Abstract
Aim
Community‐acquired pneumonia (CAP) is presumed to be bacterial in origin and empirical antibiotics are almost always given on admission. However, early detection of viral infection is also very important for hospital infection control and timely use of antiviral agents. The present study aimed to compare patients with viral and bacterial pneumonia, and identify independent predictors of viral pneumonia.
Methods
A prospective cohort study was carried out in a tertiary teaching hospital in a 1‐year period. Older patients (aged ≥65 years) were recruited if they were admitted for CAP confirmed by chest radiographs.
Results
A cohort of 488 patients was analyzed. Infective causes were found in 137 (28.1%) patients. Bacterial, viral and mixed infections were detected in 86 (17.6%), 41 (8.4%) and 10 (2.0%) patients, respectively. Bacteriology was established mostly by sputum culture and virology by nasopharyngeal aspirate (NPA) viral culture. The commonest bacterial isolates were Haemophilus influenzae (31), Pseudomonas aeruginosa (15), Mycobacterium tuberculosis (14), Klebsiella spp. (9) and Streptococcus pneumoniae (6). Influenza A virus (28, 8 were pandemic 2009 A/H1N1 subtype) and respiratory syncytial virus (16) were the most frequent viral causes. Independent predictors of viral pneumonia included nursing home residence (RR 3.056, P = 0.009) and absence of leukocytosis (RR 0.425, P = 0.026).
Conclusions
All nursing home residents hospitalized for CAP should undergo NPA viral testing because of infection control, early antiviral treatment and discharge planning. We suggest that empirical antiviral agents might be considered for nursing home residents hospitalized for CAP if outbreaks of influenza‐like illness are reported in nursing homes. Geriatr Gerontol Int 2013; 13: 949–957.
Keywords: community‐acquired pneumonia, nursing home residence, viral pneumonia
Introduction
The metaphor of pneumonia as an old man's friend was invented by Sir William Osler a century ago. Pneumonia was a frequent and potentially life‐threatening illness of the aged.1 Nowadays, it is still an important cause of mortality and morbidity of older persons. Community‐acquired pneumonia (CAP) is presumed to be infective in origin. The rates of identifying infective etiology varied considerably, from 28% to 53%, in previous studies on CAP in older patients.2, 3, 4, 5, 6, 7, 8, 9 In other words, most of the studies showed that more than half of older patients had “pneumonia of unknown etiology”.
Bacterial infection was evident in 28–38% of all study patients in previous studies on CAP in older patients.3, 6, 9 Common bacterial pathogens include Streptococcus pneumoniae and Haemophilus influenzae. Gram‐negative bacteria (GNB; Klebsiella spp, H. influenzae, Pseudomonas aeruginosa and Moraxella catarrhalis) are more common in patients with chronic lung diseases. Staphylococcus aureus, GNB and anaerobes were more frequent in nursing‐home‐acquired pneumonia (NHAP).10, 11 Pulmonary tuberculosis is not an uncommon cause of CAP in older patients, in particular those who are immunocompromised and are living in institutions.3, 7, 9
The importance of viral infection was underevaluated in previous epidemiological studies of CAP in older patients. The prevalence of viral pneumonia was not reported in three of the aforementioned studies.3, 8, 9 Respiratory viruses were detected in 1–23% of all study patients using serology in the other studies.2, 5, 6, 7 Common respiratory viruses are influenza A virus, influenza B virus, respiratory syncytial virus (RSV), adenovirus and rhinovirus.
Bacterial and viral pneumonia are treated with entirely different antimicrobials. CAP is presumed to be caused by bacterial pathogens, and empirical antibiotics are almost always given on admission. However, early detection of viral pneumonia is also very important for hospital infection control, timely use of antiviral therapy and discharge planning. This is particularly relevant for nursing home residents, because they might be hospitalized for isolation to avoid spreading viral infection in nursing homes.
The present study aimed to compare patients with viral and bacterial pneumonia, and to identify independent predictors of viral pneumonia.
Methods
Patients
We prospectively studied older patients admitted for CAP to the Prince of Wales Hospital in Hong Kong from October 2009 to September 2010. It is the teaching hospital affiliated with the Chinese University of Hong Kong. The diagnosis of CAP was made if patients had two of three clinical features confirmed by chest radiograph. Clinical features were fever (tympanic temperature ≥37.8°C), chest symptoms (shortness of breath, cough and increase in sputum production or purulence) and chest signs on physical examination (crepitations, bronchial breathing or pleural effusion). Radiographic features included abnormal infiltrates (interstitial shadowing or consolidation) or pleural effusion. Patients were excluded if they had been hospitalized in the 14 days before the index admission, were on tube feeding or had predominantly congestive heart failure on clinical examination or chest radiograph.
The study was approved by the Research Ethics Committee of the hospital.
Data collection
Investigators monitored patients' progress until death or discharge, and did not provide any intervention. If patients were confused or were not able to communicate, their relatives or caregivers might provide supplementary information
Background characteristics
Demographics, comorbidities, premorbid functional status (Katz Index) and physical findings were collected. Results of baseline blood tests and chest radiographs were also retrieved.
Confusion, blood urea nitrogen, respiratory rate and low blood pressure score
Confusion, blood urea nitrogen, respiratory rate and low blood pressure (CURB) are the core clinical adverse prognostic factors of CAP. The CURB score, ranging from 0 (least severe) to 4 (most severe), assesses the severity of pneumonia. One point is given for each parameter if the patient has confusion, blood urea level is >7.0 mmol/L, respiratory rate is ≥30/min or the patient is in shock (systolic blood pressure <90 mmHg or diastolic blood pressure ≤60 mmHg). Pneumonia with CURB ≥2 is associated with a high risk of 30‐day mortality.
Microbial investigations
Respiratory specimens were sent for analysis within 48 h after admission. Sputum smear was examined for white blood cells and epithelial cells. Gram stain examination was carried out on request. Specimens with more than 10 epithelial cells per low power field were not cultured unless they appeared purulent or blood‐stained. Common lower respiratory tract pathogens (such as S. pneumoniae and H. influenzae) were reported semi‐quantitatively when isolated. Potential pathogens (including P. aeruginosa, M. catarrhalis, Klebsiella spp. and other Enterobacteriaceae, Acinetobacter spp., Burkholderia spp., Stenotrophomonas maltophila, S. aureus, Candida spp., β‐hemolytic streptococci etc.) were reported only when they were present in heavy growth or were the predominant organism in culture. In vitro susceptibility testing on these isolates was carried out by the disc diffusion method. Acid‐fast staining and culture for Mycobacterium species onto the Löwenstein–Jensen medium was carried out after decontamination.
All nasopharyngeal aspirate (NPA) samples were collected within 8 h of hospitalization. They were obtained by catheter aspiration from the posterior pharyngeal space through the nostril when the patient was in a sitting position. The sample was saved in 3 mL of viral transport medium and was processed within 12 h. The aspirate was centrifuged, washed with saline solution and then coated on glass slides. The supernatant was incubated. Viral culture was screened initially by hemadsorption and then identified by immunofluorescence if positive. The cell suspension coated on the slide was stained with panels of monoclonal antibodies against respiratory viruses including influenza virus types A and B, parainfluenza virus types 1, 2 and 3, adenovirus, and RSV.
Blood cultures were collected before initiation of antibiotic treatment. If the bottles were flagged positive, a Gram smear was carried out and blood samples underwent subculture on the following blood agars: chocolate blood agar, MacConkey agar and vitamin K1‐supplemented blood agar.
Acute and convalescent‐phase sera were used for serological assays. Serology for influenza virus types A and B, RSV, parainfluenza virus types 1, 2 and 3, adenovirus, Chlamydia spp. (including C. psittaci and C. pneumoniae), Mycoplasma pneumoniae, and Coxiella burnetii were carried out by the complement fixation tests. Legionella serology was carried out using immunofluorescence test.
Infective etiology
The etiological diagnosis of pneumonia was established if one of the following criteria was met after excluding other sources of infection: (i) sputum or pleural fluid cultures yielding one or more bacteria; (ii) blood cultures yielding bacterial pathogen; (iii) a fourfold rise in paired immunoglobulin G titers or a single increased immunoglobulin M titer; and (iv) urinary antigen test positive for L. pneumophila.
Clinical outcomes
Relevant data during the clinical course were recorded: length of stay in hospital, use of non‐invasive mechanical ventilation and in‐hospital mortality.
Statistical analysis
Categorical variables were expressed as counts (frequency) and continuous variables as mean (±standard deviation) or median (interquartile range). Patients with viral and bacterial pneumonia were compared by χ2‐test (or Fisher's exact test) for categorical variables, and by Student's t‐test (or Mann–Whitney U‐test) for continuous variables. Covariates with P ≤ 0.05 were selected into logistic regression analysis for independent predictors of viral pneumonia. Two‐tailed tests with a significance level of 5% (α = 0.05) were used for all analyses. The Statistical Package for Social Sciences 13.0 (spss 13.0; SPSS, Chicago, IL, USA) was used for statistical analysis.
Results
Baseline characteristics
We screened 612 patients, and 124 patients were excluded for the following reasons: on nasogastric tube feeding (16), recent hospitalization (91) and predominant congestive heart failure (17). The study population consisted of 488 patients. Table 1 shows their baseline characteristics.
Table 1.
Demographic data, clinical characteristics, laboratory investigation and pneumonia severity of the study population
| Demographics | |
| Age, years (mean ± SD) | 81.0 ± 7.9 |
| Female sex, n (%) | 206 (42.2%) |
| Nursing home residents, n (%) | 116 (23.8%) |
| Charlson's Comorbidity Index, median (IQR) | 2 [1–3] |
| Katz score, median (IQR) † | 6 [2–6] |
| Comorbid illnesses, n (%) | |
| Diabetes mellitus | 140 (28.7%) |
| Congestive heart failure | 65 (13.3%) |
| Ischemic heart disease | 78 (16.0%) |
| Chronic lung diseases | 186 (38.1%) |
| Cerebrovascular accident | 93 (19.1%) |
| Chronic kidney disease | 54 (11.1%) |
| Chronic liver disease | 12 (2.5%) |
| Dementia | 124 (25.4%) |
| Active malignancy | 36 (7.4%) |
| Chest symptoms, n (%) | |
| Shortness of breath | 249 (51.0%) |
| Cough | 335 (68.6%) |
| Expectoration | 254 (52.0%) |
| Hemoptysis | 89 (18.2%) |
| Chest pain | 187 (38.3%) |
| Physical findings | |
| Fever, n (%) | 251 (51.4%) |
| Hypotension, n (%) | 19 (3.9%) |
| Tachypnoea, n (%) ‡ | 68 (13.9%) |
| MAC, cm (mean ± SD) § | 23.1 ± 3.6 |
| Confusion, n (%) ¶ | 99 (20.3%) |
| Routine blood tests | |
| Sodium (mmol/L), median (IQR) | 138 (134–141) |
| Urea > 7 mmol/L, n (%) | 257 (52.7%) |
| Creatinine (μmol/L), median (IQR) | 94 (74–124) |
| Albumin (g/L), median (IQR) | 35 (31–38) |
| Leukocytosis, n (%) † † | 248 (50.8%) |
| Alanine transaminase (U/L), median (IQR) | 18 (13–29) |
| Chest radiographs, n (%) | |
| Multilobar consolidation | 79 (16.2%) |
| Pleural effusion | 33 (6.8%) |
| CURB score, n (%) | |
| 0–1 | 376 (77.0%) |
| 2–4 | 112 (23.0%) |
n = 488. †Katz Index measures six basic activities of daily living (ADL): bathing, dressing, toileting, transferring, continence and feeding. For each ADL, one point is given for full independence and zero for partial or total dependence. ‡Tachypnoea is defined as respiratory rate ≥30 per min. §Mid‐arm circumference (MAC) is the circumference of the mid‐point between the acromial process of scapula and the olecranon process of elbow of right upper arm. Left upper arm was chosen for patients with right hemipledgia or right upper limb amputation. ¶Confusion is defined as acute delirium or depressed conscious level. † †Leukocytosis is defined as white cell counts ≥10.8 × 109/L. CURB, confusion, blood urea nitrogen, respiratory rate and low blood pressure; IQR, interquartile range.
Infective etiology
Descriptive analysis
Microbial investigations were ordered in 475 (97.3%) patients within 48 h after hospitalization. Sputum samples were collected in 294 (60.2%) patients. Patients with and without sputum specimens collected are compared in Table 2. Sputum routine microscopy, and culture and acid‐fast staining and culture were carried out in 263 (53.9%) and 241 patients (49.4%), respectively. NPA samples were collected in 453 (92.8%) patients. Blood cultures and paired serological tests for viral and atypical pathogens were carried out in 329 (67.4%) and 69 patients (14.1%), respectively. Urine antigen tests for L. pneumophila were ordered in 20 patients.
Table 2.
Comparison of patients with and without sputum samples collected
| With sputum (n = 294) | Without sputum (n = 194) | P‐value | |
|---|---|---|---|
| Demographics | |||
| Age, years (mean ± SD) | 79.7 ± 7.4 | 83.0 ± 8.1 | <0.001 |
| Female sex, n (%) | 109 (37.1%) | 97 (50%) | 0.005 |
| Nursing home residence, n (%) | 48 (16.3%) | 68 (35.1%) | <0.001 |
| CCI, median (IQR) | 1 (1–3) | 2 (1–3) | 0.005 |
| Katz score, median (IQR) | 6 (4–6) | 5 (1–6) | <0.001 |
| Comorbid illnesses, n (%) | |||
| Diabetes mellitus | 80 (27.2%) | 60 (30.9%) | 0.374 |
| Congestive heart failure | 31 (10.5%) | 34 (17.5%) | 0.026 |
| Ischemic heart disease | 46 (15.6%) | 32 (16.5%) | 0.802 |
| Chronic lung diseases | 129 (43.9%) | 57 (29.4%) | 0.001 |
| Cerebrovascular accident | 50 (17.0%) | 43 (22.2%) | 0.156 |
| Chronic kidney disease | 26 (8.8%) | 28 (14.4%) | 0.054 |
| Chronic liver disease | 11 (3.7%) | 1 (0.1%) | 0.033 |
| Dementia | 53 (18.0%) | 71 (36.6%) | <0.001 |
| Active malignancy | 24 (8.2%) | 12 (6.2%) | 0.413 |
| Chest symptoms, n (%) | |||
| Shortness of breath | 152 (51.7%) | 97 (50%) | 0.713 |
| Cough | 203 (69.0%) | 132 (68.0%) | 0.815 |
| Expectoration | 171 (58.2%) | 93 (47.9%) | 0.026 |
| Hemoptysis | 49 (16.7%) | 40 (20.6%) | 0.269 |
| Chest pain | 113 (38.4%) | 74 (38.1%) | 0.948 |
| Physical findings | |||
| Fever, n (%) | 158 (53.7%) | 93 (47.9%) | 0.209 |
| Hypotension, n (%) | 9 (3.06%) | 10 (3.06%) | 0.242 |
| Tachypnoea, n (%) | 35 (11.9) | 33 (17.0%) | 0.111 |
| MAC, cm,(mean ± SD) | 23.4 ± 3.5 | 22.7 ± 3.8 | 0.049 |
| Confusion, n (%) | 39 (13.3%) | 60 (30.9%) | <0.001 |
| Routine blood tests | |||
| Sodium (mmol/L), median (IQR) | 137 (134–140) | 138 (134–141) | 0.288 |
| Urea >7 mmol/L, n (%) | 137 (46.6%) | 112 (57.7%) | 0.016 |
| Creatinine (μmol/L), median (IQR) | 94 (75–118) | 96 (72–133) | 0.794 |
| Albumin (g/L), median (IQR) | 35 (32–38) | 34 (30–38) | 0.087 |
| Leukocytosis, n (%) | 148 (50.3%) | 100 (51.5%) | 0.794 |
| ALT (U/L) | 18 (13–28) | 19 (13–29) | 0.837 |
| Chest radiographs, n (%) | |||
| Multi‐lobar pneumonia | 39 (13.3%) | 40 (20.6%) | 0.031 |
| Pleural effusion | 19 (6.5%) | 14 (7.2%) | 0.745 |
| CURB score, n (%) | |||
| 0–1 | 240 (81.6%) | 136 (70.1%) | |
| 2–4 | 54 (18.4%) | 58 (29.9%) | 0.003 |
ALT, alanine transaminase; CCI, Charlson's Comorbidity Index; CURB, confusion, blood urea nitrogen, respiratory rate and low blood pressure; MAC, mid‐arm circumference.
Sputum routine bacteriology was positive in 78 patients (29.7%). Five patients had co‐infections with two bacteria. The most frequent bacteria were H. influenzae (31), P. aeruginosa (14), Klebsiella spp. (8) and S. pneumoniae (6). A total of 14 patients had infection with Mycobacterium tuberculosis. Concomitant infection with Klebsiella spp was found in one of them. Multidrug‐resistant bacteria were grown in sputum samples of six patients: methicillin‐resistant S. aureus (MRSA; 3) and extended‐spectrum beta‐lactamase (ESBL)‐producing Escherichia coli (3). Atypical mycobacterial infection was likely a result of contamination and not counted as infective cause. They were identified in four patients: M. fortuitium (2) and M. Kanassi (2).
NPA samples were positive in 50 patients (10.2%). The most common viruses were influenza A virus (20), H1N1 (human swine flu) virus (8) and RSV (16). Co‐infections with one bacterium were present in 10 patients.
Blood cultures were positive in 12 patients (3.6%). The isolated organisms were believed to be the culprit pathogens for pneumonia in seven patients: Klebsiella spp. (3), S. pneumoniae (2), P. aeruginosa (1) and Acinetobacter spp. (1). Four patients had identical bacteria grown in their sputum cultures: Klebsiella spp. (2) and S. pneumoniae (2). The other three patients had negative sputum culture. The remaining five patients had skin flora identified in their blood cultures: diphtheroids (3) and Bacillus species (2).
Serology was positive in three patients: M. pneumoniae (1), C. pneumoniae (1) and influenza A virus (1).
One patient had infection with L. pneumophila diagnosed by a urine antigen test.
Establishing etiological diagnosis
Infective causes were found in 137 patients (28.1%): 34 nursing home residents and 103 community‐living older adults (29.3% vs 27.7%, P = 0.734). The results are summarized in Figures 1 and 2. Bacteriology was established mostly by sputum culture and virology by NPA tests. Bacterial, viral and mixed infections were detected in 86 (17.6%), 41 (8.4%) and 10 patients (2.0%), respectively. They were found in 14, 19 and one nursing home residents as well as in 72, 22 and nine community‐living older patients, respectively. NHAP was most commonly caused by viral infection (55.9%). Meanwhile, bacterial infection was the most frequent (69.9%) cause of CAP in older patients living in the community.
Figure 1.
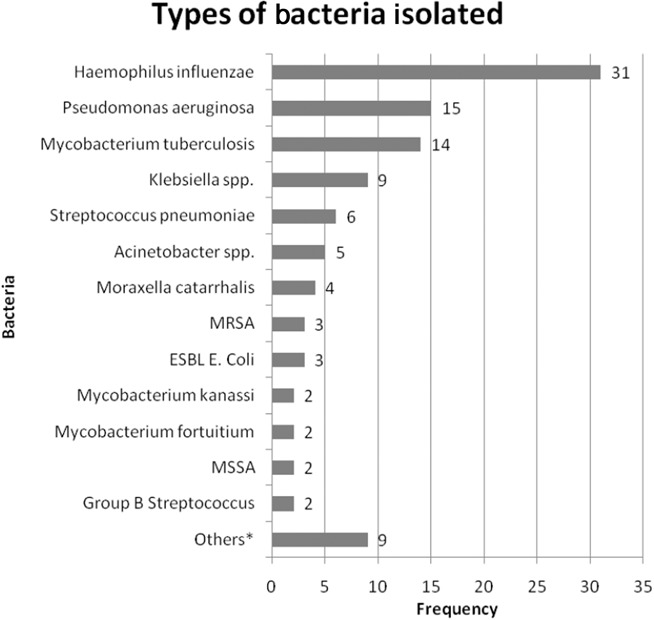
Summary of the types of bacteria isolated. *“Others” refers to one case for each of the following: Escherichia coli, Proteus, Group G Streptococcus, Citrobacter spp., Morganella morganii, Enterococcus, M. pneumoniae, C. pneumoniae and Legionella pneumophila serogroup type 1. ESBL, extended‐spectrum beta‐lactamase; MRSA, methicillin‐resistant Staphylococcus aureus; MSSA, methicillin‐sensitive Staphylococcus aureus.
Figure 2.
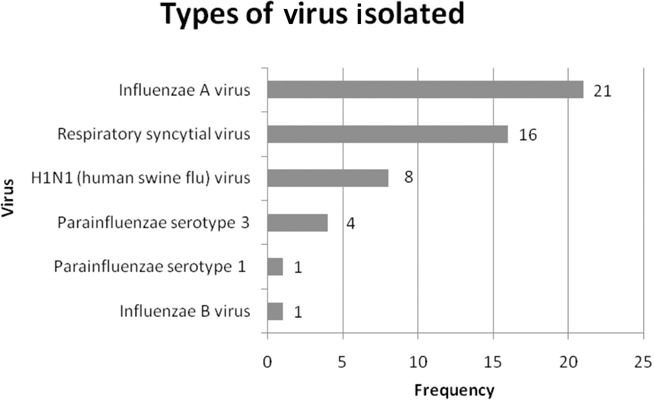
Summary of the types of viruses isolated.
Viral pneumonia
Table 3 shows the comparison between patients with confirmed viral and bacterial pneumonia. The viral group (51) consisted of patients with isolated viral infection (41) and mixed infections (10). Univariate analysis showed that many factors were associated viral pneumonia. Compared with patients living in the community, nursing home residents had a higher mean age, were predominantly female and were more likely to have a cerebrovascular accident and confusion (data not shown). Thus, these factors were not selected into regression analysis. Other variables (nursing home residence, ischemic heart disease and leukocytosis) were then entered into backward stepwise logistic regression analysis. Independent predictors of viral pneumonia in older patients were nursing home residence (RR 3.056, P = 0.009) and absence of leukocytosis (RR 0.425, P = 0.026).
Table 3.
Comparison of patients with viral and bacterial pneumonia
| Viral (n = 51) | Bacterial (n = 86) | P‐value | |
|---|---|---|---|
| Demographics | |||
| Age, years (mean ± SD) | 83.2 ± 8.4 | 79.8 ± 7.6 | 0.015 |
| Female sex, n (%) | 29 (56.9%) | 24 (27.9%) | 0.001 |
| Nursing home residence, n (%) | 20 (39.2%) | 14 (16.3%) | 0.003 |
| CCI, median (IQR) | 2 (1–3) | 1 (1–3) | 0.072 |
| Katz score, median (IQR) | 6 (1–6) | 6 (4–6) | 0.077 |
| Comorbid illnesses, n (%) | |||
| Diabetes mellitus | 16 (31.4%) | 21 (24.4%) | 0.376 |
| Congestive heart failure | 10 (19.6%) | 9 (10.5%) | 0.134 |
| Ischemic heart disease | 12 (23.5%) | 8 (9.3%) | 0.023 |
| Chronic lung diseases | 17 (33.3%) | 42 (48.8%) | 0.076 |
| Cerebrovascular accident | 15 (29.4%) | 12 (14.0%) | 0.028 |
| Chronic kidney disease | 4 (7.8%) | 10 (11.6%) | 0.480 |
| Chronic liver disease | 0 (0%) | 3 (3.5%) | NC* |
| Dementia | 15 (29.4%) | 14 (16.3%) | 0.069 |
| Active malignancy | 6 (11.8%) | 8 (9.3%) | 0.646 |
| Chest symptoms, n (%) | |||
| Shortness of breath | 28 (54.9%) | 45 (52.3%) | 0.770 |
| Cough | 35 (68.6%) | 59 (68.6%) | 0.998 |
| Expectoration | 25 (49.0%) | 35 (40.7%) | 0.343 |
| Hemoptysis | 13 (25.5%) | 16 (18.6%) | 0.340 |
| Chest pain | 21 (41.2%) | 37 (43.0%) | 0.833 |
| Physical findings | |||
| Fever, n (%) | 34 (66.7%) | 44 (51.2%) | 0.076 |
| Hypotension, n (%) | 0 (0%) | 4 (4.7%) | NC* |
| Tachypnoea, n (%) | 8 (15.7%) | 8 (9.3%) | 0.261 |
| MAC, cm (mean ± SD) | 22.5 ± 2.9 | 23.2 ± 3.1 | 0.137 |
| Confusion, n (%) | 14 (27.5%) | 11 (12.8%) | 0.032 |
| Routine blood tests | |||
| Sodium (mmol/L), median (IQR) | 137 (133–140) | 138 (134–141) | 0.257 |
| Urea >7 mmol/L, n (%) | 21 (41.2%) | 48 (55.8%) | 0.098 |
| Creatinine (μmol/L), median (IQR) | 89 (77–117) | 99 (77–128) | 0.289 |
| Albumin (g/L), median (IQR) | 36 (33–38) | 35 (30–37) | 0.067 |
| Leukocytosis, n (%) | 17 (33.3%) | 47 (54.7%) | 0.016 |
| Neutrophil count (×109/l) | 7.8 (7.2–9.2) | 8.4 (7.7–10.4) | 0.456 |
| Lymphocyte count (×109/l) | 3.4 (3.1–3.9) | 2.9 (2.5–3.3) | 0.223 |
| ALT (U/L) | 19 (11–28) | 18 (12–30) | 0.755 |
| Chest radiographs, n (%) | |||
| Multilobar pneumonia | 7 (13.7%) | 13 (15.1%) | 0.824 |
| Pleural effusion | 3 (5.9%) | 5 (5.8%) | 0.987 |
| CURB score, n (%) | |||
| 0–1 | 41 (80.4%) | 69 (80.2%) | |
| 2–4 | 10 (19.6%) | 17 (19.8%) | 0.982 |
n = 137. *Non‐calculable. ALT, alanine transaminase; CCI, Charlson's comorbidity index; CURB, confusion, blood urea nitrogen, respiratory rate and low blood pressure; MAC, mid‐arm circumference.
In‐hospital mortality
In‐hospital mortality rates were not significantly different in patients with and without established etiology (14.6% vs 11.7%, P = 0.381) as well as those with isolated viral and bacterial pneumonia (17.1% vs 12.8%, P = 0.518). Patients with mixed infections and pneumonia of unknown etiology had in‐hospital mortality rates of 20.0% and 11.7% respectively.
Discussion
The present study showed that in‐hospital mortality was not related to the results of microbial investigation. GNB and tuberculosis were the most frequent bacterial isolates. Nursing home residence and absence of leukocytosis were independent predictors of viral pneumonia.
A small minority (2.7%) of patients did not undergo any microbial investigation during hospitalization. The rate of identifying the infective etiology in the present study was 28.1%, which was relatively low compared with those (28–53%) reported in the existing literature.2, 3, 4, 5, 6, 7, 8, 9 The present study was pragmatic in nature; so much so that microbial investigations were ordered for clinical care rather than research purposes. Sputum and blood culture samples were collected in over half of all patients, and NPA viral samples in over 90% of all patients. Serological test for viral and atypical pathogens, however, was carried out in only 14% of all patients. The low availability was explained by its limited usefulness in clinical management. First, NPA viral cultures are superior to serological tests in allowing a more accurate and rapid diagnosis of viral infections. Second, serological tests do not help in the immediate medical treatment, because empirical antibiotics for atypical bacterial pathogens might be started much earlier than the results of test are available. Third, serological tests require paired samples to be collected 10–14 days apart. The acute titers are collected shortly after admission, and the convalescence titers might not be collected because patients have recovered and been discharged. Despite their limitations, serological tests provide important epidemiological data on CAP caused by atypical pathogens (AP). The present study cannot evaluate the importance of AP in older patients hospitalized for CAP, which can be supplemented by the results of another prospective cohort study on adult patients hospitalized for CAP. That study found that M. pneumoniae and C. pneumoniae were the most common (97.8%) AP. The majority of atypical infections involved older patients (63.4%) with multiple medical comorbidities (41.8%). In comparison with bacterial pneumonia, AP infection resulted in lower 30‐day mortality (1.1% vs 6.6%, P = 0.04).12
In the present study, frail older patients with multiple comorbidities and poorer nutritional status, especially those with chronic cardiorespiratory diseases and dementia, were less likely to expectorate for bacteriology. Nursing home residents, with a preponderance of female sex and a higher mean age (results were not shown here), were less likely to produce sputum than their community‐living counterparts. The sickest (CURB ≥2) and dehydrated (plasma urea level >7 mmol/L) patients were also less able to produce sputum. Thus, expectoration is a good clinical stress test for older patients hospitalized for CAP.
Chronic lung diseases, such as chronic obstructive pulmonary diseases (COPD), bronchiectasis and asthma, was the commonest comorbidity of our study population. These diseases predisposed our patients to infection with GNB.10, 11 Our findings were consistent with another study of our center that H. influenzae (26.0%), P. aeruginosa (5.5%) and S. pneumoniae (3.4%) were the commonest bacterial isolates in routine sputum cultures of COPD patients admitted for CAP.13
Tuberculosis is an endemic, statutory notifiable respiratory disease in our locality. It was not surprising that it ranked third in the list of bacterial isolates. The conventional culture on the Löwenstein–Jensen medium usually takes 6–8 weeks to yield, so the diagnosis of tuberculosis infection can be easily delayed. We suggest that, in the places where tuberculosis is endemic, molecular tests such as polymerase chain reaction (PCR) of respiratory samples should be used to make rapid diagnosis within hours if pulmonary tuberculosis is highly suspected.14
In‐hospital mortality of CAP depends on the interaction among factors of patient, disease and treatment. Our group has shown that in‐hospital mortality was related to pneumonia severity and patients' factors (comorbidities and nutritional status).15 The present study added that it was not related to the results of microbial investigation. Pneumonia severity represents the end result of adding numerous disease factors. In other words, it is pneumonia severity, but not infective cause, that determines the case mortality of pneumonia.
Chest symptoms, physical signs and pneumonia severity (CURB score) can hardly differentiate viral from bacterial pneumonia. We found that nursing home residence and absence of leukocytosis were independent predictors of viral pneumonia. Respiratory viruses were the most common infective cause of NHAP. In general, respiratory viruses have a higher infectivity than bacteria. We hypothesize that the over‐crowded living environment in nursing homes contributes to viral spreading among frail older people with multiple comorbidities. This is why outbreaks of influenza‐like illness, caused by viral but not bacterial infection, are commonly reported in the influenza peak seasons. This theory needs to be proven in future studies. Furthermore, we propose that NPA viral tests should be ordered for every nursing home patient admitted for CAP because of infection control in both hospital and nursing homes. In our hospital, the results of NPA immunofluorescence tests are available within 12 h after collection. Patients are hospitalized for isolation if their NPA viral tests are positive. Empirical antiviral agents, such as oseltamivir, might be added if outbreaks of influenza‐like illness are reported in nursing homes. This helps avoid spreading viral infection in both hospital and nursing homes.
Absence of leukocytosis is one of the few biomakers that distinguish viral from bacterial pneumonia. Others included lower serum levels of C‐reactive protein and procalcitonin levels.16 However, their results might not be available within 1 day, limiting their usefulness in acute management of pneumonia.
In the past decade, several respiratory viruses have been drawing increasing attention from clinicians and researchers. These viruses are rhinovirus, coronavirus and human metapneumovirus (hMPV). Rhinovirus can be isolated in viral culture, but not serology; whereas coronavirus and hMPV can be detected by serology, but not viral cultures. However, all these tests take time to yield a diagnosis and are not helpful in immediate clinical management. Real‐time PCR tests are able to detect all of these viruses within hours. Outbreaks of respiratory tract infections in long‐term care facilities caused by hMPV and rhinoviruses were reported.17, 18, 19 These PCR tests are not routine investigations. We suggest that real‐time PCR tests of nasopharyngeal specimens should be carried out in all nursing home residents hospitalized for CAP because of infection control in hospital and nursing homes.
One of the strengths of the present study was its large sample size with the focus on older patients with the mean age of 80 years. Furthermore, NPA viral tests were carried out in over 90% of the study population, of which the results highlighted the importance of viral pneumonia in clinical practice.
The present study has a few limitations. First, the importance of AP was underinvestigated. Second, aspiration is an important cause of pneumonia in older patients. When patients were in an acute medical condition, we could accurately assess their premorbid swallowing ability. Third, we did not evaluate the relationship between influenza vaccination status and viral pneumonia. Fourth, further studies are required to compare the efficacy of influenza vaccination and NPA viral testing.
To conclude, routine NPA viral testing should be considered for older patients hospitalized for NHAP. In addition to antibiotic therapy, antiviral agents might be considered as empirical treatment if outbreaks of influenza‐like illness are reported in nursing homes.
Disclosure statement
No potential conflicts of interest were disclosed.
Acknowledgment
We express our thanks to Miss Kathy Chow for her statistical support.
References
- 1. Osler W. The Principles and Practice of Medicine. New York: D Appleton and Co, 1898; 109–112. [Google Scholar]
- 2. Zalacain R, Torres A, Celis R et al. Community‐acquired pneumonia in the elderly: Spanish multicentre study. Eur Respir J 2003; 21: 294–302. [DOI] [PubMed] [Google Scholar]
- 3. Vila‐Corcoles A, Ochoa‐Gondar O, Rodriguez‐Blanco T, Raga‐Luria X, Gomez‐Bertomeu F, EPIVAC Study Group . Epidemiology of community‐acquired pneumonia in older adults: a population‐based study. Respir Med 2009; 103: 309–316. [DOI] [PubMed] [Google Scholar]
- 4. Ruiz M, Ewig S, Marcos MA et al. Etiology of community‐acquired pneumonia: impact of age, comorbidity and severity. Am J Respir Crit Care Med 1999; 160: 397–405. [DOI] [PubMed] [Google Scholar]
- 5. Capelastegui A, Espana PP, Quintana JM et al. Development of a prognostic index for 90‐day mortality in patients discharged after admission to hospital for community‐acquired pneumonia. Thorax 2009; 64: 496–501. [DOI] [PubMed] [Google Scholar]
- 6. Venkatesan P, Gladman J, Macfarlane JT et al. A hospital study of community acquired pneumonia in the elderly. Thorax 1990; 45: 254–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. El‐solh AA, Sikka P, Ramadan F, Davis J. Etiology of severe pneumonia in the very elderly. Am J Respir Crit Care Med 2001; 163: 645–651. [DOI] [PubMed] [Google Scholar]
- 8. Kaplan V, Angus DC, Griffin MF, Clermont G, Watson RS, Linde‐zwirble WT. Hospitalized community‐acquired pneumonia in the elderly. Am J Respir Crit Care Med 2002; 165: 766–772. [DOI] [PubMed] [Google Scholar]
- 9. Song JH, Oh WS, Kang CI et al. Epidemiology and clinical outcomes of community‐acquired pneumonia in adult patients in Asian countries: a prospective study by the Asian network for surveillance of resistant pathogens. Int J Antimicrob Agents 2008; 31: 107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. BTS guidelines for the management of community‐acquired pneumonia in adults. Thorax 2001; 56 (Suppl 4): 1–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guidelines for the management of adults with community‐acquired pneumonia: diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med 2001; 163: 1730–1754. [DOI] [PubMed] [Google Scholar]
- 12. Lui G, Ip M, Lee N et al. Role of “atypical pathogens” among adult hospitalized patients with community‐acquired pneumonia. Respirology 2009; 14: 1098–1105. [DOI] [PubMed] [Google Scholar]
- 13. Ko FWS, Lam RKY, Li TST et al. Sputum bacteriology in patients hospitalized with acute exacerbation of chronic obstructive pulmonary disease and concomitant pneumonia in Hong Kong. Intern Med J 2005; 35: 661–667. [DOI] [PubMed] [Google Scholar]
- 14. Drobniewski FA, Caws M, Gibson A, Young D. Modern laboratory diagnosis of tuberculosis. Lancet Infect Dis 2003; 3: 141–147. [DOI] [PubMed] [Google Scholar]
- 15. Ma HM, Tang WH, Woo J. Predictors of in‐hospital mortality for older patients admitted for community‐acquired pneumonia. Age Ageing 2011; 40: 736–741. [DOI] [PubMed] [Google Scholar]
- 16. Ruuskanen O, Lahti E, Jennings LC, Murdoch DR. Viral pneumonia. Lancet 2011; 377: 1264–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boivin G, Serres GD, Hamelin ME et al. An outbreak of severe respiratory tract infection due to human metapneumovirus in a long term care facility. Clin Infect Dis 2007; 44: 1152–1158. [DOI] [PubMed] [Google Scholar]
- 18. Louie JK, Yagi S, Nelson FA et al. Rhinovirus outbreak in a long term care facility for elderly persons associated with unusually high mortality. Clin Infect Dis 2005; 42: 262–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hicks LA, Shepard CW, Britz PH et al. Two outbreaks of severe respiratory disease in nursing homes associated with rhinovirus. J Am Geriatr Soc 2006; 54: 284–289. [DOI] [PubMed] [Google Scholar]


