Abstract
Middle East respiratory syndrome coronavirus (MERS‐CoV) is an emerging zoonotic pathogen discovered in 2012. The purpose of this scoping review was to summarize the empirical evidence for MERS‐CoV in animals in order to map knowledge gaps and to extract data for modelling disease transmission in dromedary camels. A review protocol was developed a priori, and a systematic search, data extraction and summary were conducted using the Arksey and O'Malley framework. Ninety‐nine publications were identified for full review out of 1,368 unique records. Of these publications, 71 were articles in scientific journals. Ninety of the studies were observational and the remaining nine were experimental. We summarize characteristics of animal studies including study design, study population and outcomes of interest for future transmission modelling in the reservoir population. The majority of field studies reported measures of prevalence, while experimental studies provided estimates of transmission parameters that pertain to the natural course of disease.
Keywords: camels, disease reservoirs, dromedary, MERS‐CoV, Middle East respiratory syndrome, review, zoonoses
Impacts
MERS‐CoV is an emerging zoonotic disease that is maintained in dromedary camels with sporadic transmission to humans. Since it was first reported in 2012, numerous studies have been conducted to identify and better understand the virus in reservoir animal populations.
We conducted a scoping review in order to summarize the empirical evidence of MERS‐CoV in animals, including knowledge gaps and data for disease transmission models.
Gaps in the evidence base of MERS‐CoV in animals include epidemiological field studies that are generalizable beyond the sample population, and studies that examine questions of immunity in dromedary camels, especially long‐term immunity. Tackling these two challenges would greatly advance our understanding of zoonotic risk and improve our ability to develop sound surveillance and disease prevention strategies.
1. INTRODUCTION
Middle East respiratory syndrome, caused by the eponymous coronavirus (MERS‐CoV), is an emerging zoonotic disease that was first isolated in 2012 from a human case in Saudi Arabia (Zaki, van Boheemen, Bestebroer, Osterhaus, & Fouchier, 2012). Subsequent investigations pointed to dromedary camels as the putative source of human infections (Azhar et al., 2014; Al Hammadi et al., 2015; Farag et al., 2015). The early implication of livestock (and dromedary camels in particular, Haagmans et al., 2014) in MERS‐CoV transmission rapidly led to a number of experimental and field studies that aimed to improve our understanding of the epidemiology of this virus in animal hosts (Adney et al., 2014; Alagaili et al., 2014; Hemida et al., 2013; Meyer et al., 2015; Reusken et al., 2013). These studies have led to the consensus that dromedary camels are the natural reservoir. They have furthermore provided some insight about the host and geographical range of the virus and have suggested some epidemiological characteristics, including the clinical picture and age distribution in dromedary camels (Wernery, Lau, & Woo, 2017). The evidence base that builds from these experimental and field studies provides the foundation for more complex epidemiological analyses, including statistical and mathematical modelling, risk assessments and meta‐analyses. Rigorous, detailed epidemiological data based on pragmatic research questions are crucial to these analyses and ultimately for sound policy and health interventions.
In the 6 years since the discovery of MERS‐CoV, several reviews have been published that have described key advances in understanding the virus in animal populations, and identified research gaps, such as the zoonotic modes of transmission (Arabi et al., 2017; Mackay & Arden, 2015; Mohd, et al., 2016). However, no formal mapping of the literature has yet been attempted. Scoping reviews provide the means to summarize and communicate findings, evaluate the existing body of literature and identify research gaps in a way that is replicable and minimizes bias (Levac, Colquhoun, & O'Brien, 2010). High‐impact emerging diseases, upon which there is typically a high degree of research activity in a short amount of time, could benefit from early and iterative synthesis research. Formal scoping reviews and/or systematic reviews can provide improved clarity for targeting research needs and therefore improve the effective and efficient use of limited resources. A scoping review of the MERS‐CoV animal literature will generate a detailed map of epidemiological and experimental knowledge, assess the suitability of the evidence for systematic review and chart outcomes for informing disease transmission models.
The purpose of this scoping review was to summarize the empirical evidence for MERS‐CoV in animals in order to map knowledge gaps and to extract data for modelling disease transmission in dromedary camels. This was achieved by conducting a systematic search of epidemiological characteristics of MERS‐CoV in animal host populations and answering the following questions: (a) What primary research studies or surveys have been conducted on animal hosts? and (b) What are the general, epidemiological and methodological characteristics of these studies?
2. MATERIALS AND METHODS
The review team consisted of one person (EG) developing the research questions, search strategy, screening criteria, data characterization forms, screening and extraction, and synthesis in consultation with AG, DK, ZP and SvD.
The review was guided by the five phases outlined by Arksey and O'Malley's (2005) framework for scoping reviews: defining the research question; identifying relevant studies; study selection; charting the data; collating, summarizing and reporting the results. In order to more accurately identify knowledge gaps (Pham et al., 2014), methodological questions were included in the data extraction forms. The consultation exercise recommended as an optional sixth phase in the Arksey and O'Malley framework was not conducted. While at least two reviewers are recommended to reduce reporting bias, only one reviewer (EG) conducted the citation screening and data extraction (Peters, Godfrey, McInerney, Baldini Soares, & Khalil, 2017).
A review protocol was developed a priori according to the research question “What are the general, epidemiological and methodological characteristics of MERS‐CoV in animal host populations?” Searches were restricted to 2012 or later, and to publications in English or French. Publications were included if they described primary research that measured animal‐level outcomes of MERS‐CoV in non‐human hosts, including laboratory animal models of non‐human hosts.
The initial search was conducted on 26 April 2017 using five electronic databases: PubMed via NCBI, Web of Science, Agricola via Proquest, CAB direct and Medline via Ovid. The search was limited to 2012 or later, given that MERS‐CoV was previously unrecognized (Zaki et al., 2012). Search terms and strategies were tailored to the requirements and structure of each database, and consisted of “MERS‐CoV” OR “Middle East respiratory syndrome coronavirus.” The search was conducted again on 24 August 2017. At this time, bibliographies of review articles were searched for any articles missed by the initial electronic search (Arabi et al., 2017; Hemida et al., 2015; Mackay & Arden, 2015; Mohd, Al‐Tawfiq, & Memish, 2016). Conference proceedings, and government and university websites in the Middle East were hand searched for citations on 11 September 2017. Two citations were added from searching conference proceedings. Although government and university websites in the Middle East were searched for reports and academic theses, no records were found. This could be due to language differences, as many of the websites were in Arabic and the search was conducted using online translation utilities. The World Organization for Animal Health (OIE) publishes reports of notifiable and reportable diseases that have been submitted by national governments. MERS‐CoV reports were accessed through the Food and Agriculture Organization's disease event database, EMPRES‐i, on 11 September 2017 (Food and Agriculture Organization of the United Nations (FAO) (2014)).
Search results were downloaded to Mendeley for removal of duplicates and initial and full‐text screening, and Excel was used for data extraction and summarization. One reviewer (EG) completed these steps. The full scoping review protocol can be found in the Supporting information Appendix S1: Technical Appendix.
3. RESULTS
A total of 1,368 unique citations were screened for relevance. Title and abstract screening removed 1,254 records, while full‐text screening removed an additional 15 records, leaving 99 for full‐text characterization. Figure 1 depicts the article identification and screening process following PRISMA reporting guidelines (Moher, Liberati, Tetzlaff, Altman, & Group, 2009).
Figure 1.

PRISMA chart of the flow of search results through the scoping review. 1Each OIE MERS‐CoV animal event entered in EMPRES‐i was treated as a separate record in the review, but were included under one bibliographic entry, per FAO citation protocol. Therefore, there were 74 citations containing 99 studies.
The general characteristics of the studies included in the scoping review are listed in Table 1. All of the articles included in this review were in English. The majority of publications (71/99) were scientific journal articles, with 27 of these (38.0%) published in Emerging Infectious Diseases (EID). OIE reports of positive findings were the source for 26 publications included in this scoping review (26/99). Ninety of the included publications were field reports (e.g., observational studies, case reports), while 9 were experimental (Table 1). The most common study design was cross‐sectional (51 articles). Three articles described both cross‐sectional and longitudinal studies. Other types of study designs included case reports, outbreak investigations and field sampling for diagnostic test validation or phylogenetic analysis. Experimental studies consisted of challenge and vaccine experiments. Two of the seven challenge experiments included a component that examined transmission to susceptible hosts. Two of the three vaccine experiments conducted post‐vaccination pathogen challenges (Table 1).
Table 1.
General characteristics of included records, with the number and per cent of overall publications
| Characteristic | N = 99 | % | Appendix S1: Technical appendix reference[Link] |
|---|---|---|---|
| Publication year | |||
| 2012 | 0 | 0.0 | |
| 2013 | 11 | 11.1 | 1–11 |
| 2014 | 27 | 27.3 | 11–29 |
| 2015 | 17 | 17.2 | 30–46 |
| 2016 | 26 | 26.3 | 11, 47–61 |
| 2017 | 18 | 18.2 | 11, 62–74 |
| Publication type | |||
| Scientific journal article | 71 | 71.7 | 1–10, 12–15, 17–26, 28–74 |
| Conference proceeding | 2 | 2.0 | 16, 27 |
| OIE report | 26 | 26.3 | 11 |
| Other (government reports, etc.) | 0 | 0.0 | |
| Study type | |||
| Observational | 90 | 90.9 | |
| Cross‐sectional | 51 | 51.5 | 1–10, 12, 14–17, 20, 21, 23–27, 29–36, 38–42, 44, 47, 51, 53, 56, 58, 59, 63, 65–72 |
| Longitudinal | 8 | 8.1 | 12, 22, 35, 37, 48, 61, 63, 73 |
| Outbreak investigation | 28 | 28.3 | 11, 13, 52 |
| Other | 6 | 6.1 | 18, 19, 43, 45, 46, 60 |
| Experimental | 9 | 9.1 | |
| Challenge only | 5 | 5.1 | 28, 50, 54, 57, 64 |
| Challenge with transmission | 2 | 2.0 | 55, 57 |
| Vaccine with challenge | 2 | 2.0 | 49, 62 |
| Vaccine without challenge | 1 | 1.0 | 74 |
| Journal | |||
| Emerging Infectious Diseases | 27 | 27.3 | 1, 2, 5, 7, 15, 19, 20, 22–26, 28–30, 35, 37, 38, 46, 47, 51, 53–55, 61, 64, 68 |
| Eurosurveillance | 10 | 10.1 | 3, 9, 10, 17, 21, 39, 44, 45, 69, 70 |
| Emerging Microbes and Infections | 5 | 5.1 | 40, 43, 60, 63, 73 |
| Virus Genes | 3 | 3.0 | 8, 31, 52 |
| EMPRES‐i | 26 | 26.3 | 11 |
| Other[Link] | 28 | 28.3 | 4, 6, 12–14, 16, 18, 27, 32–34, 36, 41, 42, 48–50, 56–59, 62, 65–67, 71, 72, 74 |
Each OIE MERS‐CoV animal event entered in EMPRES‐i was treated as a separate record in the review, but were included under one bibliographic entry, per FAO citation protocol.
Other journals included Epidemiology and Infection; Infection Ecology and Epidemiology; Infection Genetics and Evolution; Japanese Journal of Infectious Diseases; Journal of Veterinary Medicine and Animal Health; Journal of Virology; MBIO; NEJM; One Health; PLOS One; Science China; Scientific Reports; American Journal of Tropical Medicine and Hygiene; The Lancet Infectious Diseases; Transboundary and Emerging Diseases; Vaccine; Vector‐borne and Zoonotic Diseases; Virology Journal; Virus Genes; Science; Viruses.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Saudi Arabia has the highest human MERS burden (World Health Organization, 2018) and has conducted more observational studies in animals than any other country (Table 2). Ten publications included studies conducted in more than one country. Approximately two‐thirds of the studies completed sampling within a one‐year period. Most of the multiple‐year studies included the use of archived serum samples to investigate historical animal exposure to MERS‐CoV (as far back as 1983), and bat studies that collected samples seasonally over the course of multiple years (Table 2). Figure 2 presents a map of countries included in this scoping review with the presence or absence of reported positive findings of either infection or exposure in animals.
Table 2.
The number of observational studies conducted in each country, and the study duration for each observational study with the per cent of overall observational studies
| Characteristic | Positive findings reported (antigen and/or antibody) | N = 90 | % | Appendix S1: Technical appendix reference[Link] |
|---|---|---|---|---|
| Country sampled | ||||
| Saudi Arabia | Yes | 25 | 27.78 | 7, 9, 11, 14, 18, 19, 22, 37, 45, 48, 71, 73 |
| United Arab Emirates | Yes | 11 | 12.22 | 11, 12, 15, 30, 31, 35, 43, 46, 52, 60, 61 |
| Qatar | Yes | 9 | 10.00 | 11, 13, 21, 25, 27, 36, 53 |
| Egypt | Yes | 7 | 7.78 | 3, 20, 29, 45, 47, 63, 70 |
| Jordan | Yes | 4 | 4.44 | 10, 11, 65 |
| Ethiopia | Yes | 3 | 3.33 | 26, 67, 69 |
| Kenya | Yes | 3 | 3.33 | 24, 41, 72 |
| Oman | Yes | 3 | 3.33 | 6, 11, 17 |
| Iran | Yes | 3 | 3.33 | 11 |
| Other[Link] | 42 | 46.67 | 1, 2, 4–6, 8, 11, 12, 16, 23, 26, 29, 30, 32–34, 38–40, 42, 44, 45, 47, 51, 56, 58, 59, 66, 68, 69 | |
| Asia | Yes[Link] | 15 | ||
| Africa | Yes[Link] | 11 | ||
| Europe | Yes[Link] | 5 | ||
| Americas | No | 3 | ||
| Multiple countries | 10 | 11.11 | ||
| Single country | 80 | 88.89 | ||
| Study duration (years) | ||||
| <1 | 61 | 67.78 | 3, 10–14, 16–21, 23, 25, 31, 35, 36, 38–42, 44, 46, 48, 51–53, 58, 65–67, 69–73 | |
| 1–5 | 20 | 22.22 | 1, 2, 4, 5, 7–9, 22, 26, 33, 37, 47, 56, 59–61, 63, 68 | |
| >5 | 5 | 5.56 | 15, 24, 29, 34, 45 | |
| Not reported | 5 | 5.56 | 6, 27, 30, 32, 43 | |
Each OIE MERS‐CoV animal event entered in EMPRES‐i was treated as a separate record in the review, but were included under one bibliographic entry, per FAO citation protocol.
Other countries included Australia; China; Japan; Korea; Laos; Cambodia; Mongolia; Pakistan; Thailand; Kazakhstan; Taiwan; Lebanon; Kuwait; Burkina Faso; Morocco; Ghana; Madagascar; Mali; Nigeria; Tunisia; South Africa; Sudan; Somalia; Germany; Romania; Ukraine; Italy; Spain; Netherlands; Chile; Canada; USA.
Other countries in Asia with positive findings in animals are Pakistan and Kuwait.
Other countries in Africa with positive findings in animals are Burkina Faso, Mali, Morocco, Nigeria, Somalia, Sudan, Tunisia.
Country in Europe with positive findings in animals is the Canary Islands.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Figure 2.
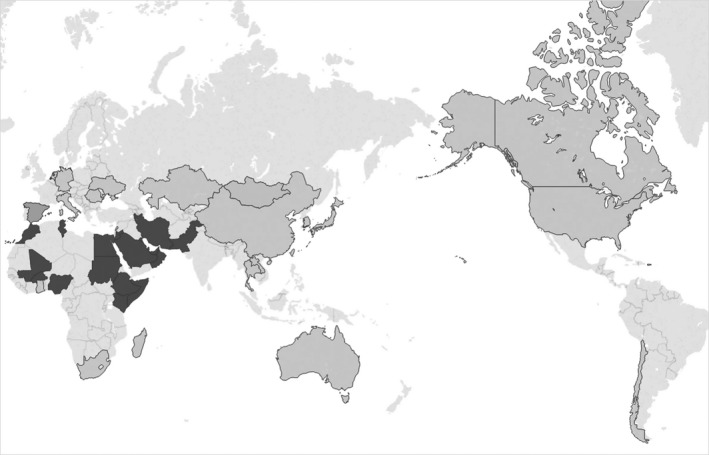
Countries with reported MERS‐CoV exposure or infection in animals, based on publications included in this scoping review. Countries with light shading indicate where samples were collected, but none tested positive to MERS‐CoV. Countries with dark shading indicate where samples collected from animals in at least one study tested positive to MERS‐CoV either by antigenic or antibody testing
Study sample sizes according to study type and species group were summarized (Table 3). Ruminant species sampled included sheep, goats and cattle. Camelids other than dromedaries were also included in the observational studies and included alpacas and llamas, as well as bactrian camels and guanacos (wild camelids sampled in a zoo). The other domestic animals sampled in observational studies were chickens, while mice and pigs fell under this category in experimental studies. Equids include horses, donkeys and mules. One observational study sampled a species of baboon (Papio hamadryas hamadryas). The sample size was reported in all studies except two OIE reports, while calculations for sample size or power were provided in three of ninety observational studies (data not shown). Experimental studies suggest that camelids, pigs, goats and bats may act as hosts, although field studies have demonstrated natural infection through exposure only in dromedaries and a single flock of alpacas housed near exposed dromedaries in Saudi Arabia (Table 3).
Table 3.
Characteristics of sample sizes by study type and animal category
| Animal category | No. of studies that reported positive antigen or antibody findings (observational)—or seroconversion (experimental) | N studies | Sample size | Appendix S1: Technical appendix reference[Link] | |
|---|---|---|---|---|---|
| Median | Range | ||||
| Observational | |||||
| Dromedaries | 67 | 70 | 82 | 3–7,803 | 3, 6, 9–15, 17–22, 24–27, 29, 31, 32, 35–37, 39, 41–46, 48, 51–53, 60, 61, 63, 65–70, 72, 73 |
| Bats | 1 (RNA segment isolated from faeces) | 15 | 194 | 32–5,030 | 1, 2, 4, 5, 7, 8, 16, 23, 33, 34, 47, 56, 58, 59, 70 |
| Ruminants | 1 (One sheep was seropositive) | 10 | 89 | 3–276 | 3, 6, 9, 10, 12, 14, 52, 65, 66, 70 |
| Cattle N = 6 | |||||
| Sheep N = 9 | |||||
| Goats N = 5 | |||||
| Other camelids | 1 (Alpacas housed near dromedaries in Saudi Arabia) | 6 | 65 | 6–200 | 6, 32, 38, 40, 51, 55 |
| Bactrian n = 5 | |||||
| Alpacas n = 2 | |||||
| Llamas, Guanacos n = 1 | |||||
| Equids | 0 | 3 | 19 | 3–889 | 12, 30, 70 |
| Horses n = 3 | |||||
| Donkeys, mules = 2 | |||||
| Other domestic | 0 | 1 | 240 | n/a | 9 |
| Chickens n = 1 | |||||
| Other wildlife | 0 | 1 | 50 | n/a | 71 |
| Hamadryas baboons | |||||
| Experimental | |||||
| Other camelids | 3 | 3 | 8 | 3–9 | 54, 55, 64 |
| Alpacas n = 2 | |||||
| Llamas n = 1 | |||||
| Other domestic | 3 | 3 | 15 | 14–24 | 62, 64, 74 |
| Mice n = 2 | |||||
| Pigs n = 1 | |||||
| Ruminants | 1 (seroconversion of inoculated kids but no transmission to their susceptible dams) | 2 | 12 | 10–14 | 57, 64 |
| Sheep n = 2 | |||||
| Goats n = 1 | |||||
| Dromedaries | 2 | 2 | 6 | 3–8 | 28, 49 |
| Equids | 0 | 2 | 6 | 4–8 | 57, 64 |
| Horses n = 2 | |||||
| Bats | 1 | 1 | 12 | n/a | 50 |
Each OIE MERS‐CoV animal event entered in EMPRES‐i was treated as a separate record in the review, but were included under one bibliographic entry, per FAO citation protocol.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
In general, details about the dromedary camel populations being studied were reported more frequently than those of other species (Table 4). Age or age group of the animals was reported in 53% of the dromedary camel studies, although almost all of the publications that did not report age were OIE reports. The group size refers to whether the number of camels in the epidemiological unit being sampled was reported, such as herd size, the number of camels at a market or the number of camels grouped together awaiting slaughter (Table 4). Most field studies sampled animals at primary production sites such as ranches, pastoralist herds or pleasure herds. Ten studies sampled multiple sites along the livestock production chain.
Table 4.
Variables reported and sampling points of observational studies by dromedary camels and all other animals
|
Dromedaries N = 72 |
% | Appendix S1: Technical appendix reference[Link] |
Other species N = 37 |
% | Appendix S1: Technical appendix reference[Link] | |
|---|---|---|---|---|---|---|
| Characteristics reported | ||||||
| Age | 38 | 52.78 | 6, 9, 10, 12, 14, 15, 18–22, 24, 26, 27, 35–37, 39, 41, 43, 44, 46, 48, 51–53, 60, 61, 63, 65–70, 72, 73 | 13 | 35.14 | 1, 5, 8, 10, 14, 30, 32, 38, 51–53, 65, 71 |
| Sex | 22 | 30.56 | 10, 12, 15, 21, 22, 24, 26, 32, 39, 46, 51–53, 60, 63, 66, 67–70, 72, 73 | 9 | 24.32 | 1, 5, 8, 10, 32, 51–53, 71 |
| Breed | 1 | 1.39 | 29 | 2 | 5.41 | 10, 14 |
| Location (below country level) | 68 | 94.44 | 3, 6, 9–15, 18–22, 24–26, 29, 31, 32, 35–37, 39, 41–45, 48, 51–53, 60, 63, 65–70, 72, 73 | 22 | 59.46 | 1–10, 12, 14, 16, 32–34, 38, 40, 51–53, 56, 58, 59, 65, 70, 71 |
| Group size | 42 | 58.33 | 11, 13, 18, 19, 21, 22, 41–44, 46, 51–53, 61, 69, 72, 73 | 5 | 13.51 | 32, 38, 51–53 |
| Animal contact/herd structure described | 13 | 18.06 | 6, 18, 21, 22, 24, 37, 41, 43, 53, 61, 65, 69, 73 | 4 | 10.81 | 6, 53, 59, 71 |
| Sampling points | ||||||
| Primary production (farms/herds/backyard) | 51 | 70.83 | 6, 11, 13, 18, 19, 21, 22, 24, 26, 29, 31, 35, 41, 43, 48, 51–53, 61, 63, 65, 67, 69, 70, 72, 73 | 7 | 18.92 | 3, 38, 40, 51–53, 65 |
| Abattoir | 15 | 20.83 | 3, 16, 20, 26, 29, 31, 36, 37, 42, 44, 45, 48, 63, 69, 70 | 1 | 2.70 | 3 |
| Live animal market | 4 | 5.56 | 37, 48, 63, 70 | 1 | 2.70 | 59 |
| Other[Link] | 8 | 11.11 | 31, 32, 37, 42, 45, 46, 63, 70 | 11 | 29.73 | 2, 4–7, 30, 32, 33, 47, 58, 71 |
| Multiple sampling points | 10 | 13.89 | 26, 29, 31, 37, 42, 45, 48, 63, 69, 70 | 1 | 2.70 | 59 |
Each OIE MERS‐CoV animal event entered in EMPRES‐i was treated as a separate record in the review, but were included under one bibliographic entry, per FAO citation protocol.
Other sampling points included wild habitat; zoo; border crossing and trade‐related gathering point; hunting village; wild meat restaurant; veterinary hospital; quarantine.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
All experimental studies that involved livestock included in their methodology the testing of animals prior to challenge or vaccination. Two studies that used purpose‐bred white mice did not report testing the animals for MERS‐CoV prior to intervention. The duration of experimental studies ranged from 24 to 84 days after the first intervention (pathogen or vaccine inoculation), and six out of nine studies reported sampling subjects for greater than one month. One study used positive controls, while four studies used negative control subjects (none reported both types of controls).
Three studies examined animal vaccine candidates by experimental inoculation. The studies used MVA, ChAdOx1 and inactivated rabies virus vaccines, and all vaccines expressed full or partial MERS‐CoV spike protein. One study was conducted on dromedaries, while the other two were conducted on mice (Tables 1 and 3). All three administered the vaccine via intramuscular injection, while one additionally administered intranasally with the injection. Two of the studies gave a second booster vaccine after 28 days, while one study boosted after 7 and 21 days after the first immunization.
Many different outcomes were reported in the studies characterized here. However, this review categorized outcomes of interest for understanding pathogen transmission and public health risk, and according to epidemiological inputs that would be useful for disease transmission modelling, and is by no means an exhaustive list (Table 5). Outcome categories were defined a priori. Prevalence refers specifically to active infection and was defined as any proportion of virus‐positive field samples over a denominator, usually the number of animals tested. Seroprevalence was similarly defined as a proportion of antibody‐positive field samples over a denominator. The immunity outcome was defined as any study that described or inferred dynamics of natural or vaccine immunity from collected data. A study was counted as measuring pathogen transmission from one animal to another if this was documented or inferred from the data, for example, transmission to susceptible animals during an experimental study, or seroconversion during longitudinal studies. If studies described the duration of one or more stages of infection, such as exposure, shedding or immunity, either as measured experimentally or estimated from repeated field measures, it was listed under the “duration” outcome (Table 5).
Table 5.
Frequency of outcome measures categorized according to relevance to transmission modelling, frequency of outcome variables, specimens collected and whether raw data were provided
| Characteristic | N | % | |
|---|---|---|---|
| Outcome measures | |||
| Observational | 90 | ||
| Prevalence | 69 | 69.70 | 1, 2, 4, 5, 7, 8, 11, 13–21, 23, 25, 27, 31–38, 43, 44, 46–48, 52, 53, 56, 58, 59, 61, 63, 65, 67, 69, 70, 73 |
| Seroprevalence | 39 | 39.39 | 3, 6, 9, 10, 12–15, 18–21, 24, 26, 29, 30, 32, 36, 38–46, 51, 53, 63, 65–73 |
| Immunity | 5 | 5.05 | 22, 36, 61, 63, 73 |
| Transmission | 5 | 5.05 | 22, 35, 61, 63, 73 |
| Duration | 3 | 3.03 | 35, 52, 61 |
| Clinical signs reported | 2 | 2.02 | 18, 22 |
| Experimental | 9 | ||
| Immunity | 5 | 5.05 | 49, 50, 55, 62, 74 |
| Transmission | 2 | 2.02 | 57, 55 |
| Duration | 7 | 7.07 | 28, 49, 50, 54, 55, 57, 64 |
| Clinical signs reported | 6 | 6.06 | 28, 49, 50, 55, 57, 64 |
| Outcome variables | 99 | ||
| Antibodies—quantified | 29 | 29.29 | 3, 6, 12, 13, 15, 18–22, 24, 29, 36, 42, 45, 46, 49, 50, 53–55, 57, 61–64, 66, 73, 74 |
| Antibodies—dichotomous | 31 | 31.31 | 3, 6, 9, 10, 14, 15, 20, 21, 24, 26, 28, 30, 32, 35, 36, 38–41, 43, 44, 50, 51, 65, 67–73 |
| Antigen—quantified | 17 | 17.18 | 17–19, 25, 27, 28, 36, 48, 49, 53–55, 57, 61, 62, 64, 73 |
| Antigen—dichotomous | 67 | 67.68 | 1, 2, 4, 5, 7, 8, 11, 13–17, 20–23, 27, 31–38, 40, 43, 44, 46, 47, 52, 53, 56, 58, 59, 61, 63, 65, 67, 69, 70, 73 |
| Infectious virus—all measures | 14 | 14.14 | 18, 25, 28, 35, 43, 48–50, 54, 55, 57, 61, 62, 64 |
| Specimen | |||
| Serum/blood | 50 | 50.51 | 3, 6, 9, 10, 12–15, 18–22, 24, 26, 28–30, 32, 35, 36, 38–47, 49, 50, 51, 53–55, 57, 61–74 |
| Nasal swab | 36 | 36.36 | 13, 14, 17–22, 25, 27, 28, 31, 32, 35–38, 40, 43, 44, 46, 48, 49, 52, 53–55, 57, 60, 61, 63–65, 67, 69, 70, 73 |
| Faeces | 13 | 13.13 | 1, 2, 4, 5, 7, 15, 16, 27, 28, 32, 34, 58, 59 |
| Rectal swabs | 23 | 23.23 | 7, 8, 13, 14, 18, 21–23, 27, 33, 34, 36, 47, 49, 50, 52–54, 56, 59, 63, 64, 73 |
| Urine | 3 | 3.03 | 18, 28, 63 |
| Milk | 4 | 4.04 | 18, 21, 52, 63 |
| Oropharyngeal | 11 | 11.11 | 22, 27, 28, 33, 47, 50, 53, 54, 56, 59, 70 |
| Other | 19 | 19.19 | 4, 17, 18, 22, 27, 28, 37, 43, 47, 49, 50, 52, 54, 55, 57, 59, 62, 64, 74 |
| Raw data provided | 20 | 20.20 | 3, 12–14, 18, 19, 21, 22, 28, 32, 41, 42, 46, 49, 50, 53–55, 65, 73 |
Each OIE MERS‐CoV animal event entered in EMPRES‐i was treated as a separate record in the review, but were included under one bibliographic entry, per FAO citation protocol.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Study outcomes were measured using several different variables. Results of antigenic testing were reported as continuous measurements and/or dichotomous outcomes based upon a prespecified cut‐off for positive and negative reactions (Table 5). Numerous studies considered both dichotomous and quantified (continuous or categorical) test results. Almost all studies collected serum or blood and/or nasal swabs, generally corresponding to antibody or antigen outcome variables. Those studies that did not report collecting these samples were sampling bats non‐invasively (Table 5). One‐fifth of the studies provided access to the raw data.
4. DISCUSSION
The aim of this scoping review was to identify and characterize the literature that explored MERS‐CoV in animal hosts. Field studies have provided compelling evidence that dromedary camels act as the reservoir host for MERS‐CoV. Experimental evidence has confirmed the susceptibility of dromedary camels and provided key details regarding the course of infection in camelids (Table 3). A challenge and transmission study conducted on goats suggests they may act as dead‐end hosts; however, this has not been demonstrated in the field. One experimental study provided evidence that pigs may also act as a host for MERS‐CoV (Table 3); however, the production range of domestic pigs does not overlap with camelids and is unlikely to be a risk factor where the disease is currently endemic. Bats present a unique challenge in determining their role in the ecology of MERS‐CoV. Although a study of Jamaican fruit bats (Artibeus jamaicensis) demonstrates the potential of this species as a host, field sampling of bats has found a single fragment of MERS‐CoV RNA from a faecal sample of an Egyptian tomb bat (Taphozous perforates; Table 3). The immunology of bats presents a unique challenge in drawing conclusions regarding their role as a MERS‐CoV host (Brook & Dobson, 2015). While all known zoonotic transmission events have occurred in the Arabian Peninsula (World Health Organization, 2018), dromedary camels are raised across Africa and South Asia, and it is evident that the virus is also circulating in dromedary camels across their production range in these regions (Figure 2). While no autochtonous human cases of MERS‐CoV have been reported from African countries, the endemnicity of the virus in dromedary camel populations presents on the one hand a public health risk, and on the other, an opportunity for better understanding reservoir dynamics. African countries are underrepresented in the published body of literature, with only one‐fifth of the observational studies in this review conducted there. Likewise, only one study was published from the South Asia region (Saqib et al., 2017), which has a large dromedary camel population. Camel raising varies between the Middle East and other regions, and further epidemiological research coupled with anthropological and value chain studies that reflect these differences would potentially enhance our understanding of the risks for zoonotic spillover and how they differ across the regions.
Almost all the publications in this review provided a measure of prevalence, including seroprevalence and prevalence of infection. Study results suggested heterogeneity around these values, especially with respect to the prevalence of infection, with point prevalence estimates ranging from zero to almost 90%. Nine studies provided variability estimates (e.g., confidence intervals) around at least one prevalence value, indicating additional post hoc calculations would be required to assess the precision of prevalence estimates. Two studies presented results of multivariable models, which can help explain apparent variability in prevalence by identifying risk factors for infection and other determinants of virus activity. Dromedary sampling strategy within studies was often not reported (n = 30), reported as census sampling of a single group (e.g., case study, outbreak investigation) (n = 30), or convenience (n = 4), rather than random (n = 2), indicating that the majority of studies were not designed to be generalizable to a target population. An understanding of how MERS‐CoV fluctuates within and between host populations cannot be extracted from these data. It is recommended that future research uses existing evidence on MERS‐CoV in animal populations to inform sample size calculations, sampling strategies and research questions in order to improve on the strength of the evidence and address more sophisticated study objectives.
Seroprevalence data can be useful for estimating transmissibility, with accuracy improved with detailed age data (Keeling & Rohani, 2008). Age is an important factor in dromedary transmission (Mackay & Arden, 2015) and was reported in years or months in fifteen of the studies included in this review, while twenty‐one studies provided age data as dichotomous or categorical variables.
Studies thus far have generated a multitude of hypotheses around MERS‐CoV prevalence and risk factors in animals, especially dromedaries. However, there is a lack of studies that test these hypotheses, and a sizeable gap in our knowledge of when and where infection rates differ, and which factors are important for infection in dromedaries.
The question of camel immunity to MERS‐CoV has important implications for public health risk, infection control and disease prevention. The dynamics of immunity (e.g., duration of immunity) inform intervention strategies such as vaccination, as well as research questions such as the structure of disease transmission models. Longitudinal studies have been used to infer that natural infection in dromedaries confers either waning or partial immunity to MERS‐CoV (Ali, Shehata, et al., 2017; Hemida et al., 2017; Hemida, Perera, et al., 2014). One experimental study demonstrated partial immunity following natural infection in alpacas (Adney, Bielefeldt‐Ohmann, Hartwig, & Bowen, 2016). Longitudinal field studies may be capturing second infections or persistently infected camels with intermittent shedding. Examples of both exist among coronaviruses (Dowell & Ho, 2004; Isaacs, Flowers, Clarke, Valman, & MacNaughton, 1983), and the duration of natural immunity, and if or how reinfection differs from first infection are important characteristics to know. Molecular epidemiologic analyses, longer‐term field studies or experimental studies may help to answer these questions.
Vaccine studies included here have demonstrated short‐term efficacy in reducing viral shedding, but further studies that examine long‐term efficacy under field conditions are required. The unanswered questions surrounding natural and vaccine‐induced immunity in dromedaries have important implications for the efficacy and planning of interventions targeted at animal hosts.
Experimental challenge and transmission studies provided key data regarding the time course of infection. Field studies have also provided valuable information, including evidence supporting the duration of pathogen shedding (Al Muhairi et al., 2016), and documenting transmission among dromedaries (Ali, Shehata, et al., 2017; Meyer et al., 2016).
Although the upper respiratory tract is now understood to be the primary site of viral replication and shedding, it is important to understand the role of other potential routes of transmission for understanding risk. Therefore, the negative results of observational studies (Al‐Muhairi et al., 2016; Azhar et al., 2014) are as important as the positive findings (Ali, El‐Shesheny, et al., 2017; Reusken et al., 2014) as they provide a more complete picture of possible routes of transmission.
This review has several limitations. It was conducted in English, which likely led to the omission of Arabic literature, introducing a language bias. Article screening and data extraction was conducted by one author which may have introduced reporting bias.
This scoping review describes the general and epidemiologic characteristics of published primary studies of MERS‐CoV in animal hosts. MERS‐CoV is a newly discovered zoonotic disease, and there is a need to assess the evidence base so that future research strategically fills the knowledge gaps. Dromedary camels are the reservoir host for MERS‐CoV; disease is mild and predominantly affects young animals. Improving our understanding of how the virus circulates in the reservoir population including dynamics of immunity, temporal and geographic variation, and key risk factors for infection would provide important insights to inform research and policy such as transmission modelling and disease prevention strategies with the ultimate goal of reducing this public health threat.
CONFLICT OF INTEREST
None.
Supporting information
ACKNOWLEDGEMENTS
EG is supported by an Ontario Veterinary College (OVC) Fellowship and a Natural Sciences and Engineering Council of Canada (NSERC) Graduate Scholarship (PGSD2 ‐ 505055 – 2017). This work was also supported by the Canada Research Chairs Program (ALG). This article was also made possible by the generous support of the American people through the United States Agency for International Development (USAID). The views expressed in this publication are those of the authors and do not necessarily reflect the views of the Food and Agriculture Organization, USAID or the United States government. We acknowledge the support of Juan Lubroth, Subhash Morzaria, Ahmed El Idrissi and Eran Raizman of the Food and Agriculture Organization of the United Nations (FAO).
Gardner EG, Kelton D, Poljak Z, von Dobschuetz S, Greer AL. A rapid scoping review of Middle East respiratory syndrome coronavirus in animal hosts. Zoonoses Public Health. 2019;66:35–46. 10.1111/zph.12537
REFERENCES
- Adney, D. R. , Bielefeldt‐Ohmann, H. , Hartwig, A. E. , & Bowen, R. A. (2016). Infection, replication, and transmission of middle east respiratory syndrome coronavirus in Alpacas. Emerging Infectious Diseases, 22(6), 1031–1037. 10.3201/eid2206.160192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adney, D. R. , van Doremalen, N. , Brown, V. R. , Bushmaker, T. , Scott, D. , de Wit, E. , … Munster, V. J. (2014). Replication and shedding of MERS‐CoV in upper respiratory tract of inoculated dromedary camels. Emerging Infectious Diseases, 20(12), 1999–2005. 10.3201/eid2012.141280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Hammadi, Z. M. , Chu, D. K. W. , Eltahir, Y. M. , Al Hosani, F. , Al Mulla, M. , Tarnini, W. , … Poon, L. L. M. (2015). Asymptomatic MERS‐CoV infection in humans possibly linked to infected dromedaries imported from Oman to United Arab Emirates, May 2015. Emerging Infectious Diseases, 21(12), 2197–2200. 10.3201/eid2112.151132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Muhairi, S. , Al Hosani, F. , Eltahir, Y. M. , Al Mulla, M. , Yusof, M. F. , Serhan, W. S. , … Abdelazim, A. S. (2016). Epidemiological investigation of Middle East respiratory syndrome coronavirus in dromedary camel farms linked with human infection in Abu Dhabi Emirate. United Arab Emirates. Virus Genes, 52(6), 848–854. 10.1007/s11262-016-1367-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagaili, A. n. , Briese, T. , Mishra, N. , Kapoor, V. , Sameroff, S. c. , de Wit, E. , … Lipkin, W. i. (2014). Middle East Respiratory Syndrome Coronavirus Infection in Dromedary Camels in Saudi Arabia. Mbio, 5(2), e00884‐14 10.1128/mBio.00884-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali, M. , El‐Shesheny, R. , Kandeil, A. , Shehata, M. , Elsokary, B. , Gomaa, M. , … Makonnen, Y. J. (2017). Cross‐sectional surveillance of Middle East respiratory syndrome coronavirus (MERS‐CoV) in dromedary camels and other mammals in Egypt, August 2015 to January 2016. Eurosurveillance, 22(11), 30487 10.2807/1560-7917.ES.2017.22.11.30487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali, M. A. , Shehata, M. M. , Gomaa, M. R. , Kandeil, A. , El‐Shesheny, R. , Kayed, A. S. , … Kayali, G. (2017). Systematic, active surveillance for Middle East respiratory syndrome coronavirus in camels in Egypt. Emerging Microbes & Infections, 6(1), e1 10.1038/emi.2016.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al‐Muhairi, S. , Al‐Hosani, F. , Eltahir, Y. M. , Al‐Mulla, M. , Yusof, M. F. , Serhan, W. S. , … Abdelazim, A. S. (2016). Epidemiological investigation of Middle East respiratory syndrome coronavirus in dromedary camel farms linked with human infection in Abu Dhabi Emirate. United Arab Emirates. Virus Genes, 52(6), 848–854. 10.1007/s11262-016-1367-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabi, Y. M. , Balkhy, H. H. , Hayden, F. G. , Bouchama, A. , Luke, T. , Baillie, J. K. , … Fowler, R. A. (2017). Middle east respiratory syndrome. New England Journal of Medicine, 376(6), 584–594. 10.1056/NEJMsr1408795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azhar, E. I. , El‐Kafrawy, S. A. , Farraj, S. A. , Hassan, A. M. , Al‐Saeed, M. S. , Hashem, A. M. , & Madani, T. A. (2014). Evidence for camel‐to‐human transmission of MERS coronavirus. New England Journal of Medicine, 370(26), 2499–2505. 10.1056/NEJMoa1401505. [DOI] [PubMed] [Google Scholar]
- Brook, C. E. , & Dobson, A. P. (2015). Bats as “special” reservoirs for emerging zoonotic pathogens. Trends in Microbiology, 23(3), 172–180. 10.1016/j.tim.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell, S. F. , & Ho, M. S. (2004). Seasonality of infectious diseases and severe acute respiratory syndrome–what we don’t know can hurt us. The Lancet Infectious Diseases, 4(11), 704–708. 10.1016/S1473-3099(04)01177-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farag, E. A. B. A. , Reusken, C. B. E. M. , Haagmans, B. L. , Mohran, K. A. , Stalin Raj, V. , Pas, S. D. , … Koopmans, M. P. G. (2015). High proportion of MERS‐CoV shedding dromedaries at slaughterhouse with a potential epidemiological link to human cases, Qatar 2014. Infection Ecology & Epidemiology, 5, 28305 10.3402/iee.v5.28305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO) . (2014). EMPRESi ‐ Global Animal Disease Information System. Retrieved from https://empres-i.fao.org/eipws3g/
- Haagmans, B. L. , Al Dhahiry, S. H. S. , Reusken, C. B. E. M. , Raj, V. S. , Galiano, M. , Myers, R. , … Koopmans, M. P. G. (2014). Middle East respiratory syndrome coronavirus in dromedary camels: An outbreak investigation. The Lancet Infectious Diseases, 14(2), 140–145. 10.1016/S1473-3099(13)70690-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemida, M. G. , Alnaeem, A. , Chu, D. K. W. , Perera, R. A. P. M. , Chan, S. M. S. , Almathen, F. , … Peiris, M. (2017). Longitudinal study of Middle East Respiratory Syndrome coronavirus infection in dromedary camel herds in Saudi Arabia, 2014–2015. Emerging Microbes & Infections, 6, 10.1038/emi.2017.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemida, M. G. , Elmoslemany, A. , Al‐Hizab, F. , Alnaeem, A. , Almathen, F. , Faye, B. , … Peiris, M. (2015). Dromedary Camels and the Transmission of Middle East Respiratory Syndrome Coronavirus (MERS‐CoV). Transboundary and Emerging Diseases, 64(2), 344–353. 10.1111/tbed.12401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemida, M. G. , Perera, R. A. , Al Jassim, R. A. , Kayali, G. , Siu, L. Y. , Wang, P. , … Peiris, M. (2014). Seroepidemiology of Middle East respiratory syndrome (MERS) coronavirus in Saudi Arabia (1993) and Australia (2014) and characterisation of assay specificity. Euro Surveillance, 19(23). 10.2807/1560-7917.ES2014.19.23.20828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemida, M. G. , Perera, R. A. , Wang, P. , Alhammadi, M. A. , Siu, L. Y. , Li, M. , … Peiris, M. (2013). Middle East Respiratory Syndrome (MERS) coronavirus seroprevalence in domestic livestock in Saudi Arabia, 2010 to 2013. Euro Surveillance, 18(50), 20659 10.2807/1560-7917.ES2013.18.50.20659 [DOI] [PubMed] [Google Scholar]
- Isaacs, D. , Flowers, D. , Clarke, J. R. , Valman, H. B. , & MacNaughton, M. R. (1983). Epidemiology of coronavirus respiratory infections. Archives of Disease in Childhood, 58(7), 500–503. 10.1136/ADC.58.7.500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeling, M. J. , & Rohani, P. (2008). Modeling infectious diseases in humans and animals ‐ Matt J. Keeling, Pejman Rohani ‐ Google Books. Princeton, NJ: Princeton University Press. [Google Scholar]
- Levac, D. , Colquhoun, H. , & O'Brien, K. K. (2010). Scoping studies: Advancing the methodology. Implementation Science, 5, 69 10.1186/1748-5908-5-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay, I. M. , & Arden, K. E. (2015). Middle East respiratory syndrome: An emerging coronavirus infection tracked by the crowd. Virus Research, 202, 60–88. 10.1016/j.virusres.2015.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, B. , García‐Bocanegra, I. , Wernery, U. , Wernery, R. , Sieberg, A. , Müller, M. A. , … Eckerle, I. (2015). Serologic assessment of possibility for MERS‐CoV infection in equids. Emerging Infectious Diseases, 21(1), 181–182. 10.3201/eid2101.141342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, B. , Juhasz, J. , Barua, R. , Das Gupta, A. , Hakimuddin, F. , Corman, V. M. , … Nagy, P. (2016). Time course of MERS‐CoV infection and immunity in dromedary camels. Emerging Infectious Diseases, 22(12), 2171–2173. 10.3201/eid2212.160382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohd, H. A. , Al‐Tawfiq, J. A. , & Memish, Z. A. (2016). Middle East Respiratory Syndrome Coronavirus (MERS‐CoV) origin and animal reservoir. Virology Journal, 13(1), 87 10.1186/s12985-016-0544-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher, D. , Liberati, A. , Tetzlaff, J. , Altman, D. G. , & Group, T. P. (2009). Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. PLoS Medicine, 6(7), e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, M. D. J. , Godfrey, C. , McInerney, P. , Baldini Soares, C. , Khalil, H. P. D. (2017). Chapter 11: Scoping reviews In Aromataris E., & Munn Z. (Eds.), Joanna Briggs Institute Reviewer’s Manual. Adelaide, SA: The Joanna Briggs Institute. [Google Scholar]
- Pham, M. T. , Rajić, A. , Greig, J. D. , Sargeant, J. M. , Papadopoulos, A. , & Mcewen, S. A. (2014). A scoping review of scoping reviews: Advancing the approach and enhancing the consistency. Research Synthesis Methods, 5(4), 371–385. 10.1002/jrsm.1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusken, C. B. , Farag, E. A. , Jonges, M. , Godeke, G. J. , El‐Sayed, A. M. , Pas, S. D. , … Koopmans, M. P. (2014). Middle East respiratory syndrome coronavirus (MERS‐CoV) RNA and neutralising antibodies in milk collected according to local customs from dromedary camels, Qatar, April 2014. Euro Surveillance, 19(23), 20810. [DOI] [PubMed] [Google Scholar]
- Reusken, C. B. E. M. , Haagmans, B. L. , Mueller, M. A. , Gutierrez, C. , Godeke, G.‐J. , Meyer, B. , … Koopmans, M. P. G. (2013). Middle East respiratory syndrome coronavirus neutralising serum antibodies in dromedary camels: A comparative serological study. Lancet Infectious Diseases, 13(10), 859–866. 10.1016/S1473-3099(13)70164-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saqib, M. , Sieberg, A. , Hussain, M. H. , Mansoor, M. K. , Zohaib, A. , Lattwein, E. , … Corman, V. M. (2017). Serologic evidence for MERS‐CoV infection in dromedary camels, Punjab, Pakistan, 2012–2015. Emerging Infectious Diseases, 23(3), 550–551. 10.3201/eid2303.161285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernery, U. , Lau, S. K. P. , & Woo, P. C. Y. (2017). Middle East respiratory syndrome (MERS) coronavirus and dromedaries. Veterinary Journal., 63(9), 262–272. 10.1016/j.tvjl.2016.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . (2018). WHO | Middle East respiratory syndrome coronavirus (MERS‐CoV). Retrieved from https://www.who.int/emergencies/mers-cov/en/ [Google Scholar]
- Zaki, A. M. , van Boheemen, S. , Bestebroer, T. M. , Osterhaus, A. D. M. E. , & Fouchier, R. A. M. (2012). Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. New England Journal of Medicine, 367(19), 1814–1820. 10.1056/NEJMoa1211721 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


