Summary
AKT1, a serine/threonine kinase, plays a critical role in the controlling of intracellular growth of Mycobacterium tuberculosis. In this study, we investigated whether polymorphisms in AKT1 affect susceptibility to tuberculosis (TB). Two single nucleotide polymorphisms of AKT1, IVS3+18C>T and +726G>A were genotyped in Chinese patients with pulmonary TB by polymerase chain reaction‐restriction fragment length polymorphism. Patients with pulmonary TB had significantly lower IVS3+18 C/C genotype and higher C/T genotype compared with age‐, gender‐ and ethnically matched controls (P < 0.05). The T–A haplotype frequency was significantly higher in patients than in controls (P < 0.05). In conclusion, our result indicates that AKT1 polymorphisms are associated with susceptibility to pulmonary TB.
Introduction
It is estimated that one‐third of the world population is infected with Mycobacterium tuberculosis, with 8.8 million new cases and 2 million deaths annually (Raviglione, 2003). Long‐time epidemiological observation indicates that only 5–10% of latent tuberculosis (TB) infection progress to active TB in their lifetime. Whether a person develops TB depends on host–pathogen interactions. There are several lines of evidence indicating that susceptibility to TB is determined partly by the host genetic factors, and possibly influenced by environmental factors as well (Schurr, 2007). Mutations in genes of the interferon‐γ signalling pathway result in increased susceptibility to TB. Several other susceptibility genes, such as SLC11A1 (NRAMP1), NOD2, MCP1, ALOX5, TLR genes, have also been identified (Schurr, 2007; Austin et al., 2008).
Mycobacterium tuberculosis primarily infects macrophages. Once inside the cells, the bacteria can modulate endosomal/phagosomal maturation pathway, block the fusion of mycobacterial phagosome with lysosome and avoid bactericidal mechanisms of the host cells (Houben et al., 2006).
Recently, AKT1 (V‐akt murine thymoma viral oncogene homolog 1), a serine/threonine kinase, also known as protein kinase B (PKB) was identified to be a key kinase that involves in the controlling of intracellular growth of M. tuberculosis, through regulating host cell signalling pathways (Kuijl et al., 2007). A specific small molecule inhibitor of AKT1, H‐89, could significantly inhibit proliferation of M. tuberculosis strain H37Rv and multi‐drug resistant strain MDR16319 in primary human macrophages, without inducing apoptosis of the host cells (Kuijl et al., 2007). Specific knockdown of AKT expression with AKT1/2‐siRNA prevents intracellular growth of Mycobacterium smegmatis in human macrophages. Previous study has found that activation of AKT prevents phagosome maturation (Hernandez et al., 2004). Inhibition of AKT1 can induce phagosome maturation to phagolysosomes, resulting in killing of intracellular bacteria (Kuijl et al., 2007). Therefore, AKT1 inhibitors have the potential to be used as antibiotics for the treatment of TB, especially drug‐resistant TB.
It is not clear whether polymorphisms in AKT1 gene affect susceptibility to TB. In this study, we investigated two single nucleotide polymorphisms (SNPs) of AKT1 gene and related haplotypes in case–control sample from Chinese population and found association with susceptibility to pulmonary TB.
Materials and methods
Subjects
A total of 101 Chinese patients of Han ethnic group (58 men and 43 women; mean age 41.6 ± 12.7 years) with pulmonary TB were enrolled in the study. They were inpatients at the Institute of Tuberculosis, 309 Hospital, Beijing, China, and were diagnosed as pulmonary TB based on results from acid fast staining of sputum smear, bacterial culture of mycobacteria, chest X‐ray examination, as well as clinical symptoms and responses to standard anti‐TB chemotherapy regimens. A total of 106 age‐ and gender‐matched people (64 men and 42 women; mean age 39.2 ± 11.7 years) who underwent regular health check‐up were enrolled as controls. The criteria for selecting controls were as follows: (1) only people of Chinese Han ethnic group were selected; (2) with age between 20 and 60 years; (3) male/female ratio was similar to TB patient group; (4) people with obesity (body mass index, BMI > 25 kg m−2) were excluded from controls; (5) control individuals were selected randomly. All of the control individuals had normal chest X‐ray appearance and had no history of TB. The patients and controls were HIV negative and none was known to present any immunosuppressive condition. The study was approved by the Ethics Committee of the 309 Hospital and informed consent was obtained from all patients.
SNPs and genotyping
The whole blood was treated with ethylenediaminetetraacetic acid to prevent coagulation. The DNA was isolated from whole blood with a blood DNA extraction kit, according to the protocol provided by the manufacturer (Tiangen, China). The genomic DNA was dissolved in TE buffer and stored at −20°C until needed.
We assessed two AKT1 SNPs, rs3730358 and rs1130233, in the study. rs3730358, located at +18 position at intron 3 of AKT1 genomic sequence (Genbank accession no. NC_000014), was designated as IVS3+18C>T SNP (Fig. 1). rs1130233 was located at +726 site of the AKT1 gene‐coding sequence and was designated +726G>A SNP (Fig. 1).
Figure 1.
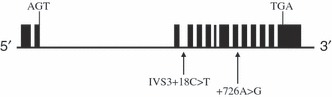
Genomic structure of the AKT1 locus and location of SNPs and haplotype. Filled box represents exons of Akt1 genomic sequence. The distance between the two SNPs is 6513 bp.
Single nucleotide polymorphism genotyping was performed by polymerase chain reaction amplification (PCR)‐restriction fragment length polymorphism assays, following the protocol provided by Dr Nadao Iwata, Fujita Health University School of Medicine, Nagoya, Japan (Ikeda et al., 2004), with modification in the PCR amplification procedure. For genotyping of IVS3+18C>T, a nested PCR was applied. The inner primer sets were 5′‐ATGGCACCTTCATTGGCTAC‐3′ and 5′‐AGAGGGCTCCAGCCAACC‐3′, and the outer primer sets were 5′‐AGTCTGCCTTCCCGTTGACCCA‐3′ and 5′‐TAGGACTCAGCCTGGAGACTCCCA‐3′. The size of the PCR product amplified with the inner primers is 138 bp. The primer sets for 726G>A SNP were 5′‐TCCTGCTGAGTTAGGGCTTC‐3′ and 5′‐CACAATCTCAGCGCCATAGA‐3′ and the resulting PCR product is 236 bp long. The restriction enzyme HaeIII was used for IVS3+18C>T genotyping and HpyCH4IV for 726G>A.
Haplotypes were analysed by UNPHASED program that are based on likelihood ratio tests in a log‐linear model for association study (Dudbridge, 2008).
Statistical analysis
Chi‐square test or t test was used to evaluate the differences in demographic variable with statistical package spss 13 for windows (SPSS Inc., Chicago, IL, USA). Statistical corrections of P values in multiple tests were performed by the Bonferroni corrections. The odds ratio (OR) and P values were calculated by unconditional logistic regression with statistical package spss 13. Hardy–Weiberg equilibrium was assessed with HAPLOVIEW program v3.2 (Barrett et al., 2005). Marker‐trait association analysis including genotype analysis and allele frequencies were evaluated with UNPHASED program v3.0.12 (Dudbridge, 2008). In haplotypic and single‐marker allelewise analysis, the permutation analysis was used. We performed 10 000 permutations for each analysis. The significance level for all statistical tests was 0.05.
Results and discussion
This study included 101 TB patients (58 men and 43 women; mean age 41.6 ± 12.7 years) and 106 age‐, gender‐ and ethnically matched control subjects (64 men and 42 women; mean age 39.2 ± 11.7 years). All of them are Chinese Han ethnic group. The demographic characteristics of patients with TB and healthy control individuals are given in Table 1. Occurrence of pulmonary TB was not significantly associated with age (P = 0.652), gender (P = 0.774), exposure to TB patients (P = 0.240) and BMI (P = 0.457) in the population studied.
Table 1.
The demographic data of TB patients and controls
| TB patients | Controls | P‐values | |
|---|---|---|---|
| Age (years), mean ± SD | 41.6 ± 12.7 | 39.2 ± 11.7 | 0.652 |
| Gender, n (%) | |||
| Male | 58 (57.4%) | 64 (60.4%) | 0.774 |
| Female | 43 (42.6%) | 42 (39.6%) | |
| Exposure to TB patients | |||
| Yes | 13 (12.9%) | 8 (7.5%) | 0.240 |
| No | 88 (87.1) | 98 (92.5%) | |
| BMI | |||
| <21 kg m−2 | 20 (19.8%) | 16 (15.1%) | 0.457 |
| 21–25 kg m−2 | 81 (80.2%) | 90 (84.9%) | |
BMI, body mass index; TB, tuberculosis.
The IVS3+18C>T and +726G>A SNPs were selected for association study for two reasons (Fig. 1). First, previous study has demonstrated that the IVS3+18C>T–+726G>A haplotype was associated with different expression level of AKT1 protein. The CC/GG major haplotype had significantly higher AKT1 expression and lower apoptosis of the cells than the minor TT/AA haplotype (Harris et al., 2005). Secondly, they are located in the middle part of AKT1 genomic sequence with five exons and a distance of 6513 bp between the two SNPs. Therefore, these polymorphisms might relate to functional changes of AKT1 in cells.
The genotype frequencies of the two SNPs were in Hardy–Weinberg equilibrium. Analysis of IVS3+18C>T SNP showed that the patients with pulmonary TB had significantly lower C/C homozygous genotype (P = 0.031) and higher C/T heterozygous genotype (P = 0.028) compared with age‐, gender‐ and ethnically matched controls (Table 2). The minor T/T homozygous genotype had one person in each group and its significant could not be determined. Allele frequencies of IVS3+18 C and T also demonstrated significant difference between the case and control groups (P = 0.043) (Table 2). However, the difference of allele frequencies was not statistically significant when P value was adjusted by Bonferroni correction. The result indicates that IVS3+18 C/C homozygous genotype is negatively associated with pulmonary TB.
Table 2.
Distributions and allele frequencies of the IVS3+18C>T and +726G>A genotypes in patients with pulmonary tuberculosis
| SNPs | Case (n = 101) | Control (n = 106) | P‐valuesa | |
|---|---|---|---|---|
| IVS3+18C>T | Genotypes | |||
| C/C | 0.7624 | 0.8774 | 0.031 | |
| C/T | 0.2277 | 0.1132 | 0.028 | |
| T/T | 0.0099 | 0.0094 | nd | |
| Allele frequencies | ||||
| C | 0.8762 | 0.934 | 0.043 | |
| T | 0.1238 | 0.066 | ||
| +726G>A | Genotypes | |||
| G/G | 0.2079 | 0.3019 | 0.259 | |
| G/A | 0.5941 | 0.434 | 0.021 | |
| A/A | 0.198 | 0.2642 | 0.1215 | |
| Allele frequencies | ||||
| G | 0.505 | 0.5189 | 0.777 | |
| A | 0.495 | 0.4811 | ||
aUNPHASED program v3.0.12 was used for statistical analysis (Barrett et al., 2005).
nd, not determined; SNPs, sigle nucleotide polymorphisms.
+726G>A SNP is a synonymous polymorphism located in exon 9 of AKT1 genomic sequence. Although the +726 G/G homozygous genotype was much lower in patients with pulmonary TB than in controls (0.2079 vs. 0.3019), the difference was not statistically significant (P = 0.259; Table 2). In contrast, the patient group had significantly higher G/A heterozygous genotype than controls (0.5941 vs. 0.434; P = 0.021). The overall G and A allele frequencies were very similar in the two groups (P > 0.05; Table 2). The results are mixed and do not support an association of +726G>A SNP with pulmonary TB.
Next, we analysed the frequencies of IVS3+18C>T–+726G>A haplotype by UNPHASED program (Dudbridge, 2008; Fig. 1). There are four different haplotypes, and the major C–G and C–A, minor T–G haplotypes had similar frequency among case and controls (P > 0.05; Table 3). The minor T–A haplotype had significantly higher frequency in patients than in controls (P = 0.0216; Table 3), indicating a positive association with pulmonary TB.
Table 3.
Haplotype analysis of IVS3+18C>T and +726G>A in patients with pulmonary tuberculosis
| IVS3+18C>T–+726G>A haplotype | Haplotype frequencies | P‐valuesa | |
|---|---|---|---|
| Case | Control | ||
| C–G | 0.4183 | 0.4609 | 0.2862 |
| C–A | 0.4579 | 0.473 | 0.8844 |
| T–G | 0.0866 | 0.0579 | 0.1134 |
| T–A | 0.0372 | 0.0081 | 0.0216 |
aUNPHASED program v3.0.12 was used for statistical analysis (Barrett et al., 2005).
We performed logistic regression analysis on both the SNPs and TB patients’ clinical characteristics, including age, sputum smear/culture result, lung cavity and extra‐pulmonary TB. Only C/T heterozygous genotype on IVS3+18C>T SNP site was negatively associated with extra‐pulmonary TB in the patient group, although not significantly (OR = 2.714, 95% CI: 0.54–13.68).
The serine/theronine kinase AKT is a central node in cell signalling of cytokines and other cellular stimuli, and it functions downstream of phosphatidylinositol 3‐kinase (PI3K) (Manning & Cantley, 2007). Activated PI3K phosphorylates phosphatidylinositol‐4,5‐bisphosphate (PIP2) to generate phosphatidylinositol‐3,4,5‐trisphosphate (PIP3). PIP3 recruits AKT to the cell membrane that results in phosphorylation and activation of AKT and initiation of downstream signalling cascade. There are three isoforms of AKT: AKT1 (also known as PKBα), AKT2 (PKBβ) and AKT3 (PKBγ), which are encoded by separate genes. Targeted knock‐out of each of these isoforms in mice revealed both specific and redundant functions.
Recent studies have identified PI3K/Akt signalling pathway as a critical regulator of replication of a number of viruses, including influenza A virus, parainfluenza virus 5, myxoma virus, severe acute respiratory syndrome‐coronavirus (SARS‐CoV) and hepatitis B virus (Mizutani et al., 2005; Wang et al., 2006; Guo et al., 2007; Shin et al., 2007; Sun et al., 2008). Suppression of PI3K/Akt activation by specific small molecular inhibitor resulted in reduction of influenza A virus yield in human lung carcinoma cells (Shin et al., 2007). Akt pathway was shown to be a key restriction determinant for the permissiveness of the human cancer cells by myxoma virus (Wang et al., 2006). Inhibitors of PI3K/Akt and c‐Jun N‐terminal protein kinase (JNK) prevented establishment of persistent infection of SARS‐CoV in Vero E6 cells (Mizutani et al., 2005).
The Akt signalling pathway also plays an important role in invasion and replication of some bacteria. The group B streptococcus requires the PI3K/Akt signalling pathway for invasion of epithelial cells (Burnham et al., 2007). Akt1 inhibitors could significantly reduce growth of Salmonella typhimurium and M. tuberculosis in host cells through inducing phagosome maturation to phagolysosomes (Kuijl et al., 2007) and might be used as antibiotics for the treatment of infection.
Of the numerous AKT1 SNPs identified in humans, six of them, designated SNP1‐5 and A, are mostly studied and have been reported to be associated with several diseases, such as Parkinson’s disease (Xiromerisiou et al., 2008), Schizophrenia (Ikeda et al., 2004; Schwab et al., 2005) and biopolar pedigrees (Yoyota et al., 2003). Recently an AKT1 E17K somatic mutation was identified. This mutation leads to pathological localization to the plasma membrane and activates AKT1, and is implicated in human breast, colorectal and ovarian cancers (Carpten et al., 2007). To our knowledge, there is no any report on relationship of human AKT1 polymorphisms with susceptibility to TB. In this study, we found association of the IVS3+18C>T SNP and the T–A haplotype with pulmonary TB. Since +726G>A is a synonymous SNP, it is possible that IVS3+18C>T polymorphism might involve in the regulation of AKT1 expression and should be studied further. Previous studies have proved that SNPs can significantly alter the expression or activity of AKT1 (Harris et al., 2005; Carpten et al., 2007), it is possible that SNPs on the AKT1 locus might influence controlling of M. tuberculosis replication in humans and ultimately development of active disease.
The IVS3+18C>T–+726G>A haplotype result was based on comparison of haplotype frequencies in the case–control sample. Because there is high percentage of heterozygous genotypes in the patients and controls, the difference in frequencies may not directly related to the AKT1 expression level in cells as expected and interpretation of the result should be cautious.
In conclusion, two SNPs of AKT1 gene and the related haplotypes were investigated in a case–control sample from Chinese Han ethnic population, and the IVS3+18C>T SNP and the T–A haplotype were found to be associated with susceptibility to pulmonary TB. It might be a good candidate of genetic marker for the risk evaluation of TB.
Conflict of interest
The authors do not have a commercial or other association that might pose a conflict of interest.
Acknowledgements
The authors wish to thank Dr Nadao Iwata, Fujita Health University School of Medicine, Nagoya, Japan for providing us with the protocol used for genotyping of AKT1 SNPs. The study was supported by a grant for infectious diseases from the Ministry of Health and the Ministry of Science and Technology, China (2008ZX10003‐012) and a grant from Beijing Natural Science Foundation, China (7092100).
References
- Austin, C.M. , Ma, X. & Graviss, E.A. (2008) Common nonsynonymous polymorphisms in the NOD2 gene are associated with resistance or susceptibility to tuberculosis disease in African Americans. Journal of Infectious Diseases, 197, 1713. [DOI] [PubMed] [Google Scholar]
- Barrett, J.C. , Fry, B. , Maller, J. & Daly, M.J. (2005) Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics, 21, 263. [DOI] [PubMed] [Google Scholar]
- Burnham, C.A. , Shokoples, S.E. & Tyrrell, G.J. (2007) Invasion of HeLa cells by group B streptococcus requires the phosphoinositide 3‐kinase signaling pathway and modulates phosphorylation of host cell Akt and glycogen synthase kinase 3. Microbiology 153, 4240. [DOI] [PubMed] [Google Scholar]
- Carpten, J.D. , Faber, A.L. , Horn, C. , Donoho, G.P. , Briggs, S.L. , Robbins, C.M. et al. (2007) A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature, 448, 439. [DOI] [PubMed] [Google Scholar]
- Dudbridge, F. (2008) Likelihood‐based association analysis for nuclear families and unrelated subjects with missing genotype data. Human Heredity, 66, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, H. , Zhou, T. , Jiang, D. , Cuconati, A. , Xiao, G.H. , Block, T.M. et al. (2007) Regulation of hepatitis B virus replication by the phosphatidylinositol 3‐kinase‐akt signal transduction pathway. Journal of Virology, 81, 10071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, S.L. , Gil, G. , Robins, H. , Hu, W. , Hirshfield, K. , Bond, E. et al. (2005) Detection of functional single‐nucleotide polymorphisms that affect apoptosis. Proceedings of the National Academy of Sciences of the United States of America, 102, 16297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez, L.D. , Hueffer, K. , Wenk, M.R. & Galan, J.E. (2004) Salmonella modulates vesicular traffic by altering phosphoinositide metabolism. Science, 304, 1805. [DOI] [PubMed] [Google Scholar]
- Houben, E.N. , Nguyen, L. & Pieters, J. (2006) Interaction of pathogenic mycobacteria with the host immune system. Curr Opin Microbiol, 9, 76. [DOI] [PubMed] [Google Scholar]
- Ikeda, M. , Iwata, N. , Suzuki, T. , Kitajima, T. , Yamanouchi, Y. , Kinoshita, Y. et al. (2004) Association of AKT1 with Schizophrenia confirmed in a Japanese population. Biological Psychiatry, 56, 698. [DOI] [PubMed] [Google Scholar]
- Kuijl, C. , Savage, N.D.L. , Marsman, M. , Tuin, A.W. , Janssen, L. , Egan, D.A. et al. (2007) Intracellular bacterial growth is controlled by a kinase network around PKB/AKT1. Nature 450, 725. [DOI] [PubMed] [Google Scholar]
- Manning, B.D. & Cantley, L.C. (2007) AKT/PKB signaling: navigating downstream. Cell, 129, 1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani, T. , Fukushi, S. , Saijo, M. , Kurane, I. & Morikawa, S. (2005) JNK and PI3k/Akt signaling pathways are required for establishing persistent SARS‐CoV infection in Vero E6 cells. Biochimica et Biophysica Acta, 1741, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raviglione, M.C. (2003) The TB epidemic from 1992 to 2002. Tuberculosis (Edinb), 83, 4. [DOI] [PubMed] [Google Scholar]
- Schurr, E. (2007) Is susceptibility to tuberculosis acquired or inherited? Journal of Internal Medicine, 261, 106. [DOI] [PubMed] [Google Scholar]
- Schwab, S.G. , Hoefgen, B. , Hanses, C. , Hassenbach, M.B. , Albus, M. , Lerer, B. , Trixler, M. , Maier, W. & Wildenauer, D.B. (2005) Further evidence for association of variants in the AKT1 gene with Schizophrenia in a sample of European sib‐pair families. Biological Psychiatry, 58, 446. [DOI] [PubMed] [Google Scholar]
- Shin, Y.K. , Liu, Q. , Tikoo, S.K. , Babiuk, L.A. & Zhou, Y. (2007) Effect of the phosphatidylinositol 3‐kinase/Akt pathway on influenza A virus propagation. Journal of General Virology, 88, 942. [DOI] [PubMed] [Google Scholar]
- Sun, M. , Fuentes, S.M. , Timani, K. , Sun, D. , Murphy, C. , Lin, Y. , August, A. , Teng, M.N. & He, B. (2008) Akt plays a critical role in replication of non‐segmented negative stranded RNA viruses. Journal of Virology, 82, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, G. , Barrett, J.W. , Stanford, M. , Werden, S.J. , Johnston, J.B. , Gao, X. , Sun, M. , Cheng, J.Q. & McFadden, G. (2006) Infection of human cancer cells with myxoma virus requires Akt activation via interaction with a viral ankyrin‐repeat host range factor. Proceedings of the National Academy of Sciences of the United States of America, 103, 4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiromerisiou, G. , Hadjigeorgiou, G.M. , Papadimitriou, A. , Katsarogiannis, E. , Gourbali, V. & Singleton, A.B. (2008) Association between AKT1 gene and Parkinson’s disease: a protective haplotype. Neuroscience Letters, 436, 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoyota, T. , Yamada, K. , Detera‐ Wadleigh, S.D. & Yoshikawa, T. (2003) Analysis of a cluster of polymorphisms in AKT1 gene in bipolar pedigrees: a family‐based association study. Neuroscience Letters, 339, 5. [DOI] [PubMed] [Google Scholar]


