Abstract
Abstract
Porcine epidemic diarrhoea virus (PEDV) causes severe diarrhoea in neonatal suckling piglets with a high mortality. Maternal vaccines that can induce lactogenic immunity to protect suckling piglets via colostrums and milk are pivotal for the prevention and control of PEDV infection in neonatal suckling piglets. In this study, a group of pregnant sows were first orally immunized with coated PEDV‐loaded microspheres and boosted with killed PEDV vaccines (heterologous prime‐boost). It has been detected that the levels of PEDV‐specific antibodies (IgG and IgA) in their sera and milks were higher than other negative groups (P < 0·001 or P < 0·05). Furthermore, it has been proved by the neutralization assay that the induced antibodies could significantly inhibit virus infection as compared to other negative groups (P < 0·01 or P < 0·05). Importantly, after PEDV challenge, more than 90% of the suckling piglets delivered by the sows in the heterologous prime‐boost group were completely protected. Overall, the results show that ‘heterologous prime‐boost’ form is an efficient and effective way to provide protection for suckling piglets against PEDV through lactogenic immunity.
Significance and Impact of the Study
As a widespread swine pathogen, PEDV affects the swine industry enormously. It causes enteritis in swine of all ages and is often fatal in neonatal piglets. Our data show that pregnant sows were immunized with ‘coated PEDV‐loaded microspheres + killed PEDV vaccines’ (heterologous prime‐boost immunization) could protect more than 90% suckling piglets delivered by the sows against the virus. These findings provide a new model of developing safe and effective immunizations for newborn animals against established and emerging enteric infections.
Keywords: coated PEDV‐loaded microspheres, heterologous prime and boost, pig, porcine epidemic diarrhoea virus (PEDV), vaccine
Significance and Impact of the Study: As a widespread swine pathogen, PEDV affects the swine industry enormously. It causes enteritis in swine of all ages and is often fatal in neonatal piglets. Our data show that pregnant sows were immunized with ‘coated PEDV‐loaded microspheres + killed PEDV vaccines’ (heterologous prime‐boost immunization) could protect more than 90% suckling piglets delivered by the sows against the virus. These findings provide a new model of developing safe and effective immunizations for newborn animals against established and emerging enteric infections.
Introduction
Porcine epidemic diarrhea (PED), an acute and highly contagious enteric disease only seen in pigs, is caused by Porcine epidemic diarrhoea virus (PEDV). PEDV, a member of the genus Alphacoronavirus within the family Coronaviridae (Pensaert and de Bouck 1978), is an enveloped, single‐stranded, positive‐sense RNA virus with a genome of appropriately 28 kb in length (Pensaert and de Bouck 1978). Its genome is sequentially composed of 5' untranslated region (UTR), open reading frame 1a/1b (ORF1a/1b), spike (S), ORF3, envelope (E), membrane (M), nucleocapsid (N) and 3' UTR (Liu et al. 2017). The clinical signs of PED are characterized by severe watery diarrhea with subsequent dehydration in all ages of swine, and a high mortality rate in suckling piglets (Stevenson et al. 2013). A case of PED was first reported in Belgium in 1978, and since then the disease has been rapidly spreading to other regions in Europe and Asia (Pensaert and de Bouck 1978; Song and Park 2012). Since 2010, a large number of PED outbreaks have been reported in China, with a mortality rate of 50–100% among infected suckling piglets (Sun et al. 2016). In 2013, PED spread rapidly to the United States, causing high rates of death among piglets and substantial economic losses to the porcine industry there (Vlasova et al. 2014).
It has been widely acknowledged that multiple immunizations (the ‘prime‐boost’) are indispensable for a vaccine to be effective. This means that the immunized subjects have to go through several immunizations before ideal efficacy can be expected. Research has proved that in many cases, priming immunizations and boost immunizations (heterologous prime‐boost) using different types of vaccines targeting the same antigens, is more immunogenic than using the same types of vaccines (homologous prime‐boost) (Lu 2009). The efficacy of the heterologous prime‐boost has been further proved by Wang et al. in their study where the combination of a fusion expression plasmid of CTLA4 and (Porcine Reproductive and Respiratory Syndrome Virus) PRRSV GP5 and inactivated PRRSV vaccines in the ‘prime‐boost’ format are found to be able to induce significant humoral and cellular responses (Wang et al. 2013). In the study of Pan et al. after sequentially immunized with a haemagglutinin (HA) DNA vaccine and an inactivated avian influenza (AI) vaccine, the Specific‐Pathogen Free (SPF) chickens were completely protected after intranasally challenged with H9N2 AIV (Pan et al. 2009). Furthermore, it has been supported by the study of Wang et al. that the use of HA DNA vaccines as the prime combined with inactivated flu vaccines as the boost is more effective in eliciting protective antibody responses than the use of DNA vaccines or inactivated vaccines alone (Wang et al. 2008). In addition, several other studies that also used the ‘heterologous prime‐boost’ strategy have demonstrated better enhanced immune responses in the ‘heterologous prime‐boost’ group compared with the use of only one type of vaccines alone (Schneider et al. 2001; Boyer et al. 2005; Ranasinghe et al. 2006), indicating that the heterologous prime‐boost is indeed a more effective way of immunization.
Although coated PEDV‐loaded microspheres could serve as an effective way to induce PEDV‐specific immunity in weaned pigs (Wen et al. 2018), detailed information about the protection of this oral vaccine against PEDV remains unclear. In this study, we used the ‘heterologous prime‐boost’ strategy to investigate whether using coated PEDV‐loaded microspheres and inactivated PEDV vaccines respectively as the priming immunizations and the boost immunizations could induce specific immune responses in pregnant sows to provide protection for neonatal suckling piglets against PEDV.
Results and discussion
As a widespread swine pathogen, PEDV affects the swine industry enormously. It causes enteritis in swine of all ages and is often fatal in neonatal piglets (Stevenson et al. 2013). Some reports show that the only effective way to protect neonatal suckling piglets from PEDV infection is maternal vaccines through lactogenic immunity (Chattha et al. 2015). In this study, coated PEDV‐loaded microspheres combined with inactivated PEDV vaccines were used to immunize pregnant sows to protect their newborn piglets against PEDV infection.
In our previous study, we found that coated PEDV‐loaded microspheres induced PEDV‐specific immunity via oral administration one time in weaned piglets (Wen et al. 2018). It is not unusual that multiple immunizations (i.e. ‘prime‐boost’) are required for many vaccines to be successful (Lu 2009). To enhance the immune responses against PEDV, we have tried the prime‐boost immunization regimen. Pregnant sows were primed with coated PEDV‐loaded microspheres and then boosted with coated PEDV‐loaded microspheres or inactivated PEDV vaccines. As shown in Fig. 1, we detected higher levels of PEDV‐specific IgG in serum samples collected from sows in the ‘coated PEDV‐loaded microspheres + inactivated vaccines’ group at day 35 after the first immunization (P < 0·05), compared to other negative controls (Fig. 1a). Moreover, we also observed higher PEDV‐specific IgA titres in milk samples collected from sows in the ‘coated PEDV‐loaded microspheres + inactivated PEDV vaccines’ group (P < 0·001), compared to other negative groups (Fig. 1b), indicating the prime‐boost strategy can enhance humoral responses in pregnant sows. In addition, we also found that the pregnant sows in the group of ‘coated PEDV‐loaded microspheres + inactivated PEDV vaccines’ are with significantly higher levels of antibodies compared with the pregnant sows boost immunized with coated PEDV‐loaded microspheres, indicating that the heterologous prime‐boost immunization can be more immunogenic than the homologous prime‐boost. This result is supported by other similar studies (Wang et al. 2008; Pan et al. 2009). Virus neutralizing antibodies are very important for the protective immunity against PEDV, as it can inhibit PEDV replication in intestinal epithelial cells by blocking PEDV attachment and internalization in the cells (Li et al. 2017). Considering that neutralizing antibodies play pivotal roles in protecting neonates from enteric infections, we tested whether serum and milk samples collected from the sows in different vaccines groups can inhibit PEDV infection in target cells by detecting the number of neutralizing antibodies in these serum and milk samples. As shown in Fig. 2, sera collected from sows immunized by coated PEDV‐loaded microspheres + inactivated PEDV vaccines have higher neutralizing antibody titres than the sera collected from sows in other negative groups (P < 0·05 or P < 0·01) (Fig. 2a). Similarly, milk samples collected from the sows after their delivery also have higher neutralizing antibody titers in the ‘coated PEDV‐loaded microspheres + inactivated PEDV vaccines’ group (P < 0·05), compared to other negative groups (Fig. 2b). Our data show that the titres of neutralizing antibodies in the serum and milk of the sows were significantly increased after they were immunized with inactivated PEDV vaccines. In addition, we found that all the inoculated sows and those in the control group did not show any clinical problems (data not shown), proving that the coated PEDV‐loaded microspheres are safe for sows.
Figure 1.
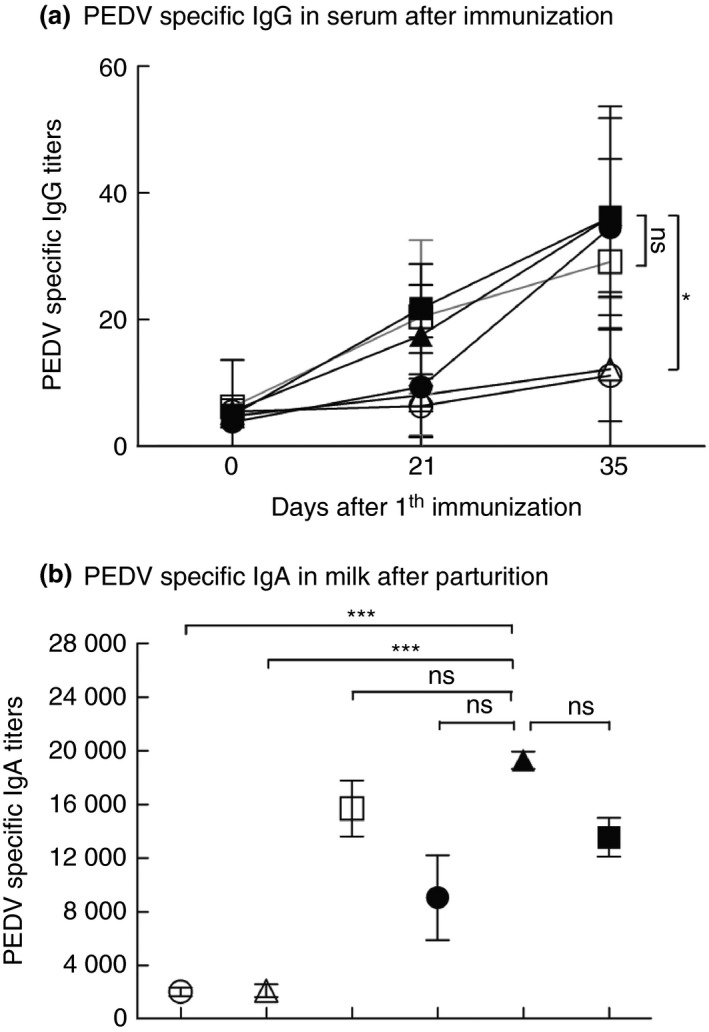
Specific anti‐PEDV antibody titres in the serum and milk collected from pregnant sows after prime‐boost immunizations. (a) Serum was collected from the pregnant sows of all groups on days 0, 21 and 35 post first immunization, and was assayed for anti‐PEDV‐specific IgG antibodies through ELISA. (b) Milk was collected from the pregnant sows of all groups on day 1 postfarrowing and was assayed for anti‐PEDV‐specific IgA antibodies through ELISA. Data are represented as mean ± SD, n = 5. * stand for P < 0·05, and *** stand for P < 0·001. Groups: (○) DMEM + DMEM; (△) PEDV + PEDV; (□) coated PEDV‐loaded microspheres + coated PEDV‐loaded microspheres; (●) PEDV + inactivated PEDV vaccines; (▲) coated PEDV‐loaded microspheres + inactivated PEDV vaccines; (■) inactivated PEDV vaccines + inactivated PEDV vaccines
Figure 2.
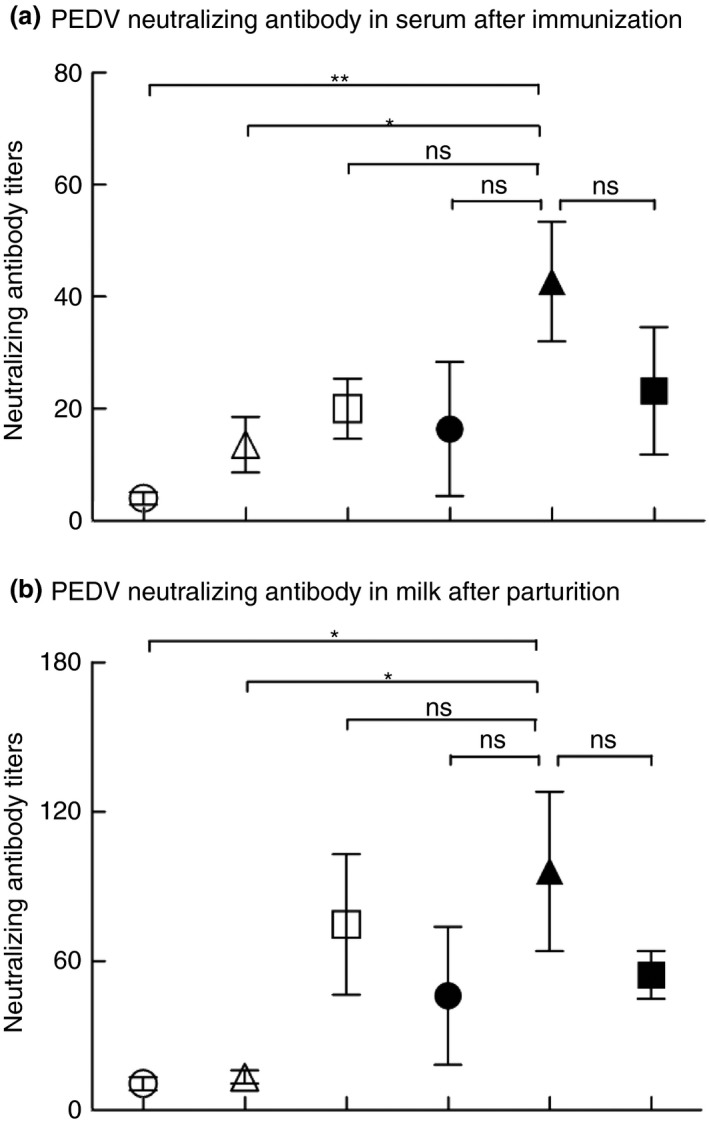
Neutralizing antibody titres in the serum and milk collected from the pregnant sows after prime‐boost immunizations. (a) Serum was collected from the pregnant sows of all groups on day 35 post first immunization and (b) milk was collected on day 1 postfarrowing and their neutralizing antibody titres were analysed. Neutralizing antibody titres were expressed as dilutability at 50% inhibition of PEDV infection in Vero cells. Data are represented as mean ± SD, n = 5. ** stand for P < 0·01 and * stand for P < 0·05. Groups: (○) DMEM + DMEM; (△) PEDV + PEDV; (□) coated PEDV‐loaded microspheres + coated PEDV‐loaded microspheres; (●) PEDV + inactivated PEDV vaccines; (▲) coated PEDV‐loaded microspheres + inactivated PEDV vaccines; (■) inactivated PEDV vaccines + inactivated PEDV vaccines
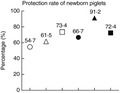
As PEDV can cause severe diarrhoea and a high mortality rate in suckling piglets (Stevenson et al. 2013), and the immune system of the neonatal suckling piglets are fragile and immature, maternal vaccines which can induce lactogenic immunity to protect suckling piglets via milk are pivotal for the prevention and control of enteric viral infection in newborn piglets (Wesley and Lager 2003; Chattha et al. 2015). In this study, all the suckling piglets delivered by the sows were challenged with PEDV via oral feeding, and we found that suckling piglets from the ‘coated PEDV‐loaded microspheres + inactivated PEDV vaccines’ group had the highest protection rate (91·2%) (Fig. 3) after the PEDV challenge without any clinical problem displayed, indicating that the heterologous prime‐boost immunization can most efficiently induce lactogenic immunity and protect suckling piglets against PEDV infection. However, there are still several important questions that need to be addressed. For example, what is the protective mechanism of the heterologous prime‐boost immunization? Can the protection rate in neonatal suckling piglets be increased by vaccines combine with adjuvants? Does the vaccination provide cross‐protection against other PEDV strains? The further elucidation of these questions will help us better control PED.
Figure 3.
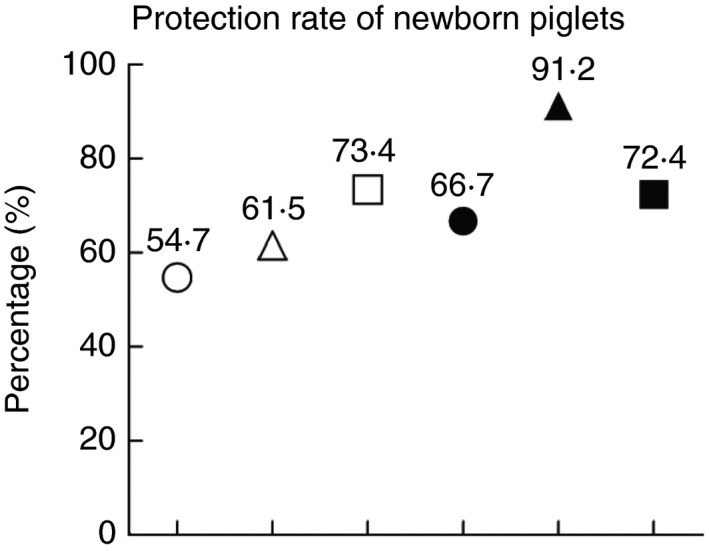
Protection efficacy of the prime‐boost immunizations in suckling piglets against the challenge of PEDV. Pregnant sows were first orally immunizated with attenuated PEDV vaccines or coated PEDV‐loaded microspheres or intramuscular injected with inactivated PEDV vaccines, and boosted with attenuated PEDV vaccines or coated PEDV‐loaded microspheres or inactivated PEDV vaccines, and the control group was orally inoculated with maintenance medium in the ‘prime‐boost’ form. All neonatal suckling piglets were orally challenged with a PEDV strain on the seventh day after delivery. The mortality rate of suckling piglets in different immunizated groups was recorded daily from the first day to the 10th day after challenge for protection rate analysis. Data are represented as mean ± SD, n = 5. Groups: (○) DMEM + DMEM; (△) PEDV + PEDV; (□) coated PEDV‐loaded microspheres + coated PEDV‐loaded microspheres; (●) PEDV + inactivated PEDV vaccines; (▲) coated PEDV‐loaded microspheres + inactivated PEDV vaccines; (■) inactivated PEDV vaccines + inactivated PEDV vaccines
In summary, our data show that the ‘coated PEDV‐loaded microspheres + inactivated PEDV vaccines’ immunization can induce higher levels of PEDV‐specific antibodies (IgG and IgA) and neutralizing antibodies in the serum and milk of treated sows than the other immunization. Importantly, after PEDV challenge, more than 90% suckling piglets delivered by the sows in the heterologous prime‐boost group were completely protected against the virus. Our findings provide a new model of developing safe and effective immunizations for newborn animals against established and emerging enteric infections.
Materials and methods
Cells and virus
Vero cells were obtained from ATCC (ATCC number: CCL‐81) (USA), and cultured in DMEM (Dulbecco's modified eagle medium) (Hyclone, Logan, UT) supplemented with penicillin (100 U ml−1), streptomycin (100 U ml−1) and 10% foetal bovine serum (FBS) (BOVOGEN, Melbourne, Australia). The maintenance medium for PEDV propagation was DMEM supplemented with 7 μg ml−1 trypsin (Gibco, Carlsbad, CA). PEDV strain Changtang, PEDV attenuated vaccines and inactivated vaccines were obtained from Wen's Foodstuffs Group Co, Ltd (Guangdong, China). Coated PEDV‐loaded microspheres were generated by centrifugal granulation‐fluidized bed coating as described previously (Wen et al. 2018).
Immunization of pregnant Landrace sows and collection of samples
The animal study was approved by the Institutional Animal Care and Use Committee of the Sun Yat‐sen University (Guangdong, China) and animals were treated in accordance with the regulations and guidelines of this committee. Thirty pregnant landrace sows were obtained from Wen's Foodstuffs Group Co, Ltd (Guangdong, China) and randomly divided into six groups (5 sows per group) and were housed in six separate rooms. All sows were maintained in our animal facility with food and water ad libitum for a minimum of 7 days before experimentation. Prior to inoculation, sows were confirmed negative for the major porcine enteric viruses (PDCoV, PEDV, TGEV, PRoV) by testing the rectal swabs collected from the sows as previously described (Xu et al. 2018). After 7‐day acclimation, sows in group 1 set as the control group were orally inoculated with 50 ml of maintenance medium twice in the ‘prime and boost’ form. Sows in group 2 and group 4 were orally vaccinated with 50 ml of maintenance medium containing a total of 1 × 106·5 TCID50 of attenuated PEDV vaccines at 79 days after pregnancy, and then respectively boosted with 50 ml of maintenance medium containing a total of 1 × 106·5 TCID50 of attenuated PEDV vaccines (oral immunization) or 2 ml of maintenance medium containing a total of 1 × 106·5 TCID50 of inactivated PEDV vaccines (intramuscular injection) at 100 days after pregnancy. Sows in group 3 and group 5 were orally vaccinated with 60 g coated PEDV‐loaded microspheres containing a total of 1 × 106·5 TCID50 of attenuated PEDV vaccines at 79 days after pregnancy, and then respectively boosted with 60 g coated PEDV‐loaded microspheres (oral immunization) or 2 ml of maintenance medium containing a total of 1 × 106·5 TCID50 of inactivated PEDV vaccines (intramuscular injection) at 100 days after pregnancy. Sows in group 6 were intramuscularly injected with 2 ml of maintenance medium containing a total of 1 × 106·5 TCID50 of inactivated PEDV vaccines twice in the ‘prime and boost’ form. After immunization, sows were observed daily for clinical signs such as vomiting, diarrhoea, lethargy and weight loss. Serum was collected from all pregnant sows on days 0, 21 and 35 after the first immunization for IgG analysis, and their milk was collected on day 1 after delivery for IgA analysis.
ELISA for the detection of PEDV‐specific antibodies
To measure the quantity of PEDV‐specific antibodies in vivo, the concentrations of IgG in serum or IgA in milk were detected by using commercial ELISA kits (BIONOTE, Korea) in accordance with the manufacturer’s instructions. The concentrations of IgG in serum were detected by using rabbit anti‐pig as secondary antibody (Invitrogen, Carlsbad, CA).
Virus neutralization assays
The neutralizing antibody titres of PEDV in sera after the second boost and in milks after delivery were examined according to the method put forward by Chunhua Li et al. with some modifications (Li et al. 2017). Briefly, the sera and milks of the sows were serial twofold diluted in serum‐free DMEM medium before mixing with an equal volume of 200 TCID50 PEDV Changtang strain and incubated for 1 h at 37°C. A positive control and a negative control were included on each plate. The mixture was added to washed (three times with sterile 1 × phosphate buffer saline (PBS)) Vero cell monolayers grown in microtitre plates (Nunc 96‐well tissue culture plate, Corning) and incubated for 1 h at 37°C. Cells were then washed again and incubated in the maintenance medium at 37°C in 5% CO2. After 2 days, CPE was observed using an inverted microscope and the neutralizing concentration was defined as the lowest concentration of antibodies in the serum and milk that prevented the occurrence of CPE.
Experimental infection with the PEDV Changtang strain in the suckling piglets
The effect of passive immunity was examined through the protection rate among the suckling piglets born by the pregnant sows in the previous experiments after being orally inoculated with the PEDV Changtang strain according to previous studies with some modifications (Kim and Chae 2003). Briefly, prior to inoculation, the newborn piglets were allowed to suckle in the first 7 days after birth. Afterwards, all piglets were orally challenged with 1 ml maintenance medium containing 1 × 105 TCID50 of the PEDV Changtang strain. The piglets were observed daily for clinical signs of vomiting, diarrhoea, lethargy, body loss. The mortality of suckling piglets born by sows in different immunizated groups was recorded daily 1 to 10 after challenge to calculate the protection rate.
Statistical analysis
Statistical comparisons were performed using GraphPad Prism software. The differences between the treatment group and the control group in antibodies (IgG, IgA and neutralizing antibody) were measured by the anova or Mann–Whitney accordingly.
Compliance with ethical standards
Ethical approval: The animal study was supervised by the Institutional Animal Care and Use Committee of the Sun Yat‐sen University (IACUC DD‐18‐0111) and used in accordance with regulation and guidelines of this committee.
Informed consent: Informed consent was obtained from all individual participants included in the study.
Author contributions
YC, ZW and ZX conceived and designed the experiments; ZW and ZX performed the experiments; YC, ZW and ZX analysed the data; QZ, WL, YW, YD and LC contributed reagents/materials/analysis tools; ZX wrote the paper; YC and ZW checked and finalized the manuscript. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that they have no conflict interest.
Acknowledgements
This work was supported by grants from National Key Research and Development Program, China (2016YFD0500101).
References
- Boyer, J.D. , Robinson, T.M. , Maciag, P.C. , Peng, X. , Johnson, R.S. , Pavlakis, G. , Lewis, M.G. , Shen, A. et al (2005) DNA prime Listeria boost induces a cellular immune response to SIV antigens in the rhesus macaque model that is capable of limited suppression of SIV239 viral replication. Virology 333, 88–101. [DOI] [PubMed] [Google Scholar]
- Chattha, K.S. , Roth, J.A. and Saif, L.J. (2015) Strategies for design and application of enteric viral vaccines. Annu Rev Anim Biosci 3, 375–395. [DOI] [PubMed] [Google Scholar]
- Kim, O. and Chae, C. (2003) Experimental infection of piglets with a Korean strain of porcine epidemic diarrhoea virus. J Comp Pathol 129, 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, C. , Li, W. , Lucio de Esesarte, E. , Guo, H. , van den Elzen, P. , Aarts, E. , van den Born, E. , Rottier, P.J.M. et al (2017) Cell attachment domains of the porcine epidemic diarrhea virus spike protein are key targets of neutralizing antibodies. J Virol 91, e00273–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , Zhang, Q. , Fang, Y. , Zhou, P. , Lu, Y. , Xiao, S. , Dong, Z. , Zhang, Y. et al (2017) Complete genome sequence of variant porcine epidemic diarrhea virus strain CH/HNZZ47/2016 isolated from suckling piglets in China. Genome Announc 5, e01744–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, S. (2009) Heterologous prime‐boost vaccination. Curr Opin Immunol 21, 346–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, Z. , Zhang, X. , Geng, S. , Cheng, N. , Sun, L. , Liu, B. , Huang, J. and Jiao, X. (2009) Priming with a DNA vaccine delivered by attenuated Salmonella typhimurium and boosting with a killed vaccine confers protection of chickens against infection with the H9 subtype of avian influenza virus. Vaccine 27, 1018–1023. [DOI] [PubMed] [Google Scholar]
- Pensaert, M.B. and de Bouck, P. (1978) A new coronavirus‐like partiele associated with diarrhea in swine. Arch Virol 58, 243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranasinghe, C. , Medveczky, J.C. , Woltring, D. , Gao, K. , Thomson, S. , Coupar, B.E. , Boyle, D.B. , Ramsay, A.J. et al (2006) Evaluation of fowlpox‐vaccinia virus prime‐boost vaccine strategies for high‐level mucosal and systemic immunity against HIV‐1. Vaccine 24, 5881–5895. [DOI] [PubMed] [Google Scholar]
- Schneider, J. , Langermans, J.A.M. , Gilbert, S.C. , Blanchard, T.J. , Twigg, S. , Naitza, S. , Hannan, C.M. , Aidoo, M. et al (2001) A prime‐boost immunisation regimen using DNA followed by recombinant modified vaccinia virus Ankara induces strong cellular immune responses against the Plasmodium falciparum TRAP antigen in chimpanzees. Vaccine 19, 4595–4602. [DOI] [PubMed] [Google Scholar]
- Song, D. and Park, B. (2012) Porcine epidemic diarrhoea virus: a comprehensive review of molecular epidemiology, diagnosis, and vaccines. Virus Genes 44, 167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson, G.W. , Hoang, H. , Schwartz, K.J. , Burrough, E.R. , Sun, D. , Madson, D. , Cooper, V.L. , Pillatzki, A. et al (2013) Emergence of Porcine epidemic diarrhea virus in the United States: clinical signs, lesions, and viral genomic sequences. J Vet Diagn Invest 25, 649–654. [DOI] [PubMed] [Google Scholar]
- Sun, D. , Wang, X. , Wei, S. , Chen, J. and Feng, L. (2016) Epidemiology and vaccine of porcine epidemic diarrhea virus in China: a mini‐review. J Vet Med Sci 78, 355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasova, A.N. , Marthaler, D. , Wang, Q. , Culhane, M.R. , Rossow, K.D. , Rovira, A. , Collins, J. and Saif, L.J. (2014) Distinct characteristics and complex evolution of PEDV strains, North America, May 2013‐February 2014. Emerg Infect Dis 20, 1620–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S. , Parker, C. , Taaffe, J. , Solorzano, A. , Garcia‐Sastre, A. and Lu, S. (2008) Heterologous HA DNA vaccine prime–inactivated influenza vaccine boost is more effective than using DNA or inactivated vaccine alone in eliciting antibody responses against H1 or H3 serotype influenza viruses. Vaccine 26, 3626–3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Zhao, H. , Ma, Z. , Wang, Y. and Feng, W.H. (2013) CTLA4 mediated targeting enhances immunogenicity against PRRSV in a DNA prime/killed virus boost strategy. Vet Immunol Immunopathol 154, 121–128. [DOI] [PubMed] [Google Scholar]
- Wen, Z. , Xu, Z. , Zhou, Q. , Li, W. , Wu, Y. , Du, Y. , Chen, L. , Zhang, Y. et al (2018) Oral administration of coated PEDV‐loaded microspheres elicited PEDV‐specific immunity in weaned piglets. Vaccine 36, 6803–6809. [DOI] [PubMed] [Google Scholar]
- Wesley, R. and Lager, K. (2003) Increased litter survival rates, reduced clinical illness and better lactogenic immunity against TGEV in gilts that were primed as neonates with porcine respiratory coronavirus (PRCV). Vet Microbiol 95, 175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Z. , Zhong, H. , Zhou, Q. , Du, Y. , Chen, L. , Zhang, Y. , Xue, C. and Cao, Y. (2018) A highly pathogenic strain of porcine deltacoronavirus caused watery diarrhea in newborn piglets. Virol Sin 33, 131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]


