Abstract
Using four criteria proposed a decade ago by Brooks & McLennan to identify a case of adaptive radiation indicates that the evolutionary history of the viviparous clade of the Gyrodactylidae is dominated by nonvicariant processes. The viviparous clade, with 446 species, has significantly more species than its sister clade (one species), and high species richness was shown to be an apomorphic trait of only the viviparous gyrodactylids within the Gyrodactylidae. Reconciliation of the phylogenetic tree of the viviparous Gyrodactylidae with that of its hosts showed a low probability for cospeciation suggesting that adaptive modes of speciation and not vicariance were predominant during the historical diversification of the clade. The proposed hypothesis suggests that the Gyrodactylidae originated on the South American continent about 60 Mya after geographical dispersal and host switching of its common ancestor to demersal freshwater catfishes by a marine ancestor. Development of hyperviviparity and the consequent loss of ‘sticky’ eggs in conjunction with other symplesiomorphic and apomorphic features allowed rapid diversification coupled with high dispersal to new host groups and geographical areas by viviparous members of the Gyrodactylidae.
Introduction
Most members of the Gyrodactylidae are ectoparasitic on actinopterygian fishes and reproduce by hyperviviparity (Cohen 1977) in which a parent worm may concomitantly bear several generations in its uterus. At birth, the progenetic offspring is usually a pregnant worm with subsequent generations of worms already present in its uterus (see Cable & Harris 2002; and references therein). Gyrodactylids have a direct monoxenic life cycle, but contrary to most monogenoid species, transmission is via the preadult/adult stage. A free‐swimming ciliated larva, the oncomiracidium of most other monogenoids is absent in their life cycles.
Until recently, the Gyrodactylidae included only viviparous species. Harris (1983) proposed the Oogyrodactylidae for two species of egg‐laying monogenoids with otherwise general morphology similar to that of viviparous gyrodactylids; additional oviparous species (currently totalling nine species) have since been described (Kritsky & Boeger 1991; Boeger et al. 1994). The limits of the Gyrodactylidae were expanded by Boeger et al. (1994) after phylogenetic analysis indicated that the Oogyrodactylidae was paraphyletic and that its members should be included in the Gyrodactylidae as basal clades.
Oviparous gyrodactylids are restricted to South American freshwater catfishes mainly of the relatively recent Loricariidae (Siluriformes). Viviparous gyrodactylids, on the other hand, represent one of the most diverse and widespread taxons of Monogenoidea, with Gyrodactylus including 402 species worldwide and parasitizing fishes representing 19 teleost orders (Bakke et al. 2002). Viviparous gyrodactylids are found in freshwater, brackish‐water and marine environments and also occur on some cephalopods, crustaceans, and amphibians. Agnathans, chondrichthyans and terrestrial animals apparently do not serve as natural hosts.
The wide host and geographical distribution of the viviparous species and the occurrence of oviparous species as basal clades in the family pose an interesting evolutionary question. If the Gyrodactylidae originated relatively recently in continental South America as suggested by members of its basal clades occurring on loricariid catfishes that date back to only 66 Mya (Berg 1958), how did the viviparous lineage that developed from an oviparous ancestor become so diverse and widely distributed on different host groups in the various geographical regions and environments throughout the world? This paper tests the hypothesis that the viviparous Gyrodactylidae shows high diversification primarily as a consequence of extensive adaptive radiation. We accomplish this by evaluating the four criteria proposed by Brooks & McLennan (1993b) that they hypothesize must be met to identify a case of adaptive radiation:
‘(1) the group in question contains more species than its sister group; (2) species richness is a derived characteristic within the larger clade; (3) an apomorphic character present in the more species‐rich group enhances the potential that adaptively driven speciation (i.e. sympatric speciation or speciation by peripheral isolation) will occur, and (4) adaptively driven speciation modes played the dominant role in the speciation of the more species‐rich group.’ (Brooks & McLennan 1993b)
If met, the first two criteria indicate that the group shows high species richness that is a result of a historical increase in diversification (speciation minus extinction). That the increase in diversification is a consequence of adaptive radiation is supported if the last two criteria are met. The third criterion also allows recognition of possible key innovations linked to the increase in diversification of the clade.
Materials and methods
The phylogenies used in testing and development of the hypothesis on adaptive radiation in the clade of viviparous Gyrodactylidae were those of Boeger & Kritsky (2001) for the families of Gyrodactylidea, of Boeger et al. (1994) for the basal evolution of the Gyrodactylidae, and of Kritsky & Boeger (2003) for species of viviparous Gyrodactylidae. The number of species in each taxon was determined from the literature (Fig. 1; Table 1); corrections for taxonomic artifacts such as synonyms, misidentifications and nomina nuda, were not made. The host phylogeny is based on Lauder & Liem (1983) with modifications of internal clades made according to hypotheses offered by Forey et al. (1996), Fink & Fink (1996), and Johnson & Patterson (1996). Composite cladograms were constructed using the method of taxon substitution as described by Wilkinson et al. (2001).
Figure 1.
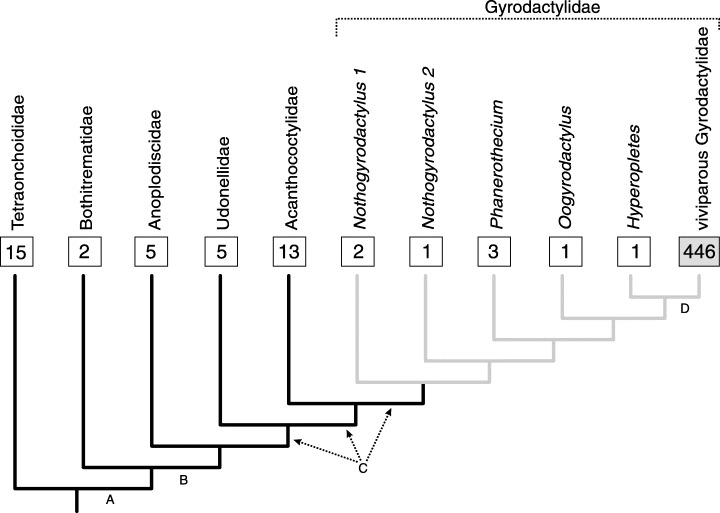
Composite cladogram for the Gyrodactylidea based on the phylogenetic hypotheses of Boeger & Kritsky (2001; black lines) and Boeger et al. (1994; grey lines). Numbers in squares refer to the determined number of described species for respective taxa; letters under branches of the cladogram refer to putative synapomorphies indicated in Table 5.
Table 1.
Number of species currently assigned to each of the genera* containing viviparous species within the Gyrodactylidae.
| Genus | Number of species |
|---|---|
| Acanthoplacatus | 7 |
| Accessorius | 1 |
| Anacanthocotyle | 1 |
| Archigyrodactylus | 3 |
| Fundulotrema | 6 |
| Gyrodactyloides | 5 |
| Gyrodactylus | 402 |
| Gyrdicotylus | 1 |
| Isancistrum | 1 |
| Lamniscus | 2 |
| Macrogyrodactylus | 5 |
| Metagyrodactylus | 1 |
| Paragyrodactylus | 2 |
| Polyclithrum | 5 |
| Scleroductus | 2 |
| Swingleus | 2 |
| TOTAL | 446 |
All listed genera exclusively comprise viviparous species.
Two methods were used to evaluate whether or not the viviparous lineage of Gyrodactylidae is associated with a significantly higher diversification (Criterion 1). The methods rely on phylogenetic relationships within the Gyrodactylidae (Fig. 1) since they represent statistical comparisons of the number of species of one clade with that of its respective sister group(s).
The first method to test Criterion 1 is based on the Markov model of lineage splitting proposed by Slowinski & Guyer (1993). Under this method, that centres on the comparative sizes of sister clades, the probability (P) of species richness in two sister groups being equal is given by the equation P = n−r/(n−1), where r is the number of species in the larger sister group, and n is the total number of species in both groups. The second set of analyses was performed using the estimation of diversification rates using maximum likelihood (ML). In this model, ML estimates the mean diversification rate of a clade based on its age (t) and current species richness (N), by treating clade growth as a pure birth process. The ML estimation (e) of diversification rate is e = ln(N)/t (see Purvis 1996); 95% confidence intervals for each estimate of diversification were calculated by −ln(1–0.975 1/N) and −ln(1–0.025 1/N) (equations suggested by S. Nee in Purvis 1996).
Sensitivity analyses were conducted to reduce the possibility of artifacts associated with potential error in the sister‐group relationships within the phylogenetic hypotheses, i.e. that synapomorphies may not be presently apparent that would support monophyly of the oviparous gyrodactylids. Three such sensitivity analyses were performed with each method where the number of species in the clade of viviparous Gyrodactylidae was compared to: (1) the number of species in its sister group (Hyperopletes); (2) the number of species in its sister group combined with that in the first immediate clade (Oogyrodactylus) towards the root of the phylogeny; and (3) the number of species in its sister group combined with those in the first two immediate clades (Oogyrodactylus and Phanerothecium) towards the root of the phylogeny (Fig. 1). An individual error rate (p) was calculated by applying the Bonferroni adjustment (P = p′/j) to the stepwise error rate (p′ = 0.05), where j= the number of hypothesis tests performed (see Morrison 2002).
The apomorphic status of the largest diversification of viviparous Gyrodactylidae was tested (Criterion 2) by optimizing species richness of clades comprising the Gyrodactylidae. The relative richness of each clade was determined by using the equation of Slowinski & Guyer (1993), but instead, comparing sister clades from the base of the cladogram towards the tip. A Bonferroni adjustment of the stepwise error rate (p′ = 0.05) was made. According to the result of the application of the equation on each sister‐group pair, the state of each clade was defined as low or high. The ancestral state of species richness for the inclusive clades was determined by optimizing the relative number of species (species richness) onto the phylogenetic tree of the Gyrodactylidae (Fig. 1) (method described in Brooks & McLennan 1991).
The relative amount of adaptive speciation among the viviparous Gyrodactylidae (Criterion 4) was estimated using Treemap (Page 1994). Treemap uses the method of tree reconciliation to maximize the number of cospeciation events (nonadaptive speciation) between the host and parasite trees (Page 1994; Paterson & Banks 2001). The calculated maximum number of cospeciation events resulting from tree reconciliation of the host and parasite phylogenies was then compared to the number of maximum cospeciation events obtained from 10 000 randomized cladograms of each of the host and parasite phylogenies.
Results
Does the clade of viviparous gyrodactylids include significantly more species than its sister group (Criterion 1)?
A total of 446 viviparous species of Gyrodactylidae has been described, whereas three or fewer species are known in each of the five basal clades of oviparous forms (Fig. 1). Tests with both the lineage splitting model and the estimation of diversification rates support the hypothesis that diversification in the viviparous clade is significantly higher than that of its sister group (Hyperopletes); sensitivity tests using the various combinations of possible sister groups also indicate significantly smaller species richness in these hypothetical sister groups than in the viviparous clade (2, 3).
Table 2.
Probability of observing symmetry in species number between the clade of viviparous species of Gyrodactylidae and its hypothesized sister groups.
| Sister‐groups compared | Probability* |
|---|---|
| Viviparous Gyrodactylidae vs. Hyperopletes | 0.0022 |
| Viviparous Gyrodactylidae vs. Hyperopletes + Oogyrodactylus | 0.0045 |
| Viviparous Gyrodactylidae vs. Hyperopletes + Oogyrodactylus + Phanerothecium | 0.011 |
Bonferroni Correction = 0.017.
Table 3.
Maximum likelihood estimates of diversification rates (*) of viviparous gyrodactylids and their postulated sister groups. Confidence Interval (CI) = 95%.
| Clade | No. of species | e | CI |
|---|---|---|---|
| Viviparous Gyrodactylidae | 446 | 6.1 | 4.80–9.78 |
| Hyperopletes | 1 | 0 | 0–3.69* |
| Hyperopletes + Oogyrodactylus | 2 | 0.69 | 0.172–4.38 |
| Hyperopletes + Oogyrodactylus + Phanerothecium | 5 | 1.61 | 0.65–5.29 |
Direct application of the formula provided in the text would generate a lower limit for the CI that is slightly higher than the ML estimate of the diversification rate. This occurs because the formula is an approximation which treats the ML estimate as a continuous random variable, whereas in reality the variable can only take on discrete values. As a result, the formula is appropriate for ‘reasonably’ sized clades but breaks down at the edges when very small clades are included (modified from a statement provided by S. Nee, pers.comm.).
While the number of species of viviparous gyrodactylids is large (n = 446) compared to its sister groups, the majority (90%) of these species is allocated to Gyrodactylus (n = 402); the number of species in the remaining viviparous genera varies between one and seven species each (Table 1). This may suggest that the diverse group is, in fact, Gyrodactylus, and not the entire lineage of viviparous species. However, taxonomy of the viviparous clade is problematic, and all available evidence suggests that while most of the other generic groups are monophyletic, i.e. supported by well‐defined synapomorphies, the same is not true for Gyrodactylus (see Kritsky & Boeger, 2003). Species presently allocated to Gyrodactylus do not share any exclusive derived character that would suggest immediate common ancestry.
Kritsky & Boeger (2003) included 35 species of Gyrodactylus and one species each of Gyrodactyloides, Gyrdicotylus, Acanthoplacatus and Fundulotrema as ingroup taxa in a preliminary phylogenetic analysis of the viviparous clade to test monophyly of Gyrodactylus. This analysis showed that Gyrodactylus lacks monophyly, with species of Acanthoplacatus and Fundulotrema having developed from respective ancestors within Gyrodactylus. In this analysis, which used molecular data, the clade Gyrdicotylus+Gyrodactyloides appears to be the sister group of the clade including species of Gyrodactylus, Acanthoplacatus and Fundulotrema (Fig. 2). Since the current classification scheme of viviparous gyrodactylids is apparently unnatural and Gyrodactylus paraphyletic, it appears likely that the entire viviparous group represents the diverse taxon rather than Gyrodactylus alone.
Figure 2.
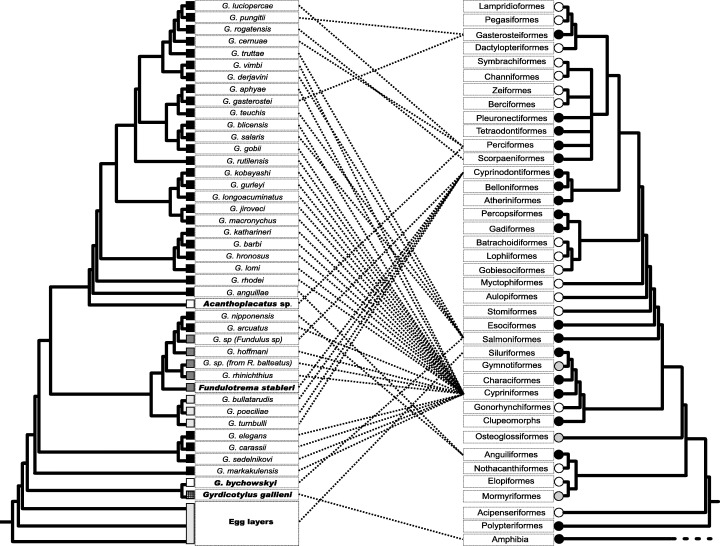
Host–parasite associations (dotted lines) of selected Gyrodactylidae. The parasite cladogram is a composite of the hypotheses of Kritsky & Boeger (2003) and Boeger et al. (1994). Bold indicates gyrodactylid species presently allocated to gyrodactylid genera other than Gyrodactylus; black squares indicate gyrodactylid species from Eurasia; white squares indicate species from the marine environment; dark grey squares indicate species from the Nearctic; light grey squares and rectangle indicate Neotropical species; cross‐hatched square indicates an Ethiopian species. The host cladogram is a composite developed from Lauder & Liem (1983), Forey et al. (1996), Fink & Fink (1996), and Johnson & Patterson (1996). Black circles refer to host taxa from which species of Gyrodactylidae have been reported and described; grey circles indicate host taxa from which species of Gyrodactylidae are reported but not described; white circles indicate host taxa from which no species of Gyrodactylidae has been reported or described.
Is species richness of the viviparous clade apomorphic within the Gyrodactylidae (Criterion 2)?
Stepwise application of the equation of Slowinski & Guyer (1993) for species richness for the oviparous and viviparous clades of Gyrodactylidae shows that the only taxon showing high species richness is the clade of viviparous gyrodactylids (Table 4). All other clades depict species richness significantly lower than their respective sister group. Optimization of the relative number of species of respective clades of the Gyrodactylidae (Fig. 1) shows that high species richness of the viviparous clade is apomorphic.
Table 4.
Probability of observing symmetry in species richness between sister groups within the Gyrodactylidae.
| Taxa Compared | n | r | n−r | P * |
|---|---|---|---|---|
| Viviparous vs. Hyperopletes | 447 | 446 | 1 | 0.0022 |
| Sister vs. Oogyrodactylus | 448 | 447 | 1 | 0.0022 |
| Sister vs. Phanerothecium | 451 | 448 | 3 | 0.0067 |
| Sister vs. Nothogyrodactylus – 2 | 452 | 451 | 1 | 0.0022 |
| Sister vs. Nothogyrodactylus– 1 | 454 | 452 | 2 | 0.0044 |
Bonferroni correction = 0.010.
Do apomorphic traits of the viviparous gyrodactylids enhance the potential for adaptively driven modes of speciation (Criterion 3)?
Boeger et al. (1994) identified three synapomorphies, all associated with reproduction and/or the reproductive system, that define the hypothetical common ancestor of the viviparous gyrodactylids: (1) copulatory sac modified into a muscular bulb; (2) mode of reproduction shifted from oviparity to viviparity (hyperviviparity); and (3) loss of Mehlis’ gland. While it is difficult to visualize how modification of the copulatory organ into a muscular bulb and loss of Mehlis’ gland could lead to adaptive speciation, the remaining synapomorphy in conjunction with other plesiomorphic and apomorphic features (Table 5) appear to form a suite of traits that could enhance adaptively driven modes within the viviparous clade. Pre‐existing (symplesiomorphic) features of viviparous gyrodactylids, i.e. ectoparasitism, loss of the free‐living oncomiracidium, a monoxenic life cycle and transmission by preadult/adult stages, did not together or individually lead to or promote adaptive speciation but apparently were important antecedents.
Table 5.
Putative plesiomorphic and apomorphic traits considered liable for adaptive radiation of the viviparous gyrodactylids.
| Character change (See Fig. 1) | Character | Evolutionary status (viviparous clade) | Relative adaptive advantage |
|---|---|---|---|
| A | Ectoparasitism | Symplesiomorphic | Direct access to new hosts |
| A | Monoxenic life cycle | Symplesiomorphic | Less constraint on host switching |
| B | Loss of oncomiracidium | Symplesiomorphic | Increased survival of offspring; quick maturation |
| C | Preadult/adult transmission | Symplesiomorphic | Reduced mortality during transmission |
| D | Progenetic development | Apomorphic | Accelerated reproductive activity |
| D | Loss of sticky egg* | Apomorphic | Release of constraints associated with host groups |
| D | Hyperviviparity | Apomorphic | Accelerated establishment of reproductively active infrapopulation |
| D | Protogyny | Apomorphic | Accelerated maturation and reproductive activity |
| D | Parthenogenesis | Apomorphic | Release of required exchange of gametes when population levels are small; colonization of new host accomplished by single worm |
A consequence of development of hyperviviparity.
Species of Udonellidae, Acanthocotylidae and the basal oviparous groups of Gyrodactylidae produce an egg with an adhesive droplet at or near the tip of the proximal polar filament (Boeger & Kritsky 2001). The droplet serves to secure eggs individually or as clusters on hard surfaces where embryonic development occurs (Kearn 1986, 1998). Hard surfaces may include those provided by the host, such as the exoskeleton of a parasitic copepod in the case of udonellids (Aken’Ova & Lester 1996) and the dermal bones of catfishes in oviparous gyrodactylids (Boeger et al. 1994); species of Acanthocotylidae may utilize other substrates such as sand grains or rocks or the eggs may be retained on the body of the parent worm (Macdonald & Llewellyn 1980; Kearn 1993). In all of these families, the ecloded larva lacks cilia and thus is incapable of actively seeking a new host. [It is not clear if the morphological stage that eclodes from the eggs of udonellids, acanthocotylids and oviparous gyrodactylids is a semaphoront comparable to the oncomiracidum or represents a stage more advanced in ontogeny. The term ‘larva’ is used to represent the stage of ontogenetic development that eclodes from the egg regardless of developmental stage.] It either autoinfests the host of its parent or is passively transmitted. In the latter case, natural hosts are usually demersal, and/or either the larva or the adult parasite may show behavioural adaptations that increase the chance of transmission (see Kearn 1998; Cable et al. 2002).
Development of the adhesive droplet on the filament of the egg in the common ancestor of the Udonellidae + Acanthocotylidae + Gyrodactylidae apparently increased survival potential of the larva and thereby success of transmission to another natural host. However, this character likely reduced ability for dispersal to new host species by limiting the spectrum of possible target hosts to those with hard surfaces or with specific behavioural characteristics. This hypothesis is supported by the limited array of host groups parasitized by members of the Udonellidae (caligid copepods), Acanthocotylidae (demersal/gregarious rays, teleosts and agnathans) and the oviparous Gyrodactylidae (demersal/armored catfishes). In those taxa in which the eggs are cemented to the hard surfaces of the host (udonellids and oviparous gyrodactylids), host switching appears to be even more limited than in the Acanthocotylidae where the eggs are deposited on hard surfaces not necessarily associated with the host. The egg‐laying behaviour of acanthocotylids likely increases somewhat the spectrum of demersal fishes that could serve as hosts for the group through host switching.
The symplesiomorphic features of the Gyrodactylidae did not promote adaptive speciation probably because of the strong host‐switching constraint conferred by the ‘sticky’ egg. However, development of viviparity with the consequent loss of the egg shell and adhesive droplet and which may also reflect the loss of Mehlis’ gland, released descendant species from the dispersal limitations associated with the egg. No longer constrained by egg type, the viviparous lineage of gyrodactylids apparently increased its ability to disperse to new host groups that exhibit a wider range of morphologic and ecological characteristics.
Absence of a free‐swimming oncomiracidium in life cycles increases the chances of autoinfestation (Llewellyn 1981; Tinsley 1983; Kearn 1986). Hatching delayed until embryos are well developed decreases exposure to hazards of the external environment and those associated with transfer by short‐lived larvae to new hosts (Llewellyn 1968, 1981). Since the transmission to new hosts by udonellids, acanthocotylids and gyrodactylids is accomplished by preadult or adult forms, transmission is not limited to a single event in the worm's lifetime, a clear adaptive advantage over the plesiomorphic once‐in‐a‐life‐time transmission by a larval stage.
Among other putative advantages of transmission by the preadult/adult stage (over larval transmission) is the ability of a worm population to survive host‐defence mechanisms or death of the host. Acquired host‐defence mechanisms are known to exist against monogenoids (Buchmann & Lindenstrøm 2002; and references therein) and have been held responsible for the observed declines in infrapopulations of gyrodactylids on individual hosts (Scott & Anderson 1984). Further, Gyrodactylus salaris, and probably most gyrodactylids, can survive for extended periods as adults on host carcasses (Harris 1980) or on the bottoms of lakes and rivers, while retaining a potential to re‐infest new host specimens (Bakke et al. 1992). This ability of moving from one host to another in order to evade consequences of host defensive responses or its death (see also Cable et al. 2002), could be an important attribute that favours colonization of a new host species by increasing probability of survival of the founder/seed and its descendant population.
The success of host switching with subsequent acquisition of identity (speciation) was apparently increased by development of new features (apomorphies) in the ancestor of the viviparous lineage. Viviparity (hyperviviparity) appears to have greatly facilitated successful colonization of new hosts by accelerating growth of the invading infrapopulation. Compared to that of their oviparous sister groups, embryonic development in viviparous gyrodactylids is extended and protected in the uterus, while progenesis and protogyny of these helminths accelerate their maturation and reproduction. Offspring are born as adult or near adult females and may reproduce as early as one day after birth (Scott 1982). These apomorphic characters (hyperviviparity, progenesis and protogyny) could have enhanced the probability for successful new host invasions during the evolutionary development of the viviparous clade.
Hyperviviparity appears especially significant in light of recent studies suggesting that initial reproduction of viviparous species is asexual, probably parthenogenetic, when invading a new uninfected natural host (Harris 1989, 1998; Cable & Harris 2002). Parthenogenesis releases the need for exchange of gametes with sexual partners to reproduce and is thus considered an important adaptation that enables generation of populations in the event of migrations to new areas (or hosts) by a single individual (White 1978). For example, an individual of G. bullatarudis can rapidly ‘colonize’ its host, Poecilia reticulata, producing infrapopulations that may reach up to 100 parasites in 14 days (Scott 1982); other experiments have suggested similar rapid establishment on previously uninfected hosts (Cusack 1986; Buchmann & Uldal 1997).
Although parthenogenesis is generally considered a ‘dead‐end’ in long‐term evolution, the adaptive capacity and ability to diversify in relatively brief periods is considerable for parthenogenetic organisms (White 1978). The dominant mode of reproduction in the populations of many species of Gyrodactylus appears to be density dependent, with asexual modes being more prevalent in populations with low densities and sexual reproduction becoming more prevalent when infrapopulational densities increase (Harris 1989). As suggested by White (1978): ‘in theory, genetical systems including both thelytokous (= parthenogenesis) and sexual reproduction might be expected to combine the evolutionary advantages of each system.’ Indeed, species of viviparous Gyrodactylidae are likely capable of taking advantage of asexual reproduction when initially colonizing a new host (low density) and then switching to sexual reproduction as the infrapopulation increases.
A single compatible worm would be capable of rapidly colonizing a new susceptible host species by producing an infrapopulation that, without further immigration, is composed of genetically similar organisms produced through asexual means (see Harris 1998). Genetic drift would likely result in differentiation following host switching, since colonization can be obtained by one or few members from an original population. While parthenogenesis and inbreeding may have a negative impact on the evolution of a population in the long term, they also may lead to early conservation of highly specialized characters that would allow continued infestation of the new host species (Llewellyn 1981; Tinsley 1983). As the new population grows, sexual reproduction between members of the clone would become more prevalent, eventually resulting in desired genetic diversification.
The combination of plesiomorphic and apomorphic characters coexisting in the common ancestor appears to have provided the viviparous Gyrodactylidae with a high potential for speciation by host switching. While survival of the offspring and success of transmission are greatly increased, colonization of new host specimens (autoinfestation or transmission) and species (host switching) is facilitated by rapid populational growth and by the potential that colonization can be successfully attained by a single worm due to its ability to reproduce asexually. Isolation of infrapopulations may be instantaneous if the newly colonized host possesses ecological or behavioural differences from those of the ancestral host species. Genetic drift associated with asexual fixation of characters and followed by sexual inbreeding, would certainly be important for differentiation and rapid definition of identity (sensu Wiley 1980) of the potential new species.
Have adaptively driven modes of speciation played the dominant role in speciation of the viviparous Gyrodactylidae (Criterion 4)?
Nine cospeciation events were postulated through tree reconciliation with Treemap for the phylogenetic hypothesis of the viviparous Gyrodactylidae and that of the orders of their hosts. Although Treemap optimizes cospeciation, the probability of obtaining nine or fewer cospeciation events was only P= 0.0134 in the 10 000 randomizations. The low probability for cospeciation suggests that adaptive modes of speciation and not vicariance were predominant in historical diversification of the viviparous clade.
Contrasting the phylogenetic hypothesis for gyrodactylids with the composite host cladogram suggests that formation of peripheral isolates through both host switching and geographical dispersal were important during the evolutionary development of the viviparous clade (Fig. 2). Despite obvious sample bias in the parasite tree (i.e. nearly all available molecular sequences are of European species; the number of gyrodactylid species is small [< 10%] compared to the total number known; and only 5 of 16 genera of viviparous gyrodactylids are represented in the analysis), instances of host switching and geographical dispersal by viviparous gyrodactylids are suggested in many of the minor clades in the cladogram (2, 3). Examples include the clades formed by Gyrodactyloides bychowskii and Gyrdicotylus gallieni and by Gyrodactylus nipponensis, G. arcuatus, Gyrodactylus sp. (from F. kansae), G. hoffmani, Gyrodactylus sp. (from R. balteatus), G. rhinichthius, Fundulotrema stableri, G. bullatarudis, G. poeciliae and G. turnbulli; respective species of these two clades occur in both marine and freshwater environments, different continents or oceans, and in distantly related host groups. In addition, species in the terminal clade comprising G. luciopercae, G. pungitii, G. rogatensis, G. cernuae, G. truttae, G. vimbi and G. derjavini are from hosts representing five distantly related orders of fishes. Although cospeciation is possible in several clades [(G. sedelnikova + G. carassii + G. elegans) (G. lomi + G. hronosus + G. barbi + G. katharineri) (G. macronychus + G. jiroveci + G. longoacuminatus + G. gurleyi + G. kobayashi), and (G. turnbulli + G. poeciliae + G. bullatarudis)], host switching and geographical dispersal cannot be totally discounted in these lineages as well.
Figure 3.
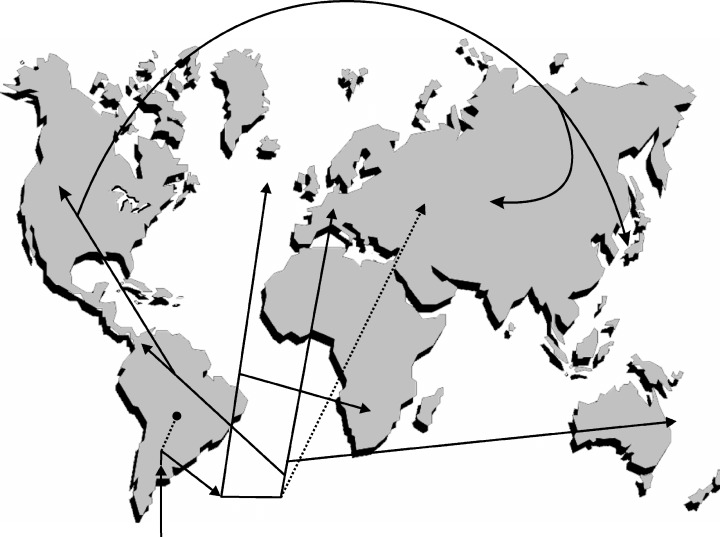
Postulated dispersal pathways for the clades of gyrodactylids depicted in Figure 2. Origin of the Gyrodactylidae is thought to have occurred after dispersal of a marine ancestor to a freshwater catfish in South America; early diversification of the family likely occurred in the continental waters of South America with subsequent dispersals to oceans and other land masses. Solid lines indicate putative monophyletic clades; dotted lines indicate paraphyletic grades.
Discussion
An explanation for the ubiquity of viviparous gyrodactylids rests within the context of their historical development as one of the most successful groups of platyhelminth parasites of vertebrates. In the present paper, a hypothesis is presented which states that the success of the group lies primarily with an evolutionary history dominated by adaptive processes linked to development of a suite of morphological and ecological characters in their ancestors. These features, both symplesiomorphic and apomorphic for the viviparous clade, interacted and fostered host switching and geographical dispersal followed by peripheral speciation. The characters included the symplesiomorphic features of ectoparasitism, a monoxenic life cycle, loss of the oncomiracidium and development of a preadult/adult transmission strategy; apomorphies included progenetic development, hyperviviparity, protogyny, parthenogenesis, and the loss of the ‘sticky’ eggs (Table 5).
The proposed model for the mechanisms involved in the adaptive radiation of viviparous gyrodactlids is provided in Fig. 4. The model suggests that hosts harbouring one (or more) genetically diverse species of viviparous Gyrodactylidae have ample opportunities for infesting other sympatric host species (1st level, upper portion). In most cases, these host transfers result in extinction of the invading lineage because of the inability of the invader or its descendants to adapt to the environmental conditions imposed by the new host (2nd level, upper portion). However, should the invader possess appropriate genetic and/or morphological attributes for compatibility, the parasite rapidly colonizes the newly invaded host, undergoing genetic drift by parthenogenesis followed by sexual reproduction to form a less genetically diverse, albeit adapted, population. Features that allowed successful host switching quickly become fixed in the parasite population. Subsequent mutation and selection eventually results in genetic diversification and speciation as age of the new relationship increases (3rd level, upper portion); cospeciation between the new parasite lineage and that of the acquired host could subsequently occur. This scenario of host switching followed by peripheral speciation in other hosts is often repeated during development of respective gyrodactylid clades (4th level, upper portion). A similar scenario also occurs if a host and its parasites disperse geographically (lower portion of Fig. 4). Initial reduction in genetic and/or morphological variability due to inability of most variants to survive in the new environment occurs. However, as the parasite‐host system ages in the new area, the same mechanisms described above will promote variability, host switching and peripheral speciation among gyrodactylid lineages.
Figure 4.
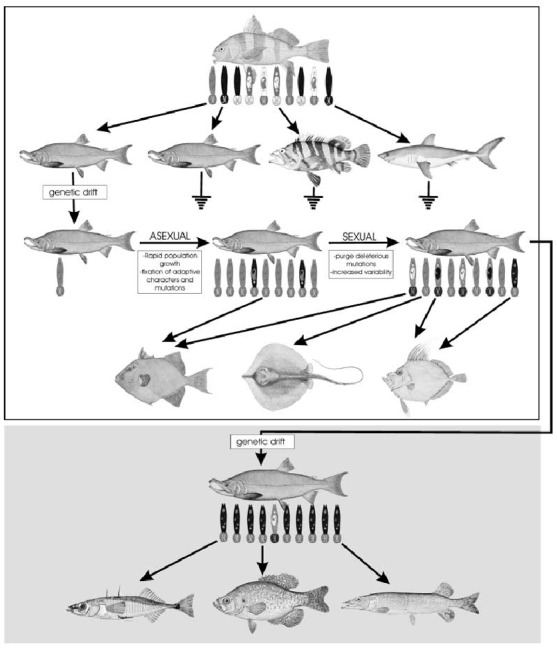
Proposed model for the mechanisms involved in the adaptive radiation of the viviparous gyrodactylids. See Discussion for explanation. Images of fishes are from the National Oceanic and Atmospheric Administration Photo Library, Historic Image Collection, United States Department of Commerce (http://www.nmfs.noaa.gov/image_gallery.htm).
According to available phylogenetic hypotheses, the Gyrodactylidae originated in the freshwater environments of South America as parasites of armoured catfishes (Loricariidae), a primary freshwater family restricted to the Neotropical Region. Origin of the Gyrodactylidae was apparently associated with a host‐switching event by an oviparous marine ancestor that colonized freshwater after the separation of South America from Africa. That the invading ancestor was marine and oviparous is supported by the phylogenetic hypothesis of Boeger & Kritsky (2001), who indicated that all other gyrodactylidean families (Tetraonchoididae, Bothitrematidae, Anoplodiscidae, Udonellidae, and Acanthocotylidae), which include only oviparous monogenoids parasitic on marine hosts, are either basal to or form the sister group of the Gyrodactylidae. Berg (1958) indicates that the earliest known fossils of loricariid catfishes (hosts to members of the basal oviparous groups of Gyrodactylidae) date back to only 66 Mya. By this time, South America was isolated from Africa as well as from all other continental landmasses. While oviparous gyrodactylids from non‐Neotropical hosts would be expected if the Gyrodactylidae had originated prior to the fragmentation of Gondwanaland, no oviparous species is known from native hosts outside the Neotropics. Extinction could explain the absence of oviparous gyrodactylids in Africa and/or other regions outside South America, but such an extensive event by parasite species is unlikely to have occurred.
The viviparous lineage of gyrodactylids is apparently monophyletic (Kritsky & Boeger, 2003). Unlike their oviparous counterparts, viviparous gyrodactylids occur on fishes representing a large number of families and orders from all of the world's oceans and continents (except perhaps Antarctica). If oviparous gyrodactylids gave rise to the viviparous clade as the phylogeny suggests (Fig. 1), origin of the viviparous clade probably also occurred in South America. Once freed of the limiting features associated with their egg‐laying ancestors, the early viviparous gyrodactylids became capable of dispersing into new hosts and new environments where peripheral speciation became possible. Indeed, phylogenetic analyses of comparatively few of the total number of viviparous species known to science (Fig. 2), suggests a complex pattern of dispersal and speciation of the viviparous gyrodactylids throughout the world (Fig. 3).
Brooks & McLennan (1993a) postulated that the evolution of direct life cycles and autoinfection influenced the rates of adaptively driven speciation in monogenoids and suggested that development of viviparity in gyrodactylids provides additional support for this hypothesis. However, Lydeard (1993) found little evidence to support viviparity influencing diversification in actinopterygean fishes when comparing several clades of fishes in which viviparity had independently developed. He (1993) suggested that ‘… adherence to a particular taxon's success being due to the evolution of viviparity may be nothing more than wishful thinking.’ Although viviparity is a synapomorphy of the viviparous clade of gyrodactylids, we also cannot say that this mode of reproduction by itself represents the key innovation for diversification in the clade. It appears, however, that the success of the viviparous gyrodactylids is probably due to a suite of characters, both plesiomorphic and apomorphic, that fortuitously coexisted in the common ancestor of the clade (Table 5).
The above hypothesis for the evolutionary history of the Gyrodactylidae allows some interesting predictions that could be used to test its validity. The hypothesis suggests that the oviparous gyrodactylids are more constrained than their viviparous counterparts regarding their ability to undergo host switching. It would be expected therefore that coevolutionary analyses will show that cospeciation (vicariant speciation) will be more prevalent in the diversification of the oviparous grade than in the viviparous clade. Further, the basal lineages of the viviparous species should be found on freshwater hosts in South America, while the oviparous gyrodactylids should be restricted to the Neotropics unless, in the unlikely event, dispersal to other regions by their demersal loracariid hosts can be demonstrated.
The hypothesis further suggests that the sister groups of monophyletic clades of viviparous gyrodactylids that occur on continents other than South America should be found among parasites of marine and/or brackish water hosts because most dispersion to these regions probably occurred via the marine environment. The general pattern of distribution and phylogeny resulting from the fragmentation of Pangea and Gondwanaland is not expected to occur. In addition, the freshwater species of each continent are expected to be more closely related to each other than to species of another continent irrespective of host occurrence. However, cases of dispersal between contiguous continents and multiple freshwater invasion of individual land masses may obscure this predicted pattern. In any case, spatial and ecological barriers will likely be more important than phylogenetic distance of host species in explaining extant host–parasite associations.
Acknowledgements
The authors gratefully acknowledge the following individual and agencies for assistance and support during the present study. Dr Sean Nee, University of Edinburgh, Scotland, provided valuable consultation concerning the use of the confidence intervals he proposed and which we used in our maximum likelihood analyses. CAPES/Brasilia, Brazil, supported MRP and the Conselho Nacional de Desenvolvimento Cientifíco e Tecnólogico (CNPq), Brazil, provided financial support to WAB during conduct of this study.
References
- Aken’Ova, T. O. & Lester, R. J. G. (1996). Udonella myliobati n. comb. (Platyhelminthes: Udonellidae) and its occurrence in eastern Australia. Journal of Parasitology, 82, 1017–1023. [PubMed] [Google Scholar]
- Bakke, T. A. , Harris, P. D. & Cable, J. (2002). Host specificity dynamics: observations on gyrodactylid monogeneans. International Journal for Parasitology, 32, 281–308. [DOI] [PubMed] [Google Scholar]
- Bakke, T. A. , Harris, P. D. , Jansen, P. A. & Hansen, L. P. (1992). Host specificity and dispersal strategy in gyrodactylid monogeneans, with particular reference to Gyrodactylus salaris (Platyhelminthes, Monogenea). Diseases of Aquatic Organisms, 13, 63–74. [Google Scholar]
- Berg, L. S. (1958). System de Rezenten und Fossilen Fischaratigen und Fische. Berlin: Veb. Deutscher Verlag der Wissenschaften. [Google Scholar]
- Boeger, W. A. & Kritsky, D. C. (2001). Phylogenetic relationships of the Monogenoidea In Littlewood D. T. J. & Bray R. A. (Eds) Interrelationships of the Platyhelminthes, pp. 92–102. London: Taylor & Francis. [Google Scholar]
- Boeger, W. A. , Kritsky, D. C. & Belmont‐Jégu, E. (1994). Neotropical Monogenoidea. 20. Two new species of oviparous Gyrodactylidea (Polyonchoinea) from loricariid catfishes (Siluriformes) in Brazil and the phylogenetic status of Ooegyrodactylidae Harris, 1983. Journal of the Helminthological Society of Washington, 61, 34–44. [Google Scholar]
- Brooks, D. R. & McLennan, D. A. (1991). Phylogeny, Ecology, and Behavior A Research Program in Comparative Biology. Chicago: The University of Chicago Press. [Google Scholar]
- Brooks, D. R. & McLennan, D. A. (1993a). Parascript Parasites and the Language of Evolution. Washington DC: Smithsonian Institution Press. [Google Scholar]
- Brooks, D. R. & McLennan, D. A. (1993b). Comparative study of adaptive radiation with an example using parasitic flatworms (Platyhelminthes: Cercomeria). American Naturalist, 142, 755–778. [DOI] [PubMed] [Google Scholar]
- Buchmann, K. & Lindenstrøm, T. (2002). Interactions between monogenean parasites and their fish hosts. International Journal for Parasitology, 32, 309–319. [DOI] [PubMed] [Google Scholar]
- Buchmann, K. & Uldal, A. (1997). Gyrodactylus derjavini infections in four salmonids: comparative host susceptibility and site selection of parasites. Diseases of Aquatic Organisms, 28, 201–209. [Google Scholar]
- Cable, J. & Harris, P. D. (2002). Gyrodactylid developmental biology: historical review, current status and future trends. International Journal for Parasitology, 32, 255–280. [DOI] [PubMed] [Google Scholar]
- Cable, J. , Scott, E. C. G. , Tinsley, R. C. & Harris, P. D. (2002). Behavior favoring transmission in the viviparous monogenean Gyrodactylus turnbulli . Journal of Parasitology, 88, 183–184. [DOI] [PubMed] [Google Scholar]
- Cohen, J. (1977).Reproduction. London: Butterworth. [Google Scholar]
- Cusack, R. (1986). Development of infections of Gyrodactylus colemanensis Mizelle and Kritsky, 1967 (Monogenea) and the effect on fry of Salmo gairdneri Richardson. Journal of Parasitology, 72, 663–668. [PubMed] [Google Scholar]
- Fink, S. V. & Fink, W. L. (1996). Interrelationships of ostariophysan fishes (Teletostei) In Stiassny M. L. J., Parenti L. R. & Johnson G. D. (Eds) Interrelationships of Fishes, pp. 209–249. San Diego: Academic Press. [Google Scholar]
- Forey, P. L. , Littlewood, D. T. J. , Ritchie, P. & Meyer, A. (1996). Interrelationships of elopomorph fishes In Stiassny M. L. J., Parenti L. R. & Johnson G. D. (Eds) Interrelationships of Fishes, pp. 175–191. San Diego: Academic Press. [Google Scholar]
- Harris, P. D. (1980). The behavior of Gyrodactylus on living hosts. Proceedings of the 3rd European Multicolloqium of Parasitology. Cambridge.
- Harris, P. D. (1983). The morphology and life cycle of the oviparous Oögyrodactylus farlowellae gen. et sp. n. (Monogenea, Gyrodactylidea). Parasitology, 87, 405–420. [Google Scholar]
- Harris, P. D. (1989). Interactions between population growth and sexual reproduction in the viviparous monogenean Gyrodactylus turnbulli Harris, 1986 from the guppy Poecilia reticulata Peters. Parasitology, 98, 245–251. [Google Scholar]
- Harris, P. D. (1998). Ecological and genetic evidence for clonal reproduction in Gyrodactylus gasterostei Glaser, 1974. International Journal for Parasitology, 28, 1595–1607. [DOI] [PubMed] [Google Scholar]
- Johnson, G. D. & Patterson, C. (1996). Relationships of lower euteleostean fishes In Stiassny M. L. J., Parenti L. R. & Johnson G. D. (Eds) Interrelationships of Fishes, pp. 251–332. San Diego: Academic Press. [Google Scholar]
- Kearn, G. C. (1986). The eggs of monogeneans. Advances in Parasitology, 25, 175–273. [DOI] [PubMed] [Google Scholar]
- Kearn, G. C. (1993). A new species of the genus Enoplocotyle (Platyhelminthes: Monogenea) parasitic on the skin of the moray eel Gymnothorax kidako in Japan, with observations on hatching and the oncomiracidium. Journal of Zoology, London, 229, 533–544. [Google Scholar]
- Kearn, G. C. (1998).Parasitism and the Platyhelminths. London: Chapman & Hall. [Google Scholar]
- Kritsky, D. C. & Boeger, W. A. (1991). Neotropical Monogenea. 16. New species of oviparous Gyrodactylidea with proposal of Nothogyrodactylus gen. n. (Oogyrodactylidae). Journal of the Helminthological Society of Washington, 58, 7–15. [Google Scholar]
- Kritsky, D. C. & Boeger, W. A. (2003). Phylogeny of the gyrodactylidae and the phylogenetic status of Gyrodactylus Nordmann, 1832 (platyhelminthes: monogenoidea). In Combes C. & Jourdane, J. (Eds) Taxonomie, écologie et évolution des métazoaires parasites. Taxonomy, ecology and evolution of metazoan parasites (Livre hommage à Louis Euzet). Tome 2. PUP Perpignan: 37–58.
- Lauder, G. V. & Liem, K. F. (1983). The evolution and interrelationships of the actinopterygian fishes. Bulletin of the Museum of Comparative Zoology, 150, 95–197. [Google Scholar]
- Llewellyn, J. (1968). Larvae and larval development of monogeneans. Advances in Parasitology, 6, 373–383. [DOI] [PubMed] [Google Scholar]
- Llewellyn, J. (1981). Evolution of viviparity and invasion by adults. Parasitology, 82, 64–68. [Google Scholar]
- Lydeard, C. (1993). Phylogenetic analysis of species richness: Has viviparity increased the diversification of actinopterygian fishes? Copeia, 1993, 514–518. [Google Scholar]
- Macdonald, S. & Llewellyn, J. (1980). Reproduction in Acanthocotyle greeni n. sp. (Monogenea) from the skin of Raia spp. at Plymouth. Journal of the Marine Biology Association of the United Kingdom, 60, 81–88. [Google Scholar]
- Morrison, D. A. (2002). How to improve statistical analysis in parasitology research publications. International Journal for Parasitology, 32, 1065–1070. [DOI] [PubMed] [Google Scholar]
- Page, R. D. M. (1994). Maps between trees and cladistic analysis of historical associations among genes, organisms, and areas. Systematic Biology, 43, 58–77. [Google Scholar]
- Paterson, A. M. & Banks, J. (2001). Analytical approaches to measuring cospeciation of host and parasites: through a glass, darkly. International Journal for Parasitology, 31, 1012–1022. [DOI] [PubMed] [Google Scholar]
- Purvis, A. (1996). Using interspecies phylogenies to test macroevolutionary hypotheses In Harvey P. H., Leigh Brown A. J., Smith J. M. & Nee S. (Eds) New Uses for New Phylogenies, pp. 153–168. Oxford: Oxford University Press. [Google Scholar]
- Scott, M. E. (1982). Reproductive potential of Gyrodactylus bullatarudis (Monogenea) on guppies (Poecilia reticulata). Parasitology, 85, 217–236. [Google Scholar]
- Scott, M. E. & Anderson, R. M. (1984). The population dynamics of Gyrodactylus bullatarudis (Monogenea) within laboratory populations of the fish host Poecilia reticulata . Parasitology, 89, 159–194. [DOI] [PubMed] [Google Scholar]
- Slowinski, J. B. & Guyer, C. (1993). Testing whether certain traits have caused amplified diversification: an improved method based on a model of random speciation and extinction. American Naturalist, 142, 1019–1024. [DOI] [PubMed] [Google Scholar]
- Tinsley, R. C. (1983). Ovoviviparity in platyhelminth life‐cycles. Parasitology, 86, 161–196. [DOI] [PubMed] [Google Scholar]
- White, M. J. D. (1978). Modes of Speciation. New York: W.H. Freeman. [Google Scholar]
- Wiley, E. O. (1980). Phylogenetics: The Theory and Practice of Phylogenetic Systematics. New York: John Wiley & Sons. [Google Scholar]
- Wilkinson, M. , Thorley, J. L. , Littlewood, D. T. J. & Bray, R. A. (2001). Towards a phylogenetic supertree of Platyhelminthes In Littlewood D. T. J. & Bray R. A. (Eds) Interrelationships of Platyhelminthes, pp. 292–301. London: Taylor & Francis. [Google Scholar]


