Abstract
Group A rotavirus (RV) and coronavirus (CV) are common viral pathogens associated with neonatal diarrhoea in numerous animal species. The purpose of this work was to investigate the presence of these viral agents in two farm populations of captured guanacos (Lama guanicoe) in the Argentinean Patagonia region, that developed severe diarrhoea outbreaks. Stool and serum samples were analysed for RV and bovine CV antigen and antibody by enzyme‐linked immunosorbent assay. Rotavirus was detected in faeces from two new‐born guanacos with acute diarrhoea, one in each farm. After electrophoretic analysis, each isolated strain, showed a distinctive long dsRNA electropherotype characteristic of group A rotaviruses (4:2:3:2). In addition, 95% (38 of 40) of the sampled animals were positive for RV antibodies, suggesting a high prevalence of RV infection in the populations tested. No evidence of CV circulation by antigen or antibody analysis was observed. To our knowledge, this is the first report of the detection and isolation of RV associated with neonatal diarrhoea in Lama guanicoe.
Introduction
Lama guanicoe commonly known as guanaco is one of the four species of South American camelids that include: llama (Lama glama), alpaca (Lama pacos), guanaco (Lama guanicoe) and vicuña (Lama vicugna). Guanaco exist as a wild species in most of the Argentinean arid areas, particularly in the Patagonia region. As there is increasing interest in Camelidae fibres, in recent years, well‐controlled programmes for capture, temporary housing, shearing and release of young guanacos have been implemented.
There are relatively few reports concerning viral diseases of South American camelids. In particular, in Lama guanicoe there is only one report in which, sera from 20 free‐ranging guanacos from Chubut province, Argentina, were tested for antibodies against bluetongue, bovine respiratory syncytial virus, bovine viral diarrhoea virus, parainfluenza‐3, equine herpesvirus‐1, vesicular stomatitis virus and foot and mouth disease virus, and all animals were seronegative for all the antigens tested (Karesh et al., 1998). However, there is no information regarding the circulation of enteric viral agents such as rotavirus (RV) and coronavirus (CV) among these animals. Rotaviruses are a major cause of neonatal diarrhoea in humans and numerous animal species world‐wide (Kapikian and Chanock, 1996). In Argentina, RV is considered one of the most important causes of diarrhoea in calves (Barrandeguy et al., 1988; Bellinzoni et al., 1989, 1990; Costantini et al., 1999), and its presence has been reported in piglets and foals (Mattion et al., 1989; Parreño et al., 1997).
Coronavirus is commonly associated with calf diarrhoea and winter dysentery in adult cattle in countries of the northern hemisphere (Saif, 1990; Clark, 1993). Although serologic surveys of adult cattle indicate that bovine CV circulates among Argentinean cattle (Panighi, 1990), its incidence associated with neonatal calf diarrhoea in Argentina is very low (Parreño et al., 1996).
Coronaviruses have been detected by electron microscopy in the faeces of llama with diarrhoea (Mattson, 1994). There are also two previous reports of the detection of antibodies against RV in alpacas in Peru (Rivera et al., 1987) and llamas in Argentina (Puntel et al., 1999), but to the authors knowledge there have been no reports of the detection or isolation of RV.
The aim of this study was to investigate the presence of RV and CV as possible agents associated with severe diarrhoea outbreaks, with high morbidity and mortality, affecting young animals during the calving season of 1998, in two farms dedicated to Lama guanicoe domestication in the Argentinean Patagonia region.
Material and Methods
This investigation was conducted in two farms working under the permission of the regulatory agency for ‘controlled capture of young guanacos’. The farms were located 700 km apart, in the Provinces of Rio Negro (farm A) and Chubut (farm B), in the Patagonia region.
Young wild guanacos (1 day to 4 months old) were captured, maintained in small yards and fed with bovine milk substitute twice a day. By November/December 1998, outbreaks of severe acute diarrhoea with 100% morbidity and 83% mortality rates were observed in both farms. The affected animals were from 7 to 40 days old, and all developed an acute dark‐green diarrhoea, hypothermia (rectal temperature lower than 38°C) and anorexia, followed by dehydration and death in a period of 2–6 days. Initial diagnosis was bacterial diarrhoea, but specific antibiotic treatment proved to be ineffective and no reduction in morbidity or mortality rates were observed. However, in the analysed cases, necropsy results indicated the presence of Escherichia coli (in three dead animals in farm A) and Salmonella sp. (in one new‐born guanaco in farm B), with septicaemia as the final causes of death.
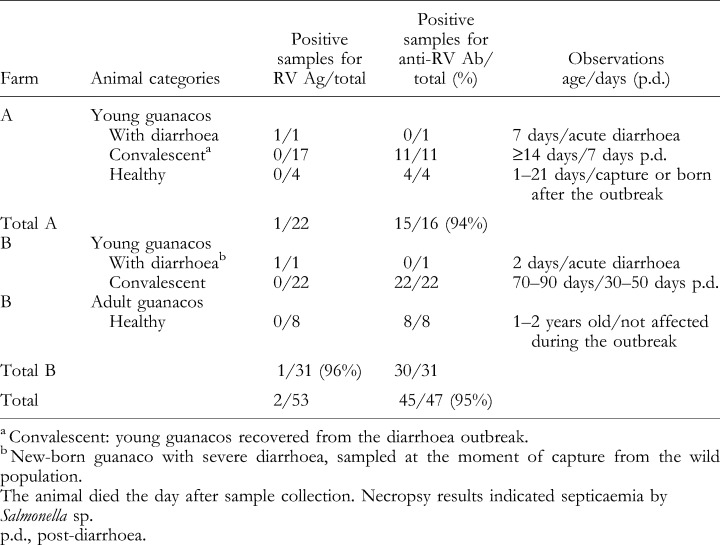
Both farms were sampled approximately 30 days after the peak of the outbreak. A total of 22 faecal and 16 serum samples were collected in farm A and 30 faecal and serum samples were obtained in farm B, belonging to the animal categories described in Table 1.
Table 1.
Summary results obtained after first screening for rotavirus antigen (Ag) detection in faecal samples and anti‐RV antibody (Ab) detection in serum samples by ELISA in the two guanaco populations under study
All faecal samples were initially screened for the presence of RV and bovine coronavirus (BCV) antigen by enzyme‐linked immunosorbent assay (ELISA), using the reagents and techniques previously described by Cornaglia et al. (1989) for RV antigen detection; and Smith et al. (1996) for CV antigen detection (source: L. J. Saif, Food Animal Health Research Program, The Ohio State University, Wooster, Ohio, USA).
Additionally, both ELISA techniques were adapted for RV and CV antibody detection in guanaco serum samples. Clarified supernatants of NCDV‐Lincoln BRV or Mebus BCV were used for the antigen‐coated wells and mock‐infected MA‐104 or HRT 18 cell culture control lysates for the control‐coated wells (cell source: L.J. Saif). Each guanaco serum sample was assayed in serial four‐fold dilutions (starting at 1:16). A 1:2000 dilution of commercial peroxidase‐labelled polyclonal goat anti‐llama IgG(H + l) (Bethyl Labs Inc, Montgomery, TX, USA) was used as the conjugate.
Results
Rotavirus antigen was detected in two of 53 faecal samples tested, corresponding to two new‐born guanacos at 7 and 2 days of age, showing severe acute diarrhoea at the time of sample collection. The former animal belonged to farm A, was captured 5 days before sampling, and was fed with commercial milk. The latter animal was captured in farm B and died the day after sample collection and necropsy results indicated septicaemia by Salmonella sp. (data not shown). Both animals were negative for serum RV antibodies by ELISA. (Table 1, Fig. 1) and showed low levels of total protein in serum determined by a glutaraldehyde test (data not shown).
Figure 1.
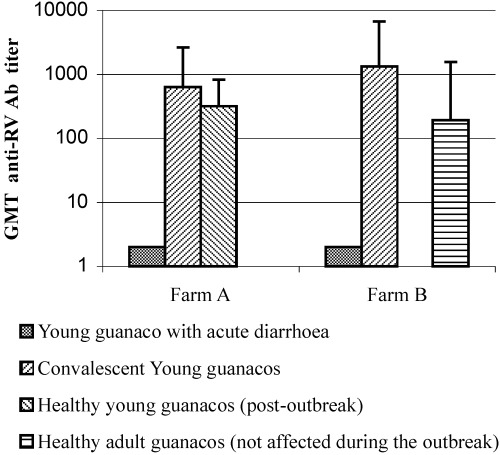
Antibody titres against group A rotavirus in guanaco serum samples assayed by ELISA in farm A and B. Each bar represents the geometric mean antibody titre (GMT) for each animal category. Errors bars indicate the standard deviation (STD) of each animal population tested.
The rest of the young guanacos had recovered from the disease at the time of sample collection (7–50 days after the outbreak), and the adult animals did not show any clinical signs during the outbreak. In agreement with this observation no RV shedding was detected in any of the convalescent animals or in the healthy young guanacos (none of 51). However, the serum of all sampled animals, except for the sick new‐borns, was positive for RV antibodies, including the adult guanacos (45 of 45) (Table 1). The high antibody prevalence detected in both farms (95%) adds evidence indicating that guanacos, as observed in previous studies of other South American camelids, such as Lama pacos (Rivera et al., 1987) and Lama glama (Puntel et al. 1999) are susceptible to rotavirus infection.
Further serologic analysis indicate that RV antibody titres in convalescent animals in farm A ranged from 64 to 4096 [geometric mean titre (GMT)=637], whereas in farm B, they ranged from 256 to 16 384 (GMT=1326). Young guanacos captured after the outbreak in farm A had an RV antibody GMT of 320 (64–1024), whereas the antibody titres of healthy adults in farm B ranged from 64 to 4096 (GMT=193) (Fig. 1). These results showed a similar distribution of high antibody titres in the groups of convalescent and healthy guanacos. As it was not possible to obtain paired serum samples from the affected animals, corresponding to the acute and convalescent phase of the disease, serological confirmation of the viral infection by seroconversion was not possible. However, the presence of very high antibody titres in some of the convalescent animals (1:16 384) suggests recent exposure to RV. In contrast, all animals tested were negative for BCV antigen in faeces (none of 53) and BCV antibodies in serum (none of 47), suggesting that this pathogen was not circulating in the guanaco population under study and was not involved in the diarrhoea outbreak.
The two RV strains detected by ELISA were successfully propagated in roller tubes containing confluent monolayers of MA‐104 cells in the presence of pancreatin (5 μg/ml) (Hoshino et al., 1983). Viral cytopathic effects were detected 24 h post‐inoculation and the culture supernatants were positive for RV by ELISA after the first and second passages.
In order to confirm RV diagnosis in stool specimens and its presence in tissue culture supernatants, both materials were examined by immuno‐electron‐microscopy (IEM) using described methods (Saif et al., 1977) to evaluate virus morphology. Typical rotavirus particles in size and morphology (60–75 nm icosahedral particles) were observed as shown in 2Fig. 2a,b.
Figure 2.

Typical rotavirus particles observed in guanaco stool samples by immuno‐electron microscopy. Bovine hyperimmune antiserum to RV diluted 1:5 (source: L. J. Saif). Magnification: × 50 000. (a) RV isolated in farm A; (b) RV isolated in farm B.
In addition, to investigate the electropherotype of each virus strain, RV genomic ds‐RNA was extracted from the original faecal samples and tissue culture, using conventional techniques (Sambrook et al., 1989). Double‐stranded RNA segments were analysed by electrophoresis in 7.5% polyacrylamide gels (PAGE) and visualized by silver staining (Laemmli, 1970). The detected strains showed 11 dsRNA segments with a band distribution pattern (4:2:3:2) that is characteristic of group A RV. However, each strain had a distinctive long electropherotype (minor migration differences), suggesting the circulation of different RV strains at each farm. Additionally, both RV strains had different electropherotypes compared with the BRV reference strains NCDV‐Lincoln, IND and NCDV‐Cody (I‐801) (Fig. 3).
Figure 3.

Rotavirus electropherotypes. Lane A, guanaco RV strain detected in the faecal sample of a new‐born guanaco at farm A; lane B, first passage in MA‐104 of guanaco RV strain isolated at farm A; lane C, guanaco RV strain detected in the faecal sample of a new‐born guanaco at farm B; lane D, first passage in MA‐104 of guanaco RV strain isolated at farm B; lane E, BRV NCDV‐Cody (I801) (P[1]G8); lane F, BRV IND (P[5]G6); and lane G, BRV NCDV Lincoln (P[1]G6) (source of reference RV strains: L. J. Saif).
Discussion
In the present study, in addition to previous reports made in llamas and alpacas, the circulation of RV was confirmed among another species of South American camelids, Lama guanicoe, not only by positive serology but also by the detection and isolation of RV from faecal samples from new‐born guanacos with acute diarrhoea. No evidence for the circulation of other viral agents associated with neonatal diarrhoea, such as BCV was observed by ELISA or IEM. However the detection of E. coli in farm A and Salmonella sp. in farm B indicated that several enteric pathogens were involved in these two diarrhoea outbreaks.
The absence of RV antibodies in the two sick animals could be related to a failure of passive transfer of maternal antibodies from the dam to the new‐born. Previous studies in camelids have shown that intestinal absorption of immunoglobulins occurs during the first 24 h after birth, being maximal between 8 and 12 h post‐partum (Murphy, 1989; Adams and Garry, 1994), and that failure of passive transfer is a major factor leading to mortality in neonatal camelids (Drew and Fowler, 1995). The present data suggests an absence of adequate levels of passive protection that may be associated with the high mortality observed after the diarrhoea outbreak. The capture of animals at several months of age, instead of a few days of age was recommended to the farmers to avoid the possible interruption of proper colostral and lactogenic antibody intake.
Rotavirus diagnosis was confirmed by IEM and PAGE. As infections with group A RV have been detected in animal species in Argentina (Barrandeguy et al., 1988; Bellinzoni et al., 1989, 1990; Mattion et al., 1989; Parreño et al., 1997; Costantini et al., 1999), it would be interesting to investigate whether these rotavirus outbreaks in Lama guanicoe were due to an autochthonous guanaco RV strain or transmission from a domestic species. Characterization assays and sequence analysis of the isolated strains are in progress to investigate these possibilities. To the authors’ knowledge, this is the first report of detection and isolation of RV associated with neonatal diarrhoea in Lama guanicoe.
Acknowledgements
The authors are indebted to Adriana McGuirre from Fundación Habitat, D. Garrido from ‘Chacay’ farm, Dr C. Cabral and personnel from ‘Dirección de Fauna de la Secretaría de Recursos Renovables’ for their valuable collaboration. This work was supported with Grants from UBACyT/I2623/99‐IVO1, Academia Nacional de Agronomia y Veterinaria, FONCyT 08/01740 and PICT‐08/04687 from ScyT‐Argentina and a grant from The Ohio Agricultural Research and Development Center (OARDC) for International Collaboration, The Ohio State University‐USA. A.A.S. is member of the CONICET Research Career (CONICET‐Argentina). V.P.’s work is supported by a CONICET internal post‐degree scholarship and V.C. by a CIC PBA scholarship.
References
- 1. Adams, R. & Garry F., 1994: Llama neonatology. Vet Clin. North Am., Food Anim. Pract. 10 , 201–208. [DOI] [PubMed] [Google Scholar]
- 2. Barrandeguy, M. E. , Cornaglia E. M., Gottschalk M., Fitjman N., Pasini M. I., Gomez Yafal A., Parraud J., Schudel A. A., 1988: Rotavirus, enterotoxigenic Escherichia coli and other agents in the feces of dairy calves with and without diarrhoea. Rev. Lat. Am. Microbiol. 30 , 239–245. [Google Scholar]
- 3. Bellinzoni, C. , Blackhall J., Terzolo H. R., Moreira A. R., Auza N., Mattion N., Micheo G. I., La Torre J., Scodeller E., 1990: Mycrobiology of diarrhoea in young beef and dairy calves in Argentina. Revista Argentina Microbiol. 22 , 130–137. [PubMed] [Google Scholar]
- 4. Bellinzoni, C. , Blackhall J., Mattion N., Estes M., Snodgrass D., La Torre J., Scodeller E., 1989: Serological characterization of bovine rotaviruses isolated from dairy and beef herds in Argentina. J. Clin. Microbiol. 27 , 2619–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clark, M. A. , 1993: Bovine coronavirus. Br. Vet. J. 149 , 51–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cornaglia, E. M. , Barrandeguy M., Fijtman N., Schudel A., 1989: Enzyme‐linked immunosorbent assay, immunofluorescence test and electrophoretic analysis of rotaviral RNA in the diagnosis and characterization of the bovine rotavirus. Rev. Lat. Am. Microbiol. 31 , 59–62. [Google Scholar]
- 7. Costantini, V. , Parreño V., Combessies G., Bardón J. C., Leunda M., Saif L., Fernández F., 1999: Diagnostic and antigenic characterization of group A bovine rotavirus in Argentina. 1994–98. In. Ellis, R. P. (ed), Proceedings of the 80th Annual Meeting of the CRWAD November, Chicago, IL, USA, (Poster 56P). Iowa State University Press, Ames.
- 8. Drew, M. L. & Fowler M. E., 1995: Comparison of methods for measuring serum immunoglobulin concentrations in neonatal llamas. JAVMA 206 , 1374–1380. [PubMed] [Google Scholar]
- 9. Hoshino, Y. , Wyatt R. G., Greenberg H., Kalica A., Flores J., Kapikian A., 1983: Isolation, propagation and characterization of a second equine rotavirus serotype. Infect. Immun. 41 , 1031–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kapikian, A. Z. & Chanock R. M., 1996: Rotaviruses. In: Fields, B., N. Knipe, D. M. Howley, et al. (eds), Fields Virology 3rd edn, Chapter 55, pp. 1657–1708. Lippincott‐Raven Publishers, Philadelphia, PA.
- 11. Karesh, W. B. , Uhart M. M., Dierenfeld E. S., Breselton W. E., Torres A., House C., Puche H., Cook R. A., 1998: Health evaluation of free‐ranging guanaco (Lama guanicoe). J. Zoo Wild Med. 29 , 134–141. [PubMed] [Google Scholar]
- 12. Laemmli, U. K. , 1970: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227 , 680–685. [DOI] [PubMed] [Google Scholar]
- 13. Mattion, N. , Bellinzoni R. C., Blackhall J. O., La Torre J., Scodeller E. A., 1989: Antigenic characterization of swine rotavirus in Argentina. J. Clin. Microbiol. 27 , 795–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mattson, D. E. , 1994: Update of llama medicine, viral diseases. Vet. Clin. North Am.: Food Anim. Pract. 10 , 345–351. [PubMed] [Google Scholar]
- 15. Murphy, P. J. , 1989: Obstetrics, neonatal care and congenital conditions. Vet. Clin. North Am., Food Anim. Pract. 5 , 183–202. [DOI] [PubMed] [Google Scholar]
- 16. Panighi, M. , 1990: Coronavirus detección de anticuerpos en bovinos de la Republica Argentina. MS Thesis. Universidad Nacional de Tucuman, Cuidad de Tucuman, Argentina.
- 17. Parreño, V. , Ricci L., Ruiz M., Fernandez F., 1996: Coronavirus Bovino: Diagnóstico, aislamiento y caracterización. In: Sociedad Argentina de Virologica (SAV) Division de AAM (eds), Proceeding of El V Congreso Argentino de Virología – Tandil, Buenos Aires, Argentina, (Poster P80). Sociedad Argentina de Virologica (SAV) Division de AAM, Buenos Aires.
- 18. Parreño, V. , Barrandeguy M., Craig M. I., Saif L. J., Fernandez F., 1997: Equine rotaviruses in thoroughbred diarrheic foals in Argentina. In: Ellis, R. P. (ed), Proceedings of the 78th Annual Meeting of the Conference of Research Workers in Animal Diseases, Chicago, IL, USA, (Poster 56P). Iowa State University Press, Ames.
- 19. Puntel, M. , Fondevila N. A., Blanco Viera J., O'Donnel V. K., Marcovechio F., Carrillo B. J., Schudel A. A., 1999: Serological survey of viral antibodies in llamas (Lama glama) in Argentina. J. Vet. Med. B. 46 , 157–161.DOI: 10.1046/j.1439-0450.1999.00215.x [DOI] [PubMed] [Google Scholar]
- 20. Rivera, H. , Madewell B. R., Ameghino E., 1987: Serologic survey of viral antibodies in the Peruvian alpaca (Lama pacos). Am. J. Vet. Res. 48 , 189–191. [PubMed] [Google Scholar]
- 21. Saif, L. J. , Bohl E. H., Kohler E. M., Hughes J., 1977: Immune electron microscopy of tge virus and rotavirus (reovirus‐like agent) of swine. Am. J. Vet. Res. 38 , 13–20. [PubMed] [Google Scholar]
- 22. Saif, L. J. , 1990: A review of evidence implicating bovine coronavirus in the etiology of winter disentery in cows: an enigma resolved? Cornell Vet. 80 , 303–311. [PubMed] [Google Scholar]
- 23. Sambrook, J. , Fritsch E. F., Maniatis T., 1989: Molecular Cloning. A Laboratory Manual, 2nd edn. Cold Spring Harbor Laboratory Press, New York, USA.
- 24. Smith, D. R. , Tsunemitsu H., Heckert R., Saif L. J., 1996: Evaluation of two antigen‐capture ELISAs using polyclonal or monoclonal antibodies for the detection of bovine coronavirus. J. Vet. Invest. 8 , 99–105. [DOI] [PubMed] [Google Scholar]


