Abstract
Zaldivar‐Riverón, A., Areekul, B., Shaw, M. R. & Quicke, D. L. J. (2004). Comparative morphology of the venom apparatus in the braconid wasp subfamily Rogadinae (Insecta, Hymenoptera, Braconidae) and related taxa. —Zoologica Scripta, 33, 223–237.
The morphology of the venom apparatus intima in representatives of 38 genera of the problematic braconid wasp subfamily Rogadinae and other cyclostome braconids was investigated and a preliminary phylogenetic analysis for the group was performed with the information obtained. Despite the limited number of characters, the data suggest several relationships at various taxonomic levels. The venom apparatus in the Clinocentrini and the Stiropiini is relatively unmodified and similar to that found in other genera previously placed within a broader concept of the Rogadinae (e.g. genera of Lysitermini, Pentatermini, Tetratermini, Hormiini) and also to that of the Betylobraconinae. The presence of a cone of filaments located inside the secondary venom duct near to its insertion on the venom reservoir/primary venom duct is proposed as a synapomorphy for the tribe Rogadini to the exclusion of Stiropiini, Clinocentrini and Yeliconini. Other features of the secondary venom duct and its insertion on the venom reservoir/primary venom duct support a number of relationships between the genera of the Rogadini and also within the large genus Aleiodes. A clade containing 15 Rogadini genera (Bathoteca, Bathotecoides, Bulborogas, Canalirogas, Colastomion, Conspinaria, Cystomastacoides, Macrostomion, Megarhogas, Myocron, Pholichora, Rectivena, Rogas, Spinaria and Triraphis) is supported by the presence of a thickened and short secondary venom duct, whereas the different members of Aleiodes (excluding members of the subgenus Heterogamus) and Cordylorhogas are distinguished by having a recessed secondary venom duct with well‐defined and numerous internal filaments. New World Rogas species exhibit a unique venom apparatus and may not be closely related to the Old World ones. Features of the venom apparatus of the enigmatic genus Telengaia and the exothecine genera Shawiana and Colastes suggest that the Telengainae and Exothecinae are both closely related to the Braconinae, Gnamptodontinae, and possibly to the Opiinae and Alysiinae. An unsculptured venom reservoir was found in one specimen of the type species of Avga, A. choaspes, which is consistent with it occupying either a very basal position within the cyclostome braconids or belonging to a recently recognized ‘Gondwanan’ clade that also includes the Aphidiinae.
Introduction
In recent years, attempts to discover evolutionary relationships between different groups of parasitic braconid wasps have made increasing use of novel characters and character systems. Among these are morphological studies of the final instar larval head capsule (e.g. Capek 1970), male genitalia (e.g. Belokobylskij 1987; Quicke 1998), internal and external ovipositor features (1992a, 1992b, 1994; Rahman et al. 1998a,, b), and venom apparatus (Edson & Vinson 1979; 1997, 1992c). Information derived from such studies has proved useful in phylogenetic analyses at a range of taxonomic levels, from subfamily (e.g. Quicke & van Achterberg 1990; Whitfield 1992; van Achterberg & Quicke 1993; Quicke & Belshaw 1999; Dowton et al. 2002) to genus level (e.g. Belshaw et al. 2001; S. A. Belokobilskij, A. Zaldivar‐Riveron and D. L. J. Quicke, personal observation).
As regards venom apparatus morphology in the Braconidae, Edson & Vinson (1979) first noted a major difference between that of the cyclostome group of subfamilies which have a heavily muscularized (type 1) venom reservoir whose intima is strongly spirally sculptured, and that of the non‐cyclostomes which is far less muscularized and has a smooth intima. Quicke & van Achterberg (1990) provided additional data and scored additional features in their phylogenetic analysis of braconid subfamilies. Different component structures of the venom apparatus were subsequently shown to vary greatly both within and between two large groups of cyclostome braconid wasps, the Doryctinae (Quicke et al. 1992c) and the Opiinae + Alysiinae (Quicke et al. 1997), in each case providing indications of previously unsuspected relationships. Moreover, although in these two works no variation was encountered in the venom apparatus of the large subfamily Braconinae, they suggested that it might provide phylogenetic information concerning the members of an ill‐defined group of subfamilies that has been referred to as the Rogadinae sensu lato. The Rogadinae sensu lato constitutes one of the most problematic groups of braconids in terms of its taxonomy due to the considerable morphological and biological heterogeneity shown by its members (Shaw & Huddleston 1991). More recently, some authors have split the Rogadinae sensu lato into several small subfamilies, e.g. Exothecinae, Hormiinae, Lysiterminae, Mesostoinae (in part, namely Hydrangeocolini), Pambolinae, Rhyssalinae, and Rhysipolinae (see Quicke & van Achterberg 1990; van Achterberg 1995; Belshaw & Quicke 2002), although some of these are still morphologically heterogeneous and also dubiously monophyletic because of the absence of clear synapomorphies.
We recognize the subfamily Rogadinae, hereinafter referred to as the Rogadinae sensu stricto, to include only four tribes composed exclusively of koinobiont endoparasites of larval Lepidoptera which mummify their hosts: Clinocentrini van Achterberg 1991, Stiropiini van Achterberg 1993, Yeliconini van Achterberg 1991, and Rogadini Foerster 1862 (cf. 1991, 1993, 1995). Although mummification of the host larva is regarded as a synapomorphy that distinguishes members of the Rogadinae sensu stricto, the lack of morphological synapomorphies among its members has not allowed a proper definition for it (although most taxa have fairly distinctive Gestalt). In the present work, we describe several previously unreported features of venom apparatus morphology in various genera of the Rogadinae sensu lato and other potentially related cyclostome braconids. Based on the information obtained, we performed a preliminary phylogenetic analysis for this group. Despite the limited amount of information, we show that this character system seems to provide valuable phylogenetic information at a variety of taxonomic levels.
Methods
Material examined
The venom apparatus intimae (the chitinous part that is visible after the soft tissue is removed) of 122 specimens belonging to 100 species were examined. These comprised 24 genera representing each of the four Rogadinae sensu stricto tribes, including a large number of species of the cosmopolitan genus Aleiodes Wesmael. Furthermore, specimens of some genera belonging to the Rogadinae sensu lato (e.g. Allobracon Gahan, Lysitermus Foerster, Pentatermus Hedqvist, Hormius Blanchard), and specimens from other potentially related subfamilies (e.g. Telengainae) whose venom apparatus was not previously known, were incorporated and discussed. Two of the venom apparatus preparations included in this study [Hormius moniliatus (Nees) and Aleiodes ruficornis (Herrich‐Schaeffer) = A. dimidiatus (Spinola)] were those previously examined by Falco Gari (1991). Voucher specimens are deposited in the Natural History Museum, London, UK and in the National Museums of Scotland, Edinburgh, UK.
The venom apparatus from dry and 100% alcohol preserved specimens were prepared by sinking the metasoma in a c. 10% w/v KOH solution for at least 2 days at room temperature to dissolve all the soft tissue, followed by dissection in water, staining with Chlorazole Black E, dehydration through graded alcohols, and mounting on microscope slides using Histomount™ via Histoclear™ (National Diagnostics, New Jersey, USA) (see Quicke et al. 1992c for details). This technique makes possible a detailed observation of the fine sculpture and individual secretory ductules of the venom glands and reservoir (Quicke et al. 1992c). Terminology of the different venom apparatus structures follows 1997, 1992c. A list of the specimens examined together with their subfamilial and tribal placements follows.
Rogadinae sensu stricto
Tribe Clinocentrini. Clinocentrus cunctator (Haliday), UK; C. exsertor (Nees), UK, two specimens; C. vestigator (Haliday), UK.
Tribe Rogadini. Aleiodes albitibia (Herrich‐Schaeffer), UK; A. aciculatus Cresson, USA (two specimens); A. aestuosus (Reinhard), Cyprus; A. alternator (Nees), UK; A. apicalis (Brullé), Cyprus; A. assimilis (Nees), France; A. aterrimus (Ratzeburg), UK; A. borealis (Thomson), UK, two specimens; A. breviradialis (Granger), Zambia; A. burrus Cresson, USA; A. circumscriptus (Nees), UK; A. compressor (Herrich‐Schaeffer), UK; A. coxalis (Spinola), France; A. dispar (Haliday), UK; A. dissector (Nees), UK; A. flavistigma Shaw, Brazil; A. gasterator (Jurine), France; A. cf. gastritor (Thunberg), agg., UK [two specimens, one reared from Theria primaria (Haworth), univoltine, delayed ovigenesis; the other one reared from Eupithecia assimilata Doubleday, plurivoltine]; A. grassator (Thunberg), UK; A. malacosomatos (Mason), USA, two specimens; A. melanopterus (Erickson), Argentina, two specimens; A. modestus (Reinhard), UK; A. nigricornis Wesmael, UK; A. pallidator (Thunberg), UK; A. parasiticus Norton, USA (two specimens); A. pictus (Herrich‐Schaeffer), UK; A. plurilineatus (Cameron), South Africa; A. praetor (Reinhard), UK; A. politiceps (Gahan), USA, two specimens; A. pulchripes Wesmael, UK; A. ruficornis (Herrich‐Schaeffer) no data; A. rugulosus (Nees), UK; A. scrutator (Say), USA, two specimens; A. seriatus (Herrich‐Schaeffer), UK; A. similis (Curtis), UK; A. simillimus (Ashmead), USA; A. sp. 1, Turkey (two specimens); A. sp. 2 [bicolor (Spinola) group], no data; A. sp. 3 [tricolor (Haliday) group], Australia; A. sp. 4, Thailand; A. sp. 5, Thailand; A. sp. 6 (bicolor group), USA; A. stigmator (Say), USA (three specimens); A. testaceus (Telenga), France; A. terminalis Cresson, USA (two specimens); A. unipunctator (Thunberg), UK; Batotheca sp., Singapore; Batothecoides sp., India; Bulborogas compressifemur van Achterberg, Panama (two specimens); Canalirogas balgooyi van Achterberg & Chen, Malaysia; Colastomion concolor (Szépligeti), Benin; Conspinaria sp., Thailand; Cordylorhogas trifasciatus Enderlein, Kenya; Cystomastacoides sp. Papua New Guinea; Cystomastax sp. 1, Ecuador; Cystomastax sp. 2, Brazil; Cystomastax sp. 3, Costa Rica (two specimens); Hemigyroneurone sp., Gambia; Macrostomion gnathothlibi Shaw, Papua New Guinea; Megarhogas sp., Malaysia; Myocron antefurcale van Achterberg, Kenya (two specimens); Pholichora bipanna van Achterberg, Kenya; Rectivena sp., Sierra Leone; Rogas luteus Nees, the Netherlands; Rogas sp. 1, Peru; Rogas sp. 2, Peru; Rogas sp. 3, Costa Rica; Rogas sp. 4, Costa Rica; Rogas sp. 5, Costa Rica; Rogas sp. 6, Tanzania; Scoporogas sp., Mali; Spinaria sp., no data; Triraphis sp., Brazil; Xenolobus sp., Nyasaland.
Tribe Stiropiini. Stiropius longicarinatus van Achterberg, Panama (two specimens).
Tribe Yeliconini. Yelicones belokobilskiji Quicke, Chishti, Chen & Kruft, Thailand; Yelicones contractus Papp, Nepal; Yelicones delicatus (Say), USA; Yelicones glabromaculatus Belokobylskij, Taiwan.
Subfamily Lysiterminae
Tribe Lysitermini. Acanthormius sp., Madagascar; Aulosaphes sp., South Africa; Afrotritermus capensis (Hedqvist), South Africa.
Tribe Tetratermini. Tetratermus sp., Australia; Katytermus palmicola van Achterberg, Malaysia.
Subfamily Betylobraconinae
Mesocentrus sp. 1, Australia; Mesocentrus sp. 2, Australia.
Subfamily Exothecinae
Shawiana catenator (Haliday) no data, Mongolia (two specimens).
Subfamily Horminae
Tribe Hormiini. Allobracon sp., Brazil; Hormius moniliatus (Nees), no data; Hormius sp., Thailand; Parahormius sp., Madagascar; Parahormius sp., no data.
Tribe Avgini. Avga choaspes Nixon, no data; Avga sp., Madagascar.
Subfamily Pambolinae
Cedria africana Belokobylskij, South Africa.
Subfamily Telengainae
Telengaia ventralis Tobias, Turkmenia.
Unplaced taxa
Tribe Pentatermini. Pentatermus striatus (Szépligeti), Nigeria (three specimens); Pentatermus striatus, no data; Pentatermus sp., Thailand (two specimens).
Phylogenetic analysis
A preliminary phylogenetic analysis for the included Rogadinae and related taxa was conducted based on those characters of the venom apparatus that varied consistently. In order to discuss the variation found at different taxonomic levels, the analysis included species, genera, and subfamilies as terminal taxa. In addition to the material examined here, the subfamilies Doryctinae and Braconinae, and the genera Colastes Haliday (Exothecinae), Betylobracon (Betylobraconinae), Gnamptodon Haliday (Gnamptodontinae), and Rhysipolis Foerster (Rhysipolinae) were also included as terminal taxa, with the Doryctinae selected as the outgroup. Information on these additional taxa was extracted from previous published studies (1997, 1992c).
A maximum parsimony analysis followed by successive approximation weighting (Farris 1969) was performed with paup* 4.0b10 (Swofford 2001). The initial search with equally weighted, unordered characters used 10 000 replicates with tree bisection reconnection swapping, holding only one tree from each addition. Subsequent iterative searches, employing the maximum retention index (RI) as the reweighting function and setting the maxtrees at 30 000, were performed until weights became stable. Owing to the scarce amount of information used to construct this phylogeny, support for the clades was not evaluated. Instead, characters that appeared to support a clade with RI = 0.3 were mapped on to the topology obtained.
Results and discussion
Variation in a number of qualitative and quantitative venom apparatus features was noted at different taxonomic levels, including between currently recognized subfamilies, both between and within the four tribes of the Rogadinae sensu stricto, and between species of the genus Aleiodes (1, 2). Among the variable features that could be scored as discrete character states were: (i) sculpture (e.g. Fig. 1A–H cf. Fig. 1J), size (e.g. 2, 3cf. Fig. 2A–C), and form (e.g. Fig. 2B cf. Fig. 2C) of the venom reservoir; (ii) width (e.g. Fig. 2A cf. Fig. 3E,G) and sculpture (e.g. Fig. 2C,F cf. Fig. 3C,E) of the primary venom duct; and (iii) width and length (e.g. Fig. 3A–H cf. Fig. 1E,F), insertion site (e.g. 1, 2, 4cf. Fig. 2A–C), and structure (e.g. Fig. 4B–D cf. Fig. 4E,F) of the secondary venom duct.
Table 1.
Characters and character states from the venom apparatus used in this study.
| Character number | Morphological characters and states |
|---|---|
| 1 | Venom reservoir: (0) essentially unsculptured (e.g. Fig. 1J); (1) totally covered with spiral sculpture (e.g. Fig. 2A–I); (2) with |
| spiral sculpture but unsculptured in the anterior part of the reservoir (e.g. Fig. 1H). | |
| 2 | Venom reservoir: (0) undivided (e.g. Fig. 3A–H); (1) divided into two sections (e.g. Fig. 2D). |
| 3 | Posterior end of venom reservoir: (0) without a neck (e.g. Fig. 2A,C,F); (1) with a distinct, very narrow neck (e.g. Fig. 2B,E). |
| 4 | Venom reservoir: (0) less than 2× longer than wide (e.g. Fig. 1D); (1) 2–4× its width (e.g. Fig. 1A,B); (2) 4–6× its width (e.g. |
| Fig. 1C,E); (3) more than 6× its width (e.g. 1, 2). | |
| 5 | Primary venom duct: (0) narrow (e.g. Fig. 2B,I); (1) wide (e.g. 1, 2). |
| 6 | Primary venom duct: (0) without sculpture or with only poorly defined sculpture (e.g. 1, 2); (1) with spiral or |
| annular sculpture (e.g. 1, 2); (2) with hexagonal‐like sculpture (e.g. 2, 4); (3) globular with reticulate | |
| sculpture (e.g. Fig. 6E); (4) with evident flask‐shaped protuberances (e.g. Fig. 1I); (5) with evident secretory ductules (e.g. | |
| Quicke et al. 1992c: 3, 4, 5). | |
| 7 | Insertion of secondary venom duct: (0) anteriorly or medially on reservoir (e.g. 1, 2); (1) posteriorly on venom |
| reservoir (e.g. Fig. 3A–H); (2) distinctly on primary venom duct (e.g. 1, 6). | |
| 8 | Base of secondary venom duct: (0) without internal filaments (e.g. 1, 4); (1) with internal filaments (e.g. Fig. 4B–D). |
| 9 | Secondary venom duct insertion on to reservoir: (0) not recessed (e.g. Fig. 4A,E,F); (1) recessed, with well‐defined and |
| numerous internal filaments (e.g. Fig. 4B–D). | |
| 10 | Secondary venom duct: (0) soft (e.g. Fig. 4A–D); (1) hard, with a distinct flange (e.g. Fig. 4E,F). |
| 11 | Secondary venom duct: (0) simple or with no evident sculpture (e.g. Fig. 4E,F); (1) with spiral sculpture (e.g. Fig. 4B,C); (2) with hexagonal‐type sculpture (e.g. Fig. 4D); (3) with flask‐shaped protuberances; (4) with evident ductules (e.g. 2, 6). |
| 12 | Secondary and following venom ducts: (0) tubular and distinctly narrow, terminating abruptly in globular glandular sacks |
| (e.g. Fig. 1F,G); (1) tubular and swollen, terminating abruptly in globular glandular sacks (e.g. Fig. 3E–H); (2) swollen, more | |
| or less gradually developing into gland filaments (e.g. Fig. 1I). | |
| 13 | Secondary venom duct: (0) considerably long, at least 2× as long as the venom reservoir length (e.g. Fig. 3A,B,F); (1) moderately long, not more than 0.5× length of the venom reservoir (e.g. Fig. 1E–G); (2) distinctly short, not more than 2× as long as wide (e.g. 1, 3). |
| 14 | Secondary venom duct: (0) branched into tertiary and subsequent gland ducts, without insertion of venom glands (e.g. |
| Fig. 1A–C); (1) with numerous venom gland sacks inserted along its length and bifurcating it into the tertiary ducts near to | |
| its end (e.g. Fig. 3A,B); (2) not branched, with two venom gland bunches, one at its middle and the other at its end (e.g. Fig. 3F). | |
| 15 | Tertiary venom duct: (0) parallel‐sided (e.g. Fig. 3A–E); (1) medially expanded (e.g. 1, 6). |
Table 2.
Distribution of character states from the venom apparatus characters. See Table 1 for character descriptions. Polymorphism coded as a = 01; b = 12.
| Characters | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinocentrus | 1 | 0 | 0 | 2 | 0 | 1 | b | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Aleiodes type A | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 2 | 0 | 0 |
| Aleiodes type B | 1 | 0 | 1 | 2 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 2 | 0 | 0 |
| Aleiodes type C | 1 | 0 | 0 | 1 | 1 | 2 | 1 | 1 | 1 | 0 | 2 | 1 | 2 | 0 | 0 |
| Aleiodes type D | 1 | 1 | 0 | 3 | 0 | 2 | 1 | 1 | 1 | 0 | 1 | 1 | 2 | 0 | 0 |
| Aleiodes type E | 1 | 0 | 1 | 2 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 2 | 0 | 0 |
| Aleiodes type F | 1 | 0 | 1 | 2 | 1 | 2 | 0 | 1 | 1 | 0 | 0 | 1 | 2 | 0 | 0 |
| Batotheca | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 2 | 0 | 0 |
| Batothecoides | 1 | 0 | 0 | 2 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 2 | 0 | 0 |
| Bulborogas | 1 | 0 | 0 | 1 | 0 | 2 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 2 | 0 |
| Canalirogas | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 2 | 0 | 0 |
| Colastomion | 1 | 0 | 0 | 1 | 1 | 2 | 1 | 1 | 0 | 1 | 0 | 1 | 2 | 0 | 0 |
| Conspinaria | 1 | 0 | 0 | 1 | 1 | 2 | 1 | 1 | 0 | 1 | 0 | 1 | 2 | 0 | 0 |
| Cordylorhogas | 1 | 0 | 1 | 2 | 1 | 2 | 1 | 1 | 1 | 0 | 1 | 1 | 2 | 0 | 0 |
| Cystomastacoides | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 2 | 0 | 0 |
| Cystomastax | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 2 | 0 | 0 |
| Hemigyroneuron | 1 | 0 | 0 | 2 | 1 | 2 | 0 | 1 | 0 | 0 | 1 | 1 | 2 | 0 | 0 |
| Macrostomion | 1 | 0 | 0 | 1 | 1 | 2 | 1 | 1 | 0 | 1 | 0 | 1 | 2 | 0 | 0 |
| Megarhogas | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 2 | 0 | 0 |
| Myocron | 1 | 0 | 0 | 1 | 1 | 2 | 1 | 1 | 0 | 1 | 0 | 1 | 2 | 0 | 0 |
| Pholichora | 1 | 0 | 0 | 1 | 1 | 3 | 2 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| Rectivena | 1 | 0 | 0 | ? | 1 | 1 | ? | 1 | 0 | 1 | 0 | 1 | 2 | 0 | 0 |
| Rogas New World | 1 | 0 | 0 | 2 | 1 | 2 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 |
| Rogas Old World | 1 | 0 | 0 | 2 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 2 | 0 | 0 |
| Scoporogas | 1 | 0 | 0 | 2 | 1 | 2 | 1 | 1 | 0 | 0 | 0 | 1 | 2 | 0 | 0 |
| Spinaria | 1 | 0 | 1 | 2 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 2 | 0 | 0 |
| Triraphis | 1 | 0 | 0 | 2 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 2 | 0 | 0 |
| Xenolobus | 1 | 0 | 0 | 1 | 1 | 2 | 1 | 1 | 0 | 0 | 2 | 1 | 2 | 0 | 0 |
| Stiropius | 1 | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Yelicones New World | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 4 | 0 | 1 | 0 | 0 |
| Yelicones Asia | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 4 | 1 | 1 | 0 | 1 |
| Acanthormius | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 |
| Afrotritermus | 1 | 0 | 0 | 1 | 0 | ? | 1 | 0 | 0 | 0 | ? | 0 | 1 | 0 | 0 |
| Aulosaphes | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 0 |
| Katytermus | 1 | 0 | 0 | 2 | 0 | 1 | ? | 0 | 0 | 0 | ? | 0 | 2 | 0 | 0 |
| Tetratermus | 1 | 0 | 0 | 2 | 0 | 2 | 2 | 0 | 0 | 0 | 3 | 0 | 2 | 0 | 0 |
| Betylobracon | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Mesocentrus | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Rhysipolis | 0 | 1 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 |
| Allobracon | 2 | 0 | 0 | 3 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 |
| Avga choaspes | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Avga sp. | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 |
| Hormius | 1 | 0 | 0 | 2 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Parahormius | 1 | 0 | 0 | 2 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 |
| Cedria | 1 | 0 | 0 | 2 | 0 | 1 | ? | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Pentatermus | 1 | 0 | 0 | 2 | 0 | 0 | 1 | a | 0 | a | a | a | 1 | 0 | 0 |
| Telengaia | 1 | 0 | 0 | 1 | 0 | 4 | 1 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 1 |
| Colastes | 1 | 1 | 0 | 2 | 0 | 4 | 0 | 0 | 0 | 0 | 1 | 2 | 1 | 0 | 0 |
| Shawiana | 1 | 0 | 1 | 2 | 0 | 4 | 1 | 0 | 0 | 0 | 1 | 2 | 1 | 0 | 0 |
| Braconinae | 1 | 0 | 0 | 2 | 0 | 4 | 1 | 0 | 0 | 0 | 0 | 2 | ? | ? | 0 |
| Gnamptodon | 1 | 0 | 0 | 2 | 0 | 5 | 1 | 0 | 0 | 0 | 1 | 2 | 1 | 0 | 0 |
| Doryctinae | 1 | 1 | 0 | 3 | 0 | 5 | 2 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 |
Figure 1.
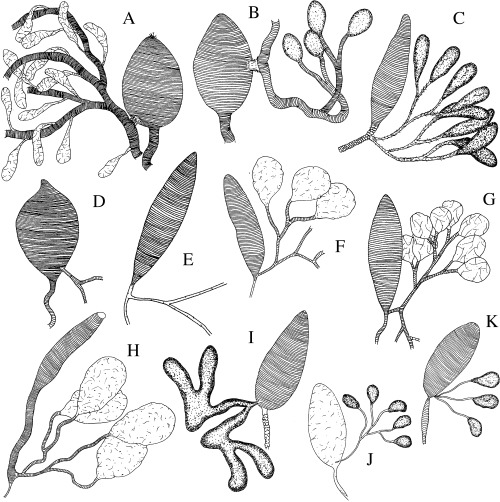
A–K. Interpreted drawings of the chitinous intimas of venom glands and reservoirs of Rogadini, Tetratermini, Betylobraconinae, Stiropiini, Pentatermini, Hormiinae, Telengainae and Avgini. —A. Megarhogas sp. —B. Batotheca sp. —C. Tetratermus sp. —D. Mesocentrus sp. —E. Stiropius longicarinatus. —F. Pentatermus striatus. —G. Hormius moniliatus. —H. Allobracon sp. —I. Telengaia ventralis. —J. Avga choaspes. —K. Avga sp.
Figure 2.
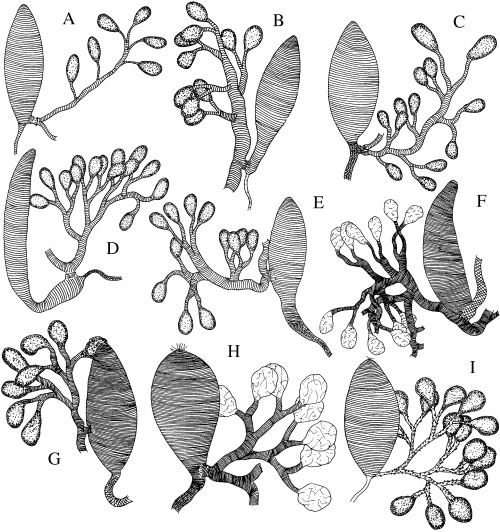
A–I. Interpreted drawings of the chitinous intimas of venom glands and reservoirs of Rogadini and Yeliconini. —A. Aleiodes dispar. —B. A. circumscriptus. —C. A. unipunctator. —D. A. albitibia. —E. A. parasiticus. —F. Scoporogas sp. —G. Hemigyroneurone sp. —H. Xenolobus sp. —I. Yelicones delicatus.
Figure 3.
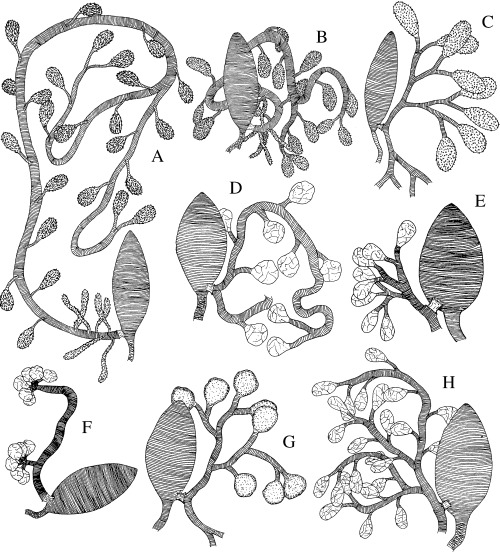
A–H. Interpreted drawings of the chitinous intimas of venom glands and reservoirs of Rogadini. —A. Rogas sp. 1. —B. Rogas sp. 4. —C. R. luteus. —D. Colastomion concolor. —E. Cystomastacoides sp. —F. Bulborogas compressifemur. —G. Canalirogas balgooyi. —H. Macrostomion gnathothlibi.
Figure 4.
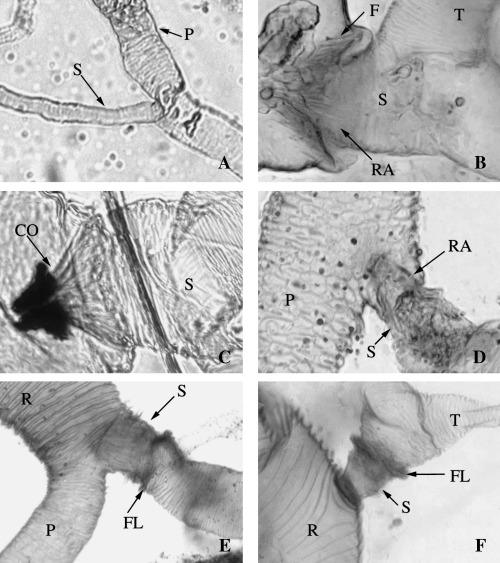
A–F. Automontage‚ light photomicrographs of the secondary venom duct. —A. Clinocentrus cunctator. —B. Aleiodes albitibia. —C. Cordylorhogas sp. —D. A. pulchripes. —E. Rogas sp. 1. —F. Batotheca sp. CO, cone of filaments; F, filaments; FL, flange; P, primary duct; R, reservoir; RA, recessed area; S, secondary duct; T, tertiary duct.
In total, 15 characters were recorded (Table 1), of which 14 were parsimony informative. The initial heuristic search resulted in 1714 most parsimonious trees with length = 69, RI = 0.76, and consistency index (CI) = 0.39. The final analysis using the successive approximation weighting approach reached the limit imposed of 30 000 most parsimonious trees with length = 40.62, RI = 0.85, and CI = 0.47. The strict consensus of these trees is shown in Fig. 5. The most relevant features observed in the venom apparatus within each of the taxa included are described below and their phylogenetic relationships based on the analysis performed are discussed.
Figure 5.
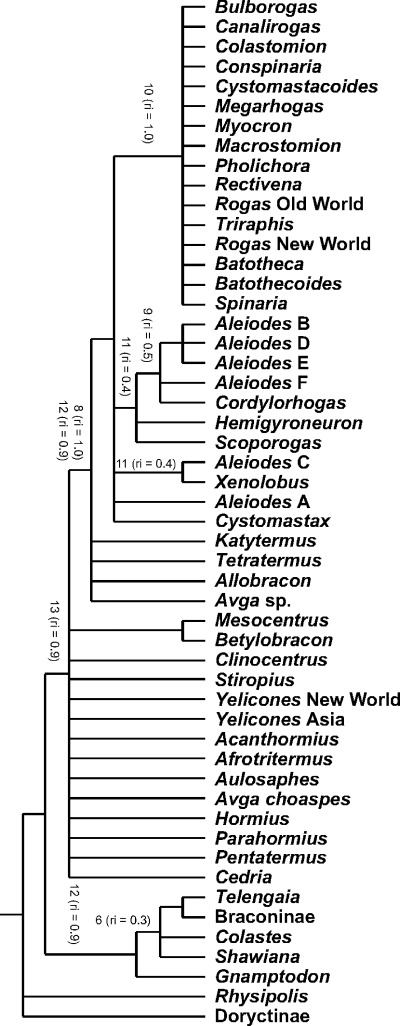
Strict consensus of the 30 000 most parsimonious trees recovered using a successive approximation approach for the venom apparatus characters examined in this study. The numbers refer to characters supporting recovered clades with a retention index = 0.33 (see Table 1 for character descriptions).
Rogadinae sensu stricto
Within the Rogadinae sensu stricto there are considerable differences in the venom apparatus among its four recognized tribes, with no evident synapomorphies that help to define this subfamily with respect to the other cyclostome subfamilies and in particular the Doryctinae (Quicke et al. 1992c). Nevertheless, there are some characters that distinguish members of the Rogadini from the remaining tribes of the Rogadinae sensu stricto. The Rogadini, which is by far the most diverse tribe in the Rogadinae sensu stricto, currently comprises two subtribes, the Rogadina and the Spinariina, the first being much more diverse and widespread than the second. Members of the Rogadini are defined by having the inner sides of the eyes emarginate, the second tergite usually with a triangular or semicircular mediobasal area, and a short ovipositor sheath (van Achterberg 1991). The venom apparatus in the specimens of the Rogadini examined usually consists of a wide venom reservoir (e.g. 2, 3), a wide primary venom duct (e.g. Figs 2F–H, 6E; except some Aleiodes, e.g. Fig. 2A,B), a short and wide secondary venom duct (e.g. Fig. 1A,B; except Pholichora van Achterberg and some Rogas Nees, see below), and glands well differentiated and often numerous (e.g. 2, 3). Although a short and wide secondary venom duct is also present in most species of the Opiinae and Alysiinae, the gland ducts in these taxa are not tubular and the venom glands are often undifferentiated with respect to their ducts (Quicke et al. 1997).
Figure 6.
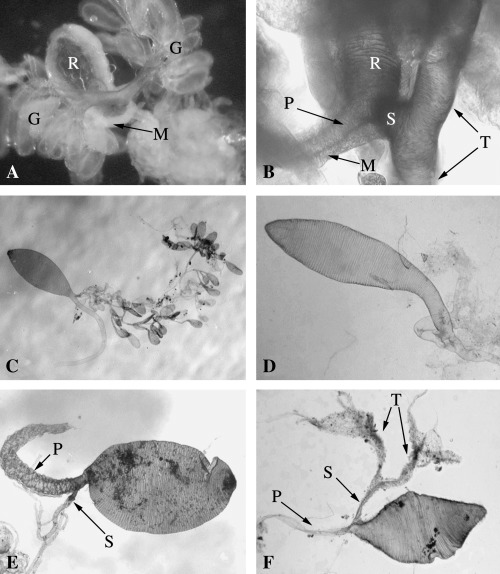
A–F. Automontage‚ light photomicrographs of Rogadinae venom apparatus. —A. Muscularized venom apparatus of Aleiodes borealis, dark field illumination showing the thick muscle layer over a brown pigmented reservoir intima, and symmetrical branching of the venom glands. —B. Muscularized secondary venom duct and part of the venom reservoir of A. borealis. —C–F. Chlorazol Black stained venom reservoir and part of the gland ducts of A. pulchripes, A. coxalis, Pholichora bipanna, and Yelicones contractus, respectively. G, venom glands; M, muscle layer; P, primary duct; R, reservoir; S, secondary duct; T, tertiary duct.
Despite the above differences, the synapomorphy that consistently supports the monophyly of the Rogadini in our analysis, apart from the presence of a swollen secondary venom duct (RI = 0.9), is the presence of a ring of distally directed filaments within the secondary venom duct just at or before its insertion into the venom reservoir (RI = 1.0; Fig. 4B–F). These filaments are arranged in a cone which we suggest acts as a valve for controlling the flow of venom from the glands to the reservoir, potentially limiting back‐flow when the reservoir muscles contract to expel venom during host manipulation (Fig. 7A–C). The presence of thick muscle around the secondary venom duct (Fig. 6A,B) suggests that the wasp may be able to control the opening of the filament cone. No similar structure has been observed in any other group of braconids and therefore in our phylogenetic hypothesis it appears as a synapomorphy that defines this tribe.
Figure 7.
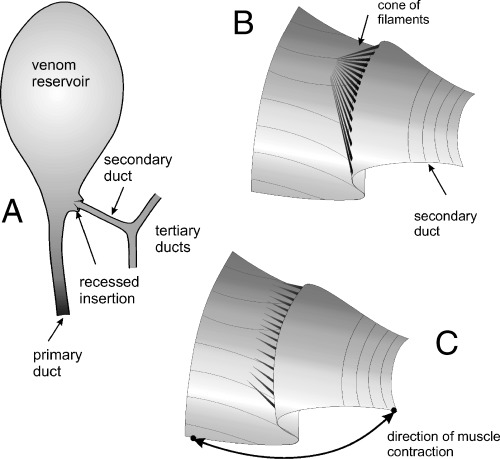
A–C. Diagrammatic representations of venom apparatus features in the Rogadinae. —A. Showing terminology of venom ducts. —B, C. Detail of the internal structure of the recessed insertion of the secondary duct on to the reservoir, showing filaments in a cone arrangement (B) and indicating the hypothesized result of the contraction of muscles associated with insertion causing an increase in the degree of recess and consequent separation of filaments (C).
Some relationships within the Rogadini can be proposed based on features of the secondary venom duct. A number of genera of the subtribe Rogadina (Bulborogas van Achterberg, Canalirogas van Achterberg & Chen, Colastomion Baker, Conspinaria Schulz, Cystomastacoides van Achterberg, Myocron van Achterberg, Macrostomion Szépligeti, Pholichora, Rectivena van Achterberg, Rogas, and Triraphis Ruthe) and the three examined species representing the subtribe Spinariina (Spinaria Brullé, Bathoteca Enderlein, and Bathotecoides Watanabe) are nested within a clade supported by the presence of a thickened, apparently rigid, and distinctly differentiated secondary venom duct with a distinct flange (RI = 1.0; e.g. Fig. 4E,F). Although Pholichora has a narrow and moderately large secondary venom duct (Fig. 6E), its modification is essentially similar. On the other hand, the six remaining examined genera of the Rogadina (Aleiodes, Cordylorhogas Enderlein, Cystomastax Szépligeti, Hemigyroneurone Baker, Scoporogas van Achterberg, and Xenolobus Cameron) lack the above feature, instead having a soft, undifferentiated secondary venom duct (Fig. 4B–D).
The relationships recovered between the Rogadini genera are also supported by some external morphological characters. A setal comb on the inner side of the apex of the hind tibia is found in most of those genera with a thickened and short secondary venom duct, and is absent in those with a soft secondary venom duct and also in Pholichora, which has a distinctive duct (Fig. 6E). The only exception is A. seriatus, which also has a setal comb but has a soft secondary venom duct as in the other examined species of Aleiodes. Furthermore, in two subsets of genera, two other external morphological characters show a high degree of congruence with the thickened secondary venom duct, these being a basally widened first metasomal tergite which is present in Canalirogas, Colastomion, Macrostomion, and Myocron, and curved and largely glabrous hind tibial spurs in Colastomion, Cystomastacoides, Macrostomion, Megarhogas, Myocron, and Rectivena. Finally, although not recovered as monophyletic in our analysis, members of the Spinariina, which are characterized by a disproportionately small and transverse head and by the metasomal tergites being united to form a carapace and bearing spines (Chen & He 1997), are the only genera which have a thickened secondary venom duct inserted at the midlength of the reservoir (Fig. 1B; see also Quicke et al. 1992c: fig. 8C). However, because these genera share the probably derived thickened hard secondary venom duct with several other Rogadini genera, it seems that the Spinariina is just a derived genus group within a larger group of genera.
With more than 300 described species, the cosmopolitan genus Aleiodes is the largest genus of the Rogadinae sensu stricto (Chen & He 1997). Members of Aleiodes are known usually to oviposit into second or early third instar larvae of several macrolepidopteran families and kill the host before it becomes fully grown (Shaw 1983; Shaw & Huddleston 1991). Although many species of Aleiodes have in the past been placed in Rogas (e.g. Shaw 1983), the latter name is correctly applied to a different group and both generic names are considered as valid (van Achterberg 1991; Shaw 1997). However, owing to the occurrence of aberrant character states within Aleiodes, the distinction between even the true Rogas and Aleiodes can be problematic, with few consistent or convincing characters to separate them, such that keys to genera rely heavily or entirely on the presence of a large pointed basal lobe to the claws in Rogas. From our study, based on the differences in the secondary venom duct mentioned above, it seems that Aleiodes and Rogas are each more closely related to other genera of Rogadini than to each other. Unlike all other Rogadini, Cordylorhogas and all species of Aleiodes, except for A. dispar and A. spp. 4 and 5 (see below), have the secondary venom duct evidently recessed and with numerous, well‐defined internal filaments (Fig. 4B–D). Despite the fact that one of the terminal taxa with a recessed secondary venom duct is nested outside the clade comprising the rest of the taxa with this condition (species of Aleiodes with type C venom apparatus, see below), this arrangement is apparently unique within the Braconidae and probably represents a synapomorphy for these two genera. Although not recovered in our phylogenetic hypothesis, variation in the venom apparatus between the species of Rogas from Old and New Worlds has also revealed a marked dichotomy. Whereas the gland ducts in European and Afrotropical Rogas are symmetrically branched in a similar way to those of the other Rogadini genera (Fig. 3C), those of all five New World Rogas species examined possess an extremely long tubular secondary venom duct that has numerous venom gland sacks inserted along its length and which only bifurcates into the tertiary ducts near to its end (Fig. 3A,B). The relative length of the secondary venom duct varies among the New World species, being relatively longer in larger species.
Examination of the venom apparatus in 46 species of Aleiodes from different continents resulted in the recognition of six different venom apparatus types (2, 3), although most of the species displayed either type B or type C. It is notable that the type B species all belong to the subgenus Aleiodes (sensu van Achterberg 1991), while those of type C all fall into the subgenera Chelonorhogas Enderlein or Neorhogas Szépligeti. Furthermore, our species groupings based on venom apparatus type are generally congruent with the phylogeny proposed by Fortier & Shaw (1999), where the 208 examined species of Aleiodes were placed in 18 species groups distributed among three sections (basal, intermediate and derived). Our observations revealed that: (1) the only described species we examined with type A venom apparatus, A. dispar (Fig. 2A), and the species with type E venom apparatus are present in the intermediate A. dispar species group; (2) species with type B venom apparatus (2, 6) fall within basal groups or within the intermediate coxalis species group; and (3) species with types C, D, and F venom apparatus (Fig. 2C,D,E, respectively) are placed in derived species groups.
Table 3.
Classification of the species of Aleiodes examined in this study and their respective venom apparatus types. Character states for the venom apparatus types are listed in Table 2.
| Venom apparatus types | Aleiodes species |
|---|---|
| Type A | A. dispar, A. sp. 4, A. sp. 5 |
| Type B | A. aciculatus, A. alternator, A. assimilis, A. borealis, A. circumscriptus, A. compressor, A. coxalis, A. cf. gastritor, A. |
| malacosomatos, A. modestus, A. nigricornis, A. pallidator, A. pictus, A. scrutator, A. seriatus, A. similis, A. | |
| simillimus, A. sp. 1, A. sp. 2 (bicolor group), A. sp. 6 (bicolor group), A. stigmator, A. testaceus | |
| Type C | A. aestuosus, A. apicalis, A. aterrimus, A. burrus, A. dissector, A. flavistigma, A. gasterator, A. grassator, A. |
| melanopterus, A. politiceps, A. praetor, A. pulchripes, A. ruficornis, A. rugulosus, A. sp. 3 (tricolor group), | |
| A. terminalis, A. unipunctator | |
| Type D | A. albitibia |
| Type E | A. breviradialis, A. plurilineatus |
| Type F | A. parasiticus |
Although our data are not sufficient to resolve fully the relationships within Aleiodes, it is interesting that in A. dispar and related species, the venom apparatus differs from the others in being least modified. Although type A venom apparatus possesses a series of filaments at the insertion of the secondary venom duct, these are not so well defined in this group and lack a distinctly recessed part of the secondary duct. Van Achterberg (1991) synonymized four small genera with Aleiodes, including Heterogamus Wesmael of which dispar is the type species, because he considered that recognition of all of them as genera would leave the remainder of Aleiodes paraphyletic. The differences in the venom apparatus between A. dispar, with its closely related species, and the other Aleiodes studied suggest that these species represent a basal lineage within Aleiodes, and in the future it may be desirable to resurrect the generic name Heterogamus for them (M. Mori, M. R. Shak & D. L. J. Quicke, unpublished results).
Based on their morphological and venom action characteristics, the tribes Clinocentrini and Stiropiini have been proposed as basal members within the Rogadinae sensu stricto (Shaw 1983; Whitfield 1988; Shaw & Huddleston 1991). Species of these taxa are known as endoparasitoids of web‐inhabiting or semiconcealed well‐grown microlepidoptera (Shaw 1983; Shaw & Huddleston 1991), and of leaf‐mining Lyonetiidae and Gracillariidae larvae (Whitfield 1988; Shaw 1997), respectively (cf. 1991, 1995). The unmodified venom apparatus morphology in the examined specimens of these tribes compared with members of the Rogadini suggests a basal placement of them within the Rogadinae sensu stricto, although their relationships were not recovered here. Typically, the venom apparatus in these taxa consists of an undivided and sculptured venom reservoir, a narrow primary venom duct, a narrow secondary venom duct without any traces of filaments at its insertion with the venom reservoir, and well‐differentiated and not numerous venom glands (1, 4).
Yelicones Cameron, the only genus of the tribe Yeliconini, is a moderately extensive, cosmopolitan group whose species are known as solitary endoparasites of lepidopteran larvae which they also mummify (Capek 1970; Quicke & Kruft 1995). Furthermore, some morphological features present in Yelicones (e.g. short, robust tarsi; longitudinal row of pegs on the fore tibia; form of hypoclypeal depression) resemble those seen in members of other groups (e.g. Betylobraconinae, various Doryctinae such as Stenocorse Marsh, and Opiinae). The venom apparatus in the four examined species of Yelicones does not show any obvious relationship with other taxa (2, 6; see also Quicke et al. 1992c). However, our observations do suggest a possible biogeographical trend within Yelicones. In the Asian species of Yelicones examined, the venom ducts show evident ductules and have a very distinctive form with the secondary ducts medially expanded, which could terminate either in a few or no distinct venom glands (Fig. 6F). In contrast, the only Yelicones examined from the New World possesses tubular and strongly branched ducts with numerous, well‐differentiated glands that are more typical of members of the Rogadini (Fig. 2I).
Other taxa
Considerable confusion has existed with respect to the placement of some groups of cyclostome genera that exhibit intermediate morphological characteristics between the Doryctinae and the Rogadinae sensu lato (Wharton 1993). Most of these genera were originally grouped by Shenefelt (1975) in four tribes of the subfamily Exothecinae (namely Exothecini, Pambolini, Hormiini, and Lysitermini). Currently, despite extensive taxonomic work on these genera, their placement has not been adequately solved, mainly because of the vast amount of homoplasy present in their external morphological characters.
In general, the venom apparatus in Betylobraconinae and in most of the examined genera that were previously in the Rogadinae sensu lato and are now excluded from the Rogadinae sensu stricto (i.e. in the Hormiini, Lysitemiini, Pambolini, Pentatermini, Tetratermini) resemble those seen in the examined specimens of the Clinocentrini and Stiropiini (e.g. Fig. 1C,D,F,G), showing no derived features. The only exception involves one specimen of Pentatermus striatus, which differs from the rest of the specimens assigned to this genus by having a hard secondary venom duct with filaments, as in some members of the Rogadina. Currently, six species of Pentatermus have been described, P. austrini Belokobylskij, P. carinatus Hedqvist, P. concolor Szépligeti, P. medvedewi Belokobilskij, P. parnarae He & Chen, and P. striatus Szépligeti. Because they cannot be readily differentiated morphologically, P. carinatus had previously been considered as a synonym of the widespread Afrotropical and oriental P. striatus (van Achterberg 1991). However, in the last revision of the genus, Belokobylskij (2002) considered P. striatus and P. carinatus as separate species, and P. medvedewi and P. parnarae as junior synonyms of P. striatus. Although, unfortunately, our specimen with a hard secondary venom duct has no locality data, this finding emphasizes the necessity of more detailed taxonomic studies on this poorly known genus and poses the possibility that it is not monophyletic as currently constituted. Indeed, this genus is not defined by any obvious autapomorphy.
Wharton (1993) placed the genus Allobracon Gahan within the Hormiinae based mainly on the presence of a desclerotized metasomal tergite and the narrow separation of the submedius from the hind wing margin. However, differences in the petiole and propodeum with respect to the Hormiinae noted by Wharton leave the placement of Allobracon still uncertain. As in some members of the Doryctinae, Alysiinae and Opiinae (the last two not included in this study), the venom reservoir of Allobracon is divided and, as in some opiines and alysiines, has a distinctly unsculptured posterior area (Fig. 1H). However, the secondary and tertiary ducts in Allobracon are tubular and with well‐differentiated glands, whereas in the Opiinae they are swollen and more or less gradually developing into gland filaments (Quicke et al. 1997). Moreover, although some alysiines have similar secondary and tertiary gland ducts, they never occur in combination with a sculptured anterior bulb to the reservoir (Quicke et al. 1997).
The Telengainae and the Exothecinae are two small braconid subfamilies, the first one represented only by the enigmatic genus Telengaia Tobias, which is restricted to Central Asia (Quicke & van Achterberg 1990; van Achterberg 1993), whereas the second one comprises few genera which contain idiobiont ectoparasites of mining larvae of holometabolous insects (Shaw 1983; 1983, 1993). Although Quicke & van Achterberg (1990) stated that the Telengainae lacks any clear morphological synapomorphy with other braconid subfamilies, they hypothesized that it may be closely related to the Gnamptodontinae and to the Braconinae, mainly because of the presence of posteriorly diverging grooves on the third metasomal tergite. Moreover, in this work, the authors demonstrated that the group currently referred to as the Exothecinae does not form part of the Rogadinae sensu stricto. These suggestions are consistent with the features of the venom apparatus observed for the specimens examined belonging to the Telengainae and the Exothecinae. Telengaia and the two members of the Exothecinae (Shawiana van Achterberg and Colastes) were recovered in a clade containing the Braconinae and Gnamptodon of the Gnamptodontinae (Fig. 5). The synapomorphy that supports this clade is the presence of swollen secondary and following venom ducts, more or less gradually developing into gland filaments (RI = 0.33; e.g. Fig. 1I.) This type of gland duct also resembles those observed in most members of the Opiinae and Alysiinae (Quicke et al. 1997). Furthermore, within the latter clade there is a subclade containing the Braconinae, Telengainae and Exothecinae. This clade is supported by the presence of a primary duct densely supplied with ductules that open into a fairly large dome or vase‐shaped chamber (RI = 0.33; e.g. Fig. 1I). This same apomorphic condition has also been observed in some species of the Alysiinae (Quicke et al. 1997).
The presence of an unsculptured venom reservoir is a feature apparently fixed in the non‐cyclostome braconids with the marked exception of the putatively basal Trachypetinae (Quicke & van Achterberg 1990; 1999, 1992c; Jiménez et al. 1997), although it has also been seen in some species of the Aphidiinae, which has proved to be part of a ‘Gondwanan’ clade that is the sister group of the cyclostome braconids (Belshaw et al. 2000; Dowton et al. 2002). In contrast, all the cyclostome braconids present a pattern of spiral annulation on the venom reservoir which seems to be a derived characteristic that distinguishes this group (Quicke & van Achterberg 1990; 1997, 1992c). In this study, all the taxa included present a venom reservoir with spiral sculpture except one of the two examined species of Avga, the type species A. choaspes (Fig. 1J). Our analysis did not include non‐cyclostome taxa, and hence this feature was uninformative. However, it can be observed that the overall morphology of the venom apparatus in the two examined species of Avga (Fig. 1J,K) is more similar to those seen in the Aphidiinae than to those of the Hormiinae. Thus, the possibility that Avga does not form part of the cyclostome group needs to be considered. This finding seems to agree with Wharton's (1993) review of the Hormiini, who observed that A. choaspes and closely related species present some morphological differences with respect to the rest of hormiines. Future critical taxonomic studies are needed to test the monophyly of Avga and the actual taxonomic position of A. choaspes and related species.
Acknowledgements
We thank Kees van Achterberg, Scott Shaw, Sura Pimpasalee, Georg Georgen, John LaSalle, Jenö Papp, Vladimir Tobias, Shen‐Horn Yen, and Doug Yu for providing some of the specimens examined in this study; Gavin Broad for collecting living material; Andrew Polaszek for use of Automontage®; and David Orme for help with editing the figures. This work was made possible by a National Council of Science and Technology (CONACyT, Mexico) scholarship for PhD studies to AZR, and a Royal Thai Government scholarship for PhD studies to BA.
References
- Van Achterberg, C. (1983). Revisionary notes on the Palaearctic genera and species of the tribe Exothecini Foerster (Hymenoptera: Braconidae). Zoologische Mededelingen, 57, 339–355. [Google Scholar]
- Van Achterberg, C. (1991). Revision of the genera of the Afrotropical and W. Palearctic Rogadinae Foerster (Hymenoptera: Braconidae). Zoologische Verhandelingen, 273, 1–102. [Google Scholar]
- Van Achterberg, C. (1993). Illustrated key to the subfamilies of the Braconidae (Hymenoptera: Ichneumonoidea). Zoologische Verhandelingen, 283, 1–189. [Google Scholar]
- Van Achterberg, C. (1995). Generic revision of the subfamily Betylobraconinae (Hymenoptera: Braconidae) and other groups with modified fore tarsus. Zoologische Verhandelingen, 298, 1–242. [Google Scholar]
- Van Achterberg, C. & Quicke, D. L. J. (1993). Phylogeny of the subfamilies of the family Braconidae: a reassessment assessed. Cladistics, 8, 237–264. [DOI] [PubMed] [Google Scholar]
- Belokobylskij, S. A. (1987). Structure of the male genitalia of the braconid subfamily Doryctinae (Hymenoptera: Braconidae), their evolution and meaning for the classification of the group. Nauka, 69, 209–219. [Google Scholar]
- Belokobylskij, S. A. (2002). On the genus Pentatermus Hedqvist (Hymenoptera: Braconidae). Zoosystematica Rossica, 10, 387–396. [Google Scholar]
- Belshaw, R. , Dowton, M. , Quicke, D. L. J. & Austin, A. D. (2000). Estimating ancestral geographic distributions: a Gondwanan origin for aphid parasitoids. Proceedings of the Royal Society of London, Series B, 267, 491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belshaw, R. , Lopez‐Vaamonde, C. , Degerli, N. & Quicke, D. L. J. (2001). Paraphyletic taxa and taxonomic chaining: evaluating the classification of braconine wasps (Hymenoptera: Braconidae) using 28S, D2–3 rDNA sequences and morphological characters. Biology Journal of the Linnean Society, 73, 411–424. [Google Scholar]
- Belshaw, R. & Quicke, D. L. J. (2002). Robustness of ancestral state estimates: evolution of life history strategy in ichneumonoid parasitoids. Systematic Biology, 51, 450–477. [DOI] [PubMed] [Google Scholar]
- Capek, M. (1970). A new classification of the Braconidae (Hymenoptera) based on the cephalic structures of the final instar larva and biological evidence. Canadian Entomologist, 102, 846–875. [Google Scholar]
- Chen, X. & He, J. (1997). Revision of the subfamily Rogadinae (Hymenoptera: Braconidae) from China. Zoologische Verhandelingen, 308, 1–187. [Google Scholar]
- Dowton, M. , Belshaw, R. , Austin, A. D. & Quicke, D. L. J. (2002). Simultaneous molecular and morphological analysis of braconid relationships (Insecta: Hymenoptera: Braconidae) indicates independent mt‐tRNA gene inversions within a single wasp family. Journal of Molecular Evolution, 54, 210–226. [DOI] [PubMed] [Google Scholar]
- Edson, K. M. & Vinson, S. B. (1979). A comparative morphology of the venom apparatus of female braconids (Hymenoptera: Braconidae). Canadian Entomologist, 111, 1013–1024. [Google Scholar]
- Falco Gari, J. V. (1991). Contribución al estudio de las subfamilias Braconinae, Doryctinae y Rogadinae (Hymenoptera, Braconidae) de España. PhD Thesis, Universitat de Valencia.
- Farris, J. S. (1969). A successive approximations approach to character weighting. Systematic Zoology, 18, 374–385. [Google Scholar]
- Fortier, J. C. & Shaw, S. R. (1999). Cladistics of the Aleiodes lineage of the subfamily Rogadinae (Hymenoptera: Braconidae). Journal of Hymenoptera Research, 8, 204–237. [Google Scholar]
- Jiménez, R. , Falco‐Gari, J. V. & Moreno, J. (1997). Descripción del aparato del veneno de Cosmophorus cembrae Ruschka (Hymenoptera, Braconidae). Bulletin de la Societè Entomologique de France, 102, 385–389. [Google Scholar]
- Quicke, D. L. J. (1998). Inter‐generic variation in the male genitalia of the Braconinae (Insecta, Hymenoptera, Braconidae). Zoologica Scripta, 17, 399–409. [Google Scholar]
- Quicke, D. L. J. & Van Achterberg, C. (1990). Phylogeny of the subfamilies of the family Braconidae (Hymenoptera). Zoologische Verhandelingen, 258, 1–95. [Google Scholar]
- Quicke, D. L. J. , Van Achterberg, C. & Godfray, H. C. J. (1997). Comparative morphology of the venom gland and reservoir in opiine and alysiine braconid wasps (Insecta, Hymenoptera, Braconidae). Zoologica Scripta, 26, 23–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quicke, D. L. J. , Basibuyuk, H. H. , Fitton, M. G. & Rasnitsyn, A. P. (1999). Morphological, paleontological and molecular aspects of ichneumonoid phylogeny (Hymenoptera, Insecta). Zoologica Scripta, 28, 175–202. [Google Scholar]
- Quicke, D. L. J. & Belshaw, R. (1999). Incongruence between morphological data sets: an example from the evolution of endoparasitism among parasitic wasps (Hymenoptera: Braconidae). Systematic Biology, 48, 436–454. [Google Scholar]
- Quicke, D. L. J. , Ficken, L. & Fitton, M. G. (1992a). New diagnostic ovipositor characters for doryctine wasps (Hymenoptera, Braconidae). Journal of Natural History, 26, 1035–1046. [Google Scholar]
- Quicke, D. L. J. , Fitton, M. G. & Ingram, S. (1992b). Phylogenetic implications of the structure and distribution of ovipositor valvilli in the Hymenoptera. Journal of Natural History, 26, 587–608. [Google Scholar]
- Quicke, D. L. J. , Fitton, M. G. , Tunstead, J. R. , Ingram, S. N. & Gaitens, P. V. (1994). Ovipositor structure and relationships within the Hymenoptera, with special reference to the Ichneumonoidea. Journal of Natural History, 28, 635–682. [Google Scholar]
- Quicke, D. L. J. & Kruft, R. A. (1995). Species of Yelicones (Hymenoptera: Braconidae: Rogadinae) in North America with descriptions of two new species. Annals of the Entomological Society of America, 88, 129–138. [Google Scholar]
- Quicke, D. L. J. , Tunstead, J. , Falco, J. V. & Marsh, P. M. (1992c). Venom gland and reservoir morphology in the Doryctinae and related braconid wasps (Insecta, Hymenoptera, Braconidae). Zoologica Scripta, 21, 403–416. [Google Scholar]
- Rahman, H. , Fitton, M. G. & Quicke, D. L. J. (1998a). Ovipositor internal microsculpture in the Braconidae (Insecta, Hymenoptera). Zoologica Scripta, 27, 319–331. [Google Scholar]
- Rahman, H. , Fitton, M. G. & Quicke, D. L. J. (1998b). Ovipositor internal microsculpture and other features in doryctine wasps (Insecta, Hymenoptera, Braconidae). Zoologica Scripta, 27, 333–343. [Google Scholar]
- Shaw, M. R. (1983). On[e] evolution of endoparasitism: the biology of some genera of Rogadinae. Contributions of the American Entomological Institute, 20, 307–328. [Google Scholar]
- Shaw, S. R. (1997). Rogadinae In Wharton R. A., Marsh P. M. & Sharkey M. J. (Eds) Manual of the New World Genera of the Family Braconidae (Hymenoptera). International Society of Hymenopterists, Special Publication 1 (pp. 403–412). Washington DC, WA: International Society of Hymenopterists. [Google Scholar]
- Shaw, M. R. & Huddleston, T. (1991). Classification and biology of braconid wasps (Hymenoptera: Braconidae). Handbooks for the Identification of British Insects, 7 (11), 1–126. [Google Scholar]
- Shenefelt, R. D. (1975). Braconidae 8. Exothecinae. Rogadinae. Hymenopterum Catalogus, 12, 1115–1262. [Google Scholar]
- Swofford, D. L. (2001). paup*: Phylogenetic Analysis Using Parsimony (*and Other Methods), version 4.0b10. Sunderland, MA: Sinauer. [Google Scholar]
- Wharton, R. A. (1993). Review of the Hormiini (Hymenoptera: Braconidae) with a description of new taxa. Journal of Natural History, 27, 107–171. [Google Scholar]
- Whitfield, J. B. (1988). Revision of the Nearctic species of the genus Stiropius Cameron (=Bucculatriplex Auct.) with the description of a new related genus (Hymenoptera: Braconidae). Systematic Entomology, 13, 373–385. [Google Scholar]
- Whitfield, J. B. (1992). The polyphyletic origin of endoparasitism in the cyclostome lineages of Braconidae (Hymenoptera). Systematic Entomology, 17, 273–286. [Google Scholar]


