Summary
Antibody detection against selected potentially zoonotic vector‐borne alphaviruses and flaviviruses was conducted on sera from bats from all six parishes in Grenada, West Indies. Sera were tested for (i) antibodies to flaviviruses West Nile virus, St. Louis encephalitis virus, Ilhéus virus, Bussuquara virus (BSQV), Rio Bravo virus and all four serotypes of dengue virus (DENV) by plaque reduction neutralization test (PRNT); (ii) antibodies to alphaviruses western equine encephalitis virus, Venezuelan equine encephalitis virus and eastern equine encephalitis virus by epitope‐blocking enzyme‐linked immunosorbent assay (ELISA); and (iii) antibodies to the alphavirus chikungunya (CHIKV) by PRNT. Two species of fruit bats were sampled, Artibeus jamaicensis and Artibeus lituratus, all roosting in or within 1,000 m of human settlements. Fifteen (36%) of the 42 bats tested for neutralizing antibodies to CHIKV were positive. The CHIKV‐seropositive bats lived in localities spanning five of the six parishes. All 43 bats tested for epitope‐blocking ELISA antibody to the other alphaviruses were negative, except one positive for Venezuelan equine encephalitis virus. All 50 bats tested for neutralizing antibody to flaviviruses were negative, except one that had a BSQV PRNT 80 titre of 20. The CHIKV serology results indicate that bats living close to and within human settlements were exposed to CHIKV in multiple locations. Importantly, bats for this study were trapped a year after the introduction and peak of the human CHIKV epidemic in Grenada. Thus, our data indicate that bats were exposed to CHIKV possibly during a time of marked decline in human cases.
Keywords: alphavirus, antibodies, arbovirus, bats, flavivirus, Grenada
Impacts.
Neutralizing antibodies to chikungunya virus (CHIKV) that was introduced into Grenada in 2014 were detected in 15 of the 42 bats tested (36%). These bats inhabited five of the six parishes in Grenada.
Grenada has been endemic for the dengue flavivirus for decades, but neutralizing antibodies to this virus were not detected in any of the 50 bats tested.
Results suggest that bats may be a reservoir host for chikungunya in the Caribbean. The detection of antibodies to CHIKV indicates exposure to infected mosquitoes, suggesting replication of the virus in bats and warrants further investigation to determine whether levels of viremia in bats could infect mosquitoes.
1. INTRODUCTION
Bats are of great importance for healthy ecosystems, as they are abundant mammals that contribute to seed dispersion, flower pollination and insect control (Calisher, Childs, Field, Holmes, & Schountz, 2006; Hahn et al., 2014; Kunz, Braun de Torrez, Bauer, Lobova, & Fleming, 2011). However, they can also harbour viruses that have zoonotic potential (Moratelli & Calisher, 2015; Vasconcelos & Calisher, 2016; Wynne & Wang, 2013). Due to the possible impact on human health, there is increasing interest in determining which viruses infect bats and clarifying the role that these mammals play in the ecology of zoonotic viruses.
Bats have been identified as primary reservoir hosts for several high impact zoonotic viruses, including several variants of the rabies virus and other lyssaviruses (Rhabdoviridae), SARS and MERS coronaviruses (Coronaviridae), Nipah and Hendra viruses (Paramyxoviridae) and Ebola and Marburg viruses (Filoviridae). As is frequently observed with reservoir hosts, and except for the rabies virus variants, these viruses appear to cause little to no pathology in bats (Calisher et al., 2006).
Of particular interest for the Caribbean region is the role bats may play in the ecology of mosquito‐borne zoonotic arboviruses, as several of these viruses are either endemic or have been recently introduced (Gibson, Fitzpatrick, Stone, Noel, & Macpherson, 2016). It is unknown whether or not bats can play a role in inter‐epidemic viral maintenance in sylvatic cycles. A first step in investigating whether bats may be important in the ecology of any of these viruses is to determine whether exposure occurs under natural conditions. Several studies have documented bat exposure to mosquito‐transmitted zoonotic alphaviruses and flaviviruses in various parts of the world. For example, a recent study in Trinidad identified bats that were positive for antibodies to Venezuelan encephalitis virus (VEEV) and St. Louis encephalitis virus (SLEV) by epitope‐blocking enzyme‐linked immunosorbent assay (ELISA) and to an undetermined flavivirus by hemagglutination inhibition assay (HIA) (Thompson et al., 2015). A study of insectivorous bats in New Jersey and New York detected antibodies that neutralized West Nile virus (WNV) and SLEV (Pilipski, Pilipskl, & Risley, 2004). Bats with neutralizing antibodies to VEEV, eastern equine encephalitis virus (EEEV), western equine encephalitis virus (WEEV) and SLEV have also been reported in Guatemala (Ubico & McLean, 1995), and bats with neutralizing antibodies to SLEV have been found in Ohio (Herbold, Heuschele, Berry, & Parsons, 1983). A study on bats in Indonesia detected HIA antibodies against both flaviviruses (Japanese encephalitis virus and Zika virus [ZIKV]) and alphaviruses (Ross River virus and chikungunya virus [CHIKV]), but failed to detect any neutralizing antibodies to these viruses (Olson et al., 1983).
Currently, the mosquito‐borne alphaviruses and flaviviruses with highest public health significance in the Caribbean are dengue virus (DENV), CHIKV and ZIKV (Gibson et al., 2016). Several studies have found evidence of bat exposure to, or infection with DENV under natural conditions, across a wide range of endemic areas and utilizing various detection methods. Dengue virus has been detected in bats in the Gulf and Pacific coasts of Mexico by RT‐PCR and by DENV NS1 protein detection (Aguilar‐Setien et al., 2008), and in south‐eastern Mexico by RT‐PCR (Sotomayor‐Bonilla et al., 2014). Antibodies that neutralized DENV were detected in bats in Mexico (Machain‐Williams et al., 2013), Costa Rica and Ecuador (Platt et al., 2000). However, all titres in these bats were low, indicating that they could have been infected with a DENV‐like virus not included in the plaque reduction neutralization test (PRNT) analysis. Furthermore, experimental infection of the Jamaican fruit bat (Artibeus jamaicensis) with DENV serotypes 1 and 4 did not demonstrate viremia or seroconversion (Cabrera‐Romo et al., 2014), and some studies failed to detect seropositive bats in areas where DENV is known to circulate (Cabrera‐Romo et al., 2016; Maguire, Macnamara, Miles, Spears, & Mataika, 1971). These contrasting results may be the product of confounding factors such as bat species, DENV serotype, geographic region and the type of antibody assay used. Antibodies to CHIKV have been detected in wild rodents (Darwish, Hoogstraal, Roberts, Ahmed, & Omar, 1983; Vourc'h et al., 2014) and non‐human primates (Vourc'h et al., 2014) exposed under natural conditions. Experimental infection of bats with CHIKV has been performed with no apparent clinical signs (Bedekar & Pavri, 1969; Bosco‐Lauth, Nemeth, Kohler, & Bowen, 2016), but one of these studies demonstrated that bats can generate neutralizing antibodies to CHIKV (Bosco‐Lauth et al., 2016). With the exception of one report describing the isolate of CHIKV from the fruit bat Rousettus leschenaultia in China (Chen & Tao, 1996) and phylogenetic evidence indicating the involvement of bats in the zoonotic potential of alphaviruses (Olival et al., 2017), there have been no published reports that confirm exposure of bats to CHIKV under natural conditions. Of note is that ZIKV was introduced into the Caribbean after samples for this study were collected, and thus, exposure to this virus was not examined.
Grenada is a small island state in the southernmost region of the Caribbean Windward Islands. As is common in the Caribbean, humans in Grenada live in close proximity to bats, and both bats and mosquitos are abundant and active year around. Grenada reflects much of the rest of the Caribbean in that DENV has been endemic for decades and by experiencing the introduction and epidemic of CHIKV in 2014. Thus, Grenada provides an ideal setting within which to determine whether bats are exposed to these, and possibly other, zoonotic mosquito‐transmitted alphaviruses and flaviviruses under natural conditions. Importantly, the study reported here reflects data on bats sampled in the fall of 2015, a year after the peak chikungunya epidemic in Grenada (PAHO, [Link]) and before ZIKV was detected on this island (PAHO, [Link]).
2. MATERIALS AND METHODS
2.1. Bat capture and sampling
Bats were captured using mist nets and hand nets and placed in individual clean cloth bags to prevent cross‐contamination after capture (for a concurrent virus study). Bats were captured next to inhabited locations from all six parishes of Grenada from mid‐August into the first week of September of 2015. Bats were transported live to the necropsy laboratory at St. George's University, School of Veterinary Medicine, where they were euthanized using isoflurane overdose and exsanguination. The Animal Care and Use Committee of the American Society of Mammalogists (Sikes & Gannon, 2007) and St. George's University Institutional Animal Care and Use Committee (IACUC‐14008R) approve these methods as humane. Digital images were collected, and a written report was generated on each bat to document location of capture, species, sex, age (juvenile, subadult or adult) and gross findings. Cardiac blood collection was performed immediately after isoflurane overdose to obtain serum samples. Serum volume varied depending on the bat's size, from 0.1 ml in the smaller insectivorous bats (weight 10–12 g) to 1 ml in the larger fruit bats (weight 40–60 g).
2.2. Epitope‐blocking ELISA
Sera were tested for antibodies to WEEV, VEEV and EEEV by epitope‐blocking ELISA following procedures previously described (Thompson et al., 2015; Wang et al., 2005). The antigens used were VEEV‐TC83 (vaccine) strain for VEEV, North American Strain (NJ/60) for EEEV and Fleming strain for WEEV. The monoclonal antibodies (anti‐WEEV—MAB8746; anti‐VEEV—MAB8767) (EMD Millipore Corporation, Temecula, CA, USA) and anti‐EEEV (IBT Bioservices, Gaithersburg, MD, USA) were used in the assay.
The seropositivity of the tested alphaviruses was calculated by use of the formula: % inhibition = 100 − (TS − B)/(CS − B) × 100, where TS = optical density (OD) of test sera, CS = OD of control sera and B = background OD (Hall, Broom, Hartnett, Howard, & Mackenzie, 1995). Each new batch of CS, normal equine serum, is tested by PRNT using all the flaviviruses tested for in the laboratory to ensure that it is negative. For TS, CS and B, the mean optical densities from duplicate tests were used. An inhibition value of 30% or greater was considered to indicate the presence of viral antibodies (Blitvich et al., 2003). Each sample was tested in duplicate, so an average optical density was first calculated. Normal control equine sera (Vector Laboratories Inc, Burlingame, CA, USA) were utilized with the ELISAs.
2.3. PRNT for flaviviruses
Sera were assayed by PRNT following standard protocols (Beaty, Calisher, & Shope, 1995). Plaque reduction neutralization tests were performed using nine flaviviruses: Bussuquara virus (BSQV; strain BeAn‐4073), dengue virus type 1 (DENV‐1; strain Hawaii), dengue virus type 2 (DENV‐2; strain NGC), dengue virus type 3 (DENV‐3; strain H‐87), dengue virus type 4 (DENV‐4; strain 241), Ilhéus virus (ILHV; original strain), Rio Bravo virus (RBV; strain M64), St. Louis encephalitis virus (SLEV; strain TBH‐28) and West Nile virus (WNV; strain NY99‐35261‐11). Bussuquara virus, ILHV, SLEV, WNV and the four DENV serotypes were obtained from the World Health Organization Center for Arbovirus Reference and Research at the US Centers for Disease Control and Prevention, Division of Vector‐Borne Infectious Diseases in Fort Collins, Colorado; RBV was obtained from the World Arbovirus Reference Collection at the University of Texas Medical Branch in Galveston, Texas. Plaque reduction neutralization tests were performed in six‐well plates using African green monkey kidney (Vero) cells. Sera were initially tested at a dilution of 1:20. Those that reduced the number of plaques by ≥80% (PRNT80) were titrated. Final titres were expressed as the reciprocal of serum dilutions yielding ≥80% reduction in the number of plaques (PRNT80). For aetiological diagnosis, the PRNT80 antibody titre to the respective virus was required to be at least 4‐fold greater than that of the other flaviviruses tested.
2.4. PRNT for CHIKV
Plaque reduction neutralization test was performed to demonstrate the presence of neutralizing antibodies against CHIKV. Chikungunya virus 181‐25 strain, which belongs to the Asian genotype, was used in study. The strain was chosen because of the endemic status of the Asian genotype of CHIKV in the New World. Sera were tested in duplicate at each dilution with a starting dilution of 1:10 or higher if sufficient serum was not available. Neutralization of antibodies was performed at 37°C for 1 hr. Mixtures of serum and virus were plated on Vero 76 cells in 24‐well plates and incubated at 37°C for 5 days. PRNT end‐point titres were expressed as the reciprocal of the last serum dilution showing the desired per cent reduction in plaque counts. Currently, no international CHIKV antibody reference sera are available for routine testing. The PRNT titre was calculated based on a 50% or greater reduction in plaque counts (PRNT50).
The PRNT assays for the flaviviruses and for CHIKV were performed in different laboratories with minor discrepancies on diagnostic standards. Both laboratories used the same cell line, providing the same standards for comparison of neutralizing antibody titres. The PRNT50 standard was used in the serological diagnosis of CHIKV because the relative short period of its circulation in the New World might limit the incidence of repeated exposure and result in lower antibody titres in naturally infected bats.
2.5. Ethical approval and permits
The capture of bats was approved by the Grenada Ministry of Agriculture, Forestry and Fisheries. The study design and all methods were approved by the Institutional Animal Care and Use Committee of St. George's University for the capture, euthanasia and sampling of bats.
3. RESULTS
One hundred and twenty‐three bats were captured. Based on external morphological features, the four species collected were the insectivore Molossus molossus (velvety free‐tailed bat; n = 26), the frugivores A. jamaicensis (Jamaican fruit bat; n = 51), Artibeus lituratus (great fruit‐eating bat; n = 2) and the nectarivore Glossophaga longirostris (Miller's long‐tongued bat; n = 43). Sera were obtained from 111 bats, and a subset (n = 50) was selected for this study. These 50 individuals represented bats from all six parishes of Grenada and were all fruit bats (A. jamaicensis, n = 48 and A. lituratus, n = 2). Figure 1 shows the number of bats serologically tested from each of the parishes of Grenada.
Figure 1.
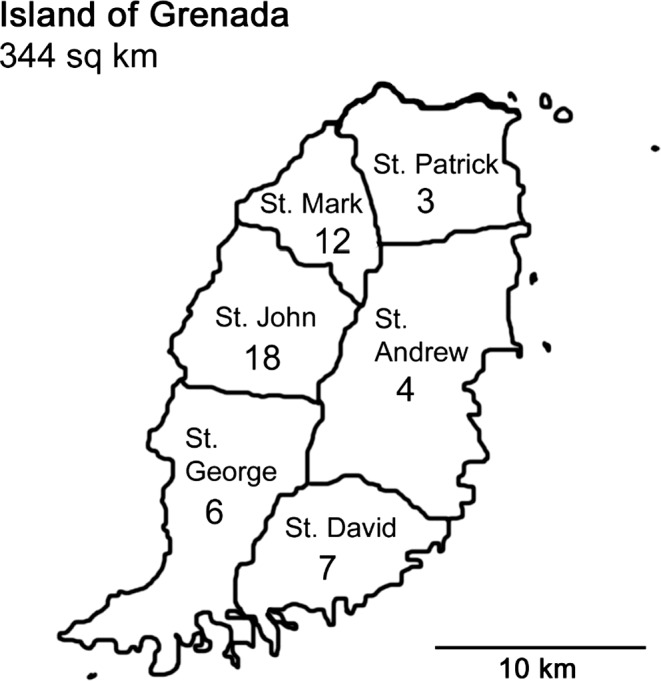
Island of Grenada. Number of bats sampled in each parish
The results of the serology analyses for the alphaviruses and flaviviruses tested are shown in Table 1. Fifteen of 42 bats (36%) were seropositive by PRNT for CHIKV. These bats represented both species of fruit bats tested and were trapped in five of the six parishes. Eight bats were not tested for antibodies to CHIKV because an insufficient amount of sera was available. Of the 38 bats tested by epitope‐blocking ELISA for antibodies to VEEV, WEEV and EEEV, all were negative except one A. jamaicensis that was seropositive for VEEV. Twelve bats were not tested for antibodies to these viruses due to insufficient sera. All 50 bats were tested by PRNT for evidence of flavivirus infection, and all were negative except one A. jamaicensis that had a BSQV PRNT80 titre of 20.
Table 1.
Seropositivity for alpha and flavivirus antibodies in bats from Grenada
| Virus | Test | Species (# positive/# tested) Parishes Total % positive | Titres |
|---|---|---|---|
| CHIKV | PRNT50 |
Artibeus lituratus (1/2) St. George Artibeus jamaicensis (14/40) All parishes except St. Andrew 36% |
Positive at 1:10–>1:640 dilution |
| Bussuquara virus | PRNT80–90 |
A. jamaicensis (1/48) A. lituratus (0/2) St. David 2% |
Pos at PRNT80 (Neg at PRNT90) |
| DEN 1, 2, 3, 4 | PRNT80–90 | 0/0.0% | NA |
| WNV | PRNT80–90 | 0/0.0% | NA |
| SLEV | PRNT80–90 | 0/0.0% | NA |
| Rio Bravo virusa | PRNT80–90 | 0/0.0% | NA |
| VEEV | Epitope‐blocking ELISA |
A. jamaicensis (1/36) A. lituratus (0/2) St. John 2.6% |
30.38% inhibition |
| WEEV | Epitope‐blocking ELISA | 0/0.0% | NA |
| EEEV | Epitope‐blocking ELISA | 0/0.0% | NA |
CHIKV, chikungunya virus; EEEV, eastern equine encephalitis virus; ELISA, enzyme‐linked immunosorbent assay; PRNT, plaque reduction neutralization test; SLEV, St. Louis encephalitis virus; VEEV, Venezuelan encephalitis virus; WEEV, western equine encephalitis virus; WNV, West Nile virus.
Although not mosquito‐transmitted, neutralizing antibodies for Rio Bravo virus were also assayed. Rio Bravo virus is a flavivirus associated with bats and, if present, can influence the interpretation of antibody responses to other flaviviruses.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Details on the 15 bats with antibodies that neutralized CHIKV are shown in Table 2. Chikungunya virus PRNT50 titres and the number of bats with these titres were as follows: 10 (n = 3), 20 (n = 5), 40 (n = 3), 80 (n = 1), 160 (n = 1), ≥320 (n = 1) and ≥640 (n = 1). Eight of the 15 seropositive bats, all A. jamaicensis (seven males and one female), were trapped at the same time from a cave on the western coast of Grenada in St. John parish. This cave is located approximately 500 m from human habitation. Three of the 15 seropositive bats, all female A. jamaicensis, were trapped at the same time from an abandoned hotel in the St. David parish, approximately 1,000 m from human habitation. All other positive bats for CHIKV represented single findings at their respective locations, and all of these locations were within 100 m of human settlements.
Table 2.
Chikungunya virus PRNT50 titres by bat species, sex (F: female; M: male), parish and capture conditions
| Species/(Sex/#) | Parish | Capture conditions | CHIKV titre PRNT50 |
|---|---|---|---|
| Artibeus lituratus (M/1) | St. George | Captured on mist net near golf course and houses | 1:80 |
| Artibeus jamaicensis (M/1) | St. George | Captured on mist net near river and houses | 1:10 |
| A. jamaicensis (M/7, F/1) | St. John | Cave roost hand net | 1:10–>1:640 |
| A. jamaicensis (F/3) | St. David | Abandoned hotel hand net | 1:10–1:40 |
| A. jamaicensis (M/1) | St. Patrick | School roof mist net | 1:20 |
| A. jamaicensis (F/1) | St. Mark | Abandoned house hand net | 1:20 |
CHIKV, chikungunya virus; PRNT, plaque reduction neutralization test.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
4. DISCUSSION
Little is known about bat immune responses to viruses (Schountz, 2014) including the type, duration and protection of antibody responses generated to infection with specific zoonotic alphaviruses and flaviviruses. Further complicating the interpretation of serologic data on bats under natural conditions are coinfections, cross‐reacting antibodies and lack of information on which viruses are currently circulating in a particular geographic area. Of the alphaviruses and flaviviruses included in the study reported here, only DENV and CHIKV are known to circulate in Grenada. ZIKV also occurs in Grenada (PAHO, 2017), but the bats were sampled prior to the introduction of the virus onto the island. The only other virus included in this study that has been evaluated for exposure in any animal in Grenada is WNV. In 2006, 45 donkeys were tested by WNV IgM ELISA and all were negative (Chikweto et al., 2008). Thus, other than DENV and CHIKV, there is limited information on which mosquito‐borne alphaviruses and flaviviruses occur in Grenada.
Chikungunya virus was introduced into Grenada in 2014, and the first documented human case was in May 2014 (PAHO, [Link]). Serologic evidence of bat exposure to CHIKV needs to be interpreted with caution because of the possible cross‐reactivity with Mayaro virus (MAYV), a related alphavirus endemic to Latin America (Garcia de Figueiredo & Figueiredo, 2014). Although serological cross‐reactivity has been observed in human humoral immune responses, it remains unclear if a similar cross‐reactivity occurs in bats (Fox et al., 2015). Mayaro virus is mostly confined to forest ecosystems (Vasconcelos & Calisher, 2016), but urban human cases have been reported, most recently from the Caribbean island of Haiti (Lednicky et al., 2016). The presence of MAYV in Grenada is unknown. In support of our positive CHIKV serologic results, a concurrent study on the molecular detection of alphaviruses in a larger bat sample confirmed the presence of CHIKV in one of the 15 CHIKV‐seropositive bats included in this study, and in seven of 73 bats (9.6%) not serologically tested (Cheetham, manuscript in preparation). It is possible that a subset of the CHIKV‐seropositive bats in this study, particularly those with low PRNT50 titres, could have been exposed to a CHIKV‐like virus not included in the PRNT analysis. This is unlikely because the only alphavirus identified by RT‐PCR and Sanger sequencing was CHIKV. Importantly, bats for this study were trapped a year after the peak of the human CHIKV epidemic on the island (PAHO, [Link]), and seropositive bats were identified from five of the six parishes of the island of Grenada. Thus, our results indicate that bats living close to and within human settlements were exposed to CHIKV in multiple locations and may have been exposed during a time of marked decline in human cases (PAHO fall 2014 and 2015 data). Future studies will determine if bats continue to be infected with CHIKV even in the absence of human infections.
The only other alphavirus for which antibodies were detected was VEEV in one bat, as determined by blocking ELISA. Of interest is that the per cent positive for VEEV antibodies due to this one bat (one bat = 2.6% of those tested) is in close agreement with results (2.9%) from a larger number of tested bats in Trinidad (Thompson et al., 2015), a much larger island located 103 miles from Grenada, and a region where VEEV is known to circulate (Jonkers, Aitken, Spence, & Worth, 1966). The same epitope‐blocking ELISA was used in both studies. The detection of one seropositive bat constitutes the first report of antibodies to VEEV in any animal in Grenada. Additional testing will be required to confirm this observation and determine whether VEEV is actively circulating in Grenada.
Bussuquara virus was the only flavivirus that neutralized bat serum. The BSQV neutralizing antibodies were detected in a single bat, and it had a low PRNT80 titre (20) indicating it may have been exposed to another, perhaps unrecognized, flavivirus not included in the PRNT analysis. The only flavivirus known to be circulation in Grenada at the time of this study is DENV. Historically, DENV has been endemic in Grenada for decades and all four serotypes have been documented on the island at various times between 2000 and 2013 (Schioler & Macpherson, 2009). Thus, the lack of detection of antibodies that neutralized DENV in the 50 bats tested in this study was an unexpected result. In an endemic area of Mexico, antibodies that neutralized DENV were detected in the two species of fruit bat that were also evaluated in this study, A. jamaicensis and A. lituratus (Machain‐Williams et al., 2013). In endemic areas of Costa Rica and Ecuador, DENV serotype 2 neutralizing antibodies were also detected in an unspecified Artibeus species (Platt et al., 2000). Importantly, the concurrent molecular study (Cheetham, manuscript in preparation) confirmed the presence of DENV RNA in six of the 50 DENV‐seronegative bats in this study and in 35 bats not serologically tested. Two of the sequenced bands confirmed DENV‐3. Our negative DENV serology results may reflect lack of, or undetectable levels of, neutralizing antibody responses developed against the DENV serotype circulating in Grenada at the time the bats sampled in this study became infected. It will be important to determine the type of immune responses to DENV in naturally exposed Grenada bats, including whether there is a consistent lack of neutralizing antibodies to some serotypes in some bat species, and whether or not bats generate non‐neutralizing antibodies. Answering these questions will shed light on the potential that bats have to establish persistent infections and high‐titre viraemias, with implications for the role of bats in the ecology of specific DENV serotypes.
As in the Grenada bats, all bats tested in a recent study in Trinidad were negative for antibody to EEEV, WNV and WEEV. However, out of the 308 bats tested in Trinidad, a few were found positive for RBV (1.0%) and SLEV (or a closely related virus: 1.8%) by HIA and blocking ELISA, respectively (Thompson et al., 2015). It is possible that RBV and SLEV are either not circulating in Grenada or are present at very low levels and that the smaller sample size in this study did not allow for detection. It is also possible that bats do not make neutralizing antibodies in response to infection with these viruses and thus were missed by the PRNT used in this study.
In conclusion, the CHIKV serologic results reported here, which are supported by the detection of CHIKV RNA in some of the bats, indicate that there was widespread exposure of at least two species of fruit bats, A. jamaicensis and A. lituratus, to CHIKV in Grenada. Future studies will determine whether bats continue to be exposed to CHIKV in Grenada in the absence of human infections, the types and duration of antibody responses to CHIKV and whether bats under natural conditions can develop high‐level viraemias sufficient to infect the primary vector in Grenada, Aedes aegypti.
CONFLICT OF INTEREST
None.
ACKNOWLEDGEMENTS
We are grateful to the St. George's University Small Research Grant Initiative for its financial support (Grant number 14018). We thank the government of Grenada Ministry of Agriculture, Forestry and Fisheries and Dr. Bowen Louison, Grenada Chief Veterinary Officer, for their assistance and cooperation. We thank St. George's University veterinary students Kathleen Parker, Cassandra Tang Wing and Christine Cornish and Grenada veterinarian Dr. Kenrith Carter for their assistance with bat capturing and sample preparation. We also thank Dr. Kathryn Gibson for her assistance with the figure for this manuscript.
Stone D, Lyons AC, Huang Y‐JS, et al. Serological evidence of widespread exposure of Grenada fruit bats to chikungunya virus. Zoonoses Public Health. 2018;65:505–511. 10.1111/zph.12460
REFERENCES
- Aguilar‐Setien, A. , Romero‐Almaraz, M. L. , Sanchez‐Hernandez, C. , Figueroa, R. , Juarez‐Palma, L. P. , Garcia‐Flores, M. M. , … Ramos, C. (2008). Dengue virus in Mexican bats. Epidemiology and Infection, 136, 1678–1683. 10.1017/S0950268808000460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty, B. J. , Calisher, C. H. , & Shope, R. E. (1995). Diagnostic procedures for viral and rickettsial diseases. Washington, DC: American Public Health Association. [Google Scholar]
- Bedekar, S. D. , & Pavri, K. M. (1969). Studies with chikungunya virus. Part I. Susceptibility of birds and small mammals. Indian Journal Medical Research, 57, 1181–1192. [PubMed] [Google Scholar]
- Blitvich, B. J. , Bowen, R. A. , Marlenee, N. L. , Hall, R. A. , Bunning, M. L. , & Beaty, B. J. (2003). Epitope‐blocking enzyme‐linked immunosorbent assays for detection of west nile virus antibodies in domestic mammals. Journal of Clinical Microbiology, 41, 2676–2679. 10.1128/JCM.41.6.2676-2679.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco‐Lauth, A. M. , Nemeth, N. M. , Kohler, D. J. , & Bowen, R. A. (2016). Viremia in North American mammals and birds after experimental infection with chikungunya viruses. American Journal of Tropical Medicine and Hygiene, 94, 504–506. 10.4269/ajtmh.15-0696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera‐Romo, S. , Max Ramirez, C. , Recio‐Totoro, B. , Tolentino‐Chi, J. , Lanz, H. , Del Angel, R. M. , … Ludert, J. E. (2016). No evidence of dengue virus infections in several species of bats captured in central and southern Mexico. Zoonoses and Public Health, 63, 579–583. 10.1111/zph.12276 [DOI] [PubMed] [Google Scholar]
- Cabrera‐Romo, S. , Recio‐Totoro, B. , Alcala, A. C. , Lanz, H. , del Angel, R. M. , Sanchez‐Cordero, V. , … Ludert, J. E. (2014). Experimental inoculation of Artibeus jamaicensis bats with dengue virus serotypes 1 or 4 showed no evidence of sustained replication. American Journal of Tropical Medicine and Hygiene, 91, 1227–1234. 10.4269/ajtmh.14-0361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calisher, C. H. , Childs, J. E. , Field, H. E. , Holmes, K. V. , & Schountz, T. (2006). Bats: Important reservoir hosts of emerging viruses. Clinical Microbiology Reviews, 19, 531–545. 10.1128/CMR.00017-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, B. , & Tao, S. (1996). Arbovirus survey in China in recent ten years. Chinese Medical Journal, 109, 13–15. [PubMed] [Google Scholar]
- Chikweto, A. , Sharma, R. N. , Matthew, V. , Stratton, G. , Thorne, K. , Parke, L. , & Dummer, D. (2008). Seroprevalence of West Nile virus in donkeys in Grenada and Carriacou. West Indian Veterinary Journal, 8, 32–33. [Google Scholar]
- Darwish, M. A. , Hoogstraal, H. , Roberts, T. J. , Ahmed, I. P. , & Omar, F. (1983). A sero‐epidemiological survey for certain arboviruses (Togaviridae) in Pakistan. Transactions of the Royal Society of Tropical Medicine and Hygiene, 77, 442–445. 10.1016/0035-9203(83)90106-2 [DOI] [PubMed] [Google Scholar]
- Fox, J. M. , Long, F. , Edeling, M. A. , Lin, H. , van Duijl‐Richter, M. K. , Fong, R. H. , … Diamond, M. S. (2015). Broadly neutralizing alphavirus antibodies bind an epitope on E2 and inhibit entry and egress. Cell, 163, 1095–1107. 10.1016/j.cell.2015.10.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia de Figueiredo, M. L. , & Figueiredo, L. T. M. (2014). Emerging alphaviruses in the Americas: Chikungunya and mayaro. Revista da Sociedade Brasileria de Medicina Tropical, 47, 677–683. 10.1590/0037-8682-0246-2014 [DOI] [PubMed] [Google Scholar]
- Gibson, K. E. , Fitzpatrick, D. M. , Stone, D. M. , Noel, T. P. , & Macpherson, C. N. L. (2016). Vector‐borne diseases in the Caribbean: History and current status. CAB Reviews, 11, 1–28. [Google Scholar]
- Hahn, M. B. , Epstein, J. H. , Gurley, E. S. , Islam, M. S. , Luby, S. P. , Daszak, P. , & Patz, J. A. (2014). Roosting behaviour and habitat selection of Pteropus giganteus reveals potential links to Nipah virus epidemiology. Journal of Applied Ecology, 51, 376–387. 10.1111/1365-2664.12212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, R. A. , Broom, A. K. , Hartnett, A. C. , Howard, M. J. , & Mackenzie, J. S. (1995). Immunodominant epitopes on the NS1 protein of MVE and KUN viruses serve as targets for a blocking ELISA to detect virus‐specific antibodies in sentinel animal serum. Journal of Virological Methods, 51, 201–210. 10.1016/0166-0934(94)00105-P [DOI] [PubMed] [Google Scholar]
- Herbold, J. R. , Heuschele, W. P. , Berry, R. L. , & Parsons, M. A. (1983). Reservoir of St. Louis encephalitis virus in Ohio bats. American Journal of Veterinary Research, 44, 1889–1893. [PubMed] [Google Scholar]
- Jonkers, A. H. , Aitken, T. H. , Spence, L. , & Worth, C. B. (1966). Ecological studies of Venezuelan equine encephalitis virus (VEEV) in the Bush Bush wilderness of Trinidad. Revista Venezolana de Sanidad y Asistencia Social, 3, 929. [PubMed] [Google Scholar]
- Kunz, T. H. , Braun de Torrez, E. , Bauer, D. , Lobova, T. , & Fleming, T. H. (2011). Ecosystem services provided by bats. Annals of the New York Academy of Sciences, 1223, 1–38. 10.1111/j.1749-6632.2011.06004.x [DOI] [PubMed] [Google Scholar]
- Lednicky, J. , De Rochars, V. M. , Elbadry, M. , Loeb, J. , Telisma, T. , Chavannes, S. , … Morris, J. G. Jr (2016). Mayaro virus in child with acute febrile illness, Haiti, 2015. Emerging Infectious Diseases, 22, 2000–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machain‐Williams, C. , Lopez‐Uribe, M. , Talavera‐Aguilar, L. , Carrillo‐Navarrete, J. , Vera‐Escalante, L. , Puerto‐Manzano, F. , … Lorono‐Pino, M. A. (2013). Serologic evidence of flavivirus infection in bats in the Yucatan Peninsula of Mexico. Journal of Wildlife Diseases, 49, 684–689. 10.7589/2012-12-318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire, T. , Macnamara, F. N. , Miles, J. A. , Spears, G. F. , & Mataika, J. U. (1971). Mosquito‐borne infections in Fiji. II. Arthropod‐borne virus infections. The Journal of Hygiene, 69, 287–296. 10.1017/S0022172400021513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moratelli, R. , & Calisher, C. H. (2015). Bats and zoonotic viruses: Can we confidently link bats with emerging deadly viruses? Memorias do Instituto Oswaldo Cruz, 110, 1–22. 10.1590/0074-02760150048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olival, K. J. , Hosseini, P. R. , Zambrana‐Torrelio, C. , Ross, N. , Bogich, T. L. , & Daszak, P. (2017). Host and viral traits predict zoonotic spillover from mammals. Nature, 546, 646–650. 10.1038/nature22975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson, J. G. , Ksiazek, T. G. , Gubler, D. J. , Lubis, S. I. , Simanjuntak, G. , Lee, V. H. , … See, R. (1983). A survey for arboviral antibodies in sera of humans and animals in Lombok, Republic of Indonesia. Annals of Tropical Medicine and Parasitology, 77, 131–137. 10.1080/00034983.1983.11811687 [DOI] [PubMed] [Google Scholar]
- PAHO (2014, 2015, 2016). Number of reported cases of chikungunya fever in the Americas‐cumulative cases. washington, DC: Pan American Health Organization (PAHO, WHO). [Google Scholar]
- PAHO (2017). Zika: Countries and Territories with autochthonous transmission in the Americas reported in 2015‐2017. Retrieved from http://www.paho.org/hq/index.php?option=com_content&view=article&Id=11603&Itemid=41696&lang=en.
- Pilipski, J. D. , Pilipskl, L. M. , & Risley, L. S. (2004). West nile virus antibodies in bats from New Jersey and New York. Journal of Wildlife Diseases, 40, 335–337. 10.7589/0090-3558-40.2.335 [DOI] [PubMed] [Google Scholar]
- Platt, K. B. , Mangiafico, J. A. , Rocha, O. J. , Zaldivar, M. E. , Mora, J. , Trueba, G. , & Rowley, W. A. (2000). Detection of dengue virus neutralizing antibodies in bats from Costa Rica and Ecuador. Journal of Medical Entomology, 37, 965–967. 10.1603/0022-2585-37.6.965 [DOI] [PubMed] [Google Scholar]
- Schioler, K. L. , & Macpherson, C. N. L. (2009). Dengue transmission in the small‐island setting: Investigations from the Caribbean Island of Grenada. American Journal of Tropical Medicine and Hygiene, 81, 280–286. [PubMed] [Google Scholar]
- Schountz, T. (2014). Immunology of bats and their viruses: Challenges and opportunities. Viruses, 6, 4880–4901. 10.3390/v6124880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikes, R. , & Gannon, W. (2007). Guidelines of the American Society of Mammalogists for the use of wild mammals in research. Journal of Mammalogy, 88(3), 809–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotomayor‐Bonilla, J. , Chaves, A. , Rico‐Chavez, O. , Rostal, M. K. , Ojeda‐Flores, R. , Salas‐Rojas, M. , … Suzan, G. (2014). Dengue virus in bats from southeastern Mexico. American Journal of Tropical Medicine and Hygiene, 91, 129–131. 10.4269/ajtmh.13-0524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, N. N. , Auguste, A. J. , Travassos da Rosa, A. P. , Carrington, C. V. , Blitvich, B. J. , Chadee, D. D. , … Adesiyun, A. A. (2015). Seroepidemiology of selected alphaviruses and flaviviruses in bats in Trinidad. Zoonoses and Public Health, 62, 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubico, S. R. , & McLean, R. G. (1995). Serologic survey of neotropical bats in Guatemala for virus antibodies. Journal of Wildlife Diseases, 31, 1–9. 10.7589/0090-3558-31.1.1 [DOI] [PubMed] [Google Scholar]
- Vasconcelos, P. F. , & Calisher, C. H. (2016). Emergence of human arboviral diseases in the Americas, 2000‐2016. Vector‐Borne and Zoonotic Diseases, 16, 295–301. 10.1089/vbz.2016.1952 [DOI] [PubMed] [Google Scholar]
- Vourc'h, G. , Halos, L. , Desvars, A. , Boue, F. , Pascal, M. , Lecollinet, S. , … Bremont, M. (2014). Chikungunya antibodies detected in non‐human primates and rats in three Indian Ocean islands after the 2006 ChikV outbreak. Veterinary Research, 45, 52 10.1186/1297-9716-45-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, E. , Paessler, S. , Aguilar, P. V. , Smith, D. R. , Coffey, L. L. , Kang, W. , … Weaver, S. C. (2005). A novel, rapid assay for detection and differentiation of serotype‐specific antibodies to Venezuelan equine encephalitis complex alphaviruses. American Journal of Tropical Medicine and Hygiene, 72, 805–810. [PubMed] [Google Scholar]
- Wynne, J. W. , & Wang, L. F. (2013). Bats and viruses: Friend or foe? PLoS Pathogens, 9, e1003651 10.1371/journal.ppat.1003651 [DOI] [PMC free article] [PubMed] [Google Scholar]


