrich RS. Evidence based environmental design for improving medical outcomes In: Proceedings of the Healing by Design: Building for Health Care in the 21st Century Conference, Montreal, Quebec, Canada; 2000:20:3.1‐3.10. [Google Scholar]
- 15. Devlin AS, Arneill AB. Health care environments and patient outcomes: A review of the literature. Environ Behav. 2003;35:665–694. [Google Scholar]
- 16. Jonas WB, Chez RA. Toward optimal healing environments in health care. J Altern Complement Med. 2004;10:S‐1. [DOI] [PubMed] [Google Scholar]
- 17. Schweitzer M, Gilpin L, Frampton S. Healing spaces: elements of environmental design that make an impact on health. J Altern Complement Med. 2004;10:S‐71. [DOI] [PubMed] [Google Scholar]
- 18. Sklavou E, Tzouvadakis I. Healing environment and evidence‐based design (EBD): the international experience and the case of Greece. Archiv Hellenic Med/Arheia Ellenikes Iatrikes. 2012;29:154–161. [Google Scholar]
- 19. Katz JD. Control of the environment in the operating room. Anesth Analg. 2017;125:1214–1218. [DOI] [PubMed] [Google Scholar]
- 20. Chaudhury H, Mahmood A, Valente M. The effect of environmental design on reducing nursing errors and increasing efficiency in acute care settings: a review and analysis of the literature. Environ Behav. 2009;41:755–786. [Google Scholar]
- 21. Choi JH, Beltran LO, Kim HS. Impacts of indoor daylight environments on patient average length of stay (ALOS) in a healthcare facility. Build Environ. 2012;50:65–75. [Google Scholar]
- 22. Codinhoto R, Tzortzopoulos P, Kagioglou M, Aouad G, Cooper R. The impacts of the built environment on health outcomes. Facilities. 2009;27:138–151. [Google Scholar]
- 23. Dijkstra K, Pieterse M, Pruyn A. Physical environmental stimuli that turn healthcare facilities into healing environments through psychologically mediated effects: systematic review. J Adv Nurs. 2006;56(2):166–181. [DOI] [PubMed] [Google Scholar]
- 24. Ghazali R, Abbas MY. Paediatric community: healing environment conducive enough? Procedia‐Soc Behav Sci. 2012;42:42–54. [Google Scholar]
- 25. Huisman ER, Morales E, van Hoof J, Kort HS. Healing environment: a review of the impact of physical environmental factors on users. Build Environ. 2012;58:70–80. [Google Scholar]
- 26. Joseph A. The impact of light on outcomes in healthcare settings. Center Health Des. 2006;https://www.healthdesign.org/chd/research/impact-light-outcomes-healthcare-settings. Accessed Jannuary 17, 2018 [Google Scholar]
- 27. Joseph A. The Impact of the Environment on Infections in Healthcare Facilities. Concord, CA: Center for Health Design; 2006:19. [Google Scholar]
- 28. Joseph A. The Role of the Physical Environment in Promoting Health, Safety, and Effectiveness in the Healthcare Workplace. Research Reports & Papers. Concord, CA: Center for Health Design; 2006. [Google Scholar]
- 29. Joseph A, Ulrich R. Sound Control for Improved Outcomes in Healthcare Settings. Concord, CA: Center for Health Design; 2007. [Google Scholar]
- 30. Rubin HR, Owens AJ, Golden G. Status Report (1998): An Investigation to Determine Whether the Built Environment Affects Patients' Medical Outcomes. Martinez, CA: Center for Health Design; 1998. [PubMed] [Google Scholar]
- 31. Stichler JF. Creating healing environments in critical care units. Crit Care Nurs Q. 2001;24:1–20. [DOI] [PubMed] [Google Scholar]
- 32. Zimring C, Joseph A, Choudhary R. The Role of the Physical Environment in the Hospital of the 21st Century: A Once‐In‐A‐Lifetime Opportunity. Concord, CA: The Center for Health Design; 2004. [Google Scholar]
- 33. Ulrich RS, Zimring C, Zhu X, et al. A review of the research literature on evidence‐based healthcare design. Health Environ Res Des J. 2008;1:61–125. [DOI] [PubMed] [Google Scholar]
- 34. Van DenBerg AE. Health Impacts of Healing Environments: A Review of Evidence for Benefits of Nature, Daylight, Fresh Air, and Quiet in Healthcare Settings. Groningen, Netherlands: Foundation 200 years University Hospital Groningen; 2005. [Google Scholar]
- 35. Khodakarami J, Nasrollahi N. Thermal comfort in hospitals—a literature review. Renew Sustain Energy Rev. 2012;16:4071–4077. [Google Scholar]
- 36. Calkins MP. Evidence‐based long term care design. NeuroRehabilitation. 2009;25:145–154. [DOI] [PubMed] [Google Scholar]
- 37. Nimlyat PS, Kandar MZ. Appraisal of indoor environmental quality (IEQ) in healthcare facilities: a literature review. Sustain Cities Soc. 2015;17:61–68. [Google Scholar]
- 38. Andrade C, Lima ML, Fornara F, Bonaiuto M. Users' views of hospital environmental quality: validation of the perceived hospital environment quality indicators (PHEQIs). J Environ Psychol. 2012;32:97–111. [Google Scholar]
- 39. Hendron R, Leach M, Bonnema E, Shekhar D, Advanced Pless S. Energy Retrofit Guide (AERG): Practical Ways to Improve Energy Performance; Healthcare Facilities (Book). Retrieved from U.S. Department of Energy: https://www.nrel.gov/docs/fy13osti/57864.pdf; 2013. [Google Scholar]
- 40. Singer BC. Hospital Energy Benchmarking Guidance. Retrieved from Lawrence Berkeley National Laboratory, Berkeley CA. 2009; https://www.osti.gov/servlets/purl/974318-kOtEMd/.
- 41. Azizpour F, Moghimi S, Salleh E, Mat S, Lim CH, Sopian K. Thermal comfort assessment of large‐scale hospitals in tropical climates: A case study of University Kebangsaan Malaysia Medical Centre (UKMMC). Energy Build. 2013;64:317–322. [Google Scholar]
- 42. Lomas KJ, Giridharan R. Thermal comfort standards, measured internal temperatures and thermal resilience to climate change of free‐running buildings: a case‐study of hospital wards. Build Environ. 2012;55:57–72. [Google Scholar]
- 43. Melhado MA, Hensen JL, Loomans M. Literature review of staff thermal comfort and patient thermal risks in operating rooms In 8th International Healthy Buildings Conference. 2006:11–14. [Google Scholar]
- 44. Pourshaghaghy A, Omidvari M. Examination of thermal comfort in a hospital using PMV–PPD model. Appl Ergon. 2012;43:1089–1095. [DOI] [PubMed] [Google Scholar]
- 45. Skoog J, Fransson N, Jagemar L. Thermal environment in Swedish hospitals: summer and winter measurements. Energy Build. 2005;37:872–877. [Google Scholar]
- 46. Xie H, Kang J, Mills GH. Clinical review: the impact of noise on patients' sleep and the effectiveness of noise reduction strategies in intensive care units. Crit Care. 2009;13:208 10.1186/cc7154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dascalaki EG, Lagoudi A, Balaras CA, Gaglia AG. Air quality in hospital operating rooms. Build Environ. 2008;43:1945–1952. [Google Scholar]
- 48. Hellgren U. Indoor Air Problems in Finnish Hospitals‐From the Occupational Health Perspective. Helsinki: University of Helsinki; 2012. [Google Scholar]
- 49. Hellgren U, Hyvärinen M, Holopainen R, Reijula K. Perceived indoor air quality, air‐related symptoms and ventilation in Finnish hospitals. Int J Occup Med Environ Health. 2011;24:48–56. [DOI] [PubMed] [Google Scholar]
- 50. Mendes ACP. Indoor air quality in hospital environments. 20th Congress of IFHE XXVI Seminario de IH, Congreso Nacional. 2008.
- 51. Gesler W, Bell M, Curtis S, Hubbard P, Francis S. Therapy by design: evaluating the UK hospital building program. Health Place. 2004;10:117–128. [DOI] [PubMed] [Google Scholar]
- 52. Rollins JA. Evidence‐based hospital design improves health care outcomes for patients, families, and staff. Pediat Nurs. 2004;30:338–339. [PubMed] [Google Scholar]
- 53. Whitehouse S, Varni JW, Seid M, et al. Evaluating a children's hospital garden environment: utilization and consumer satisfaction. J Environ Psychol. 2001;21:301–314. [Google Scholar]
- 54. Ulrich RS. Effects of interior design on wellness: theory and recent scientific research. J Healthc Interior Des. 1991;3:97–109. [PubMed] [Google Scholar]
- 55. Sadler BL, DuBose J, Zimring C. The business case for building better hospitals through evidence‐based design. Health Environ Res Des J. 2008;1:22–39. [DOI] [PubMed] [Google Scholar]
- 56. Zimring C, Denham ME, Jacob JT, et al. Evidence‐based design of healthcare facilities: opportunities for research and practice in infection prevention. Infect Control Hosp Epidemiol. 2013;34:514–516. [DOI] [PubMed] [Google Scholar]
- 57. Campion N, Thiel CL, Focareta J, Bilec MM. Understanding green building design and healthcare outcomes: evidence‐based design analysis of an oncology unit. J Archit Eng. 2016;22:04016009. [Google Scholar]
- 58. Mahbob NS, Kamaruzzaman SN, Salleh N, Sulaiman R. A correlation studies of indoor environmental quality (IEQ) towards productive workplace In: 2nd International Conference on Environmental Science and Technology IPCBEE. 2011;6:434–438. [Google Scholar]
- 59. Sadek AH, Nofal EM. Effects of indoor environmental quality on occupant satisfaction in healing environments. Build Simul Cairo‐Towards Sustain Green Life. 2013; https://www.researchgate.net/profile/Eslam_Nofal/publication/258510534_Effects_of_Indoor_Environmental_Quality_on_Occupant_Satisfaction_in_Healing_Environments/links/02e7e5286929065e1d000000.pdf. Accessed April 25, 2018. [Google Scholar]
- 60. Fisk WJ. How IEQ affects health, productivity. ASHRAE J. 2002;44:56–60. [Google Scholar]
- 61. Norbäck D. An update on sick building syndrome. Curr Opin Allergy Clin Immunol. 2009;9:55–59. [DOI] [PubMed] [Google Scholar]
- 62. Hodgson M. The sick‐building syndrome. Occup Med. 1995;10:167–175. PMID: 7792673. [PubMed] [Google Scholar]
- 63. Kameel R, Khalil E. Thermal comfort vs air quality in air‐conditioned healthcare applications. 36th AIAA Thermophysics Conference. 2003;4199.
- 64. Lutz BD, Jin J, Rinaldi MG, Wickes BL, Huycke MM. Outbreak of invasive Aspergillus infection in surgical patients, associated with a contaminated air‐handling system. Clin Infect Dis. 2003;37:786–793. [DOI] [PubMed] [Google Scholar]
- 65. Rashid M, Zimring C. A review of the empirical literature on the relationships between indoor environment and stress in health care and office settings: problems and prospects of sharing evidence. Environ Behav. 2008;40:151–190. [Google Scholar]
- 66. Ulrich RS, Berry LL, Quan X, Parish JT. A conceptual framework for the domain of evidence‐based design. Health Environ Res Des J. 2010;4:95–114. [DOI] [PubMed] [Google Scholar]
- 67. Srikanth P, Sudharsanam S, Steinberg R. Bio‐aerosols in indoor environment: composition, health effects and analysis. Indian J Med Microbiol. 2008;26:302‐312. [DOI] [PubMed] [Google Scholar]
- 68. Kramer A, Guggenbichler P, Heldt P, et al. Hygienic relevance and risk assessment of antimicrobial‐impregnated textiles In: Biofunctional Textiles and the Skin. Karger Publishers; 2006;33:78–109. [DOI] [PubMed] [Google Scholar]
- 69. Kramer A, Schwebke I, Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis. 2006;6:130 10.1186/1471-2334-6-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Memarzadeh F. Literature review of the effect of temperature and humidity on viruses. ASHRAE Trans. 2012;118:1049–1060. [Google Scholar]
- 71. Kramer A, Assadian O. Survival of microorganisms on inanimate surfaces In: Borkow G, ed. Use of Biocidal Surfaces for Reduction of Healthcare Acquired Infections. Springer: Cham; 2014:7–26. [Google Scholar]
- 72. Ramos T, Dedesko S, Siegel JA, Gilbert JA, Stephens B. Spatial and temporal variations in indoor environmental conditions, human occupancy, and operational characteristics in a new hospital building. PLoS ONE. 2015;10:e0118207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lowen AC, Mubareka S, Steel J, Palese P. Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathog. 2007;3:e151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Shaman J, Kohn M. Absolute humidity modulates influenza survival, transmission, and seasonality. Proc Natl Acad Sci. 2009;106:3243–3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tang JW. The effect of environmental parameters on the survival of airborne infectious agents. J R Soc Interface. 2009;06:S737–S746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. ASHRAE . ASHRAE Standard 55‐2013, Thermal Environmental Conditions for Human Occupancy. Atlanta: ASHRAE;2013. [Google Scholar]
- 77. Lowen AC, Steel J, Mubareka S, Palese P. High temperature (30°C) blocks aerosol but not contact transmission of influenza virus. J Virol. 2008;82:5650–5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Abad FX, Villena C, Guix S, Caballero S, Pintó RM, Bosch A. Potential role of fomites in the vehicular transmission of human astroviruses. Appl Environ Microbiol. 2001;67:3904–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Vonberg R, Gastmeier P. Nosocomial aspergillosis in outbreak settings. J Hosp Infect. 2006;63:246–254. [DOI] [PubMed] [Google Scholar]
- 80. Hardin BD, Kelman BJ, Saxon A. Adverse human health effects associated with molds in the indoor environment. J Occupat Environ Med. 2003;45:470–478. [DOI] [PubMed] [Google Scholar]
- 81. Khan NN, Wilson BL. An environmental assessment of mold concentrations and potential mycotoxin exposures in the greater Southeast Texas area. J Environ Sci Health Part A. 2003;38:2759–2772. [DOI] [PubMed] [Google Scholar]
- 82. Hollins PD, Kettlewell PS, Atkinson MD, et al. Relationships between airborne fungal spore concentration of Cladosporium and the summer climate at two sites in Britain. Int J Biometeorol. 2004;48:137–141. [DOI] [PubMed] [Google Scholar]
- 83. Schoen L, Hodgson M, McCoy W, Miller S, Li Y, Olmsted R. ASHRAE Position Document on Airborne Infectious Diseases. 2014.
- 84. Hwang RL, Lin TP, Cheng MJ, Chien JH. Patient thermal comfort requirement for hospital environments in Taiwan. Build Environ. 2007;42:2980–2987. [Google Scholar]
- 85. Fossum S, Hays J, Henson MM. A comparison study on the effects of prewarming patients in the outpatient surgery setting. J PeriAnesthesia Nurs. 2001;16:187–194. [DOI] [PubMed] [Google Scholar]
- 86. Salonen H, Kurnitski J, Kosonen R, Hellgren UM, Lappalainen S, Peltokorpi A, Reijula K, Morawska L. The effects of the thermal environment on occupants’ responses in health care facilities: A literature review In: 9th International Conference on Indoor Air Quality, Ventilation & Energy Conservation in Buildings (IAQVEC2016). 2016. [Google Scholar]
- 87. Memarzadeh F, Manning A. Thermal comfort, uniformity, and ventilation effectiveness in patient rooms: performance assessment using ventilation indices. ASHRAE Trans. 2000;106:748–761. [Google Scholar]
- 88. Verheyen J, Theys N, Allonsius L, Descamps F. Thermal comfort of patients: objective and subjective measurements in patient rooms of a Belgian healthcare facility. Build Environ. 2011;46:1195–1204. [Google Scholar]
- 89. Verheyen J. Thermal comfort of patients in healthcare facilities [Dissertation]. Faculty of engineering sciences, Department of architectural engineering, 353. Vrije Universiteit Brussel. 2012.
- 90. Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical‐wound infection and shorten hospitalization. New Engl J Med. 1996;334:1209–1216. [DOI] [PubMed] [Google Scholar]
- 91. Wagner VD. Impact of perioperative temperature management on patient safety. Surg Serv Manag. 2003;9:38–43. [Google Scholar]
- 92. Wagner D, Byrne M, Kolcaba K. Effects of comfort warming on preoperative patients. AORN J. 2006;84:427–448. [DOI] [PubMed] [Google Scholar]
- 93. Sessler DI, Schroeder M, Merrifield B, Matsukawa T, Cheng C. Optimal duration and temperature of prewarming. Anesthesiol. 1995;82:674–681. [DOI] [PubMed] [Google Scholar]
- 94. Vanni SM, Braz JR, Módolo NS, Amorim RB, Rodrigues GR Jr. Preoperative combined with intraoperative skin‐surface warming avoids hypothermia caused by general anesthesia and surgery. J Clin Anesth. 2003;15:119–125. [DOI] [PubMed] [Google Scholar]
- 95. Melhado MA, Loomans MG, Hensen JL, Lamberts R. Design of air distribution system in operating rooms‐theory versus practice In: 14th International Conference on Indoor Air Quality and Climate (Indoor Air 2016), Ghent, Belgium; 2016. [Google Scholar]
- 96. Khodakarami J, Knight I. Measured thermal comfort conditions in Iranian hospitals for patients and staff In: Proceedings of CLIMA2007: Wellbeing Indoors, Helsinki. 2007. [Google Scholar]
- 97. Parsons KC. The effects of gender, acclimation state, the opportunity to adjust clothing and physical disability on requirements for thermal comfort. Energy Build. 2002;34:593–599. [Google Scholar]
- 98. Mora R, English MJ, Athienitis AK. Assessment of thermal comfort during surgical operations/discussion. ASHRAE Trans. 2001;107:52–62. [Google Scholar]
- 99. Just B, Trévien V, Delva E, Lienhart A. Prevention of intraoperative hypothermia by preoperative skin‐surface warming. Anesthesiology. 1993;79:214–218. [DOI] [PubMed] [Google Scholar]
- 100. Hynson JM, Sessler DI, Moayeri A, McGuire J, Schroeder M. The effects of preinduction warming on temperature and blood pressure during propofol/nitrous oxide anesthesia. Anesthesiology. 1993;79:219–228. [DOI] [PubMed] [Google Scholar]
- 101. Macario A, Dexter F. What are the most important risk factors for a patient’s developing intraoperative hypothermia? Anesth Analg. 2002;94:215–220. [DOI] [PubMed] [Google Scholar]
- 102. El‐Gamal N, El‐Kassabany N, Frank SM, et al. Age‐related thermoregulatory differences in a warm operating room environment (approximately 26°C). Anesth Analg. 2000;90:694–698. [DOI] [PubMed] [Google Scholar]
- 103. Morris RH. Influence of ambient temperature on patient temperature during intraabdominal surgery. Ann Surg. 1971;173:230–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Morris RH. Operating room temperature and the anesthetized, paralyzed patient. Arch Surg. 1971;102:95–97. [DOI] [PubMed] [Google Scholar]
- 105. Morris RH, Wilkey BR. The effects of ambient temperature on patient temperature during surgery not involving body cavities. Anesthesiology. 1970;32:102–107. [DOI] [PubMed] [Google Scholar]
- 106. Frank SM, El‐Rahmany HK, Cattaneo CG, Barnes RA. Predictors of hypothermia during spinal anesthesia. Anesthesiology. 2000;92:1330–1334. [DOI] [PubMed] [Google Scholar]
- 107. Kongsayreepong S, Chaibundit C, Chadpaibool J, et al. Predictor of core hypothermia and the surgical intensive care unit. Anesth Analg. 2003;96:826–833. [DOI] [PubMed] [Google Scholar]
- 108. Kasai T, Hirose M, Matsukawa T, Takamata A, Kimura M, Tanaka Y. Preoperative blood pressure and intraoperative hypothermia during lower abdominal surgery. Acta Anaesthesiol Scand. 2001;45:1028–1031. [DOI] [PubMed] [Google Scholar]
- 109. Leslie K, Sessler DI. Perioperative hypothermia in the high‐risk surgical patient. Best Pract Res Clin Anaesthesiol. 2003;17:485–498. [DOI] [PubMed] [Google Scholar]
- 110. Buggy DJ, Crossley AW. Thermoregulation, mild perioperative hypothermia and post‐anaesthetic shivering. Br J Anaesth. 2000;84:615–628. [DOI] [PubMed] [Google Scholar]
- 111. Flores‐Maldonado A, Medina‐Escobedo CE, Ríos‐Rodríguez et alMild perioperative hypothermia and the risk of wound infection. Arch Med Res. 2001;32:227–231. [DOI] [PubMed] [Google Scholar]
- 112. Frank SM, Fleisher LA, Breslow MJ, et al. Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events: a randomized clinical trial. JAMA. 1997;277:1127–1134. [PubMed] [Google Scholar]
- 113. Lenhardt R, Marker E, Goll V, et al. Mild intraoperative hypothermia prolongs postanesthetic recovery. Anesthesiology. 1997;87:1318–1323. [DOI] [PubMed] [Google Scholar]
- 114. Rajagopalan S, Mascha E, Na J, Sessler DI. The effects of mild perioperative hypothermia on blood loss and transfusion requirement. Anesthesiology. 2008;108:71–77. [DOI] [PubMed] [Google Scholar]
- 115. National Collaborating Centre for Nursing and Supportive Care (UK) . The Management of Inadvertent Perioperative Hypothermia in Adults [Internet]. London: Royal College of Nursing (UK); 2008. (NICE Clinical Guidelines, No. 65.) Available from: https://www.ncbi.nlm.nih.gov/books/NBK53797/. [PubMed] [Google Scholar]
- 116. Torossian A, Bräuer A, Höcker J, Bein B, Wulf H, Horn EP. Preventing inadvertent perioperative hypothermia. Deutsches Ärzteblatt Int. 2015;112:166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Torossian A, Bein B, Bräuer A. Summary S3 Guideline, Avoidance of perioperative hypothermia. 2014. Available at.https://www.the37company.com/summary-s3-guideline-avoidance-of-perioperative-hypothermia.
- 118. Scott EM, Buckland R. A systematic review of intraoperative warming to prevent postoperative complications. AORN J. 2006;83:1090–1113. [DOI] [PubMed] [Google Scholar]
- 119. Melling AC, Ali B, Scott EM, Leaper DJ. Effects of preoperative warming on the incidence of wound infection after clean surgery: a randomised controlled trial. Lancet. 2001;358:876–880. [DOI] [PubMed] [Google Scholar]
- 120. Odom M. Maintaining intraoperative normothermia: a meta‐analysis of outcomes with costs. AANA J. 1999;67:155–164. [PubMed] [Google Scholar]
- 121. Sessler DI. Complications and treatment of mild hypothermia. Anesthesiology. 2001;95:531–543. [DOI] [PubMed] [Google Scholar]
- 122. Deren ME, Machan JT, DiGiovanni CW, Ehrlich MG, Gillerman RG. Prewarming operating rooms for prevention of intraoperative hypothermia during total knee and hip arthroplasties. J Arthroplasty. 2011;26:1380–1386. [DOI] [PubMed] [Google Scholar]
- 123. Inaba K, Berg R, Barmparas G, et al. Prospective evaluation of ambient operating room temperature on the core temperature of injured patients undergoing emergent surgery. J Trauma Acute Care Surg. 2012;73:1478–1483. [DOI] [PubMed] [Google Scholar]
- 124. Forbes SS, Eskicioglu C, Nathens AB, et al. Evidence‐based guidelines for prevention of perioperative hypothermia. J Am Coll Surg. 2009;209:492–503. [DOI] [PubMed] [Google Scholar]
- 125. Lyon AJ, Freer Y. Goals and options in keeping preterm babies warm. Arch Dis Childhood Fetal Neonatal Ed. 2010;96:F71–F74. [DOI] [PubMed] [Google Scholar]
- 126. Bell EF, Acarregui MJ. Restricted versus liberal water intake for preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev. 2008;1:1465–1858. UR: 10.1002/14651858.CD000503 [DOI] [PubMed] [Google Scholar]
- 127. Sinclair JC. Servo‐control for maintaining abdominal skin temperature at 36°C in low birth weight infants. Cochrane Database Syst Rev. 2000;2:CD001074. [DOI] [PubMed] [Google Scholar]
- 128. Balaras CA, Dascalaki E, Gaglia A. HVAC and indoor thermal conditions in hospital operating rooms. Energy Build. 2007;39:454–470. [Google Scholar]
- 129. Obbard JP, Fang LS. Airborne concentrations of bacteria in a hospital environment in Singapore. Water Air Soil Pollution. 2003;144:333–341. [Google Scholar]
- 130. Shaman J, Pitzer VE, Viboud C, Grenfell BT, Lipsitch M. Absolute humidity and the seasonal onset of influenza in the continental United States. PLoS Biol. 2010;8:e1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Noti JD, Blachere FM, McMillen CM, et al. High humidity leads to loss of infectious influenza virus from simulated coughs. PLoS ONE. 2013;8:e57485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Coughenour C, Stevens V, Stetzenbach LD. An evaluation of methicillin‐resistant Staphylococcus aureus survival on five environmental surfaces. Microb Drug Resist. 2011;17:457–461. [DOI] [PubMed] [Google Scholar]
- 133. Makison C, Swan J. The effect of humidity on the survival of MRSA on hard surfaces. Indoor Built Environ. 2006;15:85–91. [Google Scholar]
- 134. Tang JW, Li Y, Eames I, Chan PK, Ridgway GL. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. J Hosp Infect. 2006;64:100–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. WHO Regional Office Europe . In: Heseltine E, Rosen J, eds. WHO Guidelines for Indoor Air Quality: Dampness and Mould. WHO Regional Office Europe; 2009. [PubMed] [Google Scholar]
- 136. El‐Sharkawy MF, Noweir ME. Indoor air quality levels in a University Hospital in the Eastern Province of Saudi Arabia. J Family Community Med. 2014;21:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Sadrizadeh S, Loomans MG. Thermal comfort in hospital and healthcare facilities: a literature review In: IAQVAC 2016, the 9th International Conference on Indoor Air Quality Ventilation & Energy Conservation in Buildings, 23‐26 October 2016, Seoul, South Korea. 2016. [Google Scholar]
- 138. Noskin GA, Peterson LR. Engineering infection control through facility design. Emerg Infect Dis. 2001;7:354–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Srinivasan A, Beck C, Buckley T, et al. The ability of hospital ventilation systems to filter Aspergillus and other fungi following a building implosion. Infect Control Hosp Epidemiol. 2002;23:520–524. [DOI] [PubMed] [Google Scholar]
- 140. Araujo R, Cabral JP. Fungal air quality in medical protected environments In: Kumar A, ed. Air quality. In Tech 10.5772/9766. (http://www.intechopen.com/books/air-quality/fungal-air-quality-in-medical-protected-environmentshttp://www.intechopen.com/books/air-quality/fungal-air-quality-in-medical-protected-environments). 2010. [DOI] [Google Scholar]
- 141. Méheust D, Le Cann P, Gangneux JP. Rapid quantification of viable fungi in hospital environments: analysis of air and surface samples using solid‐phase cytometry. J Hosp Infect. 2013;83:122–126. [DOI] [PubMed] [Google Scholar]
- 142. Caggiano G, Napoli C, Coretti C, et al. Mold contamination in a controlled hospital environment: a 3‐year surveillance in southern Italy. BMC Infect Dis. 2014;14:595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Fernstrom A, Goldblatt M. Aerobiology and its role in the transmission of infectious diseases. J Pathogens. 2013;2013:13 10.1155/2013/493960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Cox CS. The microbiology of air In: Collier L, Balows A, Sussman M, eds. Topley & Wilson's Microbiology and Microbial Infections, 9th edn Arnold, London: Oxford University Press; 1998:339–350. [Google Scholar]
- 145. Skoog J. Relative air humidity in hospital wards–user perception and technical consequences. Indoor Built Environ. 2006;15:93–97. [Google Scholar]
- 146. Berglund LG. Comfort and humidity. ASHRAE J. 1998;40:35–41. [Google Scholar]
- 147. Sinclair L, Crisp J, Sinn J. Variability in incubator humidity practices in the management of preterm infants. J Paediatr Child Health. 2009;45:535–540. [DOI] [PubMed] [Google Scholar]
- 148. Knobel R, Holditch‐Davis D. Thermoregulation and heat loss prevention after birth and during neonatal intensive‐care unit stabilization of extremely low‐birthweight infants. J Obstet Gynecol Neonatal Nurs. 2007;36:280–287. [DOI] [PubMed] [Google Scholar]
- 149. Lindsley WG, Blachere FM, Davis KA, et al. Distribution of airborne influenza virus and respiratory syncytial virus in an urgent care medical clinic. Clin Infect Dis. 2010;50:693–698. [DOI] [PubMed] [Google Scholar]
- 150. Tellier R. Review of aerosol transmission of influenza A virus. Emerg Infect Dis. 2006;12:1657–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Tellier R. Aerosol transmission of influenza A virus: a review of new studies. Interface. 2009;6(Suppl. 6):S783–S790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Li Y, Leung GM, Tang JW, et al. Role of ventilation in airborne transmission of infectious agents in the built environment—a multidisciplinary systematic review. Indoor Air. 2007;17:2–18. [DOI] [PubMed] [Google Scholar]
- 153. Jiang Y, Zhao B, Li X, Yang X, Zhang Z, Zhang Y. Investigating a safe ventilation rate for the prevention of indoor SARS transmission: An attempt based on a simulation approach. Build Simul. 2009;2:281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Menzies D, Fanning A, Yuan L, FitzGerald JM. Hospital ventilation and risk for tuberculous infection in Canadian health care workers. Ann Internal Med. 2000;133(10):779–789. [DOI] [PubMed] [Google Scholar]
- 155. Yu IT, Li Y, Wong TW, et al. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N Eng J Med. 2004;350:1731–1739. [DOI] [PubMed] [Google Scholar]
- 156. Yu IT, Wong TW, Chiu YL, Lee N, Li Y. Temporal‐spatial analysis of severe acute respiratory syndrome among hospital inpatients. Clin Infect Dis. 2005;40:1237–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Leung M, Chan AH. Control and management of hospital indoor air quality. Med Sci Monit. 2006;12:SR17–SR23. [PubMed] [Google Scholar]
- 158. Li Y, Huang X, Yu IT, Wong TW, Qian H. Role of air distribution in SARS transmission during the largest nosocomial outbreak in Hong Kong. Indoor Air. 2005;15:83–95. [DOI] [PubMed] [Google Scholar]
- 159. Eames I, Tang JW, Li Y, Wilson P. Airborne transmission of disease in hospitals. J R Soc Interface. 2009;6:S697–S702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Qian H, Li Y, Seto WH, Ching P, Ching WH, Sun HQ. Natural ventilation for reducing airborne infection in hospitals. Build Environ. 2010;45:559–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Aliabadi AA, Rogak SN, Bartlett KH, Green SI. Preventing airborne disease transmission: review of methods for ventilation design in health care facilities. Adv Prev Med. 2011;2011:124064–124064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Mousavi E. Airborne Infection in Healthcare Environments: Implications to Hospital Corridor Design. The University of Nebraska‐Lincoln; 2015. [Google Scholar]
- 163. Sundell J, Levin H, Nazaroff WW, et al. Ventilation rates and health: multidisciplinary review of the scientific literature. Indoor Air. 2011;21:191–204. [DOI] [PubMed] [Google Scholar]
- 164. Memarzadeh F. Literature Review: Room Ventilation and Airborne Disease Transmission. Chicago, IL: American Society for Healthcare Engineering; 2013. [Google Scholar]
- 165. Luongo JC, Fennelly KP, Keen JA, Zhai ZJ, Jones BW, Miller SL. Role of mechanical ventilation in the airborne transmission of infectious agents in buildings. Indoor Air. 2016;26:666–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. English TR. A brief history of health‐care ventilation. ASHRAE J. 2016;58:52–60. [Google Scholar]
- 167. ASHRAE, ANSI . ANSI/ASHRAE/ASHE Standard 170–2013, Ventilation of Health Care Facilities. Atlanta, GA: American Society of Heating, Refrigerating and Air‐Conditioning Engineers, Inc., 2013. [Google Scholar]
- 168. Facility Guideline Institute (FGI) . Guidelines for Design and Construction of Health Care Facilities. Dallas: Facility Guidelines Institute; 2010. [Google Scholar]
- 169. American Institute of Architects (AIA) . Guidelines for Design and Construction of Hospital and Health Care Facilities. Washington, DC: American Institute of Architects; 2006. [Google Scholar]
- 170. ASHRAE . Health Care Facilities In: Handbook 2013 Applications –HVAC Applications. The American Society of Heating, Refrigerating and Air‐Conditioning Engineers (ASHRAE). 2013; Chapter 7. [Google Scholar]
- 171. ASHRAE . HVAC Design Manual for Hospitals and Clinics. 2nd Edition. Atlanta, GA: American Society of Heating, Refrigerating, and Air‐conditioning Engineers; 2013. [Google Scholar]
- 172. Chartier Y, Pessoa‐Silva CL. In: Atkinson J, Chartier Y, Pessoa‐Silva CL, Jensen P, Li Y, Setp W, eds. Natural Ventilation for Infection Control in Health‐Care Settings. Canberra: World Health Organization; 2009. [PubMed] [Google Scholar]
- 173. Bolashikov ZD, Melikov AK, Kierat W, Popiołek Z, Brand M. Exposure of health care workers and occupants to coughed airborne pathogens in a double‐bed hospital patient room with overhead mixing ventilation. HVAC&R Res. 2012;18:602–615. [Google Scholar]
- 174. Memarzadeh F, Manning AP. Comparison of operating room ventilation systems in the protection of the surgical site/discussion. ASHRAE Trans. 2002;108:3–15. [Google Scholar]
- 175. Memarzadeh F, Jiang J. Methodology for minimizing risk from airborne organisms in hospital isolation rooms. ASHRAE Trans. 2000;106:731–747. [Google Scholar]
- 176. Wong LT, Chan WY, Mui KW, Lai AC. An experimental and numerical study on deposition of bioaerosols in a scaled chamber. Aerosol Sci Technol. 2010;44:117–128. [Google Scholar]
- 177. Memarzadeh F, Xu W. Role of air changes per hour (ACH) in possible transmission of airborne infections. Build Simul. 2012;5:15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Faulkner WB, Memarzadeh F, Riskowski G, Hamilton K, Chang CZ, Chang JR. Particulate concentrations within a reduced‐scale room operated at various air exchange rates. Build Environ. 2013;65:71–80. [Google Scholar]
- 179. Pantelic J, Tham KW. Adequacy of air change rate as the sole indicator of an air distribution system's effectiveness to mitigate airborne infectious disease transmission caused by a cough release in the room with overhead mixing ventilation: a case study. HVAC&R Res. 2013;19:947–961. [Google Scholar]
- 180. Mousavi ES, Grosskopf KR. Ventilation rates and airflow pathways in patient rooms: A case study of bioaerosol containment and removal. Ann Occupat Hygiene. 2015;59:1190–1199. [DOI] [PubMed] [Google Scholar]
- 181. Olmedo I, Nielsen PV, de Adana MR, Jensen RL, Grzelecki P. Distribution of exhaled contaminants and personal exposure in a room using three different air distribution strategies. Indoor Air. 2012;22:64–76. [DOI] [PubMed] [Google Scholar]
- 182. Kierat W, Bolashikov ZD, Melikov AK, Popiołek Z, Brand M. Exposure to coughed airborne pathogens in a double bed hospital patient room with overhead mixing ventilation: Impact of posture of coughing patient and location of doctor In Proceedings of ASHRAE IAQ 2010. 2010. TOPC‐00128‐2010. [Google Scholar]
- 183. Grosskopf K, Mousavi E. Bioaerosols in health‐care environments. ASHRAE J. 2014;56:22–31. [Google Scholar]
- 184. Memarzadeh F, Jiang J. Effect of operation room geometry and ventilation system parameter variations on the protection of the surgical site. IAQ Conference 2004. http://www.txap.com/wp-content/uploads/2017/01/Effect-of-Operation-Room-Geometry.pdf. Accessed August 5, 2016.
- 185. King MF, Noakes CJ, Sleigh PA. Modeling environmental contamination in hospital single‐and four‐bed rooms. Indoor Air. 2015;25:694–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Yu HC, Mui KW, Wong LT, Chu HS. Ventilation of general hospital wards for mitigating infection risks of three kinds of viruses including Middle East respiratory syndrome coronavirus. Indoor Built Environ. 2017;26:514–527. [Google Scholar]
- 187. Ninomura P, Bartley J. New ventilation guidelines for health‐care facilities. ASHRAE J. 2001;43:29–33. [Google Scholar]
- 188. Wan MP, Chao CY. Transport characteristics of expiratory droplets and droplet nuclei in indoor environments with different ventilation airflow patterns. J Biomech Eng. 2007;129:341–353. [DOI] [PubMed] [Google Scholar]
- 189. Beggs CB, Kerr KG, Noakes CJ, Hathway EA, Sleigh PA. The ventilation of multiple‐bed hospital wards: review and analysis. Am J Infect Control. 2008;36:250–259. [DOI] [PubMed] [Google Scholar]
- 190. Buus M, Winther FV, Thilageswaran M. Contaminant flow in the microenvironment between people under different ventilation conditions. ASHRAE Trans. 2008;114:632–638. [Google Scholar]
- 191. Zhao B, Zhang Y, Li X, Yang X, Huang D. Comparison of indoor aerosol particle concentration and deposition in different ventilated rooms by numerical method. Build Environ. 2004;39:1–8. [Google Scholar]
- 192. Qian H, Li Y, Nielsen PV, Hyldgaard CE, Wong TW, Chwang AT. Dispersion of exhaled droplet nuclei in a two‐bed hospital ward with three different ventilation systems. Indoor Air. 2006;16:111–128. [DOI] [PubMed] [Google Scholar]
- 193. Yin Y, Xu W, Gupta JK, et al. Experimental study on displacement and mixing ventilation systems for a patient ward. HVAC&R Res. 2009;15:1175–1191. [Google Scholar]
- 194. Guity A, Gulick B, Marmion P. Healthcare ventilation research collaborative: displacement ventilation research: phase II summary report. Healthcare Without Harm. 2009. [Google Scholar]
- 195. Mazumdar S, Yin Y, Guity A, Marmion P, Gulick B, Chen Q. Impact of moving objects on contaminant concentration distributions in an inpatient ward with displacement ventilation. HVAC&R Res. 2010;16:545–563. [Google Scholar]
- 196. Zhang Z, Chen Q. Experimental measurements and numerical simulations of particle transport and distribution in ventilated rooms. Atmos Environ. 2006;40:3396–3408. [Google Scholar]
- 197. Wan MP, Chao CY, Ng YD, Sze To GN, Yu WC. Dispersion of expiratory droplets in a general hospital ward with ceiling mixing type mechanical ventilation system. Aerosol Sci Techn. 2007;41:244–258. [Google Scholar]
- 198. Kao PH, Yang RJ. Virus diffusion in isolation rooms. J Hosp Infect. 2006;62:338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Khalil EE. Air‐conditioning systems’ design in hospitals for comfort, air quality, and energy utilization In: Proceedings CLIMA. 2005. [Google Scholar]
- 200. Sadrizadeh S. Design of Hospital Operating Room Ventilation using Computational Fluid Dynamics . Doctoral dissertation, KTH Royal Institute of Technology. 2016.
- 201. Diab‐Elschahawi M, Berger J, Blacky A, et al. Impact of different‐sized laminar air flow versus no laminar air flow on bacterial counts in the operating room during orthopedic surgery. Am J Infect Control. 2011;39:e25–e29. [DOI] [PubMed] [Google Scholar]
- 202. Hansen D, Krabs C, Benner D, Brauksiepe A, Popp W. Laminar air flow provides high air quality in the operating field even during real operating conditions, but personal protection seems to be necessary in operations with tissue combustion. Int J Hygiene Environ Health. 2005;208:455–460. [DOI] [PubMed] [Google Scholar]
- 203. Edmond MB, Wallace SE, McClish DK, Pfaller MA, Jones RN, Wenzel RP. Nosocomial bloodstream infections in United States hospitals: a three‐year analysis. Clin Infect Dis. 1999;29:239–244. [DOI] [PubMed] [Google Scholar]
- 204. Azimi P, Stephens B. HVAC filtration for controlling infectious airborne disease transmission in indoor environments: predicting risk reductions and operational costs. Build Environ. 2013;70:150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Sautour M, Sixt N, Dalle F, et al. Prospective survey of indoor fungal contamination in hospital during a period of building construction. J Hosp Infect. 2007;67:367–373. [DOI] [PubMed] [Google Scholar]
- 206. Korves TM, Piceno YM, Tom LM, et al. Bacterial communities in commercial aircraft high‐efficiency particulate air (HEPA) filters assessed by PhyloChip analysis. Indoor Air. 2013;23:50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Alberti C, Bouakline A, Ribaud P, et al. Relationship between environmental fungal contamination and the incidence of invasive aspergillosis in haematology patients. J Hosp Infect. 2001;48:198–206. [DOI] [PubMed] [Google Scholar]
- 208. Dharan S, Pittet D. Environmental controls in operating theatres. J Hosp Infect. 2002;51:79–84. [DOI] [PubMed] [Google Scholar]
- 209. Friberg S, Ardnor B, Lundholm R, Friberg B. The addition of a mobile ultra‐clean exponential laminar airflow screen to conventional operating room ventilation reduces bacterial contamination to operating box levels. J Hosp Infect. 2003;55:92–97. [DOI] [PubMed] [Google Scholar]
- 210. Hahn T, Cummings KM, Michalek AM, Lipman BJ, Segal BH, McCarthy PL. Efficacy of high‐efficiency particulate air filtration in preventing aspergillosis in immunocompromised patients with hematologic malignancies. Infect Control Hosp Epidemiol. 2002;23:525–531. [DOI] [PubMed] [Google Scholar]
- 211. Oren I, Haddad N, Finkelstein R, Rowe JM. Invasive pulmonary aspergillosis in neutropenic patients during hospital construction: before and after chemoprophylaxis and institution of HEPA filters. Am J Hematol. 2001;66:257–262. [DOI] [PubMed] [Google Scholar]
- 212. Araujo R, Carneiro A, Costa‐Oliveira S, Pina‐Vaz C, Rodrigues AG, Guimaraes JE. Fungal infections after haematology unit renovation: evidence of clinical, environmental and economical impact. Eur J Haematol. 2008;80:436–443. [DOI] [PubMed] [Google Scholar]
- 213. Nihtinen A, Anttila VJ, Richardson M, Meri T, Volin L, Ruutu T. The utility of intensified environmental surveillance for pathogenic moulds in a stem cell transplantation ward during construction work to monitor the efficacy of HEPA filtration. Bone Marrow Transplant. 2007;40:457–460. [DOI] [PubMed] [Google Scholar]
- 214. Eckmanns T, Rüden H, Gastmeier P. The influence of high‐efficiency particulate air filtration on mortality and fungal infection among highly immunosuppressed patients: a systematic review. J Infect Dis. 2006;193:1408–1418. [DOI] [PubMed] [Google Scholar]
- 215. Mantadakis E, Samonis G. Novel preventative strategies against invasive aspergillosis. Med Mycol. 2006;44(Supplement_1):S327–S332. [DOI] [PubMed] [Google Scholar]
- 216. Richardson MD, Rennie S, Marshall I, et al. Fungal surveillance of an open haematology ward. J Hosp Infect. 2000;45:288–292. [DOI] [PubMed] [Google Scholar]
- 217. Boswell T. Use of HEPA filters to reduce airborne concentrations of Pseudomonas aeruginosa. 2006 Federation of Infection Societies Meeting, Cardiff, Wales, UK. 2006;29.
- 218. Dee SA, Deen J, Cano JP, Batista L, Pijoan C. Further evaluation of alternative air‐filtration systems for reducing the transmission of porcine reproductive and respiratory syndrome virus by aerosol. Can J Vet Res. 2006;70:168–175. [PMC free article] [PubMed] [Google Scholar]
- 219. Cole EC, Cook CE. Characterization of infectious aerosols in health care facilities: an aid to effective engineering controls and preventive strategies. Am J Infect Control. 1998;26:453–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Beggs CB, Donnelly JK, Kerr KG, Sleigh PA, Mara DD, Cairns G. The use of engineering controls to disinfect Mycobacterium tuberculosis and airborne pathogens in hospital buildings. Indoor Built Environ. 2000;9:17–27. [Google Scholar]
- 221. Chuaybamroong P, Chotigawin R, Supothina S, Sribenjalux P, Larpkiattaworn S, Wu CY. Efficacy of photocatalytic HEPA filter on microorganism removal. Indoor Air. 2010;20:246–254. [DOI] [PubMed] [Google Scholar]
- 222. Jankowska E, Reponen T, Willeke K, Grinshpun SA, Choi KJ. Collection of fungal spores on air filters and spore reentrainment from filters into air. J Aerosol Sci. 2000;31:969–978. [Google Scholar]
- 223. Salam ZH, Karlin RB, Ling ML, Yang KS. The impact of portable high‐efficiency particulate air filters on the incidence of invasive aspergillosis in a large acute tertiary‐care hospital. Am J Infect Control. 2010;38:e1–e7. [DOI] [PubMed] [Google Scholar]
- 224. Siegel JD, Rhinehart E, Jackson M, Chiarello L. 2007 guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35:S65–S164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Centers for Disease Control and Prevention (CDC) . Guidelines for preventing the transmission of Mycobacterium tuberculosis in health‐care settings . MMWr. 2005;54. [PubMed]
- 226. Jensen PA, Lambert LA, Iademarco MF, Ridzon R, Centers for Disease Control and Prevention . Guidelines for preventing the transmission of Mycobacterium tuberculosis in health‐care settings, 2005. MMWR. 2005;54:1–141. [PubMed] [Google Scholar]
- 227. Cubi Montanya E, Salom Tormo J, Garrido Soriano N. Indoor environmental quality and infection control in surgery rooms: Code requirements vs. performance motivation. A critical review. HVAC&R Res. 2014;20:643–654. [Google Scholar]
- 228. Streifel AJ. Health‐care IAQ: guidance for infection control. Heating/piping/air Condit Eng. 2000;72:28–36. [Google Scholar]
- 229. Gore B, Smith K. Tuberculosis infection control: a practical manual for preventing TB, 2011. [Curry International Tuberculosis Center]. 2011. http://www.currytbcenter.ucsf.edu/sites/default/files/ic_book_2011.pdf. Accessed November 10, 2017.
- 230. Cubi Montanya E, Salom Tormo J, Garrido Soriano N. Energy‐efficient ventilation control strategies for surgery rooms. Sci Technol Built Environ. 2015;21:228–237. [Google Scholar]
- 231. Tung YC, Hu SC, Tsai TI, Chang IL. An experimental study on ventilation efficiency of isolation room. Build Environ. 2009;44:271–279. [Google Scholar]
- 232. Emmerich SJ, Heinzerling D, Choi JI, Persily AK. Multizone modeling of strategies to reduce the spread of airborne infectious agents in healthcare facilities. Build Environ. 2013;60:105–115. [Google Scholar]
- 233. Rydock JP, Eian PK. Containment testing of isolation rooms. J Hosp Infect. 2004;57:228–232. [DOI] [PubMed] [Google Scholar]
- 234. Adams NJ, Johnson DL, Lynch RA. The effect of pressure differential and care provider movement on airborne infectious isolation room containment effectiveness. Am J Infect Control. 2011;39:91–97. [DOI] [PubMed] [Google Scholar]
- 235. De Witte J, Sessler DI. Perioperative shivering physiology and pharmacology. Anesthesiology. 2002;96:467–484. [DOI] [PubMed] [Google Scholar]
- 236. Hooper VD, Chard R, Clifford T, et al. ASPAN’s evidence‐based clinical practice guideline for the promotion of perioperative normothermia. J PeriAnesthesia Nurs. 2010;25:346–365. [DOI] [PubMed] [Google Scholar]
- 237. Association of periOperative Registered Nurses . Recommended practices for the prevention of unplanned perioperative hypothermia. AORN J. 2007;85:972–988. [DOI] [PubMed] [Google Scholar]
- 238. Wang CS, Chen CL, Huang CJ, et al. Effects of different operating room temperatures on the body temperature undergoing live liver donor hepatectomy. Transplant Proc. 2008;40:2463–2465. [DOI] [PubMed] [Google Scholar]
- 239. Knobel RB, Wimmer JE Jr, Holbert D. Heat loss prevention for preterm infants in the delivery room. J Perinatol. 2005;25:304–308. [DOI] [PubMed] [Google Scholar]
- 240. Knobel R, Holditch‐Davis D. Thermoregulation and heat loss prevention after birth and during neonatal intensive‐care unit stabilization of extremely low‐birthweight infants. Adv Neonatal Care. 2010;10:S7–S14. [DOI] [PubMed] [Google Scholar]
- 241. Robine E, Derangere D, Robin D. Survival of a Pseudomonas fluorescens and Enterococcus faecalis aerosol on inert surfaces. Int J Food Microbiol. 2000;55:229–234. [DOI] [PubMed] [Google Scholar]


