Abstract
It has been described that polymorphonuclear neutrophils (PMNs) enhance the replication of CC‐chemokine receptor 5/macrophage‐tropic (R5) HIV in cultures of monocyte‐derived macrophages (MDMs). In this study, the inhibitory effect of glycyrrhizin (GL) on R5 HIV replication influenced by PMNs was investigated in MDM cultures. The replication of R5 HIV in MDMs was greatly enhanced when cells were co‐cultured with freshly isolated PMNs (syngeneic to MDMs). When GL was added to this culture, however, the viral replication enhanced by PMNs was completely inhibited. CCL2 and interleukin 10 (IL‐10) were produced in cultures of PMNs exposed to R5 HIV, and the replication of R5 HIV was greatly enhanced in MDM cultures supplemented with a mixture of recombinant CCL2 and IL‐10. However, CCL2 and IL‐10 were not produced by PMNs exposed to R5 HIV, when GL was added to the cultures. In the presence of GL, these soluble factors were not detected in co‐cultures of MDMs and PMNs exposed to R5 HIV. In addition, the replication of R5 HIV in MDMs stimulated with CCL2 and IL‐10 was not directly influenced by GL. These results indicated that GL suppresses the PMN‐dependent increase of R5 HIV replication in MDMs through inhibiting CCL2/IL‐10 production by PMNs stimulated with R5 HIV.
Keywords: glycyrrhizin, HIV, macrophages, neutrophils, CCL2, IL‐10
The major targets for HIV infection are cells expressing CD4 molecules and HIV entry co‐receptors on the cell surface. The difference in chemokine receptors is important for the cellular tropism of HIV. The β‐chemokine receptor, CCR5, is the major co‐receptor for macrophage‐tropic strains of HIV (R5 HIV), 1 , 2 whereas the α‐chemokine receptor, CXCR4, facilitates entry of T‐tropic HIV strains (X4 HIV). 3 The R5X4 HIV uses both CCR5 and CXCR4 co‐receptors. 4 , 5 , 6 R5 HIV replicates in monocyte‐derived macrophages (MDMs), but not in primarily isolated monocytes. 7 , 8 , 9 As primarily isolated monocytes do not express CCR5 on their surface, R5 HIV cannot enter into the cells. 10 , 11 X4 HIV replicates in cultures of T cells. 12
Recently, we showed that polymorphonuclear neutrophils (PMNs) stimulate R5 HIV replication in MDMs. 13 Greatly increased replication of R5 HIV was shown in co‐cultures or transwell cultures between MDMs exposed to R5 HIV and freshly isolated PMNs (syngeneic to MDMs). CCL2 and interleukin 10 (IL‐10) were produced by PMNs exposed to R5 HIV, and the replication of R5 HIV was not accelerated in MDM/PMN transwell cultures supplemented with a mixture of mAbs (monoclonal antibodies) for CCL2 and IL‐10. In contrast, the replication of R5 HIV was accelerated in MDM cultures supplemented with a mixture of recombinant CCL2 and IL‐10. These results indicate that, through the production of CCL2 and IL‐10, PMNs influenced by R5 HIV enhance the replication of R5 HIV in MDM cultures.
Earlier, we have shown that the HIV replication is inhibited by glycyrrhizin (GL, an active component of licorice) in CD8+ T cell‐depleted HIV patient peripheral blood mononuclear cells (PBMCs) co‐cultured with healthy donor PBMCs. 14 CCL4 and CCL5 produced by healthy donor PBMCs are identified as effector molecules for the anti‐HIV activity of GL. 15 In this study, the inhibitory effect of GL on the PMN‐associated enhancement of R5 HIV replication in MDMs was investigated. In Japan, GL has been used clinically for more than 20 years in patients with chronic hepatitis. 16 , 17 , 18 , 19 The antiviral activities of GL against human cytomegalovirus, herpes simplex virus type 2, influenza virus and coronavirus that are related to severe acute respiratory syndrome have been reported. 20 , 21 , 22 , 23
RESULTS
Effect of GL on R5 HIV replication influenced by PMNs
To determine the inhibitory effect of GL on the PMN‐associated R5 HIV replication, MDMs (5 × 105 cells ml−1) exposed to 15.8 TCID50 per ml of R5 HIV were co‐cultured with PMNs (5 × 106 cells ml−1, syngeneic to MDMs), in the presence of GL (100 μg ml−1). Viral replication was not shown in cultures of MDMs exposed to 15.8 TCID50 per ml of R5 HIV, whereas R5 HIV was shown to be replicated in MDMs co‐cultured with PMNs. The viral replication in MDMs co‐cultured with PMNs was not shown when cultures were performed with GL (Figure 1a). In the next experiments, MDMs were exposed to 1580 TCID50 per ml of R5 HIV and co‐cultured with PMNs. As compared with the control (the growth of R5 HIV in MDMs), the viral replication in MDMs co‐cultured with PMNs enhanced eightfold (Figure 1b). At this time, the viral replication was completely inhibited by GL in co‐cultures between MDMs and PMNs (Figure 1b). A dose–response inhibitory effect of GL in the co‐cultures between MDMs exposed to 1580 TCID50 per ml of R5 HIV and healthy PMNs was examined. As shown in Figure 1c, significant inhibition of the viral replication was shown, when GL at doses ranging from 1 μg ml−1 to 100 μg ml−1 was added to the cultures. From the figure, a 50% inhibition was indicated when the cultures were performed with 1 μg ml−1 of GL.
Figure 1.
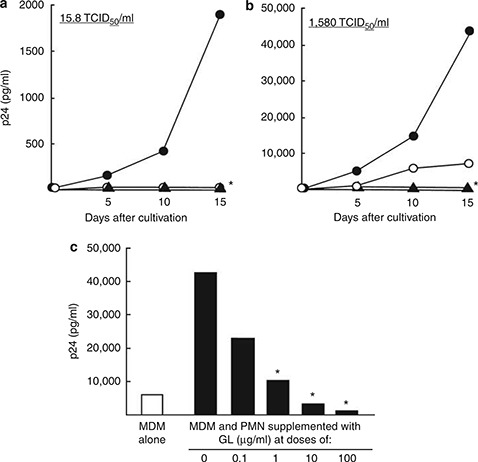
Effect of GL on the replication of R5 HIV in MDMs co‐cultured with PMNs (syngeneic to MDMs). MDMs (5 × 105 cells ml−1) were exposed to 15.8 TCID50 per ml (a) or 1580 TCID50 per ml (b) of R5 HIV for 3 h. After washing excess viruses, these cells were co‐cultured with healthy donor PMNs (5 × 106 cells ml−1, syngeneic to MDMs) in the presence (filled triangles) or absence (filled circles) of GL (100 μg ml−1). As a control, MDMs exposed to R5 HIV were cultured with media (open circles). The co‐cultivation (c) was performed in the presence of various doses of GL for 15 days. Fifty percent of the medium was changed every 5 days. The quantity of p24 antigen in culture fluids of these cells was determined by ELISA. The results shown are representative of three replicate experiments. * P<0.01 compared with the untreated group.
Next, the inhibitory effect of GL on R5 HIV replication in MDMs transwell cultured with PMNs was examined. MDMs (lower chamber) exposed to 1580 TCID50 per ml of R5 HIV were cultured with PMNs (5 × 106 cells ml−1, upper chamber) in double chamber transwells supplemented with 100 μg ml−1 of GL. At 24 h after cultivation, the upper chamber was removed and MDMs in the lower chamber were cultured for an additional 14 days. Culture fluids harvested were assayed for p24 antigen. In the results, 42 860±4640 pg ml−1 of p24 antigen was detected in the culture fluids of MDMs, whereas 2200 pg ml−1 of the antigen (95% decrease) was detected in the same cultures supplemented with GL (Figure 2).
Figure 2.
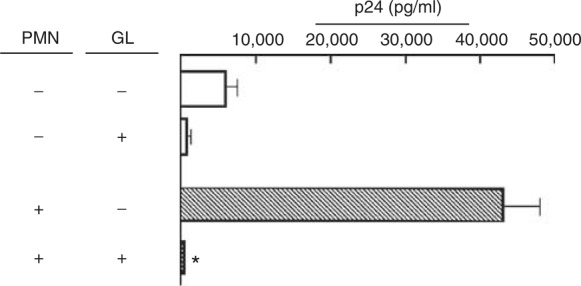
Effect of GL on R5 HIV replication in MDMs transwell cultured with PMNs. MDMs (5 × 105 cells ml−1, lower chamber) were exposed to 1580 TCID50 per ml of R5 HIV for 3 h. After washing, these cells were cultured with PMNs (5 × 106 cells ml−1, upper chamber) in double chamber transwells supplemented with or without GL (100 μg ml−1). At 24 h after cultivation, the upper chamber was removed and cells in the lower chamber were cultured for an additional 14 days. As controls, with or without 100 μg ml−1 of GL, MDMs exposed to the virus were cultured alone. The quantity of p24 antigen in the culture fluids of these cells was determined by ELISA. The results shown are representative of three replicate experiments. * P<0.01 compared with the untreated group.
Inhibition of CCL2 and IL‐10 production by GL in cultures of PMNs exposed to R5 HIV
Polymorphonuclear neutrophils influenced by R5 HIV produced CCL2 and IL‐10, whereas these factors were not produced by healthy donor PMNs. Therefore, the effect of GL on the production of IL‐10 and CCL2 by PMNs previously exposed to R5 HIV was examined. Thus, PMNs (2 × 106 cells ml−1) stimulated with 1580 TCID50 per ml of R5 HIV for 3 h, were washed with media, and cultured with GL (100 μg ml−1) for an additional 18 h. Culture fluids harvested were assayed for CCL2 and IL‐10 by ELISA. In the results, 3.4 ng ml−1 of CCL2 and 158 pg ml−1 of IL‐10 were detected in the culture fluids of PMNs pulse‐treated with the virus. However, the production of CCL2 and IL‐10 by PMNs exposed to the virus was greatly decreased (85–90% reduction), when cultures were performed with 100 μg ml−1 of GL (Figure 3).
Figure 3.
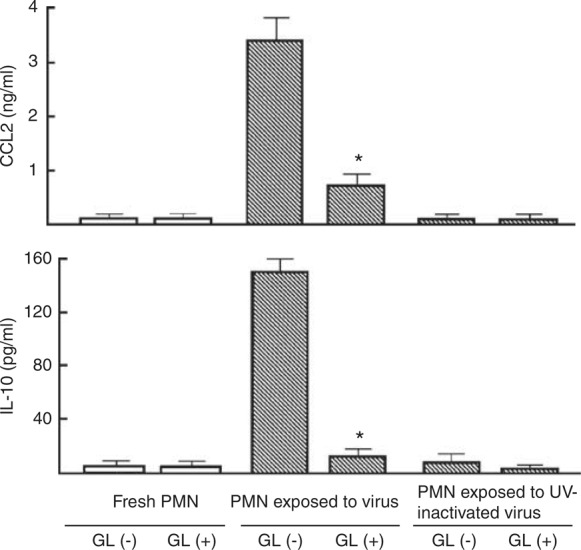
Effect of GL on CCL2 and IL‐10 production by PMNs exposed to R5 HIV. PMNs (2 × 106 cells ml−1) were exposed to 1580 TCID50 per ml of R5 HIV or ultraviolet (UV)‐inactivated R5 HIV for 3 h. PMNs not exposed to the virus were cultured as a control. The cells were washed with media and re‐cultured with or without 100 μg ml−1 of GL for 18 h. Culture fluids harvested were assayed for CCL2 and IL‐10. The data are displayed as the mean±s.e.m., and are representative of three experiments. * P<0.05 compared with control.
Effect of GL on R5 HIV replication in MDMs stimulated with a mixture of rCCL2 and rIL‐10
In the presence of a mixture of CCL2 and IL‐10, the effect of GL on R5 HIV replication in MDMs was examined. The replication of R5 HIV in cultures of MDMs was greatly increased when a mixture of rCCL2 (2 ng ml−1) and rIL‐10 (0.2 ng ml−1) was added to these cultures. In these cultures, however, viral replication was not suppressed by GL (Figure 4). These results indicate that R5 HIV replication enhanced by a mixture of CCL2 and IL‐10 is not influenced by GL.
Figure 4.
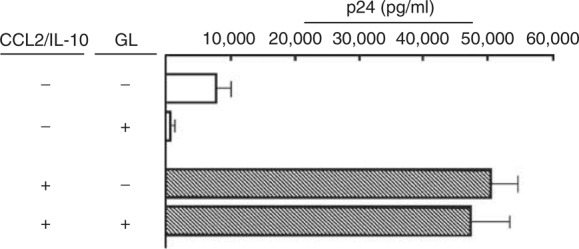
Effect of GL on HIV replication in MDM cultures supplemented with a mixture of CCL2 and IL‐10. MDMs (5 × 105 cells ml−1) exposed to 1580 TCID50 per ml of R5 HIV were cultured for 3 days. Then, cells were washed and re‐cultured with fresh medium added with a mixture of rCCL2 (2 ng ml−1), rIL‐10 (0.2 ng ml−1) and GL (100 μg ml−1) for 12 days. The quantity of p24 antigen in the culture fluids of these cells was determined by ELISA. Data shown in this figure are derived from three independent experiments using different donors. The data are displayed as the mean±s.e.m.
DISCUSSION
In our earlier studies, 14 , 15 GL was shown to be inhibitory on the replication of R5 HIV in PBMCs from patients with R5 HIV infection, or MDMs exposed to R5 HIV. In these studies, the anti‐HIV activity of GL was displayed through the induction of CCL4 and CCL5 production. One of the cellular entry sites of R5 HIV (CCR5) on the surface of target cells is blocked by these chemokines. 1 , 2 , 3 In addition, anti‐R5 HIV activity of GL was shown in monocytes, which were previously treated with 1‐methyladenosine. 15 CCR5 expression in monocytes was stimulated by CCL2 and IL‐10, which were released from the same monocytes treated with 1‐methyladenosine. However, in the presence of GL, CCL2 and IL‐10 were not produced by monocytes treated with 1‐methyladenosine. 15 In this paper, the inhibitory effect of GL on R5 HIV replication in MDMs co‐cultured with PMNs was examined. The replication of R5 HIV in MDMs was greatly enhanced when these cells exposed to the virus were co‐cultured with PMNs. Enhanced R5‐HIV replication in MDMs co‐cultured with PMNs was inhibited by 1–100 μg ml−1 of GL. Over 95% inhibition of the viral replication was shown when GL at a dose of 100 μg ml−1 was added to the co‐cultures. A 50% inhibition was shown when 1 μg ml−1 of GL was added to the co‐cultures. The non‐cytotoxic properties of GL have been reported in many papers. 24 , 25 , 26 GL at concentrations ranging from 1 to 200 μg ml−1 is not cytotoxic against various murine and human tissue culture cells. In addition, we tested the toxicity of GL on human PMNs. By trypan blue dye‐exclusion test, 90% or more of PMNs were shown to be viable, after cultivation of healthy donor peripheral blood PMNs (1 × 106 cells ml−1) for 18 h with RPMI‐1640 medium supplemented with 10% fetal bovine serum and 300 μg ml−1 GL. Under microscopic examination, morphological changes of 18 h‐cultured PMNs with GL were not shown. These previous descriptions and our data indicate that cytotoxic properties of GL are not involved in its antiviral activity against R5 HIV replication in MDMs co‐cultured with PMNs.
The antiviral mechanism of GL against enhanced replication of R5 HIV in MDMs co‐cultured with PMNs was examined next. Some soluble factors released from PMNs have been indicated as effector molecules to enhance the replication of R5 HIV in MDMs, because the viral replication has been greatly enhanced in MDMs transwell cultured with PMNs. Indeed, the enhanced viral replication was not shown when a mixture of mAbs directed against CCL2 and IL‐10 was added to this transwell culture. 13 In contrast, without PMNs, replication of R5 HIV was greatly enhanced in MDM cultures supplemented with a mixture of rCCL2 and rIL‐10. In this study, the replication of R5 HIV was not enhanced in MDMs and PMNs transwell cultures supplemented with GL. However, the enhanced R5 HIV replication in MDM cultures supplemented with a mixture of rCCL2 and rIL‐10 was not influenced by GL. GL suppressed the released of CCL2 and IL‐10 from PMNs influenced by R5 HIV. These results indicate that GL suppresses the enhanced replication of R5 HIV in MDMs transwell cultured with PMNs through the inhibition of CCL2 and IL‐10 production from PMNs influenced by R5 HIV.
In our assay system, MDMs previously exposed to 1580 TCID50 per ml of R5 HIV (bottom chamber) were cultured with PMNs (upper chamber) in a double chamber transwell for 24 h. There is a possibility that, in this transwell culture (0.4 μm pore), small amounts of HIV could pass through the transwell membrane from the lower chamber to the upper chamber, and viral particles that pass through the membrane could stimulate PMNs to induce IL‐10/CCL2 production. In addition, it has been described that HIV‐infected monocytes have a function to produce high levels of tumor necrosis factor α (TNF‐α) and IL‐6. 27 , 28 Although in our system, we did not test the function of HIV‐infected MDMs to produce TNF‐α and IL‐6, there is a possibility that MDMs exposed to HIV may have an ability to produce TNF‐α and IL‐6. In addition, small numbers of monocytes contaminating the PMN preparation may have a possibility to produce TNF‐α and IL‐6. As our PMN preparations utilized in the experiments were not completely pure (93% pure cells), further studies for the clarification of a role of TNF‐α/IL‐6 will be needed in our transwell‐cultivation system.
It has been described in many papers 15 , 29 , 30 , 31 , 32 , 33 that CCL2 and IL‐10 enhance the replication of R5 HIV in tissue culture cells. Thus, the replication of R5 HIV in patient PBMCs depleted of CD8+ T cells was increased by CCL2, 29 and intracellular growth of R5 HIV in MDMs was enhanced by rCCL2. 30 In addition, IL‐10 increases R5 HIV replication in cultures of monocytes 31 and MDMs. 32 , 33 In our earlier study, 15 R5 HIV was grown in freshly isolated monocytes previously treated with 1‐methyladenosine. CCL2 and IL‐10 were produced by monocytes stimulated with 1‐methyladenosine.
Further, we examined the mechanism by which R5 HIV stimulates CCL2 and IL‐10 production by PMNs. When PMNs were stimulated with ultraviolet (UV)‐inactivated HIV (corresponds to 1580 TCID50 of viable HIV), significant amounts of CCL2 and IL‐10 were not detected in their culture fluids, whereas these soluble factors were produced by PMNs stimulated with viable R5 HIV. This indicates that CCL2 and IL‐10 are inducible by viable R5 HIV (but not inactivated HIV) in cultures of PMNs. Although peripheral blood PMNs are known to unconventionally express endogenous CD4 and bind to HIV, it remains unclear what receptors on PMNs were triggered by R5 HIV. Further studies will be required.
METHODS
Reagents
Recombinant human IL‐10 and CCL2 were purchased from PeproTech (Rocky Hill, NJ, USA). mAbs for IL‐10 and CCL2 were obtained from BD PharMingen (San Diego, CA, USA). GL was supplied by Minophagen Pharmaceutical Co., Ltd, Tokyo, Japan. According to our previous papers, 14 , 15 1 mg of GL was dissolved in 1 ml of culture medium at room temperature, and it was further diluted to appropriate concentrations when used in experiments.
Media and MDMs
The PBMCs were prepared from heparinized whole blood by Ficoll–Hypaque sedimentation. 15 Monocytes were isolated from PBMCs using fibronectin‐coated plates, as previously described. 15 Briefly, PBMCs (1 × 107 cells ml−1) were placed into fibronectin‐coated 24‐well plates. The plates were incubated for 15 min at 37 °C to allow monocytes to attach, and then floating cells were removed by washing with warm medium. The attached monocytes were maintained in culture for 7 days with RPMI‐1640 medium supplemented with 10% heat‐inactivated human AB serum (Sigma‐Aldrich, St Louis, MO, USA), 2 mm l‐glutamine and antibiotics to allow differentiation into MDMs, and then used for HIV infection.
R5 HIV
R5 HIV (SF162 strain) was propagated in healthy donor PBMCs stimulated with PHA (5 μg ml−1) for 3 days. Cell‐free supernatant was harvested at peak viral infection, and stored at −80 °C, as previously described. 15
Isolation of PMNs
Polymorphonuclear neutrophils were isolated from whole peripheral blood using Ficoll–Hypaque and dextran sedimentations. 34 Thus, after the Ficoll–Hypaque sedimentation of whole blood, precipitates were obtained as a PMN‐rich fraction. To eliminate erythrocytes, precipitates were suspended in 1% dextran and kept for 30 min at room temperature. The resulting PMN fraction was further treated with erythrocyte‐lysing buffer (R&D Systems, Minneapolis, MN, USA) to eliminate the remaining erythrocytes. Contaminating monocytes and lymphocytes were eliminated using magnetic beads coated with anti‐CD14 and anti‐CD3 mAbs. The purity of PMNs finally obtained was 93% or more, when it was analyzed by Wright–Giemsa staining. The cell viability was higher than 99%, when it was determined by trypan blue dye‐exclusion test.
R5 HIV replication in tissue cultures
Co‐cultivation
Monocyte‐derived macrophages (5 × 105 cells ml−1) were exposed to R5 HIV (15.8 or 1580 TCID50 per ml) for 3 h. Afterwards, excess viruses were removed by washing with warm medium, and these cells were co‐cultured with PMNs (5 × 106 cells ml−1). Every 5 days, culture fluids were harvested after co‐cultivation, and the amounts of HIV p24 antigen in the culture fluids were measured by ELISA (Beckman Coulter, Somerset, NJ, USA).
Transwell cultivation
Monocyte‐derived macrophages (lower chamber) exposed to 15.8 TCID50 per ml of R5 HIV were cultured with PMNs (5 × 106 cells per ml, upper chamber) in double chamber transwells (0.4 μm pore, Corning Costar, NY, USA). At 24 h after cultivation, the upper chamber was removed and the cells in the lower chamber were re‐cultured for 14 days. The quantity of p24 antigen in the culture fluids of MDMs was determined by ELISA.
Assay of GL antiviral activities
Monocyte‐derived macrophages exposed to R5‐HIV were co‐cultured or transwell cultured with PMNs as mentioned above, in media supplemented with 0.1–100 μg ml−1 of GL (test group) or media (control group). A total of 5–15 days after cultivation, culture fluids were harvested from both groups and the amounts of HIV p24 antigen in the culture fluids were measured by ELISA.
Effect of CCL2 and IL‐10 on R5 HIV replication in MDMs
A mixture of rCCL2 (2 ng ml−1) and rIL‐10 (0.2 ng ml−1) was added to MDMs treated with 100 μg ml−1 of GL 72 h after R5 HIV infection (15.8 TCID50 per ml). The quantity of p24 antigen in the culture fluids of MDMs was determined by ELISA.
CCL2 and IL‐10 production by PMNs exposed to R5 HIV
Polymorphonuclear neutrophils (2 × 106 cells ml−1) were exposed to 1580 TCID50 per ml of R5 HIV. At 3 h after cultivation, the cells were washed with media, and re‐cultured with 1–100 μg ml−1 of GL. At 18 h after cultivation, the amounts of CCL2 and IL‐10 in the culture fluids were measured by ELISA. The detection limits for IL‐10 and CCL2 were 8 and 16 pg ml−1, respectively. Each assay was performed three times.
Statistical analysis
Data shown in each figure are derived from three independent experiments using different donors. Data are presented as mean±s.e.m. All results were statistically analyzed by ANOVA (analysis of variance) using Statview 4.5 (Brain Power, Calabasas, CA, USA). If a P‐value was lower than 0.05, the result was considered to be significant.
ACKNOWLEDGEMENTS
We thank Minophagen Pharmaceutical Co., Ltd (Tokyo, Japan) for their generous donation of GL.
References
- 1. Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PK, Murphy PM et al CC CKR5: a RANTES, MIP‐1α, MIP‐1β receptor as a fusion cofactor for macrophage‐tropic HIV‐1. Science 1996; 272: 1955–1958. [DOI] [PubMed] [Google Scholar]
- 2. Dragic T, Litwin V, Allaway GP, Martin SR, Huang Y, Nagashima KA et al HIV‐1 entry into CD4+ cells is mediated by the chemokine receptor CC‐CKR‐5. Nature 1996; 381: 667–673. [DOI] [PubMed] [Google Scholar]
- 3. Feng Y, Broder CC, Kennedy PE, Berger EA. HIV‐1 entry cofactor: functional cDNA cloning of a seven‐transmembrane, G protein‐coupled receptor. Science 1996; 272: 872–877. [DOI] [PubMed] [Google Scholar]
- 4. Doranz BJ, Rucker J, Yi Y, Smyth RJ, Samson M, Peiper SC et al A dual‐tropic primary HIV‐1 isolate that uses fusin and the beta‐chemokine receptors CKR‐5, CKR‐3, and CKR‐2b as fusion cofactors. Cell 1996; 85: 1149–1158. [DOI] [PubMed] [Google Scholar]
- 5. Collman R, Balliet JW, Gregory SA, Friedman H, Kolson DL, Nathanson N et al An infectious molecular clone of an unusual macrophage‐tropic and highly cytopathic strain of human immunodeficiency virus type 1. J Virol 1992; 66: 7517–7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhu T, Mo H, Wang N, Nam DS, Cao Y, Koup RA et al Genotypic and phenotypic characterization of HIV‐1 patients with primary infection. Science 1993; 261: 1179–1181. [DOI] [PubMed] [Google Scholar]
- 7. Valentin A, Von Gegerfelt A, Matsuda S, Nilsson K, Asjo B. In vitro maturation of mononuclear phagocytes and susceptibility to HIV‐1 infection. J Acquir Immune Defic Syndr 1991; 4: 751–759. [PubMed] [Google Scholar]
- 8. Mosborg‐Petersen P, Toth FD, Zachar V, Villadsen JA, Norskov‐Lauritsen N, Aboagye‐Mathiesen G et al Differential HIV replication and HIV‐induced interferon production in mononuclear phagocytes: relationship to cell maturation. Res Virol 1991; 142: 353–361. [DOI] [PubMed] [Google Scholar]
- 9. Sonza S, Maerz A, Deacon N, Meanger J, Mills J, Crowe S. Human immunodeficiency virus type 1 replication is blocked prior to reverse transcription and integration in freshly isolated peripheral blood monocytes. J Virol 1996; 70: 3863–3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fear WR, Kesson AM, Naif H, Lynch GW, Cunningham AL. Differential tropism and chemokine receptor expression of human immunodeficiency virus type 1 in neonatal monocytes, monocyte‐derived macrophages, and placental macrophages. J Virol 1998; 72: 1334–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Naif HM, Li S, Alali M, Sloane A, Wu L, Kelly M et al CCR5 expression correlates with susceptibility of maturing monocytes to human immunodeficiency virus type 1 infection. J Virol 1998; 72: 830–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Collman R, Hassan NF, Walker R, Godfrey B, Cutilli J, Hastings JC et al Infection of monocyte‐derived macrophages with human immunodeficiency virus type 1 (HIV‐1). Monocyte‐tropic and lymphocyte‐tropic strains of HIV‐1 show distinctive patterns of replication in a panel of cell types. J Exp Med 1989; 170: 1149–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yoshida T, Jones VC, Kobayashi M, Li XD, Pollard RB, Suzuki F. The acceleration of R5 HIV replication by neutrophils in cultures of macrophages. Immunol Cell Biol 2007; 84: 551–556. [DOI] [PubMed] [Google Scholar]
- 14. Sasaki H, Takei M, Kobayashi M, Pollard RB, Suzuki F. Effect of glycyrrhizin, an active component of licorice roots, on HIV replication in cultures of peripheral blood mononuclear cells from HIV‐seropositive patients. Pathobiology 2002; 70: 229–236. [DOI] [PubMed] [Google Scholar]
- 15. Takei M, Kobayashi M, Li XD, Pollard RB, Suzuki F. Glycyrrhizin inhibits R5 HIV replication in peripheral blood monocytes treated with 1‐methyladenosine. Pathobiology 2005; 72: 117–123. [DOI] [PubMed] [Google Scholar]
- 16. Suzuki H, Ohta Y, Takino T, Fujisawa K, Hirayama C. Effects of glycyrrhizin on biomedical tests in patients with chronic hepatitis‐double blind trial. Asian Med J 1983; 26: 423–438. [Google Scholar]
- 17. van Rossum TG, Vulto AG, de Man RA, Brouwer JT, Schalm SW. Glycyrrhizin as a potential treatment for chronic hepatitis C. Aliment Pharmacol Ther 1998; 12: 199–205. [DOI] [PubMed] [Google Scholar]
- 18. Arase Y, Ikeda K, Murashima N, Chayama K, Tsubota A, Koida I et al The long term efficacy of glycyrrhizin in chronic hepatitis C patients. Cancer 1997; 79: 1494–1500. [DOI] [PubMed] [Google Scholar]
- 19. Kumada H. Long‐term treatment of chronic hepatitis C with glycyrrhizin [stronger neo‐minophagen C (SNMC)] for preventing liver cirrhosis and hepatocellular carcinoma. Oncology 2002; 62: 94–100. [DOI] [PubMed] [Google Scholar]
- 20. Numazaki K, Nagata N, Sato T, Chiba S. Effect of glycyrrhizin, cyclosporin A, and tumor necrosis factor α on infection of U‐937 and MRC‐5 cells by human cytomegalovirus. J Leukoc Biol 1994; 55: 24–28. [DOI] [PubMed] [Google Scholar]
- 21. Pompei R, Flore O, Marccialis MA, Pani A, Loddo B. Glycyrrhizic acid inhibits virus growth and inactivates virus particles. Nature 1979; 281: 689–690. [DOI] [PubMed] [Google Scholar]
- 22. Utsunomiya T, Kobayashi M, Pollard RB, Suzuki F. Glycyrrhizin, an active component of licorice roots, reduces morbidity and mortality of mice infected with lethal doses of influenza virus. Antimicrob Agents Chemother 1997; 41: 551–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cinatl J, Morgenstern B, Bauer G, Chandra P, Rabenau H, Doerr HW. Glycyrrhizin, an active component of liquorice roots, and replication of SARS‐associated coronavirus. Lancet 2003; 361: 2045–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shinada M, Azuma M, Kawai H, Sazaki K, Yoshida I, Yoshida T et al Enhancement of interferon‐γ production in glycyrrhizin‐treated human peritoneal lymphocytes in response to concanavalin A and to surface antigen of hepatitis B virus. Proc Soc Exp Biol Med 1986; 181: 205–210. [DOI] [PubMed] [Google Scholar]
- 25. Zhang YH, Yoshida T, Isobe K, Rahman SMJ, Nagase F, Ding L et al Modulation by glycyrrhizin of the cell‐surface expression of H‐2 class I antigens on murine tumor cell lines and normal cell populations. Immunology 1990; 70: 405–410. [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang YH, Isobe K, Nagase F, Lwin T, Kata M, Hamaguchi M et al Glycyrrhizin as a promoter of the late signal transduction for interkeukin‐2 production by splenic lymphocytes. Immunology 1993; 79: 528–534. [PMC free article] [PubMed] [Google Scholar]
- 27. Nakajima K, Martínez‐Maza O, Hirano T, Breen EC, Nishanian PG, Salazar‐Gonzalez JF et al Induction of IL‐6 (B cell stimulatory factor‐2/IFN‐beta 2) production by HIV. J Immunol 1989; 142: 531–536. [PubMed] [Google Scholar]
- 28. Merrill JE, Koyanagi Y, Chen IS. Interleukin‐1 and tumor necrosis factor alpha can be induced from mononuclear phagocytes by human immunodeficiency virus type 1 binding to the CD4 receptor. J Virol 1989; 63: 4404–4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vicenzi E, Alfano M, Ghezzi S, Gatti A, Veglia F, Lazzarin A et al Divergent regulation of HIV‐1 replication in PBMC of infected individuals by CC chemokines: suppression by RANTES, MIP‐1α, and MCP‐3, and enhancement by MCP‐1. J Leukoc Biol 2000; 68: 405–412. [PubMed] [Google Scholar]
- 30. Fantuzzi L, Spadaro F, Vallanti G, Canini I, Ramoni C, Vicenzi E et al Endogenous CCL2 (monocyte chemotactic protein‐1) modulates human immunodeficiency virus type‐1 replication and affects cytoskeleton organization in human monocyte‐derived macrophages. Blood 2003; 102: 2334–2337. [DOI] [PubMed] [Google Scholar]
- 31. Sozzani S, Ghezzi S, Iannolo G, Luini W, Borsatti A, Polentarutti N et al Interleukin 10 increases CCR5 expression and HIV infection in human monocytes. J Exp Med 1998; 187: 439–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Weissman D, Poli G, Fauci AS. IL‐10 synergizes with multiple cytokines in enhancing HIV production in cells of monocytic lineage. J Acquir Immune Defic Syndr Hum Retrovirol 1995; 9: 442–449. [PubMed] [Google Scholar]
- 33. Finnegan A, Roebuck KA, Nakai BE, Gu DS, Rabbi MF, Song S et al IL‐10 cooperates with TNF‐α to activate HIV‐1 from latently and acutely infected cells of monocyte/macrophage lineage. J Immunol 1996; 156: 841–851. [PubMed] [Google Scholar]
- 34. Yamashiro S, Kamohara H, Yoshimura T. MCP‐1 is selectively expressed in the late phase by cytokine‐stimulated human neutrophils: TNF‐α plays a role in maximal MCP‐1 mRNA expression. J Leukoc Biol 1999; 65: 671–679. [DOI] [PubMed] [Google Scholar]


