Abstract
Faecal samples from suckling (n=205) and weaned piglets (n=82) with diarrhoea from 24 farms in Southern Germany were examined for shedding of important metazoic parasitic, viral and bacterial pathogens using culture, microscopic and electronmicroscopic methods. Escherichia coli isolates were tested further for the enterotoxin genes est‐Ia and elt‐I by colony blot hybridization. Isospora suis was diagnosed in 26.9 % and Cryptosporidium parvum in 1.4 % of the piglets investigated. The proportion of coronavirus‐positive animals was 13.4 % and 4 % were positive for rotavirus. It was found that 17.6 % of the animals were infected with enterotoxigenic E. coli (ETEC; 10.1 % ETEC‐ST‐Ia and 8.6 % ETEC‐LT‐I, respectively). The occurrence of the pathogens was significantly associated with the age of the animals examined (P < 0.001). Isospora suis was predominantly isolated from suckling piglets (in the second and third week of life), while in weaned piglets (fourth week of life) rotavirus and ETEC were most prevalent. On 22 of the 24 piglet production farms examined at least one of the investigated pathogens was detected. Coronavirus was diagnosed in 66.7 %, I. suis in 62.5 %, rotavirus in 20.8 % and C. parvum in 8.3 % of the farms. These results underline the fact that despite the hygienic, technical and immune preventive efforts during the last years, enteropathogens are still common in German piglet production units.
Introduction
Diarrhoea caused by infectious agents is responsible for large economic losses in pig production farms, especially in suckling and weaned piglets. Coronavirus and rotavirus, as well as enterotoxigenic Escherichia coli (ETEC), are described as the most frequent viral and bacterial enteropathogens, respectively. Of the metazoic parasites of piglets, Isospora suis and Cryptosporidium parvum are considered the most prevalent.
Investigations determining the prevalence of these pathogens have been conducted world‐wide. However, in many studies faecal samples were not representative because they had been taken either by practicing veterinary surgeons or by the owners of the animals for routine diagnostic purposes only (Bergeland and Henry, 1982; Morin et al., 1983; Fitzgerald et al., 1988). In addition, most investigations concentrated on single pathogens only, leaving a lack of information about other relevant enteropathogens (Roepstorff and Jorsal, 1989; Driesen et al., 1993; Otten et al., 1996; Meyer et al., 1999). Occasionally, samples were investigated that had been collected from representative animals, however, their number was only small (Guscetti, 1991). Another concern is that faecal samples are not usually sent to the diagnostic laboratory directly, but the same sample is passed from one diagnostic unit to another for virological, bacteriological and parasitological examinations. Usually, the results from such studies leave the different survival rates of the pathogens unconsidered. Furthermore, much of the currently available data is based on results from more than 10 years ago and does not necessarily reflect the current epidemiological situation.
To circumvent some of these problems, in the study presented here, faecal samples were taken from the rectum of piglets with diarrhoea and within a few hours were transported to the virological, bacteriological and parasitological laboratories, respectively. The prevalences were recorded with regard to the animals (percentage of infected piglets) as well as to the farms (percentage of positive production units). The data presented will give new clues for the improvement of therapeutic intervention as well as for prophylactic and metaphylactic strategies. Preventive measures, such as vaccination or the application of anti‐microbial therapies, can thus be used in a more focused manner.
Materials and Methods
Animals
Faecal samples from 205 suckling piglets and 82 weaned piglets from 24 different pig production farms in Southern Germany were included in the investigation. The farms comprised eight small facilities (less than 60 mother sows), 16 medium‐sized facilities (61–120 mother sows) and two large operations (121–250 mother sows). The piglets were raised on either concrete or perforated pen floors, with or without straw. Three farms were managed by the ‘all in – all out’ procedure, while on the other 23 farms the animals were raised continuously.
Sampling and sample handling
Samples were only taken from piglets with diarrhoea. Diarrhoea was diagnosed when piglets shed aqueous or even bloody faeces and/or defecated more often. Faecal samples from 287 piglets were examined parasitologically and from 278 piglets bacteriologically. In addition, 149 single and 53 pooled faecal samples from piglets were investigated for viruses by electron microscopy. Only faecal samples within one suckling litter or within one distinct pen were pooled. All samples were collected from animals that had not been previously treated with antibiotics. The number of samples collected in one farm ranged from four to 43, representing approximately 10 % of the piglets with diarrhoea at the time of sampling.
Parasitological examination
Faecal samples from all the piglets were examined for Cryptosporidium oocysts by carbolfuchsin‐staining of faecal smears (Heine, 1982) and for other intestinal parasites by the merthiolate iodine formalin concentration technique (Rommel et al., 1992). Faecal samples taken from piglets after necropsy were also examined by a modified sedimentation–flotation technique using a zinc sulphate solution (Rommel et al., 1992).
Bacteriological examination
Faecal samples were streaked on blood and Gassner‐agar (waterblue metachromic lactose agar; Merck, Darmstadt, Germany) and incubated for 18–24 h at 37°C. Coliforms were identified by the in vitro Diagnostical Bactident® (Merck, Darmstadt, Germany) technique. Escherichia coli isolates were screened for E. coli enterotoxin genes by colony blot hybridization, as described by Franke et al. (submitted for publication). The E. coli strains B41 (O101:H‐, est‐la), E57 (O138:K81, est‐la, stx2e), H10407 (O78:H11, est‐la, est‐Ib, elt‐Ib) and P307 (O8:K87, est‐II, elt‐Ib) were used as positive controls. All DNA probes were generated and labelled with Digoxigenin‐11‐dUTP (Boehringer, Mannheim, Germany) by a polymerase chain reaction technique using recombinant plasmids pRIT10220 (est‐la) (Lathe et al., 1980), pEWD299 (elt‐Ib) (Moseley et al., 1983) and pSLM004 (est‐Ib) (Moseley et al., 1983) as templates. The following oligonucleotide primers were used: SK5 (5′ AAT CAC TTG ACT CTT CAA AAG 3′) and SK6 (5′ CCC TCT ATG CTT TTT AAT AAC 3′) for amplification of est‐la; SK7 (5′ CGG AGG TAA TAT GAA GAA ATC 3′) and SK8 (5′ GTA CAA GCA GGA TTA CAA CAC 3′) for amplification of est‐Ib; SK9 (5′ AAT AAA GTA AAA TGT TAT GTT 3′) and SK10 (5′ GTT TTT CAT ACT GAT TGC CGC 3′) for amplification of stx. Amplification was performed on a thermal cycler (Eppendorf‐Nether, Hamburg, Germany). After an initial denaturation for 1 min at 94°C, the samples were subjected to 30 cycles, each consisting of 60 s at 94°C, 60 s at 45°C and 80 s at 72°C. Amplification was terminated with a final elongation step for 300 s at 72°C. PCR products were separated by electrophoresis through 1.5 % agarose gels supplemented with 0.5 μg/ml ethidium bromide (80–120 V, 30–60 min) and visualized by ultraviolet illumination Sambrook et al., 1989).
Virological examination
Coronaviruses and rotaviruses were examined by electron microscopy as described by Krauss and Arens (Kraus and Arens, 1981). Each grid was microscopically investigated for at least 10 min.
Statistical analysis
If pathogens were detected in a sample from a farm, then that farm was recorded as being positive. To analyse the qualitative features, frequency charts were established with the statistical program package bmdp/ dynamic release 7.0 (Dixon, 1993). Significances were calculated with the Fisher/χ2‐test of the statistical program bmdp4 f (Dixon, 1993) as well as with the statistical program package statxact‐ turbo (Dixon, 1993). Logistic regression analyses were performed with the program bmdplr or with the program complex logxact turbo. Significances were displayed as follows: P < 0.001 highly significant, P < 0.01 significant, P ≤ 0.05 low significance and P > 0.05 not significant (NS).
Results
Prevalence of enteropathogens in pig production farms
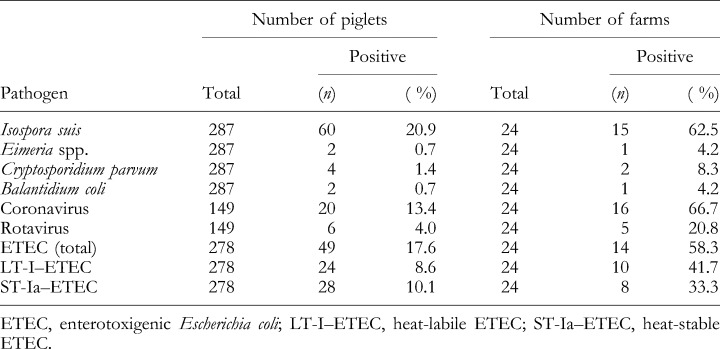
Table 1 summarizes the prevalences of the various enteropathogens examined at the farm level. Isospora suis could be detected in samples from 15 of the 24 farms tested, and C. parvum was found for two of the farms. By electron microscopy, coronaviruses were detected in samples from 16 of the farms investigated, while only five farms were positive for rotavirus. A total of 14 farms were recorded positive for enterotoxigenic E. coli (ETEC), eight of which harboured ETEC–ST‐Ia, while ETEC–LT‐I was isolated from 10 farms. In the majority of the production farms, two or more different pathogens were detected. However, no combination of pathogens was predominant. In two farms, none of the enteropathogens was detectable in the piglets with diarrhoea by the diagnostic procedures elaborated. Neither eggs of Strongyloides ransomi nor eggs of any other helminths were found in any of the faecal samples investigated.
Table 1.
Prevalences of diarrhoeagenic pathogens in piglets with diarrhoea on 24 farms in Southern Germany
Prevalence of enteropathogens in piglets with diarrhoea
The results of the microbiological analyses at the level of single piglets are summarized in Table 1. Isospora suis (10.9 %), enterotoxigenic E. coli (17.6 %) and coronavirus (13.4 %) were the most prevalent enteropathogens. The other diarrhoeagenic pathogens were detected more rarely, namely 4 % for rotavirus, 1.4 % for C. parvum and 0.7 % for Eimeria spp. Of the samples from the 144 piglets with diarrhoea that were tested with all the available methods described in this study, 57.7 % were negative for all of the pathogens investigated. The majority of the positive piglets (33.3 %) were infected with one pathogen only, and 9.1 % proved positive for two or more pathogens. In those samples that harboured more than one pathogen, no preferential combination of pathogens was observed.
Prevalence of enteropathogens as a function of the age of the piglets
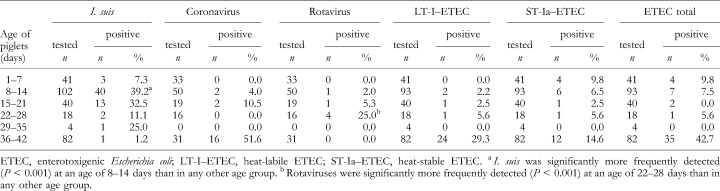
In order to define an association between prevalence of pathogens and age of the animals, the results were further analysed by matching different age groups. In Table 2, the results are displayed at weekly intervals ranging from the first to the sixth week of life. At the age of 5 weeks, the piglets were usually weaned. These analyses revealed that I. suis was determined more frequently during the second (P < 0.001) and third week of life, while rotaviruses were detected more frequently in piglets at an age of 22–28 days (P < 0.001). In contrast, coronaviruses or ETEC were observed more frequently in weaned than in suckling piglets (P < 0.001).
Table 2.
Prevalences of diarrhoeagenic pathogens in piglets at various ages; piglets tested/positive for the diarrhoeagenic pathogens shown
Discussion
The results displayed in our study reveal that enteropathogens are still very common in German pig production farms. In 22 of 24 farms examined, at least one of the well‐known enteropathogens could be isolated from faecal samples of piglets with diarrhoea. The prevalences determined in this study confirm the results of comparable investigations, in which Coccidia, coronavirus and rotavirus, as well as ETEC, were the predominant diarrhoeagenic pathogens in piglets.
Coccidia, in particular I. suis, are one of the most important diarrhoeagenic pathogens of piglets (Morin et al., 1983; Biehl and Hoefling, 1986; Driesen et al., 1993). In our investigation, 62.5 % of the farms were contaminated with I. suis. Otten et al. (1996) and Meyer et al. (1999) reported an I. suis prevalence of 100 % in Germany, Roberts and Walker (1982) of 78 % in Great Britain and Eyskers et al. (1994) one of 68 % in the Netherlands. Besides I. suis, Eimeria spp. were also found frequently in these pig production units. However, Eimeria spp. are shed more frequently from sows than from piglets (Lindsay et al., 1984; Nilsson et al., 1984). Stuart et al. (1982) postulate that older animals develop a lifelong immunity against I. suis. According to Tubbs (1986), passive immunity acquired via the colostrum protects piglets against Eimeria infections during the first weeks of life. In contrast, the lactogenic immunity against I. suis is insufficient. In addition, the short pre‐patency (5–6 days) compared to that of Eimeria spp. and the short sporulation time of I. suis cause higher concentrations of infectious I. suis stages in the litters (Tubbs, 1986). These considerations are confirmed by the high prevalence of I. suis (20.9 %) and the low prevalence of Eimeria spp. (0.7 %) detected in the present study. The highest prevalences for I. suis were observed in the second and third weeks of life. These data correspond to, and thus confirm, findings from earlier investigations (Henriksen and Christensen, 1989; Henriksen, 1991). In general I. suis appears to be the most prevalent enteropathogen of young piglets, as was also shown by studies in the USA (Bergeland and Henry, 1982; Lindsay et al., 1984; Biehl and Hoefling, 1986; Fitzgerald et al., 1988), Canada (Morin et al., 1983), Australia (Driesen et al., 1993), Great Britain (Roberts and Walker, 1982), Sweden (Nilsson, 1988), Italy (Trotti et al., 1987) and Germany (Otten et al., 1996; Meyer et al., 1999). Guscetti (1991) examined both healthy piglets and those with diarrhoea for the shedding of I. suis, and found a difference in the prevalence of 61 % to 4.3 %, respectively. These findings underline the importance of I. suis as a cause of diarrhoea.
In contrast to I. suis, oocysts of C. parvum were found only rarely (0.7 %) in the faecal samples examined. This result is in accordance with previously published data (Bergeland and Henry, 1982; Trotti et al., 1987; Sanford, 1987; Villacorta et al., 1991; Otten et al., 1996). Higher prevalences have only been reported if older animals were investigated (Sanford, 1987; Otten et al., 1996). Oocysts of C. parvum are frequently isolated from healthy piglets (Villacorta et al., 1991; Otten et al., 1996) and C. parvum is therefore not regarded as an obligate pathogen for piglets (Sanford, 1987). Nor does Balantidium coli appear to be an aetiologically relevant pathogen, although in comparison to the results gained in the present study (prevalence 0.7 %) higher prevalences have been published (5–15 %) (Indermühle, 1978; Morris et al., 1984; Otten et al., 1996). Apart from protozoal infections it is remarkable that helminth infections were completely absent in the present study. Particularly, S. ransomi had been reported in previous years to be a common parasite in young piglets in Germany (Hörchner et al., 1980; Muss and Hasslinger, 1989; Otten et al., 1996). The weaners, at least, were old enough also to shed eggs of other nematodes such as Ascaris suum or Oesophagostomum spp. Clearly more data are needed on a broader scale to gain evidence as to whether the situation has changed generally, possibly due to improved hygiene standards, or not.
Studies describing the prevalences of coronaviruses and rotaviruses in faecal samples of piglets with diarrhoea have been published only rarely and the data published varied widely. In the few comparable studies, prevalences ranged between 20 and 60 % for coronaviruses and between 1 and 40 % for rotaviruses. It is an interesting finding that the numbers found in this study and those of other investigators from Germany are very low in comparison with studies from other countries (Morin et al., 1983; Fitzgerald et al., 1988; Biermann et al., 1989a, 1989b; 15Herbst et al., 1989; Otten et al., 1996). However, the finding that rotavirus infections were increasing during the third and fourth week of life was also observed in other studies (Nilsson et al., 1984; Fitzgerald et al., 1988; Svensmark et al., 1989). In contrast, infections with coronaviruses were detected particularly in weaned piglets. In these cases, the piglets suffered from a mild form of the enzootic coronavirus infection (Pritchard, 1987).
The results concerning infections with ETEC are comparable with those of the coronavirus infections. In particular, ETEC were shed by weaned piglets preferentially (42.7 %). In suckling piglets, the prevalences were lower and they decreased from 9.5 to 5.6 % between the first and third weeks of life. To our knowledge, a similar low level prevalence (7.4 %) has so far only been reported by Guscetti (1991). In most of the studies published previously the prevalences in suckling piglets ranged between 30 and 50 % (Bergeland and Henry, 1982; Morin et al., 1983; Biehl and Hoefling, 1986; Evans et al., 1986; Nakazawa et al., 1987; Fitzgerald et al., 1988; Driesen et al., 1993). In piglets with diarrhoea, isolation rates even reached 88.6 % (Nagy et al., 1990). Since most of these high prevalences were measured more than 10 years ago, the low prevalence detected in our study is presumably due to the increasing use of ETEC vaccines in pregnant sows during the last years. In agreement with the results of Wilson and Francis (1986), ST‐I‐positive ETEC were found more frequently than LT‐I‐positive strains in suckling piglets. However, the finding that LT‐I‐positive ETEC strains are highly prevalent in weaned piglets has not been observed previously (Moon et al., 1986; Nakazawa et al., 1987; Ojeniyi et al., 1992; Wray et al., 1993). These results warrant further investigations, because the rearing practice has changed to early weaning at 3–5 weeks of life and this may have contributed to the higher infection rate with these particular subsets of ETEC.
It is noticeable that a relatively large proportion of the animals (57.6 %) was found to be negative for all of the enteropathogens we looked for, although more than 60 % of the production units were contaminated with coccidia, coronaviruses, or ETEC and although only piglets with diarrhoea were investigated. Since the procedures of sampling, transport to the diagnostic laboratory and analyses of the samples were to a large extent optimized, one must assume that either a large proportion of the cases was caused by non‐infectious factors or that currently unknown pathogens were involved. However, the latter is unlikely, since a much broader spectrum of microbiological pathogens is detectable with the diagnostic techniques applied. It was also surprising that in contrast to the situation in calves with diarrhoea (Baljer et al., 1987) only 9.1 % of the 42.4 % positive faecal samples harboured more than one pathogen.
The analysis of the association between the age of the animals and the shedding of the pathogens revealed that, with the exception of I. suis and rotavirus, the prevalences of each single pathogen were lower before than after weaning at the age of 5 weeks. Presumably, the lactogenic transfer of maternal immunity against these pathogens to the litters is very efficient. However, under this protective shield of maternal immunity suckling piglets are not able to build up a sufficiently active immunity, which would be protective after weaning. This protection gap after weaning, in connection with an increased susceptibility to infections caused by the stress of weaning, seems to be a major cause of the economic losses from diarrhoea during the raising of piglets.
Bibliography
- 1. Baljer, G. , Eichhorn W., Göbel G., Wolf M. & Bachmann P. A., 1987: Vorkommen und Verbreitung wichtiger Durchfallerreger bei neugeborenen Kälbern in Süddeutschland im Zeitraum 1984–86. Tierärztl. Umschau 42, 56–63. [Google Scholar]
- 2. Bergeland, M. E. & Henry S. C., 1982: Infectious diarrhoeas of young pigs. Vet. Clin. North Am. Large Anim. Practice 4, 389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Biehl, L. G. & Hoefling D. C., 1986: Diagnosis, treatment and prevention of diarrhoea in 7–14 day old pigs. J. Am. Vet. Med. Assoc. 188, 1144–1146. [PubMed] [Google Scholar]
- 4. Biermann, U. , Herbst W., Krauss H. & Schliesser T., 1989a: Elektronenmikroskopische Nachweisrate enteraler Viren bei Durchfallkrankheiten von Hund, Katze, Kalb, Schwein und Fohlen im Jahre 1988. Berl. Münch. Tierärztl. Wochenschr. 102, 412–414. [PubMed] [Google Scholar]
- 5. Biermann, U. , Schmitt H. & Krauss H., 1989b: Elektronenmikroskopische Virusdiagnostik bei Hund, Kalb, Schwein und Fohlen im Jahre 1989. Berl. Münch. Tierärztl. Wochenschr. 104, 117–119. [PubMed] [Google Scholar]
- 6. Dixon, W. J. , 1993: BMDP Statistical Software Manual, Vol. 1 and 2. University of California Press, Berkeley, Los Angeles.
- 7. Driesen, S. J. , Carland P. G. & Fahy V. A., 1993: Studies on preweaning diarrhoea. Austr. Vet. J. 70, 259–262. [DOI] [PubMed] [Google Scholar]
- 8. Evans, M. G. , Waxler G. L. & Newman J. P., 1986: Prevalence of K88, K99 and 987P pili of Escherichia coli in neonatal pigs with enteric coli‐bacillosis. Am. J. Vet. Res. 47, 2431–2434. [PubMed] [Google Scholar]
- 9. Eyskers, M. , Boerdam G. A., Hollanders W. & Verheijden J. H., 1994: The prevalence of Isospora suis and Strongyloides ransomi in suckling piglets in The Netherlands. Vet. Q. 16, 203–205. [DOI] [PubMed] [Google Scholar]
- 10. Fitzgerald, G. R. , Barker T., Welter M. W. & Welter C. J., 1988: Diarrhoea in young pigs: comparing the incidence of the five most common infectious agents. Vet. Med. 83, 80–86. [Google Scholar]
- 11. Guscetti, F. , 1991: Untersuchungen zum Erregerspektrum und zur Morphologie des Darmes bei 1 bis 4 Wochen alten Saugferkeln mit Durchfall. Thesis, University of Zürich, Zürich.
- 12. Heine, J. , 1982: Eine einfache Nachweismethode für Kryptosporidien im Kot. Zentralblatt Vet. Med. B 29, 324–327. [PubMed] [Google Scholar]
- 13. Henriksen, S. A. & Christensen J. P. B., 1989: Coccidiosis in piglets in Denmark. Shedding of oocysts of Isospora suis in relation to the age of host. In: Yvoré, P. (ed.), Coccidia and Intestinal Coccidiomorphs, Proceedings of the 5th International Coccidiosis Conference, Tours (France), 17–20 October, Colloques de l’INRA vol. 49, pp. 489–492. INRA Publishers, Toulouse.
- 14. Henriksen, S. A. , 1991: Untersuchungen bezüglich der Coccidiose bei Ferkeln. Slutrapport Over NKJ.-Projekt 59, 7–1012. [Google Scholar]
- 15. Herbst, W. , Lange H., Danner K., Krauss H. & Schliesser T., 1989: Elektronenmikroskopischer Virusnachweis in Kotproben von enteritisch erkrankten Schweinen zwischen 1981 und 1987. Dtsch. Tierärztl. Wochenschr. 96, 294–296. [PubMed] [Google Scholar]
- 16. Hörchner, F. , Grelck H., Unterholzner J., Heydorn K. P. & Tunger G., 1980: Helminthosen im Schweinebetrieb. Berl. Münch. Tierärztl. Wochenschr. 93, 370–373. [PubMed] [Google Scholar]
- 17. Indermühle, N. A. , 1978: Endoparasitenbefall beim Schwein. Schweiz. Arch. Tierheilk. 120, 513–525. [PubMed] [Google Scholar]
- 18. Krauss, H. & Arens M., 1981: Die elektronenmikroskopische Untersuchung von Kot‐ und Organmaterial als diagnostischer Schnellnachweis bei der Parvovirusinfektion der Hunde. Prakt. Tierarzt 62, 38–41. [Google Scholar]
- 19. Lathe, P. , Hirth M., DeWilde N., Harford J. P. & Lecocq S. A., 1980: Cell‐free synthesis of enterotoxin of E. coli from a cloned gene. Nature 284, 473–474. [DOI] [PubMed] [Google Scholar]
- 20. Lindsay, D. S. , Current W. L., Ernst J. V., Stuart B. P. & Stewart B., 1984: Prevalence of oocysts of Isospora suis and Eimeria species from sows on farms with and without a history of neonatal coccidiosis. J. Am. Vet. Med. Ass. 185, 419–421. [PubMed] [Google Scholar]
- 21. Meyer, C. , Joachim A. & Daugschies A., 1999: Occurrence of Isospora suis in larger piglet production units and on specialized piglet rearing farms. Vet. Parasitol. 83, 277–284. [DOI] [PubMed] [Google Scholar]
- 22. Moon, H. W. , Schneider R. A. & Moseley S. L., 1986: Comparative prevalence of four enterotoxin genes among Escherichia coli isolated from swine. Am. J. Vet. Res. 47, 210–212. [PubMed] [Google Scholar]
- 23. Morin, M. , Turgeon D., Jolette J., Robinson Y., Phaneuf J. B., Saufageau R., Beauregard M., Teuscher E., Higgens R. & Lariviere S., 1983: Neonatal diarrhoea of pigs in Quebec: infectious causes of significant outbreaks. Can. J. Comp. Med. 47, 11–17. [PMC free article] [PubMed] [Google Scholar]
- 24. Morris, R. G. , Jordan H. E., Luce W. G., Coburn C. T. & Maxwell C. V., 1984: Prevalence of gastrointestinal parasitism in Oklahoma swine. Am. J. Vet. Res. 45, 2421–2423. [PubMed] [Google Scholar]
- 25. Moseley, S. L. , Hardy J. W., Huq M. I., Echeverria P. & Falkow S., 1983: Isolation and nucleotide sequence determination of a gene encoding for a heat stable enterotoxin of Escherichia coli . Infect. Immun. 39, 1167–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Muss, C. & Hasslinger M. A., 1989: Epidemiologische Untersuchungen zur Wurmbürde in Ferkelerzeuger‐ und Mastbetrieben Schwabens. Dtsch. Tierärztl. Wochenschr. 96, 45–84. [PubMed] [Google Scholar]
- 27. Nagy, B. , Casey T. A. & Moon H. W., 1990: Phenotype and genotype of Escherichia coli isolated from pigs with postweaning diarrhea in Hungary. J. Clin. Microbiol. 28, 651–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nakazawa, M. , Sugimoto C., Isayama Y. & Kashiwazaki M., 1987: Virulence factors in Escherichia coli isolated from piglets with neonatal and post‐weaning diarrhea in Japan. Vet. Microbiol. 13, 291–300. [DOI] [PubMed] [Google Scholar]
- 29. Nilsson, O. , 1988: Isospora suis in pigs with postweaning diarrhoea. Vet. Rec. 122, 310–310. [DOI] [PubMed] [Google Scholar]
- 30. Nilsson, O. , Martinsson K. & Persson E., 1984: Epidemiology of porcine neonatal steatorrhoea in Sweden: Prevalence and clinical significance of coccidial and rotaviral infections. Nord Vet. Med. 36, 103–110. [PubMed] [Google Scholar]
- 31. Ojeniyi, B. , Ahrens P., Jorsal S. E. & Meyling A., 1992: Detection of enterotoxigenic Escherichia coli from pigs with diarrhea using colony hybridization and S labelled probe. In: Proceedings of the 12th International Pig Veterinary Society Congress, The Hague, p. 246. Pig Veterinary Society.
- 32. Otten, A. , Takla M., Daugschies A. & Rommel M., 1996: The epizootiology and pathogenic significance of infections with Isospora suis in ten piglet production operations in Nordrhein‐Westfalen. Berl. Münch. Tierärztl. Wochenschr. 109, 220–223. [PubMed] [Google Scholar]
- 33. Pritchard, G. C. , 1987: Transmissible gastroenteritis in endemically infected breeding herds of pigs in East Anglia. Vet. Rec. 120, 226–230. [DOI] [PubMed] [Google Scholar]
- 34. Roberts, L. & Walker E. J., 1982: Field study of coccidial and rotaviral diarrhoea in unweaned piglets. Vet. Rec. 110, 11–13. [DOI] [PubMed] [Google Scholar]
- 35. Roepstorff, A. & Jorsal S. E., 1989: Relationship of the prevalence of swine helminths to management practises and anthelminthic treatment in Danish sow herds. Vet. Parasitol. 36, 245–257. [DOI] [PubMed] [Google Scholar]
- 36. Rommel, M. , Eckert J. & Kutzer E., 1992: Untersuchungsmethoden. In: Eckert, J., E. Kutzer, M. Rommel, H.‐J. Bürger, and W. Körting (eds), Veterinärmedizinische Parasitologie, 4th edn, pp. 46–69. Paray, Berlin.
- 37. Sambrook, J. , Fritsch E. F. & Maniatis T., 1989: Molecular Cloning, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York.
- 38. Sanford, S. E. , 1987: Enteric cryptosporidial infection in pigs. 184 Cases (1981–85). J. Am. Vet. Med. Assoc. 190, 695–698. [PubMed] [Google Scholar]
- 39. Stuart, B. P. , Gosser H. S., Allen C. B. & Bedell D. M., 1982: Coccidiosis in swine: dose and age response to Isospora suis . Can. J. Comp. Med. 46, 317–320. [PMC free article] [PubMed] [Google Scholar]
- 40. Svensmark, B. , Nielsen K., Dalsgaard K. & Willeberg P., 1989: Epidemiological studies of piglet diarrhea in intensively managed Danish sow herds. 3. Rotavirus infection. Acta Vet. Scand. 30, 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Trotti, G. C. , Pampiglione S. & Visconti S., 1987: Cryptosporidium species and Isospora suis in pigs in Italy. Parasitology 26, 299–304. [PubMed] [Google Scholar]
- 42. Tubbs, R. C. , 1986: A review of porcine neonatal Coccidiosis. Mod. Vet. Pract. 67, 899–903. [Google Scholar]
- 43. Villacorta, I. , Ares‐Mazas E. & Lorenzo M. J., 1991: Cryptosporidium parvum in cattle, sheep and pigs in Galicia. Vet. Parasitol. 38, 249–252. [DOI] [PubMed] [Google Scholar]
- 44. Wilson, R. A. & Francis D. H., 1986: Fimbriae and enterotoxins associated with Escherichia coli serogroups isolated from pigs with colibacillosis. Am. J. Vet. Res. 47, 213–217. [PubMed] [Google Scholar]
- 45. Wray, C. , McLaren I. M. & Carroll P. J., 1993: Escherichia coli isolated from farm animals in England and Wales between 1986 and 1991. Vet. Rec. 133, 439–442. [DOI] [PubMed] [Google Scholar]


