Abstract
An outbreak of dual infection in dogs with canine adenovirus type 1 (CAV‐1) and canine coronavirus (CCV) infection is reported in an animal shelter that comprised approximately 200 adults stray dogs and 30 puppies. Twenty puppies died 7–8 days after the onset of the clinical signs (severe enteritis, leucopoenia, respiratory distress and dehydration). Both CAV‐1 and CCV were isolated from tissue or swab samples. Antibodies to CCV and, at high levels, to CAV‐1 also were detected in several puppies. The principal histological findings were atrophy of small intestinal villi, lymphoid depletion, hepatitis and bronchopneumonia. The persistence of CCV in the faeces, observed by the polymerase chain reaction assay, was longer than previously reported. Results demonstrated the serious consequences which may occur with dual infections by CAV‐1 and CCV in assembled groups of dogs that are housed in poorly managed kennels with inadequate vaccination programmes.
Introduction
Canine adenovirus type 1 (CAV‐1) is the causal agent of infectious canine hepatitis (ICH). The disease is characterized by fever, often above 40°C, apathy, anorexia, abdominal pain or tenderness, vomiting and diarrhoea. Dogs may develop bronchopneumonia, conjunctivitis, photophobia and a transient corneal opacity, ‘blue eye’, which may occur after clinical recovery as result of anterior uveitis and corneal oedema (Appel, 1987a; Green, 1990). ICH is now uncommon in vaccinated populations. However, it is observed more frequently in unvaccinated populations, especially in puppies less than 1 year old. Clinical signs of uncomplicated CAV‐1 infection usually lasts 5–7 days, with rapid recovery; however, they may last longer in dogs with concurrent infections, such as canine distemper virus (CDV) or, rarely, in animals that develop chronic active hepatitis (Green, 1990; Kobayashi et al., 1993). Although ICH occurs sporadically, the disease has been well controlled since the 1950s when attenuated viral vaccines became available (Appel, 1987a). Serological surveys conducted in various parts of the world prior to the widespread use of vaccines indicated that the seroprevalence of CAV‐1 antibodies ranged from 30 to 60% in the dogs tested (Rubarth, 1947; Brunner et al., 1951; Cabasso, 1953; Sasaki et al., 1956).
Canine coronavirus (CCV) infection is the causative agent of a generally mild, usually self‐limiting enteric illness in dogs characterized by sudden onset of vomiting and malodorous diarrhoea. Although CCV infections appear to be mainly asymptomatic, young puppies may be more severely affected as well as dogs with mixed infections with other viruses or bacteria (Hoskins, 1998).
This article describes an outbreak of CAV‐1 infection in puppies in an animal shelter in Bari, Italy, with concurrent infection by canine coronavirus (CCV).
Materials and Methods
Animals and clinical findings
The puppies were located in a kennels which accommodates approximately 200 adult stray dogs and 30 puppies less than 4 months old. The sanitary conditions of the kennels were poor; there was severe overcrowding, with 15–20 animals in each room which had area 12–13 m2. Although the kennels were washed daily with tap water, detergents and disinfectants were only applied irregularly. In addition, vaccination schedules were not systematically carried out and none of the sick puppies had been vaccinated.
In the present outbreak, puppies developed severe enteritis that was sometimes haemorrhagic, shortly after their introduction into the kennels. There was no vomiting. The puppies examined (n=15) had a moderate leucopoenia, dehydration, abdominal tenderness and were reluctant to move. Twenty puppies died 7–8 days after the onset of clinical signs. The puppies that recovered, about 10 days after the onset of the enteritis, had moderate to severe dehydration lasting 1–3 weeks, and three puppies developed a bilateral keratitis (‘blue eyes’), respiratory distress, cough and accelerated respiratory rates.
Sample collections
The collection of the samples for laboratory study could not be carried out systematically because of the uncontrolled movement of dogs within the kennels and poor record keeping. Nevertheless, blood samples and nasal and rectal swab specimens were obtained from 15 puppies at the onset of enteritis and, thereafter, at 4–7 day intervals until the puppies had either died or recovered. Over the 37 day observation period, 10–15 puppies died, but necropsy examinations were only possible on two animals (no. 19, 31).
Histological examinations
Tissue samples were obtained from the lungs, small intestine, mesenteric lymph nodes and livers of the dead puppies (no. 19, 31) and fixed in 10% buffered formalin. Fixed tissue for histological examination were cut in 5 μm sections, mounted on glass slides, and stained with haematoxylin and eosin (HE).
Virus isolation
Faecal and nasal swabs were collected from the sick puppies for a period of 1 month at 4–7 day intervals. Nasal, faecal, and ocular swab samples, as well as tissue samples from the small intestine, rectum and liver, were collected from the dead puppies. Madin–Darby canine kidney (MDCK) and the A‐72 dog cell lines were used for virus isolation attempts. Cells were propagated in Dulbecco minimal essential medium (D‐MEM) that contained 10% (v/v) foetal bovine serum. Swab and tissue samples were homogenized (10% w/v) in D‐MEM and centrifuged at 4000 g for 20 min at 4°C. The supernatant portion of each sample was then treated with antibiotics (5000 IU/ml penicillin, 2500 μg/ml streptomycin, 10 μg/ml amphotericin) for 30 min at 37°C, and inoculated onto partially confluent monolayers of A‐72 and MDCK cells. The inoculated cell cultures were then incubated at 37°C in a 5% CO2 incubator and observed daily for cytopathic effects. If cytopathic effects were absent after 4 days of incubation, cell cultures were frozen and thawed three times and four additional passages were made. Inoculated A‐72 cells were also examined at 2‐day intervals by indirect immunofluorescence tests using CCV monoclonal antibodies (generously supplied by Dr Gilles Chappuis, Merial, France) and a canine parvovirus (CPV‐2) antiserum. Cell cultures which developed cytopathic effects were stained with HE to reveal the presence of the inclusion bodies.
Nested‐polymerase chain reaction for CCV
Faecal and nasal samples collected at the onset of illness, and at 4–7 day intervals thereafter for 37 days, were examined for CCV in a nested‐polymerase chain reaction (n‐PCR) assay, as previously reported (Pratelli et al., 1999a). Briefly, genomic RNA was extracted from 1 ml of the supernatant fraction of each sample, using the Rneasy Total RNA Kit (Qiagen GmbH, Hilden, Germany). The target sequence for amplification was a segment of the gene encoding for the transmembrane protein M of CCV. The above sequence of 230 bp straddles nucleotides 535 and 746. The following primers were used: CCV1 (5′‐TCC AGA TAT GTA ATG TTC GC‐3′), CCV2 (5′‐TCT GTT GAG TAA TCA CCA GCT‐3′) and CCV3 (5′‐GGT GTC ACT CTA ACA TTG CTT‐3′).
CAV endonuclease analysis
The following CAV strains were examined: (1) CAV‐1, field strain (Buonavoglia et al., 1993); (2) CAV‐2, Toronto A26/61 strain (Appel, 1987a); (3) puppy isolate (no. 30); (4) puppy isolate (no. 19). Endonuclease analysis of CAV DNA was carried out as previously reported (Buonavoglia et al., 1993). Briefly, DNA was extracted from MDCK cells with 80% cytopathic effects using phenol/chloroform and isoamyl alcohol mixture. The digestion was performed with HpaII and PstI endonucleases (Amersham Pharmacia Biotech, Milan, Italy). A typical reaction, containing 2–3 μl of DNA in a 25‐μl reaction, and 8–10 units of enzyme per microgram of DNA, was applied. The fragments of the digestion were separated by electrophoresis in a 1.2% agarose gel containing Tris‐acetate (90 mm pH 8.2), EDTA (2.5 mm) and boric acid (90 mm), at 70 V and 40 mA for 60 min. Visualization of the ethidium bromide‐stained DNA fragments was performed using a UV transluminator at 302 nm.
Serological studies
Sera were not available from the dogs prior to the development of disease; however, serum samples were collected from 10 dogs at the onset of the clinical signs, and after 12 and 34 days. All sera were tested for CAV‐1, CCV and CDV antibodies by conventional serum neutralization tests using MDCK cells for CAV‐1, A‐72 cells for CCV and VERO cells for CDV. CPV‐2 antibodies were evaluated in haemoagglutination inhibition test.
Results
Gross post‐mortem examination of the two puppies that were available for necropsy revealed haemorrhagic enteritis involving the entire small intestine. Necrosis and haemorrhages also were observed in the liver and tonsils of one pup. Gross changes were not observed in the lungs of either pup. Histologically, there was moderate atrophy of epithelial villi and an increase in cellularity of the lamina propria in the small intestine. In addition, lymphoid depletion was observed in the mesenteric lymph nodes. The prominent microscopic changes in the liver were hydropic degeneration of the hepatocytes, areas of centrolobular necrosis and the presence of occasional basophilic intranuclear inclusion bodies (Fig. 1). The prominent histological lesions in the lungs were moderate purulent bronchopneumonia with fibrous thickening of the peribronchial tissues.
Figure 1.
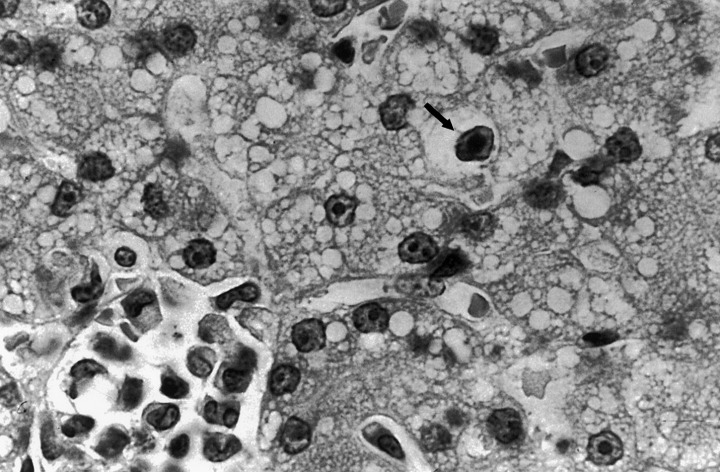
Inclusion bodies typical of adenovirus infection in the liver of puppy no. 19. Haematoxylin and eosin stain.
Faecal samples from all puppies examined were negative to CPV‐2 by the IFA test. However, MDCK cells inoculated with nasal, rectal and ocular swabs of one puppy (no. 30) with keratitis, developed a cytopathic effect that consisted of clumps of rounded and refractile cells 48–72 h post‐inoculation. Similar results were observed in MDCK cells inoculated with small intestine and rectal swab samples from the other dead puppy (no. 19). Intranuclear inclusion bodies also were observed in MDCK cell monolayers with cytopathic effects. No cytopathic effects or inclusions were observed in MDCK cells inoculated with liver homogenates from either of the dead puppies. Only A‐72 cells inoculated with rectal swab samples collected at the onset of the clinical signs from two puppies (no. 21, 30), had cytopathic effects at the third and the fourth serial passages, respectively. The immunofluorescence test was also positive for CCV.
Table 1 reports the results of n‐PCR analysis for CCV on faecal and nasal swab samples. CCV shedding was shown to occur over a period of 7–37 days in faecal swabs; nasal swabs were positive only for 1–13 days.
Table 1.
Evaluation by PCR of CCV shedding in rectal and nasal swabs from puppies
Restriction enzyme patterns of the CAV isolates digested with PstI (lines 2, 3, 4 and 5) and with HpaII (lines 7, 8, 9 and 10) are shown in Fig. 2. The two isolates (no. 30 and 19; lines 4, 5 and 9, 10, respectively) had migration patterns that were similar to the control CAV‐1 field strain (lines 2, 7), but were different from CAV‐2 (lines 3, 8). The PstI patterns revealed only a very slight difference between the CAV‐1 field strain (line 2) and the two isolates (no. 30 and 19; lines 4, 5).
Figure 2.
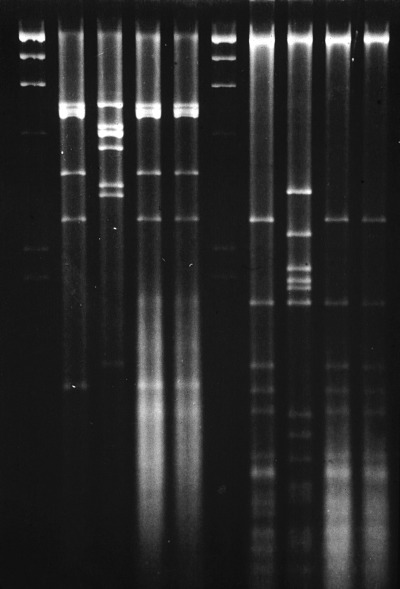
Restriction patterns of CAV isolates digested with PstI (lines 2, 3, 4 and 5) and with HpaII (lines 7, 8, 9 and 10). Molecular size markers (lanes 1, 6): Lambda DNA × HindIII. Lanes 2, 7: CAV‐1 strain; lanes 3, 8: CAV‐2 Toronto/A2 6/61 strain; lanes 4, 9: puppy no. 30 isolate; lanes 5, 10: puppy no. 19 isolate.
Serological results on 10 of the 15 dogs examined at the initial bleeding, and in surviving dogs at 12 and 34 days after the outbreak was reported, demonstrated high serum neutralization antibody titres to CAV‐1 (1:3200). Three puppies had significant increases (>four‐fold) in antibody titres during the observation period. Antibodies to CCV were demonstrated in eight puppies and an increase in antibody titres was observed over the observation period in all puppies tested. Serum neutralization antibody titres to CCV were never high (maximum 1:16). There were no elevations in titres to CPV‐2, and low antibodies titres to CDV (1:8) were only observed in two puppies.
Discussion
ICH is a severe disease of the dog which has rarely been reported during the past few years, except for occasional cases in unvaccinated dogs. On the other hand, CCV infections are common in dogs, but most are mild, or asymptomatic, except in puppies or adult dogs suffering from stress or other infections (Appel et al., 1979; Appel, 1987b; Hoskins, 1998). The outbreak described in the present report provides evidence that CAV‐1 is still circulating in the canine population and that serious health problems may result where CCV is endemic, management is poor and systematic vaccination is not carried out. The investigated kennels, an animal shelter, was very poorly managed with respect to record keeping and sanitation; moreover, vaccinations had not been methodically carried out. The above factors very likely contributed to the severity of the outbreak of CAV‐1 in the kennels. Another unusual finding revealed in this study was the presence of CCV in the same kennels, often in the puppies that were infected with CAV‐1.
Some of the clinical signs constantly observed, for example, the severe haemorrhagic enteritis, are not typical of ICH but of other infections such as CPV‐2 or CCV.
CCV infection in dogs is usually self‐limited and infected animals usually recover after a brief period of illness. Nevertheless, puppies with secondary bacterial infections, parasites or other viral infections may suffer from severe, even fatal, disease (Evermann et al., 1980; Appel, 1987b; Buonavoglia et al., 1993). Dual infections by both CCV and CPV‐2 have been reported previously (Appel et al., 1979; Yasoshima et al., 1983; Martin and Zeidner, 1992), and it has been shown that CCV enhances the severity of a sequential CPV‐2 infection. Recently, a severe CCV infection was reported in three puppies that recovered from a CPV‐2b infection (Pratelli et al., 1999b). Other viruses, such as rotaviruses or caliciviruses, also might enhance the pathogenicity of CAV‐1 since mixed infections have been observed in dogs with enteritis (Hammond and Timoney, 1983; Marshall et al., 1984).
The n‐PCR for CCV led to another result which seems important from an epidemiological point of view and is linked to the duration of the period of virus shedding in the faeces. CCV shedding in faeces has been reported from 6 to 14 days post‐infection (Keenan et al., 1976; Tennant et al., 1991). In the present study, CCV was isolated in cell cultures only from the faecal samples collected at the onset of the clinical signs. Therefore, by means of a highly sensitive test (n‐PCR) CCV faecal shedding was detected for periods up to 37 days. Although not proved, dual infections by both CCV and CAV‐1 may have influenced the prolonged shedding of CCV in the faeces of infected puppies.
In summary, it is believed likely that the dual infections (CCV/CAV‐1) that were observed presented clinical signs similar to those of infectious hepatitis (CAV‐1), but the enteritis was augmented by the presence of both viruses. Infection with CAV‐1 may also favour the persistence of CCV in the intestine of infected dogs.
References
- 1. Appel, M. J. , 1987a: Canine adenovirus type 1 (infectious canine hepatitis virus). In: Horzinek, M. C. (series ed.), Virus Infections of Vertebrates, Vol. I. Virus Infections of Carnivores, pp. 29–43. Elsevier Science Publishers, Amsterdam, The Netherlands.
- 2. Appel, M. J. , 1987b: Canine coronavirus. In: Horzinek, M. C. (series ed.), Virus Infections of Vertebrates, Vol. I. Virus Infections of Carnivores, pp. 115–122. Elsevier Science Publishers, Amsterdam, The Netherlands.
- 3. Appel, M. J. , Cooper B. J., Greisen H., Scott F., Carmichael L. E., 1979: Canine viral enteritis. I. Status report on corona‐ and parvo‐like viral enteritides. Cornell Vet. 69 , 123–133. [PubMed] [Google Scholar]
- 4. Brunner, K. T. , Scheitlin M., Stunzi H., 1951: Zum serologischen Nachweis der Hepatitis Contagiosa Canis. Schweiz Arch. Tierheilk. 93 , 443–458. [Google Scholar]
- 5. Buonavoglia, D. , Ferrara G., Marsilio F., Cavalli A., Voigt V., 1993: Tipizzazione di uno stipite di adenovirus. O.D.V. 10 , 39–41. [Google Scholar]
- 6. Cabasso, V. J. , 1953: Canine viruses. II. Infectious canine hepatitis and rabies control. Southwest. Vet. 6 , 137–141. [Google Scholar]
- 7. Evermann, J. F. , Foreyt W., Maag‐Miller L., Leathers C. W., McKeirnan A. J., LeaMaster B., 1980: Acute hemorrhagic enteritis associated with canine coronavirus and parvovirus in a captive coyote population. J. Am. Vet. Med. Assoc. 177 , 784–786. [PubMed] [Google Scholar]
- 8. Green, C. E. , 1990: Infectious canine hepatitis. In: Green, C. E. (ed.), Infectious Diseases of the Dog, and Cat, pp. 242–251. W. B. Saunders, Philadelphia, PA.
- 9. Hammond, M. M. & Timoney P. J., 1983: An electron microscopic study of viruses associated with canine gastroenteritis. Cornell Vet. 73 , 82–97. [PubMed] [Google Scholar]
- 10. Hoskins, J. D. , 1998: Canine coronaviral enteritis. In: Green, C. E. (ed.), Infectious Diseases of the Dog, and Cat, pp. 45–47. W. B. Saunders, Philadelphia, PA.
- 11. Keenan, K. P. , Jervis H. R., Marchwicki R. H., Binn L. N., 1976: Intestinal infection of neonatal dogs with canine coronavirus 1–71: studies by virologic, histologic, histochemical and immunofluorescent techniques. Am. J. Vet. Res. 37 , 247–256. [PubMed] [Google Scholar]
- 12. Kobayashi, Y. , Ochiai K., Itakura C., 1993: Dual infection with canine distemper virus and infectious canine hepatitis virus (canine adenovirus type 1) in a dog. J. Vet. Med. Sci. 55 , 699–701. [DOI] [PubMed] [Google Scholar]
- 13. Marshall, J. N. , Healey D. S., Studdert M. J., Scott P. C., Kennett M. L., Ward B. K., Gust I. D., 1984: Viruses and virus‐like particles in the faeces of dogs with and without diarrhoea. Austral. Vet. J. 61 , 33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martin, H. D. & Zeidner N. S., 1992: Concomitant cryptosporidia, coronavirus and parvovirus infection in a raccoon (Procyon lotor). J. Wildl. Dis. 28 , 113–115. [DOI] [PubMed] [Google Scholar]
- 15. Pratelli, A. , Tempesta M., Greco G., Martella V., Buonavoglia C., 1999a: Development of a nested PCR assay for the detection of canine coronavirus. J. Virol. Meth. 80 , 11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pratelli, A. , Tempesta M., Roperto F. P., Sagazio P., Carmichael L. E., Buonavoglia C., 1999b: Fatal coronavirus infection in puppies following canine parvovirus 2b infection. J. Vet. Diagn. Invest. 11 , 550–553. [DOI] [PubMed] [Google Scholar]
- 17. Rubarth, S. , 1947: An acute virus disease with liver lesions in dogs (hepatitis contagiosa canis). A pathologico‐anatomical and aetiologic investigation. Acta Pathol. Microbiol. Scand. (Suppl. 69) 24, 1–222. 20269221 [Google Scholar]
- 18. Sasaki, N. , Nakai M., Iwamoto I., Konishi S., Ikegami T., 1956: Studies on infectious hepatitis of dogs. II. The distribution of the disease in Japan, and its immunization. Jpn. J. Vet. Sci. 18 , 113–118. [Google Scholar]
- 19. Tennant, B. J. , Gaskell R. M., Kelly D. F., Carter S. D., 1991: Canine coronavirus infection in the dog following oronasal inoculation. Res. Vet. Sci. 51 , 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yasoshima, A. , Fucinami F., Doi K., Dojima A., Takada H., Okaniwa A., 1983: Case report on mixed infection of canine parvovirus and canine coronavirus. Electron microscopy and recovery of canine coronavirus. Jpn. J. Vet. Sci. 45 , 217–225. [DOI] [PubMed] [Google Scholar]


