Abstract
Destruction of target cells by cytotoxic T lymphocytes (CTLs) or natural killer (NK) cells requires the coordinated action of the pore forming protein perforin (Pfp) and the granzyme (Gzm) family of serine proteases. The activation of a number of serine proteases, including GzmA and B, is predominately mediated by cathepsin C (CatC). Deficiencies in CatC‐null mice were therefore expected to replicate the defects observed in GzmAB‐deficient mice. We have previously determined that GzmAB‐deficient mice exhibit increased susceptibility to murine cytomegalovirus (MCMV) infection. Here, we have compared the ability of CatC−/− mice to control MCMV infection with that of GzmAB‐deficient animals. We found that CatC−/− mice have organ‐specific defects in the ability to control MCMV replication, a phenotype that is distinct to that observed in GzmAB−/− mice. Significantly, the cytolytic function of CatC‐deficient NK cells and CTLs elicited during infection was indistinguishable from that of wild‐type cells. Hence, CatC is involved in limiting MCMV replication; however, this effect is independent of its role in promoting effector cytolytic activity. These data provide evidence for a novel and unexpected role of CatC during viral infection.
Keywords: cytomegalovirus, cathepsin c, granzyme
Cytotoxic T lymphocyte (CTL) and natural killer (NK) cells are critical for protecting higher organisms against intracellular pathogens, including viruses. Following the formation of a stable conjugate, major histocompatibility complex‐restricted CD8+ T cells secrete granule‐bound toxins that induce apoptosis in the infected cell. The pore‐forming protein perforin (Pfp) coalesces into transmembrane channels that permit access for a family of serine proteases (granzymes, Gzms) to proapoptotic substrates in the target cell cytosol. 1 Pfp, which is encoded by a single copy gene, is critical for granule‐mediated cytotoxicity, and its deficiency results in severe susceptibility to many viruses, including the poxvirus ectromelia and the herpesvirus cytomegalovirus (CMV). 2 , 3 , 4 , 5 , 6 In contrast, deficiency of more than one Gzm (typically both GzmA and GzmB) is required for significant viral susceptibility to become evident in vivo. 4 , 7
Given the potency of granule toxins, cytotoxic lymphocytes have unsurprisingly developed strategies to limit their own susceptibility to Gzm‐mediated apoptosis. In particular, Gzms are synthesized as zymogens and become activated by the removal of an N‐terminal dipeptide only at the time of packaging into secretory vesicles. Cathepsin C (CatC) (dipeptidylpeptidase 1) is known to be the major mediator of pro‐Gzm activation, as mice deficient in this enzyme have reduced Gzm activity. 8 CatC is also critical for activation of additional families of serine proteases, including those expressed in mast cells and neutrophils. 9 Recently, we made the surprising observation that the CTLs and NK cells of CatC‐deficient mice still retain potent killing capacity. Both in vitro‐activated alloreactive CTLs and interleukin (IL‐2) activated NK cells from these mice were able to kill target cells more efficiently than cells isolated from mice with genetic deletion of both the GzmA and GzmB genes. 10 In the absence of CatC, we found that GzmA activity was negligible; however, there was sufficient residual GzmB activity to permit significant Pfp‐dependent apoptosis to continue. It is therefore likely that proteases other than CatC are also capable of activating pro‐GzmB, although with reduced efficiency.
Mouse models have proved very useful to dissect the roles of specific effector pathways in complex immune responses, such as those elicited during viral infection. In particular, infection of mice with murine CMV (MCMV) is one of the best systems for this type of analysis. NK cells and CD8+ CTLs are the principal cellular mediators of anti‐MCMV activities during acute infection, with NK cells having the main antiviral role in the C57BL/6 mouse strain. 11 NK cells and CTLs deliver most of their cytolytic effects through granule exocytosis. We have previously shown that mice lacking GzmAB display increased susceptibility to MCMV, characterized by higher viral titers in several of the target organs during acute infection. 4 As CatC‐deficient mice possess reduced Gzm activity, we set out to investigate how CatC−/− mice respond to MCMV infection. Surprisingly, we found that although CatC‐deficient mice showed increased susceptibility to MCMV infection, this was not as overt as that observed in mice lacking GzmAB. Furthermore, the effector function of both NK cells and antiviral CD8+ CTLs from CatC‐deficient mice was equivalent to that of wild‐type mice. Thus, CatC has an important role in limiting acute MCMV infection, but this is independent of its function as a proactivator of Gzm A and GzmB cytolytic activity. To our knowledge this is the first evidence that effector cells generated in vivo in response to a viral infection exert their cytolytic functions independently of CatC.
RESULTS
Role of CatC in controlling MCMV replication in vivo
Several studies have assessed the role of CatC activity on aspects of the immune response. The phenotype of CatC‐deficient mice was proposed to mirror that of GzmAB‐null mice as lymphocytes isolated from these animals were found to have minimal amounts of GzmB activity and no detectable GzmA activity. 8 However, alloreactive CTLs and IL‐2‐activated NK cells isolated from CatC−/− mice were shown to induce apoptosis in target cells as efficiently as wild‐type CTL, and, unlike GzmAB−/− mice, CatC‐deficient animals were not susceptible to ectromelia virus infection. 10 These data suggest that despite CatC having a role in the generation of active Gzm, immune effectors in CatC−/− mice may be capable of generating sufficient Gzm activity to kill target cells. However, the lytic activity of CatC−/− effectors generated during a physiological response in vivo has not been assessed.
We have previously determined that Pfp−/− or GzmAB−/− mice are more sensitive to MCMV infection than wild‐type B6 mice. 4 , 5 As cytotoxic activity mediated by NK cells and CD8+ T cells is critical to effectively limit MCMV infection, this model provides an ideal setting to compare the impact of CatC deletion with that caused by specific deletion of Gzm A and B. MCMV replication was assessed in the indicated target organs at days 4 and 10 post infection (p.i.). As expected, 4 GzmAB−/− mice showed raised viral titers in both the spleen and the lungs (Figure 1a). Conversely, CatC−/− mice could limit MCMV replication as effectively as wild‐type mice in both the spleen and the lungs at the time points tested (Figure 1a). However, like GzmAB−/− mice, CatC‐deficient mice exhibited increased MCMV titers in the liver and salivary glands (Figure 1b). Thus, the absence of CatC results in organ‐specific defects in the ability to limit MCMV infection, a phenotype that differs significantly from that observed in GzmAB−/− mice.
Figure 1.
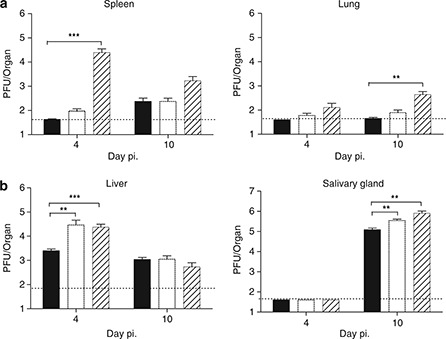
Increased MCMV replication in mice lacking CatC. B6 (solid bar), B6.CatC−/− (open bar) or B6.GzmAB−/− (hatched bar) mice were infected with 104 plaque forming units (PFU) of MCMV‐K181‐Perth and viral load in spleen and lung (a) and liver and salivary gland (b) was determined by plaque assay on days 4 and 10 p.i. Viral titers were determined in three separate experiments and the data pooled, mean ± s.e.m. are plotted (n⩾8). Statistical analysis was performed using the nonparametric Mann–Whitney U‐test. ∗∗ P<0.01; ∗∗∗ P<0.001. Dotted line represents the limit of detection of the assay.
Depletion of neutrophils has no impact on MCMV replication in vivo
The processing and activation of several serine proteases expressed by neutrophils is dependent on CatC activity, and the recruitment of CatC‐deficient neutrophils to sites of inflammation is impaired in some experimental settings. 9 , 12 These findings prompted us to examine the role of neutrophils in the immune response to MCMV infection. Before infection with MCMV, B6 mice were injected with an anti‐Ly6G‐specific antibody (NIMP‐R14) according to the protocol outlined in the methods section. Fluorescence‐activated cell sorting (FACS) analysis confirmed that treatment with the NIMP‐R14 antibody resulted in the specific depletion of neutrophils (data not shown). Importantly, depletion of neutrophils had no impact on the ability of B6 mice to control MCMV infection in any of the organs examined (Figure 2). This finding indicates that the increased sensitivity to MCMV infection observed in CatC−/− mice is not consistent with a defect in neutrophil function.
Figure 2.
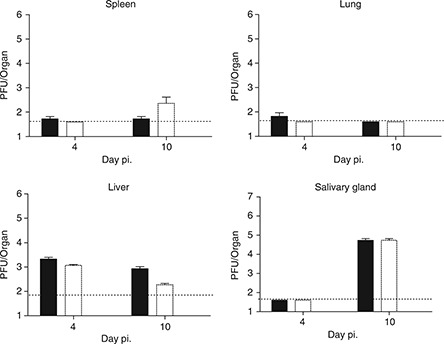
Control of MCMV replication does not require neutrophils. B6 (solid bar) or neutrophil depleted B6 mice (open bar) were infected with 1 × 104 PFU of MCMV‐K181‐Perth and the viral load in the indicated organs determined by plaque assay. Mean ± s.e.m. are plotted (n=3). Dotted line represents the limit of detection of the assay.
CatC is not essential for NK cell‐mediated cytotoxicity
Some mouse strains, such as B6, successfully limit acute MCMV infection due to an effective NK‐cell response. As CatC‐deficient mice are more sensitive to MCMV infection than wild‐type B6 animals, we assessed the ability of NK cells isolated from CatC−/− mice to kill sensitive targets. Pfp‐, GzmAB‐ and CatC‐deficient mice (all on a B6 background) were infected with MCMV and splenocytes isolated 3 days p.i., a time when maximal NK‐cell cytotoxic activity is expected. Splenocytes were incubated with 51Cr‐labeled YAC‐1 target cells and specific lysis was quantified after 4 h. In agreement with previous reports, 13 , 14 Pfp−/− NK cells were unable to lyse sensitive targets (Figure 3). In contrast, CatC−/− NK cells were as effective as wild‐type or GzmAB−/− NK cells in killing YAC‐1 target cells (Figure 3). Therefore, deletion of CatC does not impair the cytotoxic function of NK cells. Indeed, the fact that deficiency of both GzmA and GzmB produced no reduction in NK‐cell killing indicates that, in this model, NK cell‐dependent target cell death involves Pfp‐mediated pathways that utilize alternative Gzms or effector proteases.
Figure 3.
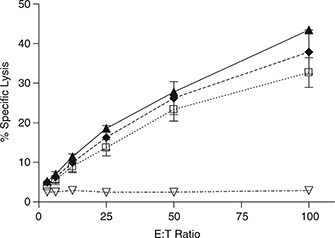
NK cell cytotoxic function does not require CatC. B6 mice (filled triangle), B6.CatC−/− (empty square), B6.GzmAB−/− (filled diamond) or B6.Pfp−/− (empty triangle) mice were infected with 5 × 103 PFU of MCMV‐K181‐Perth and spleens collected 3 days p.i. Splenocytes were incubated with 51Cr‐labeled Yac‐1 target cells for 4 h and specific lysis determined as described in methods section. Data from three independent experiments have been pooled, mean ± s.e.m. are plotted (n=9).
CatC deletion does not inhibit antigen processing
The Cat family of proteases are involved in a number of biological processes. As a number of Gzms are involved in antigen processing (reviewed in Zavasnik‐Bergant and Turk 15 ), the capacity of CatC−/− dendritic cells (DCs) to process exogenous antigen was assessed. Antigen processing was assessed using DQ‐ovalbumin (DQ‐OVA). DQ‐OVA is a self‐quenched OVA conjugate that fluoresces only after proteolytic processing. Splenocytes were isolated from naïve B6, CatC−/− or GzmAB−/− mice and incubated with DQ‐OVA at either 37 °C or 4 °C. The incubation at 4 °C provides a control for nonenzymatic processing of DQ‐OVA. Splenocytes were then stained with antibodies against CD11c and MHCII, and cells analyzed by FACS. The percentage of DCs (CD11c+MHCII+) in the spleen was equivalent in all the mouse strains tested (Figure 4a). Furthermore, processing of DQ‐OVA by DCs was equivalent in the three mouse strains (Figure 4b). Thus, deletion of CatC or GzmAB does not impair the capacity of DCs to process exogenous antigen.
Figure 4.
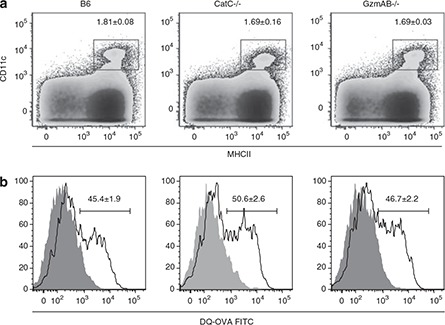
Processing of antigen by DCs is not impaired in the absence of CatC. Splenocytes from naive B6, B6.CatC−/− and B6.GzmAB−/− mice were isolated and incubated with 10 μg ml−1 DQ‐OVA for at 37 or 4 °C for 2 h. Cells were then stained with CD11c and MHCII antibodies and analyzed by FACS (a). DQ‐OVA processing in DCs (CD11c+ MHCII+) was assessed by comparing the intensity of FITC signal from cells incubated at 37 °C (open histogram) with that obtained when cells were incubated at 4 °C (filled histogram) (b). Data represents mean ± s.e.m. (n=3).
Impact of CatC on the generation of CTL
Although CatC−/− mice were able to process exogenous antigen normally and possessed similar numbers of DCs as wild‐type mice, the DCs of CatC−/− mice expressed lower levels of the β2‐integrin CD11c (Figure 4a). Indeed the mean fluorescent intensity of CD11c staining of CatC−/− DCs was significantly lower than that of wild‐type or GzmAB−/− DCs (Table 1). β2‐integrins consist of a heterodimer between a unique α‐subunit associated with the common β2 chain (CD18). The β2 family is composed of four members: LFA‐1 (CD11a/CD18), Mac‐1 (CD11b/CD18), p150,95 (CD11c/CD18) and αdβ2 (CD11d/CD18). 16 The β2‐integrins are leukocyte‐specific proteins that are critical for cell–cell interactions during immune responses. 16 Hence, the reduction in CD11c expression observed on CatC−/− DCs could potentially impact on the capacity of DCs to interact with naïve T cells, thereby inhibiting the generation of the subsequent adaptive response to MCMV. Some support for this hypothesis comes from the observation that CatE‐deficient macrophages have a reduced capacity for adhesion correlating with decreased expression of CD18. 17
Table 1.
Expression of β2‐integrins on DC, CD8+ T cells and NK cells after MCMV infection
| B6 | B6.CatC −/− | B6.GzmAB −/− | |
|---|---|---|---|
| Dendritic cells a | |||
| CD11c | 5523±206 | 3298±195∗∗∗ | 4972±350 |
| CD8 T cells b | |||
| CD11a | 1603±239 | 1668±183NS | 2675±119 |
| CD11b | 477±66 | 207±15∗∗ | 540±28 |
| CD11c | 303±22 | 138±4∗∗∗ | 275±17 |
| NK cells c | |||
| CD11b | 1338±134 | 639±68∗∗ | 1361±325 |
Abbreviations: DC, dendritic cell; MCMV, murine cytomegalovirus; NK, natural killer; NS, not significant.
Values are the geometric mean fluorescence intensity ± s.d. (n=3). Statistical analysis was performed using an unpaired t‐test. ∗∗ P<0.005, ∗∗∗ P<0.0005.
Naïve spleens.
Day 6‐infected spleens.
Day 3‐infected spleens.
The ability of CatC−/− mice to generate an effective adaptive antiviral immune response was initially examined by evaluating the expression of β2‐integrin proteins on CD8+ T cells. Several studies have determined that expression of both CD11b and CD11c is increased on activated CTL. 18 , 19 , 20 Following MCMV infection of wild‐type or GzmAB−/−, mice expression of CD11b and CD11c was upregulated on a significant proportion of splenic CD8+ T cells (Figure 5 and Table 1). In contrast, fewer CD8+ T cells upregulated expression of either CD11b or CD11c in CatC−/− mice after MCMV infection (Figure 5 and Table 1). Importantly, expression of CD11a on CD8+ T cells was equivalent in wild‐type, GzmAB−/− and CatC−/− mice (Figure 5 and Table 1). Thus, deletion of CatC prevents the upregulation of CD11b and CD11c, β2‐integrins associated with T‐cell activation.
Figure 5.
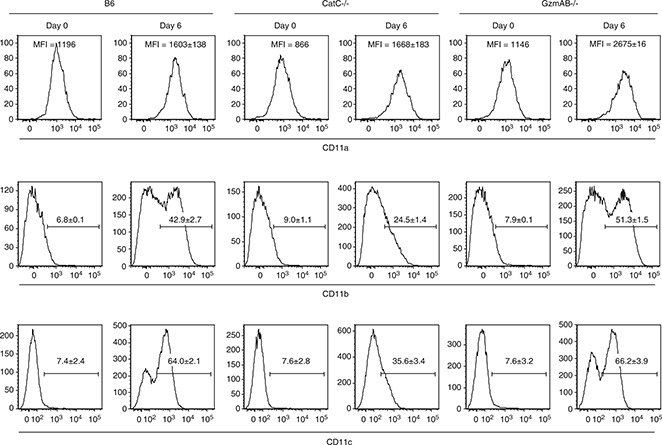
Upregulation of CD11b and CD11c on CD8 T+ cells is impaired in CatC−/− mice. B6, B6.CatC−/− and B6.GzmAB−/− mice were infected with 5 × 103 PFU MCMV‐K181‐Perth and splenocytes isolated 6 days p.i. Expression of the β2‐integrins CD11a, CD11b and CD11c on CD8+ T cells was determined by FACS analysis. The percentages of CD8+ T cells positive for the various β2‐integrins are shown. Two independent experiments were performed with three mice per group, representative plots are shown.
Following MCMV infection, the frequency and absolute number of CD8+ T cells increased in wild‐type mice (Figures 6a and b). Importantly, expansion of the splenic CD8+ T‐cell pool in CatC−/− was equivalent to that observed in wild‐type mice. Although the percentage of CD8+ T cells in the spleens of GzmAB−/− was equivalent to that of wild‐type mice, the absolute number was significantly lower (Figure 6b). This finding reflects a general loss of splenic cellularity associated with the inability to control MCMV replication in the spleen of GzmAB−/− mice. 4
Figure 6.
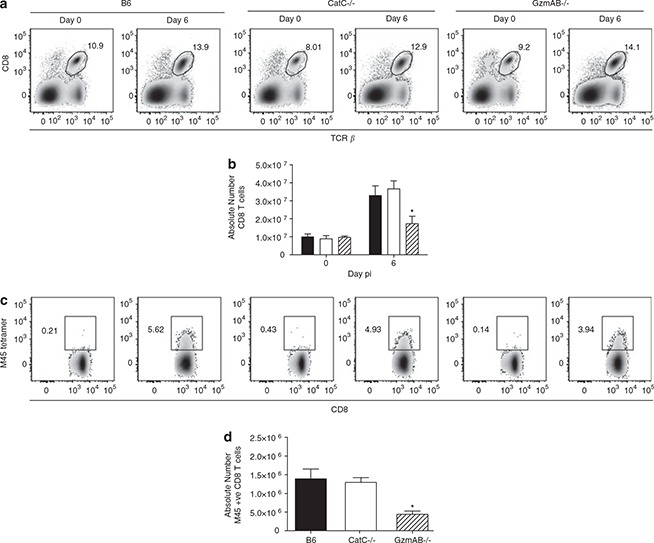
Expansion of antigen‐specific CTL does not require CatC. B6, B6.CatC−/− and B6.GzmAB−/− mice were infected with 5 × 103 PFU of MCMV‐K181‐Perth. On day 6 p.i, splenocytes were collected and stained with CD8 and TCR‐β antibodies and M45 tetramers. Representative FACS plots showing percentages of CD8+ T cells in the spleens of the various mouse strains are shown (a). Total numbers of CD8+ T cells are shown in (b). Representative FACS plots showing percentage of M45‐specific CD8+ T cells per spleen are shown in (c) and total numbers of M45‐specific CD8+ T cells are shown in (d). B6 mice (solid bar), B6.CatC−/− (open bar) and B6.GzmAB−/− (hatched bar). Data from two independent experiments have been pooled, mean ± s.e.m. are plotted (n⩾4).
Despite the potential for a large pool of virally derived peptides to be presented to CD8+ T cells, the immune response to many viruses is often directed against a few immunodominant epitopes. During the adaptive immune response to MCMV infection in B6 mice, an epitope derived from the M45 viral protein is immunodominant. 21 The expansion of CD8+ T cells directed against M45 was monitored by staining splenic T cells with a fluorescently conjugated M45 tetramer. By day 6 post‐MCMV infection, a significant expansion of CD8+ T cells directed against M45 had occurred in wild‐type mice (Figures 6c and d). Importantly, a similar expansion of M45‐specific CD8+ T cells occurred in CatC−/− mice, suggesting that the loss of CatC does not impair the capacity to generate antigen‐specific T‐cell responses. A significant, but lower expansion of M45‐specific CD8+ T cells was observed in MCMV‐infected GzmAB−/− mice (Figures 6c and d).
CTL function is not affected by CatC deletion
We have previously determined that ex vivo activated CatC−/− CD8+ T cells are capable of producing active GzmB. 10 We therefore sought to determine if CD8+ T cells in CatC−/− mice were capable of producing active GzmB in response to viral infection in vivo. CD8+ T cells were purified from the spleen of wild‐type or relevant knockout mice at day 6 p.i. with MCMV. The capacity of lysates prepared from purified CD8+ T cells to cleave a GzmB‐specific substrate was then assessed. Significant GzmB activity was detected in lysates from wild‐type CD8+ T cells (Figure 7a). The specificity of the assay is demonstrated by the finding that no significant cleavage of the substrate occurred when lysates prepared from GzmAB−/− CD8+ T cells were used (Figure 7a). Importantly, although the GzmB activity of CatC−/− CD8+ T cells is below that found in wild‐type cells, active GzmB is clearly present in these cells (Figure 7a). Thus, although CatC has a role in the production of active GzmB in vivo, other means of processing GzmB into its active form clearly exist. These data are the first to establish that CatC−/− cells have the capacity to produce active GzmB in vivo, in response to infection with a viral pathogen.
Figure 7.
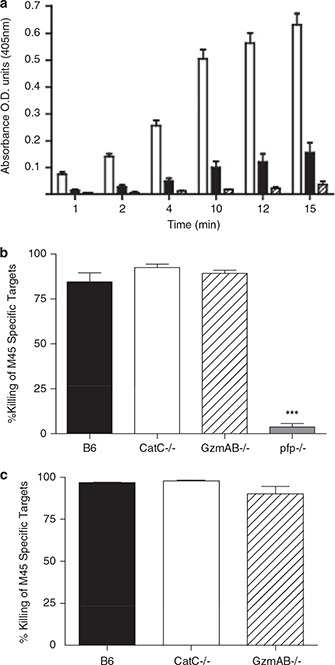
Antiviral CTL function is normal in CatC mice. (a) B6 mice (open bar), B6.CatC−/− (solid bar) and GzmAB−/− (hatched bar) mice were infected with MCMV and CD8+ T cells purified at day 6 p.i. Lysates prepared from these cells were assayed for GzmB activity by measuring the hydrolysis of peptide substrate. Data are pooled from two independent experiments (n=4). (b) The indicated mouse strains were infected with 5 × 103 PFU of MCMV‐K181‐Perth. At day 6 p.i., mice were injected intravenously with a 1:1 mixture of CFSE‐labeled M45‐pulsed targets cells and nonpulsed targets. A single‐cell suspension from the spleens of the infected mice was prepared 4 h after target cell transfer and loss of peptide‐pulsed targets measured by FACS analysis. B6 mice (solid bar), B6.CatC−/− (open bar), B6.GzmAB−/− (hatched bar) and B6.Pfp−/− (grey bar) mice. Data from two independent experiments have been pooled, mean±s.e.m. are plotted (n⩾8). ∗∗∗ P=0.0004. (c) Mice were infected with MCMV and peptide‐labeled target cells injected as described. A single‐cell suspension was prepared from livers of infected mice 16 h after target cell transfer and loss of peptide pulsed targets measured by FACS analysis. B6 mice (solid bar), B6.CatC−/− (open bar), B6.GzmAB−/− (hatched bar).
Expansion of M45‐antigen‐specific CD8+ T cells in CatC−/− occurred to the same extent as in wild‐type mice, implying that this response occurs independently of CatC. However, expression of CD11b and CD11c on the total CD8+ T‐cell pool in CatC−/− mice was lower than that observed in CD8+ T cells from either wild‐type or GzmAB−/− mice (Figure 5 and Table 1). Expression of integrins is essential for some aspects of T‐cell function, such as homing to sites of inflammation. 19 Hence, although expansion of antigen‐specific T cells occurs normally in CatC−/− mice, it is possible these cells may be functionally impaired. Furthermore, GzmB activity in CatC−/− CD8+ T cells is lower than that of wild‐type cells (Figure 7a). We therefore tested the possibility that the function of CatC−/− CD8+ T cells may be impaired; this was achieved by measuring the in vivo CTL response of CatC−/− mice. Wild‐type mice, or the relevant knockout mouse strains, were infected with MCMV. On day 6 p.i., a 1:1 mixture of 5,6‐carboxyfluorescein diacetate succinimidyl ester (CFSE)‐labeled M45 peptide‐pulsed target cells and nonpulsed targets were injected into mice. Mice were killed 4 h later, a single‐cell suspension prepared from the spleen and the specific loss of M45‐pulsed targets determined by FACS analysis. Mice deficient in Pfp were unable to kill M45‐pulsed target cells (Figure 7b). In contrast, CatC−/− and GzmAB−/− mice were as efficient as wild‐type mice in clearing M45‐peptide‐pulsed targets in the spleen (Figure 7b). Similarly, the clearance of peptide‐pulsed targets in the liver of MCMV‐infected CatC−/− or GzmAB−/− mice was equivalent to that of wild‐type mice (Figure 7c). Together these data demonstrate that CatC−/− mice are capable of generating functional antiviral CD8+ T‐cell responses in vivo.
DISCUSSION
The cysteine protease CatC is critical for the processing and activation of several serine proteases, including GzmA and GzmB. We have previously demonstrated that control of MCMV infection is impaired in GzmAB−/− mice, 4 therefore, the capacity of CatC‐null mice to control MCMV would be predicted to mirror the defect seen in GzmAB−/− mice. However, we found that unlike GzmAB−/− mice, CatC‐deficient mice were able to efficiently control MCMV infection in the spleen and the lungs. In B6 mice, control of acute MCMV infection is mediated by NK cells that use a combination of cytotoxic activity and production of interferon‐γ to limit viral replication in the various target organs. 5 , 22 , 23 In the spleen and the lungs of B6 mice, NK cells exert their effects through Pfp‐mediated cytolytic activities. 5 , 22 , 23 CTL have an antiviral role later in infection. We found that NK cells and CTL from MCMV‐infected CatC‐deficient mice were as effective as wild‐type B6 effectors in killing sensitive targets in vitro and in vivo. Furthermore, CD8+ T cells isolated from MCMV‐infected CatC−/− mice contain active GzmB. These findings are consistent with data demonstrating that CatC−/− alloreactive CTLs or IL‐2 NK cells retain cytotoxic function in vitro. 10 These results are the first to show that the capacity of NK cells and CTL to lyse virally infected cells in vivo in the spleen of MCMV‐infected mice is not impaired in the absence of CatC activity.
Unlike CatC‐deficient mice, GzmA/B−/− mice cannot control MCMV replication in the spleen. 4 However, NK cells or MCMV‐specific CTLs isolated from GzmA/B−/− mice were as effective as those isolated from wild‐type mice in lysing target cells in vitro. Indeed, independent studies have reported that cytotoxic effector cells from GzmAB−/− mice efficiently lyse various target cells. 13 , 17 , 24 Thus, there is an apparent discrepancy between the ex vivo killing efficiency of GzmAB−/− effectors, which is equivalent to that of wild‐type cells, and the inability of GzmAB−/− mice to control MCMV replication in the spleen. Although GzmAB−/− effectors are capable of killing target cells, the type of cell death induced is distinct from that induced by wild‐type effector cells. 13 , 14 Hence, it is possible that the killing of MCMV‐infected cells in vivo may require a specific form of cell death that GzmAB−/− effectors are incapable of inducing. Alternatively, the Gzms may have a role in inhibiting some aspect of viral replication completely independently of their role as mediators of cell death. For example, the TSP‐1 proteinase secreted by CTL is able to inactivate the reverse transcriptase derived from the Moloney murine leukemia virus. 25 Similarly, GzmB activity prevents the reactivation of HSV‐1 by degrading the ICP4 immediate early protein in neurons without inducing apoptosis, 26 and GzmH inhibits replication of adenovirus by cleaving a virally encoded DNA binding protein. 27 Therefore, there is increasing evidence that Gzms have noncytotoxic activities that may contribute to the control of viral infections.
Surprisingly, viral replication in the liver of CatC−/− mice was significantly higher than that observed in wild‐type B6 mice, despite the fact that CatC‐deficient mice could control MCMV infection in the spleen and the lungs. CatC has a role in the activation of neutrophil serine proteases, including neutrophil elastase. 9 , 12 It was, therefore, plausible that the increased viral replication observed in CatC‐deficient mice might have been the result of impaired neutrophil function. Our studies however showed that depletion of neutrophils does not lead to an increase in viral titers and does not recapitulate the inability to control viral replication observed in target organs of CatC‐deficient mice. These data provide evidence, although indirect, that the phenotype observed in CatC−/− mice after MCMV infection does not relate to a defect in neutrophil function.
As some members of the Cat family have been shown to participate in antigen processing, the possibility that defective antigen processing in CatC−/− mice may account for the inability to control MCMV infection was tested. We found that DCs purified from CatC‐deficient mice processed antigen as efficiently as wild‐type DCs. Furthermore, antigen‐specific antiviral T‐cell responses were comparable in CatC−/− and wild‐type mice. Thus, the fundamental aspects of an antiviral immune response appeared normal in CatC‐deficient mice.
We found that DCs, NK cells and CTLs from CatC−/− mice expressed reduced levels of some β2‐integrins. The β2‐integrins are involved in various aspects of lymphocyte adhesion and migration. Therefore, the inability of CatC−/− mice to control MCMV in the liver could have been due to defects in the migration of effector cells, especially NK cells which are critical to virus control in the liver during acute infection. However, the number of NK cells that accumulate in the liver of MCMV‐infected CatC‐deficient mice was equivalent to that of wild‐type mice (data not shown), indicating that migration of NK cells was not altered by the absence of CatC.
The inability of CatC−/− mice to control MCMV replication in the liver may reflect a defect in the generation of an appropriate inflammatory response. GzmA activity has been shown to initiate an inflammatory response by stimulating macrophages to produce cytokines such as IL‐1β, tumor necrosis factor‐α and IL‐6. 28 As CatC−/− mice lack GzmA activity, a reduction in the production of proinflammatory cytokines may account for the inability to control MCMV replication in the liver. However, levels of IL‐6, IL‐12 and interferon‐γ in the liver of CatC−/− mice were equivalent to those of wild‐type mice (data not shown). Furthermore, we have previously demonstrated that production of interferon‐γ and tumor necrosis factor‐α in GzmAB−/− mice after MCMV infection is equivalent to that of wild‐type mice. 4 Together the data indicate that the inability of CatC−/− mice to control MCMV infection is not because of defects in the production of proinflammatory cytokines.
Like CatC−/− mice, the mice deficient in either GzmA or GzmB only effectively control MCMV replication in the spleen but not the liver. 4 These observations suggest that the activities of both GzmA and GzmB are required to control MCMV infection in the liver, although in the spleen the activity of either protease is sufficient to limit viral replication. This hypothesis is consistent with the observation that CatC−/− mice retain residual GzmB activity, but have no GzmA activity. 10 Alternatively, some of the antiviral effects of CatC may be totally independent of its role as a proactivator of Gzms. The Cat family of endosomal proteases has been implicated in the proteolytic processing of several viruses. In the majority of instances described to date the Cats participate in activities that favour viral replication. For example, CatB and CatL are required for the efficient entry of Ebola and Severe acute respiratory syndrome coronavirus into host cells. 29 , 30 Here, we describe an antiviral role for CatC.
Previous studies excluded a role for CatC in controlling infection with ectromelia virus. 10 It is worth noting that ectromelia virus and CMV are viral pathogens with very distinct characteristics. Although ectromelia is a highly cytopathic virus which kills the cells it infects, the primary targets of MCMV infection in vivo, macrophages and DCs are not killed by viral infection. 31 Furthermore, the control of the ectromelia and MCMV in vivo relies on Gzm and Pfp to differing degrees; both Pfp‐ and GzmAB‐deficient mice succumb to infection with ectromelia, 2 whereas only Pfp‐deficient, but not GzmAB‐deficient mice die after MCMV infection. 4 It is therefore not unexpected that the role of CatC may be different in different viral infections. It is possible that the proteolytic activities of CatC may be utilized to various degrees by the host as a means to impede viral replication by interfering, for example, with appropriate viral assembly. This is an exciting possibility worthy of further investigation.
In summary, we have provided novel evidence that CatC participates in antiviral immune responses and is involved in limiting MCMV replication during acute infection, especially in the liver. Importantly, this role of CatC is independent of its known functions as an activator of Gzm A and GzmB cytolytic activity or neutrophil elastase function. To our knowledge, this is the first study to show in vivo, in a physiological setting of viral infection that the lytic function of NK cells and CTL is not affected by the absence of CatC.
METHODS
Mice
Inbred C57BL/6J (B6) mice were obtained from the Animal Resources Centre (Perth, WA, Australia). C57BL/6J.cathepsinC−/− (CatC−/−), C57BL/6J.granzymeA−/− and granzymeB−/− (GzmAB−/−) and C57BL/6J.perforin−/− (Pfp−/−) mice were bred at the Peter MacCallum Cancer Centre, Melbourne, Australia. Mice were housed under specific pathogen‐free conditions at the animal services facility of the University of Western Australia. Experiments were performed with the approval of the animal ethics and experimentation committee of the University of Western Australia and according to the guidelines of the National Health and Medical Research Council of Australia.
Cell lines and reagents
M210B4 cells were cultured in minimal essential medium (Gibco Life Sciences, Sydney, NSW, Australia) containing neonatal calf serum (Gibco Life Sciences). YAC‐1 cells were cultured in complete RPMI medium 1640 (Gibco Life Sciences) containing 10% heat‐inactivated fetal bovine serum (Gibco Life Sciences), 2 mm l‐glutamine and 50 μm 2‐mercaptoethanol.
Treatment of mice
Mice were infected intraperitoneally with mCMV‐K181‐Perth salivary gland‐propagated virus diluted in phosphate‐buffered saline (PBS)‐0.05% fetal bovine serum. A dose of 104 PFU of salivary gland‐propagated virus was used for the pathogenesis studies, whereas a 5 × 103 PFU of salivary gland‐propagated virus dose was used for phenotypic analysis of lymphocytes. To deplete neutrophils, mice were inoculated intraperitoneally with 25 μg of the NIMP‐R14 monoclonal antibody 32 diluted in PBS‐0.05% fetal bovine serum at days −1 and +1 relative to MCMV infection. Depletion of neutrophils was confirmed by FACS analysis on day 2 p.i.
Determination of viral titers
At various times p.i., spleen, liver, lungs and salivary glands were collected and processed to determine viral titers by standard plaque assay using M210B4 cells as previously described. 33
Chromium release assay
NK‐cell activity was assessed by standard Cr‐release assay. Single‐cell suspensions were prepared from the spleen. Splenocytes were diluted twofold on 96‐well plates starting with 1 × 106 cells per well and 51Cr‐labeled YAC‐1 target cells (1 × 104 cells per well) were added. Each assay was performed in triplicate. Chromium release was measure after 4 h incubation. Data are presented as percentage of specific lysis, calculated by the following formula: percentage‐specific lysis=(experimental c.p.m.−spontaneous release c.p.m.)/(total c.p.m.−spontaneous release c.p.m.) × 100.
Flow cytometry
Single‐cell suspensions prepared from the spleen were preincubated on ice for 30 min with PBS‐2% fetal calf serum containing 20% normal goat serum. To detect CD8T cells, fluorescein isothiocyanate‐conjugated T‐cell receptor (TCR; H57‐597; Biolegend, San Diego, CA, USA) was used together with Alexa Fluor‐750‐conjugated CD8α (53–6.7; eBioscience, San Diego, CA, USA) along with either PE‐conjugated H‐2‐Db‐985‐HGIRNASFI‐983 M45 tetramer (ImmunoID Tetramers, University of Melbourne,Victoria, Australia), biotin‐conjugated CD11a (2D7; BD Pharmingen, Franklin Lakes, NJ, USA), Pe‐Cy7‐conjugated CD11b (M1/70; BD Pharmingen) or PE‐conjugated CD11c (N418; Biolegend). 7‐Amino‐actinomycin D was incorporated in the final wash at 2 μg ml−1. Cells were analyzed on a FACSCanto (Becton Dickinson, San Jose, CA, USA). Appropriately stained controls were used to check compensation for all fluorochromes used. Analysis of the FACS data was performed with the FlowJo software (Stanford University, Stanford, CA, USA).
DQ‐OVA assay
Single‐cell suspensions prepared from the spleen were incubated with 10 μg ml−1 DQ‐OVA (Molecular Probes, Carlsbad, CA, USA) at 37 or 4 °C for 2 h. Cells were washed in PBS‐2% fetal calf serum and then stained with APC‐conjugated CD11c (HL3; BD Pharmingen) and PE‐conjugated MHCII (M5/114.15.2; BD Pharmingen) and analyzed by FACS.
In vivo CTL assay
Viral‐specific CTL‐mediated cytotoxicity was assessed by measuring the elimination of targets pulsed with the M45 viral peptide HGIRNASFI. The in vivo CTL assay quantifies CTL activity by measuring the loss of specific peptide‐pulsed targets in comparison with targets that have not been pulsed with peptide. Here, target cell lysis was measured using a modification of the previously described in vivo CTL assay. 34 M45 peptide‐pulsed (0.02 μg ml−1) splenocyte targets were labeled with a low concentration (final (0.025 μm)) of 5,6‐carboxyfluorescein diacetate succinimidyl ester (Molecular Probes), whereas control targets not pulsed with peptide were labeled with a high concentration (final (0.25 μm)) of CFSE. Labeled cells were washed to remove free CFSE and differential labeling confirmed by flow cytometry. CFSE‐low M45‐pulsed targets and CFSE‐high unpulsed control targets were resuspended in PBS, mixed in equal proportions, and a total of 4 × 107 cells per mouse transferred intravenously into syngeneic mice that had been infected with MCMV for various periods of time. Mice were killed 4 h later and single‐cell suspensions from spleen analyzed by flow cytometry. Transferred cells serve as targets for peptide‐specific CTL. The frequency of unpulsed targets serves as an internal control for trafficking, recovery and nonspecific cell loss. The loss of specific peptide‐pulsed targets is a measure of CTL activity against targets pulsed with the M45 viral peptide. Specific lysis was determined using the following formula: Percentage specific lysis of CFSE‐labeled target cells in each mouse is calculated as follows: (1−(r uninfected control mouse/r infected test mouse)) × 100 where r=frequency of unpulsed targets/frequency of peptide−pulsed targets. The assay is a sensitive and specific method to measure major histocompatibility complex class I‐restricted, CD8‐dependent lysis in vivo.
Quantification of GzmB activity
Mice were injected with 1 × 104 PFU of MCMV and spleens removed at day 6 p.i. A single‐cell suspension was prepared and CD8+ T cells purified by depletion of B cells and CD4+ T cells. Briefly, splenocytes were resuspended in PBS 0.5% FCS and incubated with anti‐CD19 (clone 1D3, BD) 1 μg ml−1 and anti‐CD4 (clone GK1.5) 5 μg ml−1 antibodies for 30 min on ice. Cells were resuspended at a concentration of 2 × 107 cells ml−1 and incubated with goat anti‐rat IgG beads (Qiagen, Hilden, Germany) and labeled cells removed. unlabeled cells enriched for CD8+ T cells were washed in PBS and lysed in NP40 lysis buffer (0.1% NP40, 25 mm hepes, 250 mm NaCl and 2.5 mm EDTA).
ASPase (GrB) activity: GrB activity in CD8+ T cells was assessed by the cleavage of a specific synthetic peptide thiobenzylester substrate, Boc‐Ala‐Ala‐Asp‐SBzl (SM Biochemicals LLC, CA, USA) as described previously. 35 Whole‐cell lysates were normalized for protein content and each sample was assayed in triplicate (50 μg protein per well).
Statistical analysis
For statistical analysis, the two‐tailed Mann–Whitney U‐test was performed using the statistical software package InStat (GraphPad Software, San Diego, CA, USA). All data are shown as mean ± s.e.m. ± s.d. as indicated.
ACKNOWLEDGEMENTS
We thank S Ross, P Farmer and S Griffiths for assistance with animal care.
References
- 1. Bolitho P, Voskoboinik I, Trapani JA, Smyth MJ. Apoptosis induced by the lymphocyte effector molecule perforin. Curr Opin Immunol 2007; 19: 339. [DOI] [PubMed] [Google Scholar]
- 2. Mullbacher A. Cell‐mediated cytotoxicity in recovery from poxvirus infections. Rev Med Virol 2003; 13: 223. [DOI] [PubMed] [Google Scholar]
- 3. Tay CH, Szomolanyi‐Tsuda E, Welsh RM. Control of infections by NK cells. Curr Top Microbiol Immunol 1998; 230: 193. [DOI] [PubMed] [Google Scholar]
- 4. van Dommelen SL, Sumaria N, Schreiber RD, Scalzo AA, Smyth MJ, Degli‐Esposti MA. Perforin and granzymes have distinct roles in defensive immunity and immunopathology. Immunity 2006; 25: 835. [DOI] [PubMed] [Google Scholar]
- 5. Sumaria N, van Dommelen SL, Andoniou CE, Smyth MJ, Scalzo AA, Degli‐Esposti MA. The roles of interferon‐gamma and perforin in antiviral immunity in mice that differ in genetically determined NK‐cell‐mediated antiviral activity. Immunol Cell Biol 2009; 87: 559–566. [DOI] [PubMed] [Google Scholar]
- 6. Voskoboinik I, Smyth MJ, Trapani JA. Perforin‐mediated target‐cell death and immune homeostasis. Nat Rev Immunol 2006; 6: 940. [DOI] [PubMed] [Google Scholar]
- 7. Mullbacher A, Waring P, Tha Hla R, Tran T, Chin S, Stehle T et al Granzymes are the essential downstream effector molecules for the control of primary virus infections by cytolytic leukocytes. Proc Natl Acad Sci USA 1999; 96: 13950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pham CT, Ley TJ. Dipeptidyl peptidase I is required for the processing and activation of granzymes A and B in vivo . Proc Natl Acad Sci USA 1999; 96: 8627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Adkison AM, Raptis SZ, Kelley DG, Pham CT. Dipeptidyl peptidase I activates neutrophil‐derived serine proteases and regulates the development of acute experimental arthritis. J Clin Invest 2002; 109: 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sutton VR, Waterhouse NJ, Browne KA, Sedelies K, Ciccone A, Anthony D et al Residual active granzyme B in cathepsin C‐null lymphocytes is sufficient for perforin‐dependent target cell apoptosis. J Cell Biol 2007; 176: 425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Scalzo AA, Yokoyama WM. Cmv1 and natural killer cell responses to murine cytomegalovirus infection. Curr Top Microbiol Immunol 2008; 321: 101. [DOI] [PubMed] [Google Scholar]
- 12. Akk AM, Simmons PM, Chan HW, Agapov E, Holtzman MJ, Grayson MH et al Dipeptidyl peptidase I‐dependent neutrophil recruitment modulates the inflammatory response to Sendai virus infection. J Immunol 2008; 180: 3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Simon MM, Hausmann M, Tran T, Ebnet K, Tschopp J, ThaHla R et al In vitro‐ and ex vivo‐derived cytolytic leukocytes from granzyme A × B double knockout mice are defective in granule‐mediated apoptosis but not lysis of target cells. J Exp Med 1997; 186: 1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Waterhouse NJ, Sutton VR, Sedelies KA, Ciccone A, Jenkins M, Turner SJ et al Cytotoxic T lymphocyte‐induced killing in the absence of granzymes A and B is unique and distinct from both apoptosis and perforin‐dependent lysis. J Cell Biol 2006; 173: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zavasnik‐Bergant T, Turk B. Cysteine cathepsins in the immune response. Tissue Antigens 2006; 67: 349. [DOI] [PubMed] [Google Scholar]
- 16. Evans R, Patzak I, Svensson L, De Filippo K, Jones K, McDowall A et al Integrins in immunity. J Cell Sci 2009; 122: 215. [DOI] [PubMed] [Google Scholar]
- 17. Tsukuba T, Yanagawa M, Okamoto K, Okamoto Y, Yasuda Y, Nakayama KI et al Impaired chemotaxis and cell adhesion due to decrease in several cell‐surface receptors in cathepsin E‐deficient macrophages. J Biochem 2009; 145: 565. [DOI] [PubMed] [Google Scholar]
- 18. McFarland HI, Nahill SR, Maciaszek JW, Welsh RM. CD11b (Mac‐1): a marker for CD8+ cytotoxic T cell activation and memory in virus infection. J Immunol 1992; 149: 1326. [PubMed] [Google Scholar]
- 19. Nielsen HV, Christensen JP, Andersson EC, Marker O, Thomsen AR. Expression of type 3 complement receptor on activated CD8+ T cells facilitates homing to inflammatory sites. J Immunol 1994; 153: 2021. [PubMed] [Google Scholar]
- 20. Huleatt JW, Lefrancois L. Antigen‐driven induction of CD11c on intestinal intraepithelial lymphocytes and CD8+ T cells in vivo . J Immunol 1995; 154: 5684. [PubMed] [Google Scholar]
- 21. Gold MC, Munks MW, Wagner M, Koszinowski UH, Hill AB, Fling SP. The murine cytomegalovirus immunomodulatory gene m152 prevents recognition of infected cells by M45‐specific CTL but does not alter the immunodominance of the M45‐specific CD8T cell response in vivo . J Immunol 2002; 169: 359. [DOI] [PubMed] [Google Scholar]
- 22. Tay CH, Welsh RM. Distinct organ‐dependent mechanisms for the control of murine cytomegalovirus infection by natural killer cells. J Virol 1997; 71: 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Loh J, Chu DT, O'Guin AK, Yokoyama WM, Virgin HW IV. Natural killer cells utilize both perforin and gamma interferon to regulate murine cytomegalovirus infection in the spleen and liver. J Virol 2005; 79: 661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Regner M, Pavlinovic L, Koskinen A, Young N, Trapani JA, Mullbacher A. Cutting edge: rapid and efficient in vivo cytotoxicity by cytotoxic T cells is independent of granzymes A and B. J Immunol 2009; 183: 37. [DOI] [PubMed] [Google Scholar]
- 25. Simon HG, Fruth U, Kramer MD, Simon MM. A secretable serine proteinase with highly restricted specificity from cytolytic T lymphocytes inactivates retrovirus‐associated reverse transcriptase. FEBS Lett 1987; 223: 352. [DOI] [PubMed] [Google Scholar]
- 26. Knickelbein JE, Khanna KM, Yee MB, Baty CJ, Kinchington PR, Hendricks RL. Noncytotoxic lytic granule‐mediated CD8+ T cell inhibition of HSV‐1 reactivation from neuronal latency. Science 2008; 322: 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Andrade F, Fellows E, Jenne DE, Rosen A, Young CS. Granzyme H destroys the function of critical adenoviral proteins required for viral DNA replication and granzyme B inhibition. EMBO J 2007; 26: 2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Metkar SS, Menaa C, Pardo J, Wang B, Wallich R, Freudenberg M et al Human and mouse granzyme A induce a proinflammatory cytokine response. Immunity 2008; 29: 720. [DOI] [PubMed] [Google Scholar]
- 29. Chandran K, Sullivan NJ, Felbor U, Whelan SP, Cunningham JM. Endosomal proteolysis of the Ebola virus glycoprotein is necessary for infection. Science 2005; 308: 1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Simmons G, Gosalia DN, Rennekamp AJ, Reeves JD, Diamond SL, Bates P. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc Natl Acad Sci USA 2005; 102: 11876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Andoniou CE, Andrews DM, Manzur M, Ricciardi‐Castagnoli P, Degli‐Esposti MA. A novel checkpoint in the Bcl‐2‐regulated apoptotic pathway revealed by murine cytomegalovirus infection of dendritic cells. J Cell Biol 2004; 166: 827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lopez AF, Strath M, Sanderson CJ. Differentiation antigens on mouse eosinophils and neutrophils identified by monoclonal antibodies. Br J Haematol 1984; 57: 489. [DOI] [PubMed] [Google Scholar]
- 33. Andrews DM, Andoniou CE, Granucci F, Ricciardi‐Castagnoli P, Degli‐Esposti MA. Infection of dendritic cells by murine cytomegalovirus induces functional paralysis. Nat Immunol 2001; 2: 1077. [DOI] [PubMed] [Google Scholar]
- 34. Barber GN. Host defense, viruses and apoptosis. Cell Death Differ 2001; 8: 113. [DOI] [PubMed] [Google Scholar]
- 35. Edwards KM, Kam CM, Powers JC, Trapani JA. The human cytotoxic T cell granule serine protease granzyme H has chymotrypsin‐like (chymase) activity and is taken up into cytoplasmic vesicles reminiscent of granzyme B‐containing endosomes. J Biol Chem 1999; 274: 30468. [DOI] [PubMed] [Google Scholar]


