Abstract
Background
Bromodomain and extra-terminal domain proteins are promising epigenetic anticancer drug targets. This first-in-human study evaluated the safety, recommended phase II dose, pharmacokinetics, pharmacodynamics, and preliminary antitumor activity of the bromodomain and extra-terminal domain inhibitor molibresib (GSK525762) in patients with nuclear protein in testis (NUT) carcinoma (NC) and other solid tumors.
Methods
This was a phase I and II, open-label, dose-escalation study. Molibresib was administered orally once daily. Single-patient dose escalation (from 2 mg/d) was conducted until the first instance of grade 2 or higher drug-related toxicity, followed by a 3 + 3 design. Pharmacokinetic parameters were obtained during weeks 1 and 3. Circulating monocyte chemoattractant protein-1 levels were measured as a pharmacodynamic biomarker.
Results
Sixty-five patients received molibresib. During dose escalation, 11% experienced dose-limiting toxicities, including six instances of grade 4 thrombocytopenia, all with molibresib 60–100 mg. The most frequent treatment-related adverse events of any grade were thrombocytopenia (51%) and gastrointestinal events, including nausea, vomiting, diarrhea, decreased appetite, and dysgeusia (22%–42%), anemia (22%), and fatigue (20%). Molibresib demonstrated an acceptable safety profile up to 100 mg; 80 mg once daily was selected as the recommended phase II dose. Following single and repeat dosing, molibresib showed rapid absorption and elimination (maximum plasma concentration: 2 hours; t1/2: 3–7 hours). Dose-dependent reductions in circulating monocyte chemoattractant protein-1 levels were observed. Among 19 patients with NC, four achieved either confirmed or unconfirmed partial response, eight had stable disease as best response, and four were progression-free for more than 6 months.
Conclusions
Once-daily molibresib was tolerated at doses demonstrating target engagement. Preliminary data indicate proof-of-concept in NC.
Bromodomains (BRD) are structurally conserved functional motifs that are found in components of chromatin-associated transcription factor complexes (1,2). The BRD and extra-terminal domain (BET) family of proteins (BRD2, BRD3, BRD4, and BRDT) are epigenetic readers that regulate gene expression through BRD-mediated recognition of acetylated histones (1,2) and influence transcription of genes controlling growth, cell cycle progression, and differentiation (3–5). In addition, BET proteins contribute to both carcinogenesis and treatment resistance in multiple solid and hematologic malignancies (5,6). These findings have prompted interest in the development of small-molecule BET inhibitors that use competitive acetyl-lysine binding to displace BET proteins from chromatin (7).
One tumor type potentially vulnerable to BET inhibition is nuclear protein in testis (NUT) carcinoma (NC, also referred to as NUT midline carcinoma or NMC), defined by rearrangement of the NUTM1 gene and known to be driven by BET fusion proteins, most commonly BRD4-NUT (2,5,6,8). NC is an aggressive cancer with an estimated median survival of 6.7–9.7 months, for which disease control from surgical resection, conventional chemotherapy, or radiation therapy is short-lived (9,10). Abrogation of NUT fusion protein activity in NC cell lines has been shown to irreversibly induce squamous differentiation followed by growth arrest, implicating NUT fusion proteins in a differentiation block with dysregulated proliferation (11). One target of BRD-NUT fusion proteins responsible for driving growth and blocking differentiation is myelocytomatosis viral oncogene homolog (MYC) (12). Recent preclinical studies in leukemia and myeloma models indicate that BET inhibition may reduce both c-MYC expression and its transcriptional downstream effects, resulting in antitumor activity (13,14). These findings are of potential relevance for NC therapy. BET proteins also regulate other relevant transforming proteins, including Breast Carcinoma-Amplified Sequence 1 and PDZ Domain-containing 1 (15).
Molibresib (GSK525762) is an orally bioavailable, small-molecule BET inhibitor (16) that has demonstrated antitumor activity in preclinical models of NC and other solid and hematologic malignancies (17,18). Here, we report the results of a first-in-human dose-escalation study in advanced solid tumors, with a particular focus on a 19-patient cohort with NC.
Materials and Methods
Eligibility
Patients aged 16 years or older with the following diagnoses were eligible: previously treated or treatment-naïve NC; small cell lung cancer, colorectal cancer (CRC), triple-negative breast cancer (TNBC), estrogen receptor-positive BC, castration-resistant prostate cancer (CRPC), non-small cell lung cancer, neuroblastoma, or any other MYCN-amplified solid tumor (copy number gain ≥5). Patients with solid tumors (excluding CRPC) were required to have measurable disease according to Response Evaluation Criteria in Solid Tumors (RECIST) criteria v1.1 (19). NC patients with evaluable disease could be enrolled at Medical Monitor discretion. An Eastern Cooperative Oncology Group Performance Status score of 0–2 was required for patients with NC and 0–1 for other patients. Full details are provided in the Supplementary Methods (available online).
Study Design and Treatment
This was a two-part phase I and II, open-label, multicenter, dose-escalation study (BET115521; www.gsk-clinicalstudyregister.com/study/115521; NCT01587703) conducted at centers in Canada, France, Spain, the United Kingdom, and the United States. The primary objective of part 1, reported here, was to evaluate the safety and tolerability of once-daily molibresib and to determine the recommended phase II dose (RP2D). The ongoing part 2 is exploring the activity of molibresib at the RP2D in multiple solid tumor cohorts.
Molibresib was administered orally as an amorphous free-base tablet. Single-patient dose escalation with a twofold dosing increase per escalation was conducted, followed by a 3 + 3 dose-escalation design. The starting dose was 2 mg once daily. If there were no dose-limiting toxicities (DLTs) and no grade 2 or greater drug-related adverse events (AEs) in the first 4 weeks, dose escalation was permitted. The dose at which a DLT or any grade 2 or greater drug-related AE was observed was taken forward into the 3 + 3 dose-escalation plan, which enrolled three patients per dose cohort, with twofold or less dosing increase per escalation. During the 3 + 3 phase, if one out of three patients experienced a DLT, an additional three patients were enrolled at that dose. The dose at which two or more DLTs were observed in six patients was considered to have exceeded the maximum tolerated dose (MTD). Intrapatient dose escalations were permitted in patients who did not experience a grade 2 or greater AE during accelerated dose escalation or a DLT in 3 + 3 dose escalation. Up to an additional six patients could be enrolled at any dose level below the MTD to obtain additional safety, tolerability, pharmacokinetic, and efficacy information. Additional enrollments were also permitted to confirm the RP2D. See Supplementary Methods (available online) for full details of the dose-escalation procedure.
This study was conducted in accordance with good clinical practice and the Declaration of Helsinki, and approval was given by each institution’s ethical review board. All patients provided written informed consent before participation.
Safety Evaluations
DLTs were defined within the first 4 weeks of therapy and included grade 4 neutropenia lasting 7 or more days; febrile neutropenia lasting for longer than 24 hours despite adequate treatment; grade 4 thrombocytopenia; molibresib-related grade 3–4 nonhematologic toxicity or grade 2 nonhematologic toxicity (at any time) considered to be dose limiting; grade 2 troponin T elevation on two separate occasions within 48 hours; alanine aminotransferase (ALT) five or more times the upper limit of normal (ULN); ALT three or more times the ULN and bilirubin two times the ULN (>35% direct); or ALT three to five times the ULN with bilirubin less than two times the ULN but with hepatitis symptoms or rash; or treatment delay of at least 14 days due to unresolved drug-related toxicity. The MTD was exceeded if two or more patients in a cohort of six experienced a DLT. The RP2D was defined based on the MTD and the safety and pharmacokinetic (PK) profiles as well as the biologically active dose determined from pharmacodynamic data.
AEs and serious AEs were collected from the time of the first dose of molibresib until 28 days after discontinuation and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0) (20).
Physical examination, Eastern Cooperative Oncology Group assessment, vital sign measurements, echocardiograms, safety electrocardiograms, Holter monitoring, and clinical laboratory assessments (serum chemistry, hematology, urinalysis) were also performed. Patients were hospitalized for the first 48 hours for telemetry monitoring, required because preclinical data suggested a potential increased risk of arrhythmia and myocardial damage.
Pharmacokinetic Analyses
Serial blood sampling for PK and pharmacodynamic analysis was performed after the first dose at week 1 day 1, and after repeated administration at week 3 day 4. Molibresib plasma concentrations were quantified using a validated high-performance liquid chromatography-mass spectrometry-mass spectrometry method. The two major human metabolites of molibresib (M5 [N-desethyl] and M13 [ethyl-hydroxyl]) were also quantified following administration of a molibresib 80-mg dose using a validated high-performance liquid chromatography-mass spectrometry-mass spectrometry method. Molibresib and metabolite PK parameters were analyzed using conventional noncompartmental methods (Phoenix WinNonlin, Certara, Princeton, NJ).
Pharmacodynamic Analyses
BET proteins have emerged as critical regulators of inflammatory gene expression in macrophages (21,22). Monocyte chemoattractant protein 1 (MCP-1) expression is regulated by BET proteins so that measuring circulating levels of this cytokine (Myriad RBM Inflammation MAP v1.0 assay) provides pharmacodynamic information (22). Circulating Factor VII was also assessed as part of a select cytokine panel and provided an indirect measure of safety related to hepatic events (transcriptional effect) and outcome (bleeding events independent of thrombocytopenia).
Efficacy Evaluations
Clinical response was evaluated according to RECIST 1.1 (19) or the Prostate Cancer Working Group 2 guidelines (23) and were reviewed centrally (Supplementary Methods, available online). Fluorodeoxyglucose positron emission tomography was optional during the initial dose-escalation phase until implementation of the standard 3 + 3 dose-escalation regimen.
Baseline Assessments for NC Patients
The diagnosis of NC was confirmed by immunohistochemistry (IHC), fluorescence in-situ hybridization (FISH), or next-generation sequencing for all enrolled patients. Additionally, FISH analysis or next-generation sequencing was carried out to characterize the NUT gene fusion partner and to support exploratory analysis of differential outcomes based on the NUT fusion partner.
Statistical Analyses
No formal statistical hypotheses were tested in part 1. Sample size was determined by the number of patients required to define the MTD. Descriptive safety and efficacy analyses were performed in the “all-treated patients population,” defined as all patients who received at least one dose of molibresib.
Results
Patient Characteristics and Treatment
Part 1 of the study was conducted from March 28, 2012, to April 13, 2018. Sixty-five patients were treated with molibresib at doses of 2–16 mg (n = 11), 30 mg (n = 4), 60 mg (n = 9), 80 mg (n = 32), or 100 mg (n = 9). Patient characteristics and demographics are summarized in Table 1. The most frequent primary tumors were CRC (n = 22 [34%]) and NC (n = 19 [29%]). At the time of analysis (June 2016), 38 (58%) patients had died, with 35 (92%) deaths due to disease progression (cause of death unavailable for three patients); four (6%) patients were still receiving study treatment; 22 (34%) were in follow-up; and one (2%) had withdrawn from the study at investigator discretion.
Table 1.
Patient characteristics and demographics*
| Characteristic | NC cohort | All patients |
|---|---|---|
| (n = 19) | (n = 65) | |
| Age, y | ||
| Mean (SD) | 31.5 (13.0) | 50.7 (17.4) |
| Median (range) | 27.0 (17–61) | 54.0 (17–86) |
| Female sex, no. (%) | 10 (53) | 31 (48) |
| ECOG performance status, no. (%) | ||
| 0 | 4 (21) | 22 (34) |
| 1 | 12 (63) | 40 (62) |
| 2 | 3 (16) | 3 (5) |
| ≥3 | 0 | 0 |
| Primary tumor type, no. (%) | ||
| Colon or rectum | — | 22 (34) |
| NC | 19 (100) | 19 (29) |
| Prostate | — | 9 (14) |
| Small cell lung | — | 6 (9) |
| Breast | — | 5 (8) |
| Non-small cell lung | — | 2 (3) |
| Neuroblastoma | — | 1 (2) |
| Multiple myeloma | — | 1 (2) |
| Time since initial diagnosis, median (range), d† | 95 (26–318) | 725 (26–5667) |
| Time since last progression, median (range), d‡ | 40 (9–177) | 45 (5–359) |
| Metastatic disease at screening, no. (%)§ | 15 (79) | 60 (94) |
| Prior cancer-related therapy, no. (%) | ||
| Surgery | 12 (63) | 44 (68) |
| Radiotherapy | 12 (63) | 41 (63) |
| Prior systemic therapy lines, no. (%) | ||
| 0 | 4 (21) | 4 (6) |
| 1 | 9 (47) | 10 (15) |
| 2 | 4 (21) | 5 (8) |
| ≥3 | 2 (11) | 46 (71) |
Due to some instances of missing dates, durations could not be calculated for all patients. ECOG = Eastern Cooperative Oncology Group; NC = NUT carcinoma; NUT = nuclear protein in testis.
NC: n = 18, total: n = 57.
NC: n = 9, total: n = 51.
NC: n = 19, total: n = 64.
Safety and DLTs
During dose escalation, seven (11%) of the 65 patients experienced DLTs: six instances of grade 4 thrombocytopenia (9%) and one of hepatitis (2%; drug-induced hepatitis in a patient with CRC and hepatic metastases that had increased in size on day 17). All DLTs occurred in patients receiving 60–100 mg molibresib once daily, with one patient (11%) in the 60-mg group, five patients (16%) in the 80-mg group, and one patient (11%) in the 100-mg group. The MTD was not reached.
All AEs occurring in greater than 10% of the all-treated patients population are summarized in Supplementary Table 1 (available online), and all AEs judged by the investigator to be related to treatment are summarized in Table 2. Overall, 54 (83%) of 65 patients had a treatment-related AE. The most frequent treatment-related AEs were thrombocytopenia (n = 33 [51%]), nausea (n = 27 [42%]), decreased appetite (n = 18 [28%]), vomiting (n = 15 [23%]), and diarrhea (n = 15 [23%]). AEs previously associated with BET inhibitors as a class (eg, dysgeusia, increased bilirubin, fatigue) (24,25) occurred in 17%–22% of patients. Grade 3 and 4 treatment-related AEs occurred in 31 patients (48%), with only thrombocytopenia (n = 24 [37%]) and anemia (n = 5 [8%]) occurring in more than 5% of patients (Table 2). Serious AEs regardless of attribution were reported in 29 (45%) of 65 patients, with only thrombocytopenia (n = 11 [17%]) and nausea (n = 4 [6%]) occurring in more than 5% of patients (Supplementary Table 2, available online). Despite the high incidence of thrombocytopenia, hemorrhage, hemoptysis, or other bleeding complications were not a clinically significant issue. Among the cardiac AEs of special interest, three (5%) patients experienced a QTc prolongation (two grade 1, one grade 2).
Table 2.
Summary of treatment-related AEs occurring in more than 10% of patients (all treated patients)*
| Once-daily molibresib dose |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment-related AEs, No. (%) |
Grade 3 and 4 treatment-related AEs, No. (%) |
|||||||||||
| 2–16 mg | 30 mg | 60 mg | 80 mg | 100 mg | Total | 2–16 mg | 30 mg | 60 mg | 80 mg | 100 mg | Total | |
| (n = 11)† | (n = 4) | (n = 9) | (n = 32) | (n = 9) | (n = 65) | (n = 11) | (n = 4) | (n = 9) | (n = 32) | (n = 9) | (n = 65) | |
| Any event | 5 (45) | 2 (50) | 7 (78) | 31 (97) | 9 (100) | 54 (83) | — | — | 1 (11) | 23 (72) | 7 (78) | 31 (48) |
| Thrombocytopenia | — | 1 (25) | 3 (33) | 22 (69) | 7 (78) | 33 (51) | — | — | 1 (11) | 16 (50) | 7 (78) | 24 (37) |
| Nausea | 2 (18) | 2 (50) | 2 (22) | 15 (47) | 6 (67) | 27 (42) | — | — | — | 2 (6) | — | 2 (3) |
| Decreased appetite | 1 (9) | 1 (25) | 1 (11) | 11 (34) | 4 (44) | 18 (28) | — | — | — | 3 (9) | — | 3 (5) |
| Diarrhea | — | — | 1 (11) | 12 (38) | 2 (22) | 15 (23) | — | — | — | 1 (3) | — | 1 (2) |
| Vomiting | 2 (18) | — | 1 (11) | 9 (28) | 3 (33) | 15 (23) | — | — | — | — | — | — |
| Anemia | — | — | — | 12 (38) | 2 (22) | 14 (22) | — | — | — | 5 (16) | — | 5 (8) |
| Dysgeusia | — | 1 (25) | 3 (33) | 6 (19) | 4 (44) | 14 (22) | — | — | — | — | — | — |
| Fatigue | — | — | 1 (11) | 8 (25) | 4 (44) | 13 (20) | — | — | — | 1 (3) | — | 1 (2) |
| Blood bilirubin increased | — | — | — | 7 (22) | 4 (44) | 11 (17) | — | — | — | 2 (6) | 1 (11) | 3 (5) |
| Asthenia | — | — | — | 10 (31) | — | 10 (15) | — | — | — | 3 (9) | — | 3 (5) |
| AST increased | — | — | — | 5 (16) | 2 (22) | 7 (11) | — | — | — | — | 1 (11) | 1 (2) |
| Rash | — | — | 2 (22) | 4 (13) | 1 (11) | 7 (11) | — | — | — | — | — | — |
Including all grade 3 and 4 treatment-related AEs occurring in more than one patient. AE = adverse event; AST = aspartate aminotransferase; DLT = dose-limiting toxicities; PD = progressive disease.
Early PD among patients enrolled at the lowest dose levels caused more than one enrollment to achieve one DLT-evaluable patient. The 3 + 3 rule was triggered at 16 mg after a grade 2 AE of nausea was documented.
Events leading to permanent discontinuation of the study drug or dose reductions occurred in 12 (18%) and 14 (22%) patients, respectively. The most frequent event in both cases was thrombocytopenia (discontinuation, n = 4 [6%]; dose reduction, n = 10 [15%]). The only other events leading to discontinuation or dose reduction in more than one patient were increased ALT (discontinuation, n = 2 [3%]), increased bilirubin (dose reduction, n = 2 [3%]), and dysgeusia (dose reduction, n = 2 [3%]). All events leading to discontinuation or dose reduction occurred in the 60- to 100-mg groups, except for one discontinuation event (involving hypertension, hyponatremia, and a confused state, all considered to be unrelated to treatment) that occurred in the 4-mg group). Although the incidence of discontinuation events was similar across the 60- to 100-mg groups (all 22%), the incidence of dose reduction events was markedly greater in the 100-mg group compared with both the 60-mg and 80-mg groups (n = 7 [78%] vs n = 2 [22%] and n = 5 [16%], respectively). Molibresib 80 mg was selected as the RP2D.
Pharmacokinetic Parameters
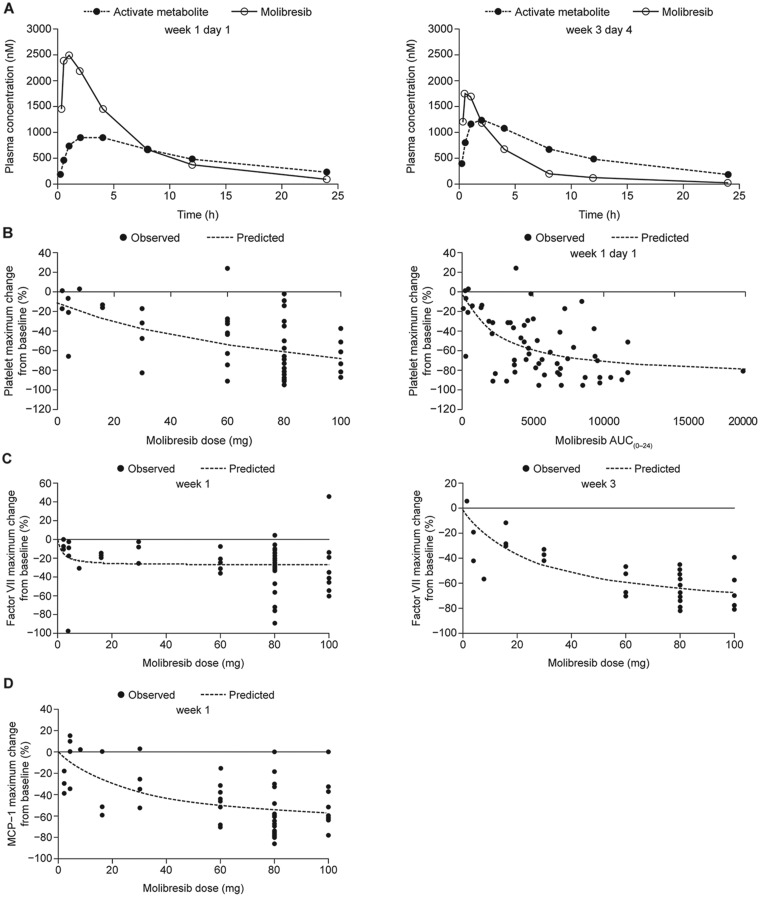
Following single- and repeat-dose oral administration of 2–100 mg (n = 64), molibresib showed rapid absorption and elimination, with the maximum plasma concentration (Cmax) occurring within 2 hours postdose and a mean terminal phase half-life of 3–7 hours (Figure 1A;Supplementary Table 3, available online). Exposure data showed large between-patient variability. Both Cmax and area under the concentration-time curve (AUC) values for molibresib appeared to increase in a dose-proportional fashion after single and repeated administration of doses less than or equal to 30 mg, but individual AUC values overlapped at doses greater than 30 mg (Supplementary Figure 1, available online). A trend for decreased exposure to molibresib over time was observed at doses of 60 mg and greater (Figure 1A;Supplementary Table 3, available online). Molibresib has two major human metabolites (M5 and M13) that have demonstrated inhibitory activity against tumor cell lines with a similar potency to the parent molecule (Supplementary Figures 2 and 3, available online). Following single oral administration of molibresib 80 mg (n = 29), M5 and M13 showed rapid formation (median time to Cmax of 2 hours) and an exposure (AUC) of approximately 85% that of molibresib (Figure 1A;Supplementary Table 3, available online). Following repeated oral administration of molibresib 80 mg (n = 14), exposure to the two major active molibresib metabolites was greater compared with single-dose administration, accounting for decreased exposure to the parent compound over time (Figure 1A;Supplementary Table 3, available online).
Figure 1.
Pharmacokinetic parameters and pharmacodynamic profiles of molibresib. A) Pharmacokinetic profiles of molibresib (80-mg dose) and its major active human metabolites M5 and M13 measured together at week 1 day 1 (left) and week 3 day 4 (right). B) Maximum change in platelet count from baseline (%) by molibresib dose (left) by exposure measured on week 1 day 1 (right). Platelet data are for the initial dose level during the first 60 days on treatment and limited to patients who received the planned first 2 weeks of treatment. C) Percent maximum change in Factor VII from baseline by molibresib dose at week 1 (left) and week 3 (right). D) Percent maximum change in MCP-1 from baseline by molibresib dose at week 1. AUC(0–24) = area under the concentration–time curve from 0 to 24 hours; MCP-1 = monocyte chemoattractant protein 1. In B–D, predicted values represent the line of best fit (ie, a simple maximum effect [Emax] model) through observed values.
Pharmacodynamic Analyses
Analysis of platelet count nadirs showed molibresib dose-dependent and exposure-dependent reductions over the first 60 days of treatment (Figure 1B). Circulating Factor VII showed dose-dependent reductions from baseline at week 3 only (Figure 1C). MCP-1 concentrations in blood samples also decreased for longer than 10 hours postdose (Supplementary Figure 4, available online) showing that the expression of MCP-1 was suppressed in a time frame consistent with the molibresib PK profile (median time to Cmax = 1–2 hours) and in a dose-dependent manner (Figure 1D), indicating target engagement. Additional circulating cytokine parameters at weeks 1 and 3 are summarized in Supplementary Table 4 (available online).
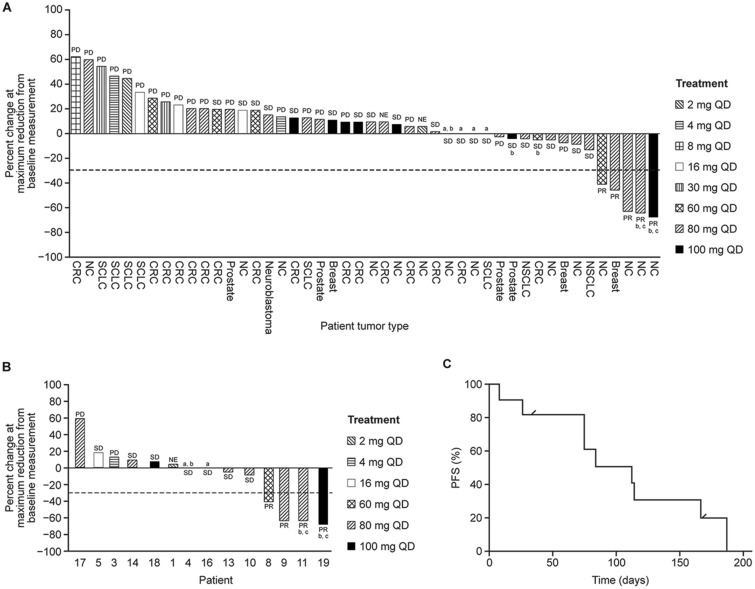
Clinical Response
Clinical and radiographic responses for all treated patients and those with NC are summarized in Figure 2, A–C and Table 3. In the NC cohort (n = 19), the investigator-assessed best overall response rate was 11% (95% confidence interval = 1.3% to 33.1%), with no complete responses (CRs) reported and two (11%) of 19 patients experiencing a confirmed partial response (PR). Scans illustrating one of these confirmed PRs are shown in Supplementary Figure 5A, available online. There were two patients with unconfirmed PRs whose disease demonstrated substantial regression; one of these patients had progressive disease (PD) as the best confirmed response, and a second patient had worsening of evaluable disease on subsequent 18F-fluorodeoxyglucose positron emission tomography, with stable disease as the best confirmed response (Supplementary Figure 5B, available online).
Figure 2.
Antitumor activity of molibresib. A) Maximum tumor reduction (% from baseline) and best clinical responses for all treated patients who had measurable disease and at least one complete postbaseline assessment (n = 46 of 65 enrolled). B) Maximum tumor reduction (% from baseline) and best clinical responses for NC patients who had measurable disease and at least one complete postbaseline assessment (n = 14 of 19 enrolled). In A and B, NE indicates patients with clinical progression, with the postbaseline scan performed before 28 days from baseline. Patients not depicted in A and B were removed from the trial for early clinical progression or progression based on evidence of new lesion(s) without reassessment of baseline target lesions or had nonmeasurable disease at outset. C) Progression-free survival (PFS) in the NC cohort for patients who received at least 60 mg daily (n = 11). Tick marks represent censored patients: If a patient received subsequent anticancer therapy before the date of documented events, PFS was censored at the last adequate assessment before the initiation of therapy. Otherwise, if a patient did not have a documented date of events, PFS was censored at the date of the last adequate assessment. aMaximum reduction from baseline is 0%. bRemained on treatment for greater than 4 months. cConfirmed response. CRC = colorectal cancer; NC = NUT carcinoma; NSCLC = non-small cell lung cancer; NUT = nuclear protein in testis; SCLC = small cell lung cancer; PD = progressive disease; PR = partial response; QD = once daily; SD = stable disease.
Table 3.
Individual patient treatment duration and clinical responses (NC cohort)
| Patient* | Age, y | Sex | Fusion protein | Primary site of origin | Dose, QD | Best response by RECIST | No. of prior treatments | Duration of treatment, d |
|---|---|---|---|---|---|---|---|---|
| 1 | 23 | Male | BRD4-NUT | Head and neck | 2 | NE† | 2 | 11 |
| 2 | 36 | Male | BRD4-NUT | Thoracic | 2 | NE | 2 | 9 |
| 3 | 43 | Female | BRD4-NUT | Other (left kidney) | 4 | PD | 0 | 17 |
| 4 | 17 | Male | BRD4-NUT | Head and neck | 4 | SD | 1 | 255 |
| 5 | 48 | Female | BRD4-NUT | Head and neck | 16 | SD | 0 | 43 |
| 6 | 19 | Male | BRD4-NUT | Head and neck | 30 | PD‡ | 1 | 42 |
| 7 | 20 | Female | BRD4-NUT | Thoracic | 60 | PD‡ | 1 | 8 |
| 8 | 23 | Male | NA | Thoracic | 60 | PR§ | 1 | 82 |
| 9 | 27 | Male | BRD3-NUT | Thoracic | 80 | PR‖ | 1 | 69 |
| 10 | 39 | Female | NSD3-NUT | Thoracic | 80 | SD | 0 | 48 |
| 11 | 36 | Female | BRD3-NUT | Head and neck | 80 | PR¶ | 1 | 196 |
| 12 | 51 | Female | BRD4-NUT | Head and neck | 80 | non-CR/non-PD# | 1 | 55 |
| 13 | 37 | Female | BRD4-NUT | Head and neck | 80 | SD | 0 | 199 |
| 14 | 39 | Female | BRD4-NUT | Head and neck | 80 | SD | 2 | 75 |
| 15 | 23 | Male | NA | Thoracic | 80 | NE | 1 | 8 |
| 16 | 22 | Male | BRD3-NUT | Thoracic | 80 | SD | 1 | 114 |
| 17 | 17 | Female | BRD4-NUT | Thoracic | 80 | PD | 1 | 28 |
| 18 | 61 | Female | BRD4-NUT | Thoracic | 100 | SD | 1 | 113 |
| 19 | 18 | Male | BRD3-NUT | Other (right scapula) | 100 | PR¶ | 2 | 186 |
All patients came off study treatment for PD except patient 10, who withdrew consent because of a disease-related pericardial effusion requiring drainage, and patient 13, who withdrew consent for multiple AEs, including periorbital edema around the resected lesion. Patient 4 underwent dose escalation from 4 to 8 to 16 mg once daily. Patients 9 and 13 had the dose increased from 80 mg to 100 mg once daily. Two patients had dose reduction due to thrombocytopenia, including patient 18 (from 100 mg to 80 mg in week 5 to 60 mg in week 13) and patient 19 (from 100 mg to 80 mg in week 12). BRD = bromodomain; CR = complete response; CT = computed tomography; FDG-PET = 18F-fluorodeoxyglucose positron emission tomography; NA = not available; NE = not evaluable due to insufficient or unavailable pre- or postbaseline data; NC = NUT carcinoma; NUT = nuclear protein in testis; PD = progressive disease; PR = partial response; QD = once daily; RECIST = Response Evaluation Criteria in Solid Tumors; SD = stable disease.
Considered to have clinical progression but was NE by RECIST 1.1.
PD defined by presence of new lesion(s) without reassessment of baseline target lesions.
Unconfirmed PR with subsequent scans demonstrating PD by RECIST 1.1.
Unconfirmed PR with subsequent FDG-PET scans demonstrating worsening bone disease without CT correlate; best response scored as stable disease.
Confirmed response of PR by RECIST 1.1.
Nonmeasurable disease at baseline.
The total number of patients with stable disease as best response was 8 (42%) of 19, and one (5%) of 19 with non-measurable disease at baseline was classified as non-CR/non-PD. Five (26%) of 19 had PD. One additional patient had PD defined by clinical progression, and two additional patients were not RECIST evaluable (Table 3;Supplementary Table 5, available online).
For the two patients with a confirmed PR, the duration of confirmed response was 105 days and 110 days, and the duration of treatment was 186 days and 196 days, respectively. In addition to the two patients with confirmed PR, two patients with a best response of stable disease were also on treatment for longer than 6 months in total. Median progression-free survival for the NC cohort was 2.5 months (95% confidence interval = 0.5 to 3.7 months). Of the 10 patients in the NC cohort who experienced a confirmed PR or stable disease, eight received molibresib 60–100 mg once daily. Among the remaining two patients, one underwent molibresib dose escalation from 4 mg through 8 mg to 16 mg once daily and achieved stable disease for 37 weeks, and the other received molibresib 16 mg once daily and achieved stable disease for 6 weeks.
The four NC patients who remained on treatment longer than 6 months all had nonthoracic primary tumors. The two confirmed PRs and one unconfirmed PR occurred in patients with tumors harboring BRD3-NUTM1 fusion, whereas the NUT fusion partner was unknown for the other patient with unconfirmed PR. However, fusion gene status did not correlate with treatment duration overall (Table 3;Supplementary Figure 6, available online).
Across other tumor types, one patient with TNBC who received molibresib at 80 mg achieved an unconfirmed PR. The remaining 41 patients with non-NC or non-BC tumors experienced stable disease or PD as the best response, including patients with CRC and CRPC who were progression free more than 4 months (Figure 2A).
Discussion
The results of this phase I study establish the safety profile and RP2D and provide evidence of target engagement and preliminary antitumor activity of the BET inhibitor molibresib. The most common dose-limiting event was thrombocytopenia, likely reflecting on-target BET inhibition (2), with all instances occurring in the 60–100-mg dose groups. Although no dose exceeded the protocol-defined MTD and the incidence of AEs leading to discontinuation was similar across the 60–100-mg dose levels, the incidence of dose-reduction events was markedly higher in the 100-mg group. Despite preclinical concerns, no specific cardiac toxicity signal was detected.
The selection of the 80-mg dose (rather than a ≤60-mg dose) as the RP2D was supported by PK, pharmacodynamic, and clinical data. Despite intrapatient variability, molibresib exposure and target engagement demonstrated dose dependence. These findings, coupled with the predominance of clinical responses observed at doses of 60–100 mg and the apparent reduced tolerability of the 100-mg dose, resulted in consideration of the highest dose less than 100 mg as having the most favorable risk–benefit profile. Interestingly, at 80 mg, exposure to the two major active metabolites of molibresib was similar to or greater than exposure to the parent drug. Because these metabolites have demonstrated equipotency to molibresib, they are anticipated to contribute substantially to the activity of molibresib in humans.
To date, this trial contains the largest prospective cohort of NC patients included in a single study. Among the 19 patients in the NC cohort, there were both confirmed and unconfirmed PRs and four patients overall who remained on study treatment for longer than 6 months. Additionally, instances of substantial clinical benefit with reduction in pain occurred, even if only for a brief duration. Most patients with PR or stable disease were in the 60–100-mg dose groups, again favoring selection of 80 mg as the RP2D. These results, along with those from the structurally related BET inhibitor birabresib (25), provide proof of concept for the activity of BET inhibition in NC.
Recently, a novel risk classification prognostic model for NC incorporating clinicopathologic and genomic features has been established based on longitudinal follow-up of 124 patients enrolled to the International NUT Midline Carcinoma registry between 2010 and 2017 (26). Although long-term prognosis was uniformly poor, a nonthoracic primary tumor (eg, head and neck origin) was associated with an improved prognosis compared with a thoracic primary. Among nonthoracic primaries, the presence of BRD3-NUT or NSD3-NUT conferred an improved prognosis compared with BRD4-NUT fusion, whereas thoracic primaries had the worst outcome regardless of the fusion protein expressed.
Interestingly, the two patients with confirmed PR lasting longer than 6 months had tumors of nonthoracic origin that expressed BRD3-NUT fusion proteins. Other patients who remained on the study longer than 6 months also had nonthoracic primaries, although these tumors expressed BRD4-NUT fusion proteins. Additionally, the two patients with unconfirmed PRs had tumors of thoracic origin, one of which expressed BRD3-NUT. Thus, clinical benefit manifested as stable disease was derived among the less favorable nonthoracic group, and at least transient improvement was observed among patients with thoracic primaries.
Of note, three of the four patients in whom substantive regressions were documented had tumors harboring BRD3-NUT fusions. A fourth patient with tumor harboring BRD3-NUT fusion had stable disease as the best response for approximately 4 months. Interestingly, of four patients with NUT midline carcinoma who received the BET inhibitor BMS-986158, one patient with a BRD3-NUT fusion achieved stable disease with a 16% reduction in tumor burden lasting 279 days (27). Notably, patients who achieved PR to the BET inhibitor RG6146 all had non-BRD4-NUT fusions (28). Nonetheless, further work will be required to determine whether non-BRD4 fusion proteins are more easily dislodged from chromatin by competitive BET inhibitors than BRD4-NUT fusion proteins.
Taken together, these data suggest that it may be reasonable to further investigate the use of molibresib monotherapy in patients with NCs of nonthoracic origin, especially those with non-BRD4-NUT (eg, BRD3-NUT or NSD3-NUT) fusions, where responses were documented. In other cases (ie, thoracic primaries or tumors carrying BRD4-NUT fusions), combinatorial approaches are likely warranted. The recent demonstration that BET inhibition can disrupt DNA repair and DNA damage response pathways (29,30) indicates opportunities for standard chemotherapy combinations in this population.
The unconfirmed PRs noted in this study, as well as the transient nature of responses observed with the small-molecule BRD inhibitor birabresib in patients with NC (25,31), suggest rapid activation of secondary resistance mechanisms, which may include restoration of MYC transcription or expression mediated by WNT/β-catenin signaling, increased binding of GLI2 to the c-MYC promoter, loss of tripartite motif-containing protein 33 (TRIM33), PI3K and/or ERK activation, as well as enhanced BRD4 phosphorylation (32). This further implies that BET inhibitor monotherapy may be sufficient only in a minority of NC patients and that additive or synergistic combinatorial approaches may be required. This should be a consideration for future BET inhibitor clinical development.
In this trial, evidence of clinical benefit was also observed in other solid tumor types, including TNBC, CRC, and CRPC. Among tumor types that often carry MYC amplification, one patient with neuroblastoma achieved stable disease as the best response. Although patients with small cell lung cancer did not respond, the majority of these patients received doses well below the RP2D. Despite preclinical evidence of the potential role of MYC downregulation in the mechanism of action of molibresib and other BET inhibitors (13,14), this is not yet established in a clinical setting. Indeed, a phase I trial of birabresib in hematologic malignancies did not demonstrate an association between c-MYC protein expression and birabresib sensitivity (33). The planned assessment of correlation between MYC amplification status and clinical outcome in part 2 of this study will shed further light on the potential relationship between the response to BET inhibition and MYC expression.
Additionally, molibresib is currently under investigation for the treatment of hematologic malignancies (34) and in hormonal combinations for BC and CRPC (35,36). Collectively, these studies will further define the role of BET inhibition in the anticancer armamentarium.
Funding
This study (BET115521; NCT01587703) was supported by GlaxoSmithKline (GSK). Financial support for this work was also provided by National Institutes of Health (NIH) Cancer Center Support Grants P30 CA016672 and P30 CA006516, as well as NIH grant R01 CA124633 to CAF.
Notes
Affiliations of authors: University of Texas MD Anderson Cancer Center, Houston, TX (SAP-P, DSH); Johns Hopkins University School of Medicine, Baltimore, MD (CLH, MCM); Department of Pathology, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (CAF); Medical Oncology, Institute Bergonié, Bordeaux, France (SC); Medical Oncology Department, Vall d’Hebron University Hospital, Barcelona, Spain (IB); Medical Oncology, Léon Bérard Cancer Center, Lyon, France (PAC); Medical Oncology, START Madrid-FJD, Fundación Jiménez Díaz Hospital, Madrid, Spain (VM); The Institute of Cancer Research and Royal Marsden Hospital, London, UK (JSdB); GSK, Research Triangle Park, NC (SDH); GSK, Collegeville, PA (GF-B, OB, AW, YW, TH, MA, AD); UCB Celltech, Slough, UK (NJP); Division of Medical Oncology, Ottawa Hospital Cancer Centre, Ottawa, ON, Canada (RKP, JH); Rubius Therapeutics, Cambridge, MA (CLC); Abramson Cancer Center at University of Pennsylvania, Philadelphia, PA (NBH, PJO); Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA (GIS) and Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA (GIS).
SAP-P has received research support from GSK, AbbVie, Curis, FivePrime, Helix Biopharma, Incyte, MedImmune, Medivation, Newlink Genetics, Novartis, Pfizer, Pieris, Principia Biopharma, Puma Biotechnology, Taiho Pharma, Tesaro, and XuanZhu Pharma. CLH has received research support from GSK. CAF has received research support and acted as a paid consultant to GSK. He has also received research support from C4 Therapeutics, Constellation Pharmaceuticals, and Curis. PAC has received research support from Novartis and Merck Sharp & Dohme. He has received nonfinancial support from Plexxikon and Merck Sharp & Dohme and travel grants from Bristol-Myers Squibb and Roche. JSdB has received research support and served as a paid advisor to GSK, AstraZeneca, Bayer, Daiichi Sankyo, Genentech, Menarini, Merck Serono, Merck Sharp & Dohme, Orion Pharma, Pfizer, Sierra Oncology, Taiho Pharma, and Terumo. SDH, GF-B, OB, AW, YW, TH, MA, RKP, and AD are employees of GSK and hold shares and/or options in the company. At the time of the study, NJP and CLC were employees of GSK and held shares and/or options in the company; NJP is now an employee of UCB Celltech, Slough, UK, and CLC is an employee of Rubius Therapeutics. JH has served as a paid consultant to AstraZeneca, Bristol-Myers Squibb, Merck, Novartis, and Pfizer and is a data monitoring committee member for Bristol-Myers Squibb. DSH is the owner of Oncoresponse Inc. He has received research grants from Adaptimmune, AbbVie, Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Daiichi Sankyo, Eisai, Genentech, Genmab, Ignyta, Infinity, Kite, Kyowa, Lilly, MedImmune, Merck, Mirati, Molecular Templates, Novartis, Pfizer. He has acted as a paid advisor to Bayer, Baxter, and Guidepoint Global, Janssen, Molecular Match, and Takeda and received travel grants from Loxo Oncology and Mirna Therapeutics. GIS has received sponsored research support and has served as a paid advisor to Lilly, Pfizer, Merck/EMD Serono (Merck KGaA), and Sierra Oncology. He has additional sponsored research support from Merck & Co. and Array BioPharma. He has also served as a paid advisor to Almac, Astex, Bayer, Bicycle Therapeutics, Cybrexa Therapeutics, Fusion Pharmaceuticals, G1 Therapeutics, Ipsen, and Roche. IB, MCM, NBH, PJO, SC, and VM have declared no conflicts of interest.
The authors thank Ursula Kees (Telethon Kids Institute, Perth, Australia) for the gift of the PER-704 and PER-403 cell lines used in the growth assays. Editorial assistance in the preparation of this manuscript was provided by Katy Tucker, PhD, CMPP, and Jennie McLean, PhD, of Fishawack Indicia Ltd, UK, and was funded by GSK.
Preliminary data for this study were presented as an oral presentation at the 107th Annual Meeting of the American Association for Cancer Research, 2016.
Clinical trial registration number: NCT01587703.
GSK makes available anonymized individual participant data and associated documents from interventional clinical studies that evaluate medicines on approval of proposals submitted to www.clinicalstudydatarequest.com. To access data for other types of GSK-sponsored research, for study documents without patient-level data, and for clinical studies not listed, please submit an inquiry via the website.
Supplementary Material
References
- 1. Mujtaba S, Zeng L, Zhou MM.. Structure and acetyl-lysine recognition of the bromodomain. Oncogene. 2007;26(37):5521–5527. [DOI] [PubMed] [Google Scholar]
- 2. French CA. Small-molecule targeting of BET proteins in cancer. Adv Cancer Res. 2016;131:21–58. [DOI] [PubMed] [Google Scholar]
- 3. Dey A, Nishiyama A, Karpova T, et al. Brd4 marks select genes on mitotic chromatin and directs postmitotic transcription. Mol Biol Cell. 2009;20(23):4899–4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yang Z, He N, Zhou Q.. Brd4 recruits P-TEFb to chromosomes at late mitosis to promote G1 gene expression and cell cycle progression. Mol Cell Biol. 2008;28(3):967–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Belkina AC, Denis GV.. BET domain co-regulators in obesity, inflammation and cancer. Nat Rev Cancer. 2012;12(7):465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Padmanabhan B, Mathur S, Manjula R, et al. Bromodomain and extra-terminal (BET) family proteins: new therapeutic targets in major diseases. J Biosci. 2016;41(2):295–311. [DOI] [PubMed] [Google Scholar]
- 7. Perez-Salvia M, Esteller M.. Bromodomain inhibitors and cancer therapy: from structures to applications. Epigenetics. 2017;12(5):323–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grayson AR, Walsh EM, Cameron MJ, et al. MYC, a downstream target of BRD-NUT, is necessary and sufficient for the blockade of differentiation in NUT midline carcinoma. Oncogene. 2014;33(13):1736–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bauer DE, Mitchell CM, Strait KM, et al. Clinicopathologic features and long-term outcomes of NUT midline carcinoma. Clin Cancer Res. 2012;18(20):5773–5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chau NG, Hurwitz S, Mitchell CM, et al. Intensive treatment and survival outcomes in NUT midline carcinoma of the head and neck. Cancer. 2016;122(23):3632–3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. French CA, Ramirez CL, Kolmakova J, et al. BRD-NUT oncoproteins: a family of closely related nuclear proteins that block epithelial differentiation and maintain the growth of carcinoma cells. Oncogene. 2008;27(15):2237–2242. [DOI] [PubMed] [Google Scholar]
- 12. Alekseyenko AA, Walsh EM, Wang X, et al. The oncogenic BRD4-NUT chromatin regulator drives aberrant transcription within large topological domains. Genes Dev. 2015;29(14):1507–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Delmore JE, Issa GC, Lemieux ME, et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell. 2011;146(6):904–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mertz JA, Conery AR, Bryant BM, et al. Targeting MYC dependence in cancer by inhibiting BET bromodomains. Proc Natl Acad Sci USA. 2011;108(40):16669–16674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Perez-Salvia M, Simo-Riudalbas L, Llinas-Arias P, et al. Bromodomain inhibition shows antitumoral activity in mice and human luminal breast cancer. Oncotarget. 2017;8(31):51621–51629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhao Y, Yang CY, Wang S.. The making of I-BET762, a BET bromodomain inhibitor now in clinical development. J Med Chem. 2013;56(19):7498–7500. [DOI] [PubMed] [Google Scholar]
- 17. Chaidos A, Caputo V, Gouvedenou K, et al. Potent antimyeloma activity of the novel bromodomain inhibitors I-BET151 and I-BET762. Blood. 2014;123(5):697–705. [DOI] [PubMed] [Google Scholar]
- 18. Wyce A, Degenhardt Y, Bai Y, et al. Inhibition of BET bromodomain proteins as a therapeutic approach in prostate cancer. Oncotarget. 2013;4(12):2419–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. [DOI] [PubMed] [Google Scholar]
- 20.National Cancer Institute. Common Terminology Criteria for Adverse Events v4.0, 2009. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.pdf. Accessed August 18, 2019.
- 21. Belkina AC, Nikolajczyk BS, Denis GV.. BET protein function is required for inflammation: Brd2 genetic disruption and BET inhibitor JQ1 impair mouse macrophage inflammatory responses. J Immunol. 2013;190(7):3670–3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nguyen TH, Maltby S, Eyers F, et al. Bromodomain and extra terminal (BET) inhibitor suppresses macrophage-driven steroid-resistant exacerbations of airway hyper-responsiveness and inflammation. PLoS One. 2016;11(9):e0163392.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26(7):1148–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Doroshow DB, Eder JP, LoRusso PM.. BET inhibitors: a novel epigenetic approach. Ann Oncol. 2017;28(8):1776–1787. [DOI] [PubMed] [Google Scholar]
- 25. Lewin J, Soria JC, Stathis A, et al. Phase Ib trial with birabresib, a small-molecule inhibitor of bromodomain and extraterminal proteins, in patients with selected advanced solid tumors. J Clin Oncol. 2018;36(30):3007–3014. [DOI] [PubMed] [Google Scholar]
- 26. Chau NG, Clement M, Danga K, et al. An anatomical site and genetic based prognostic model for patients with NUT midline carcinoma: analysis of 124 patients. JNCI Cancer Spectr. doi: 10.1093/jncics/pkz094. [DOI] [PMC free article] [PubMed]
- 27. Hilton J, Cristea MC, Voskoboynik M, et al. 4110: initial results from a phase I/IIa trial evaluating BMS-986158, an inhibitor of the bromodomain and extra-terminal (BET) proteins, in patients (pts) with advanced cancer. Ann Oncol. 2018;29(suppl 8):viii134. [Google Scholar]
- 28. Shapiro GI, Dowlati A, LoRusso PM, et al. Clinically efficacy of the BET bromodomain inhibitor TEN-010 in an open-label substudy with patients with documented NUT-midline carcinoma (NMC). Mol Cancer Ther. 2015;14(12 suppl 2):A49. [Google Scholar]
- 29. Yang L, Zhang Y, Shan W, et al. Repression of BET activity sensitizes homologous recombination-proficient cancers to PARP inhibition. Sci Transl Med. 2017;9(400):eaal1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang J, Dulak AM, Hattersley MM, et al. BRD4 facilitates replication stress-induced DNA damage response. Oncogene. 2018;37(28):3763–3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stathis A, Zucca E, Bekradda M, et al. Clinical response of carcinomas harboring the BRD4-NUT oncoprotein to the targeted bromodomain inhibitor OTX015/MK-8628. Cancer Discov. 2016;6(5):492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shu S, Polyak K.. BET bromodomain proteins as cancer therapeutic targets. Cold Spring Harb Symp Quant Biol. 2016;81:123–129. [DOI] [PubMed] [Google Scholar]
- 33. Amorim S, Stathis A, Gleeson M, et al. Bromodomain inhibitor OTX015 in patients with lymphoma or multiple myeloma: a dose-escalation, open-label, pharmacokinetic, phase 1 study. Lancet Haematol. 2016;3(4):e196–e204. [DOI] [PubMed] [Google Scholar]
- 34. Dawson M, Stein EM, Huntly BJP, et al. A phase I study of GSK525762, a selective bromodomain (BRD) and extra terminal protein (BET) inhibitor: results from part 1 of phase I/II open label single agent study in patients with acute myeloid leukemia (AML). Blood. 2017;130(Suppl 1):Abstract 1377. [Google Scholar]
- 35. Sparano JA, Cescon DW, Oliveira M, et al. A phase I/II dose escalation and expansion study to investigate the safety, pharmacokinetics, pharmacodynamics and clinical activity of GSK525762 in combination with fulvestrant in subjects with ER+ breast cancer. J Clin Oncol. 2017;35(Suppl 1):Abstract TPS 1114. [Google Scholar]
- 36. Vaishampayan UN, Narayan V, Wise D, et al. A phase Ib open-label, dose escalation and expansion study to investigate the safety, pharmacokinetics, pharmacodynamics and clinical activity of GSK525762 in combination with abiraterone or enzalutamide in metastatic castrate-resistant prostate cancer. J Clin Oncol. 2018;36(suppl):Abstract TPS391. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.