Abstract
The ecology of Ebola virus (EBV) remains largely unknown, but the previous detection of viral RNA and anti‐EBV antibodies in African bats suggests that they might play a role in the EBV reservoir. Moreover, African bats also carry other potentially zoonotic agents such as Henipah‐like viruses, coronaviruses and lyssaviruses. Today only little information is available on interactions between humans and bats. The objective of our exploratory study was to describe the extent and modes of contacts between humans and bats in southern Cameroon, considered as an area at risk for future EBV outbreaks. The survey was conducted in 11 villages of four distinct rural areas in southern Cameroon. A total of 135 respondents were interviewed using semi‐structured questionnaires, between February and May 2017. The study showed that direct contacts between bats and humans are relatively common. Bat bushmeat appeared to be an occasional meat resource; 40% of respondents consume bats with a median annual consumption of three, and 28% of respondents hunt them. About 22% of the respondents reported children catching bats. Indirect contact also appeared to be common; 55% of hunters use caves as shelters and 67% of interviewees eat fruits previously chewed by bats. Bat consumption varied significantly between regions (from 0% to 87%) and between pygmies and bantus in the extreme south‐east of Cameroon. The study revealed considerable diversity in practices among interviewees, most of them being subsistence cultivators and relying on self‐hunted bushmeat. Geographical diversity of contacts and perceptions regarding bats in Cameroon emphasizes the need to adjust zoonotic pathogen surveillance and education campaigns to the specificities of the communities and their context of interaction with wildlife.
Keywords: bat, Cameroon, Central Africa, Ebola, risk behaviour, zoonotic transmission
Impacts
Bats carry numerous potentially zoonotic pathogens such as filoviruses, Henipah‐like viruses, coronaviruses and lyssaviruses but only little information is available on interactions between humans and bats.
Direct contacts between bats and human populations of Southern Cameroon differ across communities; in general they are common, although not frequent but 40% of respondents consume bats with a median annual consumption of 3.
Danger perception is very low: 78% of the respondents do not believe bat bushmeat consumption can be associated with health risk.
1. INTRODUCTION
Since the first recognized human outbreak in 1976, Ebola viruses continue to emerge unpredictably across tropical Africa and raise growing concern in a quickly changing socio‐demographic context (Feldmann & Geisbert, 2011). Like many emerging pathogens, Ebola virus (EBV) appears to originate from wildlife (Pigott et al., 2014). During the largest outbreak in 2013–2016 in West Africa, transmission chains reached urban centres for the first time, causing more cases and deaths than all other outbreaks combined (Holmes, Dudas, Rambaut, & Andersen, 2016). Moreover, the two independent EBV outbreaks in the Equateur and Nord‐Kivu Provinces in 2018 in the Democratic Republic (DRC) provide additional evidence that these viruses can reach urban centres (WHO, 2018). Current evidence suggests that EBV reservoir is complex and might involve several species. Attention has been drawn to bats in which viral RNA was detected in a handful of wild living specimens only (Leroy et al., 2005) as well as anti‐EBV antibodies in several species (Hayman et al., 2012, 2010; Leroy et al., 2005; Ogawa et al., 2015; Pourrut et al., 2007, 2009), but knowledge about the maintenance of the virus in bat populations is scarce. The limited number of positive specimen detected so far (Leendertz, Gogarten, Düx, Calvignac‐Spencer, & Leendertz, 2016; Leroy et al., 2005), despite intensive sampling efforts, hampered by the richness of African fauna, limits our understanding of EBV ecology in wildlife.
Nevertheless, bats are suspected to be involved in zoonotic EBV transmissions. For example, it was suggested that the index case of the 2007 outbreak in Luebo in the DRC was asymptomatically infected through contact with bat bushmeat (Leroy et al., 2009). For the 2013 outbreak in West Africa, the index case was a 2‐year‐old boy suspected to have played with a colony of insectivorous bats in a hollow tree (Mari Saez et al., 2015). But for most outbreaks, the initial source of zoonotic transmission has not been identified. The only laboratory‐confirmed sources of human Ebola disease are duikers, chimpanzees and gorillas and great ape populations in which viral RNA has been identified on animal carcasses (Le Guenno et al., 1995; Leroy et al., 2004). Moreover, EBV causes high mortality rates in African apes (Bermejo et al., 2006; Leroy et al., 2004).
Meanwhile, human–wildlife interactions that increase risks of transmissions are frequent and diverse; hunting, butchering and consuming wild animals, including bats, are common in tropical Africa. These practices can potentially lead to transmission of zoonotic pathogens through animal bites, scratches, contact with infected body fluids, tissues and excrements that can also contaminate fruits (Wolfe, Daszak, Kilpatrick, & Burke, 2005).
More specifically, interactions between humans and bats, considered as a probable route of EBV transmission to humans, are very poorly documented (Mickleburgh, Waylen, & Racey, 2009). Information on bat hunting and consumption practices, as well as indirect contacts, is scarce (Kamins et al., 2011; Mickleburgh et al., 2009). Understanding of the attitudes and perceptions of communities towards bat‐related diseases is also limited, as large‐scale surveys were only conducted in Ghana (Gbogbo & Kyei, 2017; Kamins et al., 2015).
The aim of this exploratory study was to document the extent of contacts between human populations and bats as well as the contact modes, attitudes and perception regarding bats in Southern Cameroon, a country considered at risk for EBV outbreaks (Pigott et al., 2014). This information will help improve the risk assessment for transmission of viruses hosted by bats to humans, in order to reinforce prevention measures against the emergence of zoonotic pathogens from bats, as well as surveillance and response to health risks. Especially since recent studies are accumulating evidence that bats may not only host EBV, but also several other zoonotic pathogens including henipaviruses (Hayman et al., 2008) and other paramyxoviruses (Baker et al., 2013; Drexler et al., 2012), as well as lyssaviruses (Wright et al., 2010) among many others.
2. METHODS
2.1. Study sites
The study was conducted in villages in four rural forest areas of southern Cameroon (Figure 1). Three of these areas (Campo [CP], Northern periphery of Dja Reserve [BQ], Mambele [MB]) are close to protected natural parks, and have a relatively rich fauna. This is in contrast to the area of Gwap/Bipindi (BP), where bushmeat has become scarce in the last decade due to a combination of factors, including over‐hunting, deforestation and agricultural intensification. The study sites had previously been chosen for their richness in gorilla and chimpanzee populations in the context of studies on the origin of HIV, conducted by the team since 2000 (Keele et al., 2006), in addition to studies investigating the EBV reservoir in wildlife initiated in 2015. These remote areas are hardly accessible and transportation to the closest city is limited for their inhabitants. Villages are organized along the main dirt road, usually built to transport wood from logging concessions (in all four areas) or products from mining concessions (with BOCOM company exploiting iron in the area of BP). Fruit trees are located around the houses; cultivated fields are nearby the villages bordering the forest. Regarding water availability, villagers rely on one‐point source water or river water except in BP where there is tap water from a nearby underground source for almost every household. In BP, agricultural activity is not only for subsistence purpose, but also a source of income for inhabitants who sell part of their production.
Figure 1.
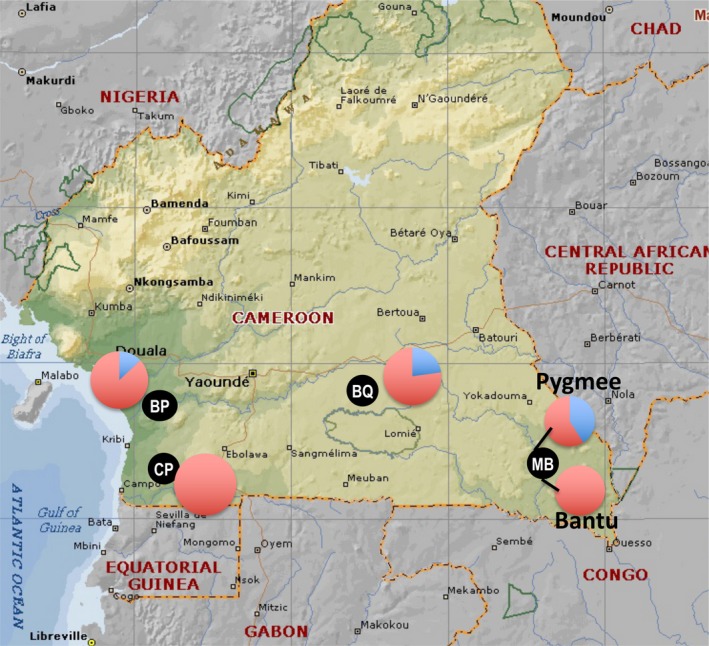
Map illustrating the proportion of respondents consuming bats (in blue) versus non consuming (red) in the four study sites (black dots) in rural southern Cameroon (Campo [CP], Gwap/Bipindi [BP], northern periphery of Dja [BQ] and Mambele [MB]) [Colour figure can be viewed at http://wileyonlinelibrary.com]
2.2. Questionnaires
Household surveys were conducted from February to May 2017 using standardized semi‐structured questionnaires, adapted empirically according to the experience of the field team. With the prior consent of the local authorities, households were selected according to convenience sampling along the only main road of each village and by choosing to interview one volunteer inhabitant of the household. All interviews were conducted in person in French first, with a villager translating in the local language punctually if the interviewee did not understand a question.
2.3. Data analysis
Responses were summarized using descriptive statistics, and Fisher's exact test as well as Pearson Chi‐squared test were used to determine differences between categories of responses. Statistical tests were considered significant at the threshold of 5%. A multivariate analysis was performed in order to identify the variables significantly correlated with the proportion of bat consumption among villagers, interactions between variables being taken into account. Sample size was too small to perform multivariate analysis in order to study the effects of explanatory variables on bat consumption, but we relied on the best‐fitted generalized linear model, selected according to the Akaike's Information Criterion, in order to identify the variables that might best explain the proportion of bat consumption among villagers, interactions between variables being taken into account. Responses in function of each of these variables were then tested separately by Fisher's exact test or Pearson Chi‐squared test. The following explanatory variables were included in the maximal model: gender, age, geographic area, education, village, participation to bat hunting, knowledge on bat roosting places, perception of dangers or benefits related to bat consumption, existence of a traditional totem, use of bat cadavers by healers. We used R version 3.3.1 for all our statistical analyses.
3. RESULTS
3.1. Socio‐demographic characteristics of surveyed populations
The demographic characteristics of the respondents are presented in Figure 2. In total, there were 135 respondents consisting of 106 (79%) men and 29 (21%) women. This imbalance was largely due to the fact that the member of the household volunteering to answer the questionnaire was generally a man. Respondent's age ranged from 15 to 80 years, with a median of 45 years old. The majority of respondents (58%, n = 78) had elementary education, 28% (n = 37) had secondary education, 8% (n = 11) went to high school, and 6% (n = 8) had no school education. Most of the respondents (74%, n = 100) were subsistence farmers, without distinction between women and men and only five respondents who were NGO employees, declared having a regular income. Twenty‐three per cent (n = 31) declared hunting or fishing as a secondary activity (culturally, only men hunt). However, the communities mostly rely on self‐hunted bushmeat as their sole source of protein as domesticated animal meat is absent or not affordable in those areas.
Figure 2.
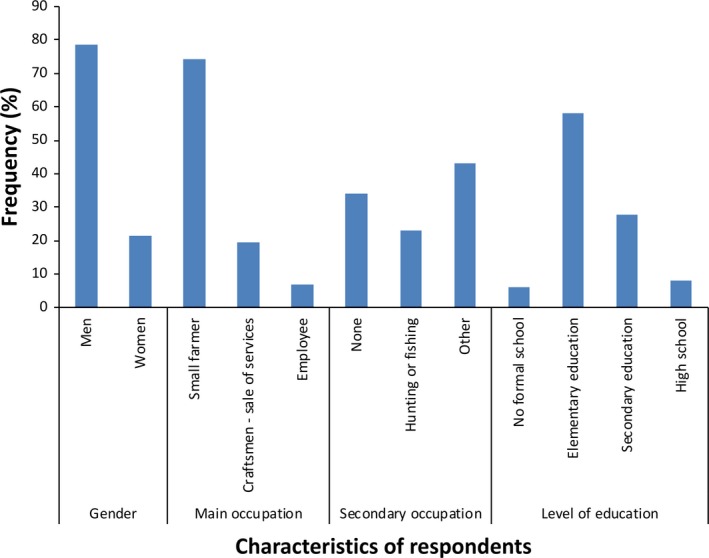
Frequency distribution of the demographic characteristics of the 135 respondents in the four study sites communities in rural southern Cameroon [Colour figure can be viewed at http://wileyonlinelibrary.com]
In total, 135 respondents declared to belong to 16 ethnic groups which can be gathered in two larger units: Pygmy (27%) and Bantu (73%). The number of respondents per site ranged from 22 in Campo (CP), 35 in Northern periphery of Dja (BQ) to 39 in Gwap/Bipindi (BP) and Mambele (MB). The number of ethnic groups per site varied from two in BQ, four in MB, five in CP and six in BP. Bajele Pygmies were present in CP as well as in BP. Since ethnic group diversity did not allow a sufficient sample size for each group, except for MB, we considered only area in our analyses.
3.2. Evaluation of contact and exposure through bat consumption
Overall, 40% (n = 54) of respondents consume bats. The best‐fitted model suggested that this consumption might be mostly associated with the area, participation to bat hunting and perception of dangers. None of the respondents in the area of CP declared eating bats (0/22), while 23% (8/35), 31% (12/39) and 87% (34/39) of the respondents did in BQ, MB and BP respectively (Fisher's exact test, p‐value < 10−12, Table 1; Figure 2). Within one study site only, MB, a clear difference was observed in consumption practices between ethnic groups, with the Baka Pygmie ethnic group consuming significantly more (12/29, 41%), compared to non‐Pygmies (0/10; Fisher's exact test, p‐value < 0.01, Figure 1).
Table 1.
Details on bat consumption practices in the study population
| Bat consumption practices | N | % |
|---|---|---|
| Bat consumption in general | ||
| CP | 0/22 | 0 |
| BP | 34/39 | 87 |
| BQ | 8/35 | 23 |
| MB | 12/39 | 31 |
| Total | 54/135 | 40 |
| Type of bats eaten | ||
| Fruit bats only | 42/54 | 78 |
| Insectivorous bats only | 3/54 | 11 |
| All bats | 9/54 | 17 |
| Who consumes bats | ||
| All respondents | 49/54 | 91 |
| Males only | 3/54 | 6 |
| Adults only | 2/54 | 4 |
| Importance of bats for subsistence | ||
| None | 37/54 | 68 |
| Comfort | 15/54 | 28 |
| Essential | 1/54 | 2 |
| Reasons for non consumption | ||
| Elders didn't eat | 56/135 | 41 |
| Other resourcesa | 9/135 | 7 |
| Hunting accessibilityb | 4/135 | 3 |
| Danger perceptionc | 4/135 | 3 |
Other resources available, not considered as food;
Don't hunt, difficult to catch;
Witchcraft, transmission of disease.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
When interviewees hunt bats themselves, they are more likely to eat bats (97%, 37/38), compared to those who did not hunt bats (18%, 17/97; Fisher's exact test, p < 10−15).
Moreover, bat consumption was significantly associated with a lower perception of danger related to this consumption. Indeed, among consumers, only 7% (4/54) thought this could be associated to some danger (20% of them didn't know), compared to 32% (26/81) among those who did not eat bats (Fisher's exact test, p‐value < 0.001).
Usually, women prepare the carcass without any specific precaution except for washing hands (with water, soap being rare).
Bats are not a major protein source for the communities: 69% of the interviewees eating bats (37/54) consider bats are not an important resource, 28% (15/54) judged it was a “comfort,” and one respondent reported bats were essential regarding how much he appreciates bat meat (Table 1). Among bat consumers, the median frequency of consumption is three times a year and 75% of them eat bats <11 times a year.
3.3. Evaluation of contact and exposure through bat hunting
In total 28% (n = 38) of respondents hunt bats, comprising men only. Bat hunters capture bats in various ways depending on the area (Figure 3). The context of hunting in BP is quite particular since villagers hunt bats exclusively in one cave that is locally well‐known and difficult and dangerous to access; only young men go there. This cave is forbidden to women and is associated with a sacred ritual for the community.
Figure 3.
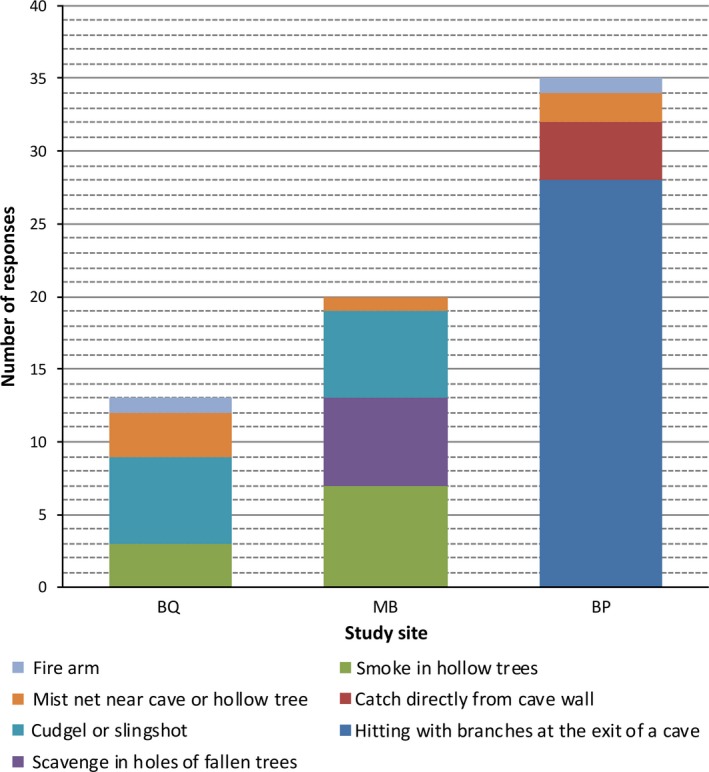
Different hunting methods reported across the study sites, except CP where no bat hunting was reported [Colour figure can be viewed at http://wileyonlinelibrary.com]
In total 17% (n = 23) of the respondents have been bitten by bats at least once: 18/38 of bat hunters, three respondents, too old to go hunting and two respondents who did not hunt bats. Hunters capturing bats in caves with sticks or bare hands are more likely to have been bitten multiple times (10/22, 45%) compared to those capturing bats in trees (2/16, 13%; Fisher's exact test, p < 0.01). Only one hunter reported using gloves as protective measure, others used none, but most of them didn't pick up the bat before killing it first.
3.4. Evaluation of direct contacts between children and bats
Twenty‐two per cent (n = 30) of respondents report that children catch bats: 14 declare it is only to play (most of them in CP where they don't eat bats), 12 to play and eat, and four to eat only. 13/30 declared children keep bats alive, usually tying them to a string and a stick. Children from bat consumers caught significantly more bats (35%, 19/54) compared to children of non‐consumers (14%, 11/81; Pearson's Chi‐squared test, p < 10−2). Two respondents reported their children had been bitten several times by bats, one in MB, the other in BP.
3.5. Evaluation of indirect contacts
Homogeneously among areas, respondents (86%, n = 116) declared bats were eating fruits on trees surrounding the village. Overall 67% (n = 90) of the respondents declared that either themselves or their children were eating fruits that were already chewed by bats. This proportion, however, varies significantly depending on the area, from 41% in MB to 89% in BQ (Fisher's exact test, p < 10−4). Most of the fruits need to be peeled before being consumed, except for plum and umbrella tree (Musanga cecropiodes) fruit. Mango is the fruit most frequently cited by respondents declaring they ate chewed fruits (59/90); other fruits reported are guava, plum, avocado, soursop and papaya. Fifty‐five per cent (21/38) of hunters used caves as shelters for protection during bad weather conditions (rain), which implies they might be in contact with guano (bat faeces) dropped on the cave's floor or with air saturated with contaminated aerosols. Respondents did not exploit bat guano from caves, for instance as fertilizer, and they had never heard of this practice.
3.6. Risk perception and traditional beliefs towards bats
Overall perception of danger related to bat consumption is low, with 78% (n = 105) of the respondents that do not believe there is any danger linked to bat consumption. Among respondents who did believe in danger, disease transmission was mentioned by 70% (21/30) of them, and 15% (5/30) believed bats were associated with witchcraft activities. The other dangers mentioned were linked to the suspiciousness towards bats due to the unfamiliarity of villagers with their “lifestyle.” During informal discussion, some respondents qualified bats of “strange creature, living at night,” “nor animal, nor bird,” “defecating through their mouth.” In contrast, 7% (n = 10) of respondents thought bat consumption is beneficial, as a food supply (n = 5), to improve health (n = 3) or for culinary satisfaction in BP (n = 2); all of them consume bats.
4. DISCUSSION
In general, we obtained preliminary information on practices related to bats. Overall, it is legal to hunt bats, and there is no taboo or stigma associated with hunting bats in southern Cameroon and interviewees were thus comfortable answering our questions. However, in places where bat consumption was associated to Pygmy ethnic groups, a minority population group often marginalized and stigmatized, it cannot be excluded that some respondents, in presence of a Bantu interpreter and interviewer, might not have been comfortable addressing this topic. Although the majority of the communities, we interviewed depend on self‐hunted bushmeat, the low rate of interviewees mentioning hunting as a secondary activity could potentially be linked to the fear of poaching repression.
Nevertheless, the results of our questionnaires show that direct human–bat contacts are substantial: 40% (n = 54) of respondents consume bats, 28% (n = 38) hunt them, 17% (n = 23) have already been bitten by bats and 22% (n = 30) report children catch them. This is especially significant as a single zoonotic disease transmission can have major consequences as illustrated by the large EBV outbreaks in West Africa with subsequent transmission chains of human‐to‐human transmissions (Baize et al., 2014). In regard to indirect contacts, communities are homogeneously exposed to fruits possibly contaminated by bat faeces or saliva, since 67% of respondents consume munched fruits. This raises concern, since there is evidence of transmission of other viruses carried by bats to humans through this route, for instance, Nipah virus spillovers caused fatal encephalitis and respiratory failure in South‐East Asia (Breed, Field, Epstein, & Daszak, 2006; Chua et al., 2000). African bats also host similar paramyxoviruses that could potentially be transmitted in the same way (Weiss et al., 2012), though zoonotic transmission and impact of these viruses on human health are still unknown.
Alarmingly, perception of danger related to disease transmission from bats is very low, as much as 78% of the respondents do not believe bat bushmeat consumption can be associated with health risk. This is not surprising considering similar results of other studies (Gbogbo & Kyei, 2017; Kamins et al., 2015), conducted in countries where awareness‐raising campaigns had been implemented previously, contrary to Cameroon. More strikingly, even when respondents spontaneously mention Ebola disease, they declare not to feel “at risk” in their country, since no outbreak has occurred yet. There is thus a need to reinforce public health education. However, previous Ebola communication in other countries has been marked by a series of errors, erroneous or inappropriate messages that have contributed to doubts and created anxiety (Seytre, 2016). This emphasizes the need to re‐think the way of interventions, through social mobilization, sanitary education and health promotion as suggested by the World Health Organization (WHO, 2014). The recent EBV epidemics of 2014, 2017 and 2018 are clear reminders that EBV disease prevention can be improved.
Although bats attract attention, contacts with bats in our study sites might be less frequent compared to contact with other species that are regularly hunted and essential for the subsistence of the communities. Indeed, although bat meat consumption is common, a majority of respondents state it has “no importance” as a food resource. Rather, it appears to be an occasional dish (with three‐quarter of consumers eating bats <11 times per year), or in some communities an appreciated delicacy. Gathering more information on the interface with other potential compartments of wildlife hosts is thus also crucial.
Importantly, this study highlights the high geographical and cultural diversity of contacts and perceptions regarding bats in Cameroon. This emphasizes the heterogeneity in terms of zoonotic transmission risks in Central Africa, and the need to adjust surveillance and education campaigns to the specificities of the communities and their context of interaction with wildlife.
CONFLICT OF INTEREST
None.
ACKNOWLEDGEMENTS
This work was supported in part by grants from INSERM/The EBOLA Task Force, REACTing; EBO‐SURSY project funded by European Union; International Mixt Laboratory “PreVIHMI” of IRD (Institut de Recherche pour le Developpement). HB received support from France Vétérinaire International and Toulouse University. We thank the staff and SIV team from Projet PRESICA (Innocent Ndong Bass, Aime Mebenga, Joseph Moudindo, Thomas Atemkem) for logistical support in Cameroon as well as people that volunteered to be interviewed.
Baudel H, De Nys H, Mpoudi Ngole E, Peeters M, Desclaux A. Understanding Ebola virus and other zoonotic transmission risks through human–bat contacts: Exploratory study on knowledge, attitudes and practices in Southern Cameroon. Zoonoses Public Health. 2019;66:288–295. 10.1111/zph.12563
REFERENCES
- Baize, S. , Pannetier, D. , Oestereich, L. , Rieger, T. , Koivogui, L. , Magassouba, N. , … Günther, S. (2014). Emergence of Zaire Ebola virus disease in Guinea. The New England Journal of Medicine, 371(15), 1418–1425. 10.1056/NEJMoa1404505 [DOI] [PubMed] [Google Scholar]
- Baker, K. S. , Todd, S. , Marsh, G. A. , Crameri, G. , Barr, J. , Kamins, A. O. , … Wang, L.‐F. (2013). Novel, potentially zoonotic paramyxoviruses from the African straw‐colored fruit bat Eidolon helvum . Journal of Virology, 87(3), 1348–1358. 10.1128/JVI.01202-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermejo, M. , Rodríguez‐Teijeiro, J. D. , Illera, G. , Barroso, A. , Vilà, C. , & Walsh, P. D. (2006). Ebola outbreak killed 5000 gorillas. Science, 314(5805), 1564 10.1126/science.1133105 [DOI] [PubMed] [Google Scholar]
- Breed, A. C. , Field, H. E. , Epstein, J. H. , & Daszak, P. (2006). Emerging henipaviruses and flying foxes – Conservation and management perspectives. Biological Conservation, 131(2), 211–220. 10.1016/J.BIOCON.2006.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua, K. B. , Bellini, W. J. , Rota, P. A. , Harcourt, B. H. , Tamin, A. , Lam, S. K. , … Mahy, B. W. (2000). Nipah virus: A recently emergent deadly paramyxovirus. Science, 288(5470), 1432–1435. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10827955 [DOI] [PubMed] [Google Scholar]
- Drexler, J. F. , Corman, V. M. , Müller, M. A. , Maganga, G. D. , Vallo, P. , Binger, T. , … Drosten, C. (2012). Bats host major mammalian paramyxoviruses. Nature Communications, 3, 796 10.1038/ncomms1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann, H. , & Geisbert, T. W. (2011). Ebola haemorrhagic fever. Lancet, 377(9768), 849–862. 10.1016/S0140-6736(10)60667-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gbogbo, F. , & Kyei, M. O. (2017). Knowledge, perceptions and attitude of a community living around a colony of straw‐coloured fruit bats (Eidolon helvum) in Ghana after Ebola virus disease outbreak in West Africa. Zoonoses and Public Health, 64, 628–635. 10.1111/zph.12357 [DOI] [PubMed] [Google Scholar]
- Hayman, D. T. S. , Suu‐Ire, R. , Breed, A. C. , McEachern, J. A. , Wang, L. , Wood, J. L. N. , & Cunningham, A. A. (2008). Evidence of henipavirus infection in West African fruit bats. PLoS ONE, 3(7), e2739 10.1371/journal.pone.0002739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman, D. T. S. , Yu, M. , Crameri, G. , Wang, L.‐F. , Suu‐Ire, R. , Wood, J. L. N. , & Cunningham, A. A. (2012). Ebola virus antibodies in fruit bats, Ghana, West Africa. Emerging Infectious Diseases, 18(7), 1207–1209. 10.3201/eid1807.111654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman, D. T. S. , Emmerich, P. , Yu, M. , Wang, L.‐F. , Suu‐Ire, R. , Fooks, A. R. , … Wood, J. L. N. (2010). Long‐term survival of an urban fruit bat seropositive for Ebola and Lagos bat viruses. PLoS ONE, 5(8), e11978 10.1371/journal.pone.0011978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes, E. C. , Dudas, G. , Rambaut, A. , & Andersen, K. G. (2016). The evolution of Ebola virus: Insights from the 2013–2016 epidemic. Nature, 538(7624), 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamins, A. O. , Rowcliffe, J. M. , Ntiamoa‐Baidu, Y. , Cunningham, A. A. , Wood, J. L. N. , & Restif, O. (2015). Characteristics and risk perceptions of ghanaians potentially exposed to bat‐borne zoonoses through bushmeat. EcoHealth, 12(1), 104–120. 10.1007/s10393-014-0977-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamins, A. O. , Restif, O. , Ntiamoa‐Baidu, Y. , Suu‐Ire, R. , Hayman, D. T. S. , Cunningham, A. A. , … Rowcliffe, J. M. (2011). Uncovering the fruit bat bushmeat commodity chain and the true extent of fruit bat hunting in Ghana, West Africa. Biological Conservation, 144(12), 3000–3008. 10.1016/j.biocon.2011.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele, B. F. , Van Heuverswyn, F. , Li, Y. , Bailes, E. , Takehisa, J. , Santiago, M. L. , … Hahn, B. H. (2006). Chimpanzee reservoirs of pandemic and nonpandemic HIV‐1. Science, 313(5786), 523–526. 10.1126/science.1126531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Guenno, B. , Formenty, P. , Formentry, P. , Wyers, M. , Gounon, P. , Walker, F. , & Boesch, C. (1995). Isolation and partial characterisation of a new strain of Ebola virus. Lancet, 345(8960), 1271–1274. 10.5555/URI:PII:S0140673695909257 [DOI] [PubMed] [Google Scholar]
- Leendertz, S. A. J. , Gogarten, J. F. , Düx, A. , Calvignac‐Spencer, S. , & Leendertz, F. H. (2016). Assessing the evidence supporting fruit bats as the primary reservoirs for Ebola viruses. EcoHealth, 13(1), 18–25. 10.1007/s10393-015-1053-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy, E. M. , Epelboin, A. , Mondonge, V. , Pourrut, X. , Gonzalez, J.‐P. , Muyembe‐Tamfum, J.‐J. , & Formenty, P. (2009). Human Ebola outbreak resulting from direct exposure to fruit bats in Luebo, Democratic Republic of Congo, 2007. Vector Borne and Zoonotic Diseases, 9(6), 723–728. 10.1089/vbz.2008.0167 [DOI] [PubMed] [Google Scholar]
- Leroy, E. M. , Kumulungui, B. , Pourrut, X. , Rouquet, P. , Hassanin, A. , Yaba, P. , … Swanepoel, R. (2005). Fruit bats as reservoirs of Ebola virus. Nature, 438(7068), 575–576. 10.1038/438575a [DOI] [PubMed] [Google Scholar]
- Leroy, E. M. , Rouquet, P. , Formenty, P. , Souquière, S. , Kilbourne, A. , Froment, J.‐M. , … Rollin, P. E. (2004). Multiple Ebola virus transmission events and rapid decline of central African wildlife. Science, 303(5656), 387–390. 10.1126/science.1092528 [DOI] [PubMed] [Google Scholar]
- Mari Saez, A. , Weiss, S. , Nowak, K. , Lapeyre, V. , Zimmermann, F. , Dux, A. , … Leendertz, F. H. (2015). Investigating the zoonotic origin of the West African Ebola epidemic. EMBO Molecular Medicine, 7(1), 17–23. 10.15252/emmm.201404792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickleburgh, S. , Waylen, K. , & Racey, P. (2009). Bats as bushmeat: A global review. Oryx, 43(02), 217 10.1017/S0030605308000938 [DOI] [Google Scholar]
- Ogawa, H. , Miyamoto, H. , Nakayama, E. , Yoshida, R. , Nakamura, I. , Sawa, H. , … Takada, A. (2015). Seroepidemiological prevalence of multiple species of filoviruses in fruit bats (Eidolon helvum) migrating in Africa. Journal of Infectious Diseases, 212(Suppl 2), S101–S108. 10.1093/infdis/jiv063 [DOI] [PubMed] [Google Scholar]
- Pigott, D. M. , Golding, N. , Mylne, A. , Huang, Z. , Henry, A. J. , Weiss, D. J. , … Hay, S. I. (2014). Mapping the zoonotic niche of Ebola virus disease in Africa. Elife, 3, e04395 10.7554/eLife.04395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourrut, X. , Délicat, A. , Rollin, P. E. , Ksiazek, T. G. , Gonzalez, J.‐P. , & Leroy, E. M. (2007). Spatial and temporal patterns of Zaire ebolavirus antibody prevalence in the possible reservoir bat species. The Journal of Infectious Diseases, 196 Suppl 2(s2), S176–S183. 10.1086/520541 [DOI] [PubMed] [Google Scholar]
- Pourrut, X. , Souris, M. , Towner, J. S. , Rollin, P. E. , Nichol, S. T. , Gonzalez, J.‐P. , & Leroy, E. (2009). Large serological survey showing cocirculation of Ebola and Marburg viruses in Gabonese bat populations, and a high seroprevalence of both viruses in Rousettus aegyptiacus. BMC Infectious Diseases, 9, 159 10.1186/1471-2334-9-159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seytre, B. (2016). Les errances de la communication sur la maladie à virus Ebola. Bulletin De La Société De Pathologie Exotique, 109(4), 314–323. 10.1007/s13149-016-0524-z [DOI] [PubMed] [Google Scholar]
- Weiss, S. , Nowak, K. , Fahr, J. , Wibbelt, G. , Mombouli, J.‐V. , Parra, H.‐J. , … Leendertz, F. H. (2012). Henipavirus‐related sequences in fruit bat bushmeat, Republic of Congo. Emerging Infectious Diseases, 18(9), 1535–1536. 10.3201/eid1809.111607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2014). Communication pour un impaCt Comportemental (ComBi) . Retrieved from http://apps.who.int/iris/bitstream/handle/10665/129391/WHO_HSE_GCR_2012.13_fre.pdf;jsessionxml:id=33DB27E41552EF1971C7FA0F922F0520?sequence=1
- WHO (2018). Ebola|Ebola situation reports: Democratic Republic of the Congo . WHO. Retrieved from http://www.who.int/ebola/situation-reports/drc-2018/en/
- Wolfe, N. D. , Daszak, P. , Kilpatrick, A. M. , & Burke, D. S. (2005). Bushmeat hunting, deforestation, and prediction of zoonotic disease. Emerging Infectious Diseases, 11(12), 1822–1827. 10.3201/eid1112.040789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, E. , Hayman, D. T. S. , Vaughan, A. , Temperton, N. J. , Wood, J. L. N. , Cunningham, A. A. , … Fooks, A. R. (2010). Virus neutralising activity of African fruit bat (Eidolon helvum) sera against emerging lyssaviruses. Virology, 408(2), 183–189. 10.1016/j.virol.2010.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]


