POSTER TOPIC 01 Cell proliferation, lineages and differentiation
T01‐01A. Function of the Nkx2.2 transcription factor in oligodendrocytes and their progenitor cells
C. Fenger1, M. Thomassen2, B. Emery3, T. Kuhlmann4, T.A. Kruse2, B. Finsen 1
1University of Southern Denmark, Department of Neurobiology Research, Odense C, Denmark 2Odense University Hospital, Human Microarray Centre, Odense C, Denmark 3University of Melbourne, Centre of Neuroscience, Melbourne, Australia 4University Hospital of Münster, Neuropathology, Münster, Germany
Background: The importance of the Nkx2.2 transcription factor during oligodendrogenesis has been documented by findings of impaired oligodendrogenesis in perinatal Nkx2.2 deficient mice. However, only four Nkx2.2 target genes have been identified so far in oligodendrocyte (Ol)‐lineage cells. In concordance with its content of activator and repressor domains, Nkx2.2 promotes the expression of Ermn and Plp1 and represses the expression of Mbp and Sirt2, although they are all essential for generation of functional myelin.
Hypothesis: Nkx2.2 promotes oligodendrogenesis and myelination of CNS by acting as activator and repressor of several genes depending on the presence of other transcription factor/co‐factors at different stages of the oligodendrogenesis.
Method: To further elucidate the function of Nkx2.2 during oligodendrogenesis, primary Nkx2.2 target genes were identified in murine Ol progenitor cells (OPC) and in vitro differentiated Ols by chromatin immunoprecipitation combined with DNA sequencing. Furthermore, the transcriptome were investigated in OPC/Ol containing CNS structures of postnatal Nkx2.2 deficient and wild type mice by microarray, qPCR, and in situ hybridization.
Results: In total, 14.470 putative primary Nkx2.2 target genes were identified in OPCs and/or Ols of which 51 were significantly lower expressed in Nkx2.2 deficient mice. These genes included Nkx2.2, the known target genes; Mbp, Plp1, and Sirt2, and several genes with known or possible roles in cell division, myelination, myelin compaction, and axonal protection suggesting that Nkx2.2 activates the expression of these genes in OPCs and/or Ols. Since many of these genes appear to be target genes in OPCs and Ols, although they are not expressed by both cell types, the results of this study open the possibility that Nkx2.2 works as activator and repressor at different stages of the oligodendrogenesis. Higher NG2 and PDGFαR mRNA levels and an apparently higher number of NG2 and PDGFαR expressing OPCs in the Nkx2.2 deficient mice suggest that Nkx2.2 is important for the terminal Ol differentiation including myelination, but not for the cell fate specification of OPCs in the developing murine CNS.
Conclusion: Nkx2.2 has impact on the expression of several genes with known or possible roles in cell division, myelination, myelin compaction, and axonal protection.
T01‐01B. Influence of ECM substrate and chemotropic molecule interactions on OPC migration and differentiation
D. Harlow, W. Macklin
University of Colorado School of Medicine, Aurora, United States
Oligodendrocyte progenitor cells (OPCs) migrate long distances before differentiating and myelinating axons. It is well established that both short and long‐range soluble guidance factors, as well as contact with the surrounding extracellular matrix (ECM) and neighboring cells, can modulate OPC migration and differentiation. However, the influence of cell‐matrix interactions on OPC responses to guidance molecules is less clear. Previous in vitro studies of OPC migration in response to semaphorins and netrin‐1 yielded inconsistent results, but these studies were performed on a variety of ECM substrates, i.e., explants, collagen, poly‐D‐lysine. In the current study, we systematically studied OPC migration in response to guidance factors on several different ECM substrates, to better understand the influence of ECM interactions on OPC responses to chemotropic molecules. Understanding the regulation of OPC migration and differentiation has important implications for the treatment of demyelinating diseases such as Multiple Sclerosis (MS). For example, semaphorins 3A and 3F are expressed in active, but not chronic, MS lesions, and in demyelination/remyelination studies in animal models, semaphorins influence OPC migration into lesions, affecting the rate at which remyelination occurs. In addition, aberrant expression of ECM ligands occurs in and around MS lesions. We performed live imaging experiments of OPC migration on different ECM substrates in response to gradients of chemotropic molecules. These studies are combined with IHC, co‐IP, Western blot and RT‐PCR analyses to determine the effects of ECM substrates on OPC migration and signaling pathway responses to chemotropic molecules. Our preliminarily results suggest that ECM substrate can regulate both the direction and rate of OPC migration. Semaphorin 3A is repulsive to OPCs on laminin 1, while on fibronectin OPC migration remains uniform in the presence of semaphorin 3A. Since laminin 1 is the ligand for α6β1 integrin heterodimers, while OPC migration on fibronectin is mediated by αVβ3 integrins, the repulsive effect of semaphorin 3A appears likely to function through β1 integrin. Thus, signaling through the Neuropilin‐Plexin receptor complex that underlies the repulsive effects of semaphorin 3A on OPC migration may be modulated by interactions with β1 integrins.
T01‐02A. Oligodendrocyte precursor cells generate astrocytes after acute cortical injury
X. Bai
University of Saarland, Homburg, Germany
Secondary injury processes after acute brain trauma involve activation of different cell types like astrocytes, oligodendrocytes and microglia cells. A complex and yet not completely understood sequence of cellular responses initiate functional recovery after the neurodegeneration process. By using double‐transgenic mice GCPB (GFAP‐EGFP × PLP‐DsRed1) mice, we were able to identify a particular type of activated glia expressing astro‐ AND oligodendroglial properties simultaneously (AO cells) that transiently appeared after three types of acute cortical injuries (stab wound injury, pial vessel disruption and middle cerebral artery occlusion). AO cells could be labeled by oligodendrocyte precursor cell (OPC) markers Olig2 and Sox10, but not for markers of mature astrocytes or neurons.
Two‐photon live imaging of GCPB mice revealed that AO cells originated from PLP‐DsRed‐positive oligodendrocyte lineage cells. Electrophysiological inspection of AO cells in acutely isolated brain slices revealed the expression of delayed rectifying, voltage gated K+ currents, a typical property of NG2 glia. Therefore, we classified AO cells as OPCs. To follow the fate of AO cells which disappeared about 15 days after the injury, we generated GFAP‐N‐Cre × PLP‐C‐Cre × Rosa26EYFP triple transgenic (CreC) mice. Under non‐injury conditions, few recombined cells were observed. However, numerous recombined cells appeared after 1 week of stab wound injury adjacent to the lesion site, and lasted over 8 weeks. We found that 55–70% of recombined cells were GFAP‐positive, 18–29% of them were PDGFRα‐positive and 19–30% were positive for the oligodendrocyte marker GSTπ. In line with that, about 46% of newly generated GFAP‐positive astrocytes were observed from NG2‐CreERT2 x Rosa26tdTomato mice after 1 week of stab wound injury. These results provide a strong evidence for OPCs giving rise to astrocytes after acute cortical injuries. To further understand the molecular mechanism, we performed intra‐cerebral injection of bone morphogenetic protein 4 (BMP4, known to promote astrocyte, but blocking oligodendrocyte differentiation) into GCPB and CreC mice. BMP4, but not leukemia inhibitory factor (LIF), significantly increased the number of AO cells as well as astrocytes in the respective mice.
In conclusion, NG2/OPCs display strong potential to differentiate into astrocytes after acute cortical injury.
T01‐02B. SOX17 affects oligodendrocyte lineage progression and myelination
M. Fauveau, C. Kerninon, M. Frah, B. Nait Oumesmar
CRICM UMRS975 UPMC, Paris, France
Transcription factors play a key role in oligodendrocyte progenitor cells (OPC) specification and differentiation, as well in the myelination process of the central nervous system (CNS). Among them, the SOX transcription factors have critical functions in oligodendrocyte development. For instance, SOX9 and SOX10 have a crucial role in OPC specification and differentiation, respectively. Using microarray analysis of oligodendrocyte lineage cells, we previously identified SOX17 as a major regulator of oligodendrocyte development (Sohn et al., 2006). In OPC cultures, SOX17 expression was maximal at developmental corresponding to cell cycle exit and onset of differentiation. These findings, suggest that SOX17 has critical functions in the controlling of OPC cell cycle exit and differentiation.
In order to decipher the functional role of Sox17 in oligodendrocyte development, we generated a Tet‐Sox17:Sox10rtTA double transgenic mouse line. This mouse strain allows a SOX17 overexpression specifically in Sox10+ cells in a doxycycline dependant manner (Tet‐On system). In the CNS of Dox‐treated transgenic animals, we showed that Sox17 overexpression was specifically targeted in Sox10+ oligodendroglial cells. In the developing CNS, Sox17 overexpression was targeted in PDGFRα+/Nkx2.2+ OPCs and in Olig2+/CC1+ differentiated oligodendrocytes. To assess the effect of Sox17 gain‐of‐function on oligodendrocyte development and myelination, we analysed OPC proliferation, apoptosis and differentiation, after doxycycline induction from E12.5 to P15. In the embryonic spinal cord, we showed that overexpression of Sox17 did not modify cell proliferation or the number of PDGFRα+ OPCs. These data strongly suggest that SOX17 is not involved in early steps of oligodendrocyte development. Interestingly, we found that Sox17 gain‐of‐function drastically reduces the density of CC1+ mature oligodendrocytes at postnatal stages, correlating with a delay in myelination in transgenic mouse spinal cords. Altogether, our data reveal critical functions of SOX17 in oligodendrocyte lineage progression and myelination.
Supports: ARSEP and NMSS (USA). M.F is a PhD fellow of the French MESR (ED3C).
T01‐03A. Glial differentiation is delayed in the hypothalamus of rats malnourished during a restrict period in early life
M. Rocha, P. Fernandes, A.C. Manhães, P.C. Barradas, F. Tenorio
Universidade do Estado Rio de Janeiro, Instituto de Biologia, Rio de Janeiro, Brazil
Nutrient restriction during the perinatal period exerts a profound influence on brain development. Previous studies using an undernutrition protocol (0% protein diet) during the first half of the lactation period in rats showed that the offspring presented an altered feeding pattern, which reflected a metabolic imprinting effect on feeding behavior. There is a delay in the pattern of NPY expression in the arcuate‐paraventricular (ARC‐PVN) pathway, reflecting an effect on the development of the hypothalamic circuitry, leading to metabolic imprinting. Here we studied the effects of undernutrition on glial differentiation using the same model. We used rats from 5 to 30 postnatal (P) days of age whose dams were either fed a 0% protein diet (DG) or a normoprotein diet (CG) from P1 to P10. We assessed Ki67, vimentin, GFAP, ED‐1 and CNPase distribution in hypothalamic nuclei using immunohistochemistry. Our results showed impairment in cell proliferation, more evident in the PVN and in the lateral hypothalamus (LH), where DG animals showed a lower number of Ki‐67positive (Ki67+) cells at P5 when compared to CG. However, at P10 and P15, the number of Ki67+ cells was significantly increased in DG. From P20 onwards, staining intensity remained relatively stable and similar in all groups. DG animals showed a delay in astroglial differentiation, presenting a decrease in GFAP expression and a peak of vimentin expression at P10 and P15 accompanied by an increase in vimentin/Ki67+ cells. An increase in the number of ED1 positive cells next to the third ventricle was also shown at the same ages in DG. CNPase staining was very faint in most nuclei and did not show any obvious difference in DG animals. Our results suggest that glial differentiation is delayed in DG, which may contribute to the changes in hypothalamic circuitry that causes metabolic imprinting.
Support: CAPES, CNPq, FAPERJ and SR2‐UERJ
T01‐03B. Spatiotemporal and genetic fate‐mapping of oligodendrogenesis following cuprizone‐induced demyelination
T. Merson, Y. Xing, P. Röth, S. Ng, T.J. Kilpatrick
The University of Melbourne, Florey Institute of Neuroscience and Mental Health, Melbourne, Australia
Oligodendrocyte progenitor cells (OPCs) are considered the principal cell type responsible for the production of new oligodendrocytes during remyelination of the central nervous system (CNS). Recent studies have demonstrated that neural precursor cells (NPCs) residing in the adult subventricular zone also exhibit the capacity to generate new oligodendrocytes following experimental demyelination. The relative capacities of these two cell types to contribute to oligodendrogenesis in this context remain largely unexplored. We have adopted transgenic approaches that enable either the specific labeling of NPCs or oligodendrocytes and the interrogation of their subsequent fate within the corpus callosi of mice subjected to central demyelination induced by cuprizone. Adult NestinCreERT2: R26R‐eYFP mice were administered tamoxifen (0.3g/kg/day) for 4 days by oral gavage, inducing the permanent expression of yellow fluorescent protein (YFP) in NPCs and their progeny. Mice were subsequently fed 0.2% cuprizone for 6 weeks followed by 6 weeks recovery on normal food to enable the analysis of remyelination. Expression of YFP and other cellular markers was examined immunohistochemically to determine the fate and migratory potential of NPCs in the remyelinating corpus callosum (CC). Rostrocaudal analyses of CC in the cuprizone‐challenged mice demonstrated that approximately 60% of YFP+ NPCs commit to an oligodendroglial fate. There was robust recruitment of NPC‐derived oligodendroglial cells, with a 14‐fold increase in YFP+Sox10+ cells compared to unchallenged mice. Most of these cells were mature oligodendrocytes (YFP+CC1+) and their density in the remyelinating CC was highest adjacent to the dorsolateral corner of SVZ and decreased proportionally with distance in the mediolateral axis. On the other hand, fate mapping of OPCs using PdgfRaCreERT2: R26R‐eYFP mice revealed that oligodendrocytes derived from parenchymal OPCs were distributed in a converse manner to SVZ‐derived oligodendrocytes. In addition, we found that, independent of the cellular source, many oligodendrocytes repopulated the corpus callosum as linear arrays of clonally derived cells, in a manner that recapitulated the postnatal development of these structures. These results demonstrate that lineage determination and maturation of oligodendroglia from NPCs occurs efficiently in situ. We also reveal that remyelination occurs in a coordinated way, most likely secondary to interactions between a restricted pool of axons and a sentinel OPC induced to proliferate in situ.
T01‐04A. Enhanced human and murine oligodendrocyte differentiation in response to a selective thyroid hormone β receptor agonist
E. Baxi, A. Fairchild, C. Pardo‐Villamizar, J. Rothstein, D.E. Bergles, P. Calabresi
Johns Hopkins University, Baltimore, United States
Demyelinating diseases such as multiple sclerosis are characterized by loss of myelin and oligodendrocytes. Oligodendrogenesis is essential for successful remyelination and repair. Thyroid hormones are known to play an important role in developmental oligodendrogenesis and myelination and mounting evidence from rodent models of demyelination suggest that they also promote remyelination. The therapeutic application of thyroid hormone to demyelinating disorders is limited by the potential for cardiac side effects mediated by thyroid hormone receptor (THR) α signaling. Here we report that GC‐1, a thyromimetic with selective THRβ1 action promotes in vitro oligodendrogenesis from both rodent and human oligodendrocyte progenitor cells. In addition, we used PDGFαR‐CreER;Rosa26‐eYFP double‐transgenic mice to examine the effect of GC‐1 on the fate of oligodendrocyte progenitor cells and find that treatment with GC‐1 during developmental myelination promotes oligodendrogenesis in vivo. These results indicate that a β receptor selective thyromimetic can enhance OL differentiation in vitro and during developmental myelination and warrants further study in demyelinating models.
T01‐04B. The Stem Cell Propeties of Adult Olfactory Horizontal Basal Cells
Y.‐I. Ohnishi 1, K. Shinzawa2, K. Iwatsuki1, T. Yoshimine1
1Osaka University Medical School, Depatment of Neurosurgery, Suita, Japan 2Osaka University Medical School, Department of Molecular genetics, Suita, Japan
The olfactory epithelial layer contains multipotent horizontal basal cells (HBCs) that differentiate into olfactory sensory neurons. We generated olfactory sphere (OS) cells in cultures that were derived from adult rat olfactory mucosa. Fluorescence‐activated cell sorting and immunofluorescence analyses showed that OS cells were derived from HBCs. OS cells underwent neuronal and glial differentiation in vitro. To examine multipotent differentiation in vivo, OS cells were transplanted into injured rat spinal cords. The transplanted cells integrated into host tissue and underwent glial differentiation. Our data showed the stem cell properties of HBCs.
T01‐05A. Intrinsic mechanisms regulating oligodendrocyte progenitor cell division: the role of Citron‐kinase
E. Boda 1, S. Piretta1, F. Bianchi2, F. Di Cunto2, A. Buffo1
1University of Turin, Dept. of Neuroscience, Neuroscience Institute Cavalieri Ottolenghi, Orbassano (Turin), Italy 2University of Turin, Molecular Biotechnology Center, Dept. of Genetics, Biology and Biochemistry, Turin, Italy
Oligodendrocyte progenitor cells (OPCs) comprise the main cycling cell population of the CNS parenchyma during the early postnatal period and at adult stages. However, the molecular mechanisms implicated in OPC divisions are still by large obscure. With the aims to unveil i) whether OPC divisions exploit the same molecular machinery of neuronal precursors, and ii) whether distinct OPC subsets exist with different cell division machineries, we focused on a mutant mouse where Citron‐kinase, a crucial regulator of cytokinesis in neuronal precursors, is germinally ablated (CIT‐K KO). These mice have severe CNS developmental abnormalities, reduction of selected neuronal populations due to apoptosis triggered by defective divisions, display ataxia, epileptic seizures and die by the third postnatal week. Interestingly, they were reported to have myelin defects, suggesting that the CIT‐KO affects oligodendroglial cells. Notably, already early after birth we found a 2‐fold decrease in OPC density in both the cortical grey (GM) and white matter (WM), compared to wild‐type mice (WT). Here, most OPCs were hypertrophic and multinucleated, indicating a cytokinesis failure after nuclear division. At later ages, OPCs progressively disappeared from the cortical GM, while in both WM and ventral areas of the telencephalon (i.e. striatum, hypothalamus), uni‐and multi‐nucleated OPCs could be detected, although at lower densities compared to WT. These data suggests OPC heterogeneity in both CIT‐K requirement for cytokinesis and susceptibility to death. However, even where normal uninucleated OPCs persisted, we could not find pre‐myelinating or myelinating cells, in line with additional differentiation defects. To discriminate the contribution of cell‐autonomous vs. environmental factors in differentiation impairment, we performed homochronic grafts of WT fluorescently tagged (GFP+) cells into perinatal CIT‐KO mice. Strikingly, GFP+ OPCs invaded the whole CIT‐K KO brain and actively divided. However, they hardly ever differentiated into more mature cells at difference with cells grafted in the WT, indicating that altered environmental signals contribute to myelination defects in CIT‐K KO. In conclusion, our results show that CIT‐K is involved also in glial cell divisions and suggest distinct CIT‐K requirement and proneness to death of dorsal and ventral OPCs. Additionally, CIT‐K ablation results in pervasive nervous tissue alterations affecting OPC maturation. Further studies will clarify the molecular mechanisms underlying these alterations.
T01‐05B. An ex‐vivo model of radial glial cell development in the spinal cord
J. Pakan, K. McDermott
University College Cork, Anatomy and Neuroscience, Cork, Ireland
CNS progenitor cells are important for repair after injury and in degenerative diseases. Knowledge of how these cells act in repair is lacking, partly due to a limited understanding of their behaviour in normal developmental processes. Radial glia cells are a unique population of neural progenitors that have recently been suggested to function in neurogenesis in the cerebral cortex. However, many questions still remain regarding their specific role in the developing spinal cord. Using an organotypic spinal cord slice culture model and two‐photon microscopy we directly visualized radial glial cell behaviour in the developing spinal cord. 400 µm slices of rat embryonic spinal cord (E12‐E16) were cultured in order to preserve the 3D cytoarchitecture of the cellular environment. Using electroporation, slices were transfected with a brain‐lipid binding protein (BLBP) driven GFP expression plasmid, which specifically labels endogenous radial glial cells. Slice cultures were then imaged using two‐photon time‐lapse microscopy, allowing for high spatially and temporally resolved 4D imaging. We were able to follow individual GFP expressing radial glial cells over time (24 hours to 6 days) and characterized precise morphological changes. At early developmental ages, GFP expressing cells initially exhibited a radial morphology with the soma located in the ventricular zone and processes extending out to the pial surface. Over time, many cells developed a multipolar morphology and migrated away from the lumen, primarily using somal translocation. Using immunohistochemistry we identified populations of transfected cells which also expressed GFAP, representing the differentiation of BLBP‐expressing radial glia cells into astrocytes. We also found that radial glial cells differentiate into oligodendrocytes (Olig2 and CNPase positive) but found little evidence for GFP co‐localization with neuronal markers (NeuN, DCX, βIIITubulin). Using this model we can directly observe complex developmental behaviours of specific cell populations and elucidate the relevant genetic regulation and molecular mechanisms that govern spinal cord development. By understanding these intricate events, we will gain insight into how a simple tube of undifferentiated neuroepithelial cells generates different cell populations that will eventually develop into the adult spinal cord.
T01‐06A. Fast repopulation of microglia after ablation
J. Bruttger 1, S. Woertge1, S. Yona2, Y. Wolf2, M. Prinz3, S. Jung2, A. Waisman1
1University Medical Centre Mainz, Mainz, Germany 2Weizmann Institute of Science, Rehovot, Israel 3University of Freiburg, Department for Neuropathology, Freiburg, Germany
Question: Unlike other glia cells, microglia originate from the myeloid cell lineage and therefore often are considered as resident brain macrophages. They act in the first response to direct injury or peripheral insults. However, their exact contribution in brain homeostasis is not yet clear. Using a novel system microglia were depleted in vivo. After optimizing depletion protocol, the role of microglia in health and disease was analyzed.
Methods: We used the new Cre‐expressing mouse strain CX3CR1CreER to express a diphtheria toxin receptor (DTR) specifically on microglia/macrophages. In these mice, administration of tamoxifen leads to Cre‐mediated excision of a loxP flanked transcriptional STOP element in both macrophages and microglia, thus allowing for the expression of the DTR as well as an YFP reporter. Due to their fast turnover macrophages are replaced by unaffected precursors whereas microglia persist in the modified stage. These mice were used for in vivo and ex vivo analysis of microglia by FACS and histology.
Results: Our optimized protocol with two tamoxifen injections at the age of 2 weeks resulted in 80–90% of reporter‐positive microglia in adulthood, whereas all peripheral macrophages were reporter‐negative. With additional three successive DT injections we could achieve a microglia ablation efficiency of 80%. Interestingly, histological as well as FACS analysis revealed 50% of microglia repopulation already 4 days after depletion. Two weeks after depletion microglia numbers were even higher compared to unaffected controls. On day 6, we found a CD45.2high Ly6C+ population, indicating replenishment by peripheral monocytes. In BM chimera experiments, where peripheral cells were labeled with a congenic marker, we obtained striking evidence showing that almost all of the repopulating cells are of peripheral origin.
Conclusions: After depletion microglia were rapidly repopulated, which emphasizes their critical role in brain homeostasis and function.
T01‐06B. Mesenchymal Stem Cells Retain Their Pro‐oligodendrogenic Activity During Aging: A Rationale For Autologous Transplantation in Multiple Sclerosis
F.J. Rivera 1,2,3, R. Wodnar1,2, M. Feichtner1,2, E. Oberbauer1,2, G. Brachtl4, R. Greil4, E. Rohde5, R.J.M. Franklin3, L. Aigner1,2
1Paracelsus Medical University, Institute of Molecular Regenerative Medicine, Salzburg, Austria 2Paracelsus Medical University, Spinal Cord Injury and Tissue Regeneration Center Salzburg (SCI‐TReCS), Salzburg, Austria 3University of Cambridge, Wellcome Trust and MRC Cambridge Stem Cell Institute & Department of Veterinary Medicine, Cambridge, United Kingdom 4Federal Hospital of Salzburg and Paracelsus Medical University, Laboratory for Immunological and Molecular Cancer Research, 3rd Medical Department for Hematology, Medical Oncology, Hemostasiology, Infectious Diseases and Rheumatology, Salzburg, Austria 5Federal Hospital of Salzburg and Paracelsus Medical University, Department of Blood Group Serology and Transfusion Medicine, Salzburg, Austria
Multiple Sclerosis (MS) is an autoimmune disease of the central nervous system (CNS) and ultimately leads to oligodendrocytes' and myelin loss and to severe neurological deficits. Mesenchymal stem cells (MSCs) display immunemodulatory and neuroprotective activities and promote oligodendrocyte differentiation of neural progenitors (NPCs) and are thus interesting candidates for autologous cell therapy in MS. Even though MS has an onset in young patients, it persists throughout life and progresses along adulthood and aging. Therefore, MSCs from aged individuals should be tested for their pro‐oligodendrogenic activity before moving into the clinical trials. This is insofar of clinical relevance, since the brain's capacity for self‐repair and remyelination strongly declines with aging. Thus, the aim of this study was to determine whether MSCs from aged individuals maintain their known pro‐oligodendrogenic activity. MSCs were obtained from bone marrow of young (2 months) as well as old (17–20 months) rats and analyzed their stem cell properties as well as their pro‐oligodendrogenic activity. As expected, MSCs derived from aged bone marrow presented diminished stem cell properties and differentiation capacity. To test the MSC pro‐oligodendrogenic activity, NPCs were incubated with MSC conditioned medium. Surprisingly, soluble factors derived from old rats MSCs maintain their ability to induce an increase of MBP‐expressing oligodendrocytes on NPCs. Moreover, aged MSCs conserve their oligodendrogenic activity also on NPCs derived from old donors (20 months). These results indicate that MSCs keep their oligodendrogenic activity regardless of age and, therefore, this study provides a rationale for clinical trials of autologous MSC transplantation in MS therapy.
T01‐07A. Proliferation of reactive astrocytes is enriched in juxtavascular positions
J. Frik 1,2, S. Bardehle1,3, M. Krüger4, I. Bechmann4, L. Dimou1,3, N. Plesnila5,6, M. Götz1,3,6
1Institute of Physiology, LMU, Physiological Genomics, Munich, Germany 2University of La Plata, Institute for Biotechnology and Molecular Biology, La Plata, Argentina 3Institute for Stem Cell Research, Helmholtz Zentrum, Munich, Germany 4Institute for Anatomy, University of Leipzig, Leipzig, Germany 5Institute for Stroke and Dementia Research (ISD), University of Munich Medical School, Experimental Stroke Research, Munich, Germany 6Munich Cluster for Systems Neurology (Synergy), Munich, Germany
Astrocytes fulfill essential functions under physiological conditions and are involved in various beneficial and adverse functions after brain injury (reviewed by Sofroniew 2009). In order to improve functional recovery after injury, it is essential to delineate the beneficial from adverse effects. However, little is yet to know which extent distinct sets of astrocytes perform distinct functions or whether all astrocytes behave in a uniform manner. Live imaging of astrocytes after stab wound injury in the adult cerebral cortex in vivo revealed a striking heterogeneity of astrocyte behaviour with distinct subsets extending long processes towards the site of injury, others proliferating and yet other subtypes undergoing only hypertrophy. Most strikingly, the vast majority of astrocytes proliferating were located with their somata directly at blood vessels (capillaries or post‐capillary vessels). This close proximity was confirmed at ultrastructural level and identified as juxtavascular positions, sometimes adjacent to pericytes located in the perivascular space.
This novel population of reactive astrocytes at juxtavascular positions is of particular interest, as the capacity of astrocytes to reactivate proliferation (Buffo et al. 2008; Simon et al. 2011) and stem cell potential (reviewed by Robel et al. 2011.) seems to be tightly linked (Sirko et al., Cell Stem Cell, in press). Therefore we examined whether this population of juxtavascular astrocytes would also preferentially proliferate in even more severe injury conditions, such as 1 hour of middle cerebral artery occlusion (MCAO). The proliferation of juxtavasular astrocyte was analyzed immunohistochemically in fixed brain sections co‐stained for S100b/GFAP/Ki67/CD31 at different time points after injury. At 3 days post injury, 18% of all astrocytes were actively dividing (Ki67+). Strikingly the majority of these was again located at juxtavascular positions (68%), suggesting a general bias of astrocytes at this position to proliferate after injury, while astrocytes further distant from blood vessels virtually fail to divide. We shall further present data from conditional knock‐out mice with reduced proliferative activity of juxtavascular astrocytes in order to elucidate the function of this highly specific astrocyte population and their proliferative reaction after injury.
T01‐07B. Lithium promotes generation of oligodendrocytes ex vivo in the adult mouse optic nerve
A.D. Rivera, A. Butt
University of Portsmouth, Portsmouth, United Kingdom
The generation of oligodendrocytes (OLs) from their precursors (OPCs) occurs throughout adult life and is particularly important following demyelinating insults, such as occurs in multiple sclerosis (MS). Glycogen synthase kinase 3‐β (GSK3β) acts as a constitutively active negative regulator of multiple pathways that regulate proliferation, survival and differentiation of OPCs. Lithium is a potent inhibitor of GSK‐3 and is used for the treatment of bipolar disorder, but in addition is neuroprotective in a wide range of diseases, including stroke, amyotrophic lateral sclerosis, and Alzheimer's disease. Lithium inhibits GSK‐3 by binding directly to the enzyme's magnesium‐sensitive site and indirectly by enhancing its phosphorylation. Notably, by inhibiting GSK3b, lithium acts as a Wnt mimetic to promote β‐catenin‐dependent transcriptional events, including enhancement of genes involved in apoptotic inhibition. Here, we have examined the effects of lithium on oligodendrocytes in the adult mouse optic nerve, a typical CNS white matter tract; all procedures were in accordance with the Animals Scientific Procedures Act (1986). Transgenic mice aged postnatal day (P) 35 were used in which oligodendrocyte specific genes drive the expression of fluorescent reporters: Sox10‐EGFP for OLs and OPCs, and PLP‐DsRed for differentiated OLs. Mice were killed humanely and optic nerves (ONs) with the retina intact (ON‐retina) were isolated intact and maintained in organotypic culture conditions for 3 days in vitro (DIV), in medium containing lithium (20mM) or control medium. Cell counts and western blot analysis showed that lithium treatment stimulated cell proliferation and survival, and dramatically increased the generation of OLs compared with controls (p < 0.05, unrelated t‐test). Whole genome microarray and western blot analyses indicated lithium down‐regulated the Wnt signalling pathway and Id2/4, which inhibit OL differentiation, and up‐regulated pro‐differentiation factors such as Olig2 and Sox10. The results provide evidence that lithium promotes the generation of OLs in the adult CNS by regulating multiple pathways, and further studies are required to identify the diverse potential targets of lithium in oligodendrocytes.
Supported by an Anatomical Society PhD Studentship.
T01‐08A. Enteric glia: S100B, GFAP and beyond
D. Grundmann 1, E. Loris1, D. Simon1, F. Markwart1, W. Huang2, F. Kirchhoff2, K.‐H. Schäfer1
1University of Applied Sciences, Zweibrücken, Germany 2University of Saarland, Molecular Physiology, Homburg, Germany
Introduction: The enteric nervous system (ENS) harbours neurons, glial cells and their precursors derived from the neural crest. For years, enteric glia cells have played a “wallflower”‐like role, although their importance was already recognised by various authors, including Michael Gershon, Georgio Gabella and Kristjan Jesssen, who demonstrated that enteric glia contain the glial fibrillary acidic protein (GFAP) like reactive astrocytes in the central nervous system. In recent years, the key role of enteric glia in supplying neurotrophic support, responding to inflammation or being the source for neural stem cells has become increasingly obvious. There must be more than one or two individual glial subtypes to fulfill the different tasks.
Objectives: To deliver a baseline for further studies on the role of enteric glia in different diseases and species, we tried to deliver a basic inventory which includes general (S100B) and reactive (GFAP) glial markers, as well as neural stem cell (Nestin) or markers for specific so far not identified glial cells. We studied the enteric nervous system using different transgenic mouse models for Nestin (GFP), NG2 (tdtomato) and PLP1 (DsRed) to evaluate the glial composition in the individual segments of the gastrointestinal tract. Whole‐mounts were dissected from stomach, small intestine and colon of the different transgenic mice and additional immunofluorescence stainings were performed to analyze the glial phenotype.
Results: We found both S100B+/Nestin+ and S100B+/Nestin‐ glial cells in the myenteric plexus of the different segments of the gastrointestinal tract.In addition, we revealed that there are S100B glial cells outside the myenteric ganglia between the ganglia and in the muscle layers which are positive for the oligodendroglial or Schwann cell marker PLP1. NG2+ cells (and their progeny) with different morphologies are present in the gastrointstinal tract. The NG2 proteoglycan did not co‐localize with the other glial markers S100B or GFAP, but partly with PDGFRα and SK3 which are shown as fibroblast‐like cell markers in the gastrointestinal tract.
Conclusion: Our data demonstrate that the enteric glial population is heterogenous. There are not only glial cells with astrocytic characteristics but also (longitutinal) glial cells with oligodendroglial or Schwann cell properties.
T01‐08B. Low density lipoprotein receptor‐related protein 1 (LRP1) is expressed on radial glia cells and controls their differentiation towards oligodendroglia
D. Safina 1, E. Hennen1, U. Huassmann2, P. Wörsdörfer3, F. Edenhofer3, A. Poetsch2, A. Faissner1
1Ruhr‐University Bochum, Department of Cell Morphology and Molecular Neurobiology, Bochum, Germany 2Ruhr‐University Bochum, Department of Plant Biochemistry, Bochum, Germany 3University of Bonn‐Life&Brain Center, Institute of Reconstructive Neurobiology, Bonn, Germany
In the developing and adult CNS multipotent neural stem cells reside in distinct niches. Specific carbohydrates and glycoproteins are expressed in these niche microenvironments that are important regulators of cell fate determination and stem cell maintenance. In previous studies we described LewisX (LeX) glycan as a specific marker of neural stem precursor cells (NSPCs) in developing brain and showed it to be involved in NSPCs migration and proliferation. Using LeX glycosylation as a biomarker we aimed to identify the LeX carrier glycoproteins which could have a functional relevance for CNS development. In addition to already known LeX carriers we have found LRP1 as new major LeX carrier and for the first time showed it's expression in NSPCs and developing brain.
LRP1 is essential for the normal neuronal function in adult CNS, whereas the role of LRP1 in development is still unclear, mainly due to the lethality of the LRP1 knock‐out in mice.
In the current study we investigated the basic properties of LRP1 knock‐out NSPCs created by means of Cre‐loxp mediated recombination. The elimination of LRP1 in vitro was induced by the addition of cell permeable Cre‐recombinase to NSPCs derived from embryonic brain of LRP1flox/flox mice. The functional status of LRP1‐deficient cells was subsequently studied using proliferation, migration and differentiation assays.
LRP1 knock‐out cells maintained as neurospheres, retained the ability to migrate and differentiate. Interestingly, LRP1 knock‐out NSPCs generated 3‐times less OPCs in comparison to LRP1wt/wt cells. This suggests that LRP1 is involved in the control of the oligodendroglial differentiation.
T01‐09A. Continuous live imaging of reactive astrocyte divisions in vitro
G. Heimann 1, M. Götz1,2,3, S. Sirko1,2
1Institute of Physiology, Ludwig‐Maximilians University Munich, Physiological Genomics, Munich, Germany 2Institute for Stem Cell Research, Helmholtz Zentrum Munich, German Research Center for Health, Munich, Germany 3Munich Cluster for Systems Neurology (SyNergy), Munich, Germany
Astrocytes are multifaceted cells in the adult central nervous system and react to pathology of the adult brain with a finely graded continuum of morphological and behavioral changes. Given that astrocytes in healthy adult brain rarely divide outside the stem cell niches, but activate both proliferation as well as a stem cell potential after injury (for review see Robel et al., 2011), it is important to understand their exact mode of cell division. Indeed, a defining hallmark of a stem cell is the capacity to self‐renew – often by asymmetric cell division. Therefore we set out to image reactive astrocyte divisions in vitro by developing a primary culture system for reactive astrocytes obtained from the somatosensory cortex of adult mouse at 5 days after stab wound injury. Continuous live imaging and single cell tracking (Costa et al., 2008, 2011) allowed us observing the mode of cell division mode as well the cell fate of their immediate progeny. Results of our study reveal (i) that reactive astrocytes can undergo multiple rounds of cell division, (ii) the SHH signaling has a profound influence on this behavior, (iii) reactive astrocytes divide with distinct modes of cell division, which can be influenced by the local microenvironment. Taken together, this new culture system allows close examination of signals on the mode and multitude of reactive astrocyte cell divisions and provides a great tool to identify signals derived from the extracellular matrix, other cell types or as diffusible signals.
T01‐09B. Probing for oligodendrocyte progenitor cell function by limiting their proliferation in the adult brain
S. Schneider 1, C. Simon1, M. Irmler2, J. Beckers2,3, G. Eichele4, M. Götz1,5,6, L. Dimou1,5
1Physiological Genomics, Institute of Physiology, Ludwig‐Maximilians University, Munich, Germany 2Institute of Experimental Genetics, Helmholtz Zentrum München, Neuherberg, Germany 3Chair of Experimental Genetics, Technical University Munich, Freising‐Weihenstephan, Germany 4Department of Genes and Behavior, Max Planck Institute for Biophysical Chemistry, Goettingen, Germany 5Institute for Stem Cell Research, Helmholtz Zentrum München, Neuherberg, Germany 6Munich Cluster for Systems Neurology (SyNergy), Munich, Germany
NG2+ cells, also known as oligodendrocyte progenitor cells (OPCs), are the only proliferating cells outside the neurogenic niches in the adult brain and constitute a major fraction (5–10%) of the cells at this age. Despite their high numbers and substantial characterization, their actual function in the adult brain is still widely unknown. To tackle this important question we used conditional deletion of the Esco2 protein triggering apoptosis of dividing cells during M‐phase (Whelan et al. 2012) in the inducible Sox10‐iCreERT2xCAG‐eGFPxEsco2fl/fl mouse line (Simon et al. 2012) to specifically delete OPCs. As the proliferation of OPCs is tremendously increased after stab wound injury (Simon et al. 2011), we reasoned this lesion might play an important role in this context. Preliminary results indicate that the Esco2 deletion in OPCs results in a transient reduction in OPC numbers resulting in alterations of other glial cell populations. Interestingly, even without injury temporary depletion of proliferating OPCs leads to behavioral deficits which will be described. To identify the molecular mechanisms by which NG2+ cells may react and influence other cells after brain injury, we isolated Sox10‐GFP+ cells by FACS either from the intact or the lesioned cerebral cortex at three days after stab wound injury. Comparative genome‐wide expression profiling revealed exciting candidates for OPC function in the physiological and pathological brain.
T01‐10A. Thyroid hormone mediated OPC differentiation is ‘Hairless'
M. Hofer 1, A. Baer2, M.J. Rossner3, M. Trotter1, M. Kotter1
1University of Cambridge, Cambridge, United Kingdom 2Medical University Vienna, Department of Neurosurgery, Vienna, Austria 3Max Planck Institute for Experimental Medicine, Göttingen, Germany
Oligodendrocytes are the myelin forming cells of the central nervous system (CNS) that enable fast salutatory signalling transduction and mediate trophic support and protection of axons. Oligodendrocytes originate from a population of precursors cells (oligodendrocyte precursor cells, OPCs) that are formed at distinct developmental stages. The biological process leading to OPC differentiation involves a cascade of molecular events that eventually constitute the complex and multifaceted cellular program that determines the generation of mature oligodendrocytes. To identify the earliest detectable transcriptional correlates of differentiation we used a microarray approach investigating changes in gene expression occurring in primary rat OPCs within the first 12 hours after purification.
Amongst the most regulated factors that were detected was hairless (Hr), a nuclear receptor co‐repressor that has been associated with thyroid hormone signalling in the neonatal CNS. Validation of the microarray results by RT‐qPCR, Western blot and immunochemistry confirmed a rapid increase of Hr expression during OPC differentiation in media containing thyroid hormone. In the absence of thyroid hormone, Hr expression was suppressed and associated with impaired OPC differentiation. Similarly, RNAi mediated gene silencing of Hr induced an impairment of OPC differentiation demonstrating an important functional role of Hr in the differentiation program. Thyroid hormones regulate Hr expression via the thyroid hormone receptors THRα and THRβ as RNAi‐mediated gene silencing of each of the receptors resulted in a decrease in Hr expression. Hr is known to negatively regulate RAR‐related orphan receptor alpha (RORα) and Vitamin D receptor (VDR). However, RNAi‐mediated gene silencing of RORα and VDR did not change the course of OPC differentiation.
Interestingly, an interaction between Hr and histone de‐acetylases (HDACs), of which HDAC1 and 2 are known regulators of OPC differentiation, has been noted in the past. Using an in situ protein‐protein interaction assay we were able to demonstrate a direct interaction of Hr with HDAC1 and HDAC2 in differentiating OPCs. Moreover, ChIP qPCR analysis revealed Hr‐HDAC1 recruitment to the OPC differentiation inhibitor Hes5. Taken together these data show that thyroid hormone promotes OPC differentiation via Hr by modulating HDAC1 and 2 function. As iodine deficiency results in hypothyriodism our results give an example of a developmental disease that is triggered by environmental factors (the absence of iodine) causing epigenetic dysregulation.
T01‐10B. Differential effects of Wnt3a and lithium on astrocytes in the adult mouse optic nerve
C. Stagni, A.D. Rivera, A. Butt
University of Portsmouth, Portsmouth, United Kingdom
Astrocytes respond to all forms of CNS insults by reactive astrogliosis, which involves changes in their molecular expression and morphology, and in most severe cases glial scar formation. Reactive astrogliosis is potentially neuroprotective, but can also act as chemical and physical barrier to axon growth and repair. However, mechanisms underlying this process are still poorly understood. The enzyme glycogen synthase kinase 3‐β (GSK‐3β) is a key regulator of many signalling pathways involved in cell proliferation, survival and differentiation, including Wnt signalling. Lithium is a potent inhibitor of GSK‐3 and acts as a Wnt mimetic to promote β‐catenin‐dependent transcriptional events. Wnt signalling is altered following CNS injury and in a range of neurodegenerative diseases, such as stroke, amyotrophic lateral sclerosis, and Alzheimer's disease. Notably, lithium is neuroprotective in these same diseases. Here, we have examined the effects of Wnt3a and lithium on astrocytes of the adult mouse optic nerve, a typical CNS white matter tract; all procedures were in accordance with the Animals Scientific Procedures Act (1986). We used transgenic mice aged postnatal day (P) 35 inwhich GFAP drives the expression of eGFP. Optic nerves (ONs) were isolated with the retina intact and maintained in organotypic culture for 3 days in vitro (DIV), in control medium, or medium containing lithium chloride, or Wnt3a. Both agents resulted in a significant increase in the number of astrocytes, compared to controls (p < 0.05, unrelated t‐tests), but lithium and Wnts3a had markedly divergent effects on astrocyte morphology. In the presence of lithium, astrocytes became highly polarised, extending long thin processes that traversed the nerve perpendicularly to the long axis. In contrast, Wnt3a treatment resulted in the generation of morphologically simple astrocytes, with short, fine branching processes that extended radially from a centrally located cell body. Genome wide microarray analysis indicated Wnt3a and lithium differentially altered chondroitin sulphate proteoglycans (CSPGs), a family of extracellular matrix molecules highly expressed at CNS injury sites. Further experiments are required to define the molecular phenotypes of the novel astrocytes generated by these treatments, but the results provide evidence that Wnt signalling regulates astrogenesis in situ and is therefore a potential molecular target to modulate astrogliosis. The contrasting effects of lithium suggest it is acting via other pathways, which require further study.
Supported by the International Spinal Research Trust.
T01‐11A. Mouse Schwann cell culture and the expression of L‐MAG in Schwann cells and in myelinating cocultures
H. Honkanen, A. Heape
University of Oulu, Oulu, Finland
The knowledge of the control of SC proliferation is essential for understanding how the PNS nerve develops, how the myelin sheath is formed by SCs, and what happens during the degeneration of myelin sheath. Here we investigated the effects of several growth factors with the aim of improving the efficiency of our published protocol for expanding mouse SCs (Honkanen et al, 2007). We have studied the expression of L‐MAG in Schwann cells alone, and during early phases of myelination in an attempt to better understand the role of L‐MAG during PNS myelination.
Studies of Mouse Schwann cell culture. The proliferation of SCs is regulated by axonal signals and several growth factors. Growth factors that are essential for rodent SC survival in culture include heregulin‐β1 and forskolin. In the case of rat SCs, these factors are also sufficient to allow a robust proliferation. In contrast, the proliferation of mouse‐derived SCs in culture requires the presence of other growth factors. We studied effect of several factors known to be expressed in SCs after nerve injury on mouse SC proliferation. These included fibroblast growth factor type 2 (FGF), pituitary extract (PE), epidermal growth factor, platelet‐derived growth factor BB, and transforming growth factor β1. We found that heregulin‐β1 and forskolin are essential for mouse SC to survive in culture, FGF and PE were the best combination of growth factors promoting the mouse SC proliferation, and that neuronal axons provide effective mitogenic environment for mouse SCs.
The expression of the Large Myelin‐Associated Glycoprotein in PNS was studied in SCs cultured alone and in cocultures of SCs and DRGNs. When Schwann cells are alone without axons, expression of the cytoplasmic domain of L‐MAG is seen in the nucleus of Schwann cells. When Schwann cells make contact with neuronal axons, the location of the expression shifts out of the nucleus to the perinuclear and cytoplasmic regions; the extracellular domain is not visible. until the Schwann cells initiate the myelination programme. These results support the hypothesis that L‐MAG might have role in the regulation of the myelination programme in the PNS.
T01‐11B. Neuroprotective properties of glia‐committed NG2‐positive cells: comparison with uncommitted mesenchymal stem cells derived from Wharton jelly
J. Sypecka, A. Sarnowska
Instytut medycyny Doświadczalnej i Klinicznej PAN, Warszawa, Poland
Oligodendrocyte progenitor cells (OPCs) have been shown to exhibit certain properties of neural stem cells. They have become the focus of intense research for the purpose of designing cell replacement therapies to be used in acquired or inherited neurodegenerative disorders characterized by oligodendrocyte depletion and ongoing demyelination. It has been postulated that trophic support provided by either stem cells or precursors is actuallyone of the most beneficial effects in the cell‐based therapies. To address this issue, a comparison between the neuroprotective properties of OPCs and the stem cells derived from Warton jelly was carried out in the co‐culture system with ischemically injured organotypic hippocampal slices. Those two cell types were selected in respect of the advancement in their differentiation process. While the mesenchymal stem cells residing in Warthon jelly are still pluripotent, the OPCs are already the glia‐committed precursors. To evaluate their neuroprotective properties, we set ‐up an ex vivo co‐culture system of either stem cells or OPCs isolated from neonatal rat brain with organotypic hippocampal slices subjected to a short oxygen‐glucose deprivation (OGD). The cell death, as well as the number of the newborn cells has been quantified in slices co‐cultured for one week. A microarray analysis of a broad spectrum of trophic factors and cytokines expressed by OPCs was performed for the purpose of finding the factor(s) contributing to the observed effect. Three of them were selected for the subsequent blocking experiments with specificl antibodies to verify their influence on the injured tissue. The results obtained in the study prove that both cell types secrete soluble factors and are potent in exerting the neuroprotective effect in a paracrine‐like manner, promoting cell survival and proliferation in damaged hippocampal slices. In conclusion, the observed advantageous qualities of stem cells and oligodendroglia‐biased progenitors significantly contribute to anticipating a clinical improvement in cell replacement therapies.
Supported by grants 0345/B/P01/2010/38 and 6430/B/P01/2011/40.
T01‐12A. Variable differentiation potential of NG2 glia during mouse development
W. Huang 1, N. Zhao2, A. Cupido2, X. Bai2, A. Scheller2, S. Goebbels3, F. Kirchhoff2
1University of Saarland, Homburg, Germany 2University of Saarland, Molecular Physiology, Homburg, Germany 3Max‐Planck‐Institute of Experimental Medicine, Department of Neurogenetics, Göttingen, Germany
NG2 is a type I transmembrane glycoprotein and also known as chondroitin sulphate proteoglycan 4 (CSPG4). In the central nervous system NG2‐expressing cells have been identified as a novel type of glia with a strong potential to generate oligodendrocytes in the developing white matter. For temporally‐controlled gene targeting of NG2 glia in vivo, we generated a mouse line in which the open reading frame of the tamoxifen‐inducible form of Cre recombinase (CreERT2) was inserted into the NG2 locus by homologous recombination.
Here, we investigated the differentiation potential of NG2 glia at different developmental stages of the forebrain. Cre recombinase activity was induced at embryonic day 17.5, postnatal day 0 (P0), P3, P8, P30 and P60 by intraperitoneal tamoxifen injections into novel TgH(NG2‐CreERT2) mice crossbred to Rosa26‐tdTomato and Rosa26‐EYFP reporter lines that helped to identify NG2 glia and its progeny. Recombination and cell identification were determined 10 to 60 days after the first tamoxifen injection.
Induction of recombination at embryonic stages revealed that embryonic NG2+ cells mainly generated more NG2 glia and oligodendrocytes, however, a significant number of astrocytes could be detected as well. Recombined cells were predominantly restricted to the ventral brain. In contrast, after tamoxifen injections at P0 and P3 recombined cells were widely found in all brain regions, thereby suggesting the presence of a distinct subpopulation of NG2+ cells after birth. Interestingly, only very few recombined astrocytes could be found, which are probably derived from few remaining embryonic NG2 glia. Starting from P8, NG2 glia stopped generating astrocytes, with the progeny largely restricted to the oligodendrocyte lineage. However, consistently, we found recombined NeuN+ cells with the morphology of neurons in the ventral cortex after administration of tamoxifen at P8. Even more of such cells were present in the whole cortex when Cre activity was induced in adult mice. The morphology, the presence of NeuN immunoreactivity and our electrophysiological characterization that demonstrated bona fide action potentials clearly identify these cells as functional neurons.
Our results suggest that NG2+ cells display a broad differentiation potential that appears to be highly age‐dependent during development.
T01‐12B. Notch Signaling in in vitro derivation of Schwann cells from bone marrow stromal cells
E.W.Y. Tai 1, G.K.H. Shea2, A.Y.P. Tsui2, K.H.Y. Leung2, Y.S. Chan1, D.K.Y. Shum2
1University of Hong Kong Li Ka Shing Faculty of Medicine, Physiology, Pokfulam, Hong Kong 2University of Hong Kong Li Ka Shing Faculty of Medicine, Pokfulam, Hong Kong
Bone marrow stromal cells can be an autologous source of Schwann cells for transplantation to treat demyelination related diseases. Our strategy of deriving Schwann cells from bone marrow stromal cells (Shea et.al, 2010) exploited dorsal root ganglia (DRG) neurons to provide Schwann cell‐like cells with juxtacrine signals that mediated commitment to the Schwann cell fate. To address if the juxtacrine signaling is served by Notch and its ligands, analysis of the DRG neurons found cell surface immunopositivities for the ligands, DLL1 and Jagged1 whereas that of bone marrow‐derived Schwann cell‐like cells found immunopositivity for the receptor, Notch‐1. In cocultures with DRG neurons, during which Schwann cell‐like cells underwent changes towards fate commitment, the nuclear localization of Notch intracellular domain (NICD) and the expression of downstream target gene hes1 by PCR, were indicative of Notch signaling. This was simultaneous with progressive increase in ErbB2/B3 expression as revealed by immunocytochemistry and Western blotting. Taken together, Notch signaling between DRG neuron and Schwann cell‐like cells in contact mediated the upregulation of ErbB receptors in Schwann cell‐like cells for signaling interaction with neuregulin as they transition into fate commitment.
T01‐13A. The effect of microglia and secreted factors on cell density in oligodendrocyte precursor cell culture
K. Kleinsimlinghaus, M. Serdar, R. Marx, I.D. Dietzel
Ruhr‐Universität Bochum, Biochemie II – Neurobiochemie, Bochum, Germany
In order to develop strategies to expand olidodendrocyte progenitor cultures (OPCs), leading to as high yields of OPCs as possible, we investigated the influence of different culture conditions on OPC proliferation rate (using a BrdU‐assay) and the number of surviving cells (by counting DAPI‐stained nuclei). After we had observed, that the choice of the basal medium (neurobasal, RPMI or DMEM) exerts a pronounced effect on OPC proliferation we here investigated the effect of cell density on OPC proliferation.
Starting from a small seeding density (10.000 cells/cm2) a tenfold increase in seeding density only resulted in a fourfold augmentation of the density of surviving cells after 7 days in culture. Using plating densities of 5.000; 20.000 and 80.000 cells/cm2 we observed, that an increase in seeding density led to a decrease in the proliferation rate, as tested with a BrdU‐assay and monitored after 3 days in culture. Similarly, the percentage of A2B5‐positive, immature, cells was lower, the higher the cell density.
We then tested whether this finding is due to proliferation inhibiting factors secreted in cultures with high seeding densities and whether the remaining microglia population could be involved. To this aim we seeded OPCs at low density (5.000 cells/cm2) and cultivated them with the medium supernatant of cultures with high density (80.000 cells/cm2). Furthermore we seeded OPCs in low density (5.000 cells/cm2 ) and co‐cultivated them with an added density of microglia as counted in cultures of high density (80.000 cells/cm2).
The resulting cell counts after 3 days showed, that the percentage of BrdU positive cells after cultivation with the supernatant was significantly reduced compared with control cultures, although the number of A2B5‐positive cells was not influenced by this treatment. The addition of microglia to the cultures lead to a strong reduction of surviving cells, even though the percentage of BrdU and A2B5 positive cells were not altered. So apoptosis, necrosis or phagocytosis might be responsible for the reduction of surviving cells.
Our findings suggest that the reduction of surviving OPCs in high density cultures is due to secreted factors inhibiting OPC proliferation and that microglia could be involved in this effect.
T01‐13B. Usefulness of glucose transporter‐5, MHC class II, CD68, and Iba‐1 as human microglia markers
K. Takahashi
National Hospital Organization Iou Hospital, Kanazawa, Japan
[Background] In neuropathological studies, it is important to detect microglial cells; however, a specific microglia marker has yet to be determined. [Methods] In this study, we examined and compared the usefulness of various microglia markers, including human glucose transporter‐5 (GLUT‐5), MHC class II, CD68, and Iba‐1. [Results] All of the microglia markers consistently stained for microglia in paraffin sections fixed with formalin. CD68 staining of microglia in the gray matter was not as well defined as the other microglia markers. Moreover, CD68 did not clearly stain the microglial processes. With regard to MHC class II, it was difficult to observe the microglial processes in the white matter. In contrast, both GLUT‐5 and Iba‐1 clearly stained for microglia in the gray and white matter, and provided detailed staining of microglial processes. Although it has previously been shown that perivascular cells are negative for GLUT‐5, we found perivascular macrophages to be GLUT‐5 positive. [Conclusions] Nevertheless, GLUT‐5 and Iba‐1 may be considered as markers suitable for routine histopathological staining procedures.
T01‐14A. Use of genetic tools to perform in vivo analysis of glia in the Enteric Nervous System
R. Lasrado 1, S. McCallum1, W. Boesmans2, P.V. Berghe2, V. Pachnis1
1National Institute for Medical Research, London, United Kingdom 2TARGID, KULeuven, Leuven, Belgium
Neurons and glia of the Enteric Nervous System (ENS) originate from a pool of Sox10+ progenitors. In addition to being a scaffold for enteric neurons, enteric glial cells (EGCs) have been suggested to function in mucosal integrity, neuroprotection, adult neurogenesis, neuro‐immune interactions and synaptic transmission. Currently, diverse glial populations are known to reside within the ENS. However, whether, specific functions are carried out by specialized glial subtypes, with characteristic morphologies and molecular markers and within specific locations in the gut remains elusive.
To address this question we used a repertoire of genetic tools, immunostaining and imaging techniques. For high single‐cell resolution study of EGCs, we Mosaic analysis with Double Markers (MADM) in conjunction with Sox10‐Cre. We analyzed adult gut tissue to characterize the different glial populations based on morphology, position in the ENS and their relationship with the intestinal epithelial and vascular system. Moreover, we used reporter mice (Sox10‐iCreERT2; R26R‐YFP and GFAP‐iCreERT2; R26R‐YFP) to quantify the expression of three glial markers – GFAP, S100β and Sox10 in myenteric plexus (MP) preparations of adult mice.
Our investigation on MADM‐MP preparations, revealed three subtypes of enteric glia in the MP of adult mice. Type I and Type II glia, present in the primary plexus of mouse ENS have been previously defined. In addition, we characterized a third subtype in the extraganglionic space of the MP of adult mice, at present termed as Type III‐extraganglionic glia. Furthermore, our analyses using 3D‐imaging, on MADM‐gut sections allowed us to characterize the relationship of enteric glia located within the mucosa, with the intestinal epithelium and vascular system of the villi.
Our marker expression analysis showed that while the majority of glial cells co‐expressed GFAP and S100β, a large fraction of glia within the myenteric ganglia was positive for either S100β (∼30%) or GFAP (∼10%). We also found that S100β+/GFAP‐ glia were abundant outside the ganglia. Most glial cells co‐expressed Sox10 with GFAP and S100β yet a significant proportion (∼10%) of mainly extra‐ganglionic glia (Type III) expressed only Sox10. Surprisingly, ∼5% of the cells that were S100β‐ and Sox10‐ displayed high GFAP expression. Use of GFAP‐iCreERT2; R26R‐YFP mice showed that about ∼30% of YFP‐labelled cells did not express GFAP, implying dynamic regulation of GFAP expression.
Overall, our high resolution studies reveal extensive heterogeneity of enteric glial cells in terms of morphology, position and expression of molecular markers suggesting differential functional roles. Our future experiments will address whether different physiological roles of EGCs can be assigned to specific subtypes.
T01‐14B. BMP‐Smad1/5/8 Signaling is Necessary for Development of Müller Glia
Y. Ueki, K. Cox, T. Reh
University of Washington, Seattle, United States
Müller glia are the major type of glia in the retina, spanning its entire thickness. It is known that BMP signaling promotes astroglial differentiation in cortex, but its role in Müller glial development in the retina has not been studied. The purpose of this study was to investigate the role of BMP‐Smad1/5/8 signaling in Müller glial development. C57BL/6 mouse retinas were collected at various ages between postnatal day 0 (P0) and P21 in order to analyze the expression and activation of Smad1/5/8. Smad1/5/8 was transiently but robustly activated in the inner nuclear layer, including developing Müller glia between P5‐P8. The timing of this transient activation of Smad1/5/8 corresponds with the timing of Müller glial differentiation and maturation. To test the hypothesis that activation of BMP‐Smad1/5/8 signaling is essential for Müller glial development, retinas were explanted at P3 or P6 and treated with BMP4 or a BMP inhibitor dorsomorphin (DM) for 5 days in order to activate or inhibit Smad1/5/8, respectively. When P3 and P6 retinal explants were treated with DM for 5 days in vitro, there was a significant reduction in Müller glial markers (CRALBP, GS, Sox9, and Sox2). Smad1/5/8 activation and CRALBP expression were also significantly attenuated in vivo 1 day after intraocular injection of a BMP inhibitor DM at P5. Our data suggest that activation of BMP‐Smad1/5/8 signaling is necessary for the development of Müller glia.
This work was supported by 1R01EY021482 to TAR.
T01‐15A. ROCK inhibition with Y27632 promotes the proliferation and cell cycle progression of cultured astrocyte from spinal cord
X. Luo
Huazhong University of Science and Technology, Wuhan, China
Rho‐associated Kinase (ROCK) has been identified as an important regulator of proliferation and cell cycle progression in a number of cell types. Although its effects on astrocyte proliferation have not been well characterized, ROCK has been reported to play important roles in gap junction formation, morphology, and migration of astrocytes. In the present study, our aim was to investigate the effect of ROCK inhibition by [(+)‐(R)‐trans‐4‐(1‐aminoethyl)‐N‐(4‐pyridyl) cyclohexanecarboxamide dihydrochloride] (Y27632) on proliferation and DNA synthesis in cultured astrocytes from rat spinal cord and the possible mechanism involved. Western blots showed that treatment of astrocytes with Y27632 increased their expression of cyclin D1, CDK4, and cyclin E, thereby causing cell cycle progression. Furthermore, Y27632‐induced astrocyte proliferation was mediated through the extracellular‐signalregulated kinase signaling cascade. These results indicate the importance of ROCK in astrocyte proliferation.
T01‐15B. Regional differences in differentiation properties highlight heterogeneity among adult oligodendrocyte progenitor cells
F. Viganò 1,2, M. Götz1,2,3, L. Dimou1,2
1Physiological Genomics, Institute of Physiology, Ludwig‐Maximilians University, Munich, Germany 2Institute for Stem Cell Research, Helmholtz Zentrum München, Neuherberg, Germany 3Munich Cluster for Systems Neurology (SyNergy), Munich, Germany
Glial cells in the adult brain are very heterogeneous and some of them represent the stem and progenitor cells of the CNS. The only proliferating cells in the healthy, adult cerebral cortex outside the neurogenic niches are the oligodendrocyte progenitor cells (OPCs), also named NG2+ cells. Adult OPCs in the mouse cerebral cortex regulate their proliferation, self‐renewal and differentiation in a rather dynamic manner, with acute invasive injury promoting increased and faster proliferation, while running leads to their fast differentiation (Simon et al., 2011; Dimou and Götz, 2012). Interestingly, also under physiological conditions OPC proliferation and differentiation are differentially regulated between the white (WM) and grey matter (GM) of the cerebral cortex (Dimou et al., 2008; Kang et al., 2010; Psachoulia et al. 2009), with OPCs resident in the WM preferentially differentiating into mature, myelinating oligodendrocytes (Dimou et al., 2008; Kang et al., 2010). Here we used cell transplantation experiments to determine to which extent this differential behavior is regulated by environmental signals differing between the WM and GM, or intrinsic fate determinants restricted to one or the other cell population. These experiments indicate that the WM promotes differentiation of OPCs to mature oligodendrocytes, as the rate of differentiation of GM cells increased when exposed to this environment. Interestingly, cells derived from the WM retained their higher differentiation rate also in a less supportive environment like the GM. These results reveal not only important environmental differences for OPC maturation, but also support the concept of intrinsic heterogeneity among adult OPCs, persisting even when exposed to more inhibitory environments. Thus, this work provides the basis to identify molecular pathways regulated by distinct niche/environmental signals and involved in the heterogeneity of adult OPCs.
T01‐16A. Inhibition of endogenous Phosphodiesterase 7 promotes oligodendrocyte precursor survival and differentiation
E.M. Medina‐Rodriguez 1, F.J. Arenzana1, J. Pastor2,3, M. Redondo4, V. Palomo4, R. Garcia de Sola2,3, C. Gil4, A. Martinez4, A. Bribian1,5, F. de Castro1
1Hospital Nacional de Paraplejicos, Grupo Neurobiologia del Desarrollo, Toledo, Spain 2Hospital Universitario La Princesa, Neurocirugia, Madrid, Spain 3Hospital Universitario La Princesa, Neurofisiologia clínica, Madrid, Spain 4CSIC, Instituto de Química Médica, Madrid, Spain 5Universidad de Barcelona, Instituto de Bioingeniería de Cataluña, Barcelona, Spain
Multiple sclerosis (MS) is a chronic inflammatory and neurodegenerative demyelinating disease of the central nervous system (CNS) characterized by inflammation, which leads to formation of demyelinating areas due to loss of oligodendrocytes, astrogliosis and, finally, axonal degeneration. Different studies have suggested that 3′‐5′‐cyclic adenosine monophosphate (cAMP) levels might play an important role in neuroprotection and neuroinflammatory response so the modulation of this nucleotide intracellular levels might control the neuroinflammatory pathological process and, consequently, to delay the progression of MS.
Intracellular cAMP levels depend on its synthesis by adenylyl cyclases, and on its degradation by cyclic nucleotide 3′,5′‐phosphodiesterases (PDEs). Specific inhibitors for the different isoforms of PDEs family, particularly cAMP‐specific PDE, emerge as putative treatments of this kind of disease. PDE7 has emerged as a new therapeutic target, not only for a variety of immunological and immunodeficiency conditions to alleviate chronic inflammation, but also for several neurodegenerative disorders, including MS, in which both immune system and CNS are implicated.
In the present work, we have detected the expression of PDE7 in oligodendrocyte precursor cells (OPCs) isolated from cerebral cortex of P0 and P15 mice and from adult human samples derived from neurosurgery of epilepsy. OPCs are abundant in the mice and human CNS during development but they also represent 5–7% of the total number of cells in the adult CNS, where they serve as a source of new oligodendrocytes to replace those which die. However, in MS these endogenous OPCs are not able to effectively replace dead oligodendrocytes. In vitro, we have demonstrated that two newly‐developed PDE7 selective inhibitors (TC 3.6 and VP 1.15) favour OPC survival and differentiation towards mature oligodendrocytes, being both more effective than the commercial PDE7 inhibitor BRL‐50481. ERK intracellular pathway has been identified as a key in these cellular processes related with the cAMP/PKA/CREB pathway. Moreover, we have observed that both specific inhibitors (VP1.15 and TC3.6) increase the differentiation of human OPCs. These findings, combined with the already known anti‐inflammatory effect carried out by these PDE7 inhibitors, point them as potential therapeutic agents to treat MS. The knowledge of factors that affect the biology of murine OPCs and even more, human OPCs, are critical for possible remyelination in this kind of pathologies.
T01‐16B. Foxj1 identifies cells other than ependymal epithelia that contribute to CNS remyelination
B. Wang, D. Ma, E. Rawlins, R.J.M. Franklin, C. Zhao
University of Cambridge, Cambridge, United Kingdom
Ependymal cells lining the central canal of spinal cord have properties of adult neural stem cells. Their role in CNS remyelination has not been fully investigated. Previous studies using genetic fate mapping techniques revealed their recruitment into injured spinal cord and differentiation into astrocytes and oligodendrocytes. In this study, we used aninducible Cre‐floxed‐Stop‐Rosa26‐YFP mediated lineage tracing strategy to characterize the fate of foxj1 expressing ependymal cells during remyelination of toxin‐induced demyelination. We found that in unlesioned spinal cord, foxj1 labelled ependymal cells. However, it also labeled a population of cells in the spinal roots with immnochemical features of fibroblasts. Following lysolecithin‐induced demyelination in the dorsal funiculus, there were few YFP expressing cells in the lesion area at 5 and 14 days post lesion (dpl) indicating that the ependymal cells were not recruited in demyelinated area. However, in ventral white matter lesions, which were adjacent to damaged nerve roots, many cells were detected expressing YFP. The distribution of YFP positive cells overlapped that of remyelinating Schwann cells and a number of YFP expressing cells colocalised with mature Schwann cell marker periaxin. These data revealed a foxj1 expressing population in peripheral nerve which migrates into demyelinated lesions andcontributes to CNS remyelinaiton.
T01‐17A. Specification and maintenance of oligodendrocyte precursor cells from neural progenitor cells: involvement of microRNA‐7a
X. Zhao
The Fourth Military Medical University, Xi'an, China
The generation of myelinating cells from multipotential neural stem cells in the CNS requires the initiation of specific gene expression programs in oligodendrocytes (OLs). We reasoned that microRNAs (miRNAs) could play an important role in this process by regulating genes crucial for OL development. Here we identified miR‐7a as one of the highly enriched miRNAs in oligodendrocyte precursor cells (OPCs), overexpression of which in either neural progenitor cells (NPCs) or embryonic mouse cortex promoted the generation of OL lineage cells. Blocking the function of miR‐7a in differentiating NPCs led to a reduction in OL number and an expansion of neuronal populations simultaneously. We also found that overexpression of this miRNA in purified OPC cultures promoted cell proliferation and inhibited further maturation. In addition, miR‐7a might exert the effects just mentioned partially by directly repressing proneuronal differentiation factors including Pax6 and NeuroD4, or proOL genes involved in oligodendrocyte maturation. These results suggest that miRNA pathway is essential in determining cell fate commitment for OLs and thus providing a new strategy for modulating this process in OL loss diseases.

POSTER TOPIC 02 Cell signalling
T02‐01A. Cell‐specific receptor expression defines the differential response of astrocytes versus microglia to oncostatin M
I. Campbell, M.‐P. Hsu
University of Sydney, Sydney, Australia
The IL‐6/gp130 family of cytokines including IL‐6, IL‐11, leukemia inhibitory factor (LIF) and oncostatin M (OSM), are pivotal mediators in a wide range of processes in the CNS ranging from neurodevelopment to neuroinflammation. Surprisingly little is known about the relative responses of glial cells to these cytokines. In addressing this problem we first examined the expression of the receptors for IL‐6, IL‐11, LIF and OSM in cultured murine astrocytes versus microglia. In astrocytes, the OSMR mRNA was most abundant while low levels of the IL‐6R, IL‐11R and LIFR mRNAs were also detectable. In comparison, in microglia, neither the OSMR nor LIFR mRNAs were detectable while the IL‐11R mRNA and IL‐6R mRNA were present at similar and higher levels, respectively. Immunoblotting for the IL‐6R and OSMR proteins revealed that both astrocytes and microglia produced IL‐6R however, the OSMR was abundant in astrocytes but not detectable in microglia. Consistent with the pattern of receptor expression, astrocytes and microglia both responded to hyper‐IL6 with strong activation of STAT3. However, in response to OSM, STAT3 activation was induced in astrocytes but not microglia. Moreover, in contrast to a previous report, OSM failed to activate NFkB or induce NOS2 in microglia. In conclusion, these findings, (1) identify notable differences in the expression of some key IL‐6/gp130 family cytokine receptors in astrocytes and microglia, and (2) provide the molecular basis for the differential response of astrocytes versus microglia to OSM. Supported by NHMRC project grant 632754.
T02‐01B. Microglial P2Y receptor‐mediated currents and ATP release in situ
C. Madry 1, R. Jolivet1, C. Eder2, D. Attwell1
1University College London, London, United Kingdom 2St. George's, University of London, London, United Kingdom
Microglia, the resident immune cells of the brain, are very sensitive to changes in their environment, and respond rapidly to brain injury. Purinergic receptor signalling is crucially involved in the communication between microglia and their surrounding cells. ATP or related compounds released from neurons evoke signalling through ionotropic P2X and metabotropic P2Y and P1 receptors. This enables microglia to find damaged cells, to which they send out highly branched and motile processes, and also triggers transformation of microglia into activated, phagocytotic cells. Due to the multitude of different P2Y receptor subtypes expressed, microglia are able to respond to a wide range of extracellular nucleotides such as ATP (possibly using P2Y2), ADP (using P2Y1,12,13), UTP (P2Y2,4) or UDP (P2Y6).
Here we show that, in addition to currents mediated directly through P2X receptors, P2Y receptor activation also causes secondary ion fluxes across the cell membrane. By whole‐cell patch‐clamping microglial cells the rat hippocampal slices, we found that low concentrations of ATP or ADP (+current which causes a long lasting hyperpolarisation of the cell. Pharmacological analysis using PSB‐0739, 2‐methylthio‐AMP and MRS‐2211, and blockade by pertussis toxin and N‐ethylmaleimide, identified Gi‐coupled P2Y12 receptors as the mediator of this current. Interestingly, in the presence of 10 mM intracellular EGTA, adding 50 mM intracellular BAPTA (which should negligibly increase calcium buffering) blocked the current. UDP and UTP also generated microglial cell hyperpolarisation.
Furthermore, we found that upon inducing a change in extracellular flow, microglia were capable of releasing their own ATP (detected using the luciferin‐luciferase assay in the slice). By pulling out individual microglial cells from the slice we confirmed that the ATP is released by microglia themselves. Subsequently, the ATP and its enzymatically produced ADP evoked a hyperpolarisation of the cell through a P2Y12 receptor‐dependent signalling cascade as outlined above.
The changes in membrane potential seen in the presence of extracellular nucleotides may be linked to microglial ATP‐induced chemotaxis, which has been shown to crucially depend on P2Y12 receptor activation.
Supported by the ERC, Wellcome Trust and a Marie Curie Fellowship to R.J.
T02‐02A. High‐resolution electrophysiological determination of unitary exocytic events in cultured astrocytes
A. Guček 1, J. Jorgačevski1,2, B. Rituper1, M. Kreft1,2,3, R. Zorec1,2
1University of Ljubljana, Ljubljana, Slovenia 2Celica, Biomedical Center, Ljubljana, Slovenia 3University of Ljubljana, Biotechnical faculty, Ljubljana, Slovenia
Bidirectional communication between neurons and astrocytes, the most abundant type of glial cells, has become a subject of numerous researches, embodying astrocytes as an important partner in synaptic modulation. Astrocytes possess Ca2+‐based cytosolic excitability, which can induce release of gliotransmitters introducing (patho)physiological impact on surrounding cells. Non‐vesicular and vesicular‐based mechanisms appear to co‐exist in astrocytes, however, the lack of understanding the basic astrocytic properties of secretion requires additional insight into regulated exocytosis. To directly monitor exocytosis of individual vesicles with the plasma membrane we employed the high‐resolution cell‐attached patch‐clamp technique on cultured astrocytes. We monitored changes in membrane capacitance (Cm), a parameter linearly related to the surface area of the membrane. Preliminary results show, that in astrocytes discrete steps in membrane capacitance reflect unitary exo‐endocytic events of single vesicles. For the first time we demonstrate direct real‐time measurements of predominantly reversible capacitance steps, reflecting transient exocytosis, and irreversible capacitance steps, reflecting full‐fusion exocytosis, in cultured astrocytes.
T02‐02B. Epicatechin promotes the generation of oligodendrocytes ex vivo in the adult mouse optic nerve
F. Pieropan, A.D. Rivera, A.V. Patel, R. Gibbs, P. Cox, A. Butt
University of Portsmouth, Portsmouth, United Kingdom
The phosphatidylinositol‐3 kinase (PI3K)‐Akt signalling pathway promotes cell proliferation and cell survival in oligodendrocyte lineage cells. The flavanol epicatechin has been shown to be neuroprotective and is believed to act in part via PI3K/Akt. Here, we examined whether epicatechin is pro‐survival for oligodendrocytes ex vivo in the mouse optic nerve. Transgenic mice aged postnatal day (P) 35 were used in which oligodendrocyte specific genes drive the expression of fluorescent reporters: the Sox10‐EGFP strain was used to identify oligodendrocytes (OLs) and their precursors (OPCs), and PLP‐DsRed to identify differentiated OLs. Mice were killed humanely in accordance with the Animal Scientific Procedures Act (1986), and optic nerves (ONs) with the retina (ON‐retina) were isolated intact and maintained in culture, either in normal medium, or medium containing epicatechin or the PI3K inhibitor LY294002. After 3 days in vitro (DIV), optic nerves were examined by confocal microscopy, cell counts were performed in a constant field of view (FOV), and significance determined by ANOVA followed by Bonferroni post hoc test. Compared to controls (Sox10+ cells 107 + 1.9 per FOV, n = 4; PLP+ cells 26.8 + 2.5 per FOV, n = 7), epicatechin (20 µM) marginally, but statistically significantly, increased Sox10+ cells (118.3 + 1.8 per FOV, n = 4; p < 0.001) and almost doubled PLP+ OLs (49.5 + 2.2 per FOV, n = 4; p < 0.001). In contrast, LY294002 (50 µM) significantly decreased Sox10+ cells (87.2 + 1 per FOV, n = 4; p < 0.001), but had no statistical effect on PLP+ OLs (47.6 +10.6 per FOV = 4, p>0.05). The results indicate that epicatechin increased OPCs and OLs, and support the possibility it may act in part through the PI3K/Akt signalling pathway. Further experiments are required to determine the mechanism of action of epicatechin, but this study supports the possibility that epicatechin could contribute to strategies for promoting the generation of OLs in adult CNS white matter.
Supported by HEIF.
T02‐03A. RXR Signalling and Regulation in Oligodendrocyte Lineage Cells
A. Guzman de la Fuente 1, J. Huang1, B. Nait Oumesmar2, C. ffrench‐Constant3, R. Franklin1
1Wellcome Trust/MRC Stem Cell Institute, Cambridge, United Kingdom 2Centre de Recherche de l'Institut du Cerveau et de la Moelle Epinière – CRICM, Paris, France 3MRC Centre for Regenerative Medicine, Queen's Medical Research Institute, Edinburgh, United Kingdom
Myelin sheaths are lost from CNS axons in demyelinating diseases. New sheaths can be regenerated following the recruitment of adult neural progenitor cells (OPC) into areas of injury and their differentiation into myelinating oligodendrocytes. Although this regenerative process (called remyelination) occurs efficiently in the early stages of Multiple Sclerosis, its efficiency gradually declines leaving axons demyelinated and vulnerable to degeneration. Several signalling pathways are involved in regulating OPC differentiation and subsequent myelination. Understanding their mechanisms is necessary to design regenerative therapies to overcome remyelination failure.
We have identified RXRγ as a key positive regulator of OPC differentiation and remyelination (Huang et al., 2011). RXRγ is a nuclear receptor forms a heterodimer with other nuclear receptors to activate its downstream signalling cascades.
Here we describe RXRγ binding partners expressed in both OPC and oligodendrocytes, and the binding of VDR, RARβ and PPARγ to RXRγ within oligodendrocyte lineage cells by coimmunoprecipitation. VDR‐ RXRγ heterodimer is necessary for the oligodendrocyte differentiation. Treatment of oligodendrocytes with VDR antagonist inhibits OPC differentiation and abrogates the effect of 9‐cis retinoic acid, an RXRγ agonist, in OPC differentiation.
Our data suggests a model in which RXRγ is an essential anchor for different nuclear receptors, including VDR, with a shifting and complex RXRγ signaling in oligodendrocyte lineage cells.
T02‐03B. Alpha and gamma secretase dependent cleavage of the glial proteoglycan NG2/Kontiki is conserved between species and mediates signalling
D. Sakry 1, A. Bustos2, F. Biname1, B. Altenhein2, K. Endres3, J. Trotter1
1Johannes Gutenberg University, Biology, Dept. of Molecular Cell Biology, Mainz, Germany 2Johannes Gutenberg University, Biology, Institute of Genetics, Mainz, Germany 3University Medical Centre Johannes Gutenberg‐University, Department of Psychiatry and Psychotherapy, Mainz, Germany
Cleavage of type I membrane proteins by α‐ and γ‐secretases to yield biologically active fragments is best exemplified for the evolutionarily conserved protein Notch. Here we show that the protein NG2, a type I membrane protein which has homologues in mouse (NG2) and Drosophila (Kontiki) and is expressed by multiple immature cell types including glia, is processed in a similar fashion to Notch. In both mouse and Drosophila this generates a major 290 kD shedded ectodomain, a c‐terminal fragment (CTF) and a small intracellular domain (ICD). The NG2 ICD is present after overexpression in cells of biochemical isolates of nuclear fractions, and can be visualized in the nucleus by immunofluorescence of cultured murine glial cells. In glial nuclei of developing Drosophila larva, a striking intranuclear staining of the Kontiki ICD is observed. Levels of expression of NG2 mRNA and protein in mouse glial cells are regulated by the neurotransmitter glutamate and overexpression of cleaved fragments modulates protein expression in murine glial cells. These results demonstrate that cleavage of NG2 takes place in mouse and Drosophila glia and that this generates fragments with signaling properties. Modulation of NG2 expression by glutamate provides a way for neuronal activity to regulate NG2 signalling.
T02‐04A. TLR‐signaling induces Type I interferon responses in microglia and astrocytes and regulates leukocyte infiltration to the CNS
R.M.H. Khorooshi 1, T. H. Holm2, C. Tue Berg1, R. Truong Dieu1, D. Dræby1, S. Lienenklaus3, T. Owens1
1Institute of Molecular Medicine, Odense, Denmark 2Medical Biochemistry, Department of Biomedicine, Århus, Denmark 3Helmholtz Centre for Infection Research, Braunschweig, Germany
Our goal is to understand the glial signaling that controls inflammatory responses in the central nervous system (CNS). This glial response can play both a detrimental and beneficial role. Toll like receptor (TLR) signaling is implicated in responses to pathogens or endogenous signals. TLR signaling mediates immune response by inducing cytokines, including type I interferons (IFN). IFN‐beta, a member of the type I IFN family, is a first‐line therapeutic for multiple sclerosis. Type I IFNs signal through a common receptor, IFNAR. The aim of the present study was to investigate the in vivo response of microglia and astrocytes to CNS administration of TLR ligand/agonist, and to examine whether this response involves type I IFN signaling. Mice were administered TLR ligands/agonists by injection to the cisterna magna. We analyzed leukocyte entry to the CNS by flow cytometry, and their localization by immunostaining. The induction of IFN‐beta was examined by in vivo imaging of an IFN‐beta reporter mouse. Astrocytes and microglia were sorted by FACS and gene expression was measured using quantitative real time‐PCR. Injection of ligands/agonists for TLR2, 3 and 4 resulted in infiltration of CD45+ leukocytes after 18 hrs, most strongly by TLR2. Immunostaining showed parenchymal localization of infiltrating cells in cerebellum. FACS sorted astrocytes expressed equivalent levels of TLR3 mRNA to microglia but lower levels of TLR2 or 4. Astrocytes were induced by TLR3 signaling to express interferon regulatory factor 7, which regulates the induction of type I IFN. The induction of IFN‐beta in CNS in response to TLR3 signaling was verified in IFN‐beta reporter mice. TLR2, 3 and 4 signaling led to increased levels of mRNA for glial CXCL10. Together these results suggest the involvement of type I IFN signaling. However, unlike CXCL10 gene expression, that was dependent on IFNAR signaling, TLR2‐induced leukocyte infiltration was not affected in IFNAR deficient mice. These studies point to a role for TLR signaling in the innate glial response that regulates CNS inflammation.
T02‐04B. Phosphoinositide 3‐kinaseγ mediates microglial phagocytosis via lipid kinase‐independent control of cAMP
C. Schmidt 1, N. Schneble1, J. Müller1, R. Bauer1, A. Perino2, R. Marone3, S.D. Rybalkin4, M.P. Wymann3, E. Hirsch2, R. Wetzker1
1Molecular Cell Biology, University Hospital, Jena, Germany 2Molecular Biotechnology Center, University of Torino, Torino, Italy 3Biochemistry and Genetics, University Basel, Basel, Switzerland 4Pharmacology, University of Washington, Seattle, United States
Microglial phagocytosis plays a key role in neuroprotective and neurodegenerative responses of the innate immune system in the brain. Here we investigated the regulatory function of the signaling protein phosphoinositide 3‐kinaseγ (PI3Kγ) in phagocytosis of bacteria and Zymosan particles by mouse brain microglia in vitro and in vivo.
Using genetic and pharmacological approaches our data revealed PI3Kγ as an essential mediator of microglial phagocytosis. Unexpectedly, microglia expressing lipid kinase deficient mutant PI3Kγ exhibited similar phagocytosis as wild‐type cells. Detailed analysis disclosed PI3Kγ dependent stimulation of cAMP phosphodiesterase as a crucial mediator of phagocytosis. Both stimulation of protein kinase A and Epac were shown to convey inhibitory effects of cAMP on phagocytosis.
Together our findings indicate PI3Kγ‐dependent suppression of cAMP signaling as an indispensible regulatory element of microglial phagocytosis.
T02‐05A. The involvement of NO‐mediated signaling in PDT‐induced injury of neurons and glial cells
V. Kovaleva, E. Berezhnaya, M. Rudkovskii, A. Uzdensky
Southern Federal University, Rostov‐on‐Don, Russian Federation
Photodynamic therapy (PDT) is currently used in oncology, particularly, in treatment of brain tumors. The possible role of NO‐mediated signaling in photodynamic injury and protection of neurons and surrounding glial cells (GC) was studied. Crayfish stretch receptor consisting of a single neuron enveloped by GC was photosensitized with alumophthalocyanine Photosens (10 nM, 30 min incubation) and irradiated with laser diode (670 nm, 0.4 W/cm2). Application of NO generators notably 10 mM sodium nitroprusside and 10 mM NONOate reduced PDT‐induced necrosis of GC and showed the same tendency in neuronal necrosis. NONOate significantly increased PDT‐induced apoptosis of GC. Inhibitor of neuronal NO‐synthase L‐NAME (1mM) significantly increased the percentage of necrotic glial cells after PDT but did not influence neuronal necrosis. This confirmed the anti‐necrotic effect of NO in glial cells and involvement of neuronal NO synthase in protection of glial cells. 1 mM L‐NAME and 1 mM L‐NNA (another inhibitor of neuronal NO‐synthase) as well as 50 μM S‐Methhylisothioharnstoff Sulfat (inhibitor of inducible NO synthase) protected GC from PDT‐induced apoptosis. Therefore NO may be involved in PDT‐induced apoptosis of glial cells. Inhibition of NO‐activated protein kinase G with 10 μM KT5823 decreased the percentage of necrotic glial cells but not neurons. Therefore protein kinase G appeared to be involved in PDT‐induced necrosis of GC, possibly independently on NO. KT5823 also increased the level of apoptosis of GC indicating the anti‐apoptotic role of protein kinase G. Thus NO is involved in regulation of PDT‐induced necrosis of neurons and glial cells as well as in apoptosis of glial cells.

T02‐05B. EphB1/ephrin‐B1 reverse signalling induces astrocyte activation
G. Tyzack, T. Cymes, N. Lau, A. Lakatos
University of Cambridge, Cambridge, United Kingdom
Background and Question. Emerging evidence suggests that signalling by damaged neurones leads to reactive astrocyte response that may have a fundamental impact on synaptic reorganisation. Soluble factors, such as cytokines, have been described to induce such astrocyte activation. However, rapid contact dependent signalling between damaged neurones and astrocytes is also a possibility. In light of recent findings of EphB1 upregulation on injured neurons closely surrounded by reactive astrocytes, we hypothesized that EphB1 can signal to ephrin‐B1 expressing astrocytes.
Methods. To test this in simplified in vitro assays, purified astrocyte cultures were treated with clustered‐EphB1‐Fc, and the characteristic features of glial activation, such as cytoskeletal protein expression, STAT3 phosphorylation, and p‐STAT3 nuclear transfer were monitored.
Results. We have demonstrated that 1) Astrocytes express ephrin‐B1, 2) EphB1 triggers a two‐fold increase in GFAP and ezrin expression, 3) EphB1 induces both STAT3 phosphorylation and nuclear transfer by a three‐fold value, and 4) all effects were significantly diminished by knocking down ephrin‐B1 in astrocytes.
Conclusions. Our data show that EphB1 induces astrocyte activation by triggering ephrinB1‐mediated reverse signalling via STAT3 activation. This work therefore suggests a potential novel route in neuron‐astrocyte communication after injury. A further characterisation of this signalling pathway may provide a better understanding of mechanisms underlying glial activation and its role in plasticity.
T02‐06A. Laquinimod reduces astrocytic but not microglial NFkB activation in vitro and in vivo
N. Kramann 1, R. Pförtner1, U.‐K. Hanisch1, K. Hagemeier2, T. Kuhlmann2, W. Brück1, C. Wegner1
1University Medical Center Göttingen, Department of Neuropathology, Göttingen, Germany 2University Hospital Münster, Institute of Neuropathology, Münster, Germany
Introduction: Laquinimod (LAQ) is an oral well‐tolerated molecule that has been shown to reduce brain atrophy, disability progression and relapse rate in patients with relapsing‐remitting multiple sclerosis. Recent experimental data indicate that LAQ minimizes demyelination, inflammation and axonal damage in mice with cuprizone challenge.
Aim: Since astrocytic NFκB activation plays a crucial role in cuprizone‐induced demyelination, we investigated the effects of LAQ on CNS cells in vitro and in vivo.
Methods: In vitro experiments used primary cells to test effects of LAQ on oligodendroglial survival as well as on cytokine secretion and NFκB activation in astrocytes and microglia. Primary mouse astrocytes and microglia were pre‐treated with 0, 0.25 and 2.5 µM LAQ and stimulated by pro‐inflammatory cytokines. A dual‐luciferase reporter assay was used to measure NFκB activity.
For in vivo experiments, 10‐week‐old male C57BL/6J mice were challenged with 0.25% cuprizone and treated daily with LAQ (25 mg/kg) or water. To assess astrocytic and microglial NFkB activation in vivo, nuclear translocation of NFκB p65 was assessed in astrocytes and microglia by double immunofluorescence with antibodies against p65 and Iba1 or GFAP.
Results: In vitro, astrocytic but not microglial NFκB activation was markedly reduced by LAQ as evidenced by NFκB reporter assay. In astrocytes, pre‐treatment with 0.25 and 2.5 µM LAQ significantly reduced the induced NFκB activity after TNFα stimulation compared to stimulated controls. Pre‐treatment with 2.5 µM LAQ also significantly reduced NFκB activation after stimulation with the combination of IL‐1β and IFNγ compared to untreated stimulated controls. Oligodendroglial viability and survival were not affected by LAQ treatment. To confirm the in vivo relevance of these findings, we also examined astrocytic and microglial p65 translocation in mice with and without LAQ treatment after cuprizone challenge. The proportion of astrocytes with nuclear p65 immunoreactivity was significantly reduced in LAQ‐treated mice (14.0% ± 0.9%) compared to untreated controls (25.8% ± 1.1%). Microglia did not display marked translocation of NFκB p65 after cuprizone challenge.
Conclusion: Our data indicate that LAQ prevents cuprizone‐induced demyelination by attenuating astrocytic NFκB activation. In vitro, LAQ reduced the astrocytic NFκB activation by up to 46% as evidenced by NFκB reporter assay. Similar quantitative findings were obtained in vivo when LAQ treatment also led to a 46% reduction of astrocytes with NFκB activation, as evidenced by nuclear translocation of p65. These findings suggest that targeting the astrocytic NFκB pathway might have therapeutic effects in demyelinating CNS disorders.
T02‐06B. Brain‐Derived Neurotrophic Factor/TrkB signaling regulates daily astroglial plasticity in the suprachiasmatic nucleus: electron‐microscopic evidence in mouse
O. Bosler 1, C. Girardet1, B. Lebrun2, M.‐J. Cabirol‐Pol1, C. Tardivel2, A.‐M. Francois‐Bellan1, D. Becquet1
1CRN2M, UMR7286, CNRS/Aix‐Marseille Université, Marseille, France 2PPSN, EA4674, Aix‐Marseille Université, Marseille, France
Synchronization of circadian rhythms to the 24‐h light/dark cycle is associated with daily rearrangements of the neuronal‐glial network of the suprachiasmatic nucleus of the hypothalamus (SCN), the central master clock orchestrating biological functions in mammals. These plastic events involve changes in the extent of astroglial enwrapping of the major neuronal integrators of photic signals in the retino‐recipient region of the SCN, namely neurons synthesizing vasoactive intestinal peptide (VIP). We asked whether brain‐derived neurotrophic factor (BDNF), known to be highly expressed in a circadian manner in SCN, might be involved in the mechanisms governing these astroglial rearrangements. Using an analog‐sensitive kinase allele murine model (TrkBF616A) and semi‐quantitative electron microscopy, we found that the pharmacological blockade of the tropomyosin‐related kinase receptor type B (TrkB), the high affinity receptor of BDNF, abolished the day/night changes in glial coverage of VIP dendrites. The BDNF/TrkB signaling pathway therefore exerts a permissive role on the ultrastructural rearrangements that occur in SCN across the day/night cycle. In contrast, the extent of glial coverage of non‐VIP dendrites was not different at daytime and nighttime in TrkBF616A mice submitted to TrkB inactivation or not receiving any pharmacological treatment. Considering the well‐established role of BDNF in the clock's photic synchronization process, these dataprovide strong support to the view that the daily astroglial rearrangements in SCN could be involved in the photic entrainment of circadian rhythms. Above all, they highly suggest that they are necessary for the modulatory action of BDNF on the SCN responses to light.
POSTER TOPIC 03 Degenerative disease, toxicity and neuroprotection
T03‐01A. In vivo imaging of inflammation, de‐and remyelination using Fluorescence Molecular Tomography (FMT)
S. Albrecht 1, C. Geyer2, L. Wachsmuth3, T. Vogl4, C. Faber3, M. Eisenblätter2, T. Kuhlmann1
1University Hospital Münster, Institute of Neuropathology, Münster, Germany 2University Hospital Münster, IZKF Münster Core Unit Optical Imaging (OPTI), Clinical Radiology, Münster, Germany 3University Hospital Münster, Experimental NMR, Clinical Radiology, Münster, Germany 4University Münster, Institute of Immunology, Münster, Germany
Multiple sclerosis (MS) is a chronic disease characterized by inflammation, demyelination, gliosis and axonal degeneration. Until now, no imaging techniques exist that allow the specific detection of these different pathologies in vivo. Therefore our aim was to establish imaging techniques to detect specifically inflammation as well as remyelination in vivo. Feeding mice with cuprizone induces demyelination in many brain regions and is a frequently used animal model to study remyelination. Using Fluorescent Molecular Tomography (FMT) we were able to follow inflammation as well as de‐and remyelination in the cuprizone model. Cuprizone induced inflammation was imaged using a fluorescent conjugated antibody against the proinflammatory macrophage marker myeloid‐related protein 14 (MRP‐14). The reduction as well as progression of myelination after cuprizone withdrawal was followed via the fluorescent myelin binding compound 3,3ß‐diethylthiatricarbocyanine iodide (DBT). Due to different fluorescence characteristics of the antibody and the myelin binding compound, we are able to longitudinally and simultaneously image the degree of inflammation and de‐ and remyelination. The FMT studies were accompanied by Diffusion Tensor Imaging (DTI) studies to visualise the changes in radial diffusivity of the corpus callosum in mice fed with cuprizone during de‐ and remyelination. Additionally, extensive histological and electronmicroscopical analyses were applied to verify the FMT and DTI results.
T03‐01B. The role of Glia in the pathogenesis of the ataxic syndrome Dentatorubro‐pallidoluysian atrophy (DRPLA)
D. Mazaud, M. Fanto
King's College London, London, United Kingdom
Several dominant ataxias are polyglutamine (polyQ) diseases caused by the expansion of polyQ stretches in the affected proteins. We investigate DRPLA, caused by polyQ Atrophin‐1. We have studied Atrophin functions in Drosophila and used this organism to develop models for DRPLA. These studies have provided important clues into the specific features of this form of neurodegenerative ataxia, including a striking reduction in lifespan when polyQ Atrophins are expressed in the glia. A sensible hypothesis is that affected glia will act non‐autonomously on neurons as in other neurodegenerative pathologies. This is an emerging field that yields new and important therapeutic possibilities.
We now wish to elucidate the mechanisms through which degenerating glia impairs organism functions, and identify genes involved cell‐autonomously and non‐autonomously in generating this aspect of the pathology.
First, we investigated the impact of glial polyQ Atrophin on lifespan. Using different glia marker, we have demonstrated that the expression of polyQ Atrophin in glia, or specifically in astrocyte‐like cortex glia, has a dramatic effect on drosophila lifespan while brood brain barrier glia do not have any phenotype. We will then investigate the effect of other subtypes of glia looking at lifespan and motility to identify the relevance of each subtype in DRPLA.
Second, to identify new regulators of gliopathology we will conduct unbiased genetic screens for mutations in neurons that modify the lifespan of DRPLA flies, expressing polyQ Atrophin within the glia.
Our studies will be paramount in deciphering the complex glia‐neurons interactions in ataxias and may be beneficial for several ataxias. They will open up new avenues for therapies aimed at targeting glia‐neuron interactions during disease progression.
T03‐02A. Beta‐amyloid induces apoptosis of phagocytic microglia and macrophages
A. Babcock 1, L. Ilkjær1, M. Wirenfeldt1, T. Krøigård1, C. Myhre1, H. Toft‐Hansen2, L. Dissing‐Olesen1, T. Deierborg3, S. Darvesh4, M. Jensen5, M. West5, B. Finsen1
1University of Southern Denmark, Neurobiology Research, Odense, Denmark 2University of Southern Denmark and Odense University Hospital, Pediatrics and Clinical Immunology, Odense, Denmark 3Lund University, Experimental Medical Science, Lund, Sweden 4Dalhousie University , Medicine, Anatomy & Neurobiology, Halifax, Canada 5University of Aarhus, Biomedicine, Aarhus, Denmark
In Alzheimer's disease, microglial clearance of beta‐amyloid (Aβ) is considered neuroprotective. Whether Aβ uptake regulates microglial cell responsiveness, and whether macrophages participate, remains unclear. Here we investigated whether in vivo phagocytosis of endogenously‐produced Aβ disturbs the turnover of microglia and macrophages by changing rates of cellular proliferation and apoptosis. Using APPswe/PS1ΔE9 Tg mice, we show that plaque‐associated microglia and CD11b+CD45high macrophages bind and ingest endogenously‐produced Aβ in vivo. Plaque‐associated microglia and CD45+ leukocyte‐like cells were also observed in brains from patients with Alzheimer's disease. Phagocytic microglia and macrophages had a reduced rate of proliferation compared to Aβ‐ cells. Instead, high proportions of apoptotic Annexin V+ microglia and macrophages were found in aged APP/PS1 Tg mice. Phagocytic microglia and macrophages had greater caspase activity and increased p53 expression after in vivo uptake of Aβ, than cells that did not phagocytose Aβ. In vitro, we found that Aβ uptake induced caspase activation in microglia, whereas blocking Aβ uptake triggered apoptosis‐induced cell death in macrophages. Our data demonstrate that Aβ uptake impairs microglial/macrophage viability and responsiveness. Amyloid clearance may fail as phagocytic microglia and macrophages undergo Aβ‐induced apoptosis.
T03‐02B. Aquaporin 4 expression in the rat hippocampus and cortex during trimethyltin‐induced neurodegeneration
F. Michetti, S. Ceccariglia, A. D'Altocolle, F. Pizzolante, M. Barba, A. DelFà, C. Gangitano
Università Cattolica del S. Cuore, Rome, Italy
Trimethyltin (TMT) is a neurotoxicant producing neuronal degeneration in the mammalian central nervous system , especially in the hippocampus (for reviews, 1,2). Magnetic resonance imaging (MRI) investigation in TMT‐treated rats has evidenced dilation of lateral ventricles, possibly correlated to alterations in blood brain barrier permeability. We have investigated in the hippocampus and cortex of rats the expression of aquaporin 4 (AQP4), a glial water channel protein which is regarded to play a role in brain oedematous conditions, to explore the molecular mechanisms involved in the phenomenon. TMT‐treated AQP4 expression was tested both by real‐time PCR and western blotting analysis in hippocampus and cortex homogenates. To confirm molecular results and visualize the AQP4 cell distribution, double‐label immunofluorescence for AQP4 and GFAP was performed. Real‐time PCR and western blotting data show a significant upregulation of AQP4 starting from 14 days of TMT treatment in the hippocampus and, at a lower degree, in the cortex. Accordingly, the immunofluorescence shows an intense astrogliosis and AQP4 immunoreactivity diffusely pronounced in the hippocampal and cortex areas starting from 14 days after intoxication. AQP4 immunolabelling was localized in astrocytic end‐feet encircling the blood vessels, as expected. The study of the Rhodamine B fluorescent tracer, intraperitoneally administered, also revealed an intense vascular reaction, characterized by hypertrophic vessels with abnormal course and dimensions in the brain of TMT‐treated rats, indicating a vascular involvement in the TMT‐induced neurodegenerative processes. AQP4 over‐expression and the concurrent astrogliosis occurring in the brain of TMT‐treated rats might be candidated to play a role in alterations of vascular permeability and brain oedema formation evidenced by MRI studies.
1. Geloso MC, Corvino V, Michetti F, Neurochem Int 2011, 58: 729–38.
2. Corvino V, Marchese E, Michetti F, Geloso MC. Neurochem Res 2013, 38: 240–53.
T03‐03A. Decreased expression of alpha1 subunit of Na+/K+‐ATPase in the ALS rat brain
D. Bataveljic 1, L. Nikolic2, P. Andjus1
1Faculty of Biology, University of Belgrade, Center for Laser Microscopy, Belgrade, Serbia 2Institute for Biological Research “Siniša Stanković”, University of Belgrade, Department of Neurophysiology, Belgrade, Serbia
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease affecting predominantly motoneurons but with a particular pathological role of non‐neuronal cells. Previous research suggested that increased concentration of extracellular potassium could lead to motoneuron dysfunction. The Na+/K+‐ATPase should regulate the homeostasis of potassium ions and thus help maintain regular neuronal activity. This homeostasis may be regulated by Na+/K+‐ATPase in both neurons and astrocytes. In order to reveal the possible role of the Na+/K+‐ATPase in ALS pathology, we explored the expression of the alpha1 catalytic subunit of Na+/K+‐ATPase in neurons and astrocytes from different brain regions of the SOD1G93A rat model of ALS. Labeling immunofluorescently the alpha1 isoform of the Na+/K+‐ATPase revealed a decreased signal in the trigeminal nucleus and cerebellum of the ALS rat. Double immunofluorecence with NeuN for neurons showed that the reduced alpha1 was on the neuronal surface in the trigeminal nucleus. In addition, the alpha1 ring‐like staining of cerebellar granule neurons was lower in ALS as compared to the wild type rat. In comparison to neurons, astrocytes from the wild type rat were characterized with a lower expression of alpha1. In the examined brain regions we did not observe a prominent difference in alpha1 expression between astrocytes of wild type and ALS rats. Based on the findings of this study the prominent reduction of Na+/K+‐ATPase level observed in neurons and not in astrocytes could contribute to further understaning of impaired ion homeostasis affecting proper neuronal physiology in ALS. The role of glial Na+/K+‐ATPase needs to be further studied with markers of other alpha subunit isoforms.
T03‐03B. Branched‐chain amino acids influence the immune properties of microglial cells and their responsiveness to pro‐inflammatory signals
M.A. Ajmone‐Cat, R. De Simone, F. Vissicchio, C. Mingarelli, C. De Nuccio, S. Visentin, L. Minghetti
Istituto Superiore di Sanità, Rome, Italy
The branched‐chain amino acids (BCAAs) valine, leucine and isoleucine are essential amino acids involved in several important brain functions. Although commonly used as nutritional supplements, excessive intake of BCAAs might favour the establishment of neurotoxic conditions as indicated by the severe neurological symptoms characterising inherited disorders of BCAA catabolism such as maple syrup urine disease (MSUD). Recent evidence indicates that BCAAs induce excitotoxicity through mechanisms that require the presence of astrocytes. In the present study, we evaluated the effects of BCAAs on microglia, the main immune cells of the brain. As an experimental model we used primary microglial cells harvested from mixed glial cultures that had been kept in normal or high BCAA medium (H‐BCAA). We show that H‐BCAA microglial cells exhibit a peculiar phenotype characterized by a partial skewing toward the M2 state, with enhanced IL‐10 expression and phagocytic activity but also increased free radical generation and decreased neuroprotective functions. We suggest that such an intermediate M1/M2 phenotype might result in a less efficient microglial response, which would promote the establishment of a low grade chronic inflammation and increase the likelihood of neurodegeneration. Although based on in vitro evidence, our study adds on to an increasing literature indicating that the increasing use of dietary integrators might deserve consideration for the possible drawbacks. In addition to excitotoxicity, the altered immune profile of microglia might represent a further mechanism by which BCAAs might turn into toxicants and facilitate neurodegeneration.
T03‐04A. Olesoxime for the treatment of hereditary dysmyelinating diseases: The importance of the therapeutic window
M. Begou 1, B. Depiets2,3, F. Giraudet3,4, J. Barbier2,3, C. Bechon2,3, M. Michaud5, R. Pruss5, T. Bordet5, O. Boespflug‐Tanguy2,6
1INSERM, U676, Paris, France 2INSERM, U676, Paris, France 3Université d'Auvergne, Faculté de Médecine, Clermont‐Ferrand, France 4INSERM, U1107, Clermont‐Ferrand, France 5TROPHOS, Marseille, France 6APHP, Hôpital Robert Debré, Paris, France
The proteolipid protein gene (PLP1), located on the X‐chromosome, undergoes alternative splicing to produce the most abundant proteins of central nervous system myelin: PLP and DM20. Related to the extent of myelin defect, mutations in the PLP1 gene in humans are associated with a spectrum of X‐linked disorders, from the severe Pelizaeus‐Merzbacher disease (PMD), to the mild form spastic paraplegia type 2 (SPG2). Phenotype‐genotype correlation exists, large duplications of PLP1 gene are predominantly found in PMD and null mutations in SPG2.
PLP is almost 100% conserved across mammalian species, and the large spectrum of phenotype severity is also found Plp transgenic mice. Experiments on these models have contributed to our understanding in the pathophysiology of PLP related disorders. In one hand, mice with extra copies of the Plp1 gene (+PlpTg mice) have neurological symptoms and CNS pathology similar to those found in PMD patients while mice with Plp1 gene inactivation (Plp null mice) share similarities with SPG2 patients. Then, these two mouse models represent very useful tools to evaluate the efficacy of a therapeutic strategy for the severe and the mild form of the PLP related disorders on the neuropathological and behavioral aspects.
Olesoxime (TRO19622), a cholesterol‐like small molecule, has been shown to rescue motor neurons from cell death and to promote axonal regeneration both in vitro and in vivo. More recently, olesoxime has also been shown to promote central remyelination by accelerating oligodendrocyte maturation both in vitro and in vivo. Altogether, these results strongly suggest that olesoxime may be a potential drug candidate for the treatment of white matter neurodegenerative disorders and notably to PLP related disorders.
Here we treated +PlpTg mice with olesoxime for 2 months starting at birth, as well as Plp null mice from 3 to 12 months of age (presymptomatic treatment) or from 9 to 15 months of age (symptomatic treatment). Behavioral and neuropathological analysis revealed that olesoxime failed to correct the development of symptoms in mice modeling the severe form of PLP related disorders. On the contrary, treatment of Plp null mice with olesoxime resulted in a 3‐month delay in the onset of abnormal behaviors related to anxiety and in the slowing of central nerve conduction. These beneficial effects are observed only when the drug is administered before disease onset. Treatment with olesoxime also reduces the effect of aging on rotarod performance decline in both WT and Plp null old mice. Altogether these results suggest that olesoxime could be of interest as a treatment to delay the onset of symptoms in PLP related disorders provided that patients suffer from a mild form of the disease and are treated before the establishment of symptoms.
T03‐04B. Pathways used for amyloid‐beta uptake by adult human astrocytes and microglia
S. Mulder, T. Aydin, M.A. Hagens, R. Veerhuis
VU University Medical Center, Amsterdam, Netherlands
Objectives: Glial cells play an important role in amyloid‐beta (Aß) clearance, however little is known about the cellular routes involved in internalization of different Aß aggregation forms by adult human microglia and astrocytes. Therefore, we evaluated the uptake mechanism of Aß 1‐42 by primary human adult microglia and astrocytes isolated from brain tissue of non‐demented control and AD cases. Methods: Cells were preincubated for 1 hour with different inhibitors, before exposure to fluorescence labelled (FAM) oligomeric and fibrillar forms of synthetic Aß. The number of amyloid positive cells was quantified by flow cytometry. Inhibitors of various pathways tested, include Cytochalasin B (inhibits actin polymerization; general endocytosis inhibitor), Nocadozole (inhibits tubulin depolymerization; general endocytosis inhibitor) and Fucoidan (scavenger receptor inhibitor). Results: Cytochalasin B inhibited Aß fibril uptake (90% reduction, p < 0.01) and to a lesser extent oligomer (50% reduction, p < 0.05) uptake in microglia, whereas no effect was seen on Aß uptake by astrocytes. In contrast, Nocodazole was found to inhibit Aß fibril uptake (54% reduction, p < 0.05) and oligomer (77% reduction, p < 0.01) uptake in astrocytes, whereas no effect was seen on Aß uptake by the microglia. Interestingly, Fucoidan prevented Aß uptake in both astrocytes (oligomers:86% (p < 0.01); fibrils: 79% (p = 0.01) and microglia (oligomers:67% (p < 0.001); fibrils: 79% (p < 0.05)). Conclusions: These data indicate that both human astrocytes and microglia use scavenger receptors to internalize Aß oligomers and fibrils. Whether different types of scavenger receptors are involved in Aß uptake by astrocytes and microglia will be further investigated.
T03‐05A. CD38 deficiency inhibits Alzheimer's disease pathology in a mouse model
E. Blacher
Tel‐Aviv University, Tel Aviv, Israel
Alzheimer's disease (AD) accounts for the majority of cases of dementia worldwide, affecting over 50% of the population over 85. The disease is characterized by a progressive memory loss, cognitive deterioration, behavioral disorders, deposit of beta‐amyloid (Ab) peptides, neurofibrillary tangles, reactive astrocytosis and activation of microglia cells. Mutations in the genes that encode APP (Amyloid Precursor Protein) and components of the proteases that generate amyloid beta cause familial forms of AD.
Microglia, the resident immune cells of the central nervous system (CNS) were suggested to play both beneficial and harmful roles in the course of the disease. Therefore, modulating the microglial response is being considered as a promising approach for the treatment of this disease.
We have recently shown using in vitro and in vivo systems that the ectoenzyme CD38, regulates microglial activation. In view of the significant role of activated microglia in AD and the important role played by CD38 in microglial activation, we hypothesized that CD38 deficiency would affect the course of the disease.
In order to test this hypothesis, we implicated the APPswePS1dE9 transgenic mouse model for AD, and compared the cognitive performance (as evident by behavioral studies) of aged APPswePS1dE9 mice, to APPswePS1dE9 Cd38‐/‐ mice. In addition, Abload, accumulation of microglia and astrocytes was assessed in the brains of these mice. Our preliminary results show that CD38 deficiency has a neuroprotective role in this AD mouse model as indicated by improvement in cognitive performance and dramatic reduction in Aβ load.
T03‐05B. Gap junction activity is necessary for Cx43‐mediated protection of astrocytes in response to oxidative stress
C. Naus 1, H.T. Le1,2, J. Bechberger1, S. Lozinsky1, J. Vega1,3,4, W.C. Sin1
1University of British Columbia, Vancouver, Canada 2Vietnam National University, College of Science, Hanoi, Vietnam 3Pontificia Universidad Católica de Chile, Santiago, Chile 4Universidad de Antofagasta, Antofagasta, Chile
Oxidative stress induced by reactive oxygen species (ROS) is associated with various pathological conditions, including aging, neurodegenerative disorders, traumatic and ischemic insults. The mechanism by which the gap junction protein connexin43 (Cx43) contributes to oxidative stress‐induced cell death or the “rescue” of the dying cells is still unclear. Cx43 is highly expressed in astrocytes. We have previously shown that astrocytic Cx43 is critical for neuroprotection during ischemic insults in vivo. To investigate the protective effect of Cx43 in oxidative stress pathways, we induced oxidative stress in wild‐type and Cx43 knockout astrocytes using hydrogen peroxide (H2O2).
Cx43 knockout astrocytes exhibited increased H2O2‐induced cell death as measured by the loss of membrane integrity using the dye Rhodamine B dextran, supporting a cell protective effect of Cx43; this effect was not observed in wild‐type astrocytes. To examine the involvement of Cx43 in H2O2‐induced cell death, we studied the expression and distribution of Cx43 in response to H2O2 in wild‐type astrocytes. H2O2 treatment altered the expression level and phosphorylation of Cx43. This was accompanied with changes in Cx43 distribution and gap junction plaque formation. Hemichannel activity was measured using dye uptake. H2O2 (0.7 mM) increased dye uptake, an effect that was blocked by GAP26 (syntethic mimetic peptide, 200 µM) or carbenoxolone (100 µM), two connexin hemichannels blockers. However, H2O2 treatment did not result in any measureable gap junction activity in Cx43 knockout cells as measured by a scrape‐loading dye transfer assay. These findings suggests that gap junction activity contributes to Cx43‐mediated protection in response to ROS.
Supported by grants from the Heart & Stroke Foundation of Canada (to CCN) and FONDECYT 3120006 of Chile (to JLV). CCN holds a Canada Research Chair.
T03‐06A. Lack of Microglial TREM2 Receptor Increases Susceptibility to Dopaminergic Degeneration Triggered by Systemic Inflammation
L.‐G. Bodea 1, M. Colonna2, H. Neumann3
1University of Bonn, Bonn, Germany 2Washington University School of Medicine, Department of Pathology & Immunology, St. Louis, United States 3University of Bonn, Institute of Reconstructive Neurobiology, Bonn, Germany
Under normal conditions, inside central nervous system the immune functions are accomplished by microglial cells. One of the receptors found on microglia is the Triggering Receptor Expressed on Myeloid cells 2 (TREM2), which signals intracellularly via an adapter molecule referred to as TYRO protein tyrosine kinase‐binding protein (TYROBP, known also as DAP12). In humans, loss of function of either TREM2 or TYROBP leads to Nasu‐Hakola disease, which is characterized by neuroinflammation and bone cysts.
By using a TREM2 knock‐out (KO) mouse line, we provided further insights on the TREM2 function in neurodegenerative diseases.
First, we observed mild signs of dopaminergic neurodegeneration and a slight increase in microglial Iba1‐immunoreactivity in different brain regions of 3 months TREM2 KO mice compared with the wild type (WT) control animals. However, the transcription levels of inflammatory cytokines (TNFα, IL1β) or inducible nitric oxide synthase (iNOS) were unaffected in mice of different ages (3, 9 or 12 months). To shorten the time required for neurodegeneration to occur, we have injected the mice intraperitoneally with lipopolysaccharides (LPS) on four consecutive days and analyzed the dopaminergic neurodegeneration in the substantia nigra after a three weeks period. While the quantification of dopaminergic neurons in the substantia nigra showed a slight decrease in number for WT animals, a higher loss was recorded in the TREM2 KO mice after systemic LPS challenge. Concomitantly, significant increase of microglia activation was detected in the LPS‐treated TREM2 KO mice compared with LPS‐treated WT animals.
Our results are pointing towards a neuroprotective effect of the microglial TREM2 receptor under chronic and systemic inflammatory conditions.
T03‐06B. Glucose transporters in multiple sclerosis
P. Nijland, I. Mihaelidou, S. van der Pol, J. Drexhage, W. Gerritsen, P. van der Valk, H. de Vries, J. van Horssen
VU University Medical Center, Pathology, Neuroscience Campus, Amsterdam, Netherlands
Increasing evidence suggest that brain energy metabolism is severely altered in multiple sclerosis patients, which may contribute to the process of neurodegeneration. Imaging studies reveal a disturbed cerebral perfusion and glucose uptake and metabolism in MS patients. Moreover mitochondrial dysfunction is recognized to play a crucial role in MS pathology. To date little is known about the distribution of glucose transporters (GLUTs), key molecules for the cellular uptake of glucose, in human brain and their role in the pathogenesis of MS. Therefore, the aim of this study was to provide a complete overview of the distribution and expression levels of glucose transporters in control and MS brain. Our data so far show that mRNA levels of GLUT1, 3 and 4 as well as their regulators hypoxia inducible factor (HIF)‐1a, HIF‐2a and peroxisome proliferator‐activated receptor gamma coactivator (PGC)‐1a, are increased in normal appearing white matter (NAWM) of MS patients compared to controls. Immunohistochemical analysis revealed that brain endothelial cells not only express GLUT1 but also GLUT3 and GLUT4. GLUT 1, 3 and 4 are present in resting microglia and highly expressed in activated microglia and macrophages in active MS lesions. Interestingly reactive astrocytes expressed increased levels of GLUT1 and GLUT4 compared to control, in addition GLUT3 positive astrocyes were observed in MS brain. Axons expressed high levels of GLUT3 in active MS lesions, which was evidently reduced in chronic lesions. Strikingly, all glucose transporters studied were down regulated in chronic MS lesions. Together, these data emphasize the extent to which glucose metabolism appears to be affected in all stages of MS. A better understanding of these metabolic changes, especially in the NAWM and chronic stage of the disease, is essential to develop strategies to improve neuronal survival and to gain more insight in the mechanisms underlying lesion formation.
T03‐07A. Pericytes are preserved in Alzheimer's Disease
L. Brandt 1, F. Fernández‐Klett1, L.W. Harris2, S. Bahn2, J. Priller1
1Charité – Universitätsmedizin Berlin, Department of Neuropsychiatry and Laboratory of Molecular Psychiatry, Berlin, Germany 2University of Cambridge, Centre for Neuropsychiatric Research, Institute of Biotechnology, Cambridge, United Kingdom
Pericytes are vascular mural cells which are enriched within the capillary basement membranes of the brain. Recent studies have provided evidence that pericytes regulate the blood‐brain barrier (BBB) and modulate cerebral blood flow. Neurovascular dysfunction and BBB damage are hallmarks of Alzheimer's disease (AD), but the role of pericytes in AD pathogenesis is still unclear.
We have examined post‐mortem samples of middle frontal gyrus in collaboration with the University of Cambridge after ethical approval by the local IRB. Ten cases with neither history of neurological disorder nor evidence of neuropathology were compared with 10 AD cases provided by the Neurological Foundation of the New Zealand Human Brain Bank. We performed immunostainings for laminin and platelet‐derived growth factor receptor (PDGFR)‐β which mark vessels and pericytes, respectively. We also stained for Aβ1–42 for evidence of amyloid pathology. Quantification of pericyte and vessel density was performed using stereological methods, which are considered as gold standard in morphometric quantification. The Spaceballs method was used to measure the density of capillaries. Pericyte density was assessed using the Optical Fractionator.
The age of the subjects did not differ between the control and AD groups (76,2 ± 4,0 vs. 75,4 ± 5,5 years). The cortex of AD cases contained more capillary vessels than controls (mean ± SD: 308 ± 40 vs. 365 ± 41 mm/mm3; p < 0,01; Fig. 1A). The capillary density increased in correlation to the total plaque load (R = 0,58; p < 0,001). In contrast, we could not observe a significant difference in the number of pericytes per mm capillary length between control and AD cases (8,56 ± 1,21 vs. 8,17 ± 0,94 cells/mm; p = 0,44; Fig. 1B). Relative to tissue volume, the cortex of AD individuals showed a tendency to contain more pericytes than controls (2624 ± 406 vs. 2985 ± 588 cells/mm3; p = 0,13; Fig. 1C).
This is the first study using state‐of‐the‐art techniques to investigate the relationship between capillaries and pericytes in AD brains. Impaired clearance of β amyloid across the pericyte‐regulated BBB and deficient microvascular regulation of blood flow may contribute causally to AD pathogenesis. In consequence, loss of pericytes could contribute to disease progression. However, our results show that AD cortical pathology is characterized by an increase in the density of capillary vessels without deficit in pericyte coverage. Thus, they do not substantiate the notion that pericyte loss is a significative feature of AD.

T03‐07B. HDAC6 is present in rat brain oligodendrocytes and modulates tau phosphorylation
M. Noack, J. Leyk, C. Richter‐Landsberg
Carl von Ossietzky Universität Oldenburg, Oldenburg, Germany
Histone deacetylase 6 (HDAC6), a member of the class II HDACs, is a unique cytoplasmic α‐tubulin deacetylase that interacts with the microtubule (MT)‐associated protein tau. In healthy human brain six tau isoforms are expressed generated by alternative mRNA splicing. These contain either three (3R‐tau) or four (4R‐tau) microtubule binding repeats regulating MT assembly and stability. Tau hyperphosphorylation impairs its MT‐binding activity. Tau deposits in nerve cells and glia are the characteristic hallmark of a number of neurodegenerative diseases termed tauopathies. In progressive supranuclear palsy and corticobasal degeneration, pathogenic glial cell inclusions are observable in oligodendrocytes, the myelin forming cells of the CNS. HDAC6 plays an important role in the degradation and accumulation of misfolded proteins by promoting transport of aggregated proteins to the aggresome, localized at the microtubule‐organizing center where protein aggregates are deposited and processed by autophagy. The present study was undertaken to elucidate the functional significance of HDAC6 in oligodendrocytes and its role in pathological protein aggregate formation.
Towards this cultured rat brain oligodendrocytes and OLN‐t40 cells, an oligodendroglial cell line expressing the longest human tau isoform, were used.
Treatment of primary oligodendrocytes with tubastatin A (TST), a selective inhibitor of the tubulin deacetylation activity of HDAC6, caused a significant increase in the level of acetylated tubulin, as determined by indirect immunofluorescence and immunoblot procedure. MTs appeared more bundled and stabilized. Inhibition of HDAC6 attenuated the phosphorylation of tau at the PHF‐1‐epitope (serine 396/404) and its enhancement at the 12E8‐epitope (serine 262, located within the microtubule binding repeat region 1). Furthermore, altered protein levels of tau isoforms containing either three or four MT binding repeats were observed. While 3R‐isoforms were reduced, 4R‐isoforms were increased in primary oligodendrocytes suggesting an impact on microtubule binding ability. HDAC6 knockdown in OLN‐t40 cells revealed no alteration in tau phosphorylation at PHF‐1‐epitope, but also augmented phosphorylation at the 12E8‐epitope. Cells were morphologically damaged and MT organization was disturbed.
The data indicate that HDCA6 modulates tau expression and phosphorylation, its inhibition leads to the accumulation of 4R‐tau which is also prominent in tauopathies with oligodendroglial pathology.
T03‐08A. Emergence of tyrosine hydroxylase‐positive cells in rat cortex after intraventricular injection of 6‐hydroxydopamine (6‐OHDA)
S. Caradonna 1,2, B. Wachter1, E. Küppers1
1University of Tübingen, Department of Cellular Neurobiology, Institute of Anatomy, Tübingen, Germany 2Eberhard Karls University, Graduate School for Cellular and Molecular Neuroscience, Tübingen, Germany
Intraventricular injection of 6‐OHDA results in increased proliferation and de‐differentiation of rat cortical astrocytes into progenitor‐like cells (Wachter et al., Cell Tissue Res. 342(2):147–60 2010). The function and relevance of these cells is not yet clear. To test the hypothesis, that these cells are part of a possible compensatory mechanism to cope with the 6‐ OHDA‐induced loss of dopaminergic neurons, we studied the expression of tyrosine hydroxylase (TH), the rate‐limiting enzyme in the catecholamine synthesis pathway, in the cortex of 6‐OHDA‐lesioned animals.
Performing immuncytochemistry at 4d after the lesion we demonstrated a 21‐fold increase in the number of TH‐positive (TH+somata in rat cortex following 6‐OHDA injection) compared to sham‐lesioned animals. TH+somata in the parietal cortex were restricted to the layers II‐V with most of the cells being bipolar.
Combining TH immuncytochemistry with classical Nissl stain yielded complete congruency. A small fraction of TH+cells co‐expressed calretinin thus pointing to an interneuron affiliation. Virtually none of the TH+cells expressed other markers of interneurons (calcium binding proteins, neuropeptides) or the glial markers GFAP and nestin.
In contrast, we found a co‐localization of TH with markers of glial progenitor cells (Sox2 and S100β) and with PSA‐NCAM, which has been shown to be expressed in immature, but not recently generated cortical neurons in cortical layer II of the cat (Varea et al.; Front. Neurosci. 5: 17 2011).
Taken together, these findings indicate that 6‐OHDA lesions might induce a glial de‐ differentiation followed by a fate re‐direction towards neuronal committed cells. Future studies have to be performed to confirm this and to find out if the TH+cells are capable of dopamine synthesis.
T03‐08B. Importance of oligodendrocytes in oxidative stress‐resistance in white matter ischemic injury
M. Noda 1, K. Fujita1, M. Hamner2, C. Higashi1, M. Yamafuji1, N. Akimoto1, M. Kido3, Y. Tanaka4, Y. Nakabeppu5, B. Ransom2
1Kyushu University, Fukuoka, Japan 2University of Washington School of Medicine, Neurology, Seattle, United States 3Kyushu University, Oral Anatomy and Cell Biology, Fukuoka, Japan 4Panasonic Corporation, Appliances Company, Kusatsu, Japan 5Kyushu University, Medical Institute of Bioregulation, Neurofunctional Genomics, Fukuoka, Japan
Background: Molecular hydrogen selectively reduces hydroxyl radicals, the most cytotoxic of reactive oxygen species (ROS). Central nervous system (CNS) white matter (WM) ischemia is an important clinical problem and may produce injury, in part, by ROS‐induced mitochondrial dysfunction.
Objective: Using the mouse optic nerve (MON) WM model, we tested whether hydrogen in drinking water reduced functional WM ischemic injury, and if it reduced cellular lipid peroxidation and mitochondrial dysfunction including mitochondrial and nuclear DNA oxidation.
Methods: Functional integrity of MON was determined by quantitatively monitoring the area of MON compound action potential (CAP) before, during and after a standardized 60 min period of oxygen and glucose deprivation (OGD), the ischemia equivalent ex vivo. Nuclear 8‐oxoguanine (nu8‐oxoG) was used as a marker of oxidative DNA damage.
Results: A 60 min period of OGD caused prompt loss of the CAP, followed by an average 20% recovery. In mice that had received hydrogen‐containing drinking water for 7–10 days, the CAP area did not disappear during ischemia and recovered to a significantly greater extent during reperfusion (normal oxygen and glucose levels). Immunostaining of axonal neurofilament by SMI‐31 showed significant protection in MONs from mice drinking hydrogen‐water. Accumulation of nu8‐oxoG was observed mainly in oligodendrocytes after OGD. The levels of 8‐oxoG after OGD were significantly reduced in optic nerves from hydrogen‐water drinking mice. The ‘protection' induced by 7–10 days of hydrogen‐water drinking lasted several days.
Conclusions: Our results show that several days of hydrogen exposure reduced the extent of CNS WM irreversible injury associated with OGD. The importance of these observations is that oligodendrocytes may play an important role in the protective effect against ischemic injury. These observations raise intriguing therapeutic options.
T03‐09A. Changes of the extracellular space diffusion parameters during aging in a triple transgenic animal model of Alzheimer's disease
M. Cicanic 1, L. Vargova1,2, M. Kulijewicz‐Nawrot2, J.J. Rodriguez2,3,4, E. Sykova1,2
1Charles University, 2nd Faculty of Medicine, Department of Neuroscience, Prague, Czech Republic 2Institute of Experimental Medicine AS CR, Department of Neuroscience, Prague, Czech Republic 3IKERBASQUE, Basque Foundation for Science, Bilbao, Spain 4University of the Basque Country UPV/EHU, Department of Neuroscience, Leioa, Spain
Amyloid‐β (Aβ) deposits in the brain extracellular space (ECS) and neurofibrillary tangles are typical features of Alzheimer's disease (AD). Both affections are expressed in triple transgenic (3xTg‐AD) mice, making them a useful model for studying the pathophysiology of AD. In these mice, significant changes in astroglial morphology appear in the limbic brain structures [1, 2], which may affect the diffusion of neuroactive substances through the ECS.
Using the real‐time iontophoretic method, we determined the ECS volume fraction α (α = ECS volume / total tissue volume) and the geometrical factor tortuosity λ (λ2 = free / apparent diffusion coefficient) in the CA1 region of the hippocampus, dentate gyrus (DG) and prelimbic cortex (PLC) in brain slices obtained from 10‐month‐old (10m) and 20‐month‐old (20m) 3xTg‐AD mice and age‐matched wild‐type (wt) controls. To study potential diffusion anisotropy, the measurements were performed along 3 orthogonal axes: medio‐lateral, rostro‐caudal and ventro‐dorsal. Astroglial morphology and the presence of Aβ were assessed using immunohistochemical staining for GFAP and Aβ.
We found no anisotropy in the CA1 region, DG, or PLC, thus the data for each structure along the three orthogonal axes were pooled. The ECS diffusion parameters measured in the CA1 are shown in table 1. In the CA1 of 10m mice, there was no significant difference in the ECS diffusion parameters between wt and 3xTg‐AD mice. In 20m mice, α decreased by 9% in wt but increased by 27% in 3xTg‐AD mice in comparison with the values found in the younger animals. During aging, there was also a significant decrease in λ in wt but not in the 3xTg‐AD mice. A similar pattern of changes in the ECS diffusion parameters during aging was also observed in the DG and PLC. Unlike in the CA1, we found significant differences in young animals: α was lower in the DG and λ was higher in the DG and PLC of 3xTg‐AD mice than in wt mice. In 20m 3xTg‐AD mice, Aβ deposits accompanied with astroglial atrophy or hypertrophy were observed.
We suggest that the amyloid load in 3xTg‐AD mice and the subsequent prevailing astroglial atrophy affect the normal aging process by inducing an increase in the ECS volume during aging instead of the normal decrease. This leads to a larger ECS space in aged 3xTg‐AD mice than in age‐matched wt mice, which may alter the efficacy of extrasynaptic as well as synaptic transmission and contribute to the impaired cognitive functions in AD.
The study was supported by the grants: GACR P304/11/0184, GACR P304/12/G069.
1. Olabarria et al. Glia 2010. 58(7): 831‐8.
2. Kulijewicz‐Nawrot et al. J Anat 2012. 221(3): 252‐62.
Table 1.
1: ECS diffusion parameters in the CA1 of 10m and 20m wt and 3xTg‐AD mice.
| 10m | 20m | |||||||
|---|---|---|---|---|---|---|---|---|
| wt | 3× tg | Wt | 3× tg | |||||
| α | 0.23±0.01 | 0.22±0.01 | 0.21±0.01 | * | 0.28±0.01 | * + | ||
| λ | 1.45±0.01 | 1.48±0.01 | 1.41±0.01 | * | 1.45±0.01 | + | ||
| n | 35 | 24 | 26 | 17 | ||||
| N | 14 | 9 | 10 | 7 | ||||
Significant differences between 10m and 20m mice of the same group are marked with *, differences between wt and 3xTg‐AD mice of the same age are marked with +.
T03‐09B. Early functional deficit and microglial disturbances in the hSOD1G93A mouse model of amyotrophic lateral sclerosis
Y. Gerber 1, J.C. Sabourin 2, H. Noristani1, M. Rabano 2, M.D.M. Vivanco 3, F. Perrin1,4,2
1INSERM U1051, Montpellier, France 2University of the Basque Country, Integrative Biology of Neurodegeneration, Leioa, Spain 3CICbioGUNE, Cell Biology, Derio, Spain 4IKERBASQUE Basque Foundation for Science, Bilbao, Spain
No effective treatment is currently available for amyotrophic lateral sclerosis (ALS), a neurodegenerative disorder characterized by selective motoneurons degeneration. There is today no clear‐cut pathogenesis sequence or any treatment. However growing evidences are in favor of the involvement, besides neurons, of several partners such as glia and muscles. To better characterize the time course of pathological events in an animal model that recapitulates human ALS symptoms, we investigated functional and cellular characteristics of hSOD1G93A mice.
We have evaluated locomotor function of hSOD1G93A mice through dynamic walking patterns and spontaneous motor activity analysis. We detected early functional deficits that redefine symptoms onset at 60 days of age, i.e. 20 days earlier than previously described. Moreover, sequential combination of these approaches allows monitoring of motor activity up to disease end stage. To tentatively correlate early functional deficit with cellular alterations we have used flow cytometry and immunohistochemistry approaches to characterize neuromuscular junctions, astrocytes and microglia. We show that (1) decrease in neuromuscular junction's number correlates with motor impairment, (2) astrocytes number is not altered at pre‐ and early‐symptomatic ages but intraspinal repartition is modified at symptoms onset, and (3) microglia modifications precede disease onset. At pre‐symptomatic age, we show a decrease in microglia number whereas at onset of the disease two distinct microglia sub‐populations emerge. We are now establishing the transcriptomic profile of microglia over the course of the disease.
In conclusion, precise motor analysis updates the onset of the disease in hSOD1G93A mice and allows locomotor monitoring until the end stage of the disease. Early functional deficits coincide with alterations of neuromuscular junctions. Importantly, we identify different sets of changes in microglia before disease onset as well as at early‐symptomatic stage. This finding not only brings a new sequence of cellular events in the natural history of the disease, but it may also provide clues in the search for biomarkers of the disease, and potential therapeutic targets.
T03‐10A. Vanishing White Matter‐mutated astrocytes impair oligodendrocyte maturation in vitro
S. Dooves, A. van de Kreeke, N. Land, G. Jacobs, M. van der Knaap, V. Heine
VU University Medical Center, Amsterdam, Netherlands
Vanishing White Matter disease (VWM) is a severe leukoencephalopathy with mainly a childhood onset. Affected children show progressive neurological symptoms like ataxia and spasticity that eventually lead to death. Pathologically, mainly the astrocytes and oligodendrocytes of the brain white matter are affected. Previous research has shown that both are in an immature state and have an abnormal morphology.
In order to gain more knowledge about the disease pathophysiology, two mouse models for VWM are developed in our lab. Co‐cultures between astrocytes (WT and VWM) and WT oligodendrocyte progenitor cells (OPCs) are done to investigate the effect of astrocytes on OPC maturation. Furthermore, induced pluripotent stem cells (iPSCs) from both VWM and WT mice are compared in their glial differentiation efficiency in vitro and in vivo.
The astrocyte‐OPC co‐cultures show that VWM astrocytes inhibit OPC maturation. This suggest that astrocytes might have a crucial role in the development of the oligodendrocyte and myelin pathology in VWM. Currently our research focuses on rescuing the maturation inhibition in vitro, which can aid the development of stem cell therapy for VWM.
T03‐10B. Inhibition of IL‐12/IL‐23 signaling reduces alzheimer's disease‐like pathology and cognitive decline
J. Obst 1, S. Prokop1, K. Miller1, J. vom Berg2, I. Lopategui‐Cabezas1, A. Wegner1, R.E. Kälin1, F. Mair2, C. Schipke3, O. Peters3, Y. Winter4, B. Becher2, F.L. Heppner1
1Charité – Universitätsmedizin Berlin, Department of Neuropathology, Berlin, Germany 2University of Zürich, Institute of Experimental Immunology, Zürich, Switzerland 3Charité – Universitätsmedizin Berlin, Department of Psychiatry, Berlin, Germany 4Humboldt University, Cognitive Neurobiology and Berlin Mouse Clinic for Neurology and Psychiatry, Berlin, Germany
Alzheimer's disease pathology displays an inflammatory component characterized by the presence of pro‐inflammatory cytokines particularly in response to β‐amyloid. Using the APPPS1 Alzheimer's disease mouse model, we observed the production of the common interleukin (IL)−12 and IL‐23 subunit p40 by microglia. Genetic ablation of various IL‐12/IL‐23 signaling molecules, of which deficiency in p40 had the strongest effect, resulted in a drastic decrease in cerebral amyloid burden. Although deletion of IL‐12/IL‐23 signaling from the radiation‐resistant glial compartment of the brain was most efficient in mitigating cerebral amyloidosis, peripheral administration of a neutralizing p40‐specific antibody likewise resulted in reduction of cerebral β‐amyloid in APPPS1 mice. Furthermore, intracerebroventricular delivery of antibodies to p40 significantly reduced soluble Aβ species and reversed cognitive deficits in aged APPPS1 mice. Our results suggest that inhibition of IL‐12/IL‐23 signaling reduces cerebral amyloidosis and cognitive dysfunction, and may pose a novel potential pharmacological target to combat Alzheimer‘s disease.
T03‐11A. Intravital characterization of microglia in Alzheimer's Disease
N.H. Drost, J.‐L. Rinnenthal, D. Pehl, F.L. Heppner
Charité – Universitätsmedizin Berlin, Berlin, Germany
Question: Microglia are attracted to and surround β‐amyloid deposits, the main hallmark of Alzheimer's disease (AD), suggesting a role for these cells in disease pathogenesis. However, recent in vivo data using the CD11b‐HSVTK system for microglial depletion suggests that resident microglia are not sufficiently capable of restricting and clearing β‐amyloid plaques. To underpin the CD11b‐HSVTK system and shed more light into the biological mechanistics of microglial depletion mediated by Ganciclovir (GCV), we aim to intravitally monitor the depletion process.
Methods: We are using CD11b‐HSVTK transgenic mice crossed to Fractalkine‐GFP+/‐ mice harboring green fluorescent microglia. A chronic cranial window is implanted onto the head of the offsprings followed by depletion of microglia and subsequent in vivo two‐photon (2P) imaging. As 2P imaging is able to visualize the upper 300 µm of the cortex, a new topical depletion approach of CD11b‐HSVTK+ microglia was established. Here, GCV is applied directly onto the brain surface in the area of the cranial window through a catheter.
Results: Manipulation of the brain skull by installation of the cranial window and placing a catheter for topic GCV application resulted in strong depletion of microglia up to 85% in Fractalkine‐GFP+/‐ mice crossed to CD11b‐HSVTK mice.
Conclusions: Taken together, we established a minimal invasive technique for visualization of the microglia depletion process in CD11b‐HSVTK mice using intravital 2P microscopy. These tools will allow the underpinning of the CD11b‐HSVTK system as well as the study of relevant biological questions involving resident and peripheral derived microglia in AD.
T03‐11B. Oxidative stress causes endoplasmic reticulum stress in X‐adrenoleukodystrophy
F.J. Ortega 1, N. Launay1,2, E.V. Ilieva1,2,3, L. Grau1,2, J.J. Martínez1,2, S. Fourcade1,2, P. Aubourg4, I. Ferrer5,6, E. Galea5,6,7, A. Pujol1,2,6,7
1IDIBELL, L'Hospitalet del Llobregat, Spain 2CIBERER U759, Centro de Investigación en Red sobre Enfermedades Raras, Spain 3University College London, Centre for Respiratory Research, London, United Kingdom 4INSERM U986, Department of Pediatric Neurology, Bicêtre‐Paris Sud Hospital, Paris, France 5Institute of Neuropathology, Pathologic Anatomy Service, Bellvitge Biomedical Research Institute (IDIBELL), Barcelona, Spain 6Institut de Neurociències, Universitat Autònoma de Barcelona, Barcelona, Spain 7Institució Catalana de Recerca i Estudis Avançats (ICREA), Barcelona, Spain
X‐linked adrenoleukodystrophy (X‐ALD) is a metabolic genetic disorder of the central nervous system characterized by demyelination in brain and/or axonopathy in the spinal cords, adrenal insufficiency and accumulation of very long‐chain fatty acids (VLCFA) in plasma and tissues. The disease is caused by inactivation of the ABCD1 transporter with the consequence production of oxidative stress mediators and damage. Here we aimed to determine: i) the existence of endoplasmic reticulum (ER) stress and the ensuing unfolded‐protein response (UPR) in brains and fibroblasts from X‐ALD patients, and in the X‐ALD mouse model (the Abcd1 null mouse); and ii) whether the early and rampant oxidative stress formerly identified accounts for the ER stress. Indeed, here we report signs of ER stress and UPR response in human and mouse tissues. A common feature is the activation of ATF6 and lower amounts of IRE1, two ER transducers constituting the core signature of UPR in X‐ALD. Chaperone GRP78 and GRP94 expression, by contrast, differ between variants and stages of the disease. We highlight how chaperones are down‐regulated in X‐ALD mice at 12 months of age, pointing to selective depletion of UPR‐related factors overtime. Treatment of the mouse model with a combination of antioxidants reversed the initial activation of transducers and chaperones, indicating that oxidative stress caused ER stress. Altogether, we postulate that oxidative‐stress elicited ER stress occurs in X‐ALD, and that a defective UPR may contribute to axonopathy progression. Thus, potentiation of the UPR may be a therapeutic avenue in X‐ALD.
T03‐12A. Alpha‐synuclein impairs differentiation of oligodendrocytic CG4 cells
B. Ettle 1, V. May1, M. Wegner2, E. Masliah3, J. Winkler1
1University Hospital Erlangen, Department of Molecular Neurology, Erlangen, Germany 2Friedrich‐Alexander University Erlangen‐Nuremberg, Institute of Biochemistry, Erlangen, Germany 3University of California, Department of Neurosciences, San Diego, La Jolla, United States
The atypical Parkinson syndrome multiple system atrophy (MSA) is a rare and rapidly progressive neurodegenerative disease. The major neuropathological hallmark of MSA is the presence of glial cytoplasmatic inclusions (GCIs) within oligodendrocytes consisting mainly of the primary neuronal protein alpha‐synuclein (α‐syn). GCI bearing oligodendrocytes become dysfunctional and thus, myelin loss accompanied by consecutive neuronal degeneration is a main characteristic of MSA observed in different regions throughout the central nervous system (CNS) including basal ganglia, pons, cerebellum, inferior olivary nuclei, and spinal cord. Although myelination is completed early after birth, oligodendrocyte precursor cells (OPCs) persist in the adult mammalian CNS. Upon injury, OPCs react with amplification and differentiate into premyelinating oligodendrocytes finally remyelinating unwrapped axons preserving them of degeneration. A recent study showed increased numbers of OPCs in MSA patients compared to control patients suggesting a disease‐related alteration of this cell population in MSA (Ahmed et al., Brain Pathol., 2012). However, detailed studies analyzing oligodendrocyte differentiation in MSA are lacking. Thus, we generated human wild‐type α‐syn expressing CG4 cells (CG4_wt‐α‐syn) representing OPC‐like cells with the potential to differentiate upon growth factor withdrawal and addition of triiodothyronine (T3) into premyelinating oligodendrocytes. Upon differentiation, significant fewer CG4_wt‐α‐syn cells express the myelin basic protein (MBP; see figure A) as a marker for terminal differentiation and additionally, mRNA levels were remarkably reduced compared to control cells (see figure B). As mRNA levels of the myelin protein proteolipid protein 1 (PLP1) as well as the major myelin‐gene regulatory factor (MRF) are also reduced in differentiated CG4_wt‐α‐syn cells (see figure B) we hypothesized that the process of differentiation is delayed upon α‐syn expression. Using various differentiation‐promoting agents targeting distinct maturation‐associated pathways we aimed to dissect the α‐syn‐mediated effect on differentiation in more detail and evaluate potential disease‐modifying approaches for this devastating neurodegenerative disorder.

T03‐12B. Intraspinal delivery of PEGylated gold nanoparticles promotes repair after spinal cord injury
F. Papastefanaki 1, I. Jakovcevski2, F. Schulz3, G. Loers2, T. Vossmeyer3, N. Bastús‐Gomez 3, H. Weller3, M. Schachner2, R. Matsas1
1Hellenic Pasteur Institute, Laboratory of Cellular and Molecular Neurobiology, Athens, Greece 2Universitätsklinikum Hamburg‐Eppendorf, Universität Hamburg, Zentrum für Molekulare Neurobiologie, Hamburg, Germany 3Universität Hamburg, Institut für Physikalische Chemie, Hamburg, Germany
The inability of the mammalian central nervous system (CNS) to regenerate after injury results in functional impairment. During the acute phase after spinal cord injury (SCI), the disruption of cell membranes leads to excitotoxicity, oxidative stress, Ca2+ influx, inflammation and cell death. This results to the detrimental events of the chronic phase, which include axonal dieback, Wallerian degeneration and demyelination thus contributing to irreversible loss of function. An interesting approach towards functional recovery would be the healing of disrupted membranes during the acute phase. Previous studies have shown that poly‐ethylene glycol (PEG) administration in CNS injury models can be effective towards restoration of action potentials and functional recovery, but with limitations. Here we have established a combinatorial approach, where we utilized the promising field of nanomedicine, in terms of drug delivery, together with the membrane‐sealing molecule of PEG. In particular, we performed intraspinal delivery of 40 nm‐diameter PEG coated gold nanoparticles (AuNPs) in a mouse model of compression SCI, at the lower thoracic level, during the acute phase. AuNPs are promising carriers for such applications due to their favorable physical and chemical properties and biocompatibility, further increased by PEG coating. Our results show for the first time that functionalized AuNPs delivered in the lesioned spinal cord caused no adverse effects, body‐weight loss, or animal death, compared to control PBS injected mice. Moreover, behavioral analysis of locomotor recovery by the Basso Mouse Scale and single‐frame motion analysis revealed significantly greater recovery of the PEG‐AuNP injected mice compared to controls, up to 8 weeks. In agreement, initial results from immunohistological analysis show a significant reduction of activated microglia in the lesion site, as estimated by Iba‐1 staining, and a significant increase in choline acetyltransferase‐positive motoneurons in the lumbar spinal cord, indicating attenuated inflammatory response and an enhanced motorneuron survival. This study underlines the potential benefit of PEG AuNPs as a drug delivery platform along with other therapeutic molecules that could be adopted clinically towards repair of the injured CNS.
Supported by EU FP7 264083 Neurosign and the Greek General Secretariat for Research and Technology GRANT 09SYN‐21–969. Also funded by the LEXI Landesexcellenzinitiative Hamburg NAME.
T03‐13A. Treatment early in life with the lectin ConA decelerates spreading depression in well‐nourished and early‐malnourished adult rats
G. S. Ferreira Soares, L. Cabral Cavalcanti, R.C. Araújo Guedes
Universidade Federal de Pernambuco, Recife, Brazil
Background and Purpose: The lectin Concanavalin A (ConA), obtained from the seeds of Canavalia ensiformis, stands out as a biochemically, biophysically and structurally well characterized lectin, commonly used as histochemical marker, due the its high selectivity to microglial cells. Early nutritional insults have important effects on neural and glial electrophysiological activity because nutrients regulate brain development during early postnatal life, when neural and glial cells present higher degree of plasticity. Herein we characterized in adult rats the electrophysiological changes caused by the systemic treatment early in life with the lectin ConA by recording the electrophysiological phenomenon of cortical spreading depression (CSD). Additionally, we evaluated the impact of early nutritional deficiency on the CSD effects of ConA.
Methods: Two nutritionally distinct groups of male newborn rats – well‐nourished (W; n = 26) and malnourished (M; n = 33) were treated from the 5th to the 24th day of postnatal life with daily intraperitoneal injections of saline (Sal) or ConA (Sigma‐Aldrich) at doses of 1 mg/kg (group W‐L1 and M‐L1) or 10 mg/kg (Groups W‐L10 and M‐L10). Two groups of “naive” (non‐injected) pups were used as additional controls. When the pups reached adult age (90–120 days), under ip anesthesia (1 g/kg urethane + 40 mg/kg chloralose) they were submitted to the CSD recording (ECoG and slow potential change) in two points of the parietal cortical surface. CSD was triggered every 20 minutes by applying 2% KCl for 1 min at a point in the frontal cortex. This study was approved by the ethics committee of our University, Case No.: 23076.031272/2010‐53.
Results: Compared to W‐Sal controls (mean ± sd velocity in mm/min = 3.41 ± 0.10), M‐Sal rats presented significantly higher velocities (4.26 ± 0.16; p < 0.05). In both nutritional conditions L1 and L10 treated groups presented significantly lower CSD velocities (3.12 ± 0.14 and 2.82 ± 0.18 for the W‐L1 and W‐L10, respectively, and 3.74 ± 0.13 and 3.25 ± 0.16 for the M‐L1 and M‐L10). This effect was dose‐dependent, and was greater in the M‐condition. The naïve‐ and Sal‐groups had similar CSD velocities.
Conclusions: The lectin ConA administered early in life to rats decelerated CSD in a dose‐dependent manner at adulthood, suggesting a long‐lasting effect. The largest relative reduction was observed in the M group, suggesting modulation of the effect by the nutritional status. The involvement of glial cells in this effect is discussed.
T03‐13B. Pharmacological inhibition of monoacylglycerol lipase and its effect on physiological parameters in vivo
N. Pasquarelli 1, J. Hanselmann1, R. Fürtig2, C. Porazik1, T. Wipp1, D. Wiesner1, B. Linkus1, P. Weydt1, B. Ferger2, A. Witting1
1Ulm University, Department of Neurology, Ulm, Germany 2Boehringer Ingelheim Pharma GmbH & Co. KG, CNS Diseases Research, Biberach an der Riß, Germany
The endocannabinoid system is of growing interest as a therapeutic target in neurological diseases. The two major endocannabinoids 2‐arachidonoylglycerol (2‐AG) and N‐arachidonoyl ethanolamine (anandamide, AEA), are the endogenous ligands for the cannabinoid receptors 1 (CB1) and 2 (CB2). CB1 is mainly expressed in the brain and associated with neuroprotective effects, whereas CB2 is primarily found in hematopoietic cells and associated with anti‐inflammatory effects. In neurodegenerative diseases, e. g. amyotrophic lateral sclerosis (ALS) and Parkinson's disease (PD), an increased CB2 expression on microglia has been reported and is therefore an interesting therapeutic target. Furthermore, an increase of endocannabinoid levels occurs in the affected neuronal tissues. Stimulating this disease‐related increase in endocannabinoids might thus be of therapeutic interest.
In our project, we evaluated the novel, highly selective inhibitor KML29 of an enzyme central to the degradation of the endocannabinoid 2‐AG, the monoacylglycerol lipase[1]. An in vitro monoacylglycerol lipase inhibitor screening assay for the comparison of the utilized inhibitor with others was performed. Additionally, behavioral experiments including body temperature measurement, pain threshold measurement and motor activity analysis in healthy control mice were performed. Furthermore, endocannabinoid system baseline levels of healthy control mice vs. SOD1 (superoxide dismutase 1) mice as an ALS mouse model were compared at the gene expression and protein level.
In vitro inhibition of the monoacylglycerol lipase by the recently published compound KML29 resulted in lower IC50 values and therefore indicated a higher potency of KML29 when compared to the other monoacylglycerol lipase inhibitors JZL184 and CPC12 applied in the assay.
In vivo administration of the reported monoacylglycerol lipase inhibitor KML29 resulted in significant decrease in body temperature, increase in pain threshold in B6SJLF1/J mice as well as in impairments in the nocturnal motor activity of C57BL/6J mice when compared to vehicle‐treated mice.
In summary, our data show a comparatively high potency in vitro and significant dose‐dependent effects on physiological parameters in vivo of the monoacylglycerol lipase inhibitor KML29 and therefore lay the foundation for a future therapeutic study targeting neuroinflammation in mouse models of ALS and PD.
[1] Chang JW et al., Highly selective inhibitors of monoacylglycerol lipase bearing a reactive group that is bioisosteric with endocannabinoid substrates, Chem Biol. 2012 May 25;19(5):579–88.
T03‐14A. Glial Lazarillo protects neurons from type I Spinocerebellar Ataxia (SCA1) degeneration by a mechanism involving the control of autophagy flow and of lipid peroxide clearance
M. del Caño‐Espinel, D. Sanchez, M.D. Ganfornina
Universidad de Valladolid – CSIC, Valladolid, Spain
Glial Lazarillo (GLaz) is a Drosophila homologue of Apolipoprotein D (ApoD), a lipocalin showing an increased expression with aging and neurodegeneration. GLaz/ApoD protects against oxidative stress and promotes axon regeneration after injury, but its mechanism of action is unknown. We hypothesize that GLaz/ApoD modulates membrane dynamics and oxidation. To contrast this hypothesis we study GLaz protective role on the polyQ‐based Spinocerebellar Ataxia type I (SCA1) model in Drosophila.
Human polyQ‐Ataxin1 expression in fly photoreceptors triggers GLaz up‐regulation. Overexpressing GLaz in photoreceptors or in retinal support cells rescues neurodegeneration. When expressed in retinal support cells, the GLaz rescue is dependent on Lipocalin‐receptor expression by photoreceptor neurons, and the GLaz‐GFP fusion protein co‐localizes with photoreceptor markers, suggesting the existence of receptor‐mediated endocytosis of GLaz.
Upon neurodegeneration, oxidative stress and induction of autophagy coexist. To test whether the GLaz beneficial effects are mediated by the modulation of autophagy we quantified Atg8a expression and monitored the accumulation of ubiquitinated proteins and p62. Overexpression of GLaz reduces Atg8a induction, but concurrently reduces the accumulation of ubiquitinated proteins and p62. Upon autophagy stimulation by rapamycin treatment, the levels of SCA1‐dependent accumulation of ubiquitinated proteins and p62 are further reduced by GLaz. In addition, GLaz decreases the SCA1‐dependent induction of Gsts1, a SCA1 genetic modifier contributing to the clearance of lipid peroxides.
Our data support that GLaz enters the degenerating neurons by a receptor‐mediated mechanism and promotes the resolution of autophagy, increasing its flow and helping to clear polyQ‐induced protein aggregates. Moreover, the beneficial effects of GLaz are linked to lipid peroxide clearance, either directly or through receptor‐mediated modulation of protective gene networks.
Support: MICINN(BFU2011‐23978); JCyL(VA180A11‐2); Fund. Rodríguez‐Pascual.
T03‐14B. Unlike physical exercise, modified environment increases the lifespan of SOD1G93A mice however both conditions induce cellular changes
Y. Gerber1,2, J.C. Sabourin2, J.‐P. Hugnot2, F. Perrin 3
1IKERBASQUE Basque Foundation for Science, Bilbao, Spain 2INSERM U 1051, Institute for Neurosciences of Montpellier, Montpellier, France 3University of the Basque Country, Leioa, Spain
Background: Amyotrophic lateral sclerosis (ALS) is characterized by a gradual muscular paralysis resulting from progressive death of motoneurons. ALS etiology remains unknown although it has been demonstrated that it is a multifactorial disease that involves several cellular partners. There is currently no effective treatment but several lines of research, including the effect of exercise, are under investigation. Whether physical exercise is beneficial or harmful is still under debate.
Methods and Findings: We investigated the effect of three different intensities of running exercises on the survival of SOD1G93A mice. At the early‐symptomatic stage (P60), males were isolated and randomly assigned to 5 conditions: 2 sedentary groups (“sedentary” and “sedentary treadmill” placed on the inert treadmill), and 3 different training intensity groups (5 cm/s, 10 cm/s and 21 cm/s; 15 min/day, 5 days/week).
We first demonstrated that an appropriate "control" of the environment is of the utmost importance since comparison of the two sedentary groups evidenced an 11.6% increase in survival in the “sedentary treadmill” group. Moreover, we showed by immunohistochemistry that this increased lifespan is accompanied with motoneurons survival and increased glial reactivity in the spinal cord. In a second step, we showed that when compared with the proper control, all three running‐based training did not modify lifespan of the animals, but resulted in glial and motoneuronal changes.
Conclusions/Significance: We demonstrate that increase in survival induced by a slight daily modification of the environment is associated with motoneurons preservation and strong glial modifications in the lumbar spinal cord of SOD1G93A. Using the appropriate control, we then demonstrate that all running intensities have no effect on the survival of ALS mice but induce cellular modifications.
Our results highlight the critical importance of the control of the environment in ALS studies and may explain discrepancy in the literature regarding the effect of exercise in ALS.
T03‐15A. N‐Arachidonoyl – Dopamine (NADA) is a novel neuroprotective Endocannabinoid
U. Grabiec 1, M. Koch2, R. Kraft3, K. Hill4, C. Merkwitz2, C. Ghadban1, B. Lutz5, A. Straiker6, F. Dehghani1
1Martin‐Luther‐Universität Halle Wittenberg, Institut für Anatomie und Zellbiologie, Halle (Saale), Germany 2Universität Leipzig, Germany, Institut für Anatomie, Leipzig, Germany 3Universität Leipzig, Carl‐Ludwig Institut für Physiologie, Leipzig, Germany 4Universität Leipzig, Rudolf‐Boehm Institut für Pharmakologie und Toxikologie, Leipzig, Germany 5Johannes‐Gutenberg‐Universität, Institut für Physiologische Chemie und Pathobiochemie, Mainz, Germany 6Gill Center for Biomolecular Medicine , Department of Psychological and Brain Sciences, Bloomington, United States
Endocannabinoids exert numerous effects in the CNS under physiological and pathological conditions. The aim of the present study was to examine whether the novel endocannabinoid N‐arachidonoyldopamine (NADA) may protect neurons in excitotoxically lesioned organotypic hippocampal slice cultures (OHSC). OHSC were excitotoxically lesioned by application of N‐methyl‐D‐aspartate (NMDA, 50 µM) for 4 h and subsequently treated with different NADA concentrations (0.1 pM – 50 µM) alone or in combination with cannabinoid receptor antagonists. NADA protected dentate gyrus granule cells and caused a faint reduction in the number of microglial cells. The number of degenerated neurons significantly decreased between 100 pM and 10 µM NADA. O‐1918, 6‐iodonordihydrocapsaicin and HC‐030031 showed no effects at all used NADA concentrations. In next step we looked at the MAP kinase signal transduction pathway activation after stimulation with NADA. For this purpose we used neuronal hippocampal cell line HT22, microglial cell line BV2 and astrocytes, because of their appearance in OHSC. NADA did not influence the activation p38 and p44/42 MAPK in analyzed cells (after 30 min, 2 h, 6 h, 24 h). Our findings demonstrate that NADA protects dentate gyrus granule cells by acting upon CB1. NADA reduced the number of microglial cells at distinct concentrations. TRPV1 was not involved in NADA mediated neuroprotection. Thus, our data implicate that NADA‐mediated activation of neuronal CB1 may serve as a novel pharmacological target to mitigate symptoms of neuronal damage.
T03‐15B. Amyloid β impairs expression, traffic and secretion of carboxypeptidase e and secretogranin III in astrocytes
V. Plá Requena 1, M. Sánchez‐Valpuesta1, E. Pozas2,3, I. Ferrer4, F. Aguado Tomás1
1Universitat de Barcelona, Biologia Cel·lular, Barcelona, Spain 2August Pi i Sunyer Biomedical Research Institute (IDIBAPS), Barcelona, Spain 3Institute of Biomedical Research of Barcelona, CSIC, Barcelona, Spain 4Universidad de Barcelona, Institut Neuropatología, IDIBELL‐Hospital Universsitari de Bellvitge, Barcelona, Spain
Alzheimer's disease is the most prevalent neurodegenerative disorder, characterized by occurrence of senile plaques, neurofibrillary tangles and aberrant function of classical neurotransmitters and peptide messengers, such as neuropeptides and growth factors. In the last years a role of astrocytes in mediating and amplifying the progression of neurodegenerative disorders has been proposed. Moreover, a growing body of evidence has shown that astrocytes can release non‐peptide and peptide transmitters to influence neuronal development, function and plasticity. Here we analyzed the impact of the main component of senile plaques, the amyloid‐β (Aβ), on two key components of peptide vesicles, Carboxypeptidase E (CPE) and Secretogranin III (SgIII), in astrocytes in vitro and in vivo. We consistently located CPE and SgIII proteins in cultured and in situ astrocytes. Traffic and secretion of astroglial CPE and SgIII were analyzed under basal and stimulated conditions in primary cultures. We found that exposure of astrocytes to Aβ1–42 markedly impairs expression, traffic and secretion of glial CPE and SgIII. Protein levels were decreased in the media, but increased into the cells, by Aβ1–42 incubations. The effect of Aβ on CPE and SgIII in glial cells was also investigated in vivo using the amyloid‐forming transgenic mice APPswe/PS1dE9. An aberrant accumulation of CPE and SgIII was found in numerous activated‐astrocytes surrounding senile plaques through all the CNS of aged transgenic mice. Taken together, the present study shows that Aβ dramatically alters expression, traffic and secretion of CPE and SgIII. Because CPE and SgIII are essential in the process and targeting of neuropeptides and growth factors, an involvement of the astroglial secretory pathway in the pathology of Alzheimer's disease is suggested.
T03‐16A. Antagonizing the TGF‐ß1 Receptor ALK5 reduces gliosis after Neonatal Hypoxia‐Ischemia
M. Guardia Clausi 1, Z. Ren1, D. Giannakidis1, S.W. Levison2
1NJMS‐UH Cancer Center, Dept of Neurology and Neurosciences, Newark, NJ, United States 2University of Medicine and Dentistry of New Jersey, Newark, United States
Our previous studies have shown that there is a regenerative response from the neural precursors in the subventricular zone after neonatal H‐I (H‐I). In vitro, neural precursors from the injured hemisphere have an increased capacity to generate oligodendrocytes and neurons. However, in vivo, after H‐I there is aberrant production of astrocytes, and there is emerging evidence that reactive astrocytes inhibit the differentiation of oligodendrocyte progenitors. We have identified TGFB1 as a cytokine that is produced subsequent to H‐I, that collaborates with other cytokines to stimulate the production of astrocytes from subventricular zone glial progenitors. The specific goal of this study was to evaluate gliogenesis after H‐I when the TGFB1 receptor ALK5 is antagonized in the Vanucci P6 H‐I rat model. By Q‐PCR, the relative amount of TGFB1 mRNA peaked 7 days after H‐I. Therefore, SB‐505124, and ALK5 antagonist, was given intraperitoneally (i.p.) 7 days after the injury to a group of rats while another group received PBS. Animals were sacrificed at different time points and brain samples processed for Western blot analysis. Validating the importance of ALK‐5 signaling, SB505124 reduced the levels of phosphorylated Smad 2/3 that increased after H‐I. In another study, SB‐50512 was administered i.p. from P10 to P15 and animals sacrificed at P20 for immunohistochemistry for GFAP, IBA‐1, GSTpi and MBP. GFAP and IBA‐1 positive cells were dramatically increased in the injured striatum and corpus callosum after H‐I and MBP staining was decreased. By contrast, both the extent of injury and the degree of reactive gliosis was decreased by SB‐505124 treatment. Altogether, our results indicate that SB‐505124 inhibits ALK5 signaling in the damaged brain. Furthermore, SB‐505124 administration decreases the extent of microgliosis and astrogliosis while preserving myelination in the damaged brain after neonatal H‐I. Supported by NIH R01 HD052064 and a grant from the LeDucq Foundation awarded to SWL.
T03‐16B. Diesel exhaust pollution affects learning abilities and leads to an altered stress response in the CNS of the honey bee (Apis mellifera)
C. Reitmayer, T.A. Newman, R. Girling, E. Farthing
University of Southampton, Southampton, United Kingdom
Question: Diesel Exhaust Pollution (DEP) is known to be detrimental to individuals with asthma and cardiovascular diseases [1]. Epidemiological, and mammalian in vivo and in vitro studies demonstrate that DEP also affects the Central Nervous System (CNS) [2]. Despite efforts to improve air quality, DEP persists as a problem and the share of diesel engined passenger cars in Western Europe is increasing steadily.
Honey bees are an important pollinator of food crops and wild flowering plants. They not only contribute to food security by providing pollination services of an enormous economic value but also play an important ecological role. Over the last five years, bee keepers world‐wide have reported a decline in honey bee populations. The reasons for this decline remain unknown, but it is thought to be multi‐factorial [3]. Honey bee colonies are confronted with a number of stressors, for example infection with the mite Varroa destructor or exposure to pesticides. To forage successfully honey bees rely on their learning and memory abilities. Our research aims to establish whether DEP might be a factor contributing to declines in honey bee populations through impairment of healthy CNS function.
Methods: We have investigated the impact of DEP exposure on the learning abilities of the honey bee using the proboscis extension reflex and classical conditioning. Western blotting and histology were used to determine regional expression levels of stress proteins in the brain in response to an acute diesel exhaust exposure. We are examining the morphological appearance of glial cells to investigate the impact of DEP in the honey bee CNS of DEP exposed, and control, forager bees.
Results and Conclusions: We hypothesize that DEP acts as a stressor in the brain of the honey bee causing changes in glial cells that are detectable using morphological and molecular techniques – similar to microglial activation or immunological responses of astroglia in the mammalian brain. This work will further our understanding of how DEP acts on the brain and how it might impact on the health of the animal. Current results indicate that diesel exhaust pollution impacts on the neurobiology of the honey bee and this may contribute to decline by impairing CNS functions.
T03‐17A. Ammonium induces an astroglial calcium dysbalance in different brain regions
N. Haack, C.R. Rose
Heinrich Heine University Düsseldorf, Duesseldorf, Germany
Hepatic encephalopathy (HE) is a serious neurological disorder caused by liver failure. The prime candidate responsible for HE pathology is an increased ammonium concentration; and following acute liver failure, ammonium can reach levels of up to 5 mM in the brain. Astrocytes are a major target of ammonium toxicity and earlier studies suggested that this toxicity includes a disturbance in intracellular calcium signaling.
In the present study, we analyzed the effect of acute hyperammonia on the intracellular calcium concentration of astrocytes in tissue slices of mouse brain, using quantitative intracellular calcium imaging with the indicator dye Fura‐2. Astrocytes were identified by staining with the vital dye SR101; cerebellar Bergmann glial cells were identified based on their morphology. In all brain regions studied, the hippocampal CA1 area, somatosensoric cortex, and cerebellar cortex, bath perfusion with 5 mM ammonium for 30 min induced a small, but persistent elevation in intracellular calcium, averaging about 50 nM. In addition, a fast and transient increase by on average 100 nM that lasted about 10 minutes was seen in a small percentage of astrocytes in hippocampal and cortical slices at the onset of ammonium perfusion. This transient increase was never observed in cerebellar Bergmann glial cells. Both the transient, as well as the plateau phase of ammonium‐induced calcium changes were unaltered in the presence of TTX, indicating that they are independent from action potential generation by neurons. Furthermore, ammonium‐induced calcium increases largely persisted upon removal of extracellular calcium, indicating that they are caused by release from intracellular stores.
Taken together, our experiments demonstrate that ammonium evokes complex changes in the intracellular calcium concentration of astrocytes in the intact tissue, which differ both between cells in one preparation as well as between different brain regions. Because of the central role of astrocyte calcium in gliotransmission, the observed ammonium‐induced calcium dysbalance might result in disturbed neuron‐glia interaction and contribute to the pathology of HE.
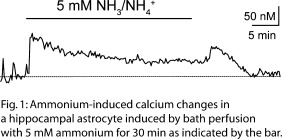
T03‐17B. Bothropic neurotoxicity in nerve terminals and Schwann cells
T. Rocha 1, L. Rodrigues Simioni2, L.A. Ponce Soto3, S. Marangoni3, M.A. Cruz Höfling4
1Universidade São Francisco, Bragança Paulista, Brazil 2Universidade Estadual de Campinas, Depto. Farmacologia, Campinas, Brazil 3Universidade Estadual de Campinas, Depto. Bioquímica, Campinas, Brazil 4Universidade Estadual de Campinas, Depto. Histologia e Embriologia, Campinas, Brazil
In Brazil, the accidents caused by Bothrops snakes are close to 90%. Although the dominant effect is marked myotoxicicity, the neurotoxicity is shown at low doses. The majority of studies characterize these effects as being pre‐ or postsynaptic then inferring that acetylcholine releases at the nerve terminal or receptors at the junctional folds are affected by the venom components. Studies dealing with the effect on Schwann cells are rare, despite evidences showing the capacity of these glial cells in modulating neuromuscular transmission. Herein, we investigate if the expression of proteins directly involved with synaptic vesicle release and Schwann cell morphology are affected by some Bothropic venoms and toxins. Myography (twitch‐tension recording), immunohistochemical and western blotting (to measure synaptophysin, synaptobrevin, SNAP25 and syntaxin expressions) and transmission electron microscopy (to evaluate Schwann cells) were used. Mouse phrenic nerve‐diaphragm preparation (PND) was incubated with B. jararacussu venom (100 μg/mL) and BthTX‐I (50 μg/mL) and chick biventer cervicis (BC) preparation was incubated with BmjeTX‐I and BmjeTX‐II (10 μg/mL). B. jararacussu venom, BthTX‐I, BmjeTX‐I, BmjeTX‐II toxins caused a total and irreversible neuromuscular blockade. Synaptophysin and synaptobrevin expressions were negative, while SNAP25 and syntaxin expressions were positive for PND and BC. Morphological analyses showed terminals with mitochondrial damage, synaptic vesicles sequestration or partial depletion. Schwann cells showed mitochondrial damage and infiltrated processes between pre‐ and postsynaptic compartments. The lack of synaptobrevin and synaptophysin expression explains synaptic vesicle depletion while the syntaxin and SNAP25 normal expression suggests absence of a postsynaptic effect by venom and toxins. The changes in the Schwann cells are evidence that these glial cells mediate alterations in both the neuromuscular transmission and proteins associated with synaptic event.
FAPESP (2011/0001‐1) and CNPq support.
T03‐18A. Astrocytes in the degenerating brain are primed to synthesize exaggerated levels of CXCL1 and CCL2 in response to IL‐1b/TNF‐a stimulation
E. Hennessy, C. Cunningham
Trinity College Dublin, Dublin, Ireland
Microgliosis and Astrogliosis are standard features of neurodegenerative disease. It has been shown in a number of animal models that microglia in the degenerating brain are primed to show exaggerated cytokine responses to subsequent stimulation with Toll‐like receptors agonists such as LPS and poly I:C. It is not clear whether the degenerating brain shows similarly exaggerated responses to pro‐inflammatory cytokines. In the current study we hypothesised that glial cells in the hippocampus of animals with chronic neurodegenerative disease (ME7 prion disease) would display abnormal responses to central cytokine challenges. In normal animals it has previously been established that intracerebral cytokine challenges elicit specific pathways, i.e. IL‐1β à CXCL‐1 à Neutrophil recruitment or TNFα à CCL‐2 à Monocyte recruitment. Unilateral 1μl doses of TNFα (300ng/μl), IL‐1β (10ng/μl) or saline were administered intrahippocampally via pulled glass microcapillary, in normal (NBH) and ME7 mice.
These animals were terminally perfused for formalin fixation and paraffin embedding at 2, 24 and 72 hours post challenge. At 2 hours post challenge ME7 microglia produced IL‐1β following either IL‐1b or TNFα challenge while NBH microglia did not. Furthermore, there was very robust nuclear localisation of the NFkB subunit p65 in the astrocyte population and this was associated with very marked astrocytic synthesis of the chemokines CXCL‐1 and CCL‐2 in response to both cytokine challenges in ME7 animals. Conversely, very limited expression of these chemokines was apparent in NBH animals similarly challenged. Thus astrocytes are the primary chemokine synthesizing brain cell and in the prion‐diseased brain are they primed to produce exaggerated chemokine responses to acute stimulation with pro‐inflammatory cytokines. This abnormal pattern of chemokine expression is predicted to have consequences for leukocyte infiltration and the ramifications of an altered cellular infiltration pathway for the degenerating brain will be discussed.
This work was supported by the Wellcome Trust (Grant no. WT 078300 to CC)
T03‐18B. Effects of in vivo selective expression of mutated huntingtin by mouse striatal astrocytes on functional properties of medium spiny neurons
A.B. Rocher 1, G. Bonvento2
1UNIL, Department of Fundamental Neurosciences, Lausanne, Switzerland 2CEA – Institut d'Imagerie Biomedicale (I2BM), MIRCen, Fontenay‐aux‐Roses, France
In patients with Huntington's disease (HD), a mutation on huntingtin (htt) gene leads to an expansion of a polyglutamine tract in N‐terminus of htt protein. Huntingtin is expressed ubiquitously in both neuronal and glial cells. In HD patients, neuropathological studies reveal major neuronal death specifically targeting efferent inhibitory medium spiny neurons (MSNs), the largest neuronal population in the striatum. The contribution of astrocytic mutated htt to the MSNs dysfunction and loss is still under investigation. In a previous study from our laboratory (Faideau et al., Human Molecular Genetics 2010), we described a novel mouse model using lentiviral vectors injected in the striatum that results in selective expression of either normal or mutated htt into striatal astrocytes. In this model, astrocytes expressing mutated htt developed a progressive phenotype of reactive astrocytes that was characterized by a marked decrease in both expression of glutamate transporters and glutamate uptake. These findings suggested that the presence of mutated htt in astrocytes alters glial glutamate transport capacity early in the disease process and may contribute to excitotoxicity. To decipher whether astrocytic mutated htt expression was directly associated with MSNs dysfunction, we investigated the functional properties of individual MSNs in proximity of mutated htt expressing astrocytes in acute slices. Results from whole‐cell patch clamp technique showed that MSNs surrounded (0 – 100 microns distance) by astrocytes expressing mutated htt vs. astrocytes expressing normal htt did not differ with regard to passive membrane properties (input resistance, resting membrane potential), action potential firing rates nor spontaneous excitatory postsynaptic current properties (averaged amplitude and frequency). Thus, astrocytic expression of mutated htt does not lead to readily apparent functional consequences in MSNs in these conditions.
T03‐19A. The L‐type voltage‐gated calcium channel subunit alpha1C (CaV1.2) is expressed in astrocytes around beta‐amyloid plaques in an Alzheimer mouse model
C. Humpel 1, N. Daschil1, G.J. Obermair2, B.E. Flucher2, B. Hutter‐Paier3, M. Windisch3, J. Marksteiner1
1Lab. Psychiatry and Exp. Alzheimers Res., Innsbruck, Austria 2Div. Physiology, Innsbruck, Austria 3JSW Lifesciences, Grambach, Austria
Growing evidence suggests that amyloid‐β peptides (Aβ) may affect the modulation of L‐type voltage‐gated calcium channels (LTCC) in Alzheimer's disease (AD). We have recently shown that the LTCC alpha1C subunit (CaV1.2, a1C) is expressed in activated astrocytes around plaques in transgenic mice expressing the human AβPP751 with the London (V717I) and Swedish (K670M/N671L) mutations (Willis et al., J Alzheimers Disease 20: 1167–1180. 2010). The aim of the present study using this Alzheimer mouse model is (1) to investigate in more detail the cellular expression of the a1C subunit in the brains in a time‐ and region specific pattern, (2) to determine quantitative mRNA expression profiles of all LTCC subunits in the hippocampus and (3) to perform a co‐localization study with the auxiliary calcium channel beta4 subunit. (4) Further we aim to culture primary astrocytes and stimulate with recombinant Aβ(42) to induce and study a1C expression in vitro.
Our data show that 11 month old tg mice displayed a significant increase of Aβ‐plaques in the brain, compared to wt mice. Furthermore, we show that the a1C subunit colocalizes with 90% of the Aβ‐positive plaques. The cellular expression of a1C was predominantly found in activated GFAP+ astroglia around plaques. qPCR profiles of the hippocampus revealed expression of the majority of calcium channel subunits, which was stable during aging. The auxiliary beta4 subunit was expressed in these mice but did not co‐localize with a1C+ astroglia around plaques. In cultures of primary astrocytes, Aβ(42) slightly enhanced the a1C mRNA expression after 3 days. We are currently underway to characterize this expression in more detail.
In summary, our data provide evidence for a largely stable expression of most LTCC subunits in Alzheimer tg mice during aging. Finally, the expression of a1C but not beta4 is upregulated in activated astrocytes located around Aβ‐plaques and Aβ(42) may directly induce astroglial CaV1.2 calcium channels.
This study was supported by the Sonderforschungsbereich SFB F4405‐B19 and F4406‐B19 of the Austrian Science Funds.
T03‐19B. Enriched environment and physical activity reverse astroglial atrophy in the hippocampus of the triple‐transgenic model of AD
J. Rodriguez Arellano 1, S. Terzieva1, M. Olabarria2, A. Verkhratsky1,2,3
1IKERBASQUE, Basque Foundation for Science, Zamudio, Spain 2The University of Manchester, Faculty of Life Science, Manchester, United Kingdom 3University of the Basque Country UPV/EHU, Department of Neuroscience, Leioa, Spain
Astrocytes are fundamental for brain homeostasis and have pivotal role in the development and the progression of the neurological diseases including Alzheimer's disease (AD). Our previous studies demonstrated reactive changes in astrocytes associated with senile plaques, whereas astrocytes not associated with plaques present a clear atrophic morphology in the relevant regions of the brain affected by the disease, including the hippocampal formation (Olabarria et al., 2010, Yeh et al., 2011).
Increasing evidence indicate that both physical and cognitive stimulation induce plastic changes in the brain, such as increase in synaptic and spine densities or neurogenesis, and counteract β amyloid (Aβ) pathology recovering cognitive function. In the present study we, for the first time, demonstrate the effects of psychostimulation on glial fibrillary acid protein (GFAP) architecture in the dentate gyrus (DG) of 3xTg‐AD, a well‐established mouse model of AD.
Starting from 3 months of age, 3xTg‐AD and their corresponding controls (non‐Tg) were housed either in the presence of a running wheel (RUN) or in the enriched environment (ENR) for 9 months. As reported previously (Olabarria et al., 2010), in standard housing, 3xTg‐AD animals showed marked atrophy of GFAP‐positive profiles, evidenced by significant reduction in GFAP surface area, by 39%, and volume, by 42%, compared with non‐Tg mice. However, physical and cognitive stimulation affected astrocyte morphology by increasing their cytoskeleton surface area and volume, both in non‐Tg and in 3xTg‐AD mice. The surface area of GFAP‐IR astrocytes increased by 145% and 154% in 3xTg‐AD mice housed in RUN and ENR, respectively, compared with transgenic mice kept in standard housing. In addition, the cell volume of GFAP‐IR astrocytes was higher by 169% and 199% in 3xTg‐AD mice housed in RUN and ENR, respectively. The hypertrophy was also evidenced by an increase in the surface area and the volume of astroglial somata and processes in both non‐Tg and 3xTg‐AD mice.
Further correlation analysis between 3xTg‐AD and their corresponding control mice housed under the same conditions, revealed that RUN and ENR not only reversed the genotype‐induced astrocytic atrophy, but even induced a hypertrophy in 3xTg‐AD mice, with GFAP positive profiles rising to the levels of non‐Tg mice.
Thus our study indicates that long‐term exercise and enriched environment restore and improve astroglial plasticity in the context of AD‐like pathology, which may recover cognitive functions by compensating disease‐associated degeneration of neuronal‐glial network.
REFERENCES
1. Olabaria et al., 2010; Glia.; 58(7):831–8.
2. Yeh et al., 2011; ASN Neuro.; 19;3(5):271–9.
T03‐20A. Neuroprotective effects of Withaferin A in three mouse models of amyotrophic lateral sclerosis
P. Patel, V. Swarup, D. Phaneuf, J. Kriz, J.‐P. Julien
Laval University, Quebec, Canada
Question: Recent studies have shown that Withaferin A (WA), an inhibitor of NF‐κB activity was efficient in reducing disease phenotype in TDP‐43 transgenic mice model of ALS. These findings led us to test WA in three mice models of ALS, SOD1G93A mice, SOD1G37R mice and TDP‐43A315T transgenic mice model.
Methods: Intraperitoneal administration of WA at a dosage of 4mg/kg of body weight was initiated from postnatal day 40 (P40) till end stage in SOD1G93A mice, from 9 months till end stage in SOD1G37R mice and from 6 months of age in TDP43A315T mice.
Results: WA was able to improve the survival by more than a week in SOD1G93A and by two weeks in SOD1G37R familial mouse model. In addition WA administration also conferred significant neuroprotection in TDP43A315T transgenic mice model. Beneficial effects of WA in SOD1G93A mice model was accompanied by reduction in loss of motor neurons and also by reduction in level of misfolded SOD1 protein in the spinal cord as detected by immunoprecipitation with antibody specific to misfolded SOD1. WA was found to be an inducer of heat shock protein 27 (HSP‐27), which could possibly explain reduced level of misfolded SOD1 and increased neuroprotection in WA treated mice. Moreover real‐time imaging with the use of biophotonic SOD1G93A transgenic mice carrying luc (luciferase) and gfp (green fluorescent protein) reporter genes under the control of the murine GAP‐43 promoter revealed that WA was able to reduce neuronal stress at post symptomatic stage.
Conclusion: Taken together, our results suggest that WA may represent a promising therapeutic drug for treatment of ALS.
T03‐20B. Astrocyte degeneration correlates with aberrant intracellular calcium signalling in Amyotrophic Lateral Sclerosis
F. Martorana1, L. Brambilla1, C. Valori 2, C. Bergamaschi1, C. Roncoroni 2, E. Aronica 3, A. Volterra 4, P. Bezzi 4, D. Rossi 1
1IRCCS Salvatore Maugeri Foundation, Pavia, Italy 2University of Milan, Milan, Italy 3Academisch Medisch Centrum, Amsterdam, Netherlands 4University of Lausanne, Lausanne, Switzerland
A major constraint to the comprehension of the pathogenesis of Amyotrophic Lateral Sclerosis (ALS) has been long represented by the conviction that this disorder selectively affects motor neurons in a cell‐autonomous manner. However, the failure to identify the events underlying the neurodegenerative process and the increased knowledge of the complex cellular interactions necessary for the correct functioning of the CNS has recently focused the attention on the contribution to neurodegeneration of glial cells, including astrocytes. Astrocytes can hurt motor neurons by secreting neurotoxic factors, but they can also play a deleterious role by losing functions that are supportive for neurons. Recently, we reported that a subpopulation of spinal cord astrocytes degenerates in the microenvironment of motor neurons in the hSOD1G93A mouse model of ALS. Mechanistic studies in vitro identified a role for the transmitter glutamate in the gliodegenerative process via the activation of its inositol 1,4,5 triphosphate (IP3)‐generating metabotropic receptor 5 (mGluR5) (1). Since non‐physiological formation of IP3 can prompt IP3 receptor (IP3R)‐mediated Ca2+ release from the intracellular stores and trigger various forms of cell death, here we investigated the intracellular Ca2+ signalling that occurs downstream of mGluR5 in hSOD1G93A‐expressing astrocytes. Contrary to wild‐type cells, stimulation of mGluR5 causes aberrant and persistent elevations of intracellular Ca2+ concentrations in the absence of spontaneous oscillations. The interaction of IP3Rs with the anti‐apoptotic protein Bcl‐XL was previously described to prevent cell death by modulating intracellular Ca2+ signals. In mutant SOD1‐expressing astrocytes, we found that the sole BH4 domain of Bcl‐XL, fused to the protein transduction domain of the HIV‐1 TAT protein (TAT‐BH4), is sufficient to restore sustained Ca2+ oscillations and cell death resistance. Furthermore, chronic treatment of hSOD1G93A mice with the TAT‐BH4 peptide reduces focal degeneration of astrocytes, slightly delays disease onset, and improves both motor performance and survival (2).
1. Rossi, D., Brambilla, L., Valori, C.F., Roncoroni, C., Crugnola, A., Yokota, T., Bredesen, D.E., and Volterra, A. 2008. Focal degeneration of astrocytes in amyotrophic lateral sclerosis. Cell Death Differ 15:1691–1700.
2. Martorana, F., Brambilla, L., Valori, C.F., Bergamaschi, C., Roncoroni, C., Aronica, E., Volterra, A., Bezzi, P., and Rossi, D. 2012. The BH4 domain of Bcl‐XL rescues astrocyte degeneration in amyotrophic lateral sclerosis by modulating intracellular calcium signals. Hum Mol Genet 21:826–840.
T03‐21A. Lysosomal enzymes in the hippocampal glial cells of kainic acid treated rats: potential implication in temporal lobe epilepsy
S. Kar, M. Banerjee, A. Sasse, Y. Wang, M. Maulik
University of Alberta, Edmonton, Canada
The temporal lobe epilepsy (TLE) is the most common form of epilepsy that originates from the hippocampus and then propagates to other limbic areas such as the amygdala and entorhinal cortex. The pathological feature associated with TLE is hippocampal sclerosis which is characterized by atrophy, induration, gliosis and loss of neurons in CA1, CA3 and the dentate hilar regions. Some animal models, albeit do not exactly match the complex etiologies identified in humans, are found to recapitulate most of the pathological features observed in TLE. There is evidence that administration of kainic acid can cause seizures in the CA3 region of the hippocampus that can lead to loss of neurons and astrogliosis characteristic of TLE, but the underlying mechanisms associated with the degeneration of neurons remain unclear. Since lysosomal enzymes, cathepsins B and D, can have important roles in the loss of neurons in a variety of experimental conditions, we evaluated their potential roles along with the insulin‐like growth factor‐II (IGF‐II) receptor, which is involved in the intracellular transport of these enzymes, in the kainic acid treated rats. Our results clearly showed that systemic administration of kainic acid evoked severe loss of neurons along with hypertrophy of astrocytes and microglia in the hippocampal region of the adult rat brain. The levels and expression of cathepsins B and D as well as IGF‐II receptor increased progressively with time in the hippocampus of kainic acid treated rats compared to control rats. The activity of both cathepsins B and D was also found to be enhanced in the hippocampus of treated rats. Our double labelling studies revealed that expression of both cathepsins were initially increased in the pyramidal neurons and then decreased with time. This was accompanied by the expression of immunoreactive IGF‐II receptors as well as cathepsins B and D in a subset of GFAP‐labelled activated astrocytes and Iba1‐labelled microglia in kainic acid treated rats. These results, taken together, suggest that enhanced levels/expression and activity of lysosomal enzymes may have a role in the loss of neurons observed in kainic acid treated rats.
T03‐21B. Gray matter oligodendroglia glia play an important role in human and rodent ALS pathogenesis
Y. Li, S. Kang, D. Bergles, J. Rothstein
Johns Hopkins University, Baltimore, United States
Question. Amyotrophic lateral sclerosis (ALS) is a fatal disease characterized by the loss of upper and lower motor neurons with unknown etiology. ALS is a non‐cell autonomous disease as deletion of mutant human SOD1 in astroglia and microglia delay disease progression in SOD1 ALS mouse models. However, whether oligodendroglia is involved in ALS pathogenesis was not clear. Our previous study showed that proliferating and reactive NG2+ glia (also known as oligodendrocyte progenitor cells) are present in the lumbar spinal cord ventral horn gray matter where motor neurons die in a SOD1 ALS mouse model. Very recently, we determined that oligodendroglia are actively degenerating during disease development leading to focal gray matter demyelination in rodent models. In addition, increased NG2 glia immunoreactivity and myelin loss were found specifically in the gray matter of patients' motor cortex and spinal cord ventral horn. Furthermore, oligodendroglial specific monocarboxylate transporter 1 expression is decreased not only in ALS mouse models but also in patients. Given that oligodendrocytes not only facilitate saltatory conduction of action potentials by myelinating axons, but also support neuronal functions metabolically through monocarboxylate transporter 1 (MCT1), the injury to oligodendroglia in ALS may have pathogenic consequences.
Methods. To further investigate whether oligodendrocytes play a role in ALS development, we selectively removed mutant human SOD1 (G37R) from postnatal NG2+ cells in PDGFαR‐CreER;loxSOD1(G37R) mice.
Results. The administration of 4‐hydorxytamoxifen (4HT) caused significant decrease in the expression of mutant human SOD1 transgene in NG2 glia as determined by quantitative PCR analysis. We found that excision of mutant hSOD1 from NG2 glia dramatically delayed disease onset and early disease, and significantly prolonged animal survival. In addition, at disease onset stage, activated astroglial and microglial responses were delayed in the animals received 4HT treatment. Moreover, the removal of mutant SOD1 helped to preserve MCT1 expression at disease onset.
Conclusions. These data indicate that expression of mutant SOD1 in NG2+ cells and their oligodendrocyte progeny has a deleterious effect on motor neuron survival, and suggest that a key negative consequence of mutant SOD1 expression in oligodendrocytes is to diminish their capacity to provide metabolic support to neurons.
This study was supported by NIH, The Robert Packard Center for ALS Research at Johns Hopkins and P2ALS.
T03‐22A. Deletion of TLR‐associated signaling adaptor TRIF significantly accelerates disease progression of ALS mice
O. Komine 1, N. Fujimori‐Tonou1, H. Yamashita2, Y. Moriwaki3, H. Misawa3, K. Yamanaka1,4
1RIKEN Brain Science Institute, Laboratory for Motor Neuron Disease, Wako‐shi, Saitama, Japan 2Kyoto University Graduate school of Medicine, Department of Neurology, Kyoto, Japan 3Keio University, Department of Pharmacology, Tokyo, Japan 4Nagoya University, Research Institute of Environmental Medicine, Nagoya, Japan
Microglial cells are central to innate immune responses against traumatic injury and bacterial infection in the central nervous system. Recent studies suggest that innate immunity might be also involved in the pathogenesis of neurodegenerative diseases, cerebral ischemia and brain injury. Although the recent works demonstrated that adaptive immune system is involved in the motor neuron disease process, the role of innate immune system in motor neuron disease was not fully investigated.
To assess the contribution of innate immunity in the pathogenesis of amyotrophic lateral sclerosis (ALS), the gene expression profile of lumbar spinal cord from symptomatic mutant SOD1 mice was obtained by microarray approach and subsequent pathway analysis indicated the involvement of innate immune pathway. Next, to test the role of innate immunity in the pathogenesis of ALS, SOD1G93A ALS model mice were mated with MyD88 and TRIF (TIR domain‐containing adaptor inducing IFNβ) deficient mice. MyD88 and TRIF are the essential adaptor proteins for Toll‐like receptor mediated signaling pathway. As compared with SOD1G93A mice, MyD88/TRIF double‐deficient and TRIF‐deficient SOD1G93A mice exhibited the substantially shorter survival times with accelerated disease progression. The disease duration was shortened by 50% in TRIF‐deficient SOD1G93A mice. In contrast, elimination of MyD88 in SOD1G93A mice showed marginal effect in survival time. In addition, the expression levels of pro‐inflammatory chemokines, CCL5 and CXCL‐10 were significantly suppressed in the spinal cord of TRIF‐deficient SOD1G93A mice, as compared with SOD1G93A mice. To determine the cell type in which TRIF‐dependent pathway contributes to the production of these chemokines, we examined the expression of chemokines in LPS‐stimulated primary microglia or astrocyte derived from TRIF‐deficient or MyD88‐deficient mice. TRIF‐dependent induction of these chemokines was observed only in LPS‐stimulated microglia. Moreover, we found that infiltration of T‐lymphocytes and other immune cells were significantly decreased in the spinal cord of symptomatic TRIF‐deficient SOD1G93A mice.
These results suggest that the basal level of TRIF‐dependent innate immune activation of microglia is beneficial to slow disease progression of ALS models through the maintenance of pro‐inflammatory chemokines and the infiltration of the immune cells to the spinal cords. The detailed analyses to clarify the role of these infiltrating immune cells are underway.
T03‐22B. Plaque associated‐GFAP+ astrocytes show increasing autophagic activity in PDAPP mice, model of Alzheimer's disease
F. Saravia 1, P. Pavia1,2, C. Pomilio1,2, A. Vinuesa1,2, R. Gorojod2, A. Alaimo2, M. Kotler2, V. Galvan3, J. Beauquis1,2
1Instituto de Biologia y Medicina Experimental, Buenos Aires, Argentina 2Facultad de Ciencias Exactas y Naturales UBA, Qca Biologica, Buenos Aires, Argentina 3University of Texas USA, San Antonio, United States
Alzheimer's disease (AD), the most common age‐dependent neurodegenerative disorder, causes a chronically progressive decline in cognitive functions. There is growing evidence that glial changes are early involved in this pathology. PDAPP mouse, a well‐ defined model of AD, accumulates toxic soluble and deposited Aβ, derived from proteolytic processing of the amyloid precursor protein (APP) and develops AD‐like synaptic deficits and cognitive impairment. On the other hand, autophagy has been associated to the neurodegenerative process, in particular with the clearance of aggregation‐prone proteins like Aβ. The amyloid plaques, mainly located in cortex and hippocampus, are closely surrounded by reactive and hypertrophic GFAP+ astrocytes. The aim of this work was to evaluate the potential astrocyte autophagic activity during the progression of AD in PDAPP mice from 5 to 20 months (m) of age. LC3II/I ratio was studied by western blot. Amyloid deposit load, stained with Congo Red, exhibited a continuing rise according to age in transgenic mice. The markers GFAP and LC3 were analyzed by immunofluorescence and confocal microscopy on hippocampal sections showing an increasing colocalization that reached the top at 14 m, where 52.5 ± 9.9% of plaque associated GFAP cells were LC3+. At 20 m, this proportion was lower. Conversely, the subpopulation of astrocytes located far from Aβ deposits were GFAP+/LC3‐, besides a decreased cell volume compared with control mice astrocytes. Additionally, the density of astroglial cells in the stratum radiatum diminished with aging (5 compared to 14 m, total GFAP+ cells, p < 0.05) along with higher plaque load, in a more prominently manner than in control mice. Our results clearly show that 1) astroglia is strongly affected during AD progression: two different morphologic subpopulations ‐close to or far from deposits‐ are distinguished in the hippocampus. Aging correlates with a glial density reduction; 2) a gradual increase of autophagic activity in plaque associated‐astrocytes is verified by the first time, suggesting a contribution to Aβ clearance until 14 m in PDAPP mice, with a significant decay after this age.
T03‐23A. Early activation of microglia has a central role in the disease pathogenesis of progressive myoclonus epilepsy, EPM1
I. Körber, T. Joensuu, A.‐E. Lehesjoki, O. Kopra
Folkhälsan Institute of Genetics and University of Helsinki, Research Programs Unit, Molecular Neurology and Neuroscience Center, Helsinki, Finland
Progressive myoclonus epilepsy of Unverricht‐Lundborg type (EPM1, OMIM 254800) is an autosomal recessively inherited neurodegenerative disorder. It is caused by loss‐of‐function mutations in the cystatin B gene (CSTB, OMIM 601145), encoding an inhibitor of lysosomal cysteine proteases. The precise role of CSTB and the mechanisms by which its loss leads to EPM1 remain poorly understood. The mouse model for EPM1, cystatin B‐deficient (Cstb‐/‐) mouse, recapitulates the key clinical features of EPM1 including myoclonic seizures and progressive ataxia. It also shows widespread neuron loss and gliosis in the brain. Interestingly, in Cstb‐/‐ mice pronounced microglial activation has been detected in selected brain areas already in presymptomatic mice, preceding astrocytosis and neuronal death.
In this study, we examine the microglial contribution to the neuronal dysfunction and death in EPM1. We aim to characterize the functional properties of Cstb‐/‐ microglia and their effects on the survival of Cstb‐/‐ neurons.
Our results show that the Cstb mRNA level in cultured wild type mouse microglia analyzed by real time quantitative PCR is high in relation to primary astrocytes or neurons. A gene‐expression profiling of microglia from Cstb‐/‐ mice and from wild type mice has been obtained by using the Affymetrix Mouse Exon 1.0 ST Array and reveals a down‐regulation of interferon‐regulated pathways in Cstb‐/‐ microglia. The stimulation of primary wild type mouse microglia with the endotoxin lipopolysaccharide (LPS) up‐regulates Cstb mRNA expression measured by real time quantitative PCR. Cstb‐/‐ microglia show an altered inflammatory response to the stimulation with LPS by increased secretion of chemokines demonstrated by cytokine array analyses. Moreover, the release of nitric oxide from Cstb‐/‐ microglia measured by Griess assay is elevated.
Our data indicate an altered response of primary Cstb‐/‐ microglia to inflammatory stimuli, which may contribute to neurodegeneration in EPM1. The data provide a basis for further detailed studies on the pathophysiology and therapy of this devastating disease.
T03‐23B. Spatial arrangement of NG2 cells and their activation in a mouse model of Alzheimer's disease
C. Schefft 1, J. Huck1, M. Füchtemeier2, J. Priller1
1Charité Berlin, Department of Neuropsychiatry, Berlin, Germany 2Charité Berlin, Department of Experimental Neurology, Berlin, Germany
Activation of astrocytes and microglia surrounding amyloid extracellular plaques in the brain is a hallmark of Alzheimer's disease (AD). Increasing evidence suggests that chronic neuroinflammation and elevated levels of several cytokines play important roles in AD development. Beside astro‐ and microgliosis, demyelination and oligodendrogenesis can be observed in human patients as well as in murine models of AD. Oligodendrocyte progenitor cells, also termed NG2 cells because they express the neuron/glial antigen 2 (NG2) proteoglycan, comprise about 5% of total cell number of the adult brain and are the main proliferating cells present. NG2 cells receive GABAergic and glutamatergic synaptic input and can secrete pro‐ and anti‐inflammatory substances upon activation by microglia‐derived cytokines. Overall, NG2 cells are highly reactive cells and one of the first cells which respond to changes in the CNS. However, their role during AD pathology is still uncompletely characterized. Using immunohistochemistry, we have analyzed their expression pattern in a transgenic murine model of AD (coexpressing a mutated form of the amyloid precursor protein (APP) and the presenilin1(PS1) gene). We focused on the spatial arrangement of NG2 cells in the hippocampus of transgenic mice at 3, 7 and 10 months of age and age‐matched controls (n = 4/time point). Our goal is to provide an anatomical and structural basis for elucidating the role of NG2 cells in AD.
The first results indicate an uniform distribution of NG2 cells in the hippocampus of APP/PS1 mice, with an accumulation at the sites of plaque deposits in close association with microglia. NG2 cells are normaly absent from the pyramidal and granular cell layers. However, when plaques deposits were found at these principal cell layers, NG2 cells could be seen surrounding them, suggesting an active migration. Changes in NG2 cell phenotype were noted from 3 months of age on. Like microglia, NG2 cells assume an activated morphology, as in a more “bushy” appearance. In numerous plaques, NG2 immunoreactivity was strongly increased, mostly in the intermingled processes that encircle the core of the plaques. Whether these activated NG2 cells proliferate and differentiate into oligodendrocytes or communicate with activated microglia in the plaque microenvironment remains to be investigated. Further, levels of the glutamatergic AMPA receptor expression will be evaluated. Ultimately we aim at understanding how NG2 cells and microglia interact in AD, which could be usefull in the development of novel therapies.
T03‐24A. Connexin hemichannels are activated in astrocytes of a murine model of Alzheimer's disease
A. Koulakoff 1, C. Yi1, X. Mei1,2, C. Giaume1,2
1Collège de France, Paris, France 2Institut de la Vision, Paris, France
Accumulating evidence supports an increasing role of astrocytes in the initiation or progression of a variety of neuropathological conditions. In Alzheimer's disease (AD), numerous data indicate that astrocyte properties are modified with potential deleterious effects on neurons. Accordingly, we have described changes in the expression of astroglial connexins, the gap junction channel and hemichannel forming proteins, in the vicinity of amyloid‐b (Ab) plaques in brains from AD patients and murine models of AD. Also Ab peptide was shown to trigger astroglial Cx43 hemichannel activation in culture and in acute slices leading to neuronal degeneration. Hence, we have investigated hemichannel function of astroglial connexins in 8–9 months old APPswe/PS1dE9 mice that exhibit Ab plaques in the cortex and hippocampus. Ethidium bromide (EtBr) uptake performed in acute brain hemisphere slices was used as an index of hemichannel activation and was quantified in astrocytes identified by GFAP immunostaining. In APPswe/PS1dE9 mice, compared to wild‐type mice of the same age, an increased uptake of EtBr was detected in the overall population of astrocytes. This uptake was blocked by carbenoxolone and lanthanum ions, indicating connexin hemichannel involvement. Such activation may be linked to the increase in resting astroglial [Ca2+]i described in this mouse model. Interestingly, EtBr uptake was higher in reactive astrocytes contacting Ab plaques than in non‐reactive astrocytes located far from (≥ 50 µm) these deposits. We are currently investigating their respective pharmacological profiles. Moreover, in APPswe/PS1dE9 mice knock‐out for Cx30 generated in our facility, EtBr uptake was comparable to that observed in APPswe/PS1dE9 mice, suggesting that Cx43 is the major contributor to the hemichannel activity observed. Altogether, these results indicate that neuroglial interactions could be affected by astroglial hemichannel activation and could account for neuronal alterations or death observed in neurodegenerative diseases.
This work was supported by the CRPCEN, FRM, LECMA and France Alzheimer.
T03‐24B. Levels of astrocyte‐derived proteins increase in the cerebrospinal fluid of Alzheimer's disease patients and inversely correlate to cognitive performance
C. Schipke 1, S. Handrick1, A. Klostermann2, B. Haas2, F.L. Heppner1, O. Peters2
1Charité Universitätsmedizin Berlin, Berlin, Germany 2Charité Universitätsmedizin Berlin, Psychiatry, CBF, Berlin, Germany
Question: The quantitative analysis of the biomarker proteins Abeta and Tau in the cerebrospinal fluid (CSF) substantiates the clinical diagnosis of Alzheimer's disease (AD). But, when the disease becomes manifest in mental disturbances, levels have become static and are unsuitable to predict disease progression. Since it is well established that amyloid plaques are associated with activated astrocytes we asked whether concentrations of the astrocyte‐derived proteins glial fibrillary acidic protein (GFAP) and S100B in human CSF might serve as additional biomarkers.
To functionally link the role of astrocytes to mechanisms involved in memory formation, we asked whether the amount of GFAP‐positive astrocytes correlates to the level of LTP in the hippocampus of aged AD transgenic mice.
Methods: We used ELISA kits for the quantitative analysis of GFAP (IBL‐International, Hamburg, Germany) and S100B (IBL). In a mixed disease cohort (n = 52 diseased patients, n = 16 controls), we correlated standard biomarkers (abeta and tau‐protein) and glia‐derived proteins. Furthermore we compared mean levels between groups of AD patients (n = 36) and healthy control patients (n = 35) and correlated levels of astroglial proteins in the CSF and cognitive performance (MMST values).
In transgenic (APP/PS1 transgenic mice), aged (10–15 months) mice (n = 10), we performed LTP experiments at the Schaffer collateral synapses in the CA1 region of the hippocampus. The number of GFAP‐positive astrocytes was quantified contralaterally.
Results: In the mixed cohort, we found a significant correlation of t‐tau and GFAP levels in CSF (r = 0.261, pbetween the S100B levels and the patients cognitive performance as measured by the MMST values (r = –0,42) as well as between the GFAP levels and the MMST values (r = –0,38). When correlating the number of astrocytes in the CA1 region to the level of LTP, 30 min after induction, we found a significant correlation of the number of astrocytes per mm3to the level of LTP (r = 0,648, p
Conclusions: The astrocyte‐derived proteins GFAP and S100B can be reliably detected in human CSF. Measurement of astroglial biomarkers might allow an improved pathobiological staging of AD, also since astrocytes appear to play a functional role in memory formation in the context of an AD‐like pathology in mice.
T03‐25A. GDNF induces secretion of Cyr61 from retinal Müller glial cells – a novel neuroprotective factor in retinal degeneration
J. Kucharska 1, P. del Rio1, B. Arango‐Gonzalez2, M. Gorza1, A. Feuchtinger3, M. Ueffing1,2, S. Hauck1
1Helmholtz Zentrum Munich, Research Unit Protein Science, Munich, Germany 2University of Tuebingen, Division of Experimental Ophthalmology, Tuebingen, Germany 3Helmholtz Zentrum Munich, Institute of Pathology, Munich, Germany
Retinitis pigmentosa is a group of inherited disorders affecting photoreceptors or retinal pigment epithelium (RPE) that leads to progressive loss of vision. The Pde6brd1 mouse model is a very well‐known animal model for retinal degeneration, in which rods carry a mutation in β subunit of rod cGMP‐phosphodiesterease. Glial cell line‐derived neurotrophic factor (GDNF) has already been shown to rescue morphology as well as function of rod cells in Pde6brd1 mouse. This effect was indirect, through stimulation of Müller glial cells (RMG). To better understand the neuroprotective effect of GDNF, primary retinal RMG were stimulated in vitro with GDNF and the secreted proteome was analyzed by proteome profiler arrays. Among others, Cyr61/CCN1, a member of CCN family, was found strongly induced upon RMG stimulation with GDNF. When applied directly to medium, Cyr61 significantly reduced photoreceptor death in organotypic ex vivo cultures of Pde6brd1 retinas. In order to identify the target cells of Cyr61 we treated, ARPE19 and MIO‐M1 cell lines with Cyr61, and observed an increase in phosphorylation of Akt and Erk1/2 signaling molecules. These results suggest that stimulation of RPE and RMG cells may have a protective influence on photoreceptor survival in organotypic ex vivo conditions. We postulate Cyr61 as a novel potential candidate for future therapeutic approaches in neurodegenerative retinal disorders.
T03‐25B. Data‐mining of genomic studies in psychiatric disorders for genes highly expressed in astrocytes
R. Schmidt‐Kastner 1, N. Pitts1, H. Steinbusch2, J. van Os2, B. Rutten2
1Florida Atlantic Univ. (FAU), Boca Raton, United States 2Maastricht Univ., School of Mental Health and Neuroscience, EURON, Maastricht, Netherlands
Question: Since astrocytes react to multiple physiological and pathological stimuli in the microenvironment of the brain, they could be mediators for gene x environment interactions in the pathogenesis of psychiatric disorders. Altered gene/protein expression and regressive changes have been reported for astrocytes in post‐mortem studies of psychiatric disorders, i.e. schizophrenia (SCZ), bipolar disorder (BP) and autism spectrum disorders (ASD). By contrast, no genetic link to astrocytes has emerged from the extensive genomic analysis of psychiatric disorders. Genetic findings mostly relate to neurons for disorders rooted in neurodevelopment (ASD, SCZ). It is timely to perform a formal analysis of genomic information emerging for psychiatric disorders for a contribution of genes expressed by astrocytes.
Methods: We generated a meta‐list of genes highly expressed in rodent astrocytes using published gene lists and literature mining. Datasets were prepared for candidate genes and genes from GWAS in attention deficit/hyperactivity disorder (ADHD), ASD, BP and SCZ, by using literature and databases (SzGene, SFARI Base, dbGaP). Datasets for copy number variations (CNVs) associated with neurodevelopmental delay were also assembled. Then the overlap between the meta‐list of genes highly expressed in astrocytes and gene datasets for psychiatric disease was determined. Overlapping genes were pooled and subjected to DAVID bioinformatics analysis.
Results: The meta‐list of genes highly expressed in astrocytes contained n = 2,680 genes (13.4% of the genome). Datasets for risk genes in psychiatric disorders showed random overlap with the meta‐list (ADHD 18%; ASD 13%; BP 12%; SCZ 13%). CNVs related to neurodevelopment were not enriched for astrocytic genes (8%). When the overlapping genes were pooled from all disorders, a small set of shared astrocytic genes emerged (n = 23). CNTNAP2, NRXN1 and SDC2 were linked by DAVID analysis (p = 0.013).
Conclusions: This correlative analysis makes it unlikely that genes highly expressed in astrocytes make a major contribution to the genetic risk in four major psychiatric disorders. No genetic link was found between astrocytes and neurodevelopmental delay. Changes in gene/protein expression and pathology in astrocytes in the adult brain in SCZ and BP may be explained by chronic neuronal dysfunction, chronic medication or acute changes.
T03‐26A. Glia impair neuron health in Juvenile Neuronal Ceroid Lipofuscinosis
J. Lange 1, L. Parviainen1, G. Anderson1, S. Dihanich2, P. Rezaie3, H. Mitchison2, B. Williams1, J. Cooper1
1King's College London, Institute of Psychiatry, London, United Kingdom 2University College London, London, United Kingdom 3Open University, Milton Keynes, United Kingdom
The neuronal ceroid lipofuscinoses (NCLs, Batten disease) are a group of autosomal recessively inherited lysosomal storage disorders affecting children and young adults, each of which is caused by a mutation in a different gene. A common feature across all NCLs is the early, localised glial activation that occurs long before the onset of neuron loss. This glial activation appears to be an accurate predictor of which neuron populations are vulnerable and will subsequently die.
Both astrocytes and microglia express the NCL gene products and it is likely that normal glial cell function may be compromised. Given the close functional relationship between neurons and glial cells it is possible that glial activation and/or dysfunction may affect neuronal health.
We have begun to explore the biology of astrocytes and microglia in the juvenile form of NCL using primary cultures from Cln3‐/‐ mice. Defects in both astrocytes and microglia, including altered protein secretion profiles and impaired morphological and proliferative responses were apparent. Co‐culturing with mutant microglia and astrocytes influenced the survival and morphology of wild type neurons, with more profound effects upon Cln3‐/‐ neurons. Intriguingly, these effects were largely reversed by substituting wild type glia, which rescue mutant neurons.
A pilot study has provided similar evidence for glial dysfunction in infantile NCL, including and altered protein secretion and response to stimulation. These changes appear to be different from those observed in juvenile NCL and we are in the process of exploring whether the glial cell phenotypes discovered so far, and their impact upon neurons are specific to the different forms of the disease.
T03‐26B. Inhibition of deubiquitination by PR‐619 leads to the formation of protein aggregates, to mitochondrial fragmentation and alters the GFAP network in astrocytes
V. Seiberlich 1, O. Goldbaum1, V. Zhukareva2, C. Richter‐Landsberg1
1Carl‐von‐Ossietzky University of Oldenburg, Oldenburg, Germany 2LifeSensors, Philadelphia, United States
The accumulation of aggregated proteins in nerve cells and glia underlie the pathogenesis of many neurodegenerative diseases. Glial pathology is characteristically observed in tauopathies, such as corticobasal degeneration and progressive supranuclear palsy, and Alexander Disease, a primary disease of astrocytes caused by mutations in the GFAP (glial fibrillary acidic protein) gene. Irreversibly damaged proteins can be degraded by the proteasomal system. For proteasomal degradation they are covalently linked to ubiquitin in a three‐step enzymatic pathway (E1, ubiquitin‐activating enzyme; E2, ubiquitin‐conjugating enzyme; and E3 ubiquitin ligases). The polyubiquitin chain needs to be removed before the translocation of the substrates to the lumen of the proteasome. This is achieved by deubiquitinating enzymes (DUBs), which oppose the functions of E3‐ligases and assist in targeting substrates to specific pathways. To assess whether impairment of DUBs may contribute to age‐related neurodegenerative diseases and the regulation of cell death and survival, we have subjected primary cultures of rat brain astrocytes to PR‐619, a DUB inhibitor with broad specificity. Immunoblot analysis demonstrates that after a 18h treatment, PR‐619 caused the induction of heat shock proteins, HSP70 and HSP32, and an increase in p62, which is considered a cargo receptor for ubiquitinated proteins and a common constituent of ubiquitinated protein inclusions. As analyzed by indirect immunofluorescence, protein aggregates positively stained by antibodies against ubiquitin and p62 assembled in the perinuclear region at the microtubule organizing center (MTOC). These aggregates were surrounded by a cage‐like network of GFAP intermediate filaments, thus resembling aggresomes. Using MitoTracker and LysoTracker staining procedures, the fluorescent images demonstrate that after DUB inhibition lysosomes and mitochondria are recruited to the MTOC. This process was dependent on an intact microtubule network, since it was interrupted after treatment with nocodazole, a microtubule destabilization drug. Furthermore, PR‐619 caused mitochondrial fragmentation which may be a stress response to enable the removal of damaged mitochondria by mitophagy. In conclusion, DUB inhibition impairs the cellular architecture, leads to protein aggregate formation and mitochondrial alterations in astrocytes. Our data sustain the hypothesis that an imbalance in DUB activities may contribute to the pathogenesis of neurodegenerative diseases.
T03‐27A. Axonal degeneration is limited in the optic nerve of EAE‐induced mice by AAV2 transduction of Retinal ganglion cells (RGCs) with a site‐specific phospho‐mutant CRMP‐2
J.Y. Lee 1, R. Kenny1, M.F. Azari1, P.M. Aui1, K. Magee1, A. Harvey2, S. Petratos1
1Monash University, Clayton, Australia 2The University of Western Australia, School of Anatomy, Physiology and Human Biology, Crawley, Australia
Question: Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system (CNS) characterised by demyelination and axonal degeneration. The molecular mechanisms that underpin axonal degeneration are relatively unexplored in MS. Studies using the mouse model of MS, experimental autoimmune encephalomyelitis (EAE), have shown that the collapsin response mediator protein 2 (CRMP‐2), which play a significant physiological role in neuronal cell bodies and axons within the CNS, is phosphorylated during the neurodegenerative phase of the inflammatory disease.
Methods: We investigated the limitation of axonal degeneration by transducing retinal ganglion neurons with a phosphorylation mutant of CRMP‐2 utilising an intraocular adeno‐associated virus 2 (AAV2) delivery system in EAE‐induced mice. The other group of mice injected with AAV2 consisting of the green flourescent protein reporter only (AAV2‐GFP) with EAE was used as a control (n = 8 per each group).
Results: We showed substantial preservation of axons of the optic nerve in the EAE‐induced mice injected with the AAV2 carrying the CRMP‐2 phospho‐mutant compared (∼10‐fold difference) with those mice injected with AAV2‐GFP. The AAV2‐GFP transduced axons showed significant degeneration during the peak stage of EAE.
Conclusion: Our data suggest that phosphorylation of CRMP‐2 may be a central mechanism that governs axonal degeneration during inflammatory demyelination of the CNS (as occurs in MS) and inhibition of phosphorylation of CRMP‐2 may be of therapeutic potential for the progressive phase of the disease in MS patients.
T03‐27B. Purinergic signalling‐regulated glial scar formation
Y. Shinozaki, S. Koizumi
Univ. of Yamanashi, Yamanashi, Japan
ATP, a major gliotransmitter integrating neuron‐glia networks, is known to be released or leaked from injured cells. In the CNS, ATP dramatically changes glial phenotypesand could affect pathogenesis of brain disorders. However, whether such glial responses facilitate or rather inhibit the diseases is still under debate. To address this issue,we studied the neuroprotective role of ATP/purinergic signaling using in vivo stab injury model on mouse cerebral cortex. Without injury or the contralateral side of the injured brain exhibited no fluoro‐jade(FJ)‐positive neuronal death, CD45+ infiltrating leukocytes or GFAP+ reactive astrocytes. Three days after injury, FJ signals, CD45+ cells and reactive astrocytes were increased in the ipsilateral side of the brain. The CD45+ cells were enclosed by reactive astrocytes‐formed glial scar. Administration of an ATP‐degrading enzyme apyrase, a broad P2 receptor antagonist PPADS, and a P2Y1 receptor antagonist MRS2179 significantly reduced the stab‐increased FJ signals. Immunohistochemical analysis revealed P2Y1 receptor was expressed in GFAP+ astrocytes. P2Y1 receptor knockout (P2Y1KO) mice also exhibited reduced FJ signals and infiltration of CD45+ cells. They exhibited higher level of GFAP expression, more remarkable hypertrophy of astrocytes and tighter glial scar formation than wild type mice did. The accelerated glial scar formation reduced CD45+ cell number and inhibition of astrocytes by fluorocitrate increased CD45+ cell number. Although glial scar formation was accelerated, no enhanced proliferation was observed. We then analyzed the mechanism of enhanced glial scar formation and neuroprotection using in vitro scratch‐wound model. In cultured astrocytes, the scratch induced a transient increase in extracellular ATP, which was followed by decrease in extracellular ATP mainly due to an increase in ectoATPase activity. Suppression of purinergic signaling by either apyrase, PPADS or MRS2179 enhanced scratch‐evoked astrocytic migration rather than proliferation. Neither activation nor inhibition of P2Y1 receptors in cultured cortical neurons affected the scratch‐induced damages. Our data suggest that the decrease in ATP/P2Y1 receptor‐mediated signal should be a trigger that transforms astrocytes into migratory phenotype, which forms tighter glial scar structure thereby suppressing neuronal death.
T03‐28A. Nrf2 activators: a novel strategy to promote oligodendrocyte survival in multiple sclerosis?
J. Lim, S. van der Pol, J. Drexhage, E. de Vries, J. van Horssen
VU University Medical Center, Amsterdam, Netherlands
Objectives: To investigate the potential of different Nrf2 activators to boost antioxidant enzyme expression in oligodendrocytes and protect them from reactive oxygen species (ROS)‐mediated cell death.
Background: Oligodendrocyte damage and loss are key features of multiple sclerosis (MS) pathology and oligodendrocytes appear to be particularly vulnerable to ROS. In vitro studies showed that ROS induce cell death and prevent the differentiation of oligodendrocyte precursor cells (OPCs) into mature myelin‐producing oligodendrocytes. Hence, a potential therapeutic strategy to protect these cells from ROS‐mediated damage is urgently needed. Here we investigated the efficacy of several compounds that are able to boost antioxidant enzyme production, including monomethyl fumarate (MMF), tert‐butylhydroquinone (tBHQ), sulforaphane (SFN) and Protandim. These compounds are thought to exert their protective function via activation of the nuclear‐factor‐E2‐related factor‐2 (Nrf2) transcriptional pathway, which is involved in the production of antioxidant enzymes necessary for oxidative stress defense.
Methods: Primary rat oligodendrocytes were treated with different concentrations of MMF, tBHQ, SFN and Protandim. Expression of antioxidant enzymes were analyzed by PCR and Western blot analyses. To study the beneficial effects of the different Nrf2 activators, oligodendrocytes were first incubated with Nrf2 activators and subsequently exposed to various concentrations of hydrogen peroxide. Oligodendrocyte cell survival was measured by a live/dead cell viability assay.
Results: SFN, MMF and Protandim are well‐tolerated and induce Nrf2‐driven antioxidant enzyme production in oligodendrocytes. Protandim was the most potent compound with regard to antioxidant enzyme induction and protected oligodendrocytes against ROS‐induced cytotoxicity.
Conclusions: Our findings indicate that several Nrf2 activators are able to induce antioxidant enzyme production in oligodendrocytes. Interestingly, Protandim, a dietary supplement consisting of herbal ingredients, was the most potent protector of primary rat oligodendrocytes. In future experiments we will determine whether Protandim can also promote the differentiation of OPCs under oxidative stress and test the clinical efficacy of Protandim in an experimental animal model for MS.
T03‐28B. Conditional ablation of Perk in Schwann cells improves myelination in S63del/CMT1B mice
M. Sidoli 1, N. Musner1,2, D. Zambroni2, S. Saccucci2, D. Ungaro3, U. Del Carro3, D. Ron4, D. Cavener5, M.L. Feltri1,2, M. D'Antonio2, L. Wrabetz1,2
1Hunter James Kelly Research Institute, University at Buffalo, Buffalo, United States 2San Raffaele Scientific Institute, Division of Genetics and Cell Biology, Milan, Italy 3San Raffaele Hospital, Neurology, Milan, Italy 4University of Cambridge, Cambridge, United Kingdom 5The Pennsylvania State University, State College, United States
Deletion of serine 63 (P0S63del) is a mutation in Myelin Protein Zero (MPZ, P0) that causes Charcot‐Marie‐Tooth 1B (CMT1B) demyelinating peripheral neuropathy, with a similar phenotype in both human and mouse. P0S63del is retained in the endoplasmic reticulum (ER) of Schwann cells (SC) where it elicits an unfolded protein response (UPR). PERK, ATF6 and IRE1 UPR transduction pathways are active in S63del nerves. In particular, PERK phosphorylates eIF2alpha, which attenuates global translation and activates CHOP, with a detrimental effect in S63del SC. Accordingly, PERK haploinsufficiency in S63del mice produces a motor improvement and complete ablation of PERK augments myelination in S63del DRG explant cultures. Nonetheless, the level of P‐eIF2alpha is reduced which should increase, not decrease, the amount of misfolded P0S63del in the ER of SCs. Moreover, the level of CHOP and the number of CHOP(+) nuclei is similar in S63del//Perk+/‐and S63del sciatic nerves. To identify the Schwann cell autonomous effect of PERK loss of function we generated S63del//P0Cre//Perkfl/fl mice. Myelin thickness and nerve conduction velocities were increased, and the number of amyelinated fibers reduced in S63del//P0Cre//Perkfl/fl compared to P0Cre//S63del//Perk+/+nerves. Moreover, chimeric co‐culture of PERK deficient DRG neurons with WT SC did not affect myelination. This data indicates that PERK ablation in S63del mice is paradoxically beneficial and this benefit derives from Schwann cells. We suggest that compensatory expression or activation of other UPR mediators, may contribute to the amelioration of S63del neuropathy.
T03‐29A. Glial pathology in the prefrontal cortex affects the cognitive function of the rat
A. Lima, M. Reis, A.F. Oliveira, V.M. Sardinha, C. Mota, L. Pinto, F. Marques, J. Cerqueira, N. Sousa, J. Oliveira
Life and Health Sciences Research Institute – ICVS, Braga, Portugal
The arising of the “tripartite synapse” concept and the discovery of the astrocytic excitability have been highlighting astrocytes as active elements concerning information flow in the brain. However, the impact of such remarkable features in complex brain function is still under‐explored mostly due to the difficulty of studying neuron‐astrocyte interactions in vivo.
The aim of this work was to use an in vivo model of astrocytic dysfunction and investigate the impact of this treatment in complex cognitive functions. The rationale consisted in studying behavioral performance that relies on the prefrontal cortex, such as behavioral flexibility and working memory in an animal model of astrocytic dysfunction. For that purpose, we used a pharmacological model in which Wistar‐Han rats were subjected to bilateral intracranial injections of aminoadipate in the prelimbic portion of the medial prefrontal cortex to cause astrocyte depletion specifically in this region, mimicking pathological states such as depression, in which marked decreases of GFAP‐positive cells are observed. This animal model was tested for its cognitive abilities by the attentional set‐shifting task (ASST) and water maze‐based tests.
A clear impairment of cognitive function was observed in the animals treated with aminoadipate. A detailed morphological and histological analysis showed that along with the astrocytic lesion, neurons were also affected at the site of lesion. This seems to contribute to the cognitive decline observed in this model and may explain similar observations under pathological states.
T03‐29B. Metabolic alteration in amyotrophic lateral sclerosis
A. Sternotte, E. Hermans
Université Catholique de Louvain, Brussels, Belgium
Amyotrophic lateral sclerosis (ALS) is an adult disease characterized by a selective loss of motor neurons, resulting in progressive paralysis and death within 3–5 years. In several inherited cases, the disease is caused by point mutations in the gene encoding for superoxide dismutase 1 (SOD1) which promotes its aggregation, leading to biochemical damages, including mitochondrial dysfunction. Alteration of mitochondrial membrane potential and/or respiratory chain leads to ATP depletion and these metabolic dysfunctions could contribute to motor neuron death in patients and animal models of ALS. In motor‐neurons, the combination of altered axonal transport with mitochondrial dysfunction likely leads to considerable decrease in ATP levels at distant neuromuscular junctions. Commonly know as the fuel gauge of mammalian cells, AMP‐activated protein kinase (AMPK) is a key enzyme in the control of cellular ATP and energy homeostasis.
In this study, the implication of AMPK in the progression of ALS was specifically investigated by measuring the expression and activity of this enzyme in the spinal cord of mice overexpressing the mutated human SOD1 (hSOD1G93A), a commonly used experimental model of ALS. No consistent modification of the enzyme was detected in spinal cord samples throughout the progression of ALS. Indeed, such experiment did not discriminate between cell types present in the tissue. Hence, a more detailed characterization of AMPK in cultured astrocytes derived from ALS animals revealed a robust induction of the enzyme activity, which correlates with decreased ATP levels in these cells.
In a second part of our study, we have investigated the consequences of ablating AMPK in the mouse model of ALS by breeding AMPK knock out mice with hSOD1G93A mice. While we did not detect any difference in the lifespan of AMPK(‐/‐)/hSOD1G93A mice as compared to hSOD1G93A mice, we observed substantial alterations in the progression of the disease in selected behavioural tests. In particular, analysis of the gait (catwalk) which enables early detection of muscle weakness revealed that the onset was delayed by up to 30 days while the progression of the disease after onset was accelerated.
Further studies will be conducted to establish the importance of ATP‐ in the disease, and to validate this enzyme as a putative pharmacological target in ALS and in other neurodegenerative diseases involving mitochondrial dysfunction.
AS is research fellow of the F.R.S.‐FNRS (National Fund for Scientific Research of Belgium).
T03‐30A. Time‐course study of NG2 glia activation in the MPTP mouse model of Parkinson's disease
Y. Liu, J. Zhou
Institute of Neuroscience, SIBS, CAS, Shanghai, China
NG2 glia is the fourth type of neuroglia in vertebrate nervous system, which also known as oligodendrocyte progenitor cell (OPC) in a developmental point of view. In adult brain, NG2 glia has a small cell body with highly branched extensions and represents a new type of glial cell differing from other glial cell types such as astrocyte, microglia and mature oligodendrocytes. Published studies by others have shown that NG2 glia is able to promptly respond to brain injury and activated in several neurodegenerative disease animal models including 6‐OHDA‐induced rat Parkinson's disease model. However, the pattern and function of these activated NG2 glia remain largely unknown. Here, we performed a time‐course study of NG2 glia activation in MPTP mouse model of Parkinson's disease using immunohistochemistry. It was found that NG2 glia was activated as early as 24 hours after the last MPTP injection in the substantia nigra (SN) of MPTP‐treated mice. This temporal character is very similar to those of microglia, indicating NG2 glia respond to MPTP challenges very quickly. The activation of NG2 glia became stronger over time and reached the peak at about 5 days after the final MPTP injection. Then, the extent of NG2 glia activation declined gradually and diminished 10 days after MPTP injection. Interestingly, we found that prominent NG2 glia activation could only be observed in the substantia nigra. The dorsolateral striatum which is the projection target of nigral dopaminergic neurons, however, was devoid of NG2 glia activation in all the time‐points examined. In summary, we characterized the NG2 glia activation in MPTP mouse model of Parkinson's disease. These data suggest that activated NG2 glia may play a role in the degenerative process of dopaminergic neurons in PD.
T03‐30B. CNS‐targeted methylprednisolone reduces pathology in mouse model of ALS
H.B. Stolp 1, M.C. Evans2, P. Gaillard3, M. de Boer3, C. Appeldoorn3, R. Dorland3, N.R. Sibson4, M.R. Turner2, D.C. Anthony2,5
1University of Oxford, Department of Physiology, Anatomy and Genetics, Oxford, United Kingdom 2University of Oxford, Nuffield Department of Clinical Neurosciences, Oxford, United Kingdom 3to‐BBB technologies BV, Leiden, Netherlands 4University of Oxford, Department of Oncology, Oxford, United Kingdom 5University of Oxford, Department of Pharmacology, Oxford, United Kingdom
Hallmarks of CNS inflammation, including microglial and astrocyte activation, are a feature of post‐mortem tissue from amyotrophic lateral sclerosis (ALS) patients and in transgenic mice overexpressing mutant superoxide dismutase‐1 (SOD1G93A). Administration of glucocorticoids does not significantly alter disease progression, but this may reflect poor CNS delivery. Here, we sought to discover whether CNS‐targeted liposomally‐packaged glucocorticoid would inhibit the CNS inflammatory response and reduce motor neuron loss. SOD1G93A mice were treated with saline, free methylprednisolone (MP, 4mg/kg/week) or glutathione PEGylated liposomal MP (2B3–201, 4mg/kg/week) and compared to saline treated wild‐type animals. Animals were treated weekly with intravenous injections for 9 weeks from 60 days of age. Animal weights and motor behaviour were monitored during this period. At the end of the experimental paradigm (116 days) mice were imaged using T2‐weighted MRI for brainstem pathology, and brain and spinal cord tissue was collected for histological analysis.
All SOD1G93A animals showed a significant decrease in motor performance compared to baseline from ∼100 days. SOD1G93A animals showed a significant increase in signal intensity on T 2 weighted MR images, which may reflect the combination of neuronal vacuolation and glial activation in these motor nuclei. Treatment with 2B3–201, but not free MP, significantly reduced T 2 hyperintensity, which correlated with significantly reduced histopathological manifestations in the brainstem motor nuclei, but not the spinal cord. Interestingly, there was a significant reduction in astrogliosis but not microglial activation following treatment with 2B3–201.
The results of this work indicated that the CNS‐targeted anti‐inflammatory agent 2B3–201 has therapeutic potential in ALS.
T03‐31A. Assessment of Cell Toxicity and Matrix Metalloproteinase‐9 expression by Antiretroviral Drugs in Cultured Primary Astrocytes
T. Latronico1, E. Raimondo1, F. Mengoni 2, M. Lichtner 3, C.M. Mastroianni 3, G.M. Liuzzi 1
1University of Bari, Bari, Italy 2Sapienza University, Rome, Italy 3Sapienza University, Polo Pontino, Rome, Italy
Background: The toxic effects of new antiretroviral drugs on the central nervous system (CNS) are unclear. Because these drugs penetrate the brain even at low concentrations, it becomes crucial to determine the doses which can be toxic for the CNS resident cells. Moreover, after the recent introduction into clinical practice, it is unclear whether the efficacy of the antiretroviral drugs of new generation may also derive from their ability to exert extravirological effects on factors responsible for the development of HIV brain injury, e.g. matrix metalloproteinases (MMPs).
Objective: To investigate on the toxicity of four different antiretroviral drugs and their ability to modulate the expression of gelatinase B (MMP‐9) in astrocyte cultures.
Methods: Primary cultures of rat astrocytes were activated by exposure to 10 mg/ml lipopolysaccaride (LPS) (positive control) and simultaneously treated for 20 h with increasing doses (1–5‐10–25. 50 mM) of: Efavirenz (EFV); Darunavir (DRV); Maraviroc (MVC) or Raltegravir (RAL). MMP‐9 mRNA expression was assessed by RT‐PCR. Quantitative determination of MMP‐9 expression was done by computerized scanning densitometry. Single drug toxicity was assessed by the MTT test. Each drug was considered toxic at the concentration able to induce a percentage of cell survival above 60%.
Results: The treatment with antiretroviral drugs inhibited MMP‐9 mRNA expression in LPS‐activated astrocytes in a dose‐dependent manner. In particular, a statistically significant inhibition of MMP‐9 expression was observed when astrocytes were treated with 25mM EFV (54% of inhibition) or with 50 mM RAL (43% of inhibition). As assessed by the MTT test, the toxicity of the antiretrovirals ranges from 10 and 50 mM. In particular, EFV was toxic for astrocytes at the concentration of 25 mM, MVC at 10 mM, while DRV and RAL were toxic at the concentration of 50mM.
Conclusions: The present results indicate that EFV and RAL directly inhibit MMP‐9 expression in LPS‐activated astrocytes with mechanisms that are independent from their antiviral activity. The toxic doses of antiretrovirals are much higher than those found in the CSF of HIV‐positive patients. Our results highlight some beneficial/deleterious extra‐viral effects of the antiretroviral drugs that may be useful to improve the development of new therapeutic strategies for the management of HIV infection.
Supported by a grant of Programma Nazionale AIDS, ISS,Italy(N. 40H8).
T03‐31B. Expression of glial potassium channel Kir4.1 and water channel aquaporin‐4 (AQP4) after status epilepticus in a mouse model of temporal lobe epilepsy
K. Szokol 1, A. Sommer2, K. Heuser3, E.A. Nagelhus1, E. Taubøll3
1University of Oslo, Institute of Basic Medical Sciences, Department of Physiology, Oslo, Norway 2University of Bonn, Bonn, Germany 3Oslo University Hospital, Rikshospitalet, Department of Neurology, Oslo, Norway
Acute glial changes after status epilepticus (SE) are not well characterized, though they may initiate an epileptogenic process that leads to neuronal cell death, reactive gliosis and neuronal hyperexcitability. Normal brain function is critically dependent on efficient mechanisms for clearance of excess K+ from the extracellular space. Recently, the astrocyte inwardly rectifying K+ channel Kir4.1 and the water channel AQP4 have been shown to regulate extracellular K+. Electrophysiological data suggest that Mesial Temporal Lobe Epilepsy with Hippocampal Sclerosis (MTLE‐HS) is associated with deficient K+ handling and have pointed to possible perturbations of K+ buffering and water transport.
We used a parahippocampal kainate injection mouse model to induce MTLE‐HS and to study early expression patterns of Kir4.1 and APQ4 after SE. Mice were sacrificed 24 h, 72 h and 7d after kainate injection. Immunhistochemistry of the hippocampus was performed to assess Kir4.1 and AQP4 expression, and GFAP staining was used for identification of astrocytes.
GFAP expression was increased compared to saline injected controls, suggesting that kainate injection induces astrogliosis. Comparison of Kir4.1 and AQP4 staining showed a similar change in the expression pattern. Our data indicate a drop in Kir4.1 and AQP4 expression within 24 hrs after SE followed by an upregulation within the first week. Altered expression of Kir4.1 and AQP4 could contribute to dysregulation of potassium and water homeostasis and play a role in the early phase of epileptogenesis.
T03‐32A. Molecular Mechanisms in Astrocytic Degradation
C. Lööv, A. Erlandsson
Uppsala University, Neuroscience/Neurosurgery, Uppsala, Sweden
We have previously shown that astrocytes effectively engulf dead cells after trauma both in vitro and in vivo, but that they store the ingested material rather than degrade it. Compared to macrophages, which degrade engulfed, dead cells within hours, our data show that astrocytes store the ingested material for weeks before the degradation is completed. To further study the routes of degradation and the possibilities to speed up this process we have used a cell culture model where UV‐treated, dead cells are added to stem cell‐derived astrocytes. By labeling the dead cells with the pH‐sensitive dye pHrodo prior to the engulfment, we demonstrate that the pH in the astrocytic phago‐lysosomes is higher than in professional phagocytes. Interestingly, Lamp1 and Lamp2, which are involved in the phago‐lysosome fusion, are both highly expressed in the astrocytes, particularly around the ingested material. Dendritic cells are known to express the inhibitory protein Rab27a that slow down the degradation in order to preserve antigen for presentation. Rab27a prolongs the actin coating around the phagosomes, which physically inhibit the phago‐lysosome fusion, and interacts with Nox‐2, which leads to an increased consumption of protons. Western blot analysis shows a high expression of Rab27a in our cell cultures which may explain the slow degradation. Moreover, dead cells in astrocytes are surrounded by actin rings for a long period of time after the ingestion. To counteract this, the astrocytes were treated with 0.1 or 1 µM of the actin inhibitor Latrunculin B. The number of actin rings were significantly lower in the cultures treated with 1 µM of Latrunculin B compared to controls, but the intensity of pHrodo was unaltered indicating that the prolonged degradation may be due to a higher pH in the lysosomes rather than a delayed actin coating. Next we will investigate the effect of Rab27a expression on the degradation process by performing siRNA experiments.
T03‐32B. Curcumin Increases Proliferation and Enhances Migration of Olfactory Ensheathing Cells
J. Tello Velasquez 1, J. St John1, R.J. Quinn1, J. Ekberg2
1Griffith University, Brisbane, Australia 2QUT, Brisbane, Australia
One of the promising strategies for the treatment of spinal cord injury is the transplantation of glial cells obtained from the olfactory system; olfactory ensheathing cells (OECs). Effective proliferation and migration of OECs are essential for optimizing clinical applications and there is a need for identification of small molecules that regulate glial cell biology. Curcumin is a natural polyphenol compound found in the spice turmeric, which is known for its neuro‐protective properties and has been reported to have an effect on neurogenesis and nerve regeneration. However, the effect of curcumin on OECs has not been determined. We have examined the effect of curcumin on OECs at the cellular level using fluorescence and timelapse microscopy. OECs were purified from S100B‐DsRed transgenic mice in which OECs express the fluorescent protein DsRed. Different treatments: (i) control medium, (ii) curcumin (0.5 to 20 µM), (iii) G5 commercial growth factor, or (iv) a combination of G5 and curcumin were used to determine the effect of curcumin on OECs proliferation and migration. Cell proliferation was quantified at two different times by cell counting and MTS proliferation assay. Timelapse microscopy was used to visualize changes in cell morphology and cell migration. Cell branching, number and area of lamellipodia and speed of migration per cell were measured for each treatment. We found that lower concentrations of curcumin (0.5 and 1 µM) increase OEC proliferation. As well, combination of G5 and curcumin showed the highest proliferative effect on OECs suggesting a possible synergistic relation between G5 and curcumin. Live cell imaging analysis showed that curcumin increased the number and area of lamellipodia resulting in faster migration of OECs. These results suggest that curcumin can regulate the proliferation, morphology and migration of OECs which could improve the therapeutic use of OECs for spinal cord injury repair.

T03‐33A. Effects of prenatal drinking on astrocytes parameters and behavior
P. Lunardi 1, G. Brolese1,2, N. B. Cunha1,2, F. L. Pedroso1, C.A. Gonçalves1,2, D. Engelke3, P. Nardin1, C. Batassini1, A.C. Tramontina1
1Universidade Federal do Rio Grande do Sul, Instituto de Ciências Básicas da Saúde, Programas de Pós‐Graduação em Bioquímica, Porto Alegre, Brazil 2Universidade Federal do Rio Grande do Sul, Instituto de Ciências Básicas da Saúde, Programas de Pós‐Graduação em Neurociências , Porto Alegre, Brazil 3Universidade Federal de São Paulo – UNIFESP, Programas de Pós‐Graduação em Neurociências, São Paulo, Brazil
Background: Although it is widely accepted that prenatal ethanol exposure (PNEE) to high doses has long‐lasting detrimental effects on brain development, the case for moderate exposure remains controversial. Recent evidence demonstrates that astrocytes are affected by PNEE, suggesting that alterations in these cells may participate in CNS disturbance associated with ethanol. The aim of this study was to investigate the effects of PNEE with moderate doses by evaluating offspring's astrocyte parameters in acute hippocampal slices. In addition, we checked anxiety‐like behavior and memory of the offspring on the thirtieth postnatal day.
Methods: The dams were divided in four groups and had received 50 mL of nonalcoholic or alcoholic beer solution – Control, Vehicle (nonalcoholic solution), EtOH 5% (nonalcoholic solution + 5%v.v ethanol) or EtOH 10% (nonalcoholic solution + 10%v.v ethanol) during gestational day 1 up to weaning (postnatal day 22). Male offspring (30 – 35 days old) were sacrificed and hippocampal acute slices were prepared. We analyzed GFAP content, S100B and glutamate uptake as astrocyte parameters. We tested the animals in the plus‐maze discriminative avoidance task to check anxiety and memory.
Results: GFAP content decreased after PNEE for EtOH 5% and EtOH 10% treatment (F3,59 = 17.75, p < 0.0001). Interestingly, we found that only the EtOH 10% treatment showed an increase of hippocampal S100B content (t = 2.38, p = 0.02) and in the CSF (F3,18 = 3.249, p = 0.0462), but no difference in secretion. Glutamate uptake was significantly decreased for EtOH 10% group (F3,20 = 4.069, p = 0.0208). To perform the plus‐maze discriminative avoidance task we selected pups from control, vehicle and EtOH 10% treatment on PND 30. Two‐way ANOVA revealed significant effects of treatment (F2,46 = 4.32, p = 0.019), arm type (F1,46 = 103.9, p < 0.0001) and treatment x arm type interaction (F2,46 = 6.66, p < 0.01). In the test session, only a significant prenatal treatment group effect (F2,46 = 6.44 p < 0.01). Bonferroni's post‐hoc test revealed that only control group presented significantly less time in the enclosed aversive arm than nonaversive one.
Conclusions: In this study we showed that PNEE with moderate doses could alter the structure and functional astrocytes balance. Thus, high levels of S100B are indicated in some neurodegenerative disorders. Taken this data together we could demonstrate that moderate ethanol exposure can be harmful to fetal brain.
T03‐33B. Litter size, age‐related memory impairments, and microglial morphological changes in the rat lateral septum analyzed by three dimensional reconstruction
L. Viana 1, C. M. Lima1, M. A. Oliveira1, I. N. F. Almeida1, D. G. Diniz1, J. Bento‐Torres1, A. Pereira1, M. Batista‐de‐Oliveira2, A. A. C. Lopes2, R. F. M. Silva2, R. Abadie‐Guedes2, A. A. Santos2, D. S. Lima2, P. F. C. Vasconcelos3, C. Cunningham4, R. C. A. Guedes2, C. C. W. P. Diniz1
1Universidade Federal do Pará – HUJBB, Lab of Investigations in Neurodegeneration and Infection, Belém – PA, Brazil 2Universidade Federal de Pernambuco, Departamento de Nutrição, Recife – PE, Brazil 3Instituto Evandro Chagas, Depto de Arbovirologia e Febres Hemorrágicas, Ananindeua – PA, Brazil 4Trinity College Institute of Neuroscience, School of Biochemistry and Immunology, Dublin, Ireland
Aging has been associated to neuroinflammation in the central nervous system, however it is not known whether microglial changes induced by aging are affected by early effects, such as litter size and sedentary life style. In addition, the lateral septum has been recognized by its complementary role in the memory processing for tasks of object recognition for identity and spatial location. In the present report, we investigated whether aging cognitive decline and microglial morphological changes in the lateral septal region are influenced by changing litter size early in life and by a sedentary life style. To assess these questions, Wistar rats suckled in litters of either six or 12 pups per dam were raised sedentarily in groups of 2 up to 3, from the 21st post‐natal day onwards. At 4 (young adult) or 23 (aged) months‐old, half of the sedentary rats underwent progressive daily treadmill exercise for five weeks, while the others remained sedentary. After performing the tests of recognition for spatial localization and object identity all of the animals were sacrificed and their brains were processed for selective microglia/macrophages immunolabeling with anti‐IBA‐1 antibodies. A representative sample of the immunolabeled cells in the lateral septum was analyzed after three‐dimensional reconstruction with Neurolucida software (Microbright Field Inc.) and morphological features of each cell were quantified by Neuroexplorer (Microbright Field Inc.). It was found that sedentary life style of Wistar rats maintained in standard laboratory cages is associated with spatial memory deficits in both mature and aged subjects no matter the litter size, and that exercise decreased these effects in aged subjects raised in small but not in large litters. On the other hand, all sedentary aged rats, despite of the litter size, presented impairment of object recognition memory for identity, and exercise decreased this effect in animals from both large and small litter size groups. Microglial morphological analysis revealed that cell soma area, perimeter and branches volume seem to be more intensely affected by aging and that these changes are mainly associated with animals raised in large litters. Furthermore, it was observed important shrinkage and thickening of the microglial branches in aged individuals on a higher proportion in the sedentary group suckled in large litters. Taken together the results suggest that litter size and sedentary life style may affect object recognition and spatial memories in both adult and aged rats and that exercise minimizes these effects on animals suckled in small but not larger litters. In addition, we suggest that these changes are related to distinct effects on the soma and branching patterns of septal microglia from young and aged subjects.
T03‐34A. Energy metabolism deficits in Huntington's disease: key role of astrocytes‐neurons interactions
L. Boussicault 1, A.‐S. Hérard1, F. Petit1, C. Malgorn1, N. Merienne1, M.C. Gaillard1, C. Escartin1, T. Delzescaux1, E. Brouillet1, P. Hantraye1, J. Mariani2, C. Vega Roiatti2, G. Bonvento1
1CEA, Fontenay‐aux‐Roses, France 2UMR CNRS 7102 UPMC, Paris, France
Huntington disease (HD) is a dominant inherited neurodegenerative disorder caused by an unstable expansion of a CAG repeat within the gene encoding for Huntingtin protein (Htt). This mutation induces formation of a poly‐glutamine tract in the mutant Htt (mHtt) protein that prones its aggregation into the cells. HD is characterized by an initial and massive degeneration of the medium spiny neurons in the striatum with later neuronal loss in cortex, globus pallidus, and other structures leading to motor and cognitive symptoms. Alterations of energy metabolism contribute to HD pathogenesis but the mechanisms responsible for these alterations are not well understood. A recent PET study provided evidence for a selective impairment of striatal glycolytic and not oxidative metabolism of pre‐symptomatic HD patients. In the brain, glycolysis is predominantly an astrocytic metabolic process, whereas oxidative metabolism is primarily neuronal raising the question that metabolic defects in HD could originates from astrocytes. The purpose of our study was to characterize (1) the metabolic alterations in vivo in a transgenic mouse model of HD (BACHD mice) and (2) the respective roles of astrocytes and neurons in metabolic defects occurring in HD using an in vitro strategy. BACHD mice express the human full length Htt protein with 97 glutamine repetitions under the human Htt promoter into a Bacterial Artificial Chromosome. BACHD mice present a slow disease progression with motor deficits occurring at 6 months and progressive neuronal aggregates from 12 months, reflecting human pathology. First, we performed [14C]‐2‐deoxyglucose autoradiography experiments in vivo to assess energy metabolism in 14 months BACHD (n = 6) and WT (n = 6) mice. We performed a 3D analysis of the whole brain glucose uptake without any regional a priori. Using Statistical Parametric Mapping (SPM), a voxel‐wise statistical analysis approach, we showed that compared to age‐matched WT mice, 14 months BACHD mice present hypometabolism in the striatum, like pre symptomatic HD patients, and also in the hippocampus. Hypermetabolism was also detected in the hypothalamus.
Second, we performed an in vitro study to dissect out the cellular mechanisms responsible for these metabolic changes. By using cell insert, we realized neurons/astrocytes co‐culture without any physical contact. This culture system allowed us to grow neurons in presence of WT or BACHD astrocytes. We showed that both BACHD and WT neurons have a decreased [14C]‐2‐deoxyglucose uptake when cultured in presence of BACHD astrocytes but not WT astrocytes. These results collectively suggest that energy defects in BACHD mice are due to non‐cell autonomous mechanisms by which astrocytes expressing mHtt induce neuronal metabolic dysfunction.
T03‐34B. The Effects of N‐acetyl‐aspartyl‐glutamate (NAAG) and N‐acetyl‐aspartate (NAA) on oligodendrocyte precursor cells
K. Volbracht, K. Evans, J.H. Stockley, R.T. Káradóttir
University of Cambridge, Cambridge, United Kingdom
NAAG and NAA are abundant metabolites in the brain which can be utilised by oligodendrocytes to make myelin. But elevated levels of NAAG and NAA are associated with myelin loss in the leukodystophies Canavan and Pelizaeus‐Merzbacher‐like disease, whereas lower levels of NAA are observed in brain tissue of MS patients. Currently, the physiological function of these two metabolites as well as their role in white matter pathology is unknown. NAAG and NAA can also act as signalling molecules as they can affect glutamate receptors. Oligodendrocyte precursor cells (OPCs) express NMDA receptors and activation of these receptors may be important for their differentiation and efficient myelination. In disease OPCs are recruited to demyelinating lesions where they proliferate and differentiate into new myelinating oligodendrocytes. We therefore investigated the effects of both physiological and pathological concentrations of NAA and NAAG on OPC biology in vitro.
We used calcium imaging to see whether NAAG or NAA can induce intracellular calcium changes in OPCs as they are known to act on neuronal NMDA receptors. 1mM NAA or 1mM NAAG evoked intracellular calcium changes in OPCs (DF[340/380] 44.6 ± 0.7 × 10−3 and 9.0 ± 3 × 10−3 respectively), but only one third of the cells responded which corresponds to the proportion of NMDA responsive cells in the cultures. However, not all OPCs that responded to NMDA did also respond to NAAG or NAA. Therefore, it seems that the NAA and NAAG evoked calcium raise is via an NMDA receptor independent pathway.
NAAG and NAA evoked a small but significant increase in intracellular calcium in OPCs, which even after prolonged application of pathological levels of NAA and NAAG did not affect the cells' viability as propidium iodide staining of OPCs showed no significant difference in viability. Over 90% of the OPCs survived 4hrs application of 1mM NAA and 1mM NAAG.
NAAG and NAA are produced and released by neurons but it is not known how OPCs take them up from the environment and how it affects them. We therefore addressed which possible transporters OPCs express and the effect NAA and NAAG have on OPCs' proliferation and differentiation.
It is unlikely that NAA and NAAG even at high concentrations have a detrimental effect on OPCs. Perhaps these high concentrations of NAA and NAAG found in Canavan and Pelizaeus‐Merzbacher‐like disease indicate rather OPCs' deficiency to transport and utilise NAA and NAAG for energy and myelin formation.
Supported by EU FP7 leukotreat, MRC and Wellcome Trust.
T03‐35B. New mouse models for Vanishing White Matter disease
M. Bugiani, L. Wisse, S. Dooves, N. Postma, V. Heine, T. Abbink, M. van der Knaap
VuMC, Amsterdam, Netherlands
Vanishing white matter (VWM) disease is a severe leukoencephalopathy, classically starting in childhood. The disease is characterized by neurological symptoms, including uncoordinated movements and balance disturbances which are slowly progressive. Episodes of rapid deterioration are triggered by febrile infections and minor head trauma, which can lead to coma. A treatment is unavailable and eventually all patients die within a few years upon diagnosis. The disease is caused by mutations in any of the five genes encoding the subunits of the eukaryotic translation initiation factor 2B (eIF2B). Although this protein has an important function in all cells, mainly the cells in the brain white matter (astrocytes and oligodendrocytes) are affected by these mutations. Pathologically the brain shows cystic degeneration with meager gliosis, dysmorphic astrocytes, lack of myelin and an increased number of oligodendrocyte and astrocyte progenitor cells. We have developed two mouse models for VWM to further unravel the disease mechanisms at molecular level and to develop and test possible treatments. The 2B4 mouse line is homozygous for a mutation in the Eif2b4 gene, encoding the eIF2Bδ subunit, and the 2B5 mouse line is homozygous for a mutation in the Eif2b5 gene, encoding the eIF2Bε subunit. Both mutations have been described in human patients and are associated with a severe form of VWM. Preliminary data show that these mice models recapitulate the clinical and neuropathological phenotype observed in VWM patients. Pathologically, the central nervous system white matter is vacuolated and shows lack of myelin while astrocytes are dysmorphic and immature. These transgenic mice appear to be a representative model for VWM.
T03‐36A. Expression and function of the late‐onset Alzheimer disease associated CD33 in human microglia
M.A. Mathews, M. Grobe‐Einsler, K. Roy, H. Neumann
University of Bonn, Bonn, Germany
Microglia are described to be involved in many neurodegenerative diseases like Alzheimer's disease (AD). CD33 (Siglec‐3) is a rapidly‐evolving, highly species specific sialic‐acid binding Ig‐like lectin expressed primarily on cells of myeloid lineage. Recent studies have shown an association between a CD33 polymorphism and late‐onset AD. The role of CD33 in human microglia was studied in induced pluripotent stem (iPS) cell‐derived microglial precursor line (iPSdM).
Microglial cell lines were differentiated from iPS cells and represent in vitro models for human microglia. RT‐PCR and flow cytometry analysis of iPSdM showed gene transcription and protein expression of CD33 respectively. The protein expression was increased by breaking the cis‐interacting sialic acids on the microglial glycocalyx using sialidases. CD33‐Fc fusion protein was designed and purified via the Fc tag to study binding patterns of CD33 to various cell types expressing different glycocalyx structures. Additionally, iPSdM exhibiting either an over expression or knockdown of CD33 will be used to determine protein functions. Furthermore, preliminary immunohistochemical analysis of control brain tissue compared to AD patients showed increased CD33 protein expression in CD68 positive cells, in the latter.
In summary, data show that CD33 is expressed by human microglial cell line enabling further characteristic and functional analysis of the protein. Furthermore, preliminary analysis on primary brain tissue corroborates the association of CD33 – expressed on microglia – to late‐onset AD.
T03‐36B. A BDNF mimetic promotes peripheral myelin development and ameliorates experimental autoimmune neuritis (EAN)
S. Murray1, R. Hughes1, G. Tran 2, S. Hodgkinson 2, A. Wong1, H. Peckham1, A. Ferner1, L. Giuffrida1, T.J. Kilpatrick1, J. Xiao 1
1The University of Melbourne, Melbourne, Australia 2University of New South Wales, Sydney, Australia
The neurotrophins are essential for peripheral nervous system development and myelination. We have previously demonstrated that the neurotrophin BDNF exerts contrasting influences upon myelination – acting through neuronal p75NTR to enhance myelination, but inhibiting it via neuronal TrkB. Recently we have generated a small peptide called cyclo‐dPAKKR that structurally mimics the region of BDNF that binds p75NTR. Here we aim to investigate whether utilising cyclo‐dPAKKR to selectively target p75NTR is an approach that could exert a unified promyelinating response. Like BDNF, cyclo‐dPAKKR promoted myelination of NGF‐dependent neurons in vitro, an effect dependent on the neuronal expression of p75NTR. Importantly, cyclo‐dPAKKR significantly enhanced the myelination of BDNF‐dependent neurons in vitro, whereas BDNF inhibited it. Local injection of cyclo‐dPAKKR adjacent to the neonatal sciatic nerve in vivo significantly enhanced myelin protein expression and increased the number of myelinated axons. We found that injection of cyclo‐dPAKKR also significantly upregulated the expression of Neuregulin 1 type‐III, a key factor required to induce peripheral myelination. Thus, our data demonstrate that cyclo‐dPAKKR promotes peripheral myelination in vitro and in vivo. To investigate whether cyclo‐dPAKKR promoted the remyelination of peripheral neurons, we utilized the EAN, a rodent model of peripheral demyelinating neuropathy. Our data show that administration of cyclo‐dPAKKR significantly delayed the disease onset and reduced clinical severity in EAN. This improved disease phenotype is supported by reduced demyelination, as cyclo‐dPAKKR substantially reduced the extent of myelin damage in myelinated peripheral nerves subjected to EAN. Collectively, our data demonstrate that using cyclo‐dPAKKR to selectively target p75NTR promotes peripheral myelin development, and is effective at ameliorating EAN disease and protecting against myelin damage. Our findings suggest that selective targeting of p75NTR is a strategy worthy of further investigation for the treatment of peripheral demyelinating diseases
T03‐37A. Oligodendrocytes, small heat shock protein HSPB5 (alpha‐B‐crystallin) and Huntington's disease: What is the link?
N. Matinyarare 1, U. Püntener1, J. Teeling1, J. Morton2, A. Wyttenbach1, V.H. Perry1, V. O'Connor1, S. Quraishe1
1University of Southampton, Southampton, United Kingdom 2University of Cambridge, Department of Pharmacology, Cambridge, United Kingdom
Introduction Huntington's disease (HD) is a neurodegenerative disease characterised by huntingtin protein misfolding and the intra‐cellular accumulation of mutant huntingtin (mHtt). Accumulation of mHtt is most pronounced in neurons and is thought to underpin neuronal dysfunction. Oligodendrocytes have been shown to accumulate mHtt aggregates, which may contribute to cellular dysfunction in these cells. Evidence from diffusion tensor MRI (DT‐MRI) studies shows that white‐matter changes are amongst the earliest pre‐symptomatic changes observed in HD. These findings have been taken to implicate axonal and or glial aberrations prior to disease onset.
Methods We have used a variety of techniques, which include, emulsion in situ hybridisation, whole brain sub‐fractionation, immunohistochemistry, meso scale cytokine assay, and western blots.
Results Our investigations using the R6/2 mouse model show that mHtt aggregates result in a specific down‐regulation of the small heat shock protein (sHSP) HSPB5 (alpha‐B‐crystallin). Localisation of HSPB5 by whole brain sub‐fractionation shows that HSPB5 is enriched in the myelin fraction. Emulsion in situ hybridisation further shows localises HSPB5 in oligodendrocytes. Detergent extraction of myelin fractions show differential HSPB5 partitioning between soluble and insoluble fractions. Cumulatively, these findings suggest that HSPB5 is constitutively expressed in oligodendrocytes and is present in two distinct pools. Recent studies implicate HSPB5 as being a negative down‐regulator of inflammation. As such down‐regulation of the sHSP in R6/2 mice may promote increased oligodendrocyte vulnerability to mHtt cytotoxicity. To investigate the role of HSPB5 as a negative regulator of inflammation, we obtained transgenic HSPB5 knockout mice. The knockout mice are viable and show very little phenotypic difference against littermate controls. We have analysed the inflammatory profiles of these mice using the meso scale. To validate our findings in the R6/2 mice, we are currently investigating human tissue from distinct Vonsattel stages of disease, to see if HSPB5 downregulation is also observed in the human disease.
Conclusion As HSPB5 has several critical cellular roles, its down‐regulation may compromise oligodendrocyte structure and function, which ultimately has negative consequences for neurons. It is therefore worthwhile to investigate it's specific role in the CNS.
T03‐37B. Alterations of juxtaparanodal domains in two rodent models of CNS demyelination
L. Zoupi 1, M. Savvaki1, K. Markoullis2, K. Kleopa2, D. Karagogeos1
1I.M.B.B. ‐FoRTH, Heraklion, Greece 2The Cyprus Institute of Neurology and Genetics, Neuroscience Laboratory & Neurology Clinics, Nicosia, Cyprus
Multiple Sclerosis (MS), a progressive autoimmune disease of the CNS, affects a large number of young adults worldwide. During the course of the disease, the myelin sheath – the axonal insulator – is destroyed, while the axon progressively degenerates. Numerous studies have indicated both myelin and axonal signals that contribute to axonal demyelination in MS, but the exact pathophysiology of MS remains unknown.
The myelin sheath is attached to the axon through multiprotein complexes that form the axon‐glial interactions. They consist of cell adhesion molecules and voltage gated ion channels and divide the axon into distinct domains: the node of Ranvier, the paranode, the juxtaparanode and the internode. This molecular organization is crucial since recent studies identified distinct perinodal proteins as autoantigens in MS patients: nodal and paranodal Neurofascin isoforms and juxtaparanodal TAG‐1/Contactin‐2. TAG‐1 is a cell adhesion molecule expressed both by axons and glial cells. In the adult nervous system, TAG‐1 organizes the juxtaparanodal domain of the fiber, where it interacts with Caspr2 and the potassium channels (VGKCs).
Question: In this study we have analyzed the alterations of the juxtaparanodal proteins Caspr2, TAG‐1 and VGKCs in relation to paranodal Caspr and nodal sodium channels upon the onset and progression of Experimental Autoimmune Encephalomyelitis (EAE) and in the Cuprizone model of toxic demyelination.
Methods: We immunohistochemically and biochemically analyzed both models of CNS demyelination.
Results‐Conclusions: Our results show a higher paranodal susceptibility to disruption compared to juxtaparanodes in EAE. As EAE progresses there is subsequent compensatory efforts for myelinated fiber restoration that include paranodal protein re‐clustering and increased heminodal formation, without subsequent juxtaparanodal protein aggregation. In contrast, juxtaparanodal organization was observed only when remyelination occured in the cuprizone model of toxic demyelination.
Supported by: IMBB‐FoRTH Scholarship, Manassaki Scholarship (University of Crete), The project “MyelinTag” (593) is implemented under the “ARISTEIA” Action of the “OPERATIONAL PROGRAMME EDUCATION AND LIFELONG LEARNING” and is co‐funded by the European Social Fund (ESF) and National Resources.
T03‐38A. In vivo therapeutic effects and oligodendrocyte protection from excitotoxicity by the magl inhibitor JZL184
S. Mato 1, A. Bernal‐Chico1,2, M. Canedo1,2, M.V. Sánchez‐Gómez1,2,3, A. Pérez‐Samartín1,2,3, R. Rodríguez‐Puertas4, C. Matute1,2,3
1University of the Basque Country‐UPV/EHU, Department of Neurosciences, Leioa, Spain 2Achucarro Basque Center for Neuroscience‐UPV/EHU, Zamudio, Spain 3Instituto de Salud Carlos III (ISCIII; CIBERNED), Leioa, Spain 4University of the Basque Country‐UPV/EHU, Department of Pharmacology, Leioa, Spain
Multiple sclerosis (MS) is a chronic disease of the human central nervous system that is characterized by focal lesions with inflammation, infiltration of immune cells, demyelination and axonal damage. Activation of cannabinoid CB1/CB2 receptors is considered a potential therapeutic strategy for the treatment of MS, based on the evidence that exogenous cannabinoid agonists exert neuroprotective and immunosuppressive effects in experimental models of the disease. Nevertheless, the therapeutic use of synthetic and/or plant‐derived agonists acting on brain cannabinoid receptors is limited by the possible adverse responses related to memory and learning impairment. An alternative approach that could avoid this limitation consists of enhancing the concentration of the main endocannabinoids (AEA, 2‐AG) by increasing their synthesis or decreasing their degradation. The main objective of this study was to analyze the effects of JZL184, a selective inhibitor of 2‐AG hydrolyzing enzyme monoacylglycerol lipase (MAGL), in the chronic EAE model of MS. Mice were treated daily with JZL184 (8 mg/kg and 32 mg/kg) or vehicle from onset of the motor symptoms to the end of the experiment. Comparison of the motor score curves indicated that both doses of JZL184 ameliorated the deficits observed in vehicle‐treated mice during the disease course. Nevertheless, the beneficial effect of the 32 mg/kg dose was no longer evident in mice scored at 40 dpi. Chronic treatment with 8 mg/kg JZL184 reduced the conduction latency of the corticospinal tract measured at the end of the experiment, as well as the number and size of inflammatory lesions in spinal cord, whereas chronic administration of the high dose of the MAGL inhibitor had no effect on these parameters. Importantly, treatment with the 32 mg/kg dose of JZL184 reduced the coupling ability of cannabinoid receptors to Gi/o proteins, measured by [35S]GTPγS autoradiography, in all the brain areas analyzed. Finally, incubation with JZL184 prevented cytotoxicity by activation of AMPA‐type glutamate receptors in oligodendrocyte precursors in vitro. This protective effect of the JZL184 was blocked by the CB1 receptor antagonist AM281. Our findings point to the involvement of CB1 receptors in the in vivo therapeutic effects of JZL184, and suggest that chronic administration of MAGL inhibitors may be a promising strategy for the treatment demyelinating disorders in which oligodendrocyte excitotoxicity plays an important role as mechanism of white matter damage.
Acknowledgements: Founded by MICINN (SAF2010‐21547), CIBERNED and Gobierno Vasco. Susana Mato, Ana Bernal and Manuel Canedo are recipients of a Ramón y Cajal contract and fellowships from the University of the Basque Country‐UPV/EHU and FPI, respectively.
T03‐38B. Mitochondrial permeability transition pores but not ryanodyne‐sensitive endoplasmic reticulum calcium channels are involved in photodynamic injury of sensory neurons and satellite glial cells in the crayfish stretch receptor
A. Uzdensky, A. Khaitin, I. Ischenko
Southern Federal University, Rostov‐on‐Don, Russian Federation
Photodynamic therapy (PDT) effect based on intense photogeneration of highly cytotoxic singlet oxygen, following oxidative stress and death in stained cells under light exposure in the presence of oxygen is used in neurooncology and various neurophysiological applications. In the crayfish stretch receptor, a simple object consisting of a single mechanosensory neuron surrounded by glial cells, PDT abolished neuron firing, induced necrosis of the sensory neuron and glial cells, and glial apoptosis. Cyclosporine A (5 µM), the inhibitor of mitochondrial permeability transition pores, prolonged firing of photosensitized neurons and reduced the levels of photo‐induced necrosis and apoptosis of glial cells. These neuro‐ and glia‐protective effects of cyclosporine A suggest involvement of Ca2+ ions that could be presumably released into cytosol from photo‐injured mitochondria through their permeability transition pores. Application of ryanodine (10 µM), the activator of Ca2+ channels in the endoplasmic reticulum, another possible Ca2+ source, showed no significant changes in the neuron activity, necrosis of neurons and glia and glial apoptosis. Therefore, photo‐injury of mitochondria but not endoplasmic reticulum in crayfish neurons and glial cells led to PDT‐induced loss of neuronal activity, necrosis and apoptosis of glial cells. Supported by Minobrnauki and RFBR grants.
T03‐39A. The role of Connexin43 gap junctions and hemichannels in the pathophysiology of Amyotrophic Lateral Sclerosis (ALS)
A. Almad, N. Maragakis
Johns Hopkins University, Baltimore, United States
Amyotrophic Lateral Sclerosis (ALS) is a progressive neurodegenerative disorder that results in the loss of motor neurons (MN) leading to paralysis and eventual death of patients. Genetic studies using animal models and in vitro studies show that in addition to neurons, astrocytes are key contributors to the disease progression. Astrocytes form a highly coupled intercellular network in the central nervous system through gap junctions (GJs). GJs are composed of connexin subunits with connexin 43 (Cx43) being the predominant astrocyte connexin, which conducts crucial homeostatic functions of regulating ions and metabolites. However, under pathological conditions GJs can perform aberrant functions such as excessive propagation of excitatory molecules such as Ca2+, glutamate and other cell death signals along the astrocyte networks. Under disease conditions, connexins can also release these signaling molecules into the extracellular milieu through hemichannels. Since astrocytes are involved in the disease progression of ALS, we are exploring if abnormal GJ activity can serve as a potential mechanism for the reported astrocyte‐mediated toxicity.
We used the SOD1G93A mutation as a ALS mouse model to address the role of connexins in ALS. We observed that at the end stage of the disease, spinal cords of SOD1G93A mice exhibited a significant increase in expression of connexin 43 protein compared to their spinal cords of control mice. This increase in Cx43 co‐localizes primarily to astrocytes in the gray matter of the spinal cord. Importantly, human post‐mortem tissue from ALS patients compared to control patients exhibited a significant increase in Cx43 RNA and protein levels in the motor cortex and a trend for increase in Cx43 in the cervical spinal cord. This is an important finding in pursuing Cx43 as a potential candidate contributing to astrocyte‐mediated toxicity in ALS. In addition, when astrocytes were isolated from WT and SOD1G93A mice, endogenous levels of Cx43 RNA and protein were elevated in SOD1G93A mice compared to the WT derived astrocytes. We are currently investigating if functional properties of astrocytes from SOD1G93A mice are altered in addition to biochemical changes. In vitro studies using Cx43 specific blockers to assess neuroprotection are also being conducted in co‐cultures of neurons and astrocytes. These findings will be novel and an important step towards harnessing therapeutic strategies for ALS.
T03‐39B. The mitochondrial serine protease OMI/HtrA2 binds to the NG2 protein expressed by oligodendrocyte progenitor cells: a role for NG2 in homoestasis and stress protection?
F. Maus 1, D. Sakry1, F. Binamé1, E.‐M. Krämer‐Albers1, K. Karram1, R. Krüger2, K. Rajalingam3, J. Stegmüller4, H. Werner4, K.‐A. Nave4, J. Trotter1
1Johannes Gutenberg University of Mainz, Department of Biology, Molecular Cell Biology, Mainz, Germany 2University of Tübingen, Hertie Institute for Clinical Brain Research, Department of Neurodegenerative Diseases, Tübingen, Germany 3Goethe University Frankfurt School of Medicine, Institute of Biochemistry II, Frankfurt on the Main, Germany 4Max Planck Institute of Experimental Medicine, Göttingen, Germany
The PDZ protein OMI/HtrA2 is localized in the intermembrane space of mitochondria. It is involved in mitochondrial homeostasis and may act as a chaperone. Mutations in the serine protease domain of OMI/HtrA2 (mnd2 mice), leads to massive loss of striatal neurons and neuromuscular disorders in mice confirming the protective function within mitochondria. Upon cellular stress, OMI/HtrA2 is translocated from mitochondria into the cytosol when the mitochondrial outer membrane is permeabilised. In the cytosol, OMI/HtrA2 blocks the action of the inhibitors of apoptosis proteins (IAPs) by competitive binding or by degradation via the protease activity. This leads to caspase activation and apoptosis induction. OMI/HtrA2 also regulates apoptosis in a caspase‐independent manner via its protease activity; recent studies have defined multiple substrates of the serine protease. In a yeast two‐hybrid screen we identified the NG2 proteoglycan as a binding partner of OMI/HtrA2. NG2 is expressed by oligodendrocyte progenitor cells (OPC). In the presence of hydrogen peroxide, which is a model of cellular oxidative stress, OMI/HtrA2 is released from mitochondria and can bind to NG2 in OPCs. Binding of NG2 to OMI/HtrA2 via the PDZ domain may thus be a way to sequester or regulate the serine protease. Oligodendrocyte‐lineage cells are known to be particularly sensitive to cellular stress and white matter diseases of new borns is a major pathology in situations where premature infants are exposed to unphysiological oxygen concentrations. It has been recently shown that glioma cells often express the NG2 Protein which may play a protective role. Our results indicate that the interaction between OMI and NG2 may help protect OPCs from cellular stress.
T03‐40A. The complex interplay between astrocytes and microglia during the development of Alzheimer's‐like symptomatology in a mouse model
D. Bouvier 1,2, E.V. Jones, R. Quirion1, K.K. Murai2
McGill University, Montreal General Hospital, Douglas Mental Health University Institute, Montréal, Canada 2 McGill University, Montreal General Hospital, Centre for Research in Neuroscience, Montréal, Canada
Microglia are the immunocompetent cells of the CNS that continuously survey the extracellular milieu for foreign antigens and play an instrumental role in brain inflammation. In contrast, astrocytes extend highly ramified processes between neurons and synapses and play a vital role in regulating brain homeostasis, synaptic function and plasticity. Interestingly, both microglia and astrocytes adopt a specialized ‘activated' or ‘reactive' state when exposed to adverse brain conditions. The changes in their molecular profiles include the release a diversity of proteins such as pro‐ and anti‐inflammatory cytokines and extracellular matrix molecules that could impact each other's behavior and regulate the properties of nearby neurons. In Alzheimer's disease (AD), activated microglia and reactive astrocytes are detected around plaques in mouse models of AD and human AD tissues. However, exactly how these glial types contribute to the onset or progression of AD still remains an open question. To gain a better understanding of the bidirectional communication and response of microglia and astrocytes in the development of AD‐like symptomatology, we investigated the properties of these cells types in the CRND8 transgenic AD mouse model by applying confocal imaging and western blot analysis. CRND8 is an early‐onset model of AD‐like symptomatology showing Aβ deposits present at 2 months of age and neuritic pathology by 5 months. Our results show complex spatio‐temporal changes in the interaction of microglia and astrocytes around Aβ plaques during the progression of the disease in both cortex and hippocampus. We found that subtle rearrangements of microglial morphology and astrocyte reactivity were detected as early as 1 month when Aβ plaque deposition is absent. By 2–3 months, low numbers of activated microglia and reactive astrocytes begin to surround small Aβ deposits. The establishment of complex ‘reactive glial domains (RGDs)' can be observed at 4 months, when amoeboid microglia fully encompass large Aβ plaques and become surrounded by reactive astrocytes forming an elaborate outer shell‐like structure. Intriguingly, after 6 months, the domains become progressively disrupted due to the reorganization and recruitment of additional microglia and astrocytes. We also observed gradated inflammatory phenotypes of glial cells. These are first restricted to RGDs in the early and middle stages of AD but spread locally in late stages when disorganized RGDs are observed. Interestingly, neuronal dystrophy was correlated with the severity of the inflammation. We conclude that communication between microglia and astrocytes may be a key process in the ability of the brain to surmount a reparative response to early neurodegenerative conditions but may be disrupted or become overwhelmed in later stages of AD. Supported by Alzheimer Society of Canada (DB) and by CIHR (KKM and RQ).
POSTER TOPIC 04 Extracellular matrix and cell adhesion molecules
T04‐01A. Roles of anosmin‐1 and FGF‐2 in the biology of adult oligodendrocyte precursor cells
A. Bribian 1,2, E.M. Medina‐Rodriguez1, F.J. Arenzana1, V. Murcia‐Belmonte1, P.F. Esteban1, F. de Castro1
1Hospital Nacional de Parapléjicos, Toledo, Spain 2Insitut de Bioenginyeria de Catalunya, Barcelona, Spain
During development, oligodendrocyte precursor cells (OPCs) migrate until their final destination and then differentiate to myelin‐forming oligodendrocytes. OPCs exist in mature rodent and human central nervous system (CNS; around 5–7% of the total cells) and constitute an interesting source regenerative therapies in demyelinating diseases, like multiple sclerosis (MS). Different molecules are involved in OPC morphofunctional development during embryogenesis and postnatal stages, and some of them are upregulated in MS lesions, suggesting their involvement in the pathogenesis of the disease.
This is de case of anosmin‐1, an extracellular matrix glycoprotein coded by the KAL1 gene and responsible for the X‐linked form of Kallmann syndrome. The best known mechanism of action of anosmin‐1 seems to be mediated through the interaction of this protein with fibroblast growth factor receptor 1 (FGFR1) and the modulation of the activation of this receptor by FGF2. This protein participates in the adhesion, migration and differentiation of various cell types in the CNS; among others, anosmin‐1 promotes the adhesion of neurons, neurite outgrowth, axonal guidance and branching of different CNS projection neurons, as well as having a role in the migration of different types of neuronal precursors, immortalized GnRH‐producing neurons and embryonic OPCs. In addition, previous results of our group also suggest a role of anosmin‐1 in demyelinated lesions from MS patients and point to the feasible pharmacological and genetic manipulation of the FGF2/FGFR‐1/anosmin‐1 system on endogenous and/or exogenous OPCs in demyelinating lesions.
In the present work we studied the functional implications of anosmin‐1 and FGF‐2 on neurobiology (cell death, proliferation, motility, migration) of postnatal/adult murine OPCs isolated from cerebral cortex (P0, P15, P60) and of OPCs isolated from adult human biopsies, using both in vivo (immunohistochemistry) and in vitro (chemotaxis chambers, migration, BrdU uptake, TUNEL, immunocytochemistry) techniques. These results would be useful for the design of effective neuroreparative therapies in MS and other demyelinating diseases.
Funded: Ministerio Economía y Competitividad (SAF2009‐07842; SAF2012‐40023, RD07‐00606‐2007, RD12‐0032‐12), partially by F.E.D.E.R.; European Union, “Una manera de hacer Europa”, Gobierno de Castilla‐La‐Mancha (PI2009‐26; PAI2010/012), Fundación Eugenio Rodríguez Pascual, Red Española de Esclerosis Múltiple (RETICS RD07/0060/2007, RD07/0060/0015). AB has a postdoctoral Sara Borrell program grant ISCIII/MINECO. EMMR has a pre‐doctoral fellowship from Ministerio Economía y Competitividad (SAF2009–07842). PFE and VM are currently hired by ADE10/0010 (MINECO). FdC is hired by Sescam.
T04‐01B. Improving outgrowth and survival of grafted iPSC‐derived dopaminergic neurons by L1 and PSA‐NCAM
S. Peng 1, M. Schachner2, R. Rössler1, E. Boddeke1, S. Copray1
1University Medical Center Groningen, Groningen, Netherlands 2Shantou University Medical College, Center of Neuroscience, Shantou, China
Grafting of induced pluripotent stem cell (iPSC) derived dopaminergic (DA) neurons has great potential for cell based therapies for Parkinson's disease (PD). So far, however, DA neurons derived either from embryonic stem cells or iPSCs, demonstrated low survival and limited outgrowth after striatal implantation in a Parkinson rat model. To improve the fate of iPSC‐derived DA neurons after implantation, we aimed to modify the extracellular environment of the cell graft with molecules that have critical roles in axon outgrowth, neuronal migration, survival, and regeneration. We have focused on L1 and PSA‐NCAM, extracellular matrix molecules which have been shown essential for proper outgrowth and survival of DA neurons during development of the nervous system.
We generated iPSC lines from fibroblasts isolated from Pitx3‐GFP knock‐in mice which allowed FACS‐purification of mature midbrain DA neurons upon in vitro differentiation based on the expression of the mature DA marker Pitx3. Subsequently, mouse Pitx3‐GFP knock‐in iPS cells were transduced with lentiviral vectors containing genes encoding for hL1 and for STX, the enzyme that add PSA onto NCAM. Next they were differentiated in vitro into DA neurons. The effects of hL1 and PSA‐NCAM on differentiation, survival and outgrowth of iPSC‐derived DA neuron were analyzed in vitro and in vivo.
Indeed, we were able to obtain a pure suspension of mature iPSC‐derived DA neurons upon in vitro differentiation and FAC sorting. Transfection of Pitx3‐GFP knock‐in iPS cells with hL1 or STX did not affect the pluripotent potential of these cells and did not interfere with the process of dopaminergic differentiation itself. We continued to establish the beneficial effect of L1 and PSA‐NCAM expression on survival and outgrowth of iPSC‐derived DA neurons in vitro and after grafting in the striatum of parkinsonian rats.
T04‐02A. Intracellular trafficking, matrix association and function of VEGF in astroglial cells
K. Egervari 1, G. Potter1, M.‐L. Guzmán‐Hernández2, P. Salmon1, T. Balla2, B. Wehrle‐Haller1, J.Z. Kiss1
1University of Geneva, Geneva, Switzerland 2National Institute of Child Health and Human Development, Bethesda, United States
Vascular endothelial growth factor (VEGF) is a major regulator of neurovascular remodeling following brain injury. While much have been learnt about VEGF functions on target endothelial cells, little is known about its intracellular trafficking, mode of secretion and matrix interactions in “source” cells, i.e. mainly reactive astrocytes. Here, we generated a VEGF::GFP fusion protein to follow the distribution of VEGF165 during its trafficking in primary astrocytes and COS7 cells. We found that VEGF::GFP forms dimers and gets glycosylated similarly to wild type, and as expected, the molecule follows the endoplasmic reticulum‐Golgi pathway. However, its post‐Golgi trafficking suggests a unique route with features that do not conform the classical constitutive secretory pathway: the secretion of VEGF165 occurs even at 19 °C and shows a Ca2+‐ and PKC‐induced increase. We investigated the distribution of VEGF in polarized primary astrocytes in an in vitro scratch wound assay. In these cells, VEGF::GFP appeared to follow a vectorial distribution and accumulated behind the leading edge. The accumulation appeared to be on the extracellular surface, moreover, ultrastructural immunogold labeling revealed that extracellular VEGF::GFP remains associated with discrete areas of the cell membrane and is accumulated in caveolae as well as in microvesicular shedding elements. This particular localization corresponds to fibrillar adhesions (FB), where VEGF::GFP is co‐localized with fibronectin. We also observed that VEGF::GFP is targeted to adherens junctions between astrocytes, however, at these sites VEGF::GFP was not co‐localized with fibronectin. Finally, we show in FRAP experiments that integrin turnover is decreased in VEGF associated FBs, which raises the possibility of an autocrine/paracrine regulation of astrocytic functions. Together, these findings have strong implications for understanding focal coordination of angiogenesis and vascular remodeling by astroglia derived VEGF.
T04‐02B. Migration of microglia in the embryonic neocortex: cellular and molecular interactions
S. Smolders 1, A. Avila1,2, N. Swinnen1,3, T. Struys1,4, I. Lambrichts1, M. Ameloot1, N. Hellings1, J.‐M. Rigo1, B. Brône1
1Biomed, Hasselt University, Diepenbeek, Belgium 2GIGA Neurosciences, University of Liège, Liège, Belgium 3UMPC, Université Paris 6, Paris, France 4KU Leuven, Imaging and Pathology, Leuven, Belgium
Question: Microglia, the immune effector cells of the central nervous system (CNS), influence synaptic development and connectivity in the normal and developing CNS. After invading the embryonic CNS, microglia migrate extensively to occupy their final positions. However, the cellular and molecular mechanisms of microglial migration are unknown. Therefore, this study aims to elucidate the microglial migration behavior, cellular interaction partners and ECM‐integrin interactions essential for migration in the developing neocortex.
Methods: Immunohistochemical stainings and immunogold labeling for transmission electron microscopy were carried out on fixed brain tissue of C57BL/6 CX3CR1+/eGFP mice embryos (Embryonic day (E) 10.5–17.5). Multicolor FACS analyses were performed on homogenized age‐pooled CX3CR1+/eGFP embryonic cortici. Microglial migration was recorded in CX3CR1+/eGFP‐hGFAP‐CFP acute brain slices using multiphoton time‐lapse excitation and analyzed using MTrackJ in ImageJ.
Results: Laminin (LM) is expressed as punctuate dots near the pia and in the ventricular zone at E10.5 and disappears at E15.5 to remain only in blood vessels and the pia. Fibronectin (FN) is present as aggregates from E10.5 until E17.5. Both ECM proteins follow the course of radial cells. Preliminary data indicate a transient upregulation of the LM receptor (LMR) on E15.5 and E16.5. In addition, the percentage of microglia expressing the FN receptor (FNR) tends to decrease during development. Ex vivo time‐lapse recordings show that embryonic microglia are dynamic cells that migrate in random patterns. These cells seem to make brief contacts with the processes of radial glia.
Conclusions: Embryonic microglia express laminin and fibronectin receptors and possibly change the expression level during development. They are dynamic cells that migrate in random patterns, and possibly make occasional contact with radial glia. Knowledge about ECM‐integrin interactions during microglial migration could reveal targets for intervention in pathological settings, such as Multiple Sclerosis and Alzheimer's disease.
T04‐03A. Astrocyte‐derived TG2 contributes to ECM production and aggregation, and cell adhesion
N. Espitia Pinzon 1, W. Baron2, J.J. Breve1, B. Drukarch1, A.‐M. van Dam1
1VU University Medical Center, Amsterdam, Netherlands 2University Medical Center Groningen, Cell Biology, Groningen, Netherlands
Multiple Sclerosis (MS) is a chronic demyelinating disease of the central nervous system in which astroglial cells become hypertrophic, produce various extracellular matrix (ECM) proteins which become aggregated when secreted. These aggregated ECM proteins contribute to a non‐permissive environment for repair. Tissue Transglutaminase (TG2) expression is enhanced in astrocytes in MS lesions. This enzyme is well‐known for protein cross‐linking capacities. Moreover, TG2 can act as a co‐receptor for β‐integrins to bind to ECM proteins, thereby mediating cell adhesion processes.
In this study we questioned whether astrocyte‐derived TG2 affects ECM protein production and/or aggregation, and if astrocyte‐derived TG2 mediates cell adhesion onto various ECM proteins. Primary rat astrocytes were transduced with an empty lentiviral vector (mock) or with a lentiviral vector expressing human wildtype TG2. Alternatively, primary rat astrocytes were transduced with a lentiviral vector expressing scrambled shRNA or TG2 shRNA to knockdown endogenous rat TG2. The rat primary astrocytes overexpressing human TG2 showed an increased production of fibronectin and collagen V. Conversely, knocking‐down TG2 showed a decrease in fibronectin and collagen production. Interestingly, overexpression of TG2 resulted in enhanced aggregation of extracellular laminin. Also, human astrocytoma cells (U373) were transduced with an empty lentiviral vector (mock) or with a lentiviral vector expressing human wildtype TG2 and allowed to adhere onto ECM‐coated wells. Astrocytoma cells overexpressing human TG2 showed increased adhesion onto laminin‐, and fibronectin‐coated wells.
We conclude that astrocyte‐derived TG2 affects production/aggregation of fibronectin and laminin. Moreover, the astrocyte‐derived TG2‐mediated elevated production/aggregation of fibronectin and laminin coincides with more adherence of astrocytes onto these ECM proteins. We hypothesize that TG2‐mediated enhanced production/aggregation of fibronectin and laminin is related to increased astrocyte adhesion which together could contribute to the non‐permissive environment for repair in the central nervous system of MS patients.
T04‐03B. Role of extracellular matrix and growth factors in oligodendrocyte cytoskeletal reorganization
A. Tripathi, Z. Parikh, P. Pillai
The M.S. University of Baroda, Vadodara, India
Background: Oligodendrocytes originate as progenitor cells (OPCs) in discrete areas of the developing brain which undergoes a complex and precisely timed program of proliferation, migration, differentiation, and myelination in CNS. During migration it interacts with its surrounding environment through integrins.
Purpose: To evaluate the interactions between integrins and growth factors (GFRs) that promote the molecular events such as proliferation, cytoskeletal reorganization and migration in oligodendrocytes leading to oligodendrocyte mediated myelination.
Methods: Cortical cells were prepared from P0 rat cerebral cortex following standard protocols. Immunocytochemistry (ICC), Western blot (WB), Immunoprecipitation (IP), migration assay and real‐time RT‐PCR analysis were performed. For dissecting out the roles of different intracellular signalling proteins in the regulation of events downstream of receptor activation, use of pharmacological inhibitors was carried out.
Results: TNFα, PDGFA and Estradiol significantly increased cell proliferation and cell processes. Data also suggest differential effects of extracellular matrix (ECM) on signalling component activation. Significant increase in ERK expression was found following GFRs‐integrin interactions. ECM proteins bind to integrin family members and mediate bidirectional signalling between the cellular interior and the external environment through the activation of focal adhesion complexes.
Conclusion: Integrin switching, oligodendrocyte proliferation, cytoskeletal reorganization and differentiation profile is highly influenced by ECM and GFRs. Integrin governs the signalling cascades underlying oligodendrocyte differentiation and myelination.
This study was performed in strict accordance with the recommendations for the care and use of laboratory animals. All the protocols were duly approved by the institutional ethical committee, The M.S. University of Baroda. Reference No: – ZD/18/2013
Source of Funding: Financial assistance from DBT‐MSUB‐ILSPARE, UGC, New Delhi & DST‐SERB, New Delhi in the form of major projects sanctioned to Dr. P. Pillai, Principal Investigator, Dept. of Zoology, MSU, Baroda is highly acknowledged.
T04‐04A. Characterization of adhesional and cytomechanical properties of single living cells and tissue slices by AFM
T. Müller, T. Neumann, A. Hermsdörfer, C. Pettersson, G. Behme
JPK Instruments AG, Berlin, Germany
Topography, roughness and mechanical properties of micro environment are crucial parameters influencing cell adhesion/motility, morphology and mechanics as well as the development of cells e.g. stem/progenitor cells, and neuronal cells [1–5]. Atomic force microscopy is a powerful tool not only to study the morphology in terms of high resolution imaging and roughness measurements, but also to map mechanical and adhesive properties of the sample/cells and tissues. Combining these remarkable abilities with advanced optical microscopy allows for extensive characterization of biomaterials and tissue slices [6–8]. However, commercially existing technical solutions are either time consuming, or only limited practicable for soft, sticky, or fragile samples. Therefore, we developed a new force curve based AFM mode – Quantitative Imaging (QI™). The novel QI tip movement algorithm prevents lateral forces and controls the vertical forces for nondestructive imaging. To demonstrate the performance and flexibility QI™ mode we investigated topography, adhesion properties, and Young's modulus local distribution of living dorsal root ganglion cells.
A specialized platform – CellHesion® – has been developed to run single cell force spectroscopy (SCFS) with a need for long‐range cell‐surface and cell‐cell binding experiments with up to 100 microns pulling length. Using single cell force spectroscopy (SCFC), cell‐cell adhesion can be quantified [e.g. 3], and the contribution of different components e.g. from the extra cellular matrix, can be assessed [9]. A new technical solution will be demonstrated if a cantilever attached cell can be transferred through the liquid‐air interface. This approach allows to measure the adhesion of the same individual single cell on different materials within different dishes as it will presented for CHO cell adhesion on plastic as well as albumin coated surfaces.
We want to present the progress in automatic data processing and display to investigate topography, Young's modulus and local adhesion phenomena on transparent and non‐transparent biomaterials like titanium, peptide functionalized surface or cell layers, and tissue slices.
REFERENCES
[1] Engler et al., Cell 126, 677–689 (2006).
[2] Docheva et al., Biochem. Biophys. Res. Com. 402, 361–366 (2010).
[3] M. Krieg et al.Nature Cell Biology 10(4), 429–436 (2008).
[4] P. Moshayedi et al., J. Phys.: Condens Matter 22, 194114pp. (2010).
[5]Li‐Y. Chianget al., Nature Neuroscience 14, 993–1000 (2011).
[6] K. Franze et al., Biophysical J. 97, 1883–1890 (2009).
[7] A.F. Christ et al., J. Biomechanics 43, 2986–2992 (2010).
[8] Kirmse et al., J. Cell Science 124, 1857–1866 (2011).
[9] J. Friedrichs et al., Nature Protocols 5(7), 1353–1361 (2010).
T04‐04B. Extracellular space diffusion parameters in the mouse thalamus in Bral2 knock‐out mice
L. Vargova 1, M. Cicanic1,2, E. Sykova1,2
1Charles University, 2nd Faculty of Medicine, Prague, Czech Republic 2Institute of Experimental Medicine, v.v.i., Department of Neuroscience, Prague, Czech Republic
Bral‐2 is a link protein that is localized in distinct brain regions, where it stabilizes the perineuronal nets of the extracellular matrix (ECM). Our previous studies showed that qualitative and quantitative ECM changes, might evoke changes in the diffusion properties of the extracellular space (ECS), thus affecting the movement of neuroactive substances in the CNS.
In the current study, we determined by the real‐time iontophoretic method the extracellular space volume fraction α (α = ECS volume / total tissue volume) and the geometrical factor tortuosity λ (λ2 = free / apparent diffusion coefficient) in coronal brain slices obtained from the sensorimotor cortex and the ventral posteromedial thalamic nucleus of sixteen‐month‐old Bral‐2 deficient mice (Bral‐2 ‐/‐), age‐matched Bral‐2 +/+ controls and young adult (5‐month‐old) Bral‐2 +/+ controls.
The diffusion parameters in the cortex of young adult Bral‐2 +/+ mice were: α = 0.21 ± 0.01 (mean ± S. E. M.) and λ = 1.48 ± 0.02, n = 6, N = 5 (n – number of slices, N – number of animals). In the thalamus, there was a smaller α and a larger λ (0.16 ± 0.01 and 1.53 ± 0.01, respectively, n = 26, N = 10). In young adult mice, we found no significant differences in the ECS diffusion parameters between Bral2 positive and negative mice in either the cortex or the thalamus.
In the cortex of aged Bral‐2+/+ mice we found a smaller α (0.18 ± 0.01, n = 10, N = 7) than in the cortex of young adult mice. During aging, there was no significant difference in the thalamic diffusion properties in wild‐type animals, but in the thalamus of aged Bral‐2 ‐/‐ mice, α significantly decreased (0.13 ± 0.01, n = 28, N = 11) in comparison to the younger Bral2‐/‐ animals as well as to age‐matched controls.
Immunohistochemical analysis of the ECM in the ventral posteromedial thalamic nucleus revealed no positivity for Bral2 protein in either group of Bral2‐/‐ mice. In the youger mice, Bral deficiency was associated with a small attenuation in the positivity of brevican and another link protein, CTRl1; however, in aged Bral2‐/‐ animals, brevican and Ctrl1 positivity was increased in comparison with the age‐matched controls.
In contrast to the cortex, we found no decrease in α in the thalamus of aged wt animals, suggesting that the process of aging may differ between the cortex and the thalamus. A decrease in the ECS volume fraction in the thalamus of Bral‐2 ‐/‐ aged mice may result from a disruption of the perineuronal nets and rearrangements of the molecular assembly, which seem to differ in young adult and aged animals.
Supported by grants: GACR 13‐11867S and GACR P304/12/G069.
POSTER TOPIC 05 Gene expression and transcription factors
T05‐01A. Cyclic AMP signaling promotes differentiation of astrocyte transcriptome
S. Paco1, M. Hummel2, V. Pla1, L. Sumoy3, F. Aguado 1
1University of Barcelona, Barcelona, Spain 2Centre for Genomic Regulation, Barcelona, Spain 3Institute for Predictive and Personalized Medicine of Cancer, Badalona, Spain
cAMP signaling produces dramatic changes in astrocyte morphology and physiology. However, its involvement in phenotype acquisition and the transcriptionally mediated mechanisms of action are largely unknown. Here we analyzed the global transcriptome of cultured astroglial cells incubated with activators of cAMP pathways. A bulk of astroglial transcripts were differentially regulated by cAMP signaling. cAMP analogs strongly upregulated genes involved in typical functions of mature astrocytes, such as homeostatic control, metabolic and structural support to neurons, antioxidant defense and communication, whereas they downregulated a considerable number of proliferating and immaturity‐related transcripts. Moreover, genes typically activated in reactive cells, such as immunological mediators and scar components, were repressed by cAMP. Gene Set Enrichment Analysis and evaluation in situ of gene expression in astrocytes in different states showed that cAMP signaling conferred a mature and in vivo‐like transcriptional profile to cultured astrocytes. These results indicate that cAMP signaling is a key pathway restricting developmental and activation features of astrocytes and promoting their maturation. A positive modulation of cAMP signaling is suggested to suppress the mechanisms of activation driven by pathological situations and to promote the physiologically normal state of differentiated astrocytes.
T05‐01B. Microglia immune priming in physiologically aged and senescence accelerated mice: gene co‐expression network analysis
I. Holtman, D. Raj, N. Brouwer, B. Eggen, E. Boddeke
University Medical Center Groningen, Groningen, Netherlands
Question: Microglia, the innate immune system of the central nervous system, are crucial to maintain homeostasis by cleaning debris and attacking stressors. In the ageing brain, increased numbers of microglia with ameboid morphology and increased inflammatory cytokine levels are reported. The altered microglial phenotype in the ageing brain is characterized by hyperresponsive to immune stimuli, which is called immune priming. Immune priming could be a potential cause of age‐associated neurodegeneration. The aim of this study is to characterize the immune pathways and biological processes that are altered in ageing‐induced immune primed microglia and to find genes potential drivers of this process.
Design/methods: The effect of ageing on microglia has been addressed in naturally aged mice (24 months old) and in ERCC1 transgenic mouse model in which accelerated ageing is induced by DNA damage accumulation. From each of these models a pure population of microglia was isolated. Microarray technology was used to obtain information about the genome wide gene expression profile. Weighted Gene Co‐Expression Network Analysis (WGCNA), uses correlational structures to find clusters of genes to make optimal use of a dataset in biological interpretation.
Results: Using Weighted Gene Co‐expression Network Analysis, a very robust microglial immune priming gene module was discovered. In this module, there is a significant enrichment of the complement system, antigen presentation, interferon type‐1 signaling and phagocytosis‐machinery. The average expression of this module is stronger up‐regulated in ERCC1 transgenic than in normally physiologically aged mice, supporting the notion that ERCC1 is characterized by excessive immune priming.
Conclusion: Microglial priming which occurs in normal ageing or in accelerated ageing models is marked by increased expression of several immune signaling pathways.
T05‐02A. The Role of FoxO3a in Oligodendrocyte Precursor Cell Differentiation
S. Ali Abdulla 1, Y. Ahmed Syed2, M. Kotter2
1Cambridge University, Cambridge, United Kingdom 2Wellcome Trust – MRC Center For Stem Cell Biology, Cambrige, United Kingdom
FoxO3a is a transcription factor that is highly expressed in the brain. However, little is known about the response of FoxO3a in regulating oligodendrocyte precursor cell (OPC) differentiation. This study tested the response of FoxO3a in regulating OPC differentiation and its role in the myelination process. A microarray analysis of early regulators of OPC differentiation showed that Foxo3a is significantly down‐regulated during the onset of OPC differentiation. In agreement, mRNA expression analysis of cell lysates collected at various differentiation time points demonstrated that the active form of FoxO3a is down‐regulated during OPC differentiation. Over expression of FoxO3a through the transfection of pure OPC cultures with adenoviral vector constructs, of a constitutively active form of FoxO3a, resulted in an inhibition of OPC differentiation. On the other hand, siRNA knock downs of FoxO3a promoted OPC differentiation. In addition, ultrastructural analysis of the corpus callosum of FoxO3a null mice showed a significant increase in the number of myelinated axons in comparison to wild type. Consequently, the data suggests that FoxO3a is an essential regulator of the myelination process.
T05‐02B. Role of the β2 adrenergic receptor in multiple sclerosis severity
C. Jensen 1, J. Stankovich2, H. Butzkueven3, J. De Keyser4
1Vrije Universiteit Brussel, Jette, Belgium 2University of Tasmania, Hobart, Australia 3University of Melbourne, Melbourne, Australia 4UZ Brussel, Neruology, Brussels, Belgium
Question: Multiple sclerosis (MS) is a chronic degenerative disease leading to physical and cognitive disability characterised by inflammatory lesions in the central nervous system. Differences have also been observed in lesions and in otherwise normal appearing white matter (NAWM) of MS cases, including the loss of staining for the β2 adrenergic receptor (ADRB2) on astrocytes.
Methods: We investigated three single nucleotide polymorphisms (SNPs) (rs1042713 (Arg16Gly), rs1042714 (Gln27Gly) and rs1042711) and five haplotypes in ADRB2 to explore whether these polymorphisms were associated with MS progression in 436 well phenotyped MS patients. Genotyping was done using Illumina bead microarray and missing genotypes were imputed using Beagle.
Results: A trend towards a decreased frequency of rs1042714C allele (27Gln) was in people with progressive MS than in people with relapsing remitting MS. This was seen in both SP‐MS (p(uncorrected) = 0.0072) and in PP‐MS (χ2 = 6.85, p(uncorrected) = 0.0325). However, those p values did not reach statistical significance after correction for multiple testing. Neither haplotypes nor SNPs were significantly associated with altered MSSS, age of onset, time between fist and second relapse, processing speed (SDT) or brain atrophy.
Conclusion: We found no evidence that polymorphisms in ADRB2 influence severity in multiple sclerosis.
T05‐03A. PGC‐1α expression in neurons and glia cells
H. Bayer, D. Pasche, I. Merdian, J. Eschbach, P. Weydt, A. Witting
Experimental Neurology, Ulm, Germany
Neurodegenerative diseases are characterized by mitochondrial and metabolic abnormalities that can be detected in neurons and non‐neuronal cells. Recent evidence from Huntington disease, Parkinson disease and amyotrophic lateral sclerosis research shows that peroxisome proliferator‐activated receptor (PPAR) gamma coactivator 1‐α (PGC‐1α), a master regulator of mitochondrial biogenesis, plays a crucial role in the mitochondrial dysfunction occurring during neurodegeneration. Of note, we recently reported specific PGC‐1α isoforms which are under the control of a brain‐specific promoter (Soyal et al., Hum Mol Gen 2012).
Here we examine the expression of these brain‐specific PGC‐1α isoforms in neurons and glia cells. We characterize the abundance of the different isoforms of PGC‐1α in primary murine cell cultures of neurons, glia cells, including oligodendrocytes, astrocytes and microglia under control conditions and metabolic stress.
We show that PGC‐1α is differentially expressed in the different cell types, e.g. neurons and oligodendrocytes express much higher levels of the novel brain‐specific isoforms of PGC‐1α. The different expression levels might reflect the different metabolic needs of the different cell types.
T05‐03B. Role of ncRNAs in oligodendrocyte precursor differentiation
S. Marques, G. Castelo‐Branco
Karolinska Institutet, Stockholm, Sweden
Oligodendrocytes (OL) are neuroepithelium‐derived cells that insulate neuronal axons through myelin‐containing membranes, essential for axonal integrity and impulse transmission. Defects in this process are present in several diseases, including the highly debilitating multiple sclerosis (MS). The process of remyelination can be promoted by OL precursor cells (OPC) endogenously and occurs during a short window of time at earlier stages of MS, ultimately failing as the disease evolve. The understanding of development and differentiation regulation of OL is essential to clarify the reasons behind the defective myelination during pathologies and to open the pave to new remyelinating cell therapies. OPCs start to be specified early during embryogenesis but regardless of the origin, their terminal differentiation and functional maturation occurs only at post‐natal stages. In this project, we are characterizing the transcriptome of OPCs during development, which will allow us to better characterize the heterogeneity of these cells and point out what might be the key factors involved in transition between states. Recent studies suggest that non‐coding RNAs can interact with proteins complexes containing chromatin modifying enzymes and transcription factors, regulating their activity, acting as scaffolds and/or recruiting them to specific loci in the genome. Therefore, we are investigating their role in OPCs by RNA interference and how they might modulate the function of key transcription factors.
T05‐04A. Gene Regulatory Networks Underlying Astrocyte Identity and Potential
A. Bithell 1, A. Michelucci2, I. Crespo2, M. Burney1, C. Johnston1, K.‐Y. Wong3, S.‐W. Teng3, B. Williams1, L. Stanton3, A. Del Sol2, N. Buckley1
1King's College London, Institute of Psychiatry, London, United Kingdom 2Luxembourg Centre for Systems Biomedicine, Luxembourg, Luxembourg 3Genome Institute of Singapore, Singapore, Singapore
Neural stem cells (NSCs) represent a potentially valuable resource when considering repair strategies for CNS damage. Unlike their embryonic counterparts, adult neural stem cells (aNSCs) in the two neurogenic niches have an astrocyte‐like identity, raising the question of what regulates this aNSC state. Some early postnatal astrocytes in the parenchyma also retain some NSC‐like characteristics and in vitro display self‐renewal and multipotency, though these properties are lost following in vivo maturation. Interestingly, following injury, adult astrocytes can become reactive and reacquire NSC‐like properties, whilst others can generate new neurons through forced expression of specific transcription factors. We are aiming to elucidate the gene regulatory networks underlying specific NSC and astrocyte states and transitions between them using transcriptomic and epigenomic tools alongside computational methods in different NSC and astrocyte populations. We will describe our latest findings from an in vitro system that aims to model immature versus mature astrocytes derived from a common progenitor. From microarray data, we have identified candidate genes and pathways involved in regulating astrocyte potential versus differentiation, including a role for specific pro‐inflammatory signalling. We will also describe the associated epigenetic signatures that accompany cell state and discuss the implications of our latest findings.
T05‐04B. The IRF8‐IRF5 transcription factor axis governs P2X4Rhi reactive microglia driving neuropathic pain
T. Masuda 1, M. Tsuda1, S. Iwamoto1, A. Nishiyama2, H. Tozaki‐Saitoh1, T. Tamura2, K. Inoue1
1Kyushu University, Dept. Mol. Syst. Pharmacol., Grad Sch. Pharm. Sci., Fukuoka, Japan 2Yokohama City University, Dept. Immunol., Grad. Sch. Med., Yokohama, Japan
Microglia are the CNS immune‐related cells and associated with the pathogenesis of several types of neurological diseases. Following peripheral nerve injury (PNI), spinal microglia become activated and have crucial roles in generating neuropathic pain. We have previously shown that interferon regulatory factor‐8 (IRF8), upregulated in spinal microglia after PNI, regulates gene expressions that switch to a reactive phenotype. Here we identified IRF5 as a crucial factor of reactive microglia that works cooperatively with IRF8. We found that PNI increased expression of IRF5 in the spinal cord, the expression of which was restricted to microglia. Forced expression of IRF8 in cultured microglia markedly increased expression of IRF5 in a manner that depended on its ability to bind DNA. Furthermore, IRF8‐deficient mice failed to increase the expression of IRF5 in spinal microglia after PNI, indicating IRF8‐dependent expression of IRF5 in microglia in vivo. Interestingly, upregulation of P2X4 receptor (P2X4R; an ATP‐gated channel crucial for producing neuropathic pain) by forced expression of IRF8 in cultured microglia was markedly suppressed by knockdown of IRF5 expression. In a model of neuropathic pain, suppressing upregulated IRF5 protein by spinal administration of siRNA targeting IRF5 alleviated pain hypersensitivity. Altogether, the IRF8‐IRF5 axis drives a program of gene expression that promotes P2X4Rhi microglia gating neuropathic pain.
T05‐05A. Analysis of astrocyte‐specific gene recombination in the brain
C. Bohn, H.M. Jahn, F. Kirchhoff
University of Saarland, Homburg, Germany
Astrocytes are the most abundant glial cell type. They express a range of neurotransmitter receptors localized along their processes that cover synapses and constitute “microdomains” for signalling pathways. To investigate astroglial purinergic P2Y1 and glutamatergic NMDA receptors we generated knockout mice in which gene recombination of “floxed” receptors was controlled by an astrocyte‐specific and tamoxifen‐inducible Cre recombinase (GLAST‐CreERT2).
Here, we present data on the DNA recombination efficiencies of GLAST‐CreERT2 x floxed P2Y1 mice and GLAST‐CreERT2 x floxed GluN1 mice in different brain regions (cerebellum, hippocampus, brainstem, optic nerve and cortex). Recombination is determined by quantitative real‐time PCR of genomic DNA using primers across the loxP sequences flanking exon 1 of the p2ry1 gene and exon 11–21 of the grin1 gene, respectively. DNA recombination was induced in young adult mice by intraperitoneal tamoxifen injection for three consecutive days and analyzed three weeks later. Primers were designed such that reduction of the PCR signal reveals increasing recombination (i.e. gene excision and knockout) compared to control. The degree of reduction in the investigated brain regions is expected to be similar in both mouse lines; allowing conclusions (1) about the reliability of the GLASTCreERT2/loxP system in general (i.e. recombination efficiency at different chromosomal locations), and (2) gives a percentage of GLAST‐positive cells (predominantly astrocytes) in the given brain area. First data demonstrate 30% recombination in the cortex, which is well in line with the percentage of astrocytes in this brain region. These results could be confirmed by using a reporter mouse line expressing the red fluorescent protein tdtomato (R26‐tdtomato) and using immunohistochemistry with astrocyte markers.
In conclusion, the combination of quantitative real‐time PCR analysis of gene recombination and cell‐specific Cre mouse lines represents a reliable approach to reveal the percentage of a given cell type in a given tissue region.
T05‐05B. Targeted CREB activation in astrocytes deeply modifies the gene‐expression profile of cortex following a focal injury
L. Pardo Fernández 1, A. Schlüter2, A. Barco3, M. Giralt4,5, J. Hidalgo4,5, R. Mateu6,4, L. Giménez‐Llort4,7, R. Masgrau6,4, A. Pujol2,8, E. Galea6,4,8
1Universitat Autònoma de Barcelona, Barcelona, Spain 2Institut de Neuropatologia de Bellvitge (IDIBELL), Neurometabolic Diseases Laboratory, Barcelona, Spain 3Instituto de Neurociencias de Alicante (UMH‐CSIC), Alicante, Spain 4Universitat Autònoma de Barcelona, Institut de Neurociències, Barcelona, Spain 5Universitat Autònoma de Barcelona, Departament de Biologia Cellular, Fisiologia i Immunologia, Barcelona, Spain 6Universitat Autònoma de Barcelona, Departament de Bioquìmica i Biologia Molecular, Barcelona, Spain 7Universitat Autònoma de Barcelona, Departament de Psiquiatria i de Medicina Legal, Barcelona, Spain 8Institució Catalana de Recerca i Estudis Avançats (ICREA), Barcelona, Spain
The transcription factor CREB is protective in injury and neurodegeneration, but the contribution of neuronal vs astrocytes is unknown. We have generated a mouse with targeted over‐expression of a constitutively active CREB (VP16‐CREB) in astrocytes by crossing TetO‐VP16 and TtA‐GFAP mice. VP16‐CREB/GFAP mice show expression of VP16 in astrocytes ‐as detected by immunohistochemistry – restricted to cerebellum, hippocampus and outer layers of cortex. We determined the role of astrocytic CREB in the outcome of brain injury by subjecting wild type (WT) and VP16‐CREB/GFAP (Tg) mice to a focal cryolesion (C) in the frontal cortex. At 3 days post‐lesion, WT mice presented a necrotic region filled with macrophages (labeled with lectin) surrounded by a rim of tissue with robust gliosis (labeled with GFAP). Tg mice showed reduced macrophage infiltration as compared with WT mice. A DNA array analysis of the damaged zone (Agilent) has revealed differentially expressed genes in paired comparisons. WTC/WT: 13,984; WTC/TgC: 5,821; TgC‐T:913; Tg/WT:0 (statistical linear model in LIMMA., P values computed by empirical Bayes moderated t‐statistics at 0.05). There were 159 significantly enriched KEGG pathways regulated by the cryolesion, according to GSEA and hypergeometric tests set at p < 0.05. VP16‐CREB had an effect on 127 of the pathways. Upon increasing the analysis stringency by setting the cut‐off at p < 0.0001, the number of significantly enriched KEGG pathways regulated by VP16‐CREB was reduced to 16. We selected 10 of these pathways related to 5 functions: energy metabolism, inflammation, structural reorganization, genomic stability and apoptosis, which we define as the core signature of the VP16‐CREB‐elicited change. The differential expression of representative genes related to this signature was validated by Real‐time PCR. Thus, VP16‐CREB rescued the decreased expression of enzymes regulating energy metabolism, while decreasing the expression of pathways related to apoptosis, extracellular matrix and inflammation. The latter is consistent with the decreased macrophage infiltration detected by immunohistochemistry in Tg mice. We posit that VP16‐CREB protects the brain against bionergetic failure and secondary damage due to macrophage infiltration, while rendering the tissue more conducive to neurite regeneration by reducing extracellular matrix components. The study supports the notion of astrocytes as therapeutic targets in brain injury.
Table 2.
1:
| Paired comparisons | Number of differentially expressed genes p<0.05 | What does the comparison mean? |
| WT‐WTC | 13.984 | Genes regulated by the cryolesion in WT |
| WTC‐TC | 5.821 | Genes regulated by VP16 in the cryolesion |
| TC‐T | 913 | Genes regulated by the cryolesion in VP16 mice |
| T‐WT | 0 | Genes regulated by VP16 in normal conditions |
T05‐06A. NFAT‐c3 promotes calcium‐dependent MMP3 expression in activated astroglial cells
E. Cano 1, F. Neria1, M. Serrano‐Pérez2, P. Velasco1, P. Tranque2
1Instituto de Salud Carlos III, Majadahonda, Madrid, Spain 2University of Castilla‐La Mancha, Facultad de Medicina and Instituto de Investigación en Discapacidades Neurológicas, Albacete, Spain
Increase in intracellular calcium ([Ca2+]i) is a key mediator of astrocyte signalling, important for activation of the calcineurin (CN)/nuclear factor of activated T cells (NFAT) pathway, a central mediator of inflammatory events. We analysed the expression of matrix metalloproteinase 3 (Mmp3) in response to increases in [Ca2+]i and the role of the CN/NFAT pathway in this regulation. Astrocyte Mmp3 expression was induced by overexpression of a constitutively‐active form of NFATc3, whereas other MMPs and tissue inhibitor of metalloproteinases (TIMP) were unaffected. Mmp3 mRNA and protein expression was also induced by calcium ionophore (Io) and 2′(3′)‐O‐(4‐benzoylbenzoyl) adenosine 5'‐triphosphate (Bz‐ATP). Mmp3 upregulation was prevented by the CN inhibitor cyclosporin A (CsA). Ca2+‐dependent astrocyte Mmp3 expression was also inhibited by actinomycin D, and a Mmp3 promoter luciferase reporter was efficiently activated by increased [Ca2+]i, indicating regulation at the transcriptional level. Furthermore, Ca2+/CN/NFAT dependent Mmp3 expression was confirmed in pure astrocyte cultures derived from neural stem cells (Ast‐NSC), demonstrating that the induced Mmp3 expression occurs in astrocytes, and not microglial cells. In an in vivo stab‐wound model of brain injury, MMP3 expression was detected in NFATc3‐positive scar‐forming astrocytes. Since [Ca2+]i increase is an early event in most brain injuries, these data support an important role for Ca2+/CN/NFAT‐induced astrocyte MMP3 expression in the early neuroinflammatory response. Understanding the molecular pathways involved in this regulation could provide novel therapeutic targets and approaches to promoting recovery of the injured brain.
T05‐06B. Circadian Variations of Gene Expression Related to Neuro‐Metabolic Coupling in Astrocytes of Anterior and Posterior Hypothalamus of the Mice
J.‐M. Petit 1,2, A. Mikhaleva2, J. Gyger2, P.J. Magistretti3
1Centre de Neurosciences Psychiatriques‐CHUV, Prilly, Switzerland 2LNDC, Brain Mind Institute , Life Science Faculty, EPFL, Lausanne, Switzerland 3Division of Biological and Environmental Sciences and Engineering, KAUST, Thuwal, Saudi Arabia
The posterior hypothalamus (PH) is crucial for maintaining wakefulness whereas the anterior hypothalamus (AH) constitutes a sleep‐promoting region. Consequently, the energy needs of AH and PH should probably change throughout the sleep‐wake cycle. The glycogen mobilization altogether with the astrocyte‐neuron lactate shuttle (ANLS) likely constitutes two major mechanisms by which astrocytes ensure the neurometabolic coupling. Therefore, we hypothesized that astrocytes of AH and PH may display differences in genes expression involved in these mechanisms during the sleep‐wake cycle.
To investigate this, we measured the gene expression levels in astrocytes obtained from transgenic mice expressing the fluorescent protein eGFP under the control of the astrocyte‐specific gene GFAP promoter. Mice were sacrificed at four time, ZT0, ZT6, ZT12 and ZT18 (ZT0 corresponding to light‐on). After brain dissection, “punches” of AH and PH were micro‐dissected from slices. Cell suspensions were obtained from a pool of 3 samples by enzymatic digestion and cell trituration. Then, GFP‐positive cells, which were highly enriched in astrocytes, were sorted by FACS. Total RNA was extracted from these cells and gene expression levels were assayed by qRT‐PCR and normalized by cyclophylin mRNA levels. Seven genes related to ANLS were tested: the alpha 2 sub‐unit of the Na‐K ATPase (Atp1a2), the two sub‐unit of the lactate dehydrogenase (LDHa and LDHb), the glutamate transporters (GLAST and GLT1) and two monocarboxylate transporters (MCT1 and MCT4). Levels of mRNA encoding genes related to glycogen metabolism such as PTG, the glycogen synthase (GS) and the glycogen phosphorylase (GPhos) were also measured Results showed that the levels of mRNA encoding LDHa, MCT1 and MCT4 expressed significant circadian variations which can reach 200% for MCT4 at ZT0, compared to ZT6 levels in AH. The GLT1 mRNA levels as well as MCT1 and MCT4 mRNA levels also displayed significant circadian variations in PH. Levels of expression of genes encoding the Gphos, GS and PTG did not display major regional circadian variation.
This study shows that astrocytes displayed some transcriptional regulation in hypothalamic areas involved in the sleep‐wake cycle. The difference in the pattern of expression of ANLS related genes in AH and PH, such as GLT1, suggests that neuro‐metabolic coupling capacity of each structure might be different and might play a functional role in the sleep‐wake regulation.
T05‐07A. Identification and characterization of novel subtypes of astrocytes
Z. Chen 1, M. Ghosh2, R. Sattler1, M. Robinson2, J. Rothstein1
1Johns Hopkins University, Baltimore, United States 2University of Pennsylvania, Philadelphia, United States
Astrocytes, the most abundant cell type in the central nervous system (CNS), are a heterogeneous cell population that is despite its functional importance still not well defined. While astrocytes are no longer just considered the supporting cells in the CNS and are recognized to play a significant functional role in e.g. synapse formation, regulation of the blood brain barrier and synaptic transmission, little is known whether specific subtypes of astrocytes fulfill these functional tasks differently. Studies in our laboratory described brain region specific differences in the expression of astrocytic glutamate transporter 1 (GLT‐1) with significantly increased levels of GLT‐1 in the spinal cord compared to brain. The mechanisms of glutamate transporter regulation are not well defined, and to date little is known about the factors that are responsible for regulating protein expression and transporter activity and whether this regulation is specific for certain subtypes of astrocytes. To study GLT‐1 regulation in more detail, we generated a family of transgenic mice using increasing lengths of the 5′ non‐coding region of the GLT‐1 gene controlling expression of tdTomato. These mice were crossed with full length BAC‐GLT‐1‐EGFP mice to compare astrocytic expression of the reporter genes. Interestingly, only a subpopulation of GFP‐positive astrocytes was found to have strong tdTomato fluorescence signal, when we crossed an 8.3 kb EAAT2 promoter fragment mouse with the BAC‐GLT‐1 mouse, suggesting the existence of astroglial subtypes that are differentially regulated for EAAT2 expression. Such differences might account for variance in local glutamate‐dependent excitotoxicity to motor neurons. To this end, we purified both tdTomato+/GFP+ and tdTomato‐/GFP+ astrocytes from multiple CNS regions by fluorescence‐assisted cell sorting (FACS) and analyzed their transcriptomic profiles using microarray analysis. A list of candidate genes, mainly transcription factors and cell membrane molecules, was complied from this analysis and validated by both quantitative RT‐PCR and immunofluorescence. Pax6 and Kir4.1 were found to be highly expressed in astrocytes, consistent with previous findings. More importantly, Kir4.1 mRNA was noted to be selectively enriched in cortical tdTomato+/GFP+ cells, compared to GFP+ only cells. It suggests Kir4.1 may serve as an astroglial subtype marker.
T05‐07B. Selective microglial depletion of the transcription factor C/EBPβ in LysM‐Cre/C/EBPβfl/fl mice
M. Pulido‐Salgado 1,2, T. Valente1,2, M. Straccia1,2, J.M. Tusell2, C. Solà2, J. Saura1
1University of Barcelona, IDIBAPS, School of Medicine, Barcelona, Spain 2CSIC, IDIBAPS, IIBB, Barcelona, Spain
We have demonstrated that the transcription factor CCAAT/enhancer binding protein β (C/EBPβ) regulates proinflammatory gene expression in activated microglia (Straccia et al, 2011). We have hypothesized that microglial C/EBPβ is a potential target to attenuate the neurotoxic effects of neuroinflammation. In order to test this hypothesis in vivo, C/EBPβ knockout mice are not suitable because they show multiple phenotypic alterations as a result of the widespread expression of C/EBPβ. Mice with specific C/EBPβ deletion in microglia would be a better model. Unlike GFAP promoter for astrocytes, there is not a gold standard promoter for Cre expression in microglia. CD11b, CX3CR1 or Lysozime M (LysM), among other promoters, have been used to this end. We have generated LysM‐Cre/C/EBPβfl/fl mice with two main aims:
1) To validate the use of LysM as a suitable promoter for microglial gene deletion by Cre‐Lox recombination
2) To analyze the effects of the absence of microglial C/EBPβ in vitro and in vivo
LysM‐Cre/C/EBPβfl/fl mice which were fertile and viable and did not show the female infertility and high perinatal mortality described in C/EBPβ knockout mice. Western blot experiments revealed the presence of C/EBPβ in protein extracts from wild‐type but not from LysM‐Cre/C/EBPβfl/fl microglial cultures. Immunocytochemistry experiments confirmed this observation. Thus, C/EBPβ immunoreactivity was present in all cells in wild‐type microglial cultures and it was absent in all microglial cells in LysM‐Cre/C/EBPβfl/fl microglial cultures. The microglial specificity of LysM‐Cre recombination was demonstrated in primary mixed glial cultures. Whereas in wild‐type primary mixed glial cultures C/EBPβ immunoreactivity was observed both in astrocytes and microglia, in LysM‐Cre/C/EBPβfl/fl mixed glial cultures it was detected in astrocytes, but never in microglia,. To assess the functional outcome of microglial C/EBPβ deficiency, we analyzed LPS+IFNγ‐induced NO production and we observed a marked decrease in LysM‐Cre/C/EBPβfl/fl microglial cultures. This decrease was also observed in mixed glial cultures in fitting with the microglial origin of NO production in this model. In summary, these findings show that LysM‐Cre promoter causes recombination in 100% of microglial cells in primary culture and it is therefore suitable for microglial‐specific gene deletion in vitro. Experiments are in progress to determine the degree and specifity of microglial C/EBPβ deletion in vivo in these mice. Supported by grants PI10/378 and PI12/00709, Instituto de Salud Carlos III, Spain.
T05‐08A. Overexpression of CPEB3 leads to astrocyte dysfunction
T. Deshpande, V. Vangoor, S. Turimella, L. Kaczmarczyk, P. Bedner, E. von Staden, A. Derouiche, R. Jabs, G. Seifert, C. Steinhäuser, M. Theis
Institute of Cellular Neurosciences, Bonn, Germany
Cytoplasmic polyadenylation and element binding (CPEB) proteins are a class of translational regulators expressed in brain. CPEB1 in astrocytes regulates the translation of β‐catenin and cyclin B1 mRNA. In the present study the expression and functions of other CPEB (2–4) proteins were investigated. The expression of CPEB proteins in astroglial cell types was analyzed by single cell RT‐PCR, in situ hybridization, FACS analysis and immunostaining. To study the functional role of CPEB3, transgenic mice overexpressing CPEB3 in astrocytes were generated. CPEB3 overexpression without the endogenous 3′ untranslated region (UTR) led to the downregulation of astrocytic connexins (Cx43 & Cx30). Consistently, in the hippocampus of CPEB3 overexpressing mice, intercellular gap junction coupling was strongly impaired. CPEB3 overexpression also led to downregulation of Glutamate transporter 1 (GLT‐1) and glutamine synthetase (GS), key players involved in extracellular glutamate clearance. We have now raised mice overexpressing CPEB3 with the endogenous 3′ UTR. In these mice, we observed the downregulation of Cx43, Cx30, GLT‐1 and GS. Since interastrocytic coupling and astrocytic GLT‐1 and GS activities are downregulated in epilepsy patients with hippocampal sclerosis, we hypothesize that upregulation of CPEBs in astrocytes may contribute to the pathogenesis of epilepsy by causing astrocyte dysfunction.
T05‐08B. New role of p53 in retinal astroglia outgrowth and signaling
A.I. Ramírez 1, R. Gallego‐Pinazo2,3, R. de Hoz4,5, M.D. Pinazo‐Durán3,6, B. Rojas4,7, J.J. Salazar4,5, M. Serrano8, J. Ramírez4,7
1Universidad Complutense (UCM), Madrid, Spain 2Hospital La Fe, Departamento oftalmologia (Universidad Politecnica), Valencia, Spain 3Unidad Invest Oftalmol Santiago Grisolia, Valencia, Spain 4Universidad Complutense, Inst Invest Oftalmol Ramón Castroviejo, Madrid, Spain 5Universidad Complutense, Facultad de Optica y Optometria, Madrid, Spain 6Universidad de Valencia, Departamento Cirugia. Fac Medicina, Valencia, Spain 7Universidad Complutense, Departamento Oftalmologia. Facultad Medicina, Madrid, Spain 8Centro Nacional de Investigaciones Oncologicas (CNIO), Madrid, Spain
Purpose: To study the influence of the p53 gene in the structural and quantitative characteristics of astrocytes in the mice retina
Methods: Adult mice of the C57BL/6 strain (12 months old) were distributed into two groups: 1) mice with two extra copies of p53 (“super p53”; n = 6) and 2) wild‐type p53 age‐matched control, as the control group (WT; n = 6). Retinas were immunohistochemically processed with GFAP. GFAP+ astrocytes were quantified.
Results: In comparison con WT: i) retinal‐astrocyte distribution followed established patterns; however, morphological changes were seen through the retinas in relation to p53 availability: astrocytes were more robust and secondary processes were more evident; ii) astroglial density was significantly higher in the sp53 retinas, both in the whole‐retina (p < 0,01 Student's t‐test) and in the intermediate and peripheral concentric areas of the retina (p < 0.05 Student's t‐test).
Conclusion: The astrocyte changes in the sp53 retinas might improve the resistance of the retinal cells against oxidative stress and its downstream signalling pathways.
T05‐09A. Understanding the role of microRNAs in microglia‐mediated neuroinflammation
S. Jadhav1, M. Choolani1, E.‐A. Ling1, J. Lu 2, T. Dheen 1
1National University of Singapore, Singapore, Singapore 2DSO National Laboratories, Singapore, Singapore
Microglia are the resident immune cells of the central nervous system (CNS) and respond to detrimental signals such as neuronal injury and infection by releasing proinflammatory cytokines and chemokines. However, neuronal damage caused due to excessive microglial activation is a well known phenomenon in neurodegenerative diseases.
MiRNAs are novel regulators of gene function, controlling several biological processes. Recent reports show altered miRNA expression in immune‐mediated pathologies, suggesting that miRNAs have roles in modulating immune responses. Thus, the present study was initiated to identify microRNAs and their target mRNAs which can modulate microglial inflammatory responses.
A pilot study was designed to understand the role of miRNA‐200b in modulating microglia‐mediated immune response as this was found to be localized in the microglia. Recently, miRNA 200b has been shown to target several proteins including c‐Jun, the substrate of JNK MAPK (mitogen‐activated protein kinase) which mediates the release of proinflammatory cytokines in activated microglia. qRT‐PCR revealed the decrease of miR‐200b expression level in activated BV2 microglia. Loss‐of‐function and gain‐of‐function studies confirmed c‐Jun to be the target of miR‐200b in microglia. Overexpression of miR‐200b in activated microglia resulted in a decrease in c‐Jun and JNK expression and activity, thereby dysregulating the MAPK‐JNK pathway and proinflammatory cytokines. Further, treatment of neuronal cells, MN9D with conditioned medium obtained from activated microglial cells, resulted in increased inflammatory‐mediated cell death upon knockdown of miR‐200b. Overexpression of miRNA‐200b also reduced the phagocytic ability in activated microglia.
Taken together, these results demonstrate the important role of miR‐200b in modulating the MAPK pathway via c‐Jun which in turn affects different aspects of the inflammatory process accompanying microglia activation including cytokine response, NO production, phagocytosis and neuronal cell death. Thus, miR‐200b may prove to be a useful target for developing therapeutic strategies to control microglial mediated inflammation in neurodegenerative diseases.
Further in order to understand the global miRNA changes contributing to microglial activation, a global miRNA microarray was carried out using control, LPS‐activated and Amyloid β‐activated primary microglia. This screen identified several families of miRNAs differentially expressed between the different treatment groups enabling us to analyze the miRNA signature in activated microglia.
T05‐09B. SUMO‐1 regulates the inflammatory response of activated microglia via NFκB‐mediated TNF‐α release
P. Rangarajan, B. Tan, E.‐A. Ling, T. Dheen
National University of Singapore, Singapore, Singapore
An inflammatory process in the brain as a result of an injury or infection is mediated by the microglia, the prime immune effector cells of the central nervous system (CNS). Microglia have also been shown to display chronic inflammatory responses leading to neurodegenerative disorders. Small ubiquitin‐like modifier‐1 (SUMO‐1) is a post‐translational protein modifier, which has been shown to be associated with nuclear factor kappa B (NFκB)‐mediated inflammatory pathway. In this study, we showed the expression of Sumo‐1 in microglia and characterized the roles of SUMO‐1 in activated microglia. We found that Sumo‐1 is specifically expressed in the amoeboid microglial cells of the early postnatal rat brain. Secondly, lipopolysaccharide‐mediated activation of microglia led to a significant increase in the expression as well the nuclear translocation of Sumo‐1 in microglia in vitro. In order to establish a function for Sumo‐1 in activated microglia, we sought to silence its expression using siRNA‐mediated gene knockdown. SUMO‐1 knockdown in LPS‐treated BV‐2 microglia in vitro subsequently showed the decreased nuclear translocation of NFκB and expression of tumour necrosis factor‐alpha (TNF‐α) level. Further, we performed an in silico screening to identify the targets of Sumo‐1 in the NFκB pathway. Our study thus, suggests that SUMO‐1 regulates NFκB translocation into the nucleus for the transcription of pro‐inflammatory genes including TNF‐α in activated microglia.
T05‐10A. Transcriptional remodeling in microglia from the penumbra area in an experimental mouse stroke model
H. Hirbec 1, C. Rey 2, A. Menteyne3, N. Nazaret2, M. Boulpicante1, J. Lachuer2, E. Audinat3, F. Rassendren1
1IGF, Montpellier, France 2ProfileXpert, Lyon, France 3INSERM U603, Paris, France
Microglia are the main resident immunological cells the CNS. In the healthy brain, microglial cells are in a surveillance state whereas upon rupture of CNS homeostasis they enter activated states. In ischemic stroke, activated microglia is one of the most important cellular components of post‐stroke neuroinflammation, which mainly occurs in and around the area of the infarct. Microglia activation is characterized by morphological alterations, functional and transcriptional remodeling, which account for the acquisition of immune phenotypes. Purinergic receptors are known to play important roles in microglia activation, and P2X4R have been suggested as potential therapeutic target to limit microglia‐mediated inflammatory responses associated with brain diseases.
In the present study, we have developed an experimental approach based on laser micro‐capture to isolate microglia from the penumbra area at different post‐lesion times (from 4h to 7 days post‐lesion). The repertoire of genes expressed by microglial cells was determined through cDNA microarray analysis. With this approach we have been able to determine clusters of genes that are co‐regulated thus revealing the time course of microglia activation in this model.
In addition, we have compared the transcriptional remodeling of microglia in wild‐type and P2X4‐deficient mice. Our results suggest that wild‐type and P2X4R KO mice present specific transcriptional profiles, which only partially overlapped. Thus our data suggest that P2X4R play significant roles in the regulation of microglial cells functions.
T05‐10B. Identification and characterization of novel subtypes of astrocytes
Z. Chen1, M. Ghosh2, R. Sattler 1, M. Robinson2, J. Rothstein1
1Johns Hopkins University, Baltimore, United States 2University of Pennsylvania, Philadelphia, United States
Question: Astrocytes, the most abundant cell type in the central nervous system (CNS), are a heterogeneous cell population that is despite its functional importance still not well defined. While astrocytes are no longer just considered the supporting cells in the CNS and are recognized to play a significant functional role in e.g. synapse formation, regulation of the blood brain barrier and synaptic transmission, little is known whether specific subtypes of astrocytes fulfill these functional tasks differently. Studies in our laboratory described brain region specific differences in the expression of astrocytic glutamate transporter 1 (GLT‐1) with significantly increased levels of GLT‐1 in the spinal cord compared to brain. The mechanisms of glutamate transporter regulation are not well defined, and to date little is known about the factors that are responsible for regulating protein expression and transporter activity and whether this regulation is specific for certain subtypes of astrocytes.
Methods: To study GLT‐1 regulation in more detail, we generated a family of transgenic mice using increasing lengths of the 5′ non‐coding region of the GLT‐1 gene controlling expression of tdTomato. These mice were crossed with full length BAC‐GLT‐1‐EGFP mice to compare astrocytic expression of the reporter genes.
Results: Interestingly, only a subpopulation of GFP‐positive astrocytes was found to have strong tdTomato fluorescence signal, when we crossed an 8.3 kb EAAT2 promoter fragment mouse with the BAC‐GLT‐1 mouse, suggesting the existence of astroglial subtypes that are differentially regulated for EAAT2 expression. Such differences might account for variance in local glutamate‐dependent excitotoxicity to motor neurons. To this end, we purified both tdTomato+/GFP+ and tdTomato‐/GFP+ astrocytes from multiple CNS regions by fluorescence‐assisted cell sorting (FACS) and analyzed their transcriptomic profiles using microarray analysis. A list of candidate genes, mainly transcription factors and cell membrane molecules, was complied from this analysis and validated by both quantitative RT‐PCR and immunofluorescence. Pax6 and Kir4.1 were found to be highly expressed in astrocytes, consistent with previous findings. More importantly, Kir4.1 mRNA was noted to be selectively enriched in cortical tdTomato+/GFP+ cells, compared to GFP+ only cells. It suggests Kir4.1 may serve as an astroglial subtype marker.
Conclusions: Our data clearly suggest the existence of molecular subtypes of astrocytes.
T05‐11A. The RNA helicase DDX5 binds MBP mRNA and regulates MBP expression on a posttranscriptional level
P. Hoch‐Kraft, C. Gonsior, R. White, E.‐M. Kraemer‐Albers, J. Trotter
Johannes Gutenberg‐University, Mainz, Germany
Myelin Basic Protein (MBP) is one of the major components of the myelin sheath and has been shown to be essential for the proper assembly of compact myelin. Consequently, animal models, lacking MBP, like the shiverer mutant mouse, show a severe CNS dysmyelination phenotype. The regulation of MBP synthesis is thereby an important step during the maturation of oligodendrocyte progenitor cells (OPCs) to myelinating oligodendrocytes (OL). In the polarized OL, MBP mRNA is translocated to the myelin compartment and its translation en route is precisely regulated in time and space mediated by the hnRNP A2‐dependent RNA‐trafficking pathway. hnRNP A2 binds the A2 response element (A2RE) in the MBP 3′UTR and recruits RNP complexes that mediate the fate of the mRNA, according to extracellular stimuli. This results in the localised translation of MBP at the axonal contact site. Analysis of proteins co‐precipitating with hnRNP A2 yielded the RNA binding protein Dead Box RNA Helicase 5 (DDX5), as a novel potential component of MBPmRNA‐associated protein complexes. Our observations show that DDX5 is associated with MBP mRNA and is capable of regulating the levels of MBP Protein on a posttranscriptional level. In line with its decreasing expression levels during OL development in vitro, DDX5 may act as an inhibitor of MBP expression and subsequently initiation of OL myelination or remyelination in the adult brain.
T05‐11B. The transcription factor C/EBPδ participates in the pro‐inflammatory and neurotoxic response of activated glia. In vitro and in vivo evidence
T. Valente1,2, M. Straccia1,2, N. Gresa‐Arribas1,2, G. Dentesano1,2, J.M. Tusell2, J. Serratosa2, C. Solà2, J. Saura 1
1University of Barcelona, IDIBAPS, School of Medicine, Barcelona, Spain 2CSIC, IDIBAPS, IIBB, Barcelona, Spain
CCAAT/enhancer binding protein δ (C/EBPδ) is a b‐zip transcription factor expressed by activated astrocytes and microglia. Studies with non‐glial cells have shown that C/EBPδ is able to regulate the expression of proinflammatory genes. However, the role of C/EBPδ in neuroinflammation has not been established. We have here analyzed the effects of C/EBPδ absence on glial activation in vitro and in vivo. In primary mixed glial and microglial‐enriched cultures the expression of the pro‐inflammatory genes NOS‐2, COX‐2 and IL‐6 induced by LPS+IFNγ, but not by LPS alone, was attenuated in the absence of C/EBPδ. ChIP experiments revealed binding of C/EBPδ to the promoters of these genes in activated, but not in control, mixed glial cultures. In neuronal‐microglial co‐cultures the neurotoxicity elicited by LPS+IFNγ‐activated microglia was completely abolished when microglial cells were devoid of C/EBPδ suggesting that this transcription factor plays a key regulatory role of the expression of potentially detrimental proinflammatory genes by activated microglia.
To analyze the role of C/EBPδ in neuroinflammation in vivo mice were treated systemically with LPS (100 ug/mouse; i.p.). This treatment induced a marked increase in C/EBPδ mRNA and protein brain levels. Double immunofluorescence experiments showed the presence of C/EBPδ in astrocytes and microglia in LPS‐treated mouse brain. In C/EBPδ ‐/‐ mice, systemic LPS‐induced brain expression of NOS‐2, TNF‐α, IL‐1β and IL‐6 was attenuated. Finally, in mouse experimental autoimmune encephalitis (EAE) C/EBPδ was upregulated in the spinal cord and the severity of EAE symptoms was reduced in C/EBPδ ‐/‐ mice. Altogether these findings demonstrate that C/EBPδ plays a major role in the regulation of proinflammatory gene expression in neuroinflammation and point to microglial C/EBPδ inhibition as a novel strategy to reduce the detrimental effects of neuroinflammation. Supported by PI10/378 and PI12/709 from Instituto de Salud Carlos III, Spain
T05‐12A. Promises and pitfalls of Pannexin1 transgenic mice
R. Hanstein1, H. Negoro 2, N. Patel1, A. Charollais 3, P. Meda 3, D.C. Spray1, S. Suadicani1, E. Scemes
1Albert Einstein College of Medicine, New York, United States 2Albert Einstein College of Medicine, Urology, New York, United States 3University of Geneva, Cell Physiology and Metabolism, Geneva, Switzerland
Gene targeting strategies have become a powerful technology for elucidating mammalian gene function. The recently generated knockout (KO)‐first strategy produces a knockout at the RNA processing level and also allows for the generation of conditional KO alleles by combining FLP/FRT and Cre/loxP systems, thereby providing high flexibility in gene manipulation. However, this multipurpose KO‐first cassette might produce hypomorphic rather than complete knockouts if the RNA processing module is bypassed. Moreover, the generation of a conditional phenotype is also dependent on specific activity of Cre recombinase. Here, we report the use of an efficient molecular biological approach to test pannexin1 (Panx1) mRNA expression in global and conditional Panx1 KO mice derived from the KO‐first mouse line, Panx1 tm1a(KOMP)Wtsi. Using qRT‐PCR, we demonstrate that tissues from wild‐type mice show a range of Panx1 mRNA expression levels, with highest expression in trigeminal ganglia, bladder and spleen. Unexpectedly, we found that in mice homozygous for the KO‐first allele, Panx1 mRNA expression is not abolished but reduced by 70% compared to that of wild‐type tissues. Thus, Panx1 KO‐first mice present a hypomorphic phenotype. Crosses of Panx1 KO‐first with FLP deleter mice generated Panx1 f/f mice. Further crosses of the later mice with mGFAP‐Cre or NFH‐Cre mice were used to generate astrocyte‐ and neuronal‐specific Panx1 deletions, respectively. A high incidence of ectopic Cre expression was found in offspring of both types of conditional Panx1 KO mice. Our study demonstrates that Panx1 expression levels in the global and conditional Panx1 KO mice derived from KO‐first mouse lines must be carefully characterized to ensure modulation of Panx1 gene expression. The precise quantitation of Panx1 expression and its relation to function is expected to provide a foundation for future efforts aimed at deciphering the role of Panx1 under physiological and pathological conditions.
POSTER TOPIC 06 Glial‐neuronal interactions
T06‐01A. Novel conditional GCaMP3 mouse lines for imaging Ca2+ signals in astrocytes
A. Agarwal, E. Hughes, D. Bergles
Johns Hopkins University, Baltimore, United States
Astrocytes elevate intracellular Ca2+ in response to stimulation of metabotropic receptors. These Ca2+ transients are linked to processes as diverse as synaptic plasticity and functional hyperemia, but the relevance of this signaling remains uncertain. Although most studies of Ca2+ signaling in astrocytes have focused on somatic events, anatomical and functional studies indicate that Ca2+ signaling in astrocytes may be compartmentalized into functionally isolated “microdomains.” To facilitate analysis of Ca2+ signaling, we generated transgenic mice in which the cytosolic and membrane anchored variant of genetically encoded calcium indicator GCaMP3, referred as cGCaMP3 and mGCaMP3 respectively, can be expressed conditionally in a cell specific manner. A ubiquitous CAG promoter is used to control cGCaMP3 and mGCaMP3 expression, and a “stopper” sequence flanked by loxP sites was placed upstream of the coding sequence, preventing expression until Cre excises this DNA. These constructs were targeted to ROSA26 locus to ensure widespread expression. To evaluate whether cGCaMP3 and mGCaMP3 were expressed at levels sufficient to resolve Ca2+ transients, we bred R26‐lsl‐cGCaMP3 and R26‐lsl‐mGCaMP3 mice to GLAST‐CreER mice. Double transgenic offspring were injected with tamoxifen at 3 weeks of age and analyzed 2–3 weeks later. Histology revealed that cGCaMP3 and mGCaMP3 were expressed in astrocytes throughout the brain. Astrocytes in acute cortical slices exhibited large amplitude, spontaneous increases in both cGCaMP3 and mGCaMP3 fluorescence. The signal to noise ratio of the fluorescence intensity was greatly improved in astyrocytes expressing mGCaMP3, partially due to the ability of mGCaMP3 to better resolve near membrane Ca2+ flux. Ca2+ transients were typically restricted to small regions of an individual astrocyte, consistent with Ca2+ elevations within microdomains. Spontaneous Ca2+ transients were occasionally observed in the soma of astrocytes expressing cGCaMP3 but not mGCaMP3, and were not coincident with activity in the processes, suggesting that these regions are functionally uncoupled. To determine if GCaMP3 expression is sufficient to visualize Ca2+ signaling in vivo, we made cranial windows over the somatosensory cortex and performed 2‐photon imaging. Microdomain Ca2+ transients were prominent in cortical astrocytes, indicating that these mice provide new opportunities for understanding the significance of this form of signaling.
T06‐01B. Activated Satellite Glial Cells Induce Peripheral Sensitization by Increasing Glutamate Concentration in the Trigeminal Ganglion
J.C. Laursen 1, X.‐D. Dong2, B.E. Cairns2,1, U. Kumar2, R. Somvanshi2, L. Arendt‐Nielsen1, P. Gazerani1
1Aalborg University, Department of Health Science and Technology, Aalborg, Denmark 2University of British Columbia, Pharmaceutical sciences, Vancouver, Canada
Question: Glutamate (Glu) is the principal excitatory transmitter found in trigeminal afferent fibres. In the trigeminal ganglion (TG), satellite glial cells (SGCs) surround neurons and are able to release substances that may affect sensory transmission through the TG and contribute to mechanisms underlying craniofacial pain. The aims of this study were 1) to investigate whether SGCs could be provoked to release Glu in vitro and 2) to study the in vivo response properties of trigeminal afferent fibres upon artificial elevation of Glu concentration in the TG.
Methods: Confocal microscopy was used to assess Glu content in and the expression of excitatory amino acid transporter 1 (EAAT1) and EAAT2 by SGCs of the TG. Glu release was investigated in vitro, where SGCs were isolated from the TG of 9 adult male Sprague‐Dawley (SD) rats and treated with control medium or medium containing 10 mM KCl together with 0, 0.1, 1, 10 µM of the EAAT1/2 inhibitor TFB‐TBOA. In vivo electrophysiology experiments were conducted in 6 additional anaesthetized adult male SD rats to determine the effect of repeated intraganglionic injections of Glu (500 mM, 3 µl × 2, 30 min apart) on the response properties of 6 TG neurons that innervated either the temporalis or masseter muscle. Cumulative afferent discharge was calculated as the sum of action potentials (AP) 10 min post injection subtracted from the sum of AP 10 min prior to injection. An electronic von‐Frey hair was used to measure afferent mechanical threshold (MT) at 1 min intervals for 10 min prior to the first and again 20 min after the second microinjection of Glu.
Results: Most SGCs were found to be Glu positive and express EAAT1 and EAAT2. Treatment with 10 mM KCl resulted in a ∼2‐fold increase in Glu concentration, compared to control (10.6 ± 1.1 vs. 4.8 ± 0.1 µM, respectively; p = 0.038). Treatment with 1 µM TFB‐TBOA significantly reduced the Glu concentration to 5.8 ± 1.4 µM (p = 0.047), which was further lowered to 3.0 ± 0.8 µM when 10 µM was applied (p = 0.001). Cumulative afferent discharge evoked in TG neurons by the second injection of Glu (20 ± 9 AP) was not significantly different from that evoked by the initial injection (21 ± 10 AP; p = 0.92). Glu injections significantly reduced muscle afferent MT (baseline: 35 ± 19 g) by 30 ± 3% (p = 0.03).
Conclusion: These results indicate that Glu can be released from SGCs through EAATs, and that increased Glu concentrations in the TG excite neurons and induce a state of peripheral sensitization. This may hypothetically be a mechanism, whereby activated TG SGCs could contribute to e.g. migraine headache.
T06‐02A. Lactate modifies neuronal excitability through both NMDA and KATP receptors: importance for plasticity genes expression
I. Allaman 1, J. Yang1, J.‐M. Petit1,2, G. Grenningloh1, E. Ruchti1, P. Jourdain1, P. Magistretti1,2
1EPFL, Lausanne, Switzerland 2Centre de Neuroscience Psychiatriques, Département de Psychiatrie, Prilly/Lausanne, Switzerland
Release of glycogen‐derived L‐lactate from astrocytes has been shown to play an important role in the establishment of long term memory (Suzuki et al, Cell, 2011). In an accompanying poster, we present data demonstrating that in cultured neurons L‐lactate induces plasticity‐related genes expression through a NMDA‐dependent mechanism (i.e. Arc, Zif268 and c‐Fos). Here, we describe the effect of L‐lactate on neuronal excitability by performing electrophysiological recordings of cultured cortical neurons. We observed that application of L‐lactate (10 mM) triggers an inward current with an amplitude of −0.55 +/‐ 0.1 nA and slow kinetics, with a peak reached at 189 +/‐ 55s (called Ilac). The membrane current decreases gradually to reach a plateau, with a mean inward current of −0.13 +/‐ 0.04 nA persisting up to 780s (13 minutes) after addition of L‐lactate (called Ipyr). In order to assess L‐lactate specificity on the generation of these currents L‐pyruvate, another monocarboxylate, as well as D‐lactate, the non‐metabolized enantiomer of L‐lactate) were tested. In contrast to L‐lactate, L‐pyruvate (10 mM) induced a low‐amplitude sustained current (−0.07 +/‐ 0.03 nA) similar to the late‐phase slow current evoked by L‐lactate (i.e Ipyr), but no current similar to Ilac. D‐lactate on its side did not elicit any specific current. Pharmacological characterization of Ilac and Ipyr currents demonstrate that Ilac is generated by NMDA receptors (as it is blocked by MK801) and relies on active NMDA receptors (as it is blocked by either glutamate or glycine binding sites inhibitors). In contrast, generation of Ipyr by both L‐lactate and L‐pyruvate is prevented by diazoxide, a KATP opener, whereas Ilac was unaffected by the treatment. In order to determine the importance of KATP for plasticity‐related gene expression, the KATP closer glibenclamide (200 uM) was applied to neurons and mRNA gene expression of Arc and Zif268 quantified. Results obtained demonstrate that Arc and Zif268 mRNA expression are unaffected by glibenclamide. As a whole this set of data demonstrates that L‐lactate generates two types of inward currents in neurons i.e. NMDA‐dependent (Ilac) or KATP‐dependent (Ipyr). Moreover, they highlight the NMDA‐dependent Ilac current as the key mediator, in contrast to KATP, of L‐lactate‐induced plasticity gene expression. This is further sustained by the observation that L‐pyruvate does not reproduce the effect of L‐lactate on gene expression (see accompanying poster). This set of results provides novel insights into the mechanisms of action of L‐lactate as an inducer of plasticity gene expression, and an important signaling molecule for neuronal plasticity.
T06‐02B. Roles of Neuregulin 1 Type III in the Ex Vivo Generation of Fate Committed Schwann cells from Bone Marrow Stromal Cells
H.Y. Leung, A.Y.P. Tsui, G.K.H. Shea, E.W.Y. Tai, Y. Chan, D.K.Y. Shum
The University of Hong Kong, Hong Kong
Schwann cell transplantation accelerates functional recovery from nerve injury by promoting axonal regrowth and remyelination. Our group had generated fate committed Schwann cells from bone marrow stromal cells (Shea et. al, 2010). It was also demonstrated that co‐culture with dorsal root ganglia (DRG, E14/15 rats) neurons was essential for the fate commitment of bone marrow derived Schwann cell‐like cells (SCLCs) (Shea et. al, 2010). Signals from the DRG neurons are therefore thought to direct the switch of SCLCs to fate committed Schwann cells.
The reliance on DRG neuron co‐culture for SCLCs fate commitment stands as a major hurdle for the therapeutic application of this finding. Thus, we aim to unravel the mechanisms underlying SCLCs fate commitment in order to develop a DRG neuron‐free condition for generating fate committed Schwann cells.
We hypothesised that the switch is brought about by the membrane bound neuregulin (NRG), NRG 1 Type III, but not the other soluble isoforms of neuregulin. As our results show that SCLCs treated with soluble neuregulin did not show significant changes in morphology nor marker expression when compared with untreated SCLCs. NRG 1 Type III expression was observed on purified DRG neurons by immunocytochemistry, Western blot analysis as well as reverse transcription PCR for the mRNA. Mammalian expression constructs for NRG 1 Type III were made by transfection into human embryonic kidney cells, HEK 293T, and mouse embryonic fibroblasts (MEF) with the aim of generating surrogate cell types for co‐culture with SCLCs to pursue cell‐specific effects of NRG 1 Type III on differentiation of SCLCs. The findings promise a way for generating fate committed Schwann cells for autologous transplantation for recovery after nerve injury.
T06‐03A. Contribution of different carbonic anhydrase isforms to proton dynamics in mouse cerebellar glial cells and neurons
M.D. Alt, J.W. Deitmer
TU Kaiserslautern, Kaiserslautern, Germany
Cell survival and proper enzyme function require a strictly regulated intracellular pH. The intracellular buffering of protons is accomplished by (1) intrinsic buffers and (2) the CO2/HCO3 ‐ buffer system. The latter being an open buffer system, because biomembranes are usually permeable to CO2, and, in addition, most cells express HCO3 ‐ transporting membrane proteins. The enzyme family of carbonic anhydrases (CAs) catalyses the reversible reaction from CO2 and H2O to HCO3 ‐ and H+, and thereby modulates the intracellular H+ buffer dynamics.We have performed calibrated in situ live‐cell imaging with the proton‐sensitive fluophore BCECF in glial cells and neurons of acute cerebellar slices of wild‐type and knockout mice. The studies were used to quantify the intracellular buffer capacity and to dissect the contribution of the intracellular CAII and the extracelluar CAIV by comparing intracellular H+ shifts in glial cells and neurons from wild‐type mice and mice deficient in their CAII or CAIV gene. We found that only one proton in 400000 is unbound and thereby chemically active, and that about 50% of this buffer capacity is mediated by the CO2/HCO3 ‐ buffer system and the other 50% by intrinsic buffers. The rate of CO2‐induced change in intracellular H+ concentration was increased by intracellular CAII in both glial cells and neurons. The extracellular CAIV on the other hand affected primarily neurons, but also showed unexpected intracellular activity (Schneider et al. 2013, PNAS 110). The rates of acidification and alkalinisation in wild‐type and knockout mice were significantly reduced in the presence of the CA blocker 6‐Ethoxy‐2‐benzothiazolsulfonamid (10 µM). We confirmed CA protein expression by Western blot and could also show that the expression of the remaining CA is not upregulated. These results provide new insights into cellular proton‐coupled processes in acute neural tissue, which shows some new complex roles of carbonic anhydrases in shaping proton dynamics in cells and tissues.
Supported by the Deutsche Forschungsgemeinschaft De 231/24‐1.
T06‐03B. TGF‐β1‐Induced Astrocytic Release of Interleukin‐6: A Possible Role in Epielsptogenesis
N. Levy 1, D. Milikovsky2, G. Baranuskas2, Y. David2, M. Ketzef2, S. Abutbul3, I. Weissberg2, I. Fleidervish2, A. Friedman2, A. Monsonego3
1Ben‐Gurion University, Beer‐Sheva, Moshav Benaya, Israel 2Ben‐Gurion University, The Dept. of Physiology and Neurobiology, Faculty of Health Sciences, The Zlotowski Center for Neuroscience, Beer‐Sheva, Israel 3Ben‐Gurion University, The Shraga Segal Dept. of Microbiology and Immunology, Faculty of Health Sciences and The National Institute of Biotechnology in the Negev, Beer‐Sheva, Israel
Objective: It is becoming increasingly evident that inflammatory mechanisms promote neuronal hyper‐synchronization and epileptogenesis following brain insults. Our recent studies identified serum albumin as a key player in the inflammatory cascade leading to epilepsy, at least partially through the activation of TGF‐β signaling pathway in astrocytes. In this study we set out to explore the mechanisms by which TGF‐β1 induces glial activation and epileptogenesis.
Methods: Primary cultures of microglia and astrocytes were treated with TGF‐β1 and gene expression analysis along with protein quantification were performed by quantitative PCR and ELISA. Electrophysiological response to IL‐6 was obtained in acute brain slices (field potential recordings) and in‐vivo (sub‐dural corticoencephalography) following to a continuous administration of IL‐6 into the lateral ventricle for 7 days.
Results: In glia cultures TGF‐β1 induced early and rapid up‐regulation of IL‐6 at both mRNA and protein levels whereas the upregulation of other pro‐inflammatory cytokines such as IL‐1β and TNF‐α was milder and at a later time point. Notably, SMAD2/3‐dependent TGF‐β1 signaling induced the expression of IL‐6 primarily in astrocytes and to a significantly lesser extent in microglia. IL‐6 was sufficient to induce epileptiform activity in acute brain slices and recurrent seizures within 3–4 days a following continuous intracerebroventricular injection of IL‐6.
Conclusions: We thus suggest astrocytic release of IL‐6 as a potential key mechanism in epileptogenesis.
T06‐04A. Astrocytes in the striatum act as a reservoir of L‐DOPA but less convert to dopamine
M. Asanuma, I. Miyazaki
Okayama Univ., Dept. of Brain Science, Okayama, Japan
Dopamine transporter (DAT) is expressed not only in catecholaminergic neurons but also in astrocytes. We previously showed that repeated L‐DOPA treatment markedly induced expression of DAT and apparent dopamine (DA)‐immunoreactivity in the reactive astrocytes in the striatum of animal models of Parkinson's disease. It is thought that astrocytes can uptake both L‐DOPA and DA via neutral amino acid transporter LAT and DAT, respectively. Therefore, uptake and metabolism of L‐DOPA and DA in the striatal astrocytes may influence their availability in dopaminergic system of parkinsonian patients. To clarify uptake and metabolism of L‐DOPA and DA in striatal astrocytes, the contents of L‐DOPA, DA and their metabolites were measured after the L‐DOPA/DA treatment using primary cultured astrocytes. First, we revealed the expression of neutral amino acid transporter LAT and aromatic amino acid decarboxylase (AADC) in the striatal astrocytes. The level of L‐DOPA in astrocytes was markedly increased after 4‐hr L‐DOPA exposure, but DA was not detected in the astrocytes 4 or 8 hr after the treatment. On the other hand, the DA treatment for 4 hr increased levels of DA and its metabolites, especially DOPAC. These results indicate that uptaken DA into astrocytes is rapidly metabolized and that uptaken L‐DOPA never be converted to DA in astrocytes. Furthermore, level of uptaken L‐DOPA in cultured striatal astrocytes was rapidly decreased after removing extracellular L‐DOPA. Taken together, the present results suggest that striatal astrocytes act as a reservoir of L‐DOPA to uptake or release L‐DOPA depending on the extracellular L‐DOPA concentration, but have less ability to convert L‐DOPA to dopamine.
T06‐04B. The role of glial cells in nutrient homeostasis of the Drosophila brain
S. Limmer, C. Klämbt
Universität Münster, Münster, Germany
Neuronal function consumes a large amount of energy and thus the brain requires a constant but regulated supply of metabolites. In invertebrates, such as Drosophila, the nervous system is floating in the hemolymph and all metabolites that enter the brain have to be transported across the blood brain barrier (BBB). As in primitive vertebrates, the Drosophila BBB is generated by glia. A layer of so‐called subperineurial glial cells completely encapsulates the nervous system and thus, all metabolites reach the neurons en route through glia.
The main energy supply of the inner organs of Drosophila is provided by trehalose that is found in high concentrations in the hemolymph. Trehalose is a nonreducing disaccharide, in which two D‐glucose units are linked via a a,a‐1,1‐glycosidic bond. The uptake of trehalose is mediated by two trehalose transporters, which are members of the SLC2A gene family. The subperineurial glial cells then either secrete C6 sugars to the neurons – or alternatively supply energy to neurons by secreting C3 metabolites as described for the bee retina. To discriminate between these possibilities we have followed a number of different approaches. The relevance of trehalose transporters in the different cell types has been determined by mutant analysis and RNAi studies. Furthermore, we have studied the role of all other putative sugar and monocarboxylate transporters in glial cells by RNAi knockdown. To determine whether C6 or C3 carbohydrates are secreted by glial cells we suppressed the expression of core enzymes regulating glycolysis or components of the respiratory chain in either glial cells or neurons. Moreover, we have ablated mitochondria specifically in glia or in neurons through expression of a restriction enzyme targeted to mitochondria. We will summarize our results and discuss approaches towards identifying neuronal signals that control glial carbohydrate secretion during energy homeostasis of the brain.
T06‐05A. Axon Initial Segment Associated Microglia
K. Baalman, M.N. Rasband
Baylor College of Medicine, Houston, United States
Microglia have long been known to respond to virtually any insult to the central nervous system. They quickly migrate to the site of the insult, and play many important roles, such as barricading the injury site, phagocytosing debris, and releasing cytokines. The role of microglia in the normal brain, however, has only recently begun to be appreciated. With the advent of in vivo imaging, microglia have been shown to be constantly surveying their environment with rapid extensions and retractions of their processes. Subsequent studies have shown that these ‘resting' microglia (now known as ‘surveying microglia') are indeed active throughout development and adulthood. During development, microglia have been shown to be involved in synaptic monitoring and pruning as well as synaptic stripping after injury. Thus far, many studies have focused on the role of microglia at the synapse. However, we report here, for the first time, that in the cortex of normal adult rodents, a small percentage of microglia are specifically associated with the axon initial segment (AIS). The AIS is characterized by a high density of voltage‐gated ion channels and plays an important role in initiation of the action potential and maintenance of neuronal polarity. This interaction seems to be limited to the ‘surveying' phenotype of microglia and much less frequent in ‘activated' microglia. This overlap of processes appears early in development and continues throughout adulthood although the function of microglia at the AIS is still currently unknown.
T06‐05B. Perturbation of sleep‐wake cycle affects astroglial networking
X. Liu 1, J.‐M. Petit2,3, P.J. Magistretti2,3, C. Giaume1
1CIRB, Collège de France, Paris, France 2LNDC, Brain Mind Institute, EPFL, Lausanne, Switzerland 3Centre de Neurosciences Psychiatriques, CHUV, Prilly, Switzerland
Astrocytes act as modulators of neuronal activity through mechanisms such as neurotransmitter uptake, ‘gliotransmitters' release, and metabolic supply. Recently, astrocytes were shown to modulate sleep by vesicular release of ATP. Besides the cellular mechanism, we are interested in the network behavior of astrocytes in sleep‐wake cycle. Indeed, astrocytes form networks of communicating cells via gap junction channels constituted by connexins (Cxs). Recently, we have demonstrated that astroglial networking is regulated by neuronal activity and that neuronal activity is affected by deletion of the two main astroglial Cxs (Cx43, Cx30). To investigate how astroglial networks behave in sleep‐wake cycle, we used pharmacological treatments and sleep deprivation to manipulate sleep‐wake status of mice and determined whether astroglial networks would change in response. Astroglial networks in acute cortical slices were revealed by “dye coupling”, i.e. by loading of a patch‐clamped astrocyte with sulforhodamine B that diffuses into neighboring astrocytes through gap junction channels. Also, Cxs expression at mRNA and protein levels was measured as an index of connections among astrocytes. The results are as follows. First, I.P. injection of modafinil, a potent wakefulness‐promoting drug, increased both mRNA and protein expression of Cx 30 in the mouse cortex. Superfusion of modafinil also enhanced dye coupling among astrocytes in acute coronal slices of the mouse somatosensory cortex. This effect was abolished by TTX treatment, which demonstrates neuronal activity is necessary for the effect of modafinil. In contrast, γ‐Hydroxybutyric acid (GHB), a sleep‐promoting agent, and propofol, a general anesthetic, decreased astroglial coupling in cortical slices. Interestingly, the effect of GHB was not affected by TTX, suggesting a different pathway of action from modafinil. Next, we used a “gentle” sleep deprivation model to sleep deprive mice for 6 hours from the onset of the light period. As a result, mRNA of Cx30, but not Cx43, was increased in both the cortex and hippocampus. Finally, dye coupling in cortical astrocytes was enhanced in mice after sleep deprivation compared to mice after normal sleep. This increase in dye coupling, however, was not observed in slices from Cx30 knock out mice after the same sleep deprivation treatment. Altogether these results indicate that astroglial networks are bidirectionally regulated by perturbations in sleep‐wake cycle and that Cx 30 is the major Cx sensitive to such perturbations.
T06‐06A. Postnatal down‐regulation of the γ2 subunit of GABAA receptors in NG2 cells precedes synaptic‐to‐extrasynaptic change in GABAergic transmission mode
M. Balia 1, M. Vélez‐Fort1, S. Passlick2, C. Schäfer2, E. Audinat1, C. Steinhäuser1, G. Seifert2, M.C. Angulo1
1INSERM U603, Paris, France: 2University of Bonn, Institute of Cellular Neurosciences, Bonn, Germany
In neurons, synaptic and extrasynaptic GABAA receptors (GABAARs) differ in their subunit composition, conferring them distinct functional and pharmacological properties. Oligodendrocyte precursor cells, also called NG2 cells, are contacted by bona fide neuronal GABAergic synapses. However, we recently showed a synaptic to extrasynaptic switch in the mode of transmission between GABAergic interneurons and NG2 cells during postnatal development of the somatosensory cortex. We therefore hypothesized that the postnatal switch of GABAergic transmission in NG2 cells is accompanied by changes in the expression of GABAAR subunits. To test for this hypothesis, we stimulated neuronal fibers to evoke GABAAR‐mediated responses in NG2 cells recorded in acute slices of the somatosensory cortex of NG2‐DsRed transgenic mice. The effect of zolpidem and α5IA on evoked GABAergic responses reveals the predominance of functional α1‐ and α5‐containing GABAA receptors, respectively, at interneuron‐NG2 cell synapses early in development. However, the expression level of α5 decreases when responses rely exclusively in extrasynaptic transmission at more mature developmental stages. More importantly, specific pharmacology for the γ2 subunit, a crucial molecular component for the clustering of GABAA receptors at postsynaptic sites, demonstrated a down‐regulation of this subunit in NG2 cells prior to the complete loss of GABAergic synaptic activity. In keeping with the synaptic nature of the γ2 subunit in neurons, this molecular change in NG2 cells correlates with the switch from synaptic to extrasynaptic transmission. To corroborate these functional data, we performed single cell multiplex RT‐PCR of α1–5, β1–3, γ1–3 and δ subunit mRNAs in NG2 cells of the second and fourth postnatal weeks. Despite the large heterogeneity of subunit mRNA expression at both developmental stages, we found that the number of cells expressing γ2 mRNAs dramatically decreases in the fourth postnatal week. In conclusion, the expression loss of the γ2 subunit is an important molecular determinant impacting the change of transmission modes between interneurons and NG2 cells during cortical development.
Financial support: FRC, ANR blanche, ARSEP, DFG (SFB/TR3) and EU (FP7‐202167 Neuroglia).
T06‐06B. Alterations in Microglial Behavior During Ocular Dominance Plasticity
R. Lowery, C. Lamantia, A. Majewska
University of Rochester, Medical Center, Rochester, United States
Synaptic plasticity is a critical process throughout the lifespan for achieving and maintaining normal and efficient operation of the nervous system. While much is known about the functional and structural changes that occur at the synapse during synaptic plasticity, the mechanisms implementing these changes are poorly understood. Despite being classically characterized as immune cells, microglia have recently been shown to play a role in normal brain function by restructuring and removing synapses. Given the novelty of this role, few details are known about how microglia behave to implement such a role during periods of plasticity. To characterize the changes in microglial behavior during ocular dominance plasticity in the developing visual cortex, we examined microglial morphology in fixed brain sections as well as in vivo using two‐photon microscopy. For analysis of microglia in fixed sections, C57/Bl6 mice were monocularly deprived for either 12 hours, 1, 2, 4, or 7 days during the visual critical period (P23‐P30). Fixed coronal sections were stained with Iba1, a specific marker for microglia, imaged on a confocal microscope and analyzed using ImageJ. Microglial density decreased rapidly (within 12 hours) and remained low for the 7 day deprivation period. Characterization of microglial morphology revealed an increase in the complexity of the microglial process arbor after 12 hours, which steadily decreased back to baseline by 7 days. This suggests that microglia do undergo a rapid change in morphology during plastic events that is reminiscent but distinct from the traditional activation pattern observed during pathological events. For analysis of microglia in vivo, heterozygous Cx3Cr1/GFP (Jung 2000) mice were monocularly deprived directly preceding the first imaging session, the binocular segment of primary visual cortex was imaged, and the same cortical area was re‐imaged 2 and 4 days later. While no change in microglial density was observed, analysis of microglial process motility showed a decrease in motility after periods of monocular deprivation. These results, combined with the observed morphological changes, show that microglia undergo behavioral changes after a period of monocular deprivation that are inconsistent with pathological activation, suggesting that microglia take on different roles and activities during normal brain function.
T06‐07A. Role of neuronal injury in the control of microglia reactivity exerted by GDNF
G. Baltazar, J. Oliveira, T. Roxo, C. Fonseca
Faculty of Health Sciences, CICS‐UBI, Covilhã, Portugal
Neuroinflammation is a pathological hallmark in patients and experimental models of Parkinson's disease. Both present the classical features of inflammation, with evidence of an uncontrolled process. Moreover, microglia may become activated early in the disease process and remain primed, responding strongly to subsequent stimuli, and thereby enhancing inflammation‐induced oxidative stress and cytokine‐dependent toxicity in vulnerable neuronal populations.
We have previously demonstrated that glial cell line‐derived neurotrophic factor (GDNF), released by ventral midbrain astrocyte cultures, potently prevents microglial activation induced by a pro‐inflammatory agent. Therefore, GDNF is an important mediator in the astrocyte‐microglia crosstalk keeping microglia in a resting state. However, in the brain, microglia and astrocytes are not alone and other cell types, e.g. neurons, may interfere and influence this astrocytic control of inflammation. Therefore, it is important to investigate if the presence of neurons alters GDNF‐mediated astrocytic control of microglial activity. In this work we aimed at investigating whether the presence of neurons, injured or not, changes the ability of astrocyte‐derived GDNF to prevent microglial activation. For that purpose, we used primary cultures of ventral midbrain astrocytes and microglia, as well as neuron‐astrocyte mixed cultures from the same brain region.
Neuron‐astrocyte cocultures were exposed to vehicle (control) or to the DA neurotoxin MPP+. The media conditioned by control and MPP+‐challenged neuron‐astrocyte mixed cultures, or by astrocyte cultures, was collected and transferred to ventral midbrain microglia cultures, which were then exposed to the pro‐inflammatory agent lipopolysaccharide. The effect of conditioned media obtained from the different cell culture systems on microglial activity was determined by analyzing the production of nitric oxide by the Griess reaction and of the phagocytic activity by determining the engulfment of fluorescent microspheres by fluorescence microscopy.
T06‐07B. Lactate modulates network activity in primary cortical neurons
L. Bozzo, J.‐Y. Chatton
University of Lausanne, Lausanne, Switzerland
Lactate is produced by astrocytes and used by neurons as metabolic substrate during activity. Recent evidence indicates that lactate may not only serve as energy substrate but also as modulator of glutamatergic or GABAergic neurotransmission. We tested this hypothesis using primary cultures of cortical neurons obtained from wild type and GAD67‐GFP knock‐in mice (C57BL/6). Neuronal excitability was monitored by electrophysiological recordings and calcium imaging using the fluorescent probe Fluo‐4 AM. Spontaneous action potentials and rhythmic calcium transients were present in more than 50% of cultured neurons, which were either GABAergic or glutamatergic cells. In the presence of 5mM glucose, L‐lactate application reversibly diminished calcium transient frequency in a concentration‐dependent manner in both cell types (glutamatergic neurons IC50 4.23 ± 1.9mM and GABAergic neurons IC50 4.18 ± 2.8mM). To test whether lactate effects were dependent on metabolism, we applied the closely related substrate pyruvate (5mM) or switched to different glucose concentrations (0.5 or 10mM). None of these conditions altered the calcium transient frequency. In contrast, the application of D‐lactate, thought not to be metabolized by neurons, decreased the spiking activity in the same concentration range as L‐lactate (IC50 4.58 ± 1.2mM). We determined that D‐lactate was taken up by neurons, however more than two‐fold less efficiently than L‐lactate. These results suggest that the mechanisms of lactate sensitivity are not purely metabolic. Since D‐lactate has the same potency as L‐lactate but it is less internalized, it is likely that lactate acts as an extracellular ligand that triggers the reduction of neuronal activity. This hypothesis is currently under investigation. This modulating effect of lactate represents a novel role for the compound that has to be taken into account in the context of the interactions between astrocytes and neurons.
T06‐08A. Volume coverage by microglial processes is reduced in the aging brain and occurs significantly earlier in mouse models of Alzheimer's disease
R. Baron, A. Nemirovsky, A. Monsonego
Ben‐Gurion University, Ber‐Sheva, Israel
Microglia integrate within the neural tissue with a distinct ramified morphology through which they scan the surrounding neuronal network, a process which appears to contribute to the integrity, maintenance and functioning of the brain. Since microglia are long‐lived, they are subjected to senescence processes which may severely compromise their function with age. Here we used a digital tool for the quantitative morphometric characterization of fine cortical microglia structures in mice. We thus followed the morphological changes microglia underwent with aging and with the progression of Alzheimer's‐like disease. Compared with microglia in young mice, microglia in old mice are less ramified and possess less branches and fine processes, a phenomenon which was associated with increased expression of pro‐inflammatory genes. Notably, a similar microglial pathology appeared 6–12 months earlier in mouse models of Alzheimer's disease (AD). We thus demonstrate that in addition to promoting neurotoxic inflammation, amyloid plaques attract the microglia and modify their structure. This, in turn, causes a severe microglial process deficiency, which possibly results in compromised neuronal function and repair.
T06‐08B. Estimation of glycolytic rates of single cells in vivo
P. Mächler 1, M. Wyss1, R. Guiterrez1,2, M. Zünd1, S. Lengacher3, P.J. Magistretti3, F. Barros2, B. Weber1
1University of Zurich, Institute of Pharmacology and Toxicology, Zurich, Switzerland 2CECS, Valdivia, Chile 3Brain Mind Institute, EPFL, Lausanne, Switzerland
Blood‐borne glucose is the main energy substrate for the brain under physiological conditions. However, the question remains unresolved if the two main cell types in the brain – neurons and astrocytes – consume glucose upon their individual needs or if glucose is mainly taken up by one cell group and glycolytically degraded to energy‐rich substrates (e.g. lactate), which are then shuttled to the other cell group. A novel genetically encoded FRET glucose sensor (Bittner et al., 2010, 2011) together with two‐photon microscopy now provides for the first time the opportunity to determine intracellular glucose concentrations in single cells in the intact organism.
Cortical microinjections of an adenoviral vector (AAV9 and AAV6) coding for the sensor FLII12Pglu600µΔ6 with the promoter sGFAP for astrocytes and synapsin for neurons were performed in mice and chronic windows were implanted above the somatosensory cortex. After two weeks, specific expression in astrocytes and neurons was observed.
The ratiometric FRET signal in both cell types increased after intraperitoneal glucose injection and decreased after insulin application demonstrating the functionality of the construct in vivo. The astrocytic sensor displayed a large linear range at blood glucose levels ranging from 3 to 20 mmol/l.
In a second set of experiments, we examined the dynamic behavior of cellular glucose concentrations upon increased brain activity. During electrical hindpaw stimulation 21% of examined astrocytes showed a 0.5% decrease of the FRET signal, whereas neuronal glucose levels did not exhibit activity‐dependent fluctuations.
To be able to draw conclusions about metabolic rates of substrate oxidation we recently started with experiments where cellular glucose uptake was transiently inhibited using different blockers of glucose transport. Drugs were applied systemically and intracortically. We could demonstrate this principle on first measurements on astrocytes.
Glucose FRET sensors allow measurements of the dynamics of glucose concentrations of single neurons and astrocytes in the intact organism. Measurements of cellular glucose transients during transport blockade hold the potential to compare glycolytic rates of neurons and astrocytes. This tool may substantially contribute to uncover the complex organization of brain energy metabolism.
T06‐09A. Synaptically evoked calcium signals in astrocytic processes enhance the stability of excitatory synapses
Y. Bernardinelli 1, E. Janett2, I. Nikonenko1, E.V. Jones3, C.E. Flores1, B. Boda1, K.K. Murai3, C. Bochet2, D. Muller1
1University of Geneva, Basic Neurosciences, Geneva, Switzerland 2University of Fribourg, Chemistry, Fribourg, Switzerland 3McGill University, Center for Research in Neurosciences, Montreal, Canada
Neuron‐glial communication in the CNS is fundamentally important for many brain processes including synaptic transmission and plasticity. Perisynaptic astrocytic processes (PAP) contact excitatory synapses, forming tripartite structures with neurons. In the hippocampus, the morphology of PAP has been shown to remodel rapidly and continuously but the mechanisms and roles of this form of structural plasticity remain unknown. This study investigated the physiological mechanisms driving PAP movements and their role during long‐term potentiation (LTP). PAP and spines contacts were labeled by viral gene delivery of farnesylated fluorescent proteins in hippocampal slice cultures and imaged by confocal microscopy. Electron‐microscopy of infected slices confirmed that PAP‐synapse morphology is preserved in organotypic cultures. PAP movements adjacent to dendritic spines were evaluated with an index of motility (MI). Increasing neuronal activity by Schaffer collaterals (SC) stimulation elevated PAP MI and this was prevented by both TTX and mGluR inhibition, suggesting that neuronal activity modulates PAP movements. We next investigated whether intracellular calcium (Ca2+ i) in astrocytes influenced PAP motility. BAPTA‐AM bulk‐loading specifically chelated Ca2+ i in astrocytes and reduced PAP movements. When exogenous Gq‐coupled receptors MrgA1 or MrgC11 were specifically targeted to astrocytes, their agonist FMRFa induced Ca2+ i increases and accelerated PAP motility. Moreover, FMRFa delivery at the synaptic level by two‐photons flash photolysis was sufficient to elevate PAP MI. We then investigated PAP dynamics in relation to LTP. Application of theta‐burst SC stimulation initially increased PAP motility, but then resulted 30min later in a decrease of PAP motility. Interestingly, the reduction of PAP movements correlated with both spine enlargement and increased PAP's spine coverage. To assess the consequences of these changes, we then specifically triggered elevation of PAP movements at the synaptic level by flash photolysis. Spine stability analysis 24 hour after uncaging revealed that spines in contact with activated PAPs were more stable than others. This study suggests that excitatory synapses control the motility of surrounding PAPs through the triggering of neurotransmitter‐evoked astrocytic Ca2+ i elevations. Increased PAP motility seems to be necessary to elevate their coverage of the synapse during LTP, leading to higher synapse stability.
T06‐09B. Astrocyte reactivity is associated with changes in the concentration of multiple striatal metabolites in situ
M.‐A. Carrillo‐de Sauvage, Y. Bramoulle, L. Ben Haim, F. Aubry, M. Guillermier, G. Auregan, J. Valette, C. Escartin
CEA, Fontenay‐aux‐Roses, France
Astrocyte reactivity occurs in response to many pathological brain situations. Reactive astrocytes display morphological and functional changes that could result in concentration variations of some brain metabolites localized both in astrocytes and in neurons. Such changes could be detected by magnetic resonance spectroscopy (MRS), allowing non‐invasive monitoring of astrocyte reactivity and thereby neuronal dysfunction in vivo.
One of the major peak detected by MRS in the brain corresponds to neuronal metabolite N‐Acetyl‐Aspartate (NAA). Although its function is unclear, it is considered as a biomarker for neurodegeneration and a decrease in its concentration is interpreted as neuronal death or dysfunction. However, there is not clear evidence that other cell types, specifically astroglia, could not contribute to change in NAA levels in the brain.
In this study, we used a rat model of astrocyte activation by stereotaxic injections of lentiviral vectors encoding for either the cytokine ciliary neurotrophic factor (lenti‐CNTF) into the right striatum or beta‐galactosidase (lenti‐LacZ) into the left striatum, as a control. MRS data were acquired on a 7T magnet from two voxels placed over each injected striatum. A diffusion‐weighted LASER sequence with echo time TE = 40 ms was used. Concentrations were measured using LCModel software for total N‐acetyl‐aspartate (NAA + NAAG), myo‐inositol (Ins), total choline (Cho), glutamate and taurine relative to total creatine.
Lenti‐CNTF injection promotes a sustained, extensive and selective activation of astrocytes, as evidenced by overexpression of GFAP and vimentin and cellular hypertrophy (Fig.1). Importantly, neurons displayed unaltered morphological, molecular and electrophysiological features, as described previously (Escartin et al., 2006; Beurrier et al., 2010). MRS evidences changes in metabolite concentration: in the lenti‐CNTF injected striatum, levels of the astrocyte metabolites Ins and Cho were increased, whereas levels of the neuronal metabolite NAA and glutamate were decreased. Increased levels of Ins and Cho are generally found associated with astrocyte reactivity, however, the decrease in NAA is unexpected given that neurons are not dysfunctional with CNTF. To understand how astrocyte reactivity can influence NAA levels, we are currently characterizing the molecular changes occurring in the NAA metabolism using quantitative PCR, biochemistry, histology and HPLC.
Our data suggest that reactive astrocytes alone result in alterations of metabolite concentrations detectable by NMR. Although the mechanisms by which activated astrocytes regulate neuronal metabolism remain to be elucidated, our work challenges the MRS dogma that decreased neuronal metabolites can be used as a biomarker for neurodegeneration.

T06‐10A. Histamine triggers microglial phagocytosis
L. Bernardino, T. Saraiva, A.C. Cristóvão, M. Esteves, G. Baltazar, S.M. Rocha
University of Beira Interior, Covilhã, Portugal
Microglial phagocytosis is a vital phenomenon for the clearance of damaged and death cells or infectious agents in a context of brain injury or infection, respectively. In addition, microglia can boost synaptic plasticity by the phagocytosis of axon terminals and dendritic spines. Therefore, it is crucial to better understand the mechanisms involved in microglia clearance in order to devise new strategies to promote an efficient brain repair. Recently, we showed that histamine modulates microglia motility and cytokines release. In this work, we aimed to investigate the role of this molecule and its receptors in microglia‐induced inflammation by evaluating microglial phagocytic activity and Reactive Oxygen Species (ROS) production. For that purpose, an IgG‐opsonized latex bead assay was performed in N9 murine microglial cell lines exposed to lipopolysaccharide (LPS) or histamine 1, 10 and 100 µM. We showed that histamine significantly stimulated phagocytosis of opsonized latex beads via H1 receptor activation. This effect was accompanied by the rearrangement of cytoskeleton analyzed by phaloidin and acetylated tubulin expression and cellular distribution. The phagocytic activity was significantly reduced after pretreatment with apocynin, a putative NAPDH oxidase (Nox) inhibitor. All Nox isoforms were expressed by microglial cells, but only the expression of Nox1 was increased by treatment of histamine. Rac1, an important subunit for the activation of Nox1, was also activated by histamine. In addition, histamine induced ROS production via H1 and H4 receptor activation. In conclusion, histamine plays an important role in the regulation of microglial phagocytosis, which is mediated by Nox1 and Rac1 activation.
T06‐10B. Alterations of Gray Matter Gap Junctions in Multiple Sclerosis and in EAE
K. Markoullis 1, I. Sargiannidou2, N. Schiza2, R. Reynolds3, K. A. Kleopa4
1The Cyprus Institute of Neurology and Genetics, Nicosia, Cyprus 2The Cyprus Institute of Neurology and Genetics, Neuroscience Laboratory, Nicosia, Cyprus 3Imperial College Faculty of Medicine, Wolfson Neuroscience Laboratories, London, United Kingdom 4The Cyprus Institute of Neurology and Genetics, Neuroscience Laboratory and Neurology Clinics, Nicosia, Cyprus
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system characterized by demyelination and axonal damage, involving both white (WM) and gray matter (GM) areas. Furthermore, cortical pathology correlates with MS progression and cognitive dysfunction. We previously found altered expression of gap junction (GJ) proteins connexin32 (Cx32) and Cx47 in oligodendrocytes and Cx43 in astrocytes in WM lesions and normal appearing WM (NAWM) in brain samples from MS patients, and in a mouse model of experimental autoimmune encephalomyelitis (EAE), implicating uncoupling of myelinating cells as an important aspect of MS pathology.
Here we studied Cx32 and Cx47 and their astrocytic partners Cx30 and Cx43 in and around cortical lesions and the normal appearing cortex (NACx) in MS samples and non‐MS controls. We examined their expression by real‐time PCR, quantitative immunoblot and immunohistochemichal analysis.
Compared to non‐MS controls, expression levels of Cx32 and Cx47 mRNA were reduced within lesions whereas Cx30 and Cx43 were increased. In NACx, all four connexins were upregulated with Cx47 being significantly increased. Immunoblot analysis of MS cortex revealed reduced Cx32 and increased Cx43 levels, while Cx30 and Cx47 showed no significant change. Immunohistochemical analysis showed severely reduced Cx47 GJ plaques within lesions with gradual increase toward NACx. In contrast to NAWM, Cx47 GJ plaques were not increased in NACx. Both astrocytic GJ proteins were increased in MS, but in distinct patterns: Cx30 GJ plaque counts were higher within lesions and perilesional areas. Cx43 was increased in MS cortex compared to non‐MS controls, but in contrast to NAWM, Cx43 GJ numbers were higher in NACx than in lesions. Similar alterations were found in the EAE mice, in which Cx32 and Cx47 GJ counts were reduced whereas Cx43 and Cx30 GJs were increased.
Our results indicate that oligodentrocytic as well as astrocytic GJs are affected not only in MS WM but also in the GM. Loss of oligodendrocyte GJs and in parallel increase of astrocytic GJs reflecting astrogliosis occurs throughout the MS brain and may negatively affect the prospects of repair and remyelination, worsening disease progression.
Acknowledgements: This project was funded by the Cyprus Research Promotion Foundation (Grants ACCESS/0308/11 and HEALTH/BIOS/0308/01 to KAK) and by Cyprus Telethon (2010‐14 grant to KAK). Post‐mortem human brain tissues were kindly provided by the UK Multiple Sclerosis Society Tissue Bank, Imperial College London, funded by the UK Multiple Sclerosis Society.
T06‐11A. Strongly reduced density of gray matter glutamine synthetase expressing astroglial cells in major depression but not bipolar disorder
H.‐G. Bernstein, G. Meyer‐Lotz, M. Walter, H. Dobrowolny, J. Steiner, B. Bogerts
University of Magdeburg, Magdeburg, Germany
Introduction: Glutamate and GABA are the chief excitatory and inhibitory neurotransmitters in the adult CNS. The mainly astroglia‐located enzyme glutamine synthetase is required to synthesize glutamine from the re‐uptake of either glutamate or GABA. Thereby, this enzyme significantly contributes to the control of brain glutamate and GABA levels. There is good evidence that both neurotransmitter systems are dysregulated in depression. Hence, we decided to study the cellular expression of this enzyme in subjects with major depression and bipolar disorder.
Material and Methods: We investigated the numerical density of glutamine synthetase immunoreactive astroglial cells in different brain areas (prefrontal cortex, anterior insula) of 14 subjects with major depression, 15 subjects with bipolar disorder and 19 matched control cases.
Results and Discussion: We found that compared with controls in cases with major depression there was a significant reduction of the density of immunostained glial cells in all brain regions studied, whereas bipolar cases showed a normal expression of the enzyme.
Our findings point to a profound disturbance of the glutamate‐glutamine‐GABA cycle in major depression, which is in good agreement with neuroimaging observations and molecular biologic data of others.
T06‐11B. Glutamine transport in perisynaptic astrocytes and presynaptic terminals supports glutamatergic transmission at the calyx of Held synapse
M.‐C. Marx, D. Billups, B. Billups
University of Cambridge, Cambridge, United Kingdom
Perisynaptic astrocytes are thought to play a key role in the recycling pathway of glutamate by supplying the precursor glutamine to the presynaptic terminal. Using fluorescent pHi measurements of astrocytes and direct patch‐clamp recordings from the calyx of Held synapse in rat brain slices, we have investigated the transport mechanisms that may mediate this release of glutamine from astrocytes in situ and assessed the role of glutamine in maintaining glutamatergic neurotransmission.
Glutamine transporter currents were elicited by puff application of 10 mM glutamine from a nearby pipette. For imaging astrocytic pHi, HPTS was included in the patch pipette. System A (SA) transporter function was probed using the substrate inhibitors MeAIB, alanine and serine, while system N (SN) function was isolated using its unique ability to transport Li+ in place of Na+. Histidine was used as a substrate inhibitor of both SA and SN.
Glutamine‐induced membrane currents Igln (−20.0 ± 2.4 pA, n = 21) were blocked by bath applied alanine (10 mM) and MeAIB (20 mM), as well as substitution of external Na+ with Li+. Fluorescent pHi measurement revealed an alkalinisation during puff application of glutamine. While bath application of histidine (20 mM) abolished the pH change, MeAIB and serine (20 mM) only attenuated it. Most of the pH change (63 ± 11%; n = 7) was insensitive to the substitution of Na+ with Li+.
In presynaptic terminals, puff application of glutamate did not induce a membrane current indicating a lack of direct glutamate uptake; however, puff application of glutamine resulted in a Igln which was eliminated by the removal of external Na+ and by MeAIB. Miniature EPSCs recorded from postsynaptic neurons (−38.8 ± 3.5 pA) were inhibited by MeAIB (−28.6 ± 3.4 pA; n = 5) following turnover of the presynaptic glutamate pool. Single EPSCs were unaffected by MeAIB indicating no direct effect on postsynaptic AMPA receptors. Together, these data identify two glutamine transport systems within astrocytes. Properties of the glial Igln indicate expression of SA while pH imaging reveals a non‐electrogenic glutamine transporter with properties identifying it as SN. SN is known to be readily reversible under physiological conditions and we propose that this mediates the astrocytic glutamine release mechanism within the glutamate‐glutamine cycle. Furthermore, system A mediates glutamine transport in glutamatergic nerve terminals and is important in maintaining the supply of glutamate for excitatory neurotransmission during periods of high frequency stimulation.
T06‐12A. Astrocytes uptake extracellular plasminogen and plasmin to control their levels
A. Briens, M. Schwalm, F. Docagne, D. Vivien
INSERM U919, Caen, France
Astrocytes are key players in the brain, cooperating with neurons to modulate crucial processes. In particular, they are able to uptake and release neurotransmitters and neuromodulators to control their synaptic level.
In our study, we hypothesize that astrocytes can regulate some effects of the serine protease plasmin in the CNS through a mechanism of uptake.
Conversion of the zymogen plasminogen to the serine protease plasmin by the activators (tPA or uPA) is the basis of fibrinolysis in the vasculature. But cerebral functions for the plasminogen activator/plasmin system have recently arisen. It has been shown that plasmin was essential to activate the Brain Derived Neurotrophic Factor (BDNF) in its mature form, promoting long‐term hippocampal plasticity. In Alzheimer's disease, plasmin plays a protective role by participating in beta‐Amyloid clearance. Plasmin can also lead to neuronal injury by degrading extra‐cellular matrix proteins. In animal models of multiple sclerosis, plasmin can also help removing intraparenchymal deposits of fibrin, thus limiting axonal damage. These data suggest that a system of regulation of plasmin effects in the brain is necessary.
In this study, we showed that astrocytes can actively and specifically uptake plasminogen and plasmin in a time‐dependent and a dose‐dependent manner. This mechanism involves clathrin pits formation in astrocytes and the lysine‐binding site of plasminogen/plasmin. Plasminogen and plasmin, following endocytosis are found in early endosomes, late endosomes, lysosomes and recycling endosomes. Finally, we noticed that astrocytes uptake plasmin more efficiently than plasminogen.
This study brings out a mechanism of glial uptake of plasminogen and plasmin. This could control their extra‐cellular levels and regulate their effects within the brain. Further investigations should determine whether this mechanism can modulate plasminogen activation and synaptic plasmin activity. Besides, plasminogen activators (tPA) and cerebral substrates of plasmin (pro‐BDNF) are also taken up by astrocytes. A link could thus exist between these processes to regulate the amount of synaptic mature BDNF. This would reveal a cross‐talk between neurons, releasing precursors in the synapse (pro‐BDNF and plasminogen) and astrocytes, regulating their activation and their clearance. Understanding and modulating plasmin uptake could be very important in pathological conditions where the plasminogen/plasmin system is involved, such as Alzheimer's disease, multiple sclerosis or stroke.
T06‐12B. Neuroprotective effects of serotonin 1A agonist target astrocytes
I. Miyazaki, S. Murakami, M. Takeshima, M. Asanuma
Okayama Univ., Dept. of Brain Science, Okayama, Japan
Astrocytes are abundant neuron‐supporting glial cells that harbor a powerful arsenal of neuroprotective antioxidative molecules and neurotrophic factors. Here we examined whether enrichment with healthy astrocytes can provide neuroprotection against dopaminergic neurodegeneration. Serotonin 1A agonist 8‐OH‐DPAT induced astrocyte proliferation and increased antioxidative molecules such as glutathione (GSH) and metallothionein (MT) in cultured astrocytes and striatum of mice. The treatment with 8‐OH‐DPAT significantly ameliorated the reduction of dopamine neurons in neuron‐astrocyte coculture after exposure to H2O2 or 6‐hydroxydopamine (6‐OHDA), but not in enriched neuronal culture alone. The conditioned media from 8‐OH‐DPAT‐treated striatal astrocytes conferred significantly higher protective action against H2O2‐ or 6‐OHDA‐induced neurotoxicity. These protective effects were cancelled by 5‐HT1A antagonist or MT‐1/‐2‐specific antibody. Furthermore, reduction of nigrostriatal DA neurons in 6‐OHDA‐lesioned parkinsonian model mice was significantly abrogated by repeated injections of 8‐OH‐DPAT. Treatment with 8‐OH‐DPAT markedly increased the expression of MT in astrocytes on the hemi‐parkinsonian mice. These results suggest that stimulation of 5‐HT1a receptor in astrocytes can protect dopaminergic neurons against oxidative stress, and that MT secreted from astrocytes may be the major contributor to the 8‐OH‐DPAT‐induced neuroprotective effects.
T06‐13A. Microglia reactivity to amyloid‐β oligomers (AβO) changes according to experimental ageing
C. Caldeira 1, A. Frederico1, A.R. Vaz1, A. Fernandes1,2, D. Brites1,2
1University of Lisbon, Faculty of Pharmacy, Lisbon, Portugal 2University of Lisbon, Faculty of Pharmacy, Biochemistry and Human Biology, Lisbon, Portugal
Healthy brain aging is characterized by neuronal loss and cognitive decline being inflammation a major causative factor. Microglial activation is considered to be a major driver for the neuropathological findings in Alzheimer's disease (AD). We evaluated whether young vs. aged microglia responded differently to Aβ soluble oligomers (AβO) and to conditioned media from AβO‐treated hippocampal neurons.
Microglia from 1 day CD1 pups were incubated for 24 h at 2 (young) and 16 (old) days in vitro (DIV) with AβO (Aβ1–42 at 50 and 1000 nM). In parallel, E16 mice hippocampal neurons were treated with AβO at 4 (young) and 18 (old) DIV for 24 h, and the incubation media used as conditioned media to treat microglia, at respective DIV, for further 24h. Cell migration (Boyden chamber), extracellular content in glutamate (commercial kit), and activity of matrix metalloproteinases (MMP)−2 and −9 (gelatin zymography) were assessed.
An increased migration towards AβO and ATP was observed in young microglia but almost undetectable in aged cells (p < 0.01). Interestingly, microglia exposure to conditioned media from 1000 nM AβO‐treated neurons markedly decreased both young and old microglia migration, while treatment with media from 50 nM AβO‐treated neurons enhanced migration, mainly in the young microglia (p < 0.05). Concerning glutamate, aged cells released less than young ones upon AβO treatment (p < 0.01). In addition, when exposed to neuronal conditioned media, only the young microglia were able to exert a neuroprotective role by reducing the media glutamate content. Curiously, while AβO promoted a microglia release of MMP‐9, independently of cell age, it only induced MMP‐2 secretion in old cells in an AβO concentration‐dependent manner (p < 0.05 for 1000 nM AβO). In contrast, conditioned media from AβO‐treated neurons elicited MMP‐2 release only from young microglia.
Together, our data point to a loss of microglia function towards AβO with ageing and are unique in suggesting that senescent microglia may release high levels of MMP‐2 in AD. In addition, our results indicate that AβO‐treated neurons enhance the release of MMP‐2 even by young microglia. Whether this finding may contribute to enhance inflammatory stress and the onset of AD will be the aim of further studies.
Funded by Edgar Cruz e Silva‐2012 from GEECD and Pest‐OE/SAU/UI4013/2011 from FCT (to DB) and Amadeus Dias from the University of Lisbon (to AF).
T06‐13B. Effects of URMC‐099 on Tat‐treated BV‐2 Microglia Cells
T. Mockus, J. Puccini, D. Marker, J. Barbieri, S.‐M. Lu, H. Gelbard
University of Rochester, Rochester, United States
Human Immunodefeciency Virus‐1 (HIV‐1) productively infects microglia within the central nervous system (CNS). Viral replication within microglia and the resulting immune response and neurotoxicity leads to significant cognitive impairments not limited to dementia, neurocognitive abnormalities, and memory deficits. Combination antiretroviral therapy reduces the cognitive impairments associated with HIV‐1, but the regulatory HIV‐1 Tat protein is still produced in the CNS during treatment, even in the absence of productive infection. Our laboratory analyzes the effect of Tat on the murine CNS by stereotactically injected into cortex, which mimics in part the neuroinflammation and neurodegeneration characteristic of HIV‐1 associated neurocognitive disorders (HANDs). Additionally, in vitro Tat‐treated BV‐2 microglial cells exhibit activation associated with increased cytokine expression and phagocytosis. Mixed lineage kinase 3 (MLK3) has recently been implicated in the Tat‐mediated activation of microglia. MLK3 activity is increased by the presence of Tat protein, which results in microglial activation and increased production of inflammatory cytokines. Conversely, the brain penetrable, small molecule, new chemical entity URMC‐099 inhibits the MLK3 pathway. We hypothesize that URMC‐099 attenuates Tat‐induced microglial activation as measured by microglia process length, phagocytosis assay and cytokine expression. In vitro application of URMC‐099 decreased Tat‐induced production of CCL2 and IL‐6, microglia process length, and phagocytosis of charged and uncharged beads in BV‐2 microglia cells. These data, in aggregate, strongly support a role for the MLK3 pathway in neuroinflammation and suggest that inhibition of this pathway with URMC‐099 may have significant anti‐inflammatory effects in an inflammatory CNS milieu. In conclusion, this work will advance our understanding of MLK3 activity and may provide a viable drug therapy for HANDs.
T06‐14A. Proteomic identification of astrocyte proteins involved in synaptic plasticity
K. Carney 1, P. van Nierop2, K.W. Li2, A.B. Smit2, S. Oliet1, M. Verheijen2
1Université Bordeaux Segalen, Glia‐Neuron Relations, Bordeaux, France 2Vrije Universiteit, Molecular and Cellular Neurobiology, Amsterdam, Netherlands
The contributions of glial cells to the modulation of adult synaptic plasticity are becoming increasingly more appreciated. Astrocytes in the supraoptic nucleus (SON) of the hypothalamus demonstrate prominent temporary structural changes during physiological events such as dehydration and lactation. Such structural remodeling is characterized by a reduction in the astrocytic coverage of oxytocin neurons and their synapses. These astrocyte morphological changes also contribute to the observed functional changes in synaptic activity during dehydration and lactation. In order to elucidate the underlying mechanisms regulating the astrocytic contributions to synaptic plasticity we have established an astrocyte protein expression profile for structural changes in the SON during lactation and hyperosmotic challenge. For this, SON from virgin, lactating, and hyperosmotic rats were collected from acute hypothalamic slices and analyzed by mass spectrometry to identify proteins differentially regulated by the changes in synaptic structure induced by lactation and hyper‐osmolarity. Our mass spectrometry data analysis has placed particular focus on regulation of astrocyte‐specific proteins and the roles of these proteins in molecular pathways known to be involved in SON plasticity, such as cytoskeleton remodeling, cell adhesion, and cell signaling. Gene ontology analysis revealed over representation of pathways known to be highly active in astrocytes including metabolism and antioxidant activity. Promising target proteins for future functional studies will be discussed.
T06‐14B. The role of merlin isoform 2 in neurofibromatosis type 2‐associated polyneuropathy
A. Schulz1, S. Baader 2, M. Niwa‐Kawakita 3, R. Bauer 4, J. Jung1, A. Zoch1, S. Schacke1, C. Hagel 5, V. Mautner 5, O. Hanemann 6, D. Parkinson 6, J. Weis 7, D. Gutmann 8, M. Giovannini9, H. Morrison 1
1Fritz‐Lipmann Institue for Age Research, Jena, Germany 2University of Bonn, Bonn, Germany 3Inserm U674 and Université Paris, Paris, France 4Friedrich Schiller University Jena, Jena, Germany 5University Medical Center Hamburg–Eppendorf, Hamburg, Germany 6Plymouth University Peninsula Schools of Medicine and Dentistry, Plymouth, United Kingdom 7University Hospital Aachen, Aachen, Germany 8Washington University School of Medicine, St. Louis, United States 9House Ear Institute, Center for Neural Tumor Research, Los Angeles, United States
The autosomal dominant disorder neurofibromatosis type 2 (NF2) is a hereditary tumor syndrome caused by inactivation of the nf2 tumor suppressor gene encoding merlin. Apart from tumors affecting the peripheral and central nervous systems, most NF2 patients develop peripheral neuropathies. This peripheral nerve disease can occur in the absence of nerve damaging tumors, suggesting an etiology independent of gross tumor burden.
Here we elucidate a novel merlin isoform 2 (merlin‐iso2)‐specific function in maintaining axonal integrity and propose that reduced axonal nf2 gene dosage leads to NF2‐associated polyneuropathy. We identify a merlin‐iso2‐dependent complex corresponding to activation of the GTPase RhoA, enabling downstream Rho‐associated kinase to promote neurofilament heavy chain phosphorylation. In vivo, specifically merlin‐iso2 knockout mice exhibit impaired locomotor capacities, delayed sensory reactions, and electrophysiological signs of axonal neuropathy. Sciatic nerves from these mice and sural nerve biopsies from NF2 patients show reduced NF‐H phosphorylation, decreased interfilament spacings and irregular‐shaped axons.
T06‐15A. Axonal degeneration is assisted cell non‐autonomously by glial cells
A. Catenaccio, P. Silva, F. Court
Pontificia Universidad Católica de Chile, Santiago, Chile
Axonal degeneration is an active process involved in a variety of neurodegenerative conditions triggered by diverse stimuli. This degenerative process can cause permanent loss of function, so it represents a focus for neuroprotective strategies. We have recently shown that axonal degeneration triggered by distinct mechanical and toxic damage is dependent on the activation of the mitochondrial permeability transition pore (mPTP), which probably represents a central execution program of axonal degeneration, upon which several pathways converge (Barrientos et. al., Journal of Neuroscience 2011). Nevertheless, the participation of other cellular types in this degenerative process has been largely overlooked.
After axonal damage in the peripheral nervous system (PNS), Schwann cells enveloping axons have been regarded as responsible for clearing degenerating axonal debris and to mount an inflammatory response for tissue clearance by invading macrophages. Nevertheless, a possible function of glial cells in modulating axonal degeneration has not been addressed so far. Here we explored the possibility that glial cells actively participates not only in the clearance of degenerating axons, but directly in the axonal degeneration program during Wallerian degeneration.
We found that axonal injury activates an early response of Schwann cells that results in the fragmentation of their associated axons by actin‐rich cytoplasmic domains of Schwann cells known as Schmidt‐Lanterman incisures (SLI). This first step of Schwann cell fragmentation of axons is dependent on the ERK signaling pathway, wich control the dedifferentiation program initiated in Schwann cells after nerve injury (Napoli et. al., Neuron 2012). In the axonal compartment, injury triggers a parallel cell autonomous degenerating program dependent on mitochondrial mPTP activation, which is cell non‐autonomously assisted by Schwann cells through the activation of a cell extrinsic pathway of axonal destruction by TNF‐alpha secretion.
We showed for first that time that axonal degeneration in the PNS proceeds by axonal autonomous mechanisms, as well as cell non‐autonomous ones dependent on SCs. Therefore, effective targets for neuroprotection should consider not only the axonal compartment, but also the associated glial cell.
T06‐15B. Surface dynamics of the astroglial glutamate transporter GLT‐1
C. Murphy‐Royal 1, J. Dupuis2,3, B. Pinson3,4, J. Baufreton5,3, L. Groc2,3, S. Oliet1,3
1INSERM U862 Institute F. Magendie, Bordeaux, France 2IINS, CNRS UMR 5297, Bordeaux, France 3Université de Bordeaux, Bordeaux, France 4Institut de Biochemie et Génetique Cellulaires, UMR 5095, Bordeaux, France 5Institut des Maladies Neurodégénératives, Bordeaux, France
Glutamate is the major excitatory neurotransmitter in the brain. Its concentration is the synaptic area is highly regulated in order to maintain point‐to‐point transmission and to prevent excitotoxicity associated with chronic activation of glutamate receptors. As there is no endogenous extracellular enzyme to degrade glutamate, the clearance of glutamate is ensured by transporters located on the surface of astrocytes. Among these surface transporters GLT‐1 ensures almost 90% of glutamate uptake at synapses.
It is widely accepted that many receptors and transporters on the surface of neurons and glia display surface trafficking properties and are not simply static proteins. The basic surface trafficking properties of glutamate receptors at the synapse have been studied in depth and have allowed us to understand many important trafficking events that occur during synaptic plasticity. The action of GLT‐1 is fundamentally important in synaptic communication. Surface trafficking of this transporter may play a pivotal role in the spatial and temporal properties of glutamate diffusion at the synapse. However, it is still unknown whether GLT‐1 is dynamically regulated on the surface of astrocytes. Thus we set out to uncover the surface trafficking of GLT‐1 in astrocytes by using a combination of high‐resolution imaging, such as single particle (Quantum Dot [QD]) tracking, molecular biology and electrophysiological approaches.
Here we have demonstrated that GLT‐1 is indeed subject to surface trafficking on astrocytes. Using pharmacological approaches we have shown that this diffusion is subject to regulation by the activity of the transporter itself as well as neuronal activity. Furthermore, we have demonstrated that the speed of GLT‐1 diffusion is greatly reduced at excitatory synapses. This leads us to the conclusion that GLT‐1 surface diffusion is highly regulated at the synapse where it can effectively carry out its role as the principal glutamate transporter at the synapse.
Finally, to elucidate the physiological role of GLT‐1 surface diffusion, we have carried out electrophysiological experiments in which GLT‐1 trafficking is effectively reduced using a cross‐link (X‐link) protocol. We observed that a reduction in the surface trafficking of GLT‐1 resulted in an increase of neuronal excitability. Thus GLT‐1 surface trafficking on astrocytes has an implicit role in regulating basal neuronal activity through glutamate uptake at the synapse.
T06‐16A. Astroglial networks set the dynamics of neuronal bursting activity
O. Chever, E. Dossi, N. Rouach
Collège de France, Paris, France
Astrocytes play crucial roles in brain physiology by dynamic interactions with neurons. They form plastic and extensive networks mediated by gap junction channels. It has recently been shown that astroglial networks limit neuronal network activity. However, it is currently unclear how astroglial networks influence neuronal excitability and population activity. To investigate how astroglial assembly regulates neuronal network activity, we performed electrophysiological recordings in hippocampal slices (CA3 and CA1 areas) from mice with disconnected astrocytes, in which both astroglial gap junction forming proteins, connexin 30 (Cx30) and connexin 43 (Cx43), are knocked out (GFAP‐Cre Cx30‐/‐Cx43fl/fl, dKO). Synchronized excitatory discharges were recorded in an acute pharmacological model of epileptic‐like activity. In this model, spontaneous bursting discharges are characterized by a sharp and large amplitude shift in field potential, generated by a major depolarization and firing of all putative neurons. Pyramidal cells depolarized up to 40 mV during seconds and such depolarization is followed by a long‐lasting undershoot of the membrane potential. We found that the frequency of bursting activity in dKO hippocampal slices is drastically increased. However, the duration of neuronal depolarizing bursts is severely reduced. Furthermore, pyramidal neurons are more depolarized due to an increase of synaptic bombardment. This suggests that increased synaptic background promoting resting membrane potential depolarization facilitates triggering of neuronal bursts, but compromises the strength of synchronized events. To investigate local dynamics of bursts generation, we performed multielectrodes arrays recordings in the CA3 area of the hippocampus. In slices from dKO mice, bursting activity in the CA3 region is generated within a more restricted area, indicating that the number of neurons to be recruited is highly decreased. Altogether, these results indicate that gap junction‐mediated astroglial networks strengthen the coordination of neurons during synchronized events.
T06‐16B. Post‐hypoxic potentiation of breathing is mediated by astrocytes
Y. Okada 1, K. Takeda1, Y. Oyamada2, Y. Oku3, F. Miwakeichi4, H. Someya5, M. Ishiguro4, Y. Tamura4, M. Pokorski1,6
1Murayama Medical Center, Clinical Research Center, Tokyo, Japan 2Keio University School of Medicine, Division of Pulmonary Medicine, Tokyo, Japan 3Hyogo College of Medicine, Department of Physiology, Hyogo, Japan 4The Institute of Statistical Mathematics, Department of Data Science, Tokyo, Japan 5Tokai University, School of Information Science and Technology, Kanagawa, Japan 6Polish Academy of Sciences, Medical Research Center, Warsaw, Poland
Acutely loaded hypoxia increases ventilation, and increased ventilation does not immediately return to the pre‐hypoxic level, but persists for a while after resuming room air inhalation. This phenomenon is referred to as short term potentiation or plasticity of breathing, and is thought to contribute to the stabilization of ventilation. Although extensive studies have been conducted to clarify the cellular mechanism of respiratory plasticity focusing on neurons and synapses, its precise mechanism remains unknown. On the basis of the recent discovery that astrocytes are actively involved in neural plasticity in various brain regions, we hypothesized that the post‐hypoxic potentiation of breathing is mediated by astrocytes. We used unanaesthetized unrestrained adult mice. Ventilatory parameters (respiratory frequency, tidal volume and minute ventilation) were measured by whole body plethysmography. To test the hypoxic response, mice breathed room air, hypoxic gas (7% O2, 93% N2), and again room air. We also analyzed the hypercapnic response that was conducted under a hyperoxic condition to eliminate the influence of the carotid body. To test the hypercapnic response, mice breathed pure O2, hyperoxic hypercapnic gas (95% O2, 5% CO2), and again pure O2. After recording of the hypoxic and hypercapnic responses with their recovery time courses, arundic acid (ONO‐2506) was injected intraperitoneally. Arundic acid is a compound that suppresses the function of astrocytes through the inhibition of S100B production in astrocytes. Arundic acid did not affect ventilatory augmentation induced by either hypoxia or hypercapnia. However, arundic acid suppressed the short term potentiation induced by hypoxia (but not by hypercapnia) in a dose‐dependent fashion. High dose arundic acid completely eliminated hypoxia‐induced short term potentiation. These results suggest that the post‐hypoxic potentiation of breathing is mediated by astrocytes with the involvement of S100B. The mechanism of hypercapnia‐induced potentiation is different from that of hypoxia. We propose a model to explain the cellular mechanism of post‐hypoxic potentiation: Hypoxia stimulates the respiratory neuronal network in the brainstem through the excitation of the carotid body. Neurotransmitters spilled from excited neuronal synapses activate nearby astrocytes. The activation of astrocytes is sustained, and these astrocytes persistently excite respiratory neuronal network by releasing gliotransmitters.
T06‐17A. Microglial modulation of synaptic strength at the first synapse in the nociceptive pathway
A.K. Clark 1,2, D. Gruber‐Schoffnegger 3, J. Sandkühler3
1Medical University of Vienna, Centre for Brain Research, Vienna, Austria 2King's College London, Wolfson Centre for Age Related Diseases, London, United Kingdom 3Medical University of Vienna, Vienna, Austria
Long‐term potentiation of synaptic strength (LTP) in nociceptive pathways represents a cellular model for some forms of pain amplification. Extensive evidence indicates that microglia contribute to hypersensitivity in chronic pain models. Recent evidence also suggests that glial cell activity is required for spinal LTP (Gruber‐Schoffnegger et al., 2013). Here we investigated whether stimulation of microglia is sufficient to induce an amplification of synaptic strength at the first synapse in the nociceptive pathway. We utilised the chemokine Fractalkine (FKN) in order to specifically stimulate a pro‐nociceptive spinal microglial phenotype via the CX3CR1 receptor (Clark et al., 2007), and examined whether FKN was sufficient to modulate synaptic transmission between primary afferent C‐fibres and spinal lamina I neurons in vitro.
Monosynaptic C‐fibre evoked excitatory postsynaptic currents (EPSCs) were recorded in lamina I neurons using whole‐cell patch‐clamp in rat transverse spinal cord dorsal‐root slice preparations. Spontaneous EPSCs were recorded simultaneously. In all experiments Bicuculline and Strychnine were added to the bath solution to block GABAA‐ and glycine‐mediated synaptic currents.
The superfusion of spinal cord slices in vitro with FKN results in a rapid facilitation of evoked EPSCs in spinal lamina I neurons receiving monosynaptic input from primary afferent C‐fibres. Both the microglial cell inhibitor Minocycline and a FKN neutralising antibody, completely prevented FKN‐induced changes in synaptic transmission. In addition, the inclusion of the Ca2+ chelator BAPTA or the NMDA receptor antagonist MK801 in the pipette solution completely prevented FKN‐induced enhancement of synaptic strength, suggesting a post‐synaptic Ca2+‐dependent mechanism of LTP induction. Analysis of paired‐pulse ratio (PPR) indicated that FKN‐induced facilitation of synaptic strength is accompanied by a decrease in PPR suggesting a pre‐synaptic mechanism of LTP expression. In support of this finding, FKN increases the number of spontaneous EPSCs recorded from lamina I neurons.
These data indicate that stimulation of microglial cells in order to induce a pro‐nociceptive activity state is sufficient for facilitation of synaptic strength within the dorsal horn. Both pre‐ and post‐synaptic mechanisms contribute to FKN‐induced facilitation of synaptic strength.
This work was funded by a Wellcome Trust Flexible Travel Fellowship to AKC.
REFERENCES
Clark AK, Yip PK, Grist J, Gentry C, Staniland AA, Marchand F, Dehvari M, Wotherspoon G, Winter J, Ullah J, Bevan S, Malcangio M (2007). Proc Natl Acad Sci U S A 19;104:10655–10660.
Gruber‐Schoffnegger D, Drdla‐Schutting R, Hönigsperger C, Wunderbaldinger G, Gassner M, Sandkühler J (2013). J. Neurosci., In Press.
T06‐17B. Unitary synaptic connections between GABAergic interneurons and NG2 cells in the developing somatosensory cortex
D. Orduz 1, P.P. Maldonado1, M. Velez‐Fort1, Y. Yanagawa2, M.C. Angulo1
1Physiology of NG2 cells group. INSERM U603, CNRS UMR 8154, Univ. Paris Descartes, Paris, France 2Gunma University, Dept. of Genetic and Behavioral Neuroscience, Maebashi, Japan
The organization and connectivity of the developing neocortex remains largely unresolved. The establishment of neuronal microcircuits is a complex process that partly depends on the diversity of neuronal cell types and their ultimate role in the mature brain. This complexity is increased by the existence of bona fide synaptic connections between neurons and oligodendrocyte precursor cells, also called NG2 cells, which properties and function remains largely unknown. To unravel the physiological characteristics of GABAergic synapses between interneurons and NG2 cells, we performed paired recordings in the developing somatosensory cortex. Experiments were carried out during the second postnatal week in vGAT‐venus;NG2‐DsRed double transgenic mice that allowed us to identify interneurons (venus+) and OPCs (DsRed+) in acute slices. Our results demonstrated that NG2 cells are synaptically contacted by fast‐spiking (FSI) and non‐fast‐spiking (NFSI) interneurons. However, a predominant input was received by FSI, a class of interneuron that constitutes only a minor population in the second postnatal week. On the contrary, only a small proportion of the abundant NFSI were connected to NG2 cells. All the connections showed paired‐pulse depression, low probability of response, and preliminary analyses suggest that each interneuron form a single synaptic contact onto NG2 cells. Paired recordings, extensively used to examine the synaptic properties of neuronal microcircuits, may open a new perspective to understand in detail the mechanisms of GABA release and signaling between interneurons and NG2 cells in the healthy and pathological developing brain.
T06‐18A. Exploring motor neuron signaling dynamics to microglia in ALS
J.C. Cunha 1, A.R. Vaz1, D. Brites1,2
1University of Lisbon, Faculty of Pharmacy, Lisbon, Portugal 2University of Lisbon, Faculty of Pharmacy, Department of Biochemistry and Human Biology, Lisbon, Portugal
Amyotrophic Lateral Sclerosis (ALS) is a fatal neurodegenerative disease characterized by motor‐neuron (MN) degeneration, mainly in motor cortex, brainstem and spinal cord. Mutations in superoxide dismutase‐1 (SOD1) are commonly used to study familial forms of ALS. It has been suggested that neuroinflammation associated with microglia activation is imperative for ALS progression. Indeed, microglia may become activated and proliferate during disease onset, before MN degeneration.
This study aimed to characterize the reactivity of a microglial cell line towards LPS and their response to soluble factors released by degenerating MNs.
First, we characterized a microglial cell line obtained from CD1 mouse cortex (N9), in order to clarify the mechanisms involved in the activation pattern of these cells. For that, we incubated N9 microglia with 300 ng/ml of lipopolysaccharide (LPS) for 24 h. Morphology (anti‐Iba1 staining), phagocytic ability (fluorescent latex beads) and chemotaxis to ATP (Boyden Chamber) were evaluated. We observed that N9 microglia is ramified in control conditions and after LPS treatment they change to an amoeboid morphology, which is accompanied by increased phagocytosis and decreased migratory ability, indicating that LPS induces activation of N9 cell line.
Next, we investigated the role of the released factors from a MN‐like cell line expressing human SOD1 with G93A mutation (NSC‐34/hSOD1G93A) on microglia functions. NSC‐34 expressing human SOD1 wt were used as control. Thus, N9 microglia were incubated for 4 and 24 h with NSC‐34 conditioned media that had been collected at 1 and 4 days of differentiation (DIV) and reactivity tests were performed as above. Although after 4 h of incubation no significant changes were observed, data evidenced that incubation for 24 h with NSC‐34/hSOD1G93A conditioned media collected at 4 DIV changed the bipolar morphology of the N9 microglia into an active amoeboid state, decreased phagocytic ability (22%, p < 0.05) and induced apoptosis (50%) (Fig.1). Moreover, microglia have shown to not be attracted by the released factors from degenerating MN, as determined by the chemotaxis assay.
Together, our results evidence that released factors from NSC‐34/hSOD1G93A degenerating cells although inducing microglia morphological activation trigger a reduction in cell phagocytic ability and loss of viability, suggesting a role for microglia along ALS progression.
Funded by PTDC/SAU‐FAR/118787/2010 (to DB) and PEst‐OE/SAU/UI4013/2011 (iMed.UL). from Fundação para a Ciência e Tecnologia.

T06‐18B. Alterations of NG2 cell synaptic connectivity following demyelination of corpus callosum
F.C. Ortiz 1, A. Sahel2, C. Kerninon2, P.P. Maldonado1, M.C. Angulo1, B. Nait Oumesmar2
1INSERM U603, Paris, France 2Cerveau et de la Moelle Epinière (ICM), Cellular and Molecular Approaches for Myelin Repair, Paris, France
Oligodendrocyte progenitor cells (OPCs) expressing the proteoglycan NG2, also named NG2 cells, persist in the adult central nervous system (CNS). NG2 cells represent the main proliferating cell type that serve as a major source of remyelinating oligodendrocytes in demyelinating diseases, such as multiple sclerosis (MS).In the last years, the finding of functional and morphological targeted synaptic contacts from neurons on NG2 cells has motivated a growing interest in the physiology of these cells. Although it has been shown that myelination of the CNS depends on electrical activity, underlying mechanisms demonstrating the potential role of these synaptic contacts on (re)‐myelination is still lacking. Our study aims at deciphering the relevance of synapses between neurons and NG2 cells and their potential involvement in myelin repair of demyelinated lesions of the adult corpus callosum. In order to investigate whether glutamatergic innervation of NG2 cells change during remyelination, we performed lysolecithin (LPC) induced focal demyelination of the adult mouse corpus callosum. We performed electrophysiological recordings on acute slices, immunostainings and electron microscopyat 4, 7, 14 and 30 days post‐injection (dpi) alongside controls in different transgenic mouse strains. Intrinsic electrophysiological properties of NG2 cells did not changefollowing demyelination, indicating that they are not affected by LPC‐induced lesion. Nevertheless, our data demonstrated that these cells are partially disconnected at 4 dpi, during the phase of active recruitment and proliferation into the lesion. After 7dpi, the number of connected cells recovered virtually to 100%, butthe frequency of spontaneous synaptic events never reached control values.The presence of synaptic contacts on putative NG2 cells in the lesion has been also confirmed by electron microscopy.To corroborate our functional analysis, we quantifiedneuron‐NG2 cell glutamatergic connections using immunostainings against NG2 and the vesicular transporter for glutamate vGluT1 in both LPC‐induced lesions and MS lesions.Our preliminary data already showed the presence of vGluT1 puncta in apposition with NG2 cells in both types of lesions.Altogether, our results showed the presence of functional neuron‐NG2 cell synapses in demyelinated lesions andsuggest a regulation of their synaptic properties during remyelination.
FCO and AS are co‐first authors; MCA and BNO are co‐last authors.
This study is supported by ANR, FRC and ARSEP foundation. A. S. and P.P.M. are supported by Ecole des Neurosciences de Paris (ENP).
T06‐19A. Tailoring substrates for long‐term organotypic culture of adult neuronal tissue
V. Dallacasagrande 1, M. Zink2, S. Huth3, A. Jacob3,4, M. Müller3, J. Käs2, S. Mayr3,4, A. Reichenbach1
1Paul Flechsig Institute, Leipzig, Germany 2University Leipzig, Physics, Leipzig, Germany 3Leibniz Institute for Surface Modification, Leipzig, Germany 4Translational Centre for Regenerative Medicine, Leipzig, Germany
Organotypic explants culture has a pivotal role in studying the complex structure of neuronal tissue. Although embryonic tissue explants and slices are used to obtain long term culture, most applications such as drug testing require adult tissue for reliable results. In this study, we show that nanostructured metal‐oxide arrays can be employed to yield long‐term organotypic culture of adult neuronal tissue explants as demonstrated for the adult guinea pig retina and adult murine brain slices. Even after 14 days of culture, the thickness and the layered structure of the retina were well maintained. Quantitatively, the number of nuclei within the inner and outer nuclear layers was comparable to freshly isolated retinae and no significant change in the densities of the nuclei within the layers was observed. The outer plexiform layer, as the site of synaptic connection between photoreceptors and neurons, was still well preserved. Moreover, the thickness of the photoreceptor layer was conserved, indicating that the inner and the outer segments of the photoreceptors survived well during two weeks culture. This is hardly observed in long‐term cultures since the outer segments were cultured without retinal pigment epithelium. Concerning the adult murine brain slices from neo‐cortex, after 9 days of culture, the neurofilaments and cell nuclei were still well preserved and the neuronal network displayed the typical arrangement of long axons. However, preservation of the tissue depends strongly on the geometry of the nanostructured array surface such as roughness and spacing, whereby organotypic brain slice culture requires different geometries than retina culture. Since organotypic culture of adult tissue could only be obtained for about 7 days with conventional techniques, our new substrate material could be the breakthrough for in vitro studies on tissue regeneration, neuroplasticity, as well as drug testing.
T06‐19B. Glial and neuronal mechanisms underlying the label‐free intrinsic optical signal
I. Pál, G. Nyitrai, J. Kardos, L. Héja
Institute of Molecular Pharmacology, RCNS, HAS, Budapest, Hungary
The intrinsic optical signal (IOS) is widely used for mapping neuronal activity in afferent activated brain areas. IOS evoked by afferent stimulation in vitro is generally attributed to glial cell swelling via activation of Na+/K+/Cl‐ cotransporter that follows postsynaptic activation. Despite the phenomenon is known for decades, the relative contribution of different cell types and the underlying molecular mechanisms have not been disclosed in detail. Our goal was to explore the glial and neuronal correlates underlying in vitro IOS genesis.
We characterized IOS initiated by Schaffer collateral stimulation in the rat hippocampal slice using simultaneous high‐frequency imaging and field potential recordings. We used a 464‐element photodiode‐array device (PDA) that enables IOS detection with 0.6 ms time resolution, making it achievable to align optical and electrophysiological signals. IOS was primarily observed in the proximal region of the stratum radiatum and stratum pyramidale of the hippocampus. IOS was decreased by blockade of neuronal activity by voltage‐gated Na+ channel inhibitor tetrodotoxin and was significantly enhanced by suppressing inhibitory signaling with the GABAA antagonist picrotoxin. We found that IOS was predominantly initiated by postsynaptic glutamate receptor activation and progressed by the activation of astroglial glutamate transporters and Mg2+‐independent astroglial NMDA receptors. In agreement with previous studies, furosemide, the blocker of both the neuronal K+/Cl‐ cotransporter KCC2 and the glial Na+/K+/Cl‐ cotransporter NKCC1 decreased the IOS amplitude. To decide which cotransporter is responsible for this effect of furosemide, we applied selective blockers of NKCC1 and KCC2, bumetanide and (+)[R]‐DIOA, respectively. We evidenced, that NKCC1 did not contribute to the IOS generation, in contrast to previous suggestions. Enhancement and slight inhibition of IOS through anion and volume‐regulated anion channels, respectively, were also depicted.
Major players of IOS mechanisms disclosed by high‐frequency IOS imaging imply that spatiotemporal IOS reflects glutamatergic neuronal activation and astroglial response, as observed within the hippocampus. Our model may help to better interpret in vivo IOS and support diagnosis in the future.
This work was supported by grants ERA‐Chemistry OTKA 102166, KMR_12‐1‐2012‐0112 TRANSRAT and TECH‐09‐AI‐2009‐0117 NKFP NANOSEN9.
T06‐20A. The age‐related hippocampal alterations of glutamatergic neurotransmission are aggravated by ω3 PUFA deficiency and reduced by fish oil supplementation in rats
I. Denis 1, B. Potier2, A. Latour1, G. Champeil‐Potokar1, M. Hennebelle1, E. Maximin1, B. Langelier1, M. Lavialle1, J.‐M. Billard2, C. Heberden1, S. Vancassel1
1INRA, Jouy‐en‐Josas, France 2INSERM, Centre de Psychiatrie et Neuroscience, Paris, France
Question: A poor ω3 polyunsaturated fatty acids (ω3 PUFA) status, favored by the low ω3/ω6 ratio in western diets, seems to contribute to cognitive decline in the elderly, but mechanistic evidence is lacking. We therefore explored the impact of ω3 status on the evolution of glutamatergic transmission, astroglial regulation and neurogenesis in the hippocampus during ageing in rats. These processes are involved in memory formation and their dysregulation participates to the age‐related brain damage leading to cognitive decline.
Methods: We have compared 6 groups of rats aged 6 to 22 months fed ω3‐deficient, ω3/ω6‐balanced, or ω3 (fish oil) supplemented diets: Young ω3 Balanced (YB), Deficient (YD) or Supplemented (YS), and Old ω3 Balanced (OB), Deficient (OD) or Supplemented (OS) rats. We have evaluated synaptic efficacy and plasticity (electrophysiological recording), astroglial regulations (glutamate uptake and GFAP expression), neuronal markers (glutamate transporters and receptors), neurogenesis (proliferation of neuronal precursors in the sub granular zone), and analyzed brain fatty acids composition.
Results: Dietary modulation of ω3 intakes efficiently modified the incorporation of docosahexaenoic acid (DHA, the main ω3 in cell membranes) in brain (−50% deficient vs balanced, +10% supplemented vs balanced). Ageing induced a 35% reduction of synaptic efficacy due to decreased pre‐synaptic glutamate release, and a 30% decrease in the astroglial glutamate uptake associated to a marked astrogliosis (+100% GFAP). ω3 deficiency further decreased these hallmarks of ageing (OD vs OB rats: −35% synaptic efficacy, −15% glutamate uptake, +30% GFAP). On the opposite, ω3 supplementation increased synaptic efficacy (+25% OS vs OD) and seems to abolish astrogliosis (OS vs YS : no change in GFAP). Neurogenesis was altered by ω3 deficiency but not by supplementation.
Conclusion: Our results characterize some specific age‐related alterations of the glutamatergic synapse in the hippocampus that are aggravated by a dietary deficit in ω3 and attenuated by ω3 supplementation.
T06‐20B. Ibudilast and SKF modulate cisplatin‐evoked PGE2 release from isolated trigeminal satellite glial cells
J.N. Poulsen 1, F. Larsen1, F.L. Kristiansen1, J.C. Laursen2, M. Duroux3, P. Gazerani2
1Aalborg University, Aalborg, Denmark 2Aalborg University, Center for Sensory‐Motor Interaction, Aalborg, Denmark 3Aalborg University, Laboratory for Cancer Biology, Aalborg, Denmark
Question: Cisplatin (CIS) is an alkylating‐like chemotherapeutic agent commonly employed in the treatment of different types of cancer. CIS is associated with the development of chemotherapy‐induced peripheral neuropathy (CIPN), a severe debilitating condition manifested by neuropathic pain. It has recently been proposed that satellite glial cells (SGCs), non‐neuronal cells found in sensory and autonomic ganglia, may play a role in development of CIPN. However, little is known about the effect of CIS on SGCs with a potential link to CIPN. The aims of this study were to investigate prostaglandin E2 (PGE2) release from isolated SGCs following treatment by CIS and to test whether phosphodiesterase inhibitor Ibudilast (Ibu) and the p38 inhibitor SKF86002 (SKF) would modulate the PGE2 release.
Methods: SGCs were isolated from the trigeminal ganglion (TG) of adult male Wistar rats (N = 9). Animals were deeply anaesthetized and both TG were extracted and digested first in 5 mg/ml collagenase for 15 min and then in 0.125% trypsin containing DNAse for 10 min. In initial experiments, isolated SGCs were treated with control medium or medium containing 16.5, 33, or 66 µM CIS for 8h. In the subsequent experiments, SGCs were pretreated for 24h with control medium or medium containing 100 µM Ibu or 1 µM SKF. Afterwards, SGCs were stimulated for 8 hours with medium containing 66 µM CIS. PGE2 concentration was determined by ELISA.
Results: CIS caused a concentration‐dependent increase in PGE2 release from SGCs. Treatment with 33 µM CIS significantly increased PGE2 concentration to 354.8 ± 87.3 pg/ml, compared with control 211.3 ± 39.0 pg/ml (p = 0.018). PGE2 concentration was further increased to 464.1 ± 77.0 pg/ml (p = 0.002) following 66 µM CIS. Pretreatment with Ibu significantly lowered the PGE2 concentration compared with CIS‐stimulated SGCs (197. 5 ± 85.2 pg/ml vs. 415.8 ± 143.8 pg/ml, respectively; p = 0.05). A more pronounced inhibitory effect was observed following SKF pretreatment, where it reduced the PGE2 concentration to 19.7 ± 1.8 pg/ml (p = 0.003, compared with CIS‐stimulated SGCs).
Conclusion: These findings suggest that CIS contributes to an inflammatory response in the TG by provoking release of PGE2 from SGCs, which may lead to development or maintenance of peripheral sensitization involved in CIPN. The data also suggest that treatment with agents that target PGE2 release cascade may prove beneficial for preventing development or maintenance of CIPN.
T06‐21A. The “Yin and Yang” in depression: how astrocytes and neurons differently respond to antidepressants to remodel neuronal synaptic contacts
B. Di Benedetto, S. Giusti, A. Vogl, E. Butz, T. Rein, D. Refojo, R. Rupprecht
Max Planck Institute of Psychiatry, Munich, Germany
Functional alterations in synaptic contacts have often been described as a hallmark of major depressive disorder (MDD). Antidepressants (ADs) have been shown to restore neuronal circuits through an enhancement of synaptogenesis in some regions of the brain. Nevertheless, the underlying mechanisms are still unclear. Glia cells have been since long acknowledged as active partners of neurons in orchestrating molecular signals crucial for the proper arrangement of neuronal circuits in the developing and adult brain. Therefore, understanding how both neurons and astrocytes respond to antidepressants (AD) is of high interest.
Using rat C6 glioma cells and primary cultures of astrocytes and neurons, we showed a time‐dependent cell‐autonomous modulation of the ERK/MAPK signalling pathway after acute treatment with various classes of ADs in both cell types. Specifically, glia cells responded to ADs with the simultaneous and fast activation (after 10 min treatment) of both ERK1/2, in contrast to treatment with antipsychotics or mood stabilizers. This activation induced 48 hrs later an increased release of GDNF, a factor involved in synapse formation and axonal wiring, which was MAPK‐dependent. On the contrary, hippocampal neurons showed a reduction in ERK activity that correlated with neuronal activity inhibition after short term (10 min) ADs administration, as demonstrated by quantification of c‐Fos expression after KCl stimulation. Interestingly, this inhibitory effect was reversible after long term (48 hrs) ADs treatment.
To further identify whether GDNF released from astrocytes treated with ADs might be responsible for long term changes in neuronal synapses, we examined how ADs influenced synaptic densities in neurons alone or co‐cultured with astrocytes. Strikingly, we found that the number of synapses was reduced at 48hrs after AD treatments, but only in the presence of intact astrocytes and not in cultures of neurons alone or neurons treated with astrocyte‐conditioned media. This effect was reversed after 120 hrs treatment and rescued by the soluble form of the specific GDNF receptor GFRalpha1, but not by GDNF alone. Moreover, live imaging of astrocytes showed dramatic morphologic changes of their processes in response to ADs that might underlie the observed synaptic remodelling. Our analysis of changes occurring at the neuron‐glia interface upon AD treatments might elucidate novel mechanisms of ADs action that may open the avenue for unravelling the role of astrocytes in psychiatric diseases and implement pharmacologic treatment regimens for MDD patients.
T06‐21B. VMAT2‐positive astrocytes affect brain monoamine levels.
L. Pucci, C. Calì, P. Bezzi
Université Lausanne, Lausanne, Switzerland
Classical monoamines, such as dopamine, noradrenaline, adrenaline and serotonin, serve as major neuromodulators controlling a variety of functions in the brain, including locomotor activity, reward attention and memory. Evidence that astrocytes express the whole enzymatic apparatus for the metabolism of monoamines (Gasser et al., J Neurosci, 2006; Youdim et al., Nature Review Neurosci, 2006) strongly suggested a possible involvement of glial cells in mechanisms governing monoaminergic homeostasis. Here we describe the physiological implication of astrocytes located in monoaminergic pathways on monoamines homeostasis. We report that astrocytes located in frontal cortex (FCx) and striatum (ST) express VMAT2, a vesicular transporter essential to normal monoamines release into the synaptic cleft (Edwards R.H., Neuron, 2007), whose impairment can contribute to symptoms of depression, anxiety, restless leg syndrome and social anxiety (Fon et al., Neuron 1997; Takahashi et al., PNAS, 1997; Wang et al., Neuron, 1997). At ultrastructural level, VMAT2 is located on intracellular organelles and cisterns possibly representing the sites of monoamines storage in astrocytes. The physiological relevance of VMAT2 in astrocytes is then investigated by generating conditional transgenic mice in which VMAT2 is specifically deleted in GFAP expressing cells. Mice are generated by crossbreeding tamoxifen (TAM)‐inducible cre recombinase transgene driven by the human astrocytic glial fibrillary acidic protein (hGFAP) promoter (here referred to as CreTAM mice, Hirrlinger et al., Glia, 2006) with mice containing Cre‐excisable loxP sequences in the endogenous VMAT2 gene. The progeny inheriting both alleles are referred to as CreLox mice. To achieve gene recombination mice are treated for 8 days with tamoxifen, which is converted by the liver into the principal active of CreERT2‐binding metabolites 4‐hydroxytamoxifen. Western blot analysis of VMAT2 expression in FCx and ST of CreLoxTAM mice, confirm a significant reduction of the protein (−19% for FCx, −17% for ST). Interestingly, the observed down regulation of VMAT2 in astrocytes is followed by a more generalized dysregulation of monoamines turnover; indeed, both the tissue and the extracellular levels of monoamines are significantly reduced in the brain areas expressing VMAT2‐positive astrocytes. These results raise new and intriguing perspective about the role of astrocytes in the brain functions
T06‐22A. The role of glia cells in thyroid hormone induced regulation of neuronal sodium current and Na+/K+‐ATPase density
I. Dietzel‐Meyer, B. Igelhorst, V. Niederkinkhaus, S. Mohanasundaram
Ruhr‐Universität, Bochum, Germany
Thyroid hormone has been shown to induce the secretion of proteins such as, FGF‐2, EGF and neurotrophins from glial cells (see e.g. Lima et al., J. Endocrinol. 1997, 167). On the other hand, treatment with thyroid hormone (T3) up‐regulates Na+‐current density (NavD) in neonatal neurons (Hofmann and Dietzel, Neurosci, 2004; 125: 369). The resulting increase in neuronal excitability could explain the effects of this hormone on mental speed and some of the neurological symptoms of hyperthyroidism such as irritability and seizure susceptibility. The effects of T3 on neuronal NavD were mimicked by incubating neuron enriched cultures with conditioned medium from T3‐treated astrocytes, and were absent in neuron‐enriched cultures suggesting that the factors secreted by glial cells by T3 treatment up‐regulate NavD in neurons. The effect was mimicked by the addition of FGF‐2 to the culture medium and blocked by antibodies against FGF‐2, suggesting the involvement of this factor in the up‐regulation of NavD by T3 (Niederkinkhaus et al., Mol.Endocrinol 2009; 23 :1494). Here we extend these findings showing that the neurotrophin NT‐3, which also belongs to the putative factors secreted from glial cells after T3‐stimulation, also increases the NavD in neuron enriched cultures. The effects of NT‐3 and FGF‐2 are, however, not additive. Antibodies against NT‐3 potentiated the effects of FGF‐2, suggesting that by converging signal cascades NT‐3 could reduce the effect of FGF‐2. In neuron‐glia mixed cultures antibodies against NT‐3 enhanced the T3‐induced up‐regulation of NavD, suggesting, that a co‐secretion of NT‐3 from glial cells could limit the effects of FGF‐2. In a second series of experiments we investigated, whether the well‐known effect of T3 on the expression of Na+/K+‐ATPases, thought to contribute to the effect of thyroid hormone on metabolism, is also influenced by glial cells. We found, that the effect of T3 on 3[H]ouabain‐binding and on the expression of Na+/K+‐ATPase alpha2 subunits detected by western‐blots was highest in neuron‐mixed cultures, suggesting that a neuron‐glia interaction is also involved in the T3 effects on Na+/K+‐ATPase expression.
T06‐22B. Refining Methods for Studying Microglial Diversity in the Healthy and Ageing Brain
K. Renault, J. Vincenti, K. Summers, B. McColl
The Roslin Institute – The University of Edinburgh, Easter Bush, United Kingdom
In the healthy brain microglia engage in bi‐directional interactions with neurons and other brain cells, which is crucial for maintaining normal physiology and cognitive function of the brain. Disturbances in homeostasis and cell‐cell communication may predispose to age‐related dysfunction and neurodegenerative disease. For decades it has been speculated that microglia exhibit phenotypic diversity allowing specialisation to a variety of brain microenvironments. Previous observations of regional variations in microglial densities and morphologies support the existence of microglial heterogeneity. However it is clear that a more comprehensive understanding, in particular of the molecular basis of microglial phenotypic and functional diversity on a global scale, is required. The limitation of recent gene expression studies is the use of mixed whole brain or dissected mixed cell extracts which preclude the detection of cell‐specific gene expression profiles.
Thus, our aim was to validate an efficient extraction process for microglia, ideally minimising the impact on the resting state. The method developed is based on existing procedures and was refined to ensure consistency and to maximise cell yield and purity. The extraction of highly pure microglia as a single cell type was achieved by a two‐step isolation technique using density‐gradient and magnetic bead separation. Purity between 90–95% was verified by flow cytometry and immunohistochemistry. Quantitative PCR also demonstrated expression of specific microglial genes indicating that isolated cells are microglia. Cytokine assays suggest that no or only minimal activation of cells was induced by the isolation. Hence, the microglia retained their ability to respond to immunogenic stimuli and produce IL‐1β as a key inflammatory cytokine. Overall, the purification procedure was shown to achieve consistent high purities and retain crucial functional properties. Thus, the extracted microglia are likely to adequately represent the real in vivo state and enable the study of microglial phenotypes and functionality in the healthy and ageing whole brain and accordingly brain regions. Early diversity experiments showed region‐specific microglia phenotypes in the brain of healthy and immune challenged animals as well as differences between unchallenged and challenged animals.
T06‐23A. D‐Serine released by astrocyte can modulate the respiratory rhythm in neonatal mice
J. Eugenin, S. Beltran‐Castillo, I. Llona
USACH, Santiago, Chile
Question: Activation of NMDA receptors increase the respiratory frequency in mammals. Astrocytes are in intimate contact with neurons, specially in glutamatergic synapses, and are able to sense neuronal activity, react to, and even influence nearby neurons. Furthermore, they can sense changes in H+ or PCO2, and in response to these stimuli, they can release molecules like ATP and D‐serine, which will activate neuronal circuits. Then D‐serine, an agonist of the glycine site of the NMDA receptor, can affect the respiratory rhythm during hypercapnia. Our aim was to study the probable role of D‐serine in the modulation of the respiratory rhythm in mice neonates.
Methods: Fictive respiration was recorded with suction electrodes from C4‐C5 ventral roots in “en bloc” (brainstem‐spinal cord) preparations from P0‐P3 CF1 mice. Superfusion was done with artificial cerebrospinal fluid equilibrated with O2:CO2 = 95%: 5%, (pH 7.4,28°C) containing different D‐serine concentrations (0.1–100 µM) in presence or absence of D‐amino acid oxidase (DAAO), which degrades D‐serine. In addition, hypercarbic acidosis (switching equilibration from 5 to 10% CO2, changing pH from 7.4 to 7.2) was done in absence or presence of DAAO.
Results: Application of D‐serine increased the frequency of the respiratory rhythm in a concentration‐dependent way. Application of DAO attenuated the effects of D‐serine application. Likewise, DAAO reduced slightly the effect of hypercarbia on the respiratory rhythm.
Conclusions: Our preliminary experiments indicate that D‐serine is a potent agent increasing the respiratory rhythm frequency in in vitro neonatal preparations, and likely, in association with ATP, is mediating the respiratory response to hypercarbia.
T06‐23B. Microglia protect neurons at the onset of motoneuron developmental cell death and of neuronal network formation in the spinal cord of mouse embryo in vivo
C. Rigato 1, N. Swinnen1,2, J.‐M. Rigo2, H. Le Corronc1,3,4, P. Legendre1,3,4
1Université Pierre et Marie Curie, Paris, France 2Hasselt University, BIOMED‐Cell Physiology, Diepenbeek, Belgium 3INSERM U952, Université Pierre et Marie Curie, Paris, France 4CNRS, UMR 7224, Université Pierre et Marie Curie, Paris, France
We previously analysed the interaction between microglia and neurons in the developing spinal cord of CX3CR1eGFP mouse embryos in vivo (Rigato et al., 2011; Rigato et al. 2012). There, we showed that MAC‐2 positive microglia accumulate within the motoneuron area and phagocyted apoptotic bodies at the onset of motoneuron developmental cell death (from embryonic day 12.5‐ E12.5). Motoneuron developmental cell death and microglia accumulation in the motoneuron area increased at E13.5, decreased at E14.5 and disappeared at E15.5. Since it was supposed that microglia promote developmental cell death in the developing central nervous system, at least in vitro, we determined to what extent it was also the case in the embryonic spinal cord in vivo. To address this issue, we analysed in vivo the progression of developmental neuronal death in the spinal cord of transgenic mouse embryos lacking microglia (PU.1‐KO mice). As opposed to what is observed in the SC of wild type mouse embryo, we found that activated caspase‐3 staining was detectable before E12.5 in PU.1‐KO mouse embryos. In PU.1‐KO mouse embryos, activated caspase‐3 staining was not restricted to the motoneuron area, as several positive cells are also present in the dorsal region of the spinal cord and in the progenitor zone. At E13.5, activated caspase‐3 staining was 10 times greater in the spinal cord ventral area of PU.1‐KO mouse embryos when compared to wild type littermates. Moreover, and opposed to what occurs in wild type animals, caspase‐3 staining in the motoneuron area of PU.1‐KO mouse embryos increased at E14.5 and persisted at E15.5, being likely partly due to the accumulation of apoptotic bodies in the absence of microglia. Our results suggest that, in addition to their phagocytic role, embryonic microglia are likely to protect neuron from death at the onset of developmental cell death and to promote survival of progenitor‐like cells in the embryonic SC in vivo. Accordingly, microglia could have an opposite effect on cell survival in the embryonic SC when compared to postnatal SNC developmental stages and pathological conditions in the adult.
T06‐24A. Role of the α‐secretase TACE in Central Nervous System myelination
E. Fredrickx 1,2, G. Dina1, K.‐A. Nave3, A. Quattrini1, C. Taveggia1
1San Raffaele Scientific Institute, Division of Neuroscience – INSPE, Milan, Italy 2Università Vita‐Salute San Raffaele, PhD Program in Molecular Medicine – Cellular and Molecular Biology, Milan, Italy 3Max‐Planck‐Institute of Experimental Medicine, Goettingen, Germany
The ADAM family of proteins belongs to the zinc family of proteases that are involved in the ectodomain shedding of several growth factors. We recently demonstrated a key role of the α‐secretase TACE, also known as ADAM17, in peripheral nervous system (PNS) myelination by TACE‐mediated cleavage and subsequent inhibition of Neuregulin1 (NRG1) type III activity. Unlike the PNS in which NRG1 type III is an essential instructive signal for myelination, oligodendrocyte (OL) development and myelination in the central nervous system (CNS) are likely controlled by several growth factors some of which undergo cleavage by secretases.
To study the role of TACE on OPC development, immunopanned A2B5+ OPCs were cultured in proliferating or differentiating medium and treated with a soluble form of TACE (rhTACE). When OLs were scored according to their morphology, we observed enhanced OPC differentiation upon addition of rhTACE to proliferating OPCs. Addition of rhTACE to differentiating OPCs also increased the number of OLs with a complex morphology and already presenting membrane sheets, suggesting that TACE might promote OPC differentiation. To further investigate the role of neuronal TACE in CNS, we used an in vitro OL myelinating coculture system. When cocultured with TACE null DRG neurons, rat wild type OPCs never myelinate, suggesting that neuronal TACE might control OPC differentiation. In agreement, preliminary ultrastructural analyses of transgenic mice lacking TACE in CNS neurons showed hypomyelination and signs of myelin degeneration. Accordingly, conditional transgenic mice lacking TACE in OL are normally myelinated.
These studies suggest that in the CNS the α‐secretase TACE promotes OPC differentiation and might regulate CNS myelination. A better understanding of the role of TACE will provide novel insights into the mechanism regulating CNS myelination.
T06‐24B. Rapid control of glutamate transport and energy metabolism by extracellular potassium in astrocytes
T. Rimmele, J.‐Y. Chatton
Université Lausanne, Lausanne, Switzerland
In the excitatory network glutamate is released in the synaptic cleft during synaptic activity. Glutamate clearance by astrocytes is mainly performed by Na+‐dependent glutamate transporters. The Na+ load during glutamate uptake evokes an activation of the Na+/K+ ATPase, which dramatically increases energy demands in astrocytes. Astrocytes are also involved in the regulation of extracellular potassium (Ke +) which significantly increases during the repolarization of excitatory neurons. In this study we evaluated how these fundamental functions of astrocytes coexist and if they interact. We investigated the impact of altering Ke + concentrations on glutamate transporter activity measuring intracellular sodium (Nai +) using the Na+ sensitive fluorescent dye Asante Sodium Green loaded in cultured astrocytes. We observed that glutamate uptake caused a reversible rise in Nai +, which was tightly modulated in amplitude, slope and recovery rate by Ke + levels. In order to evaluate the impact of physiologically relevant Ke + fluctuations on the kinetics of the glutamate transporter the Na+/K+ ATPase was inhibited. Therefore we found that low Ke + evoked a significantly faster influx of Na+, whereas high Ke + markedly slowed down glutamate capture, indicating that Ke + directly modulates the kinetics of glutamate transporters. We then investigated the energy demands of astrocytes caused by Ke + alterations. ATP hydrolysis was indirectly measured through the changes in intracellular free Mg2+ using the Mg2+ sensitive fluorescent probe Magnesium Green. We found that low Ke + led to higher energy demands of astrocytes during glutamate uptake. Overall these results indicate that Ke + acts as a negative feedback on glutamate uptake in astrocytes. The physiological consequences of these findings have to be considered in the context of K+ buffering and of maintenance of neurotransmitter and energy homeostasis.
T06‐25A. Neurofilaments protect oligodendrocytes from lysolecithin toxicity in vitro
C. Fressinaud, J. Eyer
University Hospital, Angers, France
Neurofilaments (NF) and co‐isolated proteins increase the proliferation and differentiation of oligodendrocytes (OL) in vitro (Fressinaud et al., 2012). Since NF are released in the cerebrospinal fluid in MS, and their concentration correlates with disease severity, they might then regulate remyelination by OL. Thus, NF effects were determined in a model of OL cultures treated with lysophosphatidyl choline (LPC) (Fressinaud, 2005). LPC decreases the proliferation of OL progenitors (OLP) and the differentiation of surviving cells, and it destroys myelin‐like membranes. Recovery was significantly improved by NF fractions, as well as tubulin (TUB), added after LPC removal: compared to cultures treated with LPC alone the number of OLP (A2B5+ cells), and their proliferation increased significantly, as well as the number of differentiated (CNP+) and mature (MBP+) OL. Moreover, NF and TUB protected OL from LPC toxicity when added at the time of LPC treatment: they increased OLP proliferation, as well as the number of CNP+ and MBP+ OL significantly, compared to cultures treated only with LPC. On the contrary irrelevant proteins (actin, and skin proteins) were ineffective, demonstrating the specificity of the cytoskeleton proteins effects. Importantly, NF and TUB increase OLP survival and proliferation when challenged with LPC, without delaying differentiation and maturation. We hypothesize that release of NF and TUB following axonal damage in MS could participate in the regulation of remyelination through this process. This putative phenomenon might be complexified by alterations of axon protein expression, or of proteolysis, known to occur in MS models.
T06‐25B. A new algorithm for counting microglial cells in wholemount mice retinas
J.J. Salazar 1, B.I. Gallego1,2, A.I. Ramírez1,2, B. Rojas1,3, R. de Hoz1,2, A. Triviño1,3, J. Ramírez1,3, P. De Gracia4
1Universidad Complutense (UCM), Inst Invest Oftalmol Ramón Castroviejo, Madrid, Spain 2 Universidad Complutense (UCM), Facultad Optica y Optometria, Madrid, Spain 3 Universidad Complutense (UCM), Departamento Oftalmologia. Facultad Medicina, Madrid, Spain 4Consejo Superior de Investigaciones Científicas (CSIC), Instituto de Optica Daza de Valdes, Madrid, Spain
Purpose: Microglial proliferation is commonly accepted as a hallmark of glial activation. An automatic method that allows assessing the number of microglial cells to analyze the proliferative microglial behavior in a laser‐induced ocular hypertension (OHT) model has been developed.
Methods: Adult albino Swiss mice were organized in two groups: naive (age‐matched control; n = 6) and lasered (n = 6). Retinal wholemounts were immunostained with anti‐Iba1 and images of Iba‐1+ microglias were recorded with a fluorescence microscope. New algorithms of segmentation and control of distances were developed in Matlab to obtain the number of Iba‐1 microglial cells. Automatic results were compared with those obtain by direct human observation of the samples.
Results: The automatic method detected the number of Iba‐1+ cells in the inner and outer plexiform layers of the retina in both, naïve and OHT‐retinas. The number of cells present in the samples was not an obstacle for the program to run properly. The time required for counting Iba‐1+ cells decreased from the human guide to the program based counting method from days to one hour. Results show a strong correlation between both automatic and manual method (Pearson correlation test, R = 0.979; P = 0.000 and R = 0.942; P = 0.000 for outer and inner plexiform layer respectively) indicating the consistency of the automatic counting.
Conclusions: A new, reliable and quick algorithm was developed with Matlab to obtain the number of Iba‐1+ microglial cells and also cellular density maps through retinal wholemounts in both naïve and other proliferative conditions (i.e. glaucoma). Due to this new automatic method, a bigger set of images or samples could be included in future studies to analyze the behavior of microglial cells.
T06‐26A. Characterization of Schwann cells in mouse sciatic nerve slice: electrophysiological properties and neurotransmitter receptor expression
N. Fröhlich, D. Eißler, M. Kukley
Centre for Integrative Neuroscience (CIN), Tübingen, Germany
Schwann cells (SCs) are the myelinating cells in the peripheral nervous system (PNS). Results of few immunohistochemical and pharmacological studies available to date suggest that SCs in the PNS express ionotropic and metabotropic receptors for glutamate and ACh (Dememes et al., 1995; Kinkelin et al., 2000; Loreti et al., 2006; Vrbova et al., 2009). Furthermore, neurotransmitters are able to induce changes of SC membrane potential in the giant squid axons (Villegas et al., 1987), and these changes are abolished by application of glutamate or ACh receptor antagonists (Lieberman et al., 1989; Lieberman and Sanzenbacher, 1992). These findings indicate that neurotransmitter receptors on SCs in the nerve are functional.
We are interested to understand whether SCs in the peripheral nerves of mammals express functional ionotropic glutamate receptors, and whether SCs use these receptors for communication with axons. To start addressing this question, we established a preparation of mouse live sciatic nerve slices employing “know‐how” of live brain slices technique. Using this preparation, we performed whole‐cell patch‐clamp recordings of SCs at different stages of development (embryonic and early postnatal). We included a fluorescent dye into the pipette solution in order to perform post‐recording morphological analysis of single SCs. First, we recorded current responses of SCs to a series of depolarizing voltage steps, aiming to test whether all SCs in the nerve are electrophysiologically akin. We found that mouse SCs differ in their passive membrane properties and expression of voltage‐gated K+ channels: depending on the age, 3 to 4 cell types could be distinguished. Post‐recording morphological characterization of the recorded cells showed that SCs in the mouse sciatic nerve differ in their size, as well as in the number and length of their processes. Next, we used fast pressure‐induced application of glutamate to investigate whether SCs possess ionotropic glutamate receptors, and whether electrophysiologically distinct SC types differ in their expression of glutamate receptors. Application of 1 mM glutamate caused an inward current in at least two types of SCs, and preliminary analysis revealed a peak current amplitude in the range of 14–250 pA (n = 18). Glutamate‐induced currents in SCs were blocked by GYKI53655, indicating that mouse SCs carry ionotropic glutamate receptors of AMPA/kainate type. Currently we are studying which subunits of AMPA receptors are present in SCs, and how these receptors get activated in situ.
T06‐26B. The role of myelin gap junctions in the regulation of axonal cytoskeleton
N. Schiza, I. Sargiannidou, K. Kleopa
The Cyprus Institute of Neurology and Genetics, Nicosia, Cyprus
Question‐ Mutations affecting the gap junction (GJ) protein connexin32 (Cx32) cause the X‐linked Charcot‐Marie‐Tooth disease (CMT1X). Although Cx32 is expressed by myelinating Schwann cells in the peripheral nerves, patients with CMT1X develop relatively early axonal degeneration, which correlates best with chronic disability. We found in previous studies of Gjb1‐null mice, a model of CMT1X, that the diameter of myelinated axons was progressively reduced at the age of 2 months compared to wild type (WT) littermates, resulting from progressive dephosphorylation of axonal neurofilaments and increased packing density. Fast axonal transport was slower in distal axons of mutant compared to WT animals, with impaired mobility of synaptic vesicle‐associated proteins and accumulation of beta‐amyloid precursor protein and tau. How Cx32‐formed GJs are involved in the regulation of axonal cytoskeleton dynamics remains unclear.
Methods‐ Here we investigated the signaling mechanisms regulating axonal cytoskeleton focusing on major kinases and phosphatases involved in neurofilament phosphorylation by immunohistochemistry and immunoblot. In addition we examined the localization of axonal mitochondrial, which also depend on axonal transport. We focused on nerve pathology in 2 month‐old Gjb1‐null mice, when demyelination and inflammation is minimal, in order to identify the direct effects of GJ loss on the axon.
Results‐ Our results show that ERK1/2 kinase and PP2A phosphatase were localized in non‐compact myelin areas, including the Schmidt‐Lantermann incisures and paranodes, where GJs are normally formed by Cx32. Another kinase, Cdk5, was more diffusely localized along the axon. Biochemical analysis showed altered amounts of ERK1/2, PP2A and Cdk5 as well as their upstream activators in Gjb1‐null nerves. The PP2A inhibitor PHAPI was also localized in non‐compact myelin areas and significantly increased in Gjb1‐null nerves. Assessment of axonal mitochondria by porin immunostaining showed significantly increased density in Gjb1‐null nerves, consistent with slower axonal transport.
Conclusion‐ Our findings clarify some mechanisms of early axonal pathology that are independent of demyelination in this mouse model of CMT1X. Several components of the signaling pathway regulating axonal cytoskeleton and axonal transport appear to be functionally related to GJs at the non‐compact myelin sheath. Loss of myelin GJs results in impaired regulation of axonal cytoskeleton, energy supply, and axonal transport.
T06‐27A. Transfer of Exosomes from Oligodendrocytes to Neurons
D. Fröhlich 1, W.P. Kuo1, C. Frühbeis1, W. Möbius2, K.‐A. Nave2, A. Schneider2, M. Simons2, M. Klugmann3, S. Pinto4, B. Kyewski4, J. Trotter1, E.‐M. Krämer‐Albers1
1University of Mainz, Molecular Cell Biology, Mainz, Germany 2Max Planck Institute of Experimental Medicine, Department of Neurogenetics, Göttingen, Germany 3University of New South Wales, Department of Physiology, Sydney, Australia 4German Cancer Research Center, Division of Developmental Immunology, Germany
Introduction: Exosomes are small membranous vesicles of endocytic origin which are released by almost every cell type and have been implicated to play important roles in intercellular communication. We have recently discovered that oligodendrocytes secrete exosomes containing a distinct set of proteins as well as mRNA and microRNA. Intriguingly, oligodendroglial exosome release is stimulated by the neurotransmitter glutamate indicating that neuronal electrical activity controls glial exosome release. In this study we examined the role of exosomes in neuron‐glia communication and its implications in glial support.
Results: To analyze the transfer of oligodendroglial exosomes to neurons, we exposed cultured cortical neurons to fluorescently labeled oligodendroglial exosomes. Indeed, cultured cortical neurons internalized and accumulated oligodendroglial exosomes in the neuronal cell soma in a time‐dependent manner. Addition of endocytosis inhibitors or expression of dominant negative dynamin interfered with neuronal exosome internalization indicating that exosome uptake is mediated by clathrin‐dependent endocytosis. Furthermore, neuronal internalization of exosomes resulted in functional retrieval of exosomal cargo in vitro and in vivo upon stereotactic injection of exosomes. To investigate the influence of oligodendroglial exosomes on neuronal gene expression we performed a microarray screen of neuronal mRNAs after exosome exposure. Interesting candidates were further validated by qRT‐PCR.
Conclusion: Taken together, our results represent a proof of principle of exosome transmission from oligodendrocytes to neurons suggesting a new route of horizontal transfer in the CNS.
T06‐27B. The Principle of Ca2+ Integration in Single Astrocytes
Y.‐W. Wu1, X. Tang1, M. Arizono1, H. Bannai1, P.‐Y. Shih1, M. Tanaka1, S. Itohara1, K. Mikoshiba1, A. Semyanov 2
1RIKEN Brain Science Institute, Wako‐shi, Japan 2RIKEN Brain Science Institute, Tokyo, Japan
Astrocytes are essential for the regulation of neuronal excitability, synaptic plasticity, and the vascular coupling of brain metabolism. Unlike neurons, they are electrically silent, but produce a complex repertoire of Ca2+ events that coordinate their major functions. However, the canonical cellular mechanism for Ca2+ integration in astrocytes is unknown. Using cultured astocytes and astrocytes in brain slices expressing Ca2+sensor, GCaMP2, we report a candidate principle for Ca2+ integration in single astrocytes. Analysis of the spatiotemporal dynamics of Ca2+ signaling in cultured astrocytes demonstrated that Ca2+ event distribution can be described by a power law. We show that metabotropic glutamate receptor activation or changes in extracellular Ca2+ regulate the power law exponent. These findings demonstrate scale‐free Ca2+ dynamics in astrocytes that can provide a potential computational theory for brain operation and metabolism.
T06‐28A. Neurotransmitter signaling controls exosome secretion from oligodendrocytes
C. Frühbeis 1, D. Fröhlich1, S. Tenzer2, W.P. Kuo1, W. Möbius3, A. Saab3, F. Kirchhoff4, J. Trotter1, E.‐M. Krämer‐Albers1
1University of Mainz, Molecular Cell Biology, Mainz, Germany 2University of Mainz, Immunology, Mainz, Germany 3MPI of Experimental Medicine, Neurogenetics, Göttingen, Germany 4University of Saarland, Homburg, Germany
The biogenesis of the axon‐myelin unit is controlled by reciprocal interactions between oligodendrocytes and neurons, which continue throughout life. Oligodendrocytes secrete endosome‐derived microvesicles termed exosomes, implicated in intercellular communication. These exosomes carry a specific set of proteins as well as RNA species. Here, we show that the neurotransmitter glutamate stimulates exosome secretion from oligodendrocytes by provoking Ca2+ entry through oligodendroglial NMDA and AMPA receptors. Furthermore, active neurons evoke exosome release from oligodendrocytes, indicating that neuronal activity controls oligodendroglial exosome release. In turn, neurons internalize oligodendroglial exosomes and functionally retrieve the exosomal cargo. Thus, oligodendrocytes may influence the neuronal metabolism by an exosome‐dependent transfer of bioactive substances to neurons. Axonal degeneration resulting from lack of glial support occurs in PLP and CNP deficient mice. Intriguingly, both proteins are components of oligodendroglial exosomes. A proteomic approach revealed that amount and composition of exosomes derived from PLP‐/‐ and CNP‐/‐ oligodendrocytes are altered, supporting the hypothesis that disturbed intercellular transfer of substances by exosomes may contribute to axonal degeneration in these mouse models.
T06‐28B. Function of human‐specific sialic acid binding receptor Siglec‐11 in amyloid‐β mediated neurotoxicity
A. Shahraz, J. Kopatz, H. Neumann
University of Bonn, Bonn, Germany
Microglial cells are the resident macrophages of the brain, which are derived from the embryonic haematopoiesis of the yolk sac. Recently, we established a protocol for in vitro differentiation of pluripotent stem cells into microglial precursors by mimicking the embryonic haematopoiesis in a neural microenvironment. We now succeeded to produce microglia from human induced pluripotent stem (iPS) cells. These human microglial cells were responsive to amyloid‐β by increasing reactive oxygen species (ROS) production. In addition, we produced primitive neural stem cells (pNSC) from iPS cells that differentiated with suitable growth factors towards neurons. In human microglia‐neuron co‐culture, amyloid‐β treatment resulted in shorter neural branches length compare to the control group. Furthermore, results showed that the inhibitory human lineage specific receptor sialic acid binding immunoglobulin‐like lectin‐11 (Siglec‐11) was expressed on human microglial lines. We are now studying the neuroprotective role of Siglec‐11 in the amyloid‐β‐mediated microglia‐neuron co‐culture model. By expressing the human‐lineage specific receptors, these microglial cells will be a suitable model to investigate these receptors in a human microglia‐neurons co‐culture system.
T06‐29A. Characterization of glial cells in organotypic cultures of rat retina
B.I. Gallego 1, A.I. Ramírez1,2, M. Dierstein3, R. de Hoz1,2, B. Rojas1,4, A. Triviño1,4, J. Ramírez1,4, M. Ueffing3, J.J. Salazar1,2, B. Arango‐Gonzalez3
1Universidad Complutense (UCM), Inst Invest Oftalmol Ramón Castroviejo, Madrid, Spain 2Universidad Complutense (UCM), Facultad Optica y Optometria, Madrid, Spain 3Eberhard Karls Universität Tübingen, Institute for Ophthalmic Research , Tübingen, Germany 4Universidad Complutense (UCM), Departamento Oftalmologia. Facultad Medicina, Madrid, Spain
Purpose: Organotypic retinal culture is a useful tool to perform preclinical drug testing, in which cellular interactions as well as tissue three‐dimensionality are preserved. The present study aimed to provide a detailed characterization of the morphologic changes of macro and microglial cells in this culture system.
Methods: Retinas were isolated from 7 days old Crl: CD(SD) rats with the retinal pigment epithelium attached as described previously (Arango‐Gonzalez et al. 2010). Explants were cultured for 4, 10 and 14 days (DIV4, DIV10 and DIV14). Glial cell populations were characterized by immunostaining cryosections and wholemounts of age‐matched control and cultured retinas using specific antibodies against GFAP, vimentin and CD11‐b.
Results: In DIV4 and DIV10 cultures, GFAP‐positive astrocytes were more robust and not homogeneously distributed as observed in vivo: in some retinal areas the astrocytic network was thicker and in other regions astrocytes were sparsely distributed. At DIV14 only few thinned GFAP‐positive astrocytes remained. GFAP immunoreactivity of Müller cells was up‐regulated in culture. However, no major difference was observed in vimentin staining between both, in vivo and in vitro retinas. Müller cells extended processes from the inner to the outer limiting membrane were observed for all examined retinas. Microglial cells stained with CD11‐b at DIV4 and DIV10 had somas more robust than in vivo and cell processes were thicker and retracted. Same cells at DIV14 exhibited mainly a rounded morphology.
Conclusions: Progressive morphological changes were observed in glial cell populations in retina explants. These changes include reactive macrogliosis, rearranged astrocytic distribution and microglial activation. Since glial response is a hallmark of several retinal diseases, our organotypic retinal culture is a valuable resource for future investigations in retinal degenerative processes and therapy.
T06‐29B. Ultrastructural investigation of lateralized experience‐dependent synaptic plasticity in rat hippocampal CA1 stratum radiatum
Y. Shinohara, A. Hosoya, H. Hirase
RIKEN, Wako, Japan
It is widely recognized that grouped housing in enriched environment (ENR) impacts the animals' brain structure and function. For instance, rats reared in ENR exhibit enhanced neurogenesis in the dentate gyrus, improved hippocampus‐dependent spatial memory task performance, and spine density increases of CA3 and CA1 pyramidal neurons. We found that that ENR housing for four weeks after weaning induces an enhancement in gamma band local field potential oscillation power and an increase in spine density in the right CA1 stratum radiatum of Long‐Evans rats. The mean spine size, as assessed by serial measurements of postsynaptic density areas, did not change substantially by laterality or rearing condition. One standing question is how glial processes are organized in ENR treated rats. Unlike cerebellar parallel fiber synapses, the astrocytic coverage of a hippocampal synapse varies from synapse to synapse. Our preliminary electron microscopic observation suggests that right CA1 str. radiatum excitatory synapses tend to have larger degrees of astrocytic coverage in ENR treated rats than singly caged rats.We are currently making morphometric measurements to quantitatively assess the peri‐synaptic glial changes.
T06‐30A. Neuron‐astrocyte‐microglia interactions in a rat model of chronic cerebral ischemia
M.G. Giovannini 1, D. Lana1, A. Melani2, F. Pedata2
1University, DSS, Firenze, Italy 2University, Neurofarba, Firenze, Italy
It is becoming more and more evident that proper functioning of the neuron‐astrocyte‐microglia triad is fundamental for the functional organization of the brain, and thus it is of the utmost importance to better characterize their interactions in physiological and pathological processes. We recently demonstrated, in rat models of normal brain aging and LPS‐induced acute inflammation, that astrocytes and microglia actively collaborate in the clearance of apoptotic neurons and neuronal debris associated with programmed cell death. Here we studied the interactions between neurons, microglia and astrocytes within the CA1 region of the hippocampus after bilateral common carotid artery occlusion (bCCAo) in the rat, a valid model of chronic cerebral hypoperfusion which leads to persistent ischemic conditions and ultimately to neuronal death. Male Wistar rats were subjected to permanent bCCAo. A group of rats was infused into the jugular vein with dipyridamole (7 days, 4 mg/kg/day by an osmotic minipump). Sham‐operated animals were used as controls. Three months after bCCAo, immunohistochemical studies were performed on brain coronal slices, focussing on the hippocampal CA1 region. Using an antibody against glial fibrillary acidic protein (GFAP) no astrogliosis was detected. We found a significant increase in the number of total microglia, visualized using the IBA1 antibody, in bCCAo‐treated rats in comparison to the sham group (+ 18%, P < 0.01, one way ANOVA and Newman‐Keuls) and this effect was completely reverted by dipyridamole (P < 0.01, one way ANOVA and Newman‐Keuls). As exemplified in Figure 1, in the CA1 and Str. Radiatum of bCCAo‐treated rats, many neurons (anti‐NeuN antibody, red) showing signs of degeneration were closely apposed to and infiltrated by astrocyte branches (anti‐GFAP antibody, green) which appeared to be bisecting the cell body into cellular debris, and microglia cells (anti‐IBA1, antibody, blue) were actively phagocytosing the damaged neurons. This finding is consistent with the scavenging activity of microglia upon dying neurons or debris, a possible mechanism that prevents further injury to neighboring neurons. It will be interesting to investigate which intercellular communication mechanisms allow the recruitment and activation of different glial cells in a well‐organized reciprocal interaction to scavenge the damaged neurons and to verify the mechanisms of the protective effects of dipyridamole found in this chronic cerebral ischemic model.
Partly funded by Banco S. Paolo and Boehringer Ingelheim.

T06‐30B. Super‐resolution optical microscopic measurements of synaptobrevin 2 in vesicles of cultured astrocytes
P. Singh 1, M. Potokar1,2, M. Kreft1,2, R.F. Stout3,4, J. Jorgačevski1,2, V. Parpura4,5 , R. Zorec1,2
1University of Ljubljana, Ljubljana, Slovenia 2Celica Biomedical Center, Ljubljana, Slovenia 3Albert Einstein College of Medicine, New York, United States 4University of Alabama, Department of Neurobiology, Alabama, United States 5University of Rijeka, Department of Biotechnology, Rijeka, Croatia
Astrocytes are glial cells which provide important metabolic support to neurons, actively tune synaptic activity and influence brain microcirculation [1]. One of the key processes which sustain astrocyte communication with neighbouring cells is regulated exocytosis mediating the release of gliotransmitters (peptides, amino acids, ATP), delivery of membrane transporters, channels and other molecules to the plasma membrane. The exocytic gliotransmitter release is thought to be associated with SNARE complexes. These consist of four α‐helices where one of these is contributed by syntaxin‐1, one by synaptobrevin‐2 (sb2) and two by SNAP‐23 in astrocytes. Out of these, sb2 is a trans‐membrane protein present on the secretory vesicle [2]. However, the subvesicular nano‐architecture and the number of sb2 molecules per vesicle are unclear. Moreover, it is also unknown how many sb2 molecules are involved in the fusion between the vesicle and the plasma membrane in astrocytes. To study single vesicles and their association with sb2 in live astrocytes, we generated a pH‐sensitive indicator yellow synaptopHluorin (YspH) as a marker for sb2 and as a functional readout for monitoring the properties of fusion pores, which are formed following the merger between the vesicle and plasma membranes. In an acidic environment YspH is non‐fluorescent and becomes fluorescent upon alkalinisation, permitting the study of fusion of vesicles with the membrane during gliotransmitter release [3]. Our preliminary results, obtained by confocal fluorescence microscopy (with the resolution limit of about 200 nm) and by a super‐resolution microscopic technique structured illumination microscopy (SIM; with the resolution limit of about 120 nm) show that the new probe (i.e. YspH) efficiently reports the fusion pore establishment in astrocytes and the configuration of sb2 molecules in a vesicle.
REFERENCES
[1] Halassa MM, Fellin T and Haydon PG (2007) The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med 13(2):54–63.
[2] Crippa D, Schenk U, Francolini M, Rosa P, Verderio C, Zonta M, Pozzan T, Matteoli M and Carmignoto G (2006) Synaptobrevin2‐expressing vesicles in rat astrocytes: insights into molecular characterization, dynamics and exocytosis. J Physiol 570(3):567–582.
[3] Miesenbock G, De Angelis DA, & Rothman JE (1998) Visualizing secretion and synaptic transmission with pH‐sensitive green fluorescent proteins. Nature 394(6689):192–195.
T06‐31A. Epigenetic induction of the Ink4a/Arf locus prevents Schwann cell overproliferation during nerve regeneration and after tumorigenic challenge
C. Gomis Coloma 1, J.A. Gomez‐Sanchez2,1, C. Morenilla‐Palao1, G. Peiro2, E. Serra3, M. Serrano4, H. Cabedo Marti2,1
1Instituto Neurociencias Alicante UMH‐CSIC, San Juan de Alicante, Spain 2Hospital General Universitario Alicante, Alicante, Spain 3Institut de Medicina Predictiva i Personalitzada del Cancer (IMPPC), Barcelona, Spain 4Centro Nacional de Investigaciones Oncologicas (CNIO), Madrid, Spain
The number of Schwann cells is fitted to the axonal length in the peripheral nerves. This setting is lost when tumorigenic stimuli induce uncontrolled Schwann cell proliferation, generating tumours such us neurofibromas and schwannomas. Schwann cells proliferate as well during Wallerian degeneration. In both cases proliferation is finally arrested. We show that in neurofibroma, the induction of Jmjd3 removes trimethyl groups on lysine‐27 of histone‐H3 and epigenetically activates the Ink4a/Arf‐locus, forcing Schwann cells towards replicative senescence. Remarkably, loss of function of this mechanism allows unrestricted proliferation, inducing malignant transformation of neurofibromas. Interestingly, our data suggest that in injured nerves, Schwann cells epigenetically activate the same locus and go as well into the senescence program. Indeed, when this pathway is genetically blocked, cell proliferation results increased after nerve injury. We postulate that the Ink4a/Arf‐locus is expressed as part of a physiological response that prevents uncontrolled proliferation of the de‐differentiated Schwann cells generated during nerve regeneration, a response that is also activated to avoid overproliferation after tumorigenic stimuli in the peripheral nervous system.
T06‐31B. Effects of P2Y12 Signaling on Microglial Morphology and Motility in Visual Cortex
G. Sipe, A. Majewska
University of Rochester Medical Center, Rochester, United States
Synaptic plasticity is critical for normal neurodevelopment and proper circuit function in the adult nervous system. Recent evidence suggests that microglia, classically studied in neuroinflammation, play critical roles in neurodevelopment and synaptic plasticity. However, the mechanisms driving these roles are not well understood. To start elucidating the molecular players involved in microglial communication with neurons during plasticity, we decided to first explore the role of purinergic signaling in this process. While purinergic signaling has been implicated in microglial behaviors, studies have primarily focused on neuroinflammatory roles. However, non‐inflamed microglia highly express the purinergic receptor, P2Y12, which has known functions in microglial chemotaxis in early inflammation and can stimulate the release of cytokines implicated in synaptic plasticity. Thus, we posited that purinergic signaling could contribute to the microglial motility that underlies synapse surveillance in the non‐inflamed brain. This in turn may be critical for microglial roles in synaptic refinement during development. To explore this possibility, we took advantage of the P2Y12 knock‐out (KO) mouse and examined the morphology and motility of microglia in the visual cortex as compared to microglia in wildtype mice. First, we used immunohistochemistry for the microglia specific ionized calcium‐binding adapter (Iba‐1) protein to stain microglia in fixed sections. We then used confocal imaging and Scholl analysis to investigate the complexity of the microglial processes. We also used 2‐photon microscopy to monitor microglial motility in P2Y12 KO mice by crossing them with CX3CR1‐GFP knock‐in mice that allow visualization of microglia including fine processes in vivo. Our preliminary evidence suggests that P2Y12 disruption alters basal microglial morphology and behavior making microglia less complex and altering their motility patterns. We are now investigating whether microglia‐synapse interactions, as well as functional plasticity elicited by visual experience are altered in P2Y12 KO mice. Our studies will expand our understanding of emerging roles for microglia in neurodevelopment and will pioneer new non‐pathological roles for microglial purinergic signaling.
T06‐32A. Tonic suppression of synaptic and extrasynaptic inhibition in the striatum as a consequence of GLT‐1 deficiency in mice carrying a mutant form of huntingtin
A. Dvorzhak, A. Wojtowicz, R. Grantyn
Charité – University Medicine, Berlin, Germany
In neurodegenerative diseases, the afflicted brain is both an important object of study and an opportunity to characterize a given cellular interaction from a pathophysiological perspective.
This dual approach is particularly advantageous when human disease is based on a monogenetic defect and an appropriate animal model becomes available for detailed investigation, as in case of Z_Q175_KI (Q175), a new knock‐in mouse expressing a mutant form of murine huntingtin.
Electrophysiological recordings of GABAergic unitary IPSCs from striatal output neurons (SONs) in sagittal slices from wild‐type and homozygous Q175 challenged the current viewpoint that GABAergic transmission is enhanced in the HD striatum. Quantal analysis in combination with high frequency stimulation and paired pulse tests revealed that synaptic GABA release is in fact tonically suppressed resulting in disinhibition of striatal output activity (Dvorzhak et al J Physiol 2013).
The underlying mechanism involves a retrograde endocannabinoid signalling pathway linking postsynaptic mGluR5 with presynaptic CB1 and GABA release.
In addition to this deficit, Z‐Q175_KI homozygotes exhibited a significant reduction of tonic inhibition via extrasynaptic GABA(A) receptors.
Using pharmacological tools to alter the ratio of ambient glutamate and GABA concentrations made clear that the HD‐related depression of synaptic and extrasynaptic GABAergic actions depends on the state of both the astrocytic glutamate transporter GLT‐1 and the GABA transporter GAT‐3.
In support of Héja and colleagues, our imaging experiments suggest that in normal striatal astrocytes GAT‐3 is in a releasing mode and driven by the activity of GLT‐1.
However, the latter activity is much lower in the HD striatum. SBFI recordings of GLT‐1‐related Na transients and patch clamp recordings of GLT‐1 transporter currents revealed a marked reduction (less than 50% of WT level) in SR‐labelled astrocytes from symptomatic HD mice.
Together, our results suggest that the deficiency of astrocytic glutamate uptake might represent a pathophysiological key mechanism underlying the observed disinhibition in the striatum of mice affected by Huntington's disease.
T06‐32B. Nascent nodes of Ranvier are formed on hippocampal GABAergic neurons prior to myelination
N. Sol‐Foulon 1, S. Freeman1, A. Desmazières1, M. Gatta1, J. Simonnet1, P. Michel1, S. Guerreiro1, D. Fricker1, Y. Yanagawa2, C. Lubetzki1
1CRICM, UMR7225, Paris, France 2University, Tokyo, Japan
Nodes of Ranvier are highly enriched in voltage‐gated sodium channels (Nav), allowing rapid action potential propagation on myelinated axons. Cell adhesion molecules (neurofascin‐186 (Nfasc186) and contactin) and scaffold proteins (ankyrinG and bIVspectrin), which are also enriched at the nodes, have a critical role in assembly and/or stabilization of Nav clusters. Oligodendrocytes have been described to induce Nav channel clustering at nodes in the central nervous system (CNS); however, the nature of the signal(s) involved in nodal formation remains mostly unknown in the CNS.
To gain insight into CNS node assembly, we developed hippocampal neuronal cultures from E18 rat embryos. During the first week, as expected, Nav channel accumulation was detected at the axon initial segment (AIS), co‐localized with ankyrinG and Nfasc186, whereas expression along the axon was diffuse and hardly visible. In contrast, at 14 days in vitro (DIV), regularly spaced clusters of Nav channels, ankyrinG and Nfasc186 were detected along the axon. The percentage of hippocampal axons with clusters increased from 4.1 ± 3.1% at 14 DIV, to 15.8 ± 3.5% and 17.1±7.1% at 17 and 21 DIV, respectively (mean ± sd). Importantly, these clusters were formed in the absence of myelin and no accumulation of the paranodal protein Caspr was detected at this stage. To study the role of extrinsic versus intrinsic pro‐aggregating factors, we analyzed nearly pure hippocampal neuronal cultures. These purified neurons still formed AIS, while only few axons formed Nav clusters. Interestingly, when these purified neurons were co‐cultured with oligodendrocytes or with conditioned medium from oligodendrocytes, the percentage of neurons with nascent nodes was greatly enhanced. Taken together, these results confirm that some glial soluble factor(s) might be involved in Nav channels clustering, as previously shown for retinal neurons (Kaplan et al., 2001). Furthermore, nascent nodes were detected on GABAergic neurons, but not on glutamatergic hippocampal neurons, thus arguing also for the role of intrinsic neuronal factors in their establishment. Using hippocampal sections from GAD67‐GFP mice, we showed that some GABAergic interneurons were myelinated in vivo. Moreover, we observed that nascent node formation prior to myelination also takes place in vivo, strengthening physiological relevance. The electrophysiological properties of neurons with nascent nodes will be studied.
T06‐33A. Basal forebrain lesions reduce acetylcholinergic tone, induce microglial priming and predispose mice to inflammation‐induced cognitive deficits
E.W. Griffin, R. Field, C. Cunningham
Trinity College Dublin, Dublin, Ireland
Microglia in the degenerating or aging brain are primed by aspects of pathology to produce exaggerated pro‐inflammatory responses to subsequent central and systemic inflammatory insults. However, the mechanisms by which microglia become primed, rather than adopting an M1 phenotype, are not clear. Triggers for priming may include altered expression of complement, recognition of amyloid or neuronal/synaptic debris for phagocytosis, decreased engagement of microglial regulatory molecules such as CD200R, TREM2 or fractalkine receptor. There is also evidence that loss of basal neurotransmitter tone may contribute to this state. Microglia are known to express the nicotinic cholinergic a7 receptor, which has been shown to influence macrophage and microglial reactivity. In the current study we performed limited lesions of the basal forebrain cholinergic system using the murine‐p75‐saporin immunotoxin (mu‐p75‐sap), to investigate the degree to which loss of cholinergic tone predisposes the microglia to subsequent inflammatory stimulation and to assess the consequences of this for cognitive function in the vulnerable brain. Intracerebroventricular injection of mu‐p75‐sap (0.08 μg) depleted cholinergic neurons in the basal forebrain and decreased cholinergic innervation of the hippocampus, but left performance on hippocampal‐dependent reference and working memory tasks relatively intact. However, decreased cholinergic innervation of the hippocampus conferred increased susceptibility to cognitive deficits induced by systemic LPS (100 μg/kg) 40 days after lesioning, but microglia were not primed 40 days after 20% lesions. To investigate the regional, temporal and neurochemical basis of this we induced more severe lesions of the basal forebrain (using 1.2 μg mu‐p‐75 sap) and assessed microglial priming at 14 or 40 days post‐lesion. These data demonstrate that microglia are primed by low dose challenge at 14 but not 40 days and, when more robust lesions are induced, priming remains even at 40 days. These responses are observed at the site of injury (medial septum) but more profoundly at the site of denervation, the hippocampus. Indicating that neuronal injury and, particularly, loss of cholinergic tone have a profound effect on the reactivity of the microglia to subsequent inflammatory challenge. These data expand our knowledge of microglial priming and have clear implications for the interaction of cholinergic tone and inflammation in cognitive dysfunction such as dementia and delirium in which cholinergic dysfunction is implicated.
T06‐33B. K+ and glutamate trigger the release of lactate from astrocytes within seconds, as detected by a lactate sniffer cell
T. Sotelo Hitschfeld 1,2, I. Ruminot1,2, A. San Martin1,2, L.F. Barros1
1Centro de Estudios Científicos, Valdivia, Chile 2Universidad Austral de Chile, Valdivia, Chile
Synaptic activity is accompanied by a fast increase in interstitial lactate concentration whose source remains unclear. Astrocytes increase their release of lactate within minutes of exposure to glutamate (Pellerin and Magistretti, PNAS 1994). Using fluorescent glucose analogs and a genetically‐encoded FRET glucose nanosensor, we have observed in astrocytes that within seconds of exposure, glutamate stimulates the glucose transporter GLUT1 while K+ stimulates hexokinase (Bittner et al., J. Neurosci. 2011). However, glucose may also be metabolized to glycogen or oxidized to CO2, so it is not obvious that the stimulation of GLUT1 and hexokinase leads to lactate release.
The objective of this work was to investigate whether lactate may be released by cultured mouse astrocytes within the time frame of the interstitial lactate rise observed in vivo. A first approach with an enzymatic assay showed a significant increase in extracellular lactate after 1 minute of exposure to 12 mM K+ or 50 μM glutamate. To obtain better time resolution we developed a “lactate sniffer”, a HEK293 cell that expresses the FRET lactate nanosensor Laconic (San Martín et al., PLOS ONE 2013). Seeded on top of an astrocytic monolayer, the sniffer cell responded within 10 seconds of exposure to elevated K+ 12 mM and a similar response was elicited by 3 mM Ba2 +. The response to K+ and Ba2+ supports a role for the Na+/bicarbonate co‐transporter NBCe1, previously shown to mediate the stimulation of astrocytic glycolysis (Ruminot et al., J. Neurosci., 2011). The sniffer cell detected an equally fast increase in extracellular lactate when the culture was exposed to 50 μM glutamate. Previous work had shown that glutamate does not stimulate glucose consumption in the short‐term (Bittner et al., J. Neurosci. 2011), and therefore the rapid release of lactate came as a surprise. This phenomenon may relate to glycogen mobilization or perhaps to inhibition of astrocytic oxygen consumption (Azarias et al., J. Neurosci. 2011). K+, Ba2+ and glutamate did not produce detectable effects on the sniffer cell in the absence of astrocytes.
The rapid release of lactate by astrocytes exposed to K+ and glutamate supports a role for these cells in the fast increase in interstitial lactate concentration that accompanies synaptic activity.
T06‐34A. A specific role for the Na,K‐ATPase α2 isoform in the support of astrocyte glutamate uptake
N. Illarionova, H. Brismar, A. Aperia, E. Gunnarson
Karolinska Institutet, Stockholm, Sweden
A tightly regulated intracellular sodium (Nai) homeostasis is of fundamental importance for all cells. In astrocytes pulsative changes in the Nai concentration ([Na+]i) are regularly occurring as a result of uptake of glutamate from the synaptic space. Glutamate uptake occurs via the glutamate/Na+ co‐transporters GLAST and GLT‐1, where one glutamate is accompanied by 3 Na+ and 1 H+ in exchange for 1 K+. The salt pump, Na,K‐ATPase (NKA), is responsible for the maintenance of the trans‐membrane Na+ gradient by exporting 3 Na+ and importing 2 K+ for each ATP. NKA is dose‐dependently inhibited by ouabain. Astrocytes express two isoforms of the catalytic NKA α subunit, the ubiquitous α1 and in the CNS the glia‐specific α2. The isoforms differ with regard to Na+ affinity, which is lower for α2 and with regard to ouabain affinity, which is higher for α2 than for α1. Although α2 mutations give rise to neurological symptoms – familial hemiplegic migraine type 2, the relative roles of the α1 and α2 isoforms in astrocytes are still incompletely understood.
We hypothesized that the low Na affinity of α2 makes it more suitable to cope with transient large increases in Nai. To test this, we compared the effect of 200 µM glutamate for 10 min on real time changes of [Na+]i in primary astrocytes, which, following transient transfection, predominantly expressed either the α1 or the α2 isoform. In α1 cells glutamate caused a significantly larger increase in [Na+]i and longer recovery time to base line [Na+]i than in cells expressing α2. Glutamate uptake dependence on either α isoform was determined by aspartate uptake in astrocytes expressing endogenous α1 and α2. In the presence of ouabain concentrations that selectively inhibit α2, we found a modest (1.9mM) increase in [Na+]i, accompanied by a disproportionately large decrease (24%) in glutamate uptake.
It has been suggested that astrocyte glutamate transporters are clustered in microdomains where they may interact with NKA. In GST pull down assays on rat brain we found that both GLT‐1 and GLAST interacted with the 1st intracellular loop of both α1 and α2, but the interaction was significantly stronger for the α2 isoform. No interaction was found with other segments of the α molecule.
The data indicate that the NKA α2 isoform, which has a more restricted expression than α1, plays a specific role for the interaction with the glutamate transporters and for sodium homeostasis in astrocytes. This specificity may be attributed to low sodium affinity of α2 and to the relatively high capacity of α2 to interact with the astrocyte glutamate transporters.
T06‐34B. Remodeling of glial coverage of rat NTS glutamatergic synapses after ozone inhalation
C. Strube, K. Chounlamountry, V. Penalba, F. Gackiere, J.‐P. Kessler
CRN2M, UMR7286, CNRS/Aix‐Marseille Université, Marseille, France
Ozone, a major component of air pollution, has considerable impact on public health. Besides its well described inflammatory and dysfunction effects on the respiratory tract, there is accumulating evidence indicating that ozone exposure also affects brain functions. However, the mechanisms through which ozone exerts toxic effects on the CNS remain poorly understood. We previously showed that in addition to lung inflammation,ozone exposure caused a neuronal activation in the dorsolateral regions of the rat nucleus tractus solitarius (NTS, a sensory nucleus involved in visceral information processing) overlapping terminal fields of lung primary afferent running in the vagus nerves (Gackière et al. 2011).Knowing that neuronal activity can induce glial remodeling in different brain structures (Reichenbach et al. 2010) and taking into account the highlevel of expression of plasticity marker PSA‐NCAM in the NTS (Bouzioukh at al. 2001), we hypothesized that ozone induced neuronal activation could lead to astroglial remodeling. To investigate this hypothesis, we used electron microscopy and immunoblot techniques. In ozone‐exposed animals, the astrocytic coverage of NTS glutamatergic synapses is increased while the astrocyte volume fraction and the astrocyte membrane densities are not significantly increased. Moreover, the expression of specific astrocyte markers GFAP, S100beta and ezrin did not change in ozone‐exposed animals. Altogether, our results indicate that ozone inhalation induces glial plasticity, which is restricted to the peri‐synaptic coverage.
T06‐35A. Astrocyte TNFα‐dependent alteration of hippocampal excitatory synaptic transmission in a mouse model of Multiple Sclerosis
S. Habbas 1, M. Santello1, H. Stubbe1, G. Zappia1, N. Liaudet1, G. Kollias2, A. Fontana3, T. Suter3, A. Volterra1
1University of Lausanne, Lausanne, Switzerland 2Institute for Immunology, Vari, Greece 3University Hospital Zurich, Zurich, Switzerland
Astrocytic signalling via purinergic P2Y1R reversibly potentiates excitatory transmission at perforant path‐granule cell (PP‐GC) hippocampal synapses. The effect, mediated via glutamate release and activation of presynaptic NR2B‐NMDAR, requires constitutive levels of TNFα as permissive factor (Jourdain et al., 2007 and Santello et al., 2011). Here we show that when TNFα levels increase the cytokine becomes instructive and directly evokes glutamatergic gliotransmission. Thus, brief application of high TNFα causes persistent potentiation of excitatory transmission, measured as increased mEPSC frequency in GCs. This effect is both TNFR1‐ and NR2B‐dependent because it is blocked in TNFR1‐/‐ mice and by ifenprodil, a selective NR2B antagonist. In order to establish if the effect depends on TNFR1 signaling in astrocytes, we created mice where TNFR1, knocked out in all cells, can be conditionally re‐expressed only in astrocytes. With this model, we observed that astrocyte TNFR1 expression is sufficient to reestablish the synaptic effect of TNFα. Next, we examined whether a similar TNFα‐dependent effect could be observed in defined pathological conditions. We selected Multiple Sclerosis (MS) because: 1) >50% of the MS population suffers from cognitive impairment possibly due to hippocampal dysfunction (Benedict and Zivadinov, 2011), and 2) TNFα and TNFR1 are key players in both the human pathology (Gregory et al., 2012) and in its murine model, Experimental Auto‐immune Encephalomyelitis (EAE) (Probert et al., 2000). We recorded PP‐GC synaptic activity after EAE induction via Adoptive Transfert (AT‐EAE) and found that mEPSC frequency was increased compared to control animals. Moreover synaptic alteration in AT‐EAE mice depended on astrocytic TNFR1 because the effect was absent in TNFR1‐/‐ mice but reappeared upon TNFR1 re‐expression in astrocytes. NR2B receptors are also necessary because in vivo ifenprodil injection prevented synaptic alteration. Overall, our study reveals a specific mechanism responsible for hippocampal synaptic dysfunction possibly relevant for cognitive impairment in MS.
Research supported by SNSF grant 31003A‐140999 and NCCR “Synapsy” to AV.
T06‐35B. Somatic integration of synaptic inputs in NG2 cells
W. Sun, D. Dietrich
University Clinic Bonn, Department of Neurosurgery, Bonn, Germany
It is well established that neurons form direct synaptic contacts with NG2 cells and that NG2 cells express prominent levels of voltage gated ion channels (VGCs). However, it is unclear whether and how presynaptic activities trigger important signaling pathways for the development and differentiation of NG2 cells. In particular, as both GABAergic and glutamatergic inputs are depolarizing the NG2 cell membrane, it is unclear how NG2 cells differentiate synaptic signals from distinct populations of presynaptic neurons.
We patched NG2 cells in the hippocampal CA1 region of NG2‐DsRed mice (8–20 days old) and investigated in current clamp mode how sodium and potassium channels affected synaptic depolarizations. EPSPs and IPSPs were evoked from a resting membrane potential of −85mV by injecting mock EPSC or IPSC waveforms derived from miniature currents.
EPSPs which depolarized NG2 cells to more than −45 mV were increasingly shortened by VGCs. At −16 ± 1 mV, corresponding to a quantal content of 640, the half width of EPSPs was shortened to 47 ± 3% while the amplitude was hardly altered. Application of TTX, 4‐AP and TEA showed that most of the observed shaping of EPSPs was primarily due to activation of A‐type K+ current rectifying the depolarizing input. Na+ currents only slightly (∼10%) amplified EPSP input, but this effect was outcompeted by the dampening effect contributed by A‐type current (∼15%). TEA was almost without effect on EPSPs.
Due to their slower time course IPSPs were very differently shaped by VGCs: while recruitment of VGCs by mock IPSPs started at a similar threshold voltage, the maximal suppression of IPSP amplitude was much stronger. When depolarizing NG2 cells to −35 ± 1 mV, corresponding to a quantal content of 235, IPSP amplitudes were suppressed to 52 ± 4% and the duration of IPSPs was not shortened but increased to 193 ± 5%. A‐type K+ current was the primary contributor for rectifying as well. Interestingly, if A‐type K+ current was blocked, IPSPs with a quantal content of 105 unmasked a small TTX‐sensitive spike‐like depolarization of 20 ± 2 mV (duration: 7 ± 1 ms).
For comparison, while synaptic responses to simultaneous release of 100 glutamatergic vesicles were hardly altered by VGCs, the synaptic response of 100 GABAergic vesicles was substantially low‐pass filtered by A‐type potassium currents. Altogether, our results suggest that VGCs in NG2 cells normalize the synaptic strength of excitatory and inhibitory inputs and support their differentiation by NG2 cells through further emphasizing their pre‐existing kinetic differences.
T06‐36A. The P2X7R‐Panx1 complex in glia: Role in orofacial hypersensitivity
R. Hanstein 1, M. Gulinello2, M. Hanani3, D.C. Spray1
1Albert Einstein College of Medicine, Neuroscience, Bronx, United States 2Albert Einstein College of Medicine, Rodent Behavioral Core, Bronx, United States 3Hadassah University Hospital, Laboratory of Experimental Surgery, Jerusalem, Israel
Pain is caused by aberrant neuronal responses to peripheral stimuli and glial cells are emerging as contributors to the neuronal hyperactivity. Recent work in sensory ganglia where satellite glial cells (SGCs) have close interactions with neurons has shown that SGCs can actively modulate neuronal activity. During pain, excessive amounts of ATP are released by neurons and activate purinergic receptors (P2R), in particular P2X7R that functionally interact with pannexin1 (Panx1). P2X7R activation leads to opening of Panx1 channels, providing an efflux pathway for ATP, to which neurons respond with excessive firing. To explore the involvement of this pathway in pain and analyze its impact separately in sensory neurons and SGCs, we tested pharmacological inhibitors and transgenic mice in an orofacial pain model. Transient inflammation in the submandibular region of mice was induced using complete Freund's adjuvant (CFA) and tactile sensitivity was quantified using von Frey filaments. Although inflammation was resolved by 28 days after injection, tactile hypersensitivity persisted in wildtype mice for at least 53 days, consistent with chronic post‐inflammatory pain. Tactile hypersensitivity in mice was reversed by systemic injection of the Panx1/gap junction blockers mefloquine or carbenoxolone at 7 days (peak inflammation) and at 28 days after CFA‐injection, suggesting the contribution of these channels to tactile hypersensitivity. In dissociated trigeminal ganglia (TG), which innervate the submandibular skin, BzATP‐induced YoPro uptake into neurons and glia was prevented by mefloquine, demonstrating the functional presence of the P2X7R‐Panx1 complex in TG. Moreover, TG of CFA‐injected mice showed more ATP release and immunostaining of TG revealed higher expression of Panx1 at 1 week after CFA injection compared to controls. Development of hypersensitivity was prevented in P2X7R‐null and in Panx1‐null mice, and was attenuated in mice with glia‐specific deletion of Panx1 (GFAPcre‐Panx1f/f) but not with neuron‐specific deletion (mNFHcre‐Panx1f/f), emphasizing the importance of glial Panx1 signaling in pain. Because P2X7R and Panx1 both have a key role in the immune response by activating the inflammasome, we are currently dissecting the relative importance of neuron‐glial communication compared to inflammatory responses in the development and maintenance of orofacial pain. Overall, our results show that the P2X7R‐Panx1 complex likely plays a major role in signaling events contributing to tactile hypersensitivity.
T06‐36B. Inhibitory avoidance learning is associated with altered expression of genes involved in neuron‐glia metabolic coupling
M. Tadi 1, G. Grenningloh1, S. Lengacher1, I. Allaman1, P. Magistretti1,2
1Ecole Polytechnique Federale de Lausanne, Lausanne, Switzerland 2Centre de Neurosciences Psychiatriques, CHUV, Département de Psychiatrie, Site de Cery, Prilly/Lausanne, Switzerland
Synaptic plasticity is central to the process of learning and memory and is accompanied by strengthening of existing synapses and/or formation of new synapses. Numerous studies have investigated the molecular mechanisms of learning and memory with neurons as the primary interest. Given the tight coupling between synaptic activity and brain metabolism, it seems reasonable to assume that synaptic plasticity changes occurring at the synaptic level may also occur at the metabolic level in order to keep up with the augmented demand at the synapse.
In this study we investigated the importance of genes involved in brain energy metabolism, and in particular those involved in neuron‐glia metabolic coupling during fear‐motivated inhibitory avoidance learning. Using (14C) 2‐Deoxyglucose (2‐DG) technique we first defined the brain areas that are involved in this learning process. Context‐dependent avoidance behavior was tested in C57BL/6 mice using the step‐through inhibitory avoidance paradigm (IA). Regional brain metabolic activity, as measured by the 2DG uptake, was quantified in several brain regions. Brain metabolic mapping revealed increased glucose utilization in hippocampus, amygdala [(basolateral complex (BLA) and the central nucleus (CEA)], and anterior cingulate cortex and mammillary bodies.
Quantitative mRNA levels were assessed in dorsal hippocampal tissue at different time points following IA learning. Results obtained demonstrate that learning modulates the expression pattern of genes involved in brain energy metabolism, i.e. glycogen synthesis and degradation, pyruvate metabolism, the pentose phosphate shunt and the astrocyte‐neuron lactate shuttle (ANLS) in a time dependent manner. We found a late‐phase of enhanced dorsal hippocampal gene expression, particularly for ANLS related genes, including Monocarboxylate transporter 1 (MCT1). Furthermore, we found that mice deficient in MCT1 transporter display impaired long term memory in the inhibitory avoidance and spatial learning in Morris water maze. These observations indicate that metabolic adaptation shown by neuron‐glia metabolic coupling might be a relevant phenomenon following learning in conjunction with synaptic plasticity.
T06‐37A. Quantitative profiling of retinal Müller glial cell surface proteome changes in response to LPS treatment
S. Hauck 1, C. von Toerne1, J. Behler1, J. Merl1, M. Ueffing1,2
1Helmholtz Zentrum München, Neuherberg, Germany 2University Medical Centre, Centre of Ophthalmology, Tübingen, Germany
Retinal Müller glial cells (RMG) are the main glial cell type in the retina and are essential players providing neurotrophic, metabolic and structural support for retinal neurons as well as mediating inflammatory responses in the retina. While some key neuroprotective molecules in the context of retinal degeneration have been demonstrated to be of RMG origin, there is yet no comprehensive understanding on the multifaceted role of RMG in orchestrating diverse intercellular functions. In order to systematically as well as functionally analyse RMG reactivity to retinal degeneration and thus explore the RMG‐derived signalling molecules in depth, we aim at comprehensively profiling proteome‐wide cellular responses to stimuli. We thus developed a proteomics approach screening specifically for cell surface and membrane proteins as well as secreted protein expression profiles and in addition monitor quantitative changes induced by prototype inflammatory inducer lipopolysaccharide LPS.
This workflow utilises sugar residues of cell surface proteins on intact RMG which are mildly oxidized and then covalently coupled to biotin. Biotinylated proteins are affinity purified and glycosylated peptides are specifically released by PNGaseF followed by protein identification and quantification by label‐free LC‐MSMS. This workflow resulted in the identification of more than 500 proteins on cell surfaces complemented by more than 700 proteins identified in the RMG secretome. Bioinformatic analysis allocates 75% of the cell surface proteins to be truly membrane or extracellular matrix proteins. This comprehensive cell surfaceome includes transmembrane receptors, transporters, adhesion molecules, signalling molecules and proteases, including 18 CD markers, 18 integrins, 41 solute carriers, two ephrins, five ephrin receptors and six plexins. Treatment of cells with LPS results in a highly reproducible significant shift of cell surface proteome with upregulation of 36 proteins and downregulation of 13 proteins. Among those LPS‐induced cell surface expression changes are proteins that suggest an active role of these glial cells in inflammatory processes. The specific changes will be discussed in detail.
Cell surface biotinylation on glycosyl‐residues in combination with label‐free LC‐MSMS is a sensitive and reproducible method to profile cell surface proteomics and sheds light on the biological properties of cells.
T06‐37B. Astrocytic Ca2+ signals affect on tripartite synapse structure and hippocampus‐related learning and memory
M. Tanaka 1, P.‐Y. Shih1, H. Gomi2, T. Yoshida1, J. Nakai3, R. Ando1, T. Furuichi4, K. Mikoshiba1, A. Semyanov1, S. Itohara1
1RIKEN Brain Science Institute, Wako, Saitama, Japan 2Nihon University, College of Bioresource Sciences, Fujisawa, Kanazawa, Japan 3Saitama University, Brain Science Institute, Sakura‐ku, Saitama, Japan 4Tokyo University of Science, Faculty of Science and Technology, Noda, Chiba, Japan
Background: Neuronal activity alters calcium ion (Ca2+) dynamics in astrocytes, but the physiologic relevance of these changes is controversial. To examine this issue further, we generated an inducible transgenic mouse model in which the expression of an inositol 1,4,5‐trisphosphate absorbent, “IP3 sponge”, attenuates astrocytic Ca2+ signaling.
Results: Attenuated Ca2+ activity correlated with reduced astrocytic coverage of asymmetric synapses in the hippocampal CA1 region in these animals. The decreased astrocytic ‘protection' of the synapses facilitated glutamate ‘spillover', which was reflected by prolonged glutamate transporter currents in stratum radiatum astrocytes and enhanced N‐methyl‐D‐aspartate receptor currents in CA1 pyramidal neurons in response to burst stimulation. These mice also exhibited behavioral impairments in spatial reference memory and remote contextual fear memory, in which hippocampal circuits are involved.
Conclusions: Our findings suggest that IP3‐mediated astrocytic Ca2+ signaling correlates with the formation of functional tripartite synapses in the hippocampus.
T06‐38A. Bidirectional expression of Lck‐GCaMP3 and DsRed in NG2‐cells as an approach for monitoring glial Ca 2 +‐microdomains
B.V. Herl, M. Theis, C. Steinhäuser, R. Jabs
Institute of Cellular Neurosciences, Bonn, Germany
NG2‐cells are the fourth type of glial cells in the mammalian brain. They are the only non‐neuronal cell type in the brain that receives synaptic input from neurons. NG2‐cells exhibit a variety of Ca2 +‐signalling pathways, which might be triggered by pre‐synaptic neuronal activity. However, no global increase in the intracellular Ca2 +‐concentration ([Ca2+]i) was detected in NG2‐cells employing the minimal stimulation technique at neuron‐glial synapses. Therefore we assume that post‐synaptic Ca2 +‐microdomains might be activated under physiological conditions. These Ca2 +‐signals are locally restricted to the plasma membrane. Membrane bound Ca2 +‐sensors with a high signal‐to‐noise ratio, such as Lck‐GCaMP3, are best suited for optimal visualisation of Ca2 +‐microdomains. Here, we set out to express Lck‐GCaMP3 in NG2‐cells together with the cytosolic reporter dye DsRed.
We generated transgenic mice which express a bidirectional promoter encoding both proteins under the control of a Tet‐off system. This strategy allows an exclusive expression of the transgenes only in the presence of a tet‐responsive transcriptional activator (tTA).
The construct was successfully cloned and expressed in HEK293 cells. As expected, Lck‐GCaMP3 was located only at the inner plasma membrane while DsRed was expressed all‐over the cytosol. Functionality of the Ca2 +‐sensor was tested in vitro. Elevation of [Ca2+]i reliably increased the fluorescence intensity of Lck‐GCaMP3. After zygote injection seven positive founder animals could be identified and will be crossbred with a NG2‐tTA mouse line.
Taken together, the presented construct appears suitable for functional imaging of Ca2 +‐microdomains in post‐synaptic NG2‐cells.
T06‐38B. Optogenetic interrogation of the mechanism of astrocyte‐neurone communication in the locus coeruleus
S. Lane, F. Tang, S. Kasparov, A.G. Teschemacher
University of Bristol, Bristol, United Kingdom
Lactate has been implicated in astrocyte to neurone communication. According to the “lactate shuttle” hypothesis, glycogen stored in astrocytes can be converted to L‐lactate and exported to neurones as preferred energy substrate. We use optogenetics to investigate signalling between astrocytes and noradrenergic (NAergic) neurones of the locus coeruleus (LC). To selectively activate Gs‐protein‐mediated signalling in astrocytes, we generated a viral vector to express a chimera of rhodopsin and β2‐adrenoceptor (Optoβ2AR). We confirmed that this construct activates cAMP‐mediated signalling in astrocytes.
In cultured astrocytes, excitation of Optoβ2AR resulted in acidification as detected using SNARF‐5 indicator, and this acidification could be blocked by pre‐incubation with an inhibitor of glycogen breakdown 1,4‐dideoxy‐1,4‐imino‐D‐arabinitol (DAB), suggesting that the acidification was due to build‐up of L‐lactate. Fast scan cyclic voltammetry (FCV) was used to measure noradrenaline (NA) release in organotypic slices cut at the level of the LC in which astrocytes were expressing Optoβ2AR. Light stimulation of astrocytes powerfully induced release of NA which could be prevented by pre‐incubation with DAB or application of D‐lactate. Bath application of L‐lactate (≥100 µM) in absence of optogenetic stimulation triggered the release of NA. In patch clamp experiments, L‐lactate depolarised LC neurones and evoked vigorous firing of action potentials. These experiments suggest that activation of Gs‐protein‐coupled receptors in astrocytes could potentiate the release of NA from LC neurones via L‐lactate. The cellular and molecular mechanisms of this signalling mechanism are currently under investigation.
Supported by the British Heart Foundation.
T06‐39A. Neuroprotection of Retinal Ganglion Cells by Müller Glia and Astrocytes
J. Higginson 1, D. Piso2, P. Veiga‐Crespo1, S. Sharma3, E. Vecino1
1Universidad del Pais Vasco, Leioa, Spain 2ESS Bilbao, Research in Control and Diagnostics, Leioa, Spain 3New York Medical College, Department of Cell Biology and Anatomy, Valhalla, NY, United States
Following retinal disease or injury, for example in glaucoma or retinal ischemia, axonal degeneration and death of retinal ganglion cells (RGC) results in irreversible blindness. The retinal glial cells, astrocytes and Müller glia, provide structural and trophic support to the RGCs in the healthy retina and may also have a function in promoting cell survival after injury. In rat models of glaucoma and ischemia, Müller glia were found to adopt a branched morphology, suggesting that there exists a degree of cell plasticity, which may have functional significance. In this study, we have investigated the relationship between glia and RGCs in the healthy and damaged retina both in vivo and in vitro. In damaged retinas, Müller glia significantly modify their branches enveloping RGCs in vivo. Moreover, in vitro Müller cells promote significant neurite elongation when they are in close contact with RGCs. In addition to this, the organization of astrocytes in the retina of a rat model of glaucoma was found to be significantly different to those of a healthy retina. These alterations in glial cell morphology may reflect changes in their relationship with RGCs and could signify profound changes in their secretome, which may influence RGC survival.
T06‐39B. Potential Physiological Role of Astrocytic Glycogen‐Derived Lactate in Energy Balance
J.P. Thaler, M.D. Dorfman, M. Matsen, M.W. Schwartz
University of Washington, Seattle, United States
Astrocytes synthesize and store glycogen, and glycogen‐derived lactate is an important fuel source for neurons. Recent evidence suggests that astrocyte‐derived lactate can affect learning behavior through modulation of hippocampal synaptic activity, but the hypothesis that astrocyte glycogen stores and neuronal lactate delivery contribute to the control of food intake and energy homeostasis is untested. Consistent with this hypothesis, we found that acute intracerebroventricular (ICV) lactate injection decreased food intake and body weight at 24 h and 48 h in rats, consistent with work from Tony K. T. Lam and colleagues, whereas levels of lactate in both plasma and hypothalamus increased during a period of spontaneous hyperphagia (the first 72 h of high fat diet (HFD) feeding). To investigate whether lactate generation within the brain constrains weight gain and energy intake, we performed a 14‐d ICV infusion of oxamate, an inhibitor of lactate dehydrogenase (which catalyzes the reversible conversion of lactate to pyruvate). Both chow‐ and HFD‐fed rats receiving oxamate manifested an increase in body weight with a corresponding tendency for food intake to increase compared with vehicle‐infused controls. Similarly, cortical and hypothalamic levels of both lactate and glycogen were reduced in rats treated with ICV oxamate, consistent with effective blockade of lactate generation and a compensatory increase of glycogenolysis (to increase substrate delivery). To test directly whether astrocyte glycogenolysis limits feeding, we infused 1,4‐dideoxy‐1,4‐imino‐D‐arabinitol (DAB), which blocks glycogenolysis by inhibiting glycogen phosphorylase. Our finding that both 2 h and 4 h food intake were increased by ICV DAB offers the first evidence that astrocyte glycogenolysis generates a supply of lactate that plays a physiological role to limit spontaneous food intake.
T06‐40A. Dehydroepiandrosterone sulfate and Sulforhodamine 101 compete for active uptake by an organic anion transporting polypeptide in hippocampal astrocytes
C. Schnell, Y. Hagos, S. Hülsmann
University of Göttingen, Göttingen, Germany
Sulforhodamine 101 (SR101) is widely used as a marker of astrocytes. In this study we investigated labeling of astrocytes by SR101 in acute slices from the ventro‐lateral medulla and the hippocampus of transgenic mice expressing EGFP under the control of an astrocyte‐specific promoter. While SR101 efficiently labeled EGFP‐expressing astrocytes in hippocampus, we found that SR101‐staining was very weak in the ventro‐lateral medulla and rather unspecific. Thus, SR101 is not a reliable marker for brainstem astrocytes. Although carbenoxolone decreased the labeling of astrocytes in the hippocampus significantly, mefloquine which blocks pannexin and connexin hemichannels, was unable to prevent SR101 uptake in hippocampal astrocytes. Time‐lapse 2‐photon imaging revealed that in hippocampus both astrocytes and neurons showed temporary SR101‐loading. In astrocytes the rise of SR101 fluorescence was slower as compared to EGFP‐negative cells and SR101 was quickly removed from non‐astrocytic cells during washout, while it was retained in astrocytes. In brainstem astrocytes, however, only a very weak and transient SR101‐labeling was observed. To test if SR101 is actively removed from astrocytes in the brainstem, we applied MK‐571 to block the multi‐drug resistance transporters MRP‐1. We did not observe any increase of SR101‐labeling in brainstem astrocytes. In contrast, astrocytic SR101 labeling was significantly reduced by substrates of organic anion transport, probenecid, estron‐3‐sulfate and dehydroepiandrosterone sulfate suggesting that SR101 is actively transported into hippocampal astrocytes by an organic anion transporting polypeptide (OATP). Additionally, the data suggest that astrocytes modulate extracellular concentration of neurosteroids in the hippocampus.
T06‐40B. A new approach to understanding the 3D structure of the CNS node of Ranvier
K. Tohyama, T. Hanasaka, K. Ogasawara, E. Matsuura, T. Nozaki, K. Ishida
Iwate Medical University, Morioka, Japan
In the last decade we have gained a wealth of molecular information concerning nodal and paranodal structures. However, the three‐dimensional (3D) ultrastructure of the node remains poorly understood. Transmission electron microscope (TEM) analyses of serial ultrathin sections have been the preferred method up to now. However, for various technical reasons it is not easy to analyze even an individual node of Ranvier by TEM, with the result that only a limited number of 3D images are available.
As an alternative approach we have used scanning electron microscopy (SEM) to obtain high‐resolution back scattered images (BSI) of serial ultrathin sections over a wide‐area. In TEM the size of the specimen is limited to less than 1 mm2, whereas in SEM it can be expanded to 20–30 mm2, allowing us to mount and examine more than 100 serial ultrathin sections on the same stage, collecting 3D information about many nodal structures simultaneously. Here we demonstrate a variety of nodal architectures assembled from BSI‐SEM (SU8010,Hitachi).
In our preliminary study using this technique, the pattern of cellular processes approaching the perinodal axolemma is quite different among axons even in only four nodes examined in optic nerve. In the present study, we analyzed perinodal elements at 25 whole nodes in rat optic nerves and reconfirmed this variety of perinodal glial elements include the followings: 1) perinodal space with extracellular matrix (20–100%); 2) closely abutting astrocytic processes (0–80%) ; 3) unidentified glial cell, presumable NG2+ glial processes (0–50%), but could not find 4) pre‐synapse‐like pseudo‐neuronal processes, which was observed at the perinode in the CNS grey matter (cerebral cortex) in the previous study.
Additionally, we found unexpected structures, small teardrop‐like protrusion(s) of axolemma at 18 out of 25 nodes examined (72%). In 16 nodes out of these 18 nodes (88.9%), glial elements encircled or densely contacted with the teardrop protrusions. A typical case of 3D reconstructed image is shown in the figure. Nodal axon (Ax, red), unidentified glial process (yellow; UG, presumable NG2 cell) and teardrop‐like protrusion (asterisks) are demonstrated. Outer part of the protrusion from the position indicated two arrows is encircled by the process of UG cell.
Based on these observations, we propose that some glial cells, including astrocytes and/or NG2 cells, might contribute to remove wasted membranous elements from the axon at the node of Ranvier, which is a new type of neuron‐glial interaction.

T06‐41A. Neuron‐astrocyte interactions during the development of the somatosensory cortex in a genetic model of absence epilepsy: from morphology to in vivo calcium imaging
G. Jarre, S. Stamboulian, A. Depaulis, J.‐C. Platel, I. Guillemain
Grenoble‐Institut des Neurosciences Inserm U 836, Grenoble, France
Epilepsy is one of the most frequent neurological diseases that affects up to 1% of the world population and which is associated with a hyper‐synchronicity and hyper‐excitability of neuronal networks. Absence epilepsy is a non‐convulsive epileptic syndrome characterized by spike‐and‐wave discharges (SWD) on the electroencephalogram (EEG) associated with a behavioral arrest. It occurs during childhood and, in most of the case, disappears before adulthood, suggesting a dysfunction of brain development and maturation. We have recently shown in the GAERS, a genetic model of absence epilepsy, that spontaneous seizures are initiated in the barrel region of the somatosensory cortex (SSC) and then spread to the rest of the cortex and the thalamus. These seizures are characterized by SWD which occur spontaneously around 25th day after birth (P25) but are preceded by EEG abnormalities as soon as P15. To understand what makes this SSC network more synchronous and excitable, we performed a morphological study of neurons and astrocytes by estimating densities of NeuN and S100‐positive cells respectively. Although we found similar densities of neurons and astrocytes between GAERS and non‐epileptic controls, the thickness of the SSC was 30% smaller in GAERS. Western blotting quantification of GFAP, another astrocytic marker, was significantly higher at P15‐P17, as compared with non‐epileptic control. Moreover, GFAP‐immunolabeling suggested that these astrocytes were reactive. We hypothesized that these modifications are linked with an alteration of astrocytic and/or neuronal physiological calcium excitability that could lead to the occurrence of epileptic seizures. To better evaluate the role of astrocytes and neurons networks during the development of SSC between P15 and P25, we used two‐photon microscopy imaging coupled with EEG recording in animal under anesthesia and neuroleptanalgesia. We first analyzed neuropile, representing ascendant projection of deeper cortical layers, astrocytes and neurons calcium activities. Preliminary results obtained from animals under isoflurane anesthesia showed similar neuropile activities. Astrocytic and neuronal activities were too weak to highlight differences between GAERS and non‐epileptic control. This is likely due to the mode of anesthesia known to strongly decrease astrocyte calcium signaling and neuronal synchronization. Current experiments are therefore performed using neuroleptanalgesia know to allow the occurrence of SWD. The expected data should lead to a better understanding of neuron‐astrocyte interactions during brain development and the mechanism underlying epileptogenesis in a genetic model of epilepsy.
T06‐41B. IOP Induces qualitative But Not quantitative Changes In Astrocytes In Mice Retina Contralateral To Experimental Glaucoma
A. Triviño 1, B.I. Gallego1,2, B. Rojas1,3, R. de Hoz1,2, A.I. Ramírez1,2, J.J. Salazar1,2, F.J. Valiente‐Soriano4, M.P. Villegas‐Pérez4, J. Ramírez1,3
1Universidad Complutense (UCM), Inst Invest Oftalmol Ramón Castroviejo, Madrid, Spain 2Universidad Complutense (UCM), Facultad Optica y Optometria, Madrid, Spain 3Universidad Complutense (UCM), Departamento Oftalmologia. Facultad Medicina, Madrid, Spain 4Universidad de Murcia, Departamento Oftalmologia, Murcia, Spain
Purpose: To study the effects of laser‐induced ocular hypertension (OHT) in the astrocytes of contralateral eyes two weeks after lasering.
Methods: Adult Swiss mice were divided into two groups: naïve (n = 6) and lasered (n = 6). Retinal whole‐mounts were immunostained with antibodies against GFAP. The GFAP‐labelled retinal area (GFAP‐RA) and the number of astrocytes were quantified.
Results: In comparison with naïve: i) astrocytes were more robust in contralateral eyes. In OHT‐eyes, the astrocyte population was not homogeneous, given that astrocytes displaying only primary processes coexisted with astrocytes in which primary and secondary processes could be recognized, the former having less intense GFAP‐IR (P<0.001). The mean percentage of astrocytes in which only primary processes could be detected was 37.8%; ii) GFAP‐RA was increased in contralateral (P<0.05) and decreased in OHT‐eyes (P <0.001); iii) the mean intensity of GFAP‐IR was higher in OHT‐eyes (P<0.01), and the percentage of the retinal area occupied by GFAP+ cells with higher intensity levels was increased in contralateral (P = 0.05) and in OHT‐eyes (P<0.01); iv) the astrocyte number did not differ significantly among the eyes analyzed. However, in OHT‐eyes the number of astrocytes in which primary and secondary processes could be observed were decreased (P<0.01).
Conclusions: Two weeks of laser‐induced OHT caused changes in the GFAP‐labelled retinal area but not in astrocyte number in both, contralateral and OHT‐eyes. On the basis of the astroglial changes detected in the present work, the use of the contralateral eye as an internal control in experimental model unilateral OHT should be reconsidered.
T06‐42A. Mapping astrocyte heterogeneity by analysis of specific cell surface marker expression
C. Kantzer, A. Bosio, M. Jungblut
Miltenyi Biotec GmbH, Bergisch‐Gladbach, Germany
Astrocytes show a high level of functional and morphological heterogeneity and are involved in many aspects of neural function, e.g., formation of the blood‐brain barrier, regulation of ion homeostasis, synaptogenesis, and synaptic plasticity. Despite the diversity of this cell type, the various astrocyte subpopulations are not well characterized, which is mainly due to the lack of markers that would allow for classification of astrocyte subsets.
Our study aimed at phenotyping of astrocyte subpopulations based on the expression of cell surface markers, which are associated with certain functions. We used two novel monoclonal antibodies, ACSA‐1 and ACSA‐2 (ACSA: astrocyte cell surface antigen), directed against extracellular epitopes of astrocyte‐specific cell surface markers to identify astrocyte subtypes. The ACSA‐1 antibody was generated by immunization of GLAST1 knockout mice and specifically detects the astrocyte‐specific L‐glutamate/L‐aspartate transporter GLAST (EAAT1, SLC1A3), whereas the ACSA‐2 antibody results from an immunization of rats with astrocytes isolated from GFAP‐EGFP transgenic mice. A mass spectrometric approach for the ligand‐based identification of the ACSA‐2 antigen pointed to a number of candidates, which have to be validated by further experiments.
The antibodies ACSA‐1 and ACSA‐2 were carefully analyzed by co‐staining experiments with commonly used intracellular astrocyte markers. We found that both antibodies specifically detect astrocytes in the developing and adult central nervous system. In contrast to antibodies against intracellular markers, such as GFAP, S100ß, or glutamine synthetase, ACSA‐1 and ACSA‐2 can be used to detect and isolate living astrocytes, which enables further analysis and culture of the separated cells. Flow cytometric analysis of ACSA‐1 and ACSA‐2 antigen expression on cells from different brain regions, as well as immunohistochemical analysis, revealed differences in the expression patterns. This allowed us to define different astrocyte subtypes especially in the cerebellum and olfactory bulb of the neonatal mouse brain.
Future work will focus on the identification of additional astrocyte‐specific cell surface proteins for a comprehensive classification of astrocyte subtypes based on cell surface marker expression or expression patterns.
T06‐42B. Role of CX3CR1 in supporting cell‐to‐cell contacts between microglia and degenerating TH+ neurons
J. Uhlrich
Lyon Neurosciences Research Centre, Lyon, France
Numerous studies have investigated the molecular cross‐talk between microglia and neurons in different models of Parkinson's disease. A major role for neuronally‐derived Fractalkine/CX3CL1 and its microglial receptor CX3CR1 has been demonstrated in the MPTP model of Parkinson's disease. Such a molecular interaction inhibits microglial activation and prevents, at least in part, microglia‐mediated neuronal cell death. However, whether CX3CL1/CX3CR1 supports cell‐to‐cell contacts between microglia and degenerating neurons is still unknown. We recently demonstrated different categories of neuron/microglia physical interactions in the murine 6‐OHDA model of Parkinson's disease. These included, in particular, microglial ramifications that penetrated the soma of TH+ neurons and supported microglial microphagocytosis of TH+ material. In the present work, we used Cx3cr1 KO mice (Cx3cr1gfp/gfp homozygous mice) and the murine 6‐OHDA model to investigate the role of CX3CR1 in neuron/microglia physical interactions under neurodegenerative conditions. In addition, we mapped microglia/neuron cell‐to‐cell contacts in the substantia nigra of autopsy samples from Parkinson's disease patients.
T06‐43A. Misfolded truncated tau protein influences neuron‐glial interaction via regulation of the “On” and “Off” signalling molecules
Z. Kazmerova 1, N. Zilka1,2, M. Zilkova1,2, T. Smolek1,2, M. Novak1,2
1Institute of Neuroimmunology, Bratislava, Slovakia 2AXON Neuroscience SE, Bratislava, Slovakia
The reciprocal communication between neuronal and glial cells represents the key component of the immunosurveillance system of the brain. It has been proposed that the neuro‐glial interaction is impaired in human Alzheimer's disease. In this study we focus on neuronal “On” and “Off” signalling molecules in the transgenic rat model for Alzheimer's disease. Using enzyme‐linked immunosorbent assay we determined the level of CD47, CX3CL1 (“Off” signalling molecules) and MMP3, TREM2 (“On” signalling molecules) in brain homogenates. Our results demonstrated significantly up‐regulated level of CD47 (p < 0,001) in our AD rat transgenic animal model. On the other hand, quantification of MMP3 level revealed significant decrease of MMP3 expression (p < 0,001) in tested transgenic animals. Previous studies showed that CD47 and MMP3 are predominantly expressed on neuronal cells in CNS where CD47 functions normally as a marker of “self” to protect intact body component or “don't eat me” molecule which protect CD47‐expresing cells from phagocytosis. Our results indicate that overexpression of CD47 on neuronal cells expressing pathologically modified truncated tau protein can be used as a neuroprotective mechanism potentiating spread and accumulation of pathological modified tau protein in neurons affected by AD pathology which in final stage support progression of the developing disease. In conclusion, we showed, that pathologically modified Tau protein can modulate specific “On and Off” signalling molecules and thus affects neuron‐glia interaction in AD.
Acknowledgement: This work was supported by Axon Neuroscience and research grants VEGA 2/0161/11, 2/0205/11, 2/0193/11, APVV 0200‐11 and structural fund 26240220046.
T06‐43B. BDNF‐mediated enhancement of LTP is modulated by astrocytes
S. Vaz, A. Sebastião
Inst. Pharmacol. & Neurosc. & Neurosciences Unit, Lisbon, Portugal
Hippocampal θ‐burst long‐term potentiation (LTP) is a form of synaptic plasticity that can be enhanced by BDNF (Fontinha et al, 2008). Astrocytes, by releasing gliotransmitters, play an active role of on synaptic plasticity (Henneberger et al., 2010). Gliotransmitters include BDNF and ATP, which is extracellularly cleaved into adenosine, known to influence synaptic actions of BDNF (Sebastião and Ribeiro, 2009). We therefore hypothesised that astrocytes could play a relevant role in the facilitatory actions of BDNF upon LTP. The present work was designed to test this hypothesis.
Field‐excitatory post‐synaptic potentials (fEPSP) were recorded from the CA1 area of hippocampal slices prepared from Wistar rats (3–5 weeks old) as before (Fontinha et al., 2008). After obtaining a stable value of the fEPSP slope for at least 30 min, LTP was induced by delivery of 3 trains of 3 pulses at 100Hz, each train being separated by 200 ms. LTP magnitude (% increase of fEPSP) was evaluated 50–60 min after induction.
LTP magnitude in control conditions was 16 ± 5.9%, whereas in a second independent pathway of the same slices but in the presence of BDNF (20ng/ml) it was 39 ± 4.7% (n = 7; P <0.05). LTP was completely abolished when hippocampal slices were superfused with the gliotoxin fluorocitrate (FC, 200 µM), which selectively reduces the astrocytic metabolism decreasing intracellular Ca2+ signalling and the release of gliotransmitters. Interestingly, in presence of FC, the facilitatory action BDNF (20 ng/ml) upon LTP was completely prevented. Noteworthy, when FC (200 µM) treated slices were superfused with the selective adenosine A2A receptor agonist, CGS21680 (30nM), the facilitatory action of BDNF (20 ng/ml) upon LTP was rescued (n = 5; P < 0.05), so that ΔLTP caused by BDNF (LTP in the presence of BDNF – LTP in the absence of BDNF in two independent pathways of the same slices) in FC + CGS 21680 treated slices (19%) was similar to that observed in control slices (23% in the absence of FC and CGS 21680).
The results suggest that the facilitatory action of BDNF upon LTP is controlled by astrocytes, and that this role of astrocytes results from their contribution to the extracellular accumulation of adenosine allowing A2A receptor activation to gate plasticity actions of BDNF.
Acknowledgements: Work and SV supported by Fundação para a Ciência e Tecnologia, Portugal; BDNF was a gift by Regeneron.
Fontinha BM et al. (2008) Neuropharmacology 54:924–933.
Henneberger C et al. (2010) Nature 463:232–6.
Sebastião AM, Ribeiro JA (2009) In: Handbook of Experimental Pharmacology, 193:471–534
T06‐44A. Glial GABA transporters downregulate enhanced neuronal activity
O. Kékesi 1, G. Nyitrai1, P. Szabó1, R. Fiáth2, I. Ulbert2, J. Kardos1, L. Héja1
1Institute of Molecular Pharmacology, Budapest, Hungary 2Institute of Cognitive Neuroscience and Psychology, Comparative Psychophysiology Group, Budapest, Hungary
Several studies demonstrated the ability of astrocytes to sense, respond to and regulate neuronal function. Among the many functions of glial proteins, glutamate (Glu) and GABA transporters play important roles in balancing excitatory and inhibitory signals in the brain. Here we show that astrocytes regulate the tonic inhibition of neurons by the concerted action of Glu and GABA transporters, thereby protecting both neurons and glial cells from overactivation.
We demonstrate that the uptake of glutamate triggers the reverse function of glial GAT‐2/3 transporters by elevating the intracellular Na+ concentration in astrocytes. The released GABA significantly contributes to the tonic inhibition of neurons in a network activity‐dependent manner. We also describe the source of the releasing GABA that is synthesized by an alternative pathway from polyamines. Moreover, in the low‐[Mg2+] model of epilepsy, we show that blockade of the glial Glu/GABA exchange mechanism increases the duration of seizure‐like events and also results in increased activity of astrocytes, demonstrating the neuroprotective impact of the mechanism. Finally, we show that the released glial GABA modulates the power of gamma range oscillation in vivo, suggesting that the Glu/GABA exchange mechanism is also functioning in the intact hippocampus under physiological conditions.
Revealing this novel molecular mechanism by which astrocytes provide an adjustable, in situ negative feedback on the excitability of neurons is expected to broaden our understanding about the regulation of neuronal activity by astrocytes and may open up new targets for the treatments of pathological conditions, such as epilepsy or ischemia.
This work was supported by grants: OTKA K 81357, TÉT NEUROGEN, TÉT MULTISCA to IU, TECH‐09‐AI 2009‐0117 NKFP NANOSEN9, ERA‐Chemistry OTKA 102166 and KMR_12‐1‐2012‐0112 TRANSRAT.
T06‐44B. Chronic stress induces profound structural remodelling of astrocytes within theprefrontal cortex: A characterization of the relationship between astrocyte morphologyand density
R. Tynan, S. Beynon, M. Nilsson, F. Walker
University of Newcastle, Hunter Medical Research Institute, Newcastle, Australia
Chronic stress is well recognised to decrease the number of GFAP+ astrocytes within the prefrontal cortex (PFC). Recent research, however, has suggested that our understanding of how stress alters astrocytes may be incomplete. Specifically, chronic stress has been shown to induce a unique form of microglial remodelling, but it is not yet clear whether astrocytes also undergo similar structural modifications. Such alterations may be significant given the role of astrocytes in modulating synaptic function. Accordingly, in the current study we have examined changes in astrocyte morphology following exposure to chronic stress in adult rats, using three‐dimensional digital reconstructions of astrocytes. Our analysis indicated that chronic stress produced profound atrophy of astrocyte process length, branching and volume. We additionally examined changes in astrocyte‐specific S100β, which is both a putative astrocyte marker, and a protein whose expression is associated with astrocyte distress. While we found that S100β levels were increased by stress, this increase was not correlated with atrophy. We further established that while chronic stress was associated with a decrease in astrocyte numbers when GFAP labelling was used as a marker, we could find no evidence of a decrease in the total number of cells, based on Nissl staining, or in the number of S100β+ cells. This finding suggests that chronic stress may not actually reduce astrocyte numbers and may instead selectively decrease GFAP expression. Together, these results provide a significantly more elaborate picture of how chronic stress alters the PFC.
T06‐45A. Protection effect of glial cell line‐derived neurotrophic factor on neurons and glial cells under photodynamic injury
M. Komandirov, E. Knyazeva, M. Rudkovsky, U. Fedorenko, G. Fedorenko, A. Uzdensky
Southern Federal University, Rostov‐on‐Don, Russian Federation
Tissue reactions on external injuries depend on intercellular interactions that are provided in particular by neurotrophic factors. Neurotrophic factors are mediators that transmit “survival signal” in nervous system. The influence of the glial cell line‐derived neurotrophic factor (GDNF, 10 ng/ml) on photo‐induced changes in neuronal activity and ultrastructure and death of neurons and glial cells (GC) was studied in this work to investigate the role of neurotrophic intercellular interactions in cell survival under the photodynamic injury. Photodynamic injury is a very effective inducer of the oxidative stress. Crayfish stretch receptor (SR) was the model object in this research. Cells were photosensitized with aluminophthalocyanine “Photosens” and illuminated with laser (640 nm). We used the method of dual fluorochroming of specimens with propidium iodide and Hoechst 33342 to visualize morphologic features of photo‐induced apoptosis of GC and necrosis of neurons and GC. Photodynamic injury caused the necrosis of neurons and GC and apoptosis of GC. GDNF significantly reduced the level of photo‐induced cell death both necrosis and apoptosis. In presence of GDNF after illumination ultrastructure of cells were more saved then without one. There was the segregation in the Nissl bodies abundant in ribosomes, cisterns of endoplasmic reticulum (ER) and mitochondria. Large number of ribosomes and polysomes means that protein synthesis was saved and proteins were stored and packed in dictyosomes to be transported to the periphery of neurite. Hereby, GDNF protected neurons and GC of SR from photo‐induced damages
T06‐45B. Regulation of nutrient transport across the BBB
A. Weiler, S. Limmer, C. Klämbt
Westfälische Wilhelms‐Universität Münster, Münster, Germany
The Drosophila nervous system is ensheathed by several types of glial cells, one of which, namely the subperineurial glia, forms pleated septate junctions (pSJ) to build a tight blood‐brain barrier (BBB). Hence, nutrients from the surrounding hemolymph must cross these glial cells to reach the neurons. The amount of nutrients entering the nervous system is likely to be tightly controlled through neuron‐glia communication to ensure optimal metabolic supply while avoiding disturbance of the extracellular homeostasis.
We aim to elucidate signals involved in this regulatory interaction. To this end, an RNAi‐based screen in glia of adult flies is performed using the Gal4/Gal80ts system. We analyze the intake of blue‐dyed food by photometric measurement. Since impairment of the metabolic supply of the brain should severely affect the organism, compensatory changes in feeding behavior are expected. For instance, knockdown of nutrient transporters specifically in glia would then induce excessive feeding in normally nurtured individuals. The first set of RNAi lines screened includes genes that have previously been found to be essential in glia for survival or locomotion of the animal. Furthermore it comprises metabolic enzymes, all carbohydrate transporters, neuropeptides and their corresponding receptors. Candidate genes will be further characterized regarding their role in controlling the energy homeostasis of the nervous system.
T06‐46A. Macrophages and microglia play distinct roles in neuropathic pain perception
S. Kraft 1, K.R. Miller1, C. Witzel2, R.E. Kälin1, G. Pfuhl3, Y. Winter3, M. Endres4, F.L. Heppner1
1Charité, Neuropathology, Berlin, Germany 2Charité, Plastic Surgery, Berlin, Germany 3Humboldt University, Cognitive Neurobiology, Berlin, Germany 4Charité, Neurology, Berlin, Germany
Question: The discovery that activation of non‐neuronal CNS microglia plays a causal role in spinal processing of nociceptive signaling has shed new light on the processes underlying neuropathic pain facilitation. However, there remains much uncertainty as to the necessary contribution of microglia to enhanced pain states. We aim to define the particular role of microglia for the initiation of neuropathic pain and by answering this question also learn if the function of peripheral myeloid cells is distinct or redundant in this process.
Methods: To specifically investigate spinal microglia and peripheral macrophages in the pathogenesis of neuropathic pain, we model chronic pain by performing partial ligature of the sciatic nerve in CD11b‐HSVTK mice engrafted with GFP bone marrow (GFP>CD11b‐HSVTK). CD11b‐HSVTK+/‐ mice allow the exchange with peripherally‐derived, GFP+ macrophages upon central depletion of endogenous CD11b+ microglia. Following this depletion/ repopulation paradigm, behavioral analyses of mechanical and thermal allodynia are conducted.
Results: We established a selective tool to exchange CNS parenchymal microglia with peripheral GFP+ myeloid cells. In chronic pain tests for mechanical and thermal hyperalgesia, GFP>CD11b‐HSVTK+/‐ mice show considerable decreases in paw withdrawal thresholds in response to mechanical, but not thermal stimuli ipsilateral to the injury, indicating distinct roles of microglia and macrophages in the facilitation of thermal hyperalgesia.
Conclusions: We identified a differential contribution of resident spinal microglia vs. peripheral myeloid cells in the development of neuropathic pain in GFP>CD11b‐HSVTK chimeras. Future studies aim to examine the exact mechanisms underlying the distinction between these two populations.
T06‐46B. Metabolic imaging in the awake mouse
M. Wyss 1, M. Zünd1, J. Mayrhofer1, P. Mächler1, F. Barros2, B. Weber1
1University of Zurich, Zurich, Switzerland 2CECS, Valdivia, Chile
A deeper understanding of the processes involved in the compartmentation of brain energy metabolism is of major interest. Not only to enwrap pathomechanisms of brain diseases associated with metabolic deficits but also for a more accurate interpretation of functional neuroimaging signals which often use metabolic signals as surrogate marker for brain activity (e.g. FDG PET). Large amount of knowledge in the field has been obtained from small animal neuroimaging studies. But due to their invasiveness they often need anesthesia. Anesthetics heavily alter physiological read‐outs. The aim of this study was 1.) to test if metabolic imaging using two‐photon microscopy (2PM) and genetically encoded glucose sensors is feasible in the awake, head‐fixed mouse and 2.) to examine the effects of isoflurane anesthesia on the signals.
In two mice, a recombinant adeno‐associated virus as a carrier for the genetically encoded glucose sensor FLII12Pglu600µΔ6 was injected into the whisker barrel cortex. By using an astrocyte‐specific promoter (short GFAP) astrocytic expression was ensured. In addition, a chronic window and a head post were implanted. Behavioral training started a few days after surgery. Animals had to learn to tolerate head fixation without spontaneous motor activity. Within a single trial animals were trained not to move for 10 seconds (imaging period) to receive a water reward. Animals were subjected to water restriction during the behavioral training. Animals were trained for three weeks before 2PM imaging started.
Animals learned to tolerate head fixation up to 90 minutes allowing acquisition of up to 400 trials per session. Upon oral glucose administration (per single water reward ∼1 mg glucose was administered) FRET signal started to increase within 5 minutes and went up to a maximum of 8%. Surprisingly, isoflurane anesthesia led to an even higher increase of the signal (mean increase of 12%) compared to the awake state. In some experiments the barrel cortex was activated by vibrotactile single whisker deflections generated by a piezo bending actuator. FRET signal did not show a difference between baseline and stimulated condition (1.133 ± 0.3 vs. 1.136 ± 0.29).
In conclusion, the presented experiments demonstrate for the first time the feasibility of awake 2PM imaging for metabolic measurements on a single cell level. The signal increase upon peroral glucose administration unambiguously supports the functionality of the sensor. Isoflurane exhibits a large effect on intracellular astrocytic glucose concentration distinctly revealing the need for experiments in awake animals for unbiased results. Future experiments will now be expanded to neuronal glucose sensors to enable comparisons between astrocytes and neurons.
T06‐47A. Impact of oligodendrocyte‐derived exosomes on neuronal metabolism: a role in neuroprotection?
W.P. Kuo 1, D. Fröhlich1, C. Frühbeis1, C. Zehendner2, H. Luhmann2, J. Trotter3, E.‐M. Krämer‐Albers3
1University of Mainz, Mainz, Germany 2University of Mainz, Physiology and Pathophysiology, Mainz, Germany 3University of Mainz, Molecular Cell Biology, Mainz, Germany
Question: Myelination in the CNS requires an intimate communication between oligodendrocytes and neurons. Since oligodendrocytes maintain axonal integrity, we examined the role of oligodendroglial exosomes in axon‐glia communication and their potential impact on the neuronal metabolism. Exosomes are nano‐sized vesicles carrying a specific set of lipids, proteins, and RNAs to target cells to functionally impact their behavior. Our previous studies indicate that neuronal electrical activity controls glial exosome release resulting in subsequent neuronal uptake and functional retrieval of the exosomal content. Proteomic analysis of oligodendroglial exosomes revealed a list of candidates with potential neurotrophic action in neurons. To elucidate the impact of oligodendroglial exosomes on the neuronal metabolism, we analyzed the metabolic activity of neurons after co‐culture with oligodendrocytes or direct treatment with exosomes.
Methods: Neurons were subjected to different stress paradigms such as oxidative stress, hypoxia, and nutrient deprivation. Neuronal vitality was assessed by MTT assay and the mitochondrial membrane potential was visualized by staining with MitoCapture.
Results: Neurons grown under optimal conditions were not affected by the presence of exosomes. Intriguingly, when neurons were subjected to stress (oxidative stress, nutrient deprivation, oxygen‐glucose deprivation) their metabolic activity was significantly increased in the presence of exosomes. When challenged with oxidative stress prior to exosome treatment, neurons were not able to recover. MitoCapture staining demonstrated that oligodendroglial exosomes prevent the breakdown of the mitochondrial membrane in nutrient‐deprived neurons.
Conclusions: Our results indicate that exosome supply is protective for neurons but is unlikely to support their recovery. We suggest that oligodendroglial exosomes carry neuroprotective substances, which protect neurons from stress.
T06‐47B. Lactate as a signaling molecule for the regulation of plasticity‐related genes expression
J. Yang 1, P. Jourdain1, J.‐M. Petit1,2, G. Grenningloh1, E. Ruchti1, I. Allaman1, P. Magistretti1,2
1EPFL, Lausanne, Switzerland 2Centre de Neuroscience Psychiatriques, Département de Psychiatrie, Prilly/Lausanne, Switzerland
Recently the central role of glycogen‐derived lactate release by astrocytes has been shown to be essential for long‐term memory (LTM) formation (Suzuki et al., Cell, 2011). Interestingly, this effect of lactate on synaptic plasticity mechanisms was not fully mimicked by glucose, suggesting that the effect was not only related to supporting the potential increase in energy demands related to plasticity, but that L‐lactate could act as a signaling molecule for the regulation of expression of plasticity‐related genes. We have followed up on these observations and show that L‐lactate significantly stimulates mRNA expression of key immediate early genes (IEGs), in a time‐ and concentration‐dependent manner, in cultured neurons. Following one hour of treatment with 20 mM L‐lactate, Arc, Zif268 and c‐Fos mRNA levels were increased by 5.2, 3.7 and 8.2 folds, respectively. In addition, the increased IEGs mRNA expression levels induced by L‐lactate are correlated at the protein level with respectively 5.5, 4.0 and 3.2 fold increase compared to control values at the same time point. These effects are specific for L‐lactate, since D‐lactate (non‐metabolized enantiomer of L‐lactate), L‐pyruvate and D‐glucose (at equicaloric concentrations) have no effect on gene expression. Interestingly, we observed that, in similar culture conditions, L‐Lactate‐induced IEGs expression is only observed in neurons but not in astrocytes, implying a cell specific effect. Characterization of the underlying molecular mechanisms of L‐lactate action on IEGs expression demonstrate the involvement of NMDA receptors. Finally, we obtained evidence that such regulatory mechanisms of IEG expression also operate in vivo as direct intracortical injections of L‐lactate (10 mM) into the somatosensory‐motor cortex areas resulted, within 1 hour of application, in a significant stimulation of Arc, Zif268 and c‐Fos expression (by 61 ± 13%, 46 ± 8% and 60 ± 12%, respectively) as compared to D‐lactate (10 mM)‐injected contralaterally areas. Taken together these findings demonstrate that L‐lactate acts as a direct signaling molecule which regulates neuronal plasticity‐related IEGs expression both in vitro and in vivo. Interestingly, the underlying mechanism of action of L‐lactate involves NMDA receptors.This set of observations therefore reveals a novel signaling role of lactate which may represent a likely mechanism to account for the role of astrocytic‐derived L‐lactate on LTM formation.
T06‐48A. Mechanisms of K+‐clearance in the brain: The Na+/K+‐ATPase as the key contributor
B.R. Larsen 1, M. Assentoft1, S.Z. Hua2, K. Kaila3, J. Voipio3, N. MacAulay1
1University of Copenhagen, Copenhagen, Denmark 2University of Buffalo, Buffalo, United States 3University of Helsinki, Helsinki, Finland
Neuronal activity in the brain is associated with a transient increase in the extracellular K+ concentration. The excess K+ is removed from the extracellular space, primarily by the surrounding glial cells, leading to an intracellular accumulation of K+ via mechanisms not fully identified and/or quantified. Post‐stimulus recovery of [K+]o has been proposed to be dependent on Kir4.1‐mediated spatial buffering and/or to Na+/K+‐ATPase activity.
To resolve the molecular mechanisms involved in K+ clearance, we initially determined the contribution from the different K+‐transporting mechanisms present in primary culture of rat astrocytes and their K0.5 for K+. The Na+/K+/2Cl‐ cotransporter, NKCC1, increased its activity within a physiological concentration range of K+, while the Na+/K+‐ATPase saturated at much lower concentrations. Consequently, a concentration‐dependent switch occurs between the two mechanisms of K+ uptake in cultured astrocytes. In addition, NKCC1 was capable of mediating robust K+‐induced astrocytic cell swelling. Thus, NKCC1 could potentially act as a molecular mechanism responsible for clearance of the stimulus‐evoked rise in [K+]o and the associated shrinkage of the extracellular space.
To determine the contribution of each of the three molecular mechanisms to K+‐clearance in native brain tissue, we used rat hippocampal brain slices and employed ion‐sensitive microelectrodes in association with high‐frequency electrical stimulation as well as focal appliances of K+ by ionophoresis. Inhibition of Kir4.1 (100 uM BaCl2) or NKCC1 (10 uM bumetanide) failed to show a significant effect on the rate of K+ removal from the extracellular space. In contrast, inhibition of the α2 and α3 isoforms of the Na+/K+‐ATPase significantly delayed post‐stimulus recovery of [K+]o and this delay was further potentiated by additional inhibition of the α1 isoform.
The Na+/K+ ATPase emerged as the primary factor responsible for stimulus‐evoked K+‐clearance with no evidence in favor of Kir4.1 and NKCC1 involvement. Further characterization of the different Na+/K+‐ATPase isoforms, by heterologous expression in Xenopus laevis oocytes, revealed that the α1 isoform reached its maximal activity already at resting K+ concentrations, hinting at a “housekeeping” function. The α2 subunit displayed voltage‐sensitivity and increased turnover rate along physiologically relevant increments in extracellular K+ concentration. These α2‐related features may render this astrocyte‐specific subunit variant specifically geared for post‐stimulus recovery of [K+]o
T06‐48B. Amino acid exchanger in the brain of Drosophila: Phylogenesis, Expression and Function
A.B. Ziegler, M. Simonnet, Y. Grosjean
Centre des Sciences du Goût et de l'Alimentation, Dijon, France
Hetero(di)meric amino acid transporters (HATs) are one of the most important exchange mechanisms for amino acids. However, only little is known about their concrete function on brain activity. This study focuses on the expression, function and phylogenesis of HATs in fruit flies. HATs are formed by a catalytic “light chain” (SLC7A5–11) and a cofactor called “heavy chain”. The genome of Drososphila melanogaster encodes five proteins homologue to mammalian light chains (genderblind, minidiscs, JhI‐21, CG9413 and CG1607). All these light chains are highly conserved among several Drosophila species (Reynolds et al, 2009). We used two complementary methods to study the expression of these five candidates in the brain of Drosophila melanogaster. First, we raised antibodies for our transporter candidates. Second, we took advantage of the UAS/Gal4 system. We cloned genomic regions, which putatively contained CIS‐regulatory elements in front of Gal4 and generated transgenic animals in which we studied Gal4 induced expression of a (UAS‐)reporter gene. The previously identified light chain homologues are expressed by different subsets of glial cells. These subsets include cortex glia, glia forming the blood‐brain‐barrier and astrocyte‐like glia. Finally, we present examples of how misexpression of light chains in glia alters neuronal signal transmission. For example, Genderblind expressing glia regulates the extracellular glutamate contend which desensitizes glutamate receptors. It has though a big impact on regulating the strength of glutamatergic signal transmission (Augustin et al.,2007; Grosjean et al, 2008). Another light chain, Minidiscs, is expressed by glia close to brain centers, which are regulating food intake. Consequently, animals mutant for minidiscs are showing deficits in food‐odor detection. Taken together our work highlights the importance of glial expressed amino acid exchangers on neuronal activity and behavior.

POSTER TOPIC 07 Ischemia and hypoxia
T07‐01A. The fate of mDach1‐expressing cells in the dorsal part of the lateral ventricles following focal cerebral ischemia
M. Anderova 1,2, H. Pivonkova1,2, P. Honsa1,2
1Institute of Experimental Medicine, AS CR, Prague, Czech Republic 2Second Medical Faculty, Charles University, Prague, Czech Republic
The mDach1 gene, involved in the development of the neocortex and the hippocampus, is expressed by neural stem cells (NSCs) during early neurogenesis, and in the adult mouse brain, mDach1 gene activity persists in particular subpopulations of neurons and astrocytes of the cortical plate, in the CA1 layer of the hippocampus and in certain cell subpopulations in the dorsal part of the lateral ventricles (LV). In this study we aimed to elucidate the role of mDach1‐expressing cells in adult neurogenesis and gliogenesis under physiological as well as post‐ischemic conditions, employing transgenic mice in which the expression of green fluorescent protein (GFP) is controlled by the D6 promotor of the mDach1 gene. Middle cerebral artery occlusion (MCAo) was used as a model of ischemic injury, and sham‐operated mice were used as controls. We isolated GFP+ cells from the dorsal part of the LV of sham‐operated‐ and post‐ischemic brains and employed a neurosphere‐forming assay in order to estimate their ability to proliferate and self‐renew. Furthermore, we followed their immunocytochemical and electrophysiological properties during in vitro differentiation and compared the differentiation potential of GFP+ cells isolated from the controls to those isolated from post‐ischemic brains. The GFP+ cells isolated from the dorsal part of the LV of controls formed neurospheres and differentiated only into a glial phenotype. The GFP+ cells isolated from the dorsal part of the LV of post‐ischemic brains were able to form neurospheres as well; however, besides their differentiation into a glial phenotype, they also gave rise to cells with the properties of neuronal precursors. We also performed in situ immunohistochemical/electrophysiological analyses of GFP+ cells in the adult brain of controls and those after MCAo. In situ analyses revealed that GFP+ cells expressed the phenotype of adult NSCs or neuroblasts in controls or following ischemia and that their number was increased in both hemispheres following MCAo. Compared to controls, we found that the number of GFP/doublecortin‐positive cells significantly increased in the dorsal part of the LV as well as the number of GFP+ cells in the olfactory bulb, where they probably differentiated into calretinin+ interneurons. Our results indicate that GFP+ cells with an active mDach1 gene in the dorsal part of the LV play an important role in the increased production of neuroblasts after injury and that this process is also enhanced in the contralateral hemisphere. Collectively, our results reveal the involvement of the mDach1 gene in adult neurogenesis. Cells expressing this gene exhibit the properties of adult NSCs or neuroblasts and respond to MCAo by enhanced neurogenesis.
Supported by GA CR P303/12/0855, GAUK 383711.
T07‐01B. Microglia express the dopaminergic D2 receptor upon activation in experimental stroke
J. Huck 1, P. Mergenthaler2, N. Gladow1, J. Priller1
1Charité – Universitätsmedizin Berlin, Department of Neuropsychiatry, Berlin, Germany 2Charité – Universitätsmedizin Berlin, Department of Experimental Neurology, Berlin, Germany
Experimental cerebral ischemia leads to activation of microglia and astrocytes, which subsequently release pro‐ and anti‐inflammatory factors. Furthermore, during the earliest periods of neuronal damage, large quantities of dopamine are released, which may exacerbate the vulnerability of the lesioned tissue. On the other hand, delayed treatment with levodopa has been proven beneficial in stroke patients, suggesting a more complex effect of dopamine. Recent studies on astrocytic involvement in neuroinflammation have shown a remarkable modulation of the inflammatory response over the dopamine D2 receptor (D2R). After stroke, reactive astrocytes in the astroglial scar were found to be immunopositive for D2R. In this study, we report the expression of D2R in activated microglia, both after experimental stroke in vivo and after oxygen‐glucose deprivation (OGD), an in vitro model of ischemia.
Using immunohistological stains, we analyzed the expression of D2R in Iba1 positive cells 1, 3, 5, 7, and 14 days after middle cerebral artery occlusion (MCAO; n = 2–4 mice/time point). For each animal, >4 pictures were taken from the ischemic core as well as from the contralateral, non‐lesioned cortex from 3 sections. A total of >300 (control) and >500 (ischemic core) Iba1+ cells were analyzed for each time point. In the ischemic core, ∼25% of cells immunopositive for Iba1 expressed D2R in contrast to <1% in control areas. A strong depletion of D2R immunoreactivity was observed in the ischemic striatum, accompanied by an increase in D2R expression in the adjoining cortex and an elevated presence and activation of microglia.
For in vitro experiments, we used fluorescence activated cell sorting to isolate CD45low/CD11+ microglial cells. No significant D2R expression at mRNA level could be detected by RT‐PCR in this population, while we were able to detect D2R in non‐microglial cells. Interestingly, cultured primary mouse microglia expressed low quantities of D2R, as revealed by Western Blotting and RT‐PCR, suggesting that culturing is sufficient to induce D2R in microglial cells. Upon in vitro activation of primary microglia through 60 minutes of OGD, we found a 1.5 fold increase in D2R expression after 24 hours (n = 4). Furthermore, treatment of primary microglia with the selective D2R agonist Pramipexole showed a dose‐dependent tendency to increase lipopolysaccharide‐induced TNFa release (n = 5).
Our studies indicate that activated microglia express a functional D2R, which may contribute to the pro‐inflammatory reaction of microglia after ischemia.
T07‐02A. Age‐dependent activity of nitric oxide synthase during ischemic white matter injury
J. Zaleski1, A. Bachleda1, A. Runkle1, S. Baltan 2
1Cleveland Clinic, Neurosciences, Cleveland, United States 2Cleveland Clinic, Shaker Heights, United States
White matter (WM) is injured in most strokes and axonal injury and dysfunction contribute to disability associated with clinical deficits. In young WM, the damage from ischemic injury involves the sequence of energy depletion (ionic pathway), excessive glutamate release (excitotoxicity), generation of reactive oxygen species and oxidative stress (oxidative pathway). In older WM the injury is mediated by Ca2+‐independent excitotoxicity due to an earlier and more robust glutamate release. Because excitotoxicity leads to oxidative stress in WM we investigated whether blocking nitric oxide synthase (NOS) activity before or after a period of oxygen glucose deprivation (OGD) promoted axon function in an age‐dependent manner.
Acutely isolated optic nerves from young and old (1 and 12 month) Swiss Webster (SW) or C57BL/6 (BL6) mice were used to ascertain quantitative measurements of WM function and structure. To support a biological basis for NOS inhibitor nitro‐L‐arginine methyl ester (L‐NAME) action in theMONpreparation, we evaluated the expression and localization of brain NOS (bNOS) using immunohistochemistry in conjunction with confocal imaging. The expression of bNOS co‐localized with GFAP (+) astrocyte nuclei, cytoplasm, end‐feet as well as NF‐200 (+) axons. The pattern of bNOS expression paralleled astrocyte morphology with age and became more punctate in appearance. Evoked compound action potentials (CAPs) recovered to 21.8 ± 2.8% (n = 18) after 60 min of oxygen glucose deprivation (OGD) in young BL6 MONs. Pretreatment of MONs with L‐NAME (200 µM) promotedCAPrecovery to 69.4 ± 11.3% (n = 8) compared to OGD while CAPs recovered to 39.9 ± 4.4% (n = 11) when L‐NAME was applied after the end of OGD. Pretreatment of MONs from 1 or 12 month old SW with L‐NAME improvedCAPrecovery to 49.6 ± 3.7% (n = 8, vs OGD 21.3 ± 3.7%, n = 12) or to 51.1 ± 9.3% (n = 7, vs OGD 5.7 ±1.7%, n = 8) respectively. L‐NAME application after the end of OGD failed to promote aging axon function (8.8 ± 3.5%, n = 6). Changes in NOS activity help unveil age‐dependent oxidative injury mechanisms in ischemic white matter.
T07‐02B. Transplantation of cerebral microvascular endothelial cells promotes remyelination in ischemic white matter damage
Y. Ishizaki 1, S. Puentes1, M. Kurachi1, K. Shibasaki1, M. Naruse1, Y. Yoshimoto1, M. Mikuni1, H. Imai2
1Gunma University Graduate School of Medicine, Maebashi, Japan 2Tokyo University Graduate School of Medicine, Tokyo, Japan
Question: Cerebral endothelial cells have been reported to exert a protective effect against brain damage. Does transplantation of healthy endothelial cells have a beneficial effect on the outcome of ischemic brain damage?
Methods: We injected endothelin‐1 (ET‐1) into the rat internal capsule to induce lacunar infarction. Seven days after ET‐1 injection, microvascular endothelial cells (MVECs) prepared from adult rat cerebral cortices were transplanted into the internal capsule. Meningeal cells prepared from adult rat cerebra or 0.2% bovine serum albumin‐Hank's balanced salt solution were injected as controls. Two weeks later, the footprint test and histochemical analysis were performed.
Results: We found that MVEC transplantation improved the behavioral outcome based on recovery of hind‐limb rotation angle (P <0.01) and induced remyelination (P<0.01) compared with the control groups. Also the inflammatory response was repressed by MVEC transplantation, judging from fewer ED‐1‐positive activated microglial cells in the MVEC‐transplanted group than in the other groups.
Conclusions: Elucidation of the mechanisms by which MVECs ameliorate ischemic damage of the white matter may provide important information for the development of effective therapies for white matter ischemia.
T07‐03A. Lactate increases TREK channel activity in CA1 stratum radiatum Astrocytes
A. Banerjee, S. Ghatak, S. Sikdar
Indian Institute of Science, Bangalore, India
Background: Astrocytes play a major role in the pathology of cerebral ischemia. In order to minimize post‐ischemic neuronal damage, astrocytes increase glutamate uptake, to reduce excitotoxicity. CA1 stratum radiatum astrocytes in the hippocampus express two pore domain potassium channel TREK1, which contributes to their characteristic linear current‐voltage profile (Zhou M et al., 2009). Inhibition of TREK1 channel in astrocytes decreases glutamate clearance capacity and increases ischemia‐evoked neuronal apoptosis (Wu X et al., 2012). Global cerebral ischemia increases expression of TREK1 channel in astrocytes (Pivonkova H et al., 2010). Astrocytes meet the energy requirements of neurons by releasing lactate. Lactate concentration reaches upto 25 – 30 mM in the ischemic hemisphere of the brain, when intravenously injected and reduces neuronal lesions (Berthet C et al., 2012).
Objective: Though lactate is a known neuroprotective agent, its mechanism of action is not known. We hypothesized that lactate can provide neuroprotection by activating TREK channels. The effect of lactate on the electrophysiology of TREK channels was studied both in an in vitro brain slice model, after blocking the activity of other ion channels and in a heterologous expression system.
Results: Whole cell patch clamp experiments, on CA1 stratum radiatum astrocytes, in acute hippocampal slices, significantly increased TREK channel activity by 23.3 ± 6.4% and hyperpolarized their resting membrane potential by 5 ± 0.6 mV, on bath application of 30 mM lactate. Comparison of rectification index at negative potentials between control and 30 mM lactate treatment showed no significant difference, suggesting that the conductance activated by lactate is outward rectifying. Lactate‐evoked increase in TREK channel activity was reversibly inhibited by 200 µM quinine, a potent inhibitor of TREK channels. Further, lactate was unable to increase TREK channel activity after disruption of intracellular lactate uptake with 2 mM α‐cyano‐4‐hydroxy cinnamate, a blocker of monocarboxylate transporter. This was confirmed by inside‐out patch clamp experiments on human TREK1 channel expressed in HEK293 cells.
Conclusion: The experimental observations suggest uptake of lactate by astrocytes to activate TREK channels intracellularly.
REFERENCES
Berthet C et al., 2012. Cerebrovasc Dis 34:329.
Pivonkova H et al., 2010. Neurochem Int 57:783.
Wu X et al., 2012. J Mol Neurosci DOI 10.1007/s12031‐012‐9875‐5.
Zhou M et al., 2009. J Neurosci 29:8551.

T07‐03B. Sirt1 regulates the regenerative response of white matter oligodendrocyte progenitor cells after neonatal hypoxia
B. Jablonska, M. Gierdalski, M. Catron, V. Gallo
Children's National Medical Center, Washington, DC, United States
Regenerative responses occurring after different types of postnatal brain injury often involve expansion of an endogenous neural progenitor cell pool, but the molecular mechanisms underlying this process are still poorly understood. We studied expansion of the glial progenitor cell pool in a mouse model of neonatal hypoxia (HX) that reproduces the hallmarks of perinatal brain injury in infants, including diffused white matter injury (DWMI). We have previously shown that HX causes proliferation of WM oligodendrocyte progenitor cells (OPCs), and that this process is regulated by the cyclin‐dependent kinase 2 (Cdk2) pathway. Our analysis in WM in vivo and in cultured cells demonstrated: i) higher expression of Cdk2, cyclinE, pRb806/811 and E2F1 in WM OPCs after HX; ii) activation of the Cdk2 pathway after HX, and iii) reduced HX‐induced OPC proliferation in Cdk2‐null mutant mice. Here, we studied the upstream molecular mechanisms that regulate activity of the Cdk2 signaling pathway resulting in OPC proliferation after HX. We show that Sirtuin 1 (Sirt1) histone deacetylase activity is a major regulator of Cdk2 signaling and of the regenerative response of OPCs after HX. Under hypoxic conditions, Sirt1 is upregulated in WM, and interacts with both Rb and Cdk2. Patterns of posttranslational modifications of Cdk2 or Sirt1 proteins after silencing either of these genes in cultured cells from normoxic and hypoxic WM indicate that Cdk2 is a substrate for deacetylation by Sirt1, and that Sirt1 is phosphorylated by Cdk2. Also, Sirt1 causes Rb deacetylation resulting in dissociation of E2F1 from the Rb/E2F1 complex, which ultimately leads to higher OPC proliferation. Knockdown of Sirt1 in hypoxic WM cell cultures suppresses OPC proliferation. Finally, inhibition of Sirt1 activity by sirtinol accelerates OPC differentiation to mature oligodendrocytes, and reduces the number of OPCs. We are currently analyzing the effects of HX on OPC proliferation and differentiation in the WM of Sirt1‐null mice. Our results provide evidence that Sirt1 is an essential regulator of the Cdk2‐dependent regenerative response observed in WM OPCs after HX, and that inhibition of Sirt1 activity facilitates oligodendrocyte regeneration from OPCs, which might ultimately promote WM recovery after HX.
Supported by NIH R01NS045702, P01NS062686 and IDDRC P30HD40677.
T07‐04A. Neuroprotection in stroke by gonadal steroids: an active role for microglia and astroglia‐microglia crosstalk
P. Habib1, J. Dang1, D. Dreymüller 2, C. Beyer 3
1RWTH Aachen, Institute of Neuroanatomy, Aachen, Germany 2RWTH Aachen, Institute of Pharmacology, Aachen, Germany 3RWTH Aachen, Aachen, Germany
Gonadal steroid hormones reveal a potential neuroprotective role in acute brain ischemia. In rat in vivo studies using the transient occlusion of the middle cerebral artery as ischemic stroke model, we have shown that 17ß‐estradiol and progesterone reduce the infarct area by more than 70% given 1h after the onset of stroke, prevent neuronal death and behavioral deficits. An intriguing aspect of this study was that the application of steroid hormones reduced the number of microglia and microglia‐related markers in the penumbra during the first 24 h after the onset of stroke. Besides microglia, penumbral astroglia coevally appeared as cellular target for steroid hormones by reducing the expression of proinflammatory‐ and molecules necessary for the attraction and activation of microglia and lymphocytes. This suggests that protective steroid hormones influence glia cell cross‐talk and activity during early damaging conditions in the lesioned brain site. By using an in vitro hypoxic approach, we have now demonstrated that microglia express steroid hormone receptors and directly respond to ischemic conditions and steroid hormone treatment by changing expression and secretion of inflammatory compounds, trans‐signaling factors and by modulating phagocytotic activity. This clearly shows that microglia is an important direct target for steroid hormones but also indirectly influenced via cross‐talk by adjacent astrocytes at the damaged brain site. This generally points at an extensive bidirectional crosstalk between both glial cell types to convey steroid‐mediated neuroprotection in ischemic brain tissue. In summary, the balance of inflammatory astroglia‐microglia interactions within the injured brain tissue during an early phase of ischemic destruction is a pivotal step for tissue repair.
T07‐04B. Cannabinoid receptor antagonists/inverse agonists, hinokiresinols regulate astroglial heme‐oxygenase‐1 expression and microglial migration
C. Ju 1, P. Prather2, W.‐K. Kim1
1Korea University College of Medicine, Department of Neuroscience, Seoul, Republic of Korea 2University of Arkansas for Medical Sciences, Department of Pharmacology and Toxicology, Little Rock, Arkansas, United States
Cannabinoids are considered as key regulators in the pathology of various diseases, including ischemic stroke. We previously demonstrated that post‐ischemic treatment of two naturally occurring phenylpropanoids, trans‐ and cis‐hinokiresinols (HRs) significantly reduced ischemic injury in rats subjected to middle cerebral artery occlusion (MCAO). In studies to screen their cellular targets, we found that HRs selectively bind to both type 1 and type 2 cannabinoid receptors (CB1R and CB2R). In the CB1R reporter gene assay, both HRs demonstrated antagonistic activity. In CB2R‐overexpressing U2 OS cell lines, both HRs increased forskolin‐induced cAMP accumulation, suggesting their inverse agonism. Since cAMP induces expression of heme oxyagenase‐1 (HO‐1), a well‐known antioxidant stress protein, we further investigated the effect of hinokiresinols on HO‐1 expression in mixed cortical neuron/glia cultures. Trans‐HR increased astroglial expression of HO‐1 greater than cis‐HR did. A selective HO‐1 inhibitor, tin protoporphyrin IX significantly reduces the neuroprotective effect of trans‐HR in cortical cultures exposed to oxygen‐glucose deprivation. In addition, both HRs reduced the number of ED‐1‐immunopositive cells (i.e., active microglia and macrophages) in ischemic lesions. Also, both HRs significantly reduced microglial migration induced by an endocannabinoid, 2‐arachidonoylglycerol. The present study suggests that HRs reduce ischemic injury via regulation of glial CBRs.
T07‐05A. Pyruvate carboxylation in astrocytes and the pentose phosphate pathway are affected after neonatal hypoxic‐ischemic brain injury – a 13C NMR spectroscopy study
E. Brekke 1, T. Morken2, M. Widerøe3, A. Håberg4, A.‐M. Brubakk2, U. Sonnewald4
1Norwegian University of Science and Technology, Trondheim, Norway 2Norwegian University of Science and Technology, Department of Laboratory Medicine, Women and Children's health, Trondheim, Norway 3Norwegian University of Science and Technology, Department of Circulation and Medical Imaging, Trondheim, Norway 4Norwegian University of Science and Technology, Department of Neuroscience, Trondheim, Norway
Question: Pyruvate carboxylase (PC), the main anaplerotic enzyme in the brain, is predominantly located in astrocytes. In the adult brain, ischemia is known to reduce pyruvate carboxylation. Moreover, reperfusion after ischemia leads to production of reactive oxygen species (ROS). The pentose phosphate pathway (PPP) is, by making NADPH necessary for the regeneration of reduced glutathione, a major pathway in the protection against ROS. However, astrocytic and neuronal metabolic function in the neonatal brain after hypoxic‐ischemic brain injury (HI) remains to be explored.
Methods: HI was induced in 7‐day‐old rats by unilateral severing of the carotid artery followed by 2 hours recovery and subsequent exposure to hypoxia (8%O2) for 90 min. 30 min after end of hypoxia (the early reperfusion phase), rats were injected with [1,2‐13C]glucose and decapitated 30 min later. One group was sham‐operated, and not exposed to hypoxia, thus reflecting normal metabolism in the neonate. Extracts of ipsilateral hemispheres from HI and sham animals were analysed with 1H‐ and 13C‐NMR spectroscopy.
Results: The sham animals had similar glutamine content, but lower amounts of 13C labelled glutamine compared to corresponding values from adult rats, reflecting a generally lower rate of glucose metabolism in the neonatal astrocytes. However, approximately equal amounts of 13C labelled isotopomers of glutamine were derived from PC and pyruvate dehydrogenase (PDH), resulting in a relatively high PC/PDH‐ratio compared the same ratio in adults. Following HI and reperfusion, a reduction in glucose metabolism and mitochondrial metabolism was seen by increased glucose and reduced incorporation of 13C labelling in glutamate, glutamine and aspartate via PC and PDH. However, the PC/PDH‐ratio in glutamine was similar in HI and sham. Total mean values of glutamate, glutamine and aspartate were decreased, but only the latter to a significant degree. Labelling in lactate via the PPP was reduced following HI.
Conclusion: The low labelling of lactate via the PPP indicates that the flux through the PPP was in fact reduced in the early reperfusion phase after HI. Moreover, mitochondrial metabolism of pyruvate from glucose was reduced in both astrocytes and neurons. Impaired anaplerosis in astrocytes was confirmed by lower amounts of aspartate. However, the proportionally similar decrease in 13C labelling in glutamate and glutamine via PC, and the maintained PC/PDH‐ratio implies that astrocytes continue to provide metabolic support for the neurons during the early reperfusion phase.
T07‐05B. Long‐term activation of the hippocampal neurogenic niche following stroke
S. Keiner, M. Storch, O.W. Witte, C. Redecker
Hans Berger Department of Neurology, Jena, Germany
Several experimental studies have shown that stroke strongly stimulates proliferation of distinct progenitor cells followed by an increase of adult neurogenesis. However, most of these studies analyzed the effects of cortical lesions in close proximity after infarct induction. In the current study we assessed the total number of progenitor cells up to 7 weeks after photothrombotic stroke in 3 and 16 month old transgenic pNestin‐eGFP mice. The distinct neural subpopulations were immunocytochemically phenotyped by confocal laser scanning microscopy using triple‐labelling of nestin‐GFP, GFAP and DAPI (4', 6‐diamidin‐2'‐phenylindoldihydrochlorid) to characterize the glia‐like type 1 progenitor cells. The type 2a, type 2b and type 3 cells (separated in short or long horizontal branches) were identified by the staining of nestin‐GFP, DCX and DAPI. Stereological evaluation revealed a significant increase in the absolute number of type 1, 2b and 3 cells in the SGZ of animals with cortical infarcts 7 weeks post ischemia compared with sham‐operated controls in 3 month old animals. In the dentate gyrus of 16 month old mice the photothrombotic stroke showed no lesion‐dependent activation of all subtypes 7 weeks post infarct. Our data indicate that small cortical lesions in the forelimb sensorimortor cortex induce a long‐lasting activation of the hippocampal neurogenic niche in young but not in old animals.
T07‐06A. The Isolectin IB4 binds RET Receptor Tyrosine Kinase in microglia
A. Casamassa 1, L. Cerchia2, C.L. Esposito2, V. de Franciscis2, L. Annunziato1, F. Boscia1
1University “Federico II" of Naples, Naples, Italy 2CNR, IEOS, Naples, Italy
Ret receptor tyrosine kinase is the signaling component of the receptor complex for the family ligands of the glial cell line‐derived neurotrophic factor (GDNF). Ret is involved in the development of enteric nervous system, of sympathetic, parasympathetic, motor and sensory neurons and it is necessary for the postnatal maintenance of dopaminergic neurons. Ret expression has been as well demonstrated on microglia and several evidence indicate that GDNF regulates not only neuronal survival and maturation but also certain functions of microglia in the brain.
Here we demonstrated that isolectin IB4, commonly used as a microglial marker in the brain, binds to the glycosylated extracellular domain of Ret both on the surface of living NIH3T3 fibroblasts cells stably transfected with Ret than in adult rat brain sections as revealed by immunoblotting. Further, confocal immunofluorescence analysis demonstrated a clear overlap staining between pRet and IB4 labeling in primary microglia cultures as well as in adult rat sections obtained from control or postischemic brain after permanent middle artery occlusion (pMCAO).
Interestingly, IB4 staining identified activated or amoeboid Ret‐expressing microglia under ischemic conditions. Collectively, our data indicate Ret receptor as one of the IB4‐reactive glycoconjugate accounting for the IB4 stain in microglia under physiological and ischemic conditions.
T07‐06B. Oxygenation regulates monocarboxylate transporter 4 (MCT4) expression via HIF‐1a in primary cultures of mouse cortical astrocytes
K. Rosafio, L. Pellerin
Université de Lausanne, Lausanne, Switzerland
Astrocytes can adapt to lower oxygen tensions by enhancing their glycolytic rate of energy production. In this case, adequate expression of monocarboxylate transporters (MCTs) may be essential to sustain prominent lactate efflux and prevent glycolysis inhibition. Here we show that oxygen level is an important factor controlling MCT4 expression in primary cultures of mouse cortical astrocytes. Indeed, while MCT4 is clearly expressed by astrocytes in vivo, its basal in vitro expression in primary cultures of mouse cortical astrocytes is undetectable under classical culture conditions (95% air, 5%CO2). Exposing astrocytes to more physiological brain oxygen tensions (hypoxic chamber flushed with 1%O2/5%CO2/94%N2, leading to a concentration of dissolved oxygen in the culture medium of 40–60mmHg) restored MCT4 expression. MCT4 mRNA expression became detectable after 12 hours under lower oxygen tension and was still present after 10 days with a maximum at 36 hours of incubation. This effect was paralleled by the appearance of the MCT4 protein which reached a plateau between 48 hours and 96 hours, with a further enhancement at 7 and 10 days of incubation. This induction was specific for MCT4 since MCT1 expression was not altered. Lactate release was significantly enhanced after 48 hours of incubation and further increased up to 10 days compared to astrocytes maintained under atmospheric conditions. Treatment with dimethyloxalylglycine (DMOG), a compound that mimics hypoxia by stabilizing the hypoxia‐inducible factor 1 alpha (HIF‐1α), produced a similar effect. Both MCT4 mRNA and protein induction reached their maxima when astrocytes were treated with a concentration of DMOG ranging between 1 to 2.5mM, which is also the range for greatest HIF‐1α expression. Again, no effect was detectable on MCT1 expression. Transfecting astrocyte cultures with a siRNA against HIF‐1α significantly reduced the effect of lower oxygen tension on MCT4 expression. Our results suggest that 1) under physiological conditions, changes in local oxygenation might regulate the capacity of astrocytes to supply lactate to neighboring cells via notably alteration in MCT4 expression 2) under pathological conditions, MCT4 may be upregulated as a consequence of hypoxia and contribute to neuroprotection by favouring the release and accumulation of lactate in the extracellular space. Indeed, this process might give rise to the beneficial effect of lactate for neurons observed during the recovery from hypoxic/ischemic conditions in vitro and in vivo.
T07‐07A. Hypoxia/ischemia increases the expression of TREM2 in gray and white matter of neonatal mice brain
M. Chertoff 1, K. Shrivastava1, L. Gimenez Llort2
1Universidad Autonoma de Barcelona, Neuroscience Institute, Department of Cell Biology, Physiology & Immunology, Medical Histology, Barcelona, Spain 2Universidad Autonoma de Barcelona, Neuroscience Institute, Department of Psychiatry and Forensic Medicine, Barcelona, Spain
Recently, rare variants or mutations of Triggering Receptor Expressed on Myeloid cells 2 (TREM2) had been associated with increased risk of Alzheimer's Disease, Frontotemporal Dementia‐like Syndrome and Early onset Dementia and. The loss‐of‐function of TREM2 or its coreceptor DAP12 is responsible for the recessively inherited Nasu‐Hakola disease (also known as PLOSL). The brains of PLOSL affected patients show strong microglial activation in the cerebral white matter. Reports demonstrate that TREM2‐mediated phagocytic function of microglia is required for debris clearance and CNS tissue homeostasis. Perinatal brain injury is the underlying etiology for a host of developmental disabilities that includes spastic motor deficits and cognitive, behavioral and learning difficulties. Hence, our aim is to characterize the expression of TREM2 in the control of neuroinflammation following hypoxia/ischemia (HI) in the newborn brain.
We performed HI brain damage in postnatal day 7 (P7) C57/BL6 mice by permanent left carotid occlusion and litters were exposed to 8% of oxygen balanced with nitrogen for 50 minutes in a hypoxic chamber with controlled humidity and temperature maintained at 37°C. Pups were then returned to their dam until sacrifice. The age‐matched controls and samples from 3 hours to 7 days after hypoxia were collected and processed for immunohistochemistry.
In control animals, TREM2 expression was observed in the corpus callosum and in the subventricular zone at P7 that almost disappeared at P10. Following HI, an increase in TREM2 staining was observed in the corpus callosum, hippocampus, caudate‐putamen, fimbria, cortex and thalamus in the ipsilateral (IL) hemisphere, following a similar regional pattern of microglia activation. The increased expression of TREM2 was observed from 24 hours to 7 days post‐hypoxia. TREM2 colocalized mainly with microglia markers, such as Iba‐1 and CD68. Oligodendrocyte expression of TREM2 was also analyzed.
In conclusion, HI produced an increase in TREM2 expression on the IL damaged regions. These results suggest that the modulation of TREM2 might be a possible target for damage control in neonatal brain.
Supported by Fundación La Maratò de TV3(2011–110531). M.C. holds a Marie Curie International Incoming fellowship (2009‐IIF‐253110).
KEYWORDS: microglia, TREM2, Hypoxia/ischemia, white matter, M1/M2 microglia
T07‐07B. Metabolic impairment of Müller glia differentially affects retinal ganglion cell survival after acute ischemia/reperfusion in the mouse eye in vivo
T. Vohra, R. Schultz, O.W. Witte, C. Schmeer
Hans Berger Department of Neurology, Jena, Germany
Increasing evidence shows that astrocytes play a critical role in neuronal protection during an ischemic insult. In the vertebrate retina, most of astrocytic functions are executed by Müller glial cells, the major macroglial cell type in this tissue. The aim of this study was to evaluate the role of Müller glia in the onset and progression of retinal ganglion cell (RGC) degeneration after acute ischemic injury in the mouse eye in vivo.
Here, we used the selective gliotoxin fluorocitrate (FC) in order to transiently impair Müller glial metabolism at different time points during and after induction of an acute retinal ischemia/reperfusion by means of elevated intraocular pressure (IOP).
The effect of FC (3 mM) on metabolism was determined by measuring retinal glutamine levels, aconitase activity and mitochondrial membrane depolarization after intravitreal injection of the gliotoxin. Retinal ischemia was unilaterally induced by elevating IOP for 45 min. FC was intravitreally injected 4 h before onset of ischemia and after 6, 12 or 24h of reperfusion. Saline was injected in the contralateral eye and served as control. Surviving RGCs were quantified by means of confocal scanning microscopy and automated cell counting‐based analysis on retinal wholemounts 7 days after lesion.
FC treatment reduced glutamine levels to 35–38% as compared to control 4–6 h after injection. Inhibition was completely reversed 24 h after injection. Aconitase activity was reduced to ∼30% from control levels 4 h after FC injection. Mitochondrial membrane potential was reduced by 50–70% as indicated by reduced intensity of the specific stain mitotracker red CMXRos. One week after ischemia, only 40% of RGCs survived as compared to unlesioned controls. Impairment of Müller glial metabolism during ischemia further reduced RGCs by 15% as compared to ischemia alone. FC treatment did not significantly modify RGCs survival 6 and 12 h after lesion.
Results support the notion for a critical neuroprotective role of Müller glial cells during ischemia. In the acute phase following ischemia, however, Müller glia inhibition does not further affect RGCs survival. Further research is required to establish the time point for this switch in Müller glial function and the underlying mechanisms.
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (DFG SCHM2639/1‐1) to CS.
T07‐08A. Post‐ischemic treatment of the standardized Cordyceps militaris extract, WIB‐801C reduces cerebral ischemic injury and improved long‐term survival in rats
G.S. Cho, S. Hwang, C. Ju, W.‐K. Kim
Korea University, College of Medicine, Department of Neuroscience, Seoul, Republic of Korea
A traditional herbal medicine, caterpillar fungus (Cordyceps militaris,) extract has been widely investigated in animal models and clinical studies for various diseases including ischemic stroke. Its major metabolite, cordycepin, has been reported to act as a selective A3 adenosine receptor agonist and to protect neurons against ischemic injury. However, their underlying mechanisms remain unclear. In the present study, we report that the standardized C. militaris extract, WIB‐801C, which contains cordycepin 8% of total dry weight of the extract, markedly reduced ischemic injury by inhibiting post‐ischemic inflammatory responses. Post‐ischemic treatment with WIB‐801C (orally administered twice at 3 and 8 h after onset of MCAO) significantly reduced infarct size and edema in rats subjected to transient middle cerebral artery occlusion (MCAO, 1.5 h) and subsequent reperfusion (22 h). WIB‐801C also significantly improved integrity of glial cells in ischemic lesions, as evidenced by reduced white matter degeneration and loss of blood‐brain barrier. Importantly, WIB‐801C significantly ameliorated neurological deficits in not only transient but also in permanent stroke models. Moreover, WIB‐801Csignificantly improved long‐term survival of MCAO rats over 30 days. As we previously reported with selective A3 receptor agonists (Choi et al., 2011, Am J Pathol), WIB‐801C also significantly inhibited the infiltration of ED‐1 and MPO‐positive inflammatory cells into ischemic lesions. The present findings indicate that a natural composite with selective A3 receptor agonist, WIB‐801C confers robust neuronal/glial protection and reduced inflammatory response, leading to long‐lasting improvement of post‐ischemic injury in brains.
#G‐S. Cho and S. Hwang contributed equally to this work.
T07‐08B. Phenotypic characterization of CD200R+ microglia/ macrophages and CD200+ neuronal cells in Neonatal C57BL/6 mice brain following hypoxia/ischemia
K. Shrivastava 1, M. Chertoff1, L. Gimenez‐Llort2, B. Gonzalez1
1Universidad Autonoma de Barcelona, Department of Cell Biology, Physiology & Immunology, Medical Histology, Neuroscience Institute, Barcelona, Spain2Universidad Autonoma de Barcelona, Department of Psychiatry and Forensic Medicine, Neuroscience Institute, Barcelona, Spain
Introduction: CD200 & CD200R are immune inhibitory molecules that have been shown to be involved in inducing immune tolerance and in contributing to immune privileged status of the CNS. The developing brain exhibits distinct morphological as well as physiological characteristics determining a peculiar response to injury showing an aggravated susceptibility to excitotoxicity and pro‐inflammatory cytokines, along with an exacerbated inflammatory response. Previous studies from our group have described the expression of CD200‐CD200R in brain during development showing a distinct pattern of expression in the cortex and the hippocampus. Hence, the aim of this study is phenotypic characterization of CD200R+ microglia/macrophages and CD200+ neuronal cells following hypoxia/ischemia (H/I) in neonatal mice brain.
Methods: Wild‐type C57/BL6 mice postnatal (P) day 1,3,5,7,10,14,21 and adult were used for developmental studies. P7 mice was used for H/I injury that was administered using Vannucci model modified for neonatal mice (8% O2, 55 min) and samples were collected 3h, 12h, 24h, 48h, 72h & 7 days after hypoxia for immunofluorescence staining.
Results: Phenotypic characterization of CD200R+ microglia/macrophages showed them to express markers of pro‐ or anti‐inflammatory phenotype in a temporal fashion post lesion. They expressed M2 phenotype as observed by colocalization with CD206+ cells throughout the time points studied but a subpopulation of CD200R+ cells exhibited M1 phenotype as exhibited by MHCII+, CD86+ cells from 24–48 hrs post lesion. Calretinin (CR) used for the characterization of CD200+ neurons showed that most CR+ neurons were CD200+ especially the interneurons in the innermolecular layer of hippocampus, Layer I of the neocortex and the hilus at most age groups studied. After H/I, CD200 immunolabeling was increased in the hippocampal fissure until 7 days post‐lesion. This followed a change in CD200R+ microglial cells described above.
To conclude, a balance between M1/M2 phenotype of CD200R+ microglia/macrophages and expression of CD200 by CR+ cells are involved in evolution of H/I induced brain injury in neonatal mice.
Support: Supported by BFU2009‐08805 from the Spanish Ministry of Science and Innovation and Fundació La Marato de TV3 (2011‐110531)
T07‐09A. Distinct subsets of interleukin‐1 receptor antagonist producing cells are neuroprotective after focal cerebral ischemia in mice
B.H. Clausen 1, K.L. Lambertsen1, A. Babcock1, C. von Linstow1, T. Deierborg1, B. Finsen1
1Institute for Molecular Medicine, Neurobiology Research, Odense, Denmark
Objective: The cytokine interleukin‐1 (IL‐1) and its naturally occurring receptor antagonist (IL‐1Ra) play a key role in determining neuronal cell death and survival in focal cerebral ischemia. The objective of this study was to determine the cellular production of IL‐1/IL‐1Ra, and to test the neuroprotective potential of post‐surgically injected IL‐1Ra overexpressing bone marrow (BM) cells in a mouse model of focal cerebral ischemia.
Materials and methods: C57BL/6 mice were injected i.v. with BM cells isolated from sIL‐1Ra overexpressing mice, 30 min after permanent middle cerebral artery occlusion (pMCAo). Physiological parameters and behaviour were recorded post‐surgically, infarct sizes were estimated, and microglial‐leukocyte expression of IL‐1/IL‐1Ra was analyzed by flowcytometry, in situ hybridization and immunohistochemistry.
Results: We identify microglia, and not recruited leukocytes as the major producers of IL‐1Ra after pMCAo in mice, and we show by using IL‐1Ra knock out mice that microglial‐produced IL‐1Ra is neuroprotective. We report that the sIL‐1Ra produced by the post‐surgically injected BM cells, potentiates the neuroprotective effect of microglial‐derived IL‐1Ra, at both 24 h and 5 days, which is consistent with behavioural improvement at 5 days, and detection of recruited BM cells in the ischemic area 1.5 h after pMCAo. Interestingly, we also find that the sIL‐1Ra overexpressing BM cells stimulate microglial production of IL‐1Ra 6 h after pMCAo, at which time both IL‐1α and IL‐1β is upregulated.
Conclusion: Our results provide proof of principle that increasing the production of IL‐1Ra by recruited BM cells can counteract the effect of IL‐1a/b, increase neuronal survival and improve motor function alone or through induction of microglial‐produced IL‐1Ra after pMCAo in mice.
T07‐09B. Glial activation following oxygen‐glucose deprivation
G. Skibo, M. Orlovsky, G. Maleeva, M. Patseva, I. Lushnikova
Bogomoletz Institute of Physiology, Kyiv, Ukraine
Short‐term oxygen‐glucose deprivation (OGD) is known to activate excitatory glutamatergic synapses, induce long‐term potentiation and result in neuronal and synaptical ultrastructural remodelling in organitypic cultured hippocampal slices (OCHS). In this work we studied changes in glial synaptic coverage following 30 min OGD episode using 3D reconstruction of OCHS confocal and electron microscopic images. Propidium iodide (PI) and MitoTracker Orange CMTMRos (MTO) were used to estimate cell viability and their mitochondrial potential. Astroglial and microglial cells were identified using immunohistochemical staining with anti‐GFAP and anti‐Iba‐1 antibodies. The study demonstrated that astroglial and microglial cells retain viability (there was no significant increase in the amount of PI‐labbelled cells). 3D reconstruction revealed significant increase in excitatory synapses glial coverage as early as 1 hour following OGD accompanied by a significant increase in number of astroglial and microglial processes. The deprivation also resulted in gradual increase of mitochondrial potential from 4 hr to 24 hr in both astroglial and microglial cells. It was previously shown that pyramidal neurons under the same conditions lose mitochondrial potential and became PI‐positive after 4 and 24 reoxygenetion, indicating damage of cytoplasmic membrane, the effect prevented by application of NMDA receptor antagonist D‐AP5.
Thus, unlike neurons astroglial and microglial cells are OGD‐resistant and demonstrate marked mitochondrial activation – a process that might be involved in maintaining neuronal function during oxygen‐glucose deficiency.
T07‐10A. Heterogeneity of GFAP‐positive glia in the cerebral cortex: from development to injury – single cell gene expression profiling
D. Dzamba 1,2, P. Honsa1,2, V. Rusnakova3, A. Stahlberg4,5, M. Kubista3,5, M. Anderova1,2
1Institute of Experimental Medicine, AS CR, Prague, Czech Republic 2Second Medical Faculty, Charles University, Prague, Czech Republic 3Institute of Biotechnology, AS CR, Prague, Czech Republic 4Sahlgrenska Academy at the University of Gothenburg, Sahlgrenska Cancer Center, Department of Pathology, Gothenburg, Sweden 5TATAA Biocenter, Gothenburg, Sweden
GFAP‐positive glial cells represent a key cell type that controls and regulates a diversity of complex functions in the central nervous system. Heterogeneity among these cells has been shown previously on the level of gene expression as well as in functional studies. To unravel astrocyte heterogeneity during postnatal development and after focal cerebral ischemia, we employed single‐cell gene expression profiling in acutely isolated GFAP/EGFP‐positive cells from the mouse cortex. Using a microfluidic high throughput qPCR platform, we measured the expression of 47 selected genes encoding astrocytic and polydendrocytic markers, ion channels, transporters and receptors that participate in maintaining K+ and glutamate homeostasis in each of 292 individual GFAP/EGFP‐positive glial cells. In this study we show how the expression of these selected genes changes during development (postnatal days 10, 20, 30 and 50) as well as 3, 7 and 14 days after middle cerebral artery occlusion (MCAO). Self‐organizing maps and principal component analyses divided the cells according to their similarity in gene expression into three subpopulations of astrocytes present within the first 10–50 days of postnatal development (P10‐P50). The first subpopulation comprises immature glia mainly from P10, characterized by the high transcriptional activity of all of the studied genes, including polydendrocytic markers. The second subpopulation is dominated by cells from P20 and displayed low transcript levels of all of the studied genes. The third subpopulation represents mature astrocytes mainly from P30 and P50. Three, seven and fourteen days after ischemia (D3, D7, D14), additional astrocytic subpopulations appear: resting astroglia (mainly from P50 and D3), transcriptionally active early reactive astroglia (mainly from D7) expressing the mRNA of polydendrocytic markers, and, presumably, permanent reactive astroglia (solely from D14). Following focal cerebral ischemia, reactive astrocytes undergo pronounced changes in their expression of aquaporins, nonspecific cationic and potassium channels, glutamate receptors and markers of reactive astrocytes and polydendrocytes. From the data obtained from single cell PCR analysis, we calculated the Spearman correlation coefficients between pairs of genes, which revealed interesting positive gene expression correlations with polydendrocytic markers (Gria2‐4, Grik1‐5, Grin3a, Kcnj16, Kcnk2 and Kcnk10 genes). This study was further supplemented by an immunohistochemical analysis of NMDA receptor subunits and PDGFαR, which confirmed that changes in gene expressions correlate with immunodetected proteins.
GA CR 13‐02154S, ASTF 110‐2011, GAUK 604212.
T07‐10B. Differentiated character of astroglial proliferative response to transient ischemia in the cerebral cortex and striatum of the rat
A. Steliga 1, M. Waskow1, S. Wójcik2, G. Lietzau2, I. Klejbor1,2, Z. Karwacki3, P. Kowiański1,2
1Pomeranian University of Slupsk, Slupsk, Poland 2Medical University, Department of Anatomy and Neurobiology, Gdansk, Poland 3Medical University, Department of Neuroanesthesiology, Gdansk, Poland
Background: Reactive astrogliosis is often regarded as universal type of astroglial response to various forms of injuries. Among its most characteristic features one may include: glial fibrillary acidic protein (GFAP) up‐regulation, cellular hypertrophy and proliferation and gliotic scar formation. An important element of glial cells response is their ability of de‐differentiation, which is related with their re‐gaining of immunological and molecular properties characteristic for earlier developmental forms. The origin of cells proliferating in response to various types of brain injury is still a subject of debate. Among others it is related to the pathomechanism of lesion, affected brain structure and developmental stage of the nervous system.
Aim: In our study we aimed to assess the differences of de‐differentiation and proliferation potential of astroglia localized in the cerebral cortex and striatum to the transient focal brain ischemia.
Material and methods: Transient focal brain ischemia was evoked in 20 adult male Wistar rats by placement of the monofilament surgical thread into the internal carotid artery and occlusion of the middle cerebral artery for 1h. The postoperative survival period was up to 6 weeks. Immunocytochemical double‐staining for astrocytic marker (GFAP), developmental (Pax6) and proliferative (Ki‐67) markers was performed and subsequent qualitative and quantitative study was done by means of confocal microscopy.
Results: Double‐labeled GFAP/Pax6 and GFAP/Ki67 reactive astroglial cells were revealed in the cerebral cortex and striatum of the ischemic region, starting from 24h after initiating the ischemia. The apparent difference in the intensity of morphological changes, quantity of de‐differentiating and proliferating astroglial cells was observed between ischemic cerebral cortex and striatum. Intensity of astroglial response and proliferative potential were much higher in the striatum than in the cerebral cortex.
Summary and conclusion: Apparent difference in proliferative response between the cerebral cortex and striatum may be consequence of metabolic and molecular differences between astroglial cells populating two mentioned above structures. It may be also consequence of uneven decrease of cerebral blood flow and resulting different metabolic conditions influencing astroglial function in various fragments of ischemic area. Reassuming, the differentiated potential of astroglial proliferative response to ischemic changes in different brain regions must be taken into account while analyzing clinical consequences of cerebral infarcts of different localization.
T07‐11A. Attenuated Inflammatory Response in Triggering Receptor Expressed on Myeloid Cells 2 (TREM2) Knock‐Out Mice following Stroke
C. Frahm 1, M.W. Sieber1, N. Jaenisch1, M. Brehm1, M. Guenther1, B. Linnartz‐Gerlach2, H. Neumann2, O.W. Witte1
1University Hospital Jena, Jena, Germany 2University Hospital Bonn, Bonn, Germany
Background
Triggering receptor expressed on myeloid cells‐2 (TREM2) is a microglial surface receptor involved in phagocytosis. Clearance of apoptotic debris after stroke represents an important mechanism to re‐attain tissue homeostasis and thereby ensure functional recovery. The role of TREM2 following stroke is currently unclear.
Methods and Results
As an experimental stroke model, the middle cerebral artery of mice was occluded for 30 minutes with a range of reperfusion times (duration of reperfusion: 6 h/12 h/24 h/2 d/7 d/28 d). Quantitative PCR (qPCR) revealed a greatly increased transcription of TREM2 after stroke (∼10fold). We subsequently analyzed the expression of pro‐inflammatory cytokines, chemokines and their receptors in TREM2‐knockout (TREM2‐KO) mice via qPCR. Microglial activation (CD68, Iba1) and CD3‐positive T‐cell invasion were analyzed via qPCR and immunohistochemistry. Functional consequences of TREM2 knockout were assessed by infarct volumetry. The acute inflammatory response (12 h reperfusion) was very similar between TREM2‐KO mice and their littermate controls. However, in the sub‐acute phase (7 d reperfusion) following stroke, TREM2‐KO mice showed a decreased transcription of pro‐inflammatory cytokines TNFα, IL‐1α and IL‐1β, associated with a reduced microglial activity (CD68, Iba1). Furthermore, TREM2‐KO mice showed a reduced transcription of chemokines CCL2 (MCP1), CCL3 (MIP1α) and the chemokine receptor CX3CR1, followed by a diminished invasion of CD3‐positive T‐cells. No effect on the lesion size was observed.
Conclusions
Although we initially expected an exaggerated pro‐inflammatory response following ablation of TREM2, our data support a contradictory scenario that the sub‐acute inflammatory reaction after stroke is attenuated in TREM2‐KO mice. We therefore conclude that TREM2 appears to sustain a distinct inflammatory response after stroke.
T07‐11B. Glial expression of metabotropic glutamate receptors in the mouse optic nerve: a role in ischemia mediated disruption in postnatal CNS white matter
I. Vanzulli, A. Butt
University of Portsmouth, Portsmouth, United Kingdom
Metabotropic glutamate receptors (mGluRs) are seven transmembrane domain G‐protein‐coupled receptors for L‐glutamate, the main excitatory neurotransmitter in the mammalian brain. In addition, glutamate‐mediated excitotoxicity plays a central role in mediating neuron and oligodendrocyte death during ischemic episodes. In vitro, developing oligodendrocytes (OLs) in particular have been shown to be highly vulnerable to excitotoxicity and oxidative stress. Notably, activation of group 1 mGluRs has been shown to attenuate OL excitotoxicity in vitro and in brain slices has been shown to reduce ischemia‐mediated impairment of astrocytes. Here, we have examined the functional expression of mGluRs and their role in acute ischemic injury in developing CNS white matter of the mouse optic nerve. Calcium imaging experiments were performed in optic nerves from P9–13 wild‐type mice. Optic nerves were isolated intact, loaded with fluo‐4 AM and visualized using a Zeiss LSM 5 Pascal confocal microscope; images were collected in optical z‐sections and changes in fluorescence intensity were measured. The application of mGluR agonists ACPD and DHPG showed that group I mGluR mediate a rise in glial [Ca2+]i. The action of the group I mGluR antagonist AIDA versus ACPD indicated that the rise in [Ca2+]i also involves group II (and possibly group III) mGluR. Immunohistochemistry confirmed glial expression of group I subunit mGluR5 and group II subunits mGluR2 and mGluR3 in the optic nerve, although further analysis of cellular localization is required. To examine the role of mGluRs in white matter ischemia, optic nerves from P8–9 mice in which fluorescent reporters are driven by astrocyte (GFAP‐EGFP) or oligodendrocyte (Sox10‐EGFP) genes were isolated intact into artificial cerebrospinal fluid (aCSF) and subjected to oxygen glucose deprivation (OGD) for 1 hour. Activation of group I or group II mGluR with the agonists ACPD, DHPG or LY379268 protected astrocytes and oligodendrocytes from ischemic cell death. Further experiments are required to determine the mechanisms involved, but these findings demonstrate functional expression of mGluR in optic nerve glia and indicate that activation of mGluR is a possible therapeutic strategy to protect against glial damage in response to ischemia.
T07‐12A. Comparative analysis of neuronal loss, glial activation and tissue degeneration in different cortical areas of adult rats following focal ischemia
W. Gomes‐Leal, R. Fernandes, R. Rodrigues Lima, E.M.N. Dos Santos
Federal University of Para, Belem, Brazil
The cerebral cortex is one of the most often affected areas after stroke, but there are no studies comparing the outcome in different cortical regions after focal ischemia. The aim of this investigation was to evaluate the patterns of glial activation, tissue degeneration and neuronal loss in different survival times following cortical ischemia. Focal ischemia was induced by stereotaxic microinjections of endothelin‐1 (ET‐1) into the somatosensory, motor and association cortices of adult rats (N = 45). The control animals were injected with the same volume of sterile saline (N = 27). The animals were perfused at 1, 3 and 7 days after ischemia. Gross histopathology was evaluated using cresyl violet staining. 20 μm sections were submitted to immunohistochemistry for astrocytes (anti‐GFAP), activated microglia/macrophages (anti‐ED1) and microglia (anti‐Iba1 and ED1). Tissue loss and glial activation were more intense in the somatosensory cortex than in other cortical areas. The motor cortex was the second more affected area. The association cortex was the less damaged area, which was confirmed by quantitative analysis (ANOVA‐Tukey). The results suggest that an ischemic lesion of the same intensity induces a differential pattern of tissue loss and neuroinflammation, depending on the cortical area, and that the primary sensory and motor areas are more susceptible to ischemia than association areas.
T07‐12B. Ischemic Preconditioning In Vivo and Hypoxia‐hypoglycemia In Vitro Induce Interferon Stimulated Gene Expression in Microglia
J. Weinstein, R. Lee, S. Noor, J. Colman, S. Murphy, T. Möller
University of Washington, Seattle, WA, United States
Ischemic preconditioning (IPC) is a robust neuroprotective phenomenon in which a brief ischemic exposure increases resistance to the injurious effects of subsequent prolonged ischemia. Characterizing the cellular and molecular mechanisms of IPC is an active area of investigation in stroke research. Several lines of evidence support a role for microglia in IPC. Microglia express Toll‐like receptors (TLRs) that mediate powerful immune responses to exogenous and endogenous agonists. TLR4 is required for IPC‐induced neuroprotection. In order to elucidate the mechanisms by which microglial TLR4 contributes to IPC, we carried out cell‐targeted genomic analyses specifically on microglia exposed to either ischemic conditions in vitro or IPC in vivo. For our in vitro model, we exposed WT and TLR4‐/‐ mouse primary microglia to hypoxic/hypoglycemic conditions for 24 hours. For our in vivo model, we carried out transient middle cerebral artery occlusion (MCAO) or sham surgery as our IPC pulse on WT and TLR4‐/‐ adult male mice. Three days later mice were sacrificed and microglia acutely isolated from ipsilateral cortex using magnetic affinity chromatography cell separation and ex vivo flow cytometry. Microarray analysis was carried out on RNA from both in vitro and in vivo microglia. Results from both datasets identified robust expression of type 1 and/or type 3 interferon (IFN)‐stimulated genes (ISGs) as the predominant transcriptomal feature of ischemia‐exposed microglia. In vitro, we found that 25 out of the 60 (40%) individual genes that were significantly up‐regulated >2‐fold by hypoxia/hypoglycemia in WT, but not TLR4‐/‐, microglia were ISGs including Mx1, Oas2, IRF7 and CCL5. Promoter analysis demonstrated that the top four most active transcription factors were ISGF3, IRF2, IRF1 and IRF7; all IFN regulatory factors (IRFs). A similar pattern emerged from the in vivo dataset with multiple IRFs again among the most activated transcription factors in sorted microglia from IPC‐exposed mice. However, unlike the ISG response in vitro, the ISG response from the in vivo dataset was not TLR4‐dependent. The IFN family of cytokines is recognized as a key component of the innate immune response to infection and other types of injury. The role of microglial IFN‐signaling in IPC is unknown. These results suggest that broad activation of ISGs may be a key feature of the microglial response to ischemic conditions.
T07‐13A. Increased expression of hyperpolarization‐activated cyclic nucleotide‐gated channels in reactive astrocytes after ischemia
P. Honsa 1,2, L. Harantova1,2, H. Pivonkova1, V. Rusnakova3, D. Dzamba1,2, M. Kubista3,4, M. Anderova3,2
1Institute of Experimental Medicine, Department of Cellular Neurophysiology, Prague, Czech Republic22nd Faculty of Medicine, Charles University, Prague, Czech Republic 3Institute of Biotechnology, Laboratory of Gene Expression, Prague, Czech Republic 4TATA Biocenter, Gothenburg, Czech Republic
Astrocytes respond to central nervous system (CNS) injury by the formation of a glial scar that develops within few days after insult and is characterized by astrocyte proliferation and cellular hypertrophy. Reactive astrocytes change their immunohistochemical profile, such as increasing expression of GFAP, nestin and vimentin; however, they also markedly alter the expression of ion channels, receptors and transporters, which participate in the maintenance of ionic‐, water‐ and neurotransmitter homeostasis, such as K+ channels, aquaporins or glutamate transporters. Here, we aimed to characterize the gene expression profile and functional properties of reactive astrocytes 5 weeks after global cerebral ischemia (GCI) or focal cerebral ischemia (FCI) with a specific focus on K+ channels or non‐specific cationic channels. GCI was induced in adult rats by bilateral, 15‐minute common carotid artery occlusion combined with low oxygen, while FCI was induced in adult EGFP/GFAP mice by permanent middle cerebral artery occlusion. Using the patch‐clamp, we investigated the membrane properties of astrocytes in situ 5 weeks after GCI in the rat hippocampal CA1 region and 5 weeks after FCI in the mouse cortex. Regardless of the type of ischemic injury, astrocytes from both CNS regions depolarized their membrane potential by ∼14 mV 5 weeks after ischemia. When compared to astrocytes from the non‐injured CA1 region or the cortex, the post‐ischemic astrocytes displayed large hyperpolarization‐activated time‐ and voltage‐dependent, non‐inactivating inward currents, the current density of which increased 3‐fold in response to GCI or FCI. In addition, these non‐specific cationic channels were sensitive to ZD7288 and to a low extracellular Na+ concentration, suggesting that they may belong to the family of hyperpolarization‐activated cyclic nucleotide‐gated (HCN) channels. We have taken advantage of fluorescently labeled astrocytes in EGFP/GFAP mice and isolated EGFP+ cells by FACS from non‐injured as well as ischemic regions and performed gene expression profiling using single‐cell RT‐qPCR. Our data revealed that cortical astrocytes after FCI express, besides the increased expression of Gfap, Gfapδ, Aqp1 and 9, extremely high levels of Hcn1–3 transcripts that encode HCN 1–3 channels. Moreover, our immunohistochemical analyses confirmed the presence of HCN1–3 in reactive astrocytes, especially HCN1, 2 and 3 channels. In summary, our results show that reactive astrocytes from post‐ischemic tissue express HCN channels and that their activity might significantly contribute to Na+ influx, possibly maintaining ATPase function and/or contributing to intracellular Na+‐dependent events, such as glutamate transporter or Na+/Ca2+ exchanger reversal.
GA CR 13–02154S, P304/12/G069 and GAUK 383711.
T07‐13B. Involvement of TREK‐1 channel activity in astrocyte function and neuroprotection under ischemia conditions
M. Xie, X. Wu, Y. Liu, X. Chen, Q. Sun
Tongji Hospital, Tongji Medical College, HUST, Wuhan, China
The optimal operation of electrogenic astrocytic transporters and exchangers for some well‐defined astrocyte brain homeostatic functions depends on the presence of K+ channels in the cell membranes and the hyperpolarized membrane potential. Our previous study showed that astrocytes functionally express two‐pore domain K+ channel TREK‐1, which helps to set the negative resting membrane potential. However, the roles of TREK‐1 on astrocytic function under normal and ischemic conditions remain unclear. In this study, we investigated the effects of TREK‐1 activity on astrocytic function and neuronal death under ischemic conditions. In an in vitro hypoxia model with astrocytes culture, TREK‐1 immunoreactivity was up‐regulated after hypoxia. Suppression of TREK‐1 activity inhibited the glutamate clearance capability, enhanced the inflammatory secretion of astrocytes derived s100β and led to increased neuronal apoptosis after ischemic insult. After middle cerebral artery occlusion induced focal ischemia in rats, the TREK‐1 expression was significantly increased which correlated with reactive astrogliosis. Application of lineonic acid, a TREK‐1 agonist which could potentiate the astrocytic conductance and hyperpolarization membrane potential, up‐regulated the expression of astrocytic glutamate transporter GLT‐1, inhibited the micro‐glial inflammatory response and attenuated neuronal damage. Our results suggest that TREK‐1 activity is involved in astrocytic function and neuronal survival. This would provide evidence showing astrocytic TREK‐1 involvement in ischemia pathology which may serve as a potential therapeutic target in stroke.
POSTER TOPIC 08 Myelin
T08‐01A. [1,6‐13C]glucose metabolism in immature and in differentiated oligodendrocytes in vitro
A. Amaral 1, U. Sonnewald2, M. Kotter1
1University of Cambridge, Cambridge, United Kingdom 2Norwegian University of Science and Technology, Trondheim, Norway
Oligodendrocytes are the myelinating cells of the central nervous system (CNS). They are responsible for ensheathing myelin layers around axons, which contributes to improve conduction of neuronal impulses and additionally provides axonal support. Oligodendrocytes are generated by oligodendrocyte precursor cells (OPCs), a self‐renewing and proliferating adult stem/precursor cell population in the CNS. This process occurs not only during developmental myelination but also mediates CNS remyelination, a regenerative process that restores myelin sheaths to demyelinated axons.
Although energy metabolism in the oligodendrocyte lineage is thought to be very active to support the lipid production specific to this cell type, it is still poorly characterized. As a first step towards a detailed characterization of the energy metabolism associated with specific lineage stages we investigated how glucose – the main cerebral energy substrate – is metabolized at an immature OPC stage and in differentiated oligodendrocytes. Primary cultures of OPCs and oligodendrocytes were incubated with medium containing [1,6‐13C]glucose for 4h and metabolites in both cell extracts and culture medium were collected and analysed for excess % 13C enrichment using Gas‐Chromatography‐Mass Spectrometry (GC‐MS) as well as for total amounts using HPLC. The significant 13C‐enrichment in citrate, malate, and aspartate in cell extracts indicate that oligodendrocytes oxidize glucose‐derived metabolites extensively in the Tricarboxylic Acid (TCA) Cycle. Moreover, they release 13C‐labelled aspartate and glutamate to the extracellular medium, although a significant consumption of aspartate, glutamate and glutamine from the medium was also observed. Enrichment of extracellular lactate and increased alanine synthesis in oligodendrocytes as compared to OPCs suggests that the rate of aerobic glycolysis is increased in immature as compared to differentiated cells.
In conclusion, these data confirm that both OPCs and oligodendrocytes are highly metabolically active and, in addition to extensively oxidizing glucose, synthesize and release distinct metabolites. We anticipate that further studies on this subject will contribute to a better understanding of the physiological role of oligodendrocytes in the CNS and the role of glycolysis and mitochondrial metabolism during their maturation.
T08‐01B. FASN‐mediated fatty acid homeostasis is critical for myelination
L. Montani 1, M. Trötzmüller2, B. von Niederhäusern1, P. Dimas1, H. Pohl1, C.F. Semenkovich3, H.C. Köfeler2, S. Jessberger4, U. Suter1
1Inst Molecular Health Sciences – ETH Zürich, Zürich, Switzerland 2Medical University of Graz, Lipidomics Center for Medical Research, Graz, Austria 3Washington University School of Medicine, Division of Endocrinology, Metabolism & Lipid Research, St. Louis, United States 4University of Zürich, Brain Research Institute, Zürich, Switzerland
Schwann cells in the peripheral and oligodendrocytes in the central nervous system require a tremendous amount of lipids to be capable of producing all cell membranes forming the myelin structure. Furthermore, when compared to other cells, myelin membrane presents an extremely high lipid content (∼80% of dry weight) coupled to an unique lipid composition. The importance of myelin lipid composition towards myelin formation and maintenance is highlighted by the frequent appearance of myelin defects in lipid‐metabolism human disorders. One of the main represented class of lipids in myelin are phospholipids, of which fatty acids (FAs) represents the building blocks. Most cells preferentially uptake circulating free FAs. However, cells under increased membrane production, e.g. highly proliferating cells, can endogenously produce them through the enzyme fatty acid synthase (FASN), which synthesize palmitate. In these cells, FASN is transcriptionally augmented via the mTORC1 pathway. Thus, we hypothesized that due to their increased lipid demand myelinating cells would require FASN activity towards myelin production. The functional role of FASN in the metabolic control of myelin membrane lipid composition and myelination is unidentified. Thus, we addressed it experimentally by conditionally deleting FASN in myelinating cells in vivo. We show that endogenous FAs biosynthesis in myelinating cells is essential to maintain myelin membrane lipid homeostasis. Furthermore, maintaining a correct lipid homeostasis is critical for myelination. Thus, we analyzed whether FASN is required to allow palmitoylation of myelin proteins and their targeting to the myelin membrane. In addition, we show that interfering with myelin FAs homeostasis results in the activation of systemic compensating metabolic loops promoting FAs uptake, although not sufficient to preserve efficient myelination. Taken together our data demonstrate that in myelinating cells FAs homeostasis is under the synergistic control of endogenous metabolic FAs production via FASN and systemic functional regulation of free FAs uptake. Most importantly, maintaining a correct homeostasis of FA‐derived lipids in myelin is critical for myelination.
T08‐02A. A vertebrate specific Glutaredoxin affects cellular functions of oligodendrocytes
C. Berndt, K. Lepka, K. Volbracht, R. Schneider, H.‐P. Hartung, T. Prozorovski, O. Aktas
Heinrich‐Heine‐Universität, Düsseldorf, Germany
Glutaredoxins, members of the Thioredoxin family of proteins, are general disulfide reductases maintaining and regulating cellular thiol homeostasis and signaling, respectively (1). In mammals, two cytosolic and two mainly mitochondrial located Glutaredoxins have been identified (2). We already described an essential role for the formation of a functional neuronal network during embryonic development of one of these proteins, the vertebrate specific Glutaredoxin 2 (3). Here, we will focus on the contribution of Glutaredoxin 2 on basic cellular functions of oligodendrocytes, the myelinating cells of the central nervous system, under physiological and pathophysiological situations, e.g. multiple sclerosis, which is characterized by inflammatory axonal demyelination (4). Oligodendrocyte progenitors and mature oligodendrocytes are very sensitive against oxidative stress induced by release of nitric oxide by activated microglia (5). Using A2B5 positive glia restricted progenitor cells as well as organotypic cerebellar slice cultures, we found that increased levels of Glutaredoxin 2 protect against cell death induced by either activated microglia or S‐nitrosoglutathione, a physiological nitric oxide donor. Under physiological conditions elevated levels of Glutaredoxin 2 increased migration of A2B5 positive cells nearly three fold. Western Blot analyses, quantitative real time PCR, as well as immunocytochemistry revealed that Glutaredoxin 2 inhibited further differentiation of NG2 positive oligodendrocyte progenitor cells. In summary, our data indicate the importance of specific thiol redox signaling during cellular protection and function in this type of glia cells.
(1) Lillig, Berndt, Antioxid Redox Signal, accepted manuscript (2013).
(2) Lillig, Berndt, Holmgren, Biochim Biophys Acta 1780: 1304–1317 (2008).
(3) Bräutigam, Schütte, Godoy, Prozorovski, Gellert, Hauptmann, Holmgren, Lillig, Berndt, Proc Natl Acad Sci USA 108: 20532–20537 (2011).
(4) Aktas, Kieseier, Hartung, Trends Neurosci 33: 140–152 (2010).
(5) Pang, Campbell, Zheng, Fan, Cai, Rhodes, Neuroscience 168: 240–245 (2010).
T08‐02B. The nootropic agent nefiracetam is a potential remyelinating therapeutic in vivo
R.P. Murphy 1, E.A. Keogh1, S.D. O'Shea1, G. Bowen1, M. Pickering2, K.J. Murphy1
1University College Dublin, College of Science, School of Biomolecular and Biomedical Science, UCD Conway Institute, Neurotherapeutics Research Group, Belfield, Dublin, Ireland 2University College Dublin, College of Health Sciences, School of Medicine and Medical Science, Belfield, Dublin, Ireland
Current multiple sclerosis therapeutics act as immunomodulators and/or immunosuppressors. This strategy is largely ineffective at preventing progression of the disease. Significant benefits to multiple sclerosis patients might be seen with a therapeutic agent which induces remyelination, however, to date no such agents have been developed. Using in vitro and in vivo models we have identified nefiracetam as a potential remyelinating therapeutic.
To study remyelination in vitro, hippocampal organotypic cultures were exposed to lysophospatidylcholine (LPC) for 18hrs to induce demyelination before being allowed to recover. Cultures were treated with nefiracetam for 24hrs of this recovery period. In all cases immunofluorescent labelling of myelin basic protein (MBP) was used as a measure of myelin. Nefiracetam was shown to increase MBP expression, relative to untreated controls, accelerating recovery from LPC.
Two different models were used to investigate the efficacy of nefiracetam in vivo; an inflammatory model, experimental autoimmune encephalomyelitis (EAE), and a toxin induced model of demyelination, achieved through the use of cuprizone chow. Nefiracetam treatment did not alleviate the symptoms of EAE. However, nefiracetam did restore the amount of myelin in cuprizone exposed animals, compared to saline‐treated controls.
In combination, these observations suggest that nefiracetam is capable of accelerating remyelination through a mechanism of action which is independent of immunomodulation.
We conclude that nefiracetam encourages remyelination both in vitro and in vivo. These pro‐myelin properties identify nefiracetam as a potential therapeutic for demyelinating disorders.
Funded by Multiple Sclerosis Ireland, Enterprise Ireland and Congressionally‐Directed Medical Research Programme.
T08‐03A. Silencing or knocking‐out of the Na+/Ca2+ exchanger 3 (NCX3) impairs oligodendrocyte differentiation
F. Boscia, C. D'Avanzo, A. Pannaccione, A. Secondo, A. Casamassa, L. Formisano, N. Guida, L. Annunziato
University of Naples “Federico”, Neuroscience, Naples, Italy
Changes in intracellular [Ca2+]i levels have been shown to influence the developmental processes that accompany the transition of human oligodendrocyte precursor cells (OPCs) into mature myelinating oligodendrocytes and are required for the initiation of myelination and remyelination processes. In the present study, we explored whether calcium signals mediated by the selective sodium calcium exchanger (NCX) family members NCX1, NCX2, and NCX3, play a role in oligodendrocyte maturation. Functional studies, as well as mRNA and protein expression analyses, revealed that NCX1 and NCX3, but not NCX2, were divergently modulated during OPC differentiation into oligodendrocyte phenotype. In fact, while NCX1 was down‐regulated, NCX3 was strongly up‐regulated during the oligodendrocyte development. The importance of calcium signaling mediated by NCX3 during oligodendrocyte maturation was supported by several findings. Indeed, whereas the knocking down of the NCX3 isoform in OPCs prevented the up‐regulation of the myelin protein markers CNPase and MBP, its overexpression induced an up‐regulation of CNPase and MBP. Furthermore, NCX3 knock‐out mice exhibited not only a reduced size of spinal cord but also a marked hypomyelination, as revealed by the decrease in MBP expression and by the accompanying increase in OPCs number. Collectively, our findings indicate that calcium signaling mediated by NCX3 plays a crucial role in oligodendrocyte maturation and myelin formation.
T08‐03B. Kif13B motor protein regulates myelination in the peripheral and central nervous system
R. Noseda 1, S. Scarlino1,2, D. Triolo1, D. Sherman3, L. Feltri4, L. Wrabetz4, K.‐A. Nave5, R.L. Huganir6, S.C. Previtali1, A. Bolino1,2
1San Raffaele Scientific Institute, Milan, Italy 2Dulbecco Telethon Institute, Milan, Italy 3University of Edinburgh, Edinburgh, United Kingdom 4State University of New York at Buffalo, Buffalo, NY, United States 5Max Planck Institute of Experimental Medicine, Goettingen, Germany 6The John Hopkins University School of Medicine, Baltimore, MD, United States
Myelination is a multistep process which includes axon recognition and contact; promotion of myelination by several intrinsic and extrinsic signals; membrane biosynthesis; compaction, and remodeling. However, the molecular mechanisms that promote and regulate myelin biogenesis during myelination and re‐myelination are at the present largely unknown.
We recently reported that the Kinesin 13B (kif13B) motor protein interacts with the Dlg1 scaffold and regulates myelination in Schwann cells. Indeed, downregulation of kif13B in Schwann cell/DRG neuron co‐cultures significantly impairs myelination (A. Bolis et al., 2009). Kif13B kinesin is a plus end motor protein that has been shown to be involved in the transport of several cargos in polarized cells (N. Asaba et al., 2003). In neurons, kif13B transports PIP3‐containing vesicle along microtubules, thus promoting neuronal polarity (K. Horiguchi et al., 2006).
To explore the role of kif13B in myelination in vivo, we generated conditional knock‐out mice with kif13B specific ablation in either Schwann cells or oligodendrocytes. We found that kif13BFl/Fl P0Cre mouse nerves are hypomyelinated with reduced myelin thickness, thus confirming in vitro data.
In the PNS, axonal Neuregulin (NRG) 1 type III is one of the main signaling promoting myelination and remyelination after injury. NRG1 type III binds to ErbB2/ErbB3 receptors in Schwann cells, which signal through a plethora of downstream signaling pathways also including the PI3K/AKT complex. The PI3K/AKT signaling pathway is attenuated by the PTEN phosphatase, which dephosphorylates PtdIns(3,4,5)P 3 (S. Goebbels et al., 2010). Dlg1 is believed to interact with PTEN and potentiate its ability to dephosphorylate PtdIns(3,4,5)P 3, thus negatively modulating the AKT‐mTOR pathway (L. Cotter et al, 2010).
As kif13B interacts with Dlg1 in Schwann cells, we investigated the PI3K/AKT pathway in kif13B‐null nerves and surprisingly, we found that Dlg1 expression levels are increased whereas AKT phosphorylation is decreased in mutant nerves. Our findings suggest that kif13B negatively regulates Dlg1 activity and acts as a promoter of myelination in the PNS.
On the contrary, when Kif13B is lost specifically in oligodendrocytes, myelin sheaths are thicker. At the molecular level, the hypermyelination is consistent with increased AKT phosphorylation. We are currently investigating the molecular mechanisms at the basis of the Kif13B/Dlg1 interaction in both Schwann cells and oligodendrocytes.
T08‐04A. Resetting translational homeostasis restores myelination in CMT1B mice
M. D'Antonio 1, N. Musner1, C. Scapin1, D. Ungaro1, U. Del Carro1, D. Ron2, M.L. Feltri1,3, L. Wrabetz1,3
1Ospedale San Raffaele, Milano, Italy 2University of Cambridge, Metabolic Research Laboratories, Cambridge, United Kingdom 3Hunter James Kelly Research Institute, Buffalo, United States
P0 glycoprotein is an abundant product of terminal differentiation in myelinating Schwann cells. The mutant P0S63del causes Charcot‐Marie‐Tooth 1B neuropathy in humans, and a very similar demyelinating neuropathy in transgenic mice. P0S63del is retained in the endoplasmic reticulum of Schwann cells where it promotes unfolded protein stress and elicits an unfolded protein response (UPR) associated with translational attenuation. Ablation of Chop, a UPR mediator, from S63del mice completely rescues their motor deficit and reduces active demyelination by half. Here we show that Gadd34 is a detrimental effector of CHOP that reactivates translation too aggressively in myelinating Schwann cells. Genetic or pharmacological limitation of Gadd34 function moderates translational reactivation, improves myelination in S63del nerves, and reduces accumulation of P0S63del in the ER. Resetting translational homeostasis may provide a therapeutic strategy in tissues impaired by misfolded proteins that are synthesized during terminal differentiation.
T08‐04B. Exploring the Mechanism of Action of the Novel Remyelinating Therapeutic Nefiracetam
S.D. O'Shea 1, E.A. Keogh1, R.P. Murphy1, G. Bowen1, M. Pickering2, K.J. Murphy1
1University College Dublin, College of Science, School of Biomolecular and Biomedical Science, UCD Conway Institute, Neurotherapeutics Research Group, Dublin, Ireland 2University College Dublin, College of Health Sciences, School of Medicine and Medical Science, Dublin, Ireland
Current multiple sclerosis therapeutics act as immunomodulators but are largely ineffective at preventing disease progression. Significant benefits to patients might be achieved by supporting myelination repair. No agents which directly promote remyelination have yet been developed. We have identified the nootropic nefiracetam as a potential novel remyelinating therapeutic using in vivo and in vitro models of demyelination. In this study we investigate the mechanism of action of nefiracetam in the context of remyelination as regulated by Wnt/β‐catenin signalling and spontaneous activity.
To investigate whether nefiracetam acts by modulating Wnt signalling, organotypic hippocampal cultures were exposed to nefiracetam or the Wnt/β‐catenin inhibitor cardamonin. Both increased immunofluorescent staining for the OPC marker NG2. Myelin formation, as assessed by MBP staining, was enhanced by nefiracetam but was not affected by cardamonin. Thus, modifying Wnt signalling appears to impact OPC development without effecting differentiation, suggesting the pro‐myelinating effects of nefiracetam involve an additional mechanism.
We next investigated the effect of nefircatam on OPC differentiation using purified OPC cultures. Nefiracetam increased the OL:OPC ratio, as assessed by immunofluorescent staining for MBP and NG2 respectively, suggesting nefiracetam is capable of promoting OL maturation through a direct action on OPCs.
Finally, we investigated the effect of nefiracetam on spontaneous activity. Organotypic hippocampal cultures were exposed to lysophospatidylcholine (LPC) for 18hrs to induce demyelination before being allowed to recover. Cultures were treated with nefiracetam for 24hrs of the recovery period. Spontaneous calcium activity was then measured using Fluo4‐AM. Cultures not exposed to LPC displayed low levels of activity which were unaffected by nefiracetam. LPC exposure caused an increase in activity which was enhanced by nefiracetam. Additionally, in these cultures, LPC decreased MBP staining compared to control while nefiracetam post‐LPC caused a return to control levels. These data suggest increased activity may be an adaptive response related to myelin repair which is enhanced by nefiracetam.
Together, these findings suggest a complex mechanism of action for nefiracetam as a remyelinating agent involving modulation of Wnt signalling, spontaneous activity and OPC differentiation.
Funded by Multiple Sclerosis Ireland, Enterprise Ireland and Congressionally‐Directed Medical Research Programme.
T08‐05A. The growth factor NRG induces NMDA receptor dependent myelination by oligodendrocytes, employing Akt and CREB signalling
K. Evans 1, I. Lundgaard1, A. Luzhynskaya1, J.H. Stockley1, Z. Wang2, C. ffrench‐Constant3, D. Attwell4, R.T. Karadottir1
1University of Cambridge, Wellcome Trust – MRC Cambridge Stem Cell Institute, John van Geest Centre for Brain Repair & Dept. of Veterinary Medicine, Cambridge, United Kingdom 2CNRS, Institut de Biologie de l'Ecole Normale Supérieure Génomique Fonctionnelle, Paris, France 3University of Edinburgh, MRC Centre for Regenerative Medicine, Centre for Multiple Sclerosis Research, Edinburgh, United Kingdom 4University College London, Department of Neuroscience, Physiology & Pharmacology, London, United Kingdom
The growth factor neuregulin (NRG) can regulate myelination by signalling to ErbB receptors, respectively, on myelinating cells. Furthermore, in CNS neurons, NRG increase the expression of NMDA receptors. Oligodendrocytes at all developmental stages in the white matter exhibit NMDA evoked currents, mediated by receptors with very weak magnesium block which are expressed in the myelin1,2, indicating that they might play a role in myelination.
In an assay in which cortical oligodendrocytes ensheath dorsal root ganglion cells3 we found that NRG and NMDA receptors interact to regulate myelination. Without NRG, blocking NMDA receptors had no effect on myelination. Adding NRG increased myelination at all time‐points analysed (2–5 weeks), suggesting that NRG did not merely accelerate myelination. Strikingly, NRG led to the majority of myelination becoming dependent on activation of NMDA receptors and action potential activity. NRG's effect was associated with a 4‐fold increase in NMDA receptor current in oligodendrocyte lineage cells. Thus, NRG switches myelination from a default programme, which is independent of neuronal activity, to a mechanism that is regulated by glutamate released from active axons. The effects of NRG were associated with an increase in the phosphorylation of Akt and the transcription factor CREB, but not associated with changes in the phosphorylation of ERK.
These data reveal a function for oligodendrocyte NMDA receptors. The absence of NRG in multiple sclerosis lesions, and enhanced remyelination by added NRG, suggest a role for neuregulin/NMDA receptor dependent remyelination after pathology.
1. Káradóttir et al., (2005) Nature 438, 1162–1166.
2. Káradóttir et al., (2008) Nature Neuroscience 11, 450–456.
3. Wang et al., (2006) Glia 55, 537–45.
Supported by the Wellcome Trust, MRC, EU FP7 Leukotreat, FP7 Marie Curie Training Network, Action Medical Research, and the Royal Society.
T08‐05B. Up‐regulation of the multiligand receptor megalin/LRP‐2 in multiple sclerosis
M. Ortega, D. Clemente, F. de Castro
1National Hospital for Paraplegics, Toledo, Spain
Low‐density lipoprotein receptor‐related proteins (LRPs) are endocytic receptors that internalize several ligands and are involved in lipoprotein homeostasis in adult brain. LRP‐2, also known as megalin, is involved in the development of the central nervous system (CNS) and in the transport of molecules across the blood brain barrier (BBB). In a previous work, our group revealed that megalin is selectively involved in Sonic hedgehog‐mediated effects on oligodendrocyte precursor cell migration and proliferation during the development of the CNS. Although there are some reports describing the presence of LRPs in acute lesions of multiple sclerosis (MS) patients and pointing to an important role of Shh in the pathogenesis of this disease, there are not data about the expression of megalin in MS tissue. In the present study, we analyse the distribution and the cellular characterisation of megalin in samples of human brain from MS patients to elucidate the role of this receptor in the pathogenesis of demyelination. Changes in megalin distribution parallel the different MS histopathological lesions: we detected megalin in active demyelinating lesions and in the periplaque of chronic‐active lesions, areas where remyelination spontaneously occurs. On the contrary, megalin was absent in the demyelinated region of chronic lesions. In addition, we observed the up‐regulation of megalin in a subpopulation of perivascular astrocytes within the normal appearing grey matter (NAGM) of MS patients. Moreover, megalin was up‐regulated in blood vessels within active lesions, in the periplaque of chronic‐active lesions and in the NAGM. In parallel, we also analysed the BBB profile to determine structural and functional alterations. Altogether, we suggest that megalin is an important receptor expressed in diverse areas of the CNS of MS patients representing different aspects of MS development: i) a potential target to improve remyelination; ii) an indicator of a functional rather than a structural disruption of the BBB. Therapeutic strategies to modulate specific LRPs would be useful to effectively treat MS.
Funded by Ministerio de Economía y Competitividad (SAF2009–07842,SAF2012‐40023, RD07‐0060‐2007 and RD12/0032/0012‐ partially financed by F.E.D.E.R.; European Union, “Una manera de hacer Europa”‐), Gobierno de Castilla‐La Mancha (PI2009/26) and Fundación Eugenio Rodríguez Pascual (Spain). DC and FDC are hired by SESCAM. MCO is currently hired by ARSEP Foundation.
T08‐06A. Effects of TrkB expression and signalling on oligodendrocyte myelination
A. Ferner, J. Xiao, L. Giuffrida, A. Wong, H. Peckham, T.J. Kilpatrick, S. Murray
1University of Melbourne, Melbourne, Australia
BDNF is known to promote central nervous system myelination both in vitro and in vivo. Adopting in vitro myelination assays, we identified that BDNF acts through oligodendroglial‐expressed TrkB receptors to promote myelination. This was verified in vivo, as deletion of TrkB in mature oligodendrocytes (in TrkBfl/fl MBP‐cre mice) resulted in a hypomyelinating phenotype during development. Question: Here we investigate the consequence of TrkB deletion in oligodendrocyte progenitor cells (in TrkBfl/fl CNPase‐cre mice) and the signalling pathways downstream of TrkB that promote myelination.
Methods and Results: TrkBfl/fl CNPase‐cre mice were born in Mendelian ratios and were phenotypically indistinguishable from littermate control mice. Surprisingly, analysis of myelinated axonal tracts in the spinal cord and optic nerve revealed normal myelin development. In addition, the number of cells in the oligodendroglial lineage was the same as control mice during postnatal development. These data indicate that the timing of TrkB deletion in the oligodendroglial lineage is critical, as deletion in OPCs results in no significant phenotype, whereas deletion in mature oligodendrocytes results in a hypomyelinating phenotype. These data suggest that OPCs are able to compensate for the loss of TrkB and myelinate normally in the absence of BDNF signalling.
Our in vitro data have shown that the promyelinating influence of BDNF strongly correlates with Erk1/2 activation. Here we show that overexpression of Erk2 in OPCs exerts a more significant promyelinating effect than Erk1. As Erk1/2 are known to exert some of their effects through direct transcription factor phosphorylation, we have undertaken an in silico analysis of myelin‐specific transcription factors and identified several that contain potential Erk1/2 binding domains and phosphorylation sites. Co‐immunoprecipitation experiments show an interaction between Erk1/2 and these transcription factors. Investigation into the nature of these interactions and their functional consequences are ongoing.
Conclusions: This work suggests a novel role for Erk1/2 signalling within oligodendrocytes that regulates CNS myelination, possibly via activation of myelin‐specific transcription factors. As Erk1/2 is activated by multiple receptors, other receptors could potentially compensate for the loss of TrkB in OPCs, resulting in a normally myelinated CNS in vivo.
T08‐06B. The adaxonal compartment of CNS myelin: septin filaments are required to prevent pathological outfoldings of myelin sheaths
J. Patzig 1, W. Möbius1, S. Tenzer2, K.‐A. Nave1, H. Werner1
1Max Planck Institute for Experimental Medicine, Göttingen, Germany 2University of Mainz, Institute for Immunology, Mainz, Germany
Myelin is vulnerable to impairment by genetic, toxic, and immunological triggers, but the molecular basis for many aspects of myelin pathology has remained unknown. To identify molecules required for the structural integrity of central nervous system myelin we have systematically analyzed its protein composition by quantitative proteome analysis. Based on the high abundance of filament‐forming septins in myelin, we hypothesized that septin filaments may constitute a cytoskeletal membrane cortex in myelin. We find that septin filaments localize to the non‐compact adaxonal myelin compartment, which is considered relevant for the metabolic maintenance and the turnover of myelin. For functional analysis, we generated mice lacking the most abundant septin of myelin, SEPT8, from myelinating glia. Our results indicate that SEPT2, SEPT4, SEPT7 and SEPT9 multimerize with SEPT8 and that this interaction is essential for their incorporation into myelin. Myelin lacking septin filaments display pathogenic outfoldings emerging from adaxonal myelin. We propose that this phenotype reflects that myelin septins provide long‐term structural integrity to the adaxonal compartment of CNS myelin.
T08‐07A. Dysregulation of GPR17, a key receptor involved in oligodendrocyte maturation, as a novel potential pathogenetic mechanism in demyelinating diseases
M. Fumagalli 1, E. Bonfanti1, S. Daniele2, D. Lecca1, G.T. Coppolino1, M.L. Trincavelli2, C. Martini2, M.P. Abbracchio1
1Università degli studi di Milano, Dep. of Pharmacological and Biomolecular Sciences, Milan, Italy 2Università di Pisa, Department of Psychiatry, Neurobiology, Pharmacology and Biotechnology, Pisa, Italy
The P2Y‐like Gi‐protein‐coupled receptor GPR17 is activated by both uracil nucleotides and cysteinyl‐leukotrienes, two families of mediators involved in trophic responses and in the repair of central nervous system lesions (Ciana et al., EMBO J, 2006). In both brain and spinal cord, GPR17 is transiently expressed by a subpopulation of neural progenitors expressing the proteoglycan NG2 (NG2 cells, also known as Oligodendrocyte Precursor Cells, OPCs) in transition from precursors to premyelinating phenotypes, whereas it is not present on mature oligodendrocytes. Previous data in cultured OPCs showed that early GPR17 activation by its endogenous ligands promotes, while inhibition by antagonists or silencing RNAs impairs, OPC differentiation (Lecca et al., Plos One, 2008; Fumagalli et al., J Biol Chem, 2011). Altogether, these data point at GPR17 as a key functional modulator of oligodendrocyte maturation.
Here, we used primary OPC cultures to investigate the mechanisms underlying GPR17 endogenous regulation. This is quite important since GPR17 is upregulated at injury sites in both a demyelinating in vivo model (Boda et al., Glia, 2011) and in multiple sclerosis (MS) patients (Chen et al., Nat Neurosci 2009), which, could, in turn, cause defective myelination. In line with these findings, we have recently shown that GPR17 forced over‐expression at late differentiation stages, obtained by transfecting cultured OPCs with a GFP‐GPR17 fusion vector, indeed impairs terminal cell maturation. This suggests that the receptor needs to be down‐regulated/desensitized to allow OPC terminal maturation. Physiologically, GPR17 downregulation may occur through agonist‐induced receptor phosphorylation via G‐protein coupled receptor kinases (GRKs) (Daniele et al., J Pharm Exp Ther, 2011; Frantangeli et al., J Biol Chem, 2013), which are, in turn, controlled by mTOR kinases, and are altered in MS. Our in vitro data show that rapamycin, an inhibitor of mTOR kinase, reduces GRK2 levels, with parallel increases in GPR17 expression and strong impairment of OPC maturation. Globally, these data suggest that dysregulation of these interconnected pathways leading to aberrant GPR17 overexpression may prematurely block OPC maturation. Analysis of GPR17 in vivo in MS models will confirm if the receptor is dysregulated during disease development and will help finding ways to re‐establish myelin repair in demyelinating diseases.
Sponsored by Fondazione Italiana Sclerosi Multipla, grant 2010/R/2 to MPA.
T08‐07B. Ganglioside GD1A: a novel molecular tool to promote remyelination in MS lesions?
J. Qin, D. Hoekstra, W. Baron
University Medical Center Groningen, Groningen, Netherlands
Loss of central nervous system myelin, and the failure of remyelination by oligodendrocytes, contributes to the functional impairment that characterizes neurodegenerative disorders and demyelinating diseases such as multiple sclerosis (MS). Since incomplete remyelination will irreversibly damage axonal connections, treatments effectively promoting (re)myelination are pivotal in precluding progression of disease. Although the reasons for remyelination failure are likely multifactorial, one of the major impediments to successful remyelination is the altered lesion microenvironment. Our previous findings suggest that fibronectin aggregates, as an environmental factor, contribute to remyelination failure. Here, we aim at elucidating whether exogenous gangliosides, i.e., cell surface lipids with a potential to modulate fibronectin‐ cell surface interactions, could overcome fibronectin‐mediated inhibition of myelination.
Of the gangliosides tested, only addition of GD1a, perturbed adherence of oligodendrocyte progenitor cells to fibronectin, but not to poly‐L‐lysine, without affecting cell viability. GD1a slightly increased growth factor‐mediated proliferation on fibronectin, but in the presence of the lipid migration of oligodendrocyte progenitor cells on fibronectin was reduced. Furthermore, addition of GD1a increased myelin‐like membrane formation on (aggregated) fibronectin, whereas GM1 reduced oligodendrocyte differentiation, as reflected by a decrease in the number of MBP positive cells. Most interestingly, GD1a counteracted the myelination inhibiting effect of aggregated fibronectin in co‐cultures of dorsal root ganglion neurons and oligodendrocytes. Also, GD1a enhanced remyelination in demyelinated cerebellar slice cultures that contained fibronectin aggregates.
Taken together, GD1a is able to overcome the inhibiting effect of aggregated fibronectin in remyelination. Given the persistent presence of these aggregates in MS lesions, GD1a might therefore act as a potentially novel molecular tool to selectively modulate the impeding signaling environment, apparently detrimental to remyelination. Its mechanism of action and exploration of GD1a's potential in the treatment of MS, are currently investigated.
T08‐08A. Role of Apolipoprotein D in macrophage recruitment and myelin phagocytosis upon peripheral nerve injury
N. Garcia Mateo 1, D. Sánchez1, C. Lillo2, M.D. Ganfornina1
1Instituto de Biologia Y Genetica Molecular, Valladolid, Spain 2Instituto de Neurociencias de Castilla y León, Universidad de Salamanca, Salamanca, Spain
Apolipoprotein D (ApoD) is a Lipocalin expressed by glial cells during development, adulthood and aging of the vertebrate nervous system. In the peripheral nervous system (PNS) it is secreted by Schwann cells and fibroblasts and its expression is strongly induced upon injury.
We are interested in the function of ApoD in the molecular events that take place after a PNS injury. We have studied in vivo the process of Wallerian degeneration following sciatic nerve crush and we have assayed ex vivo the phagocytosis of labelled myelin from wt and ApoD‐KO mice by flow cytometry.
The analysis of cellular processes and protein or mRNA expression of a group of signalling molecules induced by injury shows that the lack of ApoD results in an exacerbated MCP‐1 and TNFa‐dependent macrophage recruitment. At the injury site, free AA decreases in the wt. Lack of ApoD results in higher basal levels and a stronger injury‐triggered depletion of AA. Control by ApoD of the availability of AA to produce the lipid mediators involved in macrophage recruitment is therefore a key mechanism that conditions both Wallerian degeneration and injury resolution later on.
On the other hand, the phagocytosis of ApoD‐KO CNS myelin is less efficient than the phagocytosis of wt myelin. These results indicate that there are also genotype‐dependent differences in myelin composition and/or in the interaction between myelin and macrophages. An electron microscopy analysis of crude myelin preparations reveals that the CNS myelin from ApoD‐KO mice has abnormal periodicity and shows defective myelin compaction. Lipid analysis of ApoD‐KO myelin shows altered phospholipid composition, particularly in phosphoinositid species. A study of the function of ApoD in the myelination process is currently underway,
Our results demonstrate that ApoD function is relevant for both, myelin membrane properties that influence myelin‐macrophage interactions, and the control of the lipid‐mediated signalling events controlling the extent of macrophage recruitment.
Support: MICINN(BFU2008‐01170;BFU2011‐23978), JCyL(VA180A11‐2).
T08‐08B. The transcription factor Sip1 is required for peripheral nerve myelination
S. Quintes 1, B. Brinkmann1, M. Ebert1, T. Kungl1, V. Tarabykin2, D. Huylebroeck3, D. Meijer4, K.‐A. Nave1, M.W. Sereda1,5
1Max Planck Institute of Experimental Medicine, Department of Neurogenetics, Goettingen, Germany 2Charité – Universitätsmedizin Berlin, Neurocure Excellence Cluster, Institute of Cell and Neurobiology, Berlin, Germany 3University of Leuven, Laboratory of Molecular Biology (Celgen), Department of Development and Regeneration, Leuven, Belgium 4Erasmus MC, Department of Cell Biology, Rotterdam, Netherlands 5University of Göttingen (UMG), Department of Clinical Neurophysiology, Goettingen, Germany
The zinc‐finger protein Smad‐interacting protein‐1 (Sip1, Zeb2, Zfhx1b), a widely expressed transcription factor, exerts important functions in oligodendrocyte maturation and myelination. Here, we have specifically inactivated Sip1 in Schwann cells, the myelinating glial cells of the peripheral nervous system. Dhh‐Cre+/Sip1fl/fl mice display a striking phenotype with tremors and partial hind limb paralysis starting at the first postnatal week. Axonal sorting by Sip1‐deficient Schwann cells is severely impaired and at the age of 25 days, sciatic nerves are completely devoid of myelin. Mutant Schwann cells fail to downregulate the transcription factors that mark the immature stage and do not express key regulators of the myelination program, such as Krox‐20. Our results implicate Sip1 as a novel player in Schwann cell development and myelination.
T08‐09A. GPR56, an adhesion‐GPCR, regulates oligodendrocyte development and CNS myelination
S. Giera 1, Y. Deng1, M. Makinodan1,2, S. DeGenova 3, K.R. Monk3, G. Corfas2, X. Piao1
1Boston Children's Hospital, Boston, United States 2Boston Children's Hospital, Harvard Medical School, Kirby Neurobiology Center, Boston, United States 3Washington University School of Medicine, Department of Developmental Biology, St. Louis, United States
Adhesion G protein‐coupled receptors (ad‐GPCRs) are a subgroup of GPCRs that facilitate cell‐cell and cell‐matrix interaction. Structurally, they differ from other GPCRs by the presence of an extremely larger extracellular N‐terminal region that is separated from the 7‐transmembrane region by a GPCR autoproteolysis‐inducing (GAIN) domain that in most ad‐GPCRs facilitates an auto‐catalytic process to produce an N‐ and C‐ terminal fragments during the maturation process. GPR56 is a member of the ad‐GPCR family and its mutations are responsible for a human brain malformation known as bilateral frontoparietal polymicrogyria (BFPP). Individuals with BFPP present with changes in the white matter tracts in the form of hypomyelination, in addition to cortical malformation. Here, we show that Gpr56 knockout mice and zebrafish exhibit a reduction of myelin‐specific protein expression, fewer myelinated axons and decreased number of mature oligodendrocytes in the central nervous system (CNS). During cortical development, GPR56 functions together with its ligand collagen III to regulate neuronal migration and cortical lamination. However, it is not clear whether collagen III is also the ligand of GPR56 during CNS myelination. GPR56 has a very long and poorly characterized N‐terminal fragment, allowing for the possibility of multiple binding partners that mediate cell‐extracellular matrix interactions. Indeed, GPR56 also binds to tissue transglutaminase (TG2) in melanoma cells. We have begun to study the possible role of collagen III and TG2 in oligodendrocyte development and CNS myelination. The likelihood of these two proteins as being the ligand(s) of GPR56 during oligodendroglial development will be discussed as will our ongoing work to define the role of GPR56 in CNS myelination.
T08‐09B. Role of Jun activating binding protein 1 (Jab1) in Central Nervous System (CNS) myelination
C. Rivellini 1, E. Porrello1, G. Dina1, K.‐A. Nave2, C. Lappe‐Siefke2, R. Pardi3, A. Quattrini1, S.C. Previtali1
1San Raffaele Scientific Institute, Milan, Italy 2Max Planck Institute of Experimental Medicine, Neurogenetics, Goettingen, Germany 3San Raffaele Scientific Institute, Division of Immunology, Transplantation and Infectious Disease, Milan, Italy
Proper control of cell cycle, proliferation and differentiation is fundamental to regulate oligodendrocyte (OL) development and recruitment of oligodendrocyte precursors (OPC) after brain damage. Failure in this process results in severe dysmyelinating disorders and defective brain repair. The molecular machinery that couples differentiation to proliferation withdrawal plays therefore a critical role in brain development and repair and is regulated by multiple extracellular cues including extracellular matrix (ECM) and growth factor derived signals. However, how these signals are integrated into OL to control cell cycle progression and differentiation is only partially understood.
We are investigating the role of Jun activating binding protein 1 (Jab1), an intracellular signalling molecule, shuttling between nucleus and cytoplasm, involved in the control of cell proliferation, survival and gene transcription. Recent evidence showed that Jab1 function is regulated by ECM and growth factors. We demonstrated that Jab1 is expressed in glial cells in peripheral and central nervous system (CNS), and conditional inactivation of Jab1 in Schwann cells results in dysmyelinating neuropathy.
Here we present preliminary data showing that Jab1 plays a role in CNS development. Mice with conditional inactivation of Jab1 in OL, by CNP‐Cre tansgene, show CNS hypomyelination, including corpus callosum, optic nerve and spinal cord. Concomitant fiber degeneration was observed. Our preliminary data suggest that Jab1 expression in OL plays a role in CNS myelination and possibility axonal survival.
T08‐10A. Post‐transcriptional regulation of Myelin Basic Protein during cell stress conditions
C. Gonsior, J. Trotter
University of Mainz, Mainz, Germany
Myelin Basic Protein (MBP), a major component of central nervous system (CNS) myelin, is essential for oligodendroglial function and myelination in the CNS and its expression is tightly regulated in time and space. The mRNA of MBP is sorted to cytoplasmic RNA granules and transported up to the distal processes of oligodendrocytes in a translationally silenced state. We and others could show that, dependent on axonal signals, activation of the non‐receptor tyrosine kinase Fyn leads to a release of translational inhibition allowing synthesis of MBP protein at the axon‐glial contact site. A key factor in this pathway is the Fyn target and RNA‐binding protein heterogeneous nuclear ribonucleoprotein (hnRNP) A2, that recognizes the A2 response element (A2RE) in the MBP 3′UTR and orchestrates the dynamics of the MBP mRNA granule. Moreover, we identified hnRNP F as an additional target of Fyn kinase present in these ribonucleoprotein complexes and contributing to post‐transcriptional regulation of MBP.
Cell stress conditions, as present in various disease states such as multiple sclerosis, may result in the formation of cytoplasmic RNA‐containing stress granules (SGs), potentially influencing the fate of myelin transcripts including their translation.MBP mRNA was shown to be sorted to these cytoplasmic foci. We here identified the hnRNPs A2 and F to be associated with oligodendroglial SGs. De‐regulation of such factors involved in MBP mRNA metabolism could affect stress dependent synthesis of MBP and thus (re‐)myelination and myelin maintenance.
T08‐10B. In vivo pathogenesis of demyelination in an animal model of multiple sclerosis
E. Romanelli 1, S. Potz1, D. Merkler2, M. Weber3, D. Bishop4, T. Misgeld5, M. Kerschensteiner1
1Ludwig‐Maximilians‐Universität München, Research Unit Therapy Development, Institute of Clinical Neuroimmunology, München, Germany 2University of Geneva, Department of Pathology and Immunology, Geneva, Switzerland 3Universitätsmedizin Göttingen, Institut für Neuropathologie, Göttingen, Germany 4Indiana University School of Medicine, Department of Physiology, Muncie, Indiana, United States 5Technische Universität München, Institute of Neuroscience, München, Germany
Multiple sclerosis (MS) is an immune‐mediated demyelinating disease of the human central nervous system (CNS). Although demyelination represents the major histopathological hallmark of the disease, currently the mechanisms that mediate myelin loss are only incompletely understood.
This study is aimed to understand how myelin is removed in Experimental Autoimmune Encephalomyelitis (EAE), the most commonly used animal model of MS, by combing in vivo imaging (Kerschensteiner et al., 2005 Nature Medicine; Misgeld and Kerschensteiner, 2006 Nature Reviews Neuroscience; Nikic et al., 2011 Nature Medicine) with confocal microscopy and correlated ultrastructural analysis.
We performed the experiments in Biozzi ABH mice known to develop a chronic relapsing‐remitting form of EAE following immunization with myelin oligodendrocyte glycoprotein. Our findings suggest that in demyelinating lesions, myelin layers are pulled off from the myelin sheath and form bulb‐like structures that we call “myelinosomes”. We can detect the formation of such structures in acute and in chronic stages in the animal model, as well in biopsies from multiple sclerosis patients. We are currently investigating the molecular interactions that underlie the formation of myelinosomes to gather further insight into the mechanisms that underlie immue‐mediated demyelination.
T08‐11A. A3 adenosina receptor triggers oligodendrocyte death and myelin loss
E. González‐Fernández 1, R. Arellano1,2,3, A. Pérez‐Samartín1,2,3, M.V. Sánchez‐Gómez1,2,3, C. Matute1,2,3
1University of Basque Country‐UPV/EHU, Leioa, Spain 2Achucarro Basque Center For Neuroscience, Zamudio, Spain 3Instituto de Salud Carlos III (ISCIII; CIBERNED), Leioa, Spain
Adenosine is a potent neuromodulator which can be released from most cells and its extracellular concentration increases dramatically after brain injury. It acts on four G protein‐coupled receptors, A1, A2a, A2b and A3, and their activation is involved in myelination as well as in apoptosis linked to neurodegenerative diseases. In this study, we initially found that rat oligodendrocytes in vitro express all four adenosine receptor subtypes, adenosine transporters ENT1 and ENT2 and the adenosine degrading enzymes adenosine deaminase and adenosine kinase. Activation of adenosine receptors caused mitochondrial dysfunction and oligodendroglial apoptosis. Notably, selective activation of adenosine receptors revealed that A3 receptors are the main subtype mediating oligodendrocyte death. Thus, A3 receptor agonist 2‐CI‐IB‐MECA caused a robust increase in ROS levels, mitochondrial membrane depolarization and caspase‐dependent apoptosis. In turn, selective A3 receptor activation induced MAPK modifications compatible with cellular damage, which was prevented by the selective antagonist MRS1220. The modulation of G protein and adenylate cyclase suggested that 2‐CI‐IB‐MECA acts via cAMP‐PKA. Moreover, selective activation of A3 receptor triggered intracellular calcium mobilization via PLC pathway. Finally, we used cerebellar organotypic slices and optic nerve ex vivo to investigate adenosine‐mediated oligodendroglial death in more integral preparations. Consistent with data in dissociated cultures, A3 receptor activation induced a significant damage in the optic nerve as well as in the cerebellum white matter, an effect that was prevented by caffeine, an adenosine receptor blocker. Together, these results indicate that adenosine can trigger oligodendrocyte death via activation of A3 receptors, and suggest that this mechanism may contribute to the etiology of demyelinating diseases.
T08‐11B. Efficient Lentiviral Gene Delivery to Schwann Cells
I. Sargiannidou 1, A. Kagiava1, S. Bashardes1, J. Richter1, N. Schiza1, C. Christodoulou1, A. Gritti2, K. Kleopa1
1The Cyprus Institute of Neurology & Genetics, Nicosia, Cyprus; 2Fondazione Centro San Raffaele del Monte Tabor, Milan, Italy
Question‐ One of the commonest forms of inherited neuropathy, X‐linked Charcot Marie‐Tooth disease (CMT1X) leads to progressive distal muscle weakness and sensory loss. The disease is caused by a large number of mutations in the GJB1 gene encoding the gap junction protein connexin32 (Cx32). Cx32 is expressed by Schwann cells in peripheral nerves and forms important channels of communication between the layers of the non‐compact myelin sheath and is necessary for the homeostasis and survival of the myelinating cells and the axon. Transgenic disease models generated in our lab as well as clinical‐genetic analysis of large number of patients have demonstrated that most CMT1X mutations result in loss of Cx32 function and failure to form gap junctions. Cx32 KO mice offer a well characterized and relevant model of the disease to study future therapies for CMT1X, for which gene replacement may be an effective treatment strategy.
Methods‐ For this purpose, we have generated a novel 3rd generation lentiviral vector to allow gene delivery to peripheral nerves. In this vector, the Cx32 open reading frame is placed under the control of cell‐specific promoter for Schwann cells, the rat Mpz/P0 promoter. The expression cassette also includes a green fluorescent protein (EGFP) as a marker. We have successfully produced lentiviral vector particles and delivered them to the sciatic nerves of 2‐month old wild type (WT) and Cx32 KO mice, immediately distal to the sciatic notch. The expression of EGFP on WT background and of Cx32 on KO background was determined by Western blot analysis and immunohistochemistry of teased nerve fibers at different time points after injection.
Results‐ Our initial studies in WT mice (n = 15) show that more than 60% of Schwann cells from different nerve fascicles expressed EGFP as far as 5 mm distal to the injection site. Expression started a week after the intraneural injection and remained stable for up to 3 months. When the lentiviral construct was injected into the sciatic nerves of Cx32 KO mice (n = 5), virally delivered Cx32 was expressed and was properly localized at the non‐compact myelin areas including the paranodal regions and incisures of myelinated fibers, where gap junctions are normally formed.
Conclusions‐ Thus, we have generated lentiviral vector that leads to efficient gene expression specifically in Schwann cells. These results indicate that gene replacement therapy is feasible and may lead to correction of the gap junction loss in myelinating cells of the PNS.
Acknowledgements: This project (Grant Health/BIOS/0609/(ΒΙΕ)/09 to KAK) is co‐financed by the European Regional Development Fund and the Republic of Cyprus through the Research Promotion Foundation.
T08‐12A. Long‐term consequences of perinatal inflammation on de‐ and remyelination in the central nervous system
V. Gudi 1, K. Bénardais1,2, J. Neßler1, V. Singh1,2, L. Gai1, T. Skripuletz1, M. Stangel1,2
1Hannover Medical School, Hannover, Germany 2Center for Systems Neuroscience, Hannover, Germany
Perinatal inflammation is intensively discussed to have a long‐term impact on various cell populations and on the development of autoimmune diseases such as multiple sclerosis (MS) in adulthood. In order to determine long‐term effects of perinatal inflammation on the course of demyelination in adulthood we mimic a bacterial inflammation by exposure to lipopolysaccharide (LPS) of either pregnant or newborn mice. Demyelination as a “second hit” was induced by feeding adult mice with the oligodendrocyte toxin cuprizone (bis‐cyclohexanone oxaldihydrazone). A single prenatal LPS injection at E13.5 did not affect the course of demyelination in adulthood. In contrast, serial postnatal LPS injections lead to a delayed demyelination and a reduced number of activated microglia in the corpus callosum, suggesting a long‐lasting effect of perinatal inflammation on the function of microglia. Surprisingly, mice exposed to LPS after birth showed an enhancement of early remyelination accompanied by an increased number of newly differentiated oligodendrocytes. Furthermore, independent of cuprizone administration the perinatal LPS injections seem to induce a long‐term impact on the blood‐brain barrier (BBB) by decreasing the number of claudin‐5 positive vessels. In summary, perinatal inflammation mimicked by the administration of LPS has long‐lasting effects on the BBB, microglia activation, and remyelination.
T08‐12B. Modulation of Neuregulin‐1 signaling in S63del‐CMT1B Neuropathy
C. Scapin 1, C. Ferri1, E. Pettinato1, V. Volpi1, M. Schwab2, K.‐A. Nave2, C. Taveggia1, M.L. Feltri1,3, M. D'Antonio1, L. Wrabetz1,3
1Ospedale San Raffaele, Milan, Italy 2Max Planck Institute for Experimental Medicine, Göttingen, Germany 3Hunter James Kelly Research Institute, University of Buffalo School Of Medicine and Biomedical Sciences, Buffalo, United States
Myelin Protein Zero (P0) is a highly abundant transmembrane glycoprotein synthesized by myelinating Schwann cells. More than 120 mutations of the P0 gene (Mpz) are associated with various forms of neuropathy, many of them with dominant inheritance. In particular, the deletion of serine 63 in the extracellular domain of P0 (P0S63del) is responsible for the demyelinating Charcot‐Marie‐Tooth type 1B and it has been shown to produce a similar neuropathy in transgenic mice. P0S63del is a misfolded protein retained in the endoplasmic reticulum (ER) that fails to be incorporated into myelin. This results in the activation of a pathogenetic unfolded protein response (UPR), via a dose‐dependent, toxic gain of function mechanism (Wrabetz et al., 2006; Pennuto et al., 2008). S63del nerves manifest not only demyelination, but also a developmental hypomyelination. To explore the hypothesis that increasing a key pro‐myelinating axonal signal, such as NRG1 Type III, could ameliorate the phenotype, we crossed S63del mice with transgenic mice that over express an HA‐Nrg1 Type III (HANI) fusion protein (Velanac et al., 2011). In HANI/+// S63del sciatic nerves we found an increase of myelin thickness, more obvious in smaller caliber axons, as compared to S63del nerves. Conversely, axons with larger diameter remained hypomyelinated as compared to WT, with a g‐ratio similar to S63del nerves. Surprisingly, myelin protein levels were not increased in HANI/+// S63del sciatic nerves, and phospho‐AKT and Krox20 protein expression remained unaltered. Furthermore, the UPR was still active at a level comparable to that of S63del nerves. Myelin gene expression, electrophysiology and motor capacity analysis are ongoing. Taken together with previous results in HANI/Q215X/CMT1B mice, these data provide preliminary proof of principle that modulation of Nrg1 Type III activity could be a more general potential treatment for MPZ‐related neuropathies.
T08‐13A. Histone Methyltransferase Enhancer of Zeste Homolog 2 regulates Schwann cell differentiation
A. Heinen, N. Tzekova, N. Graffmann, K.J. Torres, M. Uhrberg, H.‐P. Hartung, P. Küry
Heinrich Heine University Düsseldorf, Düsseldorf, Germany
Question: Epigenetic control is crucial for the differentiation of a variety of cells including oligodendrocytes, the myelinating glial cells of the central nervous system. However, studies about the implication of epigenetic factors in peripheral nervous system maturation are just emerging and we were wondering whether methylation of histones is involved in this process.
Methods: We describe the function of EZH2 in primary Schwann cells and DRG cocultures using immunohistochemistry, transfection and lentiviral transduction, shRNA, qRT‐PCR analysis, morphological analysis, Western blotting and chromatin immunoprecipitation.
Results: Here, we demonstrate for the first time the impact of a histone methyltransferase, encoded by the enhancer of zeste homolog 2 (EZH2) gene, on Schwann cell differentiation. In sciatic nerves, EZH2 expression was found in Schwann cells and to peak perinatally. Suppression of EZH2 expression in cultured primary rat Schwann cells reduced the length of cell processes. These morphological changes were accompanied by widespread alterations in the gene expression pattern, including downregulation of myelin genes and induction of p57kip2, which we have recently identified as an intrinsic inhibitory regulator of Schwann cell maturation. In addition, we show that EZH2 suppression in dorsal root ganglion cocultures interferes with in vitro myelination. Chromatin immunoprecipitation analysis revealed binding of EZH2 at the p57kip2 promoter and reduction of histone H3K27 trimethylation upon gene suppression. EZH2 suppression‐dependent effects on morphology and myelin genes could be reversed by concomitant suppression of p57kip2, indicating that p57kip2 is a downstream effector of EZH2. Furthermore, we describe Hes5 as transcriptional repressor of myelin genes in Schwann cells, which was induced upon EZH2 suppression and downregulated in p57kip2‐suppressed Schwann cells.
Conclusions: We have identified a molecular link between histone methylation and control of Schwann cell differentiation and demonstrate that this epigenetic mechanism is crucial for glial differentiation to proceed.
T08‐13B. Schwann cell neuregulin‐1 constitutes an endogeneous factor of myelin repair
R.M. Stassart 1, R. Fledrich1, V. Velanac1,2, D. Meijer3, M. Schwab1, M.W. Sereda1,4, K.‐A. Nave1
1Max Planck Institute of Experimental Medicine, Göttingen, Germany 2Deutsches Zentrum für Neurodegenerative Erkrankungen, Bonn, Germany 3Erasmus MC, Rotterdam, Netherlands 4Universitätsklinik Göttingen, Göttingen, Germany
The peripheral nervous system demonstrates an exceptional plasticity after nerve injury and Schwann cells remyelinate newly regenerated axons. However, myelin sheath thickness does not reach its original thickness suggesting insufficient growth factor stimulation after injury. During peripheral nerve development, the transmembrane growth factor neuregulin‐1 type III is expressed on the axonal surface and regulates Schwann cell development as well as myelin sheath thickness. After peripheral nerve injury, Wallerian degeneration is induced and Schwann cells remain in the distal nerve stump without axons and, hence, axonal neuregulin‐1 type III stimulation. We demonstrate that during this time period, denervated Schwann cells strongly induce the expression of the paracrine type I isoform of neuregulin‐1 which is barely detectable in non‐injured nerves. Mutant mice with a specific deletion of the neuregulin‐1 gene in Schwann cells are fully myelinated during development, but exhibit impaired remyelination after experimental peripheral nerve injury. We suggest a model in which loss of axonal contact triggers denervated Schwann cells to transiently express neuregulin‐1 as an endogeneous signal that promotes Schwann cell redifferentiation and remyelination.
T08‐14A. Electrical activity‐dependent control of myelin gene expression in vivo
J. Hines, B. Appel
University of Colorado School of Medicine, Aurora, CO, United States
Question and Methods: Myelination of CNS axons is a multi‐step process beginning with the production of oligodendrocyte progenitor cells (OPCs) in the ventral neural tube. Newly specified OPCs proliferate, migrate into white matter tracts, and differentiate into mature oligodendrocytes that wrap select axons with a myelin sheath. Previous studies, performed primarily in cell culture, suggest that electrical activity may regulate multiple stages of oligodendrocyte development including proliferation, migration, initial wrapping and myelination. In this study, we have examined the requirement for electrical activity in oligodendrocyte development in vivo using zebrafish as a model system.
Results: We find that electrical activity is not required for proliferation, migration, or the initiation of axon wrapping. Rather, our data indicate a specific role for electrical activity in oligodendrocyte differentiation by regulating a subset of myelin genes, including myelin basic protein (mbp). Silencing electrical activity with tetrodotoxin reduced mRNA expression of mbp, as assessed by RNA in situ hybridization and quantitative RT‐PCR. In contrast, myelin protein zero, claudin K, claudin 11a, ugt8, and 36K levels were not regulated by electrical activity. Ongoing experiments are aimed to uncover the mechanism of activity‐dependent mbp mRNA expression during oligodendrocyte differentiation.
Conclusions: Taken together, these data indicate that electrical activity can modulate myelin gene expression in vivo but do not support the notion that electrical activity drives key axon‐glia messengers that are required for the initial stages of myelination.
T08‐14B. Schwann cell type‐specific requirements of Dynamin‐2 function revealed by gene ablation
E. Tinelli, J. Pereira, U. Suter
ETH Zurich, Zurich, Switzerland
Dynamin‐2 (Dnm2) is a ubiquitously expressed, pleiotropic GTPase protein mainly involved in clathrin‐mediated endocytosis, fission of membranes, and remodeling of different organelles. Our findings show that Dnm2 function is essential for early embryonic development of mice. In contrast, autosomal dominant mutations of Dnm2 cause slowly progressing and tissue‐specific human disorders. In particular, Dnm2 mutations are linked to either dominantly inherited forms of Charcot‐Marie‐Tooth disease (DI‐CMTB) or dominant Centronuclear Myopathy (CNM). Understanding the cell type‐specific functional roles of Dynamin 2 is a crucial basic step towards the analysis of the pathogenetic mechanisms. Thus, we have used cell‐specific conditional Dnm2 knock‐out mice to elucidate the role of Dnm2 in Schwann cells.
T08‐15A. Remyelination after Cuprizone treatment: Galectin‐3 involvement
H. Hoyos 1, M. Marder1, G.A. Rabinovich2, L. Pasquini1, J. Pasquini1
1FFYB‐UBA IQUIFIB‐CONICET, Biological Chemistry, Buenos Aires, Argentina 2FCEN‐UBA IBYME‐CONICET, Cell Biology, Buenos Aires, Argentina
Cuprizone (CPZ) is a copper chelator which induces reproducible demyelination in the mouse brain (Matsushima and Morell, 2001). CPZ‐induced demyelination is characterized by oligodendroglial cell loss, myelin sheath degeneration and astrocyte and microglia recruitment to the lesioned area. The CPZ model causes demyelination without participation of the immunological system. Galectin‐3 (Gal‐3) is a 31 kDa lectin which binds to β‐galactosides and is widely spread among different cell types and tissues. We have previously found that Gal‐3 is involved in the control of myelin integrity and function and drives oligodendroglial cell differentiation (Pasquini et al., 2011). Remyelination is a regenerative process during which new myelin sheaths wrap axons after pathological demyelination (Fancy et al., 2011). Eight‐week‐old Lgals3‐/‐ and wild type (WT) mice were fed a diet containing 0.2% CPZ w/w during 6 weeks, after which CPZ was withdrawn in order to evaluate remyelination 2 weeks after. According to our results, CPZ‐induced demyelination in Lgals3‐/‐ mice showed an exacerbated astrocytic and microglial response as compared to WT littermates. Electron microscopy showed a significant lack of myelinated axons in Lgals3‐/‐ mice as compared to controls. Furthermore, the few myelinated axons present were nearly 50% less myelinated than those of controls and were found to be collapsed. Remarkably, the remyelination process seemed to be faster in Lgals3‐/‐ mice than in WT. Remyelinated Lgals3‐/‐ mice showed a higher Myelin Basic Protein (MBP) recovery rate as compared to their controls. Flow cytometry assays showed a sharper microglial response in Lgals3‐/‐ mice, which was supported by an exacerbated number of CD11b+ and CD45+ cells. However, electron microscopy images from remyelinated Lgals3‐/‐ animals showed, again, collapsed axons with a defective myelin wrap, as compared to WT mice showing normal axons without any relevant myelin wrap disruption. Behavioral performance observed during CPZ treatment recovery correlates with alterations in the morphological studies, which show that neither Lgals3‐/‐ nor WT mice reach basal myelination levels.
T08‐15B. Oligodendrocyte production in the adult CNS: myelin remodelling or replacement?
R. Tripathi, I. McKenzie, E. Kougioumtzidou, W.D. Richardson
University College of London, London, United Kingdom
We have shown that new oligodendrocytes (OLs) continue to be generated in healthy adult mice after 8 months of age, even in the optic nerves, which are considered to be completely myelinated already [1]. This raises the question of whether the adult‐born OLs and myelin are engaged in remodeling existing myelin (e.g. intercalating among the existing myelin sheaths), or replacing OLs that die in use.
To ask whether OLs die and are replaced during healthy adulthood we generated transgenic mice that express iCreERT2 under the transcriptional control of the Myelin Basic Protein promoter (Mbp6‐iCreERT2). The ∼6kb Mbp upstream fragment that we used is reported to direct transcription to OLs but not to either OL precursors (OPs) or Schwann cells [2]. We confirmed that our Mbp transgene targets Cre recombination specifically to differentiated OLs by crossing to Ros26‐YFP conditional reporters; when tamoxifen was administered to double‐transgenic offspring, essentially all YFP+ cells in the CNS were also CC1+. When Mbp6‐iCreERT2 is crossed to Tau‐mGFP conditional reporter mice (mGFP is membrane‐tethered) we can visualize differentiated OLs and their myelin internodes in white and grey matter, allowing us to follow the fates of myelinating OLs for extended periods of time after tamoxifen labeling.
Following tamoxifen administration to Mbp6‐iCreT2: Tau‐mGFP mice on postnatal day 60 (P60) or P120, a large proportion of OLs became GFP‐labelled in the corpus callosum, optic nerve and spinal cord. Examining these mice at increasing times post‐tamoxifen will reveal if and how the number and fraction of mGFP‐labeled OLs/ internodes changes with age – whether there is net loss or gain of OLs or whether new OL synthesis is balanced by the rate of OL death (i.e. there is OL turnover). Our preliminary results indicate that new OL synthesis exceeds OL death, so the function of adult OL genesis is not simply to replace dying OLs.
1. Young KM, Psachoulia K, Tripathi RB, Dunn S‐J, Cossell L, Attwell D, Tohyama K, Richardson WD (2013) Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodelling. Neuron in press.
2. Farhadi HF, Lepage P, Forghani R, Friedman HC, Orfali W, Jasmin, L, Miller W, Hudson TJ, Peterson AC (2003) A combinatorial network of evolutionarily conserved Myelin Basic Protein regulatory sequences confers distinct glial‐specific phenotypes. J Neurosci 23:10214–10223.
T08‐16A. Unravelling protein networks involved in peripheral nerve myelination
S. Kangas 1, S. Ohlmeier2, R. Sormunen2, A. Heape1
1University of Oulu, Oulu, Finland 2University of Oulu, Biocenter Oulu, Oulu, Finland
This study aims to identify protein networks that participate in peripheral myelin formation and maintenance.
We use a cell culture system, where C57BL/6J mouse embryos (E13.5) are used for the isolation of dorsal root ganglia neuron explants. Explants are cultured in Matrigel, and endogenous Schwann cells appear in the culture within a few days. Myelin formation is induced by adding myelination‐promoting substances to the culture medium. Immunocytochemistry is used to evaluate the myelination efficiency and the developmental timetable of myelination in culture. Electron microscopy is used to study structural features of myelin in culture and to compare its ultrastructural features with those of myelin in situ. RNA microarray analysis is performed to study gene expression changes during myelin development in culture. The RNA expression data indicates the changes in the RNA expression levels, but it does not give information about the amount of final protein product, their posttranslational modifications or stability. We combine the RNA expression analysis with a proteomics approach. Proteins are extracted for 2‐dimensional electrophoresis analysis at the same time points, and protein spots exhibiting time‐dependent changes in their expression levels are analyzed by mass spectrometry.
We show that mouse dorsal root ganglion explant cultures produce substantial amounts of mature myelin in 3 weeks after the induction of myelination. Ultrastructural analysis shows that myelin in our cell culture system has the same structural characteristics as those observed in peripheral nerves in situ. RNA microarray analysis reveals changes in myelin‐, nervous system‐ and Schwann cell‐related genes. The most significant changes in gene expression occur at the induction of myelination. At the protein level, peaks of specific proteins can be observed at the first and the last time points, which complement the results obtained using microarray data.
We have developed a reproducible and efficient method to study molecular changes in myelination at the proteomic and genomic levels. This approach will be exploited further for the characterization of the molecular networks involved in the myelination process.
T08‐16B. Immunoglobilun dependent modulation of Schwann cell differentiation: implication for peripheral nerve damage and disease
N. Tzekova 1, A. Heinen1, S. Bunk2, C. Hermann3, H.‐P. Hartung1, P. Küry1
1Heinrich Heine University, Düsseldorf, Germany 2Baxter Innovations, Department of Immunology, Vienna, Austria 3Baxter Innovations, Medical Affairs EMEA, Vienna, Austria
Question: The myelinating glial cells of the peripheral nervous system (PNS), the Schwann cells, exert important regenerative functions revealing them as central repair components of many if not all peripheral nerve pathologies. Intravenous immunoglobulins (IVIG) are widely used to treat autoimmune and inflammatory diseases including immune‐related neuropathies. However, it is currently not known whether immunoglobulins can also exert additional effects on neural cells. This project aims at determining whether IVIG influence Schwann cell homeostasis and maturation and could thus be used to promote nerve regeneration in different nerve pathologies.
Methods: We performed our investigations on three different primary Schwann cell culture systems: (a) naïve (immature) Schwann cells, (b) differentiating Schwann cells and (c) myelinating co‐cultures of Schwann cells.
Results: Our findings show that primary rat Schwann cells respond to IVIG stimulation with altered cell morphologies accompanied by an accelerated growth of cellular protrusions in early stages of the differentiation process. Stimulation with IVIG was also found to reduce cellular proliferation rates without affecting cell survival. Furthermore, we observed that IVIG treatment transiently boosts myelin gene expression of immature cells, whereas myelin gene expression of differentiating Schwann cells is promoted on a long‐term scale. Importantly, myelin gene expression responses cannot be detected upon application of IgG1 control antibodies (herceptin, synagis, avastin). In addition, we could demonstrate that primary rat Schwann cells express the CD64 Fc receptor and that in differentiating Schwann cells CD64 levels were significantly increased. On the other hand, we could also reveal a specific binding of the human IVIG on the Schwann cell surface.
Conclusions: We therefore conclude that Schwann cells are not only able to respond to but also specifically bind immunoglobulins and that IVIG stimulation can promote their differentiation.
T08‐17A. 2′,3′‐cyclic nucleotide 3′‐phosphodiesterase (CNP) deficiency causes axonal loss and hypermyelination in the sensory peripheral nervous system
T. Kungl 1, T. Nientiedt1, K.J. Neufeld1, M.W. Sereda1,2, K.‐A. Nave1
1Max Planck Institute of Experimental Medicine Göttingen, Göttingen, Germany 2University of Göttingen, Neuropathology, Göttingen, Germany
CNP is expressed in myelin forming glial cells of the central (CNS) and peripheral nervous system (PNS). It is present in cytoplasmic regions like Schmidt‐Lanterman incisures and the paranodal compartments, but is excluded from compact myelin. Mice deficient of CNP display axonal loss without an obvious myelination defect in the CNS. So far the role of CNP in the PNS remains unclear. Here, we demonstrate that CNP deficient mice exhibit alterations in mechanical and thermal sensation. Histological analyses of CNP deficient mutants reveal loss of sensory axons when quantified in the dorsal root and the predominantly sensory saphenous nerve. In contrast ventral roots remain unaltered. Moreover, electron microscopical assessments of saphenous nerves display a hypermyelination of small to mid‐sized caliber axons and an overrepresentation of the smallest fibers. The myelin pathology of sensory axons is reflected by alterations of sensory nerve conduction velocities. Thus, our data demonstrate that, contrary to findings in the central nervous system, CNP has an impact on myelination and is especially important for the integrity of small to mid‐sized caliber axons within the sensory peripheral nervous system.
T08‐17B. The role of the ERAD pathway in the pathophysiology of PNS myelination
V. Volpi 1, E. Pettinato1, C. Ferri1, C. Scapin1, H.L. Ploegh2, M.L. Feltri3, L. Wrabetz3, M. D'Antonio1
1Ospedale San Raffaele, Milan, Italy 2Whitehead Institute for Biomedical Research, Cambridge, MA, United States 3Hunter James Kelly Research Institute, Buffalo, NY, United States
P0, the most abundant glycoprotein in peripheral myelin, is specifically expressed by Schwann cells and acts as an adhesive molecule holding together adjacent wraps of myelin membrane. More than 120 mutations in P0 have been described resulting in clinically heterogeneous hereditary neuropathies in Humans. In particular, deletion of serine 63 (P0S63del) results in the demyelinating Charcot Marie Tooth‐Type 1B disease (CMT1B). To unravel the pathogenetic mechanism of the S63del mutation, we generated transgenic mice that express P0S63del protein and manifest a demyelinating neuropathy similar to CMT1B. P0S63del is a misfolded protein retained in the ER, where it activates a chronic unfolded protein response (UPR). The UPR is a cellular response to ER stress, aimed to reduce the load of abnormal proteins by attenuating protein translation, increasing the ER folding capacity and stimulating protein degradation. Among the degradation strategies upregulated by the UPR is the ER‐Associated‐Degradation (ERAD) pathway. In ERAD, proteins destined for degradation are retrotranslocated from the ER to the cytosol, ubiquitylated and degraded by the proteasome. Microarray analysis of S63del nerves revealed that several ERAD genes, such as derlins, are strongly upregulated and derlin‐3 expression returns towards normal in association with the amelioration of demyelination produced by ablation of Chop, indicating a possible involvement of this pathway in CMT1B neuropathy. Derlins elicit particular interest for exerting a protective role in several ER stress‐mediated diseases. In S63del nerves, Derlins co‐immunoprecipitate with P0S63del protein and, finally, Derlin‐1 overexpression reduces the level of ER stress in COS7 cells expressing P0S63del protein, suggesting a positive role in the modulation of P0S63del protein retrotranslocation. These observations indicate that Derlins may be part of the Schwann cell adaptive response that attempts to cope with chronic ER stress by modulating P0S63del clearance. We are now in the process of investigating the role of Derlins, in the pathophysiology of PNS myelination using in vitro, ex vivo and in vivo approaches.
T08‐18A. Transport and translation of MBP MRNA is differentially regulated by distrinct hnRNP proteins
J. Torvund‐Jensen, J. Stensgaard, L. Fihl, L. Reimer, L. Laursen
Aarhus University, Aarhus, Denmark
During the myelination process, the oligodendrocyte extends processes to and wraps around multiple axons of different diameter, keeping the number of wraps proportional to the axon diameter. This is one unique example of how cells in the central nervous system establish asymmetry. Transport of mRNA and local regulation of protein synthesis represent one mechanism by which such cellular asymmetry can be generated and the different requirements for myelin sheath at each axo‐glia interaction can be controlled.
The mRNA of a key myelin sheath protein, myelin basic protein (MBP), is known to be transported into the oligodendrocyte processes, and a tight temporal and spatial control of MBP mRNA translation is thought to be required for normal myelination. The molecular basis for the tight control of mRNA transport and translation is the assembly of large mRNA‐protein complexes. Prior work has identified a number of proteins that interact with the MBP mRNA, including hnRNP‐A2, hnRNP‐K and hnRNP‐E1. However, it is currently unknown how these protein functions together to prevent translation during transport. It is also unknown how targeting of the MBP mRNA to the myelin sheets occurs.
To delineate the precise role of the individual binding protein in regulating MBP mRNA translation, we have identified regulatory elements within the 3′UTR of the MBP mRNA involved in translational inhibition, and we have identified binding sites in the mRNA for hnRNP‐K and hnRNP‐E1. Furthermore, we have analyzed the effect of siRNA‐mediated knockdown and overexpression of these proteins in primary oligodendrocytes. Together, our results allow us to propose a model, in which the individual mRNA binding proteins are assigned distinct roles for correct spatial and temporal expression of MBP.
T08‐18B. Ectopic myelination of cerebellar granule cell axons triggered by PtdIns(3,4,5)P3‐dependent neuronal signals
G.L. Wieser, A. Pieper, B. Weege, S.P. Wichert, W. Hinrichs, M.J. Rossner, K.‐A. Nave, S. Goebbels
Max Planck Institute of Experimental Medicine, Dept. of Neurogenetics, Göttingen, Germany
In the vertebrate central nervous system (CNS), the formation of myelin by oligodendrocytes enables rapid saltatory impulse propagation by decreasing the electrical capacity of axonal membranes. The failure to achieve and maintain normal myelin causes severe neurological diseases including leukodystrophies and multiple sclerosis (MS), which indicates that the identification of signals stimulating myelin formation in the CNS is of therapeutical interest. It is assumed that axons play an important role in controlling oligodendrocyte development. However it is yet largely unclear how axons affect migration, proliferation, differentiation and survival of OPC and oligodendrocytes in vivo and by what molecular signals they act. By geneticaly inactivating the lipid phosphatase PTEN selectively in cerebellar granule cell (GC) neurons and thus enhancing phosphatidylinositol (3,4,5)‐triphosphate (PtdIns(3,4,5)P3) dependent Akt/mTOR signalling, we could experimentally enlarge their normally small and unmyelinated axons to calibers well above the normal size of 0.2 μm. Remarkably, this manipulation was sufficient to achieve both the expansion of oligodendrocyte precursor cells (OPC) and the de novo myelination of a large fraction of enlarged parallel fiber axons in the cerebellar molecular layer, independent of axonal neuregulin‐1 expression. By laser‐guided microdissection and gene expression profiling of cerebellar GC, we identified neuronal factors that promote OPC proliferation and myelin synthesis in vitro. These findings suggest that oligodendrocytes and their precursors respond to multiple ‘instructive' signals expressed by CNS neurons in vivo, but only on axons with diameters >0.2 μm that became ‘permissive' for myelination.
T08‐19A. Transferrin and Thyroid hormone converge in the control of myelinogenesis
L. Marziali, P. Franco, J. Pasquini
University of Buenos Aires, Caba, Argentina
Promyelinating effects of both Apo‐Transferrin (aTf) and Thyroid Hormone (TH) on rat brain are well documented. TH effects are mediated by nuclear receptors which act as transcription factors and regulate different cell processes. Previous results from our laboratory showed that TH promotes the commitment of oligodendrocyte progenitors (OPCs) to mature myelinating oligodendrocytes (OLG). On the other hand, aTf is able to promote the commitment of neural stem cells (NSC) to cells of the oligodendroglial linage and favors OLG maturation after an intracranial injection. Our previous demonstration that TH administration is able to up‐regulate Tf mRNA expression leads us to hypothesize that both factors converge in the control of oligodendrogenesis. To test if the combined effects of both aTf and TH are required for proper myelination in the rat brain, studies were done at P10 and P20 for each experimental condition. A hyperthyroid state was rendered by daily subcutaneous TH administration from the day of birth (Hyper). Hypothyroidism was achieved by giving propylthiouracil to the mother from gestational day 18 to the end of the experiment (Hypo). Half of the hypo pups received an intracranial injection of aTf at postnatal day 3 (Hypo+aTf). A set of euthyroid animals received an intracranial injection of aTf at postnatal day 3 (aTf). Control groups received an intracranial injection or subcutaneous saline solution (C).
At P10, Hyper animals showed an up‐regulation of 55% in Tf mRNA expression and 87% in protein levels as compared to controls. Hypo showed a decrease of 25% in Tf mRNA levels. This effect was not affected by exogenous administration of aTf.
At P20, Hyper showed no differences in the expression of Tf mRNA or protein levels as compared to controls. Hypo showed a decrease of 25% in Tf mRNA and a 40% decrease in protein levels. This effect was not affected by exogenous administration of aTf. No differences were observed in the expression of Insulin Growth Factor I (IGF‐I) or IGF‐I Receptor between groups at P10 or P20.
Immunohistochemical analyses were performed at P20 in all groups usingspecific markers anti CAII, anti MBP, anti RIP and PDGFRα.
Our conclusion is that, in a hypothyroid state, Tf is not able to produce OLG and myelin maturation at the corpus callosum, which indicates that TH is necessary for aTf action. However, the finding that hyperthyroid animals showed a significant increase in Tf mRNA strongly suggests that Tf could be involved in TH effects.
New experiments are carrying out in order to knock down the Tf gen to probe this last piece of evidence.
T08‐19B. Functions of Exon‐II Containing Isoforms of Myelin Basic Protein in Oligodendrocytes
H. Yigit, L. Meyer, C. Gonsior, P. Hoch‐Kraft, J. Trotter
Johannes Gutenberg University, Mainz, Germany
Myelination is the prerequisite for fast saltatory conductance of action potentials. In the central nervous system, the myelin sheath is produced by oligodendrocytes which extend processes and wrap the axons. This structure is subsequently compacted by the help of certain proteins. Of major importance here is the myelin basic protein (MBP) which interacts with the cytoplasmic leaflet of the plasma membrane. MBP is a highly basic protein and binds to the negatively charged plasma membrane with high affinity. It has 6 isoforms in mice produced by alternative splicing. The mostly studied 4 isoforms are 14 kD, 17 kD, 18,5 kD and 21,5 kD. Interestingly, exon‐II containing 17 kD and 21,5 kD isoforms are actively transported into the nucleus while 14 kD and 18,5 kD isoforms are rather localized to the distal part of the plasma membrane. Transport to the nucleus depends on temperature and energy. Recently, it was reported that exon‐II contains a non‐canonical PY nuclear localization signal. As a cytoplasmic protein, MBP is essential for proper myelination. Furthemore MBP is supposed to have multiple additional functions, e.g. in calcium homeostasis and cytoskeleton dynamics. It was also shown that the expression level of MBP exon‐II containing is changed in Schizophrenia patients. Selectively transport of exon‐II containing isoforms to the nucleus raises the question of its nuclear functions which have not been elucidated yet. This study aims to unravel the role of exon‐II containing MBPs in the nucleus.
T08‐20B. Myelin Basic Protein synthesis is regulated by small non‐coding RNA 715
N. Bauer1, C. Müller1, J. van Horssen2, H. Luhmann1, R. White 1
1University Medical Center Mainz, Mainz, Germany 2VU University Medical Center, Amsterdam, Netherlands
Oligodendroglial Myelin Basic Protein (MBP) synthesis is essential for myelin formation in the central nervous system. MBP mRNA is kept in a translationally silenced state during oligodendrocyte differentiation and intracellular transport until neuron‐derived signals initiate localized MBP translation. The molecular mechanisms controlling translational inhibition of MBP are only poorly understood. We identified the small non‐coding RNA 715 (sncRNA 715) as a key inhibitor of MBP translation. MBP and sncRNA 715 levels inversely correlate during oligodendrocyte differentiation in culture and the optic nerve. Experimentally increased levels of sncRNA 715 reduce MBP protein levels and blocking the sncRNA 715/ MBP mRNA interaction results in higher MBP protein levels. Increased sncRNA 715 levels affect cell morphology and induce apoptosis resulting in reduced cell numbers. SncRNA 715 is found in cytoplasmic granular structures and co‐purifies with hnRNP A2 and MBP mRNA. Chronic Multiple Sclerosis (MS) lesions contain Olig2+ cells and MBP mRNA, but lack MBP protein. Compared to normal appearing white matter (NAWM), these lesions contain elevated levels of sncRNA 715 alluding to a role of this molecule in the pathology of MS.
POSTER TOPIC 09 Neural stem/progenitor cells
T09‐01A. Wnt‐signalling regulates oligodendrogenesis in the dorsal postnatal subventricular zone
K. Azim 1, B. Fischer1, K. Basler3, L. Sommer4, A. Butt2, O. Raineteau1
1University of Zurich/ETHZ, Brain Research Institute, Zurich, Switzerland 2University of Portsmouth, IBBS, School of Pharmacy and Biomedical Sciences, Portmouth, United Kingdom 3University of Zurich, IMLS, Zurich, Switzerland 4University of Zurich, Institute of Anatomy, Zurich, Switzerland
In the developing postnatal forebrain, the myelin forming cells, oligodendrocyte (OL) lineage cells originate from neural stem cells (NSCs) of the dorsal subventricular zone (SVZ) of the lateral ventricle. The mechanisms that regulate OL progenitor (OP) differentiation from postnatal SVZ sources are relatively complex but the early NSC‐intrinsic signals are unknown.
Gene expression profiling of the microdissected SVZ regions revealed that Wnt‐responsive genes were enriched in the postnatal dorsal SVZ compared to the lateral SVZ. We performed inhibition of GSK3β by intraventricular infusion of ARA to initiate Wnt‐signalling. Several approaches confirmed activation of the Wnt‐signalling pathway in the dorsal SVZ as well as within OPs. Thus, gene expression profiles of Wnt‐target genes of the dorsal SVZ indicated that Axin2, Lef1, Fzdl1 and Tcf4, but not those other pathways, were dramatically up‐regulated. Furthermore, nuclear β‐catenin was transiently up‐regulated in the dorsal SVZ including in a large population of Sox10‐EGFP expressing cells following pharmacological Wnt‐activation. Gene expression profiling and immunostainings revealed that the densities of NSCs, cycling progenitors and subsequent OP differentiation in the dorsal SVZ were augmented.
In parallel, we genetically manipulated Wnt‐signalling in stem and progenitor cells of the dorsal wall of the lateral ventricle. Targeted dorsal SVZ electroporation of Cre‐GFP plasmids in floxed stabilised β‐catenin (gain‐of‐function) and floxed β‐catenin (loss‐of‐function) transgenic mouse lines were performed and resulted in a phenocopy of the results observed following ARA‐intraventricular infusion.
In summary, our findings illustrate that Wnt‐signalling endogenously persists in the dorsal SVZ after birth. Pharmacological as well as genetic interventions reveal an important role of this signalling pathway in the regulation of OP genesis during the period of myelination.
T09‐01B. Neurogenic and gliogenic astroglial progenitors in the developing cerebellum
E. Parmigiani 1,2, K. Leto1,2, C. Rolando3, A. Buffo1,2, F. Rossi1,2
1University of Turin, Neuroscience, Turin, Italy 2NICO (Neuroscience Institute Cavalieri Ottolenghi), Orbassano, Italy 3University of Basel, Biomedicine, Basel, Switzerland
In the cerebellum the generation of neurons and glia and the relationships between these two lineages are poorly understood. All the cerebellar neuronal phenotypes derive from two distinct embryonic germinal neuroepithelia that are restricted regarding the neuronal cell fate: the rombic lip (RL) gives rise to the glutamatergic neurons, whereas the ventricular zone (VZ) generates all GABAergic neurons. In particular, different types of inhibitory interneurons are generated during the postnatal life from a common pool of progenitors expressing the transcription factor Pax2. On the other hand, the glial development is relatively unexplored. Part of the oligodendrocytes derives from extracerebellar sources, whereas some of them derive from endogenous precursors. Astrocytes, instead, may derive from progenitors that delaminate from the VZ and continue to divide in the prospective white matter (PWM) up to postnatal life. Given the VN origin of both astrocytes and interneurons, we wondered whether a lineage relationship exists between these two cell populations and whether they share the same bipotent progenitor. In order to address these issues, we performed a genetic fate mapping study of GlastCreERT2 mice. We showed that both cerebellar GABAergic interneurons and astrocytes derive from proliferating Glast+ precursors. In particular, we found that these progenitors are able to produce interneurons at earlier developmental phases, whereas after P6 they appear restricted to the glial lineage. Proliferating Glast+ cells that may produce interneurons comprise Bergmann glia and PWM progenitors. However, when recombination was specifically induced in Bergmann glia, we found that these cells were not neurogenic. Therefore, we concluded that GLAST+ progenitors reside in the PWM. To get further insight in the behaviour of these precursors, we injected in the PWM a GFP‐containing lentiviral vector with a specific tropism for astroglial cells. Some days after, infected cells generated Pax2+‐interneurons that were able to mature into interneurons of different cortical layers. Moreover, in vitro and in vivo clonal analysis of PWM derivatives indicates that the PWM is neurogenic and both astrocytes and interneurons may derive from a common progenitor.
T09‐02A. Modulation of the proliferation and differentiative potential of adult brain subventricular zone cells by purinergic signaling in vitro and in vivo: contribution of reactive astrocytes
S. Ceruti 1, M. Boccazzi1, C. Rolando2, M.P. Abbracchio1, A. Buffo2
1Università degli Studi di Milano, Milan, Italy 2Università di Torino, Neuroscience NICO, Turin, Italy
Evidence is accumulating that neurogenesis in the subventricular zone (SVZ) is boosted after trauma or ischemia, also through the interaction with surrounding parenchyma or niche cells. Nevertheless, the number of newborn neurons that survive and integrate in the damaged areas is negligible, suggesting a non‐permissive environment. Thus, understanding the complex signaling network guiding neuroblast generation/survival could help identifying strategies to limit negative inputs and promote regeneration. Extracellular nucleotides (eNTs) are among the hypothetical modulators of SVZ cell functions, especially under pathological conditions where their concentrations raise several folds and they contribute to reactive astrogliosis. Few literature data have recently pointed for a role of the P2Y1 receptor subtype (one of the 8 known G protein‐coupled nucleotide receptors) in controlling the proliferation and differentiative potential of SVZ cells. Thus, we tested the ability of ADPβS, a stable P2Y1 agonist, to modulate stem cell properties in the adult brain, with a focus on the possible modulatory effects exerted by reactive astrocytes. The administration of ADPβS in the lateral ventricle of adult mice led to reactive astrogliosis in the surrounding brain parenchima, and to a massive reaction of GFAP‐expressing precursors and astrocytes in the SVZ. Also proliferation was increased, paralleled by a significant expansion of the population of Mash1+ transit‐amplifying cells and of doublecortin+ neuroblasts. Thanks to the conditional GLAST::CreERT2 YFP mouse model, we also demonstrated that ADPβS promoted the proliferation of GLAST‐expressing progenitors in the neurogenic niche, and sustained their progression towards the generation of rapidly dividing transit‐amplifying cells. In vitro the nucleotide analog increased the proliferation of SVZ cells grown as neurospheres, and their differentiation towards neurons, fully confirming in vivo data. Interestingly, a significant enhancement in neurosphere generation was detected when SVZ cells were initially grown in the supernatant of astrocytes exposed to ADPβS, and then shifted to normal medium. This suggests that ADPβS stimulates the release of yet‐to‐be identified astrocytic mediator(s) whose removal from the culture medium boosted proliferation of SVZ cells. Our results further strengthen the notion that the purinergic system is a key regulator of the neurogenic potential of SVZ cells, both directly and through the involvement of reactive astrocytes.
T09‐02B. Generation of oligodendrocyte precursors by direct cellular reprogramming
M. Pawlowski, M. Trotter, M. Kotter
Cambridge Stem Cell Institute, Cambridge, United Kingdom
Oligodendrocytes, one of the principal glial cell‐types of the central nervous system, are critical for neuronal function and represent the primary target of many neurological diseases, including multiple sclerosis and leukodystrophies. Despite the recent progress in stem cell research and the possibility to generate various neural cell types in vitro, the derivation of oligodendrocytes from human pluripotent stem cells remains inefficient and unreliable.
We employed a new experimental approach termed “direct cellular reprogrammng" which was already succesfully applied for the generation of different types of neurons and neural stem cells. Aided by bioinformatic filtering we have generated a pool of seventeen candidate reprogramming factors that could potentially convert fibroblasts directly into oligodendrocyte lineage cells. Combinatorial testing of the candidate factors in overexpression experiments has revealed a combination of just two transcription factors that was sufficient to convert mouse embryonic fibroblasts directly into O4‐expressing oligodendrocyte precursors. Supplementation of additional factors and multicistronic gene delivery strategies render the reprogramming process more efficient and widen the applicability of this protocol.
Cellular reprogramming offers a new tool for the generation of oligodendrocyte precursors. This approach, that circumvents the protracted specification and differentiation process inherent to the oligodendrocyte lineage will foster the quest for a culture system of human oligodendrocyte lineage cells.
T09‐03A. Enteric stem cell niche in Hirschsprung's disease
C.I. Hagl, M. Theisen, S. Heumüller‐Klug, E. Wink, K.‐H. Schäfer
University of Heidelberg, Medical Faculty Mannheim, Mannheim, Germany
The transplantation of neural crest derived stem cells (NCSC) is a potent alternative for the treatment of Hirschsprung's disease (HSCR), especially as the microenvironment in the aganglionic segment is suitable for enteric stem cell survival and differentiation. Ideally, the cells transplanted are derived from progenitors within the intestine. To gain further insight in possible stem cell niches the compelling evidence that mouse enteric glia can also be neuronal precursors was related to human tissue. Human colon from infants with Hirschsprung's disease was investigated with a panel of glial and stem cell markers.
Tissue samples from infants with Hirschsprung's disease were investigated with glial and stem cell markers along the gut axis. The tissue samples from ganglionic, aganglionic and transient segments were immuonstained either for S100/nestin, S100/p75, GFAP/nestin and GFAP/p75.
In all samples investigated, nestin positive ganglia could be found, even in the distal parts with a severe hypo‐ or aganglionosis. Beside nestin positive cells in all segments, there were also different expression pattern glial markers within the ganglia, indicating that distinct phenotypes of glia cells could be found.
Neural and glial precursor cells are present in the ganglionic as well as in the hypoganglionic segments of Hirschsprung's colon, suggesting that these cells might be suitable for NCSC generation and further transplantation.

T09‐03B. Mechanisms of activation of the adult subventricular zone in response to inflammatory demyelination
F. Pourabdolhossien, C. Deboux, V. Tepavcevic, A. Baron Van‐Evercooren
CRICM UMRS975 UPMC, Paris, France
The subventricular zone (SVZ) of the lateral ventricle, the largest adult stem cell niche, has a striking pinwheel organization specific to regions of adult neurogenesis. The pinwheel's core contains the apical endings of B1 cells and in its periphery two types of ependymal cells: multiciliated (E1) and biciliated (E2) cells. Previous studies showed that SVZ cells are reactivated in response to demyelination. However, the mechanisms inducing SVZ reactivation in response to inflammatory demyelination such as in Multiple sclerosis (MS) are poorly understood. We hypothesize that B1 and E, which are in direct contact with CSF through cilia, may play a crucial role in initiation of SVZ reactivation. In the present study, we examined the SVZ reactivation in mice in which we targeted an MS‐like pathology to the forebrain white matter (t‐EAE). Animals were immunized with subclinical doses of myelin oligodendrocyte protein and after 20 days, received a stereotaxic injection of a cytokine cocktail consisting of tumour necrosis factor (TNFα) and interferon (IFN γ) into the corpus callosum. To obtain an en face view of the ventricular surface, we dissected whole mounts of lateral ventricle of control and EAE animals at 3, 7, 14 and 28 days post‐EAE induction (dpi). We investigated by immunohistochemistry whether and how the pinwheel architecture of the SVZ is modified and ependymal cells are reactivated during disease course of EAE. In SVZ niche of t‐EAE animals we found that the number of B1 cells increased and peaked at 7 dpi compared to control. The number of E2 cells and pinwheels decreased and then reached control level at 7 dpi. While the number of E1 cells remained steady, their surface area increased at 3dpi and then returned to normal size over time. In addition, the number of their ciliary basal bodies increased during EAE time courses, and E1 cells highly expressed GFAP in EAE animals compared to controls. Thus, the findings of our ongoing research suggest that neuroinflammation induces some changes in cell number and morphology of SVZ niche as well as reactivation of ependymal cells, which altogether may contribute to SVZ reactivation mechanisms. Further study will provide detailed information on the homotypic and heterotypic interactions between pinwheel cells in response to t‐EAE.
Sponsored by INSERM and MSIF.
T09‐04A. Hypothalamic tanycytes: a neurogenic population of radial‐glial like cells in the postnatal and adult brain
N. Haan1, T. Goodman1, A. Nadji‐Samieir1, C. Stratford1, R. Rice2, E. Al Agha3, S. Bellusci3, M.K. Hajihosseini 1
1University of East Anglia, Norwich, United Kingdom 2University of Helsinki, Helsinki, Finland 3University of Giessen, Giessen, Germany
Hypothalamus is emerging as the third neurogenic region of the postnatal and adult rodent brain. However, there is much debate about the identity of its stem/progenitor cells and very little is known about the dynamics and significance of hypothalamic neurogenesis.
To test whether hypothalamic tanycytes act as bona fide neural stem/ progenitor cells in vivo, we analysed their immumoprofile and proliferative potential, and lineage‐traced them in vivo. For this, we exploited our earlier findings that in the adult hypothalamus, Fibroblast growth factor 10 (Fgf10) is expressed exclusively by tanycytes (Hajihosseini et al. 2008 MCN 37: 857‐68), a radial glial‐cell like population that lines the floor and ventral walls of the third ventricle. Using a line of Fgf10‐lacZ reporter mice, we found that Fgf10+ beta tanycytes express a panel of neural/stem progenitor markers such as Nestin, Musashi1, BLBP and Sox2. Fgf10+ tanycytes also incorporated BrdU under cumulative BrdU‐labelling paradigms, and formed neurospheres in vitro. To directly test the potential of Fgf10+ve tanycytes, we lineage‐traced them at P28 and P70 by activating the constitutive expression of the marker gene, tomato‐dsred, in these cells and their descendants. This was achieved by applying tamoxifen to Fgf10‐creERT2::Rosa26R‐Tomato double transgenic mice.
After short survival time‐points, tomato was detected exclusively in tanycytes, whilst with longer survival time‐point, tomato+ cells emerged in the neighbouring parenhcyma. The majority of these were NeuN+ neurons with fine arborizations. We noted that the newly‐generated neurons become associated mainly with hypothalamic nuclei that regulate appetite and energy‐balance. Moreover, they respond to appetite/energy balance regulating signals such as acute food deprivation and leptin administration.
Our findings establish Fgf10+ beta‐tanycytes as a putative population of neural stem/ progenitor cells that generate appetite/energy balance regulating neurons in the postnatal and adult hypothalamus.

T09‐04B. GFAP‐positive cells are multipotent progenitors with limited self‐renewal potential in the adult spinal cord
R. Fiorelli, O. Raineteau
Brain Research Institute, Zurich, Switzerland
Adult neural stem cells (aNSCs) of the forebrain are GFAP‐expressing cells that are intercalated within ependymal cells of the subventricular zone (SVZ). Their glial identity has been questioned in other regions of the CNS, i.e. the spinal cord, where recent evidences propose an ependymal identity of NSCs.
We used an inducible transgenic mouse line (hGFAP‐CreERT2) to conditionally label and fate map putative GFAP(+) NSCs populations in the adult SVZ and spinal cord (SC), and compare their self‐renewal and multipotential properties. We evidence that a population of GFAP+ cells that share the morphology and antigenic properties of SVZ‐NSCs reside in the dorsal and ventral aspects of the central canal (CC) throughout the spinal cord. These cells are non‐proliferative in the intact spinal cord, but incorporate EdU, an S‐phase marker following spinal cord injury. Multipotent YFP‐expressing neurospheres (i.e. which derive from recombined GFAP‐expressing cells) were successfully obtained from both the intact and injured spinal cord, confirming their early GFAP origin. Notably, only spheres isolated from the injured spinal cord revealed long‐term self‐renewal properties resembling those of SVZ neurospheres.
Altogether, this work highlights discrepancies in the identity of NSCs that reside in distinct regions of the adult CNS. Our observations however reconcile divergent views on the location and identity of spinal cord‐NSCs by showing a population of multipotent GFAP+ progenitors with limited self‐renewal properties co‐existing alongside with multipotent, self‐renewing ependymal cells within the spinal cord central canal.
T09‐05A. microRNA regulation of neural precursor maintenance and specification
L. Hudish, B. Appel
University of Colorado Denver School of Medicine, Aurora, United States
During development neural precursors (NPs) both divide, to expand the cell population, and produce many different kinds of neurons and glia. This balance appears to be regulated by Par complex proteins, which polarize neural precursors and can thereby direct daughter cells for different fates. How Par complex proteins are regulated to appropriately polarize NPs remains unknown. In recent years, regulation of gene function by microRNAs has emerged as an important mechanism during development. Using bioinformatics we identified the polarity gene pard3 as a candidate target for microRNA219 (miR‐219). miR‐219 is specifically expressed in the developing central nervous system (CNS) of vertebrates and miR‐219‐deficient zebrafish embryos have a deficit of oligodendrocytes, the myelinating glial cells of the CNS. Because a disruption in polarity could affect the types of cell divisions that NPs undergo, thus altering the balance of cell types that arise, we hypothesize that neural precursor maintenance is regulated by modulation of polarity cues through miR‐219. By using an in vitro reporter assay we found that miR‐219 can downregulate expression of a luciferase gene fused to the pard3 3′UTR. Reduction of pard3 function in zebrafish embryos suppressed the oligodendrocyte phenotype resulting from loss of miR‐219 function and injection of a morpholino oligonucleotide designed to block binding of miR‐219 to its pard3 target sequence phenocopied miR‐219 knock down. Together, these data provide strong evidence that pard3 is a functionally relevant in vivo target of miR‐219. To further investigate the role of miR‐219 function we tested expression of NP, radial glial and neuronal markers in miR‐219‐deficient embryos. The number of cells expressing Sox2, a marker of NPs and neural stem cells, was significantly increased upon miR‐219 knock‐down. Concomitantly, miR‐219‐deficient embryos had a dramatic deficit of radial glia and late born neurons. These data provide evidence for a new mechanism of NP regulation, in which miR‐219 regulates Pard3 levels, thereby regulating the transition of dividing neural precursors to differentiated neurons and glia.
T09‐05B. Distinct Roles of Nogo‐A and Nogo Receptor 1 in the Homeostatic Regulation of Adult Neural Stem Cell Function
C. Rolando 1,2,3, R. Parolisi3, E. Boda2,3, F. Rossi2,3, V. Taylor1, A. Buffo2,3
1University of Basel, Dept of Biomedicine, Basel, Switzerland 2University of Turin, Dept of Neuroscience, Turin, Italy 3Scientific Institute of the Cavalieri‐Ottolenghi Foundation, Orbassano, Italy
The adult subventricular zone (SVZ) holds GFAP positive Neural Stem Cells (NSCs) that are able to generate neuroblasts, which migrate tangentially within astroglial tubes through the rostral migratory stream (RMS) towards the olfactory bulb (OB). In the mouse brain, we found that the Nogo‐A and Nogo receptor 1 (NgR1) are differentially expressed in the SVZ‐OB system. Nogo‐A is expressed by immature neuroblasts and NgR1 by germinal astrocytes. We examined the role of Nogo‐A and NgR1 in the regulation of neurogenesis. Neurosphere assays revealed that Nogo‐A/NgR1 interactions inhibit neural progenitor stemness, and the application of the Nogo66 antagonist NEP1–40 increased self‐renewal and proliferation of SVZ‐derived progenitors. Furthermore, the neurosphere‐forming capability of SVZ progenitors was highly impaired after exposure to Nogo‐66, which activates NgR1. In vivo, NEP1–40 application to the SVZ increased NSC proliferation and subsequent increased neuroblast formation. We addressed the signal transduction pathways mediating the effects of NgR1 activation in the SVZ. Based on the similar enhancement of SVZ neurogenesis observed upon both NgR1 antagonism in vivo and deletion of the protein phosphatase and tensin homolog (PTEN), we hypothesised that NgR1 activation stimulates PTEN signalling. In line with this view, preliminary experiments indicate that PTEN inhibition reverts the negative effect of NgR1 activation on neurosphere formation and progenitor cell proliferation. Moreover, pS6, a downstream target of PTEN signalling, appears to be decreased after activation of NgR1 signalling. In summary, we demonstrated that Nogo66/NgR1 interactions actively participate in restraining activation and proliferation of adult NSCs in vivo and in vitro.
T09‐06A. Mesenchymal stem cell conditioning promotes oligodendroglial maturation
J. Jadasz 1, D. Kremer1, P. Göttle1, N. Tzekova1, F.J. Rivera2, H.‐P. Hartung1, L. Aigner2, P. Küry1
1Heinrich Heine University, Düsseldorf, Germany 2Paracelsus Medical University, Institutes of Molecular Regenerative Medicine, Salzburg, Austria
Question: In demyelinating diseases such as multiple sclerosis myelin repair activities based on recruitment, activation and differentiation of resident progenitor and stem cells can be observed. However, the overall degree of successful remyelination is limited. It is therefore of considerable interest to understand oligodendroglial precursor cell (OPC) homeostasis and maturation processes in order to develop remyelination therapies. Mesenchymal stem cells (MSC) were shown to exert positive immunomodulatory effects, to reduce demyelination, to increase neuroprotection and to promote adult neural stem cell differentiation towards the oligodendroglial lineage. We here addressed whether MSC secreted factors can influence primary OPCs in a myelin non‐permissive environment.
Methods: To this end we analyzed cellular morphologies, expression and regulation of key genes/proteins involved in oligodendroglial cell fate and maturation upon incubation with mesenchymal stem cell conditioned medium.
Results: This demonstrated that MSC derived soluble factors promote and accelerate oligodendroglial differentiation, even under astrocytic endorsing conditions. Accelerated maturation featured elevated levels of 2′, 3′‐cyclic nucleotide 3′‐phosphodiesterase and myelin basic protein expression, reduced glial fibrillary acidic protein expression and was accompanied by downregulation of prominent inhibitory differentiation factors such as ID2 and ID4.
Conclusions: We thus conclude that besides the previously established immunomodulatory and neuroprotective roles of MSCs these cells can also positively influence oligodendrogenesis in the adult central nervous system.
T09‐06B. The impact of erythropoietin and its isoforms on murine neurogenesis
S. Rolfes, E.‐M. Lyras, C. Böttcher, J. Priller
Charité – Universitätsmedizin Berlin, Laboratory of Molecular Psychiatry, Berlin, Germany
The cytokine erythropoietin (EPO) is the main regulator of erythropoiesis. Beyond this canonical role, cytoprotective and neuroprotective effects of EPO have been widely demonstrated in neurodegenerative CNS disorders. However, long‐term application of EPO remains limited due to its undesired erythropoietic side effects. Recently discovered endogenous EPO splice variants (i.e. hS3 and ms), while lacking systemic effects on erythrocyte production, are able to induce neuroprotection in an in vitro stroke model. Thus, EPO splicing variants may represent interesting candidates as neuroprotective agents.
In this study, we evaluated the effects of EPO and the EPO splicing variant (vEPO) on murine neurogenesis ex vivo. For this purpose, we analyzed the survival of cultured neural stem cells (NSCs) either exposed transiently to recombinant protein, or transduced with lentiviral vectors to achieve stable protein expression.
In comparison to non‐exposed controls, recombinant EPO and vEPO enhanced survival of NSCs ex vivo (EPO: 150 ± 25%; vEPO: 250 ± 60% survival). NSCs transduced with pCL20‐MSCV‐EPO‐ and pCL20‐MSCV‐vEPO showed also higher viability ex vivo compared to non‐transduced NSCs (EPO: 182 ± 4% vEPO: 131 ± 4% survival). Furthermore, pCL20‐MSCV‐EPO, but not pCL20‐MSCV‐vEPO, increased the proportion of differentiated neurons when compared to non‐transduced controls. Thus, stable expressed vEPO may have rather an effect on NSC survival than on NSC differentiation.
Our results demonstrate that vEPOs are neuroprotective in vitro. vEPO may serve as therapeutic protein for treatment of neurodegenerative disease associated with impaired neurogenesis such as Huntington disease.
T09‐07A. Adult neural stem cells generate waves of oligodenrocyte progenitor cells that populate transiently the corpus callosum but do not contribute to its pool of oligodendrocytes
I. Kazanis, R. Franklin
University of Cambridge, Cambridge, United Kingdom
In the subependymal zone (SEZ) cytogenic niche of the adult mouse brain neural stem cells drive the continuous generation of new cells, mostly of olfactory bulb interneurons, but also of cells of the oligodendroglial lineage. Previous reports have shown that newly‐born cells exit the SEZ and migrate to the adjacent corpus callosum (cc) where they differentiate into oligodendroglial cells. However, the real contribution of these niche‐derived oligodendrocyte progenitor cells and oligodendrocytes (nOPCs and nOligos) to the oligodendroglial population of the cc has not been assessed so far. In this study we used the hGFAP‐CreERT2 × Rosa26‐EYFP double transgenic mice in order to label specifically the progeny of SEZ‐located adult neural stem cells. We found that cells expressing markers of OPCs, such as PDGFRα and Olig2, are constantly generated in the SEZ and migrate to the proximal fraction of the cc. Interestingly though, these cells do not remain in the cc for more than 15 days with their contribution to the total population of oligodedroglial cells remaining stably below 2% even in the ageing brain. Moreover, immunostaining for markers of cell cycle revealed that a higher fraction of nOPCs undergoes mitosis, when compared with local OPCs; hence, they constitute almost 25% of proliferating OPCs of the cc. Notably, during their transient presence in the cc, nOPCs express markers of maturing oligodendrocytes and are incorporated in established local cell structures. When the cc is challenged with a focal demyelinating insult the local and the niche‐derived oligodendroglial machineries exhibit differential cell kinetics, nOPCs increase their contribution to the total pool of oligodendroglial cells; however, their presence remains transient.

T09‐07B. Stimulation of Müller Glia Proliferation and Progeny Generation in the Mouse Retina
P. Schaefer 1, K. Anastissiadis2, M.O. Karl1,3
1Center for Regenerative Therapies Dresden (CRTD), Dresden, Germany 2TU Dresden, Biotechnology Center (BIOTEC), Dresden, Germany 3German Center for Neurodegenerative Diseases (DZNE), Dresden, Germany
In the human and mouse retina Müller glia (MG) are well known to undergo gliosis in all major types of retinal diseases – which sometimes may even lead to scar formation due to proliferative gliosis. Some studies suggest that in the mouse retina MG derived neuronal regeneration can be stimulated, but only to a very limited extent. Here, we started to find out, if conditional immortalization might stimulate MG derived proliferative gliosis and /or neuronal regeneration.
Others and we reported that in the juvenile mouse retina, after retinogenesis is finished, some Müller glia shift from a quiescent differentiated state into a proliferative state upon damage of retinal explants ex vivo. We now observed that this process is differentially inducible by mitogens like EGF (epidermal growth factor). EdU (S‐phase marker) pulse chase experiments revealed a tremendous increase in MG proliferation within the first two days, a peak of EdU positive cells at day 4 (14 +−2 SEM, N = 4) and a massive decrease until day 6 (4 +−0.4 SEM, N = 4) per 400 µm of a central retina section.
Next, we used transgenic mice with tightly and temporally controlled expression of the proto‐oncogene SV40 large T‐antigen (cSV40LT). It is well reported that cSV40LT binds several proteins including the tumor suppressor p53 and retinoblastoma and bypasses cell cycle checkpoints. Induction of the cSV40LT for 6 days ex vivo led to an overall increase in proliferation compared to control. The number of EdU+ cells was 8.5 fold increased (cSV40LT: 23 +−6 SEM, N = 4; control: 3 +−0.16 SEM, N = 4; p
Our results so far suggest that induction of cSV40LT not only overcomes the proliferative restriction of Müller glia but also maintains its progeny in the cell cycle over extended period of time. Surprisingly, major parts of the generated cell progeny formed gliotic cell clusters, which were all located within the boundaries of the retina. Further, we present data of successful Müller glia isolation at high purity by FACSorting. In our current and future work we study the Müller glia and its derived progeny to find out the underlying mechanisms that enable neuronal regeneration and prevent gliotic scar formation.
T09‐08A. Artificial cell fate regulation of the progenitor cells in the adult spinal cord
M. Kitada, J.‐I. Suzuki, M. Dezawa
Tohoku University Graduate School of Medicine, Sendai, Japan
In mammals, regeneration capacity of the central nervous system (CNS) is considered too low to recover the neurological functions after traumatic injury. In fishes and amphibians, however, the spontaneous regeneration is vigorous enough to complete the anatomical reconstruction and functional recovery even after severe damage of CNS, in which the ependymal cells play the central role to exhibit proliferation, epithelial‐mesodermal transition, cell migration, support of regenerating axons and neurogenesis to contribute to recovery from injury. These positive roles of the ependymal cells observed in fish and amphibian damaged CNS may be good candidates for treating mammalian CNS injury. In this study, we attempted to control the activity of the ependymal cells in the adult rodent spinal cord by virus‐mediated gene transfer for imitating the reactions that are observed in the ependymal cells of fish and amphibian damaged CNS. We introduced the gene known to be expressed in both the ependymal cells and neural stem cells (NSCs) and found the proliferation of ependymal cells. In this case, the ependymal cells expressed glial fibrillary acific protein suggesting that this gene regulates proliferation and differentiation into astrocytes. In addition, introduction of another gene, also known to be expressed in the ependymal cells and NSCs, caused cell proliferation without differentiation tendency. These findings indicate that the cell fate of the ependymal cells in the adult rodent spinal cord can be modulated by artificial intervention. Future studies will clarify whether cell fate modification in the ependymal cells can contribute to anatomical reconstruction and functional recovery in the adult mammalian damaged spinal cord.
T09‐08B. The human enteric nervous system: an appropriate autologous source for neural stem cells
K.‐H. Schäfer 1, C.I. Hagl2, S. Heumüller2, E. Wink1, A. Moosmann1, D. Grundmann1
1University of Applied Sciences Kaiserslautern, Zweibrücken, Germany 2University Heidelberg, Medical Faculty Mannheim, Mannheim, Germany
Question Stem cell therapies seem to be an appropriate tool for the treatment of a variety of diseases, especially when a substantial cell loss leads to a severe clinical impact. This is the case in most neurodegenerative diseases. Unfortunately, adequate neural stem cell sources are hard to find and alternatives, such as induced programmed stem cells, still have uncalculated risks. Evidence of neurogenesis in the adult human enteric nervous system (ENS) brings up a new perspective. Although these cells do show a decreased plasticity in the aging gut, they are a potential source for autologous neural stem cells. Nestin expressing glial cells are to be found in all parts of the human gut, all over the life span, together with different types of neurones and glial cells.
Methods Neural stem cells are isolated from different parts of the human gastrointestinal tract, and also from different compartments (muscle, submucous and mucous layer) using enzymatical digestion approaches. Generated neurospheres were screened for neuronal, glial, neural stem cell and pluripotency markers, prior and after differentiation. Additional experiments were performed where neurospheres were either co‐cultured with tissue blocks from different parts of the brain, or transplanted into brain slices from the rat.
Results Neurospheres could be generated from all areas investigated and differentiated on glas coverslips. Nestin and Musashi‐1 could be found in all neurospheres. Moreover Sox‐10, Nanog and Oct‐4 could be found in the differentiating cultures. After differentiation tubulin and PGP 9.5 positive neurons could be found. Transplantation experiments showed that cells from the transplanted spheres migrated into the brain slices and differentiated into neurons or glial cells. Neurospheres co‐cultured with tissue blocks from cortex, cerebellum or hippocampus showed a significant higher outgrowth than neurospheres cultured without any brain tissue.
Conclusions In general, the ENS is a suitable tissue for the isolation of neural stem cells in the individual patient and might so be an appropriate source for autologous neural stem cells.
Fig: Human myenteric ganglia. A: PGP 9.5 staining, B: Nestin staining, C: merge

T09‐09A. Isolation of radial glia‐like neural stem cells from fetal and adult mouse brain via selective adhesion to a novel adhesive peptide‐conjugate
T. Kohidi 1, K. Markó1, N. Hádinger1, T. Andrási1, G. Mező2, E. Madarász1
1IEM‐HAS, Budapest, Hungary 2Eötvös Lóránd University, HAS, Budapest, Hungary
Rapid procedure for isolating radial glia‐like cells from fetal, perinatal or adult rodent brain was established by employing the preferential adhesion of neural stem cells to a novel synthetic adhesive polypeptide (AK‐cyclo[RGDfC]). Radial glia‐like (RGl) neural stem/progenitor cells grew readily on the peptide‐covered surfaces under serum‐free culture conditions in the presence of EGF as the only growth factor supplement. Proliferating cells derived either from fetal (E 14.5) forebrain or from different regions of perinatal or adult brain maintained several radial glia‐specific features including nestin and RC2 immunoreactivity, Pax6, Sox2, Blbp and Glast gene expression. Proliferating RGl cells were obtained also from non‐neurogenic zones including the parenchyma of the adult cerebral cortex and dorsal midbrain. Regardless of origin in space and age, all RGl populations expressed positional genes (Pax6, Olig2, Dlx2, Emx2) characteristic to developing forebrain regions. Upon appropriate induction, cells cloned from different fetal or adult brain areas could generate astrocytes, oligodendrocytes and neurons with different phenotypes. All RGl lines gave rise to GABAergic neurons. Significant differences however, were found among the cell lines in generation of glutamatergic and cathecolamine‐synthesizing neurons and in the production of oligodendrocytes. Despite of uniform expression of positional genes, mature derivatives of RGl cells displayed characteristics resembling the region of their origin. Sub‐clones of channelrhodopsin‐expressing RGl cells have been also established in order to investigate stem cell responses and pretended developmental shifts in response to light‐evoked bioelectric stimuli.
T09‐09B. High efficiency transfection and survival rates of oligodendrocyte precursor cells achieved by electroporation
S. Schröder 1, B. Bertram2, A. von Holst1,2
1University Heidelberg, Institute for Anatomie and Cell Biology, Heidelberg, Germany 2Ruhr‐University Bochum, Department of Cell Morphology and Molecular Neurobiology, Bochum, Germany
The central nervous system develops through the controlled proliferation of neural stem cells (NSCs). The symmetrically division of NSCs during forebrain development causes a cell expansion followed by the distinct differentiation of radial glial cells into neurons during neurogenesis. At the end of embryogenesis, oligodendrocytes and astrocytes appear during the process of gliogenesis. Although in the last decades diverse extrinsic and intrinsic factors involved in these developmental processes were identified, our understanding of detailed mechanisms is still comparatively low.
In order to increase the knowledge about the cellular and molecular mechanisms that underlie central nervous system development, transfection of NSCs showed to be a promising method to understand the function of specific genes and proteins. To overcome the well‐known difficulties to transfect cells of the central nervous system, we improved the electroporation conditions to achieve high efficiency transfection and survival rates for embryonic and adult NSCs isolated from mouse forebrain structures. One day after electroporation transfection and viability rates of around 80% were achieved in murine NSCs (Bertram et al. J Neurosci Meth 2012).
Due to our interest in differentiation and maturation of oligodendrocytes we are currently improving the transfection conditions of oligodendrocyte precursor cells (OPCs), since a sufficient protocol for the transfection of OPCs derived from neonatal rat cortices is currently not established. The OPCs are isolated from rat cortical tissue by a shaking protocol and transfected with the 4D‐Nucleofector (Lonza). When we used the same pulse protocol that successfully works on NSCs, oligodendrocytes were sensitive to the transfection process, and many cells died or did not undergo normal differentiation. This demonstrates that other pulses are needed to electroporate OPCs. In cooperation with Lonza we tested several recommended pulses to increase the transfection and survival rates of OPCs. A dramatic increase of the viability rate has already been achieved. In further experiments the pulse protocol will be improved to obtain transfection rates above 20%. By establishing this transfection protocol we want to accomplish transfection and viability rates comparable to murine NSCs.
This improved protocol for the transfection of sensitive OPCs will allow for reliable studies of gene function by over‐expression or knock‐down experiments, and will enhance our understanding of cell biological mechanisms important for oligodendrocyte development.
T09‐10A. Endogenous retinoic acid synthesis contributes to neural stem cell differentiation
B. Orsolits, A. Borsy, E. Madarász, Z. Mészáros, T. Kőhidi, K. Markó, M. Jelitai, E. Welker, Z. Környei
Institute of Experimental Medicine, Budapest, Hungary
Retinoic acid (RA) has been widely used as a potent inducer of neuronal differentiation in various multipotent cell populations (embryonic carcinoma [EC], embryonic and neural stem [ES; NS] cells, iPS cells), in vitro. In vivo, in the central nervous system, RA action has been reported at sites of both embryonic and postnatal neurogenesis, indicating that RA signaling is involved in the regulation of neural stem cell commitment and differentiation throughout life. While NS cells respond to RA delivered to or synthetised within the brain, little is known about i; the potential of NS cells to utilize RA precursor molecules and ii; the rearrangement of the components of retinoid metabolism/signaling during NS cell development. In the presented in vitro study we monitored the changes in the retinoid machinery in differentiating NS cell populations (NE‐4C NS cell line and radial glia like (RGl) cells) derived either from embryonic or adult brain tissues. We show, that early embryonic NS cells are capable to convert both vitamin A and retinyl ester to RA which serves as an auto‐inductive signal to initiate the neuronal differentiation protocol.
T09‐10B. Two neurogenic factors with synergistic action, Cend1 and Neurogenin‐2, drive astrocytic reprogramming towards multipotency and neurogenesis
K. Aravantinou‐Fatorou1, R. Matsas1, B. Berninger2,3, D. Thomaidou 1
1Hellenic Pasteur Institute, Athens, Greece 2University Mainz, Mainz, Germany 3Ludwig‐Maximillians University, Munich, Germany
Recent studies demonstrate that astroglial cells isolated from non‐neurogenic brain regions have the potential to be reprogrammed into functional neurons through forced expression of transcription factors known to instruct neurogenesis. Based on our previous studies on the potential of the neurogenic gene Cend1 in directing neural stem/precursor cells (NSC) to exit the cell cycle and acquire a neuronal phenotype, in parallel with evidence demonstrating activation of Cend1 expression by genes of the neurogenin family, we explored the combined effect of Cend1 and Neurogenin‐2 on their reprogramming potential on postnatal cortical astrocytes. To this end, forced expression of either Cend1, Neurogenin‐2 or both, resulted in an important increase of two morphologically distinct subpopulations of GFAP‐ cells with elongated morphology, that strongly expressed the radial glial marker glutamate transporter GLT‐1. Further characterization revealed that a subpopulation of these cells differentiates towards the neuronal lineage, as they were exhibiting a differentiated neuronal morphology and expressed β‐III tubulin, as well as neuronal subtype‐specific markers, including GABA and TH. Further analysis using long time live cell imaging and BrdU cumulative labeling revealed that the majority of Cend1‐transduced astrocytes undergo 1–2 cell divisions before differentiating to GABAergic neurons, whereas most Ngn2+ astrocytes directly trans‐differentiate giving rise to TH+ neurons. Additionally, only in the double‐transduced cultures, Cend1+/Ngn2+ astrocytes seemed to take a step back forming colonies of small round Glast+/Nestin+ cells, detected 24h following transduction. A day later, these colonies grew as three‐dimensional spheres of high proliferative potential attached to the culture dish. When ‘astrospheres' were isolated and cultured under NSC conditions, they grew as neurospheres and were propagated for over ten passages. Importantly, as soon as ‘astrospheres' were cultured in the absence of growth factors, they differentiated into neurons, astrocytes and oligodendrocytes, implying that they possess neural stem cell properties. At the moment we are performing electrophysiology recordings to assess the functionality of Cend1‐derived neurons in vitro, while studies are in progress to explore the potential role of Cend1 and Ngn2 on astrocytic reprogramming in vivo following cortical brain injury.
Supported FP7 Project 264083 Neurosign, Synergasia‐Noiseplus 09SYΝ‐21‐969 and an EMBO short‐term fellowship.
T09‐11A. The Wnt signaling pathway affects the differentiation potential of neonatal neural stem cells in vitro
J. Kriska 1,2, P. Honsa1,2, D. Dzamba1,2, L. Tumova1, V. Korinek1, M. Anderova1,2
1Institute of Experimental Medicine, Prague, Czech Republic 2Charles University, 2nd Faculty of Medicine, Prague, Czech Republic
Wnt proteins regulate many processes during embryonic development and play an important role in the proliferation/differentiation of neuronal progenitors and in the establishment of neurogenic niches. Here we aimed to clarify the effect of the Wnt signaling pathway on the proliferation and differentiation of neonatal neural stem cells (NSCs) isolated from the subventricular zone (SVZ) and their membrane properties during differentiation in vitro. To suppress the Wnt signaling pathway, the Rosa26‐DN‐TCF4 mouse strain was used, allowing the manipulation of Wnt signaling in the nucleus via the Cre/lox system. Crossbreeding Rosa26‐DN‐TCF4 mice with the Rosa26‐CreERT2 strain enabled Cre‐mediated excision triggered by tamoxifen (4OHT), after which EGFP‐DN‐Tcf4 protein is constitutively produced, thus inhibiting the expression of the target genes driven by the Wnt signaling pathway. In vitro experiments were performed in proliferation medium in order to estimate the impact of Wnt signaling inhibition on the ability of NSCs to form neurospheres or during their differentiation using 4OHT untreated‐ (control) and treated cultures. To follow the fate of NSCs, the expression of neuronal/glial markers was analyzed 8 days after the onset of in vitro differentiation. The patch‐clamp and Ca2+ imaging were employed to characterize the membrane properties of differentiated NSCs. The cells were divided into 3 groups based on their electrophysiological and immunocytochemical properties. GFAP+ cells displayed passive time‐ and voltage‐independent K+ currents, and their average membrane potential (Vm) was −77 mV and input resistance (IR) was 92 MΩ, while DCX+ or MAP2+ cells expressing fast activating outwardly rectifying K+ currents (KA), and delayed outwardly rectifying K+ currents (KDR) were defined by a Vm of −72 mV and high values of IR (1943 MΩ). NG2+ cells with a complex current pattern expressed inwardly rectifying K+ (KIR) currents, in addition to KDR and KA currents, and their Vm was −76 mV and IR was 383 MΩ. The electrophysiological analysis revealed that the inhibition of the Wnt signaling pathway significantly increased the incidence of cells with a complex current pattern, while the number of cells with the marked expression of outwardly rectifying K+ currents declined. Wnt signaling inhibition also resulted in increased KIR and decreased KA current amplitudes. 4OHT‐treated cells were also hyperpolarized when compared to controls. Immunocytochemistry using antibodies against MAP2 and DCX showed that neuronal progenitors in 4OHT‐treated cultures were less developed, having fewer and less branched processes. In summary, our data imply that the canonical Wnt pathway in the neonatal SVZ promotes NSC differentiation into cells with neuronal characteristics. GACR P303/12/0855; P304/12/G069.
T09‐11B. Towards remyelination therapy: derivation of oligodendrocyte precursors from adult bone marrow stromal cells
A.Y.P. Tsui, R. Li, A. Lo, Y. Chan, D.K.Y. Shum
The University of Hong Kong, Hong Kong, Hong Kong
Myelin damage and disorders cause axon to degenerate and result in severe loss of function. With an aim towards remyelination therapy, we attempted to direct differentiation of bone marrow stromal cells (BMSCs, adult rats) along the oligodendroglial lineage in vitro. BMSCs were first maintained in non‐adherent culture to derive spheres of neural progenitors. These BMSC‐derived neural progenitors were then induced to differentiate along oligodendorglial lineage in adherent culture supplemented with β‐heregulin, PDGF‐AA and bFGF. Oligodendrocyte precursors expressing the markers – NG2, Olig2, PDGFRa and Sox10, were detectable within two weeks and can be expanded in culture for more than 3 months with no observable decline in marker expression. To test for myelination capability, BMSC‐derived oligodendrocyte precursor cells (OPCs) in a 2‐week co‐culture with dorsal root ganglion neurons extended myelin basic protein‐positive processes along axons, suggesting maturation into myelinating oligodendrocytes. In vivo myelination by BM‐OPCs was tested by exploitation of unmyelinated axons of retinal ganglion cells of adult rats. Myelin basic protein‐positive processes were also observable along retinal axons by 8 weeks post‐injection. Our findings indicate BMSCs as a possible source of OPCs for remyelination therapy.
T09‐12A. Selective ablation of CNS‐resident microglia disturbs homeostasis within the adult hippocampal neurogenic niche
K. Miller 1, C. Baufeld1, J. Winterer2, S. Prokop1, K.E. Carney3,4, D. Schmitz2,5,6,7, F.L. Heppner1,6
1Charité – Universitätsmedizin Berlin, Department of Neuropathology, Berlin, Germany 2Charité – Universitätsmedizin Berlin, Neuroscience Research Center, Berlin, Germany 3VU University, Center for Neurogenomics and Cognitive Research, Amsterdam, Netherlands 4Université de Bordeaux, Neurocentre Magendie, Iserm Unité 862, Bordeaux, France 5Bernstein Center for Computational Neuroscience Berlin, Berlin, Germany 6Charité – Universitätsmedizin Berlin, Cluster of Excellence ‘NeuroCure', Berlin, Germany 7DZNE – German Center for Neurodegenerative Diseases, Berlin, Germany
Adult neurogenesis plays a critical role in the overall health of, and repair processes occurring within the nervous system. While a great deal of information has been gained about the elements of the neurogenic niche and the factors that mediate neurogenesis, many unanswered questions remain. Specifically, studies suggest that microglia have a significant impact on adult neurogenesis, but no unifying theory exists to explain their exact role in this process. In order to determine if microglia play a pivotal role in adult hippocampal neurogenesis and to examine the mechanisms through which microglia exert their effects on the hippocampal neurogenic niche, we employed a transgenic mouse model that allows for the selective ablation of microglia in the central nervous system (CNS), the CD11b‐herpes simplex virus thymidine kinase (CD11b‐HSVTK) mouse. Using this system, we removed microglia from the adult hippocampal neurogenic niche and evaluated the effects of this manipulation on neurogenesis and hippocampal homeostasis, including immunohistochemical analyses of stem cell survival and proliferation, as well as analyses of neuroblast development, migration and electrophysiological activity. Taken together, these studies help to provide a better understanding of the factors governing adult neurogenesis and further our knowledge about the increasingly diverse role of microglia in the CNS.
T09‐12B. Subventricular glial nodules in the third and lateral ventricles of the human brain contain neural stem cells
M. van Strien 1, S. van den Berge1, J. Sluijs1, E. Hol1,2
1Netherlands Institute for Neuroscience, Astrocyte Biology & Neurodegeneration, Amsterdam, Netherlands 2Swammerdam Institute for Life Sciences, Center for Neuroscience, University of Amsterdam, Amsterdam, Netherlands
Subventricular glial nodules (SGNs) are distinct hypercellular structures in the human ventricular system that protrude into the ventricles and are denuded of the overlying ependyma. They are present in brains of individuals without a neurological disorder and have been linked to various diseases. The aetiology and cellular identity of SGNs is unclear. The current assumption is that ependymal damage, caused by viral infections may result in reactive gliosis in the subventricular zone (SVZ), leading to SGNs. However, as the SVZ is currently recognized as one of the neurogenic niches in the adult human brain, and SVZ astrocytes have been identified as neural stem cells (NSCs), the assumption of SGNs being reactive astrocytes has to be reconsidered. Therefore, we have immunophenotyped the cell types present in these structures in the brains of individuals of various ages. In addition, we assessed nodule incidence in the SVZ in cases infected with HIV, where cells in the SVZ are likely to be to be affected. We found SGNs in almost 90% of all cases, independent of age or HIV infection. We determined that cells in the SGNs express adult NSC markers, rather than markers for reactive astrocytes. We hypothesized that direct contact of the NSCs with cerebrospinal fluid (CSF) may induce nodule formation. Indeed, we could show that human ventricular CSF stimulated the proliferation of human NSCs in culture. From our data, we conclude that SGNs most likely are formed upon ependymal damage in response to exposure to CSF and represent local expansions of the SVZ.
T09‐13A. Influence of demyelination and aging on adult oligodendrocyte precursor cells RNA profil: towards an identification of new molecular cues for myelin repair
S. Moyon 1, M.‐S. Aigrot1, L. Dauphinot1, M.‐C. Potier1, M. Trotter2, J. Huang3, R. Franklin2, C. Lubetzki1
1CRICM, Paris, France 2University of Cambridge, Cambridge, United Kingdom 3Georgetown University, Washington, United States
Multiple sclerosis (MS) is a chronic demyelinating disease of the central nervous system (CNS). Spontaneous remyelination can occur in MS, but it is most often insufficient, mainly linked to defect of oligodendrocyte progenitor cells (OPCs). In experimental murine model of demyelination, the repair capacity is robust and sufficient, even if it might decrease with aging. One major therapeutic goal in MS is to favour endogenous myelin repair, to prevent neurodegeneration and disability progression. Using a transcriptomic approach on pure populations of OPCs, we try to identify mechanisms specifically involved in OPCs activation. We set up a method to isolate a purified population of OPCs, by fluorescent‐activated cell sorting from PDGFαR::GFP mouse brains. We created the gene expression profile of neonatal OPCs, adult OPCs and adult oligodendrocytes (OLs) in control conditions and of adult OPCs in cuprizone‐induced demyelinating conditions. We analyzed the transcriptomic profile of adult OPCs, neo‐natal OPCs and OLs, and identified more than 1000 differentially expressed genes. After demyelination, we identified more than 2000 adult‐OPC‐specific genes that are either up‐ or down‐regulated compared to control conditions. Preliminary analysis suggests that adult OPCs are more “mature” than neonatal OPCs and partially revert to a more immature profile after demyelination. Bio‐informatic analysis is ongoing, with the perspective of identifying key candidates for myelin repair capacity, then develop functional experiments to validate their involvement in remyelination. In parallel, we plan to compare the transcriptomic profile of adult OPCS from aged versus young mice, to get insight into age‐related decrease of repair efficacy.
T09‐13B. Neural stem cell properties of an astrocyte subpopulation sorted by sedimentation field‐flow fractionation
N. Vedrenne 1, V. Sarrazy1, S. Battu2, N. Bordeau1, F. Billet1, V. Coronas3, A. Desmoulière1
1University of Limoges, EA 6309, Limoges, France 2University of Limoges, EA 3842, Limoges, France 3University of Poitiers, CNRS FRE 3511, Poitiers, France
Question: After damage, astrocytes are activated and exert specific roles for central nervous system (CNS) repair. Among these, astrocytes within the injury site may represent a novel source of cells with stem cell potential. In this study, we used sedimentation field‐flow fractionation (SdFFF) method to rapidly sort well preserved astrocyte subpopulations and we identified stem cell properties within one of these subpopulations.
Methods: Cells were collected from newborn rat cortex, separated mechanically and subjected to SdFFF. Cell fractions obtained were analyzed by immunocytofluorescence using antibodies against specific epitopes: glial fibrillary acidic protein (GFAP), O4, β III tubulin, and CD68, labelling respectively astrocytes, oligodendrocytes, neurons, and microglial cells. Then, proteomic analysis was performed on astrocyte subpopulations and their behaviour in culture was analyzed, particularly under culture conditions usually allowing neurosphere development.
Results: Three main fractions (F1, F2, and F3) were isolated and compared with the total eluted population (TP). After one week in vitro, TP and F2 derived cell cultures contained respectively 74.5% and 11.9% of GFAP expressing astrocytes while this percentage was extremely high in F1 and F3 derived cell cultures (95.6% and 98.0% respectively). F3 derived cells displayed higher migratory capacities than those of F1. Proteomic analysis of F1 and F3 derived cells indicated a high expression of vimentin in F3 derived cells. In the presence of epidermal growth factor (EGF), F3 derived cells formed neurospheres containing cells expressing GFAP and nestin (Figure) that, when placed in appropriate conditions, were able to generate the three major cell types of the CNS (neurons, astrocytes and oligendrocytes). Moreover, colony‐forming cell assay in a collagen‐containing semi‐solid matrix allowed in the presence of EGF the formation of large colonies. In addition, 7 days after cortical lesion in adult rats, cells presenting similar stem properties were obtained after SdFFF cell sorting.
Conclusions. Our study demonstrates that SdFFF enables the isolation of almost pure astrocyte subpopulations after one week in vitro. In addition, after SdFFF cell sorting, we isolated an astrocyte subpopulation expressing vimentin and displaying the self‐renewal and multipotency properties of neural stem cells. Our data support that SdFFF is an efficient tool to explore the different astrocyte subpopulations of the CNS, particularly those involved in CNS repair.

T09‐14A. Characterization of neural precursors derived from mouse iPS cells: in vitro and in vivo after transplantation into the central nervous system
S. Mozafari 1, A. Marteyn1, C. Laterza2, C. Deboux1, G. Martino 2, A. Baron‐Van Evercooren 1
1Université Pierre et Marie Curie, Institut du Cerveau et de la Moelle épinière, Paris, France 2San Raffaele Scientific Institute, Institute of Experimental Neurology–DIBIT 2, Division of Neuroscience, Milan, Italy
Experimental studies have shown that loss of myelin results in axonal loss and disability. So, finding of an expandable and autologous source of myelin‐forming cells to enhance remyelination is required. Induced pluripotent stem cell‐derived neural progenitor cells (iPSC‐NPCs) have been developed recently. The remyelination potential and safety of these cells still remained to be well‐addressed. The main goal of this study is to characterize mouse iPS‐NPCs in vitro and in vivo after transplantation.
We used embryonic mouse neural progenitor cells (mNPCs) as control and characterized mouse iPS‐NPCs for their expression of major markers of immature or mature cells by immunostaining. RT‐PCR was performed to analyze expression of Nestin, Olig2, β3‐tubulin, Olig1, Sox10, and NKX2.2 mRNAs. Furthermore, to investigate the fate of these cells in vivo, we injected the lysolecithin to induce focal demyelination in the spinal cord of adult nude or Shiverer mice. We transplanted cells in the lesion site 2 days after demyelination. Animals were sacrificed 2 days, 1, 2, 6 and 8 weeks post transplantation to assess survival and differentiation of the grafted cells.
Our preliminary results showed that miPS‐NPCs similar to mNPCs were Nestin+, Ki67+, Olig2+, β3‐tubulin+ but O4‐ and MAP5‐. miPS‐NPCs, were more proliferative than mNPC. Moreover, miPS‐NPC expressed all mRNA transcripts except Sox10. A first line of miPS‐NPCs, was transplanted in the newborn Shiverer:Rag forebrain or the demyelinated spinal cord of immunosuppressed Shiverer mice with a constant failure in terms of cell survival beyond 2 days of transplantation, highlighting the fragility of some of the reprogrammed cell lines. Cells of a second line of miPS‐NPCs were transplanted in nude mice to be sacrificed at 1, 2 and 6 weeks. An additional serie of transplantation was performed in the demyelinated Shi:Rag spinal cord. Data at 1 and 2 weeks indicate excellent survival and integration of the miPS‐NPC at both time points. Some of the grafted cells expressed GFAP, Olig2 and Ki67 (few only) and so far, no tumors were observed at these time‐points. Immunohistochemistry with other markers and analysis of 6 weeks post transplantation animals, are in progress.
Our preliminary results show that although miPS‐NPCs have very similar immature phenotype of mNPCs in vitro, they may have different survival capacities in vivo. Future studies will reveal whether transplanted cells which showed short‐term survival after grafting are capable of differentiation into myelin‐forming cells.
Sponsored by MSIF and ELA.
T09‐14B. Adult spinal cord and adult DRG sphere‐forming cells exhibit distinct but promising features for CNS remyelination
M. Vidal, M. Maniglier, V. Zujovic, C. Deboux, A. Baron‐Van Evercooren
CRICM UMRS975 UPMC, Paris, France
We recently demonstrated that boundary cap (BC) cells, a transient cell population located at the boundary between the CNS and PNS, are embryonic stem cells with therapeutic potential of interest for CNS remyelination. However, like any embryonic sources, they raise ethical concerns about the use of human embryos. This led to seek for alternative adult stem cells sources. Previous studies suggested the presence of stem cells in adult dorsal root ganglia (DRG). However these putative stem cells have only been partly characterized in vitro and their therapeutic potential for CNS repair has not been addressed. We cultured adult mouse DRG cells in sphere‐forming conditions. In parallel, we derived spheres from adult spinal cord of the same animals and compared their properties. This last tissue contains well‐described stem/progenitor cells whose repair capacities have already been proven for spinal cord injury but not for primary demyelination. In vitro, regardless of their origins, all tissues generated self‐renewable spheres up to 10 passages. RT‐PCR and immunocytochemistry revealed that sphere‐forming cells actively proliferate and express neural stem cells markers. DRG spheres were multipotent: as they differentiated into Schwann cells, neurons and myofibroblasts, whereas spinal cord spheres differentiated into astrocytes, neurons and oligodendrocytes. We then compared the integration, differentiation potential and remyelination capacity of primary sphere‐forming cells isolated from actin‐GFP transgenic mice after their engraftment in two distinct environments: the adult nude LPC‐demyelinated spinal cord and the developing newborn Shiverer forebrain. Both cell types survived but with distinct integration and differentiation patterns. In the MBP deficient mutant, spinal cord cells exhibit a substantial tropism toward white matter tracts and differentiated mainly into myelinating oligodendrocytes while DRG cells were largely distributed in the parenchyma in close association with blood vessels and differentiated in vascular smooth muscle cells. Surprisingly, after focal demyelination, in the adult spinal cord, DRG cells migrated extensively towards the lesion and differentiated into remyelinating Schwann cells whereas adult spinal cord cells migrated less efficiently, and gave rise to oligodendrocytes and a large population of astrocytes. Our study reveals that both cell types present different but promising potential for CNS remyelination.
Funding by ELA Foudation, DIM NeRf, ARSEP Foundation.

T09‐15A. Regulation of ischemia‐induced progenitor cell proliferation in the adult mouse hippocampus by the ERK/MAPK effector ribosomal S6 kinase
K. Karelina, D. Alzate‐Correa, K. Obrietan
Ohio State University, Columbus, United States
Ischemia‐induced progenitor cell proliferation is a prominent example of the adult mammalian brain's ability to regenerate injured tissue resulting from pathophysiological processes. A key locus of regeneration is the neurogenic niche located in the subgranular zone of the dentate gyrus (SGZ). Within the SGZ, radial glia‐like progenitor cells generate neural precursor cells which migrate into the granule cell layer (GCL) of the dentate gyrus, mature, and integrate into the hippocampal synaptic network. In order to better understand and ultimately exploit the cell signaling mechanisms that regulate ischemia‐induced proliferation in the SGZ, we tested the role of the p42/44 mitogen‐activated protein kinase (MAPK) cascade effector ribosomal S6 kinase (RSK) in this process. Using the endothelin‐1 ischemia model in C57Bl/6 mice, we report that intrahippocampal cerebral ischemia triggered RSK activation in SGZ progenitor cells. Further, microinjection of a RSK inhibitor significantly reduced ischemia‐induced SGZ progenitor cell proliferation. Using the neurosphere assay, we also show that SGZ‐ and subventricular zone (SVZ)‐derived adult neural stem cells (NSC) exhibit a significant reduction in proliferation in the presence of RSK and MAPK inhibitors. Taken together, these data indicate that RSK functions as a cell‐autonomous regulator of ischemia‐induced progenitor cell proliferation.
T09‐15B. Cx3cr1‐expressing cells analysis reveals distinct features of microglia residing within the postnatal subventricular zone/rostral migratory stream
A. Xavier 1, J. Menezes1, M. Nedergaard1,2
1Federal University of Rio de Janeiro, Rio de Janeiro, Brazil 2University of Rochester Medical Center, Center for Translational Neuromedicine, Rochester, United States
This study sought to ascertain microglia characteristics within subventricular zone (SVZ), the major postnatal neurogenic niche, where interneuron precursors are continuously generated and migrate through the rostral migratory stream (RMS) towards the olfactory bulb. Sections obtained from transgenic mice expressing the enhanced green fluorescent protein (eGFP) under the chemokine receptor Cx3cr1 promoter at postnatal ages P1, P7, P14 and P60 were immunostained for glial and neuronal markers. Neuroblasts were also labeled with intraperitoneal injections of the thymidine analog bromodeoxyuridine (BrdU; 100mg/kg),1 hour and 5–7 days prior to histological analysis (n = 3 animals). Confocal imaging analysis depicts microglia as a distinct population within the SVZ/RMS, displaying typical amoeboid morphology during neonatal periods, but at later stages the immature/migratory morphology differs from cortical ramified cells. Despite displaying distinct morphology, these cells do not label for the dendritic cell marker CD11c. Microglia density differs on newborn mice: P1 – 35.2 ± 4.3; 19.3 ± 1.3; 20.0 ± 3.2 and 4.4 ± 0.6 cells/mm3 for SVZ, RMS, OB and cortex, respectively. Once established the microglia population remains stable within the SVZ/RMS/OB: P60 – 21.7 ± 2.8; 23.7 ± 2.2; 24.5 ± 3.1 and increases in the cortex: 19.9 ± 1.6 cells/mm3; mean ± SEM; p < 0.05; One‐way analysis of variance/Bonferroni's Multiple Comparison Test). Microglia appose to BLBP+/GFAP+ processes, indicating that they could establish cellular/molecular interactions with SVZ stem cell lineage. Despite the close distribution, neuroblasts do not seem to be phagocytized along the SVZ/RMS, as we do not observe colocalization of CX3CR1‐eGFP+/TREM2+ with DCX+/BrdU+. In contrast, phagocytic activity occurs at glomerular layer, where neurons are continuously replaced in adult rodents. There, CX3CR1‐eGFP+/TREM2+ cells are closely related to neuroblasts (DCX+/BrdU+ cells). ELISA analysis of media collected from cultures obtained from P1, P7 and P30 mice reveals that the anti‐inflammatory cytokine interleukin (IL) 10 and its activator IL6 are present in the SVZ at all ages, differing from cortical cells that only secrete these cytokines at P7. The presence of CX3CR1‐eGFP+ cells with distinct characteristics under physiological conditions in the SVZ/RMS could reflect intrinsic mechanisms of this neurogenic niche controlling its macrophage population, that should be addressed for a consistent understanding of adult neurogenesis.
T09‐16A. Oligodendrogliogenic and neurogenic adult subependymal zone neural stem cells constitute distinct lineages and exhibit differential responsiveness to Wnt signaling
F. Ortega 1, S. Gascon2, G. Masserdotti2, A. Deshpande2, C. Simon2, J. Fischer3, L. Dimou2, D. Chichung Lie4, T. Schroeder3, B. Berninger1
1Johannes Gutenberg University Mainz, Mainz, Germany 2Ludwig‐Maximilians‐Universität, Physiological Genomics, München, Germany 3Institute of Stem Cell Research, Helmholtz Zentrum München, ISF, München, Germany 4University Erlangen‐Nürnberg, Erlangen, Erlangen, Germany
The adult mouse subependymal zone (SEZ) harbors adult neural stem cells (aNSCs)that give rise to neuronal and oligodendroglial progeny. However it is not known whether the same aNSC can give rise to neuronal and oligodendroglial progeny or whether these distinct progenies constitute entirely separate lineages. Continuous live imaging and single cell tracking of aNSCs and their progeny isolated from the mouse SEZ revealed that aNSCs exclusively generate oligodendroglia or neurons, but never both within a single lineage. Moreover, activation of canonical Wnt signaling selectively stimulated proliferation within the oligodendrogliogenic lineage, resulting in a massive increase in oligodendrogliogenesis without changing lineage choice or proliferation within neurogenic clones. In vivo activation or inhibition of canonical Wnt signaling respectively increased or decreased the number of Olig2 and PDGFR‐a positive cells, suggesting that this pathway contributes to the fine tuning of oligodendrogenesis in the adult SEZ.
T09‐16B. Induced Neural Progenitors from somatic cells by a Novel Combination of Defined Factors: New strategy for the treatment of neurodegenerative diseases
C. Tian, K. Ma, Y. Wang, J. Zheng
University of Nebraska Medical Center, Omaha, United States
Recent reports have demonstrated that somatic cells can be directly converted to other differentiated cell types through ectopic expression of sets of transcription factors, directly avoiding the transition through a pluripotent state. Using fibroblasts and astrocytes derived from E/Nestin:EGFP transgenic mice as staring cells, we successfully screened a novel combination of five or three transcription factors which directly converted somatic cells into neural progenitor‐like cells (iNPCs). Here we demonstrated that the iNPCs not only possess NPC‐specific marker genes, but also have qualitites of primary brain‐derived NPCs (WT‐NPCs), including tripotent differentiation potential, mature neuron differentiation capability and synapse formation. Moreover, the mature neurons derived from iNPCs exhibit significant physiological properties, such as potassium channel activity and generation of action potential‐like spikes. Importantly, besides survival, the transplanted iNPCs exhibit the migratory property responding to inflammatory stimulation in vivo. These results suggest that directly reprogrammed iNPCs closely resemble WT‐NPCs, which may suggest an alternative strategy to overcome the restricted proliferative and lineage potential of induced neurons (iNCs) and broaden applications of cell therapy in the treatment of neurodegenerative disorders.
POSTER TOPIC 10 Neuroimmunology and neuroinflammation
T10‐01A. Astrocyte‐Tissue inhibitor of metalloproteinases‐1: The TIMP‐ed balance of neuroinflammation: Relevance to HIV‐1‐associated neurocognitive disorders
A. Ghorpade, J.A. Fields, C. Chao, K. Borgmann, L. Tang
University of North Texas, Health Science Center, Fort Worth, United States
The pathogenesis of neurodegenerative disorders, including Human Immunodeficiency Virus (HIV)−1 associated neurocognitive disorders (HAND), is exacerbated by an imbalance between metalloproteinases (MMPs) and their inhibitors, tissue inhibitors of metalloproteinases (TIMPs). As the TIMPs exhibit diverse non‐classical functions including anti‐apoptotic effects, the induction of TIMP‐1 in neuroinflammatory conditions likely serves multiple roles in addition to modulating MMP activity. Our work demonstrates differential TIMP‐1 expression in acute versus chronic activation of astrocytes and HIV‐1 associated dementia (HAD) brain tissues. The TIMP‐1 promoter harbors five consensus CCAAT boxes. CCAAT enhancer binding protein (C/EBP)b levels were elevated in brain specimens from HIV‐1 patients and this transcription factor regulated astrocyte TIMP‐1 expression. HIV‐relevant stimuli increased C/EBPb expression in human astrocytes and localized the factor to the nucleus. Overexpressing C/EBPb in astrocytes increased TIMP‐1 promoter activity, mRNA and protein expression, while knockdown of C/EBPb decreased TIMP‐1 mRNA and protein expression. ERK1/2 activation is critical for IL‐1b‐mediated astrocyte TIMP‐1 expression and p38K activation contributes to IL‐1b‐induced astrocyte TIMP‐1 and C/EBPb expression. These data suggest that ERK1/2 signals downstream of C/EBPb to facilitate IL‐1b‐induced astrocyte TIMP‐1 expression. The role of astrocyte‐TIMP‐1 as a neurotrophic factor was examined. Interestingly, cotreatment with TIMP‐1 protected neurons from apoptosis and reversed neuronal morphological changes induced by staurosporine (STS) and HIV‐1ADA virus. Further, the anti‐apoptotic effect was specific to TIMP‐1 and partially independent of MMP‐inhibition. Additionally, TIMP‐1 modulated the Bcl‐2 family of proteins and inhibited opening of mitochondrial permeability transition pores induced by HIV‐1 or STS. Together, these findings describe a novel function, regulatory mechanism and direct role of astrocyte‐TIMP‐1 in neuroprotection suggesting its therapeutic potential in HAD and possibly in other neurodegenerative diseases.
T10‐01B. Nogo‐66/NgR signal is involved in neuroinflammation through regulating microglia inflammatory mediators expression
J. Yan1, Z. Zhang1, X. Zhou2, H. Liao 1
1China Pharmaceutical University, New Drug Screening Center, Nanjing, China 2Saarland University, Homburg, Germany
It is well accepted that the functional repair of central neural system (CNS) following injury is severely interrupted by the presence of axon regeneration inhibitors, including Nogo A. Nogo‐66 is a 66‐amino‐acid‐residue luminal/extracellular domain of Nogo‐A. Accumulating reports have demonstrated that interrupting the function of Nogo‐A/NgR signal was beneficial for regeneration of the neurite and for the functional improvement of injured CNS. Mounting evidence and our pervious study has also confirmed that the axon outgrowth inhibition receptor NgR was also expressed on microglia, and regulated cell adhesion and migration behavior in vitro. However, Microglia has been proposed to play a pivotal role in the neuroinflammation through expressing a range of proinflammatory enzymes and proinflammatory cytokines under pathological stimulus. These findings initiate the possibility of Nogo/NgR signal on mediating neural inflammation processes during CNS injury beside neurite outgrowth inhibition. In the present study, we found that Nogo‐P4, a 25‐aa core inhibitory peptide sequence of Nogo‐66, was able to induce microglia expressing COX‐2, iNOS, as well as releasing proinflammatory cytokines including IL‐1β, NO and PGE2, which could been reversed by NEP1–40 or PI‐PLC treatment. Microglia stimulated with Nogo‐P4 increased the phosphorylation lever of Signal transducer and activator of transcription 3 (Stat3). The activation of Stat3 further mediated the promotion of Nogo‐P4 on microglia expression proinflammatory factors. Furthermore, administration NEP1–40 was capable to attenuate the inflammation response in a mouse spinal cord compression injury model by reducing the expression of several proinflammatory mediators, as well as attenuating the activation of Stat3. Taken together, our results demonstrated, for the first time, that Nogo‐66 may directly take part in CNS inflammation process by influencing microglia expressing proinflammatory factors, which was related to NgR /Stat3 signal pathway. These results imply that the interaction of Nogo‐66 with NgR expressed on microglia may participate in diverse CNS diseases related to neural inflammation, including trauma, stroke, and neurodegeneration diseases, etc.
T10‐02A. CCL‐1 in the spinal cord contributes to neuropathic pain induced by nerve injury
N. Akimoto 1, K. Honda2, D. Uta3, H. Furue3, M. Kido4, K. Imoto3, Y. Takano2, M. Noda1
1Kyushu University, Pathophysiology, Fukuoka, Japan 2Fykuoka University, Physiology and Pharmacology, Fukuoka, Japan 3 National Institute for Physiological Sciences, Okazaki, Japan 4Kyushu University, Molecular Cell Biology and Oral Anatomy, Fukuoka, Japan
Cytokines such as interleukins are known to be involved in the development of neuropathic pain through activation of neuroglia. However the role of chemokine (C‐C motif) ligand 1 (CCL‐1), a well‐characterized chemokine secreted by activated T cells, in the nociceptive transmission remains unclear. Here we examined actions of CCL‐1 on synaptic transmission, glial cells and cytokines in the spinal dorsal horn, and found that CCL‐1 is one of the key mediators involved in the development of neuropathic pain. After a unilateral and partial sciatic nerve ligation (PSNL), expression of CCL‐1 mRNA was transiently detected from the ipsilateral dorsal root ganglion (DRG) and spinal cord, and CCL‐1 receptor CCR‐8 protein was expressed in the superficial dorsal horn. Recombinant CCL‐1 injected intrathecally to naïve mice induced hyperalgesia which was prevented by the supplemental addition of N‐methyl‐D‐aspartate (NMDA) receptor antagonist, MK‐801. Patch‐clamp recordings from spinal cord slices revealed that CCL‐1 enhanced excitatory synaptic transmission in the substantia gelatinosa (lamina II). We showed that CCL‐1 induced phosphorylation of NMDA subunit, NR1 and NR2B, and increased expression of glial cells activation markers (Iba‐1, CD11b and GFAP) and cytokines (IL‐1beta, TNF‐alpha and IL‐6) in the spinal cord. The tactile allodynia induced by nerve ligation was attenuated by prophylactic and chronic administration of neutralizing antibody against CCL‐1 and in CCR‐8 knock‐down mice. Our results indicate that CCL‐1 is one of the key molecules in pathogenesis and CCL‐1/CCR‐8 signaling system can be a potential target for drug development in the treatment for neuropathic pain.
T10‐02B. FGF9 inhibits remyelination via an off target effect on astrocytes
M. Lindner 1, A. Arthur1, C. Elliott1, A. Williams2, E. Meinl3, H. Lassmann4, C. Linington1
1University of Glasgow, Glasgow, United Kingdom 2University of Edinburgh, MRC Centre for Inflammation Research, Edinburgh, United Kingdom 3Ludwig Maximillian University, Munich, Germany 4University of Vienna, Brain Research Institute, Vienna, Austria
Demyelination plays a central role in the pathophysiology of multiple sclerosis (MS) not only because it disrupts nerve conduction, but also because of effects that compromise axonal survival. There is a growing consensus that any effective treatment for MS must include a component that restores or enhances remyelination by endogenous oligodendrocyte progenitor cells (OPC). However achieving this goal requires a far better understanding of why remyelination fails in the first place. We now report that FGF9 is selectively up regulated in demyelinating MS lesions and acts to inhibit (re)myelination in vitro. To explore the mechanism involved we compared the activity of FGF9 to that of FGF2, another member of the FGF family with well characterised effects on OPC differentiation and myelination. FGF2 acts directly on OPC to stimulate proliferation, while at the same restricting their differentiation into mature oligodendrocytes. In contrast, FGF9 is neither an OPC mitogen, nor is it able to inhibit OPC differentiation directly. However, OPC differentiation and myelination in myelinating cultures were inhibited by supernatants harvested from FGF9 treated astrocytes, even in the presence of an excess of FGF9 neutralising antibody. Microarray studies were performed to identify astrocyte derived factors responsible for this inhibitory effect. This approach identified several potential candidates that were significantly up regulated by FGF9 in astrocytes. These included LIF, a member of the GP130/IL6 cytokine family that is generally described as having pro‐myelinating effects, although our own studies indicate when present in excess relative to other GP130/IL6 family members it will inhibit myelination in vitro. Our experiments demonstrate that FGF9‐mediated signal transduction in astrocytes disrupts their ability to maintain a growth factor milieu that can support myelination.
T10‐03A. Impact of microglia‐mediated inflammation on hypothalamic homeostasis
C. Baufeld, K. Miller, F.L. Heppner
Charité – Universitätsmedizin Berlin, Department of Neuropathology, Berlin, Germany
Diets high in fat are known to cause inflammation in the periphery as well as the central nervous system. In peripheral adipose tissue, it has been shown that inflammation is primarily mediated by macrophages that are recruited to the tissue. Similarly, a recent study demonstrated reactive microgliosis in the hypothalamus of mice fed a high‐fat diet. In the CNS, however, the relevance of microglial activation in response to a high fat diet remains unclear. We aim to determine if microglia activation occurring in response to overnutrition is a cause or consequence of dysregulation of energy homeostasis and obesity.
To determine if the absence of microglia (and therefore microglia‐mediated inflammation) may influence the metabolic response to a high fat diet, we used a transgenic mouse model, the CD11b‐HSVTK mouse (TK), which allows for an inducible, specific ablation of microglia in response to treatment with the nucleoside analog, gancilovir (gcv). Mice were treated with gcv for 4 weeks during which time they were maintained on a diet high in fat (60%) or a respective control diet. Body weight, food intake, locomotor activity and energy expenditure were measured and compared to wildtype mice.
Analyses revealed alterations in the metabolic phenotype of mice fed both HFD and control diet in the absence of microglia cells. Our findings point towards a physiological role of microglia in energy homeostasis, the study of which will be the focus of further experiments.
T10‐03B. Astrocyte‐targeted production of IL‐10 modifies glial phenotype
G. Llovera 1, B. Almolda1, I. Barrera1, C. de Labra1, B. Ferrer1, M.J. Hofer2, I.L. Campbell2, J. Hidalgo1, B. González1, B. Castellano1
1Universitat Autonoma de Barcelona, Institute of Neurosciences, Department of Cell Biology, Physiology and Immunology, Barcelona, Spain 2School of Molecular Bioscience, University of Sydney, Sydney, Australia
Interleukin‐10 (IL‐10), a cytokine classically related with anti‐inflammatory and protective functions in the central nervous system (CNS), has been reported to be produced by astrocytes and microglia in different neurodegenerative and neuroinflammatory conditions. In order to study the specific role of IL‐10 in the CNS, we have created a new transgenic mouse with astrocyte‐targeted production of IL‐10 (GFAP‐IL10Tg). The aim of this study was to evaluate if production of this cytokine in the CNS has any effect on the phenotype of glial cells. For this purpose, coronal cryostat free‐floating sections from the brain of both adult transgenic mice and their corresponding wild‐type (Wt) littermates, were processed for the study of astrocytes using GFAP immunohistochemistry and microglia using antibodies against Iba1 and several markers commonly related to the activated phenotype of these microglial cells, such as CD16/32 (Fc receptor), F4/80, CD11b, CD206, CD150 and MHC‐II. Our results showed that astrocyte‐targeted production of IL‐10 induces an increase in the area covered by GFAP immunolabelling. Microglial cells in these GFAP‐IL10Tg animals displayed also morphological changes in specific areas of the brain, including the hippocampus, cortex and cerebellum, characterized by coarser processes and a notable enlargement of the cell body. Distinctively, in the hippocampus, microglial cells adopted an elongated morphology parallel to the dendrites of pyramidal neurons. In addition, in the aforementioned areas, microglia exhibited a statistically significant increase in Iba1, CD16/32, F4/80 and CD11b markers and “de novo” expression of CD150, whereas no detectable levels of either CD206 or MHC‐II was found. In conclusion, these results indicate that in specific areas, glial cells are influenced by the presence of astrocyte‐targeted IL‐10. Further studies are necessary to evaluate whether after injury these transgenic animals will present changes in the microglial and astrocyte activation that can influence the evolution of the lesion.
This work was supported by Ministerio de Ciencia e Innovación (BFU2008‐04407/BFI) and (BFU2011‐27400).
T10‐04A. Therapeutic Efficacy of Suppressing the JAK/STAT Pathway in Multiple Models of Neuroinflammation
E. Benveniste
University of Alabama at Birmingham, Birmingham, United States
Increasing evidence indicates that pathogenic T helper cells and myeloid cells, including dendritic cells, macrophages and microglia, are involved in the pathogenesis of Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis, an animal model of Multiple Sclerosis. The Janus Kinase/Signal Transducer and Activator of Transcription (JAK/STAT) pathway is utilized by cytokines for signaling, and is critical for development, regulation and termination of immune responses. Dysregulation of the JAK/STAT pathway has pathological implications, especially in autoimmune and neuroinflammatory diseases. Many of the key immunoregulatory cytokines involved in Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis, including IL‐6, IL‐12, IL‐23, IFN‐γ and GM‐CSF, use the JAK/STAT pathway to induce biological responses. As such, targeting JAKs has implications for treating autoimmune inflammation of the brain. In this study, we use a novel JAK1/2 inhibitor, AZD1480, to investigate the therapeutic potential of inhibiting the JAK/STAT pathway in five different models of Experimental Autoimmune Encephalomyelitis. AZD1480 treatment effectively inhibits disease severity in MOG‐induced classical and atypical Experimental Autoimmune Encephalomyelitis models by preventing entry of immune cells into the brain, suppressing differentiation of Th1 and Th17 cells, deactivating myeloid cells such as macrophages and dendritic cells, inhibiting STAT activation in the brain, and reducing expression of pro‐inflammatory cytokines and chemokines. Furthermore, treatment of SJL/J mice with AZD1480 delays disease onset of PLP‐induced relapsing‐remitting disease, and reduces clinical severity. Suppression of the JAK/STAT pathway by AZD1480 treatment was also effective in reducing ongoing paralysis induced by adoptive transfer of either pathogenic Th1 or Th17 cells. In vivo AZD1480 treatment impairs both the priming and expansion of T‐cells in Experimental Autoimmune Encephalomyelitis development. Immune modulation induced by AZD1480 involves attenuation of antigen‐presentation function by downregulation of MHC class II and CD40 expression, as well as a direct inhibitory effect on Th1 cell polarization. These results collectively indicate that inhibition of the JAK/STAT pathway has striking clinical efficacy in multiple pre‐clinical models of Multiple Sclerosis, and suggest the feasibility of the JAK/STAT pathway as a target for immunomodulatory therapy in Multiple Sclerosis.
T10‐04B. Nutritional n‐3 polyunsaturated fatty acid deficiency impairs microglial cell activity in the developing brain
C. Madore, C. Portal, A. Aubert, A. Sere, A. Nadjar, C. Joffre, S. Layé
INRA, Bordeaux, France
Microglial cells are an integral part of central nervous system (CNS) networks. Brain‐resident macrophages, microglial cells were considered ‘‘resting,'' but it has recently become evident that they actively sense neuronal microenvironment with their motile and ramified processes. They develop specific activities (cytotoxic, neuroprotective or phagocytic) depending on brain molecular context. For example, they contribute directly to the development of neuronal networks by eliminating or maintaining synapses in an activity‐dependent manner. Morphofunctional plasticity of microglial cells is finely tuned in order to keep brain homeostasis and to ensure such developmental activity. Like macrophages at the periphery, microglial cells indeed adopt M1 or M2 phenotypes, distinguishable through their pattern of cytokine expression and of specific cell surface markers, a M1 phenotype being more pro‐inflammatory while a M2 phenotype is more related to phagocytosis.
Linoleic acid (LA, 12:2n‐6) and alpha‐linolenic acid (ALA, 18:3n‐3) are essential fatty acids that cannot be synthesized de novo by mammals and have to be provided through the diet. They are precursors of arachidonic acid (AA; 20:4n‐6) and docosahexaenoic acid (DHA; 22:6n‐3) respectively which are key structural components of brain cell membrane phospholipids and precursors of bioactive lipid messengers involved in the regulation of inflammation and microglial activity. Most AA and DHA accumulate in the brain during the perinatal period via placenta and milk. We previously demonstrated that depleting the diet in n‐3 PUFAs from the first day of gestation alters some neuronal functions of the offspring at adulthood. We here hypothesized that this is due to an alteration of microglial mrophofunctional activity in the developing brain.
To test this hypothesis, mice were submitted to a diet deficient or not in ALA throughout gestation and lactation. Microglial morphology, phenotypes and functions were determined in the brain of pups at post‐natal day 21 (PND21). We show that DHA levels is decreased in membrane phospholipids of microglia. In addition, microglial cells from mice fed with an ALA deficient diet present a drastic increase of the CD36 marker expression combined to a dysregulated cytokine/chemokine expression profile and an altered phagocytic activity. All together, our results show that dietary n‐3 PUFAs deficiency is likely to impair brain innate immune system activity at PND21. The outcome of such microglial impairment on brain function is discussed.
T10‐05A. Immune complexes of beta amyloid with specific monoclonal antibodies induce neuronal loss via microglial activation
V. Borutaite 1, R. Morkuniene1, A. Zvirbliene2, I. Dalgediene2, P. Cizas1, S. Jankeviciute1, G. Valincius3
1Lithuanian University of Health Sciences, Kaunas, Lithuania 2Vilnius University, Institute of Biotechnology, Vilnius, Lithuania 3Vilnius University, Institute of Biochemistry, Vilnius, Lithuania
Oligomeric forms of beta amyloid (Aβ) peptides are considered as the main toxic factors in pathogenesis of Alzheimer's disease (AD). In recent years, one of the most promising therapeutic means for AD was thought to be immunization with specific antibodies against Aβ. Several therapeutic antibodies were developed, however, most of the clinical trials were canceled due to unexpected deaths and neuroinflammatory responses in some patients. The causes and mechanisms of such responses are currently only partially understood. We have recently raised monoclonal antibodies against oligomeric forms of Aβ and were investigating whether these antibodies would prevent the neurotoxicity of Aβ oligomers in primary neuronal‐glial cerebellar granule cell (CGC) cultures. Antibodies were not directly toxic to CGCs when applied at concentrations relevant to concentrations reported to be in blood serum. However, surprisingly, the antibodies when in complexes with Aβ oligomers or firbrils dramatically increased the neurotoxicity of Aβ. Similar effects were also observed with antibodies to other oligomeric proteins: hamster polyomavirus major capsid protein VP1, human metapneumovirus nucleocapsid protein and measles virus nucleocapsid protein strongly potentiated the neurotoxicity of their antigens. The neurotoxicity of antibody‐oligomeric antigen complexes was abolished by removal of the Fc region from the monoclonal antibodies or by the removal of microglia from cultures, and was accompanied by inflammatory activation and proliferation of the microglia in culture. In conclusion, we find that immune complexes formed by Aβ oligomers or other oligomeric antigens and their specific monoclonal antibodies can cause neuronal death in primary neuronal‐glial cultures via Fc‐dependent microglial activation. The results suggest that therapies resulting in antibodies to oligomeric Aβ or oligomeric brain virus proteins should be used with caution or with suppression of microglial activation. Additionally, if endogenous antibodies contribute to neuronal loss in AD or viral encephalitis, suppression of microglial activation may be therapeutic.
T10‐05B. The effect of milnacipran and venlafaxine in a rat neuropathic pain model: Glia cell modulation
W. Makuch, E. Rojewska, M. Zychowska, K. Popiolek‐Barczyk, J. Mika, B. Przewłocka
Institute of Pharmacology, Krakow, Poland
Antidepressants have often been recommended for potential treatment of chronic pain, especially for neuropathic pain caused by nerve damage. It is known that nerve injury‐induced activation of microglia and astroglia can be a cause for the development of neuropathic pain. Recent data indicate that abnormal neuronal activity mediated by glia activation may influence the level of neuroimmune signaling molecules in chronic pain. However, it remains unclear whether the mechanism underlying antidepressant effects is related to glia activation. This study investigated the potential pain‐relieving properties of milnacipran and venlafaxine, selective serotonin/noradrenaline reuptake inhibitors (SNRI), in neuropathic pain and attempted to resolve how these antidepressants affect the factors synthesized by the activated glia in neuropathy. Chronic constriction injury (CCI) was performed in Wistar rats by loose ligation of the sciatic nerve. After a single intraperitoneal (i.p.) injection of antidepressants, the responses to mechanical and thermal stimuli in neuropathic rats were evaluated 7 days after CCI by the von Frey test and the cold plate test, respectively. To confirm the influence of antidepressants on microglia and astroglia, Western blot analysis of IBA‐1 (microglia marker) and GFAP (astroglia marker) in the spinal cord and dorsal root ganglions (DRG) was performed. The experiments were carried out according to the IASP and local Bioethics Committee rules. Our findings confirmed that the administration of milnacipran (20 mg/kg) and venlafaxine (10 mg/kg) elicited antiallodynic and antihyperalgesic effects in CCI‐exposed rats. Western blot analysis showed that milnacipran elevated the level of IBA‐1 protein in the spinal cord (but not in DRG) in CCI‐exposed rats. Venlafaxine decreased the CCI‐induced IBA‐1 protein level in the spinal cord and in DRG. We observed no changes in GFAP in the spinal cord and DRG after milnacipran and venlafaxine administration. These results provide a substantial evidence that both used antidepressants belonging to SNRI attenuated allodynia and hyperalgesia in an animal model of neuropathic pain, although their effect on microglial cells and astrocytes was different, namely, milnacipran increased the microglia activation, in contrast to venlafaxine. This effect can be explained by comparing the effects of these drugs during their repeated application, and relevant experiments are currently in progress.
Acknowledgements: This study was supported by statutory founds and grant POIG.01.01.02‐12‐004/09 Demeter‐1.1.2.5.4.
T10‐06A. Primary microglia lack strict regulation of inflammasome‐mediated activation as compared to myeloid macrophages
S. Burm 1, E. Zuiderwijk‐Sick1, A. 't Jong1, C. van der Putten1, L. van Straalen1, S. Amor2, P. van der Valk2, J. van Horssen3, J. Bajramovic1
1Biomedical Primate Research Centre, Rijswijk, Netherlands 2VU Medical Centre, Department of Pathology, Amsterdam, Netherlands 3VU Medical Centre, Department of Molecular Cell Biology, Amsterdam, Netherlands
Myeloid cells can respond to intra‐ and extracellular danger/stress signals by inflammasome‐mediated activation. This process consists of two steps: Toll‐like receptor‐induced production of pro‐IL‐1b followed by inflammasome‐mediated cleavage and secretion of bioactive IL‐1b. Inflammasome‐mediated activation is strictly regulated by expression of regulatory proteins and by inhibitory processes like autophagy. Recent studies describe that microglia are markedly different from other myeloid cells. They originate from a separate progenitor, are chronically exposed to the neural microenvironment and their activation may be regulated differently. The objective of this study was to determine the expression profile of inflammasome components in primary microglia and to compare inflammasome‐mediated microglial activation and its regulation with other myeloid cells.
Primary microglia, bone marrow‐ and blood‐derived macrophages (BMDMs) of adult rhesus macaques of the same donor were profiled for all NOD‐like receptors (NLRs), inflammasome‐associated adaptor proteins, caspases, and regulatory proteins by qPCR. Inflammasome function and kinetics were assessed by exposing LPS‐primed microglia to ATP, Silica or MSU (danger‐associated molecular patterns: DAMPs) followed by measuring the transcription of pro‐IL‐1b‐encoding mRNA and the secretion of IL‐1b.
Primary microglia expressed NLRs (NALP1–3, NOD1/3/4, AIM2, IPAF, NAIP), adaptor proteins (ASC), caspases (1/3–5/7/8), and regulatory proteins (A20), and the expression profile closely resembled that of BMDMs. Priming of microglia with LPS induced high levels of pro‐IL‐1b‐encoding mRNA, but IL‐1b protein was only secreted in response to subsequent stimulation with various DAMPs. Interestingly, in LPS‐primed BMDMs the window for inflammasome‐mediated activation was 4–8 hours, while LPS‐primed microglia remained sensitive for inflammasome‐mediated activation for at least 20 hours.
In conclusion, primary microglia express multiple inflammasome components closely resembling the expression profile of BMDMs and they can be induced to form functional inflammasomes. Importantly, primed microglia remain sensitive to inflammasome‐mediated activation for much longer than other macrophages. We will present data on whether this reflects a difference in negative regulation of the inflammasome, or differences in autophagy or apoptosis sensitivity.
T10‐06B. Telomere dysfunction leads to changes in microglial numbers and morphology in a mouse model of premature aging
A. Manzoor Khan 1, A. Babcock1, H. Saeed2, M. Kassem3, B. Finsen1
1Institute of Molecular Medicine, SDU, Dept of Neurobiology Research, Odense, Denmark 2Stanford University School of Medicine, University of Stanford, Dept of Endocrinology, Gerontology & Metabolism, Stanford, CA, United States 3Endocrinology Research Lab (KMEB), Dept of Endocrinology & Metabolism, Odense University Hospital and SDU, Odense, Denmark
Objective: The susceptibility of the aging brain to neurodegenerative diseases may in part be attributed to the intrinsic senescence of the microglia. Cellular aging or senescence is linked to telomere shortening/dysfunction. Evidence is accumulating that aging microglia show morphological changes, referred to as microglial dystrophy, which may be accompanied by accumulation of the iron‐binding protein, ferritin. Here, we investigated the effect of telomere shortening on microglial morphology, numbers, and activation state in telomerase deficient (Terc‐/‐) mice, a model of premature aging due to shortened telomeres.
Methods: Male Terc+/+ mice and 3rd generation Terc‐/‐ mice, which show a clear aging phenotype, were perfusion‐fixed with 4% paraformaldehyde. Brains were processed into 60 μm thick horizontal sections. Every fourth section was used for stereological estimation of CD11b+ microglia in the molecular layer of the dentate gyrus by the optical fractionator method. Sections were also stained for the microglial markers Iba‐1 to monitor microglial morphology, CD45 and CD68 to study microglial activation, and ferritin to study microglial aging. Further sections were stained for the apoptosis marker cleaved poly (ADP‐ribose) polymerase (cPARP).
Results: Unlike resting microglia in littermate Terc+/+ mice, which display thin, finely branched processes, microglia in Terc‐/‐ mice exhibited partial retraction and hypertrophy of processes. Microglial CD45 and CD68 immunoreactivity were similar in Terc+/+ and Terc‐/‐ mice. Absolute numbers of Mac‐1+ microglia were significantly reduced in young and old Terc‐/‐ versus Terc+/+ mice. An increased microglial density in old Terc‐/‐ versus Terc+/+ mice could be attributed to a volume reduction of the dentate gyrus. We also observed an increased number of cPARP + cells in old Terc‐/‐ versus Terc+/+ mice. This increase was most prominent in the sub‐granular zone, an active site of neurogenesis, but also occurred in the molecular layer of the dentate gyrus and might reflect microglial apoptosis. We observed no difference in ferritin staining in Terc‐/‐ versus Terc+/+ mice.
Conclusion: Our findings suggest that telomere shortening impairs microglial capacity for self‐renewal. Surprisingly, changes in microglial morphology in Terc‐/‐ mice occurred without differences in CD45 and CD68 expression. Ongoing studies will show if increased numbers of microglia in Terc‐/‐ mice co‐express cPARP or ferritin as evidence of increased microglial aging.
T10‐07A. Cannabigerol quinone exerts therapeutic effects in experimental autoimmune encephalomyelitis
F.J. Carrillo‐Salinas 1, M. Mecha1, A. Feliu1, L. Mestre1, I. Cantarero2, C. Navarrete3, E. Muñoz2, C. Guaza1
1Cajal Institute (CSIC), Madrid, Spain 2University, Medicine, Córdoba, Spain 3VivaCell Biotechnology España, Córdoba, Spain
Phytocannabinoids (pCBs) without psychotropic effects are considered of special interest as novel therapeutic agents in CNS diseases. These pCBs include cannabidiol (CBD), cannabigerol (CBG), D9 tetrahydrocannabivarin (D9THCV) and cannabidivarin (CBDV). We have developed a series of new cannabinoid quinones, among them the CBG quinone (VCE‐003) that shows PPARg and CB2 receptors agonism. In addition we have found that VCE‐003 activates the Nrf2/ARE pathway in neuronal cell lines. In the present study, we investigated the therapeutic potential of VCE‐003 in the experimental autoimmune encephalomyelitis (EAE) model of multiple sclerosis (MS) by immunization con MOG33–55. VCE‐003 (5mg/Kg ip, daily) was administered to susceptible C57/BL6 mice at the onset of symptomatology. Clinical score and weights of mice were daily recorded until the day of sacrifice (28 days post‐immunization). VCE‐003 treatment delayed the onset of disease and ameliorated the symptomatology. Histological analysis of spinal cord of EAE mice treated with VCE‐003 showed decreased microglia reactivity and reduced cellular infiltrates, in particular CD4+ T lymphocytes. Double labeling with Neurofilament and the myelin protein, RIP indicated that VCE‐003 diminished the axonal damage. Demyelination was evaluated by Luxol fast blue labeling. Changes in the expression of several cytokines, adhesion molecules and Nrf2‐dependent genes were determined by qRT‐PCR. The implication of PPARg and CB2 receptors in the beneficial effects of VCE‐003 in the EAE model of MS is being investigated by using specific receptors antagonists. Taken together our results support the potential of VCE‐003 for the treatment of MS and other chronic inflammatory diseases.
This work was supported by the MINECO grants IPT‐2011‐0861‐900000 (VivaCell, EM and CG), SAF2010‐17501 (CG) and SAF2010‐19292 (EM).
T10‐07B. The Lactosylceramide synthase B4galt6 controls astrocytes activation during chronic CNS inflammation
L. Mayo 1, M. Nadeau2, M. Blain3, I. Mascanfroni2, A. Yeste2, J.P. Antel3, S.A. Trauger4, H.L. Weiner4, F.J. Quintana2
1Harvard Medical School, Brigham and Women's Hospital, Center for Neurologic Diseases, Boston, United States 2Harvard Medical School, Brigham and Women's Hospital, Center for Neurologic Diseases, Boston, United States 3McGill University, Montreal, Canada 4Harvard University , FAS Center for Systems Biology, Boston, United States
In most patients, multiple sclerosis (MS) is initially manifested as a relapsing/remitting disease, but eventually most of the patients develop secondary progressive MS (SPMS), characterized by an irreversible accumulation of disability and in which most therapies are not efficacious.
We found that the lipid molecule Lactosylceramide (LacCer), is upregulated in MS patients. To better understand the role of LacCer in MS, we used an animal model of SPMS, and found that LacCer synthase (B4galt6) is upregulated during disease progression by astrocytes, and that its inhibition halts the progressive phase of the disease, attenuating cytokine and chemokine production by the astrocytes, and reducing the recruitment of inflammatory Ly6Chigh monocytes to the CNS. Moreover, it also skewed the microglia and monocytes towards a M2 phenotype, and shifted the astrocyte phenotype to support re‐myelination and axonal growth. In vitro experiments, using primary cells demonstrated that the inhibition of LacCer synthesis directly inhibits cytokines and chemokines production in astrocytes. Importantly, similar results were also obtained with human astrocytes, reinforcing the biological importance of our findings.
In summary, we identified a novel lipid‐signaling pathway that promotes the activation of local CNS immunity and neurodegeneration in experimental SPMS and is a potential therapeutic target for SPMS.
T10‐08A. Lipopolysaccharide‐induced brain activation of the indoleamine 2,3‐dioxygenase and depressive‐like behavior are impaired in obese and diabetic db/db mice
N. Castanon, A.‐L. Dinel, C. André, A. Aubert, S. Layé
Nutrition and Integrative Neurobiology, Bordeaux, France
Converging clinical studies report an increased prevalence of comorbid neuropsychiatric symptoms, particularly major depressive disorders, in a number of conditions (e.g., aging, obesity) and diseases (e.g., atherosclerosis, congestive heart failure, rheumatoid arthritis) sharing inflammation as a common denominator. By using an experimental approach in mice exposed to innate immune system (ISS) stimulation, we demonstrated that induction of depressive‐like behavior is mediated by cytokine‐induced brain activation of a tryptophan‐catabolizing enzyme, the indoleamine 2,3‐dioxygenase (IDO). In the metabolic syndrome (MetS), a widely accepted concept that identifies a cluster of individual risk factors for type 2 diabetes and cardiovascular disease (including obesity, metabolic dysregulations and basal low‐grade inflammation), neuropsychiatric symptoms emerge as significant factors for aggravation of the disease and related outcomes. Recently, we demonstrated in a model of MetS, the diabetic and obese db/db mice that cognitive alterations and increased anxiety‐like behavior are related to hippocampal inflammation. On the other hand, depressive‐like behavior is not affected by basal inflammation displayed by db/db mice. Given the data linking increased depressive‐like behavior and IDO activation by cytokines in conditions of ISS stimulation, the question arises as to whether depressive‐like behavior is increased in those conditions in db/db mice.
To answer this question, we measured in db/db mice and in their healthy db/+ littermates the effect of a lipopolysaccharide (LPS) challenge (5 µg/mouse, ip) on behavioral reactivity in a depression test (the forced swim test, FST), plasma levels and hippocampus expression of inflammatory cytokines and related neuronal targets, and brain IDO activity.
Plasma levels and hippocampus expression of IL‐1β, IL‐6, TNFα and IL‐10 are similarly increased 2h after LPS in both db/+ and db/db mice. As expected, brain IDO activity and duration of immobility in the FST are increased in LPS‐treated db/+ mice 24h after LPS. On the contrary, induction of brain IDO activity is significantly blunted in db/db mice compared to their db/+ counterparts and no increase of depressive‐like behavior is observed. Moreover, some data suggest an impairment of the neuroimmune interactions in db/db mice.
We need now to understand how obesity and related neurobiological alterations impair IDO activation by cytokines and their consequences in terms of vulnerability to infections in MetS.
T10‐08B. TLR2‐induced MMP9 activation compromise blood brain barrier and enhances brain damage in Collagenase‐induced Intracerebral Hemorrhage
H. Min 1, Y.‐H. Jang1, J. Hong1, K.I. Kwak1, S. Lee2, S.J. Lee1
1Seoul National University, School of Dentistry, Department of Neuroscience and Dental Research Institute, Seoul, Republic of Korea 2Chungnam National University, Department of Microbiology, School of Systems Biology, Daejeon, Republic of Korea
Innate immune response plays an important role in the pathogenesis of cerebral ischemia/reperfusion injuries. Recent studies have shown that Toll‐like receptor 4 (TLR4) is involved in the innate immune responses after cerebral ischemia/reperfusion injuries, yet, the role of TLR2 in stroke or the mechanisms of its function have not been resolved. In this study, we tested it in a collagenase‐induced mouse intracerebral hemorrhage (ICH) model using TLR2 knock‐out (KO) mice. To induce ICH, collagenase or blood was injected into the right caudate putamen in 8–10 week old male mice. TLR2 expression was upregulated in the ipsilateral hemorrhagic tissues of the collagenase‐injected mice. Brain injury volume and neurological deficits following ICH were reduced in the TLR2 KO mice as compared to the wild type (WT) mice. Heterologous blood‐transfer experiments show that TLR2 signaling in the brain‐resident cells, but not leukocytes, contributes to the injury. In our study to elucidate underlying mechanisms, we found that damage in the blood‐brain barrier (BBB) integrity following the ICH was attenuated in the TLR2 KO mice compared to the WT mice, which may be due to reduced MMP‐9 activation in brain astrocyte. The reduced BBB damages accompanies with reduced neutrophil infiltration and proinflammatory gene expression the injured brain parenchyma, which may account for the attenuated brain damage in the TLR2 KO mice after ICH. Conclusively, these data demonstrate that TLR2 contributes to brain injury following ICH by compromising BBB through activating MMP‐9 in brain.
T10‐09A. Complement‐independent modulation of chemokine expression by antibodies in myelinated cultures
K.J. Chapple, E.‐M. Oesau, M. Lindner, C. Linington
University of Glasgow, Glasgow, United Kingdom
A significant proportion of children with multiple sclerosis (MS) or acute disseminated encephalomyelitis (ADEM) develop autoantibody responses directed against myelin oligodendrocyte glycoprotein (MOG). The pathological relevance of this autoantibody response is unknown, but it is generally assumed that it exacerbates demyelination via activation of complement and/or cell meditated effector mechanisms. However in a majority of cases the MOG‐specific autoantibody titre is far lower than that required to induce widespread demyelination and exacerbate disease severity in animal models of MS. To explore the possibility MOG‐specific antibodies may play other more subtle roles in disease pathogenesis we investigated possible effects in myelinating cultures derived from embryonic rat spinal cord; a model system that allows us to explore antibody‐dependent effects in the absence of exogenous complement and effector cells. Myelinating cultures were treated continuously with monoclonal antibodies specific for either MOG (clone Z2, IgG2a), sulphatide (clone O4, IgM), proteolipid protein (PLP) (clone O10, IgM), or an appropriate isotype control from 18 days in vitro onwards.
In the absence of an exogenous source of complement none of these antibodies induced demyelination, but by 24 DIV had all had a significant inhibitory effect on myelination compared to cultures treated with appropriate isotype control antibodies. To investigate possible mechanisms contributing to this inhibitory effect qPCR arrays were used to determine if this complement‐independent effect on myelination was associated changes in expression of immune mediators. Unexpectedly we found recognition of antigens exposed at the surface of the myelin sheath induced a rapid increase in expression of three chemokines known to be involved in recruitment of effector T cells into the CNS. Within 24 hours of adding antibody to the cultures expression of CCL5, CCL20 and CXCL11 increased by at least three orders of magnitude and then declined to baseline over the following five days despite continuous presence of antibody. This transient increase in mRNA transcripts for these cytokines resulted in sustained protein synthesis and secretion of biological active products as demonstrated by analysis of culture supernatants.
These results challenge the traditional view that myelin‐specific autoantibodies contribute to the pathogenesis of diseases such as MS and ADEM by virtue of their ability to initiate immune‐mediated demyelination. We now demonstrate that even in the absence of recruited immune effector mechanisms myelin‐specific autoantibodies not only inhibit myelination but trigger secretion of chemokines predicted to trigger or exacerbate inflammation within the CNS.
T10‐09B. Retinoic acid, a novel regulator of blood‐brain barrier function in neuroinflammation
M. Mizee 1, S. van der Pol1, J. Drexhage1, B. van het Hof1, R. Mebius1, P. van der Valk1, J. van Horssen1, A. Reijerkerk2, E. de Vries1
1VU University Medical Center, Amsterdam, Netherlands 2to‐BBB, Leiden, Netherlands
The blood‐brain barrier (BBB), comprising specialized brain endothelial cells, is crucial in maintaining a controlled environment within the central nervous system (CNS) to safeguard normal neuronal function. In multiple sclerosis (MS) the function of the BBB is disturbed, leading to uncontrolled influx of metabolites and immune cells and neuroinflammation. Therefore, restoring the function of the BBB may be a novel therapeutic tool to halt disease progression or new lesion formation.
We previously showed the importance of the Vitamin A metabolite retinoic acid (RA) in BBB function during CNS development1, where fetal astrocyte‐derived RA is needed to induce BBB function during CNS embryogenesis. Considering the possible involvement of developmental pathways that could regenerate the disrupted BBB, we investigated the possible role for RA in the protection or reinstatement of disrupted BBB function.
We show that RA counteracts the deleterious effects of inflammatory cytokines such as tumour necrosis factor (TNF)‐α and interferon (IFN)‐γ on BBB function in vitro. Moreover, RA diminishes the induction of inflammation‐related genes in human brain endothelial cells, and induces general immune quiescence in resting brain endothelium.
Our preliminary data as well as a recently described study in the Cuprizone animal model for demyelination suggest that RA synthesis in the CNS is increased under inflammatory conditions. The impact of CNS‐derived RA on the inflamed BBB, as well as the cellular source is currently under investigation.
Insight into the RA‐synthetic pathway and its protective effect on the BBB may lead to the development of novel therapies which are aimed to restore BBB function and reduce the inflammatory cascade in MS lesions.
1M. R. Mizee, et al., “Retinoic Acid Induces Blood‐Brain Barrier Development,” J. Neurosci. 33(4), 1660 (2013).
T10‐10A. Does tissue Transglutaminase play a role in leukocyte/monocyte infiltration during experimental multiple sclerosis?
N. Chrobok 1, C. Sestito1, E.N. Bakker2, S. van der Pol3, E.H. de Vries3, K.K. Fenrich4, F. Debarbieux4, M.M. Wilhelmus1, B. Drukarch1, A.‐M. van Dam1
1VU University Medical Center, Department of Anatomy and Neurosciences, Amsterdam, Netherlands 2Academic Medical Center, Department of Biomedical Engineering and Physics, Amsterdam, Netherlands 3VU university medical center, Department of Molecular Cell Biology and Immunology, Amsterdam, Netherlands 4Université de la Méditerranée, Institut de Biologie du Developpement de Marseille‐Luminy, Marseille, France
Introduction: Multiple sclerosis (MS) is a chronic neuroinflammatory disease which manifests in neurological deficits caused by inflammation, demyelination and axonal damage. A pathological hallmark is the infiltration of lymphocytes and monocytes into the central nervous system, which subsequently serve as a source of inflammatory mediators contributing to local activation of glial cells and further production of inflammatory mediators.
Tissue Transglutaminase (TG2) is a multifunctional enzyme whose expression and activity is enhanced during inflammatory processes. We previously observed that the expression of TG2 is increased in monocytes in lesions in post‐mortem material of MS patients. Moreover, TG2 activity and expression is enhanced in chronic‐relapsing experimental autoimmune encephalomyelitis (cr‐EAE) in rats. In this same MS model, pharmacological inhibition of TG2 activity dramatically reduced clinical symptoms and attenuated the influx of monocytes. In the present study we question whether TG2 plays a role in mouse EAE, as transgenic mice have to be used for subsequent in vivo imaging of leukocytes/monocytes during EAE.
Methods and results: EAE was induced in TG2‐/‐ mice and littermate wildtype mice (C57Bl/6) using MOG35–55. During the early phase of disease (day 14–15), the clinical symptoms observed were significantly less severe in TG2‐/‐ compared to the wildtype mice. Moreover, the maximal clinical score was significantly lower in the knockout mice. There was no difference in the day of onset of disease symptoms between the two groups.
Secondly, we aimed at visualizing leukocytes/monocytes in the spinal cord of LysM‐GFP and CD11c‐YFP mice suffering from MOG35–55‐induced EAE using intravital video microscopy. A permanent window was fixed on top of the spinal cord of mice to allow reimaging of the same field of view in the same mouse over the disease course. In a pilot experiment we observed numerous GFP positive cells in the white matter around the imaged blood vessel in the spinal cord of a LysM‐GFP EAE mouse. Although equally present, this was less prominent in a CD11c‐YFP mouse suffering from EAE. Subsequent immunohistochemical analysis revealed that various cell types had infiltrated the spinal of cord of both mice.
Outlook: To further address the role of TG2 in the infiltration of leukocytes/monocytes into the spinal cord of EAE mice in vivo, specific inhibitors for TG2 activity will be administered to the transgenic mice and leukocytes/monocytes will be visualized using intravital video microscopy.
T10‐10B. ABCD2 expression modifies the phenotype in ABCD1 deficient mouse peritoneal macrophages
Z. Muneer 1, C. Wiesinger1, G. Regelsberger2, J. Berger1, S. Forss‐Petter1
1Medical Universty of Vienna, Center for Brain Research, Vienna, Austria 2Medical University of Vienna, Institute of Neurology, Vienna, Austria
X‐linked adrenoleukodystrophy (X‐ALD) is the most frequently occurring peroxisomal neurodegenerative disorder and is often associated with cerebral demyelination and inflammation. X‐ALD is caused by mutations in the ATP‐binding cassette sub‐family D member 1 (ABCD1) gene encoding adrenoleukodystrophy protein (ALDP), which is a transporter for CoA esters of very long‐chain fatty acids (VLCFA) across the peroxisomal membrane. Adrenoleukodystrophy related protein (ALDRP), another member of the ABCD sub‐family, encoded by ABCD2, is the closest homologue of ABCD1. Upon over‐expression, ABCD2 has been shown to compensate for ABCD1 deficiency in vitro and in vivo. Several lines of evidence imply an important role of microglia/macrophages in the disease progression. Here we used mouse peritoneal macrophages (MPMΦ) to correlate the gene expression levels of candidate modifiers with X‐ALD associated defects, like accumulation of VLCFA and decreased peroxisomal β‐oxidation, in Abcd1 and Abcd2 single KO mutants as well as in Abcd1/Abcd2 double deficient (DOKO) mice. By quantitative RT‐PCR analysis, in MPMΦ from wild type mice the Abcd2 mRNA was present at about half the level of the Abcd1 mRNA. Abcd1 KO MPMΦ showed elevated accumulation of VLCFA (C26:0) compared to the MPMΦ from wild type mice as measured by GC‐MS. The VLCFA levels of Abcd2 KO MPMΦ were similar to wild type amounts. Interestingly, there was much higher accumulation of VLCFA in the MPMΦ from DOKO mice. There were no differences in the mRNA expression level of the Elovl1 gene, encoding the enzyme catalyzing the elongation step of VLCFA biosynthesis. Peroxisomal β‐oxidation activity was decreased in Abcd1 KO MPMΦ compared to wild type level. This defect was strongly enhanced in MPMΦ from Abcd1/Abcd2 DOKO mice. These results show that the increased accumulation of VLCFA in MPMΦ from DOKO mice as compared to Abcd1 KO mice was due to a more severe defect in peroxisomal β‐oxidation in DOKO MPMΦ. We conclude that a substantial expression of Abcd2 mRNA prevents a more severe metabolic phenotype in Abcd1 deficient mouse peritoneal macrophages. This study also supports the validity of ABCD2 up‐regulation as a potential therapeutic target in X‐ALD.
T10‐11A. Neuroprotective function of microglial Siglec‐E
J. Claude, B. Linnartz‐Gerlach, H. Neumann
Reconstructive Neurobiology, Bonn, Germany
Microglia have innate immune receptors recognizing pathogens and disease‐associated molecular patterns but also molecules that could sense the intact tissue. A subfamily of these receptors is the inhibitory signaling sialic acid‐binding immunoglobulin‐like lectin (Siglec) group including Siglec‐E that has an immunoreceptor tyrosine‐based inhibitory motif (ITIM) in the cytoplasmic tail to suppress activatory microglial signals.
In this study, we used primary and stem cell‐derived microglia that were modified by lentiviral vectors. Here we show that Siglec‐E is expressed on microglia and is up‐regulated following interferon‐γ (IFN‐γ) treatment. We performed lentiviral knock‐down and overexpression of Siglec‐E. Lentiviral overexpression of Siglec‐E decreased, while knock‐down increased the phagocytosis of neural debris and its associated reactive oxygen burst. The extracellular domain of Siglec‐E linked to a Fc‐part of immunoglobulin bound to the sialic acid residues of the neuronal glycocalyx. Therefore, we co‐cultured these modified microglia with primary hippocampal neurons. Overexpression and knock‐down of Siglec‐E showed an increase and decrease in relative neurite‐length, respectively. The neuroprotective effect of Siglec‐E was abrogated after removal of the sialic acid residues on the neuronal glycocalyx. Treatment with the anti‐oxidant Trolox abolished the neurotoxic effect of the Siglec‐E knock‐down on neurite length.
In summary, our data suggests an immunomodulatory function of Siglec‐E on microglia which leads to a neuroprotective phenotype by decreasing the production of reactive oxygen species and a reduced phagocytosis rate of neural debris.
T10‐11B. Anti‐inflammatory properties of omega 3 supplementation restore normal microglia‐neuron interactions in the hippocampus in a model of prenatal inflammation
A. Nadjar, C. Madore, A. Sere, A. Aubert, S. Layé
University of Bordeaux 2, Bordeaux, France
Many epidemiological studies have linked maternal exposure to infections during pregnancy to later development of cognitive disorders in the descendants. These alterations are likely to originate from a generalized neuroinflammation in the fetal brain following activation of the maternal immune system. This neuroinflammatory response relies on microglial cells activation. These latter normally contribute to the development of neuronal networks especially by eliminating/maintaining synapses, a phenomenon also known as synaptic stripping, in optimal conditions of brain development. Neonatal inflammation may alter the synaptic stripping capacity of microglial cells. Thus, targeting this persistent inflammatory microglial activation could represent an original and promising strategy to improve cognitive performances in the descendants.
Omega‐3 are known as immunomodulators and target microglial cells. We thus hypothesized that enriching the diet with omega‐3 from the first day of gestation may impair the development of neurological deficits at adulthood, through limitation of microglial inflammatory response in favor of its synaptic stripping activity.
To characterize the beneficial effects of omega‐3 on microglial activity, pregnant mice were fed with an omega‐3‐deficient diet, or a balanced omega‐3/ omega‐6 diet or supplemented with omega‐3 and received a peripheral injection of either LPS or saline at E18 of gestation. Using the Golgi method, we first evaluated the morphology of dendrites and quantified the dendritic spines density as a measure of neuronal networks maturation in the hippocampus. We found that a prenatal exposure to LPS increased the number of immature spines and this was reversed by an omega‐3 supplemented diet. To correlate these data with alterations of microglia‐neurons interactions, we took advantage of the CX3CR1‐GFP mice and injected them with a neuron‐specific lentivirus containing dsRed protein, in the hippocampus, 7 days prior to two‐photon experiments. We focused on microglia general dynamic and microglia‐neuron physical interactions and found an alteration in microglial behavior and microglia‐neurons interactions in pups from LPS‐treated females. These alterations were reversed by an omega‐3 supplemented diet.
Overall, our data show that alterations of microglia‐neurons interactions in the developing brain may explain the neurological disorders observed in the descendants of females with prenatal inflammation. These deleterious effects may be prevented by nutritional strategies.
T10‐12A. The modification of myeloid‐derived suppressor cell population by the synthetic retinoid AM80 abolishes symptom recovery in a murine model of multiple sclerosis
V. Moliné‐Velázquez, F. de Castro, D. Clemente
National Hospital for Paraplegics, Toledo, Spain
Multiple Sclerosis (MS) is an inflammatory demyelinating disease of the central nervous system. The Relapsing‐Remitting (RR‐MS) is the most frequent clinical variant, which is characterized by a relapsing phase with inflammatory cell infiltrates and a remitting period, where patients partially recover. Among the different cell types involved in the necessary immunomodulation to allow the relapsing‐to‐remitting transition, the role of myeloid‐derived suppressor cells (MDSCs) gains importance. MDSCs form a heterogenic population of immature myeloid cells that is able to suppress the inflammatory response. This cells act, among other mechanisms, through Arginase‐I (Arg‐I) activity on T‐cells. A previous study of our group showed that Arg‐I+‐MDSCs transiently enter the spinal cord of EAE mice and takes part in the immune response control by inducing T cell apoptosis around the peak of the clinical score. Therefore, changes in the MDSC population during EAE should modify the evolution of the disease.
The retinoid acid family molecules are used for the treatment of different leukaemia due to their role as MDSC differentiation factors into diverse cell populations, abolishing T cell immunosuppression. AM80, a synthetic analogue of the retinoic acid with a higher bioavailability and less side effects than the natural ones, has controversial actions on EAE depending on its administration period. In this work, we administered AM80 specifically in the critical moment for immune modulation (around the maximum clinical score). Drug administration affected MDSC population and clearly worsened the EAE course. Our results point to endogenous strengthening of MDSC population as a new and promising therapeutic strategy to treat MS by speeding up the transition from the relapsing to the remitting period.
This work was supported by the Spanish Ministerio Economía y Competitividad (SAF2009‐07842; SAF2012‐40023; RD07‐0060‐2007 and RD12‐0032/0012/F.E.D.E.R., European Union, “Una manera de hacer Europa”), Gobierno de Castilla‐La Mancha (PI2009/26) and ARSEP Foundation (France). DC and FdC are hired by SESCAM.
T10‐12B. Sildenafil (Viagra®) prevents glial activation in a mouse demyelination model possibly via NFκB
A.K. Nunes 1, C. Raposo2, R. Luna1, S. Araújo1, W. Oliveira1, A.G. Oliveira1, K. Barbosa1, M.A. Hofling2, C. Peixoto1,3
1 Instituto de Pesquisas Aggeu Magalhães, Recife, Brazil 2Universidade Estadual de Campinas, Campinas, Brazil 3Centro de Tecnologias do Nordeste, Recife, Brazil
Sildenafil induces cGMP accumulation by PDE5 inhibition. We have demonstrated that sildenafil decreases microgliosis and astrogliosis and proinflammatory cytokines expression in a demyelination model. However, little is known about mechanisms of sildenafil neuroprotection. Since NFκB plays an important role in the regulation of glial activation, we examine the hypothesis that NFkB is part of the mechanism underlying the sildenafil neuroprotective effects. Five male mice (C57BL/6), 6 weeks‐old, were used per group. The groups received for four weeks: 0.2% Cuprizone (CPZ) mixed into the chow; CPZ into the chow plus sildenafil (Viagra®, Pfizer, 25 mg/kg) in the drinking water, starting concomitantly (sild‐T0) or fifteen days (sild‐T15) after initiation of CPZ; Controls received pure chow/water. Cerebella were processed for western blotting and immunofluorescence (IF). Results showed that CPZ increased GFAP expression compared to control (astrocytes activation). Sild‐T0 (but not sild‐T15) significantly decreased GFAP compared to CPZ group. In controls, microglia showed resting phenotype, with thin and branched processes positive to Iba1/NFκBp65 (inactive fraction) double labeling. CPZ treatment decreased inactive‐NFκB expression, indicating that this transcription factor was activated. In agreement, IKβα (NFκB inactivating protein) was also decreased. IF showed increase of Iba‐1 expression, indicating microglia activation. Sild‐T0 strongly increased the NFκBp65 and its inhibitory protein, IKβα, suggesting inactivation of NFκB. IF showed decreasing in Iba‐1 labeling, suggesting inactive microglia. The delayed treatment (Sild‐T15) did not decrease Iba‐1 or increase inactive‐NFκB and IKβα expression. In conclusion, treatment with sildenafil concomitant with CPZ exposure prevents micro‐ and astrogliosis in mice, possibly through IKβα‐NFκB signaling pathway. Sildenafil may be suitable to improve neuroinflammatory/neurodegenerative diseases treatment. Financial support: CNPq, CAPES, FACEPE, FAPESP.
T10‐13A. Characterization of the role of the Metallothionein‐1 in an animal mouse model of Alzheimer's disease
G. Comes, Y. Manso, M. Belfiore, J. Carrasco, J. Hidalgo
Universitat Autònoma de Barcelona, Bellaterra, Barcelona, Spain
Metallothioneins (MTs) are low molecular weight, zinc‐ and copper‐binding proteins which are involved in antioxidant, anti‐inflamatory and anti‐apoptotic processes.
MT‐1&2 levels have been found to be increased in several human neurodegenerative diseases including Alzheimer's disease (AD). Moreover, MT1+2 are also upregulated in different AD mouse models, for example Tg2576 mice, which show a significant up‐regulation of these proteins in the vicinity of the amyloid plaques.
In the present study we generated a double transgenic mouse line that develops AD‐like pathology in addition to having an overexpresion of MT1, in order to determine the role of MTs in different aspects of AD pathology.
The results show that the overexpression of MT1 does affect the mortality rate of the Tg2576 and control mice in a gender‐dependent manner and partially reverses the behavioural phenotype of young (4–5 months) Tg2576 mice, reducing the exploratory activity and improving the learning process; in contrast, MT1 does not cause relevant changes in deambulations and anxiety.
On the other hand, the amyloid cascade and neuroinflammation is increased in the hippocampus of old (15–16 months) APPTgMT mice, but the lower level of gliosis in the hippocampus of young male APPTgMT mice suggests that the overexpression of MT1 is capable of reducing inflamatory response well before amyloid plaques are formed but not afterwards.
Further molecular and immunohistochemistry analyses are underway to give further insight into the role of MTs in amyloidosis and neuroinflammation in this AD mouse line.
T10‐13B. The effect of lipopolysaccharide induced maternal inflammation on the cytoarchitectural development on the amygdala and hippocampus
E. O'Loughlin 1
1Univeristy College Cork, Western Road, Ireland
Exposure to prenatal inflammation is a risk factor for neurodevelopmental and neurobehavioural abnormalities that manifest in later life in disorders such as autism, schizophrenia and seizure development. The amygdaloid complex is composed of more than 10 nuclei with subdivisions that have different cytoarchitectonic, chemoarchitectonic, and projection characteristics and that are responsible for the processing of higher functions such as memory consolidation and emotional conditioning. It is also known that the basolateral nucleus of the amygdala is implicated in seizure propagation and initiation. Seizure onset later in life may be linked to abnormal development of the amygdalaoid complex. Neuropeptide Y (NPY) and its receptors are known to be involved in a number of important functions such as feeding behaviours, anxiety and seizure modulation. NPY Y1 and Y2 receptors are the most abundant in the brain and are expressed at different stages of development. During foetal development different brain regions are populated by neurones and glia at different times and the interface between mother and foetus plays a crucial role in the development process. Lipopolysaccharide (LPS) induced maternal inflammation is a known animal model for studying the inflammatory effects on the developing foetus. In this study we are investigating the effects of an acute prenatal systemic insult using the LPS model on amygdala morphology and structure. Embryonic day 12 (E12) c57bl6j pregnant mice receive an intraperionteal injection of LPS (50 µg/kg) or 0.9% sterile saline. Embryos at E12, E16, E18 and pups at P1, P7 P14 and P40 are taken and used to study the developing hippocampus and amygdala. For each age and treatment group foetal brains, maternal brains, maternal serum and placentas are collected. Incidence of preterm birth, resorption rate, litter size and weights are also assessed. Immunohistochemistry, western blot and RT‐PCR are then used to assess the expression of neuronal and glial markers in the amygdala and surrounding limbic structures. Specifically alterations in expression, activation and distribution of NPY and its receptors, microglia and astrocytes in the developing amygdala and hippocampus at different stages following LPS treatment are being investigated. It is hoped that this study will further enhance our understanding of how the maternal environment influences brain development and, if disturbed at a critical time in the development of the amygdala, may predispose individuals to amygdala related disorders in later life.
This research is funded by the Health Research Board.
T10‐14A. Cox‐2 inhibitors reduce microglia inflammation in vivo
A. Cupido 1, B. Catalin1,2, F. Kirchhoff1
1University of Saarland, Homburg, Germany 2University of Medicine and Pharmacology, Department of Physiology, Craiova, Romania
Question – Methods
In vivo imaging of transgenic fluorescent mice in the CNS by two‐photon laser‐scanning microscopy (2P‐LSM) has become a powerful tool in neuroscience. For in vivo imaging of the cortex a craniotomy of the skull has to be made. In addition to anaesthesia and analgesia treatment, we occasionally apply anti‐inflammatory drugs to prevent immunological activity that can obscure the images. As anti‐inflammatory drug we used Carprofen ((RS)−2‐(6‐chloro‐9H‐carbazol‐2‐yl)propanoic acid), a known cyclooxygenase‐2 (COX‐2) inhibitor.
Adult CX3CR1EGFP mice in which microglia are labeled by expression of the green fluorescent protein EGFP were treated with a single dose of Carprofen or with vehicle 12 hours before the craniotomy. In all mice we exposed the right somato‐sensory cortex, and quantified the microglial response to a laser‐induced micro‐lesion. This micro‐lesion was caused by increasing the power of the laser for 1 second in the middle of the region of interest.
Results – Conclusions
Unexpectedly, we observed that Carprofen reduced the microglial process motility significantly. The speed with which the processes approached the lesion dropped from 0.5 µm per minute in the untreated mice to 0.2 µm per minute in the treated mice.
A molecular understanding of microglia response in inflammatory processes and how anti‐inflammatory drugs modify normal microglia response will provide a strong impact in developing treatment strategies for diseases with strong inflammatory components.
T10‐14B. Neuroimmunological changes in the neurodegeneration caused by cystatin B deficiency (progressive myoclonus epilepsy)
O. Okuneva 1, Z. Li2, I. Körber1,2,3, L. Tian2, T. Joensuu1,2,3, A.‐E. Lehesjoki1,2,3, O. Kopra1,2,3
1Folkhälsan Research Center, Helsinki, Finland 2University of Helsinki, Neuroscience Center, Helsinki, Finland 3Haartman Institute, Department of Medical Genetics and Research Program's Unit, Molecular Medicine, Helsinki, Finland
Loss‐of‐function mutations in the cystatin B gene encoding cystatin B (CSTB) an inhibitor of cysteine proteases cathepsins cause neurodegenerative disorder called progressive myoclonus epilepsy of Unverricht‐Lundborg type (EPM1). Neuropathological changes in an EPM1 mouse model (Cstb‐/‐ mouce) include early microglial activation at the age of two weeks (P14) that precedes astrocytosis and neurodegeneration and, presumably, are central in initiating and amplifying the neuronal dysfunction and loss in EPM1. Here, our aim is to characterize in detail microglial cystatin B‐deficient cells using in vitro and in vivo approaches.
Resting and activated (lipopolysaccharide, LPS) cultured Cstb‐/‐ and control microglia were analyzed for cytokine production, chemotaxis and phagocytosis. Microglia extracted directly from the mouse brains were studied for expression of MHC II and M1‐M2 polarization using flow cytometry. Mouse brain cortex (P14) was analysed for M1‐M2 microglial phenotypes and the presence of other immunological cells from the periphery. Moreover, we have checked myeloid cell population in bone marrow and spleen of P14 animals as well as cytokines' level in blood serum.
Our results from cultured microglia show that secretion of proinflammatory chemokines is elevated by Cstb‐/‐ microglia. Also, Cstb‐/‐ microglia are chemotactically more active compared to the controls whereas their phagocytic activity is decreased. CSTB‐deficient microglia show decreased expression of MHC II on the cell surface indicating reduced antigen presentation. Activated Cstb‐/‐ microglia directly extracted from the brain has predominantly proinflammatory M1 phenotype. Cstb‐/‐ mice display inflammatory changes in the peripheral tissues at their early postnatal period. We have registered high concentrations of proinflammatory chemokines and cytokines in blood serum of P14 Cstb‐/‐ pups, but no difference in amount of neutrophils and lymphocytes between brains of wild‐type and knockout mice. Also, in bone marrow and spleen amount of granulocytes is enhanced in Cstb‐/‐ mice during early postnatal period as well as level of granulocyte macrophage colony‐stimulating factor in their blood serum.
Our results suggest a role for cystatin B in regulation of immune response and that CSTB‐deficiency is associated with early inflammatory processes both in the brain and the peripheral tissues. Therefore, we consider that EPM1 should be treated as a disorder combining neuropathological and immunological features.
T10‐15A. Anti‐epileptic drugs (AEDs) alter the microglial in‐/ activation state in astroglia/microglia cocultures
H. Dambach 1, D. Hinkerohe2, Z. Moinfar1,3, N. Prochnow1,3, A. Hufnagel4, P.M. Faustmann1,3
1Ruhr‐Universität Bochum, Neuroanatomie und Molekulare Hirnforschung, Bochum, Germany 2Knappschafts‐Krankenhaus, Neurologie, Bochum, Germany 3Ruhr‐Universität Bochum, International Graduate School of Neuroscience (IGSN), Bochum, Germany 4Neurologische Privatpraxis, Düsseldorf, Germany
Background:The contribution of glial cells in the pathophysiology of epilepsy is increasingly valued. Furthermore, clinical and experimental evidence suggests a direct relationship between epileptic activity and CNS inflammation, which is characterized by accumulation, activation and proliferation of microglia and astrocytes. Concomitant glia‐mediated mechanisms of action of several AEDs have been proposed. However, their direct effects on glial cells, especially microglia, have jet been hardly investigated. We aimed to investigate the influence of commonly used AEDs on the glial viability and microglial in‐/ activation state in a physiological and inflammatory modified in‐vitro astroglia/microglia coculture model.
Methods:Primary astrocytic cultures were prepared from brains of postnatal (P0‐P2) Wistar rats and cocultured with a physiological amount of 5% (M5), as well as 30% (M30) microglia in order to mimic inflammatory conditions. Cocultures were treated for 24 hours with valproic acid (VPA), carbamazepine (CBZ), phenytoin (PHE) and gabapentine (GBT) with a concentration of 10, 25, 50 and 100 µg/ml. Viability and proliferation was measured using the tetrazolium (MTT) assay. The microglial activation state was determined by immunocytochemical labeling using a monoclonal antibody to the ED1 marker.
Results:M5 and M30 cocultures showed a dose‐dependent, significant reduction in glial viability after incubation with PHE and CBZ. Furthermore, low doses of VPA led to highly significant microglial activation in M5 cocultures. However, CBZ significantly reduced the amount of activated microglial cells and increased the total number of inactivated microglia in the inflammatory modified M30 cocultures.
Conclusion: CNS inflammation is characterized by a disturbance of glial cell functions. Strong microglial activation, a typical hallmark of inflammation, was induced by VPA in M5 and continued in M30 cocultures. With regard to the direct relation between CNS inflammation and seizures, VPA seems to be unsuitable to reduce inflammatory condition. The reverse effect was achieved after CBZ. We noticed significant microglial inactivation, after incubation of the M30 cocultures. As it has been demonstrated for levetiracetam before, we assume a beneficial therapeutic effect of anti‐epileptic drugs (AED) with an anti‐inflammatory glial potential in epileptic patients with persistent inflammation.
T10‐15B. Microglial cell activation by lipopolysaccharide does not induce neurotoxicity in organotypic hippocampal slice cultures
I. Papageorgiou 1, J. Scheffel2, U.‐K. Hanisch2, O. Kann1
1Universität Heidelberg, Medizinische Fakultät, Institut für Physiologie und Pathophysiologie, Heidelberg, Germany 2Universitätsmedizin Göttingen, Institut für Neuropathologie, Göttingen, Germany
Microglial cell activation due to homeostatic imbalances of external and/or internal etiology implies, among others, secretion of proinflammatory cytokines. The fact that microglial activation, inflammation and neurodegeneration often coexist, suggests that proinflammatory cytokines might induce and/or enhance neuronal damage.
We investigated the neurotoxic impact of microglial activation in organotypic hippocampal slice cultures by exposing them to the toll‐like receptor 4 ligand, lipopolysaccharide (LPS), for 72 hours.
Microglial activation was characterized by unbiased estimation of their number (stereology) and quantification of soma and branching morphology (Neurolucida®‐based cell reconstructions). Moreover, proinflammatory cytokine and nitrite levels in the culture supernatant were estimated by ELISA and Griess. The neurotoxic impact was assessed by morphology (Nissl staining, Fluoro‐Jade® B) and extracellular electrophysiological recordings of spontaneous and evoked neuronal activity in the hippocampal CA3 subregion.
LPS exposure induced microglial population expansion, morphological changes, such as process thickening and somatic enlargement, as well as substantial secretion of proinflammatory cytokines (TNFα, IL6) and nitrite accumulation in the culture medium. However, these changes did not coincide with neurodegeneration and were associated with only minor effects on neuronal function, as demonstrated by the amplitude of evoked neuronal responses and short‐term plasticity properties.
We conclude that, in contrast to what has been observed in vivo, chronic microglial activation by LPS is not sufficient to drive neuronal death and dysfunction in organotypic hippocampal slice cultures.

T10‐16A. IOP Induces upregulation of MHC‐II and GFAP in the glia of Contralateral Mice Retina To Experimental Glaucoma
R. De Hoz Montañana 1, B.I. Gallego1,2, B. Rojas1,3, J.J. Salazar1,2, A.I. Ramirez1,2, A. Triviño1,3, F.J. Valiente‐Soriano4, M. Vidal‐Sanz4, J.M. Ramirez1,3
1Universidad Complutense (UCM), Inst Invest Oftalmol Ramón Castroviejo, Madrid, Spain 2Universidad Complutense (UCM), Facultad Optica y Optometria, Madrid, Spain 3Universidad Complutense (UCM), Departamento Oftalmologia. Facultad Medicina, Madrid, Spain 4Universidad de Murcia, Facultad de Medicina, Murcia, Spain
Purpose: To study the effects of laser‐induced ocular hypertension (OHT) in the macro‐ and microglia of eyes with OHT (OHT‐eyes) and contralateral eyes two weeks after lasering.
Methods: Adult Swiss mice were divided into two groups: naïve (n = 6) and lasered (n = 6). Retinal wholemounts were immunostained with antibodies against GFAP, Iba‐1 and MHC‐II.
Results: In the naïve retinas, weak constitutive MHC‐II expression was scarcely found in some Iba‐1+ microglial cells and rarely in GFAP+ astrocytes. Only a small dendritiform subpopulation of Iba‐1+ cells, located in the juxtapapillary area and in the marginal region of the retina, had a strong MHC‐II immunoreaction. In comparison with naïve both, in contralateral and in OHT‐eyes: i) GFAP was upregulated in Müller cells and microglia was activated; ii) MHC‐II was upregulated on macroglia and microglia. In microglia, it was similarly expressed in contralateral and OHT‐eyes. By contrast, in macroglia, MHC‐II upregulation was observed mainly in astrocytes in contralateral eyes and in Müller cells in OHT‐eyes
Conclusions: Both, contralateral and OHT‐eyes had macro‐ and microglial retinal changes in MHC‐II expression after two weeks of laser‐induced OHT. Our results suggest that the gliotic reaction in contralateral untreated eyes could be related to the immune response. On the basis of the glial changes observed, the use of the contralateral eye as a control in experimental unilateral OHT should be reconsidered.
T10‐16B. Continuous Vs Bolus exposure of Microglial cells to Hydrogen Peroxide
P. Pathipati 1, S. Mueller2, X. Jiang1, D. Ferriero1
1University of California San Francisco, Neurology, San Francisco, United States 2University of Heidelberg, Faculty of Medicine, Heidelberg, Germany
The neonatal brain is particularly susceptible to oxidative stress. Our group has previously shown that following hypoxic‐ischemic injury, hydrogen peroxide (H2O2) levels rise significantly particularly in the neonatal brain and are sustained for up to 24 hours. This rapidly accumulated H2O2 is detrimental in the iron‐rich immature brain as it can lead to the generation of dangerous free radicals that can cause extensive injury. To date, there is limited literature on the effects of increased H2O2 levels, particularly on microglial cells, which have been extensively indicated in the mediation of ensuing injury. Here we describe the effects of a continuous exposure of microglia to H2O2, as generated using the glucose oxidase‐catalase (GOX‐CAT) system. This system allows us to generate and continuously maintain for up to 24h pathophysiological levels of H2O2 >1 uM. Microglial cultures were derived from the P1 mouse brain and exposed to either bolus concentrations of H2O2 [1–100 mM] or varying concentrations of GOX‐CAT for varying lengths of time. Conditioned medium was collected from cells at 4, 18 and 24h of treatment and analyzed for secreted cytokine levels using the 25‐plex cytokine microbead array kit. Treated cell extracts were processed for protein and fixed cells were labeled with M1 and M2a phenotype markers. Continuous exposure to very low levels of H2O2 can produce 10–20‐fold higher (over control) pro‐inflammatory cytokine protein levels of IL5, IL6, IL12p40, IL12p70, IL15, IL17 and IFNg and at least a 100‐fold higher level of IL1a, IL1b, IL7 and TNFa by 18h. Anti‐inflammatory cytokine (IL4, IL10 and IL13) and chemokine (RANTES, G‐CSF and GM‐CSF) protein levels were also increased by 18h. Interestingly, no prominent cytokine responses were seen with bolus treatment at any of the time points studied. Low, continuous H2O2 promoted a predominantly M2a microglial phenotype by 24h. We conclude that studying continuous exposure effects will help delineate the various specific effects H2O2 can have on microglial cells. These specific effects can then be used to clarify when and how microglial cell responses can be beneficial or detrimental following injury in the immature brain.
T10‐17A. Peroxisome Proliferator‐Activated Receptor‐γ agonists protect oligodendrocyte from mitochondrial stress
R. De Simone, A. Bernardo, C. De Nuccio, S. Visentin, L. Minghetti
Istituto Superiore di Sanità, Rome, Italy
We have previously shown that natural (15‐deoxy‐D12,14 prostaglandin J2, 15d) and synthetic (pioglitazone) agonists of peroxisome proliferator activated receptor‐γ (PPAR) potentiate intrinsic cellular mechanisms protecting oligodendrocyte (OL) progenitors (OPs) from oxidative insults and promote their differentiation to OLs. In addition, PPAR‐γ agonists potentiate mitochondrial activities, as the mitochondrial respiratory chain activity and the regulation of cytoplasmic Ca2+ waves, which are known to be crucial for OL differentiation.
In the present study we sought to investigate whether PPAR‐γ agonists can protect OL cultures from conditions causing mitochondrial stress.
First, to specifically induce a mitochondrial impairment, we used the complex I inhibitor rotenone. As expected rotenone, at concentration not affecting cell viability, significantly inhibited OL differentiation, as indicated by the reduced number of cells expressing specific markers of differentiation (O4 and O1). In PPAR‐γ agonist‐treated OLs the inhibitory effects of rotenone were significantly attenuated, suggesting a protective effect of the agonists against the mitochondrial toxin.
We next examined a condition mimicking inflammatory stress by challenging OP cultures with TNF‐a, an inflammatory cytokine known to retard the differentiating program of OPs. In parallel with the expected reduction of the percentage of O4 and O1 positive cells, the cytokine induced a significant reduction of mitochondrial membrane potential (mMP), suggesting an impairment of the mitochondrial functions. The simultaneous treatment with TNF‐a and PPAR‐γ agonists (15d or pioglitazone) significantly reverted both TNF‐a dependent reduction of OL differentiation and mMP. At the molecular level, we found that in OP cultures, PPAR‐γ agonists increased the expression of the uncoupling protein‐2 (UCP‐2), a mitochondrial protein known to contribute to the protection of mitochondria against oxidative stress.
These findings suggest that PPAR‐γ agonists protect OLs and promote myelination through several mechanisms, including those involving mitochondrial functions. Our studies support the therapeutic potential of PPAR‐γ agonists in brain diseases in which mitochondrial alteration, oxidative stress and demyelination occur and point to the need to better understand the role of PPAR‐γ and its agonists in OL biology.
T10‐17B. Do stressed oligodendrocytes trigger microglia activation in pre‐active MS lesions?
L. Peferoen 1, D. Vogel2, M. Breur3, W. Gerritsen4, C. Dijkstra4, S. Amor5,4
1VU medical center, Amsterdam, Netherlands 2VU medical center, Pathology and Celbiology, Amsterdam, Netherlands 3VU medical center, Celbiology, Amsterdam, Netherlands 4VU Medical Center, Pathology, Amsterdam, Netherlands 5Queen Mary University of London, Neuroimmunology, London, United Kingdom
Multiple Sclerosis (MS) is a complex disease of the central nervous system (CNS). It is characterized by lesions of demyelination and inflammation, which can be classified according to the activation state of the microglia/macrophages. Well before any myelin and blood‐brain barrier damage clusters of activated microglia are noticeable. These clusters are considered to be the first stage of lesion formation and therefore called ‘pre‐active lesions. However, they do not always develop into demyelinating lesions.
Here we postulate that stressed oligodendrocytes play an crucial role in pre‐active lesion formation, by producing factors that will attract and activate microglia to either a more pro‐inflammatory (M1) or anti‐inflammatory (M2) activation status. This can contribute to the amount of damage to the environment and further lesion formation.
This study sets out to identify the best method to mimic this early lesion formation in vitro. First, human primary isolated microglia are stimulated to either a M1 or M2 phenotype and characterized by marker expression and cytokine profile. Oligodendrocytes are isolated out of the human brain. Medium of (stressed) oligodendrocytes will be used as attractant as well as activator for microglia. By immunohistochemical staining the activation status of microglia in pre‐active lesions will be determined.
Preliminary data reveal that human microglia are able to be skewed towards a pro‐ and anti‐inflammatory phenotype. First experiments of primary human oligodendrocyte cultures, showed pure oligodendrocytes. Thus far our data support our hypothesis, however further research is required to make our conclusions more robust.
T10‐18A. Quantitative and phenotypic analysis of mesenchymal stromal cell graft survival and recognition by microglia and astrocytes in mouse brain
N. De Vocht 1, D. Lin2, J. Praet3, C. Hoornaert4,5, K. Reekmans4,5, D. Le Blon4,5, J. Daans4,5, P. Pauwels6, H. Goossens5, N. Hens2, Z. Berneman4,5, A. Van der Linden3, P. Ponsaerts4,5
1University of Antwerp, Wilrijk, Belgium 2University of Hasselt, Center for Statistics, Hasselt, Belgium 3University of Antwerp, Bio‐Imaging Lab, Antwerp, Belgium 4University of Antwerp, Laboratory of Experimental Hematology, Antwerp, Belgium 5University of Antwerp, Vaccine and Infectious Disease Institute, Antwerp, Belgium 6University of Antwerp, Laboratory of Pathology, Antwerp, Belgium
Question: Although cell transplantation is increasingly suggested to be beneficial for the treatment of various neurodegenerative diseases, the therapeutic application of such intervention is currently hindered by the limited knowledge regarding central nervous system (CNS) transplantation immunology. In this study, we aimed to investigate the early post transplantation innate immune events following grafting of autologous mesenchymal stromal cells (MSC) in the CNS of immune competent mice.
Methods and results: First, the survival of grafted Luciferase/eGFP‐expressing MSC (MSC‐Luc/eGFP) was demonstrated to be stable from on day 3 post implantation using in vivo bioluminescence imaging (BLI), which was further confirmed by quantitative histological analysis of MSC‐Luc/eGFP graft survival. Additional histological analyses at week 1 and week 2 post grafting revealed the appearance of (i) graft‐surrounding/‐invading Iba1+ microglia and (ii) graft‐surrounding GFAP+ astrocytes, as compared to day 0 post grafting. While the density of graft‐surrounding astrocytes and microglia did not change between week 1 and week 2 post grafting, the density of graft‐invading microglia significantly decreased between week 1 and week 2 post implantation. However, despite the observed decrease in microglial density within the graft site, additional phenotypic analysis of graft‐invading microglia, based on CD11b‐ and MHCII‐expression, revealed > 50% of graft‐invading microglia at week 2 post implantation to display an activated status. Although microglial expression of CD11b and MHCII is already suggestive for a pro‐inflammatory M1‐oriented phenotype, the latter was further confirmed by: (i) the expression of NOS2 by microglia within the graft site, and (ii) the absence of arginase 1‐expression, an enzyme known to suppress NO activity in M2‐oriented microglia, on graft‐surrounding and ‐invading microglia.
Conclusions: In summary, we here provide a detailed phenotypic analysis of post transplantation innate immune events in the CNS of mice, and warrant that such intervention is associated with an M1‐oriented microglia response and severe astrogliosis.
T10‐18B. CD300f immunoreceptor is a novel neuron‐glia interaction molecule with a neuroprotective role after acute brain injuries
E. Taranto1, D. Alí‐Ruiz2, D. Blanco1, D. Tejera3, A. Ejarque‐Ortíz4, L. Reyes5, E. Comas‐Casellas4, M. Negro3, A. Martínez‐Barriocanal4, N. Lago3, P. Oliver5, J. Sayós4, H. Peluffo 6
1Faculty of Medicine, UDELAR, Physiopathology Department, Hospital de Clínicas, Montevideo, Uruguay 2Faculty of Medicine, UDELAR and Institut Pasteur de Montevideo, Department of Histology and Embryology and Neurodegeneration Laboratory, Montevideo, Uruguay 3Institut Pasteur de Montevideo, Neurodegeneration Laboratory, Montevideo, Uruguay 4CIBBIM‐Nanomedicine Program, Hospital Universitari Vall d'Hebrón, Institut de Recerca (VHIR), Universitat Autònoma de Barcelona, Immunobiology Group, Barcelona, Spain 5Uruguayan Center for Molecular Imaging (CUDIM), Montevideo, Uruguay 6Institut Pasteur de Montevideo, Montevideo, Uruguay
It is well known that cell surface immune receptors play a critical role in regulating immune and inflammatory processes in the central nervous system (CNS). CD300f is a novel immunoreceptor that dampens inflammatory reactions in Experimental Autoimmune Encephalitis (EAE) and diverse allergy models. We have analyzed the function of CD300f immunoreceptor in an NMDA excitotoxicity model and in a traumatic brain injury model (TBI). For this purpose, we determined the pattern of expression of both CD300f and its putative ligand(s) in the CNS, as well as the neuroprotective role of CD300f after both acute brain injuries. First, we used a human and rat CD300f‐Ig soluble protein to show by confocal microscopy the presence of endogenous ligand(s) for this receptor mainly in CNS white matter oligodendrocytes and on the surface of oligodendrocytes, fibrous astrocytes and neurons in vitro. Interestingly, when we analyzed the in vitro expression pattern of rCD300f in brain cells by Q‐PCR and immunohistochemistry, in addition to the expected expression in microglial cells, we detected expression of CD300f in oligodendrocytes and neurons. In vivo, after a TBI, CD300f is expressed at early time‐points (3d) in small round macrophages, and at later time‐points (14d) in neurons of the penumbra. Q‐PCR studies showed significant upregulation of the mRNA for rCD300f at 7 and 14d after TBI. Interestingly, a delayed (4 hours after the lesion) intraparenchymal injections of a non‐viral modular recombinant gene therapy vector termed NLSCt overexpressing hCD300f induced a decrease in the lesion volume 3d after NMDA injection or TBI when compared to control GFP over‐expression. In addition, the over‐expression of hCD300f decreased the long‐term sensitive functional deficits observed by the “sticky tape” test. In order to validate these results with the endogenous molecule, we cloned the rat ortholog of CD300f protein. On‐going Positron Emission Tomography (PET) studies using 2‐[18F]‐fluoro‐2‐desoxiglucosa (18F‐FDG) are being used to evaluate the long‐term functional recovery after TBI. The overexpression of rCD300f receptor had a comparable neuroprotective effect as the human molecule after NMDA injection. These data suggest that CD300f may be important in neuron‐glia inflammatory and trophic interactions. In addition, our results suggest that delayed CD300f overexpression by means of a modular recombinant gene therapy may constitute an interesting therapeutic strategy for acute brain injuries.
T10‐19A. N‐3 polyunsaturated fatty acids protect against the cognitive effects of a peripheral inflammation by targeting microglia morphofunctional activity
J.‐C. Delpech, C. Madore, A. Aubert, C. Joffre, S. Layé, A. Nadjar
NutriNeuro, UMR INRA 1286, Université Bordeaux, Bordeaux, France
Microglia is the main cellular component of the cerebral innate immune system. During a peripheral immune challenge, microglial cells are activated, leading to alteration of hippocampal memory. This modulation of cognitive processes is exerted by signals produced by immune‐like processes and in particular the proinflammatory cytokine Interleukin‐1 beta (IL‐1β). Depending on the type of signals produced after an inflammatory event, microglia develops specific activities, including cytotoxic, neuroprotective or phagocytic activities in order to come back to brain homeostasis. As described for macrophages, microglia can adopt M1 (proinflammatory) or M2 (phagocytic) phenotypes distinguishable through their pattern of factors expression and specific cell surface markers. Interestingly, polyunsaturated fatty acids (PUFAs) are precursors of bioactive lipid messengers involved in the regulation of inflammation and in particular, docosahexanoic acid (DHA, 22:6n‐3) an n‐3 PUFA, presents anti‐inflammatory properties. The aim of our study was thus to investigate the effect of an increase of n‐3 PUFAs on the inflammatory response and cognitive abilities after a peripheral immune challenge. To increase n‐3 PUFAs, we took advantage of transgenic mice carrying the fat‐1 gene from the roundworm Caenorhabditis elegans. This fat‐1 transgenic mouse is capable of producing n‐3 fatty acids from the n‐6 type endogenously, eliminating confounding factors of the diet. This conversion leads to abundant n‐3 fatty acids with reduced levels of n‐6 fatty acids in tissues, including the brain. To induce a neuroinflammation, we injected mice intraperitoneally with lipopolysaccharide (LPS), components of gram negative bacteria walls. We first studied the microglial phenotype 24 h after LPS injection and found that microglia in Fat‐1 mice presented a significant increase of M2 phenotype markers. We then measured cytokine expression in the hippocampus after a LPS challenge and found a significant decrease in IL‐1β mRNA in Fat‐1mice compared to wild‐type littermates. In terms of hippocampal memories abilities, only control mice presented a deficit in the Y‐maze task whereas Fat‐1 mice were able to perform the test correctly. Our results indicate that 24 h after a LPS injection, Fat‐1 mice presented a decreased of the inflammatory response and normal hippocampal memory abilities compared to wild‐type littermates.
T10‐19B. Opposing roles of nitric oxide and interleukin‐10 on CXCL12 gene expression in astrocytes
F. Petkovic 1, J. Blazevski1, M. Momcilovic1, M. Mostarica Stojkovic2, D. Miljkovic1
1Institute for Biological Research “Sinisa Stankovic”, Belgrade, Serbia 2School of Medicine, Microbiology and Immunology, Belgrade, Serbia
Question: Astrocytes have important roles in interaction between the central nervous system (CNS) and immune system. They preserve CNS integrity and homeostasis as they have prominent role in blood brain barrier formation and maintenance, as well as in active regulation of an immune response. However, astrocytes are affected in neuroinflammation, when their protective roles might be suppressed and when they might be shifted towards pro‐inflammatory phenotype. Experimental autoimmune encephalomyelitis (EAE) is a widely used model of autoimmune neuroinflammation. Chemokine CXCL12 produced by astrocytes and endothelial cells has profound anti‐inflammatory and anti‐encephalitogenic role in EAE. Nitric oxide (NO) produced by inducible NO synthase (iNOS) within the CNS is considered as disease promoting, while interleukin (IL)‐10 as protective in EAE.
The aim of this work was to investigate effects of NO and IL‐10 on CXCL12 gene expression in astrocytes.
Methods: Astrocytes were stimulated with supernatant collected from concanavalin A‐stimulated splenocytes (ConASn) and simultaneously exposed to NO donor, sodium‐nitroprusside (SNP) or anti‐IL‐10 antibody. Also, astrocytes were stimulated with IFN‐γ+IL‐17 in the absence or presence of IL‐10. Peritoneal macrophages isolated from healthy rats were co‐cultivated with astrocytes. After 24h incubation period CXCL12 gene expression in astrocytes was measured by RT‐qPCR. Phosphorylation of p38 MAPK and NF‐κB was assessed by immunoblot in astrocytes exposed to SNP.
Results: We found that NO released from SNP or macrophages significantly reduced CXCL12 gene expression in astrocytes. This reduction was mediated by inhibition of p38 MAPK activation, but not of NF‐κB signaling. On the contrary, IL‐10 stimulated and anti‐IL‐10 antibody suppressed CXCL12 gene expression in astrocytes.
Conclusions: These results imply that expression of CXCL12 in astrocytes is inversely regulated by NO and IL‐10 in the central nervous system affected by inflammation. Having in mind, profound regulatory role of CXCL12 in neuroinflammation, these data contribute to our understanding of pro‐ and anti‐inflammatory nature of NO and IL‐10, respectively.
T10‐20A. The neuroprotective effect of a PPAR‐γ agonist in microglia‐induced neuronal death is abrogated if CD200‐CD200R1 interaction is disrupted
G. Dentesano 1,2, J. Serratosa1, J.M. Tusell1, U. Perpiñà1, J. Saura2, C. Solà1
1IIBB‐CSIC, IDIBAPS, Cerebral Ischemia and Neurodegeneration, Barcelona, Spain 2University of Barcelona, IDIBAPS, Biochemistry and Molecular Biology Unit. School of Medicine, Barcelona, Spain
Microglial cells are involved in inflammatory and immune responses of the CNS. In the presence of pathological insults, they acquire reactive phenotypes aimed at re‐establishing brain homeostasis and minimizing neuronal damage. However, reactive microglia produce several factors, typical of an inflammatory response, with a potential neurotoxic effect. Consequently, the progression and resolution of microglial activation have to be tightly controlled to avoid detrimental secondary effects. Signals arising from neuronal cells play an important role in the activation state of microglial cells. Among them, inhibitory mechanisms, such as neuronal CD200 and microglial CD200R1 interaction, keep the pro‐inflammatory phenotype of microglia under control. Alterations in the expression of CD200 and CD200R1 have been described in pathological conditions, and the modulation of CD200‐CD200R signalling could be an interesting target to be considered in therapeutic approaches against neuroinflammation occurring in neurodegenerative diseases.
Little is known on the molecular mechanisms involved in the regulation of CD200 and CD200R1 expression. The aim of the present work is to study the possible modulation of CD200 and CD200R1 by PPAR‐γ agonists, and the involvement of CD200‐CD200R1 in the anti‐inflammatory and neuroprotective effects of PPAR‐γ activation. We have used mouse microglial, mixed glial and neuronal cell cultures, as well as neuron‐microglia co‐cultures. CD200R1 expression was detected in microglial cells, and it was decreased in response to pro‐inflammatory stimuli such as LPS/IFN‐γ. The PPAR‐γ agonist 15‐deoxy‐D12, 14‐prostaglandin J2 (15d‐PGJ2) abrogated the inflammatory response in LPS/IFN‐γ‐treated microglial cells and prevented CD200R1 expression inhibition. CD200 expression was detected in neuronal cultures and, at a lesser extent, in astrocytes in mixed glial cultures. LPS/IFN‐γ‐treatment did not modify CD200 expression in neuronal cultures, but it induced an increase in mixed glial cultures. This increase was inhibited by 15d‐PGJ2 pre‐treatment. 15d‐PGJ2 also protected against LPS/IFN‐γ‐induced neurotoxicity in neuron‐microglia co‐cultures, but this effect was abolished when CD200‐CD200R1 interaction was interrupted using an anti‐CD200R1 blocking antibody. These results show that PPAR‐γ agonists modulate CD200 and CD200R1 expression in reactive glial cells, and that CD200‐CD200R1 interaction is necessary for the neuroprotective effect of PPAR‐γ agonists.
Supported by La Marató de TV3 Foundation and grants PI10/378 and PI12/00709 (Instituto de Salud Carlos III, Spain).
T10‐20B. ICG‐001, but not Aspirin promotes myelin gene expression in oligodendrocytes
A. Preisner, J. Lilienbeck, A. Lürbke, E. Hoffmann, C. Kemming, T. Kuhlmann
University Hospital Münster, Münster, Germany
Multiple sclerosis (MS) is the most frequent demyelinating disease of the CNS. Histopathologically, the disease is characterized by inflammation, demyelination and gliosis. Axonal damage and loss are the underlying causes for permanent neurological disability in MS. Limited remyelination contributes to axonal damage by increased vulnerability of demyelinated axons to immune mediated damage and lack of trophic support. The continuous stimulation of the canonical Wnt/b‐catenin (cWbC) pathway impairs differentiation of oligodendrocytes (OL) and prevents thereby remyelination in demyelinating animal models of MS. To gain insight into the contribution of the cWbC pathway to remyelination in MS, we analyzed the expression pattern of TCF7L2, a downstream target of the cWbC pathway and coactivatior of b‐catenin in MS lesions. TCF7L2 was expressed in OL and astrocytes in a subset of early MS lesions, but was also observed in tissue samples from patients with inflammatory, non‐demyelinating diseases. In contrast, in chronic lesions, no expression of TCF7L2 was detected. By WB we found slightly increased b‐catenin levels in MS lesions compared to normal appearing white matter.
Aspirin (ASS), a non‐steroidal anti‐inflammatory drug, attenuates b‐catenin signaling activity by inhibition of protein phosphatase 2A (PP2A) via increased phosphorylation.
Therefore, we determined the effect of ASS on OL differentiation and myelin gene expression (MGE). As a control for the cWbC pathway activation or inhibition the selective cWbC pathway inhibitor (ICG‐001) was chosen. ICG‐001 binds specifically to CBP and causes a disruption of the b‐catenin/CBP interaction. Exposure of OL to 0.3 µM ICG‐001 for 48 hours increased MGE and had a positive effect on OL differentiation. In contrast, ASS had no positive effect on process formation and did not promote MGE in primary murine OL.
Our results suggest that ICG‐001, but not ASS increases the expression of myelin genes. Further experiments are required to determine the functional role of the cWbC pathway for OL differentiation and remyelination in OL and MS.
T10‐21A. Microglial activation beyond the Substantia Nigra in Parkinson's Disease
K. Doorn 1, P.J. Lucassen1, J.G.J. Bol2, B. Drukarch2, W.D.J. van de Berg2, A.‐M. van Dam2
1University of Amsterdam, Swammerdam Institute for Life Sciences, Amsterdam, Netherlands 2VU Medical Center, Dept. Anatomy and Neurosciences, Amsterdam, Netherlands
Introduction: It has become clear that the characterization of Parkinson's disease (PD) as an isolated disorder of the dopaminergic system is an oversimplification of the complex pathogenesis of the disease. Various brain areas are affected in PD, showing α‐synuclein (α‐syn) accumulation, a major hallmark of PD pathology. For instance, the olfactory bulb (OB) reveals α‐syn aggregates in an early stage of the disease, and the hippocampus (HC) is affected in a rather late stage of the disease (Braak et al., 2003). Furthermore, neuroinflammation, i.e. activation of microglial cells in the substantia nigra (SN), has been widely implicated in PD progression (McGeer et al., 1988). In the present study, we question whether microglial activation occurs in brain regions beside the SN which are affected in PD patients.
Methods: To this end, we studied microglial activation and protein pathology in the OB and HC of clinically and neuropathologically verified PD patients (Braak 4–6) and control subjects (Braak 0). Human post‐mortem formaldehyde‐fixed material of PD patients included SN (n = 13), OB (n = 9) and HC (n = 12), and material from age‐matched control subjects without neurological deficits included SN (n = 10),OB(n = 6), and HC (n = 8).
Results: The presence of α‐syn pathology concentrated in the anterior olfactory nucleus of the OB and in the CA1–2 region of the HC. Furthermore, using CD68 as a microglial marker, we observed a significant increase in the number of immunopositive microglial cells, with an activated, amoeboid morphology, in the SN as well as in the OB and HC of PD patients compared to control subjects. Co‐localization studies indicated that these activated microglia were found in the proximity of α‐syn inclusion bodies and neurites, but did not co‐localize suggesting that the microglial cells do not actively phagocytose α‐syn.
Conclusion: We conclude that microglial activation occurs in other brain regions beside the substantia nigra of PD patients. The functional role of the microglial cells in those brain regions and their contribution to PD pathology remains to be established.
REFERENCES
Braak, H., Tredici, K. D., Rüb, U., de Vos, R. A. I., Jansen Steur, E. N. H., and Braak, E. (2003) Staging of brain pathology related to sporadic Parkinson's disease. Neurobiology of aging 24, 197–211. McGeer, P. L., Itagaki, S.,
Boyes, B. E., and McGeer, E. G. (1988) Reactive microglia are positive for HLA‐DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology 38, 1285–6.
T10‐21B. Chemokine signalling in hippocampal lesions of Multiple Sclerosis patients
M. Prins 1, R. Dutta2, B. Trapp2, E. de Vries3, L. Janssens1, B. Drukarch1, A.‐M. van Dam1
1VU University Medical Center, Department Anatomy and Neurosciences, Amsterdam, Netherlands 2Cleveland Clinic, Lerner Research Institute, Cleveland, United States 3VU University Medical Center, Department of Molecular Cell Biology and Immunology, Amsterdam, Netherlands
Impaired cognition is present in 40–65% of Multiple Sclerosis (MS) patients, at least partly reflecting hippocampal dysfunction. Histopathological studies indicate a profound demyelination in the hippocampal grey matter together with microglial activation, which were more often found in cases with cognitive decline. We hypothesize that in MS, glial‐derived factors can directly or indirectly determine hippocampal neuronal functioning and fate. The chemokines CCL2 (MCP‐1) and fractalkine (CX3CL1), produced by glial cells in the brain are known to play a role in leukocyte infiltration. In addition, both CCL2 and CX3CL1 signalling can be neurotoxic or neuroprotective, possibly dependent on the surrounding cytokine milieu (Hughes et al., Glia, 2002). Finally, both chemokines are known to influence neurotransmission. Recently, CCL2 and CX3CL1 have been described to be associated with increased cognitive decline (Westing et al., PLoS One, 2012; Rogers et al., J. of Neuroinflammation, 2011). In the present study, we question whether CCL2 and CX3CL1 and its receptors are present in hippocampal lesions of MS patients. To this end, semi‐quantitative RT‐PCR was performed on cDNA transcribed from RNA isolated from hippocampi of MS patients and control subjects. Moreover, immunohistochemical analysis of CCL2, CX3CL1 and its receptors CCR2 and CX3CR1 was performed on post‐mortem, formalin‐fixed hippocampal lesions (PLP‐) from MS patients and on hippocampal material (PLP+) from control subjects. CCL2 and CCR2 mRNA was significantly enhanced in demyelinated MS hippocampal homogenates compared to non‐demyelinated and control hippocampal homogenates. CX3CR1 mRNA was significantly increased in demyelinated MS hippocampal homogenates compared tot non‐demyelinated hippocampal homogenates, but CX3CL1 mRNA levels showed no difference between groups. CCL2, CCR2, CX3CL1 and CX3CR1 immunoreactivity was present mainly in white matter of control and MS hippocampi and was clearly enhanced in hippocampal grey (cornu amonis) and white (stratum lacunosum, stratum radiatum, alveus) matter lesions. The intensity of the CCL2 and CX3CL1 immunoreactivity was most pronounced when active lesions were present. Co‐localization studies indicated that CCL2 and CX3CL1 are present in astrocytes, whereas CX3CR1 and CCR2 are present in or on microglial cells. We conclude that the chemokines CCL2 and CX3CL1 and their respective receptors are enhanced in hippocampal MS lesions. As no clear influx of leukocytes is observed in the grey matter lesions, we propose that astrocyte‐derived CCL2 and CX3CL1, via interaction with their receptors, affect microglial activation and/or function thereby contributing to neuronal dysfunction in the hippocampus as seen in MS patients.
T10‐22A. Characterization and modulation of HmIba1 as an activation marker for microglia in the invertebrate model, the leech Hirudo medicinalis
F. Drago 1, A. Accorsi2, P.‐E. Sautière1, F. Croq1, C. Lefebvre1, C. Van Camp1, J. Vizioli1
1University Lille 1, Villeneuve d'Ascq, France 2Universita di Modena e Reggio emilia, Dipartimento di Biologia, Modena, Italy
Question: Ionized Calcium‐binding Adapter molecule 1 (Iba1), is a 17 kDa cytokine‐inducible protein, produced by activated macrophages during chronic transplant rejection and in inflammatory reactions. In central nervous system (CNS), Iba1 is a sensitive marker associated to activated microglia and is upregulated following neuronal death or brain lesions. Iba1‐like factors have been described in several metazoan and share a well conserved amino acid primary structure throughout evolution suggesting a common, functional role.
The medicinal leech Hirudo medicinalis is able to regenerate its CNS after injury, leading to a complete functional repair. Similarly to Vertebrates, leech neuroinflammatory processes are linked to microglia activation and recruitment at the lesion site. We investigated the expression of Hirudo Iba1 to track the activation state of leech microglial cells involved in nerve repair events.
Results: We recently identified a gene, named HmIba1, coding a 17.5 kDa protein showing high similarity with Vertebrate Iba1 factor. Quantitative RT‐PCR analyses showed that HmIba1 is constitutively expressed in cultured nerve chains. A weak down regulation was observed in the days following experimental injury. Gene transcripts rise back to basal level one week later. Cultured nerve chains stimulated with ATP shows a significant increase of HmIba1 transcript 6 hours after treatment. Immunoblot analysis, performed with anti‐HmIba1 polyclonal antibodies, revealed an immunopositive band at the predicted size. The presence of HmIba1 protein in naïve and experimentally challenged tissues was evaluated by immunohistochemistry. The protein is constitutively present in spread, stellar shaped microglial cells, distributed in connective fibers and in segmental ganglia. A few hours after experimental injury of CNS, the amount of immunopositive microglial cells increases at the lesion site and at the cut end of nerve fibers until. The amount of HmIba1+ cells in connectives rapidly increases in ATP treated nerve chains. This augmentation is visible in ganglia microglia and in connective fibers, where cells located between axon fibers display an elongated and stretched shape.
Conclusion: HmIba1 is a good marker of activated microglia. Like in vertebrates, the ATP induces its expression in leech CNS. Also if the functional role of HmIba1 has to be further elucidated, this molecule appears as a good activation marker of microglia and an interesting tool to study and follow the activity of such cells during nerve repair in leech.
T10‐22B. Role of Microglia Activation in Neurogenic Hypertension
M. Santisteban1, J. Zubcevic1, S. Kim1, J.Y. Jun1, P. Shi2, E. Scott3, C. Sumners1, M. Raizada 1
1University of Florida, Physiology & Functional Genomics, Gainesville, United States 2Cedars‐Sinai Medical Center, Los Angeles, United States 3University of Florida, Molecular Genetics & Microbiology, Gainesville, United States
Hypertension is the single most important risk factor for cardiovascular disease. Despite significant advancements, 20–30% of all hypertensives remain resistant to all current available pharmacotherapy. These patients exhibit elevated sympathetic drive, increased norepinephrine spillover, and dampened parasympathetic drive. The dysfunctional autonomic nervous system of these resistant patients indicates a neurogenic origin for their pharmacotherapy resistance. Previous studies have proposed that neuroinflammation in the autonomic brain regions plays an important role in neurogenic hypertension. This coupled with the emerging interest in microglia led us to hypothesize that activation of microglial cells in the brain could be a critical event in the initiation and establishment of hypertension pathophysiology. We have previously established that chronic low dose angiotensin II (Ang II) induced hypertension involves activation of microglia, and increase in pro‐inflammatory cytokines and reactive oxygen species (ROS) in cardioregulatory brain areas, such as the paraventricular nucleus of the hypothalamus (PVN). Intracerebroventricular (ICV) delivery of minocycline, an anti‐inflammatory antibiotic that inhibits microglia activation, decreased activated microglia, inhibited increase in cytokines and ROS, and attenuated hypertension. In addition, inhibition of brain mitochondrial ROS attenuated hypertension, microglial activation, and the over‐expression of brain pro‐inflammatory cytokines. A time‐course experiment demonstrated that there was a significant increase in activated microglia before the increase in blood pressure was detectable by radiotelemetry. Furthermore, the origin of microglia in established hypertension appears to be both from resident and bone marrow derivation. These observations indicate that the activation of microglia is a critical player in the development of neurogenic hypertension. They suggest that activation of microglia in cardioregulatory regions of the brain is an early occurrence that may initiates a cascade of signaling events leading to increase sympathetic nerve activity and initiation of hypertension.
This research is supported by NIH grant (R37 HL 33610 to MKR).
T10‐23A. Inflammatory profiling of the satellite glial cells in the dorsal root ganglia of rat experimental neuropathic pain models
P. Dubovy 1, V. Brazda2, I. Hradilova‐Svizenska1, I. Klusakova1, L. Strejckova1
1CEITEC and Medical Faculty, Masaryk University, Brno, Czech Republic 2CEITEC Masaryk University, Cellular and Molecular Neurobiology, Brno, Czech Republic
The satellite glial cells (SGC) envelope the primary sensory neurons of the dorsal root ganglia (DRG) and react intensely to various types of nerve injury that induce neuropathic pain. Unilateral chronic constriction injury (CCI, n = 64) and spare nerve injury (SNI, n = 32) of the rat sciatic nerve well as i.p. application of paclitaxel (PAC, n = 12) were used as experimental models of neuropathic pain (NPP). NPP induction was tested by withdrawal threshold of mechanoallodynia and thermal hyperalgesia. Expression of TNFa, IL‐6 and their receptor and signaling (TNFR1, TNFR2, IL‐6R, gp130, STAT3) proteins and mRNA were investigated bilaterally by immunohistochemistry and in situ hybridization in both lumbar (L4–5) and cervical (C7–8) DRG 1, 3, 7 and 14 days from treatment. The SGC were identified by colocalization with GFAP or GS.
Our results demonstrate significant inflammatory activation of the neurons and their SGC not only in DRG associated but also non‐associated with injured nerve. A distinct expression of cytokines and their receptors was identified in SGC surrounding large‐sized DRG neurons. Significant inflammatory reactions of SGC were found in all DRG of PAC‐treated rats. Moreover, inflammatory activation SGC was also observed in DRG of sham‐operated rats indicating other kinds of trigger than traumatic nerve injury. The nerve injury and PAC‐treatment also triggered SOCS3 expression in SGC to control STAT3 activation. Inflammatory activation of SGC significantly contributes to ectopic activation of the DRG neurons not only associated with injured nerve, and is involved in NPP induction.
This work was supported by the project “CEITEC – Central European Institute of Technology” (CZ.1.05/1.1.00/02.0068) from European Regional Development Fund and the EU 7th FP under the “Capacities” specific programme (Contract No. 286154 – SYLICA)”.
T10‐23B. Priming of Microglia as Response to Neuronal Dysfunction in a DNA Repair Deficient Model of Accelerated Aging
D. Raj 1, D. Jaarsma2, M. Olah1, F. Ferreira2, I. Holtman1, W. Schaafsma1, N. Brouwer1, M. Meijer1, M. de Waard3, I. van der Pluijm3, R. Brandt3, K. Kreft4, J. Laman4, B. Dykstra5, G. de Haan5, B. Eggen1, K. Biber6, J. Hoeijmakers2, E. Boddeke1
1University Medical Center Groningen, Groningen, Netherlands 2Erasmus University Medical Center, Department of Neuroscience, Rotterdam, Netherlands 3Cancer Genomics Center, Erasmus University Medical Center, Department of Genetics, Rotterdam, Netherlands 4Erasmus University Medical Center and MS Center ErasMS, Department of Immunology, Rotterdam, Netherlands 5European Research Institute on the Biology of Aging, Department of Cell Biology, Groningen, Netherlands 6University Medical Center, Freiburg, Department of Psychiatry and Psychotherapy, Freiburg, Germany
Aging is associated with reduced function and degenerative changes of the central nervous system (CNS). Increasing evidence suggests that changes in microglia cells, i.e. the resident macrophages of the CNS, contribute to the age‐related deterioration of the CNS. The most prominent age‐related change of microglia concerns enhanced sensitivity to proinflammatory stimuli of microglia in mice, rats and primates, called priming. We have addressed this issue in ERCC1 mutant mice, Ercc1 Δ/‐ mice, a progeroid, DNA repair deficient mouse model that displays features of accelerated aging in multiple tissues including the CNS. In aged Ercc1 Δ/‐ mice, microglia showed increased proliferation and enhanced immune function including expression of cytokines and antigen presentation molecules, increased phagocytosis and production of reactive oxygen species (ROS). This microglial functionality was found to be surprisingly hyper‐responsive to immune stimuli indicative of microglia priming. Transcriptome analysis revealed an expression pattern that characterizes microglia priming, featuring genes associated with increased antigen pattern recognition and antigen presentation and phagocytic‐, adhesion‐ and chemotactic ability. In mice where the ERCC1‐related DNA repair deficit was targeted to forebrain neurons, microglia priming was restricted to forebrain areas, suggesting that the DNA damage‐induced changes in neurons provided the signals leading to microglial immune priming.
T10‐24A. Acidosis affects interleukin‐1β processing in glial cells
M. Edye, S. Allan, D. Brough
University of Manchester, Manchester, United Kingdom
Acidosis is a clinical consequence of many major diseases. Non‐infectious diseases are increasing in prevalence and many have been shown to have an inflammatory component, which in the absence of infection, will be sterile. The NLRP3 inflammasome, a multimeric protein complex which induces processing of IL‐1β into its active form, is a well established mediator of sterile inflammation and is known to drive the worsening of brain injury in numerous experimental disease paradigms. We sought to investigate whether acidic conditions, typical of a disease environment, affected IL‐1β processing in primary mouse glial cells.
Mixed glial cultures were grown from wild type and NLRP3 knock‐out mice. Culture media was reduced to pH6.2 and IL‐1β release was measured following addition of activators of the NLRP3 inflammasome (calcium pyrophosphate dihydrate crystals, monosodium urate crystals, ATP) with or without pre‐treatment with caspase‐1 or cathepsin D inhibitors (YVAD‐CHO or pepstatin A respectively). Subsequently, IL‐1β release was measured following addition of lactic acid with or without pre‐treatment with the above inhibitors.
At pH6.2, activators of the NLRP3 inflammasome (calcium pyrophosphate dihydrate crystals, monosodium urate crystals, ATP) induced the release of IL‐1β from mixed glial cultures. The IL‐1β released at this low pH was 20kDa in size in addition to the mature 17kDa IL‐1β. This 20kDa IL‐1β release was maintained in NLRP3 deficient cells and was not significantly altered by pre‐treatment with the caspase‐1 inhibitor YVAD‐CHO. Lactic acid, itself released during disease and a common cause of acidosis, also induced the release of 20kDa IL‐1β from mouse glial cultures. As with the NLRP3 activators under acidic conditions, the lactic acid‐induced 20kDa IL‐1β release was maintained in NLRP3 knock‐out cultures and with pre‐treatment with YVAD‐CHO. Pre‐treatment with the cathepsin D inhibitor pepstatin A, however, significantly reduced the 20kDa IL‐1β released with the NLRP3 activators and lactic acid.
Here we show that under disease relevant conditions (low pH), 20kDa IL‐1β is released from mouse glial cultures and this 20kDa IL‐1β is independent of the classical NLRP3 inflammasome/caspase‐1 pathway and likely mediated by cathepsin D. Further investigation of this caspase‐1‐independent IL‐1β pathway may in future provide novel targets for the treatment of inflammatory disease.
T10‐24B. Phoneutria nigriventer spider venom activates astrocytes and microglia through sGC‐cGMP inhibition
C. Raposo 1, J. Sant'Ana1, W.F. Cunha1, A.K. Santana Nunes2, C. Alves Peixoto2, M.A. da Cruz‐Höfling1
1Universidade Estadual de Campinas (UNICAMP), Campinas, São Paulo, Brazil 2Centro de Pesquisas Aggeu Magalhães – CPqAM/FioCruz, Laboratório de Ultraestrutura, Recife, Brazil
The Phoneutria nigriventer (Ctenidae‐Araneaeomorpha) spider venom (PNV) causes reactive gliosis, neuroinflammation and blood‐brain barrier (BBB) impairment. Nitric oxide(NO)‐soluble guanylate cyclase(sGC)‐cGMP has been implicated in PNV‐induced cavernosal relaxation. We investigate whether NO‐sGC‐cGMP signaling acts on astrocytes/microglia activation and neuroinflammation induced by PNV. Male Wistar rats (6–8‐week‐old) were pre‐treated (i.p.) with sGC inhibitor (ODQ, 10 mg/Kg), nNOS inhibitor (7‐nitroindazole‐7NI, 40 mg/Kg), NO donor (nitroprussiate‐NTP, 3 mg/Kg) or PDE5 inhibitor (sildenafil, 10 mg/Kg). After 30 minutes, the venom (0.85 mg/Kg) was injected in the tail vein (i.v.). Saline (i.v.), PNV alone or DMSO (i.p) followed by PNV were used as controls. One hour after injection, cerebella were processed for immunofluorescence or western blotting. PNV increased GFAP, Iba‐1, IFN‐γ and sGC, compared to saline control, indicating astrocytes and microglia activation, neuroinflammation and sGC‐cGMP involvement. The sGC inhibition by ODQ intensified the PNV effects, increasing even more GFAP, Iba‐1 and IFN‐γ levels. This indicates that sGC‐cGMP production can be inhibited by venom. In agreement, the cGMP accumulation by sildenafil inhibited the PNV effects, decreasing GFAP, Iba‐1, IFN‐γ and sGC. The increase of sGC by venom could result from a feedback mechanism, when sGC activity is inhibited and its expression is increased. 7NI and NTP pre‐treatment did not show difference compared to venom alone, suggesting that NO per se is not involved in the inflammatory effects of venom. However, it is not discarded NO and sGS‐cGMP coupling in the mediation of PNV effects. We suggest that PNV induces glial reaction and neuroinflammation by sGC‐cGMP inhibition. The study gives evidence that sGC‐cGMP, coupled or not to NO signaling, mediates PNV cerebellar effects including the BBB impairment. PNV can be a useful tool for studies on the mechanisms involved in glial regulation. CNPq/FAPESP/FAEPEX support.
T10‐25A. I‐TAC signalling in primary rodent astrocytes and human glioma cells requires CXCR4
J. Engele, M. Puchert, V. Ödemis
University of Leipzig, Leipzig, Germany
It is currently believed that CXCL12 predominantly signals through CXCR4. In addition, it is assumed that the alternate CXCL12 receptor, CXCR7, represents a non‐classical G protein‐coupled receptor which primarily acts as a modulator of the function of CXCR4. Discrepant from this view, we demonstrated recently that in primary rodent astrocytes and human glioma cell lines, SDF‐1 exclusively signals through CXCR7 by a G protein‐dependent mechanism. We now provide evidence that CXCR4 is essential for CXCL11/I‐TAC signalling in primary rodent astrocytes and some human glioma cells. Treatment of cultured rat astrocytes with CXCL11 for 10 min resulted in the dose‐dependent activation (phosphorylation) of Erk1/2 and Akt with maximum activation in the presence of 100 ng/ml of the chemokine. CXCL‐11‐dependent activation of both signalling molecules persisted in astrocytes in which expression of the established receptors for CXCL11, CXCR3 and CXCR7, were inhibited by RNAi. However, CXCL11‐dependent activation of Erk and Akt was abrogated following siRNA‐mediated inhibition of CXCR4. Likewise, CXCL11 failed to activate Erk and Akt in cortical astrocytes cultured from a CXCR4‐/‐ transgenic mouse line. Moreover, similar to primary astrocytes CXCL11 activated ERK and Akt in the human glioma cell line, A767. Again activation of both signalling molecules was abrogated following RNAi‐mediated inhibition of CXCR4. CXCL11‐dependent activation of Erk and Akt further remained undetectable in a non CXCR4‐expressing subpopulation of A767 cells, previously isolated by flow cytometry. Together, these findings unravel a unique processing of CXCL11 and CXCL12 signalling in primary astrocytes which is preserved at least in some malignant astroglial cells.
T10‐25B. In vivo imaging of astrocytes activation in rat hippocampus as a biomarker of epilepsy using H‐magnetic resonance spectroscopy
T. Ravizza, A. Frasca, E. Micotti, M. Filibian, A. Vezzani
Mario Negri Institute for Pharmacological Research, Milano, Italy
Long‐lasting activation of GFAP‐positive astrocytes occurs in brain tissue exposed to injuries that promote epilepsy development. Studies in experimental models of epilepsy showed that activated astrocytes release various molecules, such as proinflammatory cytokines and danger signals, that play a role in seizures generation and recurrence. These activated cells also loose their ability to buffer extracellular K+ and glutamate. This set of evidence suggests, therefore, that astrocytes may contribute to epileptogenesis (i.e. the post‐injury phase prodromal to generation of spontaneous seizures), thus representing a putative biomarker of epilepsy. To test this hypothesis, we set up an in vivo longitudinal study using 1H‐magnetic resonance spectroscopy (MRS) to measure the hippocampal levels of metabolites that could reflect the extent and the duration of astrocytes activation after an epileptogenic brain injury.
Status epilepticus (SE), which provokes epilepsy, was induced by pilocarpine in adult male rats. 1H‐MRS measurements were done in the hippocampus every 24 h for 7 d post‐SE, thus encompassing the epileptogenesis phase, and in chronic epileptic rats using a 7 Tesla Bruker Biospec. Spectra were processed and analysed using jMrui and TARQUIN freeware softwares. We studied changes in myo‐inositol (mIns) and glutathione (GSH), which reflect astrocytes activation.
We found a progressive (2‐fold, pwhich was maintained in epileptic rats. Immunohistochemical analysis (IHC) of S100ß and GFAP confirmed the concomitant activation of astrocytes in separate time‐matched groups of rats. GSH levels during epileptogenesis showed a negative correlation with the frequency of spontaneous seizures which developed after SE. A negative correlation was also found between GSH and mIns levels during epileptogenesis and the extent of neurodegeneration in hippocampus of epileptic rats. IHC done in epileptic rats at the end of the MRS experiments, showed that hippocampal S100ß levels positively correlated with spontaneous seizure frequency. Since this is a soluble protein, further investigations of its CSF and blood levels are warrented.
Our MRS findings show that GSH levels during epileptogenesis could serve as a predictive biomarker of the ensuing seizure frequency (thus of epilepsy severity) and, together with mIns levels, also predict the extent of neuronal cell loss which develops following SE in epileptic tissue. Notably, IHC‐detected S100ß levels in the epileptic tissue also reflect seizure frequency. These findings highlight the potential use of serial 1H‐MRS analysis of astrocyte activation for predicting the severity of epilepsy and the extent of neuropathology in the clinical setting.
T10‐26A. Astrocytary IL‐6 involment in EAE and traumatic lesions
M. Erta, M. Giralt, S. Jimenez, G. Comes, J. Hidalgo
Universitat Autònoma Barcelona, Bellaterra/ Barcelona, Spain
Interleukin‐6 (IL‐6) is a highly plurifunctional cytokine, with many pleitropic actions, considered one of the main cytokines controlling the immune system and coordinating it with the nervous and endocrine systems.
IL‐6 is produced in multiple cell types in the CNS, and in turn many cells do respond to it. It is therefore important to ascertain which the contribution of each cell type is in the overall role of IL‐6 during both physiological and pathological conditions. Astrocytes are major responders to IL‐6 as well as one of the main CNS producers of IL‐6. For this work we used astrocytary IL‐6 KO (Ast‐IL6 KO) mice, which we already proved to have an important role in physiological conditions (like body weight control and exploratory/ locomotion behavior), in order to test astrocytary IL‐6 role during a neuroinflammation situation. For this purpose, we induced either an extensively used animal model of Multiple sclerosis, experimental autoimmune encephalomyelitis (EAE), or a traumatic brain injury (cryolesion) in our mice.
Regarding EAE, results indicate that lack of astrocytary IL‐6 delays clinical course of EAE and ameliorate EAE symptomatology in Ast‐IL6 KO respect littermate controls in a gender‐dependent way. Further immunohistochemistry analyses confirm a decreased number of cellular and lymphocytes infiltrates and lesser demyelination, angiogenesis and gliosis in spinal cord of Ast‐IL6 KO animals. Regarding traumatic injury, astrocytary lack of IL‐6 facilitates microgliosis, lymphocytes infiltration and a faster decrease of the injured area.
All these results are likely to bring some answers to astrocyte‐secreted IL‐6 involvement in neuroinflammation pathways and EAE pathogenesis.
T10‐26B. IL‐10 induces changes in microglial reactivity following perforant pathway transection in GFAP‐IL10Tg mice
M. Recasens 1, B. Almolda1, C. de Labra1, I. Barrera1, M.J. Hofer2, I.L. Campbell2, B. Gonzalez1, B. Castellano1
1Universitat Autonoma de Barcelona, Dept. Cell Biology, Physiology and Immunology, Institute of Neurosciences, Bellaterra, Spain 2School of Molecular Bioscience, University of Sydney, Sydney, Australia
Interleukin‐10 (IL‐10) is a counterregulatory cytokine that plays an important role in controlling inflammatory and immune reactions. In the central nervous system (CNS), production of IL‐10 has been demonstrated in activated astrocytes and microglia after different types of injuries. The specific role played by IL‐10 inmodulating glial responses is however still unclear. Hence, the objective of this study was to evaluate the effects of local astrocyte‐targeted production of IL‐10 on glial reactivity using the axonal anterograde degeneration paradigm. For this purpose, unilateral perforant pathway transection (PPT) was performed on adult GFAP‐IL10 transgenic (Tg) animals and their corresponding wild types (wt) littermates. At 3 and 7 days post‐lesion (dpl), animals were intracardially perfused with 4% of paraformaldehyde, brains frozen and parallel free‐floating sections processed for glial analysis using immunohistochemistry for Iba‐1 (microglia) and GFAP (astrocytes). Our results showed that in comparison with wt animals, microglial cells in the dennervated dentate gyrus molecular layer of GFAP‐IL10Tg showed differential reactivity changes. After lesion, astrocytic reactivity presented a higher hypertrophy in GFAP‐IL10Tg animals when compared with their corresponding wt littermates. In conclusion, this study demonstrated that local production of IL‐10 inthe CNS can modify the glial response associated with PPT. Further studies are warranted to evaluate if these alterations in microglial and astrocytic reactivity have any repercussions for the evolution of the lesion and the associated axonal sprouting.
Supported by Ministerio de Ciencia e Innovación (BFU2008‐04407/BFI) and (BFU2011‐27400).
T10‐27A. Imaging reactive astrocytes in vivo by positron emission tomography with TSPO radioligands
S. Lavisse1, M. Guillermier1, A.‐S. Hérard1, F. Petit1, M. Delahaye1, N. Van Camp1, L. Ben Haim1, V. Lebon1, F. Dollé2, T. Delzescaux1, G. Bonvento1, P. Hantraye1, C. Escartin 1
1MIRCen, Fontenay‐aux‐Roses, France 2SHFJ, Orsay, France
Astrocyte and microglia become reactive under many brain pathological conditions, making this process of neuroinflammation a surrogate marker of neuronal dysfunction.
Several studies have reported that reactive microglia overexpress the translocator protein 18kDa (TSPO, formerly known as peripheral benzodiazepine receptor). Positron emission tomography (PET) using radioligands of TSPO is thus considered as a potent technique to detect reactive microglia in situ. However, it is still controversial whether TSPO PET imaging is also able to monitor reactive astrocytes in situ. This is an important question as reactive astrocytes and reactive microglia play very different roles in brain physiology and could impact disease progression in opposite ways.
To address this question, we used a model of selective astrocyte activation through unilateral lentiviral gene transfer of the cytokine ciliary neurotrophic factor (lenti‐CNTF) in the rat striatum. CNTF induced an extensive activation of astrocytes, which overexpressed GFAP and became hypertrophic. Microglia, on the contrary, displayed a minimal increase in the expression of markers of reactivity.
CNTF‐activated astrocytes overexpressed TSPO at the mRNA and protein levels. PET imaging experiments demonstrated a significant and specific binding of two TSPO radioligands [18F]DPA‐714 and [11C]SSR180575 in the lenti‐CNTF‐injected striatum.
We show that reactive astrocytes can be monitored by TSPO‐PET imaging in the rat brain. This technique is thus well suited to monitor reactive microglia as well as reactive astrocytes, the two cell types involved in neuroinflammation, but would not allow their discrimination in situ.
T10‐27B. Interferon gamma protects mice from CNS autoimmunity also in the absence of IL‐17A and IL‐17F
T. Regen 1, J. Huppert1, N. Yogev1, I. Prinz2, A. Waisman1
1University Medicine, Johannes‐Gutenberg‐University, Institute of Molecular Medicine, Mainz, Germany 2Hannover Medical School, Institute of Immunology, Hannover, Germany
Background: The role of interferon (IFN)‐gamma‐producing T helper (Th) 1 and interleukin (IL)−17‐expressing Th17 lymphocytes in multiple sclerosis (MS) and its animal model, experimental autoimmune encephalomyelitis (EAE) is still subject to intense investigations. Objective: Here we sought to decipher the specific involvement of these Th cell subsets during manifestation and the clinical phase of EAE. Special emphasis was put on IL‐17 as effector cytokine and its cellular targets in the CNS context. Methods: We used a double‐knockout mouse lacking both IL‐17A and IL‐17F (IL‐17AFKO). Additionally, this mouse was crossed to the routinely used IFN‐gamma deficient mouse, resulting in triple‐knockout IL‐17AFKO/IFNgKO animals. Mice were subjected to EAE induction with monitoring of the disease course and subsequent flow cytometric and histological analysis of CNS infiltrates. Results: As demonstrated before, following active MOG peptide immunization, IFNgKO mice developed an exacerbated EAE disease course compared to wild type C57Bl/6 controls. In contrast, IL‐17AFKO animals failed to induce prominent disease which was accompanied by a dramatic reduction of CNS‐infiltrating immune cells. This phenotype could be partially reversed in triple‐knockout animals as these mice showed a delayed onset of EAE with severity of disease reaching the level of wild type controls. Essentially, these results were confirmed in a model of passive EAE where in vitro‐restimulated mutant T cells were adoptively transferred into wild type recipients. Transfer of IFNgKO as well as triple‐knockout cells resulted in disease of comparable levels whereas IL‐17AFKO cells failed to induce any signs of EAE. In addition, pilot experiments with cell‐specific ablation of IL‐17 receptor signaling revealed an essential role for brain endothelial cells as well as microglia in the initiation and development of the EAE disease course. Conclusions: Our results argue for a non‐redundant role of IL‐17(AF) for the induction of experimental neurodegenerative disease. Moreover, the beneficial role of IFN‐gamma in EAE seems to be independent of the action of IL‐17. Finally, IL‐17 essentially targets individual cell types at the various disease stages.
T10‐28A. Cross‐talk between neurons and non‐neuronal cells within sensory neurons: effects on ATP‐gated P2X3 receptors
A. Franceschini1, S. Vilotti1, T. Bele2, A.M.J. Van Den Maagdenberg3, A. Nistri1, E. Fabbretti 2
1International School for Advanced Studies (SISSA), Trieste, Italy 2University of Nova Gorica, Vipava, Slovenia 3University Medical Centre, Dept Human Genetics, Leiden, Netherlands
Enhanced activity of trigeminal ganglion neurons is thought to underlie neuronal sensitization facilitating the onset of chronic pain attacks. Chronicity might establish a neuroinflammatory ganglion profile with inflammatory cells contributing to the hypersensitive phenotype. We first investigated whether, in trigeminal sensory ganglia, cytokines such as TNFa might contribute to a local inflammatory phenotype of a transgenic mouse model of familial hemiplegic migraine type‐1 (FHM‐1, Cacna1a R192Q knock‐in mice). With respect to wild‐type, R192Q KI trigeminal ganglia were enriched in activated macrophages and expressed higher mRNA levels of IL1b, IL6, IL10 and TNFa cytokines and the MCP‐1. Functional consequences of crosstalk between macrophages and sensory neurons were studied in primary ganglia cultures, where larger release of soluble factors and larger currents mediated by pain‐transducing ATP‐gated P2X3 receptors were found. Consistently, we observed that, following LPS injection, TNFa expression and macrophage occurrence were significantly higher in R192Q knock‐in ganglia with respect to wild‐type ganglia. Our data suggest that, complex cellular and molecular environment of sensory ganglia could support a new tissue phenotype compatible with a neuroinflammatory profile. We propose that, in selected patients, this condition might contribute to pain pathophysiology through release of soluble mediators, including TNFa and ATP that may modulate the crosstalk between sensory neurons and resident glia, underlying the sensitisation process. Supported by Telethon (GGP10082), Cariplo Foundations, ARRS grant (1000‐11‐310161) and Crossborder Cooperation Programme Italia – Slovenia grant MINA.
T10‐28B. Immune response in rat neuron‐glia co‐cultures infected with Neospora caninum: role of oxide nitric synthetase inducible
C.S. Ribeiro 1,2, M.D.F. Costa2, A. Santos2, M. Arruda2, E. Jesus2, G. Ferraz2, A. Pinheiro2, L. Jesus3, S. Costa1
1UFBA, Biochemistry, Salvador, Brazil 2UFRB, Biochemistry, Cruz das Almas, Brazil 3UFBA, Salvador, Brazil
Neospora caninum is a protozoan that infects a wide range of animal and tissues and it is encysted preferentially in the central nervous system (CNS) of their hosts. Previous studies showed that when mixed cultures of astrocyte/microglia are stimulated with IFN‐gamma and then infected with tachyzoites of Neospora caninum they release nitric oxide (NO) that controls parasite proliferation. In order of elucidating the immune response of CNS against this parasite, this study investigated the participation of inducible nitric oxide synthase (iNOS) in the control of its proliferation in co‐cultures of neuron‐glia obtained from rat brain. The cells were stimulated with IFN‐gamma (100 IU/mL‐24h) then supplemented with L‐NG‐Nitroarginine methyl ester/L‐NAME, an inhibitor of iNOS (1.5 mM/mL – 60 min) before infection with tachyzoites of N. caninum (1:1 parasite:cell). After 72h, in the cultures supplemented with L‐NAME, it was verified, a reduction of tachyzoites number in 55.7% (control cells) and 59.8% (IFN‐y stimulated cells). However, in infected co‐cultures, it was not observed any release of NO, even when cells where modulated by IFN‐g. Additionally, in cultures infected and treated with L‐NAME, the levels of IL‐10 were increased in six fold (non stimulated) and nine fold (IFN‐g stimulated). These data suggest that NO should not participate as a mediator of N.caninum control in neuron/glia co‐culture but, the regulatory pattern of immune response must play a role in its down modulation regulating the inflammatory mediators released during the CNS infection, perhaps to protect neurons.
T10‐29A. Cannabidiol provides long‐lasting protection against the deleterious effects of inflammation in a viral model of multiple sclerosis: a role for A2A receptors
A. Feliu, M. Mecha, F.J. Carrillo‐Salinas, C. Guaza
Cajal Institute, CSIC, Madrid, Spain
Inflammation in the central nervous system (CNS) is a complex process that involves a multitude of molecules and effectors, and it requires the transmigration of blood leukocytes across the blood‐brain barrier (BBB) and the activation of resident immune cells. Cannabidiol (CBD), a non‐psychotropic cannabinoid constituent of Cannabis sativa, has potent anti‐inflammatory and immunosuppressive properties. Yet, how this compound modifies the deleterious effects of inflammation in TMEV‐induced demyelinating disease (TMEV‐IDD) remains unknown. Using this viral model of multiple sclerosis (MS), we demonstrate that CBD decreases the transmigration of blood leukocytes by downregulating the expression of vascular cell adhesion molecule‐1 (VCAM‐1), chemokines (CCL2 and CCL5) and the proinflammatory cytokine IL‐1β, as well as by attenuating the activation of microglia. Moreover, CBD administration at the time of viral infection exerts long‐lasting effects, ameliorating motor deficits in the chronic phase of the disease in conjunction with reduced microglial activation and pro‐inflammatory cytokine production. Adenosine A2A receptors participate in some of the anti‐inflammatory effects of CBD, as the A2A antagonist ZM241385 partially blocks the protective effects of CBD in the initial stages of inflammation. Together, our findings highlight the anti‐inflammatory effects of CBD in this viral model of MS, and demonstrate the significant therapeutic potential of this compound for the treatment of pathologies with an inflammatory component.
T10‐29B. Microglial activation In Contralateral Mice Retina To Experimental Glaucoma
B. Rojas 1, B.I. Gallego1,2, J.J. Salazar1,2, R. de Hoz1,2, A.I. Ramírez1,2, F.J. Valiente‐Soriano3, M. Avilés‐Trigueros3, J. Ramírez1,4, A. Triviño1,4
1Universidad Complutense Madrid, Inst Invest Oftalmol Ramón Castroviejo, Madrid, Spain 2Universidad Complutense (UCM), Facultad Optica y Optometria, Madrid, Spain 3Universidad de Murcia, Departamento de Oftalmologia, Murcia, Spain 4Universidad Complutense (UCM), Departamento Oftalmologia, Facultad Medicina, Madrid, Spain
Purpose: To analyze qualitative and quantitative effects of two weeks of laser‐induced ocular hypertension (OHT) in microglial population of eyes with OHT (OHT‐eyes) and contralateral eyes.
Methods: Adult albino Swiss mice were organized in two groups: naive (age‐matched control; n = 6) and lasered (n = 6). Anti‐Iba1 was used in retinal whole‐mounts immunolabelled to analyze the distribution, morphology, density and arbor area of Iba1+ microglial cells.
Results: In naïve, contralateral and lasered eyes, Iba1+ microglias were distributed throughout the retina in the: subretinal space (SS), outer plexiform layer (OPL), inner plexiform layer (IPL), nerve‐fibre layer (NFL) and ganglion‐cell layer (GCL). In naïve eyes, Iba1+ microglias: i) had rounded bodies with fewer and shorter processes in the SS; ii) exhibited a branched morphology emanating from small cell bodies in the OPL and IPL; iii) were less ramified and were related with the retinal vessels in the NFL and in the GCL. By contrast, the morphological features exhibited by Iba1+ cells in contralateral and OHT‐eyes differed from naïve: i) somas were more robust; ii) processes were thicker and more branched in contralateral eyes and thicker and retracted in OHT‐eyes.
In OHT‐eyes and contralateral untreated eyes the Iba1+ microglial number was increased in comparison with naïve (p < 0.001 and p < 0.05 respectively, t‐test).
In comparison to naïve retinas the Iba‐1+ cell arbor in the OPL and IPL decreased in both OHT‐eyes (p < 0.001 and p < 0.001 respectively, t‐test) and in the contralateral untreated eyes (p < 0.05 and p < 0.001 respectively, t‐test).
Conclusions: Two weeks of laser induced‐OHT triggered microglial retinal changes suggestive of activation in the contralateral untreated and OHT‐eyes. The microglial activation in contralateral eyes could be related to the immune response. This behaviour in contralateral untreated eyes, lead us to suggest that the use of contralateral eyes as internal controls in experimental unilateral OHT, should be reconsidered.
T10‐30A. Lack of astrocytic interleukin‐6 enhances high‐fat diet‐induced obesity in mice
O. Fernández Gayol, M. Giralt, A. Molinero, B. Ferrer, G. Comes, J. Hidalgo
Universitat Autonòma de Barcelona, Bellaterra, Spain
Interleukin‐6 (IL‐6) is a pleiotropic cytokine involved in inflammatory and non‐inflammatory responses. Among the latter, it participates in the regulation of body weight and metabolism. IL‐6‐deficient mice develop mature onset obesity and have higher blood glucose, as well as impaired glucose tolerance and elevated blood leptin levels, suggesting that IL‐6 participates in suppressing adiposity in mice. Moreover, transgenic mice with astrocyte‐targeted production of IL‐6 challenged with a high‐fat diet are resistant to high‐fat diet‐induced obesity, highlighting the role of centrally produced IL‐6 in the regulation of body weight.
In this context, we hypothesize that mice lacking IL‐6 produced by astrocytes (ast‐IL6KO) will be more prone to develop obesity. We therefore challenged ast‐IL6KO mice (obtained with the Cre‐lox technology) with a high fat diet (58% kcal from fat) for 17 weeks and compared their body weight and food intake with those of mice fed a standard diet (18% kcal from fat). On weeks 14 and 15, the metabolic status was evaluated by an insulin tolerance test (ITT) and an oral glucose tolerance test (OGTT), respectively.
Regarding body weight gain, we see a clear increase in ast‐IL6KO females on a high‐fat diet in comparison to their controls with no apparent difference in food intake, which is also already apparent in the control diet. In males a similar tendency is observed.
Part of this difference can be attributed to the heavier subcutaneous white adipose tissue depots (relative to body weight) in ast‐IL6KO mice (Fig 1).
Insulin and oral glucose tolerance are compromised in mice fed a high‐fat diet, but no differences between genotypes are observed.
Taken together, these results indicate that centrally produced IL‐6 has indeed a major role in the regulation of body weight, affecting subcutaneous white adipose tissue, but without significantly altering peripheral glucose metabolism.
We are currently working on assessing hypothalamic neuropeptides with in situ hybridization to study the effects of astrocytic IL‐6 deficiency at the central level.

T10‐30B. The mechanism of analgesia after minocycline administration in a rat model of neuropathic pain
E. Rojewska, W. Makuch, B. Przewłocka, J. Mika
Institute of Pharmacology, Krakow, Poland
An increasing number of data suggest a significant role of the immune response in the development of neuropathic pain. The changes in biosynthesis of immune factors appear in the spinal cord and DRG parallelly to immune and glial cell activation. Minocycline is an agent with pleiotropic properties that targets multiple proteins and cellular processes associated with development of neuropathic pain, including inhibition of the injury‐induced glia activation. The aim of our study was to examine the effects of minocycline on the injury‐induced changes in immune factors and on morphine effectiveness in a rat model of neuropathic pain. Chronic constriction injury (CCI) to the sciatic nerve in rats was performed according to Bennett and Xie (1988) and 2 behavioral tests were conducted to measure allodynia (von Frey test) and hyperalgesia (cold plate test). Minocycline was administered intraperitoneally 16h and 1h before CCI and then twice daily for 7 days. The studies were performed using competitive RT‐PCR in the spinal cord and DRG from control, CCI‐exposed and minocycline‐treated CCI‐exposed rats. Morphine was administrated i.t. (chronic catheterization according to Yaksh and Rudy, 1976) and ip 1h after the last minocycline injection. The experiments were carried out according to IASP rules (Zimmermann, 1983). Repeated administration of minocycline attenuated allodynia and hyperalgesia when measured 7 days after CCI. Minocycline downregulated the spinal and DRG level of several immune factors: C1q, MMP‐9, MMP‐2, IL‐1beta, IL‐6, IL‐18, NOS1 and NOS2 which were increased in consequence of the injury. Moreover, chronic administration of minocycline improved the response to i.t. and i.p. morphine injections. Our results demonstrate that minocycline reduces the level of pro‐inflammatory factors and increases the effectiveness of morphine, which may have clinical significance for enhancing analgesic effects of opioids in neuropathic pain.
Acknowledgements: This work was supported by grant NCN 2011/03/B/NZ4/00042 and statutory funds.
T10‐31A. A novel human in vitro microglia model
L. Filgueira 1, S. Etemad2, M. Ruitenberg3
1University of Fribourg, Fribourg, Switzerland 2University of Western Australia, Perth, Australia 3University of Queensland, Biomedical Sciences, Brisbane, Australia
Microglia are the resident innate immune cells of the brain. As the brain macrophages, microglia share common characteristics with the monocyte‐macrophage lineage such as expression of surface markers, chemokine and cytokine receptors, phagocytosis and antigen presentation. Microglia responds with activation to injury, pathogen, as well as endogenous and exogenous toxins. This activation is usually followed by secretion of pro inflammatory cytokines and chemokines. Activated microglia may contribute to pathological processes, but they play also a key role in the development of the central system and in neurogenesis through secretion of neurotrophic factors.
To date, there is no standardized simple model available to investigate the biology of human microglia. The aim of this study was to establish a new in vitro microglia model using blood‐derived precursor cells. For that purpose, human peripheral blood monocytes were cultured in serum free medium in the presence of a mixture of cytokines and chemokines (M‐CSF, GM‐CSF, NGF and CCL2) to generate monocyte‐derived microglia (M‐MG). Monocyte‐derived dendritic cells (M‐DC) were also generated as a control population using GM‐CSF and IL‐4. The human microglia cell line HMC3 was used as control.
M‐MG were clearly different in morphology, phenotype and function from M‐DC, but shared many properties with HMC3 cells. M‐MG acquired a ramified morphology with primary and secondary processes, comparable to HMC3. They expressed very low levels of CD45, CD14 and HLA‐DR, CD11b and CD11c; but a distinct pattern of chemokine receptors, including CCR1, CCR2, CCR3, CCR4, CCR5, CXCR1, CXCR3, CX3CR1. Similar to HMC3, under non‐activated condition, the M‐MG secreted of IL‐8 and IL‐6. In comparison with M‐DC, M‐MG displayed lower T‐lymphocyte stimulatory capacity, as well as lower phagocytosis activity.
In summary, we have established a new protocol for the generation of human monocyte‐derived microglia, which is is feasible, well standardized and reliable, as it uses well defined culture medium and recombinant cytokines, but no serum or conditioned medium. This model will certainly be very helpful for future studies investigating the biology and pathology of human microglia.
T10‐31B. Epigenetic memory in microglia?
W. Schaafsma
University of Groningen, Neuroscience, Section Medical Physiology, Groningen, Netherlands
Background: Microglia are the innate immune cells of the CNS. Like other tissue macrophages, they can activate and commit to distinct reactive phenotypes in response to tissue damage and infections. However, the microglial response to tissue damage may change over age and by past experience. These changes in activation patterns may be caused by epigenetic modifications which in turn can result in changes in gene expression of, for example inflammatory cytokines. A well studied condition with an altered responsiveness of innate immune cells is ‘endotoxin tolerance' (ET). Innate immune cells that are pre‐exposed to endotoxins are dampened in their inflammatory response upon re‐exposure to these endotoxins. While ET is well described for peripheral macrophages/monocytes, very little is known about ET in relation to microglia.
Objective: In our experiments we set out to investigate the presence of ET in microglia and if an ‘epigenetic' memory is involved.
Design/methods: In our in vitro experiments, microglia cells were stimulated with LPS (100 ng/ml). Naive microglia only received one LPS stimulation at the end of culture, pre‐stimulated microglia received an 24h pre‐stimulation of LPS (100 ng/ml) and a second stimulation 5 days later. In vivo, male C57Bl/6 mice received an i.p. injection with either PBS or LPS (1 mg/kg) and 4 weeks later again an i.p. injection with PBS or LPS. Transcription levels and secretion of IL1‐β and TNF‐α were assessed by use of quantitative PCR and ELISA. In order to answer if there is a long lasting epigenetic memory involved in this tolerance we used chromatin immune‐precipitation (ChIP), to investigate changes in covalent histon tail modifications associated with either permissive or repressed chromatin.
Results: In vitro, we show a ‘tolerant' phenotype in microglia which receive a second LPS stimulation after 5 days. A reduced expression level of pro‐inflammatory genes IL1‐β and TNF‐α and reduced secretion of TNF‐α were observed in response to a second LPS challenge. Furthermore, with in vivo experiments we showed that mice which received a second LPS injection after 4 weeks, showed a dampened response in their microglia inflammatory reaction at the level of IL1‐β and TNF‐α gene transcription. In both in vitro and in vivo experiments we showed a corresponding pattern for histon tail modifications in the enrichment of activation marks H3K4me3 and AcH3 at the IL1‐β and TNF‐α promotors.
Conclusion: We are the first to show a long lasting ‘tolerant' phenotype in mouse microglia in vitro and in vivo and the involvement of epigenetic changes at the level of histon modifications.
T10‐32A. Expression of Calreticulin and Other Endoplasmic Reticulum Stress Molecules in a rat model of inflammatory demyelination
U. FitzGerald 1, M. Ní Fhlathartaigh1, J. McMahon1, R. Reynolds2
1National University of Ireland, Galway, Ireland 2Imperial College London, London, United Kingdom
Endoplasmic reticulum (ER) stress is a signalling pathway, linked to many neurological diseases including multiple sclerosis (MS). Studies of human tissue in our lab have shown ER stress‐associated molecules at increased levels, in early biopsy MS samples and in cortical lesions. Increasing evidence suggests that calreticulin (CRT), a multifunctional ER‐resident protein and ER stress responder, plays a role in cell clearance and it has been implicated in autoimmunity. To extend our human tissue work, we set up an EAE model of inflammatory demyelination in Dark Agouti (DA) rats and report the profile of expression of CRT and other ER stress signalling molecules in demyelinated spinal cord lesions and control samples. To induce spinal cord demyelination, the DA rats were injected with recombinant murine myelin oligodendrocyte (rmMOG) protein mixed with incomplete Freund's adjuvant (IFA). Control rats were injected with a PBS/IFA or with 100 ml of PBS only. Animals were then monitored over a 43‐day period and clinical scores assigned. Molecular analyses was carried out by real‐time PCR to determine transcriptional expression profiles of Grp78/BiP, CHOP, CRT and XBP‐1. BiP and CHOP showed a trend towards up‐regulation whereas spliced XBP1 displayed a downward trend in EAE, compared to controls, but this was not statistically significant. In contrast, immunohistochemical staining of tissue sections allowed semi‐quantitative comparison of protein expression in the lesion (L), lesion edge (LE), normal‐appearing white matter (NAWM), central canal (CC) and grey matter (GM). Statistical analyses revealed that CHOP (p < 0.001), p‐EIF2a (p < 0.01) and CRT (p < 0.05) were significantly increased in EAE spinal cord white matter lesions and in the normal‐appearing white matter compared to healthy control tissue. Interestingly, there was also an up‐regulation of CHOP (p < 0.05), and p‐eIF2a (p < 0.01) in the grey matter and central canal of diseased spinal cord tissue. Morphological criteria and dual immunofluorescent labelling confirmed expression of ER stress protein in neurons, astrocytes, microglia or oligodendrocytes. An intriguing finding was localisation of CRT to the rim of ORO‐positive myelin fragments and the ‘patchy' nature of CRT staining seen when tissue was dual‐labelled with CRT and GFAP or IBA1. These results are the first demonstration of significantly higher levels of CRT in rodent EAE. CHOP and p‐eIF2a data has also not been reported in rat EAE. This study highlights the potential importance of ER stress in inflammatory demyelination.
The authors acknowledge support from the Irish Research Council and from the Foundation Office of NUI, Galway.
T10‐32B. Differential regulation of the Wnt signaling pathway in experimental autoimmune encephalomyelitis
R. Schneider 1, B. Bosch1, F. Schröter2, J. Ingwersen1, C. Berndt1, H.‐P. Hartung1, T. Prozorovski1, O. Aktas1
1Medical Faculty Heinrich‐Heine‐University, Neurology, Düsseldorf, Germany 2Medical Faculty Heinrich‐Heine‐University, ITZ, Düsseldorf, Germany
In chronic autoimmune diseases of the central nervous system (CNS) such as multiple sclerosis (MS) clinical signs of cognitive deficit have been recently associated with structural changes such as demyelination and synaptic damage, particularly in areas such as the hippocampus (1, 2). Here we have evaluated possible underlying cellular and molecular mechanisms in a mouse model of MS, experimental autoimmune encephalomyelitis (EAE). We have recently observed that neurogenic processes are enhanced (up‐regulation of DCX+/BrdU+ cells), while neuronal differentiation is compromised (down‐regulation of NeuN+/BrdU+ cells) in the hippocampus of affected animals (3). Considering the responsible signaling pathways regulating adult neurogenesis (4), we observed differential regulation of Wnt members in the hippocampus on transcriptional level, e.g. Wnt dependent transcription factors (Axin2, TCF4) and ligands (Wnt3, 5a, 8b and 11), as well as on translational level represented by the Wnt signaling cascade (active‐bCatenin, phospo‐glycogen synthase kinase 3b) during acute and chronic states of disease. By using in vitro studies in primary hippocampal cultures, we further show the role of transforming growth factorβb1 (TGFb1), a key cytokine early involved during EAE (5), in regulation of Wnt ligand expression and Wnt signaling activity. Furthermore we are able to visualize the connection between TGFb1 and Wnt signaling by using the Axin2‐LacZ mouse, a Wnt reporter mouse, for functional and histological investigation of the hippocampus. Taken together our results suggest a cross talk of inflammation and Wnt signaling for dysregulated hippocampal neurogenesis.
(1) Dutta et al., Ann. Neurol., 2011.
(2) Aktas et al., Ann. Neurol., 2011.
(3) Huehnchen et al., Glia, 2011.
(4) Suh et al., Annu. Rev. Cell Dev. Biol. 2009.
(5) Luo et al., J. Clin. Invest. 2007.
T10‐33A. Brain response to traumatic brain injury: interleukin‐6 relevance
M. Giralt, O. Fernandez, R. Ramos, A. Molinero, J. Hidalgo
Universitat Autònoma de Barcelona, Bellaterra, Barcelona, Spain
Interleukin‐6 (IL‐6) is a cytokine with major regulating effects of the inflammatory response. Moreover, IL‐6 is a neuropoietin that has neurotrophic effects related to neuronal survival and protection. To establish the importance of IL‐6 produced only in the central nervous system, we have generated mice producing IL‐6 essentially only in the brain by crossing GFAP‐IL6 mice (transgenic mice with astrocyte‐targeted production of IL‐6) with IL‐6 KO mice (IL‐6‐deficient). We studied the inflammatory response in a traumatic brain injury model, cryolesion, after 3 and 10 days post‐lesion GFAP‐IL6‐IL6 KO mice, comparing them with appropriate controls.
In basal conditions WT and IL‐6 KO mice showed a similar phenotype. This was also the case for GFAP‐IL6 and GFAP‐IL6‐IL‐6 KO mice, which showed prominent astrogliosis, microgliosis, increased recruitment of T lymphocytes and vascularisation compared with the other two groups.
In response to cryolesion of the cortex an increased astrogliosis, microgliosis, recruitment of T lymphocytes and vascularisation was observed. IL‐6 deficiency produced an altered inflammatory response, in a time‐and gender‐dependent manner.
This study with IL‐6 KO and GFAP‐IL6 transgenic mice indicates that during an acute neuropathological insult such as traumatic brain injury IL‐6, from either the brain or the periphery, has an important role on the inflammatory response.
T10‐33B. Development of an in vitro model to study the impact of iron in inflammatory demyelinating diseases
C. Schuh 1, S. Hametner1, I. Wimmer1, M. Lindner2, C. Elliott2, M. Bradl1, C. Linington2, H. Lassmann1
1Center for Brain Research, Vienna, Austria 2University of Glasgow, Institute of Infection, Immunity and Inflammation, Glasgow, United Kingdom
Inflammatory processes play a key role in various neurodegenerative diseases such as Multiple Sclerosis (MS), Alzheimer's Disease and Parkinson's Disease. Increasing evidence suggests that iron accumulation in the brain might contribute to neurodegeneration. Iron is a potential source of free radicals as it can catalyze the production of hydroxyl radicals under oxidative conditions. This can lead to amplification of tissue injury caused by oxidative damage.
We thus characterized iron storage within the central nervous system (CNS) of animal models of different neurodegenerative diseases. We examined animals with acute inflammation mediated by CD8 or CD4 positive T cells and animals suffering from T cell and antibody mediated chronic inflammation due to active immunization. Further, we studied LPS induced lesions which represent CNS disease caused by the innate immune system. Similarly, we characterized oxidative damage in the different models for inflammation. We did not find evidence for the presence of oxidized phospholipids or oxidized DNA in the experimental lesions, which is in contrast to our results of MS lesions. None of these models showed iron accumulation in glial cells comparable to what is seen in MS tissue. However, some iron positive microglia and perivascular macrophages were observed in acute and chronic models.
In preliminary experiments we found evidence that the presence of iron exacerbates H2O2 induced cell death in purified glial cells in vitro. As a next step, we wanted to study a possible amplification of oxidative damage and neurodegeneration by iron in a more elaborate system. For this purpose we treated myelinating spinal cord cultures with iron chloride (FeCl3) or ferritin. We observed iron loading in microglia in both experimental setups. Moreover we found a selective decrease of microglia in iron chloride treated cultures but not after ferritin application. We did not find iron loaded oligodendrocytes in this in vitro model.
Iron accumulation is only minimal compared to the human brain in all the tested animal models. These observations necessitate the search for additional animal models mimicking the human situation more closely.
T10‐34A. Are microglia just macrophages? Analysis of both cell types functions after brain injury
S. Girard, D. Brough, S. Allan
University of Manchester, Manchester, United Kingdom
Objectives: Microglia are a brain resident population of immune cells, involved in homeostatic surveillance of the brain parenchyma, with high importance in the regulation of inflammatory processes after injury. There are unresolved questions regarding microglia origin, and their similarities to peripheral macrophage populations. Our objective was to compare the capacity of microglia and bone marrow derived macrophages (BMDMs) to adopt different phenotypes, and how this influenced cell death after brain injury.
Methods: We compared the phenotype of BMDMs and microglia (both BV2 microglial cell line and primary microglia) after treatment with well known polarising agents. Lipopolysaccharide (LPS) was used as an inducer of the classical M1 phenotype and IL‐4 was used to induce an alternative M2 phenotype. Microglia or BMDMs, of different phenotypes, were added to hippocampal organotypic slices (HOSC) subjected to oxygen glucose deprivation (OGD) as an in vitro model of brain injury.
Results: BMDMs and microglia (BV2 and primary microglia) both adopt M1 or M2 phenotypes after treatment with LPS or IL‐4 respectively. However, addition of polarised microglia or BMDMs onto HOSC resulted in different outcomes. Addition of activated BMDMs, of either M1 or M2 phenotypes, led to cell death in control HOSC and increased death when combined with OGD. On the other hand, addition of BV2‐microglia did not induce any cell death in control HOSC, and were protective after OGD, except for M1‐microglia which were toxic. Endogenous microglia within the HOSC were also able to adopt different phenotypes and exert neuroprotection after IL‐4 treatment. These ex vivo data correlated with the in vivo situation where we observed increases in microglial activation early after experimental stroke, although the vast majority of these activated cells did not express markers of the M1 phenotype.
Conclusions: This study highlights functional differences between macrophages and microglia, especially in response to brain injury. Although microglia, like peripheral macrophages, can adopt different phenotypes, their functions are fundamentally different in a model of brain injury. These data provide new insights into the protective role of microglia after brain injury.
T10‐34B. Inflammatory mediators increase expression of tissue Transglutaminase, but not Factor XIIIa, in primary human monocytes
C. Sestito 1, N.L. Chrobok1, J.J. Breve1, S. van der Pol2, H.E. de Vries2, P.J. van den Elsen3, M.M. Wilhelmus1, B. Drukarch1, A.‐M. van Dam1
1VU University Medical Center, Department of Anatomy and Neurosciences, Amsterdam, Netherlands 2VU University Medical Center, Department of Molecular Cell Biology and Immunology, Amsterdam, Netherlands 3Leiden University Medical Center, Department of Immunohematology and Blood Transfusion, Leiden, Netherlands
Introduction: Multiple sclerosis (MS) is a chronic neuroinflammatory disease of the central nervous system (CNS). Although the pathogenesis is still not clear, activation of the immune system at some point of the disease process plays a crucial role. It is known that myelin‐specific autoreactive T‐cells and monocytes cross the blood‐brain barrier (BBB) and migrate into the CNS. Within the CNS, these cells secrete pro‐inflammatory cytokines which stimulate local glial cells to produce inflammatory factors, including reactive oxygen species that contribute to demyelination and ultimately axonal loss. The destruction of the BBB and the influx of monocytes and T‐cells from the circulation are key events in the progression of MS pathology. Tissue Transglutaminase (TG2) is a multifunctional enzyme that has been shown to play a role in monocyte/macrophage adhesion and migration onto extra cellular matrix proteins in vitro. This binding occurs via interaction with β‐integrins. Previously, we observed the appearance of TG2 in monocytes in active human post‐mortem MS lesions in the CNS.In the present study we question whether TG2 expression is regulated in primary human monocytes by inflammatory mediators and whether it is modulated in MS patients.
Methods: To this end, TG2 in monocytes was detected by semi‐quantitative RT‐PCR.
Results and Conclusions: Our preliminary results show that activation of primary human monocytes with LPS or LPS+IFNγ results in a time‐dependent increase in TG2 mRNA whereas the expression of Factor XIIIa (another member of the Transglutaminase family) remains stable over time. Moreover, the TG2 mRNA level is higher in unstimulated monocytes derived from MS patients compared to control subjects while Factor XIIIa mRNA level remains unaffected.
These initial data are promising with respect to a possible role for monocyte‐derived TG2 being involved in adhesion to and migration across the BBB under inflammatory conditions (e.g. Multiple Sclerosis). This hypothesis has not been proven yet. In addition, by increasing the number of patients, a consistent difference in TG2 expression in monocytes between MS patients and control subjects may point to TG2 as a novel biomarker for disease.
T10‐35A. Regulation of microglial proliferation in chronic neurodegeneration
D. Gomez‐Nicola, N.L. Fransen, S. Suzzi, V.H. Perry
University of Southampton, Southampton, United Kingdom
An important aspect of chronic neurodegenerative diseases, such as Alzheimer's, Parkinson's, Huntington's and prion disease, is the generation of an innate inflammatory response within the central nervous system (CNS). Microglial and astroglial cells play a key role in the development and maintenance of this inflammatory response, showing enhanced proliferation and morphological activation. Using a laboratory model of chronic neurodegeneration (ME7 murine model of prion disease), we studied the time‐course and regulation of microglial proliferation. Our results show that resident microglial cells have an increased proliferation rate during the development of the disease, leading to a significant increase in the population, without a contribution from circulating cells. Microglial proliferation is differentially regulated in diverse regions of the CNS, pointing to a heterogeneous development of the pathology. We have identified novel molecular regulators of the proliferative response, and addressed the significance of the contribution of microglial cells to the pathological course of the disease by modifying their proliferation. We also found a correlation of our results with the scenario present in chronic human neurodegenerative conditions variant Creutzfeldt‐Jakob Disease (vCJD) and Alzheimer's disease. Our results demonstrate that microglial proliferation is an important feature of the evolution of chronic neurodegenerative disease, with direct implications for understanding the contribution of the CNS innate immune response to disease progression.

T10‐35B. TLR3 and‐4 mediated fibronectin aggregation after demyelination impairs remyelination
A.H. Sikkema, M.J. Vos, B. Mikus, P. de Boer, K. Dörenkämper, J.C. de Jonge, A. Nomden, D. Hoekstra, W. Baron
University Medical Center Groningen, Cell Biology, Groningen, Netherlands
Demyelination in the central nervous system elicits extensive extracellular matrix (ECM) remodeling. Although a local ECM response is essential in the initial steps of injury repair, remyelination is only initiated after clearance of the altered ECM. Previously, we have shown that the ECM protein fibronectin is expressed upon demyelination, while its persistent presence in multiple sclerosis (MS) lesions, almost exclusively in the form of stable aggregates, impairs remyelination. Here, we examined the underlying mechanism of fibronectin aggregation and address whether inflammatory mediators, such as toll like receptor (TLR) agonists or pro‐inflammatory cytokines induce fibronectin aggregation by astrocytes.
Our findings reveal that only TLR3 and −4 agonists, including the endogenous TLR3 agonist stathmin, increased stable fibronectin aggregation. Interestingly, only white matter‐derived astrocytes displayed fibronectin aggregation whereas in cortical astrocyte cultures fibronectin remained unaffected, suggesting regional differences in the functional response of astrocytes. Furthermore, in rat cerebellar slice cultures, the TLR3 agonists only induce aggregation of fibronectin after lysolecitin‐induced demyelination. In this manner, efficient remyelination is impeded and, consequentially, progressive neuronal decline occurs.
Taken together, TLR3 and −4 agonists promote fibronectin aggregation by primary rat astrocytes. Since TLR3 on astrocytes and stathmin are both upregulated in MS lesions, stathmin, as an endogenous TLR3 agonist, might play a role in inducing fibronectin aggregation after demyelination, thereby creating an unfavorable environment for remyelination. Exploring efficient ways to prevent or overcome TLR‐induced fibronectin aggregation by astrocytes might pave the way for new approaches in remyelination‐targeted therapeutic strategies in MS.
T10‐36A. Altered microglial response in pre‐symtomatic SOD1 mutant mediated disease
M. Gravel, J. Kriz, E. Abdelhamid
Universite Laval – CHUQ, Quebec, Canada
Although major clinical symptoms in Amyotrophic lateral sclerosis (ALS) disease arise from the degeneration and death of motor neurons, excitotoxicity from glial cells dysfunction and inflammatory processes are very likely to be involved in the neurodegenerative process. Microglial activation is associated with a significant induction of several Toll‐like receptors (TLRs). To study the TLR2 response in‐vivo, SOD1G93A mice were crossed with the TLR2‐Luc‐GFP reporter mice, a transgenic mouse bearing the dual reporter system luciferase and green fluorescent protein under the transcriptional control of the murine TLR2 promoter. Double transgenic mice and littermates controls were monitored longitudinally using in‐vivo biophotonic/bioluminescent imaging to measure microglial activation over the course of the disease.
Surprisingly, this analysis did not reveal an increase of activation in SOD1G93A mice as the disease progressed, with only weak signal coming from olfactory bulb and brain area. However, quantification of this signal suggested a lower level of TLR2 activation in the SOD1G93A mice compared to wild‐type littermates. To further study the microglial impairment observed in the SOD1G93A mice, these transgenic mice were given LPS with i.p. injections at 5 mg/kg and were followed for 48h by bioluminescence imaging, both at a pre‐symptomatic age (60 days) and closer to paralysis (115 days). The level of TLR2 activation was lower in the SOD1G93A mice than the wild‐type littermates, both at 60 days and 115 days. Immunostaining of olfactory bulbs reveal the same phenomenon, which is less Iba1 and less TLR2‐positive cells in the SOD1G93A tissue. mRNA analysis by in‐situ hybridization confirms a similar pattern of microglial response. In a parallel study, primary microglial cell culture, derived from adult mice (60days) were similarly stimulated with LPS to further understand the altered SOD1 mutant microglia response. Here again, SOD1 cells appears less responsive to LPS stimulation as observed in our mice studies. These combined results suggest that SOD1G93A microglial cells might have a pre‐symptomatic suboptimal immune response.
T10‐36B. Temporal and regional pattern of expression of pro‐inflammatory and anti‐inflammatory genes in mouse experimental autoimmune encephalomyelitis
T. Valente1, G. Dentesano1,2, U. Perpiñà1, J. Saura 2, C. Solà 1
1IIBB‐CSIC, IDIBAPS, Cerebral Ischemia and Neurodegeneration, Barcelona, Spain 2University of Barcelona, IDIBAPS, Biochemistry and Molecular Biology Unit. School of Medicine, Barcelona, Spain
The role of reactive glia in the etiology and progress of neurological diseases is still unknown. Reactive glia produce several factors, typical of an inflammatory response, with a potential neurotoxic effect, highlighting the relevance of a strict control of the progression and resolution of glial activation. Under some circumstances glial activation does not resolve, but it exacerbates or becomes chronic resulting in detrimental secondary effects. It is necessary to develop strategies to halt the negative outcome of glial activation in these situations, by controlling the pro‐inflammatory phenotype of reactive glial cells and potentiating their beneficial effects.
We studied the temporal pattern of inflammatory reaction in spinal cord and brain regions in the experimental autoimmune encephalomyelitis (EAE) model of multiple sclerosis. Special attention was paid to the involvement of members of the C/EBP family of transcription factors, which regulate the expression of pro‐inflammatory genes in reactive glia, and CD200, CD200R1 and TREM‐2, membrane‐associated inhibitors of the pro‐inflammatory response in resting/surveillant microglia. Mice were scored for signs of EAE for up to 28 days postimmunization (DPI). EAE symptoms were present from 10 DPI, reaching score peak at 14 DPI. In the spinal cord, we observed a sustained increase in C/EBPα and a transient increase in C/EBPβ and C/EBPδ expression peaking at 14 DPI. CD200 expression decreased at 9 DPI, remaining below control values at 28 DPI. CD200R1 and TREM‐2 expression increased at 14 DPI, thereafter this effect progressively attenuated. No alterations were observed in the brain. An acute increase in the expression of pro‐inflammatory genes (IL‐1β, IL‐6, TNF‐α, iNOS, COX2) occurred in the spinal cord at 14 DPI.
Our results suggest that CD200 expression decreases before onset of EAE symptoms resulting in downregulation of the microglia inhibitory signal mediated by CD200‐CD200R1, which would facilitate the pro‐inflammatory response observed after onset of the EAE symptoms. The increase in CD200R1 expression observed after the onset of EAE symptoms suggests a concomitant intend of microglia/macrophages to resolve the inflammatory response. The transient increase in C/EBPβ and C/EBPδ expression could be related to the acute increase in pro‐inflammatory gene expression, while the sustained increases of C/EBPα expression and TREM‐2 could be involved in the resolution of the inflammatory response.
Supported by grants PI10/378 and PI12/00709 (Instituto de Salud Carlos III, Spain).
T10‐37A. Stimulation of the IL‐1 Signaling Pathway by CNS‐Infiltrating Myelin Reactive T Cells in Zones of Axonal Degeneration
M. Grebing 1, H.H. Nielsen2, C. Fenger1, M. Söderman1, K.T. Jensen1, B.H. Clausen1, K.L. Lambertsen1, M. Thomassen3, T.A. Kruse3, B. Finsen1
1University of Southern Denmark, Odense, Denmark 2Odense University Hospital, Department of Neurology, Odense, Denmark 3Odense University Hospital, Department of Human Genetics, Odense, Denmark
Recently, we showed a beneficial effect of myelin reactive T cells on oligodendrocyte precursor cell differentiation in zones of axonal degeneration in the hippocampal dentate gyrus, which mirrors grey matter injury in multiple sclerosis. This effect was associated with a marked expression of T‐cell cytokines, such as Interferon‐γ and interleukin‐17, enhanced microglial clearance of myelin debris, and an enhanced sprouting of calretinergic axons. To gain better insight in which cytokines and signaling pathways are elevated in response to the T cell enhanced regenerative responses, we performed a mRNA microarray study, in which the transcript profile in hippocampi from mice with adoptively transferred proteolipid protein specific T cells combined with an axonal lesion were compared to the transcript profile from mice with axonal lesion. Thereby we identified 1446 transcripts, which were differently expressed. We identified the interleukin‐1 (IL‐1) signaling pathway as a potential target by performing pathway analysis using DAVID and AmiGO databases. We discovered that IL‐1α, IL‐1β and IL‐1 receptor antagonist (RA) mRNA as well as the IL‐1 signaling pathway were highly upregulated in response to myelin reactive T cells. In order to determine if IL‐1β mRNA was translated into protein, we performed immunohistochemical stainings on tissues from mice with 2 days post lesion survival. Expression of IL‐1β was observed in the molecular layer of T cell infiltrated, but not in T cell naïve mice with axonal lesion, suggesting that myelin reactive T cells stimulate IL‐1β protein expression. Ongoing studies will determine the expression of IL‐1α and IL‐1RA protein and determine the cellular expression of the IL‐1 signaling pathway in T‐cell infiltrated versus non‐T cell infiltrated mice with axonal lesion. Understanding the regulation of the expression of IL‐1α, IL‐1β and IL‐1RA in T cell compared to non‐T cell infiltrated CNS may lead to a better understanding of the functional consequences of inflammatory responses in the CNS.
Supported by the Danish Multiple Sclerosis Society, the Augustinus Foundation and the University of Southern Denmark.
T10‐37B. Expression of metabotropic glutamate receptor 4 (mGluR4) in glial cells and its involvement in oligodendrocyte survival
S.F. Spampinato 1, S. Merlo1, T. Sinagra1, R. Calabrese1, F. Nicoletti2, M.A. Sortino1
1University of Catania, Catania, Italy 2university of Rome Sapienza, Physiology and Pharmacology, Rome, Italy
Metabotropic glutamate receptors (mGluR) are expressed in different cell types in the CNS and are differently involved in neurodegeneration/neuroinflammation. We have focused our attention specifically on mGluR4, since L‐2‐amino‐4‐phosphonobutanoate (L‐AP4), an orthosteric agonist specific for group III mGluRs, including mGluR4, attenuates clinical signs of experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis. Furthermore, mice lacking mGluR4 are more susceptible to EAE. These effects have been ascribed to a peripheral protective action mediated by mGluR4 limiting the immune response during neuroinflammation. The aim of our study was to investigate the role of mGluR4 in in the CNS, in different glial cell types, under conditions of neuroinflammation. Immature oligodendrocytes (IO) were maintained in culture in chemically defined medium supplemented with PDGF and FGF and grown for 2–3 days. These cultures, PDGFR+/MBP‐, express mGluR4 as detected by immunocytochemical analysis. mGluR4 was also detectable in a low percentage of microglia cells and astrocytes under basal conditions and its expression increased upon activation induced by lipopolysaccharide (LPS) and/or interferon‐γ (IFN‐γ). Treatment with L‐AP4 (50 μM) reduced IO cell death induced by exposure to kainic acid (0.3‐1 mM/24 h) or hydrogen peroxide (H2O2, 100 μM), an effect prevented by the specific mGluR4 antagonist cyclopropyl‐4‐phosphonophenylglycine (CPPG; 100 μM). When IO were challenged with kainate in the presence of conditioned medium (CM) from astrocytes, susceptibility to kainate‐induced cell death was reduced, but CM from L‐AP4‐treated astrocytes reverted kainate toxicity. In contrast, treatment with CM from control or LPS‐activated microglia, either untreated or pre‐exposed to L‐AP4, was not able to affect kainate‐induced IO cell death. To establish whether mGluR4 activation could modulate the antigen presenting activity of microglia, the BV2 microglia cell line was used. Treatment with L‐AP4 (50 μM/48 h) did not affect changes in morphology induced by activation with LPS (0.1 μg/ml/48 h), but significantly reduced the percentage of cells expressing MHC class II, as detected by flow cytometry. These data suggest that during neuroinflammation, activation of mGluR4 directly on IO or through astrocytes and microglia may contribute to a protective effect resulting in survival of the oligodendrocyte lineage.
T10‐38A. Upregulation of c‐Jun in the 3xTrg AD model regulates miR‐155 expression in glial cells and contributes to neuroinflammation
J. Guedes 1,2,3, L. Pereira de Almeida3,4, M.C. Pedroso de Lima3,5, A.L. Cardoso3
1University of Coimbra, Center for Neuroscience and Cell Biology, PDBEB – PhD Programme in Experimental Biology and Biomedicine, Coimbra, Portugal 2University of Coimbra, IIIUC – Institute for Interdisciplinary Research, Coimbra, Portugal 3University of Coimbra, CNC – Center for Neuroscience and Cell Biology, Coimbra, Portugal 4University of Coimbra, Laboratory of Pharmaceutical Technology, Faculty of Pharmacy, Coimbra, Portugal 5University of Coimbra, Department of Life Sciences, Faculty of Science and Technology, Coimbra, Portugal
MiRNAs are small non‐coding RNA molecules that modulate gene expression at a post‐transcriptional level and their role in the regulation of biological processes makes them an emerging class of therapeutic targets. Dysregulation of miRNA networks has been linked to neurodegeneration and immune dysfunctions and has become a research focus in the context of Alzheimer's disease (AD).
In this work, we demonstrate that miR‐155 expression is increased in the brain of 3×Trg AD transgenic mice, prior to senile plaque formation, and co‐localized with intraneuronal APP accumulation in the cortex and hippocampus. In view of the pro‐inflammatory functions of miR‐155 and aiming at clarifying its role in AD, we evaluated the levels of miR‐155 in Aβ‐stressed astrocytes and microglia cultures, two brain‐related cell types involved in neuroinflammation. We show that miR‐155 expression levels are increased in astrocytes and microglia following activation with Aβ fibrils but not with Aβ oligomers. These findings correlate with the observed decrease in SOCS‐1 expression, a miR‐155 validated target, and with the increase in the production of TNF‐α, IL‐1β and IL‐6 by these cells. Importantly, we show that miR‐155 expression is regulated by c‐Jun, since silencing of this transcription factor led to the reduction of miR‐155 levels following astrocyte and microglia exposure to Aβ fibrils or LPS. c‐Jun was also found to be upregulated in the 3×Trg AD transgenic model since early ages, which can help to explain the observed early increase in miR‐155 expression.
Overall, our results demonstrate the important role of miR‐155 in AD and show that silencing of c‐Jun in glial cells may constitute an interesting and promising anti‐inflammatory therapeutic strategy towards this disease, by targeting miR‐155‐mediated inflammatory responses.
T10‐38B. TGFβ‐mediated regulation of classical and alternative microglia activation states
B. Spittau 1, X. Zhou1, L. Wullkopf1, J. Rilka1, D. Pfeifer2, K. Krieglstein1
1Albert‐Ludwigs‐University, Institute for Anatomy and Cell Biology/Department of Molecular Embryology, Freiburg, Germany 2Albert‐Ludwigs‐University, University Medical Center, Department of Hematology/Oncology, Freiburg, Germany
Microglia are the resident immune cells of the central nervous system and play important roles under physiological and pathophysiological conditions. Recent studies have resulted in the definition of specific microglia activation states which are referred to as classical and alternative activation. Whereas Interferon‐γ (IFNγ) promotes the classical microglia activation characterized by the release of neurotoxic molecules such as tumor necrosis factor α (TNFα) and nitrc oxide (NO), Interleukin 4 (IL4) triggers the neuroprotective alternative microglia activation. Transforming growth factor β (TGFβ) has been described as a potent immunoregulatory factor for macrophages and lymphocytes. Here we demonstrate that TGFβ is an important endogenous factor promoting quiescence of microglia in vitro. Inhibition of microglial TGFβ signalling resulted in a microglia polarisation towards the classical activation state. Moreover, TGFβ is able to significantly decrease IFNγ‐induced classical activation of primary microglia and to enhance IL4‐induced alternative microglia activation. We further provide evidence that IL4‐mediated alternative microglia activation is dependent on active TGFβ signalling. These results demonstrate the essential functions of TGFβ as an important regulator of microglia activation states and further suggest that TGFβ might be an interesting therapeutical molecule to modulate microglial functions during development and progression of neurodegenerative diseases.
T10‐39A. Ageing augments the behavioural response to systemic Salmonella Typhimurium infection in mice – do microglia play a role?
A. Hart, S.G. Booth, U. Püntener, V.H. Perry, J. Teeling
University of Southampton, Southampton, United Kingdom
Infections often induce sickness behaviour, such as lethargy, social withdrawal and anhedonia. This behavioural response is exaggerated during ageing as a consequence of microglial priming1. In this study we extended our previous work2 to examine whether S. typhimurium infection causes a prolonged behavioural response, whether ageing modulates the sickness behaviours induced by S. typhimurium infection and what involvement microglia may have in these changes.
Young (3m) and aged (18m) C57/Bl6 mice were administered 3 × 105 CFU of attenuated S. typhimurium (SL3261) via intraperitoneal injection and tested in several behavioural assays at 3 different timepoints – before infection, 1 week after infection or 3 weeks after infection. The behavioural assays used were the novel object recognition test, open field test, static rod test and the forced swim test. Body weights were recorded daily. Tissue was collected from saline treated and mice infected for 1, 8, 15, 22 or 61 days and analysed by qPCR.
We found that young mice lost weight only in the first 24h after infection, whereas aged mice exhibited a biphasic pattern of weight loss, with the second phase of weight loss beginning 1 week after infection and continuing for approximately 2½ weeks. Aged mice also demonstrated a co‐ordination and balance deficit in the static rod test 1 week after infection compared to uninfected or 3 week post infection aged mice, whereas young mice were unimpaired at any timepoint. S. typhimurium infection caused a significant, progressive reduction in open field rearing activity at 1 week and 3 weeks after infection in both young and aged mice. A similar but not significant trend was apparent in novel object performance. Forced swim test data showed a trend towards depressive behaviour at 1 week after infection, but not at 3 weeks. Analysis of pro‐inflammatory mediators in the hippocampus did not indicate significantly upregulated IL‐1β, TNF‐α, COX‐1 or COX‐2 transcript at any timepoint, suggesting that microglia might not play a significant role in mediating these behavioural effects, or that the changes in these molecules were too subtle to reliably detect using qPCR in the hippocampus. The effects of ageing on S. Typhimurium induced weight and co‐ordination changes may be a result of regional differences in the sensitivity of microglia to peripheral infection within the CNS or increased sensitivity to activation of neuronal circuits involved in weight loss or co‐ordination with age.
1. Godbout et al, FASEB, 2005, 19:1329
2. Püntener et al, J Neuroinflammation, 2012, 9:146.
T10‐39B. Presence of severe neuroinflammation does not intensify the extent of neurofibrillary degeneration
W. Streit, Q.‐S. Xue
University of Florida, Gainesville, FL, United States
We have studied microglial cells with anti‐Iba1 and anti‐CD68 immunohistochemistry in the brains of 18 subjects with pretangle neuropathology ranging from 4 to 51 years of age. Regions examined included the locus coeruleus and surrounding brainstem areas, entorhinal cortex, hippocampal formation, and other parts of the mesial temporal lobe. Ten of the subjects (median age 34) showed severe neuroinflammation secondary to infectious disease (HIV, sepsis, toxoplasmosis) and CNS trauma, while 8 subjects (median age 32.5) died from non‐infectious conditions and lacked neuroinflammatory changes (controls). We also examined two 52‐year‐old subjects with Down syndrome both of which showed Braak stage VI neurofibrillary degeneration, where one had comorbid sepsis and the other one did not. Pretangle neuropathology was staged 1a and 1b in all 18 subjects, i.e. they exhibited primarily subcortical tau pathology in the locus coeruleus and only sporadic lesions in the transentorhinal cortex. Our findings show that rampant microglial activation in the ten subjects with infections and trauma was coincident with the same level of tau pathology as seen in the 8 control subjects, demonstrating that neuroinflammation does not initiate or exacerbate neurofibrillary degeneration. In addition, our findings show that the extent of neurofibrillary pathology seen in Down syndrome is unrelated to neuroinflammation since the Down subject without comorbid sepsis revealed a complete absence of microglial activation. Overall we conclude that neuroinflammation is not causally involved in the development of neurofibrillary degeneration which is most characteristically associated with Alzheimer's disease dementia.
T10‐40A. Peripheral poly I:C‐induced neuroinflammation: role of Toll‐like receptor 3 (TLR3) in microglia
M. Ifuku 1, S. Hossain1, M. Noda2, T. Katafuchi1
1Kyushu University, Fukuoka, Japan 2Kyushu University, Laboratory of Pathophysiology, Fukuoka, Japan
It has been suggested that peripheral infection/inflammation may play a role in the onset or development of not only neurodegenerative diseases but also depression, autism and chronic fatigue syndrome through inflammatory responses in the brain. To investigate the role of microglia in this hypothesis we used a model of neuroinflammation induced by an intraperitoneal (i.p.) injection of the Toll‐like receptor 3 (TLR3) agonist, polyinosinic:polycytidylic acid (poly I:C) in rats. As we reported previously an injection of poly I:C (i.p) decreased the daily amounts of spontaneous running wheel activity to ∼60% of the preinjection levels until day 7. Microglia were morphologically activated in the prefrontal cortex (PFC) until 72 hrs after the injection of poly I:C. Pretreatment with minocycline for the 3 consecutive days (40 mg/kg/day) blocked the poly I:C‐induced decrease in the running wheel activity as well as microglial activation. Quantitative analysis of mRNA levels demonstrated that interferon‐α (IFN‐α) increased in the PFC on both days 1 and 7. We found in the present study that poly I:C injection induced increases in mRNAs relevant to TLR‐signaling such as TLR3, interferon regulatory factor 3 (IRF3) and IRF7 in the PFC. Furthermore, direct application of poly I:C to the primary cultured microglia also induced an enhanced expression of IFN‐α, TLR3, IRF3 and IRF7. It has been reported that permeability of blood‐brain barrier is increased after poly I:C injection (Wang et al., 2004). Therefore, it is possible that the peripheral poly I:C enters into the brain to induce neuroinflammation by activating microglia. Interestingly, intracerebroventricular (i.c.v.) injection of primary cultured microglia activated by poly I:C, but not by lipopolysaccharide, effectively produced a significant decrease in spontaneous activity in rats. Taken together, these findings suggest that microglial activation is important for poly I:C‐induced neuroinflammation through inducing TLR3‐related genes. Roles of each gene in the behavioral changes will be further studied.
T10‐40B. Regulation of progranulin expression and modulation of progranulin by matrix metalloproteinase‐12 (MMP‐12) and secretory leukocyte protease inhibitor (SLPI) in human microglia
H.‐S. Suh, N. Choi, S.C. Lee
Albert Einstein College of Medicine, Department of Pathology, New York, United States
Objective: Progranulin (PGRN) is a growth factor widely expressed in mammalian tissues, where it is involved in cell proliferation, wound healing and modulation of inflammation. Haploinsufficiency resulting from PGRN gene mutations causes frontotemporal lobar dementia, a fatal progressive neurodegenerative disorder. PGRN contains seven and half granulin domains. Proteolytic cleavage of PGRN by elastase generates granulins and other molecular weight peptides. This process can be inhibited by secretory leukocyte protease inhibitor (SLPI). The full‐length PGRN and granulin peptides have been shown to have opposite roles in inflammation. In addition to neurons, microglia express PGRN in the CNS, but little is known about the regulation of PGRN expression by microglia and its biological function. In the current study, we examined the regulation of expression of PGRN, its proteolytic enzyme macrophage elastase (MMP‐12), as well as the inhibitor of PGRN proteolysis, SLPI, in human CNS cells.
Methods: Cultures of primary human microglia and astrocytes were stimulated with the TLR ligands (LPS or poly IC), Th1 cytokines (IL‐1/IFNγ), or Th2 cytokines (IL‐4, IL‐13). The roles of MMP‐12 and SLPI in PGRN cleavage were also examined using recombinant protein. Results were analyzed by Q‐PCR, immunoblotting, ELISA or immunoprecipitation.
Results: Microglia produced nanogram levels of PGRN (10 fold higher in microglia than in astrocytes), and PGRN release from microglia was suppressed by the TLR ligands or IL‐1/IFNγ, but increased by IL‐4 or IL‐13. Unexpectedly, while astrocytes stimulated with proinflammatory factors released large amounts of SLPI, none were detected in microglial cultures. We also identified MMP‐12 as a PGRN proteolytic enzyme, and SLPI as an inhibitor of MMP‐12‐induced PGRN proteolysis in human microglia.
Conclusions: Our results establish microglia as a significant source of PGRN. MMP‐12 and SLPI are modulators of microglial PGRN proteolysis. Negative and positive regulation of microglial PGRN release by the proinflammatory/Th1 and the Th2 stimuli, respectively, suggests a fundamentally different aspect of PGRN regulation compared to other known microglial activation products. Microglial PGRN appears to function as an endogenous modulator of innate immune responses.
T10‐41A. Is an inflammation caused by injury necessary for the brain?
H. Ikeshima‐Kataoka 1,2, S. Inui2, M. Yasui2, Y. Matsui3, T. Uede4
1Waseda Univ., Facul. Sci. & Engin., Tokyo, Japan 2Keio Univ. Sch. Med., Dept. Pharmacol. & Neurosci., Tokyo, Japan 3Hokko Memorial Hosp., Div. Cardiovasc. Med., Hokkaido, Japan 4Hokkaido Univ., Dept. Matrix Med., Inst. Genet. Med., Hokkaido, Japan
At pathological condition such as injury or ischemia to the central nervous system (CNS), microglial cells and astrocytes become activate and inflammation occurs like proliferate, migrate and secrete some pro‐inflammatory cytokines, IL‐1ß, TNF‐α or IL‐6. However, it is unclear that the reason why inflammation is induced in the CNS, and whether or not it is necessary for the brain during recovery from pathological situation.
To know the molecular mechanisms for inflammation occurs in activated glial cells, we made a stab wound mouse model to the brain. We performed microarray analysis for RNA extracted from the brain tissue around stab wound site compared among day after 0, 1, 3 and 7. It revealed that most of the genes from top 20 genes at high expression level around lesion site were concerned in immunological or inflammatory functions. We successfully identified and focused on to osteopontin (OPN), which is an inducer of pro‐inflammatory cytokine production expressed not only in reactive microglial cells but also in reactive astrocytes around the injured CNS. Furthermore, we also found receptors against OPN functioning in the injured brain. However, functional role of OPN in reactive astrocytes is not yet clarified. To analyze the central role of OPN in reactive astrocytes, we examined primary culture of astrocytes from OPN‐deficient (OPN/KO) mice and found that the morphology of these cells was unusual. By the stimulation with lipopolysaccharide to the primary culture of astrocytes from OPN/KO mice, some of the pro‐inflammatory cytokine expression was altered. Moreover, reactivity of astrocytes caused by stab wound to the brain was examined using analogue of nucleic acid, bromo‐deoxy‐uridine (BrdU), and clarified that it was decreased in OPN/KO mice.
From these results, we conclude that OPN might play a major role in the activation of astrocytes under inflammation occurred to the CNS.
T10‐41B. A high fat diet exacerbates neuroinflammation by activation of the brain renin angiotensin system in experimental autoimmune encephalomyelitis
S. Timmermans, J. Bogie, T. Vanmierlo, P. Stinissen, N. Hellings, J. Hendriks
Hasselt University, Biomed, Diepenbeek, Belgium
A high fat diet (HFD) and obesity result in a marked increase in plasma cholesterol levels. Interestingly, disturbances in cholesterol metabolism have been linked with Multiple Sclerosis (MS) and its animal model experimental autoimmune encephalomyelitis (EAE). However, the exact influence of a HFD on MS disease progression and the underlying mechanism are still unknown. Here we determined the influence of a HFD on EAE. We show that feeding mice with a HFD exacerbates EAE severity through the induction of the brain renin angiotensin system (RAS), resulting in an increased immune cell infiltration into the CNS, oxidative stress and Th1 cytokines. Moreover, peripheral inflammation was boosted upon a HFD due to a RAS independent mechanism, indicating that a HFD exacerbates neuroinflammation in various manners. These data suggest that nutritional modulation may have an important influence on the course of MS and other neuroinflammatory conditions.
T10‐42A. Phenotype and function of CD11c (dendritic) cells within the CNS
K. Immig 1, M. Gericke1, A. Lösche2, K. Jäger2, L. Wendeburg3, U.‐K. Hanisch4, K. Biber3, I. Bechmann1
1University of Leipzig, Institute of Anatomy, Leipzig, Germany 2University of Leipzig IZKF‐FACS‐Core Unit, Leipzig, Germany 3University of Freiburg, Department of Psychiatry and Psychotherapy, Section of Molecular Psychiatry, Freiburg, Germany 4 University of Göttingen, Institute of Neuropathology, Göttingen, Germany
The brain's immune privilege has been attributed to a lack of dendritic cells (DC) within its parenchyma and the adjacent meninges implying maintenance of antigens rather than their presentation in lymphoid organs (LO). Using CD11c‐gfp mice, we have recently reported the existence of a CD11c/IBA‐1/CD11b+‐population in the juxtavascular parenchyma which extent their processes into the glia limitans, an ideal position for antigen‐presentation. Therefore, we phenotypically compared them to the CD11c+/CD45+ population (all isolated with the same protocol used for brain CD11c+ cells) from lung, liver and spleen in healthy mice using 7‐color flow cytometry. We found unique, site‐specific expression patterns of F4/80, CD80, CD86, CX3CR1, CCR2, FLT3 and MHC‐II. As described before, two different CD45+‐populations (CD45high and CD45int) in the brain can be separated, whereas liver, lung and spleen exhibit a more homogeneous CD45high population. A higher percentage of the brain's CD45+/CD11c+‐cells were F4/80‐positive compared to spleen, liver and lung, but expressed it at lower levels. Within the CD45int /CD11c+‐population most cells expressed the microglia marker CX3CR1 and CCR2low (marker for inflammatory, motile cells). Most importantly, compared to spleen and liver, CD45int/CD11c+‐cells from the brain almost completely lacked MHC‐II expression and CD45high/CD11c+‐cells from the brain have a lower percentage of MHC‐II+‐cells. Since the CD45int cells are widely regarded as the resident, intraparenchymal microglial population and CD45high as DCs and perivascular macrophages, our data confirm the view that a small subpopulation of microglia (CX3CR1high, F4/80+,CCR2low) share established immune phenotypical characteristics of DCs (CD11c+). Additionally we showed that intraparenchymal CD11c+ microglia are unique in their low expression of MHC‐II. Their weak expression of CD80 is in line with a tolerogenic phenotype.
Supported by DFG (FOR 1336/B2).
T10‐42B. The phenotypes of microglia and macrophages during experimental autoimmune encephalomyelitis
I. Vainchtein 1, J. Vinet2, N. Brouwer1, B.J.L. Eggen1, E. Boddeke1
1University Medical Center Groningen, Neuroscience, Groningen, Netherlands 2University of Modena, Biomedical Sciences, Modena, Italy
Question: Microglia, the resident macrophages of the central nervous system, have been implicated in a number of neurodegenerative disorders including the demyelinating disease Multiple Sclerosis (MS). The exact role of microglia in MS has not been elucidated. Previously, our lab has reported that in a cuprizone mouse model of MS, microglia display a gene expression profile indicative of promoting remyelination. In order to further evaluate the phenotype of microglia we used a mouse model more reminiscent of MS, experimental autoimmune encephalomyelitis (EAE). Because of its immune stimulation and extensive infiltration of peripheral immune cells it closely mimics MS. Our aim was to study the phenotype of microglia during EAE and compare it to the phenotype of infiltrating macrophages. Based on the results from the cuprizone mouse model we hypothesize that microglia (initially) try to revert the pathological condition in EAE, whereas macrophages are the driving force behind the EAE progression.
Methods: We performed a series of experiments, where we examined the expression of pro‐inflammatory factors in resident microglia and infiltrating macrophages after fluorescence‐activated cell sorting (FACS) at different EAE stages. In order to properly discriminate the two cell types we used a set of markers where resident microglia were defined as CD11b+ CD45int Ly6C‐, and infiltrating macrophages CD11b+ CD45hi Ly6C+. Using this protocol we have analyzed the RNA expression levels of MHCII (CD74, H2‐Aa), co‐stimulatory molecules (CD80, CD86, CD40, CD274), pro‐inflammatory genes (IL‐1β, TNF‐α) and inflammasome‐regulated cytokines in these immune cells with quantitative PCR. Followed by analysis of MHCII, CD80 and CD40 at the protein level with flow cytometry.
Results: Our results indicate that infiltrating macrophages have a pronounced activated (CD74 and H2‐Aa) and pro‐inflammatory phenotype (IL‐1β and TNF‐α) at RNA level. This is supported with an increase of the expression of MHCII and the key co‐stimulatory molecules CD80 and CD40 at RNA as well as protein level. In contrast, microglia display a decrease in the expression of the co‐stimulatory molecules CD80 and CD86 at the RNA level and do not up‐regulate expression of IL‐1β and TNF‐α during the progression of EAE.
Conclusion: Our data suggest that upon EAE, infiltrating macrophages display an inflammatory profile whereas microglia are only mildly immune activated.
T10‐43A. Does systemic inflammation contribute to the progression of age‐related hearing loss?
B. Impey 1, A.E. Causon2, A. Agyemang‐Prempeh2, J.L. Bailey3, C. Verschuur2, T.A. Newman3
1University of Southampton, Southampton, United Kingdom 2University of Southampton, Hearing and Balance Centre, Faculty of the Environment and Engineering, Southampton, United Kingdom 3University of Southampton, CES, IfLS, Faculty of Medicine, Southampton, United Kingdom
Background: Age‐related hearing loss (presbycusis) affects half of people by the age of 75. It has a large impact on quality of life and is associated with accelerated cognitive decline. However, presbycusis is not an inevitable process of aging, suggesting that its progression could be minimised.
Hearing relies on sound vibrations from the middle ear to elicit movement of the basilar membrane in the cochlea, resulting in excitation of hair cells. Spiral ganglion neurons relay the inner hair cell signals to the central auditory system via the vestibulocochlear nerve. Sounds are integrated in the central auditory pathway and then processed by cognitive areas of the brain.
Degeneration of cochlear structures, including hair cells and spiral ganglion cells occurs in presbycusis. In the central auditory system there are alterations in neuronal plasticity and neurodegeneration may occur.
Chronic inflammation has previously been correlated with age‐related hearing loss in humans, implicating immune cells in the progression of presbycusis. Microglia within the central nervous system are susceptible to priming by neurodegeneration or aging, both factors in presbycusis. It is feasible that microglia in the cochlea, an organ with similar immune privilege as the brain, could also become primed. Priming increases the chance of microglia becoming activated by subsequent inflammatory events, such as ‘flu, which may cause them to contribute to neurodegeneration in the cochlea and central auditory system.
Hypothesis: Our working hypothesis is that as age‐related hearing loss progresses, microglia in the cochlea and central auditory pathway will become primed. We predict that systemic inflammation will cause primed microglia to be activated to a pro‐inflammatory damaging phenotype, which will increase the rate of cochlea and auditory pathway degeneration and hence hearing loss progression.
Methods: C57BL/6J mice are genetically predisposed to develop age‐related hearing loss. Young and middle‐aged mice will be challenged with LPS or saline (control) and microglial phenotype assessed in the cochlea and central auditory system. Neurodegeneration will be evaluated at corresponding points in the cochlea and auditory pathway using molecular techniques.
Conclusion: If our hypothesis is proved this would suggest that rigorous control of infection in older individuals would reduce progression of hearing loss by minimising degeneration in the auditory system.

T10‐43B. Seropositive and Seronegative neuromyelitis optica differs in the inflammatory profile: an in vitro study on spinal cord glial cultures
T. Valente 1,2,3, L. Sabater2, F. Mannara2, F. Graus2, J. Saura3, A. Saiz2
1Institut d'Investigacions Biomèdiques de Barcelona, Barcelona, Spain 2Hospital Clinic and Institut d'Investigació Biomèdica August Pi I Sunyer (IDIBAPS), Center for Neuroimmunology, Service of Neurology, Barcelona, Spain 3University of Barcelona, School of Medicine, Biochemistry and Molecular Biology Unit, Barcelona, Spain
Neuromyelitis Optica (NMO) is an autoimmune demyelinating disease which primarily affects the spinal cord and optic nerves. Approximately 70% of the NMO patients harbor antibodies against the water‐channel aquaporin‐4, AQP4‐IgG (seropositive NMO), expressed mainly by perivascular astrocytes. However, some patients diagnosed with NMO don't have AQP4‐IgG (seronegative NMO). The aim of this work is to compare in vitro the neuroinflammatory profile of IgG from seropositive (IgG‐NMO+) and seronegative (IgG‐NMO‐) NMO patients. Pool of purified IgG from IgG‐NMO+ and IgG‐NMO‐ patients fulfilling diagnostic criteria of 2006, and from multiple sclerosis (IgG‐MS) patients and healthy controls (IgG‐HC) were include in the study. Mixed glial cell cultures of astrocytes (40%), oligodendrocytes (40%) and microglia (15%) were obtained from spinal cord of postnatal C57BL6/J mice. After 21 days in vitro, cells were treated with the IgG pools for 12 hours for RT‐PCR, and 24 hours for Western blot and immunocytofluorescence (IF). Conditioned media were used for nitric oxide (NO) assay and cytokines ELISAs. Spinal cord glial cultures presented a high co‐localization between AQP4 and GFAP on astrocytes endfeets by IF. Only IgG‐NMO+ induced loss of AQP4 and GFAP immunolabelling, and it was associated with cell loss by DAPI staining. As compared with HC‐IgG, IgG from patients induced strong microglial activation, NO production and the expression of proinflammatory cytokines: TNFα, IL‐1β and IL‐6. However, the levels of TNFα and IL‐1β were significantly higher in IgG‐NMO‐ than in IgG‐NMO+. No differences were found between IgG‐NMO‐ and IgG‐MS except for higher IL‐1β by RT‐PCR and IL‐6 by ELISA in IgG‐MS. However, IgG‐NMO+ caused a stronger upregulation of C/EBPβ mRNA and NOS2 protein than IgG‐MS and IgG‐NMO‐. All these data show that IgG‐NMO+ and IgG‐NMO‐ induce a different proinflammatory glial activation. The proinflammatory pattern of IgG‐NMO‐ is quite similar to that induced by IgG‐MS patients. These findings raise the question if IgG‐NMO‐ could be a different disease.
This research was supported by grants from the Marató‐TV3 foundation (N°101610) and from Instituto de Salud Carlos III (PI10/378).
T10‐44A. CD14 as a key regulator of TLR‐mediated responses of microglia
H. Janova 1, T. Regen2, D. van Rossum1, S. Ribes1, A. Götz1, R. Nau1, W. Brück1, U.‐K. Hanisch1
1Universitätmedizin Göttingen, Göttingen, Germany 2Universitätsmedizin der Johannes‐Gutenberg‐Universität, Mainz, Germany
Every year, hundred thousands of people die of meningitis, an infectious disease of CNS that can be caused by Gram‐negative bacteria E. coli. The survival of these people is dependent on the early recognition of the rising danger followed by a swift response by innate immune cells of the CNS, the microglia. These cells express a large arsenal of pattern‐recognition receptors (PRR), with the best‐known Toll‐like receptor 4 (TLR4) as a main sensor of lipopolysacharide (LPS), a cell wall component of Gram‐negative bacteria. This receptor triggers the response via both MyD88‐ and TRIF‐dependent signalling pathways, leading to production of cyto‐ and chemokines and thereby alarming and attracting the immune cells of the periphery to invade the brain. However, TLR4 is only fully functional in complex with several co/receptors, such as CD14. Even though CD14 has been traditionally considered simply as a LPS affinity provider, recent data indicate that CD14 is also necessary for the endocytosis of TLR4, which links TLR4 to intracellular signalling via the adapter protein TRIF. Our latest data reveal the critical role of this receptor for the profile as well as the magnitude of microglial cyto/chemokine production in response to diverse LPS variants. CD14 increases the sensitivity towards LPS in a cell type‐specific manner, making microglia far more sensitive to LPS than bone marrow and peritoneal macrophages. While striatal applications of low LPS doses reveal less incoming neutrophils into a brain of CD14ko mice, as compared to wildtypes, high doses LPS lead to an excessive neutrophil infiltration. This phenomenon is well correlating with the observations in vitro, where CD14 absence in microglia stimulated with high doses LPS causes an excessive production of selected chemokines, with the highest impact on the neutrophil chemoatractant CXCL1. These regulatory activities of CD14 require its membrane insertion and prolonged functionality, pointing to an involvement of signalling. In order to reveal candidates for such signalling mechanisms, we tested the role of Syk and PLC. Even though these enzymes were described to play a role in CD14‐dependent signalling in dendritic cells, they have no contribution in microglia, further pointing to a cell type‐specific organization of the CD14/TLR4 complex in microglia. Importantly, we identified receptor systems that impose influences on CD14 expression itself, which would thereby determine the extent and impact of a CD14‐mediated regulation of TLR4 functions. Supported by the DFG (FOR1336).
T10‐44B. Targeted cell ablation reveals dynamics of immune responses to programmed cell death in the brain
T.J. van Ham, R. Kalicharan, J. Kuipers, B.N.G. Giepmans
University of Groningen, University Medical Center Groningen, Department of Cell Biology, Groningen, Netherlands
In neurodegenerative disease misfolded proteins and cues released by dying cells can attract immune cells by chemotaxis and alter their phenotype. To better understand and differentiate between the effect of these cues, we aim to elucidate the spatiotemporal immunological events in the brain in vivo. Zebrafish are an excellent model system to study leukocytes in vivo, and we previously showed by live imaging how engulfment occurs in the developing zebrafish brain (van Ham et al., Curr Biol 2012). Here we use genetically targeted cell ablation to specifically induce cell death of target brain cells. We subsequently track dying cells and immune cells by single and multiphoton 4D imaging using fluorescent reporter genes. To analyse immunological infiltrate and resident cells in an unbiased fashion, we use large‐scale electron microscopy (EM) of complete zebrafish cross‐sections in parallel as a complementary approach. We find that within a day after onset of cell death multiple types of phagocytic cells, including microglia, accumulate in areas where cell death occurs, engulfing dying cells. Granulocytes, do not migrate towards these dying cells and are not found inside the brain at these stages. We also find total numbers of immune cells, microglia in particular, are increased. In addition to microglia, we find phagocytic peripheral cells to be involved in clearance of dying cells.
Our findings provide an initial in vivo characterization of the immune response to programmed cell death in the brain, indicating involvement of resident and peripheral immune cells. We show spatiotemporal recruitment of these different immune cell types, and proliferation of microglia. The latter together with the recruitment of peripheral leukocytes are likely triggered to increase capacity to dispose of dying cells efficiently. Our results indicate zebrafish provides a model to tease apart basic dynamic properties of immune maintenance of the diseased brain in vivo. Our current studies focus on identifying the sequence of immunological events in resolving brain injury in vivo and how this is controlled, to ultimately help identify which of these aspects are harmful during brain damage or promote tissue recovery.
T10‐45A. Immunomodulatory properties of LIF and OSM in multiple sclerosis
K. Janssens, H. Slaets, B. Vanwijmeersch, P. Stinissen, J. Hendriks, N. Hellings
Biomedical Research Institute, Diepenbeek, Belgium
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system (CNS), characterized by multifocal inflammatory infiltrates, demyelination, and axonal loss and degeneration. In the cerebrospinal fluid and lesions of MS patients leukemia inhibitory factor (LIF) and oncostatin M (OSM), members of the IL‐6 cytokine family, are upregulated. In humans, LIF and OSM both signal through the LIF receptor (LIFR), however OSM can also activate its specific receptor, OSMR. LIFR signaling promotes the survival of glial cells and neurons, and is thought to limit the development of pathogenic T helper 17 cells while promoting differentiation of protective regulatory T cells. OSMR signaling is also neuroprotective, however the effects on immune cells are not yet elucidated.
In this study, the immunomodulatory effects of LIFR and OSMR signaling in humans are investigated. We determined which immune cells express the receptors for LIF and OSM in healthy donors and compared this to the expression levels in MS patients. We found that in blood of healthy donors approximately half of the monocytes express the LIFR and OSMR, while 5–10% of the T cells and B cells express the receptors. More importantly, in untreated MS patients higher numbers of T cells and B cells express both the LIFR and OSMR as compared to healthy controls and treated MS patients. Available treatments suppress the immune system, resulting in less activated T and B cells, which could explain the lower receptor expression. Indeed, we measured the receptor expression on T cells and B cells after activation and demonstrated this strongly induces expression of both receptors.
Currently, we are investigating the effect of LIF and OSM on proliferation, cytokine production and antigen presentation of the different immune cell subsets. As blood circulating immune subsets of MS patients have a higher expression of LIF and OSM receptors, patients show increased susceptibility for modulation by these cytokines. Thus, their immunoregulating properties together with the protection of glial cells and neurons indicates that these cytokines are promising candidates for the treatment of MS and other neuroinflammatory diseases.
T10‐45B. Effects of astrocyte‐targeted IL‐6 or IL‐10 production on neuronal survival and microglial activation after facial nerve axotomy
N. Villacampa 1, B. Almolda 1, M. Hopfer2, J. Hidalgo1, I. Campbell2, B. González1, B. Castellano1
1Universitat Autonoma de Barcelona, Department of Cell Biology, Physiology and Immunology. Institute of Neuroscience, Bellaterra, Spain 2University of Sydney, School of Molecular Bioscience, Sydney, Australia
Interleukin‐6 (IL‐6) and Interleukin‐10 (IL‐10) are key cytokines with an important role in the regulation of the inflammatory and immune responses. In the central nervous system (CNS), increased expression of both IL‐6 and IL‐10 occurs in a wide range of pathological conditions. Meanwhile IL‐6 is recognized as a cytokine with a dual role acting as a pro‐inflammatory or anti‐inflammatory signal inducing glial activation, IL‐10 is well known by its ability to counteract the inflammatory and immune reactions.
The objective of the present study was to evaluate the effects of local production of either IL‐6 or IL‐10 on the microglial response and the neuronal degeneration induced by facial nerve axotomy, a sterile neuronal injury model. To accomplish that, we used two transgenic mice, GFAP‐IL6Tg and GFAP‐IL10Tg, which express either IL‐6 or IL‐10 under the GFAP promoter on astrocytes, i.e. a local production within the CNS. Unilateral facial nerve 1mm resection was performed, one set of transgenic and wild‐type (WT) axotomized animals were sacrificed at 3, 7, 14, 21 and 28 days post injury (dpi) and cryostat free‐floating sections were processed for immunohistochemical analysis. Another set of animals were sacrificed at 21dpi to study neuronal survival.
Our results showed that, at 21 days after facial nerve axotomy, in comparison with the usual neuronal death observed in WT, selective CNS IL‐6 production had a detrimental effect on neuronal survival, whereas, IL‐10 production was able to reduce neuronal death. These effects on neuronal survival correlated with changes in the expression of different molecules associated with microglial activation such as Iba1, CD11b, CD16/32, MHC class II, and some integrins like osteopontin and its receptors (CD44 and a5), along the different time points after injury. Remarkably, when compared with their WT littermates, CD11b expression was higher on GFAP‐IL10Tg mice, whereas remains lower on GFAP‐IL6Tg animals along all the time‐points analyzed. Similarly, CD44 expression, which increases after axotomy in WT mice, was higher on GFAP‐IL10Tg mice at 14 dpi and 28 dpi, whereas no induction of this molecule was detected on the GFAP‐IL6Tg axotomized animals.
In conclusion, our results indicate that astrocyte‐targeted IL‐6 and IL‐10 production has a direct impact on neuronal survival, glial activation and integrin mediated signaling, producing a specific outcome of facial nerve axotomy.
Supported by Ministerio de Ciencia e Innovación (BFU2008‐04407/BFI) and (BFU2011‐27400).
T10‐46A. Role of anti‐inflammatory lipid mediators in the resolution of inflammation in microglial cells
C. Joffre, C. Rey, A. Aubert, S. Layé
INRA, Bordeaux, France
Question: Microglial cells are the cellular components of the brain immune system. In response to systemic bacterial infection they become activated and are strongly involved in the local inflammatory response. They produce pro‐ and anti‐inflammatory cytokines, in particular IL‐1b, IL‐6 and TNFalpha, and IL‐10, respectively. Dietary polyunsaturated fatty acids (PUFA) are able to regulate neuroinflammation. N‐6 and n‐3 PUFA are precursors of anti‐inflammatory mediators such as lipoxin A4 (LXA4) synthesized from arachidonic acid and resolvin E1 (RvE1) and D1 (RvD1) produced from eicosapentaenoic acid and docosahexaenoic acid, respectively. These mediators are synthesized by 5‐ and 15‐lipoxygenases and exert their anti‐inflammatory actions by acting on specific G‐protein coupled receptors in particular on ALX and ChemR23. The aim of this study was to determine the effects of lipoxin and resolvins on the resolution of inflammation in microglial cells stimulated with lipopolysaccharide (LPS).
Methods: We used BV‐2 cells, a murine microglial cell line. In a first experiment aimed at determining the time course of the induction of expression of the lipoxygenases and receptors, they were incubated with LPS. In a second experiment aimed at determining the effects of the resolvins and lipoxin on the production of IL‐10, IL‐1b, IL‐6 and TNFalpha, they were firstly incubated with LXA4, RvD1 and RvE1 and then incubated with LPS.
Results: Our results indicated that LPS only enhanced the expression of ALX, the receptor for RvE1 and LXA4 (Í2) (p < 0.05). The expression of the 15‐lipoxygenase was also affected by LPS (p < 0.01) and varied during the time course, reaching a maximum after 6h of incubation (p < 0.05). The production of the proinflammatory cytokines decreased after 18h of incubation: IL‐1b with RvD1 (100 nM, p < 0.001) and RvE1 (10 nM, p < 0.01); IL‐6 with RvE1 (1 and 10 nM, p < 0.05 and p < 0.001); TNFalpha with RvE1 (1 and 10 nM, p < 0.001 and p < 0.01). The production of the anti‐inflammatory cytokine IL‐10 increased only when cells were incubated with LXA4 after 6h (1 and 10 nM, p < 0.01).
Conclusions: These results suggested a potential role of PUFA‐derivates in the resolution of inflammation.
T10‐46B. Macrophages in inflammatory multiple sclerosis lesions have an intermediate activation status
D. Vogel 1, E. Vereyken1, J. Glim1, P. Heijnen1, M. Moeton1, P. van der Valk2, S. Amor2, C. Teunissen3, J. van Horssen1, C. Dijkstra1
1VU University Medical Center, Amsterdam, Netherlands 2VU University Medical Center, Pathology, Amsterdam, Netherlands 3VU University Medical Center, Clinical Chemistry, Amsterdam, Netherlands
Background: Macrophages play a dual role in multiple sclerosis (MS) pathology. They can exert neuroprotective and growth promoting effects, and contribute to tissue damage by production of inflammatory mediators. The effector function of macrophages is determined by the way they are activated. Stimulation of monocyte‐derived macrophages in vitro with interferon‐γ and lipopolysaccharide results in pro‐inflammatory (M1) macrophages, and activation with interleukin 4 induces anti‐inflammatory (M2) macrophages.
Methods: The expression of a panel of M1 and M2 markers on human monocyte derived M1 and M2 macrophages was analyzed using flow cytometry. This revealed that CD40 and mannose receptor (MR) were the most distinctive markers for M1 and M2 macrophages, respectively. We next examined the activation status of macrophages/microglia in MS and control tissue using a panel of M1 and M2 markers.
Results: Our data show that M1 markers, were abundantly expressed by microglia in normal appearing white matter and by activated microglia/macrophages throughout active demyelinating MS lesions. M2 markers, MR and CD163, were expressed by myelin‐laden macrophages in active lesions and perivascular macrophages. Double stainings using anti‐CD40 and anti‐MR revealed that 70% of the CD40‐positive macrophages in MS lesions also expressed MR, indicating that the majority of infiltrating macrophages/microglia cells display an intermediate activation status.
Conclusions: Our findings show that, although macrophages in active MS lesions predominantly display M1 characteristics, a major subset of macrophages have an intermediate activation status. Ongoing research showed that granulocyte‐macrophage colony‐stimulating factor (GM‐CSF) could be an important factor triggering this intermediate activation status. GMCSF is known to play a crucial role in the development of experimental autoimmune encephalomyelitis.
T10‐47A. Correlation of binding of [ 3H]PK11195 to lesion type and presence of activated microglia in human multiple sclerosis tissues
S. Johansson, N. Fouladi, J. Goggi, P. Jones, W. Trigg
GE Healthcare, Medical Diagnostics, Amersham, United Kingdom
Background: Multiple sclerosis (MS) is a progressive neuroinflammatory disorder. During the time course of disease, there is a widespread microglial activation associated with extensive and chronic neurodegeneration and progressive loss of motoric and cognitive functions. Translocator protein 18kDa (TSPO, also known as peripheral benzodiazepine receptor) is highly expressed in vivo by activated microglia, and is an attractive target for diagnostic imaging of microglia in MS.
Results and conclusions: The aim of the present study was to evaluate the specific binding of [3H]PK11195, a compound targeting TSPO, to human tissue sections from patients with MS, and to correlate these findings with presence of TSPO+ microglia in different types of lesions. Specific binding of [3H]PK11195 was evaluated using autoradiography, and histological analysis was performed on tissue sections to evaluate lesion type, TSPO expression and presence of microglia. There was a clear correlation between level of specific binding of [3H]PK11195 and lesion type, such that there was an increased binding to tissue with active or chronic‐active lesions compared to control tissue or tissue with chronic lesions. This was concomitant with an increased TSPO+ expression and increased presence of microglia in tissue sections with active or chronic‐active lesions. In conclusion, specific binding of [3H]PK11195 is correlated with lesion type, TSPO expression and presence of microglia in tissue sections from MS patients.
T10‐47B. Scavenger receptor A (SRA) in the induction of inflammatory activation of microglia and astrocytes: its relevance for alzheimer's disease
R. von Bernhardi 1, F. Cornejo1, G. Ramirez1, J. Eugenin2
1Pontificia Universidad Catolica de Chile Medical School, Santiago, Chile 2USACH, Biology, Santiago, Chile
Question: Neuroinflammation normally restores tissue homeostasis after injury, but it is also involved in damage mechanism in Alzheimer's disease. Glia, especially microglia and astrocytes, are its main effectors; being astrocytes key regulators of microglial cell activation. During inflammatory response, glial cells secrete cytokines such as TNFα, IL1β and TGFβ, as well as nitric oxide (NO), and activate phagocytosis, chemotaxis, increased expression of receptors that bind cytokines and inflammatory ligands. Among ligand receptors are induced pattern recognition receptors such as Scavenger Receptor (SRs), including class A receptor (SRA), expressed by astrocytes and microglia. We will evaluate if SRA has active roles in Aβ clearance and inflammatory activation of Glia.
Methods: We evaluated the role played by SRA in glial cell activation, and the regulatory capacity of astrocytes upon microglia in vitro, assessing production of NO Griess assay and cytokines by ELISA; phagocytosis and activation of activity identity markers of signaling pathways by western blot.
Results: In primary glial cultures from wild type (WT) and knockout mice (KO) for SRA, it was found that LPS‐stimulated SRA KO astrocytes release 50% more NO than SRA WT astrocytes. These differences were associated with an extended activation of ERK signaling pathway. In contrast, evaluation of LPS‐induced cytokines production, only WT astrocytes released IL1β, whereas production of TNFα and TGFβ reached similar levels in WT and SRA KO animals. The abolition of IL1β release by SRA KO astrocytes appeared to be associated to the inhibition of activation of JNK and p38 signaling, and to the delayed activation of IκB/NFκB pathway in response to LPS. Microglia from SRA KO mice also showed several differences in response to LPS stimulation compared with their WT counterparts. They failed to show activation‐associated morphological changes and were unable to secrete IL1β, although they showed an increased release of NO. In terms of activation pattern, SRA KO microglia showed increased expression of MHC‐II, indicating that SRA could be involved in a mechanism inhibiting MHC‐II expression that has not been previously documented.
Conclusions: Our results suggest that SRA participates in the activation of specific inflammatory responses by astrocytes and microglia, and the activation of signaling pathways involved in the production of soluble molecules, being also needed for astrocytes in order to be able to modulate microglia activation.
Support: FONDECYT 1090353 and 1131025.
T10‐48A. Microglial P2Y receptor‐mediated motility in situ
R. Jolivet, C. Madry, D. Attwell
University College London, London, United Kingdom
Microglia are the resident macrophages of the brain and the first responders to disturbances of brain homeostasis. They exist in one of two broadly defined states, a rather inaptly named “resting state” in which they exhibit a ramified morphology and, upon tissue disturbance, an “activated state” in which they exhibit an amoeboid morphology. The characterization as “resting” is in stark contrast with the frantic morphological changes that ramified microglia undergo in brain slices and in vivo, constantly extending and retracting processes. While the purinergic receptor P2Y12 has been identified as a key receptor via which microglial processes are guided to a source of ATP or to the site of an injury, the signals and pathways guiding microglial processes in the healthy tissue remain elusive.
Here, by imaging microglia in acute hippocampal brain slices, we assessed the baseline and targeted motility of microglial processes in the presence of different pharmacological agents targeting purinergic signaling. We found that while bath application of a P2Y12 specific blocker alone (PSB‐0739 at 500 nM) or of a P2Y1 specific blocker alone (MRS2179 at 25 µM) did not affect the baseline motility of microglial processes, bath application of a P2Y1/12/13 blocker (MRS2211 at 25 µM) strongly reduced the baseline motility of microglia, leading them to retract most of their processes. Moreover, a strong rebound of motility was observed after washing out MRS2211 that did not lead to microglial activation within the time window of the experiment (up to 90 minutes). In experiments measuring the chemotactic response of microglia to a pipette containing 1 mM of ATP inserted into the slice, we found that chemotaxis was completely abolished by PSB‐0739 (bath applied at 2 µM) but not by MRS2211 (20 µM). Finally, no chemotaxis was observed in response to a pipette containing UDP‐glucose (5 mM), an agonist of the P2Y14 receptor. These results confirm that P2Y12 alone controls the targeted chemotactic response of microglial processes to ATP, with no discernable involvement of P2Y1, 13 or 14, but suggest that P2Y1, 12 and 13 might collectively play a role in controlling the baseline motility of microglial processes in the healthy tissue. How the different P2Y receptors interact with each other to control microglial movements remains to be determined.
Supported by an EU Marie Curie Fellowship, the Wellcome Trust and the ERC.
T10‐48B. Impact of the Immuno‐proteasome on the pathogenesis of Alzheimer's and Parkinson's disease
L. Wagner 1, R. Verma2, S. Prokop1, E. Krüger2, P.M. Kloetzel2, F.L. Heppner1
1Charité – Universitätsmedizin Berlin, Department of Neuropathology, Berlin, Germany 2Charité – Universitätsmedizin Berlin, Institute of Biochemistry, Berlin, Germany
The ubiquitin‐proteasome‐system (UPS) plays a central role in degradation and clearance of short lived regulatory as well as misfolded or damaged proteins. Upon stimulation by interferons (IFN) the catalytic subunits β1, β2 and β5 of the 20S proteasomes (s‐proteasomes) can be replaced with β1i/LMP2, β2i/MECL‐1 and β5i/LMP7 subunits, giving rise to the catalytically active immuno‐proteasomes (i‐proteasomes), respectively. Since i‐proteasomes play an important role in clearance of oxidant‐damaged proteins that preferentially accumulate upon inflammatory stimulation, and many neurodegenerative diseases, including Alzheimer's disease (AD) and Parkinson's disease (PD) are associated with a chronic inflammatory reaction, we tested the impact of i‐proteasome deficiency on the pathogenesis and progression of AD and PD.
While APPPS1 mice, a model for AD associated extracellular plaque pathology, exhibited increased levels of i‐proteasome subunits in the brain, genetic ablation of the β5i/LMP7 subunit, which significantly impairs i‐proteasome function, resulted in a reduction of cerebral plaque burden, indicating a disease exacerbating role of i‐proteasomes in AD.
In contrast, transgenic alpha‐Synuclein (TgaSN) mice, a mouse model for intracellular alpha‐Synuclein pathology associated with PD, crossed to β5i/LMP7 subunit deficient mice, highlight an increase of aggregated alpha‐Synuclein accompanied by worsening of pathology in TgaSN mice lacking functional i‐proteasomes, suggesting a protective role of i‐proteasomes for intracellular amyloid pathologies.
Future studies will aim at resolving the mechanistic underpinnings of the seemingly opposing effects of i‐proteasome deficiency on the pathogenesis and progression of extracellular and intracellular protein aggregation to aid better understanding and guide novel therapeutic opportunities for neurodegenerative diseases.
T10‐49A. Mesenchymal stem cells require to be in close vicinity with microglia to arrest cell cycle
S. Jose, S. Vidyadaran
Universiti Putra Malaysia, Department of Pathology, Selangor, Malaysia
We are exploring use of mesenchymal stem cells (MSC) as a tool to modulate microglia inflammatory responses and have previously demonstrated that MSC reduce BV2 microglia proliferation and increase nitric oxide (NO) secretion following lipopolysaccharide (LPS) stimulation. Here we describe that MSC modulate proliferation of BV2 microglia through a mechanism independent of NO. MSC reduce proliferation of stimulated BV2 cells by 30% (p < .05) and the reduction was unaffected even when NO were reduced to levels comparable to that of unstimulated BV2 cells (6.07 ± 0.6 µM) using the iNOS inhibitor L‐NAME. Viability of BV2 microglia determined by Annexin V/PI staining remained unaffected by MSC (≥ 94.02 ± 1.72%), ruling out apoptosis as a mode for inhibiting microglia proliferation. To then determine whether MSC arrests BV2 microglia cell cycle at any particular phase, propidium iodide (PI) staining revealed that the percentage of BV2 microglia in the S‐phase upon LPS stimulation (54.77 ± 1.5) is reduced to 48.54 ± 1.0% following direct coculture with MSC. This modulation of microglia cell cycle by MSC is dependent on cell‐cell contact. It is therefore probable that MSC and microglia require being in close vicinity to exert an anti‐proliferative effect. In an effort to examine this, migration of both cells across a semipermeable membrane in the presence of 1µg/mL LPS was analysed. Although MSC migrate marginally to an LPS stimuli (67.0 ± 15.1), they are chemoattracted towards microglia (213.3 ± 19.8 cells). This demonstrates that MSC respond to chemotactic cues by microglia, more so if the microglia are activated (272.3 ± 18.8; p < .05). Migration of cells within tissue requires digestion of extracellular matrix by proteases, and accordingly, expression of MMP2 and MMP9 increased in LPS‐stimulated cocultures. This increased expression was accompanied with increased activity, as assessed by gelatin zymography. This work suggests that MSC and microglia migrate towards each other which may enable MSC to modulate microglia proliferation in a contact‐dependent manner through cell cycle modulation.
T10‐49B. Cell types derived from CD34+ stem cells show diverging phenotypes in X‐linked adrenoleukodystrophy
F.D. Weber 1, C. Wiesinger1, S. Forss‐Petter1, G. Regelsberger2, H. Stockinger3, J. Berger1
1Medical University Vienna, Center for Brain Research, Vienna, Austria 2Medical University Vienna, Institute of Neurology, Vienna, Austria 3Medical University Vienna, Center for Pathophysiology, Infectiology and Immunology, Vienna, Austria
In the most severe form X‐linked Adrenoleukodystrophy (X‐ALD) is a fatal neurodegenerative disorder with inflammatory demyelination of the brain caused by a deficiency of the adrenoleukodystrophy protein (ALDP), encoded by the ABCD1 gene. ALDP, a member of the ABC transporter subfamily D, is located in the peroxisomal membrane and transports very long‐chain fatty acids (VLCFA) as CoA‐esters for degradation into the peroxisome. Currently, the only curative therapies are allogeneic hematopoietic cell transplantation (HCT) or genetically corrected autologous hematopoietic CD34+ stem cell therapy (HSCT). The success probably relies on the settlement of microglia and perivascular macrophages derived from the myelo‐monocytic donor cells in the brain of the patient. Therefore, we explored the phenotypes of the major immune cell types derived from the CD34+ stem cell. Immune cells from peripheral blood of controls and X‐ALD patients were isolated by magnetic activated cell sorting (MACS). Purity of cell isolations was verified by flow cytometry. In purified cells qRT‐PCR analysis was used to determine mRNA levels of the ABCD transporters and GC‐MS was used for the quantification of VLCFA levels, a diagnostic marker. The three peroxisomal ABC transporters were differentially expressed in CD34+ derived cell types of healthy controls: ABCD1 and ABCD2 mRNA were inversely expressed in all cell types, whereas ABCD3 was equally distributed. ABCD2, the closest homolog of ABCD1 could be expected to compensate for the loss of ABCD1 function. However, the expression pattern of ABCD2 was unchanged in X‐ALD patients; accordingly, cell types lacking ABCD2 mRNA (e.g. monocytes) displayed the most severe biochemical phenotype concerning VLCFA catabolism, whereas cells with substantial ABCD2 expression (e.g. T‐lymphocytes) were barely affected. Thus, not all investigated immune cells present an intrinsic metabolic defect. Therefore, we propose that the beneficial effect of HCT and HSCT may rely on the replacement of those cells lacking sufficient ABCD2 expression in ABCD1 deficiency. In addition, these findings support the concept that ABCD2 is a target gene for pharmacological induction, as an alternative treatment strategy, to rescue VLCFA metabolism and possibly halt the inflammation in X‐ALD patients.
T10‐50A. Transcriptional and epigenetic control of microglia polarization into inflammatory or alternative phenotype
B. Kaminska, M. Dabrowski, M. Maleszewska, A. Steranka, M. Smiech, A. Ellert‐Miklaszewska
Nencki Institute of Experimental Biology, Warsaw, Poland
Microglia are innate immune cells residing in the central nervous system and their activation is observed in virtually all neurological diseases. These cells accumulate at the sites of infection or injury of the central nervous system and initiate cascades of inflammation that can turn pathological when is chronic or uncontrolled. Microglia infiltrate malignant gliomas where they switch to a cytoprotective, pro‐invasive phenotype and support tumor progression. Primary rat microglia cultures stimulated with lipopolysaccharide (LPS) or glioma‐secreted factors become activated but adapt different fates and use distinct signaling pathways. Global gene expression profiling followed by functional analysis revealed activation of distinct transcriptional, signaling and metabolic pathways. Microglia stimulated with LPS up‐regulate innate/immune and inflammatory genes encoding cytokines, chemokines, immune mediators and neurotoxic factors. Many genes characteristic for classical inflammatory response failed to be induced or were down‐regulated in glioma‐activated microglia. Specific responses include induction of genes coding for transcription factors: inhibitors of DNA binding 1/3 and c‐Myc, markers of the cytoprotective phenotype and immunosuppressive cytokines/chemokines. Such global, complex and stable changes in gene expression could involve epigenetic changes such as histone modifications, DNA methylation and remodeling of chromatin structure. To identify epigenetic mechanisms associated with establishment of different phenotypes of microglia, we investigated changes in the profile of chromatin modifications of differentially expressed, candidate genes. DNA methylation was studied by methyl‐specific PCR followed by sequencing. A profile of four covalent histone modifications, characteristic for transcriptionaly active or silenced chromatin, was studied by chromatin immunoprecipitation followed by qPCR. The results suggest that in untreated microglia most of the genes, which become transcribed in inflammatory or alternatively activated cells are in open chromatin conformation that allows quick expression directly after stimulation. Most of the analyzed genes did not show DNA methylation. Changes in histone modifications were delayed and associated with gene repression that suggests their role consolidation of the polarized phenotype. Inflammatory activation of microglia seems to be “default response” that has to actively suppressed to reveal/restore cytoprotective phenotype.
T10‐50B. Interleukin‐33 in brain development and traumatic brain injury
G. Wicher 1, U. Wallenquist1, M. Enoksso23, B. Fuchs2, E. Husic1, L. Hillered3, G. Nilsson2, K. Forsberg‐Nilsson1
1Uppsala University, Department of Immunology, Genetics and Pathology, Science for Life Laboratory, Rudbeck Laboratory, Uppsala, Sweden 2Karolinska Institutet, Department of Medicine, Clinical Immunology and Allergy Unit, Stockholm, Sweden 3Uppsala University Hospital, Department of Neuroscience, Unit of Neurosurgery, Uppsala, Sweden
Interleukin‐33 (IL‐33) is a cytokine that has important functions in inflammatory and autoimmune diseases. Little is known, however, about IL‐33 in the brain. We analyzed expression of IL‐33 in the mouse brain during embryonic and postnatal development. Being undetected until late embryogenesis, IL‐33 was highly expressed during the first two weeks of postnatal life, after which expression gradually decreased and was then absent from the normal adult brain. Astrocytes and oligodendrocyte precursors expressed IL‐33, but not neural progenitors or neurons. The vast majority of IL‐33 positive cells displayed nuclear staining. Apart from its function as a pro‐inflammatory cytokine, a role for nuclear IL‐33, potentially in transcriptional regulation, is also emerging.
The important role of neuro‐inflammation in traumatic brain injury is being increasingly recognized, and local cytokine production can mediate the inflammatory response. Because of its potential for attracting inflammatory cells we analyzed IL‐33 expression in a cortical contusion infarction model (CCI). We found that IL‐33 expression was induced by injury, with a peak of expression three days after CCI, and at six days the levels declined. IL‐33 positive cells showed an overlap with astrocytic and oligodendrocytic lineage markers, but not with neurons, neural progenitors, endothelial cells or microglia/macrophages. A similar time course for IL‐33 expression in human brain trauma was found, using cerebral microdialysis, which allows continuous sampling of the parenchymal concentrations of molecules over several days in vivo. The peak of released IL‐33 for the patient analyzed was 4 days post trauma. IL‐33 binds to a heterodimeric receptor composed of ST2 and IL‐1RAcP. Following injury of ST2 knockout mice we found a general reduction of inflammatory cells at the site of injury, compared to wild type animals, and there were fewer microglia at the trauma site of mice lacking IL‐33 receptors. Our data show that IL‐33 expression is under tight regulation in the normal brain, but is induced by traumatic injury where is important for attracting inflammatory cells.
T10‐51A. Differential roles for the small heat shock protein Alpha B‐Crystallin in de‐ & remyelination
H. Kuipers 1, J. Yoon1, J. Winderl1, J. van Horssen2, T. Palmer3, L. Steinman1
1Stanford University, Neurology and Neurological Science, Stanford, CA, United States 2VU University Medical Center, Molecular Cell Biology and Immunology, Amsterdam, Netherlands 3Stanford University, Neurosurgery, Stanford, CA, United States
The small heat shock protein alpha B‐crystallin (cryab) is expressed by oligodendrocytes (OL) and astrocytes at several stages of multiple sclerosis (MS) lesions. Cryab has been shown to be protective and therapeutic in the EAE model of MS, possibly due to its anti‐apoptotic functions and regulation of inflammatory pathways. In this study, we further investigated the role of cryab in de‐ and remyelination in vivo, applying the cuprizone model of demyelination to cryab ‐/‐ mice.
Surprisingly, we found that cuprizone‐induced lesions are significantly smaller, less severe and less inflammatory in cryab ‐/‐ mice, compared to wild type (wt) controls. Staining for microglia and astrocytes revealed that astrocytes are the main inflammatory cell type that is affected by cryab expression: lesions in cryab ‐/‐ mice display less reactive astrogliosis than wt controls. Conversely, although less severe, lesions in cryab ‐/‐ mice also contain fewer oligodendrocyte progenitor cells (OPC) than in wt mice. In addition, our data suggest that remyelination is less efficient in cryab ‐/‐ mice than in wt controls and that remyelinated areas in cryab ‐/‐ mice contain fewer OL than remyelinated areas in wt mice.
Together, we hypothesize that cryab, due to its anti‐apoptotic actions, mediates protection in both astrocytes and OL, enabling on one hand reactive astrogliosis, which contributes to demyelination, and on the other hand OL survival, facilitating remyelination. Additional in vitro experiments support this hypothesis, revealing that cryab ‐/‐ astrocytes are less reactive than wt astrocytes and that siRNA‐mediated knockdown of cryab affects OL survival. Interestingly, analyzing phosphorylation patterns of cryab in cuprizone lesions revealed that cryab is differentially phosphorylated in astrocytes and OL. Furthermore, we found that cryab is differentially phosphorylated in astrocytes in active demyelinating MS lesions, indicating that phosphorylation of the protein might underlie its (pathogenic) role in active demyelination.
These paradoxical findings not only suggest a role for cryab as a potential target in a regenerative approach for MS treatment, but also point to a possible pivotal role for reactive astrogliosis in cuprizone‐induced demyelination and, more importantly, in early MS pathology.
T10‐51B. Molecular mechanisms driving tissue damage in multiple sclerosis
I. Wimmer 1, M.T. Fischer1, R. Höftberger2, S. Gerlach1, L. Haider1, T. Zrzavy1, S. Hametner1, D. Mahad3, C.J. Binder4, J. Bauer1, M. Bradl1, H. Lassmann1
1Medical University of Vienna/ Center for Brain Research, Department of Neuroimmology, Vienna, Austria 2Medical University of Vienna, Department of Neurology, Vienna, Austria 3Newcastle University/ Institute for Ageing and Heal, Newcastle, United Kingdom 4Center for Molecular Medicine (CeMM) of the Austrian Academy of Sciences, Vienna, Austria
Multiple sclerosis (MS) is an immune‐mediated demyelinating disorder of the central nervous system. Research traditionally focused on white matter lesions but within the last years, cortical lesions gained importance in MS pathology. Inflammation appears to play a fundamental role in the formation of cortical MS lesions. Nevertheless, disease‐specific mechanisms leading to plaque‐like primary demyelination and neurodegeneration remain poorly understood.
In a comprehensive immunohistochemical survey, we compared cortical MS lesions to those of inflammatory (tuberculous meningitis, Rasmussen's encephalitis, B‐cell lymphoma, and meningitis) and neurodegenerative (Alzheimer's disease) diseases. Although complex and fulminant immune responses were seen in many disease cases, primary demyelination was only detected in MS. In search of MS‐specific molecular mechanisms, we performed whole‐genome microarrays on micro‐dissected archival formalin‐fixed paraffin‐embedded material. Apart from cortical MS lesions, we also included brain tissue from patients suffering from tuberculous meningitis (inflammatory control) and Alzheimer's disease (neurodegenerative control). Additionally, control cases without brain pathology were included. Using a restrictive cut‐off, we identified 301 genes differentially expressed in MS lesions. More than 80% of these candidate genes could be assigned to T‐cell mediated inflammation, microglia activation, oxidative stress, tissue injury, DNA damage/repair as well as remyelination and regenerative processes. Subsequent neuropathological analysis confirmed that significantly more oxidatively damaged neurons (stained positive for oxidized phospholipids) were present in cortical MS lesions than in any other examined disease. TUNEL stainings for the detection of DNA strand breaks showed that neurons and oligodendrocytes with TUNEL‐positive nuclei were most abundant in MS lesions containing zones of active demyelination and tissue injury. Interestingly, immunohistochemical stainings for radical producing enzymes revealed that oxidative stress‐mediated tissue damage in cortical MS lesions seems to be driven by NADPH oxidases rather than by nitric oxide synthases.
Taken together, we were able to show that MS‐specific primary demyelination is driven by mechanisms of tissue injury that differ from any other investigated inflammatory and neurodegenerative disease. Among these mechanisms, oxidative tissue injury seems to play a major role.
T10‐52A. Progesterone attenuates astro and microgliosis and decreases inflammatory reaction following spinal cord injury
F. Labombarda 1, S. Gonzalez1, I. Jure2, A. De Nicola1
1IBYME/CONICET/UBA, Buenos Aires, Argentina 2IBYME/CONICET, Buenos Aires, Argentina
Reactive gliosis and inflammatory mediators are implicated in demyelination and secondary damage after spinal cord injury (SCI). We have previously reported that after SCI, short‐term progesterone treatment (3 days) stimulates oligodendrocyte precursor cells proliferation and decreases reactive gliosis, whereas chronic treatment (21 days) differenciates oligodendrocytes precursor cells into mature oligodendrocytes and enhances remyelination. Presently, we further studied whether progesterone was able to modulate the inflammatory reaction and cytokine production by astrocytes and microglial cells. Thus, the time‐course mRNA expression of pro‐inflammatory cytokines (IL1β, TNFα and IL‐6) and pro‐inflammatory enzymes (COX‐2 and iNOS) was studied by PCR in Real Time. Results showed that the highest increase in cytokine and pro‐inflammatory enzymes mRNA production occurred in rat spinal cord 6 h after SCI. Progesterone treatment significantly decreased the early rise of proinflammatory mediators mRNAs at 6h. As progesterone action in spinal cord involves multiple mechanisms, the role played by the classical progesterone receptor (PR) on the progesterone inhibitory effects on cytokines, was assessed in PRKO mice. In agreement with data obtained in the rat model, SCI strongly stimulated TNFα, IL1β and IL‐6 mRNA levels in spinal cord of both wild type and PRKO mice. However, whereas progesterone treatment inhibited the mRNAs of cytokines in wild type mice 6h after SCI, it was ineffective in PRKO mice, involving PR in the inhibition of cytokines production. Finally, we measured astrocyte and microglial cells density by immunohistochemsitry after 6 h of progesterone treatment. The steroid administration significantly decreases GFAP+ and Ox‐42+ cells, which correlated with cytokine and pro‐inflammatory enzymes inhibition. We conclude that progesterone attenuates reactive gliosis and inflammatory reaction, probably reducing the secondary spinal cord damage and favouring remyelination.
Supported by the University of Buenos Aires, Ubacyt 20020100200053.
T10‐52B. Antigen‐presenting cells in the central nervous system in EAE
A. Włodarczyk, M. Løbner, O. Cédile, T. Owens
University of Southern Denmark, Department of Neurobiology Research, Institute of Molecular Medicine, Odense, Denmark
Experimental autoimmune encephalomyelitis (EAE) is a mouse model for the inflammatory demyelinating disease multiple sclerosis (MS). MS is considered to be mediated by autoreactive myelin‐specific T cells. Crucial events in generating autoimmune responses in the central nervous system (CNS) are presentation of self‐antigen to naïve T cells in lymph nodes and their subsequent reactivation in the brain by infiltrating or resident antigen‐presenting cells (APC). The major APCs in the periphery are dendritic cells (DC). In the steady state they are rarely observed in the CNS parenchyma. However, during CNS inflammation they cross the blood‐brain barrier where together with microglia (CNS‐resident APC) they can present antigen to infiltrating T cells.
The goal of this study was to compare two subpopulations of microglia: CD11c‐ and CD11c+ with brain DC in terms of promoting different T cell responses.
Two subpopulations of microglia: (CD11c‐ CD45dim CD11b+) and (CD11c+ CD45dim CD11b+) as well as (CD11c+ CD45hi) bDC have been sorted from the CNS of C57BL/6 mice subjected to EAE. Sorted cells were used either for ex vivo proliferation and cytokine assays or for qRT‐PCR.
Our results show that bDC and CD11c+ microglia are similar with regard to ability to induce proliferative T cell response from both primed and naïve T cells. Nevertheless, they differ in their cytokine profile and ability to promote cytokine release by T cells.
Our findings suggest that different subtypes of APC in the CNS promote different immune responses.
This work has been supported by Lundbeckfonden.
T10‐53A. MC4R activation induces BDNF expression through ERK and PI3K in rat astrocytes
M. Lasaga, L. Carniglia, D. Durand, C. Caruso
School of Medicine – University of Buenos Aires, Buenos Aires, Argentina
Melanocortin 4 receptor (MC4R) is predominantly expressed in the brain and it is the only MCR expressed in astrocytes. Our previous results showed that a‐melanocyte‐stimulating hormone (a‐MSH) the anti‐inflammatory action is mediated by MC4R in astrocytes and in the hypothalamus of male rats and that these effects may lead to neuroprotection. We have already demonstrated that NDP‐MSH (an a‐MSH analogue) increased brain‐derived neurotrophic factor (BDNF) expression through the cAMP‐PKA‐CREB pathway in astrocytes. In the present study we examined the participation of mitogen activated protein kinases (MAPK) and phosphatidylinosotol‐3 kinase (PI3K)‐Akt pathways in MC4R signaling in astrocytes. We also investigated the effect of a‐MSH on BDNF expression in vivo in brains of male rats.We preincubated cultured rat primary astrocytes with MAPK (p38, JNK and ERK) and PI3K‐PDK1‐Akt inhibitors 15 min before addition of 1 mM NDP‐MSH for 1 h. We found that NDP‐MSH‐stimulating effect on BDNF expression assayed by qRT‐PCR was abolished only in the presence of ERK and PI3K inhibitors. Accordingly, levels of phospho‐ERK1/2 determined by western blot were increased by NDP‐MSH whereas phospho‐Akt levels were not modified at 30 min. NDP‐MSH‐induced ERK1/2 activation was decreased by adenylate cyclase and PI3K inhibitors but not by a PKA inhibitor, suggesting a cAMP and PI3K involvement in this effect. We also investigated if a‐MSH was able to induced BDNF expression in vivo. For that purpose we injected male rats with a‐MSH (0.5 mg/kg, ip) or vehicle (saline) once and sacrificed them after 3 h. Rats were also injected twice daily and for two days and killed 48 h after the first injection. We observed that BDNF mRNA levels increased after a‐MSH treatment at 3h in the hypothalamus whereas in the cortex BDNF expression was not modified. At 48 h BDNF expression was not modified by a‐MSH. We showed by immunohistochemistry that MC4R and BDNF co‐localizes with neurons and astrocytes in the brain. Since melanocortins have anti‐inflammatory and neuroprotective effects in the brain, the mechanisms described in astroglia help understand MC4R action. Our results indicate that melanocortins induce BDNF expression in astrocytes through ERK and PI3K, suggesting that this effect could be involved in the neuroprotective actions of melanocortins. The fact that BDNF expression also increases in vivo in the hypothalamus reinforce this idea.
T10‐53B. Glutamate receptors in EAE and microglia activation
S. Wörtge 1, J. Huppert1,2, S. Lacher1, J. Bruttger1, F. Kurschus1, K. Karram1, T. Regen1, C. Kuhlmann2, H. Luhmann2, A. Waisman1
1University Medical Center Mainz, Institute for Molecular Medicine, Mainz, Germany 2University Medical Center Mainz, Institute of Physiology and Pathophysiology, Mainz, Germany
Objective: Treatment of animals with antagonists of the NMDA as well as the AMPA/Kainate family of glutamate receptors ameliorates the clinical signs of EAE, the animal model for MS and results in reduced inflammatory infiltrates and blood brain barrier disruption. Microglia are highly activated during EAE and upregulate MHCII and secrete inflammatory mediators. In EAE they are thought to act as local APCs in the reactivation of the CNS‐infiltrating T cells.
Methods: We analyzed microglia activation and the role of NMDA receptors in this process using the microglia cell line BV2 and cortical slice cultures. The in vivo analysis was performed in two models: first, in oDTR mice that express the diphtheria toxin receptor specifically in oligodendrocytes (ODCs) which allows induced killing of ODCs (Locatelli et al., 2012) and second in mice that were immunized with MOG/CFA to induce EAE.
Results: The proinflammatory cytokine IL‐17A plays a pivotal role in the pathogenesis of MS and EAE. We showed IL‐17A activates microglia in vitro. Therefore, we activated the microglia cell line BV2 and cortical slice cultures with IL‐17A to and subsequently treated the cultures with the NMDA receptor antagonists MK801 and AP5 to block NMDA receptor signaling. This treatment inhibited IL‐17A‐mediated activation of microglia and in particular ROS production, proliferation and migration. Additionally, we detected increased secretion of IL‐6 and G‐CSF. Furthermore, IL‐17A enhanced activation of the NMDA receptor through phosphorylation leading to higher influx of calcium upon ligand binding. To further analyze NMDA receptor‐depended microglia activation we used two different mouse models that provoke different mechanisms of microglia activation. Whereas in EAE a strong inflammatory process activates microglia, in oDTR mice, activation occurs due to induced ODC death. Interestingly, in the context of EAE microglia activation was clearly diminished when mice were treated with MK801 whereas inhibition of the NMDA receptor had no effect on microglia activation in the oDTR model. In line with the in vivo data we show that inhibition of microglia activation takes place only when the cells were stimulated with IL‐17A and not upon stimulation with LPS.
Conclusions: Our findings show that NMDA receptors are not involved in general microglia activation but rather play a role in microglia activation in an inflammatory context with specific cytokines such as IL‐17A.
T10‐54A. Microglial CX3CR1‐CX3CL1 signaling contributes to astroglial scarring following mesenchymal stem cell grafting in mouse brain
D. Le Blon 1, C. Hoornaert1,2, N. De Vocht1, J. Daans1,2, K. Reekmans1,2, Z. Berneman1,2, P. Ponsaerts1,2
1University of Antwerp, Antwerp, Belgium 2Vaccine and Infectious Disease Institute, Antwerp, Belgium
Introduction: Mesenchymal stem cell (MSC) transplantation is widely suggested to become a promising strategy for future cell‐based therapeutic intervention following injury or disease of the central nervous system (CNS). However, preceding data by our laboratory has demonstrated the initiation of strong glial cell responses following transplantation of MSC in mouse brain. Hereby, activated microglia will invade and surround the MSC implant, whereas astrocytes will form a dense glial scar around the MSC implant (De Vocht et al., 2011 & 2012; Praet et al., 2012).
Aims of the study: To investigate whether CX3CL1‐CX3CR1 signaling contributes to microglia migratio00ctivation and subsequent astrogliosis following MSC transplantation in the CNS.
Methods: First, in order to allow localization of grafted MSC in vivo, wt C57BL/6 MSC were transduced with a lentiviral vector encoding the blue fluorescent protein (BFP). Next, BFP+ MSC were injected into the CNS (striatum) of CX3CR1+/‐ (n = 10) and CX3CR1‐/‐ (n = 7) transgenic mice. These mice have respectively one or both copies of the CX3CR1 gene replaced by the eGFP reporter gene. At 10 days post‐transplantation, histological analyses were performed to determine Iba1+ microglia and S100β+ astrocytes within and surrounding the BFP+ MSC graft site. Astrogliosis was determined based on staining for GFAP. CX3CR1 expression was evaluated based on eGFP expression. All histological evaluations were quantified using TissueQuest and/or ImageJ cell analysis software.
Results: (i) BFP+ MSC graft survival is observed in both CX3CR1‐/‐ and CX3CR1+/‐ mice; (ii) microglia migration towards grafted MSC occurs independent of functional CX3CR1‐CX3CL1 signaling; (iii) down‐regulation of eGFP gene‐expression (and thus also CX3CR1 gene expression) is observed in both CX3CR1‐/‐ and CX3CR1+/‐ mice on MSC graft‐invading microglia, but not on MSC graft‐surrounding microglia; (iv) the absence of functional CX3CR1‐CX3CL1 signaling in CX3CR1‐/‐ mice results in significantly less astrogliosis around the MSC graft site, as compared to the MSC graft site in CX3CR1+/‐ mice.
Conclusions: Here we identified CX3CR1‐CX3CL1 signaling as a potential target to modulate and/or control astroglial scarring following (stem) cell grafting in the CNS. The latter is of significant importance as grafted cells can only functionally interact with (injured) brain tissue in the absence of a graft‐surrounding astroglial scar.
T10‐54B. Inhibition of EGFR/MAPK signaling reduces microglial inflammatory response and the associated secondary damage in rats after spinal cord injury
Z. Yu, W. Qu
Huazhong University of Science and Technology, Wuhan, China
Background: Emerging evidence indicates that reactive microglia‐initiated inflammatory responses are responsible for secondary damage after primary traumatic spinal cord injury (SCI); epidermal growth factor receptor (EGFR) signaling may be involved in cell activation. In this report, we investigate the influence of EGFR signaling inhibition on microglia activation, proinflammatory cytokine production, and the neuronal microenvironment after SCI. Methods: Lipopolysaccharide‐treated primary microglia/BV2 line cells and SCI rats were used as model systems. Both C225 and AG1478 were used to inhibit EGFR signaling activation. Cell activation and EGFR phosphorylation were observed after fluorescent staining and western blot. Production of interleukin‐1beta (IL‐1β) and tumor necrosis factor alpha (TNFα) was tested by reverse transcription PCR and ELISA. Western blot was performed to semi‐quantify the expression of EGFR/phospho‐EGFR, and phosphorylation of Erk, JNK and p38 mitogen‐activated protein kinases (MAPK). Wet‐dry weight was compared to show tissue edema. Finally, axonal tracing and functional scoring were performed to show recovery of rats. Results: EGFR phosphorylation was found to parallel microglia activation, while EGFR blockade inhibited activationassociated cell morphological changes and production of IL‐1β and TNFα. EGFR blockade significantly downregulated the elevated MAPK activation after cell activation; selective MAPK inhibitors depressed production of cytokines to a certain degree, suggesting that MAPK mediates the depression of microglia activation brought about by EGFR inhibitors. Subsequently, seven‐day continual infusion of C225 or AG1478 in rats: reduced the expression of phospho‐EGFR, phosphorylation of Erk and p38 MAPK, and production of IL‐1β and TNFα; lessened neuroinflammation‐associated secondary damage, like microglia/astrocyte activation, tissue edema and glial scar/cavity formation; and enhanced axonal outgrowth and functional recovery. Conclusions: These findings indicate that inhibition of EGFR/MAPK suppresses microglia activation and associated cytokine production; reduces neuroinflammation‐associated secondary damage, thus provides neuroprotection to SCI rats, suggesting that EGFR may be a therapeutic target, and C225 and AG1478 have potential for use in SCI treatment.
T10‐55A. Microglia activation in the leech Hirudo medicinalis: HmC1q promotes the microglial accumulation through the distinct recognition of gC1qR and cC1qR receptors
C. Lefebvre, A. Bocquet‐Garcon, J. Vizioli, C. Van Camp, P.‐E. Sautière, F. Drago, M. Salzet, F. Croq
University Lille 1, Villeneuve d'Ascq, France
Question: Unlike vertebrates, the medicinal leech, Hirudo medicinalis, is able to functionally repair its central nervous system after an injury. Of interest, when the CNS is injured in H. medicinalis, microglial cells migrate to the site of lesion. This accumulation is known to be essential for the usual sprouting of injured axons and leads to a functional nerve repair. Because of the insignificant infiltration of macrophages in the injured leech CNS, we investigate the chemotactic mechanisms of the recruitment of only resident microglial cells in order to explore in fine the crosstalk between damaged neurons and activated microglia leading to the leech CNS repair.
Methods: In vitro and ex vivo chemotactic assays were performed by using recombinant form of HmC1q on microglial cells which were pre‐incubated or not with anti‐cC1qR or anti‐gC1qR antibodies. Then, affinity purification analyses using biotinylated C1q were performed from leech microglial cell protein extracts. Finally, immunohistochemistry analyses were located the production of newly characterized C1q receptors in microglial cells.
Results: We demonstrated that rHmC1q contributes to the recruitment of some leech microglial cells. Chemotaxis assays showed that the blocking of respective receptors, homologous to human gC1qR and cC1qR, inhibits the HmC1q‐dependent recruitment. Importantly, affinity purification analyses demonstrated the interaction between both receptors and HmC1q. Finally, those receptors were located on distinct microglial subsets.
Conclusion: This work is used to specify the activation processes of only resident microglial cells. The results show the importance of HmC1q in the microglial accumulation leading to the nerve repair. In H. medicinalis, HmC1q activity is driven through two different receptors which are not present on the same cells suggesting that HmC1q activates two distinct microglial cell subpopulations.
T10‐55B. The leukocytes influx is modified by genetic background in Alzheimer's disease rat models
N. Zilka 1, Z. Kazmerova1,2, M. Zilkova1,2, P. Neradil1,2, M. Novak1,2
1Institute of Neuroimmunology, Bratislava, Slovakia 2AXON Neuroscience SE, Bratislava, Slovakia
The question whether blood‐borne immune cells are infiltrating brain areas afflicted with neurodegeneration in AD and other tauopathies yielded contradictory results. Some authors showed that the chronic neuroinflammation in AD was provided almost exclusively by resident CNS cells without any apparent influx of leukocytes from the blood while others reported that hematopoietic cells can enter the brain in Alzheimer's disease and may contribute to an increased inflammatory burden. In order to address this issue we have used two independent transgenic lines expressing human misfolded truncated tau in two different genetic background (Wistar and SHR). We have shown that tau neurodegeneration can induce inflammatory responses mediated by reactive microglia in both transgenic lines, however the microglial responses showed a striking difference between the lines. We have identified two significantly different inflammatory responses to the same inducer. Moreover, we found that the genetic background significantly modified the molecular cascade influencing leukocyte influx into the brain. In both transgenic lines, the majority of the blood cells entering the brain belonged to antigen presenting cell family – monocytes and dendritic cells. At the proteomic level we found striking differences between the lines. While in the Wistar transgenic line we observed significant increase of cytokine‐induced neutrophil chemoattractant‐1, in SHR transgenic line, tissue metalloproteinase 3 was significantly reduced. These findings suggest that blood cell infiltration of the brain affected by tau neurodegeneration is modified by genetic background. This inter‐individual variability should be taken into the consideration in the development of novel drugs with anti‐inflammatory properties.
Acknowledgement: This work was supported by Axon Neuroscience and research grants VEGA 2/0161/11, 2/0205/11, 2/0193/11, APVV 0200‐11, APVV 0206‐11 and structural fund 26240220046.
T10‐56A. Expression of UNC‐93B1 in the CNS
S. Lehnardt, D. Gaessler
Charité – Universitätsmedizin, Berlin, Germany
Toll‐like receptors (TLRs) are key molecules of the innate and adaptive immune response in vertebrates. The original protein Toll in Drosophila melanogaster regulates both host defense and morphogenesis during development. TLRs recognize host‐ and pathogen‐derived stimuli. Single‐stranded RNA is sensed by TLR7 localized to the endolysosomal compartment of immune cells. We found that both extracellular viral RNA and host‐derived microRNAs induce cell‐autonomous and microglia‐mediated neuronal cell death through the endosomal receptor TLR7 in vitro and in vivo. However, the exact intracellular signalling cascade and the cellular mechanisms through which TLR7 leads to tissue injury in this context remained unclear. UNC93B1 is a molecule specifically involved in trafficking of nucleotide‐sensing TLRs, such as TLR7, in immune cells of both humans and mice. UNC93B1 physically interacts with this receptor in the endoplasmic reticulum (ER), and the function of the membrane protein UNC93B1 is to deliver the nucleotide‐sensing receptors from the ER to endolysosomes. Making use of real‐time PCR, immunocytochemistry, and histochemistry we systematically examined the expression of UNC93B1 in the murine CNS. Whereas a distinct expression for this molecule was observed in microglia and astrocytes, expression of UNC93B1 in cultured neurons was negligible. However, expression of this molecule was detected in cortical neurons of brain sections. Moreover, UNC93B1 was strongly regulated during different embryonic, postnatal, and adult stages of the developing mouse brain. Neurons of various brain regions were identified as the main cell type expressing UNC93B1 in the developing brain.
Taken together, our data reveal a specific expression pattern of UNC93B1 in the CNS, in particular in a developmental context, and lay foundation for further investigation of the pathophysiological significance of this molecule for both injurious and developmental processes in the central nervous system of vertebrates.
T10‐56B. Doxepin and fluoxetine influence neuropathic pain in rats and modulate microglia and astroglia cell activation
M. Zychowska, W. Makuch, E. Rojewska, K. Popiolek‐Barczyk, J. Mika, B. Przewłocka
Institute of Pharmacology, Krakow, Poland
Antidepressants are often used as adjuvant drugs for treatment of neuropathy. Recent studies, including our reports, have stressed an important role of glia in neuropathy, however, it is not known whether glia is implicated in antidepressants effects. The aim of our study was to determine whether the administration of selected antidepressants influences microglia and astroglia activity and neuropathic pain. To clarify this problem, a single intraperitoneal (i.p.) injection of the triciclic antidepressant doxepin at 10 mg/kg and the selective serotonin reuptake inhibitor fluoxetine at 20 mg/kg was administered to rats on day 7 after chronic constriction injury (CCI) to the sciatic nerve in Wistar rats. The von Frey test, cold plate test and tail‐flick test were used to evaluate tactile allodynia, thermal hyperalgesia and nociceptive threshold, respectively. Using Western blot analysis, we examined the markers of glia cells, IBA‐1 for microglia and GFAP for astroglia in the spinal cord and dorsal root ganglions (DRG). The experiments were carried out according to the local Bioethics Committee and in accordance with IASP rules (Zimmermann, 1983). A single i.p. injection of doxepin or fluoxetine attenuated allodynia and hyperalgesia, with no influence on the nociceptive threshold measured by the tail‐flick test. Western blot analysis demonstrated that under neuropathic pain, a single i.p. injection of doxepin increased the spinal (but not DRG) level of IBA‐1 protein. Fluoxetine decreased the level of IBA‐1 protein in the spinal cord and DRG. We observed no changes in GFAP protein level in DRG after doxepin administration but fluoxetine administration caused its downregulation. Our findings indicate that the antidepressants under study differently modulate the microglia cell activation, however, both attenuated the neuropathic pain features, allodynia and hyperalgesia. Thus, it seems that the therapeutic effects of antidepressants in neuropathic pain are not related to their influence on the activation of glial cells, however, the latter influence can affect the synergistic effect with other drugs used in polytherapy.
Acknowledgements: This study was supported by statutory founds and grant POIG.01.01.02‐12‐004/09 Demeter‐1.1.2.5.4.
T10‐57A. Periplaques form extensive lesions in multiple sclerosis spinal cords
A. Lhuillier 1, M. Chanal1, G. Androdias1, R. Reynolds2, C. Confavreux1, S. Nataf1
1Lyon Neurosciences Research Center, Lyon, France 2Imperial College of London, UK MS Tissue Bank, London, United Kingdom
QUESTION: Our knowledge of multiple sclerosis (MS) neuropathology has benefited from a number of studies that precisely depicted different types of plaques in white or grey matter areas. Also, both inflammation and diffuse axonal loss in the normal appearing white matter (NAWM) were extensively described. However, little attention was given to the so‐called periplaque, which is usually defined as a partially demyelinated ribbon of tissue surrounding the plaque. Whether periplaques correspond to expanding lesions, sites of ongoing remyelination or areas of tract degeneration remains uncertain. METHODS: In this context, our study aimed to bring quantitative insights to the neuropathology of MS periplaques in the spinal cord of primary or secondary progressive MS patients. A neuropathological quantitative assessment of inflammation, axonal loss and myelin loss was performed concurrently in the periplaques, plaques and normal‐appearing white matter (NAWM) of cervical spinal cord samples from 16 patients with progressive MS. RESULTS: Periplaques formed large areas of incomplete myelin loss that extended distant away from the border of plaques. Axonal loss was quantitatively similar in plaques and periplaques but signs of axonal dystrophy were predominantly observed in plaques. Surprisingly, axons that remained myelinated in periplaques presented a thicker myelin sheath than myelinated axons of the NAWM. Inflammation in the periplaque was mainly characterized by an accumulation of macrophages/microglia that were closely apposed to myelin sheaths but exerted poor phagocytic activity. Finally, we found that neuropathological features of periplaques were overall disconnected from that of plaques with regard to size, shape, inflammation and axonal integrity. CONCLUSIONS: Our work indicates that in MS spinal cords, periplaques correspond to demyelinating rather than remyelinating lesions. It further suggests that in progressive forms of MS, periplaques are likely to impact significantly on neurological disability. Finally, we propose that tract degeneration might not be the only cause of periplaque extension and that slowly‐expanding demyelination, temporally remote from plaque formation, might occur in periplaques. Molecular results will be presented that support these hypotheses.
T10‐57B. Netrins enhance blood‐brain barrier function and regulate immune responses at the blood‐brain barrier
C. Podjaski 1, J. Bin1, N. Lebeurrier1, S. Terouz2, L. Bourbonniere2, S. Larouche2, M. Blain3, C. Larochelle2, L.H. Hachehouche2, M. Sabbagh2, A. Nakano2, J.I. Alvarez2, P. Gris1, P. Darlington4, J.P. Antel3, T.E. Kennedy1, A. Prat2
1McGill University, Montreal Neurological Institute, Centre for Neuronal Survival, Montreal, Canada 2Montreal University, CHUM‐Hospital Notre‐Dame and CHUM Research Centre, Montreal, Canada 3McGill University, Montreal Neurological Institute, Neuroimmunology Unit, Montreal, Canada 4Concordia University, Department of Exercise Science and the PERFORM Centre, Montreal, Canada
During development, netrin guidance cues control cell motility and cell adhesion. Cell‐adhesion between endothelial cells at the blood‐brain barrier (BBB) makes the endothelium impermeable to blood‐derivatives and immune cells. To establish and maintain this barrier during development, adulthood, and during disease, brain endo‐thelial cells must develop and sustain these strong adhesive contacts, through expression of tight junction molecules. However, we do not know whether netrin supports inter‐endothelial cell adhesion at the blood‐brain barrier. Given this, we hypothesize that netrin tightens the BBB during development, adulthood, and protects it during disease.
Methods: To test this, we used both human adult primary brain‐derived endothelial cells and newborn netrin‐1 knockout mice and evaluated netrin's effect on inter‐endothelial cell adhesion and barrier permeability. We also assessed netrins' therapeutic potential to maintain the barrier and limit immune cell infiltration into the central nervous system (CNS) during experimental autoimmune encephalomyelitis (EAE).
Results: Our results demonstrate that brain endothelial cells express netrins. They help to form a tighter BBB during development. They also maintain and protect the adult barrier by increasing the expression of endothelial junction molecules, thus promoting inter‐endothelial adhesion and reducing protein leakage across the barrier. Netrins also reduce BBB breakdown and diminish initial myeloid cell infiltration into the brain and spinal cord during EAE, which delays disease onset and ameliorates disease severity.
Discussion: We conclude that netrins enhance BBB stability, and protects the BBB during neuroinflammatory disease.
T10‐58A. Myosin‐dependent functions in microglia
S. Janßen, V. Gudi, C. Trebst, M. Stangel
Medical School Hannover, Hannover, Germany
Microglial cells were shown to play an important role in many diseases of the central nervous system, not least due to their capability to become activated upon signs of brain injury. They migrate to lesion sites, proliferate, release cytokines and chemokines, and phagocytose cells or cellular debris. Therefore, the microglial cytoskeleton has to be highly rearrangeable. One major element of the contractile system in nonmuscle cells is nonmuscle myosin II (NM II). As the role of NM II in glial cells is poorly characterized, it is an aim of this project to study NM II‐dependent functions in microglia. We found that inhibition of NM II by blebbistatin, which is a highly selective inhibitor of NM II adenosine phosphatase, prevents any morphological shaping in freshly plated microglia and leads to functional deficits during migration and phagocytosis of fluorescence‐labeled beads. Using confocal microscopy, we could find diffuse expression of NM II in resting microglia in vitro, whereas activation with bacterial lipopolysaccharide (LPS) led to perinuclear accumulation of NM II protein. In an activated state, microglial cells release pro‐inflammatory cytokines and reactive oxygen species; interestingly, in the presence of blebbistatin the release of nitric oxide (NO) as well as rearrangement of NM II was prevented. Taken together, these results illustrate a key role for NM II in microglial shaping and functioning. Ongoing studies will improve our knowledge on spatial and functional aspects of the actomyosin complex during demyelinating processes and open targets for development of therapeutic strategies towards remyelination in the CNS.
T10‐58B. MMP‐independent role of TIMP‐1 in regulating CD4 T cell migration across the glia limitans during viral encephalomyelitis
C. Savarin 1, C. Bergmann1, D. Hinton2, S. Stohlman1
1Cleveland Clinic, Cleveland, United States 2Keck School of Medicine, Pathology, Los Angeles, United States
Infection of the central nervous system (CNS) with a sublethal neurotropic coronavirus (JHMV) induces a vigorous CNS inflammatory response. Neutrophils and monocytes are the first cells to enter the CNS, followed by CD8 and CD4 T cells. Whereas CD8 T cells migrate directly to the CNS parenchyma, CD4 T cells transiently accumulate within the perivascular space. A previous study identified CD4 T cells as the primary source of TIMP‐1, a tissue inhibitor of matrix metalloproteinases (MMPs). As MMPs facilitate blood brain barrier disruption and migration across the glia limitans, these data suggested that TIMP‐1 prevents CD4 T cells migration within the CNS parenchyma by limiting MMP activity. To determine if TIMP‐1 retains CD4 T cell within the perivascular space, wild type (WT) and TIMP‐1 deficient (TIMP‐1‐/‐) were infected with JHMV. Identical virus clearance was observed between the two groups, whereas TIMP‐1‐/‐ mice presented decrease disease severity. Flow cytometry analysis did not show significant difference in overall CNS inflammation between WT and TIMP‐1‐/‐ mice. However, JHMV infection of TIMP‐1‐/‐ mice resulted in increased, rather than decreased, accumulation of CD4 T cells within the perivascular space. Absence of compensation by other TIMPs and similar expression of MMPs and T cell chemokines between WT and TIMP‐1‐/‐ mice suggested an MMP‐independent role of TIMP‐1 in regulating CD4 T cell access to the CNS parenchyma.
POSTER TOPIC 11 Neurovascular interactions
T11‐01A. The Hedgehog pathway promotes optimal Blood Brain Barrier functioning
J.I. Alvarez 1, A. Dodelet‐Devillers1, H. Kebir1, P. Fabre2, I. Ifergan1, S. Terouz1, K. Wosik1, M. Sabbagh1, M. Bernard1, E. de Vries3, F. Charron2, A. Prat1
1Université de Montréal, Faculty of Medicine, CHUM Research Center, Neuroimmunology Unit, Montréal, Canada 2Institut de Recherches Cliniques de Montréal (IRCM), Laboratory of Molecular Biology of Neuronal Development, Montréal, Canada 3VU Medical Center, Department of Molecular Cell Biology and Immunology, Amsterdam, Netherlands
Blood brain barrier‐endothelial cells (BBB‐ECs) are surrounded by astrocyte endfeet known to regulate BBB permeability. Recent studies indicate that components of the Hedgehog (Hh) pathway play an important role in vascular proliferation, differentiation and tissue repair in adult tissues. As BBB disruption is early observed in Multiple Sclerosis (MS), this study aimed to determine the role of astrocyte‐secreted Sonic Hh (Shh) in the maintenance of BBB functions. We found that human astrocytes express and secrete Shh and conversely, that human BBB‐ECs bear the Hh receptor Patched‐1, the signal transducer Smoothened (Smo), as well as transcription factors of the Gli family. Transendothelial resistance and permeability experiments using primary cultures of human BBB‐ECs showed that activation of the Hh pathway stimulated expression of junctional proteins and promoted a BBB phenotype. In vivo permeability assays showed that blocking the Hh pathway increased barrier permeability and lack of Shh significantly compromised the expression of junctional proteins and the BBB phenotype. To distinctively demonstrate the role of the Hh pathway in inducing barrier properties at the CNS endothelium, we generated a conditional knockout specifically deleting the signal transducer Smo from ECs (Tie2‐Cre; Smoc/c). Analysis of these mice at E14, E18, P5, P19 and P55 showed a significant increase in BBB permeability to endogenous and exogenous tracers and these alterations correlated with a decrease in the expression of junctional proteins, altered expression of basement membrane proteins and reduced association of astrocyte endfeet with the vasculature. Our findings show that while activation of the Hh pathway restricts BBB permeability, Hh neutralization impacts on BBB formation and stability during fetal development and adulthood.
T11‐01B. Cerebral cortical blood flow response to brief basal forebrain stimulation
N. Takata, T. Nagai, K. Ozawa, K. Mikoshiba, H. Hirase
RIKEN, Brain Science Institute, Wako, Japan
Prolonged activation of the nucleus basalis of Meynert (NBM), the primary source of cholinergic projection to the cerebral cortex has been reported to cause significant increases in cerebral cortical blood flow (CBF) in rodents. While the NBM also gives rise to GABAergic and glutamatergic projections to the cerebral cortex, the NBM‐driven increase of CBF has been described to be dependent in part on muscarinic acetylcholine receptors. Lately, several groups reported that astrocytes modulate local CBF via intracellular Ca2+ signaling. Considering that cortical astrocytes express mAChRs and in vivo activation of the NBM leads to mAChR‐dependent Ca2+ surges in astrocytes, cholinergic modulation of CBF via astrocytic Ca2+ surges is conceivable. We report here that a single train stimulation of the NBM (stNBM; 100 Hz, 0.5 s, 200 µA) induces a biphasic (a rapid increase, followed by an overshooting slower decrease that goes back to baseline within a minute) laser Doppler flowmetry (LDF) response in the somatosensory cortex. Moreover, the stNBM‐induced LDF response was sensitive to the mAChR antagonist atropine. StNBM with a weaker stimulation intensity (50 µA) induces a transient decrease. Surprisingly, we find that IP3R2 knockout mice, which lack cytosolic Ca2+ surges in astrocytes, show similar LDF responses to stNBM.
T11‐02A. Deciphering the role of astroglial Cx30 at the gliovascular interface
A.‐C. Boulay, C. Giaume, M. Cohen‐Salmon
CIRB, Paris, France
Astrocytic endfeet are specialized processes, which enwrap blood vessels and express a large molecular repertoire dedicated to the physiology of the vascular system. One of the most striking properties of astrocyte endfeet is their enrichment in the gap junction proteins connexin (Cx) 43 and Cx30 allowing for direct intercellular trafficking of ions and small signaling molecules through perivascular astroglial networks. However, the role of these proteins at the gliovascular interface is not fully understood. We have recently demonstrated that astroglial perivascular Cxs expression starts after birth during blood‐brain barrier maturation and controls its integrity (Ezan et al. 2012). The aim of the present study was to decipher the role of Cx30 at the gliovascular interface, by analyzing the brain vascular transcriptome in a new mouse model of Cx30 inactivation (Cx30 Δ / Δ) (Boulay et al., 2013). Total RNAs of purified brain vessels from adult Cx30 Δ / Δand wild‐type littermates (Yousif et al., 2007) were analyzed by high throughput sequencing. Results were further validated by quantitative RT‐PCR.
Cx30 Δ / Δ purified vessels showed essentially a strong upregulation (fold change > 12) of Sgcg. This gene encodes gamma‐sarcoglycan, a membrane glycoprotein associated with the dystrophin‐dystroglycan complex which is essential for muscle integrity and is impaired in Duchenne muscular dystrophy (Petrof et al., 1993). At the gliovascular interface, the dystrophin‐dystroglycan complex anchors astrocyte endfeet to the basal lamina (Nico et al., 2010), and is crucial to the BBB integrity. However, expression of Sgcg and its role in the brain has until now only been poorly documented. Moreover, we found an upregulation of S100a8 and S100a9 (fold change 6,3 and 5,5, respectively) encoding the calgranulin A and B, two proteins present in neutrophils and monocytes, and involved in their migration to inflammatory sites, attachment to endothelial cells and extravasation (Ryckman et al., 2003).
Altogether, our results suggest that Cx30 might modify the dystrophin‐dystroglycan complex thereby influencing blood‐brain barrier properties, and control the attachement as well as the transmigration of leukocytes through the endothelium, thus contributing to the regulation of inflammatory processes in the brain.
T11‐02B. The ‘double settlement' of the perivascular glia – changes during the radial glia/astrocytes replacement. An immunohistochemical study in rats
M. Kalman 1, K. Pocsai2, Z. Bagyura1,2, I. Adorjan1,2
1Semmelweis University, Budapest, Hungary 2Semmelweis University, Anatomy, Histology and Embryology, Budapest, Hungary
The blood‐brain barrier is supported by the perivascular glia. A crucial event during the gliogenesis is the replacement of radial glia by astrocytes. Immunohistochemical staining of nestin visualized the first gliovascular connections after E16, and by E20 the radial glial endfeet cover the vessels completely. By E20 numerous nestin immunoreactive astrocyte‐like cells participated in it. In parallel, nestin immunoreactivity also appeared in the wall of the vessels. By about P10 both radial glia and nestin immunopositive astrocytes disappeared and gave place to astrocytes immunoreactive to S100 protein and glutamine synthetase, markers of mature astrocytes. In the radial glia these markers were not found, and they colocalized with nestin only scarcely. The S100 and glutamine synthetase immunoreactive cells appeared at first in the middle zone of the pallium about P2, and their population gradually extended to both the pial surface and the deep cortical zones, colonizing the whole thickness by P8, in parallel with the disappearance of nestin immunoreactive glial elements, either radial or astrocytic ones. At P6 GFAP immunoreactive cells were still confined to the most superficial and deepest cortical zones, so showed no colocalization with S100 and glutamine synthetase, unlike that found after P8. Whether this glial ‘takeover' results in a transitory increase of leakage of the blood‐brain barrier, it remains to be answered. It seems however, to underly that cerebrovascular fibronectin and laminin immunoreactivities disappear at first from the middle zone of the developing cortex and only later from the superficial and deep ones, between P2 and P10. According to Krum and Rosenstein (1991) disappearance of these immunoreactivities refers to the fusion of the glial and vascular basal laminae, the maturation of the definite gliovascular connections.
Supported by OTKA 2006/60930.
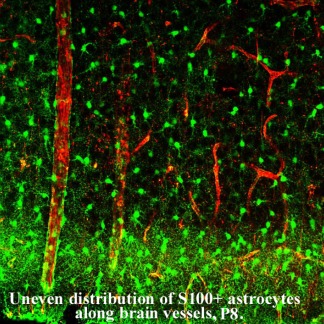
T11‐03B. The Oligodendrogenic Activity Of Brain Pericytes: A Novel Player During Myelin Repair?
S. Lange 1,2, P. Rotheneichner1,2, P. van Wijngaarden3, G. Gonzalez3, A. Guzman De La Fuente3, C. Zhao3, R.J.M. Franklin3, L. Aigner1,2, F.J. Rivera1,2,3
1Paracelsus Medical University, Institute of Molecular Regenerative Medicine, Salzburg, Austria 2Paracelsus Medical University, Spinal Cord Injury and Tissue Regeneration Center Salzburg, Salzburg, Austria 3University of Cambridge, Wellcome Trust and MRC Cambridge Stem Cell Institute & Department of Veterinary Medicine, Cambridge, United Kingdom
Multiple sclerosis (MS) is a demyelinating immune‐mediated disease of the central nervous system (CNS). It is the most common cause of acquired disability in young adults and affects over 2 Mio people worldwide. Although remyelination is a robust response upon demyelination, at least in the early phases of MS, it largely fails in the more advanced stages. Thus, remyelination is a clear target for MS therapy approaches. The endogenous cellular sources of remyelinating oligodendrocytes are oligodendroglial progenitor cells (OPCs) that are widely distributed in the CNS. We have previously shown that mesenchymal stem cells (MSCs) induce neural stem/progenitor cells (NPCs) and OPCs towards an oligodendroglial fate and thus MSCs therapy has been proposed for MS treatment. There is accumulating evidence that brain pericytes (BPCs), besides their role in regulating vascular homeostasis, might support endogenous remyelination. BPCs display MSC features and are in close contact with NPCs and OPCs. In vitro, we show that BPC‐derived soluble factors promote oligodendrocyte fate decision and differentiation in adult CNS progenitors. Furthermore, in vivo studies in different animal models reveal a significant increase in BPC density during remyelination. All these observations together strongly suggest a role of BPCs for myelin repair. Currently we aim to determine whether BPCs are involved in CNS remyelination and to study the underlying cellular mechanism(s), which might represent a novel target for developing future MS therapies.
POSTER TOPIC 12 Regeneration and repair
T12‐01A. Understanding and manipulating the astrocyte response to spinal cord injury using diblock copolypeptide hydrogels for depot delivery of bioactive molecules
M. Anderson 1, S. Zhang2, Y. Ao1, T.J. Deming2,3, M.V. Sofroniew1
1University of California, Los Angeles (UCLA), Neurobiology, Los Angeles, United States 2University of California, Los Angeles (UCLA), Chemistry, Los Angeles, United States 3University of California, Los Angeles (UCLA), Bioengineering, Los Angeles, United States
One goal for improving outcome after spinal cord injury (SCI) is to promote regrowth of injured nerve fibers across areas of tissue damage surrounded by astroglial scars that inhibit such growth. Little is known about how astrocytes assemble to form this scar, and a deeper understanding of the cellular and molecular mechanisms that control this process is needed if this goal is to be achieved. In vitro data suggest that certain bioactive molecules can alter astrocyte morphology into a more growth supporting state. Other molecules have been shown to promote axon growth. Clinical application of many of these molecules would require neurosurgical delivery directly into the spinal cord. Injectable biomaterials have the potential to serve as depots and scaffolds for in vivo delivery of bioactive molecules. We are developing di‐block co‐polypeptide hydrogels (DCH) as fully synthetic biomaterials that could safely and easily be injected into specific sites after SCI to deliver molecules in order (i) to understand better, and (ii) to manipulate, the mechanisms that control glial scar formation. We have previously shown that DCH are biocompatible after injection into brain or spinal cord and self‐assemble into structures with finely controllable properties. Our current work shows that DCH depots can deliver diffusible bioactive growth factors that influence local neurons or astrocytes in predictable ways and form gradients that are effective up to several mm away from depots. We are now testing the ability of DCH depots to deliver specific growth factors that will manipulate scar‐forming cells when DCH are injected at clinically realistic sub‐acute times after SCI. Supported by Wings for Life, CA State Roman Reed Fund, and Adelson Medical Research Foundation.
T12‐01B. Embryonic stem cell‐derived microglia and primary microglia display a similar transcriptome signature
B. Linnartz‐Gerlach 1, C. Beutner1, S. Schmidt2, M. Beyer2, M. Mallmann2, A. Staratschek‐Jox2, J.L. Schultze2, H. Neumann1
1University Bonn and Hertie‐Foundation, Institute of Reconstructive Neurobiology, Bonn, Germany 2University Bonn, Life & Medical Sciences, Bonn, Germany
Microglia originate from myeloid cells of the hematopoietic lineage and are the resident immune cells of the central nervous system. They are difficult to obtain in high numbers and purity by being isolated from the brain or mixed glial cultures. To overcome those obstacles, embryonic stem cells derived microglial lines (ESdM) have been suggested as alternative source (Beutner et al., 2010). ESdM showed expression levels of cell surface markers as well as phagocytic and migratory capacities similar to that of primary microglia. However, if ESdM can fully replace primary microglia to study microglial biology is still unknown.
To investigate the molecular phenotype of ESdM, we performed a whole transcriptome analysis of ESdM in comparison to primary cultured microglia, flow cytometry‐sorted microglia, different myeloid cells, T cells, astrocyte‐ and neuron‐enriched cultures. While being distinct from T cells, neurons and astrocytes, ESdM were closest related to primary cultured microglia followed by flow cytometry‐sorted microglia and other myeloid cells. ESdM and primary cultured microglia showed a strong overlap in their transcriptome by only having 143 gene transcripts differentially expressed. Most of these differentially expressed genes were immune‐related genes that were up‐regulated in primary cultured microglia. This indicates that ESdM were in a less activated state rather reflecting the in vivo biology of these cells. Flow cytometry analysis of cell surface markers selected from the microarray confirmed the close relationship between ESdM and primary cultured microglia.
ESdM resemble primary microglia not only in respect to surface marker expression and functional properties, but also display a molecular phenotype similar to primary microglia. Our data suggest that ESdM are a reliable tool for the replacement of primary microglia.
REFERENCE
Beutner C, Roy K, Linnartz B, Napoli I, Neumann H (2010) Generation of microglial cells from mouse embryonic stem cells. Nat Protoc 5:1481–1494.
T12‐02A. Comparative study of human mesenchymal stem cells isolated from the olfactory system and bone marrow: Effects on myelination
S. Barnett, S. Johnstone, S. Lindsay
University of Glasgow, Glasgow, United Kingdom
Mesenchymal stem cells (MSCs) have been proposed as candidates for transplant mediated repair in multiple sclerosis (MS). Clinical trials are ongoing using autologous systemic transplantation of bone marrow derived MSCs into relapsing remitting, secondary progressive and primary progressive MS. Previously we have shown that rat and adult human olfactory mucosa (OM) contain a mesenchymal‐like stem that resides in the lamina proporia (LP‐MSCs). Since the olfactory system is one of the only CNS tissues that promote neurogenesis throughout life, cells from olfactory biopsies have been proposed as excellent candidates for CNS repair. Thus, we have made a detailed comparison of MSCs isolated from bone marrow with MSCs isolated from the olfactory mucosa. Using immunocytochemistry and q‐PCR purified human LP‐MSCs expressed typical bone marrow derived (BM‐) MSC markers including CD90, CD105, CD73, CD166, CD271, NG2, fibronectin and STRO‐1, however they expressed more nestin mRNA. LP‐MSCs formed spheres, were clonogenic and differentiated into bone and fat. Comparative micro(mi) RNA‐based fingerprinting (SistemQCtm from Sistemics) demonstrated 64% homology between the two MSCs, with only 26 differentially expressed miRNAs. LP‐MSC conditioned medium (CM) promoted oligodendrocyte precursor cell proliferation and induced a highly branched morphology. Both LP and BM‐MSCs promoted OPC proliferation and differentiation, but only myelinating cultures treated with CM from LP and not BM‐MSCs had a significant increase in myelination. Comparison with fibroblasts and contaminating OM fibroblast like‐cells showed the promyelination effect was LP‐MSC specific. Preliminary in vivo cell transplantation studies suggest LP‐MSCs can promote many neurites in to the lesion and myelination around the lesion may be increased. Thus LP‐MSCs harvested from human OM biopsies may be an important candidate for cell transplantation by promoting CMS myelination.
T12‐02B. Spinal cord injury: development of a novel in vitro model
M. McGrath 1, S.D. Boomkamp1, S.C. Barnett1, M. Riehle2
1University of Glasgow, Glasgow Biomedical Research Centre, Glasgow, United Kingdom 2University of Glasgow, Centre for Cell Engineering, Glasgow, United Kingdom
Spinal cord injury (SCI) is a devastating condition which usually leads to paralysis. This outcome of injury in most cases is permanent due to the cascade of immunological factors that create an anti‐repair environment around the site of injury and due to the limited capacity of central nervous system (CNS) axons to regenerate, creates a daunting challenge to establish an effective therapy. From many years of research it is apparent that a combination of therapies would be the best course of action for treatment of SCI; this however greatly increase the animals cohorts and time to establish the most effective course of treatment. For this reason we have developed an in vitro model of spinal cord injury as a moderate throughput screen, which is in accordance with the 3R's.
This culture consist of dissociated mixed rat embryonic spinal cord cells plated onto a monolayer of astrocytes this is essential for the spinal cord cells, obtained from rats of embryonic day 15, which then develop into a carpet of neurites that over time become myelinated forming internodes of myelin interspaced with nodes of Ranvier.
This mixed CNS culture was utilized using a scalpel blade to axotomise the axons and a persistently neurite‐free area is created which is accompanied by many features of SCI, including demyelination and reduced neurite density adjacent to the lesion and the presence of and reactive astrocytes. These finding are congruent with changes seen in vivo after spinal injury.
Data will be presented regarding the effects from proven and novel pharmacological agents on neurite outgrowth and myelination looking to understand the mechanism in which they act. As well as modifications of the culture system to incorporate methods to align axons and isolate cell bodies from their processes for a more detailed analysis of regeneration.
T12‐03A. Grey Matter Astrocytes Activated by Remote Axonal Transection Mediate Structural Plasticity
D. Barson, G. Tyzack, S. Sitnikov, C. Zhao, R. Franklin, R.T. Karadottir, A. Lakatos
University of Cambridge, Cambridge, United Kingdom
The role of increased activity of astrocyte networks in structural synaptic plasticity following remote axonal injury is unexplored. We set out to investigate the efficiency of synaptic rearrangements in models where astrocyte reactivity is selectively diminished by the inhibition of the main cytokine pathway in transgenic mice (TG) in comparison to that observed in wild type mice (WT). First, synapse densities were compared in the facial nuclei two weeks after unilateral facial nerve transection by synaptotagmin‐1/PSD‐95 immunolabelling and ultrastructural analysis. We found that the recovery of synapse density was decreased by 40% in TG mice compared to WT mice, and that the reduction in synaptic density correlates with a relative loss of neuronal coverage by reactive astrocyte end‐feet in the same conditions. Specifically, we observed a 30% reduction in excitatory synapse density in the TG mice. Second, we developed an organotypic entorinho‐hippocampal slice culture system in which astrocyte activation and synaptic plasticity could be more closely monitored in real time. This was assisted by two‐photon microscopy, using glial Ca2+‐imaging and dendritic spine motility analysis after perforant pathway transection. In summary, we demonstrated that full astrocyte activation is necessary for partial structural recovery of synapses after axonal injury. Our work, using simplified models, may help experimental approaches aimed at optimizing adaptive plasticity in CNS injuries.
T12‐03B. Mobilization of progenitors in the subventricular zone to undergo oligodendrogenesis in the theiler's virus model of multiple sclerosis: implications for a remyelinating process in lesions sites
M. Mecha, A. Feliu, F.J. Carrillo‐Salinas, C. Guaza
Cajal Institute, Madrid, Spain
Pathological loss of myelin in diseases like Multiple Sclerosis (MS) is usually followed by a phenomenon of remyelination, in which oligodendrocytes synthesize new myelin sheaths to envelope exposed around axons in the adult central nervous system (CNS). The importance of remyelination arises from the fact that this process not only restores saltatory conduction, but also protects axons from different insults and thus limits clinical disability in demyelinating diseases. In this scenario, the mammalian subventricular zone (SVZ) has garnered attention as a potential source of replacement cells after injury. This zone harbours stem cells and supports long‐distance migration, and is activated in MS patients to promote gliogenesis. Although NG2+ precursor cells are the first to react to demyelination and show the highest proliferation rate in comparison with other cells, the relative contribution of the SVZ with respect to the inflammatory demyelination induced by the Theiler's virus infection and oligodendrogenesis has never been addressed. In the present study, we have investigated the behavior of the SVZ in Theiler's Murine Encephalomyelitis Virus – Induced Demyelinating Disease (TMEV‐IDD). We report that in this viral model for MS, there is a preclinical phase of the disease with demyelination in the corpus callosum that is followed later by an attempt of remyelination. This phase is accompanied by an activation of the SVZ with no apparent activation of NG2+ precursors, and a strong and clear staining for GFAP+ B cells close to the lateral ventricles of the brain that correlate with an increase of the proliferative rate in this area when measured by BrdU incorporation. Finally, we show that Theiler's infection enhances the mobilization of SVZ progenitor cells to the surrounding demyelinated corpus callosum and generate mature APC+ oligodendrocytes.
This work has been supported by grants from the MINECO SAF 2010‐17501 and REEM (Red Española de Esclerosis Múltiple, RD0700100060).
T12‐04A. ROCK inhibitors decrease actin stress fibres and GFAP while elevating stellation, L‐glutamate uptake and AHNAK expression in astrocytes on 3D nanoscaffolds
P. Beart 1, N. Zulaziz2, C.L. Lau1, D. Nisbet3, M. Horne1, R. O'Shea2
1University of Melbourne, The Florey Institutes of Neuroscience and Mental Health, Parkville, Australia 2La Trobe University, Department of Human Biosciences, Bundoora, Australia 3Australian National University, Research School of Engineering, Canberra, Australia
Astrocytes are dynamic cells performing vital roles in neuronal survival, and failure of these processes contributes to neuropathologies. Manipulation of Rho kinase (ROCK) has beneficial effects on glia, including astrocytes, where ROCK inhibitors produce a pro‐survival phenotype. Question – How such results translate to clinical actions in vivo needs further attention and we explored actions of ROCK inhibitors on astrocytes maintained under more physiological 3D primary culture. Methods – Random and aligned nanoscaffolds were engineered from poly‐ε‐caprolactone. Primary astrocytes (PND 1.5 C57Bl6 mice) were subcultured and plated at 10 days(div) in 96‐well plates, on random or aligned scaffolds in 96‐well plates (8,000 cells/well), or on glass coverslips in 24‐well plates (20,000 cells/well). Astrocytes were treated 8 div later with vehicle, dbcAMP (100 μM), or Rho kinase inhibitors Y27632 (30 μM) or Fasudil (100 μM) for another 3 div when biochemical and morphological analyses were undertaken. Results – Astrocytes possessed cobblestoned morphology in 2D culture, but were dramatically different in phenotype on scaffolds: tight clusters formed on random scaffolds, with elongated processes on aligned scaffolds. Immunoblotting showed GFAP expression was always reduced on scaffolds relative to 2D cultures. Drug treatments decreased intensity of F‐actin staining, increasing that of G‐actin (disassembly actin stress fibres) notably on aligned scaffolds, where AHNAK expression was elevated by all drugs indicative of extensive enlargesome activity. AHNAK labelling was similar to GFAP but more widespread with Y27632 and Fasudil, which produced extensive process outgrowth. Fasudil and Y27632, unlike dbcAMP, had less effect on GFAP immunolabelling in 3D cultures. Processes infiltrated both types of scaffolds, showing more growth along aligned nanofibres. On random scaffolds, Y27632 and Fasudil elevated L‐glutamate transport. Conclusions – Astrocytes flourished on biomatrices and the combination of bioengineering and ROCK inhibition produced synergism in decreasing cytoskeletal stress, elevating EAAT activity and process outgrowth, beneficial effects for brain repair.
T12‐04B. Galectin‐1/NRP1 interaction via carbohydrate binding produces regenerative response and functional recovery after spinal cord injury by blocking the Semaphorine 3A pathway
L.A. Pasquini 1, H.R. Quinta1, G.A. Rabinovich2, J.M. Pasquini1
1Universidad de Buenos Aires, Fac. Farmacia y Bioq, Buenos Aires, Argentina 2Instituto de Biología y Medicina Experimental, IBYME/CONICET, CABA, Argentina
Galectins (Gals) are a family of soluble lectins that, when binding to b‐Galactosides, form multivalent complexes with glycoconjugates of the cellular surface and induce a modulation of intracellular signaling pathways for differentiation and survival. Recently, Galectin‐1 has been described as a microglial deactivator which prevents neurodegeneration and promotes neuroprotection in an EAE model. However, its action at neuronal level is poorly understood. Spinal cord injury (SCI) also remains a major challenge to neurological research, as several factors such as extracellular matrix molecules inhibit axonal regeneration. Among them, Semaphorine 3A (Sema3A) contributes to the inhibition of axonal regeneration by acting on microtubules and actin cytoskeleton. Neuropilin‐1 (Nrp‐1) is a neuronal receptor whose binding of Sema3A generates repulsion of axonal growth. In addition, Nrp‐1 has been described as a target of Gal‐1 in non‐neuronal systems. The aim of this work was to study the role of Gal‐1 intraumatic SCI, considering that Sema3A expression is “turned on” in SCI and prevents axonal regeneration via Nrp‐1. Gal‐1 knockout mice (Lgals1 ‐/‐) were submitted to a full traumatic SCI at thoracic level (T9‐T10) and treated with different concentrations of recombinant Gal‐1. We demonstrated that Lgals1 ‐/‐ mice treated with Gal‐1 have a dose‐dependent functional recovery of motility as early as 6 days post‐treatment, which is structurally associated to neuronal repopulation and high axonal regeneration. These effects were only observed in animals treated with Gal‐1 with dimerizing capacity, but not in mice treated with a mutant Gal‐1, only present in a monomeric form. In addition, we observed that microglial response was “turned off” in Gal‐1‐treated mice. Moreover, exogenous dimeric Gal‐1 bound to the surface of injured motoneurons and promoted the spread of Nrp‐1. 3D confocal microscopy reconstruction showed Gal‐1 and Nrp‐1 spatial proximity. To confirm this result, we carried out immunoprecipitation assays, which allowed us to determine that only the dimeric form of Gal‐1 interacted with Nrp‐1 and induced a decrease in the levels of Sema‐3A bound to Nrp‐1. Our results show that Gal‐1‐based treatments combine its neuronal effect on Nrp‐1 with its deactivating effect on microglia, which leads to a better restorative process after medullar lesion. The findings presented here support the potential of dimeric Gal‐1 as a therapeutic agent for human SCI patients.
T12‐05A. Propentofylline improves oligodendrocyte remyelination following gliotoxic injury in the rat brainstem
E. Bondan 1, M.D.F. Martins1,2, D.E.M. Baliellas2, C.F.M. Menezes2, S.C. Poppe2
1Universidade Paulista, São Paulo, Brazil 2Universidade Cruzeiro do Sul, São Paulo, Brazil
Propentofylline (PROP) is a xanthine derivative with pharmacological effects distinct from those of the classical methhylxanthines theophylline and caffeine. It depresses activation of microglial cells and astrocytes which is associated with neuronal damage during inflammation and hipoxia. It is largely known that ethidium bromide (EB) injection into the CNS induces local oligodendroglial and astrocytic death, resulting in primary demyelination, blood‐brain barrier disruption and Schwann cell invasion, and thus serving as an experimental demyelinating model in animals. The aim of this study was to evaluate if PROP had the capacity of affecting glial cell behaviour during the process of demyelination and remyelination following gliotoxic injury with EB. Wistar rats were divided into 2 groups: I‐ rats injected with 10 microlitres of 0.1% EB into the cisterna pontina and treated with PROP; II‐ rats injected with EB and not treated with the xanthine. PROP (Agener) treatment was done using 12.5 mg/kg/day by intraperitonial route during all experimental period. The rats were euthanized from 7 to 31 days after EB injection and brainstem sections were collected and processed for light and transmission electron microscopy studies. Results from both groups were compared by using a semi‐quantitative method developed for documenting in semithin sections the extent and nature of remyelination in gliotoxic lesions. In general terms, by 7–11 days following gliotoxic lesion, the center of the lesion was expanded and filled with huge amounts of myelin debris among foamy macrophages and demyelinated axons. No astrocytic processes were found in this site and some lymphocytes were seen in the neuropil and around blood vessels. The most prominent feature of the 15th day was the initial association at peripheral locations between naked axons and remyelinating cells. Schwann cells were associated with one or multiple demyelinated axons or already forming thin myelin lamellae around single axons in astrocytic‐free areas. Oligodendrocytes began to form thin myelin sheaths in areas completely or partially filled with astrocytic prolongations. By 21–31 days, it became evident that rats treated with PROP presented a small improve in the repair process when compared to untreated animals, the latter presenting greater amounts of myelin‐derived membranes in the central area and a lesser extension of oligodendrocyte remyelination, but without any apparent difference regarding to Schwann cells. By 31 days results showed that PROP administration following EB injection seemed to stimulate oligodendroglial remyelination (mean remyelination scores of 3.61 ± 0.21 for oligodendrocytes and 1.20 ± 0.41 for Schwann cells) in comparison to untreated animals (scores of 2.98 ± 0.44 and 0.83 ± 0.31, respectively).

T12‐05B. High resolution microscopic analysis of the peripheral nerve regeneration using double/triple fluorescent transgenic mice
L. Politti Cartarozzi 1, A. Cupido2, R.G. Zanon3, A. Scheller2, A. Leite Rodrigues de Oliveira1, F. Kirchhoff2
1UNICAMP, Campinas, Brazil 2University of Saarland, Homburg, Germany 3University of Uberlândia, Uberlândia, Brazil
Peripheral axotomy results in a disconnection of the neuronal cell body from the target organ. A complex rearrangement of the nerve microenvironment, the so called Wallerian degeneration, takes place distally to the lesion and involves Schwann cell proliferation as well as macrophage infiltration. The process results in the formation of the Bands of Büngner which guide the axonal sprouts throughout the nerve distal stump. Although this process has been extensively studied, a thorough understanding of the three‐dimensional nerve tissue reorganization is not completely known. Such knowledge may in turn lead to more effective strategies aiming at the repair of peripheral nerves following transection or crushing. In this sense, the tetrahydrofuran (THF)‐based tissue‐clearing technique has been adapted for sciatic nerves of mice and used for subsequent observation to 2P‐LSM. Transgenic mice that express fluorescent proteins in neurons, astrocytes, Schwann cells and microglia/macrophages as well as double and triple hybrids were used. Additionally, by immunohistochemical analysis, it was possible to observe the structure of the nerve after injury by the expression of neurofilament and S100β, in non fluorescent mice. Also, increased non neural cell responsiveness after injury was studied with anti‐p75NTR immunostaining. Transmission electron microscopy allowed the evaluation of the ultrastructure of the nerve microenvironment, as well as the regenerative process by 3, 7 and 14 days after injury. Microscopic examination of cleared tissues of transgenic mice through 2P‐LSM consisted in comparing the different cell type interaction before and after injury, with particular interest on the Schwann cells, macrophages and growing axons.
T12‐06A. Sip1 expression by Schwann cells plays a role in peripheral nerve regeneration
B. Brinkmann 1, S. Quintes1, M. Ebert1, V. Tarabykin2, D. Huylebroeck3, U. Suter4, K.‐A. Nave1, M.W. Sereda1,5
1Max Planck Institute of Experimental Medicine, Department of Neurogenetics, Goettingen, Germany 2Charité – Universitätsmedizin Berlin, Neurocure Excellence Cluster, Institute of Cell and Neurobiology, Berlin, Germany 3University of Leuven, Laboratory of Molecular Biology (Celgen), Department of Development and Regeneration, Leuven, Belgium 4ETH Hönggerberg, Institute of Cell Biology, Department of Biology, Zürich, Switzerland 5University of Göttingen (UMG), Department of Clinical Neurophysiology, Goettingen, Germany
Smad‐interacting protein‐1 (Sip1, Zeb2, Zfhx1b) has been shown to be required for CNS myelination. In the present study, we show that Sip1 is also indespensible for myelination by Schwann cells, the glial cells of the peripheral nervous system. After nerve injury, Schwann cells undergo a dramatic phenotype transition that resembles a transdifferentiation process known from tumorigenesis and tissue repair. While Sip1 expression is low in mature cells, that have completed myelination, it is upregulated several days after acute nerve trauma. We have generated mutant mice that lack Sip1 specifically in mature Schwann cells by using tamoxifen inducible Cre recombination. In these conditional mutants, regeneration of crushed sciatic nerve is impaired. Functional recovery as assessed by footprint analysis is slow and remyelination is severely delayed. Our results suggest an essential role of Sip1 in Schwann cell transdifferentiation after nerve trauma.
T12‐06B. Maldi imaging mass spectrometry: a novel technology for studying neurosciences
I. Fournier1, J. Franck1, F. Croq1, D. Cizkova2, C. Lefebvre1, J. Vizioli1, P.‐E. Sautière1, M. Salzet 1
1University Lille 1, Villeneuve d'Ascq Cedex, France 2Slovak Academy of Sciences, Center of Excellence for Brain Research, Institute of Neurobiology, Kosice, Slovakia
Question: Mass Spectrometry (MS) has become nowadays an essential tool for the detection and identification of biomolecules in various contexts of biological problematic. MS can be performed from all types of biological materials ranging from biological fluids to tissues. End of 90's it was shown that with certain instrumentation such as those equipped with a MALDI ion source it was possible to record MS spectra directly in situ from tissue sections. More, point to point automated acquisition of MALDI MS spectra from a whole tissue section was shown to allow for reconstruction of the 2D images of biomolecules contained in the section distribution through ion density maps (Caprioli RM et al., Anal. Chem. 1997).
Methods: MALDI MS Imaging (MALDI MSI) is a molecular imaging modality that presents several advantages including possible imaging of hundreds of biomolecules in one step acquisition and the untargeted character of the method allowing to study molecules without any prerequisite knowledge or use of probes.
Results: Over the past decade MALDI MSI has demonstrated its potentiality in terms of applications for biology and clinics (Franck J et al., Mol. Cell. Proteomics. 2009). MALDI MSI can be use to study both endogenous molecules namely metabolites, lipids, peptides and proteins or exogenous ones such as drugs or xeniobiotics and was applied to various problematic. Understanding of physiological or physiopathological processes requires knowledge on the identity and localization of molecules within a tissue. MALDI MSI reseals a clear potential to answer this objective and give a unique contribution. Indeed, more recently MALDI MSI has gained new strategies for identification of molecules in situ on tissues. In particular, many efforts were given to develop confident identification of proteins from tissue sections.
Conclusion: Here we will review this novel technology and discuss some of its current and potential contributions for neurosciences application (Wisztorski M et al., Dev. Neurobiol. 2008). This will be illustrated by presentation of the investigation of invertebrate neuroimmune response as well as vertebrate neuropathological disorders using either fresh frozen or formalin fixed paraffin embedded tissues.
T12‐07A. S1PR1‐modulation in the convalescence period improves functional recovery and reduces reactive astrogliosis in experimental stroke
R. Brunkhorst 1, N. Kanaan1, A. Koch2, H. Steinmetz1, J. Pfeilschifter2, W. Pfeilschifter1,2
1Goethe University Hospital Frankfurt, Department of Neurology, Frankfurt, Germany 2Goethe University Hospital Frankfurt, Department of General Pharmacology and Toxicology, Frankfurt, Germany
The sphingosine 1‐phosphate (S1P) signaling pathway is known to influence astroglial reactivity in experimental autoimmune encephalomyelitis and the synthetic S1P analog FTY720 has been shown to provide neuroprotection in experimental models of acute stroke. However, the effects of a manipulation of S1P‐S1P1 signalling at later time points after experimental stroke have not yet been investigated. We examined whether a relatively late initiation of a FTY720 treatment has a positive effect on long‐term neurological outcome with a focus on reactive astrogliosis, synapses and neurotrophic factors.
We induced photothrombotic stroke (PT) in adult C57BL/6J mice and allowed them to recover for three days. Starting on post‐stroke day 3, mice were treated with FTY720 (1 mg/kg b.i.d.) for 5 days. Behavioral outcome was observed until day 31 after photothrombosis and periinfarct cortical tissue was analyzed using tandem mass‐spectrometry, TaqMan®analysis and immunofluorescence with especial emphasis on markers of astroglial reactivity.
FTY720 treatment results in a significantly better functional outcome persisting up to day 31 after PT. This is accompanied by a significant decrease in GFAP‐immunoreactivity (‐ir) and an increase in PSD‐size. However no change in GFAP‐mRNA, GS‐ir or CS56‐ir was observed. While FTY720‐treatment leads to a decrease in S1P concentration, it increases VEGF‐mRNA in the periinfarct cortex.
The initiation of FTY720 treatment in the convalescence period has a positive impact on long‐term functional outcome, probably mediated through reduced astrogliosis, a modulation in synaptic morphology and an increased expression of neurotrophic factors.
T12‐07B. Aligned Schwann cells derived from human dental pulp stem cells direct neurite growth in a tissue engineered collagen construct
K. Sanen 1, W. Martens2, M. Georgiou3, J. Phillips3, I. Lambrichts2, M. Ameloot1
1Hasselt University, Biomed, Biophysics Group, Diepenbeek, Belgium 2Hasselt University, Biomed, Morphology Group, Hasselt, Belgium 3The Open University, Department of Life, Health and Chemical Sciences, Milton Keynes, United Kingdom
A variety of traumas and diseases can cause peripheral nerve injury (PNI). In the process of spontaneous healing, Schwann cells (SC) are very important players. They not only reconstitute myelin, essential for proper nerve function, but also organize themselves into columns that lie within the endoneurial tubes to guide and support axonal regeneration from the proximal to the distal end of the injury. By contrast, large gap PNI requires artificial bridging strategies such as highly aligned scaffolds to promote directed neurite outgrowth. In the field of neural tissue engineering, hydrogels are very attractive biomaterials to encapsulate cells. Their high water content facilitates transport of oxygen, nutrients and waste and their viscoelastic properties can be relatively easy modulated. Recently, a new technique has been developed to efficiently and reproducibly align cells in a collagen type I hydrogel, thereby mimicking the environment required for nerve regeneration. This Engineered Neural Tissue (ENT) has given promising preliminary results with immortalized and primary rat SC both in vitro and in vivo. However, the use of human cells would be a significant advance in bringing this ENT from bench to bedside. Since autologous SC must be harvested from another peripheral nerve and are slowly growing in vitro, there is a high need for alternative cell types such as human dental pulp stem cells (hDPSC). hDPSC can be easily isolated from discarded teeth with very little ethical issues and their neural crest origin suggests a predisposition to differentiate into neural or glial cells. Preliminary experiments in our laboratory indicate the two‐dimensional (2D) in vitro transdifferentiation capacity of human DPSC (hPDSC) into Schwann‐like cells (SC‐hDPSC). Here, we evaluate the potential of SC‐hDPSC to align within a collagen type I hydrogel and determine the effects of this ENT on dorsal root ganglion neuron neurite outgrowth in vitro.
T12‐08A. Combination of growth factor treatment and scaffold deposition following experimental traumatic brain injury show a temporary effect on cellular regeneration
F. Clausen, A. Erlandsson
Uppsala University, Uppsala, Sweden
Due to the restrictions in regenerative capacity of the brain the tissue most severely damaged after traumatic brain injury (TBI) will end up as a fluid filled cavity. Presently, it is an impossibility to effectively restore lost brain tissue, but ongoing research on the stimulation of endogenous neural stem cells has increased the hope to viably and functionally repopulate the injured parenchyma. However, it is crucial that possible therapies can show a long‐term effect on both regeneration and functional recovery to be of clinical interest.
In this study the enhanced induction of neurogenesis in rats after experimental TBI was evaluated three or six weeks after injury. Severe focal TBI was performed using the controlled cortical impact model after which a combination therapy of intracerebroventricular administration of epidermal growth factor (EGF) for seven days followed by deposition of extracellular matrix scaffold, containing vascular endothelial growth factor, into the cortical cavity. The treatment was devised to accomplish an optimal effect on the stem cell regeneration. The animals with six weeks survival time were functionally evaluated using the Morris water maze (MWM). Before sacrifice the animals were injected with bromodeoxyuridine (BrdU) to identify newly generate cells. Sections were made and immunohistochemically double stained for BrdU and cell type markers for neurons, astrocytes and blood vessels. The sections were micrographed and analyzed for hemispheric tissue loss as well as number of new astrocytes, neurons and blood vessels.
Three weeks after injury there was a significant treatment effect as shown by an increase in neuronal and astrocytic regeneration. However, after six weeks there was no difference in the number of newly generated neurons and astrocytes. Evaluation of tissue loss and spatial learning in the MWM corroborated that the treatment had no effect at the later time point. The results clearly show the importance of long‐term studies in rodents to ensure that a promising effect on tissue regeneration and functional outcome is not merely temporary.
T12‐08B. Regulation of astrocyte activation by the cleaved P75 neurotrophin receptor
C. Schachtrup 1,2, J. Kyu Ryu2, P.M. Carlton3,4, N. Le Moan2, A. Perez5,6, E. Vagena2, M.H. Ellisman5,6, T. Wyss‐Coray7, K. Akassoglou2,8
1Albert‐Ludwigs‐University, Molecular Embryology, Freiburg, Germany 2University of California, Gladstone Institute of Neurological Disease, San Francisco, CA, United States 3University of California, Department of Biochemistry and Biophysics, San Francisco, CA, United States 4Kyoto University, Institute for Integrated Cell‐Material Sciences, Kyoto, Japan 5University of California, Department of Neurosciences, San Diego, La Jolla, CA, United States 6University of California, National Center for Microscopy and Imaging Research, San Diego, La Jolla, CA, United States 7Stanford University School of Medicine, Department of Neurology and Neurological Sciences, Palo Alto, CA, United States 8University of California, Department of Neurology, San Francisco, CA, United States
Astrocyte activation to extracellular matrix (ECM) – producing cells is inhibitory to regeneration in CNS injury or disease. Here we show that the cleaved neurotrophin receptor p75NTR controls transforming growth factor (TGF)‐beta‐mediated astrocyte activation via regulation of nucleocytoplasmic shuttling of Smad2. Loss of p75NTR completely rescues TGF‐beta‐induced hydrocephaly, astrocyte activation, and ECM production in GFAP‐TGF‐beta transgenic mice. Nuclear fractionation of TGF‐beta‐treated p75NTR knock out astrocytes revealed reduced nuclear phosphorylated Smad2 than their WT counterparts. In accordance to p75NTR depletion, inhibition of TGF‐beta‐induced gamma‐secretase‐mediated p75NTR cleavage also reduces nuclear accumulation of Smad2 and the secretion of proteoglycans that inhibit neurite outgrowth. Taken together, our results identify regulated intramembrane cleavage of p75NTR as a mechanism for astrocyte activation resulting in the regulation of TGF‐beta signaling and scar formation in the diseased brain.
Supported by the National Multiple Sclerosis Society postdoctoral fellowships to J.K.R. and N.LM., the European Commission FP7 Grant PIRG08‐GA‐2010‐276989 and the German Research Foundation Grant SCHA 1442/3‐1 to C.S., and NIH/NINDS Grants R01NS051470 and R01NS052189 to K.A. This project utilized facilities at the National Center for Microscopy and Imaging Research which is supported by grants from the National Center for Research Resources (5P41RR004050‐24) and the National Institute of General Medical Sciences (8 P41 GM103412‐24) from the National Institutes of Health to M.E.
T12‐09A. Does the developmental heterogeneity of oligodendrocyte origin influence remyelination of the adult central nervous system?
A. Crawford 1, R. Tripathi2, W.D. Richardson2, R.J.M. Franklin1
1University of Cambridge, Department of Veterinary Medicine, Neurosciences, Cambridge, United Kingdom 2University College London, Wolfson Institute for Biomedical Research, London, United Kingdom
The oligodendrocyte precursor cell (OPC) is the major cell type responsible for remyelination, a regenerative process vital to the repair of areas of demyelination in the central nervous system (CNS). OPCs originate from two discrete locations, ventral and dorsal, within the developing nervous system, raising the question of whether these may represent two functionally distinct OPC populations. The purpose of this project is to study and compare the roles of the two populations in remyelination. Using a transgenic mouse line in which ventral OPCs express green fluorescent protein, and dorsal OPCs express TdTomato, lysolecithin injection into the spinal cord was performed to create focal white matter demyelinating lesions. The remyelination process was then monitored to 21 days post lysolecithin injection. The results obtained so far have shown that both OPC populations take part in remyelination, but that the dorsal population is the most active, showing a greater proliferative response and a significant increase in cell number within the lesioned tissue. Identifying the factors that enable the dorsal population to become the major cellular player in remyelination may reveal new targets for its therapeutic enhancement.
T12‐09B. Reactive glia acquire stem cell properties in response to Sonic hedgehog in the injured brain
S. Sirko 1, G. Behrendt1, P. Johansson2, N. Plesnila3, K. Grobe4, L. Dimou1, M. Götz1,2,5
1Ludwig‐Maximilians‐University Munich, Munich, Germany 2Helmholtz Centrum Munich, German Research Center for Environmental Health, Institute for Stem Cell Research, Neuherberg/Munich, Germany 3Ludwig‐Maximilians University Munich, Institute for Stroke and Dementia Research, Munich, Germany 4Westfälische Wilhelms University Münster, Institute for Physiological Chemistry and Pathobiochemistry, Münster, Germany 5Munich Cluster for Systems Neurology (SyNergy), Munich, Germany
The reaction of glial cells toward brain injury comprises a proliferative response with some reactive astrocytes even reacquiring stem cell potential as indicated by forming long‐term self‐renewing and mutlipotent neurospheres (for review: Robel et al., 2011). Here we addressed first the question to which extent the type of injury selectively affects the proliferative response of NG2 glia and astrocytes and to which extent the proliferative reaction of astrocytes may be linked to the stem cell response. Interestingly, while both astrocytes and NG2 glia mounted a profound proliferative response after acute invasive injury, such as stab wound and MCAo, non‐invasive injury models, such as amyloid plaque deposition or widespread neuronal death could not activate the proliferation of these macroglial cells, while microglia proliferated actively in all these lesion models. Intriguingly, the proliferative response of astrocytes correlated to the activation of their potential to form self‐renewing, multipotent neurospheres with a steep decline with age. We then set‐out to determine the signals regulation reactive astrocyte proliferation and neurosphere formation after invasive injury and demonstrate that invasive lesions result in entrance of Sonic hedgehog (SHH) into the brain from extraneural sources, such as the cerebro‐spinal fluid, and activate astrocyte proliferation and neurosphere formation. This response can be blocked by systemic injection of the SHH‐signaling antagonist cyclopamine as well as inducible astrocyte‐specific deletion of the receptor smoothened by GLASTCreERT2. Interestingly, SHH‐agonists can boost the proliferative response of astrocytes in vivo and their subsequent neurosphere‐forming capacity, thereby providing a new approach how to activate this response in conditions where it is limited, as e.g. in age or non‐invasive injury conditions. Taken together, our work highlighted for the first time differences in the proliferative and stem cell response of reactive astrocytes in different injury paradigms and identified the SHH signaling pathway as a key signal in this response.
Robel S, Berninger B, Götz M. The stem cell potential of glia: lessons from reactive gliosis. Nat Rev Neurosci. 2011 Feb;12(2):88–104.
T12‐10A. Role of Tumor Necrosis Factor‐Alpha Inhibition following Spinal Cord Injury
D.G. Ellman 1, V. Bracchi‐Ricard2, H.G. Novrup1, A. Jain2, L. Lyck1, L. Lykkemark1, D.E. Szymkowski3, D.E. Pearse2, J.R. Bethea2, K.L. Lambertsen1,2
1University of Southern Denmark, Odense, Denmark 2University of Miami, Miller School of Medicine, Miami, United States 3Xencor Inc, Monrovia, United States
Tumor necrosis factor (TNF) is a pleotrophic cytokine involved in normal brain function and inflammation, apoptosis, and other responses that occur following injury and disease. There is a considerable amount of confusion as to whether or not TNF activation is beneficial or detrimental following injury to the CNS. Genetic studies in mice have suggested that inflammation in disease models involves soluble TNF (solTNF) and that maintenance of innate immune function involves transmembrane TNF (tmTNF). These findings suggest that the outcome of selective pharmacologic inhibition of TNF may depend on whether the pharmacologic intervention is targeted towards solTNF or tmTNF.
Therefore, we took advantage of a dominant‐negative inhibitor of solTNF, XPro195, that selectively inhibits solTNF and compared the effect of this inhibitor to a more widely used IgG1 Fc‐TNF receptor (TNFR) 2 fusion protein Etanercept, an inhibitor of both solTNF and tmTNF, that has been a successful treatment strategy for several autoimmune diseases. However, Etanercept has been shown to display severe side effects by increasing the risk of infections.
Female C57BL/6 mice were subjected to a spinal cord injury at T9 level. Mice were divided into three groups, receiving Etanercept, XPro1595, or saline. The drug was delivered directly onto the lesion site using Alzet mini osmotic pumps for 3 days starting immediately after introduction of the lesion. Mice were allowed to survive 35 days. Functional evaluation was performed using the Basso Mouse Scale, Rung Walk, Open Field test and Hargreaves test. In contrast to Etanercept, XPro1595 significantly ameliorated recovery of hind limb function (evaluated by motor recovery score) compared to saline when administered continuously to the lesioned cord, whereas s.c. injections every 3 days for 8 weeks following SCI had no effect.
Our study suggests that selective inhibition of solTNF is beneficial following SCI when administered directly to the contused spinal cord and raises the possibility that selective inhibition of solTNF may represent a new therapeutic strategy for the treatment of SCI without compromising the innate immune response.
T12‐10B. Oncostatin M Reduces Lesion Size and Promotes Functional Recovery after Spinal Cord Injury
H. Slaets, S. Nelissen, K. Janssens, P.‐M. Vidal, S. Hendrix, N. Hellings
Biomed‐Hasselt University, Diepenbeek, Belgium
The family of interleukin‐6 type cytokines plays a dual role in neuroinflammation by regulating both neural as well as immune responses. Here, we show that expression of oncostatin M (OSM) is upregulated after spinal cord injury (SCI). To reveal the relevance of increased OSM signaling in the pathophysiology of SCI, OSM was applied locally after hemisection. Acute OSM‐treatment significantly improved locomotor recovery after mild and severe SCI. Delayed OSM‐treatment in the chronic phase was ineffective. Improved recovery in OSM‐treated mice was associated with a reduced lesion size, less astrogliosis and diminished neuroinflammation. The underlying mechanism involves neuroprotective effects of OSM. Furthermore, OSM promoted nerve fiber regeneration both in vitro and in vivo. Thus, local production of OSM after SCI is an endogenous response that limits CNS lesion propagation and promotes recovery.
T12‐11A. Three Ca2+ channel inhibitors in combination reduce chronic secondary degeneration following neurotrauma
D. Savigni, R. O'Hare Doig, C. Szymanski, C. Bartlett, I. Lozic, N. Smith, M. Fitzgerald
The University of Western Australia, Crawley, Australia
Following neurotrauma, cells beyond the initial trauma site undergo secondary degeneration, with excess Ca2+ a likely trigger for loss of neurons, compact myelin and function. Treatment of secondary degeneration by limiting excess Ca2+ entry into cells using inhibitors of specific Ca2+ channels showed promise in preclinical studies, but clinical trials were disappointing and combinatorial approaches are acknowledged as necessary. We assessed efficacy of every possible combination of three Ca2+ channel inhibitors at reducing secondary degeneration 3 months after partial optic nerve (ON) transection in rat. We used lomerizine to inhibit voltage gated Ca2+ channels (for 3 months); oxidised adenosine‐triphosphate (oxATP) to inhibit purinergic P2X7 receptors and/ or 2‐[7‐(1H‐imidazol‐1‐yl)−6‐nitro‐2,3‐dioxo‐1,2,3,4‐tetrahydro quinoxalin‐1‐yl]acetic acid (INQ) to inhibit Ca2+ permeable α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid (AMPA) receptors (both for the first 2 weeks after injury). Only the three Ca2+ channel inhibitors delivered in combination completely preserved visual function to levels not different from normal animals.
In order to determine the mechanism/s by which the three inhibitors delivered in combination preserved function, we assessed neuroprotection, nerve swelling, myelin compaction and node/paranode complexes. Preservation of retinal ganglion cell (RGC) somata and axons is unlikely to have accounted for the full recovery of function as only partial neuroprotection of RGCs vulnerable to secondary degeneration was achieved. A range of the Ca2+ channel inhibitor combinations prevented swelling of optic nerve vulnerable to secondary degeneration, with no one agent selectively effective. Each of the treatments involving lomerizine significantly increased the proportion of axons with normal compact myelin and decreased the proportion of axons with partially decompacted myelin, indicating the importance of sustained control of Ca2+ flux via voltage gated Ca2+ channels for prevention of chronic myelin decompaction. Nevertheless, limiting decompaction of myelin or formation of atypical node/ paranode complexes was not sufficient for preservation of function in our model. It is likely that the additional prevention of the lengthening of the paranodal gap that was only achieved by treatment with the three Ca2+ channel inhibitors in combination was an important effect that contributed to the associated preservation of visual function by this combinatorial treatment strategy.

T12‐11B. EphrinB3 antibody accelerates CNS remyelination following toxin‐induced demyelination
Y.A. Syed 1, M. Hofer2, G. Gonzalez2, K.‐A. Nave1, M. Kotter2
1Max Planck Institute of Experimental Medicine, Neurogenetics, Gottingen, Germany 2Wellcome Trtust‐MRC Center for Stem Cell Biology, Cambridge, United Kingdom
Myelin regeneration (remyelination) is mediated by a multipotent adult stem/precursor cell population often referred to as oligodendrocyte progenitor cells (OPCs)
To generate myelinating oligodendrocytes OPCs undergo a distinct series of morphological and molecular changes. These are regulated by a combination of extrinsic and intrinsic factors, which have only been partially been revealed so far.
In the present study we identify a novel signaling mechanism as potent negative regulator of OPC maturation. Ephrin B3, a member of the B‐class family of ephrins, inhibits OPC process formation and blocks the formation of mature oligodendrocytes in vitro. The presence of EphrinB3 results in activation RhoA and PKCα signalling and deactivation of FAK kinase in OPCs in vitro. Moreover, EphA4 RTK, a receptor responsive to EphrinB3 in OPCs, is also expressed by OPCs in demyelinating lesions.
Infusion of recombinant Ephrin B3 into demyelinating lesions results in a failure of remyelination caused by an inhibition of OPC maturation. In contrast, antibody‐mediated masking of Ephrin B3 epitopes in vitro as well as in demyelinating lesions of aged rats promotes OPC differentiation and accelerates myelin regeneration. Our results demonstrate an important role of Ephrin B3 for the process of remyelination.
T12‐12A. Neuregulin‐1 type I enhances functional recovery after acute peripheral nerve injury and rescues axonal loss in a mouse model for Charcot Marie Tooth disease 1A
R. Fledrich 1, R.M. Stassart1, L.M. Haag2, P. Veselcic1, D. Czesnik3, K.‐A. Nave1, M.W. Sereda1,3
1Max Planck Institute of Experimental Medicine, Göttingen, Germany 2Ruhr‐Universität Bochum, Bochum, Germany 3Universitätsklinik Göttingen, Neurophysiologie, Göttingen, Germany
After peripheral nerve injury, axons rapidly degenerate and start regrowing only after a few days. Full functional recovery, however, is rare. A progressive loss of intact axons also determines the clinical phenotype of chronic demyelinating peripheral neuropathies, such as Charcot Marie Tooth disease type 1A (CMT1A). CMT1A is the commonest inherited neuropathy, caused by a duplication of the peripheral myelin protein 22kDa (PMP22) gene. We demonstrate that the transgenic neuronal overexpression of the growth factor neuregulin‐1 type I enhances axonal regeneration and functional recovery after experimental acute peripheral nerve injury. Similarly, neuregulin‐1 type I overexpression preserves axonal loss and improves nerve function in a mouse model for CMT1A. Importantly, neuregulin‐1 type I induced axonal preservation in CMT1A mice was independent of myelination, as myelin sheath thickness was not altered.
We conclude that axonal recovery and preservation relies on common supportive factors and that paracrine neuregulin‐1 type I constitutes a beneficial signal in peripheral nerve disorders.
T12‐12B. Profiling of Aquaporin 4 knockout astrocytes regarding tight junction protein expression
G. Szabó
University of Tuebingen, Tuebingen, Germany
Question: The enormous capacity for repair of the CNS after lesion is one of the most amazing property of lower vertebrates. In the mammalian central nervous system (CNS) the astrocytes reveal the so‐called orthogonal arrays of particles (OAPs); the clusterization of the AQP4 molecules at the astrocytic endfeet. In lower vertebrates these structures are lacking. In the case of the Amphibia ‐Anolis carolinensis‐ the caudal spinal cord has the capability for regeneration after loss of the tail, an ability which might be in relation with the presence of tight junctions (TJ) between the glial cells like the olfactory ensheating cells act in mammalian olfactory system through the lifespan. The growing axons of the fish optic nerve are embraced by astrocytes which are also interconnected by TJs. TJ connected glial cells could contribute to a growth‐supportive microenvironment which might reinforce our hypothesis namely the mutual exclusion of AQP4 occurrence in astrocytes and regenerative growth of nerve fiber.
Methods: We performed immunocyto‐and immunohystochemical staining, western blot, freeze‐fracture electron microscopy and PCR on cultured AQP4 knockout and wildtype astrocytes and also on formalin fixed paraffin embedded tissue sections regarding junctional proteins, such as Occludin, Claudin 1,3,5.
Result: In the light of our experiments, there is no mutual exclusiveness between the presence of AQP4 and tight junctions in astrocytes, at least in mammals. Further investigations need to be completed.
T12‐13A. Demyelinated axons regulate their own remyelination via glutamate signalling to oligodendrocyte precursor cells
H. Gautier, K. Evans, I. Lundgaard, C. Lao‐Peregrín, R.J.M. Franklin, R.T. Káradóttir
Wellcome Trust/MRC Cambridge Stem Cell Institute, Cambridge, United Kingdom
During development, some OPCs differentiate into myelinating oligodendrocytes while others remain into adult life, where they comprise approximatley 5% of CNS cells and are the main proliferating cells present. In demyelinating disease such as multiple sclerosis, adult OPCs differentiate into new oligodendrocytes that remyelinate the axons, although this process eventually fails or is incomplete, leading to sustained clinical deficits.
Glutamate and electrical activity influence OPC differentiation and myelination in normal development. OPCs receive synaptic input from unmyelinated axons, possibly to initiate myelination. Both OPCs and mature oligodendrocytes respond to glutamate via AMPA and NMDA receptors. Activation of glutamate receptors in vitro increase OPC migration but decreases proliferation.
Here we examine the role of glutamate signalling in remyelination following experimental demyelination in the adult rodent CNS. We voltage‐clamped OPCs in brain‐slices of adult rat cerebellar peduncle containing focal areas of primary demyelination and post‐identified these by NG2‐immunolabelling. Recruited OPCs mainly expressed AMPA receptors at the peak of the OPCs proliferation (5–10 days post‐lesion), as over 90% of the glutamate evoked current was blocked with NBQX (AMPA/Kainate receptor antagonist) and unaffected by AP5 (NMDA receptor antagonist).
The demyelinated axons continued to propagate action potentials with latencies similar to those in unmyelinated axons during development. Critically, the demyelinated axons established synapses with OPCs expressing voltage‐gated sodium currents. These synaptic inputs had identical decay times to those recorded during developmental myelination. Pharmacological inhibition of neuronal activity or glutamate signalling decreased remyelination efficiency by impairing differentiation.
These results indicate that 1) glutamate currents are mainly mediated via AMPA and kainate receptors during the OPC recruitment 2) OPCs establish synaptic contact with demyelinated axons and 3) the neuronal activity is essential for remyelination. Together, these data suggest that demyelinated axons remain active and communicate with OPCs through glutamate release during the regenerative process to guide their remyelination.
T12‐13B. Enhancement of stem cell integration into the retina by modulating glial reactivity in an in‐vitro stem cell transplantation model
A. Tassoni, N. Bull, K. Martin
University of Cambridge, Cambridge, United Kingdom
There has been much recent interest in the use of intraocular stem cell transplantation to slow or reverse visual loss in glaucoma. However, migration and integration of stem cells into the retina is limited by reactive gliosis, a major barrier to retinal engraftment. Our aim was to identify glial modulators of low toxicity and measure their potential to facilitate stem cell entry into the host retina.
An explant organotypic culture system was used as a stem cell transplantation model and screening tool for candidate drugs. GFP+ mouse Mesenchymal Stem Cells (mMSCs) were co‐cultured on the surface of the explants for 4 days.The microglial contribution to MSC‐induced reactive gliosis was investigated by assessing microglial activation and proliferation using immunohistochemistry for F4/80 and EdU. Distribution of chondroitin sulphate proteoglycans (CSPGs) and their role in the barrier was also explored by immunostaining for chondroitin sulphate (CS‐56) and by Chondroitinase ABC(ChABC) treatment. Stat3 inhibitor VI and Jak1 inhibitor were used to suppress Glial Fibrillary Acidic Protein (GFAP) expression. The efficacy of each drug to overcome the barrier was evaluated by immunohistochemistry for the glial markers GFAP. mMSC integration was assessed by simultaneous immunostaining for GFP. Cell viability after drug treatment was investigated by measuring the preservation of retinal thickness and by immunostaining for the neuronal marker NeuN and for Caspase3.
Co‐culture of retinal explants with mMSCs caused a 1.5 ± 0.4 fold increase in GFAP expression. Microglial activation and proliferation was not markedly affected by the presence of grafted mMSCs on retinal surface. CSPGs, highly expressed in the outer retina but only moderately in the inner retina, do not contribute substantially to the barrierto inner retinal engraftment. In contrast, interfering with the JAK/STAT pathway successfully reduced GFAP expression by 60% ± 10% compared to control and facilitated mMSCs integration into the host tissue. No toxic effect on cell viability was detected. On the contrary, over the 4 days ex‐vivo culture, STAT3 inhibitor VI treatment resulted in a neuroprotective effect on the outer nuclear layer, whose thickness (50.84 ± 4.4 µm) is better preserved compared to control (38.4 ± 9.0 µm)
The results show that microglia and CSPGs do not play a dominant role in the barrier to retinal engraftment, but the JAK/STAT pathway represents a promising pharmacological target.Targeting modulators of glia reactivity to promote stem cell integration in the host tissue may facilitate stem cell therapy in glaucoma and other neurodegenerative diseases.
T12‐14A. Olfactory ensheathing cells promote neurite outgrowth from organotypic spinal cord co‐cultures
K. Gladwin, S. Law, D. Li, D. Choi
University College London, London, United Kingdom
Olfactory ensheathing cells (OECs) are unique glia with potential for therapeutic transplantation for the repair of spinal cord injuries (SCI). OECs possess many characteristics favorable for the repair of SCI including the secretion of growth and survival factors, the expression of cell adhesion molecules and the ability to integrate with astrocytes. OECs have also been reported to promote exceptional outgrowth of severed axons across the inhibitory environment of the lesion scar. The unique combination of regenerative properties suggests OECs would be beneficial for the repair of ventral spinal root avulsion injuries. After ventral root avulsion anterior horn neurons often fail to regenerate axons back into the surgically re‐implanted nerve root resulting in poor functional recovery.
To evaluate the efficacy of OEC transplants for the repair of ventral root avulsions, the ability of OECs to promote neurite outgrowth from spinal cord neurons was investigated in a spinal cord organotypic co‐culture. The integration of OECs with the spinal cord tissue was also examined by confocal fluorescence microscopy. OECs were harvested from adult Sprauge Dawley (SD) rats, cultured for 14 days and transfected with GFP. Slices of cervical spinal cord were prepared from P8 SD pups and cultured on collagen coated membrane inserts. Each slice was surrounded by a collagen gel with or without the addition of GFP OECs and Glial derived neurotrophic factor (GDNF). Our results indicate that OECs alone and in combination with GDNF have a positive effect on neurite outgrowth and morphology.
This work was supported by the European Research Council and the International Spinal Research Trust.
T12‐14B. Netrin‐1 and central nervous system remyelination
V. Tepavcevic 1, M.‐S. Aigrot1, C. Kerninon1, E. Meppiel1, S. Mozafari1, R. Arnauld‐Laurent1, C. Ross1, P. Ravassard1, T.E. Kennedy2, B. Nait Oumesmar1, C. Lubetzki1
1CRICM, Paris, France 2McGill University, Department of Neurology and Neurosurgery, Centre for Neuronal Survival, Montreal Neurological Institute, Montreal, Canada
Stimulating remyelination in multiple sclerosis (MS) lesions is considered as a potential therapeutic strategy to reduce axonal loss, hence the development of irreversible neurological disability. Oligodendrocyte progenitor cells (OPCs) are the remyelinating cells of the central nervous system (CNS). In experimental models of demyelination, efficient repopulation of demyelinated areas is a pre‐requisite for successful myelin repair. Alteration of this process in MS lesions could explain a) absence of oligodendrocyte lineage cells within certain areas of chronic demyelination and b) presence of oligodendrocyte lineage cells but absence of myelin repair due to late OPC arrival, once the conditions required for remyelination are no longer present. Netrin‐1, a guidance molecule from laminin superfamily, is an important determinant of OPC migration during embryonic development. We have investigated the role of Netrin‐1 on remyelination of adult mouse CNS using a toxin‐induced model of demyelination. Our results show that Netrin‐1 is expressed in the demyelinating lesion only once the OPCs have repopulated the demyelinated area. If the timing of Netrin‐1 expression is accelerated using lentiviral vector injection into the lesion area immediately following demyelination, OPC recruitment into area of demyelination is diminished and remyelination delayed, while newly generated oligodendrocytes accumulate around lesion borders. We are currently investigating Netrin1 expression in different types of MS lesions.
T12‐15A. Hydrogel as a tool for vascular endothelial growth factor gradual release in peripheral nerve regeneration
S. Gnavi 1, C. Tonda Turo2, L. di Blasio3, F. Ruini2, L. Primo3, S. Geuna1,4, G. Gambarotta4, I. Perroteau4
1University of Torino, Neuroscience Institute Cavalieri Ottolenghi, Orbassano, Italy 2Polytechnic University of Torino, Department of Mechanics, Torino, Italy 3University of Torino, Institute for Cancer Research and Treatment, Candiolo, Italy 4University of Torino, Department of Clinical and Biological Science, Orbassano, Italy
The aim of this study was to obtain an hydrogel for the controlled delivery of Vascular Endothelial Growth Factor (VEGF‐A165).
Hydrogels are a class of liquid‐gel biomaterials with high water content, typically composed of cross‐linked, three‐dimensional hydrophilic water‐soluble polymer networks. The advantages of injectable hydrogel systems are: biocompatibility, biodegradability, adjustable shape, low cost and similarity to the extracellular matrix; moreover, they can be used as growth factor and cell delivery systems for tissue engineering applications.
Agarose/Gelatin (A/GL) hydrogel was cross‐linked with genepin. Rheological measurements show that A/GL hydrogel can be injected through a syringe into the inner cavity of a nerve guide. In vitro assays performed to evaluate adhesion, proliferation, viability and migration – using a fibroblast cell line (NIH3T3), Neonatal Olfactory Bulb Ensheathing Cells (NOBEC) and a Schwann cell line (RT4‐D6P2T) – show that hydrogel allows cell adhesion, proliferation, viability and migration.
VEGF‐A165 is a potent angiogenic factor and angiogenesis has long been recognized as an important and necessary step during tissue repair. VEGF is known to have a positive effect on Schwann cell proliferation and migration, neuron survival and outgrowth of regenerating nerve fibers.
The hydrogel preparation protocol was optimized to be functionally efficient at physiological conditions, allowing the loading of VEGF without the risk of its denaturation.
Different amounts of VEGF (50–100‐200 ng/ml) were incorporated in the A/GL hydrogel and the releasing rate in vitro up to 65 days was quantified by ELISA . Released VEGF bioactivity was validated in Human Umbilical Vein Endothelial Cells (HUVEC) and in NOBEC by evaluating phosphorylation of VEGF‐Receptor 2 (VEGFR2), akt and erk1–2. Moreover, dorsal root ganglia explants were cultured on hydrogel containing different amounts of VEGF, and an increased neurite outgrowth was quantitatively assessed.
These results demonstrate that VEGF can be successfully incorporated and bioactive released from A/GL hydrogel inducing VEGFR2 activation and neurite outgrowth.
In vivo test are ongoing on adult rats: one centimetre lesions on medial nerve were performed, followed by implantation of Porous Poly‐e‐caprolactone tubes filled with A/GL hydrogel containing VEGF‐A165. Functional tests and histological analysis will be carried out to evaluate peripheral nerve regeneration.
T12‐15B. Human Platelet Derived Growth Factor Responsive Neural Precursors (PRPs) Differentiate into Mature Oligodendrocytes and Produce Myelin when Transplanted into the Injured Rat Spinal Cord
G.J. Duncan1, A. Chojnacki2, J.R. Plemel3, P. Assinck3, A. Samiei1, J. Kim1, Y. Jiang1, J. Liu1, S. Weiss2, W. Tetzlaff 3
1University of British Columbia, ICORD, Vancouver, Canada 2University of Calgary, Hotchkiss Brain Institute, Calgary, Canada 3University of British Columbia, Vancouver, Canada
Spinal cord injury (SCI) often leads to death of oligodendrocytes and considerable demyelination. Demyelination of axons compromises signal conduction, and contributes to the functional deficits following SCI. Enhancing remyelination may serve to restore conduction and likely prevents axonal degeneration. Here we focussed on a potential therapeutic intervention to promote remyelination, the transplantation of human oligodendrocytes precursor cells (OPC)s capable of differentiating into mature oligodendrocytes and remyelinating denuded axons. Human platelet derived growth factor (PDGF)‐responsive neural precursor (PRP) cells were isolated from the fetal forebrain and had been previously shown to differentiate into mature oligodendrocytes with a higher efficiency in vitro than traditional EGF/FGF responsive neural stem cells (Chojnacki and Weiss 2004; Deleyrolle et al. 2006). Therefore, we tested the potential of human PRPs to differentiate into new oligodendrocytes and remyelinate axons in a rodent thoracic contusion spinal cord injury model. One week following injury, human PRPs were transplanted rostral and caudal to the lesion site. Subsequent histological examination at two, seventeen and forty‐two days post‐transplantation demonstrated that human PRPs survived and integrated well into host tissue, particularly when NK cells were depleted. Transplanted human PRP's labelled with the mature oligodendrocyte marker APC, and 45% of the cells colabelled with the oligodendroglial marker olig2. Astrocyte differentiation was also observed, suggesting human PRPs function as a multipotent glial progenitor following SCI. Human‐specific immunoreactive processes were seen in close association with axons expressing the myelin protein MBP, providing evidence of remyelination by the transplanted cells. Overall, these findings indicate that human PRPs are a source of new oligodendrocytes capable of producing myelin in response to spinal cord injury.
This research was supported by the Stem Cell Network, Multiple Sclerosis Society of Canada and CIHR.
T12‐16A. Tamoxifen promotes CNS remyelination by modulation of the PKC signalling pathway in oligodendrocyte precursor cells (OPCs)
G. Gonzalez 1, A. Baer2, J. Rundle1, Y. Syed3,4, M. Hofer3,4, R. Franklin3,5, C. Zhao5, M. Kotter3,4
1University of Cambridge, Cambridge, United Kingdom 2University of Vienna, Vienna, Austria 3University of Cambridge, Clinical Neuroscience/BRC, Cambridge, United Kingdom 4Laboratory for Regenerative Medicine (LRM), Cambridge, United Kingdom 5University of Cambridge, School of Veterinary Medicine, Cambridge, United Kingdom
Remyelination is a regenerative process during which demyelinated axons are enwrapped by newly formed myelin sheaths. In the central nervous system (CNS), this process takes place in two stages: first, during the recruitment stage, oligodendrocyte precursor cells (OPCs) divide, migrate and engage the demyelinated axons. Then, during the differentiation stage, OPCs differentiate to myelin‐forming oligodendrocytes. Under pathological conditions such as multiple sclerosis (MS), remyelination often fails and as a consequence chronic demyelinated areas accumulate in MS. Post mortem evidence suggests that OPC differentiation is often impaired in MS patients. However, the reason why OPC differentiation fails in MS is not completely understood. It has been proposed that MS lesions contain factors that act as specific inhibitors of OPC differentiation. Amongst them, myelin associated inhibitors play an important role. We have demonstrated that the inhibitory effect of myelin protein extracts (MPE) is mediated by activation of signalling cascades including PKCα‐MARCKS in vitro. Furthermore, modulation of PKCα has been shown to rescue OPC differentiation in the presence of inhibitory MPE. Here we demonstrate that tamoxifen, a drug that is used in various clinical settings because of its PKC‐inhibitory activity, is able to promote OPC differentiation in vitro.
In a series of in vivo experiments, we tested the hypothesis that inhibition of PKCα by tamoxifen promotes CNS remyelination. Demyelination was induced in the caudal cerebellar peduncle (ccp) of young‐adult Sprague‐Dawley rats by the administration of ethidium bromide. Animals received daily doses of tamoxifen. Although tamoxifen treatment did not induce changes in the number of OPCs expressing immature markers (NG2 and PDGFR‐α), a significant increase in the number of cells expressing mature marker of oligodendrocytes (CC1/Olig2 and PLP) was detected. Furthermore, analysis of resin‐embedded tissue demonstrated that tamoxifen significantly promotes remyelination in the CNS when compared to controls (Mann‐Whitney's test, p < 0.05). Although further investigations are required to elucidate the mechanisms by which tamoxifen exerts CNS remyelination, our results demonstrate that administration of tamoxifen represents a potent and clinically accessible strategy to promote endogenous myelin regeneration.
T12‐16B. Optimization of iPS‐cell based remyelination cell therapy in a nonhuman primate model for multiple sclerosis (MS)
A. Thiruvalluvan 1, M. Czepiel1, J. Boertien1, B. A. ‘t Hart2, Y. Kap2, E. Boddeke1, S. Copray2
1University Medical Center Groningen, Groningen, Netherlands 2Biomedical Primate Research Centre, Rijswijk, Netherlands
Induced pluripotent stem (iPS) cell technology now allows development of autologous oligodendrocyte precursor cells for putative remyelination cell therapy for multiple sclerosis (MS). We have successfully developed mouse and human iPS cells and converted these into oligodendrocyte precursor cells (OPCs) that can differentiate into fully mature oligodendrocytes. Furthermore, we have shown that (intracerebral) implantation of iPS‐derived OPCs in mouse MS models enhances remyelination.Before implantation‐induced remyelination therapy with autologous IPS‐derived OPC can proceed to a clinical trial for remyelination cell therapy in MS patients, we have to provide solid evidence for long term efficacy and to establish the safety thus ultimately excluding contamination of teratogenic (pluripotent) stem cells. These issues are best examined in a nonhuman primate model. The Biomedical Primate Research Center (BPRC), Rijswijk has developed a well‐documented experimental autoimmune encephalomyelitis (EAE) model in marmoset monkeys that most closely approximates the pathology of MS in humans. In this animal model we will verify that intrathecally administered iPS‐derived OPCs migrate to and remyelinate EAE lesions and determine their long‐term fate.We have developed an efficient protocol to induce marmoset IPS cells from skin fibroblasts. For that we have used a lentiviral polycistronic construct, containing the four (human) reprogramming genes Oct4, Klf4, Sox2 and cMyc, that is excisable using cre‐recombinase. The pluripotent nature of the marmoset IPS cells was established based on the endogenous expression of pluripotency transcription factors and the ability to differentiate in‐vitro (via embryoid bodies) and in‐vivo (subcutaneous injection for teratoma formation) into all the different tissues of the three germ layers. Presently we are developing protocols for the differentiation of these marmoset IPS cells into an oligodendrocyte cell lineage.
T12‐17A. Human pluripotent stem cell‐derived oligodendrocyte precursor cells for spinal cord injury repair
A. Hyysalo 1,2, M. Mäkinen1,2, L. Ylä‐Outinen1,2, T. Joki1,2, S. Narkilahti1,2
1University of Tampere, Institute of Biomedical Technology, Tampere, Finland 2BioMediTech, Tampere, Finland
Introduction: Loss of neural connections, degradation of myelin sheath, and death of oligodendrocytes arise as secondary damage in spinal cord injury (Emery et al., 1998). Currently, no effective treatment for spinal cord injury exists. However, functional recovery through remyelination by oligodendrocyte transplantation could provide a possible treatment. For that aim, human oligodendrocytes are needed in large amounts, and they can be produced from human pluripotent stem cells. Studies with animal cells suggest that electrophysiological properties of oligodendrocyte precursor cells (OPCs) can have an effect in the myelination capacity of the cells (Karadottir et al. 2008). Characteristics of developing human oligodendrocytes are not excessively studied but several studies indicate heterogeneity among cells expressing generally accepted OPC marker chondroitin sulphate proteoglycan (NG2) (Polito and Reynolds, 2005). Thus, thorough characterization of OPCs is critical for determination of safety and efficacy of these cells.
Materials and Methods: Previously, our group has developed and published protocols for differentiation of oligodendrocytes and OPCs from human embryonic stem cells (Sundberg et al. 2010, Sundberg et al. 2011). Published protocols have been modified for production of OPCs for research purposes, and produced cells are used for characterization of OPCs. Ultimately the aim is to define an OPC population with optimal myelination capacity.
Results and Discussion: Currently, human oligodendrocytes and OPCs can be produced from pluripotent stem cells. These cells are routinely characterized in protein expression level, and methods for more comprehensive characterization are under development. Produced OPCs can be purified with fluorescence‐activated cell sorting based on NG2 protein expression in order to study if this cell population is heterogeneous in terms of myelination capacity.
Conclusions: Oligodendrocytes and OPCs can be produced from human pluripotent stem cells. Studies are ongoing to determine the molecular characteristics and myelination capacity of the differentiated and purified OPC‐ population.
T12‐17B. Vascular niche provides unique microenvironment for oligodendrocyte precursor cells differentiation during CNS remyelination
J. Ulanska‐Poutanen 1, J. Mieczkowski1, B. Kaminska1, C. Zhao2, R.J.M. Franklin2, M. Zawadzka1
1Nencki Institute of Experimental Biology, Warsaw, Poland 2Wellcome Trust/MRC Stem Cell Institute, University of Cambridge, Cambridge, United Kingdom
Remyelination, the regenerative process in which myelin is restored to demyelinated axons, is carried by oligodendrocyte precursor cells (OPCs). In a previous study we have shown that oligodendrocyte precursor cells are multipotent and they can differentiate into oligodendrocytes as well as to Schwann cells and occasionally astrocytes in the remyelinating lesions. We observed that Schwann cells derived from OPCs in the central nervous system occupied almost exclusively tissue around blood vessels in astrocyte deficient areas. Therefore, we postulated the occurrence of specific niches or microenvironments that modulate OPC fate. The aim of present study was to identify the factors and their downstream effectors that significantly discriminate vascular and non‐vascular niche and determine the fate of oligodendrocyte precursor cells.
We microdissected tissue from vascular and non‐vascular regions of lesion areas at 6, 10 and 14 days after bilateral stereotaxic injection of ethidium bromide into the brain white matter of adult rats and performed microarrays to profile the whole transcriptome of both niches separately. This approach allowed us to distinguish between mRNAs that are enriched within two separate environmental niches within the same lesion area. The study identified 138 differentially expressed transcripts. Comparative bioinformatic analysis of global gene expression combined with signalling transduction pathway structure and genes function analysis revealed specific candidate signaling pathways differentially expressed in local peri‐vascular niche compared to non‐vascular one. Results of RT‐PCR‐based validation of microarray data have proven that expression gradient of BMP4, BMP7, their antagonist Sostdc1, as well as Wnt2b, Wnt6, Dhh and Scube1 act as the major regulators of OPCs fate in the vascular niche.
Moreover, we observed that blood vessels undergo substantial regeneration in the course of remyelination and postulated the important role of blood vessels reconstruction in creating the specific microenvironment of neurovascular niche during tissue regeneration. Our analysis shown that unique properties of tissue around blood vessels differ from the rest of the lesion which can be one of the factors favoring OPCs alternative differentiation in this area of lesion.
T12‐18A. HDACs in control of maintenance and regeneration in Schwann cells
V. Brügger1, C. Pattaroni1, P. Matthias2, U. Suter3, C. Jacob 1
1University of Fribourg, Fribourg, Switzerland 2FMI, Basel, Switzerland 3ETH Zurich, Zurich, Switzerland
Histone deacetylases (HDACs) are key transcriptional regulators that control gene expression by remodeling chromatin and modifying the activity of transcription factors. There are 18 known mammalian HDACs, subdivided into four classes. Using mouse genetics, we have shown that the two class I HDACs HDAC1 and HDAC2 are crucial for survival and myelination of Schwann cells, the myelinating glia of the peripheral nervous system (PNS), and for maintenance of peripheral nerve integrity in adults. Our current aim is to understand whether and how HDACs can influence the regeneration process in Schwann cells. Our data suggest important functions of HDACs in PNS regeneration.
T12‐18B. New insights into the reaction of mouse oligodendrocyte progenitor cells after brain injury by live in vivo imaging
A. von Streitberg 1, C. Straube1, M. Götz1,2,3, L. Dimou1,2
1Ludwig‐Maximilians‐University, Institute of Physiology, Physiological Genomics, Munich, Germany 2Helmholtz Zentrum, Institute for Stem Cell Research, Neuherberg/Munich, Germany 3Munich Cluster for Systems Neurology (SyNergy), Munich, Germany
After acute injury in the adult brain oligodendrocyte progenitor cells (OPCs), also referred to as NG2+‐cells, are reacting by hypertrophy and increasing their proliferation (Dimou et al., 2008; Simon et al., 2011). However, key aspects of dynamic behavior, such as cell migration or process orientation and motility, can only be monitored by live imaging. To elucidate these key aspects of OPC behavior after injury, we used repetitive in vivo two‐photon laser scanning microscopy (2pLSM) to follow NG2+‐cells after smaller and larger stab wound injuries in the mouse somatosensory cortex. We therefore used the BAC transgenic mouse line Sox10iCreERT2 (Simon et al., 2012) crossed to the inducible reporter line CAG‐GFP (Nakamura et al., 2006) selectively labeling cells of the oligodendrocyte lineage. GFP+ OPCs were imaged through a cranial window at the day of injury and at different time points thereafter. Identification of the same cells was possible based on blood vessels labeled by Rhodamine dextran as stable landmarks. Live imaging revealed that the majority of OPCs reacts within 2 days after injury with hypertrophy (enlarged soma and processes), polarization (elongation and concentration of processes towards the injury site), directed migration towards the injury site (defined as movement of the cell body for at least 10 µm) and proliferation (defined by a single cell being replaced by 2 or even more daughter cells). Only a small proportion of cells within ∼500 µm of the injury did not react in any detectable manner. We noted that polarization and hypertrophy occur rather fast after inflicting the injury, while proliferation peaks 4 days after injury. Taken together, the live observation of OPCs reacting to stab wound injury supports the concept of OPC heterogeneity and reveals new insights into the functional role of these cells after injury: the fast process orientation towards the injury site implies a contribution to wound closure and their substantial proliferation sometimes for several rounds amplifies the number of NG2+‐cells surrounding the injury site with implications for scar formation.
T12‐19A. Living art – method for labeling living human derived neural cells with fluorescent probes
T. Joki 1, M. Mäkinen1, L. Ylä‐Outinen1, H. Skottman2, R. Äänismaa1, S. Narkilahti1
1University of Tampere, Institute of Biomedical Technology, Tampere, Finland 2University of Tampere, Eye group, Tampere, Finland
Background: Fluorescent probes as such are not new but their use as tracking living cell populations in vitro is a tool that offers novel possibilities. Fluorescent probes attaching to living cells can be used e.g. in long term follow up studies with possibility to identify the labeled cell population or to enhance cell visibility in opaque cultures. These features are useful in many research areas in the field of applied sciences e.g. development of controlled neurotoxicity screening platforms, in vitro neural disease modeling, and biomaterial research for craft development.
The aim of this study was to create a non‐invasive tool for cell visualization and cell population tracking that can be used in long term studies.
Methods: This study was performed using human pluripotent stem cell lines derived in Institute of Biomedical Technology (former Regea), University of Tampere, Finland. Cells were differentiated to neural cells using neural differentiation protocol described earlier by Lappalainen and colleagues. Fluorescent probes used in this study were 1) 5‐chloromethylfluorescein diacetate (CellTracker Green CMFDA, CT) and 2) 1,1′‐dioctadecyl‐3,3,3′,3′‐tetramethylindodicarbocyanine perchlorate (DiD). Cell viability and proliferation of the labeled populations was briefly studied. Also, the labeled cells were characterized using immunocytochemistry and neuronal functionality was studied using micro electrode array (MEA).
Results: During labeling optimization a wide range of different labeling parameters was tested for both probes to obtain long term labeling. Most suitable parameters for human neural cells were 10 µM CT with 72 hours incubation time and 10 µM DiD with 2 hours incubation time. With these concentrations the labeling was visible up to 4 weeks and had no statistical effect on cell viability. CT labeling was not affecting to cell proliferation but with DiD slight decrease in proliferation was seen in long term culture. Labeled populations contained both MAP‐2 positive neurons and GFAP‐positive astrocytes. Also, the CT or DiD labeled population and their mixed‐cultures expressed normal spontaneous network activity when cultured on MEA‐platform.
Conclusions: This study indicated that fluorescent probes CT and DiD are suitable for long‐term labeling of human derived neural cultures in vitro. The labeling had no negative effects on cell behavior with the exception of DiD labeled cells having slight decrease on cell proliferation. Importantly, the labeled neural networks were electrically functional.
T12‐19B. Low‐dose‐fractionated X‐irradiation attenuates astrogliosis‐mediated inhibition of axonal regeneration and facilitates recovery of motor function after spinal cord hemi‐section in Beagle dogs
W. Wang, Q. Zhang
Huazhong University of Science and Technology, Wuhan, China
Axonal regeneration after spinal cord injury (SCI) is limited mainly due to the presence of an inhibitory microenvironment, especially reactive astrogliosis and glial scar formation. Overcoming this inhibitory barrier of reactive astrocytes and inhibitory substances making the microenvironment of the damaged neurons permissive for axonal regrowth might be crucial for CNS repair. Single‐dose irradiation has been proved to be neuroprotective in CNS trauma through elimination of reactive astrocytes, however, the irradiation conditions with single‐dose protocols are not in clinical use. In the present study, we aimed to investigate whether low‐dose‐fractionated irradiation, which is currently being used widely for clinical treatment of several tumor types, could regulate cell cycle elements of reactive astrocytes just like its use in cancers, inhibit glial scar formation, attenuate astrogliosis‐mediated inhibition of axonal regeneration and facilitate recovery of motor function after spinal cord hemi‐section in Beagle dogs. As expected, our results demonstrated that low‐dose‐fractionated irradiation reduced reactive astrogliosis, attenuated astrogliosis‐associated neural injuries, ameliorated microenvironment of axonal regeneration, and benefited recovery of the animals subjected to spinal cord trauma. Accordingly, irradiation might be a promising therapeutic strategy targeting at excessive astrogliosis for SCI in the near future.
T12‐20A. Bone marrow‐derived mesenchymal stem cell intraventricular injection in a chronically demyelinated mouse model
J. Jones, P. Cruz Martínez, S. Martinez
Univ. Miguel Hernandez, Neuroscience Institute, San Juan, Alicante, Spain
Mesenchymal stem cell transplantation has been proven to have beneficial effects in various degenerative diseases, including demyelinating models. Both in our lab as well as others have shown that they express a number of trophic factors that are capable of inducing remyelination, mainly by activating nearby oligodendrocyte progenitors towards mature oligodendrocytes that in turn remyelinate the damaged area. However, this effect was only observed locally, in the area surrounding the graft, thus in order to achieve general remyelination in various brain structures simultaneously, we decided to perform bone marrow‐derived mesenchymal stem cell injections into the lateral ventricles. In this manner, the cells may attach to various areas such as the hippocampus, corpus callosum and fornix, among others, all of which are demyelinated. Previous to the graft, the cells were incubated with iron nanoparticles. This way, it is possible to track in vivo the grafted cells by magnetic resonance imaging (MRI). As a result, the cells were observed at different time points (0–15‐30–60‐90 days) by MRI. Also, the demyelinated areas can also be visualized and myelin quantified using image analysis software. This allows the quantification myelin density in the same individual mice at different moments before and after transplantation.
T12‐20B. Investigation of de‐ and remyelination of toxin‐induced lesions in the adult zebrafish optic nerve
J. Muenzel1,2, C. Becker2, T. Becker2, A. Williams 1
1University of Edinburgh, Centre for Regenerative Medicine, Edinburgh, United Kingdom 2University of Edinburgh, Centre for Neuroregeneration, Edinburgh, United Kingdom
Central nervous system (CNS) remyelination is important for the restoration and protection of proper nervous system function in demyelinating diseases such as multiple sclerosis but is poorly understood, and inefficient in humans. In contrast, zebrafish are able to regenerate many tissues very efficiently. As zebrafish are being increasingly used to study myelination, we aimed to develop and characterise an adult zebrafish model of CNS de‐ and remyelination, to answer the question if remyelination is also very efficient, to describe the biology and to identify similarities and differences with mammalian remyelination. This then may allow us to better understand why zebrafish have a high regenerative capacity compared to rodents and humans.
Previous studies in teleost fishes have used optic nerve crush as a paradigm of myelin injury to investigate myelin repair. This, however, involves the de novo myelination of regenerated axons, rather than de‐ and remyelination of the same axons. Lysophosphatidylcholine (LPC) is a detergent‐like toxin, which is widely used in rodent models to demyelinate axons. We administer LPC to the optic nerve of adult zebrafish and observe less myelin by both immunoreactivity and electron microscopy at the lesion site at 8 days post lesion (dpl) and microglia activation along the optic pathway. Immunoreactivity and electron microscopy suggest complete regeneration of myelin at 28 dpl. We cannot identify any axonal injury in this model. In young zebrafish (aged 4–6 months), the myelin thickness of remyelinated fibres shows no difference to the pre‐lesion state, which is different to mammals, where the myelin thickness is reduced. However, in old fish (aged 18 + months), although the axon diameter is the same as pre‐lesion and in young animals, , remyelinated fibres have thinner myelin, suggesting that the regenerative capacity of zebrafish declines with age.
We believe that this new zebrafish model of CNS remyelination can be added to the suite of current models to better understand the remyelination process, why it fails, and to test for compounds to improve it, with the added benefit that zebrafish are rapid breeders, transgenesis is easy and there is a high potential for live imaging.
T12‐21A. Perineurial Glia: First Repsonders After a Peripheral Nerve Injury
S. Kucenas, W. Lewis
University of Virginia, Charlottesville, United States
Although often described as a hard‐wired component of the vertebrate body, the nervous system is a plastic and considerably fluid organ system that reacts to external stimuli in a consistent, stereotyped manner, while maintaining incredible flexibility and plasticity of its core components. Unlike the CNS, the PNS is capable of significant repair, but we have only just begun to understand the cellular and molecular mechanisms that underlie this phenomenon. Peripheral nerves are composed of axons surrounded by layers of glia and connective tissue. They are ensheathed by myelinating or non‐myelintaing Schwann cell glia, which are in turn wrapped into a fascicle by a cellular sheath called the perineurium. This structure forms from centrally‐derived glial cells and serves as a protective barrier that is essential for nerve function. Following an injury, adult peripheral nerves have the remarkable capacity to remove damaged axonal debris, regenerate and re‐innervate targets. Schwann cells have been shown to play an important role in this process by trans‐differentiating, proliferating, clearing debris, and guiding re‐growing axons, but less is known about the potential role of perineurial glia. To investigate the role of perineurial cells in PNS regeneration, we have developed an injury response assay that uses a Micropoint laser to create injuries along the motor nerves in live transgenic zebrafish. Time‐lapse imaging of injured nerves reveals that perineurial glia rapidly respond to nerve injury and extend processes toward the injury zone. This is in contrast to Schwann cells, which we observe orienting towards the distal stump where they engulf and clear axonal debris. These data demonstrate that perineurial glia respond immediately to motor nerve injuries in a manner distinct from Schwann cells, and future work is aimed at defining the molecular mechanisms that mediate the cellular responses of perineurial glia and Schwann cells, as well as determining if developmental paradigms are recapitulated in these glial populations during the regenerative process.
T12‐21B. Novel interactions between the c‐Jun and Notch signalling pathways regulate the Schwann cell response to peripheral nerve injury
D. Wilton 1, K. Malgapo1, C. Benito1, C. Gomis1,2, J. Shen3, D. Meijer4, L. Feltri5, L. Wrabetz5, A. Behrens6, R. Mirsky1, K. Jessen1
1UCL, London, United Kingdom 2Instituto de Neurosciencias de Alicante, Alicante, Spain 3Harvard Medical School, Center for Neurological Diseases, Boston, United States 4Erasmus MC, Cell Biology and Genetics, Rotterdam, Netherlands 5University of Buffalo, School of Medicine and Biomedical Science, Buffalo, United States 6Cancer Research UK, Mammalian genetics laboratory, London, United Kingdom
We have previously shown that in Schwann cells the transcription factor c‐Jun acts as a master regulator of Wallerian degeneration and is required for successful repair following nerve injury (Arthur‐Farraj et al., (2012) c‐Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron. 75(4): 633‐47). In this study we identify a novel role for c‐Jun in the activation of Notch signalling in the denervated Schwann cell. We find that c‐Jun is required to activate Notch signalling, leading to upregulation of the bHLH protein Hes1. Hes1 then plays two functions in the denervated cell, promoting myelin breakdown and acting as part of a negative feedback loop to reduce c‐Jun levels. As a result of this, ablating Notch signalling specifically in Schwann cells acts to increase c‐Jun levels. We show that this upregulation of Schwann cell c‐Jun accelerates axon outgrowth, target re‐innervation and remyelination by generating a cell, which promotes faster than normal functional recovery. These results identify novel functional links between the c‐Jun and Notch signalling pathways. They also show that not only is Schwann cell c‐Jun necessary for successful nerve regeneration, but that nerve repair can be improved by enhancing normal c‐Jun signalling.
T12‐22A. CD300f immunoreceptor contributes to peripheral nerve regeneration after crush injury
P. Solari1, N. Puig1, M. Negro1, L. Acarin2, J. Sayós3, H. Peluffo4, N. Lago 5
1Institut Pasteur de Montevideo, Neurodegeneration Laboratory, Montevideo, Uruguay 2Autonomous University of Barcelona, Faculty of Medicine, Unit of Histology, Barcelona, Spain 3Universitat Autònoma de Barcelona, Hospital Universitari Vall d'Hebrón, Institut de Recerca (VHIR), Immunobiology Group, CIBBIM‐Nanomedicine Program, Barcelona, Spain 4Faculty of Medicine, UDELAR and Institut Pasteur de Montevideo, Department of Histology and Embryology and Neurodegeneration Laboratory, Montevideo, Uruguay 5Institut Pasteur de Montevideo, Montevideo, Uruguay
It is well known that cell surface immune receptors play a critical role in regulating immune and inflammatory processes in the central nervous system (CNS). After injury of the peripheral nervous system (PNS), Schwann cells and macrophages phagocyte myelin debris in Wallerian degeneration in order to regenerate axons to distal targets. The immunoreceptor CD300f is normally expressed in the myeloid line and in nervous system in microglia, oligodendrocytes and neurons under certain conditions. However, little is known about the CD300f ligands. By using a CD300f‐Fc fusion protein we have analyzed the expression of the CD300f ligands in the PNS. Moreover, we have also analyzed the possible role of the CD300f immunoreceptor in peripheral nerve regeneration by blocking the interaction between CD300f and its ligands with the same fusion protein. Thy1‐YFP‐H mice sciatic nerves were injected with CD300f‐Fc, control mIgG2a or PBS immediately before a crush injury. The results show that CD300f ligand is expressed in Schwann cells. Moreover, after a sciatic nerve injury, animals injected with the CD300f‐Fc protein show a lower number of YFP‐positive fibers growing into the tibial nerve after 10 days post‐lesion (dpl) than control groups. Moreover, the CD300f‐Fc group shows a higher number of macrophages and CD206‐positive cells at 10 dpl when compared to control groups. We do not see these differences in axon regeneration and macrophage infiltration after 28 dpl. We have also evaluated the mRNA expression of the pro‐inflammatory cytokine IL‐1β at 24 hours after crush and injection of the fusion protein. Q‐PCR shows an up‐regulation of the mRNA in control groups and a lower mRNA expression level in the CD300f‐Fc protein group. Together these results show that the pair CD300f receptor and ligand is implicated in some aspects of Wallerian degeneration and nerve regeneration such as the modulation of both the influx and phenotype of macrophages.
T12‐22B. Hematopoetic stem/progenitor cell‐derived microglia‐like cells with potential to support regeneration in the central nervous system
B. Wylot, K. Konarzewska, M. Zawadzka
Nencki Institute of Experimental Biology, Warsaw, Poland
In response to the central nervous system (CNS) injury, hematopoetic cells migrate to the lesion where they can potentially contribute to tissue regeneration. Following CNS injury, these cells can secrete a plethora of immunomodulating cytokines and/or pro‐regenerative growth factors as well as phagocyte proinflammatory tissue debris. Nevertheless, the role of primitive hematopoetic stem/progenitor cells (HSPC) – derived from bone marrow – to support CNS regeneration has not been studied in detail. Therefore, the aim of the present study was to examine characteristics of HSPC in vitro as well as to test pro‐regenerative potential of these cells to support CNS repair in vivo.
Highly pure population of primitive hematopoetic stem/progenitor cells was isolated from the bone marrow and the CNS with fluorescence activated cell sorting (FACS). The number of HSPC in the CNS lesion was significantly increased compared to intact CNS tissue. Sorted‐bone marrow HSPC were co‐cultured with primary astrocytes to examine their phenotype according to the presence of specific antigens and gene expression as well as to test their phagocytosis potential. In the presence of astrocytes, bone marrow‐derived HSPC differentiated in vitro into microglia‐like cells expressing specific myeloid/microglia markers and showing high ability to ingest fluorescent microbeads. The in vivo potential of HPSC for supporting regeneration was examined by their transplantation into the CNS lesion.
Results of our study demonstrated that primitive hematopoetic stem/progenitor cells are the source of microglia‐like cells which can support regeneration in the central nervous system.
T12‐23A. Canonical Wnt signaling and formation of the glial scar
J. Levine, J.P. Rodriguez
Stonybrook University, Dept. of Neurobiology and Behavior, Stony Brook, United States
When the brain or spinal cord is injured, glial cells in the damaged area undergo complex changes resulting in the formation of the glial scar. Whether the scar is beneficial and detrimental to recovery remains controversial. The signals that initiate the formation of the glial scar are unknown. Because both canonical and non‐canonical Wnts are increased after spinal cord injury (SCI), we examined the role of canonical Wnt signaling in the glial reactions to CNS injury. To disrupt β‐catenin‐dependent Wnt signaling specifically in OPCs, we created transgenic mice carrying an OPC‐specific conditionally deleted β‐catenin gene. After moderate contusion injuries to the thoracic spinal cord of control mice, OPCs proliferate and accumulate in the penumbra region surrounding the injury epicenter. In the β‐catenin‐depleted mice, there is reduced proliferation and accumulation of OPCs after SCI, reduced accumulation of activated microglia/macrophages and reduced astrocyte hypertrophy. Using a crushed optic nerve model, we show that these reduced glial reactions create an environment that is permissive for axonal regeneration. These results suggest that canonical Wnt signaling in glia after CNS injury is necessary for the formation of the glial scar and they identify Wnt signaling as a new therapeutic target for promoting axon regeneration.
T12‐23B. Schwann cell c‐Jun is essential for successful peripheral nerve regeneration
R. Mirsky 1, P. Arthur‐Farraj1, M. Latouche1, D. Wilton1, S. Quintes1, E. Chabrol1, A. Woodhoo2, B. Jenkins1, M. Turmaine1, A. Behrens3, G. Raivich2, K. Jessen1
1University College London, Cell and Developmental Biology, London, United Kingdom 2CICbioGune, Metabolomics Unit, Derio, Bizcaia, Spain 3CRUK, London Research Institute, London, United Kingdom
We recently showed (Arthur‐Farraj et al., Neuron 75:633‐47, 2012) a critical role for Schwann cell c‐Jun in controlling the generation of the repair Schwann cell (Bungner cell) generated after nerve injury. This cell is generated as a result of Schwann cell plasticity and involves the conversion of mature myelinating and Remak cells, in a process that resembles transdifferentiation, to a phenotype that promotes nerve repair. In the absence of c‐Jun in Schwann cells, 172 genes are misregulated in the distal stump of the sciatic nerve 7 days after cut. Among the misregulated genes are trophic factors and signalling molecules such as GDNF, artemin, BDNF and sonic hedgehog, adhesion molecules including p75NTR, N‐cadherin and N‐CAM and the transcription factor Olig1. c‐Jun also controls the structure of the regeneration tracks. In Schwann cell c‐Jun null mice, the initial rate of axonal regeneration after injury is impeded and long term recovery of function measured by footprint analysis, toe pinching , Von Frey hairs, and the Hargreaves test is not seen 10 weeks after injury. There is widespread death of both large and small DRG neurons and although spinal cord motor neurons survive there is widespread failure of both sensory and motor neurons to reinnervate target muscles.
We conclude that a single glial transcription factor, c‐Jun, is essential for restoration of injured nerves, acting to control the transdifferentiation of myelin and Remak
Schwann cells to dedicated repair cells in damaged tissue.
POSTER TOPIC 13 Transmitter receptors, ion channels and gap junction
T13‐01A. In search of the satellite glia role: insights from their membrane properties
S. Agathou 1, I. Lundgaard2, K. Evans2, K. Volbracht2, R.T. Káradóttir2
1University of Cambridge, Cambridge, United Kingdom 2University of Cambridge, Wellcome Trust/MRC Cambridge Stem Cell Institute, John van Geest Centre for Brain Repair & Dept. of Veterinary Medicine, Cambridge, United Kingdom
The crucial role of central nervous system (CNS) glial cells in the integrity and physiology of neuronal networks, is well known. However, little is known about glial cells in the peripheral nervous system (PNS). One of the main glial cell types in the PNS is the perineuronal satellite glial cells (SGCs), that surround DRG neurons in an envelope‐like fashion. Despite their abundance in peripheral nerves, having stem cells properties and playing a role in neuropathic pain, there is limited information on their physiology. Although SGCs have similarities to astrocytes in terms of purinergic‐ and gap junction‐ mediated signaling, their membrane properties and ionotrophic glutamate receptor expression are more or less unknown.
Our aim was therefore to characterize the membrane properties and glutamatergic ion channel expression of SGCs. We performed patch‐clamp experiments on SGCs wrapped around sensory neurons in the rat dorsal root ganglion (DRG) in vitro. Whole‐cell recordings revealed that SGCs are slightly hyperpolarized in comparison to the neurons they ensheath, with a resting membrane potential of approximately ∼80mV and a high input resistance (>1GΩ). Moreover, upon membrane depolarization no detectable voltage‐gated Na+, Ca2+ or K+ currents were detected. Extracellular application of Glutamate agonist, Kainic acid, N‐Methyl‐D‐aspartic acid (NMDA) and 2‐amino‐3‐(3‐hydroxy‐5‐methyl‐isoxazol‐4‐yl) propanoic acid (AMPA) during the recording, did not evoke any response from the cells, indicating their lack of ionotropic glutamatergic receptor (iGluR) expression. Double immunocytochemistry on lucifer yellow (LY) filled SCGs revealed that they express the SCIP/Oct6 transcription factor, which is also expressed by promyelinating Schwann cells.
DRG ganglion SGCs differ from CNS perineuronal cells as they lack glutamatergic receptors, but are similar to astrocytes as they have no voltage‐gated ion channels, are gap‐junctionally coupled and express glutamine synthetase. Thus, we are currently addressing if SGCs respond to neuronal activity in a similar manner to astrocytes in the CNS with the use of calcium imaging. These studies will provide further insights into the physiological role of SGCs in the PNS.
T13‐01B. Functional Role of Potassium Channels in Oligodendroglial Lineage Cells
S. Korr 1,2, P. Ehling1,3, S. Albrecht2, T. Kuhlmann2
1University Hospital Münster, Department of Neurology – Inflammatory Disorders of the Nervous System and Neurooncology, Münster, Germany 2University Hospital Münster, Institute of Neuropathology, Münster, Germany 3University of Münster, Institute of Physiology I – Neuropathophysiology, Münster, Germany
Multiple sclerosis (MS), a chronic neuroinflammatory disease with presumed autoimmune etiology, is the most common demyelinating disorder of the central nervous system among young adults. Progressive neurological impairment of the patients are associated with accumulation of characteristic brain lesions characterized by inflammation, demyelination, gliosis and axonal damage. Demyelination and loss of oligodendrocytes contributes to axonal loss and permanent neurological deficits. Remyelination occurs but is limited; one contributing factor is the incapability of oligodendrocyte precursor cells (OPC) to differentiate and form new myelin sheaths. K+ channels are not only known to predominantly set and maintain the membrane potential but also to be important regulators of various cell functions like proliferation and maturation. Specific regulation of these cellular functions is based on high channel diversity and their ability to form heteromers as well as alternate mRNA splicing and posttranslational modifications. So far the expression and function of potassium channels in cells of the oligodendroglial lineage has not been clearly identified. Previous studies indicate that an unspecific blockade of K+ channels in OPC suppresses maturation as well as proliferation. Moreover, for one distinct channel (KIR4.1) a functional relevance has been shown, as deficiency of Kir4.1 leads to altered OPC maturation and hypomyelination in mice. Here, we elucidate the expression and functional relevance of K+ channels in oligodendrocytes. Our first electrophysiological recordings from primary murine OPCs revealed the presence of different K+ channels with both inward and outward rectification. In future experiments, we aim at identifying K+ channel subtypes that specifically regulate OPC cell functions and might influence cell maturation under neuroinflammatory conditions. Thereby, we want to offer new insights in the functional role of K+ channels in OPCs/oligodendrocytes and contribute to novel therapeutic strategies for the treatment of MS.
T13‐02A. Phosphorylation of aquaporin‐4 at Ser111 is not required for channel gating
M. Assentoft 1, S. Kaptan2, R. Fenton3, S.Z. Hua4, B. de Groot2, N. MacAulay1
1University of Copenhagen, Department of Cellular and Molecular Medicine, Copenhagen, Denmark 2Max Planck Institute for Biophysical Chemistry, Computational Biomolecular Dynamics Group, Gottingen, Germany 3Aarhus University, Department of Biomedicine and InterPrET Center, Aarhus, Denmark 4State University of New York in Buffalo, Department of Physiology and Biophysics, Buffalo, United States
Aquaporin 4 (AQP4) is the predominant water channel in the mammalian brain and is mainly expressed in the perivascular glial endfeet at the brain‐blood interface. AQP4 has been described as an important entry and exit site for water during formation of brain edema and regulation of AQP4 is therefore of broad interest. Phosphorylation of some aquaporins has been proposed to regulate their water permeability via gating of the channel itself. Protein kinase (PK)‐dependent phosphorylation of Ser111 has been reported to increase the water permeability of AQP4 expressed in an astrocytic cell line. This possibility was, however, questioned based on the crystal structure of the human AQP4. Our study aimed to resolve if Ser111 was indeed a site involved in phosphorylation‐mediated gating of AQP4. The water permeability of AQP4‐expressing Xenopus oocytes was not altered by a range of activators and inhibitors of PKG and PKA. Mutation of Ser111 to alanine or aspartate (to prevent or mimic phosphorylation) did not change the water permeability of AQP4. PKG activation had no effect on the water permeability of AQP4 in primary cultures of rat astrocytes. Molecular dynamics simulations of a phosphorylation of AQP4.Ser111 recorded no phosphorylation‐induced change in water permeability. A phospho‐specific antibody, exclusively recognizing AQP4 when phosphorylated on Ser111, failed to detect phosphorylation in cell lysate of rat brain stimulated by conditions proposed to induce phosphorylation of this residue. Thus, our data indicate a lack of phosphorylation of Ser111 and of phosphorylation‐dependent gating of AQP4.
T13‐02B. Selective permeation pathway through connexin 43 hemichannels
D. Bloch Hansen, J. Pauli Hofgaard, T. Hartig Braunstein, M. Schak Nielsen, N. MacAulay
University of Copenhagen, Copenhagen, Denmark
Gap junctions are composed of two connexons from neighboring cells. Undocked connexons may form open hemichannels in the plasma membrane when exposed to specific stimuli, e.g. reduced extracellular concentration of divalent cations, and allow passage of fluorescent molecules with masses lower than 1 kDa. A range of physiologically relevant molecules, smaller than the assumed molecular cut‐off of 1 kDa, have therefore been proposed to permeate connexin 43 (Cx43) in its hemichannel configuration. However, the permeability profile of Cx43 hemichannels remains unresolved as does the molecular substrate for hemichannel activity in astrocytes. Exposure of Cx43‐expressing Xenopus laevis oocytes to divalent cation free solution induced a gadolinium‐sensitive uptake of the fluorescent dye ethidium. In spite thereof, a range of smaller biological molecules, such as water, glutamate, lactate, glucose, and ATP, did not gain detectable access through the pore. In contrast, permeability of glutamate, glucose and ATP was observed in oocytes expressing the other major astrocytic connexin, Cx30. Exposure to low divalent cation solutions also induced a robust membrane conductance in Cx30‐expressing oocytes but none in Cx43‐expressing oocytes. Expression of Cx43 in C6 glioma cells failed to induce hemichannel activity. Our results thus call for caution when assigning molecular identity to astrocytic channel activity and when interpreting hemichannel‐mediated dye‐uptake as equal to permeability of physiologically relevant molecules.
T13‐03A. Inhibition of P2X4 function by P2Y6 UDP receptors in microglia
L.‐P. Bernier 1,2, A. Ase2, E. Boue‐Grabot3, P. Seguela2
1University of British Columbia, Vancouver, Canada 2McGill University, Montreal, Canada 3Université de Bordeaux, Bordeaux, France
ATP‐gated P2X4 receptor channels expressed in spinal microglia actively participate in central sensitization, making their functional regulation a key process in chronic pain pathologies. P2Y6 metabotropic Gq‐coupled receptors, also expressed in microglia, are involved in the initial response to nerve injury, triggering phagocytosis upon activation by UDP. It has been reported recently that expression of both P2X4 and P2Y6 is upregulated in activated microglia following nerve injury. We show here, in resting as well as LPS‐activated primary microglia, that P2Y6 decreases P2X4‐mediated calcium entry and inhibits the dilation of P2X4 channels into a large‐conductance pore measured with a YO‐PRO‐1 uptake assay. Furthermore, P2Y6 activation modulates the ATP‐dependent migration of microglia, a process likely involved in their shift from migratory to phagocytic phenotype. Reconstituting the P2X4‐P2Y6 interaction in recombinant systems shows that P2Y6 activation decreases P2X4 current amplitude, activation and desensitization rates, and reduces P2X4 channel permeability to the large cation NMDG+. Phospholipase C‐mediated hydrolysis of the phosphoinositide PI(4,5)P2, a necessary cofactor for P2X4 channel function, underlies this inhibitory crosstalk. As extracellular levels of both ATP and UDP are increased in the spinal cord following nerve injury, the control of P2X4 activity by P2Y6 might play a critical role in regulating neuropathic pain‐inducing microglial responses.
T13‐03B. Ca2+ permeable AMPA receptors are coupled to vesicular glutamate release from Bergmann glia processes
M. Marcoli 1, C. Cervetto1, M. Passalacqua1, S. Alloisio2, M. Nobile2, D. Frattaroli1, G. Maura1
1University of Genova, Genova, Italy 2CNR, Genova, Italy
Question. Bergmann glial cells are recognized to play important role in the control of cerebellar glutamatergic transmission at parallel fibers/Purkinje cell synapses although the mechanisms that mediate modulation of transmission are largely unexplored.
Methods. We here investigate on the release of the gliotransmitter glutamate from superfused isolated purified glial processes (gliosomes) obtained from adult rat cerebellum. Intracellular Ca2+ levels were measured by a microfluorimetric technique. Immunocytochemical confocal and Western blot analysis were also carried on cerebellar gliosomes.
Results. Confocal and Western blot analysis confirmed that cerebellar GFAP‐positive gliosomes are a purified preparation of glia processes. More then 80% of gliosomes were positive for brain‐specific lipid binding protein (BLBP), indicating that they derived from Bergmann cells. By measuring the release of glutamate we obtained evidence for the presence of glutamate release‐facilitatory AMPA receptors on the Bergmann glial processes. In fact, AMPA evoked [3H]D‐aspartate or endogenous glutamate release which was abolished in Ca2+‐free medium; effectiveness of the selective inhibitor naphthylacetyl spermine indicated the involvement of Ca2+‐permeable, GluA2‐lacking AMPA receptors. Ca2+ imaging confirmed the presence of Ca2+‐permeable, GluA2‐lacking AMPA receptors on Bergmann glia processes. Activation of the GluA2‐lacking AMPA receptors triggered vesicular exocytotic glutamate release: inhibition of vesicular loading by the vesicular glutamate transporter (VGLUT) inhibitors rose bengal or trypan blue, or by the H+‐ATPase inhibitor bafilomycin A1 prevented the AMPA‐evoked glutamate release (see Figure). Confocal analysis confirmed that BLBP‐ and GFAP‐positive processes expressed VGLUT1 and VGLUT2.
Conclusions. We here report evidence that: 1. functional processes of Bergmann cells are recovered in purified preparation of adult rat cerebellar gliosomes; 2. AMPA receptors located on Bergmann glia processes show features of Ca2+ permeable, GluA2‐lacking AMPA receptors; 3. activation of the AMPA receptors evokes Ca2+ entry in isolated Bergmann glia processes and Ca2+‐dependent vesicular glutamate release from the processes. Activation of Ca2+‐permeable, GluA2‐lacking AMPA receptors coupled to vesicular glutamate release might represent a mechanism by which Bergmann glia processes modulate synaptic transmission at parallel fibers/Purkinje cell synapses.
Supported by University of Genova (to C.C. and M.M.)

T13‐04A. Astrocyte volume regulation in the cortex of alpha‐syntrophin‐negative GFAP/EGFP mice
O. Butenko 1, J. Benesova1, M. Mikesova1, P. Honsa1, D. Dzamba1, J. Kriska1, V. Rusnakova2, M. Kubista2,3, M. Anderova1
1Institute of Experimental Medicine, Prague, Czech Republic 2Institute of Biotechnology, Prague, Czech Republic 3TATA Biocenter, Gothenburg, Sweden
The formation of brain edema, which accompanies ischemic or traumatic brain injuries, originates from a disruption of ionic/neurotransmitter homeostasis that leads to extracellular K+ elevation and neurotransmitter accumulation in the extracellular space. An increased uptake of these osmotically active substances, predominantly provided by astrocytes, is accompanied by intracellular water accumulation via aquaporin‐4 (AQP4). Previously, it has been shown that the removal of perivascular AQP4 via the deletion of alpha‐syntrophin, a protein responsible for anchoring AQP4 on the astrocytic membrane, delays edema formation and K+ clearance (Amiry‐Moghaddam et al.,2003, PNAS 11;100(23):13615‐20). Therefore, we aimed to elucidate the impact of alpha‐syntrophin deletion on astrocyte volume changes in the cortex during pathological states, such as hypoosmotic stress or oxygen‐glucose deprivation (OGD), using three‐dimensional (3D) confocal morphometry in situ. In addition, single cell RT‐qPCR profiling was carried out to reveal possible differences in the expression profiles of ion channels/transporters that participate in maintaining ionic/neurotransmitter homeostasis. In order to visualize individual astrocytes that lack alpha‐syntrophin, double transgenic mice were generated by crossbreeding GFAP/EGFP mice (Nolte et al., 2001, Glia. 33(1):72–86) with alpha‐syntrophin knockout mice (Amiry‐Moghaddam et al., 2003, PNAS 11; 100(23):13615‐20). 3D‐confocal morphometry revealed that alpha‐syntrophin deletion results in significantly smaller/slower astrocyte swelling when induced by 20 min hypoosmotic stress (210 mOsm), 30 min OGD or by high extracellular K+ (50 mM), while alpha‐syntrophin deletion had no effect on the astrocytic shrinkage evoked by hyperosmotic stress (350 mOsm). The volume recovery of cortical astrocytes from GFAP/EGFP/alpha‐syntrophin knockout mice was significantly slower following their exposure to hypo‐ or hyperosmotic stress, whereas no differences were found in astrocyte volume recovery following OGD or after their exposure to high extracellular K+. Compared to the cortical astrocytes of GFAP/EGFP mice, single cell RT‐qPCR analyses revealed that astrocytes from GFAP/EGFP/alpha‐syntrophin knockout mice express higher mRNA levels for two‐pore domain K+ channels (TWIK‐1, TASK‐2, TASK‐3), inwardly or outwardly rectifying K+ channels (Kir3.1, Kir5.1, Kv1.3, Kv1.6) and chloride channels (ClC1,ClC4), while mRNA expression for the glutamate transporter GLT‐1 is lower. In summary, the deletion of alpha‐syntrophin slowed down astrocyte swelling during hypoosmotic stress, OGD or high K+; however, it also resulted in alterations in astrocytic gene expression profiles.
Supported by grants GA CR 13‐02154S and P304/12/G069 from the GACR.
T13‐04B. The subpopulation of microglia expressing functional muscarinic acetylcholine receptors expands in stroke and Alzheimer's disease
M. Pannell 1, F. Szulzewsky1, V. Matyash1, M. Endres2, F.L. Heppner3, G. Kronenberg2,4, V. Prinz2, S. Prokop3, S. Waiczies4, S.A. Wolf1, H. Kettenmann1
1Max‐Delbrück‐Center for Molecular Medicine, Cellular Neuroscience, Berlin, Germany 2Charité – Universitätsmedizin Berlin, Department of Neurology, Berlin, Germany 3Charité – Universitätsmedizin Berlin, Institute of Neuropathology, Berlin, Germany 4Charité Medical Faculty and the Max‐Delbrück Center for Molecular Medicine, Experimental and Clinical Research Center, Berlin, Germany
Microglia undergo a process of activation in any type of pathology which is controlled by many factors such as cytokines, chemokines or growth factors, but also by neurotransmitters. We found that a sub‐population (16%) of freshly isolated microglial cells from the adult brain respond to the muscarinic acetylcholine receptor agonist carbachol with a Ca2+ response, indicating the expression of functional receptors. The carbachol‐sensitive population increased in microglia/brain macrophages isolated from the tissue of mouse models for stroke (57%) and Alzheimer's disease (26%), but not for glioma and multiple sclerosis. Microglia cultured from adult and neonatal brain contained a similar carbachol‐sensitive sub‐population (17% and 10%) which was increased by treatment with interferon‐γ to 60% within 12 hours, and was sensitive to blockers of protein synthesis. Carbachol was a chemoattractant for microglia and decreased phagocytosis activity, indicating that microglia are a functionally heterogeneous population.
T13‐05A. Astrocytes and S1P Receptors
K. Dev
Trinity College Dublin, Dublin, Ireland
The family of sphingosine 1‐phosphate receptors (S1PRs) are G protein‐coupled comprising five subtypes (S1P1R‐S1P5R). These receptors are expressed in cells of the immune, cardiovascular, and central nervous systems (CNS), in addition to others. S1PRs play important roles in celular proliferation, differentiation, survival and migration. Recently, the immunomodulatory drug, Gilenya® has been approved as the first oral therapy for Multiple Sclerosis (MS), after proving efficacious in clinical trials. The active ingredient of Gilenya® is the phosphorylated compound FTY720 (pFTY720), which is a potent agonist on all S1PRs, except S1P2Rs. pFTY720 has been suggested to work as a ‘functional antagonist' causing S1P1R internalisation in lymphocytes, thus limiting T cell auto‐immunity. In addition to regulating the immune system, the lipophilic nature of the pro‐drug FTY720 allows it to readily cross the blood‐brain‐barrier (BBB) where it may also activate S1PRs expressed on both neurons and glia. Here, the role of S1PRs in the CNS was investigated, by specifically investigating their role in astrocyte function. A range of methods were used to examine the effects of S1PR activation and their roles in astrocytes, including: (i) S1P1R subtype trafficking, (ii) transient and continued intracelular signalling, (iii) astrocyte cell migration, (iv) release of pro‐inflammatory cytokines, (v) oligodendrocyte cell differentiation and survival and (vi) myelination state in brain slice cultures. The data showed that activation of the S1P1R subtype leads to (i) its internalisation and (ii) continued signalling in astrocytes, and that S1P1Rs were found to (iii) promote astrocyte migration, (iv) limit release of pro‐inflammatory cytokines, (v) promote oligodendrocyte survival and (vi) limit demyelination. These studies demonstrate a role for S1P1Rs in regulating astrocyte function and suggest their use a drug targets for neuroinflammtory and neurodegenerative disorders.
T13‐05B. Expression of Kir7.1 in glia; potential glioprotective qualities
M. Papanikolaou, A. Lewis, A.M. Butt
University of Portsmouth, Portsmouth, United Kingdom
Glial cells express a variety of Kir channel subtypes, which are involved in several important functions. In particular, both astrocytes and oligodendrocytes express Kir4.1, which are essential for setting their strongly negative RMP, as well as the maintenance of [K+]o following neuronal activity. Notably, strong expression of the Kir7.1 subtype was determined during a whole genome microarray analysis of the mouse optic nerve, a typical CNS white matter that contains mainly astrocytes and oligodendrocytes. The Kir7.1 subunit is known to be involved in potassium transport in epithelial cells and has been identified in cerebellar Purkinje neurons, but has not been reported in glia. Here, we examined functional expression of Kir7.1 in mouse brain and optic nerve. RT‐PCR and western blot confirmed expression of Kir7.1 mRNA and protein expression in the brain and optic nerve. Positive Kir7.1 immunostaining was observed in astrocytes and oligodendrocytes in brain sections and in optic nerve explant cultures and the results indicated a developmental increase in Kir7.1 expression. In astrocytes, Kir7.1 was localised to perivascular end‐feet, supporting a potential role in K+ regulation. In oligodendrocytes, Kir7.1 were localised to cell somata, suggesting a developmental role in setting their RMP. In addition, oxygen and glucose deprivation (OGD) experiments were performed on isolated intact optic nerves from mice aged P10 and examined for the effects of the Kir7.1 channel blocker VU590. After 60 min OGD, blockade of Kir7.1 resulted in increased cell death of optic nerve glia, measured using propidium iodide (PI) labelling, from 80 ± 30 in controls to 189 ± 49 in VU590 (n = 4 nerves per group, 6 FOV per nerve; p < 0.001, ANOVA). Our results demonstrate expression of Kir7.1 in glial cells, and indicate they are important in protecting glial cells from ischemic damage.
T13‐06A. MLC1 dysfunction causes brain oedema with white matter vacuolation in murine brain
M. Dubey 1, M. Bugiani2, M. Ridder2, J.C. Lodder1, M. Verheijen1, H.D. Mansvelder1, I. Boor1,2, M. van der Knaap1
1VU‐University, Amsterdam, Netherlands 2VU‐Univ Medical Center, Department of Child Neurology, Amsterdam, Netherlands
MLC1 is an eight transmembrane domain protein highly expressed in brain. Human mutations in MLC1 lead to the rare genetic disorder “megalencephalic leukoencephalopathy with subcortical cyst” (MLC). Characteristic for the disease are the onset of macrocephaly within the first year of life, and a slowly progressive loss of motor skills with epilepsy and cognitive decline. At the cellular level, countless fluid‐filled vacuoles occur within myelin sheaths surrounding axons and in astrocytic endfeet. In cell culture models, MLC1 mutations are associated with defects in chloride currents and cell volume regulation. Thus far, the expression and function of MLC1 in intact murine and human brain are still controversial.
To understand the role and developmental expression of MCL1 in the brain, we have developed a MLC1 mutant null mouse model carrying an eGFP reporter gene under the MLC1 promotor. We now show the exclusive expression of MLC1 in astrocytes throughout the brain with specifically higher expression around blood vessels, at the sub‐ventricular zone and at the glia limitans. The functional loss of MLC1 in mutant mice recapitulates in part the human MLC disease. KO mice show macrocephaly with high brain wet weight. Water filled vacuoles develop in the white matter of the cerebrum and in large fiber tracts of the brainstem. By contrast, the heterozygous loss of MLC1 has no consequences on myelin structural integrity. Both heterozygous and homozygous MLC1 KO mice have dysmorphic peri‐vascular and peri‐ventricular astrocytes. In contrast to humans, KO mice have no motor deficits, but are hyperactive and show an anxiety‐like behavior.
In the MLC1 KO mouse, a dysfunction of an astrocytic protein causes loss of myelin structural integrity leading to vacuolating myelinopathy. Therefore, the MLC mutant mouse could be a key model to study the astrocytic involvement in brain water and ion homeostasis.
T13‐06B. Molecular and functional characteristics of GABAA receptors in NG2 cells of the hippocampus
S. Passlick 1, M. Grauer1, C. Schäfer2, R. Jabs1, G. Seifert1, C. Steinhäuser1
1University of Bonn, Bonn, Germany 2University of Cologne, Cologne, Germany
NG2 cells are equipped with transmitter receptors and receive direct synaptic input from glutamatergic and GABAergic neurons. The functional impact of these neuron‐glia synapses is still unclear. Here, we combined functional and molecular techniques to analyze properties of GABAA receptors in NG2 cells of the juvenile mouse hippocampus. GABA activated slowly desensitizing responses in NG2 cells which were mimicked by muscimol and inhibited by bicuculline. To elucidate the subunit composition of the receptors we tested its pharmacological properties. Co‐application of pentobarbital, benzodiazipines and zolpidem all significantly increased the GABA responses. The presence of small tonic currents indicated the presence of extrasynaptic GABAA receptors. To further analyze the subunit expression, single cell transcript analysis was performed subsequent to functional characterization of NG2 cells. The subunits α1, α2, β3, γ1 and γ2 were most abundantly expressed, matching properties resulting from pharmacological characterization. Importantly, lack of the γ2 subunit conferred a high Zn2+ sensitivity to the GABAA receptors of NG2 cells. To determine the effect of GABAA receptor activation on membrane potential, perforated patch recordings were performed. In the current‐clamp mode, GABA depolarized the cells to about −30 mV, indicating a higher intracellular Cl‐ concentration (about 50 mM) than previously reported. GABA‐induced depolarization in NG2 cells might trigger Ca2+ influx through voltage‐activated Ca2+ channels.
Supported by DFG (SFB/TR3) and EU (FP7–202167 Neuroglia).
T13‐07A. Purinergic receptors in adipose‐derived stem cells differentiated to Schwann cells
A. Faroni 1, A. Grolla2, G. Terenghi1, V. Magnaghi3, A. Verkhratsky2
1The University of Manchester, Faculty of Medical and Human Sciences, Manchester, United Kingdom 2The University of Manchester, Faculty of Life Sciences, Manchester, United Kingdom 3The University of Milan, Department of Pharmacological and Biomolecular Sciences, Milan, Italy
Schwann cells (SC) play important roles in the development and regeneration of the peripheral nervous system (PNS) following injury. Several molecules such as neurosteroids and neurotransmitters have been suggested as potential pharmacological targets in regulating SC physiology and regenerative potential. Nevertheless, the slow growth rate and difficulties in harvesting limit SC applications in regenerative medicine.
Adipose‐derived stem cells (ASC) can be differentiated into a SC‐like phenotype (dASC) sharing morphological and functional properties with SC, thus representing a valid SC alternative. We have previously shown that dASC express γ‐aminobutyric‐acid (GABA) receptors (i.e. GABA‐A and GABA‐B receptors), which modulate their proliferation and neurotrophic potential, although little is known about the role of other neurotransmitter systems in ASC.
In this study we investigated the expression of purinergic receptors in dASC. Using RT‐PCR, Western blot analyses and immunohistochemistry we have demonstrated that ASC express P2X3, P2X4 and P2X7 purinoceptors. Interestingly, differentiation of ASC towards glial phenotype was accompanied by up‐regulation of P2X4 and P2X7 receptors. Using Ca2+‐imaging techniques, we have shown that stimulation of purinoceptors with adenosine‐5′‐triphosphate (ATP) results in intracellular Ca2+ signals, indication functional activity of these receptors.. Moreover, we have shown that the increase of intracellular Ca2+ leads to SC death, an effect that can be prevented using specific P2X4 or P2X7 antagonists.
Altogether, these results show, for the first time, the presence of functional purinergic receptors in SC‐like derived from ASC and their link with critical physiological processes such as cellular death and survival. The presence of these novel pharmacological targets in dASC might open new opportunities for the management of cell survival and neurotrophic potential in tissue engineering approaches using dASC for peripheral nerve repair.
T13‐07B. Differential regulation of acid‐base transporters in glial cells following extracellular pH changes
M. Schroedl, E. Roussa
University of Freiburg, Freiburg, Germany
Intracellular pH homeostasis is a vital function shared by all cells and is mainly regulated by the coordinated action of several acid‐base transporters. In the brain, pH homeostasis is of particular importance since changes of extracellular pH (pHo) are events associated with both physiological conditions and pathological states in brain function. Transient pHo changes usually accompany physiological processes, including neuronal activity, and astrocytes respond to a rise in extracellular K+ with plasma membrane depolarization and intracellular alkalinization. On the other hand, loss of brain pH homeostasis can lead to severe pathological conditions and significant changes of pHo have been observed during seizures and spreading depression.
In the present study, we sought to investigate regulation mechanisms of the electrogenic sodium/bicarbonate cotranspoter 1, NBCe1, and of the vacuolar H+‐ATPase (V‐ATPase) in primary hippocampal astrocytes following extracellular acid‐base changes, following neuronal activity, and using the 4‐aminopyridine (4‐AP) model of epilepsy in vitro.
We show that extracellular acidosis, but not extracellular alkalosis, increased the number of activated astrocytes. Our data also show differential regulation of acid‐base transporters in the hippocampal glial cells during extracellular acid‐base changes. Intracellular pH measurements revealed a crucial role for V‐ATPase in regulation of intracellular pH, a process accompanied by increased membrane expression of the transporter, as assessed by immunofluorescence and surface biotinylation. NBCe1‐B is the NH2‐terminal variant of NBCe1 predominantly expressed in glial cells and is transcriptionally regulated followed neuronal activity or treatment of the cells with 4‐AP.
These data propose transcriptional and post‐translational modification of acid‐base transporters as putative regulatory mechanisms in astrocytes to cope with changes of extracellular pH serving the maintenance of intracellular pH homeostasis.
T13‐08A. Connexin mediated glial networks are heterogenous among brain regions
S. Griemsmann 1, L. Claus1, S. Höft1, P. Bedner1, J. Zhang1, R. Jabs1, M. Theis1, D.W. Cope 2, V. Crunelli2, G. Seifert1, C. Steinhäuser1
1University of Bonn, Bonn, Germany 2University of Cardiff, Cardiff, United Kingdom
Astrocytes are connected with each other via gap junction channels. These astroglial networks fulfil a variety of functions in the brain, e.g. potassium buffering and metabolite transport. In this study we compared glial networks in different brain regions to investigate site specific effects on network formation. We combined electrophysiology and immunohistochemstry with semi‐quantitative RT‐PCR and Western blot analysis to investigate connexin expression, gap junction coupling and antigen profiles. Experiments were performed in wild type and transgenic mice with glia specific fluorescence labelling as well as in Cx30ko mice. Astrocytes were investigated between postnatal days 12–60.
Gap junction networks in the CA1 region of the hippocampus and the ventrobasal thalamus show abundant coupling in hGFAP‐EGFP mice. Intriguingly, we found significant coupling between oligodendrocytes and astrocytes in the thalamus, while in the hippocampus panglial coupling was less abundant. Other glial cells did not participate in the networks. We also found that a fluorescent glucose analog, 2‐NBDG, propagates through the thalamic panglial network. The function of these panglial networks remains largely unclear.
In heterozygous Cx43‐ECFPki mice, deletion of one allele of the major hippocampal Cx43 significantly reduced the number of coupled astrocytes only in the hippocampus, while the thalamic networks remained unchanged.
SR101 labelling of astrocytes and subsequent 2P microscopy identified a significant subset of thalamic SR101+ cells lacking Cx43‐ECFP expression. SR101 did not label oligodendrocytes as analysed in PLP‐GFP mice. Semi‐quantitative RT‐PCR and Western blot analysis revealed stronger expression of Cx30 in thalamic nuclei while Cx43 levels were higher in the hippocampus. This indicates a minor role for Cx43 in gap junction coupling of astrocytes in the thalamus. Consistent with these findings, the thalamus of Cx30ko mice displayed a strong decrease in astrocytic cell coupling compared to wild type littermates.
Together, these results indicate that thalamic astrocytes differ in various aspects from their counterpart in other brain regions and support the emerging concept of astrocyte heterogeneity.
Supported by DFG (SFB‐TR3) and EU (FP7–202167 Neuroglia).
T13‐08B. ATP released through connexin43 hemichannels mediates secondary cellular damage spread from the trauma zone to distal zones in astrocyte monolayers
M. Rovegno 1, P.A. Soto2, P.J. Saéz2, C. Naus3, J.C. Saéz2, R. von Bernhardi4
1Pontificia Universidad Católica de Chile, Santiago, Chile 2Pontificia Universidad Católica de Chile, Fisiología, Facultad de Biología, Santiago, Chile 3University of British Columbian, Cellular and Physiological Sciences, Vancouver, Canada 4Pontificia Universidad Católica de Chile, Neurología, Laboratorio de Neurociencias, Santiago, Chile
The mechanism of secondary damage spread after brain trauma remains unresolved. In this work, we redirected the attention to astrocytic communication pathways. Using an in vitro trauma model that consists of a scratch injury applied to a rat astrocyte monolayer, we found a significant induction of connexin43 hemichannel activity, demonstrated by ethidium uptake, in regions distal from the injury (∼17 mm away and maximal ∼1 h after injury). This response was abolished by two connexin hemichannel blockers, La3+ and the peptide Gap26. In addition, the trauma‐induced increase in hemichannel activity was prevented by inhibition of purinergic P2 receptors. The scratch‐induced increase in hemichannel activity was absent in astrocytes from Cx43 knockout mice. This activity took place with a singular spatial distribution, since cells located at ∼17 mm away from the scratch gained connexin hemichannel activity. However, the functional state of gap junction channels (dye coupling) was not significantly affected in the same locations. The connexin43 hemichannel activity was also enhanced by the acute extracellular application of 60 mM K+. The increase in hemichannel activity was correlated with an increment in apoptotic cells, measured by immunofluorescence to annexin V at 24 h post‐trauma, which was totally prevented by Gap26 peptide. These findings could open a new approach to prevent or reduce the secondary cell damage due to brain trauma.
T13‐09A. K+ effects on oligodendrocytes in ischaemia
N.B. Hamilton, K. Kolodziejczyk, D. Attwell
University College London, London, United Kingdom
The myelin sheaths wrapped around axons by oligodendrocytes are crucial for brain function. In ischaemia the myelin is damaged, partly as a result of glutamate release. Glutamate is thought to activate oligodendrocyte AMPA/kainate and NMDA receptors, causing a Ca2+ influx that leads to myelin disruption and a loss of action potential propagation. We now show that, although ischaemia‐evoked membrane current changes in oligodendrocytes are triggered by glutamate release, they are dominated by a K+ influx caused by a rise of extracellular [K+]. This influx is opposed by a reduction of the membrane K+ conductance. Unexpectedly, activation of NMDA and AMPA/kainate receptors does not raise [Ca2+]i or [Na+]i in oligodendrocytes, but leads to a rise of [K+]i. Thus, myelin disruption and loss of action potential propagation may result from the activation of ionotropic glutamate receptors on cells other than oligodendrocytes, triggering an excessive influx of K+ that leads to osmotic swelling in the tiny intra‐myelin spaces. Ischaemic damage to oligodendrocytes therefore resembles the swelling occurring in astrocytes rather than the excitotoxic damage occurring in neurons
Acknowledgements: Supported by the Wellcome Trust, ERC and EU (Leukotreat).
T13‐09B. Analysis of GABAB receptor deletion in mouse astrocytes
L. Schlosser, A. Scheller, F. Kirchhoff
Institute of Physiology, Saarland University, Homburg, Germany
Current research suggests that astrocytes represent heterogeneous neuroglial populations in different regions of the brain. This is particularly evident when the expression pattern of various transmitter receptors is analyzed. Hippocampal astrocytes, for example, do not express AMPA receptors, while cerebellar Bergmann glia is loaded. Functional analysis of glial receptors requires dedicated efforts for each distinct brain region and developmental age. Unfortunately, the molecular identification using antibody approaches is often hampered by either poor antibody quality or abundant receptor expression on adjacent neuronal membranes. Therefore, we are using Cre/lox‐mediated gene ablation for proper functional analysis.
Here, we are focusing on the characterization of astrocyte‐specific gene deletion of the GABAB1 receptor. Metabotropic GABAB receptors consist of two subunits GABAB1 and GABAB2 forming a heteromeric receptor complex of which GABAB1 is essential. In contrast to neurons, activation of astroglial GABAB receptors leads to transient rises of intracellular Ca2+. The functional impact of these glial GABAB‐receptors is largely unknown.
To address the GABAB receptor function in astrocytes we took advantage of the Cre/lox system and crossbred GLAST‐CreERT2 knockin mice with floxed GABAB1 receptor mice. First immunohistochemical analysis reveals a selective deletion of GABAB1 on a majority of astroglial processes. Functional studies addressing the role of these receptors in astroglial Ca2+ signaling are in progress.
T13‐10A. Temporally controlled ablation of astroglial P2Y1 receptors
H.M. Jahn 1, A.S. Saab1, A. Verkhratsky2, F. Kirchhoff1
1University of Saarland, Homburg, Germany 2University of Manchester, Faculty of Life Science, Manchester, United Kingdom
Purinergic signaling is the most diverse system in astrocytes to communicate with other glial cells and neurons. Astrocytes express a variety of P2X (ionotropic) and P2Y (metabotropic) receptors. Especially in case of brain injury, ATP levels and receptor expression on astrocytes are increased. ATP is immediately degraded to ADP, AMP and adenosine. These nucleotides/nucleosides act back on the preferred purinoreceptor expressed on glial and neuronal cells. In the last decade researchers tried to evaluate the functional impact of astrocytic purinoreceptors on the complex information flux at the tripartite synapse. A prominent role has been suggested for the P2Y1 receptor subtype. We generated conditional mouse mutants to investigate the function of P2Y1 receptors in astrocytes in vivo and crossbred mice carrying the floxed P2Y1 gene with mice that express the inducible DNA recombinase (CreERT2) under the control of the GLAST (L‐glutamate/L‐aspartate transporter) locus.
Ablation of the P2Y1 receptor is induced in development and adulthood by intraperitoneal tamoxifen injections. Successful recombination of the targeted P2Y1 receptor is evaluated by qRT‐PCR on genomic DNA and mRNA usually 21 days after tamoxifen application. At the genomic level we find ∼25% and ∼50% recombination in astrocytes of the cerebellum and hippocampus, respectively. Similar recombination frequencies are observed in young (p11) or adult mice (6–10 weeks). When looking at the transcript level, the mRNA is reduced to 57% in the cerebellum of adult mice and to 76% in the hippocampus. In young mice we determined a reduction to 40% and 42% mRNA expression in the cerebellum and hippocampus, respectively. Given the high level of P2Y1 expression on other cells of the brain these findings indicate a successful astrocyte‐specific knockout. Further fluorescence‐activated cell sorting (FACS) of recombined cells expressing the red fluorescent protein td‐tomato will be used to verify the knockout. The potential function of these astroglial receptors in neuronal networks will be as well investigated using histological (EM and light microscopy), two‐photon Ca2+ imaging in situ and in vivo and behavioral approaches.
T13‐10B. Microglia in the embryonic cortex – mature team players or young bench sitters?
N. Swinnen 1, S. Smolders1, B. Brône1, P. Legendre2, J.‐M. Rigo1
1Hasselt University – BIOMED, Diepenbeek, Belgium 2UMPC Université Paris 06, Paris, France
Microglia are the immune cells of the central nervous system. They are suspected to play important roles in adult synaptogenesis and in the development of the neuronal network. Microglial cells originate from progenitors in the yolk sac. We recently showed that they invade the cortex at 11.5 days of embryonic age (E11.5). They first accumulate at the pial surface and within the lateral ventricles, after which they spread throughout the cortical wall, avoiding the cortical plate region in later embryonic ages. The absence of the expression of Mac‐2 and MHC II suggest these cells have a naïve/quiscent phenotype during embryonic development of the cortex.
Besides immunohistochemical markers is the presence of different K+ channels on microglia also an indication of their activation stage. However most studies have been conducted on postnatal and adult microglial cells. Therefore we aimed at determining the electrophysiological activation phenotype of these embryonic microglia.
At the age of E13.5 and E15.5, microglial cells display a small inward rectifying K+ current and this independent of their location in the embryonic cerebral cortex and their cell morphology. These cells also express functional P2X receptors, which based on the profile of the response are most probably P2X7 receptors. Time‐lapse analysis showed that embryonic microglia are highly dynamic cells. Suggesting that these cells are already very active during fetal development.
T13‐11A. Scratch‐wound induced alterations in glial glutamate transporter distribution and function
W.K. Kafitz, A.E. Schreiner, J. Langer, M.C. Stock, C.R. Rose
Heinrich Heine University Düsseldorf, Düsseldorf, Germany
Rapid extracellular removal of glutamate, the major excitatory neurotransmitter in the CNS, is essential for normal brain function. This task is primarily accomplished by the action of the sodium‐dependent, high‐affinity transporters GLAST and GLT‐1 (rodent analogous of EAAT1 and EAAT2), which are mainly expressed by astrocytes. Impairment or failure of GLAST and GLT‐1 plays an important role in many pathological conditions.
In the present study, we analysed changes in glutamate transporter expression and function following a mechanical lesion in organotypic slice cultures of the mouse hippocampus using immunohistochemistry, western blots and dynamic imaging. The lesion was positioned perpendicular to the stratum pyramidale in the CA1 area and comprised the entire hippocampus proper. After three‐six days, a glial scar had formed along the lesion site. Activated astrocytes in close proximity (100–150 µm) to the lesion (“scar cells”) directed long, palisading GFAP‐positive processes towards the lesion, had significantly swollen somata and lost their ability to take up SR101. Furthermore, some exhibited distinct clustering of GLAST and GLT‐1 immunoreactivity. Scar cells showed greatly diminished increases in intracellular sodium in response to application of D‐aspartate, an agonist for glutamate transporters. Astrocytes in the periphery to the lesion, in contrast, maintained their ability to take up SR101 and showed only slight up‐regulation of GFAP, as well as less swollen cell bodies. Cells in the periphery displayed only marginal changes in glutamate transporter immunoreactivity and unaltered amplitudes of sodium changes in response to D‐aspartate.
Taken together, our data show that mild astrogliosis in the periphery of a mechanical lesion is not accompanied by a significant change in glial glutamate uptake capacity. At the scar itself, a strong clustering of glutamate transporters is observed that apparently goes along with a severe functional reduction in glutamate uptake.
Supported by the DFG (Ro2327/4‐3).
T13‐11B. High bicarbonate sensitivity of mouse cortical astrocytes mediated by sodium‐bicarbonate cotransporter NBCe1: a novel bicarbonate sensor?
S.M. Theparambil, I. Ruminot, J.W. Deitmer
TU Kaiserslautern, Kaiserslautern, Germany
Transport of base equivalent (HCO3 –) across the membrane is a crucial process to maintain the intracellular and extracellular proton concentrations within a narrow physiological range. The electrogenic sodium‐bicarbonate cotransporter (NBCe1, SLC4 A4) is a major base transporter in eukaryotes and expressed in many tissues, especially in epithelial cells. NBCe1 may transport significant amounts of bicarbonate into and out of the cell. In the brain, NBCe1 is predominantly expressed in glial cells and operates with a transport stoichiometry of 1Na:2HCO3 ‐. We have studied the bicarbonate sensitivity of NBCe1, in primary cultured mouse cortical astrocytes (C57BL6/N) using live cell fluorescence imaging with confocal microscope and BCECF‐AM as a proton sensitive fluorescence probe. We have also used the primary cortical astrocyte culture from NBCe1‐KO mouse to compare the NBCe1‐mediated processes in WT astrocyte culture. Bicarbonate dose response protocols suggest that NBCe1 has a very high affinity for bicarbonate (Km <1 mM). Due to this high affinity for bicarbonate, NBCe1 can sensitize even residual bicarbonate concentrations of 150–300 μM present in nominally bicarbonate‐free extracellular solutions (buffered with HEPES). The removal of residual bicarbonate by saturating extracellular buffer (HEPES) with 100% O2 reduced these NBCe1‐mediated intracellular proton changes significantly. In astrocytes of NBCe1‐KO mice, cytosolic H+ changes could not be detected at HCO3 ‐ concentrations lower than 3 mM. It has been reported that astrocytic NBCe1 activity mediates the activation of astrocytic glycolysis by a mechanism dependent of intracellular bicarbonate and pH (Ruminot et al 2011 J Neurosci 40: 64–71). Using a genetically encoded FRET‐based glucose nanosensor, we show that glucose metabolism can be activated at low millimolar bicarbonate concentration, which was largely absent in astrocytes from NBCe1‐KO. Our results demonstrate the highest bicarbonate sensitivity yet described in animal cells, mediated by NBCe1 in cortical astrocytes. NBCe1 may function as a bicarbonate sensor in these cells, e.g. for modulating energy metabolism.
Supported by the Deutsche Forschungsgemeinschaft DE 231/24‐1.
T13‐12A. Oligodendrocyte‐targeted gene therapy to treat leukodystrophy
A. Kagiava 1, I. Sargiannidou1, S. Bashardes2, J. Richter2, N. Schiza1, C. Christodoulou2, A. Gritti3, K. Kleopa4
1The Cyprus Institute of Neurology and Genetics, Neuroscience Laboratory, Nicosia, Cyprus 2The Cyprus Institute of Neurology and Genetics, Department of Molecular Virology, Nicosia, Cyprus 3Fondazione Centro San Raffaele del Monte Tabor, Milan, Italy 4The Cyprus Institute of Neurology and Genetics, Neuroscience Laboratory and Neurology Clinics, Nicosia, Cyprus
Question: Pelizaeus‐Merzbacher‐like disease (PMLD) is a hypomyelinating leukodystrophy caused by mutations in the GJC2 gene encoding the gap junction (GJ) protein connexin47 (Cx47). Cx47 is expressed in oligodendrocytes and forms most GJ channels to astrocytes and to other oligodendrocytes. Since PMLD‐associated GJC2 mutations cause loss of Cx47 function, gene replacement strategies may be promising for developing future treatments. A mouse model for PLMD, the Cx32/Cx47 double knockout (KO), is characterized by early onset of severe leukodystrophy at 4 weeks of age leading to death by 6 weeks, offering the possibility to test therapies. The aim of the present study was to generate a lentiviral vector to allow gene delivery specifically to oligodendrocytes, and to examine the transduction efficacy, distribution, duration and levels of EGFP reporter gene and Cx47 expression throughout the CNS, in order to establish a method for oligodendrocyte‐targeted gene therapy.
Methods: The expression cassette with the Cx47 gene along with the IRES‐EGFP as a reporter gene under the control of oligodendrocyte‐specific CNP promoter was cloned into the lentiviral vector pCCLsin.PPT.hPGK.GFP.pre. Mock vectors lacking the Cx47 gene were also generated as controls. Viral particles were produced to high titers and purified, before injection. Lentiviral vectors were delivered into the brain of wild type (WT) and Cx47KO mice at postnatal day 1 (P1), intraventricularly and in the stratum radiatum of the dorsal hippocampus. EGFP and Cx47 expression was assessed using immunochemistry and immunoblot analysis at different time points post injection.
Results: We found widespread expression of virally delivered EGFP in different brain regions colocalizing with oligodendrocyte markers (CC1 and Olig2). The expression of EGFP was detected from P15 until 3 months post‐injection. On average 20.3 ± 2.56% of oligodendrocytes were EGFP positive, with highest rates in the subventricular zone (29.3 ± 6.31%, n = 6) and olfactory bulb (19.9 ± 4.36%, n = 8) and lower rates in the cortex (16.1 ± 2.60%, n = 6) and corpus callosum (12.3 ± 3.40%, n = 3). In Cx47KO brain expression of virally delivered Cx47 was also found after P21 with formation of GJ plaques in a subpopulation of oligodendrocytes.
Conclusions: Our results show that neonatal lentiviral gene delivery may result in stable and widespread CNS expression targeted to oligodendrocytes by using cell specific promoters. Thus, gene therapy approaches using lentiviral vectors may be feasible for future treatment of leukodystrophy and should be further studied.
Acknowledgement: This project is funded by the European Leukodystrophy Association, ELA (Grant 2011‐025I2 to KAK).
T13‐12B. The role of L‐type calcium channels subtypes 1.2 and 1.3 in NG2 glia
N. Zhao, W. Huang, A. Scheller, F. Kirchhoff
University of Saarland, Molecular Physiology, Homburg, Germany
An abundant class of glial cells in CNS white matter that express the chondroitin sulfate proteoglycan NG2 have been identified as oligodendrocyte precursor cells (OPCs). Voltage‐gated calcium channels (Cavs) that have been detected on oligodendrocyte lineage cells in vitro and in vivo are suggested to play a fundamental role at early stages of OPC maturation.
Recent reports identified mRNA coding for different Cav subtypes like 1.2 and 1.3 in hippocampal NG2 glia. Using acute brain slices prepared from developing and mature NG2‐EYFP mice, we also found that all NG2 glia upon depolarization in different brain regions elicited inward currents which were significantly blocked by the L‐type calcium channel antagonist nimodipine.
To better investigate the role of L‐type calcium channels in NG2 glia at different developmental stages of the CNS, we take advantage of the inducible Cre‐lox system by breeding homozygous Cav1.2 floxed mice and Cav1.3 flexed mice with NG2‐CreERT2 knock‐in mice. We are currently investigating how the removal of Cav1.2/1.3 alters proliferation, migration and survival of NG2 glia. A particular focus lies on the analysis of behavioral abnormalities generated by Cav1.2/1.3‐deficient NG2 glia.
T13‐13A. Astrocytic CX43 hemichannels and gap junctions play a crucial role in development of chronic neuropathic pain following spinal cord injury
M. Chen, B. Kress, X. Han, K. Moll, W. Peng, R.‐R. Ji, M. Nedergaard
University of Rochester School of Medicine and Dentistry, Center for Translational Neuromedicine and Department of Neurosurgery and Neurology, Rochester, NY, United States
Chronic neuropathic pain is a frequent consequence of spinal cord injury (SCI). Yet despite recent advances, upstream releasing mechanisms and effective therapeutic options remain elusive. Previous studies have demonstrated that SCI results in excessive ATP release to the peritraumatic regions and that purinergic signaling, among glial cells, likely plays an essential role in facilitating inflammatory responses and nociceptive sensitization. We sought to assess the role of connexin 43 (Cx43) as a mediator of CNS inflammation and chronic pain. To determine the extent of Cx43 involvement in chronic pain, a weight‐drop SCI was performed on transgenic mice with Cx43/Cx30 deletions. SCI induced robust and persistent neuropathic pain including heat hyperalgesia and mechanical allodynia in wild‐type control mice, which developed after 4 weeks and was maintained after 8 weeks. Notably, SCI‐induced heat hyperalgesia and mechanical allodynia were prevented in transgenic mice with Cx43/Cx30 deletions, but fully developed in transgenic mice with only Cx30 deletion. SCI‐induced gliosis, detected as upregulation of glial fibrillary acidic protein in the spinal cord astrocytes at different stages of the injury, was also reduced in the knockout mice with Cx43/Cx30 deletions, when compared with littermate controls. In comparison, a standard regimen of post‐SCI treatment of minocycline attenuated neuropathic pain to a significantly lesser degree than Cx43 deletion. These findings suggest Cx43 is critically linked to the development of central neuropathic pain following acute SCI. Since Cx43/Cx30 is expressed by astrocytes, these findings also support an important role of astrocytes in the development of chronic pain.
T13‐13B. Panx1 form Ca2+ permeable membrane hemichannels essential for amyloid β peptide‐induced mast cells degranulation
P.A. Harcha 1, A.A. Vargas1, J.C. Sáez1,2
1Pontificia Universidad Católica de Chile, Facultad de Ciencias Biológicas, Departamento de Fisiología, Santiago de Chile, Chile 2Centro Interdisciplinario de Neurociencias de Valparaíso, Instituto Milenio, Valparaíso, Chile
Mast cells (MCs) are immune cells that reside in normal brain tissue. They store in granules an array of pro‐inflammatory mediators, which are rapidly released to the extracellular milieu upon activation through an extracellular Ca2+‐dependent mechanism. Neurotoxic amyloid peptides are known to induce MCs degranulation but the membrane transduction mechanism remains unknown. Here, the possible role of pannexin 1 hemichannel (Panx1 HC) known to be permeable to Ca2+ was studied.
Objective: Since Ca2+ influx is essential for MCs degranulation, we evaluated if the neurotoxic amyloid fragment Aβ25–35 peptide promotes MCs degranulation via activation of Panx1 HCs.
Methods: Primary naïve MCs were differentiated from bone marrow precursors of wild type (WT) and Panx1 Knock out (Panx1‐/‐) C57BL/6 mice using IL‐3 present in WEHI3 conditioned medium. The presence of Panxs 1, 2 and 3 mRNAs presence was evaluated by RT‐PCR analyses. The HC activity was assessed using the 4',6‐diamidino‐2‐phenylindole (DAPI) uptake assays and measurements of membrane current using whole cell patch clamp while intracellular Ca2+ signal was evaluated using Fura‐2AM. Degranulation was assessed by quantification of extracellular histamine as well as release of toluidine blue (TB) from TB preloaded MCs from WT and Panx1‐/‐ mice.
Results: Only Panx1 mRNA was detected in WT MCs. Stimulation with Aβ25–35 but not Aβ35‐25 peptide induced histamine and TB release. It also enhanced DAPI uptake and total membrane current in WT MCs. These responses were drastically reduced in WT MCs pretreated with 10 µM carbenoxolone and 200 µM 10Panx1, blockers of Panx HCs and were absent in Panx1‐/‐ MCs. In WT MCs treated with Aβ25–35increase the intracellular Ca2+ signal and was drastically prevented by 10Panx1, absence of extracellular divalent cations and in Panx1‐/‐ MCs.
Conclusion: These findings indicate that MCs express functional Panx1 HCs, which are essential for Ca2+ influx required for the degranulation response induced by Aβ25–35. Thus, inhibition of Panx1 HCs might avoid spreading of MC‐dependent inflammatory mediators released during Alzheimer's disease progression.
POSTER TOPIC 14 Tumours
T14‐01A. Metabolic reprogrammation following mouse cortical astrocytes transformation in vitro: a proteomic study
A. Bentaib 1, P. De Tullio2, M.‐P. Junier3, P. Leprince1
1University of Liège, Giga Neurosciences, Liège, Belgium 2university of Liège, Drug Research Center, Liège, Belgium 3University of Paris V, Équipe Plasticité gliale, Paris, France
Gliomas are the most frequent primitive CNS tumors and are thought to derive from astrocytes or from neural progenitors/stem cells. However, the precise identity of the cells at the origin of gliomas remains a matter of debate because no pre‐neoplastic state has been yet identified. TGF alpha is frequently over‐expressed in the early stages of glioma progression. Sharif & al (Oncogene. 2007 (19):2695‐706) previously demonstrated that prolonged exposure of normal astrocytes to TGF alpha is sufficient to trigger their reversion to a neural progenitor‐like state. When astrocytes de‐differentiated with TGF alpha were submitted to oncogenic stress using gamma irradiation, they acquired cancerous properties: they were immortalized, showed cytogenomic abnormalities, and formed high‐grade glioma‐like tumors after brain grafting.
Our study aims to identify and characterize the protein signature of those in vitro transformed cells in an attempt to understand their neoplastic behavior and the effect of transformation on metabolic processes. This involves a global proteomic analysis using the 2D‐DIGE methodology and a study of the metabolism of these cells (carbon source, lactate, glucose and glutamine use, ROS metabolism…) by 1H‐ and 13C‐NMR NMR quantification of metabolites. Such approaches are expected to provide information allowing understanding the metabolic reprogrammation that occurred during transformation.
The comparative 2D‐DIGE proteomic analysis of normal and transformed astrocytes shows that during transformation, the cells increase their expression of glycolytic enzymes, thus acquiring the ability to use aerobic glycolysis (Warburg effect). Moreover, the transformed cells reduce their capacity for tricarboxylic acid oxidation and for neurotransmitters (glutamate and GABA) metabolism. Ingenuity Pathway Analysis indicates major effects on carbohydrates, amino acids and nucleotides metabolic components. Using enzymatic activity measurements and the detection of protein isoforms by 2D‐Western blot and zymography, we document a change in expression and activity of various isoenzymes that may be responsible for those metabolic reprogrammations.
T14‐01B. Migration of glioblastoma cells is modulated by KCa3.1 channel activity
A. Grimaldi, G. D'Alessandro, C. Lauro, M. Catalano, C. Limatola
Sapienza University of Rome, Rome, Italy
Glioblastoma (GBM) is one of the most aggressive tumor of the central nervous system, being characterized by a great invasiveness and a very low survival rate of patients. This work wants to better define the role of tumor microenvironment in the modulation of tumor invasiveness. It is known that tumor migration and invasiveness can be modulated by the chemokine CXCL12 and its receptor CXCR4. The expression of both these proteins has been demonstrated in various human GBM cell lines and it has been shown that, blocking this pathway, tumor cells greatly reduce their invasive ability. We have recently demonstrated that an important molecule directly implicated in tumor migration is the intermediate conductance calcium‐activated potassium channel (KCa3.1). Given the expression of these channels also in other cell types infiltrating the tumor mass, like microglia, we investigated the effect of KCa3.1 inhibition on microglia‐glioblastoma interaction. Preliminary data indicate that KCa3.1 activity on microglia is involved in the movement of these cells toward the tumor mass.
T14‐02A. Identification of epigenetic modifications associated with inhibition of the stem and tumorigenic properties of glioblastoma stem cell
A. Bogeas 1, K. Kuranda2, L.G. Dubois1, E.A. El‐Habr1, S. Sayd1, F.‐X. Lejeune1, A. Dias‐Morais1, T. Virolle3, M. Goodhardt 2, M.‐P. Junier1, H. Chneiweiss1
1Inserm U894, Paris, France 2Inserm UMRS 940, Paris, France 3Inserm U898, Nice, France
A growing body of evidence indicates that glioblastoma stem‐like cells (GSCs) play a central role in human glioblastoma development and resistance to current therapies. We recently described a cluster of micro‐RNAs, the miR‐302–367, which induces the exit of GSCs from their stem state and suppresses their tumorigenic properties in an irreversible manner (Fareh et al, 2012). To elucidate the gene networks that govern GSC exit from their stem state, we used ChIP‐seq (chromatin immunoprecipitation followed by next generation DNA sequencing), to identify gene loci showing enrichment of the active (H3K4me3) and repressive (H3K27me3) epigenetic marks in GSC‐miR‐302–367 as compared to naïve GSCs. Functional significance of the ChIP‐seq results was evaluated with confrontation of the ChiP data with the transcriptomes of the corresponding cells derived from exon array hybridization.
Western blot analysis showed that global H3K4me3 and H3K27me3 expression levels were similar in GSC and GSC‐miR‐302–367. ChIP‐seq analysis revealed similar gross number of gene loci associated with H3K4me3 or H3K27me3 (13000 and 5000 genes, respectively) in either cell type. Interestingly, 16% (2381 genes) of the analyzed genes exhibited a change in either H3K4me3 or H3K27me3, or both in miR‐302–367‐GSCs as compared to naïve GSCs. These changes resulted into a transcript variation in 14% of the cases (332/2381) considering as significant an increase or a decrease of at least 2‐fold and the 77% of these genes translated into congruent alterations in the corresponding transcript levels.
Analysis of the functional significance of the changes observed using functional annotation databases identified novel gene networks, likely to participate in the regulation of GSC properties. Studies under way focus on members of these networks specifically activated in miR‐302–367 GSCs that might alter GSC dialog with their microenvironment.
T14‐02B. Toll‐like‐receptor 2 mediates microglia/brain macrophage MT1‐MMP expression and glioma expansion
F. Hu 1, K. Vinnakota1, M.‐C. Ku1, P. Georgieva1, F. Szulzewsky1, S. Lehnardt2, U.‐K. Hanisch3, M. Synowitz2, D. Markovic4, R. Glass5, S.A. Wolf1, H. Kettenmann1
1Max Delbrueck Center, Berlin, Germany 2Charité – Universitätsmedizin, Berlin, Germany 3University of Göttingen, Göttingen, Germany 4Helios Clinic, Berlin, Germany 5University of Munich, Munich, Germany
Malignant gliomas are the most frequent primary tumors of the brain with poor clinical prognosis. Infiltrating peripheral macrophages and resident microglia contribute significantly to the tumor mass. We have previously shown that microglia as the intrinsic immune competent brain cells promote glioma expansion by up‐regulating metalloprotease MT1‐MMP through Toll‐like receptor (TLR) and its adaptor protein MyD88. In this study we identified TLR2, as the main TLR controlling MT1‐MMP expression and pro‐tumorigenic signaling in microglia. Glioma‐derived soluble factors and synthetic TLR specific ligands induced MT1‐MMP expression in microglia from WT mice but not TLR2‐/‐ mice. By using the organotypic brain slice model, we found that tumor expansion depended on both parenchymal TLR2 expression and the presence of microglia. TLR2 is also highly expressed in human gliomas and inversely correlates with patient survival. In search for an endogenous TLR2 ligand released from glioma cells, we screened glioma conditioned medium (GCM) by mass spectrometry for endogenous TLR2 agonists produced by glioma cells and found versican, an extracellular matrix proteoglycan and a reported ligand of TLR2. To examine if versican is the factor mediating the glioma‐microglia interaction, we silenced versican expression in GL261 cells. Primary microglia were then stimulated with GCM from versican siRNA and non‐target siRNA transfected GL261 cells and microglial MT1‐MMP expression was analyzed by real‐time PCR. An almost 3‐fold up‐regulation in microglial MT1‐MMP expression was observed using GCM from non‐target transfectants while the expression was reduced with GCM from versican knock‐down GL261 transfectants. Our results show that TLR2 activation is an essential part of the signaling cascade employed by glioma cells to convert microglia into a pro‐tumorigenic phenotype and TLR2 thus may be a novel target for glioma therapies.
T14‐03A. Proteomic profiling of human induced pluripotent stem cell‐derived microglia upon exposure to glioblastoma cells
J. Choi 1,2, K. Roy3, T. Kempf4, M. Schnölzer4, A. Hotz‐Wagenblatt4, H. Neumann3, A. Régnier‐Vigouroux1,2
1German Cancer Research Center, Program Infection and Cancer, Heidelberg, Germany 2Johannes Gutenberg University of Mainz, Faculty of Biology, Mainz, Germany 3University Hospital Bonn, University of Bonn, Neural Regeneration, Institute of Reconstructive Neurobiology, Bonn, Germany 4German Cancer Research Center, Protein Analysis Facility, Heidelberg, Germany
Question: Glioma‐associated microglia/macrophages contribute to at least 1/3 of the total tumor mass and are known to support glioma growth and invasion. However, there have been several studies demonstrating the potential of modulating microglia/macrophages response to reduce glioma growth or invasion (Kees et al. 2012; Synowitz et al. 2006), warranting the need to better understand the interaction between glioma and microglia. While it is the gold standard to study glioma using human specimens, research involving human microglia is often challenged with the problem of low cell yield.
Method: Recently, human induced pluripotent stem cell‐derived microglia (iPSdM) cell lines were developed as a substitute for primary microglia according to an established protocol (Beutner et al. 2010). Two iPSdMs were used to determine their responses in the presence of glioblastoma cells. Using LC‐MS/MS method, proteomic profiles were generated and compared between iPSdMs exposed to human glioblastoma cells and iPSdMs exposed to normal human astrocytes as control.
Results: Immunolabeling of the two iPSdM cell lines showed specific expression of microglial markers such as Iba‐1, CD11b, and CD68. The comparative proteomic analyses indicated that microglia exposed to glioblastoma cells showed differential protein expressions relevant for cytoskeletal activity, energy production, and cell survival compared to control.
Conclusion: This is the first study showing the proteomes of human induced pluripotent stem cell‐derived microglia exposed to glioblastoma cells, and the findings could provide further insight of the interaction between microglia and glioblastoma.
T14‐03B. Dissecting the Role of Apelin Signaling in Gliomagenesis
R.E. Kälin, A. Jarczewski, F. Apel, R. Monk, S. Kraft, J. Radke, F.L. Heppner
Charité – Universitätsmedizin Berlin, Neuropathology, Berlin, Germany
Question: Glioma are the most frequent malignant primary tumors of the brain. Glioma survival and growth is determined by the interaction with brain parenchyma, which includes intense tumor angiogenesis. This process is supported by glioma‐invading myeloid (GIM) cells, namely brain resident microglia or brain‐invading macrophages. Depletion of GIM cells leads to a significant decrease in glioma volume. In a previous study, we established the novel neuroendocrine hormone apelin as an angiogenic factor and described its upregulation in human glioblastoma multiforme. Our aim is thus to study the role of apelin signaling in tumor angiogenesis but also its possible effect on brain monocytes for the regulation of glioma growth.
Methods: To investigate apelin signaling during gliomagenesis we took advantage of an orthotopic mouse model for tumor formation. Intracerebral xenotransplantation of the human glioma cell line U87MG, expressing lentiviral control or apelin shRNA, allows us to study the specific contribution of glioma‐derived apelin to gliomagenesis. To further dissect a putative immune function of apelin, we used the isogenic mouse glioma cell line GL261 for intracerebral implantation into immunocompetent mice lacking (Apelin‐KO) or overexpressing apelin (Apelin‐Tg).
Results: Loss‐of‐Apelin expression in U87MG cells resulted in a reduced glioma volume and attenuated the formation of the xenograft vasculature. GL261 implants in Apelin‐KO mice produced a similar phenotype. In addition, invasion of GlM cells was significantly reduced. Interestingly, expression analysis showed an upregulation of the apelin receptor APJ in brain myeloid cells making them competent to respond to glioma‐derived apelin.
Conclusions: Our findings demonstrate that apelin plays an important role in tumor angiogenesis and glioma growth. We show here that both, glioma cell‐derived apelin and apelin expressed by the tumor neovasculature are contributing to tumor angiogenesis, GIM invasion and glioma growth. We anticipate that our study will provide insights whether apelin signaling may serve as a future novel target for anti‐angiogenic tumor therapy.
T14‐04A. Pro‐ versus anti‐tumor activities of microglia/macrophages: experimental and mathematical modeling approaches
L.D.R. Cisneros Castillo 1, A. Toma2, T.M. Buzug3, A. Régnier‐Vigouroux1,4
1German Cancer Research Center, Heidelberg, Germany 2University of Lübeck, Institute of Medical Engineering, Centre of Excellence for Technology and Engineering in Medicine (TANDEM), Lübeck, Germany 3University of Lübeck, Institute of Medical Engineering, Lübeck, Germany 4Johannes‐Gutenberg University Mainz, Mainz, Germany
Tumor infiltrating microglia/macrophages (TIMs) constitute the largest population of infiltrating cells in glioblastoma (GBM), the most aggressive brain tumor. Data from the clinic and from experimental work performed in murine and human models indicate that TIMs play a significant role in GBM biology as they support proliferation, migration and invasion of tumor cells. Evidence is amounting that tumor cells actually polarize TIMs towards this M2‐like, tumor‐supportive phenotype. We showed that toll‐like receptor 3 ligand reverses this M2‐ into a M1‐like phenotype. Pre‐activated, M1‐like polarized TIMs incubated with spheroids of GBM cells reduced migration, killed and phagocytosed tumor cells over a 15 day‐period, indicating a sustained M1 activation of TIMs in absence of exogenously added stimuli. In order to analyse in more detail TIMs‐GBM cells interactions, we have undertaken two approaches. We use spheroids of cells (tumor with/without TIMs) embedded in collagen matrix, in which TIMs can be implanted, as an experimental model. This three‐dimensional in‐vitro system is well suited for a qualitative and quantitative monitoring of cell proliferation, death, migration and invasion. Data experimentally generated are then implemented in a mathematical model that is, to our knowledge, the first one proposed to take into account tumor cells and TIMs in order to simulate GBM progressive behaviour. In a first step, the capacity of spheroids made of murine glioma cells to invade collagen matrices was evaluated in absence and presence of microglia. Invasion was monitored by photography of the spheroids and images were processed with Photoshop CS5 software. In order to achieve a precise determination of invasion, we chose to measure the diameter of the core plus the invasive rim as a read out of expansion rate. Untreated microglia promoted growth and invasion of tumor cells. Data generated through the proposed in‐vitro system were comparable to and reproduced the in‐silico simulations obtained with the mathematical model, hence validating our mathematical and experimental approaches. We currently evaluate the rate of proliferation and death of tumor cells in various settings that modulate TIMs polarization, using flow cytometry and confocal (live) imaging. Data contributed by these two approaches should facilitate the delineation of a predictive model for tumor progression in a TIMs‐enriched microenvironment with possible therapeutic fallouts.
T14‐04B. Siglec‐h on M1‐polarized microglia triggers uptake of cells with an altered glycocalyx
J. Kopatz, C. Beutner, J. Ackermann, H. Neumann, H. Neumann
University of Bonn, Bonn, Germany
Sialic‐acid‐binding immunoglobulin‐like lectins (Siglecs) are type 1 membrane proteins displaying an amino‐terminal immunoglobulin‐like variable (V‐set Ig‐like) domain that binds sialic acid and variable numbers of immunoglobulin‐like constant region type 2 (C2‐set Ig‐like) domains. Siglec‐h is a recently identified mouse‐specific CD33‐related Siglec that signals via the ITAM linked adaptor protein DAP12. So far the function of Siglec‐h and its ligand are unknown. In this project, we demonstrate gene transcription and protein expression of Siglec‐h by mouse microglia after interferon‐γ (IFN‐γ) treatment or polarization into a M1‐subtype. Targeting of beads to Siglec‐h by specific antibodies triggered the phagocytic uptake of the beads by the microglia. A Siglec‐h Fc fusion protein generated by our laboratory selectively bound to cells with an altered glycocalyx, particularly glioma cells, but not to normal control cells. M1‐polarized microglia phagocytosed glioma cells and also limited their proliferation in a co‐culture system. Interestingly, no signs of apoptosis were observed before phagocytosis of the glioma cells by Siglec‐h expressing microglia. Phagocytosis of glioma cells was reduced after shRNA mediated Siglec‐h knock down in microglia. Furthermore, microglial clearance of glioma in vitro was dependent on the interaction of Siglec‐h with its corresponding adaptor protein DAP12. Our data show that M1‐polarized microglial cells can engulf glioma cells via a DAP12‐mediated and Siglec‐h dependent uptake.
T14‐05A. Cytostatic and cytotoxic effects induced by M2 muscarinic receptor activation on human glioblastoma cells
M. Di Bari 1, V. Tombolillo1, F. Alessandrini1, C. Conte1, F. De Grassi2, R. Ricordy2, A.M. Tata1
1University of Rome Sapienza, Rome, Italy 2CNR, Rome, Italy
Glioblastomas (GBM) are the most common brain tumors in humans. Although advances in the chemotherapeutic management of glioblastoma have been made, almost 80% of the patients die within the first 2 years after diagnosis. On the basis of these observations, the identification of new molecules that can counteract the GBM growth and invasiveness appears relevant.
Previously, we have demostrated that M2 muscarinic receptor agonist arecaidine inhibited cell proliferation in a time and dose‐dependent manner and induced a severe apoptosis both in two glioma stable cell lines (U251 and U87) and in primary cultures derived from human biopsies. In order to clarify the mechanisms causing the decreased cell proliferation, we have evaluated the ability of M2 receptor activation to counteract the Notch‐1 and EGFR pathways. The analysis by real time PCR and western blot have demonstrated that, in both cell lines, M2 receptor caused a decreased expression of EGFR and Notch‐1 and its ligands (e.g. delta‐1, jagged‐1 and −2), suggesting that the decreased cell proliferation may dependent on altered expression and activity of these pathways. Although FACS analysis have showed that arecaidine induced an arrest of cell cycle progression, we observed, in both cell lines, a significant increase of dividing cells already after 24 hours of treatment. In particular, the number of metaphases increased significantly, while the percentage of anaphases diminished.
Furthermore we observed in both cell lines, a dis‐regulation of mitotic spindle assembly, misalignment of chromosomes and the presence of multipolar spindles. Moreover, due to prolonged activation of the promethaphase/methaphase mitotic checkpoint, chromosomes appeared more condensed in cells treated with arecaidine. FACS analysis and immunocitochemistry using anti‐histone γ‐H2AX, a marker of double strand breaks, have also demonstrated that arecaidine treatment induced DNA damage. On the other hand, M2 agonist induced also oxidative stress, followed by an increased expression of the enzyme superoxide dismutase and sirtuins (SIRT1 and SIRT2).
In conclusion, the data obtained suggest that the activation of M2 receptor induces cytostatic and cytotoxic effects in glioblastoma cell lines, opening new therapeutic perspectives for this receptor in glioblastoma therapy.
T14‐05B. Glioblastoma a fatal tumour with stem cells
V. Moura‐Neto
Univdersidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil
Glioblastoma (GBM) is one of the most aggressive human cancers. Despite current advances in multimodality therapies, such as surgery, radiotherapy and chemotherapy, the outcome for patients with high grade glioma remains fatal. There is now a growing awareness that the main limitations in understanding and successfully treating GBM might be bypassed by the identification of a distinct cell type that has defining properties of somatic stem cells, as well as cancer‐initiating capacity – brain tumor stem cells, which could represent a therapeutic target. In addition, experimental studies have demonstrated that the combination of antiangiogenic therapy, based on the disruption of tumor blood vessels, with conventional chemotherapy generates encouraging results. Emerging reports have also shown that microglial cells can be used as therapeutic vectors to transport genes and/or substances to the tumor site, which opens up new perspectives for the development of GBM therapies targeting microglial cells. Finally, recent studies have shown that natural toxins can be conjugated to drugs that bind to overexpressed receptors in cancer cells, generating targeted‐toxins to selectively kill cancer cells. Further, extensive effort is being dedicated to characterize the molecular basis of GBM resistance to chemotherapy and to explore novel therapeutic procedures that may improve overall survival. We show that a non‐cytotoxic concentration of equinatoxin II (EqTx‐II), a pore‐forming toxin from the sea anemone Actinia equina, potentiates the cytotoxicity induced by temozolomide (TMZ), a first‐line GBM treatment, and to etoposide (VP‐16), a second‐ or third‐line GBM treatment. We also suggest that this effect is selective to GBM cells and occurs via PI3K/Akt pathway inhibition. Finally, Magnetic resonance imaging (MRI) revealed that a non‐cytotoxic concentration of EqTx‐II potentiates the VP‐16‐induced inhibition of GBM growth in vivo. These combination therapies constitute a new and potentially valuable tool for GBM treatment, leading to the requirement of lower concentrations of chemotherapeutic drugs and possibly reducing, therefore, the adverse effects of chemotherapy. Curr Top Med Chem. 2012 Nov 12.,Biochim Biophys Acta. 2012 Dec;1826(2):338‐49. doi: 10.1016/j.bbcan.2012.05.004
T14‐06A. Regulation of glioblastoma stem‐like cells properties by GABA‐related metabolites
L.G. Dubois 1,2, E.A. El‐Habr2, J. Lipecka2, M. Fareh3, V. Moura‐Neto1, T. Virolle3, H. Chneiweiss2, M.‐P. Junier2
1Universidade Federal do Rio de Janeiro, Instituto de Ciências Biomédicas, Rio de Janeiro, Brazil 21. Inserm U894, Paris, France 32.INSERM U898, Nice, France
A growing body of evidence indicates that Glioblastoma stem‐like cells (GSCs) play a central role in glioblastoma development and resistance to current therapies. We recently described a cluster of micro‐RNAs, the miR‐302–367, which induces the exit of GSC from their stem state and suppresses their tumorigenic properties in an irreversible manner (Fareh et al, 2012). We postulated that such a drastic change in the cell phenotype should be accompanied with metabolic alterations that could be instrumental in the loss of functional properties of GSCs induced by the micro‐RNA cluster.
Metabolome profiling by mass‐spectrometry of GSCs and GSC‐miR‐302–367 pointed to changes in the GABA synthesis pathway (see abstract El‐Habr et al). Remarkably, exposure of naïve‐GSC to metabolites of the GABA synthesis pathway found to be overproduced in GSC‐miR‐302–367 reproduced at least in part the effects of miR‐302–367, including inhibition of clonality, down‐regulated expression of self‐renewal markers, and loss of self‐renewal properties. The metabolites studied acted by interfering with progression of the cell cycle and the nuclear localization of transcription factors crucial for maintenance of GSC stem‐like properties. Studies assaying the effects of the metabolites on GSC tumor‐initiating properties are under way.
These results demonstrate that metabolic regulations can participate in the control of GSC properties, and opens novel paths in therapeutic targeting of glioblastoma.
*LGD and EAE contributed equally.
T14‐06B. LIM domain kinases as therapeutic targets for Neurofibromatosis Type 2
A. Petrilli 1, A. Copik1, M. Posadas1, L.‐S. Chang2,3, D.B. Welling4, M. Giovannini5, C. Fernandez‐Valle1
1University of Central Florida, College of Medicine, Department of Biomedical Science, Orlando, United States 2The Ohio State University College of Medicine, Center for Childhood Cancer, The Research Institute at Nationwide Children's Hospital, Columbus, United States 3The Ohio State University College of Medicine, Nationwide Children's Hospital, Dept. of Pediatrics, Columbus, United States 4The Ohio State University College of Medicine, Nationwide Children's Hospital, Dept. of Otolaryngology, Columbus, United States 5House Research Institute, Division of Clinical and Translational Research, Los Angeles, United States
Mutations in the neurofibromatosis 2 (NF2) gene cause Neurofibromatosis Type 2 (NF2) characterized by formation of multiple schwannomas and meningiomas. NF2 encodes a tumor suppressor protein called merlin. Loss of function of merlin is associated with an increased level of active Rac and p21‐activated kinases (PAK). The LIM domain kinases (LIMK1 and 2) are Rac‐PAK substrates and modulate actin dynamics and cytoskeletal organization by phosphorylating cofilin, an actin severing and depolymerizing agent. Here we report that mouse Schwann cells (MSCs) in which merlin function is lost as a result of Nf2 exon2 deletion (Nf2ΔEx2) exhibited increased levels of LIMK1, LIMK2 and active phospho‐Thr508/505‐LIMK1/2, as well as phospho‐Ser3‐cofilin, compared to control MSCs. Similarly, human vestibular schwannomas (VS) exhibited elevated levels of LIMK1 and 2 expression and of their active phosphorylated forms compared to control human Schwann cells (SCs). Reintroduction of wild‐type NF2 into Nf2ΔEx2 MSCs normalized LIMK1 and LIMK2 levels. In addition, we show that pharmacological inhibition of LIMK with BMS‐5, decreased the viability of Nf2ΔEx2 MSCs in a dose‐dependent manner, but did not affect viability of normal MSCs. Similarly, LIMK knockdown decreased the viability of Nf2ΔEx2 MSCs. The loss of Nf2ΔEx2 MSCs viability was caused by inhibition of cell cycle progression, arresting cells in the G2/M phase. Our results suggest that LIMKs are a potential drug targets for NF2 and tumors associated with merlin deficiency.
Funding Acknowledgements: DHHS/NIH award to C.F.V. (5R01NS062825), A.P. is recipient of Young Investigator Award from the Children's Tumor Foundation.
T14‐07A. Tumor suppression effects of miRNA‐302–367 on glioblastoma stem cells are correlated with altered GABA metabolism
E.A. El‐Habr 1, L.G. Dubois1, M. Fareh2, A. Bogeas1, F.‐X. Lejeune1, J. Lipecka1, T. Virolle2, M.‐P. Junier1, H. Hervé Chneiweiss1
INSERM U894, Paris, France 2INSERM U898, Nice, France
Glioblastomas are the most frequent and aggressive form of adult primary brain tumors. Glioblastoma stem cells (GSCs) are thought to play key roles in the development and resistance of the tumor to existing radio‐ and chemotherapies. Our previous results showed that the cluster of microRNAs, miRNA‐302–367, induces loss of GSC stem‐like and tumor‐inducing properties (Fareh et al. 2012). To further understand the molecular pathways controlling the peculiar properties of GSCs, we sought for metabolic changes likely to accompany the drastic change in cell phenotype induced by miR‐302–367 expression.
We measured the intracellular and secreted levels of 271 metabolites by mass spectrometry. Reconstitution of the metabolomes revealed significant and coordinated changes in components of the Krebs cycle, and the glutamine/glutamate neuropeptide metabolic pathway that suggested altered turnover of the GABA synthesis pathway. Exon array hybridization and Western blot analysis, revealed changes in expression of several enzymes of the GABA synthesis pathway in miR‐302–367‐GSCs. Of note, none of the analyzed enzyme transcripts appeared as a direct target of miR‐302–367.
Our results could be integrated in a complex multistep schema compatible with enhanced turnover of the GABA synthesis pathways, resulting in decreased cell GABA levels and increased levels of GABA by‐products, as observed in miR‐302–367 GSCs. Further studies showed that these metabolic changes participate in the induced loss of GSC properties (see abstract by Dubois et al).
* EAE and LGD contributed equally
T14‐07B. Carcinoma cells misuse the host tissue danger response to invade the brain
H.‐N. Chuang1, D.V. vanRossum1, D. Sieger2, L. Siam1, F. Klemm1, A. Bleckmann1, M. Bayerlová1, B. Wenske1, K. Farhat1, J. Scheffel1, M. Schulz1, F. Dehghani3, C. Stadelmann1, U.‐K. Hanisch1, C. Binder1, T. Pukrop 1
1University Medicine Göttingen, Göttingen, Germany 2EMBL, Heidelberg, Germany 3University Leipzig, Institute of Anatomy, Leipzig, Germany
Colonization of the brain by carcinoma cells is barely understood, in particular when considering interactions with the host tissue. We recently demonstrated that microglia assist carcinoma cell invasion. Current findings indicate that this is part of a danger response of the entire brain tissue.
In the brain slice coculture model, contact with both benign and malignant epithelial cells induced a response by microglia as well as astrocytes comparable to that seen at the interface of human cerebral carcinoma metastases. This response tries to protect the brain from intrusion of benign epithelial cells by inducing apoptosis. However, this is ineffective against malignant cells which did not undergo apoptosis and actually exploited this reaction to invade instead. Gene expression and functional analyses revealed that C‐X‐C chemokine receptor type 4 (CXCR4) and WNT signaling are involved in this process. Furthermore, CXCR4 regulates the recruitment of microglia towards brain injury in a zebrafish model and was expressed in human stroke patients, suggesting a conserved role of CXCR4 in various danger reactions.
We propose that glia‐assisted malignant invasion takes advantage of the physiological two‐stage glial defense reaction. Carcinoma cells escape the second step of cell killing and misuse the damage response to colonize the brain.
T14‐08A. An allograft glioma model reveals the dependence of aquaporin‐4 expression on the brain microenvironment
P. Fallier‐Becker 1, S. Noell1, M. Tatagiba1, R. Ritz1, H. Woburg1, K. Wolburg‐Buchholz1
University of Tuebingen, Tuebingen, Germany
Aquaporin‐4 (AQP4), the main water channel of the brain, is highly expressed in animal glioma and human glioblastoma in situ. In contrast, most cultivated glioma cell lines do not express AQP4, and primary cell cultures of human glioblastoma lose it during the first passages. Accordingly, in two glioma cell lines of the rat (C6 cells and RG2 cells) and in SMA mouse glioma cell lines we found no AQP4 expression. This let us consider the possibility that AQP4 expression depends on brain microenvironment. AQP4 negative rat glioma cells were implanted into rat brain. Within two weeks, a tumor developed. AQP4 staining of the tumor cells was positive. However, if the identical cells were implanted into the rat's flank, they did not express AQP4. In contrast to the normal brain, where AQP4 staining is polarized in the astrocytic endfoot membranes, the AQP4 staining in C6 and RG2 tumors was distributed over the whole glioma cell as in human glioblastoma. We conclude that the micro‐environment is crucial for AQP4‐expression in brain and brain‐tumor.
T14‐08B. A vascularisation switch induced by Notch pathway activation in glioblastoma stem‐like cells
P.‐O. Guichet, M. Teigell, L. Hopp, B. Rothhut, J.‐P. Hugnot
Institute for Neurosciences, INSERM U1051, Montpellier, France
Glioblastomas (GBM) are infiltrative and highly vascularised brain tumors containing a subpopulation of multipotent stem‐like cells. Here we questioned whether Notch pathway activation in these cells influenced the extent of tumoral vascularisation and dissemination. We used two CD133+ Sox2+ GBM stem‐like cultures generating highly infiltrative but poorly vascularised tumors upon intracranial grafts. The use of a Hes5 promoter reporter construct indicated that the Notch pathway was only active in a small fraction of cells. Sustained Notch activation in these cultures, using an activated form of the Notch‐1 receptor (NICD), induced a profound alteration of their morphology, phenotype and properties. A severe decline of their migration and proliferation rates was observed in vitro and in vivo. Intracranial and subcutaneous transplantation revealed that NICD triggered an extensive vascularisation of tumoral cells indicated by the presence of well‐formed vessels with large lumens. Close interactions between GBM cells and host endothelial cells were readily observed but no evidence for endothelial transdifferentiation was found either in vitro or in vivo. Microarray and proteomic analyses showed that NICD induced the expression of vascular adhesion proteins (ICAM‐1, VCAM‐1, ITGA9), proangiogenic cytokines (PlGF, Il‐8, HB‐EGF), metalloproteinase (MMP‐9), a‐smooth muscle actin and Dll4, a Notch ligand essential for vascularisation. This was associated with a remarkable transcription factor switch whereby cells became Klf9+ Snai2+ while Ascl1, Olig2, and Sox2 were reduced or lost. Thus in addition to its well‐described role in vascular development and remodelling, the Notch pathway is also central in controlling proliferation, migration and vascularisation of GBM‐stem like cells.


