The number of natural nest‐holes is considered a crucial element for cavity‐nesting bats and birds (Newton 1994, Berthinussen et al. 2014), especially in human‐modified landscapes where modern strategies of forest management have reduced the numbers of trees containing holes (Newton 1994, Remm et al. 2006, Myczko et al. 2014). Critically important are natural holes excavated by woodpeckers (Picidae), and they are preferred by hole‐nesting mammals and birds (Czeszczewik et al. 2008, Cockle et al. 2011). Moreover, even where holes occur, not all are of sufficient quality to be inhabited by mammals and birds (Czeszczewik et al. 2008), and hence organisms compete for the best holes (Flux & Flux 1992, Juskaitis 2006, Remm et al. 2006). Important factors include security, availability and access to foraging places, for example to open habitats (Mazgajski 2000, Smith 2006, Czeszczewik et al. 2008).
Among cavity‐nesting species, selection promotes aggression and fighting for holes, both at an intra‐ and inter‐species level. A particular example of a species with marked aggression directed mainly at other species is the Starling Sturnus vulgaris, even for freshly made holes of woodpeckers (Mazgajski 2000, Smith 2006) or small mammals (Flux & Flux 1992, Juskaitis 2006, Czeszczewik et al. 2008).
Interactions between cavity roosting and nesting bats and birds are especially interesting. Although natural holes made by birds and nest‐boxes are both occupied by bats (e.g. Berthinussen et al. 2014), to date the simultaneous occurrence of bats and birds has not been reported. However, we suggest that this is a frequent occurrence with potentially profound implications. We describe four cases of spring reproductive co‐occurrence of Noctule Bats Nyctalus noctula and Starlings in western Poland, and discuss the potential mechanisms explaining this phenomenon.
The observations were made in forests of the suburban area of the city of Poznań and in the Zielonka forest complex (52°27′–52°37′N, 16°49′–17°12′E) in western Poland in spring 2016. Woodlands cover 21% of the area and are mostly patchily distributed within the agricultural matrix and built‐up areas. Most woodlands are of Scots Pine Pinus sylvestris forests, but mixed and pure deciduous stands, mostly dominated by oaks (Quercus spp.), also occur. The major land cover types surrounding the woodlands are cereal crops and grasslands (Myczko et al. 2014), but lakes and ponds also occur.
Holes in the chosen forest stands were located before deciduous leafing and we also noted which were recently excavated woodpecker holes. Later, during the Starlings’ egg incubation phase, we inspected all these holes using a digital camera. A second inspection took place before fledging. During this second phase we also monitored holes in other forest stands. In total 672 holes were checked for the presence of bats and/or birds, and all holes were of woodpecker origin (mainly Great Spotted Woodpecker Dendrocopos major).
To monitor nest‐holes we used a modified internet camera Creative® Live!® Cam (Creative Labs Ltd, Dublin, Ireland) with additional LED lighting. We connected the camera to a laptop using a USB 2.0 Repeater Cable, 15 m DIGITUS® (Assmann Electronic GmbH, Lüdenscheid, Germany). The camera was attached to a telescopic stick to reach higher holes. For videos and still pictures we used Creative® Live!® Central 3® software, version 3.01.26 (Creative Technology Ltd, Singapore).
Visits with the camera started on 21 April 2016, and were repeated within 1 month. Starling chick age was determined according to the key by Kania (1983).
Among the 672 natural holes, 271 were occupied by birds, six by bats, and four simultaneously by Bats and birds. The same combination of species occurred in all cases of simultaneous occupation, i.e. Noctule Bats and Starlings. The first coexistence was recorded on 12 May 2016, when Starling chicks were already 14–20 days old. Noctule Bats (with Starling chick numbers in parentheses) occurred in the four holes in the following numbers: 4 (3), 6 (4) 6 (4) and 7 (2) individuals, and the bats sat on top of and moved among the Starling chicks (Fig. 1, Movie S1). All four Starling broods were successful with young fledged from the nest. The other holes occupied solely by Noctules contained 1, 2, 5, 6 and 14 individuals. In addition we found one hole occupied by 11 Daubenton's Bats Myotis daubentonii.
Figure 1.
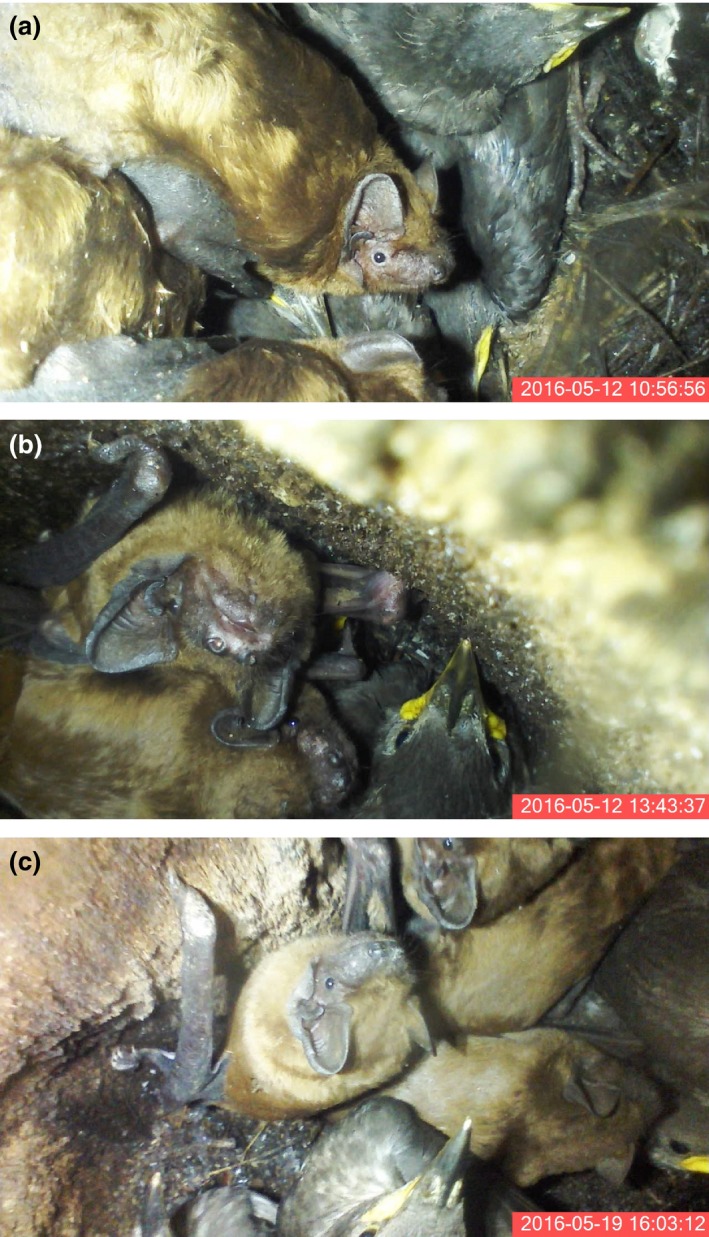
Noctule Bats and Starling chicks coexisting in a nest hole in the suburban area of Poznań (a, b) and in the Zielonka forest (c). [Colour figure can be viewed at wileyonlinelibrary.com]
The phenomenon of co‐occurrence of Noctules and Starling chicks is probably quite common, especially for Noctules, for which coexistence occurred in four out of nine holes occupied by bats. This is very surprising because previous publications on the interactions between bats and birds (reviewed in Kowalski & Lesiński 1994, Czeszczewik et al. 2008, Mikula et al. 2016) do not mention coexistence. However, the older papers were based mainly on data from artificial nest‐boxes, because detailed monitoring of natural holes is very difficult (Czeszczewik et al. 2008, Zawadzka et al. 2016), and any representative data for natural holes are not available. Here, we solved the monitoring problem by using modern technology which allows for fast collection of large amounts of high‐quality data from natural holes.
Noctules started co‐occupying holes with breeding Starlings in early May, which is a typical time for their arrival from wintering hibernation sites (Van Heerdt & Sluiter 1965, Ruczyński & Bogdanowicz 2008). This raises the question of why Noctules would choose holes already used by Starlings. A likely explanation is the thermal benefit, because holes occupied by Starlings are likely to be warmer, because incubation and then growing chicks transfer heat to the cavity (Biebach 1986, Ward et al. 1999). Additionally a study in forests in eastern Poland showed that maternity roosts chosen by Noctules during late pregnancy and lactation were warmer than unoccupied cavities (Ruczynski 2006, Ruczyński & Bogdanowicz 2008). We cannot also exclude the possibility that social information is obtained by bats from the presence of Starlings in the same natural holes. Occupying holes with successful Starlings could provide Noctules with information on the lack of predation during the breeding season, and hence the safety of the nest‐hole (Seppänen et al. 2007).
A second possibility which might explain this coexistence results from a shortage of safe and suitable nest‐holes because of intensive forest management. Currently almost all European forests are managed as intensive forestry, which significantly reduces the availability of natural holes (Newton 1994, Remm et al. 2006). Therefore, we can expect an increase in the phenomenon of coexistence of these two species. However, in the forest stands where we conducted our research, alternative nest‐holes were available. Our data show that more than half of the holes in the vicinity of where bats and Starlings coexisted were unoccupied.
Our data do not yet allow us to draw conclusions about the influence of coexisting species on reproductive success and fitness. However, these coexistences could have wider consequences. Coexistence could increase the transmission of parasites and diseases between species. Bird and bats are natural reservoirs of coronaviruses and influenza viruses (Tong et al. 2012, Chan et al. 2013). Therefore, coexistence could permit the mixing of different bird and mammal viruses for the generation of novel mutant, recombinant or reassortment of RNA viruses. Such a situation seems more likely given that both species are known to be hosts of influenza viruses – Noctules with H3N2 (L'Vov et al. 1979) and Starlings with H5N1 (Boon et al. 2007). Bats can also carry many other diseases (Petney et al. 2010, Smith & Wang 2013, Hall et al. 2016).
We would like to thank D. Czeszczewik, C. Mitrus, I. Ruczyński, G. Lesiński and T. Mazgajski for their comments on our results. Data were collected under licence to MŁ: GDOŚ Nr DZP‐WG.6401.09.3.2016.
Supporting information
Movie S1. Noctule Bats and Starling chicks coexisting in a nest hole. Four individual Noctule Bats and two Starling chicks are visible (a third chick was underneath its siblings).
References
- Berthinussen, A. , Richardson, O.C. & Altringham, J.D. 2014. Bat conservation: Global evidence for the effects of interventions (Vol. 5). Exeter: Pelagic Publishing Ltd. [Google Scholar]
- Biebach, H. 1986. Energetics of rewarming a clutch in starlings (Sturnus vulgaris). Physiol. Zool. 59: 69–75. [Google Scholar]
- Boon, A.C. , Sandbulte, M.R. , Seiler, P. , Webby, R.J. , Songserm, T. , Guan, Y. & Webster, R.G. 2007. Role of terrestrial wild birds in ecology of influenza A virus (H5N1). Emerg. Infect. Dis. 13: 1720–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, J.F.W. , To, K.K.W. , Tse, H. , Jin, D.Y. & Yuen, K.Y. 2013. Interspecies transmission and emergence of novel viruses: lessons from bats and birds. Trends Microbiol. 21: 544–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockle, K.L. , Martin, K. & Wesolowski, T. 2011. Woodpeckers, decay, and the future of cavity‐nesting vertebrate communities worldwide. Front. Ecol. Environ. 93: 77–382. [Google Scholar]
- Czeszczewik, D. , Walankiewicz, W. & Stanska, M. 2008. Small mammals in nests of cavity‐nesting birds: why should ornithologists study rodents? Can. J. Zool. 86: 286–293. [Google Scholar]
- Flux, J.E.C. & Flux, M.M. 1992. Nature red in claw: how and why starlings kill each other. Notornis 39: 293–300. [Google Scholar]
- Hall, J.S. , Ip, H.S. , TeSlaa, J.L. , Nashold, S.W. & Dusek, R.J. 2016. Experimental challenge of a peridomestic avian species, European starlings (Sturnus vulgaris), with novel influenza A H7N9 virus from China. J. Wildl. Dis. 52: 709–712. [DOI] [PubMed] [Google Scholar]
- Juskaitis, R. 2006. Interactions between dormice (Gliridae) and hole‐nesting birds in nestboxes. Folia Zool. 55: 225–236. [Google Scholar]
- Kania, W. 1983. Probability method of ageing of the passerine nestlings and its usage in breeding phenology investigations of Starling. Not. Orn. 24: 46–68. [Google Scholar]
- Kowalski, M. & Lesiński, G. 1994. Bats occupying nest boxes for birds and bats in Poland. Nyctalus 5: 19–26. [Google Scholar]
- L'Vov, D.K. , Easterday, B. , Hinshow, W. , Dandurov, I. & Arkhipov, P.N. 1979. Isolation of strains of the Hong Kong complex (H3N2) influenza virus from Nyctalus noctula bats in Kazakhstan. Vopr. Virusol. 4: 338–341. [PubMed] [Google Scholar]
- Mazgajski, T.D. 2000. Competition for nest sites between the Starling Sturnus vulgaris and other cavity nesters ‐ study in forest park. Acta Ornithol. 35: 103–107. [Google Scholar]
- Mikula, P. , Morelli, F. , Lučan, R.K. , Jones, D.N. & Tryjanowski, P. 2016. Bats as prey of diurnal birds: a global perspective. Mamm. Rev. 46: 160–174. [Google Scholar]
- Myczko, Ł. , Rosin, Z.M. , Skórka, P. & Tryjanowski, P. 2014. Urbanization level and woodland size are major drivers of woodpecker species richness and abundance. PLoS ONE 9: e94218.v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton, I. 1994. The role of nest sites in limiting the numbers of hole‐nesting birds: a review. Biol. Conserv. 70: 265–276. [Google Scholar]
- Petney, T.N. , Skuballa, J. , Pfäffle, M. & Taraschewski, H. 2010. The role of European starlings (Sturnus vulgaris L.) in the dissemination of ticks and tick‐borne pathogens in Germany. Syst. Appl. Acarol. 15: 31–35. [Google Scholar]
- Remm, J. , Lohmus, A. & Remm, K. 2006. Tree cavities in riverine forests: what determines their occurrence and use by hole‐nesting passerines?. Forest Ecol. Manag. 221: 267–277. [Google Scholar]
- Ruczynski, I. 2006. Influence of temperature on maternity roost selection by noctule bats (Nyctalus noctula) and Leisler's bats (N. leisleri) in Bialowieza Primeval Forest, Poland. Can. J. Zool. 84: 900–907. [Google Scholar]
- Ruczyński, I. & Bogdanowicz, W. 2008. Summer roost selection by tree‐dwelling bats Nyctalusnoctula and N. leisleri: a multiscale analysis. J. Mammal. 89: 942–951. [Google Scholar]
- Seppänen, J.T. , Forsman, J.T. , Mönkkönen, M. & Thomson, R.L. 2007. Social information use is a process across time, space, and ecology, reaching heterospecifics. Ecology 88: 1622–1633. [DOI] [PubMed] [Google Scholar]
- Smith, K.W. 2006. The implications of nest site competition from starlings Sturnus vulgaris and the effect of spring temperatures on the timing and breeding performance of great spotted woodpeckers Dendrocopos major in southern England. Ann. Zool. Fenn. 43: 177–185. [Google Scholar]
- Smith, I. & Wang, L.F. 2013. Bats and their virome: an important source of emerging viruses capable of infecting humans. Curr. Opin. Virol. 3: 84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong, S. , Li, Y. , Rivailler, P. , Conrardy, C. , Castillo, D.A. , Chen, L.M. , Recuenco, S. , Ellison, J.A. , Davis, C.T. , York, I.A. , Turmelle, A.S. , Moran, D. , Rogers, S. , Shi, M. , Tao, Y. , Weil, M.R. , Tang, K. , Rowe, L.A. , Sammons, S. , Xu, X. , Frace, M. , Lindblade, K.A. , Cox, N.J. , Anderson, L.J. , Rupprecht, C.E. & Donis, R.O. 2012. A distinct lineage of influenza A virus from bats. Proc. Natl. Acad. Sci. U. S. A. 109: 4269–4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Heerdt, P.F. & Sluiter, J.W. 1965. Notes on the distribution and behaviour of the noctule bat (Nyctalus noctula) in the Netherlands. Mammalia 29: 463–477. [Google Scholar]
- Ward, S. , Rayner, J.M.V. , Möller, U. , Jackson, D.M. , Nachtigall, W. & Speakman, J.R. 1999. Heat transfer from starlings Sturnus vulgaris during flight. J. Exp. Biol. 202: 1589–1602. [DOI] [PubMed] [Google Scholar]
- Zawadzka, D. , Drozdowski, S. , Zawadzki, G. & Zawadzki, J. 2016. The availability of cavity trees along an age gradient in fresh pine forests. Silva Fenn. 50: article id 1441. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie S1. Noctule Bats and Starling chicks coexisting in a nest hole. Four individual Noctule Bats and two Starling chicks are visible (a third chick was underneath its siblings).


